ABSTRACT
The regulation of gene expression is a fundamental cellular process and its misregulation is a key component of disease. Enhancers are one of the most salient regulatory elements in the genome and help orchestrate proper spatiotemporal gene expression during development, in homeostasis, and in response to signaling. Notably, molecular aberrations at enhancers, such as translocations and single nucleotide polymorphisms, are emerging as an important source of human variation and susceptibility to disease. Herein we discuss emerging paradigms addressing how genes are regulated by enhancers, common features of active enhancers, and how non-coding enhancer RNAs (eRNAs) can direct gene expression programs that underlie cellular phenotypes. We survey the current evidence, which suggests that eRNAs can bind to transcription factors, mediate enhancer-promoter interactions, influence RNA Pol II elongation, and act as decoys for repressive cofactors. Furthermore, we discuss current methodologies for the identification of eRNAs and novel approaches to elucidate their functions.
KEWORDS: Enhancer, Enhancer RNA, eRNA, Super Enhancer, Gene Regulation
Introduction
The transcriptional output of any given cell is largely dictated by the repertoire of active enhancer elements contained within its genome. Enhancers were first described in the 1980’s as a sequence of DNA that increases the expression of a linked gene in a manner that is independent of its distance and orientation [1–3]. Interestingly, the first enhancer was a 72-base pair (bp) sequence, cloned out of the simian virus 40 (SV40), that was capable of increasing the expression of the human β-globin gene [1,2]. Since then, enhancers have been found in many metazoan organisms, including humans, and have been shown to recruit specific transcription factors to dictate spatiotemporal gene expression during development, normal physiology, and even in disease [4–9].
The advent of next-generation sequencing technologies has facilitated comprehensive and unbiased identification of enhancers across the genome, and current estimates suggest that there are over 400,000 enhancers scattered throughout the genome [10,11]. Enhancers can be located within genes, in intragenic regions, and even on different chromosomes [12–15]. Interactions between enhancers and their target genes are not exclusive, and frequently promoters are found to interact with multiple enhancers and vice versa [11,16,17]. Of note, enhancers are highly cell-type specific and thus not all potential enhancers are expected to be active at the same time. Instead, within any given cell type, there are tens of thousands of active enhancers orchestrating lineage-specific gene expression [10,11,18–20].
Enhancer malfunction is emerging as a major contributor to human diseases, including cancer, as mounting evidence across many tumor types shows that enhancer networks are rewired by molecular aberrations that collectively lead to the cancer phenotype [21–27]. Translocations, deletions, and mutations within regulatory regions of the genome are frequently observed in cancer patients, which may cause loss of expression of tumor suppressors or overexpression of oncogenes [25,27,28]. For example, in Burkitt lymphoma, genomic translocations relocate the highly active enhancers of the immunoglobin heavy chain genes in close proximity to the MYC oncogene, leading to deleterious activation of MYC expression [29,30]. In B-cell lymphoma, rearrangements and duplications of benign enhancers drive the expression of typical cancer genes, such as MYC, BCL2, and NOTCH1 [31]. In addition to enhancer translocation or duplication, cell type-specific enhancers can also be activated, or “hijacked”, by cancer cells for the activation of genes that lead to tumorigenesis or drug resistance [21,23,32]. Thus, delineating the dysregulated enhancer network would certainly extend our knowledge of cancer biology, and provide alternative pathways to treat stubborn cancers lacking driver mutations [33,34]. The increasing appreciation for the role of enhancers in the etiology of disease has placed emphasis on defining the mechanisms of enhancer function, and, although many common features of enhancers have been described over the years, the order-of-events for the activation of enhancers is still unknown.
Common features of active enhancers
Recent studies have focused on the properties of enhancers beyond the binding of sequence-specific transcription factors, which might give clues to their mechanisms of action and aid in their identification. In this regard, cooperative binding of transcription factors, co-factors, chromatin remodeling enzymes, and ultimately RNA Polymerase II complex (RNA Pol II) at enhancers mediate the activation of target gene expression (Figure 1) [35–37]. However, assembly of these proteins and enzymes requires the DNA at enhancers to be open and accessible. Thus, chromatin accessibility is a defining feature of active enhancers and can be measured using various techniques, such as DNase I Hypersensitivity, Formaldehyde-Assisted Isolation of Regulatory Elements (FAIRE), or transposase assisted measurements of chromatin accessibility (ATAC) (Table 1) [3,38–40]. In addition, current models posit that communication between enhancers and their target genes typically occurs via DNA looping, which enables physical interaction between enhancers and target gene promoters (Figure 1) [41–44]. The long-range interactions between enhancers and promoters was initially confirmed using proximity ligation techniques, such as chromosome conformation capture (3C) [45–47], and later coupled to high-throughput sequencing methods such as 4C and HiC-seq [48–51]. Of note, it is thought that chromatin loops are established prior to enhancer activation, suggesting that these networks are pre-determined during the differentiation of the cell.
Figure 1.
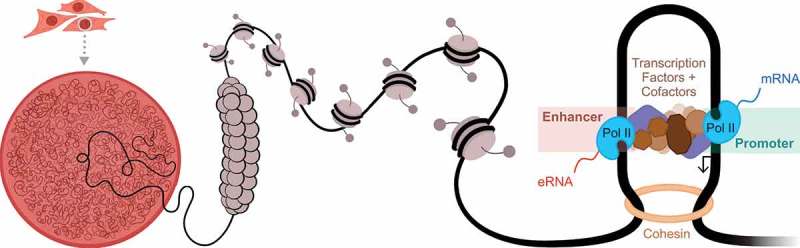
A model for enhancer-promoter interactions within the nucleus of a cell. Enhancers may be located far away in linear distance to their target genes, but are brought into close proximity to these genes via chromatin loops and higher order chromatin structure. Enhancers are bound by sequence-specific transcription factors, which in turn facilitate the cooperative binding of chromatin remodeling enzymes, histone modifying enzymes, other co-factors, and ultimately the RNA polymerase II complex (RNA pol II). Both the target genes and the enhancers are transcribed by RNA pol II. Transcription of the enhancers give rise to non-coding RNA molecules called enhancer RNAs (eRNAs).
Table 1.
Common features of an active enhancer.
| Feature | Commonly Used Methods for Detection | References |
|---|---|---|
| Chromatin accessibility | DNase-seq, ATAC-seq, and FAIRE-seq | [11,38–40] |
| Histone modification H3K4me1 | ChIP-qPCR, ChIP-chip, and ChIP-seq | [10,52,53] |
| Histone modification H3K27Ac | ChIP-qPCR, ChIP-chip, and ChIP-seq | [10,60,61] |
| Enhancer-promoter looping | 3C, 4C, HiC, and Hi-ChIP | [45–50,163,165–167,176] |
| Binding of transcription factors, co-factors, and RNA Pol II | ChIP-qPCR, ChIP-chip, and ChIP-seq | [10,18–20,52,55,56,58] |
| Production of eRNAs | GRO-seq, CAGE, PRO-seq, Total RNA-seq RT-qPCR (using random hexamer primers) | [21,97,152,156–158] |
The epigenetic state of enhancers is also fundamental to their functions and can be used to distinguish between enhancers and gene promoters (Table 1). For example, the nucleosomes surrounding enhancers typically harbor histone H3 lysine 4 monomethylation (H3K4me1) and lack histone H3 lysine 4 trimethylation (H3K4me3). In contrast, active promoters are enriched in H3K4me3 and have reduced H3K4me1 at their transcription start sites [52,53]. The status of H3K4 methylation allows us to differentiate between enhancers and promoters, but this sole criterion is not sufficient to divide all enhancers and promoters without ambiguity. Combining H3K4 methylation with other indicators of enhancer activity, for example, transcriptional coactivators p300/CBP or Mediator, can significantly improve the enhancer prediction [18,54–57]. Similarly, binding of RNA Pol II, BRD4, and other TFs, have also been utilized [10,20,52,55,58,59]. Furthermore, studies in embryonic stem cells and several primary cell types uncovered that acetylation of histone H3 lysine 27 (H3K27ac) together with H3K4me1 is typically enriched at active enhancers, whereas H3K4me1 alone designates inactive or “‘poised’” enhancer regions (Table 1) [3,60,61].
One of the most robust indicators of an active enhancer is the binding of RNA Pol II and the production of non-coding enhancer RNAs (eRNAs) (Figure 1). The discovery that enhancers are transcribed to produce non-coding RNAs has added another layer of complexity to the mechanisms of gene activation by enhancers and has given rise to an intense area of investigation. Below we detail the current state of enhancer RNAs, what is known about their potential functions, and highlight some emerging paradigms about their role in gene expression (Table 2).
Table 2.
Common features of eRNAs.
| Common features of eRNAs | Selected References | Comments |
|---|---|---|
| Produced at active enhancers | [21,82,104] | |
| Non-coding transcripts | [78–80] | |
| Transcribed by RNA Pol II | [8,78,82,88–90]. | |
| Not spliced | [81,96,97] | Spliced eRNA: [14] |
| Lack of polyadenylation | [78,90,96,100,101] | eRNAs with polyadenylation: [80,96] |
| Cell-type specific | [21,82,104] | |
| Bidirectional transcription | [79,81–84] | Unidirectional transcription: [78,80,85–87] |
| Degraded by RNA Exosome | [78,90,96,100,101] | |
| Regulated by Integrator | [95] | |
| Average size 2kb – 5kb | [78–80] | |
| Bind transcription factors | [58,89,98,117,126–131] | |
| Release paused RNA Pol II | [90,133–135] | |
| Contribute to enhancer-promoter looping | [63,89,92,94,99,108,142] | Exceptions: [82,90,143] |
| Interfere with transcriptional repressors | [90] |
Super enhancers and liquid-liquid phase separation
Advances in enhancer identification using high-throughput sequencing technologies have revealed the presence of large clusters of enhancers that exist in close proximity to each other and seem to work synergistically for the activation of target genes. These clusters of enhancers, named super-enhancers (SEs), are noted for their unusually high occupancy of interacting factors, enzymes, and histone modifications, that work together to drive the expression of prominent lineage specific genes [55,62]. Super-enhancers are larger than typical enhancers, are highly enriched in the classic features of active enhancers (such as H3K27ac, H3K4me1, mediator, p300, BRD4, and RNA Pol II), and are thought to act synergistically for the activation of target genes. Out of 10,000 to 150,000 putative active enhancers in a cell, there are only a few hundred super-enhancers. These SEs drive high levels of transcription and are strongly proposed to be cell fate determinants that dictate cell type-specific gene expression [55,63,64]. Current evidence suggests that super-enhancers contribute to the high expression of pluripotent genes in embryonic stem cells (mESCs), cell type-specific genes in terminally differentiated cells, and oncogenes in various cancer cells [65,66], and therefore have garnered significant attention.
Emerging evidence suggests that the high levels and very dense assemblies of transcription factors and co-factors at super-enhancers impart the ability of SEs to form biomolecular condensates as a result of liquid-liquid phase separation [67]. Liquid-liquid phase separation refers to the process by which a homologous solution of molecules spontaneously separates into two liquid phases with enriched or depleted molecules [68]. This concept has been widely adopted to explain the dynamics of membrane-less organelles in cells, including centrosome, nucleolus, paraspeckles, P-bodies, and stress granules [68,69]. In contrast to membrane-enclosed subcellular compartments, these membrane-less organelles solely consist of a large number of macromolecules, primarily RNAs and proteins, which “condensate” to form a complex based on protein-protein and protein-nucleic acid interactions. Phase separation at SEs are thought to allow for compartmentalization of the transcriptional components needed for robust activation of target genes [67,69–71].
However, this also makes SEs more susceptible to perturbation as compared to most typical enhancers [67]. Recent literature suggests that function of SEs relies on the proper aggregation of the participating transcription factors and coactivators, which depends on the phase-separating properties of their intrinsically disordered regions (IDRs). Chemical compounds with capacity to disrupt liquid-liquid biomolecular condensates, such as 1,6-hexanediol, have been shown to deprive occupation of BRD4, MED1 and RNA Pol II at SEs and attenuate the expression of SE-driven genes [67,70,72,73]. Since SEs are known to regulate the expression of oncogenes, they can be targeted to change the fate of cancer cells by disrupting the biomolecular condensates that mediate their function. The emerging roles of phase separation and its implications on gene expression and human health have been reviewed thoroughly in the following publications [67,68,71,74,75]. Despite the order of magnitude that separates enhancers and super-enhancers, they share many key features that govern their functions. One of the most interesting of these is the production of eRNAs.
Enhancer RNA discovery and transcriptional properties
The discovery that enhancers are transcribed and produce non-coding RNA transcripts came as a significant breakthrough in the study of enhancer function. This phenomenon was first described at the β-globin locus, where it was shown that the hypersensitive site 2 (HS2) enhancer produced transcripts when active [76,77]. It was not until 2010 that enhancer transcription was demonstrated to be a shared feature of enhancers, as opposed to a mechanism exclusive to the HS2 enhancer. Using RNA Pol II chromatin immunoprecipitation coupled with high-throughput sequencing (ChIP-seq), it was shown that enhancers and super-enhancers produce non-coding RNA molecules, termed enhancer-derived RNAs (eRNAs) [78–80]. The role of eRNAs in enhancer function remains unclear, however, numerous studies have set forth to characterize eRNAs and determine their role, which has highlighted a variety of shared and unique features that are important to enhancers (Table 2).
The simplest definition of an eRNA is a non-coding transcript produced within a validated enhancer region, though the traits that are often shared amongst them are considerably more complex. Current models posit that enhancers are transcribed bidirectionally in a manner resembling gene promoters [79,81–84], with unidirectional transcription occurring at a small subset of these elements [76,78,80,85,86]. Interestingly, a recent single-cell transcriptomic profiling technique has been used to demonstrate that enhancers are primarily transcribed unidirectionally from a single strand on a cell-to-cell basis, challenging the previously accepted belief that both strands are transcribed simultaneously [87]. This study also used single-molecule fluorescence in situ hybridization (smFISH) to show that when both strands are transcribed in the same cell, the eRNAs rarely colocalize. This suggests that enhancers may have additional functionality based on which strand is actively transcribed, though the mechanism by which a strand is selected for transcription has not been defined. Transcription of eRNAs occurs via RNA Pol II and temporally precedes the activation of target gene promoters [8,78,82,88–90]. These transcripts exist in low abundance and are primarily found in nuclear and chromatin-bound fractions [78,91–93]. Quantification of eRNAs that are thought to exist in higher abundance suggests a range of 0.5–20 copies per cell [94]. eRNA stability and 3ʹ-end processing appears to be regulated by the integrator complex [95] in a poly(A) cleavage site-mediated mechanism that leads to early termination, as compared to promoters [96,97]. Several studies have used northern blots to demonstrate that eRNAs appear as distinct bands, corroborating that they have defined termination sites and are produced at consistent lengths [89,98,99]. eRNAs are predominantly non-polyadenylated and degraded via the exosome, resulting in a high turnover rate with a half-life of approximately 7 minutes [78,90,96,100,101]. Some studies have identified polyadenylated and stable eRNAs, although there is still a debate on whether these transcripts should be classified as long non-coding RNAs instead [22,80,96]. eRNAs are largely unspliced due to a general deficit of U1 splice sites within enhancer regions, resulting in only ~5% of transcripts being spliced (Table 2) [81,96,97].
eRNA production constitutes a large portion of the human transcriptome with previous estimates indicating ~40,000–65,000 events [81,88]. eRNAs are not unique to humans, as they appear in several diverse organisms, suggesting an early evolutionary origin that is critical to enhancer function [81,88,102,103]. Indeed, the production of eRNAs has become widely accepted as one of the most robust indicators of an active enhancer [21,82,104]. Despite this, the lack of a clear mechanism for eRNA requirement has sparked debate regarding their role. Explanations for the role of eRNA production typically fall within three broad schools of thought. The first suggests that eRNAs exist as background noise that occurs as enhancer loci are incidentally transcribed based on close proximity to active gene promoters. The second supposes that transcription of an enhancer region is necessary for its function, likely to establish an open chromatin state, but that eRNAs are merely a byproduct of this and serve no additional purpose. The third model for eRNA production stipulates that eRNAs play an active role post-transcriptionally and are required for proper enhancer function. A growing body of evidence has begun to shed light on the potential functions of eRNAs, highlighting a complex and fascinating collection of transcripts that do not always fall within convenient classifications (Table 2).
Emerging paradigms for eRNA function
eRNAs are transcriptional noise
A simple explanation for the pervasive production of eRNAs, is that they may arise from leaky transcription events across the genome without significant biological function. It has been suggested that similar to other DNA-binding proteins, RNA Pol II binding to chromatin is not stringent, with only a small percentage (~10%) of RNA Pol II binding leading to productive transcription [105]. Open chromatin structures at active enhancers likely facilitates RNA Pol II binding and may accidentally promote initiation of transcription. Consequently, promiscuous RNA Pol II binding and stochastic transcription events would inevitably produce numerous transcripts as background noise with no apparent biological functions [105]. Most eRNAs are subject to destruction by the exosome complex, therefore, most eRNAs have low abundance and are rapidly degraded, suggesting that they may not have an appreciable biological function in local or long-range transcription events [106–108]. However, this idea has been evolving as improved genomic approaches have revealed that low abundance transcripts are still capable of having a profound effects on biological processes.
eRNAs are a byproduct of necessary transcription at enhancers
Instead of the eRNA transcript itself, the act of transcription could promote chromatin remodeling around the enhancer to establish and maintain an open chromatin state required for proper activation of target genes. For example, it has been shown that initiation and elongation of RNA Pol II at its binding sites leads to the remodeling of local chromatin and deposition of histone modifications necessary for active transcription [109,110]. In addition, there are several histone modifying enzymes that “piggy back” onto RNA Pol II, such as p300, which can acetylate histones co-transcriptionally [111]. Thus, perturbations of transcription at the enhancers often result in reduced target gene activation [17,112,113]. Although transcription of the enhancer region appears critical for enhancer function, it is difficult to dissociate transcription and the resulting RNA products since both are impaired upon interference. However, recent advances have made it possible to knockdown eRNAs transcripts and study their functions without affecting the enhancers from which they are produced. For example, at TLR4 signaling induced enhancer sites, RNA Pol II transcription induced changes in histone methylation, and this methylation was solely dependent on RNA PoI II transcription instead of the eRNA transcripts [83]. Beyond the chromatin remodeling capacity of RNA Pol II transcription, a recent study demonstrates that transcription induced-supercoiling drives chromatin loop extrusion, therefore, playing a role in stimulating enhancer-promoter contacts during enhancer transcription [114].
eRNA transcripts are required for enhancer function
The association between eRNA production and enhancer activity has become so widely accepted that production of eRNAs alone has been used as a method to identify novel enhancers [21,104]. Several studies have demonstrated that eRNA production temporally precedes the upregulation of target gene mRNAs [8,78,82,88–90] and occurs with similar kinetics, as shown by single-cell profiling [93]. Given that eRNAs are predominantly located within the nucleus and chromatin-bound fractions, this suggests that enhancer transcription and/or eRNAs are critical for enhancer-mediated target gene upregulation [78,91–93]. Dissecting the requirement for the eRNA transcript apart from the act of transcription has proven challenging for investigations into enhancer function. This has been primarily overcome through the use of short hairpin RNAs (shRNAs), small interfering RNAs (siRNAs), and locked nucleic acids (LNAs) that knockdown eRNAs without affecting transcription at the enhancer; providing some of the most compelling evidence for eRNA function [89,90,92,94,98,99,115–123]. These studies showed that knockdown of the eRNA was associated with a reduction in target gene expression, strongly suggesting a functional role for the eRNA. Conversely, tethering of eRNA transcripts to inactive enhancers has been shown to elicit features of target gene activation [92,98,124,125]. Taken together, these data suggest that the eRNA transcripts are required for proper enhancer function, although their mechanisms of action are less clear.
Several studies have utilized a variety of methods at individual enhancer loci to determine how eRNA transcripts might be involved in enhancer mechanisms (Figure 2). Enhancers are characterized by their ability to bind transcription factors (TFs) needed for efficient upregulation of their target genes [91]. It has therefore been suggested that eRNAs may be involved in binding these transcription factors to stabilize or trap them in place [126]. Several studies have identified interactions that occur between eRNAs and key transcription factors known to bind at a broad array of enhancers, such as mediator, CBP, BRD4, cohesin, P300, YY1, and p53 (Figure 2(b)) [58,89,98,117,126–131]. In-vitro assays have further demonstrated that eRNAs capable of interacting with CBP do so in an eRNA sequence independent manner, suggesting that CBP may recognize specific RNA secondary structures that are inherent to many eRNAs [130]. The association between eRNA transcripts and TF binding is so strong that production of eRNAs has even been used as a method to predict TF activity [132]. These observations demonstrate how eRNA functionality can be tied into a fundamental characteristic of enhancer activity, transcription factor binding and stabilization (Table 2).
Figure 2.
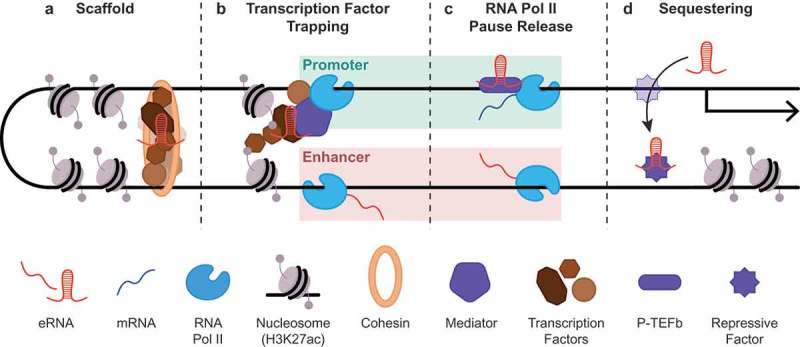
Emerging paradigms for eRNA function. eRNAs have been implicated as critical components of active enhancers in several ways. (a). eRNAs can interact with chromatin looping factors to act as scaffolding for proper enhancer-promoter loop formation. (b). Binding to various transcription factors enables eRNAs to participate in TF trapping, RNA Pol II loading, and histone modification. (c). eRNAs can facilitate RNA Pol II pause release via activation of the P-TEFb complex. (d). eRNAs can also act as decoys to sequester cofactors that repress transcription at target genes.
eRNAs have also been implicated in the release of paused RNA Pol II from target gene promoters to allow for productive elongation. Studies into this potential role have demonstrated two distinct ways that eRNAs control RNA Pol II elongation. Similar to TF trapping, eRNAs have been shown to bind and activate the positive transcription elongation factor B (P-TEFb) complex (Figure 2(c)), which uses phosphorylation to promote the release and elongation of RNA Pol II [133–135]. Specifically, the PSA eRNA has been shown to interact with the kinase CYCLIN T1, a subunit of the P-TEFb complex, and to be necessary for serine-2 phosphorylation and RNA Pol II release [134]. eRNAs have also been shown to act as decoys that bind repressive cofactors, enabling productive transcription of their target genes (Figure 2(d)). This mechanism was best demonstrated through the observation that neuronal eRNAs were required for binding and sequestering away the negative elongation factor (NELF), allowing for the release of paused RNA Pol II from cognate target promoters (Table 2) [90].
Perhaps the most notable feature of enhancers is the formation of chromatin loops that allow for direct interaction with target gene promoters. While the specific mechanisms that create these loops are not well understood, it is known that several architectural proteins are crucial for their creation, such as CTCF, YY1, cohesin, mediator, and integrator [95,136–141]. As discussed earlier, several investigations have provided considerable evidence to show that eRNAs are capable of interacting with many of these elements, suggesting that they may be required to establish and/or stabilize chromatin loops (Figure 2(a)). To further emphasize their importance, several studies have demonstrated that knockdown of eRNAs disrupt the loop structures and result in decreased target gene transcription [63,89,92,94,99,108,142]. eRNAs also exhibit the ability to bind signal regulated transcription factors to facilitate enhancer-promoter interactions, as shown with the estrogen receptor in breast cancer cells [94]. However, this should not necessarily be considered as a universal mechanism for eRNAs considering that other studies have identified enhancers that do not rely on the eRNA transcript for loop formation/stabilization [82,90,143]. It is likely that at these particular loci, the eRNAs serve a disparate purpose unassociated with the formation of chromatin loops (Table 2).
Though enhancers are commonly considered to be cis acting elements, some do have the ability to operate in trans and there is evidence to suggest that eRNAs also have trans activity in rare cases. It has recently been demonstrated that an eRNA produced at the MyoD locus work in trans to colocalize with Myogenin nascent transcripts and associate with the cohesin complex to enable recruitment to the Myogenin locus [14]. Despite the rare occurrence, this study demonstrates that eRNAs are capable of performing diverse roles and are not limited to acting in cis. Interestingly, this particular eRNA is spliced, unidirectional, and polyadenylated. This allows for speculation that the transcript is more stable than its non-polyadenylated counterparts and these features may aid in defining a subgroup of eRNAs that have trans functionality. The accumulated evidence for eRNA active participation in the enhancer mechanism is very compelling and continues to grow as the field moves forward. In fact, eRNAs have very recently been implicated in the formation of liquid-liquid phase separation [67,144]. Taken together, these studies suggest that eRNA activity should always be considered at novel enhancers unless the loss of eRNA transcript can be deemed non-essential through experimental testing. While there are some shared mechanisms of eRNA behavior, there does not appear to be a defining feature and each eRNA should be assessed on an individual basis, perhaps even within a specific enhancer locus.
Conclusions and future perspectives
The study of enhancers has gained significant interest recently as research into human diseases has shifted from the protein coding regions of the genome to the vast and primarily unexplored non-coding regions. As more mutations and genomic alterations of the non-coding genome become associated with disease, the investigation of novel enhancers and aberrantly regulated enhancers will continue to increase. These investigations will likely focus on a few key areas: (1) the identification of enhancers that drive disease, (2) the network of genes that they regulate, and (3) the mechanisms by which they function. The current body of evidence for enhancer function clearly demonstrates the necessity for enhancer transcription and their cognate eRNAs, while also depicting that enhancers lack a common unifying mechanism. Therefore, it is important to view the current models as general outlines for how eRNAs can behave and to continue searching for novel mechanisms. Furthermore, the pervasiveness of eRNA production raises several crucial questions about the nature of these RNAs and biological or pathological significance of enhancer transcription. We hypothesize that eRNAs act locally at the enhancer from which they originate, functioning as a scaffold for assembling components of an active enhancer complex. We also anticipate that eRNAs may be a critical participant in liquid-liquid phase separation of chromatin as emerging literature provides compelling evidence for this phenomenon [144,145]. In addition, it is now evident that eRNA-producing enhancers are highly cell type specific, represent the most highly active enhancers (as compared to non-eRNA-producing enhancers), and consequently drive the critical gene expression pathways of any given cell type.
The enhancers discussed in this review serve as a reminder that each locus has unique features, and most vary in how they utilize their produced transcripts. Therefore, the role of enhancer transcription and also the eRNA transcript itself should be assessed when studying a particular enhancer or set of enhancers. Some reports have identified active enhancer loci that fail to produce eRNAs [91,92,146]. However, there is still a debate regarding whether these enhancers do not produce eRNAs or simply produce them at an undetectably low level [103]. In consideration of these points, it is important to reiterate that eRNAs do not always fall within well-defined classifications. It is possible and likely that some currently assigned eRNAs are actually long non-coding RNAs (lncRNAs), and the reverse. It is also critical to remember that the current definitions for these two groups of transcripts are not exclusive and that several transcripts can be fairly labeled as both an eRNA and lncRNA. For example, linc-p21 and lockd are annotated lncRNAs that have been shown to exhibit cis-regulatory effects on target genes, independent of the RNA transcript, suggesting that the regulatory effects of these particular lncRNAs are due to DNA enhancer elements found within the locus [147,148]. These findings have prompted the idea that lncRNAs may evolve from eRNAs, whereby spurious splicing and polyadenylation of ancestral eRNAs, due to the accidental presence of splicing and 3' processing sequences in the vicinity of the respective enhancers, would lead to the stabilization of the eRNAs and the acquisition of novel biochemical properties of these RNA transcripts [149]. It is also equally likely that enhancers may simply exist within lncRNA genes, similar to how they are found within mRNA genes, and thus co-exist as “lncRNAs with enhancers within them”; negating the need to relabel these particular lncRNA as eRNAs. Thus, the boundaries that define enhancer/eRNA function remain broad and allow for several potential avenues that must be explored. Below we provide some context for methods commonly used to study enhancers and their eRNAs that will hopefully serve as a primer for those launching inaugural studies in this field.
Common methods used for detecting eRNAs
Several genomic techniques have been used to measure RNA production, however the majority of eRNA transcripts were originally missed because these genome-wide transcriptomic methods relied on polyadenylation priming. While valuable poly(A)-based datasets have been analyzed to draw worthwhile conclusions about enhancers and their transcripts, this limits the eRNAs studied to a relatively small subset of all potential eRNAs [22]. Future experiments should utilize techniques that focus on detecting nascent transcripts, such as global run-on sequencing (GRO-Seq) [21,82,94,95,97,118,150–155], precision run-on nuclear sequencing (PRO-Seq) [156], and cap analysis gene expression (CAGE) [157,158], to ensure that all eRNAs are surveyed. Total RNA-sequencing using random hexamer primers for reverse transcription, coupled to very high depth of sequencing, can also be used to measure eRNA transcripts. These techniques are particularly useful for the identification of previously unannotated eRNAs in a high-throughput manner, which has proven to be a successful approach to discover novel active enhancers [21].
Knowledge of the gene(s) regulated by a particular enhancer is necessary to draw meaningful conclusions about the role of the enhancer, but this can prove to be challenging. It was previously thought that enhancers act upon their nearest neighboring gene, however we now know that enhancers are capable of upregulating genes located farther than 1 Mbp away and can skip over closer genes [36,37,159,160]. This is further complicated by the discovery that individual enhancers can interact with and influence several different promoters [16]. Fortunately, there are several approaches to identify target gene(s) and investigate the mechanisms of enhancer function.
Chromatin loops allow physical interaction between enhancers and their target gene promoters, thus the ability to visualize the 3D structure of the genome is extremely valuable [8,42,44,89,161,162]. There are several chromatin conformation techniques that enable the mapping of enhancer-promoter interactions, such as chromatin conformation capture (3C) [46,47,163], Hi-C [164,165], and recently Hi-ChIP [166,167]. These techniques can be used to identify new enhancer-promoter interactions or describe the loss of contact following disruption of enhancer activity. As our general understanding of higher order chromatin structure and phase separation grows, these techniques will likely increase in value as they can provide a unique insight into a fundamental aspect of enhancer activity.
As with chromatin loops, we know that enhancers and eRNAs interact with key enzymes and TFs that have significant impacts on target gene upregulation. Therefore, techniques to discover the identity of proteins interacting with specific enhancers or eRNA transcripts are extremely useful. Some methods used to accomplish this are RNA immunoprecipitation (RIP) [168,169], RNA antisense purification coupled with mass spectrometry (RAP-MS) [120,170], variations of ChIP-seq [78,79], and several other RNA-protein interaction techniques, as discussed in a recent review [171]. Additionally, there are several valuable databases, such as FANTOM [172,173] and ENCODE [10,59], that contain annotated enhancers using datasets generated by several of these approaches.
Methods for eRNA perturbation
Beyond observing the enhancer-promoter physical interactions that occur, another common approach is to perturb the activity of the enhancer and assess the effects on target gene(s) expression. This can be accomplished through several avenues that typically involve either altering the DNA sequence of the enhancer, the transcriptional status of the enhancer, or the eRNA transcript produced. A classical approach is to delete the enhancer region entirely [174], or insert a transcriptional terminator sequence within the enhancer [85,112], to cause a loss of enhancer function. Both of these methods involve altering the sequence of the enhancer; however, insertion of a terminator sequence is considered to be less invasive since it inhibits transcription while having a smaller impact on the enhancer sequence. Another recently popular technique for disrupting enhancers is the use of clustered regularly interspaced palindromic repeat interference (CRISPRi) that features a catalytically dead Cas9 (dCas9) fused to a Krüppel associated box (KRAB) domain to epigenetically inhibit transcription at a targeted enhancer and thereby prevent its ability to upregulate target gene(s) [17,175–180]. This technique allows researchers to manipulate the function of the enhancer element without altering the DNA sequence. Interestingly, the reverse can also be performed using CRISPR activation (CRISPRa), a method that can specifically activate or turn on enhancers to study their effects [128,177,181–185]. In a step to further refine these approaches, several researchers have employed siRNAs [142] or antisense mediated decay [89,128] to knockdown the eRNA transcripts directly without altering the transcriptional status of the enhancer. These methods are considered to be the most targeted approaches as they only disrupt mechanisms associated with the post-transcriptional function of eRNA transcripts. The converse approach to this would be CRISPR-Display, which involves tethering an eRNA transcript to dCas9 to stimulate eRNA-based mechanisms in the absence of transcription [125].
Production of eRNAs can also be inhibited by treatment with small molecule inhibitors of RNA Pol II elongation such as flavopiridol [82], or inhibitors of transcription initiation such as Triptolide [186]. It is important to note that these small molecule inhibitors are not specific to just eRNAs and will disrupt global transcription. The rapid degradation of eRNA transcripts has thwarted the use of common RNA sequencing approaches that measure steady-state RNA levels [187,188]. To combat this issue, investigators have used exosome inhibition to stabilize eRNA transcripts and allow for further study into their molecular function [81]. Taken together, the techniques discussed here demonstrate the variety of approaches that can be taken to identify and study enhancers and their eRNAs, from classical genetic engineering to cutting edge epigenetic perturbations. The combination of several methodologies has been the greatest asset to these investigations and should be regarded as the best avenue for future studies into enhancer function. This field will continue to be shaped by the constantly evolving technology that allows researchers to answer novel questions and address past queries with greater precision.
Funding Statement
This work was supported by the Congressionally Directed Medical Research Programs [BC180450]; National Cancer Institute [T32CA2178242]; National Cancer Institute [R00CA204628].
Acknowledgments
This work was supported by grants from the NIH/National Cancer Institute (R00CA204628) and the Department of Defense Breast Cancer Research Program (BC180450) to H.L.F. Additional support was provided by a grant from the NIH/National Cancer Institute (T32CA2178242) to M.W.L.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Banerji J, Rusconi S, Schaffner W.. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27(2 Pt 1):299–308. [DOI] [PubMed] [Google Scholar]
- [2].Moreau P, Hen R, Wasylyk B, et al. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981;9(22):6047–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144(3):327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Banerji J, Olson L, Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983;33(3):729–740. [DOI] [PubMed] [Google Scholar]
- [5].Gillies SD, Morrison SL, Oi VT, et al. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983;33(3):717–728. [DOI] [PubMed] [Google Scholar]
- [6].Kong S, Bohl D, Li C, et al. Transcription of the HS2 enhancer toward a cis-linked gene is independent of the orientation, position, and distance of the enhancer relative to the gene. Mol Cell Biol. 1997;17(7):3955–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li WB, Notani D, Rosenfeld MG. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 2016;17(4):207–223. [DOI] [PubMed] [Google Scholar]
- [8].Kim T-K, Shiekhattar R. Architectural and functional commonalities between enhancers and promoters. Cell. 2015;162(5):948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heinz S, Romanoski CE, Benner C, et al. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol. 2015;16(3):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Consortium TEP An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thurman RE, Rynes E, Humbert R, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Birnbaum RY, Clowney EJ, Agamy O, et al. Coding exons function as tissue-specific enhancers of nearby genes. Genome Res. 2012;22(6):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dong X, Navratilova P, Fredman D, et al. Exonic remnants of whole-genome duplication reveal cis-regulatory function of coding exons. Nucleic Acids Res. 2010;38(4):1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsai PF, Dell’Orso S, Rodriguez J, et al. A muscle-specific enhancer RNA mediates cohesin recruitment and regulates transcription in trans. Mol Cell. 2018;71(1):129–141 e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Spilianakis CG, Lalioti MD, Town T, et al. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435(7042):637–645. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Y, Wong CH, Birnbaum RY, et al. Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504(7479):306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fulco CP, Munschauer M, Anyoha R, et al. Systematic mapping of functional enhancer-promoter connections with CRISPR interference. Science. 2016;354(6313):769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Visel A, Blow MJ, Li Z, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457(7231):854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heintzman ND, Hon GC, Hawkins RD, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459(7243):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shen Y, Yue F, McCleary DF, et al. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Franco HL, Nagari A, Malladi VS, et al. Enhancer transcription reveals subtype-specific gene expression programs controlling breast cancer pathogenesis. Genome Res. 2018;28(2):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen H, Li C, Peng X, et al. A pan-cancer analysis of enhancer expression in nearly 9000 patient samples. Cell. 2018;173(2):386–399 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Northcott PA, Lee C, Zichner T, et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature. 2014;511(7510):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Z, Zhang Q, Zhang W, et al. HEDD: human enhancer disease database. Nucleic Acids Res. 2018;46(D1):D113–d120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sur I, Taipale J. The role of enhancers in cancer. Nat Rev Cancer. 2016;16:483. [DOI] [PubMed] [Google Scholar]
- [26].Rickels R, Shilatifard A. Enhancer logic and mechanics in development and disease. Trends Cell Biol. 2018;28(8):608–630. [DOI] [PubMed] [Google Scholar]
- [27].Rheinbay E, Parasuraman P, Grimsby J, et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547(7661):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Frohling S, Dohner H. Chromosomal abnormalities in cancer. N Engl J Med. 2008;359(7):722–734. [DOI] [PubMed] [Google Scholar]
- [29].Dalla-Favera R, Bregni M, Erikson J, et al. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7824–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Taub R, Kirsch I, Morton C, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7837–7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ryan RJ, Drier Y, Whitton H, et al. Detection of enhancer-associated rearrangements reveals mechanisms of oncogene dysregulation in B-cell lymphoma. Cancer Discov. 2015;5(10):1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rodrigues P, Patel SA, Harewood L, et al. NF-kappaB-dependent lymphoid enhancer co-option promotes renal carcinoma metastasis. Cancer Discov. 2018;8(7):850–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Franco Hector L, Kraus WL. No driver behind the wheel? Targeting transcription in cancer. Cell. 2015;163(1):28–30. [DOI] [PubMed] [Google Scholar]
- [34].Bradner JE, Hnisz D, Young RA. Transcriptional addiction in cancer. Cell. 2017;168(4):629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Soutourina J. Transcription regulation by the Mediator complex. Nat Rev Mol Cell Biol. 2018;19(4):262–274. [DOI] [PubMed] [Google Scholar]
- [36].Pennacchio LA, Bickmore W, Dean A, et al. Enhancers: five essential questions. Nat Rev Genet. 2013;14(4):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15(4):272–286. [DOI] [PubMed] [Google Scholar]
- [38].Boyle AP, Davis S, Shulha HP, et al. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132(2):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Buenrostro JD, Giresi PG, Zaba LC, et al. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Giresi PG, Kim J, McDaniell RM, et al. FAIRE (Formaldehyde-assisted isolation of regulatory elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17(6):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13(19):2465–2477. [DOI] [PubMed] [Google Scholar]
- [42].Blackwood EM, Kadonaga JT. Going the distance: a current view of enhancer action. Science. 1998;281(5373):60–63. [DOI] [PubMed] [Google Scholar]
- [43].de Laat W, Klous P, Kooren J, et al. Three-dimensional organization of gene expression in erythroid cells. Curr Top Dev Biol. 2008;82:117–139. [DOI] [PubMed] [Google Scholar]
- [44].Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19(5):631–642. [DOI] [PubMed] [Google Scholar]
- [45].Cullen KE, Kladde MP, Seyfred MA. Interaction between transcription regulatory regions of prolactin chromatin. Science. 1993;261(5118):203–206. [DOI] [PubMed] [Google Scholar]
- [46].Dekker J, Rippe K, Dekker M, et al. Capturing chromosome conformation. Science. 2002;295(5558):1306–1311. [DOI] [PubMed] [Google Scholar]
- [47].Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet. 2013;14(6):390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhao Z, Tavoosidana G, Sjölinder M, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 2006;38(11):1341–1347. [DOI] [PubMed] [Google Scholar]
- [49].Simonis M, Klous P, Splinter E, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture–on-chip (4C). Nat Genet. 2006;38(11):1348–1354. [DOI] [PubMed] [Google Scholar]
- [50].Dostie J, Richmond TA, Arnaout RA, et al. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16(10):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39(3):311–318. [DOI] [PubMed] [Google Scholar]
- [53].Koch CM, Andrews RM, Flicek P, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17(6):691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ramos YFM, Hestand MS, Verlaan M, et al. Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic Acids Res. 2010;38(16):5396–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Whyte WA, Orlando D, Hnisz D, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Blow MJ, McCulley DJ, Li Z, et al. ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet. 2010;42(9):806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ghisletti S, Barozzi I, Mietton F, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32(3):317–328. [DOI] [PubMed] [Google Scholar]
- [58].Lee JE, Park YK, Park S, et al. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat Commun. 2017;8(1):2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yip KY, Cheng C, Bhardwaj N, et al. Classification of human genomic regions based on experimentally determined binding sites of more than 100 transcription-related factors. Genome Biol. 2012;13(9):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Creyghton MP, Cheng AW, Welstead GG, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107(50):21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rada-Iglesias A, Bajpai R, Swigut T, et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470(7333):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Parker SC, Stitzel ML, Taylor DL, et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc Natl Acad Sci U S A. 2013;110(44):17921–17926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hnisz D, Abraham B, Lee T, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Loven J, Hoke H, Lin C, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sengupta S, George RE. Super-enhancer-driven transcriptional dependencies in cancer. Trends Cancer. 2017;3(4):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pott S, Lieb JD. What are super-enhancers? Nat Genet. 2015;47(1):8–12. [DOI] [PubMed] [Google Scholar]
- [67].Hnisz D, Shrinivas K, Young RA, et al. A phase separation model for transcriptional control. Cell. 2017;169(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alberti S. Phase separation in biology. Curr Biol. 2017;27(20):R1097–R1102. [DOI] [PubMed] [Google Scholar]
- [69].Boija A, Klein IA, Sabari BR, et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175(7):1842–1855.e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sabari BR, Dall’Agnese A, Boija A, et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361(6400):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Boeynaems S, Alberti S, Fawzi NL, et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28(6):420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cho WK, Spille JH, Hecht M, et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361(6400):412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chong S, Dugast-Darzacq C, Liu Z, et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361(6400):eaar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30(1):39–58. [DOI] [PubMed] [Google Scholar]
- [75].Cook PR, Marenduzzo D. Transcription-driven genome organization: a model for chromosome structure and the regulation of gene expression tested through simulations. Nucleic Acids Res. 2018;46(19):9895–9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ashe HL, Monks J, Wijgerde M, et al. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 1997;11(19):2494–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tuan D, Kong S, Hu K. Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc Natl Acad Sci U S A. 1992;89(23):11219–11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].De Santa F, Barozzi I, Mietton F, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8(5):e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kim T-K, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465(7295):182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Koch F, Fenouil R, Gut M, et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat Struct Mol Biol. 2011;18(8):956–963. [DOI] [PubMed] [Google Scholar]
- [81].Andersson R, Gebhard C, Miguel-Escalada I, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507(7493):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hah N, Murakami S, Nagari A, et al. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013;23(8):1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kaikkonen MU, Spann N, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51(3):310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Melgar MF, Collins FS, Sethupathy P. Discovery of active enhancers through bidirectional expression of short transcripts. Genome Biol. 2011;12(11):R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ho Y, Elefant F, Liebhaber SA, et al. Locus control region transcription plays an active role in long-range gene activation. Mol Cell. 2006;23(3):365–375. [DOI] [PubMed] [Google Scholar]
- [86].Kim A, Zhao H, Ifrim I, et al. Beta-globin intergenic transcription and histone acetylation dependent on an enhancer. Mol Cell Biol. 2007;27(8):2980–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kouno T, Moody J, Kwon AT-J, et al. C1 CAGE detects transcription start sites and enhancer activity at single-cell resolution. Nat Commun. 2019;10(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Arner E, Daub CO, Vitting-Seerup K, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347(6225):1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hsieh CL, Fei T, Chen Y, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci U S A. 2014;111(20):7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schaukowitch K, Joo JY, Liu X, et al. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 2014;56(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mousavi K, Zare H, Dell’Orso S, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51(5):606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rahman S, Zorca CE, Traboulsi T, et al. Single-cell profiling reveals that eRNA accumulation at enhancer-promoter loops is not required to sustain transcription. Nucleic Acids Res. 2017;45(6):3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li W, Notani D, Ma Q, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498(7455):516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lai F, Gardini A, Zhang A, et al. Integrator mediates the biogenesis of enhancer RNAs. Nature. 2015;525(7569):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Andersson R, Refsing Andersen P, Valen E, et al. Nuclear stability and transcriptional directionality separate functionally distinct RNA species. Nat Commun. 2014;5:5336. [DOI] [PubMed] [Google Scholar]
- [97].Core LJ, Martins AL, Danko CG, et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat Genet. 2014;46(12):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Melo CA, Drost J, Wijchers P, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell. 2013;49(3):524–535. [DOI] [PubMed] [Google Scholar]
- [99].Pnueli L, Rudnizky S, Yosefzon Y, et al. RNA transcribed from a distal enhancer is required for activating the chromatin at the promoter of the gonadotropin alpha-subunit gene. Proc Natl Acad Sci U S A. 2015;112(14):4369–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Flynn RA, Almada AE, Zamudio JR, et al. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc Natl Acad Sci U S A. 2011;108(26):10460–10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lubas M, Andersen P, Schein A, et al. The human nuclear exosome targeting complex is loaded onto newly synthesized RNA to direct early ribonucleolysis. Cell Rep. 2015;10(2):178–192. [DOI] [PubMed] [Google Scholar]
- [102].Chen RA, Down TA, Stempor P, et al. The landscape of RNA polymerase II transcription initiation in C. elegans reveals promoter and enhancer architectures. Genome Res. 2013;23(8):1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mikhaylichenko O, Bondarenko V, Harnett D, et al. The degree of enhancer or promoter activity is reflected by the levels and directionality of eRNA transcription. Genes Dev. 2018;32(1):42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cheng JH, Pan DZ, Tsai ZT, et al. Genome-wide analysis of enhancer RNA in gene regulation across 12 mouse tissues. Sci Rep. 2015;5:12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol. 2007;14(2):103–105. [DOI] [PubMed] [Google Scholar]
- [106].LaCava J, Houseley J, Saveanu C, et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121(5):713–724. [DOI] [PubMed] [Google Scholar]
- [107].Wyers F, Rougemaille M, Badis G, et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121(5):725–737. [DOI] [PubMed] [Google Scholar]
- [108].Pefanis E, Wang J, Rothschild G, et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161(4):774–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wilson CJ, Chao DM, Imbalzano AN, et al. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell. 1996;84(2):235–244. [DOI] [PubMed] [Google Scholar]
- [110].Schwabish MA, Struhl K. The Swi/Snf Complex is important for histone eviction during transcriptional activation and RNA polymerase II elongation in vivo. Mol Cell Biol. 2007;27(20):6987–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cho H, Orphanides G, Sun X, et al. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18(9):5355–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ling J, Ainol L, Zhang L, et al. HS2 enhancer function is blocked by a transcriptional terminator inserted between the enhancer and the promoter. J Biol Chem. 2004;279(49):51704–51713. [DOI] [PubMed] [Google Scholar]
- [113].Engreitz JM, Haines JE, Perez EM, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Racko D, Benedetti F, Dorier J, et al. Transcription-induced supercoiling as the driving force of chromatin loop extrusion during formation of TADs in interphase chromosomes. Nucleic Acids Res. 2018;46(4):1648–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Alvarez-Dominguez JR, Hu W, Yuan B, et al. Global discovery of erythroid long noncoding RNAs reveals novel regulators of red cell maturation. Blood. 2014;123(4):570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Banerjee AR, Kim YJ, Kim TH. A novel virus-inducible enhancer of the interferon-beta gene with tightly linked promoter and enhancer activities. Nucleic Acids Res. 2014;42(20):12537–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lai F, Orom UA, Cesaroni M, et al. Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature. 2013;494(7438):497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lam MT, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498(7455):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Maruyama A, Mimura J, Itoh K. Non-coding RNA derived from the region adjacent to the human HO-1 E2 enhancer selectively regulates HO-1 gene induction by modulating pol II binding. Nucleic Acids Res. 2014;42(22):13599–13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hacisuleyman E, Goff LA, Trapnell C, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA firre. Nat Struct Mol Biol. 2014;21(2):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Onodera CS, Underwood JG, Katzman S, et al. Gene isoform specificity through enhancer-associated antisense transcription. PLoS One. 2012;7(8):e43511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Orom UA, Derrien T, Beringer M, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143(1):46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ounzain S, Pezzuto I, Micheletti R, et al. Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. J Mol Cell Cardiol. 2014;76:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bikard D, Jiang W, Samai P, et al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41(15):7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shechner DM, Hacisuleyman E, Younger ST, et al. Multiplexable, locus-specific targeting of long RNAs with CRISPR-display. Nat Methods. 2015;12(7):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sigova AA, Abraham BJ, Ji X, et al. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350(6263):978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bose DA, Donahue G, Reinberg D, et al. RNA binding to CBP stimulates histone acetylation and transcription. Cell. 2017;168(1–2):135–149 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Maeder ML, Linder SJ, Cascio VM, et al. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10(10):977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rahnamoun H, Lee J, Sun Z, et al. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat Struct Mol Biol. 2018;25(8):687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. [DOI] [PubMed] [Google Scholar]
- [131].Shi L, Li S, Maurer K, et al. Enhancer RNA and NFkappaB-dependent P300 regulation of ADAMDEC1. Mol Immunol. 2018;103:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Azofeifa JG, Allen MA, Hendrix JR, et al. Enhancer RNA profiling predicts transcription factor activity. Genome Res. 2018;28:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23(3):297–305. [DOI] [PubMed] [Google Scholar]
- [134].Zhao Y, Wang L, Ren S, et al. Activation of P-TEFb by androgen receptor-regulated enhancer RNAs in castration-resistant prostate cancer. Cell Rep. 2016;15(3):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhou Q, Li T, Price DH. RNA polymerase II elongation control. Annu Rev Biochem. 2012;81:119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kagey MH, Newman JJ, Bilodeau S, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nora EP, Goloborodko A, Valton AL, et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell. 2017;169(5):930–944 e922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rao SP, Huntley M, Durand N, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rubin AJ, Barajas BC, Furlan-Magaril M, et al. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet. 2017;49(10):1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tang Z, Luo O, Li X, et al. CTCF-mediated human 3D genome architecture reveals chromatin topology for transcription. Cell. 2015;163(7):1611–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Weintraub AS, Li CH, Zamudio AV, et al. YY1 is a structural regulator of enhancer-promoter loops. Cell. 2017;171(7):1573–1588 e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kim YJ, Xie P, Cao L, et al. Global transcriptional activity dynamics reveal functional enhancer RNAs. Genome Res. 2018;28(12):1799–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ivaldi MS, Diaz LF, Chakalova L, et al. Fetal gamma-globin genes are regulated by the BGLT3 long noncoding RNA locus. Blood. 2018;132(18):1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Nair SJ, Yang L, Meluzzi D, et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat Struct Mol Biol. 2019;26(3):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gibson BA, Doolittle LK, Schneider MWG, et al. Organization of chromatin by intrinsic and regulated phase separation. Cell. 2019;179(2):470–484 e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhu Y, Sun L, Chen Z, et al. Predicting enhancer transcription and activity from chromatin modifications. Nucleic Acids Res. 2013;41(22):10032–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Groff AF, Sanchez-Gomez D, Soruco ML, et al. In vivo characterization of Linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 2016;16(8):2178–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Paralkar VR, Taborda C, Huang P, et al. Unlinking an lncRNA from its associated cis element. Mol Cell. 2016;62(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Espinosa JM. On the origin of lncRNAs: missing link found. Trends Genet. 2017;33(10):660–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322(5909):1845–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hah N, Benner C, Chong LW, et al. Inflammation-sensitive super enhancers form domains of coordinately regulated enhancer RNAs. Proc Natl Acad Sci U S A. 2015;112(3):E297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hah N, Danko C, Core L, et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145(4):622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kwak H, Fuda NJ, Core LJ, et al. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science. 2013;339(6122):950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li W, Hu Y, Oh S, et al. Condensin I and II complexes license full estrogen receptor alpha-dependent enhancer activation. Mol Cell. 2015;59(2):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wang KC, Yang YW, Liu B, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mahat DB, Kwak H, Booth GT, et al. Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat Protoc. 2016;11(8):1455–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Carninci P, Sandelin A, Lenhard B, et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet. 2006;38(6):626–635. [DOI] [PubMed] [Google Scholar]
- [158].Shiraki T, Kondo S, Katayama S, et al. Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage. Proc Natl Acad Sci U S A. 2003;100(26):15776–15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet. 2006;7:29–59. [DOI] [PubMed] [Google Scholar]
- [160].van Arensbergen J, van Steensel B, Bussemaker HJ. In search of the determinants of enhancer-promoter interaction specificity. Trends Cell Biol. 2014;24(11):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Hatzis P, Talianidis I. Dynamics of enhancer-promoter communication during differentiation-induced gene activation. Mol Cell. 2002;10(6):1467–1477. [DOI] [PubMed] [Google Scholar]
- [162].Sanyal A, Lajoie BR, Jain G, et al. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].de Wit E, de Laat W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 2012;26(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hughes JR, Roberts N, McGowan S, et al. Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment. Nat Genet. 2014;46(2):205–212. [DOI] [PubMed] [Google Scholar]
- [165].Mifsud B, Tavares-Cadete F, Young AN, et al. Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet. 2015;47(6):598–606. [DOI] [PubMed] [Google Scholar]
- [166].Mumbach MR, Rubin AJ, Flynn RA, et al. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat Methods. 2016;13(11):919–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mumbach MR, Satpathy AT, Boyle EA, et al. Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. Nat Genet. 2017;49(11):1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gagliardi M, Matarazzo MR. RIP: RNA Immunoprecipitation. Methods Mol Biol. 2016;1480:73–86. [DOI] [PubMed] [Google Scholar]
- [169].Zheng X, Cho S, Moon H, et al. Detecting RNA-protein interaction using end-labeled biotinylated RNA oligonucleotides and immunoblotting. Methods Mol Biol. 2016;1421:35–44. [DOI] [PubMed] [Google Scholar]
- [170].McHugh CA, Guttman M. RAP-MS: a method to identify proteins that interact directly with a specific RNA molecule in cells. Methods Mol Biol. 2018;1649:473–488. [DOI] [PubMed] [Google Scholar]
- [171].Ramanathan M, Porter DF, Khavari PA. Methods to study RNA-protein interactions. Nat Methods. 2019;16(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Lizio M, Abugessaisa I, Noguchi S, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47(D1):D752–D758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lizio M, Harshbarger J, Shimoji H, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20(17):R754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Qi LS, Larson M, Gilbert L, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Gilbert LA, Horlbeck MA, Adamson B, et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159(3):647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Gilbert LA, Larson M, Morsut L, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kearns NA, Genga RMJ, Enuameh MS, et al. Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development. 2014;141(1):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Thakore PI, D’Ippolito AM, Song L, et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods. 2015;12(12):1143–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Chavez A, Scheiman J, Vora S, et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12(4):326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Hilton IB, D’Ippolito AM, Vockley CM, et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol. 2015;33(5):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kiani S, Chavez A, Tuttle M, et al. Cas9 gRNA engineering for genome editing, activation and repression. Nat Methods. 2015;12(11):1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Konermann S, Brigham MD, Trevino AE, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Perez-Pinera P, Kocak DD, Vockley CM, et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods. 2013;10(10):973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Galli GG, Carrara M, Yuan W-C, et al. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol Cell. 2015;60(2):328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Rabani M, Raychowdhury R, Jovanovic M, et al. High-resolution sequencing and modeling identifies distinct dynamic RNA regulatory strategies. Cell. 2014;159(7):1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Schwalb B, Michel M, Zacher B, et al. TT-seq maps the human transient transcriptome. Science. 2016;352(6290):1225–1228. [DOI] [PubMed] [Google Scholar]


