Abstract
The crystal structure of the F1-catalytic domain of the adenosine triphosphate (ATP) synthase has been determined from the pathogenic anaerobic bacterium Fusobacterium nucleatum. The enzyme can hydrolyse ATP but is partially inhibited. The structure is similar to those of the F1-ATPases from Caldalkalibacillus thermarum, which is more strongly inhibited in ATP hydrolysis, and in Mycobacterium smegmatis, which has a very low ATP hydrolytic activity. The βE-subunits in all three enzymes are in the conventional ‘open’ state, and in the case of C. thermarum and M. smegmatis, they are occupied by an ADP and phosphate (or sulfate), but in F. nucleatum, the occupancy by ADP appears to be partial. It is likely that the hydrolytic activity of the F. nucleatum enzyme is regulated by the concentration of ADP, as in mitochondria.
Keywords: Fusobacterium nucleatum, pathogen, catalytic F1-ATPase, structure, ATP hydrolysis, regulation
1. Introduction
The adenosine triphosphate (ATP) synthases, also known as F-ATPases or F1Fo-ATPases, are multi-subunit enzymes found in energy-transducing membranes in mitochondria, chloroplasts and eubacteria [1,2]. They catalyse the synthesis of ATP from ADP and inorganic phosphate by using energy from a transmembrane electrochemical gradient of protons, known as the proton motive force (or pmf). Alternatively, some eubacteria generate a sodium ion motive force (or smf) to power the generation of ATP [3].
The subunits of ATP synthases are organized into membrane intrinsic and membrane extrinsic sectors [1,2]. The membrane extrinsic sector, known as F1-ATPase, is the catalytic part where ATP is formed from ADP and inorganic phosphate. It can be detached experimentally from the membrane domain in an intact state and retains the ability to hydrolyse, but not to synthesize, ATP. The F1-catalytic domains of bacterial ATP synthases are assemblies of five polypeptides. Three α-subunits and three β-subunits are arranged in alternation around a central stalk made from single copies of the γ- and ɛ-subunits, and in the intact ATP synthase, the central stalk is associated with a ring of c-subunits in the membrane domain of the complex. Together, the γɛ-subcomplex and the c-ring constitute the enzyme's rotor. The turning of the rotor modulates the binding properties of the three catalytic sites which lie at three of the interfaces between α- and β-subunits, taking each of them through a cycle of substrate binding, and ATP formation and release. The single δ-subunit sits on top of the α3β3-hexamer and, together with two identical b-subunits (or related but non-identical b- and b′-subunits in some bacterial species and chloroplasts), forms part of the stator linking the external surface of the α3β3-domain to a single a-subunit in the membrane domain. Protons or sodium ions re-enter the bacterial cytoplasm via half channels at the interface between the c-ring and the a-subunit and deliver energy to impel the turning of the rotor.
Many ATP synthases can not only synthesize ATP, but under conditions of low pmf or smf and high intracellular ATP, they can operate in reverse, hydrolysing ATP to generate the pmf or smf, which is required for other cellular functions, such as transmembrane transport of small molecules, or, in motile bacteria, to drive the motor of the flagellum. Therefore, this hydrolytic mechanism has to be regulated in order to prevent wasteful hydrolysis of ATP. Since eubacteria live in a wide range of environments, their pmf or smf is influenced by external factors, such as pH, nutrients and oxygen tension, and can vary over a wide range [4]. Therefore, it is likely that diverse mechanisms of the regulation of bacterial ATP synthases will operate under these multifarious conditions of growth. In recent years, the need to understand these mechanisms of regulation has increased dramatically because of the increase of resistance to antibiotics of pathogenic microorganisms and the authentication of the ATP synthase of Mycobacterium tuberculosis, especially multidrug-resistant, extensively drug-resistant and totally resistant strains, as the target for treating tuberculosis with the drug bedaquiline [5,6]. By implication, other bacterial ATP synthases could also be developed as drug targets for treating infectious diseases, but a rational approach to the design of new drugs requires the detailed structures of the ATP synthases from the pathogens, and an understanding of how they are regulated, and how their structures, mechanisms and modes of regulation differ from those of the human enzyme. As the structure [7–26] and regulation of the closely related bovine enzyme by the inhibitor protein IF1 have been well studied [9,16,27–31], the bovine enzyme provides an excellent surrogate for the human complex.
As part of this endeavour to develop bacterial ATP synthases as drug targets for treating infectious diseases, we have studied the structure and regulation of the F1-catalytic domain of the ATP synthase from the opportunistic periodontal pathogen Fusobacterium nucleatum, which is associated with a wide range of diseases including oral infections, adverse pregnancy outcomes, gastrointestinal disorders and atherosclerosis [32]. Also, it has been linked recently to the development and progression of colorectal cancer via inhibition of anti-tumour immune signalling pathways and the subsequent promotion of chemoresistance [33,34]. Fusobacterium nucleatum is an obligately anaerobic bacterium that grows by fermentative metabolism with glutamate as a substrate. A membrane-bound glutaconyl-CoA decarboxylase catalyses the decarboxylation of glutaconyl-CoA to crotonyl-CoA [35] and couples the free energy of the reaction to the transport of Na+ ions across the membrane, generating an smf [36]. The ATP synthase uses this smf to drive the synthesis of ATP required for catabolic and anabolic reactions [37].
2. Results and discussion
2.1. Biochemical properties of the F1-ATPase from Fusobacterium nucleatum
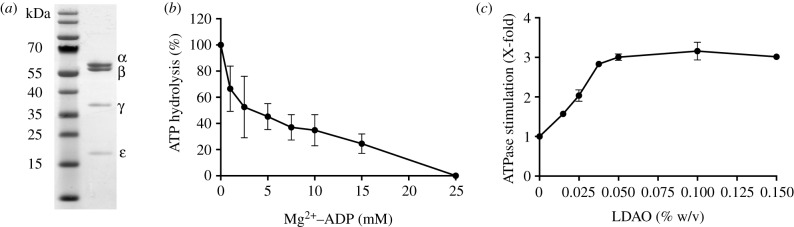
The genes atpAGDC encoding, respectively, the α-, γ-, β- and ɛ-subunits of the F1-catalytic domain of the ATP synthase from F. nucleatum (but lacking the δ-subunit) were cloned into an expression vector with a His10-tag at the N-terminus of the ɛ-subunit and an intervening site for proteolytic cleavage. The purified recombinant enzyme contained the α-, β-, γ- and ɛ-subunits (figure 1). The specific ATP hydrolytic activity at 37°C of various preparations of the enzyme ranged between 3.5 and 9.4 U mg−1 of protein (figure 1; electronic supplementary material, figures S1–S3), similar to specific activities of 4.6–5.8 U mg−1 of the intact purified ATP synthase [37]. The apparent Km value for ATP was 0.12 mM (electronic supplementary material, figure S1). The activity of the enzyme rose with increasing pH from 6.5 to 8.5 where it reached a maximum and declined at higher values (electronic supplementary material, figure S2A). At 4°C, the enzyme was stable over two weeks (electronic supplementary material, figure S2B). At temperatures of 45°C and above, the activity rose substantially, attaining a maximum value of 43.5 U mg−1 at 65°C (electronic supplementary material, figure S2C). From 65 to 75°C, the activity declined, consistent with the melting temperature of the enzyme at 72°C (electronic supplementary material, figure S2D). It is known that the c-rings of sodium-dependent ATP synthases are thermostable [37–40], but the exact molecular basis for the thermostability of c-rings and the F1-domain of the F. nucleatum enzyme remains unknown, although the general basis of thermostability of proteins is well understood [41,42]. As in F1-ATPases from other species, the ATP hydrolase activity of the enzyme was inhibited by Mg2+–ADP [43,44]. Preincubation of the enzyme with 2.5 mM Mg2+–ADP inhibited 45% of the ATP hydrolytic activity (figure 1b), and similar treatment with 25 mM Mg2+–ADP inhibited the enzyme completely. As in other species, the basal hydrolytic activity was stimulated by the addition of lauryldimethylamine oxide [LDAO; 0.05% (w/v)], by a factor of three in this instance (figure 1c). As expected, ATP hydrolysis depended on the presence of Mg2+ or Ca2+ ions, but Mg2+ at concentrations in excess of 2.5 mM was partially inhibitory (electronic supplementary material, figure S3). Both Ca2+ and Mg2+ ions stimulate the hydrolytic activity of the F1-ATPases from Escherichia coli [45,46] and from chloroplasts of Spinacia oleracea (spinach) [47]. However, the hydrolytic activities of the F-ATPases from Clostridium paradoxum [48], from the chemolithotrophic γ-proteobacterium, Acidithiobacillus ferrooxidans [49] and from the bacterial thermophile, Geobacillus stearothermophilus [43], and bovine F1-ATPases, are lower in the presence of Ca2+ than in the presence of Mg2+ [50]. By contrast, in the F1-ATPase from F. nucleatum, the apparent Km value of Ca2+ was lower, and the apparent Vmax was higher than with Mg2+. However, the intact ATP synthase complex from F. nucleatum is not active in the absence of Mg2+ or in the presence of Ca2+ [37].
Figure 1.
Characterization of F1-ATPase from F. nucleatum. (a) The subunits (1.75 µg of enzyme) were separated by SDS–PAGE and stained with Coomassie G-250 dye. Their identities (right-hand side) were verified by mass-mapping of tryptic peptides. The positions of molecular mass markers are indicated on the left. (b) The effect of ADP on ATP hydrolysis at an Mg2+ : ADP ratio of 2 : 1 was assayed by the release of inorganic phosphate. A specific activity of 3.5 U mg−1 was set to 100%. (c) The effect of LDAO on ATP hydrolysis was measured with an ATP-regenerating assay. A value of 1 corresponds to a specific activity of 3.8 U mg−1. Error bars represent the standard deviation of the mean from a biological triplicate. Where no error bars are shown, they are smaller than the diameter of the data points.
2.2. Structure of F1-ATPase from Fusobacterium nucleatum
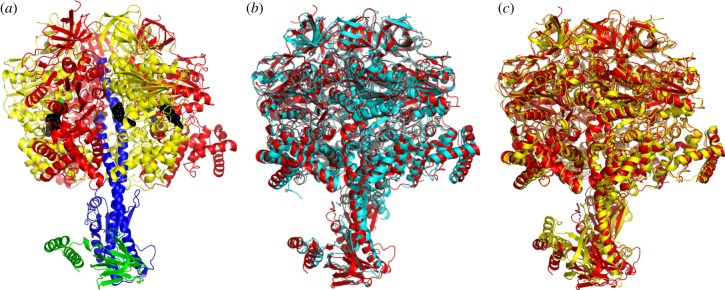
Crystals of the F1-ATPase complex from F. nucleatum have the unit cell parameters a = 111.9 Å, b = 200.2 Å, c = 201.7 Å, β = 102.2° and belong to the space group P21 with two F1-complexes in the asymmetric unit (referred to as molecules 1 and 2, respectively; figure 2a; electronic supplementary material, figure S4). The structure was solved to 3.6 Å resolution by molecular replacement with the α3β3-subcomplex, with no ligands or water molecules, taken from the structure of the F1-ATPase from Caldalkalibacillus thermarum containing the mutations Asp99Ala and Arg92Ala in the ɛ-subunit [52]. The statistics for data processing and refinement are summarized in electronic supplementary material, table S1. The quality of the electron density map is indicated in electronic supplementary material, figure S5, where representative segments and their interpretation are shown. Each of the final models of the two complexes contains three α-subunits (αE, αTP and αDP), three β-subunits (βE, βTP and βDP), and single copies of the γ- and ɛ-subunits, and a total of 6420 amino acid residues were resolved. The final model of molecule 1 (figure 2a) was better-defined than that of molecule 2 and contained 3218 amino acids distributed between the subunits as follows: αE, 25–500; αTP, 27–500; αDP, 25–397 and 403–500; βE, 1–460; βTP 1–462; βDP, 1–460; γ, 2–282; ɛ, 1–134. The final model of molecule 2 contains 3202 amino acid residues comprising αE, 27–500; αTP, 26–397 and 404–500; αDP, 27–400 and 404–495; βE, 2–460; βTP 1–460; βDP, 2–460; γ, 2–282; ɛ, 1–134. The r.m.s.d. value of the superimposed Cα atoms of all subunits of molecule 1 upon molecule 2 was 1.39 Å, and, for the superimposition of Cα atoms of the α- and β-subunits only, the value was 0.46 Å (electronic supplementary material, table S2). The difference between these two values arises from differences in lattice contacts for the two molecules (summarized in electronic supplementary material, figure S4). It results from the different position adopted by the foot of the central stalk relative to the α3β3-subcomplex in the two molecules, which in turn comes from a lattice contact involving residues 108–113 of the γ-subunit of molecule 2 that is not present in molecule 1. In molecule 2, the foot of the γ-subunit is rotated by about 12° in a clockwise direction, as viewed from above the ‘crown’ towards the membrane domain of the intact ATP synthase.
Figure 2.
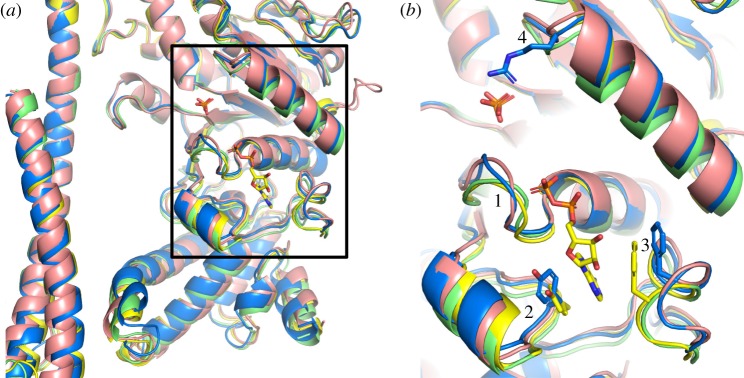
Structure of F1-ATPase from F. nucleatum. (a) Side view of the structure of molecule 1 in ribbon representation with the α-, β-, γ- and ɛ-subunits in red, yellow, blue and green, and bound nucleotides in a black space-filling representation. The green spheres represent Mg2+ ions. (b, c) Comparison of the structure of the F1-ATPase from F. nucleatum (6q45; red) with the structures of F1-ATPases from M. smegmatis [51] (6foc; cyan) and C. thermarum [52] (5ik2; yellow).
2.3. Catalytic α3β3-domain
As in other structures of F1-ATPases, each α-subunit and each β-subunit have three domains: an N-terminal domain with six β-strands, a central nucleotide-binding domain and a C-terminal domain consisting of a bundle of α-helices (seven in the α-subunits and four in the β-subunits). The six N-terminal domains associate together to form the ‘crown’ of the complex. The α3β3-domain and the entire F1-ATPase from F. nucleatum were compared with the equivalent structures from Mycobacterium smegmatis (6foc) [51] and C. thermarum (5ik2) [52] (figure 2b,c; electronic supplementary material, table S3). Similar comparisons were made with the α3β3-domains and F1-ATPases from chloroplasts in spinach (6fkf) [53], Paracoccus denitrificans (5dn6) [54], four bovine structures (4yxw, 2jdi, 1e79, 4asu) [10,12,15,23] and the α3β3-domains from G. stearothermophilus (4xd7) [55] and E. coli (3oaa) [56] and with entire F1-domains from the same species (electronic supplementary material, table S3). The most similar α3β3-domains were those from M. smegmatis (6foc) [51] and C. thermarum (5ik2) [52], and the structures of their F1-domains were the most closely related also. The least similar α3β3-domains were those from G. stearothermophilus (4xd7) [55] and E. coli (3oaa) [56]. Likewise, the least related F1-domains were also from G. stearothermophilus (4xd7) [55] and E. coli (3oaa) [56]. The high r.m.s.d. values for the F1-domains from G. stearothermophilus (4xd7) [55] and E. coli (3oaa) [56] arise because their ɛ-subunits are in the ‘up’ conformation, where the two C-terminal α-helices lie alongside the α-helical coiled-coil in the γ-subunit, resulting in the αDP–βDP interface being displaced outwards (see below).
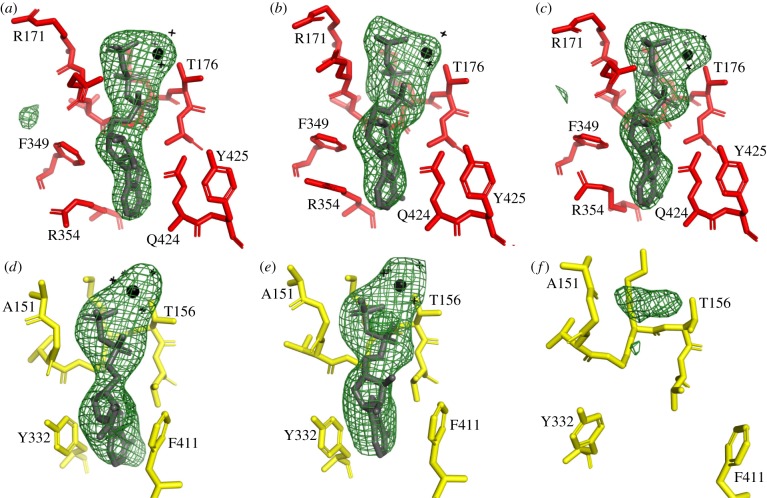
The nucleotide-binding sites in the three α-subunits have additional electron density that is compatible with each of them being occupied by an ATP molecule and an accompanying magnesium ion (figure 3a–c). Similarly, additional density in the nucleotide-binding sites of the βTP- and βDP-subunits provides strong evidence for the presence in each site of an ADP molecule with a magnesium ion (figure 3d,e). There is also some density in the nucleotide-binding site of the βE-subunit (figure 3f), which is increased slightly in molecule 2 relative to molecule 1 (electronic supplementary material, figure S6), although the amino acid side chains that form the nucleotide-binding sites in the two molecules are essentially identical positions. Possible interpretations of this density are either that it is an ADP molecule at very low occupancy, or a citrate molecule, or a mixture of both (electronic supplementary material, figure S6). Citrate was present in the crystallization buffer, and it fits the density in molecule 1 better than in molecule 2 (electronic supplementary material, figure S6E,F). It has been found to be bound to the P-loop of RecA from M. smegmatis [57]. Although the crystallization buffer contained 500 µM ADP, similar to the conditions used with the F1-ATPase from C. thermarum (5ik2) [52] and M. smegmatis (6foc) [51], no ADP was added to the buffer for harvesting the crystals of the F. nucleatum F1-ATPase, and its absence probably accounts for the low occupancy in this site compared to the C. thermarum and M. smegmatis enzymes. The interpretation of the current data is not certain, and therefore neither a nucleotide nor citrate has been included in this site in the model. There is no evidence for the binding of either a magnesium ion or phosphate in the βE-subunit of F1-ATPase in F. nucleatum.
Figure 3.
Occupancy of nucleotide-binding sites in the α- and β-subunits of the F1-ATPase from F. nucleatum. An Fo–Fc difference density map for the complex was calculated with the nucleotides, Mg2+ and water molecules at zero occupancy. The green mesh represents the difference density in the six nucleotide-binding sites contoured to 3.0 σ. In (a–c), the αDP-, αTP- and αE-subunits; in (d–f), the βDP-, βTP- and βE-subunits from molecule 1. In (a–c), the sites are occupied by an ATP molecule and an accompanying Mg2+ (black sphere) with three water ligands (black crosses); the fourth, fifth and sixth ligands are provided by O2B and O2G of the ATP and the hydroxyl of αThr-176. In (d,e), the sites are occupied by an ADP molecule and an accompanying Mg2+ (black sphere) with four water ligands (black crosses); the fifth and sixth ligands are provided by O2B of the ADP and the hydroxyl of βThr-156. In (f), the difference density in the vicinity of the P-loop cannot be interpreted with confidence, but it probably can be accounted for by an ADP molecule (without Mg2+) at low occupancy or citrate.
2.4. γ-subunit
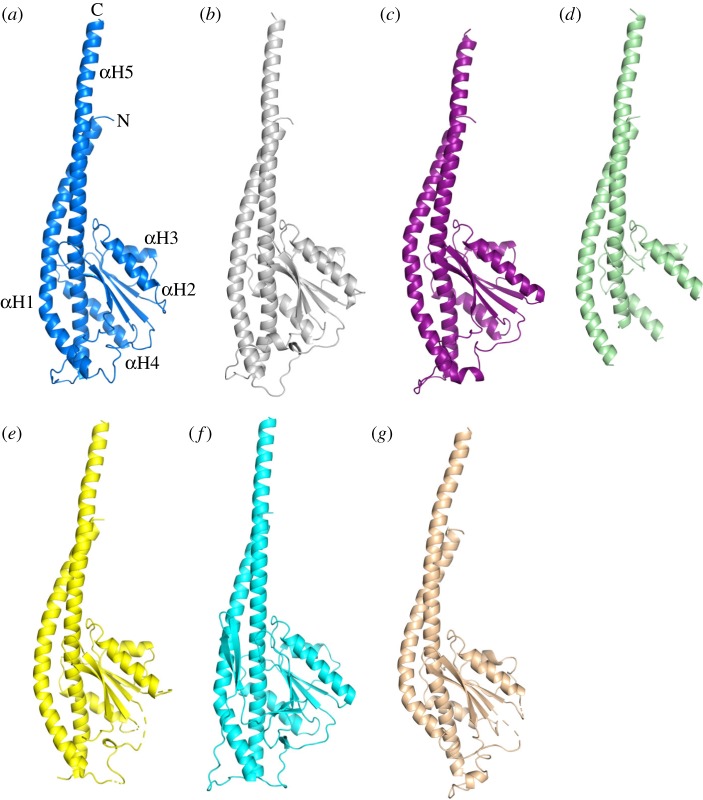
In the structure of the F1-ATPase from F. nucleatum, the γ-subunit was resolved completely. As in other F1-ATPases, it has five α-helices, αH1–αH5. Helices αH1 and αH5 make an antiparallel, α-helical coiled-coil that occupies the central axis of the α3β3-domain, and αH2–αH4 are part of a Rossmann fold with five β-strands with the three α-helices between strands 1 and 2, 2 and 3, and 3 and 4. The lower part of the coiled-coil interacts with the N-terminal domain of the ɛ-subunit. Superimposition of the F. nucleatum γ-subunit on orthologues showed that it is most similar to bacterial γ-subunits from C. thermarum (5ik2) [52], E. coli (3oaa) [56], P. denitrificans (5dn6) [54] and also to the fragmentary structure of the γ-subunit from M. smegmatis (6foc) [51], and to a lesser extent to the γ-subunit in spinach chloroplasts (6fkf) [53] where αH1 is straighter, and the subunit has the additional β-hairpin involved its redox-linked regulatory mechanism (see below). The overall fold of these bacterial γ-subunits (figure 4) is also similar to that of the γ-subunits from the enzymes from bovine (1e79) [15] and yeast (2hld) [58] mitochondria, although in the bacterial subunits αH1 extends further in a C-terminal direction and is less curved.
Figure 4.
Comparison of the structure of the γ-subunit of the F-ATPase from F. nucleatum with those of orthologues. (a) F. nucleatum (6q45; molecule 1) with the five α-helices numbered 1–5 from N- to C-terminus; (b) C. thermarum (5ik2) [52]; (c) E. coli (3oaa) [56]; (d) M. smegmatis (6foc) [51]; only the α-helices were resolved; (e) P. denitrificans (5dn6) [54]; (f) spinach chloroplasts (6fkf) [53]; and (g) bovine mitochondria (1e79) [15].
The rotation of the γ-subunit drives the synthesis of ATP in the F1-domains of F-ATP synthases with energy provided by the pmf (or smf), and each 360° rotation in three 120° steps generates three ATP molecules, one from each of the three catalytic sites of the enzyme [1,2]. During the hydrolysis of ATP, the energy provided by the ATP molecule drives rotation in the opposite sense. The hydrolytic 360° cycle also has three 120° steps [59,60], and the intervening pauses are known as the ‘catalytic dwells’, where the enzyme is poised to carry out, or is carrying out, ATP hydrolysis. At lower concentrations of ATP, a second pause, known as the ‘ATP-binding dwell’ [61,62], where the enzyme awaits the binding of the substrate, occurs 40° after the catalytic dwell, and in the mitochondrial enzyme, but not in bacterial enzymes, a third pause, the ‘phosphate release dwell’, has been observed by stopping rotation with the phosphate analogue, thiophosphate, 25° before the catalytic dwell [63].
In the wide range of high-resolution structures of F1-domains that have been resolved, the majority being structures of the bovine enzyme inhibited in a variety of ways, the ‘foot’ of the γ-subunit consisting of the Rossmann-fold domain and the associated antiparallel α-helical coiled-coil of αH1 and αH5, has rotated to a range of positions. By contrast, the ‘upper’ part of the γ-subunit, consisting of the N-terminal region of αH1, occupies the same position because of the intervention of a ‘catch loop’ provided by the adjacent βE-subunit [64]. This ‘catch loop’ holds the γ-subunit and allows torsional energy to be stored somewhere below the catch. Once a critical point is reached, the stored energy is released in a quantum to generate the rotational step or sub-step. The role of the ‘catch loop’ is illustrated in electronic supplementary material, figure S7, where a selection of mitochondrial and bacterial structures, including the current one from F. nucleatum, have been superimposed. The rotational positions of the ‘foot’ domains in the various structures are summarized in electronic supplementary material, table S4 and figure S8, with the γ-subunit in the original ‘ground-state’ structure assigned arbitrarily as having a rotation of 0° [10] (see Material and Methods for the measurement of rotation; electronic supplementary material, table S4). They include structures that can be related plausibly to rotational positions observed in ‘single-molecule’ experiments with human F1-ATPase [63]. In these rotational experiments, the phosphate release dwell is defined by the position adopted by the central stalk when rotation is inhibited by the phosphate analogue, thiophosphate, and in similar experiments, the F1-ATPase inhibitor protein IF1 stopped rotation at the catalytic dwell. Therefore, the structure of bovine F1-ATPase inhibited with thiophosphate (4yxw) [9] provides a structural representation of the phosphate release dwell, and structures of bovine F1-ATPase inhibited by the monomeric form of IF1 consisting of residues 1–60 (4tt3, 4tsf, 2v7q) [9,16] describe the catalytic dwell. Moreover, in ‘ground-state’ structures, for example (1bmf, 2jdi) [7,12], the enzyme has been arrested at approximately the same rotary position as in the thiophosphate-inhibited state [10], and therefore, it can also be interpreted as representing the phosphate release dwell. Likewise, in the structure of bovine F1-ATPase crystallized in the presence of phosphonate (4asu), rotation has been arrested at the same rotary position at approximately 30° as in the IF1-inhibited enzyme, and so it can be ascribed as representing the catalytic dwell [23]. Neither the structural data nor the ‘single-molecule’ rotary experiments [63] support alternative proposals based on simulations [65] that the structure of bovine F1-ATPase crystallized in the presence of phosphonate (4asu) [23] represents the ATP-binding dwell and that the ‘ground-state’ structures (e.g. 1bmf, 2jdi) [7,12] represent the catalytic dwell [65]. It is possible, but not certain, that the ATP-binding dwell is represented by the structure of bovine F1-ATPase inhibited by ADP and aluminium fluoride (1h8e) [19], where the γ-subunit has rotated through 105° (electronic supplementary material, figure S8 and table S4).
Based on the rotations of their γ-subunits, the structures of those bacterial F1-ATPases where their hydrolytic activities seem to be regulated by the failure to release one or more of the products of hydrolysis, namely M. smegmatis (10.5° rotation) [51] and C. thermarum (11.7° and 13.2° for molecules 1 and 2, respectively) [52], lie at the position of the phosphate release dwell in the mammalian enzyme. Fusobacterium nucleatum (19.7° and 19.1° for molecules 1 and 2, respectively) falls between the position of the phosphate release and catalytic dwells in the mammalian enzyme (electronic supplementary material, table S4). The hydrolytic activity of the P. denitrificans enzyme is inhibited by the ζ-subunit, an orthologue in its inhibitory region of the inhibitory region of bovine IF1, and therefore it is likely that the P. denitrificans structure with a γ-subunit rotation of 27° represents the catalytic dwell of the enzyme. Single-molecule experiments conducted with F1-ATPases from G. stearothermophilus and E. coli show that phosphate is released at the end of the catalytic dwell [66] and that ADP is released 25° before the catalytic dwell [67]. These positions approximate to the rotary positions in the human enzyme [63], but in the reverse order.
A comparison of the structures of the nucleotide-binding sites of the βE-subunits of F1-ATPases from F. nucleatum with those in the F1-ATPases from P. denitrificans (5dn6) [54], M. smegmatis (6foc) [51] and C. thermarum (5ik2) [52] (figure 5) illustrates that in this region, the F. nucleatum subunit is most similar to the P. denitrificans subunit, and that the C. thermarum and M. smegmatis proteins provide a second similar pair, that has a somewhat different conformation to the F. nucleatum and P. denitrificans subunits. This progression from the least open to the most open βE-subunit corresponds with the order of the extents of rotation of the γ-subunit (electronic supplementary material, table S4). The differences between the two pairs are most marked in regions 1–4 in figure 5b. Region 1 (F. nucleatum residues 151–154) is part of the P-loop, and in both F. nucleatum and P. denitrificans, it is displaced away from the γ-subunit relative to C. thermarum and M. smegmatis. Region 2 in F. nucleatum and P. denitrificans is displaced towards the γ-subunit relative to the C. thermarum and M. smegmatis subunits, and contains Tyr-332 (F. nucleatum numbering). However, this tyrosine residue (replaced by Phe-343 in M. smegmatis) contributes to one side of the adenine-binding pocket and is in approximately the same position in the four structures. By contrast, in region 3 of the F. nucleatum βE-subunit, Phe-411 (and the equivalent Phe-420 in P. denitrificans), which contributes to the opposite side of the adenine-binding pocket, is displaced away from the pocket by about 4 Å outwards relative to the equivalent residue, Phe-413, in C. thermarum, and therefore, the nucleotide would be expected to bind less strongly in F. nucleatum than in C. thermarum, as the structure of the F. nucleatum F1-ATPase suggests. The P. denitrificans enzyme was crystallized in the presence of ATP only, and ATP has never been observed bound to a βE-subunit in any structure of F1-ATPase. In region 4, the βE-subunit of F. nucleatum is more similar to the C. thermarum protein than to the P. denitrificans and M. smegmatis proteins. This region forms a loop leading into the α-helix in the top-right of figure 5b. In this loop is found residue Arg-182 (F. nucleatum numbering). In C. thermarum and M. smegmatis, this residue helps to coordinate the bound phosphate, and in F. nucleatum, the side chain is in a similar position, and yet no phosphate is evidently bound in this site. Currently, there is no clear explanation for why phosphate is not bound also in the F. nucleatum F1-ATPase.
Figure 5.
Comparison of the nucleotide-binding sites in βE-subunits in various bacterial F1-ATPases. (a) Cartoon representation of part of the α-helical coiled-coil of the γ-subunits and adjacent nucleotide-binding domains and C-terminal α-helical domains of βE-subunits based on the superimposition of F1-ATPases via their crown domains; F. nucleatum (6q45; blue); P. denitrificans [54] (5dn6; pink); M. smegmatis [51] (6foc; green); C. thermarum [52] (5ik2; yellow). An ADP molecule bound to the βE-subunit from C. thermarum, and phosphate ions bound to the βE-subunits from C. thermarum and M. smegmatis are shown in the stick representation. (b) Magnified version of the region in the box in (a); regions 1, residues 151–154 towards the N-terminal end of the P-loop (residues 149–156) in F. nucleatum; regions 2 and 3 contain aromatic residues, Tyr-332 and Phe-411 in F. nucleatum (shown in the blue stick representation), that form a pocket where the adenine ring of ADP binds; the equivalent residues in C. thermarum (Tyr-334 and Phe-413) are shown in yellow; region 4, loop containing an arginine residue (Arg-182 from F. nucleatum shown in the blue stick representation) involved in binding phosphate ions in M. smegmatis and C. thermarum, but not evidently in F. nucleatum and P. denitrificans.
2.5. ɛ-subunit
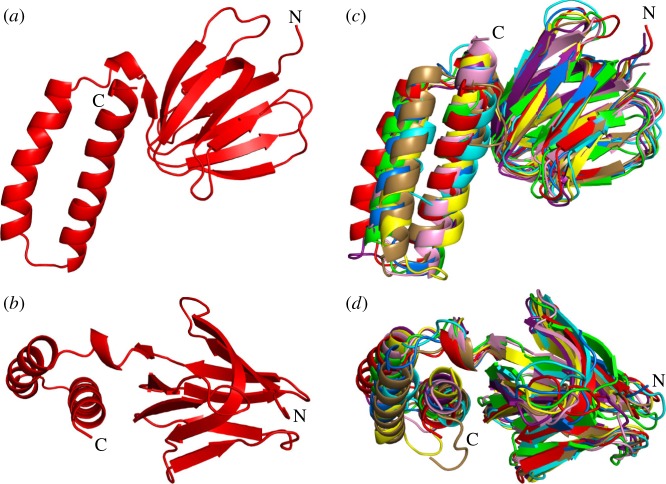
The N-terminal domain of the ɛ-subunit is folded into a 10-stranded β-sandwich, and the C-terminal domain consists of a hairpin of two α-helices, lying alongside the β-sandwich (figure 6). This conformation of two α-helices is known as the ‘down’ position. Superimposition of the structures of the ɛ-subunit from F. nucleatum via their N-terminal domains on those of orthologues demonstrates that the α-helices are in a similar position to those in E. coli (1aqt, 1bsn) [68,71,72], G. stearothermophilus (2e5y) [69] and C. thermarum (5ik2 and 5hkk) [52] (see electronic supplementary material, table S3). Similar to the structures of ɛ-subunits determined in the context of the intact F1-ATPases from E. coli (3oaa) [56] and M. smegmatis (6foc) [51], and in the structures of the intact ATP synthase from E. coli (5t4o) [73], no ATP molecule was bound to the ɛ-subunit in the F1-ATPase from F. nucleatum, and none was bound to the isolated ɛ-subunit or to the ɛ-subunit in the γɛ-subcomplex from Thermosynechococcus elongatus (5zwl) [70]. However, an ATP molecule with an accompanying Mg2+ ion has been found bound to the ɛ-subunit in the F1-ATPase from C. thermarum (5hkk) [52] and to the isolated ɛ-subunit in G. stearothermophilus (2e5y) [69]. In the ɛ-subunits in C. thermarum (5hkk) [52], E. coli (3oaa, 1aqt) [56,68] and G. stearothermophilus (4xd7, 2e5y) [55,69], four conserved amino acids are involved in binding ATP [74]. They are Ile-88, Asp-89, Arg-92 and Ala-93 (C. thermarum numbering; see electronic supplementary material, figure S9). In F. nucleatum, the first two residues are conserved, but the arginine and alanine residues are replaced by serine and glutamic acid, respectively, and hence, the F. nucleatum ɛ-subunit lacks essential features for binding an ATP molecule at this site (electronic supplementary material, figure S9). As described below, the ɛ-subunit has been studied extensively in the context of regulating the hydrolytic activity of bacterial ATP synthases.
Figure 6.
Comparison of the structure of the ɛ-subunit from F. nucleatum with those of orthologues. (a,b) The F. nucleatum ɛ-subunit (6q45, red) viewed from beneath the α3β3-domain along the axis of the central stalk and rotated by 90°, respectively; (c,d) the same views as in (a) and (b) with the structures of ɛ-subunits from the following species superimposed; M. smegmatis [51] (6foc; cyan); C. thermarum [52] (5ik2; yellow); E. coli [68] (1aqt; pink); G. stearothermophilus [69] (2e5y; purple); S. oleracea [53] (6fkf; marine blue); and T. elongatus [70] (5zwl; wheat); and with the δ-subunit from bovine mitochondrial F1-ATPase [15] (1e79;green).
2.6. Regulation of bacterial ATP synthases
Eubacteria have evolved a variety of mechanisms for regulating the hydrolytic activity of their ATP synthases. In α-proteobacteria, exemplified by P. denitrificans, ATP hydrolysis appears to be inhibited by a protein called the ζ-subunit [54,75], where the N-terminal inhibitory region binds to a catalytic interface under hydrolytic conditions in a closely related manner to the inhibitory action of the orthologous mitochondrial regulatory protein IF1 on the mitochondrial ATP synthase [9,16,76]. Cyanobacterial ATP synthases are regulated by a mechanism that appears to be similar, but not identical, to the way that ATP synthases in the chloroplasts of green plants and algae are regulated. In the absence of light, when the pmf is low, ADP–Mg2+ remains bound to one of the three catalytic sites of the chloroplast enzyme forming an inactive ADP-inhibited state of the enzyme [77,78]. This inhibited state is reinforced by the formation of an intramolecular disulfide bond in the γ-subunit of the enzyme, which is thought to stabilize a β-hairpin structure formed by a unique additional sequence (residues 198–233) in the γ-subunit. This β-hairpin wedges between the β-subunit and the central stalk, and may suppress futile ATP hydrolysis by preventing the rotation of the γ-subunit [53]. When light is restored, the pmf increases and reduction of the disulfide bond by thioredoxin unlocks the ATP synthetic activity of the enzyme. In cyanobacterial ATP synthases, the γ-subunits also contain a related insertion [79] that appears to inhibit ATP hydrolysis [70], but it lacks the segment containing the two cysteine residues, and so it cannot be regulated by a similar redox mechanism [80].
The role of the ɛ-subunit in the regulation of bacterial and chloroplast ATP synthases is an area of active study. The known structures of all bacterial [52,55,68,69,71–73,81] and chloroplast [53,70] ɛ-subunits, and the orthologous δ-subunit [15,58] in mitochondria, consist of an N-terminal domain folded into a 10-stranded β-sandwich, and a C-terminal domain folded into an α-helical hairpin. In the intact enzyme, the β-sandwich domain is involved in binding the ɛ-subunit to both the γ-subunit in the central stalk of the F1-domain and the c-ring in the membrane domain of the enzyme. In the various structures of ATP synthases and F1-ATPases, the α-helical C-terminal region has been observed in one of two different conformations. In most structures, the two α-helices are associated closely with the β-sandwich, in the ‘down’ conformation, with an ATP molecule bound between the two domains, in the case of C. thermarum (5hkk) [52] and G. stearothermophilus (2e5y) [69]. In the intact ATP synthases from E. coli (5t4o) [73] and G. stearothermophilus (6n2y) [82] and in the F1-domain from E. coli (3ooa) [56] and G. stearothermophilus (2e5y) [55], the two α-helices assume a different ‘up’ conformation, where they penetrate into the α3β3-catalytic domain along the axis of the coiled-coil of the N- and C-terminal α-helices of the γ-subunit [55,56,73,82]. In this conformation, the N-terminal domain has no bound ATP molecule. Therefore, it has been proposed that in the enzyme from G. stearothermophilus [55] but not in the E. coli enzyme, that a ‘down’-‘up’ switch might provide a physiological mechanism that operates when the pmf and the concentration of ATP are low [69,74,83,84]. Under these conditions, the ATP molecule would leave the ɛ-subunit, allowing the two α-helices to dissociate from the β-domain and form the inhibitory ‘up’ conformation. In the thermophilic cyanobacterium, T. elongatus, where no nucleotide was observed bound to the isolated subunit (2ro6) [85], and in another structure of the γɛ subcomplex where no nucleotide was present during the crystallization of the subcomplex (5zwl) [70], the ɛ-subunit was down in both instances. In this organism, it has been proposed that the ATPase is regulated by the γ-subunit, in a similar fashion to the regulation of ATP hydrolysis in the chloroplast enzyme, but without the regulation via the oxidation and reduction of a disulfide linkage that occurs in the chloroplast enzyme [70]. However, the ATP synthases from C. thermarum [52] and M. smegmatis [51] appear not to conform to this mechanism of regulation. They can both synthesize ATP under appropriate conditions, but they hydrolyse ATP very poorly. The structures of their F1-catalytic domains are very similar to each other and also to the F1-domain of the ATP synthase from F. nucleatum, but despite being inhibited in ATP hydrolysis, the ɛ-subunit of the C. thermarum enzyme is in the ‘down’ position with an ATP molecule and a magnesium ion bound to it. Moreover, the subunit remained in the ‘down’ position, and the enzyme remained inhibited when the capacity of the ɛ-subunit to bind an ATP molecule was removed by mutation [52]. In the mycobacterial enzyme, the α-helical hairpin of its ɛ-subunit is truncated and incapable of binding an ATP molecule, and it also is in the ‘down’ position [51]. However, in the structures of the F1-catalytic domains from C. thermarum and M. smegmatis, a phosphate (possibly a sulfate in M. smegmatis) is bound to the most open of the three catalytic sites, suggesting that the hydrolytic activity of this enzyme may be inhibited by the failure to release one of the products of hydrolysis. In the same catalytic site, ADP is bound also in C. thermarum and is possibly present at low occupancy in M. smegmatis. In F. nucleatum, the ɛ-subunit is ‘down’ with no bound ATP, but, in contrast to the F1-ATPases from C. thermarum and M. smegmatis, it is intrinsically active in ATP hydrolysis, and that activity can be stimulated by LDAO. This behaviour is reminiscent of the behaviour of F1-ATPases from mitochondria, which are similarly intrinsically active and their activity is also stimulated by LDAO. One explanation of the stimulatory effect of LDAO is that it releases inhibitory Mg2+–ADP from the catalytic sites [43] and it has a similar effect on the α3β3γ-subcomplex from G. stearothermophilus [86,87]. However, in the F1-ATPase from C. thermarum, this appears not to be the complete explanation as the activity of this enzyme, in addition to being stimulated by LDAO, is also partially activated by the removal of the C-terminal domain of the ɛ-subunit and could then be activated to its fullest extent by LDAO [88]. In the E. coli F1-ATPase, where the ɛ-subunit is permanently ‘up’ [56,73,89], LDAO has an additional effect as it influences interactions between the catalytic β-subunit and the ɛ-subunit. Thus, currently, the most plausible interpretation of the structure of the enzyme from F. nucleatum is that it represents the state of the enzyme that is partially inhibited by Mg2+–ADP, similar to the bovine F1-ATPase, and this partial inhibition can be relieved by LDAO. The exact molecular role of LDAO in activating these various F1-ATPases remains obscure. One possibility is that it loosens the structure of the nucleotide-binding domain so that the nucleotide is bound less tightly, and a similar explanation can be advanced to explain the activation of the F. nucleatum F1-ATPase by increased temperatures up to 65°C. Structures of LDAO-activated F1-ATPases might help to resolve this issue.
3. Material and methods
3.1. Bacterial strains
Escherichia coli DH10B [90] and MC1061, used in cloning experiments, were grown in LB medium (10 g l−1 tryptone, 5 g l−1 yeast and 5 g l−1 NaCl). The overexpression strain E. coli DK8 (Δunc) [91] was grown in medium containing 2× YT (16 g l−1 tryptone, 10 g l−1 yeast extract and 5 g l−1 NaCl) plus 0.2% [w/v] glucose to compensate for the absence of a functional ATP synthase.
3.2. Construction of expression plasmids
The genes atpAGDC from F. nucleatum encoding the α-, γ-, β- and ɛ-subunits, respectively, of ATP synthase were amplified by a polymerase chain reaction from genomic DNA with the primers FusoF1for (5′-TTTTCCATGGATGAATATTAGACCAGAAGAAG-3′) and FusoF1rev (5′-TTTTGGATCCTTAATTATTCTTAGCATCTATTTTTG-3′). The product was cloned into the expression vector pTrc99a (Amersham Biosciences). The translational initiation codons of the α- and β-subunits were changed to ATG, generating the expression construct pJP2. To facilitate the purification of enzyme, the sequence encoding either a His10-tag with a following cleavage site for the protease from tobacco etch virus (TEV) (pJP3) or a His10 followed by a 6-residue (Ser-Gly-Gly-Gly-GlyGly) linker, an intervening TEV protease cleavage site and another 6-residue (Ser-Gly-Gly-Gly-GlyGly) linker (pJP5) were introduced at the 5′-end of atpC encoding the ɛ-subunit. For pJP3, the primers FusoHis_F1for (5′-TTTTTGAATTCCATCTGCTGTTGGATATCAACC-3′) and FusoHis_F2rev (5′-AAGATTCTCATGGTGATGGTGATGGTGATGGTGATGGTGCATATTCCCTCCTTATTTTGCTAAATC-3′) were used for the first fragment and FusoHis_F3for (5′-CATCACCATCACCATCACCATGAGAATCTTTATTTTCAGGGCCCTAGTTTTGATGTAAGTGTTGTAACAC-3′) and FusoF1rev for the second fragment. For pJP5, the primers FusoHis_F1for and FusoHisLinker_F2rev (5′-ACCTGAGCC CTGAAAATAAAGATTCTCACCGCCACCGCCACCTGAATGGTGATGGTGATGGTGATGGTGATGGTGCATATTCCCT CCTTATTTTGCTAAATC-3′) for generating the first fragment and FusoHisLinker_F3for (5′-CACCATCACCATCACCATTCAGGTGGCGGTGGCGGTGAGAATCTTTATTTTCAGGGCTCAGGTGGCGGTGGCGGTCCTAGTTTTGATGTAAGTG TTGTAACAC-3′) and FusoF1rev for amplifying the second fragment. In both cases, the two fragments overlapped by 30 nucleotides and were joined by overlap extension with the external primers FusoHis_F1for and FusoF1rev. The resulting fragments were cloned into EcoRI and BamHI sites in pJP2 producing the expression vectors pJP3 and pJP5. The sequences of all four genes were verified by DNA sequence analysis. A protein expressed from pJP3 was used in all assays. However, it was found that the TEV protease was unable to cleave the His10-tag. The protein used in the crystallization trials was expressed from pJP5 where the His10-tag was able to be removed by the TEV protease.
3.3. Expression and purification of F1-ATPase from Fusobacterium nucleatum
Expression plasmids pJP3 and pJP5 were transformed into E. coli DK8 (Δunc), together with the helper plasmid pRARE (Addgene). The cells were grown at 37°C to an optical density of 0.4–0.8 at 600 nm in 2× YT medium plus ampicillin (100 µg ml–1), chloramphenicol (34 µg ml−1) and 0.2% [w/v] glucose. Expression from the trc-promoter was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside, and the culture was incubated for 3–4 h at 37°C and then for 16 h at 30°C. The cells were harvested and washed with buffer (50 mM Tris–HCl pH 8.0 and 2 mM MgCl2) and either used immediately or stored at −20°C. The yield of wet cells was 2 g l–1. Cells (approx. 7–10 g) were resuspended in the same buffer plus cOmplete EDTA-free protease inhibitor tablets (Roche) and DNase I (Roche), and disrupted by two passages through a Constant Systems cell disrupter at 31 kpsi. Cell debris was removed by centrifugation (10 000 × g, 15 min, 4°C), and the supernatant was centrifuged again (131 500 × g for 45 min at 4°C). To the resulting supernatant, 100 mM NaCl and 25 mM imidazole were added, and this solution was loaded at a flow rate of 2 ml min−1 onto a HisTrap HP nickel affinity column (5 ml; GE Healthcare). The column was washed with buffer A consisting of 20 mM Tris–HCl, pH 8.0, 10% [w/v] glycerol, 2 mM MgCl2, 100 mM NaCl, 25 mM imidazole, and 0.1 mM phenylmethylsulfonyl fluoride. The F1-ATPase was eluted with a linear gradient of 100 ml of buffer A and buffer A containing 500 mM imidazole. For use in enzymic or biophysical experiments, the enzyme was pooled and concentrated by ultrafiltration with a 100 kDa cut-off membrane, and then passed through a Superose 6 10/300 size exclusion column (GE Healthcare), equilibrated in buffer consisting of 20 mM Tris–HCl, pH 8.0, 10% [w/v] glycerol, 2 mM MgCl2, and 100 mM NaCl at a flow rate of 0.5 ml min−1. Fractions containing F1-ATPase were pooled. For use in crystallization experiments, fractions containing F1-ATPase from the HisTrap column were pooled and the His10-tag was cleaved off with the TEV protease for 18 h at 23°C in buffer containing 20 mM Tris–HCl, pH 8.0, 20% [w/v] glycerol, 2 mM MgCl2, 100 mM NaCl, and 1 mM tris(2-carboxyethyl)-phosphine). The sample was concentrated to 2–3 ml by centrifugal ultrafiltration (100 kDa molecular mass cut-off) and then re-loaded onto the HisTrap HP column at a flow rate of 1 ml min−1. The F1-ATPase eluted in the excluded volume of the column. Fractions containing the enzyme were pooled, concentrated and applied to a Superose 6 10/300 size exclusion column (GE Healthcare) equilibrated in buffer containing 20 mM Tris–HCl, pH 8.0, 10% [w/v] glycerol, 2 mM MgCl2, 100 mM NaCl, and 1 mM ADP.
3.4. Biochemical methods
The F1-ATPase from F. nucleatum was analysed by SDS–PAGE on 4–12% NuPAGE Bis–Tris Mini gels (Life Technologies). Proteins were detected with Coomassie G-250 dye. The bands from the stained gel were excised, and the identities of the proteins were verified by mass-mapping of tryptic peptides in a MALDI-TOF mass spectrometer. Protein concentrations were measured with the DC protein assay kit (Bio-Rad) with bovine serum albumin as a standard. ATP hydrolysis was measured by an ATP-regenerating assay, at 37°C unless otherwise stated, where one unit of activity is equal to 1 µmol of ADP produced per minute [92] or by the colorimetric assay of inorganic phosphate where one unit of activity is equal to 1 µmol of phosphate released per minute [93]. The influence of pH on the activity of the enzyme was examined in a three-buffer mixture composed of 50 mM each of MES–MOPS–Tris–HCl [94]. The inhibitory effect of increasing concentrations of Mg2+–ADP at a constant ratio of Mg2+ : ADP 2 : 1 (w : w) on ATP hydrolysis was examined. The enzyme was pre-incubated with the Mg2+–ADP mixture for 10 min and then ATP hydrolysis was initiated by the addition of ATP.
3.5. Thermal stability
The melting temperature of F1-ATPase was determined in a LightCycler 480 (Roche) in a reaction mixture (20 µl) containing 5 µM F1-ATPase in buffer consisting of 20 mM Tris–HCl, pH 8.0, 10% [w/v] glycerol, 2 mM MgCl2, and 100 mM NaCl, 5× SYPRO Orange Dye and 100 mM Tris–HCl, pH 8.0, at 20°C. The assay [95,96] was optimized with enzyme concentrations of 0.5–5 µM and 5–20× SYPRO Orange Dye. The optimal conditions were 5 µM F1-ATPase and 5× SYPRO Orange Dye. Samples were equilibrated at 20°C for 5 min, and then the temperature was increased by 1°C min−1 to 95°C. Fluorescence was measured at intervals of 0.588°C, and the melting point of F1-ATPase was calculated with LightCycler 480 software v.1.5.1.62.
3.6. Crystallization of F1-ATPase from Fusobacterium nucleatum
The enzyme was concentrated by ultrafiltration to 2–2.5 mg ml−1 and centrifuged (16 000 × g, 5 min) at 4°C. It was crystallized at 18°C by vapour diffusion in hanging drops in 24-well plates. The drops consisted of 1 µl of protein solution, 0.8 µl of precipitant buffer [100 mM sodium citrate, pH 6.0, 100 mM magnesium acetate and 15.5% [w/v] polyethylene glycol 5000 monomethyl ether] and 0.2 µl of low melting-point agarose (Hampton Research) (final concentration 0.2% [w/v]). The reservoir contained 1 ml of precipitant buffer. Crystals were harvested after 3–4 days' growth and washed for 2–5 min in cryoprotection buffer containing 100 mM sodium citrate, pH 6.0, 100 mM magnesium acetate, 15.5% [w/v] polyethylene glycol 5000 monomethyl ether and 30% [v/v] ethylene glycol.
3.7. Data collection, structure determination and refinement
Diffraction data were collected from two cryo-protected crystals of F1-ATPase from F. nucleatum at the Australian Synchrotron MX2 beamline [97], equipped with an ADSC Quantum 315r detector, and processed with XDS [98]. Because of radiation damage, four datasets from two crystals with the same space group and unit cell were merged with AIMLESS [99] during data reduction in CCP4 [100]. Molecular replacement was carried out with PHASER [101] using the α3β3 subcomplex from the structure of the F1-ATPase from C. thermarum containing the mutations Asp89Ala and Arg92Ala in the ɛ-subunit (5ik2) [52] with nucleotides and other ligands removed. Rigid body refinement and restrained refinement using non-crystallographic symmetry restraints were performed with REFMAC5 [102]. In between each refinement round with REFMAC5, parts of the structure were rebuilt manually with Coot [103]. The stereochemistry of the structure was assessed with MolProbity [104]. Electron density maps were calculated with FFT [100], and images of structures and electron density maps were prepared in PyMOL [105].
3.8. Rotation of the γ-subunit
Residues 23–33 of the F. nucleatum γ-subunit (and equivalent regions in other F1-ATPases) interact with the C-terminal domains of the α- and β-subunits, and this segment acts as a rigid body uninfluenced by contacts in the crystal lattice between adjacent F1-ATPase complexes. By contrast, residues 34–226 of the γ-subunit, and the associated δ- and ɛ-subunits lie outside the α3β3-domain, where their positions may be subject to such influences. Therefore, the rotations of residues 23–33 of the γ-subunit in the various aligned structures were measured relative to the position of the same segment (residues 22–32) in the ground-state structure of azide-free bovine F1-ATPase (2jdi) [12]. These measurements were made by aligning the structures via the crown domains at the N-termini of α- and β-subunits and then by calculating the centre of mass of residues 22–33 of the γ-subunit and determining the rotation angle around the pseudo-threefold axis of the α-subunits required to match its position with that of the equivalent segment in the bovine azide-free ground-state structure, which was taken as the reference point, set as 0° [10]. To calculate the rotation of the γ-subunit in molecule 2 with respect to that in molecule 1, the distances between the Cα of residues 95 and 105 (helix 2 in the γ-subunit) were measured in both molecules and then to their equivalent residues in the other model. These distances were extrapolated to a common origin giving the lengths of three sides of a triangle, allowing the angle to be calculated.
Supplementary Material
Acknowledgements
We thank the MX2 beamline staff at the Australian Synchrotron.
Data accessibility
The data available from the PDB accession code: 6q45.
Authors' contributions
Conceptualization: J.E.W and G.M.C. Methodology: J.P., Y.N., M.G.M., S.A.F., D.A., A.H. Investigation: J.P., Y.N., S.A.F., D.A., A.H. Formal analysis: J.P., Y.N., M.G.M. and A.G.W.L. Writing the original draft: J.E.W., J.P. and M.G.M. Writing review and editing: J.E.W., G.M.C., J.P., M.G.M., A.G.W.L. and S.A.F. Supervision: G.M.C. and J.E.W. Funding acquisition: G.M.C. and J.E.W.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by a James Cook Fellowship from the Royal Society of New Zealand to G.M.C. and by the Medical Research Council, U.K. by grants MC_UU_00015/8 and MR/M009858/1 to J.E.W., and MC_U105184325 to A.G.W.L.
References
- 1.Walker JE. 2013. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16. ( 10.1042/BST20110773) [DOI] [PubMed] [Google Scholar]
- 2.Walker JE. 2017. Structure, mechanism and regulation of ATP synthases. In Mechanisms of primary energy transduction in biology (ed. Wikström M.), pp. 338–373. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- 3.von Ballmoos C, Cook GM, Dimroth P.. 2008. Unique rotary ATP synthase and its biological diversity. Annu. Rev. Biophys. 37, 43–64. ( 10.1146/annurev.biophys.37.032807.130018) [DOI] [PubMed] [Google Scholar]
- 4.Dimroth P, Cook GM. 2004. Bacterial Na+- or H+ -coupled ATP synthases operating at low electrochemical potential. Adv. Microb. Physiol. 49, 175–218. ( 10.1016/S0065-2911(04)49004-3) [DOI] [PubMed] [Google Scholar]
- 5.Diacon AH, et al. 2012. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 56, 3271–3276. ( 10.1128/AAC.06126-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diacon AH, et al. 2014. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 371, 723–732. ( 10.1056/NEJMoa1313865) [DOI] [PubMed] [Google Scholar]
- 7.Abrahams JP, Leslie AGW, Lutter R, Walker JE. 1994. Structure at 2.8Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628. ( 10.1038/370621a0) [DOI] [PubMed] [Google Scholar]
- 8.Abrahams JP, Buchanan SK, Van Raaij MJ, Fearnley IM, Leslie AGW, Walker JE.. 1996. The structure of bovine F1-ATPase complexed with the peptide antibiotic efrapeptin. Proc. Natl Acad. Sci. USA 93, 9420–9424. ( 10.1073/pnas.93.18.9420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bason JV, Montgomery MG, Leslie AGW, Walker JE. 2014. Pathway of binding of the intrinsically disordered mitochondrial inhibitor protein to F1-ATPase. Proc. Natl Acad. Sci. USA 111, 11 305–11 310. ( 10.1073/pnas.1411560111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bason JV, Montgomery MG, Leslie AGW, Walker JE. 2015. How release of phosphate from mammalian F1-ATPase generates a rotary substep. Proc. Natl Acad. Sci. USA 112, 6009–6014. ( 10.1073/pnas.1506465112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowler MW, Montgomery MG, Leslie AGW, Walker JE. 2006. How azide inhibits ATP hydrolysis by the F-ATPases. Proc. Natl Acad. Sci. USA 103, 8646–8649. ( 10.1073/pnas.0602915103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowler MW, Montgomery MG, Leslie AGW, Walker JE. 2007. Ground state structure of F1-ATPase from bovine heart mitochondria at 1.9Å resolution. J. Biol. Chem. 282, 14 238–14 242. ( 10.1074/jbc.M700203200) [DOI] [PubMed] [Google Scholar]
- 13.Braig K, Menz RI, Montgomery MG, Leslie AGW, Walker JE. 2000. Structure of bovine mitochondrial F1-ATPase inhibited by Mg2+ ADP and aluminium fluoride. Structure 8, 567–573. ( 10.1016/S0969-2126(00)00145-3) [DOI] [PubMed] [Google Scholar]
- 14.Dickson VK, Silvester JA, Fearnley IM, Leslie AGW, Walker JE. 2006. On the structure of the stator of the mitochondrial ATP synthase. EMBO J. 25, 2911–2918. ( 10.1038/sj.emboj.7601177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbons C, Montgomery MG, Leslie AGW, Walker JE. 2000. The structure of the central stalk in bovine F1-ATPase at 2.4Å resolution. Nat. Struct. Biol. 7, 1055–1061. ( 10.1038/80981) [DOI] [PubMed] [Google Scholar]
- 16.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. 2007. How the regulatory protein, IF1, inhibits F1-ATPase from bovine mitochondria. Proc. Natl Acad. Sci. USA 104, 15 671–15 676. ( 10.1073/pnas.0707326104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. 2007. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl Acad. Sci. USA 104, 13 632–13 637. ( 10.1073/pnas.0706290104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagawa R, Montgomery MG, Braig K, Leslie AGW, Walker JE. 2004. The structure of bovine F1-ATPase inhibited by ADP and beryllium fluoride. EMBO J. 23, 2734–2744. ( 10.1038/sj.emboj.7600293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menz RI, Walker JE, Leslie AGW. 2001. Structure of bovine mitochondrial F1-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell 106, 331–341. ( 10.1016/S0092-8674(01)00452-4) [DOI] [PubMed] [Google Scholar]
- 20.Menz RI, Leslie AGW, Walker JE. 2001. The structure and nucleotide occupancy of bovine mitochondrial F1-ATPase are not influenced by crystallisation at high concentrations of nucleotide. FEBS Lett. 494, 11–14. ( 10.1016/S0014-5793(01)02302-X) [DOI] [PubMed] [Google Scholar]
- 21.Orriss GL, Leslie AGW, Braig K, Walker JE. 1998. Bovine F1-ATPase covalently inhibited with 4-chloro-7-nitrobenzofurazan: the structure provides further support for a rotary catalytic mechanism. Structure 6, 831–837. ( 10.1016/S0969-2126(98)00085-9) [DOI] [PubMed] [Google Scholar]
- 22.Rees DM, Leslie AGW, Walker JE. 2009. The structure of the membrane extrinsic region of bovine ATP synthase. Proc. Natl Acad. Sci. USA 106, 21 597–21 601. ( 10.1073/pnas.0910365106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees DM, Montgomery MG, Leslie AGW, Walker JE. 2012. Structural evidence of a new catalytic intermediate in the pathway of ATP hydrolysis by F1-ATPase from bovine heart mitochondria. Proc. Natl Acad. Sci. USA 109, 11 139–11 143. ( 10.1073/pnas.1207587109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Raaij MJ, Abrahams JP, Leslie AGW, Walker JE.. 1996. The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B. Proc. Natl Acad. Sci. USA 93, 6913–6917. ( 10.1073/pnas.93.14.6913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watt IN, Montgomery MG, Runswick MJ, Leslie AGW, Walker JE. 2010. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl Acad. Sci. USA 107, 16 823–16 827. ( 10.1073/pnas.1011099107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, Grigorieff N, Rubinstein JL. 2015. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. eLife 4, e10180 ( 10.7554/eLife.10180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabezon E, Arechaga I, Butler PJG, Walker JE. 2000. Dimerization of bovine F1-ATPase by binding the inhibitor protein, IF1. J. Biol. Chem. 275, 28 353–28 355. ( 10.1074/jbc.C000427200) [DOI] [PubMed] [Google Scholar]
- 28.Cabezon E, Butler PJ, Runswick MJ, Walker JE. 2000. Modulation of the oligomerization state of the bovine F1-ATPase inhibitor protein, IF1, by pH. J. Biol. Chem. 275, 25 460–25 464. ( 10.1074/jbc.M003859200) [DOI] [PubMed] [Google Scholar]
- 29.Cabezón E, Runswick MJ, Leslie AGW, Walker JE. 2001. The structure of bovine IF1, the regulatory subunit of mitochondrial F-ATPase. EMBO J. 20, 6990–6996. ( 10.1093/emboj/20.24.6990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabezón E, Montgomery MG, Leslie AGW, Walker JE. 2003. The structure of bovine F1-ATPase in complex with its regulatory protein IF1. Nat. Struct. Biol. 10, 744–750. ( 10.1038/nsb966) [DOI] [PubMed] [Google Scholar]
- 31.Bason JV, Runswick MJ, Fearnley IM, Walker JE. 2011. Binding of the inhibitor protein IF1 to bovine F1-ATPase. J. Mol. Biol. 406, 443–453. ( 10.1016/j.jmb.2010.12.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han YW. 2015. Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. ( 10.1016/j.mib.2014.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T, et al. 2017. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563. ( 10.1016/j.cell.2017.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bullman S, et al. 2017. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. ( 10.1126/science.aal5240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beatrix B, Bendrat K, Rospert S, Buckel W. 1990. The biotin-dependent sodium ion pump glutaconyl-CoA decarboxylase from Fusobacterium nucleatum (subsp. nucleatum). Comparison with the glutaconyl-CoA decarboxylases from Gram-positive bacteria. Arch. Microbiol. 154, 362–369. ( 10.1007/BF00276532) [DOI] [PubMed] [Google Scholar]
- 36.Dimroth P. 1997. Primary sodium ion translocating enzymes. Biochim. Biophys. Acta 1318, 11–51. ( 10.1016/s0005-2728(96)00127-2) [DOI] [PubMed] [Google Scholar]
- 37.Schulz S, Iglesias-Cans M, Krah A, Yildiz O, Leone V, Matthies D, Cook GM, Faraldo-Gómez JD, Meier T. 2013. A new type of Na+-driven ATP synthase membrane rotor with a two-carboxylate ion-coupling motif. PLoS Biol. 11, e1001596 ( 10.1371/journal.pbio.1001596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann S, Matthey U, Kaim G, Dimroth P. 1998. Purification and properties of the F1Fo ATPase of Ilyobacter tartaricus, a sodium ion pump. J. Bacteriol. 180, 3312–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier T, Ferguson SA, Cook GM, Dimroth P, Vonck J. 2006. Structural investigations of the membrane-embedded rotor ring of the F-ATPase from Clostridium paradoxum. J. Bacteriol. 188, 7759–7764. ( 10.1128/JB.00934-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier T, Morgner N, Matthies D, Pogoryelov D, Keis S, Cook GM, Dimroth P, Brutschy B. 2007. A tridecameric c ring of the adenosine triphosphate (ATP) synthase from the thermoalkaliphilic Bacillus sp. strain TA2.A1 facilitates ATP synthesis at low electrochemical proton potential. Mol. Microbiol. 65, 1181–1192. ( 10.1111/j.1365-2958.2007.05857.x) [DOI] [PubMed] [Google Scholar]
- 41.Walker JE, Wonacott AJ, Harris JI. 1980. Heat stability of a tetrameric enzyme, d-glyceraldehyde-3-phosphate dehydrogenase. Eur. J. Biochem. 108, 581–586. ( 10.1111/j.1432-1033.1980.tb04753.x) [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Tsai CJ, Nussinov R. 2000. Factors enhancing protein thermostability. Protein Eng. 13, 179–191. ( 10.1093/protein/13.3.179) [DOI] [PubMed] [Google Scholar]
- 43.Yoshida M, Allison WS. 1983. Modulation by ADP and Mg2+ of the inactivation of the F1-ATPase from the thermophilic bacterium, PS3, with dicyclohexylcarbodiimide. J. Biol. Chem. 258, 14 407–14 412. [PubMed] [Google Scholar]
- 44.Hyndman DJ, Milgrom YM, Bramhall EA, Cross RL. 1994. Nucleotide-binding sites on Escherichia coli F1-ATPase. Specificity of noncatalytic sites and inhibition at catalytic sites by MgADP. J. Biol. Chem. 269, 28 871–28 877. [PubMed] [Google Scholar]
- 45.Evans DJ. 1970. Membrane Mg2+–(Ca2+)-activated adenosine triphosphatase of Escherichia coli: characterization in the membrane-bound and solubilized states. J. Bacteriol. 104, 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butlin JD, Cox GB, Gibson F. 1971. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem. J. 124, 75–81. ( 10.1042/bj1240075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Digel JG, Moore ND, McCarty RE. 1998. Influence of divalent cations on nucleotide exchange and ATPase activity of chloroplast coupling factor 1. Biochemistry 37, 17 209–17 215. ( 10.1021/bi982027p) [DOI] [PubMed] [Google Scholar]
- 48.Ferguson SA, Keis S, Cook GM. 2006. Biochemical and molecular characterization of a Na+-translocating F1Fo-ATPase from the thermoalkaliphilic bacterium Clostridium paradoxum. J. Bacteriol. 188, 5045–5054. ( 10.1128/JB.00128-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakai S, Ohmori A, Kanao T, Sugio T, Kamimura K. 2005. Purification and biochemical characterization of the F1-ATPase from Acidithiobacillus ferrooxidans NASF-1 and analysis of the ATP operon. Biosci. Biotechnol. Biochem. 69, 1884–1891. ( 10.1271/bbb.69.1884) [DOI] [PubMed] [Google Scholar]
- 50.Dorgan LJ, Urbauer JL, Schuster SM. 1984. Metal dependence and thermodynamic characteristics of the beef heart mitochondrial adenosine triphosphatase. J. Biol. Chem. 259, 2816–2821. [PubMed] [Google Scholar]
- 51.Zhang AT, Montgomery MG, Leslie AGW, Cook GM, Walker JE. 2019. The structure of the catalytic domain of the ATP synthase from Mycobacterium smegmatis is a target for developing antitubercular drugs. Proc. Natl Acad. Sci. USA 116, 4206–4211. ( 10.1073/pnas.1817615116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson SA, Cook GM, Montgomery MG, Leslie AGW, Walker JE. 2016. Regulation of the thermoalkaliphilic F1-ATPase from Caldalkalibacillus thermarum. Proc. Natl Acad. Sci. USA 113, 10 860–10 865. ( 10.1073/pnas.1612035113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn A, Vonck J, Mills DJ, Meier T, Kühlbrandt W. 2018. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 360, aat4318 ( 10.1126/science.aat4318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales-Rios E, Montgomery MG, Leslie AGW, Walker JE. 2015. The structure of ATP synthase from Paracoccus denitrificans determined by X-ray crystallography at 4.0Å resolution. Proc. Natl Acad. Sci. USA 112, 13 231–13 236. ( 10.1073/pnas.1517542112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shirakihara Y, Shiratori A, Tanikawa H, Nakasako M, Yoshida M, Suzuki T. 2015. Structure of a thermophilic F1-ATPase inhibited by an ɛ-subunit: deeper insight into the ɛ-inhibition mechanism. FEBS J. 282, 2895–2913. ( 10.1111/febs.13329) [DOI] [PubMed] [Google Scholar]
- 56.Cingolani G, Duncan TM. 2011. Structure of the ATP synthase catalytic complex F1 from Escherichia coli in an autoinhibited conformation. Nat. Struct. Mol. Biol. 18, 701–707. ( 10.1038/nsmb.2058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandran AV, Prabu JR, Nautiyal A, Patil KN, Muniyappa K, Vijayan M. 2015. Structural studies on Mycobacterium tuberculosis RecA: molecular plasticity and interspecies variability. J. Biosci. 40, 13–30. ( 10.1007/s12038-014-9497-x) [DOI] [PubMed] [Google Scholar]
- 58.Kabaleeswaran V, Puri N, Walker JE, Leslie AGW, Mueller DM. 2006. Novel features of the rotary catalytic mechanism revealed in the structure of yeast F1 ATPase. EMBO J. 25, 5433–5442. ( 10.1038/sj.emboj.7601410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noji H, Yasuda R, Yoshida M, Kinosita K Jr. 1997. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302. ( 10.1038/386299a0) [DOI] [PubMed] [Google Scholar]
- 60.Yasuda R, Noji H, Kinosita K, Yoshida M. 1998. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120 degree steps. Cell 93, 1117–1124. ( 10.1016/S0092-8674(00)81456-7) [DOI] [PubMed] [Google Scholar]
- 61.Yasuda R, Noji H, Yoshida M, Kinosita K, Itoh H. 2001. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature 410, 898–904. ( 10.1038/35073513) [DOI] [PubMed] [Google Scholar]
- 62.Masaike T, Koyama-Horibe F, Oiwa K, Yoshida M, Nishizaka T. 2008. Cooperative three-step motions in catalytic subunits of F1-ATPase correlate with 80 degrees and 40 degrees substep rotations. Nat. Struct. Mol. Biol. 15, 1326–1333. ( 10.1038/nsmb.1510) [DOI] [PubMed] [Google Scholar]
- 63.Suzuki T, Tanaka K, Wakabayashi C, Saita E, Yoshida M. 2014. Chemomechanical coupling of human mitochondrial F1-ATPase motor. Nat. Chem. Biol. 10, 930–936. ( 10.1038/nchembio.1635) [DOI] [PubMed] [Google Scholar]
- 64.Greene MD, Frasch WD. 2003. Interactions among gamma R268, gamma Q269, and the β subunit catch loop of Escherichia coli F1-ATPase are important for catalytic activity. J. Biol. Chem. 278, 51 594–51 598. ( 10.1074/jbc.M309948200) [DOI] [PubMed] [Google Scholar]
- 65.Nam K, Pu J, Karplus M. 2014. Trapping the ATP binding state leads to a detailed understanding of the F1-ATPase mechanism. Proc. Natl Acad. Sci. USA 111, 17 851–17 856. ( 10.1073/pnas.1419486111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe R, Iino R, Noji H. 2010. Phosphate release in F1-ATPase catalytic cycle follows ADP release. Nat. Chem. Biol. 6, 814–820. ( 10.1038/nchembio.443) [DOI] [PubMed] [Google Scholar]
- 67.Martin JL, Ishmukhametov R, Hornung T, Ahmad Z, Frasch WD. 2014. Anatomy of F1-ATPase powered rotation. Proc. Natl Acad. Sci. USA 111, 3715–3720. ( 10.1073/pnas.1317784111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhlin U, Cox GB, Guss JM. 1997. Crystal structure of the ɛ subunit of the proton-translocating ATP synthase from Escherichia coli. Structure 5, 1219–1230. ( 10.1016/S0969-2126(97)00272-4) [DOI] [PubMed] [Google Scholar]
- 69.Yagi H, Kajiwara N, Tanaka H, Tsukihara T, Kato-Yamada Y, Yoshida M, Akutsu H. 2007. Structures of the thermophilic F1-ATPase ɛ subunit suggesting ATP-regulated arm motion of its C-terminal domain in F1. Proc. Natl Acad. Sci. USA 104, 11 233–11 238. ( 10.1073/pnas.0701045104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami S, Kondo K, Katayama S, Hara S, Sunamura EI, Yamashita E, Groth G, Hisabori T. 2018. Structure of the γ-ɛ complex of cyanobacterial F1-ATPase reveals a suppression mechanism of the γ subunit on ATP hydrolysis in phototrophs. Biochem. J. 475, 2925–2939. ( 10.1042/BCJ20180481) [DOI] [PubMed] [Google Scholar]
- 71.Wilkens S, Dahlquist FW, McIntosh LP, Donaldson LW, Capaldi RA. 1995. Structural features of the ɛ subunit of the Escherichia coli ATP synthase determined by NMR spectroscopy. Nat. Struct. Biol. 2, 961–967. ( 10.1038/nsb1195-961) [DOI] [PubMed] [Google Scholar]
- 72.Wilkens S, Capaldi RA. 1998. Solution structure of the ɛ subunit of the F1-ATPase from Escherichia coli and interactions of this subunit with β subunits in the complex. J. Biol. Chem. 273, 26 645–26 651. ( 10.1074/jbc.273.41.26645) [DOI] [PubMed] [Google Scholar]
- 73.Sobti M, Smits C, Wong AS, Ishmukhametov R, Stock D, Sandin S, Stewart AG. 2016. Cryo-EM structures of the autoinhibited E. coli ATP synthase in three rotational states. eLife 5, e21598 ( 10.7554/eLife.21598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato S, Yoshida M, Kato-Yamada Y. 2007. Role of the ɛ subunit of thermophilic F1-ATPase as a sensor for ATP. J. Biol. Chem. 282, 37 618–37 623. ( 10.1074/jbc.M707509200) [DOI] [PubMed] [Google Scholar]
- 75.Morales-Ríos E, de la Rosa-Morales F, Mendoza-Hernández G, Rodríguez-Zavala JS, Celis H, Zarco-Zavala M, García-Trejo JJ.. 2010. A novel 11-kDa inhibitory subunit in the F1Fo ATP synthase of Paracoccus denitrificans and related α-proteobacteria. FASEB J. 24, 599–608. ( 10.1096/fj.09-137356) [DOI] [PubMed] [Google Scholar]
- 76.Robinson GC, Bason JV, Montgomery MG, Fearnley IM, Mueller DM, Leslie AGW, Walker JE. 2013. The structure of F1-ATPase from Saccharomyces cerevisiae inhibited by its regulatory protein IF1. Open Biol. 3, 120164 ( 10.1098/rsob.120164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nalin CM, McCarty RE. 1984. Role of a disulfide bond in the γ subunit in activation of the ATPase of chloroplast coupling factor 1. J. Biol. Chem. 259, 7275–7280. [PubMed] [Google Scholar]
- 78.Ketcham SR, Davenport JW, Warncke K, McCarty RE. 1984. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J. Biol. Chem. 259, 7286–7293. [PubMed] [Google Scholar]
- 79.Cozens AL, Walker JE. 1987. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301: support for an endosymbiotic origin of chloroplasts. J. Mol. Biol. 194, 359–383. ( 10.1016/0022-2836(87)90667-X) [DOI] [PubMed] [Google Scholar]
- 80.Werner S, Schumann J, Strotmann H. 1990. The primary structure of the gamma-subunit of the ATPase from Synechocystis 6803. FEBS Lett. 261, 204–208. ( 10.1016/0014-5793(90)80671-5) [DOI] [PubMed] [Google Scholar]
- 81.Joon S, Priya R, Lavanya S, Nartey W, Kundu S, Manimekalai MSS, Bogdanović N, Dick T, Grüber G. 2018. The NMR solution structure of Mycobacterium tuberculosis F-ATP synthase subunit ɛ provides new insight into energy coupling inside the rotary engine. FEBS J. 285, 1111–1128. ( 10.1111/febs.14392) [DOI] [PubMed] [Google Scholar]
- 82.Guo H, Suzuki T, Rubinstein JL. 2019. Structure of a bacterial ATP synthase. eLife 8, e43128 ( 10.7554/eLife.43128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feniouk BA, Suzuki T, Yoshida M. 2007. Regulatory interplay between proton motive force, ADP, phosphate, and subunit epsilon in bacterial ATP synthase. J. Biol. Chem. 282, 764–772. ( 10.1074/jbc.M606321200) [DOI] [PubMed] [Google Scholar]
- 84.Suzuki T, Murakami T, Iino R, Suzuki J, Ono S, Shirakihara Y, Yoshida M. 2003. FoF1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of ɛ subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 278, 46 840–46 846. ( 10.1074/jbc.M307165200) [DOI] [PubMed] [Google Scholar]
- 85.Yagi H, Konno H, Murakami-Fuse T, Isu A, Oroguchi T, Akutsu H, Ikeguchi M, Hisabori T. 2010. Structural and functional analysis of the intrinsic inhibitor subunit ɛ of F1-ATPase from photosynthetic organisms. Biochem. J. 425, 85–94. ( 10.1042/BJ20091247) [DOI] [PubMed] [Google Scholar]
- 86.Matsui T, Muneyuki E, Honda M, Allison WS, Dou C, Yoshida M. 1997. Catalytic activity of the α3β3gamma complex of F1-ATPase without noncatalytic nucleotide binding site. J. Biol. Chem. 272, 8215–8221. ( 10.1074/jbc.272.13.8215) [DOI] [PubMed] [Google Scholar]
- 87.Hirono-Hara Y, Noji H, Nishiura M, Muneyuki E, Hara KY, Yasuda R, Kinosita K, Yoshida M. 2001. Pause and rotation of F1-ATPase during catalysis. Proc. Natl Acad. Sci. USA 98, 13 649–13 654. ( 10.1073/pnas.241365698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keis S, Stocker A, Dimroth P, Cook GM. 2006. Inhibition of ATP hydrolysis by thermoalkaliphilic F1Fo-ATP synthase is controlled by the C-terminus of the epsilon subunit. J. Bacteriol. 188, 3796–3804. ( 10.1128/JB.00040-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sielaff H, Duncan TM, Börsch M. 2018. The regulatory subunit ɛ in Escherichia coli FOF1-ATP synthase. Biochim. Biophys. Acta Bioenerg. 1859, 775–788. ( 10.1016/j.bbabio.2018.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grant SG, Jessee J, Bloom FR, Hanahan D. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl Acad. Sci. USA 87, 4645–4649. ( 10.1073/pnas.87.12.4645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klionsky DJ, Brusilow WS, Simoni RD. 1984. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J. Bacteriol. 160, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pullman ME, Penefsky H, Datta A, Racker E. 1960. Partial resolution of the enzymes catalysing oxidative phosphorylation. Purification and properties of soluble, dinitrophenol-stimulated adenosine triphosphatase. J. Biol. Chem. 235, 3322–3329. [PubMed] [Google Scholar]
- 93.Kobayashi H, Anraku Y. 1972. Membrane-bound adenosine triphosphatase of Escherichia coli. I. Partial purification and properties. J. Biochem. 71, 387–399. [PubMed] [Google Scholar]
- 94.Cook GM, Keis S, Morgan HW, von Ballmoos C, Matthey U, Kaim G, Dimroth P. 2003. Purification and biochemical characterization of the F1Fo-ATP synthase from thermoalkaliphilic Bacillus sp. strain TA2.A1. J. Bacteriol. 185, 4442–4449. ( 10.1128/JB.185.15.4442-4449.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lo MC, Aulabaugh A, Jin G, Cowling R, Bard J, Malamas M, Ellestad G. 2004. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 332, 153–159. ( 10.1016/j.ab.2004.04.031) [DOI] [PubMed] [Google Scholar]
- 96.Layton CJ, Hellinga HW. 2011. Quantitation of protein-protein interactions by thermal stability shift analysis. Protein Sci. 20, 1439–1450. ( 10.1002/pro.674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aragão D, et al. 2018. MX2: a high-flux undulator microfocus beamline serving both the chemical and macromolecular crystallography communities at the Australian Synchrotron. J. Synchrotron Radiat. 25, 885–891. ( 10.1107/S1600577518003120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kabsch W. 2010. XDS. Acta Cryst. D 66, 125–132. ( 10.1107/S0907444909047337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans PR, Murshudov GN. 2013. How good are my data and what is the resolution. Acta Cryst. D 69, 1204–1214. ( 10.1107/S0907444913000061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winn MD, et al. 2011. Overview of the CCP4 suite and current developments. Acta Cryst. D 67, 235–242. ( 10.1107/S0907444910045749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674. ( 10.1107/S0021889807021206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst. D 67, 355–367. ( 10.1107/S0907444911001314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Cryst. D 66, 486–501. ( 10.1107/S0907444910007493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Cryst. D 66, 12–21. ( 10.1107/S0907444909042073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schrodinger LLC. 2018. The PyMOL molecular graphics system, version 2.2.2 See www.pymol.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data available from the PDB accession code: 6q45.