Abstract
ISG15-deficient humans exhibit permanent, low-level expression of antiviral effectors that safely protect them from various viruses. Because the murine ISG15 axis functions differently, we identified animal models that recapitulate the human condition for the development of ISG15-targeting broad-spectrum antivirals. Canine, porcine, and rhesus macaque ISG15, such as human ISG15, stabilize USP18, a potent inhibitor of type I interferon (IFN)-I. Type I Interferon-primed ISG15-knockout porcine and rhesus cells demonstrate enhanced ISG expression and protection against vesicular stomatitis Indiana virus infection compared with wild type. Collectively, we unveil the interspecies diversity of the ability of ISG15/USP18 axis to control IFN-I signaling and reveal the therapeutic potential of ISG15-deficient porcine and rhesus models.
Keywords: broad-spectrum antiviral, ISG15 deficiency, USP18
Loss of ISG15 in humans leads to increased resistance to all viruses tested. We identify rhesus macaques, pigs, and dogs as species where this mechanism is also conserved that renders them optimal models for development of broad-spectrum antivirals targeting ISG15/USP18.
Type I interferon (IFN-I)-stimulated gene 15 (ISG15) and ubiquitin-specific peptidase 18 (USP18) negatively regulate the IFN-I pathway [1, 2]. By binding to IFN-I receptor 2 (IFNAR2), USP18 prevents the association and activation of downstream signaling kinases at the receptor, holding the transcription of hundreds of IFN-stimulated genes (ISGs) in check [3–5]. Free intracellular ISG15 assists this process by stabilizing USP18, preventing it from S-phase kinase-associated protein 2 (SKP2)-mediated degradation [6].
Beyond its role as a USP18 stabilizer, ISG15 functions as a ubiquitin-like protein (Ubl) that gets covalently linked to lysine residues of target molecules via an E1-3 ligase cascade termed ISGylation. It is interesting to note that the removal of ISG15 conjugates, through a process called DeISGylation, is carried out by the enzymatic activity of USP18 [7] through an interaction that is independent of ISG15-mediated stabilization of USP18 [6, 8].
We recently identified patients with complete inherited deficiencies in either ISG15 or USP18 [6, 9, 10]. Although USP18 deficiency results in complete loss of the negative regulation and, consequently, perinatal onset of lethal hyperinflammation, the adverse effects of human ISG15 deficiency are mild. ISG15-deficient individuals, some of whom are currently in their 20s, experience low-level expression of select ISGs due to unstable USP18 incapable of full resolution of IFN-I signaling [9, 10]. hTert-immortalized fibroblasts derived from ISG15-deficient individuals exhibit elevated ISG expression and consequent hyperresistance to multiple viral families—of both low and high pathogenic potential—compared with wild-type (WT) human fibroblasts. The result is consistent with the patients’ clinical history in it that these individuals have robust control of viral infections [6, 9].
Contrary to humans, murine ISG15 does not stabilize USP18. Murine USP18 resolves IFN-I signaling independent of ISG15. Upon continued stimulation with IFN-β, WT and Isg15-deficient mouse embryonic fibroblasts (MEFs) did not differ in levels of USP18, IFIT2, or phosphorylated STAT2, in contrast to human system. Likewise, ISG expression did not differ in whole blood between WT and Isg15−/− mice at steady state, nor after treatment with IFN-I. Altogether, these data suggest that, unlike human ISG15, the murine homolog does not play a crucial role in IFN-I pathway regulation [8].
In this study, we aim to identify an animal model that recapitulates human ISG15 biology for the development of broad-spectrum, prophylactic antivirals that inhibit the ISG15-USP18 interaction.
METHODS
Phylogenetic Analysis
Ubiquitin, small ubiquitin-like modifier-1 (SUMO-1), and ISG15 (Supplementary Table 1) phylogenetic analyses were performed with MUSCLE and Geneious Tree Builder (Biomatters Ltd.). Neighbor-joining tree branch lengths were calculated with Jukes-Cantro genetic distance model.
Cells
hTert-immortalized healthy donor fibroblasts (C1), WT, and Isg15-/- MEFs, PK-15, LLC-MK2, HEK293T, and Chinese hamster ovary (CHO) cells were cultured as previously described [8].
Plasmids and Transfection
For species stability assays, 3xFLAG-ISG15 and USP18-V5 were cloned into pTRIP.CMV.IVSB.iPuro-2A-TagRFP-DEST (pTRIP). Transfections were carried out as previously described [8].
Priming
Cells were stimulated with 1000 IU/mL IFNα-2b (IntronA; Merck) or 100 U/mL universal type I IFNα (PBL Assay Science) in regular growth medium. Cells were primed for 12 hours, thoroughly washed with phosphate-buffered saline, and allowed to rest for the indicated time.
Interferon-Stimulated Gene Expression Analysis
MX1 transcript levels were determined by quantitative real-time polymerase chain reaction as previously described [8]. Commercial TaqMan probes (Thermo Fisher Scientific) used are listed as follows: hMX1 (Hs00895608_m1), mMx1 (Mm00487796_m1), rhMX1 (Rh02842279_m1), pMX1 (Ss03393847_m1), and 18S ribosomal ribonucleic acid ([RNA] 4319413E).
Protein Analysis
Cells were lysed as previously described [8], and protein was detected with the following antibodies: FLAG (F1804, 1:1000; Sigma-Aldrich), V5 (R960-25, 1:500; Invitrogen), ISG15 (ab233071; Abcam), and beta-actin (8H10D10, 1:1000; Cell Signaling Technology).
CRISPR
Single-guide RNAs (sgRNAs) targeting rhesus macaque and porcine ISG15 were designed with http://crispr.mit.edu. rhISG15_gRNA: CTGAAGGTGAAGATGCTGGG GGG; pISG15_gRNA: ATCCTGGTGAGGAACGACAA GGG. Genomic deoxyribonucleic acid was analyzed using T7E1 assay (T7 Endonuclease I; NEB).
Virus
Endpoint dilution assays of recombinant vesicular stomatitis Indiana virus (rVSV)-green fluorescent protein (GFP) Indiana strain were conducted as previously described [8].
Statistical Analysis
Statistical analysis was performed in GraphPad Prism 6 with a 2-tailed, unpaired Student’s t test for gene expression analysis, and a 2-way analysis of variance corrected with Šidák’s multiple comparison test for viral assays. P < .05 was considered statistically significant.
RESULTS
Phylogenetic Analysis of ISG15 and USP18
We assessed the conservation of ISG15 in comparison to ubiquitin and SUMO-1 across several vertebrate species. Ubiquitin and SUMO-1 are almost identical, whereas the average protein identity of mature ISG15 is 66.2% across chosen species (Supplementary Figure 1A). USP18 is 70% identical within the same species cohort (Supplementary Figure 1B). Guided by the phylogeny of euteleostomi ISG15 and USP18 (Supplementary Figure 1C and D), we selected 12 species that cover a wide range of mammalian diversity to assess the capacity of ISG15 to participate in IFN-I-negative regulation across species. The list included rodents (mouse, hamster, guinea pig, chinchilla), lagomorphs (rabbit), carnivorans (canine, ferret), ungulates (swine), and primates (human, marmoset, rhesus macaque, pig-tailed macaque).
Ability of ISG15 to Stabilize USP18 Is Species-Specific
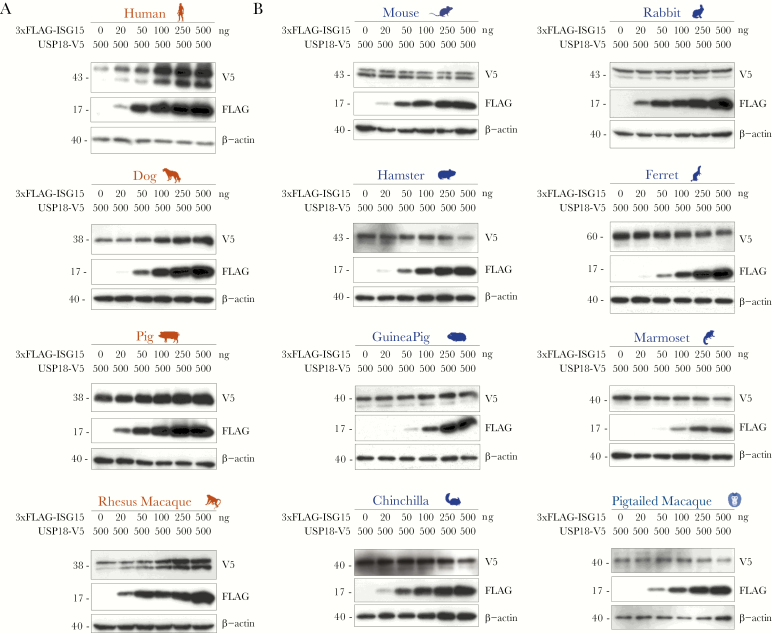
A screen for the ability of ISG15 to stabilize USP18 was conducted by overexpressing an increasing amount of 3xFLAG-ISG15 and a constant amount of USP18-V5 from each of the 12 species in HEK293T cells. Of 10 never-before tested species, only canine, swine, and rhesus macaque had an ISG15 able to sustain its respective USP18 in a dose-dependent manner (Figure 1A). We were surprised to find that, despite remarkable protein sequence identity to rhesus macaque and human ISG15 (Supplementary Figure 2A), marmoset and pig-tailed macaque ISG15 did not stabilize their respective USP18 (Figure 1B), which also share high sequence identity to the other 2 homologs (Supplementary Figure 2B). More expected was the finding that more evolutionarily distant rodent (mouse, hamster, guinea pig, and chinchilla), rabbit, and ferret ISG15 homologs did not stabilize USP18 (Figure 1B). The data, taken together, confirm that USP18 stabilization by ISG15 is species-specific and exists in humans, rhesus macaques, porcine and canine species.
Figure 1.
USP18 stabilization by ISG15 is species-specific. HEK293T cells were cotransfected with an increasing amount (0–500 ng) of 3xFLAG-ISG15 and a constant amount (500 ng) of USP18-V5 of (A) human, dog, pig, rhesus macaque, (B) mouse, golden hamster, guinea pig, chinchilla, rabbit, ferret, marmoset, or pig-tailed macaque. pTRIP-luciferase was added to keep the total amount of plasmid deoxyribonucleic acid constant (1000 ng). At 48–72 hours posttransfection, whole cell lysates were analyzed by Western blot for expression of FLAG and V5. Representative blots are presented; n = 3–5 for each transfection experiment. Color-coding of the species names is based on whether USP18 stabilization by ISG15 was observed for that species: red indicates presence and blue indicates absence of the stabilization effect, respectively.
Species Cross-Talk Suggests Roles for Both ISG15 and USP18 in Stabilization
In an attempt to identify molecular determinants crucial for establishing a stabilizing interaction between ISG15 and USP18, we proceeded to test whether ISG15 from one species is capable of stabilizing USP18 from a different species. Human, rhesus, porcine, and canine ISG15 not only sustained the levels of their own USP18, but one another’s as well (Supplementary Figure 3A–D). In contrast, these same homologs were unable to affect murine USP18 (Supplementary Figure 3E). Although murine ISG15 did not sustain human and rhesus USP18 as expected, it was, however, able to stabilize porcine and, potentially, canine USP18 (Supplementary Figure 3F). The fact that murine ISG15 is capable of shielding USP18, of some species but not that of mice, from degradation suggests that a stronger evolutionary restriction of ISG15/USP18 complex stability lies within USP18. In addition, porcine USP18 may have certain structural properties that are compatible with mouse ISG15, which may explain the mild stabilization observed with this combination. Altogether, the data imply that the species-specific functional differences in stabilization require properties, potentially structural determinants, from both ISG15 and USP18.
Persistent Elevation of Antiviral Effectors in ISG15-Knockout Rhesus and Porcine Cells
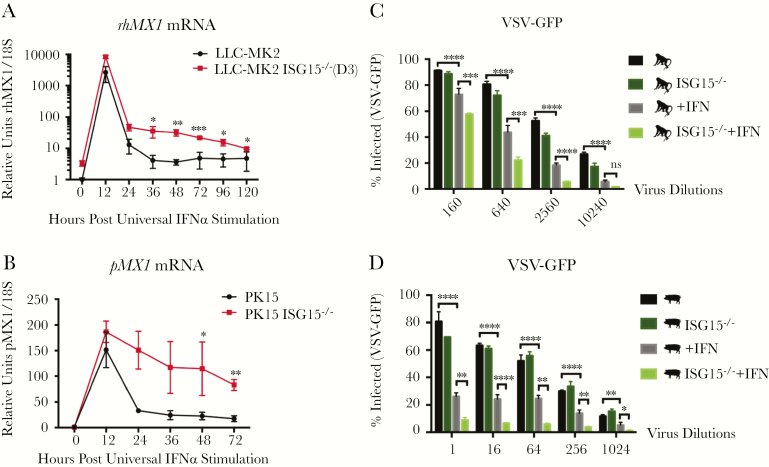
We next hypothesized that species whose ISG15 stabilizes USP18 would also exhibit persistent IFN-I signaling in the absence of endogenous ISG15, as the human homolog does. We used CRISPR/Cas9 to knockout ISG15 in rhesus macaque (LLC-MK2) and porcine (PK-15) kidney epithelial cell lines and confirmed successful targeting by immunoblot (Supplementary Figure 4A). ISG15-knockout (KO) LLC-MK2s displayed a similar phenotype to ISG15-KO human cells over a time-course after IFN priming: rhesus macaque MX1 (rhMX1) was induced to a similar extent after 12 hours of stimulation, but messenger RNA levels took longer to return to baseline in ISG15-KO cells compared with WT after cytokine clearance (Figure 2A). All 3 evaluated rhesus macaque ISG15-KO clonal lines exhibited persistent, elevated expression of rhMX1 as far out as Day 3 (Supplementary Figure 4B). Likewise, in porcine cells, MX1 (pMX1) transcripts were markedly above WT in ISG15-KOs at Day 2 and 3 after IFN-I prime (Figure 2B). As expected, ISG expression after IFN-I stimulation in human and murine ISG15-KO cells was consistent with previous reports (Supplementary Figure 4C and D). Together, these results confirm the role of rhISG15 and pISG15 as important components of IFN-I-negative regulation machinery in these species.
Figure 2.
Interferon (IFN)-primed ISG15-knockout rhesus and porcine cells show persistent induction of IFN-stimulated gene (ISG) expression and enhanced resistance against vesicular stomatitis Indiana virus (VSV). (A and B) Interferon-stimulated gene expression of IFN-primed ISG15-deficient rhesus macaque and porcine cells. Wild-type and ISG15-knockout (KO) LLC-MK2 and PK-15 were stimulated with 100 U/mL universal IFN for 12 hours, washed with phosphate-buffered saline, and allowed to rest for various lengths of time. Relative messenger ribonucleic acid (mRNA) levels of rhesus macaque MX1 (rhMX1) (A) and porcine MX1 (pMX1) (B) were analyzed. (C and D) Vesicular stomatitis Indiana virus infection of rhesus macaque and porcine cells. Wild-type and ISG15-KO LLC-MK2 (D9 clone) (C) and PK-15 cells (D) before or after universal IFN treatment (100 U/mL; 12-hour stimulation followed by 36-hour rest) were infected with serial dilutions of recombinant VSV-green fluorescent protein (GFP). The cells were fixed 16 hours postinfection, stained with 4’,6-diamidino-2-phenylindole, imaged, and analyzed for percentage of nucleus-stained cells positive for GFP. Statistical comparison was performed with unpaired t tests for ISG expression analysis and with 2-way analysis of variance corrected with Šidák’s multiple comparison test for VSV infection analysis. A shows a representative experiment with biological triplicates of 3 different experiments performed. B shows a representative experiment with biological triplicates of 2 different experiments performed. C and D show a single experiment with biological triplicates. Error bars, standard deviation. *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
Interferon-Primed ISG15-Knockout Rhesus and Porcine Cells Resist Vesicular Stomatitis Indiana Virus Infection Better Than Wild Type
To assess whether elevated ISG expression in ISG15-KO LLC-MK2 and PK-15 cells could confer heightened resistance to viral infection, we performed an end-point dilution assay of VSV. Serial dilutions of recombinant VSV expressing GFP (rVSV-GFP) were introduced to WT and ISG15-KO rhesus and porcine cells primed (12-hour stimulated and 36-hour rested) or unprimed with universal IFNα. The percentage of VSV-positive cells at 16 hours postinfection was calculated with CellProfiler [11] from fluorescent images containing thousands of cells/image. At medium and high dilutions, IFN treatment effectively reduced the level of VSV infection in WT rhesus cells up to 4-fold compared with unprimed cells, and loss of ISG15 expression enhanced the protection by an additional 2-fold beyond primed WT cells (Figure 2C). Porcine cells exhibited a similar antiviral effect at low and medium viral dilutions (Figure 2D). These data indicate that the prolonged IFN-I-mediated ISG induction in primed ISG15-KO rhesus and porcine cells improves their resistance to VSV infection. Therefore, rhesus macaques and porcine species are optimal candidate animal models for the development of ISG15/USP18-targeting, broad-spectrum antiviral therapeutics.
Discussion
The phenotypes of individuals with complete loss-of-function mutations in ISG15 lead us to conclude that the primary role of ISG15 in humans is to protect USP18 from SKP2-mediated proteasomal degradation and hence establish stable downregulation of IFN-I signaling. The lack of full negative regulation in ISG15 deficiency leads to mildly elevated ISG expression that better protects them from various viral infections. In contrast, murine ISG15 does not play a role in IFN-I-negative regulation, accounting for the absence of persistent ISG induction and enhanced viral resistance in Isg15-deficient mice [8].
The low level of ISG15 conservation led us to hypothesize that the ISG15/USP18 axis has an involved evolutionary history, and that the cause of the functional discrepancies between species lie in the sequence variation. We showed that human, rhesus macaque, porcine, and canine ISG15 can stabilize their respective USP18. However, we were surprised to find that ISG15 is not a USP18 stabilizer across all primates, with the most striking observation being that rhesus macaque and pig-tailed macaque, which share 99.4% and 89.7% protein sequence identity in ISG15 and USP18, respectively, have ISG15 homologs that function oppositely. A closer examination of the primate sequences did not result in identification of unique patterns in both ISG15 and USP18 that may be crucial for the interaction.
Discordance was also observed between canine and ferret within the order Carnivora, which, together with the primates, suggests that primary structure similarity is likely not a key determinant of stabilization. This notion was further supported by instances of interspecies stabilization in which murine ISG15 was able to stabilize porcine, and probably canine USP18 despite an inability to stabilize most species’ USP18 including its own. It then follows that ISG15 from a “negative species” (eg, mouse) possesses stabilizing ability as long as it meets a compatible USP18. Superimposition of 5 ISG15 crystal structures (human, mouse, vesper bat, canine, and bovine) illustrates that the relative orientation of the 2 Ubl domains differ greatly across species [12, 13] and may contribute to the stabilizing interaction between ISG15 and USP18. Combining these results point to convergent evolution, namely, independent development in different lineages with perhaps USP18 being more of a restricting factor than ISG15 in the formation of a stable ISG15-USP18 complex. Presence of other cellular factors is an alternative explanation for the stabilization effect. However, human and murine ortholog experiments showed the same results in human 293T and CHO cells (Supplementary Figure 3G). This suggests that ISG15 and USP18 are dominant determinants of the species-specific effect.
Conclusions
Most importantly, examination of ISG15-KO rhesus macaque and porcine cells for ISG expression and VSV resistance confirmed that a lack of ISG15 in IFN-I-primed rhesus and porcine cells sustains ISG expression above WT, resulting in enhanced antiviral protection. The ability of these species to recapitulate human biology renders them suitable model systems for the development of broad-spectrum antiviral drugs that inhibit the ISG15-USP18 interaction.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank members of the laboratory—Jennie Altman, Sofija Buta, Conor Gruber, Louise Malle, Marta Martin-Fernandez, and Ashley Richardson—for help with the project. This work represents the Master’s thesis of X. Q. as partial requirement for the fulfillment of the MS degree in Biomedical Sciences offered by the Graduate School of Biomedical Sciences at Mount Sinai.
Author contributions. D. B. conceptualized the project and wrote the manuscript. J. T. designed the constructs and helped write the manuscript. X. Q. designed and performed the experiments, analyzed the data, and wrote the original manuscript. All authors reviewed and approved the final version of the manuscript.
Financial support. This research was funded by National Institute of Allergy and Infectious Disease Grants (R01AI127372, R21AI134366, R21AI129827), the March of Dimes (awarded to D. B.), and Ruth L. Kirschstein Predoctoral Individual National Research Service Award (FAI138363A; to J. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 6th Annual Meeting of the International Cytokine & Interferon Society, October 2018, Boston MA.
References
- 1. Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity 2006; 25:373–81. [DOI] [PubMed] [Google Scholar]
- 2. van Boxel-Dezaire AH, Rani MS, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 2006; 25:361–72. [DOI] [PubMed] [Google Scholar]
- 3. Malakhova OA, Kim KI, Luo JK, et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J 2006; 25:2358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. François-Newton V, Magno de Freitas Almeida G, Payelle-Brogard B, et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon α response. PLoS One 2011; 6:e22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Francois-Newton V, Livingstone M, Payelle-Brogard B, Uzé G, Pellegrini S. USP18 establishes the transcriptional and anti-proliferative interferon α/β differential. Biochem J 2012; 446:509–16. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Bogunovic D, Payelle-Brogard B, et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 2015; 517:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang D, Zhang DE. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res 2011; 31:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speer SD, Li Z, Buta S, et al. ISG15 deficiency and increased viral resistance in humans but not mice. Nat Commun 2016; 7:11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogunovic D, Byun M, Durfee LA, et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science 2012; 337:1684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meuwissen ME, Schot R, Buta S, et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med 2016; 213:1163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carpenter AE, Jones TR, Lamprecht MR, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 2006; 7:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langley C, Goodwin O, Dzimianski JV, Daczkowski CM, Pegan SD. Structure of interferon-stimulated gene product 15 (ISG15) from the bat species Myotis davidii and the impact of interdomain ISG15 interactions on viral protein engagement. Acta Crystallogr D Struct Biol 2019; 75:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dzimianski JV, Scholte FE, Bergeron É, Pegan SD. ISG15: it’s complicated. J Mol Biol 2019; doi:10.1016/j.jmb.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.