Abstract
Background
Dental caries (tooth decay) and periodontal diseases (gingivitis and periodontitis) affect the majority of people worldwide, and treatment costs place a significant burden on health services. Decay and gum disease can cause pain, eating and speaking difficulties, low self‐esteem, and even tooth loss and the need for surgery. As dental plaque is the primary cause, self‐administered daily mechanical disruption and removal of plaque is important for oral health. Toothbrushing can remove supragingival plaque on the facial and lingual/palatal surfaces, but special devices (such as floss, brushes, sticks, and irrigators) are often recommended to reach into the interdental area.
Objectives
To evaluate the effectiveness of interdental cleaning devices used at home, in addition to toothbrushing, compared with toothbrushing alone, for preventing and controlling periodontal diseases, caries, and plaque. A secondary objective was to compare different interdental cleaning devices with each other.
Search methods
Cochrane Oral Health’s Information Specialist searched: Cochrane Oral Health’s Trials Register (to 16 January 2019), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2018, Issue 12), MEDLINE Ovid (1946 to 16 January 2019), Embase Ovid (1980 to 16 January 2019) and CINAHL EBSCO (1937 to 16 January 2019). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on the language or date of publication.
Selection criteria
Randomised controlled trials (RCTs) that compared toothbrushing and a home‐use interdental cleaning device versus toothbrushing alone or with another device (minimum duration four weeks).
Data collection and analysis
At least two review authors independently screened searches, selected studies, extracted data, assessed studies' risk of bias, and assessed evidence certainty as high, moderate, low or very low, according to GRADE. We extracted indices measured on interproximal surfaces, where possible. We conducted random‐effects meta‐analyses, using mean differences (MDs) or standardised mean differences (SMDs).
Main results
We included 35 RCTs (3929 randomised adult participants). Studies were at high risk of performance bias as blinding of participants was not possible. Only two studies were otherwise at low risk of bias. Many participants had a low level of baseline gingival inflammation.
Studies evaluated the following devices plus toothbrushing versus toothbrushing: floss (15 trials), interdental brushes (2 trials), wooden cleaning sticks (2 trials), rubber/elastomeric cleaning sticks (2 trials), oral irrigators (5 trials). Four devices were compared with floss: interdental brushes (9 trials), wooden cleaning sticks (3 trials), rubber/elastomeric cleaning sticks (9 trials) and oral irrigators (2 trials). Another comparison was rubber/elastomeric cleaning sticks versus interdental brushes (3 trials).
No trials assessed interproximal caries, and most did not assess periodontitis. Gingivitis was measured by indices (most commonly, Löe‐Silness, 0 to 3 scale) and by proportion of bleeding sites. Plaque was measured by indices, most often Quigley‐Hein (0 to 5).
Primary objective: comparisons against toothbrushing alone
Low‐certainty evidence suggested that flossing, in addition to toothbrushing, may reduce gingivitis (measured by gingival index (GI)) at one month (SMD ‐0.58, 95% confidence interval (CI) ‐1.12 to ‐0.04; 8 trials, 585 participants), three months or six months. The results for proportion of bleeding sites and plaque were inconsistent (very low‐certainty evidence).
Very low‐certainty evidence suggested that using an interdental brush, plus toothbrushing, may reduce gingivitis (measured by GI) at one month (MD ‐0.53, 95% CI ‐0.83 to ‐0.23; 1 trial, 62 participants), though there was no clear difference in bleeding sites (MD ‐0.05, 95% CI ‐0.13 to 0.03; 1 trial, 31 participants). Low‐certainty evidence suggested interdental brushes may reduce plaque more than toothbrushing alone (SMD ‐1.07, 95% CI ‐1.51 to ‐0.63; 2 trials, 93 participants).
Very low‐certainty evidence suggested that using wooden cleaning sticks, plus toothbrushing, may reduce bleeding sites at three months (MD ‐0.25, 95% CI ‐0.37 to ‐0.13; 1 trial, 24 participants), but not plaque (MD ‐0.03, 95% CI ‐0.13 to 0.07).
Very low‐certainty evidence suggested that using rubber/elastomeric interdental cleaning sticks, plus toothbrushing, may reduce plaque at one month (MD ‐0.22, 95% CI ‐0.41 to ‐0.03), but this was not found for gingivitis (GI MD ‐0.01, 95% CI ‐0.19 to 0.21; 1 trial, 12 participants; bleeding MD 0.07, 95% CI ‐0.15 to 0.01; 1 trial, 30 participants).
Very‐low certainty evidence suggested oral irrigators may reduce gingivitis measured by GI at one month (SMD ‐0.48, 95% CI ‐0.89 to ‐0.06; 4 trials, 380 participants), but not at three or six months. Low‐certainty evidence suggested that oral irrigators did not reduce bleeding sites at one month (MD ‐0.00, 95% CI ‐0.07 to 0.06; 2 trials, 126 participants) or three months, or plaque at one month (SMD ‐0.16, 95% CI ‐0.41 to 0.10; 3 trials, 235 participants), three months or six months, more than toothbrushing alone.
Secondary objective: comparisons between devices
Low‐certainty evidence suggested interdental brushes may reduce gingivitis more than floss at one and three months, but did not show a difference for periodontitis measured by probing pocket depth. Evidence for plaque was inconsistent.
Low‐ to very low‐certainty evidence suggested oral irrigation may reduce gingivitis at one month compared to flossing, but very low‐certainty evidence did not suggest a difference between devices for plaque.
Very low‐certainty evidence for interdental brushes or flossing versus interdental cleaning sticks did not demonstrate superiority of either intervention.
Adverse events
Studies that measured adverse events found no severe events caused by devices, and no evidence of differences between study groups in minor effects such as gingival irritation.
Authors' conclusions
Using floss or interdental brushes in addition to toothbrushing may reduce gingivitis or plaque, or both, more than toothbrushing alone. Interdental brushes may be more effective than floss. Available evidence for tooth cleaning sticks and oral irrigators is limited and inconsistent. Outcomes were mostly measured in the short term and participants in most studies had a low level of baseline gingival inflammation. Overall, the evidence was low to very low‐certainty, and the effect sizes observed may not be clinically important. Future trials should report participant periodontal status according to the new periodontal diseases classification, and last long enough to measure interproximal caries and periodontitis.
Keywords: Humans; Dental Devices, Home Care; Dental Caries; Dental Caries/prevention & control; Dental Plaque; Dental Plaque/prevention & control; Gingivitis; Gingivitis/prevention & control; Oral Health; Periodontal Diseases; Periodontal Diseases/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Home use of devices for cleaning between the teeth (in addition to toothbrushing) to prevent and control gum diseases and tooth decay
Review question
How effective are home‐use interdental cleaning devices, plus toothbrushing, compared with toothbrushing only or use of another device, for preventing and controlling periodontal (gum) diseases (gingivitis and periodontitis), tooth decay (dental caries) and plaque?
Background
Tooth decay and gum diseases affect most people. They can cause pain, difficulties with eating and speaking, low self‐esteem, and, in extreme cases, may lead to tooth loss and the need for surgery. The cost to health services of treating these diseases is very high.
As dental plaque (a layer of bacteria in an organic matrix that forms on the teeth) is the root cause, it is important to remove plaque from teeth on a regular basis. While many people routinely brush their teeth to remove plaque up to the gum line, it is difficult for toothbrushes to reach into areas between teeth ('interdental'), so interdental cleaning is often recommended as an extra step in personal oral hygiene routines. Different tools can be used to clean interdentally, such as dental floss, interdental brushes, tooth cleaning sticks, and water pressure devices known as oral irrigators.
Study characteristics
Review authors working with Cochrane Oral Health searched for studies up to 16 January 2019. We identified 35 studies (3929 adult participants). Participants knew that they were in an experiment, which might have affected their teeth cleaning or eating behaviour. Some studies had other problems that might make their findings less reliable, such as people dropping out of the study or not using the assigned device.
Studies evaluated the following devices plus toothbrushing compared to toothbrushing only: floss (15 studies), interdental brushes (2 studies), wooden cleaning sticks (2 studies), rubber/elastomeric cleaning sticks (2 studies) and oral irrigators (5 studies). Four devices were compared with floss: interdental brushes (9 studies), wooden cleaning sticks (3 studies), rubber/elastomeric cleaning sticks (9 studies), oral irrigators (2 studies). Three studies compared rubber/elastomeric cleaning sticks with interdental brushes.
No studies evaluated decay, and few evaluated severe gum disease. Outcomes were measured at short (one month to six weeks) and medium term (three and six months).
Key results
We found that using floss, in addition to toothbrushing, may reduce gingivitis in the short and medium term. It is unclear if it reduces plaque.
Using an interdental brush, in addition to a toothbrush, may reduce gingivitis and plaque in the short term.
Using wooden tooth cleaning sticks may be better than toothbrushing only for reducing gingivitis (measured by bleeding sites) but not plaque in the medium term (only 24 participants).
Using a tooth cleaning stick made of rubber or an elastomer may be better than toothbrushing only for reducing plaque but not gingivitis in the short term (only 30 participants).
Toothbrushing plus oral irrigation (water pressure) may reduce gingivitis in the short term, but there was no evidence for this in the medium term. There was no evidence of a difference in plaque.
Interdental brushes may be better than flossing for gingivitis at one and three months. The evidence for plaque is inconsistent. There was no evidence of a difference between the devices for periodontitis measured by probing pocket depth.
There is some evidence that oral irrigation may be better than flossing for reducing gingivitis (but not plaque) in the short term.
The available evidence for interdental cleaning sticks did not show them to be better or worse than floss or interdental brushes for controlling gingivitis or plaque.
The studies that measured 'adverse events' found no serious effects and no evidence of differences between study groups in minor effects such as gum irritation.
Certainty of the evidence
The evidence is low to very low‐certainty. The effects observed may not be clinically important. Studies measured outcomes mostly in the short term and many participants had a low level of gum disease at the beginning of the studies.
Future research
Future studies should use the new periodontal diseases classification to describe the gum health of participants, and they should last long enough to measure periodontitis and tooth decay.
Summary of findings
Summary of findings for the main comparison. Flossing plus toothbrushing compared with toothbrushing alone for periodontal diseases and dental caries in adults.
| Flossing plus toothbrushing for periodontal disease and dental caries in adults | ||||||
|
Population: adults, 16 years and older
Setting: everyday self‐care
Intervention: flossing plus toothbrushing Comparison: toothbrushing only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Toothbrushing only | Flossing plus toothbrushing | |||||
|
Gingivitis measured by gingival index SD units: investigators measured gingivitis using different scales Lower score means less severe gingivitis Follow‐up: 1 month |
The gingivitis score in the flossing group was on average 0.58 SDs lower (95% CI 0.04 lower to 1.12 lower) than the control group | ‐ | 585 (8 studies) | ⊕⊕⊝⊝ low1 | Flossing also reduced gingivitis at 3 months (‐0.33, ‐0.50 to ‐0.17, 4 studies, 570 participants) and 6 months (‐0.68, ‐0.95 to ‐0.42, 4 studies, 564 participants). | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 1 month |
The median score in the control group was 0.16 | The mean score in the intervention group was 0.03 less (0.14 less to 0.08 more) | ‐ | 158 (2 studies) | ⊕⊝⊝⊝ very low2 | 3‐month follow‐up: ‐0.14 (‐0.37 to 0.09, 2 studies, 240 participants) 6‐month follow‐up: ‐0.06 (‐0.09 to ‐0.03; 1 study, 210 participants) |
| Periodontitis | One study measured probing pocket depth but no data were reported. | |||||
| Interproximal caries | No included study assessed caries as an outcome. | |||||
|
Plaque SD units: investigators measured plaque using different scales Lower score means less plaque Follow‐up: 1 month |
The plaque score in flossing group was on average 0.42 SDs lower (0.85 lower to 0.02 higher) than the control group | ‐ | 542 (7 studies) | ⊕⊝⊝⊝ very low2 | Significant difference found for plaque at 3 months (SMD 0.20, ‐0.36 to ‐0.04, 5 studies, 594 participants), but not at 6 months (‐0.13, ‐0.30 to 0.05, 3 studies, 487 participants). | |
| Harms and adverse effects | Adverse effects were assessed and reported in seven studies. Three reported no adverse events on the oral hard or soft tissues. Four reported sporadic adverse events with mild severity, with no evidence of a difference between the flossing plus toothbrushing group and toothbrushing only group. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SD: standard deviation;SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels due to high and unclear risk of bias in the studies and substantial heterogeneity
2 Downgraded three levels due to high and unclear risk of bias in the studies, substantial heterogeneity and lack of precision in the estimate
Summary of findings 2. Interdental brushing with toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults.
| Interdental brushing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: interdental brushing plus toothbrushing Comparison: toothbrushing only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Toothbrushing only | Interdental brush plus toothbrushing | |||||
|
Gingivitis measured by gingival index SD units: investigators measure gingivitis using different scales Lower score means less severe gingivitis Follow‐up: 1 month |
The gingivitis score in interdental brush group was on average 0.53 SDs lower (0.23 to 0.83 lower) than the control group | ‐ | 62 (1 study) | ⊕⊝⊝⊝ very low1 | ||
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 1 month |
The mean score in the control group was 0.19 | The mean score in the interdental brush group was 0.05 less (0.13 less to 0.03 more) | ‐ | 31 (1 study) | ⊕⊕⊝⊝ very low2 | |
| Periodontitis | One study measured probing pocket depth but no data were reported. | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales Lower score means less plaque Follow‐up: 1 month |
The plaque score in the interdental brush group was on average 1.07 SDs lower (0.63 to 1.51 lower) than the control group | ‐ | 93 (2 studies) | ⊕⊕⊝⊝ low3 | ||
| Harms and adverse outcomes | Neither study reported any information about adverse events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to being based on only one small trial at unclear risk of bias
2 Downgraded three levels due to being based on only one small trial at unclear risk of bias
3 Downgraded two levels due to being based on only two small trials at unclear risk of bias
Summary of findings 3. Wooden cleaning stick plus toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults.
| Wooden interdental cleaning stick compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: wooden interdental cleaning stick plus toothbrushing Comparison: toothbrushing only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Toothbrushing alone | Wooden cleaning stick plus toothbrushing | |||||
| Gingivitis measured by gingival index | Not measured | ‐ | ‐ | ‐ | ‐ | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 3 months |
The mean gingivitis score in the control group was 0.90 | The mean gingivitis score in the intervention group was 0.25 lower (from 0.13 to 0.37 lower) | ‐ | 24 (1 study) |
⊕⊝⊝⊝ very low1 | 3‐month data only |
| Periodontitis | No included study assessed periodontitis as an outcome | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque
(proportion of sites with plaque) Follow‐up: 3 months |
The mean plaque in the control group was 0.22 | The mean plaque score in the intervention group was 0.03 lower (0.13 lower to 0.07 higher) | ‐ | 24 (1 study) | ⊕⊝⊝⊝ very low2 | 3‐month data only |
| Harms and adverse outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to there being only one small trial at unclear risk of bias
2 Downgraded three levels due to there being only one small trial, at unclear risk of bias, and lack of precision in the estimate
Summary of findings 4. Rubber/elastomeric cleaning stick plus toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults.
| Interdental cleaning stick compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: interdental cleaning stick plus toothbrushing Comparison: toothbrushing only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Toothbrush alone | Cleaning stick plus toothbrushing | |||||
|
Gingivitis measured by gingival index Lower score means less severe gingivitis Follow‐up: 1 month |
The mean score in the control group was 0.22 | The mean score in the intervention group was on average 0.01 lower (0.19 lower to 0.21 higher) than the control group.1 | ‐ | 12 (1 study) | ⊕⊝⊝⊝ very low1 | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 1 month |
The mean score in the control group was 0.19 | The mean score in the intervention group was 0.07 lower (0.15 lower to 0.01 higher) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Periodontitis | One study measured probing pocket depth but no data were reported. | |||||
| Interproximal caries | No included study assessed caries as an outcome. | |||||
|
Plaque (proportion of sites with plaque) Follow‐up: 1 month |
The mean plaque in the control group was 0.42 | The mean plaque score in the intervention group was 0.22 lower (0.03 to 0.41 lower) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Harms and adverse outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to being based on single small study at high risk of bias, and lack of precision in the estimate 2 Downgraded three levels due to being based on single small study at unclear risk of bias
Summary of findings 5. Oral irrigation plus toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults.
| Oral irrigation plus toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Settings: everyday self care Intervention: oral irrigation plus toothbrushing Comparison: toothbrushing only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Toothbrushing alone | Oral irrigation plus toothbrushing | |||||
|
Gingivitis measured by gingival index SD units: investigators measure gingivitis using different scales Lower score means less severe gingivitis Follow‐up: 1 month |
The gingivitis score in oral irrigation group was on average 0.48 SDs lower (0.06 lower to 0.89 lower) than the control group. | ‐ | 380 (4 studies) | ⊕⊝⊝⊝ very low1 | No significant evidence of a difference at 3 months (SMD ‐0.13, ‐0.44 to 0.17; 2 trials, 163 participants) or 6 months (MD ‐0.33, ‐0.74 to 0.08; 1 trial, 109 participants) | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 1 month |
The mean score in the control group was 0.30 | The mean score in the intervention group was the same (0.07 lower to 0.06 higher) | ‐ | 126 (2 studies) | ⊕⊕⊝⊝ low2 | Nor any evidence of a difference at 3 months (MD ‐0.04, ‐0.13 to 0.05, 1 study, 54 participants) |
| Periodontitis | Measured in one study but useable data not provided | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales. Lower score means less plaque. Follow‐up: 1 month |
The plaque score in the oral irrigation group was on average 0.16 SDs lower (0.41 lower to 0.10 higher)1 than the control group | ‐ | 235 (3 studies) | ⊕⊕⊝⊝ low3 | Nor did the evidence suggest benefit from the oral irrigator at 3 months (SMD 0.06, ‐0.25 to 0.37; 2 studies, 163 participants) or 6 months (MD 0.22, ‐0.59 to 0.15; 1 study, 109 participants) | |
| Harms and adverse outcomes | Three studies reported that there were no adverse events, one reported one incidence of aphthous ulcer in irrigator group, one reported oral lacerations but found no difference between the interventions, and one did not measure adverse events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; SMD: standardised mean difference; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels as studies at unclear risk of bias, with substantial heterogeneity and imprecise estimate 2 Downgraded two levels as studies at unclear risk of bias, with moderate heterogeneity 3 Downgraded two levels as studies at unclear risk of bias, imprecise estimate
Summary of findings 6. Interdental brushing compared to flossing for periodontal diseases and dental caries in adults.
| Interdental brushing compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: interdental brushing plus toothbrushing Comparison: flossing plus toothbrushing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flossing | Interdental brush (IDB) | |||||
|
Gingivitis measured by gingival index SD units: investigators measure gingivitis using different scales Lower score means less severe gingivitis Follow‐up: 4 to 6 weeks |
The gingivitis score in the IDB group was on average 0.40 SDs lower (0.11 to 0.70 lower)1than the flossing group | ‐ | 183 (3 studies) | ⊕⊕⊝⊝ low1 | Not measured at 3 months | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 4 to 6 weeks |
The mean score in the flossing group was 0.20 | The mean score in the IDB group was 0.06 lower (0.08 to 0.03 lower) | ‐ | 234 (6 studies) | ⊕⊕⊝⊝ low2 | Results at 3 months also indicated a small benefit for interdental brushes: MD ‐0.10 (‐0.15 to ‐0.04), 2 studies, 106 participants. |
|
Periodontitis Probing pocket depth in mm Follow‐up: 4 to 6 weeks |
The mean PPD score for the flossing group was 5.01 mm | The mean PPD score in the IDB group was0.06 lower (0.27 lower to 0.16 higher) | ‐ | 107 (3 studies) |
⊕⊕⊝⊝ low3 | Results were consistent at 3 months: MD 0.01 mm (‐0.29 to 0.31), 1 parallel‐group study, 77 participants. |
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales Lower score means less plaque Follow‐up: mean 1 month (4 to 6 weeks) |
The plaque in the IDB group was on average 0.47 SDs lower (0.84 to 0.11 lower) than the flossing group | ‐ | 290 (5 studies) | ⊕⊝⊝⊝ very low4 | This benefit for IDB compared to flossing for parallel‐group studies is not supported by the meta‐analysis of the split‐mouth studies at one month (SMD ‐0.07 (‐0.32 to 0.18), 3 studies, 66 participants). Nor by the 3‐month data (MD ‐0.12, 95% ‐0.33 to 0.10; two trials (one parallel and one split‐mouth), 106 participants). | |
| Harms and adverse outcomes | Five studies reported there were no adverse events. Two studies reported on problems with the use of interdental brushes or floss, which sometimes caused soreness. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; SMD: standardised mean difference; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels due to studies at unclear risk of bias, imprecise estimate (although consistent)
2 Downgraded two levels due to studies at unclear risk of bias, moderate heterogeneity
3 Downgraded two levels due to studies at unclear risk of bias, imprecise estimate
4 Downgraded three levels due to unclear risk of bias, imprecise estimates and moderate heterogeneity
Summary of findings 7. Wooden cleaning stick compared to flossing for periodontal diseases and dental caries in adults.
| Wooden cleaning stick compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: interdental cleaning stick plus toothbrushing Comparison: flossing plus toothbrushing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flossing plus toothbrushing | Wooden cleaning stick plus toothbrushing | |||||
| Gingivitis measured by gingival index | Not measured | |||||
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 3 months |
The mean gingivitis score in the control group was 0.64 | The mean gingivitis score in the intervention group was 0.01 higher (from 0.12 lower to 0.14 higher) | ‐ | 24 (1 study) |
⊕⊝⊝⊝ very low1 | Only 3‐month data useable |
| Periodontitis | No included study assessed periodontitis | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque
(proportion of sites with plaque) Follow‐up: 3 months |
The mean plaque in the control group was 0.88 | The mean plaque score in the intervention group was 0.02 higher (0.06 lower to 0.10 higher) | ‐ | 24 (1 study) | ⊕⊝⊝⊝ very low1 | Only 3‐month data useable |
| Harms and adverse outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to there being only one small trial, at unclear risk of bias, and lack of precision of estimate
Summary of findings 8. Rubber/elastomeric cleaning stick compared to flossing for periodontal diseases and dental caries in adults.
| Interdental cleaning stick compared to interdental brushing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: cleaning stick plus toothbrushing Comparison: interdental brushing plus toothbrushing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Floss | Cleaning stick | |||||
|
Gingivitis measured by gingival index SD units: investigators measure gingivitis using different scales Lower score means less severe gingivitis. Follow‐up: 4 to 6 weeks |
The gingivitis score in the cleaning stick group was on average 0.22 SDs lower (0.69 lower to 0.24 higher) than the floss group | ‐ | 256 (6 studies) | ⊕⊝⊝⊝ very low1 | Nor was there was evidence that one intervention performed better than the other with regards to gingivitis control at 3 months (MD 0.01, 95% CI ‐0.08 to 0.10, 1 study, 145 participants). | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 4 to 6 weeks |
The mean score in the floss group was 0.22 | The mean score in the cleaning stick group was 0.03 lower (0.08 lower to 0.03 higher) | ‐ | 212 (5 studies) | ⊕⊕⊝⊝ low2 | Nor was there was evidence that one intervention performed better than the other with regards to bleeding sites at 3 months (MD 0.01, 95% CI ‐0.03 to 0.05, 1 study, 145 participants) |
| Periodontitis | One study measured periodontitis but the data were not usable | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales Lower score means less plaque Follow‐up: 4 to 6 weeks |
The plaque score in the cleaning stick group was on average 0.08 SDs lower (0.46 lower to 0.29 higher) than the floss group | ‐ | 273 (6 studies) | ⊕⊝⊝⊝ very low3 | ||
| Harms and adverse outcomes | Five studies assessed adverse events. One did not report findings, but the others reported either no adverse events or minor adverse events that did not significantly differ between interventions. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; SMD: standardised mean difference; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to one study being at high risk of bias (others unclear), moderate heterogeneity and serious imprecision
2 Downgraded two levels due to studies at unclear risk of bias and moderate heterogeneity
3 Downgraded three levels due to studies at unclear risk of bias, moderate heterogeneity and serious imprecision
Summary of findings 9. Oral irrigation compared to flossing for periodontal diseases and dental caries in adults.
| Oral irrigation compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: oral irrigation plus toothbrushing Comparison: flossing plus toothbrushing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flossing | Oral irrigation | |||||
|
Gingivitis measured by gingival index SD units: investigators measure gingivitis using different scales Lower score means less severe gingivitis Follow‐up: 1 month |
The mean score in the floss group was 1.14 | The mean score in the irrigator group was 0.06 lower (0.12 lower to 0.00) | ‐ | 63 (1 study) | ⊕⊝⊝⊝ very low1 | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 1 month |
The mean score in the floss group was 0.56 |
The mean score in the irrigator group was 0.12 lower (0.19 lower to 0.05 lower) | ‐ | 133 (2 studies) | ⊕⊕⊝⊝ low1 | |
| Periodontitis | No included study assessed periodontitis | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales Lower score means less plaque Follow‐up: 1 month |
The plaque in the oral irrigation group was on average 0.31 SDs higher (0.08 lower to 0.70 higher) than the flossing group | ‐ | 133 (2 studies) | ⊕⊝⊝⊝ very low2 | ||
| Harms and adverse outcomes | Both studies reported there were no adverse events in either study group. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to single small study at unclear risk of bias
2 Downgraded three levels due to single small study at unclear risk of bias with serious imprecision
Summary of findings 10. Rubber/elastomeric interdental cleaning stick compared to interdental brush for periodontal diseases and dental caries in adults.
| Interdental cleaning stick compared to interdental brushing for periodontal diseases and dental caries in adults | ||||||
| Population: adults, 16 years and older Setting: everyday self care Intervention: cleaning stick plus toothbrushing Comparison: interdental brushing plus toothbrushing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IDB | Stick | |||||
|
Gingivitis measured by gingival index Lower score means less severe gingivitis Follow‐up: 4 to 6 weeks |
The mean score in the interdental brush group was 0.78 | The mean score in the cleaning stick group was 0.10 (0.32 lower to 0.52 higher) | ‐ | 61 (1 study) | ⊕⊝⊝⊝ very low1 | |
|
Gingivitis measured by proportion of bleeding sites Follow‐up: 4 to 6 weeks |
The mean score in the interdental brush group was 0.14 | The mean score in the cleaning stick group was 0.02 lower (0.10 lower to 0.06 higher) | ‐ | 31 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Periodontitis | Two studies measured periodontitis but data not presented or usable | |||||
| Interproximal caries | No included study assessed caries as an outcome | |||||
|
Plaque SD units: investigators measure plaque using different scales Lower score means less plaque Follow‐up: 4 to 6 weeks |
The plaque score in the cleaning stick group was on average 0.08 SDs higher (0.33 lower to 0.49 higher) than the IDB group | ‐ | 92 (2 studies) | ⊕⊝⊝⊝ very low3 | ||
| Harms and adverse outcomes | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IDB: interdental brushing; SMD: standardised mean difference; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded three levels due to single study at unclear risk of bias and serious imprecision
2 Downgraded three levels due to single study at unclear risk of bias and imprecision
3 Downgraded three levels due to 2 small studies at unclear risk of bias and serious imprecision
Background
Description of the condition
Periodontal diseases
Periodontal diseases are multifactorial oral health conditions (Llorente 2006; Timmerman 2006), consisting of a diverse family of pathological conditions affecting the periodontium (a collective term that comprises gingival tissue, periodontal ligament, cementum and alveolar bone). Periodontal diseases include two main conditions: gingivitis and periodontitis. Gingivitis is the presence of gingival inflammation without loss of connective tissue attachment and appears as red, puffy, shiny gums that bleed easily (Mariotti 1999). Periodontitis is inflammation and destruction of the supportive tissues of teeth and is, by its behaviour, characterised as aggressive or chronic (Armitage 1999). Periodontitis can influence quality of life through psychosocial impacts as a result of negative effects on comfort, function, appearance, and socialisation (Durham 2013; Needleman 2004), and can lead to tooth loss (Broadbent 2011).
Some form of periodontal disease affects the majority of the population, and is found in high‐, middle‐ and low‐income countries (Adult Dental Health Survey 2009; Eke 2012). A 2009 survey in the UK found only 17% of adults had healthy gums; 66% had visible plaque; and of those with plaque, 65% had bleeding gums compared with 33% with no plaque (Adult Dental Health Survey 2009). Whilst more severe forms of periodontal disease, with alveolar bone loss, are much less common, gingivitis is prevalent at all ages and is the most common form of periodontal disease (Mariotti 1999). The exact prevalence of periodontitis is difficult to establish across studies because of non‐standardised criteria, different study population characteristics, different clinical measurements, and the use of partial versus full mouth examinations (Cobb 2009; Savage 2009). Of particular concern are the differing definitions and clinical measurements used (Cobb 2009; Savage 2009). A global workshop organised by the American Academy of Periodontology and the European Federation of Periodonotology took place in 2017 to produce an updated classification scheme for periodontal and peri‐implant diseases (Caton 2018; Chapelle 2018; Papapanou 2018). This has provided "a staging and grading system for periodontitis that is based primarily upon attachment and bone loss and classifies the disease into four stages based on severity (I, II, III or IV) and three grades based on disease susceptibility (A, B or C)" (Dietrich 2019).
The primary aetiological factor in the development of periodontal diseases (and dental caries) is dental plaque (Dalwai 2006; Kuramitsu 2007; Marsh 2006; Periasamy 2009; Selwitz 2007). Dental plaque is a highly organised and specialised biofilm comprising of an intercellular matrix consisting of various micro‐organisms and their by‐products. The bacteria found within dental plaque mutually support each other, using chemical messengers, in a complex and highly evolved community, that protects them from an individual's immune system and chemical agents such as antimicrobial mouth rinses. Bacteria in biofilm are 1000 to 1500 times more resistant to antibiotics than in their free‐floating state, reducing the effectiveness of chemical agents as a solo treatment option. Therefore, disruption of the oral biofilm via mechanical methods remains one of the best treatment options (Chandki 2011). Calcified plaque (calculus) is not involved in the pathogenesis of periodontal diseases but it provides an ideal surface to collect further dental plaque and acts as a 'retention web' for bacteria, protecting plaque from appropriate preventive and therapeutic periodontal measures (Ismail 1994; Lindhe 2003).
Since periodontal diseases are inflammatory, bacterially‐mediated diseases that trigger the host's immune system, it is postulated that the individual's oral health status may influence their systemic health. Susceptibility to periodontal diseases is variable and depends upon the interaction of various risk factors, for example genetic makeup, smoking, stress, immunocompromising diseases, immunosuppressive drugs, and certain systemic diseases (Van Dyke 2005). Studies have shown some possible associations between periodontal diseases and coronary heart disease (Machuca 2012), hyperlipidaemia (Fentoğlu 2012), preterm births (Huck 2011), and lack of glycaemic control in people with diabetes mellitus (Columbo 2012; Simpson 2015). Socioeconomic factors, for instance educational and income levels, have been found to be strongly associated with the prevalence and severity of periodontal diseases (Borrell 2012).
Dental caries
Dental caries is a multifactorial, bacterially‐mediated, chronic disease (Addy 1986; Richardson 1977; Rickard 2004). It is the most common disease in the world (Frencken 2017; WHO 1990), affecting most school‐aged children and the vast majority of adults (Petersen 2003). Although the prevalence and severity of dental caries in most industrialised countries has substantially decreased in the past two decades (Marthaler 1996), this preventable disease continues to be a common public health problem in some parts of these countries (RCSEng 2018), and in other parts of the world (Burt 1998). In 2017, dental caries affected the permanent teeth of 2.3 billion people globally (GHDx 2017).
Deep pits and fissures, as well as interdental spaces, represent areas of increased risk for the collection and accumulation of dental plaque and are therefore regarded as susceptible tooth surfaces for the occurrence of carious lesions. The presence and growth of dental plaque is further encouraged by compromised host response factors, for example reduced salivary flow (hyposalivation) (Murray 1989). Fermentation of sugars by cariogenic bacteria within the plaque results in localised demineralisation of the tooth surface, which may ultimately result in cavity formation (Marsh 2006; Selwitz 2007).
People with carious teeth may experience pain and discomfort (Milsom 2002; Shepherd 1999); and, if left untreated, may lose their teeth. In the United Kingdom, tooth decay accounts for almost half of all dental extractions performed (Chadwick 1999).
Description of the intervention
Although the incidence of periodontal diseases and dental caries differs, based on regional, social, and genetic factors, the prevention of both diseases has a significant healthcare and economic benefit for society as a whole and for individuals. Prevention of dental caries and periodontal diseases is generally regarded as a priority for oral healthcare professionals because it is more cost‐effective than treating it (Brown 2002; Burt 1998). Daily mechanical disruption and removal of dental plaque is considered important for oral health maintenance (Rosing 2006; Zaborskis 2010). Additional professional plaque removal can sometimes be required, though the routine provision of this for people who regularly attend the dentist has recently been questioned (Lamont 2018). People routinely use toothbrushes at home to remove supragingival dental plaque, but toothbrushes are unable to penetrate the interdental area where periodontal diseases first develop and are prevalent (Asadoorian 2006; Berchier 2008; Berglund 1990; Casey 1988). Besides toothbrushing, which is the most common method for removing dental plaque (Addy 1986; Mak 2011; Richardson 1977), different interdental aids to plaque removal, for example, dental floss or interdental brushes, are widely available and often recommended for use in addition to toothbrushing (Bosma 2011; Särner 2010). Whilst floss can be used in all interdental spaces, the interdental brush and other interdental cleaning aids require sufficient interdental space to be used by patients. The choice of interdental cleaning aid will depend on the size of the space and the ability of the patient to use it.
Toothbrushes
Regular daily toothbrushing is a key strategy for preventing and controlling periodontal diseases and dental caries, because it disrupts supragingival dental plaque and reduces the number of periodontal pathogens in supragingival plaque (Caton 2018; Chandki 2011; Ismail 1994; Needleman 2004; Rosing 2006; Zaborskis 2010). In order to achieve highest level of dental plaque removal, various types of toothbrushes have been designed, and different toothbrushing techniques have been developed over time (Lindhe 2003). In an update of a Cochrane systematic review published in 2014 that included 56 randomised controlled trials (RCTs), moderate‐certainty evidence suggested that powered toothbrushes are more effective in reducing plaque and gingivitis than manual toothbrushes in the short and long term, with very few adverse events reported overall and no apparent differences between the two toothbrushing regimens (Yacob 2014). However, the observed likely benefit of powered toothbrushing is of unclear clinical significance, as it reduced dental plaque by 11% after one to three months of use, and by 21% after three months of use. As for clinical signs of gingivitis, there was a reduction of 6% at one month and 11% after three months of use.
Although toothbrushing is effective in removing dental plaque from buccal and lingual tooth surfaces, because of their shape, toothbrushes are not able to penetrate interdental areas and adequately clean interproximal teeth surfaces (Christou 1998). Likewise, toothbrushes are able to reach only 0.9 mm under the gingival margin, and therefore cannot reduce the rate of subgingival areas affected by periodontal pathogens (Waerhaug 1981; Xiemenez‐Fyvie 2000). Interdental plaque accumulates more quickly, is more prevalent, and more acidogenic than plaque on other tooth surfaces (Cumming 1973; Igarashi 1989; Lindhe 2003; Lovdal 1961; Warren 1996). It is important that plaque is controlled in the interdental areas because these are the sites where periodontal diseases occur more frequently, with greater severity (Asadoorian 2006; Berchier 2008; Berglund 1990; Christou 1998; Lindhe 2003; Loe 1965). Caries also occurs more often on the interproximal tooth surfaces (Berglund 1990; Casey 1988; Lindhe 2003).
Dental floss
The concept of interdental cleaning with a filamentous material was first introduced by Levi Spear Parmly, as a measure for preventing dental disease together with a dentifrice and toothbrush (Parmly 1819). Unwaxed silk floss was first produced in 1882, by Codman & Shurtleff, but it was Johnson & Johnson who made silk floss widely available from 1887, as a by‐product of sterile silk leftover from the manufacture of sterile sutures (Johnson & Johnson).
Since dental floss is able to remove some interproximal plaque (Asadoorian 2006; Waerhaug 1981), it is thought that frequent regular dental flossing will reduce the risk of periodontal diseases and interproximal caries (Hujoel 2006). Daily dental flossing in combination with toothbrushing for the prevention of periodontal diseases and caries is frequently recommended for both children and adults (Bagramian 2009; Brothwell 1998). However, patient compliance with daily dental flossing is low (Schuz 2009). People attribute their lack of dental flossing compliance to lack of motivation and difficulties using floss (Asadoorian 2006). A study of a cohort of young people at ages 15, 18, and 26 found that at age 26, only 51% of both females and males believed that using dental floss was important, with females rating flossing more important than males (Broadbent 2006).
Certain organisations, for example the American Dental Association, recommend that children’s teeth are flossed as soon as they have two teeth that touch. However, studies that measure compliance show that few children have their teeth flossed or use floss: a study in West Virginia found that only 21% of children had their teeth flossed (Wiener 2009). When measures are taken to increase compliance, for example using behavioural change techniques, then the proportion of adolescent flossing increases (Gholami 2015).
Interdental brushes
Interdental brushes are small cylindrical or cone‐shaped bristles on a thin wire that may be inserted between the teeth. They have soft nylon filaments aligned at right angles to a central stiffened rod, often twisted stainless steel wire, very similar to a bottle brush. Interdental brushes used for cleaning around implants have coated wire to avoid scratching the implants or causing galvanic shock. They are available in a range of different widths to match the interdental space and their shape can be conical or cylindrical. Most are round in section, although interdental brushes with a more triangular cross‐section can also be found on the market. Originally, interdental brushes were recommended by dental professionals to patients with large embrasure spaces between the teeth (Slot 2008; Waerhaug 1976), caused by the loss of interdental papilla mainly due to periodontal destruction. Patients who had interdental papillae that filled the embrasure space were usually recommended to use dental floss as an interdental cleansing tool. However, with the greater range of interdental brush sizes and cross‐sectional diameters now available, they are considered a potentially suitable alternative to dental floss for patients who have interdental papillae that fill the interdental space (Imai 2011). Daily dental flossing adherence is low because it requires a certain degree of dexterity and motivation (Asadoorian 2006), whereas interdental brushes have been shown as being easier to use and are therefore preferred by patients (Christou 1998; Imai 2010). Furthermore, when compared to dental floss, they are thought to be more effective in plaque removal because the bristles fill the embrasure and are able to deplaque the invaginated areas on the tooth and root surfaces (Bergenholtz 1984; Christou 1998; Imai 2011; Jackson 2006; Kiger 1991; Waerhaug 1976). However, there are conflicting study results regarding the efficacy of interdental brushes in the reduction of clinical parameters of gingival inflammation (Jackson 2006; Noorlin 2007); and whether they are only suitable for patients with moderate to severe attachment loss and open embrasures, or whether they are a suitable aid for healthy patients to prevent gingivitis who have sufficient interdental space to accommodate them (Gjermo 1970; Imai 2011).
Tooth cleaning sticks
Sticks and twigs, composed of bone, ivory, metal, plastic, quills, wood, and other substances, have been used for cleaning tooth surfaces and interdentally since prehistoric times (Christen 2003). The continuing use of hard materials for cleaning interdentally has been questioned (Mandel 1990); however, they continue to be used in different parts of the world. The meswak (or miswak) is one of the most widely used tooth cleaning sticks (Saha 2012); however, it is important to differentiate its use between cleaning tooth surfaces and interdentally (Furuta 2011). Toothpicks continue to be used, particularly in the United States and Scandinavia, predominantly in older age groups (Sarner 2010), whereas dental floss and interdental brushes are more likely to be used by younger people. Toothpicks are commonly used in East Asia such as in China, Korea, and Japan, though the main purpose is to remove food debris in the interdental areas. Interdental rubber tip stimulators, usually consisting of a carrying handle and disposable rubber tip stimulator, are readily available and are designed to stimulate gingival blood flow and remove interdental plaque.
Oral irrigators
Oral irrigation with water under pressure has been available for just over fifty years (Lyle 2012), and the benefits are described as the removal of biofilm from tooth surfaces and bacteria from periodontal pockets. Oral irrigators were first designed to be used supragingivally, using water pressure to displace and remove plaque, relying on pressure to irrigate subgingival regions (Goyal 2012). Since then, various tips have been designed that may be used subgingivally and several manufacturers provide products to do this.
How the intervention might work
Dental plaque‐induced gingivitis and incipient, non‐cavitated carious lesions are reversible (Mariotti 1999; Silverstone 1983). The progression in either disease may be attributed to a change in the environmental equilibrium that favours disease conditions. For example, gingivitis has been shown to be a risk factor in the clinical course of chronic periodontitis (Schatzle 2009); and it is important to treat gingivitis when inflammation is only in the gingival tissues and has not affected other parts of the periodontal system (Mariotti 1999). Early carious lesions can be arrested in the enamel and may or may not progress to the dentine depending on the dynamic equilibrium between demineralisation and remineralisation (Marinho 2003; Marinho 2013; Marinho 2015).
Periodontal diseases
Gingival diseases are classified as one of the periodontal diseases (Armitage 1999; Caton 2018), and are categorised as either dental plaque‐induced diseases or non‐plaque‐induced gingival lesions. Gingival inflammation, gingivitis, induced by dental plaque is an inflammatory response of the gingival tissues caused by bacteria in dental plaque (Page 1986), and characterised by swelling, redness and bleeding on probing. If dental plaque is left in place for more than two weeks, then gingivitis will occur (Loe 1965). The severity of gingivitis can be modified by factors other than plaque (Trombelli 2013).
Periodontal diseases are complex interactions of bacteria and the immune system (Page 2007; Sanz 2011); and dental plaque is the primary aetiological factor (Marsh 2006). Dental plaque may be either supragingival or subgingival and the plaque biofilm comprises different bacterial colonies at the supragingival or subgingival levels. By disrupting the plaque, the main cause of periodontal diseases can be removed. Although there is a lack of RCT evidence for the best approaches to ensuring periodontal health is maintained after treatment for periodontitis (Manresa 2015), a key aspect of supportive periodontal therapy is training in self‐administered mechanical plaque removal techiques, and this is also widely regarded as a crucial part of preventive strategies (Greenwell 2001; Lindhe 2003).
Dental caries
Dental plaque contains many bacterial species that are acidogenic. In 1890, Miller published 'The microorganisms of the human mouth' which postulated that oral bacteria found in plaque were acidogenic, but, as no specific bacteria were implicated, it became known as the "non‐specific plaque hypothesis" (Ring 2002). Later, Loesche 1976 postulated a "specific plaque theory", implicating Streptococcus mutans and Lactobacillus acidophilus as the primary bacteria involved in caries generation. Since then, the importance of the plaque biofilm has been recognised and an “ecological plaque hypothesis” proposed (Marsh 1994).
Acidogenic plaque bacteria utilise dietary sugars to demineralise dental tissues, which may progress into carious tooth lesions. The most susceptible regions of teeth to caries are the occlusal and interdental surfaces (Demirci 2010). Interdental plaque is more prevalent (Lindhe 2003), forms more readily (Igarashi 1989) and is more acidogenic than plaque on other tooth surfaces in the mouth. Therefore, interdental cleaning is often recommended as an adjunctive self care therapy, particularly when caries risk is increased (Sarner 2010; Wright 1977). Removal of dental plaque by mechanical interdental cleaning should reduce the frequency and degree of demineralisation interproximally and lead to decreased caries incidence.
Why it is important to do this review
Effective oral hygiene is a crucial factor in maintaining good oral health, which is, in turn, associated with overall health and health‐related quality of life (McGrath 2002; Sheiham 2005). Poor oral health may affect appearance in terms of stained or missing teeth; can contribute to bad breath (Morita 2001); and negatively influence self confidence, self esteem, and the ability to communicate (Exley 2009). Poor oral health is often accompanied by pain arising from carious lesions, which may lead to discomfort when eating, drinking, and speaking (Dahl 2011). Individuals with high levels of dental plaque, after accounting for sex, socioeconomic status, and dental care attendance frequency, are more likely to experience dental caries and periodontal diseases (Broadbent 2011).
The regular and effective removal of dental plaque by toothbrushing is important for the prevention and successful management of common oral diseases, in conjunction with use of fluoride toothpaste (Walsh 2019). Mechanical interdental cleaning, using either dental floss, interdental brushes, or tooth cleaning sticks, is widely recommended and advertised, but it is unclear whether there is a benefit in using interdental cleaning devices as an adjunct to toothbrushing and if a particular type of interdental cleaning device is superior to others. What the benefits may be for children and adolescents is unknown.
This review, which incorporates and expands previous reviews on flossing (Sambunjak 2011) and interdental brushing (Poklepovic Pericic 2013), was identified as a topic of clinical priority when Cochrane Oral Health undertook a comprehensive prioritisation exercise (Worthington 2015). A systematic review and meta‐analysis, combining the results of randomised controlled trials, will provide health care commissioners, practitioners, and consumers with evidence about the effectiveness of mechanical interdental cleaning at home for oral health.
Objectives
To evaluate the effectiveness of interdental cleaning devices used at home, in addition to toothbrushing, compared with toothbrushing alone, for preventing and controlling periodontal diseases, caries, and plaque. A secondary objective was to compare different interdental cleaning devices with each other.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including split‐mouth design, cross‐over trials and cluster‐randomised trials, that lasted four weeks or more. We included data from both periods of a cross‐over trial only if there was a washout period of at least two weeks before the cross‐over. Studies were included irrespective of publication status and language.
Types of participants
The review included studies of dentate participants irrespective of age, race, sex, socioeconomic status, geographical location, background exposure to fluoride, initial dental health status, setting, or time of intervention. We excluded studies if the majority of participants had any orthodontic appliances. Likewise, we excluded studies if participants were selected on the basis of special (general or oral) health conditions (for example, severely immunocompromised people), or if the majority of participants had severe periodontal disease.
Types of interventions
We included all trials that compared a combination of toothbrushing and any home‐use mechanical interdental cleaning device with toothbrushing alone, or with another mechanical interdental cleaning device.
We excluded intervention or control groups receiving any additional active agent(s) (i.e. caries‐preventive agents) as part of the study (e.g. chlorhexidine mouthwash, additional fluoride‐based procedures, oral hygiene procedures, xylitol chewing gum), in addition to interdental cleaning procedures or toothbrushing. However, we included studies using floss impregnated with active agents such as chlorhexidine or fluoride. We included studies that involved participants in both groups receiving additional measures as part of their routine oral care, such as oral hygiene advice, supervised brushing, fissure sealants, etc. We excluded studies that compared two variations of the same type of interdental cleaning device.
Types of outcome measures
Primary outcomes
Outcomes did not form part of the inclusion criteria. We included all RCTs of home‐use devices in this review, even if they did not report these outcomes.
Gingivitis ‐ assessed by gingival indices and bleeding indices in separate analyses;
Periodontitis ‐ assessed by clinical attachment loss and pocket probing depth;
Interproximal caries ‐ assessed by (a) progression of caries into enamel or dentine, (b) change in decayed, missing and filled tooth surfaces (D(M)FS) index, (c) radiographic evidence. Studies had to contain explicit criteria for diagnosing dental caries. As caries increment could be reported differently in different trials, we planned to use a set of a priori rules to choose the primary outcome data for analysis from each study (Marinho 2013; see Table 11);
Plaque – assessed by plaque scores or indices;
Harms and adverse effects.
1. A priori rules for selecting data to extract for caries increment.
| As we were aware that caries increment could be reported differently in different trials, we developed a set of a priori rules to choose the primary outcome data (decayed, missing or filled surfaces (D(M)FS)) for analysis from each study, drawing on Marinho 2013: DFS data would be chosen over DMFS data, and these would be chosen over DS or FS; data for 'all surface types combined' would be chosen over data for 'specific types' only; data for 'all erupted and erupting teeth combined' would be chosen over data for 'erupted' only, and these over data for 'erupting' only; data from 'clinical and radiological examinations combined' would be chosen over data from 'clinical' only, and these over 'radiological' only; data for dentinal/cavitated caries lesions would be chosen over data for enamel/non‐cavitated lesions; net caries increment data would be chosen over crude (observed) increment data; and follow‐up nearest to three years (often the one at the end of the treatment period) would be chosen over all other lengths of follow‐up, unless otherwise stated. When no specification was provided with regard to the methods of examination adopted, diagnostic thresholds used, groups of teeth and types of tooth eruption recorded, and approaches for reversals adopted, the primary choices described above were assumed. |
For gingivitis, plaque and adverse effects, we considered outcomes at all time points measured by the included studies except those with a duration of less than one month. We planned to use only data with at least six months' follow‐up for the outcomes of clinical attachment loss, pocket probing depth, and interproximal caries.
Secondary outcomes
Halitosis;
Patient satisfaction;
Cost of intervention.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year, or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 16 January 2019) (see Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 12) in the Cochrane Library (searched 16 January 2019) (see Appendix 2);
MEDLINE Ovid (1946 to 16 January 2019) (see Appendix 3);
Embase Ovid (1980 to 16 January 2019) (see Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 16 January 2019) (see Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6 (Lefebvre 2011).
We also initially searched Web of Science Conference Proceedings, but discontinued this search due to a poor yield of studies for inclusion (see Appendix 6 for details of the search strategy).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Trials Register (http://clinicaltrials.gov) (to 16 January 2019) (see Appendix 7);
The WHO Clinical Trials Registry Platform (http://apps.who.int/trialsearch/default.aspx) (to 16 January 2019) (see Appendix 8).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors independently carried out the selection of studies and made decisions about eligibility; one of them a methodologist and the other a topic area specialist. The search was designed to be sensitive and include controlled clinical trials; these were filtered out early in the selection process if they were not randomised. If the relevance of a study report was unclear, we read the full text and resolved disagreements by discussion with other authors.
Data extraction and management
At least two review authors independently extracted data; at least one of them a methodologist and one a topic area specialist. We compared the extracted data and identified disagreements, which we then resolved by consensus.
We extracted and entered the following data into a customised collection form. We had previously designed a data extraction form for a similar review (Sambunjak 2011).
Study characteristics: design, including details if a study differed from standard parallel‐group design, e.g. split‐mouth or cross‐over; recruitment period, setting.
Participants: number randomised and evaluated (by group); inclusion and exclusion criteria; demographic characteristics of participants: age, sex, country of origin, ethnicity, socioeconomic status, comorbidity, condition‐related health status. We recorded demographic characteristics for the study as a whole and for each intervention group, when available.
Intervention and control groups: type of interdental cleaning procedure, including type of toothbrush (powered or manual) and type of toothpaste (with or without fluoride); frequency of interdental cleaning procedure; duration of the intervention period; whether the participants were trained/instructed how to brush interdentally, floss or toothbrush, or a combination of all three, and by whom; length of follow‐up; loss to follow‐up; assessment of adherence; level of fluoride in the water supply.
Outcomes: detailed description of the outcomes of interest (both beneficial and adverse), including the definition and timing of measurement; methods of assessment; other outcomes reported in the included studies that were not outcomes of this review (we did not extract results for these outcomes).
Data on funding sources if reported.
We intended to enter the data from cross‐over studies, split‐mouth studies, and for the prevented fraction, into RevMan (Review Manager (RevMan)) using the generic inverse variance outcome type.
We extracted both gingival indices and bleeding indices (assessed as bleeding either present or absent on a site) where both were reported. We extracted data from indices assessed on the interproximal sites if available; otherwise we used the indices on the sites reported.
In studies that used both bleeding on probing (BOP) and Eastman Interdental Bleeding Index (EIBI), we included EIBI in the meta‐analyses. The suitability of the EIBI is justified by its reproducibility and high inter‐examiner and intra‐examiner reliability (Blieden 1992).
Assessment of risk of bias in included studies
We assessed the risk of bias in each study using Cochrane's 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The tool addresses seven domains: random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues. For split‐mouth and cross‐over designs, our assessment of risk of bias included additional considerations such as suitability of the design, and risk of carry‐over or spill‐over effects.
At least two review authors, a methodologist and a topic area specialist, independently carried out the assessment of risk of bias. They were not blinded to the names of the authors, institutions, journal or results of a study. We assigned a judgement of low, high or unclear risk of bias for each domain within each included study, recording in the relevant 'Risk of bias' domain the rationale for our judgement. We tested the data collection forms and assessments of the risk of bias on a pilot sample of articles. As protocols were not available for many studies, we compared the outcomes listed in the methods sections in a publication against those results reported to assess selective reporting bias. If some indications of reporting bias were found, we contacted the study authors for clarification. If information was missing from the included reports, we attempted to contact the study investigators to obtain it. If standard deviations were missing and we were unable to estimate these, we assessed selective reporting as having high risk of bias. If a study reported measured adverse effects but did not report findings, we judged it to have unclear risk of selective reporting bias.
If compliance was not assessed in a study, we judged the risk of 'other bias' to be unclear. If compliance was poor, we judged the study to be at high risk of bias. Where a study noted baseline difference, we assumed this to be an imbalance greater than what would be expected by chance and we assessed the risk of 'other bias' as high.
In our assessment of the overall risk of bias in a study, we did not include the domain of performance bias. All studies were at high risk of this because it is not possible to blind study participants to the interventions of interest in an ethical experimental situation. Removing performance bias from consideration, we assessed a study as at high risk of bias if we had judged at least one domain as having high risk of bias, unclear if at least one domain was unclear and none were high, and low if all domains were assessed as being at low risk of bias.
Measures of treatment effect
For gingivitis and plaque outcomes, we expected most measures of treatment effect to be continuous; although these measures are sometimes dichotomous at a site level, they are treated as continuous when averaged over sites within the mouth. We used the mean difference (MD) (or difference in means), or standardised mean difference (SMD) when combining different clinical indices. We calculated the corresponding 95% confidence intervals (CIs) for each result.
We intended to analyse clinical attachment loss and probing pocket depth as continuous measures; however, there were no clinical attachment data.
For caries outcomes, we intended to calculate the prevented fraction (PF), where appropriate. The PF is expressed as the mean increment in the control group minus the mean increment in the intervention group divided by the mean increment in the control group, i.e. the caries increment in the treatment group expressed as a percentage of the control group. There were no caries data reported.
Unit of analysis issues
The units of analysis were individual participants or groups of measuring sites within individual participants (e.g. interproximal sites: proportion of sites that have bleeding averaged over the number of participants). We intended to contact study authors to obtain data in the right form; however, this was not necessary. We intended to analyse split‐mouth, cross‐over and cluster trials taking the clustering into account as described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Depending on the interventions being included in multi‐arm studies, we either combined groups (if straightforward), or presented the arms separately (e.g. automated versus manual floss), ensuring that there was no double counting of participants in the control arms.
Dealing with missing data
As described in Table 16.1.a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), there are several types of missing data in a systematic review or meta‐analysis. The problems of missing studies and outcomes are addressed in the Assessment of reporting biases part of this review. A common problem was missing summary data, such as standard deviations for continuous outcomes. Missing summary data were not a reason to exclude a study from the review and we used the methods outlined in section 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions to impute missing standard deviations (Higgins 2011).
For the data judged to be 'missing at random', i.e. their being missing was unrelated to their actual values, we conducted analyses using the available data only. This was the default option for all studies, so it was unnecessary to perform a sensitivity analysis to assess how the changes in assumptions might have affected the results.
Assessment of heterogeneity
Prior to meta‐analysis, we assessed studies for clinical homogeneity with respect to the type of intervention, control group, and outcomes. We did not combine results of clinically heterogeneous studies. For studies judged as clinically homogeneous, we tested for statistical heterogeneity using the Chi² test and I² statistic. We interpreted a Chi² test resulting in a P value less than 0.10 as indicating significant statistical heterogeneity. In order to assess and quantify the possible magnitude of inconsistency (i.e. heterogeneity) across studies, we used the I² statistic, roughly interpreting values under 40% as low or no heterogeneity, values between 40 and 70% as moderate heterogeneity, and values over 70% as substantial.
Assessment of reporting biases
If there had been at least 10 studies included in a meta‐analysis, we would have created a funnel plot of effect estimates against their standard errors to assess a possible between‐study reporting bias. If an asymmetry of the funnel plot had been found either by inspection or statistical tests, we would have considered possible explanations and taken this into account in the interpretation of the overall estimate of treatment effects.
Data synthesis
We undertook meta‐analysis including only studies reporting the same outcomes. When there were a number of different indices measuring the same outcome (either plaque or gingivitis), we used the standardised mean difference (SMD), along with the appropriate 95% CI, to combine the results in meta‐analysis. Meta‐analysis of split‐mouth and cross‐over studies were combined where possible but it is inappropriate to combine these when using SMD. Some studies measured plaque and gingivitis on selected sites and we used indices based on these data if the interproximal site data were not available. We planned to combine risk ratios for binary data. As considerable heterogeneity was expected in the included studies, we undertook a random‐effects model as the primary method of meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses. We decided to conduct them if there was heterogeneity (P value < 0.1) and there were at least 10 studies in the meta‐analysis.
Age (child, adult) and dentition (primary, permanent).
Periodontal status at baseline.
Trained (instructed) versus untrained (uninstructed) interdental cleaning.
Funded versus unfunded studies.
Sensitivity analysis
The primary meta‐analyses included all eligible studies irrespective of their risk of bias. We intended to conduct sensitivity analyses by excluding studies:
at high risk of bias (excluding participant blinding from this overall study‐level assessment of risk of bias);
with estimated standard deviations;
using split‐mouth and cross‐over designs.
Summarising findings and assessing the certainty of the evidence
We adopted the GRADE system for evaluating the certainty of the evidence of systematic reviews (Guyatt 2008; Guyatt 2008a; Higgins 2011), and used it to construct 'Summary of findings' tables for the main comparisons and key outcomes: gingivitis, periodontitis, interproximal caries, plaque, adverse events (harms). We assessed the certainty of the body of evidence with reference to the overall risk of bias of the included studies (excluding performance bias), directness of the evidence, consistency of the results, precision of the estimates and the risk of publication bias. We classified the certainty of the body of evidence into four categories: high, moderate, low, and very low.
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
Results of the search
We retrieved a total of 10,203 references from electronic searches. After finding and deleting duplicates, we had 4733 references, which consisted of titles with or without abstracts. Four authors independently screened the titles and abstracts against the inclusion criteria for the review and discarded 4597 references. We identified two additional studies from screening reference lists. We obtained full‐text copies of the 138 references and four authors considered them independently. Following this, we rejected 42 records, listed 59 records as excluded studies and one as awaiting classification. We included 36 articles (see Included studies and Excluded studies). Thus, our total was 35 included studies (36 articles). Figure 1 shows the flow of studies.
1.
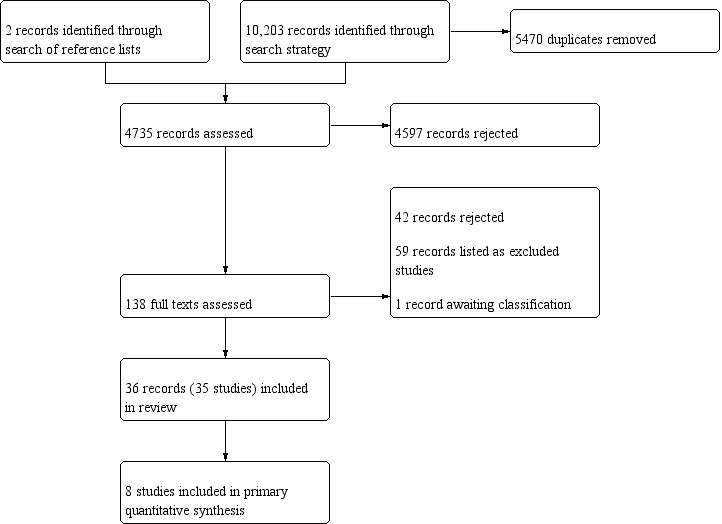
Study flow diagram
Included studies
Thirty‐five studies met the inclusion criteria for this review (see Characteristics of included studies).
Design
Three studies used a split‐mouth design (Christou 1998; Imai 2011; Ishak 2007). Gordon 1996 was a cross‐over study; however, the second period was used to measure preference, with no clinical data measured. We therefore used the data from the first period only, treating it as a parallel‐group study. We also used first‐period data only from Hague 2007 as, although it was described as a cross‐over study, the same control group was used throughout. The remaining studies were of parallel design.
Sample sizes
The studies randomised a total of 3929 participants, with individual study sample sizes ranging between 18 (Ishak 2007) and 362 (Bauroth 2003) participants. The studies evaluated approximately 3734 participants (some studies did not state the number of analysed participants), but we did not include all study arms in the review. The largest number of participants included in a single meta‐analysis was 585 (eight studies).
Setting
Twenty‐three studies were conducted in the USA; three in the Netherlands (Christou 1998; Rosema 2008; Rosema 2011); two in Canada (Goyal 2012; Imai 2011); one in Germany (Zimmer 2006); two in the UK (Ishak 2007; Jackson 2006); one in Italy (Graziani 2017); and one in Guatemala (NCT00855933). Cronin 1997 and Cronin 2005 did not report location. All the the studies that reported location were conducted in high‐income countries so we have no known data from low‐ and middle‐income countries.
Eighteen studies were conducted in an academic setting and one was conducted in a private practice dental centre (Lobene 1982). The other studies did not mention the type of setting.
Thirty‐one studies were single‐centre; four studies did not state how many centres were involved (Bauroth 2003; Biesbrock 2007; Cronin 1997; Cronin 2005).
Participants
Age and sex
No included studies were conducted with children or adolescents. In the studies reporting the age of participants, ages ranged between 18 and 78 years; 21 studies reported the mean age, which ranged from 20 to 53 years. Most studies included both males and females (two did not say, but inclusion criteria implied both were included). Twelve studies did not report the ratio of males to females. In the other studies, the proportion of males to females, in percentage, ranged from 7/53 to 60/40 (11% to 60% males). Zimmer 2006 included equal numbers of males and females. Twenty studies reported including more females than males, and more males than females were reported in three studies (Christou 1998; Goyal 2012; Schiff 2006).
Periodontal status at baseline
The studies predate the new periodontal classification system agreed at the 2017 World Workshop on the Classification of Periodontal and Peri‐implant Diseases and Conditions (Caton 2018; Chapelle 2018; Papapanou 2018). In general, it was difficult to classify and categorise participant periodontal status at baseline because insufficient information was provided by the studies.
In Christou 1998, all participants had moderate to severe periodontitis. Jackson 2006 included people with moderate periodontitis (at least one shallow pocket or at least one deep pocket > 6 mm in 4 of 6 sextants).
Smith 1988 reported that all participants were patients with periodontitis on maintenance programmes, and Walsh 1985 included participants with generalisable interproximal gingival bleeding in 25% of sites exhibiting PDs at least 4 mm or more, suggesting periodontitis; however, neither of these two studies provided a clear definition of the stage of the disease.
Two studies explicitly described that they included mixed diagnoses: Ishak 2007 stated that participants had been diagnosed with gingivitis or moderate periodontitis, and Lewis 2004 stated that included participants had either gingivitis or slight chronic periodontitis (chronic gingival inflammation with pocket depth (PD) ≥ 4 mm and clinical attachment loss ≥ 2 mm).
Participants in Graziani 2017 did not have periodontitis; they were described "periodontally healthy", defined as "absence of proximal attachment loss of > 3 mm in > 2 adjacent teeth". Graziani 2017 provided measurements of clinical attachment loss (CAL), pocket depth (PD), and inflammation to confirm this designation.
Meklas 1972 and Vogel 1975 included dental students with no information regarding their baseline oral status.
All other studies reported bleeding measurements of different values, suggesting various levels of inflammation of marginal periodontal tissues. The mean values, when presented, tended to be low, but because ranges were not usually provided, nor any further data (clinical scores, e.g. CAL, PD), periodontitis among these participants could not be ruled out.
Details per study are presented in Characteristics of included studies and Additional tables. In summary, most studies included participants with slight to moderate periodontal diseases, with the majority of studies excluding advanced periodontal diseases, such as severe periodontitis.
Smoking status
Twenty‐four studies did not report the smoking status of participants, including one that recorded smoking but did not report it (Zimmer 2006). Eight studies reported the percentage of participants who smoked: Bauroth 2003 (75%), Graziani 2017 (57%), Hague 2007 (8%), Jackson 2006 (38%), Lewis 2004 (10%), Rosema 2008 (11%), Sharma 2002 (25%) and Yost 2006 (10%). Three studies consisted only of non‐smokers (Goyal 2012; Ishak 2007; Mwatha 2017).
Socioeconomic status
None of the included studies reported the participants' socioeconomic status.
Interventions
Toothbrushing was undertaken by all participants in all studies. The participants in 33 of the 35 studies used a manual toothbrush; in Goyal 2012, participants used a sonic toothbrush and in Biesbrock 2007, participants used a powered toothbrush. The studies evaluated the use of floss (automated or manual), interdental brush, tooth cleaning stick ‐ wooden or rubber (manual or electric), and oral irrigation to remove plaque from the teeth.
The number of study arms varied from two to six; the number of arms used in our analyses varied from two to four. See Table 12. For Lobene 1982, we combined waxed, unwaxed, and minted floss arms. Comparisons evaluated in the studies are presented in the table below.
2. Study design and number of arms.
| Parallel studies | Number of study arms | Number used in review analyses |
| Graziani 2017 | 4 | 4 |
| Lobene 1982 | 4 | 4 |
| Smith 1988 | 4 | 4 |
| Finkelstein 1990* | 5 | 3 |
| Jared 2005 | 5 | 3 |
| Vogel 1975 | 4 | 3 |
| Yost 2006 | 4 | 3 |
| Barnes 2005 | 3 | 3 |
| Hague 2007 | 3 | 3 |
| Walsh 1985 | 3 | 3 |
| Biesbrock 2007 | 6 | 2 |
| Goyal 2012 | 4 | 2 |
| Mwatha 2017 | 4 | 2 |
| NCT01250769 | 4 | 2 |
| Walsh 1989 | 4 | 2 |
| Zimmer 2006 | 4 | 2 |
| Bauroth 2003 | 3 | 2 |
| Cronin 2005 | 3 | 2 |
| Rosema 2008 | 3 | 2 |
| Rosema 2011 | 3 | 2 |
| Schiff 2006 | 3 | 2 |
| Sharma 2002 | 3 | 2 |
| Cronin 1997 | 2 | 2 |
| Frascella 2000 | 2 | 2 |
| Gordon 1996 | 2 | 2 |
| Isaacs 1999 | 2 | 2 |
| Jackson 2006 | 2 | 2 |
| Kazmierczak 1994 | 2 | 2 |
| Lewis 2004* | 2 | 2 |
| Meklas 1972 | 2 | 2 |
| NCT00855933 | 2 | 2 |
| Yankell 2002 | 2 | 2 |
| Split‐mouth studies | Number of study arms | Number used in review analyses |
| Imai 2011 | 3 | 3 |
| Christou 1998 | 2 | 2 |
| Ishak 2007 | 2 | 2 |
*No data used
| Interdental cleaning device | Toothbrushing only | Floss | Interdental brush |
| Floss | 15 studies: Bauroth 2003; Biesbrock 2007; Finkelstein 1990; Graziani 2017; Hague 2007; Jared 2005; Lobene 1982; Mwatha 2017; NCT00855933; Rosema 2008; Schiff 2006; Sharma 2002; Vogel 1975; Walsh 1985; Zimmer 2006 | ‐ | ‐ |
| Interdental brush | 2 studies: Graziani 2017; Jared 2005 | 9 studies: Christou 1998; Graziani 2017; Imai 2011; Ishak 2007; Jackson 2006; Jared 2005; Smith 1988; Yankell 2002; Yost 2006 | ‐ |
| Wooden tooth cleaning sticks | 2 studies: Finkelstein 1990; Walsh 1985 | 3 studies: Finkelstein 1990; Lewis 2004; Walsh 1985 | ‐ |
| Rubber/elastomeric tooth cleaning sticks | 2 studies: Graziani 2017; Vogel 1975 | 9 studies: Cronin 1997; Cronin 2005; Gordon 1996; Graziani 2017; Isaacs 1999; Kazmierczak 1994; Smith 1988; Vogel 1975; Yost 2006 | 3 studies: Graziani 2017; Smith 1988; Yost 2006 |
| Oral irrigation | 5 studies: Frascella 2000; Goyal 2012; Meklas 1972; NCT01250769; Walsh 1989 | 2 studies: Barnes 2005; Rosema 2011 | ‐ |
Training
No specific instructions were given for the use of any of the distributed oral hygiene materials in one study (Yankell 2002), where only one brush size was used. There was no information about training in NCT00855933 and NCT01250769. In all remaining studies, participants were provided with detailed instructions on the use of the assigned product. There was often detailed information on the size of the brushes to be used, and how this was determined for each individual participant (see Characteristics of included studies).
Outcomes
Tooth sites
Twenty‐three studies provided data for the interproximal sites only (Bauroth 2003; Christou 1998; Cronin 1997; Cronin 2005; Gordon 1996; Graziani 2017; Hague 2007; Imai 2011; Isaacs 1999; Ishak 2007; Jackson 2006; Jared 2005; Kazmierczak 1994; Lewis 2004 ; Schiff 2006; Sharma 2002; Smith 1988; Vogel 1975; Yankell 2002; Yost 2006; Zimmer 2006). Goyal 2012 provided data from interproximal sites only for plaque, and from mixed sites for gingivitis. Finkelstein 1990 used interproximal sites for gingivitis and other for plaque. We were unable to use the data for Finkelstein 1990, Lewis 2004 or Smith 1988. The remaining studies only presented the indices measured on mixed sites, including the interproximal sites.
Gingivitis
Seventeen studies used more than one gingivitis index.
The most commonly used index was the Löe & Silness Gingival Index (LSGI) or a modification of it (14 studies: Barnes 2005; Biesbrock 2007; Cronin 1997; Cronin 2005; Finkelstein 1990; Hague 2007; Isaacs 1999; Lobene 1982; Schiff 2006; Smith 1988; Vogel 1975; Walsh 1989; Yost 2006). Seven studies used the Lobene Modified Interproximal Gingival Index (Bauroth 2003; Gordon 1996; Goyal 2012; Jared 2005; Mwatha 2017; Sharma 2002; Yankell 2002). Six studies used the Eastman Interdental Bleeding Index (Finkelstein 1990; Imai 2011; Jackson 2006; Lewis 2004; Yankell 2002; Yost 2006). Two studies used each of the Bleeding Index (Bauroth 2003; Kazmierczak 1994); the Lobene Modified Gingival Index (Kazmierczak 1994; NCT00855933); the Papillary Bleeding Index (Gordon 1996; Zimmer 2006); the Gingival Bleeding Index (Mwatha 2017; NCT01250769); and the Bleeding on Marginal Probing Index (Rosema 2008; Rosema 2011);
One study used each of the following: Carter & Barnes Bleeding Index (Barnes 2005); Löe & Silness Bleeding scores (when scoring 2 or 3 on the LSGI) (Cronin 2005); modified gingival index (Frascella 2000); angular bleeding index (Frascella 2000); Full Mouth Bleeding Score (Graziani 2017); Angulated Bleeding Index (Graziani 2017); Bleeding on Probing Index (Ishak 2007); Relative Interdental Papillae Level (mm) (Jackson 2006); Pocket Depth (mm) (Jackson 2006), bleeding on probing (Jackson 2006; Walsh 1989), Bleeding on probing (Van der Weijden modification) (+/‐) (Jared 2005), and one study used bleeding on probing assessed by using Angulated Bleeding Index (0/1) and Periodontal Pocket Bleeding Index and probing depth (mm) assessed using a force controlled probe (Christou 1998); Russell modified Periodontal Index (Meklas 1972); Ainamo & Bay Gingival Bleeding Index (Sharma 2002); Interproximal Bleeding on Probing Index (0/1) evaluated as percentage of bleeding interproximal surfaces (Walsh 1985); and Intracrevicular exudate sampling (Vogel 1975).
Plaque
Most studies used one plaque index. Lewis 2004 and Zimmer 2006 used more than one plaque index, while NCT00855933 and NCT01250769 did not measure plaque.
The index used most often was the Quigley‐Hein Plaque Index or a modification of it. This was used in 15 studies: original (Zimmer 2006); Turesky modification (Bauroth 2003; Cronin 1997; Hague 2007; Isaacs 1999; Jared 2005; Kazmierczak 1994; Lobene 1982; Rosema 2011; Schiff 2006; Sharma 2002; Yankell 2002); Turesky‐Gilmore‐Glickman modification (Frascella 2000); Volpe modification (Christou 1998); Benson modification (Yost 2006); and Paraskevas modification (Rosema 2008).
The Silness & Löe Plaque Index was used in five studies (Imai 2011; Jackson 2006; Smith 1988; Walsh 1985; Walsh 1989), and the Proximal/Marginal Plaque Index or a modification of it was used in four studies (Barnes 2005; Cronin 2005; Gordon 1996; Zimmer 2006). The Navy Plaque Index (Rustogi modification) was used in three studies (Biesbrock 2007; Goyal 2012; Mwatha 2017).
One study used each of the Global Plaque Index (Finkelstein 1990); Full Mouth Plaque Score (Graziani 2017); supra‐ and subgingival plaque examined using dental floss, with visible plaque deposits scored positive (Ishak 2007); O'Leary Plaque Index and Interproximal Plaque Index (Lewis 2004); a 3‐point plaque index (Meklas 1972); Podchladley's Total Plaque Index (Vogel 1975); and Modified Proximal Plaque Index (Zimmer 2006).
The indices used for gingivitis and plaque in each study are listed in Table 13, and in more detail in the Characteristics of included studies tables.
3. Gingivitis and plaque indices used in each trial.
| Study | Gingivitis index (scale) | Plaque index (scale) |
| Barnes 2005 | Löe & Silness Gingival Index (0 to 3) Carter & Barnes Bleeding Index (0/1) |
Proximal/Marginal Plaque Index (0‐5) |
| Bauroth 2003 | Lobene Modified Interproximal Gingival Index (0 to 4) Bleeding Index (0/1) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Biesbrock 2007 | Löe & Silness Gingival Index (0 to 3) | Navy Plaque Index (Rustogi modification) (0 /1) |
| Christou 1998 | Bleeding on probing assessed by using Angulated Bleeding Index (0/1) and Periodontal Pocket Bleeding Index (0/1) | Volpe modification of Quigley and Hein Plaque Index (0 to 5) |
| Cronin 1997 | Löe & Silness Gingival Index (0 to 3) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Cronin 2005 | Löe & Silness Gingival Index (0 to 3) Löe & Silness Bleeding scores (when scoring 2 or 3 on the Löe & Silness Gingival Index) |
Proximal/Marginal Plaque Index (0 to 5) |
| Finkelstein 1990 | Löe & Silness Gingival Index modified to include visual assessment only (0 to 3) Eastman Interdental Bleeding Index (0/1) |
Global Plaque Index (0 to 100%) |
| Frascella 2000 | Modified gingival index Angular bleeding index |
Turesky‐Gilmore‐Glickman modification of the Quigley‐Hein Plaque Index |
| Gordon 1996 | Lobene Modified Gingival Index (0 to 4) Papillary Bleeding Index (0/1) |
Proximal/Marginal Plaque Index (0 to 5) |
| Goyal 2012 | Lobene Modified Gingival Index (0 to 4) | Navy Plaque Index (Rustogi modification) (0/1 for each of the 9 tooth surfaces) |
| Graziani 2017 | Full Mouth Bleeding Score (0/1) Angulated Bleeding Index (0/1) |
Full Mouth Plaque Score (percentage of areas containing plaque) |
| Hague 2007 | Löe & Silness Gingival Index (0 to 3) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Imai 2011 | Eastman Interdental Bleeding Index (0/1) | Silness & Löe Plaque Index (0 to 3) (modified) |
| Isaacs 1999 | Löe & Silness Gingival Index (0 to 3) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Ishak 2007 | Bleeding on Probing Index (0/1) | Visible plaque deposits were scored as positive |
| Jackson 2006 | Eastman Interdental Bleeding Index (0/1) Bleeding on probing (0/1) Relative Interdental Papillae Level (mm) |
Silness & Löe Plaque Index (0 to 3) |
| Jared 2005 | Lobene Modified Gingival Index (0 to 4) Bleeding on probing (Van der Weijden modification) (+/‐) |
Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Kazmierczak 1994 | Lobene Modified Gingival Index (0 to 4) Bleeding Index (0/1) |
Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Lewis 2004 | Eastman Interdental Bleeding Index (0/1) | O'Leary Plaque Index (0/1) Interproximal Plaque Index (0/1) |
| Lobene 1982 | Löe & Silness Gingival Index (0 to 3) | Quigley‐Hein Plaque Index (0 to 5) |
| Meklas 1972 | Russell modified Periodontal Index (0 to 2) | 3‐point plaque index (0 to 2) |
| Mwatha 2017 | Lobene Modified Gingival Index (0 to 4) Gingival Bleeding Index (0/1) |
Navy Plaque Index (Rustogi modification) (0/1 for each of the nine tooth surfaces) |
| NCT00855933 | Lobene Modified Gingival Index (0 to 4) | ‐ |
| NCT01250769 | Modified Gingival Index (0 to 4) Gingival Bleeding Index (0 to 3) |
‐ |
| Rosema 2008 | Bleeding on Marginal Probing Index (0 to 2) | Paraskevas modification of Quigley & Hein Plaque Index (0 to 5) |
| Rosema 2011 | Bleeding on Marginal Probing Index (0 to 2) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Schiff 2006 | Löe & Silness Gingival Index (0 to 3) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Sharma 2002 | Lobene Modified Gingival Index (0 to 4) Ainamo & Bay Gingival Bleeding Index (0/1) | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Smith 1988 | Löe & Silness Gingival Index (0 to 3) | Silness & Löe Plaque Index (0 to 3) |
| Vogel 1975 | Löe & Silness Gingival Index (0 to 3) Intracrevicular exudate sampling |
Podchladley's Total Plaque Index (0/1) |
| Walsh 1985 | Interproximal Bleeding on Probing Index (0/1) evaluated as percentage of bleeding interproximal surfaces | Silness & Löe Plaque Index (evaluated as percentage of interproximal surfaces scored positive for plaque) (0/1) |
| Walsh 1989 | Löe & Silness Gingival Index (0 to 3) Bleeding on probing |
Silness & Löe Plaque Index (0 to 3) |
| Yankell 2002 | Eastman Interdental Bleeding Index (0/1) Lobene Modified Gingival Index (0 to 4) |
Turesky modification of Quigley‐Hein Plaque Index (0 to 5) |
| Yost 2006 | Eastman Interdental Bleeding Index (0/1) Löe & Silness Gingival Index (0 to 3) |
Benson modification of Quigley‐Hein Plaque Index (0 to 5) |
| Zimmer 2006 | Papillary Bleeding Index (1 to 4) | Quigley & Hein Plaque Index (0 to 5) Modified Proximal Plaque Index |
Periodontitis
Six studies measured probing pocket depth (PPD) in mm (Christou 1998; Graziani 2017; Ishak 2007; Jackson 2006; Smith 1988; Walsh 1989), most of which assessed interdental brushes versus floss. Five studies measured PPD at four to six weeks, with Smith 1988 also measuring at eight weeks, and Jackson 2006 at 12 weeks. Walsh 1989 measured at three months and six months, though were unable to use data at six months as participants received professional scale and polish after three months. We were unable to use the data from Smith 1988 and no data were reported from Graziani 2017.
Walsh 1989 also measured attachment loss but did not report results numerically.
Interproximal caries
None of the studies assessed this outcome.
Adverse effects
Adverse effects were measured by self report in five studies: questionnaire in Christou 1998, Ishak 2007 and Jared 2005, and adherence diary in Mwatha 2017 and Yost 2006. They were assessed by an examiner in 17 studies (Bauroth 2003; Biesbrock 2007; Cronin 1997; Cronin 2005; Frascella 2000; Gordon 1996; Goyal 2012; Hague 2007; Imai 2011; Isaacs 1999; Kazmierczak 1994; Meklas 1972; Mwatha 2017; Rosema 2008; Sharma 2002; Walsh 1989; Yost 2006; Zimmer 2006). NCT01250769 measured adverse events systematically but did not specify the method. Four of these studies failed to report their findings in the Results (Bauroth 2003; Jared 2005; Kazmierczak 1994; Yost 2006).
An additional seven studies that had not described how they would measure adverse effects, simply reported that there were no adverse effects (or no adverse effects related to treatment) (Barnes 2005; Frascella 2000; Jackson 2006; NCT00855933; Rosema 2011; Schiff 2006; Yankell 2002).
Seven studies did not mention anything about adverse events (Finkelstein 1990; Graziani 2017; Lewis 2004; Lobene 1982; Smith 1988; Vogel 1975; Walsh 1985).
Halitosis
None of the studies assessed this outcome.
Patient satisfaction
None of the studies assessed this outcome.
Cost of intervention
None of the studies assessed this outcome.
Timing of outcome measurement
Outcomes were most commonly measured in the short term. We did not consider measurements at less than four weeks (Barnes 2005; Goyal 2012; Graziani 2017; Hague 2007; Jared 2005; Kazmierczak 1994; Lewis 2004; Lobene 1982; Meklas 1972; Mwatha 2017; NCT01250769; Rosema 2011; Vogel 1975; Yankell 2002). Most studies measured at one month (Barnes 2005; Biesbrock 2007; Cronin 1997; Cronin 2005; Frascella 2000; Gordon 1996; Goyal 2012; Graziani 2017; Hague 2007; Ishak 2007; Jared 2005; Lobene 1982; Mwatha 2017; NCT00855933; NCT01250769; Rosema 2011; Smith 1988; Vogel 1975; Yankell 2002; Zimmer 2006) or six weeks (Christou 1998; Finkelstein 1990; Imai 2011; Jackson 2006; Kazmierczak 1994; Lewis 2004; Yost 2006). Five studies also measured at two months (Biesbrock 2007; Frascella 2000; Lobene 1982; Smith 1988; Zimmer 2006).
Twelve studies measured medium‐term outcomes: at 10 weeks (Rosema 2008) or three months (Bauroth 2003; Finkelstein 1990; Imai 2011; Isaacs 1999; Jackson 2006; Lewis 2004; Schiff 2006; Sharma 2002; Walsh 1985; Walsh 1989). Meklas 1972 measured at six time points within six months. Six studies also measured outcomes at six months (Bauroth 2003; Isaacs 1999; Rosema 2008; Schiff 2006; Sharma 2002; Walsh 1989) and nine months (Rosema 2008).
No studies measured outcomes in the long term.
We used outcomes from four to six weeks, three months and six months in our analyses.
Data considerations for exploration of heterogeneity
We did not explore heterogeneity through formal subgroup analyses due to there being fewer than 10 studies in all meta‐analyses. Informal analyses did not explain heterogeneity in the analyses.
Age and dentition
For age and dentition, none of the studies were conducted with children or on the deciduous dentition.
Baseline periodontal status
As explained above, it was difficult to categorise the periodontal disease status of participants in the included studies as they did not describe the baseline periodontal status of participants in terms of either the 1999 or 2017 classifications of periodontal diseases (Armitage 1999; Caton 2018), and many of the studies did not provide sufficient detail for the review authors to make that judgement.
Training
Most studies provided some type of training. Eighteen studies used supervised instruction (51%), but there were insufficient studies in any one meta‐analysis to make subgroup analyses meaningful.
Funding
Most studies were funded through manufacturers or grant awards. Details are given in Table 14. Eight studies did not report on funding (Gordon 1996; Imai 2011; Kazmierczak 1994; Lobene 1982; Sharma 2002; Smith 1988; Vogel 1975; Walsh 1985).
4. Details of funding.
|
Cronin 1997 and Isaacs 1999 were supported by Braun AG, Germany (Braun Oral‐B Interclean ID2); Yankell 2002 by Dental Concepts, Paramus, USA (oral hygiene devices); Jackson 2006 and Schiff 2006 by the Colgate Palmolive Company (toothbrushes, floss and toothpaste); Meklas 1972 by the General Electric Company (Aqua Pulse Oral Irrigator); Zimmer 2006 and Ishak 2007 by GlaxoSmithKline (manual toothbrush and floss); Finkelstein 1990 by Johnson & Johnson (waxed floss); Cronin 2005 by Oral‐B (manual toothbrush and flosser); Biesbrock 2007; NCT00855933 and Rosema 2008 by Procter and Gamble (sponsorship) (DE International supplied the toothpaste for Rosema 2008); Yost 2006 and Jared 2005 by Sunstar Inc. (GUM, manual toothbrush); Barnes 2005, Goyal 2012 and Rosema 2011 by Waterpik Inc., Fort Collins, USA (oral irrigator); Hague 2007 by William Getgey Company (ultra‐flosser); and NCT01250769 was sponsored by Philips Oral Healthcare. Walsh 1989 was partially funded by Xouth, Inc, Lancaster, PA, USA. In Bauroth 2003, the authors were affiliated to industry, Pfizer; in Frascella 2000, the authors were affiliated to Braun and Procter and Gamble; and in Mwatha 2017, the authors AM, MO, SS, MW and WJ were employees of Philips (Sonicare Toothbrush). The Italian Ministry of Health and Tuscan region provided a grant to Graziani 2017; the State Scholarship Foundation of Greece grant‐aided Christou 1998 (Entra‐Lactona BV provided brushes and interdental brushes); a University of Tennessee College of Dentistry Alumni Grant was given to Lewis 2004. |
We categorised 24 studies as industry funded (69%), but there were not enough studies in any one meta‐analysis to justify subgroup analysis.
Sensitivity analysis
To assess the robustness of the findings, we conducted sensitivity analyses, as planned, by removing the studies at overall high risk of bias (which did not take into account performance bias, which cannot be avoided in these type of studies), by removing studies with estimated standard deviations, and by removing split‐mouth studies when these had been combined with parallel‐group studies in meta‐analysis (see Table 15). We judged these not to undermine the findings of our main analyses, which are presented in the Effects of interventions section below. It was not necessary to conduct sensitivity analysis removing cross‐over studies as we used only first‐period data from cross‐over studies included in this review.
5. Sensitivity analyses.
| Analysis | Studies removed (and reason) | Result | Consistency with main analysis |
| Comparison 1: floss plus toothbrushing versus toothbrushing only | |||
| 1.1 GI at 1 month | Vogel (high risk of bias relating to poor compliance; estimated standard deviations) | SMD ‐0.61, 95% CI ‐1.19 to ‐0.03; high heterogeneity (I2 = 90%; P value < 0.001); 7 studies, 573 participants | Essentially the same |
| 1.2 GI at 3 months | Barouth (high risk of attrition bias; use of negative control rinse) Sharma (use of negative control rinse) | SMD ‐0.30, 95% CI ‐0.62 to 0.02; no heterogeneity (I2 = 0%; P value = 0.81); 2 studies, 151 participants | Confidence interval is larger and includes possibility of floss providing no additional benefit over toothbrushing |
| 1.3 GI at 6 months | Barouth (high risk of attrition bias and use of negative control rinse) Sharma (use of negative control rinse) | SMD ‐0.55, 95% CI ‐0.91 to ‐0.18; no/low heterogeneity (I2 = 21%; P value = 0.26); 2 studies, 151 participants | Slightly lower estimate, with larger confidence interval |
| 1.5 Bleeding at 3 months | Barouth (high risk of attrition bias; use of negative control rinse) | MD ‐0.26, 95% CI ‐0.36 to ‐0.16; 1 study, 24 participants | Shows clear benefit for floss (main analysis is equivocal) |
| 1.8 Plaque at 3 months | Barouth (high risk of attrition bias; use of negative control rinse) and Sharma (use of negative control rinse) | SMD ‐0.13, 95% CI ‐0.43 to 0.17; no heterogeneity (I2 = 0%; P value = 0.49); 3 studies, 175 participants | Slightly lower estimate, with wider confidence interval that includes the possibility of no difference or slight benefit for toothbrushing only |
| 1.9 Plaque at 6 months | Barouth (high risk of attrition bias; use of negative control rinse) Sharma (use of negative control rinse) | MD ‐0.02, ‐0.11 to 0.07; 1 study, 74 participants | Essentially the same |
| Comparison 6: interdental brush plus toothbrushing versus floss plus toothbrushing | |||
| 6.1 GI at 1 month | Yost (estimated standard deviations) | SMD ‐0.51, 95% CI ‐0.87 to ‐0.15; no/low heterogeneity (I2 = 0%, P value = 0.56); 2 studies, 121 participants | Slightly larger effect, marginally wider confidence interval |
| 6.2 Bleeding at 4 to 6 weeks | Christou, Imai, Ishak (split‐mouth studies) | MD ‐0.10, 95% CI ‐0.15 to ‐0.05; no heterogeneity (I2 = 0%, P value = 0.78); 3 studies, 169 participants | Essentially the same |
| 6.3 Bleeding at 3 months | Imai (split‐mouth study) | MD ‐0.06, 95% CI ‐0.12 to 0.00; 1 study, 77 participants | Essentially the same, though confidence interval includes zero |
| 6.4 Plaque at 1 month | Yost (estimated standard deviations) | SMD ‐0.55, 95% CI ‐1.00 to ‐0.11; moderate heterogeneity (I2 = 62%, P value = 0.05); 4 studies, 228 participants | Essentially the same |
| 6.8 Plaque at 3 months | Imai (split‐mouth study) | MD ‐0.24, 95% CI ‐0.41 to ‐0.07; 1 study, 77 participants | Shows clear benefit for interdental brush (main analysis is equivocal) |
| Comparison 9: rubber/elastomeric cleaning stick plus toothbrushing versus floss plus toothbrushing | |||
| 9.1 GI at 1 month | Vogel (high risk of bias relating to poor compliance; estimated standard deviations) Yost (estimated standard deviations) |
SMD ‐0.37, 95% CI ‐1.07 to 0.34; high heterogeneity (I2 = 80%, P value < 0.002); 4 studies, 183 participants | Slightly bigger point estimate but wider confidence interval; both analyses include all possibilities, i.e. that flossing is better or that it gives no benefit or that it is worse than toothbrushing only |
| 9.5 Plaque at 1 month | Yost (estimated standard deviations) | SMD ‐0.09, 95% CI ‐0.57 to 0.39; high heterogeneity (I2 = 65%, P value = 0.02); 5 studies, 212 participants | Essentially the same |
| Comparison 11: interdental cleaning stick plus toothbrushing versus interdental brush plus toothbrushing | |||
| 11.3 Plaque at 1 month | Yost (estimated standard deviations) | MD 0.01, 95% CI ‐0.08 to 0.09; 1 study, 31 participants | Essentially the same |
CI: confidence interval GI: gingivitis index MD: mean difference SMD: standardised mean difference
Excluded studies
After having screened 138 full texts of the studies, we rejected 42 outright, and explained the reasons for our decision in the case of 59 records. These reasons are presented in the Characteristics of excluded studies tables.
Risk of bias in included studies
Allocation
Only four studies were at low risk of selection bias (Frascella 2000; Graziani 2017; Imai 2011; Zimmer 2006).
Random sequence generation
Ten studies adequately generated the allocation sequence (Frascella 2000; Gordon 1996; Graziani 2017; Hague 2007; Imai 2011; Ishak 2007; Jackson 2006; Lewis 2004; Rosema 2008; Rosema 2011; Zimmer 2006). The rest were unclear as the reports did not provide any details of how the randomisation was performed.
Allocation concealment
Five studies adequately concealed allocation (Christou 1998; Frascella 2000; Graziani 2017; Imai 2011; Zimmer 2006). The rest were unclear as reports did not mention any attempt to conceal allocation.
Blinding
Performance bias
We assessed all included studies as being at high risk of bias as participants were not described as blinded, and would not have been blinded if they had consented to participate in the study.
Detection bias
We assessed 22 studies as being at low risk of bias as examiners did not know which group participants had been allocated to.
We did not assess any of the studies as being at high risk of detection bias; however, we considered 13 studies to be unclear as there was either no specific report on how the blinding of outcome assessors was carried out or blinding of outcome assessors was not mentioned (Barnes 2005; Finkelstein 1990; Gordon 1996; Isaacs 1999; Kazmierczak 1994; Meklas 1972; Mwatha 2017; NCT00855933; NCT01250769; Smith 1988; Vogel 1975; Yankell 2002; Yost 2006).
Incomplete outcome data
We assessed 24 studies as being at low risk of bias. We judged 10 studies to be unclear (Christou 1998; Frascella 2000; Isaacs 1999; Kazmierczak 1994; Lewis 2004; Lobene 1982; Meklas 1972; Smith 1988; Vogel 1975; Walsh 1989). We considered Bauroth 2003 to be at high risk of attrition bias as participants were excluded from analysis based on poor compliance, and the numbers per group were not reported.
Selective reporting
We judged 24 studies to be at low risk of outcome reporting bias as they reported their planned or expected outcomes (Barnes 2005; Christou 1998; Cronin 1997; Cronin 2005; Frascella 2000; Gordon 1996; Goyal 2012; Graziani 2017; Hague 2007; Imai 2011; Isaacs 1999; Ishak 2007; Jackson 2006; Lobene 1982; Meklas 1972; Mwatha 2017; NCT00855933; NCT01250769; Rosema 2008; Rosema 2011; Schiff 2006; Walsh 1989; Yankell 2002; Zimmer 2006).
Where studies mentioned adverse effects in their Methods section but did not report any findings, we judged the risk of reporting bias as unclear (Bauroth 2003, Biesbrock 2007; Jared 2005, Kazmierczak 1994; Walsh 1985; Yost 2006): Jared 2005 used diaries to collect data on possible adverse effects, and there were oral tissue assessments in Bauroth 2003, Kazmierczak 1994 and Yost 2006. Biesbrock 2007 performed assessments of oral tissue and reported that no participants were lost due to adverse events, but provided no information on whether there were any adverse events. We assessed Walsh 1985 as unclear because they used a continuous measure but interpreted it as binary.
We assessed five studies as being at high risk of outcome reporting bias: three did not report standard deviations (Finkelstein 1990; Lewis 2004; Vogel 1975); Sharma 2002 did not report means and standard deviations for bleeding outcomes; the graphs in Smith 1988 were drawn with insufficient accuracy (and no standard deviations) to use the data.
Other potential sources of bias
We assessed six studies to be at low risk of any other potential risks of bias (Frascella 2000; Hague 2007; Imai 2011; Ishak 2007; Walsh 1989; Zimmer 2006).
We considered 28 studies to be unclear in terms of their risk of other potential sources of bias as compliance was not mentioned, not assessed, or not adequately reported (Barnes 2005; Bauroth 2003; Biesbrock 2007; Christou 1998; Cronin 1997; Cronin 2005; Finkelstein 1990; Gordon 1996; Goyal 2012; Graziani 2017; Isaacs 1999; Jackson 2006; Jared 2005; Kazmierczak 1994; Lewis 2004; Lobene 1982; Meklas 1972; Mwatha 2017; NCT00855933; NCT01250769; Rosema 2008; Rosema 2011; Schiff 2006; Sharma 2002; Smith 1988; Walsh 1985; Yankell 2002; Yost 2006). In addition, Cronin 1997 and Rosema 2008 had imbalances in baseline values between the intervention groups.
We judged Vogel 1975 to be at high risk of other bias due to poor compliance in one of the study groups.
Overall bias
Aside from performance bias, which was high risk in all of these studies, we judged two studies be at low risk of bias overall (Imai 2011; Zimmer 2006). We considered 27 studies to be unclear (Barnes 2005; Biesbrock 2007; Christou 1998; Cronin 1997; Cronin 2005; Frascella 2000; Gordon 1996; Goyal 2012; Graziani 2017; Hague 2007; Isaacs 1999; Ishak 2007; Jackson 2006; Jared 2005; Kazmierczak 1994; Lobene 1982; Meklas 1972; Mwatha 2017; NCT00855933; NCT01250769; Rosema 2008; Rosema 2011; Schiff 2006; Walsh 1985; Walsh 1989; Yankell 2002; Yost 2006), and six to be at high risk of bias (Bauroth 2003; Finkelstein 1990; Lewis 2004; Sharma 2002; Smith 1988; Vogel 1975).
See Figure 2 below for a summary of the risk of bias for each included study.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Comparison 1: Toothbrushing plus flossing versus toothbrushing alone (control)
Fifteen studies compared toothbrushing plus flossing with toothbrushing alone (Bauroth 2003; Biesbrock 2007; Finkelstein 1990; Graziani 2017; Hague 2007; Jared 2005; Lobene 1982; Mwatha 2017; Rosema 2008; Schiff 2006; Sharma 2002; Vogel 1975; Walsh 1985; Zimmer 2006). We assessed four studies as being at high risk of bias and one at low risk of bias. In 10 studies, the risk of bias was unclear. One study used an automated flosser (Biesbrock 2007). Two studies used a 'negative control placebo rinse' (Bauroth 2003; Sharma 2002). Further details of the studies included in this comparison are shown in Table 16. See also Table 1.
6. Comparison 1 Flossing versus toothbrushing: included study details.
|
Study (parallel group design unless otherwise noted) |
Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Bauroth 2003 | High | Interproximal | Lobene Modified Interproximal Gingival Index (0 to 4) | Final score at 3 and 6 months | Final score at 3 and 6 months | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 3 and 6 months | N/R | Adverse events were assessed, but not reported. Performed soft‐tissue assessments at baseline, 3, and 6 months. |
| Biesbrock 2007 | Unclear | With other sites | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | ‐ | Navy Plaque Index (Rustogi modification) (0 /1) | Final score at 1 month | N/R | Reported mild gingival inflammation in the flossing plus toothbrushing group, which was resolved after few days and was not a reason to drop out |
| Finkelstein 1990 | High | Interproximal data presented for gingivitis and other sites for plaque | Löe & Silness Gingival Index modified to include visual assessment only (0 to 3) | No SDs and unable to estimate | ‐ | Global Plaque Index (0 to 100%) | No SDs and unable to estimate | N/R | Did not consider adverse effects |
| Graziani 2017 | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 1 month | Mentioned as outcome but no data reported | Did not consider adverse effects |
|
Hague 2007 (crossover design but we used only first‐period data ‐ see Characteristics of included studies for details) |
Unclear | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | ‐ | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Safety assessments were performed at each visit. Overall, two out of 76 participants enrolled in the study, both in the automated flosser group, presented with trauma of the attached gingiva in the oral or buccal areas of the posterior teeth at the second visit resulting from improper use of the flosser. |
| Jared 2005 | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | Final score at 1 month | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Adverse events were assessed, but not reported. Participants were issued a diary to keep a log of any symptoms experienced. However, no data regarding adverse events were reported in Results. |
| Lobene 1982 | Unclear | With other sites | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | ‐ | Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Did not consider adverse effects |
| Mwatha 2017 | Unclear | With other sites | Russel Modified Gingival Index (0 to 2) | Final score at 1 month | Final score at 1 month | Navy Plaque Index (Rustogi modification) (0/1 for each of the nine tooth surfaces) | Final score at 1 month | N/R | Safety assessments were carried out by clinical examinations and by evaluating participants' diary cards. Three gingival irritations and one case of gum soreness were reported in the flossing group. |
| NCT00855933 | Unclear | With other sites | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | ‐ | N/R | ‐ | N/R | None identified |
| Rosema 2008 | Unclear | With other sites | Bleeding on Marginal Probing Index (0 to 2) | Final score at 3 and 6 months | ‐ | Paraskevas modification of Quigley & Hein Plaque Index (0 to 5) | Final score at 3 months | N/R | No adverse effects on the oral hard or soft tissues observed by the examiner or reported by the participants. Used two indices to assess possible adverse effects and found no statistically significant difference in either staining or abrasion between the flossing and toothbrushing only groups at 10 weeks, 6 months and 9 months (P < 0.05). |
| Schiff 2006 | Unclear | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 3 and 6 months | ‐ | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 3 and 6 months | N/R | No adverse effects on the oral hard or soft tissues observed by the examiner or reported by the participants |
| Sharma 2002 | High | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 3 and 6 months | ‐ | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 3 and 6 months | N/R | No adverse effects on the oral hard or soft tissues observed by the examiner or reported by the participants |
| Vogel 1975 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month Imputed SD from control group of studies using this index | ‐ | Podchladley's Total Plaque Index (0/1) | Unable to impute SD for this index | N/R | Did not consider adverse effects |
| Walsh 1985 | Unclear | Interproximal | ‐ | ‐ | Final score at 3 months | Silness & Löe Plaque Index (evaluated as percentage of interproximal surfaces scored positive for plaque) (0/1) | Final score at 3 months | N/R | Did not consider adverse effects |
| Zimmer 2006 | Low | Interproximal | Papillary Bleeding Index (1 to 4) | Final score at 1 month | ‐ | Quigley & Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Participants reported mild gingival abrasions in three out of 39 participants at 1‐month time point, and in one of 39 participants at 2 months. In the toothbrush‐only arm, 1 in 39 participants at 1‐month time point reported discomfort in taste and bleeding of gingiva, respectively. No side effects were reported at 2‐month time point |
N/R: not reported SD: standard deviation
Gingivitis (Gingival Index)
Low‐certainty evidence suggested that flossing in addition to toothbrushing reduced gingivitis at one, three, and six months in comparison with toothbrushing alone (Table 1). The standardised mean difference (SMD) at one month was ‐0.58 (95% confidence interval (CI) ‐1.12 to ‐0.04; 8 trials, 585 participants; Analysis 1.1). There was substantial heterogeneity between the studies (I2 = 89%, P < 0.001). At three months, the SMD was ‐0.33 (95% CI ‐0.50 to ‐0.17; 4 trials, 570 participants; no heterogeneity; Analysis 1.2). At six months, the SMD was ‐0.68 (95% CI ‐0.95 to ‐0.42; 4 trials, 564 participants; moderate heterogeneity (I2 = 55%, P = 0.09); Analysis 1.3).
1.1. Analysis.
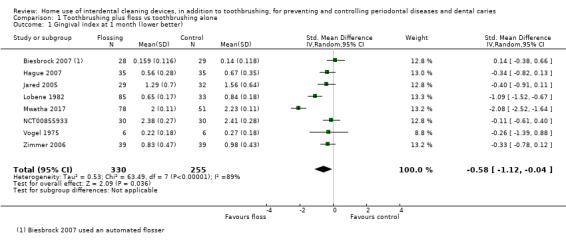
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 1 Gingival index at 1 month (lower better).
1.2. Analysis.
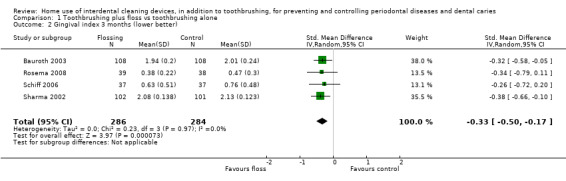
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 2 Gingival index 3 months (lower better).
1.3. Analysis.
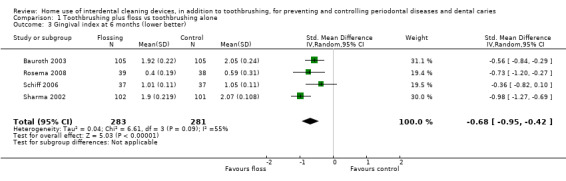
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 3 Gingival index at 6 months (lower better).
Gingivitis (proportion of bleeding sites)
Bauroth 2003, Graziani 2017, Mwatha 2017 and Walsh 1985 measured the proportion of bleeding sites. There was very low‐certainty evidence of no significant difference between flossing and toothbrushing only groups at one month (MD ‐0.03, 95% CI ‐0.14 to 0.08; 2 trials, 158 participants; substantial heterogeneity (I2 = 83%, P = 0.01); Analysis 1.4), or three months (MD ‐0.14, 95% CI ‐0.37 to 0.09; 2 trials, 240 participants; substantial heterogeneity (I2 = 95%, P < 0.001); Analysis 1.5). At six months, one trial at high risk of bias found a small difference in favour of flossing (MD ‐0.06, 95% CI ‐0.09 to ‐0.03; 210 participants; very low‐certainty evidence; Analysis 1.6).
1.4. Analysis.
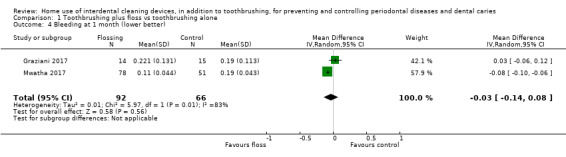
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 4 Bleeding at 1 month (lower better).
1.5. Analysis.
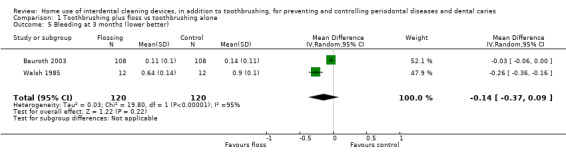
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 5 Bleeding at 3 months (lower better).
1.6. Analysis.

Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 6 Bleeding at 6 months (lower better).
Periodontitis
Graziani 2017 measured periodontitis but no data were reported.
Interproximal caries
No studies reported interproximal caries.
Plaque
Fourteen studies reported plaque data. We were unable to use the data from two studies that did not report standard deviations (Finkelstein 1990; Vogel 1975).
The pooled estimate at one month showed very low‐certainty evidence of a possible small benefit for flossing plus toothbrushing (SMD ‐0.42, 95% CI ‐0.85 to 0.02; seven trials, 542 participants; P = 0.06), with substantial heterogeneity (I2 = 83%, P < 0.0001; Analysis 1.7). Very low‐certainty evidence of a possible benefit for flossing was found at the three‐month time point (SMD ‐0.20, 95% CI ‐0.36 to ‐0.04; 5 trials, 594 participants), with no evidence of heterogeneity (I2 = 0%, P = 0.74; Analysis 1.8); however, we were unable to claim a benefit for flossing plus toothbrushing at six months (SMD ‐0.13, 95% CI ‐0.30 to 0.05; P = 0.53; 3 trials, 487 participants; no heterogeneity; Analysis 1.9).
1.7. Analysis.
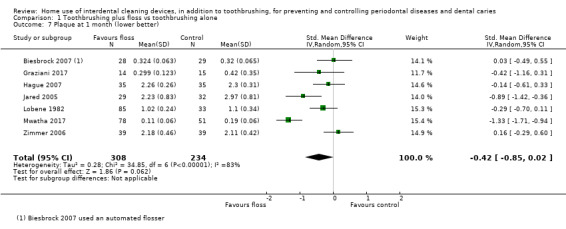
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 7 Plaque at 1 month (lower better).
1.8. Analysis.
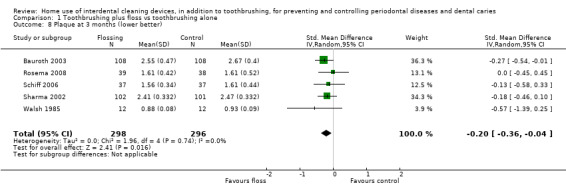
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 8 Plaque at 3 months (lower better).
1.9. Analysis.
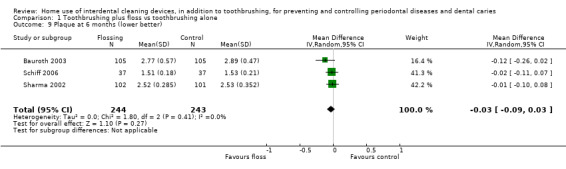
Comparison 1 Toothbrushing plus floss vs toothbrushing alone, Outcome 9 Plaque at 6 months (lower better).
Adverse effects
Overall, there were no serious adverse events reported for this comparison. Details about adverse events are described in Table 16.
Secondary outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 2: Toothbrushing plus interdental brushing versus toothbrushing alone
Two studies compared toothbrushing plus using an interdental brush with toothbrushing alone (Graziani 2017; Jared 2005), and reported data at one month. Both were at unclear risk of bias. The details of the studies included in this comparison are shown in Table 17. See also Table 2.
7. Comparison 2 Interdental brush versus toothbrushing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Graziani 2017 | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 1 month | Mentioned as outcome but no data reported | Did not consider adverse effects |
| Jared 2005 | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | ‐ | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Adverse events were assessed, but not reported. Participants were issued a diary to keep a log of any symptoms experienced. However, no data regarding adverse events were reported in Results. |
N/R: not reported
See Table 2.
Gingivitis (Gingival Index)
There was very low‐certainty evidence that interdental brushes reduced gingivitis compared to toothbrushing alone at one month (MD ‐0.53, 95% CI ‐0.83 to ‐0.23; 1 trial, 62 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2 Toothbrushing plus interdental brush versus toothbrushing alone, Outcome 1 Gingival index at 1 month.
Gingivitis (proportion of bleeding sites)
There was very low‐certainty evidence that interdental brushes did not reduce proportion of bleeding sites more than toothbrushing alone (one‐month MD ‐0.05, 95% CI ‐0.13 to 0.03; 1 trial, 31 participants; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2 Toothbrushing plus interdental brush versus toothbrushing alone, Outcome 2 Bleeding at 1 month.
Periodontitis
Graziani 2017 measured periodontitis but no data were reported.
Interproximal caries
Neither study reported interproximal caries.
Plaque
There was low‐certainty evidence that interdental brushes reduced plaque compared to toothbrushing alone at one month (SMD ‐1.07, 95% CI ‐1.58 to ‐0.69; 2 trials, 93 participants; Analysis 2.3). There was no evidence of heterogeneity (I2 = 0%, P = 0.48).
2.3. Analysis.
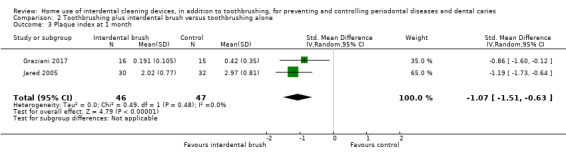
Comparison 2 Toothbrushing plus interdental brush versus toothbrushing alone, Outcome 3 Plaque index at 1 month.
Adverse effects
Graziani 2017 did not report on adverse effects. In Jared 2005, each participant was given a diary to keep a log of any symptoms experienced; however, data concerning adverse events were not reported in Results.
Secondary outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 3: Toothbrushing plus use of wooden cleaning sticks versus toothbrushing alone
Two studies made this comparison (Finkelstein 1990; Walsh 1985); however, we were only able to use the data from Walsh 1985 as Finkelstein 1990 did not provide standard deviations. Walsh 1985, which was at unclear risk of bias, measured outcomes at three months. The details of the studies included in this comparison are shown in Table 18. See also Table 3.
8. Comparison 3 Wooden cleaning stick versus toothbrushing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Finkelstein 1990 | High | Interproximal data presented for gingivitis and other sites for plaque | Löe & Silness Gingival Index modified to include visual assessment only (0 to 3) | None ‐ no SDs and unable to estimate | ‐ | Global Plaque Index (0 to 100%) | None ‐ no SDs and unable to estimate | N/R | Did not consider adverse effects |
| Walsh 1985 | Unclear | Interproximal | ‐ | ‐ | Final score at 3 months | Silness & Löe Plaque Index (evaluated as percentage of interproximal surfaces scored positive for plaque) (0/1) | Final score at 3 months | N/R | Did not consider adverse effects |
N/R: not reported
Gingivitis (Gingival Index)
We were unable to use Finkelstein 1990 data, and Walsh 1985 did not measure this.
Gingivitis (proportion of bleeding sites)
There was very low‐certainty evidence to claim a benefit for wooden cleaning sticks in reducing proportion of bleeding sites compared to toothbrushing alone at three months (MD (mean proportion of bleeding sites) ‐0.25, 95% CI ‐0.37 to ‐0.13; 1 trial, 24 participants; Analysis 3.1). This was the only time point providing useable data.
3.1. Analysis.
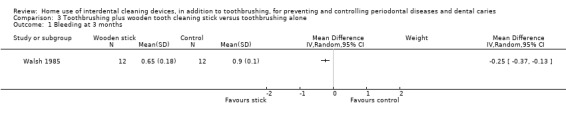
Comparison 3 Toothbrushing plus wooden tooth cleaning stick versus toothbrushing alone, Outcome 1 Bleeding at 3 months.
Periodontitis
No studies reported periodontitis.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was very low‐certainty evidence that wooden cleaning sticks did not reduce plaque more than toothbrushing alone (MD (mean proportion of sites with plaque) ‐0.03, 95% CI ‐0.13 to 0.07; 1 trial, 24 participants; Analysis 3.2). This was the only time point providing useable data.
3.2. Analysis.

Comparison 3 Toothbrushing plus wooden tooth cleaning stick versus toothbrushing alone, Outcome 2 Plaque Index at 3 months.
Adverse events
Neither of the studies assessing this comparison reported on adverse effects .
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 4: Toothbrushing plus use of rubber/elastomeric cleaning sticks versus toothbrushing alone
Two studies made this comparison (Graziani 2017; Vogel 1975), one at unclear and one at high risk of bias. The details of the studies included in this comparison are shown in Table 19. See also Table 4.
9. Comparison 4 Rubber/elastomeric toothcleaning sticks versus toothbrushing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Graziani 2017 | Unclear | Interproximal | ‐ | ‐ | Final score at one month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 1 month | Mentioned as outcome but no data reported | Did not consider adverse effects |
| Vogel 1975 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at one month | ‐ | Podchladley's Total Plaque Index (0/1) | Unable to impute for index | N/R | Did not consider adverse effects |
N/R: not reported
Gingivitis (Gingival Index)
There was no evidence that rubber/elastomeric cleaning sticks reduced plaque at one month (MD ‐0.01, 95% CI ‐0.19 to 0.21; 1 trial, 12 participants; Analysis 4.1) (very low‐certainty evidence). This was the only time point reporting data.
4.1. Analysis.

Comparison 4 Toothbrushing plus rubber/elastomeric tooth cleaning stick versus toothbrushing alone, Outcome 1 Gingival Index at 1 month.
Gingivitis (proportion of bleeding sites)
There was no evidence that rubber/elastomeric cleaning sticks reduced proportion of bleeding sites at one month (MD ‐0.07, 95% CI ‐0.15 to 0.01; 1 trial, 30 participants; Analysis 4.2) (very low‐certainty evidence). This was the only time point reporting data.
4.2. Analysis.
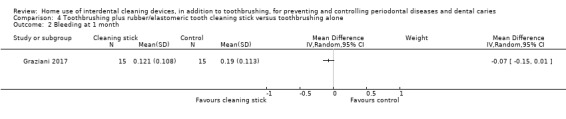
Comparison 4 Toothbrushing plus rubber/elastomeric tooth cleaning stick versus toothbrushing alone, Outcome 2 Bleeding at 1 month.
Periodontitis
No studies reported periodontitis.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was very low‐certainty evidence that wooden cleaning sticks reduced plaque at one month: MD (full mouth plaque score) ‐0.22, 95% CI ‐0.41 to ‐0.03; 1 trial, 30 participants; Analysis 4.3). This was the only time point providing useable data.
4.3. Analysis.
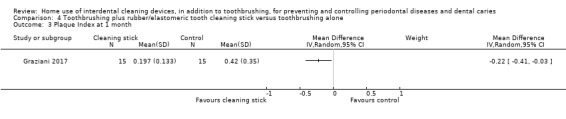
Comparison 4 Toothbrushing plus rubber/elastomeric tooth cleaning stick versus toothbrushing alone, Outcome 3 Plaque Index at 1 month.
Adverse events
Neither study reported on adverse effects (Graziani 2017; Vogel 1975).
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 5: Toothbrushing plus oral irrigation versus toothbrushing alone
Five studies, all at unclear risk of bias, compared toothbrushing plus oral irrigation versus toothbrushing alone (Frascella 2000; Goyal 2012; Meklas 1972; NCT01250769; Walsh 1989). The details of the studies included in this comparison are shown in Table 20. See also Table 5.
10. Comparison 5 Oral irrigation versus toothbrushing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Frascella 2000 | Unclear | With other sites | Modified gingival index (0 to 3) | Final score at 1 month | Final score at 1 month | Turesky‐Gilmore‐Glickman modification of the Quigley‐Hein Plaque Index | Final score at 1 month | N/R | Safety mentioned and "no problems" reported; not clear how this was assessed |
| Goyal 2012 | Unclear | Interproximal for plaque; other sites for gingivitis | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | Final score at 1 month | Navy Plaque Index (Rustogi modification) (0/1 for each of the nine tooth surfaces) | Final score at 1 month | N/R | Reported that there were no adverse effects |
| Meklas 1972 | Unclear | With other sites | Russell modified Periodontal Index (0 to 2) | Final score at 1, 3, and 6 months | ‐ | 3‐point plaque index (0 to 2) | Final score at 1, 3 and 6 months | N/R | Reported adverse events in terms of oral lacerations, with no significant difference between the study arms (toothbrushing and oral irrigation 8/55; toothbrushing only 7/54) |
| NCT01250769 | Unclear | With other sites | Modified Gingival Index (0 to 4) | Final score at 1 month | ‐ | N/R | ‐ | N/R | 1 serious (arm deep vein thrombosis) in Gp C ‐ unrelated to treatment, and 1 minor in Gp D ‐ aphthous ulcer above tooth #7 on attached gingiva |
| Walsh 1989 | Unclear | With other sites | Löe & Silness Gingival Index (0 to 3) | Final score at 3 months (6‐month data not used) | Final score at 3 months (6‐month data not used) | Silness & Löe Plaque Index (visible plaque or not ‐ 0, 1) | Final score at 3 months (6‐month data not used) |
‐ | No injury to hard or soft tissues. No soft tissue changes. |
N/R: not reported
Gingivitis (Gingival Index)
Goyal 2012 and NCT01250769 provided gingivitis data for one month, Frascella 2000 for one and two months, Meklas 1972 for one, two, three, four, five, six months, and Walsh 1989 for three months. The meta‐analysis for one month indicated that the water irrigator may reduce gingivitis (SMD ‐0.48, 95% CI ‐0.89 to ‐0.06; 4 trials, 380 participants; Analysis 5.1). There was substantial heterogeneity (I2 = 73%, P value = 0.01). At three and six months, there was no significant difference between groups (3‐month SMD ‐0.13, 95% CI ‐0.44 to 0.17, 2 trials, 163 participants; no heterogeneity; Analysis 5.2; 6‐month MD ‐0.33, 95% CI ‐0.74 to 0.08, 1 trial, 109 participants; Analysis 5.3). The evidence was very low‐certainty.
5.1. Analysis.
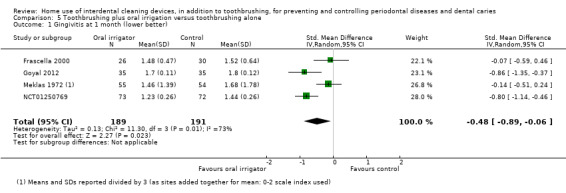
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 1 Gingivitis at 1 month (lower better).
5.2. Analysis.
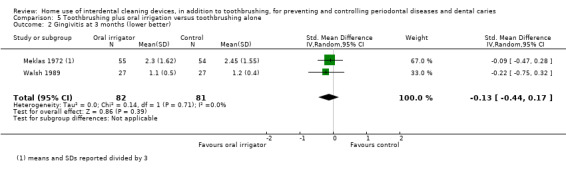
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 2 Gingivitis at 3 months (lower better).
5.3. Analysis.
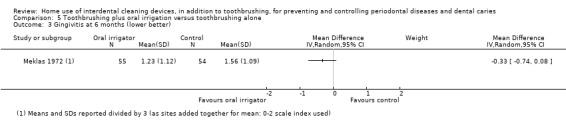
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 3 Gingivitis at 6 months (lower better).
Gingivitis (proportion of bleeding sites)
The mean score in the oral irrigation group was the same as the toothbrushing‐only group at one month (MD ‐0.00, 95% CI ‐0.07 to 0.06; 2 trials, 126 participants; moderate heterogeneity (I2 = 48%, P = 0.16); Analysis 5.4) (low‐certainty evidence). At three months, the MD was ‐0.04 (95% CI ‐0.13 to 0.05, 1 trial, 54 participants) (Analysis 5.5).
5.4. Analysis.
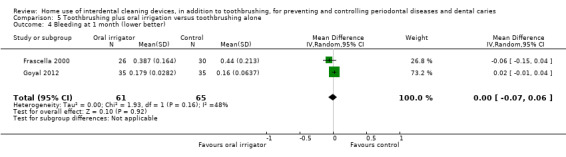
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 4 Bleeding at 1 month (lower better).
5.5. Analysis.
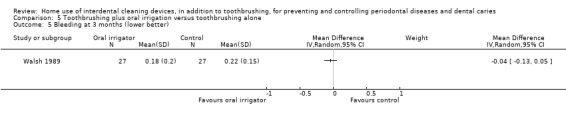
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 5 Bleeding at 3 months (lower better).
Periodontitis
Walsh 1989 reported the proportion of sites with > 4 mm pocket depth at three months, but not mean PD measurements. Walsh 1989 also measured attachment loss, but did not provide data. The authors stated that, "there was essentially no change in attachment loss in any of the groups during the experimental period".
Interproximal caries
No studies reported interproximal caries.
Plaque
Goyal 2012 provided plaque data for one month, Frascella 2000 provided data for one and two months, Meklas 1972 provided data for one, two, three, four, five, six months and Walsh 1989 provided data at three and six months. The meta‐analysis for one month indicated no evidence that the use of the oral irrigator reduced plaque more than toothbrushing alone (SMD ‐0.16, 95% CI ‐0.41 to 0.10; 3 trials, 235 participants; no heterogeneity; Analysis 5.6). There was also no evidence of a change in plaque at three months (SMD 0.06, ‐0.25 to 0.37; 2 trials, 163 participants; no heterogeneity; Analysis 5.7) or six months (MD 0.22, ‐0.59 to 0.15; 1 trial, 109 participants; Analysis 5.8). The certainty of the evidence was low.
5.6. Analysis.
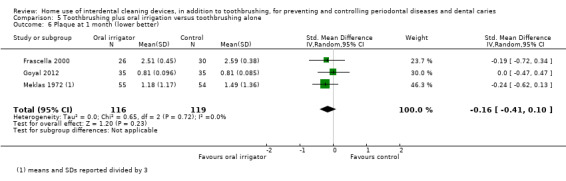
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 6 Plaque at 1 month (lower better).
5.7. Analysis.
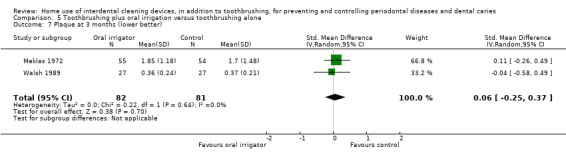
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 7 Plaque at 3 months (lower better).
5.8. Analysis.
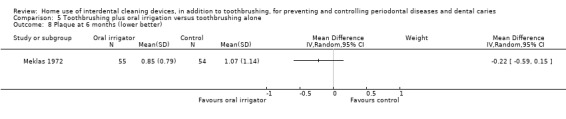
Comparison 5 Toothbrushing plus oral irrigation versus toothbrushing alone, Outcome 8 Plaque at 6 months (lower better).
Adverse events
Some participants in both groups in Meklas 1972 had oral lacerations (with no difference between intervention arms). Frascella 2000, Goyal 2012 and Walsh 1989 reported that there were no adverse events. NCT01250769 found one serious event (arm deep vein thrombosis) that was unrelated to treatment, and one minor event in one of the oral irrigator groups, an aphthous ulcer. See Table 20.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 6: Interdental brush versus floss
Nine studies compared toothbrushing plus use of an interdental brush with toothbrushing plus flossing (Christou 1998; Graziani 2017; Imai 2011; Ishak 2007; Jackson 2006; Jared 2005; Smith 1988; Yankell 2002; Yost 2006). Yankell 2002 used an automated flosser. We included the six‐week data from Yost 2006 in the one‐month analysis. Imai 2011 was at low risk of bias; Smith 1988 was at high risk; and the rest were unclear. We were unable to use the data from Smith 1988. We analysed the parallel‐group and split‐mouth studies (Christou 1998; Imai 2011; Ishak 2007) separately when using SMD. The details of the studies included in this comparison are shown in Table 21. See also Table 6.
11. Comparisons 6 Interdental brush versus flossing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Graziani 2017 | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 1 month | Mentioned PPD in mm as outcome but no data reported | Did not consider adverse effects |
| Jackson 2006 | Unclear | Interproximal | ‐ | ‐ | Final score at 1 and 3 months | Silness & Löe Plaque Index (0 to 3) | Final score at 1 and 3 months | PPD in mm | No adverse effects observed or reported during the study in either group |
| Jared 2005 | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | ‐ | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Adverse events were assessed, but not reported. Participants were issued a diary to keep a log of any symptoms experienced. However, no data regarding adverse events were reported in Results. |
| Smith 1988 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Unable to use data | ‐ | Silness & Löe Plaque Index (0 to 3) | Unable to use data | PPD in mm but unable to use data | Did not consider adverse effects |
| Yankell 2002 | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | Final score at 1 month | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | ‐ | Study reported "There were no untoward side effects, reported or observed, at any time during the study, attributed to any of the dental products distributed in this study." |
| Yost 2006 | Unclear | Interproximal | Eastman Interdental Bleeding Index (0/1) | Final score at 1 month | ‐ | Benson modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Examinations of the oral soft tissue were performed at the final visit, but were not reported. |
| Christou 1998 (split‐mouth design) | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Volpe modification of Quigley and Hein Plaque Index (0 to 5) | Final score at 1 month | PPD in mm | Participants reported significantly more problems when using the floss than IDB. The most common problem was difficulty in flossing posterior areas of mouth. |
| Imai 2011 (split‐mouth design) | Low | Interproximal | ‐ | ‐ | Final score at 1 and 3 months | Silness & Löe Plaque Index (0 to 3) | Final score at 1 and 3 months | N/R | No adverse effects observed or reported during the study in either group |
| Ishak 2007 (split‐mouth design) | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Visible plaque deposits were scored as positive | Final score at 1 month | PPD in mm | Participants encountered problems with both interventions. The IDBs tended to bend, buckle and distort, whereas floss got stuck between teeth and was thought to cause soreness. |
N/R: not reported PPD: pocket probing depth
Gingivitis (Gingival Index)
There was low‐certainty evidence of a reduction in gingivitis at one month in the parallel‐group studies when interdental brushes were used rather than floss (SMD ‐0.40, 95% CI ‐0.70 to ‐0.11; 3 trials, 183 participants; no heterogeneity; Analysis 6.1).
6.1. Analysis.
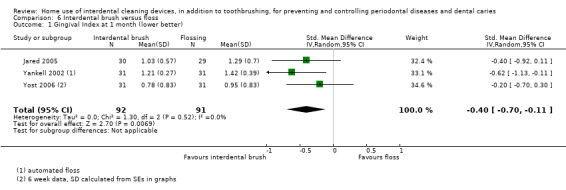
Comparison 6 Interdental brush versus floss, Outcome 1 Gingival Index at 1 month (lower better).
Gingivitis (proportion of bleeding sites)
There was low‐certainty evidence of a reduction in bleeding sites at four to six weeks when interdental brushes were used rather than floss (MD ‐0.06, 95% CI ‐0.08 to ‐0.03; 6 trials (3 parallel and 3 split‐mouth), 234 participants; Analysis 6.2). There was moderate heterogeneity (I2 = 41%, P = 0.13).
6.2. Analysis.
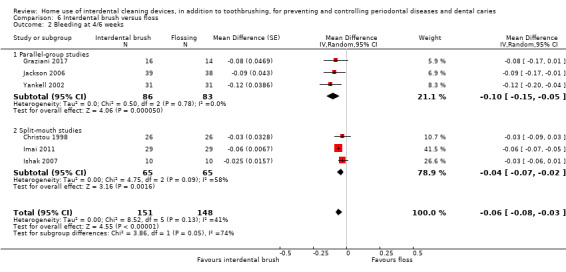
Comparison 6 Interdental brush versus floss, Outcome 2 Bleeding at 4/6 weeks.
At three months, low‐certainty evidence from the combined results of one parallel‐group study (Jackson 2006) and one split‐mouth study (Imai 2011) also indicated a possible benefit for interdental brushes (MD ‐0.10, 95% CI ‐0.15 to ‐0.04); 2 trials, 106 participants; moderate heterogeneity (I2 = 69%, P = 0.07); Analysis 6.3).
6.3. Analysis.
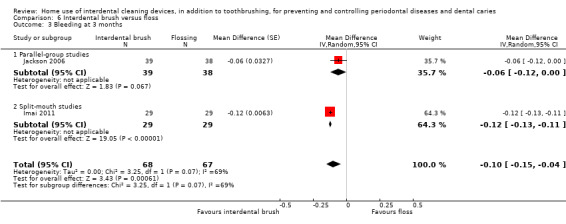
Comparison 6 Interdental brush versus floss, Outcome 3 Bleeding at 3 months.
Periodontitis
Five studies measured mean probing pocket depth scores (PPD) in mm (Christou 1998; Graziani 2017; Ishak 2007; Jackson 2006; Smith 1988). We were unable to use the data presented from Smith 1988, and data were not presented for Graziani 2017. Graziani 2017 stated there was no evidence of a difference in PPD measurements between the interdental brush and floss groups. There was no evidence of a difference between interdental brushes and floss with respect to mean PPD at four to six weeks (MD ‐0.06, 95% CI ‐0.27 to 0.16; 3 trials, 107 participants; no heterogeneity; Analysis 6.4) (low‐certainty evidence). One parallel‐group study also presented 12‐week data for PPD (MD 0.01 mm, 95% CI ‐0.29 to 0.31, 77 participants; Analysis 6.5), which provided no evidence of a difference (very low‐certainty evidence).
6.4. Analysis.
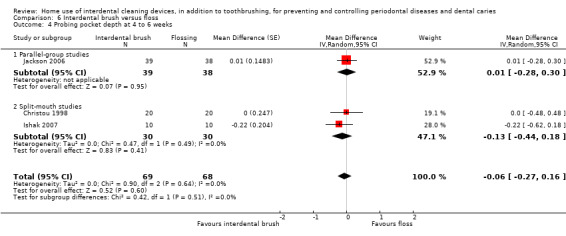
Comparison 6 Interdental brush versus floss, Outcome 4 Probing pocket depth at 4 to 6 weeks.
6.5. Analysis.
Comparison 6 Interdental brush versus floss, Outcome 5 Probing pocket depth at 12 weeks.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was very low‐certainty evidence of a reduction in plaque at one month in the parallel‐group studies when interdental brushes were used (SMD ‐0.47, 95% CI ‐0.84 to ‐0.11; 5 trials, 290 participants; moderate heterogeneity (I2 = 57%, P = 0.05); Analysis 6.6). This finding, however, was not supported by the data from the three split‐mouth studies (SMD ‐0.07, 95% CI ‐0.32 to 0.18; substantial heterogeneity (I2 = 90%, P < 0.001; Analysis 6.7), nor from the data available for three months (MD ‐0.12, 95% ‐0.33 to 0.10; 2 trials, 106 participants; substantial heterogeneity (I2 = 80%, P = 0.02); Analysis 6.8).
6.6. Analysis.
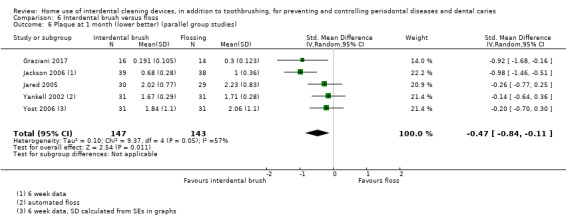
Comparison 6 Interdental brush versus floss, Outcome 6 Plaque at 1 month (lower better) (parallel group studies).
6.7. Analysis.
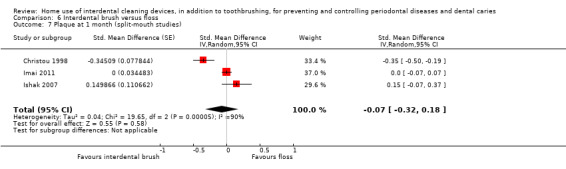
Comparison 6 Interdental brush versus floss, Outcome 7 Plaque at 1 month (split‐mouth studies).
6.8. Analysis.
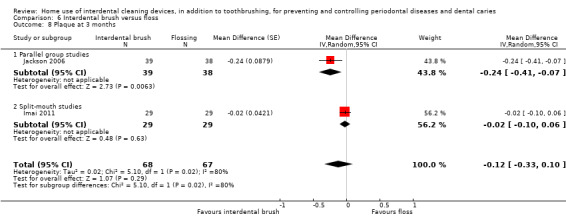
Comparison 6 Interdental brush versus floss, Outcome 8 Plaque at 3 months.
Adverse events
Six studies reported on adverse events, none of which identified clinical problems. Two reported difficulties using the devices. Further details are described in Table 21.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 7: Wooden cleaning stick versus floss
Three studies made this comparison (Finkelstein 1990; Lewis 2004; Walsh 1985); however, we were only able to use the data from Walsh 1985 as Finkelstein 1990 and Lewis 2004 did not provide standard deviations. The details of the studies included in this comparison are shown in Table 22. See also Table 7.
12. Comparison 7 Wooden cleaning sticks versus flossing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Finkelstein 1990 | High | Interproximal data presented for gingivitis and other sites for plaque | Löe & Silness Gingival Index modified to include visual assessment only (0 to 3) | No SDs and unable to estimate | ‐ | Global Plaque Index (0 to 100%) | No SDs and unable to estimate | N/R | Did not consider adverse effects |
| Lewis 2004 | Unclear | Interproximal | Eastman Interdental Bleeding Index (0/1) | No SDs and unable to estimate | ‐ | O'Leary Plaque Index (0/1) | No SDs and unable to estimate | N/R | Did not consider adverse effects |
| Walsh 1985 | Unclear | Interproximal | ‐ | ‐ | Final score at 3 months | Silness & Löe Plaque Index (evaluated as percentage of interproximal surfaces scored positive for plaque) (0/1) | Final score at 3 months | N/R | Did not consider adverse effects |
N/R: not reported
Gingivitis (Gingival Index)
Not measured.
Gingivitis (proportion of bleeding sites)
There was no evidence to claim a benefit for either wooden cleaning sticks or floss in reducing gingivitis at three months (MD (mean proportion of bleeding sites) 0.01, 95% CI ‐0.12 to 0.14; 1 trial, 24 participants; Analysis 7.1) (very low‐certainty evidence). This was the only time point providing useable data.
7.1. Analysis.
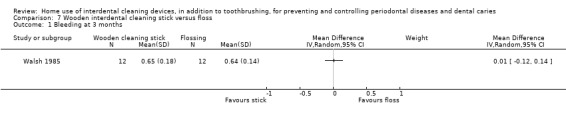
Comparison 7 Wooden interdental cleaning stick versus floss, Outcome 1 Bleeding at 3 months.
Periodontitis
No studies reported periodontitis.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was no evidence that wooden cleaning sticks reduced plaque (MD (mean proportion of sites with plaque) 0.02, 95% CI ‐0.06 to 0.10; 1 trial, 24 participants; Analysis 7.2) (very low‐certainty evidence). This was the only time point providing useable data.
7.2. Analysis.
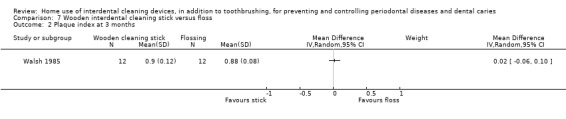
Comparison 7 Wooden interdental cleaning stick versus floss, Outcome 2 Plaque index at 3 months.
Adverse events
Neither of the studies assessing this comparison reported on adverse effects.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 8: Rubber/elastomeric interdental cleaning stick versus floss
Nine trials compared toothbrushing plus rubber interdental cleaning sticks with toothbrushing plus flossing. Five used a manual cleaning stick (Graziani 2017; Kazmierczak 1994; Smith 1988; Vogel 1975; Yost 2006), and four were powered (Cronin 1997; Cronin 2005; Gordon 1996; Isaacs 1999). We are unable to use the data from Smith 1988. We used six‐week data from Yost 2006. The details of the studies included in this comparison are shown in Table 23. See Table 8.
13. Comparison 8 Rubber/elastomeric cleaning sticks versus flossing: included study details.
|
Study (parallel group design unless noted) |
Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Cronin 1997 | Unclear | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | Final score at 1 month | Turesky modification of Quigley Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | There was no significant difference in soft tissue pathology between the groups. |
| Cronin 2005 | Unclear | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | Final score at 1 month | Proximal/Marginal Plaque index (0 to 5) | Final score at 1 month | N/R | No significant differences in the proportion of hard and soft tissue abnormalities between groups were found |
| Gordon 1996 (crossover but first‐period data only used ‐ see Characteristics of included studies for details) | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4) | Final score at 1 month | Final score at 1 month | Proximal/Marginal Plaque index (0 to 5) | Final score at 1 month | N/R | No significant soft tissue pathology was noted in any of the participants in either group |
| Graziani 2017 | Unclear | Interproximal | ‐ | ‐ | Final score at 1 month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 1 month | Mentioned as outcome but no data reported | Did not consider adverse effects |
| Isaacs 1999 | Unclear | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 3 months | Final score at 3 months | Turesky modification of Quigley Hein Plaque Index (0 to 5) | Unable to use data | N/R | There was no difference in the soft tissue status of the participants in the study groups. At 6 months, healthy soft tissue was found in 66/73 and 65/72 participants in cleaning sticks versus floss groups, respectively |
| Kazmierczak 1994 | Unclear | Interproximal | Lobene Modified Gingival Index (0 to 4 | Final score at 1 month | Final score at 1 month | Turesky modification of Quigley Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Did not consider adverse effects |
| Smith 1988 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Unable to use data | ‐ | Silness & Löe Plaque Index (0 to 3) | Unable to use data | PPD in mm but unable to use data | Did not consider adverse effects |
| Vogel 1975 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | ‐ | Podchladley's Total Plaque Index (0/1) | Unable to impute for index | N/R | Did not consider adverse effects |
| Yost 2006 | Unclear | Interproximal | Eastman Interdental Bleeding Index (0/1) | Final score at 6 weeks | ‐ | Benson modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 6 weeks | N/R | Examinations of the oral soft tissue were performed at the final visit, but were not reported. |
N/R: not reported PPD: pocket probing depth
Gingivitis (Gingival Index)
There was no evidence that one intervention performed better than the other with regards to gingivitis control at one month to six weeks (SMD ‐0.22, 95% CI ‐0.69 to 0.24; 6 trials, 256 participants) or three months (SMD 0.01, 95% CI ‐0.08 to 0.10; 1 trial, 145 participants; very low‐certainty evidence) (Analysis 8.1; Analysis 8.2). There was substantial heterogeneity in the one‐month result (I2 = 67%, P = 0.009).
8.1. Analysis.
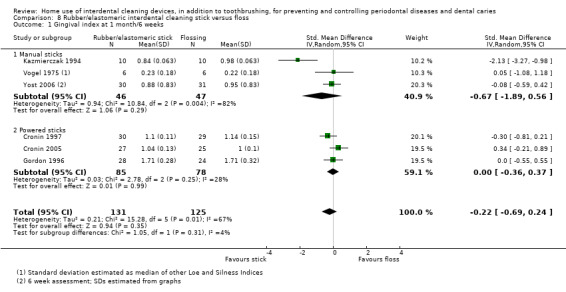
Comparison 8 Rubber/elastomeric interdental cleaning stick versus floss, Outcome 1 Gingival index at 1 month/6 weeks.
8.2. Analysis.

Comparison 8 Rubber/elastomeric interdental cleaning stick versus floss, Outcome 2 Gingival index at 3 months.
Gingivitis (proportion of bleeding sites)
Neither rubber/elastomeric cleaning sticks or floss were superior for reducing proportion of bleeding sites at one month (MD (mean proportion of bleeding sites) 0.03, 95% CI ‐0.08 to 0.03; 5 trials, 212 participants; Analysis 8.3) (low‐certainty evidence). There was moderate heterogeneity (I2 = 59%, P = 0.04). The result was similar at three months (MD 0.01, ‐0.03 to 0.05, 1 trial, 145 participants; very low‐certainty evidence; Analysis 8.4).
8.3. Analysis.
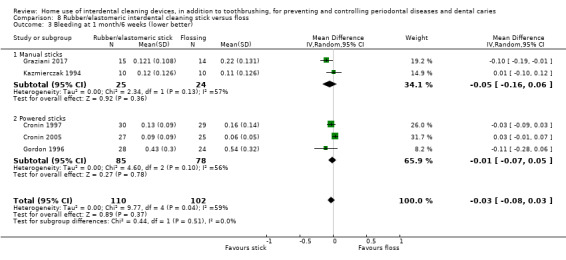
Comparison 8 Rubber/elastomeric interdental cleaning stick versus floss, Outcome 3 Bleeding at 1 month/6 weeks (lower better).
8.4. Analysis.
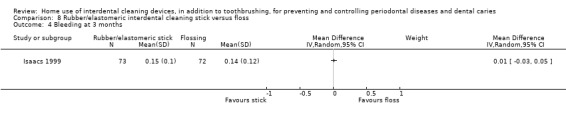
Comparison 8 Rubber/elastomeric interdental cleaning stick versus floss, Outcome 4 Bleeding at 3 months.
Periodontitis
Smith 1988 measured PPD but we were unable to use the data presented.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was no evidence that one intervention performed better than the other with regards to plaque control at one month (SMD ‐0.08, 95% CI ‐0.46 to 0.29; 6 trials, 273 participants; moderate heterogeneity (I2 = 57%, P value = 0.04); very low‐certainty evidence; Analysis 8.5).
8.5. Analysis.
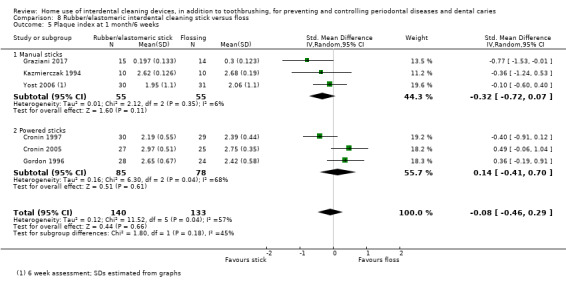
Comparison 8 Rubber/elastomeric interdental cleaning stick versus floss, Outcome 5 Plaque index at 1 month/6 weeks.
Adverse events
Two studies did not report adverse events and the other two reported adverse events as described in Table 23.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 9: Oral irrigation versus floss
Two trials (Barnes 2005; Rosema 2011), both at unclear risk of bias, provided gingivitis and plaque data at one month comparing oral irrigation with flossing. The details of the studies included in this comparison are shown in Table 24. See Table 9.
14. Comparison 9 Oral irrigation versus flossing: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Barnes 2005 | Unclear | With other sites | Löe & Silness Gingival Index (0 to 3) | Final score at 1 month | Final score at 1 month | Proximal/Marginal Plaque Index (0 to 5) | Final score at 1 month | N/R | Reported that there were no adverse events in any study group. |
| Rosema 2011 | Unclear | With other sites | Bleeding on Marginal Probing Index (0 to 2) | Final score at 1 month | Final score at 1 month | Turesky modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 1 month | N/R | Reported that there were no adverse events in any study group. |
N/R: not reported
Gingivitis (Gingival Index)
There was very low‐certainty evidence of a possible reduction in gingivitis at one month when oral irrigation was compared to flossing, though the result was also compatible with no difference between the interventions (MD ‐0.06, 95% CI ‐0.12 to ‐0.00; 1 trial, 63 participants; Analysis 9.1).
9.1. Analysis.
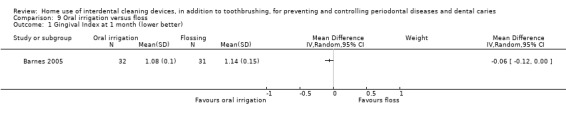
Comparison 9 Oral irrigation versus floss, Outcome 1 Gingival Index at 1 month (lower better).
Gingivitis (proportion of bleeding sites)
There was low‐certainty evidence of a reduction in proportion of bleeding sites at one month when oral irrigation was compared to flossing (MD ‐0.12, 95% CI ‐0.19 to ‐0.05; 2 trials, 133 participants; no heterogeneity (I2 = 1%, P = 0.34); Analysis 9.2).
9.2. Analysis.
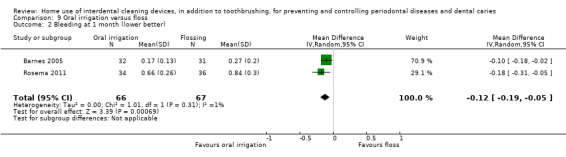
Comparison 9 Oral irrigation versus floss, Outcome 2 Bleeding at 1 month (lower better).
Periodontitis
No studies reported periodontitis.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was no evidence of a difference in plaque at one month for either oral irrigation or flossing (SMD 0.31, 95% CI ‐0.08 to 0.70; 2 trials, 133 participants; low heterogeneity (I2 = 22%, P = 0.26); very low‐certainty evidence; Analysis 9.3).
9.3. Analysis.
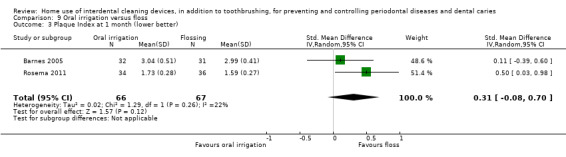
Comparison 9 Oral irrigation versus floss, Outcome 3 Plaque Index at 1 month (lower better).
Adverse events
Both Barnes 2005 and Rosema 2011 reported that there were no adverse events in any study group. See Table 24.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Comparison 10: Interdental cleaning stick versus interdental brush
Three trials compared rubber/elastomeric interdental cleaning sticks with interdental brushes (Graziani 2017; Smith 1988; Yost 2006). We were unable to use data from one trial (Smith 1988). We used six‐week data from Yost 2006. The studies were at unclear risk of bias. The details of the studies included in this comparison are shown in Table 25. See Table 10.
15. Comparison 10 Rubber/elastomeric cleaning sticks versus IDB: included study details.
| Study (parallel group design unless otherwise noted) | Risk of bias assessment | Interproximal sites only or with other sites | Gingivitis index (scale) | Gingivitis final score or change in score, time points | Bleeding Index (0 or 1) time points | Plaque index (scale) | Plaque final score or change in score, time points | Probing depth change (mm) | Adverse events |
| Graziani 2017 | Unclear | Interproximal | Full Mouth Bleeding Score (0/1) | Final score at 6 weeks | Final score at 1 month | Full Mouth Plaque Score (percentage of areas containing plaque) | Final score at 6 weeks | Mentioned as outcome but no data reported | Did not consider adverse effects |
| Smith 1988 | High | Interproximal | Löe & Silness Gingival Index (0 to 3) | Unable to use data | ‐ | Silness & Löe Plaque Index (0 to 3) | Unable to use data | PPD in mm but unable to use data | Did not consider adverse effects |
| Yost 2006 | Unclear | Interproximal | Eastman Interdental Bleeding Index (0/1) | Final score at 6 weeks | ‐ | Benson modification of Quigley‐Hein Plaque Index (0 to 5) | Final score at 6 weeks | N/R | Examinations of the oral soft tissue were performed at the final visit, but were not reported. |
IDB: interdental brush N/R: not reported PPD: pocket probing depth
Gingivitis (Gingival Index)
There was no evidence that one intervention performed better than the other with regards to gingivitis control at six weeks (MD 0.10, 95% CI ‐0.32 to 0.52; 1 trial, 61 participants; very low‐certainty evidence; Analysis 10.1).
10.1. Analysis.
Comparison 10 Interdental cleaning stick versus interdental brush, Outcome 1 Gingival index at 1 month/6 weeks.
Gingivitis (proportion of bleeding sites)
There was no evidence that one intervention performed better than the other with regards to reducing proportion of bleeding sites at one month (MD ‐0.02, 95% CI ‐0.10 to 0.06; 1 trial, 31 participants; very low‐certainty evidence; Analysis 10.2).
10.2. Analysis.
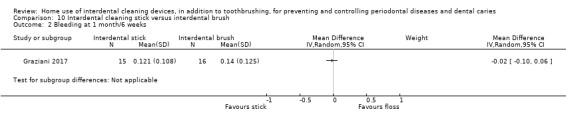
Comparison 10 Interdental cleaning stick versus interdental brush, Outcome 2 Bleeding at 1 month/6 weeks.
Periodontitis
Smith 1988 measured PPD but we were unable to use the data presented. Graziani 2017 also measured PPD but did not provide data.
Interproximal caries
No studies reported interproximal caries.
Plaque
There was no evidence that one intervention performed better than the other with regards to plaque control at one month to six weeks (SMD 0.08, 95% CI ‐0.33 to 0.49; 2 trials, 92 participants; no heterogeneity; very low‐certainty evidence; Analysis 10.3).
10.3. Analysis.
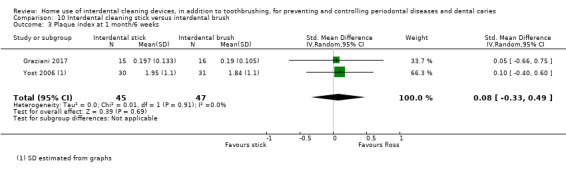
Comparison 10 Interdental cleaning stick versus interdental brush, Outcome 3 Plaque index at 1 month/6 weeks.
Adverse events
Graziani 2017 and Smith 1988 did not measure adverse effects. In Yost 2006, examinations of the oral soft tissue were performed at the final visit, but were not reported. See Table 25.
Other outcomes
Halitosis, patient satisfaction, and cost of intervention were not measured in these studies.
Discussion
Summary of main results
This review found five devices that were used in addition to toothbrushing and compared with toothbrushing alone: floss (15 trials), interdental brushes (2 trials), wooden cleaning sticks (2 trials), rubber/elastomeric cleaning sticks (2 trials), oral irrigators (5 trials). Four devices were compared with flossing: interdental brushes (9 trials), wooden cleaning sticks (3 trials), rubber/elastomeric cleaning sticks (9 trials), oral irrigators (2 trials). The other comparison was between rubber/elastomeric cleaning sticks and interdental brushes (3 trials).
Most of the trials presented results for gingivitis and plaque, which we analysed at one, three, and six months. Six studies evaluated periodontitis, but data were only useable for one comparison: interdental brushes versus flossing. Some studies considered possible harm from the interventions. None of the studies measured interproximal caries. Nor did they measure our secondary outcomes of halitosis, patient satisfaction and costs.
Participants in most studies had a low level of gingival inflammation at baseline, and outcomes were measured most commonly in the short term. Overall, the evidence was low to very low‐certainty, and the effect sizes observed may not be clinically important.
Comparisons with toothbrushing alone
For flossing plus toothbrushing compared to toothbrushing alone, there was low‐certainty evidence of flossing reducing gingivitis at one month. This was confirmed at three and six months. There was very low‐certainty evidence that did not show that flossing reduced plaque more than toothbrushing alone at one month or six months; there was some evidence for an effect at three months.
Using an interdental brush in addition to cleaning the teeth with a toothbrush may reduce gingivitis (measured by gingival index) and plaque, but not proportion of bleeding sites. The evidence was low to very low‐certainty, being based on only one or two small studies, and only measured at the one‐month time point.
Using wooden interdental toothcleaning sticks in addition to toothbrushing may reduce gingivitis measured by proportion of bleeding sites, but not plaque, when measured at three months. The evidence was very low‐certainty, being based on only 24 participants.
Using rubber/elastomeric interdental toothcleaning sticks in addition to toothbrushing did not appear to reduce gingivitis at one month any more than toothbrushing alone, but there may be a reduction in plaque. The evidence was very low‐certainty, being based on 12 or 30 participants.
Toothbrushing plus oral irrigation (pulsing water) may reduce gingivitis measured by a gingival index more than toothbrushing alone at one month, but the evidence was very low‐certainty, and this effect was not seen at three or six months. Low‐certainty evidence did not show a clear difference between groups for reduction in proportion of bleeding sites at one or three months, or plaque at one, three, or six months.
Comparisons between different interdental cleaning aids
Interdental brushes may be better than flossing for reducing gingivitis at one and three months (low‐certainty evidence). The evidence for a reduction in plaque was inconsistent (very‐low certainty evidence). There was no evidence that either device was superior for reducing periodontitis (low‐certainty evidence).
Wooden cleaning sticks or rubber/elastomeric cleaning sticks did not seem to be better or worse than flossing at controlling gingivitis or plaque at three months (low‐ to very low‐certainty evidence).
There was some evidence that oral irrigation may be better than flossing for reducing gingivitis at one month (low‐ to very low‐certainty evidence). The evidence did not show either intervention to be superior for reducing plaque (very low‐certainty evidence).
There was no evidence that rubber/elastomeric interdental cleaning sticks were better or worse than interdental brushes for controlling gingivitis and plaque (very low‐certainty evidence).
Adverse events
Adverse events were presented for some of the trials; however, there were no severe adverse events reported and no evidence of differences between study groups. One study reported on problems using the interventions (interdental brush versus floss), in particular, soreness caused when interdental brushes become stuck between the teeth.
Overall completeness and applicability of evidence
The evidence was limited in applicability and was incomplete. The included studies only presented data on gingivitis, plaque, and adverse events; most did not measure or report other important outcomes such as periodontitis or interproximal caries. One reason for this is that larger, longer term studies are needed to measure these outcomes. Costs were another outcome not reported although this may be an important consideration for patients.
Another weakness in the included trials in terms of the completeness and applicability of evidence is the low level of gingivitis and plaque in many of the participants. For example, if studies reporting gingivitis at one month using the Löe and Silness Gingival Index are examined, the mean values for the toothbrushing‐only group for the four studies varied between 0.14 and 0.84 (median 0.47), which is very low considering that this index is on a 0‐ to 3‐point scale. This means that even large effect sizes on a SMD scale are probably clinically unimportant, and that trialists may not be selecting the right participants to answer questions about the efficacy of these interventions. We also considered bleeding data measured as the proportion of bleeding sites per participant, as we thought this would make the clinical interpretation of data presented easier, along with a judgement of clinical importance. Surprisingly, however, there was little correlation between the two, which made the interpretation more complex and challenging. In addition, we are not aware that a minimally important clinical difference has been established for the commonly used gingival and plaque scales.
We included only studies on adults. There have been no studies of mechanical interdental cleaning for children at home, though there has been some research on supervised interventions delivered in schools and professional interventions delivered in the primary dental care setting. This was summarised in the systematic review by Hujoel (Hujoel 2006).
Quality of the evidence
We included 35 trials that randomised 3929 participants and evaluated approximately 3734 participants; however, many of the meta‐analyses only included a small number of trials and participants. All trials were at high risk of performance bias as participants knew which interdental aids they were using. While recognising this performance bias risk, we omitted this domain from the overall 'risk of bias' assessment that informed our assessment of the certainty of the evidence because lack of blinding is unavoidable and any behaviour change related to knowledge of these interventions can be regarded as an integral part of the intervention, and incorporated into estimates of 'real world' effectiveness. When performance bias was omitted from the overall 'risk of bias' assessment, we judged two trials (6%) to be at low risk of bias, six trials (17%) to be at high risk and 27 trials (77%) to be unclear.
The largest body of evidence we identified was for flossing and toothbrushing compared with toothbrushing only (15 studies). The body of evidence for this comparison for both gingivitis and plaque was low‐ and very low‐certainty, respectively, due to the risk of bias in the studies, substantial unexplained heterogeneity, and lack of precision in the effect estimates. We assessed the body of evidence for all comparisons and outcomes as low‐ or very low‐certainty.
The studies included in this review used many different gingivitis and plaque indices. This meant that we had to estimate a standardised mean difference (SMD) effect estimate in some cases. We did not back‐translate this to a common index as there were only two trials at low risk of bias (excluding performance bias). It would be sensible for clinicians to agree on a common index to use for both these measures; this would enable results of future studies to be pooled, which would aid precision and interpretability of effect estimates, and also help establish minimal clinically important differences. Support to select the most appropriate outcome measurement is available through the COSMIN initiative (COSMIN 2018).
Potential biases in the review process
We estimated the standard deviations for some trials that did not report these. We undertook this only for the most commonly reported gingivitis (Löe and Silness Gingival Index) and plaque indices (Turesky modification of the Quigley Hein Index). When we undertook sensitivity analyses, by removing studies with estimated standard deviations, the effect estimates were similar.
Two review authors (P Imai and HV Worthington) were each authors on an included trial; however, the assessment of these two trials was independently undertaken by other members of the review team.
The toothbrushing‐only group in two trials that compared this group to a toothbrushing plus flossing group, also included use of a 'placebo' negative control rinse. We included these studies as the rinse may help to counteract any performance bias, and our sensitivity analyses omitting these studies led to similar effect estimates.
There were some studies of manual cleaning sticks, while others were automated. There were some studies that used electric toothbrushes in both arms. We conducted meta‐analyses even if it meant combining automated and manual devices; we would have conducted subgroup analyses had there been sufficient studies.
Many of the included studies were funded by pharmaceutical companies who made the intervention being evaluated. We are unsure whether or not this has introduced publication bias into the effect estimates. It is, however, noted that there are similar numbers of head‐to‐head studies and studies comparing the intervention with toothbrushing alone.
We excluded studies that evaluated use of multiple devices, supervised use of interdental cleaning devices, or dental health professional delivery of mechanical interdental cleaning. In the update of this review, we may consider including these studies to gain a greater understanding of the best use of interdental cleaning devices for preventing or controlling periodontal diseases and dental caries.
Agreements and disagreements with other studies or reviews
This review includes updates of two previously published Cochrane reviews on flossing and interdental brushing (Sambunjak 2011; Poklepovic Pericic 2013), conducted by some of the same authors. The flossing review included a section that compared the findings to Berchier 2008, and the findings of the interdental brushing review were compared to those of the reviews by Slot 2008 and Imai 2012.
Berchier 2008 had reported that "both plaque and gingivitis values show no significant effects", and noted "a trend in favour of brushing and floss", questioning whether lack of statistically significant findings might be due to a lack of power. The current review found that toothbrushing plus flossing reduced gingivitis scores at one, three, and six months, compared to toothbrushing alone, with effects on plaque being less clear.
Slot 2008 looked at the effect of interdental brushing with toothbrushing compared to toothbrushing alone or another interdental device, on plaque and "parameters of gingival inflammation". The findings were broadly similar to this review; however, the meta‐analyses were conducted on specific indices for plaque and gingivitis rather than combining them using standardised mean differences. The authors concluded that use of interdental brushes compared to toothbrushing alone showed "a positive significant difference with respect to plaque, bleeding and probing pocket depth", which is in agreement with this review for plaque and gingivitis; however, we did not find any PPD data for this comparison. The authors also reported that interdental brushes appeared to reduce plaque when compared with flossing, which is also in agreement with this review; however, we found interdental brushes also appeared to reduce gingivitis, but not PPD. The overall findings of Imai 2012 were similar to our review, i.e. that interdental brushing is more effective than floss in reducing gingivitis and plaque scores; however, Imai 2012 included only four studies looking at this comparison, compared to nine in our review.
Hoenderdos 2008 is a systematic review that assessed the efficacy of wood sticks, used for interdental cleaning, on plaque levels and gingival inflammation. They found that wood sticks had "no visible effect on interdental plaque and did not reduce the gingival index. However, woodsticks were effective in reducing interdental gingival inflammation when tendency to bleeding was investigated". We also found some evidence for an effect on bleeding at three months, albeit based on just 24 participants. There was no RCT evidence to assess gingivitis measured by a gingival index. Hoenderdos 2008 included CCTs as well as RCTs, and studies with shorter outcome assessment time points than this review did, with more restricted types of handheld wooden toothpicks, so the two reviews are not directly comparable.
A systematic review published in 2008 looked at the effect of oral irrigation as an adjunct to brushing (Husseini 2008). This review included seven studies, both RCTs and CCTs, and reached the conclusion that as an adjunct to brushing "the oral irrigator does not have a beneficial effect in reducing visible plaque, however there is a positive trend in favour of oral irrigation improving gingival health". This aligned with our review, which found that there may be an effect of oral irrigators on gingivitis measured by gingival index at one month, but did not find this at any other time point, or for the outcomes of bleeding or plaque.
A recent network meta‐analysis included different interproximal cleaning aids of oral hygiene methods (Kotsakis 2017), with the aim of ranking them in order of importance for reducing gingival inflammation. The results included 22 trials looking at 10 interdental oral hygiene aids as adjuncts to toothbrushing. Interdental brushes yielded the largest reduction in the Gingival Index (GI) followed by water‐jet. The authors reported that all the aids except toothpicks reduced the Gingival Index when compared to toothbrushing alone. This did not align entirely with our findings. We did note that the Kotsakis 2017 review did not assess heterogeneity or transitivity, discuss the impact of sparse networks, or consider results with respect to the certainty of the evidence.
Authors' conclusions
Implications for practice.
Additional use of floss or interdental brushes compared to toothbrushing alone may reduce gingivitis or plaque, or both, and interdental brushes may be more effective than floss. The evidence is low to very low‐certainty, and the effect sizes observed may not be clinically important. Available evidence for cleaning sticks and oral irrigation aids is limited and inconsistent. Adverse events reported were minor; there were no serious adverse events and no evidence of a difference between study arms. The long‐term significance of the findings is unclear as few of the studies evaluated pocket probing depth as a measure of periodontitis and none assessed interproximal caries.
Implications for research.
The findings do not allow us to be certain whether or not home use of interdental cleaning devices makes a clinically significant impact on periodontal diseases, and they provide no information about the impact on dental caries. Most of the trials in this review were of short duration and involved many participants with only a low level of gum inflammation at baseline. In addition, all studies were at risk of performance bias, and 33 of the 35 included trials were at risk of other types of bias. If future trials are of a similar nature to those included in this review, they may not be able to add meaningfully to the current evidence base. We believe future trials should be long‐term, sufficiently powered to assess the effects of interdental cleaning devices or oral hygiene regimens on caries and periodontitis, and should include estimates of costs. Although performance bias is inevitable, it is possible to undertake randomised controlled trials of home‐use interdental cleaning devices that are otherwise at low risk of bias, and to report them according to the CONSORT statement (Consolidated Standards of Reporting Trials). Any future trials should report on the extent of gingivitis and the stage of periodontitis at baseline, according to the new periodontal diseases classification. An agreement on preferred indices for the measurement of gingivitis and plaque, along with differences considered clinically important, would aid future evidence synthesis and interpretation.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2020 | Amended | Minor edit to description of GRADE in 'Summary of findings' tables |
Acknowledgements
We would like to thank Cochrane Oral Health, especially Dr Phil Riley (Editor), Ana Jeroncic (Editor), Anne Littlewood (Information Specialist), and Professor Tanya Walsh (Editor). We also thank Derek Richards, Dagmar Slot, Professor Ian Needleman, Professor Jane Forrest, and Dr Nuala Livingstone for their comments on earlier drafts of the review. We thank Anne Lethaby for final copy editing.
For their input to the protocol for this review, we would like to thank Cochrane Oral Health (especially Anne Littlewood, Phil Riley, Zipporah Iheozor‐Ejiofor, Helen Wakeford), Jason Elliot‐Smith, Dagmar Slot, Thomas Lamont, and Edward Lo.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials
1 (caries or carious):ti,ab 2 ((teeth or tooth or dental or enamel or dentin* or root*) and (cavit* or decay* or lesion* or deminerali* or reminerali*)):ti,ab 3 ((teeth or tooth or dental or enamel or dentin) and plaque):ti,ab 4 ((tooth or teeth or dental) and (stain* or discolor* or discolour* or calculus or tartar)):ti,ab 5 (dental and deposit*):ti,ab 6 periodont*:ti,ab 7 gingivit*:ti,ab 8 (gingiva* and pocket*):ti,ab 9 ((blood or bleed*) and prob*):ti,ab 10 (gingival* and (disease* or blood* or bleed* or inflamm* or index or hemorrhag* or haemorrhag*)):ti,ab 11 (papilla* adj3 (bleed* or index*)):ti,ab 12 "bleeding index*":ti,ab 13 ((pocket* or probe or probing) and depth):ti,ab 14 "attachment loss":ti,ab 15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 16 ((interdental and brush*) or (inter‐dental and brush*) or (interspace and brush*) or (inter‐space and brush*) or (interproximal and brush*) or (inter‐proximal and brush*)):ti,ab 17 ((interdental and clean*) or (inter‐dental and clean*) or (interspace and clean*) or (inter‐space and clean)):ti,ab 18 ((interproximal and clean*) or (inter‐proximal and clean*)):ti,ab 19 ((interdental and aid*) or (inter‐dental and aid*)):ti,ab 20 (toothbrush* or tooth‐brush* or "tooth brush*"):ti,ab 21 (floss* or Superfloss or Ultrafloss or Airfloss):ti,ab 22 (dental and tape*):ti,ab 23 (miswak* or meswak* or woodstick* or toothpick* or "wood stick*" or "tooth pick*" or woodpoint* or "wood point*"):ti,ab 24 ("gingival stimulator*" or "rubber tip stimulator*" or "gum stimulator*" or "Butler GUM" or Stimu‐gum or "interproximal stimulator*" or "wedge stimulator*" or "wooden stimulator*" or "interdental stimulator" or "subgingival tip*"):ti,ab 25 ((oral or water or subgingival or dental) and irrigat*):ti,ab 26 ("water pick*" or waterpick*):ti,ab 27 (Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket* or Softpick or Softpik):ti,ab 28 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 29 (#15 and #28) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh "tooth demineralization"] #2 (caries or carious) #3 (teeth near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #4 (tooth near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #5 (dental near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #6 (enamel near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #7 (dentin* near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #8 (root* near/5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) #9 [mh ^"Dental plaque"] #10 [mh ^"dental deposits"] #11 ((teeth or tooth or dental or enamel or dentin) and plaque) #12 ((tooth or teeth or dental) near/5 (stain* or discolor* or discolour* or calculus or tartar)) #13 [mh "dental health surveys"] #14 ("DMF Index" or "Dental Plaque Index" or "Periodontal Index" or "Papillary Bleeding Index") #15 (dental near/2 deposit*) #16 [mh "Periodontal Diseases"] #17 periodont* #18 gingivit* #19 (gingiva* near/3 pocket*) #20 ((blood or bleed*) near/4 prob*) #21 (gingival* near/5 (disease* or blood* or bleed* or inflamm* or index or hemorrhag* or haemorrhag*)) #22 (papilla* near/3 (bleed* or index*)) #23 "bleeding index" #24 ((pocket* or probe or probing) near/2 depth) #25 "attachment loss" #26 {or #1‐#25} #27 [mh "Dental Devices, Home Care"] #28 [mh ^Toothbrushing] #29 ((interdental near/3 brush*) or (inter‐dental near/3 brush*) or (interspace near/3 brush*) or (inter‐space near/3 brush*) or (interproximal near/3 brush*) or (inter‐proximal near/3 brush*)) #30 ((interdental near/3 clean*) or (inter‐dental near/3 clean*) or (interspace near/3 clean*) or (inter‐space near/3 clean)) #31 ((interproximal near/3 clean*) or (inter‐proximal near/3 clean*)) #32 ((interdental near/3 aid*) or (inter‐dental near/3 aid*)) #33 (toothbrush* or tooth‐brush* or "tooth brush*") #34 Proxabrush #35 (floss* or Superfloss or Ultrafloss or Airfloss) #36 (dental near/5 tape*) #37 (miswak* or meswak* or woodstick* or toothpick* or "wood stick*" or "tooth pick*" or woodpoint* or "wood point*") #38 ("gingival stimulator*" or "rubber tip stimulator*" or "gum stimulator*" or "Butler GUM" or Stimu‐gum or "interproximal stimulator*" or "wedge stimulator*" or "wooden stimulator*" or "interdental stimulator" or "subgingival tip*") #39 ((oral or water or subgingival or dental) near/2 irrigat*) #40 ("water pick*" or waterpick*) #41 (Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket* or Softpick or Softpik) #42 {or #27‐#41} #43 #26 and #42
Appendix 3. MEDLINE Ovid search strategy
1. exp TOOTH DEMINERALIZATION/ 2. (caries or carious).mp. 3. (teeth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 4. (tooth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 5. (dental adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 6. (enamel adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 7. (dentin$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 8. (root$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 9. Dental plaque/ 10. Dental deposits/ 11. ((teeth or tooth or dental or enamel or dentin) and plaque).mp. 12. ((tooth or teeth or dental) adj5 (stain$ or discolor$ or discolour$ or calculus or tartar)).mp. 13. exp DENTAL HEALTH SURVEYS/ 14. ("DMF Index" or "Dental Plaque Index" or "Periodontal Index" or "Papillary Bleeding Index").mp. 15. (dental adj2 deposit$).mp. 16. exp Periodontal Diseases/ 17. periodont$.mp. 18. gingivit$.mp. 19. (gingiva$ adj3 pocket$).mp. 20. ((blood or bleed$) adj4 prob$).mp. 21. (gingival$ adj5 (disease$ or blood$ or bleed$ or inflamm$ or index or hemorrhag$ or haemorrhag$)).mp. 22. (papilla$ adj3 (bleed$ or index$)).mp. 23. "bleeding index".mp. 24. ((pocket$ or probe or probing) adj2 depth).mp. 25. "attachment loss".mp. 26. or/1‐25 27. exp Dental Devices, Home Care/ 28. Toothbrushing/ 29. ((interdental adj3 brush$) or (inter‐dental adj3 brush$) or (interspace adj3 brush$) or (inter‐space adj3 brush$) or (interproximal adj3 brush$) or (inter‐proximal adj3 brush$)).mp. 30. ((interdental adj3 clean$) or (inter‐dental adj3 clean$) or (interspace adj3 clean$) or (inter‐space adj3 clean)).mp. 31. ((interproximal adj3 clean$) or (inter‐proximal adj3 clean$)).mp. 32. ((interdental adj3 aid$) or (inter‐dental adj3 aid$)).mp. 33. (toothbrush$ or tooth‐brush$ or "tooth brush$").mp. 34. Proxabrush.mp. 35. (floss$ or Superfloss or Ultrafloss or Airfloss).mp 36. (dental adj5 tape$).mp. 37. (miswak$ or meswak$ or woodstick$ or toothpick$ or "wood stick$" or "tooth pick$" or woodpoint$ or "wood point$").mp. 38. ("gingival stimulator$" or "rubber tip stimulator$" or "gum stimulator$" or "Butler GUM" or Stimu‐gum or "interproximal stimulator$" or "wedge stimulator$" or "wooden stimulator$" or "interdental stimulator" or "subgingival tip$").mp. 39. ((oral or water or subgingival or dental) adj2 irrigat$).mp. 40. ("water pick$" or waterpick$).mp. 41. (Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket$ or Softpick or Softpik).mp. 42. or/27‐41 43. 26 and 42
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐ maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Dental caries/ 2. (caries or carious).mp. 3. (teeth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 4. (tooth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 5. (dental adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 6. (enamel adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 7. (dentin$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 8. (root$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp. 9. Tooth plaque/ 10. Tooth calculus/ 11. ((teeth or tooth or dental or enamel or dentin) and plaque).mp. 12. ((tooth or teeth or dental) adj5 (stain$ or discolor$ or discolour$ or calculus or tartar)).mp. 13. ("DMF Index" or "Dental Plaque Index" or "Periodontal Index" or "Papillary Bleeding Index").mp. 14. (dental adj2 deposit$).mp. 15. exp Periodontal Disease/ 16. periodont$.mp. 17. gingivit$.mp. 18. (gingiva$ adj3 pocket$).mp. 19. ((blood or bleed$) adj4 prob$).mp. 20. (gingival$ adj5 (disease$ or blood$ or bleed$ or inflamm$ or index or hemorrhag$ or haemorrhag$)).mp. 21. (papilla$ adj3 (bleed$ or index$)).mp. 22. "bleeding index".mp. 23. ((pocket$ or probe or probing) adj2 depth).mp. 24. "attachment loss".mp. 25. or/1‐24 26. Dental floss/ 27. Toothbrush/ 28. Tooth brushing/ 29. ((interdental adj3 brush$) or (inter‐dental adj3 brush$) or (interspace adj3 brush$) or (inter‐space adj3 brush$) or (interproximal adj3 brush$) or (inter‐proximal adj3 brush$)).mp. 30. ((interdental adj3 clean$) or (inter‐dental adj3 clean$) or (interspace adj3 clean$) or (inter‐space adj3 clean)).mp. 31. ((interproximal adj3 clean$) or (inter‐proximal adj3 clean$)).mp. 32. ((interdental adj3 aid$) or (inter‐dental adj3 aid$)).mp. 33. (toothbrush$ or tooth‐brush$ or "tooth brush$").mp. 34. Proxabrush.mp. 35. (floss$ or Superfloss or Ultrafloss or Airfloss).mp. 36. (dental adj5 tape$).mp. 37. (miswak$ or meswak$ or woodstick$ or toothpick$ or "wood stick$" or "tooth pick$" or woodpoint$ or "wood point$").mp. 38. ("gingival stimulator$" or "rubber tip stimulator$" or "gum stimulator$" or "Butler GUM" or Stimu‐gum or "interproximal stimulator$" or "wedge stimulator$" or "wooden stimulator$" or "interdental stimulator" or "subgingival tip$").mp. 39. ((oral or water or subgingival or dental) adj2 irrigat$).mp. 40. ("water pick$" or waterpick$).mp. 41. (Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket$ or Softpick or Softpik).mp. 42. or/26‐41 43. 25 and 42
This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see https://www.cochranelibrary.com/central/central‐creation for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. CINAHL EBSCO search strategy
S43 S26 and S42 S42 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 S41 (Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket* or Softpick or Softpik) S40 ("water pick*" or waterpick*) S39 ((oral or water or subgingival or dental) N2 irrigat*) S38 ("gingival stimulator*" or "rubber tip stimulator*" or "gum stimulator*" or "Butler GUM" or Stimu‐gum or "interproximal stimulator*" or "wedge stimulator*" or "wooden stimulator*" or "interdental stimulator" or "subgingival tip*") S37 (miswak* or meswak* or woodstick* or toothpick* or "wood stick*" or "tooth pick*" or woodpoint* or "wood point*") S36 (dental N5 tape*) S35 (floss* or Superfloss or Ultrafloss or Airfloss) S34 Proxabrush S33 (toothbrush* or tooth‐brush* or "tooth brush*") S32 ((interdental N3 aid*) or (inter‐dental N3 aid*)) S31 ((interproximal N3 clean*) or (inter‐proximal N3 clean*)) S30 ((interdental N3 clean*) or (inter‐dental N3 clean*) or (interspace N3 clean*) or (inter‐space N3 clean)) S29 ((interdental N3 brush*) or (inter‐dental N3 brush*) or (interspace N3 brush*) or (inter‐space N3 brush*) or (interproximal N3 brush*) or (inter‐proximal N3 brush*)) S28 (MH Toothbrushing) S27 (MH Dental Devices, Home Care+) S26 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 "attachment loss" S24 ((pocket* or probe or probing) N2 depth) S23 "bleeding index" S22 (papilla* N3 (bleed* or index*)) S21 (gingival* N5 (disease* or blood* or bleed* or inflamm* or index or hemorrhag* or haemorrhag*)) S20 ((blood or bleed*) N4 prob*) S19 ((gingiva* N3 pocket*) S18 gingivit* S17 periodont* S16 (MH Periodontal Diseases+) S15 (dental N2 deposit*) S14 ("DMF Index" or "Dental Plaque Index" or "Periodontal Index" or "Papillary Bleeding Index") S13 (MH dental health surveys) S12 ((tooth or teeth or dental) and (stain* or discolor* or discolour* or calculus or tartar)) S11 ((teeth or tooth or dental or enamel or dentin) and plaque) S10 (MH dental deposits) S9 (MH Dental plaque) S8 (root* N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S7 (dentin* N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S6 (enamel N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S5 (dental N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S4 (tooth N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S3 (teeth N5 (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) S2 (caries or carious) S1 (MH Tooth demineralization+)
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in CINAHL EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. Web of Science Conference Proceedings search strategy
Searches of the Web of Science Conference Proceedings database were undertaken to 18 January 2018, but this search was discontinued due to poor yield.
# 35 #21 and #34 # 34 #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 # 33 TS=(Oxyjet or Waterpik or "Water Pik" or "Oral Breeze" or PowerFloss or "Hydro Floss" or "Water Jet" or Aquajet or Interplak or h2ofloss or "Perio Pik" or "Pik Pocket" or Pickpocket* or Softpick or Softpik) # 32 TS=("water pick*" or waterpick*) # 31 TS=((oral or water or subgingival or dental) AND irrigat*) # 30 TS=("gingival stimulator*" or "rubber tip stimulator*" or "gum stimulator*" or "Butler GUM" or Stimu‐gum or "interproximal stimulator*" or "wedge stimulator*" or "wooden stimulator*" or "interdental stimulator" or "subgingival tip*") # 29 TS=(miswak* or meswak* or woodstick* or toothpick* or "wood stick*" or "tooth pick*" or woodpoint* or "wood point*") # 28 TS=(dental AND tape*) # 27 TS=(floss* or Superfloss or Ultrafloss or Airfloss or Proxabrush) # 26 TS=(toothbrush* or tooth‐brush* or "tooth brush*") # 25 TS=((interdental AND aid*) or (inter‐dental AND aid*)) # 24 TS=((interproximal AND clean*) or (inter‐proximal AND clean*)) # 23 TS=((interdental AND clean*) or (inter‐dental AND clean*) or (interspace AND clean*) or (inter‐space AND clean)) # 22 TS=((interdental AND brush*) or (inter‐dental AND brush*) or (interspace AND brush*) or (inter‐space AND brush*) or (interproximal AND brush*) or (inter‐proximal AND brush*)) # 21 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 # 20 TS="attachment loss" # 19 TS=((pocket* or probe or probing) AND depth) # 18 TS="bleeding index" # 17 TS=(papilla* AND (bleed* or index*)) # 16 TS=(gingival* AND (disease* or blood* or bleed* or inflamm* or index or hemorrhag* or haemorrhag*)) # 15 TS=((blood or bleed*) AND prob*) # 14 TS=(gingiva* AND pocket*) # 13 TS=gingivit* # 12 TS=periodont* # 11 TS=(dental AND deposit*) # 10 TS=("DMF Index" or "Dental Plaque Index" or "Periodontal Index" or "Papillary Bleeding Index") # 9 TS=((tooth or teeth or dental) AND (stain* or discolor* or discolour* or calculus or tartar)) # 8 TS=((teeth or tooth or dental or enamel or dentin) and plaque) # 7 TS=(root* AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 6 TS=(dentin* AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 5 TS=(enamel AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 4 TS=(dental AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 3 TS=(tooth AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 2 TS=(teeth AND (cavit* or caries* or carious or decay* or lesion* or deminerali* or reminerali*)) # 1 TS=(caries or carious)
Appendix 7. US National Institutes of Health Trials Registry (ClinicalTrials.gov) search strategy
interdental or interproximal
interspace or floss
miswak or toothpick
Appendix 8. World Health Organization International Clinical Trials Registry Platform search strategy
Interdental brush
Interproximal brush
floss
miswak or toothpick
Data and analyses
Comparison 1. Toothbrushing plus floss vs toothbrushing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival index at 1 month (lower better) | 8 | 585 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.12, ‐0.04] |
| 2 Gingival index 3 months (lower better) | 4 | 570 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.50, ‐0.17] |
| 3 Gingival index at 6 months (lower better) | 4 | 564 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.95, ‐0.42] |
| 4 Bleeding at 1 month (lower better) | 2 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.14, 0.08] |
| 5 Bleeding at 3 months (lower better) | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.37, 0.09] |
| 6 Bleeding at 6 months (lower better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Plaque at 1 month (lower better) | 7 | 542 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.85, 0.02] |
| 8 Plaque at 3 months (lower better) | 5 | 594 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.36, ‐0.04] |
| 9 Plaque at 6 months (lower better) | 3 | 487 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
Comparison 2. Toothbrushing plus interdental brush versus toothbrushing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival index at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Bleeding at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Plaque index at 1 month | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.51, ‐0.63] |
Comparison 3. Toothbrushing plus wooden tooth cleaning stick versus toothbrushing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Plaque Index at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 4. Toothbrushing plus rubber/elastomeric tooth cleaning stick versus toothbrushing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival Index at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Bleeding at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Plaque Index at 1 month | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 5. Toothbrushing plus oral irrigation versus toothbrushing alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingivitis at 1 month (lower better) | 4 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.89, ‐0.06] |
| 2 Gingivitis at 3 months (lower better) | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.44, 0.17] |
| 3 Gingivitis at 6 months (lower better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Bleeding at 1 month (lower better) | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
| 5 Bleeding at 3 months (lower better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Plaque at 1 month (lower better) | 3 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.41, 0.10] |
| 7 Plaque at 3 months (lower better) | 2 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.25, 0.37] |
| 8 Plaque at 6 months (lower better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 6. Interdental brush versus floss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival Index at 1 month (lower better) | 3 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 2 Bleeding at 4/6 weeks | 6 | 299 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.08, ‐0.03] |
| 2.1 Parallel‐group studies | 3 | 169 | Mean Difference (Random, 95% CI) | ‐0.10 [‐0.15, ‐0.05] |
| 2.2 Split‐mouth studies | 3 | 130 | Mean Difference (Random, 95% CI) | ‐0.04 [‐0.07, ‐0.02] |
| 3 Bleeding at 3 months | 2 | 135 | Mean Difference (Random, 95% CI) | ‐0.10 [‐0.15, ‐0.04] |
| 3.1 Parallel‐group studies | 1 | 77 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 3.2 Split‐mouth studies | 1 | 58 | Mean Difference (Random, 95% CI) | ‐0.12 [‐0.13, ‐0.11] |
| 4 Probing pocket depth at 4 to 6 weeks | 3 | 137 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.27, 0.16] |
| 4.1 Parallel‐group studies | 1 | 77 | Mean Difference (Random, 95% CI) | 0.01 [‐0.28, 0.30] |
| 4.2 Split‐mouth studies | 2 | 60 | Mean Difference (Random, 95% CI) | ‐0.13 [‐0.44, 0.18] |
| 5 Probing pocket depth at 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Plaque at 1 month (lower better) (parallel group studies) | 5 | 290 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.84, ‐0.11] |
| 7 Plaque at 1 month (split‐mouth studies) | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.07 [‐0.32, 0.18] | |
| 8 Plaque at 3 months | 2 | 135 | Mean Difference (Random, 95% CI) | ‐0.12 [‐0.33, 0.10] |
| 8.1 Parallel group studies | 1 | 77 | Mean Difference (Random, 95% CI) | ‐0.24 [‐0.41, ‐0.07] |
| 8.2 Split‐mouth studies | 1 | 58 | Mean Difference (Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
Comparison 7. Wooden interdental cleaning stick versus floss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Plaque index at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 8. Rubber/elastomeric interdental cleaning stick versus floss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival index at 1 month/6 weeks | 6 | 256 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.69, 0.24] |
| 1.1 Manual sticks | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.89, 0.56] |
| 1.2 Powered sticks | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.36, 0.37] |
| 2 Gingival index at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Bleeding at 1 month/6 weeks (lower better) | 5 | 212 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.08, 0.03] |
| 3.1 Manual sticks | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 3.2 Powered sticks | 3 | 163 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 4 Bleeding at 3 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Plaque index at 1 month/6 weeks | 6 | 273 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.46, 0.29] |
| 5.1 Manual sticks | 3 | 110 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.72, 0.07] |
| 5.2 Powered sticks | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.41, 0.70] |
Comparison 9. Oral irrigation versus floss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival Index at 1 month (lower better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Bleeding at 1 month (lower better) | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.19, ‐0.05] |
| 3 Plaque Index at 1 month (lower better) | 2 | 133 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.08, 0.70] |
Comparison 10. Interdental cleaning stick versus interdental brush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gingival index at 1 month/6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Bleeding at 1 month/6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Plaque index at 1 month/6 weeks | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.33, 0.49] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnes 2005.
| Methods | Trial design: parallel, 3 arms Location: University of Nebraska Medical Center, College of Dentistry, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults in good general health, with a minimum of 20 evaluable teeth, not including third molars, toothbrushing at least once a day Exclusion criteria: systemic disease (AIDS, leukaemia, cirrhosis, sarcoidosis, diabetes mellitus, hepatitis), a history of rheumatic fever or the need for antibiotic prophylaxis (heart valve replacement, heart valve dysfunction, heart valve prosthesis, or other artificial joints), prophylactic or therapeutic antibiotic use within two months prior to the start of the study; pregnancy or hormone therapy; visual signs of rampant caries or advanced periodontitis; fixed orthodontic or removable prosthodontic appliances, and lack of dexterity required for tooth brushing, flossing, or irrigating Baseline plaque status: minimum mean plaque score of 2.0 Baseline periodontal status: 50% bleeding sites Age at baseline (years): 19 to 70 (age distribution across intervention groups not reported) Sex: not reported Number randomised: 105 (Gp A 35; Gp B 35; Gp C 35) Number evaluated: 95 (Gp A 31; Gp B 32; Gp C 32) Smoking status not reported |
|
| Interventions |
Comparison: manual toothbrushing and flossing versus manual toothbrushing and water jet Group A (n = 31 evaluated): twice‐daily toothbrushing for 2 minutes using a standard soft‐bristle manual toothbrush and once‐daily flossing with unwaxed and mint‐flavoured dental floss; Gp B (n = 32 evaluated): standard soft‐bristle manual toothbrush and the use of water jet (Waterpik) once daily in the evening with 500 ml of lukewarm water; Other intervention not included in analysis: Gp C (n = 32 evaluated): twice daily brushing for 2 minutes using Waterpik sonic toothbrush and use of water jet (Waterpik) once daily in the evening with 500 ml of lukewarm water Training: verbal and written instructions on irrigating technique, correct flossing technique, and on Modified Bass toothbrushing technique. Participants were to refrain from using any additional oral hygiene aid, including therapeutic mouthrinses. Baseline cleaning: not reported Compliance assessment: not reported Duration of intervention: 28 days |
|
| Outcomes | Measurements: at baseline, 14 days, and 28 days Dental plaque: Proximal/Marginal Plaque Index after disclosing plaque with disclosing solution Periodontal disease ‐ gingivitis: gingival bleeding measured at interproximal sites using Carter & Barnes Bleeding Index; gingivitis scored at six sites per tooth using the Löe & Silness Gingival Index Caries: not reported Adverse outcomes: none, although method of assessing adverse events was not reported Attrition: 10 participants lost, 9 requiring treatment with antibiotics, 1 participant dismissed due to illness requiring corticosteroid treatment, random across groups |
|
| Funding | Supported by Waterpik Technologies (manufacturer of the sonic toothbrush and water jet). One author Waterpik Techologies Fort employee | |
| Notes | Examinations performed by 2 experienced examiners who were calibrated by consensus. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "35 subjects randomly assigned to each of three groups" Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blinded" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "single‐blinded" Comment: examiners may have been blinded but method of blinding not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants failed to complete, roughly equivalent across all three groups Reasons for attrition were illnesses requiring treatment with antibiotics (9 participants) or corticosteroids (1 participant). Attrition reportedly random across the groups and unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes mentioned in Methods were reported in Results |
| Other bias | Unclear risk | Compliance not assessed |
Bauroth 2003.
| Methods | Trial design: parallel group (3 arms) Location: USA Number of centres: not reported Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults with at least 20 intact natural teeth with scorable facial and lingual surfaces Exclusion criteria: significant oral soft‐tissue pathology (other than gingivitis), gross dental caries, history of allergy to oral care products, treatment with antibiotic or anti‐inflammatory drugs, history of a condition requiring antibiotic coverage before undergoing invasive dental procedures, moderate or advanced periodontitis and pregnancy, third molars, orthodontically banded teeth or abutment teeth Baseline plaque status: minimum mean Plaque Index score of 1.95 Baseline periodontal status: minimum mean interproximal Modified Gingival Index score of 1.75 Age at baseline (years): range 18 to 65; mean (SD): 39.9 (10.66); age distribution across groups: Gp A 40.1 (10.65), Gp B 39.6 (10.97), Gp C 39.9 (10.44) Sex: 122 males/204 females; Gp A 42/66, Gp B 42/68, Gp C 38/70 Number randomised: 362 Number evaluated: 326 (Gp A 108, Gp B 110, Gp C 108) Number evaluated: 324 at 3 months; 314 at 6 months (numbers for each group not reported) Smoking status: 246 non‐smokers (75.5%) and 80 smokers (24.5%) |
|
| Interventions |
Comparisons: manual toothbrushing and flossing versus manual toothbrushing and negative control rinsing Gp A (n = 108 evaluated) manual toothbrushing twice daily plus once daily use of waxed dental floss (Reach waxed dental floss, Johnson & Johnson) Gp B (n = 110 evaluated) manual toothbrushing plus twice‐daily rinsing with 20 millilitres of a 5% hydro‐alcohol negative control rinse for 30 seconds All participants given a soft‐textured toothbrush (Oral‐B 35, Gillette, Boston) and a dentifrice (Colgate MFP, Colgate‐Palmolive, New York) Duration of intervention: 6 months Other interventions (not included in the review): Gp C, manual toothbrushing with a soft‐textured toothbrush plus twice‐daily rinsing for 30 seconds with 20 millilitres of an essential oil mouthrinse (Cool Mint Listerine Antiseptic) Baseline cleaning: included a complete dental prophylaxis to remove plaque, stain, and calculus Training: participants instructed in assigned regimens, and supervised during first use Participants in Gp B given written flossing instructions Compliance: participants given diaries to record daily product use; participants returned to the clinical site monthly during which compliance was monitored by measuring returned supplies and reviewing daily diaries |
|
| Outcomes | Measurements: at baseline, 3 months, and 6 months Dental plaque: Turesky modification of Quigley‐Hein Plaque Index Periodontal disease ‐ gingivitis: Modified Interproximal Gingival Index (MGI), Bleeding Index (BI) Caries: not reported Adverse effects: oral soft tissue assessment undertaken at baseline, at three and six months, but not reported Attrition: 38 nonevaluable at 3 months and 48 at 6 months. Deemed nonevaluable for protocol infractions, failure to comply with produce usage instructions, or initiation of systemic drug therapy Numbers not given by group |
|
| Funding | Not reported. Three authors affiliated to industry (Pfizer) | |
| Notes | All examinations were performed by 2 trained dental examiners. This study used the same protocol design as Sharma 2002. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We assigned each enrolled subject to one of three groups according to a randomization schedule." Comment: no description of the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "We designed a randomized, controlled, observer blind, parallel‐group, six‐month clinical trial..." Comment: not possible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "staff at the study site instructed subjects to refrain from using their test products for at least four hours before these examinations to eliminate potential bias resulting from residual product odor" Comment: study was observer blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Subjects deemed non‐evaluable for protocol infractions, failing to comply with produce usage instructions or initiating systemic drug therapy." Comment: overall 48 out of 362 participants were considered nonevaluable at six months. Number of participants lost to follow‐up in each group could not be ascertained from the report. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Oral soft‐tissue examinations performed but not reported in Results |
| Other bias | Unclear risk | Compliance: participants were issued diaries to record product use. Non‐compliance was a factor in decision to omit some participants.from evaluation. Specific numbers of those failing to comply with product use was not reported. |
Biesbrock 2007.
| Methods | Trial design: parallel group, (6 arms) Location: USA Number of centres: not reported Recruitment period: not reported |
|
| Participants | Inclusion criteria: healthy adult participants between 18 and 70 years of age, brushing at least twice daily Exclusion criteria: less than 16 natural teeth, orthodontic appliances, removable partial dentures, extensive dental treatment needs, pre‐medication needs for dental care, history of antibiotic usage two weeks prior to study initiation, pregnancy, or nursing Baseline plaque: not reported Baseline periodontal status: at least 15 Löe‐Silness bleeding sites at screening; at least twice‐daily brushing Age: range 18 to 69 years (numbers for each group not reported) Sex: 31% males/69% females, numbers for each group not reported Number randomised: 179 (Gp A 28; Gp B 29 ; Gp C 30; Gp D 29; Gp E 30; Gp F 28) Intervention groups relevant to review: Gp A and Gp B Number evaluated: 174 (numbers for each group not reported) Smoking status: not reported |
|
| Interventions |
Comparison: powered toothbrushing and automated flossing versus powered toothbrushing Gp A (n = 28 evaluated) oscillating/rotating power toothbrush (Oral‐B Professional) and Crest® Pro‐Health™ dentifrice plus power flosser (Oral‐B Hummingbird, Procter & Gamble Co) twice a day Gp B (n = 29 evaluated) oscillating/rotating power toothbrush (Oral‐B Professional) and Crest® Pro‐ Health™ twice a day Duration of intervention: 8 weeks Other interventions (not included in the review): Gp C (n = 30) manual toothbrush Colgate Wave plus Colgate Total toothpaste Gp D (n = 29) manual toothbrush Colgate Wave plus Colgate Total toothpaste plus essential oil rinse (Listerine) Gp E (30): manual toothbrush Oral‐B CrossAction plus Crest® Pro‐Health™ dentifrice Gp F (n = 28) manual toothbrush Oral‐B CrossAction + Pro‐Health™ cetylpyridinium chloride rinse Baseline cleaning: dental prophylaxis administered after assessment of eligibility Training: participants received written (test kit) and verbal (supervised) instructions on product usage. Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 4 weeks, and 8 weeks Dental plaque and calculus: Navy Plaque Index (Rustogi Modification) on buccal and lingual surfaces Periodontal disease ‐ gingivitis: Löe & Silness Gingival Index on six surfaces Adverse effects: product‐related adverse events recorded at each visit; assessed by blinded oral examination Attrition: 5 participants lost to follow‐up; however, it was stated that: "no subject discontinued treatment due to product‐related adverse events" |
|
| Funding | Supported by Procter & Gamble. Three authors P & G employees | |
| Notes | Crest® Pro‐Health™ dentifrice contains 0.454% stannous fluoride/sodium hexametaphosphate. Examiners training or calibration not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible subjects were stratified based on gender and the number of baseline bleeding sites... and randomly assigned to one of six test regimens." Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All test products were distributed in blinded kit boxes, instructions were provided remotely from examination" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No subject discontinued treatment due to product‐related adverse events." Comment: 5 participants did not complete the eight‐week study. Number of participants lost to follow‐up in each of the groups could not be ascertained from the report, but can be estimated at 1 to 2 per group. Attrition was low (5 out of 179) and balanced between groups, therefore unlikely to affect results. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Adverse events were recorded at each visit and the study reported that no participant discontinued treatment due to product‐related adverse events, but did not state whether there were any adverse events.. |
| Other bias | Unclear risk | Compliance not assessed |
Christou 1998.
| Methods | Trial design: split‐mouth, (2 arms) Location: Academic Centre for Dentistry, Amsterdam, The Netherlands Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adult patients not previously treated for periodontitis, 25 years old or older, at least 3 natural teeth present in each quadrant Exclusion criteria: use of antibiotics over last 3 months before baseline, use of interdental cleaning aids on a regular basis Baseline plaque: not reported Baseline periodontal status: generalised moderate to severe periodontitis, the presence of at least 1 site in each quadrant for fulfilling all following criteria: probing depths > 5 mm, bleeding on probing and radiographic evidence of alveolar bone loss, gingiva with little or no recession showing overt signs of inflammation Age at baseline (years): age range 27 to 72, mean age 37.4 Sex: 14 males/12 females Number randomised: 26 (Gp A and Gp B both had 26 participants as this was a split‐mouth study) Number evaluated: 26 (Gp A and Gp B both had 26 participants as this was a split‐mouth study) Attrition per group: none lost to follow‐up Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and interdental brushing versus manual toothbrushing and flossing Gp A (n = 26 evaluated) interdental brushes (frequency of use not reported) Gp B (n = 26 evaluated) dental floss (frequency of use not reported) All participants received a manual toothbrush Duration of intervention: 6 weeks Training: participants received detailed instructions for use of a manual toothbrush, dental floss and interdental brushes by a dental hygienist and were provided with by take‐home written instructions. Baseline cleaning: supragingival calculus was removed at sites where interference with interdental cleaning occurred. Compliance assessment: compliance was confirmed by a telephone call after a week of treatment. After 3 weeks, oral hygiene instructions were reinforced by the dental hygienist. |
|
| Outcomes | Measurements: at baseline and 6 weeks Dental plaque: Volpe modification of Quigley and Hein Plaque Index Periodontal disease ‐ gingivitis: measured by bleeding on probing (BOP) assessed by Angulated Bleeding Index (ABI) and Periodontal Pocket Bleeding Index (PPBI) Probing depth (PD) evaluated using a force controlled probe Adverse effects: self‐reported; participants completed a questionnaire concerning any problems with dental floss (DF) or interdental brushes (IDB), level of comfort in handling the 2 devices and their perception of efficacy of the devices. 14 participants experienced problems with use of dental floss, 2 with use of interdental brushes, 2 with both, and 8 did not encounter any problems. Attrition: no participants were lost from the study. |
|
| Funding | State Scholarship Foundation of Greece gave a grant; Entra ‐ Lactona BV provided brushes and interdental brushes. | |
| Notes | Trial authors recorded interdental spaces that could not be entered by the assigned interdental device and excluded them from the analysis (12 sites for any size of the IDB and 2 sites for the DF). All measurements were carried out by the same examiner under the same conditions; examiner reliability was not reported, but a force‐controlled probe was used allowing confidence in the outcome assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The use of DF was randomly assigned to the left or the right side of the mouth and the use of IDB to the other side" Comment: no further information given |
| Allocation concealment (selection bias) | Low risk | Split‐mouth study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All procedures concerning instruction, cleaning and exclusion of sites from the analyses were performed in the absence of the examiner, keeping these recordings blind throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no losses to follow‐up. 12 sites not accessible to any size of IDB and 2 sites not accessible to DF were excluded from the analysis. Total number of assessed sites not reported |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes stated in the Methods section were addressed in the Results. |
| Other bias | Unclear risk | Compliance confirmed by a telephone call after a week of treatment, but not reported. |
Cronin 1997.
| Methods | Trial design: parallel group, 2 arms Location: not reported Number of centres: not reported Recruitment period: not reported |
|
| Participants | Inclusion criteria: healthy dentate adults with sufficient levels of plaque and gingivitis, more than 20 natural teeth, brushing teeth at least twice daily, flossing no more than once a week Exclusion criteria: gross carious lesions, fully crowned or restored teeth, orthodontically banded teeth, abutment teeth and third molars, major hard or soft tissue lesions, taking medication affecting gingival health (hormones, antisialologues, steroids), antibiotics intake within 30 days of enrolment, a history of rheumatic heart disease, diabetes mellitus or hepatitis. Females who were pregnant, lactating or planning a pregnancy. A physical condition limiting manual dexterity, dental prophylaxis within 30 days of enrolment, grossly neglected oral hygiene, advanced periodontitis, calculus sufficient to interfere with scoring plaque, inflammation, wide embrasure areas or advanced gingival recession. Baseline plaque status: supragingival plaque score (Turesky modification of Quigley & Hein Plaque Index) score > 2.0 Baseline periodontal status: Löe & Silness Gingival Index score within the range 1.0 to 1.6 Age at baseline: Gp A: 20 to 59 years, mean age 35.7 (10.9); Gp B, range 22 to 65 years, mean age 36.6 (10.4) Sex: 16 males/43 females (Gp A 8/22), (Gp B 8/21) data presented only for evaluated participants Number randomised: 60 (Gp A 30, Gp B 30) Number evaluated: 59 (Gp A 30, Gp B 29) Attrition: 1 participant in Gp B (floss) failed to attend for the final examination Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing plus rubber/elastomeric tooth cleaning stick (electric interdental cleaning device, ID2) versus manual toothbrushing and floss Gp A (n = 30 evaluated) Braun Oral‐B interclean with Flexi‐Tip attachment (ID2 ‐ electric interdental cleaning device) Toothbrush twice a day and interdental device (ID2 with Flexi‐tip attachment) once a day Gp B (n = 29 evaluated) manual waxed floss (Johnson & Johnson) All participants used manual toothbrushes twice daily and Colgate Regular toothpaste Toothbrush twice a day and floss once a day Duration of intervention: 4 weeks Baseline cleaning: at day 1, all participants received supragingival scaling and a prophylaxis Training: written and verbal instructions given to each participant, told not to use any additional mechanical or chemical plaque removing agents during the study Compliance assessment: not reported |
|
| Outcomes | Measurements: at day 1 and week 4 Dental plaque: plaque index, Turesky modification of Quigley & Hein Plaque Index Periodontal disease ‐ gingivitis: Löe & Silness Gingival Index (GI) (bleeding index scores derived from the gingival index data) Adverse effects: at each study visit, safety was assessed by examinations of intra‐ and extra‐oral tissues; safety analyses revealed no evidence of irritation or gingival abrasion in either group, no adverse events were observed or reported Attrition: 1 participant in the floss group did not attend the week 4 assessment. |
|
| Funding | Not reported. One author Braun employee | |
| Notes | At baseline, the floss group had statistically significant higher gingival and bleeding indices compared to the ID2 group. All clinical examinations were performed by the same (blinded) examiner. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible subjects were randomized to receive either dental floss or the ID2 with Flexi‐Tip attachment" Comment: participants were randomised to groups, but the paper did not indicate the means of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No description of how the allocation sequence was concealed. Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all clinical examinations were performed by the same (blinded) investigator"; "to ensure that the study investigator remained blinded, instructions for the use of the respective devices were given independently by a licensed, registered dental hygienist" Comment: the examiner did not know which groups the participants had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported, including 1 participant in the control group who did not return for the week 4 measurements |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes stated in the Methods section were addressed in the Results. |
| Other bias | Unclear risk | Compliance not assessed Baseline difference noted by trial authors |
Cronin 2005.
| Methods | Trial design: parallel group, 3 arms Location: not reported Number of centres: not reported Recruitment period: not reported |
|
| Participants | Inclusion criteria: healthy non‐smoking dentate adults with sufficient levels of plaque and gingivitis and a minimum of 18 scorable teeth without third molars, use of manual toothbrush at least once daily, infrequent use of dental floss Exclusion criteria: orthodontic appliances, bridges, crowns, implants, neglected dental health, major hard or soft tissue lesions, excess calculus, wide embrasure areas or advanced gingival recession, physical condition limiting manual dexterity, antibiotics or anti‐inflammatory medication intake for three consecutive days in the previous 28 days, need for antibiotic prophylaxis, pregnant or lactating females Baseline plaque status: whole mouth score of Proximal/Marginal Plaque Index (PMI) ≥ 2.0 Baseline periodontal status: whole mouth score of Löe and Silness Gingival Index (LSGI) score ≥ 1.1 Age at baseline: range 18 to 70 years (interdental pick group mean age 34.7; floss group mean age 35.2) Sex: 23 males/55 females (Gp A 8/17; Gp B 7/20; Gp C 8/18) Number randomised: 84 (Gp A 28; Gp B 28; Gp C 28) Number evaluated: 78 (Gp A 25, Gp B 27, Gp C 26) Attrition: Gp A 3; Gp B 1; Gp C 2, none related to the test products Smoking status: all non‐smokers |
|
| Interventions |
Comparison: manual toothbrushing plus rubber/elastomeric tooth cleaning stick (electric device including pick) versus manual toothbrushing and floss Gp A (n = 25 evaluated) manual waxed floss (Johnson & Johnson) Gp B (n = 27 evaluated) Oral‐B OB2040 interdental cleaning device with a cleaning pick (ID/P) attachment All participants received a manual toothbrush (Oral B Indicator) and Colgate Cavity Protection toothpaste Toothbrush twice a day and use of interdental cleaning devices (floss, ID/P) once a day in the evening before manual toothbrushing Duration of intervention: 30 days Other interventions (not included in the review): Gp C: Oral‐B OB2040 interdental cleaning device with a flossette attachment Baseline cleaning: not reported as having been undertaken Training: participants were given written and verbal instructions about their devices by a dental hygienist and were able to demonstrate the correct cleaning procedures; no brushing instructions were given Compliance assessment: participants reported on a diary form the times of tooth brushing and interdental cleaning, together with the number of picks (or flossettes) used. |
|
| Outcomes | Measurements: at day 1 and day 30 Dental plaque: Proximal/Marginal Plaque Index (PMI) Periodontal disease ‐ gingivitis: Löe & Silness Gingival Index (LSGI), Löe & Silness bleeding scores Adverse effects: safety evaluations of hard and soft tissue performed and all adverse events were recorded; adverse events of mild to moderate intensity were reported by 17 participants (Gp A 7; Gp B 4; Gp C 6), none of which were related to product use or study procedure Attrition: six participants discontinued the study for reasons unrelated to the test products. Two failed to attend Day 30 visit, 1 received antibiotics, 1 became pregnant, 1 failed to use the study product for more than 2 consecutive days and 1 had pain related to an endodontic treatment. |
|
| Funding | Funded by Oral‐B and three authors employees | |
| Notes | All examinations were performed by the same examiner, who was familiar with the measured indices and had been calibrated for intraexaminer reliability. There was wide variability in data for the ID/P group, which may have been a weakness in the study design. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible subjects were randomly assigned to use with the OB2040 with the flossette (ID/F) or pick (ID/P), or manual floss" Comment: the study was described as stratified, randomised. Stratified according to sex and initial plaque and gingival mean scores. Groups were not statistically significantly different at baseline. No description of method of generating the random sequence for allocation |
| Allocation concealment (selection bias) | Unclear risk | There was no description of how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "examiner blinded, parallel group study" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiner blinded, parallel group study", "all examinations were performed by the same examiner who was blinded to treatment randomization" Comment: the examiner did not know which group the participants they were assessing had been assigned to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts (n = 6) noted and reasons provided. None related to test products. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Compliance assessed by diaries in which participants recorded the times of using the assigned products, but the data were not reported in Results. |
Finkelstein 1990.
| Methods | Trial design: parallel group, 5 arms Location: New Jersey, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults with at least 20 uncrowned teeth and a commitment to adhere to the test protocol, occasional flossing (1 to 3 times per week) Exclusion criteria: removable prostheses, gross oral pathology, dental prophylaxis within the last 3 months Baseline plaque: not reported Baseline periodontal status: at least 10 interdental bleeding sites measured by the EIBI Age at baseline: not reported Sex: not reported Number randomised: 161 (Gp A 31; Gp B 30; Gp C 32; Gp D 33; Gp E 32) (although 161 participants were randomised, only 158 started the study) Number evaluated: 158 (Gp A 31; Gp B 30; Gp C 32; Gp D 33; Gp E 32) Smoking status: not reported |
|
| Interventions |
Comparisons: manual toothbrushing versus manual toothbrushing and floss versus manual toothbrushing and a wooden interdental cleaner Gp A (n = 31 evaluated) wooden interdental cleaner (Stim‐U‐Dent), Johnson & Johnson Gp B (n = 30 evaluated) manual waxed floss (Johnson & Johnson) Gp E (n = 32 evaluated) manual toothbrushing All participants received a manual toothbrush (Oral B Indicator) and Colgate Cavity Protection toothpaste Toothbrushing was carried out "ad lib" throughout Duration of intervention: 12 weeks Other interventions (not included in the review): Gp C: essential oil mouthrinse (Listerine Antiseptic) Gp D: cetylpyridinium chloride mouthrinse (Cepacol, Merrel Dow Pharmaceutical) Training: no training was reported as having been provided. It was stated that each product was used according to the manufacturers directions. Baseline cleaning: not reported as having been undertaken Compliance assessment: not reported |
|
| Outcomes | Measurements: at week 0, week 6, and week 12 Dental plaque: Global Plaque Index Periodontal disease ‐ gingivitis: Löe & Silness Gingival Inflammation Index (VGI) and Eastman Interdental Bleeding Index (EIBI) Adverse effects: not reported Attrition: 3 randomised participants did not start the study. |
|
| Funding | Funded by a grant from Johnson & Johnson Dental Care Company; lead author J & J employee | |
| Notes | Examiner training or calibration not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of the five test groups…" Comment: no further information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants did not complete the study with no information on the reasons for lost to follow‐up; however we considered it unlikely to affect the results. |
| Selective reporting (reporting bias) | High risk | No standard deviations reported; unable to use data |
| Other bias | Unclear risk | Compliance not assessed. |
Frascella 2000.
| Methods | Trial design: parallel group Location: New Jersey, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | 64 adults in good general health with mild to moderate gingivitis Inclusion criteria: at least 18 natural teeth (excluding third molars) without crowns or orthodontic appliances Exclusion criteria: severe periodontal disease, excessive caries, major hard or soft tissue trauma or lesions, severe gingival recession or bone loss; regular use of an oral irrigator; use of drugs that could affect results less than 28 days before the baseline visit; systemic conditions that could affect gingival assessment; need for prophylactic antibiotics for dental treatment Baseline plaque: not reported Baseline periodontal status: > 30% of bleeding sites Age at baseline: Gp A (oral irrigator group) mean 42.2 years (range 26 to 61); Gp B mean 36.8 years (range 18 to 55 years) Sex: 22 males (Gp A 10, Gp B 12); 42 females (Gp A 22, Gp B 20) Number randomised: 64 (32 in each group) Number evaluated: Gp A 26; Gp B 30 (at 4 weeks) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and oral irrigator versus manual toothbrushing Gp A: (n = 26 evaluated at 4 weeks) manual toothbrushing and oral irrigator (Braun Oral‐B Oxyjet MD15) Gp B: (n = 30 evaluated at 4 weeks) manual toothbrushing All participants received standard ADA‐approved manual toothbrush and Crest Regular toothpaste and were asked to brush twice a day, with oral irrigator group instructed in use of the device and asked to use it once daily in the evening after brushing (on rotating, non‐pulsating mode with 600 ml water at pressure level 3). Duration of intervention: 8 weeks Training: oral irrigator group instructed by the dental therapist who had conducted the baseline clinical exam, and also given written instructions Baseline cleaning: participants asked not to do any oral hygiene activities after midnight on night before visit. Assessed at baseline visit for gingivitis, plaque and bleeding Compliance assessment: yes, "only those subjects who completed all procedures and complied with all areas of the protocol were deemed to have completed the study and were included in the data analysis" |
|
| Outcomes | Clinical assessments were made at 6 sites per tooth (not third molars), i.e. 168 sites per participant, at baseline, week 4, and week 8 Gingival inflammation: modified gingival index (1 to 4) Bleeding: Angular Bleeding Index (% of bleeding sites) Plaque: Turesky‐Gilmore‐Glickman modification of the Quigley‐Hein Plaque Index (1 to 5) Adverse events: "three subjects in the MD15 group and 1 subject in the control group reported adverse events, but these events were not considered by the investigator to be related to study treatment" Compliance: "only those subjects who completed all procedures and complied with all areas of the protocol were deemed to have completed the study and were included in the data analysis" Attrition: "in total, eight subjects (six from the MD15 group and two from the control group discontinued the study prior to the visit at week 4". Reasons: 3 brushed their teeth before baseline visit; 1 inconvenience; 1 stopped using oral irrigator; 3 did not return for post baseline visit." 2 participants did not return for 4‐week visit but did for 8‐week visit. |
|
| Funding | Authors worked for Braun or Procter and Gamble | |
| Notes | Participants "randomly selected by the investigator from the general population" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned to each group using "pre‐determined computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Use of "pre‐determined computer‐generated randomization schedule" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded ‐ participants aware which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Examiner‐blind" Clinical assessments made by the same assessor at the same time points |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "In total, eight subjects (six from the MD15 group and two from the control group) discontinued the study prior to the visit at week 4". Reasons: 3 brushed their teeth before baseline visit; 1 inconvenience; 1 stopped using oral irrigator; 3 did not return for post baseline visit." 2 participants did not return for 4‐week visit but did for 8‐week visit. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Baseline difference in age unlikely to be relevant: MD15 group mean 42.2 years, control group mean 36.8 years |
Gordon 1996.
| Methods | Trial design: reported as parallel study, but after 30 days each group crossed over to the other interdental cleaner for an additional 30 days; 2 arms (data from the first period only ‐ see notes) Location: New Jersey, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults with more than 20 natural teeth Exclusion criteria: grossly carious, fully crowned or restored, orthodontically banded, abutment teeth or third molars, use of medication affecting gingival health (hormonal therapy, antisialogogues, steroids), antibiotic intake within 30 days of enrolment, history of rheumatic fever, diabetes mellitus or hepatitis, physical condition limiting manual dexterity, dental prophylaxis in the 30 days prior to enrolment, grossly neglected oral hygiene, advanced periodontitis or calculus sufficient to interfere with scoring plaque or inflammation, female participants who were either pregnant, planning a pregnancy or lactating Baseline plaque: minimum Proximal/Marginal Plaque Index score of 2.0 Baseline periodontal status: gingivitis ‐ Modified Gingival Index (MGI) score within the range 1.5 to 2.3 Age at baseline: range 24 to 45 years Sex: both male and female participants, but numbers not reported Number randomised: 60 (Gp A 30; Gp B 30) Number evaluated: 52 (Gp A 24; Gp B 28) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing and a powered interdental cleaning device Gp A: waxed floss (Johnson & Johnson), used at night prior to brushing their teeth Gp B: powered interdental cleaning device (Braun Oral‐B Interclean, ID2), used at night prior to brushing their teeth All participants used a manual toothbrush, Oral‐B P35, and were instructed to brush twice daily with Colgate Regular Toothpaste Duration of intervention: 30 days (second period crossover of 30 days not considered as there was no washout period) Training: participants were given written and verbal instructions for interdental cleaning, using either floss or the ID2 Baseline cleaning: all participants underwent a dental prophylaxis of supragingival scaling and a rubber cup polishing Compliance assessment: not reported |
|
| Outcomes | Measurements: at day 1 and day 30 Dental plaque: Proximal/Marginal Plaque Index Periodontal disease ‐ gingivitis: Lobene Modified Gingival Index and Modified Papillary Bleeding Index Adverse effects: at each visit (day 1, day 15, day 30) safety evaluations including intra and extraoral tissues were performed and all areas were scored and recorded as "normal" or "abnormal"; there were no adverse effects reported in any of the participants in either group. Four participants dropped out due to other adverse events, non treatment‐related adverse events. Attrition: 8 participants were lost to follow‐up, 4 failed to report on Day 30 and 4 others had non‐treatment related adverse events. |
|
| Funding | Not stated | |
| Notes | All clinical examinations were performed by the same investigator. The cross‐over part of the study was conducted to assess preference, and the clinical measurements only measured for the first period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomized to receive products in three strata, represented by gingival scores in the ranges 1.5‐1.7, 1.7‐2.0 and 2.0‐2.3. Within each stratum, the randomization was structured in blocks of four subjects" "before being randomly assigned to one of the two experimental groups" Comment: block randomisation was done using a random number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated that it was a single‐blinded study, but it was unclear whether the examiner was blind to the groups the participants were assigned to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants dropped out; reasons for dropout provided and groups from which they withdrew; four participants discontinued the study due to adverse events, none of which were related to treatment; 52 participants who completed the study were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | None identified. All outcomes mentioned in Methods were addressed in Results section. |
| Other bias | Unclear risk | Compliance not assessed |
Goyal 2012.
| Methods | Trial design: parallel group, 4 arms Location: Canada Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: healthy non‐smoking adults with at least 20 scorable teeth (excluding third molars), no hard or soft tissue lesions Exclusion criteria: visible signs of periodontal disease, probing depth > 5 mm, any systemic disease such as diabetes or autoimmune disease, pregnancy, use of medications that impact gingival health, antibiotics use within six months of the study, orthodontic appliances, implants, crowns, bridges, veneers, removable appliances Baseline plaque status: minimum score of 0.60 for the Rustogi Modified Navy Plaque Index (RMNPI) Baseline periodontal status: minimum score of 1.75 for the Lobene Modified Gingival Index (MGI), 50% Bleeding on Probing (BOP) Age at baseline: age range 25 to 65 years Sex: male/female: 44/96, (Gp A 15/20, Gp B 11/24, Gp C 8/27, Gp D 10/25) Number randomised: 140 (Gp A 35, Gp B 35, Gp C 35, Gp D 35) Number evaluated: 139 (Gp A 35, Gp B 35, Gp C 35, Gp D 34) Smoking status: all non‐smokers |
|
| Interventions |
Comparison: sonic toothbrush plus water irrigator versus sonic toothbrush Gp A: sonic toothbrush twice daily plus water irrigator once daily (Waterpik Complete Care: device that combines water irrigator and powered toothbrush, Sensonic Professional Plus Toothbrush) Gp B: sonic toothbrush twice daily (Sensonic Professional Plus Toothbrush) Duration of intervention: 4 weeks Other interventions not included in the review: Gp C: powered sonic toothbrush (Sonicare FlexCare toothbrush) Gp D: ADA standard manual toothbrush (Oral‐B Indicator 35) Training: Gps A, B and C received written and verbal instructions based on the recommendations of the manufacturers; Gp D received no instructions. Baseline cleaning: none performed Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, week 2, and week 4 Dental plaque: Rustogi Modified Navy Plaque Index (RMNPI) by dividing the tooth into nine sections Periodontal disease ‐ gingivitis: Lobene Modified Gingival Index at facial and lingual surfaces and scored using a 0 to 4 scale; bleeding on probing was scored binary as "positive" or "negative" Adverse effects: examinations of oral tissue performed; there were no adverse effects during the study. Attrition: 1 participant was lost to follow‐up due to a death in the family. |
|
| Funding | Research grant from Waterpik Inc., Fort Collins, Colorado, and 1 author employee | |
| Notes | No information about the examiner | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly assigned to one of four treatment groups" Comment: trial report did not indicate how participants were randomised into groups |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single masked, parallel clinical study" Comment: participants knew which group they were assigned to |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "data collection was completed by one examiner who was blinded to the group assignment and product use for all indices and time points. Subjects were instructed not to discuss their product with the examiner" Comment: stringent steps were taken to ensure the examiner did not know which group the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant dropped out from Gp D, reason provided. |
| Selective reporting (reporting bias) | Low risk | None identified. All outcomes mentioned in Methods were addressed in the Results section. |
| Other bias | Unclear risk | Compliance not assessed |
Graziani 2017.
| Methods | Trial design: parallel group, 4 arms Location: University of Pisa, Italy Number of centres: 1 Recruitment period: study conducted between May 2011 and May 2016 |
|
| Participants | Inclusion criteria: 18 years and older, at least 20 natural teeth, periodontally healthy as defined by the absence of proximal attachment loss of > 3 mm in > 2 adjacent teeth, intact interdental papilla with no loss of interdental attachment, interdental area completely filled with the papillary tissue Exclusion criteria: pregnancy, lactation, contraceptives, systemic diseases, smoking over 20 cigarettes, pipes or cigars a day, systemic disease, pregnant or lactating females, females using contraceptive methods, inability to attend all time points Baseline plaque status: Full Mouth Plaque Score (FMPS) (%): Gp A 55.8 (23.2); Gp B 49.0 (23.0); Gp C 38.5 (17.9); Gp D 36.2 (24.5); in general below 50% Baseline periodontal status: Full Mouth Bleeding Score (FMBS) (%): Gp A 26.6 (20.6); Gp B 27.7 (15.4); Gp C 22.6 (19.5); Gp D 21.2 (19.0); Angulated Bleeding Index (AngBI) (%): Gp A 28.3 (18.8); Gp B 27.0 (24.5); Gp C 17.7 (16.7); Gp D 17.3 (16.1); Probing Pocket Depths (PPD) Age at baseline: mean age in years (SD), Gp A 28.7 (9.8); Gp B 26.1 (3.7); Gp C 26.4 (5.2); Gp D 26.4 (5.4) Sex: 29 males/31 females; (Gp A 9/6; Gp B 6/8; Gp C 7/9; Gp D 7/8) Number randomised: 60 Number evaluated: not reported |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and floss versus manual toothbrushing and interdental brushes versus manual toothbrushing and rubber interdental picks Gp A: manual toothbrush Gp B: dental floss (TePe Dental Tape) Gp C: interdental brushes (TePe interdental brush) Gp D: interdental sticks (GUM Soft‐Picks, Sunstar) All groups used manual toothbrush (TePe Select, TePe Munhygienprodukter AB) Duration of intervention: 28 days Training: training was given after randomisation at the start of the 'unclean phase' (at T‐7), followed by in‐mouth demonstration. Toothbrushing was instructed according to the modified bass technique. Participants were encouraged to practise for as long as they needed. Baseline cleaning: carried out one week after enrolment at T‐0, supragingival scaling and polishing using piezoelectric instruments and rubber cups Compliance assessment: not reported Smoking status: a mixture of smokers and non‐smokers equally distributed across intervention groups |
|
| Outcomes | Measurements: at baseline (7 days before start of 'clean phase'), time points T‐0 (day zero), T‐14 (14 days) and T‐28 (28 days) Dental plaque: Full Mouth Plaque Score (FMPS) recorded dichotomously (presence or absence of plaque) Periodontal disease ‐ gingivitis: Full Mouth Bleeding Score (FMBS) assessed dichotomously; Angulated Bleeding Index: using a probe running along the marginal gingiva at the angle of approximately 60°; Probing Pocket Depths (PPD) and Gingival Recession (GR) All measurements were taken at 6 sites per tooth, excluding third molars Adverse effects: not reported Attrition: all participants completed the study. |
|
| Funding | Partly funded by the Italian Ministry of Health and the Tuscan Region | |
| Notes | Examiners training or calibration not reported. Plaque reported to be unevenly represented among groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned using a computer generated table" Comment: adequate method of sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation to treatment was concealed to the clinical examiner and statistician with sealed opaque envelopes which were opened by a clinical staff member on the day of the allocation" Comment: steps to conceal participation allocation concealment were clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "allocation to treatment was concealed to the clinical examiner" Comment: the examiner did not know which groups the participants had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not reported, but based on the response from the lead author: two participants did not attend 28‐day examination: 1 in group 3, and 1 in group 4 |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods were addressed in the Results section. |
| Other bias | Unclear risk | Compliance assessed using diaries given to participants to record their adherence to oral hygiene regimen, but were poorly reported by participants. |
Hague 2007.
| Methods | Trial design: 2‐treatment period, pseudo‐crossover design*, (2 treatment periods, and a 14‐day wash‐out), 3 arms Location: OSU Dental Clinic, Ohio State University, USA Number of centres: 1 Recruitment period: Autumn 2005 to Spring 2006, recruitment incentives included preventive dental care and monetary compensation |
|
| Participants | Inclusion criteria: adults in good medical and dental health with ≥ 24 teeth in proximal contact, and able to attend 6 consecutive study visits 2 weeks apart Exclusion criteria: significant medical history, pregnancy, treatment with antiinflammatory or antibiotic drugs, periodontitis, gross caries, oral soft tissue pathology, crowns, implants, orthodontic appliances and dental prostheses Baseline plaque: moderate plaque formation after refraining from oral hygiene for 24 hours, measured using the Quigley‐Hein Plaque Index (Turesky modification), overall mean score 2.30 ± 0.33 Baseline periodontal status: overall mean score 0.62 ± 0.36 Age at baseline: mean age, 23.3 ± 5.0 years (Gp A: 23.8; Gp B: 23.0; Gp C: 23.2) Sex: 33 males/67 females (Gp A 14/21; Gp B 7/28; Gp C 13/19) (report presented data only for participants who completed the study) Number randomised: 102 (Gp A 35; Gp B 35; Gp C 32) Number evaluated: 89 (Gp A 31; Gp B 32; Gp C 26) Smoking status: "9% of the participants used tobacco products" |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and floss versus manual toothbrushing and an automated flossing device Gp A: manual toothbrush Gp B: manual dental floss (Glide Floss Comfort Plus, Procter & Gamble Co) once a day Gp C: battery‐operated automated flossing device (Ultra Flosser, William Getgey Co) once a day All groups used soft manual toothbrush for two minutes twice a day (Oral‐B Indicator 35 with Crest Cavity Protection Regular Toothpaste) Duration of intervention: 30 days (*the first period data only was used) Baseline cleaning: none carried out Training: each participant received toothbrushing instruction and instructions in the use of manual floss and the automated flosser. A dental health educator provided oral hygiene instruction using a typodont and written/visual instructions. After the instructions, each participant showed the appropriate techniques intraorally. Compliance assessment: participants were given a log to record frequency of brushing and flossing along with measurements of returned supplies; by reviewing the daily participant logs and returned dental products it was stated that the rate of compliance was comparable among participants. |
|
| Outcomes | Measurements: at baseline and days 15 and 30 Dental plaque: Quigley‐Hein Plaque Index (Turesky modification) based on 0 to 5 scoring system and using a disclosing solution Periodontal disease ‐ gingivitis: Löe & Silness Gingival Index based on 0 to 3 scoring system Adverse effects: at each visit, safety assessments of oral tissues were performed; soft tissue trauma in two participants resulted from improper use of the automated flossing device and was observed at day 15 of the first treatment period. Attrition: 13 participants withdrew from the study because of scheduling conflicts (n = 11). Out of these, 4 were from the control group, 3 from the manual flossing group and 6 from the automated flossing group. Two participants refused to use the products assigned. |
|
| Funding | Industry funded by William Getgey Company | |
| Notes | *Described as a cross‐over study but the same control group was used throughout. We used data from the first period only for both manual and automated flossing groups compared with the non‐flossing control group. 9% of the participants used tobacco products and half the women (n = 32) used oral contraceptives. One research examiner was responsible for all scoring and data collection; intraexaminer reliability tested before the trial began and good reproducibility was shown for both the plaque index (PI; k = 0.73) and gingival index (GI; k = 0.52) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the initial baseline visit, subjects were randomly assigned to a control, manual, or automated floss group using computer‐generated‐randomized sequencing to ensure a balanced design" Comment: adequate method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher examiner was blind to the subjects' group assignments" Comment: the examiner did not know which groups the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals reported were unlikely to affect the results as they were balanced between the groups. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes stated in the 'Methods' section were addressed in the 'Results'. |
| Other bias | Low risk | To assess compliance, participants were given a log to record product use, and were asked to return unused products at the end of the trial. It was stated that the rate of compliance was determined by a review of the daily participant logs and returned dental products and was comparable among participants. |
Imai 2011.
| Methods | Trial design: split‐mouth, 2 arms Location: University of British Columbia, Canada Number of centres: 1 Recruitment period: between September 2008 to February 2009 |
|
| Participants | Inclusion criteria: adult participants, a minimum of 4 interproximal areas per side with intact interdental papillae that could accommodate a minimum 0.6 mm interdental brush width; a minimum of 4 interproximal bleeding sites per side upon stimulation; dexterity to use floss; ability to attend 5 visits Exclusion criteria: required antibiotics premedication, use of tobacco products, chlorhexidine or over‐the‐counter mouthwashes, currently having full mouth orthodontic treatment, antibiotics intake within 1 month prior to the study Baseline plaque status: not reported Baseline periodontal status ‐ gingivitis: bleeding type 1 Embrasures Age at baseline: range 19 to 53 years Sex: 10 males, 20 females evaluated at 12 weeks Number randomised: 33 Number evaluated: 29 at 6 weeks and 30 at 12 weeks Smoking status: all non‐smokers |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing and interdental brushes All participants used manual toothbrush, twice a day (soft manual toothbrush, Curaprox CS 5460 Prime) Gp A1: waxed dental floss (Johnson & Johnson) on one side of the mouth Gp A2: interdental brush (Cupraprox Prime Series) on the other side Duration of intervention: 12 weeks Baseline cleaning: non‐surgical debridement using ultrasonic and hand scaling was performed 2 weeks prior to the baseline visit to allow for tissue healing and to stabilise baseline scores. Training: participants were instructed to brush their teeth in the morning and again at night using the modified Bass method and to use the floss and interdental brush once a day on the assigned side, preferably at night. They were instructed in dental flossing and interdental brush use by the study organiser. Compliance assessment: self‐assessment, participants were given a daily journal at baseline to self report their daily compliance with interdental brushing and dental flossing and compliance was found to be good. |
|
| Outcomes | Measurements: at baseline, 6 weeks, and 12 weeks Dental plaque: Silness & Löe Plaque Index measured on four interproximal surfaces Periodontal disease ‐ gingivitis: Eastman Interdental Bleeding Index (EIBI) Adverse effects: throughout the study, the examiner assessed the participants for soft tissue trauma, indicated by clinically visible gingival cuts, redness, abraded areas or damaged interdental papilla; there were no adverse events at any time point for floss or interdental brush. Attrition: 3 participants lost at 3‐week time point, and 4 participants were lost at 6‐week time point: 1 for a family emergency, 2 were not interested any longer, 1 started taking antibiotics and was dismissed. However, 1 participant returned to the study for the 12‐week assessment. |
|
| Funding | Study supported by Grants from the Canadian Foundation of Dental Hygiene Research and Education and the British Columbia Dental Hygienists Association; toothbrushes supplied by Enterprise Dentalink Inc. | |
| Notes | All participants were found to be right‐handed. Examiner training and intra‐examiner reliability was not reported but the EIBI was used, which is believed to have high reproducibility. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation of the products to left or right of the mouth was determined by a flip of coin by the study organizer" Comment: method of random sequence generation was simple (coin tossing), but valid |
| Allocation concealment (selection bias) | Low risk | Quote: "The interdental brush was randomly assigned to the left or right side of the subject's mouths with the dental floss assigned to the remaining side" Comment: interventions allocated simultaneously |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "determined by a flip of coin by the study organizer" Comment: personnel were not aware which side of the mouth had been chosen, but participants would have been aware |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "this was an examiner blinded trial" "Blinding was achieved by keeping all the clinical records collected by the examiner separate from the enrollment and randomization process conducted by the study organizer. Only the examiner, who was unaware of the product randomization throughout the study, collected the clinical measurements at baseline, 6, and 12 weeks." Comment: the examiner was unaware of product randomisation throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition adequately reported and explained; unlikely to affect the results |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported in the abstract and in the Methods section of the article were addressed in the Results. |
| Other bias | Low risk | Compliance assessed by participants' self‐reported journal entries and estimation of product use, which was approximated as high, with numbers provided for each group. |
Isaacs 1999.
| Methods | Trial design: parallel group, 2 arms Location: Indiana University, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: general healthy participants, at least 16 natural teeth, free of extensive periodontal disease or caries, dental floss users no more than once a week Exclusion criteria: anti‐inflammatory drugs, analgesics or anticoagulants at the time of recruitment, antibiotics within 7 days of recruitment, history of hepatitis, tuberculosis, rheumatic fewer or any condition requiring antibiotic premedication, pregnancy, lactation Baseline plaque status: interproximal plaque scores of greater than 2 (Turesky Modification of the Quigley‐Hein index) Baseline periodontal status: not reported Age at baseline: 18 years and older (not specified by range or per group) Sex: 43 males/127 females (Gp A 21/64, Gp B 22/63) Number randomised: 170 (Gp A 85; Gp B 85) Number evaluated: 147 reported, but the data provided in Table 1 indicated 145 participants completing the study (Gp A 73; Gp B 72) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and electrical cleaning device (ID2) (rubber/elastomeric cleaning stick) versus manual toothbrushing and floss Gp A: (n = 73 evaluated) manual toothbrush, twice a day, (Oral‐B 35) and a Braun Interclean ID2 interdental cleaning device Gp B: (n = 72 evaluated) manual toothbrush, twice a day, (Oral‐B 35) plus waxed dental floss Duration of intervention: 6 months Training: participants were instructed in the manual flossing technique or the use of the ID2, instructions were reviewed after 1 week Baseline cleaning: after the baseline examination, dental prophylaxis was performed to remove supragingival plaque, stain and calculus Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 3 months, and 6 months (6‐month data not usable) Dental plaque: interproximal surfaces only, using the Turesky modification of the Quigley‐Hein Index Periodontal disease ‐ gingivitis: Löe & Silness Gingivitis Index (GI) For both indices all teeth, except for third molars were examined on four interproximal areas Adverse effects: oral soft‐tissue examinations made at 3 and 6 months of product use; total of 26 adverse events reported, 16 in Gp A and 10 in Gp B, none considered treatment‐related. Attrition: 23 reported, (but 25 from the data). reasons were pregnancy in four participants, one participant used medications, 18 either failed to adhere to examination schedule (8 participants), requested withdrawal (4 participants), did not comply with the study protocol (2 participants), or were not seen by all examiners (4 participants). |
|
| Funding | Study supported financially by Braun AG, Germany | |
| Notes | Discrepancy in loss to follow‐up, but both groups had a similar number of participants at the end of the study (Gp A 73, Gp B 72). Intra‐examiner reproducibility was judged as excellent with intraclass correlation coefficient of 0.95 or higher for all parameters. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we randomly assigned 21 men and 64 women to the interdental device group and 22 men and 63 women to the floss group" Comment: method of sequence generation not described in sufficient detail |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single blinded, parallel‐group study" Comment: participants and personnel not involved in assessment unlikely to be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "single blinded, parallel‐group study" Comment: study described as single‐blind but it is unclear if or how the examiner was blinded to which group the participants had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 participants lost from the study, reasons not directly related to the use of assigned devices; the attrition rate may not have affected the results as both Gp A and Gp B had a similar number of participants at the end of the study, but the dropout rate does seem very high. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes mentioned in Methods were reported in Results and no key outcomes are missing. |
| Other bias | Unclear risk | Compliance assessment not stated in the Methods. However, it was stated in the Results that out of the 23 participants not completing the study, only 2 did not comply with the study protocol. |
Ishak 2007.
| Methods | Trial design: split‐mouth, 2 arms Location: Kings College, London Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults 18 to 60 years old, visible proximal plaque deposits present, lifetime non‐smokers, at least 6 teeth present in each quadrant from lateral incisor distally, with proximal contact areas in contact or not separated by more than 1 mm, and accessible to an interdental brush Exclusion criteria: gingival enlargement or regrowth; local plaque retention factors; drugs affecting the gums, e.g. phenytoin, cyclosporin, calcium‐channel blockers in the past 6 months; systemic disease that could affect periodontal tissue, e.g. diabetes; pregnancy Baseline plaque status: visible proximal plaque deposits present (no indices specified) Baseline periodontal status: people diagnosed with gingivitis or moderate adult periodontitis and not having received periodontal treatment in the past 6 months Age at baseline: range 33 to 56 years (mean age 43.6) Sex: 3 males/7 females Number randomised: 11 Number evaluated: 11 (10 with data) Smoking status: all non‐smokers |
|
| Interventions |
Comparison: manual toothbrushing and interdental brushing versus manual toothbrushing and floss Gp A1: (n = 10 evaluated) interdental brush (cylindrical bottle brush) (IDB) Gp A2: (n = 10 evaluated) dental floss (DF) All participants used manual toothbrushes, twice a day and all materials used were GlaxoSmithKline UK (Sensodyne brand). Duration of intervention: 1 month Baseline cleaning: as much supragingival calculus as necessary for application of the assigned device was removed Training: participants received detailed instruction on the use of a manual toothbrush, the Bass toothbrushing technique, and on the use of interdental cleaning devices. Training was accompanied by written instructions. Compliance assessment: self reported; each participant was given a printed reminder to fix on bathroom mirror; participants were also given a diary sheet on which they were asked to tick off each day they had cleaned their teeth; all participants returned the diary assigned to them at the beginning of the study; 9 participants had ticked all days; 1 participant had omitted 1 day |
|
| Outcomes | Measurements: at baseline and 1 month Dental plaque: supragingival and subgingival plaque examined using dental floss; visible plaque deposits scored as positive Periodontal disease ‐ gingivitis: Bleeding on Probing (BOP) Index, Probing Depth (PD) and Recession were all scored using a force‐controlled probe (Brodontic); attachment level was obtained by adding PD to recession Adverse effects: a questionnaire was given to all participants concerning any problems with the use of the interdental brush and floss; as for IDB it tended to buckle or distort, and DF sometimes stuck between teeth and caused soreness Attrition: 1 participant excluded due to lack of baseline data |
|
| Funding | GlaxoSmithKline UK provided all materials | |
| Notes | Intra‐examiner reliability was assessed by weighted kappa statistics indicating a reasonable level. A force‐controlled probe used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "in which the use of IDB was randomly assigned to the left or right half of the mouth and the use of DF to the other side" Comment: a statistician who was not directly involved in recruiting participants generated the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "To ensure allocation concealment, the allocation methods were not revealed to the examiner (TW)...Recruitment and assignment of patients to their groups was carried out by NI". Comment: not mentioned whether the person assigning the participants was unaware of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: 'All measurements were carried out at baseline and one month by one experienced examiner (TW), who was blinded'. All procedures performed in the absence of the examiner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 11 participants completed the trial; one excluded due to lack of baseline data Attrition adequately reported and explained |
| Selective reporting (reporting bias) | Low risk | No protocol available. All primary outcomes in Methods section were addressed in Results. |
| Other bias | Low risk | Compliance assessed by participants self‐reported diary entries. All but 1 fully complied; 1 participant missed 1 day. |
Jackson 2006.
| Methods | Trial design: parallel group, 2 arms Location: Department of Periodontology, Leeds Dental Institute, UK Number of centres: 1 Recruitment period: 5 months |
|
| Participants | Inclusion criteria: adults, a minimum of 18 teeth Exclusion criteria: non‐consent, unavailability for the study duration, pregnancy, antibiotics, warfarin, drugs associated with gingival overgrowth, requirement for antibiotic prophylaxis, oral infection such as periodontal‐endodontic lesion and any medical problem that might affect the results of the study Baseline plaque status: not reported Baseline periodontal status: presence of at least 1 shallow pocket of 4 to 5 mm or at least 1 deep pocket > 6 mm in 4 of 6 sextants, suggesting moderate periodontitis Age at baseline: range 26 to 75 years, with most aged from 46 to 55 Sex: 31 males/46 females (Gp A 16/23, Gp B 15/23) Number randomised: 88 (Gp A 44; Gp B 44) Number evaluated: 77 (Gp A 39; Gp B 38) Smoking status: of the 77 participants who completed the study, 29 were smokers (Gp A 8/10; Gp B 6/5). |
|
| Interventions |
Comparison: manual toothbrushing and interdental brushes versus manual toothbrushing and floss Gp A: (n = 39 evaluated) interdental brush (IDB) (Curaprox LSR; MACRO "P" plastic coated); "Subjects were instructed to begin with the largest size and move down to the smallest size in turn to select the brush that provided the most snug interdental fit." Gp B: (n = 38 evaluated) dental floss (DF) (Colgate Non‐Shredding Floss) All participants used manual toothbrush (Colgate Total Professional) and a Colgate Regular Flavour Toothpaste Duration of intervention: 12 weeks Training: participants received a demonstration of both interdental cleaning methods and toothbrushing; full details of oral instructions were given in leaflets for home reference; at 2 weeks, written reminders were sent to each participant, and oral hygiene instructions were repeated for both interdental cleaning methods and toothbrushing. Baseline cleaning: scaling using a single double‐ended sickle scaler hand instrument was provided to remove easily accessible calculus and plaque deposits, to facilitate access for subsequent interdental cleaning. Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 6 weeks, 12 weeks, by 1 dental hygienist Dental plaque: Plaque Index (PI) at 4 sites per tooth excluding third molars Periodontal disease ‐ gingivitis: Eastman Interdental Bleeding Index (EIBI); Relative Interdental Papillae Level (RIPL) in millimetres; Eastman Interdental Bleeding Index (EIBI) scored as present or absent; Pocket Depths (PD) at 4 sites per tooth, and Bleeding On Probing (BOP) on same 4 sites. Adverse effects: assessment method not described; as stated in the Results none were reported from either of the groups. Attrition: Gp A (IDB), 5 participants were lost: 1 not having required number of sites and excluded subsequently, 2 took antibiotics for non‐dentally related reasons, and 2 failed to complete the 3 visits of the study; Gp B (DF), 6 participants were lost: 1 withdrawn due to periodontal‐endodontic lesion that required emergency treatment, and 5 failed to complete the 3 visits of the study |
|
| Funding | Financial support not declared. Colgate provided toothbrushes, floss and toothpastes, Dental Health Boutique, Leatherhead, UK provided interdental brushes, and Dentsply provided dental instruments. | |
| Notes | Intra‐examiner reliability tested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "single‐blind randomized controlled clinical trial", "using computer‐generated random numbers", "Patients were randomly allocated to a floss or interdental brush group by the research assistant after all oral hygiene advice was delivered and after the appointment time with the hygienist operator concluded". Comment: satisfactory method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "four allocation envelopes were prepared and labeled for gender and smoking habit" Comment: allocation concealment not described in sufficient detail |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "at all times the hygienist examiner was unaware of which group to which the patient was allocated" Comment: examiner did not know which group participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition 11 out of 88, equally distributed between the study arms. Reasons for attrition adequately reported and explained |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes in the Methods section addressed in Results |
| Other bias | Unclear risk | Compliance not assessed |
Jared 2005.
| Methods | Trial design: parallel group, 5 arms Location: The University of North Carolina, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults > 18 years old, at least 1 "test site" defined as an interproximal space of 1.0 mm that exhibited bleeding from the facial and lingual sides, excluding third molars Exclusion criteria: current use of interdental cleaning devices (dental floss, proxy brush, stimudent) or in the past 6 months, no appropriately sized interdental space, participants that have brushed their teeth less than once a day in the past 6 months, oral disease requiring immediate treatment; smoking within the last 6 months, pregnancy, current use of antibiotics or any other medication known to cause gingival enlargement, chronic use of non steroidal anti‐inflammatory medications, immunocompromised patients, patients with a disease that affects the gingiva, need for antibiotic prophylaxis, orthodontic patients, patients who have undergone scaling in the last 6 months, presence of interproximal calculus sufficient enough to interfere with interdental cleaning, participation in another study Baseline plaque status: Turesky modification of the Quigley‐Hein Index, mean Interproximal Plaque Score (IPS) value range from 2.82 to 2.99 Baseline periodontal status: Lobene modification of the Gingival Index (mean Interproximal Gingival Score (IGS) value range from 2.09 to 2.30 Age at baseline: mean age: 36.38 to 42.20 Sex: 60 males/92 females Number randomised: 162 (not reported across groups) Number evaluated: 152 (Gp A 31; Gp B 30; Gp C 30; Gp D 29; Gp E 32) Smoking status: all non‐smokers (smoking within preceding 6 months was an exclusion criterion) |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and an interdental brush versus manual toothbrushing and floss Gp C: (n = 30 evaluated) interdental brush (Sunstar Inc. Japan), used nightly after toothbrushing Gp D: (n = 29 evaluated) dental floss (GUM Easy‐through Floss Sunstar Inc.) used nightly before toothbrushing Gp E: (n = 32 evaluated) standard toothbrush alone Other interventions (not included in the review): Gp A: (n = 31 evaluated) interdental brush (Sunstar Inc. Japan) plus an 0.05% cetylpyridinium gel Gp B: (n = 30 evaluated) interdental brush (Sunstar Inc. Japan) plus a placebo gel All participants used manual toothbrush (GUM #409, Sunstar Inc) twice a day Duration of intervention: 4 weeks Baseline cleaning: before clinical data were collected, participants were asked to brush their teeth. After the baseline data collection, dental plaque was removed from all teeth using a rubber cup and fine grit prophylaxis paste Training: participants received verbal and written oral hygiene instructions, as well as appropriate demonstrations of the mechanical cleaning procedures Compliance assessment: participants were asked to keep a log of their dental cleaning habits, but data were not reported |
|
| Outcomes | Measurements: at baseline, 2 weeks, and 4 weeks Dental plaque: Quigley‐Hein Plaque Index (Turesky modification) Periodontal disease ‐ gingivitis: Lobene modification of the Gingival Index; bleeding upon probing using the Van der Wijden modification of the Bleeding on Marginal Probing Adverse effects: a questionnaire was given to all participants concerning any symptoms experienced; adverse effects were not reported in the Results. Attrition: of the 10 participants who did not complete the study, 9 withdrew prior to baseline, and 1 was lost due to health issues. None of the withdrawals were product‐related. |
|
| Funding | Supported by Sunstar Inc., Japan, and 3 authors were employees | |
| Notes | Almost all dropouts (9/10) occurred before baseline assessment. Chairside calibration of the examiner was conducted by an external gold‐standard examiner. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization was used, and was based on baseline dental plaque scores to assure greater baseline comparability among treatment groups" Comment: details of method not provided |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "single‐blind randomized clinical trial" No other details provided on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported and explained: "Of the 10 subjects who did not complete the study, nine withdrew prior to baseline, and one was dismissed due to health issues. None of the withdrawals were product‐related." We judged it unlikely to affect the results. |
| Selective reporting (reporting bias) | Unclear risk | Previously published abstract available. All primary outcomes in the Methods section were addressed in the Results section. However, data on possible adverse effects were not reported, although the participants were asked to keep logs. |
| Other bias | Unclear risk | Compliance was not reported, although participants were asked to keep a log of their dental cleaning habits. |
Kazmierczak 1994.
| Methods | Trial design: parallel group, 2 arms Location: Buffalo School of Dental Medicine, New York, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults Exclusion criteria: pregnancy, antibiotic use within one month prior to the baseline, chronic illness such as cardiovascular disease, diabetes mellitus, influenza, history of rheumatic fever, kidney or liver disorder, chronic use of steroids or anti‐inflammatory drugs, professional prophylaxis within one month of entry into the study. Modified Gingival Index (MGI) interproximal score > 1.7 and plaque score > 2.0 Baseline plaque status: Interproximal Plaque Score < 2 Baseline periodontal status: Modified Gingival Index (MGI) interproximal score < 1.7 Age at baseline: 20 to 65 years Sex: males and females included, numbers not specified Number randomised: 20 Number evaluated: not reported Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing and a rubber/elastomeric interdental cleaning stick Gp A: interdental cleaning stick used nightly Gp B: dental floss used nightly All participants used manual toothbrush twice a day Duration of intervention: 6 weeks Training: not reported Baseline cleaning: none reported, but participants were excluded if they exceeded certain limits for plaque and gingivitis Training: participants were given a manual toothbrush and dentifrice to use as well as the floss or cleaning stick, but no training was reported to have been undertaken. Compliance assessment: participants were asked to complete a diary of their product use, but this was not reported in the Results. |
|
| Outcomes | Measurements: at baseline, 3 weeks, and 6 weeks (we used 6‐week data) Dental plaque: Turesky modification of the Quigley‐Hein Plaque Index on six surfaces of all teeth present (mesio‐buccal, buccal, distal‐buccal, mesio‐lingual, lingual, disto‐lingual) Periodontal disease ‐ gingivitis: Lobene modification of the Löe‐Silness Gingival Index on facial and lingual margins and papillae of the entire mouth; Bleeding Index (BI) assessed buccally and lingually in the interproximal areas on the Ramfjord teeth. Adverse effects: safety assessments were made at each measurement period; adverse effects were not reported in the Results. Attrition: not reported |
|
| Funding | Not reported | |
| Notes | Study dates not reported. Oral massage device type and manufacturer not described. Participants were not instructed on how to use the assigned devices. Examiner reliability testing not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Ten subjects were randomly assigned dental floss, and ten subjects were randomly assigned the massage device" Comment: insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of examiner(s) not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how many randomised participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported in the Results although mentioned in the Methods |
| Other bias | Unclear risk | Compliance not assessed |
Lewis 2004.
| Methods | Trial design: parallel group, 2 arms Location: University of Tennessee College of Dentistry, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults who were either patients, students, faculty or employees at the University of Tennessee, College of Dentistry who had gingivitis associated with dental plaque or slight chronic periodontitis Exclusion criteria: medical conditions requiring antibiotic use within 6 months prior to the study, communicable diseases Baseline plaque status: O'Leary Plaque Index Baseline periodontal status: gingivitis associated with dental plaque or slight chronic periodontitis. Plaque‐induced gingivitis was defined as generalised clinical gingival inflammation with sulcus probing depths (PDs) no greater than 3 mm, while slight chronic periodontitis was described as generalised gingival inflammation with PD less than 4 mm and clinical attachment loss less than 2 mm. Age at baseline: age range 18 to 50 years Sex: 13 males, 42 females (not reported by group) Number randomised: 55 (Gp A 25; Gp B 30) Number evaluated: 47 (Gp A 20; Gp B 27) Smoking status: smokers were identified through a questionnaire: Gp A (toothpick) 10% (2/20); Gp B (floss) 11% (3/27) |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing and an interdental cleaning stick (wooden toothpick) Gp A: (n = 20 evaluated) interdental cleaning stick (Stim‐u‐Dent, Johnson & Johnson) Gp B: (n = 27 evaluated) dental floss (Reach, Johnson & Johnson) Interdental procedures were to be performed once daily, preferably in the evening together with brushing. All participants used manual toothbrush. Duration of intervention: 12 weeks Training: participants were instructed in the use of toothpicks, trained in the arming of the handle of the holder and issued a box of toothpicks and disclosing solution; participants in the flossing group were instructed how to use the dental floss; following instruction, participants were observed performing the prescribed method to ensure comprehension; participants were not trained in a method of toothbrushing. Baseline cleaning: not reported Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 2 weeks, 6 weeks, and 12 weeks Dental plaque: O'Leary Plaque Index, Interproximal Plaque Index (IPI) Periodontal disease ‐ gingivitis: Eastman Interdental Bleeding Index (EIBI) Adverse effects: none reported Attrition: 8 participants dropped out, 5 in Gp A (toothpick) and 3 in Gp B (floss). There was a disparity in the text between those randomised and completed: toothpick group finished with 20 participants and floss group with 27. |
|
| Funding | Study supported through the University of Tennessee College of Dentistry Alumni Clinical Research Grant Fund | |
| Notes | Examiner reliability not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly determined by coin toss" Comment: method of random sequence generation was simple but valid. |
| Allocation concealment (selection bias) | Unclear risk | No information presented about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the examiner for the study was blind to the participant's study group" Comment: examiner did not know which group the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants dropped out for various reasons, 5 from the toothpick group and 3 from the floss group. The toothpick group finished with 20 participants and the floss group finished with 27. Specific reasons for dropout not provided. |
| Selective reporting (reporting bias) | High risk | No standard deviations reported |
| Other bias | Unclear risk | Compliance not assessed |
Lobene 1982.
| Methods | Trial design: parallel group, 4 arms Location: Forsyth Dental Center, Boston, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adult participants who brushed daily, had at least 20 interproximal sites to floss, used floss less than once a week and who had an average Löe and Silness Gingival Index score of 0.8 to 1.5 Exclusion criteria: regular floss users (at least once a week) Baseline plaque status: not reported Baseline periodontal status: average gingival inflammation between 0.8 and 1.5 using Löe & Silness Gingival Index Age at baseline: age range 20 to 50 years Sex: not reported Number randomised: 118 Number evaluated: 118 |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and flossing Gp A: (n = 33 evaluated) manual toothbrush Gp B: (n = 31 evaluated) waxed dental floss (Johnson & Johnson) Gp C: (n = 25 evaluated) unwaxed dental floss (Johnson & Johnson) Gp D: (n = 29 evaluated) mint flavoured dental floss (Johnson & Johnson) Flossing was performed once daily 5 days per week by reporting to the clinic, and once daily during weekends at home. All participants used manual toothbrush. Duration of intervention: 12 weeks Baseline cleaning: complete oral prophylaxis, which reduced plaque to zero Training: participants using dental floss viewed a video tape on the proper flossing technique, which was followed by personal supervised instruction for those participants who experienced difficulty in flossing. They were also given written instructions and an illustrated brochure on the proper method of flossing. Compliance assessment: participants reported during weekdays to the clinic to have their compliance observed and at weekends flossed at home; participants kept a daily log of floss use including weekends. |
|
| Outcomes | Measurements: at baseline, 2 weeks, 4 weeks, and 8 weeks Dental plaque: Quigley‐Hein Plaque Index Periodontal disease ‐ gingivitis: Löe & Silness Gingival Index Adverse effects: not reported Attrition: not reported |
|
| Funding | Financial support not declared. Dental floss used was Johnson & Johnson, New Bruswick, New Jersey. | |
| Notes | Practice‐based study. Smoking status not reported. Examiner reliability testing not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation mentioned only in an earlier conference abstract: "Groups were balanced with respect to age, sex and gingivitis at the baseline examination and randomly assigned to the control or treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examinations were conducted so that the examiner was blind to the subject's treatment group". Comment: examiner did not know which group the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear how many participants were randomised; attrition not addressed |
| Selective reporting (reporting bias) | Low risk | Previously published abstract available. All outcomes reported in the Abstract, and in the Methods section of the article, were addressed in the Results section. |
| Other bias | Unclear risk | Compliance assessed, but not reported, although participants kept a daily log of product use. |
Meklas 1972.
| Methods | Trial design: parallel group, 2 arms Location: Louisiana State University School of Dentistry, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: dental students Exclusion criteria: none reported Baseline plaque status: not reported Baseline periodontal status: not reported Age at baseline: age range not reported (first year dental students) Sex: not reported Number randomised: 109 (Gp A: 55; Gp B: 54) Number evaluated: 109 (Gp A: 55; Gp B: 54) |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and an oral irrigator Gp A: (n = 55 evaluated) oral irrigator (#AP2 Aqua Pulse oral irrigator, General Electric Company) Gp B: (n = 54 evaluated) manual toothbrush All participants were supplied identical toothbrushes and toothpaste; all continued to brush in their usual manner. Duration of intervention: 6 months Baseline cleaning: all participants’ teeth scaled to remove hard deposits, then polished a week later Training: the water irrigator group was told to follow the manufacturer’s directions for the oral irrigation device. Compliance assessment: participants were instructed to record the number of times they used an irrigating device each day during study; charts in the form of calendars were issued to each participant at the beginning of study and collected at the end of each month; only mean data reported: mean use of oral irrigator was 1.114 times per day; not clear how many participants returned diaries Smoking status: not reported. |
|
| Outcomes | Measurements: at baseline, 2 weeks, and then 6 more examinations during the following 6 months Dental plaque: 2‐point plaque index Periodontal disease ‐ gingivitis: Russell modified Periodontal Index (2‐point scale) Plaque and gingivitis were scored on Ramfjord teeth. Adverse effects: recorded after 48 hours of use; participants were examined for oral lacerations, there were 8 new lacerations on 8 participants in the oral irrigator group and seven new lacerations on 5 participants in the toothbrush group. Attrition: not reported |
|
| Funding | Grant was given by the General Electric Company (the #AP2 Aqua Pulse oral irrigator was used in this study, manufactured by the General Electric Company). | |
| Notes | The principal investigator examined all teeth for plaque and gingivitis. Examiner reliability testing not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were assigned numbers and randomly divided into two groups" Comment: insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the text |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the results of the study were not revealed to the clinical examiners until the data collection portion of the study was completed" Comment: it was unclear whether the examiner knew which group the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | Compliance assessed by calendars that were given and collected at the end of each month, but not reported in detail |
Mwatha 2017.
| Methods | Trial design: parallel group, 4 arms Location: Las Vegas, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults, informed consent, non‐smokers who routinely used manual toothbrushes but used floss or other interdental cleaning devices less than once per week and a population with mild to moderate gingivitis Exclusion criteria: insulin dependent diabetes, advanced periodontal disease or gingival recession, xerostomia, rampant caries, routine power toothbrush users, use of professional dispensed bleaching products, orthodontic bands or extensive crown or bridgework, professional prophylaxis within four weeks of the study Baseline plaque status: a minimum plaque score of ≥ 0.5 measured by the Rustogi Modified Navy Plaque Index (RMNPI) following 2 to 6 hours of plaque accumulation Baseline periodontal status: mild to moderate gingivitis with a minimum of 10 sites with scores of ≥ 1 on Gingival Bleeding Index (GBI) Age at baseline: age range 18 to 65 years, mean ages per group, (Gp A 35.1; Gp B 34.9; Gp C: 35.2; Gp D 36.9) Sex: 104 males/186 females (Gp A 18/33, Gp B 28/51, Gp C 29/51, Gp D 29/51) Number randomised: 290 (Gp A 51; Gp B 79; Gp C 80; Gp D 80) Number evaluated: 287 (Gp A 51; Gp B 79; Gp C 78; Gp D 79) model‐based estimate presented in Tables with 287 participants, although 286 completed the day 28 visit Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and flossing Gp A: (n = 51 evaluated) manual toothbrush Gp B: (n = 78 evaluated) dental floss (Reach unflavoured Wax Floss, Johnson & Johnson) All participants used manual toothbrush (ADA reference manual toothbrush) with Crest Cool Mint gel dentrifice (Procter and Gamble) Duration of intervention: 28 days Other interventions (not included in the review): Gp C: manual toothbrush and Philips Sonic Airfloss Pro (air and water flosser) with BreathRx mouthrinse (cetylpyridinium chloride) Gp D: manual toothbrush and Philips Sonic Airfloss Pro (air and water flosser) with Listerine Cool Mint Antiseptic mouthrinse Training: all groups were instructed on product use with participants demonstrating their understanding of their study products to an assigned instructor; step‐by‐step illustrated instructions were also provided. Baseline cleaning: not reported Compliance assessment: diary cards were provided for participants to keep a record of product use. |
|
| Outcomes | Measurements: at baseline, 14 days, and 28 days Dental plaque: Rustogi Modified Navy Plaque Index (RMNPI) Periodontal disease ‐ gingivitis: Modified Gingival Index (MGI) and Gingival Bleeding Index (GBI) Adverse effects: safety assessments including gingival abrasions, irritations or ulcerations at baseline, repeated on subsequent visits; any incidents noted on participants' home diaries were also evaluated; four events in total were reported, one in the floss group (Gp B), one in the Listerine group (Gp D), and two in the BreathRx mouthrinse (cetylpyridinium chloride) (Gp C), all reported as gingival irritations or soreness, but were mild in severity and resolved. No serious adverse events reported Attrition: 3 participants failed to report for the 14‐day assessment and 1 more participant failed to report for the 28‐day assessment. |
|
| Funding | Authors AM, MO, SS, MW and WJ were employees of Philips Healthcare, USA, at the time of the study, which was stated in the Conflict of Interest section. Study was sponsored by Philips Oral Healthcare. | |
| Notes | Examiners were trained in visual assessment of plaque and gingivitis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "290 were enrolled and randomized" Comment: method of sequence generation was unclear |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a randomized, single‐blind, parallel‐design study" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "single‐blind" Unclear whether the examiner knew which group the participants had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants were lost to follow‐up, but only one in the groups used for our comparison |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | Compliance was assessed, but not reported. |
NCT00855933.
| Methods | Trial design: parallel group, 2 arms Location: Guatemala City, Guatemala Number of centres: 1 Recruitment period: not reported Duration: 4 weeks (January to February 2009) |
|
| Participants | Inclusion criteria: at least 18 years of age; physically able to floss his/her teeth; refrained from performing oral hygiene the morning of the baseline visit; have measurable gingivitis on at least 5 test sites; in good general health Exclusion criteria: severe periodontal disease; atypical discolouration or pigmentation in the gingival tissue; meaningful malocclusion of the anterior teeth; fixed facial orthodontic appliances; use of antibiotics within 2 weeks of the baseline visit and at any time during the study; any diseases or conditions that could be expected to interfere with safe completion of the study Baseline plaque status: not reported Baseline periodontal status: Mean Lobene Modified Gingival Index score: 2.40 (SD 0.27) Age at baseline: mean 28.7 years (Gp A 29.5; Gp B 27.8) Sex: 7 males/53 females (Gp A 4:26; Gp B 3:27) Number randomised: 60 (Gp A 30; Gp B 30) Number evaluated: 60 (1 from Gp B did not complete, but all participants included in analysis in trial results) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and flossing versus manual toothbrushing Gp A: (n = 30 evaluated) manual toothbrushing once daily Gp B: (n = 30 evaluated) manual toothbrushing once daily, plus once daily flossing using Glide® floss with cetylpyridinium chloride All participants used Crest Cavity Protection toothpaste and an Oral‐B® Indicator soft, manual toothbrush Experimental participants used Glide® floss with cetylpyridinium chloride Duration of intervention: 4 weeks Training: not mentioned Baseline cleaning: not mentioned Compliance assessment: not mentioned |
|
| Outcomes | Measurements: at baseline, 4 weeks Dental plaque: not measured Periodontal disease ‐ gingivitis: whole‐mouth average Lobene Modified Gingival Index (summing the scores and dividing by the number of sites graded (excludes missing teeth & sites not graded)): 0 (normal) to 4 (severe inflammation) Adverse effects: none identified Attrition: 1 participant withdrew from floss group. |
|
| Funding | Sponsored by Procter and Gamble | |
| Notes | Study director: Aaron Biesbrock, Procter and Gamble Contact: Jon Witt witt.jj.2@pg.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Masking: Single (Outcome Assessor)” ‐ method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data presented on all randomised participants, but 1 dropout reported |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Not published and only brief details of study contained in trial registration. Compliance not assessed |
NCT01250769.
| Methods | Trial design: parallel group, 4 arms Location: Indiana, US Number of centres: 1 Recruitment period: not reported Duration: 4 weeks (January to February 2009) |
|
| Participants | Inclusion criteria: 18 to 70 years of age; good/excellent health; minimum of 20 natural teeth (excluding 3rd molars); sufficient test sites; ≥ 20 bleeding sites; willing and able to participate Exclusion criteria: systemic diseases such as Down's syndrome, or known AIDS/HIV; insulin‐dependent diabetes; cardiac pacemaker; pregnant or nursing; undergoing or requiring extensive dental or orthodontic treatment; requiring antibiotic treatment for dental appointments; heavy deposits of calculus; severe gingivitis or periodontitis; extensive crown or bridge work and/or rampant decay; currently using bleaching trays; any oral or extraoral piercing on lips or in mouth; have had a professional prophylaxis within 4 weeks of study; participation in a prior study ≤ 20 days Baseline plaque status: not reported Baseline periodontal status: not reported Age at baseline: 39.6 mean years (Gp A 38.5; Gp B 39.6; Gp C 39.6; Gp D 41.2) Sex: 56 males/112 females analysed at baseline (Gp A 3:8; Gp B 24:48; Gp C 26:48; Gp D 3:8) Number randomised: 170 (Gp A 11; Gp B 73; Gp C 75; Gp D 11) Number evaluated: 167 at day 28 (Gp A 11; Gp B 72; Gp C 73; Gp D 11) Smoking status: not reported |
|
| Interventions |
Comparison: air/water cleaning device versus manual toothbrushing Gp B: (n = 72 evaluated) manual toothbrush used for 2 minutes twice a day Gp C: (n = 73 evaluated) manual toothbrush used twice a day for 2 minutes plus interproximal cleaning device used once a day All participants used Crest Cavity Protection toothpaste and an Oral‐B® Indicator soft, manual toothbrush Duration of intervention: 4 weeks Study arms not included in the review Gp A: manual toothbrush used twice a day for 1 minute Gp D: manual toothbrush used twice a day for 2 minutes plus interproximal cleaning device used twice a day Training: not mentioned Baseline cleaning: not mentioned Compliance assessment: not mentioned |
|
| Outcomes | Measurements: at baseline, 14 days, and 28 days Dental plaque: not directly measured (residual protein concentration of interproximal plaque) Periodontal disease ‐ gingivitis: Gingival Bleeding Index: evaluation using an ordinal scale of 0 to 3 (0 was best; 3 was worst) Adverse effects: measured. Found 1 serious (arm deep vein thrombosis) in Gp C ‐ unrelated to treatment, and 1 minor in Gp D ‐ aphthous ulcer above tooth #7 on attached gingiva Attrition: 3 participants |
|
| Funding | Sponsored by Philips Oral Healthcare | |
| Notes | Study director and contact: Wendy Jenkins, Director of Clinical Operations, Philips Oral Healthcare wendy.jenkins@philips.com | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: Single (Outcomes Assessor)" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Masking: Single (Outcomes Assessor)" Comment: method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis of efficacy data was performed using a modified intent‐to‐treat population (MITT). The MITT Population included all randomized subjects with both a baseline and endpoint evaluation. Missing data were not imputed". Comment: 3 participants did not complete ‐ 1 withdrawal from Gp B and 1 withdrawal from Gp C (reasons not given), 1 serious non‐treatment related adverse event in Gp C |
| Selective reporting (reporting bias) | Low risk | Planned outcomes reported |
| Other bias | Unclear risk | Not published and only brief details of study contained in trial registration Compliance not assessed |
Rosema 2008.
| Methods | Trial design: parallel group, 3 arms The study had a 3‐week pre‐experimental phase to improve oral health followed by a 9‐month study period. Location: Academic Center for Dentistry, Amsterdam, The Netherlands Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults ≥ 18 years of age, a minimum of five evaluable teeth per quadrant Exclusion criteria: oral lesions and/or periodontal pockets > 5 mm, pregnancy, systemic disease, e.g. diabetes and any adverse medical history or long‐term medication, partial dentures or orthodontic appliances and floss users Baseline plaque status: not reported Baseline periodontal status: level of gingival bleeding < 40%, periodontal pockets < 5 mm Age at baseline: age in years (± SD) Gp A 21.6 ± 2.54, Gp B 22.2 ± 3.25, Gp C 22.4 ± 2.93 Sex: 22 males/92 females: Gp A 6/32, Gp B 7/32, Gp C 9/28 Smokers/non‐smokers: Gp A 5:33, Gp B 5:34, Gp C 2:35 Number randomised: 118 (Gp A 40; Gp B 40; Gp C 38) (122 were recruited, but 4 failed to attend for randomisation) Number evaluated: 114 (Gp A 38; Gp B 39; Gp C 37) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and floss Gp A: (n = 38 evaluated) manual toothbrush (ADA Soft reference toothbrush) Gp B: (n = 39 evaluated) manual toothbrush and floss (Oral‐B Satin waxed floss, Procter & Gamble) Duration of intervention: 9 months Other interventions (not included in the review): Gp C: (n = 37) powered toothbrush (Oral‐B Triumph Professional Care 9000, Procter & Gamble) Training: professional instruction in the use of a manual toothbrush (Bass technique) and floss. The assigned brushing and flossing techniques were reinforced at 6 and 10 weeks. Powered toothbrush was to be used according to manufacturers' instructions. Baseline cleaning: 3‐week pre‐experimental toothbrushing using the Bass technique twice daily for 2 minutes plus rinsing with hydrogen peroxide solution and chlorhexidine 0.2% mouthwash. Professional dental scale and polish provided after these 3 weeks, at baseline Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 10 weeks, 6 months, and 9 months Dental plaque: modified Quigley and Hein Plaque Index (QHPI) as described in detail by Paraskkevas Periodontal disease ‐ gingivitis: Bleeding on Marginal Probing Index (BOMP) Adverse effects: throughout the study gingival abrasion lesions (GAS) were scored, and staining using the Gruendemann Modification of the Staining Index; no significant differences from the beginning of the trial were noted, nor differences between groups; overall no adverse effects were noted in the main 9‐month study period. Attrition: 2 participants (1 in the floss group and 1 in the powered toothbrush group) failed to attend the baseline visit because of scheduling conflicts, 2 participants were lost at 9‐month visit; 1 participant (manual toothbrush group) was hospitalised due to a leg injury, and 1 had moved to a different part of the country |
|
| Funding | Procter and Gamble sponsored the study, GlaxoSmithKline provided chlorhexidine and DE International provided the toothpaste; 2 authors received lectures or advising fees from Procter and Gamble | |
| Notes | All examinations performed by the same experienced examiners under the same conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed using true random numbers that are generated by sampling and processing a source of entropy outside the computer" Comment: method of sequence generation was clear and adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "examiner masked" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "examiner masked" Comment: blinding of examiner not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants lost to follow‐up in each of the groups could not be ascertained from the report. However, the total number of participants lost to follow‐up was low, so attrition was unlikely to affect the results. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | Compliance was not assessed during the experimental period, only for the pre‐experimental phase of the trial. Baseline values between groups appeared to lack balance. |
Rosema 2011.
| Methods | Trial design: parallel group, 3 arms Location: Academic Center for Dentistry, Amsterdam, The Netherlands Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults ≥ 18 years of age, a minimum of 5 evaluable teeth per quadrant and a level of gingival bleeding > 50% on marginal probing Exclusion criteria: oral lesions and/or periodontal pockets > 5 mm and/or generalised recession, pregnancy, systemic disease like AIDS, cirrhosis, diabetes, any adverse medical history or long‐term medication, conditions limiting manual dexterity, partial dentures or orthodontic appliances Baseline plaque status: not reported Baseline periodontal status: moderate gingival inflammation with 50% Bleeding on Marginal Probing Index (BOMP), periodontal pockets < 5 mm Age at baseline: age in years ± SD; Gp A 21.9 ± 3.2; Gp B 21.1 ± 2.3; Gp C 22.4 ± 3.1 Sex: 30 males/74 females (Gp A 10/24; Gp B 7/27; Gp C 13/23) Number randomised: 108 (112 were recruited, but 4 failed to attend for randomisation) Number evaluated: 104 Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and an oral irrigator with a prototype tip versus manual toothbrushing and an oral irrigator with a standard tip versus manual toothbrushing and floss Gp B: (n = 34 evaluated) oral irrigator (Waterpik Ultra Water Flosser with a standard jet tip) once a day in the evening Gp C: (n = 34 evaluated) standard waxed floss (Johnson & Johnson) once a day in the evening All participants used manual toothbrush Oral B 35 indicator 35 twice a day Duration of intervention: four weeks Other interventions (not included in the review): Gp A: (n = 36 evaluated) manual toothbrush (Oral B 35 indicator 35) plus an oral irrigator (Waterpik Ultra Water Flosser with a prototype jet tip) Baseline cleaning: not reported Training: each participant received professional advice about toothbrushing and floss usage, when applicable; verbal instructions and demonstrations were given to follow the manufacturer’s instructions. Compliance assessment: participants were asked to note when they used their products on a calendar record chart. |
|
| Outcomes | Measurements: at baseline, 2 weeks, and 4 weeks Plaque: Turesky modification of Quigley‐Hein Plaque Index Periodontal disease – gingivitis: Bleeding On Marginal Probing Index (BOMP), by Van der Weijden Adverse effects: assessment not reported; however, it was stated in the Results that no adverse events were reported by any of the participants who participated in this study. Attrition: 2 participants dropped out before 2 weeks and another 2 before 4 weeks, 2 from Gp B and 2 from Gp C. |
|
| Funding | Waterpik Inc (USA) provided study products (oral irrigators). Study performed in commission of ACTA Research BV | |
| Notes | All assessments made by experienced examiners and under same conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to one of three groups using a randomization list" Comment: method of sequence generation was clear and adequate. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the allocation of products was carried out by the study coordinator who was responsible for allocation concealment". Comment: allocation concealment not described in sufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "all products were distributed in such a way that blindness of the examiners was assured". Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all products were distributed in such a way that blindness of the examiners was assured". Quote: "at the last visit the study coordinator assured blindness of the examiners". Comment: examiners did not know which groups the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were lost to follow‐up (2 each from groups B and C). |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | To assess compliance, participants were asked to record the product use on a calendar record chart, and to return it together with all products provided. However, no data on compliance were reported. |
Schiff 2006.
| Methods | Trial design: parallel group, 3 arms Location: San Francisco, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults, 18 to 70 years, a minimum of 20 uncrowned teeth (excluding 3rd molars), available for the study duration and able to sign a consent form, in good health, with no allergies to triclosan or oral care products. An initial gingivitis index of at least 1.0 on the Löe and Silness Gingival Index and at least 1.5 on the Quigley‐Hein Plaque Index, Turesky modification Exclusion criteria: people with removable prostheses, orthodontic bands, hard or soft tissue tumours, advanced periodontal disease, more than five active carious lesions, pregnancy or lactation, and individuals taking any prescription medication Baseline plaque: Quigley‐Hein Plaque Index, Turesky modification of at least 1.5 Baseline periodontal status: Löe & Silness Gingival Index of at least 1.0 Age at baseline: mean/age range in years: Gp A 28.3, 22 to 46; Gp B 25.9, 18 to 43; Gp C 27.1, 20 to 50 Sex: 68 males/46 females (Gp A 20/17; Gp B 26/11; Gp C 22/18) Number randomised: 120 (Gp A 40; Gp B 40; Gp C 40) Number evaluated: 114 (Gp A 37; Gp B 37; Gp C 40) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing (with a triclosan‐containing toothpaste) versus manual toothbrushing (with a triclosan‐containing toothpaste) and floss Gp A: (n = 37 evaluated) floss (Colgate Dental Floss) Gp B: (n = 37 evaluated) manual toothbrush All participants used soft‐bristled adult toothbrush (Colgate Plus), for one minute twice daily, with a triclosan‐containing toothpaste (Colgate Total) Duration of intervention: 6 months Other interventions (not included in the review): Gp C: (n = 40 evaluated) soft‐bristled adult toothbrush (Colgate Plus), brushing for one minute twice daily, with a standard toothpaste (Crest Fluoride, Procter & Gamble) and floss Baseline cleaning: complete oral prophylaxis, verified for thoroughness by the use of a red disclosing solution Training: all participants were instructed to use only the dentifrice and floss provided, and to refrain from using any other oral hygiene products for the entire 6 months of the study. Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 3 months, and 6 months Plaque: Quigley‐Hein Plaque Index, Turesky modification Periodontal disease – gingivitis: Löe & Silness Gingival Index Adverse effects: oral soft tissue assessments were repeated at baseline, three, and six months; throughout the study, no adverse events of the oral hard or soft tissues of the oral cavity were observed or reported by participants when questioned. Attrition: 6 participants were lost to follow‐up, (Gp A 3; Gp B 3; Gp C 0), who did not complete the 6‐month examinations; they dropped out for reasons unrelated to the use of the treatments. |
|
| Funding | Study was supported by Colgate Palmolive Company. Three authors Colgate employees | |
| Notes | No details about examiner provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Qualifying subjects were stratified into three balanced groups according to their baseline supragingival plaque scores. These groups were then randomly assigned to one of the three treatment regimens". Comment: method of sequence generation was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "examiner blind clinical study" Comment: examiner blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Those subjects who did not complete the six‐month examinations dropped out for reasons unrelated to the use of the treatments." 6 participants were lost to follow‐up, 3 each in the first 2 groups (those used for the comparison) and none from the 3rd group. |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes stated in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | Compliance was not assessed. |
Sharma 2002.
| Methods | Trial design: parallel group, 3 arms Location: USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults, a minimum of 20 intact natural teeth, mean Modified Gingival Index ≥ 1.75 and Plaque Index ≥ 1.95. Third molar teeth, orthodontically banded or abutment teeth were not included. Exclusion criteria: significant oral soft tissue pathology other than gingivitis, treatment with antibiotic or anti‐inflammatory drugs, history of condition requiring antibiotic prophylaxis prior to invasive dental procedures, moderate or advanced chronic periodontitis, and pregnancy Baseline plaque status: Turesky modification of the Quigley‐Hein Plaque Index ≥ 1.95 Baseline periodontal status: mean Lobene Modified Gingival Index ≥ 1.75 Age at baseline: range 18 to 63 years; mean: Gp A 35.5 (9.61); Gp B 35.0 (9.58); Gp C: 37.0 (9.68) Sex: 104 males/197 females (Gp A 36/66; Gp B 31/70; Gp C 37/61) Smokers/non‐smokers: 74/227 (Gp A 22/80; Gp B 27/74; Gp C 25/73) Number randomised: 319 (numbers not reported by group) Number evaluated: 301 (Gp A 102; Gp B 101; Gp C 98) Smoking status: 24.6% of participants were smokers. |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing with a negative control rinse Gp A: (n = 102 evaluated) floss (Reach Waxed Dental Floss, Johnson & Johnson) Gp B: (n = 101 evaluated) 5% hydroalcohol negative control rinse All participants used manual toothbrush (Oral‐B 35) Duration of intervention: 6 months Other interventions (not included in the review): Gp C: (n = 98) manual toothbrush (Oral‐B 35) and an essential oil mouthrinse (Listerine Antiseptic) Training: first rinse or use of floss performed with instruction and supervision; participants in the floss group received flossing instruction from a dental hygienist and were required to demonstrate their ability to floss all regions of the mouth. The participants were also provided written flossing instructions. Baseline cleaning: complete dental prophylaxis to remove plaque, stain, and calculus Compliance assessment: participants provided with diaries to record daily use; self‐reported, measurements of returned supplies |
|
| Outcomes | Measurements: at baseline, 3 months, and 6 months Plaque: Quigley and Hein Plaque Index, Turesky modification Periodontal disease – gingivitis: Lobene modification of the Gingival Index and Ainamo & Bay Gingival Bleeding Index Adverse effects: examinations included oral soft‐tissue examination; during the course of the study, no adverse reactions occurred that could be attributed to either test regimen. Attrition: 18 participants lost to follow‐up; participants were deemed nonevaluable if they did not return for post‐baseline examinations, failed to comply with usage instructions, or were taking concomitant medications that could influence results during the time of the 3‐ or 6‐month examination. Specific reasons for dropouts, and the groups they were in, were not reported. |
|
| Funding | Source of funding, if any, was not reported. Three authors were Pfizer employees. | |
| Notes | This study protocol design was used in Bauroth 2003. All examinations were performed by a single trained examiner. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each subject was assigned to one of three groups according to a randomization schedule." Comment: method of sequence generation was not clear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "observer‐blind" Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomized, controlled, observer‐blind, parallel group 6‐month clinical trial" and "subjects refrained from use of their test products for at least 4 hours prior to the 3 and 6 month examinations to eliminate potential bias resulting from residual product odour" Comment: examiner did not know which groups the participants had been allocated to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was unclear in each of the study arms. However, loss to follow‐up was relatively low (18 of 319) and demographic characteristics of randomised participants were similar to those of evaluable participants. |
| Selective reporting (reporting bias) | High risk | Means and standard deviations for the bleeding outcome were not reported. |
| Other bias | Unclear risk | Compliance was assessed by measurements of returned supplies and review of diaries provided to participants to record daily product use, but was not reported. |
Smith 1988.
| Methods | Trial design: parallel group, 4 arms Location: University of Michigan, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults, with a minimum of 12 teeth. Oral hygiene was not a factor and maxillary and mandibular premolars and molars were required that had spaces large enough to accommodate an interdental brush Exclusion criteria: none stated Baseline plaque status: not reported Baseline periodontal status: periodontitis patients on maintenance programme after periodontal treatment Age at baseline: mean in years 53.5, range 24 to 78 Sex: 26 males/34 females Number randomised: 60 (Gp A 15; Gp B 15; Gp C 15; Gp D 15) Number evaluated: not reported; sites analysed and numbers of sites reported Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing and floss versus manual toothbrushing and superfloss versus manual toothbrushing and an interdental brush versus manual toothbrushing and a rubber tip stimulator Gp A: (n = 15) lightly waxed floss Gp B: (n = 15) Superfloss (Oral‐B) Gp C: (n = 15) interdental brush (Proxabrush, John O Butler and Co.) Gp D: (n = 15) rubber tip stimulator (John O Butler and Co) All participants used standardised manual toothbrush. Duration of intervention: 56 days Baseline cleaning: after the preliminary examination, a thorough prophylaxis was delivered to all participants 7 to 10 days before baseline assessments. Training: each participant received individual instruction in toothbrushing and in the use of assigned interdental aid. Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 28 days, and 56 days Plaque: Silness & Löe Plaque Index Periodontal disease – gingivitis: Löe & Silness Gingival Index Periodontal disease: pocket probing depth Adverse effects: not reported Attrition: not reported |
|
| Funding | Not stated | |
| Notes | No details about experience of examiners or their calibration was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly assigned into four groups". Comment: the method of sequence generation was unclear. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate not reported |
| Selective reporting (reporting bias) | High risk | Quote: "Mean scores reported only in graphs, with no exact numbers and standard deviations" |
| Other bias | Unclear risk | Compliance not assessed |
Vogel 1975.
| Methods | Trial design: parallel group, 4 arms Location: New Jersey Dental School, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: dental students after thorough scaling and prophylaxis Exclusion criteria: none stated Baseline plaque status: not reported Baseline periodontal status: not reported Age at baseline: not reported Sex: not reported Number randomised: 24 (Gp A 6; Gp B 6; Gp C 6; Gp D 6) Number evaluated: not reported Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and floss versus manual toothbrushing and a rubber tip stimulator (tooth cleaning stick) Gp A: (n = 6) manual toothbrush Gp B: (n = 6) manual toothbrush and floss Gp C: (n = 6) manual toothbrush and rubber tip stimulator (tooth cleaning stick) All participants used the modified Bass intrasulcular brushing technique with a soft nylon multi‐tufted rounded bristle brush. Other interventions (not included in the review): Gp D: (n = 6) manual toothbrush and floss and rubber tip stimulator Duration of intervention: 33 days Baseline cleaning: thorough scaling and prophylaxis Training: each participant was instructed to use unwaxed floss, rubber tip stimulator and the modified Bass intrasulcular brushing technique once a day at a specific time; additionally, individual home care techniques were reinforced on assessment days during the trial. Compliance assessment: self reported; anonymous questionnaires were given to participants at the end of trial in order to determine their compliance with the given instructions; the results of the questionnaire indicated approximately 90% adherence to the prescribed regimens; in the dental floss group, 2 of 6 participants did not follow the prescribed regimen after day 15. |
|
| Outcomes | Measurements: at baseline, and days 9, 15, and 33 Plaque: Podchladley's total plaque index Periodontal disease – gingivitis: Löe & Silness Gingival Index and Intracrevicular exudate sampling Adverse effects: not reported Attrition: not reported |
|
| Funding | Funding was not reported. | |
| Notes | Participants were dental students. Details about examiners not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the 24 subjects were randomly divided into four equal groups". Comment: insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel blinding unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported |
| Selective reporting (reporting bias) | High risk | No standard deviations reported. We were able to impute them for gingivitis but not for plaque. |
| Other bias | High risk | Compliance assessment was based on an anonymous questionnaire given to participants at the end of trial. Compliance in the flossing group after 15 days was poor. |
Walsh 1985.
| Methods | Trial design: parallel group, 3 arms Location: University of California School of Dentistry, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults Exclusion criteria: systemic illness, pregnancy, professional tooth cleaning, use of medication, antibiotics or inflammatory drugs during past 6 months Baseline plaque status: not reported Baseline periodontal status: generalised interproximal gingival inflammation and bleeding on probing with no furcation involvement; 25% of sites had probing depths of 4 mm or more Age at baseline: 30 to 70 years (mean 36) Sex: 15 males/21 females Number randomised: 36 (Gp A 12; Gp B 12; Gp C 12) Number evaluated: 36 (Gp A:12; Gp B:12; Gp C: 12) Smoking status: not reported |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and a wooden tooth cleaning stick versus manual toothbrushing and floss Gp A: (n = 12 evaluated) manual toothbrush (soft), once a day Gp B: (n = 12 evaluated) toothbrushing (not specified) and tooth cleaning stick (round toothpick) once a day Gp C: (n = 12 evaluated) toothbrushing (not specified) and unwaxed floss, once a day Duration of intervention: 3 months During the 3‐month pre‐experimental period, participants were to use toothbrush only, without interproximal cleaning devices so that the level of health participants achieved using toothbrush only could be evaluated. Training: at baseline of the pre‐experimental phase, instructions were given on sulcular toothbrushing. Instructions at the beginning of the experimental phase included a demonstration of the assigned interdental plaque control procedure in the participant's own mouth followed by guided intraoral practice by participants until they were able to perform the procedure correctly. Also, a written and illustrated handout was given, and sulcular toothbrushing was reinforced. Baseline cleaning: all participants received an oral prophylaxis at the beginning of the pre‐experimental phase (3 months of toothbrushing only), and again after 3 months at the begriming of the experimental phase before the randomisation. Compliance assessment: not reported |
|
| Outcomes | Measurements: at the beginning of the study, after the pre‐experimental phase (at 3 months), i.e. baseline, at 3 months of experimental phase (6 months from the beginning of the study) Dental plaque: Silness and Löe Plaque Index evaluated as percentage of interproximal surfaces scored positive for plaque (scored positive with a visible plaque score of 2 or 3) at 4 sites per tooth Periodontal disease – gingivitis: bleeding upon probing using Marquis X2 periodontal probe, evaluated as percentage of interproximal surfaces scored positive for bleeding Adverse effects: not reported Attrition: no participants were reported to be lost to follow‐up |
|
| Funding | Not reported | |
| Notes | Examinations performed by a single blinded examiner; no other information given on the examiner. Toothbrushing only performed once per day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly divided into three groups of 12 subjects each". Comment: insufficient information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"One examiner, functioning on a blind basis and having no access to previously recorded scores, performed all clinical examinations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not explicitly addressed, but it seemed that all randomised participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Surfaces were scored positive for plaque if they demonstrated visible plaque with a score of 2 or 3 by the Silness & Löe and positive for bleeding after probing. These scores were not recorded, but were interpreted as binary outcomes. |
| Other bias | Unclear risk | Compliance was not assessed during the experimental period. |
Walsh 1989.
| Methods | Trial design: parallel group, 4 arms Location: University of California School of Dentistry, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: 18 to 65 years of age, minimum of 20 natural teeth with gingivitis, defined as bleeding on probing (GI index > 1) on a minimum of 6 sites at the 18 sites probed on the Ramfjord teeth Exclusion criteria: oral lesions or systemically related gingival enlargement, history of organic heart valve damage or prosthetic implants, history of an oral prophylaxis or use of antibiotics within two weeks of start of study, orthodontic or extensive restorative treatment at start of study, pregnant or taking oral contraceptives Baseline plaque status: mean PI score ranged from 1.3 to 1.5 Baseline periodontal status: minimum of 20 natural teeth with gingivitis, defined as bleeding on probing (GI index > 1) on a minimum of 6 sites at the 18 sites probed on the Ramfjord teeth Age at baseline: not reported Sex: not reported Number randomised: 108 (27 per group) Number evaluated: not reported (assumed to be all) Smoking status: not reported |
|
| Interventions | Gp 1 (n = 27): manual toothbrushing (Oral B 40) Gp 3 (n = 27): manual toothbrushing plus oral irrigation (Oral B 40 plus Broxojet) All groups educated about the importance of plaque removal, instructed in use of devices (with fluoridated toothpaste) and advised to use devices twice daily. Sticky notes as reminders were provided, participants received a phone call every 2 weeks to reinforce the oral hygiene instructions, and they kept a diary of record device use and duration. Other interventions (not included in the review): Gp 2 (n = 27): powered toothbrushing (LPA/Broxo SA) Gp 4 (n = 27): powered toothbrushing plus oral irrigation (LPA/Broxo SA plus Broxojet) |
|
| Outcomes | Measurements at baseline, 3 months (the study also assessed at 6 months but we did not use this data as participants received professional scale and polish after the 3‐month assessment) Plaque Index (Silness & Löe) Tooth stain (Yankell et al 1982 method) Periodontal disease – gingivitis: Gingival Index (Löe & Silness); bleeding on probing; probing pocket depth, and attachment loss Adverse effects: inspected for soft tissue changes ‐ there were none. |
|
| Funding | Xouth, Inc., Lancaster, PA, USA | |
| Notes | Trial authors calculated interrater reliability and reported it to be "excellent" and "never lower than 0.6" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated to groups in consecutive order by time and data of entry into the study" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiners did not know to which group the patients belonged". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated if any participants dropped out or were excluded from the data analysis (we assumed that all participants were included at the 3‐month assessment) |
| Selective reporting (reporting bias) | Low risk | No protocol available; all expected outcomes reported |
| Other bias | Low risk | Compliance assessed as "excellent" |
Yankell 2002.
| Methods | Trial design: parallel group, 2 arms Location: Philadelphia, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: adults, aged between 18 and 60 years, at least 18 natural teeth present, informed consent signed Exclusion criteria: antibiotic use, use of steroidal or non‐steroidal anti‐inflammatory agents, acute illness, orthodontic treatment, pregnancy, sensitivity to or history of oral or perioral tissue reactions or allergies to dentifrice, any kind of disease or lesion of the hard or soft tissues of the mouth upon examination, prophylaxis within 4 weeks prior to baseline examination Baseline plaque status: not reported Baseline periodontal status: not reported (but we were aware from interaction with the trial author for a previous review on interdental brushing that most participants had mild gingivitis) Age at baseline: 18 to 60 years Sex: not reported Number randomised: 63 (Gp A 31; Gp B 32) Number evaluated: 62 (Gp A 31; Gp B 31) Smoking: not reported |
|
| Interventions |
Comparison: manual toothbrushing and interdental brushing versus manual toothbrushing and floss Gp A: (n = 32 evaluated) interdental brush (BrushPicks, Dental Concepts, Paramus, NJ, USA) Gp B: (n = 31 evaluated) Glide floss (W.L.Gore and Associates, Flagstaff, USA) All participants used manual toothbrush (Oral‐B 35) and a fluoride‐containing dentifrice (Crest regular), twice a day, in the morning and in the evening. Duration of intervention: 4 weeks Training: participants received a toothbrush and a fluoride‐containing dentifrice and were requested to brush their teeth twice a day, in the morning and in the evening; BrushPicks or Glide floss were to be used after toothbrushing. No specific instructions were given for any of the products distributed. Participants were not allowed to use any other tooth‐cleaning products or devices during the study. Baseline cleaning: not reported Compliance assessment: not reported |
|
| Outcomes | Measurements: at baseline, 2 weeks, and 4 weeks Plaque: plaque area scored using the Quigley‐Hein Plaque Index, Turesky modification on the facial and lingual sites of the Ramfjord teeth that were not crowned or clasp‐bearing using a disclosing agent Periodontal disease – gingivitis: evaluated using the Lobene modification of the Gingival Index at the facial and lingual margins of the Ramfjord teeth; bleeding on probing evaluated using the Eastman Interdental Bleeding Index at the mesial and distal gingival margins of all natural teeth anterior to the third molars One participant in Gp B could not have the bleeding on probing index performed, therefore there were only 31 participants assessed for that measure. Adverse effects: safety assessments including examinations of hard and soft oral tissues performed at each measurement period; investigators also recorded opinions regarding adverse reactions on study treatments; there were no adverse events reported or observed at any time during the study. Attrition: one participant in Gp B (the Glide Floss group) could not have the bleeding on probing index performed due to medical reasons and did not report for the 2‐ and 4‐week assessment. Dropout was not reported to be caused by the use of any of the products. |
|
| Funding | Funding source not reported; Industry provided oral hygiene devices: BrushPicks TM: Dental Concepts, Paramus NJ, USA. Glide floss: W.L. Gore Associates, Inc., Flagstaff, AZ, USA. Toothbrush: Oral‐B P35. Oral‐B Laboratories, Belmont, CA, USA | |
| Notes | Examiner training and intra‐examiner reliability was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty three subjects from the Philadelphia, Pennsylvania area were randomly assigned to either the ADA‐Accepted Glide floss or the BrushPicks group". Comment: no further description given on the method used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double‐blind, four week study" Comment: blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind, four week study" Comment: not clear who exactly was blinded and how |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: 1 out of 63, adequately reported and explained |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes in the Methods section were addressed in the Results section. |
| Other bias | Unclear risk | Compliance was not assessed. |
Yost 2006.
| Methods | Trial design: parallel group, 4 arms Location: Florida, USA Number of centres: 1 Recruitment period: not reported |
|
| Participants | Inclusion criteria: at least 5 interproximal sites that could accommodate the interdental brush with adjacent teeth being natural dentition, ability to floss, but not a current floss user Exclusion criteria: use of antibiotics, anticoagulants, steroids or other anti‐inflammatory products (except acetaminophen and 81 mg daily aspirin), diabetes, rheumatic fever, hepatic or renal disease, gross caries or other hard tissue pathology, transmissible diseases, heavy calculus, orthodontics, prosthodontics, piercings, allergy to red food dye Baseline plaque status: Benson modification of the Quigley‐Hein index (mean plaque score ≥ 1.5) Baseline periodontal status: Löe and Silness Gingival Index (mean gingival score ≥ 1.0) Age at baseline: mean and range (years), males (35.1; 19 to 57), females (39.6; 18 to 63) Sex: 37 males/83 females Number randomised: 128 Number evaluated: 120 Smoking status: of evaluated participants, 12 were smokers (10%) |
|
| Interventions |
Comparison: manual toothbrushing and interdental brushing versus manual toothbrushing and floss versus manual toothbrushing and interdental cleaning sticks Gp A: (n = 31 evaluated) soft manual toothbrush (GUM, Sunstar) and Crest Regular toothpaste plus an interdental brush (GUM, Go‐Betweens) Gp B: (n = 31 evaluated) soft manual toothbrush (GUM, Sunstar) and Crest Regular toothpaste plus dental floss (Crest Glide) Gp C: (n = 30 evaluated) soft manual toothbrush (GUM, Sunstar) and an interdental cleaner (GUM Soft‐Picks, interdental plastic cleaners with elastomeric tips) Duration of intervention: 6 weeks Other interventions (not included in the review): Gp D: (n = 28 evaluated) soft manual toothbrush (GUM, Sunstar) and Flosser (Butler) Training: participants were given instructions on product use and diary instructions. Product use by the participants was supervised to ensure that product was used correctly. Baseline cleaning: participants were given a prophylaxis to remove all supragingival calculus and plaque. Compliance assessment: diary and compliance review performed at 3 weeks |
|
| Outcomes | Measurements: at baseline and 6 weeks Participants returned at 3 months for medical/dental history update, diary and compliance. Plaque: Benson modification of the Quigley‐Hein Index Periodontal disease – gingivitis: Löe and Silness Gingival Index and Eastman Interdental Bleeding Index (EIBI) Adverse effects: oral soft tissue examinations performed at baseline, 3, and 6 weeks time points; none reported on in the Results Attrition: 8 participants lost from the study, but it was not reported from which study arms; no reasons provided |
|
| Funding | Study supported by the product manufacturer, Sunstar America, Inc. The first author employed by the manufacturer |
|
| Notes | Intra‐examiner reliability not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that participants were randomly assigned to 1 of the 4 test products, but no further information was given on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Examiner blinding not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: 8 out of 128. Although reasons and breakdown by study arms were not provided, we judged attrition as unlikely to affect the results. |
| Selective reporting (reporting bias) | Unclear risk | Standard deviations missing but we were able to estimate them. Examinations of the oral soft tissue were performed at 6 weeks (i.e. at the final visit), but results not reported |
| Other bias | Unclear risk | Diary and compliance review mentioned in Methods, but not reported in Results |
Zimmer 2006.
| Methods | Trial design: parallel group, 4 arms Location: Dusseldorf, Germany Number of centres: 1 Recruitment period: July to August 2004 |
|
| Participants | Inclusion criteria: no participants with good oral hygiene under normal conditions as they had to have a Modified Proximal Plaque Index (MPPI) per tooth of ≥ 1.5 and a Papillary Bleeding Index (PBI) per tooth of ≥ 0.5 Exclusion criteria: fixed orthodontic appliances, severe periodontitis, long‐term use of anti‐inflammatory drugs within 1 month, prior to, or during the study, removable dentures, less than 20 natural teeth, regular use of dental floss or antimicrobial mouthwash during the past 3 months, clinical attachment loss > 5 mm in a minimum of 3 teeth, furcation involvement or pathological tooth mobility and any dentists, dental students, dental assistants and hygienists Baseline plaque status: Modified Proximal Plaque Index (MPPI) per tooth ≥ 1.5 Baseline periodontal status: Papillary Bleeding Index (PBI) per tooth ≥ 0.5 Age at baseline: mean and range (years), 31.7 (20.0 to 64.4) Sex: 78 males/78 females Number randomised: 156 Number evaluated: 156 Smoking status: 33 smokers in the floss group and 6 in the control group |
|
| Interventions |
Comparison: manual toothbrushing versus manual toothbrushing and floss Gp A: (n = 39 evaluated) manual toothbrush used in usual manner Gp B: (n = 39 evaluated) dental floss (Odol med 3 dental floss, GlaxoSmithKline), once a day All participants used manual toothbrush (Dr Best flex plus medium, GlaxoSmithKline) and a silica‐based toothpaste with 1350 ppm fluoride as NaF (Dr Best Multi Aktiv, GlaxoSmithKline) Duration of intervention: 4 weeks Other interventions (not included in the review): Gp C: (n = 39) toothbrushing and mouth rinsing (0.06% chlorhexidine and 0.025% fluoride as sodium fluoride) Gp D: (n = 39) toothbrushing and mouth rinsing (0.1% cetylpyridiniumchloride and 0.025% F as NaF) Baseline cleaning: calculus removal in the lower front teeth Training: participants received brief instructions for dental floss and mouthrinse; 2‐minute instruction on flossing using a plastic tooth model was demonstrated; no instructions were given on toothbrushing technique nor time (participants told to brush in the usual manner). Compliance assessment: at the intermediate and final examination, participants were interviewed as to whether they used the assigned devices as requested; all stated that they performed oral hygiene as requested. |
|
| Outcomes | Measurements: at baseline, 4 weeks, and 8 weeks Plaque: Modified Proximal Plaque Index (MPPI), Quigley‐Hein Plaque Index (QHI) Periodontal disease – gingivitis: PBI Adverse effects: side effects were registered at the final examination; side effects mainly occurred in the mouthrinse groups, mostly in terms of staining of teeth and tongue Attrition: no participants were lost to follow‐up |
|
| Funding | GlaxoSmithKline, Buhl, Germany | |
| Notes | All examinations performed by 1 examiner; intra‐examiner reliability was tested. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By using the stratification by gender and PBI…the 156 participants were randomly assigned to four groups with 39 subjects in each group...In a box containing 156 envelopes in four strata...each participant had to draw one envelope containing the number of the attributed product." Comment: randomisation appears to have been performed by the participants each selecting an envelope from a box |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignment of subjects to groups was performed by a person not involved in the experimentation... box containing 156 envelopes on four strata...each participant had to draw one envelope..." Comment: allocation concealment was addressed satisfactorily |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all examinations performed by a single blinded examiner" Comment: examiner did not know which groups the participants had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes stated in the Methods section were addressed in the Results section |
| Other bias | Low risk | Compliance was self‐reported. All participants stated that they had performed oral hygiene as requested during the trial. |
ADA: Amercian Dental Association AIDS: Acquired Immune Deficiency Syndrome BI: bleeding index GI: Gingival Index Gp: group HIV: Human Immunodeficiency Virus IDB: interdental brush ID‐2: a make of interdental brush PI: Plaque Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anaise 1976 | Study length less than 4 weeks |
| Anaise 1977 | Cluster randomised by class, but no information on the number of classes |
| Anderson 1995 | Compared 2 different types of floss: electrical versus traditional |
| Arora 2014 | Study length less than 4 weeks |
| Ashwath 2014 | Not an RCT |
| Axelsson 1976 | Inappropriate study design |
| Axelsson 1981 | Inappropriate study design |
| Axelsson 1994 | Inappropriate study design |
| Bader 1997 | Inappropriate intervention |
| Baeshen 2008 | Study length less than 4 weeks |
| Barlow 2004 | Inappropriate study design |
| Barth 1990 | Study length less than 4 weeks |
| Bassiouny 1981 | Study length less than 4 weeks |
| Bergenholtz 1980 | Study length less than 4 weeks and inappropriate study design |
| Bernier 1966 | Unclear means of randomisation |
| Biesbrock 2006 | Study length less than 4 weeks |
| Blanck 2007 | Study length less than 4 weeks |
| Carter‐Hanson 1996 | Compared 2 different types of floss: floss holder (Quik Floss) versus traditional |
| Caton 1993 | Not an RCT |
| Ciancio 1992 | Inappropriate study design (floss comparison only) |
| Cronin 1996 | Insufficient data (no standard deviations) |
| Duan 1995 | Not an RCT |
| Elliott 1972 | Inappropriate study design |
| Finkelstein 1979 | Inappropriate intervention |
| Friel 1980 | Not an RCT |
| Gisselsson 1988 | Inappropriate study design |
| Gisselsson 1999 | Inappropriate study design |
| Gjermo 1970 | Insufficient follow‐up time. Appeared to be a cross‐over study, with the first period lasting only 2 weeks |
| Glickman 1964 | Toothbrushes were not the same in both arms ‐ 1 appeared to use a manual brush and the other, a powered brush |
| Goyal 2013 | Inappropriate study design |
| Goyal 2015 | Inappropriate study design (2 types of water jet compared) |
| Granath 1979 | Use of floss in school context. 12‐ and 13‐year old children grouped by different combinations of dietary and oral hygiene habits. Split‐mouth study |
| Gupta 1973 | Study length less than 4 weeks |
| Hennequin‐Hoenderdos 2018 | Participants started with experimental gingivitis built up over 21 days "After familiarization and prophylaxis, participants refrained from brushing mandibular teeth for 21 days..." |
| Hill 1973 | Not an RCT |
| Hoover 1971 | Not an RCT |
| Imai 2007 | Inappropriate study design (2 flosses compared, 1 with chlorhexidine impregnation) |
| Imai 2010 | Inappropriate study design |
| Karimi 2014 | Compared 2 types of floss |
| Kiger 1991 | Cross‐over design, no first‐period data and no washout period |
| Kleber 1988 | Cross‐over design, no first‐period data and no washout period |
| Koch 1965 | Inappropriate study design |
| Lamberts 1982 | Not an RCT |
| Larsen 2017 | Compared 2 types of interdental brush: conical versus cylindrical |
| Lobene 1969 | Inappropriate study design (different toothbrushes used in the control and intervention) |
| Lyle 2016 | Compared single use of a water flosser versus interdental brush |
| Mayfield 1998 | Inappropriate study design |
| Nayak 1977 | Study length less than 4 weeks |
| NCT01307358 | All groups used a Sonicare Interproximal cleaning prototype. They selected only 4 interproximal sites per participant and no results posted |
| Newbrun 1980 | Inappropriate study design (floss comparison only) |
| Pucher 1995 | Compared 2 different types of floss: electrical vibrating floss holder (Floss Plus easy flosser) versus traditional |
| Rich 1989 | Inappropriate study design |
| Robinson 1976 | Inappropriate study design (toothbrushing comparison only) |
| Schwarz 1990 | Inappropriate study design (powered toothbrushing comparison only) |
| Sharma 2012 | Inappropriate study design (comparison of 2 similar devices only) |
| Spolsky 1993 | Compared new flossing aid (Flosser) with finger flossing; cross‐over study |
| Steinberg 1963 | Study length less than 4 weeks |
| Wright 1976 | Inappropriate study design |
| Wright 1977 | Inappropriate study design |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02836223.
| Methods | RCT, single‐blinded Location: Canada |
| Participants | 72 participants Inclusion criteria: between 25 and 70 years of age; able to provide written informed consent prior to participation; agreed to not participate in any other oral/dental products clinical study for the study duration; good general health and a non‐smoker; minimum of 50% bleeding on probing sites; minimum pre‐brushing plaque score of 0.6; minimum of 1.75 gingivitis score; have no probing depths greater than 5 mm; a minimum of 20 teeth (not including 3rd molars); no partial dentures, orthodontic brackets, wires or other appliances; agreed to refrain from the use of any non‐study dental device or oral care product for the study duration; agreed to return for the scheduled visits and follow study procedures; agreed to delay dental prophylaxis until study completion; minimum pre‐brushing plaque score of 0.6; minimum of 1.75 gingivitis score Exclusion criteria: probing depth greater than 5 mm; systemic disease (e.g. diabetes, autoimmune disease); advanced periodontitis; taking medication that can influence gingival health such as seizure medication, calcium channel blockers, Cyclosporine, anticoagulants; orthodontic appliances or removable partial dentures; pregnant at time of study; use of antibiotics within 6 months of study |
| Interventions | Water flosser and manual toothbrush versus manual toothbrush |
| Outcomes | Reduction of gingival bleeding, reduction of gingival inflammation, reduction in dental plaque (measurement at 4 weeks) |
| Notes | Sponsor: Water Pik Inc Collaborator: All Sum Research Center Ltd |
Differences between protocol and review
Title changed to clarify that we were interested in devices used by individuals at home, in addition to regular toothbrushing.
Byline changed to involve new authors.
Background edited and updated as it had been written in 2015.
Edit to objectives to reduce word count ‐ removing specific mention of adverse effects.
Harms and adverse effects had been listed as a secondary outcome in the protocol, but we considered it important to balance benefits and harms and so we recategorised it as a primary outcome.
Although our search strategy contained 'miswak', we did not include studies evaluating this device as these sticks clean the whole mouth rather than the interdental spaces.
Contributions of authors
Helen Worthington: writing protocol, screening search results, undertaking data analysis, assessment of the certainty of the evidence, writing review Laura MacDonald: screening results of 2019 'top‐up' search, undertaking data extraction, 'Risk of bias' assessment, assessment of the certainty of the evidence, writing review Tina Poklepovic Pericic: writing protocol, screening search results, undertaking 'Risk of bias' assessment, writing review Dario Sambunjak: writing protocol, undertaking data extraction, 'Risk of bias' assessment, writing review Trevor Johnson: writing protocol, screening search results, undertaking data extraction, 'Risk of bias' assessment, writing review Pauline Imai: writing protocol, screening search results, undertaking data extraction, 'Risk of bias' assessment, writing review Jan Clarkson: writing protocol, assessment of the certainty of the evidence, writing review
Sources of support
Internal sources
School of Dentistry, The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC), the NIHR Manchester Biomedical Research Centre, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
Helen Worthington: none known. Co‐ordinating Editor of Cochrane Oral Health. Author on one included study (assessed by other review authors) Laura MacDonald: none known. Managing Editor with Cochrane Oral Health Tina Poklepovic Pericic: none known Dario Sambunjak: none known Trevor Johnson: none known. Editor with Cochrane Oral Health Pauline Imai: none known. Author on one included study (assessed by other review authors) Jan Clarkson: none known. Co‐ordinating Editor of Cochrane Oral Health
Edited (no change to conclusions)
References
References to studies included in this review
Barnes 2005 {published data only}
- Barnes CM, Russell CM, Reinhardt RA, Payne JB, Lyle DM. Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. Journal of Clinical Dentistry 2005;16(3):71‐7. [PUBMED: 16305005] [PubMed] [Google Scholar]
Bauroth 2003 {published data only}
- Bauroth K, Charles CH, Mankodi SM, Simmons K, Zhao Q, Kumar LD. The efficacy of an essential oil antiseptic mouthrinse vs. dental floss in controlling interproximal gingivitis: a comparative study. Journal of the American Dental Association 2003;134(3):359‐65. [DOI] [PubMed] [Google Scholar]
Biesbrock 2007 {published data only}
- Biesbrock AR, Bartizek RD, Gerlach RW, Terezhalmy GT. Oral hygiene regimens, plaque control, and gingival health: a two‐month clinical trial with antimicrobial agents. Journal of Clinical Dentistry 2007;18(4):101‐5. [PubMed] [Google Scholar]
Christou 1998 {published data only}
- Christou V, Timmerman MF, Velden U, Weijden FA. Comparison of different approaches of interdental oral hygiene: interdental brushes versus dental floss. Journal of Periodontology 1998;69(7):759‐64. [DOI] [PubMed] [Google Scholar]
Cronin 1997 {published data only}
- Cronin M, Dembling W, Warren P. The safety and efficacy of gingival massage with an electric interdental cleaning device. Journal of Clinical Dentistry 1997;8(5):130‐3. [PUBMED: 9487832] [PubMed] [Google Scholar]
Cronin 2005 {published data only}
- Cronin MJ, Dembling WZ, Cugini M, Thompson MC, Warren PR. A 30‐day clinical comparison of a novel interdental cleaning device and dental floss in the reduction of plaque and gingivitis. Journal of Clinical Dentistry 2005;16(2):33‐7. [PUBMED: 16170973] [PubMed] [Google Scholar]
Finkelstein 1990 {published data only}
- Finkelstein P, Yost KG, Grossman E. Mechanical devices versus antimicrobial rinses in plaque and gingivitis reduction. Clinical Preventive Dentistry 1990;12(3):8‐11. [PubMed] [Google Scholar]
Frascella 2000 {published data only}
- Frascella JA, Fernandez P, Gilbert RD, Cugini M. A randomized clinical evaluation of the safety and efficacy of a novel oral irrigator. American Journal of Dentistry 2000;13(2):55‐8. [PubMed] [Google Scholar]
Gordon 1996 {published data only}
- Gordon JM, Frascella JA, Reardon RC. A clinical study of the safety and efficacy of a novel electric interdental cleaning device. Journal of Clinical Dentistry 1996;7(3 Spec No):70‐3. [PUBMED: 9238868] [PubMed] [Google Scholar]
Goyal 2012 {published data only}
- Goyal CR, Lyle DM, Qaqish JG, Schuller R. The addition of a water flosser to power tooth brushing: effect on bleeding, gingivitis, and plaque. Journal of Clinical Dentistry 2012;23(2):57‐63. [PUBMED: 22779218] [PubMed] [Google Scholar]
Graziani 2017 {published data only}
- Graziani F, Palazzolo A, Gennai S, Karapetsa D, Giuca MR, Cei S, et al. Interdental plaque reduction after use of different devices in young subjects with intact papilla: a randomized clinical trial. International Journal of Dental Hygiene 2017 [Epub ahead of print]. [DOI: 10.1111/idh.12318; PUBMED: PMID: 28971569] [DOI] [PubMed]
Hague 2007 {published data only}
- Hague AL, Carr MP. Efficacy of an automated flossing device in different regions of the mouth. Journal of Periodontology 2007;78(8):1529‐37. [DOI] [PubMed] [Google Scholar]
- Hague Al, Carr MP, Rashid RG. Evaluation of the safety and efficacy of an automated flossing device: a randomized controlled trial. Journal of Clinical Dentistry 2007;18(2):45‐8. [PubMed] [Google Scholar]
Imai 2011 {published data only}
- Imai PH, Hatzimanolakis PC. Interdental brush in type I embrasures: examiner blinded randomized clinical trial of bleeding and plaque efficacy. Canadian Journal of Dental Hygiene 2011;45(1):13‐20. [Google Scholar]
Isaacs 1999 {published data only}
- Isaacs RL, Beiswanger BB, Crawford JL, Mau MS, Proskin H, Warren PR. Assessing the efficacy and safety of an electric interdental cleaning device. Journal of the American Dental Association 1999;130(1):104‐8. [PUBMED: 9919039] [DOI] [PubMed] [Google Scholar]
Ishak 2007 {published data only}
- Ishak N, Watts TLP. A comparison of the efficacy and ease of use of dental floss and interproximal brushes in a randomised split mouth trial incorporating an assessment of subgingival plaque. Oral Health & Preventive Dentistry 2007;5(1):13‐8. [PubMed] [Google Scholar]
Jackson 2006 {published data only}
- Jackson MA, Kellett M, Worthington HV, Clerehugh V. Comparison of interdental cleaning methods: a randomized controlled trial. Journal of Periodontology 2006;77(8):1421‐9. [DOI] [PubMed] [Google Scholar]
Jared 2005 {published data only}
- Jared H, Zhong Y, Rowe M, Ebisutani K, Tanaka T, Takase N. Clinical trial of a novel interdental brush cleaning system. Journal of Clinical Dentistry 2005;16(2):47‐52. [PubMed] [Google Scholar]
Kazmierczak 1994 {published data only}
- Kazmierczak M, Mather M, Anderson TM, Ciancio SG. An alternative to dental floss in a personal dental hygiene program. Journal of Clinical Dentistry 1994;5(1):5‐7. [PUBMED: 8031487] [PubMed] [Google Scholar]
Lewis 2004 {published data only}
- Lewis MW, Holder‐Ballard C, Selders RJ Jr, Scarbecz M, Johnson HG, Turner EW. Comparison of the use of a toothpick holder to dental floss in improvement of gingival health in humans. Journal of Periodontology 2004;75(4):551‐6. [PUBMED: 15152819] [DOI] [PubMed] [Google Scholar]
Lobene 1982 {published data only}
- Lobene RR, Soparkar PM, Newman MB. Use of dental floss. Effect on plaque and gingivitis. Clinical Preventive Dentistry 1982;4(1):5‐8. [PubMed] [Google Scholar]
Meklas 1972 {published data only}
- Meklas JF, Stewart JL. Investigation of the safety and effectiveness of an oral irrigating device. Journal of Periodontology 1972;43(7):441‐3. [PUBMED: 4504530] [DOI] [PubMed] [Google Scholar]
Mwatha 2017 {published data only}
- Mwatha A, Olson M, Souza S, Ward M, Jenkins W, Amini P, et al. Gingival health and plaque regrowth response following a four‐week interdental hygiene intervention. Journal of Clinical Dentistry 2017;1 Spec No A:A36‐44. [PUBMED: PMID: 28422463] [PubMed] [Google Scholar]
NCT00855933 {published data only}
- NCT00855933. A controlled clinical study to determine the gingivitis benefit of flossing. clinicaltrials.gov/show/NCT00855933 (first received 5 March 2009).
NCT01250769 {published data only}
- NCT01250769. Evaluating the effect of a novel interproximal cleaning device on oral health. clinicaltrials.gov/ct2/show/NCT01250769 (first received 1 December 2010).
Rosema 2008 {published data only}
- Rosema NA, Timmerman MF, Versteeg PA, Palenstein Helderman WH, Velden U, Weijden GA. Comparison of the use of different modes of mechanical oral hygiene in prevention of plaque and gingivitis. Journal of Periodontology 2008;79(8):1386‐94. [DOI] [PubMed] [Google Scholar]
Rosema 2011 {published data only}
- Nanning AM, Nienke LH, Berchier CE, Slot DE, Weijden GA. The effect of different interdental cleaning devices on gingival bleeding. Journal of the International Academy of Periodontology 2011;13(1):2‐10. [PubMed] [Google Scholar]
Schiff 2006 {published data only}
- Schiff T, Proskin HM, Zhang YP, Petrone M, DeVizio W. A clinical investigation of the efficacy of three different treatment regimens for the control of plaque and gingivitis. Journal of Clinical Dentistry 2006;17(5):138‐44. [PubMed] [Google Scholar]
Sharma 2002 {published data only}
- Sharma NC, Charles CH, Qaqish JG, Galustians HJ, Zhao Q, Kumar LD. Comparative effectiveness of an essential oil mouthrinse and dental floss in controlling interproximal gingivitis and plaque. American Journal of Dentistry 2002;15(6):351‐5. [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith BA, Armstrong CS, Morrison EC, Caffesse RG. Effectiveness of four interproximal cleaning devices in plaque removal and gingival health. American Journal of Dentistry 1988;1(2):57‐62. [PUBMED: 3179006] [PubMed] [Google Scholar]
Vogel 1975 {published data only}
- Vogel RI, Sullivan AJ, Pascuzzi JN, Deasy MJ. Evaluation of cleansing devices in the maintenance of interproximal gingival health. Journal of Periodontology 1975;46(12):745‐7. [DOI] [PubMed] [Google Scholar]
Walsh 1985 {published data only}
- Walsh MM, Heckman BL. Interproximal subgingival cleaning by dental floss and the toothpick. Dental Hygiene 1985;59(10):464‐7. [PubMed] [Google Scholar]
Walsh 1989 {published data only}
- Walsh M, Heckman B, Leggott P, Armitage G, Robertson PB. Comparison of manual and power toothbrushing, with or without adjunctive oral irrigation, for controlling plaque and gingivitis. Journal of Clinical Periodontology 1989;16:419‐27. [DOI] [PubMed] [Google Scholar]
Yankell 2002 {published data only}
- Yankell SL, Shi X, Emling RC. Efficacy and safety of BrushPicks, a new cleaning aid, compared to the use of Glide floss. Journal of Clinical Dentistry 2002;13(3):125‐9. [PubMed] [Google Scholar]
Yost 2006 {published data only}
- Yost KG, Mallatt ME, Liebman J. Interproximal gingivitis and plaque reduction by four interdental products. Journal of Clinical Dentistry 2006;17(3):79‐83. [PubMed] [Google Scholar]
Zimmer 2006 {published data only}
- Zimmer S, Kolbe C, Kaiser G, Krage T, Ommerborn M, Barthel C. Clinical efficacy of flossing versus use of antimicrobial rinses. Journal of Periodontology 2006;77(8):1380‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anaise 1976 {published data only}
- Anaise JZ. Plaque‐removing effect of dental floss and toothpicks in children 12‐13 years of age. Community Dentistry and Oral Epidemiology 1976;4(4):137‐9. [DOI] [PubMed] [Google Scholar]
Anaise 1977 {published data only}
- Anaise JZ. A comparison of two oral hygiene indexes for measuring the effectiveness of dental floss in plaque removal. Journal of Public Health Dentistry 1977;37(1):62‐7. [PUBMED: 264564] [DOI] [PubMed] [Google Scholar]
Anderson 1995 {published data only}
- Anderson NA, Barnes CM, Russell CM, Winchester KR. A clinical comparison of the efficacy of an electromechanical flossing device or manual flossing in affecting interproximal gingival bleeding and plaque accumulation. Journal of Clinical Dentistry 1995;6(1):105‐7. [PUBMED: 8694982] [PubMed] [Google Scholar]
Arora 2014 {published data only}
- Arora V, Tangade P, Ravishakar RTL, Tirth A, Pal S, Tandon V. Efficacy of dental floss and chlorhexidine mouth rinse as an adjunct to toothbrushing in removing plaque and gingival inflammation ‐ a three way cross over trial. Journal of Clinical and Diagnostic Research 2014;8(10):ZC01‐4. [PUBMED: 25478436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashwath 2014 {published data only}
- Ashwath B, Vijayalakshmi R, Arun D, Kumar V. Site‐based plaque removal efficacy of four branded toothbrushes and the effect of dental floss in interproximal plaque removal: a randomized examiner‐blind controlled study. Quintessence International 2014;45(7):577‐84. [PUBMED: 24847496] [DOI] [PubMed] [Google Scholar]
Axelsson 1976 {published data only}
- Axelsson P, Lindhe J, Waseby J. The effect of various plaque control measures on gingivitis and caries in schoolchildren. Community Dentistry and Oral Epidemiology 1976;4(6):232‐9. [PUBMED: 1069613] [DOI] [PubMed] [Google Scholar]
Axelsson 1981 {published data only}
- Axelsson P, Lindhe J. Effect of oral hygiene instruction and professional toothcleaning on caries and gingivitis in schoolchildren. Community Dentistry and Oral Epidemiology 1981;9(6):251‐5. [PUBMED: 6955123] [DOI] [PubMed] [Google Scholar]
Axelsson 1994 {published data only}
- Axelsson P, Buischi YA, Barbosa MF, Karlsson R, Prado MC. The effect of a new oral hygiene training program on approximal caries in 12‐15‐year‐old Brazilian children: results after three years. Advances in Dental Research 1994;8(2):278‐84. [PUBMED: 7865087] [DOI] [PubMed] [Google Scholar]
Bader 1997 {published data only}
- Bader H, Williams R. Clinical and laboratory evaluation of powered electric toothbrushes: comparative efficacy of two powered brushing instruments in furcations and interproximal areas. Journal of Clinical Dentistry 1997;8(3 Spec No):91‐4. [PUBMED: 9238893] [PubMed] [Google Scholar]
Baeshen 2008 {published data only}
- Baeshen HA, Kjellberg H, Lingstrom P, Birkhed D. Uptake and release of fluoride from fluoride‐impregnated chewing sticks (miswaks) in vitro and in vivo. Caries Research 2008;42(5):363‐8. [PUBMED: 18728368] [DOI] [PubMed] [Google Scholar]
Barlow 2004 {published data only}
- Barlow AP, Zhou X, Roberts J, Colgan P. Effect of a novel integrated power toothbrush and toothpaste oral hygiene system on gingivitis. Compendium of Continuing Education in Dentistry 2004;25(10 Suppl 1):15‐20. [PUBMED: 15637976] [PubMed] [Google Scholar]
Barth 1990 {published data only}
- Barth F, Barth A. Efficiency of a new instrument: the Jet‐Floss for the elimination of dental plaque [Efficacite d'un nouvel instrument: le Jet‐Floss dans l'elimination de la plaque dentaire]. Journal de Parodontologie 1990;9(4):337‐43. [PUBMED: 2269930] [PubMed] [Google Scholar]
Bassiouny 1981 {published data only}
- Bassiouny MA, Grant AA. Oral hygiene for the partially edentulous. Journal of Periodontology 1981;52(4):214‐8. [PUBMED: 6939841] [DOI] [PubMed] [Google Scholar]
Bergenholtz 1980 {published data only}
- Bergenholtz A, Bjorne A, Glantz PO, Vikstrom B. Plaque removal by various triangular toothpicks. Journal of Clinical Periodontology 1980;7(2):121‐8. [PUBMED: 6929793] [DOI] [PubMed] [Google Scholar]
Bernier 1966 {published data only}
- Bernier JL, Sumnicht RW, Lancaster JE, Monahan JL. A comparison of three oral hygiene measures. Journal of Periodontology 1966;37(4):267‐73. [PUBMED: 5220892] [DOI] [PubMed] [Google Scholar]
Biesbrock 2006 {published data only}
- Biesbrock A, Corby PM, Bartizek R, Corby AL, Coelho M, Costa S, et al. Assessment of treatment responses to dental flossing in twins. Journal of Periodontology 2006;77(8):1386‐91. [PUBMED: 16937589] [DOI] [PubMed] [Google Scholar]
Blanck 2007 {published data only}
- Blanck M, Mankodi S, Wesley P, Tasket R, Nelson B. Evaluation of the plaque removal efficacy of two commercially available dental floss devices. Journal of Clinical Dentistry 2007;18(1):1‐6. [PUBMED: 17410948] [PubMed] [Google Scholar]
Carter‐Hanson 1996 {published data only}
- Carter‐Hanson C, Gadbury‐Amyot C, Killoy W. Comparison of the plaque removal efficacy of a new flossing aid (Quik Floss) to finger flossing. Journal of Clinical Periodontology 1996;23(9):873‐8. [PUBMED: 8891940] [DOI] [PubMed] [Google Scholar]
Caton 1993 {published data only}
- Caton JG, Blieden TM, Lowenguth RA, Frantz BJ, Wagener CJ, Doblin JM, et al. Comparison between mechanical cleaning and an antimicrobial rinse for the treatment and prevention of interdental gingivitis. Journal of Clinical Periodontology 1993;20(3):172‐8. [PUBMED: 8450082] [DOI] [PubMed] [Google Scholar]
Ciancio 1992 {published data only}
- Ciancio SG, Shibly O, Farber GA. Clinical evaluation of the effect of two types of dental floss on plaque and gingival health. Clinical Preventive Dentistry 1992;14(3):14‐8. [PUBMED: 1499246] [PubMed] [Google Scholar]
Cronin 1996 {published data only}
- Cronin M, Dembling W. An investigation of the efficacy and safety of a new electric interdental plaque remover for the reduction of interproximal plaque and gingivitis. Journal of Clinical Dentistry 1996;7(3 Spec No):74‐7. [PUBMED: 9238869] [PubMed] [Google Scholar]
Duan 1995 {published data only}
- Duan Q, Zhang Q, Cao C. The periodontal clinical effects of the fluoride‐coated dental floss. Chinese Journal of Conservative Dentistry 1995;3:144‐5. [Google Scholar]
Elliott 1972 {published data only}
- Elliott JR, Bowers GM, Clemmer BA, Rovelstad GH. A comparison of selected oral hygiene devices in dental plaque removal. Journal of Periodontology 1972;43(4):217‐20. [PUBMED: 4505608] [DOI] [PubMed] [Google Scholar]
Finkelstein 1979 {published data only}
- Finkelstein P, Grossman E. The effectiveness of dental floss in reducing gingival inflammation. Journal of Dental Research 1979;58(3):1034‐9. [PUBMED: 284036] [DOI] [PubMed] [Google Scholar]
Friel 1980 {published data only}
- Friel I, Seefeld G. Comparative clinical studies on reducing plaque and gingivitis [Vergleichende klinische untersuchungen zur reduktion von plaque und gingivitis]. Stomatologie der DDR 1980;30(8):545‐8. [PUBMED: 6936950] [PubMed] [Google Scholar]
Gisselsson 1988 {published data only}
- Gisselsson H, Birkhed D, Bjorn AL. Effect of professional flossing with chlorhexidine gel on approximal caries in 12‐ to 15‐year‐old schoolchildren. Caries Research 1988;22(3):187‐92. [PUBMED: 3163527] [DOI] [PubMed] [Google Scholar]
Gisselsson 1999 {published data only}
- Gisselsson H, Birkhed D, Emilson CG. Effect of professional flossing with NaF or SnF2 gel on approximal caries in 13‐16‐year‐old schoolchildren. Acta Odontologica Scandinavica 1999;57(2):121‐5. [PUBMED: 10445367] [DOI] [PubMed] [Google Scholar]
Gjermo 1970 {published data only}
- Gjermo P, Flotra L. The effect of different methods of interdental cleaning. Journal of Periodontal Research 1970;5(3):230‐6. [PUBMED: 4254187] [DOI] [PubMed] [Google Scholar]
Glickman 1964 {published data only}
- Glickman I, Petralis R, Marks RM. The effect of powered toothbrushing plus interdental stimulation upon the severity of gingivitis. Journal of Periodontology 1964;35(6):519‐24. [DOI] [PubMed] [Google Scholar]
Goyal 2013 {published data only}
- Goyal CR, Lyle DM, Qaqish JG, Schuller R. Evaluation of the plaque removal efficacy of a water flosser compared to string floss in adults after a single use. Journal of Clinical Dentistry 2013;24(2):37‐42. [PUBMED: 24282867] [PubMed] [Google Scholar]
Goyal 2015 {published data only}
- Goyal CR, Lyle DM, Qaqish JG, Schuller R. Efficacy of two interdental cleaning devices on clinical signs of inflammation: a four‐week randomized controlled trial. Journal of Clinical Dentistry 2015;26(2):55‐60. [PUBMED: 26349127] [PubMed] [Google Scholar]
Granath 1979 {published data only}
- Granath LE, Martinsson T, Matsson L, Nilsson G, Schroder U, Soderholm B. Intraindividual effect of daily supervised flossing on caries in schoolchildren. Community Dentistry and Oral Epidemiology 1979;7(3):147‐50. [PUBMED: 287584] [DOI] [PubMed] [Google Scholar]
Gupta 1973 {published data only}
- Gupta OP, O'Toole ET, Hammermeister RO. Effects of a water pressure device on oral hygiene and gingival inflammation. Journal of Periodontology 1973;44(5):294‐8. [PUBMED: 4512220] [DOI] [PubMed] [Google Scholar]
Hennequin‐Hoenderdos 2018 {published data only}
- Hennequin‐Hoenderdos NL, Sluijs E, Weijden GA, Slot DE. Efficacy of a rubber bristles interdental cleaner compared to an interdental brush on dental plaque, gingival bleeding and gingival abrasion: a randomized clinical trial. International Journal of Dental Hygiene 2018;16(3):380‐8. [DOI] [PubMed] [Google Scholar]
Hill 1973 {published data only}
- Hill HC, Levi PA, Glickman I. The effects of waxed and unwaxed dental floss on interdental plaque accumulation and interdental gingival health. Journal of Periodontology 1973;44(7):411‐3. [PUBMED: 4576511] [DOI] [PubMed] [Google Scholar]
Hoover 1971 {published data only}
- Hoover DR, Robinson HB. The comparative effectiveness of a pulsating oral irrigator as an adjunct in maintaining oral health. Journal of Periodontology 1971;42(1):37‐9. [PUBMED: 5276430] [DOI] [PubMed] [Google Scholar]
Imai 2007 {published data only}
- Imai PH. The Effects of Flossing with a Chlorhexidine Solution on Interproximal Gingivitis: a Randomized Controlled Trial [Masters thesis]. University of British Columbia, 2007. [Google Scholar]
Imai 2010 {published data only}
- Imai PH, Hatzimanolakis PC. Encouraging client compliance for interdental care with the interdental brush: the client's perspective. Canadian Journal of Dental Hygiene 2010;44(2):56‐60. [Google Scholar]
Karimi 2014 {published data only}
- Karimi MR, Naseri M, Behtoei AH. Effect of SUAB2 impregnated dental floss on periodontal indices. Journal of Research in Dental Sciences 2014;10(4):1. [Google Scholar]
Kiger 1991 {published data only}
- Kiger RD, Nylund K, Feller RP. A comparison of proximal plaque removal using floss and interdental brushes. Journal of Clinical Periodontology 1991;18:681‐4. [DOI] [PubMed] [Google Scholar]
Kleber 1988 {published data only}
- Kleber CJ, Putt MS. Evaluation of a floss‐holding device compared to hand‐held floss for interproximal plaque, gingivitis, and patient acceptance. Clinical Preventive Dentistry 1988;10(4):6‐14. [PUBMED: 3267496] [PubMed] [Google Scholar]
Koch 1965 {published data only}
- Koch G, Lindhe J. The effect of supervised oral hygiene on the gingiva of children. The effect of toothbrushing. Odontologisk Revy 1965;16(4):327‐35. [PUBMED: 5216254] [PubMed] [Google Scholar]
Lamberts 1982 {published data only}
- Lamberts DM, Wunderlich RC, Caffesse RG. The effect of waxed and unwaxed dental floss on gingival health. Part I. Plaque removal and gingival response. Journal of Periodontology 1982;53(6):393‐6. [PUBMED: 6955502] [DOI] [PubMed] [Google Scholar]
Larsen 2017 {published data only}
- Larsen HC, Slot DE, Zoelen C, Barendregt DS, Weijden GA. The effectiveness of conically shaped compared with cylindrically shaped interdental brushes ‐ a randomized controlled clinical trial. International Journal of Dental Hygiene 2017;15(3):211‐8. [PUBMED: 26751602] [DOI] [PubMed] [Google Scholar]
Lobene 1969 {published data only}
- Lobene RR. The effect of a pulsed water pressure cleansing device on oral health. Journal of Periodontology 1969;40(11):667‐70. [PUBMED: 5260628] [DOI] [PubMed] [Google Scholar]
Lyle 2016 {published data only}
- Lyle DM, Goyal CR, Qaqish JG, Schuller R. Comparison of water flosser and interdental brush on plaque removal: a single‐use pilot study. Journal of Clinical Dentistry 2016;27(1):23‐6. [PUBMED: 28390213] [PubMed] [Google Scholar]
Mayfield 1998 {published data only}
- Mayfield L, Attatrom R, Soderholm G. Cost‐effectiveness of mechanical plaque control. European Workshop on Mechanical Plaque Control; 1988. 1998:177‐89.
Nayak 1977 {published data only}
- Nayak RP, Wade AB. The relative effectiveness of plaque removal by the Proxabrush and rubber cone stimulator. Journal of Clinical Periodontology 1977;4(2):128‐33. [PUBMED: 266505] [DOI] [PubMed] [Google Scholar]
NCT01307358 {published data only}
- NCT01307358. A study comparing the interproximal plaque and gingivitis effects of three interdental cleaning modalities. clinicaltrials.gov/show/NCT01307358 (first received 2 March 2011).
Newbrun 1980 {published data only}
- Newbrun E, Heiblum R, Mayeda A. Effect of flossing, with and without iodine, on human interproximal plaque flora. Caries Research 1980;14(2):75‐83. [PUBMED: 6927968] [DOI] [PubMed] [Google Scholar]
Pucher 1995 {published data only}
- Pucher J, Jayaprakash P, Aftyka T, Sigman L, Swol R. Clinical evaluation of a new flossing device. Quintessence International 1995;26(4):273‐8. [PUBMED: 7568747] [PubMed] [Google Scholar]
Rich 1989 {published data only}
- Rich SK, Friedman JA, Schultz LA. Effects of flossing on plaque and gingivitis in third grade schoolchildren. Journal of Public Health Dentistry 1989;49(2):73‐7. [PUBMED: 2709366] [DOI] [PubMed] [Google Scholar]
Robinson 1976 {published data only}
- Robinson E. A comparative evaluation of the Scrub and Bass Methods of toothbrushing with flossing as an adjunct (in fifth and sixth graders). American Journal of Public Health 1976;66(11):1078‐81. [PUBMED: 984277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 1990 {published data only}
- Schwarz P, Benz C, Sonnabend E. Comparative study of the Interplak tooth cleansing instrument [Vergleichende untersuchung des interplak‐zahnreinigungsgerates]. Deutsche Zahnarztliche Zeitschrift 1990;45(9):557‐8. [PUBMED: 2269193] [PubMed] [Google Scholar]
Sharma 2012 {published data only}
- Sharma NC, Lyle DM, Qaqish JG, Schuller R. Comparison of two power interdental cleaning devices on the reduction of gingivitis. Journal of Clinical Dentistry 2012;23(1):22‐6. [PUBMED: 22435321] [PubMed] [Google Scholar]
Spolsky 1993 {published data only}
- Spolsky VW, Perry DA, Meng Z, Kissel P. Evaluating the efficacy of a new flossing aid. Journal of Clinical Periodontology 1993;20(7):490‐7. [PUBMED: 8354723] [DOI] [PubMed] [Google Scholar]
Steinberg 1963 {published data only}
- Steinberg GJ. Periodontal disease ‐ a continued report on the efficacy of a new home care technique. Journal of Periodontology 1963;34:293. [Google Scholar]
Wright 1976 {published data only}
- Wright GZ, Banting DW, Feasby WH. Dorchester dental flossing study. Preliminary report. Caries Research 1976;10(5):379‐85. [PUBMED: 1067905] [DOI] [PubMed] [Google Scholar]
Wright 1977 {published data only}
- Wright GZ, Banting DW, Feasby WH. Effect of interdental flossing on the incidence of proximal caries in children. Journal of Dental Research 1977;56(6):574‐8. [PUBMED: 268338] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02836223 {published data only}
- NCT02836223. Evaluation of the addition of an interdental cleaning device to manual brushing on gingival health. clinicaltrials.gov/show/nct02836223 (first received 18 July 2016).
Additional references
Addy 1986
- Addy M, Dummer PM, Griffiths G, Hicks R, Kingdon A, Shaw WC. Prevalence of plaque, gingivitis and caries in 11‐12‐year‐old children in South Wales. Community Dentistry and Oral Epidemiology 1986;14(2):115‐8. [DOI] [PubMed] [Google Scholar]
Adult Dental Health Survey 2009
- HSCIC. The adult dental health survey 2009. www.hscic.gov.uk/catalogue/PUB01086 (accessed prior to 17 March 2019).
Armitage 1999
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology 1999;4(1):1‐6. [DOI] [PubMed] [Google Scholar]
Asadoorian 2006
- Asadoorian J, Locker D. The impact of quality assurance programming: a comparison of two Canadian dental hygienist programs. Journal of Dental Education 2006;70(9):965‐71. [PubMed] [Google Scholar]
Bagramian 2009
- Bagramian RA, Garcia‐Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry 2009;22(1):3‐8. [PubMed] [Google Scholar]
Berchier 2008
- Berchier CE, Slot DE, Haps S, Weijden GA. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: a systematic review. International Journal of Dental Hygiene 2008;6(4):265‐79. [DOI] [PubMed] [Google Scholar]
Bergenholtz 1984
- Bergenholtz A, Olsson A. Efficacy of plaque‐removal using interdental brushes and waxed dental floss. Scandinavian Journal of Dental Research 1984;92(3):198‐203. [DOI] [PubMed] [Google Scholar]
Berglund 1990
- Berglund LJ, Small CL. Effective oral hygiene for orthodontic patients. Journal of Clinical Orthodontics 1990;24(5):315‐20. [PubMed] [Google Scholar]
Blieden 1992
- Blieden TM, Caton JG, Proskin HM, Stein SH, Wagener CJ. Examiner reliability for an invasive gingival bleeding index. Journal of Periodontology 1992;19(4):262‐7. [DOI] [PubMed] [Google Scholar]
Borrell 2012
- Borrell LN, Crawford ND. Socioeconomic position indicators and periodontitis: examining the evidence. Periodontology 2000 2012;58(1):69‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosma 2011
- Bosma ML. Maintenance of gingival health post professional care. International Dental Journal 2011;61(Suppl 3):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broadbent 2006
- Broadbent JM, Thomson WM, Poulton R. Oral health beliefs in adolescence and oral health in young adulthood. Journal of Dental Research 2006;85(4):339‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broadbent 2011
- Broadbent JM, Thomson WM, Boyens JV, Poulton R. Dental plaque and oral health during the first 32 years of life. Journal of the American Dental Association 2011;142(4):415‐26. [DOI] [PubMed] [Google Scholar]
Brothwell 1998
- Brothwell DJ, Jutai DK, Hawkins RJ. An update of mechanical oral hygiene practices: evidence‐based recommendations for disease prevention. Journal of the Canadian Dental Association 1998;64(4):295‐306. [PubMed] [Google Scholar]
Brown 2002
- Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontology 2000 2002;29(1):223‐34. [DOI] [PubMed] [Google Scholar]
Burt 1998
- Burt BA. Prevention policies in the light of the changed distribution of dental caries. Acta Odontologica Scandinavica 1998;56(3):179‐86. [DOI] [PubMed] [Google Scholar]
Casey 1988
- Casey GR. Maintenance of oral hygiene and dental health during orthodontic therapy. Clinical Preventive Dentistry 1988;10(1):11‐3. [PubMed] [Google Scholar]
Caton 2018
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions – introduction and key changes from the 1999 classification. Journal of Clinical Periodontology 2018;45(S20):S1‐8. [DOI] [PubMed] [Google Scholar]
Chadwick 1999
- Chadwick BL, Dummer PMH, Dunstan FD, Gilmour ASM, Jones RJ, Phillips CJ, et al. What type of filling? Best practice in dental restorations. Quality in Health Care 1999;8:202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandki 2011
- Chandki R, Banthia P, Banthia R. Biofilms: a microbial home. Journal of Indian Society of Periodontology 2011;15(2):111‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapelle 2018
- Chapple ILC, Mealey BL, Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology 2018;45(Suppl 20):S68‐S77. [DOI] [PubMed] [Google Scholar]
Christen 2003
- Christen AG, Christen JA. A historical glimpse of toothpick use: etiquette, oral and medical conditions. Journal of the History of Dentistry 2003;51(2):61‐9. [PUBMED: 12846259] [PubMed] [Google Scholar]
Cobb 2009
- Cobb CM, Williams KB, Gerkovitch MM. Is the prevalence of periodontitis in the USA in decline?. Periodontology 2000 2009;50(1):13‐24. [DOI] [PubMed] [Google Scholar]
Columbo 2012
COSMIN 2018
- COSMIN Initiative. cosmin.nl/ (accessed 23 March 2019).
Cumming 1973
- Cumming BR, Löe H. Consistency of plaque distribution in individuals without special home care instruction. Journal of Periodontal Research 1973;8(2):94‐100. [DOI] [PubMed] [Google Scholar]
Dahl 2011
- Dahl KE, Wang NJ, Skau I, Ohrn K. Oral health‐related quality of life and associated factors in Norwegian adults. Acta Odontologica Scandinavica 2011;69(4):208‐14. [DOI] [PubMed] [Google Scholar]
Dalwai 2006
- Dalwai F, Spratt DA, Pratten J. Modeling shifts in microbial populations associated with health or disease. Applied and Environmental Microbiology 2006;72(5):3678‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demirci 2010
- Demirci M, Tuncer S, Yuceokur AA. Prevalence of caries on individual tooth surfaces and its distribution by age and gender in university clinic patients. European Journal of Dentistry 2010;4(3):270‐9. [PUBMED: 20613915] [PMC free article] [PubMed] [Google Scholar]
Dietrich 2019
- Dietrich T, Ower P, Tank M, West NX, Walter C, Needleman I, et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions ‐ implementation in clinical practice. British Dental Journal 2019;226(1):16‐22. [DOI] [PubMed] [Google Scholar]
Durham 2013
- Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health‐related quality of life. Journal of Dentistry 2013;41(4):370‐6. [DOI: 10.1016/j.jdent.2013.01.008] [DOI] [PubMed] [Google Scholar]
Eke 2012
- Eke PI, Dye BA, Wei L, Thornton‐Evans GO, Genco RJ, CDCPDS work group. Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research 2012;91(10):914‐20. [DOI] [PubMed] [Google Scholar]
Exley 2009
- Exley C. Bridging a gap: the (lack of a) sociology of oral health and healthcare. Sociology of Health and Illness 2009;31(7):1093‐108. [DOI] [PubMed] [Google Scholar]
Fentoğlu 2012
- Fentoğlu O, Kirzioğlu F, Ozdem M, Koçak H, Sütçü R, Sert T. Proinflammatory cytokine levels in hyperlipidemic patients with periodontitis after periodontal treatment. Oral Diseases 2012;18(3):299‐306. [DOI] [PubMed] [Google Scholar]
Frencken 2017
- Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Globalepidemiology of dental caries and severe periodontitis – a comprehensive review. Journal of Clinical Periodontology 2017;44(Suppl. 18):S94–S105. [DOI: 10.1111/jcpe.12677] [DOI] [PubMed] [Google Scholar]
Furuta 2011
- Furuta M, Ekuni D, Irie K, Azuma T, Tomofuji T, Ogura T, et al. Sex differences in gingivitis relate to interaction of oral health behaviours in young people. Journal of Periodontology 2011;82(4):558‐65. [PUBMED: 20936916] [DOI] [PubMed] [Google Scholar]
GHDx 2017
- Global Health Data Exchange ‐ Global Burden of Disease Study 2017 Results Tool. ghdx.healthdata.org/gbd‐results‐tool (accessed 1 April 2019).
Gholami 2015
- Gholami M, Knoll N, Schwarzer R. A brief self‐regulatory intervention increases dental flossing in adolescent girls. International Journal of Behavioural Medicine 2015;22(5):645‐51. [DOI] [PubMed] [Google Scholar]
Greenwell 2001
- Greenwell H, Committee on Research, Science and Therapy of American Academy of Periodontology. American Academy of Periodontology Guidelines for periodontal therapy (position paper). Journal of Periodontology 2001;72(11):1624‐8. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoenderdos 2008
- Hoenderdos NL, Slot DE, Paraskevas S, Weijden GA. The efficacy of woodsticks on plaque and gingival inflammation: a systematic review. International Journal of Dental Hygiene 2008;6(4):280‐9. [DOI] [PubMed] [Google Scholar]
Huck 2011
- Huck O, Tenebaum H, Davideau JL. Relationship between periodontal diseases and preterm birth: recent epidemiological and biological data. Journal of Pregnancy 2011;164654:1‐8. [DOI: 10.1155/2011/164654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hujoel 2006
- Hujoel PP, Cunha‐Cruz J, Banting DW, Loesche WJ. Dental flossing and interproximal caries: a systematic review. Journal of Dental Research 2006;85(4):298‐305. [DOI] [PubMed] [Google Scholar]
Husseini 2008
- Husseini A, Slot DE, Weijden GA. The efficacy of oral irrigation in addition to a toothbrush on plaque and the clinical parameters of periodontal inflammation: a systematic review. International Journal of Dental Hygiene 2008;6(4):304‐14. [DOI] [PubMed] [Google Scholar]
Igarashi 1989
- Igarashi K, Lee IK, Schachtele CF. Comparison of in vivo human dental plaque pH changes within artificial fissures and at interproximal sites. Caries Research 1989;23(6):417‐22. [DOI] [PubMed] [Google Scholar]
Imai 2012
- Imai PH, Yu X, MacDonald D. Comparison of interdental brush to dental floss for reduction of clinical parameters of periodontal disease: a systematic review. Canadian Journal of Dental Hygiene 2012;46(1):63‐78. [Google Scholar]
Ismail 1994
- Ismail AI, Lewis DW, Dingle JL. Chapter 37. Prevention of periodontal disease. The Canadian Guide to Clinical Preventive Health Care. Canadian Task Force on Preventive Health Care, 1994:420‐31. [Google Scholar]
Johnson & Johnson
- Johnson, Johnson. About us: company history. www.jnj.ch/en/about‐us/company‐history.html (accessed 23 March 2019).
Kotsakis 2017
- Kotsakis GA, Lian Q, Ioannou AL, Michalowicz BS, John MT. A network meta‐analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. Journal of Periodontology 2018;89(5):558‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuramitsu 2007
- Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiology and Molecular Biology Reviews 2007;71(4):653‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamont 2018
- Lamont T, Worthington HV, Clarkson JE, Beirne PV. Routine scale and polish for periodontal health in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD004625.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lindhe 2003
- Lindhe J, Karring T, Lang NP. Clinical Periodontology and Implant Dentistry. 4th Edition. Copenhagen: Blackwell Munksgaard, 2003. [Google Scholar]
Llorente 2006
- Llorente MA, Griffiths GS. Periodontal status among relatives of aggressive periodontitis patients and reliability of family history report. Journal of Clinical Periodontology 2006;33(2):121‐5. [DOI] [PubMed] [Google Scholar]
Loe 1965
- Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. Journal of Periodontology 1965;36:177‐87. [PUBMED: 14296927] [DOI] [PubMed] [Google Scholar]
Loesche 1976
- Loesche WJ. Chemotherapy of dental plaque infections. Oral Sciences Reviews 1976;9:65‐107. [PUBMED: 1067529] [PubMed] [Google Scholar]
Lovdal 1961
- Lovdal A, Arno A, Schei O, Waerhaug J. Combined effect of subgingival scaling and controlled oral hygiene on the incidence of gingivitis. Acta Odontologica Scandinavica 1961;19:537‐55. [DOI] [PubMed] [Google Scholar]
Lyle 2012
- Lyle DM. Relevance of the water flosser: 50 years of data. Compendium of Continuing Education in Dentistry 2012;33(4):278‐80, 282. [PUBMED: 22536661] [PubMed] [Google Scholar]
Machuca 2012
- Machuca G, Segura‐Egea JJ, Jiménez‐Beato G, Lacalle JR, Bullón P. Clinical indicators of periodontal disease in patients with coronary heart disease: a 10 years longitudinal study. Medicina Oral, Patología Oral y Cirugía Bucal 2012;17(4):e569‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mak 2011
- Mak KK, Day JR. Dental health behaviours among early adolescents in Hong Kong. International Journal of Dental Hygiene 2011;9(2):122‐6. [DOI] [PubMed] [Google Scholar]
Mandel 1990
- Mandel ID. Why pick on teeth?. Journal of the American Dental Association 1990;121(1):129‐32. [PUBMED: 2196296] [DOI] [PubMed] [Google Scholar]
Manresa 2015
- Manresa C, Sanz‐Miralles EC, Twigg J, Bravo M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD009376.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2003
- Marinho VCC, Higgins JPT, Logan S, Sheiham A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2013
- Marinho VCC, Higgins JPT, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD002279] [DOI] [PubMed] [Google Scholar]
Marinho 2015
- Marinho VCC, Higgins JPT, Logan S, Sheiham A. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD002280.pub2] [DOI] [PubMed] [Google Scholar]
Mariotti 1999
- Mariotti A. Dental plaque‐induced gingival diseases. Annals of Periodontology 1999;4(1):7‐19. [DOI] [PubMed] [Google Scholar]
Marsh 1994
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Advances in Dental Research 1994;8(2):263‐71. [PUBMED: 7865085] [DOI] [PubMed] [Google Scholar]
Marsh 2006
- Marsh PD. Dental plaque as a biofilm and a microbial community ‐ implications for health and disease. BMC Oral Health 2006;15(6 Suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 1996
- Marthaler TM, O'Mullane DM, Vrbic V. The prevalence of dental caries in Europe 1990‐1995. ORCA Saturday afternoon symposium 1995. Caries Research 1996;30(4):237‐55. [DOI] [PubMed] [Google Scholar]
McGrath 2002
- McGrath C, Bedi R. Understanding the value of oral health to people in Britain ‐ importance to life quality. Community Dental Health 2002;19(4):211‐4. [PubMed] [Google Scholar]
Milsom 2002
- Milsom KM, Tickle M, Blinkhorn AS. Dental pain and dental treatment of young children attending the general dental service. British Dental Journal 2002;192(5):280‐4. [DOI] [PubMed] [Google Scholar]
Morita 2001
- Morita M, Wang HL. Association between oral malodor and adult periodontitis: a review. Journal of Clinical Periodontology 2001;28(9):813‐9. [DOI] [PubMed] [Google Scholar]
Murray 1989
- Murray JJ. The Prevention of Dental Disease. 2nd Edition. Oxford, UK: Oxford University Press, 1989. [Google Scholar]
Needleman 2004
- Needleman I, McGrath C, Floyd P, Biddle A. Impact of oral health on the life quality of periodontal patients. Journal of Clinical Periodontology 2004;31(7):454‐7. [DOI] [PubMed] [Google Scholar]
Noorlin 2007
- Noorlin I, Watts TL. A comparison of the efficacy and ease of use of dental floss and interproximal brushes in a randomised split mouth trial incorporating an assessment of subgingival plaque. Oral Health and Preventive Dentistry 2007;5(1):13‐8. [PubMed] [Google Scholar]
Page 1986
- Page RC. Gingivitis. Journal of Clinical Periodontology 1986;13(5):345‐59. [PUBMED: 3522644] [DOI] [PubMed] [Google Scholar]
Page 2007
- Page RC, Eke PI. Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology 2007;78(7S):1387‐99. [DOI] [PubMed] [Google Scholar]
Papapanou 2018
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology 2018;45(Suppl 20):S162‐S170. [DOI] [PubMed] [Google Scholar]
Parmly 1819
- Parmly LS. A Practical Guide to the Management of the Teeth; Comprising a Discovery of the Origin of Caries, or Decay of the Teeth, with its Prevention and Cure. Philadelphia: Collins & Croft, 1819. [Google Scholar]
Periasamy 2009
- Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with porphyromonas gingivalis and initial, early, and late colonizers of enamel. Journal of Bacteriology 2009;191(22):6804‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2003
- Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century ‐ the approach of the WHO Global Oral Health Programme. Community Dentistry and Oral Epidemiology 2003;31(Suppl 1):3‐23. [DOI] [PubMed] [Google Scholar]
Poklepovic Pericic 2013
- Poklepovic Pericic T, Worthington HV, Johnson TM, Sambunjak D, Imai P, Clarkson JE, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD009857.pub2] [DOI] [PubMed] [Google Scholar]
RCSEng 2018
- Royal College of Surgeons. Tooth decay in 5‐year‐olds now increasing in some parts of England [Press release]. rcseng.ac.uk/news‐and‐events/media‐centre/press‐releases/phe‐oral‐health‐2017/ (accessed 1 April 2019).
Review Manager (RevMan) [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Richardson 1977
- Richardson AS, Boyd MA, Conry RF. A correlation study of diet, oral hygiene and dental caries in 457 Canadian children. Community Dentistry and Oral Epidemiology 1977;5(5):227‐30. [DOI] [PubMed] [Google Scholar]
Rickard 2004
- Rickard GD, Richardson RJ, Johnson TM, McColl DC, Hooper L. Ozone therapy for the treatment of dental caries. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004153.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ring 2002
- Ring ME. W. D. Miller. The pioneer who laid the foundation for modern dental research. New York State Dental Journal 2002;68(2):34‐7. [PUBMED: 11898270] [PubMed] [Google Scholar]
Rosing 2006
- Rosing CK, Daudt FA, Festugatto FE, Oppermann RV. Efficacy of interdental plaque control aids in periodontal maintenance patients: a comparative study. Oral Health and Preventive Dentistry 2006;4(2):99‐103. [PUBMED: 16813138] [PubMed] [Google Scholar]
Saha 2012
- Saha S, Mohammad S, Saha S, Samadi F. Efficiency of traditional chewing stick (miswak) as an oral hygiene aid among Muslim school children in Lucknow: a cross‐sectional study. Journal of Oral Biology and Craniofacial Research 2012;2(3):176‐80. [PUBMED: 25737862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sambunjak 2011
- Sambunjak D, Nickerson JW, Poklepovic T, Johnson TM, Imai P, Tugwell P, et al. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008829.pub2] [DOI] [PubMed] [Google Scholar]
Sanz 2011
- Sanz M, Winkelhoff AJ. Periodontal infections: understanding the complexity ‐ consensus of the Seventh European Workshop on Periodontology. Journal of Clinical Periodontology 2011;38 Suppl 11:3‐6. [PUBMED: 21323698] [DOI] [PubMed] [Google Scholar]
Sarner 2010
- Sarner B, Birkhed D, Andersson P, Lingstrom P. Recommendations by dental staff and use of toothpicks, dental floss and interdental brushes for approximal cleaning in an adult Swedish population. Oral Health and Preventive Dentistry 2010;8(2):185‐94. [PUBMED: 20589254] [PubMed] [Google Scholar]
Savage 2009
- Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. Journal of Clinical Periodontology 2009;36(6):458‐67. [DOI] [PubMed] [Google Scholar]
Schatzle 2009
- Schatzle M, Faddy MJ, Cullinan MP, Seymour GJ, Lang NP, Burgin W, et al. The clinical course of chronic periodontitis: V. Predictive factors in periodontal disease. Journal of Clinical Periodontology 2009;36(5):365‐71. [PUBMED: 19419434] [DOI] [PubMed] [Google Scholar]
Schuz 2009
- Schuz B, Wiedemann AU, Mallach N, Scholz U. Effects of a short behavioural intervention for dental flossing: randomized‐controlled trial on planning when, where and how. Journal of Clinical Periodontology 2009;36(6):498‐505. [DOI] [PubMed] [Google Scholar]
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Sheiham 2005
- Sheiham A. Oral health, general health and quality of life. Bulletin of the World Health Organization 2005;83(9):644. [PMC free article] [PubMed] [Google Scholar]
Shepherd 1999
- Shepherd MA, Nadanovsky P, Sheiham A. The prevalence and impact of dental pain in 8‐year‐old school children living in Harrow, England. British Dental Journal 1999;187(1):38‐41. [DOI] [PubMed] [Google Scholar]
Silverstone 1983
- Silverstone LM. Remineralization and enamel caries: new concepts. Dental Update 1983;10(4):261‐73. [PubMed] [Google Scholar]
Simpson 2015
- Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor‐Ejiofor Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD004714.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slot 2008
- Slot DE, Dörfer CE, Weijden GA. The efficacy of interdental brushes on plaque and parameters of periodontal inflammation: a systematic review. International Journal of Dental Hygiene 2008;6(4):253‐64. [DOI] [PubMed] [Google Scholar]
Särner 2010
- Särner B, Birkhed D, Andersson P, Lingström P. Recommendations by dental staff and use of toothpicks, dental floss and interdental brushes for approximal cleaning in an adult Swedish population. Oral Health and Preventive Dentistry 2010;8(2):185‐94. [PubMed] [Google Scholar]
Timmerman 2006
- Timmerman MF, Weijden GA. Risk factors for periodontitis. International Journal of Dental Hygiene 2006;4(1):2‐7. [DOI] [PubMed] [Google Scholar]
Trombelli 2013
- Trombelli L, Farina R. A review of factors influencing the incidence and severity of plaque‐induced gingivitis. Minerva Stomatologica 2013;62(6):207‐34. [PUBMED: 23828258] [PubMed] [Google Scholar]
Van Dyke 2005
- Dyke TE, Sheilesh D. Risk factors for periodontitis. Journal of the International Academy of Periodontology 2005;7(1):3‐7. [PUBMED: 15736889] [PMC free article] [PubMed] [Google Scholar]
Waerhaug 1976
- Waerhaug J. The interdental brush and its place in operative and crown and bridge dentistry. Journal of Oral Rehabilitation 1976;3(2):107‐13. [DOI] [PubMed] [Google Scholar]
Waerhaug 1981
- Waerhaug J. Healing of the dento‐epithelial junction following the use of dental floss. Journal of Clinical Periodontology 1981;8(2):144‐50. [DOI] [PubMed] [Google Scholar]
Walsh 2019
- Walsh T, Worthington HV, Glenny A‐M, Marinho VCC, Jeroncic A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database of Systematic Reviews 2019, Issue 3. [DOI: 10.1002/14651858.CD007868.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 1996
- Warren PR, Chater BV. An overview of established interdental cleaning methods. Journal of Clinical Dentistry 1996;7(3 Spec No):65‐9. [PubMed] [Google Scholar]
WHO 1990
- World Health Organisation. Diet, nutrition and prevention of chronic diseases. Geneva: World Health Organization, 1990. WHO Technical Report Series, No 797. [PubMed]
Wiener 2009
- Wiener RC, Crout RJ, Wiener MA. Toothpaste use by children, oral hygiene, and nutritional education: an assessment of parental performance. Journal of Dental Hygiene 2009;83(3):141‐5. [PUBMED: 19723433] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
Xiemenez‐Fyvie 2000
- Ximenez‐Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, Socransky CC. The effect of repeated professional supragingival plaque removal on the composition of the supra‐ and subgingival microbiota. Journal of Clinical Periodontology 2000;27(9):637‐47. [DOI] [PubMed] [Google Scholar]
Yacob 2014
- Yaacob M, Worthington HV, Deacon SA, Deery C, Walmsley AD, Robinson PG, et al. Powered versus manual toothbrushing for oral health. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002281.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaborskis 2010
- Zaborskis A, Milciuviene S, Narbutaite J, Bendoraitiene E, Kavaliauskiene A. Caries experience and oral health behaviour among 11‐13‐year‐olds: an ecological study of data from 27 European countries, Israel, Canada and USA. Community Dental Health 2010;27(2):102‐8. [PubMed] [Google Scholar]
References to other published versions of this review
Johnson 2015
- Johnson TM, Worthington HV, Clarkson JE, Poklepovic Pericic T, Sambunjak D, Imai P. Mechanical interdental cleaning for preventing and controlling periodontal diseases and dental caries. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD012018] [DOI] [PMC free article] [PubMed] [Google Scholar]


