Highlights
► Virus-like particles (VLPs) are a class of recombinant subunit vaccines. ► VLPs resemble native viruses but lack infectious genetic material. ► VLPs are promising vaccines due to strong immunogenicity and safety. ► VLPs can be produced in prokaryotic or eukaryotic expression systems, or in vitro. ► VLP-based vaccine candidates targeting many diseases are in clinical development.
Keywords: Virus-like particle, Subunit vaccine, Clinical development, Heterologous expression system, Virosome
Abstract
Virus-like particles (VLPs) are a class of subunit vaccines that differentiate themselves from soluble recombinant antigens by stronger protective immunogenicity associated with the VLP structure. Like parental viruses, VLPs can be either non-enveloped or enveloped, and they can form following expression of one or several viral structural proteins in a recombinant heterologous system. Depending on the complexity of the VLP, it can be produced in either a prokaryotic or eukaryotic expression system using target-encoding recombinant vectors, or in some cases can be assembled in cell-free conditions. To date, a wide variety of VLP-based candidate vaccines targeting various viral, bacterial, parasitic and fungal pathogens, as well as non-infectious diseases, have been produced in different expression systems. Some VLPs have entered clinical development and a few have been licensed and commercialized. This article reviews VLP-based vaccines produced in different systems, their immunogenicity in animal models and their status in clinical development.
1. Introduction
To date, vaccination remains the most effective way for control and prevention of infectious diseases. The majority of vaccines currently available are based on inactivated or live attenuated pathogens. Although these vaccines are highly effective, and for smallpox in humans and rinderpest in cattle have even led to the eradication of target pathogens, they often induce side effects at some frequency in populations [1], dictating the need for the development of safer vaccines. Over recent years, advances in recombinant DNA technologies and genetic engineering have led to the development of subunit vaccines (SUVs) [2]. SUVs are based on specific components of pathogens, often located on their surface. Therefore, SUVs are considered safer than full pathogen-based inactivated or live attenuated vaccines. However, immunogenicity of SUVs is generally low compared to that of full pathogen-based inactivated or live attenuated vaccines, thus requiring higher doses, booster administrations and co-administration of adjuvants, or developing alternative approaches for enhancing target-specific immunity. Virus-like particles (VLPs) represent a major advancement in the development of SUVs with enhanced immunogenicity. VLPs are formed by structural viral proteins which have an inherent property for self-assembly, and mimic the morphology of the pathogen. In contrast to live viruses, VLPs are non-infective and non-replicating, since they are essentially devoid of infectious genetic material. VLPs display antigenic epitopes in the correct conformation and in a highly repetitive manner, leading to cross-linking of B cell immunoglobulin receptors and B cell activation [3], [4]. As exogenous antigens, VLPs are efficiently taken up by professional antigen presenting cells, particularly dendritic cells (DCs), followed by antigen processing and presentation by MHC class II molecules, DC activation and maturation through up-regulation of co-stimulatory molecules and cytokine production, and stimulation of CD4+ T helper cells, leading to the induction of strong humoral and cellular immune responses [3], [4], [5]. Similar to native viruses, VLPs are processed in the cytosol of DCs and are presented by MHC class I molecules to cytotoxic CD8+ T cells by the cross-presentation mechanism, inducing potent cytotoxic immune responses [3], [4], [5], [6], [7], [8], [9].
Thus far, VLPs have been shown to be highly immunogenic and have recently come into focus for their diverse applications in vaccination, targeted drug delivery, gene therapy and immune therapy. All four recombinant vaccines that are on the market, GlaxoSmithKline (GSK)’s Engerix® (hepatitis B virus [HBV]) [10] and Cervarix® (human papillomavirus [HPV]) [11] and Merck and Co., Inc.’s Recombivax HB® (HBV) [12] and Gardasil® (HPV) [13], are based on highly purified VLPs. Additionally, a number of VLP-based vaccine candidates, including GSK's anti-malaria vaccine RTS,S, are in clinical development [14], while many others, targeting pathogens such as influenza virus, rotavirus (RV) and human immunodeficiency virus (HIV), are undergoing pre-clinical evaluation [15], [16], [17], [18], [19], [20]. To date, VLPs, non-enveloped and enveloped, have been produced for a number of targets using mammalian [21], plant [22], insect [23], yeast [14] or bacterial cells [24], and cell-free platforms [25], [26]. Additionally, vaccine antigens can be produced as genetic fusions or chemical conjugates to viral structural proteins, resulting in chimeric VLPs. Key aspects of licensed or advanced stage VLP vaccines are presented in Table 1 .
Table 1.
Key aspects of selected prophylactic VLP vaccines.
| VLP type | Production system | Antigen | Indication | Product name | Status | Reference |
|---|---|---|---|---|---|---|
| Non-envelopeda | Mammalian | Virus structural protein | HBV | GenHevac B® | Licensed | [21] |
| Non-envelopeda | Plant | Virus structural protein | HBV | Phase 1 | [27], [28] | |
| Non-envelopeda | Yeast | Virus structural protein | HBV | Engerix-B®, Recombivax HB® | Licensed | [10], [12] |
| Non-enveloped | Insect/baculovirus | Virus structural protein | HPV | Cervarix® | Licensed | [11] |
| Non-enveloped | Yeast | Virus structural protein | HPV | Gardasil® | Licensed | [13] |
| Non-enveloped (chimeric) | Yeast | Parasite protein | Malaria | RTS,S | Phase 1 | [14] |
| Non-enveloped (chimeric) | Bacteria | Parasite protein | Malaria | MalariVax | Phase 3 | [24] |
| Non-enveloped (chimeric) | Bacteria | Virus matrix protein | Influenza A | ACAM-FLU-A | Phase 1 | [33] |
| Enveloped | Plant | Virus envelope protein | Influenza A | Phase 1/2 | [22] | |
| Enveloped | Insect/baculovirus | Virus envelope proteins | Influenza A | Phase 2 | [23] | |
| Enveloped (virosome) | Cell-free | Virus envelope proteins | Influenza A | Inflexal® V | Licensed | [26] |
| Enveloped (virosome, chimeric) | Cell-free | Parasite protein | Malaria | PEV3 | Phase 1/2 | [29], [30], [31], [32] |
Abbreviations: HBV, hepatitis B virus; HPV, human papillomavirus.
Contain host cell lipids.
This review focuses on different approaches to the design, engineering, production and development of VLP-based vaccines.
2. Division of VLPs by structure
Based on the structure of their parental viruses, VLPs can be divided into two major categories: non-enveloped VLPs and enveloped VLPs.
2.1. Non-enveloped VLPs
Non-enveloped VLPs are typically composed of one or more components of a pathogen with the ability to self-assemble into particles [34], [35], [36] and do not include any host components (Fig. 1A). This approach has been used to develop vaccines against such pathogens as HPV [37] and RV [38]. Non-enveloped VLPs continue to be explored as a preferred approach for developing SUVs against a number of pathogens, including canine, mink and porcine parvoviruses, using VP2 protein expressed in insect cells [39], [40], [41], [42], [43], and Norwalk virus (NV), using the self-assembling 58 kDa capsid protein produced in in vitro transcription–translation and baculovirus recombinant expression systems [44]. VLPs for hepatitis E virus (HEV), resulting from self-assembly of the 50 kDa N-terminally truncated capsid protein of the virus expressed in insect cells, have been shown to be highly immunogenic [45].
Fig. 1.
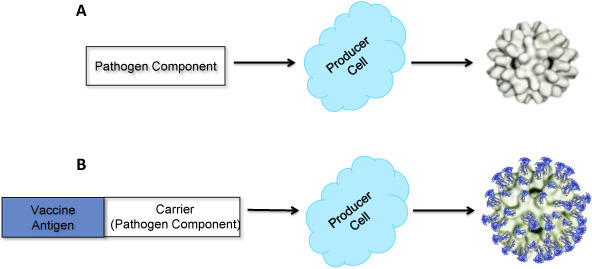
Schematic diagram of non-enveloped VLP production. (A) VLP composed of a pathogen component. (B) Chimeric VLP composed of a pathogen component carrier fused to a vaccine target antigen.
VLPs formed by HBV surface antigen (HBsAg) somewhat differ from typical non-enveloped VLPs due to the high content of host cell lipids (specifically, acidic phospholipids) which have been shown to interact with HBsAg facilitating conformationl integrity and antigenicity [46], [47], [48], [49]. HBsAg tends to assemble into water-soluble micelles [46], [50], [51] in association with the endoplasmic reticulum (ER) originated lipids [52]. Using recombinant DNA technology, HBsAg has been successfully produced in yeast [53], [54], [55] and mammalian cell [56] expression systems, resulting in VLPs similar to the 22-nm highly immunogenic HBsAg subviral particles isolated from the plasma of individuals chronically infected with HBV [57], [58]. These recombinant HBsAg VLPs varied in molecular weight, size, monomer composition, glycosylation and immunogenicity [59], [60] as well as in the site of assembly [61], [62] (for more details, see Sections 3.2, 3.4).
This approach has been further developed to engineer and produce chimeric non-enveloped VLPs where vaccine target is expressed as fusion partner on the surface of particles consisting of a component of animal or plant pathogens with the ability to self-assemble (carrier) (Fig. 1B). For example, an anti-malaria vaccine, RTS,S, is based on a malaria antigen expressed as a fusion partner on the surface of VLPs containing HBsAg [63]. During the last two decades, coat proteins (CPs) of several plant viruses have been successfully engineered to produce target antigens from a range of mammalian pathogens. For example, chimeric particles formed by CPs of tobacco mosaic virus (TMV) fused to Plasmodium falciparum epitopes [64]; alfalfa mosaic virus (AlMV) presenting rabies virus, HIV-1 [65], [66] and respiratory syncytial virus (RSV) [67], [68] epitopes; cowpea mosaic virus (CPMV) displaying epitopes of HIV-1 [69], HPV16 [70], Bacillus anthracis [71] and P. falciparum [72]; and potato virus X (PVX) carrying epitopes of HIV-1 [73] and influenza virus [74] have been shown to stimulate target-specific immune responses.
More structurally complex non-enveloped VLPs contain multiple interacting viral capsid proteins organized in several concentric layers and are represented by VLPs engineered for viruses of the family Reoviridae. Icosahedral virions of bluetongue virus contain seven capsid proteins; however, only four of them – VP2, VP3, VP5 and VP7 – are necessary for VLP formation [75]. Bluetongue VLPs containing two to four major capsid proteins have been produced in insect cells using baculovirus expression vectors [76], [77], [78]. Stable double-layered and triple-layered RV VLPs have been produced by co-expressing different combinations of major structural proteins of RV – VP2, VP4, VP6 and VP7 – in insect cells [79], [80]. RV VLPs maintained the structural and functional characteristics of native virus particles as well as the interaction between VP4 and VP6 [79].
In summary, non-enveloped VLPs can consist of a single or multiple components of a target pathogen or a single or multiple vaccine target antigens displayed on the VLP surface as a fusion to a heterologous viral protein with the ability to self-assemble.
2.2. Enveloped VLPs
Enveloped VLPs are relatively complex structures consisting of the host cell membrane (an envelope) with integrated target antigens displayed on the outer surface (Fig. 2 ) [81], [82], [83], [84]. Enveloped VLPs provide a higher degree of flexibility for integration of more antigens from the same or heterologous pathogens [19], [85], [86], [87]. The most prominent examples of enveloped VLPs are VLPs engineered to express vaccine target antigens from influenza viruses [88], [89], retroviruses [81], [90] and hepatitis C virus (HCV) [91]. Production of enveloped VLPs requires co-expression of several structural viral proteins, their assembly into particles, incorporation into host membranes and release of particles (budding) from the cell membrane. For example, a successful assembly and budding of influenza A/Udorn/72 (H3N2) VLPs have been achieved by co-expressing four structural proteins (hemagglutinin [HA], neuraminidase [NA], matrix protein M1 and ion channel protein M2) of the virus. The resulting VLPs, produced in insect cells, closely resembled native influenza virus particles by size and morphology, with the same fine structure of surface spikes [89]. Expression of M1 protein alone, but not HA, NA or nucleocapsid protein (NP), results in the formation and release of particles [89]. In other studies, using both baculovirus- and vaccinia virus-based expression systems, influenza VLPs containing HA, NA and M1 [92], HA and M1 [93], or M1 only [94] have been produced, supporting the key role for M1 protein in the enveloped VLP formation and release. In contrast, a more recent study by Chen et al., using plasmid DNA-transfected mammalian cells, demonstrated assembly and budding of influenza VLPs comprising HA and NA only [88].
Fig. 2.
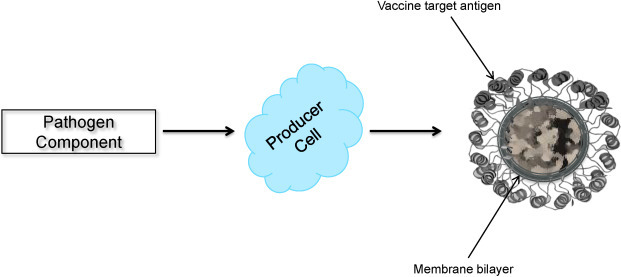
Schematic diagram of enveloped VLP production.
In addition to influenza VLPs, the correct assembly and release of enveloped VLPs have been reported for retroviruses including HIV and simian immunodeficiency virus (SIV) whose Gag proteins have been shown to form VLPs in insect cells [81], [90]. Moreover, enveloped VLPs containing target antigens from heterologous viruses, such as SIV Gag protein and HIV Env protein [95], have been produced. Furthermore, expression in insect cells of HCV core protein, envelope proteins E1 and E2, and p7 protein has been shown to result in the formation and release of enveloped VLPs of 40–60 nm in diameter, presumably derived from the ER [91]. The morphology, immunoreactivity and biophysical characteristics of HCV VLPs were similar to those of putative HCV virions isolated from HCV-infected humans. The results also suggested that HCV core and envelope proteins are sufficient for VLP assembly, while p7 protein is not required.
The enveloped VLP approach is being increasingly used to produce recombinant SUVs. There are reports on the efficient assembly and release from insect cells of human severe acute respiratory syndrome coronavirus (SARS CoV)-like particles. While expression of M and E proteins is sufficient for smooth-surfaced VLP assembly [96], [97], high-level accumulation of co-expressed S, E and M proteins of SARS-CoV from a single baculovirus vector results in a very efficient formation of VLPs that morphologically mimic native SARS-CoV virions [97]. Also, VP40 matrix protein of Ebola virus has been shown to form VLPs when produced in mammalian cells [98] and could be a potential vaccine for preventing infections with Ebola virus. Inclusion of other Ebola virus proteins such as NP, GP and VP35 enhances the VP40-based VLP formation approximately 40-fold [99] without any major changes in VLP morphology [100]. Another example of enveloped VLPs are VLPs against Newcastle disease virus (NDV) consisting of HA-NA (HN), fusion (F), NP and M proteins of the virus. These VLPs, produced in avian cells, structurally and functionally resemble NDV virions, preserve biological activity of the VLP-associated NDV glycoproteins, and are released with an efficiency of 84%, which allows for preparing large quantities of the particles needed for vaccine manufacturing [101], [102]. More recent studies have demonstrated the utility of plants as another alternative for producing VLP-based vaccines against animal pathogens [22].
In conclusion, enveloped VLPs represent complex structures consisting of multiple components of target pathogens and host membrane components and resemble the pathogen.
3. Expression systems and immunogenicity of VLPs in pre-clinical and clinical studies
VLP-based vaccines are safe and immunogenic. Testimony to this is the fact that all currently licensed recombinant SUVs are based on VLPs. Both enveloped and non-enveloped VLPs are efficient in generating humoral and cell-mediated immune responses. Different cell substrates including bacterial [24], yeast [14], insect [23], mammalian [21] and plant [22] expression systems have been used to produce VLPs.
3.1. Bacteria
Bacteria, the most widely used expression system for manufacturing recombinant proteins, is not a preferred platform for VLP production due to a number of factors, including the absence of a mammalian-like post-translational modification (PTM) system. Nevertheless, bacteria are used to produce non-enveloped VLPs based on components of a pathogen with the ability to self-assemble in the bacterial host or as fusions of vaccine target antigens to bacteriophage surface proteins [24], [103], [104].
Of many types of sexually transmitted HPV, types 6, 11, 16 and 18 are the highest-risk carcinogenic types causing cervical cancer and related genital neoplasias [105]. Bacteria-derived HPV VLPs have been produced by expressing the major capsid protein L1 of HPV types 11 and 16. Also, the intracellular assembly of HPV16 L1 VLPs has been documented in Lactobacillus casei, a lactose-inducible expression system. These VLPs have been shown to display conformational epitopes and elicit HPV16 L1-specific serum antibodies in mice [106].
Sominskaya et al., on the other hand, have engineered and produced, in Escherichia coli, chimeric VLPs consisting of the HBV core antigen (HBcAg), used as a carrier of both HBV (pre-S1) and HCV (highly conserved N-terminal core epitope) antigens [107]. Mice immunized with these bivalent chimeric VLPs generated high-titer anti-pre-S1 antibody responses and cytotoxic T lymphocyte (CTL) responses specific to both targets. VLPs were also generated following expression of truncated version of HCV core antigen (HCcAg), consisting of the N-terminal 120 amino acids, in E. coli with an average diameter of 30 nm [108]. Similarly, HCV E2 epitope fused to the C-terminus of papaya mosaic virus (PapMV) CP, following expression in E. coli, assembled into chimeric PapMV VLPs [109], [110]. In another study, PapMV CP was used to produce chimeric VLPs displaying lymphocytic choriomeningitis virus (LCMV) immunodominant CTL p33 epitope [111]. The chimeric PapMV-HCV VLP-vaccinated mice developed long-lasting (more than 120 days) epitope-specific antibody responses [109], while the PapMV-LCMV VLPs induced maturation of dendritic cells which efficiently processed and cross-presented p33 epitope to CTLs in vitro and in vivo, and protected immunized mice against LCMV challenge [111].
Another example of chimeric non-enveloped VLPs is a malaria vaccine candidate engineered and produced in E. coli by Apovia, Inc. (San Diego, CA). These chimeric VLPs consist of recombinant HBcAg carrying genetically fused central repeat regions from P. falciparum circumsporozoite protein (CSP) containing an immunodominant B cell epitope, an HLA-restricted CD4+ T cell epitope and a universal T cell epitope. The vaccine (MalariVax) has been shown to elicit anti-CSP antibodies in mice and monkeys and CSP-specific CD4+ T cell responses in mice [112], [113]. The vaccine was well tolerated by humans, and generated CSP-specific antibodies and cellular immune responses in vaccinees in Phase 1 clinical trials when administered in the presence of different adjuvants (Table 2 ) [24], [114]. This strategy was also employed by Sanofi Pasteur (Lyon, France) for developing a universal influenza vaccine based on M2e, a highly conserved epitope in both human and avian influenza A viruses, displayed on the surface of HBcAg VLPs. Animals immunized, parenterally or intranasally (IN), with these chimeric M2e VLPs were protected against lethal challenge with various influenza A virus subtypes, and protection was enhanced by the use of adjuvants, which correlated with higher anti-M2e antibody responses. The immunogenicity of the HBcAg VLP-based M2e vaccine was confirmed in a Phase 1 clinical trial (Table 2) [115].
Table 2.
VLP vaccines on the market and in the clinical development.
| Vaccine name | Company/institution | Route of administration (adjuvant) | Expression system | VLP platform | VLP type | Vaccine antigen | Clinical development stage | Reference |
|---|---|---|---|---|---|---|---|---|
| Allergic rhinoconjuctivitis and asthma | ||||||||
| CYT003-QβG10 | Cytos Biotechnology | SC (none) | Bacteria (E. coli) | Qβ | Non-enveloped | G10 (CpG DNA) | Phase 2 | [412], [413], [414], [415] |
| Alzheimer's disease | ||||||||
| CAD106 | Cytos Biotechnology/Novartis | SC, IM (unspecified) | Bacteria (E. coli) | Qβ | Non-enveloped | Aβ1-6 | Phase 2 | [117], [416], [417], [418] |
| Breast cancer | ||||||||
| Pevion Biotech | IM (none) | Cell-free | Influenza virosome | Enveloped | Her2/neu | Phase 1 | [399] | |
| C. albicans | ||||||||
| PEV7 | Pevion Biotech | IM (none) | Cell-free | Influenza virosome | Enveloped | C.a. SAP2 | Phase 1 | [398], [419] |
| Type II diabetes mellitus | ||||||||
| CYT013-IL1bQβ | Cytos Biotechnology | SC (none) | Bacteria (E. coli) | Qβ | Non-enveloped | IL-1β | Phase 1/2a | [420], [421] |
| Hepatitis A | ||||||||
| Epaxal® | Crucell | IM (none) | Cell-free | Influenza virosome | Enveloped | Inactivated HAV RG-SB | Licensed | [25] |
| Hepatitis B | ||||||||
| GenHevac B® | Pasteur-Merieux Aventis | IM (aluminum hydroxide) | Mammalian (CHO cells) | HBsAg | Non-envelopeda | HBsAg | Licensed | [21], [407], [422] |
| Bio-Hep-B® (Sci-B-Vac®) | BTG (SciGen, FDS Pharma) | IM (aluminum hydroxide) | Mammalian (CHO cells) | HBsAg | Non-enveloped | HBsAg | Licensed | [423] |
| DTP-Hep B® | P.T. Bio Farma | IM (aluminum hydroxide) | Yeast (P. pastoris) | HBsAg | Non-enveloped | HBsAg | Licensed | [424], [425] |
| Engerix-B® | GSK | IM (aluminum hydroxide) | Yeast (S. cerevisiae) | HBsAg | Non-enveloped | HBsAg | Licensed | [53], [408], [426] |
| Enivac HB® | Panacea Biotec | IM (aluminum hydroxide) | Yeast (P. pastoris) | HBsAg | Non-enveloped | HBsAg | Licensed | [427] |
| Euvax B® | LG Life Sciences | IM (aluminum hydroxide) | Yeast (S. cerevisiae) | HBsAg | Non-enveloped | HBsAg | Licensed | [428] |
| Gene Vac-B® | Serum Inst. of India | IM (aluminum hydroxide) | Yeast (H. polymorpha) | HBsAg | Non-enveloped | HBsAg | Licensed | [429] |
| Heberbiovac HB® | CIGB-Heber Biotec | IM (aluminum hydroxide) | Yeast (P. pastoris) | HBsAg | Non-enveloped | HBsAg | Licensed | [430] |
| Hepavax-Gene® | Crucell | IM (aluminum hydroxide) | Yeast (H. polymorpha) | HBsAg | Non-enveloped | HBsAg | Licensed | [431] |
| Recombivax HB® | Merck | IM (aluminum hydroxide) | Yeast (S. cerevisiae) | HBsAg | Non-enveloped | HBsAg | Licensed | [12], [432], [433] |
| Revac-B® | Bharat Biotech International | IM (aluminum hydroxide) | Yeast (P. pastoris) | HBsAg | Non-enveloped | HBsAg | Licensed | [434] |
| Shanvac-B® | Shantha Biotechnics | IM (aluminum hydroxide) | Yeast (P. pastoris) | HBsAg | Non-enveloped | HBsAg | Licensed | [435] |
| Polish Academy of Sciences/TJU | Oral (none) | Plant (Tg lettuce) | HBsAg | Non-enveloped | HBsAg | Phase 1 | [27] | |
| ASA | Oral (none) | Plant (Tg potato) | HBsAg | Non-enveloped | HBsAg | Phase 1 | [28] | |
| Hepatitis C | ||||||||
| Pevion Biotech | IM (none) | Cell-free | Influenza virosome | Enveloped | HCV peptides | Phase 1 | [391] | |
| HIV | ||||||||
| British Biotech Pharmaceuticals/NIAID | SC/IM, IM (aluminum hydroxide)/oral or rectal (none) | Yeast (S. cerevisiae) | Ty p1 | Non-enveloped | HIV-1 Gag p17/p24 | Phase 2 | [130], [131], [132] | |
| MYM-V101 | Pevion Biotech/Mymetics Corporation | IM, IN (none) | Cell-free | Influenza virosome | Enveloped | HIV-1 gp41 | Phase 1 | [401] |
| HPV | ||||||||
| Gardasil® | Merck | IM (aluminum hydroxyphosphate sulphate) | Yeast (S. cerevisiae) | HPV | Non-enveloped | HPV6/11/16/18 L1 | Licensed | [13] |
| Cervarix® | GSK | IM (aluminum hydroxide & MPL) | Insect (High Five™ cells) | HPV | Non-enveloped | HPV16/18 L1 | Licensed | [11] |
| V503 | Merck | IM (none) | Yeast (S. cerevisiae) | HPV | Non-enveloped | HPV6/11/16/18/31/33/45/52/58 L1 | Phase 3 | [436] |
| Human parvovirus B19 | ||||||||
| VAI-VP705 | NIH/Meridian Life Science | IM (MF59) | Insect (Sf-9 cells) | B19 | Non-enveloped | B19 VP1, VP2 | Phase 1/2 | [247], [248], [249], [250], [437] |
| Hypertension | ||||||||
| CYT006-AngQβ | Cytos Biotechnology | SC (none) | Bacteria (E. coli) | Qβ | Non-enveloped | Ang II | Phase 2 | [118], [119], [120], [438], [439], [440] |
| Influenza | ||||||||
| Novavax | IM (none) | Insect (Sf-9 cells) | Influenza virus | Enveloped | A/California/04/09 (H1N1) HA, NA | Phase 2 | [23] | |
| Medicago | IM (none) | Plant (transient N. benthamiana) | Influenza virus | Enveloped | A/California/04/09 (H1N1) HA | Phase 1 | [378] | |
| Novavax | IM (none) | Insect (Sf-9 cells) | Influenza virus | Enveloped | A/Indonesia/05/05 (H5N1) HA, NA | Phase 1/2a | [228] | |
| Medicago | IM (aluminum hydroxide) | Plant (transient N. benthamiana) | Influenza virus | Enveloped | A/Indonesia/05/05 (H5N1) HA | Phase 2 | [22], [377], [441] | |
| Novavax | IM (none) | Insect (Sf-9 cells) | Influenza virus | Enveloped | A/Brisbane/59/07 (H1N1), A/Brisbane/10/07, B/Florida/04/06 (H3N2) HA, NA | Phase 2a | [442] | |
| Inflexal® V | Crucell | IM (none) | Cell-free | Influenza virosome | Enveloped | A (H1N1), A (H3N2), B, HA, NA | Licensed | [26] |
| ACAM-FLU-A | Sanofi Pasteur | IM (Stimulon QS21) | Bacteria (E. coli) | HBcAg | Non-enveloped | Influenza A M2e | Phase 1 | [33], [115] |
| Malaria (P. falciparum) | ||||||||
| MalariVax (ICC-1132) | Apovia | IM (aluminum hydroxide, Montanide) | Bacteria (E. coli) | HBcAg | Non-enveloped | P.f. CSP | Phase 1 | [24], [114] |
| RTS,S | GSK/PATH Malaria Vaccine Initiative & Gates Found | IM (AS01, AS02) | Yeast (S. cerevisiae) | HBsAg | Non-enveloped | P.f. CSP | Phase 3 | [14], [443] |
| PEV3 | Pevion Biotech | IM (none) | Cell-free | Influenza virosome | Enveloped | P.f. CSP, AMA-1 | Phase 1/2 | [29], [30], [31], [32] |
| Malignant melanoma | ||||||||
| CYT004-MelQβG10 | Cytos Biotechnology | IM (±Montanide, Imiquimod) | Bacteria (E. coli) | Qβ | Non-enveloped | Melan-4, G10 DNA (CpG) | Phase 2 | [444], [445] |
| Nicotine addiction | ||||||||
| NIC002 | Cytos Biotechnology/Novartis/Duke University | SC (±aluminum hydroxide) | Bacteria (E. coli) | Qβ | Non-enveloped | Nicotine | Phase 2 | [446], [447], [448], [449], [450] |
| NV | ||||||||
| Baylor College of Medicine | Oral (none) | Insect (Sf-9 cells) | NV | Non-enveloped | NV CP | Phase 1 | [189], [190] | |
| LigoCyte Pharmaceuticals | IM (MPL, alum) | Insect (Sf-9 cells) | NV | Non-enveloped | NV CP | Phase 1 | [193] | |
| LigoCyte Pharmaceuticals | IN (±MPL, chitosan, mannitol, sucrose) | Insect (Sf-9 cells) | NV | Non-enveloped | NV CP | Phase 1; Phase 1/2 | [191], [192] | |
| Center for Vaccine Development (University of Maryland) | Oral (none) | Plant (Tg potato) | NV | Non-enveloped | NV CP | Phase 1 | [362], [363] | |
| Rabies | ||||||||
| TJU | Oral (none) | Plant (Tg spinach) | AlMV | Non-enveloped | Rabies GP/NP | Phase 1 | [66] | |
| RSV | ||||||||
| Novavax | IM (aluminum phosphate) | Insect (Sf-9 cells) | RSV | Non-enveloped | RSV-F | Phase 1 | [236], [237] | |
Abbreviations: AlMV, alfalfa mosaic virus; AMA-1, apical membrane antigen-1; Ang II, angiotensin II; CHO, Chinese hamster ovary cells; CP, coat protein; CSP, circumsporozoite protein; GP, glycoprotein; HA, hemagglutinin; HAV, hepatitis A vaccine; HBcAg, hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; IM, intramuscular; IN, intranasal; MPL, monophosphoryl lipid A; NA, neuraminidase; N/A, not applicable; NDV, Newcastle disease virus; NP, nucleocapsid protein; NV, Norwalk virus; RSV, respiratory syncytial virus; RSV-F, RSV F protein; SC, subcutaneous; Tg, transgenic; TJU, Thomas Jefferson University.
HBsAg VLPs contain host cell lipids.
Several groups are focusing on developing bacteriophage-based VLP vaccines. For example, Cytos Biotechnology AG (Zurich-Schlieren, Switzerland) has developed a number of VLP vaccine candidates based on RNA bacteriophage Qβ. These chimeric VLP vaccines target self-antigens and are directed against non-infectious diseases such as allergies, neurodegenerative (Alzheimer's disease) and autoimmune disorders (type II diabetes mellitus), cancer (melanoma) and hypertension, and nicotine (Table 2). The candidate vaccine against Alzheimer's disease (CAD-106) was designed to present chemically coupled Aβ1-6 peptide derived from the N-terminal B cell epitope of amyloid beta (Aβ) protein, the main component of amyloid plaques found in the brains of patients with Alzheimer's disease, on the surface of VLPs formed by Qβ CP. The vaccine has been shown to induce Aβ-specific IgG antibodies and reduce amyloid accumulation in transgenic mice and in rhesus monkeys expressing Aβ precursor protein, without stimulating T cells and causing microhaemorrhages or inflammatory reactions in amyloid-containing brain tissue [116]. In a randomized, placebo-controlled trial in patients with mild-to-moderate Alzheimer's disease, CAD-106 was well tolerated and did not induce meningoencephalitis. CAD-106 vaccinated patients developed substantial titers of anti-Aβ IgG antibodies but exhibited no difference compared with placebo in cerebrospinal fluid Aβ levels or whole brain volume assessed by magnetic resonance imaging [117]. Phase 2 clinical trials of CAD-106 are ongoing (Table 2). Another VLP vaccine candidate, directed against hypertension, represents an angiotensin II-derived peptide conjugated to Qβ CP VLPs. The chimeric VLPs elicited angiotensin-specific antibodies and reduced blood pressure in hypertensive rats [118]. In a Phase 2a study in patients with mild-to-moderate hypertension, the vaccine, when administered subcutaneously (SC) in a conventional regimen with injections at weeks 0, 4 and 12, was found to be well tolerated and significantly reduce blood pressure during the daytime, especially in the morning, compared with placebo [119]. This vaccine was further evaluated in two independent trials using an alternative regimen (injections at weeks 0, 2, 4, 6 and 10) in an attempt to elicit higher antibody titers leading to the stronger reduction of blood pressure [120]. Although vaccine elicited higher antibody titers compared to the first study, due to low affinity of these antibodies the extent of decrease in blood pressure was not significant compared to placebo. Thus, a higher dose of vaccine (2.7 mg vs. 1.5 mg) was not found to be more efficacious.
A panel of chimeric VLP vaccine candidates has also been developed based on RNA bacteriophage AP205, displaying peptides of self-antigens (angiotensin II and gonadotropin releasing hormone) or pathogens (Salmonella typhi outer membrane protein [D2], HIV Nef and influenza A M2 protein) fused to either the N- or C-terminus of AP205 CP [103]. AP205-derived VLPs were highly immunogenic in mice: influenza M2 VLPs induced a strong, rapid-onset M2-specific antibody response and complete protection against lethal influenza virus challenge [103].
VLP formation in E. coli from VP1 structural proteins has been also documented for murine polyomavirus [121], [122] and simian papovavirus SV40 [123]. Furthermore, cowpea chlorotic mottle virus CP has been shown to be expressed and self-assembled into native virus-like VLPs in Pseudomonas fluorescens, suggesting a potential use of this CP as a vaccine carrier. However, no foreign epitope has been reported as being introduced to these VLPs [124].
3.2. Yeast
Yeast, a well-established platform for recombinant protein expression, continues to be used for VLP production. Two Merck and Co., Inc.’s licensed VLP-based vaccines, Recombivax HB® and Gardasil®, have been manufactured using this system. Despite the successes, the system has some limitations. It differs from mammalian cells in the PTM of proteins, particularly in protein glycosylation [125], [126]. Due to this and some other limitations, the system is generally used for the production of non-enveloped VLPs. Nevertheless, production of enveloped HIV-1 Pr55Gag VLPs in yeast, using Saccharomyces cerevisiae spheroplasts, has been achieved. The released spherical VLPs were encapsulated with the yeast cell plasma membrane and morphologically resembled immature HIV particles [127]. The yeast-derived HIV VLPs were efficiently taken up by DCs in vitro via both macropinocytosis and endocytosis mediated by mannose-recognizing receptors but not the mannose receptor, inducing DC maturation and secretion of IL-12 (p70). DC maturation was also enhanced by yeast membrane components, likely via Toll-like receptor 2 signaling, contributing to stimulation of Gag-specific immune responses. Furthermore, DCs loaded with yeast-derived HIV VLPs have been shown to convert Gag-specific memory CD8+ T cells into effector cells in chronically HIV-infected individuals via cross-presentation, although some Gag-specific T cell populations in these patients remained unresponsive [128]. An alternative HIV-1 VLP vaccine candidate has been produced in yeast as a chimeric non-enveloped VLP by fusing HIV-1 p17 and p24 proteins to p1 protein of S. cerevisiae retrotransposon Ty [129]. The resulting HIV-1 p17/p24:Ty chimeric VLPs elicited HIV-specific serum antibodies in rabbits [129] and were immunogenic when evaluated in prophylactic clinical studies [130], [131], but were not effective as a therapeutic vaccine (Table 2) [132].
A number of studies have demonstrated the expression and self-assembly of HPV L1 protein into VLPs in yeast [37], [133], [134], [135], [136], [137], [138], [139], [140]. HPV L1 VLPs have been shown to be immunogenic in mice [135] and also to induce both systemic and mucosal neutralizing antibodies as well as Th1 and Th2 cytokine responses in vaccinated non-human primates [136], [141]. Furthermore, in a proof-of-principle study, cottontail rabbit papillomavirus L1 VLPs produced in S. cerevisiae successfully protected animals from virus-induced papilloma formation [142]. The first licensed HPV vaccine approved by the U.S. FDA, Gardasil® (Merck, USA), is a quadrivalent vaccine containing L1 VLPs from HPV types 6, 11, 16 and 18 produced in S. cerevisiae and administered intramuscularly (IM) in three doses with aluminum hydroxyphosphate sulphate as an adjuvant (Table 2) [13]. Several randomized, placebo-controlled Phase 2 and 3 clinical trials evaluating Gardasil® have demonstrated safety, strong immunogenicity and excellent prophylactic efficacy against persistent HPV infection and genital diseases associated with the four types of HPV present in the vaccine in young uninfected women [143], [144], [145], [146], [147], [148], [149].
CP of norovirus (or NV), the leading etiological cause of outbreaks of non-bacterial gastroenteritis in humans, expressed in Pichia pastoris has also been demonstrated to self-assemble into VLPs. Oral immunization of mice with raw yeast extract containing these VLPs stimulated both systemic and mucosal immune responses [150].
In a similar approach, production of VLPs consisting of structural proteins VP2, VP6 and VP7 of RV, the most common cause of severe gastroenteritis in infants and young children worldwide, in S. cerevisiae has been achieved by optimizing molecular and process strategies, representing the first report on the co-expression of the three RV proteins and their assembly into multilayered VLPs [151].
One of the antigens frequently used for VLP-based vaccine production is HBsAg. HBsAg has been expressed in P. pastoris, S. cerevisiae and Hansenula polymorpha [53], [54], [55], [152], [153], [154], [155]. Although high-level retention and accumulation of HBsAg in the ER have been documented, neither secretion of the protein nor intracellular VLP formation were observed, suggesting that VLP assembly took place during downstream processing of yeast biomass [62]. A number of hepatitis B vaccines licensed worldwide are based on HBsAg S protein-formed VLPs expressed in yeast, including the U.S. FDA-approved Engerix-B® (GSK, Belgium) [10] and Recombivax HB® (Merck, USA) [12], both manufactured in S. cerevisiae, that generate potent protective anti-hepatitis B antibody responses (Table 2). Another HBsAg VLP-based vaccine candidate, malaria CSP antigen fused to HBsAg, is undergoing clinical development. GSK (Rixensart, Belgium), along with the PATH Malaria Vaccine Initiative and the Bill and Melinda Gates Foundation, manufactured a malaria VLP vaccine candidate, RTS,S, by producing a mixture of native HBsAg and chimeric HBsAg, containing a 200-amino acid fragment of CSP, in S. cerevisiae. Clinical efficacy of the adjuvanted RTS,S vaccine – from protection against experimental challenge with P. falciparum sporozoites in healthy adults to protection against clinical and severe malaria in young children – has been demonstrated in several trials (Table 2) [14], [63].
Similarly, VLPs consisting of entire HCcAg, produced in methylotrophic yeast P. pastoris, have been demonstrated to resemble authentic HCV nucleocapsid particles at a mature stage, judged by size and morphology, and were specifically recognized by serum from a chronic HCV carrier patient [156].
The P. pastoris expression system, based on the constitutive promoter of glyceraldehyde-3-phosphate dehydrogenase, has been used for high-level expression of the pre-membrane and envelope (E) glycoproteins of dengue virus type 2 (DENV-2) [157] and HBsAg [158], [159], leading to the assembly and release of VLPs similar to authentic virions, as confirmed by electron microscopy (EM). In immunized mice, DENV-2 VLPs have been shown to induce strong antibody responses with neutralizing activity at similar titers to that achieved following immunization with inactivated DENV-2 virus [157].
VLPs formed by self-assembly in S. cerevisiae of the Gag protein of non-infectious dsRNA mycovirus L-A have also been exploited as a vaccine platform for displaying a C-terminally fused truncated immunodominant pp65 protein from human cytomegalovirus (HCMV) inside the particle. When tested in vitro, these chimeric VLPs were able to activate antigen-specific memory CD8+ T cells from blood cells collected from HCMV-positive donors, suggesting epitope cross-presentation to the MHC class I pathway [160].
VP1 proteins of polyomaviruses from humans (JC polyomavirus and serotypes AS and SB of BK polyomavirus), rhesus monkeys (SV40), hamsters (hamster polyomavirus), mice (murine polyomavirus) and birds (budgerigar fledgling disease virus and goose hemorrhagic polyomavirus) have also been expressed at high levels in S. cerevisiae and have shown varying cross-reactivity with heterologous mouse and rabbit sera [161] and an ability to cause hemagglutination of erythrocytes [162].
3.3. Insect cells
Another system that has been extensively utilized for VLP production is the baculovirus-insect cell expression system. Similar to yeast, insect cells have been used to produce a number of VLP-based vaccines, in particular, one of the current HPV vaccines, Cervarix®. Insect cells can be used for manufacturing both non-enveloped and enveloped VLPs. Enveloped VLP vaccines produced in insect cells are among the most advanced in clinical development. The insect cell system possesses eukaryotic-type PTMs including glycosylation, accommodates high-level accumulation of foreign proteins and lacks mammalian pathogens [75]. Contamination of target with co-produced enveloped baculovirus particles [163] is the main limitation of this system, requiring the development of more complex schemes for VLP purification.
Insect cell-produced VLPs have been targeted for developing a vaccine against HIV. When expressed in recombinant baculovirus-infected Spodoptera frugiperda (Sf)-derived cells, the precursor Pr55Gag polyprotein of HIV-1 self-assembled into large enveloped VLPs [81], [164] which were highly immunogenic eliciting both innate and acquired immune responses [5], [165]. In particular, Pr55Gag VLPs induced potent and long-lived (>8.5 months) Gag-specific CTL responses against multiple naturally processed HIV-1 Pr55Gag epitopes in non-human primates [7]. Cellular immune responses were substantially enhanced when HIV-1 Gag VLPs were administered to mice or baboons as a boost after priming with an HIV-1 gag DNA vaccine [166], [167]. In addition, in baboons the prime/boost vaccination induced high titers of serum p24 antibodies which were several-fold higher than those induced after a single administration of the DNA vaccine or VLPs [167].
Additionally, the ability of Gag protein to serve as an antigen carrier has been utilized for the construction of various chimeric VLPs expressing antigens fused to non-structural HIV Env with the aim to broaden immune responses. Chimeric Gag VLPs that have been produced using baculovirus vectors contain HIV-1 gp120 glycoprotein [168] or its CD4-binding domain [169]; gp120, Nef and Pol [85]; reverse transcriptase (RT), Tat and Nef [86], [170]; or gp140 or gp41 proteins [87]. Furthermore, co-expression of furin along with SIV Pr56Gag resulted in the incorporation of gp120 and gp41, the cleavage products of Env gp160, into assembled VLPs [90]. With the fused antigens, the employment of the chimeric VLP approach broadened the breadth and magnitude of immune responses. Chimeric HIV Pr55Gag VLPs carrying either the whole gp120 protein or its CD4-binding domain (the third variable domain [V3]), in the absence of adjuvants, have been shown to raise a strong antibody response against Pr55Gag, a neutralizing antibody response against gp120 and a potent CTL response against a gp120 epitope located in the V3 domain in mice [168], [169]. Immunization of rhesus macaques with chimeric simian-human immunodeficiency virus (SHIV) VLPs containing SIV Pr56Gag and HIV-1 gp120 proteins generated gp120-specific neutralizing antibodies and cellular immune responses and resulted in accelerated viral clearance in monkeys infected with SHIV-4 compared with non-vaccinated controls [171]. HIV-2 Pr54Gag VLPs carrying the V3 region of HIV-1 gp120 induced both anti-gp120 antibodies with neutralizing activity in rabbits and a strong gp120 epitope-specific CTL response [172]. DNA priming followed by boosting with chimeric HIV-1 VLPs expressing RT, Tat and Nef induced strong, broad Gag- and RT-specific cellular immune responses in mice that were two- to three-fold greater than after two administrations of DNA vaccine and stimulated both antigen-specific CD8+ and CD4+ T cells in contrast to non-chimeric HIV-1 Gag VLPs that induced predominantly robust Gag-specific CD4+ T cell responses [86], [170]. VLPs expressing HIV Env gp140 or gp41 glycoproteins with the native trimeric conformation elicited neutralizing antibody responses, and combining these sera had a synergistic effect on the neutralization of HIV pseudoviruses [87].
Immunogenicity of VLPs can also be enhanced by incorporating immunostimulatory adhesion molecules and cytokines. For example, membrane-bound CD40 ligand (CD40L) was expressed on the surface of SIV and HIV-1 VLPs to target and activate DCs via the CD40–CD40L interaction with the aim of enhancing CD4+ and CD8+ T cell responses [16], [173]. The activity of CD40L-displaying HIV-1 VLPs was monitored by recording an increase in IL-12 cytokine production by DCs in vitro and enhanced levels of both humoral and cellular immune responses against HIV-1 p24 protein in immunized mice, an effect not observed in immunized CD40-knockout mice [16]. In another study, chimeric SIV VLPs containing Gag, Env and membrane-anchored granulocyte-macrophage colony-stimulating factor (GM-CSF) have been generated and used to immunize mice. They significantly enhanced the production of SIV Env-specific antibodies with neutralizing activity and CD4+ and CD8+ T cell responses, by comparison with non-chimeric SIV VLPs, SIV VLPs containing CD40L, non-chimeric SIV VLPs mixed with soluble GM-CSF, or non-chimeric SIV VLPs [173].
Insect cells are equally effective in producing non-enveloped VLPs. Expression of HPV11 L1 or HPV16 L1 or L1 plus L2 proteins in baculovirus-infected Sf-9 insect cells has been demonstrated to result in the successful self-assembly of VLPs [174], [175] that elicited high titers of serum neutralizing antibodies in mice and rabbits following IM or SC immunization with no adjuvant present [174], [176]. Furthermore, HPV16 L1 VLPs have been shown to induce both systemic and cellular immunity in mice when administered IN or orally in the presence of cholera toxin or CpG, respectively, as mucosal adjuvants [177], [178]. Cervarix® manufactured by GSK (Belgium) [11] is a bivalent HPV L1 VLP-based vaccine against HPV types 16 and 18 produced in Trichoplusia ni (High Five™) insect cells and approved by the U.S. FDA in 2009 (Table 2). Similar to the situation with Gardasil®, randomized clinical trials showed excellent prophylactic efficacy of Cervarix® in young women [179], [180], [181].
Because of HPV type-specific immunogenicity and a lack of therapeutic efficacy in licensed HPV vaccines in HPV-infected individuals, attempts are being made to develop cross-protective and therapeutic HPV vaccine candidates. Chimeric HPV16 VLPs containing E7 protein fused to the C-terminus of either truncated L1 protein or L2 protein were produced in Sf-9 and High Five™ insect cells and shown to protect mice from E7-expressing tumor challenge even in the absence of adjuvant [182], [183]. The protection appears to be mediated by MHC class I-restricted CTLs, since it did not occur in β2-microglobulin- or perforin-knockout mice [182]. In contrast to tumor protection observed in animal studies, only a trend to a histological improvement has been observed in HPV16-infected patients with high-grade cervical intraepithelial neoplasia who received SC chimeric HPV16 L1-E7 VLPs, although both humoral and cellular immune responses were induced [184]. In another approach, insertion of the cross-reactive epitopes from HPV16 L2 into L1 VLPs has been found to result in the induction of cross-neutralizing antibodies against other types of HPV in immunized rabbits [185], [186].
VLPs consisting of the 58 kDa NV CP were similar to native capsids in size, appearance and antigenicity, and elicited high levels of NV-specific serum antibodies in mice, rabbits and guinea pigs following IM immunization [44]. Subsequent studies demonstrated that oral and IN immunization with NV CP VLPs induced both systemic and mucosal antibody responses in mice [187], [188], and orally administered NV CP VLPs were safe and immunogenic in humans, inducing both humoral and cellular immune responses [189], [190]. In several clinical trials, administration of a dry powder comprising NV VLP vaccine adjuvanted with monophosphoryl lipid A (MPL) and mucoadherent chitosan (LigoCyte Pharmaceuticals, Bozeman, MT) has been shown to be well tolerated, elicit antibodies and antibody-secreting cells expressing homing receptors for mucosal and peripheral lymphoid tissues [191], and confer protection against experimental human NV illness [192]. In another Phase 1 clinical trial, a bivalent Norovirus VLP vaccine formulation containing MPL and alum (LigoCyte Pharmaceuticals) was delivered via an IM route and was demonstrated to be highly immunogenic, stimulating both IgG and IgA antibodies after the first vaccine dose (Table 2) [193].
Another important pathogen targeted for developing a VLP-based vaccine is RV. Several reports have demonstrated self-assembly of different types of RV VLPs from capsid proteins expressed in baculovirus-infected insect cells. Expression of single VP2 protein resulted in the formation of empty core-like particles, while co-expression of VP2 and VP6 from heterologous RV strains (bovine and simian) yielded assembled, double-layered VLPs [38]. The central role of VP2 in RV VLP assembly was further confirmed by expressing different combinations of VP proteins and characterizing the resultant VLPs [194]. In another study, double- and triple-layered VLPs were observed when VP2 was co-expressed with VP6 or with VP6 and VP7, respectively, with or without VP4 [79], [195], [196]. The triple-layered VLPs were morphologically similar to native RV virions, and the VP2/4/6/7 VLPs retained both neutralizing and non-neutralizing epitopes on VP4 and VP7 as well as hemagglutination activity [79].
Double-layered VP2/6 RV VLPs delivered via IN, oral, IM, intraperitoneal (IP) or intrarectal routes have been demonstrated to induce systemic and mucosal immune responses and confer protection against RV challenge in mice [20], [197], [198], [199], [200], [201], [202], [203], suggesting a key role for VP6 in protective immunity against RV. In contrast, in the gnotobiotic piglet model VP2/6 VLPs with adjuvant induced antibody responses but did not confer protection [204], and when the animals were primed with live attenuated human RV, the protection rate was only 70% as determined by reduction in RV shedding [205]. As an alternative strategy, partial protection (up to 40%) against RV was achieved by IN vaccination of piglets with adjuvanted RV VLPs containing VP7 and VP8 antigens [15].
Similarly, infection of insect cells with recombinant baculoviruses encoding HBcAg has been shown to result in high-level expression of the protein and self-assembly into VLPs in Sf cells [206]. The assembly of HCV VLPs in insect cells has been achieved by expressing HCV core protein, envelope proteins E1 and E2, and p7 [91], as described above in Section 2.2. Immunization with the HCV VLPs induced both high titers of virus-specific antibodies and virus-specific CD4+ and CD8+ T cell responses in mice [207] and baboons [208], and conferred protection against recombinant HCV-vaccinia virus infection in mice, which was CD4+ and CD8+ T cell dependent [6]. VLPs composed of C, E1 and E2 proteins have also been produced and elicited HCV-specific serum antibodies in immunized mice [209].
Furthermore, expression of an N-terminally truncated version of the 50 kDa capsid protein of HEV has been shown to result in VLP assembly in Sf-9 and T. ni-derived Tn5 cells, and the release of VLPs was confirmed by EM. These HEV VLPs were a little smaller than native HEV virions but possessed similar antigenicity and bound to HEV-specific IgG and IgM antibodies [45]. Oral administration of mice with HEV VLPs without adjuvant generated systemic IgG and intestinal IgA antibodies which reacted with native HEV antigen [210]. In cynomolgus monkeys, oral immunization with HEV VLPs induced systemic antibody production and protection against infection and hepatitis following HEV challenge [211].
Significant effort, particularly during the last several years, has been placed on developing VLP-based influenza vaccines. As described above in Section 2.2, A/Udorn/72 (H3N2) and A/Hong Kong/1073 (H9N2) VLPs based on the self-assembly of influenza M1 protein in different combinations with other influenza proteins (HA, NA and M2) have been produced in Sf-9 cells [89], [92], [93]. Subsequent studies reported the production of M1-based VLPs for other influenza strains, including A/Fujian/411/2002 (H3N2) [212], A/PR/8/34 (H1N1) [213], the highly pathogenic strains 1918 pandemic H1N1 [214] and avian influenza H5N1 [215], [216], and swine influenza A/California/04/2009 (H1N1) [217], [218], [219]. In addition to the listed monovalent influenza VLP vaccines, bivalent VLPs have also been prepared by individually producing and admixing VLPs containing HA antigens from A/PR/8/34 (H1N1) and A/Aichi/2/68 (H3N2) influenza viruses [220], and trivalent VLPs have been produced by co-expressing three HA subtypes from H5N1, H7N2 and H2N2 or H1N1, H3N2 and type B influenza viruses [221], [222]. Furthermore, co-expression of a membrane-anchored form of flagellin, the Toll-like receptor 5 ligand, with M1 and HA from A/PR/8/34 (H1N1) influenza resulted in assembly of VLPs containing an internal adjuvant component [223]. All of these insect cell-produced influenza VLPs have been shown to contain uncleaved HA molecules [82]. Binding of influenza VLPs to the cell membrane was shown to result in the VLP-cell fusion (at fusogenic pH) and VLP endocytosis mediated by interaction of HA and the cell sialylated receptor, contributing to VLP antigen presentation [224].
The immunogenicity of influenza VLPs has been explored in a number of animal studies and also evaluated ex vivo. IM or IN immunization with VLPs derived from influenza A/Udorn/72 (H3N2) elicited HA-specific serum antibody titers comparable to those induced by sublethal doses of live virus, and completely protected against lethal H3N2 influenza virus challenge [93]. VLPs derived from the avian A/Hong Kong/1073 (H9N2) strain given IM or SC to mice, rats and ferrets generated high antibody titers as detected by an enzyme-linked immunosorbent assay (ELISA) and the hemagglutination-inhibition (HAI) assay, and provided partial protection against H9N2 influenza virus challenge [92], [225]. IN immunization with VLPs based on the A/PR/8/34 (H1N1) influenza strain induced high serum and mucosal antibody titers, including neutralizing antibodies, against both homologous and heterologous viruses, and mice were protected against lethal challenge with either homologous or heterologous virus, even five months post-vaccination. Moreover, immune sera also conferred complete protection upon IN transfer. Protection was associated with suppression of pro-inflammatory cytokine release [213]. Similar long-term protective immune responses in mice have been reported for H5N1 VLPs derived from A/Vietnam/1203/04 influenza virus [226]. These H5N1 VLPs and H1N1 VLPs derived from A/California/04/2009 influenza virus each elicited high HAI antibody titers and provided highly effective protection against lethal virus challenge in mice or ferrets after a single dose vaccination [218], [219], [227]. In blinded, randomized, placebo-controlled Phase 1/2a and Phase 2 clinical trials, the unadjuvanted H5N1 (A/Indonesia/5/05) and H1N1 (A/California/04/2009) VLP influenza vaccine candidates, respectively, composed of HA, NA and M1 and manufactured by Novavax, Inc. (Rockville, MD), were found to be safe and well tolerated and to elicit robust HAI and neutralizing antibody responses in healthy adults (Table 2) [23], [228]. Notably, antibodies induced by H5N1 VLPs have been shown to cross-react with multiple clades of avian H5N1 influenza virus, which correlates with preferential antibody binding to influenza HA trimers [228].
In an attempt to extrapolate the results of animal studies to humans, organ cultures of human skin explants were prepared to evaluate changes in Langerhans cells (LC) following intradermal injection of VLPs containing HA and M1 from A/PR/8/34 (H1N1) influenza virus. The results demonstrated that LCs responded to H1 influenza VLPs by morphologic changes and migration through and out of the epidermis, which is an attribute of antigen-induced LC activation [229].
The immunogenicity of influenza VLP vaccines was further explored in studies comparing VLPs with the recombinant soluble HA antigen and an inactivated influenza virus. IM vaccination of mice and ferrets with seasonal influenza strain A/Fujian/411/2002 (H3N2) VLPs generated stronger ELISA and HAI antibody responses than those induced by recombinant HA antigen or by inactivated influenza virus, and the antibodies had broader cross-reactivity with drifted H3N2 strains [212]. Mice vaccinated with H5N1 VLPs derived from highly pathogenic avian influenza clades A/Vietnam/1203/04 and A/Indonesia/5/05 also showed stronger antibody responses compared with recombinant HA, significantly increased cross-clade antibody reactivity and IFNγ responses to specific peptides, as well as enhanced protection against both homologous and cross-clade virus challenge [215]. These H5N1 VLPs also induced complete protection in ferrets against lethal challenge with either of the parental H5N1 influenza strains [216].
While VLPs demonstrated greater immunogenicity compared with recombinant HA antigen, their vaccination efficacy can be further improved by incorporating an internal adjuvant. For example, flagellin-containing H1N1 VLPs (A/PR/8/34) administered via IM or IN routes elicited stronger antibody responses in mice than standard VLPs, which were predominantly of the IgG2a subclass, and they also enhanced greater cytokine production. In addition, while both types of VLP protected mice against homologous virus challenge, only flagellin-containing VLPs protected against a heterosubtypic challenge with influenza A/Philippines (H3N2) virus [223], [230].
Another approach for achieving broad and improved protection against multiple subtypes of influenza virus is constructing VLPs containing M2 protein and using them as supplements to the inactivated influenza vaccine. Thus, in a recent study, inclusion of M2 protein into M1 protein-based VLPs resulted in complete long-term protection against lethal challenge with heterologous and heterosubtypic influenza A viruses including H1N1 (swine influenza), H3N2 and H5N1 strains, when these VLPs were used along with inactivated influenza vaccine [231].
In addition to native-like VLPs, chimeric retrovirus Gag-based VLPs containing influenza proteins were also produced in insect cells at greater quantities compared to a mammalian cell system, and resembled retroviruses by size and morphology [19], [232]. For example, murine leukemia virus Gag VLPs pseudotyped with HA and NA from H1N1, H3N2 or H5N1 influenza strains have been shown to be highly immunogenic in mice following IM immunization. H5N1-pseudotyped VLPs induced strong cross-clade HAI antibody responses in mice and ferrets and conferred complete protection against homologous virus challenge. Notably, H1N1-pseudotyped VLPs also induced H5-specific neutralizing antibodies and significant heterosubtypic protection in ferrets, the mechanism of which is not known [232]. Another VLP, a SIV-HA chimera, has been demonstrated to directly bind to B cells in vitro and stimulate their activation, proliferation, class switch recombination and somatic mutation [19]. Following IP mouse immunization, these VLPs induced germinal center formation, conventional B cell proliferation and preferential IgG2a antibody production, confirming results obtained in vitro.
Human RSV is the most important cause of acute respiratory infection in infants and children [233] as well as elderly and immunocompromised adults [234], with potential vaccines under development. Chimeric RSV VLPs have been prepared by expressing the influenza virus matrix (M1) protein core displaying F or G protein of RSV on the surface and were shown to induce IgG2a-dominant RSV-specific antibody responses in both serum and lungs and provide effective protection against RSV-A2 infection in IM vaccinated mice, as determined by the levels of lung virus loads and morbidity post-challenge [235]. However, RSV-G VLPs were found to have greater efficacy than RSV-F VLPs. Another RSV vaccine candidate, manufactured by Novavax Inc. (Rockville, MD), represents nanoparticles composed of the near full-length RSV F protein expressed in insect cells and assembled in trimers [236]. In a Phase 1, blinded, randomized, placebo-controlled clinical trial, the RSV-F VLP vaccine candidate generated strong, dose-dependent RSV-F-specific and plaque-reduction neutralizing antibody responses that were augmented in the presence of adjuvant (aluminum phosphate) [237].
Insect cell-produced VLP-based vaccine development studies are underway to target a number of other pathogens, including dengue virus [238], Rift Valley virus [239], Ebola virus [240], [241], [242], [243], [244], SARS CoV [96], [97], [245], [246], human parvovirus [247], [248], [249], [250], poliovirus [251], enterovirus 71 [252], [253], herpes simplex virus [254] and polyoma viruses [255], [256], [257], [258], [259], [260].
The approach has also been applied to developing veterinary vaccines. A variety of potential veterinary vaccines based on VLPs have been produced in insect cells, including bluetongue virus [75], [76], [78], [261], [262], chicken anemia virus [263], [264], goose hemorrhagic polyoma virus [162], infectious bursal disease virus [265], [266], [267], [268], NDV [269], porcine, mink and canine parvoviruses [39], [40], [41], [42], [43], porcine circovirus [270], rabbit hemorrhagic disease virus [271] and swine vesicular stomatitis virus [272]. Some VLP vaccine candidates, including bluetongue virus [273], bovine papillomavirus type 1 [274], chicken anemia virus [263], infectious bursal disease virus [268], porcine, mink and canine parvoviruses [39], [40], [41], and porcine encephalomyocarditis virus [275], have been evaluated in the natural target hosts and found to be protective against live virus challenge. The only approved veterinary VLP-based vaccine, Porcilis® PCV (Intervet International B.V., The Netherlands; currently Merck Animal Health), is composed of porcine circovirus type 2 Cap-2 antigen produced in baculovirus-infected Sf-9 cells [276], [277], and shown to protect pigs against porcine circovirus type 2 infections [278], [279]. The vaccine has been on the market since 2009 [280].
3.4. Mammalian and avian cells
The ability to perform complex mammalian-type PTMs of recombinant proteins is the main advantage of avian and mammalian cell expression systems, while high production costs and potential safety concerns remain a challenge [281], [282]. Similar to other recombinant expression systems, mammalian cells are increasingly being used for producing VLP-based vaccine candidates against an array of pathogens.
As mentioned earlier in Sections 2.1, 3.2, HBsAg VLPs have been successfully produced in mammalian cells (Chinese hamster ovary [CHO] cell line) [56], [61]. The mammalian cell-produced HBsAg VLPs were similar to the plasma-derived HBsAg VLPs in composition and protein glycosylation and to the yeast-produced VLPs by the lipid content. They were larger in size and consisted of greater number of monomers compared to the yeast-derived VLPs, contributing to higher immunogenicity of mammalian-origin VLPs [59], [60]. Additionally, in contrast to HBsAg VLPs of yeast origin [62], CHO cell-produced HBsAg VLPs were shown to undergo intracellular assembly and packaging in the ER and to have a tubular structure [61].
In addition to single-antigen VLPs containing only HBsAg S protein, multi-antigen VLP vaccines, containing either HBsAg M and S (pre-S2/S domains) or HBsAg M, L and S proteins (pre-S1/pre-S2/S domains), have been produced in CHO cells and were demonstrated to be safe and immunogenic in neonates [21], [283], [284]. Due to the presence of broader-range HBsAg epitopes, these vaccines were believed to be more immunogenic and fast acting and to provide better protection compared with VLP vaccines containing HBsAg S protein alone [285], [286]. Two such vaccines, GenHevac B® and Sci-B-Vac® (formerly, Bio-Hep-B™), developed in France and Israel, respectively, are on the market (Table 2). Hepacare®, another multi-antigen HBsAg VLP vaccine, indicated for immunization of non-immune adults of ≥18 years of age, was developed by Medeva Pharma Ltd (United Kingdom) and received marketing authorization in Europe, which was subsequently voluntarily withdrawn by the company for commercial reasons [287].
Another target of mammalian cell-produced VLP vaccines is DENV, a causative agent of dengue fever. Production of DENV VLPs resembling DENV virions by size and morphology has been achieved by co-expressing prM and E structural proteins in COS-1, CHO or 293T mammalian cell lines [288], [289], [290] and includes incorporation of the ER retention signal into the stem-transmembrane region of E protein [289], [291], [292]. However, the immunogenicity and protective efficacy of these VLPs were assessed by using DNA vaccines encoding the proteins [288]. A similar vaccination approach has been applied towards other flaviviruses including Murray Valley encephalitis virus, Japanese encephalitis virus and West Nile virus [293], [294], [295], [296]. Subviral particles composed of prM and E proteins of Murray Valley encephalitis virus were able to protect vaccinated mice against lethal challenge with the virulent wild-type virus strain and protection correlated with the development of neutralizing antibodies. In contrast, cell-associated recombinant E protein neither protected mice nor induced neutralizing antibodies [297]. In a recent study, VLPs of four different DENV serotypes, DENV1-4, were evaluated as vaccine candidates in mice, and monovalent VLPs of each serotype stimulated specific IgG responses and potent neutralizing antibodies against homotypic virus, while tetravalent VLPs induced specific IgG and neutralizing antibodies against all four serotypes of DENV. In addition, both monovalent and tetravalent VLPs generated virus-specific CTL responses [290].
Other challenging disease agents for which mammalian cell expression systems have been applied for vaccine candidate production include Ebola and Marburg filoviruses, infection with which results in manifestation of deadly, rapidly progressive hemorrhagic fever, and for which no approved vaccines are available. VLPs targeting these viruses have been generated using the cDNA-transfected human embryonic kidney (HEK) cell expression system. Both types of VLPs comprised viral matrix VP40 protein or VP40 and an envelope glycoprotein, and closely resembled distinctively filamentous parental viral particles as shown by EM [298], [299], [300], [301], [302], [303]. VLPs targeting either Ebola or Marburg fever were highly immunogenic and protective against viral challenge in animal models. Ebola VLPs have been shown to activate mouse DCs in vitro and CD4+ and CD8+ T cells as well as CD19+ B cells in vivo [302]. Vaccination of mice with VLPs targeting Ebola fever elicited high levels of Ebola virus-specific antibodies, including neutralizing antibodies, and conferred complete protection against lethal Ebola virus challenge [302], [304]. Protection depended on activated natural killer cells [305] and required both humoral and CTL Ebola virus-specific responses [306]. VLPs targeting Marburg fever induced CD4+ T cell-dependent proliferative responses in vitro upon re-exposure to the homologous antigen, and in vaccinated guinea pigs elicited high titers of Marburg virus-specific antibodies, including neutralizing antibodies, and conferred complete protection against lethal Marburg virus challenge [304]. Taken together, strong immunogenicity and protective capacities of Ebola and Marburg VLPs make them promising vaccine candidates for deadly filovirus infections.
Hantaan virus, an etiologic agent of hemorrhagic fever with renal syndrome, is another pathogen that requires development of medical countermeasures including vaccines. To this point, VLPs containing NP and two envelope glycoproteins of Hantaan virus have been produced in recombinant vaccinia virus-infected Vero E6, baby hamster kidney (BHK) or CHO cells [307], [308]. The resulting VLPs have demonstrated immunogenicity in a mouse model, inducing antibody responses against Hantaan virus NP and glycoproteins and a cellular response against NP, following IM or SC administration [308].
Chikungunya virus, transmitted by mosquitoes, is a cause of tropical infection similar to dengue fever. There is no specific treatment for this disease and no vaccine has been licensed. Chikungunya VLPs formed by C and E1/E2 proteins were produced in HEK cells and exhibited morphology similar to Sindbis virus. Following IM vaccination, the VLPs elicited high titers of neutralizing antibodies that reacted with both homologous and heterologous strains of Chikungunya virus in mice and rhesus macaques. Furthermore, the vaccinated monkeys were protected against viremia and inflammation associated with high-dose viral challenge, and protection was antibody-mediated, as shown by passive transfer of purified total IgG from the protected monkeys to immunodeficient mice followed by sublethal viral challenge [309].
NDV is an economically significant poultry pathogen, with the currently available live vaccine being insufficiently safe and protective [310]. To explore the potential of ND VLPs as an ND vaccine, they have been produced in the ELL-0 avian fibroblast cell line transfected with cDNAs encoding HN, F, NP and M proteins of NDV strain AV [101]. Protein concentrations and ratios in the purified VLPs were comparable to those of UV-inactivated NDV, and the glycoproteins incorporated into the VLPs exhibited intact functional activity. Adjuvant-free administration of ND VLPs elicited equal or greater levels of NDV-specific and neutralizing serum antibodies and IFNγ-producing CD4+ and CD8+ T cells in immunized mice compared to inactivated NDV [101]. VLPs also formed when HN and F proteins from NDV strain B1 were incorporated [101], suggesting broad-spectrum NDV vaccine potential for ND VLPs, including for exotic NDV strains [311].
Nipah virus (NiV) has been determined to cause zoonotic disease outbreaks in South Asia associated with highly lethal febrile encephalitis in humans and a predominantly respiratory disease in pigs [312], [313], and is a potential agent of agro-terrorism [314]. To develop a NiV vaccine, chimeric NDV-based VLPs have been constructed in which NiV G protein was fused to the transmembrane (TM) and cytoplasmic (CT) domains of NDV HN protein and produced using cDNA-transfected avian fibroblasts (ELL-0 cells) [101]. NiV VLPs formed by NiV G, F and M proteins were also produced in substantial quantities in cDNA-transfected HEK cells [315]. The assembled VLPs resembled parental NiV virions by size (40–500 nm), morphology and surface composition as shown by transmission EM and cryoEM. NiV VLPs were fusogenic inducing syncytia formation, and activated innate immune defense mechanisms (NFKB2, TBK1, IL-8 and MAPK8 genes). Immunization with these VLPs without adjuvant elicited neutralizing antibody responses in mice, indicating their vaccine potential.
An approach similar to that used for NDV-based NiV VLPs has been employed to produce a recombinant RSV VLP vaccine candidate. Specifically, RSV G and F proteins were fused to the TM and CT domains of NDV HN and F proteins, respectively, and chimeric VLPs were produced in cDNA-transfected ELL-0 cells [316], [317]. The resulting NDV/RSV chimeric VLPs have been shown to stimulate Th1-biased anti-RSV antibody responses in mice and confer protection against RSV challenge [316], [317]. Alternative RSV vaccine candidates have been produced by expressing full-length RSV F or G glycoprotein on live recombinant Sendai virus (SV) in vaccinia virus-infected mammalian cells, followed by propagating the recombinant virus in eggs. SV-RSV-G VLPs elicited RSV-specific antibody and conferred protection against RSV challenge following IN inoculation of cotton rats [318]. The preparation of a recombinant SV vaccine expressing RSV F protein has also been reported, and these chimeric particles elicited RSV-specific neutralizing antibody and T cell responses in cotton rats following IN immunization. Furthermore, the vaccine also conferred protection against challenge with RSVs, even those with a mismatched origin or sequence or of different subtypes [319]. Similarly, the SV platform has been used to produce vaccines against human parainfluenza viruses (hPIVs) by displaying F protein or hemagglutinin-neuraminidase of hPIV-2 or hPIV-3, and these recombinant SV particles generated long-lasting binding and neutralizing antibody responses as well as T cell responses, and protected against hPIV challenge [320], [321]. Two other groups have constructed RSV vaccines by displaying the soluble core domain of RSV G glycoprotein (amino acids 130–230) on recombinant replication-deficient adenovirus (AdV) particles, or RSV G or F full-length proteins on defective non-propagating Venezuelan equine encephalitis virus (VEEV) replicon particles, and produced the resulting chimeric particles in mammalian cells. A single IN immunization with either of these chimeric particles induced strong serum IgG and mucosal IgA responses in mice and cotton rats and provided long-term protection against RSV challenge [322], [323]. VEEV-based RSV vaccines also induced Th1/Th2-balanced T cell responses and were more protective when displaying RSV-F [322].
Emerging strains of influenza virus are responsible for seasonal epidemics and, more rarely, pandemics. These underscore the importance of rapid, large-scale development of vaccines against novel virus strains. Influenza VLPs have been generated in a mammalian system by co-expressing HA and NA proteins (with or without M1 protein) in plasmid-transfected HEK cells [88] and HA, NA, M1 and M2 proteins in transfected Vero cells [324]. H3N2 and H5N1 influenza VLPs produced in Vero cells resembled the parental influenza virions by particle size and morphology, as demonstrated by transmission EM and dynamic light scattering, and by the HA glycosylation pattern, as revealed by N-deglycosylation assays [324]. Vaccination of mice with H5N1 VLPs generated H5-specific IgG1 antibodies and HAI antibodies, and conferred full protection against lethal challenge with homologous virus. In addition to single pathogen target influenza VLPs, chimeric VLPs have been produced via pseudotyping of influenza viral proteins onto retroviral Gag VLPs. Thus, pseudotyped chimeric MLV Gag particles carrying HA (mostly cleaved into HA1 and HA2 products), NA and M2 from highly pathogenic A/Chicken/FPV/Rostock/1934 (H7N1) and A/Thailand/KAN-1/04 (H5N1) avian influenza strains have been produced in transiently co-transfected HEK cells [325]. Mice vaccinated with these VLPs developed a rapid and robust subtype-specific neutralizing antibody response even after a single immunization, with no cross-reactivity between H5 and H7, as determined by immune serum neutralization of VLP transduction titers [325]. However, neutralization of live H5N1 and H7N1 viruses using an HAI assay has not yet been reported.
3.5. Plant cells
During the last two decades, plants have demonstrated great potential for the production of recombinant proteins for industrial or pharmaceutical purposes, including vaccine development. As production platforms, plant expression systems are rapid, highly scalable, cost-effective and free of mammalian pathogens. Furthermore, protein structure, assembly and PTMs in plants are similar to those in mammalian cells. Expression of recombinant proteins in plants can be achieved either via stable introduction of a transgene into the nuclear or plastid genome or by means of transient transformation using plant viral vectors [125], [126]. Similar to mammalian, insect and yeast cells, plants can be used for producing both non-enveloped and enveloped VLPs [326], [327]. Both enveloped and non-enveloped plant-produced VLP vaccine candidates have been produced at large scale under current Good Manufacturing Practice (cGMP) conditions and progressed into clinical development [22], [328].
HIV-1 VLPs formed by Gag protein have been produced in plants via stable or transient transformation. Biolistic-mediated transformation of Nicotiana tabacum with a DNA vector containing the sequence of HIV-1 Pr55Gag resulted in the high-level expression of Pr55Gag polyprotein in transgenic chloroplasts and formation of spherical Gag VLPs [329]. VLPs of 110–120 nm in diameter have also been observed following high-level transient expression of Pr55Gag in Nicotiana benthamiana agroinfiltrated using a TMV-based vector [330]. In both studies, Gag VLPs were morphologically similar to VLPs produced in insect cells. Furthermore, transgenic chloroplast-derived Gag VLPs induced a Gag-specific antibody response in mice after three IP injections, that was comparable to that elicited by the same dose of insect cell-produced Gag VLPs [331].
In addition to VLPs assembled from HIV Gag protein, bivalent HBV-HIV vaccine candidates have also been engineered. HBsAg VLPs displaying immunogenic epitopes from HIV Env-Gag were produced in transgenic tomato [332], and HBsAg VLPs carrying HIV Pol-Env-Gag epitopes were produced in transgenic N. tabacum and Arabidopsis thaliana [333]. Both types of chimeric HBV-HIV VLPs were produced at low levels. Following oral administration to mice, the Env-Gag epitope-containing VLPs generated weak humoral immune responses [333] and the Pol-Env-Gag epitope-containing VLPs induced both cellular immune responses and tolerance [334].
In transiently transformed plants, HIV immunogenic epitopes have been expressed in the form of fusions to plant virus CP that formed non-enveloped chimeric VLPs. For example, a 22-amino acid long peptide from the gp41 envelope protein of HIV-1 strain IIIB (amino acids 731–752), known to induce cross-reactive neutralizing antibodies in HIV-infected humans [335], was inserted into the βB-βC loop of S protein of CPMV [69]. The resulting chimeric VLPs were demonstrated to induce gp41-specific neutralizing serum antibodies in mice following SC immunization in the presence of alum adjuvant [69]. In addition, mice vaccinated with these VLPs in the presence of cholera toxin mucosal adjuvant via IN (but not oral) administration developed both mucosal and systemic HIV-1-specific IgA and IgG2a antibody responses [336]. In another study, a highly conservative ELDKWA epitope from gp41 has been genetically fused to the N-terminus of PVX CP, and the purified chimeric VLPs induced high titers of neutralizing HIV-1-specific IgG and IgA antibodies in mice following IP or IN delivery [73]. Notably, the VLPs were also shown to stimulate human peripheral blood lymphocytes (hu-PBL) to produce antibodies against the gp41-derived epitope as shown by immunizing hu-PBL-reconstituted severe combined immunodeficient mice with VLP-pulsed autologous DCs. Sera from both normal and humanized mice possessed an anti-HIV-1 neutralizing activity. In another study, the V3 loop from HIV-1 gp120 protein has been fused to AlMV CP and cloned into a TMV-based vector. Inoculation of N. benthamiana with the infectious in vitro synthesized transcript resulted in accumulation of chimeric viral particles that were shown to elicit antigen-specific virus-neutralizing antibodies in IP immunized mice [65]. Similarly, chimeric AlMV CP VLPs have been engineered to express a 21-amino acid peptide representing amino acids 170–190 of RSV G protein (VMR-RSV) in plants. These chimeric particles generated strong humoral and cellular immune responses in vitro, when used to pulse human DCs, as well as in vivo, when introduced into non-human primates [68]. In an earlier study, AlMV VLPs displaying this peptide elicited a potent antibody response and protection against viral challenge infection in mice [67].
A 34-member library of 24-amino acid long peptides of RSV F protein fused to the N-terminus of AlMV CP (8-amino acid overlap, spanning the whole protein) was screened for potential B cell and T cell epitopes using a DC stimulation assay and human peripheral blood mononuclear cells (PBMC). Five of these peptides have been found to elicit IFNγ secretion (CD4+T cell stimulation), one of which was recognized by all five PBMC donors used in the study [337]. An epitope-based vaccine development approach has also been employed against anthrax by fusing target peptides to CPs of plant viruses. In one study, a 25-amino acid long peptide from protective antigen (PA) of B. anthracis was fused to a surface loop of the large subunit of CPMV CP and propagated in cowpea. The resulting chimeric CPMV CP VLPs displayed 60 copies of the anthrax peptide and were generated in multi-gram quantities [71]. Another group fused the small loop 15-amino acid long epitope from domain 4 of PA (PA-D4s) to the N-terminal region of AlMV CP, and infectious transcripts of recombinant AlMV RNA3 were used to inoculate transgenic N. tabacum expressing AlMV P1 and P2 replicase genes [338]. The chimeric AlMV particles accumulated in the plants in large quantities for an extended time period. IP administration of these AlMV-anthrax VLPs elicited a detectable anthrax-specific antibody response in two out of five immunized mice, when compared with mice immunized with control recombinant AlMV or purified PA [338].
The chimeric AlMV particle platform has also been used for the development of a plague vaccine candidate. A 22-member library of 24-amino acid long peptides of Yersinia pestis F1 protein fused to the N-terminus of AlMV CP (with an 8-amino acid overlap, spanning the whole protein) was screened for potential B cell and T cell epitopes using a DC stimulation assay and human PBMC. All the ten tested Y. pestis F1 constructs stimulated CD4+ T cells, and three were recognized by PBMC from seven out of the ten donors used in the study [337]. In another study, PA-D4 was integrated into the c/e1 loop of HBcAg and the resulting chimeric protein was shown to be immunogenic in mice; however, no HBcAg-PA-D4 VLP formation was observed [339].
Fusion of a chimeric peptide containing a B cell epitope from GP and a T cell epitope from NP of rabies virus to CP of AlMV, and introduction of the sequence into N. benthamiana or spinach leaves using TMV-based vectors, resulted in replication of recombinant AlMV and transient expression of the peptides on chimeric viral particles [65], [66], [340]. Chimeric VLPs displaying the rabies peptide have been shown to accumulate in the infected leaves, and purified VLPs elicited rabies-specific neutralizing antibody responses in mice following IP immunization [65]. Notably, ingestion of raw leaf material containing these VLPs also induced anti-rabies antibody responses in human volunteers previously immunized with a conventional rabies vaccine, indicating potential as a supplementary oral booster for rabies vaccination (Table 2) [66]. A new VLP vaccine candidate for rabies has been produced in agroinfiltrated N. benthamiana plants and is under development by Medicago Inc. (Quebec, QC, Canada). In pre-clinical studies, two doses of the vaccine have been demonstrated to induce protective levels of neutralizing antibodies in mice [341].
In several studies, attempts have been made to develop a malaria vaccine using chimeric VLPs based on plant viruses such as TMV and CPMV. Selected malarial B cell epitopes have been fused to either the surface loop region or the C-terminus of TMV CP using the leaky stop signal mechanism, and co-assembly of VLPs of the CP-malaria epitope fusions and wild-type CP in tobacco plants has been demonstrated [64]. In another study, malaria merozoite peptide P109 was displayed on a small CP of CPMV, and chimeric viral particles were shown to induce peptide-specific serum antibodies in rabbits [72].
CPMV was also used to engineer and produce a human rhinovirus 14 (HRV-14) vaccine candidate. Insertion of the 14-amino acid long NIm-1A epitope from VP1 of HRV-14 into the βB-βC loop of CPMV S protein to maintain the epitope closed native-like conformation and immunogenicity [342] resulted in the production of chimeric VLPs in cowpea inoculated with infectious in vitro RNA transcripts. Rabbits immunized IM or SC with two doses of the purified chimeric VLPs in the presence of Freund's adjuvant generated VP1-specific serum antibodies [343].
Chimeric non-replicating CPMV particles expressing a peptide derived from the fibronectin-binding protein B (FnBP) D2 motif (the first 30 amino acids of the D2 domain) of Staphylococcus aureus fused to the βB-βC loop of S protein were shown to work as a mucosal vaccine against S. aureus. Mice vaccinated IN with these VLPs without adjuvant generated FnBP-specific systemic and mucosal antibody responses with a bias towards the Th1 type, including serum IgG and mucosal IgA and IgG, even at distant mucosal sites [344]. Sera of the immunized mice completely inhibited binding of human fibronectin to S. aureus FnBP. In contrast, oral immunization induced only lower antibody titers in serum and no intestinal IgA [344]. In a subsequent study, immunization with these VLPs protected rats against the development of S. aureus-associated endocarditis (P < 0.05) [345]. Opsonization of S. aureus with antibodies raised against the chimeric CPMV VLPs stimulated both neutrophil activity and macrophage phagocytosis in vitro. Furthermore, intravenous passive transfer of these antibodies protected recipient mice from bacteremia-related weight loss [345].
The surface-exposed linear B cell epitope (peptide 10) from outer membrane (OM) protein F of Pseudomonas aeruginosa was expressed in tandem with protein F peptide 18 on each of the two CPs of CPMV, small (S) and large (L). Chimeric VLPs expressing the peptides on S CP (CPMV-PAE4) and L CP (CPMV-PAE5) elicited peptide-specific antibodies in immunized mice, and CPMV-PAE5 was able to induce P. aeruginosa-specific opsonic IgG2a and confer protection against challenge with two different immunotypes of P. aeruginosa in a mouse model of chronic pulmonary infection, indicating potential for further development as a protective vaccine against the bacterium [346], [347].
CPMV-based VLPs have also been successfully used to produce a vaccine against mink enteritis virus, canine parvovirus and feline panleukopenia virus. For this, chimeric CPMV VLPs have been engineered to display the VP2 epitope shared by mink enteritis virus, canine parvovirus and feline panleukopenia virus, and produced in black-eyed bean, Vigna unguiculata. SC vaccination with these VLPs was found to protect minks against clinical disease and virus shedding after challenge with virulent mink enteritis virus [348], and dogs against lethal challenge with canine parvovirus [349]. In another study, a 17-amino acid long peptide from canine parvovirus fused to small or large subunits of CP was displayed on the chimeric CPMV particles [350]. Mice vaccinated SC or IN with these chimeric VLPs developed peptide-specific antibody responses. The immune responses were markedly Th1 biased, as determined by a predominantly IgG2a isotype secretion and the release of IFNγ but not IL-4 or IL-5 from antigen-stimulated lymphocytes in vitro. Notably, both SC and IN vaccination induced serum neutralizing antibodies and peptide-specific mucosal IgA antibodies in intestinal and bronchial lavage fluids [350]. Combination of systemic and mucosal routes for priming and boosting vaccinations did not alter the Th1 bias of the immune responses, but resulted in optimal serum and intestinal antibody production [351].
Several publications have reported the production of HPV VLPs in plants using transgenic, transplastomic or transient expression systems. HPV16 L1 was stably expressed in N. tabacum cv. Xanthi and demonstrated to assemble into capsomers and VLPs retaining the neutralizing and conformational epitopes. Rabbits immunized with these plant-produced particles developed weak anti-HPV16 L1 immune responses [352]. HPV11 L1 protein without the C-terminal nuclear localization signal sequence (to enhance mRNA stability and protein accumulation) has been produced at a high level in transgenic A. thaliana and N. tabacum cv. Xanthi. Immunization of rabbits with transgenic plant extracts induced low titers of serum antibodies that neither reacted with native HPV11 L1 VLPs nor neutralized HPV11 infectious pseudovirions [353]. Expression of plant codon-optimized HPV11 L1 protein with a deleted C-terminal nuclear localization signal sequence in transgenic potato resulted in the formation of abundant 55-nm diameter spherical VLPs resembling authentic HPV virions as shown by an EM analysis of tuber extract. Feeding mice with transgenic HPV11 L1 potato tubers elicited an anti-HPV antibody response that was markedly enhanced following oral boosting with purified HPV11 L1 VLPs produced in insect cells [354]. In another study, HPV16 L1 protein was expressed at low to modest levels in transgenic tobacco and potato and self-assembled into 55-nm VLPs, with a small portion of capsomers, in transgenic tobacco, as confirmed by EM and sucrose gradient analyses [355]. The immunogenicity of HPV16 L1 VLPs was comparable with that of HPV VLPs produced in insect cells. A few mice fed with tubers from transgenic potatoes (three out of 24 mice) developed weak anti-L1 antibody responses, but the responses were significantly enhanced following IP or oral boosting with a subimmunogenic dose of purified insect cell-derived VLPs, indicating efficient priming by plant-produced VLPs [355]. In contrast to L1 VLP formation in transgenic potato and tobacco, modified HPV16 L1 protein self-assembled into capsomers in transplastomic tobacco [356].
Transient expression of HPV16 L1 VLPs in N. benthamiana plants has been achieved using TMV, bean yellow dwarf virus (BYDV) or CPMV vectors. Inoculation of plants with infectious in vitro RNA transcripts of a TMV-based vector has been shown to result in the assembly of HPV16 L1 capsomers and VLPs retaining the neutralizing and conformational epitopes and eliciting weak anti-HPV16 L1 immune responses in rabbits [357]. A shuttle vector combining a viral replicon of BYDV, single-stranded DNA geminivirus, and a Ti plasmid of A. tumefaciens was used to rapidly produce HPV16 L1 protein [358]. The CPMV vector system was also employed to produce chimeric HPV16 L1 VLPs and capsomers bearing the M2e influenza epitope, representing the first record of successful expression of chimeric HPV16 L1 particles carrying an epitope of a heterologous virus in plants [70].
As for mammalian and insect cells, plants have been used to produce VLPs composed of NV CP. These NV CP VLPs were shown to be highly immunogenic. NV CP VLPs expressed in potato tubers were 38-nm icosahedral (T = 3) particles similar to those produced in insect cells and consistent with NV virion size, while the predominant VLP form in tomato fruit was the small 23-nm particle also observed in insect cell-derived NV CP preparations [359]. Feeding of raw transgenic potato tubers or a lyophilized powder of transgenic tomato fruits containing properly assembled NV CP VLPs elicited potent serum IgG and intestinal IgA antibody responses in mice [359], [360], [361], and feeding of uncooked transgenic potato tubers resulted in positive safety and immunogenicity data in human volunteers, inducing serum IgG and IgA responses (Table 2) [362], [363].
NV CP VLPs were also transiently expressed in N. benthamiana using either the TMV-based MagnICON deconstructed vector system or the BYDV-based shuttle vector [327], [364]. Oral immunization of mice with partially purified NV CP VLPs prepared using the MagnICON vectors has been shown to induce strong systemic and mucosal virus-specific antibody responses [327]. A recent report has demonstrated successful production of this VLP vaccine candidate under cGMP conditions for testing in a Phase 1 clinical trial [328].
VLPs composed of RV structural proteins VP2 and VP6 or VP2, VP6 and VP7 were produced in transgenic tomato and tobacco and shown to be morphologically similar to native RV particles [365], [366]. The rate of VLP assembly in tomatoes was, however, low [365]. Plant extracts containing VP2/6 and VP2/6/7 VLPs, in adjuvant, generated RV-specific systemic and mucosal antibody responses in mice when delivered orally [366], and systemic responses when administered IP [365]. Protection against RV infection was not examined in these studies.
VLPs formed by HBsAg or HBcAg were produced in both transgenic and transiently transformed plants. Naïve mice fed with HBsAg VLP-containing transgenic tobacco or potato material developed both cellular and humoral immune responses [367], [368], [369], [370]. Feeding with HBsAg-expressing transgenic lettuce or potatoes elicited anti-HBsAg serum antibodies in human volunteers who received two doses of transgenic plant material or were previously vaccinated with a licensed HBV vaccine (Table 2) [27], [28].
Other studies explored the assembly and immunogenicity of HBsAg S and M polypeptides expressed in plants. In transgenic tobacco and soybean cell suspensions as well as potato leaves and tubers, S polypeptide has been found to self-assemble into tubular membrane complexes derived from the ER, similar to the HBsAg S particles assembled in CHO cells [61], and display extensive disulfide-bond cross-linking and immunogenic epitopes [371] . Complex size distribution of these particles differentiated them from VLP preparations observed in the commercial HBsAg VLP vaccine derived from recombinant H. polymorpha [371]. Furthermore, HBsAg M polypeptide containing an additional 55-amino acid long pre-S2 region at the N-terminus of S protein was expressed in both transiently and stably transformed N. benthamiana and shown to form VLPs [359]. IP administration of purified VLPs elicited stronger serum antibody responses against HBsAg in mice compared with S protein [359].
Recombinant HBcAg expressed in transgenic tobacco leaves has been shown to assemble into spherical particles of 25–30 nm diameter which maintained two antigenic determinants of HBcAg, HBc/α and HBc/β [372]. Transient expression of HBcAg in N. benthamiana was achieved using PVX and CPMV-based vectors [373]. Although both vector systems allowed for the correct assembly of HBcAg VLPs, as confirmed by immunoEM, expression levels were low and wild-type plant virus particles were co-produced. In contrast, using the MagnICON vector system and gene optimization for plant expression, a dramatically increased level of HBcAg VLP production was achieved (up to 7% of total soluble protein or 2% of fresh weight), and 30 nm particles were observed, similar to HBcAg VLPs produced in E. coli [374]. Partially purified HBcAg VLPs were immunogenic in mice when administered IP with Imject alum adjuvant (serum IgG) or orally or IN without adjuvant (serum IgG and mucosal IgA), suggesting strong potential for HBcAg VLPs, not only as an HBV vaccine but also as a foreign epitope presentation system for mucosal delivery. Indeed, HBcAg with a fused neutralizing epitope of HPV16 L2 protein formed chimeric VLPs [375]. The BYDV-based shuttle vector has also been used to rapidly produce HBcAg VLPs in N. benthamiana [364].
Recently, enveloped VLP vaccine candidates against influenza have been transiently produced in plants using both plant viral and binary vector systems. PVX-based chimeric virus particles displaying an H-2Db-restricted epitope of influenza A NP were constructed and produced in N. benthamiana, and shown to stimulate MHC class I-restricted peptide-specific IFNγ-secreting CD8+ T cells in mice when administered without adjuvant [74].
Transient expression of HA from A/Indonesia/5/05 (H5N1), A/New Caledonia/20/99 (H1N1) and A/California/04/09 influenza virus strains has been performed by Medicago Inc. using a binary vector agroinfiltration of N. benthamiana, and HA assembly into VLPs morphologically similar to those produced in mammalian and insect cells was confirmed by EM [326], [376]. Notably, the VLP-associated lipids were shown to derive from the plant cell plasma membrane [326], whereas no budding from plasma membrane has been demonstrated for any plant viruses [376]. IM immunization with two low doses of H5-VLPs with adjuvant induced HAI antibody responses in mice that were stronger than those achieved with soluble HA antigen [326] and also induced cross-reactive HAI antibodies in ferrets [22], and conferred complete protection against lethal challenge with a heterologous H5N1 influenza virus strain in both mice and ferrets [22], [326]. Evaluation of adjuvanted H5-VLPs administered IM in two doses to healthy adults in Phase 1 and Phase 2 clinical trials demonstrated a good safety profile and strong immunogenicity, with a clear dose response (Table 2) [22], [377].
An H1-VLP vaccine candidate based on the H1N1 swine influenza virus strain has also been tested in a Phase 1 randomized placebo-controlled clinical trial involving healthy adults who received a single injection of the non-adjuvanted H1N1 VLP vaccine. At all doses tested, the vaccine was shown to be safe and well tolerated, and its strong immunogenicity was confirmed by HAI, single radial hemolysis and microneutralization antibody assays (Table 2) [378]. As with insect cell-produced VLPs, plant-derived H1N1 and H5N1 influenza VLPs are potentially of value as veterinary vaccines in swine and birds, respectively.
Foot-and-mouth disease is a severe viral infection affecting farm animals. The complete open reading frame (ORF) coding for VP1, the major immunogenic protein of foot-and-mouth disease virus (FMDV), was expressed on the surface of recombinant TMV particles via inoculation of in vitro RNA transcripts into N. benthamiana. IP immunization of mice with foliar extracts from infected leaves elicited antibody responses and conferred protection against virulent FMDV challenge [379].
Two immunogenic dominant epitopes of FMDV serotype O VP1, 11 and 14 amino acids long, were fused to the TMV CP ORF between amino acid residues 154 and 155 and expressed in tobacco. The resulting TMVF11 and TMVF14 chimeric particles were able to systemically infect tobacco and produce large quantities of stable progeny viral particles assembled from the modified CP subunits. These VLPs conferred full protection in guinea pigs after parenteral immunization and partial protection after oral administration, and serum of the immunized animals contained virus-neutralizing antibodies. Protection was also demonstrated in a swine model [380].
Similarly, chimeric bamboo mosaic virus (BaMV)-based VLPs expressing an epitope of FMDV VP1 have been produced in Chenopodium quinoa. ImmunoEM confirmed the presence of BaMV CP and FMDV VP1 on the surface of these particles. Purified VLPs induced both cellular and humoral immune responses in pigs and protected the animals against FMDV challenge [381].
Recently, TNV-AC has been used as a vector to express peptides from FMDV serotype O VP1 cloned downstream of the CP ORF in Chenopodium amaranticolor via inoculation of in vitro transcripts. IM administration of the purified chimeric VLPs elicited strong systemic anti-VP1 antibody responses, whereas IN immunization induced both systemic and mucosal antibody responses [382].
Using another plant virus, cucumber mosaic virus (CMV), an HCV vaccine candidate was engineered as chimeric CMV CP-based VLPs displaying a 27-amino acid long synthetic peptide derived from the hypervariable region 1 (HVR1) sequences of HCV envelope protein E2 (the so-called R9 mimotope) fused to CMV CP (R9-CMV) [383]. The VLPs demonstrated stability in vitro under simulated gastrointestinal conditions. Rabbits fed with R9-CMV-infected lettuce developed an R9-specific antibody response, suggesting efficacy of the edible CMV VLP-based HCV vaccine [384].
The PVX/CMV CP transient expression system was used to produce VLPs presenting epitopes of NDV in N. benthamiana using an in vitro transcript inoculation approach. In this system, CMV CP that was expressed under the control of PVX subgenomic promoters self-assembled into CMV VLPs encapsidating subgenomic RNA encoding CMV CP and PVX RNA downstream of ORF3 of the hybrid virus [385]. Two short neutralizing NDV epitopes were chosen to be fused to the βH-βI loop (motif 5) of CMV CP: (1) 17-amino acid epitope of NDV F protein (amino acids 65–81), (2) 8-amino acid epitope of NDV HN protein (amino acids 346–353), and (3) both epitopes fused in tandem. The chimeric VLPs were stably expressed in plants, and correct presentation and folding of the epitopes were confirmed using NDV-specific antisera [385]. Furthermore, transmission EM of sucrose gradient-purified VLP preparations showed their morphological identity with wild-type CMV particles [386]. Immunization with NDV epitope-carrying CMV CP VLPs induced antigen-specific antibodies in chickens [386].
In addition to targeting infectious diseases, VLPs produced in plants can be used as a platform for developing vaccines against self-antigens involved in the pathogenesis of neurodegenerative diseases. Thus, fusion of a fragment of amyloid β (Aβ) protein, Aβ1-15 peptide, to CMV CP in position 248 or 392 resulted in complete peptide decoration of chimeric virus particles as shown by immunoEM using either anti-Aβ1-15 or anti-Aβ1-41 polyclonal antibodies. The results suggest potential of these chimeric VLPs as vaccine candidates against Alzheimer's disease [387].
3.6. Cell-free systems
VLPs are also made under in vitro cell-free conditions and can be assembled into virosomes using membrane-like structures, similar to membranes of enveloped VLPs, or using polypeptide scaffolding to give nano-VLPs, similar to non-enveloped VLPs. Nano-VLPs are one of the latest developments in this field and have only been tested in a pre-clinical setting [388], [389], [390]. The approach uses natural properties of amino acids of a polypeptide to self-assemble and allows for inserting immunogenic epitopes. Although at an early stage of development, this approach shows promise for recombinant vaccine design and development. Virosomes, on the other hand, have been used for presenting target antigens from a number of pathogens, and some of them have entered clinical development (see below in this section). Virosomes essentially represent enveloped VLPs assembled in vitro rather than inside a host cell. For the production of virosomes, a purified and inactivated parental virus undergoes detergent dissociation to separate the envelope fraction containing lipids and membrane-associated viral proteins from the core complex containing internal proteins and genetic material. Upon removal of detergent, the envelope fraction components assemble into empty virosomal particles. Depending on the desired type of immune response to be induced by the virosomal vaccine, the recombinant or synthetic antigen can be either displayed on the virosome surface (by integration or anchorage into the lipid bilayer, cross-linkage to membrane-associated viral proteins via a lipid anchor, or absorbance to the bilayer surface) or incorporated into its lumen, stimulating antibody production or CTL responses, respectively [391].
Virosomes carrying HA and NA of influenza virus were produced and evaluated as vaccine candidates as early as in the 1970s [392], [393], leading to the commercialization of two licensed human vaccines, Crucell's Epaxal® against hepatitis A [25] and Inflexal® V against seasonal influenza [26]. Several influenza virosome-based vaccines produced by Pevion Biotech AG (Bern, Switzerland) are also at different stages of clinical development (Table 2). A therapeutic virosomal HCV vaccine candidate has been developed to clear chronic hepatitis C infection. The vaccine was engineered by encapsulating two viral peptides – CTL epitopes – into the virosome, and displaying one viral peptide – a CD4+ T cell epitope – on the virosome surface, and thus is intended to induce both cellular and humoral immune responses [394], [395]. In a Phase 1 study the vaccine was well tolerated, but levels of T cell responses were not satisfactory [391]. A bivalent virosomal malaria vaccine displaying two synthetic peptides from two P. falciparum proteins – CSP and the apical membrane antigen 1 – was designed to induce protective antibody responses against both sporozoites and merozoites and has been tested in four Phase 1/2 clinical trials, demonstrating safety, immunogenicity and ability to produce long-lasting sporozoite-inhibitory antibody titers [29], [30], [31], [32]. As a further example, a vaccine against Candida albicans was engineered by linking a recombinant truncated form of Sap2 protein, the virulence factor of the fungus, to a virosome to induce protective mucosal antibodies [391], [396] and has been demonstrated to be safe and highly immunogenic in an ongoing Phase 1 clinical trial [397], [398].
Similarly, a breast cancer vaccine has been designed to display three synthetic peptides from Her-2/neu protein on a virosome. In a Phase 1 clinical trial conducted in patients with metastatic breast cancer, the vaccine was shown to be very safe, elicited high antibody titers against these self-peptides, even at a low dose, and induced antibodies that recognized not only the synthetic peptides but also full-length Her-2/neu protein [399]. Pevion's monovalent virosomal HIV vaccine candidate, displaying a recombinant HIV-1 gp41 peptide on an influenza virosome and delivered via combined IM and IN routes, has been shown to protect non-human primates against vaginal SHIV challenge [400]. Protection was mediated by vaginal gp41-specific HIV-1 transcytosis-blocking vaginal IgA antibodies and vaginal IgG antibodies with neutralizing and/or antibody-dependent cellular cytotoxicity activities [400]. This vaccine is currently being tested for safety and prophylactic efficacy in healthy female volunteers in a Phase 1 clinical trial (Table 2) [401].
In addition to the virosomal vaccines that have been licensed or are in clinical development, some virosomal vaccine candidates are undergoing pre-clinical evaluation. These include RSV virosomal vaccines that either display a recombinant RSV-F protein on an influenza virosome [402], [403], [404] or represent a reconstructed RSV virosome carrying toll-like receptor-binding immunomodulating lipid adjuvant in an envelope [405], [406]. In proof-of-concept animal studies, these RSV vaccines elicited both humoral and cell-mediated immune responses of the Th1 type and conferred protection against live RSV challenge [402], [403], [404], [405], [406]. Based on these results, Pevion's RSV vaccine candidate is expected to enter a combined clinical Phase 1/2 clinical study in healthy volunteers, including a controlled virus challenge [404].
4. Conclusions
During the last four decades, there has been a significant increase in the development of recombinant SUVs using heterologous expression systems. Antigens from many viral, bacterial, parasitic and fungal pathogens have been tested for effective and safe vaccine development. In a number of cases, specific vaccine antigens have been produced using different expression systems and have performed comparably well to licensed recombinant vaccines [407], [408], suggesting the critical importance of design and representation of specific antigens and their ability to generate protective immune responses. Although many antigens have been produced and evaluated as components of candidate SUVs, only a few have reached the market. Today, all recombinant SUVs on the market are based on VLPs. The only SUV approaching the market that is not based on a VLP is Protein Sciences’ influenza vaccine [409], [410]. However, even in this case, the vaccine consists of trimers or higher-order aggregates of HA. As we gain more knowledge about each pathogen, the human immune system, host-pathogen interactions and mechanisms of enhancing target-specific immune responses, it is anticipated that a broader range of SUVs will be developed, potentially including non-VLP-based examples. To date, however, VLP-based SUVs appear superior in eliciting target-specific protective responses in humans. Although the underlying mechanisms of protective immune responses elicited by VLP-based SUVs are not fully understood, the critical importance of VLP greater structural complexity has been emphasized by many studies. VLP designs allow for producing multicomponent vaccines that structurally resemble human or animal pathogens, and are thus recognized by the human immune system in a similar manner to pathogens. Each protein component is presented in the VLP's structure similarly to presentation on the pathogen. Reproducing this effect using other, non-VLP, approaches is currently not feasible.
Particulate structure and multivalent epitope organization are not the only reasons for stronger immunogenicity and protective efficacy of VLPs compared to non-assembled SUVs. While purification procedures result in highly pure soluble proteins, VLPs produced in different expression systems are often contaminated with residual host cell components such as lipids, nucleic acids and proteins that may stimulate the innate immunity and augment the adaptive immune response [5]. For example, yeast cell membrane components contaminating the preparations of yeast-derived HIV-1 Pr55Gag VLPs were shown to contribute to DC activation and the effect was partially mediated by signaling through Toll-like receptors [128]. Similarly, components of insect cells and recombinant baculoviruses induced activation of DCs and other antigen presenting cells, promoting strong Th1 type-biased immune responses [5]. According to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use regulatory guidelines, however, host cell contaminants that affect the immune system represent a major safety concern [411], which may have a significant impact on the vaccine development path based on this approach. Lessons learned from the development and use of current VLPs as well as full pathogen-based vaccines would be highly valuble in continued use of this approach or successful product development.
Additionally, VLPs offer an economic approach to producing SUVs. Several recombinant systems have been successfully used for VLP production, with promising efficacy reported for many of these VLP vaccine candidates. Thus, VLPs produced in recombinant systems are anticipated to be very prominent among approaches to commercialize further SUVs.
References
- 1.Plotkin S.A. Vaccines: past, present and future. Nat Med. 2005;11(4 Suppl.):S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray K. Application of recombinant DNA techniques in the development of viral vaccines. Vaccine. 1988;6:164–174. doi: 10.1016/s0264-410x(88)80022-7. [DOI] [PubMed] [Google Scholar]
- 3.Grgacic E.V., Anderson D.A. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Deml L., Speth C., Dierich M.P., Wolf H., Wagner R. Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol Immunol. 2005;42:259–277. doi: 10.1016/j.molimm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Murata K., Lechmann M., Qiao M., Gunji T., Alter H.J., Liang T.J. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci U S A. 2003;100:6753–6758. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paliard X., Liu Y., Wagner R., Wolf H., Baenziger J., Walker C.M. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res Hum Retroviruses. 2000;16:273–282. doi: 10.1089/088922200309368. [DOI] [PubMed] [Google Scholar]
- 8.Schirmbeck R., Böhm W., Reimann J. Virus-like particles induce MHC class I-restricted T-cell responses. Lessons learned from the hepatitis B small surface antigen. Intervirology. 1996;39:111–119. doi: 10.1159/000150482. [DOI] [PubMed] [Google Scholar]
- 9.Win S.J., Ward V.K., Dunbar P.R., Young S.L., Baird M.A. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol Cell Biol. 2011;89:681–688. doi: 10.1038/icb.2010.161. [DOI] [PubMed] [Google Scholar]
- 10.Engerix-B®. Prescribing information. GlaxoSmithKline; March 2012.
- 11.Cervarix®. Prescribing information. GlaxoSmithKline; July 2011.
- 12.Recombivax HB®. Prescribing information. Merck; July 2011.
- 13.Gardasil®. Prescribing information. Merck; April 2011.
- 14.Agnandji S.T., Lell B., Soulanoudjingar S.S., Fernandes J.F., Abossolo B.P., Conzelmann C. RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 15.El-Attar L., Oliver S.L., Mackie A., Charpilienne A., Poncet D., Cohen J. Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease. Vaccine. 2009;27:3201–3208. doi: 10.1016/j.vaccine.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Franco D., Liu W., Gardiner D.F., Hahn B.H., Ho D.D. CD40L-containing virus-like particle as a candidate HIV-1 vaccine targeting dendritic cells. J Acquir Immune Defic Syndr. 2011;56:393–400. doi: 10.1097/QAI.0b013e31820b844e. [DOI] [PubMed] [Google Scholar]
- 17.Pillay S., Shephard E.G., Meyers A.E., Williamson A.L., Rybicki E.P. HIV-1 sub-type C chimaeric VLPs boost cellular immune responses in mice. J Immune Based Ther Vaccines. 2010;8:7. doi: 10.1186/1476-8518-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J.M., Wang B.Z., Park K.M., Van Rooijen N., Quan F.S., Kim M.C. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S., Cubas R., Li M., Chen C., Yao Q. Virus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cells. Mol Immunol. 2009;46:1988–2001. doi: 10.1016/j.molimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H., Guo L., Wang M., Qu J., Zhao Z., Wang J. Prime immunization with rotavirus VLP 2/6 followed by boosting with an adenovirus expressing VP6 induces protective immunization against rotavirus in mice. Virol J. 2011;8:3. doi: 10.1186/1743-422X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soulié J.C., Devillier P., Santarelli J., Goudeau A., Vermeulen P., Guellier M. et al. Immunogenicity and safety in newborns of a new recombinant hepatitis B vaccine containing the S and pre-S2 antigens. Vaccine. 1991;9:545–548. doi: 10.1016/0264-410x(91)90240-7. [DOI] [PubMed] [Google Scholar]
- 22.Landry N., Ward B.J., Trépanier S., Montomoli E., Dargis M., Lapini G. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One. 2010;5:e15559. doi: 10.1371/journal.pone.0015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Macías C., Ferat-Osorio E., Tenorio-Calvo A., Isibasi A., Talavera J., Arteaga-Ruiz O. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29:7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardin E.H., Oliveira G.A., Calvo-Calle J.M., Wetzel K., Maier C., Birkett A.J. Phase I testing of a malaria vaccine composed of hepatitis B virus core particles expressing Plasmodium falciparum circumsporozoite epitopes. Infect Immun. 2004;72:6519–6527. doi: 10.1128/IAI.72.11.6519-6527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bovier P.A. Recent advances with a virosomal hepatitis A vaccine. Expert Opin Biol Ther. 2008;8:1177–1185. doi: 10.1517/14712598.8.8.1177. [DOI] [PubMed] [Google Scholar]
- 26.Herzog C., Hartmann K., Künzi V., Kürsteiner O., Mischler R., Lazar H. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, et al. A plant-derived edible vaccine against hepatitis B virus. FASEB J 1999;13:1796–9 [Erratum in: FASEB J 1999;13:2339]. [DOI] [PubMed]
- 28.Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci U S A. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cech P.G., Aebi T., Abdallah M.S., Mpina M., Machunda E.B., Westerfeld N. Virosome-formulated Plasmodium falciparum AMA-1 & CSP derived peptides as malaria vaccine: randomized phase 1b trial in semi-immune adults & children. PLoS One. 2011;6:e22273. doi: 10.1371/journal.pone.0022273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genton B., Pluschke G., Degen L., Kammer A.R., Westerfeld N., Okitsu S.L. A randomized placebo-controlled phase Ia malaria vaccine trial of two virosome-formulated synthetic peptides in healthy adult volunteers. PLoS One. 2007;2:e1018. doi: 10.1371/journal.pone.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okitsu S.L., Silvie O., Westerfeld N., Curcic M., Kammer A.R., Mueller M.S. A virosomal malaria peptide vaccine elicits a long-lasting sporozoite-inhibitory antibody response in a phase 1a clinical trial. PLoS One. 2007;2:e1278. doi: 10.1371/journal.pone.0001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, et al., Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS One 2008;3:e1493 [Erratum in: PLoS One 2011;6]. [DOI] [PMC free article] [PubMed]
- 33.U.S. Clinical Trials. Safety Study of Recombinant M2e Influenza-A Vaccine in Healthy Adults (FLU-A). http://www.clinicaltrials.gov/ct2/show/NCT00819013?term=sanofi+and+influenza+and+m2e&rank=1 [accessed 12 April, 2012].
- 34.Phelps D.K., Speelman B., Post C.B. Theoretical studies of viral capsid proteins. Curr Opin Struct Biol. 2000;10:170–173. doi: 10.1016/s0959-440x(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 35.Steven A.C., Trus B.L., Booy F.P., Cheng N., Zlotnick A., Caston J.R. The making and breaking of symmetry in virus capsid assembly: glimpses of capsid biology from cryoelectron microscopy. FASEB J. 1997;11:733–742. doi: 10.1096/fasebj.11.10.9271358. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnick A., Mukhopadhyay S. Virus assembly, allostery and antivirals. Trends Microbiol. 2011;19:14–23. doi: 10.1016/j.tim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasagawa T., Pushko P., Steers G., Gschmeissner S.E., Hajibagheri M.A., Finch J. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology. 1995;206:126–135. doi: 10.1016/s0042-6822(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 38.Labbé M., Charpilienne A., Crawford S.E., Estes M.K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J Virol. 1991;65:2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonis A.F., Bruschke C.J., Rueda P., Maranga L., Casal J.I., Vela C. A novel recombinant virus-like particle vaccine for prevention of porcine parvovirus-induced reproductive failure. Vaccine. 2006;24:5481–5490. doi: 10.1016/j.vaccine.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 40.Christensen J., Alexandersen S., Bloch B., Aasted B., Uttenthal A. Production of mink enteritis parvovirus empty capsids by expression in a baculovirus vector system: a recombinant vaccine for mink enteritis parvovirus in mink. J Gen Virol. 1994;75(Pt 1):149–155. doi: 10.1099/0022-1317-75-1-149. [DOI] [PubMed] [Google Scholar]
- 41.López de Turiso J.A., Cortés E., Martínez C., Ruiz de Ybáñez R., Simarro I., Vela C. Recombinant vaccine for canine parvovirus in dogs. J Virol. 1992;66:2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maranga L., Rueda P., Antonis A.F., Vela C., Langeveld J.P., Casal J.I. Large scale production and downstream processing of a recombinant porcine parvovirus vaccine. Appl Microbiol Biotechnol. 2002;59:45–50. doi: 10.1007/s00253-002-0976-x. [DOI] [PubMed] [Google Scholar]
- 43.Martínez C., Dalsgaard K., López de Turiso J.A., Cortés E., Vela C., Casal J.I. Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine. 1992;10:684–690. doi: 10.1016/0264-410x(92)90090-7. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X., Wang M., Graham D.Y., Estes M.K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T.C., Yamakawa Y., Suzuki K., Tatsumi M., Razak M.A., Uchida T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavilanes F., Gonzalez-Ros J.M., Peterson D.L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982;257:7770–7777. [PubMed] [Google Scholar]
- 47.Gavilanes F., Gomez-Gutierrez J., Aracil M., Gonzalez-Ros J.M., Ferragut J.A., Guerrero E. Hepatitis B surface antigen. Role of lipids in maintaining the structural and antigenic properties of protein components. Biochem J. 1990;265:857–864. doi: 10.1042/bj2650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gómez-Gutiérrez J., Rodríguez-Crespo I., Peterson D.L., Gavilanes F. Reconstitution of hepatitis B surface antigen proteins into phospholipid vesicles. Biochim Biophys Acta. 1994;1192:45–52. doi: 10.1016/0005-2736(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Gutiérrez J., Rodríguez-Crespo I., Peterson D.L., Gavilanes F. Antigenicity of hepatitis B surface antigen proteins reconstituted with phospholipids. Biochim Biophys Acta. 1995;1233:205–212. doi: 10.1016/0005-2736(94)00255-n. [DOI] [PubMed] [Google Scholar]
- 50.Howard C.R., Young P.R., Lee S., Dixon J., Zuckerman A.J., McAleer W.J. Hepatitis B surface antigen polypeptide micelles from antigen expressed in Saccharomyces cerevisiae. J Virol Methods. 1986;14:25–35. doi: 10.1016/0166-0934(86)90004-2. [DOI] [PubMed] [Google Scholar]
- 51.Skelly J., Howard C.R., Zuckerman A.J. Hepatis B polypeptide vaccine preparation in micelle form. Nature. 1981;290:51–54. doi: 10.1038/290051a0. [DOI] [PubMed] [Google Scholar]
- 52.Eble B.E., Lingappa V.R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986;6:1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harford N., Cabezon T., Crabeel M., Simoen E., Rutgers A., De Wilde M. Expression of hepatitis B surface antigen in yeast. Dev Biol Stand. 1983;54:125–130. [PubMed] [Google Scholar]
- 54.Hitzeman R.A., Chen C.Y., Hagie F.E., Patzer E.J., Liu C.C., Estell D.A. Expression of hepatitis B virus surface antigen in yeast. Nucleic Acids Res. 1983;11:2745–2763. doi: 10.1093/nar/11.9.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valenzuela P., Medina A., Rutter W.J., Ammerer G., Hall B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 56.Michel M.L., Pontisso P., Sobczak E., Malpièce Y., Streeck R.E., Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc Natl Acad Sci U S A. 1984;81:7708–7712. doi: 10.1073/pnas.81.24.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson W.S., Lutwick L.I. The virus of hepatitis, type B (first of two parts) N Engl J Med. 1976;295:1168–1175. doi: 10.1056/NEJM197611182952105. [DOI] [PubMed] [Google Scholar]
- 58.Robinson W.S., Lutwick L.I. The virus of hepatitis, type B (Second of two parts) N Engl J Med. 1976;295:1232–1236. doi: 10.1056/NEJM197611252952206. [DOI] [PubMed] [Google Scholar]
- 59.Diminsky D., Schirmbeck R., Reimann J., Barenholz Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997;15:637–647. doi: 10.1016/s0264-410x(96)00239-3. [DOI] [PubMed] [Google Scholar]
- 60.Zhou W., Bi J., Janson J.C., Li Y., Huang Y., Zhang Y. Molecular characterization of recombinant Hepatitis B surface antigen from Chinese hamster ovary and Hansenula polymorpha cells by high-performance size exclusion chromatography and multi-angle laser light scattering. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:71–77. doi: 10.1016/j.jchromb.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 61.Patzer E.J., Nakamura G.R., Simonsen C.C., Levinson A.D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986;58:884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lünsdorf H., Gurramkonda C., Adnan A., Khanna N., Rinas U. Virus-like particle production with yeast: ultrastructural and immunocytochemical insights into Pichia pastoris producing high levels of the hepatitis B surface antigen. Microb Cell Fact. 2011;10:48. doi: 10.1186/1475-2859-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoute J.A., Slaoui M., Heppner D.G., Momin P., Kester K.E., Desmons P. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 64.Turpen T.H., Reinl S.J., Charoenvit Y., Hoffman S.L., Fallarme V., Grill L.K. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology (N Y) 1995;13:53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- 65.Yusibov V., Modelska A., Steplewski K., Agadjanyan M., Weiner D., Hooper D.C. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc Natl Acad Sci U S A. 1997;94:5784–5788. doi: 10.1073/pnas.94.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yusibov V., Hooper D.C., Spitsin S.V., Fleysh N., Kean R.B., Mikheeva T. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine. 2002;20:3155–3164. doi: 10.1016/s0264-410x(02)00260-8. [DOI] [PubMed] [Google Scholar]
- 67.Belanger H., Fleysh N., Cox S., Bartman G., Deka D., Trudel M. Human respiratory syncytial virus vaccine antigen produced in plants. FASEB J. 2000;14:2323–2328. doi: 10.1096/fj.00-0144com. [DOI] [PubMed] [Google Scholar]
- 68.Yusibov V., Mett V., Mett V., Davidson C., Musiychuk K., Gilliam S. Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine. 2005;23:2261–2265. doi: 10.1016/j.vaccine.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 69.McLain L., Porta C., Lomonossoff G.P., Durrani Z., Dimmock N.J. Human immunodeficiency virus type 1-neutralizing antibodies raised to a glycoprotein 41 peptide expressed on the surface of a plant virus. AIDS Res Hum Retroviruses. 1995;11:327–334. doi: 10.1089/aid.1995.11.327. [DOI] [PubMed] [Google Scholar]
- 70.Matić S., Rinaldi R., Masenga V., Noris E. Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in Nicotiana benthamiana plants. BMC Biotechnol. 2011;11:106. doi: 10.1186/1472-6750-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phelps J.P., Dang N., Rasochova L. Inactivation and purification of cowpea mosaic virus-like particles displaying peptide antigens from Bacillus anthracis. J Virol Methods. 2007;141:146–153. doi: 10.1016/j.jviromet.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yasawardene S.G., Lomonossoff G.P., Ramasamy R. Expression & immunogenicity of malaria merozoite peptides displayed on the small coat protein of chimaeric cowpea mosaic virus. Indian J Med Res. 2003;118:115–124. [PubMed] [Google Scholar]
- 73.Marusic C., Rizza P., Lattanzi L., Mancini C., Spada M., Belardelli F. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J Virol. 2001;75:8434–8439. doi: 10.1128/JVI.75.18.8434-8439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lico C., Mancini C., Italiani P., Betti C., Boraschi D., Benvenuto E. Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine. 2009;27:5069–5076. doi: 10.1016/j.vaccine.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 75.Roy P., Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Adv Exp Med Biol. 2009;655:145–158. doi: 10.1007/978-1-4419-1132-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belyaev A.S., Roy P. Development of baculovirus triple and quadruple expression vectors: co-expression of three or four bluetongue virus proteins and the synthesis of bluetongue virus-like particles in insect cells. Nucleic Acids Res. 1993;21:1219–1223. doi: 10.1093/nar/21.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.French T.J., Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990;64:1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.French T.J., Marshall J.J., Roy P. Assembly of double-shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol. 1990;64:5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crawford S.E., Labbé M., Cohen J., Burroughs M.H., Zhou Y.J., Estes M.K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68:5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabara M., Parker M., Aha P., Cosco C., Gibbons E., Parsons S. Assembly of double-shelled rotaviruslike particles by simultaneous expression of recombinant VP6 and VP7 proteins. J Virol. 1991;65:6994–6997. doi: 10.1128/jvi.65.12.6994-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 82.Haynes J.R. Influenza virus-like particle vaccines. Expert Rev Vaccines. 2009;8:435–445. doi: 10.1586/erv.09.8. [DOI] [PubMed] [Google Scholar]
- 83.Li M., Schmitt P.T., Li Z., McCrory T.S., He B., Schmitt A.P. Mumps virus matrix, fusion, and nucleocapsid proteins cooperate for efficient production of virus-like particles. J Virol. 2009;83:7261–7272. doi: 10.1128/JVI.00421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tseng Y.T., Wang S.M., Huang K.J., Lee A.I., Chiang C.C., Wang C.T. Self-assembly of severe acute respiratory syndrome coronavirus membrane protein. J Biol Chem. 2010;285:12862–12872. doi: 10.1074/jbc.M109.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buonaguro L., Buonaguro F.M., Tornesello M.L., Mantas D., Beth-Giraldo E., Wagner R. High efficient production of Pr55(gag) virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res. 2001;49:35–47. doi: 10.1016/s0166-3542(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 86.Halsey R.J., Tanzer F.L., Meyers A., Pillay S., Lynch A., Shephard E. Chimaeric HIV-1 subtype C Gag molecules with large in-frame C-terminal polypeptide fusions form virus-like particles. Virus Res. 2008;133:259–268. doi: 10.1016/j.virusres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Visciano M.L., Diomede L., Tagliamonte M., Tornesello M.L., Asti V., Bomsel M. Generation of HIV-1 Virus-Like Particles expressing different HIV-1 glycoproteins. Vaccine. 2011;29:4903–4912. doi: 10.1016/j.vaccine.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Chen B.J., Leser G.P., Morita E., Lamb R.A. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J Virol. 2007;81:7111–7123. doi: 10.1128/JVI.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Latham T., Galarza J.M. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol. 2001;75:6154–6165. doi: 10.1128/JVI.75.13.6154-6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamshchikov G.V., Ritter G.D., Vey M., Compans R.W. Assembly of SIV virus-like particles containing envelope proteins using a baculovirus expression system. Virology. 1995;214:50–58. doi: 10.1006/viro.1995.9955. [DOI] [PubMed] [Google Scholar]
- 91.Baumert T.F., Ito S., Wong D.T., Liang T.J., Hepatitis C. virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827–3836. doi: 10.1128/jvi.72.5.3827-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pushko P., Tumpey T.M., Bu F., Knell J., Robinson R., Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 93.Galarza J.M., Latham T., Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 94.Gómez-Puertas P., Albo C., Pérez-Pastrana E., Vivo A., Portela A. Influenza virus matrix protein is the major driving force in virus budding. J Virol. 2000;74:11538–11547. doi: 10.1128/jvi.74.24.11538-11547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao Q., Kuhlmann F.M., Eller R., Compans R.W., Chen C. Production and characterization of simian–human immunodeficiency virus-like particles. AIDS Res Hum Retroviruses. 2000;16:227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- 96.Ho Y., Lin P.H., Liu C.Y., Lee S.P., Chao Y.C. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem Biophys Res Commun. 2004;318:833–838. doi: 10.1016/j.bbrc.2004.04.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mortola E., Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576:174–178. doi: 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jasenosky L.D., Neumann G., Lukashevich I., Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J Virol. 2001;75:5205–5214. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Licata J.M., Johnson R.F., Han Z., Harty R.N. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol. 2004;78:7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson RF, Bell P, Harty RN. Effect of Ebola virus proteins GP, NP and VP35 on VP40 VLP morphology. Virol J 2006;3:31. [DOI] [PMC free article] [PubMed]
- 101.McGinnes L.W., Pantua H., Laliberte J.P., Gravel K.A., Jain S., Morrison T.G. Assembly and biological and immunological properties of Newcastle disease virus-like particles. J Virol. 2010;84:4513–4523. doi: 10.1128/JVI.01931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pantua HD, McGinnes LW, Peeples ME, Morrison TG. Requirements for the assembly and release of Newcastle disease virus-like particles. J Virol 2006;80:11062–73 [Erratum in: J Virol 2007;81:1537]. [DOI] [PMC free article] [PubMed]
- 103.Tissot A.C., Renhofa R., Schmitz N., Cielens I., Meijerink E., Ose V. Versatile virus-like particle carrier for epitope based vaccines. PLoS One. 2010;5:e9809. doi: 10.1371/journal.pone.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W., Carmichael J., Ferguson J., Inglis S., Ashrafian H., Stanley M. Expression of human papillomavirus type 16 L1 protein in Escherichia coli: denaturation, renaturation, and self-assembly of virus-like particles in vitro. Virology. 1998;243:423–431. doi: 10.1006/viro.1998.9050. [DOI] [PubMed] [Google Scholar]
- 105.Paavonen J. Human papillomavirus infection and the development of cervical cancer and related genital neoplasias. Int J Infect Dis. 2007;11(Suppl 2):S3–S9. doi: 10.1016/S1201-9712(07)60015-0. [DOI] [PubMed] [Google Scholar]
- 106.Aires K.A., Cianciarullo A.M., Carneiro S.M., Villa L.L., Boccardo E., Pérez-Martinez G. Production of human papillomavirus type 16 L1 virus-like particles by recombinant Lactobacillus casei cells. Appl Environ Microbiol. 2006;72:745–752. doi: 10.1128/AEM.72.1.745-752.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sominskaya I., Skrastina D., Dislers A., Vasiljev D., Mihailova M., Ose V. Construction and immunological evaluation of multivalent hepatitis B virus (HBV) core virus-like particles carrying HBV and HCV epitopes. Clin Vaccine Immunol. 2010;17:1027–1033. doi: 10.1128/CVI.00468-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lorenzo L.J., Dueñas-Carrera S., Falcón V., Acosta-Rivero N., González E., de la Rosa M.C. Assembly of truncated HCV core antigen into virus-like particles in Escherichia coli. Biochem Biophys Res Commun. 2001;281:962–965. doi: 10.1006/bbrc.2001.4449. [DOI] [PubMed] [Google Scholar]
- 109.Denis J., Majeau N., Acosta-Ramirez E., Savard C., Bedard M.C., Simard S. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: evidence for the critical function of multimerization. Virology. 2007;363:59–68. doi: 10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 110.Tremblay M.H., Majeau N., Gagné M.E., Lecours K., Morin H., Duvignaud J.B. Effect of mutations K97A and E128A on RNA binding and self assembly of papaya mosaic potexvirus coat protein. FEBS J. 2006;273:14–25. doi: 10.1111/j.1742-4658.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 111.Lacasse P., Denis J., Lapointe R., Leclerc D., Lamarre A. Novel plant virus-based vaccine induces protective cytotoxic T-lymphocyte-mediated antiviral immunity through dendritic cell maturation. J Virol. 2008;82:785–794. doi: 10.1128/JVI.01811-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Birkett A., Lyons K., Schmidt A., Boyd D., Oliveira G.A., Siddique A. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect Immun. 2002;70:6860–6870. doi: 10.1128/IAI.70.12.6860-6870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milich D.R., Hughes J., Jones J., Sällberg M., Phillips T.R. Conversion of poorly immunogenic malaria repeat sequences into a highly immunogenic vaccine candidate. Vaccine. 2001;20:771–788. doi: 10.1016/s0264-410x(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 114.Oliveira G.A., Wetzel K., Calvo-Calle J.M., Nussenzweig R., Schmidt A., Birkett A. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect Immun. 2005;73:3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fiers W., De Filette M., El Bakkouri K., Schepens B., Roose K., Schotsaert M. M2e-based universal influenza A vaccine. Vaccine. 2009;27:6280–6283. doi: 10.1016/j.vaccine.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Wiessner C., Wiederhold K.H., Tissot A.C., Frey P., Danner S., Jacobson L.H. The second-generation active Aβ immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winblad B., Minthon L., Floesser A., Imbert G., Dumortier T., He Y. Results of the first-in-man study with the active Aβ immunotherapy CAD106 in Alzheimer patients. Alzheimers Dement. 2009;5(4 Suppl. 1):P113. [Google Scholar]
- 118.Ambühl P.M., Tissot A.C., Fulurija A., Maurer P., Nussberger J., Sabat R. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 119.Tissot A.C., Maurer P., Nussberger J., Sabat R., Pfister T., Ignatenko S. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 120.Cytos Biotechnology Ltd. Press Release November 10, 2009. Cytos Biotechnology updates on the development of the hypertension vaccine CYT006-AngQb. http://www.cytos.com/userfiles/file/Cytos_Press_E_091110.pdf [accessed 12 April, 2012].
- 121.Leavitt A.D., Roberts T.M., Garcea R.L. Polyoma virus major capsid protein, VP1. Purification after high level expression in Escherichia coli. J Biol Chem. 1985;260:12803–12809. [PubMed] [Google Scholar]
- 122.Liew M.W., Rajendran A., Middelberg A.P. Microbial production of virus-like particle vaccine protein at gram-per-litre levels. J Biotechnol. 2010;150:224–231. doi: 10.1016/j.jbiotec.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 123.Wróbel B., Yosef Y., Oppenheim A.B., Oppenheim A. Production and purification of SV40 major capsid protein (VP1) in Escherichia coli strains deficient for the GroELS chaperone machine. J Biotechnol. 2000;84:285–289. doi: 10.1016/s0168-1656(00)00369-2. [DOI] [PubMed] [Google Scholar]
- 124.Phelps J.P., Dao P., Jin H., Rasochova L. Expression and self-assembly of cowpea chlorotic mottle virus-like particles in Pseudomonas fluorescens. J Biotechnol. 2007;128:290–296. doi: 10.1016/j.jbiotec.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 125.Mett V., Farrance C.E., Green B.J., Yusibov V. Plants as biofactories. Biologicals. 2008;36:354–358. doi: 10.1016/j.biologicals.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 126.Yusibov V., Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev Vaccines. 2008;7:1173–1183. doi: 10.1586/14760584.7.8.1173. [DOI] [PubMed] [Google Scholar]
- 127.Sakuragi S., Goto T., Sano K., Morikawa Y. HIV type 1 Gag virus-like particle budding from spheroplasts of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2002;99:7956–7961. doi: 10.1073/pnas.082281199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsunetsugu-Yokota Y., Morikawa Y., Isogai M., Kawana-Tachikawa A., Odawara T., Nakamura T. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8(+) T cells by cross-presentation of DCs. J Virol. 2003;77:10250–10259. doi: 10.1128/JVI.77.19.10250-10259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adams S.E., Dawson K.M., Gull K., Kingsman S.M., Kingsman A.J. The expression of hybrid HIV:Ty virus-like particles in yeast. Nature. 1987;329:68–70. doi: 10.1038/329068a0. [DOI] [PubMed] [Google Scholar]
- 130.Peters B.S., Cheingsong-Popov R., Callow D., Foxall R., Patou G., Hodgkin K. A pilot phase II study of the safety and immunogenicity of HIV p17/p24:VLP (p24-VLP) in asymptomatic HIV seropositive subjects. J Infect. 1997;35:231–235. doi: 10.1016/s0163-4453(97)92814-0. [DOI] [PubMed] [Google Scholar]
- 131.Weber J., Cheinsong-Popov R., Callow D., Adams S., Patou G., Hodgkin K. Immunogenicity of the yeast recombinant p17/p24:Ty virus-like particles (p24-VLP) in healthy volunteers. Vaccine. 1995;13:831–834. doi: 10.1016/0264-410x(94)00061-q. [DOI] [PubMed] [Google Scholar]
- 132.Smith D., Gow I., Colebunders R., Weller I., Tchamouroff S., Weber J. et al., 006 Study Group, Therapeutic vaccination (p24-VLP) of patients with advanced HIV-1 infection in the pre-HAART era does not alter CD4 cell decline. HIV Med. 2001;2:272–275. doi: 10.1046/j.1468-1293.2001.00080.x. [DOI] [PubMed] [Google Scholar]
- 133.Bazan S.B., de Alencar Muniz Chaves A., Aires K.A., Cianciarullo A.M., Garcea R.L., Ho P.L. Expression and characterization of HPV-16 L1 capsid protein in Pichia pastoris. Arch Virol. 2009;154:1609–1617. doi: 10.1007/s00705-009-0484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coimbra E.C., Gomes F.B., Campos J.F., D’arc M., Carvalho J.C., Mariz F.C. Production of L1 protein from different types of HPV in Pichia pastoris using an integrative vector. Braz J Med Biol Res. 2011;44:1209–1214. doi: 10.1590/s0100-879x2011007500141. [DOI] [PubMed] [Google Scholar]
- 135.Kim S.N., Jeong H.S., Park S.N., Kim H.J. Purification and immunogenicity study of human papillomavirus type 16 L1 protein in Saccharomyces cerevisiae. J Virol Methods. 2007;139:24–30. doi: 10.1016/j.jviromet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Lowe R.S., Brown D.R., Bryan J.T., Cook J.C., George H.A., Hofmann K.J. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J Infect Dis. 1997;176:1141–1145. doi: 10.1086/514105. [DOI] [PubMed] [Google Scholar]
- 137.Neeper M.P., Hofmann K.J., Jansen K.U. Expression of the major capsid protein of human papillomavirus type 11 in Saccharomyces cerevisae. Gene. 1996;180:1–6. doi: 10.1016/s0378-1119(96)00388-5. [DOI] [PubMed] [Google Scholar]
- 138.Park M.A., Kim H.J., Kim H.J. Optimum conditions for production and purification of human papillomavirus type 16 L1 protein from Saccharomyces cerevisiae. Protein Expr Purif. 2008;59:175–181. doi: 10.1016/j.pep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 139.Patel M.C., Patkar K.K., Basu A., Mohandas K.M., Mukhopadhyaya R. Production of immunogenic human papillomavirus-16 major capsid protein derived virus like particles. Indian J Med Res. 2009;130:213–218. [PubMed] [Google Scholar]
- 140.Woo M.K., An J.M., Kim J.D., Park S.N., Kim H.J. Expression and purification of human papillomavirus 18 L1 virus-like particle from saccharomyces cerevisiae. Arch Pharm Res. 2008;31:205–209. doi: 10.1007/s12272-001-1142-1. [DOI] [PubMed] [Google Scholar]
- 141.Palker T.J., Monteiro J.M., Martin M.M., Kakareka C., Smith J.F., Cook J.C. Antibody, cytokine and cytotoxic T lymphocyte responses in chimpanzees immunized with human papillomavirus virus-like particles. Vaccine. 2001;19:3733–3743. doi: 10.1016/s0264-410x(01)00093-7. [DOI] [PubMed] [Google Scholar]
- 142.Jansen K.U., Rosolowsky M., Schultz L.D., Markus H.Z., Cook J.C., Donnelly J.J. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine. 1995;13:1509–1514. doi: 10.1016/0264-410x(95)00103-8. [DOI] [PubMed] [Google Scholar]
- 143.Ault K.A. Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 144.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915–27. [DOI] [PubMed]
- 145.FUTURE II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 2007;196:1438–46. [DOI] [PubMed]
- 146.Garland S.M., Hernandez-Avila M., Wheeler C.M., Perez G., Harper D.M., Leodolter S. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 147.Koutsky L.A., Ault K.A., Wheeler C.M., Brown D.R., Barr E., Alvarez F.B. Proof of Principle Study Investigators. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 148.Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Ault K.A., Giuliano A.R. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 149.Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Paavonen J., Iversen O.E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia M., Farkas T., Jiang X. Norovirus capsid protein expressed in yeast forms virus-like particles and stimulates systemic and mucosal immunity in mice following an oral administration of raw yeast extracts. J Med Virol. 2007;79:74–83. doi: 10.1002/jmv.20762. [DOI] [PubMed] [Google Scholar]
- 151.Rodríguez-Limas W.A., Tyo K.E., Nielsen J., Ramírez O.T., Palomares L.A. Molecular and process design for rotavirus-like particle production in Saccharomyces cerevisiae. Microb Cell Fact. 2011;10:33. doi: 10.1186/1475-2859-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cregg J.M., Tschopp J.F., Stillman C., Siegel R., Akong M., Craig W.S. High level expression and efficient assembly of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Bio/Technology. 1987;5:479–485. [Google Scholar]
- 153.Gu M.B., Park M.H., Kim D.I. Growth rate control in fed-batch cultures of recombinant Saccharomyces cerevisiae producing hepatitis B surface antigen (HBsAg) Appl Microbiol Biotechnol. 1991;35 doi: 10.1007/BF00180634. 46–30. [DOI] [PubMed] [Google Scholar]
- 154.Hardy E., Martínez E., Diago D., Díaz R., González D., Herrera L. Large-scale production of recombinant hepatitis B surface antigen from Pichia pastoris. J Biotechnol. 2000;77:157–167. doi: 10.1016/s0168-1656(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 155.Kee G.S., Jin J., Balasundaram B., Bracewell D.G., Pujar N.S., Titchener-Hooker N.J. Exploiting the intracellular compartmentalization characteristics of the S. cerevisiae host cell for enhancing primary purification of lipid-envelope virus-like particles. Biotechnol Prog. 2010;26:26–33. doi: 10.1002/btpr.307. [DOI] [PubMed] [Google Scholar]
- 156.Acosta-Rivero N., Aguilar J.C., Musacchio A., Falcón V., Viña A., de la Rosa M.C. Characterization of the HCV core virus-like particles produced in the methylotrophic yeast Pichia pastoris. Biochem Biophys Res Commun. 2001;287:122–125. doi: 10.1006/bbrc.2001.5561. [DOI] [PubMed] [Google Scholar]
- 157.Liu W., Jiang H., Zhou J., Yang X., Tang Y., Fang D. Recombinant dengue virus-like particles from Pichia pastoris: efficient production and immunological properties. Virus Genes. 2010;40:53–59. doi: 10.1007/s11262-009-0418-2. [DOI] [PubMed] [Google Scholar]
- 158.Bitter GA, Egan KM. Expression of heterologous genes in Saccharomyces cerevisiae from vectors utilizing the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Gene 1984;32:263–74. [DOI] [PubMed]
- 159.Vassileva A., Chugh D.A., Swaminathan S., Khanna N. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter. J Biotechnol. 2001;88:21–35. doi: 10.1016/s0168-1656(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 160.Powilleit F., Breinig T., Schmitt M.J. Exploiting the yeast L-A viral capsid for the in vivo assembly of chimeric VLPs as platform in vaccine development and foreign protein expression. PLoS One. 2007;2:e415. doi: 10.1371/journal.pone.0000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sasnauskas K., Bulavaite A., Hale A., Jin L., Knowles W.A., Gedvilaite A. Generation of recombinant virus-like particles of human and non-human polyomaviruses in yeast Saccharomyces cerevisiae. Intervirology. 2002;45:308–317. doi: 10.1159/000067922. [DOI] [PubMed] [Google Scholar]
- 162.Zielonka A., Gedvilaite A., Ulrich R., Lüschow D., Sasnauskas K., Müller H. Generation of virus-like particles consisting of the major capsid protein VP1 of goose hemorrhagic polyomavirus and their application in serological tests. Virus Res. 2006;120:128–137. doi: 10.1016/j.virusres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 163.Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol 2007;178:2361–9 [Erratum in: J Immunol 2007;178:6653]. [DOI] [PubMed]
- 164.Overton H.A., Fujii Y., Price I.R., Jones I.M. The protease and gag gene products of the human immunodeficiency virus: authentic cleavage and post-translational modification in an insect cell expression system. Virology. 1989;170:107–116. doi: 10.1016/0042-6822(89)90357-7. [DOI] [PubMed] [Google Scholar]
- 165.Buonaguro L., Tornesello M.L., Tagliamonte M., Gallo R.C., Wang L.X., Kamin-Lewis R. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol. 2006;80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jaffray A., Shephard E., van Harmelen J., Williamson C., Williamson A.L., Rybicki E.P. Human immunodeficiency virus type 1 subtype C Gag virus-like particle boost substantially improves the immune response to a subtype C gag DNA vaccine in mice. J Gen Virol. 2004;85(Pt 2):409–413. doi: 10.1099/vir.0.19396-0. [DOI] [PubMed] [Google Scholar]
- 167.Chege G.K., Shephard E.G., Meyers A., van Harmelen J., Williamson C., Lynch A. HIV-1 subtype C Pr55gag virus-like particle vaccine efficiently boosts baboons primed with a matched DNA vaccine. J Gen Virol. 2008;89(Pt 9):2214–2227. doi: 10.1099/vir.0.83501-0. [DOI] [PubMed] [Google Scholar]
- 168.Deml L., Schirmbeck R., Reimann J., Wolf H., Wagner R. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology. 1997;235:26–39. doi: 10.1006/viro.1997.8668. [DOI] [PubMed] [Google Scholar]
- 169.Wagner R., Deml L., Schirmbeck R., Niedrig M., Reimann J., Wolf H. Construction, expression, and immunogenicity of chimeric HIV-1 virus-like particles. Virology. 1996;220:128–140. doi: 10.1006/viro.1996.0293. [DOI] [PubMed] [Google Scholar]
- 170.Pillay S., Meyers A., Williamson A.L., Rybicki E.P. Optimization of chimeric HIV-1 virus-like particle production in a baculovirus-insect cell expression system. Biotechnol Prog. 2009;25:1153–1160. doi: 10.1002/btpr.187. [DOI] [PubMed] [Google Scholar]
- 171.Notka F., Stahl-Hennig C., Dittmer U., Wolf H., Wagner R. Accelerated clearance of SHIV in rhesus monkeys by virus-like particle vaccines is dependent on induction of neutralizing antibodies. Vaccine. 1999;18:291–301. doi: 10.1016/s0264-410x(99)00200-5. [DOI] [PubMed] [Google Scholar]
- 172.Kang C.Y., Luo L., Wainberg M.A., Li Y. Development of HIV/AIDS vaccine using chimeric gag-env virus-like particles. Biol Chem. 1999;380:353–364. doi: 10.1515/BC.1999.047. [DOI] [PubMed] [Google Scholar]
- 173.Skountzou I., Quan F.S., Gangadhara S., Ye L., Vzorov A., Selvaraj P. Incorporation of glycosylphosphatidylinositol-anchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007;81:1083–1094. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kirnbauer R., Booy F., Cheng N., Lowy D.R., Schiller J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kirnbauer R., Taub J., Greenstone H., Roden R., Dürst M., Gissmann L. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Christensen N.D., Höpfl R., DiAngelo S.L., Cladel N.M., Patrick S.D., Welsh P.A. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994;75(Pt 9):2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 177.Dupuy C., Buzoni-Gatel D., Touzé A., Bout D., Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thönes N., Müller M. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology. 2007;369:375–388. doi: 10.1016/j.virol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 179.Harper D.M., Franco E.L., Wheeler C., Ferris D.G., Jenkins D., Schuind A. GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 180.Harper D.M., Franco E.L., Wheeler C.M., Moscicki A.B., Romanowski B., Roteli-Martins C.M. HPV Vaccine Study group. Sustained efficacy up to 4. 5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 181.Hildesheim A., Herrero R., Wacholder S., Rodriguez A.C., Solomon D., Bratti M.C. Costa Rican HPV Vaccine Trial Group. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 182.Greenstone H.L., Nieland J.D., de Visser K.E., De Bruijn M.L., Kirnbauer R., Roden R.B. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci U S A. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schäfer K., Müller M., Faath S., Henn A., Osen W., Zentgraf H. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles: induction of cytotoxic T cells and specific tumor protection. Int J Cancer. 1999;81:881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 184.Kaufmann A.M., Nieland J.D., Jochmus I., Baur S., Friese K., Gabelsberger J. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007;121:2794–2800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 185.Kondo K., Ochi H., Matsumoto T., Yoshikawa H., Kanda T. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J Med Virol. 2008;80:841–846. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- 186.Slupetzky K., Gambhira R., Culp T.D., Shafti-Keramat S., Schellenbacher C., Christensen N.D. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007;25:2001–2010. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ball J.M., Hardy M.E., Atmar R.L., Conner M.E., Estes M.K. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guerrero R.A., Ball J.M., Krater S.S., Pacheco S.E., Clements J.D., Estes M.K. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ball J.M., Graham D.Y., Opekun A.R., Gilger M.A., Guerrero R.A., Estes M.K. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 190.Tacket C.O., Sztein M.B., Losonsky G.A., Wasserman S.S., Estes M.K. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 191.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 2010;202:1649–58 [Erratum in: J Infect Dis 2011;203:1036]. [DOI] [PMC free article] [PubMed]
- 192.Atmar R.L., Bernstein D.I., Harro C.D., Al-Ibrahim M.S., Chen W.H., Ferreira J. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Frey S, Treanor JJ, Atmar RL, et al. Phase 1 dosage escalation, safety and immunogenicity study of a bivalent norovirus VLP vaccine by the intramuscular route. Presented at the Annual Meeting of the Infectious Diseases Society of America, Boston, October 20–23; 2011 [abstract 717].
- 194.Zeng C.Q., Wentz M.J., Cohen J., Estes M.K., Ramig R.F. Characterization and replicase activity of double-layered and single-layered rotavirus-like particles expressed from baculovirus recombinants. J Virol. 1996;70:2736–2742. doi: 10.1128/jvi.70.5.2736-2742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang B., Barniak V., Smith R.P., Sharma R., Corsaro B., Hu B. Synthesis of rotavirus-like particles in insect cells: comparative and quantitative analysis. Biotechnol Bioeng. 1998;60:369–374. doi: 10.1002/(sici)1097-0290(19981105)60:3<369::aid-bit14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 196.Vieira H.L., Estêvão C., Roldão A., Peixoto C.C., Sousa M.F., Cruz P.E. Triple layered rotavirus VLP production: kinetics of vector replication, mRNA stability and recombinant protein production. J Biotechnol. 2005;120:72–82. doi: 10.1016/j.jbiotec.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 197.Agnello D., Hervé C.A., Lavaux A., Darniot M., Guillon P., Charpilienne A. Intrarectal immunization with rotavirus 2/6 virus-like particles induces an antirotavirus immune response localized in the intestinal mucosa and protects against rotavirus infection in mice. J Virol. 2006;80:3823–3832. doi: 10.1128/JVI.80.8.3823-3832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bertolotti-Ciarlet A., Ciarlet M., Crawford S.E., Conner M.E., Estes M.K. Immunogenicity and protective efficacy of rotavirus 2/6-virus-like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine. 2003;21:3885–3900. doi: 10.1016/s0264-410x(03)00308-6. [DOI] [PubMed] [Google Scholar]
- 199.Coste A., Sirard J.C., Johansen K., Cohen J., Kraehenbuhl J.P. Nasal immunization of mice with virus-like particles protects offspring against rotavirus diarrhea. J Virol. 2000;74:8966–8971. doi: 10.1128/jvi.74.19.8966-8971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fromantin C., Jamot B., Cohen J., Piroth L., Pothier P., Kohli E. Rotavirus 2/6 virus-like particles administered intranasally in mice, with or without the mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, induce a Th1/Th2-like immune response. J Virol. 2001;75:11010–11016. doi: 10.1128/JVI.75.22.11010-11016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McNeal M.M., Rae M.N., Conner M.E., Ward R.L. Stimulation of local immunity and protection in mice by intramuscular immunization with triple- or double-layered rotavirus particles and QS-21. Virology. 1998;243:158–166. doi: 10.1006/viro.1998.9060. [DOI] [PubMed] [Google Scholar]
- 202.O’Neal C.M., Clements J.D., Estes M.K., Conner M.E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin. Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shuttleworth G., Eckery D.C., Awram P. Oral and intraperitoneal immunization with rotavirus 2/6 virus-like particles stimulates a systemic and mucosal immune response in mice. Arch Virol. 2005;150:341–349. doi: 10.1007/s00705-004-0447-z. [DOI] [PubMed] [Google Scholar]
- 204.Yuan L., Geyer A., Hodgins D.C., Fan Z., Qian Y., Chang K.O. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J Virol. 2000;74:8843–8853. doi: 10.1128/jvi.74.19.8843-8853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.González A.M., Nguyen T.V., Azevedo M.S., Jeong K., Agarib F., Iosef C. Antibody responses to human rotavirus (HRV) in gnotobiotic pigs following a new prime/boost vaccine strategy using oral attenuated HRV priming and intranasal VP2/6 rotavirus-like particle (VLP) boosting with ISCOM. Clin Exp Immunol. 2004;135:361–372. doi: 10.1111/j.1365-2249.2004.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takehara K., Ireland D., Bishop D.H. Co-expression of the hepatitis B surface and core antigens using baculovirus multiple expression vectors. J Gen Virol. 1988;69(Pt 11):2763–2777. doi: 10.1099/0022-1317-69-11-2763. [DOI] [PubMed] [Google Scholar]
- 207.Lechmann M., Murata K., Satoi J., Vergalla J., Baumert T.F., Liang T.J. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417–423. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 208.Jeong S.H., Qiao M., Nascimbeni M., Hu Z., Rehermann B., Murthy K. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78:6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhao W., Liao G.Y., Jiang Y.J., Jiang S.D. Expression and self-assembly of HCV structural proteins into virus-like particles and their immunogenicity. Chin Med J (Engl) 2004;117:1217–1222. [PubMed] [Google Scholar]
- 210.Li T., Takeda N., Miyamura T. Oral administration of hepatitis E virus-like particles induces a systemic and mucosal immune response in mice. Vaccine. 2001;19:3476–3484. doi: 10.1016/s0264-410x(01)00059-7. [DOI] [PubMed] [Google Scholar]
- 211.Li T.C., Suzaki Y., Ami Y., Dhole T.N., Miyamura T., Takeda N. Protection of cynomolgus monkeys against HEV infection by oral administration of recombinant hepatitis E virus-like particles. Vaccine. 2004;22:370–377. doi: 10.1016/j.vaccine.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 212.Bright R.A., Carter D.M., Daniluk S., Toapanta F.R., Ahmad A., Gavrilov V. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 213.Quan F.S., Huang C., Compans R.W., Kang S.M. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Matassov D., Cupo A., Galarza J.M. A novel intranasal virus-like particle (VLP) vaccine designed to protect against the pandemic 1918 influenza A virus (H1N1) Viral Immunol. 2007;20:441–452. doi: 10.1089/vim.2007.0027. [DOI] [PubMed] [Google Scholar]
- 215.Bright R.A., Carter D.M., Crevar C.J., Toapanta F.R., Steckbeck J.D., Cole K.S. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mahmood K., Bright R.A., Mytle N., Carter D.M., Crevar C.J., Achenbach J.E. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 217.Krammer F., Nakowitsch S., Messner P., Palmberger D., Ferko B., Grabherr R. Swine-origin pandemic H1N1 influenza virus-like particles produced in insect cells induce hemagglutination inhibiting antibodies in BALB/c mice. Biotechnol J. 2010;5:17–23. doi: 10.1002/biot.200900267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pushko P., Kort T., Nathan M., Pearce M.B., Smith G., Tumpey T.M. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010;28:4771–4776. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 219.Quan F.S., Vunnava A., Compans R.W., Kang S.M. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One. 2010;5:e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Quan F.S., Steinhauer D., Huang C., Ross T.M., Compans R.W., Kang S.M. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–3361. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pushko P., Pearce M.B., Ahmad A., Tretyakova I., Smith G., Belser J.A. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011;29:5911–5918. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 222.Ross T.M., Mahmood K., Crevar C.J., Schneider-Ohrum K., Heaton P.M., Bright R.A. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4:e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang B.Z., Quan F.S., Kang S.M., Bozja J., Skountzou I., Compans R.W. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pan Y.S., Wei H.J., Chang C.C., Lin C.H., Wei T.S., Wu S.C. Construction and characterization of insect cell-derived influenza VLP: cell binding, fusion, and EGFP incorporation. J Biomed Biotechnol. 2010;2010:506363. doi: 10.1155/2010/506363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pushko P., Tumpey T.M., Van Hoeven N., Belser J.A., Robinson R., Nathan M. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25:4283–4290. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 226.Kang S.M., Yoo D.G., Lipatov A.S., Song J.M., Davis C.T., Quan F.S. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Song J.M., Hossain J., Yoo D.G., Lipatov A.S., Davis C.T., Quan F.S. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Khurana S., Wu J., Verma N., Verma S., Raghunandan R., Manischewitz J. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol. 2011;85:10945–10954. doi: 10.1128/JVI.05406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pearton M, Kang SM, Song JM, Anstey AV, Ivory M, Compans RW, et al. Changes in human Langerhans cells following intradermal injection of influenza virus-like particle vaccines. PLoS One 2010;5:e12410 [Erratum in: PLoS One 2010;5]. [DOI] [PMC free article] [PubMed]
- 230.Wang B.Z., Xu R., Quan F.S., Kang S.M., Wang L., Compans R.W. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One. 2010;5:e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Song J.M., Van Rooijen N., Bozja J., Compans R.W., Kang S.M. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Haynes J.R., Dokken L., Wiley J.A., Cawthon A.G., Bigger J., Harmsen A.G. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27:530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 233.Hall C.B., McCarthy C.A. Respiratory syncytial virus. In: Mandell J.L., Bennett J.E., Dolin R., editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 6th ed. Churchill Livingstone; London: 2004. pp. 2009–2021. [Google Scholar]
- 234.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 235.Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, et al. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 2011;204:987–95 [Erratum in: J Infect Dis 2012;205:520]. [DOI] [PMC free article] [PubMed]
- 236.Glenn G, Lu H, Rahgundran R, Kpamegan E, Massare M, Thomas N, et al. Immunogenicity of a recombinant RSV F protein nanoparticle vaccine manufactured in insect cells: induction of palivizumab-like activity. http://www.novavax.com/download/file/presentations/ISRVI_poster_final.pdf [accessed 28 May, 2012].
- 237.Novavax, Inc. Press Release October 3, 2011. NOVAVAX Reports Positive Results from Phase I Trial of Respiratory Syncytial Virus Vaccine Candidate. http://www.novavax.com/download/releases/10-03-2011%20Novavax%20RSV%2010-1-final%20clean-gg3.pdf [accessed 15 March, 2012].
- 238.Amexis G., Young N.S. Parvovirus B19 empty capsids as vaccine platform for presentation of antigenic determinants of dengue 2 virus. J Inf Dis. 2006;194:790–794. doi: 10.1086/506361. [DOI] [PubMed] [Google Scholar]
- 239.Liu L., Celma C.C., Roy P. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol J. 2008;5:82. doi: 10.1186/1743-422X-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bosio C.M., Moore B.D., Warfield K.L., Ruthel G., Mohamadzadeh M., Aman M.J. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 241.Sun Y, Carrion R Jr, Ye L, Wen Z, Ro YT, Brasky K, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology 2009;383:12–21 [Erratum in: Virology 2010;399:186]. [DOI] [PMC free article] [PubMed]
- 242.Warfield K.L., Posten N.A., Swenson D.L., Olinger G.G., Esposito D., Gillette W.K. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis. 2007;196(Suppl. 2):S421–S429. doi: 10.1086/520612. [DOI] [PubMed] [Google Scholar]
- 243.Warfield K.L., Swenson D.L., Olinger G.G., Kalina W.V., Aman M.J., Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 244.Ye L., Lin J., Sun Y., Bennouna S., Lo M., Wu Q. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 245.Bai B., Hu Q., Hu H., Zhou P., Shi Z., Meng J. Virus-like particles of SARS-like coronavirus formed by membrane proteins from different origins demonstrate stimulating activity in human dendritic cells. PLoS One. 2008;3:e2685. doi: 10.1371/journal.pone.0002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lu X., Chen Y., Bai B., Hu H., Tao L., Yang J. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology. 2007;122:496–502. doi: 10.1111/j.1365-2567.2007.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bernstein D.I., El Sahly H.M., Keitel W.A., Wolff M., Simone G., Segawa C. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine. 2011;29:7357–7363. doi: 10.1016/j.vaccine.2011.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brown C.S., Van Lent J.W., Vlak J.M., Spaan W.J. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J Virol. 1991;65:2702–2706. doi: 10.1128/jvi.65.5.2702-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L.J. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A. 1991;88:4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shelly DA, van Cleave V. Parvovirus B19 VLP vaccine manufacturing. Genet Eng Biotechnol News 2009;29(16). http://www.genengnews.com/gen-articles/parvovirus-b19-vlp-vaccine-manufacturing/3042/.
- 251.Bräutigam S., Snezhkov E., Bishop D.H. Formation of poliovirus-like particles by recombinant baculoviruses expressing the individual VP0, VP3, and VP1 proteins by comparison to particles derived from the expressed poliovirus polyprotein. Virology. 1993;192:512–524. doi: 10.1006/viro.1993.1067. [DOI] [PubMed] [Google Scholar]
- 252.Chung Y.C., Huang J.H., Lai C.W., Sheng H.C., Shih S.R., Ho M.S. Expression, purification and characterization of enterovirus-71 virus-like particles. World J Gastroenterol. 2006;12:921–927. doi: 10.3748/wjg.v12.i6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 254.Tatman J.D., Preston V.G., Nicholson P., Elliott R.M., Rixon F.J. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J Gen Virol. 1994;75(Pt 5):1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]
- 255.Caparrós-Wanderley W., Clark B., Griffin B.E. Effect of dose and long-term storage on the immunogenicity of murine polyomavirus VP1 virus-like particles. Vaccine. 2004;22:352–361. doi: 10.1016/j.vaccine.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 256.Chang D., Fung C.Y., Ou W.C., Chao P.C., Li S.Y., Wang M. Self-assembly of the JC virus major capsid protein. VP1, expressed in insect cells. J Gen Virol. 1997;78(Pt 6):1435–1439. doi: 10.1099/0022-1317-78-6-1435. [DOI] [PubMed] [Google Scholar]
- 257.Goldmann C., Petry H., Frye S., Ast O., Ebitsch S., Jentsch K.D. Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J Virol. 1999;73:4465–4469. doi: 10.1128/jvi.73.5.4465-4469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ishizu K.I., Watanabe H., Han S.I., Kanesashi S.N., Hoque M., Yajima H. Roles of disulfide linkage and calcium ion-mediated interactions in assembly and disassembly of virus-like particles composed of simian virus 40 VP1 capsid protein. J Virol. 2001;75:61–72. doi: 10.1128/JVI.75.1.61-72.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kosukegawa A., Arisaka F., Takayama M., Yajima H., Kaidow A., Handa H. Purification and characterization of virus-like particles and pentamers produced by the expression of SV40 capsid proteins in insect cells. Biochim Biophys Acta. 1996;1290:37–45. [PubMed] [Google Scholar]
- 260.Montross L., Watkins S., Moreland R.B., Mamon H., Caspar D.L., Garcea R.L. Nuclear assembly of polyomavirus capsids in insect cells expressing the major capsid protein VP1. J Virol. 1991;65:4991–4998. doi: 10.1128/jvi.65.9.4991-4998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Roy P., French T., Erasmus B.J. Protective efficacy of virus-like particles for bluetongue disease. Vaccine. 1992;10:28–32. doi: 10.1016/0264-410x(92)90415-g. [DOI] [PubMed] [Google Scholar]
- 262.Roy P., Bishop D.H., LeBlois H., Erasmus B.J. Long-lasting protection of sheep against bluetongue challenge after vaccination with virus-like particles: evidence for homologous and partial heterologous protection. Vaccine. 1994;12:805–811. doi: 10.1016/0264-410x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 263.Koch G., van Roozelaar D.J., Verschueren C.A., van der Eb A.J., Noteborn M.H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine. 1995;13:763–770. doi: 10.1016/0264-410x(94)00034-k. [DOI] [PubMed] [Google Scholar]
- 264.Noteborn M.H., Verschueren C.A., Koch G., Van der Eb A.J. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J Gen Virol. 1998;79(Pt 12):3073–3077. doi: 10.1099/0022-1317-79-12-3073. [DOI] [PubMed] [Google Scholar]
- 265.Fernández-Arias A., Risco C., Martínez S., Albar J.P., Rodríguez J.F. Expression of ORF A1 of infectious bursal disease virus results in the formation of virus-like particles. J Gen Virol. 1998;79(Pt 5):1047–1054. doi: 10.1099/0022-1317-79-5-1047. [DOI] [PubMed] [Google Scholar]
- 266.Hu Y.C., Bentley W.E. Effect of MOI ratio on the composition and yield of chimeric infectious bursal disease virus-like particles by baculovirus co-infection: deterministic predictions and experimental results. Biotechnol Bioeng. 2001;75:104–119. doi: 10.1002/bit.1170. [DOI] [PubMed] [Google Scholar]
- 267.Kibenge F.S., Qian B., Nagy E., Cleghorn J.R., Wadowska D. Formation of virus-like particles when the polyprotein gene (segment A) of infectious bursal disease virus is expressed in insect cells. Can J Vet Res. 1999;63:49–55. [PMC free article] [PubMed] [Google Scholar]
- 268.Martinez-Torrecuadrada J.L., Saubi N., Pagès-Manté A., Castón J.R., Espuña E., Casal J.I. Structure-dependent efficacy of infectious bursal disease virus (IBDV) recombinant vaccines. Vaccine. 2003;21:1952–1960. [PubMed] [Google Scholar]
- 269.Nagy E., Huber P., Krell P.J., Derbyshire J.B. Synthesis of Newcastle disease virus (NDV)-like envelopes in insect cells infected with a recombinant baculovirus expressing the haemagglutinin-neuraminidase of NDV. J Gen Virol. 1991;72(Pt 3):753–756. doi: 10.1099/0022-1317-72-3-753. [DOI] [PubMed] [Google Scholar]
- 270.Kim Y., Kim J., Kang K., Lyoo Y.S. Characterization of the recombinant proteins of porcine circovirus type2 field isolate expressed in the baculovirus system. J Vet Sci. 2002;3:19–23. [PubMed] [Google Scholar]
- 271.Laurent S., Vautherot J.F., Madelaine M.F., Le Gall G., Rasschaert D. Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into viruslike particles and induces protection. J Virol. 1994;68:6794–6798. doi: 10.1128/jvi.68.10.6794-6798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ko Y.J., Choi K.S., Nah J.J., Paton D.J., Oem J.K., Wilsden G. Noninfectious virus-like particle antigen for detection of swine vesicular disease virus antibodies in pigs by enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2005;12:922–929. doi: 10.1128/CDLI.12.8.922-929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pérez de Diego A.C., Athmaram T.N., Stewart M., Rodríguez-Sánchez B., Sánchez-Vizcaíno J.M., Noad R. Characterization of protection afforded by a bivalent virus-like particle vaccine against bluetongue virus serotypes 1 and 4 in sheep. PLoS One. 2011;6:e26666. doi: 10.1371/journal.pone.0026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hainisch E.K., Brandt S., Shafti-Keramat S., Van den Hoven R., Kirnbauer R. Safety and immunogenicity of BPV-1 L1 virus-like particles in a dose-escalation vaccination trial in horses. Equine Vet J. 2012;44:107–111. doi: 10.1111/j.2042-3306.2011.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jeoung H.Y., Lee W.H., Jeong W., Shin B.H., Choi H.W., Lee H.S. Immunogenicity and safety of virus-like particle of the porcine encephalomyocarditis virus in pig. Virol J. 2011;8:170. doi: 10.1186/1743-422X-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Porcilis PCV. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/veterinary/000135/WC500061523.pdf [accessed 15 August, 2012].
- 277.Porcilis PCV. Scientific Discussion. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/veterinary/000135/WC500061520.pdf [accessed 15 August, 2012].
- 278.Fort M., Sibila M., Pérez-Martín E., Nofrarías M., Mateu E., Segalés J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine. 2009;27:4031–4037. doi: 10.1016/j.vaccine.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 279.Martelli P., Ferrari L., Morganti M., De Angelis E., Bonilauri P., Guazzetti S. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet Microbiol. 2011;149:339–351. doi: 10.1016/j.vetmic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 280.Merck Animal Health. News Release 26 January 2010. Intervet/Schering-Plough Animal Health receives European approval for a single-shot dosage scheme of PORCILIS PCV. http://www.merck-animal-health.com/news/2010-01-26-porcilis-pcv-eu-single-shot.aspx [accessed 15 August, 2012].
- 281.Grillberger L., Kreil T.R., Nasr S., Reiter M. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J. 2009;4:186–201. doi: 10.1002/biot.200800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Walter J.K., Werz W., Berthold W. Process scale considerations in evaluation studies and scale-up. Dev Biol Stand. 1996;88:99–108. [PubMed] [Google Scholar]
- 283.Yerushalmi B, Raz R, Blondheim O, Shumov E, Koren R, Dagan R. Safety and immunogenicity of a novel mammalian cell-derived recombinant hepatitis B vaccine containing Pre-S1 and Pre-S2 antigens in neonates. Pediatr Infect Dis J 1997;16:587–92 [Erratum in: Pediatr Infect Dis J 1997;16:1037]. [DOI] [PubMed]
- 284.Hellström U.B., Madalinski K., Sylvan S.P. PreS1 epitope recognition in newborns after vaccination with the third-generation Sci-B-Vac vaccine and their relation to the antibody response to hepatitis B surface antigen. Virol J. 2009;6:7. doi: 10.1186/1743-422X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bucher B., Francioli P., Geudelin B., Fritzell B., Lavanchy D., Frei P.C. Immunogenicity of a recombinant Pre-S2-containing hepatitis B vaccine versus plasma-derived vaccine administered as a booster. Eur J Clin Microbiol Infect Dis. 1994;13:212–217. doi: 10.1007/BF01974539. [DOI] [PubMed] [Google Scholar]
- 286.Shapira M.Y., Zeira E., Adler R., Shouval D. Rapid seroprotection against hepatitis B following the first dose of a Pre-S1/Pre-S2/S vaccine. J Hepatol. 2001;34:123–127. doi: 10.1016/s0168-8278(00)00082-9. [DOI] [PubMed] [Google Scholar]
- 287.The European Agency on the Evaluation of Medicinal Products. Public Statement on Hepacare. London, 17 December 2002. http://www.emea.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018349.pdf [accessed 15 August, 2012].
- 288.Chang G.J., Hunt A.R., Holmes D.A., Springfield T., Chiueh T.S., Roehrig J.T. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology. 2003;306:170–180. doi: 10.1016/s0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 289.Purdy D.E., Chang G.J. Secretion of noninfectious dengue virus-like particles and identification of amino acids in the stem region involved in intracellular retention of envelope protein. Virology. 2005;333:239–250. doi: 10.1016/j.virol.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 290.Zhang S., Liang M., Gu W., Li C., Miao F., Wang X. Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice. Virol J. 2011;8:333. doi: 10.1186/1743-422X-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hsieh S.C., Liu I.J., King C.C., Chang G.J., Wang W.K. A strong endoplasmic reticulum retention signal in the stem-anchor region of envelope glycoprotein of dengue virus type 2 affects the production of virus-like particles. Virology. 2008;374:338–350. doi: 10.1016/j.virol.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 292.Hsieh S.C., Tsai W.Y., Wang W.K. The length of and nonhydrophobic residues in the transmembrane domain of dengue virus envelope protein are critical for its retention and assembly in the endoplasmic reticulum. J Virol. 2010;84:4782–4797. doi: 10.1128/JVI.01963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chang G.J., Hunt A.R., Davis B. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J Virol. 2000;74:4244–4252. doi: 10.1128/jvi.74.9.4244-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chang G.J., Davis B.S., Hunt A.R., Holmes D.A., Kuno G. Flavivirus DNA vaccines: current status and potential. Ann N Y Acad Sci. 2001;951:272–285. [PubMed] [Google Scholar]
- 295.Davis B.S., Chang G.J., Cropp B., Roehrig J.T., Martin D.A., Mitchell C.J. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Martin J.E., Pierson T.C., Hubka S., Rucker S., Gordon I.J., Enama M.E. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kroeger M.A., McMinn P.C. Murray Valley encephalitis virus recombinant subviral particles protect mice from lethal challenge with virulent wild-type virus. Arch Virol. 2002;147:1155–1172. doi: 10.1007/s00705-002-0809-3. [DOI] [PubMed] [Google Scholar]
- 298.Kolesnikova L., Berghöfer B., Bamberg S., Becker S. Multivesicular bodies as a platform for formation of the Marburg virus envelope. J Virol. 2004;78:12277–12287. doi: 10.1128/JVI.78.22.12277-12287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu Y., Cocka L., Okumura A., Zhang Y.A., Sunyer J.O., Harty R.N. Conserved motifs within Ebola and Marburg virus VP40 proteins are important for stability, localization, and subsequent budding of virus-like particles. J Virol. 2010;84:2294–2303. doi: 10.1128/JVI.02034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Noda T., Sagara H., Suzuki E., Takada A., Kida H., Kawaoka Y. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol. 2002;76:4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Swenson D.L., Warfield K.L., Negley D.L., Schmaljohn A., Aman M.J., Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–3042. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 302.Warfield K.L., Bosio C.M., Welcher B.C., Deal E.M., Mohamadzadeh M., Schmaljohn A. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yamayoshi S., Kawaoka Y. Mapping of a region of Ebola virus VP40 that is important in the production of virus-like particles. J Infect Dis. 2007;196(Suppl. 2):S291–S295. doi: 10.1086/520595. [DOI] [PubMed] [Google Scholar]
- 304.Warfield K.L., Swenson D.L., Negley D.L., Schmaljohn A.L., Aman M.J., Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine. 2004;22:3495–3502. doi: 10.1016/j.vaccine.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 305.Warfield K.L., Perkins J.G., Swenson D.L., Deal E.M., Bosio C.M., Aman M.J. Role of natural killer cells in innate protection against lethal ebola virus infection. J Exp Med. 2004;200:169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Warfield K.L., Olinger G., Deal E.M., Swenson D.L., Bailey M., Negley D.L. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175:1184–1191. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- 307.Betenbaugh M., Yu M., Kuehl K., White J., Pennock D., Spik K. Nucleocapsid- and virus-like particles assemble in cells infected with recombinant baculoviruses or vaccinia viruses expressing the M and the S segments of Hantaan virus. Virus Res. 1995;38:111–124. doi: 10.1016/0168-1702(95)00053-s. [DOI] [PubMed] [Google Scholar]
- 308.Li C., Liu F., Liang M., Zhang Q., Wang X., Wang T. Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine. 2010;28:4294–4300. doi: 10.1016/j.vaccine.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 309.Akahata W., Yang Z.Y., Andersen H., Sun S., Holdaway H.A., Kong W.P. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Alexander D.J. Newcastle disease. other avian Paramyxoviruses and pneumovirus infections: Newcastle disease. In: Saif Y.M., editor. Diseases of poultry. Iowa State University Press; USA: 2003. pp. 64–87. [Google Scholar]
- 311.Morrison T.G. Newcastle disease virus-like particles as a platform for the development of vaccines for human and agricultural pathogens. Future Virol. 2010;5:545–554. doi: 10.2217/fvl.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chua K.B., Goh K.J., Wong K.T., Kamarulzaman A., Tan P.S., Ksiazek T.G. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 313.Chua K.B., Bellini W.J., Rota P.A., Harcourt B.H., Tamin A., Lam S.K. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 314.Lam S.K. Nipah virus—a potential agent of bioterrorism? Antiviral Res. 2003;57:113–119. doi: 10.1016/s0166-3542(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 315.Walpita P., Barr J., Sherman M., Basler C.F., Wang L. Vaccine potential of Nipah virus-like particles. PLoS One. 2011;6:e18437. doi: 10.1371/journal.pone.0018437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.McGinnes L.W., Gravel K.A., Finberg R.W., Kurt-Jones E.A., Massare M.J., Smith G. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011;85:366–377. doi: 10.1128/JVI.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Murawski M.R., McGinnes L.W., Finberg R.W., Kurt-Jones E.A., Massare M.J., Smith G. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010;84:1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Takimoto T., Hurwitz J.L., Coleclough C., Prouser C., Krishnamurthy S., Zhan X. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78:6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhan X., Hurwitz J.L., Krishnamurthy S., Takimoto T., Boyd K., Scroggs R.A. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jones B., Zhan X., Mishin V., Slobod K.S., Surman S., Russell C.J. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27:1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhan X., Slobod K.S., Krishnamurthy S., Luque L.E., Takimoto T., Jones B. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008;26:3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mok H., Lee S., Utley T.J., Shepherd B.E., Polosukhin V.V., Collier M.L. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81:13710–13722. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yu J.R., Kim S., Lee J.B., Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wu C.Y., Yeh Y.C., Yang Y.C., Chou C., Liu M.T., Wu H.S. Mammalian expression of virus-like particles for advanced mimicry of authentic influenza virus. PLoS One. 2010;5:e9784. doi: 10.1371/journal.pone.0009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Szécsi J., Boson B., Johnsson P., Dupeyrot-Lacas P., Matrosovich M., Klenk H.D. Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic H5N1 and H7N1 influenza viruses. Virol J. 2006;3:70. doi: 10.1186/1743-422X-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.D’Aoust M.A., Lavoie P.O., Couture M.M., Trépanier S., Guay J.M., Dargis M. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 327.Santi L., Batchelor L., Huang Z., Hjelm B., Kilbourne J., Arntzen C.J. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lai H., Chen Q. Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current Good Manufacture Practice regulations. Plant Cell Rep. 2012;31:573–584. doi: 10.1007/s00299-011-1196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Scotti N., Alagna F., Ferraiolo E., Formisano G., Sannino L., Buonaguro L. High-level expression of the HIV-1 Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta. 2009;229:1109–1122. doi: 10.1007/s00425-009-0898-2. [DOI] [PubMed] [Google Scholar]
- 330.Meyers A., Chakauya E., Shephard E., Tanzer F.L., Maclean J., Lynch A. Expression of HIV-1 antigens in plants as potential subunit vaccines. BMC Biotechnol. 2008;8:53. doi: 10.1186/1472-6750-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Scotti N., Buonaguro L., Tornesello M.L., Cardi T., Buonaguro F.M. Plant-based anti-HIV-1 strategies: vaccine molecules and antiviral approaches. Expert Rev Vaccines. 2010;9:925–936. doi: 10.1586/erv.10.79. [DOI] [PubMed] [Google Scholar]
- 332.Shchelkunov S.N., Salyaev R.K., Pozdnyakov S.G., Rekoslavskaya N.I., Nesterov A.E., Ryzhova T.S. Immunogenicity of a novel, bivalent, plant-based oral vaccine against hepatitis B and human immunodeficiency viruses. Biotechnol Lett. 2006;28:959–967. doi: 10.1007/s10529-006-9028-4. [DOI] [PubMed] [Google Scholar]
- 333.Greco R., Michel M., Guetard D., Cervantes-Gonzalez M., Pelucchi N., Wain-Hobson S. Production of recombinant HIV-1/HBV virus-like particles in Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent plant-based vaccine. Vaccine. 2007;25:8228–8240. doi: 10.1016/j.vaccine.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 334.Guetard D., Greco R., Cervantes Gonzalez M., Celli S., Kostrzak A., Langlade-Demoyen P. Immunogenicity and tolerance following HIV-1/HBV plant-based oral vaccine administration. Vaccine. 2008;26:4477–4485. doi: 10.1016/j.vaccine.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 335.Dalgleish A.G., Chanh T.C., Kennedy R.C., Kanda P., Clapham P.R., Weiss R.A. Neutralization of diverse HIV-1 strains by monoclonal antibodies raised against a gp41 synthetic peptide. Virology. 1988;165:209–215. doi: 10.1016/0042-6822(88)90674-5. [DOI] [PubMed] [Google Scholar]
- 336.Durrani Z., McInerney T.L., McLain L., Jones T., Bellaby T., Brennan F.R. Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J Immunol Methods. 1998;220:93–103. doi: 10.1016/s0022-1759(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 337.Yusibov V, Streatfield SJ, Kushnir N, Roy G, Padmanaban A. Hybrid viral vectors for vaccine and antibody production in plants. Curr Pharmaceut Des; in press. [DOI] [PubMed]
- 338.Brodzik R., Bandurska K., Deka D., Golovkin M., Koprowski H. Advances in alfalfa mosaic virus-mediated expression of anthrax antigen in planta. Biochem Biophys Res Commun. 2005;338:717–722. doi: 10.1016/j.bbrc.2005.09.196. [DOI] [PubMed] [Google Scholar]
- 339.Bandurska K., Brodzik R., Spitsin S., Kohl T., Portocarrero C., Smirnov Y. Plant-produced hepatitis B core protein chimera carrying anthrax protective antigen domain-4. Hybridoma (Larchmt) 2008;27:241–247. doi: 10.1089/hyb.2008.0008. [DOI] [PubMed] [Google Scholar]
- 340.Modelska A., Dietzschold B., Sleysh N., Fu Z.F., Steplewski K., Hooper D.C. Immunization against rabies with plant-derived antigen. Proc Natl Acad Sci U S A. 1998;95:2481–2485. doi: 10.1073/pnas.95.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Medicago, Inc. News Release January 19, 2012. Medicago Announces Expansion of Pipeline with the Development of a Vaccine for Rabies. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2012/Medicago-Announces-Expansion-of-Pipeline-with-the-Development-of-a-Vaccine-for-Rabies1127985/default.aspx [accessed 24 March, 2012].
- 342.Taylor K.M., Lin T., Porta C., Mosser A.G., Giesing H.A., Lomonossoff G.P. Influence of three-dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J Mol Recognit. 2000;13:71–82. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<71::AID-JMR489>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 343.Porta C., Spall V.E., Loveland J., Johnson J.E., Barker P.J., Lomonossoff G.P. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology. 1994;202:949–955. doi: 10.1006/viro.1994.1417. [DOI] [PubMed] [Google Scholar]
- 344.Brennan F.R., Jones T.D., Longstaff M., Chapman S., Bellaby T., Smith H. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine. 1999;17:1846–1857. doi: 10.1016/s0264-410x(98)00485-x. [DOI] [PubMed] [Google Scholar]
- 345.Rennermalm A., Li Y.H., Bohaufs L., Jarstrand C., Brauner A., Brennan F.R. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine. 2001;19:3376–3383. doi: 10.1016/s0264-410x(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 346.Brennan F.R., Jones T.D., Gilleland L.B., Bellaby T., Xu F., North P.C. Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology. 1999;145(Pt 1):211–220. doi: 10.1099/13500872-145-1-211. [DOI] [PubMed] [Google Scholar]
- 347.Brennan F.R., Gilleland L.B., Staczek J., Bendig M.M., Hamilton W.D., Gilleland H.E., Jr. A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology. 1999;145(Pt 8):2061–2067. doi: 10.1099/13500872-145-8-2061. [DOI] [PubMed] [Google Scholar]
- 348.Dalsgaard K., Uttenthal A., Jones T.D., Xu F., Merryweather A., Hamilton W.D. Plant-derived vaccine protects target animals against a viral disease. Nat Biotechnol. 1997;15:248–252. doi: 10.1038/nbt0397-248. [DOI] [PubMed] [Google Scholar]
- 349.Langeveld J.P., Brennan F.R., Martínez-Torrecuadrada J.L., Jones T.D., Boshuizen R.S., Vela C. Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parvovirus. Vaccine. 2001;19:3661–3670. doi: 10.1016/s0264-410x(01)00083-4. [DOI] [PubMed] [Google Scholar]
- 350.Nicholas B.L., Brennan F.R., Martinez-Torrecuadrada J.L., Casal J.I., Hamilton W.D., Wakelin D. Characterization of the immune response to canine parvovirus induced by vaccination with chimaeric plant viruses. Vaccine. 2002;20:2727–2734. doi: 10.1016/s0264-410x(02)00200-1. [DOI] [PubMed] [Google Scholar]
- 351.Nicholas B.L., Brennan F.R., Hamilton W.D., Wakelin D. Effect of priming/booster immunisation protocols on immune response to canine parvovirus peptide induced by vaccination with a chimaeric plant virus construct. Vaccine. 2003;21:2441–2447. doi: 10.1016/s0264-410x(03)00054-9. [DOI] [PubMed] [Google Scholar]
- 352.Varsani A., Williamson A.L., Rose R.C., Jaffer M., Rybicki E.P. Expression of Human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv Xanthi. Arch Virol. 2003;148:1771–1786. doi: 10.1007/s00705-003-0119-4. [DOI] [PubMed] [Google Scholar]
- 353.Kohl T.O., Hitzeroth I.I., Christensen N.D., Rybicki E.P. Expression of HPV-11 L1 protein in transgenic Arabidopsis thaliana and Nicotiana tabacum. BMC Biotechnol. 2007;7:56. doi: 10.1186/1472-6750-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Warzecha H., Mason H.S., Lane C., Tryggvesson A., Rybicki E., Williamson A.L. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J Virol. 2003;77:8702–8711. doi: 10.1128/JVI.77.16.8702-8711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Biemelt S., Sonnewald U., Galmbacher P., Willmitzer L., Müller M. Production of human papillomavirus type 16 virus-like particles in transgenic plants. J Virol. 2003;77:9211–9220. doi: 10.1128/JVI.77.17.9211-9220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Waheed M.T., Thönes N., Müller M., Hassan S.W., Razavi N.M., Lössl E. Transplastomic expression of a modified human papillomavirus L1 protein leading to the assembly of capsomeres in tobacco: a step towards cost-effective second-generation vaccines. Transgenic Res. 2011;20:271–282. doi: 10.1007/s11248-010-9415-4. [DOI] [PubMed] [Google Scholar]
- 357.Varsani A., Williamson A.L., Stewart D., Rybicki E.P. Transient expression of Human papillomavirus type 16 L1 protein in Nicotiana benthamiana using an infectious tobamovirus vector. Virus Res. 2006;120:91–96. doi: 10.1016/j.virusres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 358.Regnard G.L., Halley-Stott R.P., Tanzer F.L., Hitzeroth I.I., Rybicki E.P. High level protein expression in plants through the use of a novel autonomously replicating geminivirus shuttle vector. Plant Biotechnol J. 2010;8:38–46. doi: 10.1111/j.1467-7652.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 359.Huang Z., Elkin G., Maloney B.J., Beuhner N., Arntzen C.J., Thanavala Y. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005;23:1851–1858. doi: 10.1016/j.vaccine.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 360.Mason H.S., Ball J.M., Shi J.J., Jiang X., Estes M.K., Arntzen C.J. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhang X., Buehner N.A., Hutson A.M., Estes M.K., Mason H.S. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol J. 2006;4:419–432. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 362.Tacket C.O., Mason H.S., Losonsky G., Estes M.K., Levine M.M., Arntzen C.J. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 363.Tacket C.O. Plant-based vaccines against diarrheal diseases. Trans Am Clin Climatol Assoc. 2007;118:79–87. [PMC free article] [PubMed] [Google Scholar]
- 364.Huang Z., Chen Q., Hjelm B., Arntzen C., Mason H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol Bioeng. 2009;103:706–714. doi: 10.1002/bit.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Saldaña S., Esquivel Guadarrama F., Olivera Flores Tde J., Arias N., López S., Arias C. Production of rotavirus-like particles in tomato (Lycopersicon esculentum L.) fruit by expression of capsid proteins VP2 and VP6 and immunological studies. Viral Immunol. 2006;19:42–53. doi: 10.1089/vim.2006.19.42. [DOI] [PubMed] [Google Scholar]
- 366.Yang Y., Li X., Yang H., Qian Y., Zhang Y., Fang R. Immunogenicity and virus-like particle formation of rotavirus capsid proteins produced in transgenic plants. Sci China Life Sci. 2011;54:82–89. doi: 10.1007/s11427-010-4104-3. [DOI] [PubMed] [Google Scholar]
- 367.Kong Q., Richter L., Yang Y.F., Arntzen C.J., Mason H.S., Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci U S A. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Mason H.S., Lam D.M., Arntzen C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci U S A. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Richter L.J., Thanavala Y., Arntzen C.J., Mason H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- 370.Thanavala Y., Yang Y.F., Lyons P., Mason H.S., Arntzen C. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1995;92:3358–3361. doi: 10.1073/pnas.92.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Smith M.L., Richter L., Arntzen C.J., Shuler M.L., Mason H.S. Structural characterization of plant-derived hepatitis B surface antigen employed in oral immunization studies. Vaccine. 2003;21:4011–4021. doi: 10.1016/s0264-410x(03)00268-8. [DOI] [PubMed] [Google Scholar]
- 372.Tsuda S., Yoshioka K., Tanaka T., Iwata A., Yoshikawa A., Watanabe Y. Application of the human hepatitis B virus core antigen from transgenic tobacco plants for serological diagnosis. Vox Sang. 1998;74(3):148–155. [PubMed] [Google Scholar]
- 373.Mechtcheriakova I.A., Eldarov M.A., Nicholson L., Shanks M., Skryabin K.G., Lomonossoff G.P. The use of viral vectors to produce hepatitis B virus core particles in plants. J Virol Methods. 2006;131:10–15. doi: 10.1016/j.jviromet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 374.Huang Z., Santi L., LePore K., Kilbourne J., Arntzen C.J., Mason H.S. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi: 10.1016/j.vaccine.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 375.Santi L., Huang Z., Mason H. Virus-like particles production in green plants. Methods. 2006;40:66–76. doi: 10.1016/j.ymeth.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.D’Aoust M.A., Couture M.M., Charland N., Trépanier S., Landry N., Ors F. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8:607–619. doi: 10.1111/j.1467-7652.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 377.Medicago Inc. News Release June 30, 2011. Medicago reports positive Phase II final results for its avian flu pandemic vaccine. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-reports-positive-Phase-II-final-results-for-its-avian-flu-pandemic-vaccine1125833/default.aspx [accessed 18 March, 2012].
- 378.Medicago, Inc. News Release September 12, 2011. Medicago to present add itional positive clinical data at the 2011ESWI influenza conference. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-to-present-additional-positive-clinical-data-at-the-2011ESWI-influenza-conference1126562/default.aspx [accessed 12 April, 2012].
- 379.Wigdorovitz A., Pérez Filgueira D.M., Robertson N., Carrillo C., Sadir A.M., Morris T.J. Protection of mice against challenge with foot and mouth disease virus (FMDV) by immunization with foliar extracts from plants infected with recombinant tobacco mosaic virus expressing the FMDV structural protein VP1. Virology. 1999;264:85–91. doi: 10.1006/viro.1999.9923. [DOI] [PubMed] [Google Scholar]
- 380.Wu L., Jiang L., Zhou Z., Fan J., Zhang Q., Zhu H. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine. 2003;21:4390–4398. doi: 10.1016/s0264-410x(03)00428-6. [DOI] [PubMed] [Google Scholar]
- 381.Yang C.D., Liao J.T., Lai C.Y., Jong M.H., Liang C.M., Lin Y.L. Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol. 2007;7:62. doi: 10.1186/1472-6750-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Zhang Y., Li J., Pu H., Jin J., Zhang X., Chen M. Development of Tobacco necrosis virus A as a vector for efficient and stable expression of FMDV VP1 peptides. Plant Biotechnol J. 2010;8:506–523. doi: 10.1111/j.1467-7652.2010.00500.x. [DOI] [PubMed] [Google Scholar]
- 383.Nuzzaci M., Bochicchio I., De Stradis A., Vitti A., Natilla A., Piazzolla P. Structural and biological properties of Cucumber mosaic virus particles carrying hepatitis C virus-derived epitopes. J Virol Methods. 2009;155:118–121. doi: 10.1016/j.jviromet.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 384.Nuzzaci M., Vitti A., Condelli V., Lanorte M.T., Tortorella C., Boscia D. In vitro stability of Cucumber mosaic virus nanoparticles carrying a Hepatitis C virus-derived epitope under simulated gastrointestinal conditions and in vivo efficacy of an edible vaccine. J Virol Methods. 2010;165:211–215. doi: 10.1016/j.jviromet.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 385.Natilla A., Hammond R.W., Nemchinov L.G. Epitope presentation system based on cucumber mosaic virus coat protein expressed from a potato virus X-based vector. Arch Virol. 2006;151:1373–1386. doi: 10.1007/s00705-005-0711-x. [DOI] [PubMed] [Google Scholar]
- 386.Natilla A., Nemchinov L.G. Improvement of PVX/CMV CP expression tool for display of short foreign antigens. Protein Expr Purif. 2008;59:117–121. doi: 10.1016/j.pep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 387.Vitti A., Piazzolla G., Condelli V., Nuzzaci M., Lanorte M.T., Boscia D. Cucumber mosaic virus as the expression system for a potential vaccine against Alzheimer's disease. J Virol Methods. 2010;169:332–340. doi: 10.1016/j.jviromet.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 388.Ghasparian A., Riedel T., Koomullil J., Moehle K., Gorba C., Svergun D.I. Engineered synthetic virus-like particles and their use in vaccine delivery. Chembiochem. 2011;12:100–109. doi: 10.1002/cbic.201000536. [DOI] [PubMed] [Google Scholar]
- 389.Kaba S.A., Brando C., Guo Q., Mittelholzer C., Raman S., Tropel D. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183:7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pimentel T.A., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Moser C., Amacker M., Zurbriggen R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev Vaccines. 2011;10:437–446. doi: 10.1586/erv.11.15. [DOI] [PubMed] [Google Scholar]
- 392.Almeida J.D., Edwards D.C., Brand C.M., Heath T.D. Formation of virosomes from influenza subunits and liposomes. Lancet. 1975;2:899–901. doi: 10.1016/s0140-6736(75)92130-3. [DOI] [PubMed] [Google Scholar]
- 393.Morein B., Helenius A., Simons K., Schirrmacher V. Virus spike protein complexes and virosomes as effective subunit vaccines. Adv Exp Med Biol. 1979;114:811–816. doi: 10.1007/978-1-4615-9101-6_133. [DOI] [PubMed] [Google Scholar]
- 394.Amacker M., Engler O., Kammer A.R., Vadrucci S., Oberholzer D., Cerny A. Peptide-loaded chimeric influenza virosomes for efficient in vivo induction of cytotoxic T cells. Int Immunol. 2005;17:695–704. doi: 10.1093/intimm/dxh249. [DOI] [PubMed] [Google Scholar]
- 395.Kammer A.R., Amacker M., Rasi S., Westerfeld N., Gremion C., Neuhaus D. A new and versatile virosomal antigen delivery system to induce cellular and humoral immune responses. Vaccine. 2007;25:7065–7074. doi: 10.1016/j.vaccine.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 396.De Bernardis F., Liu H., O’Mahony R., La Valle R., Bartollino S., Sandini S. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- 397.Pevion Biotech AG. News Release November 11, 2010. Pevion's breakthrough candida vaccine demonstrates safety and immunogenicity in Phase I clinical study. http://www.pevion.com/index.php?section=news&cmd=details&newsid=218 [accessed 18 March, 2012].
- 398.Pevion Biotech AG. News Release January 17, 2012. Pevion's therapeutic Candida vaccine generates functional B cell memory in all vaccinees. http://www.pevion.com/index.php?section=news&cmd=details&newsid=224 [accessed 18 March, 2012].
- 399.Wiedermann U., Wiltschke C., Jasinska J., Kundi M., Zurbriggen R., Garner-Spitzer E. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat. 2010;119:673–683. doi: 10.1007/s10549-009-0666-9. [DOI] [PubMed] [Google Scholar]
- 400.Bomsel M., Tudor D., Drillet A.S., Alfsen A., Ganor Y., Roger M.G. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 401.U.S. Clinical Trials. Investigation of the Safety of an HIV-1 Vaccine Given Intra-muscularly and Intra-nasally to Healthy Female Subjects. http://www.clinicaltrials.gov/ct2/show/NCT01084343?term=pevion&rank=3 [accessed 12 April, 2012].
- 402.Cusi M.G., Zurbriggen R., Correale P., Valassina M., Terrosi C., Pergola L. Influenza virosomes are an efficient delivery system for respiratory syncytial virus-F antigen inducing humoral and cell-mediated immunity. Vaccine. 2002;20:3436–3442. doi: 10.1016/s0264-410x(02)00353-5. [DOI] [PubMed] [Google Scholar]
- 403.Nallet S., Amacker M., Westerfeld N., Baldi L., König I., Hacker D.L. Respiratory syncytial virus subunit vaccine based on a recombinant fusion protein expressed transiently in mammalian cells. Vaccine. 2009;27:6415–6419. doi: 10.1016/j.vaccine.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 404.Pevion Biotech AG. PEV4: Subunit vaccine against RSV. November 2010. http://pevion.com/images/content/Pevion%20PEV4%20RSV%20vaccine.pdf [accessed 12 July, 2012].
- 405.Stegmann T., Kamphuis T., Meijerhof T., Goud E., de Haan A., Wilschut J. Lipopeptide-adjuvanted respiratory syncytial virus virosomes: a safe and immunogenic non-replicating vaccine formulation. Vaccine. 2010;28:5543–5550. doi: 10.1016/j.vaccine.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 406.Kamphuis T., Meijerhof T., Stegmann T., Lederhofer J., Wilschut J., de Haan A. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS One. 2012;7:e36812. doi: 10.1371/journal.pone.0036812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Michel M.L., Tiollais P., Hepatitis B Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris) 2010;58:288–295. doi: 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 408.Stephenne J. Production in yeast versus mammalian cells of the first recombinant DNA human vaccine and its proved safety, efficacy, and economy: hepatitis B vaccine. Adv Biotechnol Processes. 1990;14:279–299. [PubMed] [Google Scholar]
- 409.Cox M.M., Patriarca P.A., Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respi Viruses. 2008;2:211–219. doi: 10.1111/j.1750-2659.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Protein Sciences Corporation. FluBlok®. http://www.proteinsciences.com/FVAC.htm [accessed 12 April, 2012].
- 411.European Medicines Agency. June 2011. ICH guideline S6 (R1)–preclinical safety evaluation of biotechnology-derived pharmaceuticals. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002828.pdf [accessed 05 October, 2012].
- 412.Cytos Biotechnology Ltd. CYT003-QbG10: a Novel Immunotherapy for Allergies. http://www.cytos.com/userfiles/file/QbG10_Facts_0908.pdf [accessed 12 April, 2012].
- 413.U.S. Clinical Trials. CYT003-QbG10 for Treatment of Allergic Asthma Bronchial. http://www.clinicaltrials.gov/ct2/results?term=CYT003-QbG10 [accessed 12 April, 2012].
- 414.Cytos Biotechnology Ltd. Press Release July 31, 2009. CYT003-QbG10 monotherapy for the treatment of allergic rhinoconjunctivitis is safe and efficacious in phase IIb study. http://www.cytos.com/userfiles/file/Cytos_Press_E_090731.pdf [accessed 12 April, 2012].
- 415.Cytos Biotechnology Ltd. Press Release May 21, 2010. Placebo-controlled phase II study shows CYT003-QbG10 is safe and efficacious for the treatment of allergic asthma. http://www.cytos.com/userfiles/file/Cytos_Press_E_100521.pdf [accessed 12 April, 2012].
- 416.Cytos Biotechnology Ltd. Highlights Q2 2008. http://www.cytos.com/userfiles/file/excerpt%20Q2_NVS.pdf [accessed 12 April, 2012].
- 417.U.S. Clinical Trials. Safety, Tolerability and Abeta-specific Antibody Response of Repeated i.m. Injections of Adjuvanted CAD106 in Mild Alzheimer Patients. http://www.clinicaltrials.gov/ct2/results?term=CAD106 [accessed 12 April, 2012].
- 418.Chackerian B. Virus-like particle based vaccines for Alzheimer disease. Hum Vaccin. 2010;6:926–930. doi: 10.4161/hv.7.1.12655. [DOI] [PubMed] [Google Scholar]
- 419.Pevion Biotech AG. News Release October 11, 2011. Pevion's therapeutic Candida vaccine shows robust systemic and mucosal immunogenicity, while epidemiological survey confirms excellent market potential. http://www.pevion.com/index.php?section=news&cmd=details&newsid=223 [accessed 12 April, 2012].
- 420.Cytos Biotechnology Ltd. Press Release June 11, 2009. Cytos Biotechnology initiates combined phase I/IIa study with a novel anti-interleukin-1 beta vaccine in patients with type II diabetes mellitus. http://www.cytos.com/userfiles/file/Diabetes_phI_IIa_start_E_Final.pdf [accessed 12 April, 2012].
- 421.U.S. Clinical Trials. Safety, Tolerability and Pharmacodynamics of CYT013-IL1bQb in Patients With Type 2 Diabetes. http://www.clinicaltrials.gov/ct2/show/NCT00924105?term=CYT013-IL1bQb&rank=1 [accessed 12 April, 2012].
- 422.Assateerawatt A., Tanphaichitr V.S., Suvatte V., Yodthong S. Immunogenicity and efficacy of a recombinant DNA hepatitis B vaccine. GenHevac B Pasteur in high risk neonates, school children and healthy adults. Asian Pac J Allergy Immunol. 1993;11:85–91. [PubMed] [Google Scholar]
- 423.SciGen Ltd. Sci-B-Vac™. www.scigenltd.com/products_sci_b_vacs.htm [accessed 24 April, 2012].
- 424.World Health Organization 2001. Hepatitis B immunization: introducing hepatitis B vaccine into national immunization services. http://whqlibdoc.who.int/hq/2001/WHO_V&B_01.28.pdf [accessed 12 April, 2012].
- 425.Bio Farma. Company Profile 2011. http://biofarma.co.id/tl_files/data/compro.pdf [accessed 12 April, 2012].
- 426.Hauser P., Voet P., Simoen E., Thomas H.C., Pêtre J., De Wilde M. Immunological properties of recombinant HBsAg produced in yeast. Postgrad Med J. 1987;63(Suppl 2):83–91. [PubMed] [Google Scholar]
- 427.Panacea Biotech. Hepatitis B Virus Vaccine IP Recombinant (Genetically Engineered): Enivac HB. http://www.panaceabiotec.com/product-pdf/vaccine1.pdf [accessed 12 April, 2012].
- 428.Sanofi Pasteur Ltd. Euvax B®. Summary of product characteristics. http://drug.fda.moph.go.th/zone_search/files/EUVAX%20B_1C%20223_41.pdf [accessed 12 April, 2012].
- 429.Serum Institute of India Ltd. Gene Vac-B®. http://www.seruminstitute.com/content/products/product_genevac.htm [accessed 12 April, 2012].
- 430.Díaz González M., Rodríguez Lay L., López-Chávez A.U., Bravo González J.R., Pedroso Flaquet P. Adverse reactions and immune response to Heberbiovac-HB vaccine administered to infants simultaneously with DPT and VA-MENGOC-BC. Rev Cubana Med Trop. 1997;49:196–203. [PubMed] [Google Scholar]
- 431.Diethelm Ltd./Berna Biotech Korea Corp. Hepavax-Gene®. Summary of product characteristics. http://drug.fda.moph.go.th/zone_search/files/Hepavax_1C%2022_49.pdf [accessed 12 April, 2012].
- 432.McAleer W.J., Buynak E.B., Maigetter R.Z., Wampler D.E., Miller W.J., Hilleman M.R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 433.Ellis R.W., Gerety R.J. Plasma-derived and yeast-derived hepatitis B vaccines. Am J Infect Control. 1989;17:181–189. doi: 10.1016/0196-6553(89)90214-9. [DOI] [PubMed] [Google Scholar]
- 434.Bharat Biotech. Revac-B+™. http://www.bharatbiotech.com/p_revac-b.htm.
- 435.Shantha Biotechnics Ltd. Shanvac®-B. http://www.shanthabiotech.com/files/Shanvac%20B%20Domestic%20Pack%20insert.pdf [accessed 12 April, 2012].
- 436.U.S. Clinical Trials. V503. http://www.clinicaltrials.gov/ct2/results?term=V503.
- 437.Meridian Life Science, Inc. Recombinant Human Parvovirus B19 Virus-Like Particle Vaccine. http://meridianlifescience.com/products/cgmp%20web%20pages/parvovirus_b19.aspx [accessed 12 April, 2012].
- 438.U.S. Clinical Trials. CYT006-AngQb. http://www.clinicaltrials.gov/ct2/results?term=CYT006-AngQb [accessed 12 April, 2012].
- 439.Cytos Biotechnology Ltd. Fact Sheet November 2007. CYT006-AngQb: a Novel Vaccine for Hypertension. November 2007. http://www.cytos.com/doc/Hypertension_Facts_Nov2007_FINAL.pdf [accessed 12 April, 2012].
- 440.Cytos Biotechnology Ltd. Press Release June 24, 2009. Cytos Biotechnology reports biochemical findings from phase IIa study with hypertension vaccine CYT006-AngQb. http://www.cytos.com/userfiles/file/Cytos_Press_E_090624.pdf [accessed 12 April, 2012].
- 441.Medicago, Inc. News Release February 1, 2011. Medicago reports positive Phase II interim results for its avian flu pandemic vaccine. http://www.medicago.com/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-reports-positive-Phase-II-interim-results-for-its-avian-flu-pandemic-vaccine/default.aspx [accessed 12 April, 2012].
- 442.U.S. Clinical Trials. Evaluate the Safety and Immunogenicity of a Seasonal Influenza VLP Vaccine (Recombinant) in Healthy Adults. http://clinicaltrials.gov/ct2/show/NCT00754455 [accessed 12 April, 2012].
- 443.World Health Organization 2012. Safety of Experimental Malaria Vaccine RTS,S/AS01. http://www.who.int/vaccine_safety/topics/malaria_exp/Jun_2009/en/index.html [accessed 12 April, 2012].
- 444.U.S. Clinical Trials. CYT004-MelQbG10. http://www.clinicaltrials.gov/ct2/results?term=CYT004-MelQbG10 [accessed 12 April, 2012].
- 445.Cytos Biotechnology Ltd. Press Release November 20, 2007. Cytos Biotechnology presents encouraging phase IIa study results for its melanoma vaccine CYT004-MelQbG10. http://www.drugs.com/clinical_trials/cytos-biotechnology-presents-encouraging-phase-iia-study-results-melanoma-vaccine-cyt004-melqbg10-2759.html [accessed 12 April, 2012].
- 446.Maurer P., Jennings G.T., Willers J., Rohner F., Lindman Y., Roubicek K. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 447.Cornuz J., Zwahlen S., Jungi W.F., Osterwalder J., Klingler K., van Melle G. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Cytos Biotechnology Ltd. Fact Sheet August 2007. NIC002 (formerly CYT002-Nic002): a Novel Vaccine for Nicotine Addiction. http://www.cytos.com/doc/NIC002_Nicaddiction_Facts_August07.pdf [accessed 12 April, 2012].
- 449.U.S. Clinical Trials. NIC002. http://www.clinicaltrials.gov/ct2/results?term=NIC002 [accessed 12 April, 2012].
- 450.Cytos Biotechnology Ltd. Press Release October 15, 2009. Interim analysis of an ongoing phase II study with nicotine vaccine shows primary endpoint not achieved. http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf [accessed 12 April, 2012].


