Key Points
HGAL and Gb2 proteins directly interact upon BCR stimulation.
HGAL and Gb2 interaction plays a role in BCR clustering in signalosomes and regulates BCR-induced biochemical signaling.
Abstract
Human germinal center (GC)–associated lymphoma (HGAL) is an adaptor protein expressed in GC B cells. HGAL regulates cell motility and B-cell receptor (BCR) signaling, processes that are central for the successful completion of the GC reaction. Herein, we demonstrate phosphorylation of HGAL by Syk and Lyn kinases at tyrosines Y80, Y86, Y106Y107, Y128, and Y148. The HGAL YEN motif (amino acids 107-109) is similar to the phosphopeptide motif pYXN used as a binding site to the growth factor receptor–bound protein 2 (Grb2). We demonstrate by biochemical and molecular methodologies that HGAL directly interacts with Grb2. Concordantly, microscopy studies demonstrate HGAL-Grb2 colocalization in the membrane central supramolecular activation clusters (cSMAC) following BCR activation. Mutation of the HGAL putative binding site to Grb2 abrogates the interaction between these proteins. Further, this HGAL mutant localizes exclusively in the peripheral SMAC and decreases the rate and intensity of BCR accumulation in the cSMAC. Furthermore, we demonstrate that Grb2, HGAL, and Syk interact in the same complex, but Grb2 does not modulate the effects of HGAL on Syk kinase activity. Overall, the interplay between the HGAL and Grb2 regulates the magnitude of BCR signaling and synapse formation.
Visual Abstract
Introduction
The germinal center (GC) reaction is the hallmark of antibody-mediated immune responses to T-cell–dependent antigens and is necessary for immune defense.1 GCs represent morphologic and functional structures within secondary lymphoid organs in which B-cell responses to antigens are amplified and refined in specificity. Multiple orchestrated processes contribute to the successful completion of the GC reaction. These include propagating differentiation and survival signals via the B-cell receptor (BCR), regulating B-cell motility to interact with antigen-presenting cells, and the generation of plasma cells. Aberrations in the GC reaction and its underlying molecular processes may predispose patients to immune deficiency, autoimmune disorders, and lymphoma. Completely elucidating the mechanisms that control the GC reaction is of paramount importance. The Meeting on Lymphoma Biology organized by the American Society of Hematology in August 2014 proposed a Roadmap for Discovery and Translation in Lymphoma that specifically emphasized the need to “define all molecules necessary to initiate and sustain the GC response and identify all key protein-protein interactions and post-translational modifications that regulate BCR signaling.”2
We have cloned the human GC-associated lymphoma (HGAL, also known as GC-expressed transcript 2 or GC-associated signaling and motility) gene, which is specifically expressed in GC B cells and GC-derived lymphomas.3 High expression of HGAL is an independent predictor of prolonged survival of diffuse large B-cell lymphoma (DLBCL) and classical Hodgkin lymphoma patients, as demonstrated by us and other groups.3-6 We showed that HGAL is an adaptor protein that regulates both cell motility and BCR signaling, processes that are central for the successful completion of the GC reaction.7-10 We demonstrated that HGAL localizes to cellular membrane raft microdomains and increases BCR signaling by binding to and enhancing Syk kinase activity.7,10 However, our previous studies also suggested that other proteins may be involved in the HGAL-mediated regulation of BCR signaling.
Examination of the HGAL protein sequence revealed the presence of a putative growth factor receptor–bound protein 2 (Grb2) binding motif (YEN) at position amino acids 107-109. Grb2 is a ubiquitously expressed adapter protein that plays a pivotal role in BCR signaling11-15 and controls lymphoid follicle organization and the GC reaction.16 Specifically, Grb2 is an integral component of the BCR signalosome11-14,17 and plays a role in the formation of the central supramolecular activation clusters (cSMAC), the immunological synapse. In the absence of Grb2, BCR microclusters remain in the periphery and fail to form the central BCR cluster.12 Previous studies suggested an interaction between HGAL and Grb2 but did not comprehensively analyze if it is direct and its biological effects.18,19
Herein, we demonstrate that Grb2 directly interacts with phosphorylated HGAL and that both proteins colocalize in the cSMAC, but not the peripheral SMAC (pSMAC), where only HGAL can be found. Mutation of the Grb2 binding motif in the HGAL protein prevents this interaction and leads to (1) exclusive localization of HGAL in the pSMAC and (2) slower dynamics and decreased BCR accumulation in the cSMAC. Furthermore, we demonstrate that Grb2, HGAL, and Syk proteins interact with each other, but Grb2 does not modulate the effects of HGAL on Syk kinase activity.
Materials and methods
Antibodies, reagents, and plasmids
Mouse monoclonal anti-HGAL antibody was generated in our laboratory.3,20 Antibodies to Grb2 (C23), Syk (D-3), Btk (DFS), B-cell linker (BLNK; 2B11), phospholipase Cγ2 (PLCγ2) (Q-20), p-PLCγ2 (Tyr753), extracellular signal-regulated kinase 2 (ERK2) (C-14), JNK (FL), p-ERK (E-4), p-JNK (G-7), Dok-3 (F-7), and glutathione S-transferase (GST) (1E5) were from Santa Cruz Biotechnology (Santa Cruz, CA); p-p38 MAPK (Thr180/Tyr182) (28B10), p38 MAPK (5F11) were from Cell Signaling Technology (Danvers, MA); pSyk (pY352), pSyk (pY353)-PE, p-Btk (pY551), p-BLNK (pY84) were from BD Biosciences (San Jose, CA). Alexa Fluor 488–conjugated pSyk (pY352) was from BioLegend (San Diego, CA), and Alexa Fluor 647 goat anti-mouse immunoglobulin G (H+L) and goat F(ab′)2 anti-human immunoglobulin M (IgM) antibodies were from Invitrogen (Carlsbad, CA). β-Actin antibody was from Sigma (St. Louis, MO).
Recombinant Trx-HGAL proteins were generated in our laboratory.9 Recombinant GST-Grb2 was from Abnova (Taipei, Taiwan), λ protein phosphatase was from NEB (Ipswich, MA), recombinant Syk kinase was from OriGene (Rockville, MD), and recombinant Lyn kinase was from Invitrogen (Waltham, MA). Grb2 ON-TARGETplus SMARTpool, HGAL ON-TARGETplus SMARTpool, and ON-TARGETplus nontargeting pool small interfering RNA (siRNA) were from Dharmacon RNA Technologies (Lafayette, CO); siRNAs for HGAL and Grb2 were from Santa Cruz Biotechnology, Fluo-4 am was from Invitrogen, and Protein G beads were from Invitrogen.
pcDNA3.1-HGAL-GFP, pcDNA3.1-HGAL(FEN)-GFP, pCDH-Cuo-HGAL-GFP, and pCDH-Cuo-HGAL(FEN)-GFP were generated in our laboratory using standard cloning protocols. The pcDNA3-Flag-Grb2 was a generous gift from Jakub M. Swiercz’s laboratory (Department of Pharmacology, Max Planck Institute for Heart and Lung Research) and was used as a template to generate the pcDNA3.1-Grb2-mCherry and dominant-negative pcDNA3.1-Grb2(W193K)-mCherry plasmids in our laboratory.
Cell cultures and cell transfection and transduction
Human lymphoma cell lines Raji, Bjab, VAL, OCILY19, U2932, TMD8, and Mino were grown as previously reported.21,22 Amaxa Nucleofector Kits (Amaxa, Gaithersburg, MD) were used for transfection of plasmids or siRNA in accordance with the manufacturer’s instructions, as previously reported by us.22 pCDH-Cuo-HGAL-GFP and pCDH-Cuo-HGAL(FEN)-GFP were packaged into virus particle and used to transduce TMD8 and Mino cell lines, as described briefly in supplemental Materials and methods.
Preparation of supported planar membrane
Supported planar lipid bilayers were used to stimulate U2932, Bjab, and Raji cells and were formed as previously described by others.23,24 Vesicle solutions of 1,2-dioleoyl-sn-glycero-3-phosphocholine lipids (Avanti Polar Lipids) with 0.2% to 1% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(cap biotinyl) were fused onto a cleaned glass surface of a parallel plate chamber (sticky-Slide VI; ibidi USA, Madison, WI) to form the supported fluid bilayer. The supported bilayers were labeled with Alexa Fluor (405, 555, or 647)–conjugated streptavidin (Life Technologies) and subsequently functionalized with mono-biotinylated goat anti-human IgM F(ab′)2 (Southern Biotech, Birmingham, AL) (supplemental Figure 1A).
All other methods are described in supplemental Materials and methods.
Results
HGAL is phosphorylated by Lyn and Syk
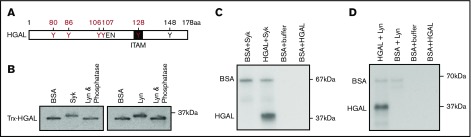
HGAL, by binding to Syk and increasing its kinase activity,7 markedly increases ligand-induced and mildly increases tonic BCR signaling (supplemental Figure 2). HGAL protein harbors 6 tyrosines, some of which are part of a modified immunoreceptor tyrosine-based activation motif (Figure 1A). We previously demonstrated that HGAL undergoes tyrosine phosphorylation in response to BCR or interleukin-6 stimulations.7,25 However, which kinases are involved in this process and what tyrosines they phosphorylate need to be defined more precisely. To address this question, we performed in vitro kinase assays using recombinant Trx-HGAL and Syk or Lyn kinases. Immunoblotting of HGAL demonstrated a shift in the HGAL band position upon Syk and Lyn kinases treatment that was absent in the presence of λ-phosphatase, suggesting HGAL phosphorylation (Figure 1B). Indeed, kinase assays using radiolabeled 32P-adenosine triphosphate confirmed HGAL phosphorylation upon exposure to active Syk or Lyn kinases (Figure 1C-D). Microcapillary reverse-phase high-performance liquid chromatography nanoelectrospray tandem mass spectrometry demonstrated HGAL phosphorylation on tyrosines Y80, Y86, Y106Y107, Y128, and Y148; Syk and Lyn kinases induced phosphorylation of tyrosines Y80, Y86, Y106Y107, and Y128, while tyrosine Y148 was already phosphorylated before exposure to these kinases (Figure 1A and not shown).
Figure 1.
Syk and Lyn kinases phosphorylate tyrosine residues in recombinant HGAL protein in vitro. (A) A schematic diagram of HGAL protein showing location of 6 tyrosines, a putative SH2 domain binding motif (YEN), and a first tyrosine (Y128) of modified immunoreceptor tyrosine-based activation motif (ITAM). Microcapillary reverse-phase high-performance liquid chromatography nanoelectrospray tandem mass spectrometry demonstrated that recombinant HGAL protein is phosphorylated on Y80, Y86, Y106Y107, and Y128 (in red) by Syk and Lyn kinase in vitro. Y148 (in black) was already phosphorylated before the addition of kinases. (B) Western blot of HGAL protein following incubation of Trx-HGAL recombinant protein with recombinant Lyn, Syk, or bovine serum albumin (BSA) in a kinase assay. Upward shift of the band in the presence of kinases suggests HGAL phosphorylation, eliminated by the addition of γ-phosphatase. (C-D) Recombinant Trx-HGAL was incubated with Syk kinase or Lyn kinase or bovine serum albumin in a kinase assay cocktail containing 32P-adenosine triphosphate. Samples were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, dried, and exposed to radiographic film. Data in panels B-D are representative of 3 independent experiments. kDa, kilodaltons.
HGAL directly interacts with Grb2 protein
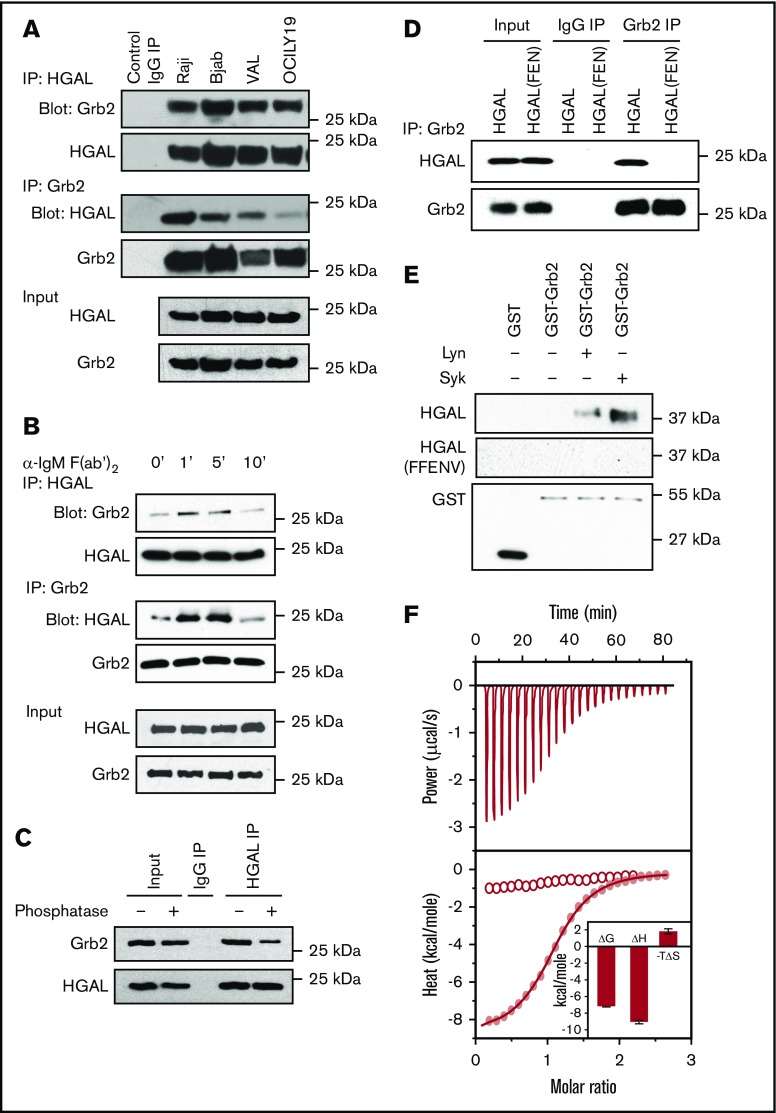
The Y107 tyrosine of HGAL comprise a YEN motif (amino acids 107-109) (Figure 1A), similar to the phosphopeptide motif pYXN frequently used as a binding site to Grb2.26,27 An interaction between HGAL and Grb2 was previously suggested by Pan et al18 but was not conclusively confirmed, since an interaction between recombinant proteins was not shown. Grb2 plays important roles in BCR signaling.11-15 The presence of the YEN motif in HGAL suggested that HGAL directly binds to Grb2 and that this interaction may play a role in regulating BCR signaling. To address this possibility, we performed reciprocal coimmunoprecipitation (co-IP) experiments in multiple lymphoma cell lines. Endogenous Grb2 was detected in immunoprecipitates of endogenous HGAL or exogenous HGAL-GFP from unstimulated Raji, Bjab, VAL, OCILY19, U2932, and TMD8 lymphoma cells (Figure 2A; supplemental Figure 3A). BCR stimulation increased HGAL and Grb2 co-IP (Figure 2B).
Figure 2.
Grb2 directly binds to HGAL via the pYEN motif. (A) Reciprocal co-IP of HGAL and Grb2 from unstimulated Raji, Bjab, VAL, and OCILY19 lymphoma cells. (B) BCR stimulation of Raji cells with goat anti-human IgM F(ab′)2 increases HGAL and Grb2 co-IP at the indicated times. (C) γ-Phosphatase decreases co-IP of Grb2 with HGAL in Raji cells. (D) Grb2 is not interacting with HGAL(FEN) mutant. HeLa cells were transfected with plasmids encoding wild-type HGAL or its mutant HGAL (FEN) and 48 hours later subjected to Grb2 IP followed by HGAL western blotting. (E) GST-Grb2 pull-down assays with recombinant Trx-HGAL and Trx-HGAL(FEN) mutant proteins in the presence or absence of active Syk or Lyn kinases demonstrate that the HGAL-Grb2 interaction is direct and occurs only with phosphorylated HGAL protein. (F) The SH2 domain of Grb2 binds to the phosphorylated (pYEN) 12-mer peptide derived from HGAL with an affinity of 5 μM using isothermal titration calorimetry (red curve with solid dots), but not the unphosphorylated (YEN) peptide (curve with empty dots). In the inset, Grb2 SH2 domain binding to HGAL is governed by favorable enthalpic contributions accompanied by entropic penalty to the overall free energy, implying that the Grb2-HGAL interaction is largely governed by electrostatic interactions with a minor hydrophobic force contribution. Data in panels A-F are representative of 3 independent experiments. IgG, immunoglobulin G.
Grb2 usually binds to phosphorylated tyrosine in the pYEN motif of its cognate binding proteins via its SH2 domain. Indeed, the interaction between HGAL and Grb2 is dependent on phosphorylation of the YEN motif, since binding was decreased in the presence of λ-phosphatase (Figure 2C) and no binding was detected in cells expressing an HGAL mutant in which tyrosine 107 of the YEN motif was mutated to phenylalanine (FEN) (Figure 2D).
A GST-Grb2 pull-down assay with recombinant Trx-HGAL protein in the presence or absence of active Syk or Lyn kinases confirmed that the HGAL-Grb2 interaction is direct and occurs only if HGAL tyrosines are phosphorylated (Figure 2E). In contrast, a GST-Grb2 pull-down assay with a recombinant Trx-HGAL(FEN) mutant protein in the presence or absence of active Syk or Lyn kinases failed to demonstrate a direct interaction, indicating that HGAL-Grb2 binding is dependent on phosphorylation of the YEN tyrosine (Figure 2E).
To corroborate these findings, we directly measured the binding of the SH2 domain of Grb2 to phosphorylated (pYEN) or unphosphorylated (YEN) 12-mer peptides derived from HGAL using isothermal titration calorimetry (Figure 2F). Our analysis reveals that while no binding to YEN peptides was observed, the pYEN peptide bound with an affinity of 5 μM (Figure 2F). Furthermore, the binding of Grb2’s SH2 domain to HGAL is governed by favorable enthalpic contributions accompanied by an entropic penalty to the overall free energy, implying that the Grb2-HGAL interaction is largely governed by electrostatic interactions with a minor hydrophobic force contribution. These observations are in excellent agreement with the binding of the SH2 domain of Grb2 to other known cellular partners of Grb2 harboring the pYXN motif.28,29 Taken together, our results demonstrate that the Grb2-HGAL interaction is direct and driven in a phospho-tyrosine (pY)–dependent manner by virtue of the ability of the SH2 domain of Grb2 to recognize the pYEN motif within HGAL in a canonical fashion.
The interaction between HGAL and Grb2 reciprocally modulates their effects on BCR-induced intracellular signaling
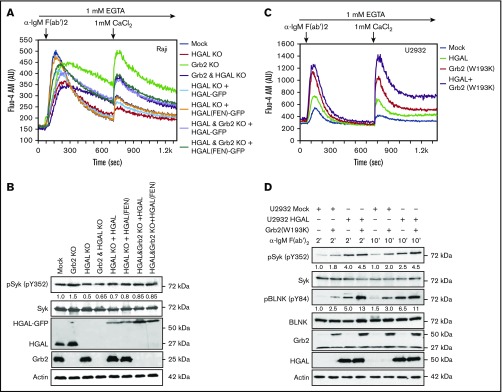
Unique adaptor protein complexes can differently modulate BCR signaling at distinct stages of B-cell differentiation. In immature DT40 chicken B cells, Grb2 has been reported to decrease BCR-induced Ca2+ influx by attenuating Lyn kinase–dependent activation of Syk via its interaction with Dok-3.11,13 While GC derived lymphomas express Dok-3, HGAL does not coimmunoprecipitate with Dok-3 (supplemental Figure 3B-C). Consequently, we next examined the functional significance of the HGAL-Grb2 interaction on BCR biochemical activation. To this end, we completely knocked out HGAL, Grb2 or both proteins using clustered regularly interspaced short palindromic repeats/Cas9 in Raji cells (named Raji HGAL KO and Grb2 KO, respectively; Figure 3A-B). As previously reported by us,7 knockout of HGAL significantly decreased BCR-induced total Ca2+ flux due to decreases in both intracellular Ca2+ influx and transmembrane Ca2+ mobilization. Knockout of Grb2 significantly increased BCR-induced total Ca2+ flux due to more sustained intracellular Ca2+ influx and transmembrane Ca2+ mobilization, as reported previously.13 Knockout of Grb2 also increased tonic BCR-induced Syk phosphorylation (supplemental Figure 4). Concomitant knockout of both HGAL and Grb2 markedly decreased the initial peak of intracellular Ca2+ influx in comparison with mock and Grb2 knockout cells while increasing the transmembrane Ca2+ mobilization compared with mock cells, but to a lesser extent than Grb2-only knockout. These effects were due to the opposing effects of each of these proteins on BCR-induced Ca2+ flux. Alterations in Ca2+ flux were accompanied by appropriate changes in phosphorylation of Syk (Figure 3B). We observed similar results in Raji and Bjab cells in which expression of Grb2, HGAL, or both proteins was knocked down by specific siRNAs (supplemental Figure 5) and in U2932 DLBCL cells upon concomitant HGAL expression and Grb2 knockdown (supplemental Figure 6).
Figure 3.
Opposite effects of HGAL and Grb2 on BCR-induced intracellular signaling. (A) BCR-induced intra- and extracellular Ca2+ mobilization of the indicated Raji cells recorded by flow cytometry. Different colors indicate different cell lines used in the experiments, as shown in the figure. (B) Raji cells used in panel A were stimulated with anti-human IgM F(ab′)2 for 5 minutes. Whole-cell lysates were prepared, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. Actin was blotted to demonstrate equal loading. Densitometry was measured for phosphorylated Syk normalized for actin content. The value 1 was assigned to each protein in Raji mock cells. No phosphorylation is observed in unstimulated cells (not shown). (C) BCR-induced intra- and extracellular Ca2+ mobilization of the indicated U2932 cells recorded by flow cytometry. Lines represent U2932 cells transfected with the following plasmids: mock (blue), HGAL (green), Grb2(W193K) (red), and a combination of HGAL and Grb2(W193K) (purple). (D) U2932 cells used in panel C were stimulated with anti-human IgM F(ab′)2 for 2 and 10 minutes. Whole-cell lysates were prepared, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. Actin was blotted to demonstrate equal loading. Densitometry was measured for phosphorylated Syk and BLNK normalized for actin content. The value 1 for each protein was assigned to U2932 cells transfected with mock plasmid and stimulated for 2 minutes. Data in panels A-D are representative of 3 independent experiments. AU, arbitrary units.
To further confirm our findings, we employed a dominant-negative Grb2 mutant protein that harbors an inactivated C-terminal SH3 domain (W193K).17 Similar to wild-type Grb2 protein, HGAL coimmunoprecipitated with the Grb2(W193K) mutant (supplemental Figure 7). Expression of Grb2(W193K) in U2932 cells overcame the inhibitory function of endogenous wild-type Grb2 and allowed increased BCR-induced intracellular influx and transmembrane Ca2+ mobilization (Figure 3C). Furthermore, in comparison with individual expression of either HGAL or Grb2(W193K) proteins, concomitant expression of both HGAL and Grb2(W193K) further increased BCR-induced total Ca2+ flux and especially transmembrane Ca2+ mobilization, as well as Syk and BLNK phosphorylation (Figure 3D). Overall, these experiments corroborated our findings with Grb2 knockout cells.
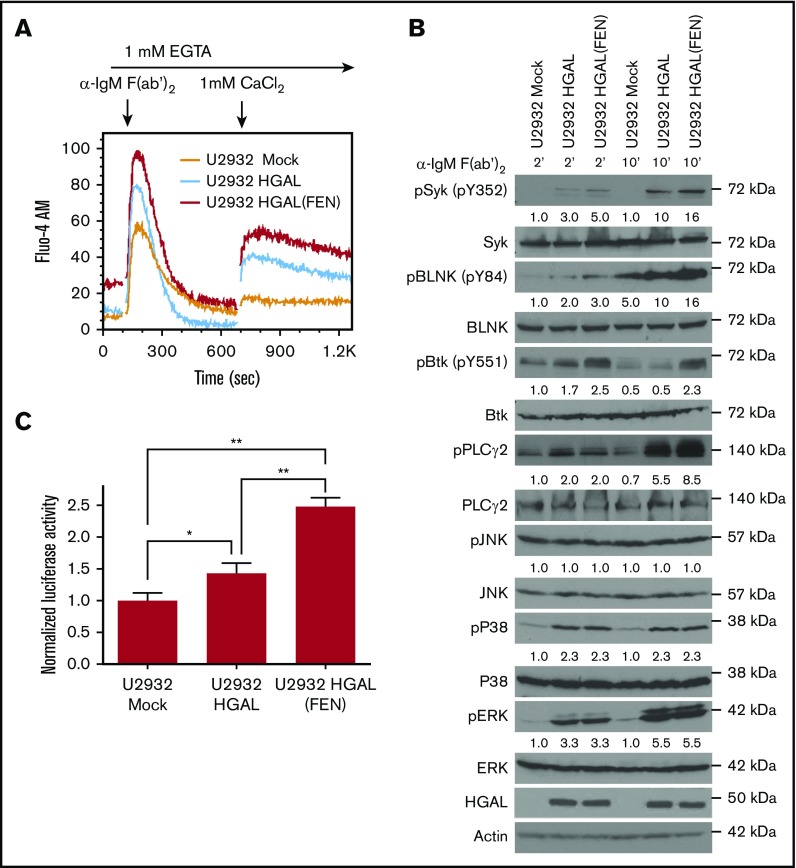
To further examine the role of the HGAL-Grb2 interaction on BCR signaling, we assessed the effects of disrupting Grb2-HGAL binding by mutating the Grb2 binding motif in HGAL. In comparison with Raji HGAL knockout cells, reconstitution with an HGAL (FEN) mutant increased total Ca2+ flux more than reconstitution with wild-type HGAL at similar protein expression levels. In contrast, there was no difference in the total Ca2+ flux between Raji cells with concomitant HGAL and Grb2 knockouts reconstituted at similar expression levels with either wild-type or HGAL (FEN) mutant (Figure 3A), suggesting a similar effect of wild-type and mutant HGAL in the absence of Grb2 protein. Similarly, expression of an HGAL (FEN) mutant in U2932 (Figure 4), TMD8, and Mino cells (supplemental Figure 8A) not expressing endogenous HGAL enhanced BCR-induced total Ca2+ influx in comparison with cells stably expressing wild-type HGAL. This was accompanied by enhanced phosphorylation of the BCR proximal effectors Syk (Y352), BLNK (Y84), BTK (Y551), and PLCγ2 (Y753) (Figure 4B) and NF-κB reporter activity (Figure 4C; supplemental Figure 8B-C), with minor differences in the dynamics and extent of phosphorylation of individual proteins between specific cell lines. However, no differences in the activation of JNK, p38, or ERK were observed between BCR-stimulated U2932 cells expressing wild-type and the HGAL (FEN) mutant (Figure 4B). Overall, these findings demonstrate that HGAL and Grb2 adaptor proteins directly modulate each other’s effects on BCR-induced intracellular signaling.
Figure 4.
HGAL (FEN) mutant not interacting with the Grb2 protein exhibits enhanced effects on BCR signaling compared with wild-type HGAL. (A) BCR-induced intra- and extracellular Ca2+ mobilization of the indicated U2932 cells recorded by flow cytometry. Lines represent U2932 cells transfected with the following plasmids: mock (orange), HGAL (blue), and HGAL (FEN) (red). (B) U2932 cells used in panel A were stimulated with anti-human IgM F(ab′)2 for 2 and 10 minutes. Whole-cell lysates were prepared, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. Actin was blotted to demonstrate equal loading. Densitometry was measured for phosphorylated Syk, BLNK, BTK, PLCγ2, pJNK, pP38, and pERK normalized for actin content. The value 1 was assigned for each protein in U2932 cells transfected with mock plasmid and stimulated for 2 minutes. Data in panels A-B are representative of 3 independent experiments. (C) U2932 cells used in panel A were cotransfected with both pNF-κB-Luc reporter and pRL-TK plasmids for 24 hours and stimulated with anti-human IgM F(ab′)2 for 30 minutes. Whole-cell lysates were prepared and used for determination of luciferase activity. Numbers refer to relative luciferase activities (Luc/Rlu) representing means + standard deviation of the mean of 3 independent experiments, each performed in triplicate. Asterisks indicate statistically significant differences (*P = .02; **P < .0005).
Interplay between HGAL, Grb2, and Syk proteins
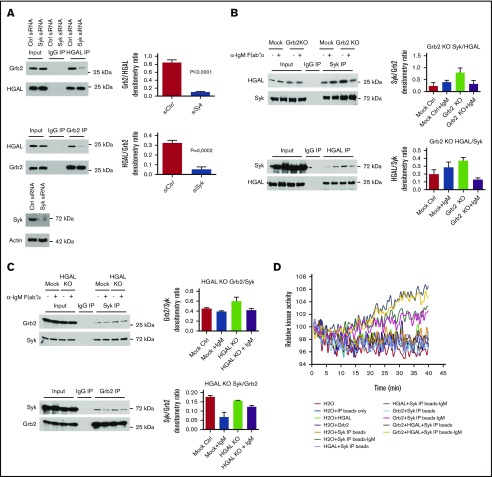
We previously reported that HGAL directly binds to Syk and increases its kinase activity, resulting in enhanced BCR signaling.7 Grb2 is also known to interact with Syk, possibly indirectly, and attenuates its activation by Lyn.11,30 We first analyzed the interplay among Syk, HGAL, and Grb2 on binding to each other and complex formation. Concordant to our data demonstrating the necessity of HGAL phosphorylation for binding to Grb2 (Figure 2), decreased Syk expression via siRNA, which mediates HGAL phosphorylation, led to decreased in vivo binding between HGAL and Grb2 proteins (Figure 5A). As previously reported by us, BCR stimulation increased Syk co-IP with HGAL7 while tending to decrease co-IP with Grb2 (Figure 5B-C). Knockout of HGAL did not affect binding between Syk and Grb2, while knockout of Grb2 enhanced the co-IP between HGAL and Syk in the resting cells but decreased it in the BCR stimulated vs nonstimulated cells. These data suggest that upon BCR stimulation, Grb2 facilitates interaction between Syk and HGAL. However, we observed inconsistencies in the quantifications of co-IP interactions between Grb2 and Syk, likely due to distinct immunoprecipitation efficiencies of the individual antibodies or because the interaction is indirect.30 Therefore, we next analyzed the effect of Grb2 on in vitro Syk kinase activity in the presence or absence of HGAL. To this end, Syk protein was immunoprecipitated from BCR-stimulated or unstimulated Raji cells and used in the kinase assay, alone or with purified HGAL and/or Grb2 proteins (Figure 5D). No Syk kinase activity was observed in unstimulated cells, even in the presence of HGAL and/or Grb2 proteins. In stimulated cells, Syk kinase activity was detected and markedly increased by the addition of HGAL, but not Grb2, protein. Grb2 did not affect HGAL-induced increases in Syk activity in vitro. Overall, these findings suggest that Grb2 may decrease HGAL and Syk binding in unstimulated cells and thus may negate HGAL effects on tonic BCR signaling, but it facilitates their binding following BCR stimulation. However, Grb2 does not affect HGAL-enhanced Syk kinase activity following BCR stimulation.
Figure 5.
Interplay among Grb2, HGAL, and Syk proteins. (A) Knockdown of Syk decreases the Grb2 and HGAL interaction in co-IP experiment in unstimulated Raji cells. Bar graphs show the relative densitometry of Grb2 protein in HGAL immunoprecipitates or HGAL protein in Grb2 immunoprecipitates; western blots demonstrate expression of Syk following Syk knockdown. (B) Effect of Grb2 knockout on HGAL and Syk interaction in a co-IP experiment in unstimulated and BCR-stimulated Raji cells. Bar graphs show the relative densitometry of HGAL protein in Syk immunoprecipitates or Syk protein in HGAL immunoprecipitates. (C) Effect of HGAL knockout on Grb2 and Syk interaction in co-IP experiment in unstimulated and BCR-stimulated Raji cells. Bar graphs show the relative densitometry of Grb2 protein in Syk immunoprecipitates or Syk protein in Grb2 immunoprecipitates. (D) Syk kinase activity assay. Syk was immunoprecipitated from unstimulated or stimulated Raji cells and used in a Syk kinase activity assay, either alone or with purified HGAL and/or Grb2 proteins. Immunoprecipitates with control antibody and beads only were used as negative controls. Data in panels A-D are representative of 3 independent experiments. Error bars represent standard deviation.
Effects of HGAL and Grb2 on BCR synapses
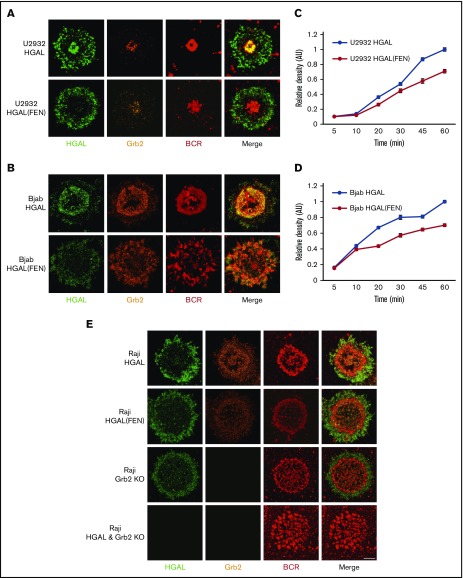
In addition to the Grb2 effects on the BCR-induced intracellular signaling, it also plays a central role in B-cell synapse formation by regulating movement of BCR microclusters to gather antigen in the cSMAC.11-14,17 In immature DT40 chicken B cells, which express Dok-3, but not HGAL, Grb2 colocalizes with Dok-3 and the BCR in the cSMAC. In these cells, BCR microclusters remain in the periphery and fail to form cSMACs in the absence of Grb2.12 We observed increased BCR synapse intensity and density following BCR stimulation of HGAL-expressing lymphocytes from Rosa26HGAL/Mb1-Cre mice in comparison with lymphocytes not expressing HGAL originating from wild-type control littermate mice (supplemental Figure 9). These observations suggest that HGAL may also regulate B-cell synapse formation in normal lymphocytes. Consequently, we examined the BCR distribution and localization of HGAL and Grb2 in human DLBCL originating from mature B cells. To this end, U2932, Bjab, and Raji HGAL KO cells expressing GFP-labeled HGAL and mCherry-labeled Grb2 were settled on planar lipid bilayers containing laterally mobile anti-human IgM F(ab′)2 and visualized with confocal microscopy (supplemental Figure 1). In U2932, Bjab, and Raji HGAL KO cells expressing wild-type HGAL, BCR microclusters formed and eventually accumulated in a dense well-circumscribed synapse area representing the cSMAC that differed in size among the cell lines (Figure 6A-B,E, top panels; supplemental Video 1). HGAL colocalized with BCR and Grb2 in the cSMAC (supplemental Figure 10), corroborating our immunoprecipitation and other biochemical studies (Figure 2). However, HGAL also localized in the pSMAC (Figure 6A-B,E; supplemental Figure 10), where Grb2 was only minimally present. In the pSMAC, HGAL colocalized with actin (supplemental Figure 11), corroborating our previous studies demonstrating HGAL’s interaction with actin that results in regulation of actomyosin function.8 In contrast, in U2932, Bjab, and Raji HGAL KO cells expressing an HGAL (FEN) mutant, the BCR and Grb2, but not HGAL, colocalized in the cSMAC. HGAL was only present in the pSMAC following BCR stimulation (Figure 6A-B,E; supplemental Figure 10; supplemental Video 2). Concordantly, in Raji Grb2 KO cells, BCR localization to the cSMAC was disrupted, resulting in dispersed and less circumscribed cSMACs characterized by a decrease in the total BCR fluorescence signal, as reported previously.12 This was associated with failure of HGAL to localize to the cSMAC. These findings suggest that Grb2 is necessary for cSMAC formation and HGAL localization to the cSMAC. Further, in U2932 and Bjab cells expressing the HGAL (FEN) mutant, we observed slower dynamics of cSMAC formation compared with cells expressing wild-type HGAL, with less circumscribed cSMACs characterized by a decrease in the total BCR fluorescence signal at all time points (Figure 6C-D; supplemental Videos 3 and 4). These observations are similar to normal mouse lymphocytes not expressing HGAL (supplemental Figure 9). Moreover, concomitant knockout of HGAL and Grb2 in Raji cells prevented BCR localization in the cSMAC to a larger extent than Grb2 knockout alone. These observations suggest cooperativity between Grb2 and HGAL in regulating BCR microcluster movement from the periphery to form the cSMAC. Overall, our findings demonstrate that in lymphoma cells, Grb2’s interaction with HGAL controls the dynamics of BCR synapse formation.
Figure 6.
HGAL colocalizes with Grb2 at cSMACs and facilitates antigen aggregation in a planar lipid bilayer model. (A-B) Upon antigen stimulation of wild-type HGAL expressing U2932 (A) and Bjab (B) cells, BCR microclusters formed and eventually accumulated in cSMACs at 30 minutes. HGAL colocalizes with the BCR in cSMACs; Grb2 redistributes to the membrane area and colocalizes with HGAL in cSMACs (top panels). In HGAL (FEN) mutant–expressing cells, HGAL mutant exhibits only pSMAC distribution and lost cSMAC colocalization with Grb2 and results in less defined cSMACs with decreased BCR accumulation (lower panels). (C-D) BCR microcluster/cSMAC formation as a function of time in U2932 (C) and Bjab (D) cells. Error bars denote ±1 standard error of the mean. Two-way analysis of variance was performed to determine if the kinetic responses of U2932 and Bjab cells transfected with HGAL and HGAL (FEN) are different. P = .0001 for U2932 and P = .0001 for Bjab cell lines. (E) Distribution of HGAL, HGAL(FEN), Grb2, and BCR in Raji HGAL KO cells reconstituted with wild-type HGAL-GFP and HGAL(FEN) GFP, Riaji Grb2 KO, and Raji HGAL and Grb2 KO cells 30 minutes after stimulation. Each image shown in panels A-B,E is 20 microns wide.
Discussion
Antigen binding to the BCR leads to 2 processes that are required to mount an effective antibody response: transmembrane signaling and antigen internalization and processing. Both processes are dependent on BCR-induced Syk activation, which initiates a cascade of protein tyrosine phosphorylation that permits inducible protein–protein interactions, mediated by adaptor proteins linking activated BCRs to cytoplasmic effectors. Comprehensively elucidating the mechanisms controlling the magnitude of BCR signaling may identify new therapeutic targets for autoimmune disorders and lymphoma treatment.
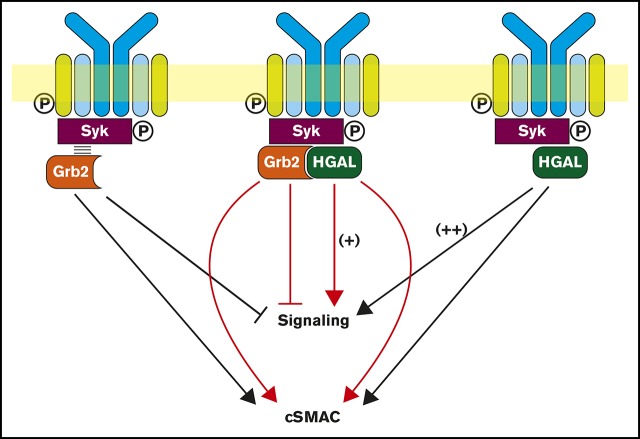
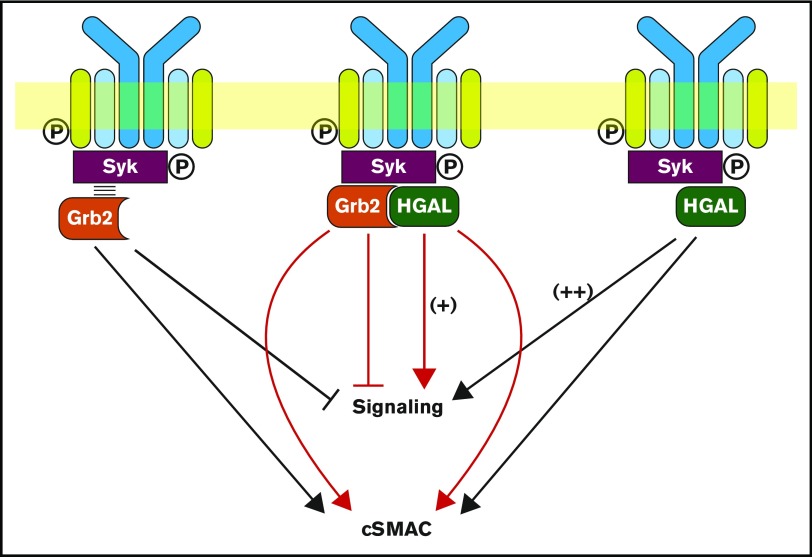
Grb2 is a ubiquitously expressed adaptor protein that contains 2 flanking SH3 domains and a central SH2 domain that preferentially binds to the pYXN motif.26,27 Grb2 may regulate BCR signaling.11-13,17 Grb2 localizes to BCR microclusters and decreases BCR-induced Ca2+ influx by interacting with Dok-3 via attenuation of Lyn kinase–dependent activation of Syk,11,13 to which it directly binds. Herein, we demonstrate that the adaptor protein HGAL, specifically expressed in GC lymphocytes and GC-derived lymphomas, directly binds to Grb2 upon BCR activation and negates the inhibitory effects of Grb2 on the BCR-induced biochemical signaling cascade. Simultaneously, it enhances the kinetics of BCR accumulation in the cSMAC during Grb2-dependent BCR synapse formation (Figure 7).
Figure 7.
Schematic diagram of the biological effects attributed to the interaction between HGAL and Grb2 proteins. Grb2 is known to interact with Syk and attenuates its activation by Lyn, leading to decreased BCR-induced intracellular signaling, while promoting cSMAC and immunological synapse formation (left). HGAL directly binds to Syk and increases its kinase activity, leading to enhanced BCR signaling (right). Further, HGAL contributes to faster dynamics of cSMAC formation and increases BCR accumulation in cSMACs. Grb2-HGAL binding counteracts the effects of individual proteins on BCR-induced biochemical signaling while cooperating to regulate cSMAC formation (middle).
Both Grb2 and HGAL interact with Syk.7,30 Our data suggest that these 3 proteins may be part of the same multiprotein complex and that Syk-mediated HGAL phosphorylation may permit HGAL binding to Grb2. Grb2 coimmunoprecipitated with Syk from nonstimulated cells, as previously reported in mast cells, suggesting that this interaction is not phosphorylation dependent or may be indirect.30 We demonstrate that Grb2 facilitates HGAL and Syk binding following BCR stimulation but does not affect the HGAL-mediated increase in Syk kinase activity. Previous studies showed that Grb2 inhibits BCR signaling by decreasing the activation of Syk by Lyn.11 Thus, while HGAL and Grb2 oppositely affect Syk kinase activity, this is not due to direct Grb2 effects on HGAL-mediated Syk kinase activation. However, HGAL binding to Grb2 may attenuate its effects on inhibiting Syk activation by Lyn.
Indeed, the opposing effects of HGAL and Grb2 on BCR biochemical signaling are due to a direct interaction between these proteins, since expression of an HGAL (FEN), which does not bind to Grb2, in HGAL-nonexpressing cells induces enhanced BCR signaling when compared with the wild-type HGAL protein. While additional proteins that bind to HGAL via the YEN motif might potentially mediate this differential effect,19 there was no difference in the magnitude of BCR-induced intracellular signaling in Grb2 knockout cells reconstituted with wild-type or HGAL (FEN) mutant, suggesting that the direct HGAL binding to Grb2 is responsible for the observed changes in BCR signaling. However, whether HGAL directly ameliorates the inhibitory effects of Grb2 on BCR signaling or simply enhances Syk activity that negates Grb2 inhibitory effects is currently unknown. Whether HGAL interacts with additional proteins that participate in this process is also unknown and will need to be studied in the future.
In immature B cells, Grb2 is also required for formation of cSMACs and signalosomes following BCR stimulation.12 Upon BCR stimulation, Grb2 initially localizes to microsignalosomes, indirectly recruits dynein, and subsequently moves together with antigen to gather in the cSMAC.12 Herein, we show that in DLBCL cells, BCR activation also leads to Grb2 colocalization with BCR and HGAL in cSMACs. Further, we show that while the interaction between Grb2 and HGAL is not required for Grb2’s localization to the cSMAC, it enhances the rate of cSMAC formation and increases BCR accumulation in the cSMAC. Concomitant knockout of both Grb2 and HGAL prevents cSMAC formation to a larger extent than individual knockout of these proteins. cSMAC formation is dependent on Syk, myosin II, and the actin cytoskeleton,12,31-34 all of which interact with and are regulated by HGAL. However, the molecular mechanism by which HGAL regulates cSMAC formation is currently unknown and needs further studies. These observations suggest that HGAL’s interaction with Grb2 may be important for the formation of functional signalosomes and BCR synapses. The faster kinetics of cSMAC formation in GC lymphocytes may be important for effective selection of lymphocytes expressing higher affinity BCRs and mediate shorter interaction with follicular dendritic cells, allowing more effective antigen sampling. Further studies will be needed to examine HGAL’s effects on antigen selection, internalization, and presentation.
The seemingly opposite effects of HGAL’s interaction with Grb2 on biochemical signaling and cSMAC formation are not mutually exclusive, since intracellular calcium signaling and phosphorylation of downstream effectors following BCR ligation are observed more rapidly than the formation of mature immunological synapses, as well as recent observations that microclusters of BCR actively signal in the periphery before cSMAC formation.35
In summary, we have demonstrated that Grb2 directly interacts with phosphorylated HGAL, collaborating in cSMAC formation while oppositely regulating BCR-induced intracellular biochemical signaling. These interactions may play an important function in regulating the magnitude of BCR signaling and antigen presentation.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the University of Miami flow cytometry core facility for cell sorting and calcium analysis.
X.J. is supported by the Stanley J. Glaser Foundation (research award UM SJG 2017-8), the American Cancer Society (institutional research grant 98-277-13), and the University of Miami Sylvester Comprehensive Cancer Center. A.F. is supported by the National Institutes of Health, National Institute of General Medical Sciences (grant R01-GM083897) and the Sylvester Comprehensive Cancer Center. V.T.M. is supported by a BioNIUM Pilot Project Award. I.S.L. is supported by the American Society of Hematology (bridge grant), the National Institutes of Health, National Cancer Institute (grant R01CA233945), the Dwoskin Family Foundation, the Recio Family Foundation, the Anthony Rizzo Family Foundation, the Jaime Erin Follicular Lymphoma Research Consortium, and the University of Miami Sylvester Comprehensive Cancer Center.
Authorship
Contribution: X.J. and V.T.M. designed and performed experiments, analyzed data, and wrote the manuscript; X.L. and A.F. performed experiments and analyzed data; Y.Z., L.L., B.J.S., D.C.M., M.M., R.E.V., and M.H.A. performed experiments; I.G.-R. and I.S.-G. provided valuable materials; I.S.L. conceptualized the study, analyzed data, and wrote the manuscript; and all authors read the manuscript and approved its content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, Division of Hematology and Oncology, Department of Medicine, University of Miami, Sylvester Comprehensive Cancer Center, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu; and Xiaoyu Jiang, Division of Hematology and Oncology, Department of Medicine, University of Miami, Sylvester Comprehensive Cancer Center, Batchelor Children Research Institute, 4th Floor, 1580 NW 10th Ave, Miami, FL 33136; e-mail: xjiang@med.miami.edu.
References
- 1.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12(1):117-139. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock DM, Dalla-Favera R, Gascoyne RD, et al. . A roadmap for discovery and translation in lymphoma. Blood. 2015;125(13):2175-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lossos IS, Alizadeh AA, Rajapaksa R, Tibshirani R, Levy R. HGAL is a novel interleukin-4-inducible gene that strongly predicts survival in diffuse large B-cell lymphoma. Blood. 2003;101(2):433-440. [DOI] [PubMed] [Google Scholar]
- 4.Natkunam Y, Hsi ED, Aoun P, et al. . Expression of the human germinal center-associated lymphoma (HGAL) protein identifies a subset of classic Hodgkin lymphoma of germinal center derivation and improved survival. Blood. 2007;109(1):298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azambuja D, Lossos IS, Biasoli I, et al. . Human germinal center-associated lymphoma protein expression is associated with improved failure-free survival in Brazilian patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2009;50(11):1830-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green TM, Jensen AK, Holst R, et al. . Multiplex polymerase chain reaction-based prognostic models in diffuse large B-cell lymphoma patients treated with R-CHOP. Br J Haematol. 2016;174(6):876-886. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Camarero I, Jiang X, Natkunam Y, et al. . Germinal centre protein HGAL promotes lymphoid hyperplasia and amyloidosis via BCR-mediated Syk activation. Nat Commun. 2013;4(1):1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Kazmierczak K, Jiang X, et al. . Germinal center-specific protein human germinal center associated lymphoma directly interacts with both myosin and actin and increases the binding of myosin to actin. FEBS J. 2011;278(11):1922-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Lu X, McNamara G, et al. . HGAL, a germinal center specific protein, decreases lymphoma cell motility by modulation of the RhoA signaling pathway. Blood. 2010;116(24):5217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Sicard R, Jiang X, et al. . HGAL localization to cell membrane regulates B-cell receptor signaling. Blood. 2015;125(4):649-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lösing M, Goldbeck I, Manno B, et al. . The Dok-3/Grb2 protein signal module attenuates Lyn kinase-dependent activation of Syk kinase in B cell antigen receptor microclusters. J Biol Chem. 2013;288(4):2303-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnyder T, Castello A, Feest C, et al. . B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity. 2011;34(6):905-918. [DOI] [PubMed] [Google Scholar]
- 13.Stork B, Neumann K, Goldbeck I, et al. . Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J. 2007;26(4):1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang IK, Zhang J, Gu H. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol Rev. 2009;232(1):150-159. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann JA, Radtke D, Maurberger A, Winkler TH, Nitschke L. Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 2011;30(8):1621-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang IK, Cronshaw DG, Xie LK, et al. . Growth-factor receptor-bound protein-2 (Grb2) signaling in B cells controls lymphoid follicle organization and germinal center reaction. Proc Natl Acad Sci USA. 2011;108(19):7926-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stork B, Engelke M, Frey J, et al. . Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity. 2004;21(5):681-691. [DOI] [PubMed] [Google Scholar]
- 18.Pan Z, Shen Y, Ge B, Du C, McKeithan T, Chan WC. Studies of a germinal centre B-cell expressed gene, GCET2, suggest its role as a membrane associated adapter protein. Br J Haematol. 2007;137(6):578-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlQuraishi M, Koytiger G, Jenney A, MacBeath G, Sorger PK. A multiscale statistical mechanical framework integrates biophysical and genomic data to assemble cancer networks. Nat Genet. 2014;46(12):1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natkunam Y, Lossos IS, Taidi B, et al. . Expression of the human germinal center-associated lymphoma (HGAL) protein, a new marker of germinal center B-cell derivation. Blood. 2005;105(10):3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt S, Matthews J, Parvin S, et al. . Direct and immune-mediated cytotoxicity of interleukin-21 contributes to antitumor effects in mantle cell lymphoma. Blood. 2015;126(13):1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews JM, Bhatt S, Patricelli MP, et al. . Pathophysiological significance and therapeutic targeting of germinal center kinase in diffuse large B-cell lymphoma. Blood. 2016;128(2):239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremer PS, Boxer SG. Formation and spreading of lipid bilayers on planar glass supports. J Phys Chem B. 1999;103(13):2554-2559. [Google Scholar]
- 24.Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006;25(4):889-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Chen J, Malumbres R, Cubedo Gil E, Helfman DM, Lossos IS. HGAL, a lymphoma prognostic biomarker, interacts with the cytoskeleton and mediates the effects of IL-6 on cell migration. Blood. 2007;110(13):4268-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann K, Oellerich T, Urlaub H, Wienands J. The B-lymphoid Grb2 interaction code. Immunol Rev. 2009;232(1):135-149. [DOI] [PubMed] [Google Scholar]
- 27.Tari AM, Lopez-Berestein G. GRB2: a pivotal protein in signal transduction. Semin Oncol. 2001;28(5 suppl 16):142-147. [DOI] [PubMed] [Google Scholar]
- 28.de Mol NJ, Catalina MI, Fischer MJ, Broutin I, Maier CS, Heck AJ. Changes in structural dynamics of the Grb2 adaptor protein upon binding of phosphotyrosine ligand to its SH2 domain. Biochim Biophys Acta. 2004;1700(1):53-64. [DOI] [PubMed] [Google Scholar]
- 29.McNemar C, Snow ME, Windsor WT, et al. . Thermodynamic and structural analysis of phosphotyrosine polypeptide binding to Grb2-SH2. Biochemistry. 1997;36(33):10006-10014. [DOI] [PubMed] [Google Scholar]
- 30.de Castro RO, Zhang J, Groves JR, Barbu EA, Siraganian RP. Once phosphorylated, tyrosines in carboxyl terminus of protein-tyrosine kinase Syk interact with signaling proteins, including TULA-2, a negative regulator of mast cell degranulation. J Biol Chem. 2012;287(11):8194-8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208(5):1055-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005;16(5):2275-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Miller H, Orlowski G, Hang H, Upadhyaya A, Song W. Actin reorganization is required for the formation of polarized B cell receptor signalosomes in response to both soluble and membrane-associated antigens. J Immunol. 2012;188(7):3237-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vascotto F, Lankar D, Faure-André G, et al. . The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol. 2007;176(7):1007-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harwood NE, Batista FD. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb Perspect Biol. 2011;3(2):a002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.