Abstract
The gustatory system serves as a critical line of defense against ingesting harmful substances. Technological advances have fostered the characterization of peripheral receptors and have created opportunities for more selective manipulations of the nervous system, yet the neurobiological mechanisms underlying taste-based avoidance and aversion remain poorly understood. One conceptual obstacle stems from a lack of recognition that taste signals subserve several behavioral and physiological functions which likely engage partially segregated neural circuits. Moreover, although the gustatory system evolved to respond expediently to broad classes of biologically relevant chemicals, innate repertoires are often not in register with the actual consequences of a food. The mammalian brain exhibits tremendous flexibility; responses to taste can be modified in a specific manner according to bodily needs and the learned consequences of ingestion. Therefore, experimental strategies that distinguish between the functional properties of various taste-guided behaviors and link them to specific neural circuits need to be applied. Given the close relationship between the gustatory and visceroceptive systems, a full reckoning of the neural architecture of bad taste requires an understanding of how these respective sensory signals are integrated in the brain.
I. INTRODUCTION
The gustatory system is the gatekeeper of the alimentary tract, permitting and promoting the entry of nutrients while preventing and rejecting ingestion of potentially harmful substances. This review is primarily concerned with the sensory, integrative, physiological, and behavioral processes that subserve the latter; what we collectively and informally refer to here as “bad” taste. Yet, we would be remiss to simply exclude “good” taste from the present review, because complete understanding of the bad necessitates comparison with the good–the yin that helps define the yang. Moreover, there are many instances in which the distinction between good and bad taste is blurred and/or contingent upon other factors in the framework presented here. Ultimately, we hope that the foregoing reconsideration of bad taste in this broader context will help to elucidate its functional and neural organization and effectively guide future experimental endeavors.
The lion’s share of animal model research on the gustatory system (and feeding, for that matter), to date, has been conducted in rodents, specifically rats and mice. Like most humans, rats and mice are generalist omnivores and possess many of the same basic features of the gustatory system (81, 396, 448, 520, 611). That is not to say there are not some important differences among rodents and humans or that the properties of the gustatory system in all other species is different (71, 296, 350, 504, 515, 806).1 From an experimental perspective, one obvious difference is that humans can verbalize experience, perceptions, needs, feelings, and so on; rodents cannot. As such, we must depend on rigorous measurements of the behavior in non-human animals, while being careful not to take too many liberties with our inferences about subjective experience. This is something that has been easier said than done in animal research, especially with sensory research like taste. Moreover, while we can glean much about the gustatory system by comparing its organization across different species, it is equally important to link stimulus inputs to brain processes and, in turn, to physiological and behavioral outputs within the same model species to advance our understanding of the neural basis of taste function (683). For these reasons, this review concentrates on the findings gleaned from rodent models, although we have done our best to note important differences with humans where especially pertinent.
The first part of this review outlines the functional properties of bad taste, indeed of taste itself. The second part reviews some of the known neurobiological mechanisms. However, as will become clear, despite the fact that bad taste serves such a vital role in survival, very little is actually known about its underlying neural substrates. Thus some limitations of the conventional approaches to studying these phenomena are also discussed.
II. THE FUNCTIONAL PROPERTIES OF BAD TASTE
A. What Is Taste?
Before the specific topic of bad taste can be addressed, it is necessary to understand the functional role of taste, in general. The consumption of a food or fluid2 is highly dependent on the initial sensations and responses it evokes (169, 317, 692, 696, 757, 771). With its peripheral receptors at the threshold of the alimentary tract, the sense of taste is a critical modality for health and survival. Taste comprises the sensations and responses, be they behavioral, physiological, or even emotional (affective), roused when chemical compounds stimulate specialized chemoreceptors located within the oral cavity. This input gives rise to the basic perceptual qualities we discern in foods,3 triggers physiological reflexes that prepare the body to appropriately handle ingested substances (e.g., salivation upon biting into an acidic lemon), and yields positive and negative affect (e.g., pleasure, displeasure). Although the system comes hardwired to respond in specific ways to subsets of chemical classes (e.g., sugars or plant alkaloids) (224, 274, 705), responses can, under certain conditions (e.g., nutritional deficiency) or through experience, be temporarily or permanently modified (e.g., Refs. 34, 48, 68, 191, 241, 286, 438, 496, 594, 649, 703, 710, 810); thus there is flexibility in the system as well.
It is important to point out that what we generally refer to as taste (as in “I don’t like the way that dish tasted”) is actually flavor. Flavor is a percept synthesized from the taste, smell, texture, and temperature of a food (33). Indeed, much of the pleasure (or displeasure, for that matter) that we derive from eating as well as the finer-tuned discriminations we make among foods are based on flavor, not taste alone. Nevertheless, some have argued that taste is the sensory component that critically links the distal exteroceptive sensory cues associated with feeding (e.g., visual, odor) to the eventual postingestive sensory, metabolic, and/or physiological consequences (e.g., nutrition, food poisoning) in the generation of adaptive behavioral and physiological responses (226, 363, 650). Accordingly, the contributions of the gustatory system are not always at the level of awareness, but they are nevertheless pivotal and pervasive.
Taste signals contribute to several different functions, which can be categorized into three primary domains (683). One is sensory-discriminative, whereby information is extracted about the quality of the stimulus (i.e., what is it?) and its intensity (i.e., how much is there?). This chemospecific information can be used to associatively link particular foods with other sensory signals (e.g., odors) and/or postingestive consequences. Taste also has a motivational domain of function, whereby it elicits or reinforces certain behavioral responses that facilitate or thwart ingestion. These motivated behaviors can be further subdivided into appetitive and consummatory processes (134, 655). Taste-guided appetitive behaviors are those that bring the animal into contact with potentially nutritious foods (i.e., approach, food seeking) or away from potentially dangerous foods (i.e., avoidance, escape) (683). Taste-guided consummatory behaviors are those that promote or maintain the ingestive responses to, or effect rejection of, foods taken into the oral cavity. Certain chemical compounds unconditionally elicit such responses. For example, quinine is mostly avoided and rejected, while sugars are generally approached and ingested by humans and rodents alike (274, 606, 706, 799).4 Yet, as we will discuss in greater detail below, the motivational valence associated with a taste stimulus is tractable; that is, it changes as a function of physiology and/or experience (e.g., Refs. 48, 68, 227, 594). The third domain pertains to physiological reflexes. Taste stimulation elicits chemospecific physiological reflexes that aid in the postingestive handling of the food and minimize homeostatic deviation (e.g., Refs. 60, 240, 252, 268, 449, 554, 555, 718).
In some cases, the outputs associated with these domains of taste function are correlated, but oftentimes they are not. For example, there are a variety of foods (e.g., sugar and low concentrations of NaCl) that are readily approached and ingested, and thus share some motivational properties, but are nevertheless easily discriminated on the basis of their sensory properties (e.g., Refs. 279, 673, 690). Many phytoalkaloids and other organic toxins taste “bitter” to humans and are avoided by rodents (73, 193, 225). Yet, acids, which generate a sour taste, and some salts, which generate complex tastes of sourness, bitterness and/or saltiness depending on their ionic composition, are also avoided by rodents. These compounds do not stimulate the same classes of taste receptors and can generate perceptually distinguishable taste qualities (as detailed in sect. IIIA), yet they all can lead to affectively negative reactions (234, 279, 606, 687, 697).5
After an animal experiences a bout of food poisoning (a phenomenon discussed in detail in later pages), the motivational properties of the offending food changes from neutral, or even positive, to repulsive, but the taste quality of that food is thought to remain the same. Theoretically, this dissociation between motivational and sensory-discriminative properties allows the subject to recognize that same food upon future encounters and ensure it is promptly avoided. There is growing evidence that (some of) these functional domains of taste are governed by partially segregated brain circuits (6, 237, 252, 284, 288, 360, 553, 681, 697). As will hopefully become clear in the course of this review, making sense of taste, both in how it is encoded by neuronal activity and how the activity in a given neuron, or population of neurons, is related to behavioral/physiological outputs, has been a challenge.
The difficulty stems, in part, from a rather constrained functional framework applied to the analysis and interpretation of neuronal activity in the gustatory system. Historically, the examination of the neural signals associated with taste has largely focused on unveiling a taste quality code, one that presumably underlies sensory-discriminative function. However, by and large, the study of taste-driven outputs has used tasks that tap into the motivational domain.6 The fact that taste quality is oftentimes orthogonal to motivational valence makes it difficult to link the neural process to a qualitative perception per se. A given taste stimulus may evoke a neural response encoding its motivational properties and/or a neural response encoding its sensory-discriminative properties; measurement of the neural response alone cannot discern which is which. Thus progress in discerning the functional organization of the gustatory system will ultimately depend on linking taste-generated neural activity to a specific output: sensory-discriminative, motivational, and/or physiological.
B. What Is Bad Taste?
The term bad taste comes from the Latin word dis-gustus or disgust meaning a visceral sense of revulsion roused by the taste properties of a food.7 Strictly speaking then, disgust is associated with a highly conserved host of behavioral and physiological reflexes like gaping, retching, gagging, nausea, and vomiting (611, 613).8 These defensive mechanisms are geared towards ridding the body of a substance that is associated with adverse consequences. Some of these stimulus-driven responses appear to be innate (or unconditioned). Indeed, the taste system has evolved distinct mechanisms for detecting and responding to a broad class of chemicals associated with potentially toxic outcomes. Rodents, monkeys, and even newborn humans will reject a quinine solution upon the very first exposure (224, 705, 707). Such a response can also be acquired through experience; the taste of a food that has been followed by a bad case of food poisoning evokes this disgust reaction (79).
From a behavioral perspective, not all bad tastes are necessarily disgusting or aversive. Pelchat et al. (550) provided an intuitive illustration of two different types of bad taste. Borrowing from their example: one person eats shrimp for the first time and then has that serious case of food poisoning. Thereafter, encounters with the orosensory properties of shrimp will evoke those negative visceral and affective reactions and that person will avoid eating shrimp. This is known as a conditioned taste aversion (CTA). Yet, a different person eats the shrimp and suffers no such poisoning, but breaks out in hives from an allergic reaction to the shellfish. This person will also avoid eating shrimp, but is not repulsed by the taste; in most cases, the allergic individual still appreciates the taste of shrimp. Thus, in the latter case, the gustatory system signals potential dangers and, in turn, discourages the ingestion of shrimp (avoidance), but it does not render the taste of shrimp disgusting (aversion).9 Such experientially based changes in behavior are thought to be forms of Pavlovian and/or instrumental learning.
C. Taste Aversion Versus Taste Avoidance
Arguably then, there are at least two types of bad taste: one corresponding to aversion and one corresponding to avoidance. Although these phenomena can be in register, there are clear discrepancies in their underlying behavioral characteristics. Theoretically, aversion is associated with the active rejection of a tastant (conditionally or unconditionally) and, as such, belongs to the consummatory subdomain of taste-based motivation. Avoidance refers to behavioral processes whereby an animal will limit its approach toward and ingestion of a particular tastant (conditionally or unconditionally) and, as such, belongs to the appetitive subdomain of taste-based motivation (229, 419, 537, 550, 611, 613). Avoidance is not necessarily accompanied by a fundamental change in oromotor reflexes (i.e., consummatory behaviors). This can be seen if the subject is forced to sample the tastant (i.e., under experimentally contrived conditions).10 Accordingly, even in cases where the primary taste inputs are identical, the downstream central processing and certainly the motor outputs are different.11
Intake, as total mass or volume consumed over a designated period, is a common measure of taste-based and/or viscerally based inputs. CTAs,12 for example, are typically measured as intake of the taste solution in a single or two-bottle (vs. water or another solution) test after conditioning. However, intake is merely an outcome, which is influenced by a myriad of different types of stimulus inputs and motor outputs (appetitive and consummatory). Consequently, intake measures alone are interpretively limited (153, 278, 681, 769). The shrimp poisoning versus shrimp allergy case above provides just one example of how two fundamentally different processes can look the same in a final outcome. Grill and Norgren (274) developed a procedure, the taste reactivity (TR) test, capable of probing taste-guided consummatory behaviors, in the absence of appetitive response requirements (see FIGURE 1; procedure described in detail in the legend). Indeed, changes in intake are not always accompanied by a change in TR. For example, in rats, intake of a neutral to normally preferred stimulus is reduced by pairing its ingestion with LiCl or a whole host of other types of viscerally related experiences, like radiation exposure, motion/vestibular disturbance, chemotherapy drug treatment, gastrointestinal (GI) pain, exteroceptive pain/paralysis, indigestion, bacterial toxins, stimuli associated with satiation (more on these in sect. IIIE), and even administration of psychoactive drugs (e.g., Refs. 22, 32, 41, 44, 137, 139, 151, 179, 227, 228, 412, 423, 445, 450, 535, 546, 550, 583, 672). Yet, only some of these are known to produce a downward shift in taste-elicited ingestive TR and/or an upward shift in taste-elicited aversive TR (21, 79, 133, 136, 138, 151, 179, 180, 184, 456, 528, 533, 542, 543, 545, 550, 685). In fact, many unconditioned stimuli (USs) that lead to a learned suppression of conditioned stimulus (CS) intake do not condition a change in TR [e.g., (535, 536, 538–541, 550, 802)]. The qualitative and/or quantitative properties of the US that lead to aversion and/or avoidance need to be more fully elucidated. Nevertheless, there is mounting evidence to suggest that events that engage the emetic system lead to changes in taste-based consummatory behaviors, whereas those that engage pain systems do not (and are perhaps working through appetitive mechanisms).13,14 The use of behavioral measures that distinguish aversion from avoidance will be crucial for advancing our understanding of the underlying motives and their neural bases. Key differences in stimuli and responses in conditioned taste aversion and conditioned taste avoidance are illustrated in FIGURE 2.
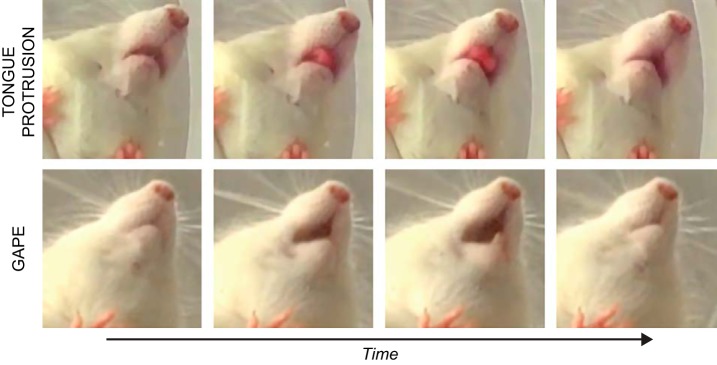
FIGURE 1.
Taste reactivity (TR) refers to the stereotypic oromotor and somatic reflexes that animals, including rodents and humans, elicit to taste stimulation (274, 705). In many applications of this procedure, animals are surgically fitted with intraoral cannulae through which taste stimuli can be directly infused under experimenter control; this effectively circumvents the appetitive component of ingestive behavior and thus provides a pure readout of consummatory responses. TR can be generally categorized as ingestive or aversive. Ingestive responses include reflexes that are associated with the act of consumption. In the rat, this consists of mouth movements, tongue protrusions, lateral tongue protrusions, and paw licking. The incidence of these ingestive responses increases with the concentration of a readily ingested substance, like sucrose. Top panel shows successive frames of a rat in the act of a tongue protrusion. Aversive responses include reflexes that are associated with stimulus rejection; in the rat, this includes gapes, chin rubs, forelimb flails, and head shakes. The incidence of these negative responses increases with the concentration of a representative “aversive” stimulus, like quinine. Bottom panel shows successive frames of a rat in the act of a gape. After a taste stimulus that unconditionally produces mainly ingestive responses, such as sucrose, is paired with the emetic agent LiCl (for CTA), rats exhibit a clear change in sucrose-elicited TR (79, 685). That is, following such conditioning, the responses to sucrose comprise more aversive behaviors and fewer ingestive behaviors. It should be noted that there are discrepancies across laboratories with respect to how ingestive and aversive responses are categorized and quantified. Such differences likely have some impact on the experimental result and interpretation.
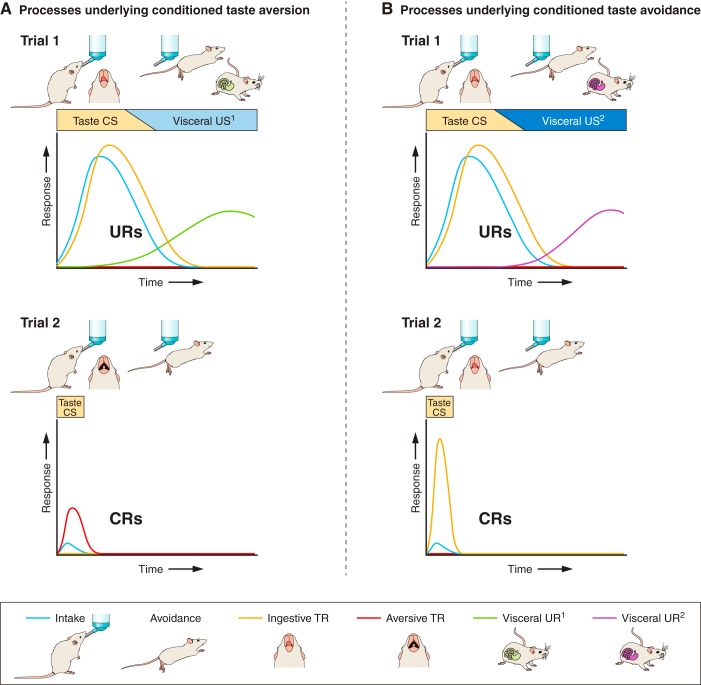
FIGURE 2.
This schematic illustrates key differences in the stimuli and responses that underlie conditioned taste aversion (A) vs. conditioned taste avoidance (B); these are broken down in the schematic as unconditioned and conditioned stimuli (USs and CSs) and responses (URs and CRs). A US is a stimulus that automatically evokes a biologically significant response (UR). When a previously neutral or unassociated stimulus (CS) is paired with the US/UR, that CS comes to evoke a response (CR) to deal with the impending US/UR. Although these nomenclatures are typically affiliated with Pavlovian conditioning, their use here is not meant to imply that other processes (e.g., Instrumental) are not involved in conditioned taste aversion and avoidance. Those theoretical distinctions are beyond the scope of this review. Conditioned taste aversion (A, trial 1): a naive rat happens upon a novel tasting fluid (CS) and consumes it; the CS promotes consumption and elicits ingestive TR (both URs). Most tastes are USs in and of themselves, compelling either approach or avoidance and/or ingestive or aversive TR, depending on the stimulus. Initially undetected by the rat, the fluid is laced with a visceral US type 1 (US1), which eventually produces a negative postingestive effect (UR1). If detected while the rat is still consuming, these USs may cause the rat to stop drinking or reject the CS (URs). A, trial 2: searching for nourishment again, the rat comes across the same fluid (CS). However, having experienced the negative US1/UR1 associated with the fluid in that bottle, the rat now avoids intake and displays aversive TR to its taste. These CRs, avoidance of further intake and a shift to an aversive TR, are the hallmark distinguishing features of the conditioned taste aversion. This is contrasted with conditioned taste avoidance shown in B. Conditioned taste avoidance (B, trial 1): in this case, the naive rat approaches and begins consuming the novel fluid CS, just like that shown in A, trial 1, except that the visceral stimulus (in this case designated by US2) lacing that fluid and the negative postingestive responses (UR2) it evokes differ. Ultimately, these events render a different conditioned response profile the next time the rat encounters the CS (trial 2). B, trial 2: here, the rat samples the fluid CS and exhibits an ingestive TR profile to the taste, but does not consume it. Thus the conditioned responses profile is largely determined by the visceral US/UR. It is also important to note that we have plotted intake here, but intake is an outcome measure resulting from other behaviors (e.g., appetitive, consummatory). Aside from the TR test, little effort has attempted to discern the specific appetitive and consummatory mechanisms related to taste aversion vs. avoidance; other behavioral measures may distinguish these responses or reveal more subcategories for aversion vs. avoidance. Moreover, both of these learned response profiles are presumptively modulated by physiological state, a feature that is not shown in the accompanying schematic. For example, extreme food deprivation may stimulate consumption of an avoided, but nutritious food. Animals can also learn to consume foods that have taste properties that initially stimulate rejection and avoidance via pairing with beneficial visceral consequences (e.g., calories).
Grill and colleagues have argued that TR represents a measure of an animal’s hedonic evaluation of a taste stimulus, a view that has since been widely adopted (47, 51, 64, 79, 270, 274, 529, 767, 777).15 Indeed, we present findings throughout this review that offer significant support for this perspective. However, we think it is important to keep in mind that these taste-elicited responses are fundamentally motor reflexes. In fact, rats that have undergone a supracollicular decerebration, which neurally disconnects the forebrain from the hindbrain, and anencephalic human infants, who are sadly born without a forebrain, still display relatively normal TR to basic chemical compounds placed on the tongue (202, 275, 705). So then, do we infer that these are generating a sense of pleasure or displeasure in the decerebrate animal? With respect to the domains of taste function laid out above, it is reasonable to ask whether TR is related to the motivational domain (i.e., hedonics) or the physiological domain. Some unconditionally bad-tasting foods (e.g., bitter plant alkaloids) trigger physiological reflexes (e.g., slowing of gastric emptying) that are thought to mitigate potential poisoning. Put another way, what is the practical difference between the motor reflexes that slow gastric emptying and the motor reflexes that lead to gaping in response to a disgusting taste?
All of that said, a response (or motor output) can be controlled by different types of inputs and through varied neural pathways (270). Case in point, decerebrate rats display the full range of unconditioned stimulus-appropriate TR, but they are unable to express a conditioned change in TR for a taste stimulus paired with LiCl (more on this in sect. IIID) (272). Nor do decerebrate rats change their consummatory TR for hypertonic salt solutions in a state of sodium depletion the way normal intact rats do (more on sodium appetite in sect. IID) (276). Accordingly, at more fundamental levels of the gustatory system, TR appears to reflect a set of hard-wired reflexive actions that adaptively serve animals by facilitating or averting ingestion of oral stimuli based on their chemical properties. However, TR can be modulated by forebrain circuits affording a practical degree of flexibility in the behavior and seemingly allowing for higher order processing to exert its control (see sect. IIIF4).
Often, rodents will suppress intake of a food/taste solution that was previously paired with substances for which rodents will work vigorously to obtain (e.g., amphetamine, morphine, cocaine, concentrated sucrose), indicating they are effective reinforcers of operant behavior (254, 395, 411, 565, 774, 783). Some have posited that these counterintuitive effects may be due to privileged associations between the taste CS and negative side effects of these drugs, effectively establishing a taste avoidance (e.g., Refs. 142, 152, 536; but see Ref. 334). In studies where TR has been measured, the data generally suggest that many psychoactive drugs suppress intake of the associated CS (i.e., avoidance), without a concomitant flip in the elicited TR profile (i.e., no aversion) (536).16
Still, considering the presumptively overwhelming positive consequences of these drugs, some researchers hypothesized that reductions in CS intake could be due to some other psychological processes (259). Stimulus contrast was proposed as one such mechanism. Stimulus contrast refers to a reduction in intake of one stimulus because it is reliably followed by a stimulus of higher value (198). Accordingly, a solution with a comparatively weaker valence is further devalued if a solution, food, or drug with more positive properties is imminent. For instance, experience with two different sucrose concentrations results in a gradual reduction in ingestive TR for the lower concentration, relative to the higher concentration (261).17 This phenomenon represents yet another basis through which taste-guided intake is reduced through experience.18
In fact, because the principal proxy of avoidance is willingness to approach, ingest, or choose one substance over another (preference/avoidance), there are a number of different phenomena that generate “avoidance” despite the fact that they have seemingly very different motivational bases. In addition to contrast, foods are avoided when the animal is satiated, sick, or stressed and when the food is novel (e.g., neophobia)19 or associated with poisoning, indigestion, GI pain/inflammation, or motion sickness. The list can go on. Therefore, it will become important to develop strategies to decipher among these different motives in behavior.
Bad taste has been most extensively studied and considered in terms of the motivational domain described here (i.e., aversion and avoidance), but the other two domains likely also significantly influence how putative bad tastes are handled. We have already mentioned some inherent physiological responses to bad tastes (e.g., slowing of gastric emptying), and there is evidence that the cephalic phase release of insulin in response to the taste of sugar is attenuated if the taste is associated with negative consequences (e.g., LiCl; Ref. 45). Despite their obvious significance for survival and homeostatic control, very little attention has been paid to these aspects of taste. Sensory-discriminative function also possibly undergoes some changes with physiological and experiential conditions (e.g., sodium deprivation, taste learning), but these too have not been explicitly interrogated. For example, does a taste stimulus become more salient or detectable in time of need or when it predicts a biologically significant outcome?20 Future work will need to find ways to study these processes and examine how they interact with the other domains of taste function with comparable experimental rigor.
D. Turning Bad Tastes Good?
Physiological state and experience are significant determinants in how a given taste signal is processed by central circuits that lead to aversion versus avoidance, and presumably to outputs related to other domains of function (e.g., physiological reflexes). Negative outcomes associated with even a highly palatable food can redirect those signals into responses that prevent contact with the food (as in the case of a shrimp allergy) or can produce disgust reactions (as in the case of food poisoning) on future occasions. Can initially aversive and avoided foods likewise undergo revisions, if they are encountered in times of physiological need or associated with positive outcomes, like the bitter, yet nutritious vegetable? Many people report having acquired tastes for foods and fluids that were initially disgusting and/or avoided. Popular examples include coffee, beer, and cruciferous vegetables. Theoretically, it would seem feasible that parallel physiological state- and/or learning-dependent mechanisms exist to make bad tastes more acceptable as indicated by appetitive and/or consummatory processes. In the case of the former, we might expect that some inherently bad tasting foods can eventually promote approach and initiate ingestion, despite the taste; this would be a parallel mechanism to avoidance. Alternatively, such need states or positive experiences could produce a fundamental change from taste-elicited aversive consummatory responses to ingestive ones; this would be a parallel mechanism to aversion. Although less empirical attention has been paid to this aspect of taste-guided behavior in the rodent literature, there are nevertheless some revealing findings.
We have already alluded to one such phenomenon in the previous section—sodium appetite. Under normal conditions, highly concentrated sodium has an aversive taste, which is readily avoided.21 Loss of bodily sodium, however, stimulates positive appetitive and consummatory behaviors towards highly salty foods, ultimately promoting the ingestion of sodium (and lithium) salts (77, 80, 162, 167, 235, 293a, 344, 454, 496, 559, 579, 580, 642, 643, 701, 712, 724, 784). Notably, whereas normal (sodium-replete) rats display primarily aversive TR towards hypertonic salt solutions, sodium-deplete rats display mainly ingestive TR to even very highly concentrated salt solutions (50, 267, 276). Interestingly, the TR change is evident upon the very first oral contact with the solution. In other words, it does not require learning or experience with sodium repletion (50). Following sodium repletion, the baseline responses are largely returned to normal (rejection and avoidance of high sodium foods and fluids), a feature that is thought to preempt the consumption of too much sodium, which has detrimental effects on the body (50, 131, 700).22 Thus, unlike some conditions in which the positive consequences of a food will make for lasting increases in intake or preference for that food, sodium appetite is strictly state-dependent. Moreover, transection of the primary taste afferents, particularly the chorda tympani nerve, severely disrupts the ability to detect and respond appropriately to sodium salts in this state, as does topical pharmacological blockade or specific genetic deletion of epithelial sodium channels (ENaCs) in taste cells, indicating that this is a taste-based phenomenon (42, 78, 80, 84, 112, 212, 235, 439, 595, 678, 700). In this case, the state of sodium balance reversibly affects appetitive and consummatory responses to sodium (and lithium) salts to maintain bodily levels within a safe range.
Like sodium, essential amino acid (EAA) levels are highly defended. Because amino acids are not stored in the body and EAAs cannot be synthesized, they must be constantly replenished through intake. When rats are given a diet devoid or deficient in a specific EAA, they learn to associate that diet with its adverse consequences and will avoid it, especially when subsequently given an alternative diet (190–192, 215, 326, 327, 381, 382, 414, 612, 750).23 Rats will also learn to specifically enhance their consumption of diets or fluids that contain the needed nutrient (69, 241, 498, 665). Interestingly, unlike sodium appetite, this does not appear to be because the taste of the specific nutrient is unconditionally recognized. For example, rats maintained on a diet deficient in l-lysine do not lick more for solutions of this EAA in a brief access taste test, but they do take more trials of all amino acids presented (trials initiated being an index of appetitive behavior), relative to replete controls (438). The general increase in appetitive behavior represents one strategy for enhancing contact with (and putatively intake of) the needed nutrient. Throughout the course of a longer-term exposure, rats can learn to selectively consume more of the needed EAA (i.e., l-lysine) over water or an alternative EAA. However, this experience does not translate into lasting increases in their responsivity to the taste of l-lysine, as measured in a subsequent brief access taste test (438). Close inspection of the drinking patterns in a long-term two-bottle choice test revealed that the deficient rats begin to consume more l-lysine over water after ~30 min and more l-lysine over another EAA, l-threonine, after 90 min. During these tests, l-lysine-deficient rats took many small bouts of the l-lysine solutions, which is reminiscent of the way rats normally lick for quinine in a short-term test (when water-deprived) (440, 694). Collectively, these results suggest that, at least with respect to this type of EAA deficiency, it is the appetitive aspects of taste function that are unconditionally modulated to yield an increase in intake, whereas the consummatory aspects appear to be unaffected.24 Considering that many of EAAs have potentially toxic side effects, it seems prudent to limit the rate of their ingestion within a meal, even in times of need. Accordingly, perhaps these behavioral results reflect a strategy in which the frequency with which the stimulus, l-lysine is approached is increased, while the accumulated levels ingested are closely monitored by postoral mechanisms; the merit of this hypothesis remains to be fully evaluated. Nevertheless, one thing is clear: both sodium and l-lysine depletion lead to increased intake of the needed substance even at concentrations that are normally avoided by the rat.25 However, it would appear that these two physiological conditions affect taste-guided behavior in fundamentally different ways.
Conditioned taste preference (CTP), or conditioned flavor preference, is often regarded as a complementary learning mechanism to CTA (reviews in Refs. 99, 646). Indeed, there is an extensive literature demonstrating that pairing a neutral or accepted flavor with certain nutritive outcomes (e.g., some carbohydrates, fats, protein, and certain amino acids) leads to lasting increases in intake of and preference for that flavor (e.g., Refs. 3, 4, 173, 431, 649). To date, we are aware of only a handful of studies that have directly examined whether a conditioned increase in intake of and/or preference for a flavor associated with positive nutritive outcomes is accompanied by a fundamental change in taste-elicted oromotor reactivity (205, 493–495). One study found that rats displayed more ingestive TR for a sweetened Kool Aid solution (CS+) that was previously paired with intragastric glucose infusions relative to a different sweetened Kool Aid solution (CS−) that had been paired with intragastric water infusions instead. Interestingly, a companion paper found that when rats were trained under similar conditions, except that the flavor CS+ was either sucrose octa-acetate (SOA, described as “bitter tasting”) or citric acid (described as “sour tasting”), rats increased their intake of and preference for the CS+ solution paired with the nutritious consequence, but did not evince an increase in CS+-elicited ingestive TR (or decrease in aversive TR, for that matter) (494). Thus the authors interpreted these results to suggest that when the CS+ is a normally preferred stimulus to start, flavor-nutrient conditioning can enhance the palatability of the CS+, as indexed by increased ingestive TR. However, if the CS+ is a normally avoided stimulus, such conditioning can increase intake, but does so without a fundamental change in palatability. Considering l-lysine is thought to have a “bitter” taste, it may be that, like SOA and citric acid, it is resistant to changes in consummatory behaviors when presented to rats that have been depleted, as described above.26 Still, other types of nutrients or other positive USs such as medicinal or pharmacological substances may be capable of flipping the affective value of tastants, even those that are normally aversive (see below).27 Thus, while inherently aversive taste stimuli can become motivationally positive through experience and/or physiological need, there appears to be biases in the system, such that the inherent responses are more difficult to overcome. Factors such as the strength and the type of physiological and/or visceral stimuli may be important determinants of how the varied motivated responses are impacted.
It is worth mentioning that the postoral actions of certain sugars (and other select nutrients) rapidly reinforce licking of a neutral or modestly accepted stimulus within minutes of the start of a single drinking episode; this phenomenon has been termed appetition (see Ref. 645 for a review). Two recent studies have examined whether sugar stimulation of ingestion rate occurs when the stimulus being ingested is inherently bad (i.e., quinine) (635, 720). In other words, is the postoral detection of nutrition yielded from an otherwise aversive food sufficient to overcome its negative orosensory properties (as might be the case with vegetables)? In principle, that could be a useful strategy to rapidly resolve some of the ambiguities between the taste of something and its actual contents to ensure adequate nutrient intake within a meal. On the other hand, the harmful effects of an ingested food may be rather delayed from the immediate nutritive ones and, therefore, increasing the amount of a substance consumed in a given meal may potentially put the animal at greater risk (for later poisoning). With respect to these two theoretical strategies, one study found that intragastric infusion of glucose rapidly stimulated licking for a sucralose-quinine solution (720), while another study found that rats preloaded intraduodenally with sucrose did not exhibit any enhancement in short-term licking for quinine or sucrose that had been previously paired with LiCl (635). Interestingly, in the case of aversively conditioned sucrose, although there was no effect on licking behavior, the gut preload of sucrose did suppress the number of trials initiated on the test. Sucrose preloads in the gut also significantly suppressed ingestive TR to intraorally delivered “aversive” sucrose in a separate set of rats. Together, these results suggest that the postoral actions of some stimuli may rapidly impact taste-guided consummatory and appetitive behaviors. Again, the properties of the taste and postoral stimuli involved may be critical determinants of whether ingestive behaviors are positively modified.
Are non-nutritive postingestive USs that have medicinal or positive pharmacological properties capable of flipping a bad taste into a good taste? The results are rather mixed. Some have found that rats given normally avoided tastes paired with drugs or other medicinal substances come to exhibit less aversive TR and/or more ingestive TR when the tastes are presented alone (e.g., Refs. 128, 204, 347, 513, 534, 545, 803). Yet, as reviewed above, there are numerous studies that have found that rodents will come to avoid even initially palatable flavors or tastants associated with pharmacological agents28 and, in some cases, increase their aversive TR (e.g., Refs. 100, 254, 411, 542, 546, 715, 773). There are numerous potential reasons for the discrepancies. It could be that the initial hedonic value of the taste/flavor stimulus is a key factor in determining the content of the learning and the adopted response strategy, or it could be dependent on experiential parameters,29 and/or the particular visceral US (including the dose, frequency, need state, withdrawal).
In the wild, animals voluntarily sample plants and other organic matter with taste properties that are normally avoided (e.g., bitter) (249, 332). Laboratory animals have also been known to consume low concentrations of “bitter” chemicals (25, 432, 733, 768). This behavior is thought to reflect a means of medicating an infection or disease state, as many plants also contain curative compounds. To explicitly test this hypothesis in a laboratory setting, Vitazkova et al. (760) gave mice with or without malaria infection the opportunity to consume a solution containing a “bitter” plant alkaloid known to treat malaria, namely, chloroquine, over a period of a week. Interestingly, both infected and uninfected mice consumed comparable amounts of the chloroquine over water across this treatment period. The finding was taken to suggest that neither the infection nor the consequent physiological symptoms of the infection were driving chloroquine consumption in the first place. Moreover, intake remained fairly stable across treatment days. Although the medicinal solution was effectively reducing the infection, the mice with malaria did not gradually increase their consumption of the solution, which would effectively increase the dose, following such experience with its beneficial postingestive outcome.30 Accordingly, the authors speculated that voluntary ingestion of these and like compounds may reflect a proactive strategy for forestalling disease, but the response strategy may not be modified through learning about the taste of the solution and its postingestive consequences. The generalizability of these results to other infectious states and medicinal compounds await empirical examination.
Clearly more studies are needed on the topic, but the available data are nevertheless consistent with the view that inherently bad tastes are more resistant to undergoing lasting and/or profound changes in a positive direction (e.g., enhancing ingestive TR). For sodium, there is a definitive change in consummatory responding, but this is largely state-dependent. For EAAs, intake and choice for a particular EAA can be increased within limits, but this does not appear to be accompanied by even a temporary change in the hedonic taste properties of the stimulus.31 Other nutrients like carbohydrates, which, when delivered postorally, can robustly enhance intake and preference for associated CS tastants that are initially good, do not appear to likewise produce lasting quantitative or qualitative shifts in the consummatory oromotor responses to CS tastants that are unconditionally bad, despite promoting increases in their intake. The generality of these effects, including, for example, the use of different types of tastants, postingestive stimuli, and training procedures needs to be experimentally evaluated. Moreover, we have concentrated on the motivational domain of taste function, on which, with the exception of sodium appetite, the empirical studies to date have focused. Nevertheless, it seems possible that such experience fosters changes in sensory-discriminative processing (e.g., increased/decreased sensitivity to the stimulus) via physiology and/or learning. Although speculative, we reason that in the case of unconditionally bad tastes, positive postingestive feedback may be especially important to allow animals to attend to the distinct sensory-discriminative features that identify a food as safe (its color, odor, etc.), while precluding the inadvertent generalization to other similar tasting but potentially toxic substances.
Animals that subsist on diets high in “bitter-tasting” nutritious compounds may have evolved other ways to deal with them too. Prolonged exposure to normally avoided or bad-tasting compounds produces physiological changes that make the foods more tolerable (e.g., Refs. 144, 248, 383, 667, 734, 735). For example, exposure to a diet enriched with tannic acid, which is bitter to humans, leads to an upregulation of salivary proline-rich proteins (735). These proteins, in turn, bind the tannins and thereby prevent them from being absorbed from the gastrointestinal tract. This adaptive strategy allows the animal to consume large amounts of foods containing tannin and to extract the essential nutrients, without being exposed to the toxic elements. These salivary proteins may also prevent the tannins from binding to taste receptors in the oral cavity, effectively dampening their bitter taste and helping promote and maintain the ingestion of some essential nutrients and medicines that have inherently aversive tastes (248, 249, 735). The mechanisms underlying these diet-induced alterations in salivary protein expression represent a new and promising experimental frontier in understanding how dietary experience can influence taste and thus food choice via physiological adaptations.
We recognize that perhaps the simplest way to affect the acceptability of an aversive stimulus is to adulterate it with a highly preferred stimulus: “a spoonful of sugar makes the medicine go down.” Indeed, in the human psychophysical literature, there is evidence of mixture suppression in which one taste compound attenuates the perceived intensity of another contained in the same solution. It is known, for example, that the perceived bitterness of quinine can be suppressed by the addition of sucrose or NaCl to the solution (82, 93, 214, 257, 388, 406, 558, 637). While the phenomenon of mixture interactions is significant from the standpoint of peripheral and central mechanisms underlying taste perception and has relevant applications for the food and beverage industry as well as to the important problem of formulating orally administered medicines, it does not necessarily represent an example of a fundamental shift in the inherent aversiveness of the target compound in isolation.
The foregoing review of bad taste, if nothing else, illustrates three fundamental principles. First, taste contributes to various functions. An understanding of the significance of a particular neural signal or the contribution of a particular neural component requires linking them to specific inputs and functional outputs. Second, with respect to the motivational domain, taste drives at least two different classes of responses: appetitive and consummatory. There are certain conditions under which taste will influence one and not the other, which appears to reflect different strategies for balancing nutritive needs against cost (e.g., pain, homeostatic imbalance) or the risk of lethal poisoning associated with ingestion. Third, it follows, therefore, that the visceral and/or physiological consequences of ingestion play a significant role in the way a food tastes. As such, any comprehensive identification of the operating principles of the gustatory system requires a complementary recognition of how visceral signals associated with feeding are functionally and neurally organized.
III. THE NEUROBIOLOGICAL MECHANISMS OF BAD TASTE
A. Taste Receptors
Taste begins, so to speak, where the chemical constituents of ingested foods and fluids interact with receptor proteins or ion channels located on the apical surfaces of specialized epithelial cells called taste receptor cells (TRCs) (see Ref. 604 for a review).32 The TRCs, along with support cells, are arranged in clusters of ~50–100 cells, called taste buds. Depolarization of the TRC generates a signal in the peripheral gustatory system in two main ways. So-called type II cells, which express G protein-coupled receptors (GPCRs) that serve as taste receptors, release ATP via a nonvesicular-related process (through Calhm1), which, in turn, stimulates P2X2/P2X3 receptors expressed on the membrane of closely apposed afferent nerve fibers (196, 378, 755). As a result of these nonconventional synapses, a given taste afferent may potentially receive input from a number of different TRCs. Type III cells, which are thought to express ion channel-based taste receptors, possess conventional synapses, in which serotonin is released from the TRC and, in turn, activates the postsynaptic serotonin type 3 (5-HT3) receptors on afferent fibers (331, 398, 732).33 A single afferent fiber can innervate various TRCs, including those from proximate but discrete taste buds (471, 472).
1. Taste type 2 receptors
The largest class of taste receptors is a family of GPCRs known as the taste type 2 receptors (T2Rs), which in rodents consists of ~30 members that bind with ligands that are considered bitter by humans and are avoided and rejected by rodents and other animals (5, 113, 448). FIGURE 3 illustrates the canonical signal transduction arising from activation of T2Rs. Most T2Rs bind with more than one bitter-tasting ligand but at the same time display some degree of selectively. Likewise, most bitter-tasting ligands bind with more than one T2R. These receptors are heavily, but not completely, coexpressed in single type II TRCs, which also express phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta-2 (PLCβ2) and transient receptor potential melastatin type 5 (TRPM5), two intracellular signaling intermediaries critical in taste transduction involving GPCRs. Recently, it was found that TRPM4 deletion has similar outcomes, suggesting a role for this channel in the signaling transduction cascade in type II cells (177). Thus, if one T2R is expressed in a type II cell, it is likely that others, but not all as initially thought, will be expressed as well (5, 37, 92, 113, 448, 469).
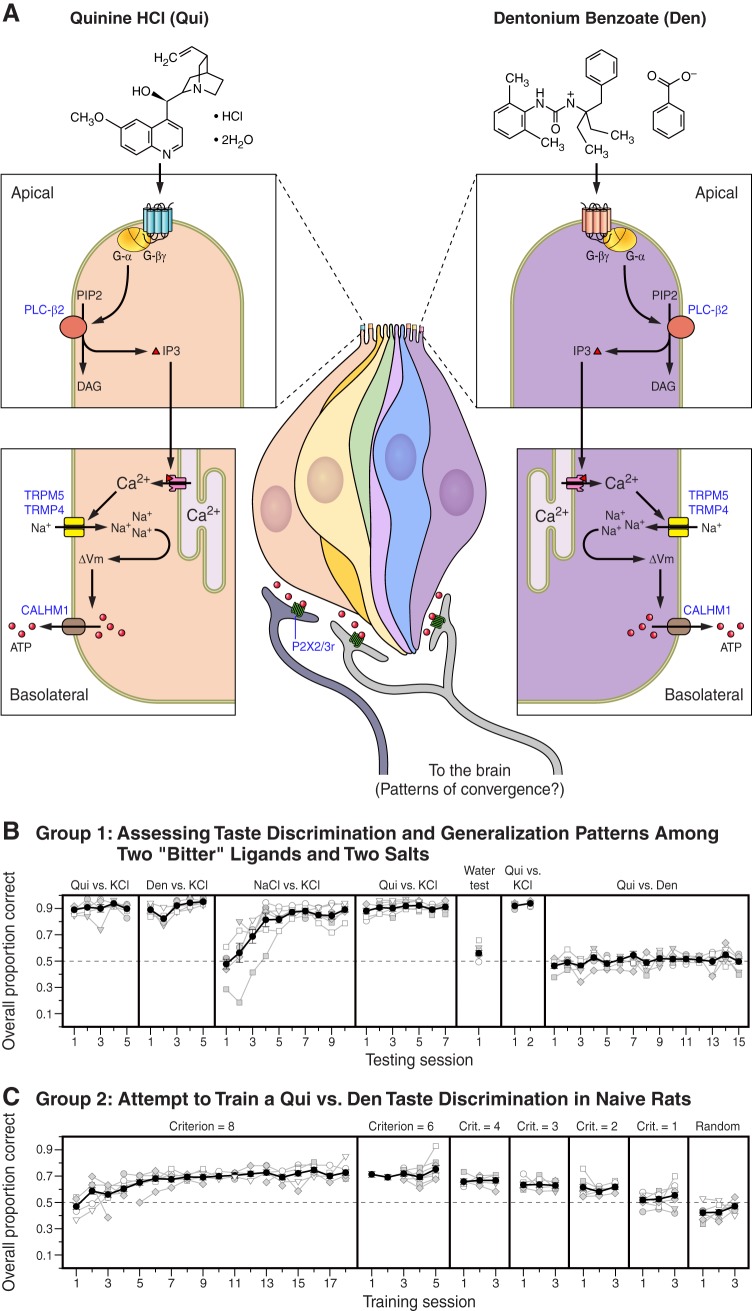
FIGURE 3.
A family of ~30 seven-transmembrane G protein-coupled receptors, known as the type 2 receptors or T2Rs, can be found in the apical membranes of mammalian type II taste receptor cells (TRCs) and are thought to be the principal mechanisms through which signals related to a structurally diverse class of bitter ligands are transduced from the periphery. The relative specificity of these receptors with respect to their ligands and the breadth of receptors belonging to this superfamily have led many to wonder whether they all yield a unitary “bitter” taste sensation or whether there are various distinguishable “bitter” sensations. On the one hand, a single TRC can express multiple different T2Rs (but not other non-T2R taste receptors). A contains a taste bud schematic with two TRCs (shown here at the lateral sides of the bud). Each of these TRCs expresses 2 or 3 different T2R receptors at their apical membranes. All T2Rs appear to employ a common intracellular signaling cascade that ultimately leads to the depolarization of the cell and release of the neurotransmitter ATP, as detailed in A, insets. Such an arrangement has led some to hypothesize that there is a singular percept for these chemically diverse substances because any given TRC may respond to a wide variety of bitter-tasting ligands in a comparable manner (113). On the other hand, using calcium imaging of a collection of TRCs, Caciedo and Roper (92) demonstrated that there are distinct patterns of ligand responsivity among individual TRCs. That is, some cells respond to 5 representative ligands; others respond to only 2 and so on. In particular, in that paper (92), the authors identified ligands [e.g., quinine (Qui) and denatonium benzoate (Den)] that stimulated largely nonoverlapping populations of TRCs. It was, therefore, hypothesized that such an arrangement could underlie discrimination among certain bitter ligands. In the schematic in A, Qui binds to its receptor (blue receptor) on the leftmost TRC, which in turn stimulates the standard signaling pathway and ultimately leads to the release of ATP. This ATP, in turn, activates P2X2/X3 receptors on the proximal afferent terminal. Den stimulates its receptor (pink receptor) on a different TRC (rightmost) and through the same signaling pathway leads to the release of ATP from that particular cell that activates P2X2/X3 receptors on a separate afferent. Thus, if these two signals run in separate lines on through the ascending neuraxis, then they should be discriminable, as hypothesized by Spector and Kopka (689). Spector and Kopka (689) explicitly tested this using a two-response operant taste discrimination task. In this task, the water-deprived rat was given a sample taste stimulus and then had to respond on the left lever if it was one stimulus (e.g., Qui) or on the right lever if it was an alternative stimulus (potassium chloride, KCl). Correct responses were reinforced with access to water. Stimulus concentration was varied to prevent the rat from using intensity as a cue. B displays the resultant data [adapted from Spector and Kopka (689)]. First, rats (group 1) were able to discern Qui from a salt, KCl, in this manner near perfectly. These same rats were similarly able to discriminate Den from KCl, even on the first session and, after some additional training, were even able to discriminate two different salts from one another (NaCl vs. KCl). However, these highly trained group 1 rats were simply unable to discriminate Qui from Den (Qui vs. Den); in fact, they were essentially guessing (at chance), even after extensive exposures to the two bitterants. C shows a separate group of rats (group 2) received Qui vs. Den discrimination training from the beginning, but were wholly unable to perform above chance when the two ligands were presented in a randomized fashion (random). Thus, although Qui and Den act at separate receptors and appear to activate distinct cells in the periphery, these two ligands do not appear to be discriminable to the rat. Other ligands remain to be tested, but the results nevertheless underscore the fact that higher-order processing plays a significant role in taste function. This may be because the signals ultimately converge (as suggested in this example), or because their significance is dependent on physiological state inputs, or by past experience. PLC-β2, phospholipase C-β2; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; PKC, protein kinase C; IP3, inositol trisphosphate; TRPM5, transient receptor potential melastatin 5; TRPM4, transient receptor potential melastatin 4; ΔVm, change in membrane potential; Calhm1, calcium homeostasis modulator 1; P2X2/3r, purinergic receptor 2, subtype 2, and subtype 3.
Although it is uncontested that T2Rs represent the primary receptor for “bitter” ligands, there is also evidence that these GPCRs are not the only way these ligands are transduced. Deletion of key transduction intermediaries in mice does not completely abolish neural and behavioral responsivity to high concentrations of certain bitter-tasting ligands, suggesting alternative peripheral receptor mechanisms exist (145, 170, 177, 250).
Given that there are many different members of the T2R family, some have wondered whether there is a unitary “bitter” sensation or different types of “bitter” sensations. In other words, do all T2R-binding ligands taste alike? On the one hand, the fact that a single TRC can express multiple types of T2Rs is at least consistent with the idea that a common signal (and therefore perceptual quality) is generated insofar as activation of any of these receptors by their respective ligands presumably produces similar downstream events (5, 113). On the other hand, a given taste receptor cell does not express all members of the T2R family and some taste cells respond rather narrowly to prototypical “bitter” compounds, as indexed by in situ calcium imaging, an arrangement that could permit discernable signals (92). FIGURE 3 further represents these alternatives.
Spector and Kopka (689) used a two-response operant taste discrimination task to help sort this out and assess whether rats were capable of distinguishing between two structurally diverse ligands: quinine and denatonium benzoate. These two ligands were previously shown to stimulate separate populations of taste receptor cells (92). In their discrimination task, Spector and Kopka (689) demonstrated that rats reliably distinguished quinine from a salt, KCl, but, interestingly, performance dropped to chance when the rats were asked to discriminate the two “bitters,” especially when the concentrations of the ligands were varied to render intensity irrelevant to the task. These behavioral findings strongly suggest that even though quinine and denatonium stimulate separate taste receptor cells in the periphery, the signals they elicit must converge at some point along the neuraxis making them indiscriminable (see FIGURE 3). Indeed, even at the first level of the central gustatory neuraxis (the nucleus of the solitary tract, NTS), some cells respond to both quinine and denatonium (237, 778). It remains to be seen whether rats would be able to discriminate denatonium/quinine from other bitter-tasting ligands such as or SOA, cycloheximide, or 6-n-propylthiouracil (PROP).34 Hamsters do not cross-generalize conditioned taste avoidances/aversions between ionic and nonionic bitters, suggesting that there may be some peripheral signal that distinguishes between these two classes of bitter ligands (211). More behavioral work of this kind should help elucidate the neural and perceptual properties of “bitter” taste.
2. Taste type 1 receptors
The other major class of taste GPCRs is the taste type 1 receptor (T1R) family consisting of three members (T1R1, T1R2, and T1R3) that form two distinct heterodimers. The T1R2+T1R3 binds sugars and other sweet-tasting ligands, and the T1R1+T1R3 binds l-glutamate and other l-amino acids and is associated with “umami” taste (24, 145, 146, 321, 380, 416, 451, 480, 481, 501, 502, 617, 806). In taste buds, all of these proteins appear to be exclusively expressed in type II cells, and, like T2Rs, their activation leads to a transduction cascade that depends on PLCβ2 and TRPM5. Indeed, studies have shown that genetic deletion of PLCβ2 or TRPM5 leads to severely blunted or abolished electrophysiological responses to ligands of the T1R heterodimers and T2Rs (170, 502, 805). For the most part, while there is obvious overlap of expression of T1R3 with either T1R1 or T1R2, the latter two proteins are rarely found in the same TRC. Moreover, T2Rs are not coexpressed with T1Rs. Thus the respective receptors that are involved in the mediation of taste sensations that humans call sweetness, bitterness, and umami are generally not found together in single TRCs. This fact buttresses the view that the cells expressing these proteins are selective sensors that are part of a labeled-line system signaling the presence of groups of compounds that fall into perceptual classes of taste stimuli, at least at the first stage of stimulus processing in the gustatory system (111, 695).
3. Ion channel taste receptors
There are a variety of ion channels that appear to serve as sensors for ionic stimuli such as salts and acids. In particular, the type III cells, which exclusively (at least in taste buds) express the PKD2L1 channel, appear to be essential for acid taste (322, 329, 341, 362). While it is generally accepted that “sour” taste depends on both dissociation of weak organic acids after passing through the cell membrane of type III cells (581) in addition to an apical proton channel, both causing decreases in intracellular pH, the identification of the apical channel has been elusive. Several candidates have been proposed including polycystic kidney disease 2-like 1 protein (PKD2L1), acid-sensing ion channels (ASICs), and hyperpolarization-activated cyclic nucleotide-gated channels (HCNs; Refs. 86, 164, 582). However, recently, evidence has been provided suggesting that the key proton channel is the Otopetrin1 (OTOP1), at least in mouse type III cells (752).
Salt transduction involves at least two receptor mechanisms, one that is selective for sodium cations and another that is not. At least in the rodent gustatory system, the sodium-selective pathway depends on the ENaC through which sodium (and lithium) cations passively diffuse and, in doing so, can depolarize the cell (72, 112, 304, 306, 311, 425, 508, 638, 798). It still remains unclear what TRC type is involved with selective sodium sensing, but the type I cell, typically considered a supporting cell, has been suggested as a candidate (754). The exact mechanism of the nonselective pathway is not completely understood.35
As reviewed above, high concentrations are generally aversive and avoided, except in times of sodium depletion. One study found that high sodium concentrations activate T2R-expressing taste cells (523). Deletion or blockade of key signaling components in the T2R transduction cascade diminished, but did not completely obliterate, neural and behavioral responsiveness to high sodium, while rescue of select transduction intermediaries restored the response. Similarly, disruption of PKD2L1 (“sour”)-expressing cells attenuated neural and behavioral responsiveness to high sodium solutions. The silencing of the T2R-signaling pathway (via TRPM5 genetic knockout) coupled with ablation of PKD2L1-expressing TRCs severely disrupted responsivity to high concentrations of sodium. Precisely how sodium stimulates the PKD2L1 cells is unclear. Moreover, rodents can discriminate sodium salts from prototypical “bitter” and “sour” stimuli, even when the sodium is presented at a high concentration. Thus it could be that the sodium-specific ENaC provides a distinguishable cue for sodium, even when alternative pathways are stimulated (211, 279, 314, 520, 687, 689).
One interesting conundrum regarding taste receptor mechanisms is the exceptional difficulty that mice have behaviorally discriminating citric acid from the bitter-tasting ligands quinine and propylthiouracil (747). Rats and even humans also display some “bitter-sour” confusion at low concentrations, but in general appear quite able to discriminate between such classes of compounds at higher concentrations (171, 279, 312, 463, 489). In mice, the discrimination difficulty is reminiscent of the failure of rats to discriminate between denatonium and quinine and suggests that the signals generated in the periphery by acids and bitter-tasting ligands converge somewhere along the gustatory neuraxis subserving sensory-discriminative taste function (747).36
4. Unconventional taste receptors
Finally, it is worth noting that there is evidence for the existence of other taste receptors beyond those discussed above. For example, splice variants of the metabotropic glutamate receptor type 4 (mGluR4) as well as metabotropic glutamate receptor type 1 (mGluR1) have also been implicated in the mediation of “umami” taste (114, 115, 390, 623, 796). The G protein-coupled receptor type 120 (GPR120) and G protein-coupled receptor type 40 (GPR40) along with the fatty acid translocator cluster of differentiation 36 (CD36) appear to be involved in fat taste (61, 106, 220, 405, 441). Although yet to be identified, maltodextrins appear to activate a TRPM5-dependent taste receptor mechanism that gives rise to a preferred taste sensation in rodents that is qualitatively distinguishable from “sweetness” (i.e., sucrose-like taste) (511, 673, 745, 748, 809). Recent data suggest that glucose might engage a taste receptor type 1 (T1R)-independent receptor pathway (252, 268, 522, 634). The point is that there are other receptors that have been proposed for some of the common ligands discussed above and likely still others that remain to be revealed.
5. Extra-oral “taste” receptors
Although they were originally discovered in taste tissue, some taste receptor type 2 (T2Rs), as well as other classes of taste receptors (e.g., T1Rs), and their associated signaling intermediaries, have since been found in tissues outside the oral cavity (for review, see Ref. 197). The growing list of T2R-expressing tissues includes the GI tract, the upper and lower airways (i.e., nasal respiratory epithelium, larynx, trachea, bronchi, bronchioles), the brain, the kidneys, and the heart (163, 195, 208, 348, 427, 485, 610, 666, 726, 727, 788). To date, these extra-oral T2Rs have been most extensively studied in the respiratory system, where they are activated by some of the prototypical T2R ligands, as well as some types of bacteria, and other irritants (for a recent review, see Ref. 408). There is still much to be learned about these extra-oral receptors and their respective functions, but in the absence of a link to the central gustatory system, it is unlikely that stimulation of these respiratory T2Rs with ligands such as denatonium benzoate produce a “bitter” sensation, like we typically ascribe to oral T2Rs. Thus, if nothing else, the existence of these receptors outside the gustatory system that are serving other functions underscores the fact that bitter taste is not in the receptor per se, but rather in how the signal generated from that receptor is channeled to and processed in the central nervous system (CNS).37
Given the extensive interplay among taste and postoral visceral signals, the population of T2Rs in the GI tract merits further attention here. Members of the T2R family and their affiliated signaling proteins (e.g., α-gustducin) are found along the gastric and intestinal mucosa, including in enteroendocrine cells (62, 63, 283, 316, 348, 349, 446, 557, 708, 713, 756, 788). The functional roles of these receptors are not well understood yet, but there are some hints. For example, incorporation of T2R ligands onto enteroendocrine cell lines in vitro stimulates the release of hormones typically associated with satiation (e.g., cholecystokinin). Administration of T2R ligands in the gut in vivo leads to enzyme secretion and slows gastric emptying (18, 117, 161, 253, 346, 348, 349, 369, 426, 618). Postoral administration of T2R agonists elicits c-Fos in hindbrain and forebrain structures known to be involved in feeding and other GI reflexes (294, 354), and this response is attenuated in rats systemically pretreated with cholecystokinin (CCK) or peptide YY (PYY) receptor antagonists and completely abolished in vagotomized mice (295). While these findings imply postoral T2Rs contribute to behavioral and physiological responses that limit ingestion and absorption, what remains to be understood is whether the T2R-generated signal is received and processed by the brain like other satiation signals or whether it is associated with dissociable consequences such as visceral malaise or pain.
The data are limited, but there are a couple of studies that have begun to examine these questions. Two studies have shown that pairing consumption of a novel flavored solution with intragastric infusions of the T2R agonist denatonium benzoate leads to subsequent avoidance of that flavor, as measured in one- and two-bottle intake tests (253, 294). Using a different approach, one study showed that rats will come to rapidly curb ongoing ingestion of a hypotonic salt solution when denatonium is infused directly into the duodenum. Moreover, this early phase reduction in drinking in response to intestinal denatonium grows more profound with experience (632). This suggests that the early sensory consequences of denatonium can become associatively linked with the more delayed consequences of this ligand. Once learned, the early phase response was partially attenuated by the CCK-A receptor antagonist devazepide, consistent with mediation, albeit partial, through a CCK-dependent signaling pathway. Whether the changes in ingestion produced by the postoral effects of T2R ligands impacts the appetitive and/or consummatory domains of function has not been addressed.
B. Brief Overview of the Anatomical Organization of the Ascending Gustatory System
With respect to taste function and the topic of bad taste, what ultimately matters is how this information is channeled through the gustatory system. FIGURE 4 shows the major anatomical pathways of the ascending gustatory system in the rodent model. TRCs are located throughout the oral cavity, including on the tongue,38 soft palate, nasoincisor ducts (only in certain species), epiglottis/laryngeal epithelium (summary distribution table provided in Ref. 740; 19, 473, 474, 476, 743). At one time, it was thought the TRCs located in different regions of the oral cavity selectively expressed receptors for certain types of ligands, comprising a specific taste topography or map, but later data dispelled this theory (5, 130, 210, 370, 410, 521). Generally speaking, the various types of taste receptors previously mentioned are found in cells across the gustatory epithelium, oftentimes within the same taste bud, though the overall oral pattern of expression is not uniform, with some regions displaying greater expression of some receptor proteins relative to others (90, 242, 321).
FIGURE 4.
The major ascending routes through which gustatory and visceral signals are transmitted from the periphery to the hindbrain and forebrain are illustrated. Blue lines represent the putative gustatory pathways. Red lines represent the putative visceroceptive pathways. Although these two sources of input ascend the central nervous system (CNS) in a roughly parallel manner, there are some notable distinctions. For instance, it appears that neurons originating in the caudal (visceral) NTS project to key structures in the forebrain in two distinct ways. One comprises a set of direct projections to several subnuclei in hypothalamus, the amygdala, the ventral tegmental area (VTA), the nucleus accumbens, just to name a few. The other pathway ultimately reaches many of these same ventral forebrain structures, but through a relay in lateral parabrachial nucleus (lPBN). A second putatively visceroceptive pathway emerges from the lPBN as well. These afferents project to the thalamus [ventroposterolateral parvicellular (VLMpc)] and, from there, to insular cortex (IC) [i.e., visceral cortex (VC)]. The functional relevance of this, both with respect to the separate pathways and to the redundancies, remains to be more fully elucidated, but there is some evidence that these populations are involved in different aspects of ingestive behavior. Additionally, cNTS neurons project to medullary motor nuclei, presumably to subserve various digestive reflexes. A subpopulation of gustatory neurons in the rostral nucleus of the solitary tract (rNTS) likewise project locally to motor nuclei, which are involved in oromotor, salivatory, and digestive reflexes, while a separate population of gustatory neurons originating in the rNTS terminate in the parabrachial nucleus (PBN), primarily the medial PBN (mPBN). From there, two forebrain-projecting gustatory pathways emerge. Some neurons in the mPBN project to the thalamus [ventroposteromedial parvicellular (VPMpc)] and then onto the gustatory cortex (GC), terminating in close proximity to VC. In general, the nucleus tractus solitarius (NTS)→PBN→thalamus→IC pathway maintains a rough topographic organization throughout. The second pathway that emerges from the mPBN targets various ventral forebrain structures, with particularly dense inputs to the central amygdala (CeA) and the lateral hypothalamus (LH). Spinal afferents from the gut appear to be highly connected to many of these same structures (here represented in dark red arrows). These neurons synapse in the caudal NTS, reticular formation (RF), lPBN, and several subnuclei with the thalamus (and then onto various cortical structures including IC). The visceroceptive afferents of the spinal system reach the amygdala (in particular, the CeA) and the hypothalamus by way of these various tracts. There is also suggestion of direct spino-hypothalamic and spino-amygdalar tracts. Blood-borne signals (e.g., physiological, metabolic, inflammatory, and toxin-related) are also sensed in circumventricular organs (CVOs) of the hindbrain and forebrain [e.g., area postrema (AP), median eminence (me), subfornical organ, and the vascular organ of lamina terminalis]. For clarity’s sake, some projections were omitted, including projections from cNTS to ventrolateral medulla, mPBN and lPBN to the RF, PBN to zona incerta, PBN to substantia inominata, VPMpc to amygdala, among others. It is important to note these routes are color-coded according to their presumptive role (gustatory vs. visceral), but in many cases, this has yet to be confirmed with functional measures. Moreover, this schematic does not show pathways of cross-talk among cells within a particular brain area; such local connections likely represent a significant means of integrative processing. CT, chorda tympani; GSP, greater superficial petrosal; GL, glossopharyngeal; SLN, superior laryngeal nerve; DMNX, dorsal motor nucleus of the vagus; HG, hypoglossal nucleus; NA, nucleus accumbens; VTA, ventral tegmental area; ARC, arcuate nucleus; PVH, paraventricular nucleus; DMH, dorsomedial nucleus; BNST, bed nucleus of the stria terminalis; BLA, basolateral amygdala.
Nevertheless, there is a topographic organization of inputs from the oral cavity, particularly with respect to how the three different taste afferents are channeled into the first-order central relay in the brain. The taste buds in the anterior two-thirds of the tongue and palate (including nasoincisor ducts) are innervated by the chorda tympani (CT) and greater superior petrosal (GSP) nerves, respectively, both branches of CN VII (geniculate ganglion). The cells of the posterior one-third of the tongue, on the other hand, are innervated by the lingual branch of CN IX (petrosal ganglion), the glossopharyngeal (GL) nerve. The sparse taste buds located in the laryngeal epithelium are innervated by the superior laryngeal branch of CN X (nodose ganglion).39 While all three of these afferents make their first central synapse in the ipsilateral rostral one-third of the nucleus tractus soliatrius (rNTS)40 in the brain stem via three cranial nerve branches, their terminal fields are somewhat segregated. That is, the branches of CN VII (CT and GSP), providing input from the anterior tongue and palate, respectively, terminate in the most rostral pole of the rNTS. Just caudal to that are the terminal fields of the CN IX and then the superior laryngeal nerve (SLN) branch of CN X, providing input from the posterior tongue and laryngeal epithelium, respectively. Although there is a large degree of overlap among these projections, a rough orotopic organization is maintained in the rNTS (132, 290, 399, 452, 453, 740, 744).
The second-order neurons arising from the rNTS have two major output pathways (288, 403, 513, 515, 516). The first is to medullary sites such as the reticular formation, caudal NTS, and the salivatory nuclei. This pathway is thought to be involved with taste-triggered oromotor and physiological reflexes. The second pathway constitutes the ascending gustatory system, and, in rodents, the second-order neurons project to taste-responsive third-order neurons situated in the ventrolateral and medial subdivisions of the caudal parabrachial nucleus (PBN) as well as within the brachium conjuctivum itself: these three areas are collectively referred to as the “waist” region of the PBN. Taste responsive neurons are also found in portions of the external lateral and external medial subdivisions, which cap the lateral margin of the brachium caudally (166, 287, 300, 307, 516, 517, 728, 731, 762).
From the PBN, two major gustatory afferent tracts emerge: the ventral forebrain pathway and the thalamocortical pathway (217, 284, 319, 360, 400, 402, 512, 514, 515, 625, 730). The ventral forebrain projections, sometimes called the limbic pathway, terminate in areas of the brain known to be involved with feeding, drinking, and reward/hedonics. This includes the lateral hypothalamus (LH), the central nucleus of the amygdala (CeA), the bed nucleus of the stria terminalis (BNST), and the substantia innominata (SI). Oddly, a projection from the PBN to the ventral tegmental area (VTA), a region of the brain considered so critical in the mesolimbic reward system, has not been widely documented, but a recent report suggested that such an output pathway may exist in mice (512, 729). The thalamocortical pathway is the traditional lemniscal-like relay to sensory cortex. The waist area projects primarily ipsilaterally, whereas the external lateral and external medial subdivisions project primarily contralaterally to the parvicellular subdivision of the ventroposteromedial nucleus (VPMpc) of the thalamus (515, 728, 762). The functional significance of the differences in laterality of thalamic projections from these different PBN areas remains to be understood. The thalamic neurons, in turn, project to the dysgranular/agranular subdivisions of the insular cortex (IC) roughly surrounding the middle cerebral artery just dorsal to the rhinal fissure (more on this below; Refs. 109, 385, 519). There is also evidence of direct projections to IC from the PBN that bypass the thalamus, but it is unclear whether these are taste-responsive (10, 217, 402, 624, 625, 661). Based on its lemniscal character, the thalamocortical taste pathway has been proposed to be involved in sensory-discriminative taste function (553).
C. Brief Overview of the Anatomical Organization of the Ascending Visceroceptive System
The gustatory system is, in some sense, just a specialized extension of the general viscerosensory system. Consistent with this, taste and visceroceptive neurons are found in close proximity to one another at nearly every level of the ascending neuraxis, as highlighted in FIGURE 4.41 This anatomical arrangement is thought to facilitate extensive integration of information. Given that so much of the processing of, and ultimately the responses to, the taste properties of a food depend on the associated visceral consequences, insight into the organization of gustatory function necessitates consideration of the visceral system too. Unfortunately, at present, our understanding of the anatomical and functional organization of the viscerosensory system, especially at the higher levels of the neuraxis, is still in its infancy. Nevertheless, a brief overview of the basic anatomy is included here and referenced in later sections of this review. The purpose is to provide a broader context for understanding taste (especially bad taste) and point to key voids in our knowledge to be filled by future research.42
The abdominal viscera involved in food assimilation are innervated by two main nerves—the vagus, with its cell bodies in the nodose ganglia 43 and the splanchnic nerves, with their cell bodies in the spinal dorsal root ganglia (57). Collectively, these nerves form the general visceral afferent system. While they are thought to transduce different sorts of stimuli and, hence, contribute to separate functions, there are certainly overlaps among their sensory and functional domains (e.g., Refs. 218, 280, 281, 320, 564). In general, vagal afferents yield the sensory input for nutrient handling and splanchnic afferents appear to be more involved in sensing pain and inflammation.44 Humoral factors that are released in response to food from cells that line the GI lumen provide another major route through which stimulus-specific information is transmitted from the viscera to the CNS.
Vagal afferents are divided into two broad submodalities: mechanoreceptors and chemoreceptors.45 Vagal mechanoreceptors terminate in the smooth muscle of the esophagus, stomach, and proximal intestines and are principally responsive to low intensity distension,46 as is experienced during the normal filling and emptying that occurs with a meal (531). Spinal mechanoreceptors also respond to distension, but notably include a subpopulation of fibers that are particularly responsive to high intensity stretch (i.e., mechano-nociceptors) (530).
Chemoreceptors, prominent in the proximal segments of the GI tract, “sense” the chemical properties47 of ingested foods. These fibers, by and large, terminate within the lamina propria, just below the GI epithelium, and around the crypts (58, 462, 464, 556). Subpopulations of epithelial cells (e.g, enteroendocrine cells, brush cells) express specific receptor proteins and/or transporter channels at their apical surface (122, 123, 172, 181, 182, 186, 315, 316, 404, 433, 436, 592, 593). Activation of these “sensors” by their respective ligands leads to the release of neuromediators across the basolateral membrane and onto their respective receptors located on the underlying vagal afferent terminals (183, 482, 483, 506, 560, 563, 566, 571, 789, 808).48 A recent study found evidence of direct synaptic connections between subpopulations of enteroendocrine cells and vagal afferent terminals (355). Splanchnic nerves also have terminal endings in the lamina propria. Interestingly, although the splanchnic fibers are thought to be principally sensitive to mechanical stimulation, they may serve a chemosensory role as well (564, 647). For instance, splanchnic fibers around the vasculature respond to mechanical changes and blood-borne signals that are released during GI inflammation and injury (333).
The precise roles that vagal and splanchnic afferents play in transducing the signals that produce taste aversion and/or avoidance, such as those associated with toxicosis or nausea, remain elusive. Both nerves respond to peripherally administered LiCl (507). Disruption of the vagal sensory inputs generally does not affect the capacity to acquire CTA, as produced by LiCl, irradiation, or rotational motion (325, 335, 442). This does not rule out a role for the vagus; it just shows that other areas are sufficient (e.g., chemoreceptors in AP). Interestingly, lesions of the vagal afferents or capsaicin-sensitive afferents impair the ability to learn to avoid a flavor associated with hypertonic saline in a rapid discrimination paradigm (799a), suggesting that different visceroceptive pathways are involved in transducing different types of sensory information from the GI tract and perhaps for different situational demands. Serotonin, through its activation of peripheral 5-HT3 receptors has also been implicated in the peripheral origin of nausea-like symptoms that lead to reductions of intake (84a).
Generally speaking, the necessity and/or sufficiency (see FIGURE 5 for a discussion of necessity vs. sufficiency) of these receptive mechanisms has largely been examined through intake measures, which, as discussed above, are interpretatively limited when used in isolation. Thus whether the vagus is necessary for the TR profile shift that accompanies aversion, for example, ought to be examined. Moreover, it will be important to determine whether these signals (e.g., LiCl vs. hypertonic saline) remain divergent at higher orders of processing and are channeled into distinct behavioral outputs or whether they eventually converge to generate common behavioral outputs in the control of ingestion.49
FIGURE 5.
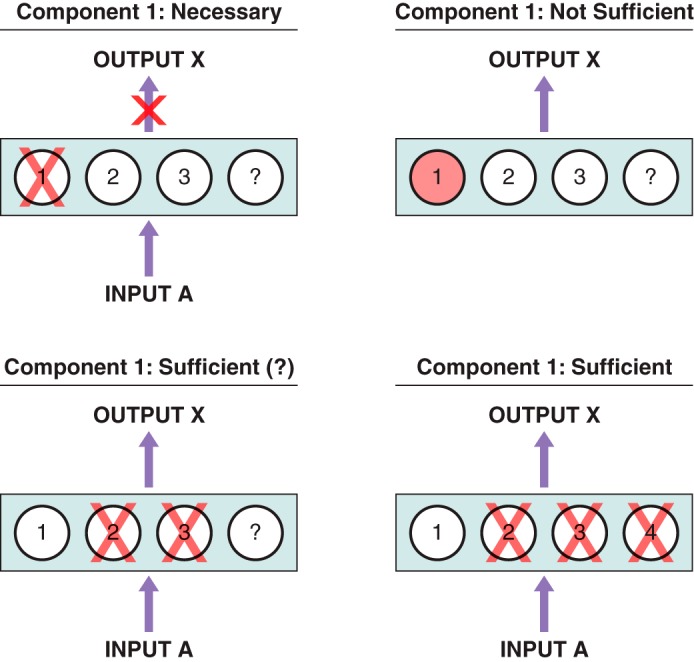
Four experiments are depicted, in which a single component or a set of components (e.g., brain site, cell type, receptor type) that are candidates for a potential neural mechanism linking Input A to Output X are silenced or activated (e.g., lesion, optogenetically, pharmacologically, etc.) to determine their necessity and/or sufficiency. Input A could be 3.0 mM quinine, for instance, and Output X could be a gape response; the intervening circles represent the different brain components implicated in the mechanism. Top left panel: if, under the condition of Input A, Component 1 is silenced, then Output X is eliminated; this clearly demonstrates that Component 1 is necessary for Input A to produce Output X. Bottom left panel: if Output X survives when Components 2 and 3 are silenced and Component 1 is left intact, then it can be concluded that both Component 2 and 3 are unnecessary. It is commonplace to further assume this means that Component 1 is sufficient. However, such a conclusion requires that all components of the system are known and have been silenced too. If parts of the system are yet unknown and simultaneously remain unsilenced (i.e., the “?” component in bottom left panel), then the sufficiency of Component 1 is not established [but the sufficiency of Component 1 in combination with remaining unknowns (?) is]. Top right panel: another common, but misguided, approach for proving sufficiency is to show that (electrophysiological, pharmacological, opto- or chemogenetic) activation of the known necessary component(s) (in this case, Component 1, indicated by red fill) in the absence of Input A, generates Output X. Importantly, this does not demonstrate the sufficiency of Component 1 in the normal mechanism evoked by Input A. Moreover, because the other candidate components (Components 2, 3, and ?) are intact, their involvement might be necessary for stimulation of Component 1 to lead to Output X. Thus it is very difficult to show sufficiency of a component. Gain-of-function approaches have their utility, but if all other areas have yet to be demonstrated unnecessary, interpretive caution is warranted. Bottom right panel: if all possible mechanistic components are indeed known and silenced, save the tested one(s), and the output remains undisturbed, then the sufficiency of the unsilenced component(s) is demonstrated. Necessary and sufficient are important concepts for understanding neural bases of function. Although these terms are buzzwords in the parlance of modern neuroscience, in some cases the terms are misused, which only leads to overgeneralizations and misattributions of structure-function relationships.
Most abdominal vagal afferents project ipsilaterally to the caudal NTS (cNTS), just behind the intermediate gustatory zone, and, to a lesser extent to the contralateral cNTS and the area postrema (AP) (16, 518). The AP is a circumventricular organ that abuts the posterior floor of the 4th ventricle (470).50 Thus, in addition to receiving neural inputs, subsets of neurons in the AP detect circulating factors, including blood-borne toxins and metabolic signals. AP neurons, in turn, project to medullary motor nuclei, including the dorsal motor nucleus of the vagus (DMNX) and the nucleus ambiguus, as well as to the cNTS and the lateral PBN (lPBN) subnuclei (307, 654). Visceroceptive neurons in the cNTS project directly to several different central targets.51 These include dense projections to the rNTS, local reflex nuclei, and the medial and lateral PBN within the hindbrain. cNTS neurons also project to forebrain structures, including various hypothalamic nuclei, amygdala, VTA, and BNST, to name a few (13, 159, 577, 585, 590).52 That said, the lPBN represents a significant visceroceptive relay for signals arising from the AP and cNTS to forebrain sites (217, 364, 589, 603, 625). In fact, in a similar fashion to the gustatory projections arising from the mPBN, two main visceroceptive routes to the forebrain emerge from the lPBN; one set of projections goes to select limbic structures such as the hypothalamus, BNST, and amygdala, and another set goes to the sensory thalamus (VPLpc) and then onto the IC in a topographic manner (15, 109). Thus, while significant portions of the central gustatory and visceral systems run in parallel, at least at a macroscopic level, there is also a large portion of the visceral system that deviates early on. In fact, in some cases, the direct projections from cNTS and the lPBN-mediated visceroceptive projections terminate within the same forebrain structures (e.g., amygdala, hypothalamus, and BNST). The functional significance of these dual overlapping and separate pathways is unknown, but at present, both are thought to contribute to ingestive behaviors. The question becomes: how do they differentially contribute to ingestive behaviors?
From the dorsal root ganglia, spinal visceral afferents cross the midline and ascend to the CNS in various tracts. The spinothalamic tract projects directly to several subnuclei within the thalamus and, from there, on to various cortical structures thought to be involved in the sensory-discriminative processing of visceral and cutaneous pain. Neurons in the spinoparabrachial tract project to the lPBN, where they converge upon and/or run in parallel with the cNTS- and AP-originating visceroceptive neurons. Yet another spinal tract terminates in the reticular formation and then on to various cortical and limbic structures (466, 467).
Finally, circumventricular organs in addition to the AP are located throughout the brain with direct access to a variety of circulating metabolic and/or toxin/infection-based peripheral signals. While there is no doubt that these “sensory” neurons play a large role in normal autonomic and physiological function, a full discussion is beyond the scope of this article (for further reading, see Refs. 31, 216, 478). Suffice it to say here that the projection patterns of these neurons are extensive and complex and generally include various limbic and cortical brain areas, as well as direct projections onto primary sensory and motor nuclei of the hindbrain. One pertinent example of this is the arcuate nucleus of the hypothalamus, in close proximity to the median eminence. Agouti-related peptide (AgRP) neurons of the arcuate nucleus of the hypothalamus are activated by circulating signals indicative of energy depletion and, in turn, influence behavioral (largely measured in the appetitive domain) and physiological responses that initiate feeding and slow energy expenditure (96). The significance of one of these outputs as it pertains to bad taste is discussed below in section IIIE.
D. Hindbrain Processing of Taste and Visceroceptive Stimuli
Considering that the first two major central structures that receive gustatory and visceral sensory signals, the NTS and PBN, and the final motor output pathways for various taste-guided behavioral and physiological responses reside in the hindbrain, it is reasonable to ask whether these areas are necessary and sufficient for the host of oral and postoral processes and the integration that underlies bad taste (necessary and sufficient are described in FIGURE 5 and the accompanying caption). As noted earlier, the anatomical organization of inputs and outputs in the rostral (or gustatory) NTS is indicative of at least two separate major circuits—one that remains local and is directly linked to motor outputs involved in reflexive responses, including oromotor, and another that ascends to the PBN. The second-order neurons in the NTS receiving visceroceptive afferent input project to a variety of third-order targets in the forebrain as well as in the PBN and also synapse with neurons in local brainstem motor outputs. Some visceroceptive signals converge upon taste-responsive neurons, both within the rNTS and PBN (28, 286, 308, 309, 361). Thus, while the NTS and PBN appear to be relays for primary sensory information to reflex arcs and higher order levels of processing, respectively, these arrangements may permit substantial taste-visceroceptive integrative processing relatively early on.
The critical roles that the NTS plays, however, have proven difficult to definitively test due in large part to its size and location. For instance, because the gustatory NTS is apposite to other sensory inputs involved in vital functions, many of the available techniques do not permit the placement of selective “gustatory lesions” that do not also impact the adjacent areas.53 Nevertheless, a handful of studies have managed. Such lesions in the rNTS blunt unconditioned concentration-dependent licking and taste reactivity for various tastants tested, but do not seem to interfere with the increased ingestion of (and ingestive TR to) normally rejected hypertonic salt solutions, following sodium depletion (203, 263, 660). Nor do they completely disrupt the capacity to express a previously acquired CTA (as measured in intake tests and TR) and acquire a new CTA (263, 264; but see Ref. 459). While these findings may seem somewhat surprising given the rNTS is at the very least an obligate relay for taste, it is important to note that spared function is likely due to incomplete damage to the structure such that a sufficient signal was received and transmitted. Once the capacity to make more selective, but complete, manipulations is sufficiently improved, the functional necessity and specificity of the rNTS is certainly worth a reexamination.
That is not to say that significant inroads are not being made towards understanding the functional organization of the rNTS through other approaches. For example, the application of “bitter” stimuli to the oral cavity elicits a distinctive pattern of c-Fos in the medial portion of the rNTS; sucrose, on the other hand, exhibits a more dispersed c-Fos response, centered lateral to the “bitter” zone (110, 297, 742). The bitter-ligand-induced c-Fos response appears primarily in local interneurons rather than neurons projecting to the PBN (168, 236, 237). Moreover, quinine-elicited c-Fos and gaping responses are virtually abolished after GL transection and restored upon nerve regeneration (372, 376, 739).54 Together, these findings suggest that these medial neurons participate in a local brain stem reflex rejection circuit. However, this certainly does not preclude the possibility that other populations of neurons in the rNTS or more central sites (e.g., PBN, forebrain) are involved in such behavioral responses as well.55 To that point, transection of the GL nerve also eliminates, and regeneration restores, the “bitter”-elicited c-Fos in the PBN, the anterior CeA, and the gustatory cortex (371, 373).56
Recently, Lee et al. (407) discovered that the semaphorin 3A (SEMA3A) protein is coexpressed with a high degree of specificity with T2Rs, and another protein, semaphorin 7A (SEMA7A) is coexpressed with T1R3 and T1R2 in TRCs. These proteins, which are also expressed in other tissues, are known to be involved in axon guidance. The experiments of Lee et al. (407) provide evidence these proteins serve as attractant molecules during development so that sugar-signaling fibers can synapse57 with the proper taste bud cells that express the T1R2+T1R3 heterodimer and that bitter ligand-signaling fibers can synapse with the proper taste bud cells that express T2Rs. Taking advantage of these expression patterns, Lee et al. (407) genetically deleted the SEMA3A protein normally found in T2R-expressing cells in a line of mice in which the expression of the human SEMA3A was transgenically targeted to T1R3-expressing cells.58 As a result, there was a striking increase in the number of geniculate ganglion cells that responded to both sweeteners and bitter-ligands. Nevertheless, there was still a sizable population of geniculate ganglion cells that responded to bitter-ligands or sweeteners alone, indicating that other axonal guidance factors must be involved as well. Nevertheless, it appeared that the peripheral gustatory system had been partially rewired so that bitter-ligands were now also stimulating pathways that signal the presence of sweeteners in the oral cavity. These animals were behaviorally assessed in a brief access test in which they were presented with different concentrations of prototypical bitter ligands in 10-s trials and licking responses were measured. The mutant mice displayed blunted avoidance of the bitter ligands relative to WT mice, but they did, nonetheless, suppress licking at the high concentrations. This was a technically stunning use of molecular biology to partially rewire the peripheral gustatory system; it will be important for future studies to extend the behavioral analysis. Given the data at hand, it is unclear whether the genetically altered mice are actually perceiving sweeteners and bitter-ligands as more similar than do wild-type mice or whether such mice are even capable of responding normally to any taste compound. Clearly, more focused psychophysical tests would help shed some light on these important questions that have bearing on the functional organization of gustatory system.
Finally, the responsivity of taste neurons in the NTS appears to be modulated in chemo- and visceroceptive-specific ways. For example, some forms of sodium depletion suppress neuronal responsivity to NaCl and other sapid stimuli, while other forms appear to lead to increases in neural responsivity to sodium or sucrose (121, 343, 499, 717).59 Treatments associated with satiation have been shown to selectively reduce neural responses to sapid stimuli (243–247).
The PBN is also a complex structure, but targeting the gustatory and/or visceral subdivisions of this region in the dorsal pons has been met with more success. With respect to unconditioned responding to basic tastants, lesions centered in the gustatory zone of the PBN attenuate, but do not entirely abolish, concentration-dependent licking and TR to a variety of tastants including the bitter stimulus quinine (203, 266, 313, 627, 682, 686). While it is tempting to conclude then that the PBN is not necessary for appetitive and consummatory responses, once again residual function may be explained by spared tissue. Indeed, one study found that the degree of shift in concentration-dependent suppression in licking to quinine in a brief access taste test was directly related to the extent of bilateral lesion damage in the medial PBN (682). That is, rats with <50% of the mPBN damaged on at least one side had an approximately half log molar rightward shift in the concentration-response function compared with prelesion performance on this same test; thus the spared tissue is sufficient to maintain some degree of competence. However, rats with >50% of mPBN damaged on both sides were far more disturbed with a rightward shift of well over an order of magnitude. When PBN lesions 60 are large and near complete, taste function is severely impaired.
When it comes to sensory-discriminative taste function, the necessity of mPBN is still unclear. In the only known experiments to examine this, lesions to mPBN produced rather variable effects (693). Whereas some rats with PBN lesions exhibited severe deficits, which essentially prohibited them from even being able to perform the task, others could perform the task, but had significantly elevated taste detection thresholds (using NaCl or sucrose as the discriminative stimulus) and still others were essentially unimpaired. The reasons underlying this variability are unknown, but the authors did note that there was no obvious relationship between lesion size and/or topography and the degree of deficit.61
The involvement of the PBN in behaviors that require the integration of taste input and visceral or physiological signals has received substantially more empirical attention, for two phenomena especially: sodium appetite and CTA. In general, dietary and pharmacological (e.g., furosemide) means of bodily sodium loss reduce the responsivity of taste neurons in the PBN, especially those that respond to sodium and reduce the proportion of neurons that respond best to sodium. Behaviorally speaking, rats with PBN lesions, including those targeted to the caudal medial/gustatory subdivision, are severely impaired with respect to the expression of a sodium appetite (203, 260, 313, 628, 693, 711).62 Moreover, one study found that some rats with gustatory PBN lesions, who failed to appropriately increase their NaCl intake in response to sodium-depleted state, were still able to detect sodium, even at low concentrations, in a taste detection task (628). The latter result indicates that it is unlikely the failure to display a depletion-induced increase in their ingestion of NaCl was related to an inability to taste the salt or differentiate it from water.63 From the available evidence, the PBN appears to be a necessary brain site for expression of sodium appetite. Moreover, some forms of acute and dietary sodium deprivation have been found to alter the responsiveness of PBN neurons to sodium taste stimulation (330, 659). The exact contribution of the PBN to this behavior is still unknown (i.e., see FIGURE 6). Some have speculated that it must be related to the actual types of visceroceptive inputs signaling physiological status since rats with mPBN lesions express sodium appetite induced by other means (e.g., calcium depletion) (232, 260).
FIGURE 6.
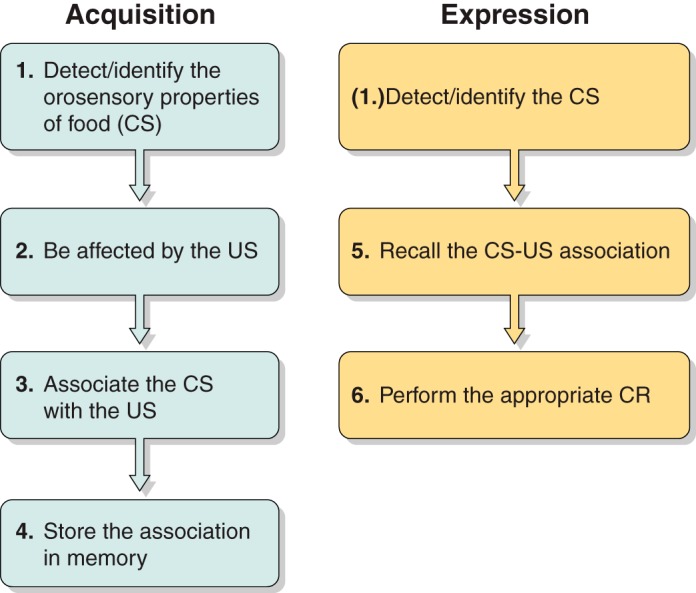
The key events that comprise the acquisition and subsequent expression of a typical conditioned taste aversion (CTA) are shown. Acquisition: first, a subject ingests or samples a distinctly flavored (and usually unfamiliar) substance typically referred to as the conditioned stimulus (CS). This is followed by an adverse postingestive consequence (the unconditioned stimulus. US), which in turn produces the unconditioned response. After such an experience (and usually after only one such experience in the case in which a moderate dose of LiCl is the US), the subject rejects and avoids the flavor upon future encounters [the conditioned response(s), CR(s)]. As such, CTA acquisition depends on 1) the ability to detect and identify the taste CS; 2) the ability to detect and process the ensuing US; 3) the ability to associate or integrate the CS and US, oftentimes over long delays; and 4) the ability to store that association in memory. Expression: the ability to then subsequently express a CTA depends on the ability to detect the taste CS (step 1 from above), as well as the ability to 5) recall the learned association and 6) perform the appropriate conditioned response. Theoretically then, manipulations that impact a brain component during and/or around CTA training could impair learning by affecting any of the processes 1–4, and manipulations that disrupt CTA expression after training could affect any one of processes 1, 5, and 6. Importantly, researchers must have confirmed that the subject successfully expressed a trained CTA to subsequently draw conclusions about the effect of neural manipulations made after the acquisition phase. The very fact that CTA is a robust and relatively well-characterized phenomenon confers advantages for its use with central manipulations. However, CTAs are rarely examined with the level of detail required to make strong inferences about structure-function relationships. Because of this, the vast CTA literature is plagued by mixed results and presumptive misattributions. These stem in large part from the fact that intake and/or choice tests are used to index a “CTA deficit.” Intake and/or choice tests measure the outcome of behavior, not the behavior itself, and thus are not capable of revealing the (impaired) processes that lead to the outcome. Moreover, many of these tests are conducted over long-term periods (e.g., 10 min or more) during which other signals besides taste (e.g., visceral) might gain significant influence over the final amount consumed. Thus the use of complementary tasks and testing procedures that probe the specific underlying processes outlined here are critical to link CNS structures with specific functions (or deficits). Although we use LiCl-induced CTA as an example here, many of these same considerations should be applied to other types of taste- and/or viscerally-based processes (e.g., conditioned taste preference, taste-odor learning, etc.) as well. Indeed, similar issues arise for physiological manipulations, like sodium appetite; the ultimate effect on taste function depends on the ability to detect the taste, detect the physiological signal or state shift, integrate these signals, and execute an appropriate response.
Lesions in the gustatory zone of the PBN also disrupt the ability to acquire and subsequently express a CTA, but do not interfere with the ability to express a CTA that was acquired before the brain damage (262, 264, 430, 568, 620, 628, 682, 691). Given the development of a CTA is dependent on the integration of taste and visceral signals, some effort has been made to localize the particular subregion of the PBN associated with this deficit. This has primarily been done by targeting lesions to the gustatory and/or visceral subregion of the PBN before CTA training. Indeed, both medial and lPBN lesions separately disrupt CTA expression (lPBN; Refs. 9, 430, 460, 569, 570, 766), but likely for different reasons. In fact, there are many ways a given brain component may contribute to CTA or to many other taste- and/or viscerally-guided behaviors (see FIGURE 6); determining the precise role a given area plays is important for uncovering its functional significance.
Given mPBN lesions do not render all rats completely aguesic (686, 693), the deficit produced by this damage cannot simply be explained by the inability of the animals to taste the CS during conditioning and testing. Not only that, but rats with mPBN lesions are able to successfully associate a taste CS with another type of unconditioned stimulus (e.g., shock). So, those results, coupled with the fact that no deficits are seen in the expression of a CTA that was acquired before the lesion, provide compelling evidence that the deficit is not related to an inability to taste per se. Nor do mPBN lesions appear to render the subject unable to detect or otherwise process the visceral US, because rats with similarly placed lesions can acquire a LiCl-induced avoidance of a non-taste CS (e.g., a place, odor, or trigeminal stimulus) (262, 568). In contrast, rats with lPBN lesions do not acquire LiCl-induced CTAs, whatever the CS modality, and fail to acquire CTA with other types of visceral USs (e.g., ethanol), suggesting that the lPBN is essential for processing or otherwise relaying at least certain types of visceral signals (140).64
Because there is oftentimes a considerable delay between the taste CS and the visceral consequences that follow, it is possible that the PBN may be a critical site for holding the taste CS information in short-term memory for later integration with the visceroceptive feedback (430, 691). One study explicitly tested this hypothesis by using a serial TR test, in which the CS was repeatedly presented in small volumes across the test period including while the visceral effects of LiCl were on board, presumably minimizing memorial demands. In that study, mPBN lesions abolished the progressive shift from ingestive to aversive TR seen in intact control rats over the 30-min test, indicating that the deficit is not a simple memorial one (691). In light of the available data, it seems likely that the mPBN is critically contributing to some aspect of the taste-visceral integrative processing that underlies CTA.
While other taste-guided behaviors are moderately affected by mPBN lesions, CTA acquisition is unequivocally eliminated by damage to this region and, as such, the PBN should be considered necessary for this phenomenon. One thing that remains to be determined though is whether the root of the deficit is the lesion-associated disruption of the flow of critical taste and visceral input to forebrain sites or whether the gustatory PBN is the actual site of the taste-visceral integration necessary for a CTA to be acquired.
Yamamoto et al. (793) reported that neuronal c-Fos induced by saccharin was concentrated in the dorsolateral PBN in naive subjects but, after CTA, shifted to the external lateral subdivision of the PBN, a site at which quinine unconditionally generates a c-Fos response. The authors interpreted these data to suggest that “hedonics” is represented in the more lateral portions of PBN, while taste quality is represented in the more medial subregions. Accordingly, one might expect that lesions that disrupt CTA should similarly disrupt behavioral responsiveness to taste stimuli that are unconditionally aversive, like quinine. Yet, Spector et al. (682) found no such correspondence, at least for rats with lesions that were targeted in the medial PBN. That is, although some rats that had the most significant CTA impairments also showed significantly blunted unconditioned concentration-licking functions for quinine, others did not show any change from their presurgical levels. The reverse was true too; some rats with significant disruptions in unconditioned licking for quinine were completely unimpaired when it came to the CTA. Whether a more direct correspondence between these two outcomes would be seen in rats with lesions specific to the lateral PBN remains to be determined. Whatever the case, this lack of correspondence between various behavioral outcomes for rats with mPBN lesions reinforces the suggestion that there appears to be anatomical segregation in the functional circuitry underlying some taste-guided behaviors in the gustatory zone of the PBN.
What about the sufficiency of the taste and visceral structures in the hindbrain to effect appropriate taste-guided responses? The chronic decerebrate (CD) rat preparation, in which the forebrain is anatomically disconnected from the remaining portion of the nervous system, is a powerful means to address this question. One very clear deficit of the CD rat is that it does not voluntarily eat and drink (202, 272, 273, 359). In other words, the CD rat lacks appetitive responsivity to foods and fluids; for this reason, the animal must be sustained via enteral gavage. However, when prototypical taste stimuli (e.g., sucrose, quinine, sodium chloride, HCl) are applied directly to the oral cavity of the CD rat, it competently expresses unconditioned and concentration-dependent stereotypic reflexive behaviors (i.e., TR) associated with ingestion and rejection of the infused solutions (202, 275). Thus it would appear that the neural circuits caudal to the midbrain are themselves sufficient to support innate taste-guided oromotor reflexive acceptance and rejection (consummatory) behaviors, but not appetitive behaviors.65,66
There are, however, two notable taste-guided phenomena that are virtually abolished in the CD rat. First, whereas intact rats normally show primarily aversive TR to hypertonic salt solutions, this response shifts to primarily ingestive TR to the same solution under conditions of bodily sodium loss. By comparison, the CD rats exhibit the normal rejection of hypertonic salt solutions in the replete state, but the behavioral response fails to shift when the CD rats are rendered sodium deplete (276). Such state-dependent adjustments in sodium consummatory behaviors must, therefore, require forebrain processing.67 The second major phenomenon affected by decerebration is CTA acquisition and expression. CTA is often accompanied by a shift from neutral/ingestive to aversive TR to the CS. CD rats show no such behavioral change; that is, they respond to the taste CS as they had before the conditioning, even after many taste-LiCl pairings (272).68
Thus, while the neural structures within the hindbrain are sufficient to maintain unconditioned reflexive responses to taste stimuli, they do not appear to be sufficient to support sodium state-dependent changes in taste-guided behavioral reactions to sodium or the type of taste-visceral integration that underlies CTA. Furthermore, while lesion studies support the notion that the gustatory PBN is necessary for CTA acquisition, the results from the CD preparation indicate the hindbrain, including the mPBN, is not sufficient for CTA acquisition. These findings point to the importance of functional connections between the mPBN and forebrain structures in these phenomena.69
Norgren and colleagues (155, 156) found a very inventive way to assess the necessity of such PBN-forebrain connections. In one series, for example, Norgren and colleagues made a selective unilateral lesion in the mPBN in one hemisphere and a selective lesion to the LH in the contralateral hemisphere, effectively disrupting all functional connections between these two sites bilaterally, while sparing their respective connections to other sites, at least unilaterally. In contrast to the lack of effect of lesions placed in the same hemisphere, the asymmetrical lesions virtually abolished the expression of furosemide-induced sodium appetite and severely retarded CTA acquisition and expedited extinction (155, 156). The fact that CTA deficits were not as severe as those following bilateral PBN lesions alone suggests that other forebrain structures contribute significantly to the underlying processes. We review some of those potential sites in later sections. In fact, it could be that the mPBN and LH are not necessary for these behaviors at all and instead the combination of lesions to these two sites disrupts the flow of information to other indispensable brain areas in the circuit. Nevertheless, this represents an approach that may eventually unveil the necessary and sufficient brain regions underlying CTA and sodium appetite.
E. Hindbrain-Forebrain Anorexigenic Circuit
Some but not all forms of taste- and/or viscerally suppressed ingestive motivation require that information is transmitted to and/or from the forebrain.70 For example, whereas CD rats fail to acquire and express a CTA, they do reduce intraoral intake in response to satiating agents, like CCK (147, 272, 277, 303). These differences suggest that the hindbrain is sufficient for some taste and taste-visceral integrative processes that result in avoidance of further consumption, but not all. One caveat to this conclusion is that the IO intake test does not involve appetitive behavior other than the termination of the ingestive bout. Nevertheless, various stimuli that shut down intake produce a strikingly similar pattern of neural activity in select subregions of the lPBN and CeA (35, 104, 294, 415, 492, 587, 589, 609, 698, 751, 758).71 Recently, a variety of anorexigenic cues were shown to activate CGRP-expressing neurons within the external lateral subdivision of the lPBN (12, 94, 95, 103, 104, 578). That is, satiation signals (e.g., CCK, amylin, GLP-1), toxic agents (e.g., LiCl, cisplatin), and infectious agents [e.g., lipopolysaccharide (LPS)] all stimulate this population of neurons; the relative activity of these lPBN CGRP+ neurons, as indexed by c-Fos response, appears to be positively correlated with the respective decrease in intake produced (i.e., LiCl > LPS > CCK ≈ amylin) (104). Artificial inhibition of lPBN CGRP+ cells attenuates the normal cessation of intake produced by LiCl, but does not impede the effectiveness of satiation-inducing drugs (at least not statistically anyway), although these treatments were also less effective at reducing intake under noninhibited conditions (i.e., floor effect). Thus it could be that the responsiveness of these neurons is graded with respect to either the intensity or aversiveness of the visceral stimulus and/or other neural mechanisms are sufficient to maintain a normal response to these types of treatments (but not LiCl/LPS) when lPBN CGRP+ neurons are rendered nonoperational.72 Together, these findings suggest a potential conduit of anorexigenic information from hindbrain to forebrain, but given that the hindbrain is sufficient to process at least some of these signals for the purposes of ingestive control and these signals vary with respect to their biological implications, the question remains: what is the functional significance of this conduit?
Lateral PBN CGRP+ neurons receive direct input from visceroceptive neurons in the cNTS and, in turn, project to primarily two distinctive sites—the CeA (in particular the lateral capsule of the CeA) and the BNST (104, 603, 644).73 Selective manipulations to each of these respective pathways have been made using opto- and chemogenetic techniques. Carter et al. (104) recently demonstrated that artificial stimulation of the CGRP+ neurons in the lPBN that project to the CeA suppresses food intake, but stimulation of those neurons that project to the BNST does not affect food intake. Chemogenetic inhibition of the LBPN CGRP+ → CeA pathway attenuated the reduction in intake normally seen in response to LiCl or LPS treatments; findings that were taken to suggest that the lBPN CGRP+ → CeA pathway is necessary for these agents to impact intake.74 Consistent with this, Alhadeff and co-workers (11, 12) found that cisplatin, a nauseo- and anorexi-genic drug used in chemotherapeutics similarly activates lPBN CGRP+ neurons and disruption of downstream glutamatergic signaling in the CeA attenuates cisplatin-induced kaolin intake (i.e., pica)75 and food intake suppression. While these studies have begun to unveil a potential singular circuit that subserves varied visceral stimuli acting as ingestive brakes,76 it would be instructive to assess how these effects on intake are rendered. In other words, what are the behavioral outputs affected by manipulation of this circuit (i.e., avoidance vs. aversion; appetitive vs. consummatory)?
Another key question is whether this circuit incorporates other input sources in a similar manner. Closer inspection of the CeA has yielded results that speak to this. The lateral subnucleus of the CeA comprises a subpopulation of neurons, PKC-δ+, that are responsive to a variety of anorexogenic stimuli (89). This includes LiCl and some satiation peptides, as well as orally applied quinine. Targeted inhibition of these neurons attenuates the effectiveness of these stimuli to reduce intake. One interesting aspect of this study was that the CeA PKC-δ+ neurons not only received input from the lPBN CGRP+ neurons, but from other lPBN (CGRP-) neurons as well as from other brain areas, such as the CeA itself, the basolateral amygdala (BLA), and the IC, among others.77 There also were some differences across these putative inputs with respect to their responsivity to the panel of anorexigenic agents tested. For instance, oral quinine and intraperitoneal LiCl evoked a c-Fos response in the lPBN, BLA, and IC, whereas CCK only evoked a response in the lPBN and BLA. An implication of this anatomical organization is that these CeA neurons act as a site of convergence for different types of information that then leads to a certain type of response output.78
Given the effectiveness of LiCl, LPS, and cisplatin to stimulate lPBN→CeA pathway, one obvious suggestion is that this circuit contributes to the development of conditioned taste avoidance and/or aversion. Indeed, Carter et al. (103) found that selective inhibition of lPBN CGRP+ neurons interfered with, but did not completely abolish, the suppression of intake of a novel (but not a familiar) food following LiCl injections. Optogenetic stimulation of the lPBN CGRP+ neurons led to a reduction in intake of the novel food as well. The effectiveness of lBPN CGRP+ neuronal stimulation to serve as a US in this paradigm was reduced in mice that had previous exposure to LiCl injections. Given that it is known that prior exposure to the US alone attenuates its ability to subsequently condition an aversion/avoidance, these results were indicative of some degree of similarity between the visceral experience induced by LiCl and stimulation of the lPBN CGRP+ neurons. The use of intake measures in this study cannot reveal whether this circuit supports the acquisition of an aversion or an avoidance response per se.79 Although some studies have found that the CeA contributes to the acquisition of taste aversion/avoidance, others have found this structure to be altogether dispensable in this regard (see below).
Moreover, if this lPBN→CeA pathway were indeed relevant to CTA, as opposed to some other process (i.e., anorexia, cessation of feeding), then why are satiation-related signals processed in this circuit, albeit perhaps to a lesser extent? Some have speculated that CCK or other satiation-related peptides reduce intake by producing a noxious or emetic side effect. CTA and related paradigms have been used to assess this possibility. Whereas high (or repeated) doses of CCK-related peptides were sufficient to condition avoidance of a taste solution, lower doses were not (165, 187, 238, 239, 318, 389, 465).80 One study more directly compared the efficacy of LiCl and CCK to produce conditioned taste avoidance (see Ref. 670). At doses that produced comparable reductions in intake, only the LiCl led to a taste avoidance. In other words, CCK-related peptides, at doses that are sufficient to significantly reduce intake, do not generate a sufficiently negative side effect to produce lasting changes in ingestive motivation for the associated taste stimulus. This is further supported by Davidson et al. (150), who found that systemic injections of CCK-related peptide and LiCl produce distinct signals or states as measured in an interoceptive discrimination paradigm. Glucagon-like peptide 1 (GLP-1) is another anorexigenic hormone that is released in response to nutrients in the GI tract (775). Whereas systemic injections of one type of GLP-1 agonist, Exendin-4, conditioned robust flavor avoidance at doses that were also sufficient to suppress food intake, a different agonist, Liraglutide, only conditioned flavor avoidance at higher doses than were required to suppress food intake (357). In the CNS, GLP-1 is principally produced by neurons localized in the NTS, which project to several structures in the forebrain, including the CeA and PVH (255, 291, 352, 397, 585, 588, 763). Infusion of GLP-1-related agents directly into the brain has been shown to produce CTA, as indexed by intake tests (379, 723), while central infusions of a GLP-1R antagonist blocks the efficacy of systemic LiCl injections to suppress food intake, stimulate pica behavior, and support CTA (379, 586, 651). Importantly, only certain central nuclei (i.e., the CeA) seem to mediate the effects GLP-1 on LiCl-induced anorexia and CTA, while others (e.g., PVH) contribute to the peptide’s influence on normal food intake (379, 457, 458). The general consensus from the available data is that the anorectic and adverse effects of negative visceroceptive cues, be they satiation- or illness-inducing, engage at least partially separable pathways (see also Ref. 585). However, these types of distinctions will need to be bolstered with closer inspections of the precise behavioral and/or physiological outputs affected by these various agents.
Importantly, hypothalamic neurons projecting to the lPBN appear to modulate the activity of this pathway according to interoceptive energy signals. That is, AgRP neurons project to a population of CGRP+ neurons in the lPBN. When the animal is in a food-deprived state, these neurons are thought to release GABA onto the CGRP+ neurons, which, in turn, suppresses the transmission of visceral signals known to inhibit food intake. Palmiter and colleagues (188, 786, 787) have nicely demonstrated that the starvation that stems from AgRP-related dysfunction may be due to a release of inhibition on this anorexigenic lPBN→CeA pathway. When, in the absence of a functioning AgRP system, the lPBN CGRP+ neurons are inhibited, mice will eat as normal. The implication is that, in times of energy depletion, the anorexic influence of potentially harmful stimuli is reduced, resulting in an animal that is more inclined to ingest substances that are conditionally or unconditionally negative.81 This represents at least one way that physiological status can interact with circuits that subserve taste- and viscerally based ingestive motivation. Although it appears that this may be a general mechanism, affecting negative food-related cues broadly, it will be important to determine whether there is a hierarchy or degree of specificity with which physiological state impacts sensory inputs.
F. Contributions of the Forebrain to Bad Taste82
1. Amygdala
The amygdala is a complex structure, comprising several cellularly and anatomically distinct subnuclei, which are interconnected with a myriad of sensory, cortical, and limbic brain areas (15, 97, 386, 552, 572, 656, 714, 801). Out of this, there are two main subdivisions, identified mainly by their distinct cellular and anatomical properties, the CeA and the BLA. These two areas have been the chief targets for understanding structure-function relationships, including taste. The amygdala was once synonymous with the fear center, but decades of research have demonstrated that the amygdala is involved in a broader array of functions, including those more typically associated with reward and reinforcement (345).
The CeA is one of the main targets of the gustatory PBN (514, 625, 730, 762). It also receives input from the gustatory thalamus (500). Based on electrophysiological measures, quinine is apparently a particularly effective stimulus (509, 510). Cells in the rostral portions of the CeA appear to be more responsive to quinine than those in the caudal portion, as revealed by taste-elicited c-Fos expression (373, 807). Although neither of these studies systematically quantified the c-Fos response along the medial-lateral axis of the CeA, the authors of one study did note that quinine-elicited c-Fos was predominately distributed in the lateral subnucleus of the rostral CeA (373). The lPBN CGRP+ neurons of the aforementioned “anorexia” circuit terminate in the nearby lateral capsular zone of CeA (104); whether these quinine-responsive cells in lateral CeA receive direct inputs from that source and whether there is a rostrocaudal gradient to the aforementioned “anorexia” pathway remains to be determined. Nevertheless, taste input is sufficient to elicit the quinine-induced c-Fos response in the rostral CeA, as GL nerve transection eliminates it and GL regeneration restores it (373). Administration of intragastric T2R agonist mixtures, which therefore bypass the oral receptors, also elicit c-Fos in the CeA, although it is unclear whether these neurons are coincident with the taste-responsive neurons or whether they are more concentrated elsewhere in this amygdalar subnucleus (294). However, bilateral lesions placed in the CeA do not dramatically affect unconditioned TR to representative taste solutions, notably including quinine delivered at a relatively high concentration, suggesting spared areas or extra-amygdalar sites are sufficient to maintain this important reflex (221, 366, 652).
We have already presented evidence that visceral signals, which are known to support CTA, as well as others, are processed in portions of CeA and, in fact, there is accumulating evidence that this area plays a critical role in the anorexiogenic effects of these agents. Yet, other amygdalar subnuclei, in particular the BLA, have also been implicated in visceral processing of noxious or aversive events underlying CTA. For instance, neurons located in BLA also respond to LiCl (89, 391, 475), but in general, the CeA appears to be more responsive to LiCl than the BLA especially at lower doses, as measured by c-Fos (43, 289, 792, 794, 795). BLA may be an alternative conduit of visceroceptive (or aversive) signals to the CeA (89, 337, 619).
Largely because the amygdala receives strong gustatory and visceral inputs, coupled with the fact that it had been long been considered the seat of fear in the brain, this cardinal limbic structure was traditionally considered one of the prime candidates for taste aversion/avoidance processing. Yet, experimentally placed lesions in (or pharmacological silencing of) the amygdala have produced rather equivocal results (7, 8, 40, 174, 221, 222, 282, 365, 393, 394, 401, 497, 599, 600, 663, 664, 790). Reilly and Bornovalova (567) published a critical review of this literature, which focused on deciphering the role of the amygdala in CTA as it relates to the issues raised in FIGURE 6. The anatomical and cellular complexity of the amygdala, coupled with the crude (by today’s standards) means for making such neural manipulations, historically made the amygdala a quagmire with respect to parsing structure-function relationships. The use of more refined techniques to disrupt neural activity revealed that the BLA may be more critically involved in CTA than the CeA (27, 223, 299, 353, 488, 699).83 More work is certainly needed to characterize the relative contributions of amygdalar nuclei to taste-guided behaviors, including CTA.
Somewhat in parallel to the use of more selective lesion-inducing tools, others began to suspect that the involvement of the amygdala may critically depend on the demands of the task (368, 629, 630). Whereas the standard practice is to present the taste CS in a bottle that the animal must approach and from which it must drink during training and testing, Schafe et al. (629) intraorally (IO) infused the CS and measured the latency for the rat to passively drip the CS solution from its mouth as an index of the strength of the CTA.84 A follow-up study pitted the two training methods (conventional bottle vs. IO) against each other (630). Two CTA tests were conducted after training: one in which the CS solution was infused via IO cannula and another in which the CS was presented in a drinking bottle in a counterbalanced order. During the IO test, intact controls let the taste CS drip from the mouth within seconds, as expected. The rats with large amygdala lesions, on the other hand, took much longer (nearly 10 min, the same as NaCl-injected controls) to passively drip the CS solution. When these same rats were tested with the taste CS in a bottle (vs. water), the ones with amygdala lesions consumed copious amounts of the CS and displayed a significant preference for the CS over water; sham-operated rats avoided the CS in the bottle tests, findings that are consistent with the view that the amygdala is critical for learned taste avoidance. However, when rats with similarly large amygdala lesions were presented with the CS in a drinking bottle during training (as opposed to the IO-conditioning method), they, like their intact counterparts, later avoided the taste CS in a two-bottle choice test, without issue. The authors interpreted these results to mean that when an active approach response was required to obtain the CS during the initial learning phase, as is the case during bottle drinking, structures other than the amygdala were sufficient to maintain the acquisition of the CTA.
But what is it about the IO method of CS presentation during conditioning that differentially recruited the amygdala? Unfortunately, other procedural differences between the IO and bottle conditioning procedures used in that study are known to affect the strength and content of learning and preclude a definitive answer. For instance, rats that drank the CS during training potentially had more exposure to the CS in the single conditioning trial; training intake volumes were not reported. TR was not explicitly measured during the IO test in that study, and thus it is unknown whether aversion was evident from other behaviors such as gapes and chin rubs. At issue is whether the IO-conditioned rats with amygdala lesions failed to learn an aversion in the first place. If they did not learn to associate the taste CS with the visceral US, then it is of no surprise that they would also fail to avoid the CS in a two-bottle choice test. Moreover, while the bottle-trained rats did successfully avoid the taste CS after training, whether they would have also showed normal rapid passive rejection (and aversive TR, for that matter) of the CS when forced to sample intraorally was not assessed.
Simbayi et al. took a different approach (664). Based on the previous work by Pelchat et al. (550), Simbayi et al. (664) sought to compare the effects of electrolytic BLA lesions on conditioned avoidance versus conditioned aversion using two different types of USs (aversion and avoidance: LiCl; avoidance: lactose).85 The rats with sham lesions in BLA successfully learned to suppress intake and preference for the CS solutions paired with either LiCl or lactose, as predicted. The rats with BLA lesions failed to learn to completely avoid the taste CS paired with LiCl, but were able to successfully avoid the taste CS paired with lactose. Taste reactivity was also assessed during the first minute of intake across training and testing.86 LiCl-conditioned rats with BLA lesions also did not display the characteristic shift from ingestive to aversive TR seen in the sham-operated conditioned rats. The implication is that the BLA may be important for the acquisition of a learned taste aversion, but not for the acquisition of a learned taste avoidance. It is important to note, though, that there were slight differences in the way that the LiCl and lactose groups were trained and tested that may have also impacted learning. For instance, rats receiving lactose were able to control their own exposure to the unconditioned stimulus (i.e., lactose) by adjusting intake via negative reinforcement; LiCl-injected rats were not.
A later study trained rats, with neurotoxic lesions placed in the BLA or CeA, to associate IO infusions of a taste CS with intraperitoneal LiCl and then quantified TR to the intraorally infused CS and measured CS intake in one- and two-bottle tests (562). The rats with CeA lesions successfully learned and subsequently expressed the CTA, both in the TR test and across the subsequent intake tests. Conditioned rats with BLA lesions consumed more of the CS in post-training intake tests compared with sham-operated controls; however, this deficit only emerged across time (i.e., expedited extinction). Importantly, these neurotoxic BLA lesions had no effect on CS-elicited TR patterns; the BLA-damaged rats displayed strong aversive reactions to the CS associated with LiCl.87 These results directly contrast with Simbayi et al. (664) and favor the opposite conclusion, that BLA lesions disrupt avoidance, as indexed by intake, but not aversion as indexed by TR. Moreover, these results contrast with Schafe et al. (630) insofar as rats with lesions placed in either amygdalar subfield were able to express a CTA in a bottle intake test, at least initially (in the case of the BLA), following IO conditioning. More work is clearly needed to unravel whether the BLA contributes to one or both aspects of these aspects of bad taste.88
Although there are substantial projections of taste and visceroceptive information to the CeA, which seem to be important for negative regulation of intake, on balance, the CeA does not seem to be necessary for CTA expression. Could it be that this anorexia pathway is specifically involved in informing real time responses to various bad events, but is not necessary for expressing learned events? It would be interesting to see whether the hindbrain to forebrain anorexia circuit also mediates a conditionally bad taste (i.e., a taste solution that was previously paired with LiCl or another adverse agent, e.g., lactose). The use of different types of visceral stimuli coupled with different types of response measurements will be useful in sorting out the relative contributions of the CeA and BLA to bad foods. Moreover, given the complexity of the amygdala and some of the data from studies that have modulated neural activity in the amygdala with more temporal specificity [not discussed here; see Reilly et al. (567), for a review], a promising strategy moving forward would be to begin to marry the two approaches to reveal how neural silencing, both within a specific subregion of the amygdala and within a specific phase of the episode (e.g., taste detection, US detection, memory consolidation, etc., FIGURE 6) affects specific behavioral outcomes.
2. Other key subcortical structures
While the amygdala has been a target of a number of studies investigating conditioned and unconditioned bad taste, there are other subcortical areas that have drawn the attention of researchers. Nearly 60 yr ago, Olds and Milner (525) performed the seminal set of experiments that showed rats would perform a response (e.g., press a lever, go to a certain location) to receive brief electrical pulses in their brains. Once trained, the rat would respond seemingly ad infinitum for contingent brain stimulation, sometimes at the expense of other basic needs (e.g., food) (608). Only select subcortical areas were efficacious in this regard, namely, the septum, LH, VTA, and nucleus accumbens, all areas that were linked to or in close proximity to the mesolimbic dopamine system of the medial forebrain bundle (65, 256, 561, 704, 782, 797). These same areas also commonly respond to other naturally reinforcing stimuli (e.g., food, drug, sex) (310, 561, 780). Moreover, the effectiveness of electrical brain stimulation was affected by physiological or experiential conditions that similarly influence behaviors associated with natural rewards. For instance, food deprivation enhanced operant responding for brain stimulation, even in the absence of any nutrition (524). This led many to speculate that activation of these areas was generating some type of ubiquitous positive affect. Decades of research have since led to some major reconsiderations of this view (e.g., see Refs. 29, 52, 102, 591, 622, 781). Moreover, some of these same experiments and others identified nearby areas that, when stimulated, either suppressed operant responding or instigated rejection or retreat (525). One possible interpretation is that these areas comprise a separate but counterbalancing “aversion” system. Nevertheless, to date, the bulk of empirical and theoretical attention has been paid towards understanding how mesolimbic areas contribute to the positive side of the affective spectrum, leaving the negative side relatively ignored.
It is uncontested that taste is a primary component of the reinforcing aspect of food. Stimulation of the oral cavity with sugars, artificial sweeteners, and fats elicit similar neurophysiological responses (e.g., phasic release of dopamine) in a concentration-dependent fashion in the ventral striatum (36, 284, 285, 417, 596, 598, 668, 725). Pharmacological manipulations that block dopamine or extensive lesions to the dopaminergic neurons significantly attenuate intake and preference, and curtail instrumental responding for reinforcing orosensory stimuli (56, 176, 230, 328, 409, 443, 444, 639–641, 657, 671, 720, 770); manipulations that enhance dopamine levels have the opposite effects (at least at certain doses) (128, 189, 534, 662, 776, 779). Although these findings provide strong evidence that dopamine plays a significant role in behavioral responsivity towards natural reinforcers in these types of tests, they do not prove that this catecholamine is essential for all forms of taste affect. For example, rats that were intraorally infused with sucrose displayed a normal ingestive TR profile when their dopamine signaling was disrupted (53, 56, 549, 749; but see Refs. 409 and 544). Dopamine agonists do not significantly enhance ingestive TR either (46, 749). Given that TR is thought to reflect the hedonic evaluation of taste stimuli in the absence of appetitive behavior, some have argued that the lack of effect of dopaminergic manipulations on these oromotor and somatic responses indicates that the dopamine system is not necessary for the “pleasures” derived from taste (52).89 The strength of that conclusion, however, depends in part on the assumption that TR is always an accurate quantitative proxy of the experience of “pleasure.” Unfortunately, this is difficult to validate because we cannot directly measure internal experiences such as pleasure, aversion, reward, affect, thirst, hunger, wanting, liking, and perception; these are all events that can only be inferred from behaviors. This caveat cannot be ignored in interpreting the outcomes of experiments attempting to reveal the contributions of neural circuits to motivational and affective processes. Nevertheless, with respect to the dopamine-related circuits of the forebrain, it is clear that they are involved in the maintenance of normal responsiveness to natural and conditioned taste reinforcers in a variety of behavioral contexts, but not all.
Part of the historic bias towards studying the neural bases of reward and positive reinforcement boils down to the simple fact that central responses to stimuli that awake and freely behaving animals actively avoid are difficult to assess (85, 679). When rats are forced to sample an inherently unacceptable solution (quinine, hypertonic saline) through direct IO infusion, there is a significant increase in the population firing rate and a rapid suppression of extracellular dopamine in the nucleus accumbens shell, opposite of a volume- and rate-matched infusion of a palatable solution (sucrose) (596, 598). Experiential conditions that dampen or otherwise devalue the expected outcome associated with normally positive taste stimuli alter the dopamine response accordingly (e.g., Refs. 36, 101, 437, 597, 772, 773). For example, after a “sweet” taste solution is paired with a negative event (e.g., LiCl, delayed cocaine access), firing rate of accumbens shell neurons is subsequently increased and/or extracellular dopamine levels are decreased in response to the paired taste solution (101, 455, 597, 772).90,91 Repeated exposure to an artificial sweetener solution, in the absence of the expected postoral nutrition, also results in a decrement in sweetener-driven dopamine response (36). On the other hand, if rats are made sodium deplete, the dopamine response to IO hypertonic saline increases above baseline (131, 207). Collectively, these data clearly demonstrate that this “reward” center also responds to bad taste stimuli 92 and that dopamine levels in this brain site reflect the current hedonic or motivational value93 of the taste stimulus, not its sensory properties.
Although the electrophysiological responses are in register with the effects of various pharmacological treatments,94 once again, dopamine does not seem to be critically involved in learning or expressing the hedonic shift that comes with LiCl-induced taste aversion. Berridge and Robinson (53) found that lesion of the dopamine system had no effect on the capacity to adopt the appropriate aversive reflexes to the associated taste CS. This is particularly meaningful given that the hindbrain alone is not sufficient to render this change. Whether the dopamine signal is necessary for rodents to learn to avoid the taste CS and subsequently express that behavior is unfortunately difficult, if not impossible, to assess because such manipulations make the subject adipsic/aphagic. That said, it would be potentially telling to test whether pairing a hedonically negative taste stimulus, like dilute quinine, with positive postingestive outcomes, like calories, which has been shown to enhance intake of (and even preference for) the stimulus without a concomitant shift towards more ingestive/less aversive TR,95 would also act to increase dopamine in the accumbens shell. The fact that positive and negative taste stimuli lead to divergent neural activity in the very same area of the brain is seemingly at odds with the general notion that oppositely valenced affective stimuli are processed in different parts of the brain. Moreover, it undermines the effectiveness of some brain imaging techniques such as fMRI to reveal sites involved in affective processes. On the basis of the available literature, it is yet unknown whether a given cell responds to the same stimulus under both positive and negative valence states. It could be that there are different cell phenotypes associated with each valence and/or potentially different sources of input and/or output (i.e., neurochemically coded).
How the signals are functionally organized is ultimately what matters. By systematically varying the target site (or lesion site) and examining the effects of different types of pharmacological and, most recently, optogenetic treatments on a variety of behavioral outcomes, Berridge and colleagues have begun to unveil a functional map of the nucleus accumbens and other brain areas (for a more comprehensive review, see Ref. 52). Importantly, these studies have shown, for example, that opioid stimulation96 of the rostrodorsal subregion of the medial accumbens shell significantly enhances ingestive TR and intake97 for sucrose and promotes a conditioned place preference. The same treatments in the area just caudal and ventral to this similarly enhance sucrose intake,98 but without any facilitative effect on sucrose-elicited ingestive TR (107, 547, 548).99 These findings offer evidence of a functional dissociation between ingestive TR and intake of a normally preferred stimulus. Berridge and colleagues believe this reflects an anatomical topography in the accumbens shell that segregates the neural circuits underlying “liking,” as measured by ingestive TR, and “wanting,” as measured by intake.100
While opioid stimulation has revealed one type of topography, pharmacological manipulation of inhibitory circuits has shown a complementary functional organization of the accumbens shell with respect to these behaviors. For example, in the caudal dorsal subregion of the medial accumbens shell, GABA-A antagonists potently enhance aversive TR, while suppressing ingestive TR, for quinine and sucrose. Moreover, GABA-A antagonism of the caudal subregion increases defensive paw-treading and leads to robust conditioned place avoidance (574–576). The results from the pharmacological manipulations of opioid and GABA systems have been taken to suggest the medial accumbens shell is topographically organized in a rostral to caudal gradient that is functionally isomorphic with positive and negative affective processing.
To the best of our knowledge, whether the accumbens shell or a more specific subregion within the shell critically contributes to taste avoidance has not been explicitly examined. Given reductions in intake could be due to any number of factors, more work is needed to systematically parse whether treatments that enhance avoidance are anatomically distinct from those that enhance aversion in the accumbens.
The ventral pallidum (VP) has been similarly characterized; the available evidence suggests that positive and negative “hotspots” may be topographically organized in the VP in an opposite gradient compared with that in the accumbens shell, with the positive “hedonic hotspot” localized caudally in the nucleus (676). This appears to explain why earlier studies involving electrolytic and (fibers-of-passage-sparing) neurotoxic lesions in LH were consistently shown to render subjects adipsic and aphagic and “finicky”101 when presented with foods and fluids that were normally readily consumed (e.g., sugars) (17, 70, 124, 491, 719). Lesions to other areas in the ventral forebrain, particularly those near the LH (i.e., globus pallidus, ventral pallidum), produced similar behavioral traits (49, 175). Recognizing this, Cromwell and Berridge (135) wondered whether these distinct structures were collectively (or separately) involved in taste-based avoidance and aversion or whether lesions that were intended to target different structures were mutually affecting a critical area. To test these possibilities, Cromwell and Berridge (135) directed small bilateral lesions targeted at the center of each these areas in separate groups of rats and then screened subjects for “aversive” responses to intraoral infusions of sucrose using a TR test. The lesions were histological quantified and characterized using a modified fractionator method to effectively yield maps of the individual lesion topographies that were compared to reveal areas of brain damage associated with the behavioral phenotype (rejection of IO sucrose). The identified region was centered at approximately −1.3 mm relative to bregma in the ventromedial portion of VP, just outside the LH. Thus lesions that destroyed LH were likely impeding on this area too. By more carefully mapping the lesion locations and volumes and comparing lesion topographies across subjects with a common behavioral deficit, Cromwell and Berridge (135) overcame a limitation of many lesion techniques (lack of control of area of damage) and corrected some longstanding misattributions about the role of the LH in “finickiness”
The VP plays a role in the expression of conditioned taste aversion in rats. Neural activity in the VP tracks the hedonic value of a taste stimulus that has been paired with LiCl (342). Injection of the GABA receptor antagonist bicuculline into the VP, just before a one-bottle CTA retention test, led to an increase in intake of the saccharin solution that had been previously paired with intraperitoneal LiCl (339). This same treatment enhanced ingestive TR and attenuated aversive TR elicited by a saccharin CS. The µ-opioid receptor agonist DAMGO recapitulated this effect. No effect was seen on intake of an innately aversive taste solution, quinine (338). Moreover, presentation of the saccharin solution intraorally in rats after taste aversion conditioning elicited a 50% increase in the release of GABA in the VP, relative to that in nonconditioned controls. Intraorally delivered quinine, although it elicited rejection responses, did not elicit a GABA response in VP (340). Together, these studies suggest that the VP may play a role in expression of the aversion towards a stimulus whose hedonic value has been modified via experience.102 With respect to the neural processes subserving avoidance versus aversion, it would be of interest to determine whether the VP would also be involved in avoidance of a taste stimulus paired with negative visceral stimuli (e.g., lactose) that do not affect TR and thus presumably leave the hedonic value of the tastant unchanged.
3. Insular cortex
Taste and visceral signals are conveyed to IC from the PBN via the thalamus in separate but roughly parallel pathways. The thalamocortical projection has been likened to a classical leminiscal system, at least for the gustatory system, leading some to hypothesize it mainly subserves sensory-discriminative function (e.g., Ref. 553). On the other hand, the hierarchical position of IC has led others to hypothesize that it underlies conscious experience of sensory pleasures and displeasures (e.g., Ref. 387). Gustatory cortex (GC) has received more attention than the adjacent visceral cortex (VC); nevertheless, the functional organization of these subregions remains largely unknown, and even controversial. The topographical organization of stimulus features is a hallmark of many sensory systems, but there is still controversy over whether GC comprises such an underlying spatial representation and, if so, whether that organization is related to sensory-discriminative and/or hedonic function. Using in vivo two-photon calcium imaging, Chen et al. (120) arguably found the most robust chemotopy in GC in the mouse. Sucrose-responsive neurons were localized in the presumptive anterior GC, and quinine-responsive neurons were localized in the presumptive posterior GC of the mouse. Discrete clusters of NaCl-responsive neurons and MSG-responsive neurons were more centrally located within mouse GC.103 However, a more recent study, also using two-photon calcium imaging, found no such spatial segregation among narrowly tuned neurons in the subregion of insular cortex receiving confirmed projections from mouse VPMpc (200), although the area examined was notably more constrained. Thus it could be that inputs from other brain areas contribute to the subfields under study in the Chen et al. study (120). Studies that have used c-Fos as a marker of taste-stimulated neuronal activation also have generally failed to reveal robust chemotopy within the GC (e.g., Refs. 373, 377). Accolla et al. (1) found that saccharin-responsive neurons tended to be located more anteriorly than quinine-responsive neurons in rat GC, although there was still substantial overlap in the center of GC. Interestingly, a follow-up study by the same group also demonstrated that after CTA training, saccharin evoked a response that was more constrained and caudally shifted into the quinine responsive subregion (2). Following extinction of the CTA, the response to saccharin reverted to its original anterior position. Because the spatial representation tracked with the learned devaluation, as opposed to sensory quality, the authors posited that GC chemotopy may relate more to the hedonic value of a stimulus than to the stimulus quality per se. This type of approach that compares the response to an unconditioned bad taste with that to a conditioned one will help to elucidate what is represented, but complementary assessments of the behavior (e.g., is it aversion vs. avoidance, both?) will importantly further refine interpretation.
Optogenetic stimulation of the “sweet” subfield of anterior insular cortex identified by Chen et al. (120) elicited appetitive and consummatory (i.e., licking) responses and conditioned a place preference in the mouse, even in the absence of taste input (551). Similarly, stimulation of the posterior GC, where “bitter” neurons were found by Chen et al. (120), inhibited licking and conditioned a place avoidance. Although stimulation of these two IC subregions was sufficient to recapitulate the ingestive behaviors associated with “sweet” and “bitter” compounds, these results alone do not reveal whether these areas are necessary. In fact, extensive bilateral lesions of GC generally do not affect normal, unconditioned, affective responses to representative taste stimuli. Not only do rats with substantial damage to the GC display fairly normal taste preference or avoidance response functions in long-term two-bottle choice tests, but rats with centrally positioned neurotoxic GC lesions exhibit normal concentration-dependent licking for sucrose or quinine solutions in brief access taste tests (38, 39, 75, 174, 301). Finally, direct intraoral infusions of low and high concentrations of sucrose and quinine solutions in rats with extensive neurotoxic lesions in and around the conventionally defined GC elicit normal ingestive and aversive TR, respectively (374). This is not to say that the GC does not play a role in affective evaluation under normal conditions. Rather it demonstrates that other brain areas are sufficient to sustain these behaviors in the absence of a functional GC (see FIGURE 5).
On the other hand, large bilateral GC lesions have been shown to disrupt the ability to detect and discriminate some taste stimuli used as discriminative cues in a two-response operant task. In particular, rats with GC lesions are less sensitive to NaCl, KCl, and quinine HCl, as indicated by shifts in an operationally defined detection threshold (30, 67). Such lesions also retard the ability to learn to discriminate among two different salts (NaCl vs. KCl) (67). Because these lesions were rather large, encompassing most, if not all of the GC, it is impossible to say whether all of the GC is necessary for these tasks or whether distinct subregions within GC are critical for some/all of these taste stimuli. Interestingly, sucrose detection was not affected by large bilateral GC lesions in rats (30). However, Peng et al. (551) trained mice to use sucrose, NaCl, and quinine as discriminative cues in a go/no-go discrimination task. Administration of a glutamate receptor antagonist in anterior GC disrupted performance on trials guided by the “sweet” cue only, whereas the same treatment targeted to posterior GC disrupted performance on trials guided by the “bitter” cue only. Moreover, mice generalized responding according to the previously trained contingencies when the “bitter” or “sweet” subfields were optogenetically activated in place of the actual sample taste solutions in separate test sessions.104 Follow-up studies will need to expand the stimulus array and the tasks to conclusively rule-out other possibilities. For instance, although sodium was also used to render hedonic valence irrelevant to the go/no-go task, there is still a possibility that these mice could have generalized along a hedonic valence or intensity dimension, as opposed to a sensory quality dimension.
A recent study found that disruption of inputs from the posterior IC to the lateral CeA impaired acquisition of a cued no-go response (reinforced with quinine) and optogenetic stimulation of this same pathway sufficiently substituted for the quinine reinforcer in this task (636). The results are suggestive of a posterior IC→lateral CeA pathway involved in taste-based avoidance (636). Additionally, Wang et al. (765) found the so-called “sweet” and “bitter” zones of murine IC project to separate subregions of the amygdala, namely, the BLA and the CeA, respectively. Moreover, inactivation of these respective amygdalar sites interfered with the capacity for optogenetic stimulation of the IC to guide licking behavior accordingly. While these exciting new data display the capacity of the IC to interact with the amygdala and perhaps other subcortical sites, clearly more work is needed to identify the nature of the signals that these projections normally transmit and link those to behavioral outcomes.
GC has been widely linked to CTA acquisition and expression (40, 67, 74, 76, 125, 141, 174, 213, 367, 424, 429, 505, 601, 602, 702, 791, but see Refs. 231, 301). Consistent with these reports, Schier et al. (633) found that, on average, a group of rats with ≥50% of GC damaged (in fact, on average, 88% of GC was destroyed) failed to completely avoid a postsurgically conditioned taste stimulus in a two-bottle choice test (near 50% CS preference vs. water) (see FIGURE 7). Although this was a considerable deficit, it was not complete. Close inspection of the behavior of individual rats with GC lesions revealed that some of the rats with extensive damage to GC were essentially normal with respect to their ability to acquire and express a CTA; the rest of the rats, also with lesions that met the criterion, were completely impaired (FIGURE 7A). Just as interesting, a subset of rats that were originally excluded because they did not meet the lesion criteria in GC (i.e., had small lesions or lesions that were outside of GC) were also severely impaired. This suggested two things.
FIGURE 7.
A: In Schier et al. (633), a CTA was trained postsurgically by pairing 0.1 M sucrose with LiCl (Li) and later tested in a series of 48-h two-bottle tests. Controls were given 0.1 M sucrose paired with equimolar NaCl (Na) during training. Mean preference scores for the CS on the last 48-h block of two-bottle tests (a total of eight 48-h test blocks were conducted) for rats with at least 50% damage to gustatory cortex (GCx-Li; GCx-Na), sham-operated controls (Sham-Li; Sham-Na), and rats that did not meet the lesion criterion (x-Li) are shown. The sucrose preference score of each rat in each of those groups is also plotted in yellow triangles. From this, it is clear that half of the rats with lesions that met the criterion were severely impaired (complete sucrose preference score = 1.0), whereas the other half were completely unimpaired (complete avoidance of sucrose score = 0.0). Additionally, some Li-injected rats that had smaller lesions within GC or around GC (x-Li) were significantly impaired. B: the size and topography of each rat’s lesion was quantified using a grid mapping system. Each 50-μm coronal section was subdivided into a ML and DV grid as shown overlaid on the photomicrograph of a representative section from a sham-operated brain (left panel). The extent of lesion was scored on a tertiary scale (color coded) for each cell in this 2-D grid (right panel shows a representative 50-μm section from a GCx lesion). C: this was repeated across the entire anterior-posterior (A-P) extent of the lesion, separately for each hemisphere (left and right, with accompanying photomicrographs of the brain/lesion at representative A-P levels). The dashed black lines demarcate the anterior (2.3 mm) and posterior (0.2 mm relative to bregma) borders of the conventionally defined GC. Other lines demark other A-P levels of reference within (solid black) and outside (dashed gray) of GC. These 2-D maps from each hemisphere were subsequently compared to derive a single lesion map for each rat, representing the bilaterally symmetrical aspects of the lesion. D: the symmetry maps of the individual rats that showed impairment were averaged (impaired). Average lesion scores were converted to a color scale. These group-wise maps were compared with that generated by averaging the symmetry maps derived from the rats that were unimpaired, revealing areas of damage associated with the behavioral deficit. Here, the solid black lines demarcate the anterior, center, and posterior levels of GC. AI, agranular insular cortex (dorsal to the rhinal fissure); D, dorsal to insular cortex; DI, dysgranular insular cortex; GI, granular insular cortex; L, lateral; M, medial; V, ventral to rhinal fissure. [Data from Schier et al. (633).]
First, it is commonplace for studies of this nature to group subjects that meet certain lesion criteria (e.g., >50% of the region of interest is damaged) for statistical comparison against a control group. In the case of the Schier et al. paper (633), a comparison of the average performance of the lesion group to that of the control group could have been taken to infer a partial impairment. However, inspection of the individual performance of each subject showed that in this group none of the rats was partially impaired; rather, some of the rats were completely impaired and others were completely normal, resulting in a 50% average preference score. Thus, in some cases, the lesion completely abolished CS avoidance, making for a very different conclusion. In those latter cases, the region damaged was indispensable for this form of CTA expression. The convention of presenting averages has perhaps inadvertently led to some confusion about the extent to which an area is necessary for or contributory to a particular function.
Second, lesions of the GC per se were not responsible for the deficit, but rather lesions that encompassed a subregion of the GC or even adjacent to the GC were responsible for impaired CTA expression. To assess this possibility and identify lesion areas associated with behavioral deficits, Schier et al. (633) devised a lesion mapping system (see FIGURE 7 for details). Using this procedure, they found that impaired subjects had damage to areas that encompassed the posterior portion of GC and the areas just posterior and dorsal to that (i.e., the anterior VC). The lesions of the unimpaired rats were slightly more anterior in GC. Thus, by exploiting the variation in lesion placement and comparing that to the individual behavioral outcomes, this approach revealed that CTA expression was not compromised by GC lesions per se, but rather by a smaller subregion of GC in addition to the adjacent VC. A follow-up study targeted lesions to this identified CTA “hotspot,” systematically varying placement in posterior GC, anterior VC, or both and used the same lesion mapping system to further home in on the precise area of insular cortex related to dysfunction of CTA expression (631). Indeed, this study confirmed that posterior GC and the overlying VC were correlated with CTA expression deficits, but lesions further posterior in VC were not.
It should be noted that other studies have attempted to elucidate a functional topography of IC by targeting pharmacological manipulations or electrolytic/neurotoxic lesions to various subregions of IC (anterior, center, posterior), spanning the gustatory and visceral cortices (434, 505, 753). One study found that central IC, but not posterior or anterior IC NMDA-induced lesions, disrupted LiCl-induced CTA acquisition (505). This “central” region identified in the Nerad et al. (505) study overlaps with the region that Schier et al. (631) considered the caudal half of GC and anterior half of VC.
The nature of the deficit does not appear to be in the ability to taste the stimulus. Neither GC lesions nor more posterior lesions render the subjects completely aguesic (30, 67, 631, 633). Having said that, stimulus intensity, both with respect to the taste CS and visceral US, is a critical determinant in the strength of learning and retention (cf. Ref. 428); thus whether the CTA expression deficit is related to a sensory impairment needs to be more fully examined. While subjects appear to be able to learn a CTA with two taste-LiCl pairings after the identified “hotspot” in posterior GC-anterior VC is damaged, retention and subsequent expression are severely affected (631). Thus the challenge now is to identify neurons or critical inputs and outputs in that “hotspot” that are responsible for normal CTA expression and the nature of their involvement (i.e., sensory, integrative, predictive, outcome-specific; see below and FIGURE 6).
A few studies have begun to delineate whether GC is required for the acquisition and/or expression of some types of taste-visceral associations, but not others. For example, Keifer and Orr (367) found that rats with large GC lesions were able to learn to avoid consuming a CS paired with LiCl in a bottle test, but when these same rats were subsequently presented with the CS through a direct IO infusion, the animals failed to show the expected aversive-type TR and, if anything, showed more ingestive TR than their untrained and sham counterparts. The authors hypothesized that perhaps the rats with GC lesions were able to learn to avoid the taste CS, but not acquire an aversion to it, per se.105 Importantly, given that the nature of the US is a critical determinant factor in whether a taste signal becomes aversive versus avoided, then one possibility is that the GC is critically involved in sensing and/or integrating those features of the visceral US with the taste CS that render a new consummatory profile. Alternatively, it may be that the GC is involved in linking or assigning taste signals to those outputs. This remains to be resolved.
Seemingly consistent with this lesion study, another study found that pharmacological disruption of 5-HT signaling in a region just posterior to GC (in VC) attenuated the development of the gaping response to a taste CS (753).106 On the other hand, pharmacological stimulation of 5-HT3 receptors in this region enhanced LiCl-induced gaping during training and in a subsequent test. It is unclear whether these treatments are affecting the visceral signal and thus impacting the development of an association between taste and LiCl or whether these treatments are affecting taste valence or both (i.e., FIGURE 6). Notably, permanent lesions in VC did not affect CTA learning and expression as measured by intake (505); TR was not measured in the former study (505). Moreover, it remains to be seen whether disruption of normal function in and around posterior GC (the lesion “hotspot” for conditioned taste avoidance, Ref. 633) also interferes with a conditioned change in TR profile after taste-LiCl pairing.
Thus, while there are hints that conditioned aversion and avoidance may be anatomically dissociated in the amygdala and in gustatory/insular cortex, methodological differences among studies and limitations within any given study preclude strong conclusions yet. Despite a very clear behavioral dissociation among these two processes, surprisingly little work has been done to systematically investigate their differential anatomical organization. Behavioral and anatomical strategies exist for probing this essential question, and it would be useful to apply them in the service of elucidating the neural circuits underlying these seemingly similar yet dissociable processes of taste aversion and taste avoidance.
Generally speaking, the fact that the GC (or portions of GC, see above) has been reliably linked to CTA deficits has fostered two main hypotheses about the broader function of this region of IC, one postulating that GC is critical for taste memory processes and the other postulating that GC is involved in learned changes in taste valence.107 Both are difficult to reconcile with the limited available data. If GC contributes to taste memory function in general, then lesions ought to produce disruptions in other types of taste-based memories, including positive forms of taste learning too.108 Very few studies have been done looking at whether GC is necessary and/or sufficient for any type of learning in which the taste serves as a positive or negative US (e.g., odor-taste and taste-taste learning).109
Interestingly, Touzani and Sclafani (736) found that rather large lesions of insular cortex, including GC and parts of VC, had no effect on the capacity of rats to acquire and subsequently express a preference for a taste stimulus paired with intragastric nutrient infusions. Moreover, in psychophysical experiments involving the use of an operant two-response taste discrimination/detection procedure, which clearly involves gustatory learning and memory, animals with large GC lesions are able to competently perform the task at least at higher concentrations (67). Clearly, more work is needed to assess other types of taste learning deficits, but as of now, the results do not implicate GC in general taste-based learning and memory. These studies also question whether GC is necessary for positive taste-visceral associations, as it (or rather an IC subfield) appears to be for some negative taste-visceral associations. Importantly, such experiments have used intake tests to assess learned taste preferences; thus it remains to be seen if other behavioral outcomes would be disrupted.
Finally, it is important to point out that although CTA-related deficits do not appear to be due to aguesia (i.e., the inability to taste the CS), the basis of the functional deficit remains to be completely understood. As noted in prior pages, manipulations can disrupt CTA in a variety of ways (CS disruption, US disruption, memory, CR disruption, CS→US integration; FIGURE 6). Because IC is a higher order sensory area, there has been a bias to interpret its organization with respect to the properties of the stimulus, like the homunculus in somatosensory cortex, rather than the processes that the stimulus might engage. There may be some of both, which makes trying to understand the organization difficult. The use of multiple tasks with multiple stimuli varying in their taste properties, coupled with selective manipulation, transient (pharmacological, optogenetics) or permanent (lesions), of carefully quantified regions of IC should go long way toward unveiling the functional topography of this key site in the forebrain gustatory system.
4. Motor outputs
We have primarily focused on taste, viscerosensory, and physiological inputs and the ascending neuraxis, but implicit in our framework is the importance of linking these to the descending motor outputs. This side of the equation has received less empirical attention. Considering behaviors are ultimately the product of activity in motor neurons, clarity on how they are functionally organized will benefit further interrogations into the neurobiological mechanisms for taste-related appetitive and consummatory phenomena. These actions and the various coordinated responses they each entail are thought to be mediated through at least partially segregated output circuits (479, 738). Appetitive responses are elicited broadly through cortico-spinal, midbrain, and hindbrain outflows; the specific effector (and likely highly complex, coordinated) pathways involved in obtaining or avoiding foods has not been fully elucidated. Nuclei within the hindbrain, particularly those within the intermediate and parvocellular reticular formation, are critical for oromotor reactivity, such as licking, chewing, and swallowing (see discussion of this in sects. IIC and IIID) (202, 275, 738). Although hindbrain sensorimotor arcs are sufficient for the expression of unconditioned taste-specific stereotypic oromotor responses (i.e., TR), these motor neurons also receive extensive centrifugal input from various forebrain structures, permitting higher order processes to affect the responses (168, 486, 653, 804). Precisely how these descending signals influence their motor targets to effect behaviors needs to be illuminated. Manipulation of certain forebrain areas (i.e., via electrodes, pharmacological, opto- and chemo-genetic techniques) can produce oromotor responses (e.g., Refs. 160, 293, 351, 356, 551, 584, 626, 658). These effects do not necessarily reveal the involvement of a given brain area, subregion, or cell in the higher order, sensory-affective determinants of the response. That will need to be determined and disambiguated from the alternative, which is that the stimulation of that brain area, subregion, or cell is simply a link in the effector chain (akin to stimulating an area of primary motor cortex). The use of approaches that combine stimulation with alternative readouts of affect or sensation will ultimately offer critical perspective as to how the system is organized.110
IV. CONCLUSIONS
Mechanisms that prevent or otherwise meter ingestion of potentially harmful substances are just as critical for health and survival as those that promote and monitor intake of nutrients. Much of taste research to date has focused on the latter, leaving the former relatively ignored, despite the fact that taste is a key component for these protective mechanisms. Taste yields the vital information that allows the body to avert or at least blunt the postoral consequences of noxious ingestants. Although the lines between the two ends of the intake control spectrum are sometimes blurred and many of the same basic principles are at work, there are clear differences in the way that animals handle good and bad tastes. The predilection of the field to focus on just one side of this continuum (or dichotomy, depending on your theoretical perspective, see Ref. 79) leads to a constrained view and hinders progress towards a fuller appreciation of the functional organization of the gustatory system and its ultimate impact on behavior. With this in mind, we summarize some of the key points in this paper that we hope will help guide further research into the functional organization of the gustatory system in general, and into the principles and mechanisms underlying bad taste in particular.
A. Taste Contributes to Multiple Domains of Function
Relatively less progress has been made towards understanding the neural bases of gustation compared with the other sensory modalities. Many different brain areas are known to respond to taste stimulation, yet, with few exceptions, it has been difficult to identify which sites are necessary or sufficient for specific types of taste function. Not only that, but there are discrepancies in the literature about how the features of a taste stimulus are coded in the brain. However, one frequently overlooked aspect of gustation that is essential to its capacity to adaptively serve at the forefront of ingestion is that taste signals are channeled into different domains of function: sensory-discriminative, motivational, and physiological. There are clear cases in which the outputs associated with these domains are not correlated. For example, two substances can elicit distinct perceptual qualities, but inhibit ingestion equally. Similarly, a normally accepted taste stimulus (e.g., sucrose) can be avoided and rejected after a bout of poisoning, yet its perceptual quality (e.g., “sweetness”) appears to remain stable and perceptually distinct from other bad tastants. As such, the functional significance of a taste-driven neural response in a given brain site or neuron may be very different than its significance in a different brain site or neuron. The key then is to link taste-driven neural activity to specific behavioral and/or physiological outputs. Importantly, this includes making neural manipulations that can be logically connected to specific behavioral and/or physiological response(s) (683). An optimal way to find such links is to assess the effects of a particular neural manipulation in a variety of functionally diverse tasks.
B. The Avoidance–Aversion Distinction
A given domain of taste function may be further subdivided into distinct sets of responses. This is very clearly the case for the motivational domain which is conventionally considered to comprise both an appetitive and consummatory phase, with each serving a different role in ingestive behavior. Along these lines, taste avoidance and taste aversion reflect distinct dimensions of bad taste. Whereas avoidance is the term commonly used to describe the behavioral responses that keep the animal from engaging with foods that are potentially harmful (in the appetitive subdomain), aversion refers to the behavioral responses associated with rejection of foods and fluids placed in the oral cavity (in the consummatory subdomain). Foods that are avoided are not necessarily aversive. A single taste stimulus can give rise to either one (or sometimes both) of these types of responses, depending on experiential and physiological conditions, proving once again that a neural response to a taste stimulus alone is not necessarily meaningful without a link to the behavioral/physiological response(s). Because outcome measures, like intake, alone do not distinguish between aversion and avoidance, the use of complementary paradigms that enable more direct measures of these behaviors, such as the TR test, provide more discriminating power in the interrogation of the underlying neural substrates. In fact, there may be other behavioral responses that further distinguish between types of avoidance or types of aversion. For example, is the avoidance of food due to satiety the same as avoidance of food associated with an inflammatory response or GI pain? Uncovering such behavioral idiosyncrasies may have great interpretive value.
C. Taste-Visceral Relationships
The distinction between conditioned aversion and avoidance underscores the importance of the postingestive consequences of a food in relation to its taste. Although the gustatory system has evolved to respond as expediently as possible to broad classes of biologically relevant chemicals (e.g., salts, sugars, acids, toxins), there are commonly discrepancies among the taste of a food and its actual content. Thus the signaling utility of taste depends heavily on the capacity of the gustatory system to adapt responses based on the specific associated visceral consequences of a food in accordance with bodily needs. If someone happens to eat ice cream for the first time that is contaminated with a bacterial agent that leads to food poisoning, then that person will not only subsequently avoid eating that flavor of ice cream, but will find it repulsive. If, in contrast, the individual eats ice cream and then experiences lactose indigestion, then that person will eventually learn to avoid consuming quantities of it that are known to produce the symptoms, but will otherwise still appreciate its flavor. The different response profiles reflect the differences in the visceral consequences, which do not carry the same risks. Different strategies are used to deal with the risks, while maximizing the potential benefit. For example, if the consequences are not potentially fatal, then it may be worth consuming some ice cream for its energy (the calories associated with lactose-containing foods). Although it appears that inherently bad tastes are less plastic, there are nevertheless conditions under which they can permit greater intake. That is, the strategies appear to be stimulus and/or state-specific, e.g., how the brain permits high sodium intake in times of need is different than how it permits l-lysine intake in times of need. Given the extensive interplay among taste, postingestive visceroceptive consequences, and physiological state, it is not surprising that there are many parallels in their underlying neural circuitry. Thus a fuller understanding of each of these input systems should come from studies of their interactions.
D. Subjective Inferences and Circular Reasoning
Because non-human animal models are nonverbal, we must rely on behavioral and physiological readouts to understand function. However, in the study of feeding and taste, there is a general tendency for investigators to infer subjective experiences from these outputs. If a rat consumes more sucrose than quinine, some might conclude that the rat must find the sucrose more pleasurable than quinine. This logic, however, is circular. Why did the rat drink more sucrose than quinine? Because it is more pleasurable. How do you know that sucrose is more pleasurable than quinine? Because the rat drank more sucrose than quinine. Moreover, many (including us) have used TR as a tool for making inferences on the hedonic value of a taste stimulus, but at their core, these behaviors represent basic reflexes. To be sure, these reflex behaviors can be heavily influenced by forebrain processes and may indeed, under certain circumstances, reflect affect, but they are not, in and of themselves, absolute proxies of hedonic experience. Even decerebrate rats display the full range of TR to prototypical taste stimuli. When all is said and done, it would be best to simply measure and describe, as plainly and directly as possible, the responses, be they behavioral, physiological, or neural, that are linked to antecedent conditions without overreliance on anthropomorphic terminology and circular reasoning. Arguably, the application of a complementary set of behavioral assays in conjunction with theoretically relevant experimental manipulations is the best way to ultimately define the motivational properties of a taste stimulus, regardless of the constructional terms used to explain the outcomes.
E. Structure-Function Relationships
The brain was once thought to have separate circuits that deal with good versus bad things. At least for the motivational domain of taste-driven functions, these models are beginning to fall out of favor. While there is evidence of segregation in some areas, these functions appear to be in close proximity to one another and, in many cases, are interconnected. Progress towards understanding the organization depends on refining our approaches to structure-function relationships. Manipulations that produce loss of function of brain components, from the cell on up to the circuit level, are the best tools available for uncovering the neural mechanisms that link stimulus inputs to functional outputs. However, as noted many times over in this review, there are often discrepancies across studies that have presumptively targeted the same brain area (or cell population, etc.) and employed comparable measures, ultimately generating a lot of equivocality and misattributions. Although the parameters of the behavioral manipulation and measurement surely account for some of these disparities (see above), shortcomings in the way in which the brain is manipulated are also to blame. For one thing, many components of the nervous system are impossible to selectively, yet completely, eliminate with the methods that have been readily available. This leaves open the possibility that the spared tissue is sufficient to maintain normal function, a caveat that must be considered in the interpretation. Newer approaches, such as chemogenetic and optogenetic techniques that target specific cell populations or projections, offer some promise in this regard.
The other side of this coin is that a given manipulation often extends beyond the brain site intended. When a deficit is observed, the tendency is to simply attribute it to the target site, but it is just as likely that more selective areas within the target or the area outside the target are responsible for the effect. This has undoubtedly undermined the discovery of a clear and consistent functional topography of taste. The common failure to rigorously quantify the size and position of a manipulation (including the bilaterality or symmetry) and relate these to the response profile(s) is one of the biggest stumbling blocks. The association of specific response outcomes with the systematic variation of the target site for a manipulation can be used to unveil structure-function relationships that are otherwise difficult to nail down. The key is to make use of the information available. We have shown the utility of some exemplar approaches in the prior pages. As such, in certain contexts, even the relationship between the inevitable spared tissue and the lack of deficit may have interpretive value.
F. Significance
Throughout this review we have highlighted some of the gaps that are yet to be filled in our understanding of the gustatory system, especially those pertaining to the neural, behavioral, and physiological mechanisms that comprise the often-neglected bad taste, while suggesting strategies that will improve our empirical frameworks. The clinical implications of such research are multitudinous. A better understanding of bad taste will aid in the development of oral medications that are more acceptable to patients (468), in devising strategies to circumvent the anorexia associated with chemotherapeutic treatments, and in finding ways to promote intake of less-acceptable but nutritious foods, all of which will enhance compliance and healthful outcomes.
GRANTS
This work was supported by National Institutes of Health Grants R01-DC004574, R01-DC009821, and R01-DK106112.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Ginger Blonde and Frank Johnson for helpful feedback on an earlier version of this review and Charles Badland for work on some of the figures.
Address for reprint requests and other correspondence: Alan C. Spector, Dept. of Psychology, 1107 West Call St., Florida State University, Tallahassee, FL 32306 (e-mail: spector@psy.fsu.edu).
Footnotes
In fact, different rodent species and even strains within a species vary in the properties of their gustatory system and such differences are thought to reflect genetic variations and/or adaptive specializations (e.g., Refs. 23, 26, 249, 251, 336). Variability is also seen between humans (e.g., Refs. 66, 88, 118, 219, 302, 721). However, those types of comparisons are outside the scope of this review.
For brevity’s sake, we often use the word “food” throughout this review, but most, if not all, of these statements are just as applicable to fluids. Moreover, food, in this sense, is not meant to refer strictly to nutritive substances, but rather to chemicals orally sampled from the environment, including toxic and/or nonrepletive matter.
Although humans and rodents can discern among major taste qualities, such as “sweet,” “salty,” “sour,” “bitter,” they are rarely experienced in isolation of one another. The typical human diet (and indeed the diet of the rodents typically used to study taste) is complex, comprising any combination of these features. The same can be said of taste-driven affect—foods can have good and bad tastes. While there have been some empirical investigations examining how various taste stimuli interact in human and rodent models, the majority of studies to date have focused on select chemicals in isolation. Once the responses to these unitary stimuli are well understood on their own, it will be important to further elucidate how they interact in more externally valid ways. This is an important caveat to keep in mind in the context of this review. The systems engaged and responses triggered by purely “bad” tastes, for instance, may be different from those engaged and triggered by good and bad tasting mixtures.
Appetitive and consummatory responses are distinct classes of behavior. The terms have been broadly adopted in the literature, although there are certain idiosyncrasies by field with respect to their meaning. Although both of these terms conjure positive aspects of feeding (appetite and consumption), we use these terms to include responses that pre-emptively prevent and limit as well as promote ingestion.
However, as will be reviewed later, there are certain stimuli and/or concentrations for which the sensory-discriminative properties of “bitter” and “sour” ligands, for example, can be confused for one another (e.g., Refs. 279, 489, 747).
Of course, there are some exceptions to this in the literature.
Although this phenomenon is thought to have its evolutionary origin in taste, human subjects report that other types of sensations and even thoughts can rouse such a sense of revulsion (149).
Not all species have the capacity for all of these reflexes. Notably, rats lack the capacity to separately control two muscles at the esophageal sphincter necessary for the vomit reflex. Rats do, however, exhibit other reflexes associated with vomiting, such as gaping (324).
To be accurate, a large part of the chemosensory experience of eating shrimp derives from the olfactory system, but this example highlights the fundamental difference between conditioned aversion and avoidance.
Pica, which is the ingestion of a non-food substrate, like dirt or clay, is a behavioral phenomenon whose impetus and purpose have been somewhat enigmatic. The fact that this behavior manifests in pregnant women and anemic patients is suggestive that it serves as a means to correct for micronutrient deficiencies. Bodily iron, calcium, or zinc shortages are associated with this behavior in laboratory rodents (677). Interestingly, gastrointestinal (GI) distress, such as that produced by chemotherapeutics, LiCl intoxication, or rotational motion, also stimulates this behavior, even in the absence of any nutritional deficits. The latter is thought to be a means for encumbering the infectious agent and quelling symptoms (158, 477). Nutritional deficiencies are known to cause nausea in humans, so whether these reflect common underlying or truly distinct etiologies is unknown. The relationship between other, non-emetic forms of GI distress and pica has been unexplored, as has the role of taste. Both conditioned taste aversion and clay intake are commonly used to measure the emetic properties of experimental pharmacological treatments (20, 716), but it is important to note that these paradigms are assessing different behaviors that may very well be subserved by different neurocircuitries. One study reported that rats previously conditioned to associate saccharin with LiCl poisoning subsequently exhibited pica when they re-encountered the saccharin stimulus alone (477a). This finding suggests that pica is not a measure of taste aversion or avoidance per se; rather, it likely reflects the response to an expected or actual visceral state.
This is one of those examples in which the taste-driven motivational circuits yield two response profiles that can only be revealed through close inspection of the behaviors. Failure to do so places limits on one’s ability to interpret experimental outcomes with respect to linking specific processes or neural substrates to function.
The term conditioned taste aversion or its acronym, CTA, is commonly used in the literature, even when “aversion” has not been directly measured. CTA is also used at times to refer to conditioned taste avoidance. To avoid further confusion, we use CTA loosely in this review. It may refer to aversion or avoidance; however, in the cases in which rejection responses were measured, then we note that in the text.
Certain psychoactive drugs have been reported to reduce intake of a paired taste solution (avoidance) without rendering it more aversive, as measured with taste reactivity (TR). However, in some of those cases, when measured, there are clear conditioned reductions in ingestive TR (e.g., Ref. 536). Whether that constitutes a change in palatability differing only in degree (quantity) and not in quality is debatable.
In the context of CTA, physiological status signals (e.g., deprivation or satiation levels) affect taste-guided consummatory processes, until the state reverses or resolves (e.g., Refs. 48, 54, 87, 185).
Aspects of licking microstructure (e.g., initial lick rate, burst size) are oftentimes considered a reflection of hedonic evaluation/palatability of the stimulus; however, appetitive and consummatory influences on licking behaviors are not entirely independent of one another in these measures (153, 178, 420, 669, 688).
The effect of these drugs on appetitive only vs. appetitive and consummatory responses may be dose-dependent (e.g., Ref. 539). In some cases, though, there were reductions in ingestive TR, which again, in our view, is a change in TR profile such that whether a particular drug changes the hedonic value of a stimulus is just a matter of degree.
Some have also observed reductions in lick burst size to the devalued tastant in negative contrast, while others have failed to see any such change in lick microstructure (e,g., Refs. 266a, 785 vs. Ref. 116).
It is interesting to note that factors such as the contingency between the two stimuli (e.g., when the positive consequences of the second stimulus are contingent on the amount of the first stimulus consumed as is arguably the case in most feeding situations) may significantly impact the types of response developed. That is, some taste stimuli that precede and are associated with positive consequences will ultimately be consumed in greater quantities, as a function of that experience (199). We discuss evidence for these processes (i.e., conditioned taste preference) in the next section.
Neophobia may represent a strategy, perhaps particularly for omnivores, to avoid ingesting foods with unknown consequences. Importantly, however, there may be different motives for an apparent lack of neophobia or the eventual ingestion of a novel food.
There have been some attempts to assess this but without much success (see Ref. 490).
With respect to physiological status, we limit our review to two examples here—sodium and l-lysine deficiencies—that differ in how they affect taste-guided behaviors, but certainly others have been examined (see Ref. 484).
Though in some cases, slighted elevated acceptance or preference for higher levels of sodium may persist beyond repletion (see Refs. 126, 487, 621).
The Feurte et al. (191) study examined whether such shifts in dietary choice were accompanied by a fundamental shift in the TR profile for the diet. While they did observe a shift towards an aversive TR profile for the deficient diet, consummatory responses were potentially confounded with appetitive responses, as rats’ oromotor responses were recorded as they freely consumed pelleted diet.
However, consummatory responses have not been measured apart from appetitive influences to date.
There has been some work done on the central processing of the visceroceptive signals that induce EAA specific appetites (for a recent review, see Ref. 305), although much less is known about how and where these are integrated with the gustatory system proper.
Of course, the possibility that initially avoided/aversive tastants are simply more resistant to changes in the motivational domain, but would be amenable with additional training and/or a stronger, more positive, US cannot be dismissed.
One early study found that simply pairing a bitter (quinine) or sour (HCl) taste stimulus with a sweet one (sucrose) led to a positive shift in TR (83). A later study implicated flavor-calorie associations in the enhancement of CS TR shifts, as opposed flavor-taste associations (205). Thus it is possible that other types of USs associated with nutrition are sufficient to modify the affective value of inherently “bad” tastants.
This includes morphine.
For instance, the rats that came to elicit ingestive TR for morphine or nicotine were given extensive exposure (i.e., many months), whereas the exposure periods in many of the other studies discussed were on the order of days to weeks.
Though, in this case, doses and responses may have been at ceiling and the cost-benefit of ingesting such substances likely follows an inverted U function.
However, this ought to be confirmed with taste reactivity tests.
Although peripheral taste transduction is a critical step in reception, yielding the initial sensory inputs, taste is ultimately rendered in the brain. Many of the examples in this review speak to this point. The same signal generated in the periphery can provoke ingestion on one occasion and rejection on another, dependent on other factors (e.g., context, physiological status, learned outcomes). Thus the significance of phenomena at the level of the periphery must take into consideration function (see FIGURE 3). For these reasons, we provide only a brief overview of the peripheral system in this section. For more comprehensive recent reviews, see Refs. 418, 605.
Type III cells are putatively involved in the transduction of “acid” stimuli. More on this below.
One study found that nicotine, which is reportedly bitter to humans, utilizes common peripheral signaling elements with T2Rs and other G protein-coupled taste receptors (namely, TRPM5), yet rats were able to discern nicotine from quinine in a behavioral discrimination task. The results suggested that distinct signaling pathways must also exist (526).
A variant of the cation nonselective channel TRPV1 has been proposed as a possible receptor, but the literature regarding the functional consequences of deletion of its associated gene on electrophysiologically and behaviorally assessed taste responsiveness to NaCl has been mixed (614, 674, 746).
More work is needed to understand this bitter-sour confusion phenomenon in humans and animal models.
Voight et al. (761) present an exciting new strategy for unveiling the function of a specific T2R intra- and extraorally.
The lingual taste receptor cells, especially those of the anterior tongue, have received the most attention in taste research, a caveat that ought to be taken into consideration in an interpretation of the literature to date for reasons that will become clearer in the sections that follow.
Based on their location and response properties, these vagally-innervated taste buds are thought to be involved with protection of airways.
Primarily in the rostrocentral subdivision of the NTS.
Notable exceptions to this are discussed below.
Accordingly, we limit our review to the extrinsic innervation.
Intestinal vagal afferents appear to be more involved in transmitting nutrient information than gastric vagal afferents.
There is emerging evidence that the vagus contributes to pain sensations as well (e.g., Refs. 98, 119, 384, 737).
Other types of vagal receptors (e.g., thermoreceptors, osmoreceptors, nocioceptors) exist, but less is known about them, particularly with respect to ingestion and digestion. Moreover, the vagus innervates the more distal GI tract as well, but in a more diffuse fashion (see Ref. 461).
Different types of vagal mechanoceptive endings appear to have partially segregated distributions along the proximal GI tract and respond to different sources of tension (see Refs. 209, 764).
This includes osmolality and pH.
Some constituents and/or byproducts of digestion/absorption may act directly on the afferent terminals as they are absorbed into the bloodstream or lymph.
Our understanding of these chemoreceptors in the GI tract has largely been gleaned by their links to physiological outputs (e.g., motility, secretion) and second to that, behavioral end points (e.g., food intake). It is important to consider the fact that, just as in the gustatory system, there may be separate channeling of some of these signals for one or the other function, though clearly, they are often related.
The AP contains a chemoreceptive field that appears to be critical for responsivity to some emetic agents. Interestingly, AP lesions disrupt learned avoidance (and conditioned changes in taste-elicited TR) of a LiCl-paired solution, but not LiCl-induced anorexia, suggesting a functional dissociation of circuits underlying the learned and unconditioned effects of LiCl (143, 184, 470, 527).
A major limitation with respect to the interpretation of these various anatomical pathways is that the type of information carried in each pathway has, in most cases, not been confirmed and could consist of gustatory/chemosensory, mechanosensory, cardiac, and barosensory signals, just to name a few. Nevertheless, the architecture of these connections does lay the framework that could be the anatomical basis for some degree of functional segregation.
The AP may also send direct, albeit minor, projections to select forebrain structures.
Newer techniques will be useful in this regard.
One caveat is that the number of quinine-stimulated c-Fos neurons in rNTS was not statistically correlated with the number of gapes elicited.
Interestingly, whereas combined CT and GL transections nearly abolish concentration-dependent licking responses to quinine, neither CT nor GL transection is as effective; this suggests that information carried by either of these nerves is sufficient to maintain these types of behaviors. This is clearly different from the gape response (233, 696).
And perhaps other sites that were not directly examined in this study.
Because these are T2R-expressing and T1R2-expressing taste receptor cells the synapses would be unconventional.
This manipulation should also promote connections to cells expressing the T1R1+T1R3 heterodimer that is an amino acid receptor.
One caveat regarding the Jacobs et al. (343) study is that the rats were depleted of sodium via presentation of sodium-deficient chow that had a high sucrose content. Control animals received laboratory chow.
Due to the anatomical complexity of the PBN including the existence of some taste-responsive neurons in external medial and external lateral PBN, it is difficult to generate absolutely complete lesions of the structure.
Though this was not quantified in anyway.
Interestingly, the behavioral response to another means of sodium imbalance such as that produced by polyethylene glycol (i.e., decreased plasma volume) was not affected by medial PBN lesions (711). Moreover, calcium deprivation, which normally enhances appetite for calcium and sodium, does not appear to be disrupted in rats with PBN lesions (260). Thus areas outside of PBN are sufficient for detecting and responding to these types of depletion signals.
This study did not evaluate whether the rats with PBN lesions were able to detect and discriminate the sodium cation specifically. Given the expression of sodium appetite is specific to this salt, it remains possible that PBN lesions interfere with sodium recognition.
That said, there are some data to suggest that other types of visceral USs are still effective in this regard in rats with lPBN lesions (i.e., hypertonic saline) (140). One interesting possibility is that the signals these USs generate are relayed to or processed in alternative sites, perhaps even within PBN.
CD rats also showed normal taste-evoked CPIR (201).
One caveat is that the CD preparation does not disrupt humoral signaling to and from the forebrain.
Notably, when challenged with excess sodium, CD rats and intact rats alike adequately excreted the surplus in the urine. Correspondingly, when bodily sodium was depleted via maintenance on a sodium-deficit diet, CD rats properly compensated their Na output. The fact that these particular adjustments to changes in sodium balance were largely unaffected in CD rats suggests that the hindbrain and/or periphery comprises sufficient mechanisms to detect the physiological pertubations and respond accordingly (276), though see footnote 66.
A number of studies using the CD preparation have nicely demonstrated that the neural circuitry within the hindbrain is sufficient for some viscerally or physiologically dependent changes in ingestive behavior, but not sufficient for other types of integrative processing. For example, the CD rat can appropriately terminate ingestion in response to the accumulation of a nutritive load in the postoral GI tract and exogenous administration of satiation hormones such as CCK, GLP-1, and bombesin and can increase ingestion in response to insulin-induced hypoglycemia. CD rats, however, do not appear to respond normally to systemically administered lipoprivic stimuli, across longer periods of time (i.e., across several meals), or following extended periods of deprivation (148, 269, 271, 277, 298, 303, 358).
However, it is important to note that it is possible that the PBN might undergo significant degeneration following the CD surgery. That said, quinine still induces c-Fos responses in the PBN of CD rats, although there are some noted differences in the expression relative to intact rats (741).
This is largely inferred from the fact that CD rats fail to show the positive appetitive behaviors towards foods, and other types of stimuli, but it is unclear whether they also are impaired when it comes to negative appetitive behaviors, such as the active avoidance of “bad” food stimuli, as distinct from negative consummatory behaviors. In other words, the fact that CD rats fail to engage in positive appetitive behaviors makes it difficult to assess whether they are able to actively avoid.
At least for select stimuli that have been tested thus far.
Recall that CD rats show normal inhibition of ingestive behavior in response to satiation (e.g., Refs. 272, 277). Although CD rats are incapable of learning to reject a taste stimulus paired with a negative visceral event, to our knowledge, it is unknown whether they exhibit rapid reductions in ingestive behavior to malaise-inducing agents.
Although there are diffuse projections to other forebrain structures, including the LH, it is unclear if individual neurons project to one or multiple structures.
Note that the anorectic response was not completely abolished by silencing the CeA-projecting neurons. Moreover, although stimulation of the neurons projecting to the BNST was not found to change short-term intake, whether silencing the PBN→BNST pathway interfered with LiCl-induced suppression of intake was not tested.
Kaolin intake has been used by some researchers as an index of a visceral aversive state. Interestingly, one study found that lateral PBN lesions significantly enhanced cisplatin-induced kaolin intake, relative to that seen in sham-operated controls (323). Horn et al. (323) speculate that the large lateral PBN lesions could diminish the strength of the negative feedback signal coming from the ingestion of kaolin, thereby allowing these rats to eat more, though the effect was specific to kaolin (chow intake was suppressed at levels comparable to controls). The precise location of the lesions and the extent to which the external lateral PBN, where the critical CGRP neurons are clustered, was damaged/spared is difficult to reconstruct; it is possible that sufficient signal was able to reach CeA (or elsewhere) to signal the presence of cisplatin. Another possibility is that the remainder of the hindbrain (or inputs from cNTS to forebrain structures) is sufficient for cisplatin to drive pica, but that the lateral PBN→CeA route is critical transmission of a signal for other purposes (e.g., anorexigenic signals). Would the lesions produced in the Horn et al. (323) study block the development of a CTA to a cisplatin-paired flavor, while simultaneously enhancing kaolin intake? Kaolin intake as proxy of a positive appetitive response to negative visceral consequences has utility in trying to decipher how a particular area is contributing to the response, especially when compared against other measures, such as aversion and avoidance.
Lesions of the PBN also interfere with the ability to learn to prefer a taste paired with postoral nutrition, at least under certain conditions; thus it will be important to determine whether positive visceroceptive signals are also mediated through the lPBN CGRP+ neurons or through alternative hindbrain-forebrain routes (157, 648, 800). A recent study implicates a separate relay in lPBN for postoral stimuli associated with nutrition (292).
PKC-δ+ neurons are reciprocally connected to nearby PKC-δ− neurons, which are thought be involved in positive ingestive motivation. Thus activation of one set and inhibition of the other set may serve as a switch between positive and negative ingestive motivation (89).
A recent study found that disruption of inputs from the posterior IC to the lateral CeA impaired acquisition of a cued no-go response (reinforced with quinine) and optogenetic stimulation of this same pathway sufficiently substituted for the quinine reinforcer in this task (636). The results are suggestive of an IC→lateral CeA pathway for taste-based avoidance (636), but the nature of this signal remains to be further described.
Rinaman and colleagues showed that a distinct population of hindbrain-originating cells contributes to LiCl-induced anorexia, but not learned avoidance (589). Selective lesions to a catecholeminergic subpopulation of neurons in cNTS resulted in an attenuation of LiCl-induced suppression of food intake. These same lesions did not disrupt the ability to learn to avoid a flavor paired with IP LiCl. Moreover, because the lesion also disrupted the c-Fos response evoked by IP LiCl in the PVH, but not in the lPBN or CeA, the authors speculated that these cells in cNTS that project to PVH are critical for the anorectic effects of LiCl, whereas an alternative pathway, presumptively the lPBN to CeA pathway, plays a role in LiCl-based CTA. Similar lesions blunted the satiation response and c-Fos response to IP CCK-8 in the PVH, but not in the lPBN or CeA (587). These findings are, on the one hand, consistent with the work of Palmiter and colleagues insofar as the CGRP positive neurons in lPBN contribute to CTA (103), but they are seemingly at odds with other findings, such as the fact that inhibition of lBPN CGRP+ neurons that project to CeA attenuates LiCl-induced food intake suppression (104). However, note that in none of these studies did lesion or inhibition completely reverse the food intake suppression, suggesting other areas may be contributing.
Recall that bitter ligands stimulate the release of CCK from enteroendocrine cells, and devazepide, a CCK-A receptor antagonist, attenuates the central c-Fos response to postoral denatonium benzoate stimulation and partially blocks the suppression of licking in response to a postoral denatonium benzoate infusion (117, 295, 632; see sect. IIIA5). It remains to be seen whether CCK is a critical signaling factor in post-oral bitter transduction or whether CCK antagonists are producing a physiological state (e.g., more deprived) that facilitates greater tolerance of “bitter” ingesta.
A recent study found that activation of AgRP neurons that project to the PBN suppress behavioral responsivity to certain types of pain (i.e., inflammatory) and not others (e.g,. acute, thermal), suggesting there is some state-sensory input specificity in this circuit (14).
We restrict our review to a few key forebrain areas that have been studied most extensively for their contributions to taste-based ingestive motivation. This is not to say that other brain areas are not important or may be the necessary component of a circuit underlying a particular behavior. We also limit our review to the behavioral phenomena that have received the most attention. As will hopefully be clear in the foregoing pages, we believe that more progress will require the addition of other behavioral and physiological measures to more completely elucidate the functional organization of the system.
Bahar et al. (27) found that pharmacological silencing of the CeA disrupted CTA acquisition, but not subsequent expression and the same manipulation to the BLA disrupted retention, but not expression.
This is also known as the intraoral intake test.
Lactose appears to produce negative lower GI distress in rats. Although rats will learn to avoid a taste solution paired with lactose, they do not appear to change their hedonic evaluation of the stimulus in a modified TR test [see Pelchat et al. (550)]. There are some methodological limitations to the approaches used by Simbayi et al. and Pelchat et al. that preclude a true distinction between aversion and avoidance (see also next footnote).
This is not an optimal way to measure TR. The rat must approach the spout and lick the solution. Thus, not only are the consummatory behaviors confounded with appetitive ones here, but because the rat is in control of intake, there are likely differences with respect to how much stimulus contacts the taste receptors across subjects and groups.
Intake tests were done after the TR tests in this study; thus it is unclear whether the aversive TR would have similarly extinguished with sufficient exposure and time. Schafe et al. (630) counterbalanced the order to the IO and consumption tests across rats.
The fact that IO-conditioned rats subsequently expressed avoidance in a bottle test suggests the CS-US association was successfully expressed in a situation with a different response requirement in rats with large lesions that damaged both BLA and CeA. It is possible that larger lesions (CeA plus BLA) would recapitulate the results of Schafe et al. (630).
Similar functional distinctions have been seen in other subcortical regions. For instance, electrical stimulation of the LH increases intake, while simultaneously evoking aversive TR (55).
Other studies suggest that aversive or negative-valenced stimuli are processed in these “reward” circuits as well (e.g., Refs. 129, 206, 392, 447).
This area includes the region typically referred to as the “hedonic hotspot,” as found in Berridge and colleagues’ studies.
Neutral (non-taste) stimuli paired with IO infusions of sucrose or quinine would eventually evoke a neural or dopamine response that resembled and took the place of the corresponding taste stimulus (596).
And, in fact, the oromotor response.
Inhibition of the nucleus accumbens shell with mu-opioid receptor agonist DAMGO or GABA agonist muscimol increases ingestive TR for sucrose (107, 547, 548, 675). Conversely, GABA antagonists enhance aversive responding to quinine (576).
Taste-taste (quinine-sucrose) associations have been shown to lead to increased ingestive TR for quinine on their own (83).
DAMGO does decrease aversive TR for quinine more caudal to the hedonic hotspot, but not in the hedonic hotspot (547).
As well as intake of chow and chocolate candies.
The region correlated with intake-enhancing effects is somewhat more extensive.
The index of wanting in many of these studies is chow or chocolate candy intake; appetitive and consummatory influences are confounded in intake measures. However, because the effects on ingestive TR are more circumscribed, the authors reason that any effect on intake in the nonoverlapping regions is through the appetitive component. Note that there were decreases in aversive TR for quinine in areas that were outside the “hedonic hotspot” and the stimuli used among the tests are not of the same sensory quality (e.g., sucrose vs. chow or M&Ms), thus one cannot rule out that different types of sensory inputs engage nonoverlapping areas. For instance, would DAMGO affect sucrose rendered aversive through conditioning in a way that accords with its effects on unconditioned sucrose or quinine?
Finicky is a term that was once widely used in this literature, but has since fallen out of favor; it generally referred to behaviors that were thought to reflect aversion and/or avoidance such as gaping, food spillage, or paw treading.
It is unclear if this is mediated via memory or CS processing (see FIGURE 6).
Interestingly, no cluster for an acidic stimulus (“sour”) was identified in this study.
It is difficult to compare the anatomical location of these lesion and pharmacological treatments, especially across species. Nevertheless, at least in the rat, this lesion study suggests that areas outside of the conventionally defined GC are sufficient to maintain normal sensory-discriminative capacity for sucrose. Whether the critical area is located in IC, but was spared with these particular lesions or whether the critical area resides outside IC awaits further examination.
Importantly, these GC lesions did not interfere with unconditioned aversive TR to quinine HCl (367).
Forebrain disruption of serotonin attenuates LiCl-induced conditioned gaping, but not taste avoidance (419a).
Note that large GC lesions do not disrupt unconditioned TR for sucrose and/or quinine. This is not entirely surprising given lower order structures (i.e,. in hindbrain) are sufficient to maintain these unconditioned reflexes (374).
Some have argued that the fact that lesions in GC disrupt taste neophobia reflects a memorial deficit (421, 422).
Saddoris et al. (616) found that non-taste punctate cues that have been paired with taste solutions come to activate the same neuron in GC as the primary taste stimulus, suggesting that the neurons may be involved in encoding expected outcomes (see also Ref. 759).
These will no doubt present some interpretive challenges.
REFERENCES
- 1.Accolla R, Bathellier B, Petersen CCH, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 27: 1396–1404, 2007. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci USA 105: 4010–4015, 2008. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackroff K, Dym C, Yiin YM, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiol Behav 97: 406–413, 2009. doi: 10.1016/j.physbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric monosodium glutamate in mice. Chem Senses 38: 759–767, 2013. doi: 10.1093/chemse/bjt042. [DOI] [PubMed] [Google Scholar]
- 5.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell 100: 693–702, 2000. doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 6.Adolphs R, Tranel D, Koenigs M, Damasio AR. Preferring one taste over another without recognizing either. Nat Neurosci 8: 860–861, 2005. doi: 10.1038/nn1489. [DOI] [PubMed] [Google Scholar]
- 7.Aggleton JP, Petrides M, Iversen SD. Differential effects of amygdaloid lesions on conditioned taste aversion learning by rats. Physiol Behav 27: 397–400, 1981. doi: 10.1016/0031-9384(81)90322-X. [DOI] [PubMed] [Google Scholar]
- 8.Agüera AD, Puerto A. Lesions of the central nucleus of the amygdala only impair flavor aversion learning in the absence of olfactory information. Acta Neurobiol Exp (Wars) 75: 381–390, 2015. [PubMed] [Google Scholar]
- 9.Agüero A, Arnedo M, Gallo M, Puerto A. The functional relevance of the lateral parabrachial nucleus in lithium chloride-induced aversion learning. Pharmacol Biochem Behav 45: 973–978, 1993. doi: 10.1016/0091-3057(93)90150-R. [DOI] [PubMed] [Google Scholar]
- 10.Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol 341: 289–314, 1994. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- 11.Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate receptors in the central nucleus of the amygdala mediate cisplatin-induced malaise and energy balance dysregulation through direct hindbrain projections. J Neurosci 35: 11094–11104, 2015. doi: 10.1523/JNEUROSCI.0440-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhadeff AL, Holland RA, Zheng H, Rinaman L, Grill HJ, De Jonghe BC. Excitatory hindbrain-forebrain communication is required for cisplatin-induced anorexia and weight loss. J Neurosci 37: 362–370, 2017. doi: 10.1523/JNEUROSCI.2714-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN. A neural circuit for the suppression of pain by a competing need state. Cell 173: 140–152.e15, 2018. doi: 10.1016/j.cell.2018.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311: 1–16, 1991. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 16.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 17.Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 77: 323–325, 1951. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 18.Andreozzi P, Sarnelli G, Pesce M, Zito FP, Alessandro AD, Verlezza V, Palumbo I, Turco F, Esposito K, Cuomo R. The bitter taste receptor agonist quinine reduces calorie intake and increases the postprandial release of cholecystokinin in healthy subjects. J Neurogastroenterol Motil 21: 511–519, 2015. doi: 10.5056/jnm15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrew BL, Oliver J. The epiglottal taste buds of the rat. J Physiol 114: 48–49, 1951. [PubMed] [Google Scholar]
- 20.Andrews PLR, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci 125: 100–115, 2006. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias C, Pautassi RM, Molina JC, Spear NE. A comparison between taste avoidance and conditioned disgust reactions induced by ethanol and lithium chloride in preweanling rats. Dev Psychobiol 52: 545–557, 2010. doi: 10.1002/dev.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthurs J, Lin J-Y, Ocampo R, Reilly S. Lactose malabsorption and taste aversion learning. Physiol Behav 180: 39–44, 2017. doi: 10.1016/j.physbeh.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2, and NH4Cl solutions by 28 mouse strains. Behav Genet 32: 445–457, 2002. doi: 10.1023/A:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmanov AA, Schlager G, Tordoff MG, Beauchamp GK. Consumption of electrolytes and quinine by mouse strains with different blood pressures. Physiol Behav 64: 323–330, 1998. doi: 10.1016/S0031-9384(98)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet 28: 117–124, 1998. doi: 10.1023/A:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci 17: 1527–1530, 2003. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- 28.Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001. doi: 10.1152/ajpregu.2001.281.5.R1581. [DOI] [PubMed] [Google Scholar]
- 29.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 191: 439–459, 2007. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 30.Bales MB, Schier LA, Blonde GD, Spector AC. Extensive gustatory cortex lesions significantly impair taste sensitivity to KCl and quinine but not to sucrose in rats. PLoS One 10: e0143419, 2015. doi: 10.1371/journal.pone.0143419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks WA. Peptides and the blood-brain barrier. Peptides 72: 16–19, 2015. doi: 10.1016/j.peptides.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker LM, Johns T. Effect of ethanol preexposure on ethanol-induced conditioned taste aversion. J Stud Alcohol 39: 39–46, 1978. doi: 10.15288/jsa.1978.39.39. [DOI] [PubMed] [Google Scholar]
- 33.Bartoshuk LM, Beauchamp GK. Chemical senses. Annu Rev Psychol 45: 419–449, 1994. doi: 10.1146/annurev.ps.45.020194.002223. [DOI] [PubMed] [Google Scholar]
- 34.Beauchamp GK, Moran M. Dietary experience and sweet taste preference in human infants. Appetite 3: 139–152, 1982. doi: 10.1016/S0195-6663(82)80007-X. [DOI] [PubMed] [Google Scholar]
- 35.Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res 1162: 76–84, 2007. doi: 10.1016/j.brainres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci 36: 2533–2546, 2012. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens M, Meyerhof W. Mammalian bitter taste perception. Results Probl Cell Differ 47: 203–220, 2009. doi: 10.1007/400_2008_5. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin RM. Cortical taste mechanisms studied by two different test procedures. J Comp Physiol Psychol 48: 119–122, 1955. doi: 10.1037/h0041257. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin RM. The effect of fluid deprivation on taste deficits following cortical lesions. J Comp Physiol Psychol 48: 502–505, 1955. doi: 10.1037/h0041873. [DOI] [PubMed] [Google Scholar]
- 40.Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res 549: 165–170, 1991. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein IL. Learned taste aversions in children receiving chemotherapy. Science 200: 1302–1303, 1978. doi: 10.1126/science.663613. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein IL, Hennessy CJ. Amiloride-sensitive sodium channels and expression of sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol 253: R371–R374, 1987. doi: 10.1152/ajpregu.1987.253.2.R371. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein IL, Koh MT. Molecular signaling during taste aversion learning. Chem Senses 32: 99–103, 2007. doi: 10.1093/chemse/bjj032. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein IL, Treneer CM, Goehler LE, Murowchick E. Tumor growth in rats: conditioned suppression of food intake and preference. Behav Neurosci 99: 818–830, 1985. doi: 10.1037/0735-7044.99.5.818. [DOI] [PubMed] [Google Scholar]
- 45.Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol 95: 363–382, 1981. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20: 1–25, 1996. doi: 10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- 47.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24: 173–198, 2000. doi: 10.1016/S0149-7634(99)00072-X. [DOI] [PubMed] [Google Scholar]
- 48.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 16: 103–120, 1991. doi: 10.1016/0195-6663(91)90036-R. [DOI] [PubMed] [Google Scholar]
- 49.Berridge KC, Cromwell HC. Motivational-sensorimotor interaction controls aphagia and exaggerated treading after striatopallidal lesions. Behav Neurosci 104: 778–795, 1990. doi: 10.1037/0735-7044.104.5.778. [DOI] [PubMed] [Google Scholar]
- 50.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci 98: 652–660, 1984. doi: 10.1037/0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 51.Berridge KC, Grill HJ. Isohedonic tastes support a two-dimensional hypothesis of palatability. Appetite 5: 221–231, 1984. doi: 10.1016/S0195-6663(84)80017-3. [DOI] [PubMed] [Google Scholar]
- 52.Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 23: 294–303, 2013. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369, 1998. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 54.Berridge KC, Schulkin J. Palatability shift of a salt-associated incentive during sodium depletion. Q J Exp Psychol B 41: 121–138, 1989. [PubMed] [Google Scholar]
- 55.Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav Neurosci 105: 3–14, 1991. doi: 10.1037/0735-7044.105.1.3. [DOI] [PubMed] [Google Scholar]
- 56.Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci 103: 36–45, 1989. doi: 10.1037/0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- 57.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16, Suppl 1: 28–33, 2004. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 58.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 191: 203–212, 1995. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 59.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319: 261–276, 1992. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 60.Berthoud HR, Trimble ER, Siegel EG, Bereiter DA, Jeanrenaud B. Cephalic-phase insulin secretion in normal and pancreatic islet-transplanted rats. Am J Physiol Endocrinol Metab 238: E336–E340, 1980. [DOI] [PubMed] [Google Scholar]
- 61.Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev 96: 151–176, 2016. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 62.Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 509: 514–525, 2008. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 63.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41–49, 2007. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 64.Bice PJ, Kiefer SW, Elder NB. Evaluating the palatability of alcohol for rats with measures of taste reactivity, consumption, and lick rate. Alcohol 9: 381–387, 1992. doi: 10.1016/0741-8329(92)90036-A. [DOI] [PubMed] [Google Scholar]
- 65.Bielajew C, Shizgal P. Evidence implicating descending fibers in self-stimulation of the medial forebrain bundle. J Neurosci 6: 919–929, 1986. doi: 10.1523/JNEUROSCI.06-04-00919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blakeslee AF, Salmon TN. Genetics of sensory thresholds: individual taste reactions for different substances. Proc Natl Acad Sci USA 21: 84–90, 1935. doi: 10.1073/pnas.21.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blonde GD, Bales MB, Spector AC. Extensive lesions in rat insular cortex significantly disrupt taste sensitivity to NaCl and KCl and slow salt discrimination learning. PLoS One 10: e0117515, 2015. doi: 10.1371/journal.pone.0117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Booth DA. Food-conditioned eating preferences and aversions with interoceptive elements: conditioned appetites and satieties. Ann N Y Acad Sci 443, 1 Experimental: 22–41, 1985. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- 69.Booth DA, Simson PC. Food preferences acquired by association with variations in amino acid nutrition. Q J Exp Psychol 23: 135–145, 1971. doi: 10.1080/00335557143000149. [DOI] [PubMed] [Google Scholar]
- 70.Boyle PC, Keesey RE. Chronically reduced body weight in rats sustaining lesions of the lateral hypothalamus and maintained on palatable diets and drinking solutions. J Comp Physiol Psychol 88: 218–223, 1975. doi: 10.1037/h0076187. [DOI] [PubMed] [Google Scholar]
- 71.Bradley RM. Tongue topography. In: Taste, edited by Acree TE, Atema J, Bardach JE, Bartoshuk LM, Beidler LM, Benjamin RM, Bradley RM, Bujas Z, Burton H, Cole LP, Farbman AI, Guth L, Kalmus H, Kare M, Kurihara K, McBurney DH, Murray RG, Nachman M, Pfaffmann C, Sato M, Shallenberger RS, Zotterman Y, Beidler LM. Heidelberg: Springer, 1971, p. 1–30. doi: 10.1007/978-3-642-65245-5_1. [DOI] [Google Scholar]
- 72.Brand JG, Teeter JH, Silver WL. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res 334: 207–214, 1985. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- 73.Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses 30: 793–799, 2005. doi: 10.1093/chemse/bji071. [DOI] [PubMed] [Google Scholar]
- 74.Braun JJ, Kiefer SW, Ouellet JV. Psychic ageusia in rats lacking gustatory neocortex. Exp Neurol 72: 711–716, 1981. doi: 10.1016/0014-4886(81)90020-0. [DOI] [PubMed] [Google Scholar]
- 75.Braun JJ, Lasiter PS, Kiefer SW. The gustatory neocortex of the rat. Physiol Psychol 10: 13–45, 1982. doi: 10.3758/BF03327004. [DOI] [Google Scholar]
- 76.Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiol Behav 9: 637–641, 1972. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- 77.Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ. Lick rate analysis of sodium taste-state combinations. Am J Physiol Regul Integr Comp Physiol 264: R312–R318, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Breslin PA, Spector AC, Grill HJ. Chorda tympani section decreases the cation specificity of depletion-induced sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol 264: R319–R323, 1993. [DOI] [PubMed] [Google Scholar]
- 79.Breslin PA, Spector AC, Grill HJ. A quantitative comparison of taste reactivity behaviors to sucrose before and after lithium chloride pairings: a unidimensional account of palatability. Behav Neurosci 106: 820–836, 1992. doi: 10.1037/0735-7044.106.5.820. [DOI] [PubMed] [Google Scholar]
- 80.Breslin PA, Spector AC, Grill HJ. Sodium specificity of salt appetite in Fischer-344 and Wistar rats is impaired by chorda tympani nerve transection. Am J Physiol Regul Integr Comp Physiol 269: R350–R356, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol 23: R409–R418, 2013. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breslin PAS, Beauchamp GK. Salt enhances flavour by suppressing bitterness. Nature 387: 563, 1997. doi: 10.1038/42388. [DOI] [PubMed] [Google Scholar]
- 83.Breslin PAS, Davidson TL, Grill HJ. Conditioned reversal of reactions to normally avoided tastes. Physiol Behav 47: 535–538, 1990. doi: 10.1016/0031-9384(90)90122-K. [DOI] [PubMed] [Google Scholar]
- 84.Brot MD, Watson CH, Bernstein IL. Amiloride-sensitive signals and NaCl preference and appetite: a lick-rate analysis. Am J Physiol Regul Integr Comp Physiol 279: R1403–R1411, 2000. doi: 10.1152/ajpregu.2000.279.4.R1403. [DOI] [PubMed] [Google Scholar]
- 84a.Browning KN. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci 9: 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience 201: 331–337, 2012. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bushman JD, Ye W, Liman ER. A proton current associated with sour taste: distribution and functional properties. FASEB J 29: 3014–3026, 2015. doi: 10.1096/fj.14-265694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabanac M, Lafrance L. Postingestive alliesthesia: the rat tells the same story. Physiol Behav 47: 539–543, 1990. doi: 10.1016/0031-9384(90)90123-L. [DOI] [PubMed] [Google Scholar]
- 88.Cabras T, Melis M, Castagnola M, Padiglia A, Tepper BJ, Messana I, Tomassini Barbarossa I. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One 7: e30962, 2012. doi: 10.1371/journal.pone.0030962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 17: 1240–1248, 2014. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caicedo A, Kim K-N, Roper SD. Individual mouse taste cells respond to multiple chemical stimuli. J Physiol 544: 501–509, 2002. doi: 10.1113/jphysiol.2002.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci 23: 9947–9952, 2003. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science 291: 1557–1560, 2001. doi: 10.1126/science.1056670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calviño AM, García-Medina MR, Cometto-Muñiz JE, Rodríguez MB, de Fisiología C. Perception of sweetness and bitterness in different vehicles. Percept Psychophys 54: 751–758, 1993. doi: 10.3758/BF03211799. [DOI] [PubMed] [Google Scholar]
- 94.Campos CA, Bowen AJ, Han S, Wisse BE, Palmiter RD, Schwartz MW. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat Neurosci 20: 934–942, 2017. doi: 10.1038/nn.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial cgrp neurons control meal termination. Cell Metab 23: 811–820, 2016. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cansell C, Denis RGP, Joly-Amado A, Castel J, Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 3: 169, 2012. doi: 10.3389/fendo.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 360: 213–245, 1995. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 98.Cao B, Zhang X, Yan N, Chen S, Li Y. Cholecystokinin enhances visceral pain-related affective memory via vagal afferent pathway in rats. Mol Brain 5: 19, 2012. doi: 10.1186/1756-6606-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Capaldi ED. Conditioned food preferences. In: Why We Eat What We Eat: The Psychology of Eating. Washington, DC: Am. Psychol. Assoc, 1996, p. 53–80. doi: 10.1037/10291-003. [DOI] [Google Scholar]
- 100.Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacology (Berl) 22: 352–356, 1971. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- 101.Carelli RM, West EA. When a good taste turns bad: neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology 76, Pt B: 360–369, 2014. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carlezon WA Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56, Suppl 1: 122–132, 2009. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci 35: 4582–4586, 2015. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci 34: 4239–4250, 2014. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castro DC, Terry RA, Berridge KC. Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal shell shifts ‘liking’ toward ‘disgust’ and ‘fear’. Neuropsychopharmacology 41: 2101–2111, 2016. doi: 10.1038/npp.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol 262: 27–45, 1987. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- 110.Chan CY, Yoo JE, Travers SP. Diverse bitter stimuli elicit highly similar patterns of Fos-like immunoreactivity in the nucleus of the solitary tract. Chem Senses 29: 573–581, 2004. doi: 10.1093/chemse/bjh062. [DOI] [PubMed] [Google Scholar]
- 111.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 112.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell 100: 703–711, 2000. doi: 10.1016/S0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 114.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 3: 113–119, 2000. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 115.Chaudhari N, Roper SD. Molecular and physiological evidence for glutamate (umami) taste transduction via a G protein-coupled receptor. Ann N Y Acad Sci 855: 398–406, 1998. doi: 10.1111/j.1749-6632.1998.tb10598.x. [DOI] [PubMed] [Google Scholar]
- 116.Chen BH, Hsiao S. Anticipatory contrast effect in rats: a new view with lick response analysis and the effect of dopamine blocking. Chin J Physiol 39: 235–243, 1996. [PubMed] [Google Scholar]
- 117.Chen MC, Wu SV, Reeve JR Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol 291: C726–C739, 2006. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 118.Chen QY, Alarcon S, Tharp A, Ahmed OM, Estrella NL, Greene TA, Rucker J, Breslin PA. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 90: 770S–779S, 2009. doi: 10.3945/ajcn.2009.27462N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen SL, Wu XY, Cao ZJ, Fan J, Wang M, Owyang C, Li Y. Subdiaphragmatic vagal afferent nerves modulate visceral pain. Am J Physiol Gastrointest Liver Physiol 294: G1441–G1449, 2008. doi: 10.1152/ajpgi.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen X, Gabitto M, Peng Y, Ryba NJP, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science 333: 1262–1266, 2011. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cho YK, Smith ME, Norgren R. Low-dose furosemide modulates taste responses in the nucleus of the solitary tract of the rat. Am J Physiol Regul Integr Comp Physiol 287: R706–R714, 2004. doi: 10.1152/ajpregu.00090.2004. [DOI] [PubMed] [Google Scholar]
- 122.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol 292: G98–G112, 2007. doi: 10.1152/ajpgi.00295.2006. [DOI] [PubMed] [Google Scholar]
- 123.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, Chen R, Jones RM, Behan DP, Leonard J. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology 149: 2038–2047, 2008. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 124.Clark AJ, Clark JM, Winn P. NMDA lesions of rat lateral hypothalamus: effects of dietary and physiological challenges. Neuroreport 1: 263–266, 1990. doi: 10.1097/00001756-199011000-00024. [DOI] [PubMed] [Google Scholar]
- 125.Clark EW, Bernstein IL. Establishing aversive, but not safe, taste memories requires lateralized pontine-cortical connections. Behav Brain Res 197: 356–363, 2009. doi: 10.1016/j.bbr.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clark JJ, Bernstein IL. Sensitization of salt appetite is associated with increased “wanting” but not “liking” of a salt reward in the sodium-deplete rat. Behav Neurosci 120: 206–210, 2006. doi: 10.1037/0735-7044.120.1.206. [DOI] [PubMed] [Google Scholar]
- 127.Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol 284: 55–67, 1978. doi: 10.1113/jphysiol.1978.sp012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clarke SN, Parker LA. Morphine-induced modification of quinine palatability: effects of multiple morphine-quinine trials. Pharmacol Biochem Behav 51: 505–508, 1995. doi: 10.1016/0091-3057(95)00042-U. [DOI] [PubMed] [Google Scholar]
- 129.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88, 2012. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collings VB. Human taste response as a function of locus of stimulation on the tongue and soft palate. Percept Psychophys 16: 169–174, 1974. doi: 10.3758/BF03203270. [DOI] [Google Scholar]
- 131.Cone JJ, Fortin SM, McHenry JA, Stuber GD, McCutcheon JE, Roitman MF. Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proc Natl Acad Sci USA 113: 1943–1948, 2016. doi: 10.1073/pnas.1519643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst 6: 303–322, 1982. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- 133.Cordick N, Parker LA, Ossenkopp KP. Rotation-induced conditioned rejection in the taste reactivity test. Neuroreport 10: 1557–1559, 1999. doi: 10.1097/00001756-199905140-00030. [DOI] [PubMed] [Google Scholar]
- 134.Craig W. Appetites and aversions as constituents of instincts. Proc Natl Acad Sci USA 3: 685–688, 1917. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res 624: 1–10, 1993. doi: 10.1016/0006-8993(93)90053-P. [DOI] [PubMed] [Google Scholar]
- 136.Cross-Mellor SK, Hoshooley JS, Kavaliers M, Ossenkopp KP. Immune activation paired with intraoral sucrose conditions oral rejection. Neuroreport 15: 2287–2291, 2004. doi: 10.1097/00001756-200410050-00029. [DOI] [PubMed] [Google Scholar]
- 137.Cross-Mellor SK, Kavaliers M, Ossenkopp KP. Comparing immune activation (lipopolysaccharide) and toxin (lithium chloride)-induced gustatory conditioning: lipopolysaccharide produces conditioned taste avoidance but not aversion. Behav Brain Res 148: 11–19, 2004. doi: 10.1016/S0166-4328(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 138.Cross-Mellor SK, Kavaliers M, Ossenkopp KP. The effects of lipopolysaccharide and lithium chloride on the ingestion of a bitter-sweet taste: comparing intake and palatability. Brain Behav Immun 19: 564–573, 2005. doi: 10.1016/j.bbi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 139.Cross-Mellor SK, Roberts S, Kavaliers M, Ossenkopp KP. Activation of the immune system in rats with lipopolysaccharide reduces voluntary sucrose intake but not intraoral intake. Pharmacol Biochem Behav 76: 153–159, 2003. doi: 10.1016/S0091-3057(03)00210-7. [DOI] [PubMed] [Google Scholar]
- 140.Cubero I, Lopez M, Navarro M, Puerto A. Lateral parabrachial lesions impair taste aversion learning induced by blood-borne visceral stimuli. Pharmacol Biochem Behav 69: 157–163, 2001. doi: 10.1016/S0091-3057(01)00494-4. [DOI] [PubMed] [Google Scholar]
- 141.Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Res 839: 323–330, 1999. doi: 10.1016/S0006-8993(99)01745-X. [DOI] [PubMed] [Google Scholar]
- 142.Cunningham CL. Flavor and location aversions produced by ethanol. Behav Neural Biol 27: 362–367, 1979. doi: 10.1016/S0163-1047(79)92440-3. [DOI] [PubMed] [Google Scholar]
- 143.Curtis KS, Sved AF, Verbalis JG, Stricker EM. Lithium chloride-induced anorexia, but not conditioned taste aversions, in rats with area postrema lesions. Brain Res 663: 30–37, 1994. doi: 10.1016/0006-8993(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 144.Da Costa G, Lamy E, Capela e Silva F, Andersen J, Sales Baptista E, Coelho AV. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J Chem Ecol 34: 376–387, 2008. doi: 10.1007/s10886-007-9413-z. [DOI] [PubMed] [Google Scholar]
- 145.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 146.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 147.Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci 24: 11457–11462, 2004. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Darling RA, Ritter S. 2-Deoxy-d-glucose, but not mercaptoacetate, increases food intake in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 297: R382–R386, 2009. doi: 10.1152/ajpregu.90827.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Darwin C. The Expression of the Emotions in Man and Animals. London: John Murray, 1872. doi: 10.1037/10001-000 [DOI] [Google Scholar]
- 150.Davidson TL, Flynn FW, Grill HJ. Comparison of the interoceptive sensory consequences of CCK, LiCl, and satiety in rats. Behav Neurosci 102: 134–140, 1988. doi: 10.1037/0735-7044.102.1.134. [DOI] [PubMed] [Google Scholar]
- 151.Davies BT, Wellman PJ. Conditioned taste reactivity in rats after phenylpropanolamine, d-amphetamine or lithium chloride. Pharmacol Biochem Behav 36: 973–977, 1990. doi: 10.1016/0091-3057(90)90108-T. [DOI] [PubMed] [Google Scholar]
- 152.Davis CM, Riley AL. Conditioned taste aversion learning: implications for animal models of drug abuse. Ann N Y Acad Sci 1187: 247–275, 2010. doi: 10.1111/j.1749-6632.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- 153.Davis JD, Levine MW. A model for the control of ingestion. Psychol Rev 84: 379–412, 1977. doi: 10.1037/0033-295X.84.4.379. [DOI] [PubMed] [Google Scholar]
- 154.Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol Regul Integr Comp Physiol 264: R97–R103, 1993. [DOI] [PubMed] [Google Scholar]
- 155.Dayawansa S, Peckins S, Ruch S, Norgren R. Parabrachial and hypothalamic interaction in sodium appetite. Am J Physiol Regul Integr Comp Physiol 300: R1091–R1099, 2011. doi: 10.1152/ajpregu.00615.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dayawansa S, Ruch S, Norgren R. Parabrachial-hypothalamic interactions are required for normal conditioned taste aversions. Am J Physiol Regul Integr Comp Physiol 306: R190–R200, 2014. doi: 10.1152/ajpregu.00333.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Araujo IE. Gustatory and homeostatic functions of the rodent parabrachial nucleus. Ann N Y Acad Sci 1170: 383–391, 2009. doi: 10.1111/j.1749-6632.2009.03923.x. [DOI] [PubMed] [Google Scholar]
- 158.De Jonghe BC, Lawler MP, Horn CC, Tordoff MG. Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiol Behav 97: 87–90, 2009. doi: 10.1016/j.physbeh.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806: 127–140, 1998. doi: 10.1016/S0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 160.Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol 215: 1–13, 1971. doi: 10.1113/jphysiol.1971.sp009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deloose E, Corsetti M, Van Oudenhove L, Depoortere I, Tack J. Intragastric infusion of the bitter tastant quinine suppresses hormone release and antral motility during the fasting state in healthy female volunteers. Neurogastroenterol Motil 30: e13171, 2018. doi: 10.1111/nmo.13171. [DOI] [PubMed] [Google Scholar]
- 162.Denton D. The Hunger for Salt. Berlin: Springer-Verlag, 1982, p. 650. [Google Scholar]
- 163.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.DeSimone JA, Lyall V, Heck GL, Feldman GM. Acid detection by taste receptor cells. Respir Physiol 129: 231–245, 2001. doi: 10.1016/S0034-5687(01)00293-6. [DOI] [PubMed] [Google Scholar]
- 165.Deutsch JA, Hardy WT. Cholecystokinin produces bait shyness in rats. Nature 266: 196, 1977. doi: 10.1038/266196a0. [DOI] [PubMed] [Google Scholar]
- 166.Di Lorenzo PM, Monroe S. Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Res 763: 167–181, 1997. doi: 10.1016/S0006-8993(97)00217-5. [DOI] [PubMed] [Google Scholar]
- 167.Dicara LV, Wilson LM. Role of gustation in sodium appetite. Physiol Psychol 2: 43–44, 1974. doi: 10.3758/BF03332988. [DOI] [Google Scholar]
- 168.DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci 17: 3826–3839, 1997. doi: 10.1523/JNEUROSCI.17-10-03826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dotson CD, Colbert CL, Garcea M, Smith JC, Spector AC. The consequences of gustatory deafferentation on body mass and feeding patterns in the rat. Am J Physiol Regul Integr Comp Physiol 303: R611–R623, 2012. doi: 10.1152/ajpregu.00633.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dotson CD, Roper SD, Spector AC. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses 30: 593–600, 2005. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Doty RL, Chen JH, Overend J. Taste quality confusions: Influences of age, smoking, ptc taster status, and other subject characteristics. Perception 46: 257–267, 2017. doi: 10.1177/0301006616685577. [DOI] [PubMed] [Google Scholar]
- 172.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 283: 13108–13115, 2008. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Drucker DB, Sclafani A. The role of gastric and postgastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav 61: 351–358, 1997. doi: 10.1016/S0031-9384(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 174.Dunn LT, Everitt BJ. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav Neurosci 102: 3–23, 1988. doi: 10.1037/0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- 175.Dunnet SB, Whishaw IQ, Jones GH, Bunch ST. Behavioural, biochemical and histochemical effects of different neurotoxic amino acids injected into nucleus basalis magnocellularis of rats. Neuroscience 20: 653–669, 1987. doi: 10.1016/0306-4522(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 176.Duong A, Weingarten HP. Dopamine antagonists act on central, but not peripheral, receptors to inhibit sham and real feeding. Physiol Behav 54: 449–454, 1993. doi: 10.1016/0031-9384(93)90234-7. [DOI] [PubMed] [Google Scholar]
- 177.Dutta Banik D, Martin LE, Freichel M, Torregrossa A-M, Medler KF. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci USA 115: E772–E781, 2018. doi: 10.1073/pnas.1718802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dwyer DM. EPS Prize Lecture. Licking and liking: the assessment of hedonic responses in rodents. Q J Exp Psychol (Hove) 65: 371–394, 2012. doi: 10.1080/17470218.2011.652969. [DOI] [PubMed] [Google Scholar]
- 179.Dwyer DM, Boakes RA, Hayward AJ. Reduced palatability in lithium- and activity-based, but not in amphetamine-based, taste aversion learning. Behav Neurosci 122: 1051–1060, 2008. doi: 10.1037/a0012703. [DOI] [PubMed] [Google Scholar]
- 180.Dwyer DM, Gasalla P, Bura S, López M. Flavors paired with internal pain or with nausea elicit divergent types of hedonic responses. Behav Neurosci 131: 235–248, 2017. doi: 10.1037/bne0000197. [DOI] [PubMed] [Google Scholar]
- 181.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305, 2005. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 182.Dyer J, Vayro S, King TP, Shirazi-Beechey SP. Glucose sensing in the intestinal epithelium. Eur J Biochem 270: 3377–3388, 2003. doi: 10.1046/j.1432-1033.2003.03721.x. [DOI] [PubMed] [Google Scholar]
- 183.Eastwood C, Maubach K, Kirkup AJ, Grundy D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci Lett 254: 145–148, 1998. doi: 10.1016/S0304-3940(98)00666-1. [DOI] [PubMed] [Google Scholar]
- 184.Eckel LA, Ossenkopp K-P. Area postrema mediates the formation of rapid, conditioned palatability shifts in lithium-treated rats. Behav Neurosci 110: 202–212, 1996. doi: 10.1037/0735-7044.110.1.202. [DOI] [PubMed] [Google Scholar]
- 185.Eckel LA, Ossenkopp KP. Cholecystokinin reduces ingestive taste reactivity responses to water in fluid-replete but not fluid-deprived rats. Physiol Behav 57: 599–603, 1995. doi: 10.1016/0031-9384(94)00327-2. [DOI] [PubMed] [Google Scholar]
- 186.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ervin GN, Teeter MN. Cholecystokinin octapeptide and lithium produce different effects on feeding and taste aversion learning. Physiol Behav 36: 507–512, 1986. doi: 10.1016/0031-9384(86)90323-9. [DOI] [PubMed] [Google Scholar]
- 188.Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, Carter ME. Agrp neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. J Neurosci 37: 8678–8687, 2017. doi: 10.1523/JNEUROSCI.0798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Evans KR, Vaccarino FJ. Intra-nucleus accumbens amphetamine: dose-dependent effects on food intake. Pharmacol Biochem Behav 25: 1149–1151, 1986. doi: 10.1016/0091-3057(86)90102-4. [DOI] [PubMed] [Google Scholar]
- 190.Even PC, Rolland V, Feurté S, Fromentin G, Roseau S, Nicolaïdis S, Tomé D. Postprandial metabolism and aversive response in rats fed a threonine-devoid diet. Am J Physiol Regul Integr Comp Physiol 279: R248–R254, 2000. doi: 10.1152/ajpregu.2000.279.1.R248. [DOI] [PubMed] [Google Scholar]
- 191.Feurté S, Nicolaidis S, Berridge KC. Conditioned taste aversion in rats for a threonine-deficient diet: demonstration by the taste reactivity test. Physiol Behav 68: 423–429, 2000. doi: 10.1016/S0031-9384(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 192.Feurté S, Tomé D, Gietzen DW, Even PC, Nicolaïdis S, Fromentin G. Feeding patterns and meal microstructure during development of a taste aversion to a threonine devoid diet. Nutr Neurosci 5: 269–278, 2002. doi: 10.1080/10284150290032003. [DOI] [PubMed] [Google Scholar]
- 193.Field KL, Beauchamp GK, Kimball BA, Mennella JA, Bachmanov AA. Bitter avoidance in guinea pigs (Cavia porcellus) and mice (Mus musculus and Peromyscus leucopus). J Comp Psychol 124: 455–459, 2010. doi: 10.1037/a0020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Finger TE. Gustatory nuclei and pathways in the central nervous system. In: Neurobiology of Taste and Smell, edtied by Finger TE, Silver WL. New York: Wiley, 1987, p. 331–354. [Google Scholar]
- 195.Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100: 8981–8986, 2003. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 197.Finger TE, Kinnamon SC. Taste isn’t just for taste buds anymore. F1000 Biol Rep 3: 20, 2011. doi: 10.3410/B3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Flaherty CF. Incentive Relativity. New York: Cambridge Univ. Press, 1996, p. 227. [Google Scholar]
- 199.Flaherty CF, Grigson PS. From contrast to reinforcement: role of response contingency in anticipatory contrast. J Exp Psychol Anim Behav Process 14: 165–176, 1988. doi: 10.1037/0097-7403.14.2.165. [DOI] [PubMed] [Google Scholar]
- 200.Fletcher ML, Ogg MC, Lu L, Ogg RJ, Boughter JD Jr. Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci 37: 7595–7605, 2017. doi: 10.1523/JNEUROSCI.0649-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Flynn FW, Berridge KC, Grill HJ. Pre- and postabsorptive insulin secretion in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 250: R539–R548, 1986. [DOI] [PubMed] [Google Scholar]
- 202.Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci 102: 934–941, 1988. doi: 10.1037/0735-7044.102.6.934. [DOI] [PubMed] [Google Scholar]
- 203.Flynn FW, Grill HJ, Schwartz GJ, Norgren R. Central gustatory lesions: I. Preference and taste reactivity tests. Behav Neurosci 105: 933–943, 1991. doi: 10.1037/0735-7044.105.6.933. [DOI] [PubMed] [Google Scholar]
- 204.Flynn FW, Webster M, Ksir C. Chronic voluntary nicotine drinking enhances nicotine palatability in rats. Behav Neurosci 103: 356–364, 1989. doi: 10.1037/0735-7044.103.2.356. [DOI] [PubMed] [Google Scholar]
- 205.Forestell CA, LoLordo VM. Palatability shifts in taste and flavour preference conditioning. Q J Exp Psychol B 56: 140–160, 2003. doi: 10.1080/02724990244000232. [DOI] [PubMed] [Google Scholar]
- 206.Fortin SM, Chartoff EH, Roitman MF. The aversive agent lithium chloride suppresses phasic dopamine release through central glp-1 receptors. Neuropsychopharmacology 41: 906–915, 2016. doi: 10.1038/npp.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fortin SM, Roitman MF. Physiological state tunes mesolimbic signaling: lessons from sodium appetite and inspiration from Randall R. Sakai. Physiol Behav 178: 21–27, 2017. doi: 10.1016/j.physbeh.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Foster S, Blank K, See Hoe L, Behrens M, Meyerhof W, Peart J, Thomas W. Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB J 28: 4497–4508, 2014. doi: 10.1096/fj.14-256305. [DOI] [PubMed] [Google Scholar]
- 209.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol 428: 558–576, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 210.Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol 65: 1452–1463, 1991. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- 211.Frank ME, Bouverat BP, MacKinnon BI, Hettinger TP. The distinctiveness of ionic and nonionic bitter stimuli. Physiol Behav 80: 421–431, 2004. doi: 10.1016/j.physbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 212.Frankmann SP, Sollars SI, Bernstein IL. Sodium appetite in the sham-drinking rat after chorda tympani nerve transection. Am J Physiol Regul Integr Comp Physiol 271: R339–R345, 1996. [DOI] [PubMed] [Google Scholar]
- 213.Fresquet N, Angst MJ, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behav Brain Res 153: 357–365, 2004. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 214.Frijters JE, Schifferstein HN. Perceptual interactions in mixtures containing bitter tasting substances. Physiol Behav 56: 1243–1249, 1994. doi: 10.1016/0031-9384(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 215.Fromentin G, Gietzen DW, Nicolaidis S. Aversion-preference patterns in amino acid- or protein-deficient rats: a comparison with previously reported responses to thiamin-deficient diets. Br J Nutr 77: 299–314, 1997. doi: 10.1079/BJN19970031. [DOI] [PubMed] [Google Scholar]
- 216.Fry M, Ferguson AV. The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav 91: 413–423, 2007. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 217.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 7: 229–259, 1984. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 218.Furness JB, Koopmans HS, Robbins HL, Clerc N, Tobin JM, Morris MJ. Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton Neurosci 92: 28–36, 2001. doi: 10.1016/S1566-0702(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 219.Fushan AA, Simons CT, Slack JP, Drayna D. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem Senses 35: 579–592, 2010. doi: 10.1093/chemse/bjq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J 22: 1458–1468, 2008. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 221.Galaverna OG, Seeley RJ, Berridge KC, Grill HJ, Epstein AN, Schulkin J. Lesions of the central nucleus of the amygdala. I. Effects on taste reactivity, taste aversion learning and sodium appetite. Behav Brain Res 59: 11–17, 1993. doi: 10.1016/0166-4328(93)90146-H. [DOI] [PubMed] [Google Scholar]
- 222.Gallo M, Roldan G, Bureš J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res 52: 91–97, 1992. doi: 10.1016/S0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- 223.Gámiz F, Gallo M. Intra-amygdala ZIP injections impair the memory of learned active avoidance responses and attenuate conditioned taste-aversion acquisition in rats. Learn Mem 18: 529–533, 2011. doi: 10.1101/lm.2253311. [DOI] [PubMed] [Google Scholar]
- 224.Ganchrow JR, Steiner JE, Canetto S. Behavioral displays to gustatory stimuli in newborn rat pups. Dev Psychobiol 19: 163–174, 1986. doi: 10.1002/dev.420190303. [DOI] [PubMed] [Google Scholar]
- 225.Garcia J, Hankins WG. The evolution of bitter and the acquisition of toxiphobia. Olfaction Taste 5: 39–45, 1975. [Google Scholar]
- 226.Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science 185: 824–831, 1974. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- 227.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122: 157–158, 1955. [PubMed] [Google Scholar]
- 228.Garcia J, Koelling RA. A comparison of aversions induced by x-rays, toxins, and drugs in the rat. Radiat Res Suppl 7: 439–450, 1967. doi: 10.2307/3583736. [DOI] [PubMed] [Google Scholar]
- 229.Garcia J, Kovner R, Green KF. Cue properties vs palatability of flavors in avoidance learning. Psychon Sci 20: 313–314, 1970. doi: 10.3758/BF03329085. [DOI] [Google Scholar]
- 230.Geary N, Smith GP. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav 22: 787–790, 1985. doi: 10.1016/0091-3057(85)90528-3. [DOI] [PubMed] [Google Scholar]
- 231.Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci 122: 1038–1050, 2008. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol 504: 379–403, 2007. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- 233.Geran LC, Garcea M, Spector AC. Nerve regeneration-induced recovery of quinine avoidance after complete gustatory deafferentation of the tongue. Am J Physiol Regul Integr Comp Physiol 287: R1235–R1243, 2004. doi: 10.1152/ajpregu.00137.2004. [DOI] [PubMed] [Google Scholar]
- 234.Geran LC, Garcea M, Spector AC. Transecting the gustatory branches of the facial nerve impairs NH(4)Cl vs. KCl discrimination in rats. Am J Physiol Regul Integr Comp Physiol 283: R739–R747, 2002. doi: 10.1152/ajpregu.00103.2002. [DOI] [PubMed] [Google Scholar]
- 235.Geran LC, Spector AC. Anion size does not compromise sodium recognition by rats after acute sodium depletion. Behav Neurosci 118: 178–183, 2004. doi: 10.1037/0735-7044.118.1.178. [DOI] [PubMed] [Google Scholar]
- 236.Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 101: 1598–1612, 2009. doi: 10.1152/jn.91168.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- 238.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495, 1973. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 239.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. 1973. Obes Res 5: 284–290, 1997. doi: 10.1002/j.1550-8528.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 240.Giduck SA, Threatte RM, Kare MR. Cephalic reflexes: their role in digestion and possible roles in absorption and metabolism. J Nutr 117: 1191–1196, 1987. doi: 10.1093/jn/117.7.1191. [DOI] [PubMed] [Google Scholar]
- 241.Gietzen DW, McArthur LH, Theisen JC, Quinton RR. Learned preference for the limiting amino acid in rats fed a threonine-deficient diet. Physiol Behav 51: 909–914, 1992. doi: 10.1016/0031-9384(92)90069-E. [DOI] [PubMed] [Google Scholar]
- 242.Gilbertson TA, Boughter JD Jr, Zhang H, Smith DV. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci 21: 4931–4941, 2001. doi: 10.1523/JNEUROSCI.21-13-04931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Giza BK, Deems RO, Vanderweele DA, Scott TR. Pancreatic glucagon suppresses gustatory responsiveness to glucose. Am J Physiol Regul Integr Comp Physiol 265: R1231–R1237, 1993. [DOI] [PubMed] [Google Scholar]
- 244.Giza BK, Scott TR. Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars. Am J Physiol Regul Integr Comp Physiol 252: R994–R1002, 1987. [DOI] [PubMed] [Google Scholar]
- 245.Giza BK, Scott TR, Antonucci RF. Effect of cholecystokinin on taste responsiveness in rats. Am J Physiol Regul Integr Comp Physiol 258: R1371–R1379, 1990. [DOI] [PubMed] [Google Scholar]
- 246.Giza BK, Scott TR, Vanderweele DA. Administration of satiety factors and gustatory responsiveness in the nucleus tractus solitarius of the rat. Brain Res Bull 28: 637–639, 1992. doi: 10.1016/0361-9230(92)90116-F. [DOI] [PubMed] [Google Scholar]
- 247.Glenn JF, Erickson RP. Gastric modulation of gustatory afferent activity. Physiol Behav 16: 561–568, 1976. doi: 10.1016/0031-9384(76)90216-X. [DOI] [PubMed] [Google Scholar]
- 248.Glendinning JI. Effect of salivary proline-rich proteins on ingestive responses to tannic acid in mice. Chem Senses 17: 1–12, 1992. doi: 10.1093/chemse/17.1.1. [DOI] [Google Scholar]
- 249.Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav 56: 1217–1227, 1994. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 250.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses 30: 299–316, 2005. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 251.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chem Senses 30: 601–614, 2005. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 252.Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol 309: R552–R560, 2015. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav 93: 757–765, 2008. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology (Berl) 114: 229–232, 1994. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- 255.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300, 1995. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 256.Goodall EB, Carey RJ. Effects of d- versus l-amphetamine, food deprivation, and current intensity on self-stimulation of the lateral hypothalamus, substantia nigra, and medial frontal cortex of the rat. J Comp Physiol Psychol 89: 1029–1045, 1975. doi: 10.1037/h0077187. [DOI] [PubMed] [Google Scholar]
- 257.Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav 101: 731–737, 2010. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett 76: 151–156, 1987. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 259.Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci 111: 129–136, 1997. doi: 10.1037/0735-7044.111.1.129. [DOI] [PubMed] [Google Scholar]
- 260.Grigson PS, Colechio EM, Power ML, Schulkin J, Norgren R. Parabrachial lesions in rats disrupt sodium appetite induced by furosemide but not by calcium deprivation. Physiol Behav 140: 172–179, 2015. doi: 10.1016/j.physbeh.2014.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Grigson PS, Kaplan JM, Roitman MF, Norgren R, Grill HJ. Reward comparison in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 273: R479–R486, 1997. [DOI] [PubMed] [Google Scholar]
- 262.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav Neurosci 112: 160–171, 1998. doi: 10.1037/0735-7044.112.1.160. [DOI] [PubMed] [Google Scholar]
- 263.Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: II. The role of the nucleus of the solitary tract in Na+ appetite, conditioned taste aversion, and conditioned odor aversion in rats. Behav Neurosci 111: 169–179, 1997. doi: 10.1037/0735-7044.111.1.169. [DOI] [PubMed] [Google Scholar]
- 264.Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: III. The role of the nucleus of the solitary tract and the parabrachial nucleus in retention of a conditioned taste aversion in rats. Behav Neurosci 111: 180–187, 1997. doi: 10.1037/0735-7044.111.1.180. [DOI] [PubMed] [Google Scholar]
- 266.Grigson PS, Spector AC, Norgren R. Lesions of the pontine parabrachial nuclei eliminate successive negative contrast effects in rats. Behav Neurosci 108: 714–723, 1994. doi: 10.1037/0735-7044.108.4.714. [DOI] [PubMed] [Google Scholar]
- 266a.Grigson PS, Spector AC, Norgren R. Microstructural analysis of successive negative contrast in free-feeding and deprived rats. Physiol Behav 54: 909–916, 1993. doi: 10.1016/0031-9384(93)90301-U. [DOI] [PubMed] [Google Scholar]
- 267.Grill HJ, Bernstein IL. Strain differences in taste reactivity to NaCl. Am J Physiol Regul Integr Comp Physiol 255: R424–R430, 1988. [DOI] [PubMed] [Google Scholar]
- 268.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol Regul Integr Comp Physiol 246: R88–R95, 1984. [DOI] [PubMed] [Google Scholar]
- 269.Grill HJ, Donahey JC, King L, Kaplan JM. Contribution of caudal brainstem to d-fenfluramine anorexia. Psychopharmacology (Berl) 130: 375–381, 1997. doi: 10.1007/s002130050253. [DOI] [PubMed] [Google Scholar]
- 270.Grill HJ, Berridge KC. Taste reactivity as a measure of the neural control of palatability. Prog Psychobiol Physiol Psychol 11: 1–64, 1985. [Google Scholar]
- 271.Grill HJ, Kaplan JM. Sham feeding in intact and chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 262: R1070–R1074, 1992. [DOI] [PubMed] [Google Scholar]
- 272.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 201: 267–269, 1978. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 273.Grill HJ, Norgren R. Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res 143: 299–312, 1978. doi: 10.1016/0006-8993(78)90570-X. [DOI] [PubMed] [Google Scholar]
- 274.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 275.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 143: 281–297, 1978. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 276.Grill HJ, Schulkin J, Flynn FW. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci 100: 536–543, 1986. doi: 10.1037/0735-7044.100.4.536. [DOI] [PubMed] [Google Scholar]
- 277.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 254: R853–R856, 1988. [DOI] [PubMed] [Google Scholar]
- 278.Grill HJ, Spector AC, Schwartz GF, Kaplan JM, Flynn FW. Evaluating taste effects on ingestive behavior. In: Techniques in the Behavioral and Neural Sciences, edited by Toates F, Rowland N. New York: Elsevier, 1987, p. 151–188. [Google Scholar]
- 279.Grobe CL, Spector AC. Constructing quality profiles for taste compounds in rats: a novel paradigm. Physiol Behav 95: 413–424, 2008. doi: 10.1016/j.physbeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 280.Grundy D. Speculations on the structure/function relationship for vagal and splanchnic afferent endings supplying the gastrointestinal tract. J Auton Nerv Syst 22: 175–180, 1988. doi: 10.1016/0165-1838(88)90104-X. [DOI] [PubMed] [Google Scholar]
- 281.Grundy D. What activates visceral afferents? Gut 53, Suppl 2: ii5–ii8, 2004. doi: 10.1136/gut.2003.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Grupp LA, Linseman MA, Cappell H. Effects of amygdala lesions on taste aversions produced by amphetamine and LiCl. Pharmacol Biochem Behav 4: 541–544, 1976. doi: 10.1016/0091-3057(76)90195-7. [DOI] [PubMed] [Google Scholar]
- 283.Gu F, Liu X, Liang J, Chen J, Chen F, Li F. Bitter taste receptor mTas2r105 is expressed in small intestinal villus and crypts. Biochem Biophys Res Commun 463: 934–941, 2015. doi: 10.1016/j.bbrc.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 284.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 285.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286: R31–R37, 2004. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 286.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182–7190, 1999. doi: 10.1523/JNEUROSCI.19-16-07182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol 78: 920–938, 1997. doi: 10.1152/jn.1997.78.2.920. [DOI] [PubMed] [Google Scholar]
- 288.Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 289.Hamamura T, Lee Y, Ohashi K, Fujiwara Y, Miki M, Suzuki H, Kuroda S. A low dose of lithium chloride selectively induces Fos protein in the central nucleus of the amygdala of rat brain. Prog Neuropsychopharmacol Biol Psychiatry 24: 285–294, 2000. doi: 10.1016/S0278-5846(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 290.Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577, 1984. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- 291.Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res 16: 97–107, 1986. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- 292.Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE. A neural circuit for gut-induced reward. Cell S0092-8674(18)31110-3, 2018. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Han W, Tellez LA, Rangel MJ Jr, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168: 311–324.e18, 2017. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293a.Handel PJ. Immediate acceptance of sodium salts by sodium deficient rats. Psychon Sci 3: 1–12, 1965. [Google Scholar]
- 294.Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 296: R528–R536, 2009. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 294: R33–R38, 2008. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 296.Harder DB, Whitney G. A common polygenic basis for quinine and PROP avoidance in mice. Chem Senses 23: 327–332, 1998. doi: 10.1093/chemse/23.3.327. [DOI] [PubMed] [Google Scholar]
- 297.Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res 711: 125–137, 1996. doi: 10.1016/0006-8993(95)01410-1. [DOI] [PubMed] [Google Scholar]
- 298.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology 148: 4623–4633, 2007. doi: 10.1210/en.2006-1565. [DOI] [PubMed] [Google Scholar]
- 299.Hashikawa K, Naka M, Nakayama D, Matsumoto N, Neve R, Matsuki N. Blockade of stimulus convergence in amygdala neurons disrupts taste associative learning. J Neurosci 33: 4958–4963, 2013. doi: 10.1523/JNEUROSCI.5462-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hashimoto K, Obata K, Ogawa H. Characterization of parabrachial subnuclei in mice with regard to salt tastants: possible independence of taste relay from visceral processing. Chem Senses 34: 253–267, 2009. doi: 10.1093/chemse/bjn085. [DOI] [PubMed] [Google Scholar]
- 301.Hashimoto K, Spector AC. Extensive lesions in the gustatory cortex in the rat do not disrupt the retention of a presurgically conditioned taste aversion and do not impair unconditioned concentration-dependent licking of sucrose and quinine. Chem Senses 39: 57–71, 2014. doi: 10.1093/chemse/bjt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses 33: 255–265, 2008. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- 303.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 305.Heeley N, Blouet C. Central amino acid sensing in the control of feeding behavior. Front Endocrinol (Lausanne) 7: 148, 2016. doi: 10.3389/fendo.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hellekant G, DuBois GE, Roberts TW, der Wel H. On the gustatory effect of amiloride in the monkey (Macaca mulatto). Chem Senses 13: 89–93, 1988. doi: 10.1093/chemse/13.1.89. [DOI] [Google Scholar]
- 307.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 308.Hermann GE, Kohlerman NJ, Rogers RC. Hepatic-vagal and gustatory afferent interactions in the brainstem of the rat. J Auton Nerv Syst 9: 477–495, 1983. doi: 10.1016/0165-1838(83)90008-5. [DOI] [PubMed] [Google Scholar]
- 309.Hermann GE, Rogers RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst 13: 1–17, 1985. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- 310.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42: 1705–1712, 1988. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 311.Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res 513: 24–34, 1990. doi: 10.1016/0006-8993(90)91085-U. [DOI] [PubMed] [Google Scholar]
- 312.Hettinger TP, Gent JF, Marks LE, Frank ME. Study of taste perception. Percept Psychophys 61: 1510–1521, 1999. doi: 10.3758/BF03213114. [DOI] [PubMed] [Google Scholar]
- 313.Hill DL, Almli CR. Parabrachial nuclei damage in infant rats produces residual deficits in gustatory preferences/aversions and sodium appetite. Dev Psychobiol 16: 519–533, 1983. doi: 10.1002/dev.420160608. [DOI] [PubMed] [Google Scholar]
- 314.Hill DL, Formaker BK, White KS. Perceptual characteristics of the amiloride-suppressed sodium chloride taste response in the rat. Behav Neurosci 104: 734–741, 1990. doi: 10.1037/0735-7044.104.5.734. [DOI] [PubMed] [Google Scholar]
- 315.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 316.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Holman GL. Intragastric reinforcement effect. J Comp Physiol Psychol 69: 432–441, 1969. doi: 10.1037/h0028233. [DOI] [PubMed] [Google Scholar]
- 318.Holt J, Antin J, Gibbs J, Young RC, Smith GP. Cholecystokinin does not produce bait shyness in rats. Physiol Behav 12: 497–498, 1974. doi: 10.1016/0031-9384(74)90127-9. [DOI] [PubMed] [Google Scholar]
- 319.Holtz SL, Fu A, Loflin W, Corson JA, Erisir A. Morphology and connectivity of parabrachial and cortical inputs to gustatory thalamus in rats. J Comp Neurol 523: 139–161, 2015. doi: 10.1002/cne.23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hölzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol Gastrointest Liver Physiol 267: G625–G629, 1994. [DOI] [PubMed] [Google Scholar]
- 321.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96: 541–551, 1999. doi: 10.1016/S0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 322.Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y. Sour taste responses in mice lacking PKD channels. PLoS One 6: e20007, 2011. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Horn CC, De Jonghe BC, Matyas K, Norgren R. Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 297: R1375–R1382, 2009. doi: 10.1152/ajpregu.00284.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. [Correction in PloS One 8(6): 2013.] PLoS One 8: e60537, 2013. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Houpt TA, Berlin R, Smith GP. Subdiaphragmatic vagotomy does not attenuate c-Fos induction in the nucleus of the solitary tract after conditioned taste aversion expression. Brain Res 747: 85–91, 1997. doi: 10.1016/S0006-8993(96)01221-8. [DOI] [PubMed] [Google Scholar]
- 326.Hrupka BJ, Lin Y, Gietzen DW, Rogers QR. Lysine deficiency alters diet selection without depressing food intake in rats. J Nutr 129: 424–430, 1999. doi: 10.1093/jn/129.2.424. [DOI] [PubMed] [Google Scholar]
- 327.Hrupka BJ, Lin YM, Gietzen DW, Rogers QR. Small changes in essential amino acid concentrations alter diet selection in amino acid-deficient rats. J Nutr 127: 777–784, 1997. doi: 10.1093/jn/127.5.777. [DOI] [PubMed] [Google Scholar]
- 328.Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacol Biochem Behav 50: 121–125, 1995. doi: 10.1016/0091-3057(95)00315-N. [DOI] [PubMed] [Google Scholar]
- 329.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature 442: 934–938, 2006. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Huang T, Yan J. Dietary sodium deprivation reduces gustatory neural responses of the parabrachial nucleus in rats. Neurosci Lett 432: 170–173, 2008. doi: 10.1016/j.neulet.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 331.Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci 25: 843–847, 2005. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Huffman MA. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc Nutr Soc 62: 371–381, 2003. doi: 10.1079/PNS2003257. [DOI] [PubMed] [Google Scholar]
- 333.Humenick A, Chen BN, Wiklendt L, Spencer NJ, Zagorodnyuk VP, Dinning PG, Costa M, Brookes SJH. Activation of intestinal spinal afferent endings by changes in intra-mesenteric arterial pressure. J Physiol 593: 3693–3709, 2015. doi: 10.1113/JP270378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev 11: 107–130, 1987. doi: 10.1016/S0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- 335.Hunt WA, Rabin BM, Lee J. Effects of subdiaphragmatic vagotomy on the acquisition of a radiation-induced conditioned taste aversion. Neurotoxicol Teratol 9: 75–77, 1987. doi: 10.1016/0892-0362(87)90073-0. [DOI] [PubMed] [Google Scholar]
- 336.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 32: 82–94, 2007. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Inui T, Inui-Yamamoto C, Yoshioka Y, Ohzawa I, Shimura T. Activation of efferents from the basolateral amygdala during the retrieval of conditioned taste aversion. Neurobiol Learn Mem 106: 210–220, 2013. doi: 10.1016/j.nlm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 338.Inui T, Shimura T. Activation of mu-opioid receptors in the ventral pallidum decreases the negative hedonic evaluation of a conditioned aversive taste in rats. Behav Brain Res 320: 391–399, 2017. doi: 10.1016/j.bbr.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 339.Inui T, Shimura T, Yamamoto T. The role of the ventral pallidum GABAergic system in conditioned taste aversion: effects of microinjections of a GABAA receptor antagonist on taste palatability of a conditioned stimulus. Brain Res 1164: 117–124, 2007. doi: 10.1016/j.brainres.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 340.Inui T, Yamamoto T, Shimura T. GABAergic transmission in the rat ventral pallidum mediates a saccharin palatability shift in conditioned taste aversion. Eur J Neurosci 30: 110–115, 2009. doi: 10.1111/j.1460-9568.2009.06800.x. [DOI] [PubMed] [Google Scholar]
- 341.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA 103: 12569–12574, 2006. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Itoga CA, Berridge KC, Aldridge JW. Ventral pallidal coding of a learned taste aversion. Behav Brain Res 300: 175–183, 2016. doi: 10.1016/j.bbr.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Jacobs KM, Mark GP, Scott TR. Taste responses in the nucleus tractus solitarius of sodium-deprived rats. J Physiol 406: 393–410, 1988. doi: 10.1113/jphysiol.1988.sp017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jalowiec JE, Stricker EM. Sodium appetite in adrenalectomized rats following dietary sodium deprivation. J Comp Physiol Psychol 82: 66–77, 1973. doi: 10.1037/h0033798. [DOI] [PubMed] [Google Scholar]
- 345.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 517: 284–292, 2015. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Janssen S, Laermans J, Verhulst P-J, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108: 2094–2099, 2011. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Jarrett MM, Scantlebury J, Parker LA. Effect of delta9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav 90: 425–430, 2007. doi: 10.1016/j.physbeh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 348.Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J 438: 33–37, 2011. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest 118: 3693–3700, 2008. doi: 10.1172/JCI36461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Jiang P, Ji Q, Liu Z, Snyder LA, Benard LMJ, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem 279: 45068–45075, 2004. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 351.Jicha GA, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: possible relation to parkinsonian symptoms. J Neurosci 11: 3822–3829, 1991. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol 271: 519–532, 1988. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 353.Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol Learn Mem 82: 159–163, 2004. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 354.Jung HY, Kim W, Yoo DY, Nam SM, Kim JW, Choi JH, Yoon YS, Kim HY, Hwang IK. Intragastric gavage with denatonium benzoate acutely induces neuronal activation in the solitary tract nucleus via the vagal afferent pathway. J Vet Sci 15: 459–464, 2014. doi: 10.4142/jvs.2014.15.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV. A gut-brain neural circuit for nutrient sensory transduction. Science 361: eaat5236, 2018. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kaku T. Functional differentiation of hypoglossal motoneurons during the amygdaloid or cortically induced rhythmical jaw and tongue movements in the rat. Brain Res Bull 13: 147–154, 1984. doi: 10.1016/0361-9230(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 357.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927, 2012. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kaplan JM, Roitman M, Grill HJ. Food deprivation does not potentiate glucose taste reactivity responses of chronic decerebrate rats. Brain Res 870: 102–108, 2000. doi: 10.1016/S0006-8993(00)02406-9. [DOI] [PubMed] [Google Scholar]
- 359.Kaplan JM, Seeley RJ, Grill HJ. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci 107: 876–881, 1993. doi: 10.1037/0735-7044.107.5.876. [DOI] [PubMed] [Google Scholar]
- 360.Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res 813: 283–302, 1998. doi: 10.1016/S0006-8993(98)00951-2. [DOI] [PubMed] [Google Scholar]
- 361.Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res 957: 193–206, 2002. doi: 10.1016/S0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- 362.Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses 33: 243–254, 2008. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Katz DB, Nicolelis MAL, Simon SA. Nutrient tasting and signaling mechanisms in the gut. IV. There is more to taste than meets the tongue. Am J Physiol Gastrointest Liver Physiol 278: G6–G9, 2000. doi: 10.1152/ajpgi.2000.278.1.G6. [DOI] [PubMed] [Google Scholar]
- 364.Kawai Y, Takagi H, Yanai K, Tohyama M. Adrenergic projection from the caudal part of the nucleus of the tractus solitarius to the parabrachial nucleus in the rat: immunocytochemical study combined with a retrograde tracing method. Brain Res 459: 369–372, 1988. doi: 10.1016/0006-8993(88)90654-3. [DOI] [PubMed] [Google Scholar]
- 365.Kemble ED, Nagel JA. Failure to form a learned taste aversion in rats with amygdaloid lesions. Bull Psychon Soc 2: 155–156, 1973. doi: 10.3758/BF03329231. [DOI] [Google Scholar]
- 366.Kiefer SW, Grijalva CV. Taste reactivity in rats following lesions of the zona incerta or amygdala. Physiol Behav 25: 549–554, 1980. doi: 10.1016/0031-9384(80)90120-1. [DOI] [PubMed] [Google Scholar]
- 367.Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci 106: 140–146, 1992. doi: 10.1037/0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- 368.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388: 377–380, 1997. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 369.Kim KS, Egan JM, Jang HJ. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. [Erratum in Diabetologia 57: 2428, 2014.] Diabetologia 57: 2117–2125, 2014. doi: 10.1007/s00125-014-3326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312: 500–506, 2003. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- 371.King CT, Deyrup LD, Dodson SE, Galvin KE, Garcea M, Spector AC. Effects of gustatory nerve transection and regeneration on quinine-stimulated Fos-like immunoreactivity in the parabrachial nucleus of the rat. J Comp Neurol 465: 296–308, 2003. doi: 10.1002/cne.10851. [DOI] [PubMed] [Google Scholar]
- 372.King CT, Garcea M, Spector AC. Glossopharyngeal nerve regeneration is essential for the complete recovery of quinine-stimulated oromotor rejection behaviors and central patterns of neuronal activity in the nucleus of the solitary tract in the rat. J Neurosci 20: 8426–8434, 2000. doi: 10.1523/JNEUROSCI.20-22-08426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.King CT, Garcea M, Spector AC. Restoration of quinine-stimulated Fos-immunoreactive neurons in the central nucleus of the amygdala and gustatory cortex following reinnervation or cross-reinnervation of the lingual taste nerves in rats. J Comp Neurol 522: 2498–2517, 2014. doi: 10.1002/cne.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.King CT, Hashimoto K, Blonde GD, Spector AC. Unconditioned oromotor taste reactivity elicited by sucrose and quinine is unaffected by extensive bilateral damage to the gustatory zone of the insular cortex in rats. Brain Res 1599: 9–19, 2015. doi: 10.1016/j.brainres.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci 19: 3107–3121, 1999. doi: 10.1523/JNEUROSCI.19-08-03107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.King MS. Distribution of Fos-immunoreactive neurons in the gustatory cortex elicited by intra-oral infusion of taste solutions in conscious rats. Brain Res 1683: 67–77, 2018. doi: 10.1016/j.brainres.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 7: 264, 2013. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283: 236–242, 2001. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- 381.Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr 133: 2331–2335, 2003. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- 382.Koehnle TJ, Stephens AL, Gietzen DW. Threonine-imbalanced diet alters first-meal microstructure in rats. Physiol Behav 81: 15–21, 2004. doi: 10.1016/j.physbeh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 383.Kohl KD, Dearing MD. Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores. Ecol Lett 15: 1008–1015, 2012. doi: 10.1111/j.1461-0248.2012.01822.x. [DOI] [PubMed] [Google Scholar]
- 384.Kollarik M, Ru F, Brozmanova M. Vagal afferent nerves with the properties of nociceptors. Auton Neurosci 153: 12–20, 2010. doi: 10.1016/j.autneu.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res 379: 342–352, 1986. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- 386.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722, 1977. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 387.Kringelbach ML, Berridge KC. The neuroscience of happiness and pleasure. Soc Res (New York) 77: 659–678, 2010. [PMC free article] [PubMed] [Google Scholar]
- 388.Kroeze JH, Bartoshuk LM. Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiol Behav 35: 779–783, 1985. doi: 10.1016/0031-9384(85)90412-3. [DOI] [PubMed] [Google Scholar]
- 389.Kulkosky PJ, Gray L, Gibbs J, Smith GP. Feeding and selection of saccharin after injections of bombesin, LiCl, and NaCl. Peptides 2: 61–64, 1981. doi: 10.1016/S0196-9781(81)80012-5. [DOI] [PubMed] [Google Scholar]
- 390.Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol 591: 1967–1985, 2013. doi: 10.1113/jphysiol.2012.236604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kwon B, Goltz M, Houpt TA. Expression of AP-1 family transcription factors in the amygdala during conditioned taste aversion learning: role for Fra-2. Brain Res 1207: 128–141, 2008. doi: 10.1016/j.brainres.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217, 2012. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem 3: 31–41, 1996. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- 394.Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci 17: 8443–8450, 1997. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol Biochem Behav 68: 603–610, 2001. doi: 10.1016/S0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 396.Landry SO., Jr The rodentia as omnivores. Q Rev Biol 45: 351–372, 1970. doi: 10.1086/406647. [DOI] [PubMed] [Google Scholar]
- 397.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 398.Larson ED, Vandenbeuch A, Voigt A, Meyerhof W, Kinnamon SC, Finger TE. The role of 5-ht3 receptors in signaling from taste buds to nerves. J Neurosci 35: 15984–15995, 2015. doi: 10.1523/JNEUROSCI.1868-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lasiter PS. Postnatal development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Bull 28: 667–677, 1992. doi: 10.1016/0361-9230(92)90245-S. [DOI] [PubMed] [Google Scholar]
- 400.Lasiter PS, Glanzman DL. Axon collaterals of pontine taste area neurons project to the posterior ventromedial thalamic nucleus and to the gustatory neocortex. Brain Res 258: 299–304, 1983. doi: 10.1016/0006-8993(83)91155-1. [DOI] [PubMed] [Google Scholar]
- 401.Lasiter PS, Glanzman DL. Cortical substrates of taste aversion learning: involvement of dorsolateral amygdaloid nuclei and temporal neocortex in taste aversion learning. Behav Neurosci 99: 257–276, 1985. doi: 10.1037/0735-7044.99.2.257. [DOI] [PubMed] [Google Scholar]
- 402.Lasiter PS, Glanzman DL, Mensah PA. Direct connectivity between pontine taste areas and gustatory neocortex in rat. Brain Res 234: 111–121, 1982. doi: 10.1016/0006-8993(82)90476-0. [DOI] [PubMed] [Google Scholar]
- 403.Lasiter PS, Kachele DL. Organization of GABA and GABA-transaminase containing neurons in the gustatory zone of the nucleus of the solitary tract. Brain Res Bull 21: 623–636, 1988. doi: 10.1016/0361-9230(88)90202-X. [DOI] [PubMed] [Google Scholar]
- 404.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58: 1058–1066, 2009. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol 93: 538–547, 1979. doi: 10.1037/h0077582. [DOI] [PubMed] [Google Scholar]
- 407.Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJP. Rewiring the taste system. Nature 548: 330–333, 2017. doi: 10.1038/nature23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Lee RJ, Cohen NA. Taste receptors in innate immunity. Cell Mol Life Sci 72: 217–236, 2015. doi: 10.1007/s00018-014-1736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Leeb K, Parker L, Eikelboom R. Effects of pimozide on the hedonic properties of sucrose: analysis by the taste reactivity test. Pharmacol Biochem Behav 39: 895–901, 1991. doi: 10.1016/0091-3057(91)90050-C. [DOI] [PubMed] [Google Scholar]
- 410.Lehman CD, Bartoshuk LM, Catalanotto FC, Kveton JF, Lowlicht RA. Effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiol Behav 57: 943–951, 1995. doi: 10.1016/0031-9384(95)91121-R. [DOI] [PubMed] [Google Scholar]
- 411.Lester D, Nachman M, Le Magnen J. Aversive conditioning by ethanol in the rat. Q J Stud Alcohol 31: 578–586, 1970. [PubMed] [Google Scholar]
- 412.Lett BT. The painlike effect of gallamine and naloxone differs from sickness induced by lithium chloride. Behav Neurosci 99: 145–150, 1985. doi: 10.1037/0735-7044.99.1.145. [DOI] [PubMed] [Google Scholar]
- 413.Lett BT, Grant VL. Conditioned taste preference produced by pairing a taste with a low dose of morphine or sufentanil. Psychopharmacology (Berl) 98: 236–239, 1989. doi: 10.1007/BF00444697. [DOI] [PubMed] [Google Scholar]
- 414.Leung PM, Rogers QR. Effect of amino acid imbalance and deficiency on dietary choice patterns of rats. Physiol Behav 37: 747–758, 1986. doi: 10.1016/0031-9384(86)90180-0. [DOI] [PubMed] [Google Scholar]
- 415.Li BH, Rowland NE. Peripherally and centrally administered bombesin induce Fos-like immunoreactivity in different brain regions in rats. Regul Pept 62: 167–172, 1996. doi: 10.1016/0167-0115(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 416.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm Genome 12: 13–16, 2001. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 291: R1236–R1239, 2006. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 418.Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron 81: 984–1000, 2014. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process 26: 371–384, 2000. doi: 10.1037/0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- 419a.Limebeer CL, Parker LA, Fletcher PJ. 5,7-Dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Neurosci 118: 1391–1399, 2004. [DOI] [PubMed] [Google Scholar]
- 420.Lin J-Y, Arthurs J, Reilly S. Conditioned taste aversion, drugs of abuse and palatability. Neurosci Biobehav Rev 45: 28–45, 2014. doi: 10.1016/j.neubiorev.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lin J-Y, Arthurs J, Reilly S. Gustatory insular cortex, aversive taste memory and taste neophobia. Neurobiol Learn Mem 119: 77–84, 2015. doi: 10.1016/j.nlm.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Lin J-Y, Reilly S. Amygdala-gustatory insular cortex connections and taste neophobia. Behav Brain Res 235: 182–188, 2012. doi: 10.1016/j.bbr.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Lin JY, Arthurs J, Reilly S. Reduced palatability in pain-induced conditioned taste aversions. Physiol Behav 119: 79–85, 2013. doi: 10.1016/j.physbeh.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Lin JY, Roman C, Reilly S. Morphine-induced suppression of conditioned stimulus intake: effects of stimulus type and insular cortex lesions. Brain Res 1292: 52–60, 2009. doi: 10.1016/j.brainres.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 426.Liszt KI, Ley JP, Lieder B, Behrens M, Stöger V, Reiner A, Hochkogler CM, Köck E, Marchiori A, Hans J, Widder S, Krammer G, Sanger GJ, Somoza MM, Meyerhof W, Somoza V. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc Natl Acad Sci USA 114: E6260–E6269, 2017. doi: 10.1073/pnas.1703728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Liu X, Gu F, Jiang L, Chen F, Li F. Expression of bitter taste receptor Tas2r105 in mouse kidney. Biochem Biophys Res Commun 458: 733–738, 2015. doi: 10.1016/j.bbrc.2015.01.089. [DOI] [PubMed] [Google Scholar]
- 428.Logue AW. Taste aversion and the generality of the laws of learning. Psychol Bull 86: 276–296, 1979. doi: 10.1037/0033-2909.86.2.276. [DOI] [Google Scholar]
- 429.Lorden JF. Effects of lesions of the gustatory necortex on taste aversion learning in the rat. J Comp Physiol Psychol 90: 665–679, 1976. doi: 10.1037/h0077237. [DOI] [PubMed] [Google Scholar]
- 430.Lorenzo PMD. Long-delay learning in rats with parabrachial pontine lesions. Chem Senses 13: 219–229, 1988. doi: 10.1093/chemse/13.2.219. [DOI] [Google Scholar]
- 431.Lucas F, Sclafani A. Flavor preferences conditioned by intragastric fat infusions in rats. Physiol Behav 46: 403–412, 1989. doi: 10.1016/0031-9384(89)90011-5. [DOI] [PubMed] [Google Scholar]
- 432.Lush IE. The genetics of tasting in mice. I. Sucrose octaacetate. Genet Res 38: 93–95, 1981. doi: 10.1017/S0016672300020425. [DOI] [PubMed] [Google Scholar]
- 433.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav 24: 71–78, 1986. doi: 10.1016/0091-3057(86)90047-X. [DOI] [PubMed] [Google Scholar]
- 435.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32: 2267–2278, 2007. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 436.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res 551: 308–310, 1991. doi: 10.1016/0006-8993(91)90946-S. [DOI] [PubMed] [Google Scholar]
- 438.Markison S, Gietzen DW, Spector AC. Essential amino acid deficiency enhances long-term intake but not short-term licking of the required nutrient. J Nutr 129: 1604–1612, 1999. doi: 10.1093/jn/129.8.1604. [DOI] [PubMed] [Google Scholar]
- 439.Markison S, St John SJ, Spector AC. Glossopharyngeal nerve transection does not compromise the specificity of taste-guided sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol 269: R215–R221, 1995. [DOI] [PubMed] [Google Scholar]
- 440.Markison S, Thompson BL, Smith JC, Spector AC. Time course and pattern of compensatory ingestive behavioral adjustments to lysine deficiency in rats. J Nutr 130: 1320–1328, 2000. doi: 10.1093/jn/130.5.1320. [DOI] [PubMed] [Google Scholar]
- 441.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6: e24014, 2011. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Martin JR, Cheng FY, Novin D. Acquisition of learned taste aversion following bilateral subdiaphragmatic vagotomy in rats. Physiol Behav 21: 13–17, 1978. doi: 10.1016/0031-9384(78)90269-X. [DOI] [PubMed] [Google Scholar]
- 443.Martínez-Hernández J, Lanuza E, Martínez-García F. Lesions of the dopaminergic innervation of the nucleus accumbens medial shell delay the generation of preference for sucrose, but not of sexual pheromones. Behav Brain Res 226: 538–547, 2012. doi: 10.1016/j.bbr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 444.Martínez-Hernández J, Lanuza E, Martínez-García F. Selective dopaminergic lesions of the ventral tegmental area impair preference for sucrose but not for male sexual pheromones in female mice. Eur J Neurosci 24: 885–893, 2006. doi: 10.1111/j.1460-9568.2006.04944.x. [DOI] [PubMed] [Google Scholar]
- 445.Masaki T, Nakajima S. Taste aversion in rats induced by forced swimming, voluntary running, forced running, and lithium chloride injection treatments. Physiol Behav 88: 411–416, 2006. doi: 10.1016/j.physbeh.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 446.Masuho I, Tateyama M, Saitoh O. Characterization of bitter taste responses of intestinal STC-1 cells. Chem Senses 30: 281–290, 2005. doi: 10.1093/chemse/bji022. [DOI] [PubMed] [Google Scholar]
- 447.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459: 837–841, 2009. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature 404: 601–604, 2000. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 449.Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 97: 406–413, 1997. doi: 10.1016/S0002-8223(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 450.Mattes RD, Arnold C, Boraas M. Learned food aversions among cancer chemotherapy patients. Incidence, nature, and clinical implications. Cancer 60: 2576–2580, 1987. doi:. [DOI] [PubMed] [Google Scholar]
- 451.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 452.May OL, Erisir A, Hill DL. Ultrastructure of primary afferent terminals and synapses in the rat nucleus of the solitary tract: comparison among the greater superficial petrosal, chorda tympani, and glossopharyngeal nerves. J Comp Neurol 502: 1066–1078, 2007. doi: 10.1002/cne.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol 497: 658–669, 2006. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.McCutcheon B, Levy C. Relationship between NaCl rewarded bar-pressing and duration of sodium deficiency. Physiol Behav 8: 761–763, 1972. doi: 10.1016/0031-9384(72)90108-4. [DOI] [PubMed] [Google Scholar]
- 455.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci 6: 137, 2012. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.McDonald RV, Parker LA, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol Biochem Behav 58: 1003–1008, 1997. doi: 10.1016/S0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- 457.McMahon LR, Wellman PJ. Decreased intake of a liquid diet in nonfood-deprived rats following intra-PVN injections of GLP-1 (7-36) amide. Pharmacol Biochem Behav 58: 673–677, 1997. doi: 10.1016/S0091-3057(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 458.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998. [DOI] [PubMed] [Google Scholar]
- 459.Mediavilla C, Bernal A, Mahía J, Puerto A. Nucleus of the solitary tract and flavor aversion learning: relevance in concurrent but not sequential behavioral test. Behav Brain Res 223: 287–292, 2011. doi: 10.1016/j.bbr.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 460.Mediavilla C, Molina F, Puerto A. The role of the lateral parabrachial nuclei in concurrent and sequential taste aversion learning in rats. Exp Brain Res 134: 497–505, 2000. doi: 10.1007/s002210000497. [DOI] [PubMed] [Google Scholar]
- 461.Mei N. Recent studies on intestinal vagal afferent innervation. Functional implications. J Auton Nerv Syst 9: 199–206, 1983. doi: 10.1016/0165-1838(83)90141-8. [DOI] [PubMed] [Google Scholar]
- 462.Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol 282: 485–506, 1978. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Meiselman HL, Dzendolet E. Variability in gustatory quality identification. Percept Psychophys 2: 496–498, 1967. doi: 10.3758/BF03210253. [DOI] [Google Scholar]
- 464.Mélone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst 17: 231–241, 1986. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- 465.Melton PM, Kopman JA, Riley AL. Cholecystokinin as a stimulus in drug discrimination learning. Pharmacol Biochem Behav 44: 249–252, 1993. doi: 10.1016/0091-3057(93)90458-6. [DOI] [PubMed] [Google Scholar]
- 466.Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol 255: 439–450, 1987. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 467.Menétrey D, De Pommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. Eur J Neurosci 3: 249–259, 1991. doi: 10.1111/j.1460-9568.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 468.Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clin Ther 35: 1225–1246, 2013. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35: 157–170, 2010. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 470.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol 15: 301–320, 1994. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- 471.Miller IJ., Jr Branched chorda tympani neurons and interactions among taste receptors. J Comp Neurol 158: 155–166, 1974. doi: 10.1002/cne.901580204. [DOI] [PubMed] [Google Scholar]
- 472.Miller IJ., Jr Peripheral interactions among single papilla inputs to gustatory nerve fibers. J Gen Physiol 57: 1–25, 1971. doi: 10.1085/jgp.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Miller IJ., Jr Taste bud distribution and regional responsiveness on the anterior tongue of the rat. Physiol Behav 16: 439–444, 1976. doi: 10.1016/0031-9384(76)90322-X. [DOI] [PubMed] [Google Scholar]
- 474.Miller JIJ Jr, Spangler KM. Taste bud distribution and innervation on the palate of the rat. Chem Senses 7: 99–108, 1982. doi: 10.1093/chemse/7.1.99. [DOI] [Google Scholar]
- 475.Miranda MI, Ferreira G, Ramírez-Lugo L, Bermúdez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc Natl Acad Sci USA 99: 11417–11422, 2002. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Mistretta CM, Baum BJ. Quantitative study of taste buds in fungiform and circumvallate papillae of young and aged rats. J Anat 138: 323–332, 1984. [PMC free article] [PubMed] [Google Scholar]
- 477.Mitchell D, Laycock JD, Stephens WF. Motion sickness-induced pica in the rat. Am J Clin Nutr 30: 147–150, 1977. doi: 10.1093/ajcn/30.2.147. [DOI] [PubMed] [Google Scholar]
- 477a.Mitchell D, Winter W, Morisaki CM. Conditioned taste aversions accompanied by geophagia: evidence for the occurrence of “physiological” factors in the etiology of pica. Psychosom Med 39: 401–412, 1977. https://www.ncbi.nlm.nih.gov/pubmed/563606. [PubMed] [Google Scholar]
- 478.Miyata S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci 9: 390, 2015. doi: 10.3389/fnins.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Mogenson GJ. Limbic‐motor intergration. Prog Psychobiol Physiol Psychol 12: 117–170, 1987. [Google Scholar]
- 480.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 481.Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol 12: 366–371, 2002. doi: 10.1016/S0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- 482.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272: R1245–R1251, 1997. [DOI] [PubMed] [Google Scholar]
- 483.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526: 95–102, 1990. doi: 10.1016/0006-8993(90)90253-8. [DOI] [PubMed] [Google Scholar]
- 484.Mori M, Kawada T, Ono T, Torii K. Taste preference and protein nutrition and L-amino acid homeostasis in male Sprague-Dawley rats. Physiol Behav 49: 987–995, 1991. doi: 10.1016/0031-9384(91)90212-7. [DOI] [PubMed] [Google Scholar]
- 485.Morice AH, Bennett RT, Chaudhry MA, Cowen ME, Griffin SC, Loubani M. Effect of bitter tastants on human bronchi. Nat Med 17: 775, 2011. doi: 10.1038/nm0711-775. [DOI] [PubMed] [Google Scholar]
- 486.Moriyama Y. Rhythmical jaw movements and lateral ponto-medullary reticular neurons in rats. Comp Biochem Physiol A 86: 7–14, 1987. doi: 10.1016/0300-9629(87)90268-4. [DOI] [PubMed] [Google Scholar]
- 487.Morris MJ, Na ES, Johnson AK. Salt craving: the psychobiology of pathogenic sodium intake. Physiol Behav 94: 709–721, 2008. doi: 10.1016/j.physbeh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Morris R, Frey S, Kasambira T, Petrides M. Ibotenic acid lesions of the basolateral, but not the central, amygdala interfere with conditioned taste aversion: evidence from a combined behavioral and anatomical tract-tracing investigation. Behav Neurosci 113: 291–302, 1999. doi: 10.1037/0735-7044.113.2.291. [DOI] [PubMed] [Google Scholar]
- 489.Morrison GR. Behavioural response patterns to salt stimuli in the rat. Can J Psychol 21: 141–152, 1967. doi: 10.1037/h0082973. [DOI] [Google Scholar]
- 490.Morrison GR. Taste thresholds, taste sensitivity and the effects of adrenalectomy in rats. Chem Senses 1: 77–88, 1974. doi: 10.1093/chemse/1.1.77. [DOI] [Google Scholar]
- 491.Morrison SD, Barrnett RJ, Mayer J. Localization of lesions in the lateral hypothalamus of rats with induced adipsia and aphagia. Am J Physiol 193: 230–234, 1958. [DOI] [PubMed] [Google Scholar]
- 492.Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol Behav 77: 723–729, 2002. doi: 10.1016/S0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- 493.Myers KP, Hall WG. Conditioned changes in appetitive and consummatory responses to flavors paired with oral or nutrient reinforcement among adult rats. Physiol Behav 68: 603–610, 2000. doi: 10.1016/S0031-9384(99)00209-7. [DOI] [PubMed] [Google Scholar]
- 494.Myers KP, Sclafani A. Conditioned acceptance and preference but not altered taste reactivity responses to bitter and sour flavors paired with intragastric glucose infusion. Physiol Behav 78: 173–183, 2003. doi: 10.1016/S0031-9384(02)00890-9. [DOI] [PubMed] [Google Scholar]
- 495.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose. II. Taste reactivity analysis. Physiol Behav 74: 495–505, 2001. doi: 10.1016/S0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- 496.Nachman M. Taste preferences for sodium salts by adrenalectomized rats. J Comp Physiol Psychol 55: 1124–1129, 1962. doi: 10.1037/h0041348. [DOI] [PubMed] [Google Scholar]
- 497.Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J Comp Physiol Psychol 87: 622–643, 1974. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- 498.Naito-Hoopes M, McArthur LH, Gietzen DW, Rogers QR. Learned preference and aversion for complete and isoleucine-devoid diets in rats. Physiol Behav 53: 485–494, 1993. doi: 10.1016/0031-9384(93)90142-3. [DOI] [PubMed] [Google Scholar]
- 499.Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am J Physiol Regul Integr Comp Physiol 269: R647–R661, 1995. [DOI] [PubMed] [Google Scholar]
- 500.Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res 36: 297–309, 2000. doi: 10.1016/S0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 501.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 416: 199–202, 2002. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 502.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 504.Nelson TM, Munger SD, Boughter JD Jr. Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses 28: 695–704, 2003. doi: 10.1093/chemse/bjg062. [DOI] [PubMed] [Google Scholar]
- 505.Nerad L, Ramírez-Amaya V, Ormsby CE, Bermúdez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol Learn Mem 66: 44–50, 1996. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- 506.Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr 130, Suppl: 971S–973S, 2000. doi: 10.1093/jn/130.4.971S. [DOI] [PubMed] [Google Scholar]
- 507.Niijima A, Yamamoto T. The effects of lithium chloride on the activity of the afferent nerve fibers from the abdominal visceral organs in the rat. Brain Res Bull 35: 141–145, 1994. doi: 10.1016/0361-9230(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 508.Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res 451: 319–325, 1988. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- 509.Nishijo H, Ono T, Uwano T, Kondoh T, Torii K. Hypothalamic and amygdalar neuronal responses to various tastant solutions during ingestive behavior in rats. J Nutr 130, Suppl: 954S–959S, 2000. doi: 10.1093/jn/130.4.954S. [DOI] [PubMed] [Google Scholar]
- 510.Nishijo H, Uwano T, Tamura R, Ono T. Gustatory and multimodal neuronal responses in the amygdala during licking and discrimination of sensory stimuli in awake rats. J Neurophysiol 79: 21–36, 1998. doi: 10.1152/jn.1998.79.1.21. [DOI] [PubMed] [Google Scholar]
- 511.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: a two-carbohydrate taste model. Neurosci Biobehav Rev 11: 187–196, 1987. doi: 10.1016/S0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- 512.Norgren R. Gustatory afferents to ventral forebrain. Brain Res 81: 285–295, 1974. doi: 10.1016/0006-8993(74)90942-1. [DOI] [PubMed] [Google Scholar]
- 513.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 514.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 515.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol 150: 217–237, 1973. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- 516.Norgren R, Leonard CM. Taste pathways in rat brainstem. Science 173: 1136–1139, 1971. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- 517.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 91: 99–117, 1975. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- 518.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 519.Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res 92: 123–129, 1975. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- 520.Nowlis GH, Frank ME, Pfaffmann C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psychol 94: 932–942, 1980. doi: 10.1037/h0077809. [DOI] [PubMed] [Google Scholar]
- 521.Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chordat typani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol 199: 223–240, 1968. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol 296: R960–R971, 2009. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature 494: 472–475, 2013. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Olds J. Brain stimulation and the motivation of behavior. Prog Brain Res 45: 401–426, 1976. doi: 10.1016/S0079-6123(08)61001-8. [DOI] [PubMed] [Google Scholar]
- 525.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419–427, 1954. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 526.Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, Desimone JA, Nicolelis MA, Simon SA. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci USA 106: 1596–1601, 2009. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Ossenkopp KP, Eckel LA. Toxin-induced conditioned changes in taste reactivity and the role of the chemosensitive area postrema. Neurosci Biobehav Rev 19: 99–108, 1995. doi: 10.1016/0149-7634(94)00024-U. [DOI] [PubMed] [Google Scholar]
- 528.Ossenkopp KP, Parker LA, Limebeer CL, Burton P, Fudge MA, Cross-Mellor SK. Vestibular lesions selectively abolish body rotation-induced, but not lithium-induced, conditioned taste aversions (oral rejection responses) in rats. Behav Neurosci 117: 105–112, 2003. doi: 10.1037/0735-7044.117.1.105. [DOI] [PubMed] [Google Scholar]
- 529.Ossenkopp KP, Parker LA, Spector AC. Behavioral, neural, and pharmacological aspects of palatability: current research on taste reactivity. In: Proceedings of a Satellite Symposium to the 23rd Annual Meeting of the Society for Neuroscience. Washington, DC: Soc. Neurosci, 1993, p. 87–88. [Google Scholar]
- 530.Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol 281: G1449–G1459, 2001. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- 531.Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol 82: 2210–2220, 1999. doi: 10.1152/jn.1999.82.5.2210. [DOI] [PubMed] [Google Scholar]
- 533.Parker L. Emetic drugs produce conditioned rejection reactions in the taste reactivity test. J Psychophysiol 12 Suppl 1: 3–13, 1998. [Google Scholar]
- 534.Parker L, Leeb K. Amphetamine-induced modification of quinine palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav 47: 413–420, 1994. doi: 10.1016/0091-3057(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 535.Parker LA. LSD produces place preference and flavor avoidance but does not produce flavor aversion in rats. Behav Neurosci 110: 503–508, 1996. doi: 10.1037/0735-7044.110.3.503. [DOI] [PubMed] [Google Scholar]
- 536.Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev 19: 143–151, 1995. doi: 10.1016/0149-7634(94)00028-Y. [DOI] [PubMed] [Google Scholar]
- 537.Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav 31: 165–172, 2003. doi: 10.3758/BF03195979. [DOI] [PubMed] [Google Scholar]
- 538.Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci 107: 118–129, 1993. doi: 10.1037/0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- 539.Parker LA. Taste reactivity responses elicited by reinforcing drugs: a dose-response analysis. Behav Neurosci 105: 955–964, 1991. doi: 10.1037/0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- 540.Parker LA, Brosseau L. Apomorphine-induced flavor-drug associations: a dose-response analysis by the taste reactivity test and the conditioned taste avoidance test. Pharmacol Biochem Behav 35: 583–587, 1990. doi: 10.1016/0091-3057(90)90294-R. [DOI] [PubMed] [Google Scholar]
- 541.Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine- and amphetamine-paired sucrose solution. Pharmacol Biochem Behav 24: 883–887, 1986. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- 542.Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci 109: 71–78, 1995. doi: 10.1037/0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- 543.Parker LA, Limebeer CL, Simpson GR. Chlordiazepoxide-induced conditioned place and taste aversion learning in rats. Pharmacol Biochem Behav 59: 33–37, 1998. doi: 10.1016/S0091-3057(97)00333-X. [DOI] [PubMed] [Google Scholar]
- 544.Parker LA, Lopez N Jr. Pimozide enhances the aversiveness of quinine solution. Pharmacol Biochem Behav 36: 653–659, 1990. doi: 10.1016/0091-3057(90)90271-I. [DOI] [PubMed] [Google Scholar]
- 545.Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci 106: 999–1010, 1992. doi: 10.1037/0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- 546.Parker LA, Rennie M. Naltrexone-induced aversions: assessment by place conditioning, taste reactivity, and taste avoidance paradigms. Pharmacol Biochem Behav 41: 559–565, 1992. doi: 10.1016/0091-3057(92)90373-N. [DOI] [PubMed] [Google Scholar]
- 547.Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25: 11777–11786, 2005. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000. doi: 10.1016/S0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 549.Peciña S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav 58: 801–811, 1997. doi: 10.1016/S0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 550.Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol 97: 140–153, 1983. doi: 10.1037/0735-7036.97.2.140. [DOI] [PubMed] [Google Scholar]
- 551.Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJP, Zuker CS. Sweet and bitter taste in the brain of awake behaving animals. Nature 527: 512–515, 2015. doi: 10.1038/nature15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 374: 387–420, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 553.Pfaffmann C, Frank M, Norgren R. Neural mechanisms and behavioral aspects of taste. Annu Rev Psychol 30: 283–325, 1979. doi: 10.1146/annurev.ps.30.020179.001435. [DOI] [PubMed] [Google Scholar]
- 554.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite 50: 194–206, 2008. doi: 10.1016/j.appet.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Powley TL, Berthoud HR. Diet and cephalic phase insulin responses. Am J Clin Nutr 42, Suppl: 991–1002, 1985. doi: 10.1093/ajcn/42.5.991. [DOI] [PubMed] [Google Scholar]
- 556.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519: 644–660, 2011. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Prandi S, Bromke M, Hübner S, Voigt A, Boehm U, Meyerhof W, Behrens M. A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS One 8: e82820, 2013. doi: 10.1371/journal.pone.0082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Prescott J, Ripandelli N, Wakeling I. Binary taste mixture interactions in prop non-tasters, medium-tasters and super-tasters. Chem Senses 26: 993–1003, 2001. doi: 10.1093/chemse/26.8.993. [DOI] [PubMed] [Google Scholar]
- 559.Quartermain D, Miller NE, Wolf G. Role of experience in relationship between sodium deficiency and rate of bar pressing for salt. J Comp Physiol Psychol 63: 417–420, 1967. doi: 10.1037/h0024611. [DOI] [PubMed] [Google Scholar]
- 560.Racké K, Schwörer H. Regulation of serotonin release from the intestinal mucosa. Pharmacol Res 23: 13–25, 1991. doi: 10.1016/S1043-6618(05)80101-X. [DOI] [PubMed] [Google Scholar]
- 561.Radhakishun FS, Korf J, Venema K, Westerink BH. The release of endogenous dopamine and its metabolites from rat striatum as detected in push-pull perfusates: effects of systematically administered drugs. Pharm Weekbl Sci 5: 153–158, 1983. doi: 10.1007/BF01961473. [DOI] [PubMed] [Google Scholar]
- 562.Rana SA, Parker LA. Differential effects of neurotoxin-induced lesions of the basolateral amygdala and central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behav Brain Res 189: 284–297, 2008. doi: 10.1016/j.bbr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 563.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284: G367–G372, 2003. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 564.Raybould HE, Hölzer H. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett 141: 236–238, 1992. doi: 10.1016/0304-3940(92)90902-J. [DOI] [PubMed] [Google Scholar]
- 565.Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Anim Learn Behav 5: 343–346, 1977. doi: 10.3758/BF03209576. [DOI] [Google Scholar]
- 566.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol 286: R1005–R1012, 2004. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- 567.Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev 29: 1067–1088, 2005. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 568.Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav Neurosci 107: 1005–1017, 1993. doi: 10.1037/0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- 569.Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: aversive and appetitive gustatory conditioning. Brain Res Bull 52: 269–278, 2000. doi: 10.1016/S0361-9230(00)00263-X. [DOI] [PubMed] [Google Scholar]
- 570.Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: neophobia and conditioned taste aversion. Brain Res Bull 55: 359–366, 2001. doi: 10.1016/S0361-9230(01)00517-2. [DOI] [PubMed] [Google Scholar]
- 571.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47: 1592–1601, 2004. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 572.Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 221: 2937–2962, 2016. doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Revusky S, Martin GM. Glucocorticoids attenuate taste aversions produced by toxins in rats. Psychopharmacology (Berl) 96: 400–407, 1988. doi: 10.1007/BF00216070. [DOI] [PubMed] [Google Scholar]
- 574.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 21: 3261–3270, 2001. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci 17: 2187–2200, 2003. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- 576.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci 22: 7308–7320, 2002. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153: 1–26, 1978. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 578.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155: 4356–4367, 2014. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol 115: 155–161, 1936. [Google Scholar]
- 580.Richter CP. Salt appetite of mammals: its dependence on instinct and metabolism. In: L'instinct dans le Comportement des Animaux et de l'homme, edited by Fondation Singer-Polignac Paris: Masson et Cie, 1956, p. 577–629. [Google Scholar]
- 581.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol 547: 475–483, 2003. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol 547: 475–483, 2003. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Riley AL, Tuck DL. Conditioned taste aversions: a behavioral index of toxicity. Ann N Y Acad Sci 443, 1 Experimental: 272–292, 1985. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- 584.Riley CA, King MS. Differential effects of electrical stimulation of the central amygdala and lateral hypothalamus on fos-immunoreactive neurons in the gustatory brainstem and taste reactivity behaviors in conscious rats. Chem Senses 38: 705–717, 2013. doi: 10.1093/chemse/bjt039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol Regul Integr Comp Physiol 277: R1537–R1540, 1999. [DOI] [PubMed] [Google Scholar]
- 587.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23: 10084–10092, 2003. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 277: R582–R590, 1999. [DOI] [PubMed] [Google Scholar]
- 589.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol 293: R1495–R1503, 2007. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 590.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 24: 2782–2786, 2004. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci 119: 5–15, 2005. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- 592.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140: 1687–1694, 1999. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 593.Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 9: e89977, 2014. doi: 10.1371/journal.pone.0089977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Rodgers WL. Specificity of specific hungers. J Comp Physiol Psychol 64: 49–58, 1967. doi: 10.1037/h0024802. [DOI] [PubMed] [Google Scholar]
- 595.Roitman MF, Bernstein IL. Amiloride-sensitive sodium signals and salt appetite: multiple gustatory pathways. Am J Physiol Regul Integr Comp Physiol 276: R1732–R1738, 1999. [DOI] [PubMed] [Google Scholar]
- 596.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45: 587–597, 2005. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 597.Roitman MF, Wheeler RA, Tiesinga PHE, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17: 539–546, 2010. doi: 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11: 1376–1377, 2008. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Roldan G, Bures J. Tetrodotoxin blockade of amygdala overlapping with poisoning impairs acquisition of conditioned taste aversion in rats. Behav Brain Res 65: 213–219, 1994. doi: 10.1016/0166-4328(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 600.Rollins BL, Stines SG, McGuire HB, King BM. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiol Behav 72: 735–742, 2001. doi: 10.1016/S0031-9384(01)00433-4. [DOI] [PubMed] [Google Scholar]
- 601.Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav Neurosci 120: 1257–1267, 2006. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- 602.Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. Eur J Neurosci 26: 2627–2632, 2007. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 603.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7: 11905, 2016. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch 454: 759–776, 2007. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497, 2017. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Dev 59: 1555–1568, 1988. doi: 10.2307/1130670. [DOI] [PubMed] [Google Scholar]
- 608.Routtenberg A, Lindy J. Effects of the availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Comp Physiol Psychol 60: 158–161, 1965. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- 609.Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul Pept 71: 171–174, 1997. doi: 10.1016/S0167-0115(97)01034-3. [DOI] [PubMed] [Google Scholar]
- 610.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–G177, 2006. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 611.Rozin P. The selection of foods by rats, humans, and other animals. In: Advances in the Study of Behavior, edited by Rosenblatt JS, Hinde RA, Shaw E, Beer C. New York: Academic, 1976, p. 21–76. [Google Scholar]
- 612.Rozin P. Specific aversions as a component of specific hungers. J Comp Physiol Psychol 64: 237–242, 1967. doi: 10.1037/h0088047. [DOI] [PubMed] [Google Scholar]
- 613.Rozin P, Fallon AE. A perspective on disgust. Psychol Rev 94: 23–41, 1987. doi: 10.1037/0033-295X.94.1.23. [DOI] [PubMed] [Google Scholar]
- 614.Ruiz C, Gutknecht S, Delay E, Kinnamon S. Detection of NaCl and KCl in TRPV1 knockout mice. Chem Senses 31: 813–820, 2006. doi: 10.1093/chemse/bjl024. [DOI] [PubMed] [Google Scholar]
- 616.Saddoris MP, Holland PC, Gallagher M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. J Neurosci 29: 15386–15396, 2009. doi: 10.1523/JNEUROSCI.3233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896–903, 2001. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 618.Sakai H, Sato K, Kai Y, Chiba Y, Narita M. Denatonium and 6-n-propyl-2-thiouracil, agonists of bitter taste receptor, inhibit contraction of various types of smooth muscles in the rat and mouse. Biol Pharm Bull 39: 33–41, 2016. doi: 10.1248/bpb.b15-00426. [DOI] [PubMed] [Google Scholar]
- 619.Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neurosci Res 35: 53–61, 1999. doi: 10.1016/S0168-0102(99)00067-X. [DOI] [PubMed] [Google Scholar]
- 620.Sakai N, Yamamoto T. Role of the medial and lateral parabrachial nucleus in acquisition and retention of conditioned taste aversion in rats. Behav Brain Res 93: 63–70, 1998. doi: 10.1016/S0166-4328(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 621.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci 101: 724–731, 1987. doi: 10.1037/0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- 622.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci 3: 13, 2009. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for l-glutamate stimuli. Chem Senses 30, Suppl 1: i25–i26, 2005. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- 624.Saper CB. Reciprocal parabrachial-cortical connections in the rat. Brain Res 242: 33–40, 1982. doi: 10.1016/0006-8993(82)90493-0. [DOI] [PubMed] [Google Scholar]
- 625.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 626.Sasamoto K, Zhang G, Iwasaki M. Two types of rhythmical jaw movements evoked by stimulation of the rat cortex. Shika Kiso Igakkai Zasshi 32: 57–68, 1990. [DOI] [PubMed] [Google Scholar]
- 627.Scalera G, Norgren R. Taste functions and Na+-appetite after excitotoxic lesions of the parabrachial nuclei in rats. In: Olfaction and Taste xi: Proceedings of the 11th International Symposium on Olfaction and Taste and of the 27th Japanese Symposium on Taste and Smell. Joint Meeting held at kosei-nenkin kaikan, Sapporo, Japan, July 12–16, edited by Kurihara K, Suzuki N, Ogawa H. Tokyo: Springer Japan, 1994, p. 533–533. doi: 10.1007/978-4-431-68355-1_221. [DOI] [Google Scholar]
- 628.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995. doi: 10.1037/0735-7044.109.5.997. [DOI] [PubMed] [Google Scholar]
- 629.Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Res 741: 109–116, 1996. doi: 10.1016/S0006-8993(96)00906-7. [DOI] [PubMed] [Google Scholar]
- 630.Schafe GE, Thiele TE, Bernstein IL. Conditioning method dramatically alters the role of amygdala in taste aversion learning. Learn Mem 5: 481–492, 1998. [PMC free article] [PubMed] [Google Scholar]
- 631.Schier LA, Blonde GD, Spector AC. Bilateral lesions in a specific subregion of posterior insular cortex impair conditioned taste aversion expression in rats. J Comp Neurol 524: 54–73, 2016. doi: 10.1002/cne.23822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Schier LA, Davidson TL, Powley TL. Ongoing ingestive behavior is rapidly suppressed by a preabsorptive, intestinal “bitter taste” cue. Am J Physiol Regul Integr Comp Physiol 301: R1557–R1568, 2011. doi: 10.1152/ajpregu.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc Natl Acad Sci USA 111: 1162–1167, 2014. doi: 10.1073/pnas.1315624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Schier LA, Spector AC. Behavioral evidence for more than one taste signaling pathway for sugars in rats. J Neurosci 36: 113–124, 2016. doi: 10.1523/JNEUROSCI.3356-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Schier LA, Spector AC. Post-oral sugar detection rapidly and chemospecifically modulates taste-guided behavior. Am J Physiol Regul Integr Comp Physiol 311: R742–R755, 2016. doi: 10.1152/ajpregu.00155.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, Li B. An insula-central amygdala circuit for guiding tastant-reinforced choice behavior. J Neurosci 38: 1418–1429, 2018. doi: 10.1523/JNEUROSCI.1773-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Schifferstein HN, Frijters JE. The effectiveness of different sweeteners in suppressing citric acid sourness. Percept Psychophys 49: 1–9, 1991. doi: 10.3758/BF03211610. [DOI] [PubMed] [Google Scholar]
- 638.Schiffman SS, Lockhead E, Maes FW. Amiloride reduces the taste intensity of Na+ and Li+ salts and sweeteners. Proc Natl Acad Sci USA 80: 6136–6140, 1983. doi: 10.1073/pnas.80.19.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Schneider LH. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann N Y Acad Sci 575: 307–320, 1989. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- 640.Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol 186: 61–70, 1990. doi: 10.1016/0014-2999(90)94060-B. [DOI] [PubMed] [Google Scholar]
- 641.Schneider LH, Gibbs J, Smith GP. D-2 selective receptor antagonists suppress sucrose sham feeding in the rat. Brain Res Bull 17: 605–611, 1986. doi: 10.1016/0361-9230(86)90231-5. [DOI] [PubMed] [Google Scholar]
- 642.Schulkin J. Behavior of sodium-deficient rats: the search for a salty taste. J Comp Physiol Psychol 96: 628–634, 1982. doi: 10.1037/h0077907. [DOI] [PubMed] [Google Scholar]
- 643.Schulkin J. SodiumH: The Search for a Salty Taste. Cambridge: Cambridge Univ. Press, 1991. [Google Scholar]
- 644.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 270: 416–426, 1988. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- 645.Sclafani A. Gut-brain nutrient signaling. Appetition vs. satiation. Appetite 71: 454–458, 2013. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite 36: 79–83, 2001. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 647.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003. doi: 10.1016/S0031-9384(02)00968-X. [DOI] [PubMed] [Google Scholar]
- 648.Sclafani A, Azzara AV, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci 115: 920–933, 2001. doi: 10.1037/0735-7044.115.4.920. [DOI] [PubMed] [Google Scholar]
- 649.Sclafani A, Nissenbaum JW. Robust conditioned flavor preference produced by intragastric starch infusions in rats. Am J Physiol Regul Integr Comp Physiol 255: R672–R675, 1988. [DOI] [PubMed] [Google Scholar]
- 650.Scott TR. The janus head of taste. Ann N Y Acad Sci 510: 600–601, 1987. doi: 10.1111/j.1749-6632.1987.tb43639.x. [DOI] [Google Scholar]
- 651.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D’Alessio D. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Seeley RJ, Galaverna O, Schulkin J, Epstein AN, Grill HJ. Lesions of the central nucleus of the amygdala. II: Effects on intraoral NaCl intake. Behav Brain Res 59: 19–25, 1993. doi: 10.1016/0166-4328(93)90147-I. [DOI] [PubMed] [Google Scholar]
- 653.Shammah-Lagnado SJ, Costa MS, Ricardo JA. Afferent connections of the parvocellular reticular formation: a horseradish peroxidase study in the rat. Neuroscience 50: 403–425, 1992. doi: 10.1016/0306-4522(92)90433-3. [DOI] [PubMed] [Google Scholar]
- 654.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol 234: 344–364, 1985. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 655.Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale Univ. Press, 1906. [Google Scholar]
- 656.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399: 440–468, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 657.Shibata R, Kameishi M, Kondoh T, Torii K. Bilateral dopaminergic lesions in the ventral tegmental area of rats influence sucrose intake, but not umami and amino acid intake. Physiol Behav 96: 667–674, 2009. doi: 10.1016/j.physbeh.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 658.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci 23: 1596–1604, 2006. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 659.Shimura T, Komori M, Yamamoto T. Acute sodium deficiency reduces gustatory responsiveness to NaCl in the parabrachial nucleus of rats. Neurosci Lett 236: 33–36, 1997. doi: 10.1016/S0304-3940(97)00745-3. [DOI] [PubMed] [Google Scholar]
- 660.Shimura T, Grigson PS, Norgren R. Brainstem lesions and gustatory function: I. The role of the nucleus of the solitary tract during a brief intake test in rats. Behav Neurosci 111: 155–168, 1997. doi: 10.1037/0735-7044.111.1.155. [DOI] [PubMed] [Google Scholar]
- 661.Shipley MT, Sanders MS. Special senses are really special: evidence for a reciprocal, bilateral pathway between insular cortex and nucleus parabrachialis. Brain Res Bull 8: 493–501, 1982. doi: 10.1016/0361-9230(82)90007-7. [DOI] [PubMed] [Google Scholar]
- 662.Sills TL, Vaccarino FJ. Individual differences in sugar consumption following systemic or intraaccumbens administration of low doses of amphetamine in nondeprived rats. Pharmacol Biochem Behav 54: 665–670, 1996. doi: 10.1016/0091-3057(96)00024-X. [DOI] [PubMed] [Google Scholar]
- 663.Simbayi LC. Effects of anterior basolateral amygdala lesions on taste aversions produced by high and low oral doses of LiCl and lactose in the rat. Behav Brain Res 25: 131–142, 1987. doi: 10.1016/0166-4328(87)90006-4. [DOI] [PubMed] [Google Scholar]
- 664.Simbayi LC, Boakes RA, Burton MJ. Effects of basolateral amygdala lesions on taste aversions produced by lactose and lithium chloride in the rat. Behav Neurosci 100: 455–465, 1986. doi: 10.1037/0735-7044.100.4.455. [DOI] [PubMed] [Google Scholar]
- 665.Simson PC, Booth DA. Olfactory conditioning by association with histidine-free or balanced amino acid loads in rats. Q J Exp Psychol 25: 354–359, 1973. doi: 10.1080/14640747308400356. [DOI] [PubMed] [Google Scholar]
- 666.Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun 406: 146–151, 2011. doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 667.Skopec MM, Hagerman AE, Karasov WH. Do salivary proline-rich proteins counteract dietary hydrolyzable tannin in laboratory rats? J Chem Ecol 30: 1679–1692, 2004. doi: 10.1023/B:JOEC.0000042395.31307.be. [DOI] [PubMed] [Google Scholar]
- 668.Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 43: 11–13, 2004. doi: 10.1016/j.appet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 669.Smith GP. John Davis and the meanings of licking. Appetite 36: 84–92, 2001. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- 670.Smith GP, Gibbs J, Kulkosky PJ. Relationships between brain-gut pepties and neurons in the control of food intake. In: The Neural Basis of Feeding and Reward, edited by Hoebel BG, Novin D. Brunswick, ME: Haer Institute, 1982, p. 149–165. [Google Scholar]
- 671.Smith GP, Smith JC. The inhibitory potency of SCH 23390 and raclopride on licking for sucrose increases across brief-access tests. Physiol Behav 101: 315–319, 2010. doi: 10.1016/j.physbeh.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 672.Smith JC. Radiationl its detection and its effects on taste preferences. In: Progress in Physiological Psychology, edited by Stellar E, Sprague JM. New York: Academic, 1971, p. 53–113. [Google Scholar]
- 673.Smith KR, Spector AC. Detection of maltodextrin and its discrimination from sucrose are independent of the T1R2 + T1R3 heterodimer. Am J Physiol Regul Integr Comp Physiol 313: R450–R462, 2017. doi: 10.1152/ajpregu.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Smith KR, Treesukosol Y, Paedae AB, Contreras RJ, Spector AC. Contribution of the TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. Am J Physiol Regul Integr Comp Physiol 303: R1195–R1205, 2012. doi: 10.1152/ajpregu.00154.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27: 1594–1605, 2007. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res 196: 155–167, 2009. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Snowdon CT. A nutritional basis for lead pica. Physiol Behav 18: 885–893, 1977. doi: 10.1016/0031-9384(77)90198-6. [DOI] [PubMed] [Google Scholar]
- 678.Sollars SI, Bernstein IL. Sodium appetite after transection of the chorda tympani nerve in Wistar and Fischer 344 rats. Behav Neurosci 106: 1023–1027, 1992. doi: 10.1037/0735-7044.106.6.1023. [DOI] [PubMed] [Google Scholar]
- 679.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81: 119–145, 1974. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 680.Spector A. Psychophysical evaluation of taste function in nonhuman mammals. In: Handbook of Olfaction and Gustation. Boca Raton, FL: CRC, 2003. doi: 10.1201/9780203911457.ch41. [DOI] [Google Scholar]
- 681.Spector AC. The functional organization of the peripheral gustatory system: lessons from behavior. In: Progress in Psychobiology and Physiological Psychology. Bingley, UK: Emerald Group Publishing Limited, 2003, p. 101–161. [Google Scholar]
- 682.Spector AC. Gustatory parabrachial lesions disrupt taste-guided quinine responsiveness in rats. Behav Neurosci 109: 79–90, 1995. doi: 10.1037/0735-7044.109.1.79. [DOI] [PubMed] [Google Scholar]
- 683.Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev 24: 391–416, 2000. doi: 10.1016/S0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 685.Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci 102: 942–952, 1988. doi: 10.1037/0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- 686.Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav 53: 277–283, 1993. doi: 10.1016/0031-9384(93)90205-T. [DOI] [PubMed] [Google Scholar]
- 687.Spector AC, Guagliardo NA, St. John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci 16: 8115–8122, 1996. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694, 1998. doi: 10.1037/0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 689.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci 22: 1937–1941, 2002. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol Regul Integr Comp Physiol 272: R1210–R1218, 1997. [DOI] [PubMed] [Google Scholar]
- 691.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci 106: 147–161, 1992. doi: 10.1037/0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- 692.Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096–1109, 1996. doi: 10.1037/0735-7044.110.5.1096. [DOI] [PubMed] [Google Scholar]
- 693.Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci 109: 939–954, 1995. doi: 10.1037/0735-7044.109.5.939. [DOI] [PubMed] [Google Scholar]
- 694.Spector AC, St. John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol Regul Integr Comp Physiol 274: R1687–R1703, 1998. [DOI] [PubMed] [Google Scholar]
- 695.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4: 143–191, 2005. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 696.St. John SJ, Garcea M, Spector AC. Combined, but not single, gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci 108: 131–140, 1994. doi: 10.1037/0735-7044.108.1.131. [DOI] [PubMed] [Google Scholar]
- 697.St. John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci 18: 4353–4362, 1998. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.St. Andre J, Albanos K, Reilly S. C-fos expression in the rat brain following lithium chloride-induced illness. Brain Res 1135: 122–128, 2007. doi: 10.1016/j.brainres.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.St. Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav Neurosci 121: 90–99, 2007. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]
- 700.St. John SJ. The perceptual characteristics of sodium chloride to sodium-depleted rats. Chem Senses 42: 93–103, 2017. doi: 10.1093/chemse/bjw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Starr LJ, Rowland NE. Characteristics of salt appetite in chronically sodium-depleted rats using a progressive ratio schedule of procurement. Physiol Behav 88: 433–442, 2006. doi: 10.1016/j.physbeh.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 702.Stehberg J, Simon F. Involvement of the insular cortex in retention of conditioned taste aversion is not time dependent. Neurobiol Learn Mem 95: 14–18, 2011. doi: 10.1016/j.nlm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 703.Stein LJ, Cowart BJ, Beauchamp GK. The development of salty taste acceptance is related to dietary experience in human infants: a prospective study. Am J Clin Nutr 95: 123–129, 2012. doi: 10.3945/ajcn.111.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One 9: e94771, 2014. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept 4: 254–278, 1973. [PubMed] [Google Scholar]
- 706.Steiner JE. Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav 13: 257–295, 1979. doi: 10.1016/S0065-2407(08)60349-3. [DOI] [PubMed] [Google Scholar]
- 707.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 25: 53–74, 2001. doi: 10.1016/S0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 708.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol 292: G457–G461, 2007. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 709.Sternini C, Anderson K. Calcitonin gene-related peptide-containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res 9: 45–59, 1992. doi: 10.3109/08990229209144762. [DOI] [PubMed] [Google Scholar]
- 710.Stratford JM, Finger TE. Taste without calories is insufficient to drive conditioned flavor preferences. FASEB J 27: 1123–1129, 2013. [Google Scholar]
- 711.Stricker EM, Grigson PS, Norgren R. Variable effects of parabrachial nucleus lesions on salt appetite in rats depending upon experimental paradigm and saline concentration. Behav Neurosci 127: 275–284, 2013. doi: 10.1037/a0031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Stricker EM, Thiels E, Verbalis JG. Sodium appetite in rats after prolonged dietary sodium deprivation: a sexually dimorphic phenomenon. Am J Physiol Regul Integr Comp Physiol 260: R1082–R1088, 1991. [DOI] [PubMed] [Google Scholar]
- 713.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1420–G1428, 2007. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 714.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci 21: 323–331, 1998. doi: 10.1016/S0166-2236(98)01265-X. [DOI] [PubMed] [Google Scholar]
- 715.Switzman L, Hunt T, Amit Z. Heroin and morphine: aversive and analgesic effects in rats. Pharmacol Biochem Behav 15: 755–759, 1981. doi: 10.1016/0091-3057(81)90018-6. [DOI] [PubMed] [Google Scholar]
- 716.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav 45: 817–821, 1993. doi: 10.1016/0091-3057(93)90126-E. [DOI] [PubMed] [Google Scholar]
- 717.Tamura R, Norgren R. Repeated sodium depletion affects gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol Regul Integr Comp Physiol 273: R1381–R1391, 1997. [DOI] [PubMed] [Google Scholar]
- 718.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite 34: 206–213, 2000. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- 719.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69: 74–90, 1962. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 720.Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci 19: 465–470, 2016. doi: 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr 28: 367–388, 2008. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 722.Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 31: 785–797, 1989. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 723.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 724.Thunhorst RL, Fitts DA. Peripheral angiotensin causes salt appetite in rats. Am J Physiol Regul Integr Comp Physiol 267: R171–R177, 1994. [DOI] [PubMed] [Google Scholar]
- 725.Tindell AJ, Smith KS, Peciña S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol 96: 2399–2409, 2006. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- 726.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11: 3, 2011. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA 107: 3210–3215, 2010. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Tokita K, Boughter JD Jr. Topographic organizations of taste-responsive neurons in the parabrachial nucleus of C57BL/6J mice: an electrophysiological mapping study. Neuroscience 316: 151–166, 2016. doi: 10.1016/j.neuroscience.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Tokita K, Inoue T, Boughter JD Jr. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161: 475–488, 2009. doi: 10.1016/j.neuroscience.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Tokita K, Inoue T, Boughter JD Jr. Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171: 351–365, 2010. doi: 10.1016/j.neuroscience.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Tokita K, Yamamoto T, Boughter JD Jr. Gustatory neural responses to umami stimuli in the parabrachial nucleus of C57BL/6J mice. J Neurophysiol 107: 1545–1555, 2012. doi: 10.1152/jn.00799.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27: 10840–10848, 2007. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr 132: 2288–2297, 2002. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Torregrossa AM, Azzara AV, Dearing MD. Testing the diet-breadth trade-off hypothesis: differential regulation of novel plant secondary compounds by a specialist and a generalist herbivore. Oecologia 168: 711–718, 2012. doi: 10.1007/s00442-011-2121-y. [DOI] [PubMed] [Google Scholar]
- 735.Torregrossa AM, Nikonova L, Bales MB, Villalobos Leal M, Smith JC, Contreras RJ, Eckel LA. Induction of salivary proteins modifies measures of both orosensory and postingestive feedback during exposure to a tannic acid diet. PLoS One 9: e105232, 2014. doi: 10.1371/journal.pone.0105232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Touzani K, Sclafani A. Insular cortex lesions fail to block flavor and taste preference learning in rats. Eur J Neurosci 26: 1692–1700, 2007. doi: 10.1111/j.1460-9568.2007.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience 74: 873–884, 1996. doi: 10.1016/0306-4522(96)00173-X. [DOI] [PubMed] [Google Scholar]
- 738.Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21: 631–647, 1997. doi: 10.1016/S0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 739.Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav Brain Res 25: 233–246, 1987. doi: 10.1016/0166-4328(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 740.Travers JB, Travers SP, Norgren R. Gustatory neural processing in the hindbrain. Annu Rev Neurosci 10: 595–632, 1987. doi: 10.1146/annurev.ne.10.030187.003115. [DOI] [PubMed] [Google Scholar]
- 741.Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 277: R384–R394, 1999. [DOI] [PubMed] [Google Scholar]
- 742.Travers SP. Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 282: R1798–R1810, 2002. doi: 10.1152/ajpregu.00590.2001. [DOI] [PubMed] [Google Scholar]
- 743.Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec 227: 373–379, 1990. doi: 10.1002/ar.1092270313. [DOI] [PubMed] [Google Scholar]
- 744.Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol 73: 2144–2162, 1995. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- 745.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855–R865, 2009. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol 292: R1799–R1809, 2007. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- 747.Treesukosol Y, Mathes CM, Spector AC. Citric acid and quinine share perceived chemosensory features making oral discrimination difficult in C57BL/6J mice. Chem Senses 36: 477–489, 2011. doi: 10.1093/chemse/bjr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol 303: R218–R235, 2012. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Treit D, Berridge KC. A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm. Pharmacol Biochem Behav 37: 451–456, 1990. doi: 10.1016/0091-3057(90)90011-6. [DOI] [PubMed] [Google Scholar]
- 750.Treneer CM, Bernstein IL. Learned aversions in rats fed a tryptophan-free diet. Physiol Behav 27: 757–760, 1981. doi: 10.1016/0031-9384(81)90038-X. [DOI] [PubMed] [Google Scholar]
- 751.Trifunovic R, Reilly S. Medial versus lateral parabrachial nucleus lesions in the rat: effects on cholecystokinin- and d-fenfluramine-induced anorexia. Brain Res 894: 288–296, 2001. doi: 10.1016/S0006-8993(01)02037-6. [DOI] [PubMed] [Google Scholar]
- 752.Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER. An evolutionarily conserved gene family encodes proton-selective ion channels. Science 359: 1047–1050, 2018. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Tuerke KJ, Limebeer CL, Fletcher PJ, Parker LA. Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT(3) receptor in the posterior and anterior insular cortex. J Neurosci 32: 13709–13717, 2012. doi: 10.1523/JNEUROSCI.2042-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1, 2008. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol 593: 1113–1125, 2015. doi: 10.1113/jphysiol.2014.281014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H, Sternini C. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS One 9: e107732, 2014. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Vigorito M, Sclafani A, Jacquin MF. Effects of gustatory deafferentation on Polycose and sucrose appetite in the rat. Neurosci Biobehav Rev 11: 201–209, 1987. doi: 10.1016/S0149-7634(87)80027-1. [DOI] [PubMed] [Google Scholar]
- 758.Viltart O, Sartor DM, Verberne AJ. Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull 71: 51–59, 2006. doi: 10.1016/j.brainresbull.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 759.Vincis R, Fontanini A. Associative learning changes cross-modal representations in the gustatory cortex. eLife 5: e16420, 2016. doi: 10.7554/eLife.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Vitazkova SK, Long E, Paul A, Glendinning JI. Mice suppress malaria infection by sampling a ‘bitter’ chemotherapy agent. Anim Behav 61: 887–894, 2001. doi: 10.1006/anbe.2000.1677. [DOI] [Google Scholar]
- 761.Voigt A, Hübner S, Döring L, Perlach N, Hermans-Borgmeyer I, Boehm U, Meyerhof W. Cre-mediated recombination in tas2r131 cells-a unique way to explore bitter taste receptor function inside and outside of the taste system. Chem Senses 40: 627–639, 2015. doi: 10.1093/chemse/bjv049. [DOI] [PubMed] [Google Scholar]
- 762.Voshart K, van der Kooy D. The organization of the efferent projections of the parabrachial nucleus of the forebrain in the rat: a retrograde fluorescent double-labeling study. Brain Res 212: 271–286, 1981. doi: 10.1016/0006-8993(81)90462-5. [DOI] [PubMed] [Google Scholar]
- 763.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149: 118–126, 2007. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 764.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol 421: 302–324, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 765.Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJP, Zuker CS. The coding of valence and identity in the mammalian taste system. Nature 558: 127–131, 2018. doi: 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Wang Y, Chambers KC. Cooling lesions of the lateral parabrachial nucleus during LiCl activation block acquisition of conditioned taste avoidance in male rats. Brain Res 934: 7–22, 2002. doi: 10.1016/S0006-8993(02)02278-3. [DOI] [PubMed] [Google Scholar]
- 767.Ward RD. Methods for dissecting motivation and related psychological processes in rodents. In: Behavioral Neuroscience of Motivation, edited by Simpson EH, Balsam PD. Cham: Springer International Publishing, 2016, p. 451–470. [DOI] [PubMed] [Google Scholar]
- 768.Warren RP, Lewis RC. Taste polymorphism in mice involving a bitter sugar derivative. Nature 227: 77–78, 1970. doi: 10.1038/227077a0. [DOI] [PubMed] [Google Scholar]
- 769.Warwick ZS, Weingarten HP. Flavor-postingestive consequence associations incorporate the behaviorally opposing effects of positive reinforcement and anticipated satiety: implications for interpreting two-bottle tests. Physiol Behav 60: 711–715, 1996. doi: 10.1016/0031-9384(96)00087-X. [DOI] [PubMed] [Google Scholar]
- 770.Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacol Biochem Behav 37: 317–323, 1990. doi: 10.1016/0091-3057(90)90341-E. [DOI] [PubMed] [Google Scholar]
- 771.Weingarten HP, Watson SD. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav 28: 401–407, 1982. doi: 10.1016/0031-9384(82)90131-7. [DOI] [PubMed] [Google Scholar]
- 772.Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry 69: 1067–1074, 2011. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron 57: 774–785, 2008. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 774.White N, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology (Berl) 52: 63–66, 1977. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- 775.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Willner P, Lappas S, Cheeta S, Muscat R. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology (Berl) 115: 454–462, 1994. doi: 10.1007/BF02245568. [DOI] [PubMed] [Google Scholar]
- 777.Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav 92: 566–573, 2009. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Wilson DM, Boughter JD Jr, Lemon CH. Bitter taste stimuli induce differential neural codes in mouse brain. PLoS One 7: e41597, 2012. doi: 10.1371/journal.pone.0041597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Winn P, Williams SF, Herberg LJ. Feeding stimulated by very low doses of d-amphetamine administered systemically or by microinjection into the striatum. Psychopharmacology (Berl) 78: 336–341, 1982. doi: 10.1007/BF00433737. [DOI] [PubMed] [Google Scholar]
- 780.Wise RA. Common neural basis for brain stimulation reward, drug reward, and food reward. In: The Neural Basis of Feeding and Reward, edited by Hoebel BG, Novin D. Brunswick, ME: Haer Institute, 1982, p. 454–455. [Google Scholar]
- 781.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14: 169–183, 2008. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science 201: 262–264, 1978. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 783.Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science 191: 1273–1275, 1976. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- 784.Wolf G. Innate Mechanisms for Regulation of Sodium Intake, edited by Pfaffmann C. New York: Rockefeller Univ. Press, 1969, p. 548–553. [Google Scholar]
- 785.Wright RL, Gilmour G, Dwyer DM. Microstructural analysis of negative anticipatory contrast: a reconsideration of the devaluation account. Learn Behav 41: 353–359, 2013. doi: 10.3758/s13420-013-0110-1. [DOI] [PubMed] [Google Scholar]
- 786.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137: 1225–1234, 2009. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483: 594–597, 2012. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99: 2392–2397, 2002. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Wu XY, Zhu JX, Gao J, Owyang C, Li Y. Neurochemical phenotype of vagal afferent neurons activated to express C-FOS in response to luminal stimulation in the rat. Neuroscience 130: 757–767, 2005. doi: 10.1016/j.neuroscience.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 790.Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neurosci Res 22: 31–49, 1995. doi: 10.1016/0168-0102(95)00875-T. [DOI] [PubMed] [Google Scholar]
- 791.Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol 44: 440–455, 1980. doi: 10.1152/jn.1980.44.3.440. [DOI] [PubMed] [Google Scholar]
- 792.Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Lett 226: 127–130, 1997. doi: 10.1016/S0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- 793.Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav 56: 1197–1202, 1994. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 794.Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport 3: 1049–1052, 1992. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 795.Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res 869: 15–24, 2000. doi: 10.1016/S0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]
- 796.Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol 590: 1155–1170, 2012. doi: 10.1113/jphysiol.2011.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J Neurosci 20: 8861–8867, 2000. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience 159: 795–803, 2009. doi: 10.1016/j.neuroscience.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 799.Young PT, Burright RG, Tromater LJ. Preferences of the white rat for solutions of sucrose and quinine hydrochloride. Am J Psychol 76: 205–217, 1963. doi: 10.2307/1419157. [DOI] [PubMed] [Google Scholar]
- 799a.Zafra MA, Prados M, Molina F, Puerto A. Capsaicin-sensitive afferent vagal fibers are involved in concurrent taste aversion learning. Neurobiol Learning Memory 86: 349–352, 2006. [DOI] [PubMed] [Google Scholar]
- 800.Zafra MA, Simón MJ, Molina F, Puerto A. The role of the external lateral parabrachial subnucleus in flavor preferences induced by predigested food administered intragastrically. Brain Res 950: 155–164, 2002. doi: 10.1016/S0006-8993(02)03032-9. [DOI] [PubMed] [Google Scholar]
- 801.Zahm DS, Jensen SL, Williams ES, Martin JR III. Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci 11: 1119–1126, 1999. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 802.Zalaquett CT, Parker LA. Further evidence that ctas produced by lithium and amphetamine are qualitatively different. Learn Motiv 20: 413–427, 1989. doi: 10.1016/0023-9690(89)90004-0. [DOI] [Google Scholar]
- 803.Zellner DA, Berridge KC, Grill HJ, Ternes JW. Rats learn to like the taste of morphine. Behav Neurosci 99: 290–300, 1985. doi: 10.1037/0735-7044.99.2.290. [DOI] [PubMed] [Google Scholar]
- 804.Zhang GX, Sasamoto K. Projections of two separate cortical areas for rhythmical jaw movements in the rat. Brain Res Bull 24: 221–230, 1990. doi: 10.1016/0361-9230(90)90209-I. [DOI] [PubMed] [Google Scholar]
- 805.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003. doi: 10.1016/S0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 806.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 807.Zhao XL, Yan JQ, Yang XJ, Chen K, Li JR, Zhang Y. Fos positive neurons in the brain stem and amygdala mostly express vesicular glutamate transporter 3 after bitter taste stimulation. Brain Res 1445: 20–29, 2012. doi: 10.1016/j.brainres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 808.Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 530: 431–442, 2001. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 809.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866–R876, 2009. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Zverev YP. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci 5: 5, 2004. doi: 10.1186/1471-2202-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]