Abstract
Thyroid hormone (TH) is critical for many aspects of neurodevelopment and can be disrupted by a variety of environmental contaminants. Sensory systems, including audition and vision are vulnerable to TH insufficiencies, but little data are available on visual system development at less than severe levels of TH deprivation. The goal of the current experiments was to explore dose-response relations between graded levels of TH insufficiency during development and the visual function of adult offspring. Pregnant Long Evans rats received 0 or 3 ppm (Experiment 1), or 0, 1, 2, or 3 ppm (Experiment 2) of propylthiouracil (PTU), an inhibitor of thyroid hormone synthesis, in drinking water from gestation day (GD) 6 to postnatal day (PN) 21. Treatment with PTU caused dose-related reductions of serum T4, with recovery on termination of exposure, and euthyroidism by the time of visual function testing. Tests of retinal (electroretinograms; ERGs) and visual cortex (visual evoked potentials; VEPs) function were assessed in adult offspring. Dark-adapted ERG a-waves, reflecting rod photoreceptors, were increased in amplitude by PTU. Light-adapted green flicker ERGs, reflecting M-cone photoreceptors, were reduced by PTU exposure. UV-flicker ERGs, reflection S-cones, were not altered. Pattern-elicited VEPs were significantly reduced by 2 and 3 ppm PTU across a range of stimulus contrast values. The slope of VEP amplitude-log contrast functions was reduced by PTU, suggesting impaired visual contrast gain. Visual contrast gain primarily reflects function of visual cortex, and is responsible for adjusting sensitivity of perceptual mechanisms in response to changing visual scenes. The results indicate that moderate levels of pre-and post-natal TH insufficiency led to alterations in visual function of adult rats, including both retinal and visual cortex sites of dysfunction.
Keywords: hypothyroidism, hypothyroxinemia, visual system, electroretinogram, visual evoked potentials, developmental, brain, neurotoxicity
INTRODUCTION
Adequate supplies of thyroid hormone (TH) are critical for normal brain development. Lack of TH during gestation and the early postnatal period results in severe neurological deficits. Concentrations of TH measured in the serum are disrupted by a variety of environmental contaminants, including polyhalogenated aromatic hydrocarbons (PHAHs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), perchlorate, and triclosan (Brucker-Davis, 1998, USEPA, 2013). The integrity of sensory organs, including the cochlea and retina, require TH for normal development and function (Jones et al., 2003, Ng et al., 2013, Ng et al., 2015). Hypothyroidism in humans and in laboratory animals leads to cochlear hair cell loss, altered neocortical cytoarchitecture, and hearing impairments (Meyerhoff, 1979, Uziel et al., 1983, Berbel et al., 1985, Goldey et al., 1995, Knipper et al., 2000, Lavado-Autric et al., 2003, Auso et al., 2004).
In the retina, developmental hypothyroidism causes a reduction of retinal progenitor cells, reduced retinal thickness, altered photoreceptor patterning, improperly formed photoreceptor outer segments, and reduced cell density in the retinal ganglion cell layer (Navegantes et al., 1996, Ng et al., 2001, Sevilla-Romero et al., 2002, Harpavat and Cepko, 2003, Roberts et al., 2006, Ng et al., 2011, Pinazo-Duran et al., 2011, Ma and Ding, 2016). Post-mitotic retinal precursor cells differentiate into all types of photoreceptors including rods, middle-wavelength sensitive (M, “green”) cones, short-wavelength sensitive (S, “blue”) cones. M-cones are formed from S-cones in response to TH signaling through the TRβ2 receptor (Ng et al., 2011). Knockout of thyroid hormone receptor TRβ2 results in a selective loss of M-cones and remaining cones all express S-cone opsin of (Ng et al., 2001).
Perturbation of the thyroid systems also negatively impacts the post-retinal components of the visual system, including the thalamus, visual and parietal cortices (Martinez-Galan et al., 2004). TH is necessary in the mature brain to maintain the neuronal spine density in the visual cortex and hippocampus (Ruiz-Marcos et al., 1979, Gould et al., 1990), whereas in the developing brain, thyroid signaling guides both the formation and refinement of CNS neural connections. Hypothyroidism beginning in early development alters the expression of trophic factors, deiodination enzymes, TH receptors, and neuronal and glial migratory patterns in the developing rodent neocortex (Berbel et al., 1985, Guadano-Ferraz et al., 1999, Martinez-Galan et al., 2004, Royland et al., 2008, Gilbert et al., 2016). Severe hypothyroidism beginning in late gestation alters axonal projections from the visual cortex to the spinal cord pyramidal tract (Li et al., 1995). Iodine deficiency resulting in reductions in maternal and offspring TH levels, reduces neuronal cell counts and synaptic number in the visual cortex (Mano et al., 1987). Developmental hypo- or hyper- thyroid conditions in mice disrupt the stability of axonal branching and synaptic boutons in layers 1 and 2 of visual cortex in grown offspring (Strobl et al., 2017). Overall, these experimental findings in rodents and nonhuman primates demonstrate the essential role of TH signaling in the structural integrity of the visual system.
Visual system processing deficits have also been reported in children with compromised thyroid status (Zoeller and Rovet, 2004). Premature infants, who are deprived of a maternal source of TH in late gestation, exhibit deficiencies in visual attention, visual contrast sensitivity, color vision, and visuomotor skills (Rovet and Simic, 2008, Simic et al., 2013). Children born to hypothyroid mothers, or suffering from thyroid gland dysgenesis at birth, exhibit both impaired color vision and visual contrast sensitivity (Mirabella et al., 2005, Simic et al., 2013, Simic and Rovet, 2017).
In support of these clinical findings, animal studies linking TH deficits with impaired visual system development have been reported. These data, however, have been largely derived from experiments with severe hormone compromise (i.e., thyroid ablation, gene deletion, high doses of TH synthesis inhibitors) causing virtually complete elimination of measurable TH. In contrast, exposures to environmental contaminants typically occur at concentrations causing only moderate dysregulation of TH, much less extreme than the existing experimental data. Previously, we demonstrated that low-moderate doses of the TH synthesis inhibitor, propylthiouracil (PTU), inducing graded levels of TH insufficiency in pregnant rats, caused brain neurochemical and structural abnormalities, hippocampal electrophysiology impairment, and learning deficits in exposed offspring (Sharlin et al., 2008, Gilbert, 2011, Lasley and Gilbert, 2011, Gilbert et al., 2013, Gilbert et al., 2014, Gilbert et al., 2016). However, it is unclear what magnitude of TH disruption presents a concern for impaired visual system development. Therefore, using the same experimental model, the present study examined dose-response relationships between moderate TH reductions during development and visual function of grown adult offspring.
METHODS
Subjects.
Pregnant Long-Evans rats were obtained from Charles River (Raleigh, NC) on gestational day (GD) 2 and housed individually in standard plastic hanging cages. The housing rooms were maintained on a 12:12 light-dark cycle. Animals were permitted free access to standard laboratory chow and, outside of the dosing period, tap water. Beginning on GD6 and continuing until postnatal day (PN) 21, dams received 0 or 3 ppm (Experiment 1) or 0, 1, 2 or 3 ppm (Experiment 2) of PTU dissolved in deionized drinking water (0–0.0003% solutions). The day of birth was designated PN0 and all litters were culled to 10 pups on PN4. Exposure to PTU terminated when pups were weaned on PN21. At weaning, offspring were transferred to plastic hanging cages (two animals of same sex/cage) and were permitted free access to food and tap water. Body weights of dams were monitored throughout gestation and the postnatal period. Body weights of offspring were monitored from PN5-PN35 and are reported elsewhere (Lasley and Gilbert, 2011). Only male pups were selected for visual function testing because of the extensive time required for electrophysiological testing, the limited time available for testing, and the principle aim of the experiment being to assess dose-response relationships between PTU treatment and visual function changes. These considerations determined a need to restrict other factors, such as sex of the offspring, in order to limit measurement variance and maximize the statistical power to detect significant differences between treatment groups.
The animal facility followed the guidelines of the National Institutes for Health for animal care, and was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the USEPA National Health and Environmental Effects Research Laboratory (NHEERL), which ensured conformance with the 2004 National Research Council “Guide for the Care and Use of Laboratory Animals, Eighth Edition”, the Animal Welfare Act and Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
Chemical Sources.
Phenylephrine hydrochloride (2.5%), Proparacaine hydrochloride (0.5%), Tropicamide (0.75%) and USP eyedrops were obtained from (Bausch and Lomb, Irvine CA). Xylazine (TranquiVed Injection, USP) was obtained from Vedco, Inc, St. Joseph MO). Ketamine (Ketaset, ketamine hydrochloride, USP) was obtained from Fort Dodge Animal Health (Fort Dodge, IA). Nembutal sodium solution (Pentobarbital sodium, USP) was obtained from Akorn, (Forest Hills IL). 6-n-Propylthiouracil was obtained from Sigma (St. Louis MO).
Experimental Designs.
Two experiments are reported. Adult male offspring of Experiment 1 (0 or 3 ppm PTU) were evaluated on a series of ERGs and pattern-elicited VEPs. Visual function tests were collected in animals PN 63–86 days of age. Each group was comprised of 6–7 rats derived from 3–4 litters/treatment group. Experiment 2 was designed to further explore dose-response relationships between developmental TH reductions and altered visual function in the adult. To accommodate the number of animals to be processed in dose-response assessment, testing was restricted to VEP recordings. Dose-response relationships for VEPs were examined in adult offspring exposed perinatally to 0, 1, 2, or 3 ppm PTU. A schematic of the timeline of the two experiments is presented in the figures to follow. Final sample sizes in Experiment 2 were: 0 ppm, n=9 litters (16 pups); 1 ppm, n= 8 litters (15 pups); 2 ppm, n=10 litters (19 pups); 3 ppm; n=8 litters (12 pups) (Supplemental Table S1). No more than 3 pups were taken from any given litter. As discussed below, the statistical analysis considered litter to be the unit of analysis, and pups within a litter were treated as repeated within-subject samples.
Electroretinograms:
ERGs were recorded using a modified UV-capable Espion E2 Colordome system (Diagnosis, LLC, Lowell, MA) as previously described (Boyes et al., 2014, Boyes et al., 2016). Briefly, three sets of ERGs were recorded including: (1) a dark-adapted (scotopic) flash series, (2) a light-adapted (photopic) UV-flicker series; and (3) a photopic green flicker series. All animals were assessed for retinal function (ERG) at least 1 week after completion of VEP testing. Animals were dark adapted overnight prior to ERG recording and all animal handling and preparations were conducted under dim red light. Rats were anesthetized (ketamine (80 mg/kg) / xylazine (8 mg/kg), placed in a flexible plastic cone, and secured on a circulating warm water blanket. Body temperatures were monitored with a rectal thermometer. Subdermal needle electrodes were placed in the back of the neck for grounding and at the lateral canthus of the left eye for reference. Proparacaine HCl (0.5%) was administered dropwise to anesthetize the left eye, while both tropicamide (0.75%) and phenylephrine HCl (2.5%) were administered dropwise to dilate the pupil. The active electrode was a loop of wire (10% iridium/90% platinum) in gentle contact with the cornea. The cornea was kept moist throughout the session with drops of USP eyedrops. ERGs were recorded from each rat in a single test session in the following order: dark-adapted ERGs proceeding from dim to bright flashes, UV flicker ERGs with a green light adaptation background and, finally, green flicker ERGs with a UV adaptation background.
The scotopic flash ERG series, designed to assess both retinal rod photoreceptor (a-wave) and bipolar cell (b-wave) functions, involved single sweep ERGs recorded at incrementing half log steps over a range of 5 log luminance values. An adaptation period of 30 s (dimmer flashes) or 1 min (brighter flashes) was allowed between each flash. Electrophysiological signals were amplified, filtered (bandpass 1.25–300 Hz) and sampled (1000 Hz). Recordings were made for each single flash. (i.e. no signal averaging). Peaks were scored automatically for a- and b-waves, and subsequently confirmed by a trained operator. The latencies and amplitudes of a- and b-wave peaks were recorded, with b-wave amplitudes calculated as the peak-to-peak difference from the a-waves.
The UV flicker ERG series, designed to assess S-cone photoreceptor function, involved stimuli (340–400 nm with a peak wavelength of 370 nm) flickered at 21 Hz (Eells et al., 2003), a rate that is above the temporal response limit of rod photoreceptors (Moses et al., 1987). The UV stimulus levels were 0.7, 2.4 and 7.8 μW/cm2/nm, against a steady green background light (515 nm, 0.93 μW/cm2/nm) to suppress any potential contribution of M-cones. Electrophysiological data were bandpass filtered (10–30 Hz) and digitally sampled at 1000 Hz. Recordings were averaged over 20 sweeps of 2000 ms each. Spectral analysis (Proc Spectra, SAS Institute Inc., Cary NC) of the resulting waveforms calculated the spectral amplitude density as the square-root of the Fourier analysis power spectrum at the stimulus rate (i.e. 21 Hz), which was used as the dependent variable for statistical analysis.
The green flicker ERG procedure, designed to assess M-cone photoreceptor function, was analogous to that for the UV flicker series, except that the green LEDs (480–560 nm with a peak of 520 nm) were flickered at 21 Hz while the UV stimulus was held steady. Green stimuli were presented at four levels of illuminance including 1, 3, 10 and 30 cd*sec/m2. Other aspects of the green flicker ERG recordings were identical to those of the UV flicker.
Visual Evoked Potentials.
Rats were prepared surgically with implanted cranial electrodes for subsequent recordings of VEPs. Adult male offspring were anesthetized with sodium pentobarbital (50 mg/kg, ip), placed in a stereotaxic device, and the skull was exposed. Indwelling screw electrodes were implanted over primary visual cortex (1 mm anterior and 4 mm left of lambda) and a ground reference over the frontal cortex (2 mm anterior and 2 mm lateral left and right of bregma) according to procedures described previously (Herr et al., 1992, Boyes et al., 2003). The electrodes were constructed from stainless steel screws (00–90 × 1/16”) soldered to Nichrome wires. The electrodes were attached to a connector and assembly encased in dental acrylic. Animals were housed singly after surgery and received 0.1 cc Rimadyl (sc) post-surgery and a 2 mg Rimadyl oral tablet the following day for analgesia. Approximately one week was allowed for surgical recovery before VEP testing.
Visual evoked potentials were recorded in a Faraday cage from awake rats, restrained in flexible tubes with their heads exposed, and with their eyes approximately 15 cm from the stimulus monitor, as described elsewhere (Hamm et al., 2000, Boyes et al., 2014, Boyes et al., 2016).
The stimuli to elicit pattern VEPs were generated with the green gun of the video monitor using a computer-based system described elsewhere (Hamm et al., 2000), and presented on a video monitor located outside the Faraday cage. The system was calibrated to achieve a linear relationship between video input signal voltage and screen luminance.
The stimulus patterns were vertical gratings with a sinusoidal spatial luminance profile, a spatial frequency of 0.16 cycles per degree visual angle, and a mean luminance of 50 lux. In Experiment 1, the stimulus temporal modulation was either pattern on/off (appearance/disappearance) or pattern reversal (phase alternation) modulated at 4.55 Hz. Stimulus peak contrast values included 0, 2, 4, 6, 8, 12, 16, 24, 32, 48 and 64 %. Thus, a series of 22 VEP waveforms was recorded from each subject (2 temporal modulation modes multiplied by 11 levels of contrast). The recording noise level was defined by the amplitude of the average evoked potential recorded to the 0% contrast stimulus (i.e. a non-modulated, pattern-free, mean luminance screen). Recordings to stimuli with contrast values between 2 – 8% were generally not different from noise and were removed from the analysis. Experiment 2 employed identical procedures except they included only pattern reversal modulation and contrast values of 0, 8, 12, 16, 24, 32, 48, 64 and 80 %. In both experiments, the different stimulus conditions were presented in a random order with a 1-minute adaptation period between each during which the non-patterned mean luminance screen was presented.
Electrophysiological potentials were amplified (10K), bandpass filtered (0.1–1000 Hz), and sampled at a rate of 1.2 KHz in five sec epochs. Each VEP was the average of 20 5-sec epochs, and each averaged waveform was submitted to a Fast Fourier Transform spectral analysis. The spectral amplitude was measured at twice the rate of the visual pattern modulation (F2).
Statistical Analyses.
Electrophysiological data from five electrophysiological protocols were analyzed using repeated measures mixed linear models fitted in the SAS MIXED procedure [SAS Institute Inc. 2015. SAS/STAT® 13.1 User’s Guide. Cary, NC: SAS Institute Inc.]. Each of the procedures included a within-subjects’ stimulus parameter in which the perceptual strength of the stimulus was manipulated to evaluate stimulus input-output functions and to demonstrate that the responses were under stimulus control. These stimulus parameters were: stimulus luminance for scotopic flash ERG and green flicker ERG; radiance for UV flicker ERGs; and visual pattern contrast for VEPs.
The model dependent variables were amplitude and latency of a-waves and b-waves for scotopic ERG data, amplitude derived from spectral analysis at the stimulation rate (F1) for UV and green flicker ERG data, and spectral amplitude at twice the stimulus rate (F2) for VEP. Repeated measures mixed linear models for amplitude and latency measurements were fitted to assess relationships with the effects of dose, the stimulus parameter (luminance, radiance or visual pattern contrast), the interaction of dose and stimulus parameter, for VEP in the first experiment temporal modulation (either on-off or pattern-reversal), and litter. The repeated measures stimulus parameters were modeled using first-order autoregressive AR(1) or banded Toeplitz TOEP(2) covariance structure with litter as the unit of analysis; the number of litters determined each experiment’s sample size. The variation in the dependent variables for a litter was modeled as two types: between-litter and within-litter variation (Dempster et al., 1984, Chen, 2006). The random effect of litter accounted for variation between litters and was modeled using variance components VC (the default) covariance structure. The electrophysiological amplitude data were log-transformed to stabilize variance, as indicated by model residuals; latency measurements were not transformed. SAS code is provided in Supplemental Table 2 to illustrate the repeated measures mixed linear model syntax used for the five protocols.
RESULTS
Body Weights and Serum Thyroid Hormones.
No significant alterations in dam or pup weights were detected in response to 1–3 ppm PTU from gestation through PN22, as previously reported (Lasley and Gilbert, 2011). Serum TH concentrations expressed here as a percent of control (Table 1) were derived from data previously reported by Lasley and Gilbert (Lasley and Gilbert, 2011) and are included here for completeness. Thyroid hormone concentrations were reduced in a dose-dependent manner in dams and pups in response to 1, 2 and 3 ppm of PTU. The primary change was seen in T4 with minimal reductions evident in T3 restricted to the highest dose group on PN14 only. Both hormones had fully recovered to control levels before the time of visual function testing (Lasley and Gilbert, 2011).
Table 1.
Mean (±SEM) serum T3 and T4 expressed as percent of control. Hormones were measured by radioimmunoassay (Diagnostic Products) in offspring on postnatal days (PN) 14, 21 and as adults at approximately PN90. Thyroid hormones were measured in dams at weaning of the pups on PN21. ANOVA followed by mean contrast tests show significant differences from 0 ppm control groups within each age group (Dunnett’s t-test, * p<0.05). A portion of these data were adapted from a previously published report and are included here for completeness (Lasley and Gilbert, 2011).
| 0 ppm | 1 ppm | 2 ppm | 3 ppm | ||
|---|---|---|---|---|---|
| Dam | T4 | 100.1±4.83 | 67.1±7.08* | 49.6±9.49* | 28.9±5.52* |
| PN21 | T3 | 100.0±4.57 | 91.6±8.18 | 106.1±10.11 | 81.2±4.76 |
| n | 13 | 10 | 12 | 14 | |
| Pup | T4 | 100.0±4.27 | 47.8±4.31* | 13.6±2.26* | 8.4±2.44* |
| PN14 | T3 | 100.0±2.44 | 104.8±7.04 | 106.2±5.61 | 77.7±5.57* |
| n | 12 | 10 | 11 | 13 | |
| Pup | T4 | 100.2±4.69 | 78.2±3.45* | 36.2±3.09* | 24.5±3.67* |
| PN21 | T3 | 100.0±2.50 | 89.49±3.61 | 86.7±3.57* | 81.4±5.02* |
| n | 12 | 10 | 10 | 10 | |
| Pup | T4 | 100.0±7.57 | 96.3±4.26 | 90.0±4.73 | 87.3±7.52 |
| PN90 | T3 | 100.0±4.38 | 94.5±3.27 | 95.2±3.19 | 99.6±6.84 |
| n | 14 | 18 | 20 | 12 | |
Electroretinograms.
Flash ERGs.
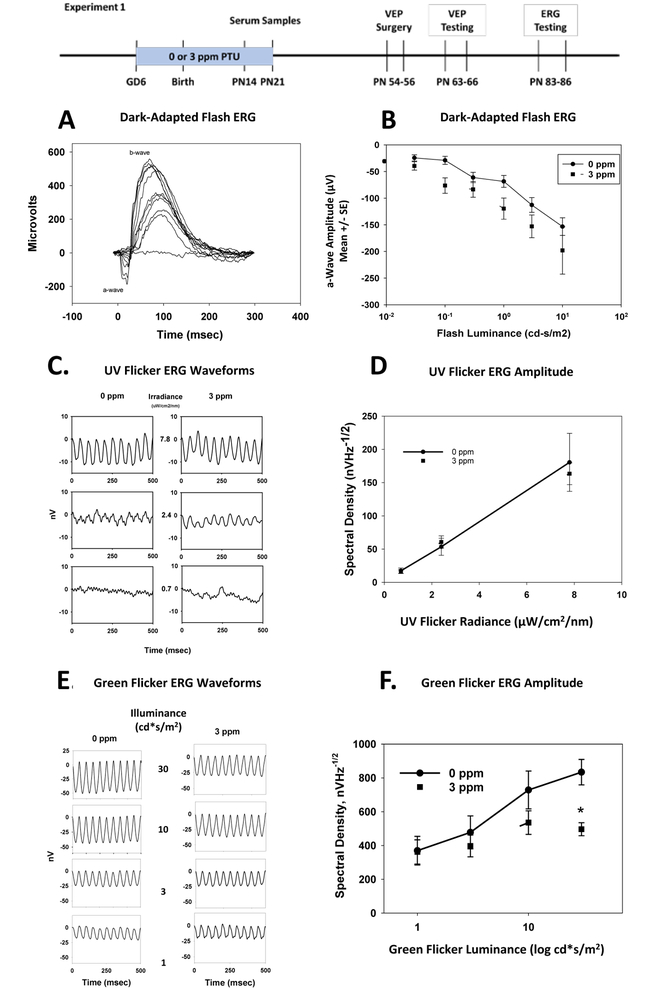
Example dark-adapted ERG waveforms from a representative control rat recorded to a series of increasing flash intensity values are presented in Figure 1A. The waveforms showed the presence of a b-wave, usually at the second or third lowest flash intensity, which grew in amplitude and reduced in latency with increasing flash luminance. The a-wave, typically observed beginning around the 5th of 11 stimulus levels, grew in amplitude and reduced in latency thereafter. Group mean a-wave amplitudes are presented in Figure 1B. In analyzing data from the 6 higher stimulus luminance values, there was a statistically significant increase in a-wave amplitude in rats treated with 3 ppm PTU. There was a main effect on a-wave amplitude of PTU treatment [F(1,68) = 4.77, p=0.0324], and a significant main effect of stimulus luminance [F(1,68) = 22.68, p<0.0001], but no significant treatment-by-luminance interaction [F(1,68) = 0.76, p=0.3849]. The latency of a-waves did not show a significant main effect of PTU treatment [F(1,69) = 1.16, p=0.2844], but did show significant effects of stimulus luminance [F(1,69) = 12.51, p=0.007] and a significant treatment-by-intensity interaction [F(1,69) = 4.03, p=0.04]. These results suggest increased function of rod photoreceptors following developmental exposure to PTU. There were no statistically significant changes in b-wave parameters (Supplemental Figure S1).
Figure 1.
Electroretinograms (ERGs) (Experiment 1).
Panel A. Dark-Adapted Flash ERG Waveforms. Waveforms are presented from a representative control rat (0 ppm PTU) recorded from a sequence of flashed stimuli that progressed from very dim to bright. The a-wave and b-wave are indicated. The response reflects primarily rod photoreceptors (a-wave) and retinal on-bipolar cells (b-wave). The response components grew systematically in amplitude and decreased in latency with increasing flash illumination.
Panel B. Group mean (± SE) dark-adapted ERG a-wave amplitudes, as a function of flash intensity. Adult offspring were previously treated with either 0 or 3 ppm PTU in drinking water from GD2-PN21. The a-wave amplitudes were significantly increased in amplitude by PTU treatment.
Panel C. UV-Flicker ERGs group mean waveforms. ERGs were recorded from light-adapted rats to a UV stimulus flickering at 21 Hz against a steady green background to isolate responses of S-cone photoreceptors. Group average responses to three levels of UV irradiance are shown for rats exposed to 0 or 3 ppm PTU.
Panel D. Mean (± SE) amplitude of the UV-Flicker ERGs as a function of UV stimulus irradiance. Amplitude is expressed as spectral density at 21 Hz, as derived from spectral analysis of the individual rat waveforms. There were no statistically significant effects of PTU treatment on UV ERG response amplitude.
Panel E. Green-Flicker ERGs group mean waveforms. ERGs were recorded from light-adapted rats to a green light flickering at 21 Hz against a steady UV background to isolate responses of M-cone photoreceptors. Group average responses to four levels of luminance are shown for rats exposed to 0 or 3 ppm PTU.
Panel F. Mean (± SE) amplitude of the Green Flicker ERGs as a function of green flicker stimulus luminance on a log scale. Amplitude is expressed as spectral density at 21 Hz, as derived from spectral analysis of the individual rat waveforms. There was a statistically significant interaction between PTU treatment and flicker illuminance. Treatment with PTU caused a statistically significant reduction of green-flicker ERG amplitude at the highest value of luminance tested (30 cd*s/m2)
UV ERGs:
The group-mean waveforms for UV flicker ERGs were sinusoidal responses with the primary frequency at the stimulus rate (Figure 1C). There were no statistically significant effects of PTU treatment on the amplitude of UV flicker ERGs [F(1,27)=0.04, p=0.8494] and no significant treatment–by-irradiance interaction [F(1,27)=0.04, p = 0.8265]. (Figure 1D; There was, however, a statistically significant main effect of stimulus irradiance [F(1,27) = 104.76, p<0.0001), indicating that the system was responsive to the manipulation of stimulus radiance, and the measurements sensitive enough to reflect different levels of stimulation. The UV flicker response is thought to be generated by S-cone photoreceptors, and the results of this experiment suggest a lack of effect on S-cones from developmental PTU treatment.
Green Flicker ERGs:
The group mean waveforms for Green Flicker ERG were sinusoidal steady state responses with a primary frequency at the rate of stimulation (21 Hz). The amplitude of the green flicker waveform grew with increasingly brighter green flicker stimuli (Figure 1E). The group-mean green flicker ERGs are presented in Figure 1F. Although there was no statistically significant main effect of PTU treatment on green flicker ERG amplitude [F(1,43) = 0.25, p=0.6202], there was a significant effect of stimulus luminance [F(1,43) = 28.54 p<0.0001], and a significant treatment-by-stimulus luminance interaction [F(1,43) = 6.22, p=0.0166]. This result indicates that there was a decreased function of M-cones in adult rats treated with PTU during development.
Visual Evoked Potentials.
Experiment 1:
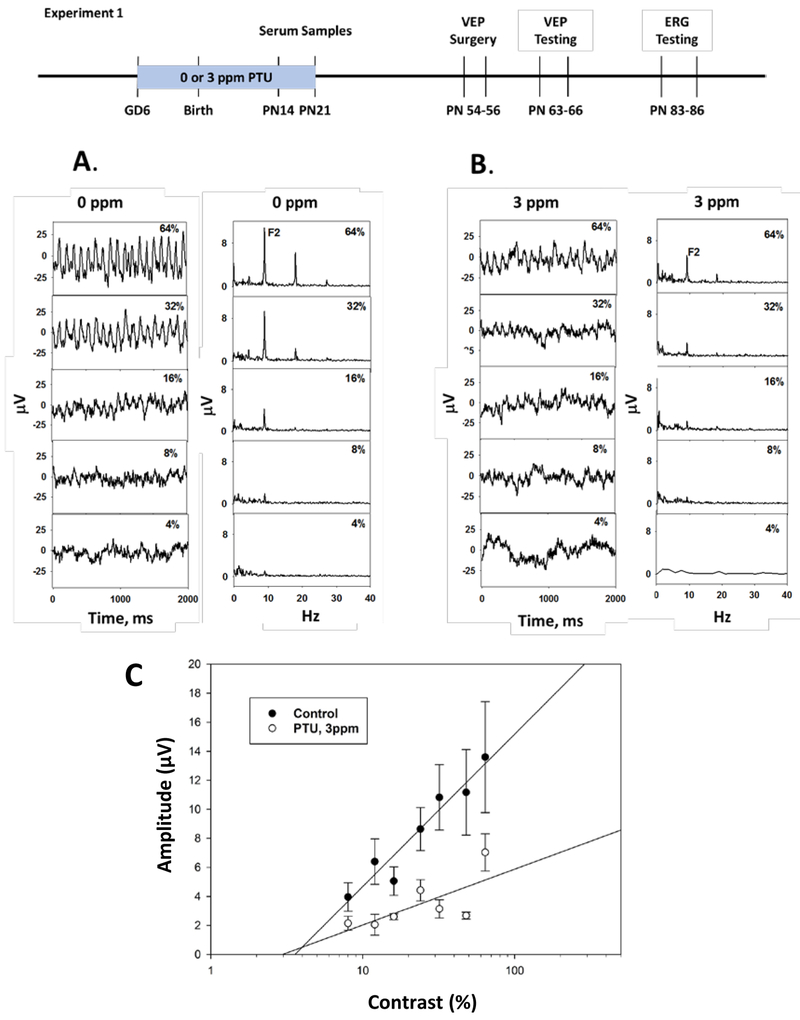
Group mean VEP waveforms are presented in Figure 2 from rats exposed to 0 (Figure 2A) or 3 ppm PTU (Figure 2B) along with a spectral analysis of each corresponding group mean waveform. For simplicity, only data from pattern reversal stimulation are presented. Data from on/off modulation showed a similar overall pattern of effects to pattern-reversal, and are presented in Supplemental Figure S2. Pattern reversal VEP waveforms and their spectral analysis showed a primary response frequency at twice the pattern modulation rate (F2) and the amplitude of the responses increased as a function of the pattern contrast. The amplitude of F2 increased as a linear function of log stimulus contrast (Figure 2C). The F2 amplitude was reduced in animals who received 3 ppm PTU during development. There was no statistically significant main effect of PTU treatment [F (1,254) = 0.39, p = 0.5330], but there was a significant PTU treatment-by-contrast interaction [F (1,254) = 18.42, p<0.0001]. There were also statistically significant effects on F2 amplitude of the temporal modulation (pattern on-off vs pattern reversal) [F(1,254) = 226.96, p<0.001], and stimulus contrast [F(1,254) = 352.85, p<0.0001].
Figure 2.
Visual Evoked Potentials (VEPs) (Experiment 1). VEPs were recorded from adult offspring to pattern-reversal stimulation with differing levels of stimulus contrast. For clarity waveforms are displayed only for values of 4, 8, 16, 32 and 64% contrast.
Panel A. Group average waveforms (left) and spectra (right) from control animals showing increased response amplitude as a function of increased stimulus contrast (bottom to top). Spectral analysis of each waveform revealed response peaks at twice the stimulus rate (F2) and higher harmonic frequencies. The amplitude at F2 was used as the principle dependent measure.
Panel B. Comparable group average waveforms from rats treated with 3 ppm PTU from GD2-PN21.
Panel C. VEP F2 amplitude (mean ± SE) as a function of stimulus contrast on a log scale recorded from adult rats treated developmentally with 0 or 3 ppm PTU. There was a statistically significant reduction of F2 amplitude following treatment with 3 ppm PTU, and a significant dose-by-contrast interaction.
Experiment 2:
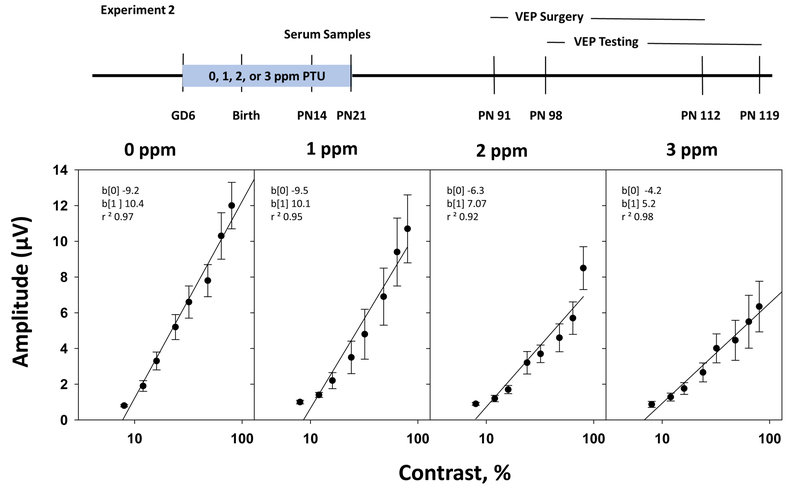
The group mean F2 amplitudes of rats treated with 0, 1, 2, or 3 ppm PTU are plotted as a function of log stimulus contrast in Figure 3, along with linear regression equations fit to the group mean data. There was no statistically significant main effect of PTU treatment on F2 amplitudes [F(3,519) = 0.03, p = 0.992]. However, F2 amplitudes were significantly altered by stimulus contrast [F(1,519) = 674.36, p<0.0001] and there was a significant PTU treatment-by-contrast interaction [F(3,519) = 5.45), p<0.0001]. By limiting the dose groups entered into the analysis, it was found that groups treated with 2 ppm PTU; [treatment-by-contrast interaction F(1,294)= 10.52, p=0.0013] and 3 ppm PTU [treatment-by-contrast interaction F(1.233)=13.05, p=0.0004] were significantly different from controls. The group treated with 1 ppm PTU did not differ significantly from control [treatment-by-contrast interaction F(1,260) = 2.66, p=0.1040].
Figure 3.
(Experiment 2). VEP Contrast-Amplitude Functions: Plots of VEP F2 amplitude vs. pattern contrast (log scale) for adult offspring treated with 0, 1, 2, or 3 ppm PTU during development. The zero-amplitude intercept of the linear regression function is interpreted as the threshold for contrast perception, and the slope is interpreted as contrast gain. The horizontal dashed lines reflect the mean recording noise levels. Mean values that were < noise + 3(SE) were eliminated when fitting the regression functions. Rats treated with 2 or 3 ppm PTU showed a statistically significant dose-by-contrast interaction, which is evident as a dose-dependent reduction of the slope of the contrast-amplitude function. No significant change was observed in contrast intercept values. The changes observed in the slope of the VEP contrast-amplitude function appeared to reflect a reduction of contrast gain.
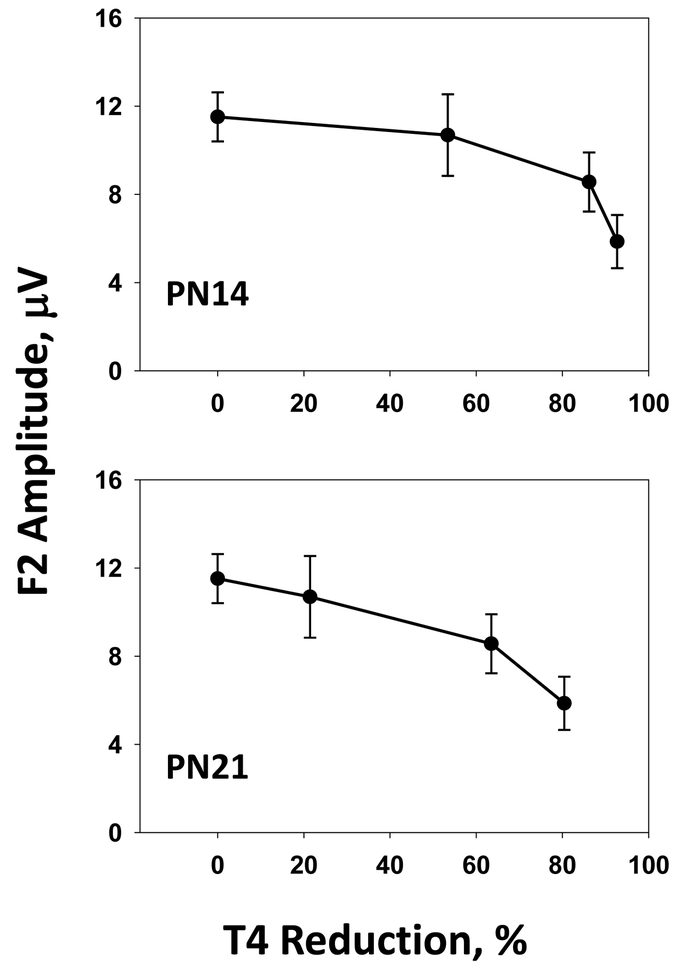
Rats exposed in utero and in the preweaning period to 2 or 3 ppm PTU were euthyroid, but showed reductions of VEP amplitude (Figure 4). The magnitude of T4 reduction was greater in pups than in dams, and greater in pups at PN14, when TH levels peak in development, than at PN21. At the 2 ppm PTU dose, a 30% reduction in serum T4 was observed in dams, an 80% reduction in littermate pups on PN14, and a 60% reduction in pups on PN21. Serum T3 concentrations were slightly reduced in dams and pups, but to a lesser extent than T4, and only at the highest dose tested. As such, the lowest dose (2 ppm) at which contrast gain impairments were seen was a condition of hypothyroxinemia where alterations in serum hormones reductions in dams and pups were restricted to T4 with no change in serum T3.
Figure 4.
(Experiment 2.). The VEP F2 amplitude (mean ± SE) recorded to 80% contrast stimuli plotted as a function of group mean T4 reduction (% control) for T4 levels measured from littermate pups on PN14 (upper panel) or PN21 (lower panel). Pups treated with 1 ppm PTU showed approximately a 50% (PN14) or a 20% (PN21) reduction of T4. Pups treated with 2 or 3 ppm PTU showed greater than 80% (PN14) or 60% (PN21) reduction of T4. Statistically significant reductions from control were seen in F2 amplitude following 2 or 3 ppm PTU, but not 1 ppm PTU (Figure 3). The results suggested that between a 60% (PN21) and 80% (PN14) reduction of developmental serum T4 was associated with a significant lowering F2 amplitude in adult offspring.
DISCUSSION
Reductions in serum TH in developing rat pups altered visual function in euthyroid adult offspring. Dose-dependent declines in circulating levels of T4 in the dams and pups were observed despite the absence of body weight reductions and eye-opening delays typical of more severe states of hypothyroidism. The compromise of visual function in adult rats was evident at both the level of the retina and visual cortex. Green-flicker ERG amplitudes were reduced in rats treated developmentally with 3 ppm PTU, indicating reduced function of retinal M-cone photoreceptors. There was also an increased a-wave amplitude of the scotopic flash ERG, which is generated by rod photoreceptors. The UV ERG response was not changed by PTU, indicating that S-cones were spared. Visual evoked potential contrast-amplitude functions recorded from visual cortex exhibited dose-dependent amplitude reductions following 2 or 3 ppm PTU. To the best of our knowledge, these are the first data to demonstrate functional alterations in the adult rodent visual system following graded degrees of developmental TH insufficiency. Using serum T4 as an internal dosimeter of effective dose, these findings are consistent with reports of TH-dependent impairments in hearing, cognition, and synaptic physiology (Crofton, 2004, Gilbert, 2011).
Retinal Dysfunction:
In electrophysiological recordings from the retina, UV flicker ERGs reflecting S-cone activation were not altered by developmental exposure to PTU. Rats exposed to 3 ppm PTU, however, had reduced amplitude of green-flicker ERGs that were indicative of impaired function of M-cone photoreceptors. These findings are consistent with selective loss of M-cones in mice following deletion of Thrb, the gene encoding thyroid hormone receptor β2 (TRβ2) (Ng et al., 2011). In contrast, upregulation of TRβ2 transcription activates the expression of M-cone and suppresses S-cone opsin in mouse retina (Yanagi et al., 2002). These data indicate that proper development of M-cones is particularly dependent on the presence of TH and TH-responsive signaling pathways. In the present study, the green flicker ERG protocol was designed to isolate and enhance M-cone function by activating retinal output at the peak spectral wavelength of M-cone sensitivity against a constant UV background to suppress S-cone response, and a stimulation frequency above the temporal response capabilities of rod photoreceptors. The green flicker responses in control animals were larger in amplitude than the UV flicker ERGs, reflecting at least in part, the larger number of M-cones over S-cones in the rat retina (Szel and Rohlich, 1992). The observation that the green flicker ERG amplitude was reduced by PTU treatment in the absence of effect on UV flicker ERGs, is consistent with a selective vulnerability of M-cones in the retina to developmental TH disruption.
In addition to reductions in M-cone driven green flicker ERG amplitudes, an increase in amplitude of the rod photoreceptor-mediated a-wave was observed in the scotopic flash ERG. There was no PTU-dependent effect on ERG b-wave amplitude. During retinal development, pluripotent retinal progenitor stem cells proliferate and differentiate into retinal photoreceptor and neuronal cell types (Pinazo-Duran et al., 2011). The default pathway for retinal precursor cells appears to be photoreceptors until extrinsic factors stimulate differentiation into neurons (Adler and Hatlee, 1989). One of these extrinsic factors modulating retinal cell differentiation is the presence of TH (Harpavat and Cepko, 2003). S-cone photoreceptors are evolutionarily older than the other photoreceptors. Rods evolve from S-cones, and the majority of rods maintain a genetic marker for S-cone opsin (Kim et al., 2016). In the presence of TH, M-cones differentiate from this pool of retinal S-cone precursor cells. It is possible that the lowered level of TH in the current studies caused fewer M-cones from the default S-opsin containing precursors, altering the organization of functional networks from the remaining progenitor cells in a manner that enhanced a-wave expression. The second larger component of the flash ERG is the b-wave. It is generated in the retinal bipolar cells and reflects spatial and temporal summation of signaling in the successive stages of retinal processing. The fact that b-waves remain unchanged suggests that second order retinal signaling was spared despite changes at the photoreceptor level. The b-wave of the dark-adapted flash ERG is driven primarily by rod input to bipolar cells. The predominance of rod photoreceptors in the rat retina contributing to the dark-adapted ERGs accounts for the lack of changes in b-wave amplitude despite TH-mediated loss of M-cone photoreceptors.
Cortical Dysfunction:
Alterations of retinal function were accompanied by reduced amplitude VEPs in response to alternating visual pattern stimuli. The visual cortex response reflects the convergence of input from the retina, optic nerve, and thalamus to activate visual cortex. Impaired M-cone function detected in green flicker ERG deficits may have contributed to the VEP amplitude deficits. Patterned stimuli for VEP recordings were elicited by the green gun of the video monitor and decreased amplitudes are consistent with lowered retinal responsiveness and reduced signal transmission from retina to visual cortex. The VEP reductions, as discussed below, likely also reflect additional impairments occurring at stages of visual processing subsequent to the retina.
The reduction of VEP amplitude and slope of the contrast-amplitude function observed in Experiment 1 was replicated in Experiment 2, and extended to lower doses of PTU. Both VEP amplitudes and the slope of VEP contrast-amplitude functions were reduced in a dose-response manner. The pattern of VEP deficits demonstrated greater reductions in amplitude as dose of PTU and stimulus contrast levels were increased, corresponding to a reduction of the slope of VEP amplitude-visual contrast functions. The linear relationship between VEP amplitude and log stimulus contrast defines visual contrast sensitivity functions (Silveira et al., 1987). According to this scheme, the zero-amplitude intercept of the function is interpreted as equivalent to contrast threshold; contrast sensitivity is defined as the inverse of contrast threshold; and the slope of the contrast-amplitude function reflects contrast gain (Bobak et al., 1988). Contrast gain is the ability of visual system neurons to adjust sensitivity in proportion to the strength (i.e. contrast) of the visual stimulus. This adjustment is achieved by modulating firing rates of cortical neurons to maintain an optimal range of sensitivity, without saturation, despite changing levels of stimulus contrast. Although there are reports of the presence of neurons with contrast gain adjustment capability in the retina and lateral geniculate nucleus of the thalamus (Scholl et al., 2012), the primary site of contrast gain adjustments resides in visual cortex. Single unit recordings in primary visual cortex neurons reveal rapid, contrast-dependent adaptations of firing rates (Ohzawa et al., 1982, Bonds, 1991).
The primary neuronal generators of pattern-elicited VEPs are the thalamocortical inputs to cortical lamina 4, with the subsequent activation of supra-granular lamina 1–3 (Schroeder et al., 1991). Instability of the structural dynamics of the synaptic connections of thalamocortical neurons in the superficial layers of visual cortex has been reported in mice following mild maternal TH insufficiency (Strobl et al., 2017). Two-photon laser scanning microscopy of visual cortex in adult mice revealed an increased number of axonal branches and synaptic boutons, and a reduction in their stability over time, in response to developmental TH reductions. These findings suggest that instability of synaptic architecture of cortical neurons following developmental TH insufficiency may compromise the brain’s ability to adapt to dynamic variations of luminance contrast in the visual world, and underlie the contrast gain reductions reported here.
Timing and Magnitude of Thyroid Hormone Insufficiency.
In utero and postnatal exposure to a range of PTU doses produced graded reductions of serum T4 in dams and offspring. All hormones had returned to control levels by the time of visual function testing in adult offspring. The results indicate that permanent visual function impairments were a consequence of perinatal TH insufficiency. The lowest dose (2 ppm), at which contrast gain impairments were seen, occurred with serum hormone reductions in T4 of dams and offspring but no change evident in circulating levels of T3.
If the contrast gain deficits are largely derived from maladaptive changes in cortical circuitry, it is likely that prenatal TH insufficiency is responsible. A 26% reduction of serum T4 in pregnant mice on GD13.5 was sufficient to disrupt thalamocortical connectivity in the adult offspring (Strobl et al., 2017). At this time in development, the dam represents the sole source of TH to the developing fetus (Morreale de Escobar et al., 2000, Postiglione et al., 2002). As such, serum concentrations of the dam and fetus at mid-gestation, rather than the postnatal assessments reported here, may be more appropriate indicators of potential contrast gain deficits in response to TH insufficiency. Expressing maternal serum T4 decrements as a function of gestational-age matched controls reveals PTU-induced decrements of 30–50% on GD10-GD15 in pregnant rats at a dose of 3 ppm PTU (Hassan et al., 2017, O’Shaughnessy et al., 2018). Together, these findings suggest that a 30% drop in maternal T4 in mid-gestation, the critical time for thalamocortical connectivity, may be sufficient to impair visual contrast encoding in the adult offspring.
Developmental timing may be different in the retina than in cortex, where in rodents much of retinal maturation occurs postnatally. S-cone opsin is detectable in the rat retina at birth, and M-cone opsin appears around PN4. S-cone photoreceptor density peaks by PN10 followed several days later by M-cone photoreceptors on PN12, shortly before eye opening (Arango-Gonzalez et al., 2010, Ebrahimi et al., 2014). In contrast, human photoreceptor development occurs primarily during the second and third trimester of pregnancy. Human S-cones appear by fetal week 11 and cover the retina by fetal week 20. Although the appearance of M cones lags that of S-cones by 3–4 weeks, M cone density in the human retina is complete by birth (Cornish et al., 2004). In rats gestational TH deficits may alter cortical development, but postnatal deficits appear necessary to impair retinal development. In humans, as in rat, the appearance of substrate upon which TH may influence visual function in the cortex precedes that in the retina, but in humans, both occur prior to birth.
Implications for Thyroid Disruption.
There is a lack of well-defined downstream apical neurodevelopmental outcomes associated with moderate degrees of TH disruption. There is also a general paucity of dose-response information on the relationship of TH in serum, and TH in brain that presumably drive these neurological outcomes. Data are particularly sparse for mild to moderate disruptions characteristic of environmental exposures to thyroid disrupting chemicals. The present findings provide a novel phenotype that has not been previously described in rat, and characterize the dose-response relationship of the observed deficits. The phenotype described has the added feature of direct translation to a non-invasive assessment of visual function that can be conducted in children or adult humans.
The present findings also underscore the importance of recognizing the stage-specific nature of TH requirements, and species differences in timing of these TH requirements. Because different brain regions require TH at different times, the relationships between serum TH and outcome measures depend on consistent timing of TH insufficiency with brain maturational stages. Xenobiotics can reduce circulating levels of TH through several distinct mechanisms (Brucker-Davis, 1998, USEPA, 2013). A chemical’s kinetics and the means whereby it disrupts the thyroid system can influence the magnitude, duration, and timing of effects on hormonal status in the dam, fetus and neonate, adding uncertainty to predictions of brain outcomes based on serum TH changes. The sampling of serum TH at multiple ages, identification of sensitive downstream markers of TH deficiencies, and linking these outcomes more directly to the timing over which the TH insufficiency occurs is likely to improve the power of peripheral markers of TH to predict brain impairment.
Implications for Vision.
The results of these studies suggest that insufficient TH during development can impair retinal and cortical development. In the retina, the selective reduction of green flicker ERGs was consistent with impaired development of M-cones, which encode middle wavelength (green) colors during daytime vision. In humans, depending on the population, about 8 % of males and 0.5 % of females have a congenital deficiency in either M-cones or L-cones, with anomaly or absence of the M-cone being the most prevalent form. These deficits cause red-green color blindness. Quality of life indicators for colorblind individuals are significantly impaired. Colorblind individuals experience variety of problems ranging from inability to read colored maps and charts, difficulty buying clothes, driving, selecting ripe fruits and so on (Barry et al., 2017). Career limitations, such as inability to become pilots or firemen, and impaired performance in jobs that do not obviously depend on color discriminations, may be particularly pernicious.
The perception of visual contrast is a vital component of pattern vision. Lower contrast sensitivity may manifest as a “washed-out” appearance of visual images, where contrast is muted and objects or patterns are less distinct or vivid than normal. Impaired contrast sensitivity may impair the ability to engage in common activities such facial recognition or reading, and is associated with restrictions of usual daily activities (Orr et al., 2011). An impaired contrast gain mechanism may translate into difficulty adjusting to conditions of changing visual contrast, such as when moving between indoors and outdoors, or with changing luminance levels at dusk or dawn. Contrast sensitivity deficits in children impair the ability to see the blackboard and are a barrier to school achievement. Loss of contrast sensitivity in adults presents other hazards, such as impaired driving and increased likelihood of falls, especially among the elderly (Ginsburg, 2003, Bansback et al., 2006, Datta et al., 2008). The long-term consequences of impairments induced by developmental TH insufficiency on visual system function can persist throughout the lifespan, potentially contributing to deficits of intellectual function (Rovet and Willoughby, 2010) and a lower quality of life in the elderly (Rubin et al., 2001, Duggan et al., 2017).
Limitations.
The current studies are limited by several factors. Only male rats were tested, making it impossible to evaluate sex-related differences in sensitivity. The exposure duration was extensive, covering both prenatal and postnatal periods, but TH measures were taken only intermittently in the preweaning period, which limited the ability to identify critical periods of vulnerability. Only one chemical agent, PTU, with a specific mechanism of thyroid disruption was assessed. The degree of T4 suppression in postnatal pups was high, despite the relatively low doses of PTU employed. It remains to be seen if environmental chemicals that lower circulating TH to a lesser degree, for a shorter duration, or by different mechanisms, will cause similar effects.
Conclusions.
In conclusion, the results of the present experiments provide the first quantitative information on the decreased function of the adult visual system in a rodent model of moderate developmental thyroid disruption. Impairments of the retina and visual cortex are implicated. Functional consequences of the retina and visual cortex are consistent with reduced M-cone expression in rodent models, and impairments of visual contrast perception reported in hypothyroid children. The results demonstrate the vulnerability of the developing visual system to moderate degrees of TH insufficiency, and suggest that life-long deficits in color or contrast perception may result from perinatal TH insufficiencies.
Supplementary Material
Acknowledgements:
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors thank Dr. David Herr, Dr. Tammy Stoker and Alice Goldstein-Plesser for comments on an earlier version of the manuscript, and Mark Bercegeay, Willard Anderson, Danielle Lyke Freeborn, and Kristen Sanders for technical assistance. The authors also express gratitude to the peer reviewers, anonymous to the authors, who’s comments during the journal peer-review process substantially improved the manuscript.
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Adler R, Hatlee M (1989) Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science 243:391–393. [DOI] [PubMed] [Google Scholar]
- Arango-Gonzalez B, Szabó A, Pinzon-Duarte G, Lukáts Á, Guenther E, Kohler K (2010) In Vivo and In Vitro Development of S- and M-Cones in Rat Retina. Investigative Ophthalmology & Visual Science 51:5320–5327. [DOI] [PubMed] [Google Scholar]
- Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P (2004) A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047. [DOI] [PubMed] [Google Scholar]
- Bansback N, Czoski-Murray C, Carlton J, Lewis G, Hughes L, Espallargues M, Brand C, Brazier J (2006) Determinants of health related quality of life and health state utility in patients with age related macular degeneration: the association of contrast sensitivity and visual acuity. Quality of Life Research 16:533. [DOI] [PubMed] [Google Scholar]
- Barry JA, Mollan S, Burdon MA, Jenkins M, Denniston AK (2017) Development and validation of a questionnaire assessing the quality of life impact of Colour Blindness (CBQoL). BMC Ophthalmology 17:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel PJ, Escobar del Rey F, Morreale de Escobar G, Ruiz-Marcos A (1985) Effect of hypothyroidism on the size of spines of pyramidal neurons of the cerebral cortex. Brain Res 337:217–223. [DOI] [PubMed] [Google Scholar]
- Bobak P, Bodis-Wollner I, Marx MS (1988) Cortical contrast gain control in human spatial vision. J Physiol 405:421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds AB (1991) Temporal dynamics of contrast gain in single cells of the cat striate cortex. Vis Neurosci 6:239–255. [DOI] [PubMed] [Google Scholar]
- Boyes WK, Bercegeay M, Ali JS, Krantz T, McGee J, Evans M, Raymer JH, Bushnell PJ, Simmons JE (2003) Dose-based duration adjustments for the effects of inhaled trichloroethylene on rat visual function. Toxicological Sciences 76:121–130. [DOI] [PubMed] [Google Scholar]
- Boyes WK, Bercegeay M, Degn L, Beasley TE, Evansky PA, Mwanza JC, Geller AM, Pinckney C, Nork TM, Bushnell PJ (2016) Toluene inhalation exposure for 13 weeks causes persistent changes in electroretinograms of Long–Evans rats. NeuroToxicology 53:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes WK, Degn LL, Martin SA, Lyke DF, Hamm CW, Herr DW (2014) Neurophysiological assessment of auditory, peripheral nerve, somatosensory, and visual system functions after developmental exposure to ethanol vapors. Neurotoxicology and Teratology 43:1–10. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F (1998) Effects of environmental synthetic chemicals on thyroid function. Thyroid : official journal of the American Thyroid Association 8:827–856. [DOI] [PubMed] [Google Scholar]
- Chen J (2006) Statistical analysis for developmental and reproductive toxicologists. Developmental and reproductive toxicology, a practical approach CRC, Boca Raton 697–711. [Google Scholar]
- Cornish EE, Hendrickson AE, Provis JM (2004) Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: early development of spatial order and density profiles. Vision Res 44:2019–2026. [DOI] [PubMed] [Google Scholar]
- Crofton KM (2004) Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Anal 24:1665–1671. [DOI] [PubMed] [Google Scholar]
- Datta S, Foss AJE, Grainge MJ, Gregson RM, Zaman A, Masud T, Osborn F, Harwood RH (2008) The Importance of Acuity, Stereopsis, and Contrast Sensitivity for Health-Related Quality of Life in Elderly Women with Cataracts. Investigative Ophthalmology & Visual Science 49:1–6. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Patel CM, Selwyn MR, Roth AJ (1984) Statistical and computational aspects of mixed model analysis. Applied Statistics 203–214. [Google Scholar]
- Duggan E, Donoghue O, Kenny RA, Cronin H, Loughman J, Finucane C (2017) Time to Refocus Assessment of Vision in Older Adults? Contrast Sensitivity but Not Visual Acuity Is Associated With Gait in Older Adults. The journals of gerontology Series A, Biological sciences and medical sciences. [DOI] [PubMed] [Google Scholar]
- Ebrahimi V, Vojoudi E, Fazel A, Ebrahimzadeh-bideskan A (2014) Histochemical study of retinal photoreceptors development during pre- and postnatal period and their association with retinal pigment epithelium. Iranian Journal of Basic Medical Sciences 17:483–489. [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT (2003) Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A 100:3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME (2011) Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci 124:432–445. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Hedge JM, Valentin-Blasini L, Blount BC, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, Fisher JW (2013) An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicol Sci 132:177–195. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, Goodman JH (2014) Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. Journal of neuroendocrinology 26:528–541. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sanchez-Huerta K, Wood C (2016) Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 157:774–787. [DOI] [PubMed] [Google Scholar]
- Ginsburg AP (2003) Contrast Sensitivity and Functional Vision. International Ophthalmology Clinics 43:5–15. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Rehnberg GL, Crofton KM (1995) Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol 135:67–76. [DOI] [PubMed] [Google Scholar]
- Gould E, Allan MD, McEwen BS (1990) Dendritic spine density of adult hippocampal pyramidal cells is sensitive to thyroid hormone. Brain Res 525:327–329. [DOI] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Escamez MJ, Rausell E, Bernal J (1999) Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm CW, Ali JS, Herr DW (2000) A system for simultaneous multiple subject, multiple stimulus modality, and multiple channel collection and analysis of sensory evoked potentials. J Neurosci Methods 102:95–108. [DOI] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL (2003) Thyroid hormone and retinal development: an emerging field. Thyroid : official journal of the American Thyroid Association 13:1013–1019. [DOI] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, Gilbert ME (2017) Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicol Sci 160:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr DW, Boyes WK, Dyer RS (1992) Alterations in rat flash and pattern reversal evoked potentials after acute or repeated administration of carbon disulfide (CS2). Fundam Appl Toxicol 18:328–342. [DOI] [PubMed] [Google Scholar]
- Jones I, Srinivas M, Ng L, Forrest D (2003) The thyroid hormone receptor beta gene: structure and functions in the brain and sensory systems. Thyroid : official journal of the American Thyroid Association 13:1057–1068. [DOI] [PubMed] [Google Scholar]
- Kim J-W, Yang H-J, Oel Adam P, Brooks Matthew J, Jia L, Plachetzki David C, Li W, Allison William T, Swaroop A (2016) Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals. Developmental Cell 37:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U (2000) Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. Journal of neurophysiology 83:3101–3112. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME (2011) Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol 33:464–472. [DOI] [PubMed] [Google Scholar]
- Lavado-Autric R, Ausó E, García-Velasco JV, del Carmen Arufe M, Escobar del Rey F, Berbel P, Morreale de Escobar G (2003) Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. Journal of Clinical Investigation 111:1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-P, Olavarria JF, Greger BE (1995) Occipital cortico-pyramidal projection in hypothyroid rats. Developmental Brain Research 89:227–234. [DOI] [PubMed] [Google Scholar]
- Ma H, Ding X-Q (2016) Thyroid Hormone Signaling and Cone Photoreceptor Viability In: Retinal Degenerative Diseases (Bowes Rickman C et al. , eds), pp 613–618 Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Mano MT, Potter BJ, Belling GB, Chavadej J, Hetzel BS (1987) Fetal brain development in response to iodine deficiency in a primate model (Callithrix jacchus jacchus). J Neurol Sci 79:287–300. [DOI] [PubMed] [Google Scholar]
- Martinez-Galan JR, Escobar del Rey F, Morreale de Escobar G, Santacana M, Ruiz-Marcos A (2004) Hypothyroidism alters the development of radial glial cells in the term fetal and postnatal neocortex of the rat. Brain research Developmental brain research 153:109–114. [DOI] [PubMed] [Google Scholar]
- Meyerhoff WL (1979) Hypothyroidism and the ear: electrophysiological, morphological, and chemical considerations. Laryngoscope 89:1–25. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J (2005) Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res 57:902–907. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F (2000) Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? The Journal of clinical endocrinology and metabolism 85:3975–3987. [DOI] [PubMed] [Google Scholar]
- Moses RA, Hart WM, (eds.) (1987) Adler’s Physiology of the eye, Clinical Application. St. Louis: The C. V. Mosby Company. [Google Scholar]
- Navegantes LC, Silveira LC, Santos GL (1996) Effect of congenital hypothyroidism on cell density in the ganglion cell layer of the rat retina. Braz J Med Biol Res 29:665–668. [PubMed] [Google Scholar]
- Ng L, Cordas E, Wu X, Vella KR, Hollenberg AN, Forrest D (2015) Age-Related Hearing Loss and Degeneration of Cochlear Hair Cells in Mice Lacking Thyroid Hormone Receptor beta1. Endocrinology 156:3853–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D (2001) A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature genetics 27:94–98. [DOI] [PubMed] [Google Scholar]
- Ng L, Kelley MW, Forrest D (2013) Making sense with thyroid hormone--the role of T(3) in auditory development. Nature reviews Endocrinology 9:296–307. [DOI] [PubMed] [Google Scholar]
- Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, Forrest D (2011) Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:11118–11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy K, Kosian P, Ford J, Oshiro W, Degitz S, Gilbert ME (2018) Developmental Thyroid Hormone Insufficiency Induces a Cortical Brain Malformation and Learning Impairments: A Cross-Fostering Study. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD (1982) Contrast gain control in the cat visual cortex. Nature 298:266–268. [DOI] [PubMed] [Google Scholar]
- Orr P, Rentz AM, Margolis MK, Revicki DA, Dolan CM, Colman S, Fine JT, Bressler NM (2011) Validation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in age-related macular degeneration. Invest Ophthalmol Vis Sci 52:3354–3359. [DOI] [PubMed] [Google Scholar]
- Pinazo-Duran MD, Pons-Vazquez S, Gallego-Pinazo R, Galbis Estrada C, Zanon-Moreno V, Vila Bou V, Sanz Solana P (2011) Thyroid hormone deficiency disrupts rat eye neurodevelopment. Brain Res 1392:16–26. [DOI] [PubMed] [Google Scholar]
- Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R (2002) Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci U S A 99:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA (2006) Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A 103:6218–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet J, Simic N (2008) The Role of Transient Hypothyroxinemia of Prematurity in Development of Visual Abilities. Seminars in Perinatology 32:431–437. [DOI] [PubMed] [Google Scholar]
- Rovet JF, Willoughby KA (2010) Maternal Thyroid Function During Pregnancy: Effects on the Developing Fetal Brain In: Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects (Zimmerman AW and Connors SL), pp 55–77 New York, NY: Springer New York. [Google Scholar]
- Royland JE, Parker JS, Gilbert ME (2008) A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. Journal of neuroendocrinology 20:1319–1338. [DOI] [PubMed] [Google Scholar]
- Rubin GS, Bandeen-Roche K, Huang GH, Munoz B, Schein OD, Fried LP, West SK (2001) The association of multiple visual impairments with self-reported visual disability: SEE project. Invest Ophthalmol Vis Sci 42:64–72. [PubMed] [Google Scholar]
- Ruiz-Marcos A, Sanchez-Toscano F, Escobar del Rey F, Morreale de Escobar G (1979) Severe hypothyroidism and the maturation of the rat cerebral cortex. Brain Res 162:315–329. [DOI] [PubMed] [Google Scholar]
- Scholl B, Latimer KW, Priebe NJ (2012) A retinal source of spatial contrast gain control. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:9824–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HG Jr.(1991) Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Res 31:1143–1157. [DOI] [PubMed] [Google Scholar]
- Sevilla-Romero E, Munoz A, Pinazo-Duran MD (2002) Low thyroid hormone levels impair the perinatal development of the rat retina. Ophthalmic research 34:181–191. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, Zoeller RT (2008) The Balance between Oligodendrocyte and Astrocyte Production in Major White Matter Tracts Is Linearly Related to Serum Total Thyroxine. Endocrinology 149:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LC, Heywood CA, Cowey A (1987) Contrast sensitivity and visual acuity of the pigmented rat determined electrophysiologically. Vision Res 27:1719–1731. [DOI] [PubMed] [Google Scholar]
- Simic N, Khan S, Rovet J (2013) Visuospatial, Visuoperceptual, and Visuoconstructive Abilities in Congenital Hypothyroidism. Journal of the International Neuropsychological Society 19:1119–1127. [DOI] [PubMed] [Google Scholar]
- Simic N, Rovet J (2017) [Formula: see text]Dorsal and ventral visual streams: Typical and atypical development. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence 23:678–691. [DOI] [PubMed] [Google Scholar]
- Strobl MJ, Freeman D, Patel J, Poulsen R, Wendler CC, Rivkees SA, Coleman JE (2017) Opposing Effects of Maternal Hypo- and Hyperthyroidism on the Stability of Thalamocortical Synapses in the Visual Cortex of Adult Offspring Cerebral cortex (New York, NY: : 1991) 27:3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, Rohlich P (1992) Two cone types of rat retina detected by anti-visual pigment antibodies. Exp Eye Res 55:47–52. [DOI] [PubMed] [Google Scholar]
- USEPA (2013) State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures. (Agency, U. S. E. P. ed). [Google Scholar]
- Uziel A, Legrand C, Ohresser M, Marot M (1983) Maturational and degenerative processes in the organ of Corti after neonatal hypothyroidism. Hear Res 11:203–218. [DOI] [PubMed] [Google Scholar]
- Yanagi Y, Takezawa S, Kato S (2002) Distinct functions of photoreceptor cell-specific nuclear receptor, thyroid hormone receptor beta2 and CRX in one photoreceptor development. Invest Ophthalmol Vis Sci 43:3489–3494. [PubMed] [Google Scholar]
- Zoeller RT, Rovet J (2004) Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. Journal of neuroendocrinology 16:809–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.