Abstract
Here we review published studies on the abundance and diversity of terrestrial rock-hosted life, the environments it inhabits, the evolution of its metabolisms, and its fossil biomarkers to provide guidance in the search for life on Mars. Key findings are (1) much terrestrial deep subsurface metabolic activity relies on abiotic energy-yielding fluxes and in situ abiotic and biotic recycling of metabolic waste products rather than on buried organic products of photosynthesis; (2) subsurface microbial cell concentrations are highest at interfaces with pronounced chemical redox gradients or permeability variations and do not correlate with bulk host rock organic carbon; (3) metabolic pathways for chemolithoautotrophic microorganisms evolved earlier in Earth's history than those of surface-dwelling phototrophic microorganisms; (4) the emergence of the former occurred at a time when Mars was habitable, whereas the emergence of the latter occurred at a time when the martian surface was not continually habitable; (5) the terrestrial rock record has biomarkers of subsurface life at least back hundreds of millions of years and likely to 3.45 Ga with several examples of excellent preservation in rock types that are quite different from those preserving the photosphere-supported biosphere. These findings suggest that rock-hosted life would have been more likely to emerge and be preserved in a martian context. Consequently, we outline a Mars exploration strategy that targets subsurface life and scales spatially, focusing initially on identifying rocks with evidence for groundwater flow and low-temperature mineralization, then identifying redox and permeability interfaces preserved within rock outcrops, and finally focusing on finding minerals associated with redox reactions and associated traces of carbon and diagnostic chemical and isotopic biosignatures. Using this strategy on Earth yields ancient rock-hosted life, preserved in the fossil record and confirmable via a suite of morphologic, organic, mineralogical, and isotopic fingerprints at micrometer scale. We expect an emphasis on rock-hosted life and this scale-dependent strategy to be crucial in the search for life on Mars.
Key Words: Subsurface life, Microbial diversity, Biosignatures, Mars, Search for life
1. Introduction
From the mid-1980s to early 1990s, evidence accumulated from both the continental and marine realms of a vast, well-populated underground biosphere that was on par with the total biomass on Earth's surface (Onstott, 2016). The discovery by Stevens and McKinley (1995) of subsurface lithoautotrophic microbial ecosystems (SLiMEs), fueled by H2 that was generated by reaction of water with Fe-bearing minerals in basaltic aquifers, had an immediate impact on the planetary science community, especially with respect to the search for extant life on Mars (McKay, 2001). Subsequently, subsurface life on Earth has been discovered at depths of 4–5 km in the continental crust (Moser et al., 2005) and 2.5 km in subseafloor sediments (Inagaki et al., 2015), at temperatures from −54°C to 122°C, at pH values ranging from 3 to 13, and in solutions with ionic strengths up to 7 M for continental crust sites (Magnabosco et al., 2018a). Subsurface life is pervasive on Earth, and rock-based microenvironments offer physical and energetic advantages to their inhabitants compared to the oceans and surface photosphere. In this paper, we refer to “rock-hosted” life, whose existence is critically dependent upon physicochemical processes within the host rock, for example, water-mineral, gaseous, or radiolytic reactions.
The most recent estimate of the mass of Earth's subsurface biosphere is ∼1030 cells, which is about 10% that of the surface biosphere (Magnabosco et al., 2018a). One key question when considering the likelihood of finding subsurface life on other planets is how the abundance of Earth's subsurface life may have changed with time, coupled with the evolution and proliferation of surface life, that is, the extensive colonization of land by plant life that began ∼450 million years ago. Some portion of Earth's current global subsurface biosphere is supported directly by or indirectly through thermocatalytic breakdown of organic photosynthate from the surface biosphere while another portion is supported by abiotically produced organic matter or autotrophic carbon fixation. In this paper, we are careful to draw the distinctions between these two types of subsurface ecosystems, focusing on the latter. Over the last decade, it has become apparent that deep subsurface microbial communities are comprised of novel subsurface species with no known closely related surface relatives and that flourish independently of the surface photosphere (Chivian et al., 2008; Osburn et al., 2014; Lau et al., 2016a; Momper et al., 2017), rather than representing the vestiges of transported or buried surface microorganisms struggling to survive on dwindling organic photosynthate (Jannasch et al., 1971). In deep crustal environments, rock-hosted life has been found to comprise entire ecosystems with multiple trophic levels built upon these species (Lau et al., 2016a).
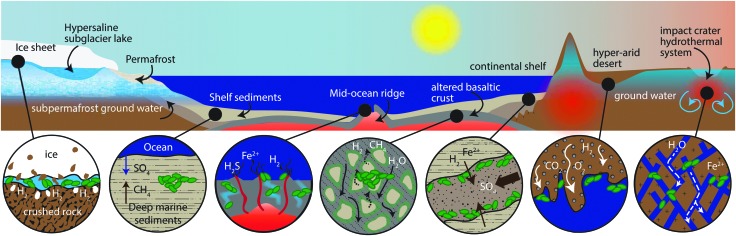
Habitats for rock-hosted life may have been—and may still be—present elsewhere in the Solar System (Fig. 1). The sub-ocean silicate crusts of Europa and Enceladus have been proposed to host low-temperature groundwater/hydrothermal systems, leading to chemical/radiolytic reactions, which could supply energy for life (Schulze-Makuch and Irwin, 2002; Hand et al., 2007; McKay et al., 2012, 2008; Pasek and Greenberg, 2012; Vance et al., 2016; Deamer and Damer, 2017; Steel et al., 2017). On Mars, throughout the first 1.5 billion years of its history, surface waters were intermittently present (Fassett and Head, 2011), whereas a more persistent and volumetrically more extensive aqueous environment existed beneath the surface, hosted in crystalline and sedimentary rocks (Clifford and Parker, 2001; Clifford et al., 2010; Des Marais, 2010; Ehlmann et al., 2011; Cockell, 2014a, 2014b), and subsurface brines may still exist today (Orosei et al., 2018). Because of the relative hostility and instability of the martian surface environment—aridity, subfreezing temperatures, frequent climate change due to obliquity cycles, and radiation—compared to Earth or Mars' clement and stable subsurface, sampling rock units that have or may have hosted groundwater warrants top priority in the search for life on Mars.
FIG. 1.
Subsurface biosphere habitats from left to right: Ice and Ice-Rock Interfaces host chemolithotrophs; Marine or Lake Sediments host primarily heterotrophic communities in a high-porosity environment with diffusive flux fueled by organic photosynthate in some places and chemolithotrophic oxidation in others; Ocean Ridges have advective fluids carrying reductants and oxidants, including dissolved gases from magma and water-rock reactions, and abiotic hydrocarbons are oxidized to carbonate mounds (magmatic, non-ridge systems may provide such fluxes on other planets); Deep Basaltic Crust has H2-fueled chemolithotrophic communities powered by water-rock reactions; Continental Sedimentary Aquifers are of lower porosity than marine sediments/crust and host mixed heterotrophic and chemolithotrophic communities; and Deep Subsurface Continental aquifers in mafic and siliceous igneous and metamorphic rocks, in some cases fractured by impacts or tectonics, host microorganisms fed by products of radiolysis and water-rock reactions.
In this review, we describe a strategy to search for past rock-hosted life on Mars by drawing on the lessons from Earth's record of extant and fossil rock-hosted life. We first describe the environmental history and habitability of Mars. We then review what is currently known about the extent, metabolic diversity, and community structure of present rock-hosted life on Earth, as well as its metabolic products. We next examine the evolutionary history of the enzymes utilized by rock-hosted versus photosynthetic life. We then address how long Earth's rock-hosted life communities, as evident in their biomarkers, have existed and what processes promote preservation of their morphological, mineralogical, isotopic, and chemical traces in the rock record. Finally, we consider the large volumes of rock that constitute past and present habitable environments on Mars and articulate an operational strategy for their exploration for the biosignatures of rock-hosted life.
2. The Case for Targeting the Search for Life on Mars to Rock-Hosted Life
On Earth, extensive plate tectonics–driven crustal recycling has removed much of the earliest geologic record and metamorphosed the rest, obscuring the history of the first billion years and extent of the biosphere. On Mars, the ancient geologic record remains largely in place with >50% of the martian rock record from earlier than 3.5 Ga preserved at the surface (e.g., Tanaka et al., 2014), including ancient units uncovered more recently by tectonics, erosion, and impact cratering. As such, if life evolved on Mars contemporaneously with Earth's life, the rocks and biosignatures recording the trajectory of its early evolution are better preserved and more easily accessible than those of time-equivalent periods on Earth.
Over the last decade, in situ exploration by rovers and high-resolution mineralogy and stratigraphy by orbiting instruments have revealed the nature of environmental conditions during the first 2 billion years. Globally widespread phyllosilicate minerals (smectites, chlorites, and other hydrated silicates) were formed by aqueous alteration of igneous materials in geologic units from the Pre-Noachian (>4.1 Ga) and Noachian (4.1–3.7 Ga) periods (Mustard et al., 2008; Carter et al., 2013; Ehlmann and Edwards, 2014). The mineral assemblages, chemistry, and geologic setting indicate much of this alteration occurred by water flowing underground (Ehlmann et al., 2011), ranging in depth from shallow sedimentary diagenesis, which depending upon location, comprised acidic, neutral, or alkaline pH fluid (Tosca et al., 2005; Bristow et al., 2015; Yen et al., 2017), to deep, hydrothermal/metamorphic fluid forming serpentine or subgreenschist facies mineral phases, including prehnite and zeolites (Ehlmann et al., 2009, 2011; McSween, 2015). Martian valley networks and open- and closed-basin lake deposits, particularly well preserved during the Late Noachian and Early Hesperian epochs (3.8–3.3 Ga) (Fassett and Head, 2008; Goudge et al., 2016), also record surface water environments. Rover exploration of sedimentary rocks from two different martian basins revealed shallow playas that experienced multiple episodes of diagenesis by acidic waters (Grotzinger et al., 2005; McLennan et al., 2005) and a Hesperian deep lake with multiple later episodes of groundwater diagenesis and/or hydrothermal alteration, possibly as late as the early Amazonian (∼3 Ga) (McLennan et al., 2014; Grotzinger et al., 2015; Martin et al., 2017; Yen et al., 2017; Rapin et al., 2018). Orbital data suggest that other sedimentary basins may have been fed by groundwater (Wray et al., 2011; Michalski et al., 2013), sometimes in communication with magmatic volatiles (Thollot et al., 2012; Ehlmann et al., 2016b). Indeed, surface expressions of impact or volcanic thermal spring systems have been located (Skok et al., 2010; Arvidson et al., 2014; Ruff and Farmer, 2016).
However, after the Late Hesperian (∼3 Ga), evidence for liquid water on Mars is sparse. While even young martian meteorites have evidence for aqueous alteration (e.g., Velbel, 2012), large lava bodies emplaced in the Hesperian and Amazonian do not have hydrated minerals in sufficient abundances to be detectable from orbit (Mustard et al., 2005). Outflow channels, lobate debris aprons, and small valleys occur only near volcanic centers or glacial-like features. Collectively, these data indicate that after a warmer and wetter first ∼1.5 billion years, frozen, arid conditions prevailed over the last ∼3 billion years (e.g., Wordsworth, 2016). Notably, even the Noachian climate may always have been relatively cold and arid (similar to the last 3–3.5 billion years throughout all of Mars' history) with punctuated intervals of higher temperatures due to volcanism (e.g., Johnson et al., 2009; Halevy and Head, 2014), large impacts (Segura et al., 2013; Tornabene et al., 2013), or punctuated release of reduced gases from water-rock reactions (Wordsworth et al., 2017).
If martian life emerged, it is possible that it might have looked like the earliest presently recognized terrestrial record of life, for example, ∼3.4 Ga laminated structures in near-shore, marine facies sediments that are believed to represent anoxygenic photosynthesizing microbial mats (Tice and Lowe, 2004; Tice, 2009) or possible benthic microorganisms in carbonate platforms (Allwood et al., 2009). However, importantly, martian surface water habitats have always been more episodic and extreme than age-equivalent surface habitats on Earth. All evidence suggests that Earth has had an ocean in continuous existence from at least 3.8 Ga and perhaps from as early as 4.4 Ga (Valley et al., 2002). In contrast, the preponderance of the martian geological and mineralogical record along with predictions from climate models suggests that no such body of water on Mars was in continuous existence (Carr and Head, 2015; Wordsworth et al., 2015; Pan et al., 2017). Unlike Earth with its stable axial tilt at 23° ± 1°, Mars' axial tilt fluctuates from 10° to 60° with changes of tens of degrees occurring on timescales of hundreds of thousands of years (Laskar et al., 2004). This has driven episodic reorganization of water reservoirs from the poles to midlatitude belts with concomitant changes in climate cyclically throughout Mars' history (Laskar et al., 2002). Occasional flood events from melting of water ice might have caused outflow channels to debouch in the Northern Lowlands of Mars, forming temporary oceans (Tanaka et al., 2003). Certainly, lakes existed for thousands and perhaps millions of years (Fassett and Head, 2008; Grotzinger et al., 2014, 2015). But by the Noachian-Hesperian boundary (∼3.7 Ga), the atmosphere was <2 bar thick and possibly only tens of millibar thick (Kite et al., 2014, 2017; Edwards and Ehlmann, 2015; Hu et al., 2015; Wordsworth et al., 2015, 2017; Bristow et al., 2017). Mars had also lost much of its protection from solar radiation and galactic cosmic rays by the loss of its dynamo-driven magnetic field at 4.1–3.9 Ga (Acuña et al., 1999) and the subsequent loss of its atmosphere (e.g., Ehlmann et al., 2016a).
Thus, certainly by ∼3.0 Ga, and perhaps earlier, Mars' surface environment had evolved to conditions different from and more challenging to life than the time-equivalent habitats on Earth (Westall et al., 2015). Early martian organisms at the surface would have faced at least seasonally subfreezing temperatures, if not nearly continuous subfreezing conditions with intermittent thaws, surface aridity, and surface radiation doses many times higher than present on early Earth. Ionizing radiation was considerably harsher than that on Earth because of the lack of magnetic field and thin atmosphere (Hassler et al., 2014), and the interaction of UV light with Fe and hydrogen peroxide would have produced photo-Fenton chemistry that is lethal to Earth bacteria (Wadsworth and Cockell, 2017). On the other hand, martian subsurface environments with water were widespread and, comparatively, stable. Evidence for groundwater extends to far more recent martian times than that for surface waters and may still be present today (Orosei et al., 2018). An example is the lake in Gale Crater whose sediments are presently being explored by the Curiosity rover. The lake persisted for up to a few million years (Grotzinger et al., 2015), but the sediments bear markers of sedimentary diagenesis long after the lake had vanished. Crosscutting geologic relationships show that at least several tens of meters of lake sediment had to be eroded, overlain by dunes, the dunes lithified to sandstone, and then crosscut by diagenetic sulfate and silica veins in multiple generations of subsurface fluid flow, persisting even into the Amazonian (Frydenvang et al., 2017; Martin et al., 2017; Rampe et al., 2017; Yen et al., 2017). Fracture networks provided a conduit between habitable subsurface aquifers and more transient surficial habitable systems. Elsewhere, fluid circulation through deep fracture networks driven by hydrothermal activity within impact craters also mobilized fluids from the surface to far beneath the cryosphere (Osinski et al., 2013).
Lastly, an important difference between habitable environments on Earth and Mars may be related to differences in communication between the surface and subsurface. Whereas on Earth warm temperatures and abundant liquid water provided a rapid pathway for recolonizing the surface from the subsurface after impacts (Abramov and Mojzsis, 2009) or global glaciation, on Mars subfreezing surface temperatures and a thick, global permafrost layer (i.e., cryosphere) might have limited communication between surface and subsurface habitats, particularly later in Mars' history (Clifford, 1993; Clifford and Parker, 2001; Harrison and Grimm, 2009; Clifford et al., 2010; Grimm et al., 2017). Therefore, if periodic warm conditions did occur at the surface, the pathways for communication with the subsurface may not have been as easily established for (re)colonization of the martian surface during brief Hesperian surface habitable periods.
Consequently, rock-hosted habitats showing evidence of persistent water warrant considerable attention in the search for martian life (Westall et al., 2015). Some of these systems may have been uninhabitable, perhaps challenged by salinity and acidity (Tosca et al., 2008). Nonetheless, the most globally widespread systems and some sites observed from orbit and explored in situ are marked by neutral to alkaline waters of low salinity, which, if on Earth today, would be habitable (Ehlmann et al., 2011; Grotzinger et al., 2015).
Several candidate martian landing sites under consideration for future exploration missions have accessible stratigraphy that may preserve rock-hosted habitats. These include aquifers in volcanic rock and in sedimentary rock. Most immediately, the volcanic rock aquifer with clay minerals, carbonate, and serpentine exposed by erosion at Northeast Syrtis was under consideration for the Mars 2020 rover mission at the time of this submission, and it is accessible in an extended mission from the chosen landing site of Jezero crater. These ancient habitats can and should be explored at high priority as available habitats for martian life, using the lessons and strategies derived from the terrestrial modern and paleorecords of the quantities, nature, and locations of biosignatures of past rock-hosted life.
3. Modern Rock-Hosted Life on Earth
3.1. Geologic settings with rock-hosted life
On Earth, microbial communities in a wide variety of rock-hosted environments occur globally. Their abundance and community structure reflect physicochemical properties of the rock/water host, the type and rates of energy and nutrient fluxes, geobiological feedbacks, and the geological history of the rock. Though most rock-hosted life is comprised of Archaea and Bacteria, active eukaryotic members exist. These range from protists in deep aquifers (Sinclair and Ghiorse, 1989) to fungi in subseafloor sediment (Orsi et al., 2013; Pachiadaki et al., 2016) and 793 m deep fracture waters in granite (Sohlberg et al., 2015) to multicellular bacteriophagous nematodes (Borgonie et al., 2011). What follows is a brief survey of the variety of rock-hosted ecosystems documented on Earth, some of which could represent terrestrial analogs to potential martian rock-hosted ecosystems (Fig. 1).
The shallowest examples of a rock-hosted ecosystem are highly concentrated cryptoendolithic communities existing millimeters beneath the rock surface, which are not truly “rock-hosted life” as we define the term here (see the introduction). The primary producers of these communities, cyanobacteria and algae, are surface-dwelling photosynthesizing organisms that have retreated to the near subsurface to reduce their exposure to moisture and temperature extremes while retaining access to a sustainable photon flux (Friedmann, 1982; Wong et al., 2010). Some shallow subsurface ecosystems do use their rock/soil hosts for metabolism, meeting our definition of rock-hosted life (Fig. 1). Chemoautotrophic aerobic and anaerobic microorganisms that fix atmospheric CO2 reside in barren polar soils and metabolize atmospheric trace gases such as H2, CO (Ji et al., 2017), and CH4 (Lau et al., 2015; Edwards et al., 2017). Though there is a significant energetic potential for such metabolisms in martian regolith, there is no detectable presence of this metabolism yet (Weiss et al., 2000; Yung et al., 2018).
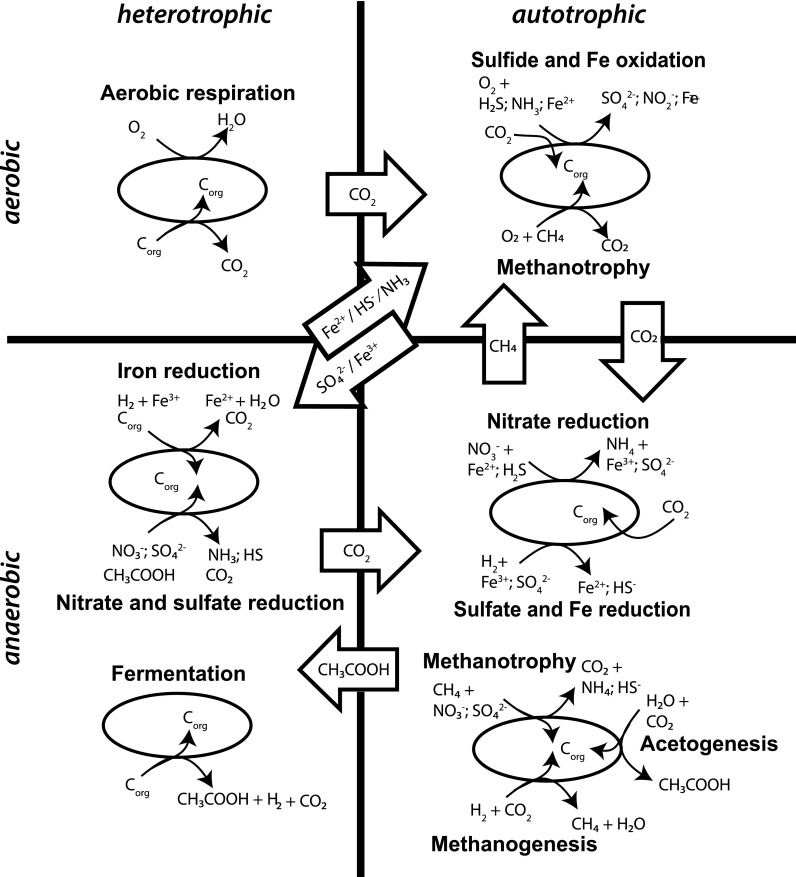
For the majority of the continental surface on Earth, heterotrophic bacteria involved in the degradation of organic photosynthate (e.g., cellulose) dominate soil communities (Federle et al., 1986), lacustrine sediments, and shallow aquifers (Balkwill and Ghiorse, 1985). Similarly, organic detritus input from the sea surface, water-column, or continental photosynthates dominate the reductant input to shallow continental margin, subseafloor sediments. Sediment pore waters host a wide variety of specialized microbes that generally use one of a succession of oxidants present in the system (in order of free energy release, O2 > Mn oxides > nitrate > Fe oxides > sulfate [Froelich et al., 1979]) to degrade organic matter. The carbon isotope composition of photosynthetic organic matter is distinctive: both terrestrial and marine derived material have a more negative δ13C value than marine inorganic carbon. Minerals precipitated in sediment pore space by these microbial metabolic processes have isotopic and chemical compositions that reflect the original organic matter and the oxidant used (e.g., reduced manganese carbonate, rhodochrosite) (Coleman et al., 1982). In organic-rich sediments, degradation processes continue with depth in the pore waters with burial until the organic oxidants are exhausted. In the lowermost oxidation zone, where the least exergonic electron acceptor, CO2, remains, methanogenic archaea and acetogenic bacteria largely rely upon abiotic or biotic subsurface H2 (Fig. 2). Positive δ13C values in carbonates reflect Rayleigh fractionated depletion of CO2 by chemolithoautotrophs.
FIG. 2.
Cartoon of different microbial metabolic processes separated into Aerobic (top), Anaerobic (bottom), Heterotrophic (left), and Autotrophic (right) bins.
The CH4 from methanogenesis may diffuse upward and itself be oxidized anaerobically by archaea, bacteria, and archaeal-bacterial consortia using sulfate or other oxidants in shallow subseafloor sediment (Orphan et al., 2001; Ettwig et al., 2010; Milucka et al., 2012; Haroon et al., 2013; Kits et al., 2015; Cai et al., 2018). Similarly, the acetate from acetogens will be oxidized anaerobically by heterotrophic bacteria (Stevens and McKinley, 1995). At greater depths and temperatures exceeding 100°C, any residual organic photosynthate is thermally matured and may be transformed to oil and/or gas or coal while the host strata become sterilized of indigenous microorganisms, a process described as “paleopasteurization” (Wilhelms et al., 2001). Thus, the microbial communities associated with oil-, gas-, or coal-endowed deposits either represent immigrants arriving with groundwater flow over geological time as the deposits cooled below their maximum temperatures (Tseng et al., 1998), residents indigenous to the sandstone reservoir when oil or gas migrated upward to be trapped (Wilhelms et al., 2001), or contaminants introduced during flooding of or production from the oil reservoirs (Dahle et al., 2008). Although these sediment-hosted microorganisms are living completely or nearly completely off the detritus of photosynthetate, organic matter from abiotic chemosynthetic or biotic chemolithoautotrophic sources would be processed in a similar fashion. The significance of these organic degradation processes is that the inorganic metabolic products may produce characteristic mineral phases, and isotopic and chemical signatures. These can be durable biosignatures that survive for very long periods of time (see Section 4, “Biosignatures of past rock-hosted life”).
In contrast to the environments above where organic photosynthate is important, in continental environments where the water table is hundreds of meters deep (Fig. 1), subsurface communities rely upon chemolithotrophs living off atmospheric or vadose zone gases (Tebo et al., 2015; Jones et al., 2016; Webster et al., 2016), redox reactions with reduced minerals (Mansor et al., 2018), and metals present in carbonate (Barton and Northup, 2007). The chemolithotrophs serve as primary producers for complex communities (Dattagupta et al., 2009; Fraser et al., 2017) and as ecosystem engineers that can excavate large caverns by dissolution of carbonate and deposition of sulfate by the sulfuric acid they produce (Mansor et al., 2018). In water-saturated environments where oxygenated water penetrates deeply into crustal rock such as mountainous terrains of North America (Sahl et al., 2008; Murdoch et al., 2012; Osburn et al., 2014), basaltic flows of the geothermal environments of Iceland (Trias et al., 2017), or taliks in 500 m thick permafrost into underlying Archean metamorphic rock (Onstott et al., 2009), heterogeneous redox conditions create highly exergonic conditions for S, Fe, N, and Mn oxidation; and subsurface microbial communities are dominated by chemolithotrophic primary producers. Where oxygenated seawater comes into contact with marine basaltic crust, chemolithoautotrophs are the primary producers fixing CO2 to support substantial biomass by mediating electron transfer at mineralogical redox interfaces from reduced forms of Fe, S, and Mn to the aerobic fluids in pore spaces (Edwards et al., 2012). Unlike the organic-rich sediments discussed above, the geochemical evidence suggests that these rock-hosted communities do not rely upon the groundwater transport of organic photosynthate (Kieft et al., 2018) (Fig. 2).
Of the bioavailable electron donors being utilized by deep, water-saturated, rock-hosted communities, H2 is probably a key fuel in the deep biosphere (Nealson et al., 2005), and several abiotic modes of formation exist within rock-hosted environments. In anaerobic volcanic aquifers (Fig. 1), basalt interacts with anaerobic groundwater releasing H2, which then supports chemolithotrophic microbial communities by the oxidation of H2 at depths of hundreds of meters (Stevens and McKinley, 1995; Mayhew et al., 2013). Even in the absence of O2, anaerobic Fe-oxidation via nitrate reduction is a metabolic process that recycles Fe2+ produced by Fe3+ reduction that can lead to a subsurface Fe-cycle, depending upon the availability of nitrate (Fig. 2; Melton et al., 2014). Given the identification of 70–1100 ppm of nitrate at Gale Crater (Stern et al., 2015) and the Fe-rich nature of the martian crust, the presence of such a metabolic network has been proposed for the martian subsurface (Price et al., 2018).
Radiolysis of groundwater also generates H2, H2O2, and O2, that has been shown to sustain subsurface chemolithoautotrophic primary producers by providing not only H2 as an electron donor but also electron acceptors, such as sulfate via oxidation of sulfides by radiolytically produced H2O2 (Lefticariu et al., 2006; Lin et al., 2006; Li et al., 2016). Metagenomic analyses combined with metaproteomic and metatranscriptomic analyses have revealed that within these radiolytically supported communities, a dynamic and temporally varying multi-tier energy pyramid of chemolithoautotrophs exists that recycles biogenic CH4 and sulfide and possibly nitrogen, while fixing CO2 using the Wood-Ljungdahl pathway and Calvin-Benson-Bassham cycle (Magnabosco et al., 2015, 2018c; Lau et al., 2016b). The bacterial biomass supports multicellular bacteriophagous nematodes at the top of the food chain (Borgonie et al., 2011). These results indicate that radiolysis combined with commensurate syntrophic interactions constantly recharges the redox couplings in these environments; they are not chemically stagnant as claimed by McMahon et al. (2018). The radiolytic H2 production rate on Mars is just as great as that found in the crustal rocks of Earth despite the lower concentrations of radiogenic isotopes, primarily because of the higher porosity at a given depth due to the lower gravity on Mars (Onstott et al., 2006; Dzaugis et al., 2018; Tarnas et al., 2018).
Cataclastic diminution of silicate minerals in the presence of water also generates H2 (Kita et al., 1982) and in the presence of CO2 generates CO and O3 (Baragiola et al., 2011). H2 release during seismic events has been recorded at 3 km depths in South Africa (Lippmann-Pipke et al., 2011), and H2 release during rock-crushing at the base on the 3 km thick Greenland ice sheet has been inferred (Telling et al., 2015). The relationship between rock fracturing and/or crushing and subsurface microbial community abundance and activity is not yet resolved and is an avenue of current research in subsurface microbiology. Nonetheless, its implications for subsurface life on Mars, which has fracturing due to impacts and tectonics, have already been proposed (McMahon et al., 2016).
At still greater depths and at temperatures >200°C in peridotite, serpentinization produces abundant H2 in high pH fluids (McCollom and Bach, 2009). This H2 as well as that generated by radiolysis in turn reacts with transition metal sulfide catalysts to produce CH4 and low-molecular-weight hydrocarbons via Fischer-Tropsch-type synthesis (Sherwood Lollar et al., 2002, 2006; McCollom, 2016). The resulting hydrocarbons can either diffuse upward to support chemolithotrophs, methanotrophs, and heterotrophic, alkane-degrading anaerobic bacteria at shallower, cooler temperatures or remain trapped until the host rock has cooled down, whereupon these microbial metabolic clades can penetrate the serpentinite during groundwater flow and utilize the hydrocarbons (Purkamo et al., 2015). These processes support the subsurface microbial communities found in continental ophiolite complexes, such as the Samail Ophiolite (Rempfert et al., 2017) and in metamorphosed komatiites of Archean greenstone belts (Sherwood Lollar et al., 2005). They have also been hypothesized to support a martian subsurface biosphere (Schulte et al., 2006; Westall et al., 2013).
These represent a few examples of the types of rock-hosted microbial communities that are globally distributed across Earth at depths ranging from millimeters to kilometers in a wide range of rock types and that are metabolically and phylogenetically diverse (Mykytczuk et al., 2013). In general, more oxic conditions nearer to the surface yield to more reduced conditions with increasing depth but with important exceptions. Despite the great abundance of organic carbon derived from the surface photosphere in marine sediments and shallow soils, chemolithotrophy is widespread and even dominant in many subsurface environments. This may explain the absence of any correlation of deep subsurface prokaryotic biomass with organic carbon content in the continental subsurface below the soil zone (Magnabosco et al., 2018a).
3.2. Fundamental physical and environmental controls on rock-hosted life
The thermal state of the crust constrains the habitable zone. The currently recognized temperature limits for metabolic activity range from −20°C for microorganisms trapped in Siberian permafrost (Rivkina et al., 2000) and −25°C for an aerobic, halophilic heterotroph in laboratory microcosm experiments utilizing 14C-labeled acetate (Mykytczuk et al., 2013) up to 122°C for a methanogen isolated from a deep sea vent plume (Takai et al., 2008). Based upon temperature alone, the habitable volume for Earth's continental and oceanic crust has been estimated to be ∼2 × 1018 m3 (Heberling et al., 2010; Magnabosco et al., 2018a), using global heat flow and surface temperature maps and thermal conductivity estimates. In the case of the continental crust, the average depth to the 122°C isotherm is 4 km; a maximum depth of 16–23 km occurs in the Siberian Craton where mean annual temperatures and heat flow are both lower than average (Magnabosco et al., 2018a). Similar types of calculations for Noachian Mars indicate an average depth to the 122°C isotherm would have been 6–8 km, and the corresponding habitable volume based on temperature constraints alone would also be ∼1018 m3 (Michalski et al., 2017).
Temperature and possibly the ionic strength of the crustal fluids play a role in constraining the abundance and activity of subsurface life since cell concentrations appear to be inversely correlated with both parameters (Magnabosco et al., 2018a). Organic markers of biodegradation of petroleum suggest that the maximum temperature of the subsurface biosphere may typically be closer to 80–85°C and that salinity >50 g L−1 may inhibit low-energy metabolisms such as methanogenesis to even lower temperatures (Head et al., 2014). High concentrations (>220 g L−1) of chaotropic salts, such as MgCl2, may even preclude life (Hallsworth et al., 2007).
The fluid-bearing (saturated or thin film) porosity that is accessible, that is, with pore throats that are greater than 0.1 μm in diameter, also controls the habitable volume. On Earth, rock strata from 3 to 5 km depth may have a matrix porosity of 0.5% to 1%, but their habitable volume could be as little as 0.05% to 0.002% (Supplementary Fig. S1; Supplementary Information available online at www.liebertonline.com/ast) due to compaction and cementation. On Mars the porosity is likely to be 10% at comparable depths due to the lower gravitational force, and as a result the subsurface habitable volume on Mars may be greater than that of Earth's.
Porosity and permeability also constrain the flux of nutrients and the degree of metabolic activity. For terrestrial life, a finite minimum quantum of energy determined by the reaction ADP + P → ATP must be available through catabolic redox or substrate-level reactions to be metabolically useful (Müller and Hess, 2017). To sustain life, the Gibbs free energy flux (energy per unit time per cell) (Hoehler, 2004; Onstott, 2004) must be equal to or exceed that required for a cell's (Hoehler and Jørgensen, 2013; Onstott et al., 2013) or a syntrophic community's (Scholten and Conrad, 2000) maintenance. Temperature is a principal control on the maintenance energy demand in part because the diffusivity of H+ through the cell membranes increases with temperature (van de Vossenberg et al., 1995). As a result, cell metabolic rates must increase with temperature to counteract these effects. The higher the temperature, the higher the nutrient flux needed to maintain a given subsurface biomass. The same may also hold true for salinity as microorganisms need to manufacture internal osmolytes to maintain osmotic pressure and osmolytic production exerts an additional energy requirement (Oren, 1999).
To the extent that higher rock permeability increases groundwater velocities that increase the rate at which reactants flow toward microorganisms hosted by rock strata, higher permeability can lead to a more prolific subsurface biomass by maintaining a nonzero Gibbs free energy (Marlow et al., 2014a). Three other abiotic processes that operate to enhance the local flux of nutrients are chemical gradients or boundaries, physical heterogeneity, and local abiotic and biotic recycling. First, the presence of high electron donor/acceptor chemical spatial gradients in rock units enhances local diffusive fluxes, which leads to higher microbial activity and biomass than in homogeneous units. On modern Earth the most dramatic examples are typically found at the contacts between organic-rich shale and sulfate-bearing sandstone (Krumholz et al., 1997) or oil and water (Bennett et al., 2013) producing higher rates of microbial metabolism than observed some distance away from these contacts. Other examples include fluid-rock redox interfaces at small scales along fractures, detailed below. On Mars an example might be the boundary between a serpentinized olivine-rich unit and an overlying sulfate-rich unit at Northeast Syrtis (Marlow et al., 2014a). Serpentinization of the olivine would generate H2 that would then diffuse into the overlying aquifer where it could be microbially oxidized using sulfate, leading to a zone with potential to support high biomass at the boundary. Second, physical heterogeneity can also act to create favorable zones, as is observed in the high cell concentrations within highly fractured and brecciated rock of the Chesapeake Bay impact structure, compared to the overlying marine sediments (Cockell et al., 2012). In this case metabolically active microorganisms are constrained to the fractured rock where they draw down the energy substrates and increase the product concentrations. Though the surrounding massive rock has pore spaces too small for microbes, the diffusive flux of energy substrates from the massive rock into the fractured zone and the diffusion of the products from the fractured zone into the massive rock enhance the biomass residing in the fracture rock (see Section 3.3, Supplementary Fig. S1). Third, abiotic recycling reactions such as radiolysis can continuously generate H2 from water and recycle metabolic waste products, such as HS- back into sulfate, and sustain subsurface microbial communities without the need for fluid transport (Lin et al., 2006). “Cryptic sulfur cycling,” in which iron oxides abiotically oxidize sulfide to more oxidized sulfur species, can support organic carbon degradation in non-stoichiometric proportions with a relatively limited sulfur supply (Holmkvist et al., 2011). Finally, syntrophic interactions between different microorganisms also act to sustain subsurface communities by converting waste products back into reactants locally without the need for advective transport (Lau et al., 2016b). Thus, obligately mutualistic metabolism (Morris et al., 2013) may be a characteristic aspect of subsurface microbial communities as a means of avoiding extinctions (Gaidos et al., 1999) because Gibbs free energy will remain nonzero.
3.3. Subsurface biomass distribution
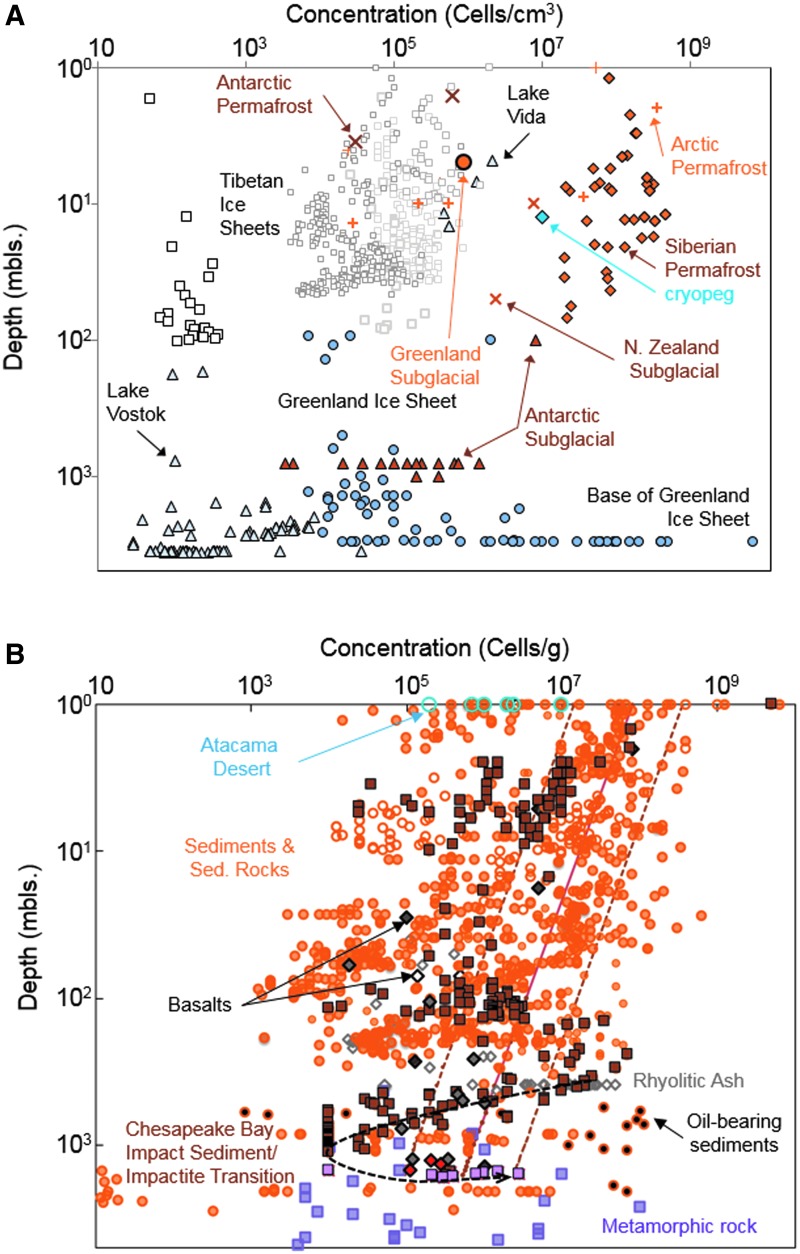
Magnabosco et al. (2018a) recently estimated the total living subsurface prokaryote biomass for Earth was 7–11 × 1029 cells of which 2–6 × 1029 cells occur in the continental subsurface, 2 × 1029 cells exist the oceanic crust, and 3 × 1029 cells reside in the subseafloor sediments. Permafrost-affected crust and continental ice sheets cover a large fraction of the continental area and are often considered terrestrial analogs to early Mars. In these, as a function of depth, biomass generally declines; but at all depths, biomass varies by orders of magnitude, depending on the sampling location, including substantial intra-site variation (Fig. 3A). The total cell concentrations for Siberian permafrost sediments can be quite high, ranging from 107 to 109 cells g−1 (brown-filled diamonds in Fig. 3A) and diminish with depth up to 100 m and with increasing permafrost age up to 2 Ma (Gilichinsky and Rivkina, 2011). The cell concentrations within the Greenland ice sheet (light blue-filled circles in Fig. 3A) are much lower, on the order of 105 cells cm−3, except at the very bottom where the ice sheet is in contact with the Precambrian bedrock and cell concentrations reach 109 cells cm−3 as a result of H2 generation at the rock-ice interface (Fig. 1). Antarctic permafrost (brown crosses) and subglacial sediments (brown-filled triangles) exhibit cell concentrations that are also greater than those of the adjacent and overlying ice sheets (open triangles). In general, cell concentrations in ice sheets (open squares, triangles, and circles) do not diminish as a function of depth and age as rapidly as observed for permafrost sediments and likely reflect a combination of airborne input flux and in situ metabolism (Chen et al., 2016). Cell concentrations are higher near rock-ice interfaces and in dust-rich ice, pointing to the importance of chemical and physical gradients.
FIG. 3.
(A) Cell concentrations versus depth for ice sheets, subglacial sediments, and permafrost. Open squares = Tibetan glacial ice sheets; brown-filled diamonds = Siberian permafrost; blue-filled diamonds = Siberian cryopeg; light gray-filled triangles = Antarctica ice sheets and lakes; brown-filled triangles = Antarctic subglacial sediments; brown crosses = Antarctic permafrost and subglacial sediment in New Zealand; orange crosses = Canadian High Arctic and Svalbard permafrost; light blue–filled circles = Greenland ice sheet; orange-filled circle = Greenland subglacial sediment. (B) Cell concentrations versus depth for rock and soil cores from nonpolar regions. Orange-filled circle = water-saturated sediments or sedimentary rock; orange open circle = vadose zone sediments or sedimentary rock; brown squares = Chesapeake Bay Impact sediments; pink squares = Chesapeake Bay Impact impactite; black-filled orange circle = oil-gas-coal-bearing sediment or sedimentary rock; gray-filled gray diamond = water saturated rhyolitic ash; open gray diamond = deep vadose zone rhyolitic ash; open black diamond = vadose zone basaltic rock; gray-filled black diamond = water-saturated basaltic rock, which includes recent Deccan Trap data from Dutta et al. (2018); red-filled diamond = Deccan Trap granite data from Dutta et al. (2018); purple square = metamorphic rock. Rest of data are from Magnabosco et al. (2018a). Blue open circles = Atacama desert soil from Connon et al. (2007) and Lester et al. (2007). Solid and dashed lines represent the best-fit power law for subseafloor sediments proposed by Parkes et al. (2014).
Unlike the ice cores, the cell concentrations from rock and sediment cores decline with increasing depth following a power law fit (Fig. 3B). Notable exceptions are the cell concentrations reported for the Chesapeake Bay Impact that increase at a depth of 1.5 km where the highly fractured basement rock exists (brown-filled squares to pink-filled squares in Fig. 3B; Cockell et al., 2012). In soil zones the concentrations range from 109 cells g−1 down to as low as 105–6 cells g−1 in the case of the Atacama Desert (blue open circles in Fig. 3B; Connon et al., 2007; Lester et al., 2007), considered by some to be a terrestrial analog site for Mars because of its aridity and low organic content. Below 10 m depth, however, cell concentrations do not correlate with water saturation of the pore space (orange open circles versus orange-filled circles in Fig. 3B). For example, the cell concentrations within unsaturated volcanic ash deposits at a depth of 400 m in central Nevada (deep vadose zone) range from 5 × 104 to 5 × 107 cells g−1 (gray open diamonds in Fig. 3B; Haldeman and Amy, 1993; Haldeman et al., 1993), which is not significantly different from the cell concentrations reported for water-saturated post-impact sediments of the Chesapeake Bay Impact (brown-filled squares in Fig. 3B; Breuker et al., 2011; Cockell et al., 2012) or Atlantic Coastal Plain Sediments (orange-filled circles in Fig. 3B; Magnabosco et al., 2018a) or Deccan Trap basalts of similar depth (gray-filled diamonds in Fig. 3B; Dutta et al., 2018).
The cell concentrations also do not correlate with the rock type, that is, sedimentary versus igneous versus metamorphic, with the possible exception of salt deposits where cell concentrations are low, ranging from 0.02 to 104 cells g−1 (Schubert et al., 2009a, 2009b, 2010; Wang et al., 2016). Despite their paucity within salt, microorganisms exhibit remarkable preservation with viable cells being isolated from salt deposited tens of thousands to hundreds of millions of years in the past (Jaakkola et al., 2016), though the older claims remain controversial (Hebsgaard et al., 2005; Lowenstein et al., 2005).
Cell concentrations on fracture or cavity surfaces are often considerably higher than those of the surrounding fluid or matrix especially if the interface acts to focus redox fluxes. For example, in deep vadose zones cell concentrations up to 107 to 108 cells cm−2 and prolific and pigmented biofilms exist on the surfaces of caverns in quartzite (Barton et al., 2014), basalt (Riquelme et al., 2015), and carbonate (Jones et al., 2016). Because of the difficulty of aseptically sampling water-saturated fracture surfaces at depth, only two studies of the cell concentrations on deep fractures have been published. Analysis of modern biofilms, occurring on fracture surfaces in 2.7 Ga metavolcanic rocks at a depth of 2.8 km, revealed 105 cells cm−2 with cells occurring in clumps of 2 to >20 (Wanger et al., 2006). Given the fracture width, such a concentration corresponds to a 100× enhancement of the living cell concentration relative to the fracture water. Much lower living cell concentrations, 40 to 2 × 103 cells cm−2, have been reported for 186 m deep groundwater-fed fractures in granite (Jägevall et al., 2011). Although these two studies would suggest that deep fracture surfaces do not harbor high biomass concentrations, examination of buried Cretaceous hydrothermal veins reveals preserved organic remains of microbial colonies in mineral surfaces, which by mass would be equivalent to ∼109 cells cm−2 (Klein et al., 2015). Similarly, observations of fossil microbial cells in veins in granite indicate a fossil biomass equivalent to 3 × 107 cells cm−2 (Pedersen et al., 1997). The higher cell concentrations in fossil biomass versus living biomass result from accumulation of necromass in biofilms on fluid-filled fracture surfaces over time, similar to what is observed in shallow subseafloor sediments (Lomstein et al., 2012).
McMahon et al. (2018) stated “the bulk of Earth's massive deep biosphere, and presumably also its fossil record, is a poor analog for any ancient or modern Martian equivalent which, in the absence of a productive surface biosphere, would be much smaller and dominated by chemoautotrophs, not heterotrophs.” This statement is not borne out by existing data. As described in Section 3.1, the cell concentrations in continental rock and groundwater do not exhibit any correlation with dissolved or particulate organic carbon concentrations and are dominantly inhabited by chemolithoautotrophs. This finding contrasts with the observations of shallow subseafloor sediments where cell concentrations do correlate with the organic photosynthate content (Lipp et al., 2008). The deep subsurface environments associated with Phanerozoic-age oil (Head et al., 2014) and coal (Kirk et al., 2015) deposits, where heterotrophic metabolisms would perhaps dominate, comprise only 1012 m3, or 0.0001%, of the total habitable volume of Earth's subsurface biosphere. The overlap in cell concentrations of the continental rocks with those of deep subseafloor sediments suggests that access to organic photosynthate has little impact on deep subsurface biomass (Fig. 3). The cell abundance data and observed metabolisms do not support the claim that most of Earth's deep biosphere is sustained by heterotrophic metabolism of surface-derived photosynthate. Rather, fracture surface concentrations of 105 to 109 cells cm2 are observed in Earth's chemolithoautotrophic communities in settings isolated from organic photosynthate and fueled by chemolithoautotrophy, which is an appropriate analog to Mars. (See Section 4 for a discussion of Earth's fossil record.)
Using simple assumptions from the chemical energy available from basalt weathering (10−13 kJ/g-yr), Jakosky and Shock (1998) estimated that over 4 billion years Mars could accumulate 10 g cm−2 of biomass from a 100 m thick basalt layer. For comparison, Earth's continental crust is estimated to contain 0.006–0.02 g of extant life cm−2, integrated from 1 m depth to the 122°C isotherm (Magnabosco et al., 2018a). The addition of fluid flow from an oxic surface to reducing subsurface, as where sulfate rocks are in contact with serpentinizing rocks, increases this estimate by an additional 10 g of biomass cm−2 by enhancing the delivery of reactants and removal products (Marlow et al., 2014a). Calculations of the energy flux from subsurface radiolytic reactions for Mars reveal an energy source comparable to that found on Earth, indicating that the subsurface biomass abundance should be comparable to that of Earth's (Onstott et al., 2006; Dzaugis et al., 2018), and allay the concerns raised about limited oxidant supply to the martian subsurface from the surface (Fisk and Giovannoni, 1999). Radiolysis releases energy into the rock at a rate of 10−9 kJ/g-yr based upon the parameters utilized by Onstott et al. (2006) of which some fraction is accessible for biomass production depending upon the porosity. This rate is greater than that estimated for weathering reactions and would be even higher on Mars during the Noachian when the radioactive parent isotopes were more abundant. These calculations suggest that adequate energy exists on Mars to support substantial biomass equivalent to that of the rock-hosted biosphere on Earth and that an inhabited Mars would accumulate organic matter over time in subsurface aquifers, completely independent of a habitable martian surface.
3.4. Biodiversity and geography
As is the case for biomass, the species richness of deep subsurface environments is highly variable. Subsurface organisms span the branches of the 16S rRNA phylogenetic tree, including deeply rooted lineages (e.g., Methanomada, Archaeoglobi, Korarchaeota, Thermotogae, and Synergistetes) (Magnabosco et al., 2018a). Reports of deep subsurface planktonic communities dominated by a single archaeal (Chapelle et al., 2002) or bacterial (Chivian et al., 2008) species are rare. More commonly, diversity estimates range from over a hundred (Marteinsson et al., 2013) to almost 100,000 (Bomberg et al., 2016) operational taxonomic units or OTUs (at 97% identity in the 16S rRNA gene) within a single fluid sample. To some extent this reflects the improvement in sequencing technology and the fact that the highly variable subregions of the 16S rRNA gene are targeted. It is not unusual to find a large number of OTUs that comprise <1% of the total population (Castelle et al., 2013; Magnabosco et al., 2014), referred to as the rare biosphere (Sogin et al., 2006). Additionally, single-cell genome sequencing of subsurface Candidatus Desulforudis audaxviator indicates significant differences in the genomes of single species (Labonté et al., 2015).
Species abundance does not correlate with its ability to influence the overall function of a subsurface community. For example, in continental subsurface environments, methanogens frequently comprise only 2% of the total community, but the primary gas phase is biogenic CH4. In the case of one deep subsurface site in South Africa, this biogenic CH4 was the principal carbon source for the remainder of the community (Simkus et al., 2015; Lau et al., 2016b). Electron microscopy (Kyle et al., 2008; Middelboe et al., 2011; Engelhardt et al., 2014), single-cell genomes (Labonté et al., 2015), and metatranscriptomic analyses (Lau et al., 2016b) have also revealed that viruses are abundant and actively infecting bacteria and altering their genomes (Paul et al., 2015) in subseafloor sediments and fractured rock aquifers. Because active viral populations can transfer genes between microbial species and control the population density, they may be a vital component of obligately mutualistic metabolic SLiMEs.
One might expect that subsurface diversity would resemble island-like behavior because of the lesser connectedness between subsurface habitats when compared to surface habitats where wind and surface water are transport agents. In island-like ecosystems, the number of species should increase with the size of the island (i.e., volume of groundwater sampled) (Locey and Lennon, 2015). However, Magnabosco et al. (2018a) did not find any such correlation. Species richness may instead correlate with greater heterogeneity of microenvironments, which are difficult to characterize in the subsurface. The lack of species richness versus habitat size could also reflect a surprisingly high degree of connectedness between habitats and motility. This is consistent with the presence of the same rare biosphere OTUs in fractures ranging from 0.6 to 3.0 km depth and separated by hundreds of kilometers in South Africa and a general lack of a distance-decay relationship (Magnabosco et al., 2018a). The presence of the same OTUs in fracture water of different isotopic compositions also suggests that these species are actively motile (Magnabosco et al., 2014). The implication of these observations for an early martian subsurface biosphere is that impacts and volcanic activity could have produced zones of sterilized rock, but these zones would have quickly become recolonized by groundwater circulation.
3.5. Subsurface metabolic activity
Estimates of the in situ metabolic rates of subsurface ecosystems offer insight into their longevity and biomass turnover and provide a better sense of how they impact biogeochemical cycles on a planetary scale, though obtaining accurate estimates has proven challenging (Orcutt et al., 2013). Geochemical estimates of the electron production rate from marine subsurface in situ microbial activity range from ∼5 mol e- L−1 yr−1 at seafloor hydrothermal vents (Wankel et al., 2011) to ∼2 to 100 pmol e- L−1 yr−1 in oligotrophic subseafloor red clays (Røy et al., 2012) to 0.004 to ∼4 pmol e- L−1 yr−1 for continental deep fractured rocks and consolidated sediments (Kieft and Phelps, 1997), estimates spanning more than 15 orders of magnitude. Recent metabolism-agnostic approaches utilize isotopic labeling and measurements of the D/L of aspartic acid of bulk subseafloor sediment (Lomstein et al., 2012) and of cells separated from deep continental fracture fluids (Onstott et al., 2013) to determine the bulk rate of growth and repair, often referred to as cell turnover. These analyses yielded cell turnover times ranging from 73,000 years for subseafloor sediments to 1.7–1.8 years for 60°C fracture water and imply that in situ metabolic rates are strongly temperature dependent (Xie et al., 2012; Onstott et al., 2013; Trembath-Reichert et al., 2017). In the case of the 60°C fracture water dominated by a single species of autotrophic sulfate-reducing bacterium, the cell turnover time corresponded to a metabolic rate of 2.6–2.8 nM of sulfate per year or 21–22 nmol e- L−1 yr−1 (Onstott et al., 2013). The shorter cell turnover times observed in deep continental fracture water are also consistent with metatranscriptome and metaproteome observations from similar environments that revealed significant intracommunity recycling of metabolic waste products (Lau et al., 2016b). Recycling of biogenic CH4, sulfide, and CO2 suggests that initial geochemical approaches for estimating metabolic rates (Phelps et al., 1994) may have underestimated the rates of cell turnover and thus metabolic rates. Despite the challenges posed in accurately estimating the metabolic rates of subsurface microbial ecosystems, recent in situ approaches utilizing natural 14C (Simkus et al., 2015) suggest that a wide range can be found that correlates with the energy fluxes and temperatures. The longer turnover times in colder ecosystems raise questions about the nature and rate of evolution in subsurface ecosystems, which are also characterized by physicochemical conditions that are more stable over time than surface ecosystems and where microorganisms are exposed to low radiation dosage rates relative to those of surface ecosystems (Teodoro et al., 2018).
3.6. Evolution of chemoautotrophic versus photosynthetic pathways
The depiction of subsurface microbial environments above as dominantly suboxic conditions near the surface yielding to more reduced conditions with increasing depth is the result of the evolutionary emergence of oxygenic photosystem II in cyanobacteria. Prior to this emergence, both surface and subsurface habitats were likely dominated by anaerobic metabolisms such as methanogenesis. Recently retrieved genomes of methanogens belonging to Crenarchaeota have revised our understanding of when methanogenic metabolisms evolved. The discovery of Verstraetearchaeota (Vanwonterghem et al., 2016) and Bathyarchaeota (Evans et al., 2015) indicates that the methanogenic metabolic pathway must have arisen after the split between Archaea and Bacteria from the Last Universal Common Ancestor, LUCA, but prior to the split between the Crenarchaeota and Euryarchaeota. This places the time for the emergence of this pathway in the Paleoarchean or Hadean. The youngest bound on age for the development of the methanogenic pathway is ∼3.25 Ga, that is, prior to or during the proposed Archean expansion of genes, based upon molecular clock analyses (David and Alm, 2011), and likely developed in the stem of Archaea (Betts et al., 2018).
Recently sequenced genomes from non-photosynthetic members of the Cyanobacteria phylum indicate that oxygenic photosynthesis arose within Cyanobacteria after the split of photosynthetic Cyanobacteria (now the class of Oxyphotobacteria) from the other non-photosynthetic Cyanobacterial lineages, Melainabacteria and Sericytochromatia (Soo et al., 2017). The age for this divergence has been estimated to be 2.6 to 2.5 Ga based upon molecular clocks (Shih et al., 2017), which lies between the ∼2.8 Ga age for the stem of the Cyanobacteria and the 2.2 Ga age for oxygenic photosynthesis emergence estimated from a different molecular clock approach (Magnabosco et al., 2018b). This is consistent with the theory that photosystem II evolved from a Mn-carbonate-oxidizing enzyme within a suboxic, neutral pH paleoocean (Johnson et al., 2013) to a HCO3−-oxidizing oxygenic photosystem and then to H2O-oxygenic photosynthesis (Dismukes et al., 2000) just prior to the rise of O2 during the Great Oxidation Event at ca. 2.3 Ga (Betts et al., 2018). During this transition, surface anaerobic ecosystems would have either started going extinct or would have adapted to higher O2 levels, perhaps incorporating aerobic metabolic pathways, whereas deep subsurface ecosystems would have remained relatively unaffected.
The molecular clock constraints on the non-oxygenic phototrophic-bearing phyla, Chloroflexi (green nonsulfur bacteria) and Chlorobi (green sulfur bacteria) suggest that the origin of their stems dates from no earlier than ∼3 Ga with Fe2+-oxidizing green sulfur bacteria likely being the most ancient (Magnabosco et al., 2018b). The inferred Fe2+ phototrophic mat structures in the 3.45 Ga Buck Reef Chert (Tice and Lowe, 2004) predate this stem age for all phototrophic lineages. The remaining phototrophic bacteria within the phyla Proteobacteria and Clostridia likely acquired their abilities by later horizontal gene transfer. The discrepancy in the timing for the emergence of phototrophy between the fossil record, 3.45 Ga, versus that of molecular clock models, 3.0 Ga, requires resolution, but the emergence of oxygenic photosynthesis is much later.
A critical nutrient to the expansion of both subsurface and surface life on any planet is the availability of nitrogen as an aqueous species. On Earth, microorganisms evolved the ability to fix N2 into ammonia with the development of nitrogenase to overcome this constraint. Nitrogenases, Nif proteins, are complex enzymes, utilizing iron, molybdenum, and/or vanadium, that exist in both bacterial and archaeal domains. Phylogenetic comparison of genes that comprise nitrogenases and a complement of proteins required for their regulation indicate that nitrogenases emerged in anoxic sulfidic environments on Earth within obligate anaerobic thermophilic methanogens and were transferred to obligate anaerobic clostridia (Boyd et al., 2015), both common subsurface microorganisms. As Nif proteins were adopted first by the aerobic diazotrophic lineage Actinobacteria and then by the more recently evolved aerobic Proteobacterial and Cyanobacterial lineages, the Nif protein suite became more complex to protect the core MoFe-bearing proteins from O2 (Boyd et al., 2015). Although it is not clear whether the emergence of the more complex protein occurred prior to or after the Great Oxidation Event, it is certain that the ancestral protein emerged in an anoxic environment when the demands for aqueous nitrogen species exceeded the abiotic supply. The implications for martian ecosystems are that nitrogenase would have also likely emerged within an anaerobic subsurface environment, not in the oxic surface environment.
Experiments on the effects of low pN2 on diazotrophic nitrogen-fixing soil bacteria have shown that they could grow in N2 partial pressures of 5 mbar but not 1 mbar (Klingler et al., 1989). This result suggests that further experiments on wild-type species are required to determine whether the evolution of pN2 in the martian atmosphere was a significant deterrent to the expansion of early life, especially after Mars lost most of its atmosphere. Analyses of the nitrogen budget and of nitrogen cycling from deep subsurface environments in South Africa indicate that the pN2 is higher at depth than on the surface, that most of this N2 originates from the rock formations through nitrogen cycling, and that N2 is being actively fixed in the subsurface by microbial communities (Silver et al., 2012; Lau et al., 2016b). Given the presence of a cryosphere barrier to diffusion on Mars, the nitrogen availability and perhaps even the pN2 of subsurface brines are likely to be higher there than on the martian surface.
4. Biosignatures of Past Rock-Hosted Life
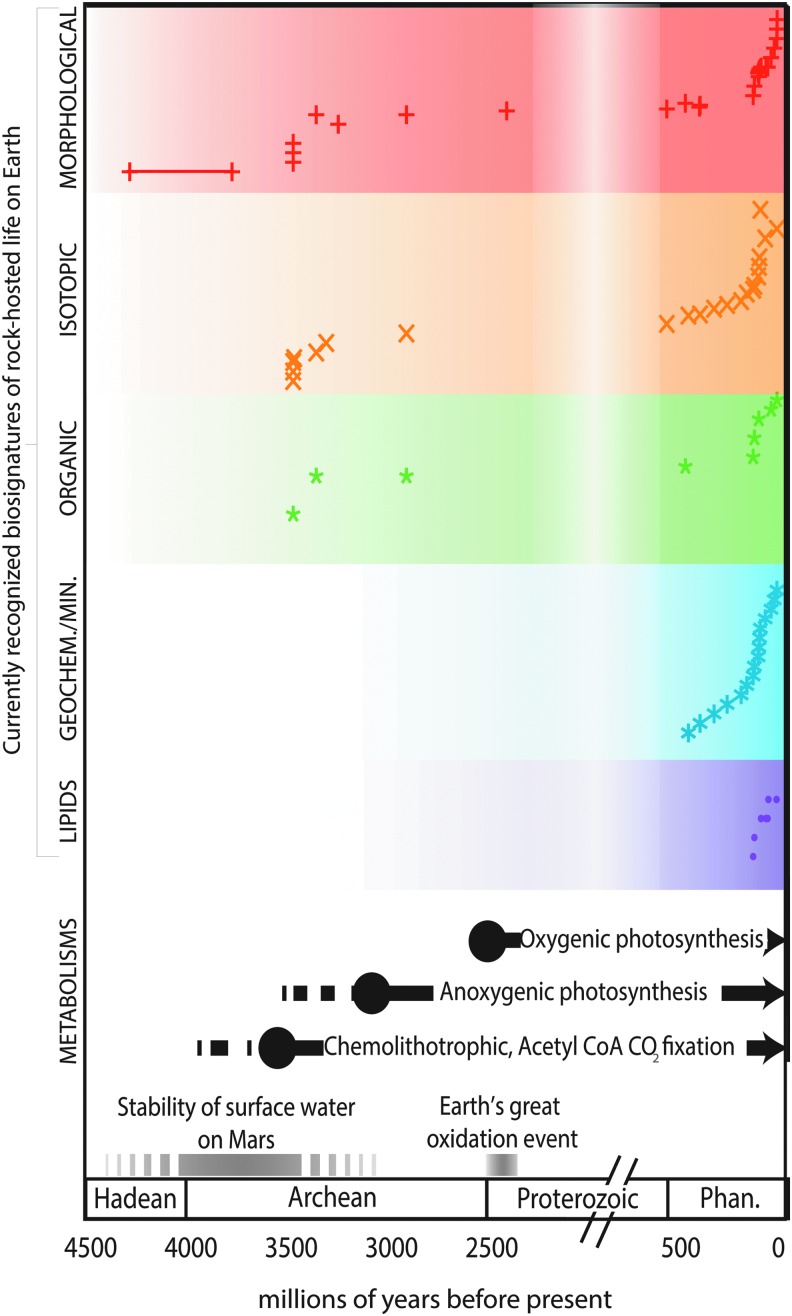
Examination of fossil evidence for life on Earth prior to ∼2 Ga is hindered by the fact that single-cell prokaryotes typically do not produce inorganic cellular components and Archean rocks have been subjected to metamorphic conditions capable of completely erasing the organic microscopic cellular remains. For those rare low-metamorphic-grade Archean rocks, molecular biosignatures such as hopanes (Eigenbrode, 2008) and their associated isotopic signatures (Williford et al., 2016) can constrain ancient metabolic processes. Examining examples of fossilized subsurface ecosystems in Phanerozoic rocks, however, provides a bridge between modern-day processes and contestable Archean examples (Table 1; Fig. 4).
Table 1.
Subsurface Biomarkers Preserved in Geological Record
| Age (Ma) | Biosignature type reported | Forms | Formation type | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Isotope | Geochemistry/mineralogy | Morphology | Organic carbon | Lipid | ||||
| 0.6 | x | filaments | Sea Mounts | 1 | ||||
| 1 | x | x | filaments | Ries Impact Crater | 2, 3 | |||
| 2 | x | x | x | concretions | Navajo Sandstone | 4 | ||
| 3.4–44 | x | Cretaceous shale | 5, 6 | |||||
| 15 | x | x | microcolonies | Columbia River Basalt | 7 | |||
| 31 | x | x | x | ichnofossils | sea floor basalt | 8 | ||
| 48 | x | x | filaments | Sea Mounts | 9, 10 | |||
| 56 | x | x | filaments | Sea Mounts | 9 | |||
| 60 | x | x | concretions | Moeraki Formation | 11 | |||
| 81 | x | x | filaments | Sea Mounts | 9 | |||
| 84 | x | x | concretions | Gammon Shale | 12 | |||
| 88.5 | x | x | x | concretions | Mancos Shale Formation | 13 | ||
| 91 | x | x | x | concretions | Frontier Formation | 13 | ||
| 95 | x | x | x | concretions | Frontier Formation | 13 | ||
| 92 | x | x | ichnofossils | Troodos ophiolite | 14, 15 | |||
| 0.115–400 | x | x | x | x | x | microcolonies | Fennoscandian shield granite | 16–18 |
| 120 | x | x | x | x | x | microcolonies | Southern Iberia Abyssal Plain | 19 |
| 152 | x | x | concretions | Kimmeridge Clay | 20 | |||
| 173 ± 8 | x | Fennoscandian shield granite | 21 | |||||
| 180 | x | x | concretions | Upper Lias | 22 | |||
| 250 | x | x | reduction spheroids | Mercia Mudstone Group | 23 | |||
| 315 | x | x | concretions | Lower Westphalian coal | 24 | |||
| 355 ± 14 | x | x | x | filaments | Fennoscandian shield granite | 21 | ||
| 358–394 | x | x | x | Fennoscandian shield granite | 21, 24 | |||
| 385 | x | x | x | filaments | Arnstein pillow basalt | 25, 26 | ||
| 388 | x | filaments | Tynet Burn limestone | 27 | ||||
| 443 | x | x | ichnofossils | Caledonian ophiolite | 28 | |||
| 458 | x | x | filaments | Lockne Impact Structure | 29 | |||
| 551 | x | x | concretions | Doushantuo Formation | 30 | |||
| 1175 | x | reduction spheroids | Bay of Stoer Formation | 23 | ||||
| 1950 | x | x | ichnofossils | Jormua ophiolite complex | 31 | |||
| 2400 | x | filaments | Ongeluk Formation sea floor basalt | 32 | ||||
| 2900–3350 | x | x | x | ichnofossils | Euro Basalt | 33, 34 | ||
| 3240 | x | filaments | Sulphur Springs Group | 35 | ||||
| 3300 | x | Barberton Greenstone Belt | 36 | |||||
| 3460 | x | Dresser Formation | 37 | |||||
| 3465 | x | x | x | microcolonies | Apex Chert | 38 | ||
| 3465 | x | microcolonies | Apex Chert | 39 | ||||
| 3465 | x | Apex Chert | 40 | |||||
| 3465 | x | x | ichnofossils | Hooggenoeg Formation | 41, 42 | |||
| 3770–4280 | x | filaments | Nuvvuagittuq belt | 43 | ||||
Ivarsson et al. (2015), 2Sapers et al. (2014), 3Sapers et al. (2015), 4Loope et al. (2010), 5Ringelberg et al. (1997), 6Elliott et al. (1999), 7McKinley et al. (2000), 8Cavalazzi et al. (2011), 9Ivarsson et al. (2009), 10Ivarsson et al. (2012), 11Thyne and Boles (1989), 12Coleman (1993), 13Mcbride et al. (2003), 14Furnes et al. (2001), 15Wacey et al. (2014), 16Pedersen et al. (1997), 17Drake et al. (2015a), 18Drake et al. (2015b), 19Klein et al. (2015), 20Irwin (1980), 21Drake et al. (2017a), 22Coleman and Raiswell (1995), 23Spinks et al. (2010), 24Curtis et al. (1986), 25Drake et al. (2018), 25Eickmann et al. (2009), 26Peckmann et al. (2007), 27Trewin and Knoll (1999), 28Furnes et al. (2002), 29Ivarsson et al. (2013), 30Dong et al. (2008), 31Furnes et al. (2005), 32Bengtson et al. (2017), 33Banerjee et al. (2007), 34McLoughlin et al. (2012), 35Rasmussen (2000), 36Ohmoto et al. (1993), 37Shen and Buick (2004), 38Schopf et al. (2018), 39Pinti et al. (2009), 40Ueno et al. (2006), 41Banerjee et al. (2006), 42Furnes et al. (2004), 43Dodd et al. (2017).
FIG. 4.
Present understanding of rock-hosted life over time. The currently recognized biosignatures of rock-hosted life from Table 1 are plotted as a function of time along with the timing of development of microbial metabolisms from molecular clock techniques, as discussed in the text. Earth's geologic timescale, Earth's oxidation, and the era of surface stability of water on Mars are also shown.
4.1. Subsurface life biosignatures in hydrothermally altered ultramafic rocks
Magma-poor paleocontinental margins expose large volumes of mantle peridotites to infiltration by seawater (Whitmarsh et al., 2001). Along the Lower Cretaceous Iberian margin, seismic data indicates 25–100% serpentinization-driven alteration of ultramafic crust to depths of 4 km (Dean et al., 2000). The upper ∼1 km is most heavily altered with serpentinized rocks crosscut by calcite-brucite assemblages, for which isotopic data indicate precipitation at temperatures from 25–40°C. The contact zone is hypothesized to represent the deep plumbing of a Cretaceous “Lost City” type hydrothermal system (Kelley et al., 2005, 2015).
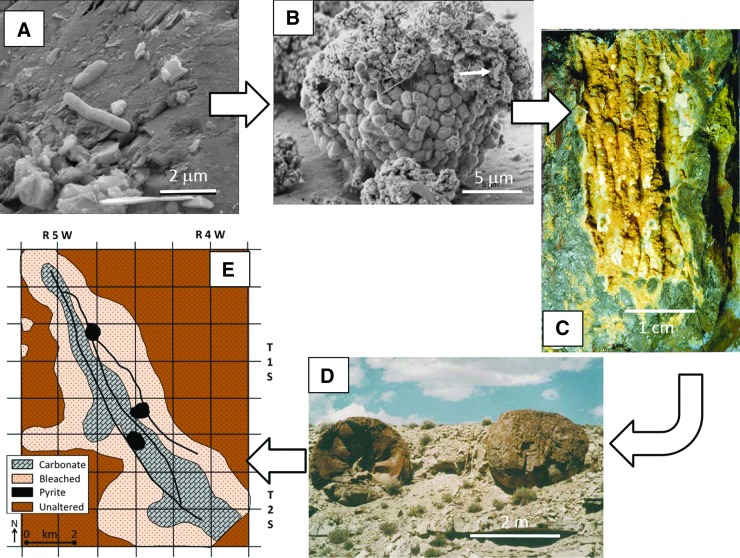
Mineralized veins in the contact zone at depths of ∼750 m are significantly enriched in organic carbon. Analysis for biosignatures revealed round to rod-shaped structures, ∼2–200 μm in diameter, which are consistent with the morphologies of microbial colonies. Analyses of these putative fossilized cells with Raman spectroscopy revealed them to be carbon-enriched, with C–H, –CH2, and –CH3 functional groups. Band positions are consistent with lipids, amino acid side chains of proteins and carbohydrates, and amide I bonds in proteins. Further analysis of lipid biomarkers revealed nonisoprenoidal dialkylglycerol diether lipids of bacterial origin and acyclic glycerol dibiphytanyl glycerol tetraether lipids of archaeal origin. Thus, hydrothermal activity ∼750 m beneath the seafloor at ∼120 Ma sustained very abundant archaeal and bacterial microbial communities, equivalent to ∼109 cells cm−2, within fractures leaving behind morphologic fossils, organic carbon, and lipids (Klein et al., 2015).
Fossilized remains of microorganisms have also been described in carbonate or serpentine veins of ∼1 Ma ultramafic peridotite rocks in the Mid-Atlantic Ridge, and characterized by a combination of morphology, chemical composition, and the presence of organic matter, sometimes including specific complex amides usually characteristic of biopolymers (Ménez et al., 2012; Ivarsson et al., 2018). In these systems, complex organics are found in either aragonite veins or within a poorly crystalline mix of serpentine, magnetite, and hydrogarnet, and remnant orthopyroxene with chemical enrichments in Ni, Co, Mo, and Mn (Ménez et al., 2012; Ivarsson et al., 2018) that are different compared to microbial preservation in basalt-hosted systems, which are instead dominated by clay minerals or Fe oxides (see Section 4.2 below).
4.2. Fracture-filling fossilized complex subsurface chasmoendolithic and cryptoendolithic communities in igneous rocks and precipitated rocks
Basaltic rocks cored from modern-day continental groundwater circulation sites show that cells are strongly concentrated within clay and oxides assemblages in fractures and pore spaces (e.g., Trias et al., 2017, their Supplementary Fig. 15). Encrustation of biological matter by mineralization is a key means of preserving the structures over geologic time, as cataloged for rocks of multiple ages and types (Hofmann and Farmer, 2000; Hofmann, 2008). Investigations of ancient, now fossilized fracture surfaces in igneous rocks show these are zones of concentration of microbial activity, sometimes including complex communities of organisms with multiple trophic levels, preserved by mineralization.
In seamount basaltic lavas ranging in age from 48 to 81 Ma, the fossilized remains of chasmoendolithic (fracture-dwelling) subsurface microorganisms, that is, coccoidal, filamentous or stromatolitic structures with elevated carbon concentration and organic matter such as lipids and rare chitin, are preserved in mixtures of clay, Fe oxides, and Mn oxides and carbonate and gypsum veins (Ivarsson and Holm, 2008; Ivarsson et al., 2009, 2012; Bengtson et al., 2014). The microstromatolitic structures found at 68–153 m below the seafloor within the fractured basalt are interpreted as the result of Fe- and Mn-oxidizing bacteria, and based on mineral succession, appear to be the initial colonizers of subseafloor basalt (Bengtson et al., 2014; Ivarsson et al., 2015). These rocks are interpreted to preserve a syntrophic community of chemolithoautotrophs, hyphae-forming fungi, and microstromatolitic Frutexites. Most filamentous and coccoidal fossils have so far been interpreted on morphological characteristics and rare chitin as fungal hyphae and yeast growth stages, respectively (Ivarsson et al., 2012, 2015). This is probably not due to a dominance of fungi in subseafloor crust but instead due to fungi being more easily recognized than prokaryotic fossils. Microstromatolites and associated single-celled features with morphologies comparable to S-cycling archaea like Pyrodictium species suggest prokaryotic remains are present as well (Bengtson et al., 2014; Ivarsson et al., 2015). The organic micron-sized coccoidal shapes occur in concentrations equivalent to ∼107 cells cm−2 on the vein walls with vein-containing tubular ichnofossils (see Section 4.3 below) and with saline fluid inclusions recording entrapment temperatures of ∼130°C.
As a second example, in continental flood basalts of Miocene age (17–6 Ma) in Oregon, secondary minerals formed within and near fractures preserve ∼1 μm sized coccoidal and rod-shaped microstructures and framboidal pyrite associated with kerogen (McKinley et al., 2000) in an aquifer where the present-day microbial communities are comprised of sulfate-reducing bacteria (Baker et al., 2003). Iron oxyhydroxides, smectites, zeolites, and silica within fractures and smectite veins were investigated because they contained framboidal pyrite, and the cell-like microstructures were discovered.
As a third example, fossilized remains of biofilms have also been found preserved within calcite-filled fractures of 1800 Ma granite at a depth of 200 m in the Fennoscandian shield using transmission electron microscopy (Pedersen et al., 1997). Stable isotope, fluid inclusion, and fission track analyses constrain the age of occupation and entrapment between 115 ka and 400–300 Ma. More recently, Drake et al. (2017a) have discovered fossil biofilms preserved in calcite veins dated at 355 ± 14 Ma that occur at 300 m depth in a similar Swedish granite and core lipids indicative of sulfate-reducing bacteria extracted from calcite veins from 80 to 920 m depth in Swedish granite and that are 358–394 million years in age (Drake et al., 2018). In the same granitic rocks, coupled bacterial sulfate reduction–anaerobic CH4 oxidation paleoactivity is recorded by the −125‰ to +36.5‰ V-PDB δ13C values and diagnostic lipid biomarkers preserved in vein-filling calcite (Drake et al., 2015a) that formed at temperatures <50°C and the −54‰ to +132‰ V-CDT δ34S values in pyrite lining open fractures (Drake et al., 2015b, 2018) over a depth range of 200–750 m. These paleobiosignatures are consistent with the present-day observations of a coupled bacterial sulfate reduction–anaerobic CH4 oxidation zone over similar depth ranges in fracture water from the granite, though in one instance the sulfate-rich zone is above the methane-rich zone (Pedersen et al., 2014), and in the other instance the opposite is true (Hallbeck and Pedersen, 2012).
Excellent preservation of fossilized fungal mycelia have also been reported from granites of the Fennoscandian shield with diagnostic morphologies like anastomosis between branches (Ivarsson et al., 2013). Drake et al. (2017b) have reported a fossilized anaerobic fungi–sulfate reducing bacteria consortium from a 740 m deep fracture in granite located at the Laxemar site, Sweden. In particular, fossils of filamentous microorganisms of fungi are within impact-induced fractures in granite from the 89 Ma Dellen impact and 458 Ma Lockne impact (Ivarsson et al., 2013). Both sites are related to the subsequent hydrothermally formed mineralization, indicating that impact-generated habitats in igneous rock can be favorable for microbial colonization and preservation.
As a fourth example, some organisms, known as “autoendoliths,” play a more active role in the formation of rock edifices, whose precipitation can result directly from microbial metabolism and encapsulate the responsible microbial constituents (Marlow et al., 2015). For example, anaerobic methanotrophs oxidize methane, increase alkalinity, and produce bicarbonate that precipitates as carbonate rock at methane seeps (Peckmann et al., 2001). Metabolic activity continues from within the rock (Marlow et al., 2014b), and biosignatures of the entombed organisms can persist for hundreds of millions of years (Peckmann and Thiel, 2004).
Other examples of fracture-filling filamentous fabrics of rock-hosted life include those preserved in chalcedony and/or zeolite in tens of terrestrial volcanic rocks ranging in age from Tertiary to Mesoproterozoic (Hofmann and Farmer, 2000); filamentous fabrics of what are interpreted as Fe-oxidizing chemotrophic bacteria of Devonian age in calcite veins cross-cutting lacustrine sedimentary rock (Trewin and Knoll, 1999); complex mineralized filamentous structures in Devonian-age pillow basalt (Peckmann et al., 2007; Eickmann et al., 2009); and fungi-like mycelial fossils in vesicles and fractures of 2.4 Ga basalt in South Africa (Bengtson et al., 2017). The interpretations that these fossils represent subsurface prokaryotes and fungi are consistent with observations of the present-day subsurface biosphere (see Section 3.1). Nonetheless, as the record is pushed backward, Earth's overprinting processes demand more sophisticated high-resolution analyses for biogenicity determination (see also Section 4.6).
4.3. Microbial trace fossils in recent and ancient glass
Complex, tubular structures that are sometimes found emanating from alteration mineral–filled fractures in basalts, basaltic glass, or impact glass may in some cases be trace fossils of microbial origin, called ichnofossils. These structures were first reported in Pleistocene volcanic glass in Iceland (Thorseth et al., 1992) and have since been documented globally in young seafloor pillow basalt glass (Thorseth et al., 1995; Furnes et al., 1996; Fisk et al., 1998; Fisk and McLoughlin, 2013) and in pillow basalt of ancient ophiolites and greenstone belts dating back 3.5 Ga (Furnes et al., 2008). The mechanisms of formation of these features combine dissolution of glass with leaching of cations and formation of clay minerals, Fe and Mn silicates, and Fe and Ti oxides (Staudigel et al., 2008).
In modern rocks, basaltic glass samples from 1.3–1.8 km depth from Hawaii Scientific Drilling Program core that were examined with Raman, deep UV fluorescence, and 16S rRNA sequencing show microorganisms are present in clays at the dissolution boundary with the glass near microtubular structures (Fisk et al., 2003). In modern seafloor basalts on the Mohns spreading ridge of the Norwegian Sea, tubular dissolution structures originate from the palagonite-glass interfaces, and evidence for bacterial processing includes the characteristic rounded and elongated, microbial-sized, 0.5–2 μm, pores, enrichments of Mn on the rims of coccoid-shaped structures with elevated concentrations of C, N, and organic carbon with a depleted isotopic signature (Kruber et al., 2008; McLoughlin et al., 2011).
Determining biotic versus abiotic origin in fossil rocks requires careful observations of textural subtleties and paired morphological-chemical criteria (e.g., McLoughlin and Grosch, 2015). As with many exothermic inorganic chemical processes that are exploited by chemolithoautotrophic microorganisms, distinguishing structure formed by abiotic reactions from biologically mediated ichnofossils is challenging (Knowles et al., 2012; Grosch and McLoughlin, 2014; Wacey et al., 2017). We describe two of the best ancient examples involving rock-hosted microbial life associated with glass dissolution.
Ichnofossils like the modern examples are found in volcanic glass of the 92 Ma Troodos ophiolite (Furnes et al., 2001). Tubular dissolution structures possess 3-D spiral morphologies with organic carbon and nitrogen enriched linings (Wacey et al., 2014). Careful analyses of the relationship between infilling clay minerals and organics shows that the organics formed first by microbial extracellular materials and were then mineralized by clays.
In the 14 Ma Ries impact crater, microtubules related to microbial life are found in the impact-generated glass within heavily altered zones with clay minerals and Fe oxides. The carbon-bearing materials have C-Hx and amide I and II absorption bands from organic materials, not observed in tubule-free areas (Sapers et al., 2014). Further analysis with Raman spectroscopy showed quinoic compounds, and STXM coupled with NEXAFS showed Fe redox patterns in these areas consistent with microbially mediated dissimilatory Fe reduction (Sapers et al., 2015).
4.4. Lipid biomarkers of rock-hosted life
Neutral lipid biomarkers have been widely utilized as a biomarker of terrestrial life in sedimentary and petroleum deposits (see review by Brocks and Summons, 2005), and their application to Archaean marine sediments had a significant impact on the understanding of the evolution of prokaryotes and eukaryotes (e.g., Brocks et al., 1999). However, aliphatic and polycyclic lipids in the metamorphosed Archean sediments containing polyaromatic hydrocarbons were later shown to be drilling contamination (French et al., 2015). Unmentioned, and unresolved, is whether some of the low concentrations of bacterial lipids and archaeal isoprenoids found in the rock matrix could in fact have originated from extant and extinct subsurface microorganisms colonizing the rock mass over billions of years.
Analyses of lipid biomarkers in modern marine sediments under anoxic conditions document how rapidly the lipid biomarkers of marine planktonic biomass, both eukaryotic and prokaryotic, are quickly replaced within the water column and the surface seafloor sediment by the bacterial lipids of the subseafloor biosphere (Schubotz et al., 2009). The archaeal lipid half-lives appear to be longer on the order of hundreds of thousands of years (Xie et al., 2012). A geological test for both the age and preservation of subsurface bacterial lipids has been documented in Cretaceous-age marine sediments that were intruded by mafic dikes 3.4 million years ago (Table 1; Fig. 4). Profiles of the phospholipids (active bacteria) and glycolipids (extinct bacteria) both stratigraphically and as a function of distance from the intrusion indicate that the glycolipids result from the decay of phospholipids of subsurface bacteria. These lipids from subsurface bacteria predate the 3.4 Ma intrusion and postdate the 44 Ma age of burial sterilization (Tmax = 125°C) of the marine shale (Ringelberg et al., 1997; Elliott et al., 1999). This persistence indicates that study of lipid biomarkers of rock-hosted life warrants considerably more attention as the microbial record of ancient terrestrial rocks is interrogated.
4.5. Putative Archean subsurface versus surface microbial biosignatures
The earliest, commonly agreed upon, preserved microbial structures are in the 3.4–3.5 Ga rock units of the Pilbara Craton, Australia. Some units contain microfossils and laminated sedimentary structures consistent with stromatolites and contain contentious carbonaceous biosignatures (e.g., Walter et al., 1980; Buick et al., 1981; Schopf, 1993; Brasier et al., 2006; Noffke et al., 2013) that are suggestive of photosynthetic microorganisms at this time, though their biogenicity has been questioned (e.g., Buick et al., 1981; Lowe, 1994; McLoughlin et al., 2008).
The 3.46 Ga Apex chert represents hydrothermal dikes with silica-mineralization containing kerogenous microfossils involved in subsurface cycling of CH4 (Schopf et al., 2018) and/or organic matter formed by abiogenic mechanisms, for example, Fischer-Tropsch-type synthesis or remobilization of other organics (Brasier et al., 2002, 2005, 2006; García-Ruiz et al., 2003). Other workers have suggested the −56‰ δ13C V-PDB value of CH4 trapped in fluid inclusions of the same veins could be the earliest evidence of methanogenesis in the subsurface (Ueno et al., 2006).
Sulfur isotope fractionation between sulfides and barite in sediments and crosscutting veins (Shen et al., 2009) and within seafloor basalt and komatiite (Aoyama and Ueno, 2018) of the Dresser Formation suggest that sulfate-reducing bacteria were also present and metabolically active at the near surface and subsurface by ∼3.46 Ga. Microfossils preserved in chertified Strelley Pool arenite and pyrite with negative δ34S V-CDT values also suggest the presence of subsurface sulfate-reducing bacteria at 3.43 Ga (Brasier et al., 2015). Pyritic filaments preserved in the 3.24 Ga deep sea volcanogenic massive sulfide deposits within the Sulphur Springs Group provide the most convincing evidence of early life in the form of thermophilic, chemotrophic prokaryotes living in hydrothermal systems beneath the seafloor (Rasmussen, 2000).
Putative microfossils in the form of mineralized tubular features in basaltic glass from the 3.47–3.46 Ga upper Hooggenoeg Formation of the Barberton Greenstone Belt, containing isotopically light carbonate, have been proposed as subsurface biosphere fossils (Furnes et al., 2004; Banerjee et al., 2006). Titanite mineralized microtubules in 3.35 Ga basaltic glass with a minimum age of 2.9 Ga have also been suggested to represent evidence of biological processing (Banerjee et al., 2007). Very negative δ34S V-CDT values from pyrite within these microtubules suggest that they were formed by microbial sulfate reduction (McLoughlin et al., 2012). More recently, however, Grosch and McLoughlin (2014) disputed the biogenicity of the microtubule textures suggesting they represent contact metamorphic textures associated with postdepositional intrusions (Table 1; Fig. 3).
Even more controversial are the earliest traces of life from 3.95–3.75 Ga amphibolite-grade metamorphic rocks in Greenland and Labrador in the form of “biogenic graphite” (Mojzsis et al., 1996; McKeegan et al., 2007; Tashiro et al., 2017) and a single graphite inclusion in 4.1 Ga zircons from Jack Hills, Australia (Bell et al., 2015) that yield negative δ13C V-PDB values comparable to those of modern life. Determining whether they represent primary organic matter versus secondary organic matter formed during later metamorphism is challenging (Papineau et al., 2011), and isotopic values do not determine whether they formed by biological fractionation or abiotic processes (van Zuilen et al., 2003; Sherwood Lollar et al., 2006), let alone whether they represent “chemofossils” of phototrophic or subsurface chemoautotrophic microbial biomass. Other evidence for Archean life is based upon textural evidence such as putative centimeter-scale stromatolites in 3.7 Ga metacarbonates in Greenland (Nutman et al., 2016) and tens-of-micron-scale hematite filaments in 4.2 Ga metasedimentary rock in Quebec (Dodd et al., 2017). The former features, however, were recently refuted as true stromatolites (Allwood et al., 2018).
In summary, the paucity of low metamorphic grade Archaean rock record hampers our ability to identify unambiguous biosignatures older than ∼3 Ga, which is approximately the time frame at which Mars' broad-scale habitability began to decline. Putative morphological biogenic structures, combined with C and S isotopic evidence, have been preserved in volcanics, dikes and quartzites that are consistent with subsurface life (methanogenesis and sulfate reduction) while those found in marine sediments are suggestive of photosynthetic life. Noteworthy is a quote from the work of Brasier et al. (2015), as follows: “Why are few cellular fossils found in rocks before 2.5 Ga? For decades, the main search image has been cyanobacteria-like assemblages as silicified algal mats and stromatolites. Have we been looking for fossils in the wrong places?” (Brasier et al., 2015). In light of new insights on the magnitude of the rock-hosted biosphere on Earth, it seems clear that while we were not looking in the wrong places, the taphonomic windows and environmental settings investigated for the biomarkers of ancient life should be expanded. The same can be said, therefore, for the >50% of the surface of Mars that is older than ∼3 Ga. As it is unmetamorphosed relative to Earth, it represents a particularly compelling exploration frontier (see Section 5).
4.6. The footprint of fossil rock-hosted life
The confirmation of biosignatures often takes place at the micrometer scale by a suite of integrated techniques described in the examples above. Nevertheless, the “footprint” of fossil rock-hosted life can far exceed this scale as the mineralogical, chemical, and isotopic signatures for the presence of life can often be observed in bulk rock samples, sometimes over enormous volumes ranging from millimeters to kilometers. Indeed, the impact of rock-hosted life on biogeochemical cycling on Earth is significant and a current major topic of research (Colwell and D'Hondt, 2013).
An example of just how large this footprint can be is the magnetic anomalies on the scale of tens of kilometers detected around oil fields by aeromagnetic surveys (Fig. 5). These anomalies are characteristic of freshwater oil reservoirs and result from the oxidation of hydrocarbon coupled to the reduction of Fe(III) in the sediment by Fe(III)-reducing bacteria producing fine-grained magnetite that records the ambient magnetic field at the time of crystallization over the geological life span of the oil reservoir (Liu et al., 2004; Schumacher, 1996).
FIG. 5.
Increasing scale of metabolic footprint of subsurface life. (A) Single microbial cells attached to clay minerals of a 2.8 km deep fracture zone (Wanger et al., 2006). (B) Framboidal pyrite sack with organic mineralization from 1.5 km deep borehole. White arrow points to single bacterial cell (Maclean et al., 2008). (C) Centimeter-scale “Pseudostalactite” of quartz and goethite cemented by biogenic filaments occurring in Tertiary volcanic rocks in California (Hofmann and Farmer, 2000). (D) Ferroan carbonate septarian concretions from 88.5 Ma in the Ferron Sandstone Member of the Mancos Shale Formation in Utah that are 1–4 m in diameter (McBride et al., 2003). (E) Surface diagenetic alteration zones and traces of pre-Permian faults over Velma field, Stephens County, Oklahoma, 1 mile scale bar (Al-Shaieb et al., 1994).
An example more relevant to planetary sciences is the footprint left behind by the subsurface microbial processes in pore waters in sediments that successively consume organic matter and give mineralogically, chemically, and isotopically characteristic products that are much larger than the microorganisms forming them. For example, the association of pyrite with nonferroan calcite with δ13C values of approximately −20‰ V-PDB is a clear indication of the former presence of sulfate-reducing bacteria, as has been shown in modern-day deposits (Coleman et al., 1993). The likely δ13C value for each of the processes, which may vary from −20‰ to +15‰ V-PDB, is summarized in Fig. 1 of Coleman (1985). Any part of a sedimentary succession might have evidence of one process only, and this results from the rate of burial of the sediment (or changes in rate), which controls in which zone the organic matter resides for the longest time (Irwin et al., 1977). The characterization is sufficiently specific that contributions from different processes can be identified in a single mineral, for example, Mn reduction, Fe reduction, and methanogenesis in siderite (Fe carbonate) minerals in 315 Ma sediments (Curtis et al., 1986). These mineral biosignatures occur as intergranular pore-filling cements in many sediment rock types ranging from shale to coarse sandstone (Curtis, 1977). However, the most spectacular occurrences are large spherical or subspherical nodular concretions ranging in size from a few millimeters up to 2 m diameter (Thyne and Boles, 1989). Their visibility makes them excellent pathfinders for other more detailed analyses to confirm their origin, and they can preserve biosignatures for more than 550 million years (Dong et al., 2008).
Other examples of carbonate/oxide concretions produced by anaerobic and micro-aerophilic subsurface bacteria are found in ancient sandstones at scales of up to meters (Coleman, 1993; Abdel-Wahab and McBride, 2001; McBride et al., 2003). These sandstone concretions form as a result of microbially mediated redox reactions occurring during fluid flow long after deposition of the sediment. Meter-sized Fe(II)-rich carbonate/iron oxide concretions (Fig. 4) are found in Jurassic sandstone deposits of southwest Colorado that were formed at hundreds of meters' depth between 2 and 0.5 Ma as the Colorado River Basin was uplifted (McBride et al., 2003; Loope et al., 2010). Similar-sized ferroan calcite and siderite concretions occur in Late Paleocene/Early Eocene Wasatch Group sandstones, and siderite nodule-bearing cores from the formation (Lorenz et al., 1996) yielded thermophilic Fe(III)-reducing bacteria that were capable of producing prodigious quantities of siderite (Roh et al., 2002). In subaqueous systems unconstrained by rock matrix, authigenic carbonate mounds at CH4 and hydrocarbon seeps, formed from carbon mobilized by methane- and alkane-oxidizing microorganisms (Greinert et al., 2001; Formolo et al., 2004; Ussler and Paull, 2008), can be hundreds of meters tall and more than a kilometer wide (Klaucke et al., 2008).
At smaller scales but still larger than individual microorganisms, filamentous bacteria often form mats centimeters in size that later are silicified into stalactite-like cavity-filling textures (Hofmann and Farmer, 2000). Smaller still are framboidal pyrites generated by sulfate-reducing bacteria in sizes ranging up to tens of micrometers in diameter (Popa et al., 2004; Maclean et al., 2008). Framboidal pyrites of similar size and texture are seen in Archean sedimentary deposits (e.g., Guy et al., 2012). However, framboidal pyrite alone is not an infallible biosignature, as pyrite with similar microcrystalline textures can be produced abiotically in the laboratory if the solutions are extremely supersaturated with respect to pyrite and/or the temperatures are greater than 60°C (Ohfuji and Rickard, 2005) and occur naturally in ore deposits formed at 150°C to 320°C (Halbach et al., 1993), temperatures well above the limit of hyperthermophiles. Nevertheless, true framboidal pyrites are widely associated with microbial sulfate reduction and can act as a valuable pathfinder so that other characterizations can be performed.
Reduction spheroids were formerly believed to be created by detrital organic matter abiotically reducing Fe(III) minerals to Fe(II) minerals in red beds, but additional mechanisms, such as radiolysis and subsurface bacteria, have been advanced to explain their occurrence (Keller, 1929; Hofmann, 1990, 1991, 2008). Reduction spheroids that range in diameter from millimeters to decimeters with a core enriched in uranium and vanadium have been found throughout the geological record reaching back to the oldest red beds in the Mesoproterozoic and are believed to record the activity of subsurface microorganisms often pre-dating peak metamorphism and deformation (Spinks et al., 2010).
Thus, the footprint of subsurface metabolic activity can greatly exceed the organic content of the microorganisms responsible. To quantify some of the examples above, 1–20 cells occurring within a cluster or patch in the rock would be comprised of only 2 × 10−15 to 4 × 10−14 mol of organic carbon in a 10−11 cm3 volume. Microbially generated framboidal pyrite ranges in size and mass up to ∼10−9 cm3 of 53 wt % S, representing ∼10−10 mol e- of sulfate reduction. Reduction spheroids are 10−3 to 103 cm3 volumes, depleted in Fe(III) by 1–3 wt % Fe2O3 compared to the rock host (Hofmann, 1990), and represent 10−6 to 5 mol e- of Fe(III) reduction. Carbonate concretions attain volumes of 103 to 106 cm3, enriched in carbonate by as much as 50–70 wt % (Coleman and Raiswell, 1995), and represent 10 to 104 mol e- from organic carbon oxidation although isotopic profiles suggest that some of the carbonate volume could accrue from increased alkalinity due to anaerobic respiration (McBride et al., 2003). These estimates correspond to a total metabolic conversion of ∼1 (reduction spheroids) to 100 (pyrite framboids) mol e- L−1 over timescales of millions of years (Thyne and Boles, 1989; Abdel-Wahab and McBride, 2001; Mcbride et al., 2003). Such rates are consistent with the metabolic rates reported for near-shore, deep subseafloor sediment microbial communities (Orcutt et al., 2013), and the size of these mineral footprints reflects the environmental stability of the subsurface environment over geological time intervals.
5. An Exploration Strategy for Past Rock-Hosted Life Biosignatures on Mars
5.1. Lessons from Earth
Earth's crust has harbored and preserved subsurface life since at least 3.2 Ga, possibly 3.45 Ga, and the fossil remains are preserved in rock with low metamorphic grade, which is promising for tracking the terrestrial fossil record as well as searching for fossils in similar-age rocks from Mars. The types of biosignatures of rock-hosted life include morphologic structures, organic carbon, spatial patterns in geochemistry, gases, minerals, and their isotopic signatures. When available in concert they can distinguish rock-hosted life from abiotic footprints (e.g., McLoughlin et al., 2011). The criteria for recognizing such life are not fundamentally different from those articulated for more “classic” near-surface sedimentary environments (Summons et al., 2011). As summarized by Grosch et al. (2014), textural, chemical, and isotopic information (about both reservoir composition and fractionation patterns) is required, initially at submillimeter scale and then micrometer scale with NanoSims, FIB-TEM, and X-ray synchrotron-based studies. The nature of the rock types that warrant investigation for fossil rock-hosted life and the methods for finding and then characterizing the most promising samples are, however, different. Crystalline igneous rocks altered by groundwater and impact-altered rocks are of high priority in the search for rock-hosted life on Mars. Several heuristic principles can be extracted, based on the terrestrial experience in finding and characterizing biosignatures of rock-hosted life, discussed in Sections 3–4. These scale down from the landscape scale to the microscopic scale (Fig. 4).
First, suitable host rock formations must be identified within the environmental parameters that support life. These include zones with a suitable temperature range during water-rock interaction (< ∼120°C) and sufficient permeability for fluid flow or porosity for diffusive transport (can be highly heterogeneous), combined with redox couples that yield sufficient energy to provide adequate power for sustaining significant biomass concentrations. Many martian rock formations may be suitable (see Section 2). Even rocks identified with higher-temperature water-rock alteration are of interest because there will exist some contact zone or gradient where the higher-temperature waters cool toward Mars surface ambient. Rocks should not have excessive overprinting by later chemical or thermal processes, which might obfuscate or destroy the interpretation of the origin of the rock-hosted life biosignatures. However, even rocks with low-grade hydrothermal or metamorphic overprinting have yielded subsurface biosignatures on Earth, although the duration of such modifications is an important consideration. Ancient martian rocks are generally far less metamorphosed than ancient terrestrial rocks due to the absence of plate tectonics.
Second, finding specific locales to search for biosignatures relies on seeking interfaces at a variety of spatial scales. Studies of terrestrial rock-hosted life (and ice-hosted life) have revealed there are two types of interfaces conducive to rock-hosted life: zones with redox disequilibria gradients or high-permeability zones of fluid flow. The former provide energy for life, and the latter ensure sufficient delivery of new material to support metabolism and removal of waste products. Fault zones, fractured rock, connected vesicles and voids, and alteration zones are locations where rock-hosted life, present and fossil, is found on Earth. Indeed, the cell count is often at least an order of magnitude higher at the interfaces in comparison to surrounding rock (Fig. 3, Section 3.3). Meter-scale and centimeter-scale analyses of morphology, mineralogy, and chemistry can identify these key interfaces for investigation. Detection of the biosignatures themselves relies on smaller spatial scale (submillimeter) analyses of the patterning in morphology, chemistry, and isotopes.
Third, bulk rock organic carbon content over large spatial scales does not track as a key indicator of the richness of the microbial community. Heterogeneity along interfaces is expected, and most subsurface cell concentrations are clustered rather than diffuse. Based upon a review of terrestrial biomass distribution (see Section 3.3), any search for cell-like materials requires searching rock fracture surfaces for ∼10 cell clusters (> ∼103 cells g−1) occupying hundreds of μm2 areas or identifying seams with carbonaceous material where cell concentrations can reach 109 cells g−1. Thus, the ability to detect 1000 cells g−1 at 100 μm spatial sampling may be a rule of thumb for evaluating candidate techniques for in situ biosignature prospecting for rock host.
Finally, a crucial lesson from the terrestrial record of fossil rock-hosted life is that the initially detected potential biosignature is more likely to be a suggestive mineralogical, chemical or isotopic composition, possibly in a particular shape or texture, rather than a direct detection of organic carbon enrichment from such life. This is because the products of life are more volumetrically significant than life itself (Section 4.6). Phases that may be metabolic products of rock-hosted life or be by-products of metabolic reactions include sulfide, carbonate, sulfate, oxides as well as gases trapped in fluid inclusions. The ability to interrogate their microscale textures, isotopic signatures, the presence or absence of organic carbon, and trace element patterns all support the ability to identify possible biosignatures that later might be confirmed with still smaller-scale analyses in terrestrial laboratories.
5.2. The exploration strategy for Mars
On Mars, rocks preserving ancient subsurface habitats are accessible to surface exploration today. Their interrogation does not require the type of large or complex drilling operations that have been proposed to search for modern deep subsurface life beneath kilometers of rock or ice (Stamenković et al., 2019). The search for paleo-rock-hosted life is considerably more straightforward and can be conducted efficiently at many locations with rovers or other mobile surface explorers. This is because faulting, impacts, and erosion have exposed scarps of rocks from the subsurface and ongoing wind erosion continually renews the upper surface to expose rocks less affected by radiation and oxidation. Crustal loading by emplacement of large volcanic edifices at Tharsis and unloading around large impact basins around Isidis and Argyre have created extensional faults that expose thick strata of crust that once hosted groundwater flow (Ehlmann et al., 2011; Ehlmann and Edwards, 2014). Active erosional processes have exposed hundreds of meters of materials that once hosted aquifers in a form accessible to rovers. Examples include mineralized ridges at centimeter- to kilometer-length-scale, which were conduits of groundwater flow (Thollot et al., 2012; Saper and Mustard, 2013; Siebach and Grotzinger, 2014; Quinn and Ehlmann, 2019). At Gale Crater, erosion rates that refresh the surface and expose materials less affected by radiation are ∼0.75 m My−1 (Farley et al., 2014). Impact craters also provide direct exposure of subsurface material by their walls, ejecta, and uplift of materials in the central peak (Cockell and Barlow, 2002; Cockell et al., 2012). The complicating factors affecting preservation of rock-hosted life biosignatures on Earth such as organic matter degradation by modern organisms and overprinting of chemical/mineralogical/isotopic signatures by metamorphic fluids billions of years later are likely absent or reduced in the near subsurface of Mars. Rock-hosted-life biosignatures sealed in reduced mineral phases in the martian subsurface may also be less susceptible to secondary oxidation during uplift and exposure to surface oxidation than surficial porous sediments comprised of oxidized mineral phases.
The exploration strategy for searching for evidence of martian rock-hosted life parallels that employed on Earth, but with the need to narrow progressively the spatial scale of the exploration zone to target efficiently, access, and explore the best sites, given the prevalence of orbital data and—relative to the terrestrial situation—the paucity of opportunities for data collection from landed missions (Table 2). Additionally, many biosignatures are (at present) only detected with advanced laboratory analyses necessitating parsimonious sample selection coupled with acquisition of contextual data for return of those samples with promising preservation of biosignatures for rock-hosted life.
Table 2.
Steps to Search for Rock-Hosted Life on Mars
| Step | Spatial scale | Key measurement requirements |
|---|---|---|
| 1. Identify rocks with ancient subsurface habitats | <100 m sampling | Ability to identify water-related mineral deposits from orbit and determine stratigraphic context |
| 2. Locate interfaces that represent favorable locations for rock life | Meter- to centimeter-scale | Ability to identify redox and permeability interfaces by identification of distinct lithologic units |
| 3. Search for mineralization from fluid flow at interfaces | Centimeter- and millimeter-scale | Ability to identify silica, carbonate, sulfate, phyllosilicate, and oxides that may mineralize microbial life |
| 4. Search for organics, mineralization, and isotopic anomalies at the interface | <100 μm sampling | Ability to detect organics, chemical, mineralogic, and/or isotopic differences between interface rocks and surrounding rocks indicative of biosignatures |
| 5. Map putative biosignatures in 3-D, tracking chemical and organic variations with texture | <1 μm sampling in 3 dimensions | Ability to identify microbial textures and distinguish biotic and abiotic processes to confirm definitively fossil rock-hosted life |
The scaled exploration strategy for rock-hosted life relies on seeking interfaces and boundaries (Table 2). Redox interfaces, indicated by mineralogy with contrasting oxidation states, can be manifest at a range of spatial scales indicating the potential for past thermodynamic disequilibria that drive metabolism. Lithological interfaces that indicate zones of focused fluid flow—fault zones, dikes, fracture networks, and connected vesicles—also are required for exchange of materials with the environment. Because of the importance of subsurface hydrology in establishing and maintaining habitable conditions, reconstructing fluid flow regimes through martian aquifers is a key priority. Volcanic rocks inherit large-scale fractures during cooling. Dike swarms also produce kilometers-scale fracture conduits due to the difference in rock properties at their contacts with bedrock. Sediment compaction and closing of pore space is less pronounced on Mars than it is on Earth due to its lower gravity. Meteorite impacts represent one of many reliable modes of fracturing rock and creating reactive surface area and permeability enhancement—as demonstrated at the Haughton and Chesapeake Bay Impact Structures (Pontefract et al., 2016)—all of which improve habitability prospects (Cockell et al., 2012).
As an example of the exploration strategy for Mars, orbit-based data can identify rocks' lithologies, recording the paleoenvironmental conditions with groundwater flowing through sulfate- and serpentine-containing rocks at Northeast Syrtis Major. The presence of serpentine alongside oxidized sulfur indicates the presence of redox interfaces. Abundant fracturing in the area and the presence of secondary minerals (Quinn and Ehlmann, 2019) suggest lithological interfaces and substantial fluid flow. Calculations of Gibbs free energy suggest that these martian habitats had the necessary energy to support anaerobic oxidation of CH4 (Marlow et al., 2014a). Mobile surface explorers with camera and instruments for remote assessment of mineralogy and chemistry can then pinpoint lithologic and mineralogical interfaces such as fractures, redox fronts, and zones of low-temperature aqueous mineralization: for example, crosscutting serpentine veins, serpentine-carbonate contacts, and zones of intense magnetite precipitation to meters then centimeters in scale. Advanced instruments for petrology employed in contact with the rock then examine a variety of initial observables, characteristic of sites hosting signatures of paleo-rock-hosted life, including organics and organic-mineral associations (Tables 1 and 2). In select cases (e.g., complex mineralized filaments, reduction spheroids, concretions or framboidal pyrite), a biosignature may be deemed highly likely, especially if concentrations of organic matter are associated with it. However, the best confirmation of biogenicity would require further higher-resolution laboratory analyses on Earth that include significant sample preparation and nanometer-scale analyses.
In addition to the igneous and sedimentary rocks at Northeast Syrtis and Nili Fossae, igneous and sedimentary rocks altered by groundwater at Valles Marineris (Thollot et al., 2012), sediments in the Terra Sirenum craters (Wray et al., 2011; Ehlmann et al., 2016b), and even Gale Crater present opportunities for searching for subsurface life. Organics have been found in diagenetically altered Gale Crater sediments, though their origin as sedimentary detritus or from later fluids cannot be established from the bulk sample composition reported from the Curiosity rover's instruments (Eigenbrode et al., 2018). As of this writing, the long-lived subsurface habitats on Mars have not yet been targeted in geological or astrobiological investigations of the Mars exploration program, which has instead targeted depositional basins, following an Earth environmental model. As subsurface habitats for rock-hosted life are the most promising sites for preservation of ancient martian life (Section 2), the best prospects for life on our neighboring world await future exploration by in situ missions or sample return.
6. Summary and Recommendations for Future Directions
A review of the published studies on abundance and diversity of extant terrestrial subsurface life, the diverse environments in which it is found, their fossil remains and biomarkers, and a comparison of the evolution of key metabolic pathways for phototrophic versus chemolithoautotrophic microorganisms provide guidance to the search for biomarkers of subsurface life on Mars. First, the metabolic pathways for microorganisms found in the terrestrial subsurface evolved much earlier in Earth's history than those of surface-dwelling phototrophic microorganisms. Second, time-equivalent environments on Mars were much less stable than on Earth, and martian surface environments were challenged by radiation, aridity, freezing temperatures, and frequent obliquity-driven climate change that reduced the availability of water.
Subsurface environments inhabited by rock-hosted life are common, not rare, on Earth. Rock-hosted life and its preserved remains are found in ultramafic serpentinizing systems, deep groundwater systems, hydrothermal systems, and shallow aquifer and diagenetic environments. Terrestrial subsurface biomass concentration tends to be highest at chemical redox gradients and at permeability interfaces; it does not correlate directly with the abundance of organic carbon. Rock-hosted life does not rely upon metabolizing organic photosynthate supplied by Earth's phototrophic organisms but rather upon subsurface energy sources and fluxes (e.g., water-rock chemical reactions, radiolysis) and the abiotic and biotic recycling of carbon and metabolic waste products. The terrestrial rock record reveals examples of subsurface biomarkers at least back hundreds of millions of years and likely to 3.45 Ga. Several excellent examples of rock-hosted life with high-quality preservation are found in rocks quite different from those traditionally explored for fossils from the photosynthetically supported biosphere.
These findings suggest a well-defined exploration strategy for rock-hosted life on Mars (Table 2):
-
(1)
locate rocks preserving aquifers, that is, the plumbing of hydrothermal and groundwater systems;
-
(2)
then, identify redox interfaces and permeability/porosity boundaries preserved within the rock outcrop;
-
(3)
search for locations where these interfaces exhibit mineralization that may have entombed cells;
-
(4)
at submillimeter scale, interrogate these zones of mineralization for patterns in organic molecule concentration, morphologies suggestive of microbial filaments or cells, changes in isotopic signatures (particularly of C, N, S, and Fe), and associations between these putative biosignatures.
Armed with this strategy, evidence of the biosignatures of rock-hosted life can be found in situ on Mars and the best samples identified for return to Earth and further interrogation. The search for rock-hosted life is essential to understanding whether Mars was once inhabited, and the search for life on Mars will only be complete once its subsurface habitats are targeted for exploration.
Supplementary Material
Acknowledgments
This work has greatly benefited from the discussions and intellectual contributions of the 20 participants in the Rock Hosted Life Workshop, February 8–10, 2017, at Caltech as well as the >100 participants in the four pre-workshop telecons open to the community. Thanks to Mary Voytek and Michael Meyer at NASA Headquarters for workshop funding and to NASA Astrobiology Institute director Penny Boston and the NASA Ames Research Center meeting support team for providing web-hosting for the telecons and their recording; all the information can be found at http://web.gps.caltech.edu/~rocklife2017. We thank partial support of T.C.O. through a subcontract supported by NASA Exobiology Award NASA NNX17AK87G to Andrew Schuerger of the University of Florida and partial support to B.L.E. by the NASA Mars Data Analysis Program award 80NSSC17K0444. The contribution of M.C. was carried out partly at the Jet Propulsion Laboratory (JPL), California Institute of Technology, under contract with the National Aeronautics and Space Administration (NASA), and via Grant NNA13AA94A issued through the NASA Science Mission Directorate and supported by the NASA Astrobiology Institute.
Abbreviations Used
- OTUs
operational taxonomic units
- SLiMEs
subsurface lithoautotrophic microbial ecosystems
Author Disclosure Statement
No competing financial interests exist.
References
- Abdel-Wahab A. and McBride E.F. (2001) Origin of giant calcite-cemented concretions, Temple Member, Qasr El Sagha Formation (Eocene), Faiyum Depression, Egypt. Journal of Sedimentary Research 71:70–81 [Google Scholar]
- Abramov O. and Mojzsis S.J. (2009) Microbial habitability of the Hadean Earth during the Late Heavy Bombardment. Nature 459:419–422 [DOI] [PubMed] [Google Scholar]
- Acuña M.H., Connerney J.E.P., Ness N.F., Lin R.P., Mitchell D., Carlson C.W., McFadden J., Anderson K.A., Rème H., Mazelle C., Vignes D., Wasilewski P., and Cloutier P. (1999) Global distribution of crustal magnetization discovered by the Mars Global Surveyor MAG/ER experiment. Science 284:790–793 [DOI] [PubMed] [Google Scholar]
- Allwood A.C., Grotzinger J.P., Knoll A.H., Burch I.W., Anderson M.S., Coleman M.L., and Kani I. (2009) Controls on development and diversity of Early Archean stromatolites. Proc Natl Acad Sci USA 106:9548–9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood A.C., Rosing M.T., Flannery D.T., Hurowitz J.A., and Heirwegh C.M. (2018) Reassessing evidence of life in 3,700-million-year-old rocks of Greenland. Nature 563:241–244 [DOI] [PubMed] [Google Scholar]
- Al-Shaieb Z., Cairns J., and Puckette J. (1994) Hydrocarbon induced diagenetic aureoles: indicators of deeper leaky reservoirs. Association of Petroleum Geochemical Explorationists Bulletin 10:24–48 [Google Scholar]
- Aoyama S. and Ueno Y. (2018) Multiple sulfur isotope constraints on microbial sulfate reduction below an Archean seafloor hydrothermal system. Geobiology 16:107–120 [DOI] [PubMed] [Google Scholar]
- Arvidson R.E., Squyres S.W., Bell J.F., III, Catalano J.G., Clark B.C., Crumpler L.S., de Souza P.A., Jr, Fairén A.G., Farrand W.H., Fox V.K., Gellert R., Ghosh A., Golombek M.P., Grotzinger J.P., Guinness E.A., Herkenhoff K.E., Jolliff B.L., Knoll A.H., Li R., McLennan S.M., Ming D.W., Mittlefehldt D.W., Moore J.M., Morris R.V., Murchie S.L., Parker T.J., Paulsen G., Rice J.W., Ruff S.W., Smith M.D., and Wolff M.J. (2014) Ancient aqueous environments at Endeavour Crater, Mars. Science 343, doi: 10.1126/science.1248097 [DOI] [PubMed] [Google Scholar]
- Baker B.J., Moser D.P., MacGregor B.J., Fishbain S., Wagner M., Fry N.K., Jackson B., Speolstra N., Loos S., Takai K., Sherwood-Lollar B., Fredrickson J.K., Balkwill D., Onstott T.C., Wimpee C.F., and Stahl D.A. (2003) Related assemblages of sulphate-reducing bacteria associated with ultradeep gold mines of South Africa and deep basalt aquifers of Washington State. Environ Microbiol 5:1168–1191 [DOI] [PubMed] [Google Scholar]
- Balkwill D.L. and Ghiorse W. (1985) Characterization of subsurface bacteria associated with two shallow aquifers in Oklahoma. Appl Environ Microbiol 50:580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N., Furnes H., Muehlenbachs K., Staudigel H., and de Wit M. (2006) Preservation of ca. 3.4–3.5 Ga microbial biomarkers in pillow lavas and hyaloclastites from the Barberton Greenstone Belt, South Africa. Earth Planet Sci Lett 241:707–722 [Google Scholar]
- Banerjee N., Simonetti A., Furnes H., Muehlenbachs K., Staudigel H., Heaman L., and Van Kranendonk M.J. (2007) Direct dating of Archean microbial ichnofossils. Geology 35:487–490 [Google Scholar]
- Baragiola R.A., Dukes C.A., and Hedges D. (2011) Ozone generation by rock fracture: earthquake early warning? Appl Phys Lett 99, doi: 10.1063/1.3660763 [DOI] [Google Scholar]
- Barton H.A. and Northup D.E. (2007) Geomicrobiology in cave environments: past, current and future perspectives. Journal of Cave and Karst Studies 69:163–178 [Google Scholar]
- Barton H.A., Giarrizzo J.G., Suarez P., Robertson C.E., Broering M.J., Banks E.D., Vaishampayan P.A., and Venkateswaran K. (2014) Microbial diversity in a Venezuelan orthoquartzite cave is dominated by the Chloroflexi (Class Ktedonobacterales) and Thaumarchaeota Group I.1c. Front Microbiol 5, doi: 10.3389/fmicb.2014.00615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E.A., Boehnke P., Harrison T.M., and Mao W.L. (2015) Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc Natl Acad Sci USA 112:14518–14521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson S., Ivarsson M., Astolfo A., Belivanova V., Broman C., Marone F., and Stampanoni M. (2014) Deep-biosphere consortium of fungi and prokaryotes in Eocene sub-seafloor basalts. Geobiology 12:489–496 [DOI] [PubMed] [Google Scholar]
- Bengtson S., Rasmussen B., Ivarsson M., Muhling J., Broman C., Marone F., Stampanoni M., and Bekker A. (2017) Fungus-like mycelial fossils in 2.4-billion-year-old vesicular basalt. Nat Ecol Evol 1, 0141. [DOI] [PubMed] [Google Scholar]
- Bennett B., Adams J.J., Gray N.D., Sherry A., Oldenburg T.B.P., Huang H., Larter S.R., and Head I.M. (2013) The controls on the composition of biodegraded oils in the deep subsurface—Part 3. The impact of microorganism distribution on petroleum geochemical gradients in biodegraded petroleum reservoirs. Org Geochem 56:94–105 [Google Scholar]
- Betts H.C., Puttick M.N., Clark J.W., Williams T.A., Donoghue P.C.J., and Pisani D. (2018) Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin. Nat Ecol Evol 2:1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberg M., Lamminmäki T., and Itävaara M. (2016) Microbial communities and their predicted metabolic characteristics in deep fracture groundwaters of the crystalline bedrock at Olkiluoto, Finland. Biogeosci Discuss 13:6031–6047 [Google Scholar]
- Borgonie G., García-Moyano A., Litthauer D., Bert W., Bester A., van Heerden E., and Onstott T.C. (2011) Nematoda from the terrestrial deep subsurface of South Africa. Nature 474:79–82 [DOI] [PubMed] [Google Scholar]
- Boyd E.S., Costas A.M.G., Hamilton T.L., Mus F., and Peters J.W. (2015) Evolution of molybdenum nitrogenase during the transition from anaerobic to aerobic metabolism. J Bacteriol 197:1690–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier M.D., Green O.R., Jephcoat A.P., Kleppe A.K., Van Kranendonk M.J., Lindsay J.F., Steele A., and Grassineauk N.V. (2002) Questioning the evidence for Earth's oldest fossils. Nature 416:76–81 [DOI] [PubMed] [Google Scholar]
- Brasier M.D., Green O.R., Lindsay J.F., McLoughlin N., Steele A., and Stoakes C. (2005) Critical testing of Earth's oldest putative fossil assemblage from the ∼3.5 Ga Apex Chert, Chinaman Creek, Western Australia. Precambrian Res 140:55–102 [Google Scholar]
- Brasier M.D., McLoughlin N., Green O., and Wacey D. (2006) A fresh look at the fossil evidence for early Archaean cellular life. Philos Trans R Soc Lond B Biol Sci 361:887–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier M.D., Antcliffe J., Saunders M., and Wacey D. (2015) Changing the picture of Earth's earliest fossils (3.5–1.9 Ga) with new approaches and new discoveries. Proc Natl Acad Sci USA 112:4859–4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuker A., Köweker G., Blazejak A., and Schippers A. (2011) The deep biosphere in terrestrial sediments in the Chesapeake Bay area, Virginia, USA. Front Microbiol 2:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow T.F., Bish D.L., Vaniman D.T., Morris R.V., Blake D.F., Grotzinger J.P., Rampe E.B., Crisp J.A., Achilles C.N., Ming D.W., Ehlmann B.L., King P.L., Bridges J.C., Eigenbrode J.L., Sumner D.Y., Chipera S.J., Moorokian J.M., Treiman A.H., Morrison S.M., Downs R.T., Farmer J.D., Des Marais D., Sarrazin P., Floyd M.M., Mischna M.A., and McAdam A.C. (2015) The origin and implications of clay minerals from Yellowknife Bay, Gale Crater, Mars. Am Mineral 100:824–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow T.F., Haberle R.M., Blake D.F., Des Marais D.J., Eigenbrode J.L., Fairén A.G., Grotzinger J.P., Stack K.M., Mischna M.A., Rampe E.B., Siebach K.L., Sutter B., Vaniman D.T., and Vasavada A.R. (2017) Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars. Proc Natl Acad Sci USA 114:2166–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocks J.J. and Summons R.E. (2005) Sedimentary hydrocarbons, biomarkers for early life. In Biogeochemistry, edited by Holland H.D., Schlesinger W.H., and Turekians K.K., Elsevier Ltd., Oxford, UK, pp 64–103 [Google Scholar]
- Brocks J.J., Logan G.A., Buick R., and Summons R.E. (1999) Archean molecular fossils and the early rise of Eukaryotes. Science 285:1033–1036 [DOI] [PubMed] [Google Scholar]
- Buick R., Dunlop J.S.R., and Groves D.I. (1981) Stromatolite recognition in ancient rocks: an appraisal of irregularly laminated structures in an early Archean chert-barite unit from North Pole, Western Australia. Alcheringa 5:161–181 [Google Scholar]
- Cai C., Leu A.O., Xie G.-J., Guo J., Feng Y., Zhao J.-X., Tyson G.W., Yuan Z., and Hu S. (2018) A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J 12:1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M.H. and Head J.W. (2015) Martian surface/near-surface water inventory: sources, sinks, and changes with time. Geophys Res Lett 42, doi: 10.1002/2014GL062464 [DOI] [Google Scholar]
- Carter J., Poulet F., Bibring J.-P., Mangold N., and Murchie S. (2013) Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: updated global view. J Geophys Res Planets 118, doi: 10.1029/2012JE004145 [DOI] [Google Scholar]
- Castelle C.J., Hug L.A., Wrighton K.C., Thomas B.C., Williams K.H., Wu D., Tringe S.G., Singer S.W., Eisen J.A., and Banfield J.F. (2013) Extraordinary phylogenetic diversity and metabolic versatility in aquifer sediment. Nat Commun 4, doi: 10.1038/ncomms3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalazzi B., Westall F., Cady S.L., Barbieri R., and Foucher F. (2011) Potential fossil endoliths in vesicular pillow basalt, Coral Patch Seamount, eastern North Atlantic Ocean. Astrobiology 11:619–632 [DOI] [PubMed] [Google Scholar]
- Chapelle F.R., O'Neill K., Bradley P.M., Methe B.A., Clufe S.A., Knobel L.I., and Lovley D.R. (2002) A hydrogen-based subsurface microbial community dominated by methanogens. Nature 415:312–314 [DOI] [PubMed] [Google Scholar]
- Chen Y., Li X.-K., Si J., Wu G.-J., Tian L.-D., and Xiang S.-R. (2016) Changes of the bacterial abundance and communities in shallow ice cores from Dunde and Muztagata Glaciers, Western China. Front Microbiol 7:1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivian D., Alm E., Brodie E., Culley D., Dehal P., DeSantis T., Gihring T., Lapidus A., Lin L.-H., Lowry S., Moser D., Richardson P., Southam G., Wanger G., Pratt L., Andersen G., Hazen T., Brockman F., Arkin A., and Onstott T. (2008) Environmental genomics reveals a single species ecosystem deep within the Earth. Science 322:275–278 [DOI] [PubMed] [Google Scholar]
- Clifford S.M. (1993) A model for the hydrologic and climatic behavior of water on Mars. J Geophys Res 98:10973–11016 [Google Scholar]
- Clifford S.M. and Parker T.J. (2001) The evolution of the martian hydrosphere: implications for the fate of a primordial ocean and the current state of the Northern Plains. Icarus 154:40–79 [Google Scholar]
- Clifford S.M., Lasue J., Heggy E., Boisson J., McGovern P., and Max M.D. (2010) Depth of the martian cryosphere: revised estimates and implications for the existence and detection of subpermafrost groundwater. J Geophys Res 115, doi: 10.1029/2009JE003462 [DOI] [Google Scholar]
- Cockell C.S. (2014a) The subsurface habitability of terrestrial rocky planets: Mars. In Microbial Life of the Deep Biosphere, edited by Kallmeyer J. and Wagners D., DeGruyter, Berlin, pp 225–260 [Google Scholar]
- Cockell C.S. (2014b) Trajectories of martian habitability. Astrobiology 14:182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell C.S. and Barlow N. (2002) Impact excavation and the search for subsurface life on Mars. Icarus 155:340–349 [Google Scholar]
- Cockell C.S., Voytek M.A., Gronstal A.L., Finster K., Kirshtein J.D., Howard K., Reitner J., Gohn G.S., Sanford W.E., Horton J.W., Jr, Kallmeyer J., Kelly L., and Powars D.S. (2012) Impact disruption and recovery of the deep subsurface biosphere. Astrobiology 12:231–246 [DOI] [PubMed] [Google Scholar]
- Coleman M.L. (1985) Geochemistry of diagenetic non-silicate minerals: kinetic considerations. Philos Trans A Math Phys Eng Sci 315:39–56 [Google Scholar]
- Coleman M.L. (1993) Microbial processes: controls on the shape and composition of carbonate concretions. Mar Geol 113:127–140 [Google Scholar]
- Coleman M.L. and Raiswell R. (1995) Source of carbonate and origin of zonation in pyritiferous carbonate concretions: evalution of a dynamic model. Am J Sci 295:282–308 [Google Scholar]
- Coleman M.L., Fleet A., and Donson P. (1982) Preliminary studies of manganese-rich carbonate nodules from Leg 68, Site 503, eastern equatorial Pacific. Initial Reports of the Deep Sea Drilling Program 68:481–489 [Google Scholar]
- Coleman M.L., Hedrick D.B., Lovley D.R., White D.C., and Pye K. (1993) Reduction of Fe(III) in sediments by sulphate reducing bacteria. Nature 361:436–438 [Google Scholar]
- Colwell F.S. and D'Hondt S. (2013) Nature and extent of the deep biosphere. In Carbon in Earth, edited by Hazen R.H., Jones A.P., and Barosss J.A., Mineralogical Society of America and Geochemical Society, Chantilly, VA, pp 547–566 [Google Scholar]
- Connon S.A., Lester E.D., Shafaat H.S., Obenhuber D.C., and Ponce A. (2007) Bacterial diversity in hyperarid Atacama Desert soils. J Geophys Res 112 doi: 10.1029/2006JG000311 [DOI] [Google Scholar]
- Curtis C.D. (1977) Sedimentary geochemistry: environments and processes dominated by involvement of an aqueous phase. Philos Trans A Math Phys Eng Sci 286:353–372 [Google Scholar]
- Curtis C.D., Coleman M.L., and Love L.G. (1986) Pore water evolution during sediment burial from isotopic and mineral chemistry of calcite, dolomite and siderite concretions. Geochim Cosmochim Acta 50:2321–2334 [Google Scholar]
- Dahle H., Garshol F., Madsen M., and Birkeland N.-K. (2008) Microbial community structure analysis of produced water from a high-temperature North Sea oil-field. Antonie van Leeuwenhoek 93:37–49 [DOI] [PubMed] [Google Scholar]
- Dattagupta S., Schaperdoth I., Montanari A., Mariani S., Kita N., Valley J.W., and Macalady J.L. (2009) A novel symbiosis between chemoautotrophic bacteria and a freshwater cave amphipod. ISME J 3:935–943 [DOI] [PubMed] [Google Scholar]
- David L.A. and Alm E.J. (2011) Rapid evolutionary innovation during an Archaean genetic expansion. Nature 469:93–96 [DOI] [PubMed] [Google Scholar]
- Deamer D. and Damer B. (2017) Can life begin on Enceladus? A perspective from hydrothermal chemistry. Astrobiology 17:834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean S., Minshull T., Whitmarsh R., and Louden K. (2000) Deep structure of the ocean continent transition in the southern Iberia Abyssal Plain from seismic refraction profiles: the IAM-9 transect at 40°20′N. J Geophys Res Solid Earth 105:5859–5885 [Google Scholar]
- Des Marais D.J. (2010) Exploring Mars for evidence of habitable environments and life. Proc Am Philos Soc 154:402–421 [Google Scholar]
- Dismukes G.C., Klimov V.V., Baranov S.V., Kozlov Y.N., DasGupta J., and Tyryshkin A. (2000) The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc Am Philos Soc 98:2170–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M.S., Papineau D., Grenne T., Slack J.F., Rittner M., Pirajno F., O'Neil J., and Little C.T.S. (2017) Evidence for early life in Earth's oldest hydrothermal vent precipitates. Nature 543:60–64 [DOI] [PubMed] [Google Scholar]
- Dong J., Zhang S., Jiang G., Zhao Q., Li H., Shi X., and Liu J. (2008) Early diagenetic growth of carbonate concretions in the upper Doushantuo Formation in South China and their significance for the assessment of hydrocarbon source rock. Science in China Series D-Earth Science 51:1330–1339 [Google Scholar]
- Drake H., Åström M.E., Heim C., Broman C., Åström J., Whitehouse M., Ivarsson M., Siljeström S., and Sjövall P. (2015a) Extreme 13C depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat Commun 6, doi: 10.1038/ncomms8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H., Tullborg E.-L., Whitehouse M., Sandberg B., Blomfeldt T., and Åström M.E. (2015b) Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in deep groundwater systems. Geochim Cosmochim Acta 161:1–18 [Google Scholar]
- Drake H., Heim C., Roberts N.M.W., Zack T., Tillberg M., Bromane C., Ivarsson M., Whitehouse M.J., and Åströma M.E. (2017a) Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet Sci Lett 470:108–118 [Google Scholar]
- Drake H., Ivarsson M., Bengtson S., Heim C., Siljeström S., Whitehouse M.J., Broman C., Belivanova V., and Åström M.E. (2017b) Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat Commun 8, doi: 10.1038/s41467-017-00094-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H., Whitehouse M.J., Heim C., Reiners P.W., Tillberg M., Hogmalm K.J., Dopson M., Broman C., and Åström M.E. (2018) Unprecedented 34S-enrichment of pyrite formed following microbial sulfate reduction in fractured crystalline rocks. Geobiology 16:556–574 [DOI] [PubMed] [Google Scholar]
- Dutta A., Gupta S.D., Gupta A., Sarkar J., Roy S., Mukherjee A., and Sar P. (2018) Exploration of deep terrestrial subsurface microbiome in Late Cretaceous Deccan traps and underlying Archean basement, India. Sci Rep 8, doi: 10.1038/s41598-018-35940-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzaugis M., Spivack A.J., and D'Hondt S. (2018) Radiolytic H2 production in martian environments. Astrobiology 18:1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.R., Onstott T.C., Miller J.M., Wiggins J.B., Wang W., Lee C.K., Cary S.C., Pointing S.B., and Laua M.C.Y. (2017) Draft genome sequence of uncultured Upland Soil Cluster Gammaproteobacteria gives molecular insights into high-affinity methanotrophy. Genome Announc 5, doi: 10.1128/genomeA.00047-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.S. and Ehlmann B.L. (2015) Carbon sequestration on Mars. Geology 43:863–866 [Google Scholar]
- Edwards K.J., Becker K., and Colwell F. (2012) The deep, dark energy biosphere: intraterrestrial life on Earth. Annu Rev Earth Planet Sci 40:551–568 [Google Scholar]
- Ehlmann B.L. and Edwards C.S. (2014) Mineralogy of the martian surface. Annu Rev Earth Planet Sci 42 doi: 10.1146/annurev-earth-060313-055024 [DOI] [Google Scholar]
- Ehlmann B.L., Mustard J.F., Swayze G.A., Clark R.N., Bishop J.L., Poulet F., Des Marais D.J., Roach L.H., Milliken R.E., Wray J.J., Barnouin-Jha O., and Murchie S.L. (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: geologic context near Nili Fossae and implications for aqueous alteration. J Geophys Res Planets, 114, doi: 10.1029/2009JE003339 [DOI] [Google Scholar]
- Ehlmann B.L., Mustard J.F., Murchie S.L., Bibring J.P., Meunier A., Fraeman A.A., and Langevin Y. (2011) Subsurface water and clay mineral formation during the early history of Mars. Nature 479:53–60 [DOI] [PubMed] [Google Scholar]
- Ehlmann B.L., Anderson F.S., Andrews-Hanna J., Catling D.C., Christensen P.R., Cohen B.A., Dressing C.D., Edwards C.S., Elkins-Tanton L.T., Farley K.A., Fassett C.I., Fischer W.W., Fraeman A.A., Golombek M.P., Hamilton V.E., Hayes A.G., Herd C.D.K., Horgan B., Hu R., Jakosky B.M., Johnson J.R., Kasting J.F., Kerber L., Kinch K.M., Kite E.S., Knutson H.A., Lunine J.I., Mahaffy P.R., Mangold N., McCubbin F.M., Mustard J.F., Niles P.B., Quantin-Nataf C., Rice M.S., Stack K.M., Stevenson D.J., Stewart S.T., Toplis M.J., Usui T., Weiss B.P., Werner S.C., Wordsworth R.D., Wray J.J., Yingst R.A., Yung Y.L., and Zahnle K.J. (2016a) The sustainability of habitability on terrestrial planets: insights, questions, and needed measurements from Mars for understanding the evolution of Earth-like worlds. J Geophys Res Planets 121, doi: 10.1002/2016JE005134 [DOI] [Google Scholar]
- Ehlmann B.L., Swayze G.A., Milliken R.E., Mustard J.F., Clark R.N., Murchie S.L., Breit G.N., Wray J.J., Gondet B., Poulet F., Carter J., Calvin W.M., Benzel W.M., and Seelos K.D. (2016b) Discovery of alunite in Cross Crater, Terra Sirenum, Mars: evidence for acidic, sulfurous waters. Am Mineral 101:1527–1542 [Google Scholar]
- Eickmann B., Bach W., Kiel S., Reitner J., and Peckmann J. (2009) Evidence for cryptoendolithlc life in Devonian pillow basalts of Variscan orogens, Germany. Palaeogeogr Palaeoclimatol Palaeoecol 283:120–125 [Google Scholar]
- Eigenbrode J.L. (2008) Fossil lipids for life-detection: a case study from the early Earth record. Space Sci Rev 135:161–185 [Google Scholar]
- Eigenbrode J.L., Summons R.E., Steele A., Freissinet C., Millan M., Navarro-González R., Sutter B., McAdam A.C., Franz H.B., Glavin D.P., Archer P.D., Jr, Mahaffy P.R., Conrad P.G., Hurowitz J.A., Grotzinger J.P., Gupta S., Ming D.W., Sumner D.Y., Szopa C., Malespin C., Buch A., and Coll P. (2018) Organic matter preserved in 3-billion-year-old mudstones at Gale Crater, Mars. Science 360:1096–1101 [DOI] [PubMed] [Google Scholar]
- Elliott W.C., Edenfield A.M., Wampler J.M., Matisoff G., and Long P.E. (1999) The kinetics of the smectite to illite transformation in Cretaceous bentonites, Cerro Negro, New Mexico. Clays Clay Miner 47:286–296 [Google Scholar]
- Engelhardt T., Kallmeyer J., Cypionka H., and Engelen B. (2014) High virus-to-cell ratios indicate ongoing production of viruses in deep subsurface sediments. ISME J 8:1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettwig K., Butler M., Le Paslier D., Pelletier E., Mangenot S., Kuypers M., Schreiber F., Dutilh B., Zedelius J., de Beer D., Gloerich J., Wessels H., van Alen T., Luesken F., Wu M., van de Pas-Schoonen K., Op den Camp H., Janssen-Megens E., Francoijs K., Stunnenberg H., Weissenbach J., Jetten M., and Strous M. (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548 [DOI] [PubMed] [Google Scholar]
- Evans P.N., Parks D.H., Chadwick G.L., Robbins S.J., Orphan V.J., Golding S.D., and Tyson G.W. (2015) Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350:434–438 [DOI] [PubMed] [Google Scholar]
- Farley K.A., Malespin C., Mahaffy P., Grotzinger J.P., Vasconcelos P.M., Milliken R.E., Malin M., Edgett K.S., Pavlov A.A., Hurowitz J.A., Grant J.A., Miller H.B., Arvidson R., Beegle L., Calef F., Conrad P.G., Dietrich W.E., Eigenbrode J., Gellert R., Gupta S., Hamilton V., Hassler D.M., Lewis K.W., McLennan S.M., Ming D., Navarro-González R., Schwenzer S.P., Steele A., Stolper E.M., Sumner D.Y., Vaniman D., Vasavada A., Williford K., Wimmer-Schweingruber R.F., and the MSL Science Team (2014) In situ radiometric and exposure age dating of the martian surface. Science 343, doi: 10.1126/science.1247166 [DOI] [PubMed] [Google Scholar]
- Fassett C.I. and Head J.W. (2008) Valley network-fed, open-basin lakes on Mars: distribution and implications for Noachian surface and subsurface hydrology. Icarus 198:37–56 [Google Scholar]
- Fassett C.I. and Head J.W. (2011) Sequence and timing of conditions on early Mars. Icarus 211:1204–1214 [Google Scholar]
- Federle T.W., Dobbins D.C., Thornton-Manning J.R., and Jones D.D. (1986) Microbial biomass, activity, and community structure in subsurface soils. Ground Water 24:365–374 [Google Scholar]
- Fisk M.R. and Giovannoni S.J. (1999) Sources of nutrients and energy for a deep biosphere on Mars. J Geophys Res 104:11805–11815 [Google Scholar]
- Fisk M.R. and McLoughlin N. (2013) Atlas of alteration textures in volcanic glass from the ocean basins. Geosphere 9:317–341 [Google Scholar]
- Fisk M.R., Giovannoni S.J., and Thorseth I.H. (1998) Alteration of oceanic volcanic glass: textural evidence of microbial activity. Science 281:978–980 [DOI] [PubMed] [Google Scholar]
- Fisk M.R., Storrie-Lombardi M.C., Douglas S., Popa R., McDonald G., and Di Meo-Savoie C. (2003) Evidence of biological activity in Hawaiian subsurface basalts. Geochem Geophys Geosyst 4, doi: 10.1029/2002GC000387 [DOI] [Google Scholar]
- Formolo M.J., Lyons T.W., Zhang C., Kelley C., Sassen R., Horita J., and Cole D.R. (2004) Quantifying carbon sources in the formation of authigenic carbonates at gas hydrate sites in the Gulf of Mexico. Chem Geol 205:253–264 [Google Scholar]
- Fraser C.I., Connell L., Lee C.K., and Cary S.C. (2017) Evidence of plant and animal communities at exposed and subglacial (cave) geothermal sites in Antarctica. Polar Biol 41:417–421 [Google Scholar]
- French K.L., Hallmann C., Hope J.M., Schoon P.L., Zumberge J.A., Hoshino Y., Peters C.A., George S.C., Love G.D., Brocks J.J., Buick R., and Summons R.E. (2015) Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc Natl Acad Sci USA 112:5915–5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann E.I. (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053 [DOI] [PubMed] [Google Scholar]
- Froelich P.N., Klinkhammer G.P., Bender M.L., Leudtke N.A., Heath G.R., Cullen D., Dauphin P., Hammond D., Hartman B., and Maynard V. (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090 [Google Scholar]
- Frydenvang J., Gasda P.J., Hurowitz J.A., Grotzinger J.P., Wiens R.C., Newsom H.E., Edgett K.S., Watkins J., Bridges J.C., Maurice S., Fisk M.R., Johnson J.R., Rapin W., Stein N.T., Clegg S.M., Schwenzer S.P., Bedford C.C., Edwards P., Mangold N., Cousin A., Anderson R.B., Payré V., Vaniman D., Blake D.F., Lanza N.L., Gupta S., Beek J.V., Sautter V., Meslin P.-Y., Rice M., Milliken R., Gellert R., Thompson L., Clark B.C., Sumner D.Y., Fraeman A.A., Kinch K.M., Madsen M.B., Mitrofanov I.G., Jun I., Calef F., and Vasavada A.R. (2017) Diagenetic silica enrichment and late-stage groundwater activity in Gale Crater, Mars. Geophys Res Lett 44, doi: 10.1002/2017GL073323 [DOI] [Google Scholar]
- Furnes H., Thorseth I.H., Tumyr O., Torsvik T., and Fisk M.R. (1996) Microbial activity in the alteration of glass from pillow basalts from Hole 896A. In Proceedings of the Ocean Drilling Program, Scientific Results, edited by Alt J.J., Kinoshita H., Stokking L.B., and Michaels P.J., Ocean Drilling Program, College Station, Texas, pp 191–206 [Google Scholar]
- Furnes H., Muehlenbachs K., Tumyr O., Torsvik T., and Xenophontos C. (2001) Biogenic alteration of volcanic glass from the Troodos ophiolite, Cyprus. J Geol Soc London 158:75–84 [Google Scholar]
- Furnes H., Muehlenbachs K., Torsvik T., Tumyr O., and Shi L. (2002) Bio-signatures in metabasaltic glass of a Caledonian ophiolite, West Norway. Geology Magazine 139:601–608 [Google Scholar]
- Furnes H., Banerjee N.R., Muehlenbachs K., Staudigel H., and de Wit M. (2004) Early life recorded in Archean pillow lavas. Science 304:578–581 [DOI] [PubMed] [Google Scholar]
- Furnes H., Banerjee N.R., Muehlenbachs K., and Kontinen A. (2005) Preservation of biosignatures in metaglassy volcanic rocks from the Jormua ophiolite complex, Finland. Precambrian Res 136:125–137 [Google Scholar]
- Furnes H., McLoughlin N., Muehlenbachs K., Banerjee N., Staudigel H., Dilek Y., deWit M., Kranendonk M.V., and Schiffman P. (2008) Oceanic pillow lavas and hyaloclastites as habitats for microbial life through time—a review. In Links Between Geological Processes, Microbial Activities & Evolution of Life, edited by Dilek Y., Furnes H., and Muehlenbachss K., Springer Science+Business Media, Dordrecht, the Netherlands, pp 1–68 [Google Scholar]
- Gaidos E.J., Nealson K.H., and Kirschvink J.L. (1999) Life in ice-covered oceans. Science 284:1631–1632 [DOI] [PubMed] [Google Scholar]
- García-Ruiz J.M., Hyde S.T., Carnerup A.M., Christy A.G., Van Kranendonk M.J., and Welham N.J. (2003) Self-assembled silica-carbonate structures and detection of ancient microfossils. Science 302:1194–1197 [DOI] [PubMed] [Google Scholar]
- Gilichinsky D. and Rivkina E. (2011) Permafrost microbiology. In Encyclopedia of Geobiology, edited by Reitner J. and Thiels V., Springer, Dordrecht, the Netherlands, pp 726–732 [Google Scholar]
- Goudge T.A., Fassett C.I., Head J.W., Mustard J.F., and Aureli K.L. (2016) Insights into surface runoff on early Mars from paleolake basin morphology and stratigraphy. Geology 44:419–422 [Google Scholar]
- Greinert J., Bohrmann G., and Suess E. (2001) Gas hydrate-associated carbonates and methane-venting at Hydrate Ridge: classification, distribution and origin of authigenic lithologies. In Natural Gas Hydrates: Occurrence, Distribution, and Detection, Vol. 124, Geophysical Monograph Series, American Geophysical Union, Washington, DC, pp 99–114 [Google Scholar]
- Grimm R.E., Harrison K.P., Stillman D.E., and Kirchoff M.R. (2017) On the secular retention of ground water and ice on Mars. J Geophys Res Planets 122:94–109 [Google Scholar]
- Grosch E.G. and McLoughlin N. (2014) Reassessing the biogenicity of Earth's oldest trace fossil with implications for biosignatures in the search for early life. Proc Natl Acad Sci USA 111:8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch E.G., McLoughlin N., Lanari P., Erambert M., and Vidal O. (2014) Microscale mapping of alteration conditions and potential biosignatures in basaltic-ultramafic rocks on early Earth and beyond. Astrobiology 14:216–228 [DOI] [PubMed] [Google Scholar]
- Grotzinger J.P., Arvidson R.E., Bell J.F., Calvin W., III, Clark B.C., Fike D.A., Golombek M., Greeley R., Haldemann A.F.C., Herkenhoff K.E., Joliff B.L., Knoll A.H., Malin M.C., McLennan S.M., Parker T., Soderblom L.A., Sohl-Dickstein J.N., Squyres S.W., Tosca N.J., and Watters W.A. (2005) Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns Formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:11–72 [Google Scholar]
- Grotzinger J., Sumner D.Y., Kah L.C., Stack K., Gupta S., Edgar L., Rubin D., Lewis K., Schieber J., Mangold N., Milliken R., Conrad P.G., Des Marais D., Farmer J., Siebach K., Calef F., Hurowitz J., III, McLennan S.M., Ming D., Vaniman D., Crisp J., Vasavada A., Edgett K.S., Malin M., Blake D., Gellert R., Mahaffy P., Wiens R.C., Maurice S., Grant J.A., Wilson S., Anderson R.C., Beegle L., Arvidson R., Hallet B., Sletten R.S., Rice M., Bell J., Griffes J., III, Ehlmann B., Anderson R.B., Bristow T.F., Dietrich W.E., Dromart G., Eigenbrode J., Fraeman A., Hardgrove C., Herkenhoff K., Jandura L., Kocurek G., Lee S., Leshin L.A., Leveille R., Limonadi D., Maki J., McCloskey S., Meyer M., Minitti M., Newsom H., Oehler D., Okon A., Palucis M., Parker T., Rowland S., Schmidt M., Squyres S., Steele A., Stolper E., Summons R., Treiman A., Williams R., andYingst A., MSL Science Team. (2014) A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1242777 [DOI] [PubMed] [Google Scholar]
- Grotzinger J.P., Gupta S., Malin M.C., Rubin D.M., Schieber J., Siebach K., Sumner D.Y., Stack., K.M., Vasavada A.R., Arvidson R.E., Calef F., III., Edgar L., Fischer W.F., Grant J.A., Griffes J., Kah L.C., Lamb M.P., Lewis K.W., Mangold N., Minitti M.E., Palucis M., Rice M., Williams R.M., Yingst R.A., Blake D., Blaney D., Conrad P., Crisp J., Dietrich W.E., Dromart G., Edgett K.S., Ewing R.C., Gellert R., Hurowitz J.A., Kocurek G., Mahaffy P., McBride M.J., McLennan S.M., Mischna M., Ming D., Milliken R., Newsom H., Oehler D., Parker T.J., Vaniman D., Wiens R.C., and Wilson S.A. (2015) Deposition, exhumation, and paleoclimate of an ancient lake deposit Gale Crater, Mars. Science 350, doi: 10.1126/science.aac7575 [DOI] [PubMed] [Google Scholar]
- Guy B.M., Ono S., Gutzmer J., Kaufman A.J., Lin Y., Fogel M.L., and Beukes N.J. (2012) A multiple sulfur and organic carbon isotope record from non-conglomeratic sedimentary rocks of the Mesoarchean Witwatersrand Supergroup, South Africa. Precambrian Res 216–219:208–231 [Google Scholar]
- Halbach P., Pracejus B., and Märten A. (1993) Geology and mineralogy of massive sulfide ores from the Central Okinawa Trough, Japan. Econ Geol 88:2210–2225 [Google Scholar]
- Haldeman D.L. and Amy P.S. (1993) Bacterial heterogeneity in deep subsurface tunnels at Rainier Mesa, Nevada Test Site. Microb Ecol 25:183–194 [DOI] [PubMed] [Google Scholar]
- Haldeman D.L., Amy P.S., Ringelberg D., and White D.C. (1993) Characterization of the microbiology within a 21 m3 section of rock from the deep subsurface. Microb Ecol 26:145–159 [DOI] [PubMed] [Google Scholar]
- Halevy I. and Head J.W. (2014) Episodic warming of early Mars by punctuated volcanism. Nat Geosci 7:865–868 [Google Scholar]
- Hallbeck L. and Pedersen K. (2012) Culture-dependent comparison of microbial diversity in deep granitic groundwater from two sites considered for a Swedish final repository of spent nuclear fuel. FEMS Microbiol Ecol 81:66–77 [DOI] [PubMed] [Google Scholar]
- Hallsworth J.E., Yakimov M.M., Golyshin P.N., Gillion J.L.M., D'Auria G., de Lima Alves F., Cono V.L., Genovese M., McKew B.A., Hayes S.L., Harris G., Giuliano L., Timmis K.N., and McGenity T.J. (2007) Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol 9:801–813 [DOI] [PubMed] [Google Scholar]
- Hand K.P., Carlson R.W., and Chyba C.F. (2007) Energy, chemical disequilibrium, and geological constraints on Europa. Astrobiology 7:1006–1022 [DOI] [PubMed] [Google Scholar]
- Haroon M.F., Hu S., Shi Y., Imelfort M., Keller J., Hugenholtz P., Yuan Z., and Tyson G.W. (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570 [DOI] [PubMed] [Google Scholar]
- Harrison K.P. and Grimm R.E. (2009) Regionally compartmented groundwater flow on Mars. J Geophys Res 114, doi: 10.1029/2008JE003300 [DOI] [Google Scholar]
- Hassler D.M., Zeitlin C., Wimmer-Schweingruber R.F., Ehresmann B., Rafkin S., Eigenbrode J.L., Brinza D.E., Weigle G., Böttcher S., Böhm E., Burmeister S., Guo J., Köhler J., Martin C., Reitz G., Cucinotta F.A., Kim M.-H., Grinspoon D., Bullock M.A., Posner A., Gómez-Elvira J., Vasavada A., Grotzinger J.P., and the MSL Science Team (2014) Mars' surface radiation environment measured with the Mars Science Laboratory's Curiosity Rover. Science 343, doi: 10.1126/science.1244797 [DOI] [PubMed] [Google Scholar]
- Head I.M., Gray N.D., and Larter S.R. (2014) Life in the slow lane: biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front Microbiol 5, doi 10.3389/fmicb.2014.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberling C., Lowell R.P., Liu L., and Fisk M.R. (2010) Extent of the microbial biosphere in the oceanic crust. Geochem Geophys Geosyst 11, doi: 10.1029/2009GC002968 [DOI] [Google Scholar]
- Hebsgaard M., Phillips M., and Willerslev E. (2005) Geologically ancient DNA: fact or artefact? Trends Microbiol 13:212–220 [DOI] [PubMed] [Google Scholar]
- Hoehler T. (2004) Biological energy requirements as quantitative boundary conditions for life in the subsurface. Geobiology 2:205–215 [Google Scholar]
- Hoehler T.M. and Jørgensen B.B. (2013) Microbial life under extreme energy limitation. Nat Rev 11:83–94 [DOI] [PubMed] [Google Scholar]
- Hofmann B.A. (1990) Reduction spheroids from northern Switzerland: mineralogy, geochemistry and genetic models. Chem Geol 81:55–81 [Google Scholar]
- Hofmann B.A. (1991) Mineralogy and geochemistry of reduction spheroids in red beds. Mineral Petrol 44:107–124 [Google Scholar]
- Hofmann B.A. (2008) Morphological biosignatures from subsurface environments: recognition on planetary missions. Space Sci Rev 135:245–254 [Google Scholar]
- Hofmann B.A. and Farmer J.D. (2000) Filamentous fabrics in low-temperature mineral assemblages: are they fossil biomarkers? Implications for the search for a subsurface fossil record on the early Earth and Mars. Planet Space Sci 48:1077–1086 [Google Scholar]
- Holmkvist L., Ferdelman T.G., and Jørgensen B.B. (2011) A cryptic sulfur cycle driven by iron in the methane zone of marine sediment (Aarhus Bay, Denmark). Geochim Cosmochim Acta 75:3581–3599 [Google Scholar]
- Hu R., Kass D.M., Ehlmann B., and Yung Y.L. (2015) Tracing the fate of carbon and the atmospheric evolution of Mars. Nat Commun 6, doi: 10.1038/ncomms10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki F., Hinrichs K.-U., Kubo Y., Bowles M.W., Heuer V.B., Hong W.-L., Hoshino T., Ijiri A., Imachi H., Ito M., Kaneko M., Lever M.A., Lin Y.-S., Methé B.A., Morita S., Morono Y., Tanikawa W., Bihan M., Bowden S.A., Elvert M., Glombitza C., Gross D., Harrington G.J., Hori T., Li K., Limmer D., Liu C.-H., Murayama M., Ohkouchi N., Ono S., Park Y.-S., Phillips S.C., Prieto-Mollar X., Purkey M., Riedinger N., Sanada Y., Sauvage J., Snyder G., Susilawati R., Takano Y., Tasumi E., Terada T., Tomaru H., Trembath-Reichert E., Wang D.T., and Yamada Y. (2015) Exploring deep microbial life in coal-bearing sediment down to ∼2.5 km below the ocean floor. Science 349:420–424 [DOI] [PubMed] [Google Scholar]
- Irwin H. (1980) Early diagenetic carbonate precipitation and pore fluid migration in the Kimmeridge Clay of Dorset, England. Sedimentology 27:577–591 [Google Scholar]
- Irwin H., Curtis C., and Coleman M.L. (1977) Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 269:209–213 [Google Scholar]
- Ivarsson M. and Holm N.G. (2008) Microbial colonization of various habitable niches during alteration of oceanic crust. In Links Between Geological Processes, Microbial Activities and Evolution of Life, edited by Dilek Y., Furnes H., and Muehlenbachss K., Springer Publications, Dordrecht, the Netherlands, pp 69–111 [Google Scholar]
- Ivarsson M., Broman C., Lindblom S., and Holm N.G. (2009) Fluid inclusions as a tool to constrain the preservation conditions of sub-seafloor cryptoendoliths. Planet Space Sci 57:477–490 [Google Scholar]
- Ivarsson M., Bengtson S., Belivanova V., Stampanoni M., Marone F., and Tehler A. (2012) Fossilized fungi in subseafloor Eocene basalts. Geology 40:163–166 [Google Scholar]
- Ivarsson M., Broman C., Sturkell E., Ormö J., Siljeström S., van Zuilen M., and Bengtson S. (2013) Fungal colonization of an Ordovician impact-induced hydrothermal system. Sci Rep 3, doi: 10.1038/srep03487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson M., Peckmann J., Tehler A., Broman C., Bach W., Behrens K., Reitner J., Böttcher M.E., and Ivarsson L.N. (2015) Zygomycetes in vesicular basanites from Vesteris Seamount, Greenland Basin—a new type of cryptoendolithic fungi. PLoS One 10, doi: 10.1371/journal.pone.0133368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson M., Bach W., Broman C., Neubeck A., and Bengtson S. (2018) Fossilized life in subseafloor ultramafic rocks. Geomicrobiol J 35:460–467 [Google Scholar]
- Jaakkola S.T., Ravantti J.J., Oksanen H.M., and Bamford D.H. (2016) Buried alive: microbes from ancient halite. Trends Microbiol 24:148–159 [DOI] [PubMed] [Google Scholar]
- Jägevall S., Rabe L., and Pedersen K. (2011) Abundance and diversity of biofilms in natural and artificial aquifers of the Äspö Hard Rock Laboratory, Sweden. Microb Ecol 61:410–422 [DOI] [PubMed] [Google Scholar]
- Jakosky B.M. and Shock E.L. (1998) The biological potential of Mars, the early Earth, and Europa. J Geophys Res 103:19359–19364 [DOI] [PubMed] [Google Scholar]
- Jannasch H.W., Eimhjell K., Wirsen C.O., and Farmanfa A. (1971) Microbial degradation of organic matter in deep sea. Science 171:672–675 [DOI] [PubMed] [Google Scholar]
- Ji M., Greening C., Vanwonterghem I., Carere C.R., Bay S.K., Steen J.A., Montgomery K., Lines T., Beardall J., van Dorst J., Snape I., Stott M.B., Hugenholtz P., and Ferrari B.C. (2017) Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 552:400–403 [DOI] [PubMed] [Google Scholar]
- Johnson J.E., Webb S.M., Thomas K., Ono S., Kirschvink J.L., and Fischer W.W. (2013) Manganese-oxidizing photosynthesis before the rise of cyanobacteria. Proc Natl Acad Sci USA 110:11238–11243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.S., Pavlov A.A., and Mischna M.A. (2009) Fate of SO2 in the ancient martian atmosphere: implications for transient greenhouse warming. J Geophys Res 114, doi: 10.1029/2008JE003313 [DOI] [Google Scholar]
- Jones D.S., Schaperdoth I., and Macalady J.L. (2016) Biogeography of sulfur-oxidizing Acidithiobacillus populations in extremely acidic cave biofilms. ISME J 10:2879–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W.D. (1929) Experimental work on red bed bleaching. Am J Sci 18:65–70 [Google Scholar]
- Kelley D.S., Karson J.A., Früh-Green G.L., Yoerger D.R., Shank T.M., Butterfield D.A., Hayes J.M., Schrenk M.O., Olson E.J., Proskurowski G., Jakuba M., Bradley A., Larson B., Ludwig K., Glickson D., Buckman K., Bradley A.S., Brazelton W.J., Roe K., Elend M.J., Delacour A.L., Bernasconi S.M., Lilley M.D., Baross J.A., Summons R.E., and Sylva S.P. (2005) A serpentinite-hosted ecosystem: the Lost City Hydrothermal Field. Science 307:1428–1434 [DOI] [PubMed] [Google Scholar]
- Kieft T. and Phelps T. (1997) Life in the slow lane: activities of microorganisms in the subsurface. In The Microbiology of the Terrestrial Subsurface, edited by Haldeman D.L. and Amy P., CRC Press, Boca Raton, FL, pp 137–163 [Google Scholar]
- Kieft T.L., Walters C.C., Higgins M.B., Mennito A.S., Clewett C.F.M., Heuer V., Pullin M.J., Hendrickson S., van Heerden E., Sherwood Lollar B., Lau M.C.Y., and Onstott T.C. (2018) Dissolved organic matter compositions in 0.6–3.4 km deep fracture waters, Kaapvaal Craton, South Africa. Org Geochem 118:116–131 [Google Scholar]
- Kirk M.F., Wilson B.H., Marquart K.A., Zeglin L.H., Vinson D.S., and Flynn T.M. (2015) Solute concentrations influence microbial methanogenesis in coal-bearing strata of the Cherokee Basin, USA. Front Microbiol 6, doi: 10.3389/fmicb.2015.01287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita I., Matsuo S., and Wakita H. (1982) H2 generation by reaction between H2O and crushed rock: an experimental study on H2 degassing from the active fault zone. J Geophys Res Solid Earth 87:10789–10795 [Google Scholar]
- Kite E.S., Williams J.-P., Lucas A., and Aharonson O. (2014) Low palaeopressure of the martian atmosphere estimated from the size distribution of ancient craters. Nat Geosci 7:335–339 [Google Scholar]
- Kite E.S., Gao P., Goldblatt C., Mischna M.A., Mayer D.P., and Yung Y.L. (2017) Methane bursts as a trigger for intermittent lake-forming climates on post-Noachian Mars. Nat Geosci 10:737–740 [Google Scholar]
- Kits K.D., Klotz M.G., and Stein L.Y. (2015) Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ Microbiol 17:3219–3232 [DOI] [PubMed] [Google Scholar]
- Klaucke I., Masson D.G., Petersen C.J., Weinrebe W., and Ranero C.R. (2008) Multifrequency geoacoustic imaging of fluid escape structures offshore Costa Rica: implications for the quantification of seep processes. Geochem Geophys Geosyst 9, doi: 10.1029/2007GC001708 [DOI] [Google Scholar]
- Klein F., Humphris S.E., Guo W., Schubotz F., Schwarzenbach E.M., and Orsi W.D. (2015) Fluid mixing and the deep biosphere of a fossil Lost City–type hydrothermal system at the Iberia Margin. Proc Natl Acad Sci USA 112:12036–12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler J.M., Mancinelli R.L., and White M.R. (1989) Biological nitrogen fixation under primordial martian partial pressure of dinitrogen. Adv Space Res 9:173–176 [DOI] [PubMed] [Google Scholar]
- Knowles E., Wirth R., and Templeton A. (2012) A comparative analysis of potential biosignatures in basalt glass by FIB-TEM. Chem Geol 330–331:165–175 [Google Scholar]
- Kruber C., Thorseth I.H., and Pedersen R.B. (2008) Seafloor alteration of basaltic glass: textures, geochemistry, and endolithic microorganisms. Geochem Geophys Geosyst 9, doi: 10.1029/2008GC002119 [DOI] [Google Scholar]
- Krumholz L.R., McKinley J.P., Ulrich G.A., and Suflita J.M. (1997) Confined subsurface microbial communities in Cretaceous rock. Nature 386:64–66 [Google Scholar]
- Kyle J.E., Eydal H.S., Ferris F.G., and Pedersen K. (2008) Viruses in granitic groundwater from 69 to 450 m depth of the Äspö hard rock laboratory, Sweden. ISME J 2:571–574 [DOI] [PubMed] [Google Scholar]
- Labonté J., Field E., Lau M., Chivian D., van Heerden E., Wommack K.E., Kieft T.L., Onstott T.C., and Stepanauskas R. (2015) Single cell genomics indicates horizontal gene transfer and viral infections in a deep subsurface Firmicutes population. Front Microbiol 6, doi: 10.3389/fmicb.2015.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskar J., Levrard B., and Mustard J.F. (2002) Orbital forcing of the martian polar layered deposits. Nature 419:375–377 [DOI] [PubMed] [Google Scholar]
- Laskar J., Correia A.C.M., Gastineau M., Joutel F., Levrard B., and Robutel P. (2004) Long term evolution and chaotic diffusion of the insolation quantities of Mars. Icarus 170:343–364 [Google Scholar]
- Lau M.C.Y., Stackhouse B.T., Layton A.C., Chauhan A., Vishnivetskaya T.A., Chourey K., Mykytczuk N.C.S., Bennett P.C., Lamarche-Gagnon G., Burton N., Ronholm J., Pollard W.H., Omelon C.R., Medvigy D.M., Hettich R.L., Pfiffner S.M., Whyte L.G., and Onstott T.C. (2015) An active atmospheric methane sink in high Arctic mineral cryosols. ISME J 9:1880–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.C.Y., Kieft T.L., Kuloyo O., Linage B., van Heerden E., Lindsay M.R., Magnabosco C., Wang W., Wiggins J.B., Guo L., Perlman D.H., Kyin S., Shwe H.H., Harris R.L., Oh Y., Yi M.J., Purtschert R., Slater G.F., Ono S., Wei S., Li L., Sherwood Lollar B., and Onstott T.C. (2016a) Deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers. Proc Natl Acad Sci USA 113:E7927–E7936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.C.Y., Kieft T.L., Kuloyo O., Linage B., van Heerden E., Lindsay M.R., Magnabosco C., Wang W., Wiggins J.B., Guo L., Perlman D.H., Kyin S., Shwe H.H., Harris R.L., Oh Y., Yi M.J., Purtschert R., Slater G.F., Ono S., Wei S., Li L., Sherwood Lollar B., and Onstott T.C. (2016b) Deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers. Proc Natl Acad Sci USA 113:E7927–E7936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefticariu L., Pratt L.M., and Ripley E.M. (2006) Mineralogic and sulfur isotopic effects accompanying oxidation of pyrite in millimolar solutions of hydrogen peroxide at temperatures from 4 to 150 degrees C. Geochim Cosmochim Acta 70:4889–4905 [Google Scholar]
- Lester E.D., Satomi M., and Ponce A. (2007) Microflora of extreme arid Atacama Desert soils. Soil Biol Biochem 39:704–708 [Google Scholar]
- Li L., Wing B.A., Bui T.H., McDermott J.M., Slater G.F., Wei S.W., Lacrampe-Couloume G., and Sherwood Lollar B. (2016) Mass-independent sulfur fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks. Nat Commun 7, doi: 10.1038/ncomms13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.H., Wang P.-L., Rumble D., Lippmann-Pipke J., Boice E., Pratt L.M., Sherwood Lollar B., Brodie E., Hazen T., Andersen G., DeSantis T., Moser D.P., Kershaw D., and Onstott T.C. (2006) Long term biosustainability in a high energy, low diversity crustal biome. Science 314:479–482 [DOI] [PubMed] [Google Scholar]
- Lipp J.S., Morono Y., Inagaki F., and Hinrichs K.-U. (2008) Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 454:991–994 [DOI] [PubMed] [Google Scholar]
- Lippmann-Pipke J., Erzinger J., Zimmer M., Kujawa C., Boettcher M., Heerden E.V., Bester A., Moller H., Stroncik N.A., and Reches Z. (2011) Geogas transport in fractured hard rock—correlations with mining seismicity at 3.54 km depth, TauTona gold mine, South Africa. Appl Geochem 26:2134–2146 [Google Scholar]
- Liu Q., Chan L., Liu Q., Li H., Wang F., Zhang S., Xia X., and Cheng T. (2004) Relationship between magnetic anomalies and hydrocarbon microseepage above the Jingbian gas field, Ordos basin, China. American Association of Petroleum Geologists 88:241–251 [Google Scholar]
- Locey K.J. and Lennon J.T. (2015) Scaling laws predict global microbial diversity. Proc Natl Acad Sci USA 113:5970–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomstein B.A., Langerhuus A.T., D'Hondt S., Jørgensen B.B., and Spivack A.J. (2012) Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484:101–104 [DOI] [PubMed] [Google Scholar]
- Loope D.B., Kettler R.M., and Weber K.A. (2010) Follow the water: connecting a CO2 reservoir and bleached sandstone to iron-rich concretions in the Navajo Sandstone of south-central Utah, USA. Geology 38:999–1002 [Google Scholar]
- Lorenz J., Nadon G., and LaFreniere L. (1996) Geology of the Molina Member of the Wasatch Formation, Piceance Basin, Colorado. In Sandia Report, Sandia National Laboratories, Albuquerque, NM, p 30 [Google Scholar]
- Lowe D.R. (1994) Abiological origin of described stromatolites older than 3.2 Ga. Geology 22:387–390 [DOI] [PubMed] [Google Scholar]
- Lowenstein T., Satterfield C., Vreeland R., Rosenzweig W., and Powers D. (2005) New evidence for 250 Ma age of halotolerant bacterium from a Permian salt crystal: comment and reply: REPLY. Geology 33:e93–e94 [Google Scholar]
- Maclean L.C.W., Tyliszczak T., Gilbert P.U.P.A., Zhou D., Pray T.J., Onstott T.C., and Southam G. (2008) A high-resolution chemical and structural study of framboidal pyrite formed within a low-temperature bacterial biofilm. Geobiology 6:471–480 [DOI] [PubMed] [Google Scholar]
- Magnabosco C., Tekere M., Lau M.C.Y., Linage B., Kuloyo O., Erasmus M., Cason E., van Heerden E., Borgonie G., Kieft T.L., and Onstott T.C. (2014) Comparisons of the composition and biogeographic distribution of the bacterial communities occupying South African thermal springs with those inhabiting deep subsurface fracture water. Front Microbiol 5:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnabosco C., Ryan K., Lau M.C.Y., Kuloyo O., Sherwood B., Kieft T.L., van Heerden E., and Onstott T.C. (2015) A metagenomic window into carbon metabolism at 3 km depth in Precambrian continental crust. ISME J 10:730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnabosco C., Lin L.-H., Dong H., Bomberg M., Ghiorse W., Stan-Lotter H., Pedersen K., Kieft T.L., van Heerden E., and Onstott T.C. (2018a) The biomass and biodiversity of the continental subsurface. Nat Geosci 11:707–717 [Google Scholar]
- Magnabosco C., Moore K.R., Wolfe J.M., and Fournier G.P. (2018b) Dating phototrophic microbial lineages with reticulate gene histories. Geobiology 16:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnabosco C., Timmers P.H.A., Lau M.C.Y., Borgonie G., Linage-Alvarez B., Kuloyo O., Alleva R., Kieft T.L., Slater G.F., van Heerden E., Sherwood Lollar B., and Onstott T.C. (2018c) Fluctuations in populations of subsurface methane oxidizers in coordination with changes in electron acceptor availability. FEMS Microbiol Ecol 94, doi: 10.1093/femsec/fiy089 [DOI] [PubMed] [Google Scholar]
- Mansor M., Harouaka K., Gonzales M.S., Macalady J.L., and Fantle M.S. (2018) Transport-induced spatial patterns of sulfur isotopes (δ34S) as biosignatures. Astrobiology 17:59–72 [DOI] [PubMed] [Google Scholar]
- Marlow J., Peckmann J., and Orphan V. (2015) Autoendoliths: a distinct type of rock-hosted microbial life. Geobiology 13:303–307 [DOI] [PubMed] [Google Scholar]
- Marlow J.J., LaRowe D.E., Ehlmann B.L., Amend J.P., and Orphan V.J. (2014a) The potential for biologically catalyzed anaerobic methane oxidation on ancient Mars. Astrobiology 14:292–307 [DOI] [PubMed] [Google Scholar]
- Marlow J.J., Steele J.A., Ziebis W., Thurber A.R., Levin L.A., and Orphan V.J. (2014b) Carbonate-hosted methanotrophy represents an unrecognized methane sink in the deep sea. Nat Commun 5, doi: 10.1038/ncomms6094 [DOI] [PubMed] [Google Scholar]
- Marteinsson V.T., Runarsson A., Stefansson A., Thorsteinsson T., Johannesson T., Magnusson S.H., Reynisson E., Einarsson B., Wade N., Morrison H.G., and Gaidos E. (2013) Microbial communities in the subglacial waters of the Vatnajokull ice cap, Iceland. ISME J 7:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.E., Farley K.A., Baker M.B., Malespin C.A., Schwenzer S.P., Cohen B.A., and Navarro-González R. (2017) A two-step K-Ar experiment on Mars: dating the diagenetic formation of jarosite from Amazonian groundwaters. J Geophys Res Planets 122:2803–2818 [Google Scholar]
- Mayhew L.E., Ellison E.T., McCollom T.M., Trainor T.P., and Templeton A.S. (2013) Hydrogen generation from low-temperature water–rock reactions. Nat Geosci 6, doi: 10.1038/ngeo1825 [DOI] [Google Scholar]
- McBride E.F., Picard M.D., and Milliken K.L. (2003) Calcite-cemented concretions in Cretaceous sandstone, Wyoming and Utah, U.S.A. Journal of Sedimentary Research 73:462–483 [Google Scholar]
- McCollom T.M. (2016) Abiotic methane formation during experimental serpentinization of olivine. Proc Natl Acad Sci USA 113:13965–13970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollom T.M. and Bach W. (2009) Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim Cosmochim Acta 73:856–875 [Google Scholar]
- McKay C. (2001) The deep biosphere: lessons for planetary exploration. In Subsurface Microbiology and Biogeochemistry, edited by Fredrickson J.K. and Fletchers M., John Wiley & Sons, Inc., New York, pp 316–327 [Google Scholar]
- McKay C.P., Porco C.C., Altheide T., Davis W.L., and Kral T.A. (2008) The possible origin and persistence of life on Enceladus and detection of biomarkers in the plume. Astrobiology 8:909–919 [DOI] [PubMed] [Google Scholar]
- McKay C.P., Khare B.N., Amin R., Klasson M., and Kral T.A. (2012) Possible sources for methane and C2–C5 organics in the plume of Enceladus. Planet Space Sci 71:73–79 [Google Scholar]
- McKeegan K., Kudryavtsev A., and Schopf J. (2007) Raman and ion microscopic imagery of graphitic inclusions in apatite from older than 3830 Ma Akilia supracrustal rocks, West Greenland. Geology 35:591–594 [Google Scholar]
- McKinley J.P., Stevens T.O., and Westall F. (2000) Microfossils and paleoenvironments in deep subsurface basalt samples. Geomicrobiol J 17:43–54 [Google Scholar]
- McLennan S.M., Bell J.F., III, Calvin W.M., Christensen P.R., Clark B.C., de Souza P.A., Farmer J., Farrand W.H., Fike D.A., Gellert R., Ghosh A., Glotch T.D., Grotzinger J.P., Hahn B., Herkenhoff K.E., Hurowitz J.A., Johnson J.R., Johnson S.S., Jolliff B., Klingelhöfer G., Knoll A.H., Learner Z., Malin M.C., McSween H.Y., Jr, Pocock J., Ruff S.W., Soderblom L.A., Squyres S.W., Tosca N.J., Watters W.A., Wyatt M.B., and Yen A. (2005) Provenance and diagenesis of the evaporite-bearing Burns Formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:95–121 [Google Scholar]
- McLennan S.M., Anderson R.B., Bell J.F., III., Bridges J.C., Calef F., III, Campbell J.L., Clark B.C., Clegg S., Conrad P., Cousin A., Des Marais D.J., Dromart G., Dyar M.D., Edgar L.A., Ehlmann B.L., Fabre C., Forni O., Gasnault O., Gellert R., Gordon S., Grant J.A., Grotzinger J.P., Gupta S., Herkenhoff K.E., Hurowitz J.A., King P.L., Le Mouélic S., Leshin L.A., Léveillé R., Lewis K.W., Mangold N., Maurice S., Ming D.W., Morris R.V., Nachon M., Newsom H.E., Ollila A.M., Perrett G.M., Rice M.S., Schmidt M.E., Schwenzer S.P., Stack K., Stolper E.M., Sumner D.Y., Treiman A.H., VanBommel S., Vaniman D.T., Vasavada A., Wiens R.C., Yingst R.A.; MSL Science Team. (2014) Elemental geochemistry of sedimentary rocks at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1244734 [DOI] [PubMed] [Google Scholar]
- McLoughlin N. and Grosch E.G. (2015) A hierarchical system for evaluating the biogenicity of metavolcanic- and ultramafic-hosted microalteration textures in the search for extraterrestrial life. Astrobiology 15:901–921 [DOI] [PubMed] [Google Scholar]
- McLoughlin N., Wilson L.A., and Brasier M.D. (2008) Growth of synthetic stromatolites and wrinkle structures in the absence of microbes—implications for the early fossil record. Geobiology 6:95–105 [DOI] [PubMed] [Google Scholar]
- McLoughlin N., Wacey D., Kruber C., Kilburn M.R., Thorseth I.H., and Pedersen R.B. (2011) A combined TEM and NanoSIMS study of endolithic microfossils in altered seafloor basalt. Chem Geol 289:154–162 [Google Scholar]
- McLoughlin N., Grosch E.G., Kilburn M.R., and Wacey D. (2012) Sulfur isotope evidence for a Paleoarchean subseafloor biosphere, Barberton, South Africa. Geology 40:1031–1034 [Google Scholar]
- McMahon S., Parnell J., and Blamey N.J.F. (2016) Evidence for seismogenic hydrogen gas, a potential microbial energy source on Earth and Mars. Astrobiology 16:690–702 [DOI] [PubMed] [Google Scholar]
- McMahon S., Bosak T., Grotzinger J.P., Milliken R.E., Summons R.E., Daye M., Newman S.A., Fraeman A., Williford K.H., and Briggs D.E.G. (2018) A field guide to finding fossils on Mars. J Geophys Res Planets 123, doi: 10.1029/2017JE005478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSween H.Y. (2015) Petrology on Mars. Am Mineral 100:2380–2395 [Google Scholar]
- Melton E.D., Swanner E.D., Behrens S., Schmidt C., and Kappler A. (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808 [DOI] [PubMed] [Google Scholar]
- Ménez B., Pasini V., and Brunelli D. (2012) Life in the hydrated suboceanic mantle. Nat Geosci 5:133–137 [Google Scholar]
- Michalski J.R., Cuadros J., Niles P.B., Parnell J., Rogers A.D., and Wright S.P. (2013) Groundwater activity on Mars and implications for a deep biosphere. Nat Geosci 6:133–138 [Google Scholar]
- Michalski J.R., Onstott T.C., Mojzsis S.J., Mustard J., Chan Q., Niles P.B., and Johnson S.S. (2017) Seeking signs of chemosynthetic life in ancient subsurface and hydrothermal settings on Mars. Nat Geosci 11:21–26 [Google Scholar]
- Middelboe M., Glud R.N., and Filippini M. (2011) Viral abundance and activity in the deep sub-seafloor biosphere. Aquat Microb Ecol 63:1–8 [Google Scholar]
- Milucka J., Ferdelman T.G., Polerecky L., Franzke D., Wegener G., Schmid M., Lieberwirth I., Wagner M., Widdel F., and Kuypers M.M.M. (2012) Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491:541–546 [DOI] [PubMed] [Google Scholar]
- Mojzsis S.J., Arrhenius G., McKeegan K.D., Harrison T.M., Nutman A.P., and Friend C.R.L. (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384:55–59 [DOI] [PubMed] [Google Scholar]
- Momper L., Jungbluth S.P., Lee M.D., and Amend J.P. (2017) Energy and carbon metabolisms in a deep terrestrial subsurface fluid microbial community. ISME J 11:2319–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B.E.L., Henneberger R., Huber H., and Moissl-Eichinger C. (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37:384–406 [DOI] [PubMed] [Google Scholar]
- Moser D.P., Gihring T., Fredrickson J.K., Brockman F.J., Balkwill D., Dollhopf M.E., Sherwood Lollar B., Pratt L.M., Boice E., Southam G., Wanger G., Welty A.T., Baker B.J., and Onstott T.C. (2005) Desulfotomaculum spp. and Methanobacterium spp. dominate 4–5 km deep fault. Appl Environ Microbiol 71:8773–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V. and Hess V. (2017) The minimum biological energy quantum. Front Microbiol 8, doi: 10.3389/fmicb.2017.02019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch L., Germanovich L., Wang H., Onstott T.C., Elsworth D., Stetler L., and Boutt D. (2012) Hydrogeology of the vicinity of DUSEL Homestake. Hydrogeol J 20:27–43 [Google Scholar]
- Mustard J.F., Poulet F., Gendrin A., Bibring J.-P., Langevin Y., Gondet B., Mangold N., Bellucci G., and Altieri F. (2005) Olivine and pyroxene diversity in the crust of Mars. Science 307:1594–1597 [DOI] [PubMed] [Google Scholar]
- Mustard J.F., Murchie S.L., Pelkey S.M., Ehlmann B.L., Milliken R.E., Grant J.A., Bibring J.-P., Poulet F., Bishop J., Noe Dobrea E., Roach F., Seelos F., Arvidson R.E., Wiseman S., Green R., Hash C., Humm D., Malaret E., McGovern J.A., Seelos K., Clancy T., Clark R., Des Marais D., Izenberg N., Knudson A., Langevin Y., Martin T., McGuire P., Morris R., Robinson M., Roush T., Smith M., Swayze G., Taylor H., Titus T., and Wolff M. (2008) Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 454:305–309 [DOI] [PubMed] [Google Scholar]
- Mykytczuk N., Foote S., Omelon C., Southam G., Greer C., and Whyte L. (2013) Bacterial growth at −15°C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J 7:1211–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K.H., Inagaki F., and Takai K. (2005) Hydrogen-driven subsurface lithoautotrophic microbial ecosystems (SLiMEs): do they exist and why should we care? Trends Microbiol 13:405–410 [DOI] [PubMed] [Google Scholar]
- Noffke N., Christian D., Wacey D., and Hazen R.M. (2013) Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia. Astrobiology 13:1103–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman A.P., Bennett V.C., Friend C.R.L., Van Kranedonk M.J., and Chivas A.R. (2016) Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537:535–538 [DOI] [PubMed] [Google Scholar]
- Ohfuji H. and Rickard D. (2005) Experimental syntheses of framboids—a review. Earth-Science Reviews 71:147–170 [Google Scholar]
- Ohmoto H., Kakegawa T., and Lowe D.R. (1993) 3.4-billion-year-old biogenic pyrites from Barberton, South Africa: sulfur isotope evidence. Science 262:555–557 [DOI] [PubMed] [Google Scholar]
- Onstott T.C. (2004) Impact of CO2 injections on deep subsurface microbial ecosystems and potential ramifications for the surface biosphere. In The CO2 Capture and Storage Project, edited by Thomas D.C. and Bensons S.M., Lawrence Berkeley National Laboratory, Berkeley, CA, pp 1207–1239 [Google Scholar]
- Onstott T.C. (2016) Deep Life: The Hunt for the Hidden Biology of Earth, Mars and Beyond, Princeton University Press, Princeton, NJ [Google Scholar]
- Onstott T.C., McGown D., Kessler J., Sherwood Lollar B., Lehmann K.K., and Clifford S. (2006) Martian CH4: sources, flux and detection. Astrobiology 6:377–395 [DOI] [PubMed] [Google Scholar]
- Onstott T.C., McGown D.J., Bakermans C., Ruskeeniemi T., Ahonen L., Telling J., Boettiger C., Ho R., Soffientino B., Pfiffner S.M., DiFurio S., Sherwood Lollar B., Frape S., Stotler R., Pratt L.M., and Vishnivetskaya T.A. (2009) Microbial sulfur cycling in subpermafrost saline fracture water at the Lupin Gold Mine, Nunavut, Canada. Microb Ecol 58:786–807 [DOI] [PubMed] [Google Scholar]
- Onstott T.C., Magnabosco C., Aubrey A.D., Burton A.S., Dworkin J.P., Elsila J.E., Grunsfeld S., Cao B.H., Hein J.E., Glavin D.P., Kieft T.L., Silver B.J., Phelps T.J., van Heerden E., Opperman D.J., and Bada J.L. (2013) Does aspartic acid racemization constrain the depth limit of the subsurface biosphere? Geobiology 12:1–19 [DOI] [PubMed] [Google Scholar]
- Orcutt B.N., LaRowe D.E., Biddle J.F., Colwell F.S., Glazer B.T., Reese B.K., Kirkpatrick J.B., Lapham L.L., Mills H.J., Sylvan J.B., Wankel S.D., and Wheat C.G. (2013) Microbial activity in the marine deep biosphere: progress and prospects. Front Microbiol 4, doi: 10.3389/fmicb.2013.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:334–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosei R., Lauro S.E., Pettinelli E., Cicchetti A., Coradini M., Cosciotti B., Paolo F.D., Flamini E., Mattei E., Pajola M., Soldovieri F., Cartacci M., Cassenti F., Frigeri A., Giuppi S., Martufi R., Masdea A., Mitri G., Nenna C., Noschese R., Restano M., and Seu R. (2018) Radar evidence of subglacial liquid water on Mars. Science 361:490–493 [DOI] [PubMed] [Google Scholar]
- Orphan V.J., House C.H., Hinrichs K.U., McKeegan K.D., and DeLong E.F. (2001) Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 293:484–487 [DOI] [PubMed] [Google Scholar]
- Orsi W., Biddle J.F., and Edgcomb V. (2013) Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS One 8, doi: 10.1371/journal.pone.0056335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn M.R., LaRowe D.E., Momper L.M., and Amend J.P. (2014) Chemolithotrophy in the continental deep subsurface: Sanford Underground Research Facility (SURF), USA. Front Microbiol 5, doi: 10.3389/fmicb.2014.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinski G., Tornabene L., Banerjee N., Cockell C., Flemming R., Izawa M., McCutcheon J., Parnell J., Preston L., Pickersgill A., Pontefract A., Sapers H., and Southam G. (2013) Impact-generated hydrothermal systems on Earth and Mars. Icarus 224:347–363 [Google Scholar]
- Pachiadaki M.G., Rédou V., Beaudoin D.J., Burgaud G., and Edgcomb V.P. (2016) Fungal and prokaryotic activities in the marine subsurface biosphere at Peru Margin and Canterbury Basin inferred from RNA-based analyses and microscopy. Front Microbiol 7, doi: 10.3389/fmicb.2016.00846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Ehlmann B.L., Carter J., and Ernst C.M. (2017) The stratigraphy and history of Mars' northern lowlands through mineralogy of impact craters: a comprehensive survey. J Geophys Res Planets 122, doi: 10.1002/2017JE005276 [DOI] [Google Scholar]
- Papineau D., Gregorio B.T.D., Cody G.D., O'Neil J., Steele A., Stroud R.M., and Fogel M.L. (2011) Young poorly crystalline graphite in the >3.8-Gyr-old Nuvvuagittuq banded iron formation. Nat Geosci 4:376–379 [Google Scholar]
- Parkes R.J., Cragg B., Roussel E., Webster G., Weightman A., and Sass H. (2014) A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere:geosphere interactions. Mar Geol 352:409–425 [Google Scholar]
- Pasek M.A. and Greenberg R. (2012) Acidification of Europa's subsurface ocean as a consequence of oxidant delivery. Astrobiology 12:151–159 [DOI] [PubMed] [Google Scholar]
- Paul B.G., Bagby S.C., Czornyj E., Arambula D., Handa S., Sczyrba A., Ghosh P., Miller J.F., and Valentine D.L. (2015) Targeted diversity generation by intraterrestrial archaea and archaeal viruses. Nat Commun 6, doi: 10.1038/ncomms7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckmann J. and Thiel V. (2004) Carbon cycling at ancient methane seeps. Chem Geol 205:443–467 [Google Scholar]
- Peckmann J., Reimer A., Luth U., Luth C., Hansen B.T., Heinicke C., Hoefs J., and Reitner J. (2001) Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar Geol 177:129–150 [Google Scholar]
- Peckmann J., Bach W., Behrens K., and Reitner J. (2007) Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology 6:125–135 [DOI] [PubMed] [Google Scholar]
- Pedersen K., Ekendahl S., Tullborg E.-L., Furnes H., Thorseth I., and Tumyr O. (1997) Evidence of ancient life at 207 m depth in a granitic aquifer. Geology 25:827–830 [DOI] [PubMed] [Google Scholar]
- Pedersen K., Bengtsson A.F., Edlund J.S., and Eriksson L.C. (2014) Sulphate-controlled diversity of subterranean microbial communities over depth in deep groundwater with opposing gradients of sulphate and methane. Geomicrobiol J 31:617–631 [Google Scholar]
- Phelps T.J., Murphy E.M., Pfiffner S.M., and White D.C. (1994) Comparison between geochemical and biological estimates of subsurface microbial activities. Microb Ecol 28:335–349 [DOI] [PubMed] [Google Scholar]
- Pinti D.L., Mineau R., and Clement V. (2009) Hydrothermal alteration and microfossil artefacts of the 3,465-million-year-old Apex Chert. Nat Geosci 2:640–643 [Google Scholar]
- Pontefract A., Osinski G.R., Cockell C.S., Southam G., McCausland P.J.A., Umoh J., and Holdsworth D.W. (2016) Microbial diversity of impact-generated habitats. Astrobiology 16:775–786 [DOI] [PubMed] [Google Scholar]
- Popa R., Kinkle B.K., and Badescu A. (2004) Pyrite framboids as biomarkers for iron-sulfur systems. Geomicrobiol J 21:193–206 [Google Scholar]
- Price A., Pearson V.K., Schwenzer S.P., Miot J., and Olsson-Francis K. (2018) Nitrate-dependent iron oxidation: a potential Mars metabolism. Front Microbiol 9, doi: 10.3389/fmicb.2018.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkamo L., Bomberg M., Nyyssönen M., Kukkonen I., Ahonen L., and Itävaara M. (2015) Heterotrophic communities supplied by ancient organic carbon predominate in deep Fennoscandian bedrock fluids. Microb Ecol 69:319–332 [DOI] [PubMed] [Google Scholar]
- Quinn D. and Ehlmann B. (2019) The deposition and alteration history of the Northeast Syrtis layered sulfates. J Geophys Res Planets, in press, doi: 10.1029/2018JE005706 [DOI] [Google Scholar]
- Rampe E.B., Ming D.W., Blake D.F., Bristow T.F., Chipera S.J., Grotzinger J.P., Morris R.V., Morrison S.M., Vaniman D.T., Yen A.S., Achilles C.N., Craig P.I., Des Marais D.J., Downs R.T., Farmer J.D., Fendrich K.V., Gellert R., Hazen R.M., Kah L.C., Morookian J.M., Peretyazhko T.S., Sarrazin P., Treiman A.H., Berger J.A., Eigenbrode J., Fairén A.G., Forni O., Gupta S., Hurowitz J.A., Lanza N.L., Schmidt M.E., Siebach K., Sutter B., and Thompson L.M. (2017) Mineralogy of an ancient lacustrine mudstone succession from the Murray Formation, Gale Crater, Mars. Earth Planet Sci Lett 471:172–185 [Google Scholar]
- Rapin W., Chauviré B., Gabriel T.S.J., McAdam A.C., Ehlmann B.L., and Hardgrove C. (2018) In situ analysis of opal in Gale Crater, Mars. J Geophys Res Planets 123:1955–1972 [Google Scholar]
- Rasmussen B. (2000) Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 405:676–679 [DOI] [PubMed] [Google Scholar]
- Rempfert K.R., Miller H.M., Bompard N., Nothaft D., Matter J.M., Kelemen P., Fierer N., and Templeton A.S. (2017) Geological and geochemical controls on subsurface microbial life in the Samail Ophiolite, Oman. Front Microbiol 8, doi: 10.3389/fmicb.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelberg D., Sutton S., and White D.C. (1997) Biomass, bioactivity and biodiversity: microbial ecology of the deep subsurface: analysis of ester-linked phospholipid fatty acids. FEMS Microbiol Rev 20:371–377 [Google Scholar]
- Riquelme C., Hathaway J.J.M., Dapkevicius M.L.N.E., Miller A.Z., Kooser A., Northup D.E., Jurado V., Fernandez O., Saiz-Jimenez C., and Cheeptham N. (2015) Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front Microbiol 6, doi: 10.3389/fmicb.2015.01342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkina E.M., Friedmann E.I., McKay C.P., and Gilichinsky D.A. (2000) Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol 66:3230–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh Y., Liu S.V., Li G., Huang H., Phelps T.J., and Zhou J. (2002) Isolation and characterization of metal-reducing Thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl Environ Microbiol 68:6013–6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røy H., Kallmeyer J., Adhikar R.R., Pockalny R., Jørgensen B.B., and D'Hondt S. (2012) Aerobic microbial respiration in 86-million-year-old deep-sea red clay. Science 336:922–925 [DOI] [PubMed] [Google Scholar]
- Ruff S.W. and Farmer J.D. (2016) Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat Commun 7:13554–13564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl J.W., Schmidt R., Swanner E.D., Mandernack K.W., Templeton A.S., Kieft T.L., Smith R.L., Sanford W.E., Callaghan R.L., Mitton J.B., and Spear J.R. (2008) Subsurface microbial diversity in deep-granitic-fracture water in Colorado. Appl Environ Microbiol 74:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper L. and Mustard J.F. (2013) Extensive linear ridge networks in Nili Fossae and Nilosyrtis, Mars: implications for fluid flow in the ancient crust. Geophys Res Lett 40:245–249 [Google Scholar]
- Sapers H.M., Osinski G.R., Banerjee N.R., and Preston L.J. (2014) Enigmatic tubular features in impact glass. Geology 42:471–474 [Google Scholar]
- Sapers H.M., Banerjee N.R., and Osinski G.R. (2015) Potential for impact glass to preserve microbial metabolism. Earth Planet Sci Lett 430:95–104 [Google Scholar]
- Scholten J.C.M. and Conrad R. (2000) Energetics of syntrophic propionate oxidation in defined batch and chemostat cocultures. Appl Environ Microbiol 66:2934–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf J.W. (1993) Microfossils of the early Archean Apex Chert: new evidence of the antiquity of life. Science 260:640–646 [DOI] [PubMed] [Google Scholar]
- Schopf J.W., Kitajima K., Spicuzza M.J., Kudryavtsev A.B., and Valley J.W. (2018) SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions. Proc Natl Acad Sci USA 115:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert B., Lowenstein T., Timofeeff M., and Parker M. (2009a) How do prokaryotes survive in fluid inclusions in halite for 30 k.y.? Geology 37:1059–1062 [Google Scholar]
- Schubert B., Lowenstein T., and Timofeeff M. (2009b) Microscopic identification of prokaryotes in modern and ancient halite, Saline Valley and Death Valley, California. Astrobiology 9:1–17 [DOI] [PubMed] [Google Scholar]
- Schubert B., Lowenstein T., Timofeeff M., and Parker M. (2010) Halophilic Archaea cultured from ancient halite, Death Valley, California. Environ Microbiol 12:440–454 [DOI] [PubMed] [Google Scholar]
- Schubotz F., Wakeham S.G., Lipp J.S., Fredricks H.F., and Hinrichs K.-U. (2009) Detection of microbial biomass by intact polar membrane lipid analysis in the water column and surface sediments of the Black Sea. Environ Microbiol 11:2720–2734 [DOI] [PubMed] [Google Scholar]
- Schulte M., Blake D., Hoehler T., and McCollom T. (2006) Serpentinization and its implications for life on the early Earth and Mars. Astrobiology 6:364–376 [DOI] [PubMed] [Google Scholar]
- Schulze-Makuch D. and Irwin L.N. (2002) Energy cycling and hypothetical organisms in Europa's ocean. Astrobiology 2:105–121 [DOI] [PubMed] [Google Scholar]
- Schumacher D. (1996) Hydrocarbon-induced alteration of soils and sediments. In Hydrocarbon Migration and Its Near-Surface Expression, AAPG Memoir 66, edited by Schumacher D. and Abrams M.A., American Association of Petroleum Geologists, Tulsa, OK, pp 71–89 [Google Scholar]
- Segura T.L., Zahnle K., Toon O.B., and McKay C.P. (2013) The effects of impacts on the climates of terrestrial planets. In Comparative Climatology of Terrestrial Planets, edited by Mackwell S.J., Simon-Miller A.A., Harder J.W., Bullock M.A., and Dotson R., University of Arizona Press, Tucson, AZ, pp 417–437 [Google Scholar]
- Shen Y. and Buick R. (2004) The antiquity of microbial sulfate reduction. Earth-Science Reviews 64:243–272 [Google Scholar]
- Shen Y., Farquhar J., Masterson A., Kaufman A.J., and Buick R. (2009) Evaluating the role of microbial sulfate reduction in the early Archean using quadruple isotope systematics. Earth Planet Sci Lett 279:383–391 [Google Scholar]
- Sherwood Lollar B., Westgate T.D., Ward J.A., Slater G.F., and Lacrampe-Couloume G. (2002) Abiogenic formation of alkanes in the Earth's crust as a minor source for global hydrocarbon reservoirs. Nature 416:522–524 [DOI] [PubMed] [Google Scholar]
- Sherwood Lollar B., Lacrampe-Couloume G., Slater G.F., Ward J., Moser D.P., Lin L.-H., and Onstott T.C. (2005) Abiogenic gases support H2-based autotrophy and methanogenesis in the deep subsurface. Chem Geol 226:328–339 [Google Scholar]
- Sherwood Lollar B., Lacrampe-Couloume G., Slater G.F., Ward J., Moser D.P., Gihring T.M., Lin L.-H., and Onstott T.C. (2006) Unravelling abiogenic and biogenic sources of methane in the Earth's deep subsurface. Chem Geol 226:328–339 [Google Scholar]
- Shih P.M., Hemp J., Ward L.M., Matzke N.J., and Fischer W.W. (2017) Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology 15:19–29 [DOI] [PubMed] [Google Scholar]
- Siebach K.L. and Grotzinger J.P. (2014) Volumetric estimates of ancient water on Mount Sharp based on boxwork deposits, Gale Crater, Mars. J Geophys Res Planets 119:189–198 [Google Scholar]
- Silver B.J., Raymond R., Sigman D., Prokopenko M., Sherwood Lollar B., Lacrampe-Couloume G., Fogel M., Pratt L., Lefticariu L., and Onstott T.C. (2012) The origin of NO3- and N2 in deep subsurface fracture water of South Africa. Chem Geol 294–295:51–62 [Google Scholar]
- Simkus D.N., Slater G.F., Sherwood Lollar B., Wilkie K., Kieft T.L., Magnabosco C., Lau M.C.Y., Pullin M.J., Hendrickson S.B., Wommack K.E., Sakowski E.G., van Heerden E., Kuloyo O., Linage B., Borgonie G., and Onstott T.C. (2015) Variations in microbial carbon sources and cycling in the deep continental subsurface. Geochim Cosmochim Acta 173:264–283 [Google Scholar]
- Sinclair J. and Ghiorse W. (1989) Distribution of aerobic bacteria, protozoa, algae, and fungi in deep subsurface sediments. Geomicrobiol J 7:15–31 [Google Scholar]
- Skok J.R., Mustard J.F., Ehlmann B.L., Milliken R.E., and Murchie S.L. (2010) Silica deposits in the Nili Patera caldera on the Syrtis Major volcanic complex on Mars. Nat Geosci 3:838–841 [Google Scholar]
- Sogin M.L., Morrison H.G., Huber J.A., Welch D.M., Huse S.M., Neal P.R., Arrieta J.M., and Herndl G.J. (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere.” Proc Natl Acad Sci USA 32:12115–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg E., Bomberg M., Miettinen H., Nyyssönen M., Heikki Salavirta, Vikman M., and Itävaara M. (2015) Revealing the unexplored fungal communities in deep groundwater of crystalline bedrock fracture zones in Olkiluoto, Finland. Front Microbiol 6, doi: 10.3389/fmicb.2015.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo R.M., Hemp J., Parks D.H., Fischer W.W., and Hugenholtz P. (2017) On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science 355:1436–1440 [DOI] [PubMed] [Google Scholar]
- Spinks S.C., Parnell J., and Bowden S.A. (2010) Reduction spots in the Mesoproterozoic age: implications for life in the early terrestrial record. Int J Astrobiol 9:209–216 [Google Scholar]
- Stamenković V., Beegle L.W., Zacny K., Arumugam D.D., Baglioni P., Barba N., Baross J., Bell M.-S., Bhartia R., Blank J.G., Boston P.J., Breuer D., Brinckerhoff W., Burgin M., Cooper I., Cormarkovic V., Davila A., Edwards C., Fischer W.W., Glavin D.P., Grimm B., Inagaki F., Komarek T., Malaska M., Ménez B., Michalski J., Mischna M., Moser D., Mustard J., Onstott T.C., Orphan V.J., Osburn M.R., Plaut J., Plesa A.-C., Putzig N., Rogers K.L., Rothschild L., Russell M., Sapers H., Sherwood Lollar B.S., Spohn T., Tarnas J.D., Tuite M., Viola D., Ward L.M., Wilcox B., and Woolley R. (2019) The next frontier for planetary and human exploration. Nat Astron 3:116–120 [Google Scholar]
- Staudigel H., Furnes H., McLoughlin N., Banerjee N., Connell L., and Templeton A.S. (2008) 3.5 billion years of glass bioalteration: volcanic rocks as a basis for microbial life? Earth-Science Reviews 89:156–176 [Google Scholar]
- Steel E.L., Davila A., and McKay C.P. (2017) Abiotic and biotic formation of amino acids in the Enceladus ocean. Astrobiology 17:862–875 [PubMed] [Google Scholar]
- Stern J., Sutter B., Freissinet C., Navarro-González R., McKay C.P., Archer P.D., Jr, Buch A., Brunner A.E., Coll P., Eigenbrode J.L., Fairen A.G., Franz H.B., Glavin D.P., Kashyap S., McAdam A.C., Ming D.W., Steele A., Szopa C., Wray J.J., Martín-Torres F.J., Zorzano M.-P., Conrad P.G., Mahaffy P.R., and the MSL Science Team. (2015) Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale Crater, Mars. Proc Natl Acad Sci USA 112:4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T.O. and McKinley J.P. (1995) Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270:450–454 [Google Scholar]
- Summons R.E., Amend J.P., Bish D., Buick R., Cody G.D., Des Marais D.J., Dromart G., Eigenbrode J.L., Knoll A.H., and Sumner D.Y. (2011) Preservation of martian organic and environmental records: final report of the Mars Biosignature Working Group. Astrobiology 11:157–181 [DOI] [PubMed] [Google Scholar]
- Takai K., Nakamura K., Toki T., Tsunogai U., Miyazaki M., Miyazaki J., Hirayama H., Nakagawa S., Nunoura T., and Horikoshi K. (2008) Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA 105:10949–10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K.L., Skinner J.A., Jr, Hare T.M., Joyal T., and Wenker A. (2003) Resurfacing history of the Northern Plains of Mars based on geologic mapping of Mars Global Surveyor data. J Geophys Res 108, doi: 10.1029/2002JE00190 [DOI] [Google Scholar]
- Tanaka K.L., Skinner J.A., Jr, Dohm J.M., Irwin R.P., III, Kolb E.J., Fortezzo C.M., Platz T., Michael G.G., and Hare T.M. (2014) Geologic Map of Mars, edited by USIM 3292s, U.S. Geological Survey, Flagstaff, AZ. Available online at http://pubs.usgs.gov/sim/3292/pdf/sim3292_map.pdf
- Tarnas J.D., Mustard J.F., Sherwood Lollar B., Bramble M.S., Cannon K.M., Palumbo A.M., and Plesa A.-C. (2018) Radiolytic H2 production on Noachian Mars: implications for habitability and atmospheric warming. Earth Planet Sci Lett 502:133–145 [Google Scholar]
- Tashiro T., Ishida A., Hori M., Igisu M., Koike M., Méjean P., Takahata N., Sano Y., and Komiya T. (2017) Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549:516–519 [DOI] [PubMed] [Google Scholar]
- Tebo B.M., Davis R.E., Anitori R.P., Connell L.B., Schiffman P., and Staudigel H. (2015) Microbial communities in dark oligotrophic volcanic ice cave ecosystems of Mt. Erebus, Antarctica. Front Microbiol 6, doi: 10.3389/fmicb.2015.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling J., Boyd E.S., Bone N., Jones E.L., Tranter M., MacFarlane J.W., Martin P.G., Wadham J., Lamarche-Gagnon G., Skidmore M.L., Hamilton T.L., Hill E., Jackson M., and Hodgson D.A. (2015) Rock comminution as a source of hydrogen for subglacial ecosystems. Nat Geosci 8:851–855 [Google Scholar]
- Teodoro L., Davila A., Elphic R.C., Hamilton D., McKay C., and Quinn R. (2018) Habitability and biomarker preservation in the martian near-surface radiation environment. In Habitability to Life on Mars, edited by Cabrol N.A. and Grins E.A., Elsevier, Amsterdam, pp 211–231 [Google Scholar]
- Thollot P., Mangold N., Ansan V., Mouélic S.L., Milliken R.E., Bishop J.L., Weitz C.M., Roach L.H., Mustard J.F., and Murchie S.L. (2012) Most Mars minerals in a nutshell: various alteration phases formed in a single environment in Noctis Labyrinthus. J Geophys Res 117, doi: 10.1029/2011JE004028 [DOI] [Google Scholar]
- Thorseth I.H., Furnes H., and Heldal M. (1992) The importance of microbiological activity in the alteration of natural basaltic glass. Geochim Cosmochim Acta 56:845–850 [Google Scholar]
- Thorseth I.H., Torsvik T., Furnes H., and Muehlenbachs K. (1995) Microbes play an important role in the alteration of oceanic crust. Chem Geol 126:137–146 [Google Scholar]
- Thyne G.D. and Boles J.R. (1989) Isotopic evidence for origin of Moeraki septarian concretions, New Zealand. J Sediment Petrol 59:272–279 [Google Scholar]
- Tice M.M. (2009) Environmental controls on photosynthetic microbial mat distribution and morphogenesis on a 3.42 Ga clastic-starved platform. Astrobiology 9:989–1000 [DOI] [PubMed] [Google Scholar]
- Tice M.M. and Lowe D.R. (2004) Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 431:549–552 [DOI] [PubMed] [Google Scholar]
- Tornabene L.L., Osinski G.R., McEwen A.S., Wray J.J., Craig M.A., Sapers H.M., and Christensen P.R. (2013) An impact origin for hydrated silicates on Mars: a synthesis. J Geophys Res Planets 118:994–1012 [Google Scholar]
- Tosca N.J., McLennan S.M., Clark B.C., Grotzinger J.P., Hurowitz J.A., Knoll A.H., Schröder C., and Squyres S.W. (2005) Geochemical modeling of evaporation processes on Mars: insight from the sedimentary record at Meridiani Planum. Earth Planet Sci Lett 240:122–148 [Google Scholar]
- Tosca N.J., Knoll A.H., and McLennan S.M. (2008) Water activity and the challenge for life on early Mars. Science 320:1204–1207 [DOI] [PubMed] [Google Scholar]
- Trembath-Reichert E., Morono Y., Ijiri A., Hoshino T., Dawson K.S., Inagaki F., and Orphan V.J. (2017) Methyl-compound use and slow growth characterize microbial life in 2-km-deep subseafloor coal and shale beds. Proc Natl Acad Sci USA 114:E9206–E9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewin N.H. and Knoll A.H. (1999) Preservation of Devonian chemotrophic filamentous bacteria in calcite veins. Palaios 14:288–294 [Google Scholar]
- Trias R., Ménez B., le Campion P., Zivanovic Y., Lecourt L., Lecoeuvre A., Schmitt-Kopplin P., Uhl J., Gislason S.R., Alfreðsson H.A., Mesfin K.G., Snæbjörnsdóttir S.Ó., Aradóttir E.S., Gunnarsson I., Matter J.M., Stute M., Oelkers E.H., and Gérard E. (2017) High reactivity of deep biota under anthropogenic CO2 injection into basalt. Nat Commun 8, doi: 10.1038/s41467-017-01288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H.-Y., Person M., and Onstott T.C. (1998) Hydrogeologic constraint on the origin of deep subsurface microorganisms within a Triassic basin. Water Resources Res 34:937–948 [Google Scholar]
- Ueno Y., Yamada K., Yoshida N., Maruyama S., and Isozaki Y. (2006) Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 440:516–519 [DOI] [PubMed] [Google Scholar]
- Ussler W., III, and Paull C.K. (2008) Rates of anaerobic oxidation of methane and authigenic carbonate mineralization in methane-rich deep-sea sediments inferred from models and geochemical profiles. Earth Planet Sci Lett 266:271–287 [Google Scholar]
- Valley J.W., Peck W.H., King E.M., and Wilde S.A. (2002) A cool early Earth. Geology 30:351–354 [Google Scholar]
- Vance S.D., Hand K.P., and Pappalardo R.T. (2016) Geophysical controls of chemical disequilibria in Europa. Geophys Res Lett 43:4871–4879 [Google Scholar]
- van de Vossenberg J.L.C.M., Trees Ubbink-Kok, Elferink M.G.L., Driessen A.J.M., and Konings W.N. (1995) Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol 18:925–932 [DOI] [PubMed] [Google Scholar]
- Vanwonterghem I., Evans P.N., Parks D.H., Jensen P.D., Woodcroft B.J., Hugenholtz P., and Tyson G.W. (2016) Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat Microbiol 1, doi: 10.1038/nmicrobiol.2016.170 [DOI] [PubMed] [Google Scholar]
- van Zuilen M.A., Lepland A., Teranes J., Finarelli J., Wahlen M., and Arrhenius G. (2003) Graphite and carbonates in the 3.8 Ga old Isua Supracrustal Belt, southern West Greenland. Precambrian Res 126:331–348 [Google Scholar]
- Velbel M.A. (2012) Aqueous alteration in martian meteorites: comparing mineral relations in igneous-rock weathering of martian meteorites and in the sedimentary cycle of Mars. In Sedimentary Geology of Mars, edited by Grotzinger J.P. and Millikens R.E., SEPM Special Publication 102. Society for Sedimentary Geology, Tulsa, OK, pp 97–117 [Google Scholar]
- Wacey D., McLoughlin N., Sauders M., and Kong C. (2014) The nano-scale anatomy of a complex carbon-lined microtube in volcanic glass from the ∼92 Ma Troodos ophiolite, Cyprus. Chem Geol 363:1–12 [Google Scholar]
- Wacey D., Fisk M., Saunders M., Eiloart K., and Kong C. (2017) Critical testing of potential cellular structures within microtubes in 145 Ma volcanic glass from the Argo Abyssal Plain. Chem Geol 466:575–587 [Google Scholar]
- Wadsworth J. and Cockell C.S. (2017) Perchlorates on Mars enhance the bacteriocidal effects of UV light. Sci Rep 7, doi: 10.1038/s41598-017-04910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Buick R., and Dunlop J. (1980) Stromatolites, 3,400–3,500 Myr old from the North Pole area, Western Australia. Nature 284:443–445 [Google Scholar]
- Wang J., Lowenstein T.K., and Fang X. (2016) Microbial habitability and Pleistocene aridification of the Asian interior. Astrobiology 16:379–388 [DOI] [PubMed] [Google Scholar]
- Wanger G., Onstott T.C., and Southam G. (2006) Structural and chemical characterization of a natural fracture surface from 2.8 kilometers below land surface: biofilms in the deep subsurface. Geomicrobiol J 23:443–452 [Google Scholar]
- Wankel S.D., Germanovitch L.N., Lilley M.D., Gence G., DiPerna C.J., Bradley A.S., Olson E.J., and Girguis P.R. (2011) Influence of subsurface biosphere on geochemical fluxes from diffuse hydrothermal vents. Nat Geosci 4:461–468 [Google Scholar]
- Webster K.D., Mirza A., Deli J.M., Sauer P.E., and Schimmelmann A. (2016) Consumption of atmospheric methane in a limestone cave in Indiana, USA. Chem Geol 443:1–9 [Google Scholar]
- Weiss B.P., Yung Y.L., and Nealson K.H. (2000) Atmospheric energy for subsurface life on Mars? Proc Natl Acad Sci USA 97:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall F., Loizeau D., Foucher F., Bost N., Betrand M.N., Vago J., and Kminek G. (2013) Habitability on Mars from a microbial point of view. Astrobiology 13:887–897 [DOI] [PubMed] [Google Scholar]
- Westall F., Foucher F., Bost N., Bertrand M., Loizeau D., Vago J.L., Kminek G., Gaboyer F., Campbell K.A., Bréhéret J.-G., Gautret P., and Cockell C.S. (2015) Biosignatures on Mars: what, where, and how? Implications for the search for martian life. Astrobiology 15:998–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh R., Manatschal G., and Minshull T. (2001) Evolution of magma-poor continental margins from rifting to seafloor spreading. Nature 413:150–154 [DOI] [PubMed] [Google Scholar]
- Wilhelms A., Larter S.R., Head I., Farrimond P., di-Primio R., and Zwach C. (2001) Biodegradation of oil in uplifted basins prevented by deep-burial sterilization. Nature 411:1034–1037 [DOI] [PubMed] [Google Scholar]
- Williford K.H., Ushikubo T., Lepot K., Kitajima K., Hallmann C., Spicuzza M., Kozdin R., Eigenbrode J., Summons R., and Valley J. (2016) Carbon and sulfur isotopic signatures of ancient life and environment at the microbial scale: Neoarchean shales and carbonates. Geobiology 14:105–128 [DOI] [PubMed] [Google Scholar]
- Wong F.K.Y., Lau M.C.Y., Lacap D.C., Aitchison J.C., Cowan D.A., and Pointing S.B. (2010) Endolithic microbial colonization of limestone in a high-altitude arid environment. Microb Ecol 59:689–699 [DOI] [PubMed] [Google Scholar]
- Wordsworth R., Kalugina Y., Lokshtanov S., Vigasin A., Ehlmann B., Head J., Sanders C., and Wang H. (2017) Transient reducing greenhouse warming on early Mars. Geophys Res Lett 44, doi: 10.1002/2016GL071766 [DOI] [Google Scholar]
- Wordsworth R.D. (2016) The climate of early Mars. Annu Rev Earth Planet Sci 44:381–408 [Google Scholar]
- Wordsworth R.D., Kerber L., Pierrehumbert R.T., Forget F., and Head J.W. (2015) Comparison of “warm and wet” and “cold and icy” scenarios for early Mars in a 3-D climate model. J Geophys Res Planets 120:1201–1219 [Google Scholar]
- Wray J.J., Milliken R.E., Dundas C.M., Swayze G.A., Andrews-Hanna J.C., and Baldridge A.M. (2011) Columbus Crater and other possible groundwater-fed paleolakes of Terra Sirenum, Mars. J Geophys Res Planets 116, doi: 10.1029/2010JE003694 [DOI] [Google Scholar]
- Xie S., Lipp J.S., Wegener G., Ferdelman T.G., and Hinrichs K.-U. (2012) Turnover of microbial lipids in the deep biosphere and growth of benthic archaeal populations. Proc Natl Acad Sci USA 110:6010–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen A.S., Ming D.W., Vaniman D.T., Gellert R., Blake D.F., Morris R.V., Morrison S.M., Bristow T.F., Chipera S.J., Edgett K.S., Treiman A.H., Clark B.C., Downs R.T., Farmer J.D., Grotzinger J.P., Rampe E.B., Schmidt M.E., Sutter B., Thompson L.M., and the MSL Science Team. (2017) Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. Earth Planet Sci Lett 471:186–198 [Google Scholar]
- Yung Y.L., Chen P., Nealson K., Atreya S., and Beckett P. (2018) Methane on Mars and habitability: challenges and responses. Astrobiology 18:1221–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.