Abstract
Introduction
The prevalence of psychological disorders remains stable despite steady increases in pharmacological treatments suggesting the need for auxiliary treatment options. Consideration of the brain–gut–microbiota axis (BGMA) has made inroads into reconceptualizing psychological illness from a more holistic perspective. While our understanding of the precise role of gut microbiota (GM) in psychological illness is in its infancy, it represents an attractive target for novel interventions.
Method
An extensive review of relevant literature was undertaken.
Results
Gut microbiota are proposed to directly and indirectly influence mood, cognition, and behavior which are key components of mental health. This paper outlines how GM may be implicated in psychological disorders from etiology through to treatment and prevention using the Four P model of case formulation.
Conclusion
Moving forward, integration of GM into the conceptualization and treatment of psychological illness will require the discipline of psychology to undergo a significant paradigm shift. While the importance of the GM in psychological well‐being must be respected, it is not proposed to be a panacea, but instead, an additional arm to a multidisciplinary approach to treatment and prevention.
Keywords: allostatic load, gut microbiota, precipitating factors, predisposing factors, protective factors, psychology
Gut microbiota are proposed to influence mood, cognition, and behavior both directly and indirectly. This offers a new approach to conceptualizing psychological disorders from predisposition through to treatment. While not suggesting that targeting the gut microbiota is the panacea to all psychological conditions, it does offer an exciting new arm to a holistic and multidisciplinary approach to promoting psychological well‐being.
1. INTRODUCTION
Burgeoning research regarding the role of the gut and its microbial inhabitants in the pathophysiology of psychological illness is gaining momentum. Early evidence points to gut microbiota (GM) as a possible missing link in the conceptualization and treatment of psychological illness that sees disparities between conventional treatment methods and prevalence rates. When discussing the role of GM in behavior, health, and disease, it is important to pay respect to the intertwined coevolution between humans and our resident microbes. It is suggested that the sharp increase in various disease states over the last 50–100 years (Campell, 2014; Linneberg et al., 2000) can be, at least in part, explained by relatively recent dietary and lifestyle changes in the context of human evolution (Broussard & Devkota, 2016). Currently, humans, particularly those in industrialized countries, are living in an environment to which they have not adaptively evolved (Gluckman, Low, Buklijas, Hanson, & Beedle, 2011). An unintended consequence of industrialization, these changes are putting the GM under evolutionary pressure to shift from a previously mutualistic relationship with their human host to a more antagonistic one (Broussard & Devkota, 2016; Quercia et al., 2014). This is due to human evolution requiring significantly more time (Uyeda, Hansen, Arnold, & Pienaar, 2011) compared to single‐celled organisms such as GM that evolve and adapt to environmental and internal states much more rapidly (within as little as 24 hr; David et al., 2014; Wu et al., 2011). These variations in the evolutionary pressures and capabilities of the two components (host and GM) of a single ecosystem (the holobiont; Theis et al., 2016) result in systemic disharmony.
This systemic disharmony leads to symptomatology and disease states that are the primary target for intervention in current medical models, which precludes effective etiology‐focused prevention (Marvasti & Stafford, 2012). This is exacerbated given that current medical models are heavily skewed toward treatment over prevention (Singh, 2010). While there is disagreement over whether the occurrence of common mental disorders are increasing or whether their prevalence remains consistent (Friedrich, 2017; Harvey et al., 2017), the consumption of antidepressant drugs doubled in most, if not all, Organisation for Economic Co‐operation and Development (OECD) countries between 2000 and 2015 (OECD, 2017). Despite this, depression has recently taken over as the leading cause of disability worldwide with anxiety also in the top 10 leading causes of disability (World Health Organisation (WHO) 2017). Furthermore, subclinical psychological symptoms that do not meet the full Diagnostic and Statistical Manual for Mental Disorders (DSM) or International Classification of Diseases (ICD) diagnostic criteria are also prevalent (Angst, Merikangas, & Preisig, 1997; Haller, Cramer, Lauche, Gass, & Dobos, 2014; Mathieson, Collings, & Dowell, 2009). These subclinical symptoms lead to impairment in psychosocial and work functioning, are a major determinant of sick leave, contribute to the use of psychotropic drugs and primary health care services, and reduce quality of life (Haller et al., 2014; Johansen & Dittrich, 2013; Mendlowicz & Stein, 2000).
The conceptualization of the human body as being made up of separate and distinguishable systems is likely to have contributed to our current poor appreciation of the complexity of mechanisms that underlie the etiology and progression of disease. Psychology, just like many other healthcare professions and medical science disciplines, specialize and focus on specific body systems. For example, treatment of psychological symptoms and disorders tends to be central nervous system (CNS)‐centric with the brain being the principle target for both psychotherapy and psychopharmacology while peripheral systems, such as the gut, receive little attention. While there is no doubt that specialization has its benefits and has contributed to the progression of medical knowledge, it also has its drawbacks. Operating within these constrictive distinctions imposed by disciplinary specialization means that the complex interplay between various body systems essential to proper functioning is overlooked. The true etiology of disease is likely to come from dysregulations in this interplay which may also go some way to explaining high levels of comorbidity, particularly between functional gastrointestinal disorders such as IBS and psychological conditions (Garakani et al., 2003; Lee et al., 2015).
Within the field of psychology, an integral part of intervention is case formulation. Essentially, case formulation involves the synthesis of information about a patient (typically gained through clinical interviews and formalized cognitive testing) in a meaningful way to facilitate the development of a treatment plan. A commonly used model in structuring a case formulation is that of the Four Ps which represent the predisposing, precipitating, perpetuating, and protective factors related to a client's presenting problem and thus the targets of psychological intervention. As information is drawn from clinical interviews and cognitive testing, the functioning of a client's gut is seldom considered in their formulation; thus, important diagnostic and treatment options may be missed. It is the contention of this paper to demonstrate that GM are intimately linked with each of these four pillars of psychological intervention and thus each stage of disease, from etiology through to treatment and prevention. This paper adds to the burgeoning research into the brain–gut–microbiota axis (BGMA; Kelly, Clarke, Cryan, & Dinan, 2016) demonstrating that the discipline of psychology is on the cusp of a significant paradigm shift, moving away from CNS‐centric approaches toward a more holistic conceptualization of health and disease which integrates other body systems. We echo the sentiment of Allen, Dinan, Clarke, and Cryan (2017) who call for a challenge to the reductionist approaches in psychology in favor of a multidisciplinary approach to conceptualizing and treating psychological disorders. In taking the unique approach of the Four P model of case formulation, this paper intends to review existing research on associations between GM and psychological outcomes, compiling it in a way that is more accessible to psychologists, especially those who have little previous knowledge regarding the BGMA.
1.1. The brain–gut–microbiota axis
The BGMA is increasingly being recognized as playing an important role in homeostasis and consequentially, health and disease states (e.g., Mu, Yang, & Zhu, 2016; Rea, Dinan, & Cryan, 2016). The BGMA refers to the relationship between the brain and the gut while acknowledging the important moderating role of GM (e.g., Carabotti, Scirocco, Maselli, & Severi, 2015; Grenham, Clarke, Cryan, & Dinan, 2011). Largely recognized as a bidirectional relationship (e.g., Rhee, Pothoulakis, & Mayer, 2009), research continues to uncover the true complexities of this communication network which Rea et al. (2016) more accurately define as a multidirectional relationship. It is multidirectional in the sense that each component of this extensive communication network has the ability to moderate and manipulate the function of the other systems involved. Communication between the brain and the gut is maintained via a complex network including the CNS, autonomic nervous system (ANS), enteric nervous system (ENS), hypothalamic–pituitary–adrenal (HPA) axis, neural, endocrine and immune systems (e.g., Carabotti et al., 2015; Cryan & Dinan, 2012; Mayer, 2011; Moloney, Desbonnet, Clarke, Dinan, & Cryan, 2014). Essentially, the BGMA provides a network for signals from the brain to influence the motor, sensory, and secretory functions of the gut while simultaneously allowing signals and metabolites from the GM to influence brain development, biochemistry, function, and behavior (e.g., Cryan & O'Mahony, 2011; Grenham et al., 2011; Marques et al., 2014). This communication system presents an exciting and novel target for psychological intervention, providing a deeper understanding of the biological underpinnings of psychological illnesses. It is hoped that developing a greater understanding of the BGMA as it relates to the Four Ps of case formulation will provide psychologists with an additional tool in the treatment of their clients.
1.2. Is diet the chicken, or the egg?
The relationship between diet and GM is an intriguing one, ironically reminiscent of the idiom regarding which came first, the chicken or the egg. Once thought to be unidirectional (diet influencing the composition of the microbiota), recent evidence suggests the relationship could in fact be bidirectional, with microbes also being able to influence food choice and dietary‐related behaviors (Alcock, Maley, & Aktipis, 2014).
Diet is arguably one of the most important environmental factors in shaping the composition and metabolic activities of GM (De Filippo et al., 2017; Garcia‐Mantrana, Selma‐Royo, Alcantara, & Collado, 2018; Voreades, Kozil, & Weir, 2014). As such, diet must be taken into consideration when discussing potential interventions involving GM. An extensive review of the relationship between diet and GM is beyond the scope of this paper, but can be found elsewhere (e.g., Sheflin, Melby, Carbonero, & Weir, 2017; Singh et al., 2017; Wu et al., 2011). Here, diet is discussed insofar as to highlight to psychologists the importance of this environmental factor in the formulation and treatment of their client's presenting problem. While it is not being suggested that psychologists become well versed in dietetics, gathering general information on a client's diet may provide further insight into the development and perpetuation of their presenting problem, and presents as an additional arm to a multidisciplinary and holistic treatment approach.
Both the content and diversity of an individual's diet are believed to be important in maintaining a well‐balanced GM (Heiman & Greenway, 2016; Oriach, Robertson, Stanton, Cryan, & Dinan, 2016). Diet quality has also been highlighted as a potential risk or protective factor for conditions such as depression (Jacka et al., 2017; Koopman & El Aidy, 2017; Lai et al., 2014). In regards to dietary content, the industrial revolution saw a significant increase in highly processed cereals rich in carbohydrates, refined sugars, sodium, omega‐6, and trans‐fatty acids. Concurrently, potassium, complex carbohydrates, fiber, omega‐3, and unsaturated fatty acids were considerably reduced (Rubio‐Ruiz, Peredo‐Escárcega, Cano‐Martínez, & Guarner‐Lans, 2015). These changes are reflective of what is today termed the “Western‐style diet,” one that has been shown to impair immune function and promote inflammation (Myles, 2014). This is concerning given that inflammation is believed to underlie and perpetuate many, if not all, psychological and neurodegenerative illnesses (Almond, 2013; Miller & Raison, 2016; Rea et al., 2016). Worryingly, dietary diversity has been further reduced over the past 50 years with an ever increasing preference for convenience and taste (Glanz, Basil, Maibach, Goldberg, & Snyder, 1998; Heiman & Greenway, 2016; Poti, Mendez, Ng, & Popkin, 2015). Essentially, this means that current human diets are not providing GM with the resources they require to perform their myriad of complex tasks involved in host homeostasis and consequently health and disease. Adding support to this contention, Jacka et al. (2017) conducted a clinical trial which demonstrated that adherence to a modified Mediterranean diet resulted in significantly greater improvement in depression ratings from baseline compared to a social support control group.
While diet is one way through which a host can modulate their GM, microbes are themselves able to influence eating behaviors of their host (Alcock et al., 2014). Microbes in the gut must cooperate and share limited resources (space and nutrients) to promote stable coexistence and ecological diversity (Allen & Nowak, 2013). This means that these microorganisms are under selective pressure to ensure their own survival and must therefore compete for available resources (Hibbing, Fuqua, Parsek, & Peterson, 2010). As such, microbes are proposed to manipulate the eating behavior of the host by either generating cravings for foods that they thrive on or those which suppress their competitors, or by influencing mood which leads to the intake of foods that enhance that species' fitness (Alcock et al., 2014; Leitao‐Goncalves et al., 2017).
1.2.1. Dietary‐derived short‐chain fatty acids
A continued loss of fiber from the Western diet will inevitably lead to continued depletion of short‐chain fatty acids (SCFAs; Broussard & Devkota, 2016) with downstream effects on the development and perpetuation of psychological illnesses through their immunoregulatory effects (Rogers et al., 2016). SCFAs (acetic, butyric, and propionic acids in particular) are one of the main metabolites of GM (Carabotti et al., 2015; Smith et al., 2013). They are the end products of dietary fiber fermentation and have been shown to have many beneficial effects on host health (Bourassa, Alim, Bultman, & Ratan, 2016; den Besten et al., 2013).
In the brain, SCFAs demonstrate neuroprotective properties (Sun et al., 2015) with butyrate in particular having a protective effect on psychological and neurodegenerative disorders (Bourassa et al., 2016). Peripherally, SCFAs are believed to influence the size and function of regulatory T cells which play a crucial role in regulating inflammation and immune homeostasis (Hakansson & Molin, 2011; Smith et al., 2013). Additionally, SCFAs (together with enzymes also produced by the GM) enhance intestinal barrier functioning through their regulation of tight junction (TJ) proteins (e.g., Anderson et al., 2010; Bischoff et al., 2014; Peng, Li, Green, Holzman, & Lin, 2009). Abnormal intestinal permeability (leaky gut) results in increased translocation of toxins and GM across the epithelial barrier which consequently trigger an inflammatory immune response that can dysregulate ENS and systemic immune functioning (Berkes, Viswanathan, Savkovic, & Hecht, 2003; Carabotti et al., 2015; Fasano, 2012; Smith et al., 2013). This immune response is believed to be the instigator of resultant symptom expression including psychological disorders such as depression (e.g., Maes et al., 2013; Mulak & Bonaz, 2015; Sheedy et al., 2009).
Also via their influence on TJ proteins, SCFAs are believed to regulate the permeability of the blood–brain barrier (BBB; Braniste et al., 2014). Dysregulation of the BBB has been associated with neuropsychological conditions including Alzheimer's disease (Kuhnke et al., 2007) and autism (Fiorentino et al., 2016). Schoknecht and Shalev (2012) suggest that depression and schizophrenia may also be related to BBB dysfunction. Although further research is needed, these associations are highly plausible given the BBB is responsible for regulating access of circulating macromolecules and potential neurotoxins to the brain (Fiorentino et al., 2016; Patel & Frey, 2015). As evidence of microbial involvement, Braniste et al. (2014) found that germ‐free (GF) mice (those devoid of bacterial colonization and therefore lacking conventional gut flora) have increased BBB permeability compared to specific pathogen‐free (SPF) mice that have conventional GM colonization free of any known pathogens. Colonization of GF mice with known SCFA‐producing bacterial strains (Clostridium tyrobutyricum and Bacteroides thetaiotaomicron) was found to normalize BBB function (Braniste et al., 2014). There is also evidence to suggest that SCFAs are involved in glucose metabolism, reducing adiposity, appetite regulation, and energy homeostasis (Byrne, Chambers, Morrison, & Frost, 2015; Chambers et al., 2015; Kondo, Kishi, Fushimi, Ugajin, & Kaga, 2009; Morrison & Preston, 2016).
1.3. Behavior
Studies using GF mice have provided the greatest depth of information regarding the influence of GM on host behavior. Such preclinical work gives useful insights into the physiological mechanisms through which the BGMA functions. For example, GF mice demonstrate altered expression of brain‐derived neurotrophic factor (BDNF) and SCFA while also exhibiting altered HPA axis functioning, anxious and depressive behaviors, and social functioning (Arentsen, Raith, Qian, Forssberg, & Diaz Heijtz, 2015; Luczynski et al., 2016; Neufeld, Kang, Bienenstock, & Foster, 2011; Sudo et al., 2004). In both animal and human models, manipulation of GM has also been demonstrated to alter levels of stress hormones corticotropin‐releasing factor (CRF) and cortisol (Yarandi, Peterson, Treisman, Moran, & Pasricha, 2016). Many of these abnormalities have been shown to be rectified by colonization with the feces from SPF mice or with specific probiotics (e.g., Bercik et al., 2011; Desbonnet et al., 2010; Sudo et al., 2004). However, Bravo et al. (2011) found that ingestion of probiotics was only beneficial in mice which had an intact vagus nerve, demonstrating the importance of the vagal pathway in brain–gut communication. Additionally, Sudo et al. (2004) demonstrated that recolonization was only effective if it occurred within a critical period, providing evidence for a fundamental role of GM in the development of crucial systems involved in behavioral outcomes.
A recently proposed way in which GM may manipulate host behavior is via their relationship with personality traits. Kim et al. (2018) found an increased abundance of Gammaproteobacteria in those with high neuroticism, as well as those with low extraversion. Low conscientiousness was associated with an increased abundance of Proteobacteria and a decreased abundance of Lachnospiraceae while those with high levels of openness demonstrated greater phylogenic diversity and richness (Kim et al., 2018). As this was the first study to have investigated the link between GM and personality directly, further research is required to elucidate these relationships. The relationship between personality and GM presents as an intriguing area of exploration, given that personality traits have a strong association with behavioral patterns in addition to physiological and psychological health outcomes (e.g., Ferguson, 2013; Kim et al., 2018; Srivastava & Das, 2015).
Additional evidence linking GM composition and behavior comes from the study of patients following gastric bypass surgery. Behavioral changes following gastric bypass surgery include patients feeling less hungry and having a preference for healthier foods (Behary & Miras, 2015) which is likely related to changes in neural responses to food (particularly high‐calorie foods) in key areas of the mesolimbic reward pathway (Ochner et al., 2011, 2012). It is tempting to speculate that these changes are associated with the compositional changes in GM following gastric bypass surgery (Furet et al., 2010; Liou et al., 2013; Zhang et al., 2009). Additionally, improvements in quality of life and levels of depression have been shown to persist two years after surgery (Karlsson, Sjostrom, & Sullivan, 1998; Mokhber, Shaghayegh, Talebi, & Tavassoli, 2016). These improvements may be the result of reduced adipose tissue which has downstream effects on GM and their role in inflammation and other related functions. While causational evidence is currently unavailable, correlational research linking GM and mood (Jiang et al., 2015) suggest that changes in GM composition following gastric bypass surgery may also have a direct influence on mood. Improvements in mood may consequently encourage healthier food choices and eating behaviors (Christensen & Brooks, 2006), exemplifying the potential for a cyclical relationship involving diet, GM, and mood that is beneficial to overall health.
Given the possible role of GM in eating behaviors, and their ability to influence hunger and satiety (Cani et al., 2009) and neuropeptide and endocrine regulation (Holzer & Farzi, 2014), a relatively new line of inquiry has emerged investigating GM involvement in eating disorders which are traditionally recognized as psychological disorders (Kleiman et al., 2015; Lam, Maguire, Palacios, & Caterson, 2017). Associations between GM composition and eating disorder psychopathology were also found by Kleiman et al. (2015), further suggesting that the GM play a role in the psychology of food choice and eating behaviors. Given that diet is a key determinant of GM composition and that eating disorders are categorized by extreme dietary changes, the GM present as a logical target for inclusion in multifaceted intervention.
While this paper focuses mainly on unconscious mechanisms underlying the relationship between GM and psychological outcomes (such as interoceptive processes and neurotransmitter production), it is acknowledged that conscious mechanisms also have potential psychological implications. For example, gastrointestinal symptoms can be noticeably unpleasant and, particularly in IBS sufferers, can lead to impairment in daily functioning (Ballou, Bedell, & Keefer, 2015), anxiety and depression (Roohafza et al., 2016), avoidance behaviors (Van Oudenhove et al., 2016), and poor quality of life (Canavan, West, & Card, 2015). Conscious mechanisms can also lead to positive psychological outcomes as exemplified by patients following gastric bypass surgery. For example, noticeable changes in body composition can result in more positive body image which in itself is related to psychological well‐being, particularly after body contouring surgery (Jumbe, Hamlet, & Meyrick, 2017; Sarwer & Steffen, 2015; Song et al., 2016). These changes can then encourage long‐term weight loss maintenance behavior (Palmeira et al., 2010).
1.4. Neurotransmitters
Perhaps the most obvious association between GM and psychological illnesses is the ability of GM to manipulate the production and action of several key neurotransmitters (e.g., Anderson & Maes, 2015; Lyte, 2011; O'Mahony, Clarke, Borre, Dinan, & Cryan, 2015). GM regulate the metabolism and concentration of amino acids which serve as precursors for several neurotransmitters including gamma‐aminobutyric acid (GABA), serotonin, melatonin, and dopamine, among others (Clarke et al., 2014; Evrensel & Ceylan, 2015; Jenkins, Nguyen, Polglaze, & Bertrand, 2016; Zagajewski et al., 2012). As such, it is highly plausible that GM are able to influence brain chemistry, which consequentially regulates cognition, mood, and behavior. As depicted in Figure 1, demonstrating bidirectionality, GM can also be directly affected by neurochemicals which alter bacterial growth and pathogenicity (Lyte, 2011). Table 1 displays some of the key neurotransmitters synthesized by GM whose dysregulation is associated with psychological disorders. While neurotransmitters produced in the gut may not directly influence brain chemistry as they do not pass through the BBB, they are able to influence the CNS through mechanisms including direct stimulation of the vagus nerve, as well as using indirect circulatory and immune pathways (Sampson & Mazmanian, 2015). For example, tryptophan, the precursor molecule to serotonin (which is itself the precursor to melatonin), is able to pass through the BBB and as such it is likely that metabolites of GM directly influence brain chemistry (Sampson & Mazmanian, 2015).
Figure 1.
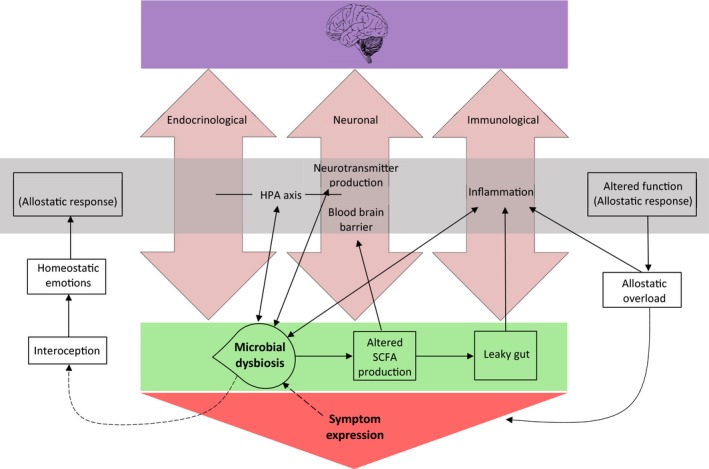
Factors influencing the multidirectional communication between the brain and the gut. Double‐headed arrows demonstrate a bidirectional relationship, with broken arrows demonstrating proposed but not yet established relationships. The figure demonstrates the three main well‐established pathways of communication between the brain and the gut, being endocrinological, neuronal, and immunological. The figure also illustrates the bidirectional relationships between microbial dysbiosis and the HPA axis, neurotransmitter production, the function of the blood–brain barrier, and inflammation which are believed to alter their functioning as an allostatic response to homeostatic emotions. It is proposed that microbial dysbiosis itself is able to be detected via the interoceptive system which then triggers these homeostatic emotions
Table 1.
Gut bacteria associated with the synthesis of key neurotransmitters
| Genus/species | Neurotransmitter (precursor) | CNS effect | Peripheral effect | Psychiatric conditions related to dysregulation | References |
|---|---|---|---|---|---|
| Candida; Streptococcus; Escherichia; Enterococcus; Lactobacillus bulgaricus | Serotonin (tryptophan) | Motor control, cerebellar regulation, synaptogenesis, addiction, emotion, memory, stress | Circadian rhythm, gut motility, body temperature, visceral pain, appetite, modulation of immune response | Depression, IBS, autism, Down's syndrome | Arreola et al., (2015), Gulesserian, Engidawork, Cairns, and Lubec (2000), Halford and Blundell, (2000), Hood et al. (2006), Jenkins et al. (2016), Leonard, (2010), Mazzoli and Pessione (2016), Marks et al. (2009), Meneses and Liy‐Salmeron (2012), Müller and Homberg (2015), Rogers et al. (2016), Stasi, Rosselli, Zignego, Laffi, and Milani (2014), Warren and Singh, (1996), Whitaker‐Azmitia (2001) |
| Corynebacterium glutamicum; Lactobacillus plantarum; Lactobacillus paracasei; Lactobacillus lactis; Brevibacterium lactofermentum; Brevibacterium flavum | l‐glutamate | Excitatory, brain development, synaptic plasticity | Generalized anxiety disorder, depression, bipolar, schizophrenia, neurodegeneration | Abdou et al. (2006), Boonstra et al. (2015), Cherlyn et al. (2010), Femenía, Gómez‐Galán, Lindskog, and Magara (2012), Hyland and Cryan (2010), Meldrum (2000), Yoto et al. (2012) | |
| Lactobacillus; Bifidobacterium; Escherichia coli; Pseudomonas | GABA (l‐glutamate) | Inhibitory, anxiolytic | Myorelaxant, moderates intestinal motility, gastric emptying, gastric acid secretion, and inhibits GI carcinogens and tumor growth | ||
| Bacillus, Serratia, E. coli | Dopamine (l‐Dopa) | Reward‐motivated behavior, motor behavior, cognition, emotion | Stimulates exocrine secretion, inhibits gut motility, and modulates sodium absorption and mucosal blood flow | Schizophrenia, Parkinson's disease, depression, anxiety, addiction | Di Chiara and Bassareo (2007), Eisenhofer et al. (1997), Freestone (2013), Grace (2016), Lyte (2011), Meyer and Feldon (2009), Scheperjans et al. (2015), Shishov, Kirovskaya, Kudrin, and Oleskin (2009) |
| Bacillus; E. coli; Saccharomyces | Norepinephrine (dopamine) | Stress hormone, attentiveness, emotion, sleep, learning | Mediates growth and virulence of potentially pathogenic bacteria | Depression, schizophrenia | Freestone (2013), Moret and Briley (2011), Yamamoto and Hornykiewicz (2004) |
| (dependent on tryptophan production and serotonin synthesis) | Melatonin (serotonin) | Circadian rhythm, mood | Gastrointestinal function, protects against gut permeability, anti‐inflammatory, antioxidant, analgesic | IBS, multiple sclerosis, autism, Alzheimer's, mood disorders | Fornaro, Prestia, Colicchio, and Perugi (2010), Ghorbani, Salari, Shaygannejad, and Norouzi (2013), Ortiz, Benítez‐King, Rosales‐Corral, Pacheco‐Moisés, and Velázquez‐Brizuela (2008), Veatch, Goldman, Adkins, and Malow (2015), Wong, Yang, Song, Wong, and Ho (2015) |
1.5. Interoception and allostatic responses
It is currently unknown whether the interoceptive system is able to detect microbial dysbiosis (imbalances resulting from the under‐ or overabundance of certain microbial species; DeGruttola, Low, Mizoguchi, & Mizoguchi, 2016), but considering that gut microbes are an essential part of human physiology which moderate several homeostatic emotions (Craig, 2002; Mayer, Naliboff, & Craig, 2006; Noakes, 2012; Paulus & Stein, 2010) it is a strong possibility. Homeostatic emotions are background emotions that may or may not enter conscious awareness but influence an individual's energy levels, mood, and disposition (Mayer et al., 2006). Signals from internal organs, particularly the gut, continuously communicate with various regions of the brain including the limbic system, autonomic and neuroendocrine centers in the hypothalamus, brainstem, and cortex (Craig, 2002; Holzer & Farzi, 2014; Mayer & Tillisch, 2011). It is plausible that through the process of interoception, GM are able to influence human cognition, emotion, and mood through their involvement in systemic functioning through their various metabolites (Holzer, 2017; Paulus & Stein, 2010). As such, increasing rates of disease might be explained by the allostatic load hypothesis (McEwen, 1998). The allostatic load hypothesis proposes that rather than having a stable set point, body systems have a range of set points allowing them to actively adapt to environmental and internal states. Allostatic load is a term used to refer to the cumulative cost of allostasis to the body (“wear and tear”; McEwen & Wingfield, 2003). While adaptive in the short term, allostasis can become maladaptive, leading to disease, when allostatic measures are required to vary widely and frequently, or are at extreme values for long periods of time (James, 2013). Additionally, allostatic systems can become dysfunctional when they lose their ability to change or regulate change (James, 2013). The result of either of these scenarios is allostatic overload which can lead to symptom expression, as depicted in Figure 1 (Berger, Juster, & Sarnyai, 2015; McEwen, 2005; McEwen & Wingfield, 2003). The fact that research has failed to define the precise composition of a healthy GM due to immense interindividual differences (Lloyd‐Price, Abu‐Ali, & Huttenhower, 2016) suggests that the GM may in fact be the most allostatic system within the body. Microbial dysbiosis then could be considered an extreme and prolonged shift away from what would be considered a relatively healthy composition which loses its ability to regulate change in various other host systems and functions. It is perhaps this dysregulation that manifests itself in psychological illness.
Figure 1 depicts the three overarching pathways (endocrinological, neuronal, and immunological) of bidirectional communication between the brain and the gut, each of which is altered during a state of microbial dysbiosis. Although it remains unconfirmed, it is proposed that microbial dysbiosis can be detected via interoception which ultimately leads to altered functioning of factors (e.g., inflammation) that mediate these pathways. These alterations are believed to be an allostatic response to a deviation from a “typical” microbiome, which over time, leads to symptom expression as a result of allostatic overload. The bidirectional relationship between microbial dysbiosis and the factors which alter the three main communication pathways between the gut and the brain illustrate the multidirectional nature of the BGMA.
2. GM THROUGH THE LENS OF A CASE FORMULATION FRAMEWORK
It is not the intention of this paper to propose that the BGMA must be at the forefront of consideration for each and every client. Instead, it is proposed that the relevance of the BGMA is determined on a case‐by‐case basis. The role of the BGMA in a client's presenting problem may be less relevant when there are clear social and emotional etiological factors, such as the presence of significant stressors for a client presenting with anxiety or depression. However, it is worth noting that stress can alter the composition of a person's GM (Bailey et al., 2011), which may or may not be clinically relevant, but should be considered if comorbidities are present. It may also be less relevant for clients who respond well to traditional psychological treatments such as cognitive behavioral therapy. Alternatively, cases in which the role or the BGMA may be more pertinent are when social and emotional etiological factors are less clear or absent, and also for clients who do not respond well to conventional psychological approaches. In cases where a treating clinician considers investigation of the GM to be appropriate, clients should be referred for stool sampling and analysis and an open dialogue established between the clinician and the microbiologist performing the analysis. The following information serves to highlight possible associations between GM and each of the Four Ps.
2.1. Predisposing
As part of their first line of questioning, mental health professionals attempt to explore a client's family history of psychological illness to establish whether that individual has an underlying genetic predisposition (vulnerability) to developing a psychological condition(s). Genetic predisposition to a multitude of psychological conditions has been well established (Hyman, 2000). While research into the role of GM in genetic predisposition is scarce, several lines of evidence suggest that GM may play an integral part in a person's vulnerability to the development of psychological illnesses. Firstly, host genetics have been demonstrated to influence the composition and metabolic activities of GM (Goodrich et al., 2014; Ussar, Fujisaka, & Kahn, 2016) which have important consequences on host physiology, brain development, and health (e.g., Krishnan, Alden, & Lee, 2015; Sekirov, Russell, Antunes, & Finlay, 2010). However, the specific mechanisms behind this relationship remain unclear (Dąbrowska & Witkiewicz, 2016).
2.1.1. Vertical transmission
Additionally, there is evidence to suggest that much like genetics are passed down from parents to offspring, GM are vertically transmitted from mother to infant (e.g., Asnicar et al., 2017; Mueller, Bakacs, Combellick, Grigoryan, & Dominguez‐Bello, 2015). The transmission of microbiota from mother to infant during birth represents the most important point of microbial colonization in the infant gut, which continues over the first three years of life (Yatsunenko et al., 2012). This critical establishment period of GM is in line with the critical development period of the human host (Rea et al., 2016). It is during this time that GM play key roles in the development of the CNS, HPA axis, and immune system (Borre et al., 2014; Cox et al., 2014; Furusawa et al., 2013; Houghteling & Walker, 2015). As such, aberrations in typical colonization of GM during this critical period may also result in abnormal development of these key systems (Tamburini, Shen, Wu, & Clemente, 2016). Factors resulting in aberrations to conventional microbial colonization of the gut during this period, such as birth by cesarean section and antibiotic treatment during infancy, have been associated with increased rates of chronic and atopic diseases (Kolokotroni et al., 2012; Sevelsted, Stokholm, Bonnelykke, & Bisgaard, 2015; Vangay, Ward, Gerber, & Knights, 2015). A recent study by Polidano, Zhu, and Bornstein (2017) highlights the microbiota as a potentially important factor in the negative relationship they found between cesarean birth and a range of cognitive outcomes compared to those born vaginally.
There is a growing consensus that maternal GM may have long‐term health consequences for the child (Stanislawski et al., 2017). It is therefore reasonable to suggest that vertically transmitted GM may act as a mechanism for intergenerational predisposition to psychological disorders. Further research is required as it is difficult to determine whether these intergenerational patterns are due to the vertical transmission of GM or whether they are due to learned behaviors and lifestyle factors shared among family members. This is evidenced by same‐household members showing a higher similarity of GM composition to those outside of the household (Abeles et al., 2016; Song et al., 2013; Yatsunenko et al., 2012).
2.1.2. Aging
Aging, in and of itself, can also predispose an individual to several psychological illnesses. For example, neurodegenerative illnesses, such as Alzheimer's disease, are considered an evolutionary accident occurring as a result of increased longevity (Giunta et al., 2008; Gluckman et al., 2011; Niccoli & Partridge, 2012). There is evidence to suggest that psychological disorders are also more prevalent in the elderly (e.g., Andreas et al., 2017). A contributing factor toward the increased prevalence of disease in the elderly is that the normal aging process is associated with compositional changes and reduced diversity of GM (Biagi et al., 2010). In parallel, normal aging is characterized by chronic low‐grade inflammation, a phenomenon commonly referred to as “inflammaging” (Franceschi et al., 2007). It is believed that this inflammation is, at least in part, attributable to alterations in GM (Buford, 2017). Additionally, aging is associated with changes in the serotonergic system which is also believed to contribute to increased prevalence of psychological disorders in the elderly (O'Mahony et al., 2015). The serotonergic system is regulated by GM (Table 1), therefore making it vulnerable to compositional and metabolic changes (O'Mahony et al., 2015; Rogers et al., 2016).
Given the sheer complexity of the systemic functioning of the human body, there are likely to be several other processes through which GM may be involved in predisposing individuals to the development of psychological illnesses. Further research into how GM influence predisposition to psychological illness will be useful in informing preventative strategies to circumvent the growing burden of such conditions. However, GM not only play a role in predisposing an individual to certain psychological illnesses as highlighted above, but also in the onset and maintenance of those negative health outcomes.
2.2. Precipitating and perpetuating
While psychologists tend to focus heavily on social and environmental factors involved in the onset and maintenance of psychological disorders, biological factors, such as GM composition, also contribute to these stages of disease. The maintenance of a diverse microbial ecosystem in the gut is essential for optimal host function (Moloney et al., 2014; Sekirov et al., 2010). On the other hand, reduced diversity and microbial dysbiosis have been implicated in various psychological, neurological, metabolic, functional gastrointestinal disorders, and autoimmune disease states (Blumstein, Levy, Mayer, & Harte, 2014). These include, but are not limited to, IBS (Jeffery et al., 2012; Tana et al., 2010), autism (Finegold et al., 2002), schizophrenia (Castro‐Nallar et al., 2015), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS; Fremont, Coomans, Massart, & Meirleir, 2013; Wallis, Butt, Ball, Lewis, & Bruck, 2016), multiple sclerosis (Jangi et al., 2016), dementia (Alkasir, Li, Li, Jin, & Zhu, 2017), stress (Knowles, Nelson, & Palombo, 2008), anxiety (Burch, 2016), depression (e.g., Jiang et al., 2015), obesity (Ley, Turnbaugh, Klein, & Gordon, 2006), diabetes (Larsen et al., 2010), coronary artery disease (Cui, Zhao, Hu, Zhang, & Hua, 2017; Emoto et al., 2017), and cancer (particularly colorectal cancer; Gagniere et al., 2016; Garrett, 2015). Current findings linking GM and disorders are, at this stage, associative and causative links are yet to be established. Additionally, it is unknown whether alterations in GM composition are the cause or consequence of disease. Given available evidence however, it is likely that this is a bidirectional, and cyclical, relationship as depicted in Figure 1.
2.2.1. Stress
Stress has long been recognized as both a precipitating and perpetuating factor to various psychological conditions (Anisman & Zacharko, 1982; Corcoran et al., 2003). Given the crucial role of GM in the development and functioning of the HPA axis (Sudo, 2012), as well as their modulation of stress hormones CRF and cortisol (Carabotti et al., 2015), the involvement of GM in the stress response is increasingly evident. This is further demonstrated by the ability to transfer stress‐prone phenotypes from one mouse to another via fecal transplantation (Collins, Kassam, & Bercik, 2013). Stress also activates an inflammatory response via the promotion of inflammatory cytokines (Liu, Wang, & Jiang, 2017). Given that inflammation is recognized as underlying many psychological and neurodegenerative disorders (Almond, 2013; Miller & Raison, 2016; Rea et al., 2016), stress then appears to play a role in both the etiology and maintenance of psychological illness via biological pathways, all of which are regulated by the GM. While this is reflective of a bottom‐up process whereby GM influence stress, substantial evidence also suggests the occurrence of a top‐down process through which stress regulates GM composition (Bailey et al., 2011; Gur, Worly, & Bailey, 2015; Knowles et al., 2008). The fact that both top‐down and bottom‐up processes have been well established demonstrates the complex cyclical and multidirectional relationship between GM, stress, and psychopathology.
2.2.2. Socioeconomic status
There is an established association between low SES and several factors negatively affecting health, one of which is poor diet (Darmon & Drewnowski, 2008; Shahar, Shai, Vardi, Shahar, & Fraser, 2005). Given that diet is the strongest environmental contributor to a properly functioning GM (e.g., De Filippo et al., 2017; Garcia‐Mantrana et al., 2018), it is possible that the composition and/or metabolic activities of the GM is one of the mediating factors of the relationship between low SES and mental health outcomes. Another factor associated with low SES is lower educational achievement (Sirin, 2005) which may also be mediated by diet‐related changes in GM. Individuals who eat a poor‐quality diet (Western diet) have poorer performance on cognitive tasks (Khan et al., 2015) and poorer mental health (Jacka, Kremer, et al., 2011; Jacka, Mykletun, Berk, Bjelland, & Tell, 2011; Markus et al., 1998) compared to those who eat high‐quality diets. In addition, an association has been found between the consumption of a Western diet and decreased left hippocampal volume (Jacka, Cherbuin, Anstey, Sachdev, & Butterworth, 2015) with hippocampal volume being related to cognition (Choi et al., 2016) and mood (Frodl et al., 2006). It is likely the GM mediate this relationship through their production of SCFAs and BDNF which have been found to be involved in neurogenesis and neuronal protection in mouse models (Canani, Di Costanzo, & Leone, 2012; Lee, Duan, & Mattson, 2002; Sun et al., 2015). Ultimately, poorer performance on cognitive tasks and poorer mental health limit a person's educational attainment (Eisenberg, Golberstein, & Hunt Justin, 2009; Fletcher, 2010; McLeod & Fettes, 2007).
2.3. Protective
Considering the protective abilities of GM or indeed, specific microorganisms, has the potential to revolutionize the treatment of psychological conditions (Kali, 2016; Mazzoli & Pessione, 2016; Sampson & Mazmanian, 2015). The addition of GM modulation to an individual's treatment plan may be the missing link in explaining and counteracting the alarming increase in the disease burden of common mental disorders.
2.3.1. Lifestyle factors
Healthy eating and exercise have long been promoted as being protective factors against both physiological and psychological conditions. Evidence suggests that one of the physiological mechanisms through which healthy eating and exercise affect health is the influence these factors have on the composition and metabolic activity of GM (Mika et al., 2015; Welly et al., 2016). Essentially, a high‐quality diet and exercise provide the GM with the resources they require to maintain optimal host function. While the influence of diet on GM composition is widely researched, that of exercise on GM receives less attention. However, exercise has been shown to enrich microbial diversity, improve the Bacteroidetes‐to‐Firmicutes ratio, and support the growth of SCFA‐producing bacteria which have immunomodulatory effects (Monda et al., 2017). This suggests that like diet, the beneficial outcomes of exercise may be mediated by GM which have downstream effects on mental health.
2.3.2. Pre‐ and probiotics
Both pre‐ and probiotics have also been demonstrated to have psychotropic like effects in healthy volunteers as well as those suffering from conditions such as depression and chronic fatigue syndrome (CFS; Akkasheh et al., 2016; Messaoudi et al., 2011; Rao et al., 2009). Probiotics showing positive effects on mental health are referred to as psychobiotics (Dinan, Stanton, & Cryan, 2013). Studies have demonstrated that various probiotic formulations (mostly including Lactobacillus and Bifidobacterium species) have the ability to improve mood in healthy (no reported diagnoses of allergic, neurological, or psychological conditions) men and women (Benton, Williams, & Brown, 2007; Messaoudi et al., 2011; Steenbergen, Sellaro, Hemert, Bosch, & Colzato, 2015). In a placebo‐controlled study, Yamamura et al. (2009) found a probiotic formulation to improve sleep efficacy and number of awakenings (as measured by actigraphy) in an elderly (60‐ to 81‐year‐old) sample. Moreover, a study using fMRI revealed altered activity in brain regions responsible for emotion and sensation processing in women following four weeks of probiotic formulation intake compared to women who received a placebo (Tillisch et al., 2013). Also in a placebo‐controlled study, participants who took a prebiotic (Bimuno‐galactooligosaccharides) daily for three weeks showed significantly lower salivary cortisol levels and decreased attentional vigilance to negative versus positive information (Schmidt et al., 2015). These findings were similar to those of a study that involved the administration of an SSRI (Murphy, Yiend, Lester, Cowen, & Harmer, 2009).
2.3.3. Fecal microbial transplant
The increasing popularity of fecal microbial transplant (FMT) in treating various conditions including but not limited to GI disorders (Brandt & Aroniadis, 2013), Parkinson's disease (Ananthaswamy, 2011), autism (Aroniadis & Brandt, 2013), and ME/CFS (Borody, Nowak, & Finlayson, 2012) is perhaps due to the proliferation of research associating microbial dysbiosis to a range of disorders. In humans, the efficacy of FMT has been shown for conditions such as ulcerative colitis (Shi et al., 2016), but has not yet been demonstrated in treating psychological conditions. It does however offer a promising avenue given strong evidence suggesting a role of GM in the pathogenesis of psychological conditions (Evrensel & Ceylan, 2016). Animal models suggest that FMT is an effective way to ameliorate abnormal physiology and function (e.g., Sudo et al., 2004); however, clinical trials are required to demonstrate whether this approach is equally effective in humans. Additionally, further research is required into the possible risks associated with FMT. While FMT has promising therapeutic potential, Alang and Kelly (2015) present a case study of a patient who developed obesity following FMT treatment from an overweight, but otherwise healthy donor. As GM are associated with numerous physiological and psychological conditions, FMT could theoretically result in the transference of any such condition from donor to recipient (Bunnik, Aarts, & Chen, 2017). As such, it is clear that great care must be taken when screening and selecting potential donors. There is still much to learn about the associations between GM and both physiological and psychological conditions, and therefore, potential long‐term risks of FMT may yet to emerge.
3. CRITICISMS OF CONVENTIONAL TREATMENT WITH RESPECT TO GM
Conventional treatment of psychological disorders typically involves pharmacological intervention such as psychotropics and/or other medications to alter brain chemistry (e.g., Bystritsky, Khalsa, Cameron, & Schiffman, 2013; Lieberman et al., 2005). Although beneficial, such treatments may induce undesirable side effects including, but not limited to, nausea, sleep disturbance, weight gain, and sexual dysfunction (Ferguson, 2001) all of which may themselves be a result of microbe‐mediated drug metabolism (Enright, Joyce, Gahan, & Griffin, 2017). Despite the ever increasing reliance on pharmacotherapy (Kallivayalil, 2008; Vozeh, 2003), disease states remain relatively stable which suggests the need for auxiliary treatment options and/or targets which take into account several body systems, including the GM. Many psychotropic drugs, known for their influence on CNS receptor function, also demonstrate antimicrobial effects (Kalayci, Demirci, & Sahin, 2014) which may have unintended consequences on the BGMA. This is particularly true of many SSRIs commonly used to treat depression and anxiety disorders.
In addition to having direct antimicrobial effects, Ayaz et al. (2015) found that sertraline (an SSRI) augments the effectiveness of several antibiotics by increasing their inhibitory zone. As such, chronic use of these drugs can induce potentially deleterious alterations in GM (Macedo et al., 2017). This may partially explain treatment resistance, although further research is needed to support this notion. Likewise, psychological interventions (e.g., cognitive behavior therapy) also target the brain via a focus on cognitions to affect behavioral change. This top‐down process has demonstrated efficacy in the treatment of functional gastrointestinal disorders such as IBS (Boersma et al., 2016; Palsson & Whitehead, 2013); however, research is needed to determine whether purely psychological interventions can enact changes in GM. By continuing to treat various disorders and symptoms through pharmacological intervention without considering the etiology of initial chemical imbalances, it is unlikely that rates of morbidity will decline. The net effect of ignoring etiology at the expense of treatment is therefore an increased burden upon individuals who are affected by disease, and wider society.
4. REDEFINING WHO WE ARE
There is currently a shift away from thinking of host–microbe interactions in such binary terms toward appreciating the complexity of the relationship between the two. Binary distinctions between host and microbiota remain useful only in so far as to aid our understanding of the role of GM in psychological well‐being, which is still in its infancy. However, emerging nomenclature such as “holobiont” acknowledges that GM are not a separate entity but are instead an integral and inseparable part of human biology (Schnorr, Sankaranarayanan, Lewis, & Warinner, 2016; Theis et al., 2016). This concept is supported by the coevolution of humans and their microbes. The concept that the ratio of bacterial cells to human cells is approximately 1:1 (recently revised down from previous estimations of 10:1; Sender, Fuchs, & Milo, 2016) pays homage to the importance of respecting GM in the conceptualization of human health and well‐being. This reconceptualization of what makes us human also provides a biological and tangible basis for explaining and treating psychological illnesses that can often be considered abstract.
5. CHALLENGES AND THE WAY FORWARD
Understanding the multidirectional relationship between GM and the nervous system is hindered by its inherent complexity (Mazzoli & Pessione, 2016). However, while still in its infancy, interdisciplinary research has uncovered novel ways of conceptualizing disease. While theoretically relevant, research into the BGMA also has important practical implications, offering a more holistic approach to treatment and prevention of psychological illness. While this new field of research is promising, it remains unclear which factors, and in which combination, alter the balance between symptomatic and asymptomatic outcomes.
The sheer number of confounding variables makes it difficult to establish causational links between GM and symptomatology. However, as understanding of the GM advances, so too will research methodology and technology. It is only with continued research into the link between GM and psychological illness that we will be able to elucidate the true extent to which our resident microbes contribute to mental health. As the majority of studies regarding the role of GM in both physiological and psychological health and disease have been conducted using animal models, clinical trials with human samples are imperative to the advancement of knowledge and ultimately practical application.
As the burgeoning research into the relationship between GM and psychological health outcomes gathers momentum, so too does the call to action for psychologists to embrace the microbiome as a potential factor in explaining, treating, and preventing mental illness. This is an important paradigm shift which must occur within the discipline of psychology in order to keep up to date with the most current and complete knowledge of the human body and mind which translates into providing the best possible care for clients. While it is unnecessary and impractical to expect psychologists to develop a detailed understanding of the influence of GM on mental health, it is important that the role of the GM is acknowledged, especially in the absence of clear social and emotional factors. Facilitating this paradigm shift may require change at a “grass roots” level, where psychobiology is better integrated into higher education psychology degrees. Additionally, professional development courses regarding the role of the GM in mental health should be established and promoted to current practicing psychologists who can use this information to provide more complete care for their clients.
The fact that GM are able to influence psychological functioning is an exciting and encouraging prospect, which begs for multidisciplinary approaches to both research and practice. In terms of practical implications, increasing our understanding of the mechanisms that mediate communication processes between GM and host has the potential to inform strategies to limit the damaging aspects of this communication. This will provide new avenues of treatment for a wide range of symptoms and disorders (Freestone, 2013) as well as to promote good health. This will require a substantial shift away from the reductionist approaches that see us working exclusively in our specific field. This is not to suggest that psychologists should become expert in areas outside of their field, but instead to understand and acknowledge that the best way forward is a multidisciplinary approach to the treatment and prevention of mental illness. A shift toward a multidisciplinary, and therefore more holistic, approach will provide an opportunity to better understand the etiology of disease which requires the expertise of several disciplines and a consideration of key body systems, including the GM, as intertwined and inseparable. In light of the evidence research has provided thus far, psychologists working as part of multidisciplinary teams with other professionals such as nutritionists, gastroenterologists, and microbiologists must seriously consider the inclusion of dietary plans, pre‐ and probiotics, and potentially even FMT in the treatment plans of their clients, in conjunction with conventional psychological treatments.
It is not the contention of this paper to claim that ameliorating gut health is the panacea to all psychological disorders and symptomatology. Instead, it is to demonstrate the complex interconnectivity between multiple body systems in disease processes, from etiology through to treatment, and ideally prevention. In support of the call to action by Allen et al. (2017), the discipline of psychology must shift away from its CNS‐centric conceptualization of disease and symptom‐centered disease treatment models toward a multidisciplinary approach to treatment and prevention. This paradigm shift will empower psychologists to better treat and care for their clients. A multidisciplinary approach where healthcare professionals across a variety of disciplines have a united approach to treatment and prevention will also empower the public to better understand and take control of their physiological and psychological health. It is only through this shared awareness that the healthcare community can make inroads into improving the mental health of current and future generations.
CONFLICT OF INTEREST
None declared.
Ganci M, Suleyman E, Butt H, Ball M. The role of the brain–gut–microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019;9:e01408 10.1002/brb3.1408
Funding information
This paper did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. However, the first author was supported by a postgraduate scholarship with no restrictions on publications cofunded by Bioscreen, an industry partner of Victoria University.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abdou, A. M. , Higashiguchi, S. , Horie, K. , Kim, M. , Hatta, H. , & Yokogoshi, H. (2006). Relaxation and immunity enhancement effects of gamma‐aminobutyric acid (GABA) administration in humans. BioFactors, 26(3), 201–208. [DOI] [PubMed] [Google Scholar]
- Abeles, S. R. , Jones, M. B. , Santiago‐Rodriguez, T. M. , Ly, M. , Klitgord, N. , Yooseph, S. , … Pride, D. T. (2016). Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome, 4(1), 39 10.1186/s40168-016-0187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh, G. , Kashani‐Poor, Z. , Tajabadi‐Ebrahimi, M. , Jafari, P. , Akbari, H. , Taghizadeh, M. , … Esmaillzadeh, A. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double‐blind, placebo‐controlled trial. Nutrition, 32(3), 315–320. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Alang, N. , & Kelly, C. R. (2015). Weight gain after fecal microbiota transplantation. Open Forum Infectious Diseases, 2(1), ofv004 10.1093/ofid/ofv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcock, J. , Maley, C. C. , & Aktipis, C. A. (2014). Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays, 36(10), 940–949. 10.1002/bies.201400071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkasir, R. , Li, J. , Li, X. , Jin, M. , & Zhu, B. (2017). Human gut microbiota: The links with dementia development. Protein Cell, 8(2), 90–102. 10.1007/s13238-016-0338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. P. , Dinan, T. G. , Clarke, G. , & Cryan, J. F. (2017). A psychology of the human brain‐gut‐microbiome axis. Social and Personality Psychology Compass, 11(4), e12309 10.1111/spc3.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, B. , & Nowak, M. A. (2013). Cooperation and the fate of microbial societies. PLoS Biology, 11(4), e1001549 10.1371/journal.pbio.1001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond, M. (2013). Depression and inflammation: Examining the link. Current Psychiatry, 12(6), 24–32.24443645 [Google Scholar]
- Ananthaswamy, A. (2011). Faecal transplant eases symptoms of Parkinson's disease. New Journal of Science, 209, 8–9. [Google Scholar]
- Anderson, G. , & Maes, M. (2015). The gut–brain axis: The role of melatonin in linking psychiatric, inflammatory and neurodegenerative conditions. Advances in Integrative Medicine, 2(1), 31–37. 10.1016/j.aimed.2014.12.007 [DOI] [Google Scholar]
- Anderson, R. C. , Cookson, A. L. , McNabb, W. C. , Park, Z. , McCann, M. J. , Kelly, W. J. , & Roy, N. C. (2010). Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiology, 10, 316 10.1186/1471-2180-10-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas, S. , Schulz, H. , Volkert, J. , Dehoust, M. , Sehner, S. , Suling, A. , … Härter, M. (2017). Prevalence of mental disorders in elderly people: The European MentDis_ICF65+ study. The British Journal of Psychiatry, 210(2), 125–131. 10.1192/bjp.bp.115.180463 [DOI] [PubMed] [Google Scholar]
- Angst, J. , Merikangas, K. R. , & Preisig, M. (1997). Subthreshold syndromes of depression and anxiety in the community. Journal of Clinical Psychiatry, 58(Suppl 8), 6–10. [PubMed] [Google Scholar]
- Anisman, H. , & Zacharko, R. (1982). Depression: The predisposing influence of stress. Behavioral and Brain Sciences, 5(1), 89–99. 10.1017/S0140525X00010633 [DOI] [Google Scholar]
- Arentsen, T. , Raith, H. , Qian, Y. , Forssberg, H. , & Diaz Heijtz, R. (2015). Host microbiota modulates development of social preference in mice. Microbial Ecology in Health & Disease, 26, 29719 10.3402/mehd.v26.29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadis, O. C. , & Brandt, L. J. (2013). Fecal microbiota transplantation: Past, present and future. Current Opinion in Gastroenterology, 29(1), 79–84. 10.1097/MOG.0b013e32835a4b3e [DOI] [PubMed] [Google Scholar]
- Arreola, R. , Becerril‐Villanueva, E. , Cruz‐Fuentes, C. , Velasco‐Velázquez, M. A. , Garcés‐Alvarez, M. E. , Hurtado‐Alvarado, G. , … Pavón, L. (2015). Immunomodulatory effects mediated by serotonin. Journal of Immunology Research, 2015, 354957 10.1155/2015/354957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnicar, F. , Manara, S. , Zolfo, M. , Truong, D. T. , Scholz, M. , Armanini, F. , … Segata, N. (2017). Studying vertical microbiome transmission from mothers to infants by strain‐level metagenomic profiling. mSystems, 2(1), e00164‐16 10.1128/mSystems.00164-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz, M. , Subhan, F. , Ahmed, J. , Khan, A.‐U. , Ullah, F. , Ullah, I. , … Hussain, S. (2015). Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. Journal of Biological Research‐Thessaloniki, 22(1), 4 10.1186/s40709-015-0028-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, M. T. , Dowd, S. E. , Galley, J. D. , Hufnagle, A. R. , Allen, R. G. , & Lyte, M. (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor‐induced immunomodulation. Brain, Behavior, and Immunity, 25(3), 397–407. 10.1016/j.bbi.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou, S. , Bedell, A. , & Keefer, L. (2015). Psychosocial impact of irritable bowel syndrome: A brief review. World Journal of Gastrointestinal Pathophysiology, 6(4), 120–123. 10.4291/wjgp.v6.i4.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behary, P. , & Miras, A. D. (2015). Food preferences and underlying mechanisms after bariatric surgery. The Proceedings of the Nutrition Society, 74(4), 419–425. 10.1017/s0029665115002074 [DOI] [PubMed] [Google Scholar]
- Benton, D. , Williams, C. , & Brown, A. (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. European Journal of Clinical Nutrition, 61(3), 355–361. 10.1038/sj.ejcn.1602546 [DOI] [PubMed] [Google Scholar]
- Bercik, P. , Park, A. J. , Sinclair, D. , Khoshdel, A. , Lu, J. , Huang, X. , … Verdu, E. F. (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut‐brain communication. Neurogastroenterology and Motility, 23(12), 1132–1139. 10.1111/j.1365-2982.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, M. , Juster, R. P. , & Sarnyai, Z. (2015). Mental health consequences of stress and trauma: Allostatic load markers for practice and policy with a focus on Indigenous health. Australas Psychiatry, 23(6), 644–649. 10.1177/1039856215608281 [DOI] [PubMed] [Google Scholar]
- Berkes, J. , Viswanathan, V. K. , Savkovic, S. D. , & Hecht, G. (2003). Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut, 52(3), 439–451. 10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi, E. , Nylund, L. , Candela, M. , Ostan, R. , Bucci, L. , Pini, E. , … De Vos, W. (2010). Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE, 5(5), e10667 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, S. C. , Barbara, G. , Buurman, W. , Ockhuizen, T. , Schulzke, J.‐D. , Serino, M. , … Wells, J. M. (2014). Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterology, 14(1), 189 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein, D. T. , Levy, K. , Mayer, E. , & Harte, J. (2014). Gastrointestinal dysbiosis. Evolution, Medicine, and Public Health, 2014(1), 163 10.1093/emph/eou029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma, K. , Ljótsson, B. , Edebol‐Carlman, H. , Schrooten, M. , Linton, S. J. , & Brummer, R. J. (2016). Exposure‐based cognitive behavioral therapy for irritable bowel syndrome. A single‐case experimental design across 13 subjects. Cognitive Behaviour Therapy, 45(6), 415–430. 10.1080/16506073.2016.1194455 [DOI] [PubMed] [Google Scholar]
- Boonstra, E. , de Kleijn, R. , Colzato, L. S. , Alkemade, A. , Forstmann, B. U. , & Nieuwenhuis, S. (2015). Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Frontiers in Psychology, 6, 1520 10.3389/fpsyg.2015.01520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody, T. J. , Nowak, A. , & Finlayson, S. (2012). The GI microbiome and its role in chronic fatigue syndrome: A summary of bacteriotherapy. Journal of the Australasian College of Nutritional and Environmental Medicine, 31(3), 3–8. [Google Scholar]
- Borre, Y. E. , O'Keeffe, G. W. , Clarke, G. , Stanton, C. , Dinan, T. G. , & Cryan, J. F. (2014). Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine, 20(9), 509–518. 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Bourassa, M. W. , Alim, I. , Bultman, S. J. , & Ratan, R. R. (2016). Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neuroscience Letters, 625, 56–63. 10.1016/j.neulet.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, L. J. , & Aroniadis, O. C. (2013). An overview of fecal microbiota transplantation: Techniques, indications, and outcomes. Gastrointestinal Endoscopy, 78(2), 240–249. 10.1016/j.gie.2013.03.1329 [DOI] [PubMed] [Google Scholar]
- Braniste, V. , Al‐Asmakh, M. , Kowal, C. , Anuar, F. , Abbaspour, A. , Toth, M. , … Pettersson, S. (2014). The gut microbiota influences blood‐brain barrier permeability in mice. Science Translational Medicine, 6(263), 263ra158 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo, J. A. , Forsythe, P. , Chew, M. V. , Escaravage, E. , Savignac, H. M. , Dinan, T. G. , … Cryan, J. F. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences USA, 108(38), 16050–16055. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard, J. L. , & Devkota, S. (2016). The changing microbial landscape of Western society: Diet, dwellings and discordance. Molecular Metabolism, 5(9), 737–742. 10.1016/j.molmet.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford, T. W. (2017). (Dis)Trust your gut: The gut microbiome in age‐related inflammation, health, and disease. Microbiome, 5(1), 80 10.1186/s40168-017-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnik, E. M. , Aarts, N. , & Chen, L. A. (2017). Physicians must discuss potential long‐term risks of fecal microbiota transplantation to ensure informed consent. The American Journal of Bioethics, 17(5), 61–63. 10.1080/15265161.2017.1299816 [DOI] [PubMed] [Google Scholar]
- Burch, J. D. (2016). Intestinal infection associated with future onset of an anxiety disorder: Results of a nationally representative study. Brain, Behaviour, and Immunity, 57, 222–226. 10.1016/j.bbi.2016.05.014 [DOI] [PubMed] [Google Scholar]
- Byrne, C. S. , Chambers, E. S. , Morrison, D. J. , & Frost, G. (2015). The role of short chain fatty acids in appetite regulation and energy homeostasis. International Journal of Obesity, 39(9), 1331–1338. 10.1038/ijo.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky, A. , Khalsa, S. S. , Cameron, M. E. , & Schiffman, J. (2013). Current diagnosis and treatment of anxiety disorders. Pharmacology & Therapeutics, 38(1), 30–57. [PMC free article] [PubMed] [Google Scholar]
- Campell, A. W. (2014). Autoimmunity and the gut. Autoimmune Diseases, 2014, 1–12. 10.1155/2014/152428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani, R. B. , Di Costanzo, M. , & Leone, L. (2012). The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clinical Epigenetics, 4(1), 4 10.1186/1868-7083-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan, C. , West, J. , & Card, T. (2015). Change in quality of life for patients with irritable bowel syndrome following referral to a gastroenterologist: A cohort study. PLoS ONE, 10(10), e0139389 10.1371/journal.pone.0139389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D. , Lecourt, E. , Dewulf, E. M. , Sohet, F. M. , Pachikian, B. D. , Naslain, D. , … Delzenne, N. M. (2009). Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. American Journal of Clinical Nutrition, 90(5), 1236–1243. 10.3945/ajcn.2009.28095 [DOI] [PubMed] [Google Scholar]
- Carabotti, M. , Scirocco, A. , Maselli, M. A. , & Severi, C. (2015). The gut‐brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology, 28(2), 203–209. [PMC free article] [PubMed] [Google Scholar]
- Castro‐Nallar, E. , Bendall, M. L. , Pérez‐Losada, M. , Sabuncyan, S. , Severance, E. G. , Dickerson, F. B. , … Crandall, K. A. (2015). Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ, 3, e1140 10.7717/peerj.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, E. S. , Viardot, A. , Psichas, A. , Morrison, D. J. , Murphy, K. G. , Zac‐Varghese, S. E. K. , … Frost, G. (2015). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut, 64(11), 1744–1754. 10.1136/gutjnl-2014-307913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlyn, S. Y. , Woon, P. S. , Liu, J. J. , Ong, W. Y. , Tsai, G. C. , & Sim, K. (2010). Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: A decade of advance. Neuroscience and Biobehavioral Reviews, 34(6), 958–977. 10.1016/j.neubiorev.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Choi, M.‐H. , Kim, H.‐S. , Gim, S.‐Y. , Kim, W.‐R. , Mun, K.‐R. , Tack, G.‐R. , … Chung, S.‐C. (2016). Differences in cognitive ability and hippocampal volume between Alzheimer's disease, amnestic mild cognitive impairment, and healthy control groups, and their correlation. Neuroscience Letters, 620, 115–120. 10.1016/j.neulet.2016.03.044 [DOI] [PubMed] [Google Scholar]
- Christensen, L. , & Brooks, A. (2006). Changing food preference as a function of mood. Journal of Psychology, 140(4), 293–306. 10.3200/jrlp.140.4.293-306 [DOI] [PubMed] [Google Scholar]
- Clarke, G. , Stilling, R. M. , Kennedy, P. J. , Stanton, C. , Cryan, J. F. , & Dinan, T. G. (2014). Minireview: Gut microbiota: The neglected endocrine organ. Molecular Endocrinology, 28(8), 1221–1238. 10.1210/me.2014-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S. M. , Kassam, Z. , & Bercik, P. (2013). The adoptive transfer of behavioral phenotype via the intestinal microbiota: Experimental evidence and clinical implications. Current Opinion in Microbiology, 16(3), 240–245. 10.1016/j.mib.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Corcoran, C. , Walker, E. , Huot, R. , Mittal, V. , Tessner, K. , Kestler, L. , & Malaspina, D. (2003). The stress cascade and schizophrenia: Etiology and onset. Schizophrenia Bulletin, 29(4), 671–692. 10.1093/oxfordjournals.schbul.a007038 [DOI] [PubMed] [Google Scholar]
- Cox, L. M. , Yamanishi, S. , Sohn, J. , Alekseyenko, A. V. , Leung, J. M. , Cho, I. , … Blaser, M. J. (2014). Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell, 158(4), 705–721. 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Cryan, J. F. , & Dinan, T. G. (2012). Mind‐altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701–712. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- Cryan, J. F. , & O'Mahony, S. M. (2011). The microbiome‐gut‐brain axis: From bowel to behavior. Neurogastroenterology and Motility, 23(3), 187–192. 10.1111/j.1365-2982.2010.01664.x [DOI] [PubMed] [Google Scholar]
- Cui, L. , Zhao, T. , Hu, H. , Zhang, W. , & Hua, X. (2017). Association study of gut flora in coronary heart disease through high‐throughput sequencing. BioMed Research International, 2017, 3796359 10.1155/2017/3796359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska, K. , & Witkiewicz, W. (2016). Correlations of host genetics and gut microbiome composition. Frontiers in Microbiology, 7, 1357 10.3389/fmicb.2016.01357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon, N. , & Drewnowski, A. (2008). Does social class predict diet quality? American Journal of Clinical Nutrition, 87(5), 1107–1117. 10.1093/ajcn/87.5.1107 [DOI] [PubMed] [Google Scholar]
- David, L. A. , Maurice, C. F. , Carmody, R. N. , Gootenberg, D. B. , Button, J. E. , Wolfe, B. E. , … Turnbaugh, P. J. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo, C. , Di Paola, M. , Ramazzotti, M. , Albanese, D. , Pieraccini, G. , Banci, E. , … Lionetti, P. (2017). Diet, environments, and gut microbiota: A preliminary investigation in children living in rural and urban Burkina Faso and Italy. Frontiers in Microbiology, 8, 1979 10.3389/fmicb.2017.01979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGruttola, A. K. , Low, D. , Mizoguchi, A. , & Mizoguchi, E. (2016). Current understanding of dysbiosis in disease in human and animal models. Inflammatory Bowel Diseases, 22(5), 1137–1150. 10.1097/MIB.0000000000000750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten, G. , van Eunen, K. , Groen, A. K. , Venema, K. , Reijngoud, D. J. , & Bakker, B. M. (2013). The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 54(9), 2325–2340. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet, L. , Garrett, L. , Clarke, G. , Kiely, B. , Cryan, J. F. , & Dinan, T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience, 170(4), 1179–1188. 10.1016/j.neuroscience.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. , & Bassareo, V. (2007). Reward system and addiction: What dopamine does and doesn't do. Current Opinion in Pharmacology, 7(1), 69–76. 10.1016/j.coph.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Dinan, T. G. , Stanton, C. , & Cryan, J. F. (2013). Psychobiotics: A novel class of psychotropic. Biological Psychiatry, 74(10), 720–726. 10.1016/j.biopsych.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Eisenberg, D. , Golberstein, E. , & Hunt Justin, B. (2009). Mental health and academic success in college. The B.E. Journal of Economic Analysis & Policy, 9(1), 1–37. 10.2202/1935-1682.2191 20098633 [DOI] [Google Scholar]
- Eisenhofer, G. , Åneman, A. , Friberg, P. , Hooper, D. , Fåndriks, L. , Lonroth, H. , … Mezey, E. (1997). Substantial production of dopamine in the human gastrointestinal tract. The Journal of Clinical Endocrinology & Metabolism, 82(11), 3864–3871. 10.1210/jcem.82.11.4339 [DOI] [PubMed] [Google Scholar]
- Emoto, T. , Yamashita, T. , Kobayashi, T. , Sasaki, N. , Hirota, Y. , Hayashi, T. , … Hirata, K.‐I. (2017). Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: Gut microbiota could be a diagnostic marker of coronary artery disease. Heart and Vessels, 32(1), 39–46. 10.1007/s00380-016-0841-y [DOI] [PubMed] [Google Scholar]
- Enright, E. F. , Joyce, S. A. , Gahan, C. G. , & Griffin, B. T. (2017). Impact of gut microbiota‐mediated bile acid metabolism on the solubilization capacity of bile salt micelles and drug solubility. Molecular Pharmaceutics, 14(4), 1251–1263. 10.1021/acs.molpharmaceut.6b01155 [DOI] [PubMed] [Google Scholar]
- Evrensel, A. , & Ceylan, M. E. (2015). The gut‐brain axis: The missing link in depression. Clinical Psychopharmacology and Neuroscience, 13(3), 239–244. 10.9758/cpn.2015.13.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrensel, A. , & Ceylan, M. E. (2016). Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clinical Psychopharmacology and Neuroscience, 14(3), 231–237. 10.9758/cpn.2016.14.3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano, A. (2012). Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clinical Gastroenterology and Hepatology, 10(10), 1096–1100. 10.1016/j.cgh.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenía, T. , Gómez‐Galán, M. , Lindskog, M. , & Magara, S. (2012). Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Research, 1476, 58–70. 10.1016/j.brainres.2012.03.053 [DOI] [PubMed] [Google Scholar]
- Ferguson, E. (2013). Personality is of central concern to understand health: Towards a theoretical model for health psychology. Health Psychology Review, 7(Suppl 1), S32–S70. 10.1080/17437199.2010.547985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, J. M. (2001). SSRI antidepressant medications: adverse effects and tolerability. Primary Care Companion to the Journal of Clinical Psychiatry, 3(1), 22–27. 10.4088/PCC.v03n0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold, S. M. , Molitoris, D. , Song, Y. , Liu, C. , Vaisanen, M.‐L. , Bolte, E. , … Kaul, A. (2002). Gastrointestinal microflora studies in late‐onset autism. Clinical Infectious Diseases, 35(Suppl 1), S6–S16. 10.1086/341914 [DOI] [PubMed] [Google Scholar]
- Fiorentino, M. , Sapone, A. , Senger, S. , Camhi, S. S. , Kadzielski, S. M. , Buie, T. M. , … Fasano, A. (2016). Blood‐brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Molecular Autism, 7, 49 10.1186/s13229-016-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, J. M. (2010). Adolescent depression and educational attainment: Results using sibling fixed effects. Health Economics, 19(7), 855–871. 10.1002/hec.1526 [DOI] [PubMed] [Google Scholar]
- Fornaro, M. , Prestia, D. , Colicchio, S. , & Perugi, G. (2010). A systematic, updated review on the antidepressant agomelatine focusing on its melatonergic modulation. Current Neuropharmacology, 8(3), 287–304. 10.2174/157015910792246227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi, C. , Capri, M. , Monti, D. , Giunta, S. , Olivieri, F. , Sevini, F. , … Salvioli, S. (2007). Inflammaging and anti‐inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development, 128(1), 92–105. 10.1016/j.mad.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Freestone, P. (2013). Communication between bacteria and their hosts. Scientifica, 2013, 1–15. 10.1155/2013/361073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont, M. , Coomans, D. , Massart, S. , & De Meirleir, K. (2013). High‐throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe, 22, 50–56. 10.1016/j.anaerobe.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Friedrich, M. (2017). Depression is the leading cause of disability around the world. JAMA, 317(15), 1517 10.1001/jama.2017.3826 [DOI] [PubMed] [Google Scholar]
- Frodl, T. , Schaub, A. , Banac, S. , Charypar, M. , Jäger, M. , Kümmler, P. , … Meisenzahl, E. M. (2006). Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of Psychiatry and Neuroscience, 31(5), 316–325. [PMC free article] [PubMed] [Google Scholar]
- Furet, J.‐P. , Kong, L.‐C. , Tap, J. , Poitou, C. , Basdevant, A. , Bouillot, J.‐L. , … Clement, K. (2010). Differential adaptation of human gut microbiota to bariatric surgery‐induced weight loss: Links with metabolic and low‐grade inflammation markers. Diabetes, 59(12), 3049–3057. 10.2337/db10-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa, Y. , Obata, Y. , Fukuda, S. , Endo, T. A. , Nakato, G. , Takahashi, D. , … Ohno, H. (2013). Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504(7480), 446–450. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Gagniere, J. , Raisch, J. , Veziant, J. , Barnich, N. , Bonnet, R. , Buc, E. , … Bonnet, M. (2016). Gut microbiota imbalance and colorectal cancer. World Journal of Gastroenterology, 22(2), 501–518. 10.3748/wjg.v22.i2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani, A. , Win, T. , Virk, S. , Gupta, S. , Kaplan, D. , & Masand, P. S. (2003). Comorbidity of irritable bowel syndrome in psychiatric patients: A review. American Journal of Therapeutics, 10(1), 61–67. 10.1097/00045391-200301000-00014 [DOI] [PubMed] [Google Scholar]
- Garcia‐Mantrana, I. , Selma‐Royo, M. , Alcantara, C. , & Collado, M. C. (2018). Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Frontiers in Microbiology, 9, 890 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, W. S. (2015). Cancer and the microbiota. Science, 348(6230), 80–86. 10.1126/science.aaa4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani, A. , Salari, M. , Shaygannejad, V. , & Norouzi, R. (2013). The role of melatonin in the pathogenesis of multiple sclerosis: A case‐control study. International Journal of Preventive Medicine, 4(Suppl 2), S180–S184. [PMC free article] [PubMed] [Google Scholar]
- Giunta, B. , Fernandez, F. , Nikolic, W. V. , Obregon, D. , Rrapo, E. , Town, T. , & Tan, J. (2008). Inflammaging as a prodrome to Alzheimer's disease. Journal of Neuroinflammation, 5, 51 10.1186/1742-2094-5-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz, K. , Basil, M. , Maibach, E. , Goldberg, J. , & Snyder, D. (1998). Why Americans eat what they do: Taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. Journal of the American Dietetic Association, 98(10), 1118–1126. 10.1016/s0002-8223(98)00260-0 [DOI] [PubMed] [Google Scholar]
- Gluckman, P. D. , Low, F. M. , Buklijas, T. , Hanson, M. A. , & Beedle, A. S. (2011). How evolutionary principles improve the understanding of human health and disease. Evolutionary Applications, 4(2), 249–263. 10.1111/j.1752-4571.2010.00164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, J. K. , Waters, J. L. , Poole, A. C. , Sutter, J. L. , Koren, O. , Blekhman, R. , … Ley, R. E. (2014). Human genetics shape the gut microbiome. Cell, 159(4), 789–799. 10.1016/j.cell.2014.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, A. A. (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews Neuroscience, 17(8), 524–532. 10.1038/nrn.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenham, S. , Clarke, G. , Cryan, J. F. , & Dinan, T. G. (2011). Brain‐gut‐microbe communication in health and disease. Frontiers in Physiology, 2, 94 10.3389/fphys.2011.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulesserian, T. , Engidawork, E. , Cairns, N. , & Lubec, G. (2000). Increased protein levels of serotonin transporter in frontal cortex of patients with Down syndrome. Neuroscience Letters, 296(1), 53–57. 10.1016/S0304-3940(00)01624-4 [DOI] [PubMed] [Google Scholar]
- Gur, T. L. , Worly, B. L. , & Bailey, M. T. (2015). Stress and the commensal microbiota: Importance in parturition and infant neurodevelopment. Frontiers in Psychiatry, 6, 5 10.3389/fpsyt.2015.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson, A. , & Molin, G. (2011). Gut microbiota and inflammation. Nutrients, 3(6), 637–682. 10.3390/nu3060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford, J. C. , & Blundell, J. E. (2000). Separate systems for serotonin and leptin in appetite control. Annals of Medicine, 32(3), 222–232. 10.3109/07853890008998829 [DOI] [PubMed] [Google Scholar]
- Haller, H. , Cramer, H. , Lauche, R. , Gass, F. , & Dobos, G. J. (2014). The prevalence and burden of subthreshold generalized anxiety disorder: A systematic review. BMC Psychiatry, 14(128), 1–13. 10.1186/1471-244X-14-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, S. B. , Deady, M. , Wang, M. , Mykletun, A. , Butterworth, P. , Christensen, H. , & Mitchell, P. B. (2017). Is the prevalence of mental illness increasing in Australia? Evidence from national health surveys and administrative data, 2001–2004. Medical Journal of Australia, 206(11), 490–493. 10.5694/mja16.00295 [DOI] [PubMed] [Google Scholar]
- Heiman, M. L. , & Greenway, F. L. (2016). A healthy gastrointestinal microbiome is dependent on dietary diversity. Molecular Metabolism, 5(5), 317–320. 10.1016/j.molmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbing, M. E. , Fuqua, C. , Parsek, M. R. , & Peterson, S. B. (2010). Bacterial competition: Surviving and thriving in the microbial jungle. Nature Reviews Microbiology, 8(1), 15–25. 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer, P. (2017). Interoception and gut feelings: Unconscious body signals' impact on brain function, behavior and belief processes In Angel H.‐F., Oviedo L., Paloutzian R. F., Runehov A. L. C., & Seitz R. J. (Eds.), New approaches to the scientific study of religion: Vol. 1. Processes of believing: The acquisition, maintenance, and change in creditions (pp. 435–442). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Holzer, P. , & Farzi, A. (2014). Neuropeptides and the microbiota‐gut‐brain axis. Advances in Experimental Medicine and Biology, 817, 195–219. 10.1007/978-1-4939-0897-4_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, S. D. , Hince, D. A. , Robinson, H. , Cirillo, M. , Christmas, D. , & Kaye, J. M. (2006). Serotonin regulation of the human stress response. Psychoneuroendocrinology, 31(9), 1087–1097. 10.1016/j.psyneuen.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Houghteling, P. D. , & Walker, W. A. (2015). Why is initial bacterial colonization of the intestine important to the infant's and child's health? Journal of Pediatric Gastroenterology and Nutrition, 60(3), 294–307. 10.1097/MPG.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland, N. P. , & Cryan, J. F. (2010). A gut feeling about GABA: Focus on GABA(B) receptors. Frontiers in Pharmacology, 1, 124 10.3389/fphar.2010.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, S. E. (2000). The genetics of mental illness: Implications for practice. Bulletin of the World Health Organization, 78(4), 455–463. [PMC free article] [PubMed] [Google Scholar]
- Jacka, F. N. , Cherbuin, N. , Anstey, K. J. , Sachdev, P. , & Butterworth, P. (2015). Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Medicine, 13, 215 10.1186/s12916-015-0461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka, F. N. , Kremer, P. J. , Berk, M. , de Silva‐Sanigorski, A. M. , Moodie, M. , Leslie, E. R. , … Swinburn, B. A. (2011). A prospective study of diet quality and mental health in adolescents. PLoS ONE, 6(9), e24805 10.1371/journal.pone.0024805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka, F. N. , Mykletun, A. , Berk, M. , Bjelland, I. , & Tell, G. S. (2011). The association between habitual diet quality and the common mental disorders in community‐dwelling adults: The Hordaland Health study. Psychosomatic Medicine, 73(6), 483–490. 10.1097/PSY.0b013e318222831a [DOI] [PubMed] [Google Scholar]
- Jacka, F. N. , O'Neil, A. , Opie, R. , Itsiopoulos, C. , Cotton, S. , Mohebbi, M. , … Berk, M. (2017). A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Medicine, 15(1), 23 10.1186/s12916-017-0791-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, G. D. (2013). Ambulatory blood pressure variation: Allostasis and adaptation. Autonomic Neuroscience, 177(2), 87–94. 10.1016/j.autneu.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Jangi, S. , Gandhi, R. , Cox, L. M. , Li, N. , von Glehn, F. , Yan, R. , … Weiner, H. L. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nature Communications, 7, 12015 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, I. B. , O'Toole, P. W. , Ohman, L. , Claesson, M. J. , Deane, J. , Quigley, E. M. , & Simren, M. (2012). An irritable bowel syndrome subtype defined by species‐specific alterations in faecal microbiota. Gut, 61(7), 997–1006. 10.1136/gutjnl-2011-301501 [DOI] [PubMed] [Google Scholar]
- Jenkins, T. A. , Nguyen, J. C. , Polglaze, K. E. , & Bertrand, P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut‐brain axis. Nutrients, 8(1), 56 10.3390/nu8010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Ling, Z. , Zhang, Y. , Mao, H. , Ma, Z. , Yin, Y. , … Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Johansen, T. , & Dittrich, W. H. (2013). Cognitive performance in a subclinical obsessive‐compulsive sample 1: Cognitive functions. Psychiatry Journal, 1, 1–10. 10.1155/2013/565191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbe, S. , Hamlet, C. , & Meyrick, J. (2017). Psychological aspects of bariatric surgery as a treatment for obesity. Current Obesity Reports, 6(1), 71–78. 10.1007/s13679-017-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayci, S. , Demirci, S. , & Sahin, F. (2014). Determination of antimicrobial properties of picaridin and DEET against a broad range of microorganisms. World Journal of Microbiology & Biotechnology, 30(2), 407–411. 10.1007/s11274-013-1456-4 [DOI] [PubMed] [Google Scholar]
- Kali, A. (2016). Psychobiotics: An emerging probiotic in psychiatric practice. Biomedical Journal, 39(3), 223–224. 10.1016/j.bj.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallivayalil, R. A. (2008). Are we over‐dependent on pharmacotherapy? Indian J Psychiatry, 50(1), 7–9. 10.4103/0019-5545.39750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, J. , Sjostrom, L. , & Sullivan, M. (1998). Swedish obese subjects (SOS)–an intervention study of obesity. Two‐year follow‐up of health‐related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. International Journal of Obesity and Related Metabolic Disorders, 22(2), 113–126. [DOI] [PubMed] [Google Scholar]
- Kelly, J. R. , Clarke, G. , Cryan, J. F. , & Dinan, T. G. (2016). Brain‐gut‐microbiota axis: Challenges for translation in psychiatry. Annals of Epidemiology, 26(5), 366–372. 10.1016/j.annepidem.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Khan, N. A. , Raine, L. B. , Drollette, E. S. , Scudder, M. R. , Kramer, A. F. , & Hillman, C. H. (2015). Dietary fiber is positively associated with cognitive control among prepubertal children. Journal of Nutrition, 145(1), 143–149. 10.3945/jn.114.198457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. , Yun, Y. , Ryu, S. , Chang, Y. , Kwon, M. , Cho, J. , … Kim, H. (2018). Correlation between gut microbiota and personality in adults: A cross‐sectional study. Brain, Behavior, and Immunity, 69, 374–385. 10.1016/j.bbi.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Kleiman, S. C. , Watson, H. J. , Bulik‐Sullivan, E. C. , Huh, E. Y. , Tarantino, L. M. , Bulik, C. M. , & Carroll, I. M. (2015). The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosomatic Medicine, 77(9), 969–981. 10.1097/PSY.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, S. R. , Nelson, E. A. , & Palombo, E. A. (2008). Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biological Psychology, 77(2), 132–137. 10.1016/j.biopsycho.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Kolokotroni, O. , Middleton, N. , Gavatha, M. , Lamnisos, D. , Priftis, K. N. , & Yiallouros, P. K. (2012). Asthma and atopy in children born by caesarean section: Effect modification by family history of allergies – a population based cross‐sectional study. BMC Pediatrics, 12, 179–179. 10.1186/1471-2431-12-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, T. , Kishi, M. , Fushimi, T. , Ugajin, S. , & Kaga, T. (2009). Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Bioscience, Biotechnology, and Biochemistry, 73(8), 1837–1843. 10.1271/bbb.90231 [DOI] [PubMed] [Google Scholar]
- Koopman, M. , & El Aidy, S. (2017). Depressed gut? The microbiota‐diet‐inflammation trialogue in depression. Current Opinion in Psychiatry, 30(5), 369–377. 10.1097/yco.0000000000000350 [DOI] [PubMed] [Google Scholar]
- Krishnan, S. , Alden, N. , & Lee, K. (2015). Pathways and functions of gut microbiota metabolism impacting host physiology. Current Opinion in Biotechnology, 36, 137–145. 10.1016/j.copbio.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke, D. , Jedlitschky, G. , Grube, M. , Krohn, M. , Jucker, M. , Mosyagin, I. , … Vogelgesang, S. (2007). MDR1‐P‐glycoprotein (ABCB1) mediates transport of Alzheimer's Amyloid‐β peptides—implications for the mechanisms of Aβ clearance at the blood‐brain barrier. Brain Pathology, 17(4), 347–353. 10.1111/j.1750-3639.2007.00075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, J. S. , Hiles, S. , Bisquera, A. , Hure, A. J. , McEvoy, M. , & Attia, J. (2014). A systematic review and meta‐analysis of dietary patterns and depression in community‐dwelling adults. American Journal of Clinical Nutrition, 99(1), 181–197. 10.3945/ajcn.113.069880 [DOI] [PubMed] [Google Scholar]
- Lam, Y. Y. , Maguire, S. , Palacios, T. , & Caterson, I. D. (2017). Are the gut bacteria telling us to eat or not to eat? Reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients, 9(6), 602 10.3390/nu9060602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, N. , Vogensen, F. K. , van den Berg, F. W. J. , Nielsen, D. S. , Andreasen, A. S. , Pedersen, B. K. , … Jakobsen, M. (2010). Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS ONE, 5(2), e9085 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Duan, W. , & Mattson, M. P. (2002). Evidence that brain‐derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry, 82(6), 1367–1375. 10.1046/j.1471-4159.2002.01085.x [DOI] [PubMed] [Google Scholar]
- Lee, Y.‐T. , Hu, L.‐Y. , Shen, C.‐C. , Huang, M.‐W. , Tsai, S.‐J. , Yang, A. C. , … Hung, J.‐H. (2015). Risk of psychiatric disorders following irritable bowel syndrome: A nationwide population‐based cohort study. PLoS ONE, 10(7), e0133283 10.1371/journal.pone.0133283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão‐Gonçalves, R. , Carvalho‐Santos, Z. , Francisco, A. P. , Fioreze, G. T. , Anjos, M. , Baltazar, C. , … Ribeiro, C. (2017). Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biology, 15(4), e2000862 10.1371/journal.pbio.2000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, B. E. (2010). The concept of depression as a dysfunction of the immune system. Current Immunology Reviews, 6(3), 205–212. 10.2174/157339510791823835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R. E. , Turnbaugh, P. J. , Klein, S. , & Gordon, J. I. (2006). Microbial ecology: Human gut microbes associated with obesity. Nature, 444(7122), 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Lieberman, J. A. , Stroup, T. S. , McEvoy, J. P. , Swartz, M. S. , Rosenheck, R. A. , Perkins, D. O. , … Hsiao, J. K. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine, 353(12), 1209–1223. 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- Linneberg, A. , Nielsen, N. H. , Madsen, F. , Frølund, L. , Dirksen, A. , & Jørgensen, T. (2000). Increasing prevalence of specific IgE to aeroallergens in an adult population: Two cross‐sectional surveys 8 years apart: The Copenhagen Allergy Study. The Journal of Allergy and Clinical Immunology, 106(2), 247–252. 10.1067/mai.2000.108312 [DOI] [PubMed] [Google Scholar]
- Liou, A. P. , Paziuk, M. , Luevano, J.‐M. , Machineni, S. , Turnbaugh, P. J. , & Kaplan, L. M. (2013). Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science Translational Medicine, 5(178), 178ra141 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. Z. , Wang, Y. X. , & Jiang, C. L. (2017). Inflammation: The common pathway of stress‐related diseases. Frontiers in Human Neuroscience, 11, 316 10.3389/fnhum.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Price, J. , Abu‐Ali, G. , & Huttenhower, C. (2016). The healthy human microbiome. Genome Medicine, 8(1), 51 10.1186/s13073-016-0307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski, P. , McVey Neufeld, K. A. , Oriach, C. S. , Clarke, G. , Dinan, T. G. , & Cryan, J. F. (2016). Growing up in a bubble: Using germ‐free animals to assess the influence of the gut microbiota on brain and behavior. International Journal of Neuropsychopharmacology, 19(8), pyw020 10.1093/ijnp/pyw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays, 33(8), 574–581. 10.1002/bies.201100024 [DOI] [PubMed] [Google Scholar]
- Macedo, D. , Filho, A. J. , Soares de Sousa, C. N. , Quevedo, J. , Barichello, T. , Junior, H. V. , & Freitas de Lucena, D. (2017). Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. Journal of Affective Disorders, 208, 22–32. 10.1016/j.jad.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Maes, M. , Kubera, M. , Leunis, J. C. , Berk, M. , Geffard, M. , & Bosmans, E. (2013). In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS‐damaged neoepitopes. Acta Psychiatrica Scandinavica, 127(5), 344–354. 10.1111/j.1600-0447.2012.01908.x [DOI] [PubMed] [Google Scholar]
- Marks, D. M. , Shah, M. J. , Patkar, A. A. , Masand, P. S. , Park, G. Y. , & Pae, C. U. (2009). Serotonin‐norepinephrine reuptake inhibitors for pain control: Premise and promise. Current Neuropharmacology, 7(4), 331–336. 10.2174/157015909790031201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus, C. R. , Panhuysen, G. , Tuiten, A. , Koppeschaar, H. , Fekkes, D. , & Peters, M. L. (1998). Does carbohydrate‐rich, protein‐poor food prevent a deterioration of mood and cognitive performance of stress‐prone subjects when subjected to a stressful task? Appetite, 31(1), 49–65. 10.1006/appe.1997.0155 [DOI] [PubMed] [Google Scholar]
- Marques, T. M. , Cryan, J. F. , Shanahan, F. , Fitzgerald, G. F. , Ross, R. P. , Dinan, T. G. , & Stanton, C. (2014). Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut–brain axis. Innovative Food Science & Emerging Technologies, 22, 239–247. 10.1016/j.ifset.2013.10.016 [DOI] [Google Scholar]
- Marvasti, F. F. , & Stafford, R. S. (2012). From sick care to health care — reengineering prevention into the U.S. system. New England Journal of Medicine, 367(10), 889–891. 10.1056/NEJMp1206230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson, F. , Collings, S. , & Dowell, A. (2009). Sub‐threshold mental health syndromes: Finding an alternative to the medication of unhappiness. Journal of Primary Health Care, 1(1), 74–77. [PubMed] [Google Scholar]
- Mayer, E. A. (2011). Gut feelings: The emerging biology of gut‐brain communication. Nature Reviews Neuroscience, 12(8), 453–466. 10.1038/nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, E. A. , Naliboff, B. D. , & Craig, A. D. (2006). Neuroimaging of the brain‐gut axis: From basic understanding to treatment of functional GI disorders. Gastroenterology, 131(6), 1925–1942. 10.1053/j.gastro.2006.10.026 [DOI] [PubMed] [Google Scholar]
- Mayer, E. A. , & Tillisch, K. (2011). The brain‐gut axis in abdominal pain syndromes. Annual Review of Medicine, 62, 381–396. 10.1146/annurev-med-012309-103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoli, R. , & Pessione, E. (2016). The neuro‐endocrinological role of microbial glutamate and GABA signaling. Frontiers in Microbiology, 7, 1934 10.3389/fmicb.2016.01934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2005). Stressed or stressed out: What is the difference? Journal of Psychiatry and Neuroscience, 30(5), 315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. , & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- McLeod, J. D. , & Fettes, D. L. (2007). Trajectories of failure: The educational careers of children with mental health problems. American Journal of Sociology, 113(3), 653–701. 10.1086/521849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum, B. S. (2000). Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. Journal of Nutrition, 130(4S Suppl), 1007s–1015s. 10.1093/jn/130.4.1007S [DOI] [PubMed] [Google Scholar]
- Mendlowicz, M. V. , & Stein, M. B. (2000). Quality of life in individuals with anxiety disorders. The American Journal of Psychiatry, 157(5), 669–682. 10.1176/appi.ajp.157.5.669 [DOI] [PubMed] [Google Scholar]
- Meneses, A. , & Liy‐Salmeron, G. (2012). Serotonin and emotion, learning and memory. Reviews in the Neurosciences, 23(5–6), 543–553. 10.1515/revneuro-2012-0060 [DOI] [PubMed] [Google Scholar]
- Messaoudi, M. , Violle, N. , Bisson, J. F. , Desor, D. , Javelot, H. , & Rougeot, C. (2011). Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes, 2(4), 256–261. 10.4161/gmic.2.4.16108 [DOI] [PubMed] [Google Scholar]
- Meyer, U. , & Feldon, J. (2009). Prenatal exposure to infection: A primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl), 206(4), 587–602. 10.1007/s00213-009-1504-9 [DOI] [PubMed] [Google Scholar]
- Mika, A. , Van Treuren, W. , Gonzalez, A. , Herrera, J. J. , Knight, R. , & Fleshner, M. (2015). Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male f344 rats. PLoS ONE, 10(5), e0125889 10.1371/journal.pone.0125889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. H. , & Raison, C. L. (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhber, N. , Shaghayegh, H. , Talebi, M. , & Tavassoli, A. (2016). Comparison of levels of depression in patients with excessive obesity before and after gastric bypass surgery. Journal of Minimally Invasive Surgical Sciences, 5(4), e34947 10.17795/minsurgery-34947 [DOI] [Google Scholar]
- Moloney, R. D. , Desbonnet, L. , Clarke, G. , Dinan, T. G. , & Cryan, J. F. (2014). The microbiome: Stress, health and disease. Mammalian Genome, 25(1–2), 49–74. 10.1007/s00335-013-9488-5 [DOI] [PubMed] [Google Scholar]
- Monda, V. , Villano, I. , Messina, A. , Valenzano, A. , Esposito, T. , Moscatelli, F. , … Messina, G. (2017). Exercise modifies the gut microbiota with positive health effects. Oxidative Medicine and Cellular Longevity, 2017, 8 10.1155/2017/3831972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret, C. , & Briley, M. (2011). The importance of norepinephrine in depression. Neuropsychiatric Disease and Treatment, 7(Suppl 1), 9–13. 10.2147/NDT.S19619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, D. J. , & Preston, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes, 7(3), 189–200. 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, C. , Yang, Y. , & Zhu, W. (2016). Gut microbiota: the brain peacekeeper. Frontiers in Microbiology, 7, 345 10.3389/fmicb.2016.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, N. T. , Bakacs, E. , Combellick, J. , Grigoryan, Z. , & Dominguez‐Bello, M. G. (2015). The infant microbiome development: Mom matters. Trends in Molecular Medicine, 21(2), 109–117. 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulak, A. , & Bonaz, B. (2015). Brain‐gut‐microbiota axis in Parkinson's disease. World Journal of Gastroenterology, 21(37), 10609–10620. 10.3748/wjg.v21.i37.10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, C. P. , & Homberg, J. R. (2015). The role of serotonin in drug use and addiction. Behavioral Brain Research, 277, 146–192. 10.1016/j.bbr.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Murphy, S. E. , Yiend, J. , Lester, K. J. , Cowen, P. J. , & Harmer, C. J. (2009). Short‐term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. International Journal of Neuropsychopharmacology, 12(2), 169–179. 10.1017/S1461145708009164 [DOI] [PubMed] [Google Scholar]
- Myles, I. A. (2014). Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutrition Journal, 13, 61 10.1186/1475-2891-13-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, K. M. , Kang, N. , Bienenstock, J. , & Foster, J. A. (2011). Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterology and Motility, 23(3), 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Niccoli, T. , & Partridge, L. (2012). Ageing as a risk factor for disease. Current Biology, 22(17), R741–752. 10.1016/j.cub.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Noakes, T. D. (2012). Fatigue is a brain‐derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Frontiers in Physiology, 3, 82 10.3389/fphys.2012.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner, C. N. , Kwok, Y. , Conceição, E. , Pantazatos, S. P. , Puma, L. M. , Carnell, S. , … Geliebter, A. (2011). Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Annals of Surgery, 253(3), 502–507. 10.1097/SLA.0b013e318203a289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner, C. N. , Stice, E. , Hutchins, E. , Afifi, L. , Geliebter, A. , Hirsch, J. , & Teixeira, J. (2012). Relation between changes in neural responsivity and reductions in desire to eat high‐calorie foods following gastric bypass surgery. Neuroscience, 209, 128–135. 10.1016/j.neuroscience.2012.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2017). Health at a glance 2017: OECD indicators. Paris, France: OECD Publishing. [Google Scholar]
- O'Mahony, S. M. , Clarke, G. , Borre, Y. E. , Dinan, T. G. , & Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain‐gut‐microbiome axis. Behavioral Brain Research, 277, 32–48. 10.1016/j.bbr.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Oriach, C. S. , Robertson, R. C. , Stanton, C. , Cryan, J. F. , & Dinan, T. G. (2016). Food for thought: The role of nutrition in the microbiota‐gut–brain axis. Clinical Nutrition Experimental, 6, 25–38. 10.1016/j.yclnex.2016.01.003 [DOI] [Google Scholar]
- Ortiz, G. G. , Benítez‐King, G. A. , Rosales‐Corral, S. A. , Pacheco‐Moisés, F. P. , & Velázquez‐Brizuela, I. E. (2008). Cellular and biochemical actions of melatonin which protect against free radicals: role in neurodegenerative disorders. Current Neuropharmacology, 6(3), 203–214. 10.2174/157015908785777201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira, A. L. , Branco, T. L. , Martins, S. C. , Minderico, C. S. , Silva, M. N. , Vieira, P. N. , … Teixeira, P. J. (2010). Change in body image and psychological well‐being during behavioral obesity treatment: Associations with weight loss and maintenance. Body Image, 7(3), 187–193. 10.1016/j.bodyim.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Palsson, O. S. , & Whitehead, W. E. (2013). Psychological treatments in functional gastrointestinal disorders: A primer for the gastroenterologist. Clinical Gastroenterology and Hepatology, 11(3), 208–216; quiz e222–203. 10.1016/j.cgh.2012.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, J. P. , & Frey, B. N. (2015). Disruption in the blood‐brain barrier: The missing link between brain and body inflammation in bipolar disorder? Neural Plasticity, 2015, 12 10.1155/2015/708306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, M. P. , & Stein, M. B. (2010). Interoception in anxiety and depression. Brain Structure and Function, 214(5–6), 451–463. 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. , Li, Z. R. , Green, R. S. , Holzman, I. R. , & Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP‐activated protein kinase in Caco‐2 cell monolayers. Journal of Nutrition, 139(9), 1619–1625. 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidano, C. , Zhu, A. , & Bornstein, J. C. (2017). The relation between caesarean birth and child cognitive development. Scientific Reports, 7(11483), 1–10. 10.1038/s41598-017-10831-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poti, J. M. , Mendez, M. A. , Ng, S. W. , & Popkin, B. M. (2015). Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? American Journal of Clinical Nutrition, 101(6), 1251–1262. 10.3945/ajcn.114.100925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quercia, S. , Candela, M. , Giuliani, C. , Turroni, S. , Luiselli, D. , Rampelli, S. , … Pirazzini, C. (2014). From lifetime to evolution: Timescales of human gut microbiota adaptation. Frontiers in Microbiology, 5, 1–9. 10.3389/fmicb.2014.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A. V. , Bested, A. C. , Beaulne, T. M. , Katzman, M. A. , Iorio, C. , Berardi, J. M. , & Logan, A. C. (2009). A randomized, double‐blind, placebo‐controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens, 1(1), 6 10.1186/1757-4749-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea, K. , Dinan, T. G. , & Cryan, J. F. (2016). The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress, 4, 23–33. 10.1016/j.ynstr.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, S. H. , Pothoulakis, C. , & Mayer, E. A. (2009). Principles and clinical implications of the brain‐gut‐enteric microbiota axis. Nature Reviews Gastroenterology & Hepatology, 6(5), 306–314. 10.1038/nrgastro.2009.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, G. B. , Keating, D. J. , Young, R. L. , Wong, M. L. , Licinio, J. , & Wesselingh, S. (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21(6), 738–748. 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohafza, H. , Bidaki, E. Z. , Hasanzadeh‐Keshteli, A. , Daghaghzade, H. , Afshar, H. , & Adibi, P. (2016). Anxiety, depression and distress among irritable bowel syndrome and their subtypes: An epidemiological population based study. Advanced Biomedical Research, 5, 183 10.4103/2277-9175.190938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio‐Ruiz, M. E. , Peredo‐Escárcega, A. E. , Cano‐Martínez, A. , & Guarner‐Lans, V. (2015). An evolutionary perspective of nutrition and inflammation as mechanisms of cardiovascular disease. International Journal of Evolutionary Biology, 2015, 1–10. 10.1155/2015/179791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson, T. R. , & Mazmanian, S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell Host & Microbe, 17(5), 565–576. 10.1016/j.chom.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwer, D. B. , & Steffen, K. J. (2015). Quality of life, body image and sexual functioning in bariatric surgery patients. European Eating Disorders Review, 23(6), 504–508. 10.1002/erv.2412 [DOI] [PubMed] [Google Scholar]
- Scheperjans, F. , Aho, V. , Pereira, P. A. B. , Koskinen, K. , Paulin, L. , Pekkonen, E. , … Auvinen, P. (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement Disorders, 30(3), 350–358. 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- Schmidt, K. , Cowen, P. J. , Harmer, C. J. , Tzortzis, G. , Errington, S. , & Burnet, P. W. (2015). Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl), 232(10), 1793–1801. 10.1007/s00213-014-3810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr, S. L. , Sankaranarayanan, K. , Lewis, C. M. Jr , & Warinner, C. (2016). Insights into human evolution from ancient and contemporary microbiome studies. Current Opinion in Genetics & Development, 41, 14–26. 10.1016/j.gde.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoknecht, K. , & Shalev, H. (2012). Blood‐brain barrier dysfunction in brain diseases: Clinical experience. Epilepsia, 53(Suppl 6), 7–13. 10.1111/j.1528-1167.2012.03697.x [DOI] [PubMed] [Google Scholar]
- Sekirov, I. , Russell, S. L. , Antunes, L. C. , & Finlay, B. B. (2010). Gut microbiota in health and disease. Physiological Reviews, 90(3), 859–904. 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- Sender, R. , Fuchs, S. , & Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biology, 14(8), e1002533 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelsted, A. , Stokholm, J. , Bonnelykke, K. , & Bisgaard, H. (2015). Cesarean section and chronic immune disorders. Pediatrics, 135(1), e92–e98. 10.1542/peds.2014-0596 [DOI] [PubMed] [Google Scholar]
- Shahar, D. , Shai, I. , Vardi, H. , Shahar, A. , & Fraser, D. (2005). Diet and eating habits in high and low socioeconomic groups. Nutrition, 21(5), 559–566. 10.1016/j.nut.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Sheedy, J. R. , Wettenhall, R. E. , Scanlon, D. , Gooley, P. R. , Lewis, D. P. , McGregor, N. , … De Meirleir, K. L. (2009). Increased d‐lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo, 23(4), 621–628. [PubMed] [Google Scholar]
- Sheflin, A. , Melby, C. L. , Carbonero, F. , & Weir, T. L. (2017). Linking dietary patterns with gut microbial composition and function. Gut Microbes, 8(2), 113–129. 10.1080/19490976.2016.1270809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Dong, Y. , Huang, W. , Zhu, D. , Mao, H. , & Su, P. (2016). Fecal microbiota transplantation for ulcerative colitis: A systematic review and meta‐analysis. PLoS ONE, 11(6), e0157259 10.1371/journal.pone.0157259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishov, V. A. , Kirovskaya, T. A. , Kudrin, V. S. , & Oleskin, A. V. (2009). Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K‐12. Applied Biochemistry and Microbiology, 45(5), 494–497. 10.1134/S0003683809050068 [DOI] [PubMed] [Google Scholar]
- Singh, A. R. (2010). Modern medicine: Towards prevention, cure, well‐being and longevity. Mens Sana Monographs, 8(1), 17–29. 10.4103/0973-1229.58817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. K. , Chang, H.‐W. , Yan, D. I. , Lee, K. M. , Ucmak, D. , Wong, K. , … Liao, W. (2017). Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 15(1), 73 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin, S. R. (2005). Socioeconomic status and academic achievement: a meta‐analytic review of research. Review of Educational Research, 75(3), 417–453. 10.3102/00346543075003417 [DOI] [Google Scholar]
- Smith, P. M. , Howitt, M. R. , Panikov, N. , Michaud, M. , Gallini, C. A. , Bohlooly‐Y, M. , … Garrett, W. S. (2013). The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341(6145), 569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, P. , Patel, N. B. , Gunther, S. , Li, C.‐S. , Liu, Y. , Lee, C. Y. G. , … Wong, M. S. (2016). Body image and quality of life: changes with gastric bypass and body contouring. Annals of Plastic Surgery, 76(Suppl 3), S216–S221. 10.1097/SAP.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. J. , Lauber, C. , Costello, E. K. , Lozupone, C. A. , Humphrey, G. , Berg‐Lyons, D. , … Knight, R. (2013). Cohabiting family members share microbiota with one another and with their dogs. eLife, 2, e00458 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, K. , & Das, R. C. (2015). Personality and health: Road to well‐being. Industrial Psychiatry Journal, 24(1), 1–4. 10.4103/0972-6748.160905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski, M. A. , Dabelea, D. , Wagner, B. D. , Sontag, M. K. , Lozupone, C. A. , & Eggesbø, M. (2017). Pre‐pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome, 5(1), 113 10.1186/s40168-017-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi, C. , Rosselli, M. , Zignego, A. L. , Laffi, G. , & Milani, S. (2014). Serotonin and its implication in the side‐effects of interferon‐based treatment of patients with chronic viral hepatitis: Pharmacological interventions. Hepatology Research, 44(1), 9–16. 10.1111/hepr.12116 [DOI] [PubMed] [Google Scholar]
- Steenbergen, L. , Sellaro, R. , van Hemert, S. , Bosch, J. A. , & Colzato, L. S. (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity, 48, 258–264. 10.1016/j.bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Sudo, N. (2012). Role of microbiome in regulating the HPA axis and its relevance to allergy. Chemical Immunology and Allergy, 98, 163–175. 10.1159/000336510 [DOI] [PubMed] [Google Scholar]
- Sudo, N. , Chida, Y. , Aiba, Y. , Sonoda, J. , Oyama, N. , Yu, X.‐N. , … Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. Journal of Physiology, 558(Pt 1), 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Wang, F. , Li, H. , Zhang, H. , Jin, J. , Chen, W. , … Liu, C. (2015). Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. BioMed Research International, 2015, 395895 10.1155/2015/395895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini, S. , Shen, N. , Wu, H. C. , & Clemente, J. C. (2016). The microbiome in early life: Implications for health outcomes. Nature Medicine, 22(7), 713–722. 10.1038/nm.4142 [DOI] [PubMed] [Google Scholar]
- Tana, C. , Umesaki, Y. , Imaoka, A. , Handa, T. , Kanazawa, M. , & Fukudo, S. (2010). Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterology and Motility, 22(5), 512–519, e114–515. 10.1111/j.1365-2982.2009.01427.x [DOI] [PubMed] [Google Scholar]
- Theis, K. R. , Dheilly, N. M. , Klassen, J. L. , Brucker, R. M. , Baines, J. F. , Bosch, T. C. G. , … Bordenstein, S. R. (2016). Getting the hologenome concept right: An eco‐evolutionary framework for hosts and their microbiomes. mSystems, 1(2), e00028‐16 10.1128/mSystems.00028-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch, K. , Labus, J. , Kilpatrick, L. , Jiang, Z. , Stains, J. , Ebrat, B. , … Mayer, E. A. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology, 144(7), 1394–1401, 1401.e1391–1394. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussar, S. , Fujisaka, S. , & Kahn, C. R. (2016). Interactions between host genetics and gut microbiome in diabetes and metabolic syndrome. Molecular Metabolism, 5(9), 795–803. 10.1016/j.molmet.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda, J. C. , Hansen, T. F. , Arnold, S. J. , & Pienaar, J. (2011). The million‐year wait for macroevolutionary bursts. Proceedings of the National Academy of Sciences USA, 108(38), 15908–15913. 10.1073/pnas.1014503108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oudenhove, L. , Crowell, M. D. , Drossman, D. A. , Halpert, A. D. , Keefer, L. , Lackner, J. M. , … Levy, R. L. (2016). Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology, 150(6), 1355–1367. 10.1053/j.gastro.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangay, P. , Ward, T. , Gerber, J. S. , & Knights, D. (2015). Antibiotics, pediatric dysbiosis, and disease. Cell Host & Microbe, 17(5), 553–564. 10.1016/j.chom.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch, O. J. , Goldman, S. E. , Adkins, K. W. , & Malow, B. A. (2015). Melatonin in children with autism spectrum disorders: How does the evidence fit together? Journal of Nature and Science, 1(7), e125. [PMC free article] [PubMed] [Google Scholar]
- Voreades, N. , Kozil, A. , & Weir, T. L. (2014). Diet and the development of the human intestinal microbiome. Frontiers in Microbiology, 5(494), 1–9. 10.3389/fmicb.2014.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozeh, S. (2003). Is the increasing use of evidence‐based pharmacotherapy causing the renaissance of complementary medicine? British Journal of Clinical Pharmacology, 56(3), 292–296. 10.1046/j.0306-5251.2003.01879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, A. , Butt, H. , Ball, M. , Lewis, D. P. , & Bruck, D. (2016). Support for the microgenderome: Associations in a human clinical population. Scientific Reports, 6, 1–9. 10.1038/srep19171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, R. P. , & Singh, V. K. (1996). Elevated serotonin levels in autism: Association with the major histocompatibility complex. Neuropsychobiology, 34(2), 72–75. 10.1159/000119295 [DOI] [PubMed] [Google Scholar]
- Welly, R. J. , Liu, T.‐W. , Zidon, T. M. , Rowles, J. L. , Park, Y.‐M. , Smith, T. N. , … Vieira‐Potter, V. J. (2016). Comparison of diet vs. exercise on metabolic function & gut microbiota in obese rats. Medicine and Science in Sports and Exercise, 48(9), 1688–1698. 10.1249/MSS.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker‐Azmitia, P. M. (2001). Serotonin and brain development: Role in human developmental diseases. Brain Research Bulletin, 56(5), 479–485. 10.1016/S0361-9230(01)00615-3 [DOI] [PubMed] [Google Scholar]
- Wong, R. K. , Yang, C. , Song, G.‐H. , Wong, J. , & Ho, K.‐Y. (2015). Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double‐blinded placebo study. Digestive Diseases and Sciences, 60(1), 186–194. 10.1007/s10620-014-3299-8 [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) (2017). Depression (Fact sheet No. 369). Retrieved from http://www.who.int/mediacentre/factsheets/fs369/en/ [Google Scholar]
- Wu, G. D. , Chen, J. , Hoffmann, C. , Bittinger, K. , Chen, Y.‐Y. , Keilbaugh, S. A. , … Lewis, J. D. (2011). Linking long‐term dietary patterns with gut microbial enterotypes. Science, 334(6052), 105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, K. , & Hornykiewicz, O. (2004). Proposal for a noradrenaline hypothesis of schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 28(5), 913–922. 10.1016/j.pnpbp.2004.05.033 [DOI] [PubMed] [Google Scholar]
- Yamamura, S. , Morishima, H. , Kumano‐go, T. , Suganuma, N. , Matsumoto, H. , Adachi, H. , … Takeda, M. (2009). The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. European Journal of Clinical Nutrition, 63(1), 100–105. 10.1038/sj.ejcn.1602898 [DOI] [PubMed] [Google Scholar]
- Yarandi, S. S. , Peterson, D. A. , Treisman, G. J. , Moran, T. H. , & Pasricha, P. J. (2016). Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. Journal of Neurogastroenterology and Motility, 22(2), 201–212. 10.5056/jnm15146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko, T. , Rey, F. E. , Manary, M. J. , Trehan, I. , Dominguez‐Bello, M. G. , Contreras, M. , … Gordon, J. I. (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222–227. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoto, A. , Murao, S. , Motoki, M. , Yokoyama, Y. , Horie, N. , Takeshima, K. , … Yokogoshi, H. (2012). Oral intake of gamma‐aminobutyric acid affects mood and activities of central nervous system during stressed condition induced by mental tasks. Amino Acids, 43(3), 1331–1337. 10.1007/s00726-011-1206-6 [DOI] [PubMed] [Google Scholar]
- Zagajewski, J. , Drozdowicz, D. , Brzozowska, I. , Hubalewska‐Mazgaj, M. , Stelmaszynska, T. , Laidler, P. M. , & Brzozowski, T. (2012). Conversion L‐tryptophan to melatonin in the gastrointestinal tract: The new high performance liquid chromatography method enabling simultaneous determination of six metabolites of L‐tryptophan by native fluorescence and UV‐VIS detection. Journal of Physiology and Pharmacology, 63(6), 613–621. [PubMed] [Google Scholar]
- Zhang, H. , DiBaise, J. K. , Zuccolo, A. , Kudrna, D. , Braidotti, M. , Yu, Y. , … Krajmalnik‐Brown, R. (2009). Human gut microbiota in obesity and after gastric bypass. Proceedings of the National Academy of Sciences USA, 106(7), 2365–2370. 10.1073/pnas.0812600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


