Abstract
The Scarr-Rowe hypothesis predicts that the heritability of cognitive abilities is higher in more privileged socioeconomic conditions, meaning that genetic potential can be more fully expressed in environments characterized by high socioeconomic status (SES) compared to low SES. This gene × SES interaction, however, has been replicated mostly in the United States, but not in other Western nations like the United Kingdom. In the current study, we tested the interaction between childhood SES and the heritability of cognitive ability in 3,074 German twin pairs comprising three age cohorts at different developmental stages (mean ages of 11, 17, and 23 years). Higher SES was associated with significantly higher mean cognitive ability scores in the two younger cohorts, with reduced variances at higher SES levels. Results further support the Scarr-Rowe hypothesis in middle childhood, and to some degree in adolescence, but not in adulthood. This indicates that the role of family SES as a moderator of the heritability of cognitive ability changes as children grow older. Moreover, children’s shared experiences appear to be explain more variance in cognitive ability at the lower end of the SES distribution in middle childhood and adolescence.
Keywords: gene-environment interaction, cognitive ability, socioeconomic status, TwinLife, modified twin correlation model
Introduction
In the average Western population - where individuals differ with respect to environmental resources and educational experiences - cognitive abilities (e.g., verbal abilities, visuospatial skills, attention, and memory) are heritable (Kan, Wicherts, Dolan, & van der Maas, 2013; Tucker-Drob, Briley, & Harden, 2013). Extant research also suggests that the magnitude of the genetic variance in cognitive ability depends upon other variables (moderators), such as age or socioeconomic status (SES). Although the general trend for age, that is increasing genetic variance from early childhood to adulthood (e.g., Deary, Spinath, & Bates, 2006; Tucker-Drob et al., 2013), has been well replicated, the findings for SES are more diverse.
One prominent hypothesis, known as the Scarr-Rowe hypothesis of gene × SES interaction, predicts that the heritability of cognitive abilities is higher in privileged socioeconomic conditions (Turkheimer, Harden, D’Onofrio, & Gottesman, 2009). The effect was first observed by Sandra Scarr in a sample of twins from the Philadelphia school system in 1971 (Scarr-Salapatek 1971), the finding was then replicated in Sweden by Fischbein (1980), and the United States by Rowe and colleagues (Rowe, Jacobson, & Van den Oord, 1999). The underlying assumption is that enriched environments provide more opportunities to match one’s genotype, which implies that the heritable potential for cognitive abilities is more fully expressed in these contexts (Bronfenbrenner & Ceci, 1994; Fischbein, 1980). Enriched environments not only encompass high levels of material resources, human, and social capital (Bradley & Corwyn, 2002), but are also assumed to provide more enduring forms of social interactions between children and caretakers (i.e., proximal processes; Bornstein & Bradley, 2003; Guo & Stearns, 2002). Scarr (1992) also assumed that, above a certain threshold in the range of environmental quality, the environment would be ‘good enough’, and consequently would not exert a meaningful influence for explaining phenotypic variance.
Gene × SES interactions that lead to greater genetic variance at the higher end of the SES distribution have been identified in (early) childhood (Rhemtulla & Tucker-Drob, 2012; Scarr-Salapatek, 1971; Tucker-Drob, Rhemtulla, Harden, Turkheimer, & Fask, 2011; Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003), adolescence (Fischbein, 1980; Harden, Turkheimer, & Loehlin, 2007; Kirkpatrick, McGue, & Iacono, 2015; Rowe et al.,1999; Turkheimer, Beam, Sundet, & Tambs, 2017), and adulthood (Bates, Lewis, & Weiss, 2013; Zavala et al., in press) with the vast majority of samples from the U.S. However, the Scarr-Rowe interaction has not always been replicated in both, U.S. and non-U.S. samples. A number of studies reported non-significant or null findings in samples from the U.S. (Grant et al., 2010; Kremen et al., 2005), the U.K. (Hanscombe et al., 2012), the Netherlands (Bartels, van Beijsterveldt, & Boomsma, 2009; van der Sluis, Willemsen, de Geus, Boomsma, & Posthuma, 2008), and Australia (Bates, Hansell, Martin, & Wright, 2016). Also, a recent study based on data from Florida did not find evidence of SES moderation of genetic influences on test scores (Figlio, Freese, Karbownik, & Roth, 2017). However, without knowledge of zygosity, this latter study had to rely on matched birth and school records from twins and siblings. A recent study investigated parental education as a moderator of genetic and environmental effects on verbal and nonverbal cognitive abilities in a German sample of young twins (Spengler et al., 2018). This study only revealed a significant nonshared environmental x parental education interaction for verbal abilities. However, parental education as a proxy of SES might not adequately reflect the socioeconomic conditions in Germany, as discussed by the authors (Spengler et al., 2018). Other than gene × SES interactions, some studies also report significant environmental interactions of SES with shared-environmental variance being lower at higher levels of SES especially in young samples (Tucker-Drob et al., 2011; Turkheimer et al., 2003).
With a recent meta-analysis suggesting that a gene × SES interaction can reliably be found in U.S. samples but not in other Western nations (Turkheimer et al., 2017; Tucker-Drob & Bates, 2016), the question of why these differing findings among studies occur has gained new prominence. Potential factors may be the age of the participants, the operationalization of cognitive abilities and SES, or both. Yet, Tucker-Drob and Bates (2016) did not observe evidence for such a moderation in their meta-analysis. Other explanations focus on differences in the magnitude of socioeconomic disparities within a country (e.g., Nisbett et al., 2012; Tucker-Drob & Bates, 2016), the educational system, or the access to high quality education.
A third possible factor is statistical methodology. Turkheimer and colleagues (2017) have put a possible methodological explanation forward that might explain some of the null findings. In a sample of Norwegian conscripts, the authors used a re-parameterization of the standard Purcell (2002) model, which is most often used to test gene × environment interactions, and reported a meaningful gene × parental education interaction on the heritability of cognitive abilities. Turkheimer et al. (2017) argue that moderation models often imply values for the DZ correlation that are less than half the MZ correlation, producing negative shared environmental variances that violate the classical twin model. Therefore, the modified twin-correlation model (MTCM; Turkheimer et al., 2017) focuses on the twin correlations rather than the additive genetic and environmental parameters (e.g., variances) derived from them.
In the present study, we extend the existing literature by testing for gene × SES interaction effects in a large representative sample of German twins in childhood (age 11), adolescence (age 17) and young adulthood (age 23) who were assessed with the same cognitive test battery. This cohort design also allows us to investigate differences in the gene × SES interaction on cognitive abilities depending on the respective developmental stage.
Method
Participants
The sample consisted of data from the first assessment of the German twin family study, TwinLife, a longitudinal cross-sequential study comprising a total of four cohorts of same-sex twins and their families (Diewald et al., 2016; Hahn et al., 2016; Lang & Kottwitz, 2017). The cohorts each consist of approximately 1,000 pairs of monozygotic (MZ) and dizygotic (DZ) twins (on average, twins were five, 11, 17, and 23 years of age at the first measurement occasion in 2014–15). Particular attention was placed on the sampling procedure to achieve a high representativeness of TwinLife compared to the German population. Lang and Kottwitz (2017) report high comparability with the German Microcensus Survey conducted by the Federal Statistical Office (Destatis, 2014a, 2014b) regarding key socio-demographic indicators (e.g., region of residence, community size, citizenship status, income and occupational status). Solely low-income groups seem to be slightly underrepresented in TwinLife, especially in cohort 1 (which was not included in the present study). It has also been reported that participation in TwinLife is to some extent selective with respect to parental education (Lang & Kottwitz, 2017). However, compared to other studies, these restrictions can be evaluated as rather minor. All families provided informed consent prior to their participation. The procedures, protocol, and informed consent were approved by the ethic committee of the German Research Foundation (DGPS).
For the present analysis, we used data from cohort 2 (Mage = 10.99, SD = 0.33), cohort 3 (Mage = 16.99, SD = 0.35), and cohort 4 (Mage = 23.04, SD = 0.85) because these age groups were tested with the same cognitive test battery. We excluded families with triplets (n = 11 pairs), with missing zygosity information (n = 7 pairs), and with missing cognitive test data (n = 6 pairs). The final sample comprised a total of 3,074 twin pairs (1,441 MZs, 1,633 DZs; see Table 1). For the present analyses, we only included families in which both twins were still living in the household with at least one parent to ensure that SES was the same for both members of a pair, resulting in a reduced number of available pairs especially in cohort 4 (see Table 1).
Table 1.
Sample statistics
| Cohort 2 | Cohort 3 | Cohort 4 | |
|---|---|---|---|
| Sample characteristics | |||
| Pairs total | 1,036 | 1,058 | 980 |
| MZ twin pairs (% male) | 420 (45.5%) | 498 (43.8%) | 523 (40.5%) |
| DZ twin pairs (% male) | 616 (49.8%) | 560 (41.8%) | 457 (43.3%) |
| Included pairs1 | 1,035 | 1,043 | 540 |
| Age twins (SD) | 10.99 (0.33) | 16.99 (0.35) | 23.04 (0.82) |
| Age mothers (SD) | 42.94 (4.99) | 47.68 (4.60) | 52.45 (4.97) |
| Age fothers (SD) | 46.54 (5.36) | 50.20 (5.20) | 55.33 (5.51) |
Notes. MZ = monozygotic, DZ = dizygotic, SD = standard deviation. The percentage of male twin pairs relates to the total number of MZ, and DZ twin pairs, respectively;
Families in which both twins were living with at least one parent; excluded cases did not differ significantly from included cases in terms of the distribution of the SES indicators and cognitive abilities.
Measures
Zygosity.
Zygosity was established via physical similarity questionnaires (e.g., eye color, hair structure, time of getting first teeth), typically yielding accuracies around 95% compared to DNA genotyping (e.g., Heath et al., 2003). The Zygosity Questionnaire for Young Twins (Goldsmith, 1991) was administered in parent-report form in the two youngest birth cohorts in TwinLife, while the Self-Report Zygosity Questionnaire (Oniszczenko, Angleitner, Strelau, & Angert, 1993) was used for adolescent and young adult twins. In addition, DNA samples of n = 328 twin pairs were collected via buccal swabs to validate the results of the zygosity questionnaires. This procedure yielded correct classification rates of 97% for the parent-report, and 92% for the self-report similarity questionnaire (Lenau et al., 2017).
Cognitive ability.
Non-verbal (fluid) intelligence was assessed based on the subtests “figural reasoning”, “figural classification”, “matrices”, and “reasoning” of the Grundintelligenztestskala 2 (CFT 20-R [Culture Fair Intelligence Test], Revision; Weiß, 2006). All tests were computer-administered with a standardized test time of five minutes for the subtests “figural reasoning”, and “figural classification”, and four minutes for the subtests “matrices”, and “reasoning”. The first three tests each encompassed 15 items, while “reasoning” encompassed 11 items. The reliability and validity of the CFT 20-R is well established in German samples of children and adults with different educational background and across different age groups (Weiß, 2006). A detailed description of the TwinLife cognitive testing procedure is available in (Gottschling, 2017).
We winsorized the subtest scores prior to analyses to reduce the risk of significant results caused by outliers (i.e., scores of six cases in cohort 2, five cases in cohort 3, and two cases in cohort 4 were rescored to equal the next highest value; Sheskin, 2011). The subtests showed a significant positive manifold of correlations ranging between .24 to .59, with generally higher correlations in the older cohorts (average of .36 in cohort 2, .47 in cohort 3, and .51 in cohort 4). We applied a confirmatory factor analysis (CFA) approach to raw data with the four subtests as indicators of a latent general (g) factor of cognitive ability. The model fit evaluated based on the standard criteria χ2, and RMSEA was excellent in all three cohorts (see Table 2).
Table 2.
Fit Statistics for CFA models of cognitive ability and family SES
| Fit indices | Unstandardized parameter estimates (p) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | χ2 (df) | P | RMSE A [95% CI] | AIC | ||||
| Cohort 2 | 6.396 (2) | .041 | .033 [.006; .063] | 35992.773 | 1.539 (.000) | 1.548 (.000) | 2.137 (.000) | 0.919 (.000) |
| Cohort 3 | 7.854 (2) | .020 | .037 [.013; .066] | 35793.681 | 1.605 (.000) | 1.570 (.000) | 1.895 (.000) | 1.431 (.000) |
| Cohort 4 | 2.799 (2) | .247 | .014 [.000; .050] | 33455.941 | 1.787 (.000) | 1.857 (.000) | 1.963 (.000) | 1.470 (.000) |
| Socioecomic status | ISCED | ISEI | EGP | Income | ||||
| Cohort 2 | 15.288 (2) | .000 | .057 [.003; .085] | 19113.147 | .708 (.000) | .945 (.000) | .719 (.000) | .612 (.000) |
| Cohort 3 | 48.292 (2) | .000 | .104 [.070; .131] | 19359.299 | .652 (.000) | .983 (.000) | .700 (.000) | .514 (.000) |
| Cohort 4 | 66.037 (2) | .000 | .063 [.051; .077] | 74495.052 | .673 (.000) | .967 (.000) | .719 (.000) | .504 (.000) |
Socioeconomic status.
We used three different indicators available in TwinLife in addition to income to operationalize family SES: Parental education was scaled by the International Standard Classification of Education (ISCED; Schneider, 2008). Parental occupation was measured by the International Socioeconomic Index of Occupational Status (ISEI; Ganzeboom, De Graaf, & Treiman, 1992), and the Erikson-Goldthorpe-Portocarero Class Schedule (EGP; Erikson, Goldthorpe, & Portocarero, 1979). ISCED, ISEI and EGP were determined for each parent. The corresponding status of the children was set to the maximum of their maternal and paternal value for each pair of twins. Family net income (i.e., all the money available per month including e.g. state benefits) was operationalized according to the “Organization for Economic Cooperation and Development (OECD)-modified equivalence scale” (for a detailed description see Hagenaars, de Vos, & Zaidi, 1998). Since income data were based on self-reports, we carefully screened the data for inconsistencies based on available household information (e.g., government benefits, occupational status, number of working person in the household). Cases identified as implausible were implemented using Markov Chain Monte Carlo multiple imputation function, producing m = 10 imputations. The resulting income variable was winsorized and square-root transformed to better approximate normality.
The results reported in this paper are a composite factor score of these four SES indicators derived from a CFA (see Table 2). SES indicators were residualized for age of the parents.
Analytical approach
Traditional twin studies compare the trait similarity of reared together MZ and DZ twins. MZ twins share all of their genetic variation, whereas DZ twins share on average 50% of their segregating genes; MZs and DZs both share their family environment. This makes it possible to estimate the heritability by doubling the difference between the MZ and DZ correlation. The twin correlation also forms the basis to derive parameter estimates for additive genetic (A; the sum of all genetic influences), shared environmental (C; common environmental influences for twins), and nonshared environmental (E; individual specific environmental influences, also includes measurement error) variance components. The classical twin model assumes uniform genetic and environmental influences over the range of a given trait, thus making the additional (strong) assumptions that gene-environment correlation and gene-by-environment interaction are absent.
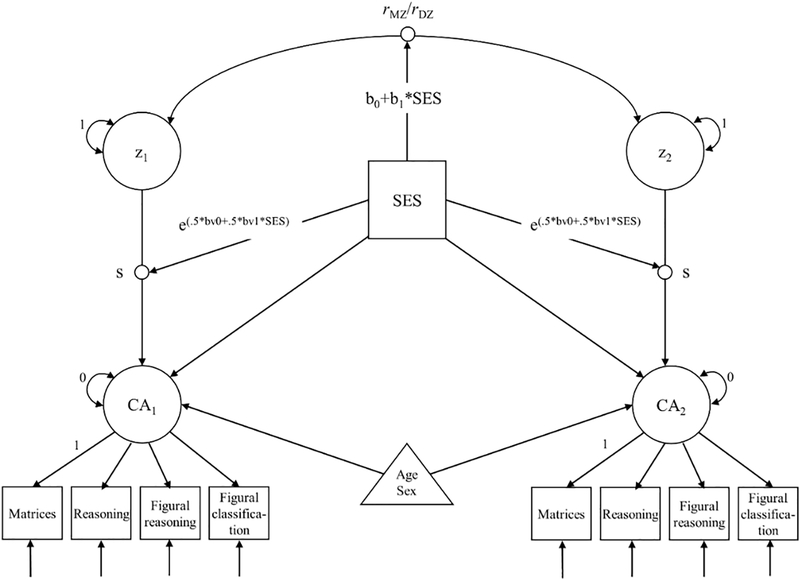
Moderating effects of family SES on the genetic and environmental architecture of cognitive abilities were tested using the MTCM (Turkheimer et al., 2017) depicted in Figure 1. The interaction models were fit on raw data in Mplus 8.0 (Muthén & Muthén, 1998-2017) using maximum likelihood estimation. The latent cognitive ability (or g) factor (denoted CA in Figure 1) was residualized for age and sex within the model to avoid inflated twin similarities and family-differences (McGue & Bouchard, 1984). The MTCM differs from the standard Purcell (2002) model in two main ways. First, the outcome variable (cognitive ability) is standardized within the model, making the raw standard deviation of the outcome available as a parameter for modeling. This allows us to test the hypothesis that the phenotypic variance of the outcome varies as a function of the moderator. Second, rather than modifying the ACE components of the outcome variable, the MTCM models linear effects on the MZ and DZ twin correlations themselves. The A and C variances, as well as their moderation, can be estimated as linear combination of the MZ and DZ twin correlations. In the model, s is the standard deviation of CA. Z is a latent variable that standardizes CA to a mean of 0 and a standard deviation of 1, so that the MZ and DZ covariances are correlations. The model also contains a main effect of family SES on twins’ CA. We then fit separate linear models of family SES to the MZ and DZ twin correlations to detect changes in twin correlations as a function of family SES and present plots of the twin correlations as a function of family SES.
Fig. 1.
Modified twin-correlation model. CA1 = twin 1 cognitive ability score, CA2 = twin 2 cognitive ability score; z1 = twin 1 latent CA score, z2 = twin 2 latent CA score; SES family SES, S random effect of latent CA on observed CA in standard deviation units; rMZ/rDZ MZ and DZ twin CA correlations, respectively, moderated by family SES. The 0.5 terms in the exponential expressions for the variance are included because s1 and s2 represent the standard deviations of GA, not the variances.
Results
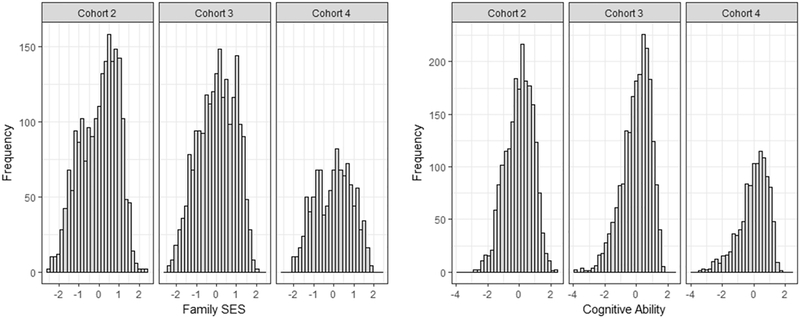
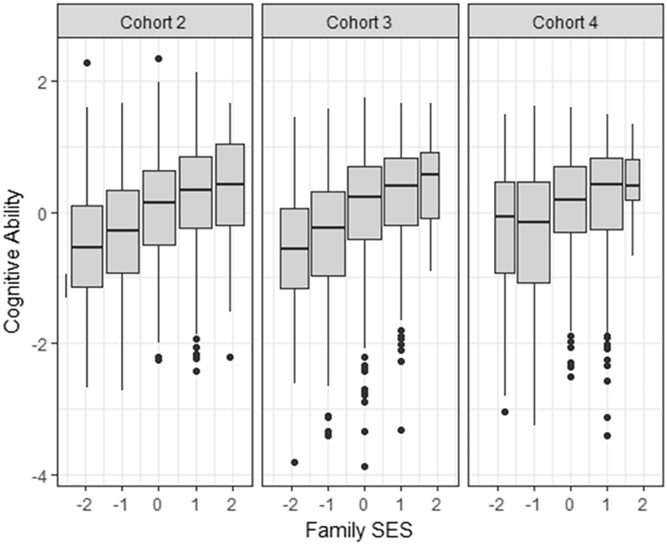
Basic statistical procedures and data preparation were conducted in R (R Core Team, 2016). The distributions of the CA latent factor scores and the family SES latent factor scores within each cohort are given in Figure 2. The skewness of the latent factor of cognitive abilities ranged between −0.32 (cohort 2) to −0.90 (cohort 4), the skewness of the latent factor of family SES ranged between −0.07 (cohort 4) to −0.34 (cohort 2). The multivariate distributions, therefore, can be assumed to be multivariate normal (Tabachnick & Fidell, 2007). Family SES and child cognitive ability showed a moderate sized relationship (r = .26, cohort 4 to r = .32, cohort 2; see Figure 3). The twin correlations for cognitive abilities were .79 (cohort 2), .84 (cohort 3), and .87 (cohort 4) in MZ twins, and .53 (cohort 2), .44 (cohort 3), and .54 (cohort 4) in DZ twins. Derived estimates for additive genetic variance were .53 in cohort 2, .81 in cohort 3, and .67 in cohort 4.
Fig. 2.
Histograms of cognitive abilities and family SES.
Fig. 3.
Box plot of cognitive ability by SES.
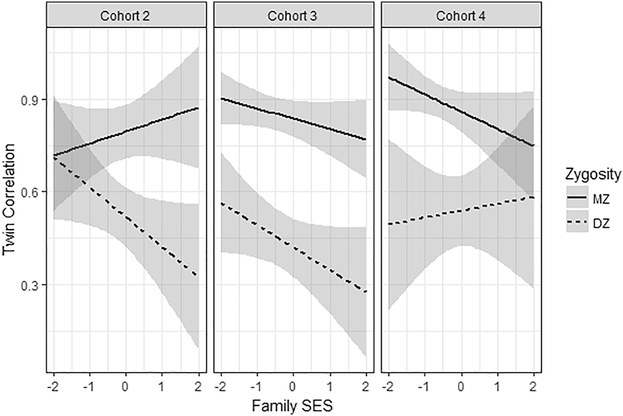
Table 3 provides the results of the MTCM. The main effect of family SES on the CA latent factor score was significant in cohorts 2 and 3. The variance of CA was significantly reduced at higher levels of family SES in all three cohorts (log(σ2CA) in Table 3). The MZ correlation in cohort 2 increased slightly with increasing family SES, whereas the DZ correlation decreased significantly [MZ: slope = 0.04 (SE = 0.04); DZ: slope = −0.10 (SE = 0.05)]. The significant positive slope of A [0.27 (SE = 0.13)], and the significant negative slope for C [−0.23 (SE = 0.18)] reflect this pattern of results. In cohort 3, both, MZ and DZ correlations showed decreases, with only the DZ slope being significant [slope = −0.08 (SE = 0.05)]. The direction of the A and C slope were the same as in cohort 2, but no longer significant in cohort 3. In cohort 4, the slope of MZ and DZ correlations both were non-significant, as were the A and C slopes. The pattern of MZ and DZ correlations across the SES continuum is illustrated in Figure 4.
Table 3.
Maximum likelihood estimates from the MTCM with family SES as moderator (significant slopes p < .05 in bold).
| Moderation by family SES | ||||||
|---|---|---|---|---|---|---|
| Main effect of family SES | log(σ2CA) | rMZ | rDZ | A slope | C slope | |
| Cohort 2 | b0 | 0.706 | 0.795 | 0.518 | ||
| (SE) | (0.080) | (0.044) | (0.049) | |||
| b1 | −0.124 | 0.039 | −0.097 | 0.272 | −0.233 | |
| (SE) 0.731 (0.115) | (0.052) | (0.043) | (0.050) | (0.130) | (0.108) | |
| Cohort 3 | b0 | 0.795 | 0.837 | 0.419 | ||
| (SE) | (0.065) | (0.029) | (0.048) | |||
| b1 | −0.250 | −0.036 | −0.079 | 0.086 | −0.122 | |
| (SE) 0.783 (0.267) | (0.051) | (0.025) | (0.045) | (0.102) | (0.093) | |
| Cohort 4 | b0 | 1.097 | 0.859 | 0.539 | ||
| (SE) | (0.084) | (0.033) | (0.058) | |||
| b1 | −0.318 | −0.056 | 0.022 | −0.156 | 0.100 | |
| (SE) 0.249 (0.340) | (0.069) | (0.034) | (0.067) | (0.148) | (0.138) | |
Notes. SE = Standard Error
Fig. 4.
Line plots of MZ and DZ correlation coefficients. Correlations modelled as a function of the intercept and slope of family SES along with Standard Errors.
Discussion
We tested for gene × SES interaction using the MTCM in three cohorts of German twins, aged 11, 17, and 23 with a reliable measure of cognitive ability. The results of the present study are noteworthy because previous studies in non-U.S. samples have not found a meaningful gene × SES interaction in middle childhood. Our results further suggest that the occurrence of a gene × SES interaction may also depend on the age of the participants under study, as the interaction was diminished in adolescence and not present in early adulthood.
We found a main effect of SES on cognitive ability scores in the two younger cohorts, accompanied by a decrease of total phenotypic variance, as is typical in European samples (e.g., Bartels et al., 2009; Hanscombe et al., 2012; Spengler et al., 2018). However, in contrast to other European samples spanning middle childhood (Bartels et al., 2009; Hanscombe et al., 2012), our results in the youngest cohort support the Scarr-Rowe hypothesis. This effect occurs because MZ correlations stay stable as a function of family SES, whereas DZ twin correlations show a significant linear decline, hence, the difference between MZs and DZs increases. This is reflected by the significant increase of A, and the significant decrease of C as a function of family SES. Interestingly, although the interaction between family SES and MZ and DZ twin correlations was not significant in early adolescence, the pattern of MZ and DZ correlations matched those reported by Turkheimer et al. (2017) in a Norwegian adolescent twin sample: both MZ and DZ correlations decline linearly as a function of family SES in cohort 3, with a steeper decline for DZ twins. We note, however, that the parameter estimates (e.g., rDZ slope, A slope, and C slope) are in the same direction in cohorts 2 and 3, suggesting some continuity of gene × SES effects from middle childhood through adolescence. The finding for the between-family (C) × SES interaction is consistent with data from other studies showing a C × SES interaction in early and middle childhood (Hanscombe et al., 2012; Tucker-Drob et al., 2011; Turkheimer et al., 2003), but typically become non-significant by adolescence (Harden et al., 2007). In the oldest cohort of early adulthood, no indication for a gene × SES interaction was found in the present data. Although the results for cohort 4 should be interpreted with caution because of the limited sample size, it is interesting to note that the MZ correlation declines over the range of SES, as is the case in cohort 3. At this point, we can only speculate about the causes of this pattern. Possible reasons might lie in a challenging process of individuation especially in MZ twins (e.g., Åkerman & Suurvee, 2003), or that, by the age of 23, young adults in Germany have already settled on a pathway, irrespective (though originally influenced) by family SES.
One prominent explanation for the differing results between U.S. studies, and studies from Europe and Australia lies in the variability of socioeconomic status, and differing educational systems in the respective societies. There are indeed some indications supporting this assumption. For one thing, the German social security system ensures a primary health and financial care which implies a higher poverty threshold compared to the U.S. In the same vein, the Gini index indicates that social disparities are more pronounced in the U.S. (Gini index of 40.64 in 2010; World Development Indicators | DataBank, 2018) than in Germany (Gini index of 31.14 in 2010; World Development Indicators | DataBank, 2018), or other Western European countries. Moreover, child poverty rates, rates of educational deprivation, and rates of children living in overcrowded home conditions are all higher in the U.S. compared to other Western nations (Chapple & Richardson, 2009), a fact that may be relevant given that the exposure to multiple risks was reported to be associated with adverse cognitive development in the U.S. (Evans, 2004; Evans & English, 2002). Another potentially meaningful difference between Germany and the U.S. lies in the differing access to high quality education, which is seemingly less dependent on social class and income in Germany. Tracking decisions at the end of elementary school (i.e. 4th grade) are based on teachers’ recommendations considering the overall achievement level of a student (Hanushek & Wössmann, 2006). This system might lower the obstacles for entry into higher educational tracks for bright, but underprivileged children.
Our analyses further indicate that family SES is a moderator of genetic variance in cognitive ability in middle childhood and possibly to some degree in adolescence (though current results were not statistically significant). Although our data is not longitudinal, this result may suggest that family level influences, including SES, diminishes as children grow older. This assumption would be compatible with the observation that the importance of the shared environment for explaining phenotypic variance decreases over time for most traits (Plomin, DeFries, Knopik, & Neiderhiser, 2013; Turkheimer, 2000). The results can be interpreted in light of ‘reciprocal effects models’ (or ‘phenotype to environment models’; see (Beam & Turkheimer, 2013; Dolan, de Kort, van Beijsterveldt, Bartels, & Boomsma, 2014). Reciprocal effects models propose that individuals will select environments suited to their ability level and that these environments in turn reinforce the individual’s ability level (Turkheimer et al., 2017). One result of this mechanism is that gene-environment correlation underlying cognitive ability - the phenomenon that environmental exposures are systematically associated with genetic differences between people - increases over time (Beam, Turkheimer, Dickens, & Davis, 2015), and also more quickly for pairs with greater within-family genetic differences (i.e., DZ pairs compared to MZ pairs). The DZ correlations in cohorts 2 and 3, thus, may have decreased with higher SES because of within-family gene-environment correlative processes, not gene-x-environment interactive processes.
The observed pattern of overall MZ and DZ twin correlations in these two different age groups, that is, stable MZ correlations and decreasing DZ correlations, is consistent with the prediction of reciprocal effects models. One implication of the current findings, then, is that the influence of SES for the development of children suggests that high SES homes can offer more opportunities to select the environment that suits the ability level of an individual (e.g., Conger, Conger, & Martin, 2010). Stronger person-environment match should accelerate the divergent development of sibling pairs with greater within-family differences (be it genetic or environmental in nature). This assumption would fit the pattern of decreasing DZ correlations across SES levels that we observed in the present sample. One easy to observe environment that would potentially mirror this reciprocal mechanism is whether twins attend the same class and/or school types. We therefore examined the frequency of same vs. different class attendance separately in MZ and DZ twins, and at different SES levels in a post-hoc analysis. The results of this analysis further support the previously made assumptions: first, the overall percentage of pairs that are not in the same class (or educational track) is not only considerably higher for DZ twins compared to MZ twins in general, but it increases with age (cohort 2: 41% MZ vs. 55% DZ; cohort 3: 56% MZ vs. 73% DZ). In addition, while the distribution of same vs. different classes/tracks across SES groups is rather equal in cohort 2, we observed a higher percentage of pairs in different classes (MZs as well as DZs) at higher levels of SES in cohort 3.
Several limitations of the present study should be noted. Although we were able to test for gene × SES interaction in three cohorts from different developmental stages, the present data are cross-sectional. Also, the sample size in cohort 4 was limited due to the chosen inclusion criteria. Given the conservative nature of gene × environment analyses (van der Sluis, Posthuma, & Dolan, 2012), large samples are required to detect interaction. Further, our results are limited to fluid cognitive abilities. Although existing evidence is mixed, some studies show differing results for abilities that are presumably more closely related to family SES (Grant et al., 2010; Spengler et al., 2018). Our results may also be limited by the fact that the ‘TwinLife’ sample is somewhat underrepresented with regard to lower SES levels compared to the German population (see Lang & Kottwitz, 2017). Further, we were not able to explicitly test for gene-environment correlations that may be present across cognitive development, given that the moderator was measured at the family level. However, modeling the main effect of the measured environment prevents biased gene × environment interactions resulting from unspecified gene-environment correlation (Purcell, 2002).
In conclusion, our results provide additional insight into how and when SES moderates the heritability of cognitive ability. Understanding the pathways and influences that increase the possibility of unfolding the full intellectual potential is of great importance. We therefore encourage future studies to also investigate more proximal factors of the home environment (e.g., family structure, parenting style, home chaos) in order to further inform our understanding of the complex interplay between genes and environments for cognitive ability.
Highlights:
Examined Scarr-Rowe interaction with the modified twin correlation model in Germany
Large cohort sample of 3,074 German twin pairs aged 11, 17, and 23
Higher SES associated with higher mean cognitive ability and less variance
Evidence for Scarr-Rowe interaction in middle childhood and adolescence
Evidence for C x SES interaction with higher C at lower SES levels
Acknowledgements
We thank the twins and their families for participation. This work was supported by the German Research Foundation (grant numbers DI 759/11–1, RI 595/8–1, SP 610/6–1); the funding from the National Institute On Aging (T32 AG000037) and the Alzheimer’s Association (AARF-17–505302); the John Templeton Foundation (58792).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åkerman BA, & Suurvee E (2003). The cognitive and identity development of twins at 16 years of age: a follow-up study of 32 twin pairs. Twin Research, 6(4), 328–333. 10.1375/twin.6.4.328 [DOI] [PubMed] [Google Scholar]
- Bartels M, van Beijsterveldt CEM, & Boomsma DI (2009). Breastfeeding, maternal education and cognitive function: a prospective study in twins. Behavior Genetics, 39(6), 616 10.1007/s10519-009-9293-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TC, Hansell NK, Martin NG, & Wright MJ (2016). When does socioeconomic status (SES) moderate the heritability of IQ? No evidence for g × SES interaction for IQ in a representative sample of 1176 Australian adolescent twin pairs. Intelligence, 56, 10–15. 10.1016/j.intell.2016.02.003 [DOI] [Google Scholar]
- Bates TC, Lewis GJ, & Weiss A (2013). Childhood socioeconomic status amplifies genetic effects on adult intelligence. Psychological Science, 24(10), 2111–2116. 10.1177/0956797613488394 [DOI] [PubMed] [Google Scholar]
- Beam CR, & Turkheimer E (2013). Phenotype–environment correlations in longitudinal twin models. Development and Psychopathology, 25(1), 7–16. 10.1017/S0954579412000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E, Dickens WT, & Davis DW (2015). Twin differentiation of cognitive ability through phenotype to environment transmission: the Louisville Twin Study. Behavior Genetics, 45(6), 622–634. 10.1007/s10519-015-9756-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, & Bradley RH (2003). Socioeconomic status, parenting, and child development Mahwah, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Bradley RH, & Corwyn RF (2002). Socioeconomic status and child development. Annual Review of Psychology, 53(1), 371–399. 10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Ceci SJ (1994). Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychological Review, 101(4), 568–586. [DOI] [PubMed] [Google Scholar]
- Chapple S, & Richardson D (2009). Doing better for children (OECD Publishing, Paris: ). Retrieved from http://www.oecd.org/els/family/43570328.pdf [Google Scholar]
- Conger RD, Conger KJ, & Martin MJ (2010). Socioeconomic status, family processes, and individual development. Journal of Marriage and Family, 72(3), 685–704. 10.1111/j.1741-3737.2010.00725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Spinath FM, & Bates TC (2006). Genetics of intelligence. European Journal of Human Genetics, 14(6), 690–700. 10.1038/sj.ejhg.5201588 [DOI] [PubMed] [Google Scholar]
- Destatis. (2014a). Bevölkerung und Erwerbstätigkeit – Haushalte und Familien: Ergebnisse des Mikrozensus 2013. Wiesbaden. [Google Scholar]
- Destatis. (2014b). Mikrozensus 2013: Qualitätsbericht. Wiesbaden. [Google Scholar]
- Diewald M, Riemann R, Spinath FM, Gottschling J, Hahn E, Kornadt AE, … Peters A-L (2016). TwinLife. GESIS Data Archive. 10.4232/1.12665 [DOI] [Google Scholar]
- Dolan CV, de Kort JM, van Beijsterveldt CEM, Bartels M, & Boomsma DI (2014). GE covariance through phenotype to environment transmission: an assessment in longitudinal twin data and application to childhood anxiety. Behavior Genetics, 44(3), 240–253. 10.1007/s10519-014-9659-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R, Goldthorpe JH, & Portocarero L (1979). Intergenerational class mobility in three western European societies: England, France and Sweden. The British Journal of Sociology, 30(4), 415–441. 10.2307/589632 [DOI] [PubMed] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59(2), 77–92. 10.1037/0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development, 73(4), 1238–1248. 10.1111/1467-8624.00469 [DOI] [PubMed] [Google Scholar]
- Figlio DN, Freese J, Karbownik K, & Roth J (2017). Socioeconomic status and genetic influences on cognitive development. Proceedings of the National Academy of Sciences, 114(51), 13441–13446. 10.1073/pnas.1708491114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbein S (1980). IQ and social class. Intelligence, 4(1), 51–63. 10.1016/0160-2896(80)90006-9 [DOI] [Google Scholar]
- Ganzeboom HBG, De Graaf PM, & Treiman DJ (1992). A standard international socio-economic index of occupational status. Social Science Research, 21(1), 1–56. 10.1016/0049-089X(92)90017-B [DOI] [Google Scholar]
- Goldsmith HH (1991). A zygosity questionnaire for young twins: a research note. Behavior Genetics, 21(3), 257–269. [DOI] [PubMed] [Google Scholar]
- Gottschling J (2017). Documentation TwinLife data: Cognitive abilities TwinLife Technical Report Series (Vol. 02). Bielefeld: Project TwinLife “Genetic and social causes of life chances” (Universität Bielefeld / Universität des Saarlandes). [Google Scholar]
- Grant MD, Kremen WS, Jacobson KC, Franz C, Xian H, Eisen SA, … Lyons MJ (2010). Does parental education have a moderating effect on the genetic and environmental influences of general cognitive ability in early adulthood? Behavior Genetics, 40(4), 438–446. 10.1007/s10519-010-9351-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, & Stearns E (2002). The social influences on the realization of genetic potential for intellectual development. Social Forces, 80(3), 881–910. 10.1353/sof.2002.0007 [DOI] [Google Scholar]
- Hagenaars A, de Vos K, & Zaidi A (1998). Patterns of poverty in Europe In Jenkins SP, Kapteyn A, & van Praag BMS (Eds.), The distribution of welfare and household production: international perspectives. Cambridge University Press. [Google Scholar]
- Hahn E, Gottschling J, Bleidorn W, Kandler C, Spengler M, Kornadt AE, … Spinath FM (2016). What drives the development of social inequality over the life course? The German TwinLife study. Twin Research and Human Genetics, 19(6), 659–672. 10.1017/thg.2016.76 [DOI] [PubMed] [Google Scholar]
- Hanscombe KB, Trzaskowski M, Haworth CMA, Davis OSP, Dale PS, & Plomin R (2012). Socioeconomic status (SES) and children’s intelligence (IQ): in a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. PLOS ONE, 7(2), e30320 10.1371/journal.pone.0030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanushek EA, & Wössmann L (2006). Does educational tracking affect performance and inequality? Differences-in-differences evidence across countries. The Economic Journal, 116, C63–C76. 10.1111/j.1468-0297.2006.01076.x [DOI] [Google Scholar]
- Harden KP, Turkheimer E, & Loehlin JC (2007). Genotype by environment interaction in adolescents’ cognitive aptitude. Behavior Genetics, 37(2), 273–283. 10.1007/s10519-006-9113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Nyholt DR, Neuman R, Madden PAF, Bucholz KK, Todd RD, … Martin NG (2003). Zygosity diagnosis in the absence of genotypic data: an approach using latent class analysis. Twin Research: The Official Journal of the International Society for Twin Studies, 6(1), 22–26. [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Kan K-J, Wicherts JM, Dolan CV, & van der Maas HLJ (2013). On the nature and nurture of intelligence and specific cognitive abilities: the more heritable, the more culture dependent. Psychological Science, 24(12), 2420–2428. 10.1177/0956797613493292 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick RM, McGue M, & Iacono WG (2015). Replication of a gene–environment interaction via multimodel inference: additive-genetic variance in adolescents’ general cognitive ability increases with family-of-origin socioeconomic status. Behavior Genetics, 45(2), 200–214. 10.1007/s10519-014-9698-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Jacobson KC, Xian H, Eisen SA, Waterman B, Toomey R, … Lyons MJ (2005). Heritability of word recognition in middle-aged men varies as a function of parental education. Behavior Genetics, 35(4), 417–433. 10.1007/s10519-004-3876-2 [DOI] [PubMed] [Google Scholar]
- Lang V, & Kottwitz A (2017). The sampling design and socio-demographic structure ot the first wave of the TwinLife panel study: a comparison with the Microcensus TwinLife Technical Report Series (Vol. 03 updated version). Bielefeld: Project TwinLife “Genetic and social causes of life chances” (Universität Bielefeld / Universität des Saarlandes). [Google Scholar]
- Lenau F, Hahn E, Peters A-L, Gottschling J, Thiel W, & Spinath FM (2017). Zygosity determination in twin studies: a validation of zygosity questionnaires using DNA in the German TwinLife study TwinLife Working Paper Series (Vol. 01). Bielefeld: Project TwinLife “Genetic and social causes of life chances” (Universität Bielefeld / Universität des Saarlandes). [Google Scholar]
- McGue M, & Bouchard TJ (1984). Adjustment of twin data for the effects of age and sex. Behavior Genetics, 14(4), 325–343. 10.1007/BF01080045 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nisbett RE, Aronson J, Blair C, Dickens W, Flynn J, Halpern DF, & Turkheimer E (2012). Intelligence: New findings and theoretical developments. American Psychologist, 67(2), 130–159. 10.1037/a0026699 [DOI] [PubMed] [Google Scholar]
- Oniszczenko W, Angleitner A, Strelau J, & Angert T (1993). The questionnaire of twins’ physical resemblance. University of Warsaw, Poland, and University of Bielefeld, Germany: Unpublished manuscript. [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, & Neiderhiser J (2013). Behavioral genetics. Palgrave Macmillan. [Google Scholar]
- Purcell S (2002). Variance components models for gene–environment interaction in twin analysis. Twin Research and Human Genetics, 5(6), 554–571. 10.1375/twin.5.6.554 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing [R Foundation for Statistical Computing; ]. Vienna, Austria: Retrieved from https://www.R-project.org/ [Google Scholar]
- Rhemtulla M, & Tucker-Drob EM (2012). Gene-by-socioeconomic status interaction on school readiness. Behavior Genetics, 42(4), 549–558. 10.1007/s10519-012-9527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, Jacobson KC, & Van den Oord EJCG (1999). Genetic and environmental influences on vocabulary IQ: parental education level as moderator. Child Development, 70(5), 1151–1162. 10.1111/1467-8624.00084 [DOI] [PubMed] [Google Scholar]
- Scarr S (1992). Developmental theories for the 1990s: development and individual differences. Child Development, 63(1), 1–19. 10.1111/j.1467-8624.1992.tb03591.x [DOI] [PubMed] [Google Scholar]
- Scarr-Salapatek S (1971). Race, social class, and IQ. Science, 174(4016), 1285–1295. 10.2307/1733128 [DOI] [PubMed] [Google Scholar]
- Schneider SL (2008). The International Standard Classification of Education (ISCED-97): an evaluation of content and criterion validity for 15 European countries. Mannheim: MZES; Retrieved from http://ub-madoc.bib.uni-mannheim.de/25411 [Google Scholar]
- Sheskin DJ (2011). Handbook of parametric and nonparametric statistical procedures (5 Revised edition). Boca Raton: Taylor & Francis Ltd. [Google Scholar]
- Spengler M, Gottschling J, Hahn E, Tucker-Drob EM, Harzer C, & Spinath FM (2018). Does the heritability of cognitive abilities vary as a function of parental education? Evidence from a German twin sample. PLoS ONE 13(5): e0196597 10.1371/journal.pone.0196597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2007). Using multivariate statistics (5th ed.). Needham Height, MA: Allyn & Bacon. [Google Scholar]
- Tucker-Drob EM, & Bates TC (2016). Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 27(2), 138–149. 10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA, & Harden KP (2013). Genetic and environmental influences on cognition across development and context. Current Directions in Psychological Science, 22(5), 349–355. 10.1177/0963721413485087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Rhemtulla M, Harden KP, Turkheimer E, & Fask D (2011). Emergence of a gene × socioeconomic status interaction on infant mental ability between 10 months and 2 years. Psychological Science, 22(1), 125–133. 10.1177/0956797610392926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E (2000). Three laws of behavior genetics and what they mean. Current Directions in Psychological Science, 9(5), 160–164. 10.1111/1467-8721.00084 [DOI] [Google Scholar]
- Turkheimer E, Beam CR, Sundet JM, & Tambs K (2017). Interaction between parental education and twin correlations for cognitive ability in a Norwegian conscript sample. Behavior Genetics, 47(5), 507–515. 10.1007/s10519-017-9857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, & Gottesman II (2003). Socioeconomic status modifies heritability of IQ in young children. Psychological Science, 14(6), 623–628. 10.1046/j.0956-7976.2003.psci_1475.x [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Harden KP, D’Onofrio B, & Gottesman II (2009). The Scarr–Rowe interaction between measured socioeconomic status and the heritability of cognitive ability. In K. McCartney & R. A. Weinberg, Experience and development: A festschrift in honor of Sandra Wood Scarr (pp. 81–97). New York: Psychology Press. [Google Scholar]
- van der Sluis S, Posthuma D, & Dolan CV (2012). A note on false positives and power in G × E modelling of twin data. Behavior Genetics, 42(1), 170–186. 10.1007/s10519-011-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S, Willemsen G, de Geus EJC, Boomsma DI, & Posthuma D (2008). Gene-environment interaction in adults’ IQ scores: measures of past and present environment. Behavior Genetics, 38(4), 348–360. 10.1007/s10519-008-9212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß R (2006). CFT 20-R. Grundintelligenztest Skala 2. Manual. Göttingen: Hogrefe. World Development Indicators | DataBank; (2018). Retrieved from http://databank.worldbank.org/data/reports.aspx?source=2&Topic=11 [Google Scholar]
- Zavala C, Beam CR, Finch B, Gatz M, Johnson W, Kremen WS, … Reynolds CA (in press). Testing gene-environment interaction in various cognitive domainds. Developmental Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]