Abstract
Similar to other plant-parasitic nematodes, root lesion nematodes possess an array of enzymes that are involved in the degradation of the plant cell wall. Here we report the identification of a gene encoding a cell wall-degrading enzyme, pectin methylesterase PME (EC 3.1.1.11), in the root lesion nematode Pratylenchus penetrans. Both genomic and coding sequences of the gene were cloned for this species, that included the presence of four introns which eliminated a possible contamination from bacteria. Expression of the Pp-pme gene was localized in the esophageal glands of P. penetrans as determined by in situ hybridization. Temporal expression of Pp-pme in planta was validated at early time points of infection. The possible function and activity of the gene were assessed by transient expression of Pp-pme in plants of Nicotiana benthamiana plants via a Potato virus X-based vector. To our knowledge, this is the first report on identification and characterization of a PME gene within the phylum Nematoda.
Introduction
The plant cell wall plays an important role in various fundamental physiological processes of plant growth and development, such as maintaining the integrity of cellular content, morphogenesis, and cell signaling. In addition, the cell wall is the primary interface for most plant-pathogen interactions, since it is the first physical barrier against invasion and infection [1, 2]. The primary cell wall has an intricate structure composed of a complex network of cellulose microfibrils interconnected within a matrix of polysaccharides, including pectins, hemicelluloses, and glycoproteins [3, 4]. Pectin, a highly abundant polysaccharide, is an important component of both primary and secondary cell walls, forming the main component of the middle lamella [5, 6]. Modification of the pectin network is tightly regulated by the action of pectinolytic enzymes and pectinases (e.g. pectate lyases, pectin methylesterases and polygalacturonases), whereas the cellulose/hemicellulose network is targeted by cellulolytic enzymes (e.g. endo- and exoglucanases) and hemicellulases [7, 8].
Pectin methylesterases (PMEs; EC 3.1.1.11) are a group of enzymes belonging to the carbohydrate esterase family 8 (CE8). They catalyze hydrolysis of the methyl ester of homogalacturonan, the backbone of pectin, which releases acidic pectins and methanol that facilitates the modification of the plant cell wall and its subsequent degradation [9, 10]. Pectin, de-esterified by PMEs, becomes more susceptible to degradation by other pectinases (e.g. polygalacturonase, pectate lyase and rhamnogalacturonan lyase), which alters the texture and integrity of the cell wall and contributes to its loosening [11].
PMEs are widely present in plants, which encode a large number of isoforms that play important roles in plant development and major physiological processes [9], such as microsporogenesis, pollen growth, seed germination, root development, polarity of leaf growth, stem elongation, fruit ripening, loss of tissue integrity, cell wall extension, and softening [9, 12–15]. Moreover, PMEs have also been reported to play an important role in response to fungal [15] and bacterial pathogens, and are required for the systemic spread of the tobacco mosaic virus in plants [16].
Plant pathogenic microorganisms (e.g. bacteria and fungi) are very efficient in degrading plant cell wall polysaccharides using their own battery of cell wall-degrading enzymes (CWDEs). These CWDEs are normally secreted into the host tissues and efficiently degrade plant cells, allowing pathogens access to the cells, or in some cases, to utilize these polysaccharides as a source of nutrients for their own growth and development. Among these are PMEs, which play key roles in the infection process of plant pathogens by breaking down of the plant cell wall, which is a primary requirement to successful invasion of a host plant. Significant differences between PMEs of plants and microorganisms have been found [9]. For example, fungal PMEs appear to have a broader range of adaptability to substrates [17]. Secreted PMEs of bacteria and fungi are involved in invasion of the host plant and pathogenicity. The breakdown of pectin by these PMEs can lead to the maceration and soft-rotting of plant tissues which is a characteristic phenotype of soft-rot diseases [18, 19].
Although the synthesis of PMEs has been often attributed to the free-living or endosymbiotic organisms that inhabit the gut of some insects, several studies confirmed that these phytophagous insects are also able to encode PMEs through their own endogenous genes [20–22]. In this context, PME encoding genes have been identified for a few species belonging to the family Curculionidae (weevils and bark beetles) [20, 23]. With the increase of genome and transcriptome datasets available for different animal species, additional PME-encoding genes have been recently identified for the whitefly Bemisia tabaci Gennadius, 1889 (Insecta: Hemiptera: Aleyrodidae) and the springtail soil arthropod Folsomia candida Willem, 1902 (Entognatha: Collembola) [24, 25]. Although functional analyses are still lacking for most of the animal PMEs identified, emerging data indicate that PME activity is important during insect-plant interactions. The PMEs of the rice weevil (Sitphilus oryzae L., 1763) were found to act synergistically with other pectinases to enable the breakdown of the complex polysaccharide pectin network that allows access of the cell contents [22, 26, 27]. Other than these reports, PME encoding genes have not yet been identified in other animals so far.
Root lesion nematodes (RLNs) are migratory, endoparasitic nematodes that are able to parasitize a broad host range and cause extensive root damage to plant hosts [28]. All motile stages of RLN can penetrate the roots, which feed predominately on the root cortical tissues and causes the formation of lesions, browning, and cell death [28]. The successful invasion of roots by RLNs is related to their ability to overcome the barrier imposed by the plant cell wall. Like other plant-parasitic nematodes (PPNs), RLNs are equipped with a protrusible stylet that mechanically disrupts the cell wall and through which CWDEs are secreted to facilitate penetration and migration of the nematode through host roots.
Pratylenchus penetrans (Cobb, 1917) Filipjev and Schuurmans Stekhoven, 1941 is regarded as one of the most destructive species of this genus because of its ability to parasitize a wide range of economically important host plants (e.g. alfalfa, corn, and potato), and its broad geographic distribution [29]. The core set of genes encoding CWDEs identified for P. penetrans so far comprises β-1,4-endoglucanases (GH5), pectate lyases (PL3), arabinogalactan endo-1,4-β-galactosidases (GH53), xylanases (GH30), and expansin-like genes [30]. As in other PPNs, the majority of these CWDEs are localized in the esophageal gland cells of P. penetrans, and are actively produced during the early time points of plant infection [31]. Interestingly, a refined data mining of the P. penetrans transcriptome [30] resulted in the identification of a transcript encoding a PME gene, here after named as Pp-pme. Here we report the identification and molecular characterization of this Pp-pme gene, which is, to the best of our knowledge, the first report of a PME encoding gene within the phylum Nematoda.
Material and methods
Nematode collection and extraction
Two different isolates of P. penetrans were used in this study: 1) NL 10p RH collected in Beltsville, MD, USA, and 2) A44L4 from potato (Solanum tuberosum L.) fields in Coimbra, Portugal. Both isolates were maintained and multiplied in vitro in roots of corn (Zea mays L. cv. ‘Iochief’) growing in Murashige and Skoog basal medium containing vitamins (MS) (Sigma-Aldrich, MO, USA), 3% (w/v) of sucrose (pH 5.8) and solidified with 1.5% (w/v) agar (Sigma-Aldrich, MO, USA). Nematodes were re-cultured every two months into new roots of corn and maintained in the dark at 28°C. Nematodes were extracted from infected corn roots as described in Vieira et al. [30].
Isolation of Pp-pme genomic DNA and cDNA sequences
During our previous analyses of the transcriptome assembly generated for P. penetrans [30], a partial transcript of 415 bp showing similarity to a bacterial pectin methylesterase gene (WP_090106049.1) was identified. In order to obtain the corresponding full-length coding sequence, BLAST searches were initially performed against the skimming genome assembly of P. penetrans [32]. Based on the assembly of the retrieved contigs, primers were designed to flank the in silico predicted fragment (S1 Table), as well as the putative full-length protein-encoding Pp-pme sequence, including partial sequences of both 5´and 3´regions (S1 Table). DNA extraction was performed from mixed stages (eggs, juveniles, females and males) of P. penetrans using the PureLink Genomic DNA Mini kit (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. Total RNA was extracted from mixed life stages of P. penetrans using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA was then treated with RNase-Free DNase (Qiagen, Valencia, CA, USA) before reverse transcription. The quantity and quality of the extracted RNA was assessed with a ND-1000 Nanodrop spectrophotometer (NanoDrop). The first strand cDNA was synthesized using the iScript first-strand synthesis kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s instructions. The putative Pp-pme genomic coding region of the isolate NL 10p RH was obtained by PCR amplification using 5 μl of the nematode DNA extract, 1x PCR buffer, 1 U Platinum Taq DNA polymerase (Invitrogen) and 0.2 μM of each primer in a 50 μl solution. A PTC-200 Peltier thermocycler was used for amplification using the following PCR conditions: one denaturation step at 94°C for 2 min, followed by 30 cycles at 94°C for 30 secs, 55°C for 30 secs, 72°C for 3 min, and a final extension step of 10 min at 72°C. For amplification of the cDNA sequences of NL 10p RH and A44L4 isolates, 1 μl of cDNA was used which derived from each of the corresponding mixed-stage libraries, followed by similar PCR conditions described above, with an adjusted extended time (i.e. 1 minute per kb was used). The corresponding genomic and cDNA amplicons of P. penetrans were separated on a 1% agarose gel and stained with SYBRSAFE, and the corresponding bands were recovered from the gel using the MinElute Gel Extraction kit (Qiagen, Valencia, CA, USA). Both amplicons were then ligated to the TOPO TA Cloning kit (Invitrogen, Carlsbad, CA, USA) using the protocol provided by the manufacturer, and transformed into E. coli TOP10 competent cells (Invitrogen, Carlsbad, CA, USA). PCR colony positive clones were grown in 3 mL of LB overnight at 37°C followed by plasmid DNA extraction (QIAprep Spin Miniprep kit, Qiagen, Valencia, CA, USA). DNA and cDNA clones were verified by automated sequencing using the universal M13 primers by Macrogen USA (MD, USA). Unless otherwise stated, all the following analyses were conducted with P. penetrans isolate NL 10p RH.
Sequence analysis
To identify introns in the genomic sequence, both cDNA and genomic sequences were aligned using MUSCLE [33]. Gene schematics were generated with the Exon–Intron Graphic maker available at WormWeb.org. The nucleotide and translated amino acid sequences were analyzed for similarity to other genes and proteins using BLAST analyses against the NCBI non-redundant nucleotide and protein databases (http://www.ncbi.nlm.nih.gov/), the nematode.net (http://www.nematode.net/), and NEMBASE4 database (http://www.nematodes.org/nembase4/). In addition, protein sequence analyses were conducted using the following programs: SignalP 4.0 for prediction of protein signal peptide [34]; ProteParam for determination of the protein molecular mass and theoretical isoelectric point [35]; and CLC Main Workbench v. 8.0 software for protein secondary structure predictions. InterPro scan was performed using the public available software package (https://www.ebi.ac.uk/interpro/sequencesearch/iprscan). The overall GC content (%) and codon usage (GC1, GC2 and GC3 corresponding to the 1st, 2nd and 3rd position in the codon triplet, respectively) were calculated using the on-line EMBOSS cusp package (http://www.bioinformatics.nl/cgi-bin/emboss/cusp). Modelling of the tertiary structure of the P. penetrans PME and the corresponding PME TOP hits of Bacteria/ Fungi/Plants/Insects were performed using Phyre2 in the normal mode analysis [36] (http://www.sbg.bio.ic.ac.uk/phyre2). Structural classification of the translated protein followed the convention of SCOPe (Structural Classification of Proteins—extended, http://scop.berkeley.edu/) [37].
BLASTp search was done against the non-redundant (nr) protein database at NCBI, and sequence selection was based on the TOP hits of Bacteria/Archaea/Fungi/Plants/Insects, excluding sequences of the same species with 100% identity. The percentage of protein identity (identical residues in alignment positions) between the P. penetrans PME and those of the other organisms was determined by pairwise comparison. Multiple sequence alignment of PME was conducted with MAFFT algorithm [38] with default settings, trimmed with TRIMAL [39] and manually curated and edited for the selection of protein domain. The phylogenetic relationships were estimated using maximum likelihood (ML) analysis. The best model for protein evolution, determined with AMINOSAN [40] was the “Whelan and Goldman” (WAG) model with discrete gamma distribution (number of gamma categories = 8). The robustness of ML analysis was inferred using 1,000 bootstrap replicates.
Pp-pme expression at different nematode developmental stages
RNA of different nematode developmental stages [eggs, juveniles (J2 to J4), males, and females] was obtained from 150 nematodes and then isolated using the same kit and conditions mentioned above. Expression of the Pp-pme at different developmental stages of P. penetrans was analyzed by semi-quantitative RT-PCR using a pair of primers that amplify a Pp-pme fragment of 221 bp (S1 Table). The P. penetrans 18S rDNA gene was used as the reference gene (S1 Table). PCR products were separated on a 1% agarose gel and stained with SYBR Safe.
In situ hybridization of Pp-pme transcripts
To assess the localization of Pp-pme transcripts, whole mount in situ hybridization was performed using all stages of P. penetrans NL 10p RH following the protocol of de Boer et al. [41]. For localization of the Pp-pme transcripts, the same primers described above for RT-PCR analyses were used, while a fragment of 241 bp of the Pp-eng-1 gene was used as positive control in this experiment (S1 Table). The resulting PCR products were used as a template for generation of sense and antisense DIG-labeled Pp-pme probes using a DIG-nucleotide labeling kit (Roche, Indianapolis, IN, USA). Hybridized probes within the nematode tissues were detected using anti-DIG antibody conjugated to alkaline phosphatase and its substrate. Nematode sections were then observed using a Nikon Eclipse 5i light microscope.
Pp-pme transcript expression in host roots after nematode infection
To validate the expression of Pp-pme during its interaction with plants, in vitro assays were conducted using roots of alfalfa, corn, potato, and soybean and challenged with P. penetrans as described by Vieira et al. [42]. Total RNA was then extracted from a pool of infected roots at 1, 3, 7 or 11 days after nematode infection (DAI) as mentioned previously. Semi-quantitative RT-PCR analyses were performed as described above. Reference genes included an actin gene for corn and potato, the Ubiquitin-3 (Ubi3) for soybean, and the NP_001237047 gene for alfalfa (S1 Table).
Expression of Pp-pme in planta
For expression of the Pp-pme in planta, the full-length coding sequence was amplified from a nematode cDNA library (S1 Table). RT-PCR products were cloned into the pCR TOPO II vector (ThermoFisher Scientific, MA, USA), digested with EcoRV restriction enzyme (restriction site was incorporated into both PCR primers), gel-purified and sub-cloned into the EcoRV-linearized PVX-based vector pP2C2S (obtained from D. Baulcombe, Sainsbury Laboratories, Norwich, England) [43]. The integrity of all clones was verified by automated sequencing. pP2C2S plasmids were linearized with SpeI, and capped transcripts were generated from cDNA clones using Ambion’s T7 mMessage Machine kit (ThermoFisher Scientific, MA, USA). The transcripts were mechanically inoculated onto fully expanded leaves of three N. benthamiana plants. Transcripts were also produced from pP2C2S plasmids without inserts (“empty” PVX vector) and inoculated onto three plants to serve as controls representing a wild-type PVX infection (PVX-WT). Three more plants were buffer-inoculated to serve as negative (“healthy”) controls. Inoculated plants were monitored daily for symptoms. The inoculation experiments that included all sets of plants of each variant was repeated three times. At 14 days after inoculation leaves and roots were photographed and collected, snap-frozen in liquid nitrogen and stored at -80°C until RNA extraction. cDNA was synthesized using the same protocol mentioned above. Semi-quantitative RT-PCR analyses were performed for transcript detection of Pp-pme or PVX wild-type fragments from infected N. benthamiana leaves and roots (S1 Table).
Protein identification
Symptomatic leaves from two individual plants infected with PVX-Pp-pme recombinant virus vector and two plants infected with empty vector (PVX-WT) were sent to Bioproximity LLC (Virginia, USA) for protein extraction and identification services to reveal the top most abundant protein groups expressed in the infected plants. All procedures were performed according to the company protocols and protein assays were carried out on a Thermo Q-Exactive HF-X Orbitrap mass spectrometer (https://www.bioproximity.com/protein-identification). The obtained Ultra Performance Liquid Chromatography—Tandem Mass Spectrometer (UPLC-MS/MS) peptide datasets (mzML format for proteomics mass spectrometric data) were exported as Mascot generic format (.mgf) file using PRIDE Inspector software [44]. PeptideShaker version 1.16.36 [45] was used for protein identification and validation. Protein identification was performed against a concatenated target/decoy version of N. tabacum cv. TN90 proteome (ID UP000084051) [46] retrieved from the UniProtKB (Universal Protein Resource Knowledgebase—http://www.uniprot.org/), with 73,605 proteins complemented with Pp-PME protein sequence (3 Jan, 2019). Decoy sequences were generated by reversing the target sequences in SearchGUI 3.3.11 [47]. Identifications settings were: Trypsin with a maximum of 1 missed cleavages; 10.0 ppm for precursor m/z tolerance (MS1) and 0.5 Da for fragment m/z tolerance MS2; fixed modifications: carbamidomethylation of C (+57.021464Da), and variable modifications: deamination of N (+0.984016 Da), deamination of Q (+0.984016 Da), oxidation of M (+15.994915 Da), pyrolidone from E (-18.010565 Da) and pyrolidone from Q (-17.026549 Da). Peptides and protein inferences were identified from the spectrum of identification results. Protein Inference was considered with 100% confidence when identification was based on 2 or more validated peptides. Peptide Spectrum Matches (PSMs), peptides and proteins were validated at 1.0% False Discovery Rate (FDR) estimated using the decoy hit distribution. UPLC-MS/MS data with respective protein inference were deposited at the proteomics data repository PRIDE Archive (http://www.ebi.ac.uk/pride/archive/) with the dataset identifier PXD012419.
Results
Pp-pme genomic and cDNA coding sequences
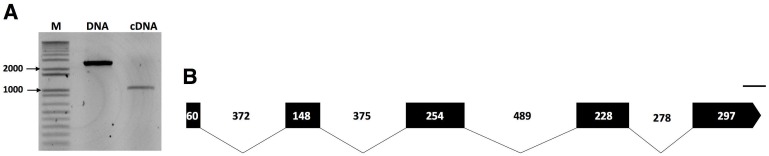
Data mining of the transcriptome assembly of P. penetrans revealed a transcript of 415 bp with high similarity to a pectin methylesterase gene from a bacterium Chitinophaga sp. CF118 (WP_090106049.1, BLAST e-value of 1,31e-111). To validate whether this transcript originated from a prokaryote or an eukaryote, BLAST search analyses were initially performed using the skimming genome assembly of P. penetrans [32], which confirmed the presence of a PME-coding gene in P. penetrans. The cloned genomic DNA amplicon revealed a transcribed genomic sequence of 2,501 bp, while the cloned cDNA amplicon contained a putative open reading frame of 987 bp for the isolate NL 10p RH (Fig 1A). The exon/intron boundaries of the genomic sequence were determined by aligning both genomic and cDNA sequences (Fig 1B and S1 Fig). The genomic sequence revealed the presence of four introns (368, 377, 493 and 282 bp long), all following the canonical GT/AG splicing site (Fig 1B). The coding sequence has an overall GC content of 56.36%, with a GC1 of 52.25%, GC2 of 45.95%, and GC3 of 70.87% (S2 Table). Using the same set of primers, the full-length cDNA sequence of a P. penetrans A44L4 isolate collected in Portugal was cloned, thus confirming the presence of a PME gene in a distant geographic isolate. The cloned Pp-pme cDNA sequence of the Portuguese isolate yielded a coding sequence of 981 bp and had 94.74% identity to the Pp-pme of the USA isolate (S2A Fig). The translated protein sequences of both isolates share 95.73% identity (S2B Fig). The cloned sequences of both isolates were deposited at NCBI as Pp-pme with the accession numbers MK295632 and MK295633, respectively. Unless otherwise stated, all the following analyses were conducted with P. penetrans NL 10p RH isolate.
Fig 1. Molecular characterization of the pectin methylesterase (Pp-pme) of Pratylenchus penetrans.
(A) Amplicons of both genomic (2,501 bp) and cDNA coding (987 bp) sequences of Pp-pme, respectively. (B) Schematic representation of the Pp-pme gene structure. Relative positions and respective sizes of the exons are indicated by dark boxes and introns by lines.
Characterization and sequence analysis of the predicted Pp-PME protein
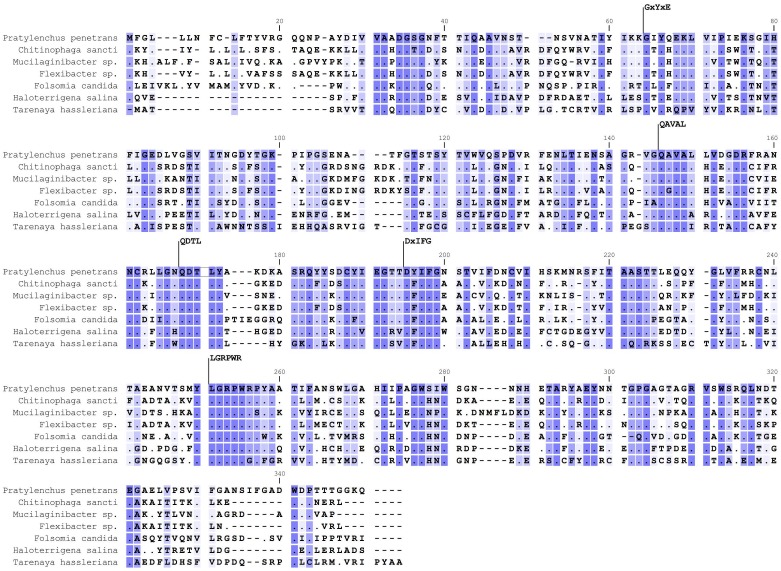
In silico translation of the Pp-pme full-length cDNA revealed a protein sequence of 328 amino acids with a predicted molecular weight of 35.815 kDA and a pI of 5.87. The protein was predicted to have an N-terminal signal peptide of 16 amino acids as determined by SignalP v. 4.0, with a cleavage site located between positions 16 and 17 (VRG-QQ), and no predicted transmembrane domain. Pfam domain search and InterPro scan confirmed this protein as a member of the CE8 family (pfam01095; InterPro IPR000070). PMEs can also be distinguished on the basis of specific signature patterns in their amino acid sequences [48]. Based on the multiple sequence alignment of Pp-PME with PMEs of other organisms, the following five conserved sequence segments typical of PMEs were identified: 64_GxYxE, 146_QAVAL, 168_QDTLY, 195_DxIFG and 251_LGRPR (Fig 2).
Fig 2. Multiple sequence alignment of the predicted Pp-PME protein of Pratylenchus penetrans with PMEs of other organisms.
Representative species of bacteria (Chitinophaga sancti, Mucilaginibacter sp., Flexibacter sp.), collembolan (Folsomia candida), Archaea (Haloterrigena salina) and plants (Tarenaya hassleriana) were used. The five conserved sequence segments typical of PME proteins are numbered according to their positions in the corresponding Pp-PME predicted protein (64_GxYxE, 146_QAVAL, 168_QDTL, 195_DxIFG, and 251_LGRPW). Conserved residues among species are indicated by dark blue shading and dots, whereas similar residues are represented in light blue using a threshold for shading of 50% similarity. The accession numbers corresponding to each species are presented in S3 Table.
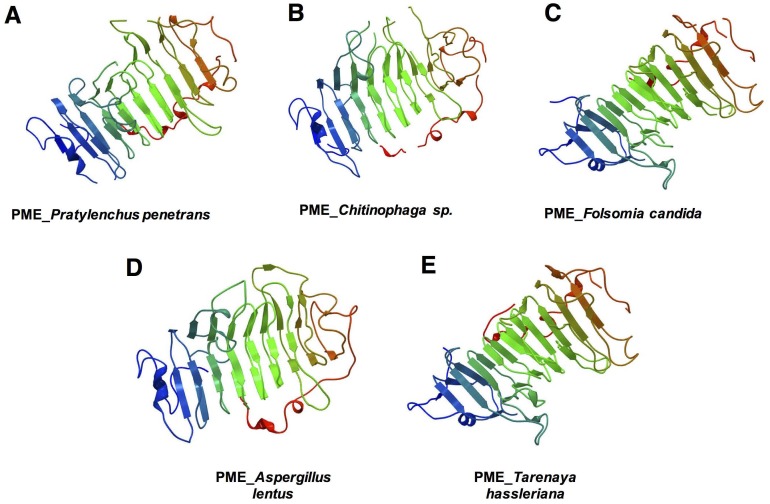
A three-dimensional model of Pp-PME of P. penetrans (Fig 3) was predicted using the PME of Erwinia chrysanthemi as template (NCBI TaxId: 556), which was the top hit species obtained by a BLASTp similarity search against the Protein Data Bank. There was 41% identity and 293 residues were covered with 100% confidence. This structure displayed a single-stranded, right-handed beta-helix fold (SCOPe: 51125) composed predominantly of beta-strands (class All beta proteins, SCOP 48124). One alpha-helix was predicted at the N-terminal end of the PME (14 amino acids) and another alpha-helix (5 amino acids) was predicted and at the C-terminal end. The latter was followed by a beta-strand (5 amino acids) and a short alpha-helix (4 amino acids). For the other organisms, the model of each corresponding PME was predicted with 100% confidence (Fig 3).
Fig 3. Three-dimensional model predicted for the pectin methylesterase of different organisms.
(A) Pratylenchus penetrans, (B) bacteria (Chitinophaga sp.), (C) collembolan (Folsomia candida), (D) fungi (Aspergillus lentus), and (E) plants (Tarenaya hassleriana). The models of P. penetrans and Chitinophaga sp. were based on the three-dimensional model of the Erwinia chrysanthemi (Phyre2 fold library ID: d1gq8a), F. candida and T. hassleriana were based on the model of Daucus carota (Phyre2 fold library ID: d1qjva), and A. lentus on the model of A. niger (Phyre2 fold library ID: c5c1cA), respectively. The N-terminal is indicated in blue and the C-terminal is shown in red.
Phylogenetic analysis of Pp-PME
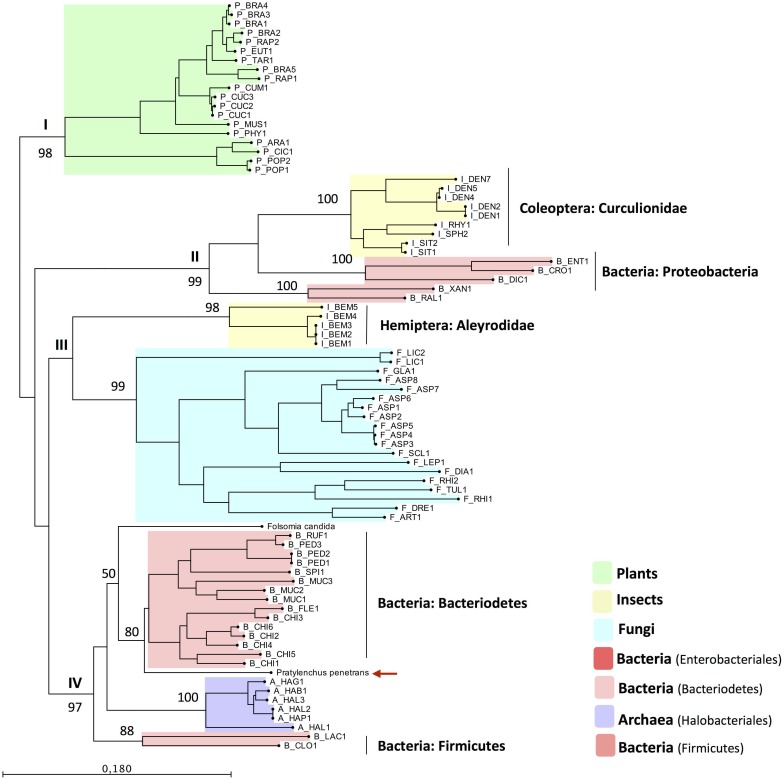
Although PMEs occur naturally in both prokaryotes and eukaryotes, prior to this work there was no record of a PME within the phylum Nematoda. BLAST searches were performed using the Pp-PME protein sequence as a query against different public databases. The top 15 BLAST hits, consisting of PMEs of bacteria origin only, are presented in Table 1. The amino acid sequence of Pp-PME was found to be most closely related to PMEs of different bacteria of the phylum Bacteriodetes (E-values ranging from 1e-111 to 1e-105), showing 56.86% identity with Chitinophaga sp. CF118, 53.65% with Mucilaginibacter sp. PPCGB 2223, and 53.54% with Flexibacter sp. ATCC35208. When BLASTp searches were performed against other organisms for which PMEs have been previously identified (i.e. archaea, fungi, insects, and plants) (S3 Table), the highest homology of the Pp-PME was found with the PME of the soil arthropod Folsomia candida (Arthropoda: Collembola: Isotomidae) (51.58% protein identity; E-value = 7e-96), and with the PME of Haloterrigena salina (Halobacteriaceae: Euryarchaeota: Archaea) (46.18% protein identity; E-value = 4e-83). BLAST results against insects and plants showed a lower identity (up to 43.17% and 40.62%, respectively) against the nematode Pp-PME (S3 Table). Despite exhaustive BLAST searches against the large number of sequences and genomes available for other RLNs or PPNs, no homologues were found in any available nematode sequence datasets. The analysis of codon usage of Pp-PME and the corresponding top hit of each group of organisms (S4 Table) indicates that Pp-PME exhibits codon usage that is more similar to other eukaryotic species, than to prokaryotes.
Table 1. List of top BLAST hit sequences obtained by BLASTp analyses (E-value <1e-5) using P. penetrans PME as query.
| Species | Accession no. | Organism (Phylum: Family) |
BLASTp (E-value) |
Identity (%) |
|---|---|---|---|---|
| Chitinophaga sp. CF118 | WP_090106049.1 | Bacteriodetes: Chitinophagaceae | 1,31e-111 | 56.86 |
| Chitinophaga sp. YR627 | WP_089809083.1 | Bacteriodetes: Chitinophagaceae | 8,21e-110 | 54.69 |
| Chitinophaga sancti | WP_072360114.1 | Bacteriodetes: Chitinophagaceae | 9,39e-113 | 54.63 |
| Chitinophaga filiformis | WP_089838976.1 | Bacteriodetes: Chitinophagaceae | 1,06e-107 | 54.37 |
| Chitinophaga sp. YR573 | WP_089782777.1 | Bacteriodetes: Chitinophagaceae | 2,05e-111 | 53.99 |
| Chitinophaga pinensis | WP_012789484.1 | Bacteriodetes: Chitinophagaceae | 9,27e-111 | 53.99 |
| Mucilaginibacter sp. PPCGB 2223 | WP_066007482.1 | Bacteriodetes: Shingobacteriaceae | 2,12e-105 | 53.65 |
| Flexibacter sp. ATCC 35208 | WP_083721311.1 | Bacteriodetes: Cytophagaceae | 2,28e-109 | 53.54 |
| Mucilaginibacter paludis | WP_008506893.1 | Bacteriodetes: Shingobacteriaceae | 5,15e-109 | 53.50 |
| Mucilaginibacter sp. OK268 | WP_090463524.1 | Bacteriodetes: Shingobacteriaceae | 1,50e-105 | 53.07 |
| Spirosoma aerolatum | WP_080054327.1 | Bacteriodetes: Cytophagaceae | 2,23e-106 | 52.98 |
| Pedobacter ginsenosidimutans KACC14530 | KRT18230.1 | Bacteriodetes: Shingobacteriaceae | 1,55e-106 | 52.12 |
| Pedobacter ginsenosidimutans | WP_083505239.1 | Bacteriodetes: Shingobacteriaceae | 2,28e-106 | 52.12 |
| Pedobacter sp. PACM 27299 | WP_062548895.1 | Bacteriodetes: Shingobacteriaceae | 6,99e-107 | 51.09 |
| Rufibacter roseus | WP_066624157.1 | Bacteroidetes: Hymenobacteraceae | 2,19e-105 | 50.61 |
The predicted pectinesterase domain sequence (pfam01095, position 23–307 aa, E-value = 1.8e-68) of Pp-PME was then aligned with the corresponding domain sequences of the top hit PME proteins of 53 eukaryotic species, including those of fungi, insects, and plants, as well as 29 prokaryotic species distributed among bacteria and Archaea (S3 Table). In order to infer the phylogenetic relationship of the Pp-PME and other organisms, ML analyses were performed (Fig 4). These analyses revealed a clear separation of the PMEs of plant origin with the remaining PMEs (i.e. archaea, bacteria, fungi, insects, and P. penetrans), and highly supported by our bootstrap results (>98%). The PMEs of the remaining taxa could be separated into three main phylogenetic clades, which is also highly supported by the bootstrap analyses. Interestingly, the PMEs of the insects of the family Curculionidae (Coleoptera) clustered together with bacterial PMEs of the Proteobacteria phylum, while the PMEs of the whitefly B. tabaci (family Aleyrodidae, Hemiptera) grouped with a monophyletic clade formed by fungi only. As denoted by the protein alignment described above, Pp-PME clustered with bacterial sequences of the phylum Bacteriodetes (80% bootstrap value), including the soil collembolan F. candida (family Isotomidae, Collembola), although with a lower bootstrap value. All remaining PME sequences of bacteria (Phylum Firmicutes) and Archaea formed well-separated sub-clusters.
Fig 4. Phylogenetic tree based on the closest homology to the catalytic domain of Pp-PME.
The PME sequences across different taxa were chosen based on the top BLAST hits against the Pp-PME predicted pectinesterase domain. The corresponding species names and range of e-values are presented in S3 Table. The red arrow indicates the position of P. penetrans PME. The phylogenetic tree was deduced by Maximum Likelihood with the “Whelan and Goldman” (WAG) model with discrete gamma distribution and 1000 bootstrap replicates.
Expression pattern profile of Pp-pme at different stages of nematode development
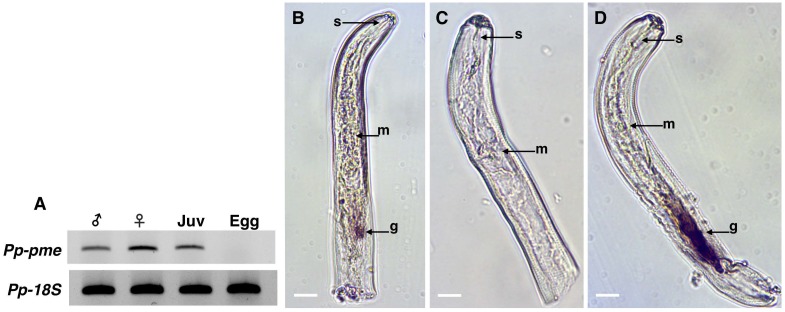
The expression pattern of Pp-pme was determined by semi-quantitative RT-PCR for different nematode developmental stages [eggs, juveniles (J2-J4), adult males and females] (Fig 5A). Pp-pme expression was only detected for nematode motile stages, whereas no expression was detected from the eggs. Amplification of 18S rDNA gene fragment was used as positive control in this experiment.
Fig 5. Expression and localization of Pratylenchus penetrans Pp-pme transcripts.
(A) Determination of Pp-pme expression in different nematode developmental stages of P. penetrans by semi-quantitative RT-PCR. As a positive control, all cDNA templates were amplified with primers derived from the 18S gene of P. penetrans. The nematode stages were separated as males, females, juveniles (J2-J4), and eggs. (B-C) Detection of the Pp-pme transcripts by in situ hybridization. Nematode sections were hybridized with antisense (B), or sense (C) Pp-pme digoxigenin-labeled cDNA probes. (D) As a positive control, in situ hybridization was performed with the antisense probe designed for the CWDE (Pp-eng-1) specifically localized within the esophageal glands g: esophageal glands; m: metacorpus; s: stylet.
Identification of the gene-specific transcripts within the nematode tissues can provide insights into putative functions of the Pp-pme. Therefore, we performed whole mount in situ hybridization for detection of transcripts within the nematode tissues. As determined by the antisense probe, the Pp-pme transcripts accumulated within the esophageal glands of the nematode (Fig 5B), while no signal was detected using the sense probe of the Pp-pme as control (Fig 5C). As a positive control, in situ hybridization was performed with the antisense probe designed for a CWDE (Pp-eng-1) that specifically localizes within the esophageal glands (Fig 5D). The localization of the Pp-pme within the glands and the presence of a N-terminal signal peptide of the deduced Pp-PME protein suggest its secretion by the nematode into the host.
Pp-pme transcript expression in host roots after nematode infection
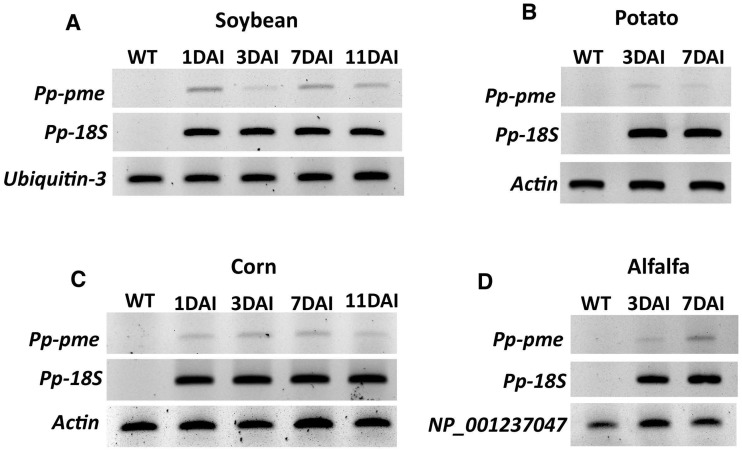
The expression of the Pp-pme was analyzed during interaction with different host plants (e.g. roots of alfalfa, corn, potato and soybean) by semi-quantitative RT-PCR at early time points after nematode infection (Fig 6). In each respective nematode-plant interaction the expression of the Pp-pme could be detected, showing that this gene is actively transcribed during the nematode’s interaction with the host plants.
Fig 6. Semi-quantitative RT-PCR showing the transcript levels of Pp-pme in different host plants at different time points after infection.
Total RNA extracted from nematode infected roots of economically important host plants (A) soybean, (B) potato, (C) corn, and (D) alfalfa was used to validate the relative expression of Pp-pme at different days after nematode infection (DAI). The nematode 18S rDNA gene was used as internal control to validate the presence of P. penetrans within the infected roots, while specific plant reference genes were used for each specific host plant. Wild-type (WT) correspond to non-infected plants.
Transient expression of Pp-pme in Nicotiana benthamiana
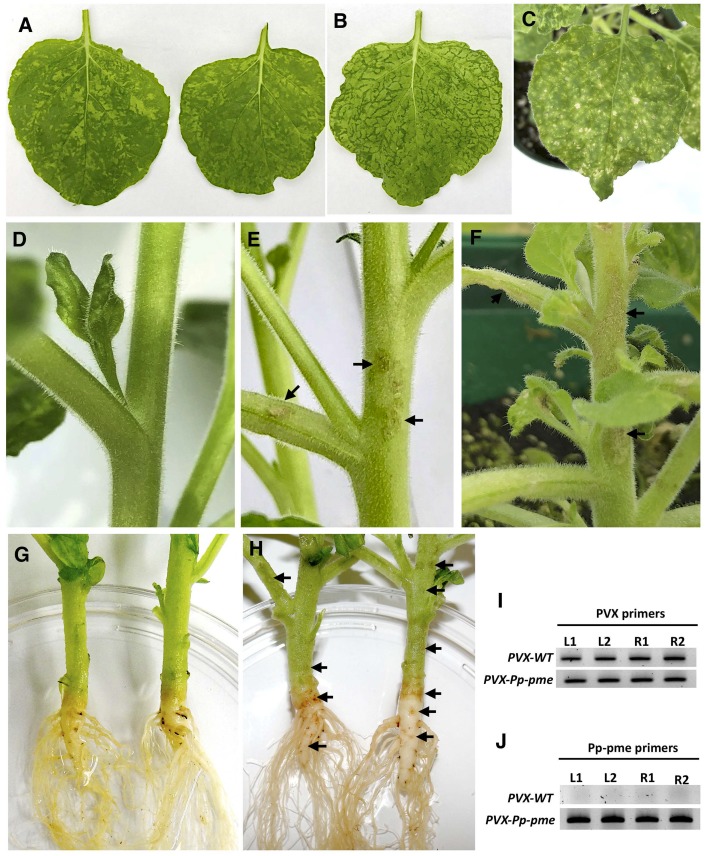
Plant-parasitic nematodes are able to secrete effector proteins directly into the apoplasm or cytoplasm of the plant cell. CWDEs are often secreted by PPNs to the apoplasm during invasion of the plant roots [49]. These CWDEs are thought to be involved in cell wall disassembly thus facilitating the penetration and migration process of the nematode into the roots. This is indicative that the Pp-PME could be also secreted into the apoplast by the nematode and be involved in the invasion of the roots. To direct the nematode PME into the secretory pathway of the host cell, and to assess putative Pp-pme functional role, the full-length coding sequence of the Pp-pme gene, including the signal peptide, was transiently expressed in N. benthamiana using a PVX-based vector. Phenotypic differences between symptoms generated by the recombinant virus containing Pp-pme and by the empty PVX vector are shown in Fig 7. Systemic expression of the Pp-pme gene via the PVX vector in N. benthamiana resulted in more pronounced chlorosis, vein clearing and yellowing of the leaves starting 10–14 days after inoculation (Fig 7B). In some cases, lesion-like symptoms were observed on the leaves of PVX-Pp-pme-infected plants (Fig 7C). Plants expressing empty PVX transcripts displayed characteristic mosaic-like symptoms (Fig 7A). In addition, all plants inoculated with PVX-Pp-pme exhibited distinct lesion-like symptoms in different areas of the stem, branches (Fig 7E and 7F) and main root (Fig 7H), which were absent in plants infected with empty PVX vector (Fig 7D and 7G). Semi-quantitative RT-PCR conducted confirmed expression of the Pp-pme gene in plants inoculated with PVX-Pp-pme (Fig 7J), while a fragment of PVX plasmid confirmed the expression of PVX transcripts in inoculated plants (Fig 7I).
Fig 7. Phenotypic changes in Nicotiana benthamiana plants infected with the recombinant PVX-Pp-pme virus.
All photos were taken 14 days after inoculation. (A) characteristic mosaic-like symptom developed in N. benthamiana plants infected with transcripts generated from the empty PVX vector. (B-C) Distinct leaf chlorosis symptoms (B) and lesion-like spots (C) developed in N. benthamiana plants infected with PVX-Pp-pme transcripts, respectively. (D) Stem and branches of the N. benthamiana plant infected with transcripts generated from the empty PVX vector displayed no symptoms. (E-F) Lesion-like symptoms observed on stems and branches of N. benthamiana plants infected with PVX-Pp-pme transcripts. (G) Stem and roots of N. benthamiana plant infected with empty PVX vector displayed no symptoms. (H) Browning in the area of the stem-root joint and lesion-like spots observed on the roots of N. benthamiana plants infected with PVX-Pp-pme transcripts. (I-J) Detection of PVX-WT and PVX-Pp-pme transcripts in inoculated N. benthamiana plants by semi-quantitative RT-PCR. L1, L2: leaves from two independently-inoculated plants; R1, R2: roots from two independently-inoculated plants.
To detect the possible accumulation of the Pp-PME in the leaves of N. benthamiana plants, UPLC-MS/MS analyses were performed with proteins extracted from the leaves of PVX-Pp-pme and PVX-WT plants. The peptides identified by mass spectrometry were matched to a set of 73,605 proteins sequences of N. tabacum, and complemented with the predicted Pp-PME protein sequence (S6A and S6B Table). In both PVX-Pp-pme plants, seven to nine peptides were identified that were 100% identical to Pp-PME (Table 2 and S3 Fig). No Pp-PME-related peptides were retrieved from two PVX-WT plants (S6C and S6D Table).
Table 2. List of Pp-PME peptides identified in the peptide datasets generated by UPLC-MS/MS from leaves expressing the PVX-Pp-PME construct.
| Library/Peptide # | Peptide Sequence | Peptide Start | Peptide End | Score (%) |
|---|---|---|---|---|
| Library 1 | ||||
| 1 | SFITAASTTLEQQYGLVFR | 206 | 225 | 100 |
| 2 | VGQAVALLVDGDRFR | 136 | 151 | 100 |
| 3 | FENLTIENSAGR | 124 | 136 | 100 |
| 4 | GQAVALLVDGDRFR | 137 | 151 | 100 |
| 5 | LLGNQDTLYAK | 156 | 167 | 100 |
| 6 | RSFITAASTTLEQQYGLVFR | 205 | 225 | 100 |
| 7 | SFITAASTTLEQQYGLVFRR | 206 | 226 | 100 |
| 8 | SGIHFIGEDLVGSVITNGDYTGK | 75 | 98 | 100 |
| 9 | VGQAVALLVDGDR | 136 | 149 | 100 |
| Library 2 | ||||
| 1 | FENLTIENSAGR | 124 | 136 | 100 |
| 2 | LLGNQDTLYAK | 156 | 167 | 100 |
| 3 | SFITAASTTLEQQYGLVFR | 206 | 225 | 100 |
| 4 | SFITAASTTLEQQYGLVFRR | 206 | 226 | 100 |
| 5 | SFITAASTTLEQQYGLVFR | 206 | 225 | 100 |
| 6 | VGQAVALLVDGDR | 136 | 149 | 100 |
| 7 | VGQAVALLVDGDRFR | 136 | 151 | 100 |
Discussion
In this work, we report the identification, characterization, and phylogenetic status of a putative pectin methylesterase gene PME that encodes a cell wall-degrading enzyme in the RLN, P. penetrans. Several previous studies have shown that PPNs possess a set of CWDEs to degrade the plant cell wall and thus facilitate their invasion and migration through the plant host tissues. The diversity and number of CWDEs endogenous to each PPN do not only reflect the complex nature of the plant cell wall and its structural components, but also indicate an elaborate parasitic strategy and adaptation of these obligatory plant-pathogens [50]. Although different families of CWDEs have been reported in a wide range of PPNs, including RLNs, prior to this work, no pectin methylesterase gene has been identified in the phylum Nematoda.
Although bacteria (e.g. Wolbachia) have been reported from within different parts of the P. penetrans body [51], the features of the Pp-pme gene exclude the possibility of a prokaryotic contamination. The gene structure of this new nematode CWDE encompasses typical eukaryotic features with four characteristic spliceosomal introns (GC/AG splice sites). The presence of a putative signal peptide in the corresponding Pp-PME sequence, coupled with its transcript expression in the esophageal glands of the nematode, highlights its potential importance in the parasitic process of P. penetrans. This is in agreement with our previous observations of other CWDEs found in this particular species [31], which were localized in the esophageal glands of the nematodes and potentially secreted into the host tissues.
Despite all PMEs being classified under the same family, their mode of action is likely different, depending on the pH and ionic environment, substrate specificity, and origin [17]. In plants, PMEs are encoded by a large family of genes, which emphasizes their functional diversity within the plant tissues [9]. In bacterial and fungal pathogens, PMEs play a critical role in their virulence [52], since their secretion has been related to enzymatic degradation of the pectin polysaccharides of the plant cell walls. P. penetrans has a wide range of host plants and is capable to parasitize on both mono- and dicotyledonous plants. The components of the cell wall in monocots and dicots vary extensively, thus requiring some plasticity in the substrate specificity of the enzymes secreted by the nematode. Our results indicate that Pp-pme is expressed during the early infection time points in different host plants. This is in line with the expression of other CWDEs identified in P. penetrans, such as pectin lyases and different glycoside hydrolase (GH) gene families [31]. Degradation of the plant cell wall pectin network requires the synergistic action of different pectolytic enzymes, such as polygalacturonases (GH28), PLs, and PMEs. PME catalyze the de-methylesterification of pectin, a major component of plant cell wall, where their activity is finely tuned through endogenous inhibitors. Plant-derived PMEs can contribute toward immunity against pathogens [53], while pathogenic PME may function to promote disease [18, 19]. The activity of PMEs can improve cell wall accessibility for other CWDEs and consequently accelerate cell wall degradation [9, 10, 11]. The correctness of the discovered Pp-pme genetic sequence was confirmed by its transient expression in planta, where it translated into a PME-like protein. Therefore, it is plausible that the synchronized expression of the nematode PME with other CWDEs during infection could induce changes in the properties of the plant cell wall, contributing to penetration and migratory activity of the nematode. Speculatively, the symptoms observed on PVX-Pp-pme infected plants could be potentially related to the activation of the plant defense reactions against this effector-like protein. Although, further experiments are needed to confirm this.
To date, PMEs are restricted to plants, bacteria, and fungi and are exclusively found in a small number of insect species belonging to the family Curculionidae [23, 26, 54], the whitefly and the springtail [24, 25]. The predicted pectinesterase amino acid domain of the Pp-PME showed significant similarity to different bacterial PMEs. Phylogenetic analyses performed in this work, indicates that this gene has most likely been acquired by horizontal gene transfer (HGT) into P. penetrans. Horizontal gene transfer events of other CWDEs from ancestral microbial donors into sedentary and migratory PPNs of the Tylenchida have been extensively reported [50, 55]. One common characteristic of these CWDE genes, which was also observed for the genomic Pp-pme sequence, is the presence of long introns [56, 57], in contrast to the average intron size reported for the available nematode genomes [58]. The functions of the transferred gene products are often linked to particular steps of nematode-plant interaction, particularly to the parasitism of PPNs. As mentioned above, Wolbachia endosymbionts have been found in different tissues of P. penetrans [51], which could be seen as a potential route for HGT of the transmissible genomic fragments to the nematode. However, the symbiotic bacteria described so far from nematodes, including the most recently characterized Wolbachia strain (wPpe) isolated from P. penetrans [46], are not known to encode any of the CWDEs that have been potentially acquired by HGT [50].
The PME sequences of the bacterial origin used in this study are highly heterogeneous and were placed by the phylogenetic analysis into different branches of the phylogenetic tree correlating with different niches and taxa. While PME sequences of flying insects of the family Curculionidae strongly correlate with Proteobacteria, the whitefly B. tabaci clustered together with PME sequences of the fungal origin. The diversity of the genes encoding PME in the different groups of insects, support the idea that these PMEs have potentially been acquired multiple times independently by their receiver taxa [23]. In many cases, genes encoding CWDEs in PPNs seemed to be most closely related to sequences found in bacteria that inhabit the soil [55]. The predominant putative ancestral donors found so far are related to bacteria of the Phyla Proteobacteria, Actinobacteria, Firmicutes, and Bacteriodetes [55, 59]. In line with these findings, P. penetrans PME is most similar to PMEs of bacterial sequences of the phyla Bacteriodetes (Chitinophagaceae, Chitinophaga), followed by the PME sequences of the soil collembolan F. candida (Collembola: Isotomidae). Species belonging to the Bacteriodetes are specialized in the degradation of complex organic matter [60, 61], particularly the family Chitinophagaceae that showed a high ability for degradation of plant-derived carbohydrates (e.g. cellulose and chitin) [62, 63]. Remarkably, this group of bacteria has been recently reported to be a part of the rhizosphere microbiome associated with the presence of P. penetrans [63] and also found in the microbiome of F. candida [64, 65]. Similarly, the genome of F. candida, one of the most abundant arthropods in soil, showed an extensive number of carbohydrate-active enzymes potentially acquired by the HGT from soil bacteria. These enzymes enable F. candida to scavenge decaying plant matter as a food source [25]. As RLN are composed of a vast number of species with all of them occurring in the soil, it will be interesting to investigate whether they also carry a PME-coding gene.
Supporting information
(XLSX)
Pp, Pratylenchus penetrans; Bacteria, B; Archaea, A; Fungi, F; Insect, I; Plant, P.
(XLSX)
These sequences of each particular taxa correspond to the top BLAST hits against P. penetrans PME sequence.
(XLSX)
The preferred codons are highlighted in red.
(XLSX)
(XLSX)
(XLSX)
Exons and introns are indicated by green and red arrows, respectively.
(PDF)
Pp-PME US isolate corresponds to the isolate NL 10p RH collected in Beltsville (Maryland, US), Pp-PME PT isolate corresponds to the isolate A44L4 collected from potato fields in Portugal (Coimbra, Portugal).
(PDF)
(A) and (B) correspond to the different Pp-PME peptides identified from two protein libraries extracted from the leaves of Nicotiana benthamiana plants inoculated with PVX-Pp-pme.
(PDF)
Acknowledgments
We thank Dr. Ivânia Esteves (NematoLab, Universidade de Coimbra, Coimbra, Portugal) for providing the Pratylenchus penetrans isolate A44L4.
Data Availability
The cloned sequences were deposited at NCBI with the accession numbers MK295632 and MK295633, and the peptide libraries were deposited at the proteomics data repository PRIDE Archive (http://www.ebi.ac.uk/pride/archive/) with the dataset identifier PXD012419.
Funding Statement
This work was supported by the FCT (Foundation for Science and Technology) postdoctoral fellowship SFRH/BPD/116030/2016 (to CSLV); the national project PTDC/AGR-PRO/2589/2014 – PratyTech (to MM), Biotechnology approaches towards control of the root lesion nematode Pratylenchus penetrans and by National Funds through FCT under the Project UID/AGR/00115/2013 (to CSLV and MM).
References
- 1.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6: 850–861. 10.1038/nrm1746 [DOI] [PubMed] [Google Scholar]
- 2.Keegstra K. Plant Cell Walls. Plant Physiol. 2010;154: 483–486. 10.1104/pp.110.161240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen I, Domozych D, Willats WGT. How have plant cell walls evolved? Plant Physiol. 2010;153: 366–372 10.1104/pp.110.154427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan DB, Bowman MJ, Braker JD, Dien BS, Hector RE, Lee CC, et al. Plant cell walls to ethanol. Biochem J. 2012;442: 241–252. 10.1042/BJ20111922 [DOI] [PubMed] [Google Scholar]
- 5.Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11: 266–277. 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Voragen AGJ, Coenen GJ, Verhoef RP, Schols HA. Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem. 2009;20: 263–275. [Google Scholar]
- 7.Protsenko MA, Buza NL, Krinitsyna AA, Bulantseva EA, Korableva NP. Polygalacturonase-inhibiting protein is a structural component of plant cell wall. Biochem Mosc. 2008;73: 1053e1062. [DOI] [PubMed] [Google Scholar]
- 8.Sénéchal F, Wattier C, Rustérucci C, Pelloux J. Homogalacturonan- modifying enzymes: structure, expression, and roles in plants. J Exp Bot. 2014;65: 5125–5160 10.1093/jxb/eru272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6: 414–419. [DOI] [PubMed] [Google Scholar]
- 10.Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci. 2014;5: 228 10.3389/fpls.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelloux J, Rustérucci C, Mellerowicz E. New insights into methylesterase structure and function. Trends Plant Sci. 2007;12: 267–277. 10.1016/j.tplants.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 12.Tieman DM, Handa AK. Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill) fruits. Plant Physiol. 1994;106: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen F, Zhu Y, Hawes MC. Effect of pectin methylesterase gene expression on pea root development. Plant Cell. 1999;11: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilling J, Willmitzer L, Bucking H, Fisahn J. Inhibition of a ubiquitously expressed pectin methyl esterase in Solanum tuberosum L. affects plant growth, leaf growth polarity, and ion partitioning. Planta. 2004;219: 32–40. 10.1007/s00425-004-1204-y [DOI] [PubMed] [Google Scholar]
- 15.Wietholter N, Graessner B, Mierau M, Mort AJ, Moerschbacher BM. Differences in the methyl ester distribution of homogalacturonans from near-isogenic wheat lines resistant and susceptible to the wheat stem rust fungus. Mol. Plant Microbe Interact. 2003;16: 945–952. 10.1094/MPMI.2003.16.10.945 [DOI] [PubMed] [Google Scholar]
- 16.Chen MH, Citovsky V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. The Plant J. 2003;35: 386–392. [DOI] [PubMed] [Google Scholar]
- 17.L’Enfant M, Domon JM, Rayon C, Desnos T, Ralet MC, Bonnin E, et al. Substrate specificity of plant and fungi pectin methylesterases: identification of novel inhibitors of PMEs. Int J Biol Macromol. 2015;81: 681–691. 10.1016/j.ijbiomac.2015.08.066 [DOI] [PubMed] [Google Scholar]
- 18.Shevchik VE, Hugouvieux-Cotte-Pattat N. Identification of a bacterial pectin acetyl esterase in Erwinia chrysanthemi. Mol Microbiol. 1997;24: 1285–1301. [DOI] [PubMed] [Google Scholar]
- 19.Valette-Collet O, Cimerman A, Reignault P, Levis C, Boccara M. Disruption of Botrytis cinerea pectin methylesterase Bcpme1 gene reduces virulence on several host plants. Mol Plant Microbe Interact. 2013;16: 360–367. [DOI] [PubMed] [Google Scholar]
- 20.Shen ZC, Manning G, Reese JC, Reeck GR. Pectin methylesterase from the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae): purification and characterization. Insect Biochem Mol. 1999;29: 209e214. [Google Scholar]
- 21.Evangelista DE, Paula FFP, Rodrigues A, Henrique-Silva F. Pectinases from sphenophorus levis vaurie, 1978 (Coleoptera: Curculionidae): Putative accessory digestive enzymes. J Insect Sci. 2015;15: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antony B, Johny J, Aldosari SA, Abdelazim MM. Identification and expression profiling of novel plant cell wall degrading enzymes from a destructive pest of palm trees, Rhynchophorus ferrugineus. Insect Mol Biol. 2017;26: 469–484. 10.1111/imb.12314 [DOI] [PubMed] [Google Scholar]
- 23.Pauchet Y, Wilkinson P, Chauhan R, ffrench-Constant RH. Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One. 2010;5: e15635 10.1371/journal.pone.0015635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Hasegawa DK, Kaur N, Kliot A, Pinheiro PV, Luan J, et al. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016;14: 110 10.1186/s12915-016-0321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faddeeva-Vakhrusheva A, Kraaijeveld K, Derks MFL, Anvar SY, Agamennone A, Suring W, et al. Coping with living in the soil: The genome of the parthenogenetic springtail Folsomia candida. BMC Genomics. 2017;18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teller DC, Behnke CA, Pappan K, Shen Z, Reese JC, Reeck GR et al. The structure of rice weevil pectin methylesterase. Acta Crystallogr F Struct Biol Commun. 2014;70: 1480–1484. 10.1107/S2053230X14020433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirsch R, Heckel DG, Pauchet Y. How the rice weevil breaks down the pectin network: Enzymatic synergism and sub-functionalization. Insect Biochem Mol Biol. 2016;71: 72–82. 10.1016/j.ibmb.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Jones MGK, Fosu-Nyarko J. Molecular biology of root lesion nematodes (Pratylenchus spp.) and their interaction with host plants. Ann Appl Bio. 2014;164: 163–181. [Google Scholar]
- 29.Castillo P, Vovlas N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management. Nematology Monographs and Perspectives. Brill; 2007.
- 30.Vieira P, Akker SEvdA, Verma R, Wantoch S, Eisenback JD, Kamo K. The Pratylenchus penetrans transcriptome as a source for the development of alternative control strategies: mining for putative genes involved in parasitism and evaluation of in planta RNAi. PLoS One. 2015;10: e0144674 10.1371/journal.pone.0144674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vieira P, Maier TR, Akker SEvdA, Howe DK, Zasada I, Baum TJ, et al. Identification of candidate effector genes of Pratylenchus penetrans. Mol Plant Pathol. 2018;19: 1887–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denver DR, Brown AMV, Howe DK, Peetz AB, Zasada IA. Genome skimming: a rapid approach to gaining diverse biological insights into multicellular pathogens. PLoS Pathog. 2016;12: e1005713 10.1371/journal.ppat.1005713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nuclei Acids Res. 2004;32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen TN, Brunak S, Von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8: 785–786 (2011). 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 35.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Identification and analysis tools on the ExPASy server The Proteomics Protocols Handbook. Humana Press; 2005 [Google Scholar]
- 36.Kelly LA, Mezulis S, Yates C, Wass M, Sternberg M. The Phyre2 web portal for protein modelling, prediction, and analysis. Nat Protoc. 2015;10: 845–858. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox NK, Brenner SE, Chandonia JM. SCOPe: Structural Classification of Proteins—Extended, integrating SCOP and ASTRAL data and classification of new structures. Nucleic Acids Res. 2014; 42: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2017; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25: 1972–1973. 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanabe AS. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analysis of multilocus sequence data. Mol Ecol Resour. 2011;11: 914–921. 10.1111/j.1755-0998.2011.03021.x [DOI] [PubMed] [Google Scholar]
- 41.de Boer JM, Yan Y, Smant G, Davis EL, Baum TJ. In-situ hybridization to messenger RNA in Heterodera glycines. J Nematol. 1998;30: 309–312. [PMC free article] [PubMed] [Google Scholar]
- 42.Vieira P, Kamo K, Eisenback JD. Characterization and silencing of the fatty acid- and retinol-binding Pp-far-1 gene in Pratylenchus penetrans. Plant Pathol. 2017;66: 1214–1224. [Google Scholar]
- 43.Chapman S, Kavanagh TA, Baulcombe DC. Potato virus X as a vector for gene expression in plants. Plant J. 1992;2: 549–557. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Riverol Y, Xu QW, Wang R, Uszkoreit J, Griss J, Sanchez A, et al. PRIDE Inspector Toolsuite: moving towards a universal visualization tool for proteomics data standard formats and quality assessment of ProteomeXchange datasets. Mol Cell Proteomics. 2016;15: 305–317. 10.1074/mcp.O115.050229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaudel M, Burkhart JM, Zahedi RP, Oveland E, Berven FS, Sickmann A, et al. PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat Biotechnol. 2015;33: 22–24. 10.1038/nbt.3109 [DOI] [PubMed] [Google Scholar]
- 46.Sierro N, Battery JN, Ouasi S, Bakaher N, Bovet L, Willig A, et al. The tobacco genome sequences and its comparison with those of tomato and potato. Nat Commun. 2014;5: 3833–3833. 10.1038/ncomms4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barsnes H, Vaudel M. SearchGUI: a highly adaptable common interface for proteomics search and de novo engines. J Proteome Res. 2018;17: 2552–2555. 10.1021/acs.jproteome.8b00175 [DOI] [PubMed] [Google Scholar]
- 48.Li P, Feng B, Wang H, Tooley PW, Zhang X. Isolation of nine Phytophthora capsici pectin methylesterase genes which are differentially expressed in various plant species. J Basic Microbiol. 2011;51: 61–70. 10.1002/jobm.201000317 [DOI] [PubMed] [Google Scholar]
- 49.Vieira P, Danchin EGJ, Neveu C, Crozat C, Juabert S, Hussey RS, et al. The plant apoplasm is an important recipient compartment for nematode secreted proteins. J Exp Bot. 2011;62: 1241–1253. 10.1093/jxb/erq352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haegeman A, Jones JT, Danchin EGJ. Horizontal gene transfer in nematodes: a catalyst for plant parasitim? MPMI. 2011;8: 879–887. [DOI] [PubMed] [Google Scholar]
- 51.Brown AMV, Wasala SK, Howe DK, Peetz AB, Zasada IA, Denver DR. Genomic evidence for plant-parasitic nematodes as the earliest Wolbachia hosts. Sci Rep. 2016;6: 34955 10.1038/srep34955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant—pathogen interactions and affects plant resistance to diseases. J Plant Physiol. 2012;169: 1623–1630. 10.1016/j.jplph.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 53.Bethke G, Grundman RE, Sreekanta S, Truman W, Katagiri F, Glazebrook J. Arabidopsis pectin methylesterases contribute to immunity against Pseudomonas syringae. Plant Physiol. 2014; 164: 1093–1110. 10.1104/pp.113.227637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keeling IC, Yuen MM, Liao NY, Docking TR, Chan SK, Taylor GA et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013;14: R27 10.1186/gb-2013-14-3-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danchin ET, Rosso MN, Vieira P, Almeida-Engler J, Coutinho PM, Henrissat B, Abad P. Multiple lateral gene transfer and duplications have promoted plant parasitism ability in nematodes. PNAS. 2010;107: 17651–17656. 10.1073/pnas.1008486107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanholme B, Haegeman A, Jacob J, Cannoot B, Gheysen G. Arabinogalactan endo-1,4-ß-galactosidase: a putative plant cell wall-degrading enzyme of plant-parasitic nematodes. Nematology 2009;11: 739–747. [Google Scholar]
- 57.Haegeman A, Vanholme B, Gheysen G. Characterization of a putative endoxylanase in the migratory plant-parasitic nematode Radopholus similis. Mol Plant Pathol. 2009;10: 389–401. 10.1111/j.1364-3703.2009.00539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kikuchi T, Eves-van den Akker S, Jones JT. Genome evolution of plant-parasitic nematodes. Annu Rev Phytopathol. 2017;55: 14.1–14.22. [DOI] [PubMed] [Google Scholar]
- 59.Quist CW, Smant G, Helder J. Evolution of Plant Parasitism in the Phylum Nematoda. Annu Rev Phytopathol. 2015;53: 289–310. 10.1146/annurev-phyto-080614-120057 [DOI] [PubMed] [Google Scholar]
- 60.Church MJ. Resource control of bacterial dynamics in the sea In: Kirchman DL (Ed.) Microbial Ecology of Oceans. Wiley & Sons; 2008. [Google Scholar]
- 61.Wolińska A, Kuzniar A, Zielenkiewicz U, Izak D, Szafranek-Nakonieczna A, Banach A, et al. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl Soil Ecol. 2017;119: 128–137. [Google Scholar]
- 62.Schulz S, Brankatschk R, Dümig AD, Kőgel-Knabner IK, Schloter M, Zeyer J. 2013. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013;10: 3983–3996. [Google Scholar]
- 63.Elhady A, Giné A, Topalovic O, Jacquiod S, Sørensen SJ, Sorribas J, et al. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS One. 2017;12: e0177145 10.1371/journal.pone.0177145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Czarnetzki AB, Tebbe CC. Diversity of bacteria associated with Collembola—a cultivation-independent survey based on PCR-amplified 16S rRNA genes. FEMS Microbiol Ecol. 2004;49: 217–27. 10.1016/j.femsec.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 65.Agamennone V, Jakupovic D, Weedon JT, Suring WJ, van Straalen NM, Roelofs D, et al. The microbiome of Folsomia candida: An assessment of bacterial diversity in a Wolbachia-containing animal. FEMS Microbiol Ecol. 2015;91: 1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Pp, Pratylenchus penetrans; Bacteria, B; Archaea, A; Fungi, F; Insect, I; Plant, P.
(XLSX)
These sequences of each particular taxa correspond to the top BLAST hits against P. penetrans PME sequence.
(XLSX)
The preferred codons are highlighted in red.
(XLSX)
(XLSX)
(XLSX)
Exons and introns are indicated by green and red arrows, respectively.
(PDF)
Pp-PME US isolate corresponds to the isolate NL 10p RH collected in Beltsville (Maryland, US), Pp-PME PT isolate corresponds to the isolate A44L4 collected from potato fields in Portugal (Coimbra, Portugal).
(PDF)
(A) and (B) correspond to the different Pp-PME peptides identified from two protein libraries extracted from the leaves of Nicotiana benthamiana plants inoculated with PVX-Pp-pme.
(PDF)
Data Availability Statement
The cloned sequences were deposited at NCBI with the accession numbers MK295632 and MK295633, and the peptide libraries were deposited at the proteomics data repository PRIDE Archive (http://www.ebi.ac.uk/pride/archive/) with the dataset identifier PXD012419.