Abstract
Objective
Urinary steroid metabolomics by GC-MS is an established method in both clinical and research settings to describe steroidogenic disorders. However, population-based reference intervals for adults do not exist.
Methods
We measured daytime and night time urinary excretion of 40 steroid metabolites by GC-MS in 1128 adult participants of European ancestry, aged 18 to 90 years, within a large population-based, multicentric, cross-sectional study. Age and sex-related patterns in adjacent daytime and night time urine collections over 24 hours were modelled for each steroid metabolite by multivariable linear mixed regression. We compared our results with those obtained through a systematic literature review on reference intervals of urinary steroid excretion.
Results
Flexible models were created for all urinary steroid metabolites thereby estimating sex- and age-related changes of the urinary steroid metabolome. Most urinary steroid metabolites showed an age-dependence with the exception of 6β-OH-cortisol, 18-OH-cortisol, and β-cortol. Reference intervals for all metabolites excreted during 24 hours were derived from the 2.5th and 97.5th percentile of modelled reference curves. The excretion rate per period of metabolites predominantly derived from the adrenals was mainly higher during the day than at night and the correlation between day and night time metabolite excretion was highly positive for most androgens and moderately positive for glucocorticoids.
Conclusions
This study gives unprecedented new insights into sex- and age-specificity of the human adult steroid metabolome and provides further information on the day/night variation of urinary steroid hormone excretion. The population-based reference ranges for 40 GC-MS-measured metabolites will facilitate the interpretation of steroid profiles in clinical practice.
Introduction
Steroid hormones mediate a wide variety of biological processes and analysis of these in serum and their metabolites in urine are used to detect steroid misuse and disorders of reproductive function, sexual development, electrolyte balance, blood pressure and stress response [1–7]. They are small hydrophobic molecules that are synthesized from cholesterol in the testis and ovary, in the adrenal cortex, the placenta and the brain. In humans, the small number of circulating sex steroids, glucocorticoids, corticosterones and mineralocorticoids are converted into a large number of metabolites predominantly excreted via the urine in which they are easily accessible for analysis [8]. Major milestones in the development of urinary steroid hormone analysis were built by the advances in transforming the steroids into suitable derivates for GC-MS analysis, e.g. by producing stable oxime-silyl-derivatives, and by the development of a derivative purification method based on lipophilic Sephadex in 1977 [9–11]. The long history of urinary steroid analysis and the current role of GC-MS in steroid analysis compared with other analytical techniques as LC-MS/MS were sufficiently addressed in detail elsewhere [12–15].
For the correct clinical interpretation of a urinary steroid profile a comparison with reference intervals is necessary. Early efforts have been made to generate such values for the 24-hour excretion of the adult urinary steroid metabolome as summarized by Cedric H. L. Shackleton in 1986 [16]. Since then, new data about urinary steroid excretion values measured by GC-MS in adults from the general population were published, however, these data are limited in several respects: 1) the completeness of characterisation of the reference population, 2) the number of analyzed individuals: it ranged from 13 to 120 women and 10 to 120 men per study with overall less than 400 women and less than 400 men in all studies on reference intervals together, 3) the number of analyzed urinary steroids, which remained below 20 in most studies, and 4) the comparability of the laboratory data if several laboratories with different analytical systems were involved in the same study. None of the published reference intervals come from a formal population-based study or was large enough to provide sex- and age-specific reference intervals according to published recommendations [17]. While recent decades have seen many new technical developments and enhancements in the field of steroid hormone diagnostics, the method of GC-MS has established itself more and more firmly and is still regarded as the gold-standard allowing a comprehensive analysis of the steroid hormone metabolome [15].
The human steroid metabolome is influenced by sex and age and this has been taken into account in the past by providing steroid excretion reference intervals for men and women and for different age groups [18–23]. Furthermore, the human steroid metabolome varies at least in part with sleep and / or with the time of day [24, 25], but data supporting this notion for the urinary steroid metabolome in humans are still sparse [26, 27].
This study aims to bridge some of these gaps. The study was conducted 1) to summarize the available data on reference intervals for the urinary steroid excretion from healthy adults of the general population measured by GC-MS during the last 30 years since 1986 and 2) to describe sex- and age-specificity and differences between day and night excretion of a large number of urinary steroid metabolites in a thoroughly characterized general population of European descent measured by GC-MS and 3) to create sex- and age-specific reference intervals for the 24-hour urinary steroid metabolome that can be used in routine clinical work.
Subjects and methods
Literature search for reference intervals of quantitative urinary steroid excretion
A literature search was conducted to find reference intervals for the urinary steroid metabolome from healthy adults of the general population measured by GC-MS that were published since 1986. Using the PubMed electronic database and Google and Google Scholar, the search included the terms “urine/urinary steroid metabolome”, “urine/urinary steroid profile/profiling”, “urine/urinary steroids”, “normative data”, “reference values/intervals”, “GC-MS/gas chromatography-mass spectrometry”, “steroidogenesis”, “steroid synthesis/metabolism” and the trivial and systematic names of the steroid compounds measured in this study.
Study population
The Swiss Kidney Project on Genes in Hypertension (SKIPOGH) is a multicenter, family-based, cross-sectional study exploring the genetic and non-genetic determinants of blood pressure and renal function in the general adult population [28]. 1128 participants were recruited in the regions of Bern and Geneva and in the city of Lausanne in Switzerland from December 2009 to March 2013 by different strategies. In Geneva, a random sample from an index list provided by the population-based Bus Santé study [29] was selected, in the city of Lausanne a random sample of volunteers were taken from the population-based CoLaus study [30], and in Bern a random sample of participants was selected from the cantonal telephone registry. Inclusion criteria for participation in the study were as follows: 1) age ≥ 18 years; 2) European ancestry; 3) at least 1 and ideally 3 first-degree family members willing to participate. The SKIPOGH study adhered to the Declaration of Helsinki and was approved by the competent institutional ethics committees in Geneva, Lausanne and Bern. All participants provided written informed consent.
Study visit
The family members were contacted separately and individual appointments for a study visit were made. All participants completed a comprehensive health questionnaire about current and past medical history, medication, nutrition and lifestyle habits. The health questionnaire was checked for completeness and accuracy during the study visit. Body weight was measured to the nearest 0.1 kg with an electronic scale in the morning after an overnight fast in light indoor clothing and height was measured to the nearest 0.5 cm with a wall-mounted stadiometer. Fasting blood venous samples were drawn and were analyzed by standard clinical laboratory methods at each center. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation was used to calculate the estimated glomerular filtration rate (eGFR)[31]. Diabetes was defined as reported, treated, or fasting glycemia ≥7 mmol/L. Hypertension was defined as either systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medications.
Urine collection procedure
A 24-hour urine sample was collected separately for day and night. Participants received two labelled sterile 3-L preservative-free polyethylene containers UriSet 24 (Sarstedt, Nümbrecht, Germany) and standardized written and precise oral instructions. Daytime urine collection started in the morning with the study visit after an overnight fast and ended before bedtime at the same day. Night time urine collection ended with the collection of the first morning urine after waking up. Participants were asked to eat and drink as usual. Containers with daytime and night time urine collection were separately mixed and weighed and aliquoted into 30 mL low density polyethylene Wide-Mouth Bottles (Thermo Scientific, Rochester, New York). Urine aliquots were immediately stored at -80°C and were sent to the steroid laboratory of the Department of Nephrology and Hypertension at the Bern University Hospital, Switzerland, for centralized steroid analysis by GC-MS. Completeness of 24-hour urine collection was assessed based on the amount of urinary creatinine excretion as recently published [32].
Quantification of steroid compounds by GC-MS
Urinary excretion of 40 steroid hormone compounds listed in Table 1 and depicted in Fig 1 were quantified separately in μg/day and in μg/night time by an in-house adapted GC-MS method previously described [13, 33]. Table 1 further provides method-validation information for all steroid analytes. The corresponding calibration curves are given in S1 Fig and an example of a selected-ion monitoring chromatogram is given in S2 Fig.
Table 1. Steroid compounds measured in urine.
| Trivial name | Systematic name | M | QIon | RT | LOD | LOQ | R2 | Rec | CV1 | CV2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Progesterones | ||||||||||
| 17α-OH-pregnanolone | 3β,17-dihydroxy-5-pregnen-20-one | 334.5 | 476 | 17.6 | 16 | 54 | 0.997 | 88 | 2.3 | 15.9 |
| pregnanetriol | 5β-pregnane-3α,17α,20α-triol | 336.5 | 435 | 19.6 | 180 | 600 | 0.999 | 107 | 5.5 | 17.3 |
| pregnenetriol | 5-pregnene-3β,17α,20α-triol | 334.5 | 433 | 23.0 | 22 | 72 | 0.997 | 101 | 5.5 | 29.9 |
| pregnanetriolone | 3α,17α,20α-trihydroxy-5β-pregnan-11-one | 350.5 | 449 | 22.0 | 487 | 1622 | 0.994 | 81 | 10.5 | 23.3 |
| pregnanediol | 5β-pregnane-3α,20α-diol | 316.5 | 269 | 19.0 | 92 | 307 | 0.995 | 108 | 8.6 | 20.2 |
| Androgens | ||||||||||
| dehydroepiandrosterone | 3β-hydroxy-5-androsten-17-one | 288.4 | 268 | 15.4 | 97 | 324 | 0.989 | 85 | 6.7 | 19 |
| 16α-OH-dehydroepiandrosterone | 3β,16α-dihydroxy-5-androsten-17-one | 304.4 | 266 | 18.3 | 614 | 2046 | 0.996 | 119 | 3.1 | 13.8 |
| androstenediol | 5-androstene-3β,17β-diol | 290.4 | 239 | 15.9 | 109 | 364 | 0.997 | 83 | 6.5 | 19.1 |
| androstenetriol | 5-androstene-3β,16α,17β-triol | 306.4 | 432 | 20.6 | 278 | 928 | 0.998 | 100 | 6.2 | 16.1 |
| testosterone | 17β-hydroxy-4-androsten-3-one | 288.4 | 389 | 16.7 | 506 | 1686 | 0.993 | 85 | 13.4 | 31.2 |
| 5α-DH-testosterone | 17β-hydroxy-5α-androstan-3-one | 290.4 | 391 | 16.0 | 544 | 1812 | 0.990 | 85 | 5.3 | 22.8 |
| androstanediol | 5α-androstane-3α,17β-diol | 292.4 | 331 | 14.6 | 258 | 860 | 0.993 | 95 | 5.9 | 16.1 |
| androsterone | 3α-hydroxy-5α-androstan-17-one | 290.4 | 270 | 14.4 | 48 | 161 | 0.996 | 85 | 6 | 20.8 |
| 11β-OH-androsterone | 3α,11β-dihydroxy-5α-androstan-17-one | 306.4 | 268 | 17.6 | 204 | 680 | 0.994 | 85 | 6.2 | 16.2 |
| etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | 290.4 | 270 | 14.6 | 29 | 98 | 0.992 | 118 | 4.8 | 18.8 |
| Estrogens | ||||||||||
| 17β-estradiol | 1,3,5(10)-estratriene-3,17β-diol | 272.4 | 416 | 16.5 | 205 | 682 | 0.999 | 81 | 17.4 | 37.3 |
| estriol | 1,3,5(10)-estratriene-3,16α,17β-triol | 288.4 | 504 | 21.3 | 96 | 320 | 0.997 | 92 | 13.2 | 29 |
| Corticosterones | ||||||||||
| TH-11-deoxycorticosterone | 3α,21-dihydroxy-5β-pregnan-20-one | 334.5 | 476 | 21.2 | 516 | 1721 | 0.995 | 96 | 16.5 | 16.9 |
| TH-11-dehydrocorticosterone | 3α,21-dihydroxy-5β-pregnane-11,20-dione | 348.5 | 490 | 23.6 | 105 | 349 | 0.996 | 88 | 21.2 | 26.9 |
| 18-OH-TH-11-dehydrocorticosterone | 3α,18,21-trihydroxy-5β-pregnane-11,20-dione | 364.5 | 457 | 26.0 | 7774 | 25913 | 0.999 | 92 | 24.5 | 30.5 |
| TH-corticosterone | 3α,11β,21-trihydroxy-5β-pregnan-20-one | 350.5 | 564 | 24.1 | 266 | 888 | 0.995 | 81 | 5.4 | 10 |
| 5α-TH-corticosterone | 3α,11β,21-trihydroxy-5α-pregnan-20-one | 350.5 | 564 | 24.5 | 60 | 201 | 0.993 | 87 | 6.4 | 14.3 |
| Mineralocorticoids | ||||||||||
| TH-aldosterone | 11β,18-epoxy-3α,18,21-trihydroxy-5β-pregnan-20-one | 364.5 | 506 | 25.5 | 1438 | 4793 | 0.988 | 83 | 12.1 | 29 |
| TH-11-deoxycortisol | 3α,17,21-trihydroxy-5β-pregnan-20-one | 350.5 | 564 | 21.0 | 388 | 1294 | 0.993 | 89 | 6.9 | 12.9 |
| Glucocorticoids | ||||||||||
| cortisol | 11β,17,21-trihydroxy-4-pregnene-3,20-dione | 362.5 | 605 | 30.8 | 336 | 1121 | 0.973 | 89 | 5.8 | 33.9 |
| 6β-OH-cortisol | 6β,11β,17α,21-tetrahydroxy-4-pregnene-3,20-dione | 378.5 | 513 | 32.0 | 1144 | 3814 | 0.989 | 81 | 6.5 | 40.3 |
| 18-OH-cortisol | 11,17,18,21-tetrahydroxy-4-pregnene-3,20-dione | 378.5 | 344 | 32.0 | 38669 | 128898 | 0.996 | 95 | 12.3 | 29.9 |
| 20α-DH-cortisol | 11β,17,20α,21-tetrahydroxy-4-pregnen-3-one | 364.5 | 296 | 32.8 | 3660 | 12199 | 0.994 | 97 | 5.2 | 19.8 |
| TH-cortisol | 3α,11β,17,21-tetrahydroxy-5β-pregnan-20-one | 366.5 | 652 | 24.8 | 122 | 408 | 0.995 | 96 | 7.1 | 12.2 |
| α-cortol | 5β-pregnane-3α,11β,17α,20α,21-pentol | 368.5 | 343 | 27.2 | 249 | 830 | 0.999 | 90 | 6.1 | 14 |
| β-cortol | 5β-pregnane-3α,11β,17α,20β,21-pentol | 368.5 | 343 | 26.0 | 228 | 758 | 0.991 | 96 | 5.9 | 14.4 |
| 11β-OH-etiocholanolone | 3α,11β-dihydroxy-5β-androstan-17-one | 306.4 | 268 | 17.9 | 127 | 423 | 0.994 | 98 | 12.9 | 15.4 |
| 5α-TH-cortisol | 3α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | 366.5 | 652 | 25.1 | 40 | 134 | 0.995 | 103 | 8.8 | 18.6 |
| cortisone | 17,21-dihydroxy-4-pregnene-3,11,20-trione | 360.5 | 531 | 29.0 | 987 | 3290 | 0.973 | 88 | 10 | 25.5 |
| 20α-DH-cortisone | 17α,20α,21-trihydroxy-4-pregnene-3,11-dione | 362.5 | 402 | 31.6 | 523 | 1744 | 0.990 | 101 | 4.9 | 19.5 |
| 20β-DH-cortisone | 17α,20β,21-trihydroxy-4-pregnene-3,11-dione | 362.5 | 402 | 30.9 | 767 | 2556 | 0.990 | 98 | 5.1 | 18.4 |
| TH-cortisone | 3α,17,21-trihydroxy-5β-pregnan-11,20-dione | 364.5 | 578 | 23.3 | 422 | 1408 | 0.996 | 96 | 8.1 | 11.1 |
| α-cortolone | 3α,17α,20α,21-tetrahydroxy-5β-pregnane-11-one | 366.5 | 449 | 25.4 | 23 | 77 | 0.995 | 101 | 5.4 | 17.1 |
| β-cortolone | 3α,17α,20β,21-tetrahydroxy-5β-pregnane-11-one | 366.5 | 449 | 26.1 | 49 | 162 | 0.984 | 106 | 5.5 | 15.3 |
| 11-keto-etiocholanolone | 3α-hydroxy-5β-androstane-11,17-dione | 304.4 | 269 | 16.1 | 435 | 1450 | 0.994 | 82 | 3.2 | 22 |
The nomenclature used for systematic names is in accordance with the recommendations of the IUPAC commission on the Nomenclature of Organic Chemistry published in 1969 [34] amended by the IUPAC–IUB Commission on Biochemical Nomenclature in 1971 [35] and again revised in 1989 [36]. M: molar mass [g/mol]. QIon: quantifier ion [m/z]. RT: retention time [min]. LOD: limit of detection [pg per sample]. LOQ: limits of quantitation [pg per sample]. R2: correlation coefficient of the linear calibration curve shown in S1 Fig. Rec: Recovery in %. CV1: intraassay coefficient of variation [%] (n = 58). CV2: interassay coefficient of variation [%] (n = 119). CV1 and CV2 were determined for a urine volume of 1.5 mL. OH: hydroxy, DH: dihydro, TH: tetrahydro.
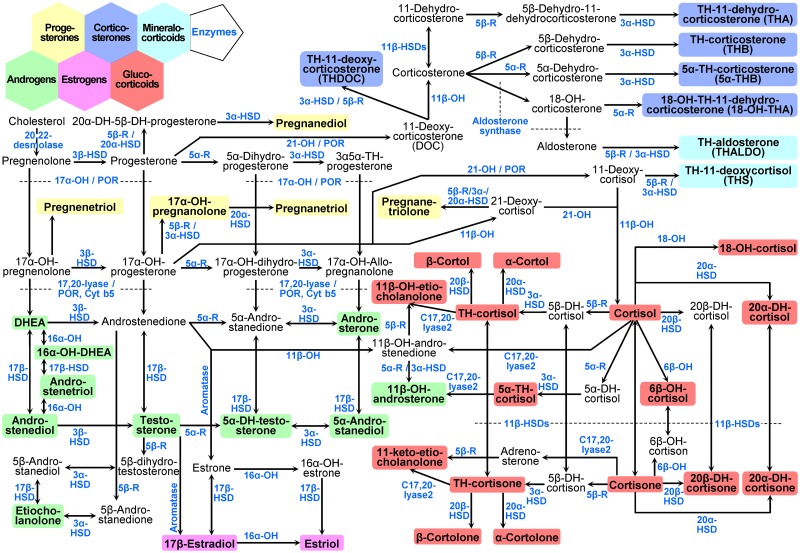
Fig 1. Pathways of human steroid hormone biosynthesis.
Pathways from multiple steroid hormone producing cell types are combined to provide an overall view about the 40 urinary steroid metabolites measured in this study and their biosynthesis processes. Steroid groups are highlighted by colored background as indicated and enzyme activities are displayed in blue color. Abbreviations: DHEA: Dehydroepiandrosterone. The OH in enzyme names indicates a hydroxylase, e.g. 17α-OH: 17α-hydroxylase. The OH in steroid names indicates a hydroxyl group, e.g. 17-OH-pregnanolone: 17-hydroxy-pregnanolone. DH: dihydro; TH: tetrahydro; HSD: hydroxysteroid dehydrogenase; POR: P450 oxidoreductase; Cyt b5: Cytochrome b5; 5α-R: 5α-reductase; 5β-R: 5β-reductase.
In brief, urine sample preparation consisted of 1) pre-extraction on a Sep-Pak C18 column with the recovery standard medroxyprogesterone, 2) enzymatic hydrolysis with sulfatase and β-glucuronidase/arylsulfatase, 3) extraction of the free steroids from the hydrolysis mixture again on a Sep-Pak C18 cartridge, 4) derivatization with methoxyamine HCl 2% in pyridine at 60°C for one hour after adding the two standards Stigmasterol and 3β5β-TH-aldosterone and derivatization with Trimethylsilylimidazole (TMSI) at 100°C for 16 hours, 5) purification by gel filtration on a Lipidex 5000 column. The derivatized samples were analyzed by mass spectrometric analyses on a gas chromatograph 7890A coupled to a mass selective detector Hewlett-Packard 5975C (both from Agilent Technologies, La Jolla, California, USA) providing selected ion monitoring.
Quality controls of the GC-MS method
The reproducibility of the applied GC-MS method is continuously monitored by an internal quality control. Urine samples of the same healthy volunteer are measured in parallel in all measurement series and the results are compared with the standard values derived from 15 measurements of this volunteer. The steroid laboratory participates monthly in an external quality control organized by the Foundation for Quality Medical Laboratory Diagnostics skml (Stichting Kwaliteitsbewaking Medische Laboratoriumdiagnostiek, Nijmegen, The Netherlands). Only analytes that fulfilled internal and external quality assessment requirements are included in steroid profiles. For internal quality control, quantitative results have to be within ±20% of the individual reference intervals. The external quality control is evaluated based on multiple of median (MOM) of reported steroids; results had to be better than ±30% of MOM of all participating laboratories for acceptance.
Selection of reference sample group
Of the 1128 participants, 33 were excluded from the analyses due to missing data for urinary steroid excretion leaving 1095 participants. Additional participants were excluded from the reference sample group for the following reasons: urine under- or over-collection, pregnancy, self-reported bilateral oophorectomy or bilateral oophorectomy with hysterectomy, Addison’s disease (the presence of other hormone disorders was not reported in the cohort), self-reported diagnosis of active malignant disease, self-reported liver disease, estimated glomerular filtration rate less than 30 mL/min per 1.73 m2 calculated by CKD-EPI formula, missing data for serum creatinine or urinary creatinine, body mass index <16 kg/ m2 or > 40 kg/m2, >3 fold elevated γ-glutamyltransferase (>120 U/L for female and >180 U/L for male), aspartate or alanine aminotransferases (>105 U/L for female and > 150 U/L for male), intake of the following drugs (current intake or in the two previous weeks): hormonal contraceptives, hormones to suppress menstrual bleeding, hormonal menopause treatment, systemic glucocorticoids or mineralocorticoids, 5α-reductase inhibitors, aromatase inhibitors, antiepileptic agents, systemic azole derivatives.
Statistical analysis
Baseline characteristics were expressed as numbers and frequency in % or as mean±SD or median;25th-75th percentile. The 24-hour urinary steroid hormone metabolite excretion was calculated in μg/24h from day and night time urine collections by the formula (metabolite[μg/day]+metabolite[μg/night])/(collection time day[min]+collection time day[min]) × 1440 min. Sex-specific differences of baseline characteristics and 24-hour excretion of steroid hormone metabolites were assessed by chi squared test or Mann–Whitney U test, where appropriate, and a p value <0.05 was considered to be significant. Day and night time excretion rates of steroid analytes expressed in μg/hour were calculated by the formula metabolite[μg/day])/collection time day[min]×60 min and by the formula metabolite[μg/night time]/collection time night time[min]×60 min, respectively. Differences between day and night time excretion rates of steroid analytes were analyzed for each sex by Wilcoxon signed-rank test and visualized by boxplots and the correlation between night and day values was further assessed by calculating the Spearman’s rank correlation coefficient. Age- and sex-related changes of 24-hour excretion of steroid hormone metabolites were modelled by linear mixed regression models taking family and center effect into account as described in detail in the S1 Text and previously by V. Rousson [34]. Using the Akaike information criterion the best model for each steroid metabolite was separately selected for men and women and was plotted for different percentiles. Reference intervals based on the 2.5th-97.5th percentiles for each metabolite were estimated from the described statistical models for men and women separately and for different age groups. All statistical analyses were conducted using the R software, version 3.3.3 [35].
Results
Literature search, measured steroid compounds and pathways of steroidogenesis
The literature search yielded twelve hits for studies that published reference intervals for the urinary steroid excretion from healthy adults of the general population measured by GC-MS since 1986 listed in S1 Table [16, 18–23, 36–40]. These studies combined included data on <800 participants, thereby precluding the detailed exploration of the relationship of steroid hormone metabolites with sex and age according to published recommendations [17] and none of them were reported to be population-based, i.e. coming from a true random sample of the general population on the strength of thoroughly described selection criteria. Studies including reference ranges for comparison purposes but not as the main objective of the publication were not considered in this list.
Characteristics of the reference population
The number of individuals for these references was 838 (459 men, 54.8%). Women were older than men (age mean±SD: 51.2±16.1 versus 47.7±17.6 years, p = 0.0054) mainly because younger women using hormonal contraceptives were excluded from the analysis (n = 118). Smoking, hypertension and diabetes were more prevalent in men than in women. Serum electrolytes, parameters of kidney and liver function and parameters of inflammation and blood count were largely within the normal range. The baseline characteristics and laboratory values are shown in S2 Table.
Sex- and age-related patterns of the urinary steroid metabolome during 24 hours
The 24-hour excretion of all urinary steroid hormone metabolites was right skewed distributed. The median metabolite excretion by sex compared by Mann–Whitney U test revealed a significant higher excretion for almost all metabolites in men than in women as shown in S3 Table, with the exception of pregnanediol (206 vs. 204 μg/24 hours, p = 0.57), of TH-aldosterone (456 vs. 378 μg/24 hours, p = 0.084), and of 18-OH-cortisol (180 vs. 173 μg/24 hours, p = 0.16). The excretion values were visualized by boxplots in S3(a) Fig. The relationship of 24-hour excretion of urinary steroid hormone metabolites to age was assessed using a nonparametric fit and revealed a different kind of relationship to age for men and for women for most metabolites as shown in S3(b) Fig. Therefore, the 24-hour excretion of urinary steroid hormone metabolites in function of age was modelled separately for men and for women. From the selected models, the 2.5th, 10th, 25th, 50th, 75th, 90th and 97.5th percentile for each metabolite was plotted on the transformed scale in function of age to get reference curves for men and women depicted in S4 Fig. No relationship to age was found for several metabolites, in particular for 11β-OH-androsterone, 18-OH-TH-11-dehydrocorticosterone, cortisol, 5α-TH-cortisol, TH-cortisone, α-cortolone, and β-cortolone in women, and for 20α-DH-cortisol and 20α-DH-cortisone in men, and for 6β-OH-cortisol, 18-OH-cortisol, and β-cortol in women and men. Lower and upper limits of sex- and age-specific reference intervals for the 24-hour excretion of urinary steroid hormone metabolites were derived from the 2.5th and 97.5th percentile for men in Table 2 and women in Table 3.
Table 2. Reference intervals for 40 steroid metabolites in 24-hour urine in men.
| Metabolite, μg/24 hours | N | Age group, years | CF to nmol/24 hours | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 18–29.9 | 30–39.9 | 40–49.9 | 50–59.9 | 60–69.9 | 70–79.9 | 80–85.7 | |||
| 17α-OH-pregnanolone | 451 | 75.3–584 | 65.8–535 | 57.4–489 | 49.8–446 | 43–406 | 37–369 | 31.7–335 | ×2.990 |
| pregnanetriol | 395 | 442–2045 | 385–1814 | 334–1607 | 289–1421 | 250–1255 | 215–1106 | 185–974 | ×2.972 |
| pregnenetriol | 448 | 54–1213 | 87.4–1580 | 47–1127 | 22.3–769 | 11.4–559 | 6.7–441 | 4.7–380 | ×2.990 |
| pregnanetriolone | 457 | 6–70.8 | 6.2–78 | 6.4–86.3 | 6.6–96 | 6.8–108 | 7–121 | 7.2–138 | ×2.853 |
| pregnanediol | 457 | 85.5–653 | 94.4–708 | 81.1–625 | 64–516 | 55.4–459 | 52.8–441 | 52.8–441 | ×3.160 |
| dehydroepiandrosterone | 439 | 36.3–8455 | 63.7–56319 | 46.1–17754 | 24.3–2776 | 15.3–907 | 11–445 | 8.9–287 | ×3.467 |
| 16α-OH-dehydroepiandrosterone | 448 | 75.2–1413 | 155–2139 | 75.7–1418 | 30.6–874 | 13.4–582 | 6.8–428 | 4.4–356 | ×3.285 |
| androstenediol | 453 | 35.4–1036 | 53.9–1878 | 42.9–1359 | 26–673 | 17–377 | 12–234 | 9–160 | ×3.444 |
| androstenetriol | 456 | 176–1652 | 214–1927 | 158–1518 | 110–1137 | 77–857 | 54.2–652 | 38.6–500 | ×3.264 |
| testosterone | 451 | 13.1–177 | 16.6–204 | 14.3–187 | 10.1–152 | 7.2–126 | 5.3–105 | 4–90.1 | ×3.467 |
| 5α-DH-testosterone | 456 | 6–91.6 | 5.4–86.6 | 4.9–81.8 | 4.4–77.3 | 4–72.9 | 3.6–68.8 | 3.2–64.8 | ×3.444 |
| androstanediol | 445 | 44.1–228 | 49.6–252 | 42.4–221 | 34.6–186 | 28.1–156 | 22.7–130 | 18.2–108 | ×3.420 |
| androsterone | 381 | 1450–5821 | 1456–5839 | 1073–4644 | 759–3594 | 541–2810 | 390–2224 | 285–1786 | ×3.444 |
| 11β-OH-androsterone | 447 | 357–1724 | 373–1771 | 376–1780 | 367–1752 | 345–1687 | 313–1589 | 273–1462 | ×3.264 |
| etiocholanolone | 390 | 883–5047 | 794–4743 | 673–4308 | 531–3770 | 385–3165 | 249–2531 | 138–1910 | ×3.444 |
| 17β-estradiol | 457 | 0.7–4.5 | 0.9–5.5 | 1–6.1 | 1.1–6.3 | 1–5.9 | 0.9–5.6 | 0.9–5.6 | ×3.671 |
| estriol | 456 | 1.8–13.8 | 1.9–14.5 | 2–15.1 | 2.2–15.8 | 2.3–16.5 | 2.4–17.3 | 2.5–18.1 | ×3.467 |
| TH-11-deoxycorticosterone | 455 | 3.2–23.3 | 3.1–22.7 | 2.9–21.4 | 2.7–19.5 | 2.4–17.3 | 2.2–16.2 | 2.2–16.2 | ×2.990 |
| TH-11-dehydrocorticosterone | 452 | 49.1–277 | 49.5–278 | 46.6–267 | 40.7–242 | 32.8–209 | 28.8–191 | 28.8–191 | ×2.869 |
| 18-OH-TH-11-dehydrocorticosterone | 433 | 14–259 | 15.1–297 | 15.7–318 | 15.7–318 | 15.1–297 | 14–259 | 12.5–212 | ×2.743 |
| TH-corticosterone | 457 | 52.6–326 | 58.3–350 | 59.8–356 | 57.1–345 | 50.5–317 | 48.6–309 | 60.1–357 | ×2.853 |
| 5α-TH-corticosterone | 457 | 155–891 | 170–976 | 147–844 | 128–738 | 119–681 | 115–663 | 115–663 | ×2.853 |
| TH-aldosterone | 456 | 5.1–79.2 | 5.4–84 | 5.5–85.6 | 5.4–83.9 | 5.1–79.1 | 4.6–71.7 | 4–62.5 | ×2.743 |
| TH-11-deoxycortisol | 457 | 27.5–145 | 28.4–152 | 29.4–160 | 30.4–168 | 31.5–176 | 32.6–185 | 33.8–194 | ×2.853 |
| cortisol | 457 | 41.6–243 | 47.1–282 | 50–302 | 49.7–300 | 47.3–282 | 48.4–291 | 54.5–335 | ×2.759 |
| 6β-OH-cortisol | 457 | 35.3–303 | 35.3–303 | 35.3–303 | 35.3–303 | 35.3–303 | 35.3–303 | 35.3–303 | ×2.642 |
| 18-OH-cortisol | 424 | 40.7–638 | 40.7–638 | 40.7–638 | 40.7–638 | 40.7–638 | 40.7–638 | 40.7–638 | ×2.642 |
| 20α-DH-cortisol | 457 | 19.2–136 | 19.2–136 | 19.2–136 | 19.2–136 | 19.2–136 | 19.2–136 | 19.2–136 | ×2.743 |
| TH-cortisol | 371 | 714–2724 | 866–3301 | 966–3682 | 992–3781 | 938–3575 | 918–3501 | 1047–3992 | ×2.729 |
| α-cortol | 452 | 127–519 | 157–638 | 161–658 | 164–668 | 169–688 | 176–717 | 186–757 | ×2.714 |
| β-cortol | 453 | 213–1171 | 213–1171 | 213–1171 | 213–1171 | 213–1171 | 213–1171 | 213–1171 | ×2.714 |
| 11β-OH-etiocholanolone | 455 | 29.7–955 | 15.1–861 | 27.7–944 | 43.7–1029 | 55–1081 | 59.1–1099 | 59.1–1099 | ×3.264 |
| 5α-TH-cortisol | 381 | 524–3424 | 631–3992 | 578–3715 | 527–3436 | 497–3278 | 488–3226 | 488–3226 | ×2.729 |
| cortisone | 456 | 69.2–378 | 74–404 | 77–420 | 77.9–425 | 76.6–418 | 73.4–401 | 68.3–373 | ×2.774 |
| 20α-DH-cortisone | 457 | 11.1–63.3 | 11.1–63.3 | 11.1–63.3 | 11.1–63.3 | 11.1–63.3 | 11.1–63.3 | 11.1–63.3 | ×2.759 |
| 20β-DH-cortisone | 457 | 21.8–127 | 22.7–131 | 23.6–135 | 24.5–140 | 25.5–145 | 26.5–149 | 27.5–154 | ×2.759 |
| TH-cortisone | 407 | 1543–6080 | 1643–6378 | 1667–6449 | 1611–6285 | 1483–5902 | 1297–5332 | 1072–4623 | ×2.743 |
| α-cortolone | 426 | 552–2214 | 587–2338 | 611–2420 | 622–2456 | 618–2443 | 600–2382 | 570–2277 | ×2.729 |
| β-cortolone | 427 | 366–1387 | 349–1328 | 332–1271 | 315–1216 | 300–1164 | 285–1114 | 271–1065 | ×2.729 |
| 11-keto-etiocholanolone | 455 | 97.7–1055 | 67.3–884 | 85–986 | 103–1082 | 109–1113 | 102–1076 | 83–975 | ×3.285 |
Reference intervals have been estimated by the described statistical models and are given as 2.5th-97.5th percentiles in the unit μg/24 hours for different age groups. N represents the sample number per analyte included in the statistical model. CF: Conversion Factor.
Table 3. Reference intervals for 40 steroid metabolites in 24-hour urine in women.
| Metabolite, μg/24 hours | N | Age group, years | CF to nmol/24 hours | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 18–29.9 | 30–39.9 | 40–49.9 | 50–59.9 | 60–69.9 | 70–79.9 | 80–90.0 | |||
| 17α-OH-pregnanolone | 375 | 18.2–383 | 28.4–704 | 27.5–672 | 16.5–337 | 9.7–166 | 8.2–132 | 8.2–132 | ×2.990 |
| pregnanetriol | 360 | 173–1156 | 216–1346 | 200–1275 | 135–975 | 80.9–696 | 56.8–554 | 48.3–501 | ×2.972 |
| pregnenetriol | 378 | 18.7–810 | 12–661 | 7.4–535 | 4.3–429 | 2.4–341 | 1.2–268 | 0.5–207 | ×2.990 |
| pregnanetriolone | 379 | 4.1–95.7 | 3–54.5 | 3.1–57.9 | 3.4–67 | 3.6–77.4 | 3.9–89.4 | 4.2–103 | ×2.853 |
| pregnanediol | 376 | 62.9–2180 | 95.2–5269 | 94.6–5192 | 61.7–2101 | 38.2–830 | 29.5–520 | 27.5–460 | ×3.160 |
| dehydroepiandrosterone | 377 | 13.8–1623 | 15.6–1989 | 13.6–1578 | 9.1–834 | 5.7–409 | 4.1–242 | 3.2–168 | ×3.467 |
| 16α-OH-dehydroepiandrosterone | 379 | 37.7–1742 | 33.9–1565 | 25–1157 | 15.2–704 | 8.9–411 | 5.8–270 | 4.3–199 | ×3.285 |
| androstenediol | 378 | 22.5–873 | 16.8–571 | 12.6–380 | 9.5–257 | 7.2–177 | 5.6–123 | 4.3–86.5 | ×3.444 |
| androstenetriol | 378 | 99.7–1351 | 72–1051 | 51.4–813 | 36.2–625 | 25.2–476 | 17.3–361 | 11.7–271 | ×3.264 |
| testosterone | 367 | 2.4–55.1 | 2.7–66.1 | 2.6–61.8 | 2.1–45.3 | 1.6–30.9 | 1.2–22.8 | 1.1–18.3 | ×3.467 |
| 5α-DH-testosterone | 377 | 2.9–75.6 | 2.5–66.9 | 2.2–59.1 | 2–52.3 | 1.8–46.3 | 1.5–41 | 1.4–36.2 | ×3.444 |
| androstanediol | 372 | 8.9–106 | 10.5–124 | 9.7–114 | 7–83.1 | 4.9–57.7 | 4–46.7 | 3.7–44 | ×3.420 |
| androsterone | 349 | 480–4482 | 414–3978 | 307–3138 | 194–2181 | 116–1462 | 75.6–1051 | 54.3–816 | ×3.444 |
| 11β-OH-androsterone | 376 | 192–1183 | 192–1183 | 192–1183 | 192–1183 | 192–1183 | 192–1183 | 192–1183 | ×3.264 |
| etiocholanolone | 351 | 543–3870 | 448–3483 | 345–3031 | 243–2540 | 153–2037 | 82.1–1551 | 33.9–1106 | ×3.444 |
| 17β-estradiol | 377 | 0.3–7.6 | 0.7–26.6 | 0.9–35.8 | 0.5–17 | 0.3–7.1 | 0.2–5.4 | 0.2–5.4 | ×3.671 |
| estriol | 374 | 0.8–36.3 | 1.6–91.8 | 1.7–99.5 | 1–45.3 | 0.6–19.5 | 0.5–15 | 0.5–15 | ×3.467 |
| TH-11-deoxycorticosterone | 378 | 1.8–34.2 | 2.5–66.1 | 2.6–69.5 | 1.9–39 | 1.4–21.5 | 1.2–17.9 | 1.2–17.9 | ×2.990 |
| TH-11-dehydrocorticosterone | 379 | 31.5–240 | 29.5–228 | 27.6–216 | 25.9–205 | 24.3–194 | 22.7–184 | 21.2–174 | ×2.869 |
| 18-OH-TH-11-dehydrocorticosterone | 342 | 10–262 | 10–262 | 10–262 | 10–262 | 10–262 | 10–262 | 10–262 | ×2.743 |
| TH-corticosterone | 379 | 28–226 | 34.8–261 | 39.7–285 | 41.7–294 | 40.4–288 | 36.2–268 | 29.7–235 | ×2.853 |
| 5α-TH-corticosterone | 379 | 60.6–556 | 57–535 | 53.5–514 | 50.2–493 | 47–474 | 44.1–454 | 41.2–436 | ×2.853 |
| TH-aldosterone | 378 | 5.3–83 | 5.6–86.3 | 5.5–84.9 | 5.1–78.8 | 4.5–69.1 | 3.7–57.3 | 2.9–44.9 | ×2.743 |
| TH-11-deoxycortisol | 379 | 15–97.3 | 17.6–114 | 19.8–128 | 21.4–138 | 22.2–144 | 22.1–143 | 21.1–137 | ×2.853 |
| cortisol | 379 | 33.7–250 | 33.7–250 | 33.7–250 | 33.7–250 | 33.7–250 | 33.7–250 | 33.7–250 | ×2.759 |
| 6β-OH-cortisol | 378 | 25.6–282 | 25.6–282 | 25.6–282 | 25.6–282 | 25.6–282 | 25.6–282 | 25.6–282 | ×2.642 |
| 18-OH-cortisol | 344 | 32.4–516 | 32.4–516 | 32.4–516 | 32.4–516 | 32.4–516 | 32.4–516 | 32.4–516 | ×2.642 |
| 20α-DH-cortisol | 379 | 16.7–176 | 16.2–169 | 15.6–163 | 15.2–156 | 14.7–150 | 14.2–144 | 13.8–138 | ×2.743 |
| TH-cortisol | 340 | 336–1677 | 416–2015 | 490–2319 | 550–2560 | 589–2714 | 603–2766 | 588–2711 | ×2.729 |
| α-cortol | 379 | 92.5–464 | 95.1–479 | 97.8–495 | 101–511 | 103–528 | 106–546 | 109–564 | ×2.714 |
| β-cortol | 378 | 125–751 | 125–751 | 125–751 | 125–751 | 125–751 | 125–751 | 125–751 | ×2.714 |
| 11β-OH-etiocholanolone | 378 | 0.5–612 | 12.2–756 | 28.4–860 | 39.1–916 | 39.3–917 | 28.9–863 | 12.7–760 | ×3.264 |
| 5α-TH-cortisol | 362 | 177–1982 | 177–1982 | 177–1982 | 177–1982 | 177–1982 | 177–1982 | 177–1982 | ×2.729 |
| cortisone | 379 | 48.3–308 | 56.3–360 | 59.4–379 | 56.7–362 | 50.2–321 | 47.9–306 | 47.9–306 | ×2.774 |
| 20α-DH-cortisone | 379 | 8.6–52.3 | 8.7–53.1 | 8.3–49.9 | 7.4–43.7 | 6.4–36.5 | 6–34.2 | 6–34.2 | ×2.759 |
| 20β-DH-cortisone | 379 | 23.9–145 | 22.5–136 | 21.2–129 | 20–121 | 18.8–114 | 17.8–107 | 16.7–101 | ×2.759 |
| TH-cortisone | 360 | 900–4654 | 900–4654 | 900–4654 | 900–4654 | 900–4654 | 900–4654 | 900–4654 | ×2.743 |
| α-cortolone | 362 | 405–1932 | 405–1932 | 405–1932 | 405–1932 | 405–1932 | 405–1932 | 405–1932 | ×2.729 |
| β-cortolone | 369 | 164–854 | 164–854 | 164–854 | 164–854 | 164–854 | 164–854 | 164–854 | ×2.729 |
| 11-keto-etiocholanolone | 379 | 23.6–652 | 43.4–778 | 60.8–870 | 70.9–918 | 71–919 | 61–871 | 43.6–779 | ×3.285 |
Reference intervals have been estimated by the described statistical models and are given as 2.5th-97.5th percentiles in the unit μg/24 hours for different age groups. N represents the sample number per analyte included in the statistical model. CF: Conversion Factor.
Sex-related differences in the urinary steroid metabolome at day and night
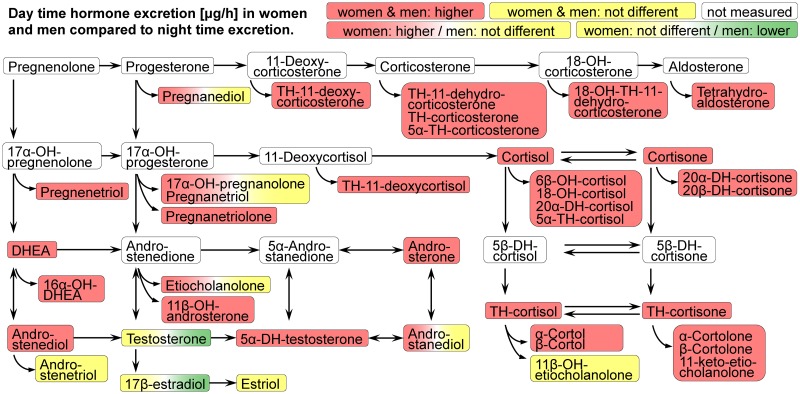
Day and night time excretion values of urinary steroid metabolites were also right skewed distributed and were compared by Wilcoxon signed-rank test. The median excreted amount in μg/hour was significantly higher during the day than at night for 31 metabolites in men and for 35 metabolites in women as shown in S4 Table. Higher urinary excretion values during the day compared to night time have been found for cortisol and cortisone and for almost all glucocorticoid metabolites in both sexes. During the day higher excretion rates for both sexes have been also found for the most abundant circulating steroid hormone dehydroepiandrosterone (DHEA) and its two metabolites 16α-OH-DHEA and androstenediol, for the most potent androgen 5α-DH-testosterone and also for androsterone, the most abundant androgen metabolite in urine. For etiocholanolone, which is the second most abundant androgen metabolite in urine, a higher night time excretion rate has been revealed in women, but not in men. Excretion rates for testosterone and 17β-estradiol have not been found to be different in women but to be lower at night time in men. The results were visualized by boxplots in S3(e) and S3(f) Fig and summarized in a comprehensive overview in Fig 2. The correlation between day and night time excretion values assessed by Spearman’s rank correlation coefficient in S3(g) and S3(h) Figs and in S5 Table revealed a moderate to high positive correlation among most of androgen and progesterone metabolites and a low to moderate positive correlation among most of glucocorticoid metabolites in men and women. Sex specific differences were found for estrogen metabolites, which were much higher positively correlated in women than in men.
Fig 2. Overview about day and nighttime steroid hormone metabolite excretion in women and men.
Day and night time excretion values of urinary steroid metabolites in μg/hour were compared by Wilcoxon signed-rank test separately for men and women. Test results are indicated by coloured boxes: a red colour indicates higher excretion values during the day compared to night time, a green colour indicates lower excretion values during the day compared to night time and a yellow colour indicates no statistically significant difference. A white colour indicates that the metabolite has not been measured in the study. The left half of each box represents the test result indicated by colour for women and the right half for men as shown in the legend. Abbreviations: DHEA: Dehydroepiandrosterone. The OH in steroid names indicates a hydroxyl group, e.g. 17-OH-pregnanolone: 17-hydroxy-pregnanolone. DH: dihydro; TH: tetrahydro.
Discussion
We quantified 40 urinary steroid metabolites by GC-MS in a large number of thoroughly characterized adults of European descent and described the impact of sex and age on each steroid compound and sex-specific differences in day and night time excretions. Furthermore, we created sex- and age-specific reference intervals for these 40 compounds that can be used in routine clinical work as diagnostic tools. According to our literature search of the hitherto available reference intervals for the urinary steroid excretion from adults of the general population measured by GC-MS during the last 30 years since 1986, this is, to our knowledge, the largest study of this kind with such a detailed phenotype and the only one large enough to explore the relationship with age, covering a broad age range in both men and women.
Due to methodological differences, a direct comparison of absolute urinary steroid excretion values obtained by our in-house adapted GC-MS method with values published by other laboratories is possible to only a limited extent. Transferability of reference ranges for the urinary steroid profile could only be guaranteed if our data could be calibrated against a certified reference material which is lacking. For the time being, approximate correction factors could be calculated based on the results of an external quality assessment scheme (available through the Foundation for Quality Medical Laboratory Diagnostics skml) in which our steroid laboratory participates.
However, an inter-methodological comparison of sex- and age-related changes of the urinary steroid metabolome is possible and the studies published by Weykamp et al. and de Jong et al. are suitable for that purpose as both studies provide sex- and age-related reference intervals including 24 or 20 individuals, respectively, per sex- and age-group summarized in S1 Table (15, 19). In line with both studies we found an age-related decline of the 24-hour excretion of most progesterones and of androgens. In addition, we obtained similar results for some glucocorticoids with an age-related increase of TH-cortisol and a slight age-related decrease of allo-TH-cortisol with higher values in men for both steroid hormones. In contrast to the study by Weykamp et al. and similarly to the study by de Jong et al, we found a moderate age-related increase for the 24-hour excretion of the 11-deoxycortisol metabolite TH-11-deoxycortisol in men whereas the results in women were more similar in all three studies with regard to age-related changes. Also for 11β-OH-etiocholanolone, which derives mainly from the metabolism of cortisol [41], our results suggest a different age-related behaviour for men and women.
Our study reveals higher urinary excretion values for almost all glucocorticoids during the day compared to night time in both sexes whereas the excretion rates of sex steroid hormones present a more heterogeneous picture. A previous study on 10 men and 10 women has shown highest excretion rates for the sum of urinary cortisol metabolites between 12:00 and 15:00 for men and women and lowest excretion rates between 24:00 and 03:00 for men and between 03:00 and 06:00 for women and suggested a delay between serum cortisol levels and the excretion of urinary cortisol metabolites of about 4–5 hours [27]. For the 3-hour urinary excretion values of the sum of androsterone and etiocholanolone the same study found a peak after noon and a trough around midnight for men and women. The results of the study are compatible to our results with regard to the circadian excretion of urinary glucocorticoids and of the main urinary androgen androsterone. In addition, our results suggest different circadian excretory behaviours of androgen metabolites, probably also because they are cleared at different rates from the plasma. It has been shown, that after injection of unconjugated radiolabelded [4-14C]-androsterone and [4-14C]-etiocholanolone both are cleared to urine very rapidly and at nearly identical rates with a half life of about 20 minutes, whereas the corresponding conjugated steroid glucuronides are excreted much less rapidly and at different rates [42].
The observed differences between day and night time steroid excretion values in our study clearly support the presence of a robust circadian rhythm of glucocorticoid synthesis in the adrenal glands [43]. Circadian rhythm refers to evolutionarily conserved biological oscillations following a roughly 24-hour cycle and comes from a genetically operated timekeeping system called the “biological clock” [44, 45]. The suprachiasmatic nucleus of the hypothalamus is considered to act as the master clock in the mammalian organism [46], Adrenal glands show a circadian rhythm of glucocorticoid synthesis [47]. We found a clear separation between day and night time urine steroid excretion, not only for glucocorticoids, but also for most other steroid hormone metabolites that are assumed to be predominantly synthesized in the adrenal glands. In contrast, no tissue-specific circadian rhythm has been so far observed in the mammalian testis [48–50], whereas a circadian clock may play a role in steroidogenesis in the mammalian ovary [51]. These tissue-specific differences may contribute to the observed heterogeneous picture of urinary androgen and estrogen excretion at day and night time.
In view of methodological aspects our study has several strengths: 1) the analyses of all urine samples in the same laboratory, ensuring comparability of the results from different participants, 2) the confirmation of gas chromatography data by MS, and 3) the high number of subjects that permits a high standard in the creation of the lower and upper limit of reference intervals [17]. Moreover, we have been recently able to validate our GC-MS method by multidimensional gas chromatography-time of flight mass spectrometry (GCxGC-TOF MS), a high-resolution method newly developed by our laboratory [52, 53]. Thereby, we are able to exclude for all 40 steroid metabolites analytical problems that may occur due to the effect of matrix interferences in urine or due to the very high chemical similarity of the measured compounds.
The collection completeness is of critical importance especially if urinary excretion is reported as absolute value per collection period (μg/24h). We have made major efforts to achieve a high level of completeness by standardising the collection procedures in all three study centres and procedures were reviewed with each participant subsequently. However, it is not possible to entirely avoid incorrect urine collections and therefore, urinary creatinine excretion was used as a criterion for collection completeness. By the detection of under- and over-collected urine samples on the basis of the lower and upper limit of the 95% CI of the 24-hour creatinine excretion at least extreme collection errors could be excluded from the analyses. The applied regression model to predict 24-hour urinary creatinine excretion was recently developed with data from more than 1000 adults of European descent from the Swiss Survey on Salt and was validated with the participants of SKIPOGH providing a good fit [32]. This comparability of the SKIPOGH population to another independent and large Swiss cross-sectional population sample group underlines the reproducibility of our reference sample group and strengthens the level of confidence in urine collection completeness in this study.
Our study has several limitations. The study was restricted to participants of European descent. Due to the cross-sectional design of the study, urinary steroid hormone excretion data from the same participants at different ages are not available. Information about the phase of menstrual cycle were not collected and therefore reference ranges established for premenopausal women cover the whole period of the menstrual cycle. However, in a study including ten healthy white women of age 20–40 years with regular endogenous menstrual cycles between 24–34 days, the range of 24-hour urinary excretion of androgens and glucocorticoids was in a similar range as measured in our study and no differences were found between menstrual, follicular and luteal phase of the menstrual cycle for the urinary excretion of androgens and of the five glucocorticoids cortisol, cortisone, TH-cortisol, allo-TH-cortisol and TH-cortisone [21]. Moreover, a study comparing salivary cortisol after awaking revealed no differences between 11 women in the luteal phase and 12 women in the follicular phase of the menstrual cycle [54] and in another study it was consciously avoided to record the phase of menstrual cycle because previous unpublished analyses did not reveal an impact on urinary glucocorticoid and androgen metabolites [27]. In contrast, levels of plasma estrogens are associated with menstrual characteristics [55] and therefore the urinary excretion of estrogens would be expected to be influenced by the menstrual cycle phase as suggested by a study on six premenopausal women with highest values during the periovulatory phase and lowest values during the early follicular phase [56]. Further studies are necessary to confirm these results and to light up the impact of menstrual cycle also on the urinary excretion of progesterones, estrogens, corticosterones, and mineralocorticoids. Additionally, it would also be preferable to use isotopically labelled internal standards for more robust and accurate quantification (e.g. one standard per compound class).
Despite these limitations, our study clearly expands the current knowledge on the urinary steroid hormone metabolome in adults from the general population. The study introduces new reference ranges for a large number of urinary steroid hormones and can serve as an important tool in clinical practice.
Supporting information
Calibration curves are shown for all analytes injected on column in the range from 39 to 20000 fmol. Crosses indicate data points, the solid line represents the linear regression curve and the dotted lines the 95% confidence interval. Axes are plotted in logarithmic scale (base 10).
(PDF)
A plot of the sum of ion abundances for the selected compound-specific ions on the vertical axis versus the retention time of each characteristic ion on the horizontal axis simultaneously obtained by a GC-MS analysis of urinary steroid derivatives is shown. Multiple chromatographic peaks indicate the elution of numerous individual steroid compounds. By knowing the retention time for a given steroid indicated in the table, the members of the urinary steroid profile can be distinguished. Some steroid compounds are labeled. QIon: quantifier ion [m/z]. RT: retention time [min].
(PDF)
A descriptive analysis of the 40 steroid compounds measured in urine is shown including one steroid compound per page. Panel (a): boxplots, steroid (log-scale) by sex; Panel (b): Gasser-Müller nonparametric fits and scatter plots, steroid (log-scale) by age and by sex; Panels (c) and (d): boxplots, transformed steroid for men and women according to optimal power transformation; outliers are plotted as black dots; Panels (e) and (f): steroid (log-scale) by day and night time for men and women; Panels (g) and (h): Spearman correlation of transformed steroid night vs day. Abbreviations used: M = men; W = women; D = day; N = night; TR = optimal power transformation; nout = number of outliers; sk = skewness; ku = kurtosis; delta = robust estimate of mean difference expressed in standard deviations; rho = Spearman correlation coefficient; n = sample size.
(PDF)
Reference curves of the 40 steroid compounds measured in urine are shown including one steroid compound per page. The percentiles 2.5, 10, 25, 50, 75, 90 and 97.5 of the steroid compounds in function of age and sex are shown on a log-scale. To improve comparison the same scale has been used for men and women.
(PDF)
Quantitative urinary steroid excretion values measured by GC-MS and published since 1986 in adults are shown. Abbreviations: GC: gas chromatography, MS: mass spectrometry, F: female, M: male, d: days, y: years.
(PDF)
The number of participants is indicated for each characteristic and sex group. Categorical variables are described by % and continuous variables by their mean±standard deviation or by their median;25th-75th percentiles. Sex-specific differences were determined by chi squared test or Mann–Whitney U test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. Metabolites in the unit μg/24 hours are described by their median;25th-75th percentile. Between-group differences were determined by Mann–Whitney U test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. Metabolites in the unit μg/hour are described by their median;25th-75th percentile. Within-sex differences were determined by Wilcoxon signed-rank test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. The correlation between day and nighttime excretion values was assessed by Spearman’s rank correlation coefficient ρ (rho). Rho values were ranked in ascending order within each sex group. Metabolites were colored by steroid groups as indicated.
(PDF)
The statistical methods applied are described in detail.
(PDF)
Acknowledgments
We thank the study nurses Marie-Odile Levy, Guler Gök-Sogüt, Ulla Schüpbach and Dominique Siminski for the data collection and Sandrine Estoppey for her invaluable help for logistics and database management.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Swiss National Science Foundation (grant number 33CM30-124087 to MB, http://p3.snf.ch/Project-124087) and by the CTU Scientific Grant of the Directorate for Education and Research, Inselspital, Bern University Hospital, University of Bern, Switzerland (grant number 84801054 to NAD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Renterghem P, Van Eenoo P, Geyer H, Schanzer W, Delbeke FT. Reference ranges for urinary concentrations and ratios of endogenous steroids, which can be used as markers for steroid misuse, in a Caucasian population of athletes. Steroids. 2010;75(2):154–63. 10.1016/j.steroids.2009.11.008 . [DOI] [PubMed] [Google Scholar]
- 2.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011;32(1):81–151. Epub 2010/11/06. 10.1210/er.2010-0013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. The Journal of clinical endocrinology and metabolism. 2011;96(12):3775–84. 10.1210/jc.2011-1565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidi SK, Shen W-J, Azhar S. Impact of aging on steroid hormone biosynthesis and secretion. Open Longevity Science. 2012;6:1–30. 10.2174/1876326X01206010001 [DOI] [Google Scholar]
- 5.Petrovic D, Pivin E, Ponte B, Dhayat N, Pruijm M, Ehret G, et al. Sociodemographic, behavioral and genetic determinants of allostatic load in a Swiss population-based study. Psychoneuroendocrinology. 2016;67:76–85. 10.1016/j.psyneuen.2016.02.003 . [DOI] [PubMed] [Google Scholar]
- 6.Dhayat NA, Marti N, Kollmann Z, Troendle A, Bally L, Escher G, et al. Urinary steroid profiling in women hints at a diagnostic signature of the polycystic ovary syndrome: A pilot study considering neglected steroid metabolites. PloS one. 2018;13(10):e0203903 10.1371/journal.pone.0203903 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochud M, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, et al. Urinary sex steroid and glucocorticoid hormones are associated with muscle mass and strength in healthy adults. The Journal of clinical endocrinology and metabolism. 2019. 10.1210/jc.2018-01942 . [DOI] [PubMed] [Google Scholar]
- 8.Bradlow HL, Frazell EL, Gallagher TF, Hellman L. Tracer studies of the absorption and fate of steroid hormones in man. The Journal of clinical investigation. 1956;35(9):1033–44. Epub 1956/09/01. 10.1172/JCI103349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fales HM, Luukkainen T. O-methyloximes as carbonyl derivatives in gas chromatography, mass spectrometry, and nuclear magnetic resonance. Anal Chem. 1965;37(7):955–7. 10.1021/ac60226a052 [DOI] [Google Scholar]
- 10.Fang K, Pan X-J, Huang B, Liu J-L, Wang Y, Gao J-P. Progress on Keto Groups Derivatization of Steroid Hormones in Gas Chromatography-Mass Spectrometry Analysis. Chinese Journal of Analytical Chemistry. 2010;38(5):743–51. 10.1016/S1872-2040(09)60045-1. [DOI] [Google Scholar]
- 11.Axelson M, Sjövall J. Analysis of unconjugated steroids in plasma by liquid-gel chromatography and glass capillary gas chromatography-mass spectrometry. J Steroid Biochem. 1977;8(6):683–92. 10.1016/0022-4731(77)90297-7. [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard P. The analysis of urinary hormonal steroids. Lipids. 1980;15(9):710–8. Epub 1980/09/01. . [DOI] [PubMed] [Google Scholar]
- 13.Shackleton C. Clinical steroid mass spectrometry: a 45-year history culminating in HPLC-MS/MS becoming an essential tool for patient diagnosis. The Journal of steroid biochemistry and molecular biology. 2010;121(3–5):481–90. Epub 2010/03/02. 10.1016/j.jsbmb.2010.02.017 . [DOI] [PubMed] [Google Scholar]
- 14.Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). The Journal of steroid biochemistry and molecular biology. 2010;121(3–5):496–504. 10.1016/j.jsbmb.2010.04.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shackleton C, Pozo OJ, Marcos J. GC/MS in recent years has defined the normal and clinically disordered steroidome: will it soon be surpassed by LC/tandem MS in this role? Journal of the Endocrine Society. 2018:js.2018-00135-js.2018-. 10.1210/js.2018-00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shackleton CH. Profiling steroid hormones and urinary steroids. Journal of chromatography. 1986;379:91–156. Epub 1986/06/20. . [DOI] [PubMed] [Google Scholar]
- 17.Siest G, Henny J, Grasbeck R, Wilding P, Petitclerc C, Queralto JM, et al. The theory of reference values: an unfinished symphony. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2013;51(1):47–64. Epub 2012/11/28. 10.1515/cclm-2012-0682 . [DOI] [PubMed] [Google Scholar]
- 18.Bevan BR, Savvas M, Jenkins JM, Baker K, Pennington GW, Taylor NF. Abnormal steroid excretion in gestational trophoblastic disease complicated by ovarian theca-lutein cysts. Journal of clinical pathology. 1986;39(6):627–34. Epub 1986/06/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weykamp CW, Penders TJ, Schmidt NA, Borburgh AJ, van de Calseyde JF, Wolthers BJ. Steroid profile for urine: reference values. Clinical chemistry. 1989;35(12):2281–4. Epub 1989/12/01. . [PubMed] [Google Scholar]
- 20.Shackleton CH. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. The Journal of steroid biochemistry and molecular biology. 1993;45(1–3):127–40. Epub 1993/04/01. . [DOI] [PubMed] [Google Scholar]
- 21.Finken MJ, Andrews RC, Andrew R, Walker BR. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11beta-hydroxysteroid dehydrogenases. The Journal of clinical endocrinology and metabolism. 1999;84(9):3316–21. Epub 1999/09/16. 10.1210/jcem.84.9.6009 . [DOI] [PubMed] [Google Scholar]
- 22.Chan AO, Taylor NF, Tiu SC, Shek CC. Reference intervals of urinary steroid metabolites using gas chromatography-mass spectrometry in Chinese adults. Steroids. 2008;73(8):828–37. Epub 2008/05/03. 10.1016/j.steroids.2008.03.004 . [DOI] [PubMed] [Google Scholar]
- 23.de Jong WHA, Buitenwerf E, Pranger AT, Riphagen IJ, Wolffenbuttel BHR, Kerstens MN, et al. Determination of reference intervals for urinary steroid profiling using a newly validated GC-MS/MS method. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2017;56(1):103–12. 10.1515/cclm-2016-1072 . [DOI] [PubMed] [Google Scholar]
- 24.Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Hormone research. 1990;34(2):45–53. 10.1159/000181794 . [DOI] [PubMed] [Google Scholar]
- 25.Parikh TP, Stolze BR, Ozarda Ilcol Y, Jonklaas J, Welsh K, Masika LS, et al. Diurnal variation of steroid hormones and reference intervals using mass spectrometric analysis. Endocrine connections. 2018. 10.1530/EC-18-0417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czekala NM, Lance VA, Sutherland-Smith M. Diurnal urinary corticoid excretion in the human and gorilla. American Journal of Primatology. 1994;34:29–34. UBHD-. [DOI] [PubMed] [Google Scholar]
- 27.Jerjes WK, Cleare AJ, Peters TJ, Taylor NF. Circadian rhythm of urinary steroid metabolites. Annals of clinical biochemistry. 2006;43(Pt 4):287–94. 10.1258/000456306777695564 . [DOI] [PubMed] [Google Scholar]
- 28.Ackermann D, Pruijm M, Ponte B, Guessous I, Ehret G, Escher G, et al. CYP17A1 Enzyme Activity Is Linked to Ambulatory Blood Pressure in a Family-Based Population Study. Am J Hypertens. 2016;29(4):484–93. 10.1093/ajh/hpv138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guessous I, Bochud M, Theler JM, Gaspoz JM, Pechere-Bertschi A. 1999–2009 Trends in prevalence, unawareness, treatment and control of hypertension in Geneva, Switzerland. PloS one. 2012;7(6):e39877 Epub 2012/07/05. 10.1371/journal.pone.0039877 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firmann M, Mayor V, Vidal PM, Bochud M, Pecoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC cardiovascular disorders. 2008;8:6 Epub 2008/03/28. 10.1186/1471-2261-8-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. Epub 2009/05/06. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forni Ogna V, Ogna A, Vuistiner P, Pruijm M, Ponte B, Ackermann D, et al. New anthropometry-based age- and sex-specific reference values for urinary 24-hour creatinine excretion based on the adult Swiss population. BMC Med. 2015;13:40 10.1186/s12916-015-0275-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhayat NA, Frey AC, Frey BM, d’Uscio CH, Vogt B, Rousson V, et al. Estimation of reference curves for the urinary steroid metabolome in the first year of life in healthy children: Tracing the complexity of human postnatal steroidogenesis. The Journal of steroid biochemistry and molecular biology. 2015;154:226–36. 10.1016/j.jsbmb.2015.07.024 . [DOI] [PubMed] [Google Scholar]
- 34.Rousson V. Monotone fitting for developmental variables. Journal of Applied Statistics. 2008;35:659–70. UBHD-. [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/; 2017. [Google Scholar]
- 36.Shamim W, Yousufuddin M, Bakhai A, Coats AJ, Honour JW. Gender differences in the urinary excretion rates of cortisol and androgen metabolites. Annals of clinical biochemistry. 2000;37 (Pt 6):770–4. Epub 2000/11/21. 10.1258/0004563001900084 . [DOI] [PubMed] [Google Scholar]
- 37.Shackleton CHJM. Hyphenated Methods. The Encyclopedia of Mass Spectrometry. 82006. [Google Scholar]
- 38.Shackleton C. Genetic Disorders of Steroid Metabolism Diagnosed by Mass Spectrometry In: Blau N, Duran M, Gibson KM, editors. Laboratory Guide to the Methods in Biochemical Genetics: Springer; Berlin Heidelberg; 2008. p. 549–605. [Google Scholar]
- 39.Taylor NF. Urinary Steroid Profiling In: Wheeler MJ, Hutchinson JSM, editors. Methods in Molecular Biology: Hormone Assays in Biological Fluids. Totowa, NJ: Humana Press Inc; 2006. p. 159–75. [DOI] [PubMed] [Google Scholar]
- 40.Taylor NF. Urinary steroid profiling. Methods in molecular biology (Clifton, NJ). 2013;1065:259–76. Epub 2013/09/03. 10.1007/978-1-62703-616-0_17 . [DOI] [PubMed] [Google Scholar]
- 41.Shackleton CH, Neres MS, Hughes BA, Stewart PM, Kater CE. 17-Hydroxylase/C17,20-lyase (CYP17) is not the enzyme responsible for side-chain cleavage of cortisol and its metabolites. Steroids. 2008;73(6):652–6. Epub 2008/03/22. 10.1016/j.steroids.2008.02.001 . [DOI] [PubMed] [Google Scholar]
- 42.Slaunwhite WR Jr, Sandberg AA. Metabolism of 4-C14-testosterone in human subjects. III. Fate of androsterone and etiocholanolone. The Journal of clinical endocrinology and metabolism. 1958;18(10):1056–66. 10.1210/jcem-18-10-1056 . [DOI] [PubMed] [Google Scholar]
- 43.Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochimica et biophysica acta. 2011;1812(5):581–91. 10.1016/j.bbadis.2011.02.003 . [DOI] [PubMed] [Google Scholar]
- 44.Foster RG. Shedding light on the biological clock. Neuron. 1998;20(5):829–32. . [DOI] [PubMed] [Google Scholar]
- 45.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96(2):271–90. . [DOI] [PubMed] [Google Scholar]
- 46.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annual review of physiology. 2001;63:647–76. 10.1146/annurev.physiol.63.1.647 . [DOI] [PubMed] [Google Scholar]
- 47.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20970–5. 10.1073/pnas.0806962106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biology of reproduction. 2003;69(1):81–91. 10.1095/biolreprod.102.011833 . [DOI] [PubMed] [Google Scholar]
- 49.Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Molecular endocrinology. 2003;17(1):141–51. 10.1210/me.2002-0184 . [DOI] [PubMed] [Google Scholar]
- 50.Liang X, Cheng S, Jiang X, He X, Wang Y, Jiang Z, et al. The noncircadian function of the circadian Clock gene in the regulation of male fertility. Journal of biological rhythms. 2013;28(3):208–17. 10.1177/0748730413486873 . [DOI] [PubMed] [Google Scholar]
- 51.Sellix MT. Circadian clock function in the mammalian ovary. Journal of biological rhythms. 2015;30(1):7–19. 10.1177/0748730414554222 . [DOI] [PubMed] [Google Scholar]
- 52.Bileck A, Verouti SN, Escher G, Vogt B, Groessl M. A Comprehensive Urinary Steroid Analysis Strategy using Two-Dimensional Gas Chromatography–Time of Flight Mass Spectrometry. Analyst In press. 2018. 10.1039/C7AN01990D [DOI] [PubMed] [Google Scholar]
- 53.Bileck A, Fluck CE, Dhayat NA, Groessl M. How high-resolution techniques enable reliable steroid identification and quantification. J Steroid Biochem Mol Biol In Press. 2018. [DOI] [PubMed] [Google Scholar]
- 54.Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28(1):35–47. . [DOI] [PubMed] [Google Scholar]
- 55.Farland LV, Mu F, Eliassen AH, Hankinson SE, Tworoger SS, Barbieri RL, et al. Menstrual cycle characteristics and steroid hormone, prolactin, and growth factor levels in premenopausal women. Cancer causes & control: CCC. 2017;28(12):1441–52. 10.1007/s10552-017-0971-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, Duncan AM, Merz-Demlow BE, Phipps WR, Kurzer MS. Menstrual cycle effects on urinary estrogen metabolites. The Journal of clinical endocrinology and metabolism. 1999;84(11):3914–8. 10.1210/jcem.84.11.6134 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calibration curves are shown for all analytes injected on column in the range from 39 to 20000 fmol. Crosses indicate data points, the solid line represents the linear regression curve and the dotted lines the 95% confidence interval. Axes are plotted in logarithmic scale (base 10).
(PDF)
A plot of the sum of ion abundances for the selected compound-specific ions on the vertical axis versus the retention time of each characteristic ion on the horizontal axis simultaneously obtained by a GC-MS analysis of urinary steroid derivatives is shown. Multiple chromatographic peaks indicate the elution of numerous individual steroid compounds. By knowing the retention time for a given steroid indicated in the table, the members of the urinary steroid profile can be distinguished. Some steroid compounds are labeled. QIon: quantifier ion [m/z]. RT: retention time [min].
(PDF)
A descriptive analysis of the 40 steroid compounds measured in urine is shown including one steroid compound per page. Panel (a): boxplots, steroid (log-scale) by sex; Panel (b): Gasser-Müller nonparametric fits and scatter plots, steroid (log-scale) by age and by sex; Panels (c) and (d): boxplots, transformed steroid for men and women according to optimal power transformation; outliers are plotted as black dots; Panels (e) and (f): steroid (log-scale) by day and night time for men and women; Panels (g) and (h): Spearman correlation of transformed steroid night vs day. Abbreviations used: M = men; W = women; D = day; N = night; TR = optimal power transformation; nout = number of outliers; sk = skewness; ku = kurtosis; delta = robust estimate of mean difference expressed in standard deviations; rho = Spearman correlation coefficient; n = sample size.
(PDF)
Reference curves of the 40 steroid compounds measured in urine are shown including one steroid compound per page. The percentiles 2.5, 10, 25, 50, 75, 90 and 97.5 of the steroid compounds in function of age and sex are shown on a log-scale. To improve comparison the same scale has been used for men and women.
(PDF)
Quantitative urinary steroid excretion values measured by GC-MS and published since 1986 in adults are shown. Abbreviations: GC: gas chromatography, MS: mass spectrometry, F: female, M: male, d: days, y: years.
(PDF)
The number of participants is indicated for each characteristic and sex group. Categorical variables are described by % and continuous variables by their mean±standard deviation or by their median;25th-75th percentiles. Sex-specific differences were determined by chi squared test or Mann–Whitney U test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. Metabolites in the unit μg/24 hours are described by their median;25th-75th percentile. Between-group differences were determined by Mann–Whitney U test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. Metabolites in the unit μg/hour are described by their median;25th-75th percentile. Within-sex differences were determined by Wilcoxon signed-rank test, and the corresponding p values are indicated.
(PDF)
The available number of participants is indicated for each metabolite stratified for sex. The correlation between day and nighttime excretion values was assessed by Spearman’s rank correlation coefficient ρ (rho). Rho values were ranked in ascending order within each sex group. Metabolites were colored by steroid groups as indicated.
(PDF)
The statistical methods applied are described in detail.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.