To the Editor:
Metabolomics of patients with chronic kidney disease (CKD) can provide a unique perspective on subclinical metabolic perturbations relative to the glomerular filtration rate (GFR) because the kidneys have a major role in maintaining metabolic homeostasis.1 Examination of metabolomics in CKD has largely focused on adults in advanced stages and centered on risk of mortality.2 However, metabolic alterations in children and adolescents with mild-moderate CKD presents a significant risk of secondary morbidities because they are undergoing periods of rapid growth and development and would otherwise not normally experience untoward issues with nutrient catabolism.
The goal of this study was to use targeted metabolomics to identify altered biochemical pathways in adolescents with mild to moderate CKD (stages 2 and 3b) in 2 cohorts matched by age, gender, and CKD etiology.3 Metabolites and their ratios were selected a priori due to previously documented alterations in CKD.4, 5, 6, 7, 8, 9
For this investigation, CKD etiology was categorized as glomerulopathy (G) or nonglomerular urologic anomalies (NG), the latter of which is defined as congenital abnormalities of the kidney and urinary tract and includes the largest proportion of the pediatric CKD population. Therefore, the 2 cohorts (CKD-2 and -3b) had equal numbers with G and NG.
Forty plasma specimens and data, including directly measured GFR (mGFR, determined by plasma iohexol clearance)10 were selected from a large heterogeneous population of children and adolescents in the ongoing, prospective, observational Chronic Kidney Disease in Children (CKiD) study in North America.4 By closely matching the cohorts, an unbiased approach to the metabolomics of mild to moderate CKD was achieved.
We posited that abnormal metabolic findings would be present in early (mild) CKD (stage 2), with some significant differences observed between CKD stages 2 and 3b (moderate).
Results
Two cohorts (CKD-2 and -3b) were defined by their respective median and interquartile range mGFR that matched the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative criteria for stages 2 and 3b.3 The CKD-2 and -3b mGFR medians and interquartile ranges were 74.3 (67.4, 82.9) and 32.8 (24.3, 35.5) ml/min per 1.73 m2 (P < 0.001), respectively. Median age was also matched between the cohorts. The racial compositions of both cohorts were 70% White, 5% Native American, and 5% “other,” whereas CKD-2 and -3b were 20% and 5% African American, respectively. Carbohydrate, total fat, and protein intake (grams per day) by food frequency did not differ appreciably between the cohorts (P = 0.19–0.63).
The cohorts (n = 20/cohort) were compared by stage for metabolites using a discrete t-test. Subgroups (n = 10/subgroup), defined by CKD stage and etiology, were analyzed using 1-way analysis of variance and post hoc testing (Table 1).
Table 1.
Subject characteristics and significant differences in plasma metabolites and metabolite ratios by CKD cohort (median and interquartile range)
| Variables | CKD stage 2 (n=20) | CKD stage 3b (n=20) | P | ||
|---|---|---|---|---|---|
| mGFR (ml/min per 1.73 m2) | 74.3 (67.4, 82.9) | 32.8 (24.3, 35.5) | < 0.001a | ||
| Age, yr | 15.0 (12.7, 15.7) | 14.8 (11.7, 16.1) | 0.59 | ||
| Body mass index (BMI) Z | 0.95 (-0.07, 1.63) | 0.34 (-0.10, 0.82) | 0.79 | ||
| Serum PTH (pg/ml) | 33.0 (26.0, 50.2) | 60.0 (37.3, 197.0) | 0.03a | ||
| Urine Pro/Cr ratio | 0.34 (0.14, 1.76) | 0.63 (0.16, 1.58) | 0.56 | ||
| Metabolites | CKD stage 2 | CKD stage 3b | T statistic | P | FDR |
| SDMA | ↑ | −5.6057 | 1.98E-06 | 3.09E-04 | |
| PC ae (C32:1) | ↑ | 4.4398 | 7.50E-05 | 5.85E-03 | |
| Kynurenine | ↑ | −4.2039 | 1.54E-04 | 7.98E-03 | |
| Creatinine | ↑ | −3.9549 | 3.23E-04 | 1.26E-02 | |
| C4:1 | ↑ | 3.5496 | 1.05E-03 | 3.27E-02 | |
| Metabolite ratios | |||||
| Tyr/Cr | ↑ | 5.3733 | 4.12E-06 | 2.47E-05 | |
| Orn/Cit | ↑ | 4.2933 | 1.17E-04 | 3.52E-04 | |
| Kyn/Trp | ↑ | −4.112 | 2.02E-04 | 4.05E-04 | |
| Pro/Cit | ↑ | 3.7856 | 5.30E-04 | 7.96E-04 | |
| SDMA/ADMA | ↑ | −3.5173 | 1.15E-03 | 1.38E-03 |
Body mass index (weight in kg/height in meters2) corrected for gender and age. The arrows indicate which metabolites and ratios were higher in CKD Stage 2 or 3b cohort.
ADMA, asymmetric dimethylarginine; Cit, citrulline; CKD, chronic kidney disease; Cr, creatinine; C4:1, butenoylcarnitine (fatty acylcarnitine); FDR, false discovery rate (5% of significant tests will result in false positives); Kyn, kynurenine; mGFR, measured glomerular filtration rate; Orn, ornithine; PCae (C32:1), phosphatidylcholine with acyl-akyl residue; Pro, proline; PTH, parathyroid hormone; SDMA, symmetric dimethylarginine; Trp, tryptophan; Tyr, tyrosine.
Differences between the CKD cohorts were considered to be significant when P < 0.001.
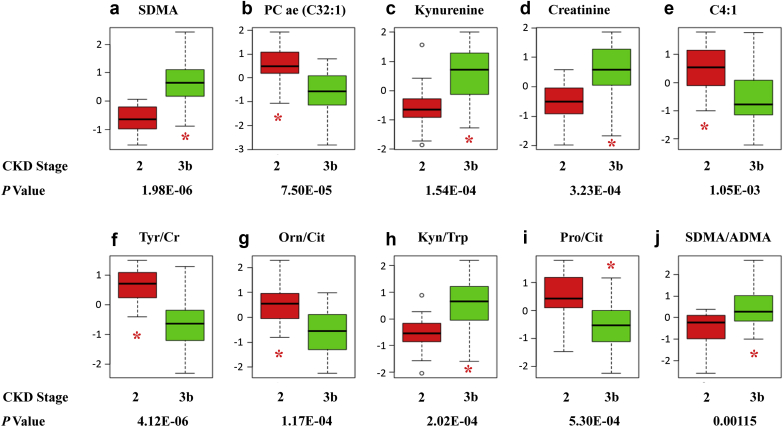
Five metabolites and ratios were significantly different between the cohorts, comprising a biogenic amine, phosphatidylcholine; metabolites, tryptophan (Trp), kynurenine (Kyn), and creatinine (Cr); and an acylcarnitine (P ≤ 0.001). Ratios included tyrosine/creatinine (Tyr/Cr), ornithine/citrulline (Orn/Cit), Kyn/Trp, proline/Cit (Pro/Cit), phenylalanine/Trp (Phe/Trp), and symmetric dimethylarginine/asymmetric dimethylarginine (SDMA/ADMA) (P ≤ 0.0012) (Figure 1a–j).
Figure 1.
(a–j) Plasma metabolites and ratios differ significantly between chronic kidney disease (CKD) cohorts (stages 2 and 3b; n = 20/group; false discovery rate <0.05). ADMA, asymmetric dimethylarginine; Cit, citrulline; Cr, creatinine; C4:1, butenoylcarnitine (fatty acylcarnitine); FDR, false discovery rate (5% of significant tests will result in false positives); Kyn, kynurenine; Orn, ornithine; PC ae (C32:1), phosphatidylcholine with acyl-akyl residue; Pro, proline; SDMA, symmetric dimethylarginine; Trp, tryptophan; Tyr, tyrosine.
Three metabolites and 5 ratios were identified as different between the subgroups (P = 0.004–0.02). Subgroup and cohort results reflected metabolite differences, whereas ratio findings were identical. Distinctions between the subgroups were consistent with biochemical pathways, catabolic alterations from declining mGFR, and disease etiology (Supplementary Material).
Discussion
Methylated arginine derivatives, SDMA and ADMA, elevated concentrations result from increased protein catabolism. SDMA increases in early CKD because it is largely excreted through the kidneys unmetabolized. Conversely, ADMA has a pronounced rise that is synonymous with advanced CKD decline in the enzyme, dimethylarginine dimethylaminohydrolase (DDAH 1) and increased oxidative stress.6, 11, 12, 13 Therefore, we expected and did observe higher concentrations of SDMA and SDMA/ADMA ratio in CKD-3b and 3b-G, respectively.
Amino acid losses in late-stage kidney disease are known and anticipated secondary to acidosis, albeit there is limited literature on mild-moderate CKD.13 Our results demonstrated some important differences in amino acid concentrations between the cohorts, whereas macronutrient intake data were not significantly different. It is well-known that there are age-dependent variations in plasma amino acid concentrations in healthy children aged 0 to 18 years, and as such emphasize the value of matching age and gender between our CKD cohorts in this investigation.14
Kyn and Kyn/Trp levels have been associated with CKD severity and known to be elevated in patients on chronic hemodialysis.5 Kyn has been suggested to infer the coexistence of proinflammatory processes. Thus, our observations of increased Kyn and Kyn/Trp in glomerulopathy (Figure 1a–j) and CKD-3b, respectively, are noteworthy.4 As such, our findings suggest upregulated Trp catabolism in glomerulopathy and moderate CKD.
The higher Tyr/Cr ratio observed in CKD-2 is consistent with an elevated Cr concentration in CKD-3b. In normal conditions, substantial conversion of Phe to Tyr occurs in the kidneys, but as GFR declines, conversion to Tyr is reduced. This explains our observation of attenuated CKD-3b Tyr/Cr levels. This is consistent with previous findings in adults and infants with CKD.7, 15 Moreover, in advanced CKD, gut microbiota metabolize Tyr into protein-bound p-Cresol, a phenolic compound and uremic toxin creating a further decline in Tyr, while Cr continues to rise.9
Cit, a urea cycle intermediary, was also higher in CKD 3b-G (mGFR median = 32.8 ml/min per 1.73 m2) (Supplementary Table S1) and is consistent with previous findings in patients with a GFR <45 ml/min per 1.73 m2.4 Cit has an important function in advancing CKD as a hydroxyl radical scavenger and is catabolized to arginine and then nitric oxide.4 However, as renal dysfunction worsens and plasma ADMA rises, nitric oxide synthesis declines.4 Cit also can be directly converted to Orn, or to arginine by addition of a second amino group, or regenerated to Orn in the urea cycle. Moreover, Orn can be enzymatically converted into proline, accounting for the higher Orn/Cit and Pro/Cit ratios observed in CKD-2 cohort and subgroups (Supplementary Table S2).
Both PC ae C32:1 and C4:1, a phosphatidylcholine and fatty acylcarnitine (butenoylcarnitine), respectively, had noticeably lower plasma concentrations in CKD-3b compared with CKD-2. Our findings are similar to another investigation that identified lower plasma phosphatidylcholine levels in healthy controls versus CKD-4.16 So too, acylcarnitines decline in advancing CKD with relative and absolute carnitine deficiencies in patients with CKD-5 on chronic dialysis.17 In end-stage disease, tissue carnitine deficiency impedes fat beta oxidation, causing generalized weakness, particularly following hemo- and peritoneal dialysis, which further deplete carnitine. The fact that lower acylcarnitine levels already exist in CKD-3b is of importance for adolescents who have not reached their full stature and anthropometric maturity.
There are 2 limitations to this pilot investigation. A small number of subjects were studied (2 cohorts of n = 20) matched by age, gender, and mGFR (cohorts of CKD-2 and -3b). Four smaller subgroups (n = 10) within the cohorts were matched by etiology and examined for stage by etiology interaction, thereby limiting the power for determining significant differences. Also, the targeted metabolite panel (Supplementary Figures S1A and B and S2A and B) was preselected, thereby limiting a complete examination of some biochemical pathways that were of interest from our results, including the Trp-Kyn catabolism, which may have provided additional valuable data.
In summary, mild metabolic derangements exist in CKD-3b (Supplementary Figure S3A–H). In this study, we were fortunate to include directly measured GFR data, which permitted us to better interpret our results in light of quantified kidney function rather than by estimation. Prospective studies are needed to determine if the metabolic alterations we have observed may contribute, in part, to growth limitations (stature and body composition) or whether they have a role in accelerating CKD progression.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Specimens and data in this article were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (DCC) (Principal Investigator) at Johns Hopkins Bloomberg School of Public Health (Alvaro Muños, PhD). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-82194, U01-DK-66116). The CKiD Web site is located at http://www.statepi.jhsph.edu/ckid. Our sincere thanks to the CKiD steering committee and staff of the DCC, including Matthew Matheson and Judith Jerry Fluker for making this ancillary pilot study possible.
Footnotes
Supplementary Material. Study methods, study design, missing value imputation and statistical analyses, and supplementary references.
Table S1. Significant differences in plasma metabolites between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Table S2. Significant differences in plasma metabolite ratios between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Figure S1. Schematic of data collection methodologies using the Biocrates p180 kit, including (A) flow-injection MS/MS and (B) LC-MS/MS.
Figure S2. Schematic depicting quantitative methodologies used for the Biocrates p180 kit using (A) flow-injection analysis and (B) LC-MS/MS analysis.
Figure S3. (A–H) Boxplots of plasma metabolites and their ratios that differ between chronic kidney disease subgroups by stage and etiology (post hoc P < 0.05).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Study methods, study design, missing value imputation and statistical analyses, and supplementary references.
Significant differences in plasma metabolites between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Significant differences in plasma metabolite ratios between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Schematic of data collection methodologies using the Biocrates p180 kit, including (A) flow-injection MS/MS and (B) LC-MS/MS.
Schematic depicting quantitative methodologies used for the Biocrates p180 kit using (A) flow-injection analysis and (B) LC-MS/MS analysis.
(A–H) Boxplots of plasma metabolites and their ratios that differ between chronic kidney disease subgroups by stage and etiology (post hoc P < 0.05).
References
- 1.Grams M.E., Tin A., Rebholz C.M. Metabolomic alterations associated with cause of CKD. Clin J Am Soc Nephrol. 2017;12:1787–1794. doi: 10.2215/CJN.02560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J.-R., Coresh J., Inker L.A. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018;94:381–389. doi: 10.1016/j.kint.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Summary of recommendation statements: definition and classification of CKD. Kidney Int Suppl. 2013;3:5–7. [Google Scholar]
- 4.Duranton F., Lundin U., Gayrard N. Plasma and urinary amino acid metabolomics profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol. 2014;9:37–45. doi: 10.2215/CJN.06000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig P., Nagl C., Neurauter G. Enhanced degradation of tryptophan in patients on hemodialysis. Clin Nephrol. 2010;74:465–470. doi: 10.5414/cnp74465. [DOI] [PubMed] [Google Scholar]
- 6.Brooks E.R., Haymond S., Rademaker A. Contribution of symmetric dimethylarginine to GFR decline in pediatric chronic kidney disease. Pediatr Nephrol. 2018;33:697–704. doi: 10.1007/s00467-017-3842-x. [DOI] [PubMed] [Google Scholar]
- 7.Møller N., Meek S., Bigelow M. The kidney is an important site for in vivo phenylalanine-to-tyrosine conversion in adult humans: a metabolic role of the kidney. Proc Natl Acad Sci U S A. 2000;97:37–45. doi: 10.1073/pnas.97.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laidlaw S.A., Berg R.L., Kopple J.D. The Modification of Diet in Renal Disease Study Group. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the modification of diet in renal disease study. Am J Kidney Dis. 1994;23:504–513. doi: 10.1016/s0272-6386(12)80371-4. [DOI] [PubMed] [Google Scholar]
- 9.Pickford J.C., McGale E.H., Aber G.M. Studies on the metabolism of phenylalanine and tyrosine in patients with renal disease. Clin Chem Acta. 1973;48:77–83. doi: 10.1016/0009-8981(73)90219-2. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz G.J., Furth S., Cole S.R. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 11.Brooks E.R., Langman C.B., Wang S. Methylated arginine derivatives in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2009;24:129–134. doi: 10.1007/s00467-008-0972-1. [DOI] [PubMed] [Google Scholar]
- 12.Nkuipou-Kenfack E., Duranton F., Gayrard N. Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS One. 2014;9:e96955. doi: 10.1371/journal.pone.0096955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goek O.N., Prehn C., Sekula P. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant. 2013;28:2131–2138. doi: 10.1093/ndt/gft217. [DOI] [PubMed] [Google Scholar]
- 14.Lepage N., McDonald N., Dallaire L., Lambert M. Age-specific distribution of plasma amino acid concentrations in a healthy pediatric population. Clin Chem. 1997;43:2397–2402. [PubMed] [Google Scholar]
- 15.Sood M.M., Murphy M.S.Q., Hawken S. Association between newborn metabolic profiles and pediatric kidney disease. Kidney Int Rep. 2018;3:691–700. doi: 10.1016/j.ekir.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Res A., Rudnitskaya A., Chariyavilaskul P. Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. J Lipid Res. 2014;56:413–422. doi: 10.1194/jlr.M055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naseri M., Mottaghi Moghadam Shahri H., Horri M. Absolute and relative carnitine deficiency in patients on hemodialysis and peritoneal dialysis. Iran J Kidney Dis. 2016;10:36–43. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study methods, study design, missing value imputation and statistical analyses, and supplementary references.
Significant differences in plasma metabolites between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Significant differences in plasma metabolite ratios between chronic kidney disease subgroups (CKD stage and etiology; significance by post hoc testing P < 0.05).
Schematic of data collection methodologies using the Biocrates p180 kit, including (A) flow-injection MS/MS and (B) LC-MS/MS.
Schematic depicting quantitative methodologies used for the Biocrates p180 kit using (A) flow-injection analysis and (B) LC-MS/MS analysis.
(A–H) Boxplots of plasma metabolites and their ratios that differ between chronic kidney disease subgroups by stage and etiology (post hoc P < 0.05).