Supplemental digital content is available in the text.
Key Words: keratoconjunctivitis sicca, dry eye disease, nanomicelles, OTX-101
Background:
Keratoconjunctivitis sicca affects 5% to 33% of the population and is often accompanied by symptoms such as burning and dryness. This pooled analysis evaluated total and central corneal fluorescein staining (CFS) in patients receiving OTX-101 0.09% or vehicle in phase 2b/3 and 3 studies and whether improvements in corneal staining correlated with improved visual acuity.
Methods:
In these randomized, vehicle-controlled studies, patients received 1 drop of OTX-101 or vehicle in both eyes twice daily. Corneal staining was performed at baseline and days 28, 56, and 84. CFS was evaluated in each zone (0-to-4 scale); total corneal staining (0-to-20 scale per eye) was averaged over both eyes. Pooled safety assessments included adverse event monitoring.
Results:
Mean baseline CFS total scores (SD) were 4.2 (2.5) and 4.3 (2.6) for the OTX-101 (n = 523) and vehicle (n = 525) groups, respectively. For total corneal staining, least squares mean changes from baseline (standard error) were −0.9 (0.08) versus −0.5 (0.08) for OTX-101 and vehicle, respectively (P = 0.0008), on day 28 and −1.4 (0.09) versus −0.9 (0.09) on day 84 (P = 0.0002). There was a significantly high correlation (P = 0.0117) between reduced central corneal staining and improved visual acuity on day 84. Treatment-related adverse events were mostly mild, with instillation site pain reported by 21.8% and 4.0% of patients receiving OTX-101 and vehicle, respectively.
Conclusions:
Treatment with OTX-101 led to greater improvements versus vehicle in corneal surface staining as early as 4 weeks, and further improvements were seen up to 12 weeks. OTX-101 was well tolerated in patients with keratoconjunctivitis sicca.
Dry eye disease, also known as keratoconjunctivitis sicca (KCS), is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and is accompanied by ocular symptoms in which tear film instability and hyperosmolarity and ocular surface inflammation and damage play etiological roles.1 Dry eye disease is a vicious cycle in which hyperosmolarity leads to an inflammatory cascade that results in ocular surface damage.2
The clinical presentation of KCS varies from patient to patient, and the relationship between signs and symptoms is not linear.3 Symptoms of KCS may include burning, stinging, grittiness, foreign body sensation, and dryness. Although the prevalence of KCS is difficult to determine because of inconsistent definitions and populations, studies have shown that the prevalence ranges from 5% to 33%.4
Treatment of KCS progresses in a stepwise approach, starting with education, lid hygiene, and modification of environmental factors and then progressing to nonpharmacologic and pharmacologic management.5 There are currently 2 topical ocular pharmacologic treatments in use to treat KCS, cyclosporine 0.05% ophthalmic emulsion (Restasis; Allergan, Irvine, CA) and lifitegrast 5% ophthalmic solution (Xiidra; Shire US Inc, Lexington, MA).6,7
The current pharmacologic treatments of KCS have several limitations. Almost 20% of patients with dry eye are dissatisfied with their overall treatment, which may include artificial tears, ocular lubricants, and prescription medications; areas of dissatisfaction include symptom relief, treatment side effects, and amount of time it takes to experience symptom relief.8 In addition, lipophilic drugs such as cyclosporine A (CsA) have low ocular tissue bioavailability and require innovative approaches to get more of the drug into ocular tissue.9,10 This highlights the unmet need for an improved ophthalmic drug delivery system.
OTX-101 (CsA 0.09%, CEQUA; Sun Pharmaceutical Industries, Inc, Cranbury, NJ) is approved by the Food and Drug Administration to increase tear production in patients with KCS.11 OTX-101 is a novel nanomicellar solution of CsA that improves the bioavailability of the active ingredient in ocular tissues, with negligible systemic exposure (see Supplemental Figure 1, Supplemental Digital Content 1, http://links.lww.com/ICO/A798).12 In the phase 2b/3 and 3 studies, OTX-101 was superior to vehicle in increasing tear production and improving conjunctival and corneal staining and demonstrated improved corneal fluorescein staining (CFS) as early as 4 weeks with an acceptable tolerability profile.13
Patients with KCS may have increased CFS because of corneal surface damage; conversely, an improvement in CFS may indicate an improvement in corneal surface integrity.14 This pooled analysis evaluated both total and central CFS in subjects receiving either OTX-101 0.09% or vehicle in the phase 2b/3 and phase 3 studies. In KCS, impaired visual function is associated with central corneal epithelial damage, which may adversely affect daily functioning.15 An improvement in central corneal staining scores may relate to an improvement in visual acuity (VA).15 Therefore, this secondary analysis also assessed whether improvements in corneal staining after treatment with OTX-101 0.09% correlate with improvements in VA in KCS and whether VA improved in patients treated with OTX-101 versus vehicle.
METHODS
Study Design
This is a pooled analysis of a phase 2b/3 and a phase 3 study that evaluated the effect of OTX-101 0.09% on CFS compared with vehicle. Both were randomized, multicenter, double-masked, vehicle-controlled studies of 12-week duration. Patients were enrolled at 29 and 45 sites across the United States for phase 2b/3 and phase 3 studies, respectively. The details of these registered studies (ClinicalTrials.gov: phase 2b, NCT02254265; phase 3, NCT02688556) were published previously.13 All study documents including informed consent were approved by a central institutional review board, and the studies were conducted in accordance with the International Council for Harmonisation guidelines.
Study Time Points and Treatments
Each study design (phase 2b/3 and phase 3) is shown in Supplemental Digital Contents 2 and 3 (see Supplemental Figure 2, http://links.lww.com/ICO/A799 and http://links.lww.com/ICO/A800). In the phase 2b/3 study, all patients first entered a run-in period for 14 to 17 days before being randomized in a 1:1:1 ratio to vehicle, OTX-101 0.05%, or OTX-101 0.09%. Patients returned for visits at baseline and days 14, 28, 42, 56, and 84 or at early discontinuation. In the phase 3 study, the run-in period was 14 to 20 days, and patients were randomized to either vehicle or OTX-101 0.09%. Patients returned for visits on days 0 (baseline), 28, 56, and 84 or at early discontinuation.
During the run-in period, all enrolled patients received 1 drop of vehicle per eye twice daily to wash out any residual effect of previous ocular medications. The composition of vehicle was identical to that of OTX-101, except for omission of CsA. The current pooled analyses focus on the FDA-approved 0.09% dosing11 and include observations from the common time points of the 2 studies: days 0, 28, 56, and 84 or early discontinuation. Patients and all study personnel were masked to randomization. Patients were instructed to administer 1 drop of treatment to both eyes twice daily for 84 days and were followed through the end of treatment.
Key Eligibility Criteria
Eligibility criteria for both studies were the same and were published previously in their entirety.13 Briefly, both studies enrolled adults aged ≥18 years. Key inclusion criteria were the following: self-reported history of ≥6 months of KCS; clinical diagnosis of bilateral KCS; lissamine green conjunctival staining sum score of 3 to 9 out of a possible score of 12, excluding the superior zones, in the same eye at both screening and baseline visits; Snellen VA better than 20/200 in each eye; and willingness to discontinue any current dry eye treatment for the duration of the study. Key exclusion criteria for both studies were the following: use of cyclosporine ophthalmic emulsion 0.05% within 3 months of screening, previous treatment failure with cyclosporine ophthalmic emulsion 0.05%, diagnosis of Sjögren disease >5 years before screening, and corneal refractive surgery within 6 months of screening.
Efficacy Assessments
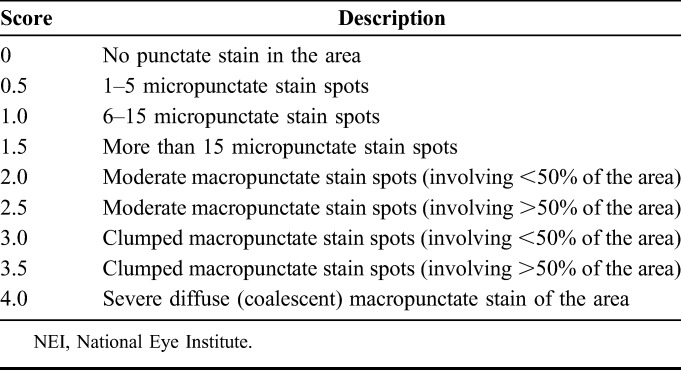
The pooled analyses included CFS measurements performed at baseline and common study days 28, 56, and 84 or early discontinuation. One drop (10 μL) of 0.5% fluorescein solution was instilled by a pipette into the conjunctival cul-de-sac, followed by adequate blinking. CFS was evaluated in each eye and graded on a 0-to-4–point scale (0.5-point increments; 0 = no staining/clear and 4 = severe diffuse macropunctate NaFl stain spots; Table 1; see Supplemental Figure 3, Supplemental Digital Content 4, http://links.lww.com/ICO/A801). Total corneal staining was scored on a 0-to-20–point scale (total possible 4 points for each of the 5 corneal zones) per eye and was averaged over both eyes at each assessment. Although the eye with higher lissamine green conjunctival staining was chosen as the study eye in the phase 2b/3 analyses, phase 3 included observations from both eyes; the pooled analysis included both eyes.
TABLE 1.
Expanded NEI/Industry Workshop CFS Scale
Other Signs and Symptoms
Additional subjective and objective assessments included Snellen VA, slit-lamp examination, lissamine green conjunctival staining, and Symptom Assessment iN Dry Eye to assess the frequency and severity of dryness and/or irritation and were performed at days 0, 28, 56, and 84; the unanesthetized Schirmer test to assess signs of KCS was performed on days 0 and 84; intraocular pressure and dilated ophthalmoscopy for fundus examination was performed on day 84. Snellen VA was collected as a safety end point in the phase 2b/3 and phase 3 studies and used in the pooled efficacy analysis in an exploratory manner under the post hoc hypotheses of a relationship to central CFS and to OTX-101 treatment. Adverse events (AEs) were monitored at each of the study visits.
Statistical Analysis
Data from randomized patients in the intent-to-treat populations from the phase 2b/3 and phase 3 studies who received either OTX-101 0.09% or vehicle were pooled at the common study time points (baseline and study days 28, 56, and 84). Summary statistics (n, mean, median, minimum, and maximum) were used to describe observed continuous variables and by-patient arithmetic changes from baseline. For the changes from baseline that could not be calculated because of missing data, values were not imputed.
The relationship between treatment and change in CFS from baseline was assessed using a restricted maximum likelihood repeated-measures mixed model on changes from baseline values as the dependent variable, treatment group as the fixed effect, baseline CFS as a covariate, and visit in relation to paired eyes within a patient as repeated measures. Differences between treatments were compared using analysis of covariance. The relationship between central CFS and Snellen VA as continuous variables was assessed in 2 steps. First, the t tests of the mean differences in CFS and VA scores between treatments were performed to test whether OTX-101 treatment influenced changes observed relative to vehicle therapy postbaseline. Second, test statistics were developed from a restricted maximum likelihood repeated-measures mixed model with VA as the dependent variable, central CFS scores as the fixed effect, and baseline VA as a covariate. Analyses and models fit using CFS and Snellen VA describe single eyes; analyses of CFS alone in relation to treatment describe single subjects.
Comparison of binary measures of response was derived from reported CFS scores, and the extent of nonrandom association between CFS and VA categories was evaluated using the Fisher exact test. Analyses and models fit using CFS and VA describe single eyes; analyses of CFS alone in relation to treatment describe single patients.
RESULTS
Patient Disposition and Demographic Characteristics
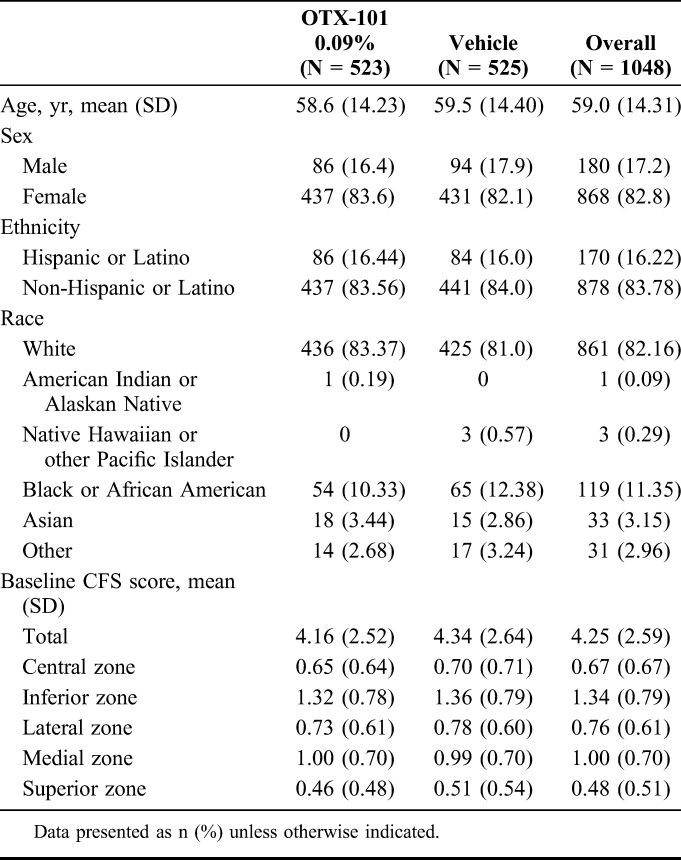
The disposition for all screened patients (N = 1508) is presented in Figure 1. Of 1200 enrolled patients, 523 patients were randomized to OTX-101 0.09% and 525 to vehicle, and 487 and 505 patients, respectively, completed the 12-week phase 2b/3 and 3 studies (Fig. 1). The mean (SD) age of patients was 58.6 (14.2) years for the OTX-101 group and 59.5 (14.4) years for the vehicle group (Table 2). The majority of the study population was female (83.6%) and white (83.4%) for the OTX-101 group and 82.1% and 81.0%, respectively, for the vehicle group. Baseline characteristics including CFS scores were similar between the 2 groups.
FIGURE 1.
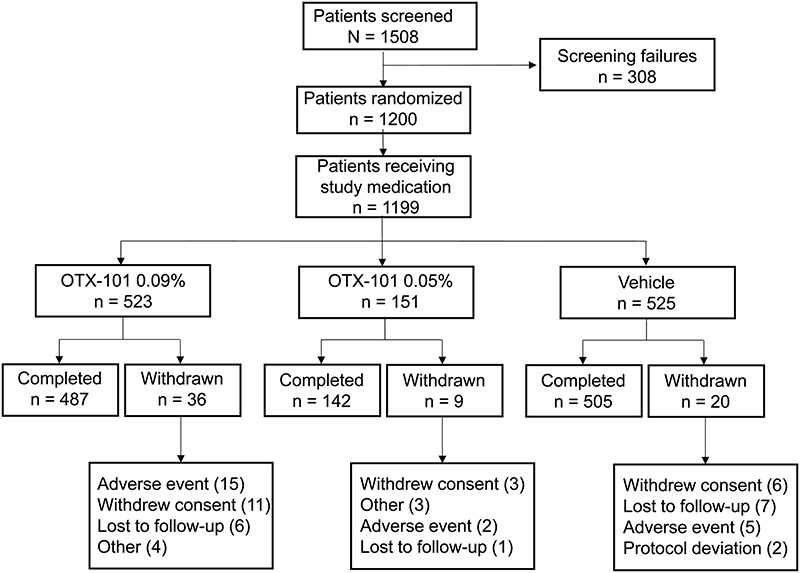
Patient disposition. Patients who received OTX-101 0.05% only in the phase 2b/3 study are not included in the present pooled analysis.
TABLE 2.
Pooled Demographic and Baseline Clinical Characteristics
Efficacy
Corneal Staining
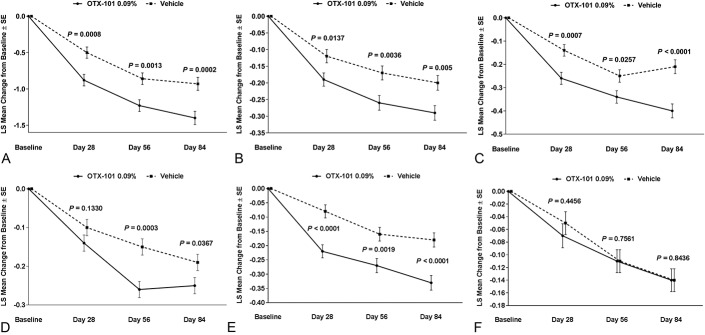
Mean baseline CFS total scores (SD) for both eyes for the OTX-101 and vehicle groups were 4.2 (2.5) and 4.3 (2.6), respectively (Table 2). For total CFS, the least squares mean changes from baseline were significant for all common postbaseline time points in OTX-101 versus vehicle (P = 0.0008, 0.0013, and 0.0002 for days 28, 56, and 84, respectively, Fig. 2A). Four of 5 zones (central, inferior, lateral, and medial) had significantly improved corneal clearing with OTX-101 versus vehicle at all time points, except for the lateral zone on day 28 (Figs. 2B–E). Superior zone CFS scores were similar for both groups at all time points (Fig. 2F). In patients with CFS >0 at baseline, OTX-101 significantly improved corneal staining at all time points versus vehicle (Fig. 3).
FIGURE 2.
Least squares mean change from baseline on days 28, 56, and 84 in corneal fluorescein staining in patients treated with OTX-101 versus vehicle in the pooled population for (A) total score, (B) central zone, (C) inferior zone, (D) lateral zone, (E) medial zone, and (F) superior zone. P values for OTX-101 0.09% versus vehicle.
FIGURE 3.
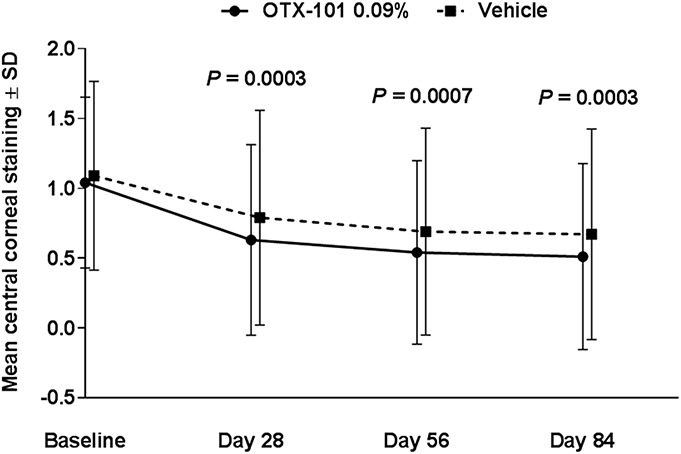
Mean central corneal staining score over time in patients with baseline scores >0. P values for OTX-101 least squares mean change from baseline versus vehicle.
There was a significantly high correlation between reduced central corneal staining and improved Snellen VA on day 84 (P = 0.0117, Fig. 4A). There was a statistically significant relationship between treatment with OTX-101 and modest improvement of VA at days 56 and 84 (P = 0.0399 and 0.0152, respectively) (Fig. 4B).
FIGURE 4.
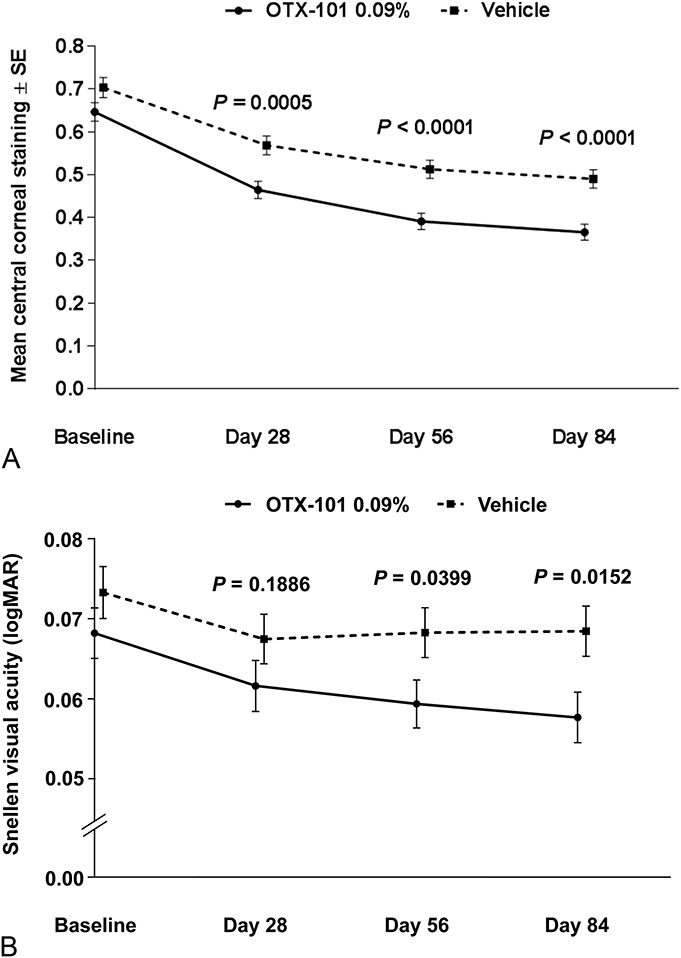
Correlation between (A) central corneal staining and (B) Snellen VA. P values for OTX-101 0.09% versus vehicle.
Other Signs and Symptoms
The primary efficacy end point of ≥10 mm increase from baseline in the Schirmer test score on day 84 was met for both studies, with a clinically meaningful and significant increase in tear production (16.6% of eyes vs 9.0% of eyes for OTX-101 0.09% and vehicle, respectively, P < 0.0001).13 Similarly, the mean decrease from baseline at day 84 in conjunctival staining was significantly greater for OTX-101 0.09% versus vehicle.13 Subjective improvement in symptoms of dryness and irritation was similar for both treatment groups.
Safety
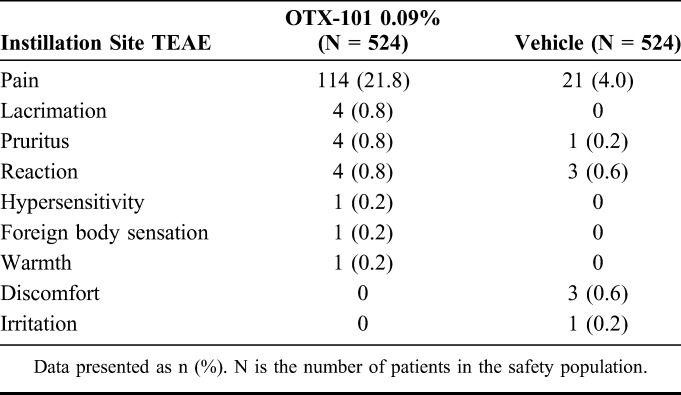
Treatment-emergent adverse events (TEAEs) in the pooled population were mostly mild to moderate in nature and resolved without any treatment. Conjunctival hyperemia was the most frequent ocular AE that occurred in 30 (5.7%) and 19 (3.6%) patients in the OTX-101 and vehicle groups, respectively. Instillation site pain (typically described as mild stinging and/or burning after each instillation) was the most frequent administration site TEAE, reported by 114 (21.8%) and 21 (4.0%) patients in the OTX-101 and vehicle groups, respectively (Table 3). Other administration site TEAEs occurred in less than 1% of the study population and were similar between the 2 groups.
TABLE 3.
Summary of Administration Site Treatment-Emergent Adverse Events
There were no clinically significant changes from baseline in VA. There were no clinically significant abnormalities noted for intraocular pressure measurements in both studies. Although the fundus examinations in the phase 2b/3 study were within the normal range, a clinically significant shift was recorded in 1 patient each from OTX-101 0.09% and vehicle groups in the phase 3 study. No serious TEAEs related to the study medications were observed in either study.
DISCUSSION
The results of this pooled analysis showed that OTX-101 0.09% improved corneal staining at 4 weeks of treatment and continued through the study end. The currently available pharmacologic dry eye treatments show statistically significant improvement versus vehicle in corneal staining scores at day 84.16,17
The nanomicelle formulation of CsA (OTX-101) showed a >10-time increase in aqueous concentrations of CsA in comparison to an oil-based formulation of CsA, which allows for a higher distribution of the drug into the target tissues to address the underlying cause of dry eye disease.12 The change from baseline for total CFS was significant at all time points for patients receiving OTX-101 versus vehicle. There was also significant improvement seen in corneal staining in 4 of the 5 corneal zones, and particularly the central corneal zone, which is integral for maintaining visual function, for patients receiving OTX-101 versus vehicle.15
There is a correlation between dry eye disease and a decrease in VA. This is believed to be due to the changes on the ocular surface regulating the factors needed to form clear visual images.18–20 This study demonstrates that OTX-101 may improve VA, along with CFS, as early as 2 months after treatment begins. Improving VA helps increase the quality of life for patients with dry eye disease. Additional studies of longer duration are needed to determine how long an improvement in VA correlates with an improvement in CFS.
Overall, OTX-101 0.09% seemed to have acceptable tolerability and a low incidence of instillation site AEs. The rate of instillation site AEs for OTX-101 0.09% was similar to the currently available pharmacologic KCS treatments.16,17 In the present studies, instillation site pain (burning and stinging) was reported by 21.8% of patients receiving OTX-101; most of the complaints of instillation site pain were transient and rated mild in intensity. In the phase 3 study for the currently marketed CsA formulation, a total of 19.7% of patients experienced burning and stinging.17 In both studies, the OTX-101 0.09% group had no incidence of dysgeusia, which is the most commonly reported nonocular AE in patients receiving lifitegrast.16
A limitation of the pooled analysis is that both studies were of relatively short duration. Although improvement in CFS was seen at day 28 and continued through day 84, it is not known whether this improvement would continue to show significance if the study length was extended. Another limitation of both studies was the use of vehicle only, rather than an active comparator. The assessment of VA as a safety end point on days 28, 56, and 84 may have been performed in a discretionary manner, thereby possibly resulting in a weaker correlation with CFS than if VA data had been collected as an efficacy end point. Finally, the vehicle was used in both studies as the comparator, rather than the currently available formulation of CsA. This limits the ability to make direct comparisons of the superiority of OTX-101 over the currently available formulation of CsA with regard to corneal staining.
Overall, treatment with OTX-101 0.09% led to greater improvements versus vehicle in corneal surface integrity in patients with KCS as early as 4 weeks. The improvements were maintained through 12 weeks, as demonstrated by reduced total and central CFS scores in both eyes. Statistically significant improvements in VA correlated with reduced central corneal staining on day 84 and are noted between treatments as early as day 56. OTX-101 0.09% was well tolerated in both studies. A faster time to efficacy in KCS treatment may help increase patient satisfaction and persistence with their medication and treatment. In addition, an improvement in corneal staining that correlates with an improvement in VA also may help increase patient satisfaction. These findings support the use of OTX-101 0.09% in patients with KCS.
Supplementary Material
Footnotes
This study was sponsored and funded by Ocular Technologies, SARL (now a wholly owned subsidiary of Sun Pharmaceutical Industries). Ocular Technologies, SARL, participated in the design, conduct, monitoring, data collection, data management, and data analysis of the study. Writing and editorial support for manuscript preparation were provided by Jennifer Meyering, RN, MS, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Ltd. All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship.
A. Kabat reports consultant fees from Bio-Tissue, Lacrivera, Shire, Ocusoft, and Vmax; speaker fees from Shire; and personal fees from Avellino, Bruder, EyeGate, TearScience, and Sun Pharmaceuticals. A. Ogundele is an employee of Sun Pharmaceuticals. B. S. Lee reports personal fees from Alcon, Essilor, Johnson & Johnson Vision, OcuSoft, and Shire and other support from Guardion Health Sciences. B. A. Schechter reports consultant fees from Sun Pharmaceuticals, Johnson & Johnson Vision, and MST; surgical and speaker fees from Bausch/Valeant and Shire Pharmaceuticals. C. Darby is a contracted consultant of Sun Pharma Advanced Research Company, Ltd. D. K. Devries reports consultant fees from Alcon, Allergan, Akorn, Bio-Tissue, Bruder, BVI Medical, Bausch & Lomb, Eye 4 Lives, Johnson & Johnson Vision (Abbott Medical Optics), Ophthalmic Resources, Revision Optics, RySurg, ScienceBased Health, Shire, Sun Pharmaceuticals, TearLab, TearScience, and Vmax Vision; personal fees from Johnson & Johnson Vision (Abbott Medical Optics); speaker fees from Alcon, Akorn, Bruder, Bausch & Lomb, Eye 4 Lives, OcuSoft, ScienceBased Health, Shire, and Vmax Vision OcuSoft; and other fees from BVI Medical and RPS. J. Bacharach reports speaker fees from Aerie, Alcon, Allergan, Bausch & Lomb, Glaukos, New World Medical, and Sun Pharmaceuticals; and consultant fees from Aerie, Alcon, Allergan, B&L, Optovue, Injectsense, and New World Medical. J. Luchs reports personal fees from Allergan, Alcon, Bausch & Lomb, Shire, Sun Pharmaceuticals, and TearLab; and other fees from Allergan, Alcon, Bausch & Lomb, Calhoun Vision, CLXO, Insightful Solutions, Shire, Sun Pharmaceuticals, and Trefoil Therapeutics. P. Karpecki reports consultant fees from Aerie Pharmaceuticals, Akorn, Alcon, Allergan, Avellino Labs, Bausch & Lomb, Beaver-Visitec, Bio-Tissue, Blephex, Bruder, Cambium, DGH Technology, EyeBrain/Neurolens, EyeGate Pharma, Eyevance, Focus Labs, Imprimis, Ivantis, Jobson Medical Information/WebMD, Johnson & Johnson Vision, Konan Medical, Lenstec, Novartis, Oasis Medical, Ocular Sciences, Oculus, OcuMedic, OcuSoft, Oyster Point Medical, Reichert/AMETEK, ScienceBased Health, Sentiss, Shire, Sight Sciences, Silk Technologies, Sun Pharmaceuticals, Tarsus Medical, TearFilm Innovations, TearLab, Total Eye Care Partners, Topcon, Visant Medical, Visionix, and Vital Tears. R. Smyth-Medina reports consultant fees from Abbott Laboratories, Alcon, Allergan, Bausch & Lomb, BioD, InSite Vision, Katena Products, Nicox, OASIS Medical, Shire, and TearScience; and speaker and other fees from Abbott Laboratories, Alcon, Allergan, Bausch & Lomb, BioD, InSite Vision, Katena Products, Nicox, OASIS Medical, Shire, and TearScience. LS and RSM report no disclosures.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
REFERENCES
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 2.Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- 3.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–365. [DOI] [PubMed] [Google Scholar]
- 5.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. [DOI] [PubMed] [Google Scholar]
- 6.Restasis® (Cyclosporine Ophthalmic Emulsion) 0.05%, for Topical Ophthalmic Use. Full Prescribing Information. Irvine, CA: Allergan; 2017. [Google Scholar]
- 7.Xiidra® (Lifitegrast Ophthalmic Solution) 5%, for Topical Ophthalmic Use. Full Prescribing information. Lexington, MA: Shire; 2016. [Google Scholar]
- 8.Schaumberg DA, Uchino M, Christen WG, et al. Patient reported differences in dry eye disease between men and women: impact, management, and patient satisfaction. PLoS One. 2013;8:e76121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cholkar K, Patel A, Vadlapudi AD, et al. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat Nanomed. 2012;2:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwano M, Ibuki H, Morikawa N, et al. Cyclosporine A formulation affects its ocular distribution in rabbits. Pharm Res. 2002;19:108–111. [DOI] [PubMed] [Google Scholar]
- 11.CEQUA™ (Cyclosporine Ophthalmic Emulsion) 0.09%. Full Prescribing Information. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; 2018. [Google Scholar]
- 12.Vaishya RD, Khurana V, Patel S, et al. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandamwar KL, Papas EB, Garrett Q. Fluorescein staining and physiological state of corneal epithelial cells. Cont Lens Anterior Eye. 2014;37:213–223. [DOI] [PubMed] [Google Scholar]
- 15.Kaido M, Matsumoto Y, Shigeno Y, et al. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci. 2011;52:9516–9522. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121:475–483. [DOI] [PubMed] [Google Scholar]
- 17.Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. [DOI] [PubMed] [Google Scholar]
- 18.Goto E, Ishida R, Kaido M, et al. Optical aberrations and visual disturbances associated with dry eye. Ocul Surf. 2006;4:207–213. [DOI] [PubMed] [Google Scholar]
- 19.Tong L, Waduthantri S, Wong TY, et al. Impact of symptomatic dry eye on vision-related daily activities: the Singapore Malay Eye Study. Eye (Lond). 2010;24:1486–1491. [DOI] [PubMed] [Google Scholar]
- 20.Ridder WH, III, Tomlinson A, Huang JF, et al. Impaired visual performance in patients with dry eye. Ocul Surf. 2011;9:42–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.