Abstract
The substantial burden of colorectal cancer and increasing trend in young adults highlight the importance of lifestyle modification as a complement to screening for colorectal cancer prevention. Several dietary and lifestyle factors have been implicated in the development of colorectal cancer, possibly through the intricate metabolic and inflammatory mechanisms. Likewise, as a key metabolic and immune regulator, the gut microbiota has been recognized to play an important role in colorectal tumorigenesis. Increasing data support that environmental factors are crucial determinants for the gut microbial composition and function, whose alterations induce changes in the host gene expression, metabolic regulation, and local and systemic immune response, thereby influencing cancer development. Here, we review the epidemiologic and mechanistic evidence regarding the links between diet and lifestyle and the gut microbiota in the development of colorectal cancer. We focus on factors for which substantial data support their importance for colorectal cancer and their potential role in the gut microbiota, including overweight and obesity, physical activity, dietary patterns, fiber, red and processed meat, marine omega-3 fatty acid, alcohol, and smoking. We also briefly describe other colorectal cancer-preventive factors for which the links with the gut microbiota have been suggested but remain to be mechanistically characterized, including vitamin D status, dairy consumption, and metformin use. Given limitations in available evidence, we highlight the need for further investigations in the relationship between environmental factors, gut microbiota, and colorectal cancer, which may lead to development and clinical translation of potential microbiota-based strategies for cancer prevention.
Over the past three decades, the incidence rate of colorectal cancer has dropped by almost 45% in the United States, from a peak of 66.3 in 1985 to 37.5 per 100,000 persons in 2013. This is mirrored by a similar decline for colorectal cancer mortality, dropping from its peak of 28.6 in 1976 to 14.1 per 100,000 in 2014.1 These trends are believed to be mainly attributable to increases in screening uptake (mainly colonoscopy) and reductions in certain risk factors (e.g., smoking and red meat consumption), with additional contributions from advances in treatment.2 Despite these advances, colorectal cancer remains the third most common cancer and the third leading cause of cancer death in each sex in the country.1 In 2018, an estimated total of 140,250 men and women will be diagnosed with colorectal cancer; 50,630 deaths will be attributable to the disease. A particularly alarming trend is the rising incidence of colorectal cancer in adults younger than 50 years old whose incidence has risen by 1.6% and mortality by 13% from 2000 to 2013–14.3 This increase has motivated the American Cancer Society to revise their recommended age to initiate colorectal cancer screening from 45 to 50 years old.4 Although the reasons for the increasing incidence of young-onset colorectal cancer have yet to be elucidated, the obesity epidemic and related changes in lifestyle patterns are thought to have played an important role. Therefore, the continued burden of colorectal cancer, particularly among young adults, highlight the critical need to consider novel prevention strategies beyond screening.
A variety of diet and lifestyle factors have been implicated in the development of colorectal cancer. Studies have consistently estimated that approximately 50–60% of incident colorectal cancer cases in the US can be prevented by lifestyle modification.5,6 Smoking, body fatness, alcohol drinks, and red and processed meat have been established to increase the risk of colorectal cancer, whereas physical activity and intake of dietary fiber, whole grains, dairy products, calcium supplements, vitamin D, and marine omega-3 fatty acid may lower disease risk.7 Furthermore, increasing data indicate a potential chemopreventive effect of metformin, an antidiabetic drug, for colorectal cancer.8
While the exact mechanisms through which each of these factors may affect colorectal cancer are likely to differ and largely remain to be elucidated, several lines of evidence suggest that the gut microbiome may represent a congregate mode of action mediating the relationship of environmental factors with colorectal cancer (Figure 1). First, the large intestine represents a vast microbial ecosystem, housing several trillion microbes that encode 100-fold greater unique genes than our own genome. The composition and function of this ecosystem appear to be largely shaped by environmental factors, particularly diet and lifestyle.9 Second, the gut microbiota plays an important role in nutrient processing and synthesis, and may affect colorectal cancer development through metabolite-mediated changes in the immune and metabolic signals.10 Finally, increasing data indicate that gut microbes are pivotal in integrating environmental cues with host physiology and metabolism, and may influence several biological processes critical to carcinogenesis, including the balance of intestinal cell proliferation and death, systemic and local immune homeostasis, and alterations of the host metabolic activities.11 Specifically for colorectal cancer, several microbes have been found to be differentially enriched in tumor versus normal tissues from the same host, or in fecal samples from patients with colorectal neoplasia versus healthy controls. Although the epidemiologic evidence remains limited and inconsistent, relatively consistent data indicate that Fusobacterium nucleatum (F. nucleatum) and Bacteroides fragilis are enriched in colorectal cancer, whereas butyrate-producing bacteria are depleted in cancer patients. The role of these and other microbes in colorectal cancer has been extensively reviewed elsewhere.12,13
Figure 1.
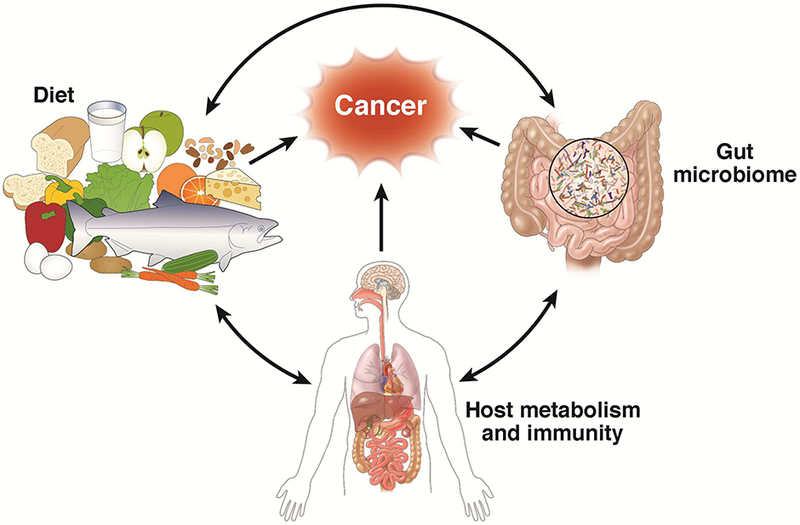
Framework for the interplay between diet and gut microbiota in colorectal cancer through influences on the host metabolism and immunity. Diet is a major determinant of the gut microbial community structure, whereas some bacteria in the gut are important for processing and synthesis of certain nutrients. The interaction between diet and gut microbiota may influence colorectal carcinogenesis through alterations in the host metabolism and immune system.
Herein, we review the evidence on how diet and lifestyle factors may influence colorectal cancer through gut microbiota-related mechanisms. We focus on factors for which substantial data support their importance for colorectal cancer and their potential role in the gut microbiota, including overweight and obesity, physical activity, dietary patterns, fiber, red and processed meat, marine omega-3 fatty acids, alcohol, and smoking. We also briefly describe other colorectal cancer-preventive factors for which the links with the gut microbiota have been suggested but remain to be mechanistically characterized, including vitamin D, dairy products, and metformin. For each factor, we review the epidemiologic evidence supporting its relationship with colorectal cancer and then summarize major findings about the interaction with the gut microbiota.
Overweight and obesity
Fueled by Western dietary patterns and sedentary lifestyle, the prevalence of obesity in US adults has almost tripled in the past few decades, rising from 13.4% in 1960–1962 to 37.7% in 2013–2014.14,15 Numerous epidemiologic studies have consistently associated higher body fatness in adulthood to increased risk of colorectal cancer (Table 1). Excess body weight has been estimated to contribute to approximately 5% of incident colorectal cancer cases in the US.5
Table 1.
Summary of evidence and potential mechanisms underlying the relationship between environmental factors, gut microbiota, and colorectal cancer.
| Risk factor | Estimated association with colorectal cancer [RR (95% Cl)]* |
Associated microbes | Potential microbiota-related mechanisms |
|---|---|---|---|
| Overweight and obesity |
1.05 (1.03–1.07) per 5 kg/m2 increase in body mass index |
↓ Bacteroidetes : Firmicutes ratio | Increased LPS levels; epigenetic changes in the colonic epithelial cells; increased production of DNA-damaging bile acids |
| Physical activity | 0.80 (0.72–0.88) comparing the highest to the lowest levels |
↑ Bacteroidetes : Firmicutes ratio; ↑ SCFAs-producing bacteria |
Increased production of SCFAs; changes in the metabolism of the microbiota. |
| Smoking | 1.26 (1.11–1.43) for current smokers and 1.18 (1.09–1.27) for former smokers, compared to never smokers† |
↓ Diversity ↑ Firmicutes, Actinobacteria; ↑ Bacteroidetes, Proteobacteria |
Altered mucin composition of the mucus layer and increased inflammatory response. |
| Diet | |||
| Western dietary pattern |
1.40 (1.26–1.56) comparing the highest to the lowest category of the Western diet score‡ |
↑ Bacteroides, Escherichia, and Acinetobacter; ↑ Prevotella |
Lower production of SCFAs from fiber deficiency |
| Fiber | 0.93 (0.87–1.00) per 10 g/day increase in dietary consumption |
↑ SCFAs-producing bacteria | Epigenetic regulation and immunomodulatory benefits of SCFAs |
| Red and processed meat |
1.12 (1.04–1.21) per 100 g/day increase in consumption |
↑ Mucin-degrading bacteria (e.g., Akkermansia muciniphila) |
Microbiota-dependent barrier dysfunction induced by heme iron; Increased production of secondary bile acids and hydrogen sulfide by microbiota associated with high red meat consumption |
| Marine omega-3 fatty acid |
0.76 (0.59–0.97) comparing the highest to the lowest category of biospecimen composition of marine omega-3 fatty acids § |
↑ Lactobacillus and Bifidobacterium; ↓ F. nucleatum and LPS- producing bacteria |
Preserved intestinal immune homeostasis due to increased abundance of immune-protective bacteria and reduced abundance of proinflammatory bacteria, as well as increased production of SCFAs and lipid mediators |
| Alcohol | 1.07 (1.05–1.08) per 10 g/day increase in consumption |
Dysbiosis; ↓ Bacteroidetes, Firmicutes, and butyrate- producing bacteria; ↑ Proteobacteria and Actinobacteria |
Intestinal bacterial overgrowth and hyperpermeability; reduced production of SCFAs; bacterial production of acetaldehyde from ethanol |
| Dairy products | 0.87 (0.83–0.90) per 400 g/day increase in consumption |
↓ Bilophila wadsworthia | Increased production of SCFAs and decreased abundance of proinflammatory pathobionts |
| Vitamin D | 0.92 (0.85–1.00) per 30 nmol/L increase in circulating vitamin D |
Variants in the vitamin D receptor gene are among the most significant loci associated with gut microbial composition |
Unclear |
| Metformin | 0.80 (0.64–1.00) comparing users to nonusers ‖ |
↑ Escherichia and Bifidobacterium; ↓ Intestinibacter |
Increased production of SCFAs. |
Abbreviations: Cl, confidence interval; LPS, lipopolysaccharides; RR, relative risk; SCFA, short-chain fatty acid.
Data are derived from the meta-analysis conducted by the World Cancer Research Fund/American Institute for Cancer Research (reference 7), unless stated otherwise.
Reference 128.
Reference 30.
Reference 84.
Reference 8.
Several metabolic and inflammatory factors, including insulin / insulin-like growth factor 1 signaling, sex hormones, adipokines, and systemic inflammation, have been proposed to underlie the relationship between obesity and colorectal cancer. On the other hand, the gut microbiota has emerged as an integral factor that modulates host metabolism and has been suggested to play a vital role in obesity-related metabolic alterations, including inflammation and insulin resistance.16 The complex, bidirectional relationship between obesity and the gut microbiota is indicated by the substantial changes in the gut microbial community induced by obesity and weight loss in animal and human studies, and recapitulation of obesity and its phenotypic features by fecal transplantation in gnotobiotic (germ-free) mouse models, as well as the positive association of antibiotic exposure in early life with childhood obesity.
Although obesity-induced changes in the gut microbiota may promote cancer development through modulation of microbe-derived proinflammatory molecules (e.g., lipopolysaccharide [LPS]) and metabolites (e.g., increased acetate and reduced butyrate) that impair gut barrier function and increase permeability,17 only recently has direct evidence emerged for the mechanisms through which the gut microbiota may mediate the relationship between obesity and cancer (Figure 2). Studies led by Wade and colleagues uncover a potential role of epigenetic alterations in the link between obesity, gut microbiota, and colorectal cancer.18–20 They showed that high-fat diet-induced obesity led to widespread remodeling of the acetylation landscape at presumptive cis-regulatory regions that likely influence the transcriptional responses integral to the initiation and progression of colon cancer.19 This epigenetic remodeling was found to be dependent on the gut microbiota as animals fed the same diet that received bacteria from non-obese donors did not show similar changes.20 Gene expression analysis indicated that the combination of high-fat diet and fecal transplantation induced a gene expression profile that had partial resemblance to that observed in human colorectal cancer.19 These findings highlight potential interactions between obesity and microbiome and their effects on the host epigenome, which prime putative enhancers in the host colon epithelium for malignant transformation. In addition to modifications of the enhancer landscape, obesity may also promote colorectal cancer through changes in DNA methylation,18 although the role of the gut microbiota in this process remains to be elucidated. Interestingly, the consequences of obesity-associated epigenetic changes may be different according to age; in young mice, obesity was associated with a colonic cellular switch favoring long-chain fatty acid oxidation that may increase the number of intestinal stem/stem-like cells; whereas in aged mice, obesity was associated with decreased expression of tumor suppressor genes and negative feedback regulators of pro-survival and pro-proliferation pathways that in turn prime for unrestrained signaling to accelerate the initiation and progression of colon tumorigenesis once oncogenic events occur.18 These findings may have implications for explaining the role of the obesity epidemic in the recent increase in the incidence of young-onset colorectal cancer.
Figure 2.
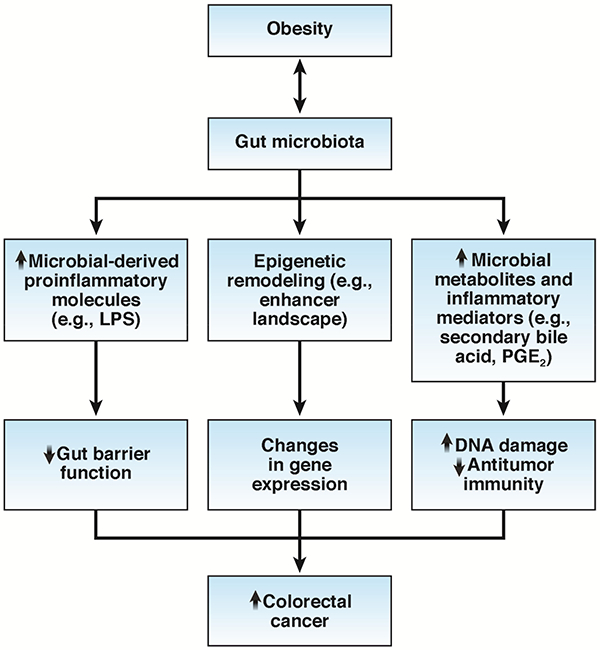
Gut microbiota-related mechanisms through which obesity may increase risk of colorectal cancer. Obesity-induced changes in the gut microbiota lead to 1) increased levels of microbial-derived proinflammatory molecules that disrupt the gut barrier function; 2) epigenetic remodeling and modification of the gene expression in colonic epithelial cells; 3) alterations in the gut microbial metabolites that can cause DNA damage and dampen antitumor immunity. LPS, Lipopolysaccharides; PGE2, Prostaglandin E2.
In addition to epigenetic mechanisms, changes in the gut microbial metabolites and constituents have also been implicated in the obesity-cancer link. Specifically, obesity enhances production of deoxycholic acid, a secondary bile acid that is produced solely by Gram-positive gut bacteria and known to cause DNA damage through reactive oxygen species production. Increased enterohepatic circulation of deoxycholic acid may promote hepatocellular carcinoma development by inducing cellular senescence and the senescence-associated secretory phenotype in hepatic stellate cells in the tumor microenvironment.21, 22 More recent evidence indicates that this effect requires cooperative induction of another Gram-positive gut microbial component, lipoteichoic acid, which upregulates the expression of prostaglandin-endoperoxide synthase 2 [also known as cyclooxygenase-2 (COX-2)] in deoxycholic acid-induced senescent hepatic stellate cells to promote cancer development in obese mice.23 As a critical enzyme in inflammation, prostaglandin-endoperoxide synthase 2 mediates production of prostaglandin E2 that suppresses antitumor immunity.23 Given the potential role of prostaglandin E2 and secondary bile acid in promoting colorectal cancer, further studies are needed to investigate whether microbial imbalance-induced metabolic change also mediates obesity-related tumor promotion in the colon.
Physical activity
Convincing evidence indicates that total and recreational physical activity reduces risk of colon cancer, whereas no benefit is found for rectal cancer. Recent animal evidence suggests that voluntary exercise can alter the composition of the gut microbiota and increase production of short-chain fatty acids (SCFAs).24–27 Exercise restored bacterial diversity in obese rats26 and increased the ratio of Bacteroidetes to Firmicutes, which was reduced in obesity in a manner that was proportional to the intensity of exercise in mice.25 Exercise also increased the relative proportion of butyrate-producing bacteria and the intestinal concentration of butyrate (Table 1).24, 25 However, whether these effects can be generalized to human remains largely unknown. A study compared the gut microbiome between 40 male elite professional rugby players and two control groups; one matched for athlete size with a comparable body mass index (n=23) and another reflecting the background age- and gender-matched population (n=23). It found that the athletes had a more diverse gut microbiota than either of the control groups and the increased gut microbiota diversity in athletes was driven by substantially higher exercise and protein intake.28 When the metabolic profile and functional capacity of the gut microbiota were examined, compared to control groups, athletes had relative increase in pathways related to amino acid and antibiotic biosynthesis, and carbohydrate metabolism, as well as increased levels of fecal metabolites (e.g., SCFAs) associated with enhanced muscle turnover and overall health.29
Dietary patterns
The hypothesis that a Western dietary pattern increases colorectal cancer risk originates from the observation that Japanese migrants to Hawaii acquire the same risk of colorectal cancer as that experienced by Caucasians in the US. This hypothesis is supported by subsequent epidemiologic studies in different countries showing an increased risk of colorectal cancer among individuals consuming a Western-style diet that is high in red and processed meats, refined grains, soda, and sweats, and low in fruits, vegetables and whole-grain products (Table 1).30 In contrast, a lower risk of colorectal cancer has been observed in individuals adhering to a prudent diet that is high in fruits, vegetables, whole grains, fish, soy, poultry, and low-fat dairy.30
A potential role of the gut microbiota in mediating the associations of dietary patterns with colorectal cancer risk is supported by the dramatic difference in the gut microbial structures and metabolite profiles between populations consuming different diets. The gut microbiota of rural Africans, whose diet is rich in fiber and low in fat, is characterized by a predominance of Prevotella genus that is involved in starch, hemicellulose, and xylan degradation, whereas the American microbiota is predominated by Bacteroides genus with a higher abundance of potentially pathogenic proteobacteria, such as Escherichia and Acinetobacter.31 These microbial structural differences parallel the lower colorectal cancer rates in Africa than Western countries. Similar differences exist in the profile of fecal metabolites, with SCFAs higher in native Africans and secondary bile acids higher in African Americans. These observational findings have been supported by an interventional study demonstrating that switching African Americans to a high-fiber, low-fat diet for 2 weeks increases production of SCFAs, suppresses secondary bile acid synthesis, and reduces colonic mucosal inflammation and proliferation biomarkers of cancer risk.32 In contrast to the pro-cancer effect of secondary bile acids, SCFAs have been shown to protect against colorectal cancer by their beneficial effects on the immune and metabolic pathways, as detailed below in the section for Fiber.
Recent mechanistic studies suggest that impairment of the colonic mucus layer, a physical barrier that separates trillions of gut bacteria from the host, may represent a potential mechanism through which a Western diet may interact with the gut microbiota to promote carcinogenesis.33, 34 A Western diet slows mucus growth rate and increases penetrability of the colonic mucus barrier, and this effect co-occurs with the shifts in microbial community characterized by gradual decrease of SCFA-producing bacteria, such as Bifidobacterium and Bacteroidales family S24–7, and increases in Firmicutes.33 Transplantation of the microbiota from chow-fed mice to Western-diet-fed mice restored the gut microbial structure and the growth and penetrability of the inner colonic mucus.
For prudent diet, we recently reported that its beneficial association with colorectal cancer was much stronger for tumors enriched with F. nucleatum.35 F. nucleatum is the most studied bacteria for colorectal cancer and clinical data have consistently implicated F. nucleatum in colorectal cancer.36–45 High abundance of F. nucleatum in tumor tissue has also been associated with poor survival in patients with established colorectal cancer.45, 46 Experimental evidence supports that F. nucleatum may promote colorectal cancer development and progression by activating the β-catenin pathway and potentiating tumoral immune evasion through recruitment of immunosuppressive myeloid cells and inhibition of natural killer cell function.47–49 Higher abundance of F. nucleatum in colorectal cancer tissue has been associated with lower density of tumor-infiltrating CD3+ T cells,50 supporting a role of F. nucleatum in attenuating antitumor immunity. Given the immunomodulatory benefits of some dietary factors (e.g., fiber and marine omega-3 fatty acid), it is possible that a healthy dietary pattern may diminish the carcinogenic effects of F. nucleatum by restoring effective immunosurveillance against malignant transformation and distant migration. Indeed, a recent dietary cross-over study observed a marked increase in stool F. nucleatum levels after rural Africans were switched to a low-fiber, high-fat diet and a decrease in F. nucleatum among African Americans who changed to a high-fiber, low-fat diet.32
Fiber
Despite the longstanding hypothesis that a high-fiber diet may protect against colorectal cancer by minimizing exposure to intestinal carcinogens, epidemiologic studies associating dietary fiber intake with subsequent risk of colorectal cancer have yielded inconsistent results.51 This inconsistency may be related to several factors, including variable degree of confounding control across studies (those with more rigorous adjustment for other dietary factors tend to report null results), large variations in fiber consumption across populations (a threshold effect has been reported in some studies for fiber intake in relation to colorectal cancer), variation in dietary sources of fiber (compared to other sources, cereal fiber has been more strongly and consistently associated with lower risk of colorectal cancer), and heterogeneity across tumor subtypes.51 Similarly, randomized clinical trials (RCTs) testing fiber supplementation among patients with a history of colorectal polyps also reported inconsistent findings.52–57 These inconsistencies may be related to the variation in the compliance and duration of the intervention and length of follow-up period.51 Nonetheless, based on existing evidence, the most recent expert report from the World Cancer Research Fund and American Institute for Cancer Research in 2017 concludes that there is probable evidence that consumption of foods containing dietary fiber protects against colorectal cancer (Table 1).7
Fiber is the major substrate of gut bacterial fermentation that produces a family of beneficial metabolites, the so-called SCFAs, which include butyrate, acetate, and propionate (Figure 3). Some,58 but not all,59 studies have shown that higher fiber intake enriches Lactobacillus spp. and butyrate-producing bacteria in the gut, such as Bifidobacterium, Clostridium, Anaerostipes, Eubacterium, and Roseburia species, and increases production of SCFAs. Laboratory data support the benefit of SCFAs for colorectal cancer. Studies using gnotobiotic mouse models have provided compelling evidence that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner via inhibition of histone deacetylase.60 The combination of high-fiber diet and butyrate-producing bacterium Butyrivibrio fibrisolvens reduced colorectal tumor incidence and multiplicity, whereas neither B. fibrisolvens nor high fiber had a protective effect on their own. Therefore, the between-individual variation in the gut microbiota may be another potential explanation for the inconsistency in epidemiologic findings relating fiber intake to colorectal cancer risk. Indeed, we recently showed that both whole grains and a prudent diet that is high in fiber were associated with lower risk of colorectal tumors that had detectable F. nucleatum, whereas no association was found for tumors without F. nucleatum.35 Future studies are warranted to test whether the variation in SCFA-producing bacteria may also modify the relationship between fiber intake and colorectal cancer risk.
Figure 3.
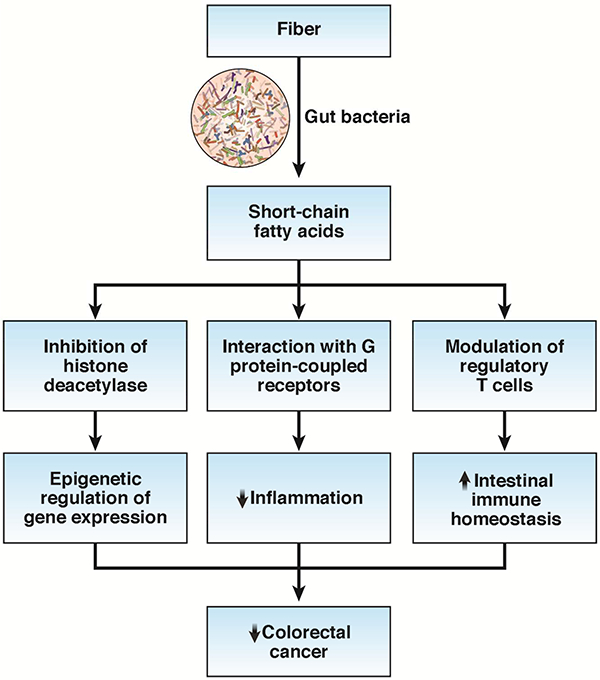
Potential benefits of short-chain fatty acids that may contribute to the anticancer effects of fiber. Short-chain fatty acids may epigenetically modulate expression of numerous cancer-related genes through inhibition of the activity of histone deacetylase, suppress inflammation by interaction with the G protein-coupled receptors (e.g., GPR43, GPR109a) at the colonic epithelial cell surface, and support intestinal immune homeostasis by modulation of the function of regulatory T cells.
In addition to epigenetic regulation, increasing data support the crucial role of butyrate and other SCFAs in intestinal immune homeostasis through modulation of regulatory T cells (Tregs).61 Tregs play a central role in suppression of inflammatory and allergic responses by limiting proliferation of effector CD4+ T cells. Butyrate and propionate have been shown to induce extrathymic generation and functional differentiation of Tregs and protect against colitis.61, 62 Possible mechanisms include inhibition of histone deacetylase, enhancement of anti-inflammatory phenotype in colonic macrophages and dendritic cells via activation of GPR109a, and a T-cell intrinsic epigenetic upregulation of the Foxp3 gene, a master regulator of Treg function.
While the role of Tregs in colorectal cancer remains controversial, partly due to its functional heterogeneity, higher levels of immune checkpoints and other immunosuppressive molecules expressed in Tregs and cancer cells has been implicated in immune evasion of tumors, adversely influencing prognosis.63–65 Antibodies targeting these immune checkpoints are the basis for immunotherapies which have revolutionized the treatment of many advanced malignancies, including colorectal cancer. Interestingly, patients with higher abundance of SCFA-producing bacteria, such as Bifidobacterium66, 67 and Faecalibacterium,68 appear to be more responsive to immunotherapy, further supporting the importance of SCFA in modulation of tumor immunity. Moreover, higher intake of fiber and whole grains after diagnosis has been associated with better survival of colorectal cancer.69 Further studies are needed to evaluate whether this beneficial association is mediated by the gut microbiota and how patients’ diet, including fiber intake, may shape and interact with their gut microbial community to influence the efficacy of immunotherapy and resistance to immune-related toxicity of immune checkpoint inhibitors in colorectal cancer.
Red and processed meat, bile acid and sulfur
Compelling data indicate that higher intake of red and processed meats is associated with increased risk of cancer, particularly colorectal cancer (Table 1).70 Therefore, the International Agency for Research on Cancer has classified processed meat as a carcinogen to humans. Several elements in red and processed meats have been suggested to have pro-cancer effects, including preservatives (e.g., nitrates and nitrites), certain nutrients enriched in meats (e.g., heme iron, sulfur, and saturated fat), and chemicals produced during meat processing and cooking (e.g., heterocyclic amines and polycyclic aromatic hydrocarbons).
Recent evidence suggests a critical role of the gut microbiota in mediating the effect of carcinogenic factors in red meats (Figure 4). For example, the gut microbiota is required for the damaging effect of heme iron on the colonic surface epithelium. Heme iron elicited an eightfold increase in the abundance of mucin-degrading bacteria Akkermansia muciniphila, leading to disruption in the mucus barrier function. Antibiotics abolished heme-induced injuries as well as the downstream carcinogenic effects, including compensatory hyperproliferation, hyperplasia, and differential expression of tumor suppressors and oncogenes.71
Figure 4.
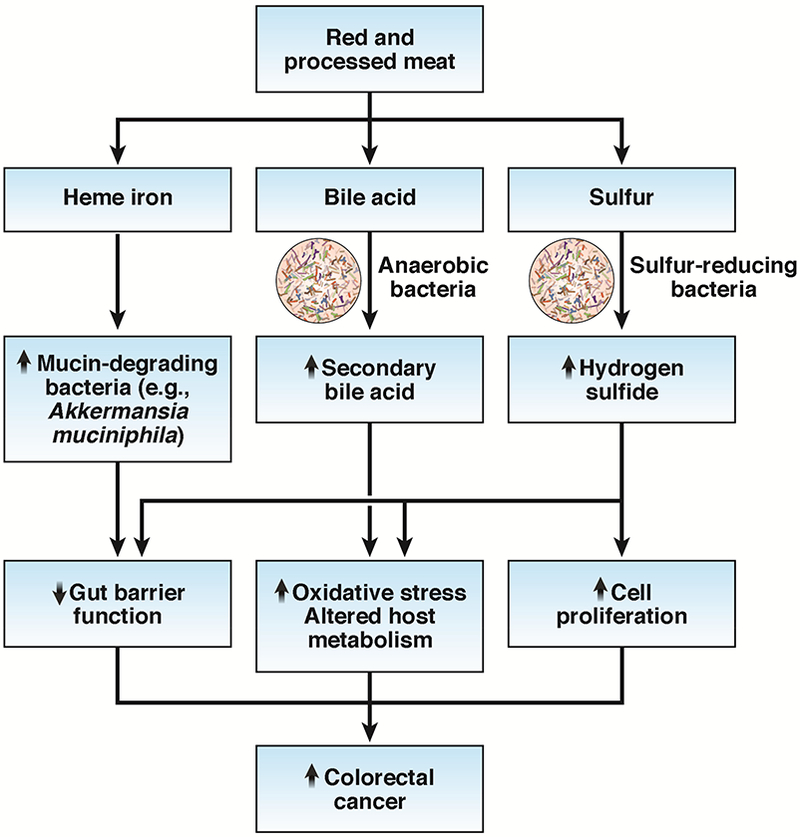
Potential role of the gut microbiota in mediating the effect of carcinogenic factors in red meats. Heme iron may damage the mucus barrier function by increasing the abundance of mucin-degrading bacteria (e.g., Akkermansia muciniphila). Increased production of secondary bile acids associated with high red meat consumption can promote carcinogenesis through increased oxidative stress and regulation of host metabolism. Excess production of hydrogen sulfide by sulfur-reducing bacteria in the colon may promote carcinogenesis through DNA damage, impaired colonocyte nutrition, reduced integrity of the mucus layer, induction of epithelial hyperproliferation, and increased inflammation.
In addition to the direct effect on the gut microbiota, compounds associated with high intake of red meat can serve as substrates for gut microbial metabolism and contribute to colorectal carcinogenesis. Two of the most studied compounds include secondary bile acids and sulfur. The high content of saturated fat in red and processed meats is associated with increased secretion of bile acids in the liver to emulsify dietary fat for absorption by the small intestine. Although the majority of bile acids are reabsorbed in the small intestine, those entering the colon are metabolized by the anaerobic bacteria (e.g., Clostridium clusters XIVa and XI and Eubacterium) into secondary bile acids, including lithocholic acid and deoxycholic acid. Secondary bile acids are potential mutagens and can induce DNA damage and apoptosis resistance by causing oxidative/nitrosative stress and liberating reactive oxygen/nitrogen species. In support of the potential role of secondary bile acids in colorectal cancer, a recent prospective cohort study found an elevated risk of proximal colon cancer among patients with ultrasound-detected gallstone disease and cholecystectomy.72 These patients are expected to have higher concentrations of fecal secondary bile acids due to a continuous bile flow into the bowel and increased activity of secondary bile acid-producing bacteria.73,74 The predominant association with proximal colon cancer may reflect a greater proximal colonic absorption of fecal secondary bile acids.75
Moreover, recent evidence indicates a crosstalk between bile acids as signaling molecules and the gut microbiota for regulation of host metabolism.76 Specifically, bile acids and gut bacteria interact with the nuclear farnesoid X receptor and the G protein-coupled membrane receptor 5 (TGR5) to regulate numerous metabolic pathways that are potentially important for cancer development. In particular, TGR5 activation in colonic L cells increases synthesis and release of glucagon-like peptide-1 (GLP-1),77 a metabolic hormone that enhances the secretion of insulin and controls glucose homeostasis. Interestingly, GLP-1 receptor agonists have been showed to promote intestinal growth and colonic tumorigenesis in mice,78 and liraglutide, a GLP-1 receptor agonist approved for treatment of type 2 diabetes, has been associated with increased risk of several cancers, including colorectal cancer, although findings remain inconsistent.79 These data additionally support a potential role of bile acids in cancer development through microbiota-dependent metabolic mechanisms.
Sulfur is another compound that is enriched in red and processed meat and can potentially influence colorectal carcinogenesis through microbial mechanisms.51 Inorganic sulfur (sulfate and sulfite) routinely used as a preservative in processed meat and sulfur contained in amino acids (e.g., cysteine and methionine) from red meat can be metabolized by sulfur-reducing bacteria to hydrogen sulfide, which has been implicated in inflammatory bowel disease and colorectal cancer.80 Patients with colitis and colorectal cancer have higher levels of fecal hydrogen sulfide than disease-free controls.81 Excess production of hydrogen sulfide in the colon may promote carcinogenesis through multiple mechanisms, including DNA damage due to its genotoxic properties, impaired colonocyte nutrition by inhibition of β-oxidation of butyrate, reduced integrity of the mucus layer for bacterial degradation, induction of epithelial hyperproliferation, and increase in inflammation by altered immune cell populations and function.51 Consistent with these mechanistic data, a recent study found that the abundance of sulfur-reducing bacteria, such as Bilophila wadsworthia and Pyramidobacter spp., was higher in colonic mucosa of patients diagnosed with colorectal cancer, compared to cancer-free individuals undergoing routine screening colonoscopy.82 Interestingly, a particularly higher abundance of sulfur-reducing bacteria was found in African Americans than non-Hispanic whites, regardless of disease status, suggesting that increased production of hydrogen sulfide may predispose African Americans to a higher risk of colorectal cancer than non-Hispanic whites. The study also identified a positive correlation between abundance of sulfur-reducing bacteria and the components of a diet high in fat and animal protein, and a negative correlation with the consumption of dairy and calcium.82
Marine omega-3 fatty acids
The anti-inflammatory activity of marine omega-3 fatty acids, including mainly eicosapentaenoic acid and docosahexaenoic acid, has been well recognized. Increasing data support a potential benefit of marine omega-3 fatty acids for colorectal cancer.83 A recent meta-analysis of prospective cohort studies indicates that individuals with the highest level of marine omega-3 fatty acids in biospecimens had 24% lower risk of colorectal cancer than those with the lowest levels (Table 1),84 although the findings on dietary intake remain inconsistent, possibly due to the long latency for any protective effect and heterogeneity across tumor subsites and subtypes.85 In a RCT of patients with familial adenomatous polyposis, supplementation of eicosapentaenoic acid at a dose of 2 g daily for 6 months reduced the number and size of polyps by 20–30%, an effect comparable to cyclooxygenase-2 inhibitors.86 In addition to protection against colorectal cancer incidence, recent data support a survival-improving benefit of high marine omega-3 fatty acid intake among patients with established colorectal cancer.87–89 These data together support that marine omega-3 fatty acids may have a beneficial effect across the entire spectrum of colorectal cancer initiation, progression, and distant metastasis.
Dietary fat composition has been suggested as a major driver of the gut microbial community structure. Compared to other types of fat, omega-3 fatty acids have been associated with higher intestinal microbiota diversity and omega-3 fatty acids-rich diet ameliorates the gut dysbiosis induced by omega-6 polyunsaturated fatty acids or antibiotics. Data from mouse models suggest that dietary omega-3 fatty acid intake or high tissue levels of omega-3 fatty acids are associated with an increased abundance of anti-inflammatory bacteria, such as lactic acid-producing bacteria (mainly Lactobacillus and Bifidobacterium), and decreased abundance of immunosuppressive and pro-inflammatory bacteria, such as F. nucleatum, LPS-producing bacteria (e.g., Escherichia coli) and Akkermansia (Figure 5).90–96 A dietary intervention that included 600mg of omega-3 fatty acid daily for 14 days has been shown in a case report to significantly enrich several SCFA-producing bacteria, including Roseburia, Blautia, Bacterioides, and Coprococcus.97 Moreover, treatment of 2 g/daily eicosapentaenoic acid for 90 days in 19 patients with long-standing ulcerative colitis has been shown to reduce mucosal inflammation, promote endoscopic and histological remission, and restore the gut microbial composition by increasing porphyromonadaceae and decreasing Ruminococcaceae.98 In addition, a recent randomized cross-over trial in 22 middle-aged, healthy volunteers found that 8 weeks’ treatment with 4 g mixed eicosapentaenoic acid/docosahexaenoic acid separated by a 12-week ‘washout’ period induced a reversible increase in several genera, including Bifidobacterium, Roseburia and Lactobacillus, although the overall α or β diversity did not significantly change.99
Figure 5.
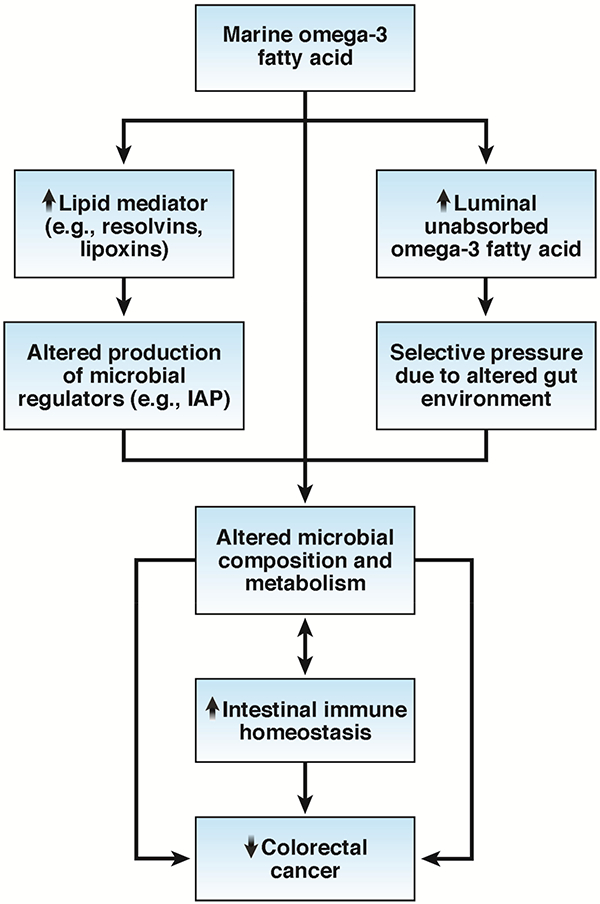
Pathways linking marine omega-3 fatty acid, gut microbiota, and immune function in colorectal cancer. Marine omega-3 fatty acid increases the abundance of Bifidobacterium and Lactobacillus genera and reduces the abundance of Fusobacterium nucleatum and lipopolysaccharide (LPS)-producing bacteria (e.g., Escherichia coli), possibly by altering the production of microbiota regulators (e.g., intestinal alkaline phosphatase [IAP]) via changes in tissue lipid mediators (e.g., resolvin) or by modifying the gut environmental conditions that in turn confer selective pressure on the microbial community. The changes in the microbial composition can, either directly or indirectly via microbial metabolites (e.g., hydroxyl fatty acids and short-chain fatty acids), help preserve intestinal immune homeostasis and protect against colorectal cancer, whereas some immune factors can conversely affect the makeup of the microbiota, promoting a reciprocal host-microbe interaction.
Several potential pathways may contribute to the microbe-modifying effect of omega-3 fatty acids. A recent study showed that high omega-3 fatty acid might alter the production of microbiota regulators in colonic tissue.100 Omega-3 fatty acid metabolite resolvin stimulates host epithelial expression of a transmissible factor, intestinal alkaline phosphatase,101 whose LPS-detoxifying activity leads to decreased abundance of LPS-producing and/or pro-inflammatory bacterial groups and increased abundance of LPS-suppressing and/or anti-inflammatory bacteria.100 Moreover, luminal unabsorbed omega-3 fatty acids may alter the gut environmental conditions and omega-3 fatty acid-induced changes in the immune response may in turn confer selective pressure on the gut microbial community.
Mechanistically, the microbiota-modulating effect of omega-3 fatty acids may lead to improved immune function that prevents against colorectal cancer. Some species from Lactobacillus and Bifidobacteria genera, which have been consistently associated with marine omga-3 fatty acids, play an important role in the host immunoprotective system,102,103 promote antitumor immunity, and facilitate cancer immunotherapy.67, 104, 105 On the other hand, higher serum levels of LPS antibodies have been associated with increased CRC risk in men,106 and higher abundance of F. nucleatum has been linked to higher risk and shorter survival of CRC.36–38, 41, 43,46 Interestingly, Lactobacillus and other anaerobic gut bacteria have been implicated in the saturation of polyunsaturated fatty acid, a detoxifying mechanism that transforms bacterial growth-inhibiting polyunsaturated fatty acid into less toxic fatty acid, such as hydroxyl fatty acid.107–113 These microbial metabolites may help preserve intestinal barrier integrity, reduce oxidative stress, and lower inflammation.114, 115 Given that Lactobacillus is selectively enriched by omega-3 fatty acids, there may exist a reciprocal mechanism by which gut microbes adapt to host dietary change with functional consequences for host health. Moreover, a cross-feeding effect has been noted between human Bifidobacterium, which produces lactate and acetate, and the butyrate-producing species, such as Eubacterium rectale, which convert lactate to butyrate.116–118 Given the potential role of butyrate in epigenetic regulation and immune function, this may represent another mechanism by which omega-3 fatty acids protect against colorectal cancer (Figure 5).
Alcohol
Alcohol consumption has been associated with increased risk of colorectal cancer in a dose-dependent manner (Table 1). The positive association has been observed for wine, beer and spirits, and does not appear to differ by tumor subsite, sex or geographic region.7
Several, albeit preliminary, lines of evidence indicate a role of the gut microbiota in the tumorigenic effect of alcohol. First, both human and animal studies have shown that chronic ethanol consumption causes dysbiosis, lowers the abundances of Bacteroidetes and Firmicutes, and enriches Proteobacteria and Actinobacteria. 119–121These structural changes in the gut microbiota have been linked to intestinal bacterial overgrowth and hyperpermeability, leading to increased translocation of gram-negative microbial bacterial products (e.g., endotoxins) from the intestinal lumen into systemic circulation.120, 122 On the other hand, abstinence from alcohol has been shown to restore gut barrier integrity in humans.123 Considering that endotoxemia has been implicated in the development of systemic inflammation, insulin resistance, and type 2 diabetes, these data suggest a link between alcohol consumption, gut microbiota, and increased metabolic risk associated with colorectal cancer. Moreover, chronic ethanol exposure has been shown to lower the abundance of butyrate-producing taxa from the Clostridiales order, including Faecalibacterium prausnitzii, Coprococcus eutactus, and Roseburia spp.124 Through comprehensive characterization of the metabolic alterations of the content of the gastrointestinal tract, a study found that rats with ethanol exposure displayed significant changes in numerous metabolic pathways critical for host physiology, including a significant decrease in SCFAs.125 Finally, in addition to colorectal mucosal cells, several intestinal aerobes and facultative anaerobes play important roles in the production of mutagen acetaldehyde from ethanol under aerobic conditions in the colon and rectum.126 Several bacteria have been identified as potential acetaldehyde accumulators, including Ruminococcus, Collinsella, Prevotella, and Coriobacterium,127 leading to the hypothesis that structural alterations of the gut microbiota in high alcohol consumers may increase oxidation of ethanol and intracolorectal levels of acetaldehyde above the minimum mutagenic concentrations to initiate carcinogenesis. Further mechanistic studies are needed to test this hypothesis.
Smoking
Smoking is associated with an increased risk of colorectal cancer with a prolonged latency period (Table 1). Compared to never smokers, former smokers remained at higher risk for up to about 25 years after quitting.128 A population-based metagenomics analysis identified a modest negative correlation between smoking and the diversity of the gut microbiome.129 A study using targeted fluorescent in situ hybridization identified higher Bacteroides-Prevotella in the fecal samples of smokers than non-smokers.130 Conversely, smoking cessation has been shown to restore, at least partly, the diversity of the gut microbiome; increase the abundance of key representatives from the phyla of Firmicutes (Clostridium coccoides, Eubacterium rectale, and Clostridium leptum subgroup) and Actinobacteria (HGC bacteria and Bifidobacteria); and decrease the abundance of Bacteroidetes (Prevotella spp. and Bacteroides spp.) and Proteobacteria.131 The smoking-related microbial changes may lead to altered epithelial mucin composition of the mucus layer and increased inflammatory response.132 In addition, some in vitro and animal studies found that cigarette smoke might decrease the fecal abundance of Bifidobacterium and reduce its production of SCFAs.133, 134
Although the causes of smoking-associated changes in the composition of the gut microbiota remain to be elucidated, preliminary evidence suggests a collective role of host, microbial, and environmental changes, such as intestinal and immune disruption, impaired clearance of pathogens, changes in the virulence of bacteria and fungi, altered growth and exopolysaccharide structure of known gut bacteria that may contribute to dysbiosis (e.g., Bifidobacterium animalis), and ingestion of bacteria that are present in cigarettes.135
Others
Several other dietary and chemopreventive agents associated with colorectal cancer have been implicated in modulation of the composition and function of the gut microbiota (Table 1).
Predominant evidence from studies on dietary and supplementary vitamin D intake and circulating 25-hydroxyvitamin D levels indicates a beneficial effect of vitamin D on colorectal cancer incidence and mortality.136, 137 These data are consistent with the mechanistic evidence for a diversity of anti-cancer effects of vitamin D, particularly through its anti-inflammatory and immune regulatory properties.51 In support of the potential mediating role of the gut microbiota, a recent genome-wide host-microbiota association study identified variants in the vitamin D receptor gene among the most significant loci that were associated with overall gut microbial variation and abundance of individual bacteria, as well as functions of gastrointestinal and immune-related tissues and cells.138
Moreover, there is strong evidence that higher intake of total dairy, milk and calcium is associated with lower risk of colorectal cancer.7 In addition to calcium and vitamin D, other constituents in dairy products have antineoplastic activity, including conjugated linoleic acid, lactose, butyrate, and lactic acid-producing bacteria.51 A recent intervention study of patients with irritable bowel syndrome showed that consumption of a fermented milk product containing dairy starters and Bifidobacterium animalis potentiated colonic production of SCFAs and decreased abundance of Bilophila wadsworthia, a pathobiont that has been linked to inflammation and colorectal cancer, suggesting the influence on the gut microbial composition and function of the microbes and other compounds in dairy.139
Finally, increasing data suggest the tumor-suppressive effect of metformin, a biguanide derivative that is widely used as the first-line treatment for type 2 diabetes.8 A recent small RCT found that 1-year administration of low-dose metformin in non-diabetic patients reduced the incidence and number of metachronous adenomas and polyps after polypectomy, providing further support for the potential of metformin in colorectal cancer chemoprevention.140 Mechanistically, metformin has been shown to reduce cellular proliferative activity through activation of AMP-activated protein kinase,141 protect against immune evasion of tumors by countering the functional exhaustion of tumor infiltrating lymphocytes,142 and accumulate in colonic tissue in a considerable concentration (approximately 150-fold higher than in plasma).143 In a recent RCT of 40 patients with type 2 diabetes, metformin altered the composition of gut microbiota by increasing the abundance of Escherichia and Bifidobacterium and decreasing the abundance of Intestinibacter, thereby increasing production of SCFAs.144
Concluding remarks
Research has begun to elucidate the interplay between environmental factors and gut microbiota in carcinogenesis. However, direct evidence linking lifestyle and gut microbiota to colorectal cancer remains lacking, and the existing evidence on the relationship of lifestyle with gut microbiota has largely been based on small crosssectional or short-term intervention studies. Given the long latency of colorectal cancer development, high-quality prospective studies with lifestyle data collected over the life course and gut microbial composition and function assessed well prior to neoplastic occurrence are critically needed to elucidate how environmental factors may influence cancer risk through modulation of the gut microbiota. Moreover, further mechanistic studies using either gnotobiotic animal models or biomarker-based human trials are needed to complement the epidemiologic investigations and uncover pathways through which exogenous factors influence the carcinogenic process by interacting with the gut microbiota and host immune and metabolic systems. These collective efforts will hopefully lead to development and clinical translation of potential microbiota-based strategies for cancer prevention.
Acknowledgments
Funding Support
This work was supported by the American Cancer Society (Grant Number MRSG-17– 220-01 - NEC to M.S.), an AACR-AstraZeneca Fellowship in Immuno-oncology Research (Grant Number 17–40-12-SONG to M.S.); and by the U.S. National Institutes of Health (NIH) grants (K99 CA215314 to M.S.; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726 to A.T.C.). Dr. Chan is a Stuart and Suzanne Steele MGH Research Scholar.
Footnotes
Conflict of interest
Andrew T. Chan previously served as a consultant for Bayer Pharma AG, Pfizer Inc., Janssen, for work unrelated to the topic of this manuscript. This study was not funded by Bayer Pharma AG, or Pfizer Inc. No other conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. Epub 2017/03/02. [DOI] [PubMed] [Google Scholar]
- 2.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006; 107(7): 1624–33. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8). Epub 2017/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018. Epub 2018/05/31. [DOI] [PubMed] [Google Scholar]
- 5.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. Epub 2017/11/22. [DOI] [PubMed] [Google Scholar]
- 6.Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol. 2016;2(9):1154–61. Epub 2016/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer. Available at: wcrf.org/colorectal-cancer-2017. All CUP reports are available at wcrf.org/cupreports. 2017. [Google Scholar]
- 8.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014;7(9):867–85. Epub 2014/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. Epub 2018/03/01. [DOI] [PubMed] [Google Scholar]
- 10.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. Epub 2011/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80–6. Epub 2015/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilg H, Adolph TE, Gerner RR, et al. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33(6):954–64. Epub 2018/04/17. [DOI] [PubMed] [Google Scholar]
- 13.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–28. Epub 2014/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. 2010. [Google Scholar]
- 15.Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. In: National Center for Health Statistics, Division of Health and Nutrition Examination Surveys, eds. 2015. [Google Scholar]
- 16.Khan MT, Nieuwdorp M, Backhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753–60. Epub 2014/09/02. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018. Epub 2018/05/31. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Grimm SA, Mav D, et al. Transcriptome and DNA Methylome Analysis in a Mouse Model of Diet-Induced Obesity Predicts Increased Risk of Colorectal Cancer. Cell Rep. 2018;22(3):624–37. Epub 2018/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Grimm SA, Chrysovergis K, et al. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. 2014;19(4):702–11. Epub 2014/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Roberts JD, Grimm SA, et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018; 19(1):7 Epub 2018/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. Epub 2013/06/28. [DOI] [PubMed] [Google Scholar]
- 22.Ohtani N, Yoshimoto S, Hara E. Obesity and cancer: a gut microbial connection. Cancer Res. 2014;74(7):1885–9. Epub 2014/03/19. [DOI] [PubMed] [Google Scholar]
- 23.Loo TM, Kamachi F, Watanabe Y, et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Inoue R, Tsukahara T, et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem. 2008;72(2):572–6. Epub 2008/02/08. [DOI] [PubMed] [Google Scholar]
- 25.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193 Epub 2014/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014; 15:511 Epub 2014/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Queipo-Ortuno MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465 Epub 2013/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. Epub 2014/07/16. [DOI] [PubMed] [Google Scholar]
- 29.Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625–33. Epub 2017/04/01. [DOI] [PubMed] [Google Scholar]
- 30.Feng YL, Shu L, Zheng PF, et al. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev. 2017;26(3):201–11. Epub 2016/03/06. [DOI] [PubMed] [Google Scholar]
- 31.Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111–20. Epub 2013/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342 Epub 2015/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder BO, Birchenough GMH, Stahlman M, et al. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018;23(1):27–40 e7 Epub 2017/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167(5):1339–53 e21 Epub 2016/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta RS, Nishihara R, Cao Y, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium Nucleatum in Tumor Tissue. JAMA Oncol. 2017. Epub 2017/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. Epub 2011/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8. Epub 2011/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11. Epub 2013/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74(5):1311–8. Epub 2014/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7(6):e39743 Epub 2012/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCoy AN, Araujo-Perez F, Azcarate-Peril A, et al. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8(1):e53653 Epub 2013/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allali I, Delgado S, Marron PI, et al. Gut Microbiome Compositional and Functional Differences between Tumor and Non-tumor Adjacent Tissues from Cohorts from the US and Spain. Gut Microbes. 2015:0 Epub 2015/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528 Epub 2015/03/12. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727 Epub 2015/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–8. Epub 2017/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973–80. Epub 2015/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–55. Epub 2015/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1(5):653–61. Epub 2015/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60 e16 Epub 2015/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J Natl Cancer Inst. 1989;81(17):1290–7. Epub 1989/09/06. [DOI] [PubMed] [Google Scholar]
- 53.McKeown-Eyssen GE, Bright-See E, Bruce WR, et al. A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps. Toronto Polyp Prevention Group. J Clin Epidemiol. 1994;47(5):525–36. Epub 1994/05/01. [DOI] [PubMed] [Google Scholar]
- 54.MacLennan R, Macrae F, Bain C, et al. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. J Natl Cancer Inst. 1995;87(23): 1760–6. Epub 1995/12/06. [DOI] [PubMed] [Google Scholar]
- 55.Alberts DS, Martinez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342(16):1156–62. Epub 2000/04/20. [DOI] [PubMed] [Google Scholar]
- 56.Bonithon-Kopp C, Kronborg O, Giacosa A, et al. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356(9238):1300–6. Epub 2000/11/10. [DOI] [PubMed] [Google Scholar]
- 57.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–55. Epub 2000/04/20. [DOI] [PubMed] [Google Scholar]
- 58.So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018. Epub 2018/05/15. [DOI] [PubMed] [Google Scholar]
- 59.Holscher HD, Caporaso JG, Hooda S, et al. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr. 2015;101(1):55–64. Epub 2014/12/21. [DOI] [PubMed] [Google Scholar]
- 60.Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4(12):1387– 97. Epub 2014/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. Epub 2013/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44(5):989–1004. Epub 2016/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27. Epub 2011/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu P, Wu D, Li L, et al. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10(6):e0131403 Epub 2015/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8. Epub 2018/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264): 1084–9. Epub 2015/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. Epub 2017/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song M, Wu K, Meyerhardt JA, et al. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2018;4(1):71–9. Epub 2017/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 71.Ijssennagger N, Belzer C, Hooiveld GJ, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112(32):10038–43. Epub 2015/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shabanzadeh DM, Sorensen LT, Jorgensen T. Association Between Screen-Detected Gallstone Disease and Cancer in a Cohort Study. Gastroenterology. 2017; 152(8): 1965–74 e1 Epub 2017/02/28. [DOI] [PubMed] [Google Scholar]
- 73.Keren N, Konikoff FM, Paitan Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7(6):874–80. Epub 2015/07/08. [DOI] [PubMed] [Google Scholar]
- 74.Pomare EW, Heaton KW. The effect of cholecystectomy on bile salt metabolism. Gut. 1973;14(10):753–62. Epub 1973/10/01. [PMC free article] [PubMed] [Google Scholar]
- 75.Linos D, Beard Cm , O’Fallon WM, et al. Cholecystectomy and carcinoma of the colon. Lancet. 1981;2(8243):379–81. Epub 1981/08/22. [DOI] [PubMed] [Google Scholar]
- 76.Wahlstrom A, Sayin SI, Marschall HU, et al. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24(1):41–50. Epub 2016/06/21. [DOI] [PubMed] [Google Scholar]
- 77.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. Epub 2009/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koehler JA, Baggio LL, Yusta B, et al. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metab. 2015;21(3):379–91. Epub 2015/03/05. [DOI] [PubMed] [Google Scholar]
- 79.Abrahami D, Yin H, Yu OHY, et al. Incretin-based Drugs and the Incidence of Colorectal Cancer in Patients with Type 2 Diabetes. Epidemiology. 2018;29(2):246–53. Epub 2017/12/29. [DOI] [PubMed] [Google Scholar]
- 80.Pitcher mC, Beatty ER, Cummings JH . The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46(1):64–72. Epub 1999/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42(8):1571–9. Epub 1997/08/01. [DOI] [PubMed] [Google Scholar]
- 82.Yazici C, Wolf PG, Kim H, et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–94. Epub 2017/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61(1):135–49. [DOI] [PubMed] [Google Scholar]
- 84.Yang B, Wang FL, Ren XL, et al. Biospecimen long-chain N-3 PUFA and risk of colorectal cancer: a meta-analysis of data from 60,627 individuals. PloS one. 2014;9(11):e110574 Epub 2014/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song M, Chan AT, Fuchs CS, et al. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int J Cancer. 2014;135(10):2413–23. Epub 2014/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–25. Epub 2010/03/30. [DOI] [PubMed] [Google Scholar]
- 87.Song M, Zhang X, Meyerhardt JA, et al. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2017;66(10):1790–6. Epub 2016/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Van Blarigan EL, Fuchs CS, Niedzwiecki D, et al. Marine omega-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol Biomarkers Prev. 2018;27(4):438–45. Epub 2018/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cockbain AJ, Volpato M, Race AD, et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63(11): 1760–8. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 90.Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell metabolism. 2015;22(4):658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487(7405): 104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosh S, DeCoffe D, Brown K, et al. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS One. 2013;8(2):e55468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patterson E, RM OD, Murphy EF, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr. 2014:1–13. Epub 2014/02/22. [DOI] [PubMed] [Google Scholar]
- 94.Mujico JR, Baccan GC, Gheorghe A, et al. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110(4):711–20. [DOI] [PubMed] [Google Scholar]
- 95.Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. The Journal of nutritional biochemistry. 2014;25(3):270–80. [DOI] [PubMed] [Google Scholar]
- 96.Kaliannan K, Wang B, Li XY, et al. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int J Obes (Lond). 2016. Epub 2016/02/16. [DOI] [PubMed] [Google Scholar]
- 97.Noriega BS, Sanchez-Gonzalez MA, Salyakina D, et al. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep Med. 2016;2016:3089303 Epub 2016/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prossomariti A, Scaioli E, Piazzi G, et al. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci Rep. 2017;7(1):7458 Epub 2017/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watson H, Mitra S, Croden FC, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2017. Epub 2017/09/28. [DOI] [PubMed] [Google Scholar]
- 100.Kaliannan K, Wang B, Li XY, et al. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Scientific reports. 2015;5:11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell EL, MacManus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107(32):14298–303. Epub 2010/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903–11. Epub 2012/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peran L, Sierra S, Comalada M, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97(1):96–103. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 104.Khazaie K, Zadeh M, Khan MW, et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2012; 109(26): 10462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong SY, Tran HQ, Gewirtz AT, et al. Serum Endotoxins and Flagellin and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(2):291–301. Epub 2016/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kishino S, Takeuchi M, Park SB, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013; 110(44): 17808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kishino S, Ogawa J, Yokozeki K, et al. Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Applied microbiology and biotechnology. 2009;84(1):87–97. [DOI] [PubMed] [Google Scholar]
- 109. Hirata A, Kishino S, Park SB, et al. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J Lipid Res. 2015;56(7):1340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Applied microbiology and biotechnology. 2010;85(6):1629–42. [DOI] [PubMed] [Google Scholar]
- 111. Sakurama H, Kishino S, Mihara K, et al. Biohydrogenation of C20 polyunsaturated fatty acids by anaerobic bacteria. J Lipid Res. 2014;55(9):1855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Druart C, Bindels LB, Schmaltz R, et al. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: Proof of concept in germ-free versus conventionalized mice. Mol Nutr Food Res. 2015;59(8):1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Druart C, Neyrinck AM, Vlaeminck B, et al. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS One. 2014;9(1):e87560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Furumoto H, Nanthirudjanar T, Kume T, et al. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicology and applied pharmacology. 2016;296:1–9. [DOI] [PubMed] [Google Scholar]
- 115.Miyamoto J, Mizukure T, Park SB, et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290(5):2902–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flint HJ, Duncan SH, Scott KP, et al. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74(1):13–22. Epub 2014/10/01. [DOI] [PubMed] [Google Scholar]
- 117.Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic crossfeeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593–9. Epub 2006/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–7. Epub 2004/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bull-Otterson L, Feng W, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS one. 2013;8(1):e53028 Epub 2013/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. Epub 2012/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuruya A, Kuwahara A, Saito Y, et al. Ecophysiological consequences of alcoholism on human gut microbiota: implications for ethanol-related pathogenesis of colon cancer. Sci Rep. 2016;6:27923 Epub 2016/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. Epub 2011/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–93. Epub 2014/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dubinkina VB, Tyakht AV, Odintsova VY, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5(1):141 Epub 2017/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie G, Zhong W, Zheng X, et al. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12(7):3297–306. Epub 2013/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nosova T, Jokelainen K, Kaihovaara P, et al. Aldehyde dehydrogenase activity and acetate production by aerobic bacteria representing the normal flora of human large intestine. Alcohol Alcohol. 1996;31(6):555–64. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 127.Tsuruya A, Kuwahara A, Saito Y, et al. Major Anaerobic Bacteria Responsible for the Production of Carcinogenic Acetaldehyde from Ethanol in the Colon and Rectum. Alcohol Alcohol. 2016;51(4):395–401. Epub 2016/01/13. [DOI] [PubMed] [Google Scholar]
- 128.Gong J, Hutter C, Baron JA, et al. A pooled analysis of smoking and colorectal cancer: timing of exposure and interactions with environmental factors. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1974–85. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–9. Epub 2016/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Benjamin JL, Hedin CR, Koutsoumpas A, et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm Bowel Dis. 2012;18(6):1092–100. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 131.Biedermann L, Brulisauer K, Zeitz J, et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20(9):1496–501. Epub 2014/07/30. [DOI] [PubMed] [Google Scholar]
- 132.Allais L, Kerckhof FM, Verschuere S, et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol. 2016;18(5):1352–63. Epub 2015/06/03. [DOI] [PubMed] [Google Scholar]
- 133.Tomoda K, Kubo K, Asahara T, et al. Cigarette smoke decreases organic acids levels and population of bifidobacterium in the caecum of rats. J Toxicol Sci. 2011;36(3):261–6. Epub 2011/06/02. [DOI] [PubMed] [Google Scholar]
- 134.Hu J, Wei T, Sun S, et al. Effects of cigarette smoke condensate on the production and characterization of exopolysaccharides by Bifidobacterium. An Acad Bras Cienc. 2015;87(2):997–1005. Epub 2015/06/11. [DOI] [PubMed] [Google Scholar]
- 135.Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. Epub 2016/11/01. [DOI] [PubMed] [Google Scholar]
- 136.Ma Y, Zhang P, Wang F, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29(28):3775–82. Epub 2011/08/31. [DOI] [PubMed] [Google Scholar]
- 137.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst. 2018. Epub 2018/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang J, Thingholm LB, Skieceviciene J, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396–406. Epub 2016/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Veiga P, Pons N, Agrawal A, et al. Changes of the human gut microbiome induced by a fermented milk product. Sci Rep. 2014;4:6328 Epub 2014/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–83. Epub 2016/03/08. [DOI] [PubMed] [Google Scholar]
- 141. Zakikhani M, Dowling RJ, Sonenberg N, et al. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila). 2008;1(5):369–75. Epub 2009/01/14. [DOI] [PubMed] [Google Scholar]
- 142.Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015; 112(6):1809– 14. Epub 2015/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paleari L, Burhenne J, Weiss J, et al. High Accumulation of Metformin in Colonic Tissue of Subjects With Diabetes or the Metabolic Syndrome. Gastroenterology. 2018; 154(5): 1543–5. Epub 2018/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017;23(7):850–8. Epub 2017/05/23. [DOI] [PubMed] [Google Scholar]


