Abstract
Objective:
Characterize virologic and immunologic outcomes of INSTI-based antiretroviral therapy (ART) in experienced patients with and without virologic failure.
Design:
Prospective clinical cohort.
Methods:
ART-experienced, INSTI-naïve participants in the University of North Carolina Center for AIDS Research HIV Clinical Cohort (UCHCC) initiating an INSTI-containing regimen 2007–2016 were followed from INSTI initiation (baseline) to the earliest of: outcome of interest, loss to follow-up (LTFU, one year without clinical visit), or death. Outcomes of interest were virologic failure (VF, first of two consecutive viral loads [VL] ≥200 copies/mL >2 weeks apart, or one VL≥200 before LTFU) and immune recovery (first CD4≥500 cells/μL). Patients with baseline VL≥50 were given 24 weeks before meeting VF criteria. Kaplan Meier curves and Cox proportional hazards models compared INSTI regimens and patient characteristics.
Results:
Of 773 patients, 32% were women, 59% African-American, and 42% had a VL≥50 at INSTI initiation. After two years, 5% of patients with baseline VL<50 experienced VF, compared to 35% of patients with baseline VL≥50 (P<0.01). Among patients with baseline VL<50, dolutegravir/NRTIs was associated with longer time to VF (adjusted hazard ratio [aHR] 0.11, 95% confidence interval [CI] 0.01, 0.80), while among patients with baseline VL≥50, raltegravir/NRTIs was associated with longer time to VF (aHR 0.35, 95% CI 0.18, 0.68), both compared to elvitegravir/NRTIs. After five years suppressed, irrespective of baseline VL, 61% of patients experienced immune recovery.
Conclusions:
In this cohort, INSTI-containing regimens led to low VF rates in patients switching ART while suppressed. Viremic patients initiating INSTIs were at high risk of VF during follow-up.
Keywords: HIV, antiretroviral therapy, integrase inhibitors, treatment experienced, observational study
INTRODUCTION
Antiretroviral therapy (ART) regimens containing an integrase strand transfer inhibitor (INSTI) are recommended in treatment-naïve and treatment-experienced patients.1 In clinical trials and observational studies, INSTI-based regimens lead to high rates of virologic success in naïve patients.2–4 Efficacy of INSTI agents has also been demonstrated in ART-experienced patients in trials,5–7 yet few large observational studies have examined their effectiveness in experienced patients in the clinic setting.8–10
INSTI agents have a high potency and more favorable safety and tolerability profiles than older ART agents.2,3,11 When introduced in 2007, the INSTI agent raltegravir was active against viruses with resistance to prior drug classes. In 2012, elvitegravir became the first INSTI agent available in a single-tablet regimen. Dolutegravir, FDA-approved in 2013, has a high barrier to resistance and is now also available as a single-tablet regimen.3 Elvitegravir and dolutegravir are dosed once-daily, compared to twice-daily for raltegravir in treatment-experienced patients. These characteristics make INSTIs appealing treatment options for virologically suppressed patients who wish to simplify their ART and for patients with virologic failure, especially, with dolutegravir, in the context of poor regimen tolerability, pre-existing resistance mutations, and suboptimal adherence. However, the anticipated benefits of INSTI agents in treatment-experienced patients should be confirmed in the clinic setting. In this study, we examined virologic and immunologic outcomes of INSTI-based therapy in ART-experienced patients with and without virologic failure.
METHODS
Study population
Participants in the University of North Carolina Center for AIDS Research Clinical Cohort (UCHCC) were eligible for this study if they were ART-experienced, INSTI-naïve, had a complete ART history available, and initiated a first INSTI-containing regimen between January 1, 2007 and December 31, 2016 while in care at the University of North Carolina (UNC). Patients were excluded if they did not have an HIV RNA viral load (VL) measured in the 90 days prior to INSTI initiation, and a CD4 cell count measured between 180 days prior to and 90 days after INSTI initiation. The UCHCC prospectively collects laboratory results via electronic health records, and genotype tests and ART information via twice-yearly health record reviews. Patients were followed from INSTI initiation (baseline) until the earliest of: outcome of interest, death, loss to follow-up (LTFU), defined as one year without a clinic or laboratory visit, or administrative censoring on August 31, 2017. Patient were stratified by baseline VL≥50 and <50 copies/mL, measured up to 90 days prior. Data collection for the UCHCC and secondary analysis for this study were both approved by UNC’s Institutional Review Board, and patients provided written informed consent to participate in the UCHCC.
Definitions
We examined two outcomes: virologic failure (VF) and immune recovery. VF was defined as the first of two consecutive viral loads (VL) ≥200 copies/mL more than two weeks apart, or one VL≥200 before being LTFU. Patients with baseline VL≥50 were given 24 weeks on INSTI therapy before meeting criteria for VF. Among patients with baseline CD4<500 cells/μL, measured up to six months prior, immune recovery was defined as the first CD4≥500 after INSTI initiation. We categorized ART regimens according to INSTI agent raltegravir (RAL), elvitegravir (EVG), or dolutegravir (DTG), taken in combination with two or more nucleoside or nucleotide analog reverse transcriptase inhibitors (NRTIs). We also examined regimens including RAL with a protease inhibitor (PI), with or without a pharmacoenhancer and with or without any NRTIs. Remaining regimens were categorized as “other.” For patients receiving an INSTI in combination only with an NRTI backbone and who had a genotypic resistance test obtained in the 90 days prior to INSTI initiation, we estimated the activity of the NRTI backbone using the Stanford algorithm based on mutations from all previously available genotypes.12 An NRTI backbone was considered fully active if no component drug had an intermediate or above resistance score. For patients with INSTI genotyping performed in the 90 days following VF on the INSTI regimen, we described the presence of major (bolded) mutations listed in the 2017 IAS-USA list.13
Analyses
We compared baseline patient characteristics using Fisher’s Exact test, the Wilcoxon Rank-Sum test, and the Kruskal-Wallis test as appropriate. We estimated time from baseline to outcome of interest and compared INSTI regimens using Kaplan-Meier curves and Cox proportional hazards models, adjusting for age, sexual risk group, race/ethnicity, CD4 count, and number of prior antiretroviral agents, all measured at baseline. Time to VF analyses among patients with baseline VL≥50 were also adjusted for baseline VL. Primary analyses were intention-to-treat and ignored changes in ART. In a secondary analysis of time to VF, patients were censored if they had any change in ART regimen or discontinued ART for longer than two weeks. Dosage or formulation changes were not considered an ART regimen change. In time to immune recovery analyses, patients were censored at VF. All P values are two-sided, and <0.05 was considered statistically significant. Analyses were conducted in SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Of 933 eligible patients, we excluded 157 (17%) missing baseline VL or CD4 count. These 157 patients were demographically and clinically comparable to patients who met inclusion criteria. Additionally we excluded 3 (<1%) patients who initiated an INSTI with only one or no NRTI. Our study population included 773 patients who were 32% women, 43% MSM, and 59% African-American patients, and at baseline had a median age of 47 years (interquartile range [IQR] 38, 54), CD4 count of 509 cells/μL (IQR 274, 739), and prior exposure to 6 (IQR 4, 8) antiretrovirals. At baseline, 327 (42%) patients had a VL≥50, with a median VL of 4.24 log10 copies/mL (IQR 2.98, 4.94). Compared to patients with baseline VL<50, patients with baseline VL≥50 were more likely African American (63% vs. 54%), younger (median age 44 vs. 50 years), had initiated ART more recently (median 9 vs. 11 years), yet been exposed to more agents (median 6 vs. 5) (Table 1, all P<0.05). Patients with baseline VL≥50 had lower CD4 counts, both nadir (median 84 vs. 158 cells/μL) and baseline (288 vs. 636 cells/μL), than patients with VL<50 (both P<0.05). In both VL groups, a majority of patients were switching from a PI-based regimen and at least one-third from an NNRTI-based regimen. Patients with baseline VL<50 most commonly initiated a first INSTI regimen containing EVG/COBI (39%) or DTG (31%) in combination with NRTIs. In contrast, the most common first INSTI regimens among patients with VL≥50 were EVG/COBI/NRTIs (20%), DTG/NRTIs (19%), RAL/NRTIs (20%), and RAL/PI (21%). When further stratifying patients with baseline VL≥50 by INSTI regimen, those receiving EVG/COBI/NRTIs were the youngest and least treatment-experienced group, with a median age of 36 years (IQR 28, 48) and prior antiretroviral drug exposure of 4 agents (IQR 3, 6) (Table 2, both P<0.01).
Table 1.
Patient characteristics stratified by HIV RNA viral load at INSTI initiation.
| Characteristic at INSTI initiation | Viral Load ≥50 (N=327) |
Viral Load <50 (N=446) |
P1 |
|---|---|---|---|
| N (%) or median (IQR) | N (%) or median (IQR) | ||
| Sexual risk group | 0.58 | ||
| MSM | 137 (42%) | 198 (44%) | |
| Women | 104 (32%) | 145 (33%) | |
| Heterosexual men | 86 (26%) | 103 (23%) | |
| IDU | 29 (9%) | 49 (11%) | 0.40 |
| Race/ethnicity | <0.05 | ||
| African American | 207 (63%) | 243 (54%) | |
| White | 88 (27%) | 161 (36%) | |
| Hispanic/other | 32 (10%) | 42 (9%) | |
| Age, years | 44 (35, 50) | 50 (41, 56) | <0.01 |
| Calendar year | 2012 (2009, 2014) | 2014 (2013, 2015) | <0.01 |
| Years since first ART | 9 (4, 13) | 11 (5, 16) | <0.01 |
| Prior ARV drugs | 6 (4, 9) | 5 (4, 8) | <0.05 |
| Prior ART regimens | 4 (2, 7) | 3 (1, 6) | <0.01 |
| Nadir CD4 count, cells/μL | 81 (15, 240) | 157 (48, 304) | <0.01 |
| CD4 count, cells/μL | 288 (113, 495) | 636 (443, 839) | <0.01 |
| HIV RNA, log10 copies/mL | 4.24 (2.98, 4.94) | N/A | N/A |
| Last prior regimen anchor class | <0.01 | ||
| NNRTI | 107 (33%) | 183 (41%) | |
| PI | 179 (55%) | 232 (52%) | |
| NNRTI and PI | 11 (3%) | 15 (3%) | |
| EI2 | 12 (4%) | 7 (2%) | |
| Other | 18 (5%) | 9 (2%) | |
| First INSTI regimen | <0.01 | ||
| EVG/COBI with ≥2 NRTI | 64 (20%) | 175 (39%) | |
| DTG with ≥2 NRTI | 61 (19%) | 141 (32%) | |
| RAL with ≥2 NRTI | 65 (20%) | 66 (15%) | |
| RAL with PI3 | 70 (21%) | 31 (7%) | |
| Other4 | 67 (20%) | 33 (7%) |
Abbreviations. ART: antiretroviral therapy. ARV: antiretroviral. COBI: cobicistat. DTG: dolutegravir. EI: entry inhibitor. EVG: elvitegravir. IDU: injection drug use. INSTI: integrase strand transfer inhibitor. IQR: interquartile range. MSM: men who have sex with men. PI: protease inhibitor. N/A: not applicable. NNRTI: non-nucleoside analog reverse transcriptase inhibitor. NRTI: nucleoside analog reverse transcriptase inhibitor. RAL: raltegravir. VL: viral load.
P value from Fisher’s Exact test for categorical variables and the Wilcoxon Rank-Sum test for continuous variables.
May include another anchor drug.
With or without any NRTI agent.
Includes patients on RAL in combination with an NNRTI (17 with VL≥50 and 14 with VL<50), RAL with a PI and an NNRTI (27 with VL≥50 and 7 with VL<50), RAL with an EI (15 with VL≥50 and 0 with VL<50), and DTG with another anchor agent (8 with VL≥50 and 12 with VL<50), all with or without any NRTI agent.
Table 2.
Characteristics of patients with HIV RNA viral load ≥50 copies/mL at INSTI initiation, stratified by ART regimen.
| Characteristic at INSTI initiation | EVG/COBI + ≥2 NRTIs (N=64) |
DTG + ≥2 NRTIs (N=61) |
RAL + ≥2 NRTIs (N=65) |
RAL + PI1 (N=70) |
Other (N=67) |
P2 |
|---|---|---|---|---|---|---|
| N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | N (%) or median (IQR) | ||
| Sexual risk group | 0.10 | |||||
| MSM | 36 (56%) | 26 (43%) | 30 (46%) | 19 (27%) | 26 (39%) | |
| Women | 16 (25%) | 18 (30%) | 21 (32%) | 28 (40%) | 21 (31%) | |
| Heterosexual men | 12 (19%) | 17 (28%) | 14 (22%) | 23 (33%) | 20 (30%) | |
| IDU | 4 (6%) | 6 (10%) | 6 (9%) | 7 (10%) | 6 (9%) | 0.95 |
| Race/ethnicity | ||||||
| African American | 46 (72%) | 37 (61%) | 33 (51%) | 48 (69%) | 43 (64%) | 0.16 |
| White | 11 (17%) | 16 (26%) | 26 (40%) | 15 (21%) | 20 (30%) | |
| Hispanic/other | 7 (11%) | 8 (13%) | 6 (9%) | 7 (10%) | 4 (6%) | |
| Age, years | 36 (28, 48) | 45 (36, 57) | 42 (33, 48) | 45 (38, 53) | 47 (42, 52) | <0.01 |
| Calendar year | 2014 (2013, 2015) | 2015 (2014, 2016) | 2011 (2009, 2012) | 2009 (2008, 2010) | 2009 (2008, 2011) | <0.01 |
| Years since first ART | 5 (2, 10) | 8 (4, 13) | 9 (2, 13) | 10 (5, 14) | 12 (9, 15) | <0.01 |
| Prior ARV drugs | 4 (3, 6) | 5 (3, 8) | 5 (4, 8) | 8 (6, 12) | 10 (7, 13) | <0.01 |
| Prior ART regimens | 2 (1, 4) | 3 (2, 5) | 3 (2, 5) | 5 (4, 9) | 9 (4, 15) | <0.01 |
| Nadir CD4 count, cells/μL | 227 (63, 355) | 152 (33, 312) | 135 (46, 297) | 28 (9, 108) | 22 (9, 15) | <0.01 |
| CD4 count, cells/μL | 388 (187, 541) | 357 (191, 545) | 315 (141, 541) | 203 (56, 397) | 178 (69, 326) | <0.01 |
| HIV RNA, log10 copies/mL | 4.04 (2.84, 4.60) | 3.75 (2.71, 4.94) | 4.05 (2.92, 4.77) | 4.35 (3.29, 5.17) | 4.51 (3.49, 4.90) | 0.20 |
| Last prior regimen anchor class | <0.01 | |||||
| NNRTI | 27 (42%) | 29 (48%) | 26 (40%) | 17 (24%) | 8 (12%) | |
| PI | 34 (53%) | 29 (48%) | 32 (49%) | 45 (64%) | 39 (58%) | |
| NNRTI and PI | 1 (2%) | 1 (2%) | 1 (2%) | 0 (0%) | 8 (12%) | |
| EI3 | 0 (0%) | 0 (0%) | 0 (0%) | 4 (6%) | 8 (12%) | |
| Other | 2 (3%) | 2 (3%) | 6 (9%) | 4 (6%) | 4 (6%) | |
| Genotype performed at INSTI initiation | 22 (34%) | 22 (36%) | 22 (34%) | 31 (44%) | 25 (37%) | 0.74 |
| Fully active NRTI backbone4 | 20 (91%) | 17 (77%) | 18 (82%) | N/A | N/A | 0.60 |
Abbreviations. ART: antiretroviral therapy. ARV: antiretroviral. COBI: cobicistat. DTG: dolutegravir. EI: entry inhibitor. EVG: elvitegravir. IDU: injection drug use. INSTI: integrase strand transfer inhibitor. IQR: interquartile range. MSM: men who have sex with men. PI: protease inhibitor. N/A: not applicable. NNRTI: non-nucleoside analog reverse transcriptase inhibitor. NRTI: nucleoside analog reverse transcriptase inhibitor. RAL: raltegravir. VL: viral load.
With or without any NRTI agent.
P values from the Monte Carlo estimate of Fisher’s Exact test for categorical variables and the Kruskal-Wallis test for continuous variables.
May include another anchor drug.
Restricted to patients on a single anchor agent and with a genotype performed at INSTI initiation. Based on mutations from all available genotype testing prior to INSTI initiation, interpreted according to the Stanford algorithm. NRTI backbones in which no agent had a resistance score of intermediate or above were considered fully active.
Time to virologic failure
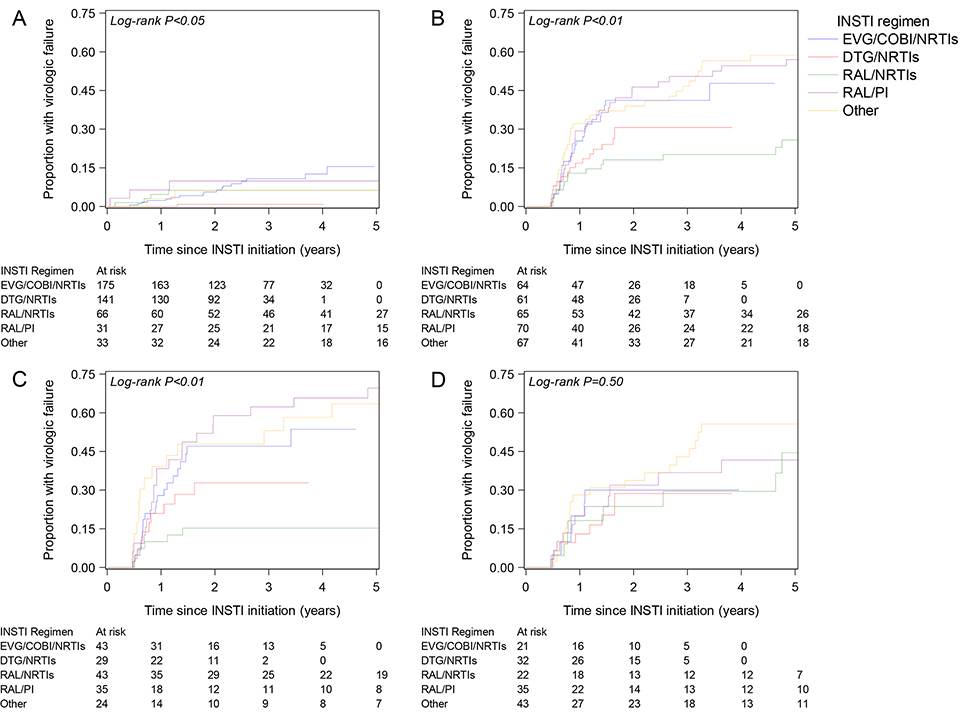
Among patients with baseline VL<50, 2% and 5% experienced VF after one and two years, respectively, compared to 23% and 35% among patients with baseline VL≥50 (log-rank P<0.01). The differences in time to VF by baseline VL persisted after stratifying by calendar period of INSTI initiation 2007–2010, 2011–2013, and 2014–2016 (Supplemental Digital Content 1, Figure, all P<0.01). Time to VF differed by INSTI regimen in both VL groups (Fig. 1 A/B, both log-rank P<0.05). Among patients with baseline VL≥50, RAL/NRTIs was associated with longer time to VF compared to EVG/COBI/NRTIs, with an adjusted hazard ratio (aHR) of 0.35 (95% confidence interval [CI] 0.18, 0.68), while there was no association with any other regimen (Table 3). Among patients with baseline VL<50, DTG/NRTIs was associated with longer time to VF compared to EVG/COBI/NRTIs, with an aHR of 0.11 (95% CI 0.01, 0.80), but there was no association with any other regimen. Regardless of baseline VL, older age was associated with longer time to VF, with an aHR of 0.74 (95% CI 0.61, 0.89) and 0.67 (95% CI 0.46, 0.98) per ten-year increase among patients with baseline VL≥50 and <50, respectively. Among patients with baseline VL≥50, a longer time to VF was associated with higher baseline CD4 count (aHR per 100-cell increase 0.82, 95% CI 0.75, 0.91) and exposure to fewer antiretrovirals (aHR per one-drug increase 1.07, 95% CI 1.01, 1.12), but not among patients with baseline VL<50. Among patients with baseline VL≥50, a one-unit increase in log10 RNA copies/mL was associated with increased VF rates in unadjusted analyses, with a HR of 1.35 (95% CI 1.16, 1.57), but not in adjusted models (aHR 1.14, 95% CI 0.96, 1.34). Patients with baseline VL>10,000 (N=182), compared to those with baseline VL 50–10,000 (N=145), had an HR of 1.78 (95% CI 1.23, 2.58) in unadjusted models and 1.19 (95% CI 0.80, 1.78) in adjusted models. Prior ART duration was not associated with time to VF in unadjusted or adjusted models (results not shown).
Figure 1.
Time from INSTI initiation to virologic failure stratified by INSTI regimen: (A) among patients with HIV viral load <50 copies/mL at INSTI initiation, (B) among patients with viral load ≥50 at INSTI initiation, (C) among patients with viral load ≥50 and aged <45 years at INSTI initiation, and (D) among patients with viral load ≥50 and aged ≥45 years at INSTI initiation.
Table 3.
Factors associated with time to virologic failure, stratified by HIV RNA viral load at INSTI initiation.
| Characteristic at INSTI initiation | Viral Load ≥50 | Viral Load <50 | ||
|---|---|---|---|---|
| Unadjusted HR1 (95% CI) | Adjusted HR2 (95% CI) | Unadjusted HR1 (95% CI) | Adjusted HR2 (95% CI) | |
| INSTI regimen | ||||
| EVG/COBI with ≥2 NRTI | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| RAL with ≥2 NRTI | 0.43 (0.22, 0.81) | 0.35 (0.18, 0.68) | 0.63 (0.23, 1.75) | 0.77 (0.27, 2.23) |
| DTG with ≥2 NRTI | 0.68 (0.37, 1.25) | 0.80 (0.42, 1.50) | 0.08 (0.01, 0.60) | 0.11 (0.01, 0.80) |
| RAL with PI | 1.10 (0.65, 1.85) | 0.59 (0.33, 1.08) | 0.78 (0.22, 2.75) | 1.03 (0.26, 4.10) |
| Other | 1.22 (0.73, 2.03) | 0.76 (0.41, 1.39) | 0.51 (0.12, 2.23) | 0.89 (0.18, 4.36) |
| Sexual risk group | ||||
| MSM | 0.74 (0.48, 1.16) | 0.58 (0.35, 0.93) | 0.74 (0.26, 2.09) | 0.61 (0.20, 1.83) |
| Women | 1.09 (0.70, 1.68) | 0.79 (0.49, 1.25) | 1.53 (0.58, 4.02) | 1.35 (0.50, 3.61) |
| Heterosexual men | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| African American race | 1.41 (0.97, 2.06) | 1.13 (0.77, 1.68) | 1.55 (0.72, 3.38) | 1.25 (0.53, 2.96) |
| Age, per 10-year increase | 0.84 (0.73, 0.98) | 0.74 (0.61, 0.89) | 0.68 (0.49, 0.94) | 0.67 (0.46, 0.98) |
| CD4, per 100-cell increase | 0.78 (0.72, 0.85) | 0.82 (0.75, 0.91) | 0.95 (0.84, 1.08) | 0.96 (0.84, 1.10) |
| Number of prior ARVs, per 1-drug increase | 1.06 (1.02, 1.11) | 1.07 (1.01, 1.12) | 0.97 (0.86, 1.09) | 0.99 (0.86, 1.12) |
| HIV RNA, per log10 increase | 1.35 (1.16, 1.57) | 1.14 (0.96, 1.34) | N/A | N/A |
Abbreviations. ARV: antiretroviral. CI: confidence interval. COBI: cobicistat. EVG: elvitegravir. DTG: dolutegravir. HR: hazard ratio. INSTI: integrase strand transfer inhibitor. MSM: men who have sex with men. N/A: not applicable. RAL: raltegravir. Ref.: referent. VL: viral load.
Estimates from separate Cox proportional models including only one characteristic.
Estimates from Cox proportional hazards models including all variables in the table, except HIV RNA for patients with baseline VL<50.
We further stratified analyses of patients with baseline VL≥50 by age <45 or ≥45 years at INSTI initiation. In Kaplan-Meier curves, we observed differences in time to VF by INSTI regimen with longer time for RAL/NRTIs in the younger group (Fig. 1C, log-rank P<0.05), but not in the older group (Fig. 1D, log-rank P=0.50). These findings were also observed in multivariable analyses stratified by age, with an adjusted HR comparing RAL/NRTIs to EVG/COBI/NRTIs of 0.21 (95% CI 0.08, 0.51) in the younger group and 0.99 (0.33, 2.96) in the older group.
Twenty-one patients had INSTI genotypes obtained at VF on the INSTI regimen. Out of 4 patients on a DTG-containing regimen, 0 (0%) had a major INSTI mutation. Out of 9 patients on a RAL-containing regimen, 1 (11%) developed N155H. Out of 8 patients on an EVG-containing regimen, 1 (13%) developed N155H, 2 (25%) developed E92Q.
Sensitivity analyses of virologic failure
In a sensitivity analysis censoring all patients after three years, estimates were similar to the main findings. Stratifying patients by baseline VL≥200 (N=282) or <200 (N=491) instead of 50 copies/ml, estimates were also comparable to our primary results. In further sensitivity analyses censoring patients who changed or discontinued ART, our results were consistent with our ITT findings, although the estimates were less precise, with an adjusted HR of 0.37 (95% CI 0.12, 1.13) comparing RAL/NRTIs to EVG/COBI/NRTIs among patients with baseline VL≥50. There were too few VF events among patients with baseline VL<50, after censoring patients at ART changes, to allow us to estimate HRs comparing different regimens. We also conducted analyses restricted to patients with baseline VL≥50, who initiated an INSTI in combination with an NRTI backbone and had a genotype at INSTI start. In this patient group, adjusting for NRTI backbone activity as well as age, sexual risk group, race, CD4 count, viral load, and number of prior agents, the aHR compared to EVG/COBI was 0.50 (95% CI 0.18, 1.35) for DTG and 0.14 (95% CI 0.04, 0.51) for RAL. In this patient group, incomplete activity of the NRTI backbone was associated with shorter time to failure, with an unadjusted HR of 3.02 (95% CI 1.33, 6.91). When we also included patients with NRTI resistance based on earlier genotypes, results comparing EVG/COBI to RAL were similar. Finally, in an analysis restricted to patients with baseline VL≥50 who initiated EVG/COBI/NRTIs or RAL/NRTIs in the years 2012–2016, the aHR comparing RAL to EVG was similar to the main findings.
Time to immune recovery
Among patients with baseline VL≥50 and <50, 246 (75%) and 134 (30%) had a baseline CD4 count <500 cells/μL, with median CD4 counts (IQR) of 202 (72, 329) and 360 (256, 426), respectively. The median age (IQR) of these patients was 44 years (36, 50) and 51 years (42, 57), respectively. In unadjusted analyses (Supplemental Digital Content 2, Figure), we observed differences in time to immune recovery by nadir CD4 count (P<0.01), but not by baseline VL, INSTI regimen, or age group. After five years of INSTI therapy, censoring patients with VF, 61% in both VL groups reached CD4 counts ≥500. Almost 80% of patients with nadir CD4>200 experienced immune recovery after five years, compared to 44% in those with nadir CD4<50.
DISCUSSION
In this clinical cohort study of ART-experienced patients initiating INSTI-based therapy, INSTI regimens were highly effective among patients who switched with undetectable HIV RNA levels, with 95% of patients remaining virologically suppressed after two years. Suppressed patients who switched to DTG had a significantly lower rate of failure compared to EVG, although the confidence interval around the point estimate was wide. On the other hand, patients with detectable viremia at INSTI initiation had high virologic failure rates, associated with younger age and lower CD4 counts at baseline. Viremic patients switching to RAL in combination with NRTIs had a lower risk of failure compared to EVG. This difference persisted in additional analyses adjusted for NRTI backbone activity, and among patients <45 years old, but not among patients 45 years or older. Irrespective of baseline VL or regimen type, patients on INSTIs experienced good immune recovery, with only observed differences by degree of prior immunosuppression.
Clinical trials have demonstrated that patients with suppressed viral loads maintain high rates of virologic suppression after switching to INSTI-containing regimens.5,14,15 Some observational studies have also shown good virologic outcomes in suppressed patients switching to RAL,16–18
one study in patients switching to EVG,9 and one in patients switching to DTG.19 One cohort study reported low failure rates over two years after switching while suppressed to an INSTI-based regimen, including RAL, DTG, and EVG, though it did not compare INSTI agents.10 Some studies have shown good virologic outcomes on RAL or DTG in heterogeneous groups comprising both suppressed and unsuppressed patients.8,20,21 However, most prior studies were small (50–150 patients), had short follow-up (24–48 weeks), or included only one INSTI (most often RAL); none compared INSTI agents.
In this larger study, we found high rates of maintained suppression among patients initiating RAL, DTG, and EVG through five years of follow-up. Further, we observed lower virologic failure rates among suppressed patients switching to DTG compared to EVG, both in combination with NRTIs. This difference may be linked to patient adherence and to DTG’s higher barrier to resistance.2,3 As a majority of patients switched from a PI-based regimen, which also have high barriers to resistance,22,23 it is possible that patients with suboptimal adherence who were able to maintain virologic suppression on a PI experienced failure after switching to an agent less forgiving of missed doses such as EVG. Our study also observed good CD4 recovery for patients who achieved and maintained suppression on INSTI-based regimens. Improvements in CD4 counts have been reported in both clinical trials and observational studies after switching to RAL-based regimens,6,8 and in clinical trials after switching to DTG- and EVG-based regimens.5,14 Our study shows a CD4 recovery in patients switching to EVG and DTG in the clinical setting as well.
High virologic failure rates in patients initiating INSTI therapy with detectable viral loads was not a surprising result. Although in clinical trials INSTI-based regimens lead to good suppression rates among patients with virologic failure, poor adherence to treatment has been shown to lead to continued lack of virologic control.6,7 Patients in our study who had previously failed several regimens, including potent ones initiated in recent years, may have been non-adherent to the new INSTI-based treatment. Two observational studies reporting 48-week outcomes of switching from a failing regimen to RAL found failure rates of 15% and 35%,17,24 while another two studies found a failure rate of 20% at 24 weeks.18,25 Only one study of RAL reported high suppression rates after 2 years in patients switching from a failing regimen.26 Our study confirms most prior reports observing high virologic failure rates following RAL initiation among patients virologically detectable at time of regimen switch, and to our knowledge is the first to report the high virologic failure risk among virologically detectable patients switching to DTG or EVG in a clinical care setting.
The lower failure rate associated with RAL/NRTIs compared to EVG was an unexpected finding. As a single-tablet regimen with once-daily dosing, adherence to EVG/COBI/FTC/TDF could be expected to be higher than adherence to RAL/NRTIs, which has a higher pill burden and required two daily doses until recently. There are several possible explanations for this result. Co-formulation of EVG with the pharmacoenhancer COBI may have led to poorer tolerance of the regimen.2 Our estimates could be affected by residual confounding due to channeling bias, if health care providers opted to prescribe the once-daily single-tablet regimen (EVG) to patients believed to be less likely to adhere. Among patients with some but incomplete adherence, it is also possible that a twice-daily regimen with some missed doses may lead to better virologic outcomes than a once-daily regimen with some missed doses.27 Younger patients may be more likely to adhere partially to treatment, explaining lower failure rates with RAL/NRTIs vs. EVG in this age group. In a German study, patients with intermittent adherence reported most commonly skipping doses because they “felt bad” or planned on consuming alcohol or drugs.28 Older patients, on the other hand, are less likely to miss doses.29 Patients in our study’s older group who were non-adherent may have had severe obstacles to adherence, such as substance use, leading to very low adherence levels and no observed difference in failure rates by regimen. Analyses restricted to 2012–2016 were similar to the main findings, suggesting that confounding by calendar time, such as better adherence in early users of RAL, is unlikely to explain regimen differences. Additionally, it is possible that patients in the RAL and EVG groups had different antiretroviral resistance profiles. In subgroup analyses of patients with a genotype at INSTI initiation, adjusting for reduced activity of the NRTI backbone, we continued to observe the RAL and EVG difference, suggesting that detected resistance could not account for the observed differences.
The appeal of INSTIs for treatment-experienced patients is likely to persist with the approval of additional once-daily single-tablet regimens (STR) containing EVG, DTG, or the new INSTI bictegravir (BIC), especially in combination with tenofovir alafenamide.30 Our findings highlight the need to continue to study INSTI outcomes in clinical settings among treatment-experienced patients, including additional follow-up time, heterogeneous patient populations, BIC use, and STR INSTI use. Comparative effectiveness studies are also needed to assess differences between INSTIs and other new STRs, such as those containing darunavir or rilpivirine, particularly in the context of incomplete patient adherence and pre-existing drug resistance.31,32 In addition, drug resistance and toxicities of INSTI agents should continue to be monitored in clinical populations.33 Finally, ongoing efforts supporting patient adherence are needed given the high virologic failure rates we observed among patients with detectable viral loads at INSTI initiation. Patients facing persistent challenges to adherence may benefit from interventions leading to even modest improvements in adherence, and from future therapies with long-acting injectable agents.
This study’s strengths include longitudinal follow-up of treatment-experienced patients on three INSTI agents including two approved in recent years, providing what is, to our knowledge, the largest and longest observational study of INSTI use in ART-experienced patients and the only one to compare INSTI agents. Our cohort also captures granular data such as complete treatment histories and genotypic resistance tests, which added valuable information to the analyses. However, our study is limited by small sample sizes, which affected our ability to obtain precise estimates, and by the lack of information on adherence or reasons for switching from a prior regimen. This study was conducted at a single center in a high-income nation and may not be directly generalizable to other settings.
In conclusion, INSTI-based regimens can be highly effective in ART-experienced patients with current virologic control. However, in patients who have detectable viremia, INSTI effectiveness is likely limited by other treatment factors such as adherence. Treatment-experienced patients who remain suppressed on INSTI regimens experience good immunologic outcomes.
Supplementary Material
Supplemental Digital Content 1. PPTX. Figure. Time from INSTI initiation to virologic failure stratified by HIV viral load <50 or ≥50 copies/mL at INSTI initiation among antiretroviral-experienced patients initiating a first INSTI-containing regimen in: (A) 2007–2010; (B) 2011–2013; (C) 2014–2016.
Supplemental Digital Content 2. PPTX. Figure. Time from INSTI initiation to CD4≥500 cells/μL among antiretroviral-experienced patients initiating a first INSTI-containing regimen with CD4<500 stratified by: (A) HIV viral load, (B) INSTI regimen, (C) nadir CD4 count, and (D) age, all measured at INSTI initiation.
ACKNOWLEDGEMENTS
Funding: This study was funded by the University of North Carolina at Chapel Hill Center for AIDS Research, an NIH-funded program (Grant Award Number P30 AI50410). Traineeship for TD was provided by the National Institute of Allergy and Infectious Diseases (Grant Award Number T32 AI007001).
REFERENCES
- 1.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448. [DOI] [PubMed] [Google Scholar]
- 3.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. [DOI] [PubMed] [Google Scholar]
- 4.Davy-Mendez T, Eron JJ, Zakharova O, Wohl DA, Napravnik S. Increased persistence of initial treatment for HIV infection with modern antiretroviral therapy. J Acquir Immune Defic Syndr. 2017;76(2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14(7):581–589. [DOI] [PubMed] [Google Scholar]
- 6.Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. [DOI] [PubMed] [Google Scholar]
- 8.Buchacz K, Wiegand R, Armon C, et al. Long-term immunologic and virologic responses on raltegravir-containing regimens among ART-experienced participants in the HIV Outpatient Study. HIV Clin Trials. 2015;16(4):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrier M, Charpentier C, Peytavin G, et al. Switch as maintenance to elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate: week 48 results in a clinical cohort. J Antimicrob Chemother. 2017;72(6):1745–1751. [DOI] [PubMed] [Google Scholar]
- 10.Gianotti N, Poli A, Nozza S, et al. Durability of switch regimens based on rilpivirine or on integrase inhibitors, both in association with tenofovir and emtricitabine, in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17(1):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806. [DOI] [PubMed] [Google Scholar]
- 12.Tang MW, Liu TF, Shafer RW. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology. 2012;55(2):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wensing AM, Calvez V, Gunthard HF, et al. 2017 Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med. 2017;24(4):132–133. [PMC free article] [PubMed] [Google Scholar]
- 14.Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, Phase IIIb study. Antivir Ther. 2017;22(4):295–305. [DOI] [PubMed] [Google Scholar]
- 15.Troya J, Montejano R, Ryan P, et al. Raltegravir plus abacavir/lamivudine in virologically suppressed HIV-1-infected patients: 48-week results of the KIRAL study. PLoS One. 2018;13(6):e0198768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caby F, Schneider L, Blanc C, et al. Efficacy of raltegravir switching strategies in HIV-infected patients with suppressed viraemia according to the genotypic sensitivity score. Infection. 2014;42(2):295–301. [DOI] [PubMed] [Google Scholar]
- 17.Naumann U, Moll A, Schleehauf D, et al. Similar efficacy and tolerability of raltegravir-based antiretroviral therapy in HIV-infected patients, irrespective of age group, burden of comorbidities and concomitant medication: Real-life analysis of the German ‘WIP’ cohort. Int J STD AIDS. 2017;28(9):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherrer AU, von Wyl V, Fux CA, et al. Implementation of raltegravir in routine clinical practice: selection criteria for choosing this drug, virologic response rates, and characteristics of failures. J Acquir Immune Defic Syndr. 2010;53(4):464–471. [DOI] [PubMed] [Google Scholar]
- 19.Todd S, Rafferty P, Walker E, et al. Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. Int J STD AIDS. 2017;28(11):1074–1081. [DOI] [PubMed] [Google Scholar]
- 20.Capetti AF, Cossu MV, Orofino G, et al. A dual regimen of ritonavir/darunavir plus dolutegravir for rescue or simplification of rescue therapy: 48 weeks’ observational data. BMC Infect Dis. 2017;17(1):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capetti AF, Sterrantino G, Cossu MV, et al. Switch to dolutegravir plus rilpivirine dual therapy in cART-experienced subjects: an observational cohort. PLoS One. 2016;11(10):e0164753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372(9639):646–655. [DOI] [PubMed] [Google Scholar]
- 23.Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23(13):1679–1688. [DOI] [PubMed] [Google Scholar]
- 24.Mata-Marin JA, Smeke AE, Rodriguez MR, et al. Effectiveness and risk factors for virological outcome of raltegravir-based therapy for treatment-experienced HIV-infected patients. Drugs R D. 2017;17(1):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittkop L, Breilh D, Da Silva D, et al. Virological and immunological response in HIV-1-infected patients with multiple treatment failures receiving raltegravir and optimized background therapy, ANRS CO3 Aquitaine Cohort. J Antimicrob Chemother. 2009;63(6):1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capetti A, Landonio S, Meraviglia P, et al. 96 Week follow-up of HIV-infected patients in rescue with raltegravir plus optimized backbone regimens: a multicentre Italian experience. PLoS One. 2012;7(7):e39222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eron JJ Jr., Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–915. [DOI] [PubMed] [Google Scholar]
- 28.Boretzki J, Wolf E, Wiese C, et al. Highly specific reasons for nonadherence to antiretroviral therapy: results from the German adherence study. Patient Prefer Adherence. 2017;11:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380–1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–2072. [DOI] [PubMed] [Google Scholar]
- 31.Eron JJ, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeJesus E, Ramgopal M, Crofoot G, et al. Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV. 2017;4(5):e205–e213. [DOI] [PubMed] [Google Scholar]
- 33.Davy-Mendez T, Eron JJ, Brunet L, Zakharova O, Dennis AM, Napravnik S. New antiretroviral agent use affects prevalence of HIV drug resistance in clinical care populations. AIDS. 2018;32(17):2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. PPTX. Figure. Time from INSTI initiation to virologic failure stratified by HIV viral load <50 or ≥50 copies/mL at INSTI initiation among antiretroviral-experienced patients initiating a first INSTI-containing regimen in: (A) 2007–2010; (B) 2011–2013; (C) 2014–2016.
Supplemental Digital Content 2. PPTX. Figure. Time from INSTI initiation to CD4≥500 cells/μL among antiretroviral-experienced patients initiating a first INSTI-containing regimen with CD4<500 stratified by: (A) HIV viral load, (B) INSTI regimen, (C) nadir CD4 count, and (D) age, all measured at INSTI initiation.