Abstract
Tolerance-inducing approaches to xenotransplantation would be optimal and may be necessary for long-term survival of transplanted pig organs in human patients. The ideal approach would generate donor-specific unresponsiveness to the pig organ without suppressing the patient’s normal immune function. Porcine thymus transplantation has shown efficacy in promoting xenotolerance in humanized mice and large animal models. However, murine studies demonstrate that T cells selected in a swine thymus are positively selected only by swine thymic epithelial cells, and therefore cells expressing human HLA-restricted TCRs may not be selected efficiently in a transplanted pig thymus. This may lead to suboptimal patient immune function. To assess human thymocyte selection in a pig thymus, we used a TCR transgenic humanized mouse model to study positive selection of cells expressing the MART1 TCR, a well-characterized human HLA-A2-restricted TCR, in a grafted pig thymus. Positive selection of T cells expressing the MART1 TCR was inefficient in both a non-selecting human HLA-A2− or swine thymus compared to an HLA-A2+ thymus. Additionally, CD8+ MART1 TCRbright T cells were detected in the spleens of mice transplanted with HLA-A2+ thymi but were significantly reduced in the spleens of mice transplanted with swine or HLA-A2− thymi. Thus, positive selection of cells expressing a human-restricted TCR in a transplanted pig thymus is inefficient, suggesting that modifications to improve positive selection of cells expressing human-restricted TCRs in a pig thymus may be necessary to support development of a protective human T cell pool in future patients.
Keywords: Tolerance, small animal models, humanized mice, thymus, thymopoiesis, T cell tolerance
Introduction
Recent progress in pig-to-nonhuman primate models suggests that human trials of clinical solid-organ xenotransplantation from pigs may be imminent.1–4 Long-term rejection-free survival of a pig organ in a human patient may require induction of tolerance in the recipient human to the swine graft.5 While improved pharmaceutical immunosuppression and genetically engineered swine source animals have dramatically increased the survival of porcine organs in non-human primates, xenogeneic immune responses are extremely strong.6,7 Therefore, the level of immune suppression needed to control anti-swine immune responses may be difficult for patients to tolerate, leaving them highly vulnerable to infections and neoplasms. Methods to induce immunological tolerance to the swine organ would be optimal for clinical xenotransplantation and would allow both recognition of the transplanted pig organ as self while maintaining robustly protective immunological function.
Inducing T cell tolerance is paramount, as they are primary mediators of xenograft rejection.8–15 Transplanting a pig thymus can tolerize human T cells to pig antigens.16–18 Over the past twenty-five years, we have demonstrated that xenogeneic thymus transplantation induces robust T cell tolerance across xenogeneic barriers in both pig-to-mouse and pig-to-humanized mouse models.16–21 Protocols incorporating swine thymus transplantation in pig-to-non-human primate models have shown promising results, with survival of recipient primates up to 193 days, with no signs of graft rejection.11,22–28 While extended survival of swine renal grafts in immunosuppressed non-human primates has been reported without swine thymus co-transplantation, explanted swine kidneys showed signs of rejection.29,30
Swine thymus transplantation allows de novo generation of T cells expressing a diverse, swine-tolerant human TCR pool.16,18,31 However, to maintain patient immune function, one of the major benefits of xenotolerance, a transplanted pig thymus must also support selection of a human TCR repertoire with robust HLA-restricted immune function.
Positive selection of developing T cells in the thymus is based on the ability of a TCR to weakly recognize selecting antigen presented in the context of major histocompatibility molecules (MHC) present on the surface of epithelial cells in the thymic stroma, or thymic epithelial cells (TECs).32,33 Experiments in the pig-to-mouse system have previously demonstrated that swine TECs are exclusively responsible for positive selection in a transplanted swine thymus.34–36 In this model, TCR transgenic mice were thymectomized, T cell depleted using antibodies, and transplanted with a fragment of fetal pig thymus. When transplanted mice were euthanized 13–19 weeks post thymus transplant and swine thymus grafts were examined, transgenic mouse cells were present at the same frequency in the grafted pig thymus as they were in a mouse thymus expressing selecting MHC. TCR transgenic cells were also present in the grafted pig thymus in the same frequency in mice that expressed MHCs that did not select the transgenic TCR as those that did, suggesting that murine thymic stroma did not contribute to positive selection of cells expressing mouse TCR transgenes in a grafted pig thymus.35 Despite exclusive positive selection on swine MHC, robust mouse-restricted vaccination responses were seen and opportunistic infections could be cleared.37
Based on these results, human T cells selected in a swine thymus would also be expected to preferentially recognize antigens in the context of swine MHC (SLA), rather than human MHC (HLA), potentially leading to poor recognition and responses to human antigen-presenting cells (APCs) in the periphery. We have previously shown that a swine thymus can select human T cells with a diverse TCR repertoire, containing at least some T cells capable of responding to antigens presented on HLA by human dendritic cells.16,18,31 However, experiments in the pig-to-mouse system showed that T cells selected in a swine thymus exhibited defects in peripheral immune homeostasis, including reduced ability to repopulate lymphopenic secondary recipients,38 which may be due to suboptimal interactions between pig-restricted TCRs and murine peripheral host APCs.38–40 Similar suboptimal homeostasis, including poor maintenance of peripheral T cells subsequent to thymic graftectomy, has been observed in the pig-to-humanized mouse model.18 These studies also suggested that the human-restricted T cell response may be reduced in animals transplanted with a pig compared to a human thymus.18
More studies in animal models are needed to assess the ability of a swine thymus to support selection and development of functional and swine-tolerant human TCRs to evaluate this strategy for immune tolerance induction pre-clinically. Previous studies did not address whether a grafted swine thymus could support the positive selection of TCRs that are known be specifically able to recognize antigen in the context of HLA, i.e. HLA-restricted TCRs. We used a TCR transgenic humanized mouse model to assess the selection and development of cells expressing the MART1 TCR, a well-characterized human HLA-A2-restricted TCR that is positively selected by HLA-A241,42 and recognizes a cancer neoantigen,11,43,44 in a grafted pig thymus. Using this system, we addressed the ability of a grafted pig thymus to support the positive selection of an HLA-restricted TCR.
Methods
Animals and human tissues and cells
NOD/SCID IL2rγko (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, NSG)45 mice transgenic for human HLA-A2 were obtained from Jackson Laboratory (Bar Harbor, ME) and crossed in our facility to NSG animals transgenic for porcine IL-3, GM-CSF, and SCF (porcine cytokine transgenic, or PCT, mice)46 to generate A2 PCT Tg mice. The porcine cytokines have been found to enhance human immune reconstitution without altering T cell phenotype in NSG mice receiving human CD34+ cells (N.Danzl, G.Nauman and M.Sykes, unpublished data) and were used here to improve efficiency of reconstitution from transduced CD34+ cells. Mice were bred, maintained, and housed in microisolator cages in a Heliobacter, Pasteurella pneumotropica and Specific Pathogen-Free animal facility. Fetal tissues (gestational age 17–21 weeks) were obtained from Advanced Biosciences Resource (Almeda, CA) and HLA typed at the HLA-A locus for the presence of HLA-A*02:01 (HLA-A2+). Fetal thymus and liver tissues were prepared as previously described.47 Briefly, 1×1×1mm fetal thymus fragments were cryopreserved in 10% DMSO (Sigma-Aldrich, St. Louis, MO) and 90% human AB serum (Gemini Bio-Products, Sacramento, CA). Cryopreservation of the thymus tissue fragments is necessary to deplete thymocytes from the tissues, which can reject other transplanted cells and limit human T cell reconstitution in recipient animals.18,48 Prior to transplantation, thymus fragments were thawed and repeatedly pipetted and agitated to remove residual thymocytes. Single cell suspensions were generated from fetal livers by Liberase digestion (Sigma-Aldrich) and CD34+ cells were isolated using positive selection by magnetic-activated cell sorting (MACS) and anti-human CD34+ microbeads according the manufacturer’s instructions (Miltenyi Biotec, Bergish Gladbach, Germany). Allele-level molecular HLA typing of fetal tissue was performed using Sanger sequencing (see S2a).
Swine thymus tissue was provided from the Massachusetts General Hospital/Columbia University SLA-defined miniature swine herd generated by Dr. David H. Sachs. Swine thymi were harvested from fetuses (60–90 days gestational age) and cryopreserved as described.48
The use of human tissues and cells and of animals was approved by the CUMC Institutional Review Board and Institutional Animal Care and Use Committee, respectively.
Lentivirus transduction
The MART1 TCR transgene49 used in these experiments was driven by the human EF1α promoter and arranged with the TCRα and TCRβ sequences separated by a P2A ribosomal skip sequence, followed by an IRES and an eGFP expression marker. This construct, encoded in the backbone of a second-generation lentiviral system, and associated packaging vectors, as previously described,50 were a kind gift from Dr. Remi Creusot. Virus was produced in HEK293 FT cells. Cells were transfected with viral plasmids using Lipofectamine 2000 according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). Supernatants were concentrated 100x by ultracentrifugation, flash frozen, and stored at −80°C prior to use. Supernatant titer was determined by transducing HEK 293 FT cells using freeze-thawed virus.
Prior to transduction, human fetal liver CD34+ cells were thawed and pre-stimulated in retronectin-coated plates (25μg/mL, Clontech, Mountain View, CA) by incubation with Stemline II medium (Sigma-Aldrich) with 10μg/mL protamine sulfate (Sigma-Aldrich) and 60ng/mL, 150ng/mL and 300ng/mL recombinant human IL-3, Flt3 Ligand, and Stem Cell Factor (PreProtech, Rocky Hill, NJ), respectively, for 3 hours. Cells were transduced overnight at a multiplicity of infection of 30, then harvested and prepared for intratibial injection. A small number of transduced CD34+ cells were cultured in stem cell medium without protamine sulfate for 4 days, then assessed for transduction efficiency by flow cytometry. Starting at four weeks post-transplantation, mice were bled every two weeks and peripheral blood chimerism was assessed by flow cytometric analysis.
Transplantation
Immunodeficient mice were surgically thymectomized as described51 and allowed to recover for at least two weeks, then irradiated with 1 Gy total body irradiation (TBI) by an X-Ray irradiator (RS-2000, Rad Source Technologies, Inc., Suwanee, GA). Mice were then transplanted with a fragment of human fetal thymus (Hu Thy, gestational week 17–21, approximately 1×1×1mm) or swine fetal thymus (Sw Thy, 60–90 gestational days) under the kidney capsule, and 2–5×105 lentivirally-transduced CD34+ positive cells were injected intratibially. In some cases, additional transduced cells were transplanted intravenously. Mice were injected intravenously with 0.4μg of the rat anti-human CD2 monoclonal antibody LoCD2b52 on the day of thymus transplantation and seven days post-transplantation to deplete remaining passenger T cells emerging from the grafted thymus, as previously described.48
Flow Cytometric (FCM) analysis
Animals were euthanized at 10–14 weeks post-transplant. Spleens were crushed to make a single cell suspension, which was passed through a 70μm nylon filter and treated with ACK lysis buffer (Life Technologies, Carlsbad, CA) prior to counting and staining. Single bone marrow cell suspensions from the tibia and femur were prepared by crushing bones using a mortar and pestle, counted, and stained for flow cytometry. Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood by density gradient separation using Histopaque 1077 (Sigma Aldrich). Grafted thymi were dissected from kidneys and crushed to single cell suspensions, and residual tissue in the anterior mediastinum above the heart was collected and processed to a single cell suspension to verify removal of the mouse native thymus. Animals with incomplete thymectomy noted at takedown were excluded from peripheral T cell analysis. MART1 TCR+ cells were detected using an A*02:01 – ELAGIGILTV tetramer (Proimmune, Oxford, UK).
Stained cells were acquired for analysis using a BD FACS Canto II, a BD LSRII, or a BD Fortessa flow cytometer (BD Biosciences, Franklin Lakes, NJ). Analysis was performed using FlowJo (TreeStar, Ashland, OR).
In vitro TCR characterization assay
Appropriate TCR expression and in vitro MART1 TCR responses were assessed using stably transfected JRT3-T3.5 cells, a variant Jurkat cell line that does not express TCRβ.53,54 Cells were cultured in complete RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (Gemini BioProducts), 10mM HEPES buffer (Gibco, Gaithersberg, MD), 20mM L-glutamine (ACROS Organics, Geel, Belgium), 1mM sodium pyruvate (Fisher BioReagents, Fair Lawn, NJ) and 0.2% 2-Mercaptoethanol (MP Biomedicals, LLC, Santa Ana, CA). MART1 TCR-transduced JRT3-T3.5 cells were incubated with human or swine PBMCs which had been irradiated with 35Gy X-ray irradiation and pulsed with the indicated concentration of MART1 peptide, the [Leu27] Melan-A MART 1 (26–35) ELAGIGILTV peptide (Anaspec, Fremont, CA). Controls were exposed to medium alone or plate-bound OKT3 (1μg/mL, eBiosciences, San Diego, CA) and soluble anti-CD28.2 (0.75μg/mL, Biolegend, San Diego, CA). After 5 days, reaction supernatants were harvested and frozen at −20°C, then assayed for IL-2 production by proliferation of CTLLs, an IL-2-sensitive murine lymphoma cell line.55,56 CTLL proliferation was assayed by incorporation of tritiated thymidine (Perkin Elmer, Waltham, MA). CTLLs were passaged in complete RPMI containing 20U/mL recombinant IL-2 (NIH AIDS Reagent Program, Bethesda, MD). CTLLs were conditioned by culturing for approximately 60 hours to allow depletion of IL-2 and then incubated with thawed supernatants for approximately 24 hours. IL-2 stimulated CTLLs were pulsed with tritiated thymidine for 12–15 hours to allow incorporation by proliferating cells. To measure thymidine uptake, CTLLs were harvested to fiberglass plates (Perkin Elmer), which were air dried, saturated with Ultima Gold liquid scintillation cocktail (Perkin Elmer) and counts per minute (cpm) were enumerated using a 1450 Microbeta counter (Perkin Elmer). IL-2 dependent proliferation of CTLLs in response to supernatants from antigen-stimulated Mart1 cells was measured in cpms and calculated as fold increase over the cpm induced by supernatants from TCRnull JRT3-T3.5 cells under the same stimulation condition.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 7 (La Jolla, CA). Groups were considered significantly different if the p value according to the appropriate test was <0.05.
Results
MART1 TCR expresses appropriately in the lentiviral expression system
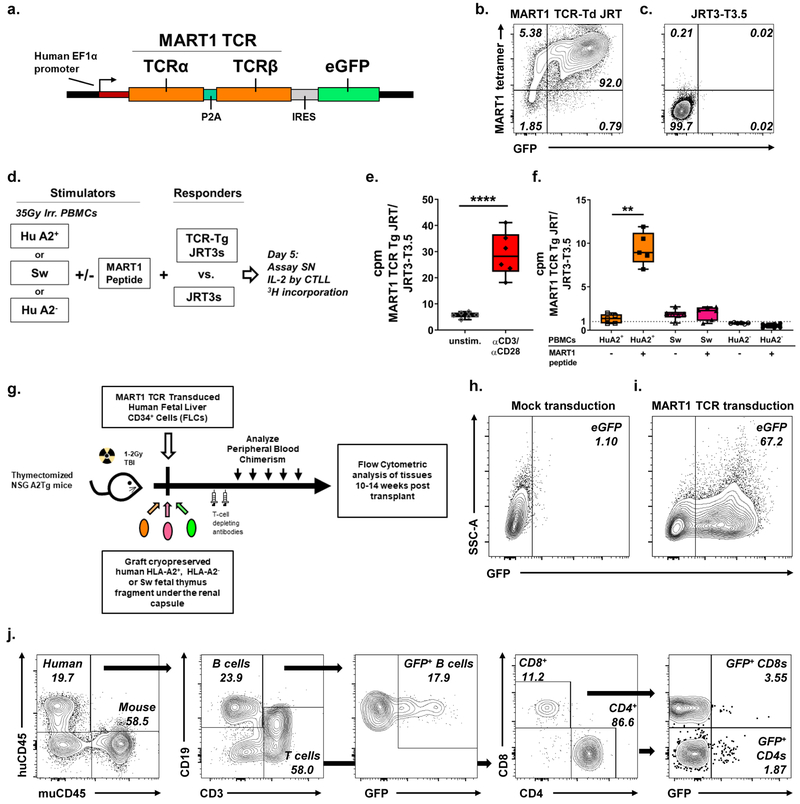
To assess the ability of a porcine thymus to support the positive selection of a known HLA-restricted TCR, we used a lentiviral system to introduce MART1 TCR, a well-characterized human TCR that recognizes a mutated Melan-A peptide in the context of the HLA-A2 MHC molecule (i.e. is HLA-A2-restricted),49 into human CD34+ cells given to NSG mice transplanted with human or swine thymus grafts.31,57 The Melan-A peptide is a neoantigen that is detected in many melanomas,58,59 and the MART1 TCR has previously been characterized as a candidate for tumor immunotherapy.44,49,60 The lentiviral construct used, diagramed in Figure 1a, consists of the MART1 TCRα and TCRβ, driven by the constitutively active human EF1α promoter and co-expressing an eGFP reporter. JRT3-T3.5 cells, a TCRβnull Jurkat line, were transduced with MART1 TCR lentivirus to validate appropriate expression of the TCR construct. We confirmed that MART1 TCR-transduced JRT3-T3.5 cells but not untransduced cells were stained positive using a commercially available MART1 tetramer (Figure 1b, 1c). Further staining showed TCRαβ expression (Figure S1a, 1b) which correlated with CD3 expression (Figure S1c, 1d). Flow cytometry of HEK 293FT cells transduced with MART1 TCR lentivirus showed that GFP-expressing CD3 negative cells did not express TCR on their surface in this lentiviral system (Figure S1e–g). Thus, while transgene expression occurs in all transduced cells, TCR surface expression occurs only in the presence of CD3.
Figure 1: MART1 TCR lentivirus drives appropriate expression of the MART1 TCR, an HLA-A2-restricted TCR, in an immortalized T cell line and in FLC CD34+ cells transplanted to immunodeficient mice.
a. Diagram of the MART1 TCR expression construct, which is driven by the human EF1α promoter and followed by an IRES and an eGFP reporter. b. MART1 TCR transduced, but not c. untransduced JRT3-T3.5 cells, which are TCRβnull, are stained by a MART1 tetramer. d. Schematic of the in vitro TCR characterization assay measuring IL-2 secretion in the supernatant (SN) of TCR transduced JRT3-T3.5 cells by tritiated thymidine incorporation of CTLLs, an IL-2-sensitive murine cell line. Response of MART1 TCRtransduced vs. untransduced JRT3-T3.5 cells to e. polyclonal stimulation and f. MART1 peptide presented by human A2+, A2− or Sw PBMCs. Representative of four separate experiments. **** p < 0.0001, ** p < 0.01 by one-way ANOVA with Sidak’s correction for multiple comparisons. g. Schematic of TCR transgenic humanized mouse model, where thymectomized immunodeficient mice are transplanted with MART1 TCR-transduced fetal liver CD34+ cells and cotransplanted subrenally with a fragment of fetal human or swine thymus. Representative h. mock transduced and i. MART1 TCR transduced CD34+ cells 96hrs post transduction. j. GFP expressing human B cells and T cells are detected in peripheral blood of mice transplanted with MART1 TCR transduced CD34+ cells 12 weeks post-transplantation. % GFP+ of CD8+ and CD4+ T cells is shown.
MART1 TCR does not respond to MART1 peptide presented by pig cells
To characterize cross-reactivity of the MART1 TCR to swine MHC, or SLA, we assessed the ability of the MART1 TCR to respond to MART1 peptide presented by SLA. As diagrammed in Figure 1d, we assessed TCR reactivity by measuring IL-2 production of JRT3-T3.5 cells transduced with MART1 TCR lentivirus (MART1 TCR Tg JRTs) cells stimulated by irradiated human or swine PBMCs (huPBMCs; swPBMCs, respectively) in the presence or absence of the MART1 peptide. IL-2 production was assayed by measuring proliferation of the IL-2 sensitive cell line CTLL in response to JRT supernatants. CTLL proliferation was measured by incorporation of radiolabeled thymidine in cpm.55,61,62 MART1 TCR Tg JRT IL-2 production is normalized to IL-2 production by TCRnull JRT3-T3.5 cells under the same conditions. The assay detected responses from TCR stimulation, as indicated by the increased IL-2 secretion of MART1 TCR Tg JRT cells in response to polyclonal stimulation by plate-bound anti-CD3 and soluble anti-CD28 antibodies compared to unstimulated cells (Figure 1e). We confirmed that, while HLA-A2+ huPBMCs could present the MART1 peptide to MART1 TCR Tg JRT cells, swPBMCs, including those sharing SLA with the swine thymus donor in selection experiments, and HLA-A2− huPBMCs did not induce a response in MART1 TCR Tg JRT cells, even when pulsed with MART1 peptide (Figure 1f). Additional swPBMCs of multiple SLA types were assayed and were also unable to present Mart1 peptide to the MART1 TCR, even at high concentrations of MART1 peptide (data not shown).
GFP+ T and non-T cells are detected in the periphery of animals transplanted with TCR-transduced HLA-A2+ CD34+ cells
We next transduced the MART1 TCR into human HLA-A2+ fetal liver CD34+ cells for transplantation to NSG mice (Figure 1g). To ensure that T cell development occurred exclusively in the human or swine thymus grafts, immunodeficient HLA-A2 transgenic NSG mice were first thymectomized and allowed to recover, since an NSG mouse native thymus can support low levels of human thymopoiesis (Figure S1h, S1i,51,63). Thymectomized mice were conditioned with irradiation and injected intratibially with transduced HLA-A2+ CD34+ cells and a fragment of cryopreserved and thymocyte-depleted human HLA-A2+, HLA-A2− or swine thymus was grafted under the renal capsule. Transduced CD34+ cells were tested for transduction efficiency 96 hours post-transduction to allow time for transgene expression. A representative transduction is shown. While mock transduced CD34+ cells did not express the eGFP reporter (Figure 1h), between 40 and 70% of fetal liver CD34+ cells were transduced in a typical experiment (Figure 1i).
Peripheral blood of recipient mice was repeatedly monitored for immune reconstitution and transduced cells. Blood chimerism from a representative animal at week 12 post-transplant is shown in Figure 1j. Human CD45+ cells, B cells and T cells were present in the blood of recipient mice and included detectable levels of GFP-expressing B cells and CD4+ and CD8+ T cells. Aggregated chimerism kinetics are depicted in Figure S2b–i, for total human chimerism, B and T cell kinetics, as well as the percentage of human, B and T cells that are GFP+ in the peripheral blood of recipient mice.
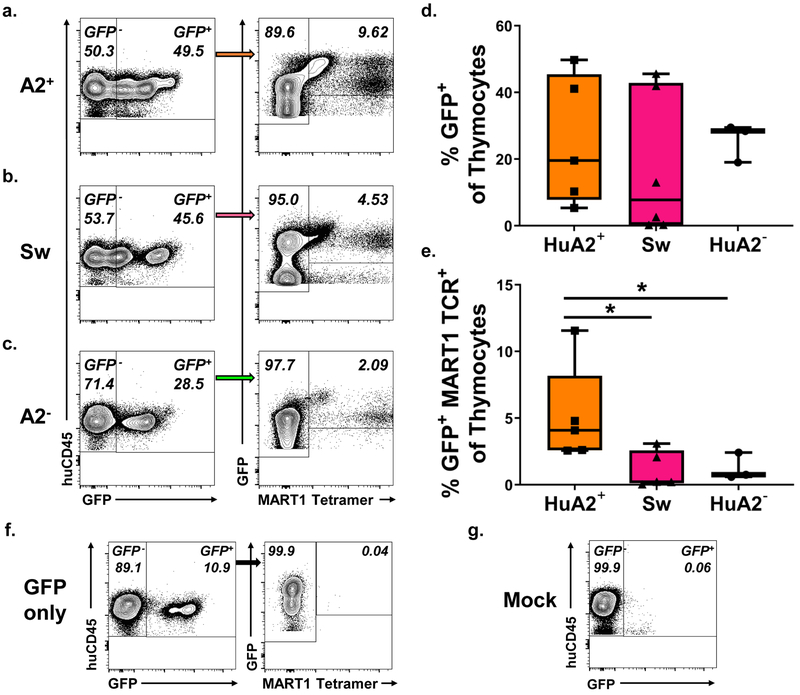
MART1 TCR+ cells are present in reduced proportions in HLA-A2− and swine thymi compared to HLA-A2+ thymi
At 10–14 weeks post-transplant, mice were sacrificed, and thymus grafts examined for human thymocyte engraftment and the presence of MART1 TCR+ cells by tetramer staining. Human CD45+ cells (Figure S3c, d), GFP+ cells and GFP+ MART1 TCR+ cells were detected in grafted human HLA-A2+ (Figure 2a), swine (2b) and human HLA-A2− thymi (2c). Thymocyte staining of thymus grafts from animals transplanted with mock transduced CD34+ cells (2G) or with eGFP-only-transduced CD34+ cells (2F) was used to set GFP+ and GFP+ MART1 TCR+ gates. We observed that all three types of thymus grafts supported similar proportions of transduced GFP+ thymocytes (2d), as well as similar total numbers of thymocytes (Figure S3b), proportions (S3c) and numbers (S3d) of human CD45+ cells, and similar numbers of GFP+ cells (S3c). However, the proportion of thymocytes that were GFP+ MART1 TCR+ was significantly greater in human HLA-A2+ thymi compared to either swine or human A2− thymi (2e). We assessed absolute numbers of GFP+ MART1 TCR+ cells in transplanted thymi and observed a similar trend, although the differences did not reach statistical significance (S3f).
Figure 2: MART1 TCR+ cells are enriched in an A2+ compared to an A2− or Swine thymus 10–14 weeks post-transplant.
Representative FCM staining of thymus grafts at takedown of transduced cells developing in a a. human HLA-A2+, b. swine or c. human HLA-A2− thymus. Proportion of d. GFP+ cells and e. GFP+ MART1 TCR+ cells in the thymus grafts of recipient mice (A2+ n = 6, Sw n = 6, A2− n=4; one A2− mouse was excluded from tetramer analysis due to too few detected GFP+ events). f., g. Example control staining of thymus grafts of animals transplanted with f. CD34+ cells transduced with a TCRnull GFP-only lentivirus and with g. untransduced CD34+ cells. * = p < 0.05% by Mann-Whitney U test.
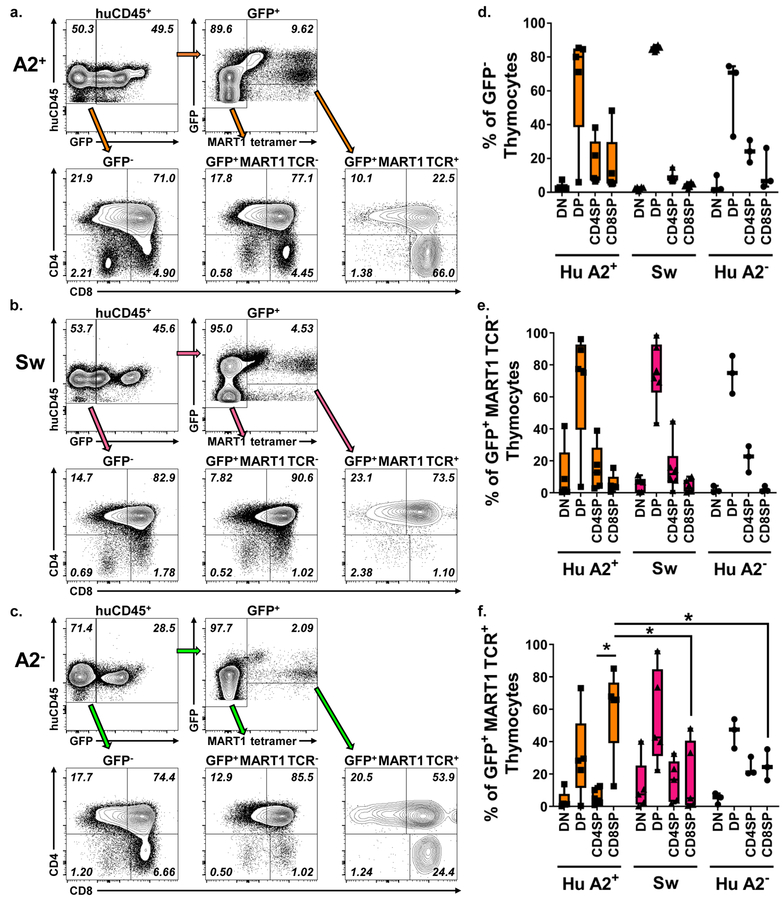
Altered development of MART1 TCR+ cells in HLA-A2− and swine thymi compared to HLA-A2+ thymi
In thymocyte development, somatic recombination leads to development of a diverse array of T cell receptor specificities. Each thymocyte expresses a TCR on its surface along with both the CD4 and CD8 coreceptor. Because HLA-A2 is a human class I MHC molecule, MART1 TCR+ thymocytes would be expected to be selected to the CD8+ lineage, as previously described in humanized mice.41,42 To assess the progression of MART1 TCR+ T cells through thymic selection in an HLA-A2+ thymus or a non-selecting HLA-A2− human or swine thymus, we gated on GFP+, GFP+ MART1 TCR−, and GFP+ MART1 TCR+ cells and analyzed their distribution among thymocyte subpopulations. Representative staining in human HLA-A2+ (Figure 3a), swine (3b) or human HLA-A2− (3c) thymus grafts is shown. GFP− and GFP+ MART1 TCR− thymocytes showed similar thymocyte distributions in terms of both proportions (Figure 3d, e) and absolute numbers (Figure S3g, h), with large proportions and numbers of CD4+CD8+ double positive thymocytes, as expected in human thymopoeisis.41 Since the MART1 TCR is class I-restricted, GFP+ MART1 TCR+ cells would be expected to be detected among double positive and enriched among CD8 single positive thymocytes as transgenic thymocytes undergo positive selection. Indeed, GFP+ MART1 TCR+ cells were enriched among CD8 single positive cells compared to CD4 single positive cells in the human HLA-A2+ thymus (Figure 3f). However, we did not see this progression among GFP+ MART1 TCR+ thymocytes in a swine or human HLA-A2− thymus (Figure 3b, c, f), in which clear preference for CD4+ or CD8+ fate was not detected. We assessed absolute numbers of GFP+ MART1 TCR+ thymocyte subpopulations and observed a similar trend, although differences did not reach statistical significance (S3i). Overall, these data suggest that MART1 TCR selection occurs as expected in a human HLA-A2+ thymus but is reduced in swine or human HLA-A2− thymi.
Figure 3: Selection of GFP+ MART1 TCR+ cells in A2+ compared to A2− or Swine thymi at 10–14 weeks post-transplant.
Representative flow staining of thymus grafts at takedown of transduced cells developing in an a. human HLA-A2+, b. swine or c. human HLA-A2− thymus. Distribution among thymocyte subpopulations of d. GFP−, e. GFP+ MART1 TCR−, and f. GFP+ MART1 TCR+ thymocytes (A2+ n = 6, Sw n = 6, A2− n=3 mice per group; * = p < 0.05% by Welch’s t-test).
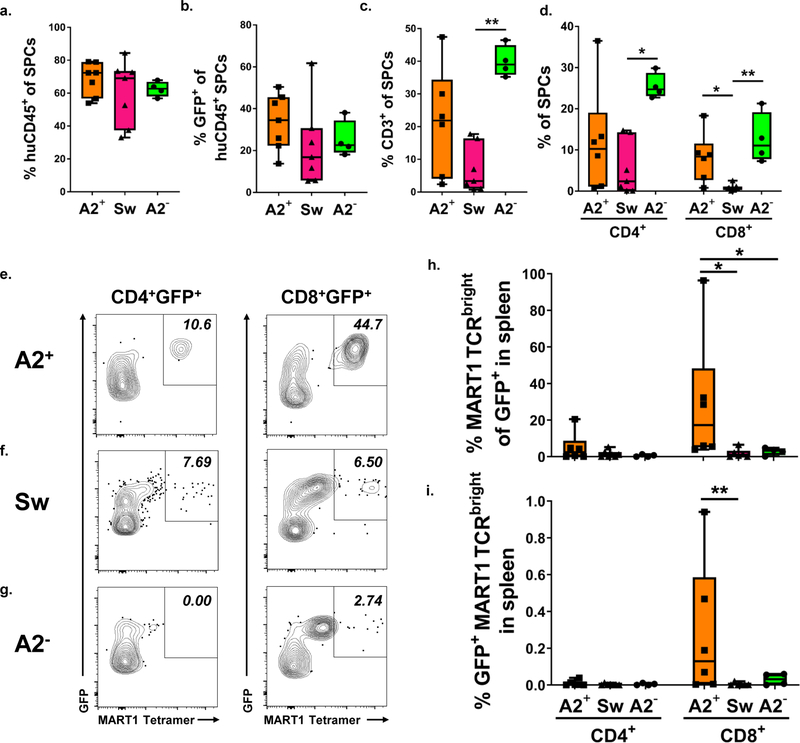
MART1bright T cells are reduced in the spleens of mice transplanted with HLA-A2− and Swine thymus grafts
Peripheral blood mononuclear cells and splenocytes of animals transplanted with Mart1 TCR-transduced cells and human or swine fetal thymus grafts were analyzed for immune cells by flow cytometry 10–14 weeks post-transplant. Tetramer staining of splenocytes confirmed that expression of the MART1 TCR was confined to T cells and not detected on B cells (Figure S3a). Human peripheral chimerism and T cell reconstitution was assessed in the spleen at 10–14 weeks post-transplant. HuCD45+ reconstitution (Figure 4a), as well as the proportion of human CD45+ cells that were GFP+ (4b) were similar regardless of the grafted thymus. By contrast, T cell reconstitution was more variable. Proportions of T cells (Figure 4c) including both CD4+ and CD8+ cells (4e) were highest in animals grafted with human HLA-A2− thymi. Similar results were observed in the blood of recipient mice (Figure S4a–d). CD8+ cells were reduced in the spleens of mice transplanted with a swine thymus compared to mice transplanted with either an HLA-A2+ or HLA-A2− human thymus (Figure 4d), consistent with previous observations that human CD8+ T cell reconstitution is reduced in humanized mice transplanted with a swine compared to a human thymus graft.18 However, no significant differences between groups was detected in absolute numbers of human T cells in the spleens of recipient animals (Figure S4h, j).
Figure 4: MART1 TCRbright T cells are reduced in the spleens of mice transplanted with A2− or swine compared to A2+ thymus. a-d.
Quantification of spleen chimerism, analyzed 10–14 weeks post-transplant, including a. proportions of huCD45+ cells among total CD45+, b. percentages of GFP+ cells within huCD45+ cells, and c. proportions of CD3+ and d. CD4+CD3+ and CD8+CD3+ splenocytes (SPCs), respectively. * = p < 0.05, ** = p < 0.01 by the Kruskal-Wallis test using Dunn’s correction for multiple comparisons. Representative FCM staining of spleens of mice transplanted with MART1 TCR-transduced cells and cotransplanted with either e. human HLA-A2+, f. swine or g. human HLA-A2− thymus, showing MART1 tetramer staining of huCD45+CD3+ CD4+ or CD8+GFP+ gated cells. Percent of MART1 TCRbright cells among h. CD4+GFP+ and CD8+GFP+ T cells and among i. splenocytes. * = p < 0.05, ** = p < 0 .01 by the Mann-Whitney U test. Data are from A2+ n = 6, Sw n = 5 and A2− n = 4 recipient mice, respectively.
Despite reduced human T cell reconstitution in the periphery of mice transplanted with swine compared to HLA-A2− human thymi, the proportion of human T cells that were GFP+ in the periphery was similar for both CD4+ and CD8+ T cells in the peripheral blood (Figure S4k) and spleen (Figure S4l), regardless of selecting thymus. Absolute numbers of GFP+ CD4+ and CD8+ T cells were also similar in the spleens of recipient mice, regardless of the thymus transplanted (Figure S4m). Thus, transduced T cells made up a similar proportion of human T cells in the periphery of all animals, including those transplanted with a swine or non-selecting human HLA-A2− thymus.
GFP+ splenic T cells were analyzed for MART1 tetramer staining. Representative examples of MART1 tetramer staining on GFP+ CD4+ and GFP+ CD8+ splenocytes from mice transplanted with HLA-A2+ (Figure 4e), swine (4f), or HLA-A2− (4g) thymi are shown. We examined proportions of GFP+ CD4+ and CD8+ cells for high expression of MART1 TCR (i.e. proportions of MART1 TCRbright cells). As expected, MART1 TCRbright cells were largely found among GFP+ CD8+ cells (Figure 4h). MART1 TCRbright cells were also enriched as a proportion of GFP+ T cells (Figure 4h) and of total splenocytes (Figure 4i) in the spleens of mice transplanted with an HLA-A2+ thymus compared to mice transplanted with a swine thymus.
Bimodal expression of MART1 TCR was noted on GFP+ T cells; a population of MART1 TCRdim cells was detected within both GFP+ CD4+ and CD8+ T cell populations, and in the spleens of mice transplanted with HLA-A2+, swine, and HLA-A2− thymi. We assessed absolute numbers of MART1 TCRdim (Figure S4n) and MART1 TCRbright (Figure S4o) cells in the spleens of transplanted mice and found that they were highly variable between animals. Representative bimodal MART1 TCR staining is shown in Figure S5a–c, as well as MART1 tetramer staining of splenocytes from a mouse transplanted with a GFP only virus (Figure S5d). We examined MART1 TCRdim and MART1 TCRbright cells and found that they had similar CD3 expression, as shown in Figure S5a–c. Because TCR and CD3 are expressed on the surface of T cells with a well-described stoichiometry,64,65 the fact that MART1 TCRdim cells showed reduced MART1 TCR expression with unchanged CD3 expression suggests that these T cells may express endogenously rearranged TCRα chains, which has been previously observed in murine66 and humanized mouse transgenic TCR systems,67 and in ordinary humanized mouse models, where 10% of normal human thymocytes selected in a human thymus graft expressed multiple TCRα chains.68 MART1 TCRdim cells were present in similar proportions among both GFP+ CD4+ and CD8+ cells (Figure S5e) and as a proportion of splenocytes (S5f) regardless of selecting thymus. In general, peripheral analysis confirms the observation that positive selection of the HLA-A2-restricted MART1 TCR is reduced in transplanted swine or human HLA-A2− thymi compared to an HLA-A2+ human thymi.
Discussion
Xenotolerance induction may be necessary for successful long-term clinical solid organ transplantation from pigs to humans, as it would allow preservation of patient immune function to protect against infections, zoonosis, and neoplasms. Xenogeneic thymus transplantation is a promising approach to xenotolerance induction. Our previous studies in the pig-to-mouse model indicated that host thymectomy19 and extensive depletion of preexisting T cells19,69 would likely be important components of a clinical regimen incorporating this approach in an immunocompetent host. In patients, either a vascularized swine thymus or a composite swine thymus/organ graft23,27,70 would support de novo development of a robustly and specifically swine-tolerant human T cell pool. However, based on the demonstration in the pig-to mouse model that porcine thymic epithelium is exclusively responsible for positive selection in thymic xenografts,35 the clinical application of this approach would be expected to generate a human T cell population with a TCR repertoire selected by transplanted swine thymic epithelium. This may lead to suboptimal patient immune function if TCRs are not capable of responding to homeostatic signals from or antigens presented on peripheral HLA. We have begun to interrogate the ability of a grafted swine thymus to support the positive selection of known human-restricted TCRs. We compared selection of the MART1 TCR, which has previously been identified for its anti-tumor activity,44,49,60 in HLA-A2+ selecting or HLA-A2− non-selecting thymus grafts or swine thymus grafts. By using lentiviral transgenesis to introduce a human TCR transgene into transplanted human fetal liver-derived HLA-A2+ CD34+ cells, we generated a TCR transgenic humanized mouse model to test the ability of a swine thymus graft to support the positive selection of a human-restricted TCR.
Overall, our results indicated that this human-restricted TCR was positively selected with poor efficiency in a swine thymus. We detected a distinct population of GFP+ MART1 TCR+ cells in transplanted HLA-A2+ thymi that, as expected, was preferentially selected into the CD8+ population. While we were able to detect GFP+ MART1 TCR+ cells in grafted swine thymus tissue, it made up a significantly smaller proportion of thymocytes in these grafts than in grafted HLA-A2+ human thymi. These smaller proportions resembled those in human HLA-A2− thymus grafts, which have been shown to not support positive selection of an HLA-A2-restricted TCR.41,42 In contrast to the HLA-A2+ thymus, preferential CD8 lineage differentiation was not seen in an HLA-A2− human or swine thymus tissue. Additionally, we found that MART1 TCRbright cells were present in reduced proportions in the spleens of mice transplanted with swine or human HLA-A2− thymi compared to those transplanted with HLA-A2+ thymi, further evidence that this human HLA-A2-restricted TCR is positively selected inefficiently in a transplanted swine thymus. Notably, peripheral T cell repopulation was reduced in mice receiving swine thymus grafts compared to those receiving human HLA-A2− thymus grafts, and CD8+ T cell repopulation was reduced in mice receiving swine thymus grafts compared to those receiving both HLA-A2+ and HLA-A2− human thymus grafts. This result is consistent with our previous observation of reduced T cell and CD8+ T cell maintenance in mice transplanted with a swine thymus compared to human thymus grafts.18 Studies in mice have shown that naïve T cells, and especially CD8+ T cells, rely on interactions with self-peptide/MHC complexes for appropriate peripheral homeostasis.71 We had previously hypothesized that poor human CD8+ T cell homeostasis in mice transplanted with a swine compared to a human thymus was due to inefficient positive selection of human-restricted TCRs that could receive appropriate homeostatic signals in the periphery of mice transplanted with human CD34+ cells. Our data showing inefficient positive selection of a known human-restricted TCR is consistent with this hypothesis.
We detected a MART1 TCRdim population in the periphery of mice that was similar between mice regardless of transplanted thymus. Such populations have been observed previously in TCR transgenic mouse66 and humanized mouse models,67 and have been shown by antibody staining or sequencing to express multiple TCRα chains. Our observation that MART1 TCRdim cells have similar CD3 expression as MART1 TCRbright cells, indicating similar expression of surface TCR65,66 but reduced expression of tetramer-stainable MART1 TCRs, suggests a similar phenomenon is taking place in our system. Thus, in the absence of HLA-A2, positive selection of MART1 TCR transgenic cells is limited to those that have rearranged an additional TCRα chain to generate a receptor that can be positively selected. MART1 TCRdim populations were observed in the spleens of mice regardless of selecting thymi and were observed among both CD4 and CD8 T cells. By contrast, MART1 TCRbright cells were mostly detectable on CD8+ T cells, as would be expected of a class I-restricted TCR. Additionally, MART1 TCRbright cells were only enriched among splenocytes of mice receiving an A2+ human thymus graft. This result suggests that only a human HLA-A2+ thymus could mediate efficient positive selection of MART1 TCRbright T cells. However, it also suggests that cells with low level MART1 TCR expression could be positively selected and emerge to the periphery due to coexpression of a second TCRα chain. More studies will be needed to see if this observation represents a generalizable phenomenon for HLA-restricted thymocytes selected in a pig thymus. T cells expressing dual TCRα chains are commonly-observed,68,72 although some data suggests they may be less functional.73 Further characterization of the MART1 TCRdim population found in these mice may allow us to determine whether coexpression of a human-restricted TCR improves human-restricted functionality of human T cells selected in a porcine thymus.
In summary, our results demonstrate inefficient positive selection of cells expressing a human-restricted TCR in a grafted swine thymus, which contrasts with previous studies of murine TCR selection in a xenogeneic thymus.34,35,40,74 In studies in our lab using the pig-to-mouse model, thymocytes expressing both murine MHC class II-restricted TCRs examined were positively selected with similar efficiency in a pig thymus compared to the native mouse thymus, but were not positively selected by a known non-selecting murine MHC.35,74 This disparity may reflect differences between human and mouse thymocyte development in a porcine thymus. However, in those experiments both mouse TCRs examined were class II-restricted, while MART1 TCR is class I-restricted. The selection criteria for thymocytes expressing class I-restricted TCRs are likely different from those for class II-restricted TCRs.75–78 The disparity may also reflect differences specific to the particular TCR transgenes studied. The current studies highlight the importance of using humanized models to study xenotolerance induction, since studies of mouse thymocyte development for xenotolerance induction may not reflect what happens to human cells. Further studies with other class I as well as some class II-restricted TCRs may be necessary to distinguish among these possibilities.
The current study provides a proof-of-concept for the utility of TCR transgenic humanized mouse models to better understand how proposed approaches to xenotolerance induction affect the human T cell pool and better predict consequences of these approaches for future patients who may receive these treatments. Our previous data had shown that a highly diverse human T cell repertoire that is specifically tolerant of the donor pig’s SLA and that can mediate a weak human-restricted vaccination response is selected in pig thymi.18,31 Adding a known TCR with defined specificity to this model allowed demonstration for the first time that cells expressing a human class I-restricted TCR with therapeutic value is not selected efficiently in a swine thymus. To overcome this limitation in positive selection, we are developing approaches to generate hybrid thymi containing both human and swine TECs, building on approaches originally developed in the pig-to-mouse model38 that have begun to be explored in a non-human primate model.79 The TCR transgenic humanized mouse described here provides an ideal in vivo model with which to assess the efficacy of this approach. This model could also be used to understand mechanisms of tolerization of xenoreactive TCRs in xenogeneic thymus transplantation and other methods for xenotolerance induction. Beyond xenotransplantation, our lab and others have used this and similar model systems to characterize the biology of human TCR deletion in central tolerance67 and are studying how cells expressing patient-derived autoreactive TCRs might escape tolerance in conditions of autoimmunity. Humanized mouse models such as this will likely be essential for assessing tolerance of human xenoreactive TCRs and testing strategies for induction of xenotolerance, with the ultimate goal of inducing patient tolerance to pig organs without immunosuppression or immune compromise to allow optimal pig-to-human solid organ xenotransplantation.
Supplementary Material
Acknowledgments
This work has been supported the following NIH grants: 5P01 AI106697, 5P01 AI045897, R01 DK103585, and UC4 DK104207. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050. GAN was supported by NIH T32 5T32AI106711. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
The authors thank Dr. Remi Creusot for the kind gift of the MART1 TCR lentiviral plasmids, and Dr. David Sachs and J. Scott Arn for generating and supplying fetal swine tissue. We also thank Dr. Kaz Yamada for his help isolating swine fetal thymus tissue. The authors also thank Dr. Robert Winchester and Jing Bi for their invaluable assistance performing Sanger allele-level HLA typing for both fetal tissue and adult peripheral blood. The authors also thank Ms. Claire Nauman for reviewing the manuscript, and Ms. Nicole Casio for her assistance with manuscript submission. We also thank Dr. Remi Creusot and Dr. David Sachs for critical review of the manuscript.
References
- 1.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah JA, Patel MS, Elias N, et al. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant. 2017;17(8):2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. [DOI] [PubMed] [Google Scholar]
- 4.Shah JA, Lanaspa MA, Tanabe T, Watanabe H, Johnson RJ, Yamada K. Remaining Physiological Barriers in Porcine Kidney Xenotransplantation: Potential Pathways behind Proteinuria as well as Factors Related to Growth Discrepancies following Pig-to-Kidney Xenotransplantation. J Immunol Res. 2018;2018:6413012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada K, Sykes M, Sachs DH. Tolerance in xenotransplantation. Curr Opin Organ Transplant. 2017;22(6):522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray AG, Khodadoust MM, Pober JS, Bothwell ALM. Porcine aortic endothelial cells activate human T cells: Direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. [DOI] [PubMed] [Google Scholar]
- 7.Yamada K, Sachs DH, DerSimonian H. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–5256. [PubMed] [Google Scholar]
- 8.Buhler LH, Cooper DK. How strong is the T cell response in the pig-to-primate model? Xenotransplantation. 2005;12(2):85–87. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwaki K, Tseng Y-L, Dor FJMF, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nature Medicine. 2005;11(1):29–31. [DOI] [PubMed] [Google Scholar]
- 11.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. [DOI] [PubMed] [Google Scholar]
- 12.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82(12):1787–1791. [DOI] [PubMed] [Google Scholar]
- 13.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. [DOI] [PubMed] [Google Scholar]
- 14.Davila E, Byrne GW, LaBreche PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13(1):31–40. [DOI] [PubMed] [Google Scholar]
- 15.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. [DOI] [PubMed] [Google Scholar]
- 16.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 17.Habiro K, Sykes M, Yang YG. Induction of human T-cell tolerance to pig xenoantigens via thymus transplantation in mice with an established human immune system. American Journal of Transplantation. 2009;9(6):1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalscheuer H, Onoe T, Dahmani A, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192(7):3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee L, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic barrier. Proc Natl Acad Sci U S A. 1994;91:10864–10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2(11):1211–1216. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Sun Z, Sun Y, Langnas AN. Achievement of cellular immunity and discordant xenogeneic tolerance in mice by porcine thymus grafts. Cellular & Molecular Immunology. 2004;1(3):173–179. [PubMed] [Google Scholar]
- 22.Wu A, Yamada K, Awwad M, et al. Experience with porcine thymic transplantation in baboons. Transplant Proc. 2000;32:1048. [DOI] [PubMed] [Google Scholar]
- 23.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75(10):1615–1624. [DOI] [PubMed] [Google Scholar]
- 24.Wu A, Yamada K, Neville DM, et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation. 2003;75(3):282–291. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, Lavelle JM, Vagefi PA, et al. Vascularized thymic lobe transplantation in a pig-to-baboon model: A novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation. 2005;80(12):1783–1790. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Huang L, Shabier Z, Wang J. Regulation of the lifespan in dendritic cell subsets. Mol Immunol. 2007;44(10):2558–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9(12):2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanabe T, Watanabe H, Shah JA, et al. Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation. Am J Transplant. 2017;17(7):1778–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams AB, Kim SC, Martens GR, et al. Xenoantigen Deletion and Chemical Immunosuppression Can Prolong Renal Xenograft Survival. Ann Surg. 2018;268(4):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SC, Mathews DV, Breeden CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu I, Fudaba Y, Shimizu A, Yang YG, Sykes M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation. 2008;86(4):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. [DOI] [PubMed] [Google Scholar]
- 33.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol. 2014;14(6):377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Sergio JJ, Swenson K, Arn JS, Sachs DH, Sykes M. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol. 1997;159:2100–2107. [PubMed] [Google Scholar]
- 35.Zhao Y, Swenson K, Sergio JJ, Sykes M. Pig MHC mediates positive selection of mouse CD4 + T Cells with a mouse MHC-restricted TCR in pig thymus grafts. J Immunol. 1998;160:1320–1326. [PubMed] [Google Scholar]
- 36.Rodriguez-Barbosa JI, Zhao Y, Zhao G, Ezquerra A, Sykes M. Murine CD4 T Cells selected in a highly disparate xenogeneic porcine thymus graft do not show rapid decay in the absence of selecting MHC in the periphery. The Journal of Immunology. 2002;169(12):6697–6710. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Fishman J, Sergio JJ, et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J Immunol. 1997;158:1641–1649. [PubMed] [Google Scholar]
- 38.Fudaba Y, Onoe T, Chittenden M, et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. J Immunol. 2008;181(11):7649–7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Y, Devos T, Yu L, et al. Pathogenesis of autoimmunity after xenogeneic thymus transplantation. The Journal of Immunology. 2003;170(12):5936–5946. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Rodriguez-Barbosa JI, Shimizu A, Sachs DH, Sykes M. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation. 2003;75(11):1832–1840. [DOI] [PubMed] [Google Scholar]
- 41.Vatakis DN, Arumugam B, Kim SG, Bristol G, Yang O, Zack JA. Introduction of exogenous T-cell receptors into human hematopoietic progenitors results in exclusion of endogenous T-cell receptor expression. Mol Ther. 2013;21(5):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Z, Xia J, Fan W, Wargo J, Yang YG. Human melanoma immunotherapy using tumor antigen-specific T cells generated in humanized mice. Oncotarget. 2015;7(6):6448–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64(6):795–800. [DOI] [PubMed] [Google Scholar]
- 44.Chodon T, Comin-Anduix B, Chmielowski B, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20(9):2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106(5):1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang YG, Chen AM, Garrett LJ, et al. Development and analysis of transgenic mice expressing porcine hematopoietic cytokines: a model for achieving durable porcine hematopoieitic chimerism across an extensive xenogeneic barrier. Xenotransplantation. 2000;7:58–64. [DOI] [PubMed] [Google Scholar]
- 47.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. [DOI] [PubMed] [Google Scholar]
- 48.Kalscheuer H, Danzl N, Onoe T, et al. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4(125):125ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole DJ, Weil DP, Shamamian P, et al. Identification of MART-1-specific T-cell receptors: T cells utilizing distinct T-cell receptor and joining regions recognize the same tumor epitope. Cancer Res. 1994;54:5265–5368. [PubMed] [Google Scholar]
- 50.Breckpot K, Dullaers M, Bonehill A, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. The journal of gene medicine. 2003;5(8):654–667. [DOI] [PubMed] [Google Scholar]
- 51.Khosravi-Maharlooei M, Hoelzl M, Li H, et al. A method for rapid thymectomy of immunodeficient NSG mice, permitting analysis of the role of native and grafted thymi in human immune system mice. Eur J Immunol. 2019. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dehoux JP TS, Dewolf N, Otsuka M, Oike F, Famar F, de la Parra B, Latinne D, Bazin H, Gianello P. Effects on human and nonhuman primate immune response of a new rat anti-CD2 monoclonal antibody. Transplantation. 2000;69(12):2622–2633. [DOI] [PubMed] [Google Scholar]
- 53.Borsotti C, Danzl N, Nauman G, et al. HSC extrinsic sex-related and intrinsic autoimmune disease–related human B-cell variation is recapitulated in humanized mice. Blood Advances. 2017;1(23):2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li HW, Vishwasrao P, Holzl MA, et al. Impact of mixed xenogeneic porcine hematopoietic chimerism on human NK cell recognition in a humanized mouse model. Am J Transplant. 2017;17(2):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–156. [DOI] [PubMed] [Google Scholar]
- 56.Gillis S, Ferm MF, Ou W, Smith KA. T Cell Growth Factor: Parameters of production and a quantitataive microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 57.Lan P, Tonomura N, Shimizu A, Wang S, Yang Y-G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. [DOI] [PubMed] [Google Scholar]
- 58.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y-G, deGoma E, Ohdan H, et al. Tolerization of anti–Galα1–3Gal natural antibody–forming B cells by induction of mixed chimerism. The Journal of Experimental Medicine. 1998;187(8):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(5):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraus AB, Shaffer J, Toh HC, et al. Early host CD8 T-cell recovery and sensitized anti-donor interleukin-2–producing and cytotoxic T-cell responses associated with marrow graft rejection following nonmyeloablative allogeneic bone marrow transplantation. Experimental Hematology. 2003;31(7):609–621. [DOI] [PubMed] [Google Scholar]
- 62.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6(9):2121–2133. [DOI] [PubMed] [Google Scholar]
- 63.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de la Hera A, Muller U, Olsson C, Isaaz S, Tunnacliffe A. Structure of the T cell antigen receptor (TCR): two CD3 epsilon subunits in a functional TCR/CD3 complex. J Exp Med. 1991;173(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Punt JA, Roberts JL, Kearse KP, Singer A. Stoichiometry of the T cell antigen receptor (TCR) complex: each TCR/CD3 complex contains one TCR alpha, one TCR beta, and two CD3 epsilon chains. J Exp Med. 1994;180(2):587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heath WR, Miller JFAP. Expression of two α chains on the surface of T cells in T cell receptor transgenic mice. J Exp Med. 1993:1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Teteloshvili N, Tan S, et al. Humanized Mice Reveal New Insights Into the Thymic Selection of Human Autoreactive CD8(+) T Cells. Front Immunol. 2019;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khosravi-Maharlooei M, Obradovic A, Misra A, et al. Crossreactive public TCR sequences undergo positive selection in the human thymic repertoire. J Clin Invest. 2019;130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, Rodriguez-Barbosa JI, Swenson K, Zhao G, Arn JS, Sykes M. Highly disparate xenogeneic skin graft tolerance induction by fetal pig thymus in thymectomized mice. Transplantation. 2001;72:1608–1615. [DOI] [PubMed] [Google Scholar]
- 70.LaMattina JC, Kumagai N, Barth RN, et al. Vascularized thymic lobe transplantation in miniature swine: I. Vascularized thymic lobe allografts support thymopoiesis. Transplantation. 2002;73(5):826–831. [DOI] [PubMed] [Google Scholar]
- 71.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. [DOI] [PubMed] [Google Scholar]
- 72.Heath WR, Carbone FR, Bertolino P, Kelly J, Cose S, Miller JFAP Expression of two T cell receptor α chains on the surface of normal murine T cells. Eur J Immunol. 1995;25:1617–1623. [DOI] [PubMed] [Google Scholar]
- 73.Blichfeldt E, Munthe LA, Ratnes JS, Bogen B. Dual T cell receptor T cells have a decreased sensitivity to physiological ligands due to reduced density of each T cell receptor. Eur J Immunol. 1996;26:2876–2884. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Y, Rodriguez-Barbosa JI, Zhao G, Shaffer J, Arn JS, Sykes M. Maturation and function of mouse T-cells with a transgeic TCR postively selected by highly disparate xenogeneic porcine MHC. Cell Mol Biol. 2001;47(1):217–228. [PubMed] [Google Scholar]
- 75.Singer A New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Current Opinion in Immunology. 2002;14:207–215. [DOI] [PubMed] [Google Scholar]
- 76.Cole DK, Pumphrey NJ, Boulter JM, et al. Human TCR-binding affinity is governed by MHC class restriction. The Journal of Immunology. 2007;178(9):5727–5734. [DOI] [PubMed] [Google Scholar]
- 77.Park JH, Adoro S, Guinter T, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011;23(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekijima M, Sahara H, Shimizu A, et al. Preparation of hybrid porcine thymus containing non-human primate thymic epithelial cells in miniature swine. Xenotransplantation. 2019:e12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.