Abstract
Background
The prevalence of obesity and metabolic diseases continues to rise globally. The increased consumption of unhealthy energy-rich diets that are high in fat and sugars results in oxidative stress and inflammation leading to hypothalamic dysfunction, which has been linked with these diseases. Conversely, diets rich in polyphenols, which are phytochemicals known for their antioxidant and anti-inflammatory properties, are associated with a reduced risk for developing metabolic diseases.
Scope of review
This review provides an overview of the effects of polyphenols against diet-induced hypothalamic dysfunction with respect to neural inflammation and mitochondrial dysfunction. Results show that polyphenols ameliorate oxidative stress and inflammation within the hypothalamus, thereby improving leptin signaling and mitochondrial biogenesis. Furthermore, they protect against neurodegeneration by decreasing the production of reactive oxygen species and enhancing natural antioxidant defense systems.
Major conclusions
The potential of polyphenols as nutraceuticals against hypothalamic inflammation, mitochondrial dysfunction, and neurodegeneration could hold tremendous value. With hypothalamic inflammation increasing naturally with age, the potential to modulate these processes in order to extend longevity is exciting and warrants exploration. The continued escalation of mental health disorders, which are characterized by heightened neuronal inflammation, necessitates the furthered investigation into polyphenol therapeutic usage in this regard.
Keywords: Nutrition, Metabolic disease, Hypothalamic inflammation, Neural mitochondrial dysfunction, Polyphenols, Longevity
Abbreviations: ARC, arcuate nucleus; BBB, blood-brain-barrier; GTE, green tea extract; HFD, high-fat diet; IKκB, inhibitor of nuclear factor kappa-B kinase subunit beta; IR, insulin resistance; NFκB, nuclear factor kappa B; NRF2, nuclear factor (erythroid-derived 2)-like 2 factor; POMC, pro-opiomelanocortin; PPs, polyphenols; ROS, reactive oxygen species; SFAs, saturated fatty acids; SOCS-3, suppressor of cytokine signaling-3; STAT-3, signal transducer and activator of transcription 3; TLR4, Toll Like Receptor 4
Graphical abstract
Highlights
-
•
Unhealthy diets high in fat and sugar can cause hypothalamic inflammation.
-
•
Hypothalamic dysfunction causes obesity, cardiovascular and neurological disorders.
-
•
Diets high in phytochemicals can reduce the risk of obesity and metabolic diseases.
-
•
Polyphenols allay harmful dietary effects by preventing hypothalamic inflammation.
-
•
Polyphenols protects against neuron damage and can improve overall human health.
1. Introduction
Obesity is a multifactorial disorder of energy balance and represents one of the single most important modifiable risk factors for the development of various metabolic diseases such as type 2 diabetes mellitus and cardiovascular and neurodegenerative disorders [1], [2], [3], [4]. The preservation of homeostasis is integral for the maintenance of health [5], with the disruption of principle components controlling these processes leading to the onset and progression of disease [6]. The hypothalamus is a key region in the brain controlling important bodily functions, such as heart rate and blood pressure, peripheral glucose and lipid energy homeostasis, as well as regulating sleep and circadian rhythms and aging [7], [8], [9], [10].
The prevalence of obesity is rising at an unprecedented rate, with unhealthy diets and physical inactivity shown to be the main contributing factors [11]. Over-nutrition related to unhealthy diets can dysregulate key hypothalamic processes, through an elicited inflammatory response. In recent years, it has been established that a sustained hypothalamic inflammatory state is regarded as the primary cause for the development of obesity, occurring in part due to molecular and functional resistance to hormonal signals such as insulin and leptin, thereby affecting whole-body energy homeostasis [12], [13], [14]. Apart from mediating the roles of leptin and insulin in controlling energy homeostasis, hypothalamic nuclei are also critically dependent on mitochondria to fulfill their appropriate functions [15]. Mitochondria are cytoplasmic double-membraned organelles that are primarily involved in energy generation as well as have a secondary role in a myriad of key cellular regulatory processes such as calcium signaling, cell growth and differentiation, cell cycle control and cell death [16], [17]. Over-consumption of unhealthy, high-energy diets can compromise mitochondrial bioenergetics, with loss of function being implicated in the pathogenesis of several human diseases including obesity and diabetes [15], [17], [18], [19].
The promotion of healthy lifestyles, such as encouraging good nutritional choices, together with maintenance of adequate levels of exercise could go a long way in managing these largely preventable metabolic disorders. A quality diet is based on a variety of foods (mostly from plant sources) that have undergone limited processing [20]. The Mediterranean diet has gained considerable popularity in this regard; it is believed that the beneficial health effects of this diet are conferred by the high polyphenol (PPs) content [21]. As such, dietary PPs are increasingly investigated as nutraceuticals against metabolic diseases [22]. It is speculated that PPs exert their beneficial health benefits in part through the modulation of hypothalamic inflammation and oxidative stress, which may facilitate and potentiate the functionality of several neural hormones acting within this brain region [23], although further research is required given the difficulty in determining these effects in humans.
Accumulating evidence shows the potential of dietary PPs to prevent or delay disease progression through the regulation of mitochondrial redox status [17]. The better a species is at protecting its mitochondria, the longer the species lives [24]. Thus, PPs may represent suitable therapeutic agents capable of protecting mitochondria, which may be useful for increasing longevity.
Nutrition can have a profound effect on brain function, and, in recent years, increasing efforts have been aimed at maximizing brain health and thereby cognitive ability. The hypothalamus is particularly vulnerable to dietary changes and dysregulation of key processes plays a meaningful role in the pathogenesis of disease. This review will describe the effect of diet-induced hypothalamic dysfunction with respect to inflammation and neural mitochondrial dysfunction in the development of metabolic syndrome. The use of PPs to mitigate the deleterious effects of diet on hypothalamic dysfunction in animal studies is reviewed, and we investigate the potential of such plant products as possible therapeutic agents.
2. Hypothalamic health and vitality
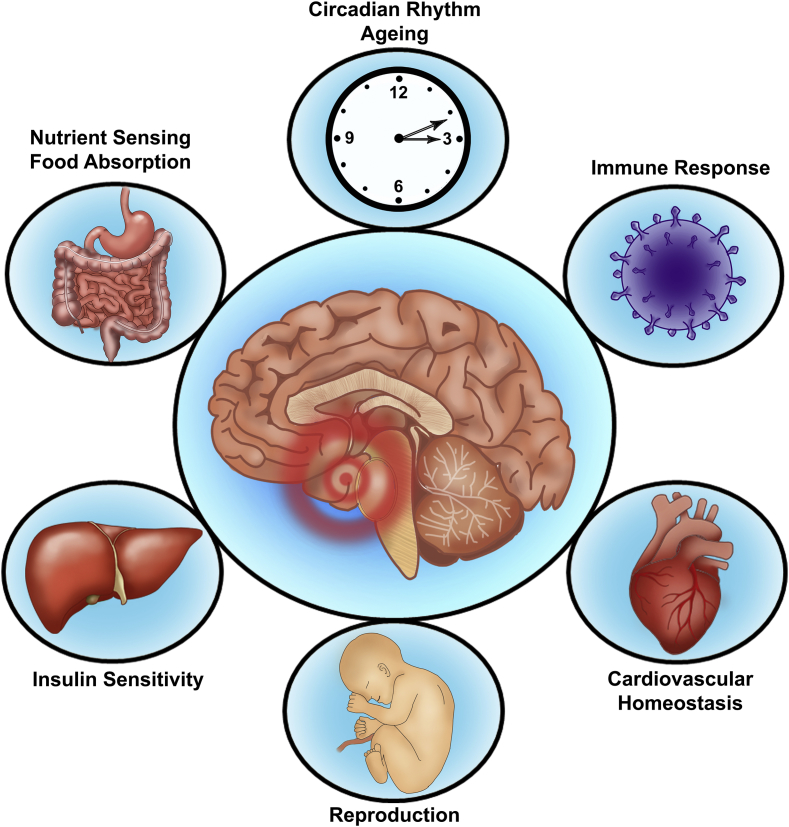
The hypothalamus is positioned at the base of the brain near the blood-brain barrier (BBB) and is perfectly poised to ‘monitor, process and then’ transmit environmental information to the peripheral organs [7]. A primary function of this neuronal center is to control energy homeostasis, through regulating feeding behavior and energy expenditure to maintain bodyweight [25]. To this end, circulating blood-borne factors of hunger and satiety in relation to nutrient availability need to traverse the BBB to reach effector neurons [26]. Specialized glial cells, called tanycytes, regulate BBB plasticity and allow the passage of key molecules such as leptin and insulin, which thereafter bind to specific neurons located within the arcuate nucleus (ARC) [27]. The ARC contains the antagonistic orexigenic and anorexigenic neurons [28], which stimulate appetite and promote satiety, respectively. The orexigenic agouti-related peptide and neuropeptide Y stimulate appetite [29], while, opposingly, the anorexigenic effect is achieved through secretion of proopiomelanocortin (POMC) and cocaine and amphetamine regulated transcript, which decreases food intake [30]. During obesity there is an imbalance between insulin stimulated leptin-induced anorexigenic and orexigenic signaling, which results in an over-expression of neuropeptide Y/agouti-related peptide with a concomitant reduction of POMC/cocaine and amphetamine regulated transcript leading to a diminished satiety signal and hyperphagia [31]. Apart from a role in nutrient sensing and energy metabolism, other key physiological processes such as thermogenesis, cardiovascular homeostasis, growth and development, as well as reproduction are also controlled by this brain region [7], [8], [27], as illustrated in Figure 1
Figure 1.
Hypothalamic physiological function. The hypothalamus plays a role in the regulation of many key physiological processes, including nutrient sensing, energy homeostasis, cardiovascular function, immune response, reproduction, circadian rhythms, and aging.
It is hypothesized that the hypothalamus is the primary regulator of aging in the entire body [7]. The aging brain exhibits increased levels of inflammation and oxidative stress [32], characteristics which are also caused by an obesogenic diet. Given that these characteristics can induce hypothalamic mitochondrial dysfunction and are involved in the onset of age-related diseases, it is possible that they accelerate the aging process [33], [34], [35].
Mitochondrial function is essential for energy production, which is required for physical and mental vitality, endurance, and the continuation of life itself. Neurons are high-energy-requiring cells, with the mitochondrial machinery being pivotal in ensuring this constant energy supply [36], but these energy-producing centers can become fragmented due to unhealthy energy-dense diets [18]. The mitochondrial network is pivotal in integrating various metabolic signals such as ATP levels, indicators of oxidative and endoplasmic reticulum stress, inflammatory responses, as well as cell signaling, all of which are key processes that become dysregulated during disease pathology [33]. Diet and nutrition represent a modifiable mechanism to optimize mitochondrial efficiency and can be useful to prevent disease.
3. The high-energy food environment
In modern times, with long work hours, reduced sleep and elevated levels of daily-stressors, the addition of a high-energy food environment incrementally increases the susceptibility of individuals to the development of obesity [37], [38], [39]. There has been a marked transition away from traditional food, to what is regarded as the “western” or “cafeteria” diet, comprised of highly processed, energy-dense foods containing elevated levels of sugar, salt, and saturated fat, which is often accompanied by a simultaneous decreased intake of protein, unprocessed fruit and vegetables, unrefined carbohydrates and fiber [40]. This type of high-energy, low-nutrient food environment is associated with the rise in obesity, which increases the risk profile for the development of several non-communicable diseases [41]. Unhealthy lifestyle habits, like reduced physical activity and increased consumption of readily available highly-palatable energy-dense foods, is a combination that amounts to excess caloric intake with reduced energy expenditure, contributing to a low-grade chronic inflammatory state, which is associated with disease pathophysiology [42].
On an evolutionary timeframe (over the last half century), the human brain is being challenged in a manner and on this scale that it has not quite experienced in the past. Already 2.1 billion people, accounting for almost 1/3 of the global population, are affected by obesity, and its prevalence is still increasing, especially among children [43]. Due to the chemosensory nature of the tanycytes, the hypothalamus is particularly susceptible to the modern-day food environment, which affects other brain regions to a lesser extent [12], [13], [44]. However, obesity induces neuro-inflammation that affects several brain regions including the amygdala, hippocampus, and cerebellum [12]. The nature of modern-day foods is that they provide both pleasurable and rewarding qualities, which can be addictive. In this regard, food is able to drive appetite beyond normal physiological metabolic requirements [45]. The consequences of over-nutrition are evident within society and can perfectly be illustrated by global obesity and related non-communicable disease prevalence, and which is projected to only continue escalating in the future [46], [47]. Non-homeostatic hedonistic cues such as those resulting from reward, motivation or pleasure arise from other non-hypothalamic regions of the brain and increases the variability of individual responses to food and in doing so adds further complexities in the quest to develop anti-obesity therapies.
4. Nutrition and brain development
The brain is particularly vulnerable to the effects of obesogenic diets, especially during early life comprising periods of rapid growth, maturation, and development [48]. It is believed that a significant amount of the brain's ultimate structure and capacity is formed by three (3) years of age [49], [50]. The failure to optimize brain development during this critical period can be catastrophic, having long term consequences that can affect an individual's learning ability, education, job potential, and mental health conditions later in life [49], [51]. Compounding matters, it is known that maternal consumption of obesogenic dietary factors, such as simple sugars or saturated fatty acids (SFAs) consumed during pregnancy, increases the risk for the development of obesity in the subsequent generations [48].
Using rodent models, it has been demonstrated that in utero exposure to such dietary factors affects numerous neurobiological processes, such as increased neuroinflammation and reduced neurotrophin mediated regulation of neurogenesis and synaptic plasticity, with epigenetic mechanisms implicated [48], [52]. Maternal consumption of a high fat diet (HFD) can alter both phenotypic and metabolic status of the offspring, modifying hypothalamic gene expression [52], [53]. These changes include increased body weight and adiposity and altered serum glucose and plasma leptin levels, while the hypothalamic neural projection formations of HFD-offspring are physically changed with aberrations in the neural circuitry controlling appetite, increasing the likelihood of these animals to develop obesity and diabetes later in life [52], [53].
5. Diet-induced hypothalamic dysfunction and metabolic diseases
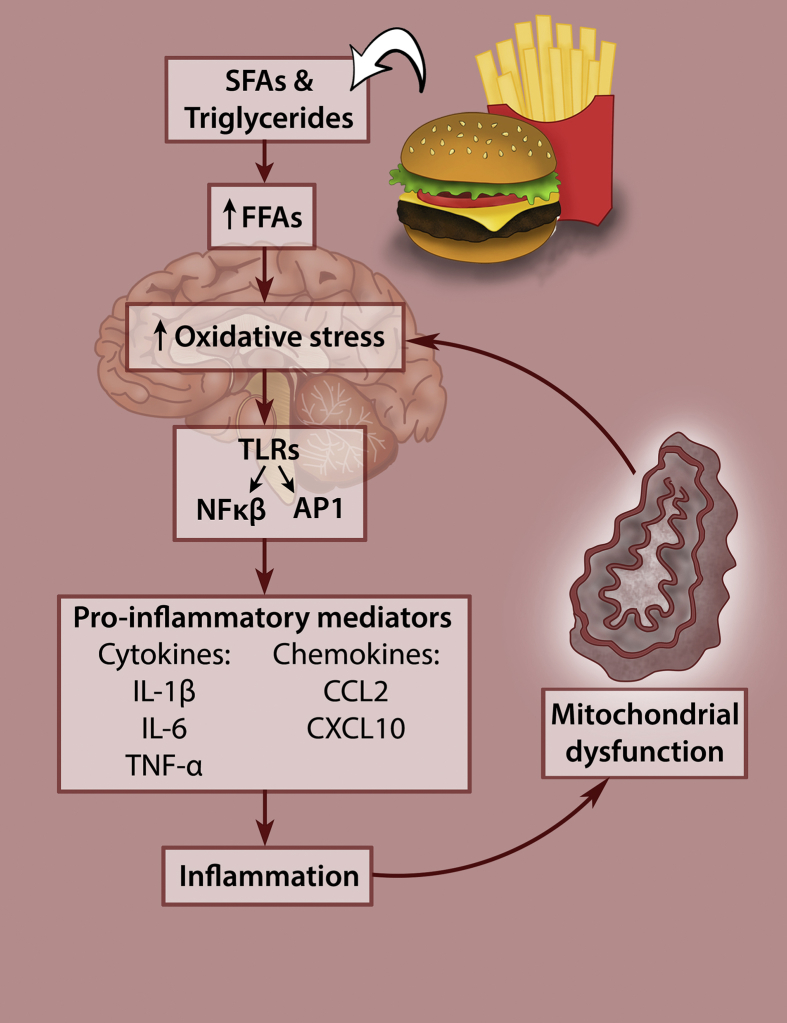
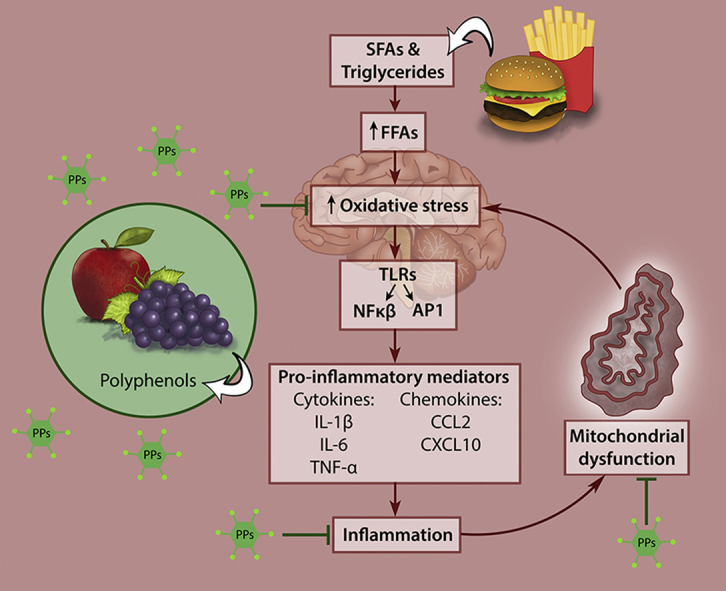
Hypothalamic neurons contain an abundance of mitochondria, with the function of this neuronal energy generating circuitry being severely compromised by obesogenic diets [25]. Diet-induced hypothalamic inflammation and mitochondrial dysfunction result in the onset and development of obesity and related metabolic diseases. It has been shown that feeding a HFD to rats directly causes brain damage [14], [54], [55], [56], [57]. Also, SFAs administration results in rapid induction of neural inflammation [55], [58], [59], with even a single high-fat meal being enough to inflict hypothalamic injury leading to impaired nutrient-sensing and energy balance functions [35], [60]. This pro-inflammatory response is regarded as one of the first causative steps involved in the onset as well as the maintenance of the obese phenotype [12], [13]. Up-regulation of pro-inflammatory mediators in the hypothalamus is a hallmark of obesity and constitutes a major component of leptin and/or insulin resistance (IR) [14].
Increased SFAs induce immune cell activation and can elicit an inflammatory response that affects the peripheral organs and tissues such as adipose, liver, pancreas, skeletal muscle, and heart [12], [61]. The innate immune response is mediated through toll-like receptors that activate two different transcription factors, Nuclear factor kappa B (NFκB) and activator protein-1, which, in turn, upregulate the expression of pro-inflammatory mediators such as cytokines, like interleuken-1β, interleuken-6, tumour necrosis factor-alpha, and chemokines, like chemokine ligand 2 and C-X-C motif 10 [12], [13]. It is noteworthy that some controversy exists regarding the involvement of toll-like receptors in the susceptibility to HFD-induced obesity. In a recent study performed by Dalby and colleagues, it was demonstrated that the hypothalamic inflammatory response was independent of TLR4 signaling [62], which was contrary to several previously reported findings [57], [63]. Furthermore, it has been shown that SFAs are not specifically TLR4 agonists but that SFA-induced inflammation is indirectly controlled by TLR4 receptors through the alteration of macrophage lipid metabolism [64].
Fructose, one of the world's most abundantly consumed sugars [65], contributes to IR, much in the same manner as fat [66]. It is metabolized by the liver to triglycerides, which then increase in circulation and contribute to the development of hypertension in rats [67], [68]. The overconsumption of unhealthy diets causes mitochondria to be overloaded with fatty acids and glucose, leading to elevated acetyl-CoA and NADH production and increasing the number of electrons able to occupy the intermembrane space, thereby producing excessive reactive oxygen species (ROS), leading to the onset of oxidative stress and activation of several TFs that drive inflammation [33].
Oxidative stress and inflammation are major contributors to cellular senescence, which prevents cell proliferation and results in excessive ROS production and the secretion of inflammatory molecules and extracellular matrix components that further drive inflammation and senescence in the surrounding tissues [69]. As a result, there is an accumulation of cellular damage, reduced activity of protective stress response pathways resulting in oxidative stress, and a low-grade systemic inflammatory state leading to the development of age-related diseases [69]. This obesogenic dietary-induced dysfunction is depicted in Figure 2.
Figure 2.
Diet-induced hypothalamic dysfunction. Increased consumption of obesogenic diets high in saturated fatty acids and simple sugars leads to hypothalamic inflammation and mitochondrial dysfunction that causes obesity and metabolic disease.
Since hypothalamic inflammation is regarded as a primary cause of obesity, it seems logical for this to be the first indicator to be targeted. It has been suggested that the inhibition of hypothalamic inflammation improves the central control of glucose homeostasis [70], [71]. The deleterious effects of sustained elevated ROS levels in the perpetuation of neural inflammation has been described in this review; however, ROS generated in response to glucose has also been shown to have a satiety promoting effect via POMC neurons [72], [73], [74], [75], [76]. The source of fuel and duration of stimulation in neuronal ROS generation (i.e. fatty acids or glucose) play an integral role in determining if the ROS-induced effect will be beneficial or harmful [72].
It is encouraging that in a study using mice fed a HFD for 8 weeks, infusing unsaturated FAs directly into the brain appeared to almost completely reverse hypothalamic inflammation and thereby prevent obesity [77]. Symptoms of metabolic disease can be reversed using lifestyle-modification strategies, and more awareness should be made to draw attention to this important detail. With a global-scale population shift in phenotype occurring as a result of increased obesity prevalence, new therapeutics are urgently required. Plant-based medications, which can accelerate weight loss could serve as a cheaper alternative for anti-obesity therapies; many nutraceuticals are proven to be safe, widely available, and often found to elicit beneficial effects on disease modulation through affecting several different mechanisms [78].
6. Dietary polyphenol therapeutics
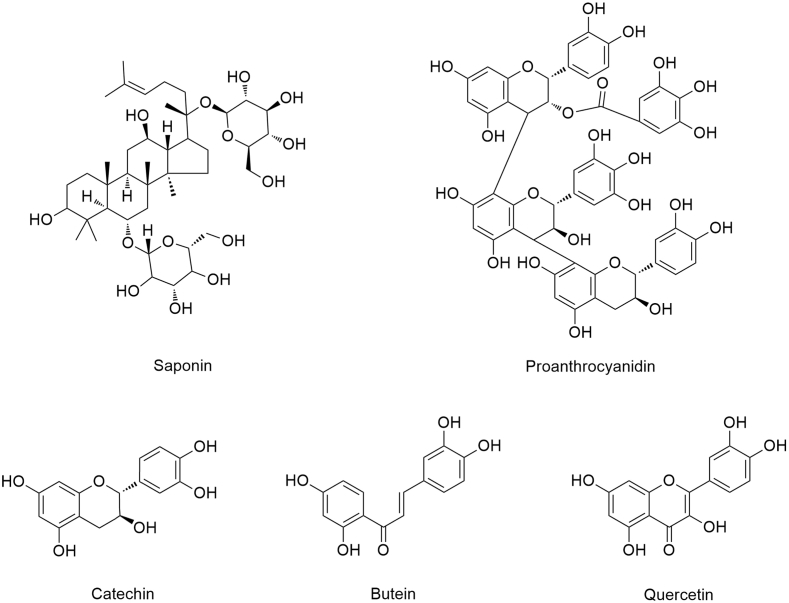
The opportunity for positively influencing the cognitive trajectory through the promotion of lifestyle modifications has been increasingly pursued, with the relationship between nutritional habits and brain health attracting special attention [22]. As such, plant-based diets have gained popularity for their health-promoting benefits, with diets high in PPs being associated with reduced incidence of age-related metabolic diseases [79], [80]. More than 8000 PPs have been identified and are found in fruit, vegetables, teas, chocolates, herbs and spices [81]. These compounds exist either as free monomers (e.g., quercetin and catechin) or oligomers (e.g., proanthocyanidins) [82]. The chemical structure of several PPs is depicted in Figure 3.
Figure 3.
Polyphenol chemical structure. PPs are characterized by diverse phenolic structural features, which contribute to significantly varied physiochemical properties and functionalities.
The increased consumption of PP-rich diets has been associated with a lower incidence of cardiovascular disease, cancer, neurodegenerative diseases, and psychiatric disorders [82]. The prevalence of neurodegenerative diseases has risen globally [83], and diseases like Alzheimer's and Parkinson's, characterized by an increased inflammatory state [84], [85], remain without a cure. Therefore, PPs could hold tremendous benefit in the treatment and prevention of these diseases [86].
Several dietary PPs exhibit the potential to cross the BBB and promote brain health due to the efficacy in protecting neurons against oxidative-stress induced injury, suppressing neuro-inflammation, which is achieved through counteracting neurotoxicity and neurodegeneration [22], [87]. Inflammation is not limited to the hypothalamus, affects other brain regions, and is believed to be linked to the increased prevalence of neuropsychiatric symptoms [88]. The interplay between obesity and mood disorders facilitate the development of both these conditions, and increase the risk of additional metabolic and cardiovascular complications [89].
Interestingly, several health benefits of cocoa and dark chocolate have been reported to be related to neurological and cardiovascular conditions, which has been attributed to the action of PPs [90]. Apart from lowering the prevalence of metabolic syndrome, PPs also hold promise as a treatment for mood disorders, particularly depression [82]; however, further research is required. The therapeutic potential of PPs in mediating obesogenic diet-induced hypothalamic dysfunction will be further discussed.
7. PP therapeutics targeting hypothalamic dysfunction
Many different plant phytochemicals have been employed as therapeutics and found to be useful in maintaining good brain health through mitigating inflammation or cellular damage induced by excessive ROS production [80]. Several studies using animal models and evaluating the effect of PPs administration on hypothalamic inflammation and mitochondrial dysfunction are summarized in Table 1.
Table 1.
Animal studies examining the effect of PPs administration in mitigating hypothalamic dysfunction in diet-induced obesity.
| Polyphenols | Study objective | Biological response | References |
|---|---|---|---|
| Tea saponin [10 mg/kg] | Examined the effect of tea saponin on obesity, inflammation, glucose metabolism, and central leptin sensitivity in obese mice fed a HFD for 16 weeks | ↓ pro-inflammatory cytokines ↓ inflammation ↑ leptin sensitivity through ↑ STAT-3 activation ↑ POMC |
[91] |
| Green tea extract [50 mg/kg] | Determined the effect of green tea treatment in male mice fed a diet high in saturated fatty acids for 8 weeks. | ↑ BW and fat depots, ↓ glucose tolerance ↑ glucose and insulin ↓ adiponectin serum levels ↑ TLR4, IKBα, NFκB p p50 and IL-6 GTE administration ↓ hypothalamic inflammation, thereby attenuating detrimental dietary effects |
[92] |
| Flavonoid: Butein [5 nmol] | Determine the effect of IKκB inhibition using butein on brain glucose and energy metabolism in mice | ↓ IKkB/NfκB activation ↓ SOCS-3 expression, ↑ leptin transduction. |
[93] |
| Pomegranate extract [150 mg/kg] | To investigate the effect of pomegranate extract administration on a spontaneous hypertensive rat model, characterized by increased oxidative stress within the hypothalamus. | ↓ blood pressure and ↓ cardiac hypertrophy. ↓ oxidative stress and ↓ inflammation ↑ NRF2 pathway activation ↓ mitochondrial superoxide anion levels ↑ antioxidant defense system ↑ mitochondrial biogenesis |
[94] |
| Flavonoid: Quercetin [50 mg/kg] | To determine the effects of quercetin on obesity-induced hypothalamic inflammation in mice, through examining the inflammatory responses in microglia treated with saturated fatty acid and/or lipid-laden microglia-conditioned medium. | ↓ oxidative stress and ↓ inflammation ↓ inflammatory cytokines ↓ microglia activation. |
[95] |
| Grape seed extract: Proanthrocyanidins [25 mg/kg] | Evaluate the effects of GSPE administered for 21 days on central and peripheral leptin resistance induced by the CD in rats. | ↑ central and peripheral leptin signaling ↓ hypothalamic inflammation ↓ hyperphagia. |
[96] |
| Tea polyphenols [2 g/L] | Determine the potential of tea polyphenols in modulating circadian rhythms to ameliorate diabetes, metabolic syndrome and memory disorders in constant-darkness mice. | Alleviate insulin resistance and disorder of glucose/lipid metabolism prevented constant darkness induced memory impairment ↓ neuron damage ↑ neurotropic factor related gene expression |
[97] |
Abbreviations: CD, cafeteria diet; GSPE, grape seed proanthocyanidins extract; GTE, green tea extract; HFD, high-fat diet; IKBα, I kappa B alpha; IKκB, inhibitor of nuclear factor kappa-B kinase subunit beta; IL-6, interleukin-6; NFκB, nuclear factor kappa B; NRF2, nuclear factor (erythroid-derived 2)-like 2; POMC, proopiomelanocortin; SOCS-3, suppressor of cytokine signaling 3; STAT-3, signal transducer and activator of transcription 3; TLR4, Toll Like Receptor 4.
In one study in which mice were fed HFD for 16 weeks together with the administration of tea saponin, the result was a decrease in both pro-inflammatory cytokines and inflammatory signals in the mediobasal hypothalamus [91]. This was accompanied by the enhanced effect of central leptin, which was achieved through restoring phosphorylated signal transducer and activator of transcription-3 (STAT-3) signaling, while also increasing POMC expression in the ARC [91].
Quercetin, a naturally occurring flavonoid known to protect against oxidative stress and inflammation, induced a reduction in the levels of inflammatory cytokines and microglia activation markers in the hypothalamus of HFD-fed obese mice [95]. Similarly, the flavonoid butein inhibited IKκB/NFκB activation within the hypothalamus and also attenuated HFD-induced obesity in mice through improved leptin function [93]. The inhibited inflammatory response was associated with reduced suppressor of cytokine signaling-3 (SOCS-3) expression, which is a known negative regulator and integral component in the development of leptin resistance [93]. Furthermore, the administration of a grape seed extract containing a high proanthocyanidin content, which is the most abundant phenolic compound occurring in the human diet, reduced hyperphagia and improved central and peripheral leptin resistance by preventing hypothalamic inflammation in rats [96]. The findings of these studies illustrate the potential of these compounds to mitigate diet-induced inflammatory response and hold promise as anti-obesity therapeutics.
Using a spontaneous hypertensive rat model, which is characterized by high levels of oxidative stress in the hypothalamic paraventricular nucleus, a pomegranate extract was able reduce both oxidative stress and inflammation [94]. The beneficial effects were achieved through an increase in the intrinsic antioxidant defense system, by the activation of the adenosine monophosphate-activated protein kinase-nuclear factor-erythroid 2 p45-related factor 2 (NRF2) pathway [94]. There was also a reduction in mitochondrial superoxide anion levels, mitochondrial biogenesis as well as an improvement in mitochondrial dynamics. An improvement in blood pressure and in cardiac hypertrophy was also observed [94]. Hypertension is a major risk factor for global cardiovascular disease morbidity and mortality, of which approximately 50% of these cardiopathic cases can be attributed to. Thus, the creation of new therapies using PP compounds could be useful and should be explored [98], [99].
In a study in which green tea extract (GTE) was administered to male mice fed a HFD diet rich in saturated FAs for 8 weeks, the animals exhibited increased body weight and fat depots mass, with impaired oral glucose tolerance tests, elevated glucose and insulin as well as decreased adiponectin serum levels [92]. Pro-inflammatory markers such TLR4, IκBα, NFκB, and Iinterleukin-6 were elevated; however, concomitant treatment with GTE ameliorated the adverse dietary effects, partially due to reduced hypothalamic inflammation [92]. In another study in which mice were subjected to constant darkness, administration of PPs in tea also had beneficial effects. Treatment abolished IR and restored lipid and glucose metabolism and prevented memory impairment by inhibiting neuron damage and increased expression of neurotrophic factor related genes. It was found that the protective mechanisms were elicited through modulation of circadian clock gene transcription and protein expression in the hypothalamus, as well as the liver [97]. The circadian rhythm allows an organism to exploit daily environmental changes and food components to adjust internal rhythms in peripheral tissues or suprachiasmatic nucleus [97]. The circadian clocks generate the circadian rhythms in behavior, physiology and metabolism, with circadian disruption being associated with reduced fitness and decreased longevity [100]. This finding is interesting especially in the context of factors that can disrupt circadian rhythms, such as reduced sleep, under-exposure to natural light and Vitamin D deficiencies [101], [102]; as such PPs administration could be useful in this regard.
These studies support a role for PPs for promoting good health outcomes through modulating the inflammatory response and oxidative stress in the hypothalamus. With several PPs available that have been shown to mitigate hypothalamic inflammation and mitochondrial dysfunction, and with these respective processes being associated cellular senescence and aging, the prospect of increasing longevity particularly through modulation of these hypothalamic processes using PPs is exciting and warrants further investigation.
8. Future considerations
Neurogenesis and neuroplasticity are receiving increased attention; the elucidation of the mechanisms underlying the adaptive response to environmental stimuli could allow for a means for modification and thus therapeutic application. It is known that diet-induced obese mice have decreased neurogenesis within the hypothalamus, resulting in fewer new neurons while older neurons are retained [103]. Similarly, neurogenesis within the adult human hypothalamus is also important and occurs in response to hormonal, nutritional and mitogenic signals; tanycytes are able to differentiate into anorexigenic or orexigenic neurons and thereby control feeding behavior [104], the manipulation of which, could have great potential in anti-obesity therapies.
Of interest, neurogenesis also occurs within the hippocampus, a brain region associated with learning and memory as well as mood, with modulation of diet-induced neurogenesis emerging as a tool to improve mental vitality [105]. Dietary restriction has been shown to improve behavioral outcomes in animal models of neurodegenerative disorders as well as enhance spatial memory, suggesting this dietary regiment can increase the brain's capacity for plasticity and self-repair [106]. With several studies investigating the effect of PPs on the hippocampus and cognitive decline [107], [108], [109], [110], it remains to be investigated whether PPs can influence hypothalamic neurogenesis, which would have significant consequences.
Another particularly alarming matter is the continued rise in the prevalence of mental disorders, and the increased occurrence of neuropsychiatric symptoms, associated with metabolic perturbations induced through inflammatory mechanisms [89]. In the field of nutritional neuroscience, it has long been known that factors such as human cognition, behavior, and emotion are intertwined with diet [111]. As such, dietary recommendations have been proposed with the aim to prevent major depressive disorder [112]. Similarly, PP-enriched diets have the potential to become medicaments in the field of mental health after a thorough study of their mechanism of action [113]. While the benefits of exercise are known for individuals diagnosed with clinical depression [114], adding PPs to such therapeutics could enhance the effect of exercise. Some scientists consider PPs to be an exercise mimetic, with a recent study showing that combining exercise with administration of grape seed PPs heightened and intensified the individual metabolic effects of exercise in rats with established IR [115]. Further studies can be done to elucidate whether the combined effects of high-PP diets in combination with exercise interventions could be useful in treating inflammatory-related neurological disorders.
9. Conclusion
To date, there is no effective therapeutic solution for curing obesity except for lifestyle modification. Since obesity and related-metabolic disease prevalence continues to increase, so too will the search for therapeutics. Plant-based diets high in PPs show great potential as a treatment in this regard and can prove to be useful in preventing metabolic disorders by limiting oxidative stress and inflammation through supplementation even in the event of poor dietary choices. With the continued increase in the prevalence of neurodegenerative and psychiatric disorders, cost-effective, functional methods of treatment are urgently required. PP-enriched diets hold the potential as natural, non-invasive, largely inexpensive therapeutic compounds that can be valuable not only for brain health but also for overall good health outcomes.
Acknowledgements
The preparation of this manuscript was supported by the National Research Foundation Professional-Development Program (NRF-PDP) grant UID 104912 and Biomedical Research and Innovation Platform (BRIP), South African Medical Research Council, Baseline funding. The authors would like to acknowledge the support of colleagues at BRIP, SAMRC.
Conflict of interest
None declared.
References
- 1.Ashrafian H., Harling L., Darzi A., Athanasiou T. Neurodegenerative disease and obesity: what is the role of weight loss and bariatric interventions? Metabolic Brain Disease. 2013;28:341–353. doi: 10.1007/s11011-013-9412-4. [DOI] [PubMed] [Google Scholar]
- 2.Burke G.L., Bertoni A.G., Shea S., Tracy R., Watson K.E., Blumenthal R.S. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Archives of Internal Medicine. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel R.H., Kahn S.E., Ferrannini E., Goldfine A.B., Nathan D.M., Schwartz M.W. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Journal of Clinical Endocrinology & Metabolism. 2011;96:1654–1663. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozza C., Isidori A.M. In: Imaging in bariatric surgery. Laghi A., Rengo M., editors. Springer International Publishing; Cham: 2018. pp. 1–8. [Google Scholar]
- 5.Huber M., Knottnerus J.A., Green L., van der Horst H., Jadad A.R., Kromhout D. How should we define health? BMJ (Clinical Research Ed) 2011;343:d4163. doi: 10.1136/bmj.d4163. [DOI] [PubMed] [Google Scholar]
- 6.Mravec B., Horvathova L., Cernackova A. Hypothalamic inflammation at a crossroad of somatic diseases. Cellular and Molecular Neurobiology. 2018 doi: 10.1007/s10571-018-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K., Choe H.K. 2018. Role of hypothalamus in aging and its underlying cellular mechanisms. Mechanisms of ageing and development. [DOI] [PubMed] [Google Scholar]
- 8.Rahmouni K. Cardiovascular regulation by the arcuate nucleus of the hypothalamus: neurocircuitry and signaling systems. Hypertension (Dallas, Tex. : 1979) 2016;67:1064–1071. doi: 10.1161/HYPERTENSIONAHA.115.06425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 10.Valdearcos M., Xu A.W., Koliwad S.K. Hypothalamic inflammation in the control of metabolic function. Annual Review of Physiology. 2015;77:131–160. doi: 10.1146/annurev-physiol-021014-071656. [DOI] [PubMed] [Google Scholar]
- 11.Romieu I., Dossus L., Barquera S., Blottiere H.M., Franks P.W., Gunter M. Energy balance and obesity: what are the main drivers? Cancer Causes & Control: CCC. 2017;28:247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillemot-Legris O., Muccioli G.G. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends in Neurosciences. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Manousopoulou A., Koutmani Y., Karaliota S., Woelk C.H., Manolakos E.S., Karalis K. Hypothalamus proteomics from mouse models with obesity and anorexia reveals therapeutic targets of appetite regulation. Nutrition & Diabetes. 2016;6:e204. doi: 10.1038/nutd.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin S., Diano S. Mitochondrial dynamics and hypothalamic regulation of metabolism. Endocrinology. 2018;159:3596–3604. doi: 10.1210/en.2018-00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osellame L.D., Blacker T.S., Duchen M.R. Cellular and molecular mechanisms of mitochondrial function. Best Practice & Research Clinical Endocrinology & Metabolism. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira J., Chavarria D., Borges F., Wojtczak L., Wieckowski M.R., Karkucinska-Wieckowska A. Dietary polyphenols and mitochondrial function: role in health and disease. Current Medicinal Chemistry. 2017 doi: 10.2174/0929867324666170529101810. [DOI] [PubMed] [Google Scholar]
- 18.Jheng H.F., Tsai P.J., Guo S.M., Kuo L.H., Chang C.S., Su I.J. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Molecular and Cellular Biology. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putti R., Sica R., Migliaccio V., Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Frontiers in Physiology. 2015;6:109. doi: 10.3389/fphys.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson J.J., Nieman D.C. Diet quality-the Greeks had it right! Nutrients. 2016;8 doi: 10.3390/nu8100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guasch-Ferre M., Merino J., Sun Q., Fito M., Salas-Salvado J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxidative Medicine and Cellular Longevity. 2017;2017:6723931. doi: 10.1155/2017/6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelli V., Grassi D., Bocale R., d'Angelo M., Antonosante A., Cimini A. Diet and brain health: which role for polyphenols? Current Pharmaceutical Design. 2018;24:227–238. doi: 10.2174/1381612824666171213100449. [DOI] [PubMed] [Google Scholar]
- 23.Panickar K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Molecular Nutrition & Food Research. 2013;57:34–47. doi: 10.1002/mnfr.201200431. [DOI] [PubMed] [Google Scholar]
- 24.Pizzorno J. Mitochondria-fundamental to life and health. Integrative Medicine (Encinitas, Calif.) 2014;13:8–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Timper K., Bruning J.C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Disease Models & Mechanisms. 2017;10:679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y., Tschop M.H., Luquet S. Hypothalamic tanycytes: gatekeepers to metabolic control. Cell Metabolism. 2014;19:173–175. doi: 10.1016/j.cmet.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. Journal of Neuroendocrinology. 2014;26:753–760. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- 28.Benite-Ribeiro S.A., Putt D.A., Soares-Filho M.C., Santos J.M. The link between hypothalamic epigenetic modifications and long-term feeding control. Appetite. 2016;107:445–453. doi: 10.1016/j.appet.2016.08.111. [DOI] [PubMed] [Google Scholar]
- 29.Varela L., Horvath T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Reports. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millington G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutrition & Metabolism. 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergen H.T., Mizuno T., Taylor J., Mobbs C.V. Resistance to diet-induced obesity is associated with increased proopiomelanocortin mRNA and decreased neuropeptide Y mRNA in the hypothalamus. Brain Research. 1999;851:198–203. doi: 10.1016/s0006-8993(99)02186-1. [DOI] [PubMed] [Google Scholar]
- 32.Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging and Disease. 2015;6:109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunarro J., Casado S., Lugilde J., Tovar S. Hypothalamic mitochondrial dysfunction as a target in obesity and metabolic disease. Frontiers in Endocrinology. 2018;9:283. doi: 10.3389/fendo.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo A.Y., Joseph A.M., Dutta D., Hwang J.C., Aris J.P., Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. Journal of Cell Science. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X.H., Qiao H., Du F., Xiong Q., Liu X., Zhang X. Quantitative imaging of energy expenditure in human brain. NeuroImage. 2012;60:2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beccuti G., Pannain S. Sleep and obesity. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaze J., Wang J., Ho L., Mendelev N., Haghighi F., Pasinetti G.M. Polyphenolic compounds alter stress-induced patterns of global DNA methylation in brain and blood. Molecular Nutrition & Food Research. 2018;62:e1700722. doi: 10.1002/mnfr.201700722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook M.A., Gazmararian J. The association between long work hours and leisure-time physical activity and obesity. Preventive Medicine Reports. 2018;10:271–277. doi: 10.1016/j.pmedr.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steyn N.P., Jaffer N., Nel J., Levitt N., Steyn K., Lombard C. Dietary intake of the urban black population of cape town: the cardiovascular risk in black South Africans (CRIBSA) study. Nutrients. 2016;8 doi: 10.3390/nu8050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atun R., Davies J.I., Gale E.A.M., Barnighausen T., Beran D., Kengne A.P. Diabetes in sub-Saharan Africa: from clinical care to health policy. The lance Diabetes & Endocrinology. 2017;5:622–667. doi: 10.1016/S2213-8587(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 42.Calder P.C., Bosco N., Bourdet-Sicard R., Capuron L., Delzenne N., Dore J. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Research Reviews. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A. Health effects of overweight and obesity in 195 countries over 25 years. New England Journal of Medicine. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolborea M., Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends in Neurosciences. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahima R.S., Antwi D.A. Brain regulation of appetite and satiety. Endocrinology and Metabolism Clinics of North America. 2008;37:811–823. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineda E., Sanchez-Romero L.M., Brown M., Jaccard A., Webber L., Jewell J. Achieving the 2025 WHO global health body-mass index targets: a modelling study on progress of the 53 countries in the WHO European region. The Lancet. 2016;388:S90. [Google Scholar]
- 47.Webber L., Divajeva D., Marsh T., McPherson K., Brown M., Galea G. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ Open. 2014;4:e004787. doi: 10.1136/bmjopen-2014-004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noble E.E., Kanoski S.E. Early life exposure to obesogenic diets and learning and memory dysfunction. Current Opinion in Behavioral Sciences. 2016;9:7–14. doi: 10.1016/j.cobeha.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cusick S.E., Georgieff M.K. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. The Journal of Pediatrics. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox S.E., Levitt P., Nelson C.A., 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker S.P., Wachs T.D., Gardner J.M., Lozoff B., Wasserman G.A., Pollitt E. Child development: risk factors for adverse outcomes in developing countries. Lancet (London, England) 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 52.Schellong K., Melchior K., Ziska T., Ott R., Henrich W., Rancourt R.C. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. The Journal of Nutritional Biochemistry. 2019;67:28–35. doi: 10.1016/j.jnutbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Barrand S., Crowley T.M., Wood-Bradley R.J., De Jong K.A., Armitage J.A. Impact of maternal high fat diet on hypothalamic transcriptome in neonatal Sprague Dawley rats. PLoS One. 2017;12:e0189492. doi: 10.1371/journal.pone.0189492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arruda A.P., Milanski M., Coope A., Torsoni A.S., Ropelle E., Carvalho D.P. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 55.Benoit S.C., Kemp C.J., Elias C.F., Abplanalp W., Herman J.P., Migrenne S. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. Journal of Clinical Investigation. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clegg D.J., Gotoh K., Kemp C., Wortman M.D., Benoit S.C., Brown L.M. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiology & Behavior. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S., Knight A.G., Gupta S., Keller J.N., Bruce-Keller A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. Journal of Neurochemistry. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posey K.A., Clegg D.J., Printz R.L., Byun J., Morton G.J., Vivekanandan-Giri A. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. American Journal of Physiology Endocrinology & Metabolism. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalby M.J., Aviello G., Ross A.W., Walker A.W., Barrett P., Morgan P.J. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein, SerpinA3N. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-33928-4. 15648–15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams L.M., Campbell F.M., Drew J.E., Koch C., Hoggard N., Rees W.D. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS One. 2014;9:e106159. doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lancaster G.I., Langley K.G., Berglund N.A., Kammoun H.L., Reibe S., Estevez E. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metabolism. 2018;27:1096–1110. doi: 10.1016/j.cmet.2018.03.014. e1095. [DOI] [PubMed] [Google Scholar]
- 65.Collino M. High dietary fructose intake: sweet or bitter life? World Journal of Diabetes. 2011;2:77–81. doi: 10.4239/wjd.v2.i6.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basciano H., Federico L., Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutrition & Metabolism. 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang I.S., Ho H., Hoffman B.B., Reaven G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension (Dallas, Tex.: 1979) 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 68.Thorburn A.W., Storlien L.H., Jenkins A.B., Khouri S., Kraegen E.W. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. American Journal of Clinical Nutrition. 1989;49:1155–1163. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 69.Serino A., Salazar G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients. 2018;11 doi: 10.3390/nu11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng Q., Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. Journal of Biological Chemistry. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purkayastha S., Zhang H., Zhang G., Ahmed Z., Wang Y., Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrews Z.B., Liu Z.W., Walllingford N., Erion D.M., Borok E., Friedman J.M. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbieri E., Sestili P. Reactive oxygen species in skeletal muscle signaling. Journal of Signal Transduction. 2012;2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diano S., Liu Z.W., Jeong J.K., Dietrich M.O., Ruan H.B., Kim E. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nature Medicine. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horvath T.L., Andrews Z.B., Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends in Endocrinology & Metabolism: TEM. 2009;20:78–87. doi: 10.1016/j.tem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S. Reactive oxygen species enhance insulin sensitivity. Cell Metabolism. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cintra D.E., Ropelle E.R., Moraes J.C., Pauli J.R., Morari J., Souza C.T. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7:e30571. doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasileva L.V., Marchev A.S., Georgiev M.I. Causes and solutions to “globesity”: the new fa(s)t alarming global epidemic. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2018;121:173–193. doi: 10.1016/j.fct.2018.08.071. [DOI] [PubMed] [Google Scholar]
- 79.Pistollato F., Giampieri F., Battino M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association. 2015;75:58–70. doi: 10.1016/j.fct.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Queen B.L., Tollefsbol T.O. Polyphenols and aging. Current Aging Science. 2010;3:34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ganesan K., Xu B. A critical review on polyphenols and health benefits of black soybeans. Nutrients. 2017;9 doi: 10.3390/nu9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez-Pinilla F., Nguyen T.T. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutritional Neuroscience. 2012;15:127–133. doi: 10.1179/1476830511Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocha N.P., de Miranda A.S., Teixeira A.L. Insights into neuroinflammation in Parkinson's disease: from biomarkers to anti-inflammatory based therapies. BioMed Research International. 2015;2015:628192. doi: 10.1155/2015/628192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freyssin A., Page G., Fauconneau B., Rioux Bilan A. Natural polyphenols effects on protein aggregates in Alzheimer's and Parkinson's prion-like diseases. Neural Regeneration Research. 2018;13:955–961. doi: 10.4103/1673-5374.233432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarubbo F., Moranta D., Pani G. Dietary polyphenols and neurogenesis: molecular interactions and implication for brain ageing and cognition. Neuroscience and Biobehavioral Reviews. 2018;90:456–470. doi: 10.1016/j.neubiorev.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fourrier C., Sampson E., Mills N.T., Baune B.T. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials. 2018;19:447. doi: 10.1186/s13063-018-2829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magrone T., Russo M.A., Jirillo E. Cocoa and dark chocolate polyphenols: from biology to clinical applications. Frontiers in Immunology. 2017;8:677. doi: 10.3389/fimmu.2017.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu Y., Wu Y., Szabo A., Wu Z., Wang H., Li D. Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology. 2013;154:3130–3140. doi: 10.1210/en.2013-1218. [DOI] [PubMed] [Google Scholar]
- 92.Okuda M.H., Zemdegs J.C., de Santana A.A., Santamarina A.B., Moreno M.F., Hachul A.C. Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. The Journal of Nutritional Biochemistry. 2014;25:1084–1089. doi: 10.1016/j.jnutbio.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 93.Benzler J., Ganjam G.K., Pretz D., Oelkrug R., Koch C.E., Legler K. Central inhibition of IKKbeta/NF-kappaB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64:2015–2027. doi: 10.2337/db14-0093. [DOI] [PubMed] [Google Scholar]
- 94.Sun W., Yan C., Frost B., Wang X., Hou C., Zeng M. Pomegranate extract decreases oxidative stress and alleviates mitochondrial impairment by activating AMPK-Nrf2 in hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Scientific Reports. 2016;6:34246. doi: 10.1038/srep34246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J., Kim C.S., Tu T.H., Kim M.S., Goto T., Kawada T. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients. 2017;9 doi: 10.3390/nu9070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibars M., Ardid-Ruiz A., Suarez M., Muguerza B., Blade C., Aragones G. Proanthocyanidins potentiate hypothalamic leptin/STAT3 signalling and Pomc gene expression in rats with diet-induced obesity. International Journal of Obesity. 2017;41:129–136. doi: 10.1038/ijo.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qi G., Mi Y., Wang Y., Li R., Huang S., Li X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food & Function. 2017;8:4421–4432. doi: 10.1039/c7fo00991g. [DOI] [PubMed] [Google Scholar]
- 98.Lawes C.M., Vander Hoorn S., Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet (London, England) 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 99.Zhou D., Xi B., Zhao M., Wang L., Veeranki S.P. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III Linked Mortality Study. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-27377-2. 9418–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaudhari A., Gupta R., Makwana K., Kondratov R. Circadian clocks, diets and aging. Nutrition and Healthy Aging. 2017;4:101–112. doi: 10.3233/NHA-160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masood T., Kushwaha R.S., Singh R., Sailwal S., Pandey H., Varma A. Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discoveries & Therapeutics. 2015;9:70–74. doi: 10.5582/ddt.2015.01002. [DOI] [PubMed] [Google Scholar]
- 102.Miller B., Norman A.W. Evidence for circadian rhythms in the serum levels of the vitamin D-dependent calcium-binding protein and in the activity of the 25-hydroxyvitamin D3-1-alpha-hydroxylase in the chick: studies on the mode of action of calciferol. FEBS Letters. 1982;141:242–244. doi: 10.1016/0014-5793(82)80057-4. [DOI] [PubMed] [Google Scholar]
- 103.McNay D.E., Briancon N., Kokoeva M.V., Maratos-Flier E., Flier J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. Journal of Clinical Investigation. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Recabal A., Caprile T., García-Robles M.D.L.A. Hypothalamic neurogenesis as an adaptive metabolic mechanism. Frontiers in Neuroscience. 2017;11 doi: 10.3389/fnins.2017.00190. 190–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stangl D., Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes & Nutrition. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattson M.P. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Research. 2000;886:47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- 107.Kumar V., Pandey A., Jahan S., Shukla R.K., Kumar D., Srivastava A. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Scientific Reports. 2016;6:28142. doi: 10.1038/srep28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu L., Zhang Q., Cai Y., Sun D., He X., Wang L. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7:56045–56059. doi: 10.18632/oncotarget.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poulose S.M., Miller M.G., Scott T., Shukitt-Hale B. Nutritional factors affecting adult neurogenesis and cognitive function. Advances in Nutrition (Bethesda, Md.) 2017;8:804–811. doi: 10.3945/an.117.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valenti D., de Bari L., de Rasmo D., Signorile A., Henrion-Caude A., Contestabile A. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochimica et Biophysica Acta. 2016;1862:1093–1104. doi: 10.1016/j.bbadis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Rao T.S., Asha M.R., Ramesh B.N., Rao K.S. Understanding nutrition, depression and mental illnesses. Indian Journal of Psychiatry. 2008;50:77–82. doi: 10.4103/0019-5545.42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Opie R.S., Itsiopoulos C., Parletta N., Sanchez-Villegas A., Akbaraly T.N., Ruusunen A. Dietary recommendations for the prevention of depression. Nutritional Neuroscience. 2017;20:161–171. doi: 10.1179/1476830515Y.0000000043. [DOI] [PubMed] [Google Scholar]
- 113.Trebaticka J., Durackova Z. Psychiatric disorders and polyphenols: can they Be helpful in therapy? Oxidative Medicine and Cellular Longevity. 2015;2015:248529. doi: 10.1155/2015/248529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Craft L.L., Perna F.M. The benefits of exercise for the clinically depressed. Primary Care Companion to the Journal of Clinical Psychiatry. 2004;6:104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambert K., Hokayem M., Thomas C., Fabre O., Cassan C., Bourret A. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Scientific Reports. 2018;8:2885. doi: 10.1038/s41598-018-21287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]