Abstract
In blood, the primary role of red blood cells (RBCs) is to transport oxygen via highly regulated mechanisms involving hemoglobin (Hb). Hb is a tetrameric porphyrin protein comprising of two α- and two β-polypeptide chains, each containing an iron-containing heme group capable of binding one oxygen molecule. In military as well as civilian traumatic exsanguinating hemorrhage, rapid loss of RBCs can lead to suboptimal tissue oxygenation and subsequent morbidity and mortality. In such cases, transfusion of whole blood or RBCs can significantly improve survival. However, blood products including RBCs present issues of limited availability and portability, need for type matching, pathogenic contamination risks, and short shelf-life, causing substantial logistical barriers to their prehospital use in austere battlefield and remote civilian conditions. While robust research is being directed to resolve these issues, parallel research efforts have emerged toward bioengineering of semisynthetic and synthetic surrogates of RBCs, using various cross-linked, polymeric, and encapsulated forms of Hb. These Hb-based oxygen carriers (HBOCs) can potentially provide therapeutic oxygenation when blood or RBCs are not available. Several of these HBOCs have undergone rigorous preclinical and clinical evaluation, but have not yet received clinical approval in the USA for human use. While these designs are being optimized for clinical translations, several new HBOC designs and molecules have been reported in recent years, with unique properties. The current article will provide a comprehensive review of such HBOC designs, including current state-of-the-art and novel molecules in development, along with a critical discussion of successes and challenges in this field.
Keywords: Blood, hemoglobin, hemorrhage, oxygen carrier, RBC, RBC surrogate
INTRODUCTION
In austere battlefield conditions and remote civilian locations, trauma-associated uncontrolled hemorrhage and acute coagulopathy remain one of the leading causes of mortality (1–6). In such scenarios, transfusion of whole blood and blood components (e.g., RBCs, platelets, and plasma), as per Damage Control Resuscitation guidelines, can significantly reduce trauma-associated morbidities and mortalities (7–9). However, the limited availability and portability, special storage requirements, and high contamination risks of these blood products often present severe logistical challenges for their prehospital application in military and civilian scenarios, for point-of-injury immediate and prolonged (e.g., en route) field care (10–17). A robust volume of research is currently being dedicated toward resolving these issues and enhancing the availability and applicability of donor-derived blood products in the field (18–21). In parallel, an exciting area of research has emerged that focuses on the development and evaluation of semisynthetic or synthetic surrogates of blood products that can be manufactured at large scale in vitro (i.e., sufficient availability), can be sterilized and stored as small volume deliverables over long periods of time at various temperature ranges (i.e., easy portability), can be reconstituted and administered “on demand” in far forward scenarios (i.e., prehospital applicability), can potentially avoid the need for type matching (universal application with minimal immunogenic risk), can circulate safely upon intravascular administration without systemic risks, and can mimic, leverage and amplify endogenous mechanisms of blood component function to mitigate the effects of traumatic exsanguinating hemorrhage (22–24). This field of research has developed in the areas of functionally mimicking blood’s cellular as well as non-cellular components and continues to focus on resolving translational challenges with regards to biocompatibility, safety, prehospital availability and universal applicability.
The research focus on preserving and transporting donor-derived blood started during World War I to treat wounded soldiers, and blood transfusions became widely available by World War II. Multiple blood banks were established in the United States from the 1950s onward and blood donation was promoted as a form of civic responsibility. Subsequent development of processes and methodologies for isolation and storage of various blood components has significantly enhanced the utilization of whole blood and its components. Currently, transfusions of whole blood as well as various isolated components are clinically approved for applications in civilian and battlefield trauma (e.g., in Damage Control Resuscitation), surgical settings (e.g., transplants), chronic and acute anemias, and disease-associated, drug-induced or congenital bleeding disorders (25–32). RBC transfusion is clinically significant in efficient mitigation of hemorrhagic shock, as part of the Massive Transfusion Protocol in hypoper-fused patients with critical levels of oxygen (33–37). It has also been demonstrated that prehospital use of RBC transfusion (if available) can significantly improve survival in critically injured subjects (38, 39). Such transfusions are dependent on donor-derived RBC products (e.g., packed Red Blood Cell [pRBC]). However, according to the Red Cross, only ~ 40% of US population is eligible to donate blood at any given time and only 10% to 15% actually donate. In addition, blood-based products have somewhat limited shelf-life due to risks of pathogenic contamination. Currently, RBCs have a shelf-life of 20 to 40 days, while platelet suspensions have a shelf-life of 3 to 5 days, at room temperature (40). Also, RBCs (and platelets) develop storage lesions in storage, which affect their stability, in vivo circulation lifetime, and bioactive functions (41, 42). Significant research is being undertaken enhancing the shelf-life of blood products by cold-storage, freezing, lyophilisation, etc., as well as, through development of pathogen reduction technologies like psoralen-based or riboflavin-based ultra-violet irradiation, extensive serological testing of donor blood, leukoreduction, and specialized storage protocols (13, 19, 31,43–48). Also, portability of blood products, especially to remote battlefield and civilian locations, especially for prehospital point-of-care use, continues to be a major logistical challenge (14, 17, 49).
Such challenges can be potentially addressed by engineering of semisynthetic or synthetic surrogates of blood components (22, 50, 51). In fact, the interest in such synthetic surrogates developed during the HIV crisis of the 1980s due to fear of contaminated blood products (4) and this research has been going on for past several decades, with several designs and products that have progressed through preclinical and clinical evaluations. However, currently no such product is clinically approved by the Food and Drug Administration for human applications in the United States, although certain products have been approved for human use in South Africa and veterinary use in the United States. A 2008 meta-analysis of 16 clinical trials of five different RBC surrogates suggested increased health risks in patients treated with such products (52). Although such analysis has resulted in some apprehension in clinical safety and utility of these products, the design of this analysis has been strongly debated and it has also directed significant re-emphasis on understanding the pros and cons of these products at fundamental physiological and mechanistic levels. To this end, the current categorization of such products has shifted from “RBC substitutes” to “oxygenation therapeutics” so as to emphasize the important role of such products in scenarios where real RBCs may not be sufficiently available (e.g., far forward military setting) as well as in perfusion of transplantable organs. In this framework, the current article will focus on reviewing “hemoglobin-based oxygen carriers” (HBOCs), comprehensively discussing relevant designs, current state-of-art and novel molecules in development, along with emphasizing successes and challenges. To this end, representative preclinical and clinical findings will be emphasized, but in vitro and in vivo findings of individual designs will not be comprehensively discussed.
Hemoglobin (Hb) function in RBCs for oxygen transport
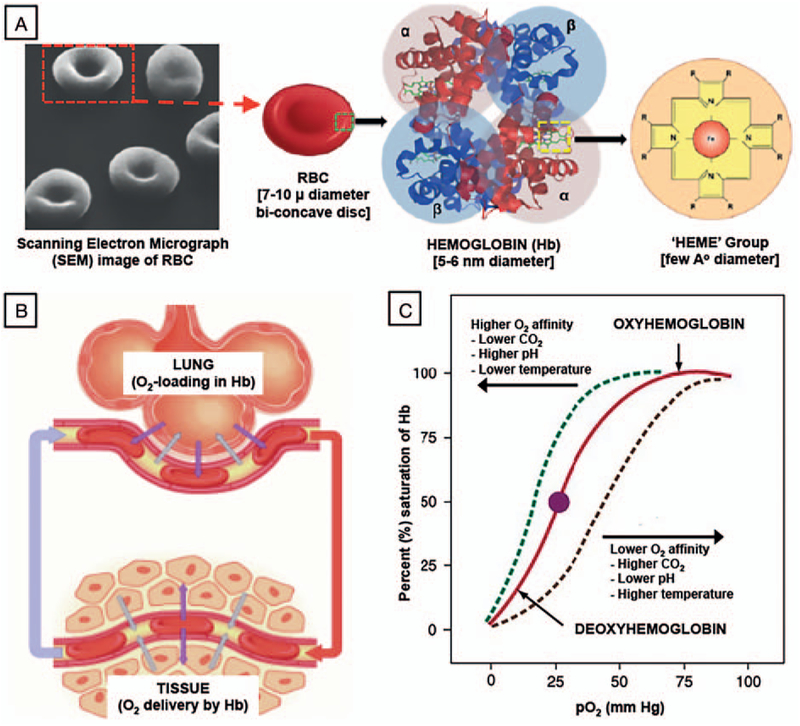
In blood, the primary function of RBCs is the transport of oxygen (O2) and to some extent carbon dioxide to and from tissues, by virtue of binding of the gases to hemoglobin (Hb) within the RBCs. The average amount of Hb in adult human RBCs (mean corpuscular hemoglobin [MCH]) is 27 to 31 picograms per cell (~250 million Hb molecules). Hb is a tetrameric protein comprising of two α- and two β-polypeptide chains, each consisting of an iron-containing heme group capable of binding one oxygen molecule (O2). Figure 1A shows a multiscale representation of RBC, Hb within RBC, and chemical structure of iron-containing “heme” group within Hb. The O2 binding kinetics to Hb is positively cooperative, such that a small variation in oxygen partial pressure as blood goes from lung to tissue (Fig. 1B) can result in a large change in oxygen bound (in lung) or released (in tissue) by Hb, as exhibited by the classic sigmoidal shape of the O2-binding equilibrium curve (OEC, Fig. 1C) (53, 54). The O2-carrying iron in Hb is in its reduced “ferrous” (Fe2+) state. When the Hb is oxidized to form methemoglobin (MetHb), the iron becomes oxidized to the “ferric” state (Fe3+), which is unable to bind oxygen (55). Due to this reason, in natural RBCs the oxygen transport mechanism of Hb is closely coupled to redox cycles (e.g., driven by enzyme NAD-cytochrome b5 reductase), such that the Fe2+-containing Hb can be maintained in its O2-binding state. Irreversible conversion of Hb to MetHb not only inhibits its oxygen-carrying capacity, but also leads to dysre-gulated vascular tone and inflammatory reactions. Furthermore, Hb in RBCs have the unique capability to undergo conformational changes to allow saturation (loading) with O2 in the lungs (higher O2 affinity) and then release O2 in the tissue capillaries (lower O2 affinity). This reversible conformational regulation of O2-binding affinity of Hb is aided by allosteric effector molecules like 2,3-diphosphoglycerate (2,3-DPG), which is formed inside RBCs as a glycolytic intermediate. Therefore, maintaining such oxygen-carrying thermodynamic and kinetic characteristics of Hb, maintaining the redox environment and minimizing irreversible MetHb formation, are some of the important and challenging design considerations in the context of developing an Hb-based RBC surrogate (56). In this context, one important factor is to maintain the physicochemical stability of Hb, since outside the protective RBC environment (i.e., cell-free or stroma-free) the Hb tetramer is prone to rapidly disintegrate into its dimeric and monomeric protein units, which in turn results in rapid clearance from circulation into extravascular space and kidneys. This results in very short circulation residence time and increased risk of nephrotoxicity. Stroma-free Hb is also devoid of oxygen affinity regulatory enzymes like 2,3-DPG, as well as, protective anti-oxidant enzymes. As a result, such Hb has a dysregulated tissue oxygenation capacity compared with RBC-encapsulated Hb and is also prone to rapid irreversible oxidation to MetHb, thereby losing its oxygen transport ability. Stroma-free Hb is also a potent scavenger of both intra- and extravascular nitric oxide (NO), which is produced from vascular endothelial cells for innate vasodilator function, and this has been implicated in hypertensive side effects of Hb. Hence, providing efficient tissue oxygenation while maintaining reasonable circulation life-time, minimization of hypertensive side-effects, and avoidance of Hb-induced toxicity are the three prominent design requirements for HBOCs. The following sections review and discuss the various design approaches that have focused on addressing these requirements.
Fig. 1. A, Multiscale representation of RBCs and hemoglobin (Hb), showing a scanning electron micrograph (SEM) image of RBC depicting the biconcave discoid structure, along with sequential schematic of RBC structure, Hb structure, and “Heme” structure.
B, It shows a schematic of RBC movement between lung (oxygen loading site) and tissue (oxygen off-loading site), while (C) shows corresponding oxygen equilibrium curve (OEC) characteristics of Hb. RBCs indicates red blood cells.
Hb-based oxygen carrier (HBOC) systems
HBOCs are semisynthetic systems that utilize natural Hb as the oxygen-carrying component, either in chemically modified cell-free suspensions or conjugated and cross-linked with polymers along with protective enzymes, or encapsulated within microparticulate or nanoparticulate vehicles (51, 57). The Hb used in these systems is usually derived from outdated human or bovine RBCs or from recombinant sources (57–63). In the case of outdated human or bovine RBCs, the Hb is isolated via cell lysis, purified by sterile filtration and chromatographic techniques and sterilized (e.g., by low heat) (64). Using cell-free Hb presents the advantage of minimum antigenicity and the ability to off-load oxygen in plasma more efficiently because of the lack of interference by cell membrane. In fact, reportedly in the early 20th century, suspension of cell-free Hb in lactated Ringer solution was used to intravenously treat 15 patients; however, a large number of them developed renal toxicity and cardiovascular complications (64). Similar results were also found in the 1950s when US Navy treated several patients with cell-free Hb (65). Cell-free Hb was also found to have a very short circulation residence time because the Hb tetramer rapidly dissociates into dimeric and monomeric forms that can bind to plasma immunoglobulins, and undergo rapid clearance by the reticulo-endothelial system into spleen and liver, as well as renal clearance into kidneys, leading to Hb-induced toxicities in these organs (66, 67). Additionally, cell-free Hb and its dissociated derivatives can also extravasate into the subendothelial domain of the circulatory system and rapidly sequester NO, resulting in its conversion into nitrate (dioxygenation reaction) and oxy-Hb to Met-Hb (68). The NO is the body’s natural vasodilator and therefore this NO-scavenging results in vasoconstriction and cardiovascular complications. Furthermore, the absence of 2,3-DPG in cell-free Hb can cause unnaturally high oxygen affinity, making O2 off-loading problematic. Cell-free Hb can also change blood osmolarity, leading to alteration of blood volumes and associated side-effects. Altogether, for these reasons cell-free human Hb has been deemed problematic for in vivo oxygen-carrying applications. Instead of human Hb, studies have also been conducted with bovine Hb, but this also presents similar issues of stability, extravasation, NO-scavenging, and renal clearance and toxicity. Another interesting way to address some of these issues is by development of recombinant Hb (e.g., in E coli) where specific mutations can enable decrease in dissociation and modulation of NO-binding capacities, but the correct combination of mutations that can lead to an ideal Hb design is yet to be established (69–71). Recombinant technologies are also substantially expensive compared with human or bovine Hb. Therefore, a substantial volume of research has been focused on in vivo stabilization and functional modulation of Hb utilizing chemical modifications like cross-linking, polymerization, and macromeric surface conjugations. The goals of these modifications are to reduce Hb dissociation, extravasation, and renal clearance, while maintaining reasonable circulation life-time and O2-transport capacities.
Chemically modified HBOCs—
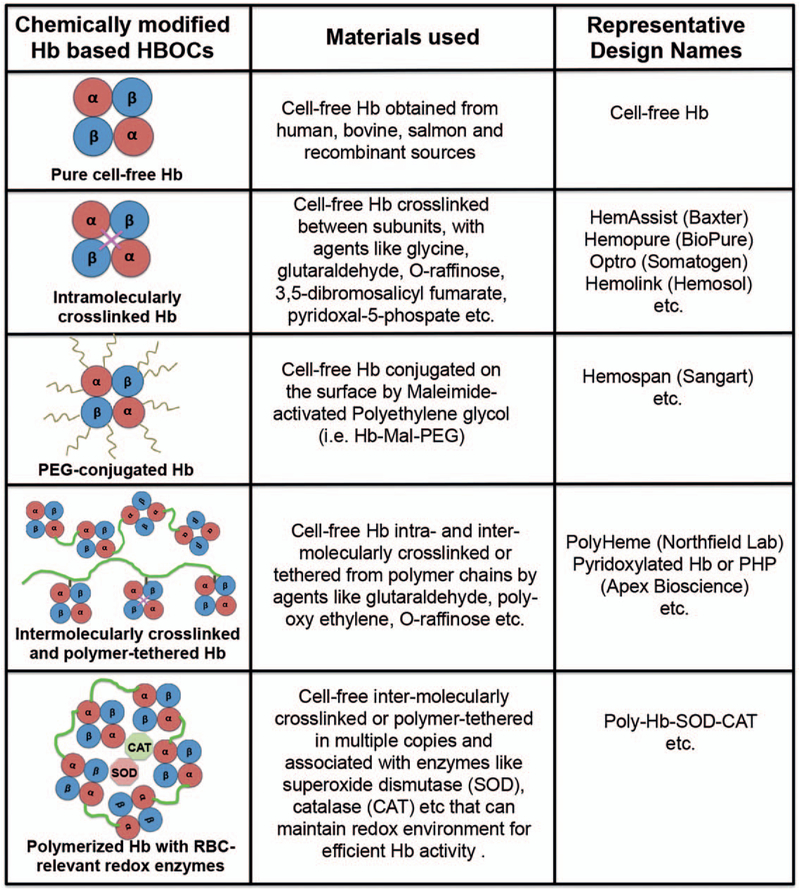
Hb can be cross-linked both intra- and intermolecularly. For example, intramolecular cross-linking in human Hb formed between its two a-subunits using acylation with bis-(3,5 dibromosalicyl)-fumarate (also known as Diaspirin) led to a product called HemAssist from Baxter (Chicago, Illinois) (57, 72, 73). This product showed an increase in circulation residence time up to 12 h compared with <6h for unmodified Hb, but the cross-linked Hb unfortunately showed a 72% increase in mortality rates in human patients compared with saline, and clinical trials were discontinued (74). An analogous approach to cross-linking the α subunits of recombinant Hb using Glycine led to a product called Optro from Somatogen (Boulder, Colo), but this also resulted in increased risks of cardiac arrest and mortality (75–77). Instead of site-specific intramolecular crosslinking only, polymerized Hb has also been created from using bifunctional cross-linking reagents like glutaraldehyde-based cross-linking of bovine Hb (e.g., Hemopure originally from Biopure, Cambridge, Mass, now HbO2 Therapeutics, Souderton, Pa) or human Hb (e.g., PolyHeme from Northfield Labs, Evanston, Ill) and o-raffinose-based cross-linking of human Hb (e.g., the product HemoLink from Hemosol, Toronto, Ontario, Canada) (78, 79). Such crosslinking allows for higher molecular weight cell-free Hb that retains oxygen-carrying properties while minimizing dissociation and rapid clearance of Hb. One challenge in these approaches is to precisely control polymer molecular weight, and rigorous purification steps are necessary to ensure product quality. PolyHeme was reported to progress into Phase III clinical trials in the United States in treating trauma-associated blood loss and showed a decreased need of natural blood transfusions (77). Clinical trials with HemoPure also showed a reduced need of additional blood transfusions in cardiac surgery (80). HemoPure has received clinical approval in South Africa for acutely anemic human patients and is under Phase III clinical trial in the United States. An analogous product from the same company (HbO2 Therapeutics, USA) called Oxyglobin is currently approved in the United States for veterinary use. HemoLink also reportedly advanced to Phase III clinical trials but was discontinued in 2003 when patients receiving treatment experienced adverse cardiac events. In fact, all of these products in their clinical studies have shown various degrees of transient hypertension, organ damage through microvascular constriction and dysfunction, gastro-intestinal distress, nephrotoxicity, neurotoxicity, and increased mortality (80–82).
Instead of intramolecular cross-linking and intermolecular polymerization, modification of Hb has also been carried out with macromeric bioconjugation to increase stability and vascular residence time while reducing immune recognition (83–85). Important examples of this approach are found in polyethylene glycol (PEG) modification of Hb (e.g., the products Hemospan from Sangart Inc, San Diego, Calif, and PEG-Hb from Enzon, South Plainfield, NJ) and poly(oxyethylene) modification of pyridoxylated crosslinked Hb (e.g., the product PHP from Apex Bioscience, Durham, NC). PEG-ylated Hb products have undergone extensive clinical trials and the studies showed risks of bradycardia and elevation of hepatic pancreatic enzymes even at low doses (86). Nonetheless, the Phase I and Phase II clinical trials showed that Hemospan was well tolerated in humans for efficient oxygen delivery, and Phase III trials in orthopedic surgery patients were carried out in Europe. The trials suggested that the risk of cardiovascular and renal dysfunctions still persisted with such chemically modified Hb products (87). Products such as PHP have also indicated such risks of cardiovascular and renal dysfunctions. During the past two decades it has been identified that cell-free Hb (including chemically modified versions) are potent scavengers of nitric oxide (NO) via rapid irreversible binding (rate constant ~ 107 M−1 s−1), which in turn can affect systemic and pulmonary vascular tone, resulting in vasoconstriction, hypertension, and lowering of cardiac output (88, 89). A resolution of this issue has been attempted by modifying Hb molecule into becoming an NO carrier through S-nitrosylation of cysteine residues in the β-subunits of Hb or imparting the ability of enzymatic transformation of Hb into a source NO donor in the presence of nitrites, but with limited success so far, in vivo (90). Natural RBCs contain enzymes like catalase (CAT) and superoxide dismutase (SOD) that help mitigate the oxidative stresses stemming from superoxide moieties in injured and ischemic tissues. Based on this rationale, in an interesting approach these enzymes have been cross-linked to polymerized Hb to form PolyHb-SOD-CAT, which has shown combined advantages of long circulation time and reduced oxidative damage (91, 92). Another interesting approach is to incorporate regulatory molecules such as 2,3-DPG and methemoglobin reductase along with Hb in appropriate HBOC systems, to prevent hemoglobin oxidation. In recent years, a product named HemoTech has been reported that uses purified bovine Hb cross-linked intra-molecularly with ATP and intermolecularly with adenosine, and conjugated with reduced glutathione (GSH) (93). This unique design allows the use of pharmacologically active molecules (ATP, adenosine, and GSH) as the chemical modifiers, where ATP regulates vascular tone through purinargic receptors, adenosine counteracts the vasoconstrictive properties of Hb via stimulating adenosine receptors, and GSH protects the “heme” from NO and various reactive oxygen species. The preclinical and early phase clinical studies have shown that HemoTech works as an effective oxygen carrier in treating blood loss, anaemia, and ischemic vascular conditions, and further studies are warranted. Another polymeric Hb reported in recent years is OxyVita, which is produced through modification of a zero-linked polymerization mechanism using car-bodiimide chemistry on bovine tetramer hemoglobin to produce “super-polymeric” macromolecules (94). In yet another recent approach, a polynitroxylated PEG-ylated hemoglobin (PNPH) nanostructure design has been reported, named VitalHeme (SynZyme Technologies LLC, Irvine, Calif), where PEG-ylated hemoglobin is covalently modified with catalytic caged NO (95). These designs reportedly allow for higher Hb stability in vivo and are currently under preclinical investigation. Figure 2 shows some of the prominent designs based on chemical modification of cell-free Hb, which have undergone (or are still undergoing) preclinical and clinical evaluation for oxygen transport. Despite promising preclinical and clinical results, many of the chemically modified Hb products have been withdrawn from clinical studies and discontinued in production, due to indication of more clinical risks than benefit, stemming from chemical heterogeneity and variable stability of final product, suboptimal vascular residence time, nonideal oxygen loading and off-loading capabilities, rapid irreversible conversion to methemoglobin, and increased cardiovascular and renal dysfunction issues. While some of the newer products are refining their design and processing to address these issues, a parallel direction of the research has focused on encapsulation of Hb within various micro- and nano-carrier vehicles, to more closely mimic the physiological encapsulated state of Hb in RBCs.
Fig. 2. Representative approaches and design schematics for HBOCs based on chemical modification (cross-linking, surface modification, polymerization, etc.) of Hb that have undergone significant preclinical and clinical evaluation.
Hb indicates haemoglobin; HBOC, Hb-based oxygen carriers.
Encapsulated HBOC systems—
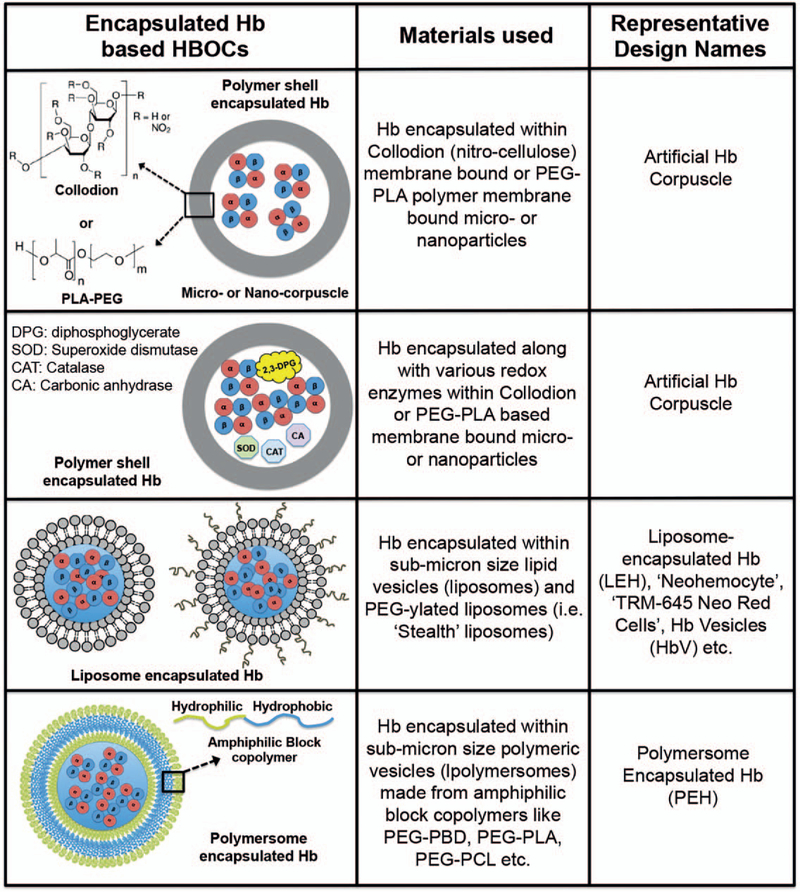
During past two decades, particulate drug delivery platform technologies (microparticles and nanoparticles) have revolutionized the packaging and delivery of pharmaceutical compounds, by encapsulating them within such particles to protect them from plasma-induced effects, increase their circulation time, and allow sustained availability to cells, tissues, and organs. This design concept has also been adapted to create HBOCs that encapsulate Hb within suitable particulate vehicles. In fact, the pioneering concept and demonstration of “bio-artificial cells” was presented as early as the 1950s and 1960s by Chang et al, by encapsulating Hb as well as other proteins and enzymes within polymeric membrane-based microvesicles. The membrane material originally used was collodion (cellulose nitrate) and later changed to biodegradable polyethylene glycol-polylactide (PEG-PLA) (96, 97). These Hb-loaded microvesicles, aptly termed “hemoglobin corpuscles,” showed oxygen equilibrium curves similar to RBCs and also allowed co-encapsulation and activity of RBC-relevant enzymes like 2,3-diphosphoglycerate (2,3-DPG), carbonic anhydrase, and CAT (98–100). However, in these systems a major challenge was posed by the rapid macrophagic uptake and clearance of these micrometer-sized vesicles from circulation, resulting in suboptimal circulation residence time for in vivo use. Reducing the diameter to ~1 μm only marginally improved the circulation lifetime, and a significant research effort has been directed toward further improving the vascular residence time by modifying the surface of the vesicles with lipids and polysaccharides. In another similar design approach, Djordjevich et al reported on encapsulation of Hb in micron and submicron size lipid vesicles (liposome-encapsulated Hb or LEH), with membrane made of phospholipids and cholesterol (101–103). This design essentially mimics the physiological state of Hb in RBCs where it is protected within the lipidic cell membrane that preserves the suitable redox mechanisms for Hb function. A number of variations of this design have followed, e.g., “neohemocytes,” “TRM-645 Neo Red Cells” etc., where the primary focus has been to maintain uniform Hb-encapsulation levels, uniform size distribution of the vesicles, minimizing vesicle destabilization or fusion over time, and enhance storage stability of the vesicles while maintaining the RBC-analogous oxygen transport properties of the encapsulated Hb (104–106). During the 1990s the “Stealth Liposome” technology was clinically established, where lipid nanovesicles (100–200 nm in diameter) were surface-functionalized with polyethylene glycol (PEG) to enhance storage stability, reduce opsonization, and prevent rapid macrophagic uptake, and this significantly enhanced the circulation residence time (107, 108). Consequently, this technology was adapted to form Hb-encapsulated PEG-ylated liposomal vesicles (HbV) (109–111). For HbV preparation, l,2-dioctadecadienoyl-sn-glycero-3-phosphatidylcholine (DODPC) was used as the major membrane phospholipid, such that γ-irradiation-induced radiolysis of water molecules in the vesicles generated hydroxy (–OH) radicals that promoted intermolecular polymerization of dienoyl groups to produce highly stable liposomes that could withstand freeze-thawing, freeze-drying, and rehydration processes. The HbV design has resulted in substantial improvement of circulation life-time (~60 h in some animal models) and several refinements of this design have been reported in recent years (112–116). The oxygen transport ability of HbVs was found to be similar to natural RBCs, with comparable oxygen saturation and release kinetics. Also, the liposomal encapsulation of Hb prevented its NO-scavenging effect and thereby reduced the associated negative effects on vasculature. The encapsulation of Hb in liposomal vesicles also prevented glomerular clearance of Hb (since liposomes are too big for renal clearance), and therefore reduced nephrotoxicity. The current optimized HbV product contains about 30,000 Hb molecules encapsulated within one PEG-ylated liposomal vesicle of ~250 nm in diameter. In comparison, a natural RBC is ~7 μm in diameter and ~2 μm in thickness, containing about 250 million Hb molecules. The HbVs have undergone extensive preclinical evaluation in suitable animal models for potential use as an RBC surrogate in transfusion and resuscitative mitigation of massive hemorrhagic shock and hemodilution incidents, and oxygenation of ischemic as well as transplanted tissues and organs. These studies have shown significant promise of HbVs as RBC-inspired oxygen carrier; however, these systems can still present issues of broad size distribution of the vehicles, variations in Hb-encapsulation efficiencies, variable pharmacokinetics, and complement-mediated immune response in vivo. Further research is currently being directed toward resolving these issues for potential clinical translation of HbV designs as well as other analogous designs of liposome-encapsulated hemoglobin (LEH) systems RBC surrogates (112–116). Interestingly, instead of encapsulating Hb, some recent research approaches have also attempted to encapsulate oxygen (O2) directly within phospholipid microvesicles (2–4 μm in diameter) to deliver O2 to deoxygenated RBCs in circulation (117, 118). Although these oxygen-loaded microbubbles were found to be stable for a few weeks in storage with only small extent of oxygen loss, in vivo they were found to have a very short circulation life-time (<1 h). Therefore, treatment with these systems would require multiple or repeated dosing, which may prompt negative effects of dysregulated oxidative stress and associated toxicity and immune response. Therefore, long-term safety profile of such technologies needs to be rigorously evaluated. Encapsulation of Hb has also been studied in other microparticle and nanoparticle systems besides lipid vesicles. In pioneering work by Chang et al, Hb was encapsulated within polymeric nanoparticles (80–200 nm in diameter) made from PEG-PLA and analogous block copolymers (119, 120). These polymeric nanoparticles could allow oxygen transport kinetics of Hb at levels similar to natural RBCs and the polymeric material could be engineered to be biocompatible and biodegradable. Furthermore, enzymes that maintain the redox environment for Hb stability and function regulation (e.g., carbonic anhydrase, CAT, SOD, MetHb reductase, etc.) could also be encapsulated within the same nanoparticles toward further mimicry of RBC action (121). This design approach has also been adopted for other polymer systems like poly(ε-caprolactone)/poly(L-lactic acid) (PCL/PLA) copolymers, poly(L-lysine) (PLL), poly(lactic-co-glycolic acid) (PLGA)/PEG copolymers, etc. (122, 123). Amphiphilic block-copolymer systems also provide the ideal building blocks for designing polymer vesicles, otherwise known as polymersomes, analogous to liposomes. These polymersome systems have been recently utilized to create polymerosome-encapsulated Hb (PEH) systems (124). The Hb loading in these PEH systems has been reported to be 1 to 2 mg mL−1, compared with human blood (i.e., within RBC) concentration of ~ 150 mg mL−1. Utilization of hollow fiber-based membrane extrusion system has provided an interesting way to manufacture these PEH systems (125). These PEH systems have been reported to be capable of encapsulating both bovine and human Hb, and have shown oxygen equilibrium kinetics and other biophysical parameters similar to RBCs. This indicates considerable promise toward the application of such PEH systems as RBC surrogates in transfusion medicine, but currently very limited in vivo evaluation data are available for these systems. A potential issue with polymersome systems may be their higher shell thickness compared with liposomes, which can lead to longer diffusion time for oxygen to saturate the encapsulated Hb or to be released from the Hb to tissues. Modulation of polymer molecular weight of the shell and therefore of the shell-thick-ness can provide a unique way to influence oxygen transport properties of the PEH systems. The higher stability of polymersomes compared with liposomes, both in storage and in vivo, may also provide additional advantages for its use as Hb-encapsulated RBC surrogate systems. Ongoing and future studies with these systems should be directed toward establishment of batch-to-batch consistency, sterilization metric and storage stability evaluation, post-sterilization Hb bioactivity determination, in vivo pharmacokinetics and biodistribution determination, and therapeutic evaluation in appropriate animal models (e.g., hemorrhagic shock, ischemia, etc.). Figure 3 shows some representative designs and components for encapsulated Hb systems that have undergone and are currently still undergoing in vitro and in vivo evaluation for RBC-mimetic oxygen carrier application.
Fig. 3. Representative approaches and design schematics for HBOCs based on encapsulation of Hb in microparticle and nanoparticle systems that have undergone significant preclinical evaluation and hold clinical promise.
Hb indicates hemoglobin; HBOC, Hb-based oxygen carriers.
Novel molecules and designs incorporating Hb as O2 carrier—
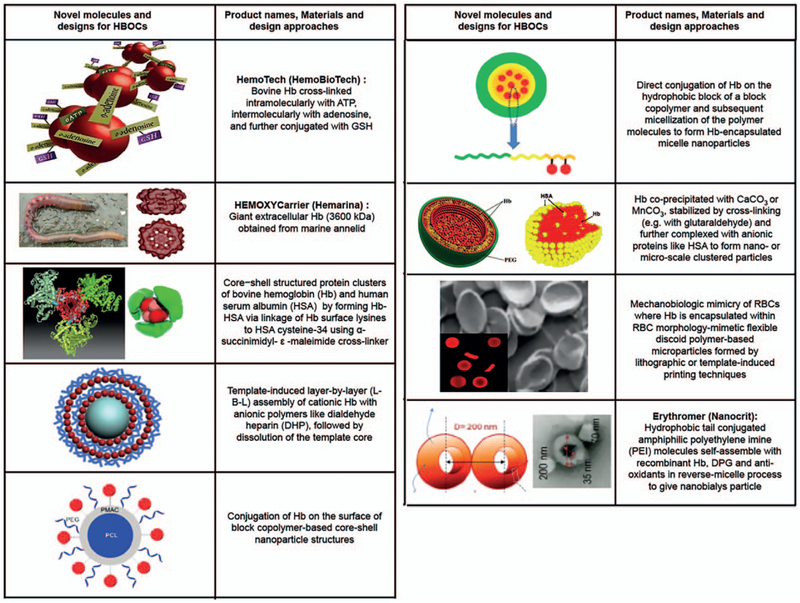
This section will not distinguish between “chemically modified” versus “encapsulated” Hb systems, but rather focus on reviewing some of the emerging novel designs and technologies that incorporate Hb for oxygen transport purposes. In one interesting approach, instead of Hb, PEG-ylation was carried out on bovine carboxyhemoglobin (CO-Hb) and the resultant PEG-CO-Hb system has been evaluated for oxygen transport (and CO transport) properties (126–128). The rationale behind this design is that, reportedly, endogenous CO produced from heme-oxygenase activity can render cytoprotective and homeostatic effects, such as inhibition of apoptosis and inflammation and reduction of oxidative stress and vasodilatory activity (129). The PEG-CO-Hb product, with the commercial name SAN-GUINATE (Prolong Pharmaceuticals, South Plainfield, New Jersey, USA), has undergone pre-clinical evaluation in small animal models, and is undergoing clinical trials in treating small groups of patients in the areas of sickle cell anemia, thrombotic thrombocytopenic purpura (TTP), and ischemia after subarachnoid hemorrhage, with promising safety profile and oxygenation parameters. In another recent approach, core-shell cluster structures were formed by conjugating human serum albumin (HSA) on Hb using Hb surface lysines conjugated to HSA cysteine-34 using α-succinimidyl-ε-maleimide cross-linker (130). These Hb-HSA clusters reportedly lower the risks of rapid clearance and extravasation, and thus improve high circulation stability and residence time. A further modification of these Hb-HSA core-shell nanoclusters was recently reported where anti-oxidant enzymes and platinum nanoparticles were embedded in the HSA pockets for protection of Hb (131). These nanocluster designs have so far been evaluated only in vitro for their oxygen-binding capacity, redox properties, and stability, with promising results. However, rigorous in vivo pharmacokinetics, toxicology, bidistribution, and oxygenation studies, along with demonstrating batch-to-batch compositional and functional consistency, would be needed to establish the in vivo applicability of these structures. In another approach, Hb has been loaded in microparticles by its coprecipitation with calcium carbonate (CaCO3), followed by crosslinking with glutaraldehyde and selective dissolution of CaCO3, resulting in Hb quantity per microparticle close to that of natural RBCs (132). These Hb-microparticles have shown oxygen equilibrium kinetics similar to free Hb, but much enhanced circulation lifetime compared with free Hb. Analogous Hb microparticles carrying about 80% Hb content compared with natural RBCs have been reported where Hb and MnCO3 were coprecipitated, immediately followed by human serum albumin addition for encapsulation and stabilization of the particles (133). These particles have shown reduced risks of NO scavenging and associated effect on vasoconstriction. In yet another recent approach, Hb was covalently conjugated directly to the hydrophobic or hydrophilic domain of block-copolymers and the resultant conjugates were self-assembled to form Hb-loaded micelles (134, 135). In another interesting design, MnCO3 nanoparticles were used as templates to deposit layer-by-layer (L-B-L) assemblies of Hb and dialdehyde heparin (DHP), followed by crosslinking to stabilize the layers and selective dissolution of the template core (136). A similar approach was also used to form L-B-L-coated nanotubes where alternate layers of Hb, DHP, and the enzyme CAT were deposited, to create systems for potential application in treating oxidative stress (137). These complex nanostructures have been characterized in vitro for their morphology, stability, cytotoxicity, and in some cases biofunctionality, but preclinical evaluation for oxygen-carrying efficacy in vivo is yet to be reported. Another recent exciting development in the area of novel HBOC molecules is the utilization of large molecular weight extracellular Hb isolated from marine invertebrates like polychaete annelid (e.g., the product HEMOXYCarrier from Hemarina, Morlaix, France) (138). Preclinical studies with this unique Hb molecule have shown reduced microvascular vasoconstriction and no significant impact on mean arterial blood pressure, compared with other HBOCs that utilize bovine or human Hb (139). Further investigation of this system is currently ongoing to establish its clinical potential as an oxygen carrier therapeutic.
In recent years, some Hb-encapsulation approaches have also focused on adapting the physico-mechanical properties of natural RBCs that significantly influence their biological functions. Natural healthy RBCs have a biconcave discoid morphology, with a diameter of ~8 μm and a thickness of ~2 μm. These RBCs are also highly flexible (Young’s modulus 0.1–0.2 kPa), which enables them to change their morphology when passing through microvascular circulation (140, 141). The mechanical integrity and viscoelastic nature of RBCs during their cyclical deformation is rendered by a two-dimensional spectrin network attached to the cytosolic side of their membrane. Oxygenated Hb results in RBCs having significantly more deformability than deoxygenated Hb, and this enables the mechanically flexible RBCs to move through microvasculature to transport oxygen. The size, shape, and flexibility of RBCs also influence their movement and distribution in the blood flow field, where they mostly reside in the center of the parabolic flow field in mid to large vessels, while in small vessels and capillaries RBCs can become distributed throughout for efficient oxygen exchange (142). These considerations have recently led to biomaterials-based mimicry of RBC’s size, shape, and flexibility attributes into Hb-encapsulating synthetic constructs. For example, poly electrolyte driven layer-by-layer assembly has been used to create microparticles that mimic the shape and deformability of natural RBCs (143). In this approach, Hb and BSA were electrostatically deposited on the surface of discoid PLGA particles of ~7 μm diameter and 400 nm shell thickness, and then the PLGA core was selectively dissolved to yield RBC-shaped Hb-loaded particles that have high elastic deformation. Similar RBC-mimetic flexible particles have been fabricated using PEG hydrogel system in a stop-flow-lithography (SFL) approach where the mechanical properties of resultant particles could be controlled by modulating cross-linking density of the hydrogel systems (144). In a different approach, RBC shape-mimetic particles were fabricated from acrylate hydrogels using a “particle replication in non-wetting templates” (PRINT) technology (145). These particles were made in 2 to 3 μm molds, such that, upon hydration, the particles swelled to disks with diameter approximately 6 μm and height approximately between 1.5 μm. Also, the meniscus effect from the molds resulted in the particles being thinner in the middle and thicker at the edges, resembling the biconcave morphology of RBCs. RBC morphology and flexibility mimicking particle designs made through these two techniques have demonstrated elastic deformation capabilities in vitro for transport through narrow channels, and controllable circulation lifetime in vivo, depending on their elastic modulus. Although these particles have been reported to be capable of Hb encapsulation via physical trapping or covalent bonding, detailed oxygen transport capabilities and associated in vivo transfusion applications have not been reported in detail yet. In another interesting approach, liposome-encapsulated actin-hemoglobin (LEAcHb) constructs were prepared using a polymerized actin core, to mimic morphology of natural RBCs (146). Although these particles were much smaller (~136.8nm) than RBCs, the biconcave shape along with the mechanical support of the membrane improved the half-life to ~72h. In natural RBCs, the negative surface charge electrostatically prevents RBC aggregation over a distance of 20 nm and this rationale has led to some research in mimicking RBC-relevant surface charge on Hb-encapsulating PEG-PLA nanoparticles (<200 nm in diameter) using cetyltrimethylammonium bromide (CTAB) or anionic sodium dodecyl sulfate (SDS) surfactants (147). Cationized particles were found to have a half-life of ~11h (8-fold higher than untreated particles), while the anionized particles were quickly eliminated, giving a half-life of <1 h. In yet another recent approach, a novel amphiphilic polymeric system was developed using polyethylene imine (PEI) modified with palmitic acid and was used to form toroidal-shaped nanoparticles (termed nanobialys, ~200nm diameter) that can encapsulate Hb, as well as, maintain redox enzymatic environment for Hb activity by co-encapsulation of 2,3-DPG and leuko methylene blue (148). These novel Hb-containing particles, termed Erythromer, have shown some promise of oxygen transport in vivo. Detailed biocompatibility studies (e.g., for PEI that can pose cytotoxicity issues), circulation lifetime and stability, Hb-loading capacity and oxygen transport capabilities, etc. would need to be further evaluated to establish the clinical potential of such designs as RBC surrogates in transfusion medicine. Figure 4 shows design schematics of these novel emerging designs and structures for Hb-based oxygen carriers.
Fig. 4. Representative schematics for novel HBOC molecules and designs, including new polymerization strategies, new sources of Hb and novel encapsulation and biomimetic strategies that are currently under development and preclinical evaluation.
Hb indicates haemoglobin; HBOC, Hb-based oxygen carriers.
DISCUSSION
In traumatic injuries and hemorrhage, tissue oxygenation is severely compromised and this can result in drastic damage to vital tissues and organs. Therefore, rapid hemorrhage control and restoration of tissue oxygen are critical for improving survival and function. To this end, transfusion of whole blood or blood components (RBCs, platelets, and plasma) has become the current clinical standard. However, these blood products currently present significant logistical challenges with regards to widespread usage in battlefield and prehospital settings, where trauma and haemorrhage-related morbidities and mortalities become significant. One potential solution is the bioengineering of semisynthetic or synthetic surrogates of blood components. In this framework, one important category of technology is that of Hb-based oxygen carriers (HBOCs), which essentially are meant to provide the oxygen transport properties of RBCs while allowing higher availability (via in vitro large-scale manufacture), universal applicability (no need for blood type matching), reduced contamination risks (due to sterilization), and longer shelf life. While a wide variety of approaches and designs have been dedicated to creating HBOCs, with some of them advancing to clinical trials with promising results, certain physiological risks and limitations associated with cell-free Hb, e.g., short circulation life-time, renal clearance, and associated toxicity, NO-scavenging and associated vasoconstrictive/hypertensive side effects, etc. have resulted in some negative clinical outcomes and concerns. As a result, FDA approval of HBOCs for human use has not happened yet in the United States, although one product (Hemo-Pure or HBOC-201) received human use approval in South Africa. This product has been evaluated in non-cardiac surgery patients and trauma patients, and is under further clinical investigation for treatment of life-threatening anaemia. Other HBOC products like PolyHeme, Hemospan, and Hemotech have all advanced to different levels of clinical trials (e.g., Phase I for Hemotech, Phase II for Hemospan, and Phase III for Poly Heme); however, further studies are needed to establish their clinical safety and efficacy profiles. In many clinical studies, the functional efficacy comparison has been with natural RBC transfusion and although the use of HBOCs has demonstrated a reduction in the number of RBC transfusions, it remains to be answered whether HBOCs are intended to be “RBC substitutes” or rather to be “oxygen carriers” in scenarios where natural RBCs are not available. Future considerations of clinical study design may utilize this framework to compare HBOCs to relevant “standard of care” (e.g., saline or plasma expanders in prehospital trauma) instead of RBCs. Other questions that remain to be answered are whether these chemically modified and polymeric HBOC designs based on cell-free Hb still present issues of NO scavenging (associated hypertensive effects) and heme toxicity. Newer HBOC designs, both as chemically modified cell-free Hb form (e.g., Hemo-Tech) and as encapsulated Hb form (e.g., LEH, PEH, Erythromer, etc.), are still undergoing rigorous preclinical evaluation to elucidate and establish batch-to-batch consistency, mechanism of action, pharmacokinetics and biodistribution, tissue oxygenation capability, and in vivo risks. In this framework, it remains to be seen if “encapsulated” Hb designs provide any advantage over the chemically modified cell-free systems, in terms of allowing co-encapsulation of oxygen affinity regulatory and redox environment-preserving molecules. It is important to note here that such multicomponent design will add substantially to manufacturing costs and thus the cost-benefit analysis needs to be rigorously validated in appropriate preclinical models, before clinical studies and translation. Going forward, there is a significant need to systematically study cell-free chemically modified polymeric Hb designs versus encapsulated Hb designs (with or without effector molecule and antioxidant enzyme co-encapsulation) in an established anatomically and physiologically relevant preclinical animal model to compare circulation residence time, tissue oxygenation efficacy, NO-scavenging associated hypertensive risks, and heme-associated toxicity. Therefore, a consensus on what HBOC design is the most beneficial remains an open question. It is also important to note that, while continued preclinical and clinical studies are being directed at identifying the best HOBOC candidate for in vivo transfusion applications in anemia, surgery, and trauma, in recent years HBOC applications have also evolved into using them for ex vivo preservation (and oxygen perfusion) of transplant tissues and organs (149, 150).
As for the source of Hb, most designs have utilized either human or bovine Hb, although some newer designs have adapted utilization of recombinant Hb where the physicochemical and biological properties can be precisely engineered, as well as, giant Hb sourced from marine invertebrates with improved properties. One critical aspect regarding “source of Hb” for efficient HBOC design is the regulation of oxygen loading/off-loading capacity of the Hb used. For human Hb, this is regulated by effector molecules like 2,3-DPG, which maintains the P50 of human Hb at 26 mm to 28 mm mercury. However, cell-free human Hb (i.e., in the absence of DPG) has a much higher oxygen affinity (OEC curve shifts to left) and this can lead to reduced tissue oxygenation (151). In contrast, oxygen affinity of bovine Hb is not critically dependent on DPG but rather on chloride ions, which are present in abundance in all mammals including humans. Bovine Hb has also been reported to have higher thermal stability than human Hb during isolation and processing (152). Furthermore, while human Hb is sourced from outdated human platelets, bovine Hb can be obtained from cow blood RBCs (e.g., from large farms and slaughterhouses) and hence has more availability. Therefore, from availability, processing and oxygen transport regulation standpoint, bovine Hb can provide benefit over human Hb and this is what has been used in design of Hemopure (or HBOC-201), which is currently the only one with veterinary approval in the United States and human approval in South Africa. Other alternative sources of Hb, like recombinant technologies, annelid supramolecular extracellular Hb, etc., should incorporate isolation and manufacturing costs, as well as physico-chemical comparison of oxygen loading/off-loading aspects (with respect to human Hb), in order to successfully translate the corresponding HBOC designs to the clinic. HBOCs designed with cell-free nonhuman Hb should also analyze immunogenicity of the products, compared with encapsulated version of the same Hb. Besides Hb-based systems, oxygen carriers based on perfluorocarbons (PFCs) and iron (Fe2+)-containing porphyrin systems have also undergone significant preclinical and limited clinical evaluation, but an ideal oxygen carrier system for safe and effective in vivo use is yet to be realized. It is also important to note that the various HBOC systems should not be categorized as “artificial blood,” but rather as a critical component of such a system. Blood contains other components like platelets and plasma, and a significant volume of research has evolved in parallel, regarding the development of platelet surrogates and plasma expanders, that have been reviewed elsewhere (23, 24, 153–156). Exciting advancements have also been made in recent years, to develop “donor independent” RBCs (and platelets) from stem cells (157–162). Therefore, a high volume of research continues to be directed in the area of in vitro manufactured “donor independent” RBCs as well as Hb-based oxygen carriers in the United States and globally. In continuing evaluation and clinical translation of these technologies, it should be very important to consider and resolve manufacturing challenges (e.g., scaling up of complex multicomponent designs while maintaining batch-to-batch consistent quality and functional efficacy, etc.), as well as meticulously designed preclinical studies in physiologically relevant animal models and clinical studies where current “standard of care” in the specific application is compared. Through such studies, it is envisioned that Hb-based oxygen carriers will revolutionize combat casualty care in prehospital and en route scenarios, as well as allow emergency management of civilian trauma in remote locations or when blood products are not immediately or sufficiently available.
ACKNOWLEDGMENTS
The literature review and work to prepare this publication was carried out using the library and facilities of Case Western Reserve University, Cleveland, Ohio.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. : Causes of Death in U.S. Special Operations Forces in the Global War on Terrorism, 2001–2004. Ann Surg 245(6):986–991, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackbourne LH, Baer DG, Eastridge BJ, Kheirabadi B, Bagley S, Kragh JF Jr, Cap AP, Dubick MA, Morrison JJ, Midwinter MJ, et al. : Military medical revolution: prehospital combat casualty care. J Trauma Acute Care Surg 76(6 Suppl 5):S372–S377, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. : Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg 75(1 suppl 1):S40–47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorlac WC, DeBakey ME, Holcomb JB, Fagan SP, Kwong KL, Dorlac GR, Schreiber MA, Persse DE, Moore FA, Mattox KL: Mortality from isolated civilian penetrating injury. J Trauma 59(1):217–222, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Smith ER, Shapiro G, Šarani B: The profile of wounding in civilian public mass shooting fatalities. J Trauma Acute Care Surg 81(1):86–92, 2016. [DOI] [PubMed] [Google Scholar]
- 6.van Oostendorp SE, Tan ECTH, Geeraedts LMG Jr: Prehospital control of life-threatening truncal and junctional haemorrhage is the ultimate challenge in optimizing trauma care; a review of treatment options and their applicability in the civilian trauma setting. Scand J Trauma Resusc Emerg Med 24(1): 110, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. : Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 313(5):471–482, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. : The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 148(2): 127–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. : Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 62(2):307–310, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Carmen R: The selection of plastic materials for blood bags. Transf Med Rev 7(1): 1–10, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG: A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion 33(10):794–797, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Blajchman MA: Bacterial contamination and proliferation during the storage of cellular blood products. Vox Sang 74(suppl 2): 155–159, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Seghatchian J, de Sousa G: Pathogen-reduction systems for blood components: the current position and future trends. Transfusion Apheresis Sci 35(3): 189–196, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Cap AP, Pidcoke HF, DePasquale M, Rappold JF, Glassberg E, Eliassen HS, Bjerkvig CK, Fosse TK, Kane S, Thompson P, et al. : Blood far forward: time to get moving! J Trauma Acute Care Surg 78(6 suppl 1):S2–S6, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB: The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 63(4):805–813, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Boscarino C, Tien H, Acker J, Callum J, Hansen AL, Engels P, Glassberg E, Nathens A, Beckett A: Feasibility and transport of packed red blood cells into Special Forces operational conditions. J Trauma Acute Care Surg 76(4): 1013–1019, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Spinella PC, Dunne J, Beilman GJ, O’Connell RJ, Borgman MA, Cap AP, Rentas F: Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion 52(5): 1146–1153, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Kauvar D, Holcomb JB, Norris GC, Hess JR: Fresh whole blood transfusion: a controversial military practice. J Trauma 61(1): 181–184, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC, et al. : Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion 53(suppl 1):137S–149S, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noorman F, van Dongen TTCF, Plat M-CJ, Badloe JF, Hess JR, Hoencamp R: Transfusion: −80°C frozen blood products are safe and effective in military casualty care. PLoS One 11(12):e0168401, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acker JP, Marks DC, Sheffield WP: Quality assessment of established and emerging blood components for transfusion. J Blood Transfusion 2016. Article ID: 4860284. doi: 10.1155/2016/4860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blajchman MA: Substitutes for success. Nat Med 5:17–18, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Modery-Pawlowski CL, Tian LL, Pan V, McCrae KR, Mitragotri S, Sen Gupta A: Approaches to synthetic platelet analogs. Biomaterials 34(2):526–541, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Sen Gupta A: Biomaterials-based strategies for blood substitutes In: Biomaterials in Regenerative Medicine and the Immune System. Ed: Santam-brogio L. Berlin, Germany: Springer, 113–137, 2015. [Google Scholar]
- 25.Giangrande PLF: The history of blood transfusion. Br J Haematol 110(4):758–767, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Blood Banking and Transfusion Medicine. Ed. Hillyer CD. Churchill Livingstone Elsevier, 2007. [Google Scholar]
- 27.Carson JL, Hill S, Carless P, Hébert P, Henry D: Transfusion triggers: a systematic review of the literature. Transfus Med Rev 16(3): 187–199, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Sharma P, Tyler LN: Transfusion of blood and blood products: indications and complications. Am Fam Physician 83(6):719–724, 2011. [PubMed] [Google Scholar]
- 29.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N: Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 56(9):2173–2183, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP: Transfusion medicine—blood transfusion. N Engl J Med 340(6):438–447, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Hess JR: An update on solutions for red cell storage. Vox Sang 91(1):13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Greening DW, Glenister K, Sparrow RL, Simpson RJ: International blood collection and storage: clinical use of blood products. J Proteomics 73(3):386–395, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Tien H, Nascimento B Jr, Callum J, Rizoli S: An approach to transfusion and hemorrhage in trauma: current perspectives on restrictive transfusion strategies. Can J Surg 50(3):202–209, 2007. [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson PI, Ostrowski SR, Secher NH: Management of major blood loss: an update. Acta Anaesthesiol Scand 54(9): 1039–1049, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Pohlman TH, Walsh M, Aversa J, Hutchison EM, Olsen KP, Lawrence Reed R: Damage control resuscitation. Blood Rev 29(4):251–262, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, et al. : Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 82(3):605–617, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, et al. : American College of Critical Care Medicine of the Society of Critical Care Medicine; Eastern Association for the Surgery of Trauma Practice Management Workgroup: Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 37(12):3124–3157, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb JB, Donathan DP, Cotton BA, Del Junco DJ, Brown G, Wenckstern TV, Podbielski JM, Camp EA, Hobbs R, Bai Y, et al. : Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care 19(1): 1–9, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX: Pretrauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg 220(5):797–808, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JW, Gilcher RO: Red blood cells, plasma, and other new apheresis-derived blood products: improving product quality and donor utilization. Transfus Med Rev 13(2): 118–123, 1999. [DOI] [PubMed] [Google Scholar]
- 41.D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, Zolla L: An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 55(1):205–219, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Devine DV, Serrano K: The platelet storage lesion. Clin Lab Med 30(2):475–487, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Jobes D, Wolfe Y, O’Neill D, Calder J, Jones L, Sesok-Pizzini D, Zheng XL: Toward a definition of “fresh” whole blood: an in vitro characterization of coagulation properties in refrigerated whole blood for transfusion. Transfusion 51(1):43–51, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Amici GM, Mirasole C, D’Alessandro A, Yoshida T, Dumont LJ, Zolla L: Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus 10(suppl 2):s46–s54, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paglia G, D’Alessandro A, Rolfsson Ó, Sigurjonsson ÓE, Bordbar A, Palsson S, Nemkov T, Hansen KC, Gudmundsson S, Palsson BO: Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 128:e43–e50, 2016. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhari CN: Frozen red blood cells in transfusion. Med J Armed Forces India 65(1):55–58, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hess JR: Red cell freezing and its impact on supply chain. Transfusion Med 14(1): 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Solheim BG: Pathogen reduction of blood components. Transfus Apher Sci 39(1):75–82, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Chang R, Eastridge BJ, Holcomb JB: Remote damage control resuscitation in austere environments. Wilderness Environ Med 28(2S):S124–S134, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Squires JE: Artificial blood. Science 295(5557): 1002–1005, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Chang TMS: Blood substitutes based on nanobiotechnology. Trends Biotechnol 24:372–377, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM: Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a metaanalysis. JAMA 299(19):2304–2312, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klotz IM: Hemoglobin-oxygen equilibria: retrospective and phenomenological perspective. Biophys Chem 100(1–3): 123–129, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Goutelle S, Maurin M, Rougier F, Barbaut X, Bourguignon L, Ducher M, Maire P: The Hill equation: a review of its capabilities in pharmacological modelling. Fund Clin Pharmacol 22(6):633–648, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Umbreit J: Methemoglobin—It’s not just blue: a concise review. Am J Hematol 82(2): 134–144, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP: Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol 30(1):39–51, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Stowell CP, Levin J, Spiess BD, Winslow RM: Progress in the development of RBC substitutes. Transfusion 41(2):287–299, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Winslow RM: Red Cell Substitutes. Semin Hematol 44(1):51–59, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Chang TMS: From artificial red blood cells, oxygen carriers, and oxygen therapeutics to artificial cells, nanomedicine, and beyond. Artif Cell Blood Substit Immobil Biotechnol 40(3): 197–199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napolitano LM: Hemoglobin-based oxygen carriers: first, second or third generation? human or bovine? where are we now? Crit Care Clin 25(2):279–301, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Piras AM, Dessy A, Chiellini F, Chiellini E, Farina C, Ramelli M, Valle ED: Polymeric nanoparticles for hemoglobin-based oxygen carriers. Biochim Biophys Acta 1784(10): 1454–1461, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Buehler PW, Alayash AI: All hemoglobin-based oxygen carriers are not created equally. Biochim Biophys Acta 1784(10): 1378–1381, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Winslow RM: Cell-free oxygen carriers: scientific foundations, clinical development, and new directions. Biochim Biophys Acta 1784(10):1382–1386, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Alayash AI: Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med 30(2):381–389, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Amberson WR, Jennings JJ, Rhode CM: Clinical experience with hemoglobin-saline solutions. J Appl Physiol 1(7):469–489, 1949. [DOI] [PubMed] [Google Scholar]
- 66.Bunn H, Jandl J: The renal handling of hemoglobin. Trans Assoc Am Phys 81:147–152, 1968. [PubMed] [Google Scholar]
- 67.Buehler PW, D’Agnillo F, Schaer DJ: Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med 16(10):447–457, 2010. [DOI] [PubMed] [Google Scholar]
- 68.Kim-Shapiro DB, Schechter AN, Gladwin MT: Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26(4):697–705, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Looker D, Abbott-Brown D, Cozart P, Durfee S, Hoffman S, Mathews AJ, Miller-Roehrich J, Shoemaker S, Trimble S, Fermi G, et al. : A human recombinant haemoglobin designed for use as a blood substitute. Nature 356(6366):258–260, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Fronticelli C, Koehler RC, Brinigar WS: Recombinant hemoglobins as artificial oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol 35(1):45–52, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varnado CL, Mollan TL, Birukou I, Smith BJZ, Henderson DP, Olson JS: Development of recombinant hemoglobin-based oxygen carriers. Antioxidants Redox Signaling 18(17):2314–2328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamy ML, Daily EK, Brichant JF, Larbuisson RP, Demeyere RJ, Vander-meersch EA, Kehot JJ, Parsloe MR, Berridge JC, Sinclair CJ, et al. : Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. Anesthesiology 92:646–656, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Saxena R, Wijnhoud A, Carton H, Hacke W, Kaste M, Przybelski R, Stern KN, Koudstaal PJ: Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 30:993–996, 1999. [DOI] [PubMed] [Google Scholar]
- 74.Sloan EP, Koenigsberg MD, Philbin NB, Gao W: DCLHb Traumatic Hemorrhagic Shock Study Group. European HOST Investigators. Diaspirin cross-linked hemoglobin infusion did not influence base deficit and lactic acid levels in two clinical trials of traumatic hemorrhagic shock patient resuscitation. j Trauma 68(5): 1158–1171, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Winslow RM: New transfusion strategies: red cell substitutes. Ann Rev Med 50:337–353, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Viele MK, Weisopf RB, Fisher D: Recombinant human hemoglobin does not affect renal function in humans: analysis of safety and pharmacokinetics. Anesthesiology 86(4):848–858, 1997. [DOI] [PubMed] [Google Scholar]
- 77.Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, DeWoskin R, Moss GS: The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg 187(2): 113–120, 1998. [DOI] [PubMed] [Google Scholar]
- 78.Jahr JS, Moallempour M, Lim JC: HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (Biopure Corporation). Expert Opin Biol Ther 8(9): 1425–1433, 2008. [DOI] [PubMed] [Google Scholar]
- 79.Cheng DC, Mazer CD, Martineau R, Ralph-Edwards A, Karski J, Robblee J, Finegan B, Hall RI, Latimer R, Vuylsteke A: A phase II dose-response study of hemoglobin raffimer (Hemolink) in elective coronary artery bypass surgery. J Thorac Cardiovasc Surg 127(1):79–86, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Alayash AI: Blood substitutes: why haven’t we been more successful? Trends Biotechnol 32(4): 177–185, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang TMS: Future generations of red blood cell substitutes. J Intern Med 253(5):527–535, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Chen J-Y, Scerbo M, Kramer G: A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics 64(8):803–813, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vandegriff KD, Winslow RM: Hemospan: design principles for a new class of oxygen therapeutic. Artif Organs 33(2): 133–138, 2009. [DOI] [PubMed] [Google Scholar]
- 84.Bobofchak KM, Tarasov E, Olsen KW: Effect of cross-linker length on the stability of hemoglobin. Biochim Biophys Act 1784(10): 1410–1414, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Caretti A, Fantacci M, Caccia D, Perrella M, Lowe KC, Samaja M: Modulation of the NO/cGMP pathway reduces the vasoconstriction induced by acellular and PEGylated haemoglobin. Biochim Biophys Act 1784(10): 1428–1434, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Jahr JS, Akha AS, Holtby RJ: Crosslinked, polymerized, and PEG-conjugated hemoglobin-based oxygen carriers: clinical safety and efficacy of recent and current products. Curr Drug Discov Technol 9(3): 158–165, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Olofsson CAT, Johansson T, Larsson S, Nellgård P, Ponzer S, Fagrell B, Przybelski R, Keipert P, Winslow N, Winslow RM: A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol-modified Hemoglobin (Hemospan) in patients undergoing major orthopedic surgery. Anesthesiology 105(6): 1153–1163, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Buehler PW, Alayash AI: Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion 44(10): 1516–1530, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Alayash AI: Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov 3:152–159, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Roche CJ, Cassera MB, Dantsker D, Hirsch RE, Friedman JM: Generating S-Nitrosothiols from Hemoglobin. Mechanisms, Conformational dependence and physiological relevance. J Biol Chem 288(31):22408–22425, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.D’Agnillo F, Chang TMS: Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotech 16(7):667–671, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Powanda D, Chang TMS: Cross-linked polyhemoglobin-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cell Blood Substit Immob Biotechnol 30(1):25–42, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Simoni J, Simoni G, Moeller JF, Feola M, Wesson DE: Artificial oxygen carrier with pharmacologic actions of adenosine-5’-triphosphate, adenosine, and reduced glutathione formulated to treat an array of medical conditions. Artif Organs 38(8):684–690, 2014. [DOI] [PubMed] [Google Scholar]
- 94.Wollocko H, Anvery S, Wollocko J, Harrington JM, Harrington JP: Zero-link polymerized hemoglobin (OxyVita(Hb) stabilizes the heme environment: potential for lowering vascular oxidative stress. Artif Cells Nanomed Biotech 45(4):701–709, 2017. [DOI] [PubMed] [Google Scholar]
- 95.Ma L, Thompson FM, Wang D, Hsia CJC. Polynitroxylated PEGylated Hemoglobin (PNPH): A nanomedicine for critical care and transfusion In: Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics. Eds: Kim HW, Greenberg AG. Berlin: Springer-Verlag, Chapter 16, 299–313, 2013. [Google Scholar]
- 96.Chang TMS. ‘Hemoglobin Corpuscles’ Report of a research project for Honours Physiology, Medical Library, McGill University 1957. Reprinted as part of ‘30 anniversary in Artificial Red Blood Cells Research’. J Biomat Artif Cells Artif Organs 16:1–9, 1988. [Google Scholar]
- 97.Chang TMS, Poznansky MJ: Semipermeable microcapsules containing catalase for enzyme replacement in acatalsaemic mice. Nature 218(5138):242–245, 1968. [DOI] [PubMed] [Google Scholar]
- 98.Chang TMS: Semipermeable microcapsules. Science 146(3643):524–525, 1964. [DOI] [PubMed] [Google Scholar]
- 99.Djordjevich L, Miller IF: Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp Hematol 8(5):584–592, 1980. [PubMed] [Google Scholar]
- 100.Hunt CA, Burnette RR, MacGregor RD, Strubbe A, Lau D, Taylor N: Synthesis and evaluation of a prototypal artificial red cell. Science 230(4730): 1165–1168, 1985. [DOI] [PubMed] [Google Scholar]
- 101.Rudolph AS, Klipper RW, Goins B, Phillips WT: In vivo biodistribution of a radiolabeled blood substitute: 99mTc-labeled liposome-encapsulated hemoglobin in an anesthetized rabbit. Proc Natl Acad Sci USA 88(23): 10976–10980, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E: Review of hemoglobin-vesicles as artificial oxygen carriers. Artif Organs 33(2): 139–145, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Pape A, Kertscho H, Meier J, Horn O, Laout M, Steche M, Lossen M, Theissen A, Zwissler B, Habler O: Improved short-term survival with polyethylene glycol modified hemoglobin liposomes in critical normovolemic anemia. Intensive Care Med 34(8): 1534–1543, 2008. [DOI] [PubMed] [Google Scholar]
- 104.Kawaguchi AT, Fukumoto D, Haida M, Ogata Y, Yamano M, Tsukada H: Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke 38(5): 1626–1632, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Agashe H, Awasthi V: Current perspectives in liposome-encapsulated hemoglobin as oxygen carrier. Adv Plan Lipid Bilayers Liposomes 9:1–28, 2009. [Google Scholar]
- 106.Ceh B, Winterhalter M, Frederik PM, Vallner JJ, Lasic DD: Stealth liposomes: from theory to product. Adv Drug Del Rev 24(2–3): 165–177, 1997. [Google Scholar]
- 107.Immordino ML, Dosio F, Cattel L: Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed 1(3):297–315, 2006. [PMC free article] [PubMed] [Google Scholar]
- 108.Philips WT, Klpper RW, Awasthi VD, Rudolph AS, Cliff R, Kwasiborski V, Goines VA: Polyethylene glycol modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J Pharm Exp Ther 288(2):665–670, 1999. [PubMed] [Google Scholar]
- 109.Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E: Hemoglobin-vesicle, a cellular artificial oxygen carrier that fulfills the physiological roles of the red blood cell structure. Adv Exp Med Biol 662:433–438, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Tsuchida E, Sou K, Nakagawa A, Sakai H, Komatsu T, Kobayashi K: Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjug Chem 20(8):1419–1440, 2009. [DOI] [PubMed] [Google Scholar]
- 111.Taguchi K, Urata Y, Anraku M, Watanabe H, Kadowaki D, Sakai H, Horinouchi H, Kobayashi K, Tsuchida E, Maruyama T, et al. : Hemoglobin vesicles, Polyethylene Glycol (PEG)ylated liposomes developed as a Red Blood Cell Substitute, do not Induce the accelerated blood clearance phenomenon in mice. Drug Metab Disposition 37(11):2197–2203, 2009. [DOI] [PubMed] [Google Scholar]
- 112.Kaneda S, Ishizuka T, Goto H, Kimura T, Inaba K, Kasukawa H: Liposome-encapsulated hemoglobin, TRM-645: current status of the development and important issues for clinical application. Artif Organs 33(2): 146–152, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Tao Z, Ghoroghchian PP: Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotech 32(9):466–473, 2014. [DOI] [PubMed] [Google Scholar]
- 114.Sakai H: Present situation of the development of cellular-type hemoglobin-based oxygen carrier (hemoglobin-vesicles). Curr Drug Discovery Tech 9(3):188–193, 2012. [DOI] [PubMed] [Google Scholar]
- 115.Yadav VR, Nag O, Awasthi V: Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif Organs 38(8):625–633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yadav VR, Rao G, Houson H, Hedrick A, Awasthi S, Roberts PR, Awasthi V: Nanovesicular liposome-encapsulated hemoglobin (LEH) prevents multi-organ injuries in a rat model of hemorrhagic shock. Eur J Pharm Sci 93:97–106, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kheir JN, Scharp LA, Borden MA, Swanson EJ, Loxley A, Reese JH, Black KJ, Velazquez LA, Thomson LM, Walsh BK, et al. : Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci Trans Med 4(140):140–188, 2012. [DOI] [PubMed] [Google Scholar]
- 118.Kheir JN, Polizzotti BD, Thomson LM, O’Connell DW, Black KJ, Lee RW, Wilking JN, Graham AC, Bell DC, McGowan FX: Bulk manufacture of concentrated oxygen gas-filled microparticles for intravenous oxygen delivery. Adv Healthcare Mater 2(8): 1131–1141, 2013. [DOI] [PubMed] [Google Scholar]
- 119.Yu WP, Chang TMS: Submicron polymer membrane hemoglobin nanocapsules as potential blood substitutes: preparation and characterization. Artif Cells Blood Substit Immobil Biotechnol 24(3): 169–184, 1996. [DOI] [PubMed] [Google Scholar]
- 120.Chang TMS, Yu WP: Nanoencapsulation of hemoglobin and RBC enzymes based on nanotechnology and biodegradable polymer In: Chang TMS, editor. Blood Substitutes: Principles, Methods, Products and Clinical Trials. Basel: Karger, 2: pp. 216–231, 1998. [Google Scholar]
- 121.Chang TMS: Blood replacement with nanobiotechnologically engineered hemoglobin and hemoglobin nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2(4):418–430, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rameez S, Alosta H, Palmer AF: Biocompatible and biodegradable polymersome encapsulated hemoglobin: a potential oxygen carrier. Bioconjug Chem 19(5): 1025–1032, 2008. [DOI] [PubMed] [Google Scholar]
- 123.Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F: In vitro macrophage uptake and in vivo biodistribution of PLA-PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. J Mater Sci Mater Med 20(9): 1881–1891, 2009. [DOI] [PubMed] [Google Scholar]
- 124.Arifin DR, Palmer AF: Polymersome encapsulated hemoglobin: a novel type of oxygen carrier. Biomacromolecules 6(4):2172–2181, 2005. [DOI] [PubMed] [Google Scholar]
- 125.Rameez S, Bamba I, Palmer AF: Large scale production of vesicles by hollow fiber extrusion: a novel method for generating polymersome encapsulated hemoglobin dispersions. Langmuir 26(7):5279–5285, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Misra H, Lickliter J, Kazo F, Abuchowski A: PEGylated carboxyhemoglobin bovine (SANGUINATE): results of a phase I clinical trial. Artif Organs 38(8):702–707, 2014. [DOI] [PubMed] [Google Scholar]
- 127.Ananthakrishnan R, Li Q, O’Shea KM, Quadri N, Wang L, Abuchowski A, Schmidt AM, Ramasamy R: Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif Cells Nanomed Biotechnol 41(6):428–436, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Abuchowski A: SANGUINATE (PEGylated Carboxyhemoglobin Bovine): Mechanism of action and clinical update. Artif Organs 41(4):346–350, 2017. [DOI] [PubMed] [Google Scholar]
- 129.Wu L: Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57(4):585–630, 2005. [DOI] [PubMed] [Google Scholar]
- 130.Tomita D, Kimura T, Hosaka H, Daijima Y, Haruki R, Ludwig K, Böttcher C, Komatsu T: Covalent core-shell architecture of hemoglobin and human serum albumin as an artificial O2 carrier. Biomacromolecules 14(6): 1816–1825, 2013. [DOI] [PubMed] [Google Scholar]
- 131.Hosaka H, Haruki R, Yamada K, Böttcher C, Komatsu T: Hemoglobin-Albumin cluster incorporating a Pt nanoparticle: artificial O2 carrier with antioxidant activities. PLoS One 9(10):e110541, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duan L, Yan X, Wang A, Jia Y, Li J: Highly loaded hemoglobin spheres as promising artificial oxygen carriers. ACS Nano 6(8):6897–6904, 2012. [DOI] [PubMed] [Google Scholar]
- 133.Xiong Y, Liu ZZ, Georgieva R, Smuda K, Steffen A, Sendeski M, Voigt A, Patzak A, Bäumler H: Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano 7(9):7454–7461, 2013. [DOI] [PubMed] [Google Scholar]
- 134.Li B, Li T, Chen G, Li X, Yan L, Xie Z, Jing X, Huang Y: Regulation of conjugated hemoglobin on micelles through copolymer chain sequences and the protein’s isoelectric aggregation. Macromol Biosci 13(7):893–902, 2013. [DOI] [PubMed] [Google Scholar]
- 135.Qi Y, Li T, Wang Y, Wei X, Li B, Chen X, Xie Z, Jing X, Huang Y: Synthesis of hemoglobin-conjugated polymer micelles by thiol Michael-addition reactions. Macromol Biosci 16(6):906–913, 2016. [DOI] [PubMed] [Google Scholar]
- 136.Jia Y, Cui Y, Fei J, Du M, Dai L, Li J, Yang Y: Construction and evaluation of hemoglobin-based capsules as blood substitutes. Adv Funct Mater 22(7): 1446–1453, 2012. [Google Scholar]
- 137.Chen B, Jia Y, Zhao J, Li H, Dong W, Li J: Assembled hemoglobin and catalase nanotubes for the treatment of oxidative stress. J Phys Chem C 117(38): 19751–19758, 2013. [Google Scholar]
- 138.Le Gall T, Polard V, Rousselot M, Lotte A, Raouane M, Lehn P, Opolon P, Leize E, Deutsch E, Zal F, et al. : In vivo biodistribution and oxygenation potential of a new generation of oxygen carrier. J Biotech 187:1–9, 2014. [DOI] [PubMed] [Google Scholar]
- 139.Tsai AG, Intaglietta M, Sakai H, Delpy E, La Rochelle CD, Rousselot M, Zal F: Microcirculation and NO-CO studies of a natural extracellular hemoglobin developed for an oxygen therapeutic carrier. Curr Drug Discov Technol 9(3):166–172, 2012. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Gao W, Peng W, Xie J, Li Y: Biorheological properties of reconstructed erythrocytes and its function of carrying-releasing oxygen. Artif Cells Blood Substit Immobil Biotechnol 37(1):41–44, 2009. [DOI] [PubMed] [Google Scholar]
- 141.Goldsmith HL, Marlow J: Flow behaviour of erythrocytes. I. Rotation and deformation in dilute suspensions. Proc R Soc Lond B 182(1068):351–384, 1972. [Google Scholar]
- 142.Charoenphol P, Mocherla S, Bouis D, Namdee K, Pinsky DJ, Eniola-Adefeso O: Targeting therapeutics to the vascular wall in atherosclerosis—carrier size matters. Atherosclerosis 217(2):364–370, 2011. [DOI] [PubMed] [Google Scholar]
- 143.Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S: Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci USA 106(51):21495–21499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haghgooie R, Toner M, Doyle PS: Squishy non-spherical hydrogel microparticles. Macromol Rapid Commun 31(2): 128–134, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, et al. : Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci USA 108(2):586–591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li S, Nickels J, Palmer AF: Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials 26(17):3759–3769, 2005. [DOI] [PubMed] [Google Scholar]
- 147.Xu F, Yuan Y, Shan X, Liu C, Tao X, Sheng Y, Zhou H: Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int J Pharm 377(1–2): 199–206, 2009. [DOI] [PubMed] [Google Scholar]
- 148.Pan D, Rogers S, Misra S, Vulugundam G, Gazdzinski L, Tsui A, Mistry N, Said A, Spinella P, Hare G, et al. ErythroMer: Nanoscale Bio-Synthetic Red Cell Substitute. Poster Abstract, Military Health System Research Symposium, Kissimmee, FL, August 15–18, 2016. [Google Scholar]
- 149.Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, Soltys K, Shiva S, Paranjpe S, Sadowsky D, et al. : Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under sub-normothermic conditions. Am J Transplant 15(2):381–394, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fontes P: The evolution of oxygen carrier solutions for machine perfusion. Transplantation 101(11):2657–2658, 2017. [DOI] [PubMed] [Google Scholar]
- 151.Muir WW, Wellman ML: Hemoglobin solutions and tissue oxygenation. J Vet Intern Med 17(2): 127–135, 2003. [DOI] [PubMed] [Google Scholar]
- 152.Sakai H, Masada Y, Takeoka S, Tsuchida E: Characteristics of bovine hemoglobin as a potential source of hemoglobin-vesicles for an artificial oxygen carrier. J Biochem 131(4):611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 153.Chan LW, White NJ, Pun SH: Synthetic strategies for engineering intravenous hemostat. Bioconj Chem 26(7): 1224–1236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lashoff-Sullivan M, Shoffstall A, Lavik E: Intravenous hemostats: challenges in translation to patients. Nanoscale 5(22): 10719–10728, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Booth C, Highley D: Crystalloids, colloids, blood, blood products and blood substitutes. Anaesthesia Intensive Care Med 11(2):50–55, 2010. [Google Scholar]
- 156.McCahon R, Hardman J: Pharmacology of plasma expanders. Anaesthesia Intensive Care Med 8(2):79–81, 2007. [Google Scholar]
- 157.Douay L, Andreu G: Ex vivo production of human red blood cells from hematopoietic stem cells: what is the future in transfusion? Transfus Med Rev 21(2):91–100, 2007. [DOI] [PubMed] [Google Scholar]
- 158.Rousseau GF, Giarratana MC, Douay L: Large-scale production of red blood cells from stem cells: what are the technical challenges ahead? Biotechnol J 9(1):28–38, 2014. [DOI] [PubMed] [Google Scholar]
- 159.Avanzi MP, Mitchell WB: Ex vivo production of platelets from stem cells. Br J Haematol 165(2):237–247, 2014. [DOI] [PubMed] [Google Scholar]
- 160.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita K, Koike T, Harimoto K, Dohda T, et al. : Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 14(4):535–548, 2014. [DOI] [PubMed] [Google Scholar]
- 161.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, Feng Q, Lu S, Lanza R, Neeves KB, et al. : Platelet bioreactor-on-a-chip. Blood 124(12): 1857–1867, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spitalnik SL, Triulzi D, Devine DV, Dzik WH, Eder AF, Gernsheimer T, Josephson CD, Kor DJ, Luban NL, Roubinian NH, et al. : State of the Science in Transfusion Medicine Working Groups. 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion 55(9):2282–2290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]