Abstract
The gene encoding eukaryotic initiation factor 5A (EIF5A) is found in diabetes-susceptibility loci in mouse and human. eIF5A is the only protein known to contain hypusine (hydroxyputrescine lysine), a polyamine-derived amino acid formed post-translationally in a reaction catalyzed by deoxyhypusine synthase (DHPS). Previous studies showed pharmacologic blockade of DHPS in type 1 diabetic NOD mice and type 2 diabetic db/db mice improved glucose tolerance and preserved beta cell mass, which suggests that hypusinated eIF5A (eIF5AHyp) may play a role in diabetes pathogenesis by direct action on the beta cells and/or altering the adaptive or innate immune responses. To translate these findings to human, we examined tissue from individuals with and without type 1 and type 2 diabetes to determine the expression of eIF5AHyp. We detected eIF5AHyp in beta cells, exocrine cells and immune cells; however, there was also unexpected enrichment of eIF5AHyp in pancreatic polypeptide-expressing PP cells. Interestingly, the presence of eIF5AHyp co-expressing PP cells was not enhanced with disease. These data identify new aspects of eIF5A biology and highlight the need to examine human tissue to understand disease.
Introduction
The mechanisms underlying the pathogeneses of type 1 diabetes (T1D) and type 2 diabetes (T2D) involve the activation of systemic and local inflammatory pathways, leading to eventual dysfunction, de-differentiation and/or death of the beta cells in the pancreatic islet. Elucidating the molecular mechanisms driving the inflammatory response is applicable to the development of therapies for both diseases. In addition, an urgent priority in T1D research is the discovery of biomarkers that can assist in the identification of individuals with pre-clinical disease so early preventative therapeutic interventions can be implemented.
Recently, our laboratories have been investigating the involvement of the hypusinated form of eukaryotic initiation factor 5A (eIF5A) in the development and progression of diabetes in mice. To date, eIF5A is the only known protein to contain hypusine (hydroxyputrescine lysine) [1], which is a polyamine-derived amino acid. This post-translational modification, formed by the process of “hypusination” [2], is catalyzed through a multi-step reaction initiated by the rate-limiting enzyme deoxyhypusine synthase (DHPS) and uses the polyamine spermidine as a cofactor to modify the Lys50 of eIF5A [2]. Previous studies using human cell lines and yeast determined that eIF5A, the hypusinated form of eIF5A (eIF5AHyp) and DHPS are vital for cell viability and proliferation [3,4]. Evolutionarily, eIF5A is highly conserved including the amino acid sequence surrounding the hypusine residue, which suggests an important role for this modification [5]. Whereas studies across species have established that eIF5AHyp efficiently binds the ribosome complex and facilitates mRNA translation [3,6,7], the exact function of eIF5A and eIF5AHyp remains unknown.
Interestingly, the gene encoding eIF5A is found in the Idd4 diabetes-susceptibility locus in non-obese diabetes (NOD) mice [8,9]. In prior studies, we showed that eIF5AHyp is expressed in the pancreatic islets of mouse [10,11], is responsible for the translation of a subset of cytokine-induced transcripts in beta cells in mouse models of diabetes [12,13], and that eIF5AHyp also appears to be required for the activation and proliferation of effector T helper cells [14]. Moreover, reducing the hypusination of eIF5A in NOD mice, a model of T1D, by pharmacological inhibition of DHPS resulted in reduced insulitis, improved glucose tolerance and preserved beta cell mass [14]. Similarly, pharmacological blockade of DHPS in db/db mice [15], a model of T2D improved glucose tolerance and enhanced beta cell mass [16]. Together these data suggest that eIF5AHyp may play a role in the pathogenesis of diabetes such that altering the expression of eIF5AHyp may improve diabetes outcomes long-term.
To translate these findings to human, a greater understanding of eIF5AHyp in the human pancreas and spleen is required. In particular, determining the expression pattern of eIF5AHyp in human tissues and whether eIF5AHyp-expressing cells stratify with characteristics of disease would be informative. In this study, we used human donor tissue samples from the Network of Pancreatic Organ Donors with Diabetes (nPOD) (www.jdrfnpod.org) to examine the expression pattern of eIF5AHyp in the human pancreas and spleen from individuals with T1D, T2D and non-diabetic controls.
Materials and methods
Pancreas and isolated islet cells from mouse
All mice were purchased from the Jackson Laboratory and maintained under a protocol approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee (OLAW Assurance Number: D16-00584 (A4091-01); USDA Certificate Number: 32-R-0025; Customer ID: 798; AAALAC Unit Number: 00083; Protocol Approval #19043). The approved method of euthanasia for mice was carbon dioxide inhalation overdose delivered using a gas cylinder, flow meter/regulator, and induction chamber; 100% CO2 delivered at a rate such that 20–30% of the volume of the chamber is displaced per minute. This was followed by the secondary method of cervical dislocation. Human donor tissues were collected and provided to the investigator by the Network of Pancreatic Organ Donors with Diabetes (https://www.jdrfnpod.org). The study was approved by the University of Florida Institutional Review Board (IRB-1); approval #IRB201600029. Written consent was obtained.
Total pancreas and isolated islets from wildtype C57BL/6 mice as well as acinar tissue and isolated islets from human donors (Table 1 details human donors) were subjected to immunoblot analysis as previously described [17]. Rabbit anti-eIF5AHyp ([13,18]; 1:1000), mouse anti-eIF5A (BD Biosciences; 1:2000) and guinea pig anti-insulin (DAKO; 1:5000) antibodies were used to confirm protein expression in the pancreas.
Table 1. Human donor information for islet and acinar tissue preparations.
| Islet Preparation | Acinar Preparation | |
|---|---|---|
| Unique identifier | SAMN11578698 | UNOS AGDB487 |
| Donor Age (years) | 57.0 | 24 |
| Donor Sex (M/F) | M | M |
| Donor BMI (kg/m2) | 25.8 | 31.7 |
| Donor HbA1c | 5.7 | not tested |
| Origin/source of islets | IIDP (Integrated Islet Distribution Program) | CORE (Center for Organ Recovery and Education) |
| Islet isolation center | The Scharp-Lacy Research Institute | Pittsburgh (AHN) |
| Donor history of diabetes? Yes/No | No | No |
| If Yes, complete the next two lines if this information is available | ||
| Diabetes duration (years) | Not Applicable | Not Applicable |
| Glucose-lowering therapy at time of death | Not Applicable | Not Applicable |
Mice containing the RIP-cre allele (B6.CG-Tg(Ins2-cre)25Mgn/J) [19] were mated with those containing the R26RTomato allele (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) [20] to produce double transgenic animals wherein all the insulin-producing beta cells in the pancreas expressed a fluorescent (Tomato) reporter. Pancreatic islets were isolated from RIP-cre;R26RTomato mice and R26RTomato mice as previously described [21]. The isolated islets from all mice were pooled together and processed for fluorescence activated cell sorting (FACS), which facilitated the separation of islet cells into two populations: Tomato-positive beta cells and Tomato-negative non-beta cells (which included islet cells expressing glucagon, somatostatin, ghrelin, and pancreatic polypeptide). Pooled islets were washed with sterile PBS (Fisher Scientific) and incubated in Accutase cell detachment solution (Sigma) for 10 minutes at 37C with constant mixing (1000 rpm). Islet cells were removed from the Accutase solution by centrifugation (500 x g for 1 min) and resuspended in cold buffer containing 2% BSA,1uM EDTA, and equal parts PBS and HBSS (Fisher Scientific). The cells were filtered, collected and incubated with APC viability dye (Zombie NIR-IR dye; BioLegend) per the manufacturer’s recommended protocol. Single-cell suspensions from RIP-cre;R26RTomato mice and R26RTomato were then sorted using an iCyt Reflection with 100 μm nozzle at 23 psi. Dead cells (NIR-IR+) were excluded; Tomato(+) cells and Tomato(-) cells were collected into tubes containing sort buffer. Data were analyzed using FlowJo software (Tree Star). Lysates from the two populations of cells were subjected to immunoblot analysis. Rabbit anti-eIF5AHyp and mouse anti-eIF5A antibodies were used as above, to evaluate the abundance of eIF5AHyp in the beta cell and non-beta cell populations. Rabbit anti-Pdx1 (Chemicon; 1:1000) antibody was used to evaluate enrichment of the beta cell (tomato+) population.
Mouse pancreas tissue and immunofluorescence analysis
Pancreas tissue was harvested from wildtype C57BL/6 mice and fixed in 4% paraformaldehyde (Fisher Scientific), cryo-preserved using 30% sucrose, embedded in OCT (Fisher Scientific) and sectioned onto glass microscope slides. Methods previously described for pancreas preservation and immunofluorescence were followed [22]. Pancreas tissue sections (8 μm) were stained using the following primary antibodies: guinea pig anti-insulin (DAKO; 1:500), goat anti-pancreatic polypeptide (abcam; 1:200), rabbit anti-eIF5AHyp ([13,18]; 1:1000). Secondary antibodies including Alexa-488, Cy3, or Alexa-647 (Jackson Immunoresearch) were used, followed by DAPI (Sigma; 1:1000) to visualize nuclei. Images were acquired with a Zeiss 710 confocal microscope.
Human pancreas and spleen tissue
Paraffin-embedded tissue sections were obtained from the nPOD consortium (www.jdrfnpod.org). A total of 10 nondiabetic donors, 4 donors with T2D, and 12 donors with T1D (6 autoantibody positive, 6 autoantibody negative) were included in this study (Tables 2 and 3). Information regarding donors’ demography, histology, and disease status were provided by nPOD. The autoantibody status was also determined by nPOD as previously described [23].
Table 2. Human donor pancreas and spleen tissue from T2D and matched controls.
| nPOD case # | Sample name | Age (years) | Gender (male/female) | Ethnicity | BMI | T2D (yes/no) | C-peptide (ng/mL) | HbA1c |
|---|---|---|---|---|---|---|---|---|
| nPOD-6097 | F1-control | 43.1 | female | Caucasian | 36.4 | no | 16.76 | 7.1 |
| nPOD-6102 | F2-control | 45.1 | female | Caucasian | 35.1 | no | 0.55 | 6.1 |
| nPOD-6132 | F1-T2D | 55.8 | female | Hispanic | 44.6 | yes | 0.8 | 9.1 |
| nPOD-6109 | F2-T2D | 48.8 | female | Hispanic | 32.5 | yes | <0.05 | 8 |
| nPOD-6060 | M1-control | 24 | male | Caucasian | 32.7 | no | 13.63 | N/A |
| nPOD-6091 | M2-control | 27.1 | male | Caucasian | 35.6 | no | 7.71 | 6.3 |
| nPOD-6114 | M1-T2D | 42.8 | male | Caucasian | 31 | yes | 0.58 | 7.8 |
| nPOD-6188 | M2-T2D | 36.1 | male | Hispanic | 30.6 | yes | 3.45 | 7.2 |
Table 3. Human donor pancreas and spleen tissue from T1D and matched controls.
| nPOD case # | Sample name | Age (years) | Gender (male/female) | Ethnicity | BMI | T1D (yes/no) | c-peptide | Auto Antibodies Detected | Duration of disease (years) |
|---|---|---|---|---|---|---|---|---|---|
| nPOD-6179 | 1-control | 21.8 | female | Caucasian | 20.7 | no | 2.74 | N/A | N/A |
| nPOD-6224 | 1-AAb-negative | 21 | female | Caucasian | 22.8 | yes | <0.05 | negative | 1.5 |
| nPOD-6070 | 1-AAb-positive | 22.6 | female | Caucasian | 21.6 | yes | <0.05 | mIAA+;1A-2A+ | 7 |
| nPOD-6034 | 2-control | 32 | female | Caucasian | 25.2 | no | 3.15 | N/A | N/A |
| nPOD-6121 | 2-AAb-negative | 33.9 | female | Caucasian | 18 | yes | 0.24 | negative | 4 |
| nPOD-6143 | 2-AAb-positive | 32.6 | female | Caucasian | 26.1 | yes | <0.05 | 1A-2A+;mIAA+ | 7 |
| nPOD-6229 | 3-control | 31 | female | Caucasian | 26.9 | no | 6.23 | N/A | N/A |
| nPOD-6208 | 3-AAb-negative | 32 | female | Caucasian | 23.4 | yes | <0.05 | negative | 16 |
| nPOD-6077 | 3-AAb-positive | 32.9 | female | Caucasian | 22 | yes | <0.05 | mIAA+ | 18 |
| nPOD-6015 | 4-control | 39 | female | Caucasian | 32.2 | no | 1.99 | N/A | N/A |
| nPOD-6038 | 4-AAb-negative | 37.2 | female | Caucasian | 30.9 | yes | 0.2 | negative | 20 |
| nPOD-6054 | 4-AAb-positive | 35.1 | female | Caucasian | 30.4 | yes | <0.05 | mIAA+ | 30 |
| nPOD-6055 | 5-control | 27 | male | Caucasian | 22.7 | no | 0.59 | N/A | N/A |
| nPOD-6041 | 5-AAb-negative | 26.3 | male | Caucasian | 28.4 | yes | <0.05 | negative | 10 |
| nPOD-6180 | 5-AAb-positive | 27.1 | male | Caucasian | 25.9 | yes | <0.05 | GADA+;1A-2A+; ZnT8A+;mIAA+ | 11 |
| nPOD-6104 | 6-control | 41 | male | Caucasian | 20.5 | no | 20.55 | N/A | N/A |
| nPOD-6173 | 6-AAb-negative | 44.1 | male | Caucasian | 23.9 | yes | <0.05 | negative | 15 |
| nPOD-6141 | 6-AAb-positive | 36.7 | male | Caucasian | 26 | yes | <0.05 | GADA+;1A-2A+; ZnT8A+;mIAA+ | 28 |
Immunofluorescence analysis of human tissues
Immunofluorescent staining was performed as previously published [22] with modifications to account for the use of paraffin embedded tissue. Briefly, tissue sections were deparaffinized through graded ethanols (100%, 95%, 85%, 75%, 50%; Fisher Scientific) and then blocked using normal donkey serum (Sigma). Primary antibodies used included guinea pig anti-insulin (DAKO; 1:500), mouse anti-glucagon (Abcam; 1:500), rat anti-somatostatin (abcam; 1:200), goat anti-pancreatic polypeptide (abcam; 1:200), goat anti-ghrelin (Santa Cruz; 1:500), mouse anti-Pax5 (DAKO; 1:200), mouse anti-CD8 (Thermo Fisher; 1:500), mouse anti-CD4 (Leica; 1:500), rabbit anti-eIF5AHyp ([13,18]; 1:1000). Secondary antibodies including Alexa-488, Cy3, or Alexa-647 (Jackson Immunoresearch) were used to visualize primary antibodies. DAPI (Sigma; 1:1000) was used to visualize nuclei. Images were acquired with a Zeiss 710 confocal microscope.
Drug treatments of cultured cells and mouse islets
HEK293T cells (ATCC #CRL-3216) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS (Hyclone; Fisher Scientific), 1% Penicillin/Streptomycin and 2 mM L-Glutamine. HEK293T cells were grown to 60–70% confluency on coverslips in a 24-well plate and treated with 10 or 100 μM N1-Guanyl-1,7-diaminoheptane (GC7) [24] (or 10 mM acetic acid; vehicle) with 0.5 mM aminoguanidine for 16 hours. Cells were fixed with 4% paraformaldehyde in PBS and blocked for 30 min in 3% bovine serum albumin (BSA) in PBS followed by permeabilization with 0.2% Triton X-100 in BSA-PBS for 10 min. All antibodies were diluted in 3% BSA-PBS and were applied in sequential order. Cells were incubated with rabbit anti-eIF5AHyp ([13,18], 1:1000) overnight at 4°C followed by a one hour incubation with anti-rabbit 488 (Jackson Immunoresearch; 1:500) at room temperature. Coverslips were washed with PBS, and DAPI (Sigma; 1:1000) used to visualize nuclei. Coverslips were mounted and images acquired with a Zeiss 710 confocal microscope.
Mouse islets were isolated as previously described [21] and subsequently cultured in RPMI 1640 media supplemented with 10% FBS (Hyclone; Fisher Scientific), and 1% Penicillin/Streptomycin. Islets were treated with 100 μM GC7 (or 10 mM acetic acid; vehicle) with 0.5 mM aminoguanidine for 72 hours; the duration of treatment to reduce hypusination was previously determined in [10]. Islet were fixed with 4% paraformaldehyde in PBS and blocked for 1 hour in 5% normal donkey serum (NDS) in PBS followed by permeabilization with 0.1% Triton X-100 in NDS-PBS for 10 min. Islets were stained as described above using chambered slides; maximum intensity projection images were processed from Z-stack image collections acquired with a Zeiss 710 confocal microscope.
Results
Beta cell and non-beta cell distribution of eIF5AHyp in mouse
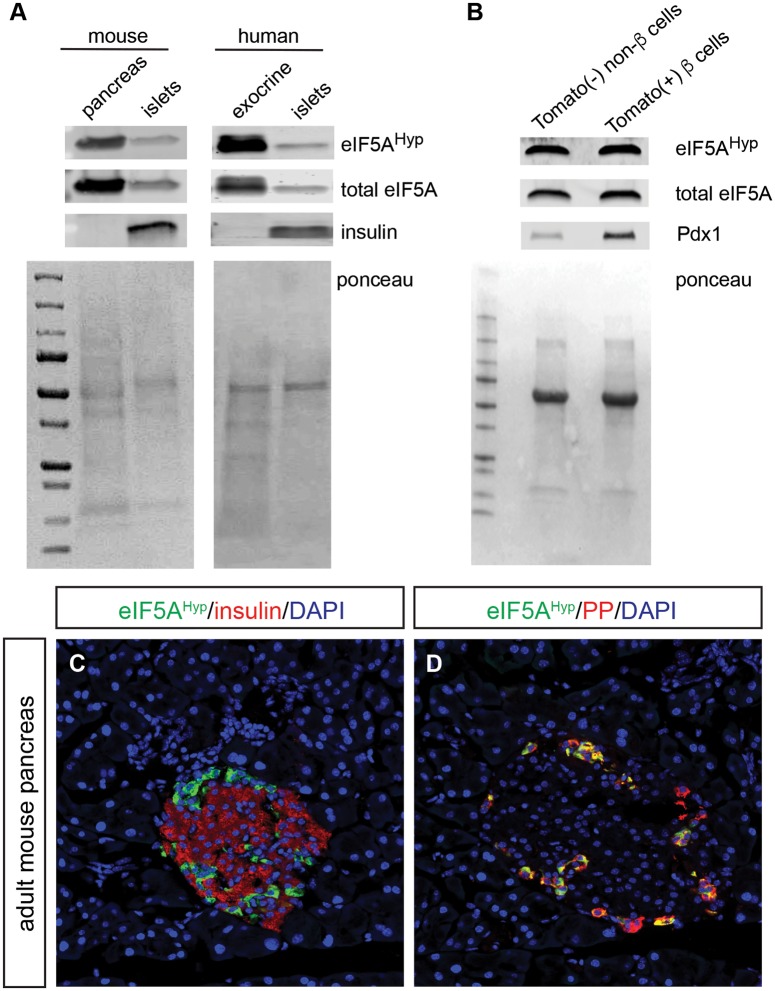
We previously developed and characterized a novel antibody that recognizes the unique amino acid hypusine, formed exclusively through posttranslational modification of the Lys50 residue of eIF5A (eIF5AHyp) [12,18]. In this study, we utilized this antibody to investigate the expression of eIF5AHyp in mouse and human pancreas tissue and isolated islets as well as human spleen tissue, to characterize the expression pattern of eIF5AHyp and determine if eIF5AHyp-expressing cells stratify with characteristics of disease. To that end, we first confirmed the presence of eIF5AHyp in islets isolated from mouse and human pancreas as well as in mouse pancreas and human acinar (exocrine) tissue (Fig 1A, S1 Fig).
Fig 1. Expression of hypusinated eIF5A (eIF5AHyp) in mouse and human pancreatic islets.
(A) Western blot from mouse and human pancreas tissue and isolated pancreatic islets. (B) Western blot from FACS sorted mouse islet cell populations. (C, D) Representative immunofluorescence images of mouse tissue demonstrating robust expression of eIF5AHyp in PP-expressing cells.
We next utilized the RIP-cre;R26RTomato mouse model wherein the insulin-expressing cells were labeled with a lineage trace, thereby generating beta cells indelibly marked with fluorescent reporter (Tomato) expression. Islet cells from RIP-cre;R26RTomato and control animals were sorted by FACS, using the presence and absence of Tomato expression to separate cells into two populations: beta cells (Tomato-positive) and non-beta cells (Tomato-negative). The cell types represented in the “non-beta cell” sample included (ordered from largest population to smallest): glucagon-expressing alpha cells, somatostatin-expressing delta cells, pancreatic polypeptide-expressing PP cells, ghrelin-expressing epsilon cells, exocrine cells (a possible contaminant from the process of islet isolation) and support cells including endothelial cells. A similar quantity of Tomato-positive beta cells (1.92x105 cells) and Tomato-negative non-beta cells (2.13x105 cells) were collected (S2 Fig). Subsequent western blot analysis identified that eIF5AHyp was present in nearly identical abundance in both the beta cell (Tomato-positive) and non-beta cell (Tomato-negative) populations (Fig 1B, S3 Fig). The expression of Pdx1 confirms the enrichment of beta cells in the Tomato positive cells; the lower level of Pdx1 expression in the non-beta cell fraction can be attributed to the presence of somatostatin-expressing delta cells. These data demonstrate that eIF5AHyp is expressed in both the beta cell and non-beta cell fractions; however, whether there is a differential expression of eIF5AHyp in a specific non-beta cell type(s) cannot be clarified from these data. Therefore, to characterize the spatial distribution of eIF5AHyp expression pattern in the islet, we performed co-immunofluorescence staining for eIF5AHyp and islet hormones in mouse pancreas tissue. Whereas relatively weak immunostaining of eIF5AHyp was found throughout the pancreas and islets, robust immunostaining of eIF5AHyp was found in the islet cell population that expressed pancreatic polypeptide (Fig 1C and 1D).
To confirm that the high expressing cell population was not an artifact and that our previously published antibody [12,18] could detect expression of eIF5AHyp by immunofluorescence, we treated HEK293T cells (human) or isolated pancreatic islets (mouse) with the DHPS inhibitor GC7 (N1-Guanyl-1,7-diaminoheptane) [24]. Cells and islets were then analyzed for eIF5AHyp by immunofluorescence. Whereas the control HEK293T uniformly expressed eIF5AHyp, the mouse islets contained cells with both weak and robust expression of eIF5AHyp. Furthermore, following treatment with the inhibitor, we observed a reduction in expression of eIF5AHyp in both the HEK293T cells and mouse islets compared with vehicle treated controls (S4 Fig), which verified the measurement of eIF5AHyp expression using our antibody.
eIF5AHyp-expressing cells in the pancreas of human type 2 diabetes
To characterize the expression pattern of eIF5AHyp in the human pancreas, we utilized tissue samples from the Network of Pancreatic Organ Donors with Diabetes (nPOD). A cohort of tissues from donors with and without T2D were provided (Table 2). Both pancreas and spleen tissues were acquired from each donor; age, gender, ethnicity and BMI were matched where possible. Given the relatively small size of the cohort, quantitative evaluations were not possible. Therefore, we evaluated the presence or absence of eIF5AHyp, its cell-type expression pattern, and its expression correlation with disease.
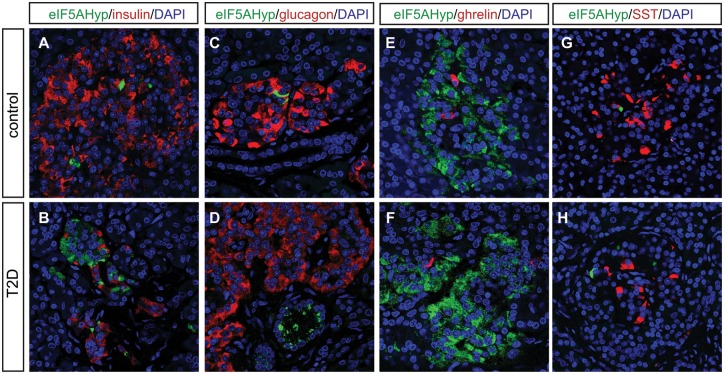
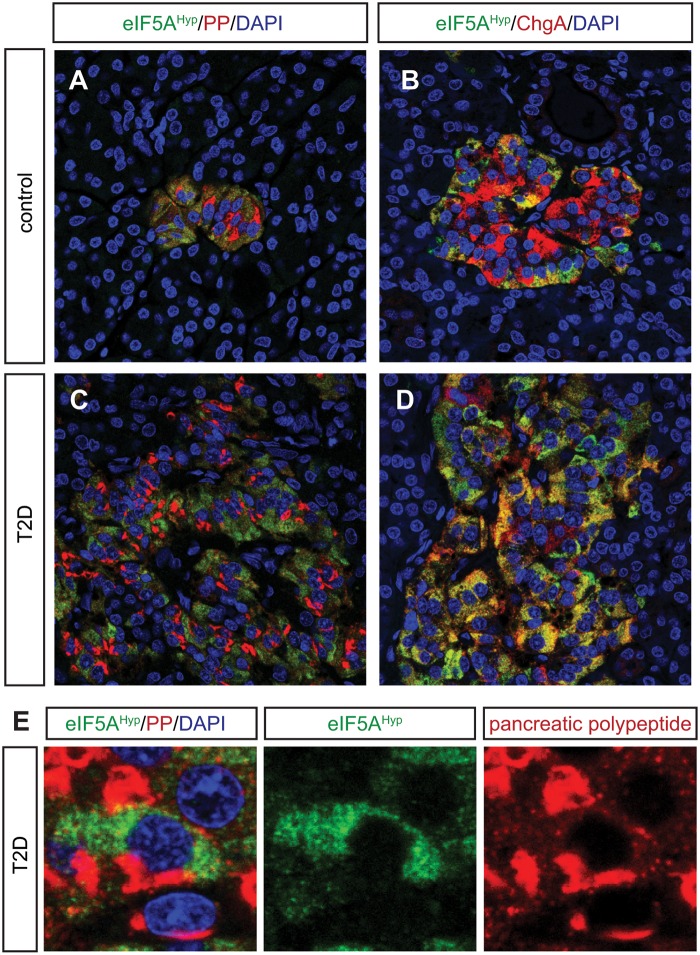
Pancreas tissue sections were co-immunostained with the eIF5AHyp-specific antibody and antibodies that recognized the hormones expressed by each of the endocrine cell populations in the islet (insulin, glucagon, somatostatin, ghrelin and pancreatic polypeptide). Robust co-localization was not observed between eIF5AHyp and insulin (Fig 2A and 2B), glucagon (Fig 2C and 2D), ghrelin (Fig 2E and 2F), or somatostatin (Fig 2G and 2H). However, as observed in the mouse pancreas, cells expressing pancreatic polypeptide were identified to co-express high levels of eIF5AHyp in control pancreas tissue (Fig 3A). These cells also expressed chromograninA, which confirms their identity as neuroendocrine cells (Fig 3B). The co-localization of eIF5AHyp with pancreatic polypeptide in the PP-expressing cells was observed in pancreas tissues from donors with T2D (Fig 3C and 3D) and non-diabetic controls, suggesting no differential expression related to disease status. Notably, whereas PP and eIF5AHyp were expressed in the same cells, the expression pattern is suggestive of localization in different compartments (Fig 3E).
Fig 2. The expression pattern of eIF5AHyp in T2D and control pancreatic tissue.
In controls (matched for age, gender and BMI) and T2D pancreas, we evaluated the co-expression of eIF5AHyp with all islet hormones and found no overlap with insulin (A,B), glucagon (C,D), ghrelin (E,F) or somatostatin (G,H).
Fig 3. eIF5AHyp is robustly expressed in the pancreatic polypeptide-expressing PP cells in the islet.
(A-D) In both controls and T2D pancreas, co-expression of eIF5AHyp with pancreatic polypeptide (PP) and chromograninA (ChgA) was observed. (E) An expression pattern of eIF5AHyp in the PP cells suggestive of localization to the ER was observed in cells in both controls and T2D pancreas.
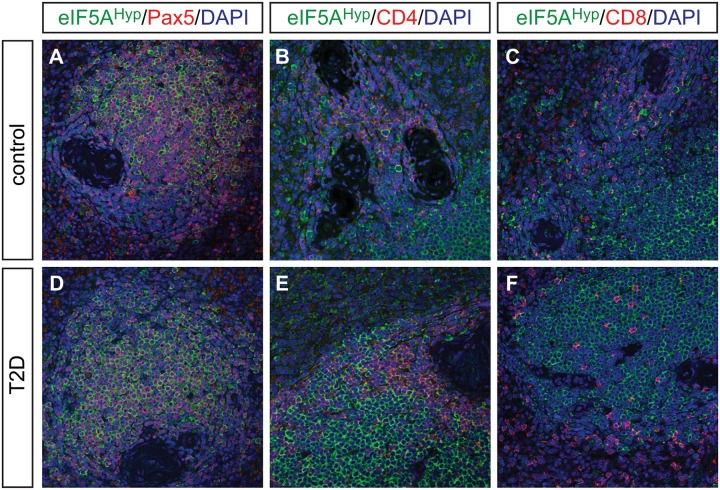
Spleen tissue sections from the same donors were co-immunostained with eIF5AHyp and markers of various cell types. In particular, Pax5-expressing B cells, CD4-expressing T cells, and CD8-expressing T cells were evaluated for co-expression of eIF5AHyp. Whereas the expression patterns observed suggest that most Pax5+ B cells expressed eIF5AHyp, only a select group of eIF5AHyp-expressing cells appear to co-expressed either CD4 or CD8 (Fig 4A–4C; S5–S7 Figs). No obvious differences in staining intensity or distribution were observed between samples from T2D and controls (Fig 4D–4E).
Fig 4. eIF5AHyp expression pattern in the spleen of control and T2D.
eIF5AHyp is expressed in immune cells in the spleen. We evaluated expression of eIF5AHyp in Pax5+ B cells, CD4+ T cells and CD8+ T cells in the spleens of donors with T2D and controls matched for age, gender and BMI. Most eIF5AHyp+ cells co-expressed Pax5+ (A,D); however, a select group of eIF5AHyp+ cells expressed either CD4+ (B,E) or CD8+ (C,F).
eIF5AHyp-expressing cells in the pancreas of human type 1 diabetes
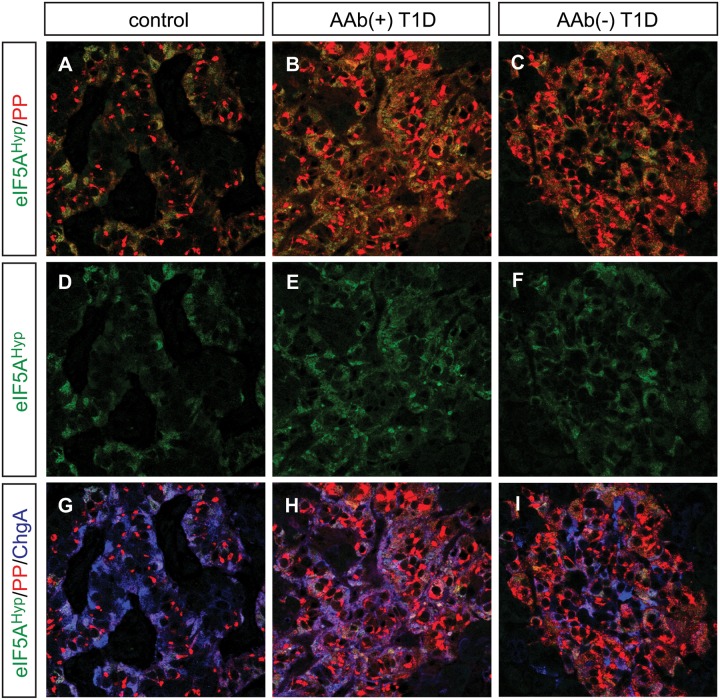
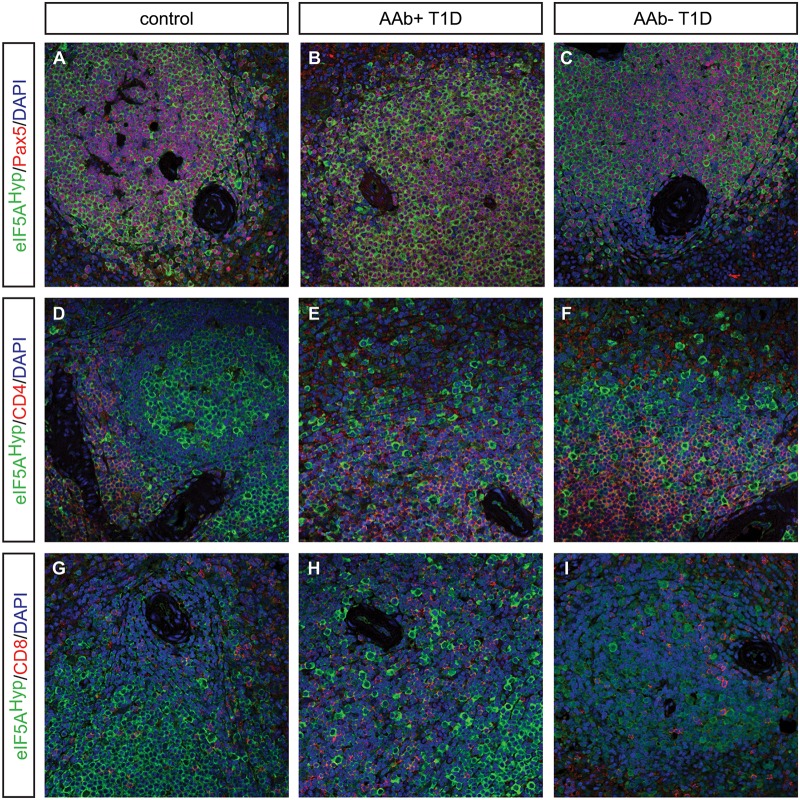
Donor pancreas and spleen tissue from individuals with T1D were also acquired from nPOD and evaluated for the expression pattern of eIF5AHyp. This cohort of samples included T1D donors that were autoantibody-positive and autoantibody-negative, with both short and long disease duration; non-diabetic controls were matched for age, gender, ethnicity and BMI (Table 3). Similar to the T2D/control samples, we identified cells co-expressing the hormone PP with high intensity eIF5AHyp immunostaining (Fig 5A–5F); robust co-expression of eIF5AHyp with other islet hormones was not observed. Moreover, the eIF5AHyp-expressing cells expressed ChromograninA (Fig 5G–5I), which again confirmed that these cells are neuroendocrine in nature. Evaluation of spleen tissue for all T1D donors and controls revealed an identical pattern of expression to that observed in the T2D donors and controls. Specifically, the majority of eIF5AHyp-expressing cells co-expressed Pax5 (Fig 6; S8–S10 Figs).
Fig 5. Expression of eIF5AHyp in the T1D pancreas.
(A-F) Identical to the pattern identified in T2D and control tissues, high expression of eIF5AHyp is observed in PP cells in the T1D, both auto-antibody positive (aAb+) and auto-antibody negative(aAb-), pancreas and controls (matched for age, gender, ethnicity and BMI). (G-I) In all cases, these cells express the endocrine cell marker ChromograninA (ChgA).
Fig 6. Expression of eIF5AHyp in spleen tissue from donors with T1D and matched control donors.
We examined spleen tissue from persons with autoantibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. As observed in T2D and matched control spleen tissue, most eIF5AHyp-expressing cells were Pax5+ (A-C); however, some eIF5AHyp+ cells expressed either CD4+ (D-F) or CD8+ (G-I).
Discussion
Previous data from mouse models identified that pharmacological modulation of the hypusination of eIF5A enhanced beta cell mass and improved glucose tolerance in mouse models of both T1D and T2D [14,16], thereby suggesting an important role for eIF5AHyp in the setting of diabetes. However, to translate these findings to human, a greater understanding of eIF5AHyp in the human pancreas and spleen would be required. This study represents the first description of eIF5AHyp expression in human organs from donors with and without diabetes. Importantly, our results reveal a heretofore unappreciated enrichment of eIF5AHyp in subsets of endocrine cells in the pancreas and immune cells in the spleen. Interestingly, the presence of eIF5AHyp co-expressing cells was not obviously enhanced in diseased tissue; however, larger cohorts are required where tissue can be sampled from across whole organs in order to precisely quantitate the presence of these cells and definitively determine correlation with disease.
Further investigation is also required as to the relative abundance of the deoxyhypusine and hypusine forms of eIF5A in both the normal and diseased setting. Currently, published work suggests that the deoxyhypusine form of eIF5A is transient and reversible, and therefore not as abundant due to its rapid modification by the enzyme DOHH (deoxyhypusine hydroxylase) to the hypusinated form of eIF5A during the process of hypusine biosynthesis [25]. Our antibody is specific for the modified forms of eIF5A over the unmodified forms [18], and the form most detectable by our antibody due to its high prevalence is the more stable hypusinated form of eIF5A (eIF5AHyp). Further investigation and tool development will be required to determine and understand the relative abundance of the deoxyhypusine and hypusine forms of eIF5A in both the normal and diseased settings.
Our findings in the pancreas demonstrate that eIF5AHyp is expressed in both the exocrine and endocrine compartments in mouse and human. Previous reports have also shown expression of eIF5AHyp in mouse islets [10,11]. However, our immunoblot of sorted mouse islet cells further defined that eIF5AHyp expression in the islet can be found in both the beta cell and non-beta cell populations. The non-beta cell populations encompass multiple hormone-expressing cell types, and our immunostaining analysis of mouse tissue clarified that the most robust expression of eIF5AHyp is in the PP cell population. Given the over-representation of PP cells in the uncinate region of the pancreas [26], we analyzed tissue sections that contained the uncinate region and found that eIF5AHyp is robustly co-expressed in PP cells of human islets. Despite evidence that PP cells have a critical secretory function in the brain-gut axis [27] and may serve as a regulator of intra-islet secretion [28], the role of PP cells in the context of diabetes has received little attention. From a developmental perspective, PP cells are predominantly derived from the ghrelin-expressing cell lineage found in the embryonic pancreas [29]; however, the function of eIF5AHyp in the PP cell population postnatally or any function for eIF5AHyp in the development of PP cells has yet to be elucidated. Interestingly, expression analysis of 12-lipoxygenase, a factor known to promote inflammation in the setting of diabetes, is also increased in the PP-expressing cell population in pancreas tissue from human donors (collected through nPOD; [30]). Clearly, a greater understanding is required for the role of PP cells in the pathogenesis of diabetes.
Given that much of the published and ongoing work on hypusine biosynthesis in mice has studied eIF5AHyp in the context of diabetes, we had hypothesized that eIF5AHyp expression would be identified predominantly in the insulin-producing beta cell population. Our western blot analysis did reveal eIF5AHyp expression in human islets. Moreover, we observed eIF5AHyp expression in a purified population of beta cells (Tomato+) from mouse islets. Interestingly, the quantitative nature of western blots indicates that the expression of eIF5AHyp must be lower in the purified beta cells compared with non-beta cells given that PP cells comprise only a small portion of the Tomato(-) non-beta cell fraction whereas the Tomato(+) fraction is composed exclusively of beta cells, and we see near equivalent expression of eIF5AHyp in both sorted populations. This finding is consistent with the immunofluorescence data, wherein we identified robust expression of eIF5AHyp in PP-expressing cells. The lack of observable co-expression of eIF5AHyp and hormones in all cell types in the islet was unexpected; however, we must consider the possibility that this may be due to the limitations in detection of low protein expression by immunofluorescence. Therefore, and considering all data together, our observations indicate the presence of eIF5AHyp in both beta cells and non-beta cells, with particularly high expression in one small non-beta cell populations, the PP cells.
Our previous finding that pharmacological inhibition of eIF5A hypusination (using the drug GC7; N1-Guanyl-1,7-diaminoheptane) in NOD mice improved glucose tolerance and preserved beta cell mass [14]. These improvements were also accompanied by reductions in insulitis, which led us to question whether the improvements in beta cell function were due to a direct effect of DHPS inhibition in beta cells, or an indirect effect related to DHPS inhibition in infiltrating immune cells. Our work and that of others suggest a role for eIF5AHyp and DHPS in promoting T cell and B cell proliferation [14,31,32], which was the basis for our hypothesis that perhaps eIF5AHyp is differentially expressed in immune cells in individuals with diabetes compared with controls. However, identical expression patterns were noted in all spleen tissue evaluated. The identical expression patterns of eIF5AHyp between healthy and disease in both the immune cell populations and islet cell populations could also suggest that it is not the abundance of eIF5AHyp that is critical for promotion of disease. Rather, the presence of eIF5AHyp facilitating the translation of different mRNAs in the disease setting compared with the healthy setting could drive pathogenesis. Given our recent findings that deletion of Dhps in adult mouse beta cells results in reduced diet-induced beta cell proliferation and subsequent glucose intolerance due to altered translation of cyclinD2 [10], we are now investigating the impact of eIF5AHyp on mRNA translation in other diabetes-related cell populations.
Supporting information
(A) Immunoblot images show expression of eIF5AHyp, insulin and total eIF5A in cell lysates from mouse whole pancreas and isolated islets. The top portions of these blots were proved with antibodies not related to this study. (B) Total protein expression as visualized by PonceauS staining. (C) Immunoblot images show expression of eIF5AHyp, insulin and total eIF5A in cell lysate from human exocrine tissue and isolated islets. One blot was probed twice, and this second antibody was not related to this study. (D) Total protein expression as visualized by PonceauS staining. The dotted box shows the lanes where the exocrine and islet samples were run; the other samples are unrelated to this study.
(PDF)
(A) Islets isolated from multiple RIP-cre;R26RTomato mice were pooled together and processed for fluorescence activated cell sorting (FACS). Islet cells were sorted into two populations: Tomato-positive beta cells (R4), and Tomato-negative non-beta cells (R3, islet cells expressing glucagon, somatostatin, ghrelin, and pancreatic polypeptide).
(PDF)
(A) Immunoblot for expression of Pdx1 and eIF5AHyp in cell lysates from Tomato-negative non-beta cells and Tomato-positive beta cells. (B) Immunoblot for expression of total eIF5A. (C) Total protein expression as visualized by PonceauS staining.
(PDF)
HEK293T cells were treated with the DHPS inhibitor GC7 (N1-Guanyl-1,7,diaminoheptane) and analyzed for eIF5AHyp expression by immunofluorescence. (A) Control HEK293T cells uniformly expressed eIF5AHyp. (B,C) Treatment with GC7 resulted in reduced expression of eIF5AHyp. (D) A secondary antibody control was also performed to confirm that the observed signal was not an artifact. Mouse pancreatic islets were also treated with GC7 and analyzed for eIF5AHyp expression by immunofluorescence. (E) Control mouse islets contained cells with both weak and robust expression of eIF5AHyp. (F, G) Islets treated with GC7 showed a reduction in expression of eIF5AHyp. Images are 20X. Inset images are higher magnification of the areas outlined with white boxes.
(PDF)
We evaluated the expression of eIF5AHyp in Pax5-expressing B cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the Pax5-expressing B cells (A, B), eIF5AHyp-expressing cells (C, D), and the overlap between the Pax5-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD4-expressing T cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD4-expressing T cells (A, B), eIF5AHyp-expressing cells (C, D), and the minimal overlap between the CD4-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD8-expressing T cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD8-expressing T cells (A, B), eIF5AHyp-expressing cells (C, D), and the minimal overlap between the CD8-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in Pax5-expressing B cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the Pax5-expressing B cells (A—C), eIF5AHyp-expressing cells (D—F), and the overlap between the Pax5-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD4-expressing T cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD4-expressing T cells (A—C), eIF5AHyp-expressing cells (D—F), and the minimal overlap between the CD4-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD8-expressing T cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD8-expressing T cells (A—C), eIF5AHyp-expressing cells (D—F), and the minimal overlap between the CD8-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
Acknowledgments
The authors wish to thank Dr. David Morris and the Flow Cytometry Core Facility at Indiana University School of Medicine for assistance with FACS. Human pancreatic islets were provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope, NIH Grant # 2UC4DK098085. Human donor acinar tissue was provided by Dr. Rita Bottino at the Center for Organ Recovery and Education (CORE), Pittsburg PA. This research was also performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant#2018PG-T1D053). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/. This manuscript was released as a preprint at bioRxiv https://www.biorxiv.org/content/10.1101/745919v1.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
TLM 5-CDA-2016-194-A-N Juvenile Diabetes Research Foundation https://www.jdrf.org/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RGM R01 DK60581 National Institute of Diabetes and Digestive and Kidney Diseases https://www.niddk.nih.gov/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981;78: 2869–2873. 10.1073/pnas.78.5.2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem. 2006;139: 161–9. 10.1093/jb/mvj034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459: 118–21. 10.1038/nature08034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park MH, Wolff EC, Lee YB, Folk JE. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem. 1994;269: 27827–32. [PubMed] [Google Scholar]
- 5.Wolff EC, Kang KR, Kim YS, Park MH. Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification. Amino Acids. 2007;33: 341–50. 10.1007/s00726-007-0525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuller AP, Wu CC-C, Dever TE, Buskirk AR, Green R. eIF5A Functions Globally in Translation Elongation and Termination. Mol Cell. 2017;66: 194–205.e5. 10.1016/j.molcel.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51: 35–45. 10.1016/j.molcel.2013.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57: 199–208. 10.2337/db07-0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serreze DV, Chapman HD, Varnum DS, Gerling I, Leiter EH, Shultz LD. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol Baltim Md 1950. 1997;158: 3978–3986. [PubMed] [Google Scholar]
- 10.Levasseur EM, Yamada K, Piñeros AR, Wu W, Syed F, Orr KS, et al. Hypusine biosynthesis in β cells links polyamine metabolism to facultative cellular proliferation to maintain glucose homeostasis. Sci Signal. 2019;12 10.1126/scisignal.aax0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tersey SA, Colvin SC, Maier B, Mirmira RG. Protective effects of polyamine depletion in mouse models of type 1 diabetes: implications for therapy. Amino Acids. 2014;46: 633–42. 10.1007/s00726-013-1560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120: 2156–70. 10.1172/JCI38924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier B, Tersey SA, Mirmira RG. Hypusine: a new target for therapeutic intervention in diabetic inflammation. Discov Med. 2010;10: 18–23. [PubMed] [Google Scholar]
- 14.Colvin SC, Maier B, Morris DL, Tersey SA, Mirmira RG. Deoxyhypusine synthase promotes differentiation and proliferation of T helper type 1 (Th1) cells in autoimmune diabetes. J Biol Chem. 2013;288: 36226–35. 10.1074/jbc.M113.473942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14: 141–148. 10.1007/bf00429772 [DOI] [PubMed] [Google Scholar]
- 16.Robbins RD, Tersey SA, Ogihara T, Gupta D, Farb TB, Ficorilli J, et al. Inhibition of deoxyhypusine synthase enhances islet {beta} cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J Biol Chem. 285: 39943–52. 10.1074/jbc.M110.170142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatanaka M, Anderson-Baucum E, Lakhter A, Kono T, Maier B, Tersey SA, et al. Chronic high fat feeding restricts islet mRNA translation initiation independently of ER stress via DNA damage and p53 activation. Sci Rep. 2017;7 10.1038/s41598-017-03869-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiki Y, Farb TB, Friedrich J, Bokvist K, Mirmira RG, Maier B. Characterization of a novel polyclonal anti-hypusine antibody. Springerplus. 2013;2: 421 10.1186/2193-1801-2-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274: 305–15. 10.1074/jbc.274.1.305 [DOI] [PubMed] [Google Scholar]
- 20.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13: 133–40. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG. Mouse Islet of Langerhans Isolation using a Combination of Purified Collagenase and Neutral Protease. J Vis Exp JoVE. 2012. [cited 30 Mar 2018]. 10.3791/4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastracci TL, Anderson KR, Papizan JB, Sussel L. Regulation of Neurod1 contributes to the lineage potential of Neurogenin3+ endocrine precursor cells in the pancreas. PLoS Genet. 2013;9: e1003278 10.1371/journal.pgen.1003278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28: 608–617. 10.1002/dmrr.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakus J, Wolff EC, Park MH, Folk JE. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268: 13151–13159. [PubMed] [Google Scholar]
- 25.Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38: 491–500. 10.1007/s00726-009-0408-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zielinski MC, Misawa R, Wen P, Wang T-Y, Wang C-Z, et al. Quantitative Analysis of Pancreatic Polypeptide Cell Distribution in the Human Pancreas. PLoS ONE. 2013;8 10.1371/journal.pone.0055501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46: 261–274. 10.1016/j.npep.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brereton MF, Vergari E, Zhang Q, Clark A. Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J Histochem Cytochem Off J Histochem Soc. 2015;63: 575–591. 10.1369/0022155415583535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnes L, Hill JT, Gross S, Magnuson MA, Sussel L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PloS One. 2012;7: e52026 10.1371/journal.pone.0052026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grzesik WJ, Nadler JL, Machida Y, Nadler JL, Imai Y, Morris MA. Expression pattern of 12-lipoxygenase in human islets with type 1 diabetes and type 2 diabetes. J Clin Endocrinol Metab. 2015;100: E387–395. 10.1210/jc.2014-3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevec D, Jaksche H, Oft M, Wöhl T, Himmelspach M, Pacher A, et al. Inhibition of HIV-1 replication in lymphocytes by mutants of the Rev cofactor eIF-5A. Science. 1996;271: 1858–1860. 10.1126/science.271.5257.1858 [DOI] [PubMed] [Google Scholar]
- 32.Schlee M, Krug T, Gires O, Zeidler R, Hammerschmidt W, Mailhammer R, et al. Identification of Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA2) target proteins by proteome analysis: activation of EBNA2 in conditionally immortalized B cells reflects early events after infection of primary B cells by EBV. J Virol. 2004;78: 3941–3952. 10.1128/JVI.78.8.3941-3952.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Immunoblot images show expression of eIF5AHyp, insulin and total eIF5A in cell lysates from mouse whole pancreas and isolated islets. The top portions of these blots were proved with antibodies not related to this study. (B) Total protein expression as visualized by PonceauS staining. (C) Immunoblot images show expression of eIF5AHyp, insulin and total eIF5A in cell lysate from human exocrine tissue and isolated islets. One blot was probed twice, and this second antibody was not related to this study. (D) Total protein expression as visualized by PonceauS staining. The dotted box shows the lanes where the exocrine and islet samples were run; the other samples are unrelated to this study.
(PDF)
(A) Islets isolated from multiple RIP-cre;R26RTomato mice were pooled together and processed for fluorescence activated cell sorting (FACS). Islet cells were sorted into two populations: Tomato-positive beta cells (R4), and Tomato-negative non-beta cells (R3, islet cells expressing glucagon, somatostatin, ghrelin, and pancreatic polypeptide).
(PDF)
(A) Immunoblot for expression of Pdx1 and eIF5AHyp in cell lysates from Tomato-negative non-beta cells and Tomato-positive beta cells. (B) Immunoblot for expression of total eIF5A. (C) Total protein expression as visualized by PonceauS staining.
(PDF)
HEK293T cells were treated with the DHPS inhibitor GC7 (N1-Guanyl-1,7,diaminoheptane) and analyzed for eIF5AHyp expression by immunofluorescence. (A) Control HEK293T cells uniformly expressed eIF5AHyp. (B,C) Treatment with GC7 resulted in reduced expression of eIF5AHyp. (D) A secondary antibody control was also performed to confirm that the observed signal was not an artifact. Mouse pancreatic islets were also treated with GC7 and analyzed for eIF5AHyp expression by immunofluorescence. (E) Control mouse islets contained cells with both weak and robust expression of eIF5AHyp. (F, G) Islets treated with GC7 showed a reduction in expression of eIF5AHyp. Images are 20X. Inset images are higher magnification of the areas outlined with white boxes.
(PDF)
We evaluated the expression of eIF5AHyp in Pax5-expressing B cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the Pax5-expressing B cells (A, B), eIF5AHyp-expressing cells (C, D), and the overlap between the Pax5-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD4-expressing T cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD4-expressing T cells (A, B), eIF5AHyp-expressing cells (C, D), and the minimal overlap between the CD4-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD8-expressing T cells in the spleens of donors with T2D and controls matched for age, gender and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD8-expressing T cells (A, B), eIF5AHyp-expressing cells (C, D), and the minimal overlap between the CD8-expressing and eIF5AHyp-expressing populations (E, F). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in Pax5-expressing B cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the Pax5-expressing B cells (A—C), eIF5AHyp-expressing cells (D—F), and the overlap between the Pax5-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD4-expressing T cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD4-expressing T cells (A—C), eIF5AHyp-expressing cells (D—F), and the minimal overlap between the CD4-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
We evaluated the expression of eIF5AHyp in CD8-expressing T cells in the spleens of donors with auto-antibody positive (AAb+) and auto-antibody negative (AAb-) T1D, and corresponding controls matched for age, gender, ethnicity, and BMI. The fluorescent channels have been separated to better display the expression patterns of the CD8-expressing T cells (A—C), eIF5AHyp-expressing cells (D—F), and the minimal overlap between the CD8-expressing and eIF5AHyp-expressing populations (G—I). All images are 20X.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.