Abstract
Objectives
To assess the effects of botulinum toxin for prevention of migraine in adults.
Design
Systematic review and meta-analysis.
Data sources
CENTRAL, MEDLINE, Embase and trial registries.
Eligibility criteria
We included randomised controlled trials (RCTs) of botulinum toxin compared with placebo, active treatment or clinically relevant different dose for adults with chronic or episodic migraine, with or without the additional diagnosis of medication overuse headache.
Data extraction and synthesis
Cochrane methods were used to review double-blind RCTs. Twelve week post-treatment time-point data was analysed.
Results
Twenty-eight trials (n=4190) were included. Trial quality was mixed. Botulinum toxin treatment resulted in reduced frequency of −2.0 migraine days/month (95% CI −2.8 to −1.1, n=1384) in chronic migraineurs compared with placebo. An improvement was seen in migraine severity, measured on a numerical rating scale 0 to 10 with 10 being maximal pain, of −2.70 cm (95% CI −3.31 to −2.09, n=75) and −4.9 cm (95% CI −6.56 to −3.24, n=32) for chronic and episodic migraine respectively. Botulinum toxin had a relative risk of treatment related adverse events twice that of placebo, but a reduced risk compared with active comparators (relative risk 0.76, 95% CI 0.59 to 0.98) and a low withdrawal rate (3%). Although individual trials reported non-inferiority to oral treatments, insufficient data were available for meta-analysis of effectiveness outcomes.
Conclusions
In chronic migraine, botulinum toxin reduces migraine frequency by 2 days/month and has a favourable safety profile. Inclusion of medication overuse headache does not preclude its effectiveness. Evidence to support or refute efficacy in episodic migraine was not identified.
Keywords: migraine disorders, botulinum toxin, botox, systematic review, randomised controlled trial
Strengths and limitations of this study.
This paper is a summary of a Cochrane review conducted using systematic and thorough methodology to identify and synthesise all available evidence for the effectiveness of botulinum toxin for prophylactic treatment of migraine.
No language or date restrictions were placed on the search strategy.
Many of the included studies were small in size and failed to fully report their data which impacted the quality ratings and the content of the meta-analyses.
Our chosen primary outcome measure, though recommended in current guidelines for controlled trials of prophylactic treatment of chronic migraine, was not commonly recorded.
Introduction
Migraine is the seventh leading cause of years lived with disability globally and is estimated to affect around 15% of the world’s population.1 Days lost from work and other activities of daily living resulting from migraines have a major economical impact.2 Many people with migraine suffer prolonged and frequent migraine attacks despite optimised acute and prophylactic treatments.3–5
Botulinum toxin type A (BTX-A) has been licensed for use in migraine in some countries, based largely on two commercially sponsored trials.6 7 The recommended reconstituted dose is 155 to 195 units, administered intramuscularly as 0.1 mL (five units) injections to between 31 and 39 sites around the head and neck.3 Cost of treatment and administration of BTX-A is much higher than standard doses of the two first line treatments for the prevention of migraine, propranolol and topiramate (around 25 times and 15 times respectively in the UK).8–10
Migraine can be categorised as chronic or episodic and these terms are commonly used in eligibility criteria for clinical trials and systematic reviews. Chronic migraine is currently defined by the International Headache Society (IHS) as headache for at least 15 days per month with migraine features on eight of those days.11 Episodic migraine is commonly used to describe patients with symptoms of migraine who have less than 15 headache days per month and according to official guidance is a term which can be used for migraine that is not covered by the definition of chronic migraine.11 Migraine can occur with medication overuse headache; the IHS definition has evolved, but currently this is defined as an interaction between a therapeutic agent used excessively and a susceptible patient.11 12 Trials recruiting participants with chronic migraine will come across many patients with this dual diagnosis. Current UK guidelines published by The National Institute for Health and Care Excellence (NICE) recommend the use of BTX-A for chronic migraine, but not for high frequency episodic migraine, and only when the condition is ‘appropriately managed’ for medication overuse.8
The aim of this evidence review was to assess the effects of botulinum toxin (BTX) versus placebo or alternative active treatment for the prophylaxis of episodic migraine or chronic migraine in adults.
This paper is a summary of key aspects from a Cochrane review first published in The Cochrane Library 2018, Issue 6 (see http://www.thecochranelibrary.com/ for information).13 Cochrane reviews are regularly updated as new evidence emerges and in response to feedback, and The Cochrane Library should be consulted for the most recent version of the review.
Methods
The protocol for this review was published in the Cochrane Database of Systematic Reviews in advance of the publication of the full review which replaced it. Deviations from the protocol are listed in the full review.13
Search strategy
A systematic search of the literature published before March 2019 was carried out. We designed a highly sensitive search strategy using methods recommended by the Cochrane collaboration to minimise publication bias. No date, language or publication status restrictions were applied. We used a combination of index terms and free text terms for headache, migraine, cephalalgia or hemicrania; and botulinum toxin, Botox, onabotulinum toxin, Oculinum or clostridium botulinum. Relevant trials were identified through electronic searches of Cochrane Central Register of Controlled Trials, MEDLINE (see full strategy in online supplementary file 1), Embase, ClinicalTrials.gov and WHO International clinical trials registry, hand-searching reference lists and citation searches on key publications and correspondence with all major manufacturers of BTX products relevant to this review.
bmjopen-2018-027953supp001.pdf (93.3KB, pdf)
Selection criteria
We included randomised, double-blind, controlled trials of people over the age of 18 years suffering from migraine as defined by any edition of the IHS criteria,11 12 14 or meeting reasonable criteria designed to distinguish between migraine and tension-type headache. Patients with both chronic migraine and episodic migraine were included in this review. Medication overuse headache was included as these types of participants have been included in large and prominent trials in this area. Trials must compare BTX (any sero-type) injected into the head and neck muscles with placebo injections, clinically relevant different dose of same treatment or active preventative agent. Trials allowing the use of concomitant preventative or rescue treatments were included.
Screening of abstracts and assessment of eligibility of full papers were carried out independently in duplicate and according to criteria predefined in the peer reviewed protocol. If disagreements occurred at any stage, a third author considered the available information or if necessary the study authors were contacted for clarification. When eligibility could not be determined through consideration of published materials or contact with trial authors the studies were excluded.
Quality assessment
Eligible material was assessed, independently by two reviewers for each trial, for methodological quality using Cochrane risk of bias methods. Publications were assessed on their method of randomisation, blinding and concealment of allocation, the number of participants lost to follow-up, evidence of selective reporting and study size.
We considered the use of funnel plots to assess the risk of publication bias but did not carry them out. We made this decision because of the small number of studies included in the individual meta-analyses and the true heterogeneity in the trial design (dose, injection paradigm) and populations studied (migraine sub-classifications), which would make it impossible to draw useful conclusions from the plots. Grading of Recommendations Assessment, Development and Evaluation (GRADE) tables were created for each comparison, this process involves assessment of the risk of publication bias for each outcome measure.
Data extraction
Data extraction was carried out independently and in duplicate onto forms designed and tested at protocol stage. The primary outcome was frequency of migraine days per month. Secondary outcomes included: frequency of headache days, frequency of migraine attacks, severity of migraine, duration of migraine, 50% responder rate, global impression scales, quality of life measures and adverse event reporting. We used risk ratios (RRs) as the preferred statistical output for dichotomous outcomes, with 95% CIs. For continuous data, we used mean differences with 95% CIs. Results with p values lower than 0.05 were considered to be statistically significant. Twelve week time-point data following final round of treatment was analysed. We sought data from the first phase for any cross-over trials identified. We attempted to contact authors and obtain missing data.
Statistical analysis
The review authors assessed trial information and baseline characteristics to identify clinical and methodological differences during the data extraction process. If clinical and methodological homogeneity were confirmed, we carried out meta-analysis of the data using Review Manager (RevMan) V.5.3.15
Heterogeneity present in doses, injection sites and participant populations led to the decision that a random-effects model should be used for the analysis. RevMan implements a version of random-effects meta-analysis that is described by DerSimonian and Laird16 and presents an estimate of the between-study variance (Tau2) at the bottom of each forest plot. We tested for statistical homogeneity of pooled estimates of effectiveness using the X2 test and the I2 statistic, for which a statistically significant (p value ≤0.1) value of the X2 test together with I2 value of at least 50% indicates heterogeneity.
Within our eligible comparisons, we split data into migraine classification subgroups in order to show results for chronic migraine, episodic migraine and a mixed group for which the diagnosis could not be split.
We planned to use the following subgroups to test for variation in the effects of the intervention:
Trials including medication overuse headache versus trials excluding this type of patient.
Different sero-types of BTX (eg, A vs B) and within sero-types (Dysport vs Botox).
Different types of agents for the prevention of migraine versus BTX.
Accepted and licensed 31 injection pattern versus other injection patterns used.
At least two trials and 200 participants per group were required for any particular subgroup analysis to be carried out.
We carried out sensitivity analyses for our primary outcome only. Prevailing evidence suggests that smaller trials are more likely to report stronger effect estimates than large trials.17 18 To assess whether these stronger effect estimates reflected the true treatment effect we carried out a sensitivity analysis in which we examined the effect of removing studies at high risk of bias from study size.
We assessed the validity of our findings as well as the level of confidence suitable to any estimates of effect generated by our analyses using the GRADE approach.19
Patient and public involvement
There was no patient or public involvement in the design or reviewing process. However, the final Cochrane manuscript including a lay summary, which is accessible to the public through the Cochrane library, was reviewed by a patient representative as part of the editorial process. Their feedback was incorporated into the final draft.
Results
Description of included studies
The flow of information through the review process is given in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart in online supplementary file 2. The characteristics of studies included in this review are given in online supplementary file 3.
bmjopen-2018-027953supp002.pdf (197.6KB, pdf)
bmjopen-2018-027953supp003.pdf (288.6KB, pdf)
We identified 28 eligible trials, involving a total of 4190 participants, which were eligible for inclusion in this review. Twenty-three of these trials compared BTX-A with placebo injections6 7 20–40 and three compared with an alternative established oral prophylactic agent.41–43
Five trials, reported in four articles, compared alternative doses of BTX-A,26 33–35 all but one of these also included a placebo arm26 and one compared with injections of histamine.44 Due to the paucity of the data, review of the dosing studies and the histamine study are included as appendices in the Cochrane review and is not repeated here.13
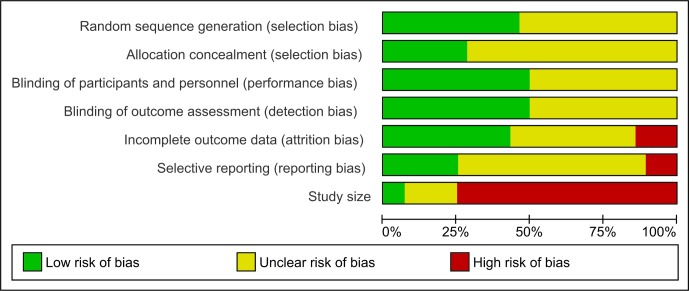
The results of the critical appraisal were mixed (figure 1). Across all domains poor reporting was an issue and in all but attrition bias and study size at least 50% of trials provided insufficient information to allow judgments about risk of bias to be made. Only two trials were at low risk of bias due to study size (at least 200 participants per trial arm) and these two trials were also at low risk of bias across all other domains.6 7
Figure 1.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Sixteen trials were commercially sponsored, including the only two trials at low risk from study size.6 7 21 22 24–27 32–35 40 41 43
For those trials providing information on the migraine diagnosis of their participants the ratio of chronic/episodic migraine was 1872/1928, leaving 392 included participants unclassified and analysed as’ Mixed’. The mean age was 42 years and 85% of all participants were female. Pregnant women were generally explicitly excluded. All included trials used BTX-A, of these 21 had at least one arm treated with the Botox formulation,6 7 20–24 26 27 30 31 33–35 38 40–44 two used Dysport,25 32 two used Prosigne28 31 and one HengLi.29 The range of doses administered in trials of Botox was 6 U to 300 U. The trials using Dysport administered doses of 80 U up to 240 U in treated arms (dose equivalency reported by trial publications: 2 to 3 U:1 U Botox). HengLi and Prosigne trials used doses ranging from 25 U to 96 U (dose equivalency reported by trial publications: 1 U:1 U Botox).
Effectiveness versus placebo
Comparison with a placebo group was made in 23 trials with 3912 participants.
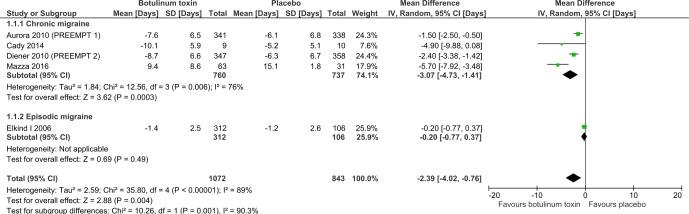
Meta-analysis of our primary outcome for the four trials in chronic migraine which reported it showed that there was a reduction of 3.1 days of migraine per month (95% CI −4.7 to −1.4) in favour of BTX-A treatment (figure 2). At least 60% of the participants in this analysis had medication overuse headache. The episodic migraine subgroup involved only one trial of 418 participants which showed no difference in the number of migraine days between treated and placebo groups (p=0.49). Insufficient data were available to carry out any of the planned subgroup analyses on the primary outcome measure. Concern about small trial effects caused us to carry out a sensitivity analysis. Removal of all chronic migraine trials at high risk of bias from study size left just the two PREEMPT trials, which gave a more conservative reduction of 2.0 days per month (95% CI −2.8 to −1.1, n=1384).
Figure 2.
Comparison of BTX-A versus placebo in relation to number of migraine days per month. BTX-A, botulinum toxin type A.
Migraine severity score on a 10 cm visual analogue scale, improved by −3.3 cm (95% CI −4.2 to −2.4) more with active treatment (figure 3). Only four small trials reported meta-analysable data for this outcome. For chronic migraine the improvement was −2.7 cm (95% CI −3.3 to −2.1, n=75), and for episodic migraine it was −4.9 cm (95% CI −6.6 to −3.2, n=34).
Figure 3.
Comparison of BTX-A versus placebo in relation to severity of migraine measured on a 10 cm visual analogue scale. BTX-A, botulinum toxin type A.
A reduction in the number headache days per month of 1.9 days (95% CI −2.7 to −1.0, two trials, n=1384) in favour of BTX-A treatment was also seen. However data for number of migraine attacks from six trials of both chronic migraine and episodic migraine participants (n=2004) showed no significant between group difference (p=0.30). Duration of migraine in hours was fully reported by only one trial showing a greater reduction of −5.1 hours (95% CI −6.2 to −4.0) for 102 chronic and episodic migraine participants. A further four trials with 420 participants reported no significant difference between groups for this outcome. Global assessment measures and quality of life measures were poorly reported and it was not possible to carry out statistical analysis of these outcome measures.
Effectiveness of BTX versus oral prophylactic agents
Three trials with 178 participants compared Botox injections with oral prophylactic agents using double dummy techniques. Two trials compared 100 U fixed dose plus optional dose of up to 100 U of Botox with topiramate maximum dose 200 mg/day.42 43 The third trial compared treatment with up to 100 U Botox with sodium valproate 250 mg twice daily.41 Fourteen of the 178 participants had episodic migraine, all other participants had chronic migraine. Where meta-analysis was possible we pooled data from these three trials as there were insufficient data to allow us to explore comparisons with individual drug types or effects on chronic migraine and episodic migraine populations.
The primary outcome, number of migraine days per month was recorded in only one of the active comparison trials. The trialists reported that there was no statistically significant difference between treatment with BTX-A and topiramate for this outcome.43
The number of headache days per month was recorded in two trials. No difference in number of headache days per month between treatment with BTX-A and sodium valproate was reported (p=0.55).35 No data were reported but it was stated that there was also no statistically significant difference between BTX-A and topiramate treated groups.42 A 5-point scale was used to compare the effect of BTX-A with alternative agents in two trials, Blumenfeld et al. reported no significant difference and Mathew et al. reported within group analysis only.41 43 Number of migraine attacks and duration of migraine were not reported by any trial. No difference between BTX-A and topiramate was stated for use of rescue medications.43
Of all the secondary outcome measures, data for meta-analysis were available only for the Migraine Disability Assessment (MIDAS) scores. Results of this showed no significant difference in change scores between the established drug treatments and injection with Botox (p=0.80, two trials, n=101).
Safety
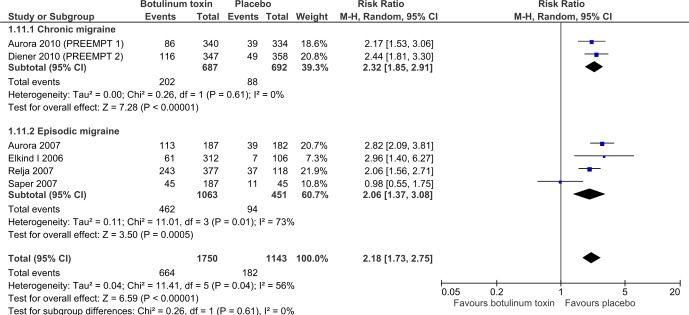
BTX-A had an RR of treatment related adverse events of twice that seen for placebo (2.2, 95% CI 1.7 to 2.8, six trials, n=2839) (figure 4). All of these events were transient and non-serious, the most common being blepharoptosis, muscle weakness, injection site pain and neck pain.
Figure 4.
Comparison of BTX-A versus placebo in relation to treatment related adverse events. BTX-A, botulinum toxin type A.
Compared with oral treatments, BTX-A showed a reduced RR of treatment related adverse events of 0.76 (95% CI 0.59 to 0.98, two trials, n=73). There was also difference in favour of BTX-A in the RR of withdrawing due to adverse events of 0.28 (95% CI 0.10 to 0.79, I2=0%) which is a RR reduction of 72%.
A low withdrawal rate of 3% for BTX-A was generated using data from all those trials treating with more than one injection cycle irrespective of the type of comparison arm.
Quality of the evidence
The quality of the evidence assessed using GRADE methods was varied but mostly low and very low; the primary outcome measure was low and very low quality evidence for the placebo and active control comparisons respectively. Small trial size, high risk of bias and unexplained heterogeneity were common reasons for downgrading the quality of the evidence. All judgements and reasons for gradings are given in online supplementary file 4 and 5.
Discussion
Evidence was identified to support the use of injections of BTX-A into the head and neck muscles, to reduce the number of migraine days experienced per month. Mean frequency of migraine days was significantly reduced by 3 days per month more by BTX-A treatment than by placebo, but this result was revised to 2 days per month as a result of sensitivity analyses. All patients included in this analysis had chronic migraine and so had a high baseline frequency with an average of 20 days per month quoted by the two largest trials in the analysis.6 7 For patients with chronic migraine, likely to be refractory to first and second line treatment, a 2 to 3 day improvement may well represent a meaningful difference. BTX-A groups also fared better than placebo in the frequency of headache days by 2 days per month. Severity of migraine measured on a visual analogue scale was improved by 3 cm for chronic migraine and 5 cm for episodic migraine on a 10 cm scale. Though these results were from few small trials and the estimate is considered to be low quality evidence, the differences in severity scores were in excess of the minimal clinically important difference of 1.2 cm determined by Kelly45 and indicate that the treatment may be reducing the impact of each migraine attack. In contrast to this no significant difference from placebo was observed for frequency of migraine attacks. Patient and clinician reported global assessment scales and quality of life scales were underused and when they were incorporated into trials they were poorly reported, so no aggregation of data of this type comparing investigative treatment with placebo was possible in this review.
It was not possible to carry out any analysis on headache diary outcomes or severity measures for head-to-head comparisons between BTX-A and other established agents due to lack of available data. MIDAS scores for 101 patients from two small trials, one comparing Botox with topiramate and one with sodium valproate were available and these showed no significant between group difference (p=0.8).
Trials included in this review commonly state that BTXs have good safety profiles and the evidence from the 23 trials included in this review which reported adverse events in some form support those assertions. Although an increased risk of experiencing treatment related adverse events was found for the BTX-A treated group compared with placebo, the event types were non-serious and transient.
A relative risk reduction (RRR) of 24% in treatment related adverse events in favour of BTX-A was found when comparing with topiramate and sodium valproate in two trials. These two trials found an RRR in favour of BTX-A of 72% for withdrawal rate due to adverse events. Percentage withdrawals due to adverse events for all of those trials included in this review which used more than one round of BTX-A injections, irrespective of the comparison arm type, was 3%. The data sets for the direct comparisons with other prophylactic agents were small, but the relationship is supported by the indirect comparison of this percentage with published rates of 20% for topiramate and 12% for sodium valproate.46 47 This result suggests that patients tolerate this treatment better than the oral alternatives.
Reporting was generally poor, with only six of 28 trials reporting data on our primary outcome in a usable format, and an additional five providing data for frequency of migraine attacks. These two outcomes are recommended as primary outcomes by the trial guidelines produced by the IHS and should be fully reported to allow individual trials to be placed in the context of the totality of the evidence.48 A large proportion of the recorded data were missing from the published reports of our included trials. Failure to fully report data in trial publications led to problems throughout the meta-analysis and greater confidence in the conclusions would have been possible if all trials that recorded our outcomes of interest had fully reported them.
Prophylactic treatments for migraine aim to reduce the frequency, duration and/or the intensity of attacks. Frequency of migraine attacks was commonly used as the primary outcome particularly in studies carried out before the publication of the PREEMPT trials. Use of this measure may mask an important improvement in symptoms seen in the form of shorter and less intense migraine attacks. Use of the more sensitive measures, number of days or hours spent with migraine per month coupled with a measure of intensity, may enable detection of such changes and could be particularly relevant to episodic migraine patients for whom attacks may be shorter at baseline. Another problem with focusing on this outcome measure was the failure generally to define what was meant by a migraine attack, and therefore, the likelihood of variation in the definitions used across the trials.
Neither efficacy nor safety data were available for long-term treatment with BTX. The longest treatment period in any of the studies included in this review was three treatments with 12 weeks between treatments, so we cannot know the implications of treating patients with BTX over a period longer than 9 months.
Most trials did not report whether or not they had included patients with medication overuse symptoms and those that did stated they had largely excluded medication overuse patients. Pooled data for the two PREEMPT trials for the chronic migraine plus medication overuse subgroup (n=906) showed that the difference between groups for both migraine and headache day frequencies was 2 days (p<0.001) in favour of treatment with BTX.49 The medication overuse subgroup result falls within the CIs of the pooled estimate generated by this review for the same outcome measure in combined populations with and without medication overuse headache. It would appear from these data that the inclusion of patients with medication overuse does not change the effectiveness of BTX for prophylactic treatment of migraine.
Conclusions
We have data which suggest that BTX effectively reduces the duration and severity of migraines in sufferers. There are however question marks over the quality of the evidence. Efficacy measures were commonly reported as showing non-inferiority of BTX to topiramate and sodium valproate and the withdrawal rate from BTX is much lower than that for first line prophylactic treatments for migraine. So should we be using more BTX?
It is currently recommended by NICE guidance that medication overuse headache should be addressed before treatment with BTX but trial data suggests it is efficacious in chronic migraine patients with untreated medication overuse headache. So although treatment of medication overuse headache is good practice, perhaps it should not be a requirement before prescription of BTX. NICE recommends the use of BTX to treat chronic migraine that has not responded to at least three prior pharmacological prophylaxis therapies. The confidence in the effectiveness of these drugs is arguably no greater than that for BTX and patients seem better able to tolerate BTX.4 5 46 47 If, as is suggested by trial data, BTX has the equivalent efficacy to other agents but lower withdrawal rates, then if it were not for the higher cost, BTX would likely be recommended as an earlier preventative treatment for chronic migraine.
The difference between chronic and episodic migraine diagnoses is arbitrary and so there is no pathophysiological reason that treatment with BTX would be efficacious in people with 15 days of headache per month and inefficacious in people with 14 days of headache per month in a stepwise fashion. The treatment may well be useful for episodic migraine, particularly in high frequency episodic migraine, but data is lacking.
bmjopen-2018-027953supp004.pdf (117.9KB, pdf)
bmjopen-2018-027953supp005.pdf (115KB, pdf)
Supplementary Material
Footnotes
Contributors: C Clarke and A Sinclair conceived the review. C Herd ran searches not covered by the review group’s information specialist. C Herd, C Tomlinson, C Rick and A Sinclair screened the search results. C Herd, C Tomlinson, C Rick, W Scotton, C Clarke and A Sinclair assessed the quality of studies and extracted data. C Herd contacted trial authors, managed data and entered it into RevMan, and carried out data analysis. N Ives provided statistical advice. C Herd, C Tomlinson, C Rick, N Ives, C Clarke and A Sinclair were involved in interpretation of the results. All authors read and edited final version of the review.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Yes, there are competing interests for one or more authors and I have provided a Competing Interests statement in my manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Full data analysis and trial summaries available in Cochrane review DOI: 10.1002/14651858.CD011616.pub2.
References
- 1. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linde M, Gustavsson A, Stovner LJ, et al. . The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012;19:703–11. 10.1111/j.1468-1331.2011.03612.x [DOI] [PubMed] [Google Scholar]
- 3. Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database of Systematic Reviews 2004. [DOI] [PubMed] [Google Scholar]
- 4. Linde M, Mulleners WM, Chronicle EP, et al. . Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013;151 10.1002/14651858.CD010609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linde M, Mulleners WM, Chronicle EP, et al. . Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013:CD010608 10.1002/14651858.CD010608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aurora SK, Dodick DW, Turkel CC, et al. . OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010;30:793–803. 10.1177/0333102410364676 [DOI] [PubMed] [Google Scholar]
- 7. Diener HC, Dodick DW, Aurora SK, et al. . OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804–14. 10.1177/0333102410364677 [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health Care Excellence. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. NICE technology appraisal guidance [TA260]. Published date 2012. http://wwwniceorguk/guidance/ta260. [Google Scholar]
- 9. National Institute for Health Care Excellence. Headaches: Diagnosis and management of headaches in young people and adults. 2012. NICE guidelines [CG150] http://wwwniceorguk/guidance/cg150.
- 10. British Medical Association, Society RP. British National Formulary (BNF). http://wwwbnforg.
- 11. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 12. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition, 1st revision. Cephalalgia 2004;24 (Suppl 1):1–160. [DOI] [PubMed] [Google Scholar]
- 13. Herd CP, Tomlinson CL, Rick C, et al. . Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev 2018;6:CD011616 10.1002/14651858.CD011616.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl. 7):1–96. [PubMed] [Google Scholar]
- 15.Review Manager (RevMan) [program]. Version 5.3 version. Copenhagen: The Cochrane Collaboration, 2014. [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17. Dechartres A, Trinquart L, Boutron I, et al. . Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304–f04. 10.1136/bmj.f2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nüesch E, Trelle S, Reichenbach S, et al. . Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515–c15. 10.1136/bmj.c3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand KS, Prasad A, Singh MM, et al. . Botulinum toxin type A in prophylactic treatment of migraine. Am J Ther 2006;13:183–7. 10.1097/01.mjt.0000212705.79248.74 [DOI] [PubMed] [Google Scholar]
- 21. Aurora SK, Gawel M, Brandes JL, et al. . Botulinum toxin type a prophylactic treatment of episodic migraine: a randomized, double-blind, placebo-controlled exploratory study. Headache 2007;47:486–99. 10.1111/j.1526-4610.2006.00624.x [DOI] [PubMed] [Google Scholar]
- 22. Barrientos N, Chana P. Botulinum toxin type A in prophylactic treatment of migraine headaches: a preliminary study. J Headache Pain 2003;4:146–51. 10.1007/s10194-003-0049-2 [DOI] [Google Scholar]
- 23. Blumenkron D, Rivera C, Cuevas C. Efficacy of botulinum toxin type A in patients with migraine. [Spanish] Eficacia del tratamiento con toxina botulinica tipo A en pacientes con migrana. Medicina Interna de Mexico 2006;22:25–31. [Google Scholar]
- 24. Cady R, Schreiber C. Botulinum toxin type A as migraine preventive treatment in patients previously failing oral prophylactic treatment due to compliance issues. Headache 2008;48:900–13. 10.1111/j.1526-4610.2007.00953.x [DOI] [PubMed] [Google Scholar]
- 25. Chankrachang S, Arayawichanont A, Poungvarin N, et al. . Prophylactic botulinum type A toxin complex (Dysport®) for migraine without aura. Headache 2011;51:52–63. 10.1111/j.1526-4610.2010.01807.x [DOI] [PubMed] [Google Scholar]
- 26. Elkind AH, O’Carroll P, Blumenfeld A, et al. . A series of three sequential, randomized, controlled studies of repeated treatments with botulinum toxin type A for migraine prophylaxis. J Pain 2006;7:688–96. 10.1016/j.jpain.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 27. Freitag FG, Diamond S, Diamond M, et al. . Botulinum Toxin Type A in the treatment of chronic migraine without medication overuse. Headache 2008;48:201–9. 10.1111/j.1526-4610.2007.00963.x [DOI] [PubMed] [Google Scholar]
- 28. Hollanda L, Monteiro L, Melo A. Botulinum toxin type a for cephalic cutaneous allodynia in chronic migraine: a randomized, double-blinded, placebo-controlled trial. Neurol Int 2014;6:70–3. 10.4081/ni.2014.5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou M, Xie JF, Kong XP, et al. . Acupoint injection of onabotulinumtoxin A for migraines. Toxins 2015;7:4442–54. 10.3390/toxins7114442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. H. Jost W. Low-dosed botulinum toxin a in the prophylactic management of unilateral migraine: a randomized double-blind placebo-controlled crossover study. Open Pain J 2011;4:4–7. 10.2174/1876386301104010004 [DOI] [Google Scholar]
- 31. Lauretti GR, Rosa CP, Kitayama A, et al. . Comparison of Botox® or Prosigne® and Facial Nerve Blockade as Adjuvant in Chronic Migraine. Journal of Biomedical Science and Engineering 2014;7:446–52. [Google Scholar]
- 32. Petri S, Tölle T, Straube A, et al. . Botulinum toxin as preventive treatment for migraine: a randomized double-blind study. Eur Neurol 2009;62:204–11. 10.1159/000228987 [DOI] [PubMed] [Google Scholar]
- 33. Relja M, Poole AC, Schoenen J, et al. . A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia 2007;27:492–503. 10.1111/j.1468-2982.2007.01315.x [DOI] [PubMed] [Google Scholar]
- 34. Saper JR, Mathew NT, Loder EW, et al. . A double-blind, randomized, placebo-controlled comparison of botulinum toxin type a injection sites and doses in the prevention of episodic migraine. Pain Med 2007;8:478–85. 10.1111/j.1526-4637.2006.00168.x [DOI] [PubMed] [Google Scholar]
- 35. Silberstein S, Mathew N, Saper J, et al. . Botulinum toxin type a as a migraine preventive treatment. Headache 2000;40:445–50. 10.1046/j.1526-4610.2000.00066.x [DOI] [PubMed] [Google Scholar]
- 36. Cady R, Turner I, Dexter K, et al. . An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache 2014;54:269–77. 10.1111/head.12250 [DOI] [PubMed] [Google Scholar]
- 37. Vo AH, Satori R, Jabbari B, et al. . Botulinum toxin type-a in the prevention of migraine: a double-blind controlled trial. Aviat Space Environ Med 2007;78(5 Suppl):B113–8. [PubMed] [Google Scholar]
- 38. Jabbari B. Investigation of Efficacy and Safety of Botulinum Toxin A (Botox-Allergan Inc) in Migraine Headaches. clinicaltrials.gov, 2008. [Google Scholar]
- 39. Mazza MR, Ferrigno G, Vescio B, et al. . Subcutaneous botulinum toxin type a treatment for prophylaxis of headaches in chronic migraine: A new therapeutic strategy. Cephalalgia 2016;36:35. [Google Scholar]
- 40. Allergan. Use of a Treatment Benefit Questionnaire in Patients With Chronic Migraine Treated With OnabotulinumtoxinA (BOTOX®). clinicaltrials.gov, 2013. [Google Scholar]
- 41. Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache 2008;48:210–20. 10.1111/j.1526-4610.2007.00949.x [DOI] [PubMed] [Google Scholar]
- 42. Cady RK, Schreiber CP, Porter JA, et al. . A multi-center double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraine. Headache 2011;51:21–32. 10.1111/j.1526-4610.2010.01796.x [DOI] [PubMed] [Google Scholar]
- 43. Mathew NT, Jaffri SF. A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study. Headache 2009;49:1466–78. 10.1111/j.1526-4610.2009.01566.x [DOI] [PubMed] [Google Scholar]
- 44. Millán-Guerrero RO, Isais-Millán S, Barreto-Vizcaíno S, et al. . Subcutaneous histamine versus botulinum toxin type A in migraine prophylaxis: a randomized, double-blind study. Eur J Neurol 2009;16:88–94. 10.1111/j.1468-1331.2008.02352.x [DOI] [PubMed] [Google Scholar]
- 45. Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 2001;18:205–7. 10.1136/emj.18.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Linde M, Mulleners WM, Chronicle EP, et al. . Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013:CD010611 10.1002/14651858.CD010611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Linde M, Mulleners WM, Chronicle EP, et al. . Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013;122 10.1002/14651858.CD010610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tfelt-Hansen P, Pascual J, Ramadan N, et al. . Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32:6–38. 10.1177/0333102411417901 [DOI] [PubMed] [Google Scholar]
- 49. Silberstein SD, Blumenfeld AM, Cady RK, et al. . OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci 2013;331(1-2):48–56. 10.1016/j.jns.2013.05.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027953supp001.pdf (93.3KB, pdf)
bmjopen-2018-027953supp002.pdf (197.6KB, pdf)
bmjopen-2018-027953supp003.pdf (288.6KB, pdf)
bmjopen-2018-027953supp004.pdf (117.9KB, pdf)
bmjopen-2018-027953supp005.pdf (115KB, pdf)