Abstract
Tissue injury leads to the release of uric acid (UA). At high local concentrations, UA can form monosodium urate crystals (MSU). MSU and UA stimulate neutrophils to release extracellular traps (NET). Here, we investigated whether these NET could be involved in the development of inflammation by stimulating cytokine release by airway epithelial cells. We found that NET significantly increased the secretion of CXCL8/IL-8 and IL-6 by alveolar and bronchial epithelial cells. These effects were not observed when NETosis was inhibited by Diphenyleneiodonium, elastase inhibitor, or Cl-amidine. Similar findings were made with NET induced by cigarette smoke extract, suggesting that NET proinflammatory capacity is independent of the inducing stimulus. Furthermore, NET affected neither the viability and morphology of epithelial cells nor the barrier integrity of polarized cells. The epithelial stimulatory capacity of NET was not affected by degradation of DNA with micrococcal nuclease, treatment with heparin, or inhibition of the elastase immobilized to DNA, but it was significantly reduced by pretreatment with an anti-HMGB-1 blocking antibody. Altogether, our findings indicate that NET exert direct proinflammatory effects on airway epithelial cells that might contribute in vivo to the further recruitment of neutrophils and the perpetuation of inflammation upon lung tissue damage.
Keywords: Neutrophil extracellular traps, Monosodium urate crystals, Uric acid, Cigarette smoke, Human epithelial cells
Introduction
The lungs are continuously exposed to exogenous pathogens and require an efficient immune surveillance to control infections [1]. The airway epithelium represents the first line of defense of the lung. It provides a mechanical barrier to prevent infections but also produces chemokines and cytokines that contribute to recruiting and activating phagocytic cells to eradicate microorganisms and infected cells [2]. Furthermore, resident pulmonary macrophages (MØ) are able to produce and release chemokines in response to microbial threats and lung injury, and are a relevant source of proinflammatory mediators [3]. Together, these mediators contribute to neutrophil recruitment, which, under physiological conditions, can be found in the lung microvasculature, being part of the so-called marginated pool [3].
Neutrophils play an essential role in the defense against bacterial and fungal infections, a fact that is highlighted by the occurrence of severe, and often fatal, infections in patients with congenital neutrophil deficiencies [4]. However, neutrophils also contribute to chronic inflammatory conditions even in the absence of microbial threats. In instances of sterile inflammation, they can be recruited through a process orchestrated by damage-associated molecular patterns (DAMPs) like monosodium urate crystals (MSU) formed from the uric acid (UA) released by disrupted cells and tissues [5, 6]. Previous studies showed that the accumulation of activated neutrophils in the alveolar space is an early step in the pulmonary inflammatory process that leads to acute lung injury (ALI), a severe clinical disorder, which may be caused by direct (e.g., pneumonia or aspiration of gastric content) or indirect insults (e.g., sepsis or transfusion) [7]. In animal models, neutrophil depletion dampens the development of ALI, whereas in neutropenic patients with lung injury, the deterioration of the pulmonary function as neutropenia resolves has been well described [8].
Neutrophil extracellular traps (NET) are fibrous structures formed by the nuclear DNA with its associated histones as well as nuclear, cytoplasmic, and granular proteins which are released during a cell death process called NETosis [9, 10, 11]. NET trap many types of microbes ex vivo and are found in various disease models in vivo; they are thought to kill microbes by exposing them to high local concentrations of antimicrobials [12]. Agents like bacteria, protozoa, fungi, and viruses as well as chemical and sterile inflammatory stimuli were shown to induce NETosis [10, 13, 14]. In the lungs, NET can be found in human allergic asthmatic airways [15], and in mice upon influenza virus pneumonitis within the alveoli and airways, and in lesions of tissue injury [16]. Huge amounts of NET have also been identified in cystic fibrosis (CF) airway fluids by confocal, atomic-force, and scanning-electron microscopy [17, 18] and in ALI [19].
UA is generated as part of the normal turnover of nucleic acids when cells break down their purines, e.g. from DNA or RNA [20]. This constitutive process results in high intracellular levels of UA which increase further, when cells are injured or die, and degrade their RNA and DNA [21]. Thus, UA is present as a normal constituent intracellularly and in biological fluids [22]. Locally, the concentration of UA can exceed the saturation point in body fluids and precipitate and form MSU [6]. Increased local UA levels are found in different pathologies, among them in patients with ALI [23]. Both UA and MSU have been shown to promote cell-death-induced inflammation [6, 21]. Based on the fact that UA and MSU are capable of stimulating the release of NET, in this study, we tested the hypothesis that these NET exert direct proinflammatory effects on alveolar and bronchial epithelial cells, and compared these effects with those induced by cigarette smoke extract (CSE), a well-known lung pathology promoter [24]. We also evaluated the impact of inhibitors of NET induction, treatment with micrococcal nuclease (MNase), and the neutralization of high-mobility group box-1 (HMGB-1) protein on the proinflammatory effects exerted by MSU-stimulated neutrophils.
Materials and Methods
The experimental protocols performed were approved by the Biosafety and Research Review Board of IMEX-CONICET-ANM and the Ethical Committee of the Institutos de la Academia Nacional de Medicina. The methods were carried out in accordance with the approved guidelines.
Reagents and Materials
RPMI 1640 and DMEM culture media were purchased from HyClone Laboratories (Logan, UT, USA). Fetal bovine serum (FBS) and bovine serum albumin (BSA) were purchased from Internegocios (Buenos Aires, Argentina). Ficoll was purchased from GE Healthcare (Munich, Germany). The anti-CD14-MicroBead isolation kit and recombinant human IL-4 were purchased from Miltenyi Biotec (Germany). Micrococcal nuclease was from Worthington. TMB substrate was from Thermo Fisher Scientific Life Technologies (Massachusetts, MA, USA). Anti-human elastase antibody was from Calbiochem (Massachusetts, MA, USA), and rabbit total IgG, mouse total IgG, and all secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). TO-PRO-3 and SYBR Gold were from Life Technologies (Carlsbad, CA, USA). Phycoerythrin-conjugated anti-CD14 antibody, mouse anti-human nucleosome antibody, and the OptEIA human CXCL8/IL-8 and IL-6 ELISA sets were purchased from BD Biosciences (Franklin Lakes, NJ, USA), and human CCL2/MCP-1 (MCP-1) ELISA from eBioscience (San Diego, CA, USA). Vitrocol and human fibronectin were purchased from Advanced Biomatrix (Carlsbad, CA, USA). Heparin was from Fondaparinux, Cl-amidine (Cl-a) was from Cayman Chemical (Ann Arbor, MI, USA), and elastase inhibitor (EI) and Diphenyleneiodonium (DPI) from Sigma Aldrich (St. Louis, MO, USA). Aqua-Poly/Mount coverslipping medium was purchased from Polysciences (Warrington, PA, USA). Cellview glass-bottom dishes were purchased from Greiner Bio One. Lab-Tek chambers were purchased from Nalge Nunc International, New York, NY, USA. CytoTox96® nonradioactive cytotoxicity assay was purchased from Promega. MSU were prepared as previously described in pyrogen-free conditions [25]. Briefly, 1.68 g of UA was added to 100 mL of heated 0.01 M NaOH (70°C). NaOH/HCl was added as required to keep the pH between 7.1 and 7.2. The solution was slowly and continuously stirred at room temperature for 24 h. The crystals were harvested by decanting the supernatant, and then washed with ethanol. They were allowed to air-dry under sterile conditions, dispensed in individual vials (10 mg each), and sterilized by autoclaving. They were then suspended in sterile PBS. The needle shape and the size of the crystals were checked by polarizing-light microscopy. Gas-phase extract of CSE was prepared by employing Camel cigarettes, using a continuous smoking protocol by a standard method previously described [26]. The concentration of CSE achieved was 13.6 mg/mL expressed in terms of its virtual tar concentration. This preparation was employed at 10-fold dilution. Unless otherwise stated, all the chemicals employed were from Sigma Aldrich. The elastase substrate used was N-methoxysuccinyl-Ala-Ala-Pro-Val. Anti-HMGB-1 (clone 2G7) was kindly provided by Dr. Kevin Tracey (The Feinstein Institute for Medical Research, USA). The FITC-dextran fluorescence was determined with a Victor 3 microplate reader (Perkin Elmer).
Human Neutrophil Isolation
Neutrophils were isolated from the heparinized human blood from healthy donors (who gave written their informed consent), by centrifugation on Ficoll-Paque, dextran sedimentation, and hypotonic lysis [27]. Cells were suspended at 5 × 106/mL in RPMI 1640 supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% FBS previously heated at 65°C for 30 min for nuclease inactivation. After isolation, neutrophil preparations were stained with an anti-CD14-PE antibody and analyzed with a FACSCalibur cytometer (Beckton Dickinson, San Jose, CA, USA) to guarantee that monocyte contamination was <0.5% (online suppl. Fig. S1; see www.karger.com/doi/10.1159/000460293 for all online suppl. material).
Differentiation of Human Monocyte-Derived Macrophages and Dendritic Cells
Peripheral blood mononuclear cells were isolated by standard density gradient centrifugation on Ficoll-Paque. Monocytes were purified by using magnetic cell-sorting with the anti-CD14-MicroBead isolation kit. Monocytes were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and 50 ng/mL M-CSF for 5 days for MØ differentiation [28], or 5–7 days with 10 ng/mL GM-CSF and 10 ng/mL IL-4 for dendritic cell (DC) differentiation, supplementing with the cytokines every other day [29]. DC differentiation was confirmed by determining the expression of high CD1a levels and low CD86 levels on flow cytometry (online suppl. Fig. S2).
Cell Cultures
Three different airway epithelial cells were employed. The A549 cell line was used to resemble type II alveolar epithelial cells [30]. This cell type is located on the boundary between the alveolar airspace and the lung interstitium, ideally situated to modulate immune responses [31]. The BEAS-2B cell line consists of transformed bronchial epithelial cells, which have been widely used to explore functional properties [32]. The 16HBE14o- cells were established from normal human bronchial epithelium, and, when polarized, retain differentiated epithelial morphology and functions [33]. The A549 and BEAS-2B cell lines were cultured in DMEM supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and glutamine (2 mM). The 16HBE14o- cell line (kindly provided by Dr. Dieter Gruenert, UCSF, USA) was grown in minimal essential medium (MEM) supplemented as described above. BEAS-2B and 16HBE14o- cells were seeded on plates coated with collagen (Vitrocol; 30 µg/mL), fibronectin (10 µg/mL), and BSA (0.01%). Cells were grown at 37°C in a humidified 5% CO2 atmosphere. For experiments on polarized epithelial cells, 16HBE14o- cells were seeded at 1 × 105 cells/cm2 on Transwell® polycarbonate filters (6.5 mm in diameter with a pore size of 3 µm, Corning, Costar, USA), precoated as described above. The integrity of the polarized monolayer was determined by measuring the transepithelial electrical resistance (TEER), using an epithelial volt-ohmmeter (MILLICELL-ERS, Millipore), and corrected for the resistance value of the cell-free filter. Only polarized monolayers with a TEER of >500 Ω/cm2 over that of cell-free filters were used.
Microscopic Assessment of NET Formation and Isolation
The formation of NET after MSU stimulation was determined both by live-cell imaging and on fixed-cell preparations. In the first case, neutrophils were live-imaged by confocal microscopy to reveal the crystals by reflection (mainly inside neutrophils; online suppl. Fig. S3) and the formation of NET by the cell-impermeable DNA dye SYTOX green (online suppl. video 1). Following the second approach, neutrophils were seeded on poly-L-lysine-coated microscope slides. Briefly, after 4 h of MSU stimulation, samples were fixed with 4% PFA, permeabilized with 0.5% Triton X-100 in PBS for 1 min, and blocked with 5% goat serum for 60 min at 37°C. They were then incubated with a rabbit anti-human elastase antibody (1 µg/mL) and a mouse anti-human nucleosome antibody (1 µg/mL), or the corresponding isotype controls for 1 h. They were then incubated with a secondary DyLight488-conjugated goat anti-rabbit IgG antibody (1:200) and a secondary DyLight549 goat anti-mouse IgG antibody (1:200) for 30 min. For DNA staining, samples were incubated with TO-PRO-3 for 5 min and mounted using Aqua-Poly/Mount coverslipping medium. Images were acquired by using a FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan) equipped with a Plapon 60X/NA1.42 objective, and then analyzed with Olympus FV10-ASW software.
NET Isolation
Neutrophils (PMN; 5 × 106/mL) were cultured with either MSU (300 µg/mL), UA (8 mg/dL), or CSE (a 10-fold dilution of a solution with a concentration corresponding to cigarettes containing 1.36 mg/mL tar) for 1 h at 37°C. Then, after gentle pipetting, stimuli were removed by a quick spin in a microcentrifuge (for 12 s), and the pellets were suspended in fresh medium and incubated for 3 additional hours at 37°C in a humidified atmosphere with 5% CO2. Afterwards, NET were detached from other cells and from the debris by a gentle ultrasonic pulse (60 s; 40 Hz; W200 ultrasonic bath; Branson) and centrifugation. NET-containing supernatants (SN NET) were collected and immediately seeded over the epithelial cell monolayers. Aliquots of these supernatants were employed to quantify the concentrations of DNA. The ultrasonic pulse applied to detach NET did not modify cell viability as determined by annexin V-FITC/propidium iodide staining and flow cytometry (online suppl. Fig. S4). For control conditions, neutrophils were either cultured in the absence of stimuli and subjected to the same procedures as for NET isolation (SN unPMN) or were heat-shocked at 45°C for 10 min and then cultured for 4 h at 37°C, centrifuged, and the supernatants then collected to attain supernatants of necrotic cells (SN necPMN) [34]. For experiments conducted to address the role of DNA in the effects exerted by NET, neutrophils were cultured either with MSU or vehicle in the presence or absence of MNase (10 U/mL), and subjected to the same procedure described above to obtain NET. In assays aimed at preventing NET release from stimulated cells, neutrophils were preincubated for 30 min with DPI (10 µM), EI (10 µM), or Cl-a (200 µM), and then subjected to the same procedure described above, maintaining the presence of NETosis inhibitors throughout the culture period to avoid NET formation. In some experiments, neutrophils were fixed with paraformaldehyde (PFA) 4%, exhaustively washed and incubated with MSU, UA, or CSE, followed by the same procedure as for NET isolation.
NET Quantification
DNA was quantified by SYBR Gold (1:2,000) staining and fluorometry detection after interpolation from a standard DNA concentration curve and subtracting the value obtained when the same sample was pretreated with a high concentration of MNase (10 U/mL) for 30 min to degrade DNA. Myeloperoxidase (MPO) concentration was determined by a reaction of the TMB substrate with NET preparations for 10 min, a detection of the change in optical density at 450 nm by spectrophotometry, and interpolation from a standard MPO concentration curve. The elastase activity of the samples was determined by spectrophotometry from their ability to cleave a specific substrate.
Epithelial Cell Stimulation
A549 and BEAS-2B epithelial cells grown at 80% confluence were cultured for 18 h at 37°C in a humidified atmosphere with 5% CO2.in the presence or absence of 200 µL of (1) NET recovered upon stimulation of 1 × 106 neutrophils; (2) supernatants of neutrophils incubated with vehicle (SN unPMN); (3) supernatants of heat-shock-induced necrotic neutrophils (SN necPMN), or (4) supernatants of PFA-fixed neutrophils incubated with MSU (fPMN + MSU), UA (fPMN + UA), or CSE (fPMN + CSE). Supernatants were then collected and CXCL8/IL-8, IL-6, and CCL2/MCP-1 concentrations were determined by ELISA. In some experiments, epithelial cells were cultured for 18 h at 37°C with supernatants of neutrophils that had been stimulated with MSU, UA, or CSE in the presence of the NETosis inhibitors (EI, DPI, or Cl-a). Twenty-four hours before stimulation of 16HBE14o- cells grown as polarized monolayers (described above), the culture medium was replaced with bronchial epithelial cell growth medium (BEGM, LONZA) to reduce the basal secretion of cytokines. Then, the cells were apically exposed for 18 h to MSU- or CSE-induced NET recovered upon stimulation of 1 × 106 neutrophils, or to supernatants of neutrophils incubated with vehicle (SN unPMN). At the end of the culture, supernatants were harvested for cytokine quantification by ELISA. TEER values were also measured at both the beginning and end of the stimulation period to check monolayer integrity. Cell permeability after NET stimulation was determined after replacing the upper-chamber culture medium with fresh MEM containing 0.2 mg/mL FITC-Dextran (40 kDa) and incubation for 2 h. Then, an aliquot of the basolateral chamber medium was collected and fluorescence was determined with a Victor 3 microplate reader (Perkin Elmer) at 492 nm excitation and 520 nm emission wavelengths.
Cytotoxicity Assay
The cytotoxic effect of SN NET on polarized 16HBE14o- epithelial cells was determined by assessing lactate dehydrogenase (LDH) release. After SN NET stimulation, culture supernatants were harvested and LDH levels were measured by using a CytoTox96® nonradioctive cytotoxicity assay (Promega). Culture supernatantsof unstimulated cells were used as basal control and lysed cells were used as a positive control of cytotoxicity.
Statistical Analysis
Data were represented as the mean ± SEM values of at least 3 different replicates of independent experiments performed in triplicate. Statistical analysis was performed using GraphPad Prism v6.00 for Windows, GraphPad Software, La Jolla, CA, USA. The D'Agostino and Pearson normality test was performed to assess the Gaussian distribution of the variables. Comparisons between groups were done by 2-way ANOVA with repeated-measures followed by Bonferroni's multiple-comparisons test. Statistical significance was defined as p < 0.05.
Results
Isolation of NET Induced by Neutrophil Stimulation with MSU, UA, and CSE
First, we evaluated the strength of MSU, UA, and CSE to induce NET generation. These agonists stimulated neutrophils to produce huge amounts of NET, whose identity was confirmed by confocal microscopy by evaluating the colocalization of DNA with elastase and MPO (online suppl. Fig. S5A, S5B). NET induced by phorbol myristate acetate (PMA) were assessed as a positive control. We then verified the efficiency of our NET isolation protocol by quantifying the concentrations of DNA and MPO, and the elastase activity. After separation from cell debris, supernatants of MSU-stimulated neutrophils contained DNA, as indicated by the reduction in the fluorescence emitted by the MNase-treated NET preparation to the levels observed in the supernatants of unstimulated neutrophils (basal) (online suppl. Fig. S5C). These NET preparations also contained MPO and exhibited elastase activity (online suppl. Fig. S5D, S5E). By confocal microscopy, in the material isolated from the supernatants of neutrophils stimulated with either MSU, UA, or CSE, we confirmed that elastase and MPO were associated with chromatin (online suppl. Fig. S6A, S6B). Having confirmed that our protocol efficiently allowed NET isolation, we then employed these NET preparations to evaluate their ability to modulate cytokine secretion by lung epithelial cells.
NET Increased Cytokine Secretion by Airway Epithelial Cells
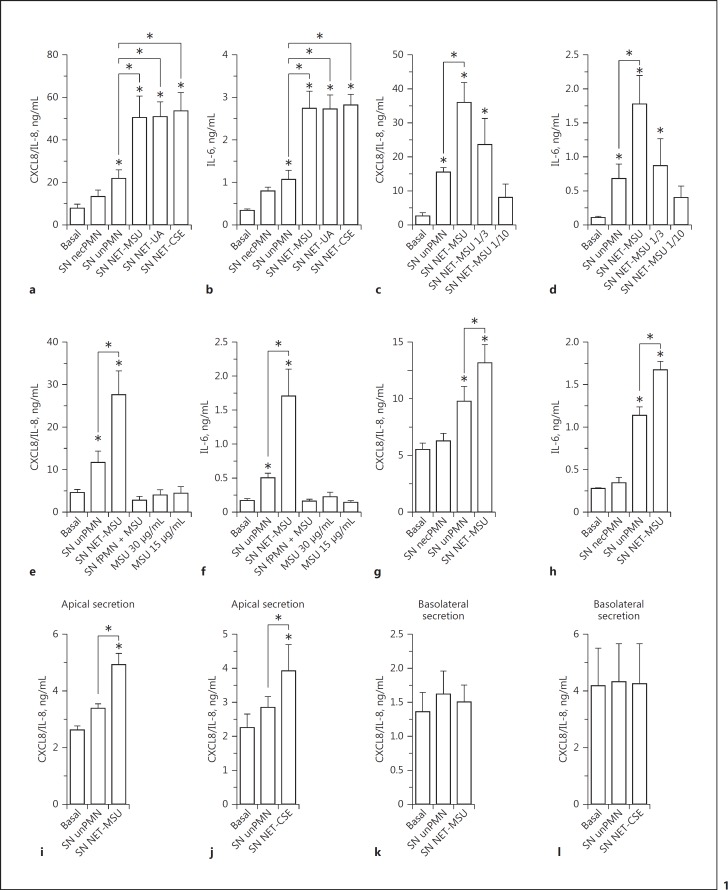
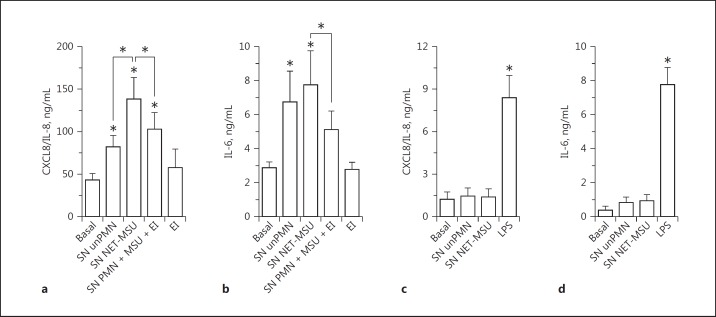
Supernatants of neutrophils containing NET induced by MSU, UA, or CSE (SN NET-MSU, SN NET-UA, and SN NET-CSE, respectively) markedly increased the production of CXCL8/IL-8 and IL-6 by A549 alveolar epithelial cells (Fig. 1a, b). By contrast, supernatants of neutrophils which were heat-shocked to undergo necrosis (SN necPMN) [34] and consequently to release their intracellular content, slightly modified cytokine release (Fig. 1a, b). On the other hand, even though culture supernatants of unstimulated neutrophils (SN unPMN) augmented epithelial cytokine secretion, this increase was significantly lower than that induced by NET. Worthy of mention, the concentrations of CXCL8/IL-8 in NET preparations were comparable to those present in supernatants of unstimulated neutrophils (online suppl. Fig. S7). Three-fold and 10-fold dilutions of MSU-induced NET obtained from 1 × 106 neutrophils showed that, as expected, the stimulatory capacity was dependent on the NET concentration (Fig. 1c, d).
Fig. 1.
NET stimulated proinflammatory cytokine release by human epithelial cells. Cytokine secretion by A549 alveolar epithelial cells (a–f), BEAS-2B bronchial epithelial cells (g, h), or polarized 16HBE14o- human bronchial epithelial cells (i–l; apical stimulation and cytokine release to the apical [i, j] or basolateral [k, l] compartment) after 18 h of culture in the absence (Basal) or presence of supernatants of necrotic neutrophils (SN necPMN), supernatants of unstimulated neutrophils (SN unPMN), or supernatant containing NET induced by MSU (SN NET-MSU); UA (SN NET-UA) or CSE (SN NET-CSE), determined by ELISA. c, d Secretion of CXCL8/IL-8 and IL-6 by A549 cells in response to the indicated dilutions of NET released by 1 × 106 neutrophils. e, f Secretion of CXCL8/IL-8 and IL-6 by A549 cells cultured in the presence of either SN NET-MSU, supernatants of PFA-fixed neutrophils (fPMN) incubated with MSU, or MSU alone. Worthy of mention, MSU was evaluated at 10- and 20-fold dilutions of that used to stimulate NETosis because, after the removal step, only a small proportion of the crystals could remain in the cultures before NETosis took place and their concentrations in NET preparations were probably lower than those tested. Data are depicted as the mean ± SEM of 7 (a), 8 (b), 3 (d, g), 4 (f, h, j, l), or 5 (e, i, k) independent experiments performed in triplicate. * p < 0.05 (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test).
We ruled out the possibility that MSU that could persist in low amounts in our NET preparations (online suppl. Fig. S3) were responsible for the effects attributed to NET, because the MSU were unable per se to stimulate cytokines production by A549 cells (Fig. 1e, f). Furthermore, supernatants that did not contain NET because they were collected from neutrophils that were first fixed with PFA and then incubated with MSU (fPMN + MSU), did not stimulate A549 cells to release either CXCL8/IL-8 or IL-6 (Fig. 1e, f). Similar findings were made when supernatants from fixed neutrophils incubated with UA (fPMN + UA) or CSE (fPMN + CSE) were employed (online suppl. Fig. S8). These findings rule out the possibility that traces of the stimuli that could persist in NET preparations were responsible for the observed proinflammatory effects on epithelial cells. In additional experiments, SN NET also stimulated cytokine secretion by BEAS-2B bronchial epithelial cells (Fig. 1g, h). To determine whether SN NET could also stimulate cytokine secretion when exposed to the luminal surface of polarized epithelial cells, we evaluated their impact on polarized 16HBE14o- bronchial epithelial cells. Both SN NET-MSU (Fig. 1i) and SN NET-CSE (Fig. 1j) significantly increased CXCL8/IL-8 secretion towards the apical compartment, not only over basal levels but also over those induced by SN unPMN. These effects were not observed in supernatants obtained from the basolateral compartment of the epithelial cell cultures (Fig. 1k, l).
Contribution of NET to the Stimulation of Cytokine Secretion by Epithelial Cells
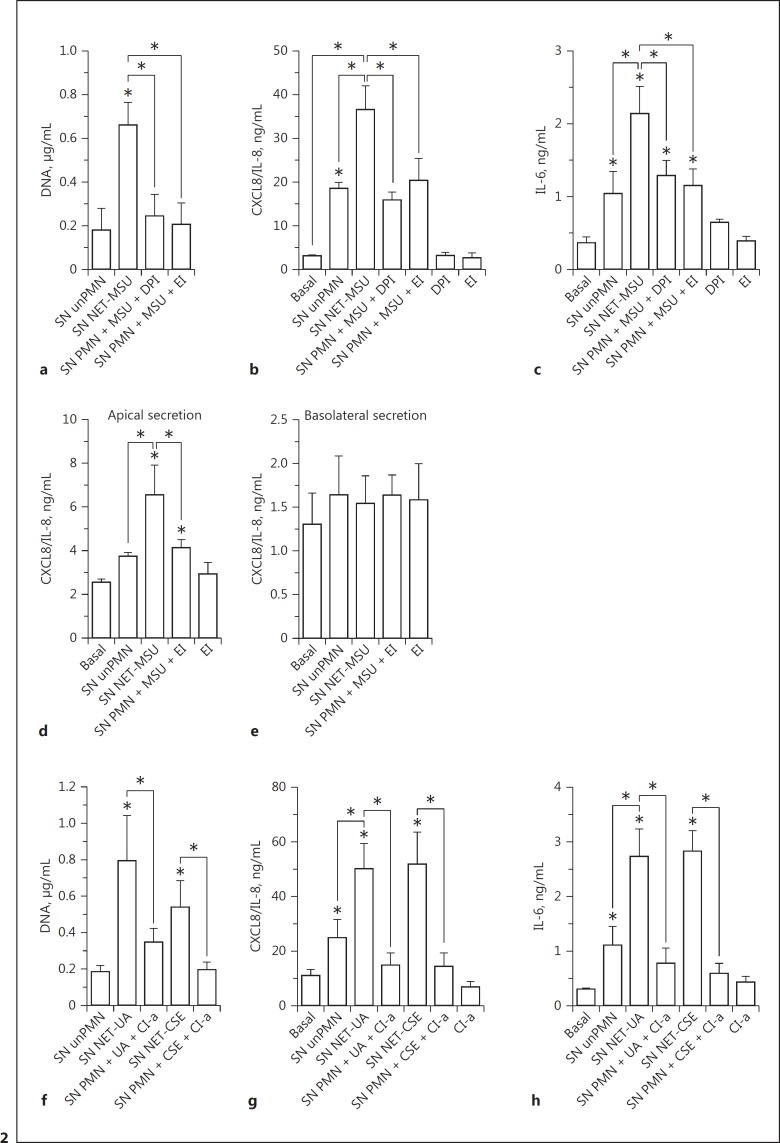
To confirm that NET were responsible for the increase in cytokine production, we first stimulated neutrophils with MSU in the presence of compounds that have been previously shown to inhibit NETosis [35, 36]. We used the NADPH oxidase inhibitor DPI because NETosis induced by MSU is dependent on ROS [35], and the neutrophil EI because this enzyme is required for the chromatin decondensation that leads to NET formation. As expected, both compounds significantly inhibited DNA release from MSU-stimulated neutrophils (Fig. 2a). When these neutrophil supernatants were added to either A549 (Fig. 2b, c) or polarized 16HBE14o- cell cultures (Fig. 2d, e), they were unable to increase CXCL8/IL-8 and IL-6 production beyond that induced by the supernatants of unstimulated neutrophils, which suggests that the presence of NET in MSU-stimulated neutrophil supernatants was responsible for this effect. Worthy of mention, as either DPI or EI had to be present throughout the stimulation period of neutrophils in order to inhibit NET release, we also tested their impact on the cytokine release by A549 cells; neither inhibited the basal release of cytokines per se (Fig. 2b-e). As NETosis induced by UA is independent of NADPH oxidase [37], and CSE-induced NETosis comprises histone H3 citrullination [38], we performed additional experiments by employing Cl-a, an inhibitor of protein arginine deiminase 4 (PAD4), as a NETosis inhibitor. PAD4 is another enzyme that is involved in chromatin decondensation for NET formation with certain agonists [39]. Cl-a significantly inhibited NET formation induced by both UA and CSE, determined as the amount of DNA released into the culture supernatant (Fig. 2f). Furthermore, the supernatants of neutrophils stimulated with NETosis agonists in the presence of Cl-a were devoid of the capacity to stimulate cytokine secretion by A549 epithelial cells (Fig. 2g, h).
Fig. 2.
NETosis inhibitors reduced the capacity of the supernatants of MSU-, UA-, or CSE-stimulated neutrophils to promote cytokine secretion by human epithelial cells. Neutrophils were stimulated with MSU in the presence or absence of DPI (10 µM), EI (10 µM), or Cl-a (200 µM) for 4 h. a, f After recovering the supernatants, the concentration of DNA was evaluated as a read-out of the presence of NET. Supernatants were added to the A549 (b, c, g, h) or 16HBE14o- (d, e) cell monolayers, which were cultured for 18 additional hours. Afterwards, cytokine concentrations in these culture supernatants were determined by ELISA. The effect of DPI, EI, and Cl-a on the epithelial cells was evaluated as a control. Data are depicted as the mean ± SEM of 6 (a, b) and 4 (c–h) independent experiments performed in triplicate. * p < 0.05 (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test.
The Epithelial Cell Stimulatory Capacity of NET Is Similar to That Exerted by Different PAMPs
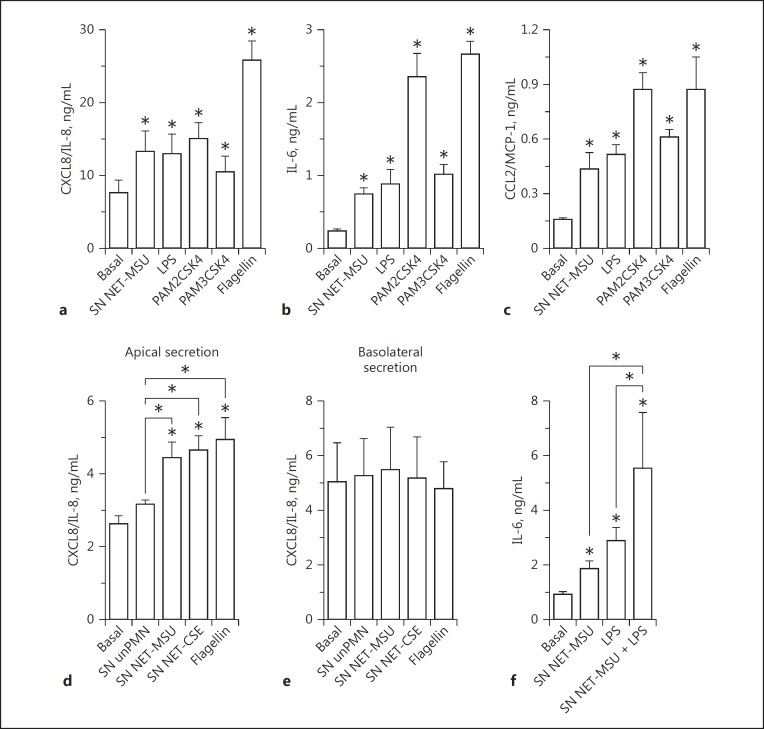
We then stimulated A549 cells with different TLR ligands which were previously shown to induce A549 and 16HBE14o- cell activation [40, 41, 42, 43]. SN NET-MSU stimulated CXCL8/IL-8 production at similar levels to LPS, PAM2CSK4, and PAM3CSK4 (Fig. 3a) and induced IL-6 and CCL2/MCP-1 release akin to LPS and PAM3CSK4 (Fig. 3b, c). However, the A549-stimulatory capacity of SN NET-MSU was always lower than that of flagellin. By contrast, MSU- and CSE-induced NET supernatants stimulated levels of CXCL8/IL-8 secretion to the apical surface of polarized 16HBE14o- cells that were similar to that induced by flagellin (Fig. 3d). These findings suggest that NET are able to stimulate cytokine responses in the range of those induced by standard concentrations of microbial agonists for these epithelial cells. On the other hand, when added together with LPS, SN NET were also able to increase, in an additive mode, the release of IL-6 by A549 cells (Fig. 3f).
Fig. 3.
Evaluation of the agonistic capacity of NET to stimulate cytokine production by human epithelial cells. a–c Monolayers of A549 epithelial cells were stimulated with SN MSU-induced NET, the TLR4 agonist LPS from E. coli O111:B4 (250 ng/mL); the TLR2 agonists PAM2CSK4 (100 ng/mL) and PAM3CSK4 (1 µg/mL), or the TLR5 agonist, flagellin (1 µg/mL) for 18 h at 37°C. Cytokine concentrations were determined in culture supernatants by ELISA. d, e Polarized 16HBE14o- cell monolayers were apically stimulated for 18 h with SN unPMN, SN MSU-, or CSE-induced NET or flagellin (1 µg/mL), and the CXCL8/IL-8 concentrations in the culture supernatants were determined by ELISA. f Monolayers of A549 epithelial cells were stimulated for 18 h with SN MSU-induced NET in the presence or absence of LPS (250 ng/mL), and the IL-6 concentration in the culture supernatants was determined by ELISA. Data are depicted as the mean ± SEM of 5 independent experiments performed in triplicate. * p < 0.05 (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test).
NET Did Not Affect Epithelial Cell Viability
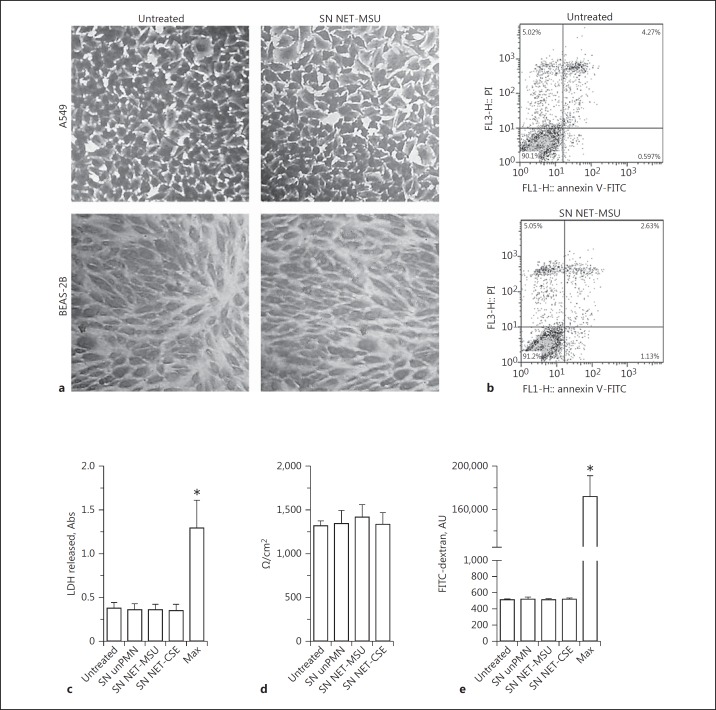
We then evaluated the impact of MSU-induced NET on the stability of epithelial cell monolayers, by means of optical microscopy, and on the viability of epithelial cells, using Annexin V/PI staining. After 18 h of culture in the presence of SN containing MSU-induced NET, neither the A549 nor BEAS-2B cell monolayers exhibited differences in their microscopic appearance (Fig. 4a) or in the levels of apoptosis or necrosis compared to untreated cells (Fig. 4b; online suppl. Fig. S9). We also evaluated whether NET affected the viability and barrier function of polarized 16HBE14o- bronchial epithelial cells. To this aim, we apically exposed polarized cells to SN NET or SN unPMN for 18 h. We then evaluated the LDH release (Fig. 4c), the TEER (Fig. 4d), and the barrier permeability of FITC-dextran applied apically over epithelial cells (Fig. 4e). None of these parameters was affected by either the unstimulated neutrophil supernatants or SN NET induced by either MSU or CSE.
Fig. 4.
Impact of NET on the viability and integrity of human epithelial cell monolayers. a The integrity of A549 or BEAS-2B cell monolayers after 18 h of culture in the absence (untreated) or presence of SN NET-MSU was evaluated by crystal violet staining and optical microscopy. Images are representative of 3 independent experiments. ×200. b A549 epithelial cell viability after culture without (untreated) or with SN NET-MSU was determined using annexin V/PI staining and flow cytometry. Data are representative of 3 experiments. c Levels of LDH were determined in the apical compartment of 16HBE14o- epithelial cell polarized cultures compared to total LDH cell content (Max) expressed in absorbance units (Abs). TEER of polarized 16HBE14o- epithelial cell monolayers (d) and permeability of FITC-dextran in fluorescence arbitrary units (AU) (e) applied apically over polarized 16HBE14o- epithelial cell monolayers exposed for 18 h at 37°C to medium (untreated), SN unPMN, SN NET-MSU, or SN NET-CSE. Permeability was compared to that evaluated in Transwells lacking epithelial cells (Max). Results are depicted as fluorescence AU. c- e Data represent the mean ± SEM of 4 independent experiments performed in triplicate. * p < 0.05 (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test).
NET Stimulates Cytokine Secretion by Human MØ but Not by Human Monocyte Derived-DC
To determine whether the stimulatory effects of NET on cytokine secretion are specific for epithelial cells or can also be exerted on other cell types, we evaluated the impact of NET on human MØ and DC. SN NET-MSU stimulated CXCL8/IL-8 and IL-6 secretion by MØ, and this effect was significantly reduced when the supernatants of neutrophils stimulated in the presence of EI were added to the epithelial cell cultures (Fig. 5a, b). However, while SN NET stimulated MØ to release significantly higher levels of CXCL8/IL-8 than the SN unPMN did (Fig. 5a), this increase was not observed when IL-6 was assessed (Fig. 5b). Worthy of mention, supernatants of neutrophils that were fixed and then incubated with MSU to mimic NETosis stimulation did not affect the secretion of cytokines by MØ, indicating that residual MSU that could remain in NET preparations were not responsible for the observed cytokine response (online suppl. Fig. S10). Supernatants containing NET did not modulate CXCL8/IL-8 or IL-6 production by DC (Fig. 5c, d) even though they were able to respond to LPS stimulation. Likewise, SN NET did not modulate DC maturation as assessed by MHC class II, CD86, and CD40 expression (data not shown). These findings suggest that the ability of NET to stimulate cytokine responses is restricted to certain cell types.
Fig. 5.
Impact of NET on cytokine secretion by macrophages (MØ) and dendritic cells (DC). CXCL8/IL-8 (a) and IL-6 (b) secretion by MØ cultured for 18 h at 37°C, with medium (Basal) or SN MSU-NET in the presence or absence of EI (10 µM) for 4 h. CXCL8/IL-8 (c) and IL-6 (d) production by DC cultured without NET (Basal) or with SN NET-MSU, SN unPMN, or LPS as a positive control (150 ng/mL). Data are depicted as the mean ± SEM of 3 independent experiments performed in triplicate. * p < 0.05 (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test).
The DAMP HMGB-1 Is Partially Responsible for NET Stimulatory Effects on Epithelial Cells
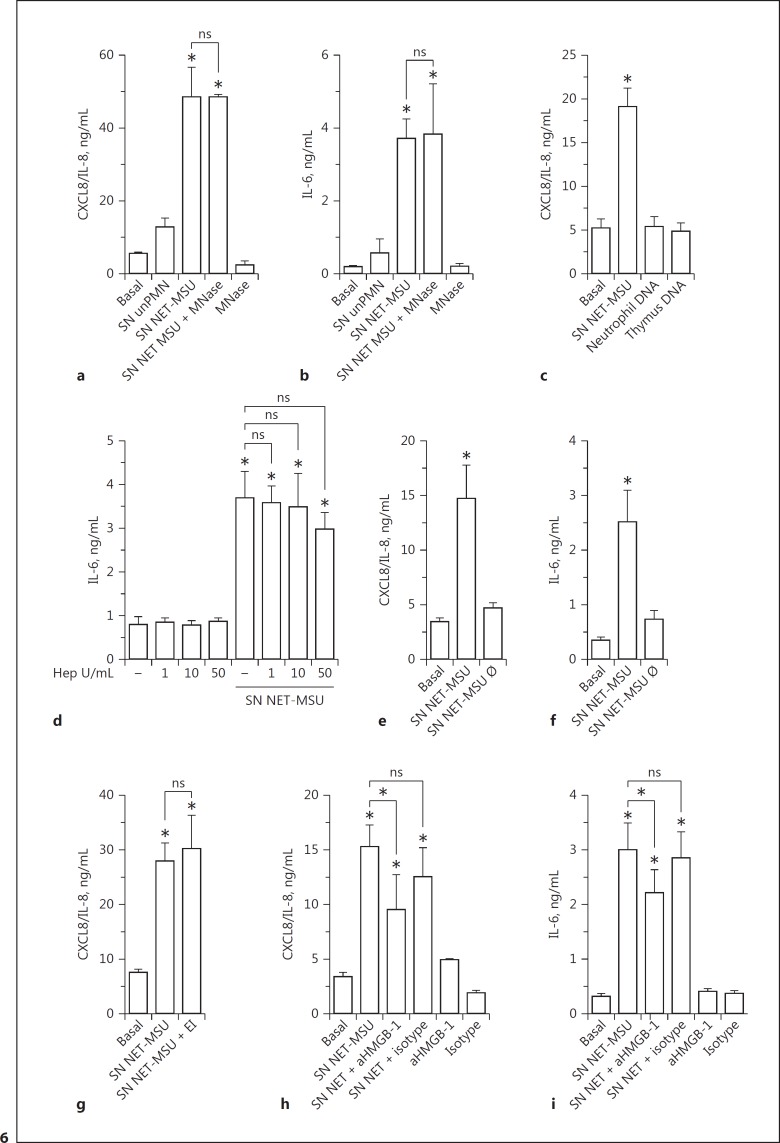
Trying to elucidate the NET component responsible for the stimulatory effects on epithelial cells, we evaluated the impact of SN NET treated with high concentrations of MNase which induced extracellular DNA degradation (online supplementary Fig. S5C, S11). Digestion of NET with MNase did not modify their ability to induce CXCL8/IL-8 and IL-6 production by A549 cells, suggesting that DNA is not responsible for these NET stimulatory capacities (Fig. 6a, b). In agreement with these findings, neither DNA isolated from human neutrophils nor from bovine thymus was able to stimulate CXCL8/IL-8 secretion by A549 cells (Fig. 6c). Moreover, the anion heparin, which is expected to complete the charge interactions of DNA [44], was unable to modulate the ability of SN NET to trigger cytokine release by epithelial cells (Fig. 6d). Taking into account that heparin has a high affinity for histones and releases histones from chromatin [45], our findings also suggest that the stimulatory effect of NET on IL-6 and CXCL8/IL-8 secretion by epithelial cells is not mediated by histones. Additional assays indicated that thermal treatment of SN NET-MSU for 30 min at 70°C abolished their ability to stimulate cytokine production, suggesting that protein denaturation could abrogate the NET stimulatory capacity (Fig. 6e, f). We then evaluated if neutrophil elastase, a serine protease which has been previously shown to stimulate epithelial cells to produce cytokines and induce lung inflammation, was responsible for the NET-mediated stimulation of lung epithelial cells [46]. However, when SN NET-MSU and EI were added together to epithelial cell cultures, the cytokine response of the A549 cells to NET was not modified (Fig. 6g).
Fig. 6.
HMGB-1 blockade but not MNase treatment reduced the cytokine stimulatory capacity of NET. CXCL8/IL-8 (a) and IL-6 (b) secretion by A549 cells cultured for 18 h at 37°C with medium (Basal), SN unPMN, SN NET-MSU, or SN NET-MSU obtained in the presence of MNase (10 U/mL). The effect of MNase on A549 cells was evaluated as a control. c CXCL8/IL-8 secretion by A549 cells stimulated with SN NET-MSU, neutrophil DNA (3 µg/mL), or thymus DNA (3 µg/mL). d The effect of heparin (Hep) on the ability of SN NET-MSU to stimulate IL-6 secretion by A549 cells. CXCL8/IL-8 (e, g) and IL-6 (f) secretion by A549 cells cultured for 18 h at 37°C in the presence or absence of SN NET-MSU (Basal) or heat-treated SN NET-MSU (SN NET-MSU Ø; 30 min at 70°C) (e, f) or SN NET together with EI (g). CXCL8/IL-8 (h) and IL-6 (i) secretion by A549 cells cultured for 18 h at 37°C with or without SN NET-MSU previously incubated for 30 min with an anti-HMGB-1 (aHMGB-1) blocking antibody (2G7) or the respective IgG2b isotype control. The effect of aHMGB-1 and its isotype control on the epithelial cells was assessed as a control. Data are depicted as the mean ± SEM of 3 (a, b), 4 (d, f, h, i), 5 (c, g), and 9 (e) independent experiments performed in triplicate. * p < 0.05 and nonsignificant (ns) (two-way ANOVA with repeated measures, followed by Bonferroni's multiple-comparisons test).
Previous studies reported that the nuclear protein HMGB-1 functions as a DAMP, inducing proinflammatory cytokine secretion when released to the extracellular medium [47]. Additional evidence involved HMGB-1 in ALI and pulmonary fibrosis [48]. HMGB-1 was previously found in NET [10], and the mRNA of the receptor for advanced glycation end-product (RAGE), which is an HMGB-1 receptor, has been reported to be expressed at high levels in alveolar epithelial cells [49]. Taking this data into account, we then evaluated whether our MSU-induced NET also contained this DAMP by confocal microscopy. As expected, we found that HMGB-1 colocalized with chromatin inside the nucleus of unstimulated neutrophils. Moreover, HMGB-1 was detected colocalizing with DNA in MSU-induced NET (online suppl. Fig. S12). More importantly, an anti-HMGB-1 neutralizing antibody significantly inhibited the secretion of CXCL8/IL-8 and IL-6 induced by SN NET-MSU, in contrast to an isotype control antibody that did not reproduce the effect (Fig. 6h, i).
Discussion
In this study, we showed that NET released upon stimulation of neutrophils with MSU, UA, and CSE increase the secretion of proinflammatory cytokines by the airway epithelial cells. NET stimulated the production of significantly higher levels of CXCL8/IL-8 and IL-6 than those induced by supernatants of heat-shocked neutrophils, indicating that the soluble intracellular components released when neutrophils undergo necrosis are not able to reproduce the effect of NET. Although SN unPMN induced some secretion of cytokines, this effect was significantly lower than that induced by SN NET, and could be due to the basal levels of CXCL8/IL-8. Of note, when neutrophils were stimulated with MSU in the presence of DPI or EI, the collected supernatants were not only, as expected, devoid of NET; more importantly, they stimulated IL-6 and CXCL8/IL-8 secretion at similar levels to SN unPMN. Similarly, when neutrophils were stimulated with UA or CSE in the presence of Cl-a, a PAD4 inhibitor, the reduction in their ability to release NET was associated with a reduced capacity to stimulate cytokine secretion by epithelial cells.
Of note, NET stimulated cytokine secretion by either alveolar A549 or bronchial (BEAS-2B and polarized 16HBE14o-) epithelial cells, indicating that the effect was exerted irrespective of the origin of the epithelial cells. However, differences in cytokines levels were observed and could have been due to the differential expression of receptors involved in the recognition of the components of NET responsible for stimulating epithelial cells.
Taking into account the ability of CXCL8/IL-8 and CCL2/MCP-1 to stimulate neutrophil and monocyte recruitment and that IL-6 is a potent proinflammatory cytokine, our findings suggest that NET generation in the airways might contribute to amplifying inflammation by stimulating airway epithelial cells to produce these proinflammatory mediators. Of note, we observed that when polarized bronchial epithelial cells were exposed on their apical surface to NET, they stimulated CXCL8/IL-8 release to the apical but not the basal compartment. Similar findings were previously described in polarized 16HBE14o- and Calu-3 cell cultures [50, 51]. It is presumed that apically polarized cytokine release could create a chemotactic gradient for neutrophil transepithelial migration. This effect could lead to neutrophil recruitment from the subepithelium into the airway lumen. These leukocytes could also release CXCL8/IL-8, in turn contributing to a positive-feedback cycle that might enhance airway inflammation [50].
We also found that the proinflammatory effects of supernatants containing MSU-induced NET were exerted on MØ but not on DC. In fact, in agreement with previous studies performed with NET induced by the calcium ionophore A23187 [52], MSU-induced NET did not induce cytokine secretion by DC, and NET were neither proapoptotic nor cytotoxic for DC (data not shown).
Our findings indicate that NET did not affect the viability of A549, BEAS-2B, and 16HBE14o- cells evaluated by annexin V/PI staining or LDH release. Moreover, NET did not modify the cell morphology evaluated by optical microscopy, the TEER, or the barrier permeability to FITC-dextran, when applied apically over polarized epithelial cells. These observations contrast with previous studies performed by Saffarzadeh et al. [53], who reported that NET exerted cytotoxic effects on the human epithelial cell lines HPAEC and A549 and the murine epithelial cell lines MlE-12 and AT-II. However, the authors employed NET obtained by 4 h of stimulation of neutrophils with PMA and after a washing step and vigorous agitation. As NET are fragile structures that could be lost during the washing step, it might be possible that part of the effects were due to residual PMA in their NET preparations. In fact, we found that, when isolated, PMA-induced NET, in contrast to MSU-induced NET, triggered marked morphological changes in DC and stimulated their adherence to the culture plate (unpubl. observations). Moreover, even though the neutrophils were extensively washed after 1 h of PMA stimulation and before NETosis would have taken place, these NET preparations maintained their ability to trigger the described effects on DC, suggesting that PMA traces were still present and were able to induce cell activation.
Previous studies indicated that UA is released as a consequence of pulmonary cell injury upon mice intranasal administration of bleomycin, leading to fibrosis [6]. At a high local concentration, UA precipitates and forms crystals that cause inflammation [54]. Gasse et al. [6, 55] reported that UA constitutes a major endogenous danger signal that activates the NALP3 inflammasome, leading to IL-1β production and neutrophil recruitment to the lung. They also showed that local administration of exogenous UA crystals recapitulates lung inflammation and repair. Local UA levels are also increased in patients with ALI, a syndrome characterized by the accumulation of neutrophils in the lung accompanied by the development of interstitial edema and an intense inflammatory response [23]. In fact, neutrophils rapidly infiltrate the pulmonary parenchyma after hypovolemic shock, intestinal ischemia/reperfusion, or endotoxin administration [8], while the depletion of neutrophils reduces pulmonary edema and proinflammatory cytokine production after hemorrhage or endotoxemia [56]. Considering that both UA and MSU induce NET formation [10, 37], our findings, which indicated that these NET markedly increased the production of proinflammatory cytokine by the airway epithelial cells, suggest that NET could contribute to the exacerbation of lung inflammation, not only upon bleomycin administration but also in ALI. Recent studies reported increased UA levels in lung lavage fluid in a murine ventilator-induced lung injury (VILI) model [23], and demonstrated that mechanical ventilation, together with LPS instillation, induced NET formation [57]. In this murine model, mechanical ventilation plus LPS was associated with increased airway levels of HMGB-1 and IL-1β, and a tendency to increase the CCL2/MCP-1 and IL-6 concentrations. Noteworthy is that the authors showed that intratracheal DNase treatment reduced NET markers but did not significantly impact on different measures of injury, concluding that NET do not play a major pathogenic role in this model of VILI [57]. However, our findings, which indicated that MNase treatment did not reduce the cytokine-stimulatory capacity of MSU-induced NET, suggest that evaluation of this parameter is not enough to rule out a role of NET in pathogenicity. Our results also warn that therapeutic strategies including the use of DNase to reduce NET-induced lung inflammation might be ineffective. In fact, we found that blockade of HMGB-1 with a specific antibody, but not MNase treatment, attenuated the NET-induced proinflammatory epithelial response. Given the incapacity of MNase to reduce the proinflammatory effects of NET, it is possible to speculate that either soluble or DNA-associated HMGB-1 stimulates cytokine secretion by the epithelial cells. However, as necrotic neutrophils do not release HMGB-1 to culture supernatants [58], it is possible that, in vivo, this molecule is released by neutrophils associated with chromatin upon NETosis, and only dissociates by the action of extracellular nucleases.
CF is caused by mutations in the CF transmembrane regulator (CFTR), resulting in dysfunctional salt and water transport across epithelia. This lung disease is the major cause of morbidity and mortality in CF patients. Abundant amounts of NET have been also found in CF airway fluids by confocal, atomic-force and scanning-electron microcopy [18, 59]. Moreover, HMGB-1 levels have also been found to be significantly elevated in the bronchoalveolar lavage fluid of CF patients [60, 61]. Aerosolized recombinant human DNase (rhDNase) is a therapeutic option used to treat patients with moderate-to-severe CF lung disease in order to reduce mucus viscosity [62]. Although rhDNase treatment has been shown to effectively reduce pulmonary exacerbations and improve lung function in some patients, it does not help with the severe neutrophilic inflammation, chronic bacterial infection, and further deterioration of the lung [63, 64]. Our findings, indicating that treatment of NET with MNase does not reduce their ability to stimulate CXCL8/IL-8 and IL-6 secretion by epithelial cells, are in agreement with these in vivo observations and suggest that other therapeutic approaches aimed at limiting the induction of NETosis or the blockade of HMGB-1 could help to mitigate the resulting lung injury in CF. In fact, previous studies have shown that the inhibition of HMGB-1 enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in CF [60].
Despite the advantageous antimicrobial properties of NET, their ineffective clearance and exacerbated production could have pathological consequences [65]. The available evidence suggests that NET are key players in many pathological situations like VILI, ALI, and CF. Our findings which indicate that NET are able per se to induce proinflammatory cytokines by airway epithelial cells, and that inhibitors of NETosis markedly reduce the ability of neutrophils to stimulate the release of these cytokines by epithelial cells, suggest that selectively targeting NET induction could hold promise for protection in these pathologies.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (PICT2010/2500 and PICT2013/2177), Universidad de Buenos Aires (grant 20020130100744BA), and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina.
References
- 1.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 2.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2013;5:348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 5.Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–323. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 7.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 9.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 12.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 13.Cui B-B, Tan C-Y, Schorn C, Tang H-H, Liu Y, Zhao Y. Neutrophil extracellular traps in sterile inflammation: the story after dying? Autoimmunity. 2012;45:593–596. doi: 10.3109/08916934.2012.719952. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–1266. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer M, Shan Q, D'Ortona S, Maurer R, Mitchell R, Olesen H, et al. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J Innate Immun. 2014;6:765–779. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock KL, Kataoka H, Lai J-J. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka H, Yang K, Rock KL. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury-induced inflammation in the liver and lung. Eur J Pharmacol. 2015;746:174–179. doi: 10.1016/j.ejphar.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuipers MT, Aslami H, Vlaar APJ, Juffermans NP, Tuip-de Boer AM, Hegeman MA, et al. Pre-treatment with allopurinol or uricase attenuates barrier dysfunction but not inflammation during murine ventilator-induced lung injury. PLoS One. 2012;7:e50559. doi: 10.1371/journal.pone.0050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 25.Schiltz C, Lioté F, Prudhommeaux F, Meunier A, Champy R, Callebert J, et al. Monosodium urate monohydrate crystal-induced inflammation in vivo: quantitative histomorphometric analysis of cellular events. Arthritis Rheum. 2002;46:1643–1650. doi: 10.1002/art.10326. [DOI] [PubMed] [Google Scholar]
- 26.Higashi T, Mai Y, Noya Y, Horinouchi T, Terada K, Hoshi A, et al. A simple and rapid method for standard preparation of gas phase extract of cigarette smoke. PLoS One. 2014;9:e107856. doi: 10.1371/journal.pone.0107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabelloni Ml, Sabbione F, Jancic C, Fuxman Bass J, Keitelman I, Iula L, et al. NADPH oxidase-derived reactive oxygen species are involved in human neutrophil IL-1β secretion but not in inflammasome activation. Eur J Immunol. 2013;43:3324–3335. doi: 10.1002/eji.201243089. [DOI] [PubMed] [Google Scholar]
- 28.Colado A, Almejún MB, Podaza E, Risnik D, Stanganelli C, Elías EE, et al. The kinase inhibitors R406 and GS-9973 impair T cell functions and macrophage-mediated anti-tumor activity of rituximab in chronic lymphocytic leukemia patients. Cancer Immunol Immunother. 2017;66:461–473. doi: 10.1007/s00262-016-1946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez D, Vermeulen M, von Euw E, Sabatté J, Maggíni J, Ceballos A, et al. Extracellular acidosis triggers the maturation of human dendritic cells and the production of IL-12. J Immunol. 2007;179:1950–1959. doi: 10.4049/jimmunol.179.3.1950. [DOI] [PubMed] [Google Scholar]
- 30.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 31.Bayer H, Müller T, Myrtek D, Sorichter S, Ziegenhagen M, Norgauer J, et al. Serotoninergic receptors on human airway epithelial cells. Am J Respir Cell Mol Biol. 2007;36:85–93. doi: 10.1165/rcmb.2006-0151OC. [DOI] [PubMed] [Google Scholar]
- 32.Amstad P, Reddel RR, Pfeifer A, Malan-Shibley L, Mark GE, Harris CC. Neoplastic transformation of a human bronchial epithelial cell line by a recombinant retrovirus encoding viral Harvey ras. Mol Carcinog. 1988;1:151–160. doi: 10.1002/mc.2940010303. [DOI] [PubMed] [Google Scholar]
- 33.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Bio. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 34.Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schorn C, Janko C, Krenn V, Zhao Y, Munoz LE, Schett G, et al. Bonding the foe - NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front Immunol. 2012;3:376. doi: 10.3389/fimmu.2012.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arai Y, Nishinaka Y, Arai T, Morita M, Mizugishi K, Adachi S, et al. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun. 2014;443:556–561. doi: 10.1016/j.bbrc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol. 2014;233:294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu T-T, Chen T-L, Loon W-S, Tai Y-T, Cherng Y-G, Chen R-M. Lipopolysaccharide stimulates syntheses of toll-like receptor 2 and surfactant protein-A in human alveolar epithelial A549 cells through upregulating phosphorylation of MEK1 and ERK1/2 and sequential activation of NF-κB. Cytokine. 2011;55:40–47. doi: 10.1016/j.cyto.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 2005;53:418–427. [PubMed] [Google Scholar]
- 42.Kondo Y, Higa-Nakamine S, Noguchi N, Maeda N, Toku S, Isohama Y, et al. Induction of epithelial-mesenchymal transition by flagellin in cultured lung epithelial cells. AJP Lung Cell Mol Physiol. 2012:1057–1069. doi: 10.1152/ajplung.00096.2012. [DOI] [PubMed] [Google Scholar]
- 43.Ferrero MC, Fossati CA, Baldi PC. Direct and monocyte-induced innate immune response of human lung epithelial cells to Brucella abortus infection. Microbes Infect. 2010;12:736–747. doi: 10.1016/j.micinf.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Grässle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaría C, Baldauf C, et al. Von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol. 2014;34:1382–1389. doi: 10.1161/ATVBAHA.113.303016. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh DE, Greene CM, Carroll TP, Taggart CC, Gallagher PM, O'Neill SJ, et al. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem. 2001;276:35494–35499. doi: 10.1074/jbc.M103543200. [DOI] [PubMed] [Google Scholar]
- 47.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 49.Katsuoka F, Kawakami Y, Arai T, Imuta H, Fujiwara M, Kanma H, et al. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- 50.Chow AWM, Ko WH, Liang JFT, Wong JSC, Fu Y, Tang NLS. Polarized secretion of interleukin (IL)-6 and IL-8 by human airway epithelia 16HBE14o- cells in response to cationic polypeptide challenge. PLoS One. 2010;5:e12091. doi: 10.1371/journal.pone.0012091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, Wu F, Sun F, Huang P. Adenosine promotes IL-6 release in airway epithelia. J Immunol. 2008;180:4173–4181. doi: 10.4049/jimmunol.180.6.4173. [DOI] [PubMed] [Google Scholar]
- 52.Barrientos L, Bignon A, Gueguen C, Chaisemartin D, Gorges R, Sandré C, et al. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J Immunol. 2014;193:5689–5698. doi: 10.4049/jimmunol.1400586. [DOI] [PubMed] [Google Scholar]
- 53.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Galuska SP, Lohmeyer J, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 55.Gasse P, Riteau N, Vacher R, Michel M-L, Fautrel A, di Padova F, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 57.Yildiz C, Palaniyar N, Otulakowski G, Khan MA, Post M, Kuebler WM, et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology. 2015;122:864–875. doi: 10.1097/ALN.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 58.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcos V, Zhou Z, Yildirim AÖO, Bohla A, Hector A, Vitkov L, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:899. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 60.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 (HMGB1) between inflammation and infection. 2015;1:1–9. doi: 10.1016/j.cmi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA. 1990;87:9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratjen F, Paul K, Van Koningsbruggen S, Breitenstein S, Rietschel E, Nikolaizik W. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: influence of treatment with dornase alpha. Pediatr Pulmonol. 2005;39:1–4. doi: 10.1002/ppul.20134. [DOI] [PubMed] [Google Scholar]
- 64.Ratjen F, Grasemann H. New therapies in cystic fibrosis. Curr Pharm Des. 2012;18:614–627. doi: 10.2174/138161212799315984. [DOI] [PubMed] [Google Scholar]
- 65.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data