Abstract
Parkinson’s disease (PD), a progressive neurodegenerative disorder, has long been associated with mitochondrial dysfunction in both sporadic and familial forms of the disease. Mitochondria are crucial for maintaining cellular homeostasis, and their dysfunction is detrimental to dopaminergic neurons. These neurons are highly dependent on mitochondrial adenosine triphosphate (ATP) and degenerate in PD. Mitochondria contain their own genomes (mtDNA). The role of mtDNA has been investigated in PD on the premise that it encodes vital components of the ATP-generating oxidative phosphorylation (OXPHOS) complexes and accumulates somatic variation with age. However, the association between mtDNA variation and PD remains controversial. Herein, we provide an overview of previously published studies on the role of inherited as well as somatic (acquired) mtDNA changes in PD including point mutations, deletions and depletion. We outline limitations of previous investigations and the difficulties associated with studying mtDNA, which have left its role unresolved in the context of PD. Lastly, we highlight the potential for further research in this field and provide suggestions for future studies. Overall, the mitochondrial genome is indispensable for proper cellular function and its contribution to PD requires further, more extensive investigation.
Keywords: Parkinson’s disease, mitochondrial DNA, mitochondrial haplogroups, somatic mtDNA variation, homoplasmic mtDNA variation, mtDNA depletion
1. The mitochondrial genome
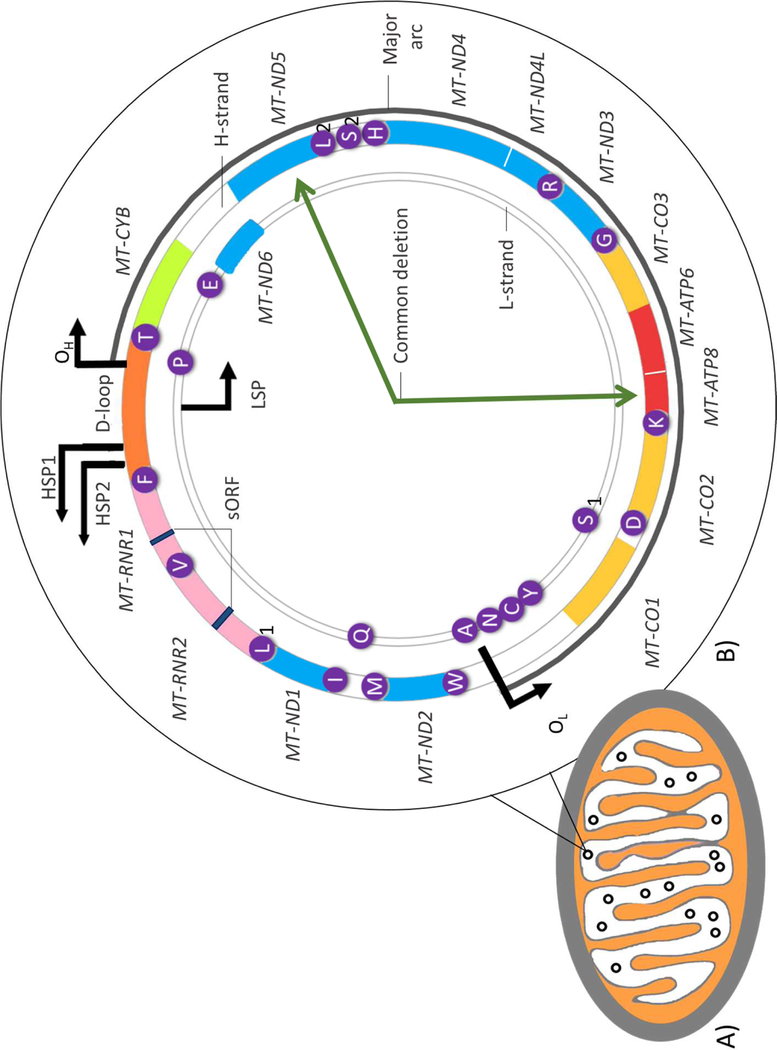
Mitochondria are primarily responsible for generating adenosine triphosphate (ATP) via oxidative phosphorylation (OXPHOS). Besides nuclei, they are the only other cellular organelles harbouring their own genome (mtDNA). mtDNA is a compact (16,569 bp), circular, double-stranded genome, comprised of 37 genes including 13 which encode essential subunits of the OXPHOS enzymes (Figure 1) [1]. More recently mtDNA has been reported to additionally encode two short open reading frames (sORFs) within the ribosomal RNA genes; MT-RNR1 and MT-RNR2 [2]. These sORFs can be translated into mitochondrial-derived peptides (MDPs) which have important functions including regulating nuclear gene expression in response to metabolic stress [3].
Figure 1. The mitochondrion and structure of human mitochondrial DNA.
A). Basic structure of a mitochondrion containing multiple copies of mitochondrial DNA (mtDNA) represented as black circles. B) Structure of mtDNA: a 16 569 bp circular, double-stranded molecule consisting of a guanine-rich heavy strand (H-strand) and a light strand (L-strand), rich in cytosine. Approximately 93% of the genome is coding, having only one significant non-coding control region which includes the displacement loop (D-loop). This stretch of DNA contains the origin of H-strand replication (OH) the H-strand transcriptional promoters (HSP1 and HSP2) as well as the L-strand promoter (LSP). The DNA 37 primarily contiguous genes, nine on the L-strand and 28 on the H-strand. Twenty-four genes encode mature RNA products: one 12 s rRNA (small ribosomal subunit) and one 16 s rRNA (large ribosomal subunit), and 22 mitochondrial tRNAs (genes highlighted in purple). The remaining 13 mtDNA genes encode polypeptide components of the electron transport chain (ETC) involved in energy production via oxidative phosphorylation (OXPHOS). These include seven subunits (MT-ND1,2,3,4,4L,5,6) of complex I, one subunit (MT-CYB) of complex III, three subunits (MT-CO1,2, and 3) of complex IV, and two subunits (MT-ATP6 and 8) of complex V. mtDNA also encodes two mitochondrial-derived peptides, MOTS-c and Humanin, as short open reading frames (sORF) in the MT-RNR1 and MT-RNR2 genes respectively. Deletions in mtDNA frequently occur in the major arc (indicated by the dark grey line) between the heavy and light strand origins of replication (OH and OL respectively). Among these deletions is the ‘common deletion’: a 4 977-base-pair deletion located between the MT-ATP8 and MT-ND5 genes as indicated by the dark green arrows.
Mitochondrial genomes differ to their nuclear counterparts with regards to their replication, repair and inheritance mechanisms. Unlike nuclear DNA (nDNA), mtDNA is primarily maternally inherited, although rare cases of biparental inheritance have recently been reported [4]. Moreover, it exists as multiple copies (known as polyploidy) within individual mitochondria. Hundreds to thousands of mtDNA copies can exist per mitochondria and thus per cell, depending on the energetic demand of the cell [5]. Compared to nDNA, mtDNA is highly susceptible to accumulating errors due to the lack of protective histones and its close proximity to damaging by-products of OXPHOS e.g. reactive oxygen species (ROS) [6]. In addition, reduced fidelity of the mtDNA polymerase, mtDNA polymerase gamma (POLG1), has been suggested to contribute to a higher mutational rate of mtDNA [7]. As a result, most individuals harbour low levels (<1%) of inherited and/or acquired mtDNA variants [8]. Most copies of mtDNA in a cell are identical, which is referred to as homoplasmy. A mix of both mutated and wild-type mtDNA is referred to as heteroplasmy. Levels of heteroplasmy can change over time through mechanisms of relaxed replication in postmitotic tissue and random segregation of mtDNA during cell division in mitotic tissues [9]. Although, the process of mammalian mtDNA replication is not yet completely understood, mtDNA is known to replicate independently of the cell cycle - undergoing life-long replication in both proliferating and post-mitotic cells [10]. Tissue-specific differences in mtDNA maintenance, replication, and expression exist [11] which, together with the unique features of mtDNA, particularly polyploidy and heteroplasmy, pose a significant challenge for studying their role in disease including Parkinson’s disease (PD).
PD is a neurodegenerative movement disorder with a complex aetiology comprising both environmental and genetic factors. Progressive dopaminergic (DA) neuronal loss in the Substantia Nigra pars compacta (SNpc) is characteristic of PD, but pathological mechanisms are poorly understood. Despite decades of research and definite links to mitochondrial dysfunction in disease susceptibility and progression, the role of mtDNA in risk and pathogenesis of sporadic PD (sPD) remains equivocal. This review aims to appraise the literature to determine the reason(s) for this. We discuss results from published studies which have investigated mtDNA changes in PD and highlight inconsistencies between studies which have left the role of mtDNA in PD largely unresolved. We also draw attention to factors which complicate the study of mtDNA in PD and may have contributed to conflicting results. In doing so, we provide suggestions to guide and possibly improve future research in this field.
2. Parkinson’s disease and mitochondrial dysfunction
The DA neuronal loss in PD results in an array of classical motor symptoms including bradykinesia, tremor and rigidity [12]. These, together with various non-motor symptoms (e.g. depression, anxiety and insomnia), arising from the loss of additional neuronal populations, significantly compromise patients’ quality of life. A pathological hallmark of PD are Lewy bodies (LB); intracellular inclusions of the aggregate-prone, alpha-synuclein protein [13]. Only approximately 10% of cases have familial PD [14] and around 30% of these have monogenic PD i.e. have mutations in nDNA-encoded genes [15]. Notably, most PD cases are sporadic and have an unknown aetiology of disease as well as an absence of a family history. However, both familial and sPD have been linked to mitochondrial dysfunction [16, 17].
Although the generation of ATP is the most recognised function of mitochondria, the organelles also participate in critical processes which help ensure cell viability, including lipid biosynthesis, calcium homeostasis and apoptosis and even play diverse roles in immune response [18, 19]. Mitochondrial dysfunction therefore impinges on a wide spectrum of cellular functions. This dysfunction is multifactorial in origin and can arise from impaired mitochondrial biogenesis, altered mitochondrial dynamics (e.g. fission and fusion), impaired mitochondrial quality control (mitophagy), compromised OXPHOS and calcium imbalances [20].
Early evidence that mitochondrial dysfunction may play a central role in sPD pathogenesis resulted from the unintentional exposure of humans to the drug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP), specifically its active derivative MPP+, causing parkinsonian symptoms [21, 22]. Primate models subsequently reported SNpc DA cell loss and other parkinsonism symptoms including akinesia, tremor and rigidity in monkeys treated with a form of MPTP [23]. Similarly, MPP+ mouse models exhibited DA neuronal loss [24, 25] and showed that MPP+ inhibits complex I activity, thereby interfering with OXPHOS [26, 27]. As such, an energy crisis was suggested as an important mechanism underlying neuronal degeneration leading to PD symptoms [26, 27]. Subsequently, in the late 1980’s and early 1990’s, defects of complex I were observed in various tissues of sPD patients, including skeletal muscle [28–32], the SNpc [33, 34] and platelets [35–38]. As a consequence of these initial studies, it was hypothesized that the observed complex I defect may be derived via the mitochondrial genome [17]. Moreover, genes linked to early-onset forms of PD (e.g. PINK1, PRKN and DJ-1), encode proteins which participate in- or mediate some form of mitochondrial function, regulation or quality control [20]. For instance, as reviewed elsewhere [39], the proteins PINK1 and Parkin are involved in mitophagy, consequently, loss of function mutations in nDNA encoding these proteins affect mitochondrial biogenesis and induction of autophagy.
3. Early evidence for mtDNA involvement in PD from cybrids
In addition to nDNA mutations contributing to familial PD onset, mtDNA variation has been linked to sPD, as demonstrated through cybrid (cytoplasmic hybrid) cell line studies (Table 1) [40]. Briefly, ρ0 cells which are devoid of mtDNA but contain identical nDNA, are fused with mtDNA-containing platelets from either PD patients or controls. Following multiple cellular replication cycles, the cybrids generated share the same environmental and nDNA background, differing only with regards to their mitochondrial genomes [41, 42]. Therefore, differences observed between cell lines with differing mtDNA content, from either a patient or control, are thought to be solely attributable to the mtDNA itself [41–43]. As a result, p0 cybrid cells have provided a useful tool to investigate the role of mtDNA in cellular health and in disease.
Table 1.
Features of mitochondrial dysfunction observed in sporadic Parkinson’s disease cybrids
| Phenotype | References |
|---|---|
| Altered mitochondrial ultrastructure: e.g. rounded, swollen mitochondria; mitochondrial matrix with few or disrupted cristae; presence of intramitochondrial inclusions. | [43, 161–163] |
| Decreased complex I activity | [42, 43, 163–165] |
| Mitochondrial depolarisation | [43] |
| Reduced mitochondrial ability to buffer cytosolic calcium/altered calcium homeostasis | [166, 167] |
| Reduced ATP levels | [43, 163, 168] |
| Apoptosis: altered levels of apoptosis-related proteins | [43, 169, 170] |
| Reduced mtDNA content | [50, 171] |
| Microtubule depolymerisation | [163, 168] |
| Increased alpha-synuclein oligomerization | [163, 166, 168] |
| Existence of fibrillar and vesicular inclusions (cybrid Lewy bodies) | [163, 172] |
| Reduced respiratory reserve capacity | [165] |
| Increased toxin susceptibility | [42, 43] |
| Significantly reduced mitochondrial axonal transport | [171] |
| Increased ROS production | [42, 163] |
ATP = Adenosine triphosphate; mtDNA = Mitochondrial DNA; ROS = reactive oxygen species
Albeit an effective model to study mtDNA background in vitro, cybrid studies have limitations which contribute to discrepancies between studies. One of the most notable is the frequent use of tumour cell lines exhibiting aneuploidy or other forms of nuclear genomic instability [44]. Despite potential limitations, sPD cybrid studies have consistently provided insight into the role which mtDNA background has on mitochondrial morphology and function between cell lines from patients and controls (Table 1).
Notably, emerging evidence implicates mitochondrial genomes in modulating nuclear gene expression by means of signalling pathways [45–47]. These include the integrated stress response, retrograde (mitochondria-nucleus) communication, proteostasis regulation and mitonuclear feedback signalling [48]. With mitochondrial biogenesis, maintenance and other mitochondrial processes heavily depending on nDNA gene expression [49], mtDNA may influence these processes and could for instance account for variable biogenesis between sPD cybrids with differing mtDNA donors [50].
Importantly, sPD cybrids have exhibited several features of mitochondrial dysfunction including decreased complex I activity, increased ROS, morphologically abnormal mitochondria and decreased maximum respiratory capacity, linking mtDNA to sPD (Table 1). These findings all highlight that mtDNA can substantially influence important mitochondrial processes and physiology. Consequently, cybrid studies spawned investigations aimed at identifying mtDNA variation which could account for mitochondrial abnormalities observed in sPD cybrids.
4. Studies investigating homoplasmic mtDNA variation
4.1. Early mtDNA sequencing studies
In the 1990’s and early 2000’s, a few studies sequenced the complex I genes of PD patients and controls to identify possible mtDNA variants associated with the disorder [51–55]. Standard Sanger sequencing techniques available during this time were primarily limited to identifying homoplasmic or high-frequency heteroplasmic variants [56]. A few studies sequenced whole mtDNA [57, 58], whilst others sequenced all of the mtDNA tRNA [54, 59, 60] and rRNA genes [60]. Furthermore, some employed restriction fragment length polymorphism (RFLP) methods to investigate whether certain mtDNA variants (e.g. m.3397A>G in MT-ND1) associate with PD [61, 62].
A handful of studies identified ‘novel’ variants in PD patients [52, 53, 59, 60] and reported variants at an increased frequency in cases compared to controls [55, 62]. However, their findings may have been coincidental. This is because the sample sizes were often too small to draw statistically-powerful conclusions. Additionally, sequencing was still in its infancy, making it more likely to identify false positives due to the limited number of known variants which had been reported. In support of this statement, most of these ‘novel’ variants have now been reported in individuals without PD on the MITOMAP database [63]; https://www.mitomap.org/MITOMAP), although not at high frequencies (Supplementary Table 1). Based on these early sequencing studies, no homoplasmic nor high-frequency heteroplasmic mtDNA variants were conclusively implicated in PD pathogenesis or risk.
4.2. Haplogroup association studies
The sequential accumulation of new variation in maternal lineages over thousands of years has generated stable mtDNA sequence variation which is homoplasmic. Subsets of such stable mtDNA variation define haplogroups which are primarily restricted to particular populations and geographic areas [64]. Details of variants which define individual mtDNA haplogroups are recorded on PhyloTree [65]; http://www.phylotree.org/).
Over the past two decades, the associations between mtDNA haplogroups and PD risk have been extensively studied using the ‘haplogroup association approach’, particularly in populations of European ancestry (Table 2 and Supplementary Table 2). This approach associates haplogroups with a disease phenotype and suggests that one or more common population variants may modify disease risk. As a result of these studies, multiple European haplogroups including J, K, U, and some super-haplogroups (e.g. UK and JT) have been associated with a reduced risk of PD [66–73]. However, a number of additional studies were unable to replicate these findings and found no significant association between PD and haplogroups or common haplogroup variants [71, 74–78] (Table 2 and Supplementary Table 2). For instance, Mehta et. al [71] were unable to replicate previous associations between PD risk and haplogroups J and K in a large Australian cohort of European ancestry. Additionally, Van Der Walt et. al [73] and Huerta et. al [75] identified the MT-ND3 single nucleotide polymorphism (SNP) m.10398A>G to be protective against PD, whilst multiple other studies could not replicate this finding [66, 68, 69, 76]. Asian haplogroup association studies have also produced somewhat inconsistent results (Table 2 and Supplementary Table 2). For instance, although Wu et. al [79] and Liou et. al [80] both reported the Asian haplogroup B5 to be protective against PD, studies done by Chen et. al [81] and Chen et. al [82] reported no significant overall association between any of the common Asian haplogroups and PD risk.
Table 2.
Summary of mtDNA haplogroup association studies in Parkinson’s disease
| Ethnicity (Ancestry) | Study participants | N | Multiple-Test Correction | Estimates of statistical power determined | Outcome/ Main findings | Reference |
|---|---|---|---|---|---|---|
| American (European) | Cases | 609 | NS | NS | Haplogroups J (P = 0,02) and K (P = 0,02) associated with reduced PD risk. SNP m.10398A>G was reported to be protective against PD (P = 0,0001). SNP m.9055G>A in MT-ATP6 reduced PD risk in women (P = 0,03). SNP m.13708G>A reduced PD risk in individuals > 70 years of age (P = 0,010). | [73] |
| Controls | 340 | |||||
| Irish (European) | Cases | 90 | Yes (Bonferroni correction) | NS | No significant association between haplogroup J and PD found. SNP m.4216T>C, in linkage with mtDNA TJ cluster, was associated with increased PD risk (P = 0,014). | [77] |
| Controls | 129 | |||||
| Finnish (European) | Cases (PD) | 210 | NS | NS | Supercluster JTWIX was associated with an increased risk of PD (P = 0,27) and PD with dementia (PDD) (P = 0,18). | [78] |
| Cases (PD) | 28 | |||||
| Controls | 104 | |||||
| Spanish from Asturias, Northern Spain (European) | Cases | 271 | Yes (Bonferroni correction) | NS | No Haplogroups were associated with PD risk. SNP m.4336T>C associated with increased PD risk only in females (P = 0,011). SNP m.10398A>G was protective against PD (P = 0,009). | [75] |
| Controls | 230 | |||||
| English (European) | Cases | 455 | NS | NS | Haplogroups J and K not significantly associated with reduced PD risk. UKJT cluster reduced PD risk (P < 0,0001). | [72] |
| Control group 1 | 269 | |||||
| Control group 2 (Birth cohort) | 178 | |||||
| Control group 3 (Post-mortem brain tissue from AD patients) | 185 | |||||
| Italian (European) | Cases | 620 | NS | NS | Haplogroup K (P = 0,048) but not J associated with reduced PD risk. SNP m.10398A>G did not significantly alter PD risk. | [68] |
| Control group 1 (CT1) | 1486 | |||||
| Control group 2 (CT2) | 509 | |||||
| Taiwanese (Asian) | Cases | 416 | Yes (Bonferroni correction) | NS | No haplogroups associated with PD risk. | [81] |
| Controls | 372 | |||||
| Polish from central, South and north-Western Poland (European) | Cases | 241 | NS | Yes | Haplogroup J (P = 0,0014) associated with reduced PD risk in males (after stratification by gender). Sub-lineages U4 +U5a1 + K+J1c + J2 also reduced PD risk (P = 0,027). SNP m.10398A>G did not significantly alter PD risk. | [66] |
| Controls | 277 | |||||
| Cases for gender Stratified haplogroup J analysis | 304 | |||||
| Controls | 316 | |||||
| Additional cases for haplogroup K analysis | 91 | |||||
| Additional controls for haplogroup K analysis | 137 | |||||
| Greek from Crete (European) | Cases | 224 | NS | NS | No haplogroups associated with PD risk. SNP m.10398A>G did not significantly alter PD risk. | [76] |
| Controls | 383 | |||||
| Russian, Tatar (European) | Cases | 157 | NS | NS | Haplogroup H associated with an increased PD risk (P = 0,0001). UK cluster associated with a decreased PD risk (P = 0,003). | [70] |
| Controls | 183 | |||||
| Australian from New South Wales and Queensland (European) | Cases | 890 | NS | Yes | No significant associations between PD risk and haplogroups J and K found, nor the pooled UJKT haplogroup cluster. | [71] |
| Controls | 3491 | |||||
| English (European) | Cases (Discovery phase) | 1719 | NS | Yes | Association study: No association between haplogroups and PD nor SNP m.10398A>G and PD. Super-haplogroup JT was associated with a protective effect against PD (P = 0,0354). Mitochondrial variants m.2158T>C (discovery: P = 0,024; replication: P = 0,0245) and m.11251A>G (discovery: P = 0,0292; replication: P = 0,0012) were associated with a reduced risk of PD in both the discovery and replication cohorts. Meta-analysis: Haplogroups J (P = 0,0122), K (P = 0,00363), T (P = 0,0245) and super-haplogroup JT (P = 0,000584) associated with reduced PD risk. Increased PD risk was associated with cluster HV (P = 0,00364). | [69] |
| Controls (Discovery phase) | 2889 | |||||
| Cases (Replication phase) | 851 | |||||
| Controls (Replication phase) | 2717 | |||||
| Cases (Meta-Analysis) | 6140 | |||||
| Controls (Meta-Analysis) | 13280 | |||||
| Spanish from Pamplona, North-East Spain (European) | Cases | 478 | Yes (NS) | NS | No haplogroups were associated with PD risk | [74] |
| Controls | 394 | |||||
| Spanish from Santiago de Compostela, North-West Spain (European) | Cases | 305 | ||||
| Controls | 293 | |||||
| Han Chinese from Southern China (Asian) | Cases (Total) | 279 | NS | NS | Overall, no association between haplogroups and PD. Haplogroup B (P =0,004) associated with a lower risk for EOPD in individuals younger than 50 years (after age stratification,). Haplogroup D associated with a higher risk of PD (P = 0,033) and Haplogroup B was associated with a lower risk of PD (P = 0,018) in individuals younger than 50. | [82] |
| EOPD (<50) | 63 | |||||
| LOPD (>50) | 216 | |||||
| Controls (Total) | 510 | |||||
| Control Team 1 | 118 | |||||
| Controls | 332 | |||||
| Han Chinese from Northern China (Asian) | Cases | 322 | Yes (Bonferroni correction) | NS | SNP m.10398A>G (P = 0,001) significantly associated with increased PD risk in females (P = 0,0036). | [173] |
| Taiwanese (Asian) | Cases | 725 | Yes (Bonferroni correction) | NS | Haplogroup B5 associated with a reduced PD risk (P = 0,002). | [80] |
| Controls | 744 | |||||
| Greek from Cypriot (European) | Cases | 230 | Yes (Bonferroni correction) | NS | Haplogroup U associated with reduced PD risk (P =0,03), supercluster LMN (P = 0,01) and cluster N(xR) (P = 0,006) were significantly protective against PD in females. | [67] |
| Controls | 457 | |||||
| Han Chinese from East China (Asian) | Cases | 500 | NS | NS | D-loop-sequencing: SNPs m.151T>C (P = 0,023), m.189G>A (P = 0,03), m.16086C>T (P = 0,007 and m.16271C>T (P = 0,0497) were associated with increased PD risk. SNPs m.318C>T and m.16134T>C (P = 0,022) were associated with decreased PD risk. Haplogroup A5 (P = 0,039) was associated with increased PD risk. Haplogroup B5 (P = 0,068) was associated with a reduced disease risk. Meta-analysis: Haplogroup B5, but not B4, was protective against PD (P = 0,0003). Haplogroup G not associated with PD (P = 0,09). | [79] |
| Controls | 505 |
AD = Alzheimer’s disease; EOPD = Early onset Parkinson’s disease; LOPD = Late onset Parkinson’s diseas e; mtDNA = Mitochondrial DNA; N = sample size; NA = Not Applicable; NS = Not specified; PDD = Parkinson’s disease with dementia; PD = Parkinson’s disease
Overall, the evident variability in results and lack of reproducibility across haplogroup studies has made it difficult to conclude whether common mtDNA population variants contribute to PD risk.
4.2.1. Limitations of past haplogroup association studies and possible sources of inter-study variability
To improve the design of future studies, it is important to identify all possible factors which are introducing variability into, and between studies in order to limit false positive or erroneous findings. In the following section we therefore highlight possible methodological concerns which may contribute to discrepancies between study findings and should be addressed in future work.
Population stratification
mtDNA haplogroup association studies are often confounded by high levels of population stratification, referring to systematic differences in allele frequencies between subpopulations in a population, due to different ancestry [83]. This is because mtDNA itself is more prone to population substructure than nDNA, given that its effective population size is four times smaller than that of nDNA due to its uni-parental inheritance. Even when cases and controls are well matched in other regards (e.g. age, sex) substructure can be a confounding factor which may result in false positives. A study done by Otaegui et. al [84] with a cohort of Spanish PD patients, from different origins (Basque and non-Basque), and non-Basque controls, highlighted the importance of conducting case-control association studies in ethnically homogeneous populations (Table 2 and Supplementary Table 2). The authors found no association between PD and the m.10398A>G SNP (P = 0.088) when comparing all cases to controls but observed a significant overrepresentation of this SNP specifically in PD patients of Basque origin when compared to the non-Basque control population (P = 0.0221).
Replication cohorts and multiple testing
Several case-control association studies, including some of the ‘PD haplogroup’ studies listed in Table 2, lack replication cohorts [85]. Such replication cohorts or even a second control group (e.g. as used by Ghezzi et. al [68], Pyle et. al [72] and Chen et. al [82]) can be useful for detecting population substructure which affects the validity of results if not taken into consideration [86].
Additionally, multiple PD haplogroup association studies failed to correct their P-values/ significance levels for multiple testing (Table 2 and Supplementary Table 2). This may also have resulted in false positive findings [87].
Power
Samuels et. al [88] demonstrated that when using the haplogroup association hypothesis, very large sample sizes of cases and controls are necessary in order to reliably detect an association between haplogroups and complex human disease. To demonstrate this, the authors gave the example that studies with 500 cases and 500 controls have 90% power to detect a greater than 35% change in the frequency of the common haplogroup H. Therefore, several of the PD haplogroup studies are underpowered (Table 2 and Supplementary Table 2). Additionally, such underpowered haplogroup association studies are frequently analysed in ways which violate the assumptions of the statistical tests used, thereby inflating Type 1 error [85].
Pseudo-haplogroups
Pseudo-haplogroups are usually composed of low frequency haplogroups present in the study cohort, clustered together in ways which cannot be justified by phylogenetic knowledge [74]. Interestingly, this artificial clustering of haplogroups is commonly observed in neurological association studies including those done by Pyle et. al [72], Ghezzi et. al [68], Gaweda-Walerych et. al [66] and Latsoudis et. al [76] on PD risk. The creation of such pseudo-haplogroups results in erroneous inferences and false positive findings [74]. This is because pseudo-haplogroups are not biologically meaningful; they do not share a set of variants characterising an exclusive phylogenetic branch. To demonstrate this, Fachal et. al [74] artificially merged haplogroups U and V of a migraine cohort from Cantabria. This resulted in the UV pseudo-haplogroup being significantly associated with migraines (P = 0.0268) even though it did not make phylogenetic sense. Thus, emphasising the risk of false associations when pseudo-haplogroups are used.
In summary, PD haplogroup studies have reported inconsistent findings. Evidently, there are many methodological concerns around haplogroup studies which may have influenced the findings of past studies. These concerns should carefully be addressed in future.
5. Somatic mtDNA changes in PD
Somatic mtDNA variation, including point mutations and deletions, accumulate over time in post-mitotic tissue, including the ageing human brain [89–91]. This gives rise to a mixed population of mutant and wild-type mtDNA molecules i.e. heteroplasmy. The acquired variants may additionally clonally expand in individual cells through relaxed replication and random intracellular drift [92]. During relaxed replication, mtDNA molecules are randomly selected for replication. Thus, the proportion of mtDNA heteroplasmy in a cell can significantly increase over time if mutant mtDNA molecules are replicated more frequently by chance than the wild-type mitochondrial genomes [92]. When the proportion of mutant mtDNA molecules with harmful mtDNA changes exceeds a critical threshold level, a cellular defect in OXPHOS will arise [93]. Consequently, a number of studies have investigated high levels of heteroplasmic mtDNA point mutations and mtDNA deletions in the post-mortem brain tissue of PD patients. Both deletions and point mutations in these studies were considered somatic rather than germline.
5.1. Somatic mtDNA point mutations and deletions
With the advent of sequencing technologies sensitive enough to detect low-level heteroplasmies (< 10%), a handful of studies sequenced mtDNA in PD cases and controls extracted from post-mortem brains (Table 3 and Supplementary Table 3). They hypothesised that multiple, individually rare mtDNA point mutations in either the entire mitochondrial genome, or subset of mtDNA genes, could collectively constitute a high variant burden in the brains of PD patients. This burden would ultimately lead to neuronal loss. However, only two studies reported significantly higher burdens of point mutations in the brains of PD cases compared to controls [94, 95]. Others reported no significant difference between case and control groups (Table 3 and Supplementary Table 3) [51, 96–98].
Table 3.
Summary of mtDNA sequencing studies on somatic variation in Parkinson’s disease
| Study participants | N | Brain region | Variants considered | Pathogenicity Scoring | Method | mtDNA region sequenced | Outcome/ Main findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Cases | 8 | FCtx (Tissue homogenate) | All point mutations; G:C to T:A and T:A to G:C transversions | NS | PCR-cloning-sequencing strategy | MT-ND4 | No significant difference in heteroplasmic point mutation burdens between cases and controls. | [97] |
| Controls (< 10 years old) | 2 | |||||||
| Controls (12–24 years old) | 6 | |||||||
| Controls (Elderly) | 7 | |||||||
| Cases | 8 | SN (Tissue homogenate) | ||||||
| Controls (< 10 years old) | 3 | |||||||
| Controls (12–24 years old) | 4 | |||||||
| Controls (Elderly) | 10 | |||||||
| Cases | 6 | FCtx (Tissue homogenate) | Nonsynonymous point mutations | Yes | PCR-cloning-sequencing strategy | MT-ND1;MT-ND2; MT-ND3; MT-ND4L; MT-ND4; MT-ND5; MT-ND6 | No significant difference in heteroplasmic point mutation burdens between cases and controls | [51] |
| Controls | 6 | |||||||
| Cases (Early PD + ILBD) | 9 | SN (Isolated dopaminergic neurons; Glial cells) | All point mutations; GT/CA transversions | NS | PCR-cloning-sequencing strategy | MT-ND5; D-loop | Significantly elevated levels of heteroplasmic point mutations in neurons of early PD + ILBD cases compared to controls (P < 0,0001) and late stage PD cases (P = 0,0006). No significant difference in mtDNA point mutation levels in SN glia from early PD + ILBD cases compared to controls (P = 0,73). | [95] |
| Cases (Late PD) | 8 | |||||||
| Controls | 23 | |||||||
| Cases | 114 | SN (Tissue homogenate) | All point mutations; nonsynonymous point mutations | Yes (MutPred scoring) | NGS (Illumina sequencing) | Whole mtDNA | Significantly higher heteroplasmic variant burden in the SN (P = 0,012) and FCtx (P = 0,005) of PD patients compared to controls. | [94] |
| Controls | 34 | |||||||
| Cases | 125 | FCtx (Tissue homogenate) | ||||||
| Controls | 30 | |||||||
| Cases | 10 | SNpc (Single dopaminergic neurons- 184 in total) | SNVs; transitions and transversions; deletions | NS | Ultra-deepNGS (Illumina sequencing) | Two rRNA (MT-RNR1, MT-RNR2); 10 tRNA (MT-TV, MT-TL1, MT- TI, MT-TQ, MT-TM, MT-TW, MT-TA, MT-TN, MT-TC, MT-TY); two Complex I genes (MT-ND1, MT-ND2) | Significantly higher levels of mtDNA deletions (P = 0,004), but not point mutations, in patients compared to controls. Significant mtDNA depletion in PD cases compared to controls (P = 0,006). | [96] |
| Controls | 10 | |||||||
| Cases (DLB-PD) | 89 | Cerebellum; Cerebral Cortex; Other Brain regions (Tissue homogenate) | All point mutations; nonsynonymous point mutations | Yes (MutPred and Polyphen-2) | NGS (Illumina sequencing) | Whole mtDNA (Extracted from Whole exome sequencing data) | No significant difference in Heteroplasmic point mutation Burdens between cases and controls. | [98] |
| Controls | 351 | |||||||
| Controls (Young) | 110 | |||||||
DLB-PD = Dementia with Lewy Bodies or Parkinson’s Disease; FCtx = Frontal cortex; ILBD = Incidental Lewy body disease; mtDNA = Mitochondrial DNA; N = Sample size; NA = Not applicable; NGS = Next Generation Sequencing; NS = Not specified; SN = Substantia Nigra; SNVs = Single nucleotide variants; PD = Parkinson’s disease
In addition, acquired mtDNA deletions have also been suggested to play an important part in the selective neuronal loss in the ageing and PD brain [90, 99–101]. Mechanisms, such as breakpoints in tandem repeats of mtDNA or replication errors [102] give rise to these deletions [100, 101]. Notably, DA metabolism has been shown to drive the generation of mtDNA deletions in in vitro and in vivo models [103]. DA is readily oxidised, leading to ROS and neurotoxic quinone production [104]. ROS, such as H2O2, have been shown to cause single and double stranded breaks in mtDNA in vitro [105]. As such, SNpc DA neurons appear to be particularly susceptible to accumulation of mtDNA deletions and most of these deletions are located between the heavy and light strand origins of replication (the major arc; Figure 1) [106].
The ‘common deletion’ is a 4,977-base-pair deletion located between the MT-ATP8 and MT-ND5 genes, spanning four genes coding for complex I (MT-ND3, MT-ND4, MT-ND4L and MT-ND5) (Figure 1). It is thought to bring about a complex I defect, thus an energy crisis [107]. The frequency of mtDNA carrying this deletion in PD cases compared to controls has been investigated using Southern blotting, competitive- and kinetic-PCR techniques as well as in situ hybridisation techniques [107–111]. Despite two studies reporting a greater burden of this deletion in patients compared to controls [107, 111], others could not replicate these findings [108–110]. Instead, they reported no difference between cases and controls, suggesting that the common deletion may be a result of the natural ageing process rather than disease pathogenesis. Gu et. al [112] later reported that the number of mtDNA deletions (not limited to the common deletion) was increased significantly the SN homogenate of PD patients compared to age-matched controls, patients with multiple system atrophy, Dementia with Lewy Bodies, and those with Alzheimer’s disease.
Additional studies compared the total burden of mtDNA carrying deletions in the major arc, between brains of PD cases and controls using quantitative PCR (qPCR) [90, 96, 113, 114]. Such qPCR methods typically measure the ratio of a gene not commonly deleted (e.g. MT-ND1) in mtDNA to one which is frequently deleted (e.g. MT-ND4) using either relative or absolute quantification methods. The latter requiring a standard curve. Two studies reported significantly higher burdens of mtDNA deletions in individual DA SNpc (P = 0.004) [96] and pedunculopontine cholinergic neurons (P < 1×10−4) [114] of PD cases compared to controls. The others reported no significant differences in deletion levels between SN neurons of cases and aged control groups [90, 113].
Based on the evidence outlined above, it is reasonable to argue for or against a role of somatic point mutations and/or deletions in PD pathogenesis. However, variability between study designs (but also small sample sizes) makes it difficult to compare findings across studies and makes it challenging to draw a reliable conclusion regarding the contribution of somatic mtDNA deletions and point mutations to PD.
5.2. Changes in mtDNA copy number
In addition to possible point mutations and deletions in mtDNA molecules, mtDNA depletion should also be considered as a possible predisposing factor for PD. The importance of adequate mtDNA in cells is highlighted by mtDNA depletion syndromes – a group of autosomal recessive disorders characterised by severe mtDNA depletion which results in impaired energy production [115]. Since mtDNA encodes essential OXPHOS complex components, a sufficient amount of mtDNA is required for the production of these OXPHOS subunits and to manage cellular energy demands [116]. Therefore, there has also been growing interest in understanding mtDNA levels (mtDNA copy number) in PD.
Frequently, studies investigating mtDNA copy number have used qPCR methods to quantify the ratio of MT-ND1 to a nuclear house-keeping gene. Recent studies have reported significant differences in mtDNA copy number values between PD patients and controls in the SN, with lower mtDNA copy numbers observed in the patients [96, 113, 117]. Notably, in one study mtDNA copy number in DA neurons was observed to increase with age in controls [96]. Therefore, despite the age-related accumulation of mtDNA deletions, the pool of wild-type mtDNA was maintained. In contrast to the controls, no compensatory increase in mtDNA copy number was observed in patients, resulting in relative depletion of wild-type mtDNA in the SNpc. This was suggested to ultimately result in respiratory deficiency and neuronal loss. Bury et. al [114], who observed increased levels of both mtDNA deletions and mtDNA copy number in pedunculopontine cholinergic neurons of PD cases compared to controls, suggested that different neurochemical cell types and brain regions in PD patients may differ in response to mtDNA deletion accumulation. However, reasons for the lack of possible compensatory mechanisms in the SNpc of the PD brain remain unclear. Hence, further research is warranted on how mtDNA levels are regulated in different cell-types.
mtDNA depletion has also been recognised as a potential biomarker for PD detection because significant reductions in mtDNA copy number have not only been observed in the SN of PD patients [96, 113, 117] but also in peripheral blood [117, 118] and cerebrospinal fluid [119]. Overall, reports on mtDNA depletion in PD appear promising, although further research in this field is required, particularly with regards to the mechanisms underlying mtDNA depletion and its utility as a biomarker.
5.3. Challenges of studying somatic mtDNA changes in PD
Studying acquired mtDNA changes in PD is particularly challenging, rendering the role of mtDNA in PD unresolved. Many factors can substantially influence study findings and can contribute to inconsistent reports such as those detailed above. In the following section we highlight important methodological and biological factors to consider when studying somatic mtDNA changes in PD case-control studies.
Techniques and technologies
Technologically, many well-established molecular techniques used to study nDNA variation may not be suitable for the study of mtDNA. A review by Moraes et. al [120] drew attention to techniques including Southern blotting, PCR amplification, and RFLP, and reviewed their respective advantages and disadvantages when analysing heteroplasmic mtDNA point mutations and deletions. The authors highlighted that these techniques can yield misinterpreted results for mtDNA given the genome’s unique properties of heteroplasmy and polyploidy. Thus, the use of Southern blotting and different PCR techniques in earlier mtDNA deletion studies could account for inconsistent findings. For instance, Ikebe et. al [107] and Ozawa et. al [111], employed PCR and kinetic-PCR techniques respectively, and reported significantly higher mtDNA common deletion levels in cases compared than controls. In contrast Mann et. al [109] and Kösel e t. al [108] who employed Southern blotting and competitive PCR techniques respectively found no significant increase of this deletion in cases compared to controls. Newer techniques used to quantify mtDNA deletions also have disadvantages. For instance, the relative quantification of levels of MT-ND1 to MT-ND4 fails to quantify rarer mtDNA deletions which extend into the minor arc and may not reflect the true extent of deleted mtDNA.
Moreover, the ability to confidently detect and quantify low levels of point mutation heteroplasmies in mtDNA varies between sequencing technologies [121, 122]. Some technologies such as Sanger sequencing are not sensitive enough to detect individually-rare mtDNA variants below 15% heteroplasmy, thereby potentially underestimating heteroplasmic variant levels [123]. Others, such as post-PCR cloning strategies, are at risk of over-estimating heteroplasmic variant loads because of DNA polymerase enzyme transcriptional errors which are indistinguishable from true variants [122]. As a result, sequencing data generated using such technologies may not be truly representative of the heteroplasmic mtDNA levels in a sample and have likely contributed to some inconsistent findings between published studies (Table 3).
When employing newer, next generation sequencing approaches (NGS), sequencing depth affects the level of heteroplasmy detected [124]. This level varies between NGS approaches. Ultra-deep sequencing of the mtDNA, with a depth of several tens of thousands is required to confidently detect very low levels of heteroplasmies (~1–10%). Additionally, technical artefacts and sequencing errors need to be controlled for in NGS to achieve accuracy of quantification and detection specificity [124]. To control for these errors and artefacts, NGS studies should integrate effective quality-control criteria. Web-based servers such as mtDNA-Server (https://mtdna-server.uibk.ac.at) are available specifically for the analysis of mtDNA NGS data. The workflow includes several quality control metrics, identification of artefacts and contamination, heteroplasmy detection, as well as variant annotation [125]. The use of such servers could aid in standardising heteroplasmy detection and quantification across studies.
Moreover, as mentioned, mtDNA in post-mitotic tissue is likely to accumulate deletions with age [99]. Consequently, mtDNA amplification in aged, post-mortem brains, prior to sequencing, could also influence results of sequencing studies. For instance, amplifying mtDNA in two fragments which overlap the deletion-prone major arc may result in mtDNA sequence data which was unrepresentative of the entire mtDNA data population. This is because mtDNA carrying deletions in the primer binding sites may not be amplified given that primers may not bind and not amplify the mtDNA effectively.
Finally, predicting the functional impact of mtDNA variants in sequencing studies is important. This is because the levels of variation accumulating with age in controls could be similar to those accumulating in PD patients but may collectively be less pathogenic. It should be stressed that studies should use in silico tools specifically developed for mtDNA variation since those developed for nDNA may not be suitable to predict the pathogenicity of mtDNA variants [126]. Examples of mtDNA tools include APOGEE [127], MToolBox [128] and MitoTIP [129]. Tools like eKLIPse [130] are also available for identifying and quantifying mtDNA deletions (and other rearrangements) and should be considered for future studies.
Tissue and brain region
There are tissue specific differences in mtDNA heteroplasmy accumulation [131] and ‘hot-spot’ regions of heteroplasmy accumulation in mtDNA [132, 133]. As a result, the brain tissue and mtDNA region selected for examination in post-mortem studies will influence results. As detailed in Table 3, published studies did not all sequence the same mtDNA region, nor examined DNA from the same brain region, which could explain conflicting results. Although multiple regions of the brain are affected in PD, the loss of DA neurons in the pathologically affected SNpc is the most extensive [134]. The SNpc, in comparison to other brain regions, may therefore be expected to show the greatest difference in mtDNA changes between cases and controls, should such changes contribute to neuronal loss in PD, as hypothesised.
Depending on cellular energy demand, the number of mitochondria per cell and the copies of mtDNA per mitochondria can differ substantially between cell types in whole tissue [135]. As such, whole blood samples frequently used for mtDNA copy number assays may be biased by cellular composition. For instance, samples with more granulocytes than lymphocytes, may show lower mtDNA copy numbers than ones with a higher proportion of lymphocytes [136]. This is because granulocytes have been observed to have fewer mitochondria, and thus less mtDNA, than lymphocytes [136].
Moreover, mtDNA deletion and point mutation studies using tissue homogenate rather than single cells are also likely biased by the cellular composition of samples. mtDNA changes identified in tissue homogenate may not reflect the true changes in mtDNA between cases and controls. For instance, although both glial cells and neurons acquire mtDNA variants, somatic variants accumulate preferentially in neurons [137]. In support of this, Lin et. al [95] reported that overall levels of mtDNA point mutations were similar in SN glia of early PD and incidental Lewy body disease (ILBD) cases (Braak stage 3) and controls. However, they were significantly elevated in the single SN neurons of early PD + ILBD cases compared to controls (Table 3). As such, a homogenised tissue sample consisting of predominantly glial cells may have less mtDNA variation than one which consists predominantly of neuronal cells. Given that post-mortem brains of PD patients typically represent advanced stages of the disease and that up to 98% of DA SNpc neurons can be lost during advanced PD [138], mtDNA molecules examined in PD brain homogenate most likely originate primarily from surrounding glial cells.
Moreover, although studying individual cells is advantageous to avoid the bias of tissue composition, it can still be problematic. The surviving neurons in post-mortem brain tissue of patients with advanced PD may have accumulated fewer somatic mtDNA changes allowing the neurons to persist. On the other hand, already degenerated neurons are likely to have accumulated very high levels of detrimental, somatic errors. Consequently, the surviving neurons of late-stage PD may not differ significantly to those of aged controls with regards to mtDNA changes. Therefore, the pathological stage of disease of the patients from whom the tissue samples originated needs to be accounted for [139]. Examining neurons from early-stage (~ Braak stage 3) post-mortem tissue may be the best strategy.
6. Future studies
To date the role of mtDNA in PD has not yet been resolved largely due to the difficulty of studying mtDNA variation but also ‘poor’ study desi gn. However, there is potential for improvement in this field. Future studies need to consider the limitations of previous studies and the challenges associated with studying mtDNA to improve future study designs. For instance, small sample sizes are a significant limitation and can be overcome with new collaborations [140]. Studies should also consider using newer, more standardised approaches, such as the variant load approach, which potentially circumvent pitfalls of past investigations. Alternatively, future studies could branch into studying nDNA variation in conjunction with mtDNA changes. These and additional options are discussed below.
6.1. Homoplasmic mtDNA variation: considering functional studies and the collective role of rare population variants
Several authors have highlighted the methodological deficiencies of haplogroup association studies, some of which have also been outlined in this paper [74, 141, 142]. Yet, the methods used in these studies have not changed significantly over the past 20 years.
The current ‘haplogroup association’ approach uses only a few mtDNA SNPs. This makes it a cheap and relatively simple method to perform, that doesn’t require more high throughput technologies such as whole exome or complete mitochondrial genome sequencing and avoids complex bioinformatics analyses. We suggest that results from future haplogroup studies should ideally be validated using functional investigations (e.g. cybrid or histochemical studies) [143] although this might be challenging given that association genetics is based on very subtle effects. Nonetheless, additional work assessing mitochondrial homeostasis regulation and function (e.g. measuring ROS production, cellular respiration etc.) can provide functional evidence to support the genetic associations made. For example, Liou et. al [80] were able to validate their findings using cybrids. In their study, cybrid results revealed that the B5 haplogroup cybrid, harbouring the m.8584G>A/m.10398A>G variants showed more resistance to rotenone than the B4 cybrid lacking these variants. Briefly, rotenone is a pesticide and a complex I inhibitor, implicated in PD pathogenesis [144]. Furthermore, low ROS production and low apoptosis rates were also observed in the B5 cybrid [80], supporting the hypothesis that the B5 haplogroup variants have a protective effect against PD. Another recent study reported that haplogroup K1 increased mtDNA copy number and demonstrated a resistance to rotenone [145]. These findings support haplogroup studies which associated haplogroup K with a PD protective effect [68, 69], and highlight the value of such studies.
Although haplogroup association studies can be improved with such additional functional work, or by using replication cohorts, correcting for multiple testing, and avoiding pseudo-haplogroup construction, the current discrepancies between studies also highlight the need for newer and more standardised models.
Future studies should study homoplasmic, rare population variants which are mildly deleterious and could collectively contribute to disease risk in an individual, instead of only investigating haplogroup-defining variants in PD risk. This hypothesis, known as the variant load hypothesis, distances itself from the study of haplogroups [146]. The most up-to-date version of the variant load hypothesis excludes variants predicted to be likely benign from the analysis, as many of these variants are common polymorphisms. Such common variants are more likely to be subject to population stratification, thus if left in the analysis they might be the cause of false positive associations. The variant load approach additionally condenses the likely impact of an individual’s mtDNA variation into a numerical value on a continuous scale rather than in the form of a letter as done in haplogroup studies. Consequently, more powerful parametric statistics can be applied and fewer comparisons are needed, thus granting this approach greater statistical power with smaller sample sizes than the traditional haplogroup association method [147]. For instance, a recent study with a total sample size of 82 employed the variant load approach to investigate the role of mtDNA in oxidative stress and inflammation [148]. This study had 80% power to detect a correlation (with moderate effect size) between markers of oxidative stress and inflammation and variant loads. Given the decreasing cost of NGS and the growing availability of pipelines which simplify mtDNA data analyses, whole mtDNA sequencing data can be used to test the variant load hypothesis.
6.2. Somatic mtDNA variation and mtDNA depletion
Studying the role of somatic mtDNA variation in PD is challenging especially since the availability of PD post-mortem brains is limited and the pathological stage of disease may vary. Moreover, not all molecular techniques used to study nDNA variation are suitable for mtDNA given the unique features of mitochondrial genomes (polyploidy and heteroplasmy). Future studies should therefore carefully consider which molecular techniques would be most suitable for the purpose of their investigation to ensure results are reliable. Moreover, somatic mtDNA studies should correct for tissue cellular composition bias where possible. A combination of the strategies employed in the herein reviewed studies could be best to study somatic mtDNA variation in PD, particularly mtDNA point mutations. For instance, ultra-deep sequencing technologies could be used to sequence mtDNA molecules of individual, early-stage PD and control SNpc neurons.
mtDNA depletion in PD is an emerging biomarker of disease pathology and may be more promising to understand PD than somatic mtDNA variation given that mtDNA depletion studies have reported few discrepancies [96, 113, 117–119]. We suggest future studies should try to replicate these reports of mtDNA depletion in PD but also correct for cellular composition when possible, to make results more reliable and reproducible [149].
Future studies should also place greater focus on studying nDNA variation, in combination with mtDNA changes, which may underlie potentially high mtDNA variant burdens in PD brains, and/or mtDNA depletion. For instance, Gui et. al [118] reported that PD patients with a particular POLG1 genotype in combination with additional POLG1 variants, had significantly lower numbers of mtDNA than those without such variants. This suggested that POLG1 variation may contribute to mtDNA depletion in PD cases. POLG1 encodes a subunit of the DNA polymerase which replicates mtDNA. mtDNA mutator mice with mutations in nDNA encoding mtDNA replication components, including Polg1, have additionally demonstrated an accelerated accumulation of mtDNA point mutations and deletions, highlighting a potential role for nDNA underlying mtDNA changes [150–153]. As a result of these mtDNA changes, the mutator mice demonstrate a progressive respiratory chain dysfunction as seen in PD patients and premature aging phenotypes (e.g. hair loss, osteoporosis, and progressive hearing loss) [151]. These studies again implicate somatic mtDNA changes, resulting from nDNA mutations, in PD which has been hypothesised to be a form of accelerated aging [154, 155]. Mitochondrial transcription factor A (TFAM) is an essential DNA-binding protein required for transcription and maintenance of mtDNA. Knockout mice with a deleted Tfam gene demonstrate a respiratory chain deficiency and reduced mtDNA expression in DA neurons [156]. These mice also exhibit parkinsonism typical phenotypes including progressive motor function impairment and DA neuronal loss [156] implicating defective mtDNA maintenance in PD, brought about by nDNA changes. Notably, components of the nDNA-encoded, mtDNA replication machinery including mitochondrial genome maintenance exonuclease 1 (MGME1), POLG1, and mtDNA replicative helicase (TWINKLE) have recently been implicated in degradation of damaged mtDNA [157]. Thus, nDNA variation in genes encoding these factors is of particular interest for further study given their dual role mtDNA synthesis and degradation [102].
6.3. Mitochondrial-derived peptides (MDPs)
Recently, MDPs, encoded as sORFs, in mtDNA have been identified as important regulatory peptides of metabolic activity and stress response [2, 158, 159]. The discovery of MDPs underscores the potential existence of more sORFs hidden in mtDNA. Recent findings have implicated the mitochondrial open reading frame of the 12S rRNA-c (MOTS-c), a 16-amino-acid MDP encoded within the MT-RNR1 gene, in the regulation of nuclear gene expression in response to metabolic stress [3]. These findings suggest that nuclear and mitochondrial genomes cross regulate each other but they also highlight the potential of MDP involvement in disease [160]. Further work needs to be conducted to better understand the role of MDPs and the mechanisms regulating their expression in PD.
7. Concluding remarks
PD is an immensely complex disorder of multifactorial origin, but one with clear links to mitochondria and their genomes. Early evidence directly implicates mtDNA of sPD patients in altering mitochondrial structure and function. However, despite a multitude of studies investigating mtDNA in PD, the contribution of the mitochondrial genome to this disorder remains elusive.
To date, studies investigating homoplasmic and heteroplasmic mtDNA changes in PD have produced conflicting results. In almost all of these studies, low statistical power resulting from small sample sizes is a significant limitation, which likely contributed to some discrepant findings. Most mtDNA haplogroup association studies additionally suffered from methodological deficiencies (e.g. population stratification, lack of- replication cohorts and correction for multiple testing) which could primarily contribute to such discrepancies. On the other hand, innate difficulties of studying somatic mtDNA changes (e.g. heteroplasmy and tissue specific differences in mtDNA) along with different methodological techniques applied have produced conflicting results in somatic mtDNA studies which either provide evidence for or against a role of somatic mtDNA changes in PD.
Consequently, a role for the involvement of mtDNA in PD cannot at this stage be ruled out and needs to be further investigated in a more critical and systematic way. Future studies should take into account limitations of past studies reviewed here to improve current study designs. Alternatively, they should consider the use of more standardised approaches such as the variant load approach. The limitations, challenges, and future prospects of studying mtDNA in PD are summarised in Supplementary Figure 1. Although not caveat-free, the study of mtDNA depletion appears most promising and we propose that further research in this area is required, particularly regarding the mechanisms and possible nDNA variation underlying mtDNA depletion in PD patients.
Albeit small, relative to the nuclear genome, the mitochondrial genome is an integral component for the production and functioning of mitochondria, and overall eukaryotic cell homeostasis. The role of mtDNA therefore should not be underestimated, and future adequately powered and well-designed studies exploring current (mtDNA variation and levels) and new avenues (including MDPs) are needed to fully assess the magnitude of its contribution to PD pathogenesis.
Supplementary Material
Supplementary Figure 1. Flow diagram summarizing the limitations and challenges of published studies investigating mtDNA variation in Parkinson’s disease as well as directions for future studies in this field. MDPs = mitochondrial derived peptides; mtDNA = mitochondrial DNA; nDNA = nuclear DNA; SNPs = single nucleotide polymorphisms
Supplementary Table 1. MITOMAP summary of mtDNA variants from early mtDNA sequencing studies and haplogroup association studies in Parkinson’s disease
Supplementary Table 2. Detailed summary of mtDNA haplogroup association studies in Parkinson’s disease
Supplementary Table 3. Detailed summary of somatic mtDNA sequencing studies in Parkinson’s disease
Highlights.
Studies investigating mtDNA variation in PD have produced conflicting results
This may be due, in part, to methodological inconsistencies between studies
Studies should consider more standardised approaches to study mtDNA variation in PD
Nuclear genes and mitochondrial copy number should be considered in future studies
Mitochondrial-derived peptides should also be further investigated
Acknowledgements
The authors are supported by the National Research Foundation of South Africa (Grant Numbers: 106052), the South African Medical Research Council (Self-Initiated Research Grant), and Stellenbosch University. This work was also supported by an NIH grant (P50NS072187) and DOD award (W81XWH-17-1-0249) to OAR, the Mayo Clinic investigators are supported in part by a Lewy Body Dementia Association (LBDA) Research Center of Excellence, an American Parkinson Disease Association (APDA) Center for Advanced Research and the Michael J. Fox Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, and Howell N, Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nature genetics, 1999. 23(2): p. 147. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim S-J, Mehta H, Hevener AL, and de Cabo R, The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell metabolism, 2015. 21(3): p. 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KH, Son JM, Benayoun BA, and Lee C, The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell metabolism, 2018. 28(3): p. 516–524. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo S, Valencia CA, Zhang J, Lee N-C, Slone J, Gui B, Wang X, Li Z, Dell S, and Brown J, Biparental inheritance of mitochondrial DNA in humans. Proceedings of the National Academy of Sciences, 2018. 115(51): p. 13039–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, and Nagley P, Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR□based assay: lack of change of copy number with age. Nucleic acids research, 2003. 31(11): p. e61–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khrapko K, Coller HA, André PC, Li X-C, Hanekamp JS, and Thilly WG, Mitochondrial mutational spectra in human cells and tissues. Proceedings of the National Academy of Sciences, 1997. 94(25): p. 13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song S, Pursell ZF, Copeland WC, Longley MJ, Kunkel TA, and Mathews CK, DNA precursor asymmetries in mammalian tissue mitochondria and possible contribution to mutagenesis through reduced replication fidelity. Proceedings of the National Academy of Sciences, 2005. 102(14): p. 4990–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye K, Lu J, Ma F, Keinan A, and Gu Z, Extensive pathogenicity of mitochondrial heteroplasmy in healthy human individuals. Proceedings of the National Academy of Sciences, 2014. 111(29): p. 10654–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinnery PF and Samuels DC, Relaxed replication of mtDNA: a model with implications for the expression of disease. The American Journal of Human Genetics, 1999. 64(4): p. 1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasukawa T and Kang D, An overview of mammalian mitochondrial DNA replication mechanisms. The Journal of Biochemistry, 2018. [DOI] [PMC free article] [PubMed]
- 11.Herbers E, Kekäläinen NJ, Hangas A, P ohjoismäki JL, and Goffart S, Tissue specific differences in mitochondrial DNA maintenance and expression. Mitochondrion, 2019. 44: p. 85–92. [DOI] [PubMed] [Google Scholar]
- 12.Gelb DJ, Oliver E, and Gilman S, Diagnostic criteria for Parkinson disease. Archives of neurology, 1999. 56(1): p. 33–39. [DOI] [PubMed] [Google Scholar]
- 13.Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, and Goedert M, α-Synuclein in Lewy bodies. Nature, 1997. 388(6645): p. 839. [DOI] [PubMed] [Google Scholar]
- 14.Elbaz A, Grigoletto F, Baldereschi M, Breteler M, Manubens-Bertran J, Lopez-Pousa S, Dartigues J, Alperovitch A, Tzourio C, and Rocca W, Familial aggregation of Parkinson’s disease A population-based case-control study in Europe. Neurology, 1999. 52(9): p. 1876–1876. [DOI] [PubMed] [Google Scholar]
- 15.Kumar KR, Djarmati-Westenberger A, and Grünewald A, Genetics of Parkinson’s Disease. Semin Neurol, 2011. 31(05): p. 433–440. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Vives-Bauza C, Yamamoto A, Tan Y, Li Y, Magrané J, Stavarache MA, Shaffer S, Chang S, and Kaplitt MG, PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and α-synuclein aggregation in cell culture models of Parkinson’s disease. PloS one, 2009. 4(2): p. e4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker WD Jr, Boyson SJ, and Parks JK, Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 1989. 26(6): p. 719–723. [DOI] [PubMed] [Google Scholar]
- 18.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K-Y, Sack MN, Kastner DL, and Siegel RM, Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). Journal of Experimental Medicine, 2011. 208(3): p. 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, and Ghosh S, TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature, 2011. 472(7344): p. 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J-S, Davis RL, and Sue CM, Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Current neurology and neuroscience reports, 2018. 18(5): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langston JW, Ballard P, Tetrud JW, and Irwin I, Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science, 1983. 219(4587): p. 979–980. [DOI] [PubMed] [Google Scholar]
- 22.Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, and Kopin IJ, Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry research, 1979. 1(3): p. 249–254. [DOI] [PubMed] [Google Scholar]
- 23.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, and Kopin IJ, A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Proceedings of the National Academy of Sciences, 1983. 80(14): p. 4546–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heikkila RE, Hess A, and Duvoisin RC, Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine in mice. Science, 1984. 224(4656): p. 1451–1453. [DOI] [PubMed] [Google Scholar]
- 25.Wallace RA, Boldry R, Schmittgen T, Miller D, and Uretsky N, Effect of 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine (MPTP) on monoamine neurotransmitters in mouse brain & heart. Life sciences, 1984. 35(3): p. 285–291. [DOI] [PubMed] [Google Scholar]
- 26.Heikkila RE, Nicklas WJ, Vyas I, and Duvoisin RC, Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neuroscience Letters, 1985. 62(3): p. 389–394. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno Y, Suzuki K, Sone N, and Saitoh T, Inhibition of ATP synthesis by 1-methyl-4-phenylpyridinium ion (MPP+) in isolated mitochondria from mouse brains. Neuroscience letters, 1987. 81(1–2): p. 204–208. [DOI] [PubMed] [Google Scholar]
- 28.Shoffner JM, Watts RL, Juncos JL, Torroni A, and Wallace DC, Mitochondrial oxidative phosphorylation defects in Parkinson’s disease. Annals of neurology, 1991. 30(3): p. 332–339. [DOI] [PubMed] [Google Scholar]
- 29.Bindoff L, Birch-Machin M, Cartlidge N, Parker W Jr, and Turnbull D, Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. Journal of the neurological sciences, 1991. 104(2): p. 203–208. [DOI] [PubMed] [Google Scholar]
- 30.Blin O, Desnuelle C, Rascol O, Borg M, Saint Paul HP, Azulay J, Bille F, Figarella D, Coulom F, and Pellissier J, Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. Journal of the neurological sciences, 1994. 125(1): p. 95–101. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa-Hattori Y, Yoshino H, Kondo T, Mizuno Y, and Horai S, Is Parkinson’s disease a mitochondrial disorder? Journal of the neurological sciences, 1992. 107(1): p. 29–33. [DOI] [PubMed] [Google Scholar]
- 32.Cardellach F, Marti M, Fernandez-Sola J, Marin C, Hoek J, Tolosa E, and Urbano-Marquez A, Mitochondria1 respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology, 1993. 43(11): p. 2258–2258. [DOI] [PubMed] [Google Scholar]
- 33.Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zo R, Boissl KW, and Reichmann H, Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neuroscience letters, 1994. 169(1–2): p. 126–128. [DOI] [PubMed] [Google Scholar]
- 34.Schapira A, Cooper J, Dexter D, Jenner P, Clark J, and Marsden C, Mitochondrial complex I deficiency in Parkinson’s disease. The Lancet, 1989. 333(8649): p. 1269. [DOI] [PubMed] [Google Scholar]
- 35.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, and Shults CW, Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Annals of neurology, 1995. 37(6): p. 714–722. [DOI] [PubMed] [Google Scholar]
- 36.Benecke R, Strümper P, and Weiss H, Electron transfer complexes I and IV of platelets are abnormal in Parkinson’s disease but normal in Parkinson-plus syndromes. Brain, 1993. 116(6): p. 1451–1463. [DOI] [PubMed] [Google Scholar]
- 37.Yoshino H, Nakagawa-Hattori Y, Kondo T, and Mizuno Y, Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson’s disease. Journal of Neural Transmission-Parkinson’s Disease and Dementia Section, 1992. 4(1): p. 27–34. [DOI] [PubMed] [Google Scholar]
- 38.Krige D, Carroll MT, Cooper JM, Marsden CD, and Schapira AH, Platelet mitochondria function in Parkinson’s disease. Annals of neurology, 1992. 32(6): p. 782–788. [DOI] [PubMed] [Google Scholar]
- 39.Trinh J and Farrer M, Advances in the genetics of Parkinson disease. Nature Reviews Neurology, 2013. 9(8): p. 445. [DOI] [PubMed] [Google Scholar]
- 40.Swerdlow RH, Does mitochondrial DNA play a role in Parkinson’s disease? A review of cybrid and other supportive evidence. Antioxidants & redox signaling, 2012. 16(9): p. 950–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, PARKER WD Jr, and Davis RE, Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer’s disease and Parkinson’s disease. Annals of the New York Academy of Sciences, 1999. 893(1): p. 176–191. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow RH, Parks JK, Miller SW, Davis RE, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, and Parker WD, Origin and functional consequences of the complex I defect in Parkinson’s disease. Annals of neurology, 1996. 40(4): p. 663–671. [DOI] [PubMed] [Google Scholar]
- 43.Esteves ARF, Domingues AF, Ferreira IL, Januário C, Swerdlow RH, Oliveira CR, and Cardoso SM, Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion, 2008. 8(3): p. 219–228. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins HM, Carl SM, and Swerdlow RH, Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies. Redox biology, 2014. 2: p. 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, Tarek M, del Carpio JC, Nesburn AB, and Boyer DS, Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2014. 1842(2): p. 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivian CJ, Brinker AE, Graw S, Koestler DC, Legendre C, Gooden GC, Salhia B, and Welch DR, Mitochondrial genomic backgrounds affect nuclear DNA methylation and gene expression. Cancer research, 2017. 77(22): p. 6202–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jandova J, Janda J, and Sligh JE, Changes in mitochondrial DNA alter expression of nuclear encoded genes associated with tumorigenesis. Experimental cell research, 2012. 318(17): p. 2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quiros PM, Mottis A, and Auwerx J, Mitonuclear communication in homeostasis and stress. Nature reviews Molecular cell biology, 2016. 17(4): p. 213. [DOI] [PubMed] [Google Scholar]
- 49.Dominy JE and Puigserver P, Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harbor perspectives in biology 5(7): p. a015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keeney PM, Dunham LD, Quigley CK, Morton SL, Bergquist KE, and Bennett JP Jr, Cybrid models of Parkinson’s disease show variable mitochondrial biogenesis and genotype-respiration relationships. Experimental neurology, 2009. 220(2): p. 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smigrodzki R, Parks J, and Parker WD, High frequency of mitochondrial complex I mutations in Parkinson’s disease and aging. Neurobiology of aging, 2004. 25(10): p. 1273–1281. [DOI] [PubMed] [Google Scholar]
- 52.Kösel S, Grasbon-Frodl EM, Mautsch U, E gensperger R, von Eitzen U, Frishman D, Hofmann S, Gerbitz K-D, Mehraein P, and Graeber MB, Novel mutations of mitochondrial complex I in pathologically proven Parkinson disease. Neurogenetics, 1998. 1(3): p. 197–204. [DOI] [PubMed] [Google Scholar]
- 53.Richter G, Sonnenschein A, Grünewald T, Reichmann H, and Janetzky B, Novel mitochondrial DNA mutations in Parkinson’s disease. Journal of neural transmission, 2002. 109(5–6): p. 721–729. [DOI] [PubMed] [Google Scholar]
- 54.Simon D, Mayeux R, Marder K, Kowall N, Beal M, and Johns D, Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson’s disease. Neurology, 2000. 54(3): p. 703–703. [DOI] [PubMed] [Google Scholar]
- 55.Kirchner SC, Hallagan SE, Farin FM, Dilley J, Costa-Mallen P, Smith-Weller T, Franklin GM, Swanson PD, and Checkoway H, Mitochondrial ND1 sequence analysis and association of the T4216C mutation with Parkinson’s disease. Neurotoxicology, 2000. 21(4): p. 441–445. [PubMed] [Google Scholar]
- 56.Howell N, Elson JL, Chinnery PF, and Turnbull DM, mtDNA mutations and common neurodegenerative disorders. Trends in Genetics, 2005. 21(11): p. 583–586. [DOI] [PubMed] [Google Scholar]
- 57.Ikebe S.-i., Tanaka M, and Ozawa T, Point mutations of mitochondrial genome in Parkinson’s disease. Molecular brain research, 1995. 28(2): p. 281–295. [DOI] [PubMed] [Google Scholar]
- 58.Ozawa T, Tanaka M, Ino H, Ohno K, Sano T, Wada Y, Yoneda M, Tanno Y, Miyatake T, and Tanaka T, Distinct clustering of point mutations in mitochondrial DNA among patients with mitochondrial encephalomyopathies and with Parkinson’s disease. Biochemical and biophysical research communications, 1991. 176(2): p. 938–946. [DOI] [PubMed] [Google Scholar]
- 59.Grasbon-Frodl EM, Kösel S, Sprinzl M, v on Eitzen U, Mehraein P, and Graeber MB, Two novel point mutations of mitochondrial tRNA genes in histologically confirmed Parkinson disease. Neurogenetics, 1999. 2(2): p. 121–127. [DOI] [PubMed] [Google Scholar]
- 60.Brown MD, Shoffner JM, Kim YL, Jun AS, Graham BH, Cabell MF, Gurley DS, and Wallace DC, Mitochondrial DNA sequence analysis of four Alzheimer’s and Parkinson’s disease patients. American Journal of Medical Genetics Part A, 1996. 61(3): p. 283–289. [DOI] [PubMed] [Google Scholar]
- 61.Bandmann O, Sweeney M, Daniel S, Marsden C, and Wood N, Mitochondrial DNA polymorphisms in pathologically proven Parkinson’s disease. Journal of neurology, 1997. 244(4): p. 262–265. [DOI] [PubMed] [Google Scholar]
- 62.Mayr-Wohlfart U, Rödel G, and Henneberg A, Mitochondrial tRNA(Gln) and tRNA(Thr) gene variants in Parkinson’s disease. European journal of medical research, 1997. 2: p. 111–113. [PubMed] [Google Scholar]
- 63.Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V, and Wallace DC, mtDNA variation and analysis using MITOMAP and MITOMASTER. Current protocols in bioinformatics, 2013: p. 1.23. 1–1.23. 26. [DOI] [PMC free article] [PubMed]
- 64.Torroni A, Achilli A, Macaulay V, Richards M, and Bandelt H-J, Harvesting the fruit of the human mtDNA tree. TRENDS in Genetics, 2006. 22(6): p. 339–345. [DOI] [PubMed] [Google Scholar]
- 65.Van Oven M and Kayser M, Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Human mutation, 2009. 30(2). [DOI] [PubMed] [Google Scholar]
- 66.Gaweda-Walerych K, Maruszak A, Safranow K, Bialecka M, Klodowska-Duda G, Czyzewski K, Slawek J, Rudzinska M, Styczynska M, and Opala G, Mitochondrial DNA haplogroups and subhaplogroups are associated with Parkinson’s disease risk in a Polish PD cohort. Journal of neural transmission, 2008. 115(11): p. 1521–1526. [DOI] [PubMed] [Google Scholar]
- 67.Georgiou A, Demetriou CA, Heraclides A, Christou YP, Leonidou E, Loukaides P, Yiasoumi E, Panagiotou D, Manoli P, and Thomson P, Mitochondrial superclusters influence age of onset of Parkinson’s disease in a gender specific manner in the Cypriot population: A case-control study. PloS one, 2017. 12(9): p. e0183444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P, Brusa L, and Bentivoglio AR, Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. European Journal of Human Genetics, 2005. 13(6): p. 748. [DOI] [PubMed] [Google Scholar]
- 69.Hudson G, Nalls M, Evans JR, Breen DP, Winder-Rhodes S, Morrison KE, Morris HR, Williams-Gray CH, Barker RA, and Singleton AB, Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology, 2013. 80(22): p. 2042–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khusnutdinova E, Gilyazova I, Ruiz‐Pesini E, Derbeneva O, Khusainova R, Khidiyatova I, Magzhanov R, and Wallace DC, A mitochondrial etiology of neurodegenerative diseases: evidence from Parkinson’s disease. Annals of the New York Academy of Sciences, 2008. 1147(1): p. 1–20. [DOI] [PubMed] [Google Scholar]
- 71.Mehta P, Mellick GD, Rowe DB, Halliday GM, Jones MM, Manwaring N, Vandebona H, Silburn PA, Wang JJ, and Mitchell P, Mitochondrial DNA haplogroups J and K are not protective for Parkinson’s disease in the Australian community. Movement Disorders, 2009. 24(2): p. 290–292. [DOI] [PubMed] [Google Scholar]
- 72.Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, Davison J, Lewis SJ, Perry RH, and Barker R, Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Annals of neurology, 2005. 57(4): p. 564–567. [DOI] [PubMed] [Google Scholar]
- 73.Van Der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Haines JL, Koller WC, and Lyons K, Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. The American Journal of Human Genetics, 2003. 72(4): p. 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fachal L, Mosquera–Miguel A, Pastor P, Ortega–Cubero S, Lorenzo E, Oterino–Durán A, Toriello M, Quintáns B, Camiña–Tato M, and Sesar A, No evidence of association between common European mitochondrial DNA variants in Alzheimer, Parkinson, and migraine in the Spanish population. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 2015. 168(1): p. 54–65. [DOI] [PubMed] [Google Scholar]
- 75.Huerta C, Castro MG, Coto E, Blázquez M,Ribacoba R, Guisasola LM, Salvador C, Martínez C, Lahoz CH, and Alvarez V, Mitochondrial DNA polymorphisms and risk of Parkinson’s disease in Spanish population. Journal of the neurological sciences, 2005. 236(1): p. 49–54. [DOI] [PubMed] [Google Scholar]
- 76.Latsoudis H, Spanaki C, Chlouverakis G, and Plaitakis A, Mitochondrial DNA polymorphisms and haplogroups in Parkinson’s disease and control individuals with a similar genetic background. Journal of human genetics, 2008. 53(4): p. 349–356. [DOI] [PubMed] [Google Scholar]
- 77.Ross OA, McCormack R, Maxwell LD, Duguid RA, Quinn DJ, Barnett YA, Rea IM, El-Agnaf OM, Gibson JM, and Wallace A, mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson’s disease in the Irish. Experimental gerontology, 2003. 38(4): p. 397–405. [DOI] [PubMed] [Google Scholar]
- 78.Autere J, Moilanen JS, Finnilä S, Soini nen H, Mannermaa A, Hartikainen P, Hallikainen M, and Majamaa K, Mitochondrial DNA polymorphisms as risk factors for Parkinson’s disease and Parkinson’s disease dementi a. Human genetics, 2004. 115(1): p. 29–35. [DOI] [PubMed] [Google Scholar]
- 79.Wu H-M, Li T, Wang Z-F, Huang S-S, Shao Z-Q, Wang K, Zhong H-Q, Chen S-F, Zhang X, and Zhu J-H, Mitochondrial DNA variants modulate genetic susceptibility to Parkinson’s disease in Han Chinese. Neurobiology of disease, 2018. 114: p. 17–23. [DOI] [PubMed] [Google Scholar]
- 80.Liou C, Chuang J, Chen J, Tiao M, Wang P, Huang S, Huang T, Lee W, Weng S, and Huang P, Mitochondrial DNA variants as genetic risk factors for Parkinson disease. European journal of neurology, 2016. 23(8): p. 1289–1300. [DOI] [PubMed] [Google Scholar]
- 81.Chen C, Kuan C, Lee-Chen G-J, and Wu Y, Mitochondrial DNA polymorphisms and the risk of Parkinson’s disease in Taiwan. Journal of neural transmission, 2007. 114(8): p. 1017. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y-F, Chen W-J, Lin X-Z, Zhang Q-J, Cai J-P, Liou C-W, and Wang N, Mitochondrial DNA haplogroups and the risk of sporadic parkinson’s disease in han Chinese. Chinese medical journal, 2015. 128(13): p. 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hellwege JN, Keaton JM, Giri A, Gao X, Velez Edwards DR, and Edwards TL, Population stratification in genetic association studies. Current protocols in human genetics, 2017. 95(1): p. 1.22. 1–1.22. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Otaegui D, Paisan C, Saenz A, Marti I, Ribate M, Marti-Masso J, Perez-Tur J, and de Munain AL, Mitochondrial polymporphisms in Parkinson’s Disease. Neuroscience letters, 2004. 370(2–3): p. 171–174. [DOI] [PubMed] [Google Scholar]
- 85.Salas A and Elson JL, Mitochondrial DNA as a risk factor for false positives in case-control association studies. Journal of Genetics and Genomics, 2015. 42(4): p. 169–172. [DOI] [PubMed] [Google Scholar]
- 86.Salas A, Fachal L, Marcos-Alonso S, Vega A, Martinón-Torres F, and ESIGEM G.d.i., Investigating the role of mitochondrial haplogroups in genetic predisposition to meningococcal disease. PLoS One, 2009. 4(12): p. e8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pardo-Seco J, Amigo J, González-Manteiga W,and Salas A, A generalized model to estimate the statistical power in mitochondrial disease studies involving 2× k tables. PloS one, 2013. 8(9): p. e73567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samuels DC, Carothers AD, Horton R, and Chinnery PF, The power to detect disease associations with mitochondrial DNA haplogroups. The American Journal of Human Genetics, 2006. 78(4): p. 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin MT, Simon DK, Ahn CH, Kim LM, and Beal MF, High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Human molecular genetics, 2002. 11(2): p. 133–145. [DOI] [PubMed] [Google Scholar]
- 90.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, and Klopstock T, High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature genetics, 2006. 38(5): p. 515. [DOI] [PubMed] [Google Scholar]
- 91.Arnheim N and Cortopassi G, Deleterious mitochondrial DNA mutations accumulate in aging human tissues. Mutation Research DNAging, 1992. 275(3–6): p. 157–167. [DOI] [PubMed] [Google Scholar]
- 92.Elson J, Samuels D, Turnbull D, and Chinnery P, Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. The American Journal of Human Genetics, 2001. 68(3): p. 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chinnery PF and Hudson G, Mitochondrial genetics. British medical bulletin, 2013. 106(1): p. 135–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coxhead J, Kurzawa-Akanbi M, Hussain R, Pyle A, Chinnery P, and Hudson G, Somatic mtDNA variation is an important component of Parkinson’s disease. Neurobiology of aging, 2016. 38: p. 217.e1–217.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzberger T, Lees AJ, Betensky RA, Beal MF, and Simon DK, Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Annals of neurology, 2012. 71(6): p. 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dölle C, Flønes I, Nido GS, Miletic H, Osuagwu N, Kristoffersen S, Lilleng PK, Larsen JP, Tysnes O-B, and Haugarvoll K, Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nature communications, 2016. 7: p. 13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simon DK, Lin MT, Zheng L, Liu G-J, Ahn CH, Kim LM, Mauck WM, Twu F, Beal MF, and Johns DR, Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson’s disease. Neurobiology of aging, 2004. 25(1): p. 71–81. [DOI] [PubMed] [Google Scholar]
- 98.Wei W, Keogh MJ, Wilson I, Coxhead J, Ryan S, Rollinson S, Griffin H, Kurzawa-Akanbi M, Santibanez-Koref M, and Talbot K, Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human brains. Acta neuropathologica communications, 2017. 5(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, and Khrapko K, Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nature genetics, 2006. 38(5): p. 518. [DOI] [PubMed] [Google Scholar]
- 100.Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, Lightowlers RN, and Turnbull DM, Nature of mitochondrial DNA deletions in substantia nigra neurons. The American Journal of Human Genetics, 2008. 82(1): p. 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nido GS, Dölle C, Flønes I, Tuppen HA, Alves G, Tysnes O-B, Haugarvoll K, and Tzoulis C, Ultradeep mapping of neuronal mitochondrial deletions in Parkinson’s disease. Neurobiology of aging, 2018. 63: p. 120–127. [DOI] [PubMed] [Google Scholar]
- 102.Nissanka N, Minczuk M, and Moraes CT, Mechanisms of Mitochondrial DNA Deletion Formation. Trends in Genetics, 2019. [DOI] [PubMed]
- 103.Neuhaus JFG, Baris OR, Hess S, Moser N, Schröder H, Chinta SJ, Andersen JK, Kloppenburg P, and Wiesner RJ, Catecholamine metabolism drives generation of mitochondrial DNA deletions in dopaminergic neurons. Brain, 2013. 137(2): p. 354–365. [DOI] [PubMed] [Google Scholar]
- 104.Halliwell B and Gutteridge J, Role of iron in oxygen radical reactions. Methods in enzymology, 1984. 105: p. 47. [DOI] [PubMed] [Google Scholar]
- 105.Shokolenko I, Venediktova N, Bochkareva A, Wilson G, and Alexeyev M, Oxidative stress induces degradation of mitochondrial DNA. Nucleic acids research, 2009. 37(8): p. 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bender A, Schwarzkopf R-M, McMillan A, Krishnan KJ, Rieder G, Neumann M, Elstner M, Turnbull DM, and Klopstock T, Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. Journal of neurology, 2008. 255(8): p. 1231–1235. [DOI] [PubMed] [Google Scholar]
- 107.Ikebe S. i., Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, and Ozawa T, Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence. Biochemical and biophysical research communications, 1990. 170(3): p. 1044–1048. [DOI] [PubMed] [Google Scholar]
- 108.Kösel S, Egensperger R, Schnopp NM, and Graeber MB, The ‘common deletion’is not increased in parkinsonian substantia nigra as shown by competitive polymerase chain reaction. Movement disorders: official journal of the Movement Disorder Society, 1997. 12(5): p. 639–645. [DOI] [PubMed] [Google Scholar]
- 109.Mann VM, Cooper JM, and Schapira AH, Quantitation of a mitochondrial DNA deletion in Parkinson’s disease. FEBS letters, 1992. 299(3): p. 218–222. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, Montine T, Smith M, Siedlak S, Gu G, Robertson D, and Perry G, The mitochondrial common deletion in Parkinson’s disease and related movement disorders. Parkinsonism & related disorders, 2002. 8(3): p. 165–170. [DOI] [PubMed] [Google Scholar]
- 111.Ozawa T, Tanaka M, Ikebe S.-i., Ohno K, Kondo T, and Mizuno Y, Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum by a kinetic PCR analysis. Biochemical and biophysical research communications, 1990. 172(2): p. 483–489. [DOI] [PubMed] [Google Scholar]
- 112.Gu G, Reyes PF, Golden GT, Woltjer RL, Hulette C, Montine TJ, and Zhang J, Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. Journal of Neuropathology & Experimental Neurology, 2002. 61(7): p. 634–639. [DOI] [PubMed] [Google Scholar]
- 113.Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, and Turnbull DM, Mitochondrial DNA Depletion in Respiratory Chain–Deficient Parkinson Disease Neurons. Annals of neurology, 2016. 79(3): p. 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bury AG, Pyle A, Elson JL, Greaves L, Morris CM, Hudson G, and Pienaar IS, Mitochondrial DNA changes in pedunculopontine cholinergic neurons in Parkinson disease. Annals of neurology, 2017. 82(6): p. 1016–1021. [DOI] [PubMed] [Google Scholar]
- 115.Suomalainen A and Isohanni P, Mitochondrial DNA depletion syndromes–many genes, common mechanisms. Neuromuscular Disorders, 2010. 20(7): p. 429–437. [DOI] [PubMed] [Google Scholar]
- 116.El-Hattab AW and Scaglia F, Mitochondrial DNA depletion syndromes: review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics, 2013. 10(2): p. 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, and Hudson G, Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiology of aging, 2016. 38: p. 216. e7–216. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gui Y-X, Xu Z-P, Lv W, Zhao J-J, and Hu X-Y, Evidence for polymerase gamma, POLG1 variation in reduced mitochondrial DNA copy number in Parkinson’s disease. Parkinsonism & related disorders, 2015. 21(3): p. 282–286. [DOI] [PubMed] [Google Scholar]
- 119.Pyle A, Brennan R, Kurzawa-Akanbi M, Yarnall A, Thouin A, Mollenhauer B, Burn D, Chinnery PF, and Hudson G, Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early□stage Parkinson’s disease. Annals of neurology, 2015. 78(6): p. 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moraes CT, Atencio DP, Oca-Cossio J, and Diaz F, Techniques and pitfalls in the detection of pathogenic mitochondrial DNA mutations. The Journal of molecular diagnostics, 2003. 5(4): p. 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pareek CS, Smoczynski R, and Tretyn A, Sequencing technologies and genome sequencing. Journal of applied genetics, 2011. 52(4): p. 413–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greaves LC, Beadle NE, Taylor GA, Commane D, Mathers JC, Khrapko K, and Turnbull DM, Quantification of mitochondrial DNA mutation load. Aging cell, 2009. 8(5): p. 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang W, Cui H, and Wong L-JC, Comprehensive one-step molecular analyses of mitochondrial genome by massively parallel sequencing. Clinical chemistry, 2012. 58(9): p. 1322–1331. [DOI] [PubMed] [Google Scholar]
- 124.Ye F, Samuels DC, Clark T, and Guo Y, High-throughput sequencing in mitochondrial DNA research. Mitochondrion, 2014. 17: p. 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weissensteiner H, Forer L, Fuchsberger C, Schöpf B, Kloss-Brandstätter A, Specht G, Kronenberg F, and Schönherr S, mtDNA-Server: next-generation sequencing data analysis of human mitochondrial DNA in the cloud. Nucleic acids research, 2016. 44(W1): p. W64–W69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bris C, Goudenege D, Desquiret-Dumas V, Charif M, Colin E, Bonneau D, Bonneau P, Lenaers G, Reynier P, and Procaccio V, Bioinformatics tools and databases to assess the pathogenicity of mitochondrial DNA variants in the field of next generation sequencing. Frontiers in genetics, 2018. 9: p. 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Castellana S, Fusilli C, Mazzoccoli G, Biagini T, Capocefalo D, Carella M, Vescovi AL, and Mazza T, High-confidence assessment of functional impact of human mitochondrial non-synonymous genome variations by APOGEE. PLoS computational biology, 2017. 13(6): p. e1005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Calabrese C, Simone D, Diroma MA, Santorsola M, Gutta C, Gasparre G, Picardi E, Pesole G, and Attimonelli M, MToolBox: a highly automated pipeline for heteroplasmy annotation and prioritization analysis of human mitochondrial variants in high-throughput sequencing. Bioinformatics, 2014. 30(21): p. 3115–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sonney S, Leipzig J, Lott MT, Zhang S, Procaccio V, Wallace DC, and Sondheimer N, Predicting the pathogenicity of novel variants in mitochondrial tRNA with MitoTIP. PLoS computational biology, 2017. 13(12): p. e1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goudenège D, Bris C, Hoffmann V, Desquiret-Dumas V, Jardel C, Rucheton B, Bannwarth S, Paquis-Flucklinger V, Lebre AS, and Colin E, eKLIPse: a sensitive tool for the detection and quantification of mitochondrial DNA deletions from next-generation sequencing data. Genetics in Medicine, 2018: p. 1. [DOI] [PubMed]
- 131.Samuels DC, Li C, Li B, Song Z, Torstenson E, Clay HB, Rokas A, Thornton-Wells TA, Moore JH, and Hughes TM, Recurrent tissue-specific mtDNA mutations are common in humans. PLoS genetics, 2013. 9(11): p. e1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M, Schönberg A, Schaefer M, Schroede r R, Nasidze I, and Stoneking M, Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. The American Journal of Human Genetics, 2010. 87(2): p. 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stoneking M, Hypervariable sites in the mtDNA control region are mutational hotspots. The American Journal of Human Genetics, 2000. 67(4): p. 1029–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brichta L and Greengard P, Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Frontiers in neuroanatomy, 2014. 8: p. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veltri KL, Espiritu M, and Singh G, Distinct genomic copy number in mitochondria of different mammalian organs. Journal of cellular physiology, 1990. 143(1): p. 160–164. [DOI] [PubMed] [Google Scholar]
- 136.Pyle A, Burn DJ, Gordon C, Swan C, Chinnery PF, and Baudouin SV, Fall in circulating mononuclear cell mitochondrial DNA content in human sepsis. Intensive Care Medicine, 2010. 36(6): p. 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cantuti-Castelvetri I, Lin MT, Zheng K, Keller-McGandy CE, Betensky RA, Johns DR, Beal MF, Standaert DG, and Simon DK, Somatic mitochondrial DNA mutations in single neurons and glia. Neurobiology of aging, 2005. 26(10): p. 1343–1355. [DOI] [PubMed] [Google Scholar]
- 138.Damier P, Hirsch E, Agid Y, and Graybiel A, The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain, 1999. 122(8): p. 1437–1448. [DOI] [PubMed] [Google Scholar]
- 139.Simon DK, Matott JC, Espinosa J, and Abraham NA, Mitochondrial DNA mutations in Parkinson’s disease brain. Acta neuropathologica communications, 2017. 5(1): p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, and Munafò MR, Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 2013. 14(5): p. 365. [DOI] [PubMed] [Google Scholar]
- 141.Herrnstadt C and Howell N, An evolutionary perspective on pathogenic mtDNA mutations: haplogroup associations of clinical disorders. Mitochondrion, 2004. 4(5): p. 791–798. [DOI] [PubMed] [Google Scholar]
- 142.Raule N, Sevini F, Santoro A, Altilia S, and Franceschi C, Association studies on human mitochondrial DNA: methodological aspects and results in the most common age-related diseases. Mitochondrion, 2007. 7(1): p. 29–38. [DOI] [PubMed] [Google Scholar]
- 143.Giannoccaro MP, La Morgia C, Rizzo G, and Carelli V, Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Movement Disorders, 2017. [DOI] [PubMed]
- 144.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, and Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature neuroscience, 2000. 3(12): p. 1301. [DOI] [PubMed] [Google Scholar]
- 145.Strobbe D, Caporali L, Iommarini L, Maresca A, Montopoli M, Martinuzzi A, Achilli A, Olivieri A, Torroni A, and Carelli V, Haplogroup J mitogenomes are the most sensitive to the pesticide rotenone: Relevance for human diseases. Neurobiology of disease, 2018. 114: p. 129–139. [DOI] [PubMed] [Google Scholar]
- 146.Pienaar IS, Howell N, and Elson JL, MutPred mutational load analysis shows mildly deleterious mitochondrial DNA variants are not more prevalent in Alzheimer’s patients, but may be under-represented in healthy older individuals. Mitochondrion, 2017. 34: p. 141–146. [DOI] [PubMed] [Google Scholar]
- 147.Venter M, Malan L, Van Dyk E, Elson JL, and Van der Westhuizen FH, Using MutPred derived mtDNA load scores to evaluate mtDNA variation in hypertension and diabetes in a two-population cohort: The SABPA study. Journal of genetics and genomics, 2017. 44(3): p. 139–149. [DOI] [PubMed] [Google Scholar]
- 148.Venter M, Malan L, Elson J, and van der Westhuizen F, Implementing a new variant load model to investigate the role of mtDNA in oxidative stress and inflammation in a bi-ethnic cohort: the SABPA study. Mitochondrial DNA. Part A, DNA mapping, sequencing, and analysis, 2019: p. 1. [DOI] [PubMed]
- 149.Guyatt AL, Brennan RR, Burrows K, Guthrie PA, Ascione R, Ring SM, Gaunt TR, Pyle A, Cordell HJ, and Lawlor DA, A genome-wide association study of mitochondrial DNA copy number in two population-based cohorts. Human genomics, 2019. 13(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, Nijtmans L, Nedergaard J, Cannon B, Larsson N-G, and Trifunovic A, Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell metabolism, 2009. 10(2): p. 131–138. [DOI] [PubMed] [Google Scholar]
- 151.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, and Wibom R, Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature, 2004. 429(6990): p. 417. [DOI] [PubMed] [Google Scholar]
- 152.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, and Loeb LA, DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nature genetics, 2008. 40(4): p. 392. [DOI] [PubMed] [Google Scholar]
- 153.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, and Larsson N-G, Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proceedings of the National Academy of Sciences, 2005. 102(50): p. 17993–17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barbeau A, Etiology of Parkinson’s disease: a research strategy. Canadian journal of neurological sciences, 1984. 11(1): p. 24–28. [DOI] [PubMed] [Google Scholar]
- 155.Collier TJ, Kanaan NM, and Kordower JH, Aging and Parkinson’s disease: different sides of the same coin? Movement Disorders, 2017. 32(7): p. 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, and Trifunovic A, Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proceedings of the National Academy of Sciences, 2007. 104(4): p. 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peeva V, Blei D, Trombly G, Corsi S, Szukszto MJ, Rebelo-Guiomar P, Gammage PA, Kudin AP, Becker C, and Altmüller J, Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nature communications, 2018. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim SJ, Xiao J, Wan J, Cohen P, and Yen K, Mitochondrially derived peptides as novel regulators of metabolism. The Journal of physiology, 2017. 595(21): p. 6613–6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee C, Yen K, and Cohen P, Humanin: a harbinger of mitochondrial-derived peptides? Trends in Endocrinology & Metabolism, 2013. 24(5): p. 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee C, Nuclear transcriptional regulation by mitochondrial-encoded MOTS-c. Molecular & Cellular Oncology, 2019. [DOI] [PMC free article] [PubMed]
- 161.Swerdlow RH, Parks JK, Davis JN, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, and Wooten GF, Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Annals of neurology, 1998. 44(6): p. 873–881. [DOI] [PubMed] [Google Scholar]
- 162.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP Jr, Miller SW, Davis RE, and Parker WD Jr, Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Experimental neurology, 2000. 162(1): p. 37–50. [DOI] [PubMed] [Google Scholar]
- 163.Esteves AR, Arduíno DM, Swerdlow RH, Oliveira CR, and Cardoso SM, Oxidative stress involvement in α-synuclein oligomerization in Parkinson’s disease cybrids. Antioxidants & Redox Signaling, 2009. 11(3): p. 439–448. [DOI] [PubMed] [Google Scholar]
- 164.Gu M, Cooper J, Taanman J, and Schapira A, Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Annals of neurology, 1998. 44(2): p. 177–186. [DOI] [PubMed] [Google Scholar]
- 165.Esteves AR, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons KE, Pahwa R, Burns JM, and Cardoso SM, Mitochondrial respiration and respiration □ associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. Journal of neurochemistry, 2010. 113(3): p. 674–682. [DOI] [PubMed] [Google Scholar]
- 166.Esteves AR, Arduíno DM, Swerdlow RH, Oliveira CR, and Cardoso SM, Dysfunctional mitochondria uphold calpain activation: contribution to Parkinson’s disease pathology. Neurobiology of disease, 2010. 37(3): p. 723–730. [DOI] [PubMed] [Google Scholar]
- 167.Sheehan J, Swerdlow R, Parker W, Miller S, Davis R, and Tuttle J, Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. Journal of neurochemistry, 1997. 68(3): p. 1221–1233. [DOI] [PubMed] [Google Scholar]
- 168.Esteves AR, Arduíno DM, Swerdlow RH, Oliveira C, and Cardoso SM, Microtubule depolymerization potentiates alpha-synuclein oligomerization. Frontiers in aging neuroscience, 2010. 1: p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Onyango IG, Tuttle JB, and Bennett JP Jr, Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson’s disease cybrids. Molecular and Cellular Neuroscience, 2005. 28(3): p. 452–461. [DOI] [PubMed] [Google Scholar]
- 170.Onyango IG, Tuttle JB, and Bennett JP Jr, Brain-derived growth factor and glial cell line-derived growth factor use distinct intracellular signaling pathways to protect PD cybrids from H2O2-induced neuronal death. Neurobiology of disease, 2005. 20(1): p. 141–154. [DOI] [PubMed] [Google Scholar]
- 171.Borland MK, Mohanakumar K, Rubinstein JD, Keeney PM, Xie J, Capaldi R, Dunham LD, Trimmer PA, and Bennett JP Jr, Relationships among molecular genetic and respiratory properties of Parkinson’s disease cybrid cells show similarities to Parkinson’s brain tissues. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 2009. 1792(1): p. 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trimmer PA, Borland MK, Keeney PM, Bennett JP, and Parker WD, Parkinson’s disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. Journal of neurochemistry, 2004. 88(4): p. 800–812. [DOI] [PubMed] [Google Scholar]
- 173.Chu Q, Luo X, Zhan X, Ren Y, and Pang H, Female genetic distribution bias in mitochondrial genome observed in Parkinson’s Disease patients in northern China. Scientific reports, 2015. 5: p. 17170–17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flow diagram summarizing the limitations and challenges of published studies investigating mtDNA variation in Parkinson’s disease as well as directions for future studies in this field. MDPs = mitochondrial derived peptides; mtDNA = mitochondrial DNA; nDNA = nuclear DNA; SNPs = single nucleotide polymorphisms
Supplementary Table 1. MITOMAP summary of mtDNA variants from early mtDNA sequencing studies and haplogroup association studies in Parkinson’s disease
Supplementary Table 2. Detailed summary of mtDNA haplogroup association studies in Parkinson’s disease
Supplementary Table 3. Detailed summary of somatic mtDNA sequencing studies in Parkinson’s disease