Abstract
Background:
Critical examination of the quality and validity of available allergic rhinitis (AR) literature is necessary to improve understanding and to appropriately translate this knowledge to clinical care of the AR patient. To evaluate the existing AR literature, international multidisciplinary experts with an interest in AR have produced the International Consensus statement on Allergy and Rhinology: Allergic Rhinitis (ICAR:AR).
Methods:
Using previously described methodology, specific topics were developed relating to AR. Each topic was assigned a literature review, evidence-based review (EBR), or evidence-based review with recommendations (EBRR) format as dictated by available evidence and purpose within the ICAR:AR document. Following iterative reviews of each topic, the ICAR:AR document was synthesized and reviewed by all authors for consensus.
Results:
The ICAR:AR document addresses over 100 individual topics related to AR, including diagnosis, pathophysiology, epidemiology, disease burden, risk factors for the development of AR, allergy testing modalities, treatment, and other conditions/comorbidities associated with AR.
Conclusion:
This critical review of the AR literature has identified several strengths; providers can be confident that treatment decisions are supported by rigorous studies. However, there are also substantial gaps in the AR literature. These knowledge gaps should be viewed as opportunities for improvement, as often the things that we teach and the medicine that we practice are not based on the best quality evidence. This document aims to highlight the strengths and weaknesses of the AR literature to identify areas for future AR research and improved understanding.
Keywords: allergen extract, allergy, allergen immunotherapy, allergic rhinitis, antihistamine, asthma, atopic dermatitis, avoidance, biologic, cockroach, conjunctivitis, consensus, corticosteroid, cough, cromolyn, decongestant, eosinophilic esophagitis, environment, epicutaneous immunotherapy, epidemiology, evidence-based medicine, food allergy, genetics, house dust mite, IgE, immunoglobulin E, immunotherapy, inhalant allergy, leukotriene, microbiome, occupational rhinitis, omalizumab, pathophysiology, perennial, pet dander, pollen, probiotic, quality of life, rhinitis, rhinosinusitis, risk factor, saline, seasonal, sensitization, sinusitis, sleep, socioeconomic, specific IgE, subcutaneous immunotherapy, sublingual immunotherapy, systematic review, rhinitis, total IgE, transcutaneous immunotherapy, validated survey
I. Introduction
The available literature on allergic rhinitis (AR) grows more quickly with each passing decade. A search of “allergic rhinitis” in the PubMed database yielded 4135 articles published between 1945 and 1979. The next 20 years (1980-2000) saw 7064 AR articles published. Each subsequent decade has surpassed this number with 8143 AR articles published between 2000 and 2010, and 8212 published from 2010 to the present day. Like many other areas of medicine, a close look at the available literature demonstrates a wide variation in the type and quality of AR publications, ranging from case reports to meta-analyses, review articles to randomized controlled trials (RCTs), and large prospective studies to small retrospective case series. As a medical professional reads the literature or hears literature quoted by others, it is important that he/she understand the quality of the evidence in order to appropriately translate the findings and recommendations into daily clinical care of the AR patient. With such vast AR literature available, developing an appropriate understanding of the relevant evidence can be daunting.
This International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis (ICAR:AR) was developed to summarize the best external evidence relating to AR, with the goal of gathering and critically reviewing the available literature on AR epidemiology, risk factors, diagnosis, management, and associated conditions/comorbidities. More than 100 international authors from various specialties utilized a structured review process to evaluate the evidence related to AR. Initial topic development and writing by a primary author or team of authors, followed by a stepwise anonymous iterative review process for over 100 AR topics held this process to extremely high standards. The resulting document provides a strong review of the existing AR literature. The recommendations for AR diagnostic modalities and treatment contained herein rely directly on this evidence, with a clear delineation of the benefit, harm, and cost considerations that supported each recommendation level.
Like the 2016 International Consensus Statement on Allergy and Rhinology: Rhinosinusitis (ICAR:RS) by Orlandi et al.,1 this ICAR:AR document places high value on the strength of the evidence in making recommendations. Therefore, for example, expert opinion receives lower value (Table II.A-1). There are limitations, however. Like ICAR:RS, this document is not a clinical practice guideline (CPG) or a meta-analysis. This document summarizes the findings of meta-analyses and other systematic reviews when those are identified in the literature for a specific AR topic area. However, a meta-analysis was not performed on the data included in this document. In addition, much of the available AR literature is not appropriate for meta-analysis due to its heterogeneous nature and inconsistent methodologies. ICAR:AR is also not a CPG, as the typical steps of a CPG (ie, medical specialty society and patient advocate review) were not employed here.
TABLE II.A-1.
Aggregate grade of evidence6
| Grade | Research quality |
|---|---|
| A | Well-designed RCTs |
| B | RCTs with minor limitations; Overwhelming consistent evidence from observational studies |
| C | Observational studies (case control and cohort design) |
| D | Expert opinion; Case reports; Reasoning from first principles |
RCT = randomized controlled trial.
Throughout this document, certain topic areas have very strong evidence whereas other topics demonstrate relatively weak evidence. Many of our common practices in the diagnosis and care of the AR patient are based upon weak external evidence. As practitioners, academicians, and scientists, we must examine this evidence and strive to increase the strength of the evidence in areas where gaps exist.
Within the ICAR:AR document, recommendations are given based on the evidence in a specific topic area. However, this document is a compilation of the best AR evidence, not a manual for the care of the AR patient. Evidence-based medicine requires that the clinician has the best evidence available, but also uses his/her expertise and takes the patient’s values and expectations into account.2 Therefore, with a background of evidence-based knowledge, the practitioner must approach each patient as an individual to determine the most appropriate diagnostic and treatment modalities for that particular patient. Given the numerous potential conditions in the AR differential diagnosis, various diagnostic and treatment options available, and diverse comorbidities and associated conditions that may accompany AR, treatment of the AR patient with an evidence-based approach requires careful consideration.
As previously stated by Orlandi et al.,1 the recommendations provided in an ICAR document must be interpreted based on the strength of the evidence that forms their foundation. The recommendations in this document are evidence-based. They do not define the standard of care or medical necessity. Recommendations written in this document, or any similar document, do not dictate the specific care of an individual patient. There are numerous other factors that enter into the treatment decisions for each individual patient. Finally, it is expected that these recommendations will change with time and with new evidence. We encourage new research, especially rigorous studies that aim to fill the identified knowledge gaps. With new evidence, recommendations will undergo necessary revisions and better patient outcomes should result.
II. Methods
II.A. Topic development
In a similar fashion to the 2016 ICAR:RS document by Orlandi et al.,1 this ICAR:AR document is formulated with the utmost reliance on published evidence. With the 2011 Rudmik and Smith3 evidence-based review with recommendations (EBRR) method as its foundation, ICAR:AR strives to analyze the existing literature on each AR topic, grading the evidence and providing literature-based recommendations where appropriate.
The subject of AR was initially divided into 103 topics or content areas. A senior author who is a recognized expert in allergy, rhinology, or the assigned topic was appointed to each topic. Authors were initially selected via online literature searches for each ICAR:AR topic. Authors of high-quality publications in each topic area were invited as ICAR:AR contributors. Other invited authors included experts in the EBRR process, experts in teaching/lecturing on specific AR topic areas, and those with knowledge of the systematic review process.
Some of the topics, such as those providing background or definitions, were assigned as literature reviews without evidence grades. Certain topics that were not appropriate for clinical recommendations were assigned as evidence-based reviews without recommendations (EBRs). Topics that had evidence to inform clinical recommendations were assigned as EBRRs.
Each topic author received specific instructions to perform a systematic review for the topic literature using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standardized guidelines.4 Ovid MEDLINE® (1947-September 2016), EMBASE (1974-September 2016), and Cochrane Review databases were included. The search began by identifying any previously published systematic reviews or guidelines pertaining to the assigned topic. Since clinical recommendations are best supported by high-quality evidence, the search focused on identifying RCTs and meta-analyses of RCTs to provide the highest level of evidence (LOE). Reference lists of all identified studies were examined to ensure all relevant studies were captured. If the authors felt as though a non-English study should be included in the review, it was instructed that the paper be appropriately translated to minimize the risk of missing important data during the development of recommendations.4
To optimize transparency of the evidence, all included studies in EBR and EBRR topic sections are presented in a standardized table format and the quality of each study was evaluated to receive a level based on the Oxford LOE (level 1a to 5).5 At the completion of the systematic review and research quality evaluation for each clinical topic, an aggregate grade of evidence was produced for the topic based on the guidelines from the American Academy of Pediatrics Steering Committee on Quality Improvement and Management (AAP SCQIM)6 (Table II.A-1).
After providing an aggregate grade of evidence for each EBRR topic (A to D), a recommendation using the AAP SCQIM guidelines was produced (Table II.A-2). It is important to note that each evidence-based recommendation took into account the aggregate grade of evidence along with the balance of benefit, harm, and costs. A summary of the EBRR development process is provided in Figure II.A-1.
TABLE II.A-2.
American Academy of Pediatrics-defined strategy for recommendation development6
| Evidence quality | Preponderance of benefit over harm |
Balance of benefit and harm | Preponderance of harm over benefit |
|---|---|---|---|
| A. Well-designed RCTs | Strong recommendation | Option | Strong recommendation against |
| B. RCTs with minor limitations; overwhelmingly consistent evidence from observational studies | |||
| C. Observational studies (case-control and cohort design) | Recommendation | Recommendation against | |
| D. Expert opinion; case reports; reasoning from first principles | Option | No recommendation |
RCT = randomized controlled trial.
FIGURE II.A-1.
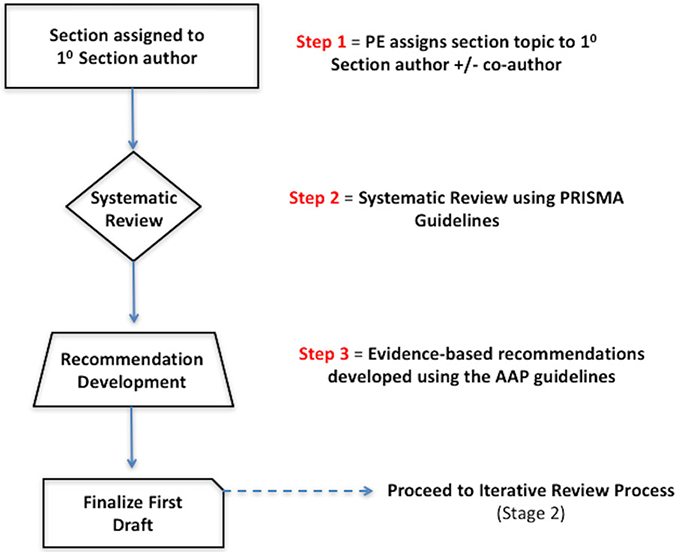
Topic development. AAP = American Academy of Pediatrics; EBRR = evidence-based review with recommendation; PE = principal editor; 10 = primary; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
II.B. Iterative review
Following the development of the initial topic text and any associated evidence tables, evidence grades, and recommendations, each section underwent a 2-stage online iterative review process using 2 independent reviewers (Fig. II.A-2). The purpose of the topic iterative review process was to evaluate the completeness of the identified literature and ensure that any EBRR recommendations were appropriate. The content of the first draft from each topic section was reviewed by a first reviewer, and all changes were agreed upon by the initial author and this first reviewer. The revised topic section was then subsequently reviewed by a second reviewer. Initial authors of the topic and both assigned reviewers agreed upon all changes before each section was considered appropriate to proceed into the final ICAR statement stage.
FIGURE II.A-2.
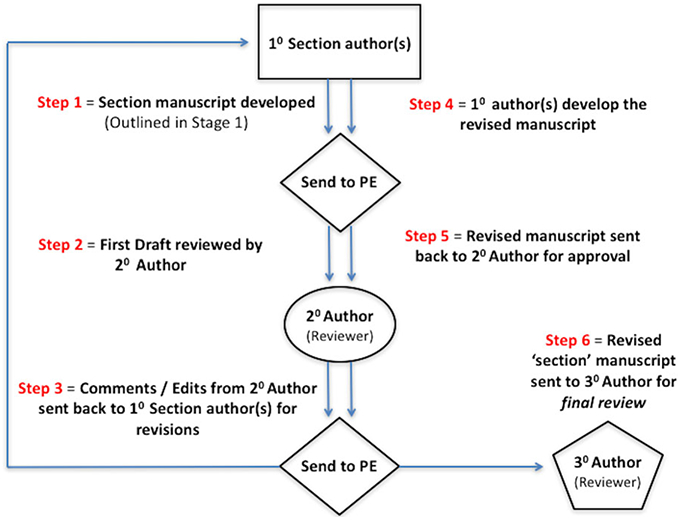
Topic EBRR iterative review. 10 = primary; 20 = secondary; 30 = tertiary; EBRR = evidence-based review with recommendation; PE = principal editor.
II.C. ICAR statement development
After the content of each of topic was reviewed and consensus reached among the initial author and 2 iterative reviewers, the principal editor (S.K.W.) compiled all topics into a single ICAR:AR statement. The first draft of each large ICAR:AR portion (ie, Evaluation and Diagnosis, Pharmacotherapy, Immunotherapy, etc.) then underwent additional reviews for consistency and understanding using a group of 6 to 8 authors. Finally, the draft ICAR:AR was circulated to all authors. The final ICAR:AR manuscript was produced when all authors agreed upon the literature and final recommendations. External peer review, with 20 reviewers, was also undertaken for the final ICAR:AR document (Fig. II.A-3).
FIGURE II.A-3.
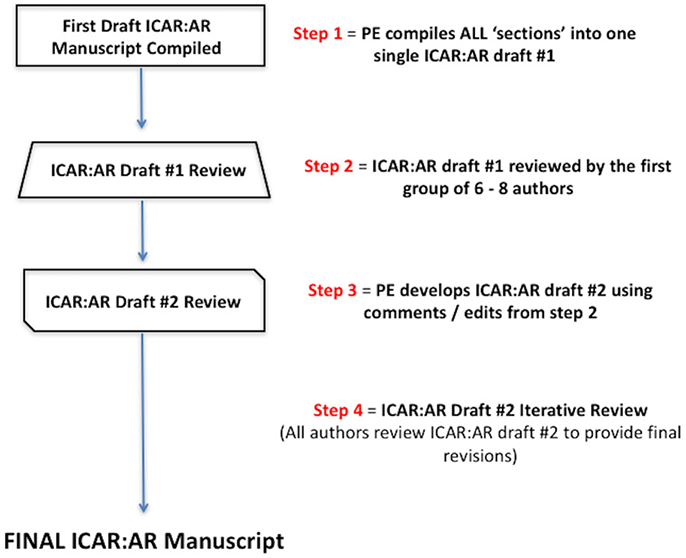
ICAR: Allergic Rhinitis statement iterative review. ICAR:AR = International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis; PE = principal editor.
II.D. Limitations of methods and data presentation
It should be noted that because each topic author individually performed the literature search for his/her assigned topic, search results may demonstrate some inherent variability despite specific and detailed search instructions. Furthermore, while aiming to be as comprehensive as possible, this document may not present every study published on every topic. For certain topics, the literature is extensive and only high-quality studies or systematic reviews are listed. If the aggregate evidence on a topic reached a high evidence grade with only high-level studies, an exhaustive list of lower level studies (or all studies ever performed) is not provided.
III. Definition and differential diagnosis
III.A. Allergic rhinitis definition
AR is an immunoglobulin E (IgE)-mediated inflammatory nasal condition resulting from allergen introduction in a sensitized individual.7 AR was defined in 1929 as a process which included 3 cardinal symptoms: sneezing, nasal obstruction, and mucus discharge.8 Symptoms occur with allergen exposure in the allergic patient. AR is a widely prevalent condition that can result in significant physical sequelae and recurrent or persistent morbidities.7
The prevalence of AR is approximately 10% to 40%, depending on geographic location,9 with the highest incidence occurring in children.10 However, AR is nearly absent in infants, typically not manifesting until the second year of life at the earliest. When AR presents in children, this is likely secondary to the rapidly evolving immune system. AR often results from an overactive response of T helper (Th) 2 lymphocytes that can initiate a systemic, IgE-driven reaction which may dominate child’s immune system until it is completely mature. During this time, a skin-prick test (SPT) or in vitro antigen-specific IgE (sIgE) test can be used to confirm the diagnosis of AR.
In the atopic individual, exposure to indoor and outdoor allergens may prompt antigen-specific IgE production. Reintroduction of the allergen triggers early-stage and late-stage reactions, leading to the clinical manifestations of AR. The early-stage reaction occurs within minutes after reintroduction of the sensitized allergen, producing nasal itching, nasal congestion, and rhinorrhea.11 The late-stage reaction occurs during the 4-hour to 8-hour period after allergen introduction and results in nasal blockage, hyposmia, increased mucus secretion, and nasal hyperresponsiveness to the same or different allergens. Additionally, even in the absence of overt symptoms, IgE has an increased presence in the lymphoid tissue of the atopic patient, which can result in persistent mucosal inflammation.12
III.B. Allergic rhinitis classification
Seasonal vs perennial allergic rhinitis
The Allergic Rhinitis and its Impact on Asthma (ARIA) proposals have categorized AR by presumed cause and seasonal vs perennial presentation. Classically, this has included seasonal AR (SAR; hay fever) and perennial allergic rhinitis (PAR).7 SAR is triggered by a wide assortment of outdoor allergens, especially pollens.7 PAR is commonly brought about by indoor allergens that are present through-out the year, such as dust mites, molds, insects (cock-roaches), and animal dander.7
Intermittent vs persistent allergic rhinitis
The classification of “seasonal” and “perennial” AR can often be in conflict, as manifestations of perennial allergy may not occur throughout the entire year. This is particularly the case for patients allergic to house dust mites (HDM), who may demonstrate mild or moderate/severe intermittent allergic rhinitis (IAR).9,13-15 In addition, because of the priming effect on the nasal mucosa initiated by low levels of pollen allergens16-21 and minimal persistent nasal inflammation in patients with “symptom-free rhinitis,”14,22,23 symptoms may not occur entirely in conjunction with the allergen season, therefore resulting in nonspecific exacerbations. Air pollution may also contribute to alterations in allergen sensitivity, resulting in varying degrees of symptoms depending on location and air quality.24 Furthermore, individuals sensitized to multiple pollens may have symptoms across several seasons while individuals with PAR may encounter symptoms for short periods of time with frequent, repetitive relapses.
Because of the issues outlined above, ARIA proposed a new method of classification based on the length and recurrence of the symptom manifestations.25 IAR is characterized by symptoms for less than 4 days per week or less than 4 consecutive weeks. Persistent AR (PER) is characterized by symptoms occurring more than 4 days per week for at least 4 consecutive weeks; therefore, PER patients are symptomatic most of the time.26 It has been recommended that the previous categories of seasonal and perennial AR (ie, SAR and PAR) not be used along with the new classification of IAR and PER, as they do not represent the same stratification of the disease state. As such, IAR and PER are not synonymous with seasonal and perennial.25,27-30 In describing AR, one should determine which classification scheme best conveys the message that he/she wishes to relay: seasonal/perennial or intermittent/persistent.
Severity of allergic rhinitis
AR can result in significant disturbances in quality of life (QOL), sleep, exercise tolerance, productivity, and social functioning. The ARIA guidelines have likewise proposed the stratification of severity (mild and moderate-severe) in view of these disabilities.13 (See section VII. Disease Burden for additional information on this topic.)
Sensitization vs clinical allergy
Monosensitization is sensitization (as indicated by positive reactions on standardized SPTs or serum sIgE levels) to only 1 allergen, such as grass pollen, tree pollen, HDM, or cat dander (even though extracts of these concentrates contain numerous diverse polypeptides).31 Monoallergy is defined as a single sensitizing allergen causing clinical allergy symptoms. Polysensitization is sensitization to 2 or more allergens. Polyallergy is affirmed clinical symptoms to 2 or more sensitizing allergens. Findings of allergy testing, either skin testing or sIgE must be correlated with clinical symptoms to identify the allergen(s) likely responsible for the symptoms.32 Allergen challenges (ie, nasal provocation testing, conjunctival challenge, or allergen challenge chambers (ACCs)) can reproducibly confirm the clinical significance of a sensitized allergen, but these tests may be difficult to perform, subjective, and limited by irritant effects.33
Allergy skin testing and sIgE titer must be carefully interpreted at the patient level, and can also be valuable at the population level when evaluating sensitization for epidemiological studies.34 With increasing availability of component-resolved diagnosis (CRD), physicians will have a more objective means of identifying clinically relevant allergens and distinguishing true co-sensitization from polysensitization due to cross-reactivity. (See section VIII.F.6. Evaluation and diagnosis - In vitro testing - Component resolved diagnosis (CRD) for additional information on this topic.)
III.C. Allergic rhinitis differential diagnosis
The symptoms of AR may be similar to symptoms of other types of sinonasal disease, and at times multiple types of rhinitis may coexist. It is important to correctly determine the etiology of rhinitis to appropriately treat the patient and have the best chance of resolving his or her symptoms. In the following sections, a discussion of the differential diagnosis of AR is presented, along with a description of how each rhinitis entity differs from AR. Of note, this section on AR differential diagnosis is specific to various etiologies of rhinitis. Other entities that may enter into the differential diagnosis of AR, such as structural sinonasal conditions (ie, deviated septum), tumors, and cerebrospinal fluid leak are not discussed here (Table III.C).
TABLE III.C.
Differential diagnosis of allergic rhinitis*
| Types of rhinitisa |
|---|
| • Drug-induced rhinitis |
| • Rhinitis medicamentosa |
| • Occupational rhinitis |
| • Chemical rhinitis |
| • Smoke-induced rhinitis |
| • Infectious rhinitis |
| • Rhinitis of pregnancy and hormonally-induced rhinitis |
| • Food- and alcohol-induced rhinitis |
| • NARES |
| • Vasomotor rhinitis (nonallergic rhinopathy) |
| • Age-related rhinitis (ie, elderly) |
| • Empty nose syndrome |
| • Atrophic rhinitis |
| • Autoimmune, granulomatous, and vasculitic rhinitis |
| • Rhinosinusitis |
For each of these conditions, the similarities and differences to allergic rhinitis are discussed within each content section.
This table is specific to various etiologies of rhinitis. Structural sinonasal conditions (ie, deviated septum), tumors, and cerebrospinal fluid leak are not listed here. NARES = nonallergic rhinitis with eosinophilia syndrome.
III.C.1. Drug-induced rhinitis
Rhinitis secondary to systemic medications can be classified into local inflammatory, neurogenic, and idiopathic types35,36 (Table III.C.1). The local inflammatory type occurs when consumption of a drug causes a direct change in inflammatory mediators within the nasal mucosa. The neurogenic type occurs after use of a drug that systemically modulates neural stimulation, leading to downstream changes in the nasal mucosa. Idiopathic drug-induced rhinitis is used to classify drugs without a well-defined mechanism contributing to symptoms. Topical nasal decongestants can cause drug-induced rhinitis, known as rhinitis medicamentosa (RM). (See Section III.C.2. Definitions, classifications, and differential diagnosis - Allergic rhinitis differential diagnosis - Rhinitis medicamentosa (RM) for additional information on this topic.)
TABLE III.C.1.
| Type of drug-induced rhinitis |
General drug category | Specific drug category | Examples |
|---|---|---|---|
| Local inflammatory |
|
||
| Neurogenic and neuromuscular | α- and β-Adrenergic receptor modulators | α Antagonists |
|
| Presynaptic α-2 agonists | Clonidine, methyldopa, guanfacine, piribedil | ||
| Beta-antagonists |
|
||
| Presynaptic depletion of norepinephrine stores | Guanethidine | ||
| Phosphodiesterase inhibitors | Phosphodiesterase-3 specific | Cilostazol | |
| Phosphodiesterase-5 specific | Sildenafil, tadalafil, vardenafil | ||
| Nonselective phosphodiesterase | Pentoxifylline | ||
| Angiotensin converting enzyme inhibitor | Ramipril, captopril, lisinopril, benazepril, quinapril, enalapril | ||
| Idiopathic | Psychotropics | Chlorpromazine, thioridazine, amitriptyline, alprazolam, reserpine, risperidone, mianserin | |
| Immunomodulators | Cyclosporine | ||
| Hormones | Estrogen, oral contraceptives | ||
| Antihypertensives | Amiloride, chlorothiazide, hydralazine, hydrochlorothiazide | ||
| Other | Gabapentin, gingko biloba |
Local inflammatory type.
Systemic ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with a disorder of eicosanoid synthesis can result in rhinitis and nasal congestion, which may also be associated with chronic rhinosinusitis (CRS) and asthma.37 In brief, NSAIDs inhibit cyclooxygenase (COX)-1 and COX-2 enzymes, shifting arachidonic acid metabolism toward the lipoxygenase pathway, with decreased production of prostaglandins and thromboxane in exchange for inflammatory leukotrienes (LT). Reduction in nasal mucosal prostaglandin E2, as well as increased LTC4, LTD4, and LTE4 causes mucus production and nasal mucosal edema, hallmarks of rhinitis.35,38
Neurogenic and neuromuscular type.
Neurogenic type non-allergic rhinitis (NAR) is caused by drug-induced modulation of the autonomic nervous system. Antihypertensives and vasodilators are among the many classes of drugs that cause drug-induced NAR. Other nonspecific drugs, such as psychotropics and immunosuppressants, have unknown mechanisms and are categorized as idiopathic, but can cause neuromodulatory effects as well. Modulation of the autonomic nervous system leads to downstream changes in nasal mucosa, blood vessels, and secretory glands.39 For example, α- and β-adrenergic antagonists, and presynaptic α-agonists, cause decreased sympathetic tone and unopposed parasympathetic stimulation producing mucosal engorgement, nasal congestion, and rhinorrhea.40-42
Phosphodiesterase (PDE)-5 specific inhibitors promote penile vasodilation and erection. PDE-3 and nonselective PDE inhibitors result in vasodilation and increased extremity blood flow, relieving symptoms of peripheral artery disease. Nitric oxide (NO)/cyclic nucleotide-mediated vasodilation occurs in the nasal mucosa as well, causing nasal mucosal engorgement and edema.43-46 Finally, angiotensin converting enzyme inhibitors (ACE-Is) inhibit the conversion of angiotensin I to angiotensin II in the lungs, resulting in a decrease in sympathetic activity. Bradykinin is also formed. Bradykinin B1 and B2 receptors have been demonstrated in nasal mucosa47; bradykinin application to the nasal mucosa has been shown to increase sneezing,44,48 suggesting a role of ACE-Is in NAR.
Illicit drug use.
The nose provides a unique portal for illicit drug use, as nasal mucosa is well vascularized and easily accessible. The illicit drug user can avoid invasive intravascular or intramuscular administration of a desired product by applying a crushed solid, liquid, or aerosolized form of the product directly to the nasal cavity. For some drugs, nasal administration increases bioavailability and shortens time to onset when compared to oral ingestion.49,50 Cocaine is most commonly associated with nasal illicit drug use and exerts its effect by modulating dopamine transporters to inhibit reuptake at the synapse, increasing dopamine available for postsynaptic stimulation.51 Cocaine-induced rhinitis is a result of vasoconstrictive events, which can be followed by rebound nasal mucosal edema and mucous production, similar to those seen in RM.52-55 In the repeat user, vasoconstriction, direct trauma compounded by anesthetic effects, and/or injury secondary to contaminants may result in nasal septal perforation.56-59 Similarly, prescription narcotics,59 antidepressants,47 anti-cholinergics, and psychostimulants can be abused by intranasal administration.47,60 Intranasal hydrocodone has been shown to induce nasal tissue necrosis and loss in a similar manner to cocaine.59 Antidepressants such as bupropion have been used to achieve a euphoria similar to that of cocaine and may induce seizures.47
In summary, systemic medications and intranasal illicit drugs affect the nasal mucosa. Increased mucosal edema, vasodilation, and inflammatory mediators are a consequence of systemic medications. Vasoconstriction and direct mucosal injury often accompanies illicit drug use. The physiologic response in drug-induced rhinitis differs from AR as it is not allergen-induced nor dependent on IgE mechanisms, although symptomatology may be similar.
III.C.2. Rhinitis medicamentosa (RM)
RM, or rebound rhinitis, is a condition induced by prolonged use of topical intranasal decongestant (IND)26,61 (Table III.C.2). Although no consensus diagnostic criteria exist, RM is classically associated with the triad of prolonged IND use, constant nasal obstruction, and poor shrinkage of the nasal mucosa61 in the setting of nasal congestion, rhinorrhea, and decreased efficacy of further INDs.55,62,63 Physical exam findings consist of mucosal edema, erythema, and hyperemia.
TABLE III.C.2.
| Sympathomimetic amines | Phenylephrine, pseudoephedrine, ephedrine, amphetamine, Benzedrine, caffeine, mescaline |
| Imidazoline derivatives | Oxymetazoline, xylometazoline, naphazoline, clonidine |
The exact physiologic mechanism causing RM is unclear. Continuous IND use may decrease endogenous norepinephrine production and cause upregulation of the parasympathetic system, leading to rebound congestion once the decongestant is discontinued.54,55 This may be further exacerbated by recurrent nasal tissue hypoxia and negative neural feedback with chronic decreased α-2 receptor responsiveness.64 Mucosal changes include ciliary damage and loss, epithelial metaplasia and hyperplasia, dilated intercellular spaces, goblet cell hyperplasia, and edema.65-67 Benzalkonium chloride (BKC), an antimicrobial preservative used in many nasal decongestants, has been implicated in the mechanism of RM. Studies have suggested that BKC is toxic to nasal epithelium and may propagate RM, although the data are inconclusive.68-71
Neither duration, nor cumulative dose of IND needed to initiate RM is known. Rebound congestion has developed after 3 to 10 days of medication use,55,66 but may not occur until after 30 days.72,73 Other studies have demonstrated a lack of rebound after 8 weeks of continuous use.72-75 Furthermore, doubling the dose of intranasal imidazoline did not increase the extent of rebound edema.72 Although inconclusive, studies suggest that IND use should be discontinued after 3 days to avoid rebound congestion.62,76,77
Treatment of RM involves discontinuation of INDs. Various medications have been used to improve nasal decongestion including nasal cromolyn, sedatives, nasal saline spray, oral antihistamines, oral decongestants, and intranasal corticosteroids (INCSs; sometimes used in conjunction with brief courses of systemic corticosteroids).50,62,78-82 Only the use of INCSs has been demonstrated to mitigate rebound congestion after discontinuation of topical INDs.67,81-83 Often there is an underlying rhinitis and/or anatomic issue that initiated the decongestant use. This underlying issue should be addressed to diminish the drive to continue to use INDs.
Importantly, RM is typically associated with repeated exposure to INDs, with increasing symptoms at times when the medication is withheld. In contrast, AR is classically associated with an allergic trigger with similar symptoms increasing upon allergen exposure, and is dependent upon IgE-mediated inflammation.
III.C.3. Occupational rhinitis
Occupational rhinitis is an inflammatory condition of the nasal mucosa, characterized by intermittent or persistent nasal congestion, sneezing, rhinorrhea, itching, and/or hypersecretion due to causes and conditions attributable to a particular work environment, and not to stimuli encountered outside the workplace.84 Occupational rhinitis is considered a form of “work-related rhinitis,” which also encompasses work-exacerbated rhinitis, which is preexisting or concurrent rhinitis that is worsened by workplace exposures84,85 (Fig. III.C.3).
FIGURE III.C.3.
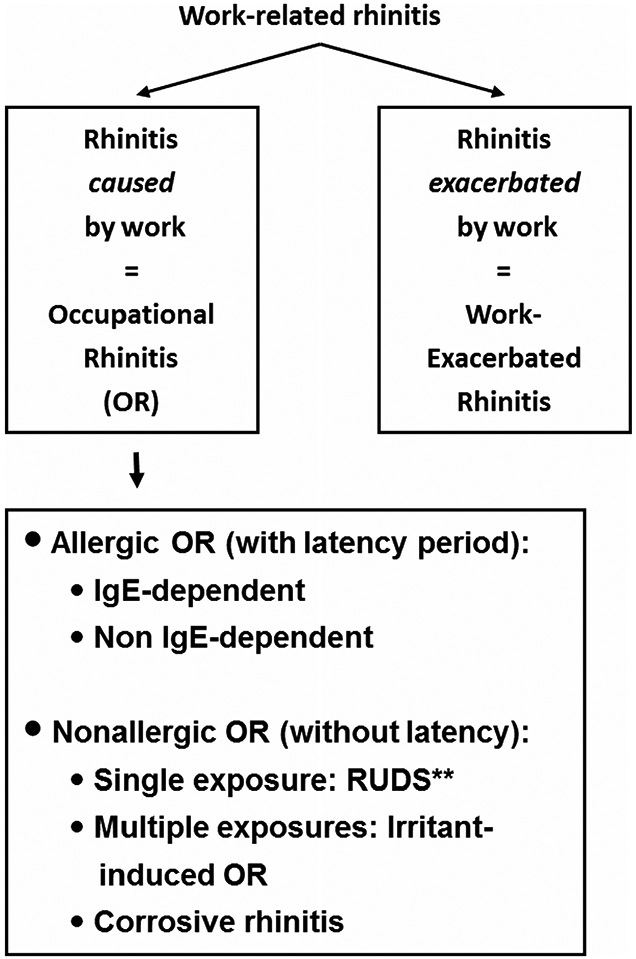
Classification of work-related rhinitis.84 Adapted from Moscato et al. Allergy. 2008;63:969-980.
Occupational rhinitis may be allergic, consequent to exposure to a sensitizing high-molecular (HMW) or low-molecular weight (LMW) compound acting through an immunological mechanism, and characterized by the presence of a latency period between beginning of exposure and symptom onset. Alternatively, occupational rhinitis may be non-allergic, mediated by and irritant or non-immunological mechanism. Symptoms occur after single or multiple exposures to irritant compounds, and usually present without a latency period. Non-allergic occupational rhinitis resulting from a single exposure to a very high concentration of irritants is also referred as reactive upper airways dysfunction syndrome (RUDS). The most severe form of irritant-induced occupational rhinitis is corrosive rhinitis, which is characterized by permanent inflammation of the nasal mucosa sometimes associated with ulcerations and perforation of the nasal septum.84,85
The results of cross-sectional studies in working groups show a wide range of prevalence of occupational rhinitis (3-87%),86 lower prevalence for LMW-agent exposure, and higher prevalence for HMW-agent exposure. Examples of occupations at increased risk are reported in Table III.C.3.87-98 Occupational rhinitis due to HMW-agents tend to be 3 times more prevalent than occupational asthma,86 with which it is often associated (up to 92% of cases).99
TABLE III.C.3.
Examples of high-risk occupations for occupational rhinitis and causal agents
| Occupation | Agent |
|---|---|
| High molecular weight agents | |
| Bakers, food industry | Cereal flours87 |
| Laboratory workers | Laboratory animals (rat, mouse)88 |
| Health care workers | Latex89 |
| Farmers | Animal-derived allergens, plant allergens, molds90 |
| Seafood workers | Shellfish, bony fish91 |
| Pharmaceutical & detergent industries | Biological enzymes92 |
| Low molecular weight agents | |
| Hairdressers | Persulphates93 |
| Carpentry, furniture making | Wood dust94,95 |
| Pharmaceutics, health care workers | Drugs96 |
| Chemical factories | Mixture of irritants96 |
| Cleaners | Mixture of irritants97,98 |
Occupational rhinitis and occupational asthma share etiologic agents and pathogenic mechanisms,100 and can be considered in the broader context of the Unified Airway Disease model.85,93,101,102 The severity of occupational rhinitis may also affect the severity of occupational asthma.103 In a high proportion (20-78%) of workers exposed to sensitizers, work-related nasal symptoms tend to develop 5 to 6 months before the onset of bronchial symptoms.84,86 Consequently, occupational rhinitis may be considered a marker of the likelihood of developing occupational asthma.
The clinical presentation of occupational rhinitis is nonspecific. Nasal symptoms do not differ from those of non-occupational rhinitis. An occupational origin should be sought for all rhinitis of new onset in adults, especially in subjects employed in high-risk occupations (Table III.C.3). The diagnostic assessment first includes a thorough clinical and occupational history, aimed to investigate type of symptoms and work-relatedness, and to collect information on occupational exposure. Typical nasal symptoms are often accompanied by crust formation, sporadic epistaxis, olfaction impairment, or conjunctivitis, or are associated with pharyngeal, laryngeal, or bronchial symptoms (which should always be evaluated). The presence of a latency period between an occupational exposure and symptom onset suggests an allergic mechanism. Documentation of noxious compounds (sensitizers and irritants) in the work-place to which the worker is more directly exposed are typically posted by the employer (ie, Material Safety Data Sheets).84,85
Nasal examinations by anterior rhinoscopy and nasal endoscopy, assessing nasal patency85,104 and inflammation in nasal secretions,105 are often performed as part of the clinical evaluation. Sensitization to a suspected HMW-agent can be evaluated through SPT and/or in vitro sIgE assessment, when standardized and validated extracts are available. A suggestive history associated with a positive immunological test for an occupational agent could be considered as probable allergic occupational rhinitis. A definitive diagnosis is obtained by objective demonstration of the causal relationship between rhinitis and the work environment through a nasal provocation test (NPT) with the suspected agent(s) in the laboratory, which is considered the gold standard for diagnosis.84,85 If NPT is negative, further evaluation of work-related changes in nasal parameters at the workplace is recommended, especially in the presence of a highly suggestive clinical history. In subjects exposed to HMW-agents with a suggestive history and negative immunological tests, the type of inflammatory response to NPT might demonstrate the presence of an occupational local allergic rhinitis (LAR).106,107 Due to the relationship between the upper and lower airways, spirometry, measurement of nonspecific airway responsiveness, and measurement of bronchial inflammation by means of exhaled NO may also be performed.84,85
Primary treatment of allergic occupational rhinitis is avoidance or reduction of culprit exposures.108 Pharmacologic treatment does not differ from that of non-occupational rhinitis.101 In allergic occupational rhinitis due to HMW-sensitizers, specific immunotherapy may be proposed when validated extracts are available.109 The prevention and early identification of occupational rhinitis during medical surveillance of exposed workers and of young apprentices may provide an excellent opportunity to prevent the development of occupational asthma.110,111
III.C.4. Chemical rhinitis
Chemical rhinitis largely falls under the category of occupational rhinitis; however, there are chemical exposures that are not necessarily occupational (and vice versa). Some chemicals may cause sensory irritation, which can include congestion, rhinorrhea, nasal discomfort, postnasal drainage, headache, and even epistaxis.112 Exposures, or exposure risk, are important elements to elicit in the history. There are many chemicals with which specific occupations are closely associated, though household chemicals and sport/leisure exposures (ie, chlorine-induced rhinitis in swimmers113) may play a role as well. Larger chemical particles are typically the culprit in this form of rhinitis as smaller particles usually pass through to the lower airways. Water soluble agents such as ammonia, formaldehyde, or sulfur dioxide may readily dissolve into the mucous membrane layer.114 These responses are non-IgE-mediated by a reflex response which is often termed neurogenic inflammation.115 A subset of these individuals involved in high-level single-exposure incidents may develop persistent symptoms. This phenomenon has been described as RUDS when only rhinitis symptoms are present, and Reactive Airways Dysfunction Syndrome when asthma-like symptoms are present.116,117
Although chemicals are not always thought of as sensitizers, some of these compounds can induce immunologic disease. Chemicals known to cause sensitization of the respiratory tract include diisocyanates, acid anhydrides, some platinum salts, reactive dyes, glutaraldehyde, plicatic acid, and chroamine.118-120 There is still much debate as to the exact mechanism behind sensitization to these chemicals. However, smaller chemical compounds must associate with larger protein molecules to induce an immune response. While specific IgE production toward chemicals causing respiratory allergy is seen, evidence to show symptoms related to chemical exposure without concomitant rise in IgE has also been documented.121 It is possible that these findings may be due to the inability to synthesize appropriate in vitro conjugates for diagnostic assays to detect serum IgE that binds these chemicals.122,123
Typically, the differential should include causes of both AR and NAR, as well as mixed rhinitis, recurrent acute rhinosinusitis (RARS), and potentially CRS. Some symptoms of chemical rhinitis may be similar to AR with nasal discharge, congestion, sneezing, and itching all being reported. Nasal discharge may be anterior or posterior with chemical rhinitis or AR but is typically not unilateral with either of these diagnoses. Chemical-induced rhinitis may be associated with olfactory dysfunction, both temporary and longlasting. These disturbances include hyposmia or anosmia, as well as dysosmia or agnosmia (inability to identify smells).112 Nasal discomfort, discharge, congestion, headaches, and sometimes epistaxis may also be present.112
III.C.5. Smoke-induced rhinitis
Environmental tobacco smoke exposure is associated with chronic rhinitis and in some cases, AR.124,125 In several studies, self-reported symptoms tend to be elicited by exposure to smoke and can correlate with serum cotinine levels.126-128 Symptoms common to both AR and smoke-induced rhinitis include rhinorrhea and congestion, but smoke-induced rhinitis does not appear to be driven by IgE-mediated hypersensitivity (which tends to exhibit a constellation of congestion, rhinorrhea, and sneezing on exposure to a specific allergen). As AR symptoms are immunologically mediated, there must be a sensitization period prior to the exposure that elicits symptoms. In contrast, smoke induced-rhinitis typically does not require sensitization, although there has been report of potential allergenic compounds in smoke.129 Interestingly, although active smokers are likely to have an elevated serum IgE, they exhibit a lower skin test reactivity to allergens than allergic nonsmokers.130
In contrast to AR, smoke-induced rhinitis is likely multi-factorial, and other mechanisms such as neurogenic or irritant etiologies play a more predominant role.131,132 Neurogenic nasal inflammation is mediated by neuropeptides such as substance P, neurokinin A, and calcitonin gene-related peptide. These mediators are released by sensory nerve fibers in the nose and result in vasodilation, edema, and inflammation.133 Patients who are reactive to tobacco exposure are identified by both subjective (congestion, rhinorrhea, sneezing) and objective response (increased nasal resistance) to controlled challenge with tobacco smoke. In a prospective study, patients were defined as demonstrating reactivity if nasal resistance on acoustic rhinometry increased by over 35% in response to tobacco smoke. Patients with less than 5% increase in nasal resistance were defined as nonreactive.131 In addition, altered mucociliary clearance (MCC) resulting from tobacco smoke exposure has been demonstrated. Congestive responses have been demonstrated on challenge with both brief and prolonged exposure to tobacco smoke. In individuals who report a history of smoke-induced rhinitis, brief smoke exposure (45 parts per million [ppm] for 15 minutes) led to increased nasal resistance as measured by posterior rhinometry. In individuals with and without a history of smoke-induced rhinitis, prolonged exposure to moderate levels of smoke (15 ppm for 2 hours) also induced a congestive response lasting for an hour or longer.134 Even though the objective response was short lived, patients reported symptoms lasting hours to days following exposure. Significant symptom overlap may exist, but a thorough history and allergy testing can help further differentiate smoke-induced rhinitis from AR. (See section VI.E. Risk factors for allergic rhinitis - Tobacco smoke for additional information on this topic.)
III.C.6. Infectious rhinitis
Infectious rhinitis may be classified into acute and chronic forms, with both bacterial and viral etiologies. Physical findings and chronicity of symptoms play an important role in differentiating between different forms of rhinitis, including infectious, allergic, and the inflammation associated with CRS. Symptoms suggestive of a noninfectious etiology include nasal itching and sneezing, while findings of mucosal inflammation and rhinorrhea may be present in either infectious or noninfectious rhinitis.26 Taken in isolation, dark or purulent rhinorrhea is not pathognomonic for bacterial rhinitis/rhinosinusitis. Additional findings suggestive of infectious etiologies include associated pharyngeal inflammation or cervical lymphadenopathy.135
Viral rhinitis typically manifests in an acute form, and accounts for up to 98% of infectious rhinitis in the young child. The incidence of viral rhinitis in young children is 6 episodes per patient-year.136 In adult viral rhinitis, the incidence is 2 to 3 episodes per year. Symptoms associated with viral rhinitis include clear rhinorrhea, nasal obstruction, and often, fever. The responsible organisms of viral rhinitis can be rhinovirus, adenovirus, influenza virus, and parainfluenza virus.81 Most viral rhinitis is self-limiting within 4 to 5 days, with prolonged symptoms lasting longer than 2 weeks suggestive of a noninfectious etiology or conversion to bacterial infection. There are instances when continued rhinitis beyond 10 days is felt to be due to worsening infection (ie, possible superimposed bacterial rhinosinusitis) and these patients should be treated more aggressively.137 Approximately 2% of viral rhinitis episodes are secondarily infected by bacterial organisms such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, with subsequent presentation of acute bacterial infection.138
III.C.7. Rhinitis of pregnancy and hormonally-induced rhinitis
The development of a type of rhinitis unique to the pregnant patient has been referred to as rhinitis of pregnancy or pregnancy rhinitis. It occurs in about 22% of pregnancies139 and, although symptoms may occur at any time, it typically starts after the second month of pregnancy and is most severe in the second trimester.26,140 Rhinitis of pregnancy has been defined as nasal congestion in the last 6 or more weeks of pregnancy, without other signs of respiratory tract infection or allergic cause, followed by complete, spontaneous resolution of symptoms within 2 weeks after delivery.141
The symptoms of rhinitis of pregnancy, like those of AR, include rhinorrhea and nasal congestion, which can be prominent and prolonged. Clinical history frequently elicits a prior history of chronic rhinitis, obscuring the extent to which pregnancy is a causal or aggravating factor.139 In addition, preexisting AR can worsen in approximately one-third of pregnant women.142
There are several etiologic factors potentially associated with the nasal symptoms in rhinitis of pregnancy. Hormonal changes, such as increased progesterone, estrogen, prolactin, vasoactive intestinal peptide, and/or placental growth hormone have been implicated,143,144 but there is little evidence to support this theory.145 Other physiologic phenomena occurring during pregnancy that may contribute to increased nasal congestion or obstruction include vasodilation, progesterone-induced smooth muscle relaxation, and a massive expansion of the circulating blood volume, which may contribute to increased nasal vascular pooling.146
Rhinitis of pregnancy does not usually require therapy, nor does it respond well to standard allergy medications. Its management is made more difficult by the lack of high-quality studies on the efficacy of treatment and fetal out-comes. In those who seek treatment, conservative non-pharmacologic measures are suggested. These can include elevation of the head of the bed,147 nasal dilator strips,148 and exercise.149,150 Saline lavage using hypertonic saline has been demonstrated to be effective without obvious deleterious effects on the fetus.151 Several medications, including INCS, have been studied in rhinitis of pregnancy but have failed to demonstrate clear efficacy.152 More recently, a systematic review by Kumar et al.153 identified only 1 RCT that failed to demonstrate any additional benefit of fluticasone compared to placebo for symptom control in this patient population. Although an extensive discussion of rhinitis of pregnancy management is beyond the scope of this document, the use of various other medications (ie, topical and oral decongestants) is controversial and should be addressed at the individual patient level, with close involvement of the obstetrician.
Direct stimulation of the nasal mucosa by estrogen may induce mucosal gland hyperactivity resulting in increased nasal secretions/rhinorrhea.154 As such, nasal symptoms can be associated with conditions other than pregnancy that affect hormone balance, such as hypothyroidism and acromegaly.155 Rhinitis may also arise as a result of changing blood hormone concentrations during puberty, menstruation, and the perimenopausal years.145 Although oral contraceptives have also been implicated as causes of nasal symptoms, a study by Wolstenholme et al.156 found no nasal physiologic effects in patients receiving oral contraceptive treatment.
In summary, there are numerous metabolic conditions with symptoms like those of AR. Accurate diagnosis can be made on history and presentation, but additional testing may be required for symptoms that are persistent or severe.
III.C.8. Food- and alcohol-induced rhinitis
Food-induced rhinitis.
Certain food ingestions may result in rhinitis based on a nonimmunologic reaction, and therefore are not characterized as an allergy. For instance, in subjects with gustatory rhinitis, shortly after ingestion of hot or spicy foods, unilateral or bilateral watery rhinorrhea develops in the absence of nasal congestion, pruritus, or facial pain. This is considered a reflex response due to an adrenergic and cholinergic neural reaction of the nose.157
The prevalence of “food-induced rhinitis” seems to be under 1%.157 While rhinitis may frequently be observed as part of systemic IgE-mediated food allergy reaction, it is rarely the only presenting symptom. In a double-blind, placebo-controlled food challenge study of 480 children, 185 children (39%) experienced ocular and upper respiratory symptoms, but only 5% had symptoms confined to the upper respiratory tract alone.158
Patients with pollen-food allergy syndrome (PFAS), also referred to as oral allergy syndrome (OAS), often experience oropharyngeal itching, tingling, and/or mild swelling of the lips, tongue, palate, and throat, and less commonly AR symptoms, after ingestion of certain raw fruits and vegetables. The assessed prevalence of this disorder ranges from 5% to 17%, and it affects up to one-half of pollen-allergic patients.159-161 It occurs in individuals who are sensitized to pollen aeroallergens through the respiratory tract, which then predisposes them to clinical symptoms of PFAS after ingestion of cross-reactive, heatlabile food proteins of plant origin. Because the antigens are heatlabile, patients are usually able to tolerate cooked forms of the causative fruits and vegetables.162 (See section X.E. Associated conditions - Food allergy and pollen-food allergy syndrome (PFAS) for additional information on this topic.)
Alcohol-induced rhinitis.
Nasal symptoms can also occur after alcohol consumption.163,164 However, very little is known about the prevalence and presentation of alcohol-induced nasal symptoms. Additionally, there is a paucity of information about the relationship between alcohol-induced nasal symptoms and other diseases, such as AR, nasal polyposis, asthma, and other chronic lower airway diseases.165
Airway symptoms are predominantly initiated by inhaled components that contact the airway mucosal membrane. However, several forms of rhinitis and asthma may not operate through this mechanism. One such example is known as alcohol-induced asthma. In these patients, alcoholic beverages, particularly red and white wines, have been shown to trigger bronchial symptoms.163,166,167
Alcohol-induced nasal symptoms are about twice as common in females as in males,165 but the basis for this predilection is not well understood.168-170 Nasal congestion is the predominant symptom, and red wine is the most common alcoholic beverage to elicit symptoms. Additionally, wine, particularly red, is also the most widely recognized trigger of alcohol-induced bronchial symptoms.163 Finally, direct alcohol utilization has also been associated with a trend toward developing SPT positivity,171 and with increased serum total IgE (tIgE) levels.172
III.C.9. Non-allergic rhinitis with eosinophilia syndrome (NARES)
Non-allergic rhinitis with eosinophilia syndrome (NARES) is a clinical disorder comprising symptoms consistent with PAR in which an absence of atopy has been demonstrated, and eosinophilia is found on nasal cytology.173 The pathophysiology of NARES is not well understood, but a key component involves an eosinophilic, self-perpetuating inflammation, with nonspecific histamine release. It is the most common type of inflammatory NAR, and was first described in 1981 by Jacobs et al.174
NARES patients report symptoms that are typical, although often more pronounced, than those of PAR. These include, nasal congestion, profuse aqueous rhinorrhea, sneezing, and nasal and ocular pruritis. A prominent feature not shared with AR is anosmia, a frequent finding in NARES patients.175 NARES is diagnosed by careful history, findings on physical exam (pale, boggy turbinates, like those found in PAR patients), and negative skin or in vitro allergy testing. Cytologic examination in NARES reveals the presence of prominent eosinophilia, usually 10% to 20%173 on nasal smear, with a diagnostic criterion (described by some) of more than 25% eosinophilia.176 In addition, nasal biopsies from these patients commonly show increased numbers of mast cells and prominent mast cell degranulation.177,178
Research has supported the role of chronic inflammation in the development of NARES. Though there is still a lack of understanding as to the exact pathophysiology, studies have shown an increased transendothelial migration of eosinophils, attracted and activated by chemokines and cytokines.179,180 Specifically, NARES is characterized by elevated nasal fluid levels of tryptase (also seen in PAR patients) and eosinophilic cationic protein (ECP) (markedly increased solely in NARES).181 In addition, increased Th2 cytokines (interleukin [IL]-6 and IL-17) appear to be a factor in the remodeling process seen in NARES.182 Other proinflammatory chemokines that have been implicated for their role in eosinophil chemotaxis and infiltration include macrophage/monocyte chemoattractant protein (MCP)-1 and regulated on activation, normal T-cell expressed and secreted (RANTES). Elevated RANTES concentrations have been found in the nasal fluid of patients with PAR and NARES.183 Recently, Peric et al.184 demonstrated a correlation between the concentration of RANTES with nasal symptoms and eosinophil counts in PAR patients. However, levels of MCP-1 and RANTES were significantly higher in the nasal fluid of NARES compared to PAR subjects, which again, correlated with nasal symptom scores and density of eosinophilia in these patients. Nasal neural dysfunction has also been described as a contributing factor to the symptomatology in NARES.185
NARES usually occurs in isolation but may be associated with aspirin-exacerbated respiratory disease (AERD), characterized by asthma, nasal polyps, and NSAID intolerance.173 NARES has also been identified as a risk factor for the induction or augmentation of obstructive sleep apnea (OSA).186
The treatment of NAR centers on its underlying cause. Given the inflammatory changes demonstrated on nasal cytology and physical exam, NARES is primarily treated with INCS sprays.154 This method of treatment is known to decrease neutrophil and eosinophil chemotaxis, reduce mast cell and basophil mediator release, and result in decreased mucosal edema and local inflammation.187 The intranasal antihistamine azelastine is U.S. Food and Drug Administration (FDA)-approved for both AR and NAR. In clinical trials, azelastine has been shown to reduce symptoms of rhinitis, including postnasal drainage, sneezing, rhinorrhea, and congestion.188 However, these multicentered, placebo-controlled trials studied azelastine for the treatment of vasomotor rhinitis (non-allergic rhinopathy) rather than NARES specifically.
III.C.10. Vasomotor rhinitis (nonallergic rhinopathy)
Vasomotor rhinitis is the most common cause of NAR, and is found in 71% of cases.189-191 The absence of an IgE-mediated immune response differentiates vasomotor from allergic forms of rhinitis.101 Therefore, the term “non-allergic rhinopathy” is recommended to replace vasomotor rhinitis, as inflammation is not regarded as a crucial part in the pathogenesis of non-allergic rhinopathy. In Europe, “idiopathic rhinitis” has also been used to describe this condition.
Non-allergic rhinopathy is a diagnosis of exclusion, and other etiologic factors for rhinopathy must be evaluated. These include CRS, NARES, AERD, infectious rhinitis, anatomical abnormalities, RM, drug side effects, cerebrospinal fluid (CSF) rhinorrhea, and rhinitis of pregnancy. Clinical characteristics of non-allergic rhinopathy have been summarized in a consensus paper by Kaliner et al.40 Non-allergic rhinopathy represents a chronic disease with primary symptoms of rhinorrhea. Associated symptoms of nasal congestion, postnasal drip in the absence of acid reflux, throat clearing, cough, Eustachian tube dysfunction, sneezing, hyposmia, and facial pressure/headache may also be present with non-allergic rhinopathy. These symptoms may be perennial, persistent, or seasonal, and are typically elicited by defined triggers, such as cold air, climate changes (ie, temperature, humidity, barometric pressure), strong smells, tobacco smoke, changes in sexual hormone levels, environmental pollutants, physical exercise, and alcohol. While often associated with non-allergic rhinopathy, the lack of a defined trigger does not preclude this diagnosis. In addition, nasal hyperreactivity to nonspecific stimuli may occur in both allergic and non-allergic rhinitis.192
Non-allergic rhinopathy is primarily found in adults, with a female-to-male ratio of 2:1 to 3:1. On physical exam, the nasal mucosa usually appears normal, but may show signs of erythema and clear rhinorrhea. While systemic allergy testing (skin or in vitro testing) is typically sufficient to differentiate between AR and non-allergic rhinopathy, a diagnosis of LAR may be considered in the setting of negative systemic testing. Individuals with LAR suffer from typical allergic symptoms upon allergen exposure, but display a lack of systemic IgE sensitization. Local provocation is necessary to definitively exclude this diagnosis.193,194
While the exact pathophysiology of non-allergic rhinopathy remains incompletely described, neurosensory abnormalities are thought to play a crucial role.40 In a prior study of central responses to olfactory stimuli, subjects with non-allergic rhinopathy underwent functional magnetic resonance imaging following exposure to different odors (vanilla and hickory smoke). Findings included increased blood flow to the olfactory cortex, leading to the hypothesis of an altered neurologic response in non-allergic rhinopathy.195,196 Patients with non-allergic rhinopathy with a predominant symptom of rhinorrhea will often respond to treatment with intranasal anticholinergics such as ipratropium bromide (IPB).
III.C.11. Age-related rhinitis (ie, elderly)
Age-related changes occur in every organ system, including the respiratory system. Specific to the nasal cavity, the physiological process of aging results in neural, hormonal, mucosal, olfactory, and histologic alterations that cause morphological and functional changes in the aging nose.197,198 This makes the elderly population more vulnerable to symptoms such as rhinorrhea, nasal congestion, postnasal drip, dry nose, intranasal crusting, and decreased olfaction.199,200 A recent publication by DelGaudio and Panella201 reviewed the literature pertaining to intranasal findings of the aging nose, which they have termed “presbynasalis.”
Age-related rhinorrhea.
Rhinitis of the older adult (ie, “drippy nose” or “senile rhinorrhea”) is a well-recognized entity. Rodriguez et al.202 used a questionnaire to demonstrate that clear rhinorrhea increases with age. Results showed that only 33% of the younger age group respondents (n = 76, mean age 19 years) regularly reported clear anterior drainage as compared to 74% of the older age group respondents (n = 82, mean age 86 years).
The physiologic reason for increased rhinorrhea with age is not entirely known. However, it is known that α and β receptors become less sensitive and autonomic function declines with age, which leads to an imbalance of sympathetic and parasympathetic tone.202-204 It is possible that decreased sympathetic tone with unopposed parasympathetic stimulation results in a rise in glandular activity in the nasal cavity, leading to increased nasal drainage.202,205 This mechanism is similar to vasomotor rhinitis/non-allergic rhinopathy, where the autonomic response to certain stimulants causes the nasal mucosal blood vessels to vasodilate and the mucus glands to become over-active, resulting in hypersecretion and drainage.206 Vasomotor rhinitis/non-allergic rhinopathy is the most common type of NAR,205 and the highest prevalence of NAR is seen in the elderly.144,189,200,207 This would suggest an autonomic dysregulation as the reason for increased rhinorrhea in the aging population.
Age-related nasal obstruction and congestion.
Factors that contribute to an increase in nasal obstruction/congestion in the aging nose include thicker mucus secondary to a decrease in body water content,208-210 nasal airflow obstruction secondary to structural changes caused by the loss of nasal cartilage elasticity and tip support,198,200,210 and mucus stasis secondary to less effective MCC.200,209 Ho et al.211 demonstrated a decline in MCC effectiveness with age in 90 healthy subjects aged 11 to 90 years. Subjects over 40 years of age had a slower ciliary beat frequency, increased microtubule disarrangement, and longer MCC times on saccharin testing. Thickened mucus and a less effective MCC system may also lead to postnasal drip, which is a common nasal complaint in the elderly population.200
Another factor contributing to nasal obstruction/congestion in the elderly is age-related central nervous system changes that affect the physiologic nasal cycle.208,212 Mirza et al.212 measured the relative airflow of the 6 nasal chambers at 15-minute intervals for 6 hours across 4 different age groups (n = 60) using liquid crystal thermography. They found that the proportion of subjects exhibiting the classic nasal cycle decreased with age, being lowest in the 70-year-old to 85-year-old group.
Age-related nasal dryness and intranasal crusting.
Nasal dryness and intranasal crusting are more common in the elderly population. This is likely due to age-related changes of the nasal mucosa,199 such as a decrease in mucosal blood flow and an increase in epithelial atrophy.213 Schrodter et al.214 evaluated nasal mucosa samples from the middle turbinate of 40 healthy subjects between the ages of 5 and 75 years, and found an age-related increase in atrophic epithelium and thickened basement membranes in patients over 40 years old.
Nasal dryness in the elderly population may also be caused by a decrease in intranasal temperature and humidity.200 Lindemann et al.199 measured these values in 80 healthy patients and found them to be significantly lower in older patients (age 61 to 84 years) than in younger patients (age 20 to 40 years). The authors attributed the difference to an increase in intranasal volume (INV) from age-related atrophy of the nasal mucosa, with INV measured by minimal cross-sectional areas and volumes of each nasal cavity. An increase in INV with age has also been demonstrated by Loftus et al.215 using 3D-volumetric analysis of computed tomography (CT) scans from subjects without sinonasal pathology. Mean INV was 15.73 mL in the 20 to 30 year age group (n = 22), 17.30 mL in the 40 to 50 year age group (n = 20), and 18.38 mL in the over 70 year age group (n = 20).
Allergic rhinitis in the elderly.
Although there is overlap between age-related rhinitis and AR in the elderly in terms of symptoms and recommended treatment with INCS,210,216 the underlying physiologic process of each is quite different. AR is a type I IgE-mediated hypersensitivity reaction,217,218 whereas allergy and allergens do not play a role in the symptoms and physiologic changes of age-related rhinitis. However, it has been shown that aging does not reduce the prevalence of AR and that AR in the elderly is likely underdiagnosed, so AR should be considered when diagnosing new-onset nasal symptoms in the elderly population.210
III.C.12. Empty nose syndrome and atrophic rhinitis
The descriptive term “empty nose syndrome” (ENS) was originally coined in 1994 by Kern and Stenkvist to describe empty space in the region of the inferior and middle turbinates on coronal CT images of patients who had partial or total inferior and middle turbinectomies.219 Today, ENS is defined as an upper airway disorder characterized by impaired nasal airflow sensation and often involves tissue loss from nasal surgery. ENS is divided into at least 3 subtypes: ENS-inferior turbinate, ENS-middle turbinate, and ENS-both, which are classified based on the site of tissue loss.219 ENS-inferior turbinate is the most common type.220 A fourth subtype is ENS-type, wherein a patient has sufficient appearing turbinate tissue but suffers ENS symptoms after surgery affecting the mucosal surface of the turbinates.
ENS typically occurs following surgery in the turbinates. Most turbinate surgery has successful outcomes, with ENS occurring after a very small percentage of sinonasal procedures.221,222 ENS occurs most frequently after total turbinate excision, but also with lesser procedures such as submucosal cautery or resection, laser therapy, and cryosurgery.223 Patients often complain of dryness and crusting, although the hallmark complaint of ENS patients is paradoxical nasal congestion that may be so severe that they feel as if they are suffocating.223 Recent research has validated that the primary physiological mechanism that produces the sensation of ample nasal airflow is activation of trigeminal cool thermoreceptors, specifically TRPM8, by nasal mucosal cooling.224-228 Beyond alterations in airflow and a reduction in surface area, aberrations in neurosensory systems likely play a major role in the abnormal sensations ENS patients experience. Not only does turbinate resection remove nasal mucosa and consequently airflow sensing thermoreceptors, such surgery causes nerve damage that if improperly healed, results in failure to return to a normal physiologic state.221 Differences in nerve recovery after surgery may explain why only some patients develop ENS despite identical turbinate surgeries. Indeed, certain surgeons have identified patients with unilateral ENS symptoms while their normal sensing side looks like a mirror image in terms of absent inferior turbinate tissue. Diagnosis is made based on history, physical exam, and the cotton test, where a piece of slightly moist cotton is placed in the nasal cavity for 10 to 30 minutes with alleviation of symptoms, validating the diagnosis.223 Other conditions that present with nasal dryness and crusting should be ruled out (ie, atrophic rhinitis, sarcoidosis, etc.). The Empty Nose Syndrome 6-Item Questionnaire has documented validity in identifying ENS patients.229 Surgery for submucosal expansion of the internal nasal mucosa can often bring relief for patients.223 It has also been reported that depression and anxiety are prevalent among ENS patients.230
Atrophic rhinitis is a chronic, degenerative condition characterized by inflammation and atrophy of the nasal and paranasal mucosa.231 Primary atrophic rhinitis runs a protracted course. It can occur spontaneously with unknown etiology, but it is also associated with a bacterial infection, almost exclusively Klebsiella ozaenae. In a study examining 45 patients diagnosed with primary atrophic rhinitis, all nasal cultures were positive for Klebsiella ozaenae.231 Mucosal injury is hypothesized to result from prolonged microvascular or ischemic injury.231-233 Secondary atrophic rhinitis is far more common and usually develops following direct injury from trauma, irradiation, reductive nasal or sinus surgery, or in certain rare granulomatous diseases.231,234 Secondary atrophic rhinitis is also associated with a bacterial infection, but Staphylococcus aureus, Proteus mirabilis, and Escherichia coli are the more common pathogens, with Klebsiella ozaenae rarely isolated.231
Atrophic rhinitis presents as thick, adherent nasal crusting, nasal congestion, foul odor, and atrophy of mucosal and turbinate surfaces, with severe cases having complete absence of recognizable anatomic landmarks, septal perforations, or saddle nose deformity.231-233 Hyposmia, epistaxis, and facial pain or pressure may also occur. Histological examination of intranasal tissue demonstrates squamous metaplasia, glandular atrophy, and diffuse endarteritis obliterans in both types of atrophic rhinitis.231 Diagnosis is established from clinical examination, nasal biopsy, and nasal cultures for associated bacteria.
Both atrophic rhinitis and ENS patients complain of nasal congestion. For atrophic rhinitis patients, this is often a result of significant nasal crusting, although as the disease progresses and mucosa and turbinate tissue is lost, the widened nasal cavity can very closely resemble that of an ENS patient. The pathophysiology of the paradoxical sensation of nasal congestion at this point is the same in both disease states, although the origin of the inciting event differs.
In the literature, ENS has repeatedly been described erroneously as a form or subset of atrophic rhinitis. ENS results from iatrogenic removal of turbinate tissue and is not associated with a bacterial infection whereas atrophic rhinitis results from a chronic, often idiopathic inflammatory process associated with bacterial infection that progresses to resorption of turbinate tissue. Atrophic rhinitis patients suffer from heavy crusting whereas ENS patients exhibit only minor crusting or no crusting.
To differentiate AR [allergic rhinitis] from atrophic rhinitis, it should be noted that AR is an immunological response to a benign substance, the allergen, that manifests primarily as nasal inflammation. AR is IgE-dependent235 and characterized by sneezing, clear rhinorrhea, watery eyes, and nasal and ocular pruritus.1 This condition has a clear distinction from ENS and atrophic rhinitis in its clinical presentation and pathophysiology.
III.C.13. Autoimmune, granulomatous, and vasculitic rhinitis
Both the upper and lower airways can be affected by systemic disorders including vasculitic, granulomatous, and autoimmune diseases. Commonly, affected patients may present with nonspecific sinonasal symptoms (nasal obstruction, rhinorrhea, facial pain, and loss of smell) mimicking AR. Allergy testing will, however, be negative or not clinically relevant. Clinicians should consider broadening the differential to consider systemic etiologies if either crusting or recurrent epistaxis is seen.236 Oral steroids are the mainstay of treatment for the entities discussed in this section, although the recent introduction of monoclonal antibodies targeting specific biomarkers represents an important hallmark for future therapy.
Granulomatosis with polyangiitis.
Previously referred to as Wegener’s disease, granulomatosis with polyangiitis (GPA) is an idiopathic disease characterized by necrotizing and granulomatous inflammation of the upper and lower airways (85%), glomerulonephritis (75%) and systemic vasculitis.237-239 Limited forms of GPA involving only the head and neck may also be seen. GPA predominantly affects small to medium sized arteries and vein walls.240 GPA affects both men and women in a similar proportion, being frequently diagnosed in the fourth to sixth decades of life.240 In the US, estimated prevalence is 13 to 30 cases permillion people per 5-year period. Nasal symptoms include obstruction, rhinorrhea, recurrent epistaxis, crusting, and pain over the nasal dorsum.237,241 Nasal mucosa disruption may lead to anosmia while tissue necrosis with secondary infection may lead to cacosmia.236 Nasal endoscopy can reveal an erythematous, friable mucosa with crusting and granulation that is seen in the septum and inferior turbinate.240 Patients with severe forms can present with nonvascular necrosis causing perforation or bony destruction of the nasal septum and/or other nasal structures.242 Diagnosis is based on clinical symptoms, physical findings, radiological examinations, laboratory tests (positive c-ANCA [anti-nuclear cytoplasmic antibody] in 60–90%), and biopsy of affected tissue for pathological examination.237,238,240 Profiling the nasal transcriptome in GPA reveals unique gene expression signatures related to innate immunity, inflammatory cell chemotaxis, extracellular matrix composition, and epithelial barrier integrity that may eventually be used clinically.243,244 Treatment includes prednisone, cyclophosphamide, or methotrexate.237,238,245 Rituximab, anti-CD20 monoclonal antibody, may be an effective therapy in refractory or relapsing c-ANCA vasculitis,246 although additional study is needed.
Eosinophilic granulomatosis with polyangiitis.
Previously known as Churg-Strauss Syndrome, eosinophilic granulomatosis with polyangiitis (EGPA) is a rare small-sized vessel vasculitis with a prevalence of 1.3 cases per 100,000,247 typically diagnosed in patients age 30 to 50 years.236 Rhinitis (75% of patients) is one of the initial manifestations of EGPA,248 in addition to CRS with nasal polyps (CRSwNP), and partial/total smell loss.249 Diagnosis should be suspected in patients with asthma, with increased peripheral blood eosinophil count (>10%) and pulmonary manifestations.238,248 EGPA is often associated with the presence of p-ANCA.247 CRSwNP is present in approximately 50% of patients.238 Nasal pain with purulent or bloody nasal discharge, nasal crusting, or nasal septal perforation can be present but are less common than in GPA patients.238,250 Treatment usually includes high doses of corticosteroids and immunosuppressants.248,251 Anti-IL-5 therapy (mepolizumab) is a potential biological treatment offering clinical benefit and stability and reducing corticosteroid needs.252
Sarcoidosis.
Sarcoidosis is a chronic multisystem disorder characterized by bilateral hilar adenopathy, pulmonary infiltration, ocular, and skin lesions.238,253 More commonly seen in young and middle-aged adults,254 females more frequently than males, and African-Americans,255 a prevalence of 50 per 100,000 individuals has been reported.236 The involvement of the upper respiratory tract epithelium is infrequent236 and nasal symptoms are nonspecific: obstruction, epistaxis, nasal pain, epiphora, and anosmia.237 The most consistent findings are erythematous, edematous, friable, and hypertrophied mucosa in the septum and inferior turbinate. Submucosal yellow nodules representative of intramucosal granulomas may be identified in mucosal biopsies, while nasal polyps, rhinophyma, and septal perforations have also been reported.238,256 Aggressive non-caseating granulomas can cause hard or soft palate erosions as well as septal perforations leading to saddle-nose deformity.257,258 The diagnosis of sinonasal sarcoidosis is based on the clinical findings with either polypoid changes or characteristic yellowish submucosal nodularity.238 Tissue for diagnosis is usually obtained by transbronchial-lung biopsy254 or nasal biopsy, as well as from skin lesions, minor salivary glands, and lymph nodes.238 The primary treatment for sarcoidosis is systemic steroids, chloroquine, immunosuppressants, and lung-transplantation.237,238,256,257 The emergence of biological therapies has increased the therapeutic options to treat refractory organ-threatening sarcoidosis, with monoclonal anti-TNF (tumor necrosis factor) agents (infliximab) being the most promising.259
Systemic lupus erythematosus.
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect any body system. SLE predominantly affects women (10:1) with an incidence of 5.6 per 100,000 people.260 The skin of the nose and nasal vestibule can also be involved in the skin rashes.237 Mucosal lesions are seen in 9% to 18% of cases, with oral, nasal, and pharyngeal mucosa being commonly affected.260 The diagnosis requires a detailed medical history, a physical examination, and laboratory tests (anti-nuclear antibody [ANA] or anti-double-stranded DNA), including a complete blood count, chemistry panel, and urinalysis.236,261 Therapy with corticosteroids, immunomodulators (prasterone, vitamin D, hydroxychloroquine), or immunosuppressants (azathioprine, cyclophosphamide, or mycophenolate) is prescribed for symptom control,238,262 while belimumab is a recent biological (anti-BAFF [B-cell activating factor] monoclonal antibody) to potentially treat SLE.263
III.C.14. Rhinosinusitis
The symptoms of AR may overlap with other forms of nasal inflammation, including rhinosinusitis. It is important to differentiate between AR and rhinosinusitis to ensure the correct diagnosis and subsequent treatment can be pursued. AR may be associated with comorbid rhinosinusitis, although whether AR increases the risk of rhinosinusitis is debatable.1 Identifying comorbid rhinosinusitis is essential to ensure the appropriate management of both conditions. Of note, these conditions are not mutually exclusive and there may be an association between rhinosinusitis and AR. It is possible to have concurrent AR and rhinosinusitis, and this possibility should be considered when patients meet diagnostic criteria for both independently and when patient symptomatology or response to treatment does not fit with a single diagnosis.1 A high degree of clinical suspicion is required; however, careful consideration of these factors may help guide clinicians to the correct diagnosis or diagnoses.
Rhinosinusitis is a broad term that includes the diagnoses of acute rhinosinusitis (ARS), RARS, and CRS, demarcated as CRSwNP or CRS without nasal polyposis (CRSsNP). Symptomatically, these conditions are characterized by nasal obstruction, nasal congestion, facial pressure or pain, anterior or posterior nasal discharge, and anosmia/hyposmia for varying durations of time.1,138 AR shares several overlapping symptoms, namely rhinorrhea and nasal congestion, which may be confused with the subtypes of rhinosinusitis.264,265 Conversely, rhinosinusitis may be mistaken for AR due to the similar symptomatology.1 Understanding the diagnostic criteria for the subtypes of rhinosinusitis will aid clinicians in solidifying the correct diagnosis, as well as identifying comorbid conditions.
ARS is defined as the sudden onset of sinonasal symptoms with associated sinonasal inflammation that lasts less than 4 weeks.1,137,138,266,267 Symptoms include nasal congestion, nasal obstruction or nasal discharge, and facial pressure or pain, or anosmia/hyposmia. Nasal discharge is often purulent and may be discolored, with a tendency to be unilateral although may be bilateral.1,138 Facial pressure and pain is described as moderate to severe.137 ARS may be viral or bacterial. In general, viral ARS is present for less than 10 days. A longer duration of illness suggests bacterial ARS.137,138 Progressive worsening over a short period of time (ie, 5 days) is also suggestive of bacterial ARS.137,138 In the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) statement, fever and elevated serum markers of inflammation (C-reactive protein or erythrocyte sedimentation rate) are also included as diagnostic criteria.138 Fever is not included in other guidelines, due to its low specificity and sensitivity.137 RARS is defined as at least 4 episodes of ARS per year, with disease-free intervals between episodes.1,137,138,266,268
CRS is an inflammatory condition of the sinonasal cavity persisting for more than 12 weeks with at least 2 symptoms of nasal obstruction and congestion, mucopurulent nasal drainage (anterior or posterior), facial pressure or pain, and anosmia/hyposmia.1,137,138,266,267 In addition, patients must have objective evidence of sinonasal inflammation on either nasal endoscopy (polyps, edema, mucopurulent rhinorrhea) or on CT scans of the sinuses.137,138,266,267 CRS is divided into 2 main phenotypic groups: CRSwNP and CRSsNP.
Comparatively, AR is characterized by nasal obstruction, nasal congestion, clear watery rhinorrhea (anterior or posterior), and allergic symptoms.264,265 The presence of these symptoms should raise suspicions of AR as either a primary or comorbid diagnosis. Conversely, AR is typically not associated with purulent or unilateral nasal discharge. Moderate to severe facial pain and/or fever would also be atypical for isolated AR and may indicate the presence of an episode of ARS or an acute exacerbation of CRS, differentiated by duration and chronicity of symptoms.1,137,138 The timing of symptoms may also help delineate between rhinosinusitis and AR as ARS symptoms typically last days to weeks (but no more than 4 weeks), CRS symptoms persist daily for greater than 12 weeks. In comparison, while AR symptoms are variable in duration, they tend to have seasonal or exposure-related fluctuations.1,137,138 AR symptoms are present for at least 1 hour on most symptomatic days; however, patients may have symptom-free intervals.264,265 AR symptoms are also exacerbated by exposure to allergens in a time dependent fashion.264 The early reaction occurs immediately after exposure and is characterized by sneezing, nasal and ocular itching and rhinorrhea, which typically resolves within 30 minutes.264 The late reaction takes place up to 6 hours after exposure and is characterized by nasal obstruction and congestion.264 Superimposed late reactions may blunt the manifestation of acute phase symptoms and make the diagnosis of AR less obvious.
When attempting to determine whether a patient has AR, ARS, RARS, or CRS, it is important to elicit a history of specific symptoms from the patient that includes onset and duration of symptoms. A history of allergic symptoms or allergen exposure-related symptoms support a possible diagnosis of AR, as these are not associated with rhinosinusitis and AR may or may not be seasonal in nature, which can also be elicited by history.264,265 The development of acute, moderate to severe symptoms, and nasal purulence may be consistent with ARS or RARS rather than AR.1,137,138 A prolonged duration of symptoms (greater than 12 weeks) should raise suspicions for CRS and prompt further investigation.1,137,138 (See section X.B. Associated conditions - Rhinosinusitis for additional information on this topic.)
IV. Pathophysiology and mechanisms of allergic rhinitis
A background understanding of the pathophysiology and underlying mechanisms of AR is necessary as we examine the clinical presentations, physical manifestations, goals of allergy testing, and response to treatment. This section addresses the cellular inflammation, soluble mediators, local allergic manifestations, and systemic effects associated with AR. While this document is not intended to provide an extensive review of the pathophysiology of AR, the following short section provides a foundation for understanding the clinical expression of AR and its treatment.
IV.A. IgE-mediated allergic rhinitis
IV.A.1. Systemic mechanisms and manifestations
The immune response leading to IgE production in AR is often a systemic phenomenon, and patients with AR demonstrate evidence of systemic atopy.269,270 One manifestation of systemic atopy in AR is the cutaneous reaction elicited during traditional allergy skin testing.271 Further evidence for the systemic nature of the IgE response in AR includes the temporal relationship of AR to a number of other allergic diseases, including atopic dermatitis (AD), food allergy, and allergic asthma, a phenomenon known as the “atopic march.”272 This pattern of atopic disease progression is well-known and supported by prospective studies.273
The immunologic processes underlying IgE-mediated AR are similar to those of other atopic conditions and involve activation of the adaptive immune system. The adaptive immune response can be broadly classified into 2 categories based upon the predominant Th lymphocyte subtype.274 The Th1 profile is responsible for defense against intra-cellular pathogens, while Th2 responses are implicated in the defense against parasitic infections as well as the IgE-mediated eosinophilic inflammation of allergy.272 Whether AR will develop as a result of inhalant allergen exposure therefore depends largely upon the balance between Th1 and Th2 effector cells.274
A number of steps in the sensitization process are responsible for eliciting the Th2-predominant response. The process begins with exposure of the nasal mucosa to inhalant allergens.275 While mucosal epithelial cells were once thought to function simply as a mechanical barrier to allergen penetration, recent research suggests that epithelial cells play a much more sophisticated role in allergy development, through the secretion of numerous inflammatory mediators including cytokines, chemokines, eicosanoids, and endopeptidases, as well as through upregulation of cellular adhesion molecules and release of matrix metalloproteinases.276 They also provide an important early stimulus toward a Th2-weighted immune response, through the secretion of thymic stromal lymphopoietin (TSLP).272,275,276 TSLP causes maturation of dendritic cells into Th2-promoting subtypes,277 which secrete chemokines that attract Th2-destined T lymphocytes, foster clonal amplification of Th2 cells, and enhance survival of memory B-cells.272 TSLP also promotes recruitment of eosinophils and enhanced activity of basophils and mast cells.272
Allergens are then engulfed by dendritic cells, which migrate to lymphoid organs where the antigen is presented to naive helper T (Th0) cells on MHC class II molecules.274 Th2 differentiation also requires co-stimulation via the interaction of CD28 on T cells with CD80 and CD86 on antigen-presenting cells (APCs).278 Additionally, the presence of the cytokine IL-4 is required.279 IL-4 binds STAT-6 on the Th0 cell, activating the master switch GATA-3.272 This stimulates IL-4, IL-5, and IL-13 production,274 which is characteristic of the Th2 response. These cytokines, produced by the newly differentiated Th2 cell, have several effects that further promote IgE-mediated eosinophilic inflammation and allergy.
IgE is produced by B-cells under the influence of Th2 effector cells and the cytokines they secrete.275 Development of an IgE-secreting B cell requires the presence of IL-4 or IL-13, which induce class switching via upregulation of ε-germline gene transcription and clonal expansion, as well as interaction between CD40 ligand on the T-cell surface and CD40 on the B-cell surface, which promotes B-cell activation and the production of IgE.279 Allergen-specific IgE (sIgE) is then released into the circulation by plasma cells.
IgE antibodies subsequently bind high-affinity receptors (FcεRI) on the surface of mast cells and basophils, rendering them sensitized.280 Future allergen exposure results in crosslinking of IgE on the surface of mast cells and basophils causing degranulation, release of inflammatory mediators such as histamine, and the classic symptoms of AR.
IV.A.2. IgE-IgE receptor cascade
IgE plays a central and defining role in the pathophysiology of acute allergic reactions as well as chronic atopic disease.281 In individuals with AR, exposure to specific allergens results in the production of allergen-specific IgE, which then binds to effector cells such as mast cells and basophils via the high-affinity receptor FcεRI. Although IgE in plasma is short-lived, IgE that is receptor-bound remains attached to these cells for weeks or months. Moreover, when IgE bound to FcεRI cross-links with a specific allergen, it induces the release of preformed inflammatory mediators from mast cells and basophils, resulting in clinical manifestations of allergic diseases.
Cytokines including IL-4 and IL-13 released from T cells and mast cells drive the differentiation of B cells into IgE-secreting plasma cells. Several studies, both in vivo and in vitro have confirmed the production of local IgE in the nasal mucosa of patients with AR.282-284 The locally produced IgE plays a key role in ongoing inflammation by up-regulating FcεRI expression in mast cells.283-285 The augmented expression of FcεRI allows them to bind greater numbers of IgE-antigen complexes, which in turn enhances the sensitivity of mast cells to allergen. This results in an increased production of immunomodulatory cytokines and chemical mediators, forming an important positive-feedback amplification loop involving the IgE-IgE receptor cascade, thus perpetuating ongoing inflammation.285,286 Interestingly, the density of IgE receptors and IgE molecules in mast cells within the nasal mucosa of patients with AR have been shown to correlate with levels of serum IgE.285 The presence of elevated levels of IgE in nasal secretions has been demonstrated in non-allergic rhinopathy as well, which potentially further highlights a significance of the IgE-IgE receptor cascade in driving the disease process of rhinitis.287
IV.A.3. Local IgE production and local allergic rhinitis (LAR)
LAR is a regional inflammatory condition defined by local symptoms and sIgE-mediated inflammation without evidence of systemic hypersensitivity.107,194,284,288 It is important to remember that conventional allergy testing, such as SPT and the radioallergosorbent test (RAST), only indicates sensitization (atopy), but not symptomatic allergy. While it is possible for a positive allergy skin or in vitro test result to lack clinical relevance, the opposite is also true, as a negative allergy skin or in vitro test result does not exclude regional IgE-mediated sensitivity, as in the case of LAR.194,288-290 LAR may affect more than 47% of children and adults previously classified as NAR,290-295 and persists throughout the years with a low rate of conversion to clinical AR.296-298 However, LAR may evolve to the development of asthma.296,297 Diagnosis of LAR is based on demonstration of a positive response to NPT and/or the detection of nasal sIgE and/or a positive basophil activation test (BAT) in the absence of systemic atopy. The pathophysiology of LAR is complex and not completely understood. Immunologic studies have revealed the existence of a Th2 inflammatory response in the nasal mucosa of LAR patients,177,299-301 with positive response to NPT,291,300-302 and local production of sIgE177,290,299-301,303-305 and inflammatory mediators.304,306,307
Nasal Th2 inflammatory response.
Flow cytometry studies in nasal secretions have confirmed that aeroallergen exposure induces a Th2 inflammatory response in the nasal mucosa of LAR patients with increased eosinophils, basophils, mast cells, CD3+, and CD4+ T cells.300,301 NPT studies have demonstrated the existence of characteristic immediate/early and late-phases of the allergic response in LAR patients with local production of sIgE, mast cell, and eosinophil activation, with mucosal secretion of tryptase and ECP.306,307 A recent study showed that 83% of LAR subjects sensitized to Olea europaea pollen responded to NPT with nOle e 1 (the most significant allergen of Olea europea), demonstrating that purified allergens can also induce an allergic response with secretion of ECP.308
Local sIgE production.
The respiratory airway mucosa is a site of IgE production during allergic inflammation, as has been demonstrated in patients with AR309-312 and LAR,299-301,303-307 with both somatic hypermutation and class switching occurring in the nasal mucosa.309,312-315 Cellular studies have confirmed the expression of ε-germline gene transcripts and messenger RNA (mRNA) for the ε heavy-chain of IgE in nasal mucosal B-cells.310 The rate of local IgE production316 is sufficient to saturate IgE receptors on local mast cells, and potentially spill over into the circulation.316,317 In LAR, the presence of sIgE in nasal secretions has been confirmed after natural allergen exposure,300,301 NPT,300,301,303-305 and periods of non-exposure.300,301 Furthermore, local sIgE in LAR has the capability of activating basophils via the high-affinity receptor FcεRI, leading to the release of inflammatory mediators characteristic of AR.308,318
IV.B. Non–IgE-mediated inflammation in allergic rhinitis
It is commonly accepted that AR is primarily an IgE-driven response.319 However, in recent years our understanding and appreciation of the important contributions of the nasal innate immune response to the pathogenesis of AR has grown substantially.320 The pathophysiologic mechanisms of inflammatory airway disease are related to large physiologic networks that influence host-environment interactions. The nasal epithelium is the first structure to encounter inhaled aeroallergens. Intrinsic proteolytic activity of allergens may disrupt the nasal epithelial barrier, facilitating allergen penetration and chronic inflammation.321 Recent data provide additional evidence that epithelial barrier dysfunction contributes to the development of inflammatory diseases such as AR, but it remains to be elucidated to what extent primary (genetic) vs secondary (inflammatory) mechanisms drive this breakdown.322 Epithelial cells not only act as a physical barrier toward inhaled allergens, but also actively contribute to airway inflammation by detecting and responding to environmental factors. The nasal epithelium expresses pattern recognition receptors in the form of toll-like receptors (TLRs) that, after activation by allergens or pathogens, lead to the production of different mediators.323,324 These mediators affect recruitment of inflammatory cells to local tissues and create a microenvironment that affects the function of immune cells, thereby propagating local inflammatory processes.325 In allergic disease, the nasal epithelium seems to be in a permanently activated state,326 potentially as a consequence of the inability to switch off the activation response.327
An interesting recent development was the discovery of innate lymphoid cells (ILCs) as potential key players in the pathogenesis of Th2-type diseases such as AR, CRSwNP, and asthma.328-330 ILCs are a family of effector cells that are important for protection against infiltrating pathogens and restoration of tissue integrity. ILCs do not express antigen-specific T-cell receptors, but can react promptly to “danger signals” and produce an array of cytokines that direct ensuing immune responses. Three major subsets have been defined based on their phenotype and functional similarities to Th1 (ILC1), Th2 (ILC2), and Th17 (ILC3) cells. Upon exposure to environmental antigens, including viruses and allergens, airway epithelial cells rapidly release the cytokines IL-25, IL-33, and TSLP which directly activate ILC2s that then produce the prototypical type 2 cytokines IL-5 and IL-13.331 Allergen challenge in AR subjects induces an increased number of peripheral serum ILC2s332,333; however, a similar increase in the nasal mucosa is yet to be demonstrated. In addition to treatments aimed at modulating IgE-mediated inflammation, novel therapies directed toward the innate immune system are in development for treatment of AR.334,335
IV.C. Unified airway concept
The upper and lower airways are linked from anatomical, histological, and immunological perspectives with inflammation in one part of the airways influencing the other part, thus forming a united airway system.336 New systemic treatment options make understanding of the relationship between upper and lower airways even more important.337
The mucosa of the upper and lower airways is similar, containing pseudostratified epithelium with ciliated columnar cells lining. Basal epithelial cells are also present, attached to the basement membrane (lamina reticularis), and have an epithelial stem cell function. In the submucosa there are vessels, mucus glands, fibroblasts, and some inflammatory cells. The main difference in mucosal components is the absence of smooth muscles in the upper airways as compared to the lower airways, and the lack of extensive subepithelial capillaries, arterial systems, and venous cavernous sinusoids in the lower airways as compared to the upper airways.
The characterization of phenotypes of rhinitis and asthma are very similar, with emphasis on allergy and eosinophilia, non-allergic phenotypes in both upper and lower airways, and the link between CRS, especially with nasal polyps, and late onset asthma.319,338,339 Both AR and asthma may also be characterized by hyperreactivity that is not correlated to the atopic state.192,340 Also in endotyping, similarities can be pointed out with emphasis on type 2 vs non-type 2 immune responses. In allergic diseases, the prominent endotype is type 2 (eg, Th2 cells, type 2 B-cells, IL-4-producing natural killer [NK]/T cells, basophils, eosinophils, mast cells, ILC2, IL-4, IL-5, IL-13, IL-25, IL-31, IL-33).319,341 In general, the type 2 profile in AR and asthma is associated with a good response to corticosteroid treatment. New targeted treatments that focus on (subgroup) type 2 elements, such as anti-IgE antibodies, anti-IL-5 (mepolizumab), and anti-IL-4/IL-13 (dupilumab) are currently used in asthma, but are not currently approved for use in the upper airways.342 Similarities are not only found in the acquired immune response, but also in the role of innate immunity like epithelial barrier function334 and innate lymphoid cells.332 Epithelial barrier leakiness, particularly tight junctions that seal the upper and lower respiratory mucosal epithelial surface, has been shown in asthma, AR and CRS.343,344
Several mechanisms may explain the influence of sinonasal inflammation on the lower airways; ie, altered breathing pattern, pulmonary aspiration of nasal contents, the nasobronchial reflex, and the uptake of inflammatory mediators in the systemic circulation.345 The nose acts as a filter and air conditioner, protecting the lower airways. Reduced filter and air-conditioning functions of the nose may lead to increased exposure of the lower airways to allergens. Mouth breathing is independently associated with asthma morbidity, indicating that air conditioning can be of major importance. The efficacy of the nasal filter depends on the size of the inhaled particles. Small molecules, such as molds and cat dander, are more associated with an increased risk for asthma, whereas larger molecules, such as tree and grass pollen, are primarily associated with upper airway symptoms. The role of preferential mouth breathing in the development of asthma is unclear.346
Although there is a relationship between postnasal drip and coughing, no direct association has been proven between overproduction of nasal secretions and bronchial hyperreactivity. Moreover, after nasal application, deposits of radioactive-labeled allergen can be found in the digestive tract but not in the respiratory tract.347 Stimulation of pharyngolaryngeal receptors is more likely to be responsible for a postnasal drip-related cough.348 Interestingly, cough is not induced in patients with rhinitis or healthy controls in simulated models of postnasal drip.349
There is not much evidence supporting the nasobronchial reflex as an important contributor to the unified airway. Nasal allergen challenge can be blocked with a vasoconstrictor but not with lidocaine. Moreover, lower airway responses after allergen challenge are in general more delayed than would be expected following a nasal-bronchial reflex.350
Allergen provocation studies represent a good model to study nasal-bronchial crosstalk in allergic airway disease. In patients with AR, segmental bronchial or nasal provocation can induce allergic inflammation in both the nasal and bronchial mucosa.347-349 Presumably, absorption of inflammatory mediators (eg, IL-5 and eotaxin) from sites of inflammation into the systemic circulation results in the release of eosinophils, basophils, and their progenitor cells from the bone marrow.351 The systemic allergic response is further characterized by increased expression of adhesion molecules, such as vascular cell adhesion molecule 1 and E-selectin on nasal and bronchial endothelium, which facilitates the migration of inflammatory cells into the tissue.352
Increases in CD34+ cells capable of eosinophil differentiation, as well as other circulatory mediators (IL-5, eotaxin, and cysteinyl leukotrienes), are associated with impaired lung function parameters and enhanced mucosal inflammation in asthmatic patients,353 and react to local corticosteroids in AR.354 Treatment with anti-IL-5 and other interleukins relevant in the eosinophilic pathway has been shown to be effective in asthma, with some beneficial results in eosinophilic upper airway disease.342
In conclusion, these studies demonstrate that the same mechanisms behind AR may be important in airway inflammation throughout the respiratory tract, even in the absence of clinical asthma. Systemic factors, such as the number of circulatory eosinophils and atopic severity are indicative of more extensive airway disease.
IV.D. Cellular inflammatory infiltrates
A variety of cells are involved in the pathophysiology of AR. Due to the nature of the disease, with different mechanisms and endotypes, it is practically impossible to comprehensively describe each of these inflammatory cells in detail. This suggests a need for an extensive endotyping and characterization of the cellular infiltrate for each endotype.355 In addition, many studies focusing on cell types in allergic diseases, including recently identified cells such as type 2 ILCs, Th17 cells, and Th22 cells, have been mostly restricted to investigations of peripheral blood cells, not tissue biopsies. There is evidence from a limited number of studies that different cells are involved at different stages of inflammation, such as exacerbation, remission, and extensive remodeling. Furthermore, different tissue sites such as sinus mucosa, polyp tissue, or inferior turbinates show a variety of different infiltrating immune and inflammatory cells.
Nasal epithelial cells are at the interface of the human body and the environment, and often act as the first line of defense against external pathogens. Epithelial cells interfere with non-self allergens and regulate infiltrating cells in AR through the production of various co-stimulatory molecules, chemokines, cytokines, and lipid mediators. These cytokines start to orchestrate a type 2 immune response characteristic of AR.356 However, when allergens have additional protease activity and/or they are accompanied by microbial components such as endotoxins or inorganic particles, epithelial secretory responses can lead to mixed type 2 and type 17 immunity, or even type 1 responses.357,358 In response to respiratory viruses, epithelial cells produce a wide range of mediators such as type I interferons, granulocyte macrophage colony-stimulating factor (GM-CSF), RANTES/C-C Motif Chemokine 5 (CCL5), and interferon gamma-induced protein 10/C-X-C Motif Chemokine 10 (IP-10/CXCL10).359 These mediators orchestrate further downstream innate and adaptive antiviral cellular immune responses.
To activate allergen-specific CD4 T-cells, adequate co-stimulation is required. Dendritic cells are professional APCs that are directly related to AR, with increased numbers and concentrations of IgE in atopic disease.360 They are in close contact with epithelial cells and ILCs and control T-cell and B-cell activation and differentiation.356 Also, elimination of dendritic cells has been shown to suppress the development of AR.360
Both innate and effector mechanisms play essential roles during the development of allergic disease.361 T-helper subset imbalance and production of typical Th2 cytokines,362 along with increased expression of GATA-3,363 is generally seen in AR nasal mucosa. Furthermore, CD4+ memory T-cells and gamma/delta-T-cells are increased in PAR patients’ mucosa.364 Effector Th2 cells produce IL-4, IL-5, IL-9, and IL-13.356,365 In addition, TSLP, IL-25, IL-31, and IL-33 contribute to the development and intensity of Th2 responses and inflammation. These cytokines have roles in production of sIgE, eosinophilia, mucus, tissue migration of Th2 cells and eosinophils, regulation of tight junctions, and epithelial barrier integrity.343,356,366,367 T-regulatory (Treg) cell subsets have distinct phenotypes and include constitutive and inducible subsets of CD4+CD25+ Forkhead box P3 (FOXP3)+ Treg cells, and type 1 Treg cells.368-370 Treg cells play a major role in allergen tolerance and allergen immunotherapy (AIT).371-373 The production of IL-10 and transforming growth factor (TGF)-β from other cells is decisive for their immune regulatory functions. The ratio between effector and regulatory cell types determines whether an allergic response is triggered by an allergen or not.
Populations of lymphoid cells that lack rearranged antigen receptors and markers for myeloid and lymphoid lineages, such as T-cells, B-cells, and NK-cells have been defined as ILCs. Type 1 ILCs (ILC1) mainly produce interferon (IFN)-γ, ILC2s produce IL-5 and IL-13,374 and ILC3s produce IL-17 and IL-22.361 Type 2 ILCs are found in AR, where they closely interact with epithelial and other cells controlling the mucosal environment. Through the production of cytokines and induction of chemokines, a type 2 immune response is favored, supporting further development of an allergic tissue inflammation.375
Although it was believed that IgE-producing B-cells reside in lymphoid follicles of the Waldeyer ring376 and antibodies were then transferred to the mucosa, newer evidence has identified B-cells and plasma cells capable of producing IgE in nasal tissue of AR patients.377 The local production of allergen-specific antibodies is further supported by the detection of secondary lymphoid tissue and IgE formation to Staphylococcus aureus in CRSwNP.378
Within the nasal epithelium of allergic individuals increased numbers of major basic protein-positive and EG2+ (activated) eosinophils can be encountered during the pollen season. Similarly, mast cells are found within the epithelium and the submucosal layer; however, no increases are observed in cell counts of T-lymphocytes or their subsets, nor of neutrophils or macrophages during seasonal allergen exposure.379 Basophil numbers in the lamina propria of the nasal mucosa increase within 1 hour of allergen provocation.380 Degranulation of both mast cells381 and basophils occurs during the early and late phases of a type I reaction after allergen encounter and crosslinking of IgE molecules as well as upon stimulation by IL-33.382
In the late phase of the allergic reaction, the influx of inflammatory cells is facilitated by chemoattractants and upregulation of adhesion molecules.383 This leads to further infiltration of the tissue by eosinophils, basophils, and T-cells. Last, those inflammatory cells driving remodeling of the mucosa in AR, and upregulating factors such as matrix metalloproteinases and angiogenic factors, remain to be identified.384
IV.E. Cytokine network and soluble mediators
Cytokines are immunomodulatory proteins important in cellular signaling. Complex interactions of innate and adaptive immune cells, as well as structural cells and their cytokines, play crucial roles in regulating allergic airway inflammation. The inflammatory process underlying AR is coordinated by a network of cytokines.
Type 2 cytokines such as IL-4, IL-5, IL-6, and IL-13 are crucial in regulating the allergic inflammatory cascade characterized by an increased presence of eosinophils and mast cells and an upregulation of IgE production. Besides their role in the induction of IgE synthesis, type 2 cytokines up-regulate the production of other cytokines and chemokines from epithelial cells and fibroblasts,283 which then leads to the influx of inflammatory cells including eosinophils and mast cells.385,386 Scadding et al.387 demonstrated the immunological aspects of rhinitis with nasal allergen challenge. After nasal challenge with grass pollen in sensitive individuals, the levels of IL-4, IL-5, and IL-13 were elevated 2 to 3 hours postchallenge and increased for up to 5 or 6 hours.387 Similarly, levels of chemokines such as thymus-regulated and activation-regulated chemokine (TARC, CCL17), macrophage derived chemokine (MDC, CCL22), eotaxin, RANTES, MCP-1, and macrophage inflammatory protein (MIP)-1α were elevated.388-391 Increases in these type 2 cytokines and associated chemokines were strongly correlated to allergic clinical responses.
Although type 2 cytokines were originally referred to as Th 2 cytokines after their suspected cellular source, several other cells have been identified as significant sources including mast cells, epithelial cells, type 2 ILCs, and eosinophils. Airway mast cells are an important source of type 2 cytokines, proinflammatory cytokines, chemokines, and the IL-7–like cytokine TSLP.283,392-394 IL-13 from mast cells plays a crucial role in mast cell–induced local IgE synthesis by B cells,286,395 which in turn upregulate FcεRI expression on mast cells.286 Further, several mast cell products heavily influence epithelial cells. TNF-α, a proinflammatory cytokine produced by mast cells, in concert with IL-4 and IL-13, enhances the production of TARC, TSLP, and eotaxin from epithelial cells.385 And chemokines such as tryptase and chymase can upregulate RANTES and GM-CSF production from epithelial cells.385 Thus, there appears to be a crucial interplay between mast cells and epithelial cells in promoting and regulating the allergic inflammatory cascade.
In addition to the cytokines and chemokines listed in the previous paragraphs, nasal epithelial cells are an important source for IL-1, IL-6, IL-8, and TNF-α. Through these signals, epithelial cells play a crucial role in the migration and activation of eosinophils, basophils, and Th2 cells.396 In addition, epithelial cells release the cytokines IL-25, IL-33, and TSLP that orchestrate both the innate and adaptive Type 2 immune response. These same cytokines are also released by tissue damage, pathogen recognition, and allergen exposure. They can regulate Th2 cell function either directly or via innate lymphoid cells, which in turn produce IL-5, IL-9, IL-13, TSLP, IL-25, and IL-33, which are all increased in the nasal mucosa of AR patients, indicating a role of these cytokines in the pathophysiology of AR.397-400 In fact, levels of IL-33 in nasal secretions have been shown to correlate with total nasal symptom scores.400 Further, TSLP has been shown to activate dendritic cells, promote Th2 responses, and activate mast cells.401
Eosinophils are another cell type that appears to play a significant role in the pathophysiology of AR. They are a major source of the inflammatory cytokines macrophage migration inhibitory factor (MIF)402 and nerve growth factor (NGF).403 Eosinophils express 5-lipoxygenase, LTC4S, and CysLT1 and CysLT2 receptors, which play a role in the arachidonic acid pathway.404 IL-5 has a key role modulating eosinophil maturation, differentiation, and survival.405 Eosinophilic chemoattractants include eotaxin, MCP4, RANTES, and cysteinyl leukotrienes, among others.406-408 As discussed in earlier paragraphs within this section, mast cells and epithelial cells either directly produce or upregulate many of these same chemoattractants.
Finally, Th17 cells are a unique subpopulation of CD4+ T cells. They produce IL-17A, IL-17F, IL-22, TNF-α, and IL-21.409 They have been demonstrated to be in the nasal mucosa of AR patients and are therefore thought to play a role in allergic inflammation.409,410 Further, IL-17A has been shown to be upregulated in SAR patients 5 hours after nasal allergen challenge.411 Finally, increased numbers of IL-17A+ cells and IL-17A mRNA were demonstrated in the nasal mucosa of patients with dust mite allergy, indicating a possible role in AR.412
In summary, AR is a type 2–mediated disease, characterized by important regulatory cytokines such as IL-4, IL-5, and IL-13. Newer type 2 cytokines have been identified in AR, including IL-17 family cytokines. Finally, Type 2 ILCs and epithelial cell-derived cytokines such as TSLP, IL-25, and IL-33 play a crucial role in the regulation of the allergic inflammatory cascade.
IV.F. Histologic and epithelial changes
Normal nasal mucosa comprises pseudostratified columnar ciliated epithelium with goblet cells over a basement membrane. The nasal submucosa contains stromal elements including fibroblasts, blood vessels, seromucinous glands, sensory nerves, and leukocytes. Leukocytes present in the nasal mucosa include CD4+ and CD8+ T lymphocytes, B lymphocytes, eosinophils, neutrophils, basophils, mast cells, and macrophages. The combined functions of ciliated and secretory cells allow for nasociliary clearance, removing pathogens and allergens as a host defense mechanism. In addition to the physical barrier, nasal epithelium plays an important role in the innate and acquired immunologic defense against pathogens359,413,414 by: (1) expressing pattern recognition receptors that recognize pathogen-associated molecular patterns; (2) secreting a vast arsenal of host defense molecules, such as antimicrobial enzymes, opsonins, permeabilizing proteins, collectins, and binding proteins; and (3) producing inflammatory cytokines in response to antigenic stimuli.
Allergy mediates epithelial change in the nasal mucosa. Nasal epithelium is thicker in patients with AR after allergen challenge,415,416 but studies on epithelial thickness in AR without allergen challenge are conflicting.415-417 While epithelial remodeling is a key feature of CRS (epithelial hyperplasia, goblet cell hyperplasia, and squamous metaplasia)418-420 and asthma (epithelial desquamation, subepithelial fibrosis, and smooth muscle hypertrophy), remodeling in AR is less marked. In general, limited studies have found no significant increase in basement membrane thickness, subepithelial fibrosis, goblet cell hyperplasia, or blood vessel volume and surface density,415,421,422 though increased vascular permeability was noted.423 In contrast to epithelial remodeling, epithelial inflammatory response to allergens is a key feature of AR. Upon allergen exposure, there is significantly higher infiltration of inflammatory cells, and increased levels of cytokines (such as IL-4, IL-5, and IL-13) in the nasal epithelium of allergic compared to non-allergic patients.182 This inflammatory response translates into mucosal edema, autonomic neural stimulation, and increased mucosal secretions, which manifest as the hallmark symptoms of nasal obstruction, pruritus, sneezing, rhinorrhea, and smell loss in severe cases.
The epithelial barrier is noted to have specific functions in allergy. Penetration of allergens through this barrier may lead to allergen sensitization and local and/or systemic inflammatory response. In the nasal mucosa, this barrier is comprised of mucus and epithelial cells, which are linked by apical junctional complexes (tight junctions and adherens junctions).367 Mechanical or infective insults to the epithelium or defective epithelium leads to barrier breach and allergen penetration.367,424-426 Loss-of-function mutations and polymorphisms in genes coding for epithelial barrier markers such as filaggrin are associated with AR and eczema.427,428 Some allergens can induce junctional dysfunction, leading to penetration of the epithelial barrier by allergens.322,429 Proteolytic allergens directly disrupt the apical junctional complex via proteolysis, leading to barrier dysfunction.430 Detection of allergens by APCs, and the ensuing Th2 responses and cytokine release (such as IL 4, IL-13, and IFN-γ) induces further “leakiness” of the apical junctional complex via various mechanisms, allowing increased levels of allergen penetration.367 Evidence suggests that this barrier impairment may be reversed with corticosteroids. Fluticasone propionate has been found to increase expression of tight junction proteins zonula occludens 1 and occludin and a more intact nasal epithelial barrier.322 Corticosteroids have not, however, been shown to cause thinning of nasal epithelium.322,431
Allergy is now considered both a systemic and local epithelial condition.337 Evidence points to the epithelium being an active participant in the development and progress of allergy, rather than as a passive barrier.432 Birch pollen has been found to rapidly bind to Bet v 1–binding proteins in sensitized nasal epithelium, and is transported through a lipid raft and caveolar-dependent process before binding to mast cells in the lamina propria.433-435 Epithelial response to allergens differs from healthy individuals in that allergic patients do not mount as robust an epithelial defense response to allergens, leading to increased penetration of allergens.432
IV.G. Microbiome
The human microbiome comprises the complex community of microorganisms that resides in and interacts with the human body. The adult intestine is a haven to approximately 100 trillion microbes and it is thought that the microbiome accounts for roughly 90% of all the cells in the human body.436,437 The microbiomes of individuals vary, likely due to the fact that the growth, development, and composition of the microbiome are affected by intricate interactions between the environment, diet, and host-related factors.437
With the advent of culture-independent high-throughput bacterial DNA sequencing techniques, a detailed description of the composition and variety of the microbiome can be described among organs and individuals.438 The Human Microbiome Project began in 2007, and as a result, extensive data have emerged examining the associations of the microbiota of the respiratory tract, oral cavity, gut, skin, and genitourinary tract to the development of disease processes including allergy and asthma.437
Increasing literature in animals and humans has implicated changes in the microbiome with the development of allergic disease.439,440 Mechanistically, a disruption in gastrointestinal bacteria is thought to alter mucosal immunological tolerance.441 Several authors have found associations of reduced gut microbial diversity with development of allergic disease in school-aged children.442,443 For example, the development of allergic symptoms in children has been associated with overall lower microbial diversity, increased prevalence of Bacteroides and Bifidobacterium adolescentis, and lower counts of Akkermansia muciniphilia, Faecalibacterium prausnitzii, and Clostridium.444 In addition, Fujimura et al.445 recently noted that a lower abundance of Bifidobacterium, Akkermansia, and Faecalibacterium were associated with a higher risk of development of polysensitization by age 2 years and physician-diagnosed asthma by age 4 years. The authors concluded that neonatal intestinal microbial dysbiosis may foster CD4+ T-cell dysfunction associated with childhood allergic disease.445,446
The most comprehensive collection of evidence evaluating a potential association between the microbiome and the development of allergic disease is from a recent systematic review by Melli et al.444 Studies included in this systematic review compared intestinal microbiota of allergic patients with healthy controls. A total of 21 studies were noted to report an association between the intestinal microbiota and allergic disease when stool collection was performed prior to the outcome assessments. Only 4 of the analyzed studies had specific outcomes related to AR or sensitization. Penders et al.447 found that the presence of Clostridium difficile at 1 month of age was associated with an increased risk for allergic sensitization (odds ratio [OR] 1.54; 95% confidence interval [CI], 1.09 to 2.31) until the age of 2 years. Adlerberth et al.448 noted an increased ratio of gram-negative to gram-positive bacteria at 1 year of age to be associated with IgE levels greater than 100 kU/L at 1.5 years of age. Bisgaard et al.449 found lower bacterial diversity was associated to higher risk of allergic sensitization (p = 0.003) and AR (p = 0.007). Johansson et al.450 reported lower frequency of colonization with Lactobacilli and Bifidobacterium bifidum in allergic children.15 Ultimately, Melli et al.444 found that most of the studies linking the microbiome to the development of atopic disease were varied and difficult to interpret due to differing methodologies, samples sizes, and culture techniques.
There are some thoughts that the composition and/or dysbiosis of the microbiota (viruses, fungi, and/or bacteria) of other sites such as the nasopharynx, lungs, and sinonasal cavities may also play a role in the development of allergic disorders. However, these studies are in their infancy and little can be concluded at this time.451
A thorough understanding of the role of the microbiome and how it influences allergic disease has not been fully elucidated. Although some data suggest associations between allergic disease and the microbiota, based on the current evidence it is difficult to distinguish between protective microorganisms and those that increase risk for allergic disease.446 Future research should provide an enriched and diverse understanding of the human microbiome and the way it impacts AR.
V. Epidemiology of allergic rhinitis
V.A. Prevalence of allergic rhinitis in adults
A variety of population-based surveys have been used to estimate the prevalence of AR within the adult population. Prevalence estimates largely rely on self-reports of “hay fever” or “nasal allergies,” or of nasal symptoms “when you did not have a cold or the flu.” Questions on seasonality (to separate seasonal from perennial rhinitis) are sometimes asked, but there are few large-scale well-conducted population-based studies that have evaluated persistent (lasting more than 4 days/week for more than 4 consecutive weeks) vs intermittent symptoms. Because many surveys differ in terms of disease definitions, geography, and seasonality prevalence estimates drawn from surveys vary widely.
One of the earliest studies, conducted in Tecumseh, Michigan, in 1959–1960 included a physician assessment and suggested that the prevalence of hay fever (diagnosed as “upper respiratory symptoms believed to be allergic in origin and occurring predominantly in either spring, summer or autumn”) was about 11% in those aged over 20 years.452 About 20 years later, the National Health and Nutrition Examination Survey (NHANES) 1976–1980 was conducted among a geographically representative sample of the U.S. population. This survey gave broadly similar estimates for prevalence of AR, defined as “physician diagnosis of hay fever or frequent nasal and/or eye symptoms that varied by both season and pollen during the last 12 months, not counting colds or the flu.”453 A more recent report based on NHANES (2005-2006), presented population prevalence figures in which two-thirds were over the age of 20 years, and showed the lifetime prevalence of physician-diagnosed hay fever was 11.3%, with 6.6% having symptoms in the last 12 months. However, reliance on physician diagnosis of AR is likely to considerably under-estimate the actual prevalence of AR, since many patients self-diagnose and self-treat. Surveys involving patient self-reporting AR have shown that one-third of the population reported “sneezing and/or nasal symptoms in the absence of cold or a flu,” with about 24% reporting that this was seasonal in nature, and a further 10% reporting these symptoms occurred year-round (ie, perennial).454
In the early 1990s, the European Community Respiratory Health Survey (ECRHS), a multicenter population-based study of adults age 20 to 44 years in 23 countries (mainly Western Europe, but also Australia and New Zealand), used a self-completed questionnaire to estimate the prevalence of “hay fever or nasal allergies.” Prevalence varied between 10% and 40% across participating centers,455 with even more participants (12-65%) reporting that they experienced a runny or stuffy nose or started to sneeze on exposure to sources of allergen.456 If a positive SPT was included in the disease definition, the prevalence of AR fell by a variable amount (absolute fall in prevalence between 4% and 16% across all centers). In the Swiss Study of Air Pollution and Lung Disease in Adults (SAPALDIA), conducted around the same time as the ECRHS, the prevalence of self-reported “nasal allergies including hay fever” in adults aged 18 to 60 years was 17.9%, and the prevalence of current symptoms (“hay fever this year or last year”) was 14.2%.457 Prevalence estimates were lower if a positive SPT was included (11.2% for current hay fever with at least 1 positive SPT and 9.1% for current hay fever with positive SPT to 1 of grass, birch, or Parietaria). More recently, the Global Allergy and Asthma Network of Excellence (GA2LEN) study suggested the prevalence of “nasal allergies and hay fever” varied between 22% and 41% in adults age 18 to 75 years living in the 12 participating European nations.458
Population-based studies have shown increases in AR prevalence in the adult population in recent decades. For example, in Renfrew Paisley, UK, the prevalence of hay fever was higher in adults and children in 1996 than in their mothers and fathers at an equivalent age in 1972.459 Hay fever prevalence doubled between 1981 and 1990 in Busselton, Australia,460 increased in Italy from 1991 to 2010,461 and increased in 8 of 11 cities in China surveyed in 2005 and again in 2011.462 In Uppsala, Umea, and Goteborg, in Sweden, “hay fever and nasal allergies” increased from 21% to 31% between 1990 and 2008,463 although recent reports from Stockholm suggest there may be a leveling off in the increase in nasal allergies over more recent years.464
From these data, the lifetime prevalence of AR in the United States can be estimated between 11% (physician-diagnosed) and approximately 33% (self-reported). In Europe, prevalence of AR in adults likely ranges between 10% and 41%, depending on the specific country.
V.B. Incidence and prevalence of allergic rhinitis in children
There are relatively few studies on the incidence of AR in children. There is evidence that AR may start as early as during the first year of life. In the Cincinnati Childhood Allergen and Air Pollution Study (CCAAPS), 9% of the 12-month-old children with a parental history of respiratory allergy fulfilled the criteria of AR.465 In the Pollution and Asthma Risk: an Infant Study (PARIS) birth cohort, 9.1% of the 18-month-old children had AR-like symptoms with a strong association with atopy and sensitization to inhalant allergens. Of these, 23.7% had rhinoconjunctivitis.466 In a study of 29,662 children from the United States that used health care records to follow participants, the incidence of physician-diagnosed AR during the first year of life was 1%. From 1 to 5 years of age, the annual incidence was between 3.6% and 4.5%, with the highest incidence between 2 and 3 years of age.467 This is broadly in line with estimates of a SAR incidence of 3% to 4% per year from 3 to 7 years of age reported in a birth cohort of 1314 German children.468
In longitudinal studies, AR often occurs for the first time in childhood and increases in prevalence with increasing age.467-471 Most children with symptoms of AR early in life have persistent symptoms for several years.469-471 The International Study of Asthma and Allergies in Childhood (ISAAC) estimated the prevalence of allergic diseases in 2 different age groups, 6 to 7 years and 13 to 14 years, through a multicenter global survey. Two cross-sectional surveys were performed approximately 7 years apart (range, 5 to 10 years). Overall, an increase in rhinoconjunctivitis prevalence was observed between the 2 surveys.10 However, there were geographical differences in both baseline prevalence and in the increases observed; therefore, it is difficult to determine whether the observed differences represented a true increase in prevalence over time. The proportion of children with symptoms of rhinoconjunctivitis was higher in the older age group. Data from the second survey (ISAAC Phase Three 1999–2004) state that the worldwide prevalence of current rhinoconjunctivitis in the 6-year to 7-year-old age group was 8.3% (range between countries, 1.8% to 24.2%) and in the 13-year to 14-year age group was 15.1% (range, 4.5% to 45.1%).472 In a more recent meta-analysis of all studies performed according to the ISAAC-protocol (1,430,329 children aged 0 to 18 years), the overall prevalence of AR was 12.66%.473
Rhinoconjunctivitis has been reported to be slightly more common among boys than girls in the 6-year to 7-year-old age group, with the opposite tendency seen in the 13-year to 14-year-old age group.474 However, gender differences were not seen in all countries in the survey. Other studies show a greater prevalence of AR among boys of all ages. For example, in the Isle of Wight (UK) birth cohort of 1456 children, the prevalence of rhinitis among boys as compared to girls was higher across all age groups (4 years 4.7% vs 2.1%, 10 years 14.9% vs 11.7%, 18 years 31.0% vs 24.0%).469
V.C. Geographic variation of allergic rhinitis
The prevalence of AR shows marked geographic variation. Many factors likely contribute to this disparity and not all are completely understood. The central difficulty in meaningfully comparing AR prevalence rates between locations is the difference in methods used to recruit participants to studies and differences in assessing the presence of disease. For example, Bauchau and Durham9 diagnosed Belgian patients via serological IgE testing after a positive telephone screen and reported that Belgium had an AR prevalence of 28.5% (the highest of the European countries evaluated). In contrast, Bousquet et al.456 skin-tested a random sample of Belgian subjects and reported a positive rate in Belgium of 16.4% (one of the lowest of 15 countries examined).
There have been major international efforts to compare variations in the national prevalence of AR using standardized methods (ie, ECRHS and ISAAC). These studies show marked geographic variation of “hay fever or nasal allergies” (adults) or “a problem with sneezing, or a runny, or a blocked nose when you DID NOT have a cold or the flu that was accompanied by itchy-watery eyes?” (children). A higher prevalence of these responses is seen in people living in “English-speaking” countries (eg, UK, Australia, New Zealand), a lower prevalence in Eastern Europe than in Western Europe, and a diagnosis of AR is more frequently seen in countries with higher asthma rates and sensitization to seasonal allergens.455,475 Because these studies have evaluated national rates based on only one or a few centers within each country, substantial intracountry variation may have been overlooked.
In understanding the effects of geographic location, differentiating between seasonal and perennial AR is an important consideration not examined in the ECRHS or ISAAC studies. Smaller studies over more limited geographic regions that examined PAR suggest increased sensitivity rates in urban settings and colder climates.476-479 Several hypotheses have been put forward for these observed differences. Li et al.477 theorized that urban dwellers participate in more indoor activities compared to their rural counterparts, amplifying their exposure to HDM, and possibly leading to increased sensitization to these perennial allergens. Additionally, some reports suggest that exposure to urban pollutants may be associated with increased risk for developing AR in children.476 Latitude may also play a role with regard to PAR. For example, the prevalence of persistent AR was found to be higher in both Northern Europe and Northern China compared to their southern counterparts.9,477
Latitude may also be an important determinant of SAR. Allergenic plant species may have a propensity for growing in certain geographic locations, and pollen concentrations of various species depend on the climate conditions of the area. Colder climates present at northern latitudes tend toward shorter growing seasons, and many allergenic species do not thrive in extreme northern climates. For instance, grass pollen, which is found across Europe, causes wide variations in atopic sensitizations across regions with different climates.480 Additionally, this increased environmental exposure has been shown to affect development of AR and patient symptoms of atopic nasal diseases.481,482
Overall, improved knowledge of the prevalence and seasonal variations in AR based on geographic location is important in that it allows patients to anticipate and better manage their symptoms through avoidance techniques and preemptive use of pharmacologic therapies.480,482 Currently, prevalence data do not fully address the different phenotypes of AR and further study is needed to expand epidemiologic understanding of this disease.
VI. Risk factors for allergic rhinitis
VI.A. Genetics
AR is well-known to run in families, and 1 of the strongest risk factors is the presence of disease in first-degree family members.483 Studies of twins support the genetic underpinnings of AR with a higher concordance rates for AR in monozygotic twins compared to dizygotic twins.484,485 The estimated heritability of AR has been suggested to be as high as 70% to 80%. Like many complex diseases, no single gene or polymorphism accounts for the hereditary effect on AR. Instead, many genes and several variants, each with small effects, are believed to contribute to disease initiation, persistence, and severity. In this section, the current literature on the genetics of AR is reviewed, including candidate gene studies and recent large-scale genome-wide association studies (GWASs). In addition, gene-environment interaction effects and epigenetics studies are briefly covered.
Single-nucleotide polymorphisms associated with AR
GWASs.
GWASs with an unbiased approach that include hundreds of thousands of common gene variants, or single-nucleotide polymorphisms (SNPs), have successfully identified important variants for complex diseases over the past decade. Five GWASs on AR (or hay fever) have been published as of September 2016, as summarized in Table VI.A. SNPs in leucine-rich repeat-containing protein 32 (LRRC32) have been strongly associated with AR in 3 of the GWASs,486-488 and with asthma,487,489 eczema,488,490 and other allergy-related comorbidities.486,489,491 At the protein level, LRRC32 is known to regulate T-cell proliferation, cytokine secretion, and TGF-β activation.492 These associations suggest shared genetic mechanisms for AR and other allergy-related diseases, evidence further supported by the large-scale GWAS on self-reported cat, HDM, and pollen allergies (as well as AR), which revealed 16 shared susceptibility loci with strong association (p < 5 × 10−8; TLR-locus top hit).487 In an accompanying GWAS on allergic sensitization, there was strong overlap between top hits for sensitization and self-reported allergies.487,493 In the GWAS by Ferreira et al.,489 11 variants were associated with the combined asthma phenotype and hay fever below the genome-wide significance level (HLA-DQB1 top hit). TLRs play a crucial role in immune regulation and SNPs in different TLRs have been associated with AR in both GWASs (TLR1, TLR6, TLR10)486,487 and candidate gene studies (TLR8), as discussed in the next paragraph.494 In addition to shared genetic effects between different allergy-related diseases, a significant overlap between susceptibility loci for allergy and autoimmune diseases has been observed.495
TABLE VI.A.
Key findings from GWASs on allergic rhinitis or hay fever
| Author (year) |
Study design | Sample size | Ethnicity | Top SNPs for AR |
p | Nearby gene(s) |
Reported association with other allergic diseases |
Protein function | LOE |
|---|---|---|---|---|---|---|---|---|---|
| Bunyavanich et al.512 (2014) | Meta-analysis of 7 cohorts | 2712 AR cases; 2921 controls | EA, L, AA | rs17133587 | 4.5E–09 (L) | AKR1E2 | No | NAD(P)H-dependent oxidoreduction | 2a |
| rs6583203 | 1.4E–08 (L) | DLG1 | No | Scaffolding protein involved in cell metabolism | |||||
| rs7780001 | 2.0E–08 (all groups) | FERD3L | No | Transcription factor | |||||
| Hinds et al.487 (2013) | Private company data (23andMe) | 46,646 total | >97% EA | rs1438673 | 3.7E–19 | WDR36 | Asthma487, 513; eczema488; atopy487 | Cellular processes and T-cell activation | 2a |
| rs2101521 | 6.0E–17 | TLR1–TLR6; TLR10 | Asthma, eczema, atopy487 | Pathogen recognition and activation of innate immunity | |||||
| rs10189629 | 9.9E–15 | IL1RL2; IL1RL1 | Asthma487, 514; eczema487; atopy487 | Proinflammatory effects, T-helper cell function | |||||
| Andiappan et al.515 (2011) | Nested case-control with replication | 1132 AR cases; 997 controls | Chinese | rs811930 | 7.3E–05 | MRPL4 | No | Protein synthesis within the mitochondrion | 2a |
| rs505101 | 1.3E–04 | BCAP (PIK3AP1) | Atopy515 | Protein tyrosine kinase | |||||
| Ramasamy et al.488 (2011) | Meta-analysis of 4 cohorts | 3933 AR cases; 8965 controls | EA | rs2155219 | 3.8E–08 | LRRC32 or C11orf30, SLCA25A46 | Co-morbidity: asthma-atopy489; asthma-eczema491; asthma-hay fever486 Eczema,487, 490 asthma, atopy487 |
LRRC32: T-cell regulation, TGF-β activity. C11orf30: regulation of viral immunity and interferon pathways | 2a |
| rs17513503 | 7.4E–07 | TMEM232 | No | Transmembrane protein | |||||
| rs1044573 | 9.7E–07 | ENTPD6 | No | Catabolism of extracellular nucleotides | |||||
| Ferreira et al.486 (2010) | Meta-analysis of 4 cohorts/datasets | 16,513 hay fever cases; 17,256 controls | EA, L, AA | rs4833095 | 4E–12 | TLR1 | Asthma, eczema, atopy487 | Pathogen recognition and activation of innate immunity | 2a |
| rs2155219 | 7E–10 | LRRC32 or C11orf30 | Co-morbidity: asthma-atopy489; asthma-eczema491; asthma-hay fever486 Eczema,487, 490 asthma, atopy487 |
See above | |||||
| rs10197862 | 2E–09 | IL1RL1 | Asthma487, 514; eczema487; atopy487 | Proinflammatory effects, T-helper cell function |
AA = African American; AR = allergic rhinitis; EA = European ancestry; GWAS = genome-wide association study; L = Latino; LOE = level of evidence; NADPH = nicotinamide adenine dinucleotide phosphate.
Candidate gene studies.
The candidate gene approach for selecting disease-relevant genes is based on previous associations reported from GWAS or biological features which could be relevant for disease risk. Studies on AR using this approach have found several well-replicated genes as summarized previously.496-498 Notably, SNPs in genes involved in antigen presentation (for example HLA-DQA1), pathogen recognition (TLR2, TLR7, TLR8), IL signaling and proinflammation (IL13, IL18, and TSLP) are considered important susceptibility variants for AR.496-502 Recently, functional evidence in blood immune cells for genetic variants in brain-derived neurotrophic factor (BDNF), a secretory proinflammatory protein implicated in AR pathogenesis, was reported.503 However, many of the candidate genes reported in the literature have not been well-replicated across studies and populations.427,504 This could be due to inadequate statistical power related to small sample sizes, inconsistent phenotype definition, or lack of true disease association. Additionally, rare variant studies focusing on candidate genes have not been particularly successful.494 The candidate gene approach is particularly necessary for hypothesis-driven analyses and functional genetic analyses, for example in populations with specific environmental exposures or with mixed ethnic backgrounds.
Gene-environment interactions and epigenetic effects
Epigenetic mechanisms, defined as changes in phenotype or gene expression caused by mechanisms (eg, methylation) other than changes in the underlying DNA sequence, have been proposed to constitute a link between genetic and environmental factors. Recent studies show that DNA methylation in children is very strongly influenced by well-known risk factors for allergic diseases such as maternal smoking during pregnancy505 and air pollution exposure.506 Currently, however, it is not known if these methylation changes are causally related to the development of AR and asthma, or if these “biomarkers” are solely markers of exposure. Several studies have convincingly linked methylation profiles to AR507-509 and IgE-related outcomes,510,511 but large-scale studies have yet to be completed.
In summary, a family history of AR remains a risk factor for disease development, and strong associations have been identified with genes involved in T-cell activation (eg, LRRC32) and innate immunity (eg, TLRs). Shared genetic mechanisms for AR and other allergy-related diseases have been very clearly identified in recent large-scale studies. There is, however, a need to functionally characterize variants in these candidate genes to understand mechanisms underlying the pathogenesis of AR. With increasing evidence for the role of epigenetics in AR, future research should also focus on investigating epigenetic mechanisms, thereby providing a functional explanation for the link between environmental exposures, genetic variants, and disease development.
Aggregate Grade of Evidence: C (Level 2a: 5 GWASs. Candidate gene studies not assessed regarding grade of evidence).
VI.B. Inhalant allergens (in utero and early childhood exposure)
AR is characterized by a loss of immunological and clinical tolerance toward a specific allergen. This involves production of sIgE which initiates allergic inflammation following allergen exposure. Therefore, sIgE is a hallmark of allergy and its production defines sensitization. Sensitization is a complex phenomenon, regulated by genetic and environmental factors, requiring a primitive exposure to a specific allergen. If a subject is never exposed to an allergen, sensitization to that allergen cannot occur. On the other hand, it is fundamental to distinguish between sensitization and allergy. Allergy, which involves the development of symptoms after the sensitizing exposure, is different from mere sensitization. Without sensitization allergy cannot exist, but not vice versa. In this section, the in utero and early childhood exposure to inhalant allergens, including mites, pollens, animal dander, and fungal allergens, will be evaluated as risk factor the development of AR.
Mites
There are 6 studies on the topic of early mite exposure and the development of AR (Table VI.B-1). Most of the studies failed to demonstrate an association between early exposure to mites and the development of AR.468,516-519 Marinho et al.520 reported that early exposure to HDM is not a protective factor for current AR, and Kim et al.521 proposed exposure to spider mites as a risk factor for AR. Interestingly, pets may be a relevant source of mites, as their fur is often settled by mites; this association may confound AR evaluation and treatment. Ultimately, the studies on early mite exposure and the development of AR are conflicting and additional research is needed.
TABLE VI.B-1.
Evidence for the effects of mite allergen exposure (in utero and early childhood exposure) on the development of allergic rhinitis*
| Study | Year | LOE | Study design | Study groups | Type of exposure | Conclusion |
|---|---|---|---|---|---|---|
| Schoos et al.518 | 2016 | 2b | Prospective birth cohort | 399 children (7–13 years old) from COPSAC study | Der p 1 in dust sample at 1 year | No association with AR at 7 years (OR 0.9; 95% CI, 0.7–1.1). |
| Der f 1 in dust sample at 1 year | No association with AR at 7 years (OR 0.9; 95% CI, 0.7–1.1). | |||||
| Illi et al.517 | 2014 | 2b | Prospective birth cohort | 513 children (5 years old) from PAULA study | Mite allergen exposure at 3 months (measured as allergen levels in the living room floor and in the mother’s or child’s mattress) | No association with current AR (OR not reported). |
| Marinho et al.520 | 2007 | 2b | Whole-population birth cohort | 815 children (5 years old) from MAAS study | Der p exposure at 0–5 years old (measured as allergen levels recovered from child’s bed, child’s bedroom floor, parental bed, and lounge floor) | Protective factor for current rhinoconjunctivitis (OR 0.8; 95% CI, 0.7–0.98). This finding failed to reach significance in multivariate analysis. |
| Corver et al.516 | 2006 | 2b | Prospective birth cohort | 416 children (4 years old) from PIAMA study | Der p 1 and Der f 1 exposure on the children’s mattresses | No association with rhinitis in 4th year (OR 0.9; 95% CI, 0.6–1.3). |
| Kulig et al.468 | 2000 | 2b | Prospective birth cohort | 587 children (7 years old) from MAAS study | Mite (Der p 1 + Der f 1) exposure at 0–18 months (measured as allergen levels obtained from carpet dust samples) | No association with SAR (OR not reported). |
| Kim et al.521 | 2002 | 3b | Cross-sectional | 16,624 children (7–18 years old) | History of spider mite exposure | Risk factor for rhinitis (OR 1.3; 95% CI, 1.2–1.5). |
ORs are unadjusted and reported with 95% CIs.
AR = allergic rhinitis; CI = confidence interval; COPSAC = Copenhagen Prospective Study on Asthma in Childhood; Der p = Dermatophagoides pteronyssinus; Der f = Dermatophagoides farinae; LOE = level of evidence; MAAS = Manchester Asthma and Allergy Study; OR = odds ratio; PAULA = Perinatal Asthma and Environment Long-term Allergy; PIAMA = Prevention and Incidence of Asthma and Mite Allergy; SAR = seasonal allergic rhinitis.
Aggregate Grade of Evidence: C (Level 2b: 5 studies; Level 3b: 1 study; Table VI.B-1).
Pollens
There are only 2 studies that addressed the impact of early pollen exposure on AR (Table VI.B-2). Kihlström et al.519 reported no association to allergic rhinoconjunctivitis whereas Erbas et al.481 showed that pollen exposure during infancy is a risk factor for hay fever.
TABLE VI.B-2.
Evidence for the effects of pollen allergen exposure (in utero and early childhood exposure) on the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Type of exposure | Conclusionb |
|---|---|---|---|---|---|---|
| Erbas et al.481 | 2013 | 2b | Prospective birth cohort | 620 children (6–7 years old) from MACS RCT (with at least 1 first-degree family member with a history of eczema, asthma, hay fever, severe food allergy) | Pollen exposurea during infancy (at 3–6 months) | Risk factor for hay fever (OR 1.1; 95% CI, 1.01–1.3) |
| Kihlström et al.519 | 2002 | 3b | Cross-sectional | 583 children with atopic heredity (4–5 years old) | High-dose exposure to birch pollen at 0–3 months | No association with allergic rhinoconjunctivitis (OR 1.0; 95% CI, 0.6–1.8) |
| High-dose exposure to birch pollen at 1 year | No association with allergic rhinoconjunctivitis (OR 1.3; 95% CI, 0.8–2.2) |
Defined as birth “inside” or “outside” the pollen season and by measuring daily 24-hour average pollen concentrations for grass and others (which include trees, weeds, and herbs).
ORs are adjusted and reported with 95% CIs in parentheses.
CI = confidence interval; LOE = level of evidence; MACS = Melbourne Atopy Cohort Study; OR = odds ratio; RCT = randomized controlled trial.
Aggregate Grade of Evidence: C (Level 2b: 1 study; Level 3b: 1 study; Table VI.B-2).
Animal dander
Numerous studies have evaluated the association between early exposure to animal dander and subsequent development of AR, with conflicting results (Table VI.B-3). Studies are divided according to the findings: positive studies (reporting a protective effect on AR development522-535), negative studies, (showing that early exposure to pets represents a risk factor for AR523,536-542), and neutral studies (reporting that early exposure to animal dander is not associated with AR468,517,518,520,524,528,530,532,536,538,539,543-554). Additional factors should be considered: pet age, gender, and species; number of household pets; home characteristics; atopic predisposition of the pet owners; and others. Considering these complex variables, debate regarding the influence of early pet exposure on developing allergic disease remains unresolved. Thus, evidence-based guidelines regarding having pets at home cannot be established. (See section VI.G.2. Risk factors for allergic rhinitis – Protective factors against allergic rhinitis – Childhood exposure to pets for additional information on this topic.)
TABLE VI.B-3.
Evidence for the effects of pet dander exposure (in utero and early childhood exposure) on the development of allergic rhinitis*
| Study | Year | LOE | Study design | Study groups | Type of exposure | Conclusiona |
|---|---|---|---|---|---|---|
| Early exposure to animal danders as a protective factor for AR (Level 2b studies listed. Level 3b studies referenced.522, 523, 525-528, 533, 535, 1530) | ||||||
| Lodge et al.534 | 2012 | 2b | Prospective birth cohort | 620 children (12 years old) with a family history of allergic diseases | Exposure to cats or dogs at birth | Borderline protective factor for hay fever (OR 0.7; 95% CI, 0.5–1.02). Stronger protective effects if children of non-sensitized fathers (OR cats alone 0.3; 95% CI, 0.2–0.8); (OR cats or dogs 0.4; 95% CI, 0.2–0.8). |
| Alm et al.531 | 2011 | 2b | Prospective birth cohort | 4465 children (4.5 years old); 246 children with current AR | Exposure to cats at 1 year | Protective factor for AR (unadjusted OR 0.5; 95% CI, 0.4–0.8, not significant in multivariate analysis). |
| Lampi et al.532 | 2011 | 2b | Prospective birth cohort | 5509 adults (31 years old) | Exposure to farm animals (cows, pigs, sheep, poultry, minks) | Borderline protective factor for AR ever (OR 0.9; 95% CI, 0.7–1.03). |
| Exposure to cats or dogs at age less than 7 years old | Borderline protective factor for AR (OR cat 0.8; 95% CI, 0.7–0.96); (OR dog 0.9; 95% CI, 0.8–1.01). | |||||
| Perzanowski et al.529 | 2008 | 2b | Birth cohort | 257 children (5 years old) from African American or Dominican mothers | Cat ownership (up to age of health outcomes) | Protective factor for AR at 5 years old (OR 0.4; 95% CI, 0.2–0.9). |
| Nafstad et al.524 | 2001 | 2b | Birth cohort | 2531 children (4 years old) | Exposure to cats at birth | Borderline protective factor for AR (OR 0.5; 95% CI, 0.2–1.4). |
| Exposure to dogs at birth | Borderline protective factor for AR to grass/pollen (OR 0.8; 95% CI, 0.4–1.6). | |||||
| Early exposure to animal dander as a risk factor for AR (All studies Level 3b.523, 530, 536-542) | ||||||
| Early exposure to animal dander is not associated with AR (Level 2b studies listed. Level 3b studies referenced.528, 530, 536, 538, 539, 543-546, 548, 551, 553, 554) | ||||||
| Schoos et al.518 | 2016 | 2b | Prospective birth cohort | 399 children (7–13 years old) from COPSAC study | Prenatal (at 3rd trimester of pregnancy) and perinatal (at 1 year) cat exposure | No association with AR at 7 years old (OR prenatal 0.4; 95% CI, 0.06–3.6); (OR perinatal 0.9; 95% CI, 0.2–3.9). |
| Prenatal (at 3rd trimester of pregnancy) and perinatal (at 1 year) dog exposure | No association with AR (OR prenatal, AR at 13 years old 0.9; 95% CI, 0.2–4.3); (OR perinatal, AR at 7 years old 0.9; 95% CI, 0.1–7.4). | |||||
| Illi et al.517 | 2014 | 2b | Prospective birth cohort | 513 children (5 years old) from PAULA study | Cat allergen exposure at 3 months (measured as allergen levels in the living room floor and in the mother’s or child’s mattress) | No association with current AR (OR not reported as value, only in figure). |
| Kellberger et al.550 | 2012 | 2b | Prospective population-based cohort | 2,810 adolescents (15–18 years old) | Pet (cat, dog, hamster, guinea pig, rabbit) ownership at 0–1 years old | No association with incidence/persistence of physician-diagnosed AR. |
| Lodrup-Carlsen et al.552 | 2012 | 2b | Prospective birth cohort | 22,840 children (6–10 years old) | Pet (cat, dog, bird, rodent) ownership at 0–2 years old | No association with AR (OR cat only 1.02; 95% CI, 0.8–1.3); (OR dog only 0.8; 95% CI, 0.6–1.1); (OR cat and dog 0.8; 95% CI, 0.4–1.4); (OR bird only 1.3; 95% CI, 0.9–1.8); (OR rodent only 0.8; 95% CI, 0.5–1.5). |
| Lampi et al.532 | 2011 | 2b | Prospective birth cohort | 5509 adults (31 years old) | Maternal work with farm animals (cows, pigs, sheep, poultry, minks) during pregnancy | No association with AR (OR 0.9; 95% CI, 0.7–1.2). |
| Sandini et al.549 | 2011 | 2b | Prospective birth cohort, RCT | 1223 children (5 years old) born to allergic families, who participated in a RCT | Dog/cat at home at 0–2 years old or 0–5 years old | No association with AR (OR 0.98; 95% CI, 0.5–1.8). |
| Chen et al.547 | 2007 | 2b | Prospective birth cohort | 2166 children (4–6 years old) (hay fever: 66/1599) from LISA study | Cat allergen exposure at 3 months (measured as Fel d 1 levels from children’s or parents’ mattress) | No association with doctor-diagnosed hay fever (OR parents’ mattress 0.9; 95% CI, 0.5–1.5); (OR children’s mattress 0.7; 95% CI, 0.4–1.1). |
| Marinho et al.520 | 2007 | 2b | Whole-population birth cohort | 815 children (5 years old) from MAAS study | Cat and dog exposure at 0–5 years old (measured as allergen levels recovered from child’s bed, child’s bedroom floor, parental bed and lounge floor) | No association with current rhinoconjunctivitis (unadjusted OR cat 1.02; 95% CI, 0.9–1.1); (unadjusted OR dog 1.03; 95% CI, 0.9–1.2). |
| Nafstad et al.524 | 2001 | 2b | Birth cohort | 2531 children (4 years old) | Cat keeping at birth | No association with AR (OR 0.5; 95% CI, 0.2–1.4). |
| Dog keeping at birth | No association with AR to grass/pollen (OR 0.8; 95% CI, 0.4–1.6). | |||||
| Kulig et al.468 | 2000 | 2b | Prospective birth cohort | 587 children (7 years old) from MAAS study | Cat (Fel d 1) exposure at 0–18 months (measured as allergen levels obtained from carpet dust samples) | No association with SAR (OR not reported). |
| Pets in household (at 18 months) | No association with SAR (OR not reported). | |||||
Level 2b studies are listed in the table. Level 3b studies are referenced.
All ORs are adjusted unless differently specified and are reported with 95% CIs in parentheses.
AR = allergic rhinitis; CI = confidence interval; COPSAC = Copenhagen Prospective Study on Asthma in Childhood; Fel d = major cat allergen; LISA = Lifestyle-Immune-System-Allergy; LOE = level of evidence; MAAS; Manchester Asthma and Allergy Study; OR = odds ratio; PAULA = Perinatal Asthma and Environment Long-term Allergy; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis.
Aggregate Grade of Evidence: C (Level 2b: 15 studies; Level 3b: 24 studies; Table VI.B-3).
Fungal allergens
Several studies have explored the role of early exposure to fungal allergens as a predisposing factor for AR (Table VI.B-4). Most studies demonstrated evidence that early exposure to fungal allergens represents a risk factor for AR development.527,538,551,553,555-560 However, 3 studies demonstrated that early exposure to fungal allergens is not associated with AR.465,542,557 Home moisture level, which is closely and positively associated with the presence of fungal allergens in the home, may be a confounding factor in interpreting the evidence on fungal exposure and AR. Ambient humidity may an intrinsic risk factor, but high moisture is also associated with increased level of mites, as mites grow in presence of elevated moisture. Moisture can be easily assessed both by direct measurement with a hygrometer and indirectly by observing the presence of mold spots on the walls.
TABLE VI.B-4.
Evidence for the effects of fungal allergens exposure (in utero and early childhood exposure) on the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Type of exposure | Conclusiona |
|---|---|---|---|---|---|---|
| Early exposure to fungal allergens as a risk factor for AR | ||||||
| Thacher et al.559 | 2016 | 2b | Birth cohort | 3798 adolescents (16 years old) from BAMSE study; 785 with AR | Visible mold at 2 months | Risk factor for AR (OR 1.3; 95% CI, 1.04–1.6) |
| Stark et al.555 | 2005 | 2b | Birth cohort | 405 children of asthmatic/allergic parents from metropolitan Boston, Massachusetts (younger than 5 years old) | Exposure to high levels of dust-borne Aspergillus at 0–3 months | Risk factor for doctor-diagnosed AR at 0–5 years (HR 3.3; 95% CI, 1.5–7.1) |
| Exposure to high levels of dust-borne Aureobasidium at 0–3 months | Risk factor for doctor-diagnosed AR at 0–5 years (HR 3.0; 95% CI, 1.3–6.9) | |||||
| Exposure to high levels of dust-borne yeasts at 0–3 months | Risk factor for doctor-diagnosed AR at 0–5 years (HR 2.7; 95% CI, 1.3–5.7) | |||||
| Deng et al.557 | 2016 | 3b | Cross-sectional | 2598 children (3–6 years old) attending kindergarten | Prenatal (whole pregnancy) or postnatal (from birth to current) exposure to indoor mold/dampness | Risk factors for rhinitis-like current symptoms: prenatal (OR 1.5; 95% CI, 1.2–1.9); postnatal (OR 2.1; 95% CI, 1.6–2.8) |
| Lin et al.558 | 2016 | 3b | Cross-sectional | 4246 children (3–8 years old) from 18 day cares | Visible indoor mold (weekly/sometimes vs never) at 0–2 years | Risk factor for new onset of rhinitis symptoms (OR 1.3; 95% CI, 1.01–1.6). Exposure was a significant risk factor for the remission of rhinitis (OR 0.6; 95% CI, 0.3–0.9) |
| Lam et al.553 | 2014 | 3b | Cross-sectional | 508 preschool children (4–6 years old) | Exposure to moisture/mold <1 year | Risk factor for rhinoconjunctivitis (OR 2.1; 95% CI, 1.2–3.8) |
| Kim et al.551 | 2012 | 3b | Cross-sectional | 4554 schoolchildren (mean age 9.50 years old, SD 1.73) | Mold exposure in house during infancy | Risk factor for current AR (OR 1.8; 95% CI, 1.4–2.4) |
| Lombardi et al.538 | 2010 | 3b | Cross-sectional | 20,016 children (median age 7 years old) from SIDRIA-2 Study | Mold exposure at 0–1 year | Risk factor for current rhinoconjunctivitis (unadjusted OR 1.4; 95% CI, 1.2–1.6) |
| Ibargoyen-Roteta et al.527 | 2007 | 3b | Cross-sectional | 3360 schoolchildren (5–8 years old) | Having mold on walls at 0–1 year | Risk factor for allergic rhinoconjunctivitis (OR 2.5; 95% CI, 1.5-4.0) |
| Kuyucu et al.556 | 2006 | 3b | Cross-sectional | 2774 children (9–11 years old) | Dampness/mold at 1 year | Risk factor for AR (OR 1.7; 95% CI, 1.3–2.3) |
| Bornehag et al.560 | 2005 | 3b | Cross-sectional | 10,851 children (1–6 years old) | Visible mold or damp spots in the child’s or parent’s bedroom at 1–6 years | Risk factor for rhinitis (OR 2.7; 95% CI, 1.4–5.4) |
| Early exposure to fungal allergens is not associated with AR | ||||||
| Biagini et al.465 | 2006 | 2b | Cross-sectional | 585 infants (1 year) born to families with at least 1 parent with positive SPT | High mold exposure (mold in 1 room (≥0.2 m2 or a combined area of visible mold and water damage on the same surface ≥0.2 m2) during early infancy (average 7.5 months) | No association with AR (OR 1.2; 95% CI, 0.6–2.5) |
| Low mold exposure (mold in one room (<0.2 m2 or a combined area of visible mold and water damage on the same surface <0.2 m2) during early infancy (average 7.5 months) | No association with AR (OR 3.2; 95% CI, 0.7–14.8) | |||||
| Deng et al.557 | 2016 | 3b | Cross-sectional | 2598 children (3–6 years old) attending kindergarten | Prenatal (during the whole pregnancy) or postnatal (from birth to the current) exposure to indoor mold or dampness | No association with AR: prenatal (OR 0.7; 95% CI, 0.4–1.1), postnasal (OR 1.0; 95% CI, 0.6–1.7) |
| Yang et al.542 | 2014 | 3b | Cross-sectional | 7389 schoolchildren (mean age 13.9 years, SD 0.9) | Mold exposure during infancy | No association with AR (OR 0.99; 95% CI, 0.8–1.3) |
ORs are adjusted unless otherwise specified.
AR = allergic rhinitis; BAMSE = Barn/Child Allergy Milieu Stockholm Epidemiology; CI = confidence interval; HR = hazard ratio; LOE = level of evidence; OR = odds ratio; SD = standard deviation; SIDRIA-2 = Studi Italiani sui Disturbi Respiratori del l’Infanzia el Ambiente; SPT = skin prick test.
Aggregate Grade of Evidence: C (Level 2b: 3 studies; Level 3b: 10 studies; Table VI.B-4).
In summary, the clinical relevance of early inhalant allergen exposure to AR development is still debated. Despite several indepth reviews and a growing body of literature,561-563 no definitive and consensus may be drawn regarding risk-benefit of early inhalant allergen exposure, and further research is welcomed to address the unmet needs on this issue.
VI.C. Food allergens (in utero and early childhood exposure)
In some studies, early sensitization to food allergens has been linked to the development of AR in childhood.468,564,565 A meta-analyses by Alduraywish et al.564 demonstrated that food sensitization in the first 2 years of life was associated with an increased risk of AR during childhood (OR = 3.0; 95% CI, 2.1 to 4.2) (Table VI.C). The relationship between sensitization to food allergens and the subsequent development of AR during childhood has been investigated in both population-based and high-risk cohorts.468,565-568 While there is a statistically significant correlation in the high-risk cohort,567 there are mixed results in the population-based studies.566,568,569 These findings prompted prospective investigation of the effects of allergen avoidance in utero and during early childhood.
TABLE VI.C.
Evidence for the effects of food allergen exposure (in utero and early childhood exposure) on the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Zeiger et al.573 | 1995 | 1b | RCT |
|
Food allergy, atopic dermatitis, AR, asthma, any atopic disease, lung function, food or aeroallergen sensitization, serum IgE level, presence of nasal eosinophils or basophilic cells at age 7 years. | No significant difference between treatment groups, though children with food allergy by 4 years had a higher 7-year prevalence of AR and asthma. |
| Lilja et al.570 | 1989 | 1b | RCT | Women with respiratory allergy to animal danders and/or pollens in the last 3 months of pregnancy randomized to:
|
Incidence of atopic diseases at 18 months of age | No significant difference in the distribution of atopic disease in relation to the maternal diet during late pregnancy. |
| Falth-Magnusson et al.571 | 1987 | 1b | RCT |
|
Skin prick, serum IgE, atopic manifestations (not AR) | Maternal elimination diet during late pregnancy does not protect the baby against atopy. Maternal elimination diet during late pregnancy is associated with low weight gain and preterm birth. |
| Alduraywish et al.564 | 2016 | 2a | Meta-analysis | Asthma, AR, eczema or sensitization against food allergens | Food sensitization in the first 2 years of life can identify children at high risk of subsequent allergic disease, including AR. | |
| Zutavern et al.572 | 2008 | 2b | Population-based, prospective birth cohort study | Asthma, AR, eczema or sensitization against food or inhalant allergens | No evidence supporting a delayed introduction of solids beyond 4–6 months. |
AR = allergic rhinitis; IgE = immunoglobulin E; LOE = level of evidence; RCT = randomized controlled trial.
In an RCT evaluating the effects of in utero exposure to food antigens and the development of AR, 162 high-risk pregnant women (history of respiratory allergy to animal danders and/or pollens) were randomized 1 of 2 diets during the last 3 months of pregnancy: either very low ingestion of hen’s egg and cow’s milk, or a daily ingestion of 1 hen’s egg and 1 [liter] of cow’s milk. A total of 163 infants were followed prospectively up to 18 months of age, at which time the incidence of atopic disease, including AR, was evaluated in a blinded fashion. There was no significant difference in the incidence of AR between the 2 groups.570 In another RCT, restricted diet during pregnancy (cow’s milk-free and egg-free diet from week 28 to delivery) was associated with a small but statistically significant lower mean gestational weight gain and did not protect the offspring from atopy.571 The pooled results of 2 trials suggest that maternal food antigen avoidance may be associated with a higher risk of preterm birth and a possible adverse effect on mean birth weight without beneficial effects on AR development in the children.570,571
Studies have also evaluated the early introduction of foods compared to food avoidance with respect to the effects on development of allergic disease. In a prospective birth cohort study of 2073 children, delayed introduction of solids (past 4 or 6 months of age) was not associated with decreased odds for AR, asthma, or sensitization against food or inhalant allergens at 6 years of age. In fact, food sensitization occurred more frequently in children who were introduced to solids later.572 In a prospective RCT of food allergen avoidance in infancy, the incidence of subsequent allergic disease, including AR, was assessed. The intervention arm of the trial required mothers to avoid cow’s milk, egg, and peanut during the last trimester of pregnancy and subsequent lactation, and required infants to avoid cow’s milk until age 1 year (casein hydrolysate supplementation before age 1), egg until age 2 years, and peanut and fish until age 3 years. Compared to maternal-infant control pairs who followed standard feeding practices, infants in the food-avoidance arm showed a significant reduction in rates food allergy and milk sensitization before age 2 years. However, by the age of 7 years, the prevalence of food allergy was no longer different between the 2 groups. Furthermore, there was no difference in rates of AR, AD, asthma, and other atopic disease at age 7 years.573
Based on the presented meta-analysis, prospective randomized studies, and a large prospective birth cohort study, there is no data to support maternal diet as a contributing factor for the development of food allergy and AR; however, there is some evidence that the presence of food allergy during childhood (greater than 2 years old) is a risk factor for AR.
Aggregate Grade of Evidence: A (Level 1b: 3 studies; Level 2a: 1 study; Level 2b: 1 study; Table VI.C).
VI.D. Pollution
The relationship between pollution and AR has received increasing attention over the past decade. Environmental air pollutants contain several compounds; however, most studies have primarily focused on particulate matter <10 μm (PM10), particulate matter <2.5 μm (PM2.5), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), and ozone (O3). These particles may potentiate atopy through multiple mechanisms, including injuring the nasal epithelium, altering the immune response, and increasing the allergenicity of certain antigens.574,575 For example, pollution may damage the nasal mucosa and impair MCC, thereby facilitating the access of inhaled allergens to cells of the immune system.576 Additionally, airborne particles, including diesel fuel exhaust, are also able to carry allergens, thus potentially increasing the spread of allergens or the duration of their exposure.574 In nasal provocation studies of HDM-sensitive individuals, a combined nasal challenge with HDM allergens and diesel exhaust particles led to enhanced mast cell degranulation and increased severity of rhinitis symptoms compared to a challenge with HDM alone.577
Numerous studies have examined the effects of air pollutants on the development of AR in both pediatric and adult patients (Table VI.D). However, 3 prospective cohort studies (the highest level of evidence identified for this topic) found no significant correlation.578-580 Codispoti et al.578 specifically looked at the relationship between exposure to diesel exhaust particles (DEP) at 1 year of age and the subsequent development of AR at 2, 3, and 4 years of age. While they found that DEP had a marginally positive association with aeroallergen sensitization at 2 and 3 years, and increased aeroallergen sensitization increased the risk of AR, they failed to identify a significant direct correlation between DEP and AR development. Additionally, Kim et al.579 evaluated exposure to NO2, SO2, CO, and PM10 in children and found no significant association with a new diagnosis of AR after 2 years. However, they did note a positive association between increased levels of O3 and an AR diagnosis in industrial areas only; O3 was also significantly associated with the development of new sensitizations to outdoor allergens, which may explain the mechanism for the related increase in AR prevalence. Finally, Gehring et al.580 pooled 4 prospective pediatric birth cohort studies with 14 to 16 year follow-up and found no indication that NO2, PM2.5, or PM10 levels influenced the development of rhinoconjunctivitis.
TABLE VI.D.
Evidence for the effects of pollution exposure on the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Codispoti et al.578 | 2015 | 2b | Prospective cohort | DEP exposure at 1 year:
|
Development of AR by age 4 years | High DEP exposure did not correlate with the development of AR. |
| Gehring et al.580 | 2015 | 2b | Pooled prospective cohort |
|
Incidence and prevalence of rhinoconjunctivitis from age 4 to 14–16 years | No association between air pollution exposure and rhinoconjunctivitis incidence or prevalence at various ages. |
| Kim et al.579 | 2011 | 2b | Prospective cohort | Concentrations of 5 air pollutants (NO2, O3, SO2, CO, PM10):
|
Development of AR in children over 2 years | Incidence of AR is not associated with air pollutants; however, there was a positive association between higher O3 levels and AR in industrial areas. |
| Chiang et al.587 | 2016 | 3b | Case-control study | Exposure to SO2 over 11 years:
|
Diagnosis of AR in children | High exposure to SO2 correlates with an increased diagnosis of AR. |
| Chung et al.588 | 2016 | 3b | Case-control study | Exposure to 5 air pollutants (PM10, NOx, SO2, CO, O3):
|
Diagnosis of AR in preschool children | Prediagnosis levels of CO and NOx were significantly related to AR diagnosis. |
| Deng et al.557 | 2016 | 4 | Cross-sectional | Exposure to 3 air pollutants (PM10, NO2, SO2):
|
Diagnosis of AR in kindergarten children | Prenatal exposure to high NO2 correlated with AR; postnatal exposure to high PM10 correlated with AR. |
| Kim et al.476 | 2016 | 4 | Cross-sectional | Exposure to 5 air pollutants (PM10, NO2, SO2, CO, O3):
|
Diagnosis of AR by the age of 6–7 years | Higher exposure to CO was associated with an increased lifetime prevalence of physician-diagnosed AR. |
| Kim et al.589 | 2016 | 4 | Cross-sectional | Exposure to 5 air pollutants (PM10, NOx, SO2, BC, O3):
|
AR treatment over the past 12 months in children | High exposure to BC, SO2, and NO2 were significantly associated with increased treatment of AR. |
| Liu et al.586 | 2016 | 4 | Cross-sectional | Exposure to 3 air pollutants (PM10, NO2, SO2):
|
Diagnosis of AR in children | High exposures to NO2 during gestation, the first year of life, second year, and throughout life correlated with the development of AR. |
| Singh et al.584 | 2016 | 4 | Cross-sectional | Frequent passage of trucks near home:
|
Diagnosis of AR in children ages 6–7 and 13–14 years | Frequent passage of trucks was correlated with the occurrence of AR in both age groups. |
| Wang et al.585 | 2016 | 4 | Cross-sectional | Exposure to 6 air pollutants (PM10, PM2.5, NO2, SO2, CO, O3):
|
Diagnosis of AR in children | High levels of PM2.5 correlate with an increased risk of AR. |
| Jung et al.582 | 2015 | 4 | Cross-sectional |
|
Lifetime AR, past-year AR symptoms, diagnosed AR, and treated AR in children | Positive correlation between distance from main road and AR symptoms, diagnosis, and treatment. |
| Shirinde et al.583 | 2015 | 4 | Cross-sectional |
|
Diagnosis of AR in 13-year-old to 14-year-old children | Diagnosis of AR is significantly associated with the frequency of trucks passing by the residence. |
| Anderson et al.581 | 2010 | 4 | Cross-sectional study |
|
Diagnosis of rhinoconjunctivitis at ages 6–7 and 13–14 years | Only significantly increased association between PM10 levels and rhinoconjunctivitis and atopy in 13-year-olds to 14-year-olds in countries with more than 1 testing center. |
AR = allergic rhinitis; BC = black carbon; CO = carbon monoxide; DEP = diesel exhaust particles; LOE = level of evidence; NO2 = nitrogen dioxide; NOx = nitrogen oxides; O3 = ozone; PM10 = particulate matter <10 μm; PM2.5 = particulate matter <2.5 μm; SO2 = sulfur dioxide.
Several international case-control and cross-sectional studies have also evaluated the relationship between pollution and AR with varied results. Anderson et al.581 performed the largest cross-sectional study evaluating the effect of PM10 levels on the development of rhinoconjunctivitis in 322,529 children from 51 countries. There was no between-country association of rhinitis with modeled pollution levels, and within countries (24 countries had more than 1 study center) there were weakly positive associations between PM10 levels and rhinoconjunctivitis symptoms in 6-year-olds to 7-year-olds and diagnosed hay fever in 13-year-olds to 14-year-olds. Interestingly, they did show a positive association between high PM10 levels and the development of atopy.581 Some pediatric studies have identified a positive correlation between increased exposure to various pollutants and an increased diagnosis of AR during childhood.476,557,582-589 Liu et al.586 and Deng et al.557 even found that prenatal/gestational exposure to high concentrations of NO2 were associated with a higher prevalence of AR diagnosis during childhood. However, almost all of these studies utilize nearby traffic density or home address geocodes to estimate local pollution exposure. In many countries, people living in more polluted areas with high levels of traffic may also be more likely to have other confounding features that influence their development of AR (ie, socioeconomic status [SES], exposure to different aeroallergens) and not all studies fully adjust for these potential confounders. Additionally, several of these studies were restricted to specific cities in Asia, in turn, limiting generalizability.
Overall, the relationship between pollution exposure and the development AR is currently unclear. More prospective pediatric and adult studies in diverse geographic locations are needed to better understand this complex relationship.
Aggregate Grade of Evidence: C (Level 2b: 3 studies; Level 3b: 2 studies; Level 4: 9 studies; Table VI.D).
VI.E. Tobacco smoke
AR has frequently been associated with both active and passive (secondhand) exposure to tobacco smoke. However, the pathophysiology behind this relationship is complex and, at times, contradictory. Studies have shown that tobacco smoke exposure can propagate the development of atopic diseases via several mechanisms including direct surface damage to nasal mucosa, altered epigenetic mechanisms through histone acetylation, expression of microRNA, and DNA methylation.590,591 Alternatively, it has also been shown that nicotine may exert an immunosuppressive effect on allergic disease by suppressing eosinophil trafficking and Th2 cytokine/chemokine responses.592
Recently, 2 large meta-analyses were published which sought to better define the relationship between tobacco and AR (Table VI.E). Saulyte et al.593 identified a significant correlation between passive smoke exposure and the development of AR, but no significant relationship between active smoking or maternal prenatal passive smoke exposure and AR. However, they did find a significant correlation between active smoking and non-allergic/chronic rhinitis. Hur et al.594 also systematically evaluated the relationship between secondhand smoke and AR and that meta-analysis of studies in adults showed an association between passive smoke and AR, while a similar analysis of pediatric studies did not. This raises the possibility that the atopic effects of secondhand smoke in the nasal mucosa may take several years to manifest. In fact, Lin et al.595 found that allergic adults were more likely to have been exposed to secondhand smoke 20 years prior when compared to non-allergic adults.
TABLE VI.E.
Evidence for the effect of active and passive tobacco smoke exposure on the development of allergic rhinitis
| Study | Year | LOE | Study design | Active vs passive smoke exposure |
Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|---|
| Saulyte et al.593 | 2014 | 2a | SR of cohort, cross-sectional, and case-control studies | Both |
|
Diagnosis of AR | No association between active smoking and maternal pre-natal passive smoking and AR. Significant association between all other passive smoking and AR. |
| Codispoti et al.599 | 2010 | 2b | Prospective cohort study | Passive |
|
Diagnosis of AR by age 3 years | Environmental tobacco exposure has no effect on the development of AR by age 3 years. |
| Keil et al.596 | 2009 | 2b | Prospective cohort study | Passive | Maternal smoking vs no smoke exposure with:
|
Diagnosis of AR over the first 10 years of life | There was no association between maternal smoking and the development of AR regardless of the allergic status of the parents. |
| Bendtsen et al.598 | 2008 | 2b | Prospective cohort study | Active |
|
Self-reported SAR or PAR | Smoking more than 15 cigarettes/day was associated with a decreased risk of SAR. |
| Annesi-Maesano et al.600 | 1997 | 2b | Prospective cohort study | Active |
|
Chronic rhinitis, SAR, or perceived nasal hyperresponsiveness | No association between smoking and seasonal AR. Significant association between chronic rhinitis and current smoking. |
| Wright et al.597 | 1994 | 2b | Prospective cohort study | Passive |
|
Physician diagnosed AR at age 6 years | No significant association between maternal smoking and physician diagnosed AR. |
| Hur et al.594 | 2014 | 3a | SR of predominantly case-control studies | Passive |
|
Diagnosis of AR | Most studies did not show a relationship between passive smoke exposure and AR. |
AR = allergic rhinitis; LOE = level of evidence; PAR = perennial allergic rhinitis; SAR = seasonal allergic rhinitis; SR = systematic review.
Five prospective cohort studies examined the effect of tobacco on the development of AR, all of which failed to find a correlation between active or passive tobacco smoke and the development of AR.596-600 Keil et al.596 found that while passive smoke was not significantly related to AR, it was strongly associated with allergic sensitization and asthma symptoms in children with a genetic predisposition (at least 1 or more atopic parents). Additionally, Wright et al.597 found that while there was no significant association between secondhand smoke exposure and AR, 63% of asthmatics born to heavy smokers developed rhinitis in the first 6 months, vs 43% of asthmatics whose mothers did not smoke. Finally, Bendtsen et al.598 found that actively smoking more than 15 cigarettes per day actually decreased a patient’s risk of developing AR.
This inverse correlation has been identified in several other studies.124,601-603 Eriksson et al.124 found that while smoking was associated with a high prevalence of chronic rhinitis in both men and women, it was correlated with a low prevalence of AR in men. Additionally, they found a significantly lower prevalence of sensitization to common airborne allergens in current and exsmokers compared to nonsmokers. In contrast, the significant positive association between tobacco and the development of non-allergic/chronic rhinitis has been repeatedly identified.124,128,604 Therefore, when discussing the effects of tobacco on rhinitis, differentiating between allergic and non-allergic/chronic is paramount.
Finally, tobacco does not appear to influence the efficacy of AR treatment. Katotomichelakis et al.605 evaluated 163 patients (both smokers and nonsmokers) receiving sublingual immunotherapy (SLIT) for AR and found that, regardless of tobacco status, total symptom scores and QOL questionnaires equally improved. Overall, while most studies evaluating AR and tobacco are case-control or cross-sectional in nature, multiple prospective cohort studies and 2 systematic reviews predominantly found no correlation between active or passive tobacco smoke and AR. Additionally, some studies suggest that tobacco may have a protective effect against the development of AR. Further investigation is needed to identify if specific patient populations (eg, asthmatics or those with atopic parents) or temporal variations (eg, exposure for 20+ years) may alter our understanding of this relationship.
Aggregate Grade of Evidence: C (Level 2a: 1 study; Level 2b: 5 studies; Level 3a: 1 study; Table VI.E).
VI.F. Socioeconomic factors
In 1829, John Bostock described 29 cases in the UK, including himself, of individuals who suffered from catarrhus aestivus or “summer cold,” which he noted occurred in patients of middle to high SES.606 During the 1870s, Blackley found no hay fever among farmers and people living in deprived areas of cities.606 The positive association between hay fever and high social class was later reported in the British 1958 and 1970 cohorts,607,608 as well as a Swedish survey of conscripts born from 1952 to 1977.609 However, during the study period, this association seemed to weaken with an OR estimate for AR among subjects with low SES changing from 0.79 to 0.92.
In 2000, an article was published from the German Multicentre Allergy Study (MAS) birth cohort including 1314 children born in 1990.610 In this study, it was found that the lifetime prevalence of hay fever was elevated in parents of high SES compared to low. However, in their children, the occurrence of hay fever was not elevated in families with high SES. Alternatively, in the Swedish birth cohort BAMSE (Swedish abbreviation for Children Allergy, Milieu, Stockholm, Epidemiology) with 4089 children born between 1994 and 1996, it was noted that high SES actually resulted in a decreased risk of AR, along with decreases in asthma and food sensitization rates.611 In a recent study from Denmark of 9720 children born between 1994 and 2006, AR was associated with low educational level of the parents.612 Interestingly, in the follow-up of the German MAS birth cohort study, SES was not associated with AR at all by the age of 20 years.613 Thus, among children born in the Western world before 1970 high SES was a risk factor, but among children born in the same regions after 1990 low SES, particularly early in life, seemed to be a risk factor614 (Table VI.F).
TABLE VI.F.
Evidence for the association between allergic rhinitis and socioeconomic factors
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Grabenhenrich et al.613 | 2015 | 2b | Prospective cohort | Parental SES:
|
Diagnosis of AR by age 20 years | No association between SES and diagnosis of AR. |
| Almqvist et al.611 | 2005 | 2b | Prospective cohort | Parental SES:
|
Diagnosis of AR at 4 years old | Parents of higher SES had children with a lower risk of AR, asthma, and food allergens. |
| Bergmann et al.610 | 2000 | 2b | Prospective cohort | Parental SES:
|
Diagnosis of AR parents and in children 3–6 years old | Parental high SES correlated to high AR rates in parents; however, SES had no correlation with AR in children 3–6 years old. |
| Lewis & Britton608 | 1998 | 2b | Prospective cohort | Level of “social advantage”:
|
Diagnosis of hay fever at ages 5, 10, and 16 years | Social advantage was significantly related to the diagnosis of AR with the “most advantaged” having the highest prevalence of AR. |
| Ahn et al.478 | 2016 | 4 | Cross-sectional survey | SES:
|
|
Significant association between higher SES and symptom-based AR; but no association between SES and allergy test–based AR. |
| Lee et al.615 | 2016 | 4 | Cross-sectional survey | Family affluence scale:
|
Diagnosis of AR in adolescents | High Family Affluence Scale was associated with higher prevalence of AR. |
| Penaranda et al.616 | 2016 | 4 | Cross-sectional survey | SES:
|
Diagnosis of AR in children and adults | Middle and high SES was associated with increased AR symptoms in children but not in adults. |
| Wronka et al.617 | 2016 | 4 | Cross-sectional survey | SES:
|
Diagnosis of AR in university students (ages 19–25 years) | Higher proportion of AR in students from high SES compared to low. |
| Hammer-Helmich et al.612 | 2014 | 4 | Cross-sectional survey | Parental SES | Diagnosis of AR in children 11–15 and 3–6 years old | No association between household income and diagnosis of AR. |
| Braback et al.609 | 2005 | 4 | Cross-sectional study | High vs low SES | Diagnosis of AR upon enrollment in military service | In the 1950s, low SES and AR were inversely related, but this association significantly decreased by 1970. |
AR = allergic rhinitis; LOE = level of evidence; SES = socioeconomic status.
More recently, 2 studies from Korea have reconfirmed the previously noted association between high SES and the development of AR. Ahn et al.478 found a positive association between higher family income and symptom-based AR diagnosis (but not allergy test-based AR diagnosis). Lee et al.615 also found family affluence, or high SES, to be a significant risk factor for AR in Korean adolescents. However, additional recent studies from South America and Europe have shown varying results. In 2016, Penaranda et al.616 found high SES to be associated with AR in children/adolescents but not in adults, while Wronka et al.617 identified a significantly higher incidence of AR in adult female university students (19 to 25 years old) from families with high SES.
Overall, SES is likely a proxy for various exposures like number of siblings, viral infections, exposure to tobacco smoke, housing conditions and location, allergen exposures, dietary factors, and nutrition including breastfeeding and general diet. Some of those exposures are associated with the hygiene hypothesis, introduced by Strachan618 in the late 1980s. However, it is worth noting that exposures relevant to the hygiene hypothesis were important predictors for the development of AR at an early age.614
Currently, there is conflicting evidence regarding the association between SES and AR. While most studies show an association between high SES and the diagnosis of AR, this is not a consistent outcome. This disparity may be explained by the additional factors evaluated in several of these studies which may confound the exact relationship between SES and AR. Additionally, there may be a temporal relationship between SES and AR considering different outcomes in children compared to adults. Additional investigation is needed to determine the true relationship between AR and SES.
Aggregate Grade of Evidence: C (Level 2b: 4 studies; Level 4: 6 studies; Table VI.F).
VI.G. Protective factors against allergic rhinitis VI.G.1. Breastfeeding
Breastfeeding is associated with several beneficial effects on mother and child health and therefore has been recommended for all infants.619 One potential benefit is the prevention of allergic disease.620 Breast milk is an immunologically complex solution, containing multiple compounds that support infant growth and facilitate development of the infant immune response.621,622 The association between breastfeeding and the prevention of allergic disease has been frequently studied and often debated.
Mimouni Bloch et al.623 performed a meta-analysis of prospective studies evaluating the effects of exclusive breastfeeding for the first 3 months of life on the development of AR (Table VI.G.1). Six prospective studies met the inclusion criteria. In their pooled analysis, they found a protective effect of exclusive breastfeeding for the first 3 months of life that approached statistical significance in the general population (OR 0.74; 95% CI, 0.54 to 1.01). Interestingly, the protective effect was not seen in children with a family history of atopic disease (OR 0.87; 95% CI, 0.48 to 1.58).
TABLE VI.G.1.
Evidence for the effects of breastfeeding on the development of allergic rhinitis*
| Study | Year | LOE | Study design |
Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lodge et al.624 | 2015 | 3a | SR | Association between breastfeeding and AR | Development of AR | Nonsignificant protective effect overall. Protective benefit for children under 5 years old, but not over 5 years old. |
| Mimouni Bloch et al.623 | 2002 | 3a | SR | Prospective studies evaluating the effects of exclusive breastfeeding for the first 3 months on AR development | Development of AR | Protective effect close to statistical significance in the general population but not in children with a family history of atopic disease. |
These systematic reviews include all published studies to date.
AR = allergic rhinitis; LOE = level of evidence; SR = systematic review.
More recently, Lodge et al.624 performed a systematic review and meta-analysis in 2015. Their analysis evaluated the association between breastfeeding and AR and included 5 cohort studies550,599,607,625,626 and 11 cross-sectional studies.627-637 The number of participants varied between 361 and 13,889 for the cohorts, and 1402 to 206,453 for the cross-sectional studies. Pooling of estimates from the various studies found a nonsignificant protective effect of breastfeeding on the development of AR (OR 0.92; 95% CI, 0.84 to 1.01). The results were then stratified by incidence of AR in different age groups. After stratification by age, a reduced risk of AR in patients under 5 years of age was associated with breastfeeding (OR 0.79; 95% CI, 0.63 to 0.98). However, there was no association after 5 years of age (OR 1.05; 95% CI, 0.99 to 1.12). While the authors of this meta-analysis argued for the benefit of breastfeeding in the prevention of AR, they do acknowledge that the protective effect of breastfeeding seen in patients less than 5 years of age may have been confounded by known protective effects of breast milk against viral respiratory infections. The authors hypothesized that, given the difficulty of differentiating between AR and viral rhinitis in young children, a reduction in viral respiratory infections have been possibly interpreted as a reduction in rhinitis symptoms.624
Aggregate Grade of Evidence: C (Level 3a: 2 studies; Table VI.G.1).
Benefit: Possible benefit from breastfeeding with reduction in AR, especially seen in young children.
Harm: None. No studies have shown harm with breast-feeding for 6 months.
Cost: Low.
Benefits-Harm Assessment: Possible benefit with no harm.
Value Judgments: There is evidence that breastfeeding may reduce the risk of AR with no perceived harm. Given the general benefits to the mother and child, breast-feeding for 4 months and possibly 6 months has been advocated.
Policy Level: Option for breastfeeding for the specific purpose of AR prevention, based upon current evidence. In general, breastfeeding has been strongly recommended due to its multiple benefits.
Intervention: Breastfeeding is generally encouraged for at least 4 months due to its multiple benefits. When specifically related to the prevention of AR, breastfeeding is an option.
VI.G.2. Childhood exposure to pets
Among subjects sensitized to pet allergens, exposure tends to exacerbate symptoms. However, the association of pet-keeping in childhood with the subsequent development of AR is more controversial, and difficult to establish. (See section VI.B. Risk factors for allergic rhinitis – Inhalant allergens (in utero and early childhood exposure) – Animal dander for additional information on this topic.)
Prevalence of household pet ownership is used to estimate pet allergen exposure. However, pet owners are frequently contaminated with pet allergens, leading to generalized exposures via social contact. Therefore, a non-exposed reference population does not exist, limiting our ability to clearly understand the relationship between exposure to pet allergens and development of AR.
The timing of pet allergen exposure early in life may be an important factor for the maturing immune system. Therefore, self-reported perinatal and newborn exposures are frequently analyzed. Few studies have measured the concentration of the major cat (Felis catus) allergen (Fel d 1) or the major dog (Canis familiaris) allergen (Can f 1) in home dust. Rather, most studies merely report exposure to cats and/or dogs, or furred pets, and some to rodents and birds. In a systemic review of epidemiologic studies of allergy and asthma, only 10 of 96 included studies reported avoidance of pets.638 Additionally, studies may often fail to account for confounding variables such as a family history of pet allergy which, in turn, may predispose likely atopic children to pet avoidance.
There is significant inconsistency with regard to pet ownership in childhood and the subsequent development of allergy. Demographic features related to pet-keeping, including race, urban vs rural environment, family size, and SES may help account for some of the conflicting results. A meta-analysis of 32 studies reported a lower prevalence of AR among subjects with furred pets in cross-sectional studies, and less asthma among cat-exposed subjects.639 An extensive systematic review of 62 studies found different associations depending on study design.640 In most of the birth cohort studies, dog exposure in early childhood was protective for sensitization against aeroallergens.640,641 On the contrary, cross-sectional studies reported inconsistent associations between cat or dog exposure and sensitization as well as the subsequent development of atopic diseases later in life562,640 (Table VI.G.2).
TABLE VI.G.2.
Evidence for the effect of early childhood pet exposure of the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Dharmage et al.562 | 2012 | 2a | SR | 19 studies (2011–2012): 9 longitudinal, 8 cross-sectional, 2 case-control | Association of AR with exposure to cats | Inconsistent association. If exposure during the first year, less AR or sensitization, or no effect. Possible protective effect until adulthood. |
| Lodge et al.642 | 2012 | 2a | SR | (2001–2008): 9 longitudinal studies; 6498 subjects aged 0–11 years | Association of physician diagnosed hay fever with exposure to pets, or cats and dogs during perinatal period in urban environment | Dogs may reduce sensitization or allergic disease in families with low risk of allergy. No association with cats. |
| Lodrup-Carlsen et al.552 | 2012 | 2a | Pooled analysis of individual data first year of recruitment | (1989–1997): 11 European birth cohorts; 11,489 participants aged 6–10 years | Association of sensitization to aeroallergens with ownership of cats only, dogs only, cats and dogs only, birds only or rodents only during 0–2 years of age | Dog and rodent exposure protective against sensitization to aeroallergens. No association with AR. |
| Smallwood & Ownby641 | 2012 | 2a | SR | 26 articles: exposure to dogs 20 weeks from gestation to 1 year. | Association of allergic symptoms with exposure to dogs | Inconsistent association. Dog exposure at birth may be protective against allergic symptoms. |
| Chen et al.640 | 2010 | 2a | SR of birth and non-birth cohort studies and cross-sectional studies | 62 articles (2000–2009); subjects 6–69 years old:
|
Association of AR with exposure to cats or dogs in cross-sectional studies | Inconsistent association. Dog exposure may be protective. Design of the study influences the association. |
| Takkouche et al.639 | 2008 | 2a | Meta-analysis | 32 studies (1985–2006); 5 studies (n = 6818) reported rhinitis | Association of AR with exposure to furred pets | Inconsistent association. Possible protective effect of furred pets on rhinitis. |
| Christensen et al.643 | 2016 | 2b | Population based cross-sectional study follow-up | RHINE cohort (2010–2012): 13,376 subjects born in Northern Europe 1945–1973 | Association of AR in adulthood with exposure to pets at birth, during childhood and to livestock farm in childhood | Exposure to pets in childhood decreases the risk of AR in adulthood independently of urban or rural upbringing. |
| Lodge et al.534 | 2012 | 2b | Prospective birth cohort | MACS cohort: 620 infants with family history of allergic disease | Association of hay fever after 7 years of age with exposure to cats and dogs at birth | In high-risk cohort, pet exposure at birth is protective against hay fever at age 7 years in children with nonsensitized fathers |
AR = allergic rhinitis; LOE = level of evidence; MACS = Melbourne Atopy Cohort Study; RHINE = Respiratory Health in Northern Europe; SR = systematic review.
The impact of pet avoidance on AR development is best evaluated via longitudinal birth cohort studies. A systematic review of 9 studies conducted solely in urban environments evaluated perinatal pet exposure.642 Six studies found that exposure to dogs, or cats/dogs protected against allergic disease. Two studies found increased risk of allergy only in highly atopic families. Furthermore, in a cohort of 620 children with family history of allergic diseases, exposure to cats or dogs was protective only in children with non-allergic fathers.534
In a pooled analysis of 11 European birth cohorts, any furred pet ownership during the first 2 years was associated with lower risk of sensitization to aeroallergens, but not with a decreased prevalence of AR later in childhood.552 In a recent study which investigated urban vs rural differences, the risk of AR in adulthood was 20% lower in subjects exposed to pets at birth or during childhood. However, pet keeping did not explain the protective effect of living on farm with livestock compared to urban dwelling.643
Overall, pet allergens are ubiquitous. There is no evidence that pet avoidance in childhood prevents the development of AR or sensitization to aeroallergens later in life. Alternatively, early pet exposure may induce immune tolerance and thus reduce the chance of development of allergic disease. This protective effect seems to be strongest in non-allergic families with dog exposure in early childhood.
Aggregate Grade of Evidence: C (Level 2a: 6 studies; Level 2b: 2 studies; Table VI.G.2).
VI.G.3. Hygiene (aka biodiversity or microflora) hypothesis
The inverse association of the number of siblings and the prevalence of hay fever was reported nearly 3 decades ago in British cohorts.618 Strachan618 proposed the term “hygiene hypothesis” and speculated that exposure to frequent infections in large families could be the protective factor. The hygiene hypothesis has evolved toward a more contemporary “biodiversity hypothesis” that looks beyond the effect of infections and single protective microbes to the potential protective effect of the colonization of mucous membranes and the skin with diverse environmental microflora.644 Recently, the term “microbiota hypothesis” has been proposed. In addition, the term “microflora” should be substituted for the term “microbiota.” Various related potential cofactors and their relationship to the development of AR are discussed in this section.
Number of siblings.
The association between number of siblings and presence of allergic diseases has been studied extensively. In a meta-analysis of 53 studies, 48 studies demonstrated that higher number of siblings was associated with decreased atopy, an effect that was more evident for AR than for sensitization and asthma645 (Table VI.G.3). A large study based on questionnaire data for children aged 6 to 7 years from 31 countries and 13 to 14 years from 52 countries confirmed that the inverse association between the number of older siblings and prevalence of hay fever was strongest in more affluent countries.646
TABLE VI.G.3.
Evidence for the hygiene hypothesis in the development of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Campbell et al.647 | 2015 | 2a | SR | 29 studies (1999–2014): 26 cross-sectional, 3 longitudinal. Meta-analysis: 8 studies | Association of farm exposure with sensitization in childhood or adulthood | Protective effect of farm exposure in infancy on allergic disease in childhood and adulthood in majority of studies. Exposure during adulthood had no consistent relationship with sensitization. |
| Karmaus & Botezan645 | 2002 | 2a | Meta-analysis | 53 studies (1986–2000). Hay fever: 17 studies (n = 253,304); Sensitization: 16 studies (n = 46,758) | Association of sensitization and AR with 3 or more siblings vs no siblings | Higher number of siblings was associated with less atopy. Effect was not explained by hygiene factors. |
| Fujimura et al.645 | 2016 | 2b | Longitudinal birth cohort study | 298 children followed until age 4 years | Association of sensitization and asthma at age 2 years with fecal microbiota in neonates targeted at age 1 month (n = 130) or 6 months (n = 168) | Reduced colonization of Bifidobacteria, Lactobacillus, Faecalibacterium, Akkermansia, and Malassezia during the neonatal period may influence the risk of multisensitization predictive for asthma. |
| House et al.648 | 2016 | 2b | Nested case-control study | Farmers and spouses: Cases: asthma (n = 1198); Controls: no asthma (n = 2031). | Association of sensitization, rhinitis, eczema, and asthma with living on a farm when born and with being exposed to farm environment when mother was performing farm activities during pregnancy | Early-life farm exposure associated with less atopy. No association with asthma. |
| Hua et al.664 | 2016 | 2b | Cross-sectional study | 1879 adult subjects | Association of seasonal allergy with fecal microbial biodiversity | Reduced fecal biodiversity and altered composition associated with more allergy. No association with asthma and eczema. |
| Arrieta et al.663 | 2015 | 2b | Longitudinal nested case-control study | 319 children followed from birth until 5 years of age | Association of sensitization and wheezing at 1 year with fecal microbiota at age 3 months and 1 year | Reduced colonization of Faecalibacterium, Lachnospira, Veillonella, and Rothia during the first 3 months of life may increase the risk of atopic asthma. |
| Strachan et al.646 | 2015 | 2b | Cross-sectional study | Children 6–7 years of age in 31 countries (n = 210,200); 13–14 years of age in 52 countries (n = 337,226) | Association of hay fever with three or more siblings vs no siblings | Protective effect of older and total number of siblings on self-reported AR. Effect was significantly stronger in affluent countries. |
| Valkonen et al.661 | 2015 | 2b | Cross-sectional stratified population study | GABRIELA study: 224 children, 6–12 years | Association of sensitization with mattress bacterial diversity | Exposure to more diverse bacterial flora associated with less sensitization. |
| Bisgaard et al.449 | 2011 | 2b | Longitudinal study | 253 high-asthma-risk children followed from birth to age 7 years | Association of sensitization and AR with high fecal microbial biodiversity | Reduced bacterial diversity associated with higher risk of sensitization and AR in childhood. |
| Ege et al.659 | 2011 | 2b | Two cross-sectional studies | PARSIFAL study: 489 rural and suburban children; GABRIELA study: 444 rural children | Association of sensitization with microbes in mattress (PARSIFAL) and in airborne dust (GABRIELA) | Farm-children had less asthma and atopy. Indoor microbial exposure much higher and diverse in farm homes. Microbial diversity related to asthma but not to atopy. |
| Tischer et al.657 | 2011 | 2b | Nested case-control study | 678 children at the age of 6 years from German (n = 346) and Dutch (n = 332) birth cohorts | Association of rhinitis and asthma with mattress dust biological components of mold and endotoxin | Inconsistent results. Microbial exposures at home had different effects on allergy in German and Dutch birth cohorts. |
| von Hertzen et al.660 | 2007 | 2b | Cross-sectional study | 563 children aged 7–16 years in Finnish and Russian Karelia | Association of sensitization with microbial content in drinking water samples from school kitchens | Microbial count much higher and sensitization much lower in Russia. High count of microbes associated with less atopy. |
| Cuello-Garcia et al.658 | 2015 | 3a | Systematic review and meta-analysis | 29 randomized controlled trials in infants | Association of AR with probiotic supplementation to pregnant mothers, breast-feeding women, or infants | No effect on allergies. |
| Simpson & Martinez656 | 2010 | 3a | Review | (2000–2007): 6 rural studies; 10 urban studies | Association of sensitization with exposure to endotoxin | Exposure to endotoxin protective in over 50% of studies. Endotoxin may be marker of other protective factors. |
| Abrahamsson et al.442 | 2014 | 3b | Longitudinal case-control study | 47 infants (n = 20 IgE-associated eczema; n = 27 healthy controls) followed until 7 years of age | Association of sensitization, asthma and AR with fecal diversity in infancy | Low microbial diversity associated with asthma later in childhood. No association with sensitization or rhinitis. |
AR = allergic rhinitis; GABRIELA = GABRIEL Advanced Survey; IgE = immunoglobulin E; LOE = level of evidence; PARSIFAL = Prevention of Allergy-Risk Factors for Sensitization Related to Farming and Anthroposophic Lifestyle; SR = systematic review.
Farming.
Since the first publications in 1999–2000, there is a growing interest in the “farm effect” on allergy. In a meta-analysis of 8 studies, the risk of sensitization, measured by sIgE or SPT in childhood or adulthood, was 40% lower (OR 0.60; 95% CI, 0.52 to 0.70) among subjects who had lived on a farm during the first year of life.647 In a recent U.S. case-control study, farm exposure in utero and in early childhood protected against allergen sensitization but not asthma in adulthood.648 The protective farm effect seems to be stronger when exposed to farm animals and stables.522,649-655 The protective effect is greatest with highest exposure occurring early in life.650
Bacterial endotoxin.
Exposure to bacterial endotoxin has been studied as a possible protective factor. Inverse association between exposure to endotoxin in infancy and childhood and the development of allergic sensitization has been shown in rural and urban environments, but the results have not been uniform between the studies.656,657
Probiotics.
A meta-analysis of 29 randomized controlled studies showed no significant association of probiotics supplementation of pregnant or breastfeeding mothers or infants with sensitization or allergic rhinitis at age 12 to 36 months.658 (See section IX.B.9. Management – Pharmacotherapy – Probiotics for additional information on this topic.)
Microbial diversity.
Changes in lifestyle, urbanization, diet, and the use of antibiotics have changed the microbiota of the environment, human skin and mucosal membranes. Differences in the microbiota may explain the difference in atopic diseases between rural and urban areas, as well as Finland and the Russian Karelia (a part of Russia geographically adjacent to Finland).659-661 Households with dogs have rich, diverse house dust microbiota with abundance of Firmicutes and Bacteroides species.662
In the GABRIEL study the mattress dust of farm children and their controls was analyzed by quantitative DNA analysis. Especially high mattress levels of Mycobacterium sp., Bifidobacteriaceae sp., and Clostridium sp. were found among farm children, and that high level was inversely associated with atopy.661
Low diversity of gut microbiota in early infancy has been related to greater risk of asthma and sensitization in some longitudinal studies with different designs in childhood.442,445,449,663 The dysbiosis of the microbiome driven by higher Bacteroides and reduced Clostridia taxa in adulthood was associated with greater prevalence of seasonal and nut allergies in adulthood in the American Gut Project.664
Skin microbiota may also be associated with protection from atopy. Compared with healthy individuals, atopic individuals have shown to have lower environmental bio-diversity at home and significantly lower generic diversity of gammaproteobacteria on their skin.665 Skin Acinetobacter (gammaproteobacteria) species were associated with anti-inflammatory immune responses only in healthy subjects.666
In summary, hygiene is important to prevent infections worldwide. Urbanization first in affluent and later in developing countries has led to reduced microbial diversity in the environment. Large microbial diversity of the skin, airways, and gut in childhood is important for the prevention of sensitization and of allergic disease in populations. More longitudinal studies are needed to show the association.
Aggregate Grade of Evidence: B (Level 2a: 2 studies; Level 2b: 10 studies; Level 3a: 2 studies; Level 3b: 1 study; Table VI.G.3).
Studies included in the Aggregate Grade of Evidence are systematic reviews and meta-analyses for the various aspects of the hygiene hypothesis discussed above. Also included are recent studies, published after the noted systematic reviews and meta-analyses. If systematic reviews and meta-analyses are not available, individual studies are listed.
VII. Disease burden
VII A. Individual burden
VII.A.1. Effect on quality of life
Two systematic reviews have evaluated the effect of AR on QOL, with both concluding that AR patients suffer from significantly decreased general and disease-specific QOL due to the impact of physical and mental health. Furthermore, both studies demonstrated that treatment of AR leads to improvement in QOL667,668 (Table VII.A.1). While the impact of AR on QOL has been suggested in the literature for decades, only recently has the effect of AR on QOL been rigorously studied. This is in part due to the development of validated general and disease-specific QOL instruments, and their use in clinical investigations and trials. The most commonly used general QOL instruments in the AR literature appear to be the Short Form 12 and 36 (SF-12/36),669,670 which measure generic physical and mental health-related QOL. The most commonly used AR disease-specific QOL tool is the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), or 1 of its variations (ie, mini-RQLQ, nocturnal RQLQ).671 However, despite the availability of these instruments, many studies in the published literature rely upon nonvalidated methods to assess QOL, leading to difficulty comparing outcomes between some studies.
TABLE VII.A.1.
Effect of allergic rhinitis on general and disease-specific quality of life
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Bousquet et al.674 | 2013 | 1b | RCT | AR (n = 716):
|
Symptoms scores, sleep questionnaire, RQLQ, WPAIAS | Desloratadine improves symptoms, QOL, and functional impairment. |
| Tatar et al.672 | 2013 | 1b | RCT | AR (n = 56):
|
Mini-RQLQ | QOL significantly affected by AR. Combination of mometasone with levocetirizine or montelukast improves QOL more than mometasone alone. |
| Yamada et al.673 | 2012 | 1b | RCT, double-blind, crossover | PAR (n = 57): mometasone | TSS, QOL score, sleep quality, nasal nitric oxide | Nasal mometasone improves nasal symptoms, QOL, and sleep quality and decreases nitric oxide. |
| Hoiby et al.678 | 2010 | 1b | RCT | AR (n = 53):
|
Symptom and medication scores | SCIT reduces symptom and medication scores compared to placebo. |
| Holmberg et al.676 | 2009 | 1b | RCT, double-blind, crossover | AR (n = 584):
|
RQLQ, symptom score | Desloratadine improves RQLQ and symptom score significantly compared to placebo. |
| Witt et al.692 | 2009 | 1b | RCT | AR (n = 981):
|
SF-36 | Acupuncture improves QOL more than control at 3 months. |
| Brinkhaus et al.680 | 2008 | 1b | RCT | AR (n = 5237):
|
RQLQ, SF-36 | QOL significantly affected by AR. Acupuncture group improves more than conventional medical care. |
| Canonica et al.677 | 2006 | 1b | RCT, double-blind | AR (n = 551):
|
RQLQ, SF-36 | QOL significantly affected by AR. Levocetirizine improves QOL compared to placebo. |
| Colas et al.679 | 2006 | 1b | RCT, double-blind | AR (n = 60):
|
RQLQ, symptoms score, medication score | QOL significantly affected by AR. SCIT improves RQLQ, symptom and medication scores. |
| Bachert et al.675 | 2004 | 1b | RCT, double-blind | PAR (n = 551):
|
SF-36, RQLQ | Levocetirizine improves QOL and decreases disease-related costs. |
| Radcliffe et al.693 | 2003 | 1b | RCT, double-blind | SAR (n = 183):
|
RQLQ, problem-free days | Enzyme potentiated desensitization does not improve QOL compared to placebo. |
| Gerth Van Wijk et al.694 | 2000 | 1b | RCT | AR and nasal capsaicin (n = 26) | VAS, RQL | Capsaicin does not sufficiently control rhinitis symptoms. |
| Juniper et al.671 | 1991 | 1b | RCT, double-blind | AR questionnaire development (n = 85); validation (n = 60) | RQLQ | In addition to local symptoms, patients experience impaired QOL through systemic, sleep, emotional symptoms, and practical/activity limitations. Beclomethasone use correlated to RQLQ. |
| Linneberg et al.667 | 2016 | 2a | SR | AR | QOL | Patients with AR suffer from decreased QOL in terms of both physical and mental health. |
| Hahn-Pedersen et al.668 | 2014 | 2a | SR | AR | QOL | AR patients have significantly worse general and disease-specific QOL with physical, practical, and activity domains most affected. SCIT improves QOL and symptoms. |
| Filanowicz et al.695 | 2016 | 2b | Observational cohort | SCIT (n = 200):
|
RQLQ | QOL is significantly affected by AR. SCIT significantly improved QOL in asthma and AR. |
| Jaruvongvanich et al.684 | 2016 | 2b | Observational cohort | AR (n = 260) | SF-12, TSS | Extranasal symptoms in AR correlate with physical and mental health QOL domains. |
| Bousquet et al.681 | 2013 | 2b | Observational cross-sectional | AR (n = 990) | VAS, RQLQ, TSS | 20% mild intermittent, 17% mild persistent, 15% moderate-severe intermittent, 48% moderate-severe persistent. Severity and duration of AR impact on QOL. Ocular symptoms impact RQLQ more than nasal obstruction. Sneezing/rhinorrhea do not impact RQLQ. |
| Demoly et al.696 | 2013 | 2b | Observational cohort | AR (n = 990) | VAS, RQLQ, TSS | 20% mild intermittent, 17% mild persistent, 15% moderate-severe intermittent, 48% moderate-severe persistent. VAS can detect QOL variations with high sensitivity. |
| de la Hoz Caballer et al.697 | 2012 | 2b | Observational cross-sectional | Primary care patients (n = 616) | SF-36, generic HRQOL, WPAI | AR impacts productivity to a greater magnitude than hypertension and DM type II, but not depression. |
| Meltzer et al.698 | 2012 | 2b | Observational cross-sectional | Nasal allergy (n = 522); no nasal allergy (n = 400) | Nonvalidated phone interview questions | AR patients rate overall health lower, have worse sleep function, and decreased productivity than those with non-AR. |
| Ciprandi et al.699 | 2010 | 2b | Observational cohort | AR undergoing SLIT (n = 167) | RQLQ | QOL is significantly affected by AR. SLIT effective at improving QOL and symptoms. |
| Stull et al.682 | 2009 | 2b | Observational cross-sectional | AR (n = 404) | Symptom scale, nocturnal RQLQ, WPAI, MOS-12 Sleep, PANAS-X | Nasal congestion is more strongly correlated to outcomes, but ocular symptoms can have significant impact of QOL. |
| Cadario et al.683 | 2008 | 2b | RCT | AR treated with SLIT (n = 40) | Nonvalidated QOL scale | QOL is significantly affected by AR. SLIT improves QOL and symptoms. |
| Petersen et al.700 | 2008 | 2b | Observational cross-sectional | AR (n = 248); AR and asthma (n = 121) | RQLQ; 15D | AR patients have worsened QOL during allergen exposure. 15D generates more comprehensive view of impact on QOL than RQLQ. |
| Ciprandi et al.701 | 2007 | 2b | Observational cohort | AR (n = 123) | RQLQ | QOL is significantly affected by AR. >2 sensitivities, eosinophil count, and nasal flow related to QOL. Eye symptoms correlate most strongly to QOL. |
| Di Rienzo et al.702 | 2006 | 2b | RCT, double-blind | AR (n = 34):
|
RQLQ | QOL is significantly affected by AR. SLIT improved QOL compared to placebo. |
| Laforest et al.703 | 2005 | 2b | Observational cohort |
|
Mini-RQLQ, SF-12 | QOL is significantly affected by SAR and asthma. Female gender, rural residence, and lower education levels associated with worse QOL in SAR. |
| Majani et al.691 | 2001 | 2b | Observational cohort | SAR (n = 33) | SF-36, SAT-P | QOL is significantly affected by AR during peak season. |
| Leynaert et al.689 | 2000 | 2b | Observational cross-sectional |
|
SF-36 | Both asthma and AR impact QOL. AR impacts emotional and mental health, social activities, and activities of daily living. Comorbid asthma caused more physical limitations than AR alone. |
| Cingi et al.704 | 2013 | 2c | Outcomes research | PAR treated with desloratadine and montelukast (n = 40) | Acoustic rhinometry, RQLQ | Desloratadine + montelukast improves nasal obstruction and QOL. |
| Bukstein et al.688 | 2016 | 3b | Observational cohort | PAR treated with beclomethasone (n = 527) | RCAT, treatment satisfaction, WPAI, PSQI, mini-RQLQ | Beclomethasone improves QOL, school-related activities, satisfaction, productivity, and sleep quality. |
| Song et al.685 | 2015 | 3b | Observational cross-sectional | Middle school students, cross-sectional stratified random sampling (n = 814) | Questionnaire | AR in 17.2%. AR impacts QOL, sleep, emotions, and memory. |
| Katelaris et al.687 | 2013 | 3b | Observational cross-sectional | AR (n = 303) | Questionnaire | AR impacts work/school performance, general QOL, and sleep quality. |
15D = Generic 15 Dimension Instrument for measuring health related quality of life; AR = allergic rhinitis; DM = diabetes mellitus; HRQOL = Health-Related Quality of Life; LOE = level of evidence; MOS-12 Sleep = Medical Outcomes Study 12-Item Sleep Scale; PANAS-X = Positive and Negative Affect Schedule-Expanded Form; PAR = perennial allergic rhinitis; PSQI = Pittsburgh Sleep Quality Index; QOL = quality of life; RCAT = Rhinitis Control Assessment Test; RCT = randomized controlled trial; RQL = rhinitis quality of life; RQLQ = rhino-conjunctivitis quality of life questionnaire; SAR = seasonal allergic rhinitis; SAT-P = satisfaction profile; SCIT = subcutaneous immunotherapy; SF-12 = short form 12; SF-36 = short form 36; SLIT = sublingual immunotherapy; SR; systematic review; TSS = total symptom score; VAS = visual analogue scale; WPAI = Work Productivity and Activity questionnaire; WPAIAS = Work Productivity and Activity Allergy Specific questionnaire.
Several high-quality studies have evaluated the impact of AR on overall and disease-specific QOL (Table VII.A.1). Most level 1b evidence includes RCTs evaluating the effect of topical nasal corticosteroids,671-673 antihistamines,672,674-677 or AIT.678,679 The general consensus of these studies is that AR has a significant negative impact on general and disease-specific QOL, and that the successful treatment of AR by any of the aforementioned therapies leads to the improvement of symptoms and QOL. One RCT that examined monotherapy vs poly-therapy showed that the combination of mometasone with either levocetirizine or montelukast led to greater symptom and QOL improvement than mometasone alone, but there was no difference between the levocetirizine and montelukast groups.672 Additionally, a RCT of acupuncture vs medical therapy showed that the improvement in QOL occurred in both groups, but the degree of improvement was larger in the acupuncture group.680
While the remaining evidence is of lower quality, it includes important and interesting findings in addition to the conclusions reached by the RCTs and systematic reviews. For example, extranasal symptoms, particularly ocular symptoms, have a significant impact on QOL and should not be ignored in the evaluation and management of AR.681-684 Furthermore, the productivity, practical/activity, emotional, social, and memory function of patients appear to be significantly impacted by AR.685-689
No high-quality studies have explicitly attempted to establish variations of QOL in AR patients over time, and most have short follow-up periods or only a single follow-up. However, some observations regarding the natural variation in QOL in AR can be extracted from the placebo arms of level 1 studies. Two RCTs have studied the effect of levocetirizine over 6 months.675,677 These RCTs show that over a 6-month period, both the placebo and treatment group experience clinically and statistically significantly improvements in generic and disease-specific QOL; however, the improvement is greater in the treatment arm. The AIT RCTs have longer follow-up periods (12 to 18 months) and show similar results, with placebo patients either staying at their baseline QOL impairment, or improving to a lesser degree than the treatment arms.678,679 As expected in patients with SAR, QOL is better outside of peak season and worsens during allergen exposure.690,691
Aggregate Grade of Evidence: B (Level 1b: 11 studies; Level 2a: 2 studies; Level 2b: 16 studies; Level 2c: 1 study; Level 3b: 3 studies; Table VII.A.1).
Benefit: Successful management of AR leads to improved overall and disease-specific QOL.
Harm: Management strategies for AR are associated with variable levels of harm and are further specified in Section IX. Management.
Cost: Management strategies for AR are associated with variable levels of cost and are further specified in Section IX. Management.
Benefits-Harm Assessment: The benefits of treating patients with AR to improve QOL may outweigh risks of treatment.
Value Judgments: Successful control of AR symptoms leads to important improvements in generic and disease specific QOL.
Policy Level: Recommend treatment of AR to improve QOL.
Intervention: AR patients may be offered various management strategies to improve general and disease-specific QOL.
VII.A.2. Effect on sleep
Like generic and disease-specific QOL, validated tools exist for the assessment of sleep-related QOL in AR, but they are not always utilized in studies reported in the AR literature. Some studies evaluating generic and disease-specific QOL suggest that AR negatively impacts patients’ sleep673,685,687 (Table VII.A.1). Several studies have specifically investigated the relationship between AR and sleep in adults and children (Table VII.A.2-1 and Table VII.A.2-2). The general conclusion from the aggregate data is that, like overall and rhinitis-specific QOL, AR negatively impacts sleep QOL and the successful treatment of AR reduces sleep disturbance. The overall quality of the data is higher for adults than for children. For the adult population, there is level 1b evidence supporting the conclusion that AR negatively impacts sleep.705-709 These data deal with subjective reporting of daytime sleepiness, sleep quality, and symptoms usually through validated tools, in the setting of testing the effect of nasal corticosteroids and/or montelukast. Results demonstrate that AR patients have improvements in sleep quality and daytime sleepiness, in addition to sinonasal symptoms and QOL after treatment with nasal corticosteroids705,706,709,710 or a combination of corticosteroids and montelukast.709 Additionally AR has been associated with worse sleep fragmentation711,712 and snoring.713,714 Treatment of AR has been also suggested to also improve continuous positive airway pressure (CPAP) compliance in patients with OSA.715 The data on the effects of AR on polysomnogram (PSG) parameters in adults is mixed. Most studies that included PSG analysis found that AR is associated with worsened PSG parameters712,714,716-719; however, 2 level 3b studies found either no difference or a modest change.720,721
TABLE VII.A.2-1.
Effect of allergic rhinitis on sleep in adults
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Shanqun et al.709 | 2009 | 1b | RCT | AR and OSA (n = 89):
|
ESS, RQLQ, TSS, CSAQLI, symptoms diary | Montelukast + budesonide improves AR and OSA QOL, sleep quality, and daytime somnolence. |
| Mansfield & Posey708 | 2007 | 1b | RCT |
|
TOVA, ESS, TSS | Fluticasone improves daytime sleepiness, cognitive performance, and nasal symptoms. |
| Gurevich et al.705 | 2005 | 1b | RCT, crossover | PAR (n = 26), nasal budesonide | ESS, sleep diary, questionnaire | Budesonide reduces nasal congestion, daytime somnolence/fatigue, and improves sleep quality in PAR. |
| Hughes et al.706 | 2003 | 1b | RCT, crossover | PAR (n = 22), nasal budesonide vs placebo | ESS, FOSQ, RQLQ, symptom diary | Budesonide improves daytime fatigue and sleep quality in PAR. |
| Craig et al.707 | 1998 | 1b | RCT, crossover | AR (n = 20), flunisolide vs placebo | Symptom and sleep diary | Nasal corticosteroids improve symptoms and subjective sleep compared to controls. |
| Parikh et al.715 | 2014 | 2b | Observational cohort | OSA and rhinitis (n = 43) | ESS, symptoms scores, CPAP compliance | Control of rhinitis (with varying regimens of steroid sprays, antihistamines, leukotrienes inhibitors, anticholinergics, etc.) important for OSA control. No difference: AR vs NAR. |
| Acar et al.716 | 2013 | 2b | Observational cohort | OSA and AR (n = 80) | ESS, PSG | Nasal corticosteroids improve sleep quality and AR symptoms. Addition of antihistamine did not have effect. |
| Lavigne et al.717 | 2013 | 2b | Observational cross-sectional |
|
PSG, nasal biopsies | In AR, nasal corticosteroids reduce nasal inflammation and improve PSG parameters. |
| Udaka et al.723 | 2007 | 2b | Observational cross-sectional | Daytime workers (n = 3442) | Questionnaire, ESS, SF-36 | Severity of nasal obstruction (nonvalidated questionnaire) correlates with worse ESS and lower QOL. |
| Mintz et al.724 | 2004 | 2b | Individual cohort | AR (n = 651) | Nocturnal RQLQ, PSQI | Treatment with triamcinolone improves nocturnal rhinitis QOL and sleep quality. |
| Camhi et al.713 | 2000 | 2b | Case-control | n = 437 from TESOAD with sleep problems/snoring | Questionnaire | AR is a risk factor for snoring. |
| Janson et al.725 | 1996 | 2b | Observational cross-sectional | n = 2661 random population of the ECRHS | SPT, methacholine challenge, questionnaire | AR independently associated with difficulty initiating sleep and daytime sleepiness (OR 2.0). |
| Colas et al.726 | 2012 | 2c | Population-based | AR (n = 2275) | TSS, RQLQ, PSQI | AR disease severity has strong relationship with sleep disturbance. |
| Leger et al.727 | 2006 | 2c | Population-based | AR (n = 591) | SDQ, ESS, symptom score | All dimensions of sleep impaired by AR, disease severity correlated with degree of sleep impairment. |
| Young et al.714 | 1997 | 2c | Population-based | Survey subjects (n = 4297); objective testing subjects (n = 911) | Questionnaire, PSG | AR and nasal obstruction associated with snoring, daytime sleepiness, and SDB. |
| Bozkurt et al.721 | 2017 | 3b | Case-control |
|
SPT, PSG | PAR did not affect PSG findings compared to controls. |
| Gadi et al.728 | 2017 | 3b | Observational cross-sectional | Sleep clinic patients (n = 157) | History, laboratory testing | 62% OSA; 53% AR in OSA. No difference in AR/atopy between OSA and non-OSA cohorts. |
| Park et al.729 | 2012 | 3b | Observational cross-sectional |
|
ESS, stress score, fatigue score, coping score, RQLQ | AR in OSA increases stress and fatigue, worsens sleepiness and QOL. |
| Meng et al.720 | 2011 | 3b | Case-control |
|
PSG | PSG parameters showed modest changes in PAR patients. |
| Rimmer et al.711 | 2009 | 3b | Observational cohort |
|
Actigraphy | AR has increased sleep fragmentation and reduced sleep quality. |
| Canova et al.730 | 2004 | 3b | Case-control |
|
Symptom score, spirometry, SPT | OSA more likely to be sensitized to perennial allergens (11% in OSA vs 2.3% COPD). |
| Stuck et al.731 | 2004 | 3b | Observational cohort |
|
ESS, SF-36, PSG | SAR leads to increased daytime sleepiness compared to controls. |
| Krouse et al.719 | 2002 | 3b | Exploratory cohort |
|
PSG, serum and nasal cytokines | Differing cytokine levels associated with variations in PSG. |
| Lavie et al.712 | 1981 | 3b | Observational cohort |
|
PSG | AR patients had 10-fold increase in microarousals compared to controls. |
| McNicholas et al.718 | 1982 | 4 | Case series | AR (n = 7) | Nasal resistance, PSG | AR patients have worse OSA symptoms when symptoms are present and have high nasal resistance. |
AR = allergic rhinitis; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; CSAQLI = Calgary Sleep Apnea Quality of Life Index; ESS = Epworth Sleepiness Scale; FOSQ = Functional Outcomes of Sleep Questionnaire; LOE = level of evidence; NAR = non-allergic rhinitis; OR = odds ratio; OSA = obstructive sleep apnea; PAR = perennial allergic rhinitis; PSG = polysomnogram; PSQI = Pittsburgh Sleep Quality Index; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; SDB = sleep disordered breathing; ECRHS = European Community Respiratory Health Survey; SDQ = Sleep Disorders Questionnaire; SF-36 = Short Form 36; SPT = skin-prick test; TESOAD = Tucson Epidemiology Study of Obstructive Airway Disease; TOVA = Test of Variables Attention; TSS = total symptom score.
TABLE VII.A.2-2.
Effect of allergic rhinitis on sleep in children
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kim et al.722 | 2015 | 2b | Individual cohort | SDB undergoing T&A (n = 70) | OSA-18, SPT, questionnaire | AR may be risk factor for deterioration of OSA QOL after T&A. |
| Koinis-Mitchell et al.732 | 2015 | 2b | Individual cohort | Non-white Latino and African American urban children (n = 195) | Clinical evaluation and follow-up | Poor AR and asthma control related to high frequency of sleep problems and poor sleep hygiene. |
| Barone et al.733 | 2009 | 2b | Case-control |
|
PSG | AR associated with OSA, OR 2.24. |
| Lin et al.734 | 2013 | 3a | Systematic review | N/A | Association between AR and SDB | Most studies show association between AR and SDB in children, but all studies were low level of evidence. |
| Di Francesco et al.735 | 2016 | 3b | Cross-sectional | SDB undergoing T&A (n = 135) | PSG | AR affected REM sleep in children with SDB without OSA. AR is not an aggravating factor in AHI severity. |
| Chimenz et al.736 | 2015 | 3b | Case-control |
|
History | AR may influence development of nocturnal enuresis. |
| Poachanukoon et al.737 | 2015 | 3b | Case-control |
|
Questionnaire | Higher incidence of sleep disturbance in AR. |
| Kwon et al.738 | 2013 | 3b | Population-based | Children with AR (n = 85,002) | National survey data | Association between late sleep time and short sleep duration with AR. |
| Li et al.739 | 2010 | 3b | Cross-sectional | Children (n = 6,349) | Questionnaire | Habitual snoring associated with AR (OR 2.9; 95% CI, 2.0–4.2). |
| Vichyanond et al.740 | 2010 | 3b | Case-control | Children with rhinitis (n = 302) | History | Upper airway obstruction associated with NAR. |
| Sogut et al.741 | 2009 | 3b | Cross-sectional | Turkish children (n = 1,030) | Questionnaire | AR associated with habitual snoring (OR 3.7; 95% CI, 1-13). |
| Liukonnen et al.742 | 2008 | 3b | Population-based | Children in Helsinki (n = 2,100) | Questionnaire | AR more common in snorers. |
| Kalra et al.743 | 2006 | 3b | Cross-sectional | Children in CCAAPS (n = 681) | Questionnaire | 29% of patients with HS have positive SPT, significant association. |
| Ng et al.744 | 2005 | 3b | Cross-sectional | School children (n = 3,047) | Questionnaire | AR associated with witnessed apnea. |
| Sogut et al.745 | 2005 | 3b | Cross-sectional | Turkish children (n = 1,198) | Questionnaire | AR associated with habitual snoring (OR 4.23; 95% CI, 2.14–8.35). |
| Chng et al.746 | 2004 | 3b | Cross-sectional | School children (n = 11,114) | Questionnaire | Snoring in 34%, AR associated with snoring (OR 2.9; 95% CI, 2.06–4.08). |
| Anuntaseree et al.747 | 2001 | 3b | Cross-sectional | Randomly selected children (n = 1,142) | PSG, questionnaire | Prevalence habitual snoring 8.5%, OSAS 0.69%; OR 5.27 in children with AR. |
| Bhattacharjee et al.748 | 2010 | 4 | Prognostic cohort | Children undergoing AT for OSA (n = 578) | PSG | AR identified in 39% of children with OSA undergoing AT. |
| Goldbart et al.749 | 2005 | 4 | Case series | SDB (n = 24) | PSG, lateral neck X-ray | Montelukast treatment for 16 weeks decreased adenoid size and respiratory sleep disturbances. |
| Kidon et al.750 | 2004 | 4 | Case series | Children with AR undergoing SPT (n = 202) | History | 17% of AR patients reported HS. |
| Mansfield et al.751 | 2004 | 4 | Case series | Children with AR (n = 14) | PSG, RQLQ | Treating AR decreases AHI. |
| McColley et al.752 | 1997 | 4 | Case series | Children with HS (n = 39) | PSG | Positive skin test associated with OSA. |
AHI = apnea-hypopnea index; AR = allergic rhinitis; AT = adenotonsillectomy; CCAAPS = Cincinnati Allergy and Air Pollution Study; CI =confidence interval; HS =habitual snoring; LOE = level of evidence; NAR = non-allergic rhinitis; OR = odds ratio; OSA = obstructive sleep apnea; OSA-18 = 18-item quality-of-life survey for obstructive sleep apnea; OSAS = obstructive sleep apnea syndrome; PSG = polysomnogram; QOL = quality of life; REM = rapid eye movement; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SDB = sleep disordered breathing; SPT = skin prick test; T&A = tonsillectomy and adenoidectomy.
Two studies looked at variations in sleep symptoms with changes in nasal inflammation over time. It seems that changes in nasal cytokine levels are associated with changes in PSG719 and that AR patients have worse PSG parameters and sleep disturbance when their symptoms are present or during their peak allergen season.718 In children, level 2 and 3 studies suggest that AR is associated with sleep disturbance in the form of increased risk of snoring, sleep disordered breathing, and OSA. Furthermore, AR has been suggested to be a risk factor for deterioration of OSA QOL after adenotonsillectomy.722 (See section X.K. Associated conditions – Sleep disturbance and obstructive sleep apnea for additional information on this topic.)
Aggregate Grade of Evidence: B (Level 1b: 5 studies; Level 2b: 10 studies; Level 2c: 3 studies; Level 3a: 1 study; Level 3b: 21 studies; Level 4: 6 studies; Tables VII.A.2-1 and VII.A.2-2).
Benefit: Successful management of AR leads to decreased sleep disturbance.
Harm: Management strategies for AR are associated with variable levels of harm and are further specified in Section IX. Management.
Cost: Management strategies for AR are associated with variable levels of cost and are further specified in Section IX. Management.
Benefits-Harm Assessment: The benefits of treating patients with AR for symptoms of sleep disturbance may outweigh risks of treatment.
Value Judgments: Successful control of AR symptoms leads to improvements in sleep.
Policy Level: Recommend treatment of AR to decrease sleep disturbance.
Intervention: AR patients may be offered various management strategies to improve sleep.
VII.B. Societal burden
As described in Section VII.A.1, AR may have significant negative effects on QOL with considerable consequences if left untreated. For many years, AR has been trivialized despite its prevalence, chronicity, and the burden it imposes on individuals and society.101,681,753 The total burden for AR lies not only in the impairment of physical and social functioning, but also in the financial burden, which is greater when its role in comorbid conditions such as asthma and rhinosinusitis are taken into account.754-756 In Europe, the total societal cost of AR and its comorbidities in 2002 was estimated at 355.06 Euros per patient per month.755 The burden of AR is now being recognized by the European Academy of Allergy & Clinical Immunology (EAACI) and also at the European Union (EU) parliament level in order to feature the dramatic impact this condition has on the QOL of patients with AR.757,758
In terms of the overall economic burden of illness, AR ranks fifth among chronic conditions in the United States.759 Estimates of the annual direct cost of AR range from $2 billion to $5 billion, with more than one-half of the AR direct costs coming from prescription medications.760-762 The direct costs attributed to AR include physician office visits, laboratory tests, medications, and AIT.763 Compared with matched controls, patients with AR have an almost 2-fold increase in medication costs and a 1.8-fold increase in visits to a healthcare provider.756,764,765 Hidden direct costs include treatment of comorbid conditions that occur at an increased incidence in patients with AR.
Recently, the TOTALL (TOTal costs of ALLergic rhinitis in Sweden) study estimated the total cost of AR using a sample representing the entire Swedish working-age population. Data from this study suggested that patients with mild AR have less impact on the health economy, with costs averaging about 25% of the costs for those with moderate to severe disease.667,766 Patients with moderate to severe AR reported visiting their primary care provider for their AR more frequently than those with mild AR (1.61 vs 1.19 times per year).753
The indirect costs of AR, such as absenteeism and presenteeism, are also significant and actually make up the majority of the cost burden of AR.767,768 Impaired productivity and/or missed work occurred as a result of AR in 52% of patients.753 In a survey of over 8000 U.S. employees at 47 employer locations, 55% reported AR symptoms for an average of 52.5 days per year. They reported missing 3.6 days of work per year because of AR and reported being unproductive 2.3 hours per workday when symptomatic. The mean total productivity losses (absenteeism and presenteeism) for AR were calculated at $593 per employee per year.769 In another UK study, patients with moderate to severe AR reported 37.7 days a year when their productivity was affected by their AR symptoms; this is almost double that reported by patients in the same study with mild AR symptoms (21.0 days).753
Health impairments associated with AR are often not severe enough to cause absenteeism, but they do interfere with cognitive functioning, resulting in fatigue and an impaired ability to learn, concentrate, and make decisions.770 In a study by Blanc et al.,771 more than one-third of AR patients reported reduced workplace performance.
In the United States, AR results in 3.5 million lost workdays and 2 million lost school days annually.772 On any given school day in the United States, approximately 10,000 children are absent from school because of AR.773 This absence from school may also affect parents’ productivity or cause them to be absent from work themselves.
In a study by Hellgren et al.,774 the average productivity loss for all Swedish workers because of absenteeism, presenteeism, and caregiver absenteeism during a year was 5.1 days, of which 2.3 days were accounted for by absenteeism and 2.0 days by presenteeism. If only those with children aged 0 to 7 years in their household were included in the analyses, the average number of days for caregiver absenteeism was 3.6 days. The cost of caregiver absenteeism comprised 19% of the mean total costs per year in this study. The cost related to caregiver absenteeism was highest for women aged 30 to 44 years.
AR is the most common chronic disorder in the pediatric population. AR can affect sleep, result in daytime sleepiness, and impair cognition and memory, which may significantly affect the learning process and impact school performance. Even when present during school hours, children with AR exhibit decreased productivity. Comorbidities associated with AR, such as like rhinosinusitis, Eustachian tube dysfunction, and associated conductive hearing loss may further contribute to learning dysfunction.775,776
AR poses a substantial burden to individuals and society. It can reduce productivity and QOL in affected patients, and contribute to comorbid conditions. This results in a significant impact to the overall health system.773
VIII. Evaluation and diagnosis
In an individual patient, the clinical suspicion for a diagnosis of AR is highlighted by the clinical history and often supported by the physical examination. The diagnosis is confirmed by objective testing, which may be performed by various means. This section reviews the existing evidence behind various aspects of evaluation and diagnosis of the AR patient.
VIII.A. Clinical examination History
Clinical history is an essential part of the evaluation of patients with a suspected diagnosis of AR.7,26,218,761,777 History taking includes the type of symptoms experienced, timing and duration of symptoms, frequency of symptoms, any environmental exposures eliciting symptoms at home/work/school, and medications or other measures that relieve or exacerbate symptoms.7,26,218,761,777,778 In addition, past medical history including comorbid conditions such as asthma or obstructive sleep apnea, family history of atopic disorders, social history (ie, pets, work exposures, home environment), and current medications should be obtained.7,26,218,761,777,778 Information regarding patient response to self-treatment with over-the-counter medications for AR is also helpful.
Nasal congestion or obstruction, nasal pruritis, clear rhinorrhea, and sneezing are classic symptoms of AR.7,26,218,761,777,778 Patients may complain of associated symptoms of ocular pruritis, erythema, and/or tearing, oral cavity or pharyngeal pruritis, and wheezing or cough (reactive airway disease and/or asthma).7,26,778 Additional associated symptoms may include hyposmia or anosmia, snoring or sleep-disordered breathing, aural congestion or pruritis, and sore throat.778,779 Commonly, patients with suspected AR will present with multiple complaints, with 96% presenting with 2 or more symptoms.778 Patients with PAR tend to report more congestive symptoms (sinus pressure, nasal block-age/congestion, and snoring) than patients with SAR. Patients with persistent AR are more likely to report the presence of sore throat, cough, sneezing, rhinorrhea, and postnasal drip.778 Rhinorrhea, sneezing, sniffing, hyposmia/anosmia, nasal obstruction, and itchy nose rank highest for diagnostic utility among symptoms of AR.779
Several guidelines suggest the diagnosis of AR be made when patients present with a history consistent with an allergic cause and 1 or more of the symptoms listed in the previous paragraph, despite the lack of high-level evidence to support such a recommendation7,26,218,761,777,780 (Table VIII.A). However, the lack of higher level evidence is not surprising as a clinical history and physical examination is essential to any medical diagnosis and randomized studies would require participants to receive an intervention without a clinical history. Using a physical examination alone to diagnose AR has been shown to have poor predictive value.781 The reliability and predictive value of the patient history alone for AR exceeds that of the physical exam alone.781 In clinical practice, the diagnosis of AR is often made by history alone.780
TABLE VIII.A.
Evidence for the role of history taking and physical examination in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Raza et al.781 | 2011 | 3b | Cross-sectional | Adults with AR | History, physical examination, SPT | Physical examination alone yields unreliable and inconsistent results in diagnosing AR. |
| Costa et al.780 | 2011 | 4 | Cohort study | Adults with AR | Physician interview and structured questionnaire | Many patients diagnosed on history alone without confirmatory testing. |
| Shatz778 | 2007 | 4 | Survey |
|
Self-completed patient questionnaire, physician patient record form | Persistent AR patients reported more symptoms than intermittent AR patients. |
| Ng et al.779 | 2000 | 4 | Case-control | Adults with AR | History, physical examination, SPT, sIgE | Rhinorrhea, sneezing, sniffing, impaired sense of smell, blocked nose, edematous nasal mucosa, and itchy nose ranked highest in diagnostic utility. Physical examination performed to eliminate other potential causes of symptoms. |
| Seidman et al.761 | 2015 | 5 | Guideline | Recommendations on diagnosis and treatment of AR | Clinical diagnosis of AR made with a history and physical examination consistent with AR. | |
| Wallace et al.26 | 2008 | 5 | Guideline | Recommendations on the diagnosis and treatment of rhinitis | Thorough allergic history remains the best diagnostic tool available. All organ systems potentially affected by AR should be examined. Typical allergic exam findings are supportive but not specific. | |
| Small et al.777 | 2007 | 5 | Guideline | Recommendations on diagnosis and treatment of rhinitis | History of allergic symptoms is essential in the diagnosis of AR. Physical exam aids in supporting the diagnosis of AR. | |
| Bousquet et al.7 | 2001 | 5 | Guideline | Recommendations on the diagnosis and treatment of AR in asthmatic patients | Symptom type and timing (obtained through history) is essential to correct diagnosis. Lung exam is recommended in asthmatic patients with symptoms of AR. |
AR = allergic rhinitis; LOE = level of evidence; sIgE = antigen-specific immunoglobulin E; SPT = skin prick test.
Physical examination
Physical examination is part of the evaluation of patients with suspected AR.7,26,218,761,777 This includes an assessment of the multiple organ systems of the head and neck, such as the integumentary system; external auditory canal, tympanic membrane, and middle ear; nasal cavities; orbits and periorbital tissues; oral cavity and pharynx; larynx via indirect laryngoscopy; and cervical tissues.26,218,761,777 It may include auscultation of the lungs, given comorbid conditions of asthma, or complaints of wheezing or coughing with exposure.7
It is not uncommon for physical examination of patients with AR complaints to be completely normal, particularly in patients with intermittent exposure.779 However, physical signs suggestive of AR may include mouth-breathing, nasal itching, or a transverse supratip nasal crease, throat clearing, periorbital edema, or “allergic shiners” (dark discoloration of the lower lids and periorbital area).26,777 Examination of the ear may reveal retraction of the tympanic membrane or transudative fluid.26,218,777 Examination of the nose may reveal inferior turbinate hypertrophy, congested/edematous nasal mucosa, purplish or bluish nasal mucosa, and clear rhinorrhea.26,218,761,777 Examination of the eyes may reveal conjunctival erythema and/or chemosis.26,777
Physical examination alone is poorly predictive and more variable when compared to history taking in the diagnosis of AR, with the average sensitivity, specificity, positive predictive value, and negative predictive values of the patient history higher than those of the physical examination.781 Most guidelines recommend a physical examination as part of the diagnosis of AR, despite a lack of high-level evidence. Without a physical examination, other potential causes of symptoms such as CRS, could not be fully evaluated or eliminated. A patient history combined with a physical examination improves diagnostic accuracy.781
Aggregate Grade of Evidence: D (Level 3b: 1 study; Level 4: 3 studies; Level 5: 4 guidelines; Table VIII.A).
Benefit: Improve accuracy of diagnosis, avoid unnecessary referrals, testing, or treatment. Possible improved diagnosis of AR with physical examination findings, evaluation/exclusion of alternative diagnoses.
Harm: Possible patient discomfort from routine examination, not inclusive of endoscopy. Potential misdiagnosis, inappropriate treatment.
Cost: Minimal.
Benefits-Harm Assessment: Preponderance of benefit over harm, potential misdiagnosis and inappropriate treatment if physical exam used in isolation.
Value Judgments: Making a presumptive diagnosis of AR on history (ideally combined with physical examination) is reasonable and would not delay treatment initiation. Confirmation with diagnostic testing is required for progression to AIT, or desirable with inadequate response to initial treatment.
Policy Level: Recommendation.
Intervention: History taking is essential in the diagnosis of AR. Physical examination is recommended in the diagnosis of AR, and when combined with patient history, it increases diagnostic accuracy and excludes alternative causes.
VIII.B. Nasal endoscopy
Diagnostic nasal endoscopy is an option for the evaluation of patients with suspected AR. Several uncontrolled observational studies evaluated the association of endoscopic findings with symptomatic rhinitis, with inconsistent results (Table VIII.B). Ameli et al.782 evaluated children with suspected AR, reporting that endoscopic findings of inferior or middle turbinate septal contact was predictive for AR, while pale turbinates were not. Conversely, Eren et al.783 evaluated a population of adult patients with rhinitis, concluding that findings of nasal endoscopy do not provide a reliable diagnosis of AR. Among adults and children with AR that is confirmed by allergy testing, no significant correlation was found between nasal endoscopy and specific nasal symptoms.784
TABLE VIII.B.
Evidence for the role of nasal endoscopy in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Hamizan et al.786 | 2016 | 3b | Cross-sectional | Adults with rhinitis and nasal obstruction | Nasal endoscopy, allergy testing | MT edema is a useful nasal endoscopic feature to predict presence of inhalant allergy. |
| White et al.785 | 2014 | 3b | Cross-sectional | Adults with isolated MT polypoid edema | Nasal endoscopy, allergy testing | Isolated MT polypoid edema is associated with positive allergy testing. |
| Eren et al.783 | 2013 | 4 | Case series | Adults with rhinitis | Nasal endoscopy, AR diagnosis | Nasal endoscopic findings do not provide reliable diagnosis of AR. |
| Ameli et al.782 | 2011 | 4 | Case series | Children with suspected AR | Nasal endoscopy, AR diagnosis | Inferior or middle turbinate septal contact was predictive for AR, whereas pale turbinates were not. |
| Jareoncharsri et al.784 | 1999 | 4 | Case series | Adults and children with PAR | Nasal endoscopy, nasal symptoms | No significant correlation between individual symptoms and endoscopic findings. |
AR = allergic rhinitis; LOE = level of evidence; MT = middle turbinate; PAR = perennial allergic rhinitis.
Central compartment atopic disease (CCAD) represents the recently described association between atopic states and centrally-located inflammation involving the middle/superior turbinates or superior nasal septum.785-787 In a recently published parallel case series (LOE = 4), Brunner et al.788 evaluated patients with CRSwNP vs isolated polypoid change of the middle turbinate. Significant findings include a higher prevalence of AR in patients with middle turbinate polypoid change (83% vs 34%, p < 0.001), further supporting CCAD as a unique atopic condition.
Although the association of endoscopic findings with AR has been shown to be inconsistent, nasal endoscopy may aid in the identification or exclusion of other possible causes of symptoms, such as nasal polyposis or CRS.
Aggregate Grade of Evidence: D (Level 3b: 2 studies; Level 4: 3 studies; Table VIII.B).*
Benefit: Possible improved diagnosis with visualization of turbinate contact or isolated central compartment edema.
Harm: Possible patient discomfort.
Cost: Moderate equipment and processing costs, as well as procedural charges.
Benefits-Harm Assessment: Equal.
Value Judgments: None.
Policy Level: Option.
Intervention: Nasal endoscopy may increase diagnostic sensitivity among children and adults with AR and may aid in ruling out other causes for nasal symptoms.
*Due to recent publication and in accordance with ICAR methodology, DelGaudio et al.787 and Brunner et al.788 are excluded from the Aggregate Grade of Evidence.
VIII.C. Radiology
Routine radiographic imaging is not recommended for the diagnosis of AR, although may be considered to rule in/out other conditions (ie, rhinosinusitis). Some recent studies have established the association between central compartment mucosal disease and aeroallergen sensitivity.787,788 However, concerns regarding unnecessary exposure to ionizing radiation, with the risk for future cancer development, preclude recommendations for routine use.789,790
Aggregate Grade of Evidence: Not applicable.*
Benefit: None appreciated.
Harm: Unnecessary radiation exposure with concern for tumor development.
Cost: High equipment and processing costs.
Benefits-Harm Assessment: Preponderance of harm over benefit.
Value Judgments: Long-term risks of unnecessary ionizing radiation exposure outweigh potential benefit.
Policy Level: Recommend against.
Intervention: Routine imaging is not recommended in the evaluation of suspected AR, but may be considered to rule in/out other sinonasal conditions.
*Due to recent publication and in accordance with ICAR methodology, DelGaudio et al.787 and Brunner et al.788 are excluded from the Aggregate Grade of Evidence.
VIII.D. Use of validated survey instruments
Validated clinical outcome surveys and questionnaires may be used as precise clinical assessment instruments to evaluate patients with suspected AR. Clinicians often use SPT, sIgE serology, and other laboratory tests to confirm or refute the diagnosis, but these tests are only useful in the context of an effective clinical history.791 Validated clinical assessment tools offer a more structured way to expose important historical elements. Furthermore, in regions where resources are scarce, SPT and laboratory testing may not be as readily available. Advancing technologies such as multiplex allergen screening, component serology, and automated SPT imaging devices may be expensive and unattainable by some clinicians.792-795 In these settings, validated surveys offer a rapid and simple point-of-care tool to formally evaluate allergic disease.
Patient reported outcome measures (PROMs) can assess a number of different aspects of how AR affects patients.796 These include symptom severity surveys, such as the Total Nasal Symptom Score (TNSS) and health-related QOL questionnaires, such as the RQLQ. Additional surveys measure aspects such as medication usage (Daily Medication Score), disease prediction (Respiratory Allergy Prediction) and disease control (Rhinitis Control Test). Each of these surveys examines slightly different, although related aspects of clinical outcomes. Several of these instruments have been used extensively in many large clinical trials to determine the effectiveness of drugs and biologics for treating AR.797-802 SPT and nasal challenge may be used to cross-validate these clinical survey tools but ultimately, how a patient reports their own symptoms could very well be the best predictor of disease control.
Validated clinical surveys for AR often include questions about congestion, rhinorrhea and/or sneezing and may either be instantaneous or reflective over a period of days or weeks. The TNSS is typically administered as an instantaneous daily survey comprised of only 4 questions about runny nose, nasal itching, sneezing, and congestion. Some studies have used the TNSS as a reflective score calculated as the average of both the 12-hour nighttime and 12-hour daytime average (rTNSS). The TNSS score can be combined with questions about rescue medication use to yield the Daily Combined Score (DCS) and the Total Combined Rhinitis Score (TCRS). Both have been used in many therapeutic intervention studies.803 The RQLQ is a more comprehensive survey that asks the patient to reflect upon the past week and includes global QOL questions. While this test can suffer somewhat from potential recall bias, it can be administered on site and avoids the possibility that self-administered daily scores could be missed periodically when the patient is home. The Control of Allergic Rhinitis and Asthma Test (CARAT10) evaluates rhinoconjunctivitis and asthma symptoms over the past 4 weeks giving a broader evaluation of seasonal symptom control.804 The Respiratory Allergy Prediction (RAP) test is a 9-question survey incorporating upper and lower respiratory queries as well as a question about medication use. If conjunctivitis is to be assessed simultaneously with rhinitis symptoms, then the Rhinitis Total Symptom Score (RTSS) can be combined with Rescue Medication Score (RMS) to yield the combined score (CS).805 Table VIII.D-1 lists several validated clinical survey tools.696,804,806-813
TABLE VIII.D-1.
Validated surveys used to diagnose AR or evaluate disease severity and treatment
| Survey | Disease targeted | Number of questions |
Symptom questions |
Medication questions |
Scoring range |
Comments and indications |
|---|---|---|---|---|---|---|
| TNSS: Total Nasal Symptom Score | AR | 4 | Yes | No | 0–12 | Simple daily symptom score to evaluate AR severity and control used in clinical trials |
| DMS: Daily Medication Score | AR, AC, asthma | Varies | No | Yes | 0–36a | Varies depending on medication scoring |
| DCS: Daily Combined Score | AR, AC, asthma | Varies | Yes | Yes | 0–48a | Combined symptom and medication score for clinical trials |
| TCRS: Total Combined Rhinitis Score | AR | Varies | Yes | Yes | 0–24a | The sum of the combined symptoms medication scores |
| Mini-RQLQ: Mini-Rhinoconjunctivitis Quality of Life Questionnaire | Rhinoconjunctivitis | 14 | Yes | No | 0–84 | Shortened version of RQLQ often used in clinical trials |
| RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire | Rhinoconjunctivitis | 28 | Yes | No | 0–168 | Reflective assessment of previous week’s symptoms often used in clinical trials |
| VAS: Visual Analogue Scale | Rhinitis | 1 or more | Yes | No | 0–10 cm | Tool may be used to evaluate multiple symptomatologies |
| RCAT: Rhinitis Control Assessment Test | AR, NAR | 6 | Yes | No | 6–30b | Self-assessment of rhinitis symptom control |
| ARCT: Allergic Rhinitis Control Test | AR | 5 | Yes | Yes | 5–25b | Self-assessment of ongoing AR symptoms control |
| CARAT10: Control of Allergic Rhinitis and Asthma Test | AR, NAR, asthma | 10 | Yes | Yes | 0–30b | Used to compare groups in clinical trials |
| ACS: Allergy Control Score | Rhinitis, AC, asthma | 10+ meds | Yes | Yes | 0–60 | Combined tool used for clinical trials and daily clinical practice |
| RC-ACS: Rhinoconjunctivitis Allergy Control Score | Rhinitis, AC | 7+ meds | Yes | Yes | 0–42 | Similar to ACS but without asthma related questions |
| RAP: Respiratory Allergy Prediction | AR, asthma | 9+ meds | Yes | Yes | 0–9 | Used to determine the need for referral and additional testing |
| SFAR: Symptom Score For Allergic Rhinitis | AR | 8 | Yes | No | 0–16 | Weighted score used to detect prevalence of AR |
| RMS: Rescue Medication Score | Rhinoconjunctivitis | Meds | No | Yes | 0–3 | Evaluates medication use only |
| RTSS: Rhinoconjunctivitis Total Symptom Score | Rhinoconjunctivitis | 6 | Yes | No | 0–18 | Evaluates symptoms only |
| CS: Combined Score | Rhinoconjunctivitis | 6+ meds | Yes | Yes | 0–3 | Combined scores of RTSS/6 + RMS/2 |
| Global Assessment: Global Assessment of Severity of Allergy | Total nasal and non-nasal symptoms | 1 | Yes | No | 1–7 | Single question about rhinitis severity |
Maximum score may vary depending on specific number of symptom related questions and specific medication score included.
Higher score equates to better control of disease. A score of 0 denotes zero control of symptoms.
AC = allergic conjunctivitis; AR = allergic rhinitis; meds = medications; NAR = nonallergic rhinitis.
The choice of which validated survey to use depends on which aspect of clinical outcomes is being studied. For example, if the goal is for a primary care physician to determine the need for referral and further testing, then the RAP test may be used because it has been scrutinized in this setting.814 The mini-RQLQ and DCS have been used extensively in clinical trials to evaluate the effectiveness of drugs and immunotherapies,797-801 and therefore may be helpful in selecting the right medication for a given population. It is important to note that some tools use a higher score to indicate severe disease whereas other tools use a higher score to indicate better control of symptoms. For example, a high score on the RCAT, ARCT, and CARAT10 indicate good control of allergic symptoms.
Unfortunately, not all studies use consistent terminology and interpretation of the scoring systems.801 Inconsistent use of questionnaires can weaken the conclusions drawn in certain therapeutic intervention studies. However, a well-executed and validated survey can be essential in research settings and help clinicians screen patients for AR and further render specific diagnostic decisions.
Overall, validated clinical survey instruments may be used as a tool to assist with the diagnosis of AR and determine the success of various therapies. This conclusion is based on review of more than 30 studies of which 9 of these reports range from level 1a to 2b (overall Grade A evidence) (Table VIII.D-2). An example approach using specific validated survey instruments is as follows. The TNSS may be used for daily symptom monitoring to determine the effectiveness of therapies and control of AR. The TNSS should be combined with a daily medication score to account for the effects of pharmaceuticals on symptomatology. Assessment of both conjunctivitis and rhinitis symptoms as well as medication use can be performed with the Combined Score (RTSS + RMS) or the Rhinoconjunctivitis Allergy Control Score (RC-ACS). The RQLQ or mini-RQLQ can be used as an additional measure to incorporate disease impact on QOL and can be administered in person by the clinician. For quick assessments or to follow a patient’s therapeutic success, a simple visual analogue scale (VAS) or global assessment is acceptable. The RAP test can be used as quick and easy tool for primary care physicians to determine the need to refer to an allergist for further testing. Many validated options are available for AR and should be tailored to the patient and clinical setting.
TABLE VIII.D-2.
Evidence for the role of validated survey instruments in the evaluation, diagnosis, and follow-up of allergic rhinitis
| Study | Year | LOE | Study design |
Study groups |
Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Di Bona et al.815 | 2015 | 1a | Systematic review | ARC | Meta-analysis of grass SLIT efficacy | Combined symptom and medication score showed efficacy of grass SLIT. |
| Calderon et al.801 | 2014 | 1a | Systematic review | AR | Comparison of scoring systems | TNSS and combined medication scores should be used in clinical trials. |
| Demoly et al.803 | 2016 | 1b | DBRPCT | AR | Efficacy of HDM SLIT tablet | TCRS confirmed efficacy of SLIT. |
| Zieglmayer et al.798 | 2016 | 1b | RCT | AR | Efficacy of B-cell vaccine | TNSS score used to determine efficacy in large study. |
| Klimek et al.805 | 2015 | 1b | RCT | ARC | Effectiveness of recombinant birch SCIT | Combined score and VAS revealed no difference between recombinant and standard birch SCIT. |
| Mosbech et al.799 | 2015 | 1b | RCT | AR | Efficacy of HDM SLIT for AR | RQLQ used effectively in this evaluation. |
| Devillier et al.802 | 2016 | 2b | Cohort | AR | Evaluation of AR by VAS, RTSS and RQLQ | Comparison of various outcome measures validates their utility. |
| Galimberti et al.814 | 2015 | 2b | Cohort | AR, AC, asthma | Evaluation of RAP test | RAP test is valid for screening allergic disease |
| Devillier et al.813 | 2014 | 2b | Cohort | ARC | Minimal clinically important difference of RTSS | RTSS vs RQLQ showed minimal clinically important difference of 1. |
| Hafner et al.806 | 2012 | 2b | Cohort | ARC | Evaluation of RC-ACS test in 81 subjects | RC-ACS is a valid test for evaluating ARC without asthma. |
AC = allergic conjunctivitis; AR = allergic rhinitis; ARC = allergic rhinoconjunctivitis; DBRPCT = double-blind randomized placebo controlled trial; HDM = house dust mite; LOE = level of evidence; RAP = Respiratory Allergy Prediction; RC-ACS = Rhinoconjunctivitis Allergy Control Score; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; RTSS = Rhinoconjunctivitis Total Symptom Score; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; TCRS = Total Combined Rhinitis Score; TNSS = Total Nasal Symptom Score; VAS = visual analog scale.
Aggregate Grade of Evidence: A (Level 1a: 2 studies; Level 1b: 4 studies; Level 2b: 4 studies; Table VIII.D-2). Note: multiple additional studies were reviewed, but Grade A evidence was reached with these 10 studies, so an extensive listing of all studies employing validated survey instruments is not provided here.
Benefit: Validated surveys offer a simple point-of-care option for screening and tracking symptoms, QOL, and control of allergic disease.
Harm: Minimal to none.
Costs: No financial burden to patients. Some fees associated with validated tests used for clinical research.
Benefits-Harm Assessment: Preponderance of benefit over harm. Low risk of misdiagnoses leading to unnecessary additional testing. Likewise, there is a low risk that false negative responses may lead to delay in testing and further management.
Value Judgments: Level 1 evidence to use validated surveys as a screening tool and primary or secondary outcome measure.
Policy Level: Strong recommendation.
Intervention: Validated surveys may be used to screen for AR, follow treatment outcomes and as a primary outcome measure for clinical trials. Specific tests are optimized for various clinicopathological scenarios and should be tailored to the patient and clinical setting.
VIII.E.1. Skin-prick testing (SPT)
SPT can be used, along with the history and physical examination, to confirm the diagnosis of AR and differentiate from non-allergic types of rhinitis. The confirmation of an IgE-mediated process guides avoidance measures and appropriate pharmacologic therapy. Skin testing is crucial to directing AIT, and therefore, should be utilized in eligible patients when AIT is being considered. According to the ARIA guidelines, patients should be considered for AIT when they have failed a 2-week to 4-week trial of moderatedose INCS combined with antihistamines.101
When an antigen is applied to the skin of a sensitized patient, the antigen cross-links IgE antibodies on the surface of cutaneous mast cells resulting in degranulation and release of mediators (including histamine), which leads to the formation of a wheal and flare reaction within 15 to 20 minutes.816,817 Given the limited depth of penetration, SPT is safe with very rare reports of anaphylaxis and no reported fatalities.818 SPT can be performed in any age group and is of particular value in pediatric populations given the speed at which multiple antigens can be applied and the limited discomfort experienced during testing.
Skin testing is not appropriate in all patients. Absolute or relative contraindications to SPT include uncontrolled or severe asthma, severe or unstable cardiovascular disease, concurrent beta-blocker therapy, and pregnancy. Certain medications and skin conditions may interfere with skin testing. These are covered in detail in section VIII.E.4. Issues that affect the performance or interpretation of skin tests: VIII.E.4.a. Medications; and VIII.E.4.b. Skin conditions, respectively.
Aside from an excellent safety profile, SPT has a reported sensitivity and specificity around 80%.818-820 It is reported to be more sensitive than serum testing with the added benefit of lower cost.818,821,822 Despite studies aimed at comparing SPT, intradermal testing, and serum testing, conclusive evidence that one type of testing is superior to the others is lacking.761
The number and choice of antigens used in testing varies considerably between clinical practices. A panel of antigens representing an appropriate geographical profile of allergens that a patient would routinely be exposed to is recommended. Positive (histamine) and negative (glycerin or saline) controls should always be included. Variability in quality and potency between commercially available allergen extracts has been demonstrated.823,824 Therefore, whenever possible, standardized allergens should be used.820
SPT is performed with lancets, which come in a variety of forms. Generally, lancets are designed to limit skin penetration depth to 1 mm. However, varying amounts of pressure applied to the delivery device can alter the depth of skin penetration, which ultimately influences the skin reaction to an antigen.825 Prick testing devices can come as single-lancet devices or multiple-lancet devices. Multiple-lancet devices have the advantage of being able to rapidly apply multiple antigens to the skin at 1 time with a more consistent amount of pressure.826,827 Wheal size, sensitivity, and reproducibility all differ from 1 device to another826-828; therefore, any healthcare provider performing SPT must thoroughly familiarize themselves with his/her testing device. Typically, the lancet is dipped into a well containing an antigen and then applied to the skin.
The volar surfaces of the forearms and the back are the most common testing sites for SPT. Choice of site is directed by the age/size of the patient. Tests should be applied 2 cm or greater apart as placing them closer to one another can cause cross-contamination.829 After 15 to 20 minutes, the results are read by measuring the size of the wheal by its greatest diameter. A wheal 3 mm or larger than the negative control is considered positive.
There is a large body of evidence detailing the use of SPT in clinical practice (Table VIII.E.1). Based upon several prospective studies and systematic reviews, SPT has been demonstrated to be a safe method of allergy testing. It is not inferior to serum or intradermal testing and is less expensive than serum testing. It does carry a risk of systemic reaction, so caution should always be exercised. It is also associated with some discomfort during testing; however, the discomfort is generally less than that experienced during intradermal testing. Reviewing the available literature, a preponderance of benefit over harm for SPT exists. Therefore, the use of SPT is recommended in situations where the diagnosis of AR needs to be supported or a patient with presumed AR has failed appropriate empiric medical therapy.
TABLE VIII.E.1.
Evidence for the role of skin-prick testing in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Nevis et al.830 | 2016 | 1a | Systematic review and meta-analysis | Not applicable | Accuracy of SPT | Pooled estimate for SPT sensitivity and specificity was 85% and 77%, respectively. SPT is accurate in discriminating subjects with or without AR. |
| Gungor et al.833 | 2004 | 3b | Prospective case-control |
|
Sensitivity and specificity of SPT vs SET for diagnosing AR | SPT more sensitive (85.3% vs 79.4%) and specific (78.6% vs 67.9%) than SET as a screening procedure for multiple antigens. SPT had a greater PPV (82.9% vs 75%) and NPV (81.5% vs 73%) than SET. None of these differences were statistically significant. |
| Krouse et al.831 | 2004 | 3b | Prospective case-control |
|
Acoustic rhinometry of minimal cross-sectional area of nasal cavity | Analysis of nasal provocation test results among groups showed a sensitivity of 42% and specificity of 44% for SPT using Alternaria antigen. |
| Krouse et al.832 | 2004 | 3b | Prospective case-control |
|
Acoustic rhinometry of minimal cross-sectional area of nasal cavity | Analysis of nasal provocation test results among groups showed a sensitivity of 87% and specificity of 86% with multi-test application of Timothy grass antigen. |
| Zarei et al.834 | 2004 | 3b | Prospective case-control |
|
Wheal size that best identifies clinical allergy to cat based on nasal provocation testing | On SPT with cat antigen, a wheal size of ≥3 mm had a sensitivity of 100% and specificity of 74.1%. This improved with increasing size of wheal. |
| Pumhirun et al.835 | 2000 | 3b | Prospective case-control | Perennial rhinitis patients | Compared sensitivity and specificity of intradermal testing to SPT and specific IgE assay for D. pteronyssinus and D. farinae | SPT for D. pteronyssinus and D. farinae were 90.4% and 86.4% sensitive and 99.5% and 93.1%, specific, respectively. This compared to sensitivity of 96.3% and 88.9% and specificity of 96.2% and 88.9% of specific IgE assay, respectively. |
| Wood et al.793 | 1999 | 3b | Prospective case-control | Patients with cat allergy determined by history and a cat-exposure model | Compared the predictive values of SPT, intradermal testing, and RASTs in the diagnosis of cat allergy | SPT and RAST values exhibited excellent efficiency in diagnosis of cat allergy. Intradermal testing added little to the diagnostic evaluation. Sensitivity and specificity of SPT were 79% and 91%, respectively. |
| Tschopp et al.822 | 1998 | 3b | Prospective case-control | A randomly selected sample of 8329 Swiss adults | Compared the sensitivity, specificity, PPV, and NPV of SPT, IgE levels, and fluoroenzyme immunoassay in diagnosing AR | Sensitivity of fluoroenzyme immunoassay was significantly higher than SPT and IgE. However, SPT was more specific and had a better PPV. SPT was the most efficient test to diagnose AR. |
| Seidman et al.761 | 2015 | 5 | Guideline | Not applicable | Not applicable | Clinicians should perform and interpret or refer for specific IgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment or the diagnosis is uncertain. |
| Heinzerling et al.836 | 2013 | 5 | Review | Not applicable | Not applicable | SPT is a reliable method to diagnose AR with specificity of 70% to 95% and sensitivity of 80% to 90% for inhalant allergies. Further standardization of SPT is needed. |
| Bernstein et al.818 | 2008 | 5 | Practice parameter | Not applicable | Not applicable | Sensitivity of SPT ranges from 85% to 87% while specificity is 79% to 86%. Many studies have verified the sensitivity and specificity of SPT. |
AR = allergic rhinitis; IgE = immunoglobulin E; LOE = level of evidence; NPV = negative predictive value; PPV = positive predictive value; RAST = radioallergosorbent test; SET = skin endpoint titration; SPT = skin prick test/testing.
Aggregate Grade of Evidence: B (Level 1a: 1 study; Level 3b: 7 studies; Table VIII.E.1).
Benefit: Supports diagnosis and directs pharmacological therapy while possibly avoiding unnecessary/ineffective treatment; guides avoidance; directs AIT.
Harm: Adverse events from testing including discomfort, pruritus, erythema, worsening of asthma symptoms, and anaphylaxis, inaccurate test results, and misinterpreted test results.
Cost: Low.
Benefits-Harm Assessment: Preponderance of benefit over harm.
Value Judgments: Patients can benefit from identification of their specific sensitivities. SPT is a quick and relatively comfortable way to test several antigens with accuracy similar to other available methods of testing.
Policy Level: Recommendation.
Intervention: SPT is recommended for evaluation of allergen sensitivities in appropriately selected patients. Regular use of the same SPT device will allow clinicians to familiarize themselves with it and interpretation of results may therefore be more consistent. The use of standardized allergen extracts can further improve consistency of interpretation.
VIII.E.2. Skin intradermal testing
The placement of allergenic proteins in the intradermal space is often used for diagnosing AR. Intradermal testing has also been described in the evaluation of sensitivities to other substances, including local anesthetic agents, neuromuscular blocking agents, antibiotics, and contrast media.837-840 While previous protocols have described the use of intradermal testing for suspected food or chemical allergies, this type of diagnostic testing is currently not recommended in routine practice.841,842 Intradermal testing may be used as a primary testing modality, or as a secondary test following SPT. Intradermal testing has also been used, primarily by otolaryngic allergists, as a method to help determine the starting point for specific AIT and as a vial safety test prior to an injection from a new treatment vial, though the level of evidence supporting these uses is low.843,844
Intradermal testing may be performed as a single injection. A short bevel needle is used to inject a diluted allergenic extract solution into the superficial dermis. Approximately 0.02 mL is used, or enough to produce a well-defined wheal, which is 4 mm in diameter.845 The wheal will expand to 5 mm by hydrostatic forces, and the reaction is observed for 10 minutes. The positive control for intradermal testing is histamine and the negative controls are typically phenolated saline and a glycerin solution that equals the concentration of glycerin in the test solution. If the diameter of the resulting wheal is at least 7 mm, and at least 2 mm wider than the glycerin control, this is considered a positive test.846 While this is a very reproducible test, it is more technically demanding than SPT, is difficult to perform in young children, and carries a higher risk of adverse reactions.847 Severe adverse events related to intradermal testing are rare. Over a 42-year period, from 1945 to 1987, only 5 fatalities were attributed to intradermal testing without prior prick/puncture testing.848
Intradermal testing may also be performed using multiple dilutions of the same allergen to more precisely quantify the level of sensitivity to that allergen and suggest a starting point for immunotherapy.849 A series of dilutions of concentrated allergenic extract (typically supplied as a 1:20 wt/vol solution) can be prepared in either a 1:5 or 1:10 ratio. Intradermal dilutional testing (IDT, previously referred to as skin endpoint titration, or SET) begins with the intradermal placement of a dilute allergen, along with appropriate controls, followed by the placement of progressively more concentrated dilutions of that allergen. The dilution producing the first positive test (defined earlier in this section as a wheal is at least 7 mm and at least 2 mm wider than the glycerin control) followed by progressively larger wheals is called the “endpoint.” To establish progression, a confirmatory wheal, produced by the next higher concentration, must be at least 2 mm wider than the suspected endpoint. IDT endpoint correlates with SPT wheal.844,850,851 While IDT endpoints have been shown to correlate with biologically relevant measures, such as basophil histamine release, a clear correlation with other measures, such as in vitro sIgE levels, has not yet been established.852,853 Currently, no studies have demonstrated a clear benefit of quantitative intradermal testing over single intradermal testing with regard to the diagnosis of clinical allergy or the outcome of specific immunotherapy (Table VIII.E.2).
TABLE VIII.E.2.
Evidence for the role of intradermal skin testing in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Nevis et al.830 | 2016 | 1a | Systematic review | AR patients who underwent skin testing (n = 430) | Sensitivity and specificity of skin testing methods | ID testing had higher sensitivity and specificity when used as a stand-alone test than when used to confirm SPT. |
| Larrabee et al.859 | 2015 | 2b | Cohort | AR patients who underwent ID testing based on high suspicion after negative SPT (n = 87) | Result of ID test | 21% were ID positive, more likely for indoor allergens. |
| Gungor et al.833 | 2004 | 2b | Cohort | Patients with SAR and ragweed sensitivity (n = 62) | Nasal provocation testing, rhinomanometry | Sensitivity and specificity of ID testing was comparable to SPT. |
| Krouse et al.832 | 2004 | 2b | Cohort | SAR (n = 37):
|
Nasal provocation with Timothy grass, rhinomanometry | ID testing after SPT increased the sensitivity from 87% to 93%. |
| Krouse et al.831 | 2004 | 2b | Cohort | AR (n = 44):
|
Nasal allergen provocation score for Alternaria, visual analog scale, rhinomanometry | ID testing after SPT increased the sensitivity from 42% to 58%. |
| Wood et al.793 | 1999 | 2b | Cohort | Patients with a history of symptoms with cat exposure (n = 120) | Cat exposure challenge, symptom scores, FEV1 | ID scores added little value beyond SPT and RAST values. |
| Nelson et al.856 | 1996 | 2b | Cohort | (n = 70):
|
Nasal challenge with Timothy grass | Positive ID along with negative SPT did not indicate the presence of clinically significant sensitivity. |
| Escudero et al.860 | 1993 | 2b | Cohort | Rhinitis patients (n = 66), 31 with Alternaria allergy | SPT, ID, challenge tests and in vitro sIgE. Clinical history and nasal/bronchial challenge considered gold standard. | For rhinitis patients, SPT, ID, and conjunctival challenge were more sensitive than serum sIgE. All testing methods had similar specificity. |
| Niemeijer et al.846 | 1993 | 2b | Cohort | Allergy patients (n = 41) | Simultaneous SPT, ID testing with varying concentrations of Phleum and D. pteronyssinus, as well as pRAST on all subjects. | Coefficient of variation of ID test histamine wheal size is 6% within patients and 12% between patients. Optimum concentration of tested allergens was 10–100 BU/mL, a 7.5 mm ID wheal is ideal cutoff value for positive result (0.83× the size of average histamine wheal). |
| Niemeijer et al.855 | 1993 | 2b | Cohort | Suspected allergy patients (n = 497) | Simultaneous ID, pRAST, and clinical history compared. Standardized grass pollen, tree pollen, cat, dust mite tested. | Ideal cutoff for positive ID test is wheal diameter 0.7 times the size of histamine control. ID has 83% predictive value vs RAST and 77% predictive value vs clinical history. |
| Reddy et al.857 | 1978 | 2b | Cohort | Patients with perennial rhinitis (n = 34), negative SPT for 60 allergens but with at least 1 positive ID test | RAST, nasal provocation and leukocyte histamine release compared to ID positivity, SPT negativity | Patients with only ID positive skin tests (SPT negative) did not have a positive RAST nor a positive leukocyte histamine release. In contrast, SPT positivity was associated with positive RAST test and leukocyte histamine release assay. When SPT are negative for perennial rhinitis patients, positive ID tests are not likely to indicate the presence of IgE-mediated allergy. |
| Perera et al.853 | 1975 | 2b | Cohort | Patients referred for allergy diagnostic testing (n = 54) | Positive clinical histories compared to RAST results and IDT results | High degrees of skin reactivity (positive ID tests at high allergen concentrations) correspond with a higher rate of positive clinical history and positive RAST results. |
| Peltier & Ryan844 | 2007 | 3b | Cohort | Volunteers underwent simultaneous SPT and IDT for 5 common allergens (n = 134) | SPT wheal size compared to IDT endpoint | IDT endpoint directly correlates with SPT wheal size for all antigens tested, especially for Bermuda, dust mite, and ragweed. |
| Peltier & Ryan850 | 2006 | 3b | Cohort | Volunteers tested simultaneously for mold allergens with SPT and IDT (n = 86) | SPT wheal size compared to IDT endpoints | In subjects with clinical symptoms of allergy there was a direct statistically significant correlation between SPT wheal size and IDT endpoint. ID tests identified 10% more positive results compared to SPT alone. |
| Purohit et al.852 | 2005 | 3b | Cohort | Patients with birch pollen allergy (n = 18) | Correlations among IDT endpoint, serum sIgE, and provocation thresholds for basophil histamine release. | IDT endpoint correlated directly with basophil histamine release in response to allergen exposure. IDT endpoint did not correlate with rBet v 1 serum sIgE level. |
| Schwindt et al.858 | 2005 | 3b | Cohort | Patients with allergy (n = 97) | Using clinical history as gold standard, prick, ID, and challenge test results compared | If SPT with multi-test II device was negative, 17% of subjects had a positive ID test that corresponded with clinical history. None of these positive ID tests corresponded with a positive nasal challenge. When multi-test II results are negative, positive ID tests are unlikely to identify clinically relevant aeroallergen sensitivity. |
| Simons et al.851 | 2004 | 4 | Retrospective cohort | Allergy clinic patients (n = 34) | Patients tested for aeroallergen sensitivity with both IDT and SPT. | A significantly greater number of patient tested positive with IDT compared to SPT. SPT wheal size and IDT endpoint correlated for several allergens. IDT may be more sensitive than SPT. |
AR = allergic rhinitis; BU = biological units; FEV1 = forced expiratory volume in 1 second; ID = intradermal; IDT = intradermal dilutional titration; LOE = level of evidence; pRAST = Phadebas radioallergosorbent test; RAST = radioallergosorbent test; SAR = seasonal allergic rhinitis; sIgE = antigen-specific immunoglobulin E; SPT = skin-prick test.
As a stand-alone diagnostic test for AR, estimates for sensitivity for intradermal testing range between 60% (95% CI, 31% to 83%) and 79% (95% CI, 63% to 90%), while estimates for specificity range between 68% (95% CI, 49% to 82%) and 69% (95% CI, 52% to 86%).793,833 This is lower than the pooled estimates of sensitivity (85-88%) and specificity (77%) for SPT, calculated from recent meta-analyses.830,854 Factors affecting the predictive value of intradermal testing include the comparator used and the concentration of allergen used with the intradermal test.855
It has been suggested that intradermal testing could potentially increase the sensitivity of SPT by injecting allergenic proteins into deeper tissue layers beneath the keratinized epidermis.847 However, the literature has not supported a clear benefit of intradermal testing for this purpose. Using intradermal testing in addition to SPT to predict a positive response from nasal challenge with Timothy grass only increased the sensitivity from 87% to 93%.832 In a similar study, Krouse et al.831 determined that adding intradermal testing to SPT as a method to predict positive nasal challenge to Alternaria increased the sensitivity from 42% to 58%. These studies suggest marginal increase in sensitivity that may vary based upon the allergen being tested.
Nelson et al.856 studied 28 individuals with a history of SAR. One group had negative SPT to Timothy and Bermuda grass, but positive intradermal testing for Timothy grass, while the other group had negative SPT and negative intradermal testing for Timothy and Bermuda grass. In both groups, 11% of individuals had a positive nasal challenge with Timothy grass. Likewise, when 39 individuals with clinical cat allergy and negative SPT underwent a cat challenge, there was no difference in the development of upper respiratory symptoms between those who had positive or negative intradermal testing (24% vs 31%, p = 0.35).793 Reddy et al.857 evaluated allergy test results in 34 patients with perennial rhinitis. Patients with only intradermal positive skin tests (SPT negative) did not have a positive RAST nor a positive leukocyte histamine release. In contrast, SPT positivity was associated with positive RAST test and leukocyte histamine release assay.857 Schwindt et al.858 studied 97 subjects with allergic rhinoconjunctivitis symptoms. Prick testing was followed by intradermal testing if prick was negative. If patients were prick-negative and intradermal-positive, a nasal challenge was performed against 5 different allergens. If SPT with the multi-test II device was negative, only 17% of subjects had a positive intradermal test that corresponded with clinical history. None of these positive ID tests corresponded with a positive nasal challenge.858 Taken together, these studies suggest that intradermal testing does not improve the diagnosis of allergy in subjects with negative SPT.
Nevis et al.830 conducted a systematic review of 4 studies to determine the sensitivity and specificity of intradermal testing when used as a confirmatory test following negative SPT. Sensitivity ranged from 27% (95% CI, 10% to 57%) to 50% (sample sizes were too small to calculate CI), while specificity ranged from 69% (95% CI, 51% to 83%) to 100% (95% CI, 83% to 100%). From a retrospective study by Larrabee and Reisacher,859 when the clinician was guided by high clinical suspicion, the incidence of positive intradermal testing following negative SPT was 36.9% for indoor allergens (D. pteronyssinus, D. farinae, cat, dog, and cockroach), 12.7% for outdoor allergens (ragweed, red birch, Timothy grass, white oak, and red maple) and 9.2% for molds (Aspergillus, Candida, Penicillium, Alternaria, and Cladosporium). However, no correlation between positive intradermal testing and nasal challenge testing was performed in this study. Escudero et al.860 found that in rhinitis patients, SPT, intradermal and conjunctival challenge were more sensitive than serum sIgE. All testing methods had the same specificity.
In summary, current evidence supports the use of intradermal testing for the diagnosis of AR due to airborne allergens as a stand-alone test, although this form of testing demonstrates no clear superiority over SPT when comparing sensitivity and specificity. There were no studies identified that directly compared single-dilution intradermal testing with IDT in terms of sensitivity, specificity, or patient outcomes. There appears to be a small gain in sensitivity when intradermal testing is used as a confirmatory test following negative SPT; however, positive intradermal test results in this setting could represent false-positive test results. It is also more likely that an intradermal test following a negative SPT will be positive when indoor allergens are being tested and least likely to be positive when testing for mold sensitivity. It is unknown whether the type of allergen has an impact on the sensitivity and specificity, as most studies examined used only 1 allergen, but intradermal testing seemed to be least sensitive and specific when mold was being tested. Other limitations of the studies identified for this review include low sample population sizes (the largest included 120 participants), variable study design, and the lack of randomized, controlled trials.
Aggregate Grade of Evidence: B (Level 1a: 1 study; Level 2b: 11 studies; Level 3b: 4 studies; Level 4: 1 study; Table VIII.E.2).
Benefit: Generally well tolerated, easy to perform, and a favorable level of sensitivity and specificity when used as a stand-alone diagnostic test.
Harm: Very low risk of severe adverse reactions.
Cost: Low.
Benefits-Harm Assessment: Benefit over harm when used as a stand-alone diagnostic test. Balance of benefit and harm when used to confirm the results of SPT, as a quantitative diagnostic test or as a vial safety test.
Value Judgments: It is important to determine the presence of IgE-mediated sensitivity for individuals with suspected AR. If SPT is negative, there is limited clinical benefit to performing intradermal testing for confirmation.
Policy Level: Option for using intradermal testing as a stand-alone diagnostic test for individuals with suspected AR. Option for using intradermal testing as a confirmatory test following negative SPT for nonstandardized allergens. The evidence for quantitative IDT is sparse and prevents a recommendation for this specific testing technique.
Intervention: Intradermal testing may be used to determine specific airborne allergen sensitization for individuals suspected of having AR.
VIII.E.3. Blended skin testing techniques
Blended allergy skin testing involves the combined use of SPT and intradermal testing to establish an “endpoint” for a specific antigen.844,847,850 The protocol, initially described by Krouse and Krouse,861 and referred to as “modified quantitative testing” (MQT), serves as an example of a blended technique. MQT involves an algorithm where a SPT is used initially to apply an antigen. Depending upon the SPT result, an intradermal test may or may not be applied.844,847,850,861 With these results, the algorithm is used to determine an endpoint for each antigen tested.844,847,850,861 The endpoint signifies the skin reactivity to the applied antigen on a graded scale and is considered to be a safe starting dose for the application of AIT.861 There is a small amount of literature on blended techniques, but AIT based upon the MQT results has been shown to be successful, with immune system alterations in line with other skin testing techniques861 (Table VIII.E.3).
TABLE VIII.E.3.
Evidence for the role of blended skin testing techniques in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lewis et al.862 | 2008 | 3b | Systematic review with cost-effectiveness analysis | Comparison of sIgE, intradermal tests, and MQT from a payer perspective | MQT most cost-effective when population prevalence of AR is 20% or higher. | |
| Fornadley847 | 2014 | 4 | Systematic review | Review of skin testing techniques | MQT is a valid form of skin testing. | |
| Peltier & Ryan844 | 2007 | 4 | Case series | Adults with AR (n = 134) |
|
MQT is a safe alternative to classic IDT for determining AIT starting doses. |
| Krouse & Krouse861 | 2006 | 4 | Case series | Adults with AR (n = 9) |
|
MQT-based AIT demonstrates immune system changes and QOL improvement. |
| Peltier & Ryan850 | 2006 | 4 | Case series | Adults with AR (n = 86) |
|
MQT-based testing is a safe method for determining starting AIT doses for fungal allergens. |
AIT = allergen immunotherapy; AOS = Allergy Outcome Survey; AR = allergic rhinitis; IDT = intradermal dilutional testing; IgG4 = immunoglobulin G4; LOE = level of evidence; MQT = modified quantitative testing; QOL = quality of life; RSDI = Rhinosinusitis Disability Index; sIgE = antigen-specific immunoglobulin E; SNOT-20 = 20-item Sino-Nasal Outcome Test; SPT = skin-prick testing;
The advantages of blended techniques, such as MQT, are that they provide the practitioner with both qualitative data (the patient demonstrates sensitivity) and quantitative data (endpoint; safe starting dose for AIT) for specific antigen sensitivities in less time than IDT.844,847,850 Disadvantages include the additional risk and time involved in placing intradermal tests. In comparison to IDT and in vitro testing methods, MQT has been shown to be more cost-effective when the prevalence of AR in a population is 20% or higher.862 While blended skin testing techniques may be considered in the evaluation of AR, especially to determine the starting point for AIT, the evidence to support this technique is not strong.
Aggregate Grade of Evidence: D (Level 3b: 1 study; Level 4: 4 studies; Table VIII.E.3).
Benefit: Ability to establish an endpoint in less time than IDT.
Harm: The additional risks, including systemic or anaphylactic reactions, of intradermal tests; additional time and discomfort.
Cost: Similar to intradermal testing.
Benefits-Harm Assessment: Benefit outweighs harm.
Value Judgments: AIT can be initiated from SPT results alone; however, endpoint-based AIT may decrease time to reaching therapeutic dose.
Policy Level: Option.
Intervention: MQT is a skin testing technique that may be used to determine a starting point for AIT.
VIII.E.4. Issues that affect the performance or interpretation of skin tests
VIII.E.4.a. Medications.
The wheal and flare reaction seen in allergy skin testing depends upon the physiologic actions of histamine released from mast cells upon degranulation. Thus, any medications that inhibit mast cell degranulation or that function as histamine H1 receptor antagonists have the potential to suppress appropriate skin test responses. The suppressive effects of H1 antihistamines on allergen and histamine induced wheal and flare responses vary greatly,863,864 and the duration of this suppression depends upon the skin tissue concentration and half-life of these agents.865,866 In fact, skin test suppression can be used as a biological assay for the onset and duration of action of antihistamines.865 Agents such as astemizole (now removed from the market due to QT prolongation) have the potential to suppress skin test reactions for a period of weeks after cessation.867 However, most antihistamines only suppress skin test responses for a period of 2 to 7 days after cessation.867,868 Topically administered antihistamines have the potential to suppress skin wheal and flare responses. One randomized placebo-controlled study showed that 14 days of azelastine nasal spray treatment reduced the histamine induced wheal and flare response, and this suppression disappeared by 48 hours after cessation869 (Table VIII.E.4.a-1).
TABLE VIII.E.4.a-1.
Evidence for the effect of medication on allergy skin test reactivity
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Kupczyk et al.871 | 2007 | 1b | DBPCT, crossover | Atopic subjects (n = 21). SPT with histamine, codeine, allergen, negative control after 5 days of ranitidine, loratadine, or placebo | Wheal, flare measured in mm. Pruritis measured with 10-point scale | Relative to placebo, ranitidine reduced histamine wheal (41%) and flare (16%); and allergen wheal (23%) and flare (22%). Loratadine reduced histamine wheal (51%) and flare (33%); and allergen wheal (40%) and flare (44%), respectively. Ranitidine and loratadine both reduced pruritis score by almost 30%. |
| Spergel et al.888 | 2004 | 1b | RDBT, within subject comparison | Atopic dermatitis and AR or asthma (n = 12 adults). Vehicle or pimecrolimus on each arm | Allergen SPT wheal and flare, before and after topical 1% pimecrolimus cream | 1% pimecrolimus cream does not significantly impact allergy skin test results. |
| Hill & Krouse876 | 2003 | 1b | RDBPCT | Atopic subjects (n = 23) | Intradermal whealing response after loratadine, montelukast, or placebo treatment | Loratadine, but not montelukast, reduced the intradermal wheal diameter after allergen injection. |
| More et al.889 | 2003 | 1b | RDBPCT | Healthy volunteers (n = 15). Single blinded dose of placebo, fexofenadine, 23 other herbal preparations. Minimum 72-hour washout period between doses | Histamine 1 mg/mL wheal at baseline and 4 hours after single dose of herbal preparation | Fexofenadine significantly reduced SPT wheal size compared to placebo. None of the 23 herbal preparations tested showed a statistically significant effect on wheal size compared to placebo. |
| Noga et al.890 | 2003 | 1b | RDBPCT | Moderate-severe asthmatics (n = 35) treated with placebo or omalizumab | SPTs for allergen before and 16 weeks after treatment | Omalizumab caused significant reduction in SPT wheal size compared to placebo. |
| Pearlman et al.869 | 2003 | 1b | RPCT | SAR patients (n = 78) | Inhibition of histamine-induced wheal after single dose or 2 weeks of azelastine nasal spray | 2 weeks of azelastine inhibited wheal and flare in some patients. Histamine skin test responses returned to baseline at 48 hours after cessation. |
| Simons & Simons865 | 1997 | 1b | RDBPCT, crossover | Adult males (n = 20) | SPT wheal and flare response after single day dosing of PO fexofenadine and loratadine | Fexofenadine and loratadine both inhibited SPT wheal and flare response for 24 hours. |
| Miller & Nelson870 | 1989 | 1b | RDBT | Healthy subjects (n = 23) | Histamine-induced and compound 48/80-induced skin prick wheal and flare after placebo or ranitidine 150 mg ×7 doses | Ranitidine reduced the histamine-induced wheal and flare by 22%. No significant reduction in compound 48/80-induced wheal and flare. |
| Pipkorn et al.891 | 1989 | 1b | RDBPCT | AR patients (n = 10) | Allergen SPT wheal and flare before and after 2 to 4 weeks of twice daily clobetasol cream applied to forearm skin test sites | Clobetasol treated skin had significantly reduced wheal and flare response to allergen. Histamine-induced wheal was reduced at 4 weeks by topical steroid. |
| Andersson & Pipkorn883 | 1987 | 1b | DBPCT | AR patients (n = 17) | Effect of topical clobetasol (BID application for 1 week) on histamine and allergen SPT response | Topical clobetasol significantly suppresses allergen-induced wheal and flare response. |
| Slott & Zweiman879 | 1974 | 1b | DBPCT, crossover | Atopic patients (n = 15) | Intradermal wheal size differences for histamine, allergen, and compound 48/80 after 7 days of methylprednisolone 24 mg per day | No effect of 7 days of methylprednisolone on intradermal wheal size. |
| Cook et al.868 | 1973 | 1b | Double blind randomized controlled study | AR patients (n = 18 adults) | Intradermal wheal size suppression after 3 day course of chlorpheniramine, tripelennamine, promethazine, hydroxyzine, and diphenhydramine | All antihistamines suppressed wheal size to varying degrees. Hydroxyzine suppressed responses for 4 days after cessation vs 2 days for diphenhydramine. |
| Isik et al.874 | 2011 | 2b | Cohort | Patients on SSRIs for depression (n = 24) | Histamine-induced and allergen-induced prick test wheal responses before and after starting SSRI treatment. | SSRIs fluoxetine, sertraline, and escitalopram did not significantly affect skin prick whealing responses. |
| Corren et al.875 | 2008 | 2b | Cohort | PAR patients (n = 40) | Dust mite allergen skin test reactivity (titrated prick tests) before during and after omalizumab therapy. | Omalizumab (anti-IgE) therapy significantly reduces allergy skin test reactivity. |
| Gradman & Wolthers885 | 2008 | 2b | Randomized crossover cohort | Atopic eczema patients (n = 12 children) | SPT for 10 allergens before and after active treatment with topical mometasone or topical tacrolimus. Skin test sites were presumably treated daily for a total of 2 weeks. | Topical mometasone and tacrolimus significantly reduced SPT wheal diameter. Topical mometasone also reduced histamine induced wheal, while tacrolimus did not. |
| Narasimha et al.882 | 2005 | 2b | Cohort | 26 subjects | Effect of topical clobetasol application on histamine-induced wheal response. | Topical clobetasol inhibited skin prick whealing response to histamine at the site of topical steroid application in a dose-dependent and duration-dependent manner. |
| Cuhadaroglu et al.877 | 2001 | 2b | Cohort |
|
SPT to histamine and allergens before and after zafirlukast 20 mg BID for at least 5 days. | Zafirlukast did not suppress histamine-induced or allergen-induced wheal and flare response. |
| Des Roches et al.878 | 1996 | 2b | Cohort |
|
Codeine and dust mite induced SPT response with or without exposure to long-term systemic steroids. | Systemic steroid therapy does not alter SPT reactivity to codeine or allergen. |
| Almind et al.867 | 1988 | 2b | Cohort | Healthy individuals (n = 23) | Effect on histamine SPT wheal size after 2-day treatment with dexchlorpheniramine, cyproheptadine, astemizole, loratadine, terfenadine. Duration of SPT wheal suppression after cessation. | All antihistamines suppressed SPT wheal response to histamine. Duration of suppression exceeded 72 hours for all agents tested. |
| Rao et al.873 | 1988 | 2b | Cohort | Healthy subjects (n = 33) | Histamine prick tests for 1 week after single dose of desipramine or doxepin. | Desipramine inhibits wheal response for 2 days; doxepin inhibits wheal response for 4 days. |
| Long et al.863 | 1985 | 2b | Cohort | 18 subjects; 10 had positive SPT to grass or ragweed allergens | Effect of 6 different antihistamines on SPT wheal and flare reaction to histamine or morphine or relevant aeroallergen. Effect of hydroxyzine and chlorpheniramine on skin test responses to other antihistamine classes. | Antihistamines varied in their ability to suppress SPT wheal response. Administration of hydroxyzine for 3 weeks leads to reduced skin test suppression for the antihistamines tested, suggesting induction to tolerance to antihistamine effects. |
| Phillips et al.864 | 1983 | 2b | Cohort | Atopic subjects (n = 10) | Inhibition of allergen-induced and histamine-induced wheals by local intradermal antihistamine and cromoglycate injection. | Antihistamines ketotifen, clemastine, and chlorpheniramine significantly inhibit skin whealing responses. Sodium cromoglycate had no effect. |
| Harvey & Schocket872 | 1980 | 2b | Cohort | Healthy subjects (n = 10) | Titrated intradermal histamine wheal before and after treatment with hydroxyzine, cimetidine, or both. | Hydroxyzine inhibited cutaneous wheal response to histamine. Cimetidine did not. However, the 2 together produced significantly reduced whealing compared to either alone. |
| Geng et al.881 | 2015 | 3b | Case-control |
|
OR that multiple clinical variables including medication use predict negative histamine control test | ICU stay, systemic steroid use, H2 blockers, and older age associated with negative histamine control test. |
| Shah et al.886 | 2010 | 4 | Retrospective cohort | Histamine SPT responses in patients with variable exposure to a variety of medications | SPT wheal area and SPT positivity as function of medication exposure and time since last dose | H1 antagonists impaired whealing responses within 3 days of discontinuation; tricyclic antidepressants, benzodiazepines, mirtazapine, quetiapine had wheal suppression; other SSRIs and SNRIs as well as H2 antagonists were not independently associated with wheal suppression. |
| Duenas-Laita et al.887 | 2009 | 4 | Cohort | Drug abusers taking alprazolam 2 mg TID (n = 42) | Histamine (10 mg/mL) SPT | All subjects taking alprazolam had negative histamine SPT. |
| Olson et al.880 | 1990 | 4 | Retrospective cohort |
|
Intradermal skin test reactivity to codeine and histamine | Chronic systemic steroid use reduces codeine-induced wheal response but not histamine-induced wheal response. |
AR = allergic rhinitis; BID = twice a day; DBPCT = double-blind placebo controlled trial; ICU = intensive care unit; IgE = immunoglobulin E; LOE = level of evidence; OR = odds ratio; PAR = perennial allergic rhinitis; PO = per os (by mouth); RDBPCT = randomized double-blind placebo controlled trial; RDBT = randomized double blind trial; RPCT = randomized placebo controlled trial; SAR = seasonal allergic rhinitis; SNRI = selective norepinephrine reuptake inhibitor; SPT = skin-prick test; SSRI = selective serotonin reuptake inhibitor; TID = 3 times a day.
Randomized, placebo-controlled trials have demonstrated that H2 receptor antagonists such as ranitidine can reduce skin whealing responses,870,871 and 1 study showed an additive effect of H1 and H2 antihistamines on skin wheal suppression.872 Some antidepressants have the potential to suppress skin wheal and flare responses, in particular the tricyclic antidepressants that have antihistaminic properties (such as doxepin).873 However, newer classes of antidepressants such as selective serotonin reuptake inhibitors (SSRI) do not appear to affect allergy skin test responses.874
Recombinant humanized anti-IgE monoclonal antibody (mAb), omalizumab, interferes with IgE-mediated mast cell degranulation reactions in the allergy skin test response. A randomized placebo-controlled trial demonstrated a significant reduction in allergen-induced skin whealing after 4 months of treatment.874 Omalizumab appears to suppress skin test reactivity in tandem with dramatic reductions in serum free IgE, and allergy skin test responses return to normal within 8 weeks of discontinuation.875
Leukotriene receptor antagonists (LTRAs) do not appear to interfere with allergy skin test results. Hill and Krouse876 as well as Simons et al.866 found no effect of montelukast on intradermal skin test results in allergic subjects. Cuhadaroglu et al.877 found no change in SPT results in allergic subjects before and treatment with zafirlukast.
In general, the highest level evidence shows that systemic steroid treatment has no effect on SPT and intradermal test results,878,879 though some less rigorous retrospective studies suggest that systemic steroid treatment could affect skin whealing responses.880,881 Topical steroid treatment has been demonstrated to suppress the wheal and flare reaction in treated skin areas, creating the possibility of false-negative test results.882-885 No studies were identified that examined the effect of intranasal or inhaled steroids on skin test results.
The effects of many classes of medications on allergy skin test responses remain inadequately studied. Benzodiazepines have been implicated as possibly suppressing skin test responses.886,887 The calcineurin inhibitor tacrolimus was shown to inhibit SPT whealing,885 whereas a study of a similar drug, pimecrolimus, did not show any effect on skin whealing responses.888 The pharmacologic effects of herbal preparations are generally unstudied, and it is unclear which of these agents could interfere with allergy skin test responses. More et al.889 performed a double-blind, placebo-controlled, single-dose crossover study in 15 healthy volunteers, examining the histamine-induced skin test response. None of the 23 herbal supplements tested caused suppression of the histamine-induced wheal response.
There are many classes of medications for which the actual impact on allergy skin testing are unknown. To mitigate against the risk of false-negative skin test results induced by medications, all allergy testing should be performed after application of appropriate positive controls (usually histamine) to ensure that the histamine-induced skin test reaction is intact at the time of testing. See Table VIII.E.4.a-1 for a comprehensive review, with Aggregate Grades of Evidence in Table VIII.E.4.a-2.
TABLE VIII.E.4.a-2.
Aggregate grades of evidence: medications that affect allergy skin testing
| H1 antihistamines | Aggregate Grade of Evidence: A (Level 1b: 2 studies, Level 2b: 3 studies) • Should be discontinued 2-7 days prior to testing. |
| H2 antihistamines | Aggregate Grade of Evidence: B (Level 1b: 2 studies) • Ranitidine suppresses skin whealing response, may result in false negatives. |
| Topical antihistamines (nasal, ocular) | Aggregate Grade of Evidence: Unable to determine from one Level 1b study. • Should be discontinued 2 days prior to testing. |
| Anti-IgE (omalizumab) | Aggregate Grade of Evidence: A (Level 1b: 2 studies) • Results in negative allergy skin test results. |
| Leukotriene receptor antagonists | Aggregate Grade of Evidence: A (Level 1b: 2 studies, Level 2b: 1 study) • May be continued during testing. |
| Tricyclic antidepressants | Aggregate Grade of Evidence: Unable to determine from one Level 2b study. • Agents with antihistaminic properties suppress allergy skin test responses. |
| Topical (cutaneous) corticosteroids | Aggregate Grade of Evidence: A (Level 1b: 2 studies, Level 2b: one study) • Skin tests should not be placed at sites of chronic topical steroid treatment. |
| Systemic corticosteroids | Aggregate Grade of Evidence: C (No effect – Level 1b: 1 study, Level 2b: 1 study; Suppression – Level 3b: 1 study, Level 4: 1 study) • Systemic corticosteroid treatment does not significantly impair skin test responses. |
| Selective serotonin reuptake inhibitors (SSRIs) | Aggregate Grade of Evidence: B (Level 2b: 1 study, Level 4: 1 study) • Does not suppress allergy skin test response. |
| Benzodiazepines | Aggregate Grade of Evidence: C (Level 4: 1 study, Level 5: 1 case report) • May suppress skin test responses. |
| Topical calcineurin inhibitors (ie. tacrolimus, picrolimus) | Aggregate Grade of Evidence: D (Level 1b: 1 study, Level 2b: 1 study – results conflicting) • Conflicting results regarding skin test suppression. |
VIII.E.4.b. Skin conditions.
The usefulness of allergy skin testing depends upon the ability to detect a Type I hypersensitivity reaction after allergen introduction into the skin. Abnormal skin (eg, dermatitis) may not respond appropriately to histamine, glycerin, or allergen. Additionally, the physical trauma of prick/puncture or intradermal testing may induce a local inflammatory response. The wheal and flare reaction also may be difficult to detect due to preexisting skin changes. Further, skin color may inhibit the ability to visualize the flare reaction, especially in darker skinned individuals.
Common sense dictates that allergy skin testing should not be performed at sites of active dermatitis, but clinical studies to investigate this phenomenon are lacking. Individuals with dermatographism may have exaggerated responses to allergy skin testing, requiring close attention to the results of negative control tests. In some cases, it may be preferable to perform in vitro specific IgE testing in patient with skin disease or dermatographism, but this is not based on data or outcomes from controlled studies.
Due to the lack of published studies on this topic, an Aggregate Grade of Evidence and evidence based recommendation cannot be provided.
VIII.F. In vitro testing
VIII.F.1. Serum total IgE (tIgE)
The literature addressing the role of serum tIgE in the evaluation and diagnosis of allergic disease offers conflicting outcomes and divergent opinions. Positive studies, demonstrating a relevant role of measuring tIgE in the evaluation and diagnosis of AR, are listed in Table VIII.F.1-1. Negative studies that report a limited role of measuring tIgE are listed in Table VIII.F.1-2. When taken together, however, this body of literature provides some information that can inform decisions related to the utility of tIgE in directing patient care decisions.
TABLE VIII.F.1-1.
Evidence supporting the use of total IgE in allergic rhinitis or allergy diagnosis
| Study | Year | LOE | Study design | Study groups | Endpoint | Conclusiona |
|---|---|---|---|---|---|---|
| Park et al.902 | 2016 | 2b | Prospective cohort | 313 school children, 2-year follow-up study | Initial examination: no allergic sensitization, serum tIgE >17.7 IU/mL | Associated with the risk for allergic sensitization (sensitivity: 46.3%; specificity: 85.3%; OR: 4.8). |
| Initial examination: allergic symptoms but negative SPT, serum tIgE >17.4 IU/mL | Associated with newly developed allergic sensitization (sensitivity: 69.9%; specificity: 100.0%). | |||||
| Demirjian et al.896 | 2012 | 2b | Prospective cohort | Patients referred to allergy clinic. Total patients (n = 358,184 with rhinitis), mean age 57 years. | Serum tIgE (IU/mL), continuous variable | tIgE levels >140 IU/mL is suggestive of an atopic etiology for patients with rhinitis. |
| Jung et al.895 | 2011 | 2b | Prospective cohort | Patients with AR symptoms (n = 442), median age 33 years. | Serum tIgE >98.7 IU/mL | tIgE cutoff: 98.7 IU/mL is a strong predictor of AR. (OR 6.93; 95% CI, 4.19–9.62; p < 0.001); AUC: 0.79 [range, 0.74–0.83]; PPV: 71.3%; NPV: 73.7%. |
| Marinho et al.893 | 2007 | 2b | Whole-population birth cohort | 478 children from MAAS | Serum tIgE (kU/L), continuous variable | Borderline association with current rhinitis (UnAdjORb 1.2; 95% CI, 1.02–1.3), not significant at multivariate analysis. Association with current rhinoconjunctivitis (UnAdjORb 1.3; 95% CI, 1.1–1.5), not significant at multivariate analysis. |
| Li et al.901 | 2016 | 3b | Retrospective case series | Patients from otolaryngology clinic. Total patients (n = 610 adults, 349 with AR), median age 27.0 years. | Serum tIgE (IU/mL), continuous variable | Serum tIgE were higher in AR (166.0 [range, 58.4–422.5] IU/mL) than in NAR pts (68.8 [range, 24.5–141.0]) IU/mL. p < 0.001 |
| Chung et al.899 | 2014 | 3b | Retrospective case series | Patients from otolaryngology clinic. Total patients (n = 1073 children and adults, 753 with rhinitis), mean age 36.9 years. | Serum tIgE level >150 IU/mL | Serum tIgE levels (cutoff value: 150 IU/mL) has good PPV (89.6%), and NPV (10%) in the in vitro diagnosis of AR (AUC: 0.88). |
| Jacobs et al.900 | 2014 | 3b | Cross-sectional | 547 children (6–14 years) from randomly selected households; 265 with skin test positive AR. | Log serum tIgE (kU/L) | Serum tIgE level are significantly associated with increased odds of skin test positive AR in children with asthma (OR 2.3; 95% CI, 1.5–3.5) but not with those without asthma (OR 1.6; 95% CI, 0.9–2.8). AR can be diagnosed if serum tIgE ≥100 kU/L both in asthmatics (AUC: 0.77 [range, 0.72–0.82], PPV: 85.1%, NPV: 68%) and in non-asthmatics (AUC: 0.84 [range, 0.79–0.89], PPV: 77.8%, NPV: 90.9%). |
| Hatcher et al.897 | 2013 | 3b | Retrospective case series, followed by a prospective study |
|
Serum tIgE (kU/mL), continuous variable | Elevated serum tIgE in the presence of a negative inhalant-specific IgE screen may suggest the presence of unidentified inhalant allergen sensitization or chronic respiratory inflammatory disease other than AR. Mean serum tIgE of the study group was 363.3 kU/L vs control group 2.2 kU/L, p < 0.0001. |
| Karli et al.898 | 2013 | 3b | Retrospective case series | Patients from otolaryngology clinic with at least 2 complaints of nasal itching, nasal obstruction, rhinorrhea, and sneezing, and/or presumed AR (n = 295), mean age 33.9 years. | Serum tIgE (U/mL), continuous variable | tIgE <20 U/mL in 23.7%, tIgE 20-100 U/mL in 38.3%, tIgE >100 U/mL 33.8%. tIgE is a factor in confirming the diagnosis, but routine use is not recommended due to high cost and testing time. |
| Salo et al.454 | 2011 | 3b | Cross-sectional | 7398 subjects (>6 years) from NHANES 2005–2006. | Serum tIgE (kU/L), continuous variable | Association with current HF (OR 1.9; 95% CI, 1.4–2.4). |
| Children (6–17 years) | Serum tIgE >40.8 kU/L (median) | Association with current HF (OR 2.1; 95% CI, 1.4–3.1). | ||||
| Serum tIgE (kU/L), continuous variable | Association with current HF (OR 2.2; 95% CI, 1.1–4.4). | |||||
| Adults (>18 years) | Serum tIgE (kU/L), continuous variable | Association with current HF (OR 1.9; 95% CI, 1.4–2.6). | ||||
| Male | Serum tIgE (kU/L), continuous variable | Association with current HF (OR 2.1; 95% CI, 1.6–2.8). | ||||
| Female | Serum tIgE (kU/L), continuous variable | Association with current HF (OR 1.7; 95% CI, 1.2–2.3). | ||||
| Kalpaklioglu et al.894 | 2009 | 3b | Retrospective case series | Consecutive and unselected pts from a tertiary care clinic (n = 323,205 with AR); mean age 31.7 years | Serum tIgE (IU/mL), continuous variable | Serum tIgE higher in AR (261) than in NAR (126), p < 0.01. |
| Ando & Shima892 | 2007 | 3b | Cross-sectional | School children (n = 98 with AR), 9–10 years old | Serum tIgE levels (IU/mL) expressed as geometric means, continuous variable | Serum tIgE higher in AR (230.4; 95% CI, 157.6–337.0) than in NAR (96.5; 95% CI, 76.9–121.1), p < 0.001 |
All reported ORs are adjusted unless differently specified and are reported with 95% CIs in parentheses.
The OR indicates an increase in the risk of current rhinitis/chronic RC per log unit increase of IgE levels.
AR = allergic rhinitis; AUC = area under the curve; CI = confidence interval; HF = hay fever; IgE = Immunoglobulin E; LOE = level of evidence; MAAS = Manchester Asthma and Allergy Study; NAR = non-allergic rhinitis; NHANES = The National Health and Nutrition Examination Survey; NPV = negative predictive value; OR = odds ratio; PPV = positive predictive value; RC = rhinoconjunctivitis; SPT = skin prick test; tIgE = total immunoglobulin E; UnAdjOR = unadjusted odds ratio.
TABLE VIII.F.1-2.
Evidence indicating a limited role for the use of total IgE in allergic rhinitis or allergy diagnosis
| Study | Year | LOE | Study design | Study groups | Endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Park et al.902 | 2016 | 2b | Prospective cohort | 313 schoolchildren, 2-year follow-up study | Initial examination: no allergic sensitization, serum tIgE <17.7 IU/mL | No association with newly developed allergic nasal symptoms. |
| Tu et al.904 | 2013 | 2b | Population-based cohort | 1321 children (5-18 years) from PATCH study | Serum tIgE (kU/L) | AUC of serum tIgE for diagnosing rhinitis: 0.70. |
| Serum tIgE >77.7 kU/L | Sensitivity: 74.7%, specificity: 56.6%, PPV: 41.9%, NPV: 84.2% | |||||
| Serum tIgE >164.3 kU/L | Sensitivity: 57.0%, specificity: 71.3%, PPV: 45.5%, NPV: 79.8% | |||||
| Serum tIgE >100 kU/L | Sensitivity: 68.1%, specificity: 62.5%, PPV: 43.2%, NPV: 82.4% | |||||
| Insufficient diagnostic accuracy of serum tIgE levels to detect allergic diseases regardless of cutoff value used. Serum tIgE is linked more to atopy than directly to symptoms. | ||||||
| Tay et al.905 | 2016 | 3b | Retrospective case series | 352 patients with serum tIgE >1000 IU/mL attributable to atopic eczema, allergic bronchopulmonary aspergillosis, helminthic infection, and rare primary immunodeficiencies. (n = 84 with AR) | serum tIgE (IU/mL) | The elevated IgE level in AR is of limited diagnostic utility. |
| Satwani et al.903 | 2009 | 3b | Cross-sectional | 258 patients (6 months-12 years) from a Pediatric Medicine Unit (n = 172 with AR) | Elevated serum tIgE | No association of tIgE and AR (UnAdjOR 1.3; 95% CI, 0.8-2.2). |
AR = allergic rhinitis; UnAdjOR = unadjusted odds ratio; AUC = area under the curve; CI = confidence interval; IgE = immunoglobulin E; LOE = level of evidence; NPV = negative predictive value; PATCH = Prediction of Allergies in Taiwanese Children; PPV = positive predictive value; tIgE = total immunoglobulin E.
Perhaps the strongest statement that can be made on behalf of tIgE is its ability to generally identify patients or populations with atopic or allergic disease. For example, Ando and Shima892 reported that tIgE is higher in children with AR than in peers with NAR. Marinho et al.893 found a borderline association between tIgE and current rhinitis. In a retrospective study, Kalpaklioglu and Kavut894 reported that tIgE is higher in AR than in NAR. Jung et al.895 conducted a prospective study that showed a tIgE cutoff of 98.7 IU/mL as a strong predictor of AR. Salo et al.454 performed a cross-sectional study reporting significant associations between tIgE levels and current hay fever in different age classes. Demirjian et al.896 demonstrated that a tIgE level over 140 IU/mL is suggestive of an atopic cause for patients with clinical symptoms of AR. Hatcher et al.897 showed that an elevated tIgE in the presence of a negative inhalant-specific IgE screen may suggest the presence of unidentified inhalant allergen sensitization or chronic respiratory inflammatory disease other than AR. Karli et al.898 reported that tIgE is helpful in confirming the diagnosis but it cannot be recommended for routine use due to its high cost and the time to perform the test. Chung et al.899 reported that tIgE (cutoff value 150 IU/mL) is a reliable biomarker for AR diagnosis. Jacobs et al.900 reported a favorable role of measuring tIgE in diagnosing AR, mainly if levels are higher than 100 IU/mL. Li et al.901 observed that tIgE is higher in AR than in NAR in a retrospective study. Finally, in a 2-year follow-up study, Park et al.902 showed that in subjects without allergic sensitization at the initial examination, tIgE greater than 17.7 IU/mL was associated with the risk for allergic sensitization, whereas in patients with allergic symptoms but negative SPT results at the initial examination, tIgE greater than 17.4 IU/mL was associated with newly developed allergic sensitization.
In contrast, there are 4 studies with negative results in the setting of tIgE and AR/allergy. Satwani et al.903 reported no association between tIgE level and AR diagnosis. Tu et al.904 demonstrated an insufficient diagnostic accuracy of tIgE levels to detect allergic diseases regardless of which cutoff value is being used; tIgE was linked more to atopy than directly to symptoms. In the same follow-up study noted above, Park et al.902 reported that in subjects without allergic sensitization at the initial examination, tIgE less than 17.7 IU/mL was not associated with newly developed allergic nasal symptoms. Finally, Tay et al.905 conducted a retrospective analysis in patients with high tIgE levels (> 1000 IU/mL) and concluded that the elevated IgE level in AR is of limited clinical/diagnostic value.
Another opportunity offered by tIgE assessment is the ratio between allergen-specific and tIgE. It has been reported that this ratio might be useful in the prediction of AIT effectiveness,906-908 as recently outlined by the EAACI Position Paper.909
In summary, tIgE is frequently increased in AR, but the clinical utility is modest in common practice. In fact, the literature is a divergent set of studies that fails to find a consistent role or value for tIgE in the management of AR patients.
Aggregate Grade of Evidence: C (Level 2b: 5 studies; Level 3b: 10 studies; Tables VIII.F.1-1 and VIII.F.1-2).
Benefit: Possibility to suspect allergy in a wide screening.
Harm: Low level does not exclude allergy.
Cost: Modest cost of test.
Benefits-Harm Assessment: Slight preponderance of benefit over harm. In addition, the ratio tIgE/sIgE may be useful.
Value Judgments: The evidence does not support a routine use.
Policy Level: Option.
Intervention: Total IgE assessment is an option to assess atopic status.
VIII.F.2. Serum antigen-specific IgE (sIgE)
sIgE testing became commercially available in 1967 with an assay reliant on radioactive anti-IgE for labeling IgE in serum.910,911 This radioactive technique, known as RAST, has largely been replaced with other technologies using enzymatically-driven reactions to produce a chemiluminescent, colorimetric, or fluorimetric reaction quantified or “read” by an autoanalyzer.910,912 The process is as follows: allergens are bound to a substrate (typically in the form of a solid or liquid phase) to which a patient’s serum is added. sIgE in the patient’s serum then binds to the allergen on the substrate. Excess serum is washed off and with it, any unbound IgE. Non-human anti-IgE antibodies tagged by a marker are subsequently added and bind any corresponding sIgE that is immobilized. Excess anti-IgE antibodies are then washed off and the autoanalyzer reads the intensity of the radioactive, chemiluminescent, colorimetric, or fluorimetric reaction. The intensity of the reaction is proportional to the amount of sIgE in the serum and a report is generated. All tests approved by the FDA are calibrated against a World Health Organization (WHO) tIgE standard serum.913 Different units are reported depending on the assay system used, but many vendors offer conversion factors.
Serum sIgE testing offers several benefits. The safety profile of serum sIgE testing is the best of all available allergy tests as the risk for anaphylaxis is nonexistent. Furthermore, the use of skin testing is limited by the presence of certain medical conditions. In patients where skin testing is contraindicated or potentially impacted by medications or skin conditions, sIgE testing offers a safe and effective option for determining the presence of sensitization as a biomarker of IgE-mediated hypersensitivities and confirming specific allergen triggers.
There are some important similarities and differences between skin testing and sIgE testing that warrant discussion. First, studies have indicated that while patients are accepting of both in vitro and in vivo allergy testing, skin testing may be preferred because it allows for immediate feedback and visible results.914 Second, neither skin or sIgE testing can definitively predict the severity of a patient’s sensitivity to an aeroallergen. Third, cross-reacting allergens and poly-sensitizations can confound both skin and in vitro testing, leading to false-positive results.915 In contrast to skin testing, sIgE tests use more extensively quality-controlled allergens and defined human serum controls. Whereas skin testing depends upon the clinician administering and interpreting the test, sIgE tests have coefficients of variation less than 15% in the College of American Pathologists diagnostic allergy proficiency survey, which is performed 3 times per year by all Clinical Immunology Laboratories licensed by the Clinical Laboratory Improvement Act of 1988. However, several reports have demonstrated poor agreement in results from testing the same sera by different commercially available assay systems.916,917 As with skin testing, sIgE results should be interpreted within the context of the patient’s clinical history.
One application of sIgE technology is multiallergen screens consisting of 10 to 15 allergens. In scenarios where a clinician wishes to either rule in or out allergy as a driving factor behind symptoms without subjecting patients to the time and cost of a full testing battery, sIgE screens are an option. Generally, either a negative or positive result is given. Screens testing for 10 to 12 allergens (ie, molds, regional pollens, cat, and mite) are positive in up to 95% of patients who would have tested positive on a larger battery.912,918 Therefore, they are effective in identifying allergic patients. Conversely, if the test is negative, there is evidence that this reliably supports an absence of allergy.910 A second application lies in the fact that levels of sIgE may correlate with severity of AR symptoms.919-923 Given that patients with more severe symptoms have been shown to respond better to AIT than those with milder symptoms, sIgE may help in the selection of candidates for AIT and possibly predict the response.919,924 Third, in polysensitized patients, it can be difficult to determine the most relevant allergen on SPT. In these situations, sIgE levels can help discriminate the most relevant allergen and guide AIT.920
Studies have shown that sIgE testing has a sensitivity between 67% and 96% and specificity of between 80% and 100%.793,822,835,925,926 Further, it has been demonstrated that sIgE shows excellent correlations with both NPT and SPT in the diagnosis of AR.793,822,835,857,911 There is good evidence to show that sIgE is, in many ways, equivalent to SPT.218,818,925 The decision to perform sIgE must be based upon a thorough history and physical examination to confirm the presence of allergy and guide therapy when necessary. It is important to note that while sIgE levels are a biomarker of allergic sensitization, this test alone cannot provide a definitive diagnosis of allergy due to the high rate of clinically irrelevant (false-positive) tests without an indicative clinical history. Based on the reviewed literature, sIgE testing is an acceptable alternative to skin testing and is safe to use in patients who are not candidates for skin testing (Table VIII.F.2).
TABLE VIII.F.2.
Evidence for the use of serum sIgE testing in the diagnosis of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Chinoy et al.927 | 2005 | 3b | Prospective cohort | Patients with AR and/or bronchial asthma (n = 118) | Compare skin test reactivity with serum sIgE antibodies | For 4 indoor allergens, skin test was more sensitive than RAST. Skin test and RAST scores showed weak to moderate correlation. |
| Pumhirun et al.835 | 2000 | 3b | Prospective cohort | Perennial rhinitis patients | Compared sensitivity and specificity of SPT to sIgE assay for D. pteronyssinus and D. farinae | sIgE for D. pteronyssinus and D. farinae had sensitivity of 96.3% and 88.9% and specificity of 96.2% and 88.9%, respectively. This compared to sensitivity of 90.4% and 86.4% and specificity of 99.5% and 93.1% for SPT, respectively. |
| Wood et al.793 | 1999 | 3b | Prospective cohort | Patients with cat allergy determined by history and a cat-exposure model | Compared the predictive values of SPT, IDT and RASTs in the diagnosis of cat allergy | SPT and RAST values exhibited excellent efficiency in diagnosis of cat allergy. IDT added little to the diagnostic evaluation. Overall sensitivity and specificity of RAST was 69% and 100%, respectively. |
| Tschopp et al.822 | 1998 | 3b | Prospective cohort | Randomly selected sample of 8329 Swiss adults | Compared the sensitivity, specificity, PPV and NPV of SPT, tIgE, and fluoroenzyme immunoassay in diagnosing AR | Sensitivity of fluoroenzyme immunoassay was significantly higher than SPT and IgE. SPT was more specific and had a better PPV. SPT was the most efficient test to diagnose AR. |
| Ferguson & Murray926 | 1986 | 3b | Prospective cohort | 168 children with clinical suspicion of allergy to cats and/or dogs | Compared the predictive values of skin tests and RASTs in children with history of allergy to cats and/or dogs | RAST sensitivity and specificity was 71%-74% and 88%-90%, respectively. SPT sensitivity and specificity 68%-76% and 83%-86%, respectively. |
| Ownby & Bailey925 | 1986 | 3b | Prospective cohort | Children age 4–19 years | Diagnostic levels by MAST and RAST were compared to skin test reactions for ragweed, grass, house dust, and mite | MAST had a sensitivity of 59%, specificity of 97%, efficiency of 72%, compared with 67%, 97%, and 78%, respectively, for RAST. Neither MAST or RAST as sensitive as skin test. |
| Reddy et al.857 | 1978 | 3b | Prospective cohort |
|
To determine the clinical relevance of positive intracutaneous test when epicutaneous test is negative | Good agreement between SPT, RAST, and NPT. Poor agreement between positive IDT at 1:1000 concentration and SPT, RAST, and NP tests. |
| Wide et al.911 | 1967 | 3b | Prospective cohort | 31 allergic patients | AcR of minimal CSA of nasal cavity | Good correlation between provocation tests and in vitro tests for allergy. |
| Seidman et al.761 | 2015 | 5 | Guideline | Not applicable | Not applicable | Clinicians should perform and interpret or refer for sIgE (skin or blood) allergy testing for patients with a clinical diagnosis of AR who do not respond to empiric treatment or the diagnosis is uncertain. |
| Bernstein et al.818 | 2008 | 5 | Review-practice parameter | Not applicable | Not applicable | Sensitivity of sIgE ranges from 50% to 90% with an average of 70% to 75%. sIgE may be used along with history and physical for diagnosis of allergy and may be preferable in certain conditions. |
AcR = acoustic rhinometry; AR = allergic rhinitis; CSA = cross-sectional area; IDT = intradermal testing; LOE = level of evidence; MAST = multiple allergosorbent test; NP = nasal provocation; NPV = negative predictive value; PPV = positive predictive value; RAST = radioallergosorbent test; SPT = skin-prick testing.
Aggregate Grade of Evidence: B (Level 3b: 7 studies; Table VIII.F.2).
Benefit: Confirms sensitization in support of an AR diagnosis and directs appropriate therapy while possibly avoiding unnecessary/ineffective treatment; guides avoidance measures; and directs AIT.
Harm: Adverse events from testing including discomfort from blood draw, inaccurate test results, false-positive test results, misinterpreted test results.
Cost: Moderate cost of testing.
Benefits-Harm Assessment: Preponderance of benefit over harm.
Value Judgments: Patients can benefit from identification of their specific sensitivities. Further, in some patients who cannot undergo skin testing, sIgE testing is a safe and effective alternative.
Policy Level: Recommendation.
Intervention: Serum sIgE testing may be used in the evaluation of AR. Using standardized allergens and rigorous proficiency testing on the part of laboratories may improve accuracy.
VIII.F.3. Correlation between skin and in vitro testing
Allergen skin testing has been used to diagnose allergic disease since first introduced by Blackley 140 years ago.791,928 The discovery of IgE in 1969 allowed for the development of in vitro serological tests which have become increasingly utilized.929 However, skin testing and sIgE serology portend unique biological functions. Therefore, the 2 tests are not fully interchangeable.
Modern SPT of aeroallergens can be up to 25% more sensitive than sIgE serology depending on the patient population and the methodologies employed.793,930-934 In the United States, SPT also generally costs about one-half as much as sIgE serology ($6.82 vs $12.50 per allergen tested).935 Other factors to consider include access to laboratory technology, comorbid disease, and the age of the patient. In vitro testing avoids the need to withhold medications that affect skin testing and allows for testing in subjects with dermatographism or other widespread skin disorders. SPT measurements are directly observable within 20 minutes, which is typically much faster than laboratory reports are obtained. Both sIgE serology and SPT are considered very safe techniques; however, SPT does carry a very small risk of anaphylaxis.
The sensitivity and specificity of SPT depends on the allergen tested, quality of reagents, the specific methodologies employed, technician expertise, and patient demographics.928,937-942 For example, SPT wheal size and sensitivity depend on the specific device selection and the choice of control reagents used for testing.928,938 Nonetheless, a recent meta-analysis indicates that SPT remains an accurate test, which when combined with a detailed clinical history, helps confirm the diagnosis of AR830 (Table VIII.F.3-1).
TABLE VIII.F.3-1.
Evidence for various allergy testing techniques
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Nevis et al.830 | 2016 | 1a | Systematic review | AR | SPT accuracy | Various factors determine SPT accuracy. |
| de Vos et al.931 | 2013 | 1b | Validating cohort | AR and asthma | Concordance of SPT and serology | SPT and serology are discordant. |
| Sharma et al.932 | 2008 | 1b | Validating cohort | Mouse allergy | RAST vs SPT vs intradermal test | Sensitivity and specificity differ across tests. |
| Carr et al.939 | 2005 | 1b | Prospective controlled trial | AR | Evaluation of 8 devices for skin testing | Consensus guidelines on skin testing. |
| Wood et al.793 | 1999 | 1b | Validating cohort | Cat allergy | RAST vs SPT vs intradermal test | Sensitivity and specificity differ across tests. |
| Nelson et al.937 | 1998 | 1b | Validating cohort | All subjects | Wheal and flare of various devices. | Results of SPT depend on device, technique, and control reagents chosen. |
| Nelson et al.856 | 1996 | 1b | Validating cohort | AR to grass | Intradermal test vs challenge | Positive intradermal test may not be relevant if SPT negative. |
| Adinoff et al.948 | 1990 | 1b | Validating cohort | AR | SPT results | SPT is accurate for various aeroallergens. |
| Jung et al.792 | 2010 | 1c | All or none case series | HDM allergies | ImmunoCAP versus SPT | Sensitivity and specificity depend on patient demographics. |
| Gendo & Larson930 | 2014 | 2a | Systematic review | AR | Utility of allergy testing | History and pretest probability determine allergy testing utility. |
| Haxel et al.947 | 2016 | 2b | Retrospective cohort | AR | Nasal challenge vs SPT vs RAST | Nasal challenge should be performed to confirm eligibility for HDM AIT. |
| Tantilipikorn et al.949 | 2015 | 2b | Individual cohort | AR | Intradermal test vs serum sIgE | Intradermal testing has higher sensitivity and lower specificity than sIgE for HDM. |
| Tversky et al.928 | 2015 | 2b | Individual cohort | All subjects | Wheal and flare of various devices | Results of SPT depend on device, technique, and control reagents chosen. |
| Choi et al.943 | 2005 | 2b | Retrospective cohort | HDM allergy | RAST vs SPT | IgE cutoff level determine sensitivity and specificity. |
| McCann & Ownby942 | 2002 | 2b | Individual cohort | AR | SPT measurements | SPT results are not reproducible across centers. |
| Pastorello et al.946 | 1995 | 2b | Exploratory case-control | AR | ImmunoCAP vs SPT | Specific IgE accuracy depend on cutoff values. |
| Westwood et al.794 | 2016 | 3a | SR | AR | Microarray results | Utility and cost of microarray testing needs further validation. |
| Mucci et al.791 | 2011 | 3a | SR | AR | Review of AR | Review of AR diagnosis and treatment. |
AIT = allergen immunotherapy; AR = allergic rhinitis; HDM = house dust mite; IgE = immunoglobulin E; LOE = level of evidence; RAST = radioallergosorbent test; sIgE = allergen-specific IgE; SPT = skin-prick test.
The performance and reliability of serum sIgE testing likewise depends on several factors including the choice of reagents, modernization of equipment, and patient demographics.932 The cutoff value for a positive test affects both the sensitivity and specificity.943 In a Korean population, SPT was found to be superior to ImmunoCAP for measuring dust mite sensitivity if the patient was less than 30 years of age.792 For the group older than age 50 years, ImmunoCAP was more sensitive.792 Intradermal or epicutaneous testing demonstrates higher sensitivity but lower specificity than SPT for several allergens.793,856,931,932,944 Based on this, intradermal tests should be selected judiciously. There is evidence to suggest that a positive intradermal reaction to grass pollen in the setting of negative prick testing may not be clinically relevant.793,856
In recent years, microarray allergy testing systems such as ImmunoCAP ISAC (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden) have been introduced in an effort to offer a comprehensive in vitro allergen test panel.794 The precision and utility of microarray testing needs more rigorous scrutiny so that consensus guidelines can be more firmly established.794,945 The cost of a single Immuno-CAP ISAC test, which includes 112 components from 51 allergens, is approximately $500 to $600 in the United States.794,945
Various studies have compared sIgE serology to allergen SPT.793,943,946,947 Both techniques are sensitive and are generally well correlated; however, interpretation of the results depends upon the gold standard reference used to define allergic status. Environmental chambers, nasal challenge, and validated questionnaires are typically used to determine the diagnostic accuracy of allergen testing. Table VIII.F.3-2 summarizes several comparative studies between skin testing for aeroallergens, specific IgE serology, and other in vitro tests.
TABLE VIII.F.3-2.
Comparative studies of allergy testing techniques
| Test | Allergen | Sensitivity | Specificity | Gold standard |
| Skin-prick test | HDM | 66.3-90.5% | 47.6-95.2% | Bronchoprovocation,943 survey,946 nasal challenge943, 947 |
| Grass | 61.6-76% | 61-85.7% | Survey856, 946 | |
| Cat | 90% | 90-92.7% | Survey,948 cat room793 | |
| Mouse | 67% | 94% | Nasal challenge932 | |
| Skin intradermal test | HDM | N/A | 85% | Nasal challenge949 |
| Grass | 78.6% | 75% | Nasal challenge856 | |
| Cat | 60% | 39.5-46.2% | Cat room793 | |
| Mouse | 100% | 65% | Nasal challenge932 | |
| sIgE (ImmunoCAP) | HDM | 61.6-76.3% | 47.6-72.8% | Bronchoprovocation,943 survey,946 nasal challenge943, 947, 949 |
| Grass | 69-75.5% | 76.5% | Survey946 | |
| Cat | 48% | 100% | Cat room793 | |
| Mouse | 74-92.2% | 91% | Nasal challenge932 |
HDM = house dust mite; N/A = not available; sIgE = allergen-specific IgE.
It is important to understand that selection and interpretation of allergen testing is not based on sensitivity and specificity alone. The intended physiological mechanism to be interrogated also needs to be considered. SPT and intradermal testing both measure end-organ pathological mechanisms associated with sIgE bound to the surface of mast cells. In contrast, serum sIgE testing and microarray approaches measure circulating IgE that may or may not represent downstream allergic inflammatory responses. Both intradermal testing and SPT rely heavily on technician skill for interpretation of the wheal and flare reaction.856,928,937 In the case of subjects with dermatographism (or other inflammatory skin conditions in the testing area), hairy arms, or darkly pigmented skin color, the interpretation of the SPT can prove to be difficult.942 Specialized imaging systems have been developed to measure the wheal reaction in an automated fashion in both light and dark skinned individuals, but additional validation is required. Until these automated systems become more widespread, in vitro testing affords the benefits of temporal and multicenter reproducibility.
The average pooled sensitivity of SPT is 85% which is often slightly higher than that of serum sIgE testing830; however, this is not universally true depending on the allergen tested and the characteristics of the patient. Based on accuracy, convenience, cost, and promptness of results, SPT is often chosen as the first line diagnostic instrument to detect sensitivity to aeroallergens. Intradermal testing can be used as a second line test to exclude reactivity if the clinical suspicion is very high. In cases where dermatographism is present and/or patients are unable to wean off medications that affect skin testing, sIgE testing may be a better choice. More studies are required to determine the role of small volume blood testing through emerging microarray technology such as the ImmunoCAP ISAC.
Aggregate Grade of Evidence: B (Level 1a: 1 study; Level 1b: 7 studies; Level 1c: 1 study; Level 2a: 1 study; Level 2b: 6 studies; Level 3a: 2 studies; Level 5: 1 study; Table VIII.F.3-1).
VIII.F.4. Nasal specific IgE
AR is classically diagnosed by clinical history and with objective testing for confirmation, usually SPT or in vitro testing with serum sIgE.301 In addition to positive systemic sIgE, AR patients have been shown to have sIgE in the nasal mucosa with evidence that class switching and antibody production occurs locally.309-312,377,950,951 However, some patients have negative SPT or serum sIgE despite a clinical history suggestive of AR and meeting ARIA clinical criteria.101,300 These patients are usually given the diagnoses of idiopathic rhinitis, vasomotor rhinitis, or NAR.300 However, it has been demonstrated that many of these patients may have local allergic phenomena or LAR, a type of rhinitis characterized by the presence of a localized allergic response in the nasal tissues, with local production of sIgE and positive response to NPT without evidence of positive SPT or serum sIgE elevation.107 LAR may affect more than 45% of patients otherwise categorized as NAR,296,302,952 and up to 25% of patients referred to allergy clinics with suspected AR.291 Like traditional AR patients, LAR can be classified as perennial or seasonal, and similar findings in the nasal mucosa have been reported in both of these populations.300,301,953 It has even been suggested that some patients with occupational rhinitis may suffer from LAR.107 Recent studies suggested a low rate of conversion of LAR to systemic AR.296,302 The first 5 years of a long-term followup study performed in a cohort of 194 patients with LAR and 130 healthy controls found that patients with LAR of recent onset (less than 18 months from the diagnosis) had a similar conversion to systemic AR when compared to controls.296 A small retrospective study performed in 19 patients with a long clinical history of LAR (greater than 7 years from the diagnosis) and negative SPT to a wide panel of allergens had a similar rate of development of systemic AR302 compared with epidemiologic data of prevalence of atopy in a healthy population from that geographic area.954 Upcoming data from the 10-year follow-up study should help to clarify the rate of a long-term conversion to systemic AR in patients with LAR. In fact, LAR can present later in life, and in elderly patients with rhinitis the incidence of LAR has been reportedly been as high as 21%.304
The diagnosis of LAR is confirmed by positive response to NPT, and evidence of sIgE in the nasal secretions. A variety of allergens have been tested in this fashion including dust mites, grasses, pollens, and molds.300,301,306,307,955 The production of nasal mast cells, eosinophils, and sIgE rapidly increases after allergen-specific stimulation in the nasal mucosa.288,294,307 Different methods have been reported regarding how to best identify nasal sIgE including nasal lavage, cellulose disks, mucosal biopsy, and brushing (Table VIII.F.4). While there is no gold standard, most of these techniques appear to yield similar results in identifying nasal sIgE in LAR patients. Additionally, normative data for nasal sIgE levels and their clinical correlations have yet to be established and agreed upon, but work has begun in this area.956
TABLE VIII.F.4.
Evidence for nasal sIgE testing
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kim et al.958 | 2016 | 2b | Cross-sectional | Collection technique: cotton ball.
|
NPT, nasal sIgE | Nasal sIgE detected in all patients, no difference between NPT groups. No comparison pre- and post-NPT was performed. |
| Lee et al.959 | 2016 | 2b | Cross-sectional | Collection technique: nasal lavage.
|
Nasal sIgE | AR with higher nasal sIgE to HDM than NAR, no difference between adults and children. Correlation between nasal and serum IgE only in children. |
| Bozek et al.304 | 2015 | 2b | Cross-sectional | Collection technique: nasal lavage.Elderly patients, (n = 219) | NPT, nasal sIgE | LAR and AR common in elderly patients. 21% with LAR, 40.2% with AR, and 38.8% with NAR. |
| Sakaida et al.960 | 2014 | 2b | Cross-sectional | Collection technique: suction of nasal secretions (n = 46 participants, 33 sensitized to allergen) | Nasal sIgE | 93% had nasal sIgE, higher levels in sensitized subjects, correlation between nasal and serum sIgE. |
| Fuiano et al.955 | 2011 | 2b | Cross-sectional | Collection technique: cellulose membrane.
|
NPT, nasal sIgE | Nasal sIgE to Alternaria detected in 69% of positive NPT. |
| López et al.306 | 2010 | 2b | Cross-sectional | Collection technique: nasal lavage.
|
Nasal tIgE, sIgE, tryptase, eosinophil cationic protein, symptoms | LAR: Nasal sIgE to D. pteronyssinus detected in 25% immediately and at 24 hours, increase mast cells/eosinophils. Controls: Negative NPT, nasal sIgE, and other markers. |
| Powe et al.950 | 2010 | 2b | Cross-sectional | Collection technique: cotton ball, immunohistochemistry.
|
Nasal Ig free light chains | Free light chains increased in AR and NAR nasal mucosa, suggesting role in hypersensitivity. |
| Rondon et al.307 | 2009 | 2b | Cross-sectional | Collection technique: nasal lavage.
|
Nasal sIgE, sIgE, tryptase, eosinophil cationic protein | 30% with nasal sIgE. LAR have local production of sIgE, mast cell/eosinophil activation. |
| Rondon et al.300 | 2008 | 2b | Cross-sectional | Collection technique: nasal lavage.
|
NPT, nasal sIgE | Nasal sIgE to grass pollen detected in 35% NAR patients with positive NPT, and with similar sIgE profile as AR. |
| Rondon et al.301 | 2007 | 2b | Cross-sectional | Collection technique: nasal lavage.
|
NPT, nasal sIgE | Nasal sIgE to HDM detected in 22% of NAR patients with positive NPT. |
| Powe et al.284 | 2003 | 2b | Cross-sectional | Collection technique: mucosal biopsy.
|
Nasal sIgE | Nasal sIgE to grass detected in 30% NAR. No nasal sIgE to HDM was detected. |
| KleinJan et al.377 | 2000 | 2b | Cross-sectional | Collection technique: mucosal biopsy.
|
Nasal B and plasma cells with IgE | sIgE produced in nasal tissue of AR patients but not healthy controls. |
| KleinJan et al.951 | 1997 | 2b | Cross-sectional | Collection technique: mucosal biopsy.
|
Nasal sIgE to grass and HDM | sIgE to grass and HDM found in SAR and PAR subjects, respectively. |
| Takhar et al.312 | 2005 | 3b | Cross-sectional, nonconsecutive | Collection technique: mucosal biopsy.
|
Nasal mRNA and gene transcripts | Allergen stimulates local class switching to IgE in the nasal mucosa. |
| Durham et al.310 | 1997 | 3b | Cross-sectional, nonconsecutive | Collection technique: mucosal biopsy.
|
NPT, nasal IgE heavy chain | Local IgE synthesis and cytokine regulation occur is the nasal mucosa of AR patients. |
| Huggins & Brostoff303 | 1975 | 3b | Cross-sectional, nonconsecutive | Collection technique: filter paper.
|
SPT, NPT, serum and nasal sIgE to HDM | Nasal sIgE in AR and NAR patients with positive NPT; but not in controls. |
| Ota et al.961 | 2016 | 4 | Descriptive | Collection technique: mucosal biopsy. AR (n = 11) | Nasal and serum sIgE | Detection of sIgE in inferior turbinate mucosa and serum. |
| Zicari et al.292 | 2016 | 4 | Descriptive | Collection technique: nasal lavage. NAR, children (n = 20) | NPT, nasal sIgE | 66% had positive NPT. Nasal sIgE present in 8% to 42%. |
| Becker et al.962 | 2015 | 4 | Descriptive | Collection technique: cotton ball. NARES (n = 19) | Nasal sIgE | No detectable nasal sIgE in any of the patients. |
| Reisacher963 | 2013 | 4 | Descriptive | Collection technique: mucosal brush. NAR (n = 20) | Nasal sIgE | Nasal sIgE detected in 100% of patients. Varied from 0% Alternaria to 90% cockroach. No association to QOL. |
| Reisacher964 | 2012 | 4 | Descriptive | Collection technique: mucosal brush. AR (n = 18) | Nasal sIgE, SPT | Nasal sIgE in 75% of subjects, association between brush testing and SPT. |
| Coker et al.309 | 2003 | 4 | Descriptive | Collection technique: mucosal biopsy. AR (n = 6) | Nasal IgE heavy chain | Somatic hypermutation, clonal expansion, and class switching occurs within the nasal mucosa of AR patients. |
| Sensi et al.965 | 1994 | 4 | Descriptive | Collection technique: nasal lavage. Children with asthma and rhinitis (n = 18) | Nasal and serum sIgE measured after allergen avoidance | Nasal sIgE may be more sensitive than serum sIgE. |
| Platts-Mills311 | 1979 | 4 | Descriptive | Collection technique: nasal lavage. AR (n = 50) | Nasal IgG, IgA, and IgE | Antibody response in AR patients is local in the nasal mucosa. |
AR = allergic rhinitis; HDM = house dust mite; Ig = immunoglobulin; IgA = immunoglobulin A; IgG = immunoglobulin G; LAR = local allergic rhinitis; LOE = level of evidence; NAR = non-allergic rhinitis; NARES = non-allergic rhinitis with eosinophilia syndrome; NPT = nasal [allergen] provocation test; PAR = perennial allergic rhinitis; SAR = seasonal allergic rhinitis; sIgE = allergen-specific immunoglobulin E; SPT = skin-prick test; tIgE = total immunoglobulin E.
When evaluating a rhinitis patient, in the setting of negative systemic testing, the differentiation of LAR from NAR can provide important information for management. While both typically respond to pharmacologic treatment, identification of offending allergens in LAR may permit allergen avoidance and immunotherapy.107 AIT is the treatment of choice for patients with AR who have failed allergen avoidance and medical therapy. Patients who are classified as NAR, would not typically be candidates for AIT. However, as previously noted, roughly 50% of patients with negative systemic testing have been shown to have LAR. In this LAR population, early studies suggest that AIT can decrease symptoms and medication usage, and improve QOL.288,957
Aggregate Grade of Evidence: C (Level 2b: 13 studies; Level 3b: 3 studies; Level 4: 8 studies; Table VIII.F.4).
Benefit: Identifying patients with LAR allows for the opportunity to treat a subset of patients who may respond to avoidance or AIT. Identification of nasal sIgE allows for diagnosis and AIT.
Harm: Measurement of nasal sIgE is minimally invasive, and no adverse effects have been reported.
Cost: Associated costs consist of the direct costs of testing, and indirect cost of increased time and effort for performing nasal sIgE diagnostic test.
Benefits-Harm Assessment: The benefits of identifying patients with an allergic component to their rhinitis may outweigh any associated risks.
Value Judgments: In patients with rhinitic symptoms and negative systemic testing, identifying nasal sIgE may assist with appropriate treatment. Standards for abnormal levels of nasal sIgE have not been established nor correlated with clinical outcomes.
Policy Level: Option.
Intervention: Nasal sIgE levels is an option in patients with suspected or known LAR to aid in diagnosis or guide allergen-specific therapy.
VIII.F.5. Basophil activation test (BAT)
The basophil activation test (BAT) is an ex vivo peripheral blood test that has been shown to be useful in the diagnosis of allergy to food and drugs, along with other hypersensitivity syndromes, when first-line tests (SPT and serum sIgE) are discordant with clinical history or do not exist, and for monitoring of AIT.966 Within the field of AR, there are small-scale trials evaluating the utility and reliability of BAT in testing for the diagnosis of specific allergens related to AR symptoms and monitoring therapy (Table VIII.F.5).
TABLE VIII.F.5.
Evidence for the use of basophil activation testing in allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Schmid et al.971 | 2014 | 1b | Open RCT | SAR to grass pollen (n = 24);
|
Clinical measures of allergy, basophil sensitivity, basophil reactivity. | Basophil sensitivity changes correspond to clinical changes in allergy symptoms in patients on SCIT. Basophil reactivity did not change. |
| Van Overtvelt et al.978 | 2011 | 1b | RCT | SAR to grass pollen (n = 89);
|
BAT using CD203c at 2 and 4 months of treatment. | BAT using CD203c did not correlate with patient response. |
| Zidarn et al.977 | 2015 | 2b | Cohort | Moderate-severe SAR to grass pollen;
|
BAT using CD63 as marker for basophil response. Evaluated after 1st pollen season, after 2nd pollen season, and 1–2 years after finishing 3–5 years of SCIT. | BAT significantly decreased with SCIT; remains decreased 1–2 years after 3–5 years of SCIT treatment. BAT is an objective measure of response to AIT and is a stable marker of allergen response over a long period. |
| Zidarn et al.976 | 2012 | 2b | Cohort |
|
CD-sens, CD63 responsiveness. Tested before and after pollen season. | CD-sens 10-fold higher in symptomatic patients. Significant difference between CD63 responsiveness in those with positive NPT vs negative NPT. CD-sens a good predictor of allergic rhinitis symptoms in those sensitized to Timothy grass pollen. |
| Lesniak et al.974 | 2016 | 3b | Case-control | Allergy patients (n = 30) diagnosed by clinical symptoms, SPT, or serum IgE.
|
BAT, basophil reactivity. | Sensitivity for basophil reactivity 83%–100%; specificity 78%–89%; PPV 75%–87%; and NPV 89%–100%. BAT may replace NPT when NPT is contraindicated. Small numbers of patients used needs to be validated in larger study. |
| Ando et al.979 | 2015 | 3b | Case-control |
|
CD203c expression on basophils when stimulated with Japanese cedar pollen. | CD203c expression has diurnal variation and should be considered when using CD203c as a marker. This was also shown in basophils derived from marrow of mice-models. |
| Campo et al.308 | 2015 | 3b | Case-control |
|
NPT, serum sIgE, BAT. | NPT positive in all AR and 10/12 LAR. Serum sIgE positive in AR, negative in LAR. BAT positive in AR and in 8/12 LAR. NPT remains the gold standard, but if unable to be done, BAT should be considered. |
| Gomez et al.318 | 2013 | 3b | Case-control |
|
BAT, nasal sIgE, NPT. | AR: BAT sensitivity 85%, specificity 93%. LAR: BAT sensitivity 50%, specificity 93%. BAT diagnosed at least 50% of cases of LAR to D. pteronyssinus and was more sensitive than detection of nasal sIgE and less time-consuming than NPTs. |
| Ozdemir et al.972 | 2011 | 3b | Case-control |
|
Discrimination of pollen allergic individuals from controls using CD203c expression as marker of allergy; cutoff values of 14%. Performed during off-season. | BAT CD203c can be used to test for grass allergens if conventional measures not available. |
| Nopp et al.969 | 2009 | 3b | Case-control |
|
CD-sens. | CD-sens decreases during early phases of treatment. No change in basophil reactivity. CD-sens good objective measure to use to assess response to AIT. |
| Ocmant et al.968 | 2007 | 3b | Case-control |
|
Tested both CD63 and CD203c expression using prescribed protocol. | 100% sensitivity for both CD63 and CD203c in cat-allergic patients. CD203 is as reliable as CD63 for diagnosis of patients with IgE-mediated allergy to cat. |
| Sanz et al.967 | 2001 | 3b | Case-control |
|
Skin tests, BAT, histamine release tests, leukotriene production. | Significant correlation between skin tests and BAT (r = 0.72, p < 0.001). Positive and significant correlation between BAT and histamine release tests (r = 0.80, p < 0.001); allergen-specific LTC4, LTD4, LTE4 production (r = 0.7, p < 0.001); and the occurrence of serum sIgE (r = 0.71, p < 0.001). BAT is a highly reliable technique in the diagnosis of allergy to inhalant allergens. BAT sensitivity = 93.3%, specificity = 98.4%, when using a cutoff point of 15% activated basophils as positive result. |
| Lesniak et al.973 | 2015 | 4 | Case series | 12 patients with AR sensitized to birch or mites | Blood sample tested 1, 4, and 24 hours after sampling compared to SPT, sIgE, and NPT. | No differences in ROC characteristics between tests. BAT can be a useful approach to determine the clinically relevant allergen in sensitized patients. |
| Nopp et al.970 | 2013 | 4 | Case series | SAR to grass pollen (n = 26) | CD-sens, nPIF. | Positive nPIF and positive CD-sens in 92%. Positive nasal symptom scores and positive CD-sens scores in 85%. Subjects tested twice: CD-sens 100% reproducible vs 78% for nasal symptom scores and 94% for nPIF. CD-sens results reproducible and correlate well with other allergen testing methods. Has potential for diagnosis and follow-up after treatment. |
| Nopp et al.975 | 2006 | 4 | Case series |
|
CD-sens, SPT, NPT, IgE antibody concentration. | CD-sens correlates significantly with SPT, NPT, and IgE antibody concentration. CD-max (reactivity) did not correlate with any sensitization measures. CD-max varies substantially between patients and does not correlate to treatment or other allergy testing measures. Using CD-sens as a quantitative measure of response to therapy or to complement other testing methods is more reliable. |
AIT = allergen immunotherapy; AR = allergic rhinitis; BAT = basophil activation test; CD-sens = EC50 for allergen concentration inverted and multiplied by 100; HDM = house dust mite; IgE = immunoglobulin E; LAR = local allergic rhinitis; LOE = level of evidence; LTC4, LTD4, LTE4 = leukotriene C4, D4, E4; nPIF = nasal peak inspiratory flow; NPT = nasal provocation test; NPV = negative predictive value; PPV = positive predictive value; RCT = randomized controlled trial; ROC = receiver operating characteristic; SAR = seasonal allergic rhinitis; SCIT = subcutaneous immunotherapy; sIgE = specific immunoglobulin E; SLIT = sublingual immunotherapy.
BAT methodology was found to be heterogeneous between trials. Most data pertaining to its accuracy used the tetraspanin CD63 (lysosome-associated membrane glycoprotein 3 [LAMP 3]) as an activation marker.967-971 CD203c (ecto-nucleotide pyrophosphatase/phosphodiesterase 3) is less frequently used.968,972 In 1 trial, it held potential as a sensitive and specific method of testing for AR as compared to CD63.968
The diagnosis of AR is a clinical decision guided by skin or serological tests; ex vivo basophil testing is rarely required. However, BAT has been shown to be comparable with traditional allergen testing methods.967,970,973,974 BAT has been shown to be useful in defining the allergen responsible for LAR in patients who have had false-negative results with first-line tests and a high suspicion for clinically-relevant allergy.308,318
Basophil reactivity (% CD63+ cells determined at 1 allergen concentration) does not reflect the effect of allergen immunotherapy. There is good evidence to suggest that basophil sensitivity (EC50, or eliciting concentration at which 50% of basophils respond; also named CD-sens if it is inverted and multiplied by 100) is a marker for treatment effect of AIT969-971,975-977 and anti-IgE treatment.975
In summary, BAT may be a useful ex vivo test when diagnosis of AR is in doubt or the allergen responsible for clinical symptoms is unknown. Basophil sensitivity is also useful for measuring response to AIT. When the methodology of BAT is more clearly standardized, it may become a more useful second line test in AR diagnosis, as using an ex vivo test is beneficial in terms of time taken to undergo testing and symptoms evoked during testing. Most studies included small samples sizes with less than 100 patients. There is an opportunity for a meta-analysis of these studies or a larger scale trial to confirm the findings of the works included in this review.
Aggregate Grade of Evidence: B (Level 1b: 2 studies; Level 2b: 2 studies; Level 3b: 8 studies; Level 4: 3 studies; Table VIII.F.5).
Benefit: Ex vivo test, patient discomfort minimal, less time consuming than nasal provocation and SPT for patient, reliable correlation between clinical symptoms and basophil sensitivity when measuring response to therapy, no risk of anaphylaxis compared to provocation testing.
Harm: None known.
Cost: Requires proximity of laboratory trained in basophil testing. Cost of testing.
Benefits-Harm Assessment: Balance of benefit over harm.
Value Judgments: Basophil sensitivity may be a useful marker for following response to immunotherapy. Differences in BAT methodology for diagnosis of AR and rare need for laboratory tests to diagnose AR make it likely to be implemented for diagnosis in tertiary care centers only.
Policy Level: Option.
Intervention: BAT is an option for AR diagnosis when first-line tests are inconclusive or for measuring response to AIT. Many small-scale studies have been completed. There is scope for meta-analysis and for larger trials to be completed.
VIII.F.6. Component resolved diagnosis (CRD)
Molecular diagnosis (MD) or component resolved diagnosis (CRD) is used in allergy to define the allergen sensitization of a patient at the individual protein level by measuring sIgE to purified natural or recombinant allergens, allowing identification of the potential disease-eliciting molecules. Overall, MD can potentially improve diagnostic accuracy (specificity), distinguish cross-reactivity phenomena from true co-sensitization, resolve low-risk markers from high-risk markers of disease activity, and may improve the indication and selection of suitable allergens for AIT when compared to diagnosis based on SPT and/or sIgE determination with raw commercial extracts.980-984 Indeed, changes in immunotherapy prescription aided by MD have been demonstrated to be cost-effective in some scenarios.985 Certain patterns of sensitization to grass or olive pollen allergens may also identify patients with higher risk of adverse reaction during immunotherapy.986,987 Nevertheless, all in vitro test results should be evaluated alongside the clinical history, since allergen sensitization does not necessarily imply clinical responsiveness.
IgE to purified or recombinant allergens is usually measured by using a fluorescence enzyme immunoassay in singleplex platforms. However, a multiplex platform with 112 allergens is also available (ISAC, Thermo Fisher Scientific, Uppsala, Sweden). Results of singleplex and multiplex platforms are not interchangeable. When comparing the singleplex and multiplex assays, concordance of results vary between allergens tested, and the sensitivity of multiplex platform is lower than that of singleplex, particularly when sIgE levels are low.983 Otherwise singleplex platforms are quantitative assays and multiplex are semiquantitative.
Specific antigens.
In the case of mite sensitivity, markers of specific sensitization include Der p 1 and Der p 2 for Dermatophagoides pteronyssinus and Dermatophagoides farinae,988 Lep d 2 for Lepidoglyphus destructor (storage mite, with limited cross-reactivity with other HDMs),989 and Blo t 5 for Blomia tropicalis (non-Pyroglyphidae mite).990 Der p 10, a tropomyosin from D. pteronyssinus, has been shown to be a good maker of clinical sensitivity to crustaceans but not a marker of sensitization to mites.991,992
Can f 1, Can f 2, and Can f 5 are specific allergen components indicating specific sensitization to dog.993 Interestingly, Can f 5, a prostatic kallikrein produced only by male dogs is responsible for monosensitivity in up to 25% to 38% of dog-allergic patients.994,995 In these cases, patients can tolerate exposure to female dogs. Fel d 1 is the major allergen component in cat allergy, indicating specific sensitization.996 Other cat allergens have some cross-reactivity with allergens from other sources; eg, Fel d 2 is likely to cross-react with other mammal albumins, such as dog Can f 3, horse Ecu c 3, pig Sus s PSA, and cow Bos d 6,997 and Fel d 4 is shown to cross-react with major allergens from horse Equ c 1, dog, or cow.998 Therefore, CRD for cat allergy provides more information about cross-reactivity and specificity of the diagnosis. Equ c 1, is the major allergen of horse dander and has some cross-reactivity with mouse Mus m 1 and cat Fel d 4.999 Equ c 3 is a serum albumin showing cross-reactivity with other mammals’ serum albumins mentioned above (i.e. Fel d 2). In summary, CRD in patients with allergy to dog, cat, and horse are not only predictive markers of allergy, but may also help clinicians to predict clinical symptoms and their severity, since some patterns of sensitization are related to more severe rhinitis and asthma.994,995
Allergens related to sensitization to cockroaches are Bla g 1, Bla g 2, Bla g 4, and Bla g 5, although in certain populations tropomyosins (Bla g 7 and/or Per a 7) can be important.1000 Alt a 1 is a major allergen that is recognized in approximately 80% to 100% of Alternaria-allergic patients.1001 Markers of sensitization to several pollen are summarized in Table VIII.F.6. Sensitization to profilin has been associated with more severe respiratory symptoms in grass-allergic patients, as well as sensitization to the minor olive allergens Ole e 7 and Ole e 9.987,1002 IgE antibodies to Phl p 1 and/or Phl p 5 can be used as specific markers of sensitization to grass pollen and Phl p 4 as a marker of sensitization to non-Pooideae grasses. However, Phl p 6 is contained only in Pooideae grasses. Allergens from groups 1, 2, 5 and 6 are only expressed in grasses but not in other plants, so they detect a genuine sensitization to grasses.981
TABLE VIII.F.6.
Pollen allergens
| Pollen | Specific components | Cross-reactivity components |
|---|---|---|
| Ragweed | Amb a 1 (pectate lyase) | |
| Mugwort | Art v 1 (defensin); Art v 3 (lipid transfer protein) |
Art v 3 (lipid transfer protein) |
| Parietaria, wall pellitory | Par j 2 (lipid transfer protein) | Par j 2 (lipid transfer protein) |
| Russian thistle or saltwort | Sal k 1 (pectinesterase) | |
| Goosefoot | Che a 1 (trypsin inhibitor) | |
| Timothy | Phl p 1 (expansin); Phl p 4 (berberine bridge enzymes); Phl p 5 (ribonuclease); Phl p 6 (Pooideae grass only) |
Phl p 4 (berberine); Phl p 7 (polcalcin); Phl p 11 (trypsin inhibitor); Phl p 12 (profilin) |
| Bermuda grass | Cyn d 1 (expansin) | Cyn d 1 and Phl p 1 |
| Alder | Aln g 1 (ribonuclease) | Aln g 1 (PR 10) |
| Birch | Bet v 1 (PR-10) | Bet v 1 (PR10); Bet v 2 (profilin); Bet v 4 (polcalcin) |
| Olive | Ole e 1 (trypsin inhibitors); Ole e 7 (lipid transfer protein); Ole e 9 (glucanase) |
|
| Japanese cedar | Cry j 1 (pectate lyases) | |
| Cypress | Cup a 1 (pectate lyases) | |
| Plane tree | Pla a 1 (invertase inhibitor); Pla a 2 (polygalacturonases) |
Pla a 3 (lipid transfer protein) |
In summary, CRD in patients with AR can help to better define the sensitization to inhalant allergens, especially in those who are polysensitized, have unclear symptoms and/or sensitization patterns, or who do not respond to treatment. On the contrary, monosensitized patients with a clear case history and symptom profile may not benefit from CRD compared to traditional diagnostic tests. Nevertheless, CRD remains a third-level approach, not to be used as a screening method in current practice. One of the most useful aspects of CRD is that it can help clinicians to better select patients and allergens for prescribing AIT,1003 and in some cases, predict the risk of adverse reactions. The pattern of sensitization to allergens may predict the severity of the disease and could potentially predict the efficacy of AIT, provided these immunotherapy products contain a sufficient amount of allergen. As there are multiple individual allergens available for CRD and several different uses for CRD, extensive evidence grading is not undertaken in this document.
VIII.G. Sensitization vs clinical allergy
Sensitization vs allergy
Although IgE-mediated sensitization has been consistently shown to be an important risk factor for rhinitis,520,1004 the strength of this association is not consistent.1005,1006 In epidemiology and clinical practice, patients are typically diagnosed as being “sensitized” based on a positive SPT (usually ≥3 mm wheal diameter), or a positive specific serum IgE (usually ≥0.35 kU/L [specific IgEs are reported in arbitrary units, thus the unit kU]).1007,1008 However, both of these tests can be positive in the absence of any symptoms, and neither positive SPT nor IgE can confirm the expression of rhinitis symptoms upon allergen exposure.1009,1010 Thus, a clear distinction has to be made between “sensitization” (which usually refers to positive allergy tests, irrespective of any symptoms), and clinical allergic disease such as AR, which denotes the presence of sensitization and related clinical symptoms.
“Positive” allergy test vs sIgE titer or SPT wheal size
Quantification of atopic sensitization by using the level of sIgE antibodies or the size of SPT wheals increases the specificity of allergy tests in relation to the presence and severity of rhinitis.893,1004 This has changed the way we interpret the results of allergy tests, with a move from dichotomization (labeling patients as being sensitized based on a “positive” test using arbitrary criteria), to quantification of blood or skin tests using sIgE titer and SPT wheal size.893,1010-1012
Whole-allergen extract vs individual allergenic molecules
Homologous proteins present in the whole-allergen extracts from different allergen sources may be cross-reactive (eg, profilins and PR-10 proteins in various plants, or tropomyosin present in mites, various insects, and shrimp). Thus, a positive test to the whole-allergen extract may reflect sensitization to a cross-reactive component.1013 Measuring sensitization to individual allergen molecules in a CRD may more be informative than standard tests using whole-allergen extracts.470,1014-1016 Current multiplex CRD platforms allow the testing for component-specific IgE to more than 100 allergenic molecules in a single assay, and in a small volume of serum.1013,1015 The patterns of component-specific IgE responses to multiple allergenic proteins have a reasonable discrimination ability for rhinoconjuinctivitis,1017 and distinct patterns of IgE responses to different protein families are associated with different clinical symptoms. For example, sensitization to proteins of plant origin strongly predicts AR, and sensitization to animal lipocalins is predictive of asthma.1018,1019 The risk of allergic disease increases with the increasing number of sensitizations to individual allergenic proteins, and IgE polysensitization to several HDM molecules strongly predicts rhinitis.1019,1020 It is important to emphasize that the age of onset of sensitization is crucially important, and that development of AR may be predicted by the unique molecular nature of IgE responses to individual allergen components.1019
Disaggregating atopic sensitization
It is becoming increasingly clear that “atopic sensitization” is not a single phenotype, but an umbrella term for several different atopic vulnerabilities which differ in their association with rhinitis and asthma.1021,1022 Different subtypes of atopy are characterized by a unique pattern of the responses to different allergens and the timing of onset of allergen-specific sensitization.1023 Translation of these findings into clinical practice requires the development of biomarkers which can differentiate between different subtypes of sensitization, and can be measured at the time of clinical evaluation.
Beyond IgE
Recent data suggest that among individuals sensitized to grass pollen, the decreasing ratio of grass allergen-specific IgG/IgE antibodies is associated with increasing risk of symptomatic SAR,1024 suggesting that the IgG/IgE ratio may help distinguish between “benign” sensitization (sensitization with no symptoms) and “pathologic” sensitization.1024 However, the measurement of allergen-specific IgG cannot as yet be recommended in a routine clinical practice.1009,1010
VIII.H. Allergen challenge testing
VIII.H.1. Allergen challenge chambers (ACCs)
Environmental exposure chambers (EECs) have been used for decades for controlled exposure of subjects to a well-defined atmosphere of a variety of substances such as allergens, particulate and gaseous air pollutants, chemicals, or climate conditions. The generation of valid exposure conditions with high temporal and spatial stability is technically demanding, and there are a limited number of EECs world-wide. Besides the opportunity to use EECs for well-designed mechanistic studies on the effect of environmental pollutants on human health, allergen challenge in the chamber setting with induction of symptoms in patients with allergic disease is an intriguing way for efficacy testing of new drugs. Therefore, several chamber facilities were installed in recent years with the focus on allergen exposure resulting in currently 15 allergen challenge chamber (ACC) facilities around the globe.1025
ACC studies have contributed to our understanding of the pathophysiology of allergic diseases. For example, it has been demonstrated that controlled allergen exposure exacerbates atopic dermatitis.1026 Also, the impact of exposure with pollen allergen fragments on AR symptoms has been shown.1027 Furthermore, the importance of the integrity of the epithelial barrier for induction of local and systemic inflammatory responses has been investigated in patients with allergic rhinoconjunctivitis using the ACC setting.1028
The use of ACCs in clinical trials for efficacy testing of investigational new drugs, and their acceptance by regulatory authorities is peremptorily dependent on the technical and clinical validation of ACCs. Many ACCs have been intensively validated regarding specificity and dose-dependency of symptom induction as well as technical aspects such as temporal stability and spatial homogeneity of the allergen exposure.1029-1037 Also, repeatability of outcome measures in the ACC has been systematically investigated and found to have excellent repeatability as measured by TNSS.1038 With the given level of technical and clinical validation, ACCs have been intensively used in clinical drug development to study pharmacological properties of new drugs during phase II trials, such as dose-finding,1039-1041 onset of action,1042-1046 and duration of action.1047-1049 In this respect, numerous randomized, placebo-controlled clinical trials have been conducted using parallel-group or crossover designs in order to test the efficacy of drugs with immediate therapeutic activity, such as antihistamines,1050-1053 or with prophylactic therapeutic potential, such as topical steroids,1054-1056 novel anti-inflammatory compounds,1057-1060 or probiotics.1061 Major advantages in the ACC setting compared to field studies are better signal-to-noise ratios, a safeguarded minimum level of symptomatology in the ACC, and repeatability of symptoms allowing intraindividual comparisons.
With availability of a variety of validated allergen atmospheres in challenge chambers,1029,1030,1034,1035 efficacy testing for dose-finding of AIT has also been performed in RCTs.1062-1066 While regulatory authorities accept the use of ACC in phase II of drug development,1067,1068 they have been reluctant to approve them in pivotal phase III studies because the clinical validation is still imperfect. Differences between natural exposure in field studies and ACC studies exist, for example with regard to exposure time (continuous vs intermittent), exposure atmosphere complexity (natural mix vs artificial purity), or selection of study population (all-comers vs allergen-challenge responders). Therefore, evaluation of efficacy during natural exposure in phase III field studies is still mandatory. However, recent joint activities of the EAACI with experts from academia, chamber owners, and regulators have defined the most relevant unmet needs and prerequisites for clinical validation to further develop the use and regulatory acceptance of ACC in pivotal phase III studies.
In summary, numerous well-designed RCTs using technically validated ACCs for efficacy testing of investigational new drugs with detailed analysis of dose-response, onset of action, and duration of action provide evidence for the use of ACCs in phase II of clinical drug development.
VIII.H.2. Local allergen challenge tests
Challenging the target organs of respiratory allergy (ie, nose, bronchi, eye) with a suspected allergen is aimed at demonstrating the actual clinical reactivity when the results of the initial allergy tests (skin tests, in vitro measurement of sIgE) are inconclusive. The NPT is designed for AR, while conjunctival provocation test (CPT) may be used in patients with rhinoconjunctivitis or AR alone.1069,1070
Nasal challenge.
The aim of nasal challenge is to reproduce the response of the upper airway upon nasal exposure to allergens.1071,1072 However, currently the only technique fulfilling this aim is the EEC (as described in the previous section), while the allergen amounts administered during an NPT usually exceed natural exposure levels, sometimes to a large extent. The allergen for NPT can be administered by various devices, including syringes, nose droppers, micropipettes, nasal sprays, or impregnated disks, none of them being free from limitations or pitfalls.1071 The result of a NPT can be assessed by several measures, including symptom scores (especially the TNSS), rhinomanometry, acoustic rhinometry, optical rhinometry, peak nasal inspiratory flow, inflammatory markers in nasal lavage fluid, and nasal NO concentration.1072 Contraindications to NPT are acute bacterial or viral rhinosinusitis, exacerbation of AR, history of anaphylaxis to allergens, severe general diseases, and pregnancy.1073 Recent studies evaluating the sensitivity and specificity of the different techniques using specific allergens are available (Table VIII.H.2). It is apparent from the contrasting findings that a standardized technique for NPT is not yet available. In fact, in the coming years, the use of NPT in the diagnosis of AR is likely to decrease, due to the diagnostic ability of emerging tools such as CRD1074 and the BAT,1075 which are able to identify the causative allergen in patients with dubious results from initial analysis.
TABLE VIII.H.2.
Recent studies evaluating the sensitivity and specificity of nasal provocation testing
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Krzych-Fałta et al.1086 | 2016 | 2b | Open controlled |
|
Sensitivity and specificity of NPT by optical rhinometry, TNSS | TNSS had a 93.3% sensitivity and a 77.4% specificity, optical rhinometry had a 100% sensitivity and specificity for diagnosis of AR. |
| de Blay et al.1085 | 2015 | 2b | Open controlled |
|
Sensitivity and specificity of a rapid NPT by clinical symptoms and rhinomanometry, safety also evaluated | Rapid NPT had a sensitivity of 83.7% and a specificity of 100%. No adverse reactions. |
| Jang & Kim1084 | 2015 | 2b | Open controlled | HDM allergy:
|
Sensitivity and specificity of NPT by acoustic rhinometry, TNSS | TNSS ≥6.5 had 90.6% sensitivity and 77.4% specificity, acoustic rhinometry had 73.4% sensitivity and 58.1% specificity for diagnosis of AR. |
| Agarwal et al.1083 | 2013 | 2b | Open controlled |
|
Results of NPT by optical rhinometry | No significant difference between allergic and control subjects. |
HDM = house dust mite; LOE = level of evidence; NPT = nasal provocation test; SPT = skin-prick test; TNSS = Total Nasal Symptom Score.
Despite its limitations, a pivotal role for NPT is currently acknowledged in diagnosis of occupational rhinitis and LAR. According to the position paper of the EAACI, occupational rhinitis “can only be established by objective demonstration of the causal relationship between rhinitis and the work environment through NPT with the suspected agent(s) in the laboratory, which is considered the gold standard for diagnosis.”84 The best time to perform a NPT is in the morning to limit the effects of common daily-life stimuli. Baseline evaluation of symptoms and nasal function should be done after adaptation to room temperature. A control test must be performed to ensure that the nasal response is specific to the tested agent.1076 A positive control test suggests rhinitis induced by irritants or nonspecific hyperresponsiveness.
In regard to LAR, the absence of sIgE in serum and in the skin requires that IgE are found locally or that they are revealed by a positive NPT.1077 Despite the introduction of techniques to detect IgE in the nose in the 1970s,1078 the ability to measure locally-present IgE in the clinic setting is not currently available. This makes NPT of critical importance, though contrasting observations have been reported. NPT with mites, pollens and Alternaria was positive in 100% of 22 adults with previously diagnosed LAR,1079 but in a case-controlled, prospective study on 28 children with a diagnosis of NAR, tested with mites and grass pollen, NPT was positive in only 25% of subjects.293
Conjunctival challenge.
While several different techniques exist for NPT, CPT is generally performed by instilling 20 to 30 μL of an allergen solution into the inferior external quadrant of the ocular conjunctiva, using diluent in the contralateral eye as a control.1069 Also, the positive response to CPT is simple to evaluate, because it consists of an immediate reaction (from 5 to 20 minutes from the instillation) with ocular itching, tearing, redness, and possibly conjunctival edema. In 1984, a study of 20 children with seasonal rhinoconjunctivitis tested 3 times with CPT reported good reproducibility.1080 In 2001, a diagnostic sensitivity and specificity of 90% and 100%, respectively, was reported in mite-allergic patients.1081 A very recent systematic review was performed and the results were published in the EAACI guidelines for daily practice of CPT, with grade B evidence for the capacity to individuate the allergen trigger.1082 The conclusion highlighted that allergists should be more familiar with CPT due to its simplicity. However, the scales to assess the symptoms need to be validated, the standardization of allergen extracts must be improved and the indication to perform CPT in patients with forms of conjunctivitis other than allergic remains uncertain.
Aggregate Grade of Evidence for Nasal Provocation Testing: C (Level 2b: 4 studies). Of note, this evidence grade is based on the studies listed in Table VIII.H.2. However, due to the variation in NPT technique and outcome measures, a reliable evidence grade for NPT is difficult to determine.
VIII.I. Nasal cytology and histology
Nasal cytology (NC) is a simple diagnostic procedure that evaluates the health of the nasal mucosa by recognizing and counting cell types and their morphology.1087 NC requires 3 steps. The first is sampling the surface cells in the nasal mucosa with an appropriate device via anterior rhinoscopy. The most commonly used collection device is the Rhino-probe (Arlington Scientific, Springville, UT, USA).1088 The second step is staining by the May-Grunwald-Giemsa method, which allows for identification of all inflammatory cells present in the nasal mucosa (ie, neutrophils, eosinophils, lymphocytes, and mast cells) as well as normal mucosal cells (ciliated and mucinous), and even bacteria or fungi. The third step is examination through an optical microscope able to magnify up to 1000×. For the analysis, at least 50 microscopic fields must be read to be sure to detect all the cells in the sample.1087 NC may detect viruses, fungi, and bacteria (including biofilms) in the nose, allowing for the diagnosis of infectious rhinitis.1089 Specific cytological patterns on NC can help in discriminating among various forms of rhinitis, including AR, NAR, idiopathic rhinitis, and overlapping forms. AR is commonly diagnosed by the combination of clinical history and results of in vivo and/or in vitro tests for sIgE antibodies.1090 When assessed by NC, the predominant cell type is the eosinophil, followed by mast cells and basophils.1091-1094 In a logistic regression model, elevated nasal eosinophil counts on NC has an OR of 1.14 (95% CI, 1.10 to 1.18) to identify AR.1092 It has been described that NC in polyallergic patients shows a more intense inflammatory infiltrate than in monoallergic patients.1093 NC has also demonstrated seasonal changes of inflammatory cells in the nose, probably mirroring the variations in allergen exposure, in patients with mite-induced rhinitis.1095
Negative allergy testing in patients with persistent rhinitis usually suggest a diagnosis of NAR.1096 The first variant of NAR, known as NARES, was described after the identification of a subset of patients with perennial rhinitis, negative skin tests, and marked eosinophilia in nasal secretions.174 In more recent years, other variants have been defined, including NAR with mast cells (NARMA), with neutrophils (NARNE), and with eosinophils and mast cells (NARESMA).1097 Idiopathic rhinitis is also characterized by high levels of eosinophils and mast cells in some patients.1098 Overlapping forms may occur.1099
NC is 1 method of diagnosing NAR and has been used to differentiate between variants in experiments.1100 However, few studies investigating the diagnostic performance of NC in diagnosing AR or NAR are available (Table VIII.I-1).
TABLE VIII.I-1.
Studies assessing the diagnostic performance of nasal cytology
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Gelardi et al.1093 | 2015 | 3b | Case-control | AR patients (n = 83):
|
Comparison of NC cell counts | Higher number of eosinophils (p = 0.005) and mast cells (p = 0.001) in polyallergy. |
| Di Lorenzo et al.1092 | 2011 | 3b | Cohort |
|
NC eosinophil count | High eosinophil count had an odds ratio of 1.14 (95% CI, 1.10-1.18) to identify AR. |
| Gelardi et al.1094 | 2011 | 3b | Case-control | AR patients (n = 62):
|
Association of cell counts with ARIA stage of disease | In moderate-severe AR there was a significantly higher number of eosinophils (p = 0.01), mast cells (p = 0.001), neutrophils (p = 0.046), and lymphocytes (p = 0.001). |
| Gelardi1099 | 2014 | 4 | Cohort | Patients with overlapping AR and NAR (n = 671) | Sneezing in response to nasal endoscopy according to type of rhinitis found on cytology | In patients with NARES, NARMA, and NARESMA there was a significantly higher rate of sneezing (p < 0.01). |
AR = allergic rhinitis; ARIA = Allergic Rhinitis and its Impact on Asthma; CI = confidence interval; LOE = level of evidence; NAR = non-allergic rhinitis; NARES = non-allergic rhinitis with eosinophilia syndrome; NARESMA = non-allergic rhinitis with eosinophils and mast cells; NARMA = non-allergic rhinitis with mast cells; NC = nasal cytology.
Aggregate Grade of Evidence: C (Level 3b: 3 studies; Level 4: 1 study; Table VIII.I-1).
Nasal histology as assessed by biopsies of the nasal cavity was the only technique to study tissues and cells in patients with AR for many decades. In the 1990s, biopsy-based investigations allowed researchers to define the role of the different inflammatory cells in AR.379 The original technique begins by spraying a local anesthetic and topical vasoconstrictor into the nasal passages. After anesthesia has taken effect, a piece of tissue is removed from the middle turbinate using small punch biopsy forceps. After immediately placing the tissue in buffered formalin, each specimen can then be stained with various reagents to detect different tissue components and cells.1101 Reagents used include Giemsa, hematoxylin/eosin, periodic acid-Schiff, Masson trichrome, azure A, and chloroacetate esterase.299,415,1101 After staining, the slides are examined by an optical double-headed light microscope, using a grid reticule divided into 100 squares to quantitate cells and tissue per square millimeter.
The introduction of NC made it possible to obtain the similar information as histology, but without the associated discomfort and potential risk for bleeding. Further, NC allows for sequential sampling where histology does not. In addition, when Lim et al.415 compared nasal histology with cytology in patients with perennial and seasonal rhinitis compared to controls, the results suggested that nasal secretions and the nasal mucosa represent 2 distinct cellular compartments. Specifically, following allergen challenge an influx of inflammatory cells was detected by cytology, while the epithelial layer assessed by histology was unchanged from baseline.415 In 2005, Howarth et al.1102 stated that, compared to simple techniques such as NC or nasal lavage, nasal biopsy requires expertise both in tissue sampling and in biopsy processing, thus being applicable only in specialist centers. This issue, as well as the previously reported drawbacks, makes nasal histology a technique of interest in the research on pathophysiology of AR but hardly feasible for routine clinical practice. Table VIII.I-2 shows the available studies on AR pathophysiology as evaluated by nasal histology.
TABLE VIII.I-2.
Studies investigating allergic rhinitis pathophysiology by nasal histology from biopsies
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Sivam et al.1103 | 2010 | 1b | DBRPCT | SAR (n = 17):
|
Measurement of olfactory function and histological analysis of the olfactory region. | Mometasone use associated with reduced olfactory eosinophilic inflammation and improved AR symptoms. |
| Uller et al.1104 | 2010 | 1b | DBRPCT | SAR to grass or birch (n = 21)
|
Mucosal eosinophilia, apoptotic eosinophils, and expression of CCL5 and CCL11 (eotaxin). | Inhibition of CCL5-dependent recruitment of cells to diseased tissue, reduced cell proliferation, and general cell apoptosis, but not increased eosinophil apoptosis, are involved in early phase steroid-induced resolution of AR. |
| Yang et al.1105 | 2010 | 1b | DBRPCT | PAR to dust mite or animal epithelia (n = 100):
|
To determine the effectiveness of Xin-yi-san in the treatment of AR and investigation of its molecular mechanism of anti-allergic activity. | Xin-yi-san exerts diverse immunomodulatory effects, including suppression of serum IgE levels and increased production of IL-10, sICAM-1, and IL-8 compared to placebo group. |
| Asai et al.1106 | 2008 | 1b | RPCT | SAR to ragweed (n = 19):
|
To determine the in vivo effect of short-course AIT on CD4+CD25+ regulatory T-cells in the nasal mucosa of ragweed-sensitive subjects. | AIT increases CD4+ CD25+ regulatory T-cell infiltration in the nasal mucosa following allergen challenge after seasonal ragweed-pollen. |
| Rak et al.1107 | 2005 | 1b | DBRPCT (double dummy) | SAR to birch (n = 41):
|
Measurement of the number of CD1a+, IgE+, and FcεRI+ cells during birch pollen season. | Treatment with budesonide, but not AIT, decreased the number of CD1a+, IgE+, and FcεRI+ cells. |
| Plewako et al.1108 | 2002 | 1b | SBRPCT | SAR to grass (n = 30):
|
Comparison of anti-CD4, CD8, anti-eosinophil peroxidase, anti-human neutrophil lipocalin, and antibodies against IgE and FcεRI. | The number of eosinophil peroxidase-positive staining cells significantly increased in the placebo-treated patients but not in the actively treated patients. |
| Pullerits et al.1109 | 2001 | 1b | RPCT | SAR to grass pollen (n = 21):
|
Comparison of IL-16 expression during the pollen season in actively vs placebo-treated patients. | Local upregulation of IL-16 expression contributes to the inflammation observed in seasonal AR. |
| Wilson et al.1110 | 2001 | 1b | RPCT | SAR to grass pollen (n = 37):
|
Relationship between symptomatic improvement after AIT and eosinophil numbers and IL-5 expression in the nasal mucosa during the pollen season. | Improvement in symptoms after grass pollen AIT may result from inhibition of IL-5-dependent tissue eosinophilia during the pollen season. |
| Kujundzić et al.1111 | 2013 | 3b | Case-control | AR (n = 90):
|
Compare by histochemical staining with anti-CD31 and VEGF-C the vascularization of the nasal mucosa of non-allergic, non-treated allergic, and allergic patients treated with mometasone. | Significantly lower values of CD31 and VEGF-C expression were observed in non-allergic compared with non-treated allergic and patients treated with mometasone. |
| Radulovic et al.1112 | 2008 | 3b | Case-control | SAR to grass pollen (n = 22):
|
Effect of AIT on the numbers of Foxp3(+) CD4(+) and Foxp3(+) CD25(+) T-cells in and out of season and expression of IL-10 in nasal mucosa. | The presence of local Foxp3(+)CD25(+) cells in the nasal mucosa, their increase after AIT, and their association with suppression of seasonal allergic inflammation support a role for T-reg cells in the induction of allergen-specific tolerance. |
| Till et al.1113 | 2001 | 3b | Case-control | SAR to grass pollen (n = 46):
|
Effect of allergen exposure on nasal antigen-presenting cell and epithelial CD1a+ Langerhans cells, CD68+ macrophages, and CD20+ B-cells. | Recruitment of CD1a+ Langerhans cells to the nasal mucosa during seasonal allergen exposure may contribute to local T-cell responses. |
AIT = allergen immunotherapy; AR = allergic rhinitis; DBRPCT = double-blind randomized placebo-controlled trial; ICAM = intercellular adhesion molecule; IgE = immunoglobulin E; IL = interleukin; LOE = level of evidence; PAR = perennial allergic rhinitis; RPCT = randomized placebo-controlled trial; SAR = seasonal allergic rhinitis; SBRPCT = single-blind randomized placebo-controlled trial; T-reg = T-regulatory cell; VEGF = vascular endothelial growth factor.
Aggregate Grade of Evidence : B (Level 1b: 8 studies; Level 3b: 3 studies; Table VIII.I-2).
IX. Management
IX.A. Allergen avoidance
Allergen avoidance and environmental controls (ECs) are frequently discussed as part of the treatment strategy for AR, along with pharmacologic management and AIT. AR patients are keen to learn about avoidance measures and ECs, especially those who wish to avoid medications or cannot commit to an AIT regimen. Considering this, it is important to examine the evidence supporting allergen avoidance and EC measures for the allergic patient.
IX.A.1. House dust mite
Techniques to reduce environmental HDM exposure have been investigated for the treatment of AR. HDMs represent 1 of the most common triggers of AR,1114 and EC measures have been advocated as a management strategy, with evaluation of both physical barriers and chemical treatments.1114-1118 Various physical techniques (eg, heating, ventilation, freezing, barrier methods, air filtration, vacuuming, and ionizers) have been evaluated for the treatment of AR, with variable findings. While several studies have demonstrated decreased concentrations of environmental HDM antigens,1119-1124 an associated reduction in clinical symptoms has not been reliably demonstrated (Table IX.A.1). Despite reductions in HDM antigen concentration, Ghazala et al.1120 and Terreehorst et al.1124 both found no clinical benefits of HDM-impermeable bedding as an isolated intervention. Similar findings were reported by Antonicelli et al.1125 following a trial of high efficiency particulate air (HEPA) filtration.
TABLE IX.A.1.
Evidence of the effectiveness of house dust mite avoidance and environmental controls in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Sheikh et al.1126 | 2010 | 1a | SR | RCTs examining the effectiveness of environmental measures for HDM | Symptoms | Acaricides are the most effective as a single measure or in combination with other measures to decrease HDM and improve symptoms. |
| Ghazala et al.1120 | 2004 | 1b | Randomized crossover study |
|
Allergen content (Der p 1, Der f 1, mite group 2), subjective clinical complaints | Impermeable encasings significantly reduce allergen concentration, without difference in subjective symptom scores. |
| Terreehorst et al.1124 | 2003 | 1b | Double-blind RCT |
|
Rhinitis-specific visual analogue scale, daily symptom score, nasal allergen provocation, Der p 1 and Der f 1 concentration | Impermeable encasings significantly reduce allergen concentration, without difference in symptoms or nasal provocation testing. |
| Nurmatov et al.1114 | 2012 | 2a | SR of RCTs |
|
HDM load, symptom scores, medication scores, disease-specific QOL | Environmental controls significantly reduced HDM load. Acaricides most effective single method. Combination therapies more effective than single interventions and may offer symptom relief. |
| Stillerman et al.1127 | 2010 | 2b | RDBPCT, crossover |
|
Reported nasal symptoms, QOL scores using the nocturnal RQLQ | PAF is associated with improved nasal symptom and QOL scores. |
| Brehler & Kneist1128 | 2006 | 2b | RDBPCT, parallel-group |
|
Allergy symptom scores, use of anti-allergic medication | HDM-impermeable bedding is associated with significant reduction in symptom scores without change in anti-allergic drug utilization. |
| Moon & Choi1122 | 1999 | 2b | Open RCT |
|
Change in HDM load, daily rhinitis symptom scores | Multimodality environmental control is associated with reductions in mean dust mite concentration and nasal symptom scores. |
| Geller-Bernstein et al.1119 | 1995 | 2b | Double-blind RCT |
|
Daily rhinitis and asthma symptom scores, medication use, twice-weekly PEF | Acaricide is associated with decreased mean symptom scores. |
| Kniest et al.1121 | 1992 | 2b | Double-blind matched pair controlled trial |
|
Daily symptoms and medication scores, physician assessment, tIgE, sIgE, serum and nasal eosinophils, guanine exposure | Acaricide associated with improvement in all outcome measures except for mite-specific IgE. |
| Antonicelli et al.1125 | 1991 | 2b | Randomized crossover study |
|
HDM concentration, rhinitis and asthma symptom score | HEPA filtration had no significant effect on rhinitis symptom scores. |
| Reisman et al.1123 | 1990 | 2b | Double-blind crossover RCT |
|
Particulate counts in bedroom air, symptom and medication scores, patients’ subjective response to treatment | HEPA filtration is associated with improved particulate counts and symptom/medication scores. |
HDM = house dust mite; HEPA = high-efficiency particulate air; IgE = immunoglobulin E; LOE = level of evidence; PAF = personal air filtration; PEF = peak expiratory flow; QOL = quality of life; RCT = randomized controlled trial; RDBPCT = randomized double-blind-placebo-controlled trial; RQLQ; Rhinoconjunctivitis Quality of Life Questionnaire; sIgE = antigen specific immunoglobulin E; SR = systematic review; tIgE = total immunoglobulin E.
Chemical techniques include the use of acaricides in household cleaners to reduce HDM concentration. Geller-Bernstein et al.1119 evaluated an acaricide spray in the bedrooms of patients with HDM sensitization, demonstrating improved mean symptom scores vs control patients without acaricide. Similar findings were reported by Kniest et al.1121 No serious adverse effects were reported from any of the evaluated interventions, and no study evaluated cost-effectiveness as an outcome measure. A 2010 Cochrane review examined the effectiveness of environmental measures for HDM including impermeable covers, HEPA filters, acaricides, or combination treatments.1126 This systematic review found acaricides to be the most effective as a single measure or in combination with other measures to decrease HDM levels and improve AR symptoms.
Aggregate Grade of Evidence: B (Level 1a; 1 study; Level 1b: 3 studies; Level 2a: 1 study; Level 2b 7 studies; Table IX.A.1).
Benefit: Reduced concentration of environmental HDM antigens with potential improvement in symptom scores and QOL.
Harm: None.
Cost: Low to moderate; however, cost-effectiveness was not evaluated.
Benefits-Harm Assessment: Benefit outweighs harm.
Value Judgments: The use of acaricides and/or bedroom-based control programs in reducing HDM concentration is promising, but further, high-quality studies are needed to evaluate clinical outcomes.
Policy Level: Option.
Intervention: Concomitant use of acaricides and EC measures, such as personalized air filtration techniques, are options for the treatment of AR.
IX.A.2. Cockroach
Cockroach infestation and allergen concentrations are often high in multi-occupant dwellings in densely populated inner city areas; although elevated levels of cockroach allergen are also found in homes in warmer, rural regions.1129-1131 Interventions are targeted at eliminating infestations and abating cockroach allergen in homes. A systematic review by Le Cann et al.,1132 identified 3 key strategies for home environmental interventions: (1) education-based methods that included instruction on house cleaning measures and sealing cracks and crevices in areas where infestation occurs (ie, kitchens); (2) physical methods using insecticides or bait traps; and (3) combination therapy containing both educational-based interventions and physical methods (Table IX.A.2).
TABLE IX.A.2.
Evidence of the effectiveness of cockroach avoidance and environmental controls on the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Le Cann et al.1132 | 2016 | 1a | SR of RCTs | Home group interventions in 3 categories:
|
Allergic and respiratory symptoms (eg, cough, daytime symptoms, wheeze, night time symptoms); lung function; medication use; urgent care use for respiratory symptoms | Overall studies supported effectiveness of home interventions in decreasing respiratory symptoms and urgent care use. |
| Sever et al.1133 | 2007 | 1b | 3-arm RCT; follow up for 12 months |
|
No direct clinical endpoints. CR trap counts and CR allergen levels (Bla g 1 and Bla g 2) | Significant reduction in CR counts in both treatment groups vs control. Insecticide bait traps more effective in reducing CR infestation than sprays. Elimination of CR populations leads to reduction in CR allergen and exposure. |
| Eggleston et al.1141 | 2005 | 1b | RCT |
|
Primary outcome: Bla g 1 CR allergen level. Secondary outcome: asthma symptoms | CR allergen reduced by 51% at 6 months. in treatment group but not sustained at 1 year; only modest effect on morbidity. |
| McConnell et al.1134 | 2005 | 1b | RCT |
|
No direct clinical endpoints; CR count and CR allergen level | Achieved 60% reduction in CR count in intervention group. Greatest reduction in allergen level in homes with heavier CR infestation but levels still higher than median level associated with severe symptoms. |
| Arbes et al.1135 | 2004 | 1b | RCT with crossover of control group |
|
No direct clinical endpoints, Bla g 1 and Bla g 2 CR allergen level | CR allergen levels reduced in 6 months with professional cleaning and insecticide bait traps; but lower CR allergen levels maintained at month 12 with bait traps alone. |
| Morgan et al.1142 | 2004 | 1b | RCT with blocked randomization |
|
Asthma symptoms, use of healthcare services | Intervention group: Reduced levels of CR allergen in bedroom were strongly correlated with decreased asthma-related morbidity. |
| McConnell et al.1136 | 2003 | 1b | RCT |
|
No direct clinical endpoints, CR count and Bla g 2 CR allergen level | Decreased CR count and allergen concentration in insecticide bait treatment was low. Homes with high initial CR counts had larger reductions in Bla g 2 CR allergen concentration. Professional cleaning may help in homes with higher CR. |
| Wood et al.1137 | 2001 | 1b | RCT |
|
No direct clinical endpoints, CR count and Bla g 1 CR allergen level | Professional extermination reduced CR numbers and median allergen levels by 80% to 90%. Cleaning solution did not add any improvements. Unclear if this level of reduction is sufficient to have clinical benefits. |
| Gergen et al.1138 | 1999 | 1b | RCT: Phase II of a multi-city study |
|
No direct clinical endpoints, Bla g 1 CR allergen level | CR allergen levels decreased within 6 months but returned or exceeded baseline levels by 12 months. Compliance with cleaning protocol was poor. |
| Williams et al.1140 | 1999 | 2b | Single-blind, non-random, stratified, placebo-controlled study |
|
No direct clinical endpoints, CR counts and CR allergen levels Bla g 1 and Bla g 2 | Treated homes had a significant decrease in number of CR compared to placebo, which continued for 6 months. Minimal reduction in Bla g 1 and Bla g 2 CR allergen. No significant difference: active vs placebo. |
| Eggleston et al.1139 | 1999 | 3b | Prospective case-control | Professional cleaning followed by pest control treatments | No direct clinical endpoints, CR counts and Bla g 1 CR allergen level | CR numbers eliminated in most inner-city homes with professionally applied insecticides. CR allergen levels decreased by 78% to 93% over 8 months; mean allergen concentrations still above threshold of asthma morbidity. |
CR = cockroach; HEPA = high-efficiency particulate air; LOE = level of evidence; RCT = randomized controlled trial; SR = systematic review.
Most studies included 1 or more interventions aimed at reducing cockroach counts and allergen (Bla g 1 and Bla g 2) levels1133-1140; however, a few focused on eliminating multiple allergens (eg, HDM, cockroach, rodent, cat, dog).1141,1142 The most effective treatment for eliminating infestation and reducing allergen load was professional pest control.1135 Sever et al.1133 found placement of insecticide bait traps to be more effective in reducing cockroach populations with a concomitant reduction in cockroach allergen compared to homes that received applications of insecticide formulations to baseboards, cracks, and crevices monitored over a 12-month period.
When cost was considered, the price of bait traps along with labor and monitoring costs were found to be less expensive than multiple commercial applications of insecticide sprays to baseboards and cracks.1133 As the expense of integrated home management consisting of professional cleaning, education, and pest control is not economically sustainable, investigations are focused on assessing the efficacy of single interventions, such as extermination alone, to assess possible cost benefits.1135,1143 In addition, family adherence to home-based interventions was generally poor, resulting in elevated cockroach concentrations over time.1138
Although there are a substantial number of RCTs that evaluated the efficacy of specific environmental control measures to eliminate the number of cockroaches and reduce cockroach allergen level, respiratory health outcomes were rarely measured. Even though cockroach count and Bla g1 and Bla g2 allergen levels were reduced in many studies with home interventions, the level of cockroach allergen following treatment remained higher than acceptable median levels associated with clinical benefits in sensitized individuals.1134,1137-1140 Although cockroach count could be significantly reduced in single-family homes using bait traps, re-infestation and high allergen levels remained an ongoing problem in multifamily buildings.1140 Thus it is difficult to dramatically reduce cockroach allergen levels in the home unless a significant reduction in cockroach counts is maintained over time.1133 Most studies did not include clinical endpoints; however, those that did evaluate clinical outcomes focused on asthma symptoms, hospitalizations or emergency room visits, and medication usage.1141,1142 No studies included any assessment of symptoms associated with AR or its treatment.
Aggregate Grade of Evidence: B (Level 1a: 1 study; Level 1b: 8 studies; Level 2b: 1 study; Level 3b: 1 study; Table IX.A.2).
Benefit: Reduction in cockroach count, but allergen levels (Bla g 1 and Bla g 2) often above acceptable levels for clinical benefits. No studies included clinical endpoints related to AR.
Harm: None reported.
Cost: Moderate. Multiple treatments applications required as well as a multi-interventional approach.
Benefits-Harm Assessment: Balance of benefit and harm, given lack of clear clinical benefit.
Value Judgments: Control of cockroach populations especially in densely populated, multifamily dwellings is important to controlling allergen levels.
Policy Level: Option.
Intervention: Combination of physical measures (such as insecticide bait traps, house cleaning) and educational-based methods are options in the management of AR related to cockroach exposure.
IX.A.3. Pets
Pet avoidance and EC represent options for the treatment of AR. Pet removal is a commonly cited strategy without high-quality outcomes evaluation.1118,1144,1145 Sánchez et al.1146 evaluated compliance rates among sensitized patients (n = 288), finding 4% of patients with direct exposure to home animals complied with removal recommendations (Table IX.A.3). EC has therefore been evaluated to decrease antigen exposure, with mixed results. Björnsdottir et al.1147 evaluated outcomes of multimodality EC among 40 patients with diagnosed cat (Fel d 1) sensitization, finding significant improvements in nasal airflow and clinical symptoms. However, despite reductions in environmental antigens, single-modality EC has not been associated with improved symptoms. Wood et al.1148 evaluated HEPA filtration in a high-quality randomized controlled study of 35 patients with Fel d 1 sensitization, finding unchanged nasal symptom scores, sleep disturbance, rescue medication usage, and spirometry following a 3-month trial. Several lower-quality studies have evaluated the duration of antigen reduction following pet washing, finding that cat and dog washing must be completed at least twice weekly to maintain significant reductions in environmental antigens.1149,1150 Furthermore, pet removal may only result in decreased allergen levels after several months1151 and Can f 1 levels in homes with “hypoallergenic” animals are generally similar to homes with non-hypoallergenic species.1152
TABLE IX.A.3.
Evidence of the effectiveness of pet avoidance and environmental controls
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Wood et al.1148 | 1998 | 1b | RCT | Cat-sensitive adults:
|
Cat allergen levels (airborne and settled dust), symptom scores, medication scores, spirometry | HEPA filters are associated with reduced airborne but not settled dust, cat allergen levels without effect on disease activity. |
| Sanchez et al.1146 | 2015 | 2b | Cohort Study | Patients with diagnosed allergy | Sensitization to household animals, compliance with avoidance recommendations and EC | Avoidance recommendations may be impractical with high rates of sensitization, indirect exposure, and low rates of compliance. |
| Björnsdottir et al.1147 | 2003 | 2ba | RCT | Cat-allergic patients:
|
Environmental (settled dust) Fel d 1 levels, nasal inspiratory flow, nasal symptoms | Multimodality EC is associated with decreased allergen concentration and significant improvements in nasal inspiratory flow and patient symptoms. |
Follow-up <80% prevents 1b.
EC = environmental control; HEPA = high-efficiency particulate air; LOE = level of evidence; RCT = randomized controlled trial.
An additional study has identified benefits of pet avoidance in the secondary prevention of asthma among previously sensitized individuals.1153 Similarly, current asthma treatment guidelines recommend pet removal from a sensitized individual’s home.1154
Aggregate Grade of Evidence: B (Level 1b: 1 study; Level 2b: 2 studies; Table IX.A.3.)
Benefit: Decreased environmental antigen exposure with possible reduction in nasal symptoms and secondary prevention of asthma.
Harm: Emotional distress caused by removal of household pets. Financial and time costs of potentially ineffective intervention.
Cost: Low to moderate.
Benefits-Harm Assessment: Equivocal.
Value Judgments: While several studies have demonstrated an association between EC and reductions in environmental antigens, only a single, multimodality RCT has demonstrated clinical improvement in nasal symptoms among patients with Fel d 1 sensitivity. The secondary prevention and treatment of asthma in sensitized individuals must also be considered.
Policy Level: Option.
Intervention: Pet avoidance and EC strategies, particularly multimodality EC among patients with diagnosed Fel d 1 sensitivity, are an option for the treatment of AR related to pets.
IX.A.4. Other (pollen, occupational)
For patients with pollen allergy, avoidance measures aim to minimize allergen exposure during the respective pollen season.101 However, pollination is a global natural phenomenon which periodically occurs, making it nearly impossible for patients to thoroughly avoid exposure. There are some practical methods to minimize patients’ exposure via EC measures. However, there is a paucity of clinical trials evaluating the clinical efficacy of therapeutic strategies. Most of the recommended strategies are based on expert consensus and clinical experience.1155
One potential EC strategy is limiting residential exposure during periods of high pollination (ie, vacationing in geographical regions with a reduced intensity of local pollen concentration).1156 Patients can get further information about the current pollen count in their respective region through internet sources (ie, the European Aeroallergen Network [EAN] database [https://ean.polleninfo.eu/Ean/]; Foundation German Pollen Information Service [http://www.pollenstiftung.de/]; American Academy of Allergy Asthma and Immunology [AAAAI] [http://www.aaaai.org/global/nab-pollen-counts]). This information may be used, for example, in avoidance of extensive outdoor exercise during peak pollen levels or timing of preventive medication.1157,1158 Although expert opinion endorses these strategies, there is no evidence to support their clinical efficacy.
In addition, patients may open their home windows when the pollen counts are low or keep windows closed and use air conditioning during times of high pollination. Special dust and pollen filters may be used in cars to reduce the pollen concentration within the car. Furthermore, pollen-allergic patients may be educated on removal of clothing and washing their hair before entering their bedrooms during pollen season as pollen grains stick to both hair and clothing. Again, expert opinion endorses these strategies, but there is no evidence to support their clinical efficacy.1159,1160
Another EC strategy utilizes physical barriers to minimize mucosal exposure to airborne allergens. In a prospective trial, 70 patients with SAR caused by grass pollen were randomized to receive wrap-around eyeglasses in addition to standard medical care (first study group) or just standard medical care (second study group) during 3 consecutive grass pollen seasons.1161 Interestingly, the authors found a significant improvement in ocular and nasal symptoms as well as RQLQ in the group provided with wraparound eyeglasses compared to the controls. Another approach is an active nasal filter by means of a membrane removing particles from the inhaled air.1162 In a prospective, single-center, randomized, double-blind, placebo-controlled, crossover study performed in an ACC, 24 adult patients with grass-pollen induced SAR were randomly assigned to either a group that received this nasal filtering membrane or to a group that did not.1162 Under repeated exposure in the ACC, patients with the membrane filter significantly improved in some of their nasal symptoms. However, the primary endpoint measuring maximum TNSS in this trial was not significant; thus, meaningful conclusions are difficult to draw from this study.1162 The small sample size was a notable limitation. A real-world, single-center, double-blind, crossover trial of 65 patients by the same researchers, however, did find significant reductions in daily TNSS and maximum TNSS with nasal filters used in-season compared to placebo1163 (Table IX.A.4).
TABLE IX.A.4.
Evidence of the effectiveness of pollen and occupational allergen avoidance and environmental controls
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Comert et al.1161 | 2016 | 1b | RCT | SAR to grass pollen (n = 70):
|
Nasal and conjunctival symptom scores, rescue medication use, RQLQ | Significant improvement of ocular/nasal symptoms and RQLQ in wrap-around eyeglass group. |
| Kenney et al.1163 | 2015 | 1b | Randomized double-blind, placebo-controlled crossover | Adults with SAR to grass pollen (n = 65):
|
In-season exposure: TNSS, individual symptoms | Daily sum TNSS and maximal TNSS were significant. Individual symptoms (sneezing, watery eyes, rhinorrhea) were also significantly decreased compared to placebo. |
| Kenney et al.1162 | 2014 | 1b | Randomized double-blind, placebo-controlled crossover | Adults with SAR to grass pollen (n = 24):
|
Following ACC exposure: nasal symptom scores, conjunctival symptom scores, throat irritation, intranasal volume, oral FeNO | Primary endpoint, TNSS, was not significant. Some secondary endpoints were positive. In the absence of natural allergen exposure, the conclusions of this trial are limited. |
| Castano et al.1165 | 2013 | 2b | Cohort, prospective, open trial | Occupational allergy (n = 20) | Nasal symptoms, disease-specific QOL, nasal patency, nasal inflammation, olfactory function | EC in occupational allergy patients results in improved QOL, rhinitis-associated symptoms, and general well-being. |
ACC = allergen challenge chamber; FeNO = fraction of exhaled nitric oxide; LOE = level of evidence; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis.
Avoidance of exposure to occupational inhalant allergens is feasible, in principle, in occupational allergic patients.112 Several modalities of reducing workers’ exposure to occupational allergens such as “engineering controls” and “administrative controls” have been described in the literature.1164 The former includes substitution of a hazardous chemical with a nonhazardous or less-hazardous alternative, isolation of the hazardous chemical, or efficient ventilation to reduce workers’ exposure. The latter includes workers’ education and personal protective equipment. A prospective controlled trial of 20 patients with confirmed diagnosis of occupational allergy demonstrated that cessation of the exposure of the causal allergen in the workplace led to a significant improvement of patients’ nasal symptom scores as well as disease-specific QOL.1165
Aggregate Grade of Evidence: B (Level 1b: 3 studies; Level 2b: 1 study; Table IX.A.4).
Benefit: Decreased allergen exposure with possible reduction in symptoms and need for allergy medication, along with improved QOL.
Harm: Financial and time costs of potentially ineffective intervention.
Cost: Low, but dependent on the EC strategy (ie, for occupational allergy ventilation measures and other “engineering controls” may be high).
Benefits-Harm Assessment: Equivocal.
Value Judgments: A limited number of studies show clinical effects of investigated EC measures. General EC recommendations are mainly based on expert opinions rather than evidence.
Policy Level: Option.
Intervention: Pollen and occupational allergen avoidance by EC strategies are an option for the treatment of AR; however, clinical efficacy has not been definitively demonstrated. More RCTs with larger samples are warranted to prospectively evaluate clinical efficacy.
IX.B. Pharmacotherapy
Whether selected by patients themselves or prescribed by medical personnel, medications are the primary modality for control of allergic symptoms. There are numerous options for oral or systemic use, topical intranasal application, and alternative therapies that can be considered. It is, therefore, imperative to understand the data supporting the efficacy and appropriate use of these pharmacotherapy options.
IX.B.1. Antihistamines
IX.B.1.a. Oral H1 antihistamines.
Histamine is a major mediator associated with the symptomatology of AR. Oral H1 antihistamines block the action of histamine by binding the histamine H1 receptor, thereby inhibiting the proinflammatory effects of histamine. Antihistamines are typically categorized by generation, such as first or second-generation agents. The older first-generation agents (ie, diphenhydramine, chlorpheniramine, brompheniramine) were lipophilic and readily crossed the blood-brain barrier. This caused unwanted side effects such as sedation, drowsiness, fatigue, and impaired concentration, and memory as well as anti-muscarinic effects. First-generation antihistamines are also inhibitors of the CYP2D6 hepatic enzymes. They may, therefore, alter the metabolism of other medicines dependent upon CYP2D6 metabolism, such as tricyclic antidepressants, some antipsychotics, β-blockers, anti-arrhythmics, and tramadol. Because of these significant side effects, in previously published guidelines and other papers, first-generation antihistamines have not been recommended for the treatment of AR.218,1166,1167 The newer-generation agents (ie, loratadine, desloratadine, fexofenadine, cetirizine, levocetirizine) were developed to minimize the adverse effects of earlier drugs. They are highly selective for the H1 receptor, lipophobic, and have limited penetration across the blood-brain barrier.
Newer-generation antihistamines, except for cetirizine, levocetirizine, bilastine, and fexofenadine, are metabolized by the hepatic cytochrome P450 CYP3A4 system. Practitioners should be cognizant that the concurrent use of other medicines (eg, macrolides, antifungals, or calcium-channel blockers) that inhibit CYP3A4 can result in accumulation of drug concentrations and increase the risk for side effects and toxicity. Furthermore, adverse cardiac effects (torsades de pointes, arrhythmia, and prolongation of the QT interval) were reported with astemizole and terfenadine, leading to their ultimate withdrawal from the market.1168,1169 RCTs have established the long-term safety and efficacy of the newer-generation H1 antihistamines cetirizine, desloratadine, fexofenadine, levocetirizine, and loratadine (Table IX.B.1.a-1).
TABLE IX.B.1.a-1.
Evidence for the role of oral H1 antihistamines in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Mullol et al.1175 | 2015 | 1a | SR | Rupatadine | Allergy symptoms, ARIA criteria, AE | Rupatadine is recommended for use in adults and children for intermittent/persistent AR and SAR/PAR. |
| Ridolo et al.1174 | 2015 | 1a | SR | Bilastine; cetirizine; desloratadine | Subjective and objective measures, TNSS, RQLQ | Bilastine at therapeutic dose has similar efficacy to other second-generation oral antihistamines. Demonstrated improvement in TNSS and RQLQ with good safety profile. |
| Scadding1176 | 2015 | 1a | Review of consensus statements: ARIA, EAACI, Royal College of Paediatrics, and Child Health | Oral antihistamines | – | Second-generation, non-sedating, antihistamines are recommended for mild to moderate AR and in combination for severe AR. Sedating antihistamines should not be used. |
| Compalati & Canonica1171 | 2013 | 1a | SR | Rupatadine | Allergy symptoms, AE | Favorable risk-benefit ratio for rupatadine in treating AR. |
| Mösges et al.1177 | 2013 | 1a | SR and meta-analysis | Desloratadine; ebastine; fexofenadine; levocetirizine | TSS and TNSS | Second-generation levocetirizine significantly improved symptom scores especially in severe AR cases. |
| Compalati et al.1178 | 2011 | 1a | SR and meta-analysis | Fexofenadine | TSS, individual symptoms (sneezing, rhinorrhea, itching congestion), AE | Fexofenadine has good efficacy with improvement in outcome measures. No significant AE compared to placebo. |
| Ferrer1179 | 2011 | 1a | SR | Levocetirizine; desloratadine; fexofenadine | TSS, PNIF, decongestion test, QOL, pruritus, ESS, wheal and flare, AE | Oral newer-generation antihistamines are well tolerated in adults and children. Efficacy and improvement in QOL and nasal obstruction. Benefits outweigh harm. Very low risk of sedation. No QT prolongation found. |
| Mösges et al.1180 | 2011 | 1a | SR and meta-analysis | Levocetirizine; loratadine | TSS, DNS, DES, in patients with persistent and SAR/PAR | Improvement in TSS, Total 5 Symptoms Score, daytime nasal symptoms, and QOL. |
| Brozek et al.1167 | 2010 | 1a | SR with consensus statement | Oral antihistamines | Evidence was graded and recommendation given | Strong recommendation to use second-generation oral antihistamines that do not cause sedation and do not interact with CYP450 enzyme. |
| Bachert1182 | 2009 | 1a | SR | Desloratadine; fexofenadine; levocetirizine; cetirizine; loratadine; terfenadine | TSS, PNIF, TSSC (with nasal obstruction), nasal congestion, and obstruction | Oral antihistamines have good efficacy for improving both subjective and objective measures, effective in relieving nasal congestion associated with AR compared to placebo. |
| Katiyar & Prakash1181 | 2009 | 1a | SR | Rupatadine; ebastine; cetirizine; loratadine; desloratadine | ARIA criteria evaluated for: intermittent/persistent, SAR/PAR. TSS, DTSSm, DSSm, QT changes | Rupatadine is a non-sedating, efficacious, and safe oral H1 antihistamine for intermittent/persistent, SAR/PAR. |
| Bachert & van Cauwenberge1183 | 2007 | 1a | SR | Desloratadine | TSS, TNSS, TNNSS, PNIF, for intermittent/persistent SAR/PAR | Desloratadine is well tolerated and efficacious for intermittent and persistent AR with reductions in congestion, TSS, TNSS, and TNNSS, with improved QOL. |
| Canonica et al.1184 | 2007 | 1a | SR and meta-analysis | Desloratadine | TSS, TNSS, nasal airflow | Reduction in TSS, TNSS, and improved nasal airflow. |
| Patou et al.1185 | 2006 | 1a | SR and meta-analysis | Levocetirizine | Nasal obstruction | Improved nasal obstruction under artificial and natural allergen exposure. |
| Schenkel1186 | 2006 | 1a | SR | Desloratadine | Morning symptoms, TSS, TNSS, TNNSS | Desloratadine improves TSS and improved QOL in patients with SAR/PAR. 24-hour action makes it effective in controlling morning symptoms. |
| Hore et al.1187 | 2005 | 1a | SR of RDBCT | H1 antihistamine vs placebo | Nasal obstruction | Oral H1 antihistamines improve nasal obstruction by 22% over placebo. |
| Passalacqua & Canonica1188 | 2005 | 1a | SR | Levocetirizine; desloratadine | Nasal symptoms, wheal-flare response, QOL, TSS | Improved QOL and TSS for SAR/PAR. Levocetirizine has a faster onset. |
| Bousquet et al.1170 | 2004 | 1a | SR with consensus statement | Desloratadine | ARIA/EAACI criteria efficacy, safety, pharmacology | Desloratadine is recommended for treating patients with AR. |
| Greisner1189 | 2004 | 1a | SR | Cetirizine; desloratadine; fexofenadine; loratadine | Onset of action | Inconsistent results. Onset of action is dependent on how it is defined and measured. |
| Limon & Kockler1190 | 2003 | 1a | SR | Desloratadine | TSS, TNSS, TNNSS, nasal congestion, nasal airflow, TASS for SAR/PAR | Desloratadine is a safe and efficacious for patients with SAR/PAR. Improved TSS, TNSS, and TNNSS, TASS, nasal congestion. Nasal congestion was excluded in the PAR group. |
| Bojkowski et al.1191 | 1989 | 1a | SR | Acrivastine (40 studies reviewed) | Rhinoconjunctivitis symptoms, nasal congestion, adverse events, drowsiness, CNS depression for SAR/PAR | Newer-generation oral H1 antihistamine acrivastine has excellent efficacy for patients with SAR/PAR. Improved nasal congestion. Small increase in drowsiness over terfenadine. No CNS depression found. |
AE = adverse effects; AR = allergic rhinitis; ARIA = Allergic Rhinitis and its Impact of Asthma; CNS = central nervous system; DES = Daytime Eye Symptoms; DNS = Daytime Nasal Symptoms; DSSm = mean Daily Symptom Score; DTSSm = mean Total Daily Symptom Score; EAACI = European Academy of Allergy and Clinical Immunology; ESS = Epworth Sleepiness Scale; H1 = histamine receptor H1; LOE = level of evidence; PAR = perennial allergic rhinitis; QOL = quality of life; QT = measure of time between the onset of ventricular depolarization and completion of ventricular repolarization; RDBCT = randomized double-blind controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; SR = systematic review; TASS = Total Asthma Symptom Score; TNNSS = Total Non-Nasal Symptom Score; TNSS = Total Nasal Symptom Score; TSS = Total Symptom Score; PNIF = peak nasal inspiratory flow; TSSC = Total Symptom Severity Complex.
Because oral antihistamines have been in use since the early 1940s, there have been many RCTs establishing oral antihistamines as an appropriate pharmacotherapy for AR.218 As such, this section does not list every published study but summarizes the highest-grade evidence that has been published. Guidelines on AR have been published, including those by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS)761 and the ARIA group.1167 The AAO-HNS concluded, based upon RCTs and a preponderance of benefit over harm, a “strong recommendation” for the use of newer-generation oral H1 antihistamines for patients with AR.218 Similar consensus came from ARIA where a “strong recommendation” was given for oral H1 antihistamines for AR.1167 Furthermore, ARIA and EAACI have published a set of recommendations that outline the pharmacological criteria that should be met by medications commonly used in the treatment of AR.1170 The main thrust of the ARIA/EAACI criteria was to assess the efficacy, safety, and pharmacology of newer-generation oral H1 antihistamines using level 1a studies. Using these criteria, a favorable risk-benefit ratio was determined for using newer-generation oral H1 antihistamines over first-generation oral antihistamines.1170 The evidence was further strengthened with several meta-analyses of the current data, where accurate and robust effect estimations can be derived from a large population1171 (Table IX.B.1.a-1).
The choice of a specific oral H1 antihistamine is often based upon the dosing, onset, drug interactions, and potential cost (Table IX.B.1.a-2). Systematic reviews evaluating multiple oral H1 antihistamines note benefits of certain drugs that may be important in deciding which drug to recommend or prescribe. Direct costs of newer-generation antihistamines are similar given the availability of many of these drugs as over-the-counter medications. In contrast, the cost of prescription-only formulations (levocetirizine and desloratadine) is much higher. Indirect costs would be expected to be similar among the newer-generation oral antihistamines given similar side-effect profiles.
TABLE IX.B.1.a-2.
List of commonly used second-generation antihistamines
| Dosage | ||||||
|---|---|---|---|---|---|---|
| Antihistamine medication |
Onset (hours) | Duration (hours) |
Drug interactions |
Elimination (hours) |
Adults | Children |
| Cetirizine | 0.7 | <24 | Unlikely | 6.5–10 | 5–10 mg QD | 2–5 years; 2.5 mg or 5 mg QD; 6–12 years: 5–10 mg QD |
| Desloratadine | 2–2.6 | >24 | Unlikely | 27 | 5 mg QD | 2–5 years: 1.25 mg QD; 6–11 years: 2.5 mg QD |
| Bilastine | 2 | 24 | Unlikely | 14.5 | 20 mg QD | 6–11 years: 10 mg QD |
| Fexofenadine | 1–3 | >24 | Unlikely | 11–15 | 60 mg BID or 180 mg QD | 2–11 years: 30 mg BID |
| Levocetirizine | 0.7 | >24 | Unlikely | 7 | 5 mg QD | 2–5 years: 1.25 mg QD; 6–11 years: 2.5 mg QD; ≥12 years: 2.5–5 mg QD |
| Loratadine | 2 | >24 | Unlikely | 7.8 | 10 mg QD or5mg BID | 2–5 years; 5 mg QD; ≥6 years; 10 mg QD |
BID = twice per day; N/A = not applicable; QD = once per day.
Aggregate Grade of Evidence: A (Level 1a: 21 studies; Table IX.B.1.a-1). There is a preponderance of high-grade investigations that have examined oral H1 antihistamines. Only level 1a studies are summarized in the table.
Benefit: Reduced nasal itching, sneezing, rhinorrhea, and nasal obstruction.
Harm: Mild drowsiness, fatigue, headache, nausea, and dry mouth.
Cost: Direct costs low (average $2 per daily dose). Indirect costs for newer generation agents lower than first-generation agents.1172,1173
Benefits-Harm Assessment: Benefits outweigh harm for use of newer-generation oral H1 antihistamines.
Value Judgments: Due to the central nervous system side effects of the first-generation oral H1 antihistamines, their use is not recommended for typical AR.
Policy Level: Strong recommendation for use of newer-generation oral antihistamines to treat AR.
Intervention: Prescribing newer-generation oral H1 antihistamines for patients with AR should be considered early in treatment.
IX.B.1.b. Oral H2 antihistamines.
The role of the H2 receptor in mediating histamine-related nasal symptoms in AR is controversial. Few small studies have investigated the impact of H2 receptor antagonism, with varied results (Table IX.B.1.b). Further, no data exists comparing H2 receptor antagonism efficacy to common modern first-line therapy such as nasal topical corticosteroids. Finally, the clinical significance of the changes associated with H2 antihistamines has not been clearly defined. Despite these caveats, some studies support the addition of an H2 antihistamine for patients with recalcitrant nasal airway obstruction while on oral H1 antihistamines. There are drug-drug interactions that can occur with H2 antihistamines through decreased gastric acidity and inhibition of P450.1192 However, due to the low cost of these medications, clinical situations may arise that would justify their use.
TABLE IX.B.1.b.
Evidence for the role of oral H2 antihistamines in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Taylor-Clark et al.1194 | 2005 | 1b | RCT | Histamine challenge with premedication:
|
Nasal airway resistance | Cetirizine alone and ranitidine alone improve nasal resistance. Cetirizine plus ranitidine improves nasal resistance more than either alone. |
| Juliusson & Bende1196 | 1996 | 1b | RCT | Allergy challenge with premedication:
|
Laser Doppler flowmeter, allergic symptoms | No difference in symptoms or flowmetry with cimetidine. No additive effect of cimetidine with terfenadine. |
| Wang et al.1195 | 1996 | 1b | RCT | Allergy challenge with premedication:
|
Symptoms (itching, sneezing, rhinorrhea, congestion), sneeze count, nasal airway resistance | Combination of cetirizine + cimetidine showed improved nasal airway resistance and nasal airflow over cetirizine alone. |
| Wood-Baker et al.1193 | 1996 | 1b | RCT | Allergy challenge with premedication:
|
Nasal lavage fluid protein concentration, nasal airway resistance | Ranitidine improved nasal resistance more than cetirizine. Cetirizine decreased total protein and albumin more than ranitidine. |
| Carpenter et al.1198 | 1983 | 1b | RCT | During allergy season medicated with:
|
Symptoms (rhinorrhea, sneezing, nasal congestion, nasal pruritus, eye discomfort), medication usage beyond study therapy | Reduced symptoms and medication scores in cimetidine plus chlorpheniramine group. |
| Brooks et al.1197 | 1982 | 1b | RCT | Allergy challenge with premedication:
|
Subjective symptoms (congestion, itch, drainage, sneeze), nasal resistance, nasal secretion weight | No difference in subjective scores. Increased secretion and sneeze count, no difference in nasal resistance. |
H2 = histamine receptor H2; LOE = level of evidence; PO = per os (medication taken orally); RCT = randomized controlled trial.
All but 1 of the RCTs investigating the efficacy of H2 antihistamines are within the context of pretreatment of a subject prior to a nasal allergen challenge. Wood-Baker et al.1193 compared oral cetirizine to oral ranitidine. Objective measures of nasal airway resistance showed greater improvement with ranitidine, yet cetirizine decreased objective measures of nasal secretion more than ranitidine. Taylor-Clark et al.1194 found similar improvement in nasal airway resistance between cetirizine and ranitidine, but a significant improvement with the use of combination therapy. Combination therapy was also shown to improve nasal airflow when cimetidine was added to cetirizine.1195 Two studies did not find improvement in nasal airflow with the addition of an H2 antihistamine.1196,1197 The clinical significance of these objective findings is unclear, and the studies that employed PROMs did not demonstrate subjective improvement in nasal obstruction.
Four studies investigated the impact of H2 antagonism on symptoms; however, these studies did not utilize standardized outcome measures as they pre-dated the development of such tools. Subjects were asked to report some combination of congestion, blockage, itching, drainage, sneezing, eye symptoms, and asthma with a categorical severity measure. Three of the 4 studies examined symptoms after nasal allergen challenge, and none demonstrated efficacy of H2 antihistamines, either alone or in conjunction with an H1 antihistamine in diminishing allergic symptoms.1195-1198 One study of 23 subjects1198 did investigate the impact of cimetidine in conjunction with chlorpheniramine in a real-world setting. Subjects with known late-summer AR were randomized during this season to receive alternating 2-week courses of either chlorpheniramine plus placebo, or chlorpheniramine plus cimetidine, and symptom scores were recorded twice daily along with adjuvant medical therapies (specifically, oral corticosteroids). Patients receiving both H1 and H2 antihistamines reported decreased medication usage (28 corticosteroid days vs 44 corticosteroid days, p < 0.02) and decreased symptoms scores during 1 of the 8 weeks when weed pollen counts were high. A caveat of this study is its utilization of a first-generation antihistamine that is no longer recommended as a first-line treatment of AR.
The data existing on the use of H2 antihistamines in AR are limited in scope and quality. The objective findings of improved nasal airway resistance suggest that the H2 histamine receptor does modulate nasal tissue response to histamine.1193-1195 However, the clinical significance of this mechanism is not clear, particularly in the context of modern treatment algorithms.1195-1198 The relatively manageable side effect profile and costs of H2 antihistamines, does offer patients with otherwise recalcitrant AR symptoms an additional treatment option.
Aggregate Grade of Evidence: B (Level 1b: 6 studies; Table IX.B.1.b).
Benefit: Decreased objective nasal resistance, and improved symptom control in 1 study when used in combination with H1 antagonists.
Harm: Drug-drug interaction (P450 inhibition, inhibited gastric secretion and absorption),
Cost: Increased cost associated with H2 antagonist.
Benefits-Harm Assessment: Unclear benefit and possible harm.
Value judgments: No studies evaluating efficacy of H2 antihistamines in context of topical nasal corticosteroids.
Policy Level: No recommendation. The data available does not adequately address the question as to the benefit of H2 antihistamines in clinical AR as part of modern treatment protocols.
Intervention: Addition of an oral H2 antagonist to an oral H1 antagonist may improve symptom control in AR; however, the evidence to support this is not strong.
IX.B.1.c. Intranasal antihistamines.
The use of intranasal antihistamine spray for AR has been well studied. Two agents are currently available in North America for intranasal use as a topical spray, azelastine hydrochloride and olopatadine hydrochloride. A systematic review of the English-language literature was performed for clinical trials of azelastine or olopatadine for the treatment of AR. A total of 44 papers were identified that reported results of RCTs of intranasal antihistamine monotherapy against either placebo or active control1046,1199-1241 (Table IX.B.1.c). Of these, 11 studies included comparison of different doses of intranasal antihistamine1204,1205,1207,1211,1212,1216,1218,1219,1231,1235,1237 and 29 studies utilized inactive placebo.1201,1202,1204,1205,1207-1209,1211-1214,1216,1218-1222,1224,1225,1227-1231,1233,1235,1237-1239 Overall, there were 38 studies of azelastine1046,1199-1201,1203,1205,1207-1213,1215,1217,1220-1241 and 10 studies of olopatadine1202,1204,1206,1208,1210,1211,1214,1216,1218,1219 as monotherapy.
TABLE IX.B.1.c.
Evidence for the role of topical intranasal antihistamines as monotherapy in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Carr et al.1199 | 2012 | 1b | DBRCT (post hoc analysis) |
|
rTNSS, rTOSS, RQLQ | Fluticasone superior to azelastine for improving rhinorrhea; comparable symptom and QOL improvement. |
| Han et al.1200 | 2011 | 1b | DBRCT |
|
rTNSS | Comparable symptom improvement. |
| Howland et al.1201 | 2011 | 1b | DBRCT |
|
rTNSS, rTOSS, RQLQ | Azelastine superior to placebo for nasal and eye symptoms and QOL. |
| Meltzer et al.1202 | 2011 | 1b | DBRCT |
|
rTNSS, rTOSS, PRQLQ, CGTSQ-AR | Olopatadine superior to placebo in reducing symptoms in children, improving QOL, and satisfying caregivers. |
| Berger et al.1204 | 2009 | 1b | DBRCT |
|
TNSS, TOSS, PRQLQ, CGTSQ, SGA | Olopatadine superior to placebo in reducing symptoms in children, improving QOL, and satisfying caregivers. |
| Bernstein et al.1205 | 2009 | 1b | DBRCT |
|
TNSS | Both azelastine spray formulations superior to placebo; dose-response effect between dosages; no difference in bitter taste between formulations. |
| Kaliner et al.1206 | 2009 | 1b | DBRCT |
|
rTNSS, rTOSS | Both treatments improve symptoms; faster onset for olopatadine. |
| Shah et al.1207 | 2009 | 1b | DBRCT |
|
TNSS | Both azelastine doses superior to placebo; greater improvement with higher dose. |
| Shah et al.1208 | 2009 | 1b | DBRCT |
|
TNSS | Both treatments superior to placebo; no difference between treatments; less bitter taste with olopatadine. |
| van Bavel et al.1209 | 2009 | 1b | DBRCT |
|
TNSS | Azelastine superior to placebo. |
| Meltzer et al.1210 | 2008 | 1b | DBRCT |
|
Sensory perception | Olopatadine favored for taste, aftertaste, and likelihood of use. |
| Pipkorn et al.1211 | 2008 | 1b | DBRCT |
|
4-item symptom score, nasal lavage | Both olopatadine doses superior to placebo for reducing symptoms; higher concentration inhibits mast cell degranulation. |
| Lumry et al.1212 | 2007 | 1b | DBRCT |
|
TNSS | Azelastine both doses superior to placebo. |
| Patel et al.1213 | 2007 | 1b | DBRCT |
|
TNSS | Azelastine superior to mometasone and placebo. |
| Patel et al.1214 | 2007 | 1b | DBRCT |
|
TNSS, patient satisfaction | Olopatadine superior to placebo and mometasone in reducing symptoms; faster onset for olopatadine. |
| Berger et al.1215 | 2006 | 1b | DBRCT |
|
TNSS, RQLQ | Azelastine superior for sneezing and nasal congestion; azelastine superior for QOL. |
| Hampel et al.1216 | 2006 | 1b | DBRCT |
|
Total Symptom Score, RQLQ | Olopatadine (both doses) superior to placebo in majority of domains for QOL improvement. |
| Horak et al.1046 | 2006 | 1b | DBRCT |
|
TNSS | Azelastine superior to desloratadine and placebo. |
| Corren et al.1217 | 2005 | 1b | DBRCT |
|
TNSS, RQLQ | Azelastine superior cetirizine for symptoms and QOL. |
| Meltzer et al.1218 | 2005 | 1b | DBRCT |
|
TNSS, RQLQ | Olopatadine (both doses) superior to placebo for symptoms and QOL improvement. |
| Ratner et al.1219 | 2005 | 1b | DBRCT |
|
TNSS | Olopatadine (both doses) superior to placebo. |
| LaForce et al.1220 | 2004 | 1b | DBRCT |
|
TNSS | Azelastine superior to placebo; no additional benefit of adding oral fexofenadine to azelastine monotherapy. |
| Berger & White1221 | 2003 | 1b | DBRCT |
|
TNSS | All treatments superior to placebo; azelastine at least as effective as desloratadine; no additional benefit of adding oral loratadine to azelastine monotherapy. |
| Saengpanich et al.1222 | 2002 | 1b | DBRCT |
|
TNSS, nasal lavage, methacholine challenge | Azelastine superior to placebo for symptoms; no effect on nasal eosinophils or cytokines; azelastine inhibits methacholine response. |
| Falser et al.1223 | 2001 | 1b | DBRCT |
|
10-item symptom score, global assessment | Azelastine superior to levocabastine. |
| Berlin et al.1224 | 2000 | 1b | DBRCT |
|
9-item symptom score | Flunisolide superior to azelastine; both treatments superior to placebo. |
| Golden et al.1225 | 2000 | 1b | DBRCT |
|
RSS, ESS | Azelastine superior to placebo for improving rhinorrhea and sleep quality. |
| Berger et al.1226 | 1999 | 1b | DBRCT |
|
5-item symptom score, global evaluation | Azelastine at least as effective as combination therapy with loratadine plus beclomethasone spray. |
| Stern et al.1227 | 1998 | 1b | DBRCT |
|
3-item symptom score | Budesonide superior to azelastine; both treatments superior to placebo. |
| Herman et al.1228 | 1997 | 1b | DBRCT |
|
TNSS | Azelastine superior to placebo for children. |
| Newson-Smith et al.1229 | 1997 | 1b | DBRCT |
|
6-item symptom score | Beclomethasone superior to azelastine for long-term symptom improvement; both treatments superior to placebo; azelastine more rapid onset. |
| Weiler & Meltzer1230 | 1997 | 1b | DBRCT |
|
13-item symptom score | Azelastine spray showed limited benefit over placebo in patients already treated with systemic azelastine. |
| LaForce et al.1231 | 1996 | 1b | DBRCT |
|
8-item symptom score | Azelastine superior to placebo at both doses; no comparison with chlorpheniramine. |
| Charpin et al.1232 | 1995 | 1b | DBRCT |
|
8-item symptom score | Azelastine superior for nasal stuffiness and rhinorrhea; no difference in other symptoms. |
| Pelucchi et al.1233 | 1995 | 1b | DBRCT |
|
8-item symptom score, nasal lavage, methacholine challenge | Azelastine superior to placebo and comparable to beclomethasone for symptom improvement; neither treatment prevented bronchial responsiveness; no effect of azelastine on eosinophils. |
| Gastpar et al.1234 | 1994 | 1b | DBRCT |
|
13-item symptom score | Comparable symptom improvement. |
| Meltzer et al.1235 | 1994 | 1b | DBRCT |
|
11-item symptom score | Azelastine comparable to chlorpheniramine and superior to placebo at both doses. |
| Passali & Piragine1236 | 1994 | 1b | DBRCT |
|
13-item symptom score | Azelastine at least as effective as cetirizine. |
| Ratner et al.1237 | 1994 | 1b | DBRCT |
|
8-item symptom score | Azelastine twice-daily superior to placebo. |
| Davies et al.1238 | 1993 | 1b | DBRCT |
|
TNSS, rhinomanometry | Azelastine superior to beclomethasone and placebo for symptoms; no change in airway resistance with either treatment. |
| Dorow et al.1239 | 1993 | 1b | DBRCT |
|
13-item symptom score | Azelastine comparable to budesonide for nasal symptoms and superior for ocular symptoms; both treatments superior to placebo. |
| Gambardella1240 | 1993 | 1b | DBRCT |
|
12-item symptom score, global assessment | Azelastine at least as effective as loratadine. |
| Gastpar et al.1241 | 1993 | 1b | DBRCT |
|
10-item symptom score, nasal flow rate | Azelastine at least as effective as budesonide for symptoms; flow rate improved in both treatment groups. |
| Kalpaklioglu & Kavut1203 | 2010 | 2b | Single-blind RCT |
|
TNSS, nPIFR, ESS, SF-36, mini-RQLQ | Comparable improvement in nasal symptoms, nPIFR, ESS and QOL; azelastine superior for ocular symptoms. |
AR = allergic rhinitis; BID = twice a day; CGTSQ = Caregiver Treatment Satisfaction Questionnaire; CGTSQ-AR = Caregiver Treatment Satisfaction Questionnaire for Allergic Rhinitis; DBRCT = double-blind randomized controlled trial; ESS = Epworth Sleepiness Scale; LOE = level of evidence; nPIFR = nasal peak inspiratory flow rate; PRQLQ = Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; QD = once daily; QOL = quality of life; RCT = randomized controlled trial; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; RSS = Rhinitis Severity Score; rTNSS = reflective Total Nasal Symptom Score; rTOSS = reflective Total Ocular Symptom Score; SF-36, 36-Item Short Form; SGA = Subjective Global Assessment; TNSS = Total Nasal Symptom Score; TOSS = Total Ocular Symptom score.
Outcome measures were predominantly patient-reported symptom scores or QOL assessments. The most common outcome measure was the TNSS (23 studies), which records the severity of runny nose, sneezing, itching, and congestion. Other outcome measures included the RQLQ (7 studies), the Total Ocular Symptom Score (TOSS, 5 studies), the Caregiver Treatment Satisfaction Questionnaire (2 studies), the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (1 study), the Short Form-36 (1 study), the Epworth Sleepiness Scale (ESS, 1 study), the Rhinitis Severity Score (1 study), and a Subjective Global Assessment (1 study). Multiple studies, particularly those published prior to 2002, used a variety of nonvalidated symptom scoring systems ranging from 5 to 13 items each (19 studies). Objective measures included nasal lavage (3 studies), response to methacholine challenge (2 studies), nasal flow rate (2 studies), and rhinomanometry (1 study).
Study duration ranged from 2 days to 8 weeks, with the most frequent duration being 14 days of treatment. The number of subjects in each study ranged from 20 to 1188. Intranasal antihistamine was compared to placebo in 29 studies,1201,1202,1204,1205,1207-1209,1211-1214,1216,1218-1222,1224,1225,1227-1231,1233,1235,1237-1239 with primary outcomes showing superiority to placebo in all studies. Intranasal antihistamine was trialed against an active treatment comparator of a different medication class in 24 studies.1046,1199,1203,1206,1213-1215,1217,1220,1221,1224,1226,1227,1229,1231-1236,1238-1241 Although not reported in all studies, the intranasal antihistamine spray consistently had a more rapid onset of action, occurring as early as 15 minutes after administration. Azelastine and olopatadine were directly compared in 3 studies, with no significant difference in symptom relief between agents.1208,1210,1211 In 2 additional studies, azelastine was compared with an experimental formulation of intranasal levocabastine, with either comparable or superior results for azelastine.1200,1223
Intranasal antihistamine was compared to INCS in 12 studies, with the primary outcome favoring antihistamine in 2 studies,1213,1214 corticosteroid in 3 studies,1224,1227,1229 and showing equivalency in 7 studies.1199,1203,1206,1233,1238,1239,1241 In 2 of the studies showing equivalency, antihistamine was superior for ocular symptoms.1203,1239 The 3 studies showing superiority of corticosteroids were all conducted prior to 2000 and used heterogeneous nonvalidated symptom scores as primary outcomes. Intranasal antihistamine was compared to oral antihistamine monotherapy in 8 studies, with the primary outcome favoring intranasal antihistamine in 3 studies1215,1217,1232 and showing equivalency in 5 studies.1221,1234-1236,1240 One study included a treatment arm with oral chlorpheniramine as a positive control without intent to compare efficacy with azelastine.1231 One study comparing azelastine spray with oral loratadine plus intranasal beclomethasone found that azelastine monotherapy was at least as effective as combination therapy.1226 Two studies comparing intranasal azelastine plus oral antihistamine to intranasal azelastine monotherapy showed no additional benefit for combination therapy.1220,1221
The minimum age of subjects in the included studies was generally 12 years or older. Children aged 6 to 12 years old were included in 3 studies, which in aggregate showed superiority of intranasal antihistamine to placebo in improving symptoms and QOL.1202,1204,1228
Serious adverse effects were not reported in any study. Intranasal antihistamine was generally well tolerated, with the most commonly reported adverse effect of an unpleasant taste. One study that compared the commercially available form of azelastine with a reformulated vehicle found no difference in taste aversion.1205 One study directly comparing olopatadine with azelastine reported better sensory attributes for olopatadine.1210 Other reported adverse effects included somnolence, headache, epistaxis and nasal discomfort, all occurring in less than 10% of cases in any study.
Aggregate Grade of Evidence: A (Level 1b: 43 studies; Level 2b: 1 study; Table IX.B.1.c). Due to the large number of studies with high level of evidence, studies of lower evidence levels are not considered here.
Benefit: Intranasal antihistamines have a rapid onset, are more effective for nasal congestion than oral antihistamines, are more effective for ocular symptoms than INCS, and show consistent reduction in symptoms and improvement in QOL in RCTs compared to placebo.
Harm: Concerns for patient tolerance, especially due to taste. Intranasal antihistamines are less effective for congestion than INCS.
Costs: Low-to-moderate financial burden; available as prescription only.
Benefits-Harm Assessment: Preponderance of benefit over harm. Intranasal antihistamine as monotherapy is consistently more effective than placebo. Most studies show intranasal antihistamines superior to INCS for sneezing, itching, rhinorrhea, and ocular symptoms. Adverse effects are minor and infrequent.
Value Judgments: Extensive level 1 evidence comparing intranasal antihistamine monotherapy to active and placebo controls demonstrates overall effectiveness and safety.
Policy Level: Recommendation.
Intervention: Intranasal antihistamines may be used as first-line or second-line therapy in the treatment of AR.
IX.B.2. Corticosteroids
IX.B.2.a. Oral corticosteroids.
The anti-inflammatory effect of oral corticosteroids in AR is well known and has been demonstrated experimentally using the nasal challenge model and clinically in the context of seasonal disease. Compared to placebo, premedication with oral prednisone for 2 days prior to an allergen challenge showed a reduction in sneezes, and levels of histamine and mediators of vascular permeability in nasal lavages during the late phase response884 (Table IX.B.2.a). Further, active treatment resulted in a reduction in the priming response to consecutive allergen challenge.884 Prednisone has also been shown to reduce the influx of eosinophils and levels of the eosinophil mediators (major basic protein and eosinophil derived neurotoxin) into nasal secretions during the late-phase response compared to placebo.1242,1243 Non–placebo-controlled studies have demonstrated efficacy of oral corticosteroids for SAR. Schwartz et al.1244 demonstrated that 15 days of cortisone 25 mg 4 times daily during the ragweed season resulted in significant relief of symptoms in 21 of 25 patients. Similarly, 100 mg of cortisone daily for 4-day courses during the pollen season showed rhinitis symptom relief in 42 of 51 patients, with 20 patients relapsing within 7 days after cessation of therapy.1245 Oral hydrocortisone 40 to 80 mg daily has also been shown to reduce symptoms of ragweed allergies.1246 Brooks et al.1247 performed a placebo-controlled study comparing the efficacy of methylprednisolone 6, 12, or 24 mg PO daily for 5 days to placebo in controlling nasal symptoms during the ragweed season. Whereas the 6-mg and 12-mg doses led to a significant reduction in some of the symptoms compared to placebo (congestion, postnasal drainage, and eye symptoms), the 24-mg dose resulted in a significant reduction of all symptoms (congestion, runny nose, sneezing, itching, postnasal drainage, and eye symptoms).
TABLE IX.B.2.a.
Evidence for the role of oral corticosteroids in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Brooks et al.1247 | 1993 | 1b | Placebo-controlled, parallel group study | SAR during season (n = 31): MP 6, 12, 24 mg QD × 5 days | Symptom scores | All doses more effective than placebo in reducing symptoms with the highest dose most effective. |
| Bascom et al.1243 | 1989 | 1b | Placebo controlled, crossover, nasal challenge study | SAR out of season (n = 13): prednisone 60 mg PO daily for 2 days | Number of eosinophils and levels of MBP and EDN in nasal lavages | Prednisone reduced the number of eosinophils and the levels of its mediators after allergen challenge. |
| Bascom et al.1242 | 1988 | 1b | Placebo controlled, crossover, nasal challenge study | SAR out of season (n = 10): prednisone 60 mg PO daily for 2 days | Number of neutrophils, eosinophils, and mononuclear cells in nasal lavages | Prednisone reduced the influx of eosinophils into nasal secretions after allergen challenge. |
| Pipkorn et al.884 | 1987 | 1b | Placebo controlled, crossover, nasal challenge study | SAR out of season (n = 13): prednisone 60 mg PO daily for 2 days | Sneezes, levels of histamine, TAME-esterase, kinins, PGD2, LTC4/D4, and albumin in nasal lavages | Prednisone inhibited the late-phase response to nasal allergen challenge. |
| Kwaselow et al.1248 | 1985 | 1b | Multicenter, randomized, double-blind, placebo-controlled study | SAR during season (n = 99):
|
Symptom scores | Intranasal preparation only one to show efficacy in reducing rhinitis symptoms. |
| Karaki et al.1249 | 2013 | 2b | Open label, parallel, randomized trial | SAR during season (n = 72):
|
Symptom scores | The groups on steroids had lower symptoms compared to loratadine alone, with no significant difference between them. |
| Schwartz1246 | 1954 | 4 | Observational case series | SAR during season (n = 10): Hydrocortisone 40-80 mg daily | Symptom relief | 7/10 patients reported symptom relief. |
| Schiller & Lowell1245 | 1953 | 4 | Observational case series | SAR during season (n = 51): cortisone 100 mg daily × 4 days | Symptom relief | 42/51 patients reported symptom relief. |
| Schwartz et al.1244 | 1952 | 4 | Observational case series | SAR during season (n = 25): cortisone 100 mg daily × 15 days | Symptom relief | 21/25 patients reported symptom relief. |
BID = twice daily; EDN = eosinophil-derived neurotoxin; LOE = level of evidence; LTC4/D4 = leukotriene C4/D4; MBP = major basic protein; MF = mometasone furoate; MP = methylprednisolone; PGD2 = prostaglandin D2; PO = per os (medication taken orally); QD = once daily; SAR = seasonal allergic rhinitis; TAME = N-a-p-tosyl-L-arginine methyl ester.
Because of the recognized systemic adverse events associated with oral corticosteroids,101 their use has been largely replaced by the intranasal preparations. In a double-blind, placebo-controlled trial, the effect of intranasal flunisolide and its oral dose bioequivalent (an oral dose that would lead to similar systemic levels) were compared in ragweed-induced SAR.1248 The intranasal preparation was shown to be efficacious in reducing rhinitis symptoms while the oral dosing was not. This suggested that INCSs achieve their benefit primarily by their local activity as opposed to systemic bioavailability. In a head-to-head comparison of the efficacy of intranasal vs systemic steroids, Karaki et al.1249 performed an open-label, parallel, randomized trial during the cedar pollen season in Japan. Patients received loratadine 10 mg daily alone, loratadine with intranasal mometasone furoate (200 μg once daily), or loratadine with oral betamethasone 0.25 mg twice daily for 1 week. The groups receiving some form of steroid in addition to loratadine had significantly lower symptoms of sneezing, rhinorrhea, and nasal obstruction compared to loratadine alone, with no significant difference between the intranasal and oral preparations. The oral steroid was more effective than the INCS in controlling allergic eye symptoms.
The above data suggest that oral corticosteroids are effective for the treatment of AR. However, given the significant systemic adverse effects related to using oral corticosteroids for prolonged periods of time these agents are not recommended for the routine treatment of AR.
Aggregate Grade of Evidence: B (Level 1b: 5 studies; Level 2b: 1 study; Level 4: 3 studies; Table IX.B.2.a).
Benefit: Oral corticosteroids can attenuate symptoms of AR.
Harm: Oral corticosteroids have known undesirable adverse effects. These include effects on the hypothalamic-pituitary axis, growth and musculoskeletal system, gastrointestinal system, hypertension, glycemic control, mental/emotional state, and others.
Cost: Low.
Benefits-Harm Assessment: The risks of using oral corticosteroids outweigh the benefits when compared to similar symptom improvement with the use of INCS.
Value Judgments: In the presence of effective symptom control using INCS, the risk of adverse effects from using oral corticosteroids for AR appears to outweigh the potential benefits.
Policy Level: Recommendation against the routine use of oral corticosteroids for AR.
Intervention: Although not recommended for routine use in AR, certain clinical scenarios warrant the use of short courses of systemic corticosteroids after a discussion of the risks and benefits with the patient. This may include patients with significant nasal obstruction that would preclude penetration of intranasal agents (INCS or antihistamines). In these cases, a short course of systemic oral corticosteroids could improve congestion and facilitate access and efficacy of the topical agents.
IX.B.2.b. Injectable corticosteroids.
Corticosteroids have been injected intramuscularly or into the turbinates for management of AR. The evidence evaluating deep intramuscular injections will be reviewed first. Overall, several early studies1250-1254 demonstrated clinical effectiveness in improving allergic symptoms; however, the safety outcomes demonstrated the risk of undesired systemic corticosteroid adverse effects. More recent evidence1255 confirms the increased risk of endogenous cortisol suppression along with other corticosteroid-related adverse effects such as osteoporosis and hyperglycemia (Table IX.B.2.b).
TABLE IX.B.2.b.
Evidence for the role of corticosteroid injections in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Yang et al.1262 | 2008 | 1b | Randomized, placebo-controlled single-blind trial | Patients with PAR received intraturbinate injections (n = 39):
|
Symptoms of rhinorrhea, nasal obstruction, sneezing and itching at 1, 4, 8, 12, 16, and 20 weeks after injections | onabotulinum toxin A controlled nasal symptoms for the longest time after injection. Steroid injection was better than placebo but the duration of action was shorter than onabotulinum toxin A. |
| Laursen et al.1253 | 1988 | 1b | Double blind, double dummy, placebo controlled, study | SAR during season (n = 30):
|
Rhinoconjunctivitis symptom scores | IM injection significantly more effective than placebo or intranasal preparation. |
| Borum et al.1254 | 1987 | 1b | Double-blind, placebo controlled, parallel study during 2 consecutive pollen seasons | SAR during 2 consecutive allergy seasons (n = 24):
|
Number of sneezes and nose blowing during the day. Symptom scores of sneezing, rhinorrhea, nasal blockage, eye itching recorded at the end of the day. | IM injection was efficacious against nasal congestion with less pronounced effects against rhinorrhea and sneezing in active vs placebo treatment irrespective of timing of administration. |
| Laursen et al.1252 | 1987 | 2b | Randomized, double-blind comparative | SAR during season (n = 37):
|
Nasal peak flow and symptom scores. ACTH test performed at 3 weeks. | IM and oral steroid resulted in a significant reduction of nasal/ocular symptoms during season. Significant suppression of adrenal function with oral steroid treatment only. |
| Ohlander et al.1251 | 1980 | 2b | Prospective, randomized, parallel group | SAR during season (n = 60). Received 1 of 3 long-acting IM injections:
|
Scores of rhinorrhea, congestion, and ocular symptoms at 1, 2, and 4 weeks after injection. Cortisol and glucose blood levels in 38 subjects. | All treatments led to significant reductions in nose and eye symptoms during season; no difference between groups. All preparations suppressed endogenous cortisol; 2 out of 3 injections caused increases in blood sugar levels. |
| Kronholm1250 | 1979 | 2b | Prospective, parallel, randomized, open label | SAR during season. IM injection at season onset (n = 42):
|
Weekly nasal and ocular symptoms for 5 weeks | Both preparations led to a significant reduction of nose and eye symptoms; betamethasone combination was more effective. |
| Aasbjerg et al.1255 | 2013 | 4 | Retrospective study of Danish National Registries between 1995 and 2011 | Patients receiving IM steroid injections in April–July or immunotherapy against grass or birch pollen (n = 47,382) | Incidence and relative risk of osteoporosis, diabetes, tendon rupture, and respiratory tract infection | Relative risk and incidence of osteoporosis and diabetes were higher in individuals receiving at least 1 depot corticosteroid injection vs those receiving immunotherapy. |
ACTH = adrenal corticotropic hormone; IM = intramuscular; LOE = level of evidence; PAR = perennial allergic rhinitis; PO = per os (medication taken orally); SAR = seasonal allergic rhinitis.
Kronholm1250 demonstrated that a single injection of either betamethasone dipropionate/betamethasone phosphate or methylprednisolone acetate given at the onset of the hay fever season led to a significant reduction of both nasal and ocular symptoms during the 5 weeks of the study, with the betamethasone combination being more effective. Ohlander et al.1251 compared 3 long-acting corticosteroid injections given at the beginning of the season, and showed that all treatments led to significant reductions in nasal and ocular symptoms during the season with no difference between groups. However, all preparations also suppressed endogenous cortisol, in some cases for more than 14 days after injection, and 2 out of the 3 injections resulted in increases in blood sugar levels.
When compared to other agents, injected corticosteroids demonstrated similar effectiveness outcomes. Specifically, there were similar clinical outcomes when comparing preseasonal steroid injections to both daily oral prednisolone1252 and daily intranasal beclomethasone dipropionate spray.1253 An adrenal corticotropic hormone (ACTH) test performed at 3 weeks showed significant suppression of adrenal function in the oral steroid treatment group and no evidence of suppression in the corticosteroid injection or topical intranasal corticosteroid groups.1252 This was probably related to the short duration of adrenal suppression expected after a single injection of corticosteroids compared to continuous administration.
When evaluating the timing of injectable corticosteroid therapy, Borum et al.1254 compared the effects of a single depot injection of methylprednisolone given either at the beginning of the allergy season or later when pollen counts peaked. Compared to placebo, intramuscular methylprednisolone was efficacious against nasal congestion with less pronounced effects against rhinorrhea and sneezing. The authors argue that depot injectable steroids may be considered after other safer medical therapy fails and may provide an effective alternative treatment even if provided late in the allergy season.
Injectable corticosteroid preparations may have significant side effects that include adrenal suppression and growth retardation.1256 In a large retrospective study of Danish National Registries, the relative risk and incidence of both osteoporosis and diabetes were higher in allergic individuals receiving at least 1 depot corticosteroid injection during the allergy season compared to those receiving immunotherapy.1255
Several early reports detailed significant improvement in symptoms of AR in a large proportion of patients who received intraturbinate injections of cortisone,1257 hydrocortisone acetate,1258 or prednisolone.1259 Similar, noncontrolled, studies showed improvement in AR symptoms after intraturbinate injections.1260,1261 A more recent randomized, placebo-controlled, single-blind trial by Yang et al.1262 compared the efficacy of intraturbinate injections of either onabotulinum toxin A, triamcinolone, or isotonic saline in patients with PAR. Both onabotulinum toxin A and triamcinolone therapy showed better control of nasal symptoms than placebo with onabotulinum toxin A efficacy lasting longest.
Orbital complications have been reported with intraturbinate but not intramuscular injections. Based on a large clinical experience, Mabry cites an estimated incidence of visual loss after intraturbinate injections to be 0.006%.1263 Other complications have included transient visual loss and diplopia,1264 blurred vision and temporary blindness,1265 temporary distorted vision, and decreased visual acuity and paresis of the medial rectus.1265 Martin et al.1266 reported the rapid onset of ocular pain, blurred vision, and decreased visual acuity after an intraturbinate injection of triamcinolone acetonide. Choroidal and retinal arterial embolization were confirmed as the cause and they resolved completely within 24 hours. The mechanism of embolization is likely related to retrograde flow from the anterior tip of the inferior turbinate to the ophthalmic artery, followed by anterograde flow with the particles lodging in the end arteries of the choroid and retinal vessels. Steroids with larger particle size (eg, methylprednisolone) are thought to present higher risk than lower-sized particles (eg, triamcinolone).
Aggregate Grade of Evidence: B (Level 1b: 3 studies; Level 2b: 3 studies; Level 4: 7 studies; Table IX.B.2.b).
Benefit: Injectable corticosteroids improve symptoms of AR in clinical studies.
Harm: Injectable corticosteroids have known adverse effects on the hypothalamic-pituitary axis, growth suppression, osteoporosis, hyperglycemia, and other systemic adverse effects. Intraturbinate corticosteroids have a small, but potentially serious, risk of ocular side effects including decline or loss of vision.
Cost: Low.
Benefits-Harm Assessment: In routine management of AR, the risk of serious adverse effects outweighs the demonstrated clinical benefit.
Value Judgments: Injectable corticosteroids are effective for the treatment of AR. However, given the risk of significant systemic adverse effects, the risk of serious ocular side effects, and the availability of effective alternatives (ie, topical INCS therapy), injectable corticosteroids are not recommended for the routine treatment of AR.
Policy Level: Recommendation against.
Intervention: None.
IX.B.2.c. Intranasal corticosteroids (INCSs).
INCSs are effective for the treatment of AR. Their potent anti-inflammatory properties directly affect the pathophysiologic mechanisms of nasal inflammation in AR. In both nasal allergen challenge models and seasonal disease, treatment with INCS results in significant reduction in mediator and cytokine release along with a significant inhibition in the recruitment of basophils, eosinophils, neutrophils, and mononuclear cells to the nasal mucosa and secretions.187,389,1267,1268 INCSs also reduce the antigen-induced hyperresponsiveness of the nasal mucosa to subsequent challenge by antigen187 and histamine.1269,1270
Multiple placebo-controlled clinical trials in adults and children have demonstrated the effectiveness of INCS in the reduction of nasal symptoms in AR, including sneezing, itching, rhinorrhea, and congestion.1271,1272 With the reduction of nasal symptoms, INCS significantly improve the QOL1272-1274 and sleep673,706,707,1275,1276 of these patients. No significant differences in efficacy between available agents have been demonstrated in studied populations1273; therefore, sensory attributes may be an important factor in patient preference and adherence to therapy.1277 These sensory attributes include aftertaste, nose runout, throat rundown, and smell. Addressing some of these concerns are 2 intranasal non-aqueous preparations with hydrofluoroalkane (HFA) aerosols recently approved for the treatment of AR in the United States. These include beclomethasone dipropionate and ciclesonide, both approved and effective for SAR and PAR in adults and children 12 years and older.688,1278-1281 Onset of action for INCS starts at time points ranging from 3 to 5 hours to 60 hours after first dosing.1282-1285 Although the recommended continuous daily use of INCS is superior to other dosing strategies,1286,1287 studies have demonstrated the efficacy of as-needed use of intranasal fluticasone propionate compared to placebo1288,1289 (Table IX.B.2.c-1).
TABLE IX.B.2.c-1.
Evidence for the clinical efficacy of intranasal corticosteroids in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Rachelefsky & Farrar1274 | 2013 | 1a | SR | SAR (n = 2290) and PAR (n = 800). Sixteen controlled clinical trials ≥2 weeks in duration. Children aged 2–18 years. |
Measures that assessed impairment and/or risk of comorbid conditions. | Intranasal steroids improved risk outcomes associated with asthma and OSA. |
| Rodrigo & Neffen1272 | 2011 | 1a | SR with meta-analysis | 16 trials (n = 5348). SAR:7 studies; PAR: 9 studies. Adults and adolescents ≥12 years: 13 studies; children: 3 studies. FFNS vs placebo. |
Primary outcomes: rTOSS, iTOSS, rTNSS, and iTNSS. Secondary outcomes: QOL, and adverse effects. | FFNS significantly improved rTOSS, iTOSS, rTNSS, and iTNSS scores compared with placebo in patients with SAR and PAR. There were greater improvements in QOL with a favorable safety profile. |
| Penagos et al.1271 | 2008 | 1a | Meta-analysis of RDBPCTs | 16 trials (n = 2998). MFNS vs placebo. |
TNSS, individual nasal symptoms, and TNNSS. | MFNS was associated with a significant reduction in TNSS and TNNSS. Significant effect was seen for nasal stuffiness/congestion, rhinorrhea, sneezing, and nasal itching. |
| Yamada et al.673 | 2012 | 1b | Randomized, placebo-controlled, double-blind, crossover study | PAR (n = 57). MFNS vs placebo for 14 days. |
Nasal symptom scores, QOL, and sleep quality, ESS. | MFNS significantly improved nasal symptoms, QOL, and sleep quality. Significant reduction of the ESS observed in the MFNS group with high sleep disturbance. |
| Meltzer et al.1276 | 2010 | 1b | Double-blind, parallel group, placebo-controlled study | Adults with PAR, moderate rhinitis and disturbed sleep (n = 30). MFNS 200 μg vs placebo, 4-week trial. |
Primary endpoint: AHI. Secondary measures: TNSS, nighttime symptom score, daytime nPIF, nighttime flow limitation index, RQLQ, ESS, WPAI-AS | AHI was not statistically significantly different between groups. MFNS significantly improved morning and evening TNSS, nasal obstruction/ blockage/congestion, daily nPIF, ESS, QOL score, and 2 of 5 WPAI–AS domains. |
| Kaiser et al.1284 | 2007 | 1b | Double-blind, parallel-group, randomized, placebo-controlled study | Adults and adolescents with SAR (n = 299). FFNS 110 μg vs placebo. |
Nasal and ocular symptoms on 4-point scale. rTNSS, iTNSS, rTOSS, iTOSS | FFNS significantly improved daily rTNSS, morning pre-dose iTNSS, daily rTOSS, and patient-rated overall response to therapy. Onset of therapeutic effect occurred at 8 hours after initial administration. |
| Craig et al.1275 | 2003 | 1b | Double-blind, placebo-controlled study | PAR (n = 32). Fluticasone NS vs placebo. |
Questionnaires, QOL instruments, daily diary, ESS, and polysomnography. | Fluticasone improved subjective sleep vs placebo. There was no difference in the AHI in treated subjects. |
| Dykewicz et al.1289 | 2003 | 1b | RDBPCT | Adults and adolescents ≥12 year (n = 241), SAR to fall allergen. FPNS 200 μg PRN vs placebo for 4 weeks. |
Mean change from baseline in TNSS. | Patients treated with FPNS PRN had a significantly greater reduction from baseline in TNSS. Individual symptoms were also significantly improved by active therapy. |
| Hughes et al.1706 | 2003 | 1b | Double-blind, placebo-controlled, crossover study | PAR (n = 22). Budesonide 128 μg/day or placebo for 8 weeks. |
ESS, Functional Outcomes of Sleep Questionnaire, RQLQ. Daily diary of nasal symptoms, sleep problems, and daytime fatigue. | Budesonide significantly improved daytime fatigue, somnolence, and quality of sleep vs placebo. |
| Fokkens et al.1283 | 2002 | 1b | RDBPCT, parallel-group, multicenter | PAR (n = 202, age 6–16 years). BANS 128 μg daily vs placebo. |
Daily nPIF, nasal symptom scores, and overall evaluation of treatment efficacy. Subset (n = 76) QOL by validated questionnaires. | BANS significantly more effective than placebo for nPIF, combined and individual nasal symptom scores, and the overall evaluation of treatment efficacy. Onset of action within the first 12-hour time interval for combined nasal symptoms and within 48 hours for nPIF. |
| Day et al.1282 | 2000 | 1b | RDBPCT, parallel-group | SAR, ragweed-sensitivity (n = 217), symptoms for at least 1 year. Challenge via chamber. BANS 64 μg vs BANS 256 μg vs placebo. |
Combined nasal score, individual nasal symptoms, overall evaluation of treatment efficacy, nPIF. | 7–12 hours: BANS better than placebo in reducing combined nasal and blocked nose symptoms. nPIF: onset of action (3 hours) was shortest for BANS 256 μg. Treatment efficacy was higher for those receiving BANS compared with placebo starting at 5 hours. All treatments well tolerated, no specific adverse events occurred. |
| Jen et al.1288 | 2000 | 1b | RDBPCT, parallel-group. | Adults, SAR, ragweed sensitivity (n = 52). FPNS PRN vs placebo for 4 weeks. |
Nasal symptom score, QOL, eosinophil count, and eosinophilic cationic protein in nasal lavage. | Nasal symptom score lower with FPNS vs placebo. QOL significantly improved with FPNS. Eosinophil count significantly lower in with FPNS. |
| Craig et al.707 | 1998 | 1b | Double-blind, placebo-controlled study | PAR (n = 20). Topical INCS vs placebo |
Daily symptom diary of nasal symptoms, sleep, and daytime sleepiness. | Nasal congestion and subjective sleep improved significantly in the INCS-treated subjects but not in the placebo group. |
| Day & Carrillo1285 | 1998 | 1b | RDBPCT, multicenter, parallel-group | Adults, PAR (n = 273). BANS and FNSP nasal sprays. Baseline: 8–14 days. 6 weeks: Active treatment. |
Mean combined nasal symptoms scores (nasal blockage, runny nose, and sneezing). | BANS significantly decreased nasal symptoms vs FPNS. Both treatments significantly decreased nasal symptoms vs placebo. Time to achieve statistically significant improvement: BANS 36 hours, FPNS 60 hours. Adverse events were mild and transient. |
| Juniper et al.1286 | 1990 | 1b | Randomized, double-blind, parallel-group | Adults, SAR, ragweed sensitivity (n = 60).
|
Sneezing, stuffy nose, and rhinorrhea, measured by a daily diary. QOL questionnaires and rescue medication use (terfenadine). | Nasal symptoms, QOL, and use of rescue medications were significantly better controlled in the regular-treated group as compared to the PRN group. |
| Herman1273 | 2007 | 2a | Review of randomized, controlled, comparison trials | SAR and PAR. 14 studies reviewed. BANS, MFNS, FPNS, or TANS. |
Different endpoints for different studies | All 4 INCSs administered once daily were effective and well tolerated in the treatment of AR in adult patients, with similar efficacy and adverse event profiles. Based on sensory attributes, patients preferred BANS and TANS vs MFNS and FPNS. |
| Juniper et al.1287 | 1993 | 2b | Randomized, non-blinded, parallel group comparison | Adults, SAR, ragweed sensitivity (n = 60). Beclomethasone dipropionate NS regular use (400 μg daily) vs PRN use. |
Daily symptoms and medication use, QOL, and patient satisfaction with symptom control. | 27% of PRN patients reported unsatisfactory control, worse QOL, and increased medication use. Patients who achieved satisfactory control in the PRN group had similar symptom and QOL scores to the regular group. |
AHI = apnea-hypopnea index; BANS = budesonide aqueous nasal spray; ESS = Epworth Sleepiness Scale; FFNS = fluticasone furoate nasal spray; FPNS = fluticasone propionate nasal spray; INCS = intranasal corticosteroid; iTNSS = instantaneous Total Nasal Symptom Score; iTOSS = instantaneous Total Ocular Symptom Score; LOE = level of evidence; MFNS = mometasone furoate nasal spray; nPIF = nasal peak inspiratory flow; NS = nasal spray; OSA = obstructive sleep apnea; PAR = perennial allergic rhinitis; PRN = as needed; QOL = quality of life; RDBPCT = randomized double-blind placebo-controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; rTNSS = reflective Total Nasal Symptom Score; rTOSS = reflective Total Ocular Symptom Score; SAR = seasonal allergic rhinitis; SR = systematic review; TANS = triamcinolone aqueous nasal spray; TNSS = Total Nasal Symptom Score; TOSS = Total Nasal Symptom Score; WPAI-AS = Work Productivity and Activities Impairment-Allergy Specific questionnaire.
Along with improved nasal symptoms, INCSs have beneficial effects on allergic eye symptoms including itching, tearing, redness, and puffiness.1290-1292 This is secondary to a reduction in the naso-ocular reflex, which contributes to these eye symptoms.1293 Most INCSs lead to improved ocular symptoms, but the evidence suggests that the effects are not equal among INCS preparations.1294 Some studies have suggested that INCSs improve asthma control measures in patients suffering from both AR and asthma1295,1296 (Table IX.B.2.c-2).
TABLE IX.B.2.c-2.
Effect of intranasal corticosteroids on comorbidities: ocular symptoms and asthma
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lohia et al.1296 | 2013 | 1a | SR and meta-analysis | Asthma and AR. 18 studies (n = 2162). Efficacy of INCS on asthma outcomes. |
Asthma outcomes: pulmonary function, bronchial reactivity, asthma symptom scores, asthma-specific QOL, and rescue medication use. | Use of INCS resulted in significant improvements in FEV1, bronchial challenge, asthma symptom scores, and rescue medication use vs placebo. INCS improved morning and evening PEF. Addition of INCS spray to orally inhaled corticosteroids did not result in additional improvement. |
| Bielory et al.1291 | 2011 | 1a | Meta-analysis of placebo-controlled RCTs | 10 studies (n = 3132). SAR: 6 studies; PAR: 4 studies; MFNS 200 μg daily. |
Severity of reflective ocular symptoms (itching/burning, redness, and tearing/watering) on a 4-point scale over 12 hours. | Overall treatment effect was significant for all 3 individual ocular symptoms in SAR and PAR studies. |
| DeWester et al.1290 | 2003 | 1a | Retrospective analysis of multicenter, RDBPCTs | 7 studies. Efficacy of FPNS 200 μg daily for nasal and ocular symptoms in patients with SAR. |
Mean change from baseline in the clinician-rated TOSS (itching, tearing, redness, and puffiness) at 7 and 14 days of therapy. | FPNS group had significantly greater mean changes from baseline in the TOSS and in all 4 individual symptom scores vs placebo at days 7 and 14. |
| Taramarcaz & Gibson1295 | 2003 | 1a | Meta-analysis of RCTs | Asthma and AR. 14 studies (n = 477). INCS vs placebo/routine asthma treatment. |
Asthma outcomes: symptom scores, FEV1, PEF, and methacholine airway responsiveness. | No statistically significant benefit of INCS in asthma. |
| Ratner et al.1292 | 2015 | 1b | Randomized, double-blind, parallel, multicenter study | SAR (n = 614). FPNS 200 μg daily vs placebo × 14 days. |
Mean change from baseline in patient-rated rTOSS. | FPNS was significantly more efficacious in reducing the ocular symptoms of AR vs placebo. |
| Baroody et al.1293 | 2009 | 1b | Double-blind, placebo-controlled, crossover trial | SAR out of season (n = 20). FFNS 110 μg daily vs placebo × 1 week. Nasal allergen challenge. |
Nasal and ocular symptoms after allergen challenge. | Pretreatment with FFNS significantly reduced eye symptoms after nasal allergen challenge. |
AR = allergic rhinitis; FEV1 = forced expiratory volume in 1 second; FFNS = fluticasone furoate nasal spray; FPNS = fluticasone propionate nasal spray; INCS = intranasal corticosteroid; LOE = level of evidence; MFNS = mometasone furoate nasal spray; PAR = perennial allergic rhinitis; PEF = peak expiratory flow; QOL = quality of life; RCT = randomized controlled trial; RDBPCT = randomized double-blind placebo-controlled trial; rTOSS = reflective Total Ocular Symptom Score; SAR = seasonal allergic rhinitis; SR = systematic review; TOSS = Total Ocular Symptom Score.
In comparative studies, INCSs have shown superior efficacy to H1 antihistamines in controlling nasal symptoms, including nasal congestion, with no significant difference in the relief of ocular symptoms.1297-1299 INCSs are more effective than LTRAs1299,1300 (Table IX.B.2.c-3).
TABLE IX.B.2.c-3.
Comparison of intranasal corticosteroids to other agents for the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Benninger et al.1299 | 2010 | 1a | SR of RCTs of at least 2-week duration, and studying U.S.-approved INCS indication/dose | SAR: 38 studies (n = 11,980 adults, 946 children); PAR: 12 studies (n = 3800 adults, 366 children). |
Median percentage changes from baseline for TNSS. | INCS produce the greatest improvements in nasal symptoms in SAR. INCS effective for PAR, but data quality variable; oral antihistamines may be equally effective for some patients. |
| Wilson et al.1300 | 2004 | 1a | SR and meta-analysis of RCTs of the effectiveness of LTRAs | SAR: 11 studies. 8 evaluating LTRAs (alone or plus other treatments) vs placebo or other treatments (n = 3924); 3 evaluating LTRAs plus antihistamine (n = 80). |
Composite daily rhinitis symptom scores and rhinitis-specific quality of life. | LTRAs are modestly better than placebo, as effective as antihistamines, but less effective than INCS in improving symptoms and QOL in patients with SAR. |
| Yanez & Rodrigo1298 | 2002 | 1a | SR of RCTs | AR: 9 studies (n = 648). INCS vs topical antihistamines. |
Total nasal symptoms, sneezing, rhinorrhea, itching, and nasal blockage. | INCS produced greater relief of nasal symptoms vs topical antihistamines. No difference between the 2 treatments for ocular symptoms. |
| Weiner et al.1297 | 1998 | 1a | Meta-analysis of RCTs | AR: 16 studies (n = 2267). INCS vs oral antihistamines. |
Nasal blockage, nasal discharge, sneezing, nasal itch, postnasal drip, nasal discomfort, total nasal symptoms, nasal resistance, and eye symptoms and global ratings. | INCS produced greater relief of nasal blockage, nasal discharge, sneezing, nasal itch, postnasal drip, and total nasal symptoms vs oral antihistamines. No difference between the 2 treatments for nasal discomfort, nasal resistance, or eye symptoms. |
AR = allergic rhinitis; INCS = intranasal corticosteroid; LOE = level of evidence; LTRA = leukotriene receptor antagonist; PAR = perennial allergic rhinitis; QOL = quality of life; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis; SR = systematic review; TNSS = Total Nasal Symptom Score.
The most common side effects of INCSs are a result of local irritation and include dryness, burning, stinging, blood-tinged secretions, and epistaxis. The incidence of epistaxis with different preparations ranges from 4% to 8% over short treatment periods (2 to 12 weeks) with no differences between placebo and active therapy.1301,1302 In studies carried over 1 year, epistaxis is as high as 20%.1303,1304 Septal perforations are rare complications of INCS.51 A systematic review of published articles looking at biopsy studies in patients with AR or CRS using INCS identified 34 studies. Of those, 21 studies included patients with AR, mixed rhinitis, and NAR, and 13 involved patients with CRS with/without polyposis.1305 None of the studies that included atrophy of the nasal mucosa as an outcome measure reported any atrophy with INCS. A meta-analysis of a subgroup of the studies showed no significant chance of developing atrophy while taking INCS, and no difference between active and control groups in basement membrane characteristics. The review also found a significant reduction in the OR for the development of squamous metaplasia in patients using INCS, suggesting a favorable effect. Studies in adults and children evaluating effects of INCS on the hypothalamic pituitary axis have assessed morning cortisol concentrations, cosyntropin stimulation, 24-hour serum cortisol and 24-hour urinary free cortisol excretion. They show no adverse effects.1304,1306-1317 Although there has been a report of an association between the use of INCS and the development of posterior subcapsular cataracts,1318 a systematic review of controlled trials did not demonstrate a clinically relevant impact of INCS on either ocular pressure, glaucoma, lens opacity, or cataract formation.1319 The effect of INCS on growth in children has been investigated in controlled studies using both knemometry in short-term studies (2 to 4 weeks) and stadiometry in long-term (12 months) studies. A meta-analysis of 8 randomized controlled trials with appropriate controls showed that, compared to children using placebo, mean growth was significantly lower among children using INCS in trials using knemometry (n = 4) and that there was no significant growth difference in studies using stadiometry (n = 4).1320 The data suggests that INCS might have deleterious effects on short-term growth in children, but the heterogeneity in the stadiometry studies makes the effects on long-term growth suppression unclear (Table IX.B.2.c-4).
TABLE IX.B.2.c-4.
Studies evaluating adverse effects of intranasal corticosteroids
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Ahmadi et al.1319 | 2015 | 1a | SR | 19 studies of INCS reporting original ocular endpoints (10 RCTs, 1 case-control, 8 case series) included. | IOP, lens opacity, glaucoma or cataract incidence. | None of the 10 RCTs reporting IOP demonstrated changes vs control. None of the 6 RCTs reporting cataract or lens opacity demonstrated changes vs control. |
| Mener et al.1320 | 2015 | 1a | SR with meta-analysis | 8 RCTs (n = 755) investigating INCS for AR in children 3-12 years. | Interval change in growth. Knemometry (n = 342 participants, duration 2–4 weeks). Stadiometry (n = 413 participants, duration 12 months). | Knemometry studies: Mean growth lower among children using INCS. Stadiometry studies: No significant growth difference in INCS vs placebo. Limitations: Difficulty in predicting longer-term or catch-up growth. |
| Verkerk et al.1305 | 2015 | 1a | SR | 34 studies (11 RCTs, 5 cohorts, 20 case series) included. INCS use with or without control group. |
Histopathology of nasal mucosa. Mucosal atrophy reported in 17 studies. | The concept of nasal mucosal atrophy is poorly defined. No histological evidence for deleterious effects from INCS use on human nasal mucosa. |
| Hampel et al.1317 | 2015 | 1b | RDBPCT | PAR, children 6–11 years. BDP 800 μg daily (n = 67) vs placebo (n = 32) for 6 weeks. |
Change in 24-hour serum cortisol from baseline. | Serum cortisol values remained stable in both groups. Concentration-time profiles similar for the placebo and BDP groups at baseline and week 6. |
| Meltzer et al.1302 | 2009 | 1b | Subanalysis of 3 RDBPCTs, focusing on the 6-11 age group | SAR: 2-week U.S. study. PAR: 12-week global study. HPA axis safety: 6-week U.S. study. FF 55 μg vs FF 110 μg vs placebo daily (n = 948). |
Different endpoints, which included: adverse event monitoring, nasal examinations, ophthalmic examinations, 24-hour urinary cortisol excretions, and serum cortisol concentrations. | Epistaxis 4% in both active and placebo groups. No differences between groups for IOP, and no posterior subcapsular cataracts. No difference in HPA measures between groups. |
| Ratner et al.1304 | 2009 | 1b | Multicenter, randomized, controlled trial | PAR, children 6–11 years (n = 255). MFNS 100 μg vs BDP 168 μg daily for 12 months. |
Symptom control and safety. | There was appropriate symptom control in both groups. Adverse events were mild. Incidence of epistaxis was 12.7% with MFNS and 9.4% for BDP. |
| Tripathy et al.1316 | 2009 | 1b | Double-blind, randomized parallel-group study | PAR, children 2–11 years (n = 112). FF 110 μg vs placebo daily for 6 weeks. |
24-hour serum and urinary cortisol. FF plasma measurements. | FF was non-inferior to placebo with respect to 24-hour serum cortisol. Urinary cortisol excretion over 24 hour at baseline and end of treatment similar between treatment groups. |
| Weinstein et al.1315 | 2009 | 1b | RDBPCT, multicenter, parallel-group | PAR, children 2–5 years (n = 474). TAA 110 μg vs placebo daily for 4 weeks. |
Adverse events, morning serum cortisol levels, and growth as measured using office stadiometry. | Adverse event rates comparable between groups. No significant change from baseline in serum cortisol levels after cosyntropin infusion. Distribution by stature-for-age percentile remained stable. |
| Maspero et al.1301 | 2008 | 1b | Double-blind, placebo-controlled study | PAR, children 2–11 years (n = 558). FF 110 μg vs FF 55 μg vs placebo daily for 12 weeks. |
Nasal symptom scores for efficacy. Nasal and ophthalmic examinations, and HPA assessments for safety. | Epistaxis 6% in all groups. There were no significant ophthalmic or HPA related side effects in the treated subjects. The lower dose of FF reduced nasal symptoms. |
| Patel et al.1314 | 2008 | 1b | RDBPCT, parallel-group | PAR, 12–65 years (n = 112). FF 110 μg daily for 6 weeks vs prednisone 10 mg daily for last 7 days of study vs placebo. |
Change in 24-hour serum cortisol and 24-hour urinary free cortisol, total 24-hour urinary free cortisol, 6-beta hydroxycortisol excretion, and plasma concentration of FF. | Ratio from baseline in serum cortisol weighted mean: FF noninferior to placebo, prednisone significantly reduced the ratio. 24-hour urinary cortisol excretion was similar in the FF and placebo groups. Plasma levels of FF were undetectable after 6 weeks of treatment. |
| Chervinsky et al.1313 | 2007 | 1b | RDBPCT | PAR patients ≥12 years (n = 663). Ciclesonide 200 μg vs placebo daily for up to 52 weeks. |
Adverse events, exam findings, 24-hour urinary free cortisol, morning plasma cortisol, IOP, lens opacification. | No clinically relevant differences observed between the ciclesonide and placebo groups. |
| Kim et al.1312 | 2007 | 1b | Two separate phase 3, double-blind, parallel-group, placebo-controlled trials | PAR, children 2–5 years. Safety, tolerability, and efficacy of intranasal ciclesonide 200 μg once daily. First study: 6 weeks. Second study: 12 weeks. |
Cortisol levels were measured at the beginning and end of each study. The systemic exposure of ciclesonide and its active metabolite measured at treatment end in the 6-week study. | Changes in plasma or urine cortisol levels showed no difference in active vs placebo group. Serum concentrations were below the lower limit of quantification, suggesting that systemic exposure to ciclesonide was low. |
| Rosenblut et al.1303 | 2007 | 1b | RDBPCT, parallel-group | PAR (n = 806). FF 110 μg vs placebo daily for 12 months. |
Adverse events, 24-hour urinary cortisol excretion, nasal and ophthalmic examinations, electrocardiograms and clinical laboratory tests. | Incidence of adverse events similar to placebo, except epistaxis (active 20%, placebo 8%). No clinically meaningful differences in ophthalmic parameters or urine cortisol excretion. |
| Galant et al.1311 | 2003 | 1b | RDBPCT | AR, children 2-3 years (n = 65). FP 200 μg vs placebo daily for 6 weeks. |
12-hour urinary free cortisol concentration at baseline and after 6 weeks of treatment. | FP group equivalent to placebo group in mean change from baseline of 12-hour urinary free cortisol at treatment end. |
AR = allergic rhinitis; BDP; beclomethasone dipropionate; FF = fluticasone furoate; FP = fluticasone propionate; HPA; hypothalamic pituitary axis; INCS = intranasal corticosteroid; IOP = intraocular pressure; LOE = level of evidence; MFNS = mometasone furoate nasal spray; PAR = perennial allergic rhinitis; RCT = randomized controlled trial; RDBPCT = randomized double-blind placebo-controlled trial; SAR = seasonal allergic rhinitis; SR = systematic review; TAA = triamcinolone acetonide.
INCSs are first-line therapy for the treatment of AR due to their superior efficacy in controlling nasal congestion and other symptoms of this inflammatory condition. Subjects with known SAR should start prophylactic treatment with INCS several days before the pollen season with an evaluation of the patient’s response in 2 weeks. In addition to making changes to the treatment regimen according to the patient’s response, a nasal exam evaluates for signs of local irritation due to the drug or mechanical trauma from the applicator itself. Aiming the spray away from the nasal septum may also reduce irritation in this area. Children receiving INCS should be on the lowest effective dose to avoid negative growth effects.
Aggregate Grade of Evidence: A (Level 1a: 15 studies; Level 1b: 33 studies; Level 2a: 3 studies; Level 2b: 1 study; Level 5: 1 study; Tables IX.B.2.c-1, IX.B.2.c-2, IX.B.2.c-3, and IX.B.2.c-4).
Benefit: INCSs are effective in reducing nasal and ocular symptoms of AR. They have superior efficacy compared to oral antihistamines and LTRAs.
Harm: INCS have known undesirable local adverse effects such as epistaxis with some increased frequency compared to placebo in prolonged administration studies. There are no apparent negative effects on the hypothalamic-pituitary axis. There might be some negative effects on short-term growth in children, but it is unclear whether these effects translate into long-term growth suppression.
Cost: Low.
Benefits-Harm Assessment: The benefits of using INCS outweigh the risks when used to treat SAR and PAR.
Value Judgments: None.
Policy Level: Strong recommendation for the use of INCS to treat AR.
Intervention: The well-proven efficacy of INCSs, as well as their superiority over other agents, make them first-line therapy in the treatment of AR.
IX.B.3. Decongestants
IX.B.3.a. Oral decongestants.
Oral decongestants, such as pseudoephedrine, act on adrenergic receptors and lead to vasoconstriction, which can relieve nasal congestion in patients with AR. With extended-release oral decongestants nasal decongestion can last up to 24 hours. Oral decongestants are available for use alone or in combination with oral antihistamines. (See section IX.B.10.a. Management – Pharmacotherapy – Combination therapy – Oral antihistamine and oral decongestant for additional information on this topic.)
Availability of pseudoephedrine in the United States has been limited to behind-the-counter at pharmacies since 2006 due to stricter control over the distribution and sale of substances that can be used to manufacture methamphetamine. In a study by Mucha et al.,1321 pseudoephedrine resulted in significant improvement in all symptoms in adults with ragweed-induced AR (Table IX.B.3.a). Phenylephrine has been marketed as an over-the-counter (OTC) medication as a substitute for pseudoephedrine for nasal decongestion. However, an RCT by Horak et al.1322 found that while pseudoephedrine was significantly more effective at reducing nasal congestion than both placebo and phenylephrine, there was no significant difference between phenylephrine and placebo. In addition, Meltzer et al.1323 performed a randomized, open-label, dose-range trial in 539 patients with SAR and found phenylephrine to be no more effective than placebo in reducing symptomatic nasal congestion.
TABLE IX.B.3.a.
Evidence for the role of oral decongestants in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Salerno et al.1324 | 2005 | 1a | SR |
|
SBP, DBP, HR | Phenylpropanolamine caused increase in SBP. |
| Salerno et al.1325 | 2005 | 1a | SR |
|
SBP, DBP, HR | Pseudoephedrine caused increase in SBP and HR. |
| Meltzer et al.1323 | 2015 | 1b | RCT |
|
Daily reflective nasal congestion score | Phenylephrine is not better than placebo at relieving nasal congestion. |
| Horak et al.1322 | 2009 | 1b | RCT |
|
Subjective evaluation of nasal congestion | Pseudoephedrine resulted in improvement in nasal congestion. Phenylephrine did not improve nasal congestion. |
| Mucha et al.1321 | 2006 | 1b | RCT |
|
Nasal symptoms, nPIF, QOL | Significant improvement from baseline in all symptoms of AR, nPIF, and QOL with both pseudoephedrine and montelukast. |
| Vernacchio et al.1327 | 2008 | 3b | Non-consecutive cohort | Pseudoephedrine use in pediatric population | Children less than 2 years of age are at the highest risk for toxicity with pseudoephedrine. Safe dosing recommendations are lacking for this age group. | |
| Kernan et al.1326 | 2000 | 3b | Case-control |
|
Association between the use of phenylpropanolamine and the risk of a hemorrhagic stroke. | Phenylpropanolamine is an independent risk factor for hemorrhagic stroke in women. |
| Roberge et al.1328 | 1999 | 4 | Case report | 2-year-old developed psychosis and ataxia after being overmedicated with pseudoephedrine/ dextromethorphan cough preparation. | ||
| Sauder et al.1329 | 1998 | 4 | Case report | 3-year-old with visual hallucinations caused by inappropriately high doses of pseudoephedrine. |
AR = allergic rhinitis; DBP = diastolic blood pressure; HR = heart rate; LOE = level of evidence; nPIF = nasal peak inspiratory flow; QOL = quality of life; RCT = randomized controlled trial; SBP = systolic blood pressure; SR = systematic review.
Known side effects of this class of medications include insomnia, nervousness, anxiety, tremors, palpitations, and increased blood pressure (BP). Two systematic reviews by Salerno et al.1324,1325 looked at the effect of oral decongestants on blood pressure. The first study showed that phenylpropanolamine significantly increased systolic blood pressure (SBP) by 5.5 mmHg (95% CI, 3.1 to 8.0) and diastolic blood pressure (DBP) by 4.1 mmHg (95% CI, 2.2 to 6.0) with no effect on heart rate as compared to placebo.1324 The second study found that pseudoephedrine also caused a small but significant increase in SBP by 0.99 mmHg (95% CI, 0.08 to 1.9) and heart rate (HR) by 2.83 beats/minute (95% CI, 2.0 to 3.6) with no effect on DBP.1325 Additionally, higher doses and immediate-release preparations of pseudoephedrine were associated with greater BP elevations.1325 Further, in a study by Kernan et al.,1326 phenylpropanolamine use in women was an independent risk factor for hemorrhagic stroke. Phenylpropanolamine is no longer available on the market. Given these cardiovascular side effects, oral decongestants should be used with caution in patients who are already at risk for hypertension and its sequelae (eg, coronary artery disease, cerebral vascular disease, hyperthyroidism, arrhythmias). Blood pressure should be closely monitored for any changes when using oral decongestants in this population.
Oral decongestants are known to be effective in children older than 6 years of age. However, care should be taken in the younger population (less than 2 years of age) as this population is more prone to toxicity, and safe dosing recommendations have not yet been established for this age group.1327 In infants and young children, oral decongestants may have central nervous system (CNS) stimulatory effects with known cases of psychosis, ataxia, and hallucinations with ingestion.1328,1329 Evaluation of risk and benefits should be considered in patients less than 6 years old.
Aggregate Grade of Evidence: B (Level 1a: 2 studies; Level 1b: 3 studies; Level 3b: 2 studies; Level 4: 2 studies; Table IX.B.3.a).
Benefit: Reduction of nasal congestion with pseudoephedrine. No benefit with phenylephrine.
Harm: Side effects include insomnia, loss of appetite, irritability, palpitations, and increased blood pressure. Risk of toxicity in young children.
Cost: Low.
Benefits-Harm Assessment: Balance of benefit and harm for pseudoephedrine. Harm likely outweighs benefit for phenylephrine.
Value Judgments: Patient’s other comorbidities and age should be considered before use.
Policy Level: Option for pseudoephedrine. Recommendation against for phenylephrine.
Intervention: Pseudoephedrine as an oral decongestant can be effective in reducing symptom of nasal congestion in patients with AR; used for short-term symptom relief. Side effects, comorbidities, and age of patient should be considered before use.
IX.B.3.b. Intranasal decongestants.
Topical decongestants, such as xylometazoline and oxymetazoline, are alpha-adrenergic stimulators delivered directly to nasal mucosal tissue that result in vasoconstriction and reduction of mucosal thickness. In an 18-day study, Barnes et al.1330 found that nasal xylometazoline was a stronger decongestant than nasal corticosteroids (Table IX.B.3.b). Topical decongestants relieve the symptom of nasal congestion, however they have no effect on other symptoms of AR, such as sneezing, rhinorrhea, or nasal itching.
TABLE IX.B.3.b.
Evidence for the role of topical intranasal decongestants in the management of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Barnes et al.1330 | 2005 | 1b | RCT | (n = 36):
|
nPIF, nasal forced inspiratory volume in 1 second, nasal blockage score | Xylometazoline was a stronger nasal decongestant than mometasone furoate. |
| Watanabe et al.1331 | 2003 | 1b | RCT | (n = 30):
|
Subjective nasal blockage, nPIF, airway resistance, airway volume | No significant nasal blockage or impaired decongestant response to oxymetazoline following 4-week treatment. |
| Morris et al.72 | 1997 | 1b | RCT | (n = 50):
|
Nasal airway resistance, subjective scaling of nasal patency, clinical examination | Evidence of rebound nasal congestion was found following 3 days of both daily and intermittent oxymetazoline treatment. |
| Yoo et al.83 | 1997 | 2b | Individual cohort study | (n = 10): Daily oxymetazoline | Subjective history, physical exam, anterior rhinomanometry | All subjects remained responsive to oxymetazoline 4 weeks and 8 weeks after the study began. |
LOE = level of evidence; nPIF = nasal peak inspiratory flow; RCT = randomized controlled trial; TID = 3 times daily.
Rhinitis medicamentosa (RM), a condition thought to result from prolonged usage of topical decongestants, involves an increase in symptomatic nasal congestion, thereby precluding a recommendation for chronic use of this medication. Studies to identify the duration of topical decongestant use that leads to rhinitis medicamentosa have shown variable results. Some studies show prolonged use up to 8 weeks does not produce any symptoms of rebound nasal congestion,83,1331 while others note development of RM within 3 days of use.72
Known adverse effects of topical decongestants include nasal burning, stinging, dryness, epistaxis, and mucosal ulceration. While topical decongestants are effective at reducing nasal congestion, short-term use of the medication, 3 days or less, is recommended to avoid the potential for rebound nasal congestion and effects on mucociliary activity. (See section III.C.2. Definitions, classifications, and differential diagnosis – Allergic rhinitis differential diagnosis – Rhinitis medicamentosa (RM) for additional information on this topic.)
Aggregate Grade of Evidence: B (Level 1b: 3 studies; Level 2b: 1 study; Table IX.B.3.b).
Benefit: Reduction of nasal congestion with topical decongestants.
Harm: Side effects include nasal burning, stinging, dryness, and mucosal ulceration. Potential for rebound congestion when used long term.
Cost: Low.
Benefits-Harm Assessment: Harm likely outweighs benefit if used more than 3 days.
Value Judgments: Topical decongestants can be helpful for short-term relief of nasal congestion.
Policy Level: Option.
Intervention: Topical decongestants can provide effective short-term nasal decongestion in patients with AR, but recommend against chronic use due to risk for RM.
IX.B.4. Leukotriene receptor antagonists (LTRAs)
LTRAs have been studied and used in the treatment of AR. Montelukast is approved by the FDA for the treatment of SAR in adults and children over 2 years of age, and for PAR in adults and children over 6 months of age. Several systematic reviews and meta-analyses of RCTs have demonstrated symptom reduction and improved QOL in patients treated with LTRA monotherapy compared to placebo.1300,1332-1335 Nevertheless, in a clinical practice guideline on AR from the AAO-HNS there was a recommendation against LTRA monotherapy, citing decreased effectiveness compared to other first-line agents.761
Systematic review identified 28 studies, of which 19 were considered level 1 evidence, examining the use of LTRA monotherapy in AR (Table IX.B.4). Multiple systematic reviews1300,1332-1335 and RCTs1336-1344 demonstrated that LTRA monotherapy was superior to placebo at improving patient symptoms and QOL. This effect was consistent in studies of SAR,1340-1344 PAR,1339 and artificial allergen exposure.1336-1338 Furthermore, in a double-blind RCT by Philip et al.1341 montelukast improved both AR and asthma disease-specific QOL in patients with concurrent SAR and asthma.
TABLE IX.B.4.
Evidence for the use of leukotriene receptor antagonists as monotherapy in the treatment of allergic rhinitis (Level 1a and 1b studies only)
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Devillier et al.1332 | 2014 | 1a | SR of RCTs, with homogeneity |
|
Symptoms | SLIT superior clinical effect to LTRA. LTRA with clinical effect compared to placebo. |
| Goodman et al.1347 | 2008 | 1a | SR of RCTs, with homogeneity |
|
Symptoms, cost | Montelukast with higher incremental cost-effectiveness ratio than levocetirizine and desloratadine. |
| Grainger & Drake-Lee1333 | 2006 | 1a | SR of RCTs, with homogeneity |
|
Symptoms, QOL | Montelukast improved symptoms and QOL compared to placebo, and was inferior to oral antihistamines and INCS. |
| Rodrigo & Yanez1334 | 2006 | 1a | SR of RCTs, with homogeneity |
|
Symptoms, QOL | LTRA improved symptoms and QOL compared to placebo, was equally effective to oral antihistamine, and inferior to INCS. |
| Wilson et al.1300 | 2004 | 1a | SR of RCTs, with homogeneity |
|
Symptoms, QOL | Montelukast improved QOL compared to placebo, and was inferior to antihistamines and INCS. |
| Gonyeau & Partisan1335 | 2003 | 1a | SR of RCTs, with homogeneity |
|
Symptoms | Montelukast was more effective than placebo in reducing symptoms, but was inferior to INCS. |
| Endo et al.1336 | 2012 | 1b | RCT |
|
Symptoms | Pranlukast prevented and reduced symptoms compared to placebo after artificial introduction of allergen. |
| Wakabayashi et al.1337 | 2012 | 1b | RCT |
|
Symptoms | Pranlukast reduced symptoms compared to placebo in children with artificial allergen exposure. |
| Day et al.1338 | 2008 | 1b | RCT |
|
Symptoms | Both montelukast and levocetirizine improved symptoms following artificial allergen exposures. Levocetirizine was more effective than montelukast. |
| Jiang1348 | 2006 | 1b | RCT |
|
Symptoms, acoustic rhinometry, rhinomanometry | All treatment groups had a significant reduction of pretreatment symptoms. Zafirlukast was superior at reduction of nasal congestion. There were no differences in acoustic rhinometry and rhinomanometry between the 3 treatment groups. |
| Mucha et al.1321 | 2006 | 1b | RCT |
|
Symptoms, QOL, nasal peak inspiratory flow | Montelukast and pseudoephedrine had equivalent improvement of symptoms (except nasal congestion for which pseudoephedrine was more effective), QOL, and nasal peak inspiratory flow. |
| Patel et al.1339 | 2005 | 1b | RCT |
|
Symptoms, QOL | Montelukast was more effective than placebo in reducing symptoms and improving QOL in patients with perennial allergic rhinitis |
| Chervinsky et al.1340 | 2004 | 1b | RCT |
|
Symptoms, pollen count | Montelukast was more effective than placebo in reducing symptoms. The effect size was related to the amount of pollen exposure. |
| Philip et al.1341 | 2004 | 1b | RCT |
|
Symptoms, rhinitis QOL, asthma QOL | Montelukast improved symptoms, rhinitis QOL, and asthma QOL compared to placebo in patients with concurrent seasonal allergic rhinitis and asthma. |
| Ratner et al.1345 | 2003 | 1b | RCT |
|
Symptoms, QOL | Fluticasone was more effective than montelukast in reducing symptoms and improving QOL. |
| van Adelsburg et al.1342 | 2003 | 1b | RCT |
|
Symptoms, QOL | Montelukast was more effective than placebo in reducing symptoms and improving QOL. Montelukast not directly compared to loratadine. |
| van Adelsburg et al.1343 | 2003 | 1b | RCT |
|
Symptoms, QOL | Montelukast was more effective than placebo in reducing symptoms and improving QOL. Montelukast not directly compared to loratadine. |
| Philip et al.1344 | 2002 | 1b | RCT |
|
Symptoms, QOL, peripheral eosinophil count | Montelukast was more effective than placebo in reducing symptoms and peripheral eosinophil count, and improving QOL. Montelukast not directly compared to loratadine. |
| Pullerits et al.1346 | 1999 | 1b | RCT |
|
Symptoms, tissue eosinophilia | Zafirlukast was not different from placebo in symptom or tissue eosinophilia reduction. Both were inferior to intranasal beclomethasone. |
INCS = intranasal corticosteroids; LOE = level of evidence; LTRA = leukotriene receptor antagonist; QOL = quality of life; RCT = randomized controlled trial; SLIT = sublingual immunotherapy; SR = systematic review.
Despite multiple studies demonstrating superior effect of LTRA monotherapy over placebo in the treatment of AR, there is consistent evidence that LTRA is inferior to INCS.1300,1333-1335,1345,1346 Multiple systematic reviews and meta-analyses have shown that INCS result in greater symptom reduction and QOL improvement compared to LTRA.1300,1333-1335 A double-blinded RCT by Pullerits et al.1346 showed decreased numbers of activated tissue eosinophils in nasal mucosa biopsies in patients treated with intranasal beclomethasone compared to zafirlukast and placebo. There is conflicting evidence on the relative effect of LTRA compared to oral antihistamines, with 2 systematic reviews demonstrating that oral antihistamines have superior symptom reduction and QOL improvement1300,1333 and a third study indicating equivalent effect.1334 Moreover, a double-blind RCT by Mucha et al.1321 indicated that montelukast and pseudoephedrine yielded equivalent symptom reduction and QOL improvement. In that study, objective measurement of nasal peak inspiratory flow was not different between the montelukast and pseudoephedrine treatment groups.
In addition to less relative effectiveness compared to other agents, the AAO-HNS clinical practice guideline on AR cited increased costs of LTRA in the recommendation against this drug class as monotherapy in patients with AR without asthma.761 Goodman et al.1347 examined the relative cost effectiveness of montelukast compared to several second-generation oral antihistamines. Montelukast was determined to have increased cost for relative effectiveness compared to levocetirizine, desloratadine, and branded and generic fexofenadine. The annual drug and incurred medical costs for montelukast were estimated to be $631.
LTRA monotherapy may be a useful alternative in rare patients with contraindications for both INCS and oral antihistamines, but this limits recommendations or options for these agents in general. In patients with concurrent AR and asthma, LTRA can contribute to symptom management of both respiratory diseases. LTRA monotherapy is not recommended as first-line treatment for patients with concurrent AR and asthma, although this may be a consideration in patients with contraindications to INCS.
Aggregate Grade of Evidence: A (Level 1a: 6 studies; Level 1b: 17 studies; Level 2a: 2 studies; Level 2b: 3 studies; Level 4: 3 studies; Table IX.B.4).
Benefit: Consistent reduction in symptoms and improvement in QOL compared to placebo, as demonstrated in RCTs and systematic review of RCTs.
Harm: Consistently inferior compared to INCS at symptom reduction and improvement in QOL in RCTs and systematic reviews of RCTs. Equivalent-to-inferior effect compared to oral antihistamines in symptom reduction and improvement of QOL.
Cost: Annual incurred drug and medical costs estimated to be $631 for generic montelukast.
Benefits-Harm Assessment: Preponderance of benefit over harm. LTRAs are effective as monotherapy compared to placebo. However, there is a consistently inferior or equivalent effect to other, less expensive agents used as monotherapy.
Value Judgments: LTRAs are equivalent to oral antihistamine alone and more effective than placebo at controlling both asthma and AR symptoms in patients with both conditions. Control of AR symptoms with LTRAs, however, is less effective than INCS, and inferior or equivalent to oral antihistamines. Therefore, evidence is lacking to recommend LTRAs as first-line or second-line monotherapy in the management of AR alone or in combination with asthma.
Policy Level: Recommendation against as first-line therapy for AR.
Intervention: LTRAs should not be used as monotherapy in the treatment of AR but can be considered as second-line therapy, such as when INCSs are contraindicated.
IX.B.5. Cromolyn
Disodium cromoglycate (DSCG) [synonyms: cromolyn sodium, sodium cromoglycate, disodium 4,4′-dioxo-5,5′-(2-hydroxytrimethylenedioxy)-di(4H-chromene-2-carboxylate)] was first used by ancient Egyptians for its spasmolytic properties. It is derived from the plant Ammi visnaga. DSCG is a mast cell stabilizer that prevents histamine release. It impedes the function of chloride channels important in regulating cell volume and prevents extracellular calcium influx into the cytoplasm of the mast cell, thus preventing the degranulation of sensitized cells.1349,1350 DSCG is best used prophylactically to prevent the onset of symptoms by interrupting the physiological response to nasal allergens.
DSCG was discovered over 50 years ago, and since that time other cromoglycate type agents (chromones) have been developed. The chromones have demonstrated the ability to inhibit the early-phase and late-phase reactions of asthma.1351 Initial studies focused on histamine and cytokine release from mast cells. More recent studies have shown anti-allergy activity unrelated to mast cell activation, but rather through the inhibition of macrophages, eosinophils, monocytes, and platelets.1352-1354
DSCG can be used in an inhaled form as a prophylactic agent in the treatment of mild to moderate asthma, as a nasal spray to treat SAR, or as an ophthalmic solution to treat allergic or vernal conjunctivitis. DSCG may also be taken orally to control allergic reactions to certain foods. It can be used for patients 2 years and older but has a short half-life requiring dosing of 3 to 6 times daily.1355 DSCG has an excellent safety profile, although the need for frequent dosing may affect compliance. Minor adverse effects include nasal irritation or burning, sneezing, epistaxis, and bad taste.1355
Most studies comparing DSCG directly to placebo have shown that it is effective in patients with SAR (Table IX.B.5). Studies on the efficacy of DSCG in PAR have been controversial.1356-1360 In a recent RCT, Lejeune et al.1356 examined the role of DSCG in monosensitized PAR patients and found that DSCG resulted in significant reduction in symptom scores for nasal obstruction, discharge, and sneezing compared to placebo. When compared to INCS, DSCG has been shown to be less effective.1357,1361-1369 To date, there have been no direct comparisons between DSCG and intranasal antihistamines. Ultimately, the role of DSCG as a primary treatment for AR is limited given its lower efficacy when compared to INCS and potential compliance challenges secondary to frequent dosing regimen.
TABLE IX.B.5.
Evidence for the use of disodium cromoglycate in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lejeune et al.1356 | 2015 | 1b | DBRCT | PAR, adults:
|
Symptom scores, nasal cytology, allergic mediators | DSCG performed better than placebo. |
| Meltzer1370 | 2002 | 1b | DBRCT | SAR, over 12 years old:
|
Nasal symptoms | DSCG performed better than placebo. |
| Schuller et al.1371 | 1990 | 1b | DBRCT | SAR, 12–65 years old:
|
Nasal symptoms | Nedocromil was equivalent to DSCG. Both performed better than placebo. |
| Chandra et al.1372 | 1982 | 1b | DBRCT, crossover | SAR, 9–41 years old (n = 47):
|
Nasal symptoms, medication use | DSCG performed better than placebo. |
| Brown et al.1367 | 1981 | 1b | RCT | SAR:
|
Nasal symptoms | Flunisolide performed better than DSCG. |
| Craig et al.1373 | 1977 | 1b | DBRCT | SAR (n = 39):
|
Nasal symptoms, medication use | No difference between DSCG and placebo. |
| Handelman et al.1374 | 1977 | 1b | DBRCT | SAR, 6–51 years old:
|
Symptom score, medication use | DSCG performed better than placebo. |
| McDowell & Spitz1358 | 1977 | 1b | DBRCT, crossover | PAR, 17–71 years old (n = 13):
|
Nasal symptoms, cytology | No significant difference in majority of patients. |
| Nizami & Baboo1375 | 1977 | 1b | DBRCT, crossover | SAR, 7–59 years old (n = 92):
|
Nasal symptoms | DSCG performed better than placebo. |
| Posey & Nelson1376 | 1977 | 1b | DBRCT | SAR, 12–54 years old:
|
Symptom score, medication use | No difference, except for in-season use of medications in DSCG group. |
| Warland & Kapstad1359 | 1977 | 1b | DBRCT, crossover | PAR, 15–57 years old (n = 17):
|
Nasal symptoms | No difference between DSCG and placebo. |
| Cohan et al.1360 | 1976 | 1b | DBRCT, crossover | PAR, 16–37 years old:
|
Symptom score, medication use | DSCG performed better than placebo. |
| Knight et al.1377 | 1976 | 1b | DBRCT | SAR:
|
Nasal symptoms | DSCG performed better than placebo. |
| Lange et al.1361 | 2005 | 2b | RCT, no placebo | SAR, 18–65 years old:
|
Symptom scores, nPIF | MF performed best. |
| Fisher1362 | 1994 | 2b | RCT, blinded, no placebo | SAR, 6–15 years old:
|
Nasal symptoms | Budesonide performed better than DSCG. |
| Bousquet et al.1363 | 1993 | 2b | DBRCT, no placebo | SAR:
|
Nasal/ocular symptoms, medication use | FP better in all except nasal discharge. No difference in medication use. |
| Welsh et al.1364 | 1987 | 2b | RCT, blinded |
|
Symptom score, medication use | All medications were better than placebo. DSCG was the least effective. |
| Bjerrum & Illum1365 | 1985 | 2b | DBRCT, no placebo | SAR, 15–55 years old:
|
Nasal symptoms | Budesonide was better than DSCG. |
| Morrow-Brown et al.1366 | 1984 | 2b | RCT, no placebo | SAR, 11–71 years old:
|
Symptom score, medication use | BDP performed better than DSCG. No difference in rescue medications. |
| Tandon & Strahan1357 | 1980 | 2b | DBRCT, crossover, no placebo | PAR, 13–45 years old (n = 14):
|
Nasal symptoms | BDP performed better than DSCG. |
| Wilson & Walker1368 | 1976 | 2b | RCT, no placebo | SAR, adults:
|
Nasal symptoms | BV performed better than DSCG. |
| Frankland & Walker1369 | 1975 | 2b | DBRCT, no placebo | SAR, adults:
|
Nasal symptoms, nPIF | BV performed better than DSCG for symptoms. The 2 medications performed the same for nPIF. |
BDP = beclomethasone dipropionate; BID = 2 times daily; BV = betamethasone valerate; DBRCT = double-blind randomized controlled trial; DSCG = disodium cromoglycate; FP = fluticasone propionate; LOE = level of evidence; MF = mometasone furoate; nPIF = nasal peak inspiratory flow; PAR = perennial allergic rhinitis; QD = once daily; QID = 4 times daily; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis.
Aggregate Grade of Evidence: A (Level 1b: 13 studies; Level 2b: 9 studies; Table IX.B.5).
Benefit: DSCG is effective in reducing sneezing, rhinorrhea, and nasal congestion.
Harm: Rare local side effects include nasopharyngeal irritation, sneezing, rhinorrhea, and headache.
Cost: Low.
Benefits-Harm Assessment: Preponderance of benefit over harm. Benefit is considered mild to moderate. Less effective than INCS.
Value Judgments: Useful for preventative short-term use in patients with known exposure risks.
Policy Level: Option.
Intervention: DSCG may be considered for the treatment of AR, particularly in patients known triggers who cannot tolerate INCS.
IX.B.6. Intranasal anticholinergics
Ipratropium bromide (IPB) nasal spray acts by controlling watery nasal secretory output from seromucous glands. IPB is used primarily to reduce rhinorrhea and is effective in adults and children with perennial rhinitis and common cold.1378,1379 It has a quick onset of action and short half-life administered up to 6 times per day, with less than 10% absorption over a range of 84 μg/day to 336 μg/day.1380 Local side effects include nasal dryness, irritation, epistaxis, and burning. Systemic side effects have not been observed with therapeutic dosing, as plasma concentrations of greater than 1.8 ng/mL are needed to produce systemic anticholinergic effects.1380 However, care should be taken to avoid over-dosage that could lead to high serum concentrations of ipratropium.
All studies have shown that the use of IPB significantly controls rhinorrhea in children and adults with PAR (Table IX.B.6). The combined use with INCS have also been shown to be more effective than either agent alone, suggesting a role of IPB for patients with persistent rhinorrhea.1381
TABLE IX.B.6.
Evidence for the use of ipratropium bromide in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Dockhorn et al.1381 | 1999 | 1b | DBRCT | PAR, 8–75 years old:
|
Rhinorrhea | Combined use of IPB with BDP is more effective than either agent alone for controlling rhinorrhea. |
| Finn et al.1382 | 1998 | 1b | DBRCT, crossover | PAR, 18–75 years old (n = 205):
|
Nasal symptoms | Control of rhinorrhea and sneezing better in IPB + terbinafine. No differences in nasal congestion. |
| Kaiser et al.1379 | 1998 | 1b | DBRCT | PAR, adults:
|
Nasal symptoms | High-dose and low-dose IPB resulted in significant reduction of nasal hypersecretion vs placebo. |
| Meltzer et al.1383 | 1997 | 1b | DBRCT | PAR and perennial NAR, 6–18 years old:
|
Nasal symptoms, medication use, QOL | In perennial NAR, IPB reduced symptoms. In PAR, a modest effect was seen. |
| Gorski et al.1384 | 1993 | 1b | DBRCT | PAR, 23–33 years old (n = 18):
|
Sneezing, albumin and total protein in nasal lavage | IPB resulted in a decrease in albumin, total protein, eosinophil count, and an increase in nasal reactivity to histamine with an increase in the number of sneezes. |
| Meltzer et al.1385 | 1992 | 1b | DBRCT | PAR, 18–70 years old:
|
Nasal symptoms, nasal cytology | IPB is effective in controlling rhinorrhea. No differences in other outcomes. |
| Sanwikarja et al.1386 | 1986 | 1b | DBRCT, crossover | SAR or PAR (n = 14), non-allergic perennial rhinitis (n = 10), 18-49 years old:
|
Nasal symptoms | IPB has suppressive effects on sneezing and hypersecretion, but no influence on nasal airway resistance. |
| Schultz Larsen et al.1387 | 1983 | 1b | RCT, crossover | PAR, 23–84 years old (n = 20):
|
Nasal symptoms | IPB is effective in controlling rhinorrhea. |
| Borum et al.1388 | 1979 | 1b | RCT, crossover | PAR, 18–82 years old (n = 20):
|
Nasal symptoms | IPB had a significant effect on rhinorrhea. No effect on other symptoms. |
| Kim et al.1378 | 2005 | 2b | Prospective | Common cold, SAR or PAR; 2–5 years old (n = 230); Allergy group: IPB 0.06%, 1 spray (42 μg) TID for 14 days (n = 187) |
Nasal symptoms | IPB is effective in controlling rhinorrhea. |
| Milgrom et al.1389 | 1999 | 2b | RCT, blinded, no placebo | PAR, non-allergic perennial rhinitis, 6–18 years old:
|
Nasal symptoms, QOL | Equally effective in controlling rhinorrhea and improving QOL. BDP more effective in controlling sneezing. |
| Kaiser et al.1390 | 1995 | 2b | Prospective | PAR, 18–75 years old (n = 219): First 6 months: 0.06% IPB TID (84 μg); 6 months to 1 year: lowest dose IPB controlling rhinorrhea | Nasal symptoms, medication use, QOL | IPB was effective in controlling rhinorrhea, congestion, postnasal drip, and sneezing. Reduction in the use of medications and improvement in QOL. |
BDP = beclomethasone dipropionate; DBRCT = double-blind randomized controlled trial; IPB = ipratropium bromide; LOE = level of evidence; NAR = non-allergic rhinitis; PAR = perennial allergic rhinitis; QID = 4 times daily; QOL = quality of life; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis; TID = 3 times daily; BID = 2 times daily.
Aggregate Grade of Evidence: B (Level 1b: 9 studies; Level 2b: 5 studies; Table IX.B.6).
Benefit: Reduction of rhinorrhea with topical anticholinergics.
Harm: Local side effects include nasopharyngeal irritation, burning, headache, pharyngitis, epistaxis, nasal dryness, nasal congestion, and dry mouth. Care should be taken to avoid over-dosage leading to systemic side effects.
Cost: Low to moderate.
Benefits-Harm Assessment: Preponderance of benefit over harm in PAR patients with rhinorrhea.
Value Judgments: No significant benefits in controlling symptoms other than rhinorrhea. Evidence for combined use with INCS is limited but encouraging for patients with persistent rhinorrhea.
Policy Level: Option.
Intervention: IPB nasal spray may be considered as an adjunct medication to INCS in PAR patients with uncontrolled rhinorrhea.
IX.B.7. Biologics (omalizumab)
Biologics have been studied in the treatment of AR, specifically omalizumab, either alone or in combination with specific AIT. Omalizumab is a humanized antibody that binds to human IgE. No biologic is currently approved by the FDA for the treatment of AR. One systematic review and meta-analysis of RCTs has demonstrated reduced symptoms, reduced rescue medication use, and improved QOL in patients treated with omalizumab.1391 However, the cost of omalizumab is very high, estimated to be over $18,000 year in the United States.
Systematic review identified 5 level 1 evidence studies examining the use of omalizumab in AR (Table IX.B.7). Four RCTs1392-1395 demonstrated that omalizumab monotherapy was superior to placebo at improving patient symptoms and QOL. The first RCT evaluating different delivery routes and dose-ranges did not show efficacy against ragweed-induced AR, but reported no significant adverse events associated with omalizumab.1396 A second study randomized birch pollen-induced SAR patients to receive either 300 mg of omalizumab (originally named rhumAb-E25) or placebo given 2 or 3 times over the season, depending on baseline IgE levels. RhemAB-E25 treatment significantly reduced nasal symptom severity scores, the average number of tablets of rescue antihistamines per day, the proportion of days with any SAR medication use, and all domains of QOL.1392 A third study applied omalizumab, 50 mg, 150 mg, or 300 mg, vs placebo subcutaneously prior to ragweed season and repeated every 3 to 4 weeks during the pollen season dependent on the patient’s base-line serum IgE.1393 At the highest dose studied, 300 mg of omalizumab significantly reduced nasal symptom severity scores and rhinitis-specific QOL scores. A significant association was observed between IgE reduction and nasal symptoms and rescue antihistamine use. The frequency of adverse events was not significantly different between omalizumab and placebo groups.
TABLE IX.B.7.
Evidence for the use of omalizumab as monotherapy in the treatment of allergic rhinitis (Level 1a and 1b studies with clinical endpoints only)
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Tsabouri et al.1391 | 2014 | 1a | SR of RCTs, with homogeneity |
|
Symptom score, rescue medication, QOL | Omalizumab was superior to placebo. Omalizumab was generally well tolerated. |
| Okubo et al.1395 | 2006 | 1b | RCT |
|
Symptom score, rescue medication | Efficacy and tolerability in cedar pollen AR. |
| Chervinsky et al.1394 | 2003 | 1b | RCT |
|
Symptom score, rescue medication, QOL | Efficacy and tolerability in PAR. |
| Casale et al.1393 | 2001 | 1b | RCT |
|
Symptom score, rescue medication, QOL | Dose-finding trial, 300-mg dose effective in improving symptoms and QOL compared to placebo. |
| Adelroth et al.1392 | 2000 | 1b | RCT |
|
Symptom score, rescue medication, QOL | Omalizumab was significantly superior to placebo in improving symptoms and QOL. Well tolerated. |
| Casale et al.1396 | 1997 | 1b | RCT |
|
Symptom score, rescue medication, QOL | First dose-finding study, safety confirmed. |
AR = allergic rhinitis; LOE = level of evidence; PAR = perennial allergic rhinitis; QOL = quality of life; RCT = randomized controlled trial; SR = systematic review.
Omalizumab was also studied in the treatment of PAR, significantly reducing the mean daily nasal severity score and the rescue medication, and improving QOL when given subcutaneously every 4 weeks for 16 weeks.1394 Omalizumab therapy was well tolerated. Similarly, effectiveness and safety of subcutaneously injected omalizumab was shown in the treatment of Japanese cedar pollen-induced SAR.1395 Omalizumab treatment markedly reduced serum free IgE and the clinical response to nasal allergen challenge in an open study, but did not affect IgE-secreting B cells and epsilon mRNA in nasal lavage fluid, suggesting that treatment for 6 months does not significantly modulate synthesis of nasal IgE.1397 The biologic also suppressed tryptase and ECP levels in nasal secretions in seasonal allergy.1398 Omalizumab showed significantly greater improvements than suplatast tosilate, a selective T-helper type 2 cytokine inhibitor, in the treatment of SAR induced by Japanese cedar pollens.1399
In 4 trials, a combination of omalizumab with AIT was studied to determine whether combined therapy could provide better efficacy and lower adverse events than AIT alone. In children and adolescents with SAR to birch or grass pollen, combination therapy significantly reduced symptom load over AIT alone independent of the allergen.1400 Anti-IgE monotherapy alone significantly diminished rescue medication use and reduced the number of symptomatic days. The combined treatment with AIT and anti-IgE showed superior efficacy on symptom severity compared with anti-IgE alone.1401 Combination therapy may, therefore, be useful for the treatment of AR, particularly for polysensitized patients. Patients receiving omalizumab and rush ragweed AIT showed a significant improvement in severity scores during season compared with AIT alone.1402 Although omalizumab carries some risk of anaphylaxis itself, addition of omalizumab resulted in a significant decrease in risk of anaphylaxis caused by AIT. Combination therapy also significantly reduced the symptom load in HDM-allergic subjects better than AIT monotherapy, and improved asthma control and QOL with respect to asthma and AR.1403 These effects were limited to the combined treatment period.1404
There are no other published studies evaluating other biologics (anti-IL5, anti-IL4, or IL-4R) as monotherapy for AR. A combination therapy of anti-IL4 with suboptimal AIT provided no additional benefit over subcutaneous immunotherapy (SCIT) alone in suppressing the allergen-induced skin late-phase response.1405
Although there is consistent evidence that omalizumab monotherapy is superior to placebo in symptom reduction and QOL improvement in AR, the benefits are relatively small over pharmacotherapy. Omalizumab is superior in combination with AIT vs AIT alone and reduces the risk of anaphylaxis associated with AIT, but the costs of the treatment preclude a widespread use. The combination therapy might be indicated in selected patients who are polysensitized and highly sensitive.
Aggregate Grade of Evidence: A (Level 1a: 1 study; Level 1b: 5 studies; Table IX.B.7).
Benefit: Consistent reduction in symptoms and rescue medication as well as improvement in QOL in RCTs and systematic review of RCTs compared to placebo.
Harm: Injection site reactions, possibility of anaphylactic reaction.
Costs: High. Annual incurred drug costs estimated to be above $18,000 per year in the United States.
Benefits-Harm Assessment: No therapy option as omalizumab is not registered for treatment of AR alone. This review was limited to evaluation of AR only; comorbid asthma was not evaluated.
Value Judgments: Omalizumab monotherapy is superior to placebo, but effects are small over pharmacotherapy. May be evaluated in exceptional cases of highly sensitive polysensitized individuals in combination with AIT.
Policy Level: No indication for the treatment of AR alone.
Intervention: Omalizumab should not be used as monotherapy in the treatment of AR but may be considered in combination with AIT for highly sensitive polyallergic rhinitis patients with increased risk of anaphylaxis. As omalizumab is not currently approved by the FDA for AR treatment, in the US this treatment approach would likely not be performed in routine clinical practice presently.
IX.B.8. Nasal saline
Nasal saline is frequently utilized in the treatment of AR. However, the term “nasal saline” encompasses a wide variety of therapeutic regimens. These can include hypertonic saline, isotonic/normal saline, seawater, buffered or nonbuffered solutions, and volumes varying from 300 μL to 500 mL per administration. Irrigation regimens are also used with varying frequency.
This review included only level 1 evidence published in the English language. The search identified 5 RCTs in adults151,1406-1409 (Table IX.B.8-1), 6 RCTs in children1410-1415 (Table IX.B.8-2), and 1 systematic review1416 encompassing all ages (included in both tables), which evaluated the efficacy of nasal saline in the treatment of AR.
TABLE IX.B.8-1.
Evidence for the use of nasal saline in the treatment of allergic in adults
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Hermelingmeier et al.1416 | 2012 | 1a | SR and meta-analysis | SAR and PAR, adults and children | Nasal symptom score, medicine use, QOL | Nasal symptoms and medicine use decreased with the use of nasal saline. Adults benefit more than children. |
| Chusakul et al.1409 | 2013 | 1b | DBRCT, crossover | AR:
|
Nasal symptom score | Nasal symptoms were improved from baseline only by buffered saline with mild alkalinity. |
| Garavello et al.151 | 2010 | 1b | RCT, no blinding | SAR, pregnant women:
|
Nasal symptom score, oral antihistamine use | Hypertonic saline irrigations during pollen season improves nasal symptoms and decreases oral antihistamine use. |
| Ural et al.1408 | 2008 | 1b | RCT, no blinding | PAR:
|
Mucociliary clearance time | Isotonic saline improved mucociliary clearance time. |
| Cordray et al.1406 | 2005 | 1b | SBRCT | SAR:
|
RQLQ | Dead Sea saline group had significant improvements but not as significant as triamcinolone group; no change in placebo group. |
| Rogkakou et al.1407 | 2005 | 1b | RCT, no blinding | PAR:
|
Nasal symptoms, QOL (Rhinasthma questionnaire) | The addition of hypertonic saline resulted in a significant improvement in symptoms and QOL. |
AR = allergic rhinitis; BID = 2 times daily; DBRCT = double-blind randomized controlled trial; LOE = level of evidence; PAR = perennial allergic rhinitis; QID = 4 times daily; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; SBRCT = single-blind randomized controlled trial; SR = systematic review; TID = 3 times daily.
TABLE IX.B.8-2.
Evidence for the use of nasal saline in the treatment of allergic rhinitis in children
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Hermelingmeier et al.1416 | 2012 | 1a | SR and meta-analysis | SAR and PAR, adults and children | Nasal symptom score, medicine use, QOL | Nasal symptoms and medicine use decreased with the use of nasal saline. Adults benefit more than children. |
| Chen et al.1415 | 2014 | 1b | RCT, no blinding | PAR:
|
Nasal symptom score, nasal signs | All groups improved. Steroid spray plus seawater had more significant improvements than other arms. |
| Marchisio et al.1413 | 2012 | 1b | SBRCT | SAR:
|
Nasal symptom score, turbinate and adenoid hypertrophy, oral antihistamine use | Hypertonic saline was significantly more effective in improving symptom score, decreasing adenoid and turbinate hypertrophy, and decreasing duration of antihistamine use. |
| Satdhabudha et al.1114 | 2012 | 1b | DBRCT | AR:
|
TNSS, QOL (Rcq-36), oral antihistamine use | Greater improvement in symptoms with buffered hypertonic saline. No significant difference in QOL or antihistamine use at 4 weeks. |
| Li et al.1412 | 2009 | 1b | RCT, no blinding | PAR:
|
Nasal symptoms | All groups improved. Steroid spray plus saline irrigations had more significant improvement than other arms. |
| Garavello et al.1411 | 2005 | 1b | RCT, no blinding | SAR:
|
Nasal symptom score, oral antihistamine use | Hypertonic saline irrigations during pollen season had significant improvement in nasal symptoms and reduction in oral antihistamine use after 5 weeks. |
| Garavello et al.1410 | 2003 | 1b | RCT, no blinding | SAR:
|
Nasal symptom score, oral antihistamine use | Hypertonic saline irrigations during pollen season improves nasal symptoms and decreases oral antihistamine use. |
AR = allergic rhinitis; BID = 2 times daily; DBRCT = double-blind randomized controlled trial; LOE = level of evidence; PAR = perennial allergic rhinitis; QOL = quality of life; Rcq-36 = rhinoconjunctivitis QOL questionnaire; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis; SBRCT = single-blind randomized controlled trial; SR = systematic review; TID = 3 times daily; TNSS = Total Nasal Symptom Score.
In adults, all 5 studies found improvements in clinical outcomes with the use of various types of nasal saline. These studies varied in their evaluation of SAR vs PAR, as well as the type and volume of saline. Studies by Garavello et al.151 and Rogkakou et al.,1407 found that the addition of hypertonic saline significantly improved nasal symptoms and QOL compared to not using saline. Ural et al.1408 further compared the efficacy of hypertonic to isotonic saline irrigations, finding improved mucociliary clearance time with the isotonic solution. They postulated that in PAR, the rheologic properties of the mucus are enhanced most by isotonic saline, thus improving mucociliary clearance. Chusakul et al.1409 also identified that buffered isotonic saline with mild alkalinity had the greatest impact on reducing nasal symptom scores and was preferred by the most patients. Finally, Cordray et al.1406 found that Dead Sea saline spray had a significant improvement in the RQLQ compared to isotonic saline. Cordray et al.1406 suggested that the magnesium in the Dead Sea saline may have anti-inflammatory properties, resulting in improved AR outcomes.
In the pediatric population, all studies evaluating either PAR or SAR found an improvement in nasal symptoms or QOL with the incorporation of nasal saline. Both studies by Garavello et al.1410,1411 showed a significant improvement after the addition of hypertonic saline irrigations TID when compared to no irrigations. Marchisio et al.1413 and Satdhabudha and Poachanukoon1414 further identified that hypertonic saline irrigations resulted in a greater improvement in nasal symptom scores in children vs isotonic saline. Finally, Li et al.1412 and Chen et al.1415 found an additive effect in the utilization of nasal saline spray as an adjunct to a nasal steroid spray when compared to either therapy independently.
The systematic review by Hermelingmeier et al.1416 included 10 studies of which 7 were RCTs evaluating both adult and pediatric patients. Several of these studies are also included above.151,1406-1408,1410-1412 This review found that almost all studies showed an improvement in nasal symptoms from 3.1% to 70.5% with the addition of nasal saline. Additionally, they identified a 24.2% to 100% reduction in medication usage, as well as an improvement in QOL of 29.8% to 37.5%. This review also suggested that isotonic saline was more effective than hypertonic saline. Perhaps surprisingly, they found that nasal saline sprays resulted in greater symptom improvement than saline irrigations. Overall, they concluded that nasal saline was as effective as other frequently utilized AR pharmacologic treatments (ie, nasal antihistamines, oral antihistamines, etc.) in treatment of both SAR and PAR.
Overall, there is substantial evidence to support the use of nasal saline as an adjunct treatment for SAR and PAR. It appears that in adults, a buffered isotonic spray may provide maximum benefit. However, in children, a hypertonic solution may be more effective. Some studies have suggested less intranasal irritation when using isotonic solutions rather than hypertonic. Hypotonic saline has not been studied as a treatment for AR. Adding mild alkalinity (pH 7.2 to 7.4) to the solution may further improve tolerability.1409 Although nasal saline has been shown to improve symptoms and QOL outcomes when used alone, it is often implemented as an adjunct to other therapies including nasal steroid, antihistamine sprays, or oral antihistamines. In both adults and children, nasal saline appears to have an additive effect when used in combination with other standard AR treatments. Further, nasal saline is of relatively low cost and has an excellent safety profile. While adverse effects are rare, they can include local irritation, ear pain, nosebleeds, headache, nasal burning, nasal drainage, and bottle contamination.1417
Aggregate Grade of Evidence: A (Level 1a: 1 study; Level 1b: 11 studies; Table IX.B.8-1 and IX.B.8-2). Lower-level studies were not considered in this review.
Benefit: Reduced nasal symptom scores, improved QOL, improved mucociliary clearance; well tolerated with excellent safety profile.
Harm: Intranasal irritation, headaches, ear pain.
Cost: Minimal.
Benefits-Harm Assessment: Preponderance of benefit over harm.
Value Judgments: Nasal saline should be used as an adjunct to other pharmacologic treatments for AR. Isotonic solutions may be more beneficial in adults, while hypertonic may be more effective in children.
Policy Level: Strong recommendation.
Intervention: Nasal saline is strongly recommended as part of the treatment strategy for AR.
IX.B.9. Probiotics
The relationship between microbiome and development of atopy is complex and incompletely understood. (See section IV.G. Pathophysiology and mechanisms of allergic rhinitis - Microbiome for additional information on this topic.) Preliminary data from observational studies suggest that microbial exposure, especially in infancy, shapes the gut and airway microbiome and affects subsequent Th2 or Th1 immunologic bias. Given the link between gut flora and atopy, manipulation of the microbiome via probiotic administration could theoretically lead to clinical improvement of allergic disease. Probiotics have been posited to elicit immunomodulatory effects on atopic disease via gut-associated lymphoid tissue. Stimulation of dendritic cells induces Th1 responses via IL-12 and IFN-γ, upregulation of Treg cells via IL-10 and TGF-β, and suppression of Th2 pathways through downregulation of IL-4, sIgE, IgG1, and IgA.1418
The optimal timing of probiotic administration for the treatment of atopy is unknown. A meta-analysis of 17 double-blind RCTs demonstrated that probiotics in pregnancy and early infancy were associated with decreased incidence of eczema but not asthma or rhinosinusitis in early childhood.1419 Many double-blind RCTs and randomized crossover studies have investigated the effects of probiotics on AR in older children and adults (Table IX.B.9). Meta-analyses of these studies have been published in 2015 by Zajac et al.1420 and 2016 by Guvenc et al.1421 with positive results. Adverse events due to probiotics were rare and minor, including diarrhea, abdominal pain, and flatulence.
TABLE IX.B.9.
Evidence for the use of probiotics in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Guvenc et al.1421 | 2016 | 1a | SR and meta-analysis | SAR and PAR, adults and children. Daily probiotic vs placebo. 22 DBRCTs (n = 2242) |
Symptom scores, QOL, immunologic parameters | 17 studies demonstrated clinical benefit of probiotics. Improvement in TNSS, TOSS, total QOL, nasal QOL, and ocular QOL. |
| Zajac et al.1420 | 2015 | 1a | SR and meta-analysis | SAR and PAR, adults and children. Daily probiotic vs placebo. 21 DBRCTs and 2 crossover studies, (n = 1919) |
Validated QOL or symptom scores, immunologic parameters | 17 studies demonstrated clinical benefit of probiotics. Improvement in RQLQ global and nasal symptom scores. |
| Costa et al.1425 | 2014 | 1b | DBRCT | SAR to grass pollen, adults (n = 425). Lactobacillus paracasei-33 × 5 weeks |
RQLQ, RTSS | Probiotic improved RQLQ. |
| Lin et al.1434 | 2014 | 1b | DBRCT | PAR to HDM, children (n = 60). Lactobacillus paracasei HF.A00232 × 8 weeks |
RTSS, PRQLQ | Probiotic improved PRQLQ, sneezing, ocular itching/swelling at 12 weeks. |
| Dolle et al.1445 | 2013 | 1b | DBRCT | SAR to grass pollen, adults (n = 34). Escherichia coli Nissle 1917 × 6 months |
Symptom-medication score | No benefit. |
| Lin et al.1426 | 2013 | 1b | DBRCT | PAR to HDM, children (n = 199). Lactobacillus salivarius × 12 weeks |
Specific symptom score, symptom-medication score, tIgE | Probiotic improved nasal, eye, medication scores. |
| Singh et al.1441 | 2013 | 1b | DBRCT | SAR to grass pollen, adults (n = 20). Bifidobacterium lactis NCC2818 × 8 weeks |
TNSS | Probiotic improved TNSS. |
| Lue et al.1422 | 2012 | 1b | Randomized crossover | PAR, children (n = 63). Lactobacillus johnsonii EM1 |
RTSS, PRQLQ | Probiotic improved RTSS. |
| Jan et al.1438 | 2011 | 1b | DBRCT | PAR to HDM, children (n = 240). Lactobacillus gasseri × 12 weeks |
SCORing Allergic Rhinitis Index: specific symptom score, symptom-medication score, tIgE, blood eosinophil count | No benefit. |
| Chen et al.1432 | 2010 | 1b | DBRCT | SAR and PAR, children (n = 105). Lactobacillus gasseri A5 × 8 weeks |
Subjective symptoms, tIgE | Probiotic decreased nasal symptoms. |
| Nagata et al.1431 | 2010 | 1b | DBRCT | SAR to JCP, adults (n = 55). Lactobacillus plantarum #14 × 6 weeks |
Symptom-medication score, tIgE, sIgE | Probiotic improved symptom-medication score and ocular itching. |
| Gotoh et al.1439 | 2009 | 1b | DBRCT | SAR, adults (n = 107). Lactobacillus gasseri × 8 weeks |
Symptom-medication score, RQLQ, tIgE, sIgE, blood eosinophil count, Th1:Th2 ratio | Probiotic improved symptom-medication score. |
| Kawase et al.1427 | 2009 | 1b | DBRCT | SAR to JCP, adults (n = 40). Lactobacillus GG and L. gasseri TMC0356 × 10 weeks |
Mean symptom score, mean symptom-medication score, tIgE, sIgE | Probiotic improved nasal blockage and medication score. |
| Nishimura et al.1444 | 2009 | 1b | DBRCT | PAR to HDM, adults (n = 45). Tetragenococcus halophilus Th221 × 8 weeks |
Disease severity, TNSS, tIgE, sIgE | Probiotic improved TNSS at high dose. |
| Ouwehand et al.1433 | 2009 | 1b | DBRCT | SAR to birch, children (n = 47). Lactobacillus acidophilus NCFM and Bifidobacterium lactis B1-04 × 4 months |
Subjective symptoms | No benefit. |
| Yonekura et al.1435 | 2009 | 1b | DBRCT | SAR to JCP, adults (n = 116). Lactobacillus paracasei KW3110 × 3 weeks |
RQLQ, sIgE | Probiotic improved QOL when pollen scattering low. |
| Ivory et al.1440 | 2008 | 1b | DBRCT | SAR to grass pollen, adults (n = 20). Lactobacillus casei × 5 months |
tIgE, sIgE, sIgG, cytokines | Probiotic decreased Th2 cytokines (IL-5, IL-6), sIgE, IFN-γ, and increased sIgG. |
| Giovannini et al.1428 | 2007 | 1b | DBRCT | SAR and PAR, children (n = 187). Lactobacillus casei × 12 months |
Time free of asthma/rhinitis, number of episodes of rhinitis, tIgE | Probiotic decreased annual rhinitis episodes. |
| Tamura et al.1429 | 2007 | 1b | DBRCT | SAR to JCP, adults (n = 120). Lactobacillus casei Shirota × 8 weeks |
Symptom-medication score | No benefit. |
| Xiao et al.1061 | 2007 | 1b | Randomized crossover | SAR to JCP, adults (n = 24). Bifidobacterium longum BB536 × 4 weeks |
Subjective symptoms | Probiotic reduced throat and ocular symptoms. |
| Xiao et al.1442 | 2006 | 1b | DBRCT | SAR to JCP, adults (n = 40). Bifidobacterium longum BB536 × 14 weeks |
Subjective symptoms | Probiotic decreased ocular symptoms. |
| Xiao et al.1443 | 2006 | 1b | DBRCT | SAR to JCP, adults (n = 44). Bifidobacterium longum BB536 × 13 weeks |
Subjective symptoms | Probiotic improved rhinorrhea, congestion, composite scores. |
| Ciprandi et al.1446 | 2005 | 1b | DBRCT | SAR, children (n = 20). Bacillus clausii × 3 weeks |
RTSS, medication use | Probiotic reduced medication use. |
| Ishida et al.1436 | 2005 | 1b | DBRCT | PAR to HDM, adults (n = 49). Lactobacillus acidophilus L-92 × 8 weeks |
Symptom-medication score, tIgE, sIgE | Probiotic improved nasal symptom-medication scores. |
| Peng & Hsu1424 | 2005 | 1b | DBRCT | PAR to HDM, children (n = 90). Lactobacillus paracasei × 30 days |
Modified PRQLQ | Probiotic improved PRQLQ (frequency, level of bother). |
| Wang et al.1423 | 2004 | 1b | DBRCT | PAR to HDM, children (n = 90). Lactobacillus paracasei-33 × 30 days |
Modified PRQLQ | Probiotic improved PRQLQ (frequency, level of bother). |
| Aldinucci et al.1437 | 2002 | 1b | DBRCT | SAR and PAR, adults (n = 20). Lactobacillus acidophilus and Bifidobacterium × 4 months |
Subjective symptoms | Probiotic decreased nasal symptoms. |
| Helin et al.1430 | 2002 | 1b | DBRCT | SAR to birch, adults and children (n = 36). Lactobacillus rhamnosus × 5.5 months |
RTSS; nose, eye, lung symptoms | No benefit. |
DBRCT = double-blind randomized controlled trial; HDM = house dust mite; IFN = interferon; IL = interleukin; JCP = Japanese cedar pollen; LOE = level of evidence; PAR = perennial allergic rhinitis; PRQLQ = Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; QOL = quality of life; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; RTSS = Rhinitis Total Symptom Score; SAR = seasonal allergic rhinitis; sIgE = antigen-specific immunoglobulin E; sIgG = antigen-specific immunoglobulin G; SR = systematic review; tIgE = total immunoglobulin E; TNSS = Total Nasal Symptom Score; TOSS = Total Ocular Symptom Score.
Guvenc et al.1421 performed a systematic review and meta-analysis of 22 double-blind RCTs comprising 2242 patients aged 2 to 65 years with SAR or PAR. Patients received daily probiotic or placebo for 4 weeks to 12 months as an adjuvant to standard allergy therapies; primary outcomes included Total Nasal/Ocular Symptom Scores and QOL. Secondary outcomes included specific nasal symptom scores and immunologic parameters. Seventeen trials demonstrated clinical benefit of probiotics, with improvement in TNSS (standardized mean difference [SMD] −1.23, p < 0.001), TOSS (SMD −1.84, p < 0.001), total QOL (SMD −1.84, p < 0.001), nasal QOL (SMD −2.30, p = 0.006), and ocular QOL (SMD −3.11, p = 0.005). Subgroup analysis demonstrated improvement in clinical parameters for SAR and PAR. Th1:Th2 ratio was improved (SMD −0.78, p = 0.045) in the probiotic group, with no difference in tIgE, sIgE, or eosinophil count.
Zajac et al.1420 published a systematic review and meta-analysis of 21 double-blind RCTs and 2 randomized crossover studies comprising 1919 adult and pediatric patients with SAR or PAR treated with 3 weeks to 12 months of probiotic vs placebo. A total of 26 level 1b studies analyzed by Guvenc et al.1421 and Zajac et al.1420 are included in Table IX.B.9. Zajac et al.1420 limited outcomes measures to validated QOL or symptom scores and immunologic variables; 17 studies demonstrated clinical benefit of probiotics in AR. Meta-analysis demonstrated improvement in RQLQ global score (SMD −2.23, p = 0.02) and RQLQ nasal symptom score (SMD −1.21, p < 0.00001). No effect was found for RTSS, tIgE, or sIgE.
The preponderance of data from meta-analyses and double-blind RCTs suggests a beneficial effect for probiotics in the treatment of SAR and PAR in both adults and children, but interpretation is limited by the heterogeneity of age and diagnosis, interventions, and outcomes included in the studies. Probiotics varied in dose, were delivered via milk, yogurt, powder, or capsules, and included a number of diverse strains: 19 studies employed Lactobacillus species1422-1440; 6 studies Bifidobacterium1061,1433,1437,1441-1443; and 1 study each Tetragenococcus halophilus,1444 Escherichia coli,1445 and Bacillus clausii.1446
Aggregate Grade of Evidence: A (Level 1a: 2 studies; Level 1b: 26 studies; Table IX.B.9).
Benefit: Improved nasal/ocular symptoms or QOL in most studies. Possible improvement in immunologic parameters (Th1:Th2 ratio).
Harm: Low.
Benefits-Harm Assessment: Balance of benefit and harm.
Value Judgments: Minimal harm associated with probiotics, but heterogeneity across studies makes magnitude of benefit difficult to quantify. Variation in organism and dosing across trials prevents specific recommendation for treatment.
Policy Level: Option.
Intervention: Consider adjuvant use of probiotics for patients with symptomatic SAR and PAR.
IX.B.10. Combination therapy
IX.B.10.a. Oral antihistamine and oral decongestant.
Oral antihistamines function as reversible competitive antagonists of the histaminic H1 receptor and prevent the binding of histamine to its receptors. Oral decongestants, such as pseudoephedrine and phenylephrine, are alpha-adrenergic stimulatory drugs which bind to precapillary and post-capillary blood vessels resulting in vasoconstriction of nasal mucosa.1447 The unrelated biologic targets of these medications’ mechanisms of action has been shown in RCTs to result in synergistic improvement in AR symptoms.1448,1449
The combination of an oral antihistamine along with an oral decongestant has been shown to be more effective than placebo in controlling sneezing, nasal itching, and reducing nasal congestion in patients with AR1044,1050,1052,1167,1450-1456 (Table IX.B.10.a). Investigations by Kaiser et al.1450 found that both once-daily or twice-daily loratadine-pseudoephedrine were consistently superior to placebo in reducing total nasal and non-nasal symptom scores with significantly higher risk of insomnia and dry mouth in both antihistamine-decongestant arms compared to placebo. Additionally, Nathan et al.1451 reported in 2006 that cetirizine-pseudoephedrine reduced AR total symptom severity scores, asthma symptom severity scores, and improved asthma QOL scores significantly vs placebo. However, they found no significant changes in pulmonary function testing in patients receiving cetirizine-pseudoephedrine or placebo and they identified similar rates of discontinuation and adverse events in both treatment arms.
TABLE IX.B.10.a.
Evidence for oral antihistamine and oral decongestant combination therapy for the treatment of allergic rhinitis
| Study | Year | LOE | Study design |
Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Badorrek et al.1050 | 2009 | 1b | RCT | (n = 49):
|
Symptoms, nasal flow, nasal secretions | Cetirizine-pseudoephedrine was more effective than the other arms in improving nasal obstruction, nasal flow, and nasal secretions after controlled pollen exposures. |
| Grubbe et al.1462 | 2009 | 1b | RCT | (n = 598):
|
TSS (without nasal congestion), nasal congestion score | Combination therapy was significantly more effective then monotherapy in reducing symptoms, including nasal congestion. |
| Chen et al.1464 | 2007 | 1b | RCT | (n = 48):
|
TSS | Both groups showed significant improvement without significant difference between groups. |
| Chiang et al.1463 | 2006 | 1b | RCT | (n = 51):
|
Nasal total symptom scores | Both groups had a significant improvement in symptoms with no statistically significant difference between groups. |
| Nathan et al.1451 | 2006 | 1b | RCT | (n = 274):
|
Symptoms (total and asthma), PFTs, asthma QOL | Combination therapy significantly reduced symptoms of SAR, asthma symptom scores, and asthma QOL scores. |
| Chervinsky et al.1461 | 2005 | 1b | RCT | (n = 650):
|
TSS without nasal congestion, TSS with nasal congestion | Nasal congestion symptoms scores were significantly reduced with desloratadine-pseudoephedrine compared to monotherapy. |
| Pleskow et al.1460 | 2005 | 1b | RCT | (n = 1047):
|
TSS, morning instantaneous TSS, nasal congestion score | Combination therapy was more effective than either drug alone in reducing TSS and nasal congestion. |
| Zieglmayer et al.1449 | 2005 | 1b | RCT | (n = 36):
|
Rhinomanometry, nasal cavity images, nasal congestion | Oral cetirizine + pseudoephedrine was superior to budesonide in reducing nasal congestion when exposed to HDM. |
| Moinuddin et al.1467 | 2004 | 1b | RCT | (n = 72):
|
RQLQ, nasal symptoms, nPIF | Fexofenadine-pseudoephedrine and loratadine-montelukast were equally effective in improving RQLQ, total symptoms, and nPIF, except for the sleep domain (loratadine-montelukast better). |
| Berkowitz et al.1044 | 2002 | 1b | RCT | (n = 298):
|
Single exposure major symptom complex, total symptom complex, individual symptoms | Fexofenadine-pseudoephedrine was more effective in reducing all symptoms following a single exposure to allergen; onset of action: 45 minutes. |
| Stubner et al.1468 | 2001 | 1b | RCT | (n = 36):
|
Nasal congestion by photographs and digital airflow, nasal secretions, nasal and ocular symptoms | Nasal congestion by photographs was similar between groups. Cetirizine-pseudoephedrine was significantly better in improving all subjective symptoms. |
| McFadden et al.1452 | 2000 | 1b | RCT | (n = 20):
|
Acoustic rhinometry, endoscopic inferior turbinate photography, QOL | Significant improvement in nasal edema and secretions and nasal/ocular symptoms of rhinoconjunctivitis in the treatment group compared to placebo. |
| Sussman et al.1457 | 1999 | 1b | RCT | (n = 651):
|
Total symptoms, nasal congestion | Combination therapy significantly more effective in improving total symptom score and nasal congestion, produced greater improvement in daily activities and work productivity. |
| Horak et al.1052 | 1998 | 1b | RCT | (n = 24):
|
Nasal obstruction, nasal patency/airflow | Cetirizine-pseudoephedrine was significantly better than placebo in improving nasal obstruction and airflow. |
| Kaiser et al.1450 | 1998 | 1b | RCT | (n = 469):
|
Total nasal and non-nasal symptom scores | Loratadine-pseudoephedrine (either dose) was superior to placebo in reducing symptom scores. |
| Serra et al.1453 | 1998 | 1b | RCT | (n = 40):
|
Nasal symptoms or signs, mean TSS | Combination drug was significantly better than placebo in improving signs and TSS; both placebo and combination drug improved nasal symptoms. |
| Corren et al.1454 | 1997 | 1b | RCT | (n = 193):
|
Nasal and chest symptoms, albuterol use, peak expiratory flow | Combination drug significantly reduced symptom scores and improved peak flow rates and FEV1 compared to placebo. |
| Grosclaude et al.1459 | 1997 | 1b | RCT | (n = 687):
|
5 daily symptoms: congestion, sneezing, rhinorrhea, nasal and ocular pruritus | Combination was significantly more effecting in controlling all symptoms and providing more comfortable days than either medication alone. |
| Bertrand et al.1458 | 1996 | 1b | RCT | (n = 210):
|
Daily symptom scores | Cetirizine-pseudoephedrine resulted in significantly reduced symptoms and more symptom-free days than either drug alone. |
| Bronsky et al.1455 | 1995 | 1b | RCT | (n = 874):
|
Composite symptom scores: total, nasal and non-nasal | Combination drug was significantly superior to either drug alone or placebo in reducing symptom scores. |
| Grossman et al.1456 | 1989 | 1b | RCT | (n = 264):
|
4 nasal and 4 non-nasal symptoms | Treatment group had significantly lower nasal and non-nasal symptom scores than the placebo group. |
FEV1 = forced expiratory volume in 1 second; HDM = house dust mite; LOE = level of evidence; nPIF = nasal peak inspiratory flow; PFT = pulmonary functiontest; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; TSS = Total Symptom score.
Oral antihistamine and oral decongestant combinations have also been shown to be more effective in controlling AR symptoms when compared to INCS or compared to treatment with either oral antihistamines or oral decongestants alone.1050,1455,1457-1460 In 2005, Zieglmayer et al.1449 found that the combination of cetirizine with prolonged release pseudoephedrine was significantly superior to budesonide nasal spray for improving nasal congestion after exposure to HDM, as measured by anterior rhinomanometry and nasal imaging. The combination of second-generation oral antihistamines and pseudoephedrine has been shown to significantly reduce symptom scores in patients with SAR more than either drug alone.1050,1455,1457-1462 Additionally, the type of second-generation antihistamine and medication dosing schedule does not seem to have a significant effect on efficacy.1463,1464
Oral decongestants have the benefit of relieving the symptoms of nasal congestion through their ability to vasoconstrict capillaries within the nasal mucosa; however, their mechanism of action can also result in unfavorable systemic adverse effects such as hypertension and urinary retention. Oral decongestants have also been linked to an increased incidence of specific birth defects including pyloric stenosis and endocardial cushion defects when utilized by pregnant women.1465 Furthermore, decongestants are not recommended for children under 4 years of age secondary to the high risk of adverse drug events associated with utilization in this age group.1466 Finally, oral decongestants have OTC sales restrictions secondary to their potential utilization in the production of methamphetamines. Therefore, caution must be applied in the utilization of these medications, particularly in children under 4 years and patients who are pregnant or have a preexisting cardiovascular condition, hypertension, or benign prostatic hypertrophy. Oral antihistamines are well tolerated, with a favorable risk-benefit ratio. However, caution should still be exercised as antihistamines have cardiac side effects, alter the metabolism of other medicines, and have been linked to a higher incidence of adverse events and drug-drug interactions in the elderly.216
It is likely because of this significant risk of adverse events and propensity for interactions with other medications that the ARIA 2010 guidelines recommended against the routine treatment of AR with a combination oral decongestant and oral antihistamine.1167 The 2010 ARIA document suggested that oral decongestants only be added in patients who are not controlled by antihistamines alone and are less averse to side effects or adverse reactions. Additionally, they suggested that oral decongestants be limited to utilization primarily as a rescue medication during periods of significant symptom exacerbations.
Overall, despite the available evidence verifying the efficacy of combination oral antihistamines and oral decongestants in improving AR symptoms, caution should still be exercised when prescribing this treatment, particularly in patients with cardiovascular or urologic comorbidities.
Aggregate Grade of Evidence: A (Level 1b: 21 studies; Table IX.B.10.a).
Benefit: Improved control of nasal congestion with combination of oral antihistamines and oral decongestants.
Harm: Oral decongestants can cause significant adverse effects, particularly in patients with hypertension, cardiovascular disease, or benign prostatic hypertrophy. Additionally, these medications should not be used in children under 4 years of age or pregnant patients. This should be weighed against the potential benefits prior to prescribing.
Cost: Low.
Benefits-Harm Assessment: Harm likely outweighs benefit when used on a routine basis.
Value Judgments: Combination therapy of oral antihistamines and oral decongestants can be helpful for relief of an acute exacerbation of AR, especially nasal symptoms, when exposed to triggers. Caution should be exercised regarding long-term use given the possibility of significant adverse effects.
Policy Level: Option, particularly for acute exacerbations of nasal congestion.
Intervention: Combination therapy with oral antihistamine and oral decongestant can provide effective reduction of nasal congestion symptoms in patients with AR; however, recommend against chronic use given the significant side effect profile of oral decongestants.
IX.B.10.b. Oral antihistamine and intranasal corticosteroid.
A combination of an oral antihistamine and INCS is often used in clinical practice for the treatment of AR. As previously mentioned, oral antihistamines function as a reversible competitive antagonist of the histamine H1 receptor and thereby prevent the binding of histamine that is present in the circulation. The newer, second-generation agents, such as fexofenadine and cetirizine, are less sedating, have fewer adverse effects, and provide good control of sneezing, rhinorrhea, and nasal itching, but with less effect on nasal congestion.1448 Additionally, INCSs, such as fluticasone or beclomethasone, have repeatedly been validated as an effective treatment option for AR while offering a good safety profile and low systemic absorption.1448
Several RCTs have examined the efficacy of combination therapy utilizing both an oral antihistamine and INCS and demonstrated no added benefit of combination therapy (Table IX.B.10.b). In 2000, Wilson et al.1469 demonstrated that oral cetirizine and intranasal mometasone were effective at improving nasal peak inspiratory flow rates as well as nasal symptoms and total daily symptoms after 4 weeks of use. However, the combination was not significantly better than cetirizine and placebo or cetirizine and montelukast. In a double-blinded crossover study, Barnes et al.1470 compared the combination of fluticasone and levocetirizine vs fluticasone and placebo and found, in most patients, that the benefits of an additional oral antihistamine to an effective nasal steroid regimen were not significant. Additionally, Ratner et al.1471 found that fluticasone monotherapy compared to fluticasone plus loratadine had comparable efficacy in nearly all clinician and patient rated symptoms. Finally, Di Lorenzo et al.1472 demonstrated similar results in patients with SAR, noting that combination therapy did not appear to offer substantial improvement in daily nasal symptom scores or in reduction of nasal lavage inflammatory markers.
TABLE IX.B.10.b.
Evidence for the use of combination oral antihistamine and intranasal corticosteroids in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Pinar et al.1473 | 2008 | 1b | RCT | (n = 95):
|
TNSS, rhinoconjunctivitis Scores, nPIF | Combination therapy resulted in better nasal symptom scores at week 2 and better QOL scores than INCS monotherapy. |
| Barnes et al.1470 | 2006 | 1b | DBRCT, crossover | (n = 27):
|
TNSS, mini-RQLQ, nPIF, nasal nitric oxide | Nasal symptom scores are equivalent with combination therapy compared to INCS. |
| Di Lorenzo et al.1472 | 2004 | 1b | DBRCT, double dummy | SAR, (n = 100):
|
DNSS, nasal lavage eosinophil count and ECP level | Combination therapy was equivocal to monotherapy INCS in reducing nasal symptoms in SAR. |
| Wilson et al.1469 | 2000 | 1b | SBRCT | SAR, (n = 38):
|
nPIF, symptom diary card | Combination of oral cetirizine and mometasone INCS was not significantly better than cetirizine alone for SAR. |
| Ratner1471 | 1998 | 1b | DBRCT, double dummy | SAR, (n = 600):
|
Symptoms | Combination therapy, although significantly better than an oral antihistamine alone, offered no significant advantage over INCS alone. |
DBRCT = double-blind randomized controlled trial; DNSS = Daily Nasal Symptom Score; ECP = eosinophil cationic protein; INCS = intranasal corticosteroid; LOE = level of evidence; mini-RQLQ = mini-Rhinoconjunctivitis Quality of Life Questionnaire; nPIF = nasal peak inspiratory flow; QOL = quality of life; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis; SBRCT = single-blind randomized controlled trial; TNSS = Total Nasal Symptom Score.
In contrast, a 2008 study by Pinar et al.1473 compared mometasone spray monotherapy to mometasone plus desloratadine and found that the combination therapy group had significantly better nasal symptom scores at the end of study week 2 and better QOL scores throughout the study. A recent systematic review and meta-analysis by Feng et al.1474 summarized the efficacy of the combination therapy of an oral antihistamine and INCS as compared to either therapy independently. They concluded that the combination demonstrated significant improvement in symptom scores in AR when compared to an oral antihistamine alone, but do not provide significant additional benefit when compared to monotherapy with an effective INCS.1474 Limitations to this data include the fact that the studies did not control for variations in the specific oral antihistamines or INCS utilized and that the studies predominantly evaluated patients with SAR, excluding patients with PAR. Additionally, the conclusions of this meta-analysis are supported by the updated 2010 ARIA guidelines, which also do not recommend the addition of an oral antihistamine to an effective INCS, in contrast to prior recommendations.1167 It should also be noted that adverse effects of oral antihistamine and INCS combination therapies include drowsiness and dry mouth (from oral antihistamines) as well as epistaxis and nasal irritation (from INCS).
Aggregate Grade of Evidence: B (Level 1b: 5 studies; Table IX.B.10.b).
Benefit: Reduction of nasal congestion with combination of oral antihistamines and INCS compared to oral antihistamines alone.
Harm: Side effects include sedative properties of antihistamines, although significantly decreased with the newer second-generation agents. Side effects of topical INCS include nasal dryness and epistaxis, burning in the nose, and with prolonged usage, possible growth suppression in the pediatric population.
Cost: Low.
Benefits-Harm Assessment: Harm likely outweighs benefit of adding the oral antihistamine unless treating symptoms other than nasal symptoms.
Value Judgments: Combination therapy of oral antihistamine and INCS can be helpful when managing the symptoms of nasal congestion.
Policy Level: Option.
Intervention: Combination therapy of INCS and oral antihistamine does not improve symptoms of nasal congestion over INCS use alone, and does risk the adverse effects of systemic antihistamine use.
IX.B.10.c. Oral antihistamine and LTRA.
Combination therapy with LTRA and oral antihistamines in the treatment of AR has been studied in a single systematic review1300 and multiple RCTs1467,1472,1475-1483 (Table IX.B.10.c). Combination therapy generally improved symptoms and QOL compared to placebo in multiple RCTs.1472,1475,1479,1482,1483 The efficacy of combination therapy compared to monotherapy with either LTRA or oral antihistamine is less clear. In the systematic review by Wilson et al.,1300 combination therapy improved patient symptoms compared to either agent as monotherapy, but there were no differences in standardized QOL measures. An RCT by Cingi et al.1477 indicated that montelukast and fexofenadine combination therapy was superior at reducing symptoms and nasal resistance measured by rhinomanometry, compared to either fexofenadine alone or fexofenadine administered concomitantly with placebo. Several other RCTs, however, did not demonstrate a difference in symptom reduction between combination therapy and oral antihistamine monotherapy.1475,1479,1482
TABLE IX.B.10.c.
Evidence for the use of combination leukotriene receptor antagonist and oral antihistamine in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Wilson et al.1300 | 2004 | 1a | SR of RCTs, with homogeneity |
|
Symptoms, QOL | Combination therapy improved symptoms vs either treatment alone. No differences in QOL measures. No difference in symptoms for combination therapy compared to INCS. |
| Ciebiada et al.1475 | 2013 | 1b | RCT |
|
Symptoms, ICAM-1 levels, eosinophilia | Active treatments were superior to placebo at reducing symptoms, ICAM-1 levels and eosinophilia. Active treatments were not statistically different from each other. |
| Yamamoto et al.1476 | 2012 | 1b | RCT |
|
Symptoms | Combination therapy improved symptom scores, specifically sneezing and rhinorrhea. |
| Cingi et al.1477 | 2010 | 1b | RCT |
|
Symptoms, rhinomanometry | Combination therapy improved symptoms and decreased nasal resistance compared to fexofenadine alone or with placebo. |
| Li et al.1478 | 2009 | 1b | RCT |
|
Symptoms, acoustic rhinometry, cytokine levels | Combination therapy improved symptoms, increased nasal volume by rhinometry. No difference in cytokine levels. |
| Lu et al.1479 | 2009 | 1b | RCT |
|
Symptoms, QOL | Combination therapy improved symptoms more than placebo or montelukast alone. There was no difference compared to loratadine alone. Combination therapy was inferior to beclomethasone INCS. |
| Watanasomsiri et al.1480 | 2008 | 1b | RCT |
|
Symptoms, turbinate hypertrophy | No difference in symptoms with combination therapy vs antihistamine alone. Turbinate swelling significantly reduced with combination therapy. |
| DiLorenzo et al.1472 | 2004 | 1b | RCT |
|
Symptoms, peripheral eosinophilia, nasal eosinophil counts | Montelukast + cetirizine improved symptoms and decreased nasal eosinophil counts compared to placebo. Generally inferior to fluticasone INCS alone or in combination. |
| Moinuddin et al.1467 | 2004 | 1b | RCT |
|
Symptoms, QOL, nPIF | No significant difference between treatment groups for symptoms, QOL, and nPIF. Montelukast + loratadine reduced sleep domain symptoms. |
| Saengpanich et al.1481 | 2003 | 1b | RCT |
|
Symptoms, nasal eosinophil count, nasal ECP level | No difference in total symptom score, but nasal symptoms reduced in the fluticasone group. Decreased eosinophil cell count and ECP level in the fluticasone group. |
| Nayak et al.1482 | 2002 | 1b | RCT |
|
Symptoms, QOL, peripheral eosinophilia | Combination therapy decreased symptoms and improved QOL compared to placebo. Effect did not reach statistical significance compared to monotherapy. Combination therapy decreased peripheral eosinophilia compared to placebo and loratadine only. |
| Meltzer et al.1483 | 2000 | 1b | RCT |
|
Symptoms, QOL | Combination therapy improved symptoms and QOL compared to placebo. Combination therapy not directly compared to monotherapy. |
ECP = eosinophil cationic protein; ICAM = intercellular adhesion molecule; INCS = intranasal corticosteroid; LOE = level of evidence; LTRA = leukotriene receptor antagonist; nPIF = nasal peak inspiratory flow; QOL = quality of life; RCT = randomized controlled trial; SR = systematic review.
Several studies also examined the relative effectiveness of combination LTRA and oral antihistamine therapy compared to INCS. Combination therapy was generally less effective than INCS monotherapy,1472,1479,1481 although some studies did not detect a statistically significant difference.1300,1484 The systematic review by Wilson et al.1300 did not discern a difference in symptom reduction between LTRA and oral antihistamine combination therapy and INCS. In contrast, 3 RCTs showed that INCS resulted in improved nasal symptoms compared to treatment with the combination,1472,1479,1481 in addition to decreased nasal mucosa eosinophil counts.1472,1481
There is conflicting evidence on whether combination therapy is more effective than oral antihistamine alone, and there appears to be relatively consistent evidence that INCS monotherapy is more effective at nasal symptom reduction than LTRA and oral antihistamine combination therapy. Therefore, combination therapy may be an option in patients whose symptoms are incompletely controlled with oral antihistamine monotherapy, and in whom INCS are not tolerated or contraindicated. This may be particularly useful in a subset of these patients with concurrent asthma. Montelukast may be effective at simultaneously reducing AR symptoms and improving asthma control.1341
Drug interaction and safety are an important consideration when using combination therapies. Reported adverse events for montelukast and loratadine in combination were similar to montelukast and loratadine monotherapy and placebo.1485 The most common reported adverse events were headache (4.5%), fatigue (1.2%), and pharyngolaryngeal pain (1.2%). There were no changes of vital signs, electrocardiogram, or physical exam findings during the monitoring period.1485 Combination LTRA and oral antihistamine therapy can be administered with minimal adverse events, and with similar frequency to either agent as monotherapy.
Aggregate Grade of Evidence: A (Level 1a: 1 study; Level 1b: 11 studies; Level 2b: 1 study; Table IX.B.10.c).
Benefit: Inconsistent evidence that combination LTRA and oral antihistamine were superior in symptom reduction and QOL improvement than either agent as monotherapy. Combination therapy is inferior in symptom reduction compared to INCS alone.
Harm: No significant safety-related adverse events from combination therapy.
Costs: Generic montelukast was more expensive than either generic loratadine or cetirizine on a per dose basis, according to weekly National Average Drug Acquisition Cost (NADAC) data provided by the Centers for Medicare & Medicaid Services (CMS).
Benefits-Harm Assessment: Balance of benefit and harm.
Value Judgments: Combination therapy of LTRA and oral antihistamines does not result in consistently improved AR symptoms compared to either agent alone. There are few reported safety-related adverse events from combination therapy. The addition of an LTRA may have a role in management of comorbid asthma.
Policy Level: Option.
Intervention: Combination therapy of LTRA and oral antihistamines is an option for management of AR, particularly in patients with comorbid asthma or those who do not tolerate INCS and symptoms are not well-controlled on oral antihistamine monotherapy.
IX.B.10.d. Intranasal corticosteroid and intranasal antihistamine.
The use of combination intranasal antihistamine and corticosteroid spray for AR has been well studied. One topical formulation is currently available in North America for intranasal use as a combination of azelastine hydrochloride and fluticasone propionate (AzeFlu; Mylan, Canonsburg, PA). This agent is also designated in the literature as MP-AzeFlu or MP29-02, and is marketed in the United States under the trade name Dymista. A systematic review of the English-language literature was performed for clinical trials of combination INCS and intranasal antihistamine for the treatment of AR. A total of 10 RCTs (9 double-blind, 1 non-blinded) evaluated combination therapy against either placebo or active control.1486-1495 An additional 2 observational studies in the allowable search date range for this document reported outcomes of AzeFlu as a single treatment arm1496,1497 (Table IX.B.10.d).
TABLE IX.B.10.d.
Evidence for the use of combination intranasal corticosteroids and intranasal antihistamine in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Berger et al.1486 | 2016 | 1b | DBRCT |
|
rTNSS, rTOSS, PRQLQ | AzeFlu superior to placebo for symptoms and QOL improvement in children; symptoms improved when children self-rate. |
| Meltzer et al.1487 | 2013 | 1b | DBRCT |
|
rTNSS, rTOSS | AzeFlu superior to placebo for all symptoms. |
| Price et al.1488 | 2013 | 1b | DBRCT |
|
rTNSS, symptom-free days | AzeFlu superior to fluticasone for symptom reduction; faster onset. |
| Carr et al.1489 | 2012 | 1b | DBRCT |
|
rTNSS, rTOSS, RQLQ | AzeFlu superior to either spray alone for symptom and QOL improvement; faster onset. |
| Meltzer et al.1490 | 2012 | 1b | DBRCT |
|
rTNSS, rTOSS, RQLQ | AzeFlu superior to either spray alone for symptom and QOL improvement. |
| Salapatek et al.1491 | 2011 | 1b | DBRCT |
|
TNSS | Both treatments superior to placebo; CDX-313 superior to suspension-type spray for symptoms and speed of onset. |
| Hampel et al.1492 | 2010 | 1b | DBRCT |
|
TNSS | AzeFlu superior to either spray alone; all treatments superior to placebo. |
| LaForce et al.1493 | 2010 | 1b | DBRCT |
|
TNSS | No difference between treatments. |
| Ratner et al.1494 | 2008 | 1b | DBRCT |
|
TNSS | Combination superior to either agent alone. |
| Berger et al.1495 | 2016 | 2b | RCT, non-blinded |
|
Total symptom score | AzeFlu superior to fluticasone for children; faster onset. |
| Klimek et al.1496 | 2016 | 2c | Prospective observational | AzeFlu | VAS | 76% of subjects had symptom control after 14 days; significant improvement from baseline. |
| Klimek et al.1497 | 2015 | 2c | Prospective observational | AzeFlu | VAS | Rapid symptom relief across all age groups. |
AzeFlu = combination spray of azelastine hydrochloride and fluticasone propionate; DBRCT = double-blind randomized controlled trial; LOE = level of evidence; PRQLQ = Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; rTNSS = reflective Total Nasal Symptom Score; rTOSS = reflective Total Ocular Symptom Score; TNSS = Total Nasal Symptom Score; VAS = visual analog
Outcome measures were predominantly patient-reported symptom scores or QOL assessments. The most common outcome measure was the TNSS (9 studies), which records the severity of runny nose, sneezing, itching, and congestion. Other outcome measures included the TOSS (4 studies), a VAS (3 studies), the RQLQ (2 studies), the Pediatric RQLQ (1 study), and a threshold/discrimination/identification (TDI) score (1 study).
The minimum age of subjects in most included studies was 12 years or older. Study duration was 14 days of active treatment in most studies, except 1 study with a 3-month duration1495 and 1 study with a 52-week duration.1488 The number of subjects in each study ranged from 47 to 3398. Combination therapy with AzeFlu was compared to placebo in 6 studies, with primary outcomes showing superiority to placebo in all studies.1486,1487,1489-1492 AzeFlu was compared to active treatment with fluticasone propionate monotherapy in 6 studies, all of which showed superiority of the combination therapy.1488-1490,1492,1494,1495 Similarly, intranasal AzeFlu was compared to active treatment with azelastine hydrochloride monotherapy in 4 studies, all of which showed superiority of the combination therapy.1489,1490,1492,1494 AzeFlu was directly compared to combination therapy with intranasal olopatadine and fluticasone in 1 study, with no significant difference in symptom relief between treatment groups.1493 One study found superiority of an experimental combination of solubilized azelastine and budesonide compared to either a suspension-type formulation of azelastine and budesonide or placebo.1491
Two studies evaluated children aged between 6 and 12 years old. Like findings in adults, AzeFlu showed superiority to placebo in improving symptoms and QOL in children.1486,1495 Several studies reporting time to onset found that AzeFlu had a more rapid effect compared to INCS alone.
Serious adverse effects were not reported in any study. Intranasal antihistamine and corticosteroid combination therapy was generally well tolerated, with the most commonly reported adverse effect being an unpleasant taste. Other reported adverse effects included somnolence, headache, epistaxis, and nasal discomfort, all occurring in less than 5% of cases in each study. One study that compared combination therapy of fluticasone propionate with either azelastine or olopatadine reported more treatment-related events for the azelastine group (16/68) than the olopatadine group (7/67).1493
Aggregate Grade of Evidence: A (Level 1b: 9 studies; Level 2b: 1 study; Level 2c: 2 studies; TableIX.B.10.d).
Benefit: Rapid onset, more effective for relief of multiple symptoms than either INCS or intranasal antihistamine alone.
Harm: Patient intolerance, especially due to taste.
Costs: Moderate financial burden. Average wholesale price of $202 USD per 23-g bottle (1-month supply when used as labeled).
Benefits-Harm Assessment: Preponderance of benefit over harm. Combination therapy with intranasal antihistamine and INCS is consistently more effective than placebo. Low risk of non-serious adverse effects.
Value Judgments: Despite level 1 evidence demonstrating that combination spray therapy (INCS plus intranasal antihistamine) is more effective than monotherapy and placebo, the increased financial cost and need for prescription limit the value of combination therapy as a routine first-line treatment for AR.
Policy Level: Strong recommendation for the treatment of AR when monotherapy fails to control symptoms.
Intervention: Combination therapy with INCS and intranasal antihistamine may be used as second-line therapy in the treatment of AR when initial monotherapy with either INCS or antihistamine does not provide adequate control.
IX.B.11. Nontraditional and alternative therapies
IX.B.11.a. Acupuncture.
In complimentary medicine, acupuncture has the distinction of being 1 of the oldest forms of healing arts practiced, with its origins dating back to the 6th to 5th centuries BC.1498 Traditional Chinese medicine holds to the concept that the body’s vital energy (Qi) flows through a network of meridians beneath the skin.1499 In a healthy state, the flow of the Qi is uninterrupted whereas disease states mark a disruption of the Qi. The aim of acupuncture is to stimulate acupuncture points (acupoints) with needles to recover equilibrium. Acupoints are specific anatomic points located along meridians that are believed to correspond to the flow of energy through the body.
There have been several blinded RCTs evaluating acupuncture as a treatment for AR. Acupuncture has an excellent safety profile with only minor side effects reported.1500,1501 Some studies have shown acupuncture to influence allergic and inflammatory mediators including IgE and IL-10 levels in AR patients significantly more than controls,1501,1502 suggesting a possible immunomodulatory effect. The clinical significance of these changes, however, remains to be seen.
Two meta-analyses addressing acupuncture have been performed (Table IX.B.11.a). The first, published in 2008 reviewed 7 RCTs and found a high degree of heterogeneity between studies with most studies being of low quality.1500 No overall effects of acupuncture on AR symptom scores or use of relief medications were identified.1500 A more recent meta-analysis of 13 studies had more favorable findings, demonstrating a significant reduction in nasal symptoms, improvement in RQLQ scores, and decreased use of rescue medications in the group receiving acupuncture.1501 This meta-analysis included 6 of the 7 studies in the 2008 review and 7 new studies. Again, a high level of heterogeneity between studies and varied quality of the studies was noted. Most important to note is that neither meta-analysis discussed the specific consideration of concomitant AR medication use during the trials, which is common in most acupuncture trials. The uncontrolled use of AR medications could have significantly impacted the outcomes in any of these studies and raises concerns when interpreting the results.
TABLE IX.B.11.a.
Evidence for the use of acupuncture in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Feng et al.1501 | 2015 | 1a | SR and metaanalysis |
|
Nasal symptom scores, RQLQ scores, rescue medication use | Significant reduction in nasal symptoms, improvement in RQLQ scores and use of rescue medications with acupuncture. |
| Roberts et al.1500 | 2008 | 1a | SR and metaanalysis |
|
AR symptom scores, rescue medication use | No overall effect on AR symptom scores or need for rescue medications. |
AR = allergic rhinitis; LOE = level of evidence; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SR = systematic review.
Aggregate Grade of Evidence: B (Level 1a: 2 studies; Level 2b: 13 studies; Table IX.B.11.a). Only level 1a studies are presented in the table.
Benefit: Unclear, as 1 meta-analysis showed no overall effects of acupuncture on AR symptoms or need for rescue medications and a second meta-analysis showed an effect of acupuncture on symptoms, QOL, and need for rescue medications.
Harm: Needle sticks associated with minor adverse events including skin irritation, pruritis, erythema, subcutaneous hemorrhage, infection, and headache. Need for multiple treatments and possible ongoing treatment to maintain any benefit gained.
Cost: Cost of acupuncture treatment with multiple treatments required.
Benefits-Harm Assessment: Balance of benefit and harm.
Value Judgments: The authors determined that the evidence was inconclusive but that acupuncture could be appropriate for some patients to consider as an adjunct therapy.
Policy Level: Option.
Intervention: In patients who wish to avoid medications, acupuncture may be suggested as possible therapeutic adjunct.
IX.B.11.b. Honey.
A long-held belief has been that honey is effective in treating symptoms of AR; however, evidence in support of this is scarce. It is postulated that environmental antigens contained within locally produced honey could, when ingested regularly, lead to the development of tolerance in a manner similar to SLIT. It is important to note that heavy, insect-borne pollens do not meet Thomen’s postulates, as they are not airborne and hence should not be able to induce allergic sensitivity.818 Studies in animals have demonstrated the ability of honey to suppress IgE antibody responses elicited against different allergens and to inhibit IgE-mediated mast cell activation.1503-1505 As yet, these same effects have not been tested for in humans; however, studies in humans have demonstrated various anti-inflammatory properties of honey which point to a potential benefit for its use in the treatment of AR.1506,1507
There have been 2 randomized, double-blind, placebo-controlled trials and 1 RCT evaluating honey in the treatment of AR (Table IX.B.11.b). The studies differed in geographic location, length of honey treatment, dose of honey, and timing regarding specific allergy seasons. One double-blind trial and 1 RCT showed a significant decrease in total symptom scores in the treatment group compared to control.1508,1509 The RCT additionally reported fewer number of severe symptom days and decreased need for antihistamines in the honey group.1509 Contradicting these findings, a randomized, double-blind, placebo-controlled trial by Rajan et al.1510 found no benefit of honey ingestion compared to controls for the relief of AR symptoms. Of note, it has been reported that higher doses (50 to 80 g daily intake) of honey are required to achieve health benefits from honey1511 and only the study by Asha’ari et al.1508 dosed patients at that level.
TABLE IX.B.11.b.
Evidence for the use of honey in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Asha’ari et al.1508 | 2013 | 1b | RDBPCT |
|
AR symptom scores | Improvement in overall and individual AR symptoms with honey. |
| Rajan et al.1510 | 2002 | 1b | RDBPCT |
|
Daily AR symptoms, rescue medication use | No significant difference in AR symptoms or need for rescue medication. |
| Saarinen et al.1509 | 2011 | 2b | RCT |
|
Daily AR symptoms, number of asymptomatic days, rescue medication use | Birch pollen honey significantly lowered total symptom scores and decreased use of rescue medications. Honey groups had significantly more asymptomatic days. |
AR = allergic rhinitis; LOE = level of evidence; RCT; randomized controlled trial; RDBPCT = randomized double-blind placebo-controlled trial.
Aggregate Grade of Evidence: B (Level 1b: 2 studies; Level 2b: 1 study; Table IX.B.11.b).
Benefit: Unclear, as studies have shown differing results. Honey may be able to modulate symptoms and decrease need for antihistamines.
Harm: Some patients stopped treatment because they could not tolerate the level of sweetness. Some patients could have an allergic reaction to honey intake, and in rare instances, anaphylaxis. Use of this therapy in prediabetics and diabetics would likely need to be avoided out of concern for elevated blood glucose levels.
Cost: Cost of honey; low.
Benefits-Harm Assessment: Balance of benefit and harm.
Value Judgments: Studies are inconclusive and heterogeneous.
Policy Level: No recommendation due to inconclusive evidence.
Intervention: None.
IX.B.11.c. Herbal therapies.
Like acupuncture and honey, herbal remedies have been used for the treatment of various physical ailments, including AR, world-wide for thousands of years. This area of complementary/alternative medicine is an attractive alternative to mainstream medicine for patients who wish to avoid traditional pharmacotherapy or who have not tolerated various anti-allergic medications in the past. There are a vast number of studies looking at the effectiveness of numerous herbs and herbal supplements in the treatment of AR; however, most are small and of poor quality. Those herbal remedies that have been subjected to more rigorous study are summarized in Table IX.B.11.C
TABLE IX.B.11.c.
Evidence for the use of herbal therapies in the treatment of allergic rhinitis
| Herb | Mechanism of action | Evidence | Side effects |
|---|---|---|---|
| Astragalus membranaceus | Unknown | RDBPCT comparing 80 mg daily × 6 weeks showed significant improvement in rhinorrhea, changes in TSS, and QOL.1512 | Pharyngitis, rhinosinusitis |
| Aller-7 | Possibly through antioxidant and anti-inflammatory pathways1513-1515 | 2 RDBPCTs showed some relief of symptoms with Aller-7. However, there were some contradictory findings.1516 | Dry mouth, gastric discomfort |
| Benifuuki green tea | Inhibits type I and type IV hypersensitivity reactions1517, 1518 | RDBPCT showed 700 mL Benifuuki green tea daily significantly reduced AR symptoms, improved QOL, and suppressed peripheral eosinophils.1519 | None reported |
| Biminne | Unknown | RDBPCT found 12 weeks of biminne significantly reduced sneezing.1520 | Not reported |
| Butterbur (Petasites hybridus) | Inhibits leukotriene and histamine synthesis and mast cell degranulation1521 | 3 RDBPCTs showed Butterbur effective in alleviating symptoms, attenuating nPIF recovery, and reducing maximum % nPIF decrease from baseline after adenosine monophosphate challenge. Butterbur similar to antihistamine for improving QOL and symptom relief.1516 1 RDBPCT demonstrated no benefit for nPIF, symptoms, or QOL.1516 | Hepatic toxicity, headache, gastric upset |
| Capsaicin | Thought to desensitize and deplete sensory C-fibers1522, 1523 | No evidence of a therapeutic effect of intranasal capsaicin in AR.1524, 1694 | Mucosal irritation, burning |
| Cinnamon bark, Spanish needle, acerola (ClearGuard) | Inhibits production of prostaglandin D21525 | RDBPCT showed 450 mg CG TID comparable to loratadine 10 mg in symptom reduction. CG prevented increase in prostaglandin D2 release following nasal allergen challenge.1525 | None reported |
| Grape seed extract | Contains catechin monomers that may inhibit allergen-induced histamine release1526 | RDBPCT showed no benefit of 100 mg grape seed extract BID on nasal symptoms, need for rescue medications, or QOL.1527 | None reported |
| Nigella sativa (Black seed) | Inhibited histamine release from rat macrophages.1528 Thymoquinone may inhibit Th2 cytokines and eosinophil infiltration in airways.1529 | 2 RDBPCTs showed N. sativa capsules and 1 RDBPCT showed N. sativa nasal drops improve AR symptoms.1530-1532 1 RDBPCT did not find significant differences between treatment and placebo.1530 | Gastrointestinal complaints with oral intake. Nasal dryness with topical drops. |
| Perilla frutescens | Polyphenolic phytochemicals such as rosmarinic acid inhibit inflammatory processes and the allergic reaction.1533-1536 | RDBPCT showed 50 mg or 200 mg P. frutescens enriched for rosmarinic acid did not significantly improve symptom scores.1537 | None reported |
| RCM-101 | Inhibits histamine release and prostaglandin E2 production1538, 1539 | RDBPCT showed 4 tablets of RCM-101 TID for 8 weeks significantly improved symptom scores and RQLQ.1540 | Mild gastrointestinal side effects |
| Spirulina | Reduces IL-4 levels,1541 inhibits histamine release from mast cells1542 | RDBPCT showed 2000 mg/day spirulina significantly improved sneezing, rhinorrhea, congestion, and nasal itching.1543 | Not reported |
| Ten-Cha (Rubus suavissimus) | Inhibits cyclooxygenase activity and histamine release by mast cells1544 | RDBPCT showed no significant improvement in symptom scores, RQLQ, or need for antihistamine with 400 mg daily of Ten-Cha extract.1545 | None reported |
| TJ-19a | Inhibits histamine signaling and IL-4 and IL-5 expression in a rat model1546 | RDBPCT showed 3 g TJ-19 TID significantly improved sneezing, stuffy nose, and runny nose.1547 | Not reported |
| Tinofend (Tinospora cordifolia) | Possibly through anti-inflammatory effects1548 | RDBPCT showed 300 mg Tinofend × 8 weeks significantly improved multiple AR symptoms and a significant decrease in eosinophil, neutrophil, and goblet cell counts on nasal smear.1548 | Leukocytosis |
| Urtica dioica (stinging nettle) | In vitro: antagonist/negative agonist activity against Histamine-1 receptor, inhibits mast cell tryptase, prevents mast cell degranulation, inhibits prostaglandin formation1549 | 1 RDBPCT showed symptom improvement over placebo at 1 hour.1550 1 systematic review showed no significant intergroup differences.1516 | Not reported |
Not available in the United States as it contains ephedra.
AR = allergic rhinitis; BID = 2 times daily; CG = ClearGuard; IL = interleukin; nPIF = nasal peak inspiratory flow; QOL = quality of life; RDBPCT = randomized double blind placebo controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; TID = 3 times daily; TSS = Total Symptom Score.
Given the lack of robust and repeated large double-blind randomized placebo-controlled trials on any 1 herbal remedy, no evidence based recommendations can be made supporting the routine use of any 1 herb or compound; this should be considered an area requiring further research before any such recommendations can be made.
Aggregate Grade of Evidence: Uncertain.
Benefit: Unclear, but some herbs may be able to provide symptomatic relief.
Harm: Some herbs are associated with mild side effects. Also, the safety and quality of standardization of herbal medications is unclear.
Cost: Cost of herbal supplements; variable.
Benefits-Harm Assessment: Unknown.
Value Judgments: The authors determined that there is a lack of sufficient evidence to recommend the use of herbal supplements in AR.
Policy Level: No recommendation.
Intervention: None.
IX.C. Surgical treatment
AR is a medical disease, but at times may become refractory to medical management. Surgery for AR is primarily aimed at reducing nasal obstruction and/or rhinorrhea, with the contributing structures being the nasal septum and turbinates.1551 Vidian neurectomy is historically a surgical technique that seeks to overcome chronic and intractable rhinitis.
No Cochrane review of septoplasty or vidian neurectomy for allergic patients currently exists. A Cochrane review of turbinate reduction in allergic patients refractory to medical management was explored, but was unable to identify any qualifying studies (selection criteria stringently required randomized controlled trials of inferior turbinate surgery vs continued medical treatment for proven AR, or comparisons between 1 technique of inferior turbinate surgery vs another technique, after maximal medical treatment).1552 Physicians must, therefore, rely upon less scientifically rigorous data when deciding upon surgery for AR patients.
The role of septoplasty for the treatment of nasal obstruction in AR is poorly understood. The nasal septum is not a major contributor to allergic disease because it does not experience the extent of dynamic change the turbinate tissue does, and therefore, there is a paucity of literature investigating septoplasty alone to improve nasal patency in AR. The nasal septal swell body may serve to alter nasal airflow and humidification, but no literature exists to implicate a role in AR.1553 Karatzanis et al.1554 found that subjective improvement in patients undergoing septoplasty was higher in those without AR than those with it. For this reason, a cautious approach to the management of nasal septal deviation in AR is warranted. On the other hand, Kim et al.1555 found that AR patients undergoing septoplasty with turbinoplasty felt more relief of nasal obstruction then those undergoing turbinoplasty alone (Table IX.C).
TABLE IX.C.
Evidence for surgery in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Jose & Coatesworth2 | 2010 | 1a | SR of RCTs | Turbinate reduction in refractory AR | No studies qualified as RCT | No conclusions could be made. |
| Chen et al.1557 | 2008 | 1b | RCT | AR patients undergoing IT:
|
VAS, anterior rhinomanometry, saccharin transit time | Significant improvement in all parameters for both treatment groups at 1, 2, and 3 years. |
| Passali et al.1236 | 1999 | 2b | RCT | AR patients undergoing IT:
|
Rhinomanometry, acoustic rhinometry, mucociliary transport time, secretory IgA levels, symptom scores | Submucosal resection with lateral displacement of the IT results in the greatest increase in nasal airflow and nasal respiratory function with the lowest risk of long-term complications. |
| Tan et al.1566 | 2012 | 3b | Observational cohort | AR patients undergoing:
|
QOL outcomes | All subjects improved, but improvement in vidian neurectomy group exceeded group undergoing turbinectomy and/or septoplasty. |
| Kim et al.1555 | 2011 | 3b | Case-control | AR patients undergoing:
|
Mean rescue medication score, Rhinasthma Questionnaire | Significant improvement in both groups but less obstruction in septoplasty group. |
| Karatzanis et al.1554 | 2009 | 3b | Case-control | Septoplasty in patients with or without AR | NOSE scores, anterior rhinomanometry | Non-AR subjects showed more improvement than AR subjects. |
| Mori et al.1556 | 2002 | 3b | Observational cohort | AR patients undergoing IT submucous turbinectomy | Standard symptom score, rhinometry, nasal challenge | Significant improvement seen at 1 and 3 years. |
| Caffier et al.1558 | 2011 | 4 | Case series | AR patients undergoing mucosal laser reduction, 95% to IT | Rhinomanometry and VAS | Objective and subjective improvement up to 2 years. |
| Aksoy et al.1564 | 2010 | 4 | Case series | AR patients undergoing IT outfracture | CT sinus preoperatively, and 1 and 6 months postoperatively | Statistically significant reductions were noted in the angle and distances in all sections. |
| Lin et al.1562 | 2010 | 4 | Case series | AR patients undergoing IT radiofrequency turbinoplasty | Symptoms per VAS | Statistically significant reductions were noted in obstruction, rhinorrhea, sneezing, and itching. |
| Siméon et al.1563 | 2010 | 4 | Case series | Children with AR undergoing IT coblation turbinoplasty | Rhinomanometry, VAS, PRQLQ | All improved per PRQLQ. |
| Li et al.1561 | 1998 | 4 | Case series | AR patients undergoing IT radiofrequency turbinoplasty | Questionnaires and VAS | 21 of 22 showed improved symptoms at 8 weeks. |
AR = allergic rhinitis = CT = computed tomography; IgA = immunoglobulin A; IT = inferior turbinate; LOE = level of evidence; NOSE = Nasal Obstruction Symptom Evaluation score; PRQLQ = Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; QOL = quality of life; RCT = randomized controlled trial; SR = systematic review; VAS = visual analog scale.
In contrast to the septum, the inferior turbinates are a prime target of allergic effects, characterized by vasodilation of capacitance vessels leading to engorgement, in turn causing nasal obstruction and congestion. Although surgery will not eliminate the inflammatory origins of AR, additional patency of the nasal cavity reduces the effects of edematous mucosa. From a surgical standpoint, inferior turbinate reduction is the most beneficial treatment for nasal obstruction in AR refractory to medical therapy.1552 The inferior turbinate consists of 3 primary components: a mucosal covering, a submucosal layer (containing the capacitance vessels), and a bony center. Surgery is typically aimed at the submucosa or bone, or total/partial turbinectomy which involves removal of all 3 components.
The submucosal tissue can be reduced through direct removal (eg, submucous bony resection or microdebrider submucosal resection) or energy applied to damage tissue with subsequent remodeling (eg, cautery, radiofrequency, laser, Coblation™). These various techniques have substantial support in the literature. Mori et al.1556 reported on long-term outcomes on patients undergoing submucous bony resection over a 5-year follow-up period and noted a significant improvement in symptoms and nasal allergen responses. Additionally, QOL was enhanced in postoperative patients and maintained long term. Microdebrider submucous reduction targets the cavernous tissue surrounding the bony turbinate. Advantages include real-time suction with precise tissue removal. Compared to submucosal bony resection, data suggests improved mucociliary time due to less tissue trauma.1557
Laser turbinate reduction seeks to induce scarring in the submucosa, though the overlying superficial mucosal layer is transgressed in the process. Caffier et al.1558 reported on the effects of diode laser turbinoplasty in 40 patients with AR. Statistically significant improvements occurred in rhinomanometry and nasal obstruction, rhinorrhea, sneezing, and nasal pruritus. The improvement in nasal obstruction was sustained at 2 years.1558
In radiofrequency ablation (RFA) for nasal obstruction, a probe is inserted directly into the inferior turbinate to deliver a low-frequency energy, causing ionic agitation of tissues.1559 The thermal effect is limited to the submucosal layer, which preserves surface epithelium and ciliary function.1560 Following RFA, coagulative necrosis occurs first, with scar contracture and tissue retraction occurring later in the healing process. Over time, portions of the fibrotic scar undergo resorption and the submucosal scar will adhere to the bony periosteum, which reduces turbinate bulk and renders it less susceptible to edema and engorgement.1560,1561 In the first long term study of its kind, Lin et al.1562 published a report on 101 patients who were followed up to 5 years postoperatively after undergoing RFA turbinoplasty for the treatment of AR. The 6-month and 5-year response rates were 77.3% and 60.5%, respectively, and statistically significant improvement was achieved in nasal obstruction, rhinorrhea, sneezing, itchy nose, and itchy eyes.1562 Coblation™ technology relies on electrodissection by molecular activation. This technology can similarly target the submucosal layers. Siméon et al.1563 investigated the efficacy of Coblation™ on 9 AR patients with a mean age of 12.7 years. Favorable decreases in nasal resistance, pruritus, sneezing, hyposmia, and rhinorrhea were observed and sustained at 6-month follow-up.1563 RFA and Coblation™ procedures are well-tolerated with minimal adverse effects and can be safely performed in the operating room or the outpatient office setting.
Bony outfracture seeks to shift the bony skeleton of the inferior turbinate laterally into the inferior meatus, thereby creating more breathing space. Aksoy et al.1564 found statistically significant reductions in the distance between the inferior turbinate and the lateral nasal wall after outfracture in 40 patients. This effect was sustained at 6 months postoperatively, which suggests that lateralization persists.1564 Radical turbinate excision might overcome obstruction, but, at the cost of dryness and possibly empty nose syndrome.1565
Vidian neurectomy is an older technique that seeks to damage the parasympathetic nerve impulses to the nasal cavity. Tan et al.1566 found significant improvement in QOL measures in a prospective group undergoing vidian neurectomy over septoplasty/partial turbinectomy or medical management groups. This technique is considered more effective for non-allergic patients and seeks to primarily address severe rhinitis.1567 Posterior nasal nerve section may also be considered for recalcitrant rhinorrhea; this technique aims to avoid the dry eye complications of vidian neurectomy.1568
Recent publications have identified isolated middle turbinate polypoid edema or frank polyps to have a significant correlation with inhalant allergy, especially in more severe cases.785,786 In cases where the polypoid changes in the middle turbinate are significant enough to cause nasal obstruction, conservative recontouring of the middle turbinate(s) can reduce nasal obstructive symptoms.
To summarize, surgical treatment of the septum, inferior and/or middle turbinates, and possibly vidian/posterior nasal neurectomy may be considered in both allergic and non-allergic patients. Outcomes of these various techniques are variable in patients with AR.
Aggregate Grade of Evidence: C (Level 1a: 1 study; Level 1b: 1 study; Level 2b: 1 study; Level 3b: 4 studies; Level 4: 5 studies; Table IX.C).
Benefit: Improved postoperative symptoms and nasal airway.
Harm: Possible septal perforation, empty nose syndrome, nasal dryness, mucosal damage, epistaxis.
Cost: Office-associated vs operating room-associated procedural costs.
Benefits-Harm Assessment: Preponderance of benefit over harm.
Value Judgments: Properly selected patients can experience an improved nasal airway with judicious surgical intervention.
Policy Level: Option.
Intervention: Turbinate reduction with or without septoplasty may be considered in AR patients that have failed medical management, and have anatomic features which explain symptoms of nasal obstruction.
IX.D. Allergen immunotherapy (AIT)
In addition to allergen avoidance and numerous pharmacotherapy options, AIT is frequently considered in the management of AR. AIT involves scheduled administration of allergen extracts at effective doses with the goal of instituting a sustained immunologic change. AIT effectiveness is often measured through control of allergy symptoms and reduction in allergy medication use. The following section reviews the specifics of allergen extract units and standardization, allergen extract adjuvants and modifications, and subcutaneous and sublingual immunotherapy (SCIT, SLIT), as well as less traditional types of immunotherapy.
IX.D.1. Allergen extract units, potency, and standardization
Historically, allergy testing began with pollen grains placed directly on the conjunctiva,1569,1570 but as skin testing and SCIT became the diagnostic and immunotherapy treatment methods of choice, injectable allergen extracts were required. Inhaled allergenic particles are composed of a complex heterogeneous mixture of allergenic and non-allergenic proteins and macromolecules. Allergen extracts are created by collecting raw material from a particular species of plant, mold, or animal and then using a solution to extract proteins from the source.1571
There are multiple sources of variance in allergen extracts. There is biologic variability in the raw material, and proteins can vary in antigenicity and composition; furthermore, the relative amounts of allergenic proteins may vary.1572,1573 Impurities in the source materials, such as mold growing on pollen granules or bacteria on cat pelts, may also be immunogenic even if nonviable. Variation occurs in the collection and processing of the raw material.1573 There is variability in the extraction process with different manufacturers using different techniques including filtration, extraction, sterilization, and preservation.1571,1572,1574,1575 Only a very small fraction of the proteins extracted are allergenic.1571 Given that the protein composition of allergen extracts is not known, producing and labeling allergen extracts that are safe and effective is challenging.
Units and potency.
Allergen extracts are labeled with an assortment of units that provide an indirect indication of the allergen content of the extract. Most allergen extracts are labeled in units that do not convey information about biological composition or potency. There are multiple types of units that can be grouped into nonstandardized, standardized, or proprietary. The difference between standardized and nonstandardized extracts is discussed later this section.
Potency of an allergen can have different meanings. Potency sometimes refers to the allergenicity of a source material’s proteins or the biologic activity. For example, grass pollens are generally more potent than tree pollens. The typical grass-allergic person would have a larger clinical reaction to grass pollen than a tree-allergic person to the same amount of tree pollen. However, a measure of potency of an allergen extract may also just refer to the strength or concentration measured in units.
Nonstandardized allergen extracts.
Most allergen extracts available in the United States are nonstandardized. Allergen extracts are regulated by the Center for Biologics Evaluation and Research (CBER) under the FDA in the United States.1576 Allergen extracts are required to list the biologic source, a potency unit, and an expiration date.
Weight/volume (wt/vol). Weight/volume refers to the ratio of grams of dry raw material to milliliters of extract solvent. Commonly this is 1/20 wt/vol, which means that for every 1 g of raw material (pollen for example) there is 20 mL of extract solvent. This does not provide direct information about the amount of allergenic proteins in the allergen extract nor its biologic activity. However, it implies a reproducible methodology was employed.1571
Protein nitrogen units (PNUs). This is the second most common nonstandardized unit currently used in the United States. PNU refers to an assay of the precipitable protein nitrogen by phosphotungstic acid which correlates with the total protein. While most of the protein is non-allergenic, the total protein is another method to quantitate an allergen extract’s content.1571
In Europe, many manufacturers use proprietary units and internal quality controls which must utilize a validated assay.1572 This European manufacturer-based quality control is known as “In House Reference Preparation.”1573 However, the European Medical Agency has been developing a standardized framework based on protein homology rather than source species.1577 The EU is also developing additional allergen standards with the WHO starting with Bet v 1 and Phl p 5a.1577
Standardized allergen extracts.
In the United States, standardized allergen extracts are tested by the manufacturer to be within a reference range (70-140%) when compares to a standard provided by the CBER. The government’s standard is referenced to the reactivity in highly allergic individuals, creating a standard of biologic activity.
The CBER creates the standard extract through testing in known “highly allergic” individuals. They use serial intradermal 3-fold titrations and measure potency by how many dilutions are needed to produce a flare reaction of a certain size. The size is determined by measuring the largest diameter and adding the length of a line 90 degrees to the largest diameter line. The orthogonal sums are plotted for each dilution and a best fit line drawn. The concentration that corresponds to where the orthogonal sum of the flare is 50 mm (ID50EAL) determines the units listed in either Allergy Units (AU) or Biologic Allergy Units (BAU). AU is used for dust mites. A mean ID50EAL of 14 threefold dilutions is defined as 100,000 BAU/mL and 12 threefold dilutions 10,000 BAU/mL.1577
The FDA allergen standards are compared to the produced allergen extracts by the manufacturers. The process is different for extracts where the major allergen reactivity correlates with overall allergen reactivity (cat and ragweed) than for extracts that do not have a major allergen that correlates as strongly. A major allergen is defined as a specific protein epitope that more than 50% of individuals allergic to that species react. If there is a major allergen that correlates strongly with the population’s clinical reactivity, the manufacturer can compare their extract to the standard extract by gel electrophoresis with the gel having monoclonal IgG antibodies to the major allergen protein. If there is not a single allergen that correlates well with the reactivity of the population, the manufactured extract and the standard are compared through competition enzyme-linked immunosorbent assay (ELISA) using pooled serum IgE from known allergic subjects. The manufacturer’s extract must fall within a 70% to 140% range of the FDA’s reference.1576 The amount of major allergen is sometimes listed in μg/mL, Fel d 1 units (cat), or Antigen E units (ragweed). Standardized inhalant allergens within the United States include cat, Dermatophagoides pteronyssinus, Dermatophagoides farinae, short ragweed, and multiple grass species.1577
Some allergen extracts in Europe use the Nordic method where 10,000 biologically standardized units/mL is comparable to a skin reaction elicited by 10 mg/mL of histamine.1577
In conclusion, an international consensus has not been established for allergen units or standardization of allergen extracts. While standardization and transparent potency assays increase manufacturing costs, it is widely agreed that greater standardization and consistency across manufacturers would be beneficial. Variations in allergen extracts between manufacturers may discourage medical providers from changing between vendors reducing the effect of price on competition. The multitude of allergen extract units and variability also complicates the interpretation and application of published studies between the United States, the EU, and other countries. The WHO has identified allergen standardization as a problem and the EU funded a project known as CREATE, “Development of Certified Reference Materials for Allergenic Products and Validation of Methods for the Quantification.”1578 But as of 2017, multiple allergen units are still in use worldwide.
IX.D.2. Modified allergen extracts
The goal of AIT is to suppress the underlying inflammatory diathesis and induce a state of clinical tolerance to the relevant allergen. This thereby attenuates, if not completely arrests, the inflammation that manifests as AR. Traditional AIT with native, unmodified extracts is successful but has several limitations. Immunotherapy can lead to adverse reactions which rarely can be life-threatening. Besides the risks, allergen extracts have significant production costs with limitations of availability and consistency between batches. Variations exist in pharmaceutical-produced native extracts in the allergen amounts, potencies, and immunogenicity of individual allergen molecules that cannot be controlled in the manufacturing process.1579
New advances in AIT have focused on redirecting the untoward allergic diathesis through upregulation of T-regulatory and B-regulatory cells, restoring the balance between Th2 and Th1 cell subtypes, and establishing T-cell immune tolerance. The use of recombinant-derived allergens, synthetic peptides, allergoids, and adjuvants has been sought to provide safer, more consistent, readily available, and effective allergens compared to commercially available native extracts1580-1582 (Table IX.D.2-1).
TABLE IX.D.2-1.
Modified allergen immunotherapy constructs*
| Injectable immunotherapy approaches |
|---|
| Recombinant allergens (SQ) |
| Peptide constructs (ID) |
| Chemical modifications (SQ) |
| Alum salts (SQ) |
| Allergoids/polymerized allergens |
| Adjuvant constructs (SQ; IM) |
| DNA vaccines |
| TLR-9 (CpG oligonucleotides) (SQ) |
| Linked to allergen; co-combined |
| Nanoparticle-based VLPs |
| TLR-4 (MPL) (SQ) |
*Modified and used with permission; from: Creticos PS. Allergen immunotherapy: vaccine modification. Immunol Allergy Clin North Am. 2016;36:103-124.
CpG = cytosine phosphorylated to guanine; ID = intradermal; IM = intramuscular; MPL = monophosphoryl lipid A; SQ = subcutaneous; TLR = toll-like receptor; VLP = viral-like particles.
The laboratory production of allergens allows for modification of extracts and epitope structures that aim to enhance immunogenicity while decreasing the risk of adverse reactions. Clinical studies have reported outcomes for AIT using recombinant-produced molecules, synthetically-produced peptides, and modifications of allergens via allergoids with adjuvant molecules or through denaturing of proteins.
Recombinant allergens.
Recombinant-derived allergens are produced by cloning of native allergen proteins with use of recombinant DNA technology. The allergy protein is reverse transcribed to yield a complimentary DNA molecule which can then be transferred into bacteria which produce copies of the incorporated DNA. This technique allows for controlled production of a high-yield product with consistent structure. Immunotherapy trials with recombinant allergens has been reported for birch pollen and Timothy grass pollen (Table IX.D.2-2). Recombinant birch AIT demonstrated equivalent clinical outcomes to native birch extract and improved symptoms over placebo.1583-1585 Recombinant Timothy grass AIT showed improved outcomes compared to placebo with a good safety profile.805,1586 Recently, a recombinant peptide carrier fusion grass vaccine has reported positive outcomes with a B-cell epitope-based vaccine for immunotherapy of grass pollen allergy.798
TABLE IX.D.2-2.
Evidence for the use of recombinant, peptide, allergoid/polymerized, and adjuvant allergen immunotherapy
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Recombinant allergens | ||||||
| Zieglmayer et al.798 | 2016 | 1b | RDBPCT |
|
Total nasal symptoms scores, ocular symptoms, skin tests | Improvement in primary endpoint for 2 higher doses but not the lower dose. |
| Nony et al.1584 | 2015 | 1b | RDBPCT |
|
Symptom scores, medication scores | SLIT with rBet v 1 resulted in a significant decrease of symptom score and medication score vs placebo. |
| Meyer et al.1063 | 2013 | 1b | RDBPCT |
|
Symptom scores, change in IgG1 and IgG4 | All dosing regimens were more effective than placebo. |
| Klimek et al.1585 | 2012 | 1b | RDBPCT | Recombinant Timothy grass antigens (Phl p 1, Phl p 2, Phl p 5a, Phl p 5b, Phl p 6):
|
Primary: systemic allergic reactions; Secondary: Improvement in symptoms, conjunctival provocation test | Recombinant allergens safe and effective even at high protein levels. |
| Pauli et al.1583 | 2008 | 1b | RDBPCT |
|
Symptoms, immunologic markers | Recombinant allergens were safe and effective for 2 seasons. |
| Jutel et al.1586 | 2005 | 1b | RDBPCT |
|
Symptoms, medication use, RQLQ, immunologic markers, conjunctival provocation test | Recombinant allergens safe and effective over 2 grass seasons. |
| Klimek et al.805 | 2015 | 2b | Open RCT |
|
Symptom scores, IgG levels | Both were safe and equally efficacious over 2 seasons. |
| Peptide constructs | ||||||
| Spertini et al.1589 | 2016 | 1b | RDBPCT |
|
Combined rhinoconjunctivitis symptom and medication scores, QOL | Improved symptom, medication, and QOL scores in both treatment groups vs placebo. |
| Couroux et al.1599 | 2015 | 1b | RDBPCT |
|
Rhinoconjunctivitis symptom scores 2 years after start of treatment, symptom scores after challenge | Significant reduction in symptoms was observed in the 6 nmol dose group but not the other groups. |
| Patel et al.1065 | 2013 | 1b | DBPCT |
|
Total rhinoconjunctivitis score at 20 weeks and 52 weeks | Durable treatment effect at 1 year with best regimen 4 × 6 nmol at 4 weeks apart. |
| Purohit et al.1600 | 2008 | 1b | DBPCT |
|
Primary: symptom medication scores; Secondary: skin and nasal sensitivities, immunoglobulins, adverse reactions | No significant difference in symptom and medication scores between the groups. |
| Oldfield et al.1601 | 2002 | 1b | RCT |
|
Development of late respiratory reaction | Increase in late respiratory reaction with treatment. Tolerance may develop with continued treatment. |
| Maguire et al.1602 | 1999 | 1b | RCT |
|
Improvement in pulmonary function, adverse events | Improvement in pulmonary function. Increased incidence of late adverse reaction. |
| Norman et al.1603 | 1996 | 1b | RDBPCT |
|
Nose, lung, and symptom scores during live cat exposure | Dose response was observed at highest dose, resulting in the most significant decrease in lung and nasal symptoms upon cat exposure. |
| Litwin et al.1604 | 1991 | 2b | Placebo-controlled trial |
|
Symptom-medication scores | Subjects receiving the peptide fragment preparation had improved scores vs other groups. |
| Allergoids/polymerized allergens | ||||||
| Klimek et al.1605 | 2014 | 1b | DBPCT |
|
Combined symptom and medication score, rescue medication use, total rhinoconjunctivitis symptom score | Improvement in symptoms and medication usage compared to placebo. |
| Pfaar et al.1594 | 2013 | 1b | DBPCT |
|
Combined symptom and medication score | Significant reduction in median combined scores at year 2 compared to placebo. |
| Pfaar et al.1593 | 2012 | ib | DBRCT |
|
Combined symptom and medication score | Significantly improved combined scores in peak season at year 2 compared to placebo. |
| Corrigan et al.1606 | 2005 | 1b | DBPCT |
|
Combined symptom and medication score | Pre-seasonal grass pollen allergoid resulted in significantly improved symptom and medication score compared to placebo. |
| Bousquet et al.1607 | 1990 | 1b | RDBPCT |
|
Symptom and medication scores during pollen season | Significant reduction in symptom and medication scores for both treatment groups compared to placebo. |
| Bousquet et al.1608 | 1989 | 1b | RDBPCT |
|
Clinical symptoms: rhinitis, conjunctivitis, asthma | High molecular weight and pollen extract were most effective, followed by unfractionated allergoid. All better than placebo. |
| Grammer et al.1592 | 1983 | 1b | RDBPCT |
|
Blocking antibodies, daily symptom scores | Significant elevations in blocking antibodies and decrease in symptoms scores in treatment group. |
| Grammer et al.1591 | 1982 | 1b | DBPCT |
|
IgE and blocking antibodies, daily symptom scores | Significant elevations in blocking antibodies and decrease in symptoms scores in treatment group. |
| Pfaar et al.1609 | 2016 | 2b | RCT |
|
Clinical response to a titrated nasal provocation test | All doses above 20,000 AUeq/mL showed improved efficacy compared to placebo. |
| Norman et al.1590 | 1981 | 2b | Open trial |
|
Daily symptom and medication scores | Significant improvement of allergoid over allergen. |
| Adjuvant constructs | ||||||
| Patel et al.1066 | 2014 | 1b | RDBPCT |
|
Rhinoconjunctivitis symptoms after exposure in a chamber | Significant improvement in symptom scores in the treatment group. |
| Dubuske et al.1598 | 2011 | 1b | RCT |
|
Symptom and medication scores | Significant improvement in subjects with severe symptoms and long-standing symptoms with treatment. |
| Creticos et al.1596 | 2006 | 1b | RDBPCT |
|
Symptoms, immune changes, adverse reactions | Efficacious, benefits lasted for 2 more seasons. |
| Tulic et al.1610 | 2004 | 1b | RCT |
|
Primary: symptom and medication scores; Secondary: tissue markers of inflammation. | No difference in primary endpoint after 1 season, chest symptoms were better in the treatment group after the second season. |
| Drachenberg et al.1611 | 2001 | 1b | RDBPCT |
|
Symptom scores, medication scores, skin reactivity, IgG and IgE antibodies | Significant improvement in nasal, ocular, and combined symptom and medication scores in treatment group. |
| Senti et al.1612 | 2009 | 2b | Open trial | 10 weekly injections of dust mite with A-type CpG oligodeoxynucleotides with virus-like particles | Symptoms, conjunctival provocation, skin-prick tests, IgG and IgE levels | Significant reduction in symptoms, improved conjunctival tolerance, increase in IgG, and decreased skin reactivity. |
DBPCT = double blond placebo controlled trial; Ig = immunoglobulin; LOE = level of evidence; QOL = quality of life; RCT = randomized controlled trial; RDBPCT = randomized double blind placebo controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; TLR = toll-like receptor.
Aggregate Grade of Evidence for birch: B (Level 1b: 3 studies; Level 2b: 1 study).
Aggregate Grade of Evidence for Timothy grass: B (Level 1b: 3 studies).
These studies of recombinant allergens for birch and Timothy grass demonstrate safety and efficacy.
Peptide constructs. Synthetic peptides for immunotherapy are linear fragments of amino acids that correspond to T-cell epitopes. These fragments lack the secondary and tertiary structure that activate IgE receptors, but can induce immunologic tolerance by targeting allergen-specific T-cells to induce tolerance. The premise with synthetic peptides is that the lack of IgE activation will eliminate the risk of IgE-mediated adverse reaction while preserving the immunogenicity that leads to desensitization. AIT trials with synthetic peptides have been reported for cat, birch, and ragweed allergens (Table IX.D.2-2). Overall, studies have shown mixed outcomes from synthetic peptides with some peptide molecules resulting in an increase in late adverse reactions. The recently completed large-scale multicenter field trial (https://clinicaltrials.gov/ct2/show/NCT01620762; Phase III Cat-PAD Study) with cat peptide failed; however, as of this writing, the HDM peptide study is ongoing.1587,1588 Newer peptide constructs under investigation include overlapping peptides that reproduce the entire sequence of the naturally-occurring allergen in an attempt to cover all T-cell epitopes and natural peptide fragments that cover a broad panel of epitopes.1589
Aggregate Grade of Evidence for cat: B (Level 1b: 5 studies).
Aggregate Grade of Evidence for birch: Indeterminate, based on only 1 Level 1b study.
Aggregate Grade of Evidence for ragweed: B (Level 1b: 1 study; Level 2b: 1 study).
Allergoids and polymerized allergens. Allergoids are chemically modified allergens which were developed for improved immunotherapy protocols via accelerated dosing and decreased side effects. Initial attempts at development of an allergoid by partial denaturing of the allergenic moiety with formalin resulted in reduced allergenicity; however, concurrent reduction in the immunogenicity of the allergoids, as defined by IgG antibody production, was seen.1590 Studies using a glutaraldehyde-linked polymerization of allergens for grass and ragweed allergens demonstrated efficacy and tolerability.1591,1592 However, standardization criteria and production factors negatively impacted regulatory approval in the United States. Clinical trials for allergoids employing ragweed, grass, and HDM allergens have been reported. Promising early results are seen for these allergoids. In addition, more recent work has focused on depigmented allergoid constructs, which are currently in use in Europe1593,1594 (Table IX.D.2-2).
Aggregate Grade of Evidence for ragweed: B (Level 1b: 1 study; Level 2b: 1 study).
Aggregate Grade of Evidence for grass: B (Level 1b: 7 studies).
Aggregate Grade of Evidence for HDM: Indeterminate, based on only 1 Level 2b study.
Allergoid or polymerized allergen products have been approved in Europe but none has received FDA approval.
Adjuvant constructs.
The addition of molecules (adjuvants) to the native allergen has been attempted to improve desensitization protocols. Alum was the first adjuvant to gain acceptance in AIT. Early studies with alum-precipitated extracts demonstrated an augmented immunologic response. However, alum induced an initial IgE immune response which hindered its therapeutic application.1595 Clinical trials with adjuvants have been reports for ragweed, grass, and HDM allergens (Table IX.D.2-2).
Creticos reported the proof-of-concept study for using bacterial DNA (CpG oligonucleotide synthetically derived from Mycobacterium bovis) to upregulate an immunostimulatory response to allergen through the corresponding ligand (TLR ligand) on a specific class of regulatory dendritic cells.1596 The TLR-9 agonist was administered in a 2-year double-blind placebo-controlled study of ragweed-allergic subjects immunized with a 6-injection regimen administered prior to the initial ragweed season. A similar magnitude of effect vs placebo was observed over both ragweed seasons indicating that the vaccine conferred meaningful long-term efficacy (clinical and immune tolerance) over 2 ragweed seasons.1596 Subsequent large-scale multicenter trials were not able to satisfy regulatory approval requirements and this specific product is not going forward in development.1597 However, the field of adjuvant approaches to immunization is moving forward.
A TLR-4 adjuvant is also currently in clinical development. This construct is comprised of monophosphoryl lipid A, derived from detoxified lipopolysaccharide of gram-negative bacterium (Salmonella minnesota, a TLR-4 inducing adjuvant), and formulated with pollen allergoids absorbed onto microcrystalline tyrosine. This compound reduces IgE-mediated allergenicity but preserves immunogenicity. A large grass study showed significant improvement in symptom and medication scores vs placebo with subgroup analysis showing greater benefit in patients with more severe symptoms.1598 An abbreviated ragweed trial showed clinical effect in the primary endpoint vs placebo.1066
These studies of adjuvant-modified extracts demonstrate potential for improved immunotherapy protocols; however, several challenges remain. Each of the modified extracts requires robust clinical outcomes data to demonstrate short and long-term improvement in both efficacy and safety over conventional allergenic extracts.
Aggregate Grade of Evidence for ragweed: B (Level 1b: 3 studies).
Aggregate Grade of Evidence for grass: B (Level 1b: 2 studies).
Aggregate Grade of Evidence for HDM: Indeterminate, based on only 1 Level 2b study.
In summary, a wide variety of immunotherapeutic agents are currently undergoing clinical development with the goal of improving safety and achieving immune tolerance with long-lasting therapeutic efficacy. This new generation of vaccines includes recombinant allergens, peptide constructs, allergoids/polymerized allergens, and adjuvant constructs—each of which must undergo rigorous clinical evaluation to demonstrate acceptable safety and meaningful clinical outcomes that meet regulatory guidelines for approval. For some of the studied preparations, there appears to be improvement over placebo and comparable outcomes to native allergens. The TLR-9 agonist trial showed 2 years of efficacy post-discontinuation of drug. However, some peptide molecules demonstrated increased late reactions as well as mixed clinical outcomes depending on the preparation. Allergoids, adjuvants, and peptides have also shown efficacy in multiyear clinical trials. There is insufficient evidence to make recommendations based on the low number of studies for each preparation and lack of long-term outcomes, as no study has examined outcomes for longer than a 2-year period.
IX.D.3. Subcutaneous immunotherapy (SCIT)
AIT is a treatment for IgE-mediated sensitivity to environmental allergens.101,1613,1614 SCIT involves the injection of increasing doses of an extract of the allergen in question, followed by repeated injections of the top or maintenance dose for periods of 3 to 5 years, to reduce symptoms on exposure to that allergen. SCIT has been practiced for over a century using aqueous extracts of the naturally occurring allergens.1615 SCIT has been shown to be effective for AR, allergic asthma, and sensitivity to hymenoptera venom, along with demonstrated benefit in selected patients with AD. Although meta-analyses conclude that AIT is effective, this positive judgment of efficacy (and safety) should be limited to products tested in the clinical trials. It is incorrect to make a general assumption that “AIT is effective,” since this may lead to the clinical use of products that have not been properly studied.1614,1616 However, as currently practiced, SCIT has the drawbacks not only of the prolonged period of treatment and multiple visits to health care facilities but also the ever-present risk of systemic reactions. There are now attempts to overcome these limitations by modifying the native allergens or using recombinant technology to produce extracts that are less reactive with sIgE, allowing higher dosing with greater safety and shorter courses of treatment.1615 (See section IX.D.2. Management – Allergen immunotherapy (AIT) – Modified allergen extracts for additional information on this topic.)
Two U.S. healthcare agencies have recently commissioned systematic reviews of the medical literature on the use of AIT in AR1617,1618 (Table IX.D.3-1). The National Institute for Health Research commissioned an update of the 2007 Cochrane Review of AIT for SAR1617 and the Agency for Healthcare Research and Quality commissioned a systematic review of the use of SCIT and SLIT for the treatment of AR and bronchial asthma.1618 The first of these systematic reviews found highly significant differences in favor of SCIT over placebo for improvement of symptoms and medication use for treatment of AR, as well as for improvement in the rhinitis QOL, all with a p value of < 0.00001.1617 The second systematic review found high-quality evidence for SCIT, compared to placebo, improving rhinitis and rhinoconjunctivitis symptoms and QOL, with moderate quality of evidence for reduction in medication use for treating AR.1618 A third systematic review using the EBRR methodology found that SCIT for SAR and PAR has Aggregate Grade of Evidence A and recommended SCIT for SAR or PAR patients not responsive to medical therapy, whose symptoms significantly affect QOL.1619
TABLE IX.D.3-1.
Recent systematic reviews and selected RDBPCTs for the use of SCIT in allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lin et al.1618 | 2013 | 1a | Systematic review | Rhinoconjunctivitis and/or asthma, adults and children | Efficacy, effectiveness, safety. Symptoms, medication use, QOL. | Rhinitis or rhinoconjunctivitis:
|
| Meadows et al.1617 | 2013 | 1a | Systematic review | SAR, adults and children. | Clinical effectiveness, cost effectiveness. Symptoms, medication use, QOL. |
|
| Purkey et al.1619 | 2013 | 1a | Systematic review | SAR and PAR, adults and children, level 1b evidence, single-extract AIT | Symptoms, medication use, QOL | SCIT for SAR and PAR has Aggregate Grade of Evidence A. SCIT is recommended for SAR or PAR patients not responsive to medical therapy, whose symptoms significantly affect QOL. |
| Bozek et al.1622 | 2016 | 1b | RDBPCT | SAR (n = 55), age 65-75 years; Maintenance dose 26.3 μg Phl p 5 |
Combined symptom-medication score | Third-year combined symptom-medication score reduced 41% from baseline (p = 0.004) and 37% vs placebo. |
| Klimek et al.1605 | 2014 | 1b | RDBPCT | SAR (n = 102), age 18-75 years; Maintenance dose 24 μg Gp 1 plus Gp 5 |
Symptoms, medication use | Reduction in symptoms: 34% (p = 0.004). Reduction in medication use: 40% (p = 0.004). |
| Pfaar et al.1594 | 2013 | 1b | RDBPCT | SAR (n = 269), age 12-70 years. Maintenance dose Betv 1 6.75 μg and Phl p 5 15.75 μg |
Symptom-medication score | Symptom-medication score reduced for grass and birch pollen seasons: 1st year 21% (NS), 2nd year 19.4% (p = 0.0385). |
| Pfaar et al.1593 | 2012 | 1b | RDBPCT | SAR (n = 179), age 11-69 years. Maintenance dose 31.5 μg Phl p 5 |
Symptom-medication score | Symptom-medication score reduced: 1st year 16% (p < 0.01), 2nd year 37% (p < 0.01). |
| Rajakulasingam1621 | 2012 | 1b | RDBPCT | SAR (n = 37), ages 22-54 years. Maintenance dose 25.2 μg group 5 |
Symptom improvement from baseline year | Improvement from baseline year of ≥2/10 in symptoms: active 65%, placebo 35% (p = 0.024). |
LOE = level of evidence; NS = not significant; PAR = perennial allergic rhinitis; QOL = quality of life; RDBPCT = randomized double-blind placebo-controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; SCIT = subcutaneous immunotherapy; SMD = standardized mean difference.
A search of the EMBASE, MEDLINE, and Cochrane Library databases for systematic reviews and randomized controlled clinical trials yielded a recent otolaryngology clinical practice guideline for AR761 and an International Consensus on Allergy Immunotherapy1577,1620 as well as 5 double-blind, placebo-controlled trials of SCIT in AR that were published since the previously discussed systematic reviews (Table IX.D.3-1). All 5 of these trials were conducted with aldehyde-modified natural pollen extracts (allergoids).1593,1594,1605,1621,1622 These trials all support the efficacy of SCIT in treating AR.
Patient selection.
There are 3 therapeutic options for patients with AR: avoidance, pharmacotherapy, and immunotherapy. The evidence supporting avoidance is reviewed in section IX.A. Management – Allergen avoidance. Pharmacotherapy is discussed in section IX.B. Management – Pharmacotherapy. There are 2 primary reasons to consider AIT.101,1623 One is that addition of AIT to pharmacotherapy alone will likely result in a more pronounced decrease of symptoms (even after a short course of AIT). The second relates to the failure of pharmacotherapy to alter the underlying immunologic process. Patients may choose AIT largely to obtain a lasting benefit, prevent the progression of AR to bronchial asthma, or prevent new sensitizations.1624-1626
Contraindications for AIT.
The 2015 EAACI Position Paper noted contraindications for instituting SCIT for AR.1627 Absolute contraindications were poorly controlled or uncontrolled asthma, active autoimmune disorders, and malignant neoplasm. Relative contraindications were partially controlled asthma, autoimmune diseases in remission, cardiovascular disease, and use of beta-adrenergic blocking agents. The Allergy Immunotherapy: Practice Parameters 3rd Update, on the other hand, found no substantive evidence that immunotherapy is harmful in patients with autoimmune diseases.1623 The Practice Parameters also list pregnancy as a contraindication to initiating SCIT.1623 It may, however, be continued if the patient is on maintenance dosing.
Extracts.
In the United States, most pollen, dander, insect, and fungal extracts are available either in a buffered saline with phenol or in 50% glycerin. The exception is those extracts that have been standardized by the FDA which only come in 50% glycerin. There is 1 line of alum-precipitated extracts, consisting solely of pollen extracts. In Europe, on the other hand, alum-precipitated extracts are commonly employed and there is increasing use of allergoid extracts consisting of natural allergens partially denatured by mixture with an aldehyde.1593,1594,1605,1621,1622,1628 (See sections IX.D.1. Management – Immunotherapy – Allergen extract units, potency, and standardization and IX.D.2. Management – Immunotherapy – Modified allergen extracts for additional information on this topic.)
Dosing.
The beneficial results of SCIT have been repeatedly shown to be dependent on administering a sufficient maintenance dose of each extract with each maintenance injection.1609,1629-1631 Reduction of the effective maintenance dose by 90% to 95% causes partial or complete loss of efficacy.1632 The results of many double-blind, placebo-controlled studies have been utilized to formulate the recommendations for dosing in Table IX.D.3-2, adapted from the Immunotherapy Practice Parameters 3rd Update.1623
TABLE IX.D.3-2.
Recommended dosing for SCIT*
| Allergenic extract | Labeled potency or concentration | Probable effective dose range | Range of estimated major allergen content in U.S. licensed extracts |
|---|---|---|---|
| House dust mites: D. farinae and D. pteronyssinus | 3000, 5000, 10,000, 30,000 AU/mL | 500–2000 AU | 10,000 AU/mL; 20–160 μg/mL Der p 1, Der f 1; 2–180 μg/mL Der p 2, Der f 2 |
| Cat hair | 5000, 10,000 BAU/mL | 1000–4000 BAU | 10,000 BAU/mL; 20–50 μg/mL Fel d 1 |
| Grass, standardized | 100,000 BAU/mL | 1000–4000 BAU | 100,000 BAU/mL; 425–1100 Phl p 5 |
| Bermuda | 10,000 BAU/mL | 300–1500 BAU | 10,000 BAU/mL; 141–422 Cyn d 1 μg/mL |
| Short ragweed | 1:10 wt/vol, 1:20 wt/vol 100,000 AU/mL | 6–12 μg Amb a 1 or 1000–4000 AU | 1:10 wt/vol; 300 μg/mL Amb a 1 |
| Acetone precipitated (AP) dog | 1:100 wt/vol | 15 μg Can f 1 | 80–400 μg/mL Can f 1 |
| Nonstandardized dog extracts | 1:10wt/vol to 1:20 wt/vol | 15 μg Can f 1 | 0.5–10 μg/mL Can f 1 |
| Nonstandardized pollen extracts | 1:10 to 1:40 wt/vol or 10,000 to 40,000 PNU/mL | 0.5 of 1:100 or 1:200 wt/vol | Not available |
| Nonstandardized fungal, cockroach extracts | 1:10 to 1:40 wt/vol or 10,000 to 40,000 PNU/mL | Highest tolerated dose | Not available |
*Adapted from Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1-S55.1623
AU = allergy units; BAU = bioequivalent allergy units; PNU = protein nitrogen unit; SCIT = subcutaneous immunotherapy; wt/vol = weight by volume.
Monosensitization vs polysensitization.
In most large studies of AR, 80% to 85% of the subjects are sensitized to more than 1 unrelated allergen. Analysis of some of these studies has shown that the polysensitized subjects respond as well to (sublingual) AIT as those with sensitivity only to the administered allergen.1633 There is no immunological rationale why this should be different in subcutaneous AIT, but this specific question is an important unmet need which should be addressed in future trials.28,1634
Single-allergen vs multiple-allergen AIT.
It is the common practice among US allergists to include in their treatment multiple allergen extracts to which the patient is sensitized. A recent survey of 670 patients in 6 practices found a mean of 18 allergen extracts in their treatment.29,1635 On the other hand, European guidelines recommend treating with the single most troublesome allergen identified clinically,1636 or if more than 1 extract is to be given they should be given at separate sites with at least 30 minutes in between administration.32 Scientific support for the U.S. allergists’ approach of using multiple allergen mixtures for SCIT can be found in 4 double-blind, placebo controlled studies, 2 in patients with AR,1629,1637 1 in children with asthma,1630 and 1 in patients with both rhinitis and asthma,1638 all of which demonstrated significant improvement in patients receiving mixtures of multiple, unrelated allergen extracts. However, a recent review concluded that multiallergen immunotherapy in polysensitized patients, whether delivered sublingually or subcutaneously, requires more supporting evidence from well-designed, well-powered, double-blind, placebo-controlled clinical trials to validate its efficacy in practice.1634
Mixing.
If multiple-allergen mixtures are to be used for SCIT, there are several considerations, in addition to ensuring that each extract in the mixture is at a concentration that will provide an effective dose when delivered with the maintenance injection. These considerations are (1) avoiding mixing extracts with strong proteolytic activity with extracts whose allergens are susceptible to this activity; (2) paying attention to allergenic cross-reactivity; and (3) using preservatives that are appropriate for the allergens.1632
All fungal and some insect body extracts (but not U.S. HDM extracts) have strong proteolytic activity to which many pollen, mite, and animal dander allergens are susceptible.1639 Fungal and cockroach extracts should not be mixed, but fungal extracts can be combined.1640
Plant pollens contain some allergens that are like the allergens of unrelated plants (pan-allergens) but generally the major allergens are unique. When the appropriate allergens are available in the testing panel, the use of molecular diagnosis or CRD can be of great use in differentiating cross-reactivity due to pan-allergens from that due to multiple related major allergens. (See section VIII.F.6. Evaluation and diagnosis - In vitro testing - Component resolved diagnosis (CRD) for additional information on this topic.) When the patient is sensitized to the major allergens of botanically related plants there are 2 approaches that can be employed.1641 One approach is to only include the locally most important member of a related group (such as ragweed or northern pasture grasses); the other approach is to use a mixtures of related allergen extracts, but treating it as if it were 1 allergen.1641
Diluents.
Diluents containing 50% glycerin are excellent at maintaining extract potency and are used in the United States routinely for extracts with high protease activity.1639,1642 The drawback to using extracts with high glycerin content is that they cause pain when injected.1633 A phenol-saline extract containing 0.3% human serum albumin is well tolerated and, in the absence of high proteolytic activity, is an excellent diluent that may be used routinely for making dilutions for initiation of SCIT in the United States.1643
Regimens.
For reasons of safety, SCIT is initiated at a dilution of the final dose and built up usually with weekly injections of increasing amounts and concentrations over a period of weeks or even months. Once maintenance doses are achieved, the interval between injections can be increased but usually not beyond 4 weeks with aqueous extracts used in the United States,1623 but up to 4 to 6 weeks for depot extracts as used in Europe.1614
Venue for administering SCIT.
SCIT in allergy practices in the United States is associated with a rate of severe systemic reactions of 0.1%.1644 For this reason the Immunotherapy Practice Parameters 3rd Update state that injections should be given only in a medical facility where prompt recognition and treatment of anaphylaxis is assured and patients should remain under observation for at least 30 minutes following the injection.1623 This is in line with the European perspective.32 There is a company in the United States that promotes the practice of home administration of SCIT.1645 Their protocol calls for administration of relatively low doses of SCIT several times per week resulting in a cumulative dose that approaches that recommended in the Practice Parameters. However, there is evidence to suggest that it is the size of the individual dose rather than the cumulative amount administered that determines efficacy,1646 and no blinded studies have been offered to support the efficacy of this low-dose approach.
Accelerated SCIT administration.
To shorten the length of the buildup, cluster dosing is sometimes employed. Two or 3 injections are given on each visit on nonconsecutive days, with a 30-minute waiting between injections. If visits are twice weekly, maintenance dosing can be achieved in 4 weeks1647 or even after a shorter period depending on the product administered and schedule followed.1648 A retrospective analysis of rates of systemic reactions in a large, multiple-physician practice1649 and a double-blind randomized trial1650 showed no increase in the rate of systemic reactions in patients, comparing cluster to conventional regimens. Another (open) trial supports these findings.1651
Rush regimens administer many injections per day on consecutive days, typically achieving maintenance dosing in 1 to 3 days. Even with the use of premedication, there is an increased rate of systemic reactions compared to conventional dosing.1652
Mechanism of action.
In general, the immunologic response to SCIT involves 2 sequential steps. The first is a generation of regulatory T-cells secreting IL-10 and TGF-β, leading to a switch from IgE to IgG4 antibody formation.1653,1654 With continued AIT the Treg response declines and an immune deviation from Th2 to Th1 responses dominates.1577,1653 (See section IV. Pathophysiology and mechanisms of allergic rhinitis for additional information on this topic.)
Modification of disease.
An advantage of SCIT over pharmacotherapy is that it alters the underlying immunologic response towards that which is seen in non-allergic individuals.1654 The results of this alteration in the underlying immune response by SCIT can be seen clinically in the reduction in new sensitizations, in the progression from AR to asthma, and in the persisting benefit following an adequate course of therapy.
In children, adolescents, and young adults, who are sensitized only to the allergen being administered, the development of new sensitizations is reduced not only during AIT but for several years following completion of the course of AIT.1625,1626 A similar protective effect has not been demonstrated in patients polysensitized at the initiation of AIT.
SCIT has also been shown to prevent the progression from AR to asthma. A total of 205 children, sensitized to grass, birch or both, and showing no evidence of asthma during an observational year, were treated with Timothy and/or birch SCIT for 3 years, or standard pharmacotherapy alone, and observed for an additional 7 years after completion of SCIT in an open trial.1624 The risk for developing asthma was significantly reduced at the end of SCIT and persisted for the 7 years of follow-up. The database of the German National Health Insurance was used to follow patients with AR without asthma who were or were not placed on AIT in 2006.1655 During a 5-year follow-up, those patients who received AIT (90% on SCIT) were significantly less likely to have developed asthma.
Duration of treatment and persistence of treatment effect.
Regarding persistence of benefit, a double-blind, randomized study was conducted in patients with AR who had received 3 or 4 years of SCIT with Timothy grass extract.1656 Subjects were randomized to continue maintenance SCIT or receive placebo for 3 years. There was no difference in symptom/medication scores over the 3 grass pollen seasons between those receiving and not receiving Timothy extract injections. In another trial, grass SCIT was discontinued in 108 grass-sensitive patients who had responded well to the treatment after 3 or 4 years of SCIT.1657 The patients were followed through up to 4 grass pollen seasons looking for relapse. Approximately 30% relapsed by the third grass pollen season, with few more subsequently relapsing.
In the 2 studies discussed in the preceding paragraph,1656,1657 3 or 4 years of SCIT with grass extract induced remissions that persisted in most of the subjects for at least 3 years. There are only a few studies that look at longer or shorter periods of treatment. A study that compared 3 or 5 years of SCIT with HDM extract found significant improvement after 3 years but added clinical improvement in rhinitis after 5 years of SCIT.1658
Safety.
Information regarding the occurrence of fatal reactions to SCIT was obtained retrospectively by the Immunotherapy Committee of the AAAAI by periodic surveys of its members from 1985 to 20011659,1660 and by an online website since 2008.1644 The earlier retrospective surveys suggested that a fatal reaction occurs with every 2 to 2.5 million injection visits.1659,1660 The online survey elicited information on 2 fatal reactions in 28.9 million injection visits, which was thought to represent an improvement due to more careful monitoring of patients with asthma.1644 The rate of systemic reactions has remained steady, with 1.9% of patients experiencing a systemic reaction, most mild, but with 0.08% experiencing a grade 3 and 0.02% a grade 4 reaction.1644 The occurrence and size of local reactions do not predict the occurrence of a systemic reaction with the next injection.1661,1662
Cost effectiveness.
SCIT can be administered for 3 to 5 years with continuing relief of symptoms for years after discontinuation. Pharmacotherapy, on the other hand, must be continued indefinitely, since it has no disease-modifying activity. Because of this difference, the initial higher cost of SCIT may be offset by the continuing benefit after it is stopped. This factored into a decision-making analysis that suggested if a patient with SAR requiring nasal steroids 6 months per year is seen before age 41 years, the cost will be less in the long term if they are placed on SCIT.1662,1663 If the patient has perennial need for nasal steroids, and they are less than 60 years of age, the most cost effective approach is SCIT. Another cost-effectiveness analysis found that SCIT for SAR may be more effective and less expensive than pharmacotherapy from the societal perspective when costs of productivity loss are considered.1664 A retrospective study compared U.S. Medicaid-treated adults and children who were newly diagnosed with AR and were or were not placed on AIT. Eighteen-month follow-up revealed 30% and 42% healthcare cost savings, respectively, in the AIT treated patients.1665
Aggregate Grade of Evidence for SCIT in the treatment of AR: A (Level 1a: 3 recent studies listed; Level 1b: 5 recent studies listed; Table IX.D.3-1). Of note, due to the large body of literature supporting SCIT as a treatment for AR, only recent systematic reviews and select double-blind, placebo-controlled RCTs are included in Table IX.D.3-1, as these achieve an Aggregate Grade of Evidence of A.
Benefit: Improvement in symptoms and decreased need for rescue medication. Decreased likelihood of progression from AR to bronchial asthma. Persistent benefit for years after completion of 3 to 5 years of SCIT.
Harm: Inconvenience of multiple visits to a medical facility to receive injections. Potential for systemic reactions, including anaphylaxis.
Cost: Cost for preparation of allergen extract for treatment. Cost of visits to medical facilities to receive injections.
Benefits-Harm Assessment: Benefit greater than harm for patients who cannot obtain adequate relief with symptomatic treatment and whose symptoms extend more than a few weeks each year.
Value Judgments: Patients who can obtain adequate relief of symptoms with medication must decide if the short-term increased cost and inconvenience of SCIT is compensated for by the long-term persisting clinical benefit and relief from need to take medication. Pharmacoeconomic studies suggest that in the long term, SCIT is cost effective over symptomatic therapy.
Policy Level: Strong recommendation for SCIT in patients unable to obtain adequate relief with symptomatic therapy.
Intervention: SCIT should be recommended to the AR patient who cannot obtain adequate relief from symptomatic medication for significant periods of time each year and to those who would benefit from its secondary disease-modifying effects (prevention of bronchial asthma and new sensitization), particularly children and adolescents.
IX.D.4. Sublingual immunotherapy (SLIT)
SLIT is an alternative application variant of SCIT, which was first practiced over a century ago by Noon and others.1570,1666 The first double-blind placebo-controlled trial with SLIT was not conducted until 1986 by Scadding and Brostoff1667 in London, UK. After that, only several small trials were conducted until the beginning of the new millennium, when several “big trials” finally demonstrated the clinical efficacy and safety of SLIT. Since then, many high-quality SLIT trials have been reported. As a result, the actual evidence for SLIT appears to be at least as solid as that for SCIT. The literature on SLIT for AR/rhinoconjunctivitis is vast and several good meta-analyses and systematic reviews have been published over the past decade; the decision was made to primarily analyze results from these reviews and to complement them with findings from large randomized trials published during 2016 (Table IX.D.4-1).
TABLE IX.D.4-1.
Evidence for the use of SLIT in the treatment of allergic rhinitis—systematic reviews and meta-analyses from the last decade
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusiona |
|---|---|---|---|---|---|---|
| Di Bona et al.815 | 2015 | 1a | Meta-analysis of RCTs | SLIT grass pollen tablets vs placebo for SAR | Symptom and medication score | Small improvement in symptom and medication scores vs placebo: (SMD −0.28; 95% CI, −0.37 to −0.19; p < 0.001) and (SMD −0.24; 95% CI, −0.31 to −0.17; p < 0.001). Adverse events: 7/2259 SLIT patients were given epinephrine. |
| Leatherman et al.1692 | 2015 | 1a | Systematic review of RCTs for SLIT doses | SLIT for AR vs placebo | Doses of the effective vs doses of non-effective SLIT | Wide dose ranges between studies. For certain antigens, effective and non-effective dose ranges often overlap. For other allergens: insufficient data. |
| Devillier et al.1332 | 2014 | 1a | Meta-analysis of RCTs | Pollen SLIT vs pharmacotherapy vs placebo for SAR | Relative clinical impactb | Clinical impact: 5-grass pollen tablet > INCS > Timothy grass pollen tablet > montelukast > antihistamines |
| Makatsori et al.1693 | 2014 | 1a | Systematic review of RCTs | SLIT vs placebo | Drop-out rates in SLIT and placebo groups | No tendency for a skewed dropout ratio between SLIT and placebo groups. Confirms trial results are unbiased and SLIT appears to be safe. |
| Lin et al.1694 | 2013 | 1a | Systematic review of RCTs | Aqueous SLIT vs placebo for SAR (and asthma) | Symptom and medication scores | Moderate evidence aqueous SLIT reduces symptoms and medication use in AR/ARC. |
| Meadows et al.1617 | 2013 | 1a | Meta-analysis of RDBPCTs, cost analysis | SCIT and SLIT vs placebo for SAR | Several efficacy variables, costs | Symptom reduction with SCIT and SLIT is greater than placebo. |
| Di Bona et al.1696 | 2011 | 1a | Meta-analysis of RDBPCTs | Grass pollen SLIT vs placebo for SAR (and asthma) | Symptom and medication scores | SLIT vs placebo: Reduction in symptoms (SMD −0.32) and medication use (SMD −0.33). No epinephrine use. |
| Radulovic et al.1695 | 2011 | 1a | Meta-analysis of RDBPCTs | SLIT vs placebo for AR | Symptom and medication scores | SLIT vs placebo: Reduction in symptoms (SMD −0.49) and medication use (SMD −0.32). No epinephrine use. |
| Durham et al.1673 | 2016 | 1b | Pooled analysis from RCTs | SAR: grass or ragweed SLIT tablet vs pharmacotherapy. PAR: HDM SLIT tablet vs pharmacotherapy. |
Total Nasal Symptom Score | SAR: SLIT numerically greater than montelukast and antihistamine; almost equal to mometasone furoate INCS. PAR: SLIT effect numerically greater than all pharmacotherapy. |
| Maloney et al.1675 | 2015 | 1b | Pooled analysis from RCTs | Grass SLIT tablet vs placebo. Grass SLIT in AR patients with (24%) and without (76%) mild asthma. | Treatment related AE frequency | Severe asthma-related adverse events due to treatment in 6/120 SLIT and 2/60 placebo. No difference between the 2 groups. Both adults and children were included. |
| Creticos et al.1676 | 2016 | 2a | Systematic review | Patients treated with SLIT, started in-season, vs out-of-season vs placebo | Serious treatment-related AE, systemic AE discontinuations | 11 SLIT trials (n = 2668 subjects total). No epinephrine administration. 0% to 4% systemic AE with in-season vs 0% out-season initiation. 2 serious treatment-related AE with co-season SLIT initiation. |
| Oykhman et al.1677 | 2015 | 3a | Systematic review of cohort studies | Pregnant women with vs without SLIT or SCIT and their offspring. 422 pregnancies continuing AIT and 31 starting AIT. | Pregnancy outcome, allergy in offspring | No difference in prematurity, proteinuria, hypertension, congenital malformations, perinatal death. No fetal complications of 10/453 systemic reactions to SCIT. No altered risk of developing atopic disease in offspring. |
| SLIT or SCIT: children only | ||||||
| Larenas-Linnemann et al.1671 | 2013 | 2a | Systematic review of RCTs | Children with AR and/or asthma treated with SLIT vs placebo/open controls | Symptom and medication scores | Strong evidence that grass pollen SLIT in children reduces symptoms of AR. Moderate-low evidence for HDM SLIT. |
| Roder et al.1670 | 2008 | 2a | Systematic review of RCTs | Children 0–18 years with AR: any form of AIT vs placebo | Symptom and medication scores | Insufficient evidence that AIT in any form has a positive effect on AR in children. |
| SLIT vs SCIT | ||||||
| Chelladurai et al.1697 | 2013 | 1a | Systematic review of RCT | SCIT vs SLIT (and vs placebo) in AR | Symptom and medication scores | Low grade evidence favors SCIT over SLIT for AR symptom and medication reduction. Moderate evidence for nasal and eye symptom reduction. |
| Di Bona et al.1698 | 2012 | 1a | Meta-analysis based comparison | Grass pollen SCIT; placebo vs grass pollen SLIT; placebo in SAR | SMD of symptom and medication scores | SCIT more effective than SLIT (drops) and SLIT (tablet) for symptom and medication score reduction. |
| Nelson et al.1699 | 2015 | 1b | Network meta-analysis of RCTs | Grass pollen SLIT tablets vs placebo. Grass pollen SLIT drops vs placebo. Grass pollen SCIT vs placebo. | Symptom and medication scores | Symptom and medication scores with SCIT, SLIT tablets and drops all reduced vs. placebo, except for symptom score with SLIT drops. |
| Aasbjerg et al.1700 | 2015 | 2a | Systematic review of RCTs, product information, registry | AR patients receiving Phleum pratense SCIT, SLIT drops, or SLIT tablets vs placebo. (including 314 children.) | Safety data | Many products without structured collection of safety data. General safety assessment: SLIT safer than SCIT. |
| Dranitsaris and Ellis1701 | 2014 | 2a | Systematic review of RCTs and indirect comparison | Timothy grass tablet, 5-grass tablet, grass pollen SCIT vs placebo in SAR | Efficacy, safety, cost for Canadian setting | Symptoms: all IT treatments better than placebo. Costs for 5-grass tablet greater than costs for Timothy grass tablet and SCIT. |
| Calderon et al.1702 | 2013 | 2a | Systematic review of RCTs | Patients allergic to HDM, with AR and asthma, treated with HDM SCIT vs SLIT vs placebo | Symptom score, IT schedule, dosing | Improved symptom score vs placebo was observed more frequently for SCIT. Data is weak as the basic treatment parameters vary widely. |
| Dretzke et al.1703 | 2013 | 2a | Systematic review of RCT and indirect comparison | SCIT and aqueous SLIT vs placebo, SCIT vs SLIT in AR | Symptom and medication scores | Trend favoring SCIT over SLIT for AR symptom and medication score reduction. No conclusive results. |
| SLIT vs SCIT: children only | ||||||
| Kim et al.1672 | 2013 | 2a | Systematic review of RCTs and indirect comparison | Children with SAR (asthma): Aqueous SLIT vs SCIT vs placebo for SAR (and asthma) | Symptom and medication scores | In children, moderate evidence that SLIT improves AR symptoms and medication use, low evidence that SCIT is superior to SLIT for both outcomes. |
| Hoeks et al.1704 | 2008 | 2a | Systematic review of RCTs | SLIT vs placebo in children with asthma/ARC | Symptom and medication scores | Not enough evidence because of poor quality of the studies. |
Only outcomes with statistically significance are mentioned here.
Clinical impact score = season-long nasal or total symptom scores: 100 × (scorePlacebo – scoreActive)/scorePlacebo.
AE = adverse event; AIT = allergen immunotherapy; AR = allergic rhinitis; ARC = allergic rhinoconjunctivitis; CI = confidence interval; HDM = house dust mite; INCS = intranasal corticosteroid; LOE = level of evidence; PAR = perennial allergic rhinitis; RCT = randomized controlled trial; RDBPCT = randomized double-blind placebo-controlled trial; SAR = seasonal allergic rhinitis; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; SMD = standardized mean difference.
Efficacy in adults.
Most systematic reviews and meta-analyses show a low to moderate efficacy of SLIT over placebo (SMD = 0.30 to 0.50), and this approaches high efficacy with longer treatment1668 (greater than 12 months’ treatment SMD = 0.70). It must be considered that all patients, both those in the SLIT and the placebo arms, have open access to rescue medication, and that SLIT results in an efficacy on top of the symptom improvement obtained with rescue medication.
Efficacy in children.
Over 5 years ago, Dutch colleagues analyzed systematic reviews of SLIT in children and concluded that the methodological quality should be improved. They especially questioned the heterogeneity of the included trials and the risk of bias.1669 Roder et al.1670 also determined in 2008 that there was not enough evidence to support the usefulness of SLIT in children. These flaws have been improved in recent studies. There is strong1671 evidence that grass pollen SLIT tablets in children reduce symptoms of AR. The evidence for aqueous SLIT is moderate.1672 The evidence for HDM SLIT is of moderate-to-low quality.
Efficacy of SLIT over pharmacotherapy.
For PAR, SLIT with HDM tablets is more effective than any single pharmacotherapy, including antihistamines, antileukotrienes and INCS.1673 For SAR, grass and ragweed tablet SLIT is almost as effective as INCS and more effective than the other pharmacotherapies.1673 These data had already been confirmed for the SLIT grass pollen tablets by a previous meta-analysis; in this publication the separate analysis of the 5-grass tablet showed its superiority over all pharmacotherapy treatments.1332
Efficacy of SLIT compared to SCIT.
Several investigators have tried to compare the efficacy of SLIT against that of SCIT. Most meta-analyses are based on indirect comparisons, as there are only a very few direct head-to-head randomized trials comparing both treatments; therefore, the evidence that SCIT is more effective than SLIT is weak. Also in children, SCIT seems more effective than SLIT, but again the quality of evidence is low.1672
Safety.
Rare systemic and serious adverse events have been reported with SLIT, but in general, meta-analyses found SLIT to be safer than SCIT. In the complete data-set of systemic reviews there were 7 reports of the use of epinephrine in the SLIT group and 1 case of eosinophilic esophagitis with a grass pollen SLIT tablet. There was no administration of epinephrine in trials outside of the United States. A 2012 review by Calderon et al.1674 estimated the anaphylaxis rate of SLIT to be 1 per 100 million doses, or 1 per 526,000 treatment years. Grass pollen SLIT tablets are just as safe in AR patients with and without mild asthma.1675 Starting SLIT in-season appeared to be safe. Although there were 2 serious treatment-related adverse events with co-seasonal SLIT initiation, none required epinephrine administration.1676 In the United States, the FDA requires patients be prescribed an epinephrine autoinjector and the first dose be given in the physician’s office for those on SLIT tablets. Continuing AIT during pregnancy did not augment the incidence of adverse outcomes during delivery nor alter the risk of developing atopic disease in the offspring. No conclusion can be drawn regarding the safety of starting SLIT in a pregnant woman, due to lack of cases.1677
Preventative effects.
There are no systematic reviews specifically addressing the preventative effects of SLIT that fall within the allowable search date range of this ICAR:AR document. The preventative effect SLIT on asthma development was investigated in an open RCT by Marogna et al.1678 involving 216 children treated with SLIT for 3 years. Mild persistent asthma was less common in patient treated with SLIT than patients receiving only pharmacotherapy. In a double-blind RCT involving 812 children with grass pollen-induced rhinoconjunctivitis, after 3 years of therapy with SQ-standardized grass pollen tablet, children in the treatment group presented a reduced risk of developing asthma compared to placebo group at 2-year follow-up (OR 0.71; p < 0.05).1679 Although these findings are interesting, the overall strength of evidence for the prevention of asthma in SLIT studies is low at present, though the evidence for asthma symptom and medication reduction is high.
Developing new allergen sensitizations frequently occurs in the natural history of respiratory allergy. Preventative effects of AIT on the onset of new sensitizations is often discussed. However, currently available SLIT data for prevention of new allergen sensitivities is also limited. The above referenced Marogna et al.1678 study did note that the rate of new sensitizations was low, corresponding to 3.1% of SLIT-treated patients and to 34.8% of controls, with an OR of 16.85 to develop new sensitizations in controls. Another study by Marogna et al.1680 prospectively evaluated the long-term effect of SLIT given for 3, 4, or 5 years in 78 SLIT patients vs 12 controls. Over a 15-year follow-up, all the control subjects developed new allergen sensitivities, while this occurred in less than 25% of the patients receiving SLIT (21% in treated for 3 years, 12%, in treated for 4 years, and 11% in treated for 5 years, respectively).
Cost-effectiveness.
The meta-analysis comparing the efficacy and cost-savings of the 5-grass SLIT tablet vs the Timothy grass SLIT tablet has several flaws, as some trials were reported in several publications and thus these publications should be analyzed as one. More importantly, the outcome variables and the precise definition of the pollen season vary between the Timothy grass SLIT tablet and the 5-grass SLIT tablet trials, so direct comparison of outcomes should not be done, as was reviewed in detail previously.1681,1682 The 5-grass SLIT tablet ($1003 Canadian dollar) was associated with cost savings against year-round SCIT (+$2471), seasonal SCIT (+$948), and the Timothy grass SLIT tablet (+$1168) during the first year of therapy and still during the second and third year of treatment. The higher costs for SCIT were due to the elevated indirect costs from missing working hours and transportation costs due to in-office SCIT administration. The higher costs for the Timothy grass SLIT tablet were due to the year-round dosing vs the preseasonal/co-seasonal 6-month total dosing of 5-grass SLIT tablet.
A UK meta-analysis of costs showed that SCIT and SLIT may be cost-effective compared with standard pharmacotherapy for 6 years (when considering a threshold of pound 20,000-30,000 per quality-adjusted life-year [QALY]). The investigators were not able to establish a clear difference between SCIT and SLIT in cost-effectiveness.1617
Additional data from double-blind placebo-controlled trials.
Some of the most important recent trials with data that add to the already presented systematic reviews are listed here:
High-dose tree pollen aqueous SLIT was effective in reducing symptom-medication scores in children in a high-quality double-blind placebo-controlled trial.1683
Double-blind, placebo-controlled trials with ragweed SLIT reduced the combined symptom-medication score when administered as drops1684,1685 and as tablets, particularly at the high dose.1686,1687
In a small, double-blind, placebo-controlled trial of moderate-high quality, Alternaria SLIT for AR (and asthma) was shown to be effective in significantly reducing the AR combined symptom-medication score.1688
As for the SLIT HDM tablets, a dose-effect for a reduction in AR symptoms-medication scores has been shown in 3 double-blind, placebo-controlled trials.1064,1689 One trial demonstrated a significant difference and a symptom score reduction of 29% only in those patients with more moderate-severe disease.799
Moderate evidence for efficacy of dual grass pollen-HDM SLIT after 12 months of treatment and 1 year after discontinuation.1690
Multi-allergen SLIT has been tested in a single-center, double-blind, placebo-controlled trial with Timothy grass monotherapy, Timothy grass plus 9 other pollen allergens, or placebo. Only the Timothy grass monotherapy group showed statistically significant improvement in the nasal challenge test, titrated SPT, sIgE (reduction), and IgG4 (increase). Due to a very low pollen season, there were no differences in symptom-medication scores between any of the groups.1691 Additional study on multi-allergen SLIT is needed.
Aggregate grade of evidence and recommendations.
In Table IX.D.4-2 the grade of evidence is shown and how this leads to recommendations in the decision-making concerning SLIT.
TABLE IX.D.4-2.
Aggregate grades of evidence for specific SLIT issues
| Issue | Aggregate grade of evidence |
Direction of impact |
Magnitude of impacta | Recommendation, considering: harm and cost |
| SLIT is effective for AR symptom reduction in adults | A | Yes | Low impact | Strong recommendation |
| LOE: Lin 1a; Radulovic 1a; Di Bona (2 studies) 1a; Nelson 1b; Calderon 2a. | ||||
| SLIT is effective for AR symptom reduction in children | B | Yes | Low impact | Recommendation |
| LOE: Kim 2a; Larenas-Linnemann 2a. Not enough evidence: Roder 2a. | ||||
| SLIT is safe for the treatment of AR in adults | A | Yes | —— | Safety profile is very good |
| Many of the systematic reviews (1a and 2a) included safety evaluation. Makatsori 1a: same dropout rates SLIT vs placebo. | ||||
| SLIT is safe for the treatment of AR in children | B | Yes | —— | Safety profile is very good |
| The systematic reviews (Kim, Larenas-Linnemann, Roder: all 2a) included safety evaluation. Makatsori 1a: same dropout rates SLIT vs placebo. | ||||
| SCIT is more effective than SLIT | A | Yes | Weak evidence | Recommendation |
| LOE: Chelladurai 1a; Dretzke 2a; Calderon 2a; Kim 2a. Grass pollen tablets/drops vs SCIT: Di Bona 2012 1a; SCIT = grass pollen tablets only, drops slightly less effective Nelson 1b. | ||||
| SLIT is safer than SCIT | B | Yes | Weak evidence | Recommendation |
| LOE: Aasbjerg 2a | ||||
| The total cost of SLIT is less than SCIT | A | Yes | Moderate evidence | Recommendation |
| LOE: Meadows 1a (UK setting); Dranitsaris 2a (Canadian setting) | ||||
| It is safe to continue SLIT during pregnancy | B | No added risk. | Moderate evidence | Recommendation |
| LOE: Oykman 3a | ||||
| It is safe to start SLIT during the season | B | Slightly added risk. | Moderate evidence | Option |
| LOE: Creticos 2a | ||||
| Tablet SLIT is more effective than pharmacotherapy. Exception in SAR: INCS are as efficacious as tablet SLIT. | A | Yes | Moderate: antihistamine, montelukast. Weak: INCS | Recommendation |
| LOE: Devillier 1a (pollen tablet SLIT); Durham 1b (grass pollen or ragweed tablet SLIT). | ||||
| SLIT is cost-effective in the 1st year | B | No | Moderate evidence | Option (considering its long-term benefit) |
| LOE: Meadows 1a; Dranitsaris 2a | ||||
| SLIT is cost-effective after several years of treatment | B | Yes | Weak-moderate | Recommendation |
| LOE: Meadows 1a; Dranitsaris 2a | ||||
| SLIT has a long-term effect beyond 3-years’ application | B | Yes | Moderate evidence | Recommendation |
| LOE: Durham 20121705 2b, Didier 20151706 2b | ||||
| SLIT with grass-pollen is effective for SAR | A | Yes | Low impact | Strong recommendationb |
| LOE: Di Bona (2 studies) 1a; Nelson 1b; Durham 1b. | ||||
| SLIT with tree-pollen is effective for SAR | A | Yes | Moderate effect | Strong recommendationb |
| LEO: Valovirta 20061683 1b | ||||
| SLIT with ragweed-pollen is effective for SAR | A | Yes | Moderate effect | Strong recommendationb |
| LOE: Durham 2016, Nolte 2013, Creticos 2013, 1b (tablet ragweed); Creticos 2014 (drop ragweed); Skoner 2010 (drop ragweed) 1b | ||||
| SLIT with HDM is effective for AR | A | Yes | Low impact | Strong recommendationb |
| LOE: Nolte 2015, Bergmann 2014, Mosbech 2015 all 1b; Calderon 2a | ||||
| SLIT with epithelia is effective for AR | — | No data | No data | Option |
| No separate data in the systematic reviews/meta-analyses; no recent trials | ||||
| SLIT with fungi is effective for AR | B | Yes | Weak evidence | Option |
| No separate data in the systematic reviews/meta-analyses. Cortellini 20101688 1b | ||||
For those variables with meta-analysis: according to Cohen’s classification: low impact SMD 0.2-0.5, moderate 0.5-0.8, high above 0.8. For those with only systematic review: strength of evidence.
Considering the added long-term posttreatment effect and the possible preventive effects on the development of asthma and new sensitizations.
AR = allergic rhinitis; INCS = intranasal corticosteroids; LOE = level of evidence; SAR = seasonal allergic rhinitis; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy.
Aggregate Grade of Evidence: A (Level 1a: 10 studies; Level 1b: 3 studies; Level 2a: 11 studies; Level 3a: 1 study; Table IX.D.4-1).
Benefit: SLIT improved patient symptom scores, even as add-on treatment on top of rescue medication. SLIT reduced medication use. The effect of SLIT lasts for at least 2 years after a 3-year course of high-dose therapy. Benefit is generally higher than with single-drug pharmacotherapy; however, it is possibly somewhat less than with SCIT. Although a very recent high-quality head-to-head trial did not show a statistically significant difference in efficacy between SCIT and SLIT, this evidence is not presented here, as the publication date is outside the review period for this manuscript.797
Harm: Minimal harm with very frequent, but mild, local adverse events. Very rare systemic adverse events. SLIT seems to be safer than SCIT.
Cost: Intermediate, SLIT becomes cost-effective compared to pharmacotherapy after several years of administration. Data on cost of SLIT compared to SCIT is variable.
Benefits-Harm Assessment: Benefit of treatment over placebo is small, but tangible. SLIT benefit is demonstrated beyond the improvement seen with rescue medications. Lasting effect at least 2 years off treatment. Minimal harm with SLIT, greater risk for SCIT.
Value Judgments: SLIT improved patient symptoms with low risk for adverse events.
Policy Level:
∘ Use of SLIT: grass pollen tablet, ragweed tablet, HDM tablet, tree pollen aqueous solution - Strong recommendation.
∘ Alternaria SLIT - Recommendation.
∘ Epithelia SLIT - Option.
∘ Dual SLIT in biallergic patients - Recommendation.
Intervention: We recommend high-dose tablet or aqueous SLIT be administered in patients (adults and children) with SAR and/or PAR who wish to reduce their symptoms and their medication use. SLIT can be continued safely in the pregnant patient.
IX.D.5. Transcutaneous/epicutaneous immunotherapy
Transcutaneous or epicutaneous immunotherapy is a noninvasive form of AIT that consists of the application of allergens to the skin. The epidermis is rich in APCs while being less vascularized potentially reducing the risk for systemic reaction.1707,1708 To improve delivery of antigens through the stratum corneum to the immune cells of the epidermis and dermis, different techniques have been used: scarification or scratching of the skin, tape stripping, microneedle arrays, and sweat accumulation through the application of a patch.1709 Epicutaneous immunotherapy has recently been investigated in a mouse model using nanoparticles containing an allergen encoding DNA.1710 Records of allergen administration via the skin date back to 1926, where 29 patients with hay fever received intradermal pollen extract administrations; all benefited after only 3 doses without significant side effects.1711 The first RCT was in 2009. To date, 4 clinical trials using this procedure have been published (Table IX.D.5)
TABLE IX.D.5.
Evidence for the use of transcutaneous/epicutaneous immunotherapy in the treatment of allergic rhinitis
| Study | Year | LOE | Study design |
Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Senti et al.1715 | 2015 | 1b | RDBPCT | Adults:
|
Subjective symptoms, conjunctival provocation test | Symptom score improved in the treatment arm in year 1, but was not significantly different from control in year 2. Conjunctival provocation improved in the treatment group. Systemic reactions occurred in 7 treatment (14.6%) and 1 control patients. |
| Senti et al.1714 | 2012 | 1b | RDBPCT | Adults:
|
Subjective symptoms, medication use, SPT, conjunctival provocation test | Symptoms improved only in the highest dose group. There was no difference in medication use, SPT, or conjunctival provocation test. Local reactions were common. Systemic reactions occurred in 8.3% of patients. |
| Agostinis et al.1713 | 2009 | 1b | RDBPCT | Children:
|
SPT endpoint, subjective symptoms, antihistamine use | No difference in SPT endpoint. Treatment group had less rhinoconjunctivitis symptoms and antihistamine use. |
| Senti et al.1712 | 2009 | 1b | RDBPCT | Adults:
|
Nasal provocation test, subjective symptom score | No significant difference in nasal provocation test. Subjective symptoms score improved. More local reactions (eczema) in treatment group. |
LOE = level of evidence; RDBPCT = randomized double-blind placebo-controlled trial; SPT = skin-prick test.
In a single-center, placebo-controlled, double-blind trial, 37 adults with positive SPT and nasal challenge to grass pollen were randomized to treatment with allergen (n = 21) or placebo patches (n = 16).1712 Treatment was started 1 month before the 2006 pollen season. The skin was tape-stripped 6 times; patches were applied weekly for 12 weeks, and removed 48 hours later. Patients were assessed before, at the beginning of, and after the 2006 pollen season, and followed up before (n = 26) and after (n = 30) the pollen season of 2007. The primary outcome was nasal provocation test with grass extract; secondary outcomes included a rhinitis questionnaire, medication use, and adverse events. In grass immunotherapy-treated patients, nasal challenge test scores significantly decreased in the first (p < 0.001) and second year (p = 0.003). In placebo-treated patients, scores decreased after year 1 (p = 0.03), but the effect diminished in year 2 (p = 0.53). However, the improvement of nasal provocation test scores was not significantly better in the treatment vs placebo groups. Patients in the treatment arm had improvement in subjective symptom scores, both after the pollen seasons of 2006 (p = 0.02) and 2007 (p = 0.005). Eczema at the application site was significantly higher in the treatment arm, and there were no serious adverse events.
A second single-center, double-blind RCT treated 15 children with grass transcutaneous immunotherapy and 15 children with placebo.1713 The adhesive patch was placed weekly from February to April 2008, and removed after 24 hours. There were no significant differences in prick tests between groups before and after treatment. Both groups had an increase in symptoms, but the treatment group had lower rhinorrhea, nasal obstruction, dyspnea, and ocular tearing. The treatment group had a significant reduction in antihistamine use (p = 0.019). There were no systemic or local reactions.
A third single-center, double-blind, placebo-controlled trial, published by the same authors enrolled 132 adults with grass pollen allergic rhinoconjunctivitis.1714 Patients received placebo, low-dose, medium-dose, and high-dose grass extract treatment (n = 33 in each arm). Weekly for 6 weeks, starting 1 month prior to the initiation of the 2008 pollen season, patches were applied with subsequent removal after 8 hours. SPT and conjunctival provocation tests were done at baseline, and after the pollen seasons of 2008 and 2009. Ninety-three of 132 patients were included in the efficacy analysis. The primary endpoint was subjective rhinoconjunctivitis symptoms using a VAS. Five months after application of the first patch, all treatment and placebo groups improved. One year later, only the high-dose treatment group had improved compared to control (p = 0.017); symptoms were reduced by more than 30% (2008 pollen season) and 24% (2009 pollen season) compared with placebo. There were no differences in rescue medication use, SPTs, or CPTs. Local reactions were more frequent with higher doses and improved with subsequent applications. Systemic reactions leading to discontinuation of treatment occurred in 11 patients (8.3%) within 45 minutes of patch application; reactions were milder (grade 1 to 2) and did not require treatment with epinephrine.
A fourth single-center, double-blind, placebo-controlled trial, published by the same authors enrolled 98 adults with grass allergic rhinoconjunctivitis; 48 received grass patches and 50 received placebo.1715 Treatment consisted of 6 weekly patches kept on for 8 hours. After treatment in the year 2009, median rhinitis symptoms improved by 48% in the treatment group vs 10% in the placebo group (p = 0.003); a year later, this was 40% compared to 18% for placebo (p = 0.43). There was no change in combined symptom and medication scores. CPT scores improved after the first year in the treatment group but not the placebo group. In the first year, allergen-specific IgG4 increased in the treatment group, while allergen-specific IgE decreased in the placebo group; there was no difference in both measures compared to baseline in the second year. Eight systemic reactions led to study exclusion. The authors concluded that this treatment strategy may have a potential role in treating IgE-mediated allergies, but further research was needed to find an optimal regimen that balances efficacy and safety.
Aggregate Grade of Evidence: B (Level 1b: 4 studies; Table IX.D.5).
Benefit: Transcutaneous immunotherapy resulted in limited and variable improvement in symptoms, medication use, and allergen provocation tests in patients with AR or conjunctivitis.
Harm: Transcutaneous immunotherapy resulted in systemic and local reactions. Systemic reactions occurred in up to 14.6% of patients receiving grass transcutaneous immunotherapy.
Cost: Unknown.
Benefits-Harm Assessment: There is limited and inconsistent data on benefit of the treatment, while there is a concerning rate of adverse effects. Three out of 4 studies on this topic were published by the same investigators from 2009 to 2015.
Value Judgments: Transcutaneous immunotherapy could offer a potential alternative to SCIT and SLIT, but further research is needed.
Policy Level: Recommend against.
Intervention: While transcutaneous immunotherapy may potentially have a future clinical application in the treatment of AR, at this juncture there are limited studies that show variable and limited effectiveness, and a significant rate of adverse reactions. Given the above and the availability of alternative treatments, transcutaneous immunotherapy is not recommended presently.
IX.D.6. Intralymphatic immunotherapy (ILIT)
Intralymphatic immunotherapy (ILIT) is a novel method for AIT, where allergen is injected directly into lymph nodes.1716 The major advantages of this route of allergen application are the markedly reduced duration of immunotherapy treatment (both time spent and number of visits) and the much lower amount of allergen required to achieve results. This lower dose of allergen also confers a lower risk of adverse allergic side effects.
Clinical trials have illustrated that a reduction in AR symptoms can be achieved with just 3 doses of injected allergen, with a dosage interval of 1 month1716-1720 (Table IX.D.6). This contrasts with subcutaneous application, where up to 70 doses may be needed over a 5-year period. ILIT involves the injection of allergen directly into inguinal lymph nodes under ultrasound guidance.
TABLE IX.D.6.
Evidence for the use of intralymphatic immunotherapy in the treatment of allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Hylander et al.1719 | 2016 | 1b | RCT, blinded | Birch-pollen-induced or grass-pollen-induced AR (n = 36):
|
Seasonal allergic symptoms by VAS, safety of injections, nasal symptom score following nasal provocation test, IgE and IgG4 levels, inflammatory cells, rescue medication use | ILIT is effective and safe; results in a marked reduction of seasonal allergic symptoms. |
| Patterson et al.1720 | 2016 | 1b | RCT, blinded | Adolescents, grass-pollen-induced AR (n = 15):
|
Patient diary score of allergy and asthma symptoms and medication use, local and systemic symptoms score after injections | ILIT is effective and safe, with notably low adverse reactions. |
| Hylander et al.1718 | 2013 | 1b | Pilot study and RCT, blinded | Birch-pollen/grass-pollen-induced AR (pilot n = 6; RCT n = 15):
|
Seasonal allergic symptoms by VAS, SPT, validated rhinitis QOL questionnaire | ILIT is effective and safe. |
| Witten et al.1721 | 2013 | 1b | RCT, blinded | Grass pollen-induced AR (n = 45):
|
Combined symptom and medication score, global seasonal assessment, RQLQ | ILIT produced immunological changes but no improvement in symptoms. |
| Senti et al.1717 | 2012 | 1b | RCT, blinded | Cat-dander-induced AR (n = 20):
|
Immunological parameters, systemic adverse effects, nasal provocation test, SPT, validated rhinitis QOL questionnaire | ILIT with MAT-Fel d 1 (Recombinant major cat dander allergen fused to a modular antigen transporter) was safe and induced allergen tolerance after 3 injections. |
| Senti et al.1716 | 2008 | 2b | RCT, open | Grass pollen-induced AR (n = 165):
|
Seasonal allergic symptoms by VAS, adverse events, safety of injections, rescue medication use, SPT, grass-specific IgE levels | ILIT enhanced safety and efficacy of immunotherapy and reduced treatment time from 3 years to 8 weeks. |
| Schmid et al.1722 | 2016 | 4 | Pilot study, open, no control group | Grass-pollen-allergy-induced AR (n = 7):
|
Combined symptom and medication score, RQLQ, number of IgE+ and IgE− plasmablasts specific for grass | ILIT may induce allergen-specific plasmablasts. Confirms an effect on provocation of mast cells in skin and nasal mucosa during the ensuing winter. |
AR = allergic rhinitis; Ig = immunoglobulin; ILIT = intralymphatic immunotherapy; LOE = level of evidence; MAT = modular antigen transporter; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SPT = skin-prick test; SQ-U = standardized quality units; VAS = visual analogue scale.
Five of the clinical trials published to date have compared ILIT with placebo. In 2008, Senti et al.1716 compared ILIT to SCIT and not to placebo. All trials have used aluminum hydroxide-adsorbed antigen as the vaccine. Most trials1716,1718-1721 used commercially available grass pollen or birch pollen allergen extract as the antigen. One trial1717 used recombinant major cat dander allergen fused to a translocation sequence and to part of the human invariant chain generating a modular antigen transporter, or “MAT,” vaccine.
The general protocol for administration was 3 injections with 1000 standardized quality units (SQ-U) of aluminum hydroxide-adsorbed allergen at 4-week intervals. Variations to this included a shorter dose interval in 1 trial1721 and no translation of allergen quantities into SQ-U in the trial using recombinant major cat dander allergen.1717
Of the 6 trials published thus far, 5 have demonstrated clinical efficacy and safety.1716-1720 In total, 127 patients have received active treatment and 45 patients have received placebo. Witten et al.1721 demonstrated immunological changes with ILIT, but no improvement in symptoms. Of note, the dose interval in this trial was shorter than in the trials that demonstrated clinical efficacy, with allergen administered at 2-week intervals instead of 4-week intervals.
The greatest variation between the trials to date is in the selection of clinical endpoints and the measurement of clinical outcomes, as illustrated in Table IX.D.6. All trials have used subjective measures to define clinical endpoints, most commonly in the form of symptom questionnaires.
Given the reduction in treatment duration, allergen dose, financial burden relative to SCIT, and the low risk of adverse effects, ILIT is a promising new therapy for AR. Before ILIT is integrated into clinical practice, a well-designed pharmacoeconomic evaluation of ILIT vs SCIT and larger RCTs are needed, as well as further studies investigating the impact of treatment protocol on outcomes.
Aggregate Grade of Evidence: B (Level 1b: 5 studies; Level 2b: 1 study; Level 4: 1 study; Table IX.D.6).
Benefit: Reduced treatment period, reduced number of injections, reduced dose of allergen injected, decreased risk of adverse events.
Harm: Risk of anaphylaxis.
Cost: ILIT might be associated with reduced costs relative to SCIT (reduced time, reduced financial burden for patients and healthcare provider). Application requires training.
Benefits-Harm Assessment: Balance of benefit over harm for ILIT relative to SCIT.
Value Judgments: ILIT appears to be efficacious in the treatment of AR. Preliminary data indicates that, relative to SCIT, the burden of treatment on the patient and on the healthcare system is lower.
Policy Level: Option, pending additional studies.
Intervention: While the research is promising, further studies are needed before ILIT can be translated into routine clinical practice.
IX.D.7. Alternative forms of immunotherapy
Oral, nasal, and inhaled (intrabronchial) AIT represent alternate options for the treatment of AR, with primarily historical significance.1623 While alternative forms of AIT have been evaluated in an effort to avoid the local discomfort and resource utilization associated with SCIT, the adoption of SLIT has largely replaced these methods.1623
Non-injectable, alternative immunotherapies involve the topical absorption of allergen extracts via oral/gastrointestinal, nasal, or inhalational exposures. SLIT, intralymphatic, and epicutaneous routes are reviewed separately in this document. Double-blind, placebo-controlled studies have evaluated oral/gastrointestinal immunotherapy for the treatment of birch,1723 cat,1724 and ragweed1725 sensitivity, without a significant decline in nasal symptoms, improvements in provocation testing, or reductions in medication utilization. Additionally, oral/gastrointestinal allergen administration requires extract concentrations approaching 200 times greater than SCIT, and is associated with adverse gastrointestinal side effects.1623,1724 However, the efficacy of oral/gastrointestinal immunotherapy has been demonstrated for the treatment of food hypersensitivity, where this approach remains investigational.1726
Oral mucosal immunotherapy (OMIT) is an alternative form of AIT that is distinctly different from SLIT and oral/gastrointestinal strategies. OMIT utilizes a glycerin based toothpaste vehicle to introduce antigen to high-density antigen processing oral Langerhans cells in the oral vestibular and buccal mucosa.1727 Theoretical benefits include induction of immune tolerance with lower antigen concentrations, decreased local side effects and higher adherence vs SLIT.1728 A recently completed pilot study of OMIT vs SLIT identified clinically meaningful improvements in disease-specific QOL measures with a significant rise in specific IgG4 over the first 6 months of treatment.1729 No adverse events were reported, and there were no significant differences between outcome measures for both treatment arms.1729 Additional study is needed to define the role of OMIT in the treatment of AR.
Local nasal immunotherapy has been established as an effective approach for the treatment of pollen and HDM sensitivity.1730 However, high rates of local adverse reactions limit patient compliance, with 1 prior study finding that 43.9% of treated children abandoned this treatment option within the first year of therapy.1731 High-quality studies of inhaled/intrabronchial immunotherapy for the treatment of AR have not yet been completed, with current studies limited to the treatment of allergic asthma.1732 In light of these findings, including poor compliance and limited efficacy, oral/gastrointestinal, nasal, and inhaled immunotherapies have limited utility in the current treatment of AR, while OMIT represents an emerging alternative to SCIT and SLIT.
IX.D.8. Combination omalizumab and SCIT
In consideration of combination therapy with concurrent biological omalizumab and AIT, each intervention targets different mechanisms in the allergic cascade. AIT desensitizes the body’s response to a specific antigen, with alteration of the Th1/Th2 balance and induction of T-cell anergy.1623 Omalizumab indiscriminately targets the humoral effector of allergic inflammation, with use of a humanized monoclonal antibody to block unbound IgE.1623 While both modalities have independently demonstrated efficacy as treatment options, improved strategies are needed, especially in patients with multiple sensitizations.1733
Two benefits of combination therapy have been described: decreased incidence of AIT-associated systemic allergic reactions and improved control of AR symptoms.1400-1402,1734-1736 Anaphylaxis is a persistent concern with AIT, with incidence of reported systemic reactions as high as 65% following rush protocols.1737,1738 Omalizumab pretreatment has therefore been evaluated as a strategy to improve AIT tolerance, with positive findings. Two multicenter, randomized, placebo-controlled studies have evaluated the incidence of AIT-induced systemic allergic reactions following pretreatment with omalizumab1402,1736 (Table IX.D.8VIII.E.4.a-1VIII.E.4.a-2). Massanari et al.1736 evaluated 248 patients with moderate persistent asthma receiving omalizumab pretreatment or placebo prior to cluster AIT, an accelerated AIT buildup schedule. A significantly lower incidence of systemic and respiratory-related reactions was reported among the omalizumab group, with an improved likelihood of reaching maintenance therapy compared to the group without preventive treatment with this biological. Casale et al.1402 evaluated 123 adult patients with ragweed-induced AR receiving omalizumab prior to 1-day rush AIT, finding a 5-fold decreased risk of systemic allergic reactions with omalizumab pretreatment (OR, 0.17). Further outcomes included significant improvement in daily symptom scores among patients receiving combination therapy (continued omalizumab + AIT) vs AIT alone. Additional study of AIT for the treatment of food1739 or insect venom1740,1741 hypersensitivity has also demonstrated improved safety with omalizumab pretreatment.
TABLE IX.D.8.
Evidence for the combination of omalizumab and subcutaneous immunotherapy in the treatment of allergic rhinitis
| Study | Year | LOE | Study design |
Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Massanari et al.1736 | 2010 | 1b | RCT | Adults with poorly controlled moderate persistent allergic asthma:
|
Incidence of systemic allergic reactions | Omalizumab pretreatment is associated with a lower incidence of systemic allergic reactions and higher likelihood of reaching maintenance AIT dose. |
| Klunker et al.1734,a | 2007 | 1b | RCT | Adults with ragweed induced AR:
|
Ragweed hypersensitivity via IgE-FAB assay, allergen-specific IgG4 | Combination therapy enhanced the inhibition of sIgE binding for 42 weeks after discontinuation. |
| Casale et al.1402,a | 2006 | 1b | RCT | Adults with ragweed induced AR:
|
Daily symptom severity, incidence of adverse events | Pretreatment with omalizumab resulted in a 5-fold decrease in risk of RIT associated anaphylaxis. Combination therapy is associated with significant reduction in symptom severity versus AIT alone. |
| Rolinck-Werninghaus et al.1401,b | 2004 | 1b | RCT | Subgroup analysis of Kuehr et al.1400 study | Daily symptom severity, rescue medication use | Combination therapy is associated with reduced symptom severity and rescue medication scores. |
| Kopp et al.1735,b | 2002 | 1b | RCT | Subgroup analysis of Kuehr et al.1400 study | In vitro leukotriene release following antigen stimulation | Combination therapy is associated with reduced leukotriene release following antigen stimulation. |
| Kuehr et al.1400,b | 2002 | 1b | RCT | Children and adolescents with SAR and:
|
Daily symptom severity, rescue medication use | Combination therapy is clinically superior to either component monotherapy, with reduced symptom severity and rescue medication scores. |
Immune Tolerance Network Group.
Omalizumab Rhinitis Study Group.
AIT = allergen immunotherapy; AR = allergic rhinitis; Ig = immunoglobulin; IgE-FAB = IgE-facilitated allergen binding; IT = immunotherapy; LOE = level of evidence; RCT = randomized controlled trial; RIT = rush immunotherapy; SAR = seasonal allergic rhinitis; sIgE = antigen-specific IgE.
The efficacy of combination therapy for the treatment of AR has been further evaluated by several iterative analyses of a single RCT.1400,1401,1735 Kuehr et al.1400 evaluated 221 adolescents (6 to 17 years) with moderate to severe AR and sensitization to birch and grass pollen. Using a randomized, controlled design, the effectiveness of combination therapy was evaluated during sequential birch and grass pollen seasons, with comparison of AIT +/− concurrent omalizumab. Significant findings included superiority of combination therapies vs AIT alone, with 48% reduction in symptom load (sum of mean daily symptom severity score plus mean daily rescue medication use) during an entire pollen season and 80% reduction in median rescue medication score. Two additional studies report unique findings generated by this trial.1401,1735 Rolinck-Werninghaus et al.1401 completed a subgroup analysis of study patients receiving specific AIT +/− concurrent omalizumab during the matched grass season. Results included decreased symptoms scores and rescue medication usage for patients receiving combination vs either therapy alone. Kopp et al.1735 evaluated a subgroup of 92 children, with findings of decreased leukotriene (LTC4, LTD4, and LTE4) release among patients receiving combination therapies following in vitro antigen stimulation of collected blood cells. An unrelated study by Klunker et al.1734 provides further evidence for the efficacy of combination therapy, with in vitro demonstration of inhibition of allergen-specific IgE binding for 42 weeks after discontinuation of combination therapy (vs 30 weeks with omalizumab alone).
While a prior study has estimated the cost of omalizumab (1,253 EUR/patient/month) and AIT therapies (425 EUR/patient/year), evaluation of economic and productivity outcomes has not been completed for patients undergoing combination therapy.1401 Finally, omalizumab has been associated with anaphylactic reactions in 0.09% to 0.2% of patients, with current recommendations to monitor patients for 30 minutes following administration.1742,1743
Aggregate Grade of Evidence: B (Level 1b: 4 studies, plus 2 additional iterative analyses of a parent study; Table IX.D.8).
Benefit: Improved safety of accelerated cluster and rush AIT protocols, with decreased symptom and rescue medication scores among a carefully selected population.
Harm: Financial cost and risk of anaphylactic reactions.
Cost: Moderate to high.
Benefits-Harm Assessment: Preponderance of benefit over harm.
Value Judgments: Combination therapy increases the safety of AIT, with decreased systemic reactions following cluster and rush protocols. Associated treatment costs and likelihood of systemic reactions must be considered, with greater consideration for omalizumab pretreatment prior to higher-risk AIT protocols. While 2 high-quality RCTs have demonstrated improved symptom control with combination therapy over AIT or omalizumab alone, not all patients will require this approach. Rather, an individualized approach to patient management must be considered, with evaluation of alternative causes for persistent symptoms, such as unidentified allergen sensitivity. The current evidence does not support the utilization of combination therapy for all patients failing to benefit from AIT alone.
Policy Level: Option, based on current evidence. However, it is important to note that omalizumab is not currently approved by the FDA for the treatment of AR.
Intervention: Omalizumab may be offered as a premedication prior to induction of cluster or rush AIT protocols. Combination therapy is an option for a carefully selected patient with persistent symptomatic AR following AIT. An individualized approach to patient management must be considered. In addition, as omalizumab is not currently approved by the FDA for AR treatment, in the United States this treatment approach would likely not be performed in routine clinical practice presently.
X. Associated conditions
Several medical conditions have been associated with AR, with varying prevalence dependent upon the specific comorbidity. In contrast, certain conditions are often associated with allergy or AR by conjecture, yet the available literature fails to identify a close association. This section examines various medical conditions that have a potential association with AR, specifically examining the evidence that supports or refutes the association
X.A. Asthma
X.A.1. Asthma definition
Asthma is a heterogeneous and complex disease, perhaps better characterized as a syndrome with overlapping phenotypes. The definition of asthma has evolved over the past several decades, combining clinical symptoms, examination findings, and functional parameters. When analyzing current international or national asthma guidelines,1744-1747 all include respiratory symptoms such as cough, shortness of breath, wheezing or chest tightness, and the presence of a variable expiratory airflow limitation that needs to be documented from bronchodilator reversibility testing or bronchial hyperreactivity tests (eg, methacholine test or other tests such as inhaled histamine, mannitol, exercise, or eucapnic hyperventilation). All guidelines also include the statement that symptoms and airflow limitation characteristically vary over time and in intensity and may resolve spontaneously or in response to medication. Discussion of chronic airway inflammation is included in all guideline documents. This has been characterized by several important cellular elements including mast cells, eosinophils, T-cells, macrophages, and neutrophils, but none of the guidelines require demonstration of inflammation by invasive or noninvasive methods. The Global Initiative of Asthma guidelines1744 specify that asthma is usually associated with bronchial hyperresponsiveness but highlight that demonstration of airway hyperresponsiveness and inflammation are not necessary or sufficient to make the diagnosis. Asthma is also classified by severity (ie, mild, moderate, severe) and by persistence (ie, intermittent vs persistent); however, the specific definitions of these categories vary dependent upon the specific guideline. Since asthma is defined as a heterogeneous disease, or rather as a syndrome, there appear to exist significant and variable etiologies that may manifest in similar phenotypes. Consequently, in the last decade, the definition of asthma has sought to include recognizable clusters of clinical and/or pathophysiological characteristics to more accurately characterize endotypes that exist.1748,1749
X.A.2. Asthma association with allergic and non-allergic rhinitis
Most patients with asthma (both allergic and non-allergic) also have rhinitis, whereas 10% to 40% of patients with AR have comorbid asthma.101,1167 Asthma and allergy may have similar underlying pathogenesis and immunologic mechanisms. IgE-mediated inflammation can involve both the upper and lower airways, suggesting an integration of the involved areas of the airway. This pattern of similarities gave rise to the concept of the unified airway model, which considers the entire respiratory system to represent a functional unit that consists of the nose, paranasal sinuses, larynx, trachea, and distal lung.1750
Some, but not all, studies suggest that asthma is more common in patients with moderate-to-severe persistent rhinitis than in those with mild rhinitis.25,1751-1753 Other large studies found a link between the severity and/or control of both diseases in children and adults.1754-1758 Adults and children with asthma and documented concomitant AR experience more asthma-related hospitalizations and doctors’ visits and also incur higher asthma drug costs than adults with asthma alone1759-1764 (Table X.A.2). Concerning changes in prevalence of rhinitis and asthma, some studies have demonstrated a parallel increasing prevalence of asthma and rhinitis,1765,1766 whereas others have not.1767-1775 It appears that in regions of highest prevalence, the proportion of subjects suffering from asthma or rhinitis may be reaching a plateau.
TABLE X.A.2.
Evidence for the association between asthma, allergic rhinitis and non-allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Ohta et al.1754 | 2011 | 3b | Case series | Asthmatic patients (n = 26,680) | Rhinitis and asthma diagnosis | Rhinitis is common in asthma and impairs asthma control. |
| Valero et al.1756 | 2009 | 3b | Case series | Patients with AR (n = 3225) | Rhinitis comorbidities | Asthma was influenced by skin sensitization and severity of AR. |
| Ponte et al.1755 | 2008 | 3b | Case series | Patients with severe asthma (n = 557) | Asthma severity | Moderate/severe rhinitis is a strong predictor for greater severity of asthma. |
| Bousquet et al.25 | 2005 | 3b | Case-control | Patients consulting ENT and allergy specialists for AR (n = 591) vs controls (n = 502) | Presence of asthma | Asthma prevalence increases with duration and severity of rhinitis. |
| Leynaert et al.1753 | 2004 | 3b | Cohort | International cross-sectional study of representative samples of young adults (n = 3000) | Rhinitis and asthma diagnosis | Association between asthma and rhinitis was not fully explained by atopy. |
| Linneberg et al.1752 | 2002 | 3b | Cohort | Follow-up on 2 occasions 8 years apart (n = 734) | Rhinitis and asthma in patients sensitized to pollen | AR and allergic asthma are manifestations of the same disease. |
| Bresciani et al.1757 | 2001 | 3b | Case series | Patients with severe steroid-dependent asthma (n = 35) | Sinonasal disease | Frequency of rhinosinusitis in patients with mild-to-moderate or severe steroid-dependent asthma is similar. |
AR = allergic rhinitis; ENT = ear, nose and throat; LOE = level of evidence.
Rhinitis and asthma are closely associated and thus AR should be evaluated in asthmatic patients, and likewise, the possibility of a diagnosis of asthma should be evaluated in patients with AR.
Aggregate Grade of Evidence: C (Level 3b: 7 studies; Table X.A.2).
X.A.3. Allergic rhinitis as a risk factor for asthma
AR and NAR are risk factors for developing asthma. This has been demonstrated in several large epidemiological studies (Table X.A.3). The Children’s Respiratory Study597 showed that physician-diagnosed AR during infancy is independently associated with a doubling of the risk of developing asthma at age 11 years. In children and adults, AR is a risk factor for asthma according to a 23-year follow-up of college students.1776 These studies were confirmed by other studies.458,1764,1777-1786 Some of these studies showed that rhinitis is a significant risk factor for adult-onset asthma in both atopic and nonatopic subjects.1779,1780,1783 Therefore, rhinitis is a risk factor independent of allergy for developing asthma in both adults1779,1780,1783 and children.597 In adulthood, the development of asthma in patients with rhinitis is often independent of allergy, whereas in childhood, it is frequently associated with allergy,597,1785 as almost all asthma in children is allergic.
TABLE X.A.3.
Evidence for allergic rhinitis as a risk factor for asthma
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Guerra et al.1779 | 2006 | 2a | Nested case-control study | Longitudinal cohort | Asthma onset | Rhinitis is a significant risk factor for adult-onset asthma in both atopic and nonatopic subjects. |
| Wright et al.597 | 1994 | 2a | Cohort | Birth cohort | Respiratory symptoms at age 6 years | Asthma in the child (OR, 4.06; 95% CI, 2.06-7.99). |
| Ibáñez et al.1764 | 2013 | 3b | Cross-sectional study | Children with AR | Associated diseases | Asthma was present in 49.5% of patients with AR. |
| Jarvis et al.458 | 2012 | 3b | Cross-sectional study | General population | Self-reported current asthma | Asthma was associated with chronic rhinosinusitis. |
| Rochat et al.1785 | 2010 | 3b | Cohort | Birth cohort | Wheezing onset | AR is a predictor for subsequent wheezing onset. |
| Shaaban et al.1783 | 2008 | 3b | Cohort | Population-based study | Frequency of asthma | Rhinitis, even in the absence of atopy, is a powerful predictor of adult-onset asthma. |
| Burgess et al.1786 | 2007 | 3b | Cohort | General population | Incident of asthma in preadolescence, adolescence, or adult life | Childhood AR increased the likelihood of new-onset asthma. |
| Shaaban et al.1784 | 2007 | 3b | Cohort | General population | Changes in bronchial hyperresponsiveness in nonasthmatic subjects | AR was associated with increased onset of bronchial hyperresponsiveness. |
| Bodtger et al.1777 | 2006 | 3b | Cohort | Population-based | Rhinitis onset | Asymptomatic sensitization, but not NAR, was a significant risk factor for later development of AR. |
| Porsbjerg et al.1781 | 2006 | 3b | Cohort | Random population sample | Prevalence of asthma | Presence of bronchial hyperresponsiveness and concomitant atopic manifestations in childhood increase the risk of developing asthma in adulthood. |
| Toren et at1780 | 2002 | 3b | Case-control | General population | Adult-onset physician-diagnosed asthma | Noninfectious rhinitis and current smoking, especially among nonatopics, are associated with increased risk for adult-onset asthma. |
| Plaschke et al.1778 | 2000 | 3b | Cohort | Random sample | Risk factors and onset or remission of AR and asthma | AR, sensitization to pets, and smoking were risk factors for onset of asthma. |
| Settipane et al.1776 | 2000 | 3b | Cohort | Follow-up of students | Asthma development | Allergic asthma depends on: elevated IgE, eosinophilia, airway hyperresponsiveness, exposure to allergens, and the predominance of the Th2 pathway of immunologic reactions. |
AR = allergic rhinitis; CI = confidence interval; IgE = immunoglobulin E; LOE = level of evidence; NAR = non-allergic rhinitis; OR = odds ratio.
Asthma and AR also share common risk factors. Sensitization to allergens is probably the most important. Most inhaled allergens are associated with nasal1787 and bronchial symptoms, but in epidemiologic studies, differences have been observed (eg, in pollen allergy). Some genetic polymorphisms are different in the case of AR and asthma. Other risk factors for asthma such as gender, obesity, viral infections in infancy, exposure to tobacco smoke (passive smoking or active smoking), diet, or stress are not found as common risk factors for AR. Outdoor or indoor air pollution is still a matter of debate as risk factor for AR or NAR.101 In summary, AR and NAR are risk factors for developing asthma.
Aggregate Grade of Evidence: C (Level 2a: 2 studies; Level 3b: 11 studies; Table X.A.3).
X.A.4. Treatment of allergic rhinitis and its effect on asthma
The 2015 AR clinical practice guideline from the AAO-HNS has highlighted the overlap of AR and asthma, specifically recommending that clinicians should assess for and document associated medical comorbid conditions including asthma.761 The guidelines also review and consider the impact of comorbid asthma on treatment decisions for AR, though the action statements may not apply to AR with comorbid asthma. However, there is a body of evidence to suggest that AR therapies, including INCS,1296,1788-1790 oral antihistamines,1791,1792 LTRAs,7,1793,1794 and AIT1672,1788,1795,1796 may benefit both conditions. Some of the most promising results in altering the course of allergic inflammation common to AR and asthma have been seen with AIT.1678,1797,1798 Given this increased understanding of the relationship between AR and asthma as similar inflammatory processes affecting the upper and lower airways, respectively, the importance of understanding the overlap of AR treatment with the treatment of asthma is increasingly evident. The studies reviewed in this section are limited to prospective randomized trials to minimize inherent biases and weaknesses of retrospective studies.1794
Allergen avoidance.
Allergen avoidance is often advocated for allergy treatment, specifically for AR and allergic asthma.7 Despite the intuitive acceptance of this and reasonable biological plausibility, the evidence for benefit of avoidance and environmental control measures in AR with associated asthma is limited. A Cochrane review examining randomized trials of subjects with asthma who underwent chemical or physical methods to reduce HDM allergen found no benefit with these methods.1799 Single allergen avoidance or elimination plans such as removing or washing pets, mattress coverings, removing carpeting, and use of HEPA filters have shown limited evidence-based clinical benefit for reducing asthma and/or AR symptoms.101,1799,1800 However, there is theoretical benefit of reducing allergen exposure, a paucity of data on multimodality approaches to reduce allergen load, and minimal negatives to attempting these various techniques; therefore, allergen avoidance could be considered as part of a multifactorial approach in the management of asthma associated with comorbid AR.1801,1802 (See section IX.A. Management – Allergen avoidance for additional information on this topic.)
Pharmacotherapy: oral H1 antihistamines.
We identified 6 RCTs which specifically evaluated oral H1 antihistamines for the treatment of asthma in the context of coexistent AR (Table X.A.4-1). There are many oral H1 antihistamine medications, but cetirizine and loratadine are the 2 most highly studied second-generation antihistamines used concomitantly in AR and asthma. There is biologic plausibility for a role of antihistamines in the treatment of allergic asthma, as elevated histamine levels after allergen challenge are associated with bronchoconstriction responses in acute asthma episodes. Cetirizine also has bronchodilatory effects which are significant both as monotherapy as well as in combination with albuterol.1803 Despite improvement in asthma symptoms, objective measures using pulmonary function testing and peak expiratory flow have failed to demonstrate significant improvements.1804-1806 Alternatively, there is growing evidence that antihistamines may have a preventive effect on the development of asthma in atopic patients, as shown in the Early Treatment of the Atopic Child trial.1807 Briefly, atopic infants were treated with 18 months of cetirizine and followed for the development of asthma. While analysis of the entire group found no significant difference between cetirizine-treated and placebo-treated patients, subgroup analysis revealed approximately 50% reduced risk of developing asthma among certizine-treated patients with grass pollen and HDM sensitivities. The authors hypothesize that variation in key genes related to histamine regulation may explain these differences.1807,1808 (See section IX.B.1.a. Management – Pharmacotherapy – Antihistamines – Oral H1 antihistamines for additional information on this topic.)
TABLE X.A.4-1.
Evidence for oral H1 antihistamines for the treatment of asthma in the context of coexistent allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Pasquali et al.1827 | 2006 | 1b | DBRCT | Persistent AR and asthma (n = 50):
|
Daily rhinitis and asthma symptoms, QOL by Rhinasthma questionnaire and SF-36 | Rhinitis and asthma symptoms reduced with levocetirizine. Rhinasthma QOL score reduced with levocetirizine. No differences in SF-36. |
| Baena-Cagnani et al.1828 | 2003 | 1b | DBRCT | SAR and asthma (n = 924):
|
TASS, FEV1, β-agonist medication use | Desloratadine vs placebo: reduction in mean TASS, improvement in FEV1, reduction in average β-agonist medication use. Desloratadine vs montelukast: No differences. |
| Berger et al.1829 | 2002 | 1b | DBRCT | AR and asthma (n = 326):
|
TSS, asthma symptom scores, β-agonist medication use | Desloratadine reduced rhinitis symptoms, asthma TSS, and β-agonist medication use. |
| Aubier et al.1804 | 2001 | 1b | DBRCT, crossover | SAR and asthma (n = 12):
|
BHR (measured as methacholine PD20). NBI (measured using peak expiratory flow meter and calculated as [oral peak flow – nasal peak flow] divided by oral peak flow). | BHR: increase with cetirizine; NBI: reduced with cetirizine compared to placebo at 6 hours. |
| Aaronson1830 | 1996 | 1b | DBRCT | AR and perennial asthma (n = 28):
|
Daily rhinitis and asthma symptoms, medication use, PEFR, PC20, PFTs, asthma management | Cetirizine reduced asthma and rhinitis symptoms. No difference in albuterol use. No difference in PFTs, PC20, and patient PEFRs. No difference in asthma management.a |
| Grant et al.1831 | 1995 | 1b | DBRCT | AR and asthma (n = 186):
|
Rhinitis and asthma symptoms, pulmonary function by spirometry | Improvement in asthma symptoms with cetirizine. No differences in objective measures. |
Note small sample size and no power-analysis or sample size calculation which limits interpretation of negative findings.
AR = allergic rhinitis; BHR = bronchial hyperresponsiveness; DBRCT = double blind randomized controlled trial; FEV1 = forced expiratory volume in 1 second; LOE = level of evidence; NBI = Nasal Blocking Index; PC20 and PD20 = provocation “concentration” or “dose” of methacholine causing a 20% decrease in FEV1 (also described as PD20FEV1); PEFR = peak expiratory flow rate; PFT = pulmonary function test; QOL = quality of life; SAR = seasonal allergic rhinitis; SF-36 = The Short Form Health Survey; TASS = Total Asthma Symptom Severity Score; TSS = Total Symptom Score.
Pharmacotherapy: oral corticosteroids.
Oral corticosteroids are an effective component of the asthma treatment algorithm, particularly for cases which are inadequately controlled with bronchodilators and inhaled corticosteroids.1809 They are also effective for symptoms of rhinitis.1247 However, oral corticosteroids have significant side effects, especially with increasing duration of use.7 Because of the side effect profile associated with these medications, they are not recommended for the routine treatment of AR, and utilization is only recommended for select cases after thorough discussion of the associated risks and benefits. (See section IX.B.2.a. Management - Pharmacotherapy - Corticosteroids - Oral corticosteroids for additional information on this topic.)
Pharmacotherapy: intranasal corticosteroids.
In the 1980s, topical INCSs were reported to improve asthma symptoms in patients with coexistent AR and asthma.1364,1810 Since then, it has been shown that very little intranasally administered corticosteroid reaches the lung (approximately 2%), suggesting this effect on the lower airway may be related to its intranasal effects.1788,1811 We have identified 2 meta-analyses and 12 RCTs that address this potential “unified airway” effect of INCS on asthma (Table X.A.4-2). A 2003 Cochrane review evaluated the efficacy of INCS on asthma outcomes in patients with coexistent rhinitis, finding no significant improvement in asthma outcomes with the use of INCS.1295 Heterogeneity in study designs may have limited the findings of this meta-analysis and explain the discrepancy of the results compared to high-quality RCTs. Alternatively, a 2013 systematic review and meta-analysis of the efficacy of INCS for asthmatics with concomitant AR demonstrated improvements in asthma outcomes with the use of INCS compared to placebo, but a lack of further improvement with INCS as an addition to inhaled corticosteroids.1296 Interestingly, patients with concomitant AR and asthma who received training on the proper use of INCS and education on the relationship of AR and asthma demonstrated significant reductions in asthma symptoms and albuterol use compared to patients receiving INCS without additional education.1812 This demonstrates the importance of patient instruction for both therapy evaluation and future trial design. (See section IX.B.2.a. Management – Pharmacotherapy – Corticosteroids – Intranasal corticosteroids (INCSs) for additional information on this topic.)
TABLE X.A.4-2.
Evidence for intranasal corticosteroids for the treatment of asthma in the context of coexistent allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Lohia et al.1296 | 2013 | 1a | SR and meta-analysis | 18 RCTs (n = 2162):
|
Asthma symptoms, rescue medication use, FEV1, PEF, PC20, QOL | INCS improved FEV1, PC20, asthma symptom scores, and rescue medication use. No asthma outcome changes with INCS plus oral inhaled CS vs oral inhaled CS alone. Nasal inhaled CS improved PEF. |
| Taramarcaz & Gibson1295 | 2003 | 1a | Meta-analysis | 14 RCTs with 3 interventions:
|
Asthma symptoms and β-agonist use, asthma exacerbation events, QOL, FEV1, PEF, PC20, and PD20, inflammatory markers | Nonsignificant symptom improvement INCS vs placebo. No difference in FEV1, PEF, PC20, and PD20. |
| Jindal et al.1832 | 2016 | 1b | RCT, single-blind | AR and asthma (n = 120):
|
Symptom scores of rhinitis and asthma, PEF | Reduction in asthma symptom severity score and increase in PEF with FP INCS vs montelukast. |
| Kersten et al.1789 | 2012 | 1b | DBRCT | AR and mild-to-moderate exercise exacerbated asthma (n = 32):
|
Change in exercise induced decrease in FEV1, change in AUC of the FEV1 curve, ACQ score, PAQLQ score, FeNO | Exercise induced decrease in FEV1 reduced with FP. No difference in FEV1, ACQ, PAQLQ, FeNO. |
| Baiardini et al.1833 | 2010 | 1b | DBRCT | Moderate/severe persistent AR with intermittent asthma (n = 47):
|
QOL by GS; symptom scores; Rhinasthma scores of RAI, LA, and UAa, rescue asthma medication use | GS score reduction with MF INCS. LA score decreased with MF INCS. No difference MFNS vs placebo for rescue medications. |
| Nair et al.1834 | 2010 | 1b | DBRCT, double-dummy, 3-way crossover | Persistent AR and asthma (n = 25):
|
Methacholine PC20, FeNO, nPIF, FEV1, asthma and rhinitis QOL | Improvement of PC20 in all groups. No PC20 improvement with INCS and inhaled steroid vs inhaled FP alone. No change in Asthma QOL. FeNO and nPIF reduced only with INCS. |
| Agondi et al.1835 | 2008 | 1b | DBRCT | AR and asthma (n = 33):
|
Rhinitis and asthma symptom scores, rescue medication use, BHR (histamine provocation) | Changes with Bdp INCS vs placebo: asthma symptoms reduced, decrease in rescue medication use, BHR reduced. |
| Pedroletti et al.1836 | 2008 | 1b | DBRCT | Perennial rhinitis and allergic asthma (n = 40):
|
FeNO, ECP in nasal lavage, PEF, FEV1 | No difference of FeNO for MF INCS vs placebo. Nasal ECP reduced. No difference in PEF or FEV1. |
| Dahl et al.1837 | 2005 | 1b | DBRCT, double dummy | Pollen-induced AR and asthma (n = 262):
|
Asthma and AR symptoms, PFTs, methacholine BHR, PEF | Increased PEF for FP INCS + inhaled FP vs other groups. PEF increase for inhaled FP vs no inhaled FP. FEV1 higher with inhaled FP. Increased BHR with FP INCS; no increase with inhaled FP. |
| Nathan et al.1838 | 2005 | 1b | RCT, plus open-label | SAR and persistent asthma (n = 863):
|
Daily PEF, daily asthma and AR symptoms, rescue albuterol use | FP INCS improved nasal symptoms. No asthma outcome improvement with FP INCS addition to inhaled FP-salmeterol. |
| Stelmach et al.1839 | 2005 | 1b | DBRCT | PAR and mild-to-moderate persistent asthma (n = 59):
|
Asthma and AR symptom scores, PEF, FEV1 and BHR (PC20), proxy indicators of asthma-related morbidity (work absence, emergency department visits, etc.) | Reductions of AR and asthma symptoms in all groups. No change PEF or BHR. Increased FEV1 for inhaled Bdp. Asthma morbidity reduced for all. |
| Thio et al.1840 | 2000 | 1b | DBRCT | Two grass pollen seasons of treatment (season 1, n = 21; season 2, n = 67):
|
Asthma scores, rescue use of salbutamol, methacholine PD20, FEV1 | No difference in asthma scores or rescue salbutamol for all groups. PD20 not significantly different. FEV1 increased with FP and BDP in season 2. |
| Watson et al.1811 | 1993 | 1b | DBRCT, crossover | AR and controlled asthma (n = 21):
|
Asthma and rhinitis symptoms, PC20, Bdp depositionb | No difference of all asthma symptoms with Bdp. PC20 improved with Bdp. Evening asthma symptoms reduced with Bdp. |
| Corren et al.1788 | 1992 | 1b | DBRCT | Mild SAR and asthma (n = 18):
|
Nasal and chest symptoms, NBI, BHR (PC20) | PC20 decreased over pollen season with placebo, not Bdp. Morning NBI decreased with placebo, improved with Bdp. No difference in symptoms. |
Rhinasthma GS includes scores from the 3 categories of RAI, LA, and UA.
Radiolabeled Bdp <2% deposition in lungs, 20%-50% in nasal cavity, and 48%-78% swallowed in 1993 Watson et al.1811 study.
ACQ = Asthma Control Questionnaire; AR = allergic rhinitis; AUC = area under the curve; Bdp = beclomethasone dipropionate; BHR = bronchial hyper-responsiveness; CS = corticosteroid; DBRCT = double-blind randomized controlled trial; ECP = eosinophil cationic protein; FeNO = fraction of exhaled nitric oxide; FEV1 = forced expiratory volume in 1 second; FP = fluticasone propionate; GS = global summary; INCS = intranasal corticosteroid; LA = lower airway; LOE = level of evidence; MF = mometasone furoate; NBI = Nasal Blocking Index; PAQLQ = Pediatric Asthma Quality of Life Questionnaire; PAR = perennial allergic rhinitis; PC20 and PD20 = provocation “concentration” or “dose” of methacholine causing a 20% decrease in FEV1 (also described as PD20FEV1); PEF = peak expiratory flow; PFT = pulmonary function test; nPIF = peak nasal inspiratory flow; QOL = quality of life; RAI = respiratory allergy impact; RCT = randomized controlled trial; SAR = seasonal allergic rhinitis; SR = systematic review; UA = upper airway.
Pharmacotherapy: leukotriene receptor antagonists.
LTRAs (montelukast and zafirlukast) have demonstrated benefit for the treatment of both asthma and AR, consistent with efficacy in addressing inflammation in the “unified airway”1813 (Table X.A.4-3). In 2008, the ARIA group reviewed the evidence for effectiveness of montelukast in treating patients with asthma and AR, finding improvement of both nasal and bronchial symptoms as well as reduction of β-agonist use.101 In fact, the LTRAs are the only class of medications specifically described in the 2008 AR management guide for primary care physicians, and in the full ARIA report, as effective for both asthma and AR.101,1814 The 2010 ARIA update further supports the recommendation of LTRAs for both AR and asthma, but specifies that LTRAs are not recommended over other first-line therapies for the respective conditions (ie, it is better to treat asthma and AR with both a nasal and inhaled steroid, than try to treat both with an LTRA). A more recent review in 2015 also identified some utility of LTRAs for patients with concomitant AR and asthma.1802 Despite this evidence, the limited additional benefit and added cost leads to a strong recommendation (based on moderate quality evidence) for inhaled glucocorticoids over LTRAs for single-modality treatment of asthma in patients with comorbid AR.1167 Based on the summarized RCTs, an evidence-based recommendation is made for LTRAs not to be used as monotherapy for AR, but LTRAs may be considered as part of the treatment of comorbid asthma and AR (See section IX.B.4. Management – Pharmacotherapy – Leukotriene receptor antagonists (LTRAs) for additional information on this topic) (Table X.A.4-3).
TABLE X.A.4-3.
Evidence for leukotriene receptor antagonists for the treatment of asthma in the context of coexistent allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Katial et al.1841 | 2010 | 1b | RCT | SAR and asthma (n = 1385):
|
PEF, rescue albuterol use, asthma and rhinitis symptoms | No additional improvements in asthma with montelukast plus FP-salmeterol. FP-salmeterol associated with improvement in all outcome measures vs montelukast. |
| Price et al.1842 | 2006 | 1b | DBRCT; analysis of COMPACT trial data | Asthma symptoms despite inhaled corticosteroid. Subgroup with coexistent AR. (n = 889).
|
Improvement in morning PEF compared to baseline | Least-squares mean difference of morning PEF greater increase from baseline in montelukast + budesonide vs double dose budesonide.a |
| Nathan et al.1838 | 2005 | 1b | RCT, plus open-label | SAR and persistent asthma (n = 863):
|
Daily PEF, daily asthma and AR symptoms, rescue albuterol use | FP INCS improved nasal symptoms. No asthma outcome improvement with FP INCS addition to inhaled FP-salmeterol. |
| Philip et al.1341 | 2004 | 1b | DBRCT | SAR and asthma (n = 831):
|
Rhinitis symptoms, RQLQ, global evaluations of asthma, β-agonist medication use | Global evaluation of asthma by patients and physicians improved with montelukast. Reduction in β-agonist medication use montelukast. |
| Baena-Cagnani et al.1828 | 2003 | 1b | DBRCT | SAR and asthma (n = 924):
|
TASS, FEV1, β-agonist medication use | Montelukast vs placebo: reduction in mean TASS, improvement in FEV1, reduction in average β-agonist medication use. Desloratadine vs montelukast: no differences. |
Least squared mean difference in Price et al. study calculated as [(montelukast + budesonide) – double dose budesonide].
AR = allergic rhinitis; COMPACT = Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy; DBRCT = double-blind randomized controlled trial; FEV1 = forced expiratory volume in 1 second; FP = fluticasone propionate; INCS = intranasal corticosteroid; LOE = level of evidence; PEF = peak expiratory flow; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SAR = seasonal allergic rhinitis; TASS = Total Asthma Symptom Severity Score.
Pharmacotherapy recommendations for the treatment of AR with coexisting asthma.
Aggregate Grade of Evidence: A (Level 1a: 2 studies; Level 1b: 23 studies). Antihistamines (Level 1b: 6 studies; Table X.A.4-1). INCS (Level 1a: 2 studies; Level 1b: 12 studies; Table X.A.4-2). LTRAs (Level 1b: 5 studies; Table X.A.4-3).
Benefit: Pharmacotherapy improves subjective and objective severity of asthma in patients with coexistent AR. Patient education and training on medication use improves compliance and benefits for INCS, and likely all patient-administered pharmacotherapy.
Harm: Pharmacotherapy other than systemic steroids—minimal harm with rare mild adverse events such as drowsiness. No serious adverse events reported in the studies reviewed. Systemic corticosteroids have significant side effects.
Cost: Generally low cost for pharmacotherapy.
Benefits-Harm Assessment: There is a benefit over placebo for asthma treatment, though no significant benefit is seen over standard asthma pharmacotherapy. Risks of routine use of systemic corticosteroids generally outweighs the benefits, though short courses for acute indications (eg, asthma exacerbation) have a favorable likelihood of benefit relative to harm.
Value Judgments: Pharmacotherapy for AR may also benefit asthma symptoms and objective parameters of pulmonary function in patients with coexisting asthma and AR, however, the benefit for asthma should be considered a positive side effect rather than an indication for use as there appears to be limited benefit compared to standard asthma therapy.
Policy Level: Use of pharmacotherapy other than systemic steroids: Recommended use for optimal control of AR, with potential additional benefit for coexistent asthma, though not recommended for primary intent of asthma treatment. Use of systemic corticosteroid: Not recommended for routine use in AR with comorbid asthma due to unfavorable risk-benefit profile, though certain situations may indicate a short course (eg, acute asthma exacerbation).
Biologics: omalizumab.
Omalizumab is an anti-IgE mAb that binds free IgE, preventing interactions with high-affinity IgE receptors and resulting in receptor down-regulation on inflammatory cells.1815 Omalizumab has demonstrated effectiveness separately for asthma as well as AR.1393,1815-1818 Despite a number of studies evaluating omalizumab in AR or asthma,1815,1819 there is only 1 double-blind RCT which specifically evaluates the efficacy of omalizumab in patients with concomitant moderate-to-severe asthma and persistent AR.1820 Additionally, another study evaluates omalizumab as an adjunct to SCIT,1403 with both studies showing a reduction in symptoms as well as an improvement in QOL measures (Table X.A.4-4). The 2010 ARIA update makes a conditional recommendation of using a mAb against IgE, such as omalizumab for treatment of asthma in patients with both AR and asthma, where there is a clear IgE-dependent allergic component and failure of other maximal therapy.1167 Additional biologics, including anti-IL5, anti-IL4, and IL-4 receptor mAbs, are currently in varying stages of development/emergence with positive findings for the treatment of asthma and other atopic diseases. Additional evaluation is needed to further evaluate their role for the treatment of coexistent AR and asthma. (See section IX.B.7. Management – Pharmacotherapy – Biologics for additional information on this topic.)
TABLE X.A.4-4.
Evidence for omalizumab for the treatment of asthma in the context of coexistent allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kopp et al.1403 | 2009 | 1b | DBRCT | AR and seasonal asthma. All patients received SCIT. (n = 140):
|
AR and asthma symptoms, rescue medication use, PEF, patient and provider GETE, asthma symptoms by ACQ, disease-specific QOL by AQLQ and RQLQ, PFTs | Omalizumab addition to SCIT: reduced symptom severity, improved QOL by ACQ and AQLQ. No difference in rescue medication use. No difference in FEV1 or mean PEF. |
| Vignola et al.1820 | 2004 | 1b | DBRCT | Moderate-to-severe persistent AR and allergic asthma (n = 405):
|
Asthma exacerbations, disease-specific QOL by AQLQ and RQLQ, rescue medication use, symptom scores, patient and investigator GETE, inhaled corticosteroid use, FEV1, FVC, and morning PEF | Omalizumab: reduced asthma exacerbations; increased AQLQ and RQLQ; reduced asthma symptoms; increased FEV1, FVC, and PEF. No difference in β-agonist use. |
ACQ = Asthma Control Questionnaire; AQLQ = Asthma Quality of Life Questionnaire; AR = allergic rhinitis; DBRCT = double-blind randomized controlled trial; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GETE = Global Evaluation of Treatment Effectiveness; LOE = level of evidence; PEF = peak expiratory flow; PFT = pulmonary function test; QOL = quality of life; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; SCIT = subcutaneous immunotherapy.
Biologics recommendations for the treatment of AR with coexisting asthma.
Aggregate Grade of Evidence: B (Level 1b: 2 studies; Table X.A.4-4). Grade A evidence with multiple 1b RCTs and 1a reviews exist for asthma and AR individually, but only 1 double-blind RCT specifically evaluating omalizumab vs placebo in patients with concurrent conditions.
Benefit: Decreased asthma exacerbations, decreased symptom scores, and improvement in disease-specific QOL in patients with coexisting asthma and AR.
Harm: There is evidence for acceptable safety for use up to 52 weeks.1821 Potential longer-term harm unknown. Minor events such as mild injection site reactions are reported. Possibility of anaphylaxis.
Cost: Substantially higher cost than conventional therapy for asthma and AR.
Benefits-Harm Assessment: Benefits appear to outweigh potential harm for the treatment of more severe/persistent coexistent AR and asthma.
Value Judgments: Added benefit of omalizumab as therapy for patients with AR and asthma that is uncontrolled despite maximal conventional interventions. However, given the significant increased cost associated with omalizumab, the value of this therapy is likely greatest for patients with severe asthma and symptoms that persist despite usual therapies.
Policy Level: Omalizumab is recommended for those patients with clear IgE-mediated allergic asthma with coexistent AR who fail conventional therapy. The significant additional cost of this therapy should be considered in evaluating its value.
Allergen immunotherapy.
Both SCIT and SLIT have been shown to improve the control of comorbid AR conditions, such as asthma1618,1788,1822 (Table X.A.4-5). AIT also appears to prevent the development of asthma.1678,1797,1798 The efficacy of SLIT for AR has been confirmed by several systematic reviews.1694,1695,1823 Both SCIT and SLIT have been shown to be efficacious for AR, though there is ongoing debate as to whether 1 form is superior.1697,1703 AIT is also thought to help halt the progression of allergic disease, including prevention of new allergic sensitivities and the development of asthma.1624,1626,1678,1797,1798,1824-1826 AIT also appears to have long-lasting effects even after discontinuing treatment, unlike pharmacotherapy. Such promising results have led to a 2010 ARIA update statement recommending both SCIT and SLIT for the treatment of asthma in patients with AR and asthma.1167 Recent systematic reviews demonstrate that SCIT and SLIT reduce both asthma and rhinitis symptoms, as well as medication use.1694,1822 These evidence-based reviews also demonstrate strong evidence for the utility of SCIT and SLIT in the treatment of asthma alone in studies that did not specifically address the condition of combined asthma and AR.1694,1822 Evidence for AIT (SCIT and SLIT) for asthma in context of comorbid asthma and AR, is reviewed in Table X.A.4-5. (See section IX.D. Management – Allergen immunotherapy (AIT) for additional information on this topic.)
TABLE X.A.4-5.
Evidence for allergen immunotherapy for the treatment of asthma in the context of coexistent allergic rhinitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Erekosima et al.1822 | 2014 | 1a | SR | Systematic review of 61 RCTs (26 specifically asthma and rhinitis):
|
|
|
| Lin et al.1694 | 2013 | 1a | SR | Systematic review of 63 RCTs (SCIT and SLIT):
|
|
|
| Marogna et al.1678 | 2008 | 1b | RCT | Rhinitis with/without intermittent asthma (n = 216):
|
Development of persistent asthma (not at baseline), symptom and medication scores, daily medication use, new sensitization | Persistent asthma incidence lower with SLIT vs control. Methacholine-positive patients after 3 years reduced with SLIT. Lower symptom and medication scores with SLIT. |
| Novembre et al.1798 | 2004 | 1b | RCT | Rhinoconjunctivitis, no asthma (n = 97):
|
Symptoms, rescue medication use, development of asthma | Rescue medication use reduced with SLIT. Relative risk of asthma after 3 years greater in control group vs SLIT. |
| Möller et al.1797 | 2002 | 1b | RCT | Rhinoconjunctivitis with or without asthma (n = 191):
|
Development of asthma (if none at trial start), BHR by PC20, VAS of symptoms | Asthma incidence greater in controls. BHR improved with SCIT after 1 year pollen season. |
| Grembiale et al.1795 | 2000 | 1b | DBRCT | HDM AR and BHR to methacholine (n = 44):
|
BHR by PD20, serum IgE levels, rescue medication use, additional visits for symptoms, development of asthma | BHR increased with SCIT. No HDM IgE difference. Increased medication use and visits with placebo. No difference in asthma incidence. |
| Inal et al.1825 | 2007 | 2b | Open, nonrandomized, prospective, parallel group | HDM AR and/or mild-to-moderate asthma (n = 147):
|
Asthma and rhinitis medication use, positive HDM skin test, development of asthma | Decreased asthma medication use with SCIT. Improved atopy scores with SCIT. Asthma incidence nearly half with SCIT. |
Strength of evidence moderate to high, for asthma-focused studies and rhinitis-focused studies, respectively.
The strength of evidence is moderate for both comparisons.
SLIT administered as sublingual drops of standardized allergen for a buildup phase and then continued for maintenance phase.
AR = allergic rhinitis; BHR = bronchial hyper-responsiveness; DBRCT = double-blind randomized controlled trial; HDM = house dust mite; IgE = immunoglobulin E; LOE = level of evidence; PC20 and PD20 = provocation “concentration” or “dose” of methacholine causing a 20% decrease in FEV1 (also described as PD20FEV1); RCT = randomized controlled trial; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; SR = systematic review; VAS = visual analogue scale.
Allergen immunotherapy recommendations for the treatment of AR with coexisting asthma.
Aggregate Grade of Evidence: A (Level 1a: 2 studies; Level 1b: 4 studies; Level 2b: 1 study; Table X.A.4-5).
Benefit: AIT (both SCIT and SLIT) has demonstrated benefit in concomitant AR and asthma, with decreased symptoms, rescue medication use, and bronchial hyperresponsiveness, as well as reduced development of asthma in patients with AR only.
Harm: Local site reactions are common and there is potential for anaphylactic events with any form of AIT.
Cost: Increased cost compared to standard therapy for AR and asthma, though the potential to treat the underlying disease process and prevent progression of disease could reduce long-term costs.
Benefits-Harm Assessment: Significant evidence to support the use of AIT for patients with AR and asthma, as well as the potential utility of AIT for preventing progression of allergic disease from AR to the development of allergic asthma. Harms are generally limited to minor local reactions, though there is a potential risk of anaphylaxis. Benefits appear to outweigh potential harm, given that anaphylaxis is rare.
Value Judgments: There appears to be unique value in AIT, as this therapy treats the underlying pathology of AR and asthma, with potential to halt the progression of allergic disease. The unique benefits of this therapy are of value, despite some uncertainty of their true magnitude.
Policy Level: AIT (SCIT and SLIT) is recommended for treatment of AR with asthma in patients following an appropriate trial of medical therapy, and may also be considered for the benefit of preventing progression of AR to asthma in patients with AR only, and for whom AIT is otherwise indicated.
X.B. Rhinosinusitis
AR may be associated with rhinosinusitis in several clinical settings. In general, AR is regarded as a disease-modifying factor for rhinosinusitis.1 Rhinosinusitis may be broadly divided into ARS, RARS, CRSwNP, or CRSsNP. The association between each of these forms of rhinosinusitis with AR will be discussed individually below. Of note, many of these studies used SPT or in vitro testing for confirmation of allergic disease. While positive testing does indicate evidence of sensitization, this does not necessarily correlate with allergic nasal disease.1843 Given the paucity of literature exclusively discussing AR and rhinosinusitis (vs allergy and rhinosinusitis), this literature will be included.
AR is thought to be a potential risk factor for the development of rhinosinusitis in general. Exposure to allergens in allergic patients has been associated with increased eosinophilia in the maxillary sinus.1844,1845 In addition, the majority of ragweed allergic patients (60%) display abnormal opacification of CT scans of the paranasal sinuses in peak allergic seasons.1846 These CT findings persist despite symptom resolution outside the allergic season.1846 These studies do not always delineate whether ARS, RARS, or CRS is the form of rhinosinusitis associated with AR.
Allergic rhinitis and acute rhinosinusitis
In addition to these more general studies, evidence exists to support the concept of an increased risk of ARS with AR. There is a significantly higher incidence of ARS in both children and adult patients with a history of AR.1847,1848 Children with AR are also more likely to experience orbital complications of ARS compared to those without AR, especially in pollinating seasons.1849 A mouse model has also shown that ongoing nasal allergy is associated with worsened episodes of ARS.1850,1851 Available data supports an association between AR and ARS. However, AR is thought to be a disease-modifying or risk-modifying factor rather than a causative one. There are no studies examining the effects of treating AR on the risk of developing an episode of ARS. For example, it is unclear whether treating AR decreases the incidence of ARS. Future study may help clarify the interaction between AR and ARS (Table X.B-1).
TABLE X.B-1.
Evidence for an association between allergic rhinitis and acute rhinosinusitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Rantala et al.1856 | 2013 | 2a | Cross-sectional | Atopic and nonatopic adults age 21-63 years (n = 1008) | Upper and lower respiratory tract infections | Individuals with atopic disease had higher risk of developing URTI, including RS. |
| Chen et al.1848 | 2001 | 2a | Questionnaire | Children in Taiwan (n = 8723) | Rhinosinusitis | Children reporting allergy are more likely to have RS. |
| Holzmann et al.1849 | 2001 | 2b | Retrospective review | Children with orbital complications of ARS (n = 102) | Prevalence of AR | Orbital complications are more common in allergy season. |
| Frerichs et al.1857 | 2014 | 3a | SR | Allergic and non-allergic patients | Prolonged course (>4 weeks) of RS | No significant increase in prolonged RS in AR patients. |
| Savolainen1847 | 1989 | 3b | Case-control | Acute maxillary sinusitis with and without allergy (n = 224) | ARS | Prevalence of AR 25% and 16.5% in non-AR patients. |
AR = allergic rhinitis; ARS = acute rhinosinusitis; LOE = level of evidence; RS = rhinosinusitis; SR = systematic review; URTI = upper respiratory tract infection.
Aggregate Grade of Evidence: C (Level 2a: 2 studies; Level 2b: 1 study; Level 3a: 1 study; Level 3b: 1 study; Table X.B-1).
Allergic rhinitis and recurrent acute rhinosinusitis
The potential link between AR and RARS is an extension of the link between AR and ARS. The increase in sinonasal inflammation associated with AR is proposed to increase mucosal edema, sinus ostium obstruction, and the retention of sinus secretions.1 This environment may support secondary bacterial overgrowth and subsequent ARS or RARS.1 Two studies have specifically examined the association between RARS and AR, with a focus on potentially altered innate immunity. The results of these 2 studies are conflicting. One study suggests there is a decrease in the antimicrobial properties of sinonasal secretions in patients with RARS and AR compared to AR only patients as well as control patients.1852 The second study identified an upregulation in toll-like receptor 9 expression, suggesting increased resistance to bacterial infection rather than susceptibility.1853 Further study is required to define the association between AR and RARS (Table X.B-2).
TABLE X.B-2.
Evidence for an association between allergic rhinitis and recurrent acute rhinosinusitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Melvin et al.1853 | 2010 | 2b | Prospective cohort | (n = 21):
|
Expression of TLR9 in sinonasal epithelium | Increased expression of TLR9 in allergic patients with RS. |
| Kalfa et al.1852 | 2004 | 2b | Cross-sectional | (n = 47):
|
Nasal secretion levels of EDN and lysozyme levels | Allergic patients with RS have elevated levels of EDN and decreased lysozyme levels. |
EDN = eosinophil-derived neurotoxin; LOE = level of evidence; RS = rhinosinusitis; TLR9 = toll like receptor 9.
Aggregate Grade of Evidence: D (Level 2b: 2 studies; conflicting evidence; Table X.B-2).
Allergic rhinitis and chronic rhinosinusitis without nasal polyposis
CRS is a condition of the sinonasal cavity characterized by persistent inflammation. The cause of the inflammation varies from patient to patient. As AR is a cause of sinonasal inflammation, many have suspected there may be an association with the pathogenesis of CRS. However, there are no controlled studies examining the role of AR in the development of CRSsNP. Additionally, there are no studies showing that the treatment or control of allergic disease alters the progression of CRSsNP, or vice versa.1 Given the varied pathophysiology of CRSsNP, it is challenging to determine the association between allergy and CRSsNP. Wilson et al.1854 performed a systematic review of allergy and CRS, excluding studies that did not differentiate between CRSsNP and CRSwNP. Their review found 4 studies that supported an association between allergy and CRSsNP and 5 that did not.1854 Because the relationship remains unclear, allergy testing is listed as an option in CRSsNP patients based on the theoretical benefit of identifying and treating comorbid allergic disease1,1854 (Table X.B-3).
TABLE X.B-3.
Evidence for allergic rhinitis and chronic rhinosinusitis without nasal polyposis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Baroody et al.1844 | 2008 | 1b | RCT | CRSsNP with or without ragweed allergy (n = 18) | Reactivity in ragweed season determined by symptoms and sinus inflammation | Allergic patients have increased reactivity and sinonasal inflammation in ragweed season. |
| Wilson et al.1854 | 2014 | 3a | SR | CRSsNP with or without allergy | Association between CRSsNP and allergy | Conflicting evidence with no clear association. |
| Tan et al.1858 | 2011 | 3b | Prospective case-control | CRSsNP with or without allergy (n = 63) | Rates of atopy in rhinitis vs CRSsNP | No significant difference in rates of atopy (72% in rhinitis, 79% in CRSsNP). |
| Pearlman et al.1859 | 2009 | 3b | Prospective case series | CRSsNP with or without allergy (n = 115) | CT scores | No difference in CT scores. |
| Gelincik et al.1860 | 2008 | 3b | Prospective case series | CRSsNP with or without allergy (n = 66) | Prevalence of CRSsNP in allergic and non-allergic rhinitis patients | CRSsNP was equally prevalent in allergic (43%) and non-allergic (50%) rhinitis patients. |
| Kirtsreesakul & Ruttanaphol1861 | 2008 | 3b | Retrospective case series | CRSsNP with or without allergy (n = 198) | Sinus X-rays, nasal endoscopy | Allergic patients had a higher incidence of abnormal sinus X-rays. |
| Robinson et al.1862 | 2006 | 3b | Prospective case series | CRSsNP with or without allergy (n = 193) | Lund-Mackay CT scores and symptoms scores | Allergy was not associated with CT findings or symptoms scores. |
| Alho et al.1863 | 2004 | 3b | Prospective case series | CRSsNP with or without allergy (n = 48) | CT findings during viral URTI, incidence of S. aureus sensitization | Allergic patients had higher CT scores and higher incidences of S. aureus sensitization. |
| Van Zele et al.1864 | 2004 | 3b | Prospective case-control | CRSsNP with or without allergy (n = 31) | Rates of S. aureus colonization | No difference in colonization rates. |
| Berrettini et al.1865 | 1999 | 3b | Prospective case-control | CRSsNP with or without allergy (n = 77) | CT scan findings, nasal endoscopy, nasal swabs, rhinomanometry | Increased CT evidence of sinusitis in allergy (68%) vs non-allergic (33%) patients. |
CRSsNP = chronic rhinosinusitis without nasal polyposis; CT = computed tomography; LOE = level of evidence; RCT = randomized controlled trial; SR = systematic review; URTI = upper respiratory infection.
Aggregate Grade of Evidence: D (Level 1b: 1 study; Level 3a: 1 study; Level 3b: 8 studies; conflicting evidence; Table X.B-3). Adapted from Wilson et al.1854
Allergic rhinitis and chronic rhinosinusitis with nasal polyposis
The pathogenesis of CRSwNP is strongly associated with Th2-mediated inflammation.1 Additionally, nasal polyps in CRSwNP have high levels of tissue eosinophilia, as well as mast cells and basophils.1 AR follows a similar inflammatory pathway and this suggests there may be a pathophysiologic similarity between CRSwNP and AR. Wilson et al.1854 examined the association between allergic disease and CRSwNP. Again, the evidence was conflicting. Ten studies supported an association while 7 did not. One study had equivocal findings.1854 Since this review, Li et al.1855 examined the association between atopy and CRSwNP and concluded that there was no correlation between atopic status and disease severity. They did note that atopy-positive patients were younger than atopy-negative patients.1855 Despite some overlapping pathophysiologic features between allergic disease and CRSwNP, conflicting evidence exists and there is no clear association between AR and CRSwNP. Allergy testing is once again an option in CRSwNP patients based on the theoretical benefit of identifying and treating comorbid allergic disease1,1854 (Table X.B-4).
TABLE X.B-4.
Evidence for allergic rhinitis and chronic rhinosinusitis with nasal polyposis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Houser & Keen1866 | 2008 | 2b | Retrospective case series | CRSwNP with or without allergy (n = 373) | Nasal polyposis | AR is associated with the development of nasal polyposis. |
| Wilson et al.1854 | 2014 | 3a | Systematic review | CRSwNP with or without allergy | Association between CRSwNP and allergy | Conflicting evidence with no clear association. |
| Al-Qudah1867 | 2016 | 3b | Prospective cohort study | CRSwNP compared to CRSsNP (n = 155) | Rates of food sensitivity | No difference between allergic and non-allergic patients. |
| Li et al.1855 | 2016 | 3b | Prospective cohort | CRSwNP with or without allergy (n = 210) | Nasal endoscopy, CT scores, serum inflammatory markers | No difference in allergic and non-allergic patients. |
| Gorgulu et al.1868 | 2012 | 3b | Prospective case-control | CRSwNP compared to controls (n = 60) | Rate of allergen sensitivity | No difference between allergic and non-allergic patients. |
| Lill et al.1869 | 2011 | 3b | Prospective case-control | CRSwNP compared to controls (n = 50) | Rates of food sensitivity | Higher rate of milk sensitivity in CRSwNP. |
| Tan et al.1858 | 2011 | 3b | Prospective case-control | CRSwNP with or without allergy (n = 62) | Rates and number of antigen sensitivity | No difference in rates of sensitivity. |
| Munoz del Castillo et al.1870 | 2009 | 3b | Prospective case-control | CRSwNP compared to controls (n = 190) | Rates of allergy compared to control | Higher rates of allergy in CRSwNP compared to controls. |
| Collins et al.1871 | 2006 | 3b | Prospective case-control | CRSwNP compared to controls (n = 40) | Rates of food sensitivity | Higher rates of food sensitivity in CRSwNP. |
| Van Zele et al.1864 | 2004 | 3b | Prospective case-control | CRSwNP compared to CRSsNP and controls (n = 55) | Rates of S. aureus colonization | Higher rates of colonization in CRSwNP. |
| Kirtsreesakul1872 | 2002 | 3b | Prospective cohort | CRSwNP with or without allergy (n = 68) | Response to budesonide nasal sprays (sneezing, oral and nasal peak flow, overall response to therapy) | Improved response in non-allergic patients. |
| Asero & Bottazzi1874 | 2001 | 3b | Prospective case-control | CRSwNP compared to non-polyp controls (n = 68) | Rates of Candida and house dust sensitivity | Higher rates of sensitivity in CRSwNP. |
| Voegels et al.1873 | 2001 | 3b | Prospective case-control | CRSwNP with or without allergy (n = 39) | Rates of asthma in allergic or non-allergic patients | Higher rates of asthma in allergic patients. |
| Asero & Bottazzi1875 | 2000 | 3b | Prospective case-control | CRSwNP compared to allergic controls (n = 20) | Rates of Candida sensitivity | Higher rates of sensitivity in CRSwNP. |
| Pang et al.1876 | 2000 | 3b | Prospective case-control | CRSwNP compared to controls (n = 80) | Rates of food sensitivity | Higher rates of food sensitivity in CRSwNP. |
| Pumhirun et al.1877 | 1999 | 3b | Prospective case-control | CRSwNP compared to controls (n = 40) | Incidence of house dust and cockroach allergy | Higher rates of allergy in CRSwNP compared to controls. |
| Keith et al.1878 | 1994 | 3b | Prospective case-control | CRSwNP with or without allergy (n = 64) | Symptom scores, serum levels of inflammatory markers | No difference except in patients with ragweed allergy. Ragweed-positive patients had increase symptom scores and serum inflammatory markers. |
| Pearlman et al.1859 | 2009 | 4 | Prospective case series | CRSwNP with or without allergy (n = 40) | Prevalence of CRSwNP in allergic or non-allergic patients | No difference between allergic and non-allergic patients. |
| Bonfils & Malinvaud1879 | 2008 | 4 | Prospective case series | CRSwNP with or without allergy (n = 63) | Postoperative course, recurrence | No difference between allergic and non-allergic patients. |
| Erbek et al.1880 | 2007 | 4 | Retrospective case series | CRSwNP with or without allergy (n = 83) | Polyp size, symptom scores, recurrence | No difference between allergic and non-allergic patients. |
| Bonfils et al.1881 | 2006 | 4 | Prospective case series | CRSwNP with or without allergy (n = 180) | Endoscopy, CT scores | No difference between allergic and non-allergic patients. |
AR = allergic rhinitis; CRSsNP = chronic rhinosinusitis without nasal polyposis; CRSwNP = chronic rhinosinusitis with nasal polyposis; CT computed tomography; LOE = level of evidence.
Aggregate Grade of Evidence: D (Level 2b: 1 study; Level 3a: 1 study; Level 3b: 15 studies; Level 4: 4 studies; conflicting evidence; Table X.B-4). Adapted from Wilson et al.1854
In summary, AR has a moderate level of evidence supporting an association with ARS (Level C). Regarding RARS, CRSsNP and CRSwNP, the preponderance of evidence does not support an association, though the evidence is highly conflicting. The available literature is also limited as it often assumes patients who test positive on allergy testing have nasal allergic disease and may not differentiate between systemic allergy and nasal allergy. Further study is needed to determine the association between AR and rhinosinusitis, as well as the impact treating 1 process has on the progression of the other. However, the diagnosis and treatment of comorbid allergic disease is an option in rhinosinusitis patients balancing the cost and low evidence with the low risk of allergic rhinosinusitis treatment and the theoretical benefits of reducing allergic sinonasal inflammation.1
X.C. Conjunctivitis
Although the burden of illness (impaired QOL) associated with allergic conjunctivitis (AC) is well established, this condition is often under recognized and consequently undertreated except when it is most severe.1882 Its frequent association with AR contributes to the substantial burden associated with AR. Although this association is well recognized clinically, its extent remains poorly defined due to methodologic differences and deficiencies of the studies which have examined this association in the literature. Further compounding this problem is the phenotypic diversity of both AR and AC, and the observation that very few studies have adequately characterized the phenotypes of their study populations. Additionally, many epidemiologic studies are limited by being based solely on questionnaire results rather than on objective clinical evidence of allergic sensitization.
The largest data source regarding the AR-AC association derives from the ISAAC study, a worldwide study established in 1991 with the aim of investigating the epidemiology and etiology of asthma, rhinitis, and atopic dermatitis in each country, using standard methodology including questionnaire and SPT. ISAAC has reported the prevalence of AC symptoms in 257,800 children aged 6 to 7 years in 91 centers in 38 countries and 463,801 children aged 13 to 14 years in 155 centers in 56 countries. Although the ISAAC survey was not validated for the diagnosis of AC, ISAAC studies support the frequent association of AR with itchy-watery eyes, reporting that ocular symptoms affect approximately 33% to 50% of children with AR1883 (Table X.C).
TABLE X.C.
Evidence for an association between allergic rhinitis and allergic conjunctivitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kim et al.1884 | 2016 | 2b | Cross-sectional survey | General population: 14,356 students, health screening 2010-2014. “Korean International Study of Asthma and Allergies in Childhood” AR defined as symptoms + SPT positivity. | SPT positivity, AR prevalence, prevalence of comorbidities | Most common comorbid allergic diseases associated with AR: pollen allergy (37.0%), AC (34.5%). |
| Han et al.1889 | 2015 | 2b | Cohort | 1020 children total, 338 with AR. “The Allergic Rhinitis Cohort Study for Kids (ARCO-kids)” | SPT, questionnaire, endoscopic examination. Evaluation of risk factors for AR. | History of AC identified as risk factor for AR (OR, 14.25; 95% CI, 4.99-40.74). |
| Alexandropoulos et al.1885 | 2012 | 3a | Case series | Adult nonrandom patients referred to a Clinical Immunology outpatient clinic 2001-2007 (n = 1851). AR defined according to ARIA. | SPT, questionnaire, sIgE. Evaluation of risk factors for AR. | AR prevalence was 38.4%. AC identified as risk factor for AR (OR, 6.16; 95% CI, 4.71-8.06). |
| Navarro et al.1890 | 2009 | 3a | Cross-sectional | n = 4991 patients selected by referral for allergy evaluation | Characteristics of patients with AR. | AR prevalence was 55%. 65% had associated AC. |
| Almaliotis et al.1888 | 2010 | 3b | Retrospective case series | n = 448 subjects selected by clinic referral and diagnosis of AC by ophthalmologist | SPT, questionnaire. Evaluation of comorbidities of ocular allergy. | 70% of patients with AC also had AR. Symptoms of ocular allergy are very common in patients with AR and asthma. |
| Gradman & Wolthers1886 | 2006 | 3b | Retrospective survey | n = 458 children (5–15 years) selected from a secondary pediatric outpatient clinic with diagnosis of AC, asthma, AR, or eczema | Prevalence of AC in children with rhinitis, asthma and eczema. | Prevalence of AC in children with rhinitis: 42%. Prevalence of AR in children with AC: 97%. |
| Kosrirukvongs et al.1887 | 2001 | 3b | Case series | n = 445 patients (mean age 24.5 ± 16.3 years) with a history of itching, foreign body sensation, lacrimation and red eyes. No control group. | Skin test. Evaluation of clinical features and risk factors of various AC types. | 73.8% of patients with perennial AC had associated AR. Most common allergen sensitization was HDM. |
AC = allergic conjunctivitis; AR = allergic rhinitis; ARIA = Allergic Rhinitis and its Impact on Asthma; CI = confidence interval; LOE = level of evidence; OR = odds ratio; sIgE = allergen-specific IgE; HDM = house dust mite; SPT = skin-prick test;
The best evidence of disease-association derives from studies of AR patients assessed for the prevalence of AC as a comorbidity.1884-1890 The evidence suggests that AR is associated with 35% to 74% prevalence of AC and that among patients with AC, the prevalence of AR may be as high as 97%.
To summarize, there is a substantial body of evidence which supports AC as a frequently occurring comorbidity of AR, particularly in children. Not only is this disease-association common, but ocular allergy symptoms also contribute significantly to the QOL impairment associated with AR. It is not surprising, therefore, that ocular symptoms of allergic rhinoconjunctivitis are among the most common symptoms which cause patients to seek allergy treatment.1891 It is advisable, when assessing patients with AR, to also assess for ocular symptoms and to consider treatment specific to providing relief of AC.
Aggregate Grade of Evidence: C (Level 2b: 2 studies; Level 3a: 2 studies; Level 3b: 3 studies; Table X.C).
X.D. Atopic dermatitis (AD)
AD is a chronic and/or relapsing skin disorder characterized by pruritus, scratching, and eczematous lesions.1892 Its burden of illness, impact on QOL, and complications are substantial.1893 AD commonly presents as the first manifestation of atopy in infants and children who later develop AR and/or asthma, a pattern that has been referred to as “the atopic march.”1894
Although the association between AR and AD has long been clinically recognized, the extent of this association remains poorly defined due to methodologic differences and limitations of the studies that have examined this association537,556,636,1895-1912 (Table X.D). Further compounding this problem is the phenotypic diversity of both AR and AD, and the observation that very few studies have adequately characterized the phenotypes of their study populations. Additionally, many epidemiologic studies are limited by being based purely on questionnaire results rather than objective evidence of allergic sensitization, such as SPT or in vitro testing.
TABLE X.D.
Evidence for the association between allergic rhinitis and atopic dermatitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Mortz et al.1901 | 2015 | 2b | Prospective cohort | The Odense Adolescence Cohort Study (TOACS). Cross-sectional study (n = 1501 8th graders); 15-year retention cohort (n = 899) |
Questionnaire, interview, clinical exam, serum IgE, patch test, SPT. Persistence of AD, comorbidities |
Lifetime prevalence of AD was 34.1%. 60.8% prevalence of AR in those with AD vs 31% in those without AD. Subjects with AD were twice as likely to develop AR. |
| Sybilski et al.1902 | 2015 | 2b | Cross-sectional | Questionnaire (n = 22,703 Polish subjects); Medical evaluation (n = 4783 patients) | Questionnaire (response rate 64.4%), SPT with 15 aeroallergens. Diagnosis of AD and comorbidities. |
AD identified in 3.91% of subjects. Comorbidities of AD included AR in 26.17%. Association of AD with rhinitis subtypes: 9.5% with perennial vs 9.3% with seasonal and 9.6% with polyvalent vs 9.0% monovalent sensitization. |
| Lowe et al.1907 | 2007 | 2b | Prospective birth cohort | n = 620 infants with family history of atopic disease; 71.5% had sufficient data for analysis. | SPT, interview. Risk of AR development amongst infants with atopic AD vs those with nonatopic AD. |
Children with atopic eczema had a substantially greater risk of AR (OR, 2.91; 95% CI, 1.48–5.71). In children with eczema within the first 2 years of life, SPT can provide information on the risk of AR. |
| Kusel et al.1909 | 2005 | 2b | Prospective birth cohort | (n = 263); 75.3% of the 263 followed for the full 5 years | SPT at 6 months, 2 years, 5 years. Evaluation of risk factors for eczema in relation to atopic status. |
Persistent eczema significantly associated with AR (OR, 2.8; 95% CI, 1.5–5.3). AR significantly associated with AD (OR, 3.5; 95% CI, 1.7–7.1). AR not associated with nonatopic dermatitis. |
| Schneider et al.1900 | 2016 | 3b | Cohort | n = 1091 infants age 3-18 months with AD followed for 3 years. | Development of comorbidities in patients with AD. | 18.5% of patients developed AR. Mean age at onset was 2.4 ± 1.3 years for AR. Comorbidities developed more often in infants with greater baseline AD severity. |
| Bozek & Jarzab1903 | 2013 | 3b | Cross-sectional | n = 7124 Polish participants; mean age 66-67 years; 70% participation | Questionnaire, examination, SPT, tIgE, sIgE. Epidemiology of allergic disease in an elderly Polish population. |
1.6% had AD/eczema (95% CI, 1.1–2.0). 12.6% had SAR (95% CI, 10.8–14.6). 17.1% had PAR (95% CI, 15.9–19.7). |
| Batlles-Garrido et al.537 | 2010 | 3b | Cross-sectional | n = 1143 participants; 10-year-old and 11-year-old school children; 49.8% response rate. Part of ISAAC II study. | Homologated questionnaire, SPT. Assessment of prevalence, severity, and factors linked to rhinitis. |
Prevalence of “rhinitis” during the previous year: 8.9%. Concomitant with atopic eczema: 3.5%. Significant association between “rhinitis” and atopic eczema (OR, 1.98; 95% CI, 1.36–2.88). |
| Batlles-Garrido et al.1905 | 2010 | 3b | Cross-sectional | n = 1143 participants; 10 and 11-year-old school children; 49.8% response rate. Part of ISAAC II study. | Homologated questionnaire, SPT, physical examination. Assessment of prevalence, severity, and factors linked to atopic eczema. |
Prevalence of atopic eczema: 11.4%. Risk factors was severe rhinitis (OR, 7.7; 95% CI, 1.79–33). |
| Peroni et al.1906 | 2008 | 3b | Cross-sectional | n = 1402 preschool children aged 3-5 years; response rate 92%. Part of ISAAC study. | SPT. Assessment of prevalence of AD, comorbidities and risk factors. |
Rhinitis symptoms present in 32.2% AD children. Allergic sensitization to egg, cat, grass pollen and mites, presence of symptoms of rhinitis, and family history of atopy were risk factors for AD. |
| Karaman et al.1908 | 2006 | 3b | Cross-sectional | n = 1217 children in 3rd, 4th, and 5th grade in Izmir, Turkey; response rate 57.6%. ISAAC II methodology. | Questionnaire, physical examination, SPT. Prevalence and etiologic factors of asthma, rhinitis, and eczema. |
Prevalence of physician-diagnosed AR: 17%. Prevalence of physician-diagnosed eczema: 4.9%. Atopic sensitization prevalence: 8.8%; HDM sensitization most frequent. |
| Kuyucu et al.556 | 2006 | 3b | Cross-sectional | n = 2774 Turkish school children aged 9-11 years; response rate: 89.2%. ISAAC II questionnaire. | Questionnaire, SPT (subset), flexural dermatitis. | Prevalence of ever rhinitis: 36.3%, current rhinitis: 30.6%, ever hay fever: 8.3%. SPT positivity: 20.4% among children with current rhinitis. Flexural dermatitis significantly associated with current rhinitis. |
| Yemaneberhan et al.1911 | 2004 | 3b | Cross-sectional | n = 12,876 participants; 95% of those eligible took part in the survey. | Questionnaire, SPT (subset). Prevalence of AD symptoms, association with rhinitis symptoms. | Lifetime cumulative prevalence of AD symptoms: 1.2%. AD symptoms strongly associated with rhinitis symptoms (OR, 61.94; 95% CI, 42.66–89.95). |
| Peroni et al.636 | 2003 | 3b | Cross-sectional | n = 1402 preschool children age 3-5 years; response rate: 92%. ISAAC questionnaire. | Questionnaire, SPT. Comparison of disease associations between rhinitic and non-rhinitic children. |
Prevalence of rhinitis in the last 12 months: 16.8%. Rhinitic children had significantly more AD (22.9% vs 13.9%, p < 0.001). |
| Rhodes et al.1898 | 2002 | 3b | Longitudinal cohort | n = 100 infants from atopic families followed for 22 years; 63% retained at last follow-up. | Examination, SPT, tIgE, bronchial hyper-responsiveness to inhaled histamine. Development of AR and asthma |
Prevalence of AD peaked at 20% of children by 1 year of age, declined to 5% at end of the study. AR prevalence slowly increased over time from 3% to 15%. |
| Min et al.1912 | 2001 | 3b | Cross-sectional | n = 71,120 randomly selected subjects from Korean otolaryngology clinics | Questionnaire, examination, SPT, serum allergy test. | Prevalence of PAR in tertiary referral hospitals in Korea is 3.93%. Associated atopic dermatitis in 20.9% subjects with PAR. |
| Gustafsson et al.1899 | 2000 | 3b | Longitudinal cohort | n = 94 children with AD followed for 8 years | tIgE, sIgE, SPT. Evaluation of development of AR and asthma. |
AD improved in 84 of 92 children; 45% developed AR. Severity of AD was a risk factor for subsequent development of AR. Consistent with atopic march. |
| Ozdemir et al.1913 | 2000 | 3b | Cross-sectional | n = 1603 college students in Eskisehir, Turkey; 94.5% response rate. | Questionnaire, physical examination and SPT (subset). Determine prevalence of asthma, AR, AD. |
Eczema rate: 5.4% among females, 6.3% among males. Rhinitis symptoms: 11.1% among females, 8.9% among males. |
| Garcia-Gonzalez et al.1914 | 1998 | 3b | Cross-sectional | n = 365 students from Malaga, Spain | Interview, SPT, tIgE, sIgE. Evaluation of prevalence of atopic disease. |
19.9% suffered from rhinoconjunctivitis, and 0.8% AD. |
| Leung & Ho1915 | 1994 | 3b | Cross-sectional | n = 2208 secondary school students; response rate over 87%. | Questionnaire, SPT (subset). Evaluation of prevalence of asthma and allergic disease. |
Hay fever prevalence: Hong Kong 15.7%; Kota Kinabalu 11.2%; San Bu 2.1%. Eczema prevalence: Hong Kong 20.1%; Kota Kinabalu 7.6%; San Bu 7.2%. |
| Kidon et al.1910 | 2005 | 4 | Prospective case series | n = 175 newly diagnosed AR patients; predominantly Chinese; mean age 7.9 years. | Questionnaire, SPT. Relative risk of sensitization and associated risk factors. |
Prevalence of AD: 48%. SPT positive for HDM in 85%. Children with AR and concomitant AD show preferential sensitization to Dermatophagoides mites. |
AD = atopic dermatitis; AR = allergic rhinitis; HDM = house dust mite; IgE = immunoglobulin E; ISAAC = International Study of Asthma and Allergies in Childhood; LOE = level of evidence; OR = odds ratio; CI = confidence interval; PAR = perennial allergic rhinitis; SAR = seasonal allergic rhinitis; sIgE = antigen-specific immunoglobulin E; SPT = skin-prick test; tIgE; total immunoglobulin E.
The largest data source regarding AR-AD association comes from the ISAAC study, investigating the epidemiology and etiology of asthma, rhinitis, and AD using standard methodology including questionnaires, SPT, and flexural dermatitis examination.1895 ISAAC reported the prevalence of AD symptoms in 256,410 children aged 6 to 7 years in 90 centers from 37 countries, and 458,623 children aged 13 to 14 years in 153 centers from 56 countries. These studies indicate that AD is a major public health problem worldwide, affecting approximately 5% to 20% of children aged 6 to 7 and 13 to 14 years.1896 While longitudinal studies demonstrate improvement or resolution of AD with age,1897 increasing severity of AD has been shown to correlate with an increased risk of developing AR, with prevalence of AR among people with AD ranging from 15% to 61%.1898-1900
The best evidence of disease association derives from studies which compare the incidence and/or prevalence of AR in populations with and without AD. In this regard, the limited evidence available suggests that AD is associated with a 2-fold increase in AR among people with AD compared with the normal population.1901 In this study, among those children with present or past AD, 60.8% reported AR compared to 31% in subjects without AD.
Aggregate Grade of Evidence: C (Level 2b: 4 studies;, Level 3b: 15 studies; Level 4: 1 study; Table X.D).
X.E. Food allergy and pollen-food allergy syndrome (PFAS)
Approximately 5% to 8% of patients with pollen allergy will develop food allergy and pollen-food allergy syndrome (PFAS).1916 Patients with pollen allergies may have allergy-related manifestations after consuming specific fruits, vegetables, nuts, or spices. The prevalence of pollen-food allergies varies with the type of pollen. As many as 70% of patients with birch allergy will manifest a food-related sensitivity.1917 PFAS is an IgE-mediated reactivity, which occurs in the oral mucosa, leading to itching, stinging pain, angioedema, and rarely systemic symptoms. The term, “oral allergy syndrome” (OAS), has also been frequently used and refers to a pollen-food allergy that occurs only at the level of the oral mucosa. OAS is, therefore, a specific manifestation of the broader PFAS. The symptoms of OAS manifest because of IgE specific for the offending pollen cross-reacting with highly homologous proteins found in a variety of fruits, vegetables, and nuts. The most common example of this cross-reactivity in western populations is birch pollen and apples. Table X.E-1 lists common pollen allergens with plant-derived foods that may demonstrate cross-reactivity. These pollen-food relationships have been observed clinically and are also demonstrated at a molecular level through identification of the homologous amino acids, cross-reactive carbohydrate determinants, and lipid transfer proteins. The birch-apple syndrome is due to the high homology of the major birch allergen Bet v 1 and the apple allergen Mal d 1.1918
TABLE X.E-1.
Pollen-food allergy cross-reactivity1928
| Pollen | Food |
|---|---|
| Birch | Apple, pear, sweet cherry, peach, plum, apricot, almond, celery, carrot, potato, kiwifruit, hazelnut, mango |
| Japanese cedar | Tomato |
| Mugwort | Celery, carrot, mango, spice |
| Grass | Melon, watermelon, tomato, potato, kiwifruit, orange, peanut |
| Ragweed | Melon, watermelon, cantaloupe, zucchini, cucumber, banana |
| Plane | Hazelnut, apple, lettuce, corn, peanut, chickpea |
The diagnosis of PFAS is typically established by a detailed history and physical exam. The history should be guided by an understanding of the patient’s underlying pollen allergy and foods that share highly homologous proteins. The clinician should elicit a detailed history of the allergic response including any systemic symptoms and history of anaphylaxis. The estimated rate of systemic reaction from a pollen-food allergy is 10% and the estimated rate of anaphylaxis is 1.7% to 10%.1742,1919,1920 Systemic symptoms are the manifestation of an allergic response by organ systems that have not come into direct contact with the ingested food and include: urticaria, nasal congestion, sneezing, flushing, wheezing, cough, diarrhea, and hypotension. The gold standard for establishing a diagnosis of PFAS is a double-blind food challenge. However, this is difficult to perform because of the bias inherent to the appearance, texture, and taste of foods.1921 Oral food challenge, SPT, and food-specific IgE levels have also been used to establish the diagnosis. The diagnostic approach should be guided by the patient’s history and severity of allergic response.
The standard recommendation for the treatment of PFAS has been elimination of the offending food. Patients should be counseled on the risk for systemic and anaphylactic reactions. Patients with a history of systemic or anaphylactic reactions should be provided with an epinephrine autoinjector. The proteins responsible for PFAS are often labile and may be denatured by heat. The denatured proteins are typically not cross-reactive with the pollen IgE. Therefore, pollen-associated foods may become edible when heated. In 1 study, food challenges were performed with cooked apple, carrot, or celery in patients with atopic dermatitis and birch pollen allergy who had OAS and dermatologic symptoms upon ingestion of the raw foods. Cooked versions of the offending foods did not cause oral allergy symptoms.1922 However, some patients did manifest a late eczematous skin reaction, which was likely T-cell–mediated (Table X.E-2).
TABLE X.E-2.
Evidence for the role of pollen allergy in pollen-food allergy syndrome
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Inuo et al.1916 | 2015 | 2b | Cohort | Children with AR to JCP and tomato sensitization (n = 23, age 6–17 | Basophil activation by tomato and JCP extract, IgE and IgG4 levels against tomato and JCP antigens | Tomato-specific basophil activation decreases after JCP-based SCIT, suggesting efficacy in treating PFAS symptoms in patients with JCP AR. |
| Bohle et al.1922 | 2006 | 2b | Case-control | Patients with birch pollen allergy and OAS | Oral challenge and basophil activation assays | T-cell cross-reactivity occurs independently of IgE cross-reactivity. The view that cooked pollen-related foods can be consumed without allergologic consequences should be reconsidered. |
| Bolhaar et al.1925 | 2004 | 2b | RCT | Patients with PFAS (birch-apple, n = 25) randomized to:
|
Double-blind placebo-controlled food challenge and SPT | Birch pollen AIT decreases allergy to foods containing homologous allergens (apple). |
| Skamstrup Hansen et al.1921 | 2004 | 2b | RCT | Patients with birch reactivity (n = 74) randomized to:
|
Oral challenge with apple before and after treatment | AIT was not accompanied by a significant decrease in the severity of reactivity to apple compared with placebo. |
| Asero1927 | 2003 | 2b | Case-control |
|
Prevalence of apple allergy at 30 months by symptoms or SPT | Most patients have propensity for apple re-sensitization. No significant difference between in prevalence of PFAS between test group and controls at 30 months. In some patients, pollen AIT can exert a long-lasting effect on PFAS. |
| Asero1924 | 1998 | 2b | Case-control | Patients with PFAS (birch-apple, n = 75) assigned to:
|
Oral apple challenge and SPT at 12, 24, and 36 months of AIT | AIT with birch pollen extracts effectively reduces clinical apple sensitivity and skin reactivity in most cases after 1 year of treatment. |
| Bircher et al.1929 | 1994 | 2b | Case-control | Serum samples from:
|
Presence of IgE for 6 pollen-associated foods | There is a high prevalence of food specific IgE in pollen allergic patients, but not in non–pollen-allergic patients. |
| Bindslev-Jensen et al.1923 | 1991 | 2b | RCT | Patients with PFAS (birch-hazelnut, n = 30) randomized to:
|
Symptom score (0–5 rating) with hazelnut provocation before and after 2 weeks of treatment | Treatment with antihistamine (astemizole) significantly reduced (but did not eliminate) the severity of local symptoms after ingestion of hazelnuts compared to placebo. |
| Mauro et al.1926 | 2011 | 4 | Cohort | Patients with birch allergy (n = 30) randomized to:
|
Oral challenge with apple before and after treatment | Different doses of birch extract may be necessary to induce apple tolerance amongst patient with birch-apple PFAS. |
AIT = allergen immunotherapy; AR = allergic rhinitis; Ig = immunoglobulin; JCP = Japanese cedar pollen; LOE = level of evidence; OAS = oral allergy syndrome; PFAS = pollen-food allergy syndrome; RCT = randomized controlled trial; SCIT = subcutaneous immunotherapy; SLIT = sublingual immunotherapy; SPT = skin-prick test.
There is also 1 RCT in a group of 30 patients evaluating the use of an antihistamine to reduce PFAS symptoms, which demonstrated a clinically significant reduction in allergy symptoms compared to placebo when ingesting offending foods.1923 The antihistamine used in this study, astemizole, has been removed from the market due to QT interval prolongation on electrocardiogram.
There have been several studies evaluating the effect of targeted immunotherapy for pollen allergy at reducing PFAS symptoms. The results are mixed. Several small cohort studies and RCTs have shown an increased tolerance to the offending food when patients are treated with pollen specific immunotherapy.1916,1924-1926 However, 1 RCT failed to demonstrate any improved tolerance to apple in birch allergic patients treated with birch specific immunotherapy compared to placebo.1921 One study evaluating the persistence of tolerance for apple after birch immunotherapy demonstrated that some patients had an increased apple tolerance for up to 30 months after immunotherapy. However, there was no statistically significant difference between the immunotherapy and control groups.1927 Immunotherapy is not currently recommended for the sole purpose of treating PFAS. Patients receiving immunotherapy for the treatment of pollen allergies should be counseled on the potential but unsubstantiated benefit for improved food tolerance.
Aggregate Grade of Evidence: B (Level 2b: 8 studies; Level 4: 1 study; Table X.E-2).
X.F. Adenoid hypertrophy
In children, adenoid hypertrophy (AH) and AR may exhibit similar symptoms including nasal obstruction and rhinorrhea. The potential relationship between AR and AH is explored in this section. Adenoid enlargement most commonly begins during infancy; it continues through the first 5 to 6 years of life and involutes with puberty.1930,1931 Symptomatic AH affects an unknown percentage of children and may contribute to a range of symptoms including nasal obstruction, nasal drainage, sleep disturbance, increased episodes of rhinosinusitis, increased lower respiratory tract infections, worsened asthma, and Eustachian tube dysfunction.1930,1932
Case series evaluating the relationship between AH and allergic sensitization fall into 2 main categories: (1) cohorts of children with allergic conditions assessed for AH; or (2) children identified with AH assessed for allergy sensitization. These may not represent the same populations.
Three studies assessing allergic children found a higher rate of AH than controls (when present). In 2015, 1322 children (mean age 5.9 ± 3.3 years) treated for “allergic conditions” were compared to 100 age-matched children with no allergic disease for AH. They found AH was more prevalent in the allergic group (12.4%) than controls (3%) (p < 0.0001). AH was statistically associated with AR and cigarette smoke exposure (p = 0.004).1933 Similarly, Dogru et al.1934 found that among 566 children with AR the prevalence of AH was 21.2% (no control group). Additionally, they reported that children with both AH and AR had a higher frequency of persistent rhinitis (p < 0.05), moderate/severe rhinitis (p = 0.005), and nasal congestion (p = 0.001) than those with AR alone. The AR-only group had a higher prevalence of asthma (p = 0.037) and “itchy nose” (0.017). In another study, adenoid size in seasonally allergic children was assessed by Modrynski and Zawisza,1935 concluding that seasonal adenoid enlargement was observed in birch pollen–allergic children more than controls not allergic during the tree-pollen season. The increased adenoid size resolved after pollen season in the study group, and the seasonal increase in adenoid size was not observed in birch-allergic children treated co-seasonally with topical nasal steroid and antihistamines. The study was small (n = 67 among 4 groups) and did not state whether it was blinded (Table X.F).
TABLE X.F.
Evidence for the association between allergic rhinitis and adenoid hypertrophy
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Dogru et al.1934 | 2017 | 4 | Retrospective, cross-sectional, nonrandomized |
|
Symptoms, allergen sensitivities, allergy comorbidities | The AR plus AH group had more severe symptoms than the group with AR alone. |
| Atan Sahin et al.1936 | 2016 | 4 | Case-control | Children from humid vs less humid locations | AH, SPT, IgE, vitamin D | High humidity group had higher prevalence of AH, higher IgE levels, and an association between AH and SPT for dust mite. |
| Eren et al.1941 | 2015 | 4 | Consecutive cohort | 155 children referred to Otolaryngology from Pediatric Allergy | Nasal endoscopy and SPT | There was a negative correlation between AH and SPT positivity (r = −0.208, p = 0.009). |
| Evicimk et al.1933 | 2015 | 4 | Retrospective, cross-sectional, nonrandomized |
|
AH, cigarette exposure, gender, age, family history of allergy, asthma, SPT | AH was more prevalent in the AR group. Cigarette smoke exposure was associated with AH. |
| Pagella et al.1947 | 2015 | 4 | Retrospective case series | Otolaryngology clinic for nasal symptoms (1-7 years, n = 582; 8-14 years, n = 213) | Allergy testing (n = 169), endoscopic adenoid size, clinical symptoms | In the whole population: AH and AR not associated age 1-7 years (p = 0.34), AH and AR associated with age in 8-year-old to 14-year-old group (p = 0.0043). |
| Ameli et al.1939 | 2013 | 4 | Consecutive cohort | 205 children with persistent upper airway obstruction | Nasal endoscopy and SPT | Adenoid volume and % with no associated allergy (p < 0.001). |
| Karaca et al.1938 | 2012 | 4 | Case series | Children with upper airway obstruction (n = 82) | Radiographic AH, clinical tonsillar hypertrophy, allergy sensitivity | Negative correlation: SPT and tonsil hypertrophy. No correlation: SPT and AH. |
| Sadeghi-Shabestari et al.1940 | 2011 | 4 | Cohort |
|
SPT for food, inhalant, and latex | Adenotonsillar hypertrophy and positive SPT 70.3%. No adenotonsillar hypertrophy and positive SPT 10%. (p = 0.04). |
| Modrzynski & Zawisza1935 | 2007 | 4 | Prospective unblinded, controlled |
|
Acoustic rhinometry, endoscopic adenoid exam | Increased adenoid size in birch allergic children during pollen season, decreased after pollen season, and prevented by allergy pharmacotherapy. |
| Cassano et al.1931 | 2003 | 4 | Cohort (recruitment not specified) | Children with nasal obstruction (n = 98, age 3–14 years) | Nasal endoscopy. “Allergic rhinitis was diagnosed by prick test and RAST in 22 patients” (20.9%) | % with “allergy” decreased with increasing adenoid size. Statistical significance not calculated. |
| Huang & Giannoni1937 | 2001 | 4 | Case-control |
|
SPT, otitis media, sinusitis, lower respiratory tract infection, secondhand smoke, sleep-disordered breathing | Higher prevalence of mold SPT positivity and lower respiratory tract infection (in some age groups) in AR plus AH group. |
AH = adenoid hypertrophy; AR; allergic rhinitis; IgE = immunoglobulin E; LOE = level of evidence; SPT = skin-prick test.
Exposure and sensitization to mold and AH has been specifically examined. Atan Sahin et al.1936 compared 242 children living in a less humid environment to 142 children living on the more humid Turkish Mediterranean coast. Mite-sensitive children in the coastal group had an increase in AH (p = 0.01). Those living in the more humid coastal location demonstrated increased mold and pollen sensitization but no significant correlation with adenoid hypertrophy was found. In contrast, Huang and Giannoni1937 compared 315 children with AH and AR to age-matched controls with AR-alone. There was a higher prevalence of positive skin tests to molds in the AH group (p = 0.013 to <0.0001). Dogru et al.1934 also reported an increased sensitization to Alternaria in children with both AH and AR compared to AR alone (p = 0.032), although a statistical correction for multiple variables was not described.
In studies where children were recruited by nasal obstruction, the degree of AH sometimes showed either no relationship or an inverse relationship with the prevalence of allergy sensitization. Cassano et al.1931 reported that the prevalence of specific inhalant IgE sensitization decreased as the AH increased: AH first degree (37% sensitized), AH second degree (35% sensitized), and AH third degree (19% sensitized). Karaca et al.1938 did SPT on 82 children who presented with upper airway obstruction to an otolaryngology clinic and compared allergy sensitization to radiographic adenoid size and clinically assessed tonsil size. They concluded that there was not a statistically significant association with adenoid size (p = 0.195) and a negative correlation with tonsil size (p = 0.045). The methods are vague on how the correlation was performed with tables showing percentages of “negative” SPT and the text incongruently stating “all of the cases were positive for at least 1 of the 14 allergens.”1938 Ameli et al.1939 assessed 205 children (mean age 6.7 years) with nasal endoscopy and SPT and found an association between negative SPT and adenoid volume (p < 0.0001). In an exception to the previously noted studies, Sadeghi-Shabestari et al.1940 compared 117 children aged 1 to 14 years with adenotonsillar hypertrophy to 100 controls of similar age for allergen SPT, total IgE, and smoking parents. They reported 70.3% of the adenotonsillar hypertrophy group had a positive SPT compared to 10% of the control group (p = 0.04); however, they included SPTs for foods (highest positive allergen subgroup) and latex.
In a study that is difficult to categorize by recruitment, 155 children (mean age 8.7 years) referred from Pediatric Allergy to Otolaryngology were assessed by rigid nasal endoscopy and SPT. Children on allergy medication were excluded. They observed a negative correlation between AH and allergen positivity (r = −0.208, p = 0.009).1941
Immunologic evidence of allergy in adenoid tissue is limited in the literature. Ni et al.1942 found a higher Th17/Treg ratio in adenoid tissue from children with AR than controls. Masieri et al.1943 reported Th1 gene expression in non-allergic adenoid tissue, Th1 and Th2 gene expression in adenoid tissue in those with AR treated with antihistamines, and a down regulation in Th1 and Th2 gene expression in adenoid tissue from children treated with SLIT. Both studies were small.
Treatment studies are also limited. One retrospective, uncontrolled study (n = 47) reported improvement in rhinitis symptoms in similar percentages for both AR (86%) and NAR (76%) after adenoidectomy.1944 The effect of INCS on reducing nasal obstruction in the setting of AH, independent of allergy, has been demonstrated in systematic reviews,1932,1945 but whether this is due to decrease in adenoid size is less clear and blinded studies are uncommon. 1946
In conclusion, there is a trend among allergic children who are assessed for AH to have increased prevalence AH compared to non-allergic controls. However, when children are selected for upper airway obstruction and then assessed for inhalant allergy sensitivity, a consistently increased prevalence of allergic sensitivity is not found. One potential explanation for this discrepancy is that symptomatic AH peaks in younger children than pediatric AR, with the allergic cohorts having a higher average age. This is supported in the literature by Pagella et al.1947 who retrospectively reviewed records of children referred to Otolaryngology for nasal symptoms (n = 795). They found an association between allergy and AH in children aged 8 to 14 years (p = 0.0043), but not for children aged 1 to 7 years (p = 0.34).
Aggregate Grade of Evidence: C (Level 4: 11 studies; Table X.F).
X.G. Otologic conditions Eustachian tube dysfunction
Ear symptoms are commonly experienced by patients with AR. Ear fullness and pressure, otalgia, popping or other sounds during swallowing, and transient hearing loss can all be manifestations of Eustachian tube dysfunction. The Eustachian tube opens into the nasopharynx and is in direct continuity with the upper respiratory tract. Inflammation of the nasal mucosa may involve the torus tubarius or Eustachian tube mucosa, resulting in obstruction that leads to negative pressure as middle ear gases are resorbed. Frequent sniffing or swallowing during nasal obstruction may transmit negative pressure to the middle ear space. The frequently observed clinical association of Eustachian tube symptoms and AR is corroborated by high-level evidence that demonstrates that in AR patients, nasal challenge with histamine or relevant aeroallergens results in transient Eustachian tube obstruction.1948-1950 These studies used the 9-step inflation-deflation swallow test of Eustachian tube function developed by Bluestone and Cantekin.1951 The development of negative middle ear pressure after allergen challenge corresponds with increases in nasal airway resistance.1952 AR appears to increase the incidence of Eustachian tube dysfunction relative to control populations,1953 and natural pollen exposure has been associated with negative middle ear pressures1954 and defects in Eustachian tube opening.1955 This body of evidence supports a direct causal role for AR in some cases of Eustachian tube dysfunction (Table X.G-1).
TABLE X.G-1.
Evidence for the role of allergic rhinitis in Eustachian tube dysfunction
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Skoner et al.1950 | 1987 | 1b | Double-blind crossover with provocation (histamine) |
|
Inflation-deflation swallow test of ET function | All AR subjects had ET obstruction after challenge. |
| Skoner et al.1949 | 1986 | 1b | Cohort with intervention (HDM nasal provocation) | HDM sensitive AR subjects with normal ET function | Inflation-deflation swallow test of ET function | 55% of ears developed ET obstruction after provocation. |
| Friedman et al.1948 | 1983 | 1b | Double-blind crossover, nasal provocation (pollen insufflation) | 8 adult AR subjects with ragweed or Timothy grass allergy | Inflation-deflation swallow test of ET function | Allergen intranasal challenge induces transient ET obstruction. |
| Osur et al.1955 | 1989 | 2b | Cohort | Children with AR, ragweed sensitive (n = 15) | 9-step ET function test | 60% of children developed ET obstruction during ragweed season. |
| Lazo-Saenz et al.1953 | 2005 | 3b | Case-control |
|
Tympanometry | AR pts had negative pressure. 15% of AR children had type B or C tympanograms. |
| Knight et al.1954 | 1992 | 4 | Cohort | SAR patients | Middle ear pressure on tympanometry, ETD symptoms during pollen season | Symptoms or tympanogram evidence of ETD in 24% of subjects. Increased to 48% in pollen season. |
| O’Connor et al.1952 | 1984 | 4 | Cohort | Children with AR (n = 37) | Middle ear pressure and nasal airway resistance after pollen challenge | 69% of children had negative middle ear pressure after challenge. |
AR = allergic rhinitis; ET = Eustachian tube; ETD = Eustachian tube dysfunction; HDM = house dust mite; LOE = level of evidence; SAR = seasonal allergic rhinitis.
Aggregate Grade of Evidence: C (Level 1b: 3 studies; Level 2b: 1 study; Level 3b: 1 study; Level 4: 2 studies; Table X.G-1).
Otitis media
The role of allergy as a causative factor in otitis media has not been clearly demonstrated. Historically, allergy was considered an important etiologic factor in otitis media. However, as clinical definitions have become more stringent and evidence expectations have evolved, it has become apparent that a clear etiopathogenic connection between AR and otitis media is yet to be demonstrated. Investigations into the connection between these 2 conditions have examined the evidence for type 1 IgE-mediated inflammation in the middle ear space, epidemiologic associations between the 2 conditions, and the effect of allergy treatment on otitis outcomes. The middle ear mucosa may behave in a manner similar to nasal mucosa and be a site of local IgE-mediated inflammatory reactions.1956-1958 However, direct intranasal allergen challenge in allergic subjects does not appear to cause otitis media.1948-1950 Studies of the epidemiologic association of AR or atopy and otitis media with effusion (OME) are widely discordant. Some studies have found no significant difference in allergic sensitization or clinical allergy in OME patients compared to control groups,1959,1960 while others have shown a dramatically increased prevalence of IgE sensitization or clinical allergy in OME patients,1961-1964 or that AR is an independent risk factor for the development of OME.1965 Finally, some studies suggest a nearly universal association of OME and allergic disease.1966-1970 These inconsistencies in the literature are likely related to highly selected patient populations in specialty practices, variability in allergy test methods, and the problems incumbent in identifying appropriate control groups. Thus, the relationship of allergy and OME remains unclear (Table X.G-2).
TABLE X.G-2.
Evidence for the role of allergic rhinitis in otitis media
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Yeo et al.1960 | 2007 | 2b | Cohort with control group |
|
History, SPT | AR was present in 28% of OME group vs 24% of control. |
| Caffarelli et al.1959 | 1998 | 2b | Cohort with control group |
|
SPT and tympanogram for all subjects | Equal rates of sensitization between OME group and controls. |
| Chantzi et al.1961 | 2006 | 3b | Case-control |
|
Allergy history and tests | IgE sensitization is independent risk factor for OME. |
| Corey et al.1964 | 1994 | 3b | Case-control |
|
RAST | Positive RAST: 61% in OME group vs 41% in controls |
| Borge1963 | 1983 | 3b | Case-control |
|
Allergy history and testing | 41% of serous OM patients had perennial rhinitis vs 11% of controls. |
| Kreiner-Moller et al.1965 | 2012 | 4 | Case series | 6-year-old children (n = 262) | Assessment for OME and allergy | 39% of cohort had OME. OR of 3.36 for AR and OME. |
| Hurst1966 | 2008 | 4 | Cohort |
|
Resolution of effusion or drainage at 2-year to 8-year follow-up | 100% of OME had positive allergy tests; 85% of AIT treated patients cured. |
| Alles et al.1969 | 2001 | 4 | Cohort | 3-year-old to 8-year-old children with OME | Assessment of AR, asthma, eczema | 57% with positive SPT, almost all with rhinitis. |
| Hurst1967 | 1996 | 4 | Cohort |
|
Allergy tests, effusion, ECP | Allergies in 97% of COME. |
| Hurst1968 | 1990 | 4 | Cohort | 20 OME patients, all allergic: 17 treated with AIT, 3 untreated controls | AIT or food elimination diet | All patients treated with AIT or food elimination resolved. |
| Tomonaga et al.1962 | 1988 | 4 | Cohort | 259 children with OME; 605 nasal allergy; 104 controls | Allergy testing; tympanometry | 50% of OME cases had nasal allergy vs 17% control. |
| Bernstein et al.1956 | 1985 | 4 | Cohort | 100 patients with OME: 35 allergic, 65 non-allergic | Total and specific IgE in MEE and serum | 23% of allergic OME patients had evidence of local IgE. |
| Bernstein et al.1957 | 1983 | 4 | Cohort | 77 children with recurrent OME and history of myringotomy tubes | Allergy evaluation, serum, nasal, MEE total IgE | Higher levels of IgE in MEE of allergic children. |
| Bernstein et al.1958 | 1981 | 4 | Cohort | 41 patients with OME: 20 allergic, 21 non-allergic | Total and specific IgE in MEE and serum | 15% of allergic OME cases had evidence of local IgE. |
| McMahan et al.1970 | 1981 | 4 | Case series | 119 COME patients | RAST test | 93% of COME positive to inhalant allergens. |
AIT = allergen immunotherapy; AR = allergic rhinitis; COME = chronic otitis media with effusion; IgE = immunoglobulin E; LOE = level of evidence; MEE = middle ear effusion; OM = otitis media; OME = otitis media with effusion; OR = odds ratio; RAST = radioallergosorbent test; SPT = skin-prick test.
In general, randomized placebo-controlled trials have shown that INCS do not improve OME outcomes.1971-1973 Also, a Cochrane systematic review found no benefit of antihistamines and/or decongestants in the treatment of OME. Thus, traditional medical treatments for AR do not appear to be an effective option for OME and recent otitis media CPGs recommend against the use of these agents.1974 Additional investigation is needed to discern the effect of allergy on the incidence or natural history of OME and to determine if AIT has beneficial effects.
Aggregate Grade of Evidence: C (Level 2b: 2 studies; Level 3b: 3 studies; Level 4: 11 studies; Table X.G-2).
Inner ear disease
Meniere’s disease is characterized by recurring episodes of tinnitus, hearing loss, aural fullness, and vertigo. The basic pathophysiologic defect in Meniere’s disease appears to be a dysregulation of endolymph in the inner ear (endolymphatic hydrops).1975 An immunologically-mediated disturbance in fluid handling by the endolymphatic sac has been postulated as 1 cause for the disease.1976 The notion that “allergy” of the inner ear is a cause of Meniere’s disease predates our modern understanding of type 1 IgE-mediated hypersensitivity, and is still evoked as a possible causative or contributing factor for the disease in some individuals. Indeed, AR has been postulated as a cause of inner ear dysfunction,1977 and a connection between allergy and inner ear disorders such as Meniere’s disease is plausible based on compiled circumstantial evidence. Derebery and colleagues have published studies suggesting that inhalant and food allergies are more common in Meniere’s patients,1978 and that allergy treatment including AIT results in improved Meniere’s disease symptoms.1979,1980 However, these studies generally provide low grade evidence, and aside from 1 small study that also found a higher prevalence of IgE-mediated hypersensitivity in Meniere’s patients,1981 these findings have not been duplicated by others. Case-control studies examining total serum IgE levels have provided conflicting results.1981,1982 A few small studies have shown changes in objective parameters such as the electrocochleographic summating potential/action potential (SP/AP) ratio in response to aeroallergen or food challenge in Meniere’s patients.1983,1984 Overall, the evidence supporting a connection between type 1 IgE-mediated hypersensitivity and Meniere’s disease is of low grade, with substantial defects in study design (Table X.G-3).
TABLE X.G-3.
Evidence for the role of allergic rhinitis in inner ear disease
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Singh et al.1977 | 2011 | 3b | Case-control |
|
Audiometry, OAE, ABR | AR subjects had evidence of inner ear dysfunction. |
| Keles et al.1981 | 2004 | 3b | Case-control |
|
Peripheral blood lymphocyte populations, cytokines, allergen-specific and total IgE levels | Meniere’s patients are more likely to have positive allergy test. 41% Meniere’s patients had elevated total IgE. |
| Derebery & Berliner1978 | 2000 | 3b | Case-control |
|
Allergy symptoms, history questionnaire | Meniere’s disease patients have more AR and food allergy. |
| Hsu et al.1982 | 1990 | 3b | Case-control |
|
Serum total IgE | No difference in serum total IgE between groups. |
| Derebery1979 | 2000 | 4 | Cohort |
|
Self-reported symptoms via post treatment survey | Allergy treatment reduced tinnitus and vertigo. |
| Gibbs et al.1983 | 1999 | 4 | Case series | 7 patients with Meniere’s and inhalant allergy | Change in ECoG after allergen challenge | 57% of subjects had >15% change in SP/AP ratio after challenge. |
| Derebery & Valenzuela1980 | 1992 | 4 | Cohort | 93 Meniere’s disease patients with suspected allergy | Intradermal test, in vitro allergy tests, serum IgE, provocative food testing, AIT response | 82% had normal serum IgE; AIT improved vertigo in 62% |
| Viscomi & Bojrab1984 | 1992 | 4 | Cohort | 5 patients with Meniere’s disease and AR | Allergen challenge with intracutaneous provocative food test. >15% change in SP/AP ratio on ECoG, provocation of Meniere’s symptoms | 6/27 intracutaneous food challenges had induction of aural symptoms and >15% change in SP/AP ratio. |
ABR = auditory-brainstem response; AIT = allergen immunotherapy; AR = allergic rhinitis; ECoG = electrocochleography; IgE = immunoglobulin E; LOE = level of evidence; OAE = oto-acoustic emissions.
Aggregate Grade of Evidence: C (Level 3b: 4 studies; Level 4: 4 studies; Table X.G-3).
X.H. Cough
Cough is a sudden reflex used to clear the breathing passage of any foreign particles or irritants. There is evidence that vagal afferent nerves regulate an involuntary cough; yet, there is also cortical control of this overall visceral reflex.1985 Cough is often considered a comorbidity of AR. The rhinobronchial reflex is 1 of the mechanisms that may explain the ability of stimuli on the nasal mucosa, such as an allergen, to result in direct bronchospasm.1986 The role of descending secretions (postnasal drip) from the upper to lower airways is a second theory. While many practitioners link postnasal drainage to cough, there is very little evidence to support this. When functioning normally, the vocal folds protect the lower airways from upper airway secretions and foreign bodies. Third, a direct mechanism due to diffuse inflammation and activation of eosinophils may be responsible for the common upper and lower airway manifestations. The American College of Chest Physicians evidence-based clinical practice guidelines on cough suggest the term upper airway cough syndrome, rather than postnasal drip syndrome, when discussing a cough originating from the upper airway due to the varying possible causes.1985
AR and asthma may coexist and may indeed produce a continuum of the same airway disease.1167 Associations with cough in AR patients can relate to their underlying asthma or a seasonal asthma during peak pollen season. The Asia Pacific Burden of Respiratory Diseases study, a 1000-person cross-sectional observational study, revealed that cough was the primary reason for a visit to the physician for patients with asthma and or COPD. However, AR patients were more likely to present with classic watery, sneezing, runny nose. The study however did find that 33.5% of patients were diagnosed with combinations of respiratory disease; the most frequent was asthma and AR1987,1988 (Table X.H).
TABLE X.H.
Evidence for the association between allergic rhinitis and cough
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| He et al.1994 | 2016 | 2b | Cohort, prospective nonrandomized | AR patients (n = 2713) | sIgE, questionnaire | D. pteronyssinus was the most common offending allergen. The occurrence cough increased with increasing AR severity. |
| Passali et al.1986 | 2011 | 2b | Individual cohort | 159 patients from 9 otolaryngology and pulmonary centers | Standardization of diagnostic approach for rhinobronchial syndrome | Increased frequency of rhinobronchial syndrome with allergic disease (37.9% vs 20.9%). Cough was a frequent symptom (96%). |
| Krzych-Falta et al.1989 | 2015 | 3b | Case-control |
|
Safety evaluation of nasal allergen challenge | In early phase of allergic reaction, extranasal symptoms were observed (cough, breathlessness), especially in PAR patients. |
| Chakir et al.1991 | 1996 | 3b | Case-control |
|
Immunohistochemical analysis of the distribution of collagens, laminin, and fibronectin in bronchial biopsy specimens | Content of type I and III collagens was increased in rhinitic subjects compared with controls, suggesting active structural remodeling in the lower airways of AR patients. |
| Cho et al.1993 | 2016 | 4 | Case series | Patients ages ≥18 years with asthma, AR, COPD, or rhinosinusitis (n = 5250) | Patient and physician surveys | Report of cough symptom: COPD (73%), followed by asthma (61%), rhinosinusitis (59%), AR (47%). Cough as the main reason for seeking medical care: COPD (43%), asthma (33%), rhinosinusitis (13%), and AR (11%). |
| Ghoshal et al.1988 | 2016 | 4 | Case series | Patients aged ≥18 years with asthma, AR, COPD, or rhinosinusitis (n = 1,000) | Survey regarding symptoms, healthcare resource utilization, work productivity, activity impairment. Cost analysis. | Asthma was the most frequent primary diagnosis followed by AR, COPD, and rhinosinusitis. 33.5% patients were diagnosed with combinations of the 4 respiratory diseases. |
| Lin et al.1987 | 2016 | 4 | Case series | Patients aged ≥18 years with asthma, AR, COPD, or rhinosinusitis (n = 1001) | Survey regarding symptoms, healthcare resource utilization, work productivity, activity impairment. | AR was the most frequent primary diagnosis (31.2%). Cough was the primary reason for the medical visit for patients with asthma and COPD. Nasal symptoms were the primary reasons for AR and rhinosinusitis. |
| Chakir et al.1990 | 2000 | 4 | Case series | Adults with SAR, nonasthmatic (n = 12) | Immunohistochemistry and cytokine expression of bronchial biopsy specimens. | Natural pollen exposure is associated with an increase in lymphocyte numbers, eosinophil recruitment, and IL-5 expression in the bronchial mucosa of nonasthmatic subjects with SAR. |
| Buday et al.1992 | 2016 | 5 | Bench research | 30 guinea pigs divided into the HDM-sensitized group, OVA-sensitized group, and control group | Symptoms of AR induced by intranasal application of 15 μL 0.5% HDM and cough challenges with citric acid. Airway resistance measurements. | Both HDM and OVA-sensitized groups showed a significantly enhanced nasal reactivity and cough response compared with controls. The airway resistance data did not show significant differences. |
AR = allergic rhinitis; COPD = chronic obstructive pulmonary disease; HDM = house dust mite; IL = interleukin; LOE = level of evidence; OVA = ovalbumin; PAR = perennial allergic rhinitis; SAR = seasonal allergic rhinitis; sIgE = allergen-specific immunoglobulin E.
While patients with AR that have concomitant chest symptoms such as cough often do have asthma, seasonal asthma, and/or a nonspecific bronchial hyperreactivity, many studies show generalized inflammation of the upper airways extending to the lower airways. There is a complex interplay between cells and inflammatory cytokines and hence one should consider the upper and lower airways as a single unique functional unit.1986 The key pathogenic mechanism is the inflammation of the upper airways with extension to the lower airways and the induction of a systemic dysregulation via a complex interaction between cells and inflammatory cytokines.1986
Many patients with AR and cough do not have the diagnostic airflow obstruction or the reversibility of forced expiratory volume in 1 second (FEV1) following bronchodilator administration to make a diagnosis of asthma.1167 Krzych-Falta et al.1989 performed a nasal challenge in 30 patients with AR. Extranasal symptoms were noted, including a cough and breathlessness, especially in those with PAR. In 2000, Chakir et al.1990 performed histochemical tests on bronchial biopsies of patients with AR but without current or history of asthma. They demonstrated increased numbers of lymphocytes, eosinophil recruitment and IL-5 expression in the bronchial mucosa after exposure with natural pollen.1990 This 2000 study followed a prior investigation of deposition of type I and III collagens and fibronectin by bronchial myofibroblasts in AR patients.1991 This is suggestive of an active structural remodeling of the lower airways in AR patients that is similar to asthma patients but less severe. In addition, Buday et al.1992 demonstrated that guinea pigs sensitized to HDM had a significantly enhanced cough response compared to those that were not sensitized; however, airway resistances did not change. This study is relevant to humans, since the neurophysiology of the vagus nerve in the guinea pig is thought to be closest to humans. These studies demonstrate that AR, unrelated to asthma, can indeed result in bronchial inflammation, possible lower airway remodeling and ultimately a symptom of cough.
A large-scale cross-sectional, multinational observational study set out to determine the symptom of cough as it relates to respiratory diseases in the Asia-Pacific region. With over 5250 patients enrolled, the study found that 47% of patients with AR frequently reported cough as a symptom; however, only 11% of these patients had cough as the main reason for seeking medical care.1993 The numbers were 61% and 33%, respectively, for patients with asthma and cough. In a prospective study with 2713 AR patients, He et al.1994 found the occurrence of comorbidities, including cough, to gradually increase from mild intermittent, to mild persistent, to moderate-severe intermittent, and moderate-severe persistent AR.
There is low level evidence that associates AR with cough or, more commonly, cough as a comorbidity of AR.1990-1992 The severity of AR may affect its manifestation toward upper airway cough syndrome.1994 AR is often a comorbidity with asthma which also has an increased correlation with cough. The exact pathways and mechanisms by which the unified airway functions continue to unfold.
Aggregate Grade of Evidence: C (Level 2b: 2 studies; Level 3b: 2 studies; Level 4: 4 studies; Level 5: 1 study; Table X.H).
X.I. Laryngeal disease
AR has been implicated as a cause of laryngeal disease. However, further understanding of its precise role has been limited. While previous research has provided anecdotal evidence of a relationship between the 2, establishing a causal relationship between AR and laryngeal dysfunction had proven difficult due to a lack of safe and effective models for studying the larynx.1995 Findings of laryngeal inflammation have largely been attributed to laryngopharyngeal reflux (LPR), but various etiologies may contribute to laryngeal dysfunction.
Vocal dysfunction can have a significant psychosocial impact on patients, including those with AR. Several studies have reported higher Voice Handicap Index (VHI) scores in patients with AR compared to control subjects.1996-1999 Dysphonia is particularly disturbing for professional voice users. Singers with self-perceived voice issues were 15% more likely to have AR than singers without vocal complaints.2000 The likelihood of AR increased as the number of vocal symptoms increased.2000 When comparing patients with AR and NAR to control patients, Turley et al.2001 found that dysphonia was more prevalent in patients with asthma. A prior study had similar overall findings in patients with AR while controlling for asthma.2002 Studies have reported the adverse effects of AR on voice-related QOL, and Turley et al.2001 validated this by showing that patients who reported poor rhinitis-related QOL on questionnaires also had poor voice-related QOL and more severe chronic laryngeal symptoms.1996,1998 The greater the degree of allergen load, the greater severity of vocal symptoms.1999 Overall, patients with vocal dysfunction have a higher than anticipated incidence of AR and vice versa1999,2001,2002 (Table X.I).
TABLE X.I.
Evidence for an association between allergic rhinitis and laryngeal disease
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Roth et al.2008 | 2013 | 2b | RCT | Patients responding to an advertisement | Effect of allergen on larynx | Relationship between allergen exposure and impaired vocal function independent of asthma or nasal exposure. |
| Dworkin et al.2010 | 2009 | 2b | RCT | Adults testing positive for HDM allergy:
|
Effect of allergen on larynx | Laryngeal abnormalities occurred secondary to lower respiratory stimulation. |
| Krouse et al.1998 | 2008 | 2b | Prospective cohort |
|
Effect of allergen on larynx | Significant changes in VHI in patients with HDM allergy. Findings present among subjects without symptomatic LPR/GERD. |
| Millqvist et al.1996 | 2006 | 2b | Case-control |
|
Prevalence of vocal dysfunction | Statistically significant differences in VHI between allergic patients and controls. |
| Reidy et al.2009 | 2003 | 2b | RCT |
|
Effect of allergen on larynx | No significant differences between antigen and placebo exposed subjects on any measure. |
| Roth & Ferguson1995 | 2010 | 3a | Systematic review | Relationship of allergy and laryngeal disease | Not applicable | Further investigations into mechanisms mediating laryngeal response to allergy are necessary. |
| Brook et al.2011 | 2015 | 3b | Retrospective case-control |
|
Endoscopic findings in AR | Findings within the nasopharynx, rather than the larynx, are predictive of positive atopic status. |
| Koc et al.1997 | 2014 | 3b | Case-control |
|
Laryngeal findings in AR | AR patients had higher incidence of dysphonia and mean VHI. |
| Turley et al.2001 | 2011 | 3b | Case-control |
|
Prevalence of dysphonia | Patients with AR or NAR had higher prevalence of dysphonia versus controls. Patients with worse rhinitis symptoms had worse voice-related QOL and more severe chronic laryngeal symptoms. |
| Hamdan et al.2000 | 2006 | 3b | Retrospective case-control |
|
Symptom prevalence | Incidence of AR in singers is high. Occult allergies may affect professional voice. |
| Brook et al.2004 | 2016 | 4 | Retrospective case series | Patients undergoing in vitro allergy testing | Symptom prevalence | Yield of in vitro allergy testing for laryngeal symptoms comparable to other common allergy testing indications. |
| Eren et al.2005 | 2014 | 4 | Case series | Patients referred from allergy clinic with SPT testing | Laryngeal findings in AR and LPR | Thick endolaryngeal mucus was a predictor of allergy. No association between allergic sensitization and presence of LPR. No significant difference in laryngeal appearance between allergy-positive and LPR-positive individuals. |
| Randhawa et al.2003 | 2010 | 4 | Case series | Patients with primary voice disorder or globus sensation | Prevalence of AR and LPR | Three times as many patients had allergies compared with LPR, no statistical significance. |
| Randhawa et al.1999 | 2010 | 4 | Cross sectional | Patients presenting to rhinology clinic, no prior voice-related symptoms | Allergy and vocal dysfunction association | The degree of allergen load correlates with the severity of vocal symptoms, as per an increase in score on the VHI. |
| Simberg et al.2002 | 2007 | 4 | Cross sectional |
|
Symptom prevalence | Individuals with allergies had more severe vocal symptoms than non-allergic controls. Patients who had undergone AIT >2 years had fewer symptoms. |
| Jackson-Menaldi et al.2012 | 1997 | 4 | Prospective cohort | Subjects referred to voice center with a voice problem | Association between AR, LPR, laryngeal findings | Could not determine causative relationship between allergy and vocal symptoms. |
| Belafsky et al.2006 | 2015 | 5 | Bench research | Guinea pigs exposed to:
|
Mean eosinophilic profile in the glottic, subglottic, and tracheal epithelium and submucosa | Iron soot and HDM resulted in eosinophilia in glottic, subglottic, and tracheal epithelium and submucosa. |
| Mouadeb et al.2007 | 2009 | 5 | Bench research | Guinea pigs exposed to intranasal HDM for 9 weeks | Histopathologic findings | Twice as much eosinophilia in supraglottis in animals exposed to HDM vs saline. |
AIT = allergen immunotherapy; AR = allergic rhinitis; GERD = gastroesophageal reflux; HDM = house dust mite; LOE = level of evidence; LPR = laryngopharyngeal reflux; NAR = non-allergic rhinitis; QOL = quality of life; RCT = randomized controlled trial; SPT = skin-prick test; VHI = Voice Handicap Index.
Allergic laryngitis can be difficult to distinguish from other laryngeal inflammatory disorders, including LPR, due to the limitations of current diagnostic methods, which overall have poor specificity and interrater reliability. In a study of patients presenting with voice complaints, Randhawa et al.2003 noted that two-thirds of patients were diagnosed with allergies whereas only one-third were diagnosed with LPR. However, allergy testing may be positive in up to 46% of the general population.2004 Laryngeal findings in AR and LPR can be indistinguishable and include laryngeal edema, excessive mucus, vocal fold erythema, and arytenoid erythema.1995,2005 A study by Eren et al.2005 supported this diagnostic challenge in demonstrating no significant difference in the appearance of the larynx between allergy-positive and LPR-positive subjects; however, thick endolaryngeal mucus has been shown to be a predictor of allergy. Belafsky et al.2006 and Mouadeb et al.2007 examined the effects of Dermatophagoides on the laryngeal mucosa of guinea pigs and found an increase in eosinophilia compared to those exposed to saline, which provides some support for etiologies other than reflux contributing to laryngeal disease. In contrast, Krouse et al.1998 were unable to demonstrate a difference in acoustic and speech aerodynamic testing or videostroboscopic evaluation between allergic patients compared to control subjects.
Despite anecdotal evidence implicating the role of allergic laryngitis in laryngeal dysfunction, there have been limited studies demonstrating a direct causal relationship between the 2. Three studies with similar design evaluated the symptoms and laryngeal appearance and function in patients with proven allergies exposed to direct laryngeal stimulationship by the nebulized allergen D. pteronyssinus.2008-2010 Initially, Reidy et al.2009 were unable to find any significant difference between antigen-challenged and placebo-challenged subjects on any of the evaluated measures, including VHI, Sinus Symptoms Questionnaire, laryngoscopic findings, and acoustic and speech aerodynamic testing. In a subsequent study, Dworkin et al.2010 increased the concentration of allergen in the antigenic suspension and noted an increase in endolaryngeal mucus in addition to coughing and throat clearing. The study was terminated prematurely due to adverse pulmonary reactions attributed to the higher antigen concentration, and it is possible that the lower airway reactivity contributed to the visualized endolaryngeal mucus.2010 Roth et al.2008 then performed a study using similar methods but isolated the larynx by utilizing a nose clip to ensure oral inhalation and by eliminating patients with reactive airways based on methacholine challenge testing. They demonstrate an apparent causal relationship between allergen stimulation and impaired vocal function.2008
There is mounting evidence suggesting a relationship between AR and laryngeal dysfunction. There have not been consistently reported laryngeal findings specific to allergic laryngitis, though thick endolaryngeal mucous should raise suspicion for allergy as a cause. Although its exact role in the pathophysiology of laryngitis has yet to be fully elucidated, AR should be considered in the differential diagnosis of patients with vocal complaints as it may have implications on treatment of laryngeal disease.
Aggregate Grade of Evidence: C (Level 2b: 8 studies; Level 3a: 1 study; Level 3b: 4 studies; Level 4: 5 studies; Table X.I).
X.J. Eosinophilic esophagitis (EoE)
Eosinophilic esophagitis is an allergic inflammatory condition of the esophagus with infiltration of eosinophils. Symptoms include dysphagia, heartburn, and vomiting. Several studies have examined the prevalence of clinician-diagnosed AR and aeroallergen sensitization in patients with eosinophilic esophagitis (EoE) (Table X.J). Among both pediatric and adult patients with EoE, it has consistently been found that 50% to 75% have AR.2013-2020 Although many of these studies were case series, the consistency of the findings strongly suggests that most patients with EoE have comorbid AR.
TABLE X.J.
Evidence for the association between allergic rhinitis and eosinophilic esophagitis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Evidence of AR prevalence in patients with EoE | ||||||
| Furuta et al.2015 | 2007 | 3a | Systematic review | Adult and pediatric patients with EoE | Demographic and clinical characteristics | 50% to 80% had AR and sensitization to aeroallergens. |
| Spergel et al.2013 | 2009 | 4 | Case series | Pediatric patients with EoE (n = 562) | Demographic and clinical characteristics | 68% were atopic and 43% had AR. |
| Roy-Ghanta et al.2014 | 2008 | 4 | Case series | Adult patients with EoE (n = 23) | Demographic and clinical characteristics | 78% had AR; 86% were sensitized to aeroallergens. |
| Assa’ad et al.2016 | 2007 | 4 | Case series | Pediatric patients with EoE (n = 89) | Demographic and clinical characteristics | 79% were sensitized to environmental allergens. |
| Plaza-Martin et al.2017 | 2007 | 4 | Case series | Pediatric patients with EoE in Spain (n = 14) | Demographic and clinical characteristics | 93% had AR and sensitization to aeroallergens. |
| Sugnanam et al.2018 | 2007 | 4 | Case series | Pediatric patients with EoE in Australia (n = 45) | Demographic and clinical characteristics | 93% had AR. |
| Remedios et al.2019 | 2006 | 4 | Case series | Adult patients with EoE in Australia (n = 26) | Demographic and clinical characteristics | 77% were atopic and 54% had AR. |
| Guajardo et al.2020 | 2002 | 4 | Case series | Adult and pediatric patients with EoE in worldwide registry (n = 39) | Demographic and clinical characteristics | 64% had AR. |
| Evidence for role of aeroallergens in EoE pathogenesis | ||||||
| Ramirez & Jacobs2025 | 2013 | 4 | Case report | A pediatric patient with EoE and dust mite allergy treated with dust mite immunotherapy | Eosinophils on esophageal biopsies | Resolution of esophageal eosinophilia was observed after course of dust mite immunotherapy. |
| Moawad et al.2021 | 2010 | 4 | Case series | Adult patients with EoE (n = 127) | Season of EoE diagnosis and correlation with pollen counts | Highest percentage (33%) diagnosed in spring and lowest (16%) in winter; significant correlation with grass pollen counts. |
| Almansa et al.2022 | 2009 | 4 | Case series | Adult patients with EoE (n = 41) | Season of EoE diagnosis | 68% diagnosed in spring and summer vs 32% in fall and winter. |
| Wang et al.2023 | 2007 | 4 | Case series | Pediatric patients with EoE (n = 234) | Season of EoE diagnosis and biopsy findings by season | Significantly fewer patients diagnosed with EoE in winter vs spring, summer, and fall; least intense esophageal eosinophilia in winter. |
| Fogg et al.2024 | 2003 | 4 | Case report | Pediatric patient with EoE | Seasonal biopsy findings | Increased esophageal eosinophilia during pollen seasons. |
AR = allergic rhinitis; EoE = eosinophilic esophagitis; LOE = level of evidence.
The evidence for an association between environmental allergies and EoE pathogenesis is less clear. A few case series, among both children and adults, have observed seasonal peaks of EoE diagnosis in the spring and summer.2021-2023 One of these studies found that EoE diagnosis was correlated with grass pollen counts.2021 Another showed that esophageal eosinophilia on biopsies was least intense in the winter.2023 There is 1 reported case of a pediatric EoE patient whose symptoms flared seasonally, in whom biopsies revealed moderate to severe esophageal eosinophilia during pollen seasons with no or mild inflammation in winter months, with no change in diet.2024 Another case report described resolution of esophageal eosinophilia in a pediatric patient with EoE and dust mite sensitization after a course of high-dose dust mite immunotherapy.2025 Therefore, there is very limited observational data suggesting a potential association between aeroallergens and EoE pathogenesis, but more study is needed.
Aggregate Grade of Evidence: C (Level 3a: 1 study; Level 4: 12 studies; Table X.J).
X.K. Sleep disturbance and obstructive sleep apnea (OSA)
Nasal congestion is reported by as many as 90% of AR patients.2026 Nocturnal nasal congestion can significantly affect sleep quality. Nasal obstruction due to AR has been well established as a cause of sleep disruption.707,714,2026 One population-based survey study of children with AR identified sleep disturbance due to AR as a significant factor affecting health-related QOL.2027 Diminished sleep quality resulting from AR has been shown to negatively impact work performance and productivity.2028 Another population-based study found that patients with AR were more likely to report suffering from insomnia, snoring and sleep apnea than control groups.727 The severity of AR symptoms was also shown to affect the duration of sleep, frequency of daytime somnolence, and sleep latency. The influence of AR on sleep is multifactorial. Upper airway resistance, biochemical and hormonal effects, and pharmacologic interventions all play a role in altering sleep. A large population-based survey of AR patients demonstrated a strong correlation between AR disease severity and sleep disturbance.679 The study showed that increasing severity of AR symptoms caused worse sleep quality.
When establishing a diagnosis of AR, the impact of allergy symptoms on sleep should be assessed by detailed history. There are several different instruments, which have been used to assess the impact of AR on sleep. These include: the ESS, Stanford Sleepiness Score, Jenkins Questionnaire, Pittsburgh Sleep Quality Index, University of Pennsylvania Functional Outcomes of Sleep, Sleep scale from the Medical Outcome Study, Sleep Disorders Questionnaire, The Pediatric Sleep Questionnaire, and The Pediatric Daytime Sleepiness Scale. These metrics may be useful in establishing baseline symptoms and monitoring a response to treatment.
There have been several studies that have investigated the relationship between AR and sleep-disordered breathing (SDB) (Table X.K). SDB refers to a spectrum of conditions including primary snoring, upper airway resistance syndrome, and obstructive sleep apnea. In a population-based analysis, Young et al.714 found that moderate-to-severe SDB were 1.8 times more frequent in participants with nasal congestion due to allergy. In a small case series of patients with SAR who underwent repeat PSG, patients with symptomatic AR had an average 1.7 occurrences of obstructive apnea per hour of sleep that decreased to 0.7 per hour when patients were symptom free.718 A 2011 case-control study assessing differences in polysomnography between persistent AR sufferers and healthy controls found no statistically significant difference in apnea-hypopnea index (AHI) between the 2 groups.720 There were modest differences in sleep efficiency, arousal index, and snoring time.
TABLE X.K.
Evidence for an association between allergic rhinitis and sleep disturbance
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Yamada et al.673 | 2012 | 1b | RCT | PAR, adults (n = 57) | ESS and RQLQ | INCS mometasone significantly improves nasal symptoms, QOL, sleep quality, and upper airway condition. |
| Meltzer et al.1276 | 2010 | 1b | RCT | PAR, adults (n = 30) | PSG, ESS, RQLQ-S, and WPAI-AS | INCS mometasone improves nasal symptoms and sleepiness. |
| Craig et al.1275 | 2003 | 1b | RCT | AR, adults (n = 32) | PSG, ESS, RQLQ, direct sleep questions in daily diary | Improvement in NC and sleep with treatment with topical nasal fluticasone. |
| Hughes et al.706 | 2003 | 1b | RCT | PAR, adults (n = 22) | ESS, SSS, FOSQ, RQLQ | INCS budesonide improved daytime fatigue, somnolence and quality of sleep. |
| Craig et al.707 | 1998 | 1b | RCT | PAR, adults (n = 20) | Direct sleep questions in daily diary | Improvement in congestion and sleep with treatment with INCS flunisolide. |
| Sherkat et al.2030 | 2011 | 2b | RCT | AR, adults (n = 14) | ESS, PSQI, FOSQ, RQLQ, NRQLQ, Pennsylvania Quality of Life, direct sleep questions in daily diary | Sleep quality is not significantly affected by pseudoephedrine. |
| Colas et al.726 | 2012 | 2c | Population-based | AR, adults (n = 2275) | PSQI RQLQ, direct sleep questions based on Epworth scale | Moderate-severe AR and NC are associated with worse sleep quality. |
| Meltzer et al.2027 | 2009 | 2c | Population-based | AR, children (n = 1004) | Direct sleep questions by telephone interviews | AR disrupts the pattern and quality of sleep. |
| Bousquet et al.2028 | 2006 | 2c | Population-based | AR, adults (n = 3052) | Jenkins Questionnaire, RQLQ, WPAI-AS | The severity of the AR has more effect on QOL and sleep, than the duration (intermittent/persistent). |
| Leger et al.727 | 2006 | 2c | Population-based | AR, adults (n = 591) | ESS, Sleep Disorders Questionnaire, Score for Allergic Rhinitis | All dimensions of sleep were impaired by AR, and more impaired in severe AR than in mild AR. |
| Young et al.714 | 1997 | 2c | Population-based | Adults (n = 4927) | PSG, direct sleep questions | Moderate-to-severe SDB was 1.8 times more frequent in participants with NC due to allergy. |
| Ishman et al.2034 | 2012 | 3b | Case-control | AR, children (n = 21) | PSQ, PDSS, Obstructive Sleep Apnea-18 | AR children have higher SDB and sleepiness scores. |
| Meng et al.720 | 2011 | 3b | Case-control | PAR, adults (n = 98) | PSG | Differences in most PSG parameters including sleep efficiency, arousal index, and snoring time, statistically significant (though clinically modest). |
| Benninger & Benninger2036 | 2009 | 3b | Case-control | AR, adults (n = 701) | RSDI and sleep question by RSDI | AR has a significant negative impact on sexual function, sleep, and fatigue. |
| Meltzer et al.2037 | 2009 | 3b | Case-control | AR, adults (n = 7024) | MOS-Sleep and mini-RQLQ | AR adversely affects QOL and sleep parameters. |
| Yuksel et al.2035 | 2009 | 3b | Case-control | SAR, children (n = 14) | PSQI and actigraphy | Sleep dysfunction scores, sleep latency and fragmentation index are significantly higher in the AR group. |
| Shedden2026 | 2005 | 3b | Case-control | AR, adults and children (n = 2355) | Direct sleep questions | >80% with NC affected in some way at night, primarily causing them to wake up or made it difficult to fall asleep. |
| Stuck et al.731 | 2004 | 3b | Controlled trial | SAR, adults (n = 50) | ESS, SF-36, PSG | SAR increases daytime sleepiness, and worsens QOL. |
| Stull et al.682 | 2009 | 4 | Case series | AR, adults (n = 404) | MOS-Sleep, NRQLQ, WPAI-AS, PANAS-X | Those with more severe NC or ocular symptoms report poorer scores on sleep domains. |
| McNicholas et al.718 | 1982 | 4 | Case- series | SAR, adults (n = 7) | PSG | In patients with SAR, obstructive sleep apneas are more frequent during a period of symptomatic nasal obstruction. |
AR = allergic rhinitis; ESS = Epworth Sleepiness Scale; FOSQ = Functional Outcomes of Sleep; INCS = intranasal corticosteroid; LOE = level of evidence; mini-RQLQ = mini-Rhinoconjunctivitis Quality of Life Questionnaire; MOS-Sleep = Sleep Scale from the Medical Outcomes Study; NC = nasal congestion; NRQLQ = Nocturnal Rhinoconjunctivitis Quality of Life Questionnaire; PANAS-X = Positive and Negative Affect Schedule-Expanded Form; PAR = perennial allergic rhinitis; PDSS = Pediatric Daytime Sleepiness Scale; PSG = polysomnogram; PSQ = Pediatric Sleep Questionnaire; PSQI = Pittsburgh Sleep Quality Index; QOL = quality of life; RCT = randomized controlled trial; RQLQ = Rhinoconjunctivitis Quality of Life Questionnaire; RQLQ-S = Standardized Rhinoconjunctivitis Quality of Life Questionnaire; RSDI = Rhinosinusitis Disability Index; SAR = seasonal allergic rhinitis; SDB = sleep disordered breathing; SF-36 = Medical Outcomes Study 36-item Short Form health survey; SSS = Stanford Sleepiness Score; WPAI-AS = Work Productivity and Activity Impairment Questionnaire-Allergy-Specific.
A standard approach to the treatment of AR should help to decrease or alleviate the symptoms that adversely impact sleep. Medications that act to treat nasal congestion are typically effective at improving sleep quality. INCS have been shown to improve nasal congestion, daytime somnolence, and sleep quality.2029 INCS are also thought to improve sleep quality by reducing proinflammatory cytokines, which have been shown to negatively impact sleep.2030 There have been 5 RCTs assessing the efficacy of INCSs on nasal congestion and sleep.673,706,707,1275,1276 The results of all 5 studies demonstrated an improvement in sleep quality and sleep-related QOL metrics. A meta-analysis by Weiner et al.1297 found that INCSs were more effective than oral antihistamines at treating nasal blockage, although there was no significant differences between treatments on nasal resistance.
The pharmacologic interventions used in the treatment of AR may also have consequences on sleep. The first-generation H1 antagonists are known to cause sedation due to the capability of crossing the blood-brain barrier and acting as a depressant on the central nervous system leading to drowsiness.2031 While this may be a desirable side effect at bedtime, it is an undesirable consequence for daytime symptom management. The second-generation H1 antagonists have less propensity for crossing the blood-brain barrier and are therefore less sedating. Fexofenadine and loratadine are reported as the least sedating oral antihistamine treatment options.2032,2033 Patients should be counseled regarding the potential for sedation when taking oral H1 antihistamines. There has been 1 RCT study looking at pseudoephedrine (taken in the morning) and the impact on sleep quality, daytime somnolence, and fatigue. The study found no significant negative or positive impact on all measures compared to placebo.2030 There was a statistically significant beneficial effect on nasal congestion.
The impact of AR on sleep should be assessed by history, sleep and QOL questionnaires, and careful physical examination. A standard treatment algorithm for symptomatic management of AR should be effective at improving the symptoms which adversely affect sleep. INCSs are the most effective pharmacologic therapy for alleviating nasal congestion. Patients treated with oral antihistamines should be mindful of the potential for sedation.
Aggregate Grade of Evidence: B (Level 1b: 5 studies; Level 2b: 1 study; Level 2c: 5 studies; Level 3b: 7 studies; Level 4: 2 studies; Table X.K).
XI. Knowledge gaps and research opportunities
The existing literature related to AR is quite deep in certain areas but notably lacking in others (Table XI). We continue to see more and more citations related to AR every year, yet the process undertaken to produce this ICAR:AR document has identified some important knowledge gaps. The sections below highlight the need for future research related to specific aspects of AR.
TABLE XI.
Aggregate grades of evidence and recommendation levels
| Topic | Number of listed studies |
Aggregate grade of evidence |
Recommendation level |
Interpretation |
|---|---|---|---|---|
| Risk factors for AR | ||||
| Genetics | 5 (GWAS) | C | — | Some genes have been associated with development of AR and other atopic diseases. |
| In utero or early exposure (mites) | 6 | C | — | Data inconclusive. |
| In utero or early exposure (pollen) | 2 | C | — | Data inconclusive. |
| In utero or early exposure (animal dander) | 39 | C | — | Data inconclusive. |
| In utero or early exposure (fungal allergens) | 13 | C | — | Data inconclusive. |
| Restricted diet (during pregnancy and early childhood) | 5 | A | — | Maternal diet restriction while the child is in utero does not influence the development of AR. Food allergy during childhood is a risk factor for AR. |
| Pollution | 14 | C | — | Data inconclusive. |
| Tobacco smoke | 9 | A | — | Most studies found no association between active or passive tobacco smoke exposure and AR. Specific patient populations and temporal variations (ie, length of exposure) should be further evaluated. |
| Socioeconomic status | 10 | C | — | Most studies show an association between high SES and AR, but this is not a consistent finding across all studies. |
| Potential protective effect on the development of AR | ||||
| Breastfeeding | 2 (SRs) | C | Option | Option for breastfeeding for the specific purpose of AR prevention. In general, breastfeeding has been strongly recommended due to its multiple beneficial effects. |
| Pet exposure | 6 | C | — | No evidence that pet avoidance in childhood prevents AR later in life. Early pet exposure, especially dog exposure in non-allergic families early in childhood, may be protective. |
| Microbial diversity (“hygiene hypothesis”) | 15 | B | — | Microbial diversity of the skin, airways, and gut is important for the prevention of sensitization and allergic disease in populations. |
| Disease burden | ||||
| QOL | 33 | B | Recommendation | AR has significant effects on general and disease-specific. QOL Treatment of AR is recommended to improve QOL. |
| Effect on sleep | 46 | B | Recommendation | AR has significant negative effects on sleep. Treatment of AR is recommended to decrease sleep disturbance. |
| Evaluation and diagnosis | ||||
| Clinical examination (history and physical) | 4 | D | Recommendation | Despite the lack of studies to address clinical examination in the diagnosis of AR, history taking is essential and physical examination is recommended. Multiple prior guideline documents support this recommendation. |
| Nasal endoscopy | 5 | D | Option | Evidence does not support the routine use of nasal endoscopy for diagnosing AR. However, it may be helpful in ruling out other causes of symptoms. |
| Radiologic imaging | 0 | N/A | Recommend against | Radiologic imaging is not recommended for the diagnosis of AR. |
| Use of validated survey instruments | 10 | A | Strong recommendation | Validated survey instruments can be used to screen for AR, follow treatment outcomes, and as an outcome measure for clinical trials. |
| Skin-prick testing | 8 | B | Recommendation | SPT is recommended for evaluation of allergen sensitivities in appropriately selected patients. The practitioner may decide whether skin or in vitro sIgE testing is best in an individual patient. |
| Skin intradermal testing | 17 | B | Option | Intradermal testing may be used to determine specific airborne allergen sensitization for individuals suspected of having AR. |
| Blended skin testing techniques | 5 | D | Option | MQT is a skin testing technique that may be used to determine a safe starting dose for AIT. |
| Serum total IgE (tIgE) | 15 | C | Option | Serum tIgE is an option to assess atopic status. |
| Serum antigen-specific IgE (sIgE) | 7 | B | Recommendation | Serum sIgE testing is recommended for evaluation of allergen sensitivities in appropriately selected patients. The practitioner may decide whether skin or in vitro sIgE testing is best in an individual patient. |
| Correlation between skin and in vitro testing | 19 | B | — | Studies differ regarding the concordance of various allergy testing methods. |
| Nasal sIgE | 24 | C | Option | Nasal sIgE is an option in patients with suspected or known LAR to aid in diagnosis or guide therapy. |
| Basophil activation test | 12 | B | Option | BAT may be used for diagnosis when first-line tests are discordant, and for monitoring response to AIT. |
| Nasal provocation testing | 4 | C | — | NPT has been employed for diagnosis of occupational rhinitis and LAR. |
| Nasal cytology | 4 | C | — | Nasal cytology is an investigational tool, rather than diagnostic. |
| Nasal histology | 11 | B | — | Nasal histology is used for research on the pathophysiology of AR but is not routinely used in clinical practice for the diagnosis of AR. |
| Management-avoidance measures and environmental controls | ||||
| House dust mite | 12 | B | Option | Concomitant use of acaricides and EC measures is an option for the treatment of AR. |
| Cockroach | 11 | B | Option | Combination of physical measures (bait traps, house cleaning) and education is an option for AR management related to cockroach exposure. |
| Pets | 3 | B | Option | Pet avoidances and EC strategies are an option for AR related to pets. |
| Pollen and occupational allergens | 3 | B | Option | Pollen and occupational allergen avoidance by EC strategies are an option for the treatment of AR. |
| Management-pharmacotherapy | ||||
| Oral H1 antihistamines | 21 | A | Strong recommendation | Newer-generation oral H1 antihistamines are strongly recommended for the treatment of AR. |
| Oral H2 antihistamines | 6 | B | No recommendation | Available data does not adequately address the question of benefit in the treatment of AR. |
| Intranasal antihistamines | 44 | A | Recommendation | Intranasal antihistamines many be used as first-line or second-line therapy for the treatment of AR. |
| Oral corticosteroids | 9 | B | Recommend against | Due to the risks of oral steroid use, along with the availability of other pharmacotherapy options, this therapy is not recommended for routine AR management. |
| Injectable corticosteroids | 13 | B | Recommend against | Due to the risks of injectable steroid use, along with the availability of other pharmacotherapy options, systemic or intraturbinate injection of corticosteroids is not recommended for the routine treatment of AR. |
| Intranasal corticosteroids | 53 | A | Strong recommendation | INCS should be used as first-line therapy in the treatment of AR. |
| Oral decongestants | 9 | B | Option | Option for pseudoephedrine for short-term treatment of AR symptoms. |
| Recommend against | Recommend against phenylephrine, as it has not been shown to be superior to placebo. | |||
| Topical decongestants | 4 | B | Option | Option for topical IND use in the short-term for nasal decongestion. Chronic use carries a risk of RM. |
| Leukotriene receptor antagonists | 31 | A | Recommend against | LTRAs should not be used as monotherapy in the treatment of AR. |
| Cromolyn (DSCG) | 22 | A | Option | DSCG may be considered in the treatment of AR, particularly for patients with known triggers who cannot tolerate INCS. |
| Intranasal anticholinergic (IPB) | 14 | B | Option | IPB nasal spray may be considered as an adjunct to INCS in PAR patients with uncontrolled rhinorrhea. |
| Omalizumab | 6 | A | No indication | Omalizumab is not approved by the FDA for the treatment of AR alone. |
| Nasal saline | 12 | A | Strong recommendation | Nasal saline is strongly recommended as part of the treatment strategy for AR. |
| Probiotics | 28 | A | Option | Probiotics may be considered in the treatment of AR. |
| Combination: oral antihistamine and oral decongestant | 21 | A | Option | Option, particularly for acute exacerbations with a primary symptom of nasal congestion. |
| Combination: oral antihistamine and INCS | 5 | B | Option | Combination equivocal over either drug alone. |
| Combination: oral antihistamine and LTRA | 13 | A | Option | Combination is an option for AR management, particularly in patients with comorbid asthma who do not tolerate INCS and are not well-controlled on oral antihistamine monotherapy. |
| Combination: INCS and intranasal antihistamine | 12 | A | Strong recommendation | Strong recommendation for combination therapy when monotherapy fails to control AR symptoms. |
| Acupuncture | 15 | B | Option | In patients who wish to avoid medications, acupuncture many be suggested as a possible therapeutic adjunct. |
| Honey | 3 | B | No recommendation | Studies are inconclusive and heterogeneous. |
| Herbal therapies | — | — | No recommendation | Multiple different herbs studied, with few studies for each specific therapy. Results are inconclusive. |
| Surgical treatment | 12 | C | Option | Turbinate reduction may be considered in AR patients with nasal obstruction who have failed medical management. |
| Management–allergen immunotherapy | ||||
| Subcutaneous immunotherapy | 8 | A | Strong recommendation | Strong recommendation for SCIT in patients unable to obtain adequate relief from pharmacotherapy and those who would benefit from secondary disease-modifying effects. |
| Sublingual immunotherapy | 25 | A | Strong recommendationa | Strong recommendation for SLIT in patients unable to obtain adequate relief from pharmacotherapy. |
| Trans/epicutaneous immunotherapy | 4 | B | Recommend against | Limited studies show variable effectiveness, along with adverse reactions. Trans/epicutaneous immunotherapy is not recommended for AR treatment. |
| Intralymphatic immunotherapy | 7 | B | Option | Pending additional studies, ILIT may be a viable option for AR treatment in the clinical population. |
| Associated conditions | ||||
| Asthma-association with rhinitis | 7 | C | — | Asthma is associated with AR and NAR. |
| Asthma–rhinitis as a risk factor | 13 | C | — | AR and NAR are risk factors for developing asthma. |
| Asthma–benefit of AR treatment | — | — | — | See section X.A.4 for specific recommendations. |
| Acute rhinosinusitis | 5 | C | — | AR is thought to be a disease-modifying factor for ARS. |
| Recurrent acute rhinosinusitis | 2 | D | — | Data inconclusive. |
| Chronic rhinosinusitis without nasal polyps | 10 | D | — | Conflicting evidence for/against an association. |
| Chronic rhinosinusitis with nasal polyps | 21 | D | — | Conflicting evidence for/against an association. |
| Conjunctivitis | 7 | C | — | AC is a frequently occurring comorbidity of AR. |
| Atopic dermatitis | 20 | C | — | There is evidence for an association between AR and AD. |
| Food allergy and PFAS | 12 | B | — | There is evidence for a link between pollen allergy and PFAS. |
| Adenoid hypertrophy | 11 | C | — | Data inconclusive. |
| Otologic conditions–Eustachian tube dysfunction | 7 | C | — | There is a causal role for AR in some cases of ETD. |
| Otologic conditions–otitis media | 16 | C | — | Relationship between AR and OTE is unclear. |
| Otologic conditions–Meniere’s disease | 8 | C | — | Evidence for an association is of low grade, with substantial defects in study design. |
| Cough | 9 | C | — | Low level evidence for an association between AR and cough. |
| Laryngeal disease | 18 | C | — | There is some evidence for an association between AR and laryngeal disease. |
| Eosinophilic esophagitis | 13 | C | — | Limited observational data suggests a potential association between aeroallergens and EoE pathogenesis. |
| Sleep disturbance and OSA | 20 | B | — | Sleep disturbance is associated with AR. |
Specific recommendations for various SLIT preparations and treatment effects are given in section IX.D.4.
AC = allergic conjunctivitis; AD = atopic dermatitis; AIT = allergen immunotherapy; AR = allergic rhinitis; ARS = acute rhinosinusitis; BAT = basophil activation test; DSCG = disodium cromoglycate; EC = environmental controls; EoE = eosinophilic esophagitis; ETD = Eustachian tube dysfunction; FDA = Food and Drug Administration; GWAS = genome-wide association study; ILIT = intralymphatic immunotherapy; INCS = intranasal corticosteroids; IND = intranasal decongestants; IPB = ipratropium bromide; LAR = local allergic rhinitis; LTRA = leukotriene receptor antagonist; MQT = Modified Quantitative Testing; NAR = non-allergic rhinitis; NPT = nasal provocation testing; OSA = obstructive sleep apnea; OTE = otitis media with effusion; PAR = perennial allergic rhinitis; PFAS = pollen-food allergy syndrome; QOL = quality of life; RM = rhinitis medicamentosa; SCIT = subcutaneous immunotherapy; SES = socioeconomic status; sIgE = antigen-specific immunoglobulin E; SLIT = sublingual immunotherapy; SPT = skin-prick test; SR = systematic review; tIgE = total immunoglobulin E.
XI.A. Epidemiology and risk factors
Studies have previously been undertaken to determine the prevalence of AR in various parts of the world. While the data from these studies is often quoted, it is limited by its methodology relating primarily to surveys (sometimes complemented by allergen sensitivity testing). Our world is better connected by technology today than it had been previously. We should leverage these capabilities to better understand the epidemiology of AR. Research opportunities include:
Improved understanding of the incidence and prevalence of AR and its phenotypes (ie, SAR, PAR, IAR, PER) worldwide.
Improved understanding of AR variation by geographic region, patient age, and sex.
Evaluation of climate change and its effect on the pattern and degree of allergen exposure.
Our understanding of the risk factors for the development of AR should also be improved. While certain areas (ie, early childhood exposure to pets as a risk factor vs protective factor) have seen numerous articles published, the data is highly conflicting. In other areas, such as early exposure to pollens and mites, the data is more limited. Genetic studies provide some notable evidence for potential AR risk but functional data needs to be expanded. Research opportunities include:
Understanding the role of candidate gene alterations in the pathophysiology of AR via functional characterization.
Investigation of epigenetic mechanisms to provide a functional explanation between gene-environment interactions and AR disease development.
Improved understanding of environmental exposures as a risk/protective factor for AR disease development, especially in diverse geographic locations.
Further study of the role of pollutants and tobacco smoke in the development of AR and in the severity of allergic rhinitis symptoms.
Greater elucidation of the environmental risk factors and protective factors for AR, particularly exposure to pets, HDM, and breastfeeding.
Longitudinal study evaluating risk factor reduction and its effect on the incidence of AR.
XI.B. Evaluation and diagnosis
Evaluation of the patient with suspected AR classically relies on a thorough history, often reinforced by findings on physical examination. The diagnosis is further supported with skin or in vitro testing methods. These techniques have been rather dependable, provided objective testing is correlated to the patient’s clinical symptoms and not used in isolation to determine a treatment plan, as there are distinct differences between sensitization and clinical allergy. As newer testing methods gain their footing, we have the opportunity to bring them to widespread clinical practice with solid supporting evidence. Research opportunities include:
Improved characterization of newer testing techniques (ie, nasal sIgE, BAT) in larger populations to provide standardization for incorporation into mainstream clinical practice.
Need for comparative studies for IDT and single-dilution intradermal testing.
Further study of the role of single intradermal testing after a negative prick test.
Development of standardized testing and interpretation of testing for LAR, as well as further defining the clinical utility of testing.
Further elucidation of clinical uses for CRD in patient management.
Need for international consensus on allergen units in antigen standardization.
XI.C. Management
There are several options for management of the AR patient. Allergen avoidance and EC strategies are often discussed, yet high-level evidence is frequently lacking, especially as it relates to AR symptom control. Many pharmacotherapy options have very high LOEs, which is helpful as we strive to choose the best drug options to control patient symptoms. SCIT and SLIT also have very high LOEs in general, yet specific issues related to AIT management could be bolstered with additional evidence. Research opportunities include:
Improved understanding of the impact of EC strategies on AR symptom control and rescue medication use, especially for cockroach, pet, and pollen allergens.
Improved understanding of the polyallergic AR patient and appropriate AIT regimens in this population.
Improved understanding and characterization of ILIT for possible routine clinical application.
Further study of comparative efficacy/effectiveness of SLIT vs SCIT.
Further study of AIT with multiple allergens.
Improved understanding of cost effective management for optimal AR control and the use of multimodality therapy, including combinations of pharmacotherapy and AIT.
Further study of the comparative effectiveness of various AR treatments.
XI.D. Associated conditions
The evidence supporting an association between AR and numerous other conditions is weak or conflicting. There is clearly a need to better define the relationship between AR and several of the comorbidities identified in this document (especially rhinosinusitis, otitis media with effusion, cough, laryngeal disease, and eosinophilic esophagitis), and to further delineate the role that AR treatment has for potential improvement of associated conditions.
XII. Conclusion
In summary, the authors of ICAR:AR have worked to collate the best external evidence for various aspects of AR, providing evidence grades and recommendations where appropriate. From this evidence, knowledge gaps and research opportunities have been identified. It is our sincere hope that the ICAR:AR document will be a reference for understanding the current AR evidence and a springboard for future investigation.
List of abbreviations
- AAAAI
American Academy of Allergy Asthma&Immunology
- AAO-HNS
American Academy ofOtolaryngology-Head and Neck Surgery
- AC
allergic conjunctivitis
- ACC
allergen challenge chamber
- ACE-I
angiotensin converting enzyme inhibitor
- ACTH
adrenal corticotropic hormone
- AD
atopic dermatitis
- AERD
aspirin exacerbated respiratory disease
- AH
adenoid hypertrophy
- AHI
apnea-hypopnea index
- AIT
allergen immunotherapy
- ANA
anti-nuclear antibody
- ANCA
anti-nuclear cytoplasmic antibody
- APC
antigen presenting cell
- AR
allergic rhinitis
- ARIA
Allergic Rhinitis and its Impact on Asthma
- ARS
acute rhinosinusitis
- BAFF
B-cell activating factor
- BAT
basophil activation test
- BDNF
brain-derived neurotrophic factor
- BKC
benzalkonium chloride
- CARAT10
Control of Allergic Rhinitis and Asthma Test
- CCAAPS
Cincinnati Childhood Allergen and Air Pollution Study
- CCAD
central compartment atopic disease
- cGMP
cyclic guanosine monophosphate
- CI
confidence interval
- CNS
central nervous system
- CO
carbon monoxide
- COX
cyclooxygenase
- CPAP
continuous positive airway pressure
- CPG
clinical practice guideline
- CPT
conjunctival provocation test
- CRD
component resolved diagnosis
- CRS
chronic rhinosinusitis
- CRSsNP
chronic rhinosinusitis without nasal polyposis
- CRSwNP
chronic rhinosinusitis with nasal polyposis
- CS
Combined Score
- CSF
cerebrospinal fluid
- CT
computed tomography
- DBP
diastolic blood pressure
- DCS
Daily Combined Score
- DEP
diesel exhaust particles
- DSCG
disodium cromoglycate
- EAACI
European Academy of Allergy&Clinical Immunology
- EAN
European Aeroallergen Network
- EBR
evidence-based review (without recommendations)
- EBRR
evidence-based review with recommendations
- EC
environmental control
- ECP
eosinophil cationic protein
- ECRHS
European Community Respiratory Health Survey
- EEC
environmental exposure chamber
- EGPA
eosinophilic granulomatosis with polyangiitis
- ENS
empty nose syndrome
- EoE
eosinophilic esophagitis
- EPOS
European Position Paper on Rhinosinusitis and Nasal Polyps
- ESS
Epworth Sleepiness Scale
- EU
European Union
- FDA
U.S. Food and Drug Administratio
- FEV1
forced expiratory volume in 1 second
- FoxP3
forkhead box P3
- GA2LEN
Global Allergy and Asthma Network of Excellence
- GM-CSF
granulocyte-macrophage colony stimulating factor
- GPA
granulomatosis with polyangiitis
- GWAS
genome-wide association study
- HD-42
Sleep Disorders Questionnaire
- HDM
house dust mite
- HEPA
high-efficiency particulate air
- HFA
hydrofluoroalkane
- HMW
high molecular weight
- HR
heart rate
- IAR
intermittent allergic rhinitis
- ICAR
International Consensus Statement on Allergy and Rhinology
- ICAR:AR
International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis
- ICAR:RS
International Consensus Statement on Allergy and Rhinology: Rhinosinusitis
- IDT
intradermal dilutional testing
- IFN
interferon
- IgE
immunoglobulin E
- IL
interleukin
- ILC
innate lymphoid cell
- ILIT
intralymphatic immunotherapy
- INCS
intranasal corticosteroid
- IND
intranasal decongestant
- INV
intranasal volume
- IPB
ipratropium bromide
- ISAAC
International Study of Asthma and Allergies in Childhood
- JSQ
The Jenkins Questionnaire
- LAR
local allergic rhinitis
- LMW
low molecular weight
- LOE
level of evidence
- LPR
laryngopharyngeal reflux
- LRRC32
leucine-rich repeat-containing protein 32
- LT
leukotriene
- LTRA
leukotriene receptor antagonist
- mAb
monoclonal antibody
- MAS
Multicentre Allergy Study
- MCC
mucociliary clearance
- MCP
macrophage/monocyte chemoattractant protein
- MD
molecular diagnosis
- MDC
macrophage-derived chemokine
- MIF
macrophage migration inhibitory factor
- MIP
macrophage inflammatory protein
- MQT
Modified Quantitative Testing
- NAR
non-allergic rhinitis
- NARES
non-allergic rhinitis with eosinophilia syndrome
- NARESMA
non-allergic rhinitis with eosinophils and mast cells
- NARMA
non-allergic rhinitis with mast cells
- NARNE
non-allergic rhinitis with neutrophils
- NC
nasal cytology
- NGF
nerve growth factor
- NHANES
National Health and Nutrition Examination Survey
- NO
nitric oxide
- NO2
nitrogen dioxide
- NPT
nasal provocation test
- NSAID
nonsteroidal anti-inflammatory drug
- O3
ozone
- OAS
oral allergy syndrome
- OME
otitis media with effusion
- OMIT
oral mucosal immunotherapy
- OR
odds ratio
- OSA
obstructive sleep apnea
- OTC
over the counter
- PAR
perennial allergic rhinitis
- PARIS
Pollution and Asthma Risk: An Infant Study
- PDE
phosphodiesterase
- PER
persistent allergic rhinitis
- PFAS
pollen food allergy syndrome
- PM10
particulate matter <10 μm
- PM2.5
particulate matter <2.5 μm
- PNU
protein nitrogen unit
- ppm
parts per million
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROM
patient-reported outcome measure
- PSG
polysomnogram
- QOL
quality of life
- RANTES
regulated on activation, normal T-cell expressed and secreted
- RAP
Respiratory Allergy Prediction test
- RARS
recurrent acute rhinosinusitis
- RAST
radioallergosorbent test
- RC-ACS
Rhinoconjunctivitis-Allergy Control Score
- RCT
randomized controlled trial
- RFA
radiofrequency ablation
- RM
rhinitis medicamentosa
- RMS
Rescue Medication Score
- RQLQ
Rhinoconjunctivitis Quality of Life Questionnaire
- rTNSS
Reflective Total Nasal Symptom Score
- RTSS
Rhinitis Total Symptom Score
- RUDS
reactive upper airway dysfunction syndrome
- SAPALDIA
Swiss Study of Air Pollution and Lung Disease in Adults
- SAR
seasonal allergic rhinitis
- SBP
systolic blood pressure
- SCIT
subcutaneous immunotherapy
- SDB
sleep-disordered breathing
- SES
socioeconomic status
- sIgE
antigen-specific immunoglobulin E
- SLE
systemic lupus erythematosus
- SLIT
sublingual immunotherapy
- SMD
standardized mean difference
- SNP
single nucleotide polymorphism
- SO2
sulfur dioxide
- SPT
skin-prick test
- SQ-U
standardized quality units
- SSRI
selective serotonin reuptake inhibitor
- SSS
Stanford Sleepiness Score
- TARC
thymus and activation regulated chemokine
- TCRS
Total Combined Rhinitis Score
- TDI
threshold, discrimination, identification
- TGF-β
transforming growth factor beta
- Th
T-helper cell
- Th0
naive T-helper cell
- tIgE
total immunoglobulin E
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TNSS
Total Nasal Symptom Score
- TOSS
Total Ocular Symptom Score
- TOTALL
TOTal Costs of ALLergic Rhinitis in Sweden
- Treg
T-regulatory cell
- TSLP
thymic stromal lymphoprotein
- VAS
Visual Analog Scale
- VHI
Voice Handicap Index
- WHO
World Health Organization
Appendix
Contributing Authors
Anand Andiappan, PhD1, Philipp Badorrek, MD2, Christopher D. Brook, MD3, Paloma Campo, MD, PhD4, Mohamad R. Chaaban, MD, MSCR, MBA5, Anna Charles-Jones, MD6, Esther Cheng, MD7, Nipun Chhabra, MD8, Daniel Cox, MD9, Pedram Daraei, MD10, Aaron M. Drucker, MD, ScM11, Kai Fruth, MD, PhD12, Canting Guo, MD13, Matthias Kopp, MD, PhD14, Patricia A. Loftus, MD15, Mauricio López-Chacón, MD16, Michael J. Marino, MD17, Jose Mattos, MD18, Nuray Bayar Muluk, MD19, Chew Lip Ng, MD20, Bright I. Nwaru, PhD21, Gianni Pala, MD22, Jono Paulin, MBChB23, Michael Pfisterer, MD24, Andrew J. Rosko, MD25, Chloe Lan Russo, MD26, Theodore Asher Schuman, MD27, Christine Segboer, MD28, Michela Silvestri, PhD29, Kristine A. Smith, MD30, Michael B. Soyka, MD31, Jeanie Sozansky Lujan, MD32, Andrew J. Thomas, MD33, Arja Viinanen, MD, PhD34, Thomas J. Willson, MD35
Contributing Author Affiliations
1Immunology, Agency for Science, Technology and Research, Singapore;2Respiratory Medicine, Fraunhofer Institute for Toxicology and Experimental Medicine, Germany; 3Otolaryngology, Boston University, USA; 4Allergy, Regional University Hospital of Málaga, Spain; 5Otolaryngology, University of Texas Medical Branch, USA; 6Medicine, University of Otago, New Zealand; 7Otolaryngology, Loyola University, USA; 8Otolaryngology, Case Western Reserve University, USA; 9Otolaryngology, University of Utah, USA; 10Otolaryngology, Emory University, USA; 11Dermatology, Alpert Medical School of Brown University, Women's College Research Institute, USA, Canada; 12Otorhinolaryngology, Helios Kliniken Wiesbaden, Germany; 13Medicine, Alpert Medical School of Brown University, USA; 14Pediatric Allergy/Pulmonology, University of Lubeck, Germany; 15Otolaryngology, University of California San Francisco, USA; 16Otolaryngology, Universitat de Barcelona; Hospital Clinic, IDIBAPS, Spain; 17Otolaryngology, McGovern Medical School at the University of Texas Health Science Center Houston, USA; 18Otolaryngology, Medical University of South Carolina, USA; 19Otolaryngology, Kirikkale University, Turkey; 20Otolaryngology, Ng Teng Fong General Hospital, Singapore; 21 Allergy/Asthma, University of Edinburgh, UK; 22Allergy/Immunology, University of Pavia, Italy; 23Medicine, University of Otago, New Zealand; 24Otolaryngology, Rutgers New Jersey Medical School, USA; 25Otolaryngology, University of Michigan, USA; 26Allergy/Immunology, Emory University, USA; 27Otolaryngology, University of North Carolina, USA; 28Otolaryngology, University of Amsterdam, Netherlands; 29Pediatric Pneumology/Allergy, Istituto Giannina Gaslini, Italy; 30Otolaryngology, University of Calgary, Canada; 31Otorhinolaryngology, University Hospital Zurich, Switzerland; 32Otolaryngology, Case Western Reserve University, USA; 33Otolaryngology, University of Utah, USA; 34Pulmonary/Allergy, Turku University Hospital, Finland;35Otolaryngology, Brook Army Medical Center, USA
XIV. Appendix: Author disclosures
Authors
| Author | Nothing to disclose | Company | Nature of relationship |
|---|---|---|---|
| Sarah K. Wise, MD, MSCR | Medtronic | Consultant | |
| Elron | Consultant | ||
| OptiNose | Advisory board | ||
| Sandra Y. Lin, MD | Agency for Healthcare Research and Quality | Research funding | |
| Elina Toskala, MD, PhD, MBA | Medtronic | Research funding | |
| Richard R. Orlandi, MD | Medtronic | Consultant | |
| BioInspire | Consultant | ||
| 480 Biomedical | Consultant | ||
| Cezmi A. Akdis, MD | Davos Diagnostics | Company shares | |
| Allergopharma | Research funding | ||
| Actellion AG | Research funding | ||
| Jeremiah A. Alt, MD, PhD | University of Utah Program in Personalized Heath and the National Center for Advancing Translational Sciences of the NIH | Research funding | |
| National Institute of Allergy and Infectious Diseases | Research funding | ||
| National Institute of Deafness and Other Communication Disorders | Research funding | ||
| Medtronic | Consultant | ||
| GlycoMira Therapeutics | Consultant | ||
| Spirox | Consultant | ||
| AngioSonic | Consultant | ||
| Antoine Azar, MD | Relez Therapeutics | Research funding | |
| Shire | Consultant | ||
| Claus Bachert, MD, PhD | Sanofi | Consultant | |
| Glaxo Smith Kline | Consultant | ||
| Novartis | Consultant | ||
| AstraZeneca | Consultant | ||
| Allakos | Consultant | ||
| Fuad M. Baroody, MD | Allergan | Consultant | |
| Glaxo Smith Kline | Consultant | ||
| MEDA | Speaker | ||
| G. Walter Canonica, MD | A. Menarini | Research or speaker or advisory | |
| ALK-Abello | Research or speaker or advisory | ||
| Anallergo | Research or speaker or advisory | ||
| AstraZeneca | Research or speaker or advisory | ||
| Boehringer Ingelheim | Research or speaker or advisory | ||
| Chiesi Farmaceutici | Research or speaker or advisory | ||
| Circassia | Research or speaker or advisory | ||
| Genentech | Research or speaker or advisory | ||
| Guidotti-Malesci | Research or speaker or advisory | ||
| Glaxo Smith Kline | Research or speaker or advisory | ||
| HAL Allergy | Research or speaker or advisory | ||
| MEDA | Research or speaker or advisory | ||
| Merck | Research or speaker or advisory | ||
| Merck Sharp & Dome | Research or speaker or advisory | ||
| Novartis | Research or speaker or advisory | ||
| Recordati-InnuvaPharma | Research or speaker or advisory | ||
| Roche | Research or speaker or advisory | ||
| Sanofi-Aventis | Research or speaker or advisory | ||
| Stallergenees | Research or speaker or advisory | ||
| UCB Pharma | Research or speaker or advisory | ||
| Uriach Pharma | Research or speaker or advisory | ||
| Teva | Research or speaker or advisory | ||
| Thermo Fisher | Research or speaker or advisory | ||
| Valeras | Research or speaker or advisory | ||
| Vibor-Pharma | Research or speaker or advisory | ||
| Thomas Chacko, MD | X | ||
| Cemal Cingi, MD | X | ||
| Giorgio Ciprandi, MD | Stallergenes | Consultant | |
| Jacquelynne Corey, MD | Greer-Stallergenes | Consultant, speaker’s bureau | |
| CSL | Advisory board | ||
| Behring | Advisory board | ||
| Intersect ENT | Advisor | ||
| Linda S. Cox, MD | X | ||
| Peter Socrates Creticos, MD | Stellergenes-Greer | Research funding, consultant | |
| Circassia | Research funding, consultant | ||
| ASIT: Allergy therapeutics | Consultant | ||
| Merck | Research funding | ||
| National Institute of Allergy and Infectious Diseases | Research funding | ||
| Patient-Centered Outcomes Research Institute | Research funding | ||
| Adnan Custovic, MSc, DM, MD, PhD | Novartis | Consultant | |
| Boehringer Ingelheim | Consultant | ||
| ALK-Abello | Consultant | ||
| Thermo Fisher | Speaker | ||
| Glaxo Smith Kline | Speaker | ||
| Cecelia Damask, DO | Audigy Medical | Consultant | |
| ALK | Speaker | ||
| Adam DeConde, MD | Intersect ENT | Consultant | |
| Stryker Endoscopy | Consultant | ||
| Olympus | Consultant | ||
| John M. DelGaudio, MD | Spirox | Research funding | |
| Intersect ENT | Stockholder | ||
| Charles S. Ebert, Jr. MD, MPH | Acclarent | Consultant | |
| Medtronic | Consultant | ||
| Jean Anderson Eloy, MD | X | ||
| Carrie E. Flanagan, MD | X | ||
| Wytske J. Fokkens, MD | MEDA | Research funding | |
| Sanofi | Research funding | ||
| BioInspire | Research funding | ||
| Glaxo Smith Kline | Research funding | ||
| Christine Franzese, MD | ALK | Advisory board | |
| Greer | Advisory board, speaker | ||
| Jan Gosepath, MD, PhD | X | ||
| Ashleigh Halderman, MD | X | ||
| Robert G. Hamilton, PhD | X | ||
| Hans Jürgen Hoffman, BSc, PhD | X | ||
| Jens Hohlfeld, MD | X | ||
| Steven M. Houser, MD | X | ||
| Peter H. Hwang, MD | Olympus | Consultant | |
| Medtronic | Consultant | ||
| 480 Biomedical | Consultant | ||
| BioInspire | Consultant | ||
| Arrinex | Consultant | ||
| Cristoforo Incorvaia, MD | Bayer | Consultant | |
| Stallergenes | Consultant | ||
| Prof. Deborah Jarvis | X | ||
| Ayesha N. Khalid, MD, MBA | Castle Creek Pharma | Consultant | |
| 480 Biomedical | Consultant and equity holder | ||
| Smith and Nephew | Consultant | ||
| Stallergenes-Greer | Consultant, speaker | ||
| Hacking Medical Institute | Consultant, co-founder | ||
| Maritta Kilpenäinen, MD, PhD | X | ||
| Todd. T. Kingdom, MD | X | ||
| Helene Krouse, PhD, ANP-BC | X | ||
| Desiree Larenas-Linnemann, MD | UCB | Advisory board, speaker | |
| Glaxo Smith Kline | Advisory board, speaker, research funding | ||
| MEDA | Advisory board, speaker | ||
| Astra-Zeneca | Advisory board, speaker, research funding | ||
| Armstrong | Advisory board, speaker | ||
| Grunenthal | Advisory board, speaker | ||
| Novartis | Advisory board, speaker, research funding | ||
| Boehringer Ingelheim | Advisory board, speaker, research funding | ||
| Pfizer | Advisory board, speaker | ||
| DBV | Advisory board, speaker | ||
| Teva | Research funding | ||
| Chiesi | Research funding | ||
| Adrienne M. Laury, MD | X | ||
| Stella E. Lee, MD | Sanofi-Aventis | Research funding | |
| Allakos | Research funding | ||
| Joshua M. Levy, MD, MPH | X | ||
| Amber U. Luong, MD, PhD | ENTvantage Diagnostics | Advisory board, research funding | |
| Aerin Medical | Consultant | ||
| 480 Biomedical | Consultant | ||
| Medtronic | Consultant | ||
| Intersect ENT | Research funding | ||
| Allakos | Research funding | ||
| Bradley F. Marple, MD | X | ||
| Edward D. McCoul, MD, MPH | Acclarent | Consultant | |
| K. Christopher McMains, MD | X | ||
| Erik Melén, MD, PhD | X | ||
| James W. Mims, MD | X | ||
| Gianna Moscato, MD | X | ||
| Joaquim Mullol, MD, PhD | ALK-Abello | Consultant, speaker | |
| Sanofi | Advisory board, consultant | ||
| Mylan | Consultant, research funding | ||
| MEDA | Consultant, research funding | ||
| UCB | Speaker | ||
| Uriakh | Consultant, research funding | ||
| Harold S. Nelson, MD | X | ||
| Monica Patadia, MD | X | ||
| Ruby Pawankar, MD, PhD | X | ||
| Oliver Pfaar, MD, PhD | ALK Abello | Consultant, advisory board, research funding | |
| Allergopharma | Consultant, speaker, research funding | ||
| Allergy Therapeutics/Bencard | Advisory board, speaker, research funding | ||
| Anergis | Consultant, research funding | ||
| Biotech Tools | Research funding | ||
| Circassia | Research funding | ||
| HAL Allergy | Consultant, advisory board, speaker, research funding | ||
| Laboratorios LETI/LETI Pharma | Consultant, speaker, research funding | ||
| Lofarma | Consultant, speaker | ||
| Mobile Chamber Experts | Advisory board | ||
| Stallergenes-Greer | Consultant, advisory board, research funding | ||
| Michael P. Platt, MD, MSc | Plural Publishing | Book royalties | |
| William Reisacher, MD | Allovate | Advisory board, stockholder | |
| Direct allergy | Advisory board | ||
| Cornell University | Patent: U.S. 8.993.347 B2 | ||
| Carmen Rondón, MD, PhD | X | ||
| Luke Rudmik, MD, MSc | BioInspire | Advisory board | |
| 480 Biomedical | Consultant | ||
| Matthew Ryan, MD | X | ||
| Joaquin Sastre, MD, PhD | Thermo Fisher | Consultant | |
| Sanofi | Consultant | ||
| ALK | Consultant | ||
| LETI | Consultant | ||
| Stallergenes | Consultant | ||
| Rodney J. Schlosser, MD | Olympus | Consultant | |
| Arrinex | Consultant | ||
| Entellus | Research funding | ||
| Intersect ENT | Research funding | ||
| Russell A. Settipane, MD | Astra Zeneca | Advisory board, speaker | |
| Boehringer Ingelheim | Speaker | ||
| Genentech/Novartis | Advisory board, research funding, speaker | ||
| Stallergenes-Greer | Research funding, speaker | ||
| Merck | Research funding, speaker | ||
| Mylan | Speaker | ||
| Teva | Advisory board, speaker, research funding | ||
| ALK | Advisory board | ||
| Circassia | Advisory board | ||
| Sanofi/Regeneron | Advisory board | ||
| CSL Behring | Advisory board | ||
| Shire | Speaker | ||
| Pharming | Speaker | ||
| Hemant P. Sharma, MD | X | ||
| Aziz Sheikh, OBE, BSc, MSc, MD | X | ||
| Timothy L. Smith, MD, MPH | X | ||
| Pongsakorn Tantilipikorn, MD, PhD | X | ||
| Jody R. Tversky, MD | X | ||
| Maria C. Veling, MD | X | ||
| De Yun Wang, MD, PhD | X | ||
| Marit Westman, MD, PhD | X | ||
| Magnus Wickman, MD, PhD | X | ||
| Mark Zacharek, MD | NOTA Laboratories | Founder, advisory board | |
| Contributing Authors | |||
| Anand Andiappan, PhD | X | ||
| Philipp Badorrek, MD | X | ||
| Christopher D. Brook, MD | X | ||
| Paloma Campo, MD, PhD | X | ||
| Mohamad R. Chaaban, MD, MSCR, MBA | X | ||
| Anna Charles-Jones, MBChB | X | ||
| Esther Cheng, MD | X | ||
| Nipun Chhabra, MD | X | ||
| Daniel Cox, MD | X | ||
| Pedram Daraei, MD | X | ||
| Aaron M. Drucker, MD, ScM | Regeneron | Research funding | |
| Sanofi | Consultant, research funding | ||
| Astellas Canada | Speaker | ||
| Prime, Inc. | Speaker | ||
| Spire Learning | Speaker | ||
| RTI Health Solutions | Consultant | ||
| Kai Fruth, MD, PhD | X | ||
| Canting Guo, MD | X | ||
| Prof. Matthias Kopp | ALK-Abello | Consultant or speaker | |
| Allergopharma | Consultant or speaker | ||
| Chiesi | Consultant or speaker | ||
| Glaxo Smith Kline | Consultant or speaker | ||
| MEDA | Consultant or speaker | ||
| Novartis | Consultant or speaker | ||
| Infectopharm | Consultant or speaker | ||
| Nutricia | Consultant or speaker | ||
| Vertex | Consultant or speaker | ||
| Patricia A. Loftus, MD | X | ||
| Edgar Mauricio López-Chacón, MD | X | ||
| Michael J. Marino, MD | X | ||
| Jose Mattos, MD | X | ||
| Nuray Bayar Muluk, MD | X | ||
| Chew Lip Ng, MD | X | ||
| Bright I. Nwaru, PhD | X | ||
| Gianni Pala, MD | X | ||
| Jono Paulin, MBChB | X | ||
| Michael Pfisterer, MD | X | ||
| Andrew J. Rosko, MD | X | ||
| Chloe Lan Russo, MD | X | ||
| Theodore Asher Schuman, MD | X | ||
| Christine Segboer, MD | X | ||
| Michela Silvestri, PhD | X | ||
| Kristine A. Smith, MD | X | ||
| Michael B. Soyka, MD | Sanofi | Consultant | |
| MEDA | Advisory board | ||
| Preclin Biosystems | Research funding | ||
| Jeanie Sozansky Lujan, MD | X | ||
| Andrew J. Thomas, MD | X | ||
| Arja Viinanen, MD, PhD | Novartis | Speaker | |
| Chiesi | Speaker | ||
| Boehringer Ingelheim | Speaker | ||
| Mundi pharma | Speaker | ||
| Astra-Zeneca | Speaker | ||
| Thomas J. Willson, MD | X | ||
Footnotes
Additional Supporting Information may be found in the online version of this article.
The American Academy of Otolaryngic Allergy Foundation provided funding for administrative support in preparation of this document but exercised no control over its content.
Potential conflicts of interest: See the Appendix at the end of this article.
XIII. References
- 1.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl)1:S22–S209. [DOI] [PubMed] [Google Scholar]
- 2.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudmik L, Smith TL. Development of an evidence-based review with recommendations using an online iterative process. Int Forum Allergy Rhinol. 2011;1:431–437. [DOI] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxford Centre for Evidence-based Medicine (CEBM). Levels of Evidence. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ Accessed December 19, 2017.
- 6.American Academy of Pediatrics Steering Committee on Quality Improvement, Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114:874–877. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Van Cauwenberge P, Khaltaev N; Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. [DOI] [PubMed] [Google Scholar]
- 8.Hansel F Clinical and histopathologic studies of the nose and sinuses in allergy. J Allergy. 1929;1:43–70. [Google Scholar]
- 9.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. [DOI] [PubMed] [Google Scholar]
- 10.Asher MI, Montefort S, Bjorksten B, et al. World-wide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. [DOI] [PubMed] [Google Scholar]
- 11.Vinke JG, KleinJan A, Severijnen LW, Hoeve LJ, Fokkens WJ. Differences in nasal cellular infiltrates between allergic children and age-matched controls. Eur Respir J. 1999;13:797–803. [DOI] [PubMed] [Google Scholar]
- 12.Papatziamos G, van der Ploeg I, Hemlin C, Patwardhan A, Scheynius A. Increased occurrence of IgE+ and FcepsilonRI+ cells in adenoids from atopic children. Allergy. 1999;54:916–925. [DOI] [PubMed] [Google Scholar]
- 13.Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2005;60:350–353. [DOI] [PubMed] [Google Scholar]
- 14.Ciprandi G, Buscaglia S, Pesce G, et al. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995;96:971–979. [DOI] [PubMed] [Google Scholar]
- 15.Platts-Mills TA, Hayden ML, Chapman MD, Wilkins SR. Seasonal variation in dust mite and grass-pollen allergens in dust from the houses of patients with asthma. J Allergy Clin Immunol. 1987;79:781–791. [DOI] [PubMed] [Google Scholar]
- 16.Connell JT. Quantitative intranasal pollen challenges. 3. The priming effect in allergic rhinitis. J Allergy. 1969;43:33–44. [DOI] [PubMed] [Google Scholar]
- 17.Wachs M, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Observations on the pathogenesis of nasal priming. J Allergy Clin Immunol. 1989;84:492–501. [DOI] [PubMed] [Google Scholar]
- 18.Juliusson S, Bende M. Priming effect of a birch pollen season studied with laser Doppler flowmetry in patients with allergic rhinitis. Clin Allergy. 1988;18:615–618. [DOI] [PubMed] [Google Scholar]
- 19.Naito K, Ishihara M, Senoh Y, Takeda N, Yokoyama N, Iwata S. Seasonal variations of nasal resistance in allergic rhinitis and environmental pollen counts. II: Efficacy of preseasonal therapy. Auris Nasus Larynx. 1993;20:31–38. [DOI] [PubMed] [Google Scholar]
- 20.Koh YY, Lim HS, Min KU, Min YG. Airways of allergic rhinitics are ‘primed’ to repeated allergen inhalation challenge. Clin Exp Allergy. 1994;24:337–346. [DOI] [PubMed] [Google Scholar]
- 21.Assing K, Bodtger U, Poulsen LK, Malling HJ. Grass pollen symptoms interfere with the recollection of birch pollen symptoms—a prospective study of suspected, asymptomatic skin sensitization. Allergy. 2007;62:373–377. [DOI] [PubMed] [Google Scholar]
- 22.Knani J, Campbell A, Enander I, Peterson CG, Michel FB, Bousquet J. Indirect evidence of nasal inflammation assessed by titration of inflammatory mediators and enumeration of cells in nasal secretions of patients with chronic rhinitis. J Allergy Clin Immunol. 1992;90:880–889. [DOI] [PubMed] [Google Scholar]
- 23.Ricca V, Landi M, Ferrero P, et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;105:54–57. [DOI] [PubMed] [Google Scholar]
- 24.Riediker M, Monn C, Koller T, Stahel WA, Wuthrich B. Air pollutants enhance rhinoconjunctivitis symptoms in pollen-allergic individuals. Ann Allergy Asthma Immunol. 2001;87:311–318. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet J, Annesi-Maesano I, Carat F, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. 2005;35:728–732. [DOI] [PubMed] [Google Scholar]
- 26.Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–S84. [DOI] [PubMed] [Google Scholar]
- 27.Van Hoecke H, Vastesaeger N, Dewulf L, Sys L, van Cauwenberge P. Classification and management of allergic rhinitis patients in general practice during pollen season. Allergy. 2006;61:705–711. [DOI] [PubMed] [Google Scholar]
- 28.Demoly P, Allaert FA, Lecasble M, Bousquet J, Pragma. Validation of the classification of ARIA (Allergic Rhinitis and its Impact on Asthma). Allergy. 2003;58:672–675. [DOI] [PubMed] [Google Scholar]
- 29.Bachert C, van Cauwenberge P, Olbrecht J, van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006;61:693–698. [DOI] [PubMed] [Google Scholar]
- 30.Todo-Bom A, Loureiro C, Almeida MM, et al. Epidemiology of rhinitis in Portugal: evaluation of the intermittent and the persistent types. Allergy. 2007;62:1038–1043. [DOI] [PubMed] [Google Scholar]
- 31.Demoly P, Passalacqua G, Pfaar O, Sastre J, Wahn U. Management of the polyallergic patient with allergy immunotherapy: a practice-based approach. Allergy Asthma Clin Immunol. 2016;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuberbier T, Bachert C, Bousquet PJ, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–1530. [DOI] [PubMed] [Google Scholar]
- 33.Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. [DOI] [PubMed] [Google Scholar]
- 34.Haahtela T, Burbach GJ, Bachert C, et al. Clinical relevance is associated with allergen-specific wheal size in skin prick testing. Clin Exp Allergy. 2014;44:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varghese M, Glaum MC, Lockey RF. Drug-induced rhinitis. Clin Exp Allergy. 2010;40:381–384. [DOI] [PubMed] [Google Scholar]
- 36.Settipane RA, Kaliner MA. Chapter 14: Nonallergic rhinitis. Am J Rhinol Allergy. 2013;27 Suppl 1:S48–51. [DOI] [PubMed] [Google Scholar]
- 37.Walgama ES, Hwang PH. Aspirin-exacerbated respiratory disease. Otolaryngol Clin North Am. 2017;50:83–94. [DOI] [PubMed] [Google Scholar]
- 38.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. [DOI] [PubMed] [Google Scholar]
- 40.Kaliner MA, Baraniuk JN, Benninger M, et al. Consensus definition of nonallergic rhinopathy, previously referred to as vasomotor rhinitis, nonallergic rhinitis, and/or idiopathic rhinitis. World Allergy Organ J. 2009;2:119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23–34. [PubMed] [Google Scholar]
- 42.Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009:CD003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiroglu AF, Bayrakli H, Yuca K, Cankaya H, Kiris M. Nasal obstruction as a common side-effect of sildenafil citrate. Tohoku J Exp Med. 2006;208:251–254. [DOI] [PubMed] [Google Scholar]
- 44.Motamed M, Sandhu D, Murty GE. Sildenafil and nasal obstruction. J Otolaryngol. 2003;32:259–261. [DOI] [PubMed] [Google Scholar]
- 45.Cingi C, Ozdoganoglu T, Songu M. Nasal obstruction as a drug side effect. Ther Adv Respir Dis. 2011;5:175–182. [DOI] [PubMed] [Google Scholar]
- 46.Togias A Unique mechanistic features of allergic rhinitis. J Allergy Clin Immunol. 2000;105:S599–604. [DOI] [PubMed] [Google Scholar]
- 47.Riccio MM, Proud D. Evidence that enhanced nasal reactivity to bradykinin in patients with symptomatic allergy is mediated by neural reflexes. J Allergy Clin Immunol. 1996;97:1252–1263. [DOI] [PubMed] [Google Scholar]
- 48.Shirasaki H, Kanaizumi E, Himi T. Immunohistochemical localization of the bradykinin B1 and B2 receptors in human nasal mucosa. Mediators Inflamm. 2009;2009:102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trimarchi M, Miluzio A, Nicolai P, Morassi ML, Bussi M, Marchisio PC. Massive apoptosis erodes nasal mucosa of cocaine abusers. Am J Rhinol. 2006;20:160–164. [PubMed] [Google Scholar]
- 50.Tan TH, Stevenson B, Yip D. Docetaxel-induced nasal septal perforation. Intern Med J. 2006;36:471–472. [DOI] [PubMed] [Google Scholar]
- 51.Lanier B, Kai G, Marple B, Wall GM. Pathophysiology and progression of nasal septal perforation. Ann Allergy Asthma Immunol. 2007;99:473–479; quiz 480-471, 521. [DOI] [PubMed] [Google Scholar]
- 52.Wang SH, Wang HW, Wang JY. Effects of cocaine on human nasal mucosa. Eur Arch Otorhinolaryngol. 1993;250:245–248. [DOI] [PubMed] [Google Scholar]
- 53.Snyder RD, Snyder LB. Intranasal cocaine abuse in an allergists office. Ann Allergy. 1985;54:489–492. [PubMed] [Google Scholar]
- 54.Hall LJ, Jackson RT. Effects of alpha and beta adrenergic agonists on nasal blood flow. Ann Otol Rhinol Laryngol. 1968;77:1120–1130. [DOI] [PubMed] [Google Scholar]
- 55.Walker JS. Rhinitis medicamentosa. J Allergy. 1952;23:183–186. [DOI] [PubMed] [Google Scholar]
- 56.Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12:158–161. [DOI] [PubMed] [Google Scholar]
- 57.Sataloff RT, Gullane PJ, Goldstein DP. Sataloff’s Comprehensive Textbook of Otolaryngology, Head and Neck Surgery. New Delhi: Jaypee Brothers Medical Publishing; 2016. [Google Scholar]
- 58.Daws LC, Callaghan PD, Moron JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. [DOI] [PubMed] [Google Scholar]
- 59.Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106:1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Prisinzano TE, Donovan MD. Permeation and metabolism of cocaine in the nasal mucosa. Eur J Drug Metab Pharmacokinet. 2012;37:255–262. [DOI] [PubMed] [Google Scholar]
- 61.Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. 2006;16:148–155. [PubMed] [Google Scholar]
- 62.Graf PM. Rhinitis medicamentosa. Clin Allergy Immunol. 2007;19:295–304. [PubMed] [Google Scholar]
- 63.Min YG, Kim HS, Suh SH, Jeon SY, Son YI, Yoon S. Paranasal sinusitis after long-term use of topical nasal decongestants. Acta Otolaryngol. 1996;116:465–471. [DOI] [PubMed] [Google Scholar]
- 64.Graf P, Juto JE. Sustained use of xylometazoline nasal spray shortens the decongestive response and induces rebound swelling. Rhinology. 1995;33:14–17. [PubMed] [Google Scholar]
- 65.Bralow L Vicks Sinex PDR. Montvale, NJ: Thomson, 2004. [Google Scholar]
- 66.Fleece L, Mizes JS, Jolly PA, Baldwin RL. Rhinitis medicamentosa. Conceptualization, incidence, and treatment. Ala J Med Sci. 1984;21:205–208. [PubMed] [Google Scholar]
- 67.Knipping S, Holzhausen HJ, Goetze G, Riederer A, Bloching MB. Rhinitis medicamentosa: electron microscopic changes of human nasal mucosa. Otolaryngol Head Neck Surg. 2007;136:57–61. [DOI] [PubMed] [Google Scholar]
- 68.Marple B, Roland P, Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions. Otolaryngol Head Neck Surg. 2004;130:131–141. [DOI] [PubMed] [Google Scholar]
- 69.Graf P Adverse effects of benzalkonium chloride on the nasal mucosa: allergic rhinitis and rhinitis medicamentosa. Clin Ther. 1999;21:1749–1755. [DOI] [PubMed] [Google Scholar]
- 70.Graf P Rhinitis medicamentosa: a review of causes and treatment. Treat Respir Med. 2005;4:21–29. [DOI] [PubMed] [Google Scholar]
- 71.Graf P Benzalkonium chloride as a preservative in nasal solutions: re-examining the data. Respir Med. 2001;95:728–733. [DOI] [PubMed] [Google Scholar]
- 72.Morris S, Eccles R, Martez SJ, Riker DK, Witek TJ. An evaluation of nasal response following different treatment regimes of oxymetazoline with reference to rebound congestion. Am J Rhinol. 1997;11:109–115. [DOI] [PubMed] [Google Scholar]
- 73.Chodirker WB. Rhinitis medicamentosa. Can Med Assoc J. 1981;124:370, 372. [PMC free article] [PubMed] [Google Scholar]
- 74.May M, West JW. The “stuffy” nose. Otolaryngol Clin North Am. 1973;6:655–674. [PubMed] [Google Scholar]
- 75.Graf P, Hallen H, Juto JE. The pathophysiology and treatment of rhinitis medicamentosa. Clin Otolaryngol Allied Sci. 1995;20:224–229. [DOI] [PubMed] [Google Scholar]
- 76.Elwany S, Abdel-Salaam S. Treatment of rhinitis medicamentosa with fluticasone propionate—an experimental study. Eur Arch Otorhinolaryngol. 2001;258:116–119. [DOI] [PubMed] [Google Scholar]
- 77.Tas A, Yagiz R, Yalcin O, et al. Use of mometasone furoate aqueous nasal spray in the treatment of rhinitis medicamentosa: an experimental study. Otolaryngol Head Neck Surg. 2005;132:608–612. [DOI] [PubMed] [Google Scholar]
- 78.Stephens AL Jr, Boggs PB. Intranasal dexamethasone: an adjunct in the treatment of chemical rhinitis. Ann Allergy. 1968;26:612–613. [PubMed] [Google Scholar]
- 79.Elwany SS, Stephanos WM. Rhinitis medicamentosa. An experimental histopathological and histochemical study. ORL J Otorhinolaryngol Relat Spec. 1983;45:187–194. [DOI] [PubMed] [Google Scholar]
- 80.Settipane RA. Other causes of rhinitis: mixed rhinitis, rhinitis medicamentosa, hormonal rhinitis, rhinitis of the elderly, and gustatory rhinitis. Immunol Allergy Clin North Am. 2011;31:457–467. [DOI] [PubMed] [Google Scholar]
- 81.Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1998;81:478–518. [DOI] [PubMed] [Google Scholar]
- 82.Akerlund A, Bende M. Sustained use of xylometazoline nose drops aggravates vasomotor rhinitis. Am J Rhinol. 1991;5:157–160. [Google Scholar]
- 83.Yoo JK, Seikaly H, Calhoun KH. Extended use of topical nasal decongestants. Laryngoscope. 1997;107:40–43. [DOI] [PubMed] [Google Scholar]
- 84.Moscato G, Vandenplas O, Gerth Van Wijk R, et al. Occupational rhinitis. Allergy. 2008;63:969–980. [DOI] [PubMed] [Google Scholar]
- 85.Moscato G, Dykewicz MS, Desrosiers M, Castano R. Occupational rhinitis. In: Malo JL, Chan-Yeung M, Bernstein D, eds. Asthma in the Workplace. New York: Taylor & Francis; 2013:344–356. [Google Scholar]
- 86.Siracusa A, Desrosiers M, Marabini A. Epidemiology of occupational rhinitis: prevalence, aetiology and determinants. Clin Exp Allergy. 2000;30:1519–1534. [DOI] [PubMed] [Google Scholar]
- 87.Brant A Baker’s asthma. Curr Opin Allergy Clin Immunol. 2007;7:152–155. [DOI] [PubMed] [Google Scholar]
- 88.Folletti I, Forcina A, Marabini A, Bussetti A, Siracusa A. Have the prevalence and incidence of occupational asthma and rhinitis because of laboratory animals declined in the last 25 years? Allergy. 2008;63:834–841. [DOI] [PubMed] [Google Scholar]
- 89.Mazurek JM, Weissman DN. Occupational respiratory allergic diseases in healthcare workers. Curr Allergy Asthma Rep. 2016;16:77. [DOI] [PubMed] [Google Scholar]
- 90.Szeszenia-Dabrowska N, Swiatkowska B, Wilczynska U. Occupational diseases among farmers in Poland. Med Pr. 2016;67:163–171. [DOI] [PubMed] [Google Scholar]
- 91.Lopata AL, Jeebhay MF. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013;13:288–297. [DOI] [PubMed] [Google Scholar]
- 92.Cullinan P, Harris JM, Newman Taylor AJ, et al. An outbreak of asthma in a modern detergent factory. Lancet. 2000;356:1899–1900. [DOI] [PubMed] [Google Scholar]
- 93.Moscato G, Pala G, Perfetti L, Frascaroli M, Pignatti P. Clinical and inflammatory features of occupational asthma caused by persulphate salts in comparison with asthma associated with occupational rhinitis. Allergy. 2010;65:784–790. [DOI] [PubMed] [Google Scholar]
- 94.Pala G, Pignatti P, Perfetti L, et al. Occupational rhinitis and asthma due to cabreuva wood dust. Ann Allergy Asthma Immunol. 2010;104:268–269. [DOI] [PubMed] [Google Scholar]
- 95.Siracusa A, Folletti I, Moscato G. Non-IgE-mediated and irritant-induced work-related rhinitis. Curr Opin Allergy Clin Immunol. 2013;13:159–166. [DOI] [PubMed] [Google Scholar]
- 96.Schyllert C, Ronmark E, Andersson M, et al. Occupational exposure to chemicals drives the increased risk of asthma and rhinitis observed for exposure to vapours, gas, dust and fumes: a cross-sectional population-based study. Occup Environ Med. 2016;73:663–669. [DOI] [PubMed] [Google Scholar]
- 97.Siracusa A, De Blay F, Folletti I, et al. Asthma and exposure to cleaning products—a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68:1532–1545. [DOI] [PubMed] [Google Scholar]
- 98.Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma. 2014;51:18–28. [DOI] [PubMed] [Google Scholar]
- 99.Malo JL, Lemiere C, Desjardins A, Cartier A. Prevalence and intensity of rhinoconjunctivitis in subjects with occupational asthma. Eur Respir J. 1997;10:1513–1515. [DOI] [PubMed] [Google Scholar]
- 100.Castano R, Malo JL. Occupational rhinitis and asthma: where do we stand, where do we go? Curr Allergy Asthma Rep. 2010;10:135–142. [DOI] [PubMed] [Google Scholar]
- 101.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. [DOI] [PubMed] [Google Scholar]
- 102.Akpinar-Elci M, Pasquale DK, Abrokwah M, Nguyen M, Elci OC. United airway disease among crop farmers. J Agromedicine. 2016;21:217–223. [DOI] [PubMed] [Google Scholar]
- 103.Moscato G, Pala G, Folletti I, Siracusa A, Quirce S. Occupational rhinitis affects occupational asthma severity. J Occup Health. 2016;58:310–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ottaviano G, Fokkens WJ. Measurements of nasal airflow and patency: a critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy. 2016;71:162–174. [DOI] [PubMed] [Google Scholar]
- 105.Pignatti P, Pala G, Pisati M, Perfetti L, Banchieri G, Moscato G. Nasal blown secretion evaluation in specific occupational nasal challenges. Int Arch Occup Environ Health. 2010;83:217–223. [DOI] [PubMed] [Google Scholar]
- 106.Gomez F, Rondon C, Salas M, Campo P. Local allergic rhinitis: mechanisms, diagnosis and relevance for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2015;15:111–116. [DOI] [PubMed] [Google Scholar]
- 107.Rondon C, Campo P, Togias A, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012;129:1460–1467. [DOI] [PubMed] [Google Scholar]
- 108.Grammer LC, 3rd. Occupational rhinitis. Immunol Allergy Clin North Am. 2016;36:333–341. [DOI] [PubMed] [Google Scholar]
- 109.Moscato G, Pala G, Sastre J. Specific immunotherapy and biological treatments for occupational allergy. Curr Opin Allergy Clin Immunol. 2014;14:576–581. [DOI] [PubMed] [Google Scholar]
- 110.Moscato G, Pala G, Boillat MA, et al. EAACI position paper: prevention of work-related respiratory allergies among pre-apprentices or apprentices and young workers. Allergy. 2011;66:1164–1173. [DOI] [PubMed] [Google Scholar]
- 111.Foss-Skiftesvik MH, Winther L, Johnsen CR, et al. High occurrence of rhinitis symptoms in hair-dressing apprentices. Int Forum Allergy Rhinol. 2017;7:43–49. [DOI] [PubMed] [Google Scholar]
- 112.Shusterman D Occupational irritant and allergic rhinitis. Curr Allergy Asthma Rep. 2014;14:425. [DOI] [PubMed] [Google Scholar]
- 113.Ottaviano G, Staffieri A, Stritoni P, et al. Nasal dysfunction induced by chlorinate water in competitive swimmers. Rhinology. 2012;50:294–298. [DOI] [PubMed] [Google Scholar]
- 114.Centers for Disease Control. 1986 Surgeon General’s report: the health consequences of involuntary smoking. MMWR Morb Mortal Wkly Rep. 1986;35:769–770. [PubMed] [Google Scholar]
- 115.Tai CF, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol. 2002;2:11–19. [DOI] [PubMed] [Google Scholar]
- 116.Meggs WJ. RADS and RUDS—the toxic induction of asthma and rhinitis. J Toxicol Clin Toxicol. 1994;32:487–501. [DOI] [PubMed] [Google Scholar]
- 117.Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985;88:376–384. [DOI] [PubMed] [Google Scholar]
- 118.Graham C, Rosenkranz HS, Karol MH. Structure-activity model of chemicals that cause human respiratory sensitization. Regul Toxicol Pharmacol. 1997;26:296–306. [DOI] [PubMed] [Google Scholar]
- 119.Kimber I, Dearman RJ. Toxicology of Chemical Respiratory Hypersensitivity. London: Taylor & Francis; 1997. [Google Scholar]
- 120.Baur X A compendium of causative agents of occupational asthma. J Occup Med Toxicol. 2013;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cartier A, Grammer L, Malo JL, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84:507–514. [DOI] [PubMed] [Google Scholar]
- 122.Kimber I, Dearman RJ. Chemical respiratory allergy: role of IgE antibody and relevance of route of exposure. Toxicology. 2002;181–182:311–315. [DOI] [PubMed] [Google Scholar]
- 123.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eriksson J, Ekerljung L, Sundblad BM, et al. Cigarette smoking is associated with high prevalence of chronic rhinitis and low prevalence of allergic rhinitis in men. Allergy. 2013;68:347–354. [DOI] [PubMed] [Google Scholar]
- 125.Reh DD, Higgins TS, Smith TL. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol. 2012;2:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abramson MJ, Schindler C, Schikowski T, et al. Rhinitis in Swiss adults is associated with asthma and early life factors, but not second hand tobacco smoke or obesity. Allergol Int. 2016;65:192–198. [DOI] [PubMed] [Google Scholar]
- 127.Pallasaho P, Kainu A, Juusela M, Meren M, Sovijarvi A. High prevalence of rhinitis symptoms with-out allergic sensitization in Estonia and Finland. Eur Clin Respir J. 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shargorodsky J, Garcia-Esquinas E, Galan I, Navas-Acien A, Lin SY. Allergic sensitization, rhinitis and tobacco smoke exposure in US adults. PLoS One. 2015;10:e0131957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gleich GJ, Welsh PW, Yunginger JW, Hyatt RE, Catlett JB. Allergy to tobacco: an occupational hazard. N Engl J Med. 1980;302:617–619. [DOI] [PubMed] [Google Scholar]
- 130.Burrows B, Halonen M, Lebowitz MD, Knudson RJ, Barbee RA. The relationship of serum immunoglobulin E, allergy skin tests, and smoking to respiratory disorders. J Allergy Clin Immunol. 1982;70:199–204. [DOI] [PubMed] [Google Scholar]
- 131.Bascom R, Kesavanathan J, Fitzgerald TK, Cheng KH, Swift DL. Sidestream tobacco smoke exposure acutely alters human nasal mucociliary clearance. Environ Health Perspect. 1995;103:1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lundblad L, Lundberg JM. Capsaicin sensitive sensory neurons mediate the response to nasal irritation induced by the vapour phase of cigarette smoke. Toxicology. 1984;33:1–7. [DOI] [PubMed] [Google Scholar]
- 133.Meggs WJ. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect. 1993;101:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bascom R, Kulle T, Kagey-Sobotka A, Proud D. Upper respiratory tract environmental tobacco smoke sensitivity. Am Rev Respir Dis. 1991;143:1304–1311. [DOI] [PubMed] [Google Scholar]
- 135.Quillen DM, Feller DB. Diagnosing rhinitis: allergic vs. nonallergic. Am Fam Physician. 2006;73:1583–1590. [PubMed] [Google Scholar]
- 136.Desrosiers M, Evans GA, Keith PK, et al. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. Allergy Asthma Clin Immunol. 2011;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152:S1–S39. [DOI] [PubMed] [Google Scholar]
- 138.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 139.Ellegard E, Hellgren M, Toren K, Karlsson G. The incidence of pregnancy rhinitis. Gynecol Obstet Invest. 2000;49:98–101. [DOI] [PubMed] [Google Scholar]
- 140.Lieberman P, Pattanaik D. Nonallergic rhinitis. Curr Allergy Asthma Rep. 2014;14:439. [DOI] [PubMed] [Google Scholar]
- 141.Ellegard EK. The etiology and management of pregnancy rhinitis. Am J Respir Med. 2003;2:469–475. [DOI] [PubMed] [Google Scholar]
- 142.Schatz M, Zeiger RS. Diagnosis and management of rhinitis during pregnancy. Allergy Proc. 1988;9:545–554. [DOI] [PubMed] [Google Scholar]
- 143.Sobol SE, Frenkiel S, Nachtigal D, Wiener D, Teblum C. Clinical manifestations of sinonasal pathology during pregnancy. J Otolaryngol. 2001;30:24–28. [DOI] [PubMed] [Google Scholar]
- 144.Georgitis JW. Prevalence and differential diagnosis of chronic rhinitis. Curr Allergy Asthma Rep. 2001;1:202–206. [DOI] [PubMed] [Google Scholar]
- 145.Ellegard EK, Karlsson NG, Ellegard LH. Rhinitis in the menstrual cycle, pregnancy, and some endocrine disorders. Clin Allergy Immunol. 2007;19:305–321. [PubMed] [Google Scholar]
- 146.Toppozada H, Michaels L, Toppozada M, El-Ghazzawi I, Talaat M, Elwany S. The human respiratory nasal mucosa in pregnancy. An electron microscopic and histochemical study. J Laryngol Otol. 1982;96:613–626. [DOI] [PubMed] [Google Scholar]
- 147.Stroud RH, Wright ST, Calhoun KH. Nocturnal nasal congestion and nasal resistance. Laryngoscope. 1999;109:1450–1453. [DOI] [PubMed] [Google Scholar]
- 148.Turnbull GL, Rundell OH, Rayburn WF, Jones RK, Pearman CS. Managing pregnancy-related nocturnal nasal congestion. The external nasal dilator. J Reprod Med. 1996;41:897–902. [PubMed] [Google Scholar]
- 149.Eccles R Nasal airflow in health and disease. Acta Otolaryngol. 2000;120:580–595. [DOI] [PubMed] [Google Scholar]
- 150.Schatz M, Zeiger RS, Falkoff R. Asthma and allergic diseases during pregnancy. In: Adkinson NF, Bochner BS, Burks AW, et al. , eds. Middleton’s Allergy: Principles and Practice. St. Louis: Mosby; 2014:951. [Google Scholar]
- 151.Garavello W, Somigliana E, Acaia B, Gaini L, Pignataro L, Gaini RM. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. 2010;151:137–141. [DOI] [PubMed] [Google Scholar]
- 152.Ellegard EK, Hellgren M, Karlsson NG. Fluticasone propionate aqueous nasal spray in pregnancy rhinitis. Clin Otolaryngol Allied Sci. 2001;26:394–400. [DOI] [PubMed] [Google Scholar]
- 153.Kumar R, Hayhurst KL, Robson AK. Ear, nose, and throat manifestations during pregnancy. Otolaryngol Head Neck Surg. 2011;145:188–198. [DOI] [PubMed] [Google Scholar]
- 154.Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86:494–507; quiz 507-498. [DOI] [PubMed] [Google Scholar]
- 155.Fokkens WJ. Thoughts on the pathophysiology of nonallergic rhinitis. Curr Allergy Asthma Rep. 2002;2:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wolstenholme CR, Philpott CM, Oloto EJ, Murty GE. Does the use of the combined oral contraceptive pill cause changes in the nasal physiology in young women? Am J Rhinol. 2006;20:238–240. [PubMed] [Google Scholar]
- 157.Raphael G, Raphael MH, Kaliner M. Gustatory rhinitis: a syndrome of food-induced rhinorrhea. J Allergy Clin Immunol. 1989;83:110–115. [DOI] [PubMed] [Google Scholar]
- 158.Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990;117:561–567. [DOI] [PubMed] [Google Scholar]
- 159.Kleine-Tebbe J, Herold DA. [Cross-reactive allergen clusters in pollen-associated food allergy]. Hautarzt. 2003;54:130–137. German. [DOI] [PubMed] [Google Scholar]
- 160.Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev-Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20:686–692. [DOI] [PubMed] [Google Scholar]
- 161.Eriksson NE, Wihl JA, Arrendal H. Birch pollen-related food hypersensitivity: influence of total and specific IgE levels. A multicenter study. Allergy. 1983;38:353–357. [DOI] [PubMed] [Google Scholar]
- 162.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016–1025.e43. [DOI] [PubMed] [Google Scholar]
- 163.Vally H, de Klerk N, Thompson PJ. Alcoholic drinks: important triggers for asthma. J Allergy Clin Immunol. 2000;105:462–467. [DOI] [PubMed] [Google Scholar]
- 164.Andersson M, Persson CG, Svensson C, Cervin-Hoberg C, Greiff L. Effects of loratadine on red wine-induced symptoms and signs of rhinitis. Acta Otolaryngol. 2003;123:1087–1093. [DOI] [PubMed] [Google Scholar]
- 165.Nihlen U, Greiff LJ, Nyberg P, Persson CG, Andersson M. Alcohol-induced upper airway symptoms: prevalence and co-morbidity. Respir Med. 2005;99:762–769. [DOI] [PubMed] [Google Scholar]
- 166.Gershwin ME, Ough C, Bock A, Fletcher MP, Nagy SM, Tuft DS. Grand rounds: adverse reactions to wine. J Allergy Clin Immunol. 1985;75:411–420. [DOI] [PubMed] [Google Scholar]
- 167.Dahl R, Henriksen JM, Harving H. Red wine asthma: a controlled challenge study. J Allergy Clin Immunol. 1986;78:1126–1129. [DOI] [PubMed] [Google Scholar]
- 168.Emery NL, Vollmer WM, Buist AS, Osborne ML. Self-reported food reactions and their associations with asthma. West J Nurs Res. 1996;18:643–654. [DOI] [PubMed] [Google Scholar]
- 169.Ryden O, Andersson B, Andersson M. Disease perception and social behaviour in persistent rhinitis: a comparison between patients with allergic and nonallergic rhinitis. Allergy. 2004;59:461–464. [DOI] [PubMed] [Google Scholar]
- 170.Bergman H, Kallmen H. Alcohol use among Swedes and a psychometric evaluation of the alcohol use disorders identification test. Alcohol Alcohol. 2002;37:245–251. [DOI] [PubMed] [Google Scholar]
- 171.Linneberg A, Petersen J, Nielsen NH, et al. The relationship of alcohol consumption to total immunoglobulin E and the development of immunoglobulin E sensitization: the Copenhagen Allergy Study. Clin Exp Allergy. 2003;33:192–198. [DOI] [PubMed] [Google Scholar]
- 172.Gonzalez-Quintela A, Gude F, Boquete O, et al. Association of alcohol consumption with total serum immunoglobulin E levels and allergic sensitization in an adult population-based survey. Clin Exp Allergy. 2003;33:199–205. [DOI] [PubMed] [Google Scholar]
- 173.Ellis AK, Keith PK. Nonallergic rhinitis with eosinophilia syndrome. Curr Allergy Asthma Rep. 2006;6:215–220. [DOI] [PubMed] [Google Scholar]
- 174.Jacobs RL, Freedman PM, Boswell RN. Nonallergic rhinitis with eosinophilia (NARES syndrome). Clinical and immunologic presentation. J Allergy Clin Immunol. 1981;67:253–262. [DOI] [PubMed] [Google Scholar]
- 175.Simola M, Malmberg H. Sense of smell in allergic and nonallergic rhinitis. Allergy. 1998;53:190–194. [DOI] [PubMed] [Google Scholar]
- 176.Moneret-Vautrin DA, Jankowski R, Bene MC, et al. NARES: a model of inflammation caused by activated eosinophils? Rhinology. 1992;30:161–168. [PubMed] [Google Scholar]
- 177.Powe DG, Huskisson RS, Carney AS, Jenkins D, Jones NS. Evidence for an inflammatory pathophysiology in idiopathic rhinitis. Clin Exp Allergy. 2001;31:864–872. [DOI] [PubMed] [Google Scholar]
- 178.Berger G, Goldberg A, Ophir D. The inferior turbinate mast cell population of patients with perennial allergic and nonallergic rhinitis. Am J Rhinol. 1997;11:63–66. [DOI] [PubMed] [Google Scholar]
- 179.De Corso E, Baroni S, Lucidi D, et al. Nasal lavage levels of granulocyte-macrophage colony-stimulating factor and chronic nasal hypereosinophilia. Int Forum Allergy Rhinol. 2015;5:557–562. [DOI] [PubMed] [Google Scholar]
- 180.De Corso E, Baroni S, Battista M, et al. Nasal fluid release of eotaxin-3 and eotaxin-2 in persistent sinonasal eosinophilic inflammation. Int Forum Allergy Rhinol. 2014;4:617–624. [DOI] [PubMed] [Google Scholar]
- 181.Kramer MF, Burow G, Pfrogner E, Rasp G. In vitro diagnosis of chronic nasal inflammation. Clin Exp Allergy. 2004;34:1086–1092. [DOI] [PubMed] [Google Scholar]
- 182.Groger M, Klemens C, Wendt S, et al. Mediators and cytokines in persistent allergic rhinitis and nonallergic rhinitis with eosinophilia syndrome. Int Arch Allergy Immunol. 2012;159:171–178. [DOI] [PubMed] [Google Scholar]
- 183.Marcella R, Croce A, Moretti A, Barbacane RC, Di Giocchino M, Conti P. Transcription and translation of the chemokines RANTES and MCP-1 in nasal polyps and mucosa in allergic and non-allergic rhinopathies. Immunol Lett. 2003;90:71–75. [DOI] [PubMed] [Google Scholar]
- 184.Peric A, Sotirovic J, Spadijer-Mirkovic C, Matkovic-Jozin S, Peric AV, Vojvodic D. Nonselective chemokine levels in nasal secretions of patients with perennial nonallergic and allergic rhinitis. Int Forum Allergy Rhinol. 2016;6:392–397. [DOI] [PubMed] [Google Scholar]
- 185.Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol. 1992;149:3309–3315. [PubMed] [Google Scholar]
- 186.Kramer MF, de la Chaux R, Fintelmann R, Rasp G. NARES: a risk factor for obstructive sleep apnea? Am J Otolaryngol. 2004;25:173–177. [DOI] [PubMed] [Google Scholar]
- 187.Pipkorn U, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987;316:1506–1510. [DOI] [PubMed] [Google Scholar]
- 188.Banov CH, Lieberman P, Vasomotor Rhinitis Study Group. Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial non-allergic) rhinitis. Ann Allergy Asthma Immunol. 2001;86:28–35. [DOI] [PubMed] [Google Scholar]
- 189.Settipane RA. Epidemiology of vasomotor rhinitis. World Allergy Organ J. 2009;2:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mullarkey MF, Hill JS, Webb DR. Allergic and non-allergic rhinitis: their characterization with attention to the meaning of nasal eosinophilia. J Allergy Clin Immunol. 1980;65:122–126. [DOI] [PubMed] [Google Scholar]
- 191.Enberg RN. Perennial nonallergic rhinitis: a retrospective review. Ann Allergy. 1989;63:513–516. [PubMed] [Google Scholar]
- 192.Segboer CL, Holland CT, Reinartz SM, et al. Nasal hyperreactivity is a common feature in both allergic and nonallergic rhinitis. Allergy. 2013;68:1427–1434. [DOI] [PubMed] [Google Scholar]
- 193.James LK, Durham SR. Rhinitis with negative skin tests and absent serum allergen-specific IgE: more evidence for local IgE? J Allergy Clin Immunol. 2009;124:1012–1013. [DOI] [PubMed] [Google Scholar]
- 194.Campo P, Rondon C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non-allergic rhinitis. Clin Exp Allergy. 2015;45:872–881. [DOI] [PubMed] [Google Scholar]
- 195.Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46:1139–1151. [DOI] [PubMed] [Google Scholar]
- 196.Bernstein JA, Hastings L, Boespflug EL, Allendorfer JB, Lamy M, Eliassen JC. Alteration of brain activation patterns in nonallergic rhinitis patients using functional magnetic resonance imaging before and after treatment with intranasal azelastine. Ann Allergy Asthma Immunol. 2011;106:527–532. [DOI] [PubMed] [Google Scholar]
- 197.Sahin-Yilmaz AA, Corey JP. Rhinitis in the elderly. Clin Allergy Immunol. 2007;19:209–219. [PubMed] [Google Scholar]
- 198.Edelstein DR. Aging of the normal nose in adults. Laryngoscope. 1996;106:1–25. [DOI] [PubMed] [Google Scholar]
- 199.Lindemann J, Sannwald D, Wiesmiller K. Age-related changes in intranasal air conditioning in the elderly. Laryngoscope. 2008;118:1472–1475. [DOI] [PubMed] [Google Scholar]
- 200.Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.DelGaudio JM, Panella NJ. Presbynasalis. Int Forum Allergy Rhinol. 2016;6:1083–1087. [DOI] [PubMed] [Google Scholar]
- 202.Rodriguez K, Rubinstein E, Ferguson BJ. Clear anterior rhinorrhea in the population. Int Forum Allergy Rhinol. 2015;5:1063–1067. [DOI] [PubMed] [Google Scholar]
- 203.Parashar R, Amir M, Pakhare A, Rathi P, Chaudhary L. Age related changes in autonomic functions. J Clin Diagn Res. 2016;10:CC11–CC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10(Suppl 1):S127–S136. [DOI] [PubMed] [Google Scholar]
- 205.Lal D, Corey JP. Vasomotor rhinitis update. Curr Opin Otolaryngol Head Neck Surg. 2004;12:243–247. [DOI] [PubMed] [Google Scholar]
- 206.Kimmelman CP, Ali GH. Vasomotor rhinitis. Otolaryngol Clin North Am. 1986;19:65–71. [PubMed] [Google Scholar]
- 207.Baptist AP, Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin North Am. 2016;36:343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Janzen VD. Rhinological disorders in the elderly. J Otolaryngol. 1986;15:228–230. [PubMed] [Google Scholar]
- 209.Ciftci Z, Catli T, Hanci D, Cingi C, Erdogan G. Rhinorrhoea in the elderly. Eur Arch Otorhinolaryngol. 2015;272:2587–2592. [DOI] [PubMed] [Google Scholar]
- 210.Bozek A Pharmacological management of allergic rhinitis in the elderly. Drugs Aging. 2017;34:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. [DOI] [PubMed] [Google Scholar]
- 212.Mirza N, Kroger H, Doty RL. Influence of age on the ‘nasal cycle’. Laryngoscope. 1997;107:62–66. [DOI] [PubMed] [Google Scholar]
- 213.Slavin RG. Treating rhinitis in the older population: special considerations. Allergy Asthma Clin Immunol. 2009;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schrodter S, Biermann E, Halata Z. Histological evaluation of age-related changes in human respiratory mucosa of the middle turbinate. Anat Embryol (Berl). 2003;207:19–27. [DOI] [PubMed] [Google Scholar]
- 215.Loftus PA, Wise SK, Nieto D, Panella N, Aiken A, DelGaudio JM. Intranasal volume increases with age: computed tomography volumetric analysis in adults. Laryngoscope. 2016;126:2212–2215. [DOI] [PubMed] [Google Scholar]
- 216.Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010;31:179–184. [DOI] [PubMed] [Google Scholar]
- 217.Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis executive summary. Otolaryngol Head Neck Surg. 2015;152:197–206. [DOI] [PubMed] [Google Scholar]
- 219.Scheithauer MO. Surgery of the turbinates and “empty nose” syndrome. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2010;9:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Coste A, Dessi P, Serrano E. Empty nose syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:93–97. [DOI] [PubMed] [Google Scholar]
- 221.Sozansky J, Houser SM. Pathophysiology of empty nose syndrome. Laryngoscope. 2015;125:70–74. [DOI] [PubMed] [Google Scholar]
- 222.Kuan EC, Suh JD, Wang MB. Empty nose syndrome. Curr Allergy Asthma Rep. 2015;15:493. [DOI] [PubMed] [Google Scholar]
- 223.Houser SM. Surgical treatment for empty nose syndrome. Arch Otolaryngol Head Neck Surg. 2007;133:858–863. [DOI] [PubMed] [Google Scholar]
- 224.Zhao K, Blacker K, Luo Y, Bryant B, Jiang J. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS One. 2011;6:e24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao K, Jiang J, Blacker K, et al. Regional peak mucosal cooling predicts the perception of nasal patency. Laryngoscope. 2014;124:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Willatt DJ, Jones AS. The role of the temperature of the nasal lining in the sensation of nasal patency. Clin Otolaryngol Allied Sci. 1996;21:519–523. [DOI] [PubMed] [Google Scholar]
- 227.Kimbell JS, Frank DO, Laud P, Garcia GJ, Rhee JS. Changes in nasal airflow and heat transfer correlate with symptom improvement after surgery for nasal obstruction. J Biomech. 2013;46:2634–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lindemann J, Tsakiropoulou E, Scheithauer MO, Konstantinidis I, Wiesmiller KM. Impact of menthol inhalation on nasal mucosal temperature and nasal patency. Am J Rhinol. 2008;22:402–405. [DOI] [PubMed] [Google Scholar]
- 229.Velasquez N, Thamboo A, Habib AR, Huang Z, Nayak JV. The Empty Nose Syndrome 6-Item Questionnaire (ENS6Q): a validated 6-item questionnaire as a diagnostic aid for empty nose syndrome patients. Int Forum Allergy Rhinol. 2017;7:64–71. [DOI] [PubMed] [Google Scholar]
- 230.Lee TJ, Fu CH, Wu CL, et al. Evaluation of depression and anxiety in empty nose syndrome after surgical treatment. Laryngoscope. 2016;126:1284–1289. [DOI] [PubMed] [Google Scholar]
- 231.Moore EJ, Kern EB. Atrophic rhinitis: a review of 242 cases. Am J Rhinol. 2001;15:355–361. [PubMed] [Google Scholar]
- 232.Goodman WS, De Souza FM. Atrophic rhinitis. Otolaryngol Clin North Am. 1973;6:773–782. [PubMed] [Google Scholar]
- 233.Cottle MH. Nasal atrophy, atrophic rhinitis, ozena: medical and surgical treatment: repair of septal perforations. J Int Coll Surg. 1958;29:472–484. [PubMed] [Google Scholar]
- 234.Hildenbrand T, Weber RK, Brehmer D. Rhinitis sicca, dry nose and atrophic rhinitis: a review of the literature. Eur Arch Otorhinolaryngol. 2011;268:17–26. [DOI] [PubMed] [Google Scholar]
- 235.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. [DOI] [PubMed] [Google Scholar]
- 236.Alobid I, Mullol J, Cid MC. Rhinitis of granulomatous and vasculitic diseases. Clin Allergy Immunol. 2007;19:221–239. [PubMed] [Google Scholar]
- 237.Sardana K, Goel K. Nasal septal ulceration. Clin Dermatol. 2014;32:817–826. [DOI] [PubMed] [Google Scholar]
- 238.Alobid I, Guilemany JM, Mullol J. Nasal manifestations of systemic illnesses. Curr Allergy Asthma Rep. 2004;4:208–216. [DOI] [PubMed] [Google Scholar]
- 239.Watts RA, Lane S, Scott DG. What is known about the epidemiology of the vasculitides? Best Pract Res Clin Rheumatol. 2005;19:191–207. [DOI] [PubMed] [Google Scholar]
- 240.Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am. 2003;36:685–705. [DOI] [PubMed] [Google Scholar]
- 241.Metaxaris G, Prokopakis EP, Karatzanis AD, et al. Otolaryngologic manifestations of small vessel vasculitis. Auris Nasus Larynx. 2002;29:353–356. [DOI] [PubMed] [Google Scholar]
- 242.Diamantopoulos II, Jones NS. The investigation of nasal septal perforations and ulcers. J Laryngol Otol. 2001;115:541–544. [DOI] [PubMed] [Google Scholar]
- 243.Grayson PC, Steiling K, Platt M, et al. Defining the nasal transcriptome in granulomatosis with polyangiitis (Wegener’s). Arthritis Rheumatol. 2015;67:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Izquierdo-Dominguez A, Cordero Castillo A, Alobid I, Mullol J. Churg-Strauss syndrome or eosinophilic granulomatosis with polyangiitis. Sinusitis. 2015;1:24–43. [Google Scholar]
- 245.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. [DOI] [PubMed] [Google Scholar]
- 246.Smith RM, Jones RB, Guerry MJ, et al. Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:3760–3769. [DOI] [PubMed] [Google Scholar]
- 247.Chaigne B, Dion J, Guillevin L, Mouthon L, Terrier B. [Pathophysiology of eosinophilic granulomatosis with polyangitis (Churg-Strauss)]. Rev Med Interne. 2016;37:337–342. French. [DOI] [PubMed] [Google Scholar]
- 248.Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med. 2015;26:545–553. [DOI] [PubMed] [Google Scholar]
- 249.Keogh KA, Specks U. Churg-Strauss syndrome: clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med. 2003;115:284–290. [DOI] [PubMed] [Google Scholar]
- 250.Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361:587–594. [DOI] [PubMed] [Google Scholar]
- 251.Gross WL. Churg-Strauss syndrome: update on recent developments. Curr Opin Rheumatol. 2002;14:11–14. [DOI] [PubMed] [Google Scholar]
- 252.Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–1343. [DOI] [PubMed] [Google Scholar]
- 253.Long CM, Smith TL, Loehrl TA, Komorowski RA, Toohill RJ. Sinonasal disease in patients with sarcoidosis. Am J Rhinol. 2001;15:211–215. [DOI] [PubMed] [Google Scholar]
- 254.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. [DOI] [PubMed] [Google Scholar]
- 255.Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. [DOI] [PubMed] [Google Scholar]
- 256.Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63–78. [DOI] [PubMed] [Google Scholar]
- 257.Lawson W, Jiang N, Cheng J. Sinonasal sarcoidosis: a new system of classification acting as a guide to diagnosis and treatment. Am J Rhinol Allergy. 2014;28:317–322. [DOI] [PubMed] [Google Scholar]
- 258.Rottoli P, Bargagli E, Chidichimo C, et al. Sarcoidosis with upper respiratory tract involvement. Respir Med. 2006;100:253–257. [DOI] [PubMed] [Google Scholar]
- 259.Chapelon-Abric C, Saadoun D, Biard L, et al. Long-term outcome of infliximab in severe chronic and refractory systemic sarcoidosis: a report of 16 cases. Clin Exp Rheumatol. 2015;33:509–515. [PubMed] [Google Scholar]
- 260.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–1888. [DOI] [PubMed] [Google Scholar]
- 261.Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford). 2017;56:i3–i13. [DOI] [PubMed] [Google Scholar]
- 262.Durcan L, Petri M. Immunomodulators in SLE: clinical evidence and immunologic actions. J Autoimmun. 2016;74:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Garcia A, De Sanctis JB. A review of clinical trials of belimumab in the management of systemic lupus erythematosus. Curr Pharm Des. 2016;22:6306–6312. [DOI] [PubMed] [Google Scholar]
- 264.Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kakli HA, Riley TD. Allergic rhinitis. Prim Care. 2016;43:465–475. [DOI] [PubMed] [Google Scholar]
- 266.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32. [DOI] [PubMed] [Google Scholar]
- 267.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shapiro DJ, Gonzales R, Cabana MD, Hersh AL. National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics. 2011;127:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Togias A Systemic effects of local allergic disease. J Allergy Clin Immunol. 2004;113:S8–S14. [DOI] [PubMed] [Google Scholar]
- 270.Pinart M, Benet M, Annesi-Maesano I, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014;2:131–140. [DOI] [PubMed] [Google Scholar]
- 271.Togias AG. Systemic immunologic and inflammatory aspects of allergic rhinitis. J Allergy Clin Immunol. 2000;106:S247–S250. [DOI] [PubMed] [Google Scholar]
- 272.Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013;3:384–392. [DOI] [PubMed] [Google Scholar]
- 273.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–1179. [DOI] [PubMed] [Google Scholar]
- 274.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sin B, Togias A. Pathophysiology of allergic and nonallergic rhinitis. Proc Am Thorac Soc. 2011;8:106–114. [DOI] [PubMed] [Google Scholar]
- 276.Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Geha RS. Regulation of IgE synthesis in humans. J Allergy Clin Immunol. 1992;90:143–150. [DOI] [PubMed] [Google Scholar]
- 280.Henry AJ, Cook JP, McDonnell JM, et al. Participation of the N-terminal region of Cepsilon3 in the binding of human IgE to its high-affinity receptor FcepsilonRI. Biochemistry. 1997;36:15568–15578. [DOI] [PubMed] [Google Scholar]
- 281.Posa D, Hofmaier S, Arasi S, Matricardi PM. Natural evolution of IgE responses to mite allergens and relationship to progression of allergic disease: a review. Curr Allergy Asthma Rep. 2017;17:28. [DOI] [PubMed] [Google Scholar]
- 282.Cameron L, Hamid Q, Wright E, et al. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000;106:46–52. [DOI] [PubMed] [Google Scholar]
- 283.Pawankar R, Yamagishi S, Yagi T. Revisiting the roles of mast cells in allergic rhinitis and its relation to local IgE synthesis. Am J Rhinol. 2000;14:309–317. [DOI] [PubMed] [Google Scholar]
- 284.Powe DG, Jagger C, Kleinjan A, Carney AS, Jenkins D, Jones NS. ‘Entopy’: localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003;33:1374–1379. [DOI] [PubMed] [Google Scholar]
- 285.Pawankar R, Ra C. IgE-Fc epsilonRI-mast cell axis in the allergic cycle. Clin Exp Allergy. 1998;28(Suppl 3):6–14. [PubMed] [Google Scholar]
- 286.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Powe DG, Jones NS. Local mucosal immunoglobulin E production: does allergy exist in non-allergic rhinitis? Clin Exp Allergy. 2006;36:1367–1372. [DOI] [PubMed] [Google Scholar]
- 288.Rondon C, Bogas G, Barrionuevo E, Blanca M, Torres MJ, Campo P. Nonallergic rhinitis and lower airway disease. Allergy. 2017;72:24–34. [DOI] [PubMed] [Google Scholar]
- 289.Scadding G, Hellings P, Alobid I, et al. Diagnostic tools in rhinology EAACI position paper. Clin Transl Allergy. 2011;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fuiano N, Fusilli S, Passalacqua G, Incorvaia C. Allergen-specific immunoglobulin E in the skin and nasal mucosa of symptomatic and asymptomatic children sensitized to aeroallergens. J Investig Allergol Clin Immunol. 2010;20:425–430. [PubMed] [Google Scholar]
- 291.Rondon C, Campo P, Galindo L, et al. Prevalence and clinical relevance of local allergic rhinitis. Allergy. 2012;67:1282–1288. [DOI] [PubMed] [Google Scholar]
- 292.Zicari AM, Occasi F, Di Fraia M, et al. Local allergic rhinitis in children: novel diagnostic features and potential biomarkers. Am J Rhinol Allergy. 2016;30:329–334. [DOI] [PubMed] [Google Scholar]
- 293.Duman H, Bostanci I, Ozmen S, Dogru M. The relevance of nasal provocation testing in children with nonallergic rhinitis. IntArch Allergy Immunol. 2016;170:115–121. [DOI] [PubMed] [Google Scholar]
- 294.Blanca-Lopez N, Campo P, Salas M, et al. Seasonal local allergic rhinitis in areas with high concentrations of grass pollen. J Investig Allergol Clin Immunol. 2016;26:83–91. [DOI] [PubMed] [Google Scholar]
- 295.Buntarickpornpan P, Veskitkul J, Pacharn P, et al. The proportion of local allergic rhinitis to dermatophagoides pteronyssinus in children. Pediatr Allergy Immunol. 2016;27:574–579. [DOI] [PubMed] [Google Scholar]
- 296.Rondon C, Campo P, Zambonino MA, et al. Follow-up study in local allergic rhinitis shows a consistent entity not evolving to systemic allergic rhinitis. J Allergy Clin Immunol. 2014;133:1026–1031. [DOI] [PubMed] [Google Scholar]
- 297.Sennekamp J, Joest I, Filipiak-Pittroff B, von Berg A, Berdel D. Local allergic nasal reactions convert to classic systemic allergic reactions: a long-term follow-up. Int Arch Allergy Immunol. 2015;166:154–160. [DOI] [PubMed] [Google Scholar]
- 298.Rondon C, Campo P, Blanca-Lopez N, Torres MJ, Blanca M. More research is needed for local allergic rhinitis. Int Arch Allergy Immunol. 2015;167:99–100. [DOI] [PubMed] [Google Scholar]
- 299.Powe DG, Huskisson RS, Carney AS, et al. Mucosal T-cell phenotypes in persistent atopic and nonatopic rhinitis show an association with mast cells. Allergy. 2004;59:204–212. [DOI] [PubMed] [Google Scholar]
- 300.Rondon C, Dona I, Lopez S, et al. Seasonal idiopathic rhinitis with local inflammatory response and specific IgE in absence of systemic response. Allergy. 2008;63:1352–1358. [DOI] [PubMed] [Google Scholar]
- 301.Rondon C, Romero JJ, Lopez S, et al. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007;119:899–905. [DOI] [PubMed] [Google Scholar]
- 302.Wedback A, Enbom H, Eriksson NE, Moverare R, Malcus I. Seasonal non-allergic rhinitis (SNAR)—a new disease entity? A clinical and immunological comparison between SNAR, seasonal allergic rhinitis and persistent non-allergic rhinitis. Rhinology. 2005;43:86–92. [PubMed] [Google Scholar]
- 303.Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975;2:148–150. [DOI] [PubMed] [Google Scholar]
- 304.Bozek A, Ignasiak B, Kasperska-Zajac A, Scierski W, Grzanka A, Jarzab J. Local allergic rhinitis in elderly patients. Ann Allergy Asthma Immunol. 2015;114:199–202. [DOI] [PubMed] [Google Scholar]
- 305.Klimek L, Bardenhewer C, Spielhaupter M, Harai C, Becker K, Pfaar O. [Local allergic rhinitis to Alternaria alternata: evidence for local IgE production exclusively in the nasal mucosa]. HNO. 2015;63:364–372. German. [DOI] [PubMed] [Google Scholar]
- 306.Lopez S, Rondon C, Torres MJ, et al. Immediate and dual response to nasal challenge with dermatophagoides pteronyssinus in local allergic rhinitis. Clin Exp Allergy. 2010;40:1007–1014. [DOI] [PubMed] [Google Scholar]
- 307.Rondon C, Fernandez J, Lopez S, et al. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J Allergy Clin Immunol. 2009;124:1005–1011.e1. [DOI] [PubMed] [Google Scholar]
- 308.Campo P, Villalba M, Barrionuevo E, et al. Immunologic responses to the major allergen of olea europaea in local and systemic allergic rhinitis subjects. Clin Exp Allergy. 2015;45:1703–1712. [DOI] [PubMed] [Google Scholar]
- 309.Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol. 2003;171:5602–5610. [DOI] [PubMed] [Google Scholar]
- 310.Durham SR, Gould HJ, Thienes CP, et al. Expression of epsilon germ-line gene transcripts and mRNA for the epsilon heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur J Immunol. 1997;27:2899–2906. [DOI] [PubMed] [Google Scholar]
- 311.Platts-Mills TA. Local production of IgG, IgA and IgE antibodies in grass pollen hay fever. J Immunol. 1979;122:2218–2225. [PubMed] [Google Scholar]
- 312.Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–5032. [DOI] [PubMed] [Google Scholar]
- 313.Ying S, Humbert M, Meng Q, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001;107:686–692. [DOI] [PubMed] [Google Scholar]
- 314.Erazo A, Kutchukhidze N, Leung M, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Cameron L, Gounni AS, Frenkiel S, Lavigne F, Vercelli D, Hamid Q. S epsilon S mu and S epsilon S gamma switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination. J Immunol. 2003;171:3816–3822. [DOI] [PubMed] [Google Scholar]
- 316.Smurthwaite L, Walker SN, Wilson DR, et al. Persistent IgE synthesis in the nasal mucosa of hay fever patients. Eur J Immunol. 2001;31:3422–3431. [DOI] [PubMed] [Google Scholar]
- 317.Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. 2012;129:635–645. [DOI] [PubMed] [Google Scholar]
- 318.Gomez E, Campo P, Rondon C, et al. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J Allergy Clin Immunol. 2013;132:975–976.e5. [DOI] [PubMed] [Google Scholar]
- 319.Papadopoulos NG, Bernstein JA, Demoly P, et al. Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy. 2015;70:474–494. [DOI] [PubMed] [Google Scholar]
- 320.Toppila-Salmi S, van Drunen CM, Fokkens WJ, et al. Molecular mechanisms of nasal epithelium in rhinitis and rhinosinusitis. Curr Allergy Asthma Rep. 2015;15:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bashir ME, Ward JM, Cummings M, et al. Dual function of novel pollen coat (surface) proteins: IgE-binding capacity and proteolytic activity disrupting the airway epithelial barrier. PLoS One. 2013;8:e53337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Steelant B, Farre R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137:1043–1053. e5. [DOI] [PubMed] [Google Scholar]
- 323.van Tongeren J, Golebski K, Van Egmond D, de Groot EJ, Fokkens WJ, van Drunen CM. Synergy between TLR-2 and TLR-3 signaling in primary human nasal epithelial cells. Immunobiology. 2015;220:445–451. [DOI] [PubMed] [Google Scholar]
- 324.Radman M, Golshiri A, Shamsizadeh A, et al. Toll-like receptor 4 plays significant roles during allergic rhinitis. Allergol Immunopathol (Madr). 2015;43:416–420. [DOI] [PubMed] [Google Scholar]
- 325.van Tongeren J, Roschmann KI, Reinartz SM, et al. Expression profiling and functional analysis of Toll-like receptors in primary healthy human nasal epithelial cells shows no correlation and a refractory LPS response. Clin Transl Allergy. 2015;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Vroling AB, Jonker MJ, Luiten S, Breit TM, Fokkens WJ, van Drunen CM. Primary nasal epithelium exposed to house dust mite extract shows activated expression in allergic individuals. Am J Respir Cell Mol Biol. 2008;38:293–299. [DOI] [PubMed] [Google Scholar]
- 327.Golebski K, van Egmond D, de Groot EJ, Roschmann KI, Fokkens WJ, van Drunen CM. EGR-1 and DUSP-1 are important negative regulators of pro-allergic responses in airway epithelium. Mol Immunol. 2015;65:43–50. [DOI] [PubMed] [Google Scholar]
- 328.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. [DOI] [PubMed] [Google Scholar]
- 329.Matsushita K, Kato Y, Akasaki S, Yoshimoto T. Proallergic cytokines and group 2 innate lymphoid cells in allergic nasal diseases. Allergol Int. 2015;64:235–240. [DOI] [PubMed] [Google Scholar]
- 330.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Karta MR, Broide DH, Doherty TA. Insights into group 2 innate lymphoid cells in human airway disease. Curr Allergy Asthma Rep. 2016;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Doherty TA, Scott D, Walford HH, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133:1203–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–1195.e4. [DOI] [PubMed] [Google Scholar]
- 334.Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology. 2016;54:195–205. [DOI] [PubMed] [Google Scholar]
- 335.Melvin TA, Ramanathan M Jr. Role of innate immunity in the pathogenesis of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20:194–198. [DOI] [PubMed] [Google Scholar]
- 336.Genuneit J, Seibold AM, Apfelbacher CJ, et al. Overview of systematic reviews in allergy epidemiology. Allergy. 2017;72:849–856. [DOI] [PubMed] [Google Scholar]
- 337.Ng CL, Wang DY. Latest developments in allergic rhinitis in Allergy for clinicians and researchers. Allergy. 2015;70:1521–1530. [DOI] [PubMed] [Google Scholar]
- 338.Hirsch AG, Yan XS, Sundaresan AS, et al. Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy. 2015;70:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shim E, Lee E, Yang SI, et al. The association of lung function, bronchial hyperresponsiveness, and exhaled nitric oxide differs between atopic and nonatopic asthma in children. Allergy Asthma Immunol Res. 2015;7:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Agache I, Sugita K, Morita H, Akdis M, Akdis CA. The complex type 2 endotype in allergy and asthma: from laboratory to bedside. Curr Allergy Asthma Rep. 2015;15:29. [DOI] [PubMed] [Google Scholar]
- 342.Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16:70. [DOI] [PubMed] [Google Scholar]
- 343.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130:1087–1096.e10. [DOI] [PubMed] [Google Scholar]
- 344.Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139:93–103. [DOI] [PubMed] [Google Scholar]
- 345.Braunstahl GJ, Fokkens W. Nasal involvement in allergic asthma. Allergy. 2003;58:1235–1243. [DOI] [PubMed] [Google Scholar]
- 346.Izuhara Y, Matsumoto H, Nagasaki T, et al. Mouth breathing, another risk factor for asthma: the Nagahama Study. Allergy. 2016;71:1031–1036. [DOI] [PubMed] [Google Scholar]
- 347.Bagnasco M, Mariani G, Passalacqua G, et al. Absorption and distribution kinetics of the major Parietaria judaica allergen (Par j 1) administered by noninjectable routes in healthy human beings. J Allergy Clin Immunol. 1997;100:122–129. [DOI] [PubMed] [Google Scholar]
- 348.Braunstahl GJ. United airways concept: what does it teach us about systemic inflammation in airways disease? Proc Am Thorac Soc. 2009;6:652–654. [DOI] [PubMed] [Google Scholar]
- 349.Rimmer J, Hellgren J, Harvey RJ. Simulated postnasal mucus fails to reproduce the symptoms of postnasal drip in rhinitics but only in healthy subjects. Rhinology. 2015;53:129–134. [DOI] [PubMed] [Google Scholar]
- 350.Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161:2051–2057. [DOI] [PubMed] [Google Scholar]
- 351.Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858–865. [DOI] [PubMed] [Google Scholar]
- 352.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–476. [DOI] [PubMed] [Google Scholar]
- 353.Allakhverdi Z, Comeau MR, Smith DE, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–478. [DOI] [PubMed] [Google Scholar]
- 354.Sergejeva S, Malmhall C, Lotvall J, Pullerits T. Increased number of CD34+ cells in nasal mucosa of allergic rhinitis patients: inhibition by a local corticosteroid. Clin Exp Allergy. 2005;35:34–38. [DOI] [PubMed] [Google Scholar]
- 355.Muraro A, Lemanske RF Jr, Hellings PW, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137:1347–1358. [DOI] [PubMed] [Google Scholar]
- 356.Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138:984–1010. [DOI] [PubMed] [Google Scholar]
- 357.Schuijs MJ, Willart MA, Vergote K, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. [DOI] [PubMed] [Google Scholar]
- 358.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. [DOI] [PubMed] [Google Scholar]
- 359.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.KleinJan A, Willart M, van Rijt LS, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–1125. [DOI] [PubMed] [Google Scholar]
- 361.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–635. [DOI] [PubMed] [Google Scholar]
- 362.Durham SR, Ying S, Varney VA, et al. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992;148:2390–2394. [PubMed] [Google Scholar]
- 363.Sogut A, Yilmaz O, Kirmaz C, et al. Regulatory-T, T-helper 1, and T-helper 2 cell differentiation in nasal mucosa of allergic rhinitis with olive pollen sensitivity. Int Arch Allergy Immunol. 2012;157:349–353. [DOI] [PubMed] [Google Scholar]
- 364.Pawankar RU, Okuda M, Okubo K, Ra C. Lymphocyte subsets of the nasal mucosa in perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152:2049–2058. [DOI] [PubMed] [Google Scholar]
- 365.Akdis M Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18:738–744. [DOI] [PubMed] [Google Scholar]
- 366.Kubo T, Wawrzyniak P, Morita H, et al. CpG-DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol. 2015;136:1413–1416.e8. [DOI] [PubMed] [Google Scholar]
- 367.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov. 2009;8:645–660. [DOI] [PubMed] [Google Scholar]
- 369.Raedler D, Ballenberger N, Klucker E, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135:81–91. [DOI] [PubMed] [Google Scholar]
- 370.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Suarez-Fueyo A, Ramos T, Galan A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133:130–138.e2. [DOI] [PubMed] [Google Scholar]
- 372.Fox EM, Torrero MN, Evans H, Mitre E. Immunologic characterization of 3 murine regimens of allergen-specific immunotherapy. J Allergy Clin Immunol. 2015;135:1341–1351.e7. [DOI] [PubMed] [Google Scholar]
- 373.Akdis CA, Akdis M. Advances in allergen immunotherapy: aiming for complete tolerance to allergens. Sci Transl Med. 2015;7:280ps286. [DOI] [PubMed] [Google Scholar]
- 374.Doherty TA, Baum R, Newbury RO, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Morita H, Arae K, Unno H, et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015;43:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ganzer U, Bachert C. Localization of IgE synthesis in immediate-type allergy of the upper respiratory tract. ORL J Otorhinolaryngol Relat Spec. 1988;50:257–264. [DOI] [PubMed] [Google Scholar]
- 377.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15:491–497. [DOI] [PubMed] [Google Scholar]
- 378.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–79. [DOI] [PubMed] [Google Scholar]
- 379.Bentley AM, Jacobson MR, Cumberworth V, et al. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992;89:877–883. [DOI] [PubMed] [Google Scholar]
- 380.KleinJan A, McEuen AR, Dijkstra MD, Buckley MG, Walls AF, Fokkens WJ. Basophil and eosinophil accumulation and mast cell degranulation in the nasal mucosa of patients with hay fever after local allergen provocation. J Allergy Clin Immunol. 2000;106:677–686. [DOI] [PubMed] [Google Scholar]
- 381.Gomez E, Corrado OJ, Baldwin DL, Swanston AR, Davies RJ. Direct in vivo evidence for mast cell degranulation during allergen-induced reactions in man. J Allergy Clin Immunol. 1986;78:637–645. [DOI] [PubMed] [Google Scholar]
- 382.Haenuki Y, Matsushita K, Futatsugi-Yumikura S, et al. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130:184–194.e11. [DOI] [PubMed] [Google Scholar]
- 383.Semik-Orzech A, Barczyk A, Wiaderkiewicz R, Pierzchala W. Eotaxin, but not IL-8, is increased in upper and lower airways of allergic rhinitis subjects after nasal allergen challenge. Allergy Asthma Proc. 2011;32:230–238. [DOI] [PubMed] [Google Scholar]
- 384.Kim TH, Lee JY, Lee HM, et al. Remodelling of nasal mucosa in mild and severe persistent allergic rhinitis with special reference to the distribution of collagen, proteoglycans, and lymphatic vessels. Clin Exp Allergy. 2010;40:1742–1754. [DOI] [PubMed] [Google Scholar]
- 385.Pawankar R Mast cells in allergic airway disease and chronic rhinosinusitis. Chem Immunol Allergy. 2005;87:111–129. [DOI] [PubMed] [Google Scholar]
- 386.Powe DG, Hiskisson RS, Carney AS, Jenkins D, Jones NS. Idiopathic and allergic rhinitis show a similar inflammatory response. Clin Otolaryngol Allied Sci. 2000;25:570–576. [DOI] [PubMed] [Google Scholar]
- 387.Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384:25–32. [DOI] [PubMed] [Google Scholar]
- 388.Erin EM, Zacharasiewicz AS, Nicholson GC, et al. Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge. Clin Exp Allergy. 2005;35:1608–1614. [DOI] [PubMed] [Google Scholar]
- 389.Erin EM, Leaker BR, Zacharasiewicz AS, et al. Single dose topical corticosteroid inhibits IL-5 and IL-13 in nasal lavage following grass pollen challenge. Allergy. 2005;60:1524–1529. [DOI] [PubMed] [Google Scholar]
- 390.Sim TC, Reece LM, Hilsmeier KA, Grant JA, Alam R. Secretion of chemokines and other cytokines in allergen-induced nasal responses: inhibition by topical steroid treatment. Am J Respir Crit Care Med. 1995;152:927–933. [DOI] [PubMed] [Google Scholar]
- 391.Terada N, Hamano N, Kim WJ, et al. The kinetics of allergen-induced eotaxin level in nasal lavage fluid: its key role in eosinophil recruitment in nasal mucosa. Am J Respir Crit Care Med. 2001;164:575–579. [DOI] [PubMed] [Google Scholar]
- 392.Wilson AM, Duong M, Crawford L, Denburg J. An evaluation of peripheral blood eosinophil/basophil progenitors following nasal allergen challenge in patients with allergic rhinitis. Clin Exp Allergy. 2005;35:39–44. [DOI] [PubMed] [Google Scholar]
- 393.Bradding P, Holgate ST. The mast cell as a source of cytokines in asthma. Ann N Y Acad Sci. 1996;796:272–281. [DOI] [PubMed] [Google Scholar]
- 394.Pawankar RU, Okuda M, Hasegawa S, et al. Interleukin-13 expression in the nasal mucosa of perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152:2059–2067. [DOI] [PubMed] [Google Scholar]
- 395.Smurthwaite L, Durham SR. Local IgE synthesis in allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2002;2:231–238. [DOI] [PubMed] [Google Scholar]
- 396.Pawankar R Epithelial cells as immunoregulators in allergic airway diseases. Curr Opin Allergy Clin Immunol. 2002;2:1–5. [DOI] [PubMed] [Google Scholar]
- 397.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. [DOI] [PubMed] [Google Scholar]
- 398.Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. [DOI] [PubMed] [Google Scholar]
- 399.Kimura S, Pawankar R, Mori S, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Asaka D, Yoshikawa M, Nakayama T, Yoshimura T, Moriyama H, Otori N. Elevated levels of interleukin-33 in the nasal secretions of patients with allergic rhinitis. Int Arch Allergy Immunol. 2012;158(Suppl 1):47–50. [DOI] [PubMed] [Google Scholar]
- 401.Okayama Y, Okumura S, Sagara H, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34:425–435. [DOI] [PubMed] [Google Scholar]
- 402.Nakamaru Y, Oridate N, Nishihira J, Takagi D, Furuta Y, Fukuda S. Macrophage migration inhibitory factor in allergic rhinitis: its identification in eosinophils at the site of inflammation. Ann Otol Rhinol Laryngol. 2004;113:205–209. [DOI] [PubMed] [Google Scholar]
- 403.Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. [DOI] [PubMed] [Google Scholar]
- 404.Figueroa DJ, Borish L, Baramki D, Philip G, Austin CP, Evans JF. Expression of cysteinyl leukotriene synthetic and signalling proteins in inflammatory cells in active seasonal allergic rhinitis. Clin Exp Allergy. 2003;33:1380–1388. [DOI] [PubMed] [Google Scholar]
- 405.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 406.Pawankar R Inflammatory mechanisms in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2007;7:1–4. [DOI] [PubMed] [Google Scholar]
- 407.Nonaka M, Pawankar R, Fukumoto A, Ogihara N, Sakanushi A, Yagi T. Induction of eotaxin production by interleukin-4, interleukin-13 and lipopolysaccharide by nasal fibroblasts. Clin Exp Allergy. 2004;34:804–811. [DOI] [PubMed] [Google Scholar]
- 408.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. [DOI] [PubMed] [Google Scholar]
- 409.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. [DOI] [PubMed] [Google Scholar]
- 410.Han D, Wang C, Lou W, Gu Y, Wang Y, Zhang L. Allergen-specific IL-10-secreting type I T regulatory cells, but not CD4(+)CD25(+)Foxp3(+) T cells, are decreased in peripheral blood of patients with persistent allergic rhinitis. Clin Immunol. 2010;136:292–301. [DOI] [PubMed] [Google Scholar]
- 411.Baumann R, Rabaszowski M, Stenin I, et al. Comparison of the nasal release of IL-4, IL-10, IL-17, CCL13/MCP-4, and CCL26/eotaxin-3 in allergic rhinitis during season and after allergen challenge. Am J Rhinol Allergy. 2013;27:266–272. [DOI] [PubMed] [Google Scholar]
- 412.Liu Y, Yu HJ, Wang N, et al. Clara cell 10-kDa protein inhibits T(H)17 responses through modulating dendritic cells in the setting of allergic rhinitis. J Allergy Clin Immunol. 2013;131:387–394.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Wang DY, Li Y, Yan Y, Li C, Shi L. Upper airway stem cells: understanding the nose and role for future cell therapy. Curr Allergy Asthma Rep. 2015;15:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Akira S Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Lim MC, Taylor RM, Naclerio RM. The histology of allergic rhinitis and its comparison to cellular changes in nasal lavage. Am J Respir Crit Care Med. 1995;151:136–144. [DOI] [PubMed] [Google Scholar]
- 416.Amin K, Rinne J, Haahtela T, et al. Inflammatory cell and epithelial characteristics of perennial allergic and nonallergic rhinitis with a symptom history of 1 to 3 years’ duration. J Allergy Clin Immunol. 2001;107:249–257. [DOI] [PubMed] [Google Scholar]
- 417.Calderon MA, Lozewicz S, Prior A, Jordan S, Trigg CJ, Davies RJ. Lymphocyte infiltration and thick-ness of the nasal mucous membrane in perennial and seasonal allergic rhinitis. J Allergy Clin Immunol. 1994;93:635–643. [DOI] [PubMed] [Google Scholar]
- 418.Gao T, Ng CL, Li C, et al. Smoking is an independent association of squamous metaplasia in Chinese nasal polyps. Int Forum Allergy Rhinol. 2016;6:66–74. [DOI] [PubMed] [Google Scholar]
- 419.Zhao L, Li YY, Li CW, et al. Increase of poorly proliferated p63+ /Ki67+ basal cells forming multiple layers in the aberrant remodeled epithelium in nasal polyps. Allergy. 2017;72:975–984. [DOI] [PubMed] [Google Scholar]
- 420.Li YY, Li CW, Chao SS, et al. Impairment of cilia architecture and ciliogenesis in hyperplastic nasal epithelium from nasal polyps. J Allergy Clin Immunol. 2014;134:1282–1292. [DOI] [PubMed] [Google Scholar]
- 421.Eifan AO, Orban NT, Jacobson MR, Durham SR. Severe persistent allergic rhinitis. Inflammation but no histologic features of structural upper airway remodeling. Am J Respir Crit Care Med. 2015;192:1431–1439. [DOI] [PubMed] [Google Scholar]
- 422.Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004;113:43–49. [DOI] [PubMed] [Google Scholar]
- 423.Svensson C, Andersson M, Greiff L, Alkner U, Persson CG. Exudative hyperresponsiveness of the airway microcirculation in seasonal allergic rhinitis. Clin Exp Allergy. 1995;25:942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Comstock AT, Ganesan S, Chattoraj A, et al. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol. 2011;85:6795–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Vercelli D Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. [DOI] [PubMed] [Google Scholar]
- 428.Portelli MA, Hodge E, Sayers I. Genetic risk factors for the development of allergic disease identified by genome-wide association. Clin Exp Allergy. 2015;45:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Post S, Nawijn MC, Hackett TL, et al. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax. 2012;67:488–495. [DOI] [PubMed] [Google Scholar]
- 430.Georas SN, Rezaee F, Lerner L, Beck L. Dangerous allergens: why some allergens are bad actors. Curr Allergy Asthma Rep. 2010;10:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Minshall E, Ghaffar O, Cameron L, et al. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitis. Otolaryngol Head Neck Surg. 1998;118:648–654. [DOI] [PubMed] [Google Scholar]
- 432.Toppila-Salmi S, Renkonen J, Joenvaara S, Mattila P, Renkonen R. Allergen interactions with epithelium. Curr Opin Allergy Clin Immunol. 2011;11:29–32. [DOI] [PubMed] [Google Scholar]
- 433.Renkonen J, Mattila P, Lehti S, et al. Birch pollen allergen Bet v 1 binds to and is transported through conjunctival epithelium in allergic patients. Allergy. 2009;64:868–875. [DOI] [PubMed] [Google Scholar]
- 434.Mattila P, Renkonen J, Toppila-Salmi S, et al. Time-series nasal epithelial transcriptomics during natural pollen exposure in healthy subjects and allergic patients. Allergy. 2010;65:175–183. [DOI] [PubMed] [Google Scholar]
- 435.Mattila K, Renkonen R. Modelling of Bet v 1 binding to lipids. Scand J Immunol. 2009;70:116–124. [DOI] [PubMed] [Google Scholar]
- 436.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. [DOI] [PubMed] [Google Scholar]
- 437.Riiser A The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Bendiks M, Kopp MV. The relationship between advances in understanding the microbiome and the maturing hygiene hypothesis. Curr Allergy Asthma Rep. 2013;13:487–494. [DOI] [PubMed] [Google Scholar]
- 439.Prince BT, Mandel MJ, Nadeau K, Singh AM. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. [DOI] [PubMed] [Google Scholar]
- 441.Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35:1511–1520. [DOI] [PubMed] [Google Scholar]
- 442.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. [DOI] [PubMed] [Google Scholar]
- 443.Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39:518–526. [DOI] [PubMed] [Google Scholar]
- 444.Melli LC, do Carmo-Rodrigues MS, Araujo-Filho HB, Sole D, de Morais MB. Intestinal microbiota and allergic diseases: a systematic review. Allergol Immunopathol (Madr). 2016;44:177–188. [DOI] [PubMed] [Google Scholar]
- 445.Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Ipci K, Altintoprak N, Muluk NB, Senturk M, Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Eur Arch Otorhinolaryngol. 2017;274:617–626. [DOI] [PubMed] [Google Scholar]
- 447.Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 2007;56:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Adlerberth I, Strachan DP, Matricardi PM, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–350. [DOI] [PubMed] [Google Scholar]
- 449.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652.e5. [DOI] [PubMed] [Google Scholar]
- 450.Johansson MA, Sjogren YM, Persson JO, Nilsson C, Sverremark-Ekstrom E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6:e23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Lan F, Zhang N, Gevaert E, Zhang L, Bachert C. Viruses and bacteria in Th2-biased allergic airway disease. Allergy. 2016;71:1381–1392. [DOI] [PubMed] [Google Scholar]
- 452.Broder I, Barlow PP, Horton RJ. The epidemiology of asthma and hay fever in a total community, Tecumseh, Michigan. I. Description of study and general findings. J Allergy. 1962;33:513–523. [DOI] [PubMed] [Google Scholar]
- 453.Turkeltaub PC, Gergen PJ. Prevalence of upper and lower respiratory conditions in the US population by social and environmental factors: data from the second National Health and Nutrition Examination Survey, 1976 to 1980 (NHANES II). Ann Allergy. 1991;67:147–154. [PubMed] [Google Scholar]
- 454.Salo PM, Calatroni A, Gergen PJ, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1226–1235.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1996;9:687–695. [DOI] [PubMed] [Google Scholar]
- 456.Bousquet PJ, Leynaert B, Neukirch F, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008;63:1301–1309. [DOI] [PubMed] [Google Scholar]
- 457.Wuthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int Arch Allergy Immunol. 1995;106:149–156. [DOI] [PubMed] [Google Scholar]
- 458.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–98. [DOI] [PubMed] [Google Scholar]
- 459.Upton MN, McConnachie A, McSharry C, et al. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ. 2000;321:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Peat JK, Haby M, Spijker J, Berry G, Woolcock AJ. Prevalence of asthma in adults in Busselton, Western Australia. BMJ. 1992;305:1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.de Marco R, Cappa V, Accordini S, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. [DOI] [PubMed] [Google Scholar]
- 462.Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Bjerg A, Ekerljung L, Middelveld R, et al. Increased prevalence of symptoms of rhinitis but not of asthma between 1990 and 2008 in Swedish adults: comparisons of the ECRHS and GA(2)LEN surveys. PLoS One. 2011;6:e16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Eriksson J, Ekerljung L, Ronmark E, et al. Update of prevalence of self-reported allergic rhinitis and chronic nasal symptoms among adults in Sweden. Clin Respir J. 2012;6:159–168. [DOI] [PubMed] [Google Scholar]
- 465.Biagini JM, LeMasters GK, Ryan PH, et al. Environmental risk factors of rhinitis in early infancy. Pediatr Allergy Immunol. 2006;17:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Herr M, Just J, Nikasinovic L, et al. Risk factors and characteristics of respiratory and allergic phenotypes in early childhood. J Allergy Clin Immunol. 2012;130:389–396.e4. [DOI] [PubMed] [Google Scholar]
- 467.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Kulig M, Klettke U, Wahn V, Forster J, Bauer CP, Wahn U. Development of seasonal allergic rhinitis during the first 7 years of life. J Allergy Clin Immunol. 2000;106:832–839. [DOI] [PubMed] [Google Scholar]
- 469.Kurukulaaratchy RJ, Karmaus W, Raza A, Matthews S, Roberts G, Arshad SH. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life. Clin Exp Allergy. 2011;41:851–859. [DOI] [PubMed] [Google Scholar]
- 470.Westman M, Lupinek C, Bousquet J, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–1206.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Westman M, Stjarne P, Asarnoj A, et al. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J Allergy Clin Immunol. 2012;129:403–408. [DOI] [PubMed] [Google Scholar]
- 472.Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D, ISAAC phase III study group. World-wide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–124. [DOI] [PubMed] [Google Scholar]
- 473.Pols DH, Wartna JB, van Alphen EI, et al. Inter-relationships between atopic disorders in children: a meta-analysis based on ISAAC questionnaires. PLoS One. 2015;10:e0131869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Mallol J, Crane J, von Mutius E, et al. The International Study of Asthma and Allergies in Childhood (ISAAC) phase three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73–85. [DOI] [PubMed] [Google Scholar]
- 475.Weinmayr G, Forastiere F, Weiland SK, et al. International variation in prevalence of rhinitis and its relationship with sensitisation to perennial and seasonal allergens. Eur Respir J. 2008;32:1250–1261. [DOI] [PubMed] [Google Scholar]
- 476.Kim J, Han Y, Seo SC, et al. Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc. 2016;37:e1–e7. [DOI] [PubMed] [Google Scholar]
- 477.Li CW, Chen DD, Zhong JT, et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou City in china. PLoS One. 2014;9:e114950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Ahn JC, Kim JW, Lee CH, Rhee CS. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean National Health and Nutrition Survey 2008–2012. JAMA Otolaryngol Head Neck Surg. 2016;142:162–167. [DOI] [PubMed] [Google Scholar]
- 479.Song WJ, Sohn KH, Kang MG, et al. Urbanrural differences in the prevalence of allergen sensitization and self-reported rhinitis in the elderly population. Ann Allergy Asthma Immunol. 2015;114:455–461. [DOI] [PubMed] [Google Scholar]
- 480.Toth I, Peternel R, Gajnik D, Vojnikovic B. Microregional hypersensitivity variations to inhalant allergens in the city of Zagreb and Zagreb County. Coll Antropol. 2011;35(Suppl 2):31–37. [PubMed] [Google Scholar]
- 481.Erbas B, Lowe AJ, Lodge CJ, et al. Persistent pollen exposure during infancy is associated with increased risk of subsequent childhood asthma and hayfever. Clin Exp Allergy. 2013;43:337–343. [DOI] [PubMed] [Google Scholar]
- 482.Beggs PJ, Katelaris CH, Medek D, et al. Differences in grass pollen allergen exposure across Australia. Aust N Z J Public Health. 2015;39:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Westman M, Kull I, Lind T, et al. The link between parental allergy and offspring allergic and nonallergic rhinitis. Allergy. 2013;68:1571–1578. [DOI] [PubMed] [Google Scholar]
- 484.Thomsen SF, Ulrik CS, Kyvik KO, et al. Genetic and environmental contributions to hay fever among young adult twins. Respir Med. 2006;100:2177–2182. [DOI] [PubMed] [Google Scholar]
- 485.Rasanen M, Laitinen T, Kaprio J, Koskenvuo M, Laitinen LA. Hay fever—a Finnish nationwide study of adolescent twins and their parents. Allergy. 1998;53:885–890. [DOI] [PubMed] [Google Scholar]
- 486.Ferreira MA, Matheson MC, Tang CS, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133:1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Hinds DA, McMahon G, Kiefer AK, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ramasamy A, Curjuric I, Coin LJ, et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol. 2011;128:996–1005. [DOI] [PubMed] [Google Scholar]
- 489.Ferreira MA, Matheson MC, Duffy DL, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Weidinger S, Willis-Owen SA, Kamatani Y, et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum Mol Genet. 2013;22:4841–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Marenholz I, Esparza-Gordillo J, Ruschendorf F, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015;6:8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–3322. [DOI] [PubMed] [Google Scholar]
- 493.Bonnelykke K, Matheson MC, Pers TH, et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013;45:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Henmyr V, Lind-Hallden C, Carlberg D, et al. Characterization of genetic variation in TLR8 in relation to allergic rhinitis. Allergy. 2016;71:333–341. [DOI] [PubMed] [Google Scholar]
- 495.Bonnelykke K, Sparks R, Waage J, Milner JD. Genetics of allergy and allergic sensitization: common variants, rare mutations. Curr Opin Immunol. 2015;36:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Davila I, Mullol J, Ferrer M, et al. Genetic aspects of allergic rhinitis. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):25–31. [PubMed] [Google Scholar]
- 497.Andiappan AK, Nilsson D, Hallden C, et al. Investigating highly replicated asthma genes as candidate genes for allergic rhinitis. BMC Med Genet. 2013;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Nilsson D, Andiappan AK, Hallden C, et al. Toll-like receptor gene polymorphisms are associated with allergic rhinitis: a case control study. BMC Med Genet. 2012;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kang I, Oh YK, Lee SH, Jung HM, Chae SC, Lee JH. Identification of polymorphisms in the Toll-like receptor gene and the association with allergic rhinitis. Eur Arch Otorhinolaryngol. 2010;267:385–389. [DOI] [PubMed] [Google Scholar]
- 500.Kormann MS, Ferstl R, Depner M, et al. Rare TLR2 mutations reduce TLR2 receptor function and can increase atopy risk. Allergy. 2009;64:636–642. [DOI] [PubMed] [Google Scholar]
- 501.Moller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Borglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63:1064–1069. [DOI] [PubMed] [Google Scholar]
- 502.Sun Q, Liu Y, Zhang S, et al. Thymic stromal lymphopoietin polymorphisms and allergic rhinitis risk: a systematic review and meta-analysis with 6351 cases and 11472 controls. Int J Clin Exp Med. 2015;8:15752–15758. [PMC free article] [PubMed] [Google Scholar]
- 503.Jin P, Andiappan AK, Quek JM, et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J Allergy Clin Immunol. 2015;135:1486–1493.e8. [DOI] [PubMed] [Google Scholar]
- 504.Nilsson D, Andiappan AK, Hallden C, et al. Poor reproducibility of allergic rhinitis SNP associations. PLoS One. 2013;8:e53975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Joubert BR, Felix JF, Yousefi P, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98:680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Gruzieva O, Xu CJ, Breton CV, et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Li JY, Zhang Y, Lin XP, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust, mite-sensitized subjects. Clin Exp Allergy. 2016;46:298–307. [DOI] [PubMed] [Google Scholar]
- 508.Nestor CE, Barrenas F, Wang H, et al. DNA methylation changes separate allergic patients from healthy controls and may reflect altered CD4+ T-cell population structure. PLoS Genet. 2014;10:e1004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sarnowski C, Laprise C, Malerba G, et al. DNA methylation within melatonin receptor 1A (MTNR1A) mediates paternally transmitted genetic variant effect on asthma plus rhinitis. J Allergy Clin Immunol. 2016;138:748–753. [DOI] [PubMed] [Google Scholar]
- 510.Liang L, Willis-Owen SA, Laprise C, et al. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature. 2015;520:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Everson TM, Lyons G, Zhang H, et al. DNA methylation loci associated with atopy and high serum IgE: a genome-wide application of recursive Random Forest feature selection. Genome Med. 2015;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Bunyavanich S, Schadt EE, Himes BE, et al. Integrated genome-wide association, coexpression network, and expression single nucleotide polymorphism analysis identifies novel pathway in allergic rhinitis. BMC Med Genomics. 2014;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Hirota T, Takahashi A, Kubo M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Andiappan AK, Wang de Y, Anantharaman R, et al. Genome-wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PLoS One. 2011;6:e19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Corver K, Kerkhof M, Brussee JE, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA-study. Pediatr Allergy Immunol. 2006;17:329–336. [DOI] [PubMed] [Google Scholar]
- 517.Illi S, Weber J, Zutavern A, et al. Perinatal influences on the development of asthma and atopy in childhood. Ann Allergy Asthma Immunol. 2014;112:132–139.e1. [DOI] [PubMed] [Google Scholar]
- 518.Schoos AM, Chawes BL, Jelding-Dannemand E, Elfman LB, Bisgaard H. Early indoor aeroallergen exposure is not associated with development of sensitization or allergic rhinitis in high-risk children. Allergy. 2016;71:684–691. [DOI] [PubMed] [Google Scholar]
- 519.Kihlstrom A, Lilja G, Pershagen G, Hedlin G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. 2002;110:78–84. [DOI] [PubMed] [Google Scholar]
- 520.Marinho S, Simpson A, Lowe L, Kissen P, Murray C, Custovic A. Rhinoconjunctivitis in 5-year-old children: a population-based birth cohort study. Allergy. 2007;62:385–393. [DOI] [PubMed] [Google Scholar]
- 521.Kim YK Chang YS, Lee MH, et al. Role of environmental exposure to spider mites in the sensitization and the clinical manifestation of asthma and rhinitis in children and adolescents living in rural and urban areas. Clin Exp Allergy. 2002;32:1305–1309. [DOI] [PubMed] [Google Scholar]
- 522.Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy. 2000;30:194–200. [DOI] [PubMed] [Google Scholar]
- 523.Leynaert B, Neukirch C, Jarvis D, et al. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med. 2001;164:1829–1834. [DOI] [PubMed] [Google Scholar]
- 524.Nafstad P, Magnus P, Gaarder PI, Jaakkola JJ. Exposure to pets and atopy-related diseases in the first 4 years of life. Allergy. 2001;56:307–312. [DOI] [PubMed] [Google Scholar]
- 525.Fasce L, Tosca MA, Silvestri M, Olcese R, Pistorio A, Rossi GA. “Early” cat ownership and the risk of sensitization and allergic rhinitis in Ligurian children with respiratory symptoms. Ann Allergy Asthma Immunol. 2005;94:561–565. [DOI] [PubMed] [Google Scholar]
- 526.Dimich-Ward H, Chow Y, Chung J, Trask C. Contact with livestock—a protective effect against allergies and asthma? Clin Exp Allergy. 2006;36:1122–1129. [DOI] [PubMed] [Google Scholar]
- 527.Ibargoyen-Roteta N, Aguinaga-Ontoso I, Fernandez-Benitez M, et al. Role of the home environment in rhinoconjunctivitis and eczema in schoolchildren in Pamplona, Spain. J Investig Allergol Clin Immunol. 2007;17:137–144. [PubMed] [Google Scholar]
- 528.Majkowska-Wojciechowska B, Pelka J, Korzon L, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 2007;62:1044–1050. [DOI] [PubMed] [Google Scholar]
- 529.Perzanowski MS, Chew GL, Divjan A, et al. Cat ownership is a risk factor for the development of anti-cat IgE but not current wheeze at age 5 years in an inner-city cohort. J Allergy Clin Immunol. 2008;121:1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Vargas C, Bustos P, Diaz PV, Amigo H, Rona RJ. Childhood environment and atopic conditions, with emphasis on asthma in a Chilean agricultural area. J Asthma. 2008;45:73–78. [DOI] [PubMed] [Google Scholar]
- 531.Alm B, Goksor E, Thengilsdottir H, et al. Early protective and risk factors for allergic rhinitis at age 4(1/2) yr. Pediatr Allergy Immunol. 2011;22:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Lampi J, Canoy D, Jarvis D, et al. Farming environment and prevalence of atopy at age 31: prospective birth cohort study in Finland. Clin Exp Allergy. 2011;41:987–993. [DOI] [PubMed] [Google Scholar]
- 533.Matheson MC, Dharmage SC, Abramson MJ, et al. Early-life risk factors and incidence of rhinitis: results from the European Community Respiratory Health Study—an international population-based cohort study. J Allergy Clin Immunol. 2011;128:816–823.e5. [DOI] [PubMed] [Google Scholar]
- 534.Lodge CJ, Lowe AJ, Gurrin LC, et al. Pets at birth do not increase allergic disease in at-risk children. Clin Exp Allergy. 2012;42:1377–1385. [DOI] [PubMed] [Google Scholar]
- 535.Perkin MR, Bader T, Rudnicka AR, Strachan DP, Owen CG. Inter-relationship between rhinitis and conjunctivitis in allergic rhinoconjunctivitis and associated risk factors in rural UK children. PLoS One. 2015;10:e0143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Tamay Z, Akcay A, Ones U, Guler N, Kilic G, Zencir M. Prevalence and risk factors for allergic rhinitis in primary school children. Int J Pediatr Otorhinolaryngol. 2007;71:463–471. [DOI] [PubMed] [Google Scholar]
- 537.Batlles-Garrido J, Torres-Borrego J, Rubi-Ruiz T, et al. Prevalence and factors linked to allergic rhinitis in 10 and 11-year-old children in Almeria. Isaac Phase II, Spain. Allergol Immunopathol (Madr). 2010;38:135–141. [DOI] [PubMed] [Google Scholar]
- 538.Lombardi E, Simoni M, La Grutta S, et al. Effects of pet exposure in the first year of life on respiratory and allergic symptoms in 7-yr-old children. The SIDRIA-2 study. Pediatr Allergy Immunol. 2010;21:268–276. [DOI] [PubMed] [Google Scholar]
- 539.Kurosaka F, Terada T, Tanaka A, et al. Risk factors for wheezing, eczema and rhinoconjunctivitis in the previous 12 months among six-year-old children in Himeji City, Japan: food allergy, older siblings, day-care attendance and parental allergy history. Allergol Int. 2011;60:317–330. [DOI] [PubMed] [Google Scholar]
- 540.Brunekreef B, Von Mutius E, Wong G, et al. Exposure to cats and dogs, and symptoms of asthma, rhinoconjunctivitis, and eczema. Epidemiology. 2012;23:742–750. [DOI] [PubMed] [Google Scholar]
- 541.Tamay Z, Akcay A, Ergin A, Guler N. Prevalence of allergic rhinitis and risk factors in 6- to 7-yearold children in Istanbul, Turkey. Turk J Pediatr. 2014;56:31–40. [PubMed] [Google Scholar]
- 542.Yang SI, Lee E, Jung YH, et al. Effect of antibiotic use and mold exposure in infancy on allergic rhinitis in susceptible adolescents. Ann Allergy Asthma Immunol. 2014;113:160–165 e161. [DOI] [PubMed] [Google Scholar]
- 543.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–617. [DOI] [PubMed] [Google Scholar]
- 544.Anyo G, Brunekreef B, de Meer G, Aarts F, Janssen NA, van Vliet P. Early, current and past pet ownership: associations with sensitization, bronchial responsiveness and allergic symptoms in school children. Clin Exp Allergy. 2002;32:361–366. [DOI] [PubMed] [Google Scholar]
- 545.Henriksen AH, Holmen TL, Bjermer L. Sensitization and exposure to pet allergens in asthmatics versus non-asthmatics with allergic rhinitis. Respir Med. 2001;95:122–129. [DOI] [PubMed] [Google Scholar]
- 546.Waser M, von Mutius E, Riedler J, et al. Exposure to pets, and the association with hay fever, asthma, and atopic sensitization in rural children. Allergy. 2005;60:177–184. [DOI] [PubMed] [Google Scholar]
- 547.Chen CM, Rzehak P, Zutavern A, et al. Longitudinal study on cat allergen exposure and the development of allergy in young children. J Allergy Clin Immunol. 2007;119:1148–1155. [DOI] [PubMed] [Google Scholar]
- 548.Sultesz M, Katona G, Hirschberg A, Galffy G. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. Int J Pediatr Otorhinolaryngol. 2010;74:503–509. [DOI] [PubMed] [Google Scholar]
- 549.Sandini U, Kukkonen AK, Poussa T, Sandini L, Savilahti E, Kuitunen M. Protective and risk factors for allergic diseases in high-risk children at the ages of two and five years. Int Arch Allergy Immunol. 2011;156:339–348. [DOI] [PubMed] [Google Scholar]
- 550.Kellberger J, Dressel H, Vogelberg C, et al. Prediction of the incidence and persistence of allergic rhinitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2012;129:397–402.e3. [DOI] [PubMed] [Google Scholar]
- 551.Kim WK, Kwon JW, Seo JH, et al. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012;130:421–426.e5. [DOI] [PubMed] [Google Scholar]
- 552.Lodrup Carlsen KC, Roll S, Carlsen KH, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS One. 2012;7:e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lam A, Wong GW, Poon CM, Lee SS. A GIS-based assessment of environmental influences on allergy development in children. Asia Pac J Public Health. 2014;26:575–587. [DOI] [PubMed] [Google Scholar]
- 554.Torfi Y, Bitarafan N, Rajabi M. Impact of socioeconomic and environmental factors on atopic eczema and allergic rhinitis: a cross sectional study. EXCLI J. 2015;14:1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Stark PC, Celedon JC, Chew GL, et al. Fungal levels in the home and allergic rhinitis by 5 years of age. Environ Health Perspect. 2005;113:1405–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Kuyucu S, Saraclar Y, Tuncer A, et al. Epidemiologic characteristics of rhinitis in Turkish children: the International Study of Asthma and Allergies in Childhood (ISAAC) phase 2. Pediatr Allergy Immunol. 2006;17:269–277. [DOI] [PubMed] [Google Scholar]
- 557.Deng Q, Lu C, Ou C, Chen L, Yuan H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere. 2016;152:459–467. [DOI] [PubMed] [Google Scholar]
- 558.Lin Z, Norback D, Wang T, et al. The first 2-year home environment in relation to the new onset and remission of asthmatic and allergic symptoms in 4246 preschool children. Sci Total Environ. 2016;553:204–210. [DOI] [PubMed] [Google Scholar]
- 559.Thacher JD, Gruzieva O, Pershagen G, et al. Mold and dampness exposure and allergic outcomes from birth to adolescence: data from the BAMSE cohort. Allergy. 2017;72:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Bornehag CG, Sundell J, Hagerhed-Engman L, et al. ‘Dampness’ at home and its association with airway, nose, and skin symptoms among 10,851 preschool children in Sweden: a cross-sectional study. Indoor Air. 2005;15(Suppl 10):48–55. [DOI] [PubMed] [Google Scholar]
- 561.Hardjojo A, Shek LP, van Bever HP, Lee BW. Rhinitis in children less than 6 years of age: current knowledge and challenges. Asia Pac Allergy. 2011;1:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Dharmage SC, Lodge CL, Matheson MC, Campbell B, Lowe AJ. Exposure to cats: update on risks for sensitization and allergic diseases. Curr Allergy Asthma Rep. 2012;12:413–423. [DOI] [PubMed] [Google Scholar]
- 563.Ji Y, Liu Y, Yang N. Pediatric rhinitis risk factors. Exp Ther Med. 2016;12:2383–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Alduraywish SA, Lodge CJ, Campbell B, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. [DOI] [PubMed] [Google Scholar]
- 565.Brockow I, Zutavern A, Hoffmann U, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. 2009;19:180–187. [PubMed] [Google Scholar]
- 566.Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos-Holzmann I. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group, Germany. Pediatr Allergy Immunol. 1998;9:61–67. [DOI] [PubMed] [Google Scholar]
- 567.Garden FL, Simpson JM, Marks GB; CAPS Investigators. Atopy phenotypes in the Childhood Asthma Prevention Study (CAPS) cohort and the relationship with allergic disease: clinical mechanisms in allergic disease. Clin Exp Allergy. 2013;43:633–641. [DOI] [PubMed] [Google Scholar]
- 568.Chiu CY, Huang YL, Tsai MH, et al. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS One. 2014;9:e102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Kjaer HF, Eller E, Andersen KE, Host A, Bindslev-Jensen C. The association between early sensitization patterns and subsequent allergic disease. The DARC birth cohort study. Pediatr Allergy Immunol. 2009;20:726–734. [DOI] [PubMed] [Google Scholar]
- 570.Lilja G, Dannaeus A, Foucard T, Graff-Lonnevig V, Johansson SG, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic diseases in infants up to 18 months of age—in-vivo results. Clin Exp Allergy. 1989;19:473–479. [DOI] [PubMed] [Google Scholar]
- 571.Falth-Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy—a randomized study. J Allergy Clin Immunol. 1987;80:868–875. [DOI] [PubMed] [Google Scholar]
- 572.Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008;121:e44–e52. [DOI] [PubMed] [Google Scholar]
- 573.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–1190. [DOI] [PubMed] [Google Scholar]
- 574.Dunlop J, Matsui E, Sharma HP. Allergic rhinitis:environmental determinants. Immunol Allergy Clin North Am. 2016;36:367–377. [DOI] [PubMed] [Google Scholar]
- 575.Diaz-Sanchez D Pollution and the immune response: atopic diseases—are we too dirty or too clean? Immunology. 2000;101:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.D’Amato G, Liccardi G, D’Amato M, Cazzola M. The role of outdoor air pollution and climatic changes on the rising trends in respiratory allergy. Respir Med. 2001;95:606–611. [DOI] [PubMed] [Google Scholar]
- 577.Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000;106:1140–1146. [DOI] [PubMed] [Google Scholar]
- 578.Codispoti CD, LeMasters GK, Levin L, et al. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann Allergy Asthma Immunol. 2015;114:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Kim BJ, Kwon JW, Seo JH, et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol. 2011;107:214–219.e1. [DOI] [PubMed] [Google Scholar]
- 580.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3:933–942. [DOI] [PubMed] [Google Scholar]
- 581.Anderson HR, Ruggles R, Pandey KD, et al. Ambient particulate pollution and the world-wide prevalence of asthma, rhinoconjunctivitis and eczema in children: phase one of the International Study of Asthma and Allergies in Childhood (ISAAC). Occup Environ Med. 2010;67:293–300. [DOI] [PubMed] [Google Scholar]
- 582.Jung DY, Leem JH, Kim HC, et al. Effect of traffic-related air pollution on allergic disease: results of the children’s health and environmental research. Allergy Asthma Immunol Res. 2015;7:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Shirinde J, Wichmann J, Voyi K. Allergic rhinitis, rhinoconjunctivitis and hayfever symptoms among children are associated with frequency of truck traffic near residences: a cross sectional study. Environ Health. 2015;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Singh S, Sharma BB, Salvi S, et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. (in press). Epub 2016. September 24 10.1111/crj.12561. [DOI] [PubMed] [Google Scholar]
- 585.Wang IJ, Tung TH, Tang CS, Zhao ZH. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. 2016;219:66–71. [DOI] [PubMed] [Google Scholar]
- 586.Liu W, Huang C, Hu Y, et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in Shanghai, China: a retrospective cohort study. Environ Int. 2016;92–93:284–293. [DOI] [PubMed] [Google Scholar]
- 587.Chiang TY, Yuan TH, Shie RH, Chen CF, Chan CC. Increased incidence of allergic rhinitis, bronchitis and asthma, in children living near a petrochemical complex with SO2 pollution. Environ Int. 2016;96:1–7. [DOI] [PubMed] [Google Scholar]
- 588.Chung HY, Hsieh CJ, Tseng CC, Yiin LM. Association between the first occurrence of allergic rhinitis in preschool children and air pollution in Taiwan. Int J Environ Res Public Health. 2016;13(3). 10.3390/ijerph13030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Kim HH, Lee CS, Yu SD, et al. Near-road exposure and impact of air pollution on allergic diseases in elementary school children: a cross-sectional study. Yonsei Med J. 2016;57:698–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Yang HJ. Impact of perinatal environmental tobacco smoke on the development of childhood allergic diseases. Korean J Pediatr. 2016;59:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Gangl K, Reininger R, Bernhard D, et al. Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009;64:398–405. [DOI] [PubMed] [Google Scholar]
- 592.Mishra NC, Rir-Sima-Ah J, Langley RJ, et al. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol. 2008;180:7655–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Saulyte J, Regueira C, Montes-Martinez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Hur K, Liang J, Lin SY. The role of secondhand smoke in allergic rhinitis: a systematic review. Int Forum Allergy Rhinol. 2014;4:110–116. [DOI] [PubMed] [Google Scholar]
- 595.Lin SY, Reh DD, Clipp S, Irani L, Navas-Acien A. Allergic rhinitis and secondhand tobacco smoke: a population-based study. Am J Rhinol Allergy. 2011;25:e66–e71. [DOI] [PubMed] [Google Scholar]
- 596.Keil T, Lau S, Roll S, et al. Maternal smoking increases risk of allergic sensitization and wheezing only in children with allergic predisposition: longitudinal analysis from birth to 10 years. Allergy. 2009;64:445–451. [DOI] [PubMed] [Google Scholar]
- 597.Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901. [PubMed] [Google Scholar]
- 598.Bendtsen P, Gronbaek M, Kjaer SK, Munk C, Linneberg A, Tolstrup JS. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy. 2008;38:1179–1185. [DOI] [PubMed] [Google Scholar]
- 599.Codispoti CD, Levin L, LeMasters GK, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. 2010;125:1054–1060.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Annesi-Maesano I, Oryszczyn MP, Neukirch F, Kauffmann F. Relationship of upper airway disease to tobacco smoking and allergic markers: a cohort study of men followed up for 5 years. Int Arch Allergy Immunol. 1997;114:193–201. [DOI] [PubMed] [Google Scholar]
- 601.Hersoug LG, Husemoen LL, Thomsen SF, Sigsgaard T, Thuesen BH, Linneberg A. Association of indoor air pollution with rhinitis symptoms, atopy and nitric oxide levels in exhaled air. Int Arch Allergy Immunol. 2010;153:403–412. [DOI] [PubMed] [Google Scholar]
- 602.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Factors related to allergic sensitization to aeroallergens in a cross-sectional study in adults: The Copenhagen Allergy Study. Clin Exp Allergy. 2001;31:1409–1417. [DOI] [PubMed] [Google Scholar]
- 603.Eriksson J, Ekerljung L, Pullerits T, et al. Prevalence of chronic nasal symptoms in West Sweden: risk factors and relation to self-reported allergic rhinitis and lower respiratory symptoms. Int Arch Allergy Immunol. 2011;154:155–163. [DOI] [PubMed] [Google Scholar]
- 604.Shargorodsky J, Garcia-Esquinas E, Navas-Acien A, Lin SY. Allergic sensitization, rhinitis, and tobacco smoke exposure in U.S. children and adolescents. Int Forum Allergy Rhinol. 2015;5:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Katotomichelakis M, Tripsianis G, Daniilidi A, et al. Smoking effects on quality of life of allergic rhinitis patients after sublingual immunotherapy. Rhinology. 2015;53:325–331. [DOI] [PubMed] [Google Scholar]
- 606.Waite KJ. Blackley and the development of hay fever as a disease of civilization in the nineteenth century. Med Hist. 1995;39:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Butland BK, Strachan DP, Lewis S, Bynner J, Butler N, Britton J. Investigation into the increase in hay fever and eczema at age 16 observed between the 1958 and 1970 British birth cohorts. BMJ. 1997;315:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Lewis SA, Britton JR. Consistent effects of high socioeconomic status and low birth order, and the modifying effect of maternal smoking on the risk of allergic disease during childhood. Respir Med. 1998;92:1237–1244. [DOI] [PubMed] [Google Scholar]
- 609.Braback L, Hjern A, Rasmussen F. Social class in asthma and allergic rhinitis: a national cohort study over three decades. Eur Respir J. 2005;26:1064–1068. [DOI] [PubMed] [Google Scholar]
- 610.Bergmann RL, Edenharter G, Bergmann KE, Lau S, Wahn U. Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin Exp Allergy. 2000;30:1740–1745. [DOI] [PubMed] [Google Scholar]
- 611.Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005;35:612–618. [DOI] [PubMed] [Google Scholar]
- 612.Hammer-Helmich L, Linneberg A, Thomsen SF, Glumer C. Association between parental socioeconomic position and prevalence of asthma, atopic eczema and hay fever in children. Scand J Public Health. 2014;42:120–127. [DOI] [PubMed] [Google Scholar]
- 613.Grabenhenrich LB, Keil T, Reich A, et al. Prediction and prevention of allergic rhinitis: a birth cohort study of 20 years. J Allergy Clin Immunol. 2015;136:932–940.e12. [DOI] [PubMed] [Google Scholar]
- 614.Matheson MC, Walters EH, Simpson JA, et al. Relevance of the hygiene hypothesis to early vs. late onset allergic rhinitis. Clin Exp Allergy. 2009;39:370–378. [DOI] [PubMed] [Google Scholar]
- 615.Lee KS, Rha YH, Oh IH, Choi YS, Choi SH. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: a cross-sectional study. BMC Pediatr. 2016;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Penaranda A, Garcia E, Barragan AM, et al. Factors associated with allergic rhinitis in Colombian sub-populations aged 1 to 17 and 18 to 59. Rhinology. 2016;54:56–67. [DOI] [PubMed] [Google Scholar]
- 617.Wronka I, Klis K, Jarzebak K. Association of allergic rhinitis in female university students with socioeconomic factors and markers of estrogens levels. Adv Exp Med Biol. 2016;884:53–59. [DOI] [PubMed] [Google Scholar]
- 618.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. [DOI] [PubMed] [Google Scholar]
- 620.Szajewska H Early nutritional strategies for preventing allergic disease. Isr Med Assoc J. 2012;14:58–62. [PubMed] [Google Scholar]
- 621.Hoppu U, Kalliomaki M, Laiho K, Isolauri E. Breast milk—immunomodulatory signals against allergic diseases. Allergy. 2001;56(Suppl 67):23–26. [DOI] [PubMed] [Google Scholar]
- 622.Friedman NJ, Zeiger RS. The role of breast-feeding in the development of allergies and asthma. J Allergy Clin Immunol. 2005;115:1238–1248. [DOI] [PubMed] [Google Scholar]
- 623.Mimouni Bloch A, Mimouni D, Mimouni M, Gdalevich M. Does breastfeeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatr. 2002;91:275–279. [DOI] [PubMed] [Google Scholar]
- 624.Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38–53. [DOI] [PubMed] [Google Scholar]
- 625.Kramer MS, Matush L, Bogdanovich N, Dahhou M, Platt RW, Mazer B. The low prevalence of allergic disease in Eastern Europe: are risk factors consistent with the hygiene hypothesis? Clin Exp Allergy. 2009;39:708–716. [DOI] [PubMed] [Google Scholar]
- 626.Strachan DP. Epidemiology of hay fever: towards a community diagnosis. Clin Exp Allergy. 1995;25:296–303. [DOI] [PubMed] [Google Scholar]
- 627.Bjorksten B, Ait-Khaled N, Innes Asher M, Clayton TO, Robertson C; ISAAC Phase Three Study Group. Global analysis of breast feeding and risk of symptoms of asthma, rhinoconjunctivitis and eczema in 6–7 year old children: ISAAC phase three. Allergol Immunopathol (Madr). 2011;39:318–325. [DOI] [PubMed] [Google Scholar]
- 628.Kurt E, Metintas S, Basyigit I, et al. Prevalence and risk factors of allergies in Turkey: results of a multicentric cross-sectional study in children. Pediatr Allergy Immunol. 2007;18:566–574. [DOI] [PubMed] [Google Scholar]
- 629.Lee SY, Kwon JW, Seo JH, et al. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012;158:168–174. [DOI] [PubMed] [Google Scholar]
- 630.Miyake Y, Arakawa M, Tanaka K, Sasaki S, Ohya Y. Cross-sectional study of allergic disorders associated with breastfeeding in Japan: the Ryukyus Child Health Study. Pediatr Allergy Immunol. 2007;18:433–440. [DOI] [PubMed] [Google Scholar]
- 631.Miyake Y, Yura A, Iki M. Breastfeeding and the prevalence of symptoms of allergic disorders in Japanese adolescents. Clin Exp Allergy. 2003;33:312–316. [DOI] [PubMed] [Google Scholar]
- 632.Selcuk ZT Caglar T, Enunlu T Topal T. The prevalence of allergic diseases in primary school children in Edirne, Turkey. Clin Exp Allergy. 1997;27:262–269. [DOI] [PubMed] [Google Scholar]
- 633.Song N, Shamssain M, Zhang J, et al. Prevalence, severity and risk factors of asthma, rhinitis and eczema in a large group of Chinese schoolchildren. J Asthma. 2014;51:232–242. [DOI] [PubMed] [Google Scholar]
- 634.Sun Y, Sundell J. Life style and home environment are associated with racial disparities of asthma and allergy in Northeast Texas children. Sci Total Environ. 2011;409:4229–4234. [DOI] [PubMed] [Google Scholar]
- 635.Ehlayel MS, Bener A. Duration of breast-feeding and the risk of childhood allergic diseases in a developing country. Allergy Asthma Proc. 2008;29:386–391. [DOI] [PubMed] [Google Scholar]
- 636.Peroni DG, Piacentini GL, Alfonsi L, et al. Rhinitis in pre-school children: prevalence, association with allergic diseases and risk factors. Clin Exp Allergy. 2003;33:1349–1354. [DOI] [PubMed] [Google Scholar]
- 637.Siriaksorn S, Suchaitanawanit S, Trakultivakorn M. Allergic rhinitis and immunoglobulin deficiency in preschool children with frequent upper respiratory illness. Asian Pac J Allergy Immunol. 2011;29:73–77. [PubMed] [Google Scholar]
- 638.Apfelbacher C, Frew E, Xiang A, Apfel A, Smith H. Assessment of pet exposure by self-report in epidemiological studies of allergy and asthma: a systematic review. J Asthma. 2016;53:363–373. [DOI] [PubMed] [Google Scholar]
- 639.Takkouche B, Gonzalez-Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63:857–864. [DOI] [PubMed] [Google Scholar]
- 640.Chen CM, Tischer C, Schnappinger M, Heinrich J. The role of cats and dogs in asthma and allergy—a systematic review. Int J Hyg Environ Health. 2010;213:1–31. [DOI] [PubMed] [Google Scholar]
- 641.Smallwood J, Ownby D. Exposure to dog allergens and subsequent allergic sensitization: an updated review. Curr Allergy Asthma Rep. 2012;12:424–428. [DOI] [PubMed] [Google Scholar]
- 642.Lodge CJ, Allen KJ, Lowe AJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies. Clin Dev Immunol. 2012;2012:176484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Christensen SH, Timm S, Janson C, et al. A clear urban-rural gradient of allergic rhinitis in a population-based study in Northern Europe. Eur Clin Respir J. 2016;3:33463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.von Hertzen L, Hanski I, Haahtela T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health. 2002;56:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Strachan DP, Ait-Khaled N, Foliaki S, et al. Siblings, asthma, rhinoconjunctivitis and eczema: a world-wide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy. 2015;45:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Campbell BE, Lodge CJ, Lowe AJ, Burgess JA, Matheson MC, Dharmage SC. Exposure to ‘farming’ and objective markers of atopy: a systematic review and meta-analysis. Clin Exp Allergy. 2015;45:744–757. [DOI] [PubMed] [Google Scholar]
- 648.House JS, Wyss AB, Hoppin JA, et al. Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J Allergy Clin Immunol. 2017;140:249–256.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–193. [DOI] [PubMed] [Google Scholar]
- 650.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. [DOI] [PubMed] [Google Scholar]
- 651.Barnes M, Cullinan P, Athanasaki P, et al. Crete: does farming explain urban and rural differences in atopy? Clin Exp Allergy. 2001;31:1822–1828. [DOI] [PubMed] [Google Scholar]
- 652.Downs SH, Marks GB, Mitakakis TZ, Leuppi JD, Car NG, Peat JK. Having lived on a farm and protection against allergic diseases in Australia. Clin Exp Allergy. 2001;31:570–575. [DOI] [PubMed] [Google Scholar]
- 653.Wickens K, Lane JM, Fitzharris P, et al. Farm residence and exposures and the risk of allergic diseases in New Zealand children. Allergy. 2002;57:1171–1179. [DOI] [PubMed] [Google Scholar]
- 654.Remes ST, Pekkanen J, Soininen L, Kajosaari M, Husman T, Koivikko A. Does heredity modify the association between farming and allergy in children? Acta Paediatr. 2002;91:1163–1169. [DOI] [PubMed] [Google Scholar]
- 655.Remes ST, Iivanainen K, Koskela H, Pekkanen J. Which factors explain the lower prevalence of atopy amongst farmers’ children? Clin Exp Allergy. 2003;33:427–434. [DOI] [PubMed] [Google Scholar]
- 656.Simpson A, Martinez FD. The role of lipopolysaccharide in the development of atopy in humans. Clin Exp Allergy. 2010;40:209–223. [DOI] [PubMed] [Google Scholar]
- 657.Tischer C, Gehring U, Chen CM, et al. Respiratory health in children, and indoor exposure to (1,3)-beta-D-glucan, EPS mould components and endotoxin. Eur Respir J. 2011;37:1050–1059. [DOI] [PubMed] [Google Scholar]
- 658.Cuello-Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. [DOI] [PubMed] [Google Scholar]
- 659.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. [DOI] [PubMed] [Google Scholar]
- 660.von Hertzen L, Laatikainen T, Pitkanen T, et al. Microbial content of drinking water in Finnish and Russian Karelia - implications for atopy prevalence. Allergy. 2007;62:288–292. [DOI] [PubMed] [Google Scholar]
- 661.Valkonen M, Wouters IM, Taubel M, et al. Bacterial exposures and associations with atopy and asthma in children. PLoS One. 2015;10:e0131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Fujimura KE, Johnson CC, Ownby DR, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126:410–412.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. [DOI] [PubMed] [Google Scholar]
- 664.Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: analysis of the American Gut Project. EBioMedicine. 2016;3:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Hanski I, von Hertzen L, Fyhrquist N, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012;109:8334–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Fyhrquist N, Ruokolainen L, Suomalainen A, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol. 2014;134:1301–1309.e11. [DOI] [PubMed] [Google Scholar]
- 667.Linneberg A, Dam Petersen K, Hahn-Pedersen J, Hammerby E, Serup-Hansen N, Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Hahn-Pedersen J, Boxall N, Maier W, Linneberg A, Serup-Hansen N. Systematic literature review assessing data on the burden of allergic rhinitis from a cost and quality of life perspective. Value Health. 2014;17:A602. [DOI] [PubMed] [Google Scholar]
- 669.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 670.McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 671.Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21:77–83. [DOI] [PubMed] [Google Scholar]
- 672.Tatar EC, Surenoglu UA, Ozdek A, Saylam G, Korkmaz H. The effect of combined medical treatment on quality of life in persistent allergic rhinitis. Indian J Otolaryngol Head Neck Surg. 2013;65:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Yamada T, Yamamoto H, Kubo S, et al. Efficacy of mometasone furoate nasal spray for nasal symptoms, quality of life, rhinitis-disturbed sleep, and nasal nitric oxide in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2012;33:e9–e16. [DOI] [PubMed] [Google Scholar]
- 674.Bousquet J, Zuberbier T, Canonica GW, Fokkens WJ, Gopalan G, Shekar T. Randomized controlled trial of desloratadine for persistent allergic rhinitis: correlations between symptom improvement and quality of life. Allergy Asthma Proc. 2013;34:274–282. [DOI] [PubMed] [Google Scholar]
- 675.Bachert C, Bousquet J, Canonica GW, et al. Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J Allergy Clin Immunol. 2004;114:838–844. [DOI] [PubMed] [Google Scholar]
- 676.Holmberg K, Tonnel AB, Dreyfus I, et al. Desloratadine relieves nasal congestion and improves quality-of-life in persistent allergic rhinitis. Allergy. 2009;64:1663–1670. [DOI] [PubMed] [Google Scholar]
- 677.Walter Canonica G, Bousquet J, Van Hammée G, et al. ; XPERT Study Group. Levocetirizine improves health-related quality of life and health status in persistent allergic rhinitis. Respir Med. 2006;100:1706–1715. [DOI] [PubMed] [Google Scholar]
- 678.Hoiby AS, Strand V, Robinson DS, Sager A, Rak S. Efficacy, safety, and immunological effects of a 2-year immunotherapy with Depigoid birch pollen extract: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2010;40:1062–1070. [DOI] [PubMed] [Google Scholar]
- 679.Colas C, Monzon S, Venturini M, Lezaun A. Double-blind, placebo-controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol. 2006;117:810–816. [DOI] [PubMed] [Google Scholar]
- 680.Brinkhaus B, Witt CM, Jena S, Liecker B, Wegscheider K, Willich SN. Acupuncture in patients with allergic rhinitis: a pragmatic randomized trial. Ann Allergy Asthma Immunol. 2008;101:535–543. [DOI] [PubMed] [Google Scholar]
- 681.Bousquet PJ, Demoly P, Devillier P, Mesbah K, Bousquet J. Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol. 2013;160:393–400. [DOI] [PubMed] [Google Scholar]
- 682.Stull DE, Schaefer M, Crespi S, Sandor DW. Relative strength of relationships of nasal congestion and ocular symptoms with sleep, mood and productivity. Curr Med Res Opin. 2009;25:1785–1792. [DOI] [PubMed] [Google Scholar]
- 683.Cadario G, Ciprandi G, Di Cara G, et al. Comparison between continuous or intermittent schedules of sublingual immunotherapy for house dust mites: effects on compliance, patients satisfaction, quality of life and safety. Int J Immunopathol Pharmacol. 2008;21:471–473. [DOI] [PubMed] [Google Scholar]
- 684.Jaruvongvanich V, Mongkolpathumrat P, Chantaphakul H, Klaewsongkram J. Extranasal symptoms of allergic rhinitis are difficult to treat and affect quality of life. Allergol Int. 2016;65:199–203. [DOI] [PubMed] [Google Scholar]
- 685.Song Y, Wang M, Xie J, et al. Prevalence of allergic rhinitis among elementary and middle school students in Changsha city and its impact on quality of life. J Laryngol Otol. 2015;129:1108–1114. [DOI] [PubMed] [Google Scholar]
- 686.Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012;33(Suppl 1):S113–S141. [DOI] [PubMed] [Google Scholar]
- 687.Katelaris CH, Sacks R, Theron PN. Allergic rhinoconjunctivitis in the Australian population: burden of disease and attitudes to intranasal corticosteroid treatment. Am J Rhinol Allergy. 2013;27:506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Bukstein D, Parikh R, Eid S, Ferro T, Morello JP. Beclomethasone Dipropionate Nasal Aerosol in Patients with Perennial Allergic Rhinitis (BALANCE) study: 6-month results. Allergy Asthma Proc. 2016;37:121–130. [DOI] [PubMed] [Google Scholar]
- 689.Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma. A population-based study of young adults. Am J Respir Crit Care Med. 2000;162:1391–1396. [DOI] [PubMed] [Google Scholar]
- 690.Juniper EF, Rohrbaugh T, Meltzer EO. A questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2003;111:484–490. [DOI] [PubMed] [Google Scholar]
- 691.Majani G, Baiardini I, Giardini A, et al. Health-related quality of life assessment in young adults with seasonal allergic rhinitis. Allergy. 2001;56:313–317. [DOI] [PubMed] [Google Scholar]
- 692.Witt CM, Reinhold T, Jena S, Brinkhaus B, Willich SN. Cost-effectiveness of acupuncture in women and men with allergic rhinitis: a randomized controlled study in usual care. Am J Epidemiol. 2009;169:562–571. [DOI] [PubMed] [Google Scholar]
- 693.Radcliffe MJ, Lewith GT, Turner RG, Prescott P, Church MK, Holgate ST. Enzyme potentiated desensitisation in treatment of seasonal allergic rhinitis: double blind randomised controlled study. BMJ. 2003;327:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Gerth Van Wijk R, Terreehorst IT, Mulder PG, Garrelds IM, Blom HM, Popering S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo-controlled study. Clin Exp Allergy. 2000;30:1792–1798. [DOI] [PubMed] [Google Scholar]
- 695.Filanowicz M, Szynkiewicz E, Cegla B, Bartuzi Z. Analysis of the quality of life of patients with asthma and allergic rhinitis after immunotherapy. Postepy Dermatol Alergol. 2016;33:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013;43:881–888. [DOI] [PubMed] [Google Scholar]
- 697.de la Hoz Caballer B, Rodriguez M, Fraj J, Cerecedo I, Antolin-Amerigo D, Colas C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: the Cross-sectional study to evAluate work Productivity in allergic Rhinitis compared with other common dIseases (CAPRI) study. Am J Rhinol Allergy. 2012;26:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Meltzer EO, Gross GN, Katial R, Storms WW. Allergic rhinitis substantially impacts patient quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract. 2012;61:S5–10. [PubMed] [Google Scholar]
- 699.Ciprandi G, Cadario G, Valle C, et al. Sublingual immunotherapy in polysensitized patients: effect on quality of life. J Investig Allergol Clin Immunol. 2010;20:274–279. [PubMed] [Google Scholar]
- 700.Petersen KD, Kronborg C, Gyrd-Hansen D, Dahl R, Larsen JN, Lowenstein H. Quality of life in rhinoconjunctivitis assessed with generic and disease-specific questionnaires. Allergy. 2008;63:284–291. [DOI] [PubMed] [Google Scholar]
- 701.Ciprandi G, Klersy C, Cirillo I, Marseglia GL. Quality of life in allergic rhinitis: relationship with clinical, immunological, and functional aspects. Clin Exp Allergy. 2007;37:1528–1535. [DOI] [PubMed] [Google Scholar]
- 702.Di Rienzo V, Pucci S, D’Alo A, et al. Effects of high-dose sublingual immmunotherapy on quiality of life in patients with cypress-induced rhinitis: a placebo controlled study. Clin Exp Allergy Rev. 2006;6:67–70. [Google Scholar]
- 703.Laforest L, Bousquet J, Neukirch F, et al. Influence of sociodemographic factors on quality of life during pollen season in seasonal allergic rhinitis patients. Ann Allergy Asthma Immunol. 2005;95:26–32. [DOI] [PubMed] [Google Scholar]
- 704.Cingi C, Oghan F, Eskiizmir G, Yaz A, Ural A, Erdogmus N. Desloratadine-montelukast combination improves quality of life and decreases nasal obstruction in patients with perennial allergic rhinitis. Int Forum Allergy Rhinol. 2013;3:801–806. [DOI] [PubMed] [Google Scholar]
- 705.Gurevich F, Glass C, Davies M, et al. The effect of intranasal steroid budesonide on the congestion-related sleep disturbance and daytime somnolence in patients with perennial allergic rhinitis. Allergy Asthma Proc. 2005;26:268–274. [PubMed] [Google Scholar]
- 706.Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy. 2003;58:380–385. [DOI] [PubMed] [Google Scholar]
- 707.Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol. 1998;101:633–637. [DOI] [PubMed] [Google Scholar]
- 708.Mansfield LE, Posey CR. Daytime sleepiness and cognitive performance improve in seasonal allergic rhinitis treated with intranasal fluticasone propionate. Allergy Asthma Proc. 2007;28:226–229. [DOI] [PubMed] [Google Scholar]
- 709.Shanqun L, Shenyuan L, Zhou J, Bai C. The role of montelukast and intranasal budesonide on OSAHS and allergic rhinitis. Allergy. 2009;64:591. [Google Scholar]
- 710.Thompson A, Sardana N, Craig TJ. Sleep impairment and daytime sleepiness in patients with allergic rhinitis: the role of congestion and inflammation. Ann Allergy Asthma Immunol. 2013;111:446–451. [DOI] [PubMed] [Google Scholar]
- 711.Rimmer J, Downie S, Bartlett DJ, Gralton J, Salome C. Sleep disturbance in persistent allergic rhinitis measured using actigraphy. Ann Allergy Asthma Immunol. 2009;103:190–194. [DOI] [PubMed] [Google Scholar]
- 712.Lavie P, Gertner R, Zomer J, Podoshin L. Breathing disorders in sleep associated with ‘microarousals’ in patients with allergic rhinitis. Acta Otolaryngol. 1981;92:529–533. [DOI] [PubMed] [Google Scholar]
- 713.Camhi SL, Morgan WJ, Pernisco N, Quan SF. Factors affecting sleep disturbances in children and adolescents. Sleep Med. 2000;1:117–123. [DOI] [PubMed] [Google Scholar]
- 714.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99:S757–S762. [DOI] [PubMed] [Google Scholar]
- 715.Parikh NG, Junaid I, Sheinkopf L, Randhawa I, Santiago SM, Klaustermeyer WB. Clinical control in the dual diagnosis of obstructive sleep apnea syndrome and rhinitis: a prospective analysis. Am J Rhinol Allergy. 2014;28:e52–e55. [DOI] [PubMed] [Google Scholar]
- 716.Acar M, Cingi C, Sakallioglu O, San T, Fatih Yimenicioglu M, Bal C. The effects of mometasone furoate and desloratadine in obstructive sleep apnea syndrome patients with allergic rhinitis. Am J Rhinol Allergy. 2013;27:e113–e116. [DOI] [PubMed] [Google Scholar]
- 717.Lavigne F, Petrof BJ, Johnson JR, et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin Exp Allergy. 2013;43:1124–1133. [DOI] [PubMed] [Google Scholar]
- 718.McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126:625–628. [DOI] [PubMed] [Google Scholar]
- 719.Krouse HJ, Davis JE, Krouse JH. Immune mediators in allergic rhinitis and sleep. Otolaryngol Head Neck Surg. 2002;126:607–613. [DOI] [PubMed] [Google Scholar]
- 720.Meng J, Xuan J, Qiao X, et al. Assessment of sleep impairment in persistent allergic rhinitis patients using polysomnography. Int Arch Allergy Immunol. 2011;155:57–62. [DOI] [PubMed] [Google Scholar]
- 721.Bozkurt B, Serife Ugur K, Karamanli H, Kucuker F, Ozol D. Polysomnographic findings in persistent allergic rhinitis. Sleep Breath. 2017;21:255–261. [DOI] [PubMed] [Google Scholar]
- 722.Kim DK, Han DH. Impact of allergic rhinitis on quality of life after adenotonsillectomy for pediatric sleep-disordered breathing. Int Forum Allergy Rhinol. 2015;5:741–746. [DOI] [PubMed] [Google Scholar]
- 723.Udaka T, Suzuki H, Fujimura T, et al. Chronic nasal obstruction causes daytime sleepiness and decreased quality of life even in the absence of snoring. Am J Rhinol. 2007;21:564–569. [DOI] [PubMed] [Google Scholar]
- 724.Mintz M, Garcia J, Diener P, Liao Y, Dupclay L, Georges G. Triamcinolone acetonide aqueous nasal spray improves nocturnal rhinitis-related quality of life in patients treated in a primary care setting: the Quality of Sleep in Allergic Rhinitis study. Ann Allergy Asthma Immunol. 2004;92:255–261. [DOI] [PubMed] [Google Scholar]
- 725.Janson C, De Backer W, Gislason T, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9:2132–2138. [DOI] [PubMed] [Google Scholar]
- 726.Colas C, Galera H, Anibarro B, et al. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAaR study). Clin Exp Allergy. 2012;42:1080–1087. [DOI] [PubMed] [Google Scholar]
- 727.Leger D, Annesi-Maesano I, Carat F, et al. Allergic rhinitis and its consequences on quality of sleep: an unexplored area. Arch Intern Med. 2006;166:1744–1748. [DOI] [PubMed] [Google Scholar]
- 728.Gadi G, Wali S, Koshak E, et al. The prevalence of allergic rhinitis and atopic markers in obstructive sleep apnea. J Epidemiol Glob Health. 2017;7:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Park CE, Shin SY, Lee KH, Cho JS, Kim SW. The effect of allergic rhinitis on the degree of stress, fatigue and quality of life in OSA patients. Eur Arch Otorhinolaryngol. 2012;269:2061–2064. [DOI] [PubMed] [Google Scholar]
- 730.Canova CR, Downs SH, Knoblauch A, Andersson M, Tamm M, Leuppi JD. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration. 2004;71:138–143. [DOI] [PubMed] [Google Scholar]
- 731.Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113:663–668. [DOI] [PubMed] [Google Scholar]
- 732.Koinis-Mitchell D, Kopel SJ, Boergers J, et al. Asthma, allergic rhinitis, and sleep problems in urban children. J Clin Sleep Med. 2015;11:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D. Nocturnal enuresis and over-weight are associated with obstructive sleep apnea. Pediatrics. 2009;124:e53–e59. [DOI] [PubMed] [Google Scholar]
- 734.Lin SY, Melvin TA, Boss EF, Ishman SL. The association between allergic rhinitis and sleep-disordered breathing in children: a systematic review. Int Forum Allergy Rhinol. 2013;3:504–509. [DOI] [PubMed] [Google Scholar]
- 735.Di Francesco RC, Alvarez J. Allergic rhinitis affects the duration of rapid eye movement sleep in children with sleep-disordered breathing without sleep apnea. Int Forum Allergy Rhinol. 2016;6:465–471. [DOI] [PubMed] [Google Scholar]
- 736.Chimenz R, Manti S, Fede C, et al. Primary nocturnal enuresis in children with allergic rhinitis and severe adenotonsillar hypertrophy: a single center pilot study. J Biol Regul Homeost Agents. 2015;29:73–79. [PubMed] [Google Scholar]
- 737.Poachanukoon O, Kitcharoensakkul M. Snoring and sleep problems in children with and without allergic rhinitis: a case control study. J Med Assoc Thai. 2015;98 Suppl 2:S138–S144. [PubMed] [Google Scholar]
- 738.Kwon JA, Lee M, Yoo KB, Park EC. Does the duration and time of sleep increase the risk of allergic rhinitis? Results of the 6-year nationwide Korea youth risk behavior web-based survey. PLoS One. 2013;8:e72507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest. 2010;138:519–527. [DOI] [PubMed] [Google Scholar]
- 740.Vichyanond P, Suratannon C, Lertbunnaphong P, Jirapongsananuruk O, Visitsunthorn N. Clinical characteristics of children with non-allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol. 2010;28:270–274. [PubMed] [Google Scholar]
- 741.Sogut A, Yilmaz O, Dinc G, Yuksel H. Prevalence of habitual snoring and symptoms of sleep-disordered breathing in adolescents. Int J Pediatr Otorhinolaryngol. 2009;73:1769–1773. [DOI] [PubMed] [Google Scholar]
- 742.Liukkonen K, Virkkula P, Aronen ET, Kirjavainen T, Pitkaranta A. All snoring is not adenoids in young children. Int J Pediatr Otorhinolaryngol. 2008;72:879–884. [DOI] [PubMed] [Google Scholar]
- 743.Kalra M, Lemasters G, Bernstein D, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest. 2006;129:942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Ng DK, Kwok KL, Cheung JM, et al. Prevalence of sleep problems in Hong Kong primary school children: a community-based telephone survey. Chest. 2005;128:1315–1323. [DOI] [PubMed] [Google Scholar]
- 745.Sogut A, Altin R, Uzun L, et al. Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3–11-year-old Turkish children. Pediatr Pulmonol. 2005;39:251–256. [DOI] [PubMed] [Google Scholar]
- 746.Chng SY, Goh DY, Wang XS, Tan TN, Ong NB. Snoring and atopic disease: a strong association. Pediatr Pulmonol. 2004;38:210–216. [DOI] [PubMed] [Google Scholar]
- 747.Anuntaseree W, Rookkapan K, Kuasirikul S, Thongsuksai P. Snoring and obstructive sleep apnea in Thai school-age children: prevalence and predisposing factors. Pediatr Pulmonol. 2001;32:222–227. [DOI] [PubMed] [Google Scholar]
- 748.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–683. [DOI] [PubMed] [Google Scholar]
- 749.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Kidon MI, See Y, Goh A, Chay OM, Balakrishnan A. Aeroallergen sensitization in pediatric allergic rhinitis in Singapore: is air-conditioning a factor in the tropics? Pediatr Allergy Immunol. 2004;15:340–343. [DOI] [PubMed] [Google Scholar]
- 751.Mansfield LE, Diaz G, Posey CR, Flores-Neder J. Sleep disordered breathing and daytime quality of life in children with allergic rhinitis during treatment with intranasal budesonide. Ann Allergy Asthma Immunol. 2004;92:240–244. [DOI] [PubMed] [Google Scholar]
- 752.McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111:170–173. [DOI] [PubMed] [Google Scholar]
- 753.Price D, Scadding G, Ryan D, et al. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Reed SD, Lee TA, McCrory DC. The economic burden of allergic rhinitis: a critical evaluation of the literature. Pharmacoeconomics. 2004;22:345–361. [DOI] [PubMed] [Google Scholar]
- 755.Bousquet J, Demarteau N, Mullol J, et al. Costs associated with persistent allergic rhinitis are reduced by levocetirizine. Allergy. 2005;60:788–794. [DOI] [PubMed] [Google Scholar]
- 756.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. [DOI] [PubMed] [Google Scholar]
- 757.European Academy of Allergy and Clinical Immunology (EAACI). Allergy Awareness Campaign. http://www.eaaci.org/outreach/eaaci-campaigns/2877-allergy-awareness-campaign.html. Accessed December 19, 2017.
- 758.Antonescu E, Childers N, Elisabeta Gardini E, et al. European Academy of Allergy and Clinical Immunology (EAACI). EAACI Campaigns. The MEP Written declaration campaign. http://www.eaaci.org/outreach/eaaci-campaigns/2670-ad.html. Accessed December 19, 2017.
- 759.Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. 2004;46:398–412. [DOI] [PubMed] [Google Scholar]
- 760.Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–S16. [DOI] [PubMed] [Google Scholar]
- 761.Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152:S1–S43. [DOI] [PubMed] [Google Scholar]
- 762.Blaiss MS. Allergic rhinitis: direct and indirect costs. Allergy Asthma Proc. 2010;31:375–380. [DOI] [PubMed] [Google Scholar]
- 763.Blaiss MS. Important aspects in management of allergic rhinitis: compliance, cost, and quality of life. Allergy Asthma Proc. 2003;24:231–238. [PubMed] [Google Scholar]
- 764.Santos R, Cifaldi M, Gregory C, Seitz P. Economic outcomes of a targeted intervention program: the costs of treating allergic rhinitis patients. Am J Manag Care. 1999;5:S225–S234. [PubMed] [Google Scholar]
- 765.Bhattacharyya N Incremental healthcare utilization and expenditures for allergic rhinitis in the United States. Laryngoscope. 2011;121:1830–1833. [DOI] [PubMed] [Google Scholar]
- 766.Cardell LO, Olsson P, Andersson M, et al. TOTALL: high cost of allergic rhinitis-a national Swedish population-based questionnaire study. NPJ Prim Care Respir Med. 2016;26:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Crystal-Peters J, Crown WH, Goetzel RZ, Schutt DC. The cost of productivity losses associated with allergic rhinitis. Am J Manag Care. 2000;6:373–378. [PubMed] [Google Scholar]
- 768.Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007;120:381–387. [DOI] [PubMed] [Google Scholar]
- 769.Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin. 2006;22:1203–1210. [DOI] [PubMed] [Google Scholar]
- 770.Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88:2–7. [DOI] [PubMed] [Google Scholar]
- 771.Blanc PD, Trupin L, Eisner M, et al. The work impact of asthma and rhinitis: findings from a population-based survey. J Clin Epidemiol. 2001;54:610–618. [DOI] [PubMed] [Google Scholar]
- 772.Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105:S622–S627. [DOI] [PubMed] [Google Scholar]
- 773.Schoenwetter WF, Dupclay L Jr, Appajosyula S, Botteman MF, Pashos CL. Economic impact and quality-of-life burden of allergic rhinitis. Curr Med Res Opin. 2004;20:305–317. [DOI] [PubMed] [Google Scholar]
- 774.Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO. Allergic rhinitis and the common cold—high cost to society. Allergy. 2010;65:776–783. [DOI] [PubMed] [Google Scholar]
- 775.Jauregui I, Mullol J, Davila I, et al. Allergic rhinitis and school performance. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):32–39. [PubMed] [Google Scholar]
- 776.Mir E, Panjabi C, Shah A. Impact of allergic rhinitis in school going children. Asia Pac Allergy. 2012;2:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Small P, Frenkiel S, Becker A. The Canadian Rhinitis Working Group. Rhinitis: a practical and comprehensive approach to assessment and therapy. J Otolaryngol. 2007;36(Suppl 1):S5–S27. http://www.allergyfoundation.ca/userfiles/Rhinitis-guidelines%202007.pdf Accessed December 19, 2017. [Google Scholar]
- 778.Schatz M A survey of the burden of allergic rhinitis in the USA. Allergy. 2007;62(Suppl 85):9–16. [DOI] [PubMed] [Google Scholar]
- 779.Ng ML, Warlow RS, Chrishanthan N, Ellis C, Walls R. Preliminary criteria for the definition of allergic rhinitis: a systematic evaluation of clinical parameters in a disease cohort (I). Clin Exp Allergy. 2000;30:1314–1331. [DOI] [PubMed] [Google Scholar]
- 780.Costa DJ, Amouyal M, Lambert P, et al. How representative are clinical study patients with allergic rhinitis in primary care? J Allergy Clin Immunol. 2011;127:920–926.e1. [DOI] [PubMed] [Google Scholar]
- 781.Raza SN, Yousuf K, Small P, Frenkiel S. Diagnosing allergic rhinitis: effectiveness of the physical examination in comparison to conventional skin testing. J Otolaryngol Head Neck Surg. 2011;40:407–412. [PubMed] [Google Scholar]
- 782.Ameli F, Brocchetti F, Tosca MA, Signori A, Ciprandi G. Nasal endoscopy in children with suspected allergic rhinitis. Laryngoscope. 2011;121:2055–2059. [DOI] [PubMed] [Google Scholar]
- 783.Eren E, Aktas A, Arslanoglu S, et al. Diagnosis of allergic rhinitis: inter-rater reliability and predictive value of nasal endoscopic examination: a prospective observational study. Clin Otolaryngol. 2013;38:481–486. [DOI] [PubMed] [Google Scholar]
- 784.Jareoncharsri P, Thitadilok V, Bunnag C, Ungkanont K, Voraprayoon S, Tansuriyawong P. Nasal endoscopic findings in patients with perennial allergic rhinitis. Asian Pac J Allergy Immunol. 1999;17:261–267. [PubMed] [Google Scholar]
- 785.White LJ, Rotella MR, DelGaudio JM. Polypoid changes of the middle turbinate as an indicator of atopic disease. Int Forum Allergy Rhinol. 2014;4:376–380. [DOI] [PubMed] [Google Scholar]
- 786.Hamizan AW, Christensen JM, Ebenzer J, et al. Middle turbinate edema as a diagnostic marker of inhalant allergy. Int Forum Allergy Rhinol. 2017;7:37–42. [DOI] [PubMed] [Google Scholar]
- 787.DelGaudio JM, Loftus PA, Hamizan AW, Harvey RJ, Wise SK. Central compartment atopic disease. Am J Rhinol Allergy. 2017;31:228–234. [DOI] [PubMed] [Google Scholar]
- 788.Brunner JP, Jawad BA, McCoul ED. Polypoid change of the middle turbinate and paranasal sinus polyposis are distinct entities. Otolaryngol Head Neck Surg. 2017;157:519–523. [DOI] [PubMed] [Google Scholar]
- 789.American College of Radiology (ACR). ACR Position Statement on Recent Studies Regarding CT Scans and Increased Cancer Risk. December 15, 2009. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/CT-Scans-and-Increased-Cancer-Risk. Accessed December 19, 2017.
- 790.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Mucci T, Govindaraj S, Tversky J. Allergic rhinitis. Mt Sinai J Med. 2011;78:634–644. [DOI] [PubMed] [Google Scholar]
- 792.Jung YG, Cho HJ, Park GY, et al. Comparison of the skin-prick test and Phadia ImmunoCAP as tools to diagnose house-dust mite allergy. Am J Rhinol Allergy. 2010;24:226–229. [DOI] [PubMed] [Google Scholar]
- 793.Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol. 1999;103:773–779. [DOI] [PubMed] [Google Scholar]
- 794.Westwood M, Ramaekers B, Lang S, et al. Immuno-CAP(R) ISAC and Microtest for multiplex allergen testing in people with difficult to manage allergic disease: a systematic review and cost analysis. Health Technol Assess. 2016;20:1–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Tversky J, MacGlashan DWJ. Short Wave Infrared (SWIR) camera as a novel approach to allergy skin testing. J Allergy Clin Immunol. 2017;139:AB156. [Google Scholar]
- 796.Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Zieglmayer P, Focke-Tejkl M, Schmutz R, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Mosbech H, Canonica GW, Backer V, et al. SQ house dust mite sublingually administered immunotherapy tablet (ALK) improves allergic rhinitis in patients with house dust mite allergic asthma and rhinitis symptoms. Ann Allergy Asthma Immunol. 2015;114:134–140. [DOI] [PubMed] [Google Scholar]
- 800.Casale TB. Anti-immunoglobulin E (omalizumab) therapy in seasonal allergic rhinitis. Am J Respir Crit Care Med. 2001;164:S18–S21. [DOI] [PubMed] [Google Scholar]
- 801.Calderon MA, Bernstein DI, Blaiss M, Andersen JS, Nolte H. A comparative analysis of symptom and medication scoring methods used in clinical trials of sublingual immunotherapy for seasonal allergic rhinitis. Clin Exp Allergy. 2014;44:1228–1239. [DOI] [PubMed] [Google Scholar]
- 802.Devillier P, Bousquet PJ, Grassin-Delyle S, et al. Comparison of outcome measures in allergic rhinitis in children, adolescents and adults. Pediatr Allergy Immunol. 2016;27:375–381. [DOI] [PubMed] [Google Scholar]
- 803.Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine-Tebbe J. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: Results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137:444–451.e8. [DOI] [PubMed] [Google Scholar]
- 804.Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65:1042–1048. [DOI] [PubMed] [Google Scholar]
- 805.Klimek L, Bachert C, Lukat KF, Pfaar O, Meyer H, Narkus A. Allergy immunotherapy with a hypoallergenic recombinant birch pollen allergen rBet v 1-FV in a randomized controlled trial. Clin Transl Allergy. 2015;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Häfner D, Reich K, Matricardi PM, Meyer H, Kettner J, Narkus A. Prospective validation of ‘Allergy-Control-SCORE™’: a novel symptom-medication score for clinical trials. Allergy. 2011;66:629–636. [DOI] [PubMed] [Google Scholar]
- 807.Demoly P, Jankowski R, Chassany O, Bessah Y, Allaert FA. Validation of a self-questionnaire for assessing the control of allergic rhinitis. Clin Exp Allergy. 2011;41:860–868. [DOI] [PubMed] [Google Scholar]
- 808.Demoly P, Calderon MA, Casale T, et al. Assessment of disease control in allergic rhinitis. Clin Transl Allergy. 2013;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the Rhinitis Control Assessment Test in patients with rhinitis. J Allergy Clin Immunol. 2013;131:379–386. [DOI] [PubMed] [Google Scholar]
- 810.Spector SL, Nicklas RA, Chapman JA, et al. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol. 2003;91:105–114. [DOI] [PubMed] [Google Scholar]
- 811.Annesi-Maesano I, Didier A, Klossek M, Chanal I, Moreau D, Bousquet J. The score for allergic rhinitis (SFAR): a simple and valid assessment method in population studies. Allergy. 2002;57:107–114. [DOI] [PubMed] [Google Scholar]
- 812.Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62:367–372. [DOI] [PubMed] [Google Scholar]
- 813.Devillier P, Chassany O, Vicaut E, et al. The minimally important difference in the Rhinoconjunctivitis Total Symptom Score in grass-pollen-induced allergic rhinoconjunctivitis. Allergy. 2014;69:1689–1695. [DOI] [PubMed] [Google Scholar]
- 814.Galimberti M, Passalacqua G, Incorvaia C, et al. Catching allergy by a simple questionnaire. World Allergy Organ J. 2015;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of grass pollen allergen sublingual immunotherapy tablets for seasonal allergic rhinoconjunctivitis: a systematic review and meta-analysis. JAMA Intern Med. 2015;175:1301–1309. [DOI] [PubMed] [Google Scholar]
- 816.Anon JB. Introduction to in vivo allergy testing. Otolaryngol Head Neck Surg. 1993;109:593–600. [PubMed] [Google Scholar]
- 817.Kim BJ Mun SK. Objective measurements using the skin prick test in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2010;136:1104–1106. [DOI] [PubMed] [Google Scholar]
- 818.Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1–S148. [DOI] [PubMed] [Google Scholar]
- 819.Oppenheimer J, Nelson HS. Skin testing: a survey of allergists. Ann Allergy Asthma Immunol. 2006;96:19–23. [DOI] [PubMed] [Google Scholar]
- 820.Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. [DOI] [PubMed] [Google Scholar]
- 821.Chafen JJ, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848–1856. [DOI] [PubMed] [Google Scholar]
- 822.Tschopp JM, Sistek D, Schindler C, et al. Current allergic asthma and rhinitis: diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests, and Phadiatop). Results from 8329 randomized adults from the SAPALDIA Study. Swiss Study on Air Pollution and Lung Diseases in Adults. Allergy. 1998;53:608–613. [DOI] [PubMed] [Google Scholar]
- 823.Sander I, Fleischer C, Meurer U, Bruning T, Raulf-Heimsoth M. Allergen content of grass pollen preparations for skin prick testing and sublingual immunotherapy. Allergy. 2009;64:1486–1492. [DOI] [PubMed] [Google Scholar]
- 824.Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–263. [DOI] [PubMed] [Google Scholar]
- 825.Brown HM, Su S, Thantrey N. Prick testing for allergens standardized by using a precision needle. Clin Allergy. 1981;11:95–98. [DOI] [PubMed] [Google Scholar]
- 826.Ates A, Kinikli G, Turgay M, Aydogan N, Duman M. The results of skin prick testing in patients with allergic rhinitis: a comparison between a multiple lancet device and a single lancet. Asian Pac J Allergy Immunol. 2004;22:109–114. [PubMed] [Google Scholar]
- 827.Phagoo SB, Wilson NM, Silverman M. Skin prick testing using allergen-coated lancets: a comparison between a multiple lancet device and a single lancet applied with varying pressures. Clin Exp Allergy. 1991;21:589–593. [DOI] [PubMed] [Google Scholar]
- 828.Rhodius R, Wickens K, Cheng S, Crane J. A comparison of two skin test methodologies and allergens from two different manufacturers. Ann Allergy Asthma Immunol. 2002;88:374–379. [DOI] [PubMed] [Google Scholar]
- 829.Piette V, Bourret E, Bousquet J, Demoly P. Prick tests to aeroallergens: is it possible simply to wipe the device between tests? Allergy. 2002;57:940–942. [DOI] [PubMed] [Google Scholar]
- 830.Nevis IF, Binkley K, Kabali C. Diagnostic accuracy of skin-prick testing for allergic rhinitis: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2016;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Krouse JH, Shah AG, Kerswill K. Skin testing in predicting response to nasal provocation with alternaria. Laryngoscope. 2004;114:1389–1393. [DOI] [PubMed] [Google Scholar]
- 832.Krouse JH, Sadrazodi K, Kerswill K. Sensitivity and specificity of prick and intradermal testing in predicting response to nasal provocation with timothy grass antigen. Otolaryngol Head Neck Surg. 2004;131:215–219. [DOI] [PubMed] [Google Scholar]
- 833.Gungor A, Houser SM, Aquino BF, et al. A comparison of skin endpoint titration and skin-prick testing in the diagnosis of allergic rhinitis. Ear Nose Throat J. 2004;83:54–60. [PubMed] [Google Scholar]
- 834.Zarei M, Remer CF, Kaplan MS, et al. Optimal skin prick wheal size for diagnosis of cat allergy. Ann Allergy Asthma Immunol. 2004;92:604–610. [DOI] [PubMed] [Google Scholar]
- 835.Pumhirun P, Jane-Trakoonroj S, Wasuwat P. Comparison of in vitro assay for specific IgE and skin prick test with intradermal test in patients with allergic rhinitis. Asian Pac J Allergy Immunol. 2000;18:157–160. [PubMed] [Google Scholar]
- 836.Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test—European standards. Clin Transl Allergy. 2013;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Kvisselgaard AD, Kroigaard M, Mosbech HF, Garvey LH. No cases of perioperative allergy to local anaesthetics in the Danish Anaesthesia Allergy Centre. Acta Anaesthesiol Scand. 2017;61:149–155. [DOI] [PubMed] [Google Scholar]
- 838.Mertes PM, Moneret-Vautrin DA, Leynadier F, Laxenaire MC. Skin reactions to intradermal neuromuscular blocking agent injections: a randomized multicenter trial in healthy volunteers. Anesthesiology. 2007;107:245–252. [DOI] [PubMed] [Google Scholar]
- 839.Mota I, Gaspar A, Chambel M, Piedade S, Morais-Almeida M. Hypersensitivity to beta-lactam antibiotics: a three-year study. Eur Ann Allergy Clin Immunol. 2016;48:212–219. [PubMed] [Google Scholar]
- 840.Berti A, Della-Torre E, Yacoub M, et al. Patients with breakthrough reactions to iodinated contrast media have low incidence of positive skin tests. Eur Ann Allergy Clin Immunol. 2016;48:137–144. [PubMed] [Google Scholar]
- 841.Trevino RJ, Gordon BR, Veling MC. Food allergy and hypersensitivity In: Krouse HJ, Chadwick SJ, Gordon BR, Derebery MJ, eds. Allergy and Immunology: An Otolaryngic Approach: Lippincott Williams & Wilkins; 2002:50–77. [Google Scholar]
- 842.Fox RA, Sabo BM, Williams TP, Joffres MR. Intradermal testing for food and chemical sensitivities: a double-blind controlled study. J Allergy Clin Immunol. 1999;103:907–911. [DOI] [PubMed] [Google Scholar]
- 843.De Asis LB, Reisacher WR. Allergen immunotherapy In: Rosenstreich DL, ed. Manual of Allergy and Clinical Immunology for Otolaryngologists. San Diego, CA: Plural Publishing; 2015:383–406. [Google Scholar]
- 844.Peltier J, Ryan MW. Comparison of intradermal dilutional testing, skin prick testing, and modified quantitative testing for common allergens. Otolaryngol Head Neck Surg. 2007;137:246–249. [DOI] [PubMed] [Google Scholar]
- 845.Trevino RJ, Veling MC. The importance of quantifying skin reactivity in treating allergic rhinitis with immunotherapy. Ear Nose Throat J. 2000;79:362–364, 366. [PubMed] [Google Scholar]
- 846.Niemeijer NR, Goedewaagen B, Kauffman HF, de Monchy JG. Optimization of skin testing. I. Choosing allergen concentrations and cutoff values by factorial design. Allergy. 1993;48:491–497. [DOI] [PubMed] [Google Scholar]
- 847.Fornadley JA. Skin testing for inhalant allergy. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S41–S45. [DOI] [PubMed] [Google Scholar]
- 848.Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol. 1987;79:660–677. [DOI] [PubMed] [Google Scholar]
- 849.King HC. Skin endpoint titration. Still the standard? Otolaryngol Clin North Am. 1992;25:13–25. [PubMed] [Google Scholar]
- 850.Peltier J, Ryan MW. Comparison of intradermal dilutional testing with the Multi-Test II applicator in testing for mold allergy. Otolaryngol Head Neck Surg. 2006;134:240–244. [DOI] [PubMed] [Google Scholar]
- 851.Simons JP, Rubinstein EN, Kogut VJ, Melfi PJ, Ferguson BJ. Comparison of Multi-Test II skin prick testing to intradermal dilutional testing. Otolaryngol Head Neck Surg. 2004;130:536–544. [DOI] [PubMed] [Google Scholar]
- 852.Purohit A, Laffer S, Metz-Favre C, et al. Poor association between allergen-specific serum immunoglobulin E levels, skin sensitivity and basophil degranulation: a study with recombinant birch pollen allergen Bet v 1 and an immunoglobulin E detection system measuring immunoglobulin E capable of binding to Fc epsilon RI. Clin Exp Allergy. 2005;35:186–192. [DOI] [PubMed] [Google Scholar]
- 853.Perera MG, Bernstein IL, Michael JG, Johansson SG. Predictability of the radioallergosorbent test (RAST) in ragweed pollenosis. Am Rev Respir Dis. 1975;111:605–610. [DOI] [PubMed] [Google Scholar]
- 854.Ontario HQ. Skin testing for allergic rhinitis: a health technology assessemnt. Ontario Health Technology Assessment Series. 2016;16:1–45. [PMC free article] [PubMed] [Google Scholar]
- 855.Niemeijer NR, Fluks AF, de Monchy JG. Optimization of skin testing. II. Evaluation of concentration and cutoff values, as compared with RAST and clinical history, in a multicenter study. Allergy. 1993;48:498–503. [DOI] [PubMed] [Google Scholar]
- 856.Nelson HS, Oppenheimer J, Buchmeier A, Kordash TR, Freshwater LL. An assessment of the role of intradermal skin testing in the diagnosis of clinically relevant allergy to Timothy grass. J Allergy Clin Immunol. 1996;97:1193–1201. [DOI] [PubMed] [Google Scholar]
- 857.Reddy PM, Nagaya H, Pascual HC, et al. Reappraisal of intracutaneous tests in the diagnosis of reaginic allergy. J Allergy Clin Immunol. 1978;61:36–41. [DOI] [PubMed] [Google Scholar]
- 858.Schwindt CD, Hutcheson PS, Leu SY, Dykewicz MS. Role of intradermal skin tests in the evaluation of clinically relevant respiratory allergy assessed using patient history and nasal challenges. Ann Allergy Asthma Immunol. 2005;94:627–633. [DOI] [PubMed] [Google Scholar]
- 859.Larrabee YC, Reisacher W. Intradermal testing after negative skin prick testing for patients with high suspicion of allergy. Int Forum Allergy Rhinol. 2015;5:547–550. [DOI] [PubMed] [Google Scholar]
- 860.Escudero AI, Sanchez-Guerrero IM, Mora AM, et al. Cost-effectiveness of various methods of diagnosing hypersensitivity to Alternaria. Allergol Immunopathol (Madr). 1993;21:153–157. [PubMed] [Google Scholar]
- 861.Krouse JH, Krouse HJ. Modulation of immune mediators with MQT-based immunotherapy. Otolaryngol Head Neck Surg. 2006;134:746–750. [DOI] [PubMed] [Google Scholar]
- 862.Lewis AF, Franzese C, Stringer SP. Diagnostic eval-uation of inhalant allergies: a cost-effectiveness analysis. Am J Rhinol. 2008;22:246–252. [DOI] [PubMed] [Google Scholar]
- 863.Long WF, Taylor RJ, Wagner CJ, Leavengood DC, Nelson HS. Skin test suppression by antihistamines and the development of subsensitivity. J Allergy Clin Immunol. 1985;76:113–117. [DOI] [PubMed] [Google Scholar]
- 864.Phillips MJ, Meyrick Thomas RH, Moodley I, Davies RJ. A comparison of the in vivo effects of ketotifen, clemastine, chlorpheniramine and sodium cromoglycate on histamine and allergen induced weals in human skin. Br J Clin Pharmacol. 1983;15:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Simons FE, Simons KJ. Peripheral H1-blockade effect of fexofenadine. Ann Allergy Asthma Immunol. 1997;79:530–532. [DOI] [PubMed] [Google Scholar]
- 866.Simons FE, Johnston L, Gu X, Simons KJ. Suppression of the early and late cutaneous allergic responses using fexofenadine and montelukast. Ann Allergy Asthma Immunol. 2001;86:44–50. [DOI] [PubMed] [Google Scholar]
- 867.Almind M, Dirksen A, Nielsen NH, Svendsen UG. Duration of the inhibitory activity on histamine-induced skin weals of sedative and non-sedative antihistamines. Allergy. 1988;43:593–596. [DOI] [PubMed] [Google Scholar]
- 868.Cook TJ, MacQueen DM, Wittig HJ, Thornby JI, Lantos RL, Virtue CM. Degree and duration of skin test suppression and side effects with antihistamines. A double blind controlled study with five antihistamines. J Allergy Clin Immunol. 1973;51:71–77. [DOI] [PubMed] [Google Scholar]
- 869.Pearlman DS, Grossman J, Meltzer EO. Histamine skin test reactivity following single and multiple doses of azelastine nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:258–262. [DOI] [PubMed] [Google Scholar]
- 870.Miller J, Nelson HS. Suppression of immediate skin tests by ranitidine. J Allergy Clin Immunol. 1989;84:895–899. [DOI] [PubMed] [Google Scholar]
- 871.Kupczyk M, Kuprys I, Bochenska-Marciniak M, Gorski P, Kuna P. Ranitidine (150 mg daily) inhibits wheal, flare, and itching reactions in skin-prick tests. Allergy Asthma Proc. 2007;28:711–715. [DOI] [PubMed] [Google Scholar]
- 872.Harvey RP, Schocket AL. The effect of H1 and H2 blockade on cutaneous histamine response in man. J Allergy Clin Immunol. 1980;65:136–139. [DOI] [PubMed] [Google Scholar]
- 873.Rao KS, Menon PK, Hilman BC, Sebastian CS, Bairnsfather L. Duration of the suppressive effect of tricyclic antidepressants on histamine-induced wheal-and-flare reactions in human skin. J Allergy Clin Immunol. 1988;82:752–757. [DOI] [PubMed] [Google Scholar]
- 874.Isik SR, Celikel S, Karakaya G, Ulug B, Kalyoncu AF. The effects of antidepressants on the results of skin prick tests used in the diagnosis of allergic diseases. Int Arch Allergy Immunol. 2011;154:63–68. [DOI] [PubMed] [Google Scholar]
- 875.Corren J, Shapiro G, Reimann J, et al. Allergen skin tests and free IgE levels during reduction and cessation of omalizumab therapy. J Allergy Clin Immunol. 2008;121:506–511. [DOI] [PubMed] [Google Scholar]
- 876.Hill SL 3rd, Krouse JH. The effects of montelukast on intradermal wheal and flare. Otolaryngol Head Neck Surg. 2003;129:199–203. [DOI] [PubMed] [Google Scholar]
- 877.Cuhadaroglu C, Erelel M, Kiyan E, Ece T, Erkan F. Role of Zafirlukast on skin prick test. Allergol Immunopathol (Madr). 2001;29:66–68. [DOI] [PubMed] [Google Scholar]
- 878.Des Roches A, Paradis L, Bougeard YH, Godard P, Bousquet J, Chanez P. Long-term oral corticosteroid therapy does not alter the results of immediate-type allergy skin prick tests. J Allergy Clin Immunol. 1996;98:522–527. [DOI] [PubMed] [Google Scholar]
- 879.Slott RI, Zweiman B. A controlled study of the effect of corticosteroids on immediate skin test reactivity. J Allergy Clin Immunol. 1974;54:229–234. [DOI] [PubMed] [Google Scholar]
- 880.Olson R, Karpink MH, Shelanski S, Atkins PC, Zweiman B. Skin reactivity to codeine and histamine during prolonged corticosteroid therapy. J Allergy Clin Immunol. 1990;86:153–159. [DOI] [PubMed] [Google Scholar]
- 881.Geng B, Thakor A, Clayton E, Finkas L, Riedl MA. Factors associated with negative histamine control for penicillin allergy skin testing in the inpatient setting. Ann Allergy Asthma Immunol. 2015;115:33–38. [DOI] [PubMed] [Google Scholar]
- 882.Narasimha SK, Srinivas CR, Mathew AC. Effect of topical corticosteroid application frequency on histamine-induced wheals. Int J Dermatol. 2005;44:425–427. [DOI] [PubMed] [Google Scholar]
- 883.Andersson M, Pipkorn U. Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticosteroids. J Allergy Clin Immunol. 1987;79:345–349. [DOI] [PubMed] [Google Scholar]
- 884.Pipkorn U, Proud D, Lichtenstein LM, et al. Effect of short-term systemic glucocorticoid treatment on human nasal mediator release after antigen challenge. J Clin Invest. 1987;80:957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Gradman J, Wolthers OD. Suppressive effects of topical mometasone furoate and tacrolimus on skin prick testing in children. Pediatr Dermatol. 2008;25:269–270. [DOI] [PubMed] [Google Scholar]
- 886.Shah KM, Rank MA, Dave SA, Oslie CL, Butterfield JH. Predicting which medication classes interfere with allergy skin testing. Allergy Asthma Proc. 2010;31:477–482. [DOI] [PubMed] [Google Scholar]
- 887.Duenas-Laita A, Ruiz-Munoz P, Armentia A, Pinacho F, Martin-Armentia B. Successful treatment of chronic drug-resistant urticaria with alprazolam. J Allergy Clin Immunol. 2009;123:504–505. [DOI] [PubMed] [Google Scholar]
- 888.Spergel JM, Nurse N, Taylor P, Parneix-Spake A. Effect of topical pimecrolimus on epicutaneous skin testing. J Allergy Clin Immunol. 2004;114:695–697. [DOI] [PubMed] [Google Scholar]
- 889.More DR, Napoli DC, Hagan LL. Herbal supplements and skin testing: the lack of effect of commonly used herbal supplements on histamine skin prick testing. Allergy. 2003;58:492–494. [DOI] [PubMed] [Google Scholar]
- 890.Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131:46–52. [DOI] [PubMed] [Google Scholar]
- 891.Pipkorn U, Hammarlund A, Enerback L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin Exp Allergy. 1989;19:19–25. [DOI] [PubMed] [Google Scholar]
- 892.Ando M, Shima M. Serum interleukins 12 and 18 and immunoglobulin E concentrations and allergic symptoms in Japanese schoolchildren. J Investig Allergol Clin Immunol. 2007;17:14–19. [PubMed] [Google Scholar]
- 893.Marinho S, Simpson A, Soderstrom L, Woodcock A, Ahlstedt S, Custovic A. Quantification of atopy and the probability of rhinitis in preschool children: a population-based birth cohort study. Allergy. 2007;62:1379–1386. [DOI] [PubMed] [Google Scholar]
- 894.Kalpaklioglu AF, Kavut AB. Allergic and nonallergic rhinitis: can we find the differences/similarities between the two pictures? J Asthma. 2009;46:481–485. [DOI] [PubMed] [Google Scholar]
- 895.Jung YG, Kim KH, Kim HY, Dhong HJ, Chung SK. Predictive capabilities of serum eosinophil cationic protein, percentage of eosinophils and total immunoglobulin E in allergic rhinitis without bronchial asthma. J Int Med Res. 2011;39:2209–2216. [DOI] [PubMed] [Google Scholar]
- 896.Demirjian M, Rumbyrt JS, Gowda VC, Klaustermeyer WB. Serum IgE and eosinophil count in allergic rhinitis—analysis using a modified Bayes’ theorem. Allergol Immunopathol (Madr). 2012;40:281–287. [DOI] [PubMed] [Google Scholar]
- 897.Hatcher JL, Cohen SD, Mims JW. Total serum immunoglobulin E as a marker for missed antigens on in vitro allergy screening. Int Forum Allergy Rhinol. 2013;3:782–787. [DOI] [PubMed] [Google Scholar]
- 898.Karli R, Balbaloglu E, Uzun L, Cinar F, Ugur MB. Correlation of symptoms with total IgE and specific IgE levels in patients presenting with allergic rhinitis. Ther Adv Respir Dis. 2013;7:75–79. [DOI] [PubMed] [Google Scholar]
- 899.Chung D, Park KT, Yarlagadda B, Davis EM, Platt M. The significance of serum total immunoglobulin E for in vitro diagnosis of allergic rhinitis. Int Forum Allergy Rhinol. 2014;4:56–60. [DOI] [PubMed] [Google Scholar]
- 900.Jacobs TS, Forno E, Brehm JM, et al. Underdiagnosis of allergic rhinitis in underserved children. J Allergy Clin Immunol. 2014;134:737–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Li Y, Wu R, Tian Y, Bao T, Tian Z. The correlation of serum eosinophil cationic protein level with eosinophil count, and total IgE level in Korean adult allergic rhinitis patients. Asian Pac J Allergy Immunol. 2016;34:33–37. [DOI] [PubMed] [Google Scholar]
- 902.Park SC, Kim JH, Lee KH, Hong SC, Lee HS, Kang JW. Association of serum eosinophilia and total immunoglobulin E concentration with the risk of allergic symptoms and allergic sensitization, respectively: A 2-year follow-up study. Int J Pediatr Otorhinolaryngol. 2016;86:167–171. [DOI] [PubMed] [Google Scholar]
- 903.Satwani H, Rehman A, Ashraf S, Hassan A. Is serum total IgE levels a good predictor of allergies in children? J Pak Med Assoc. 2009;59:698–702. [PubMed] [Google Scholar]
- 904.Tu YL, Chang SW, Tsai HJ, et al. Total serum IgE in a population-based study of Asian children in Taiwan: reference value and significance in the diagnosis of allergy. PLoS One. 2013;8:e80996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Tay TR, Bosco J, Aumann H, O’Hehir R, Hew M. Elevated total serum immunoglobulin E (≥1000 IU/mL): implications? Intern Med J. 2016;46:846–849. [DOI] [PubMed] [Google Scholar]
- 906.Huss-Marp J, Darsow U, Brockow K, et al. Can immunoglobulin E-measurement replace challenge tests in allergic rhinoconjunctivits to grass pollen? Clin Exp Allergy. 2011;41:1116–1124. [DOI] [PubMed] [Google Scholar]
- 907.Karakoc GB, Yilmaz M, Altintas DU, Kendirli SG. Can serum-specific IgE/total IgE ratio predict clinical response to allergen-specific immunotherapy in children monosensitized to house dust mite? J Allergy (Cairo). 2012;2012:694094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Di Lorenzo G, Mansueto P, Pacor ML, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123:1103–1110.e4. [DOI] [PubMed] [Google Scholar]
- 909.Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156–1173. [DOI] [PubMed] [Google Scholar]
- 910.Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010;125:S284–S296. [DOI] [PubMed] [Google Scholar]
- 911.Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2:1105–1107. [DOI] [PubMed] [Google Scholar]
- 912.Cox L Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447–453. [DOI] [PubMed] [Google Scholar]
- 913.Osguthorpe JD. In vitro allergy testing. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S46–S50. [DOI] [PubMed] [Google Scholar]
- 914.Brown CE, Jones CJ, Stuttaford L, Robertson A, Rashid RS, Smith HE. A qualitative study of the allergy testing experiences, views and preferences of adult patients. Clin Transl Allergy. 2016;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Mari A, Iacovacci P, Afferni C, et al. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999;103:1005–1011. [DOI] [PubMed] [Google Scholar]
- 916.Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99:34–41. [DOI] [PubMed] [Google Scholar]
- 917.Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121:1219–1224. [DOI] [PubMed] [Google Scholar]
- 918.Emanuel IA. In vitro testing for allergy diagnosis. Otolaryngol Clin North Am. 2003;36:879–893. [DOI] [PubMed] [Google Scholar]
- 919.Corsico AG, De Amici M, Ronzoni V, et al. Allergen-specific immunoglobulin E and allergic rhinitis severity. Allergy Rhinol (Providence). 2017;8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Ciprandi G, De Amici M, Giunta V, Marseglia GL. Comparison of serum specific IgE and skin prick test in polysensitized patients. Int J Immunopathol Pharmacol. 2010;23:1293–1295. [DOI] [PubMed] [Google Scholar]
- 921.Chen ST, Sun HL, Lu KH, Lue KH, Chou MC. Correlation of immunoglobulin E, eosinophil cationic protein, and eosinophil count with the severity of childhood perennial allergic rhinitis. J Microbiol Immunol Infect. 2006;39:212–218. [PubMed] [Google Scholar]
- 922.Ciprandi G, Comite P, Ferrero F, Fontana V, Bruzzone M, Mussap M. Serum allergen-specific IgE, allergic rhinitis severity, and age. Rhinology. 2016;54:231–238. [DOI] [PubMed] [Google Scholar]
- 923.Ciprandi G, Comite P, Ferrero F, et al. Birch allergy and oral allergy syndrome: the practical relevance of serum immunoglobulin E to Bet v 1. Allergy Asthma Proc. 2016;37:43–49. [DOI] [PubMed] [Google Scholar]
- 924.Howarth P, Malling HJ, Molimard M, Devillier P. Analysis of allergen immunotherapy studies shows increased clinical efficacy in highly symptomatic patients. Allergy. 2012;67:321–327. [DOI] [PubMed] [Google Scholar]
- 925.Ownby DR, Bailey J. Comparison of MAST with radioallergosorbent and skin tests for diagnosis of allergy in children. Am J Dis Child. 1986;140:45–48. [DOI] [PubMed] [Google Scholar]
- 926.Ferguson AC, Murray AB. Predictive value of skin prick tests and radioallergosorbent tests for clinical allergy to dogs and cats. CMAJ. 1986;134:1365–1368. [PMC free article] [PubMed] [Google Scholar]
- 927.Chinoy B, Yee E, Bahna SL. Skin testing versus radioallergosorbent testing for indoor allergens. Clin Mol Allergy. 2005;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Tversky JR, Chelladurai Y, McGready J, Hamilton RG. Performance and pain tolerability of current diagnostic allergy skin prick test devices. J Allergy Clin Immunol Pract. 2015;3:888–893. [DOI] [PubMed] [Google Scholar]
- 929.Ishizaka T, Ishizaka K, Johansson SG, Bennich H. Histamine release from human leukocytes by anti-gamma E antibodies. J Immunol. 1969;102:884–892. [PubMed] [Google Scholar]
- 930.Gendo K, Larson EB. Evidence-based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med. 2004;140:278–289. [DOI] [PubMed] [Google Scholar]
- 931.de Vos G, Nazari R, Ferastraoaru D, et al. Discordance between aeroallergen specific serum IgE and skin testing in children younger than 4 years. Ann Allergy Asthma Immunol. 2013;110:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Sharma HP, Wood RA, Bravo AR, Matsui EC. A comparison of skin prick tests, intradermal skin tests, and specific IgE in the diagnosis of mouse allergy. J Allergy Clin Immunol. 2008;121:933–939. [DOI] [PubMed] [Google Scholar]
- 933.Bernstein DI, Biagini RE, Karnani R, et al. In vivo sensitization to purified Hevea brasiliensis proteins in health care workers sensitized to natural rubber latex. J Allergy Clin Immunol. 2003;111:610–616. [DOI] [PubMed] [Google Scholar]
- 934.Koskela H, Taivainen A, Tukiainen H, Chan HK. Inhalation challenge with bovine dander allergens: who needs it? Chest. 2003;124:383–391. [DOI] [PubMed] [Google Scholar]
- 935.Centers for Medicare and Medicaid Services (CMS). Clinical Laboratory Fee Schedule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/. Accessed December 19, 2017.
- 936.Liccardi G, D’Amato G, Canonica GW, Salzillo A, Piccolo A, Passalacqua G. Systemic reactions from skin testing: literature review. J Investig Allergol Clin Immunol. 2006;16:75–78. [PubMed] [Google Scholar]
- 937.Nelson HS, Lahr J, Buchmeier A, McCormick D. Evaluation of devices for skin prick testing. J Allergy Clin Immunol. 1998;101:153–156. [DOI] [PubMed] [Google Scholar]
- 938.Andersen HH, Lundgaard AC, Petersen AS, et al. The lancet weight determines wheal diameter in response to skin prick testing with histamine. PLoS One. 2016;11:e0156211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Carr WW, Martin B, Howard RS, et al. Comparison of test devices for skin prick testing. J Allergy Clin Immunol. 2005;116:341–346. [DOI] [PubMed] [Google Scholar]
- 940.Seibert SM, King TS, Kline D, Mende C, Craig T. Reliability of skin test results when read at different time points. Allergy Asthma Proc. 2011;32:203–205. [DOI] [PubMed] [Google Scholar]
- 941.van der Veen MJ, Mulder M, Witteman AM, et al. False-positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98:1028–1034. [DOI] [PubMed] [Google Scholar]
- 942.McCann WA, Ownby DR. The reproducibility of the allergy skin test scoring and interpretation by board-certified/board-eligible allergists. Ann Allergy Asthma Immunol. 2002;89:368–371. [DOI] [PubMed] [Google Scholar]
- 943.Choi IS, Koh YI, Koh JS, Lee MG. Sensitivity of the skin prick test and specificity of the serum-specific IgE test for airway responsiveness to house dust mites in asthma. J Asthma. 2005;42:197–202. [PubMed] [Google Scholar]
- 944.de Vos G Skin testing versus serum-specific IgE testing: which is better for diagnosing aeroallergen sensitization and predicting clinical allergy? Curr Allergy Asthma Rep. 2014;14:430. [DOI] [PubMed] [Google Scholar]
- 945.Hermansson LL, Korhonen K, Silvan M, Rantanen S, Isoaho R, Savolainen J. Prospective study on cost-effectiveness of nurse interviw introducing retesting with in vitro diagnostics (IVD) to parents of children with suspected food allergy in Finland. Value Health. 2014;17:A588. [DOI] [PubMed] [Google Scholar]
- 946.Pastorello EA, Incorvaia C, Ortolani C, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol. 1995;96:580–587. [DOI] [PubMed] [Google Scholar]
- 947.Haxel BR, Huppertz T, Boessert P, Bast F, Fruth K. Correlation of skin test results and specific immunoglobulin E blood levels with nasal provocation testing for house-dust mite allergies. Am J Rhinol Allergy. 2016;30:60–64. [DOI] [PubMed] [Google Scholar]
- 948.Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to Food and Drug Administration-approved standardized extracts. J Allergy Clin Immunol. 1990;86:766–774. [DOI] [PubMed] [Google Scholar]
- 949.Tantilipikorn P, Danpornprasert P, Ngaotepprutaram P, Assanasen P, Bunnag C, Thinkhamrop B. The correlation between intradermal testing and serum specific IgE to house dust mite in negative skin prick test allergic rhinitis adult patients. Asian Pac J Allergy Immunol. 2015;33:308–311. [DOI] [PubMed] [Google Scholar]
- 950.Powe DG, Groot Kormelink T, Sisson M, et al. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J Allergy Clin Immunol. 2010;125:139–145.e3. [DOI] [PubMed] [Google Scholar]
- 951.KleinJan A, Godthelp T, van Toornenenbergen AW, Fokkens WJ. Allergen binding to specific IgE in the nasal mucosa of allergic patients. J Allergy Clin Immunol. 1997;99:515–521. [DOI] [PubMed] [Google Scholar]
- 952.Carney AS, Powe DG, Huskisson RS, Jones NS. Atypical nasal challenges in patients with idiopathic rhinitis: more evidence for the existence of allergy in the absence of atopy? Clin Exp Allergy. 2002;32:1436–1440. [DOI] [PubMed] [Google Scholar]
- 953.Reisacher WR, Bremberg MG. Prevalence of antigen-specific immunoglobulin E on mucosal brush biopsy of the inferior turbinates in patients with nonallergic rhinitis. Int Forum Allergy Rhinol. 2014;4:292–297. [DOI] [PubMed] [Google Scholar]
- 954.Nicolai T, Bellach B, Mutius EV, Thefeld W, Hoffmeister H. Increased prevalence of sensitization against aeroallergens in adults in West compared with East Germany. Clin Exp Allergy. 1997;27:886–892. [PubMed] [Google Scholar]
- 955.Fuiano N, Fusilli S, Incorvaia C. A role for measurement of nasal IgE antibodies in diagnosis of Alternaria-induced rhinitis in children. Allergol Immunopathol (Madr). 2012;40:71–74. [DOI] [PubMed] [Google Scholar]
- 956.Reisacher WR. Total and allergen-specific immunoglobulin E in the serum and nasal mucosa of a nonallergic population. Int Forum Allergy Rhinol. 2016;6:618–623. [DOI] [PubMed] [Google Scholar]
- 957.Rondon C, Blanca-Lopez N, Aranda A, et al. Local allergic rhinitis: allergen tolerance and immunologic changes after preseasonal immunotherapy with grass pollen. J Allergy Clin Immunol. 2011;127:1069–1071. [DOI] [PubMed] [Google Scholar]
- 958.Kim JH, Yoon MG, Seo DH, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016;8:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Lee KS, Yu J, Shim D, et al. Local immune responses in children and adults with allergic and nonallergic rhinitis. PLoS One. 2016;11:e0156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.Sakaida H, Masuda S, Takeuchi K. Measurement of Japanese cedar pollen-specific IgE in nasal secretions. Allergol Int. 2014;63:467–473. [DOI] [PubMed] [Google Scholar]
- 961.Ota Y, Ikemiyagi Y, Sato T, et al. Measuring local immunoglobulin E in the inferior turbinate nasal mucosa in patients with allergic rhinitis. Allergol Int. 2016;65:396–399. [DOI] [PubMed] [Google Scholar]
- 962.Becker S, Rasp J, Eder K, Berghaus A, Kramer MF, Groger M. Non-allergic rhinitis with eosinophilia syndrome is not associated with local production of specific IgE in nasal mucosa. Eur Arch Otorhinolaryngol. 2016;273:1469–1475. [DOI] [PubMed] [Google Scholar]
- 963.Reisacher WR. Detecting local immunoglobulin E from mucosal brush biopsy of the inferior turbinates using microarray analysis. Int Forum Allergy Rhinol. 2013;3:399–403. [DOI] [PubMed] [Google Scholar]
- 964.Reisacher WR. Mucosal brush biopsy testing of the inferior turbinate to detect local, antigen-specific immunoglobulin E. Int Forum Allergy Rhinol. 2012;2:69–74. [DOI] [PubMed] [Google Scholar]
- 965.Sensi LG, Piacentini GL, Nobile E, et al. Changes in nasal specific IgE to mites after periods of allergen exposure-avoidance: a comparison with serum levels. Clin Exp Allergy. 1994;24:377–382. [DOI] [PubMed] [Google Scholar]
- 966.Hoffmann HJ, Knol EF, Ferrer M, et al. Pros and cons of clinical basophil testing (BAT). Curr Allergy Asthma Rep. 2016;16:56. [DOI] [PubMed] [Google Scholar]
- 967.Sanz ML, Sanchez G, Gamboa PM, et al. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne. Clin Exp Allergy. 2001;31:1007–1013. [DOI] [PubMed] [Google Scholar]
- 968.Ocmant A, Peignois Y, Mulier S, Hanssens L, Michils A, Schandene L. Flow cytometry for basophil activation markers: the measurement of CD203c up-regulation is as reliable as CD63 expression in the diagnosis of cat allergy. J Immunol Methods. 2007;320:40–48. [DOI] [PubMed] [Google Scholar]
- 969.Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. 2009;64:811–814. [DOI] [PubMed] [Google Scholar]
- 970.Nopp A, Cardell LO, Johansson SG. CD-sens can be a reliable and easy-to-use complement in the diagnosis of allergic rhinitis. Int Arch Allergy Immunol. 2013;161:87–90. [DOI] [PubMed] [Google Scholar]
- 971.Schmid JM, Wurtzen PA, Dahl R, Hoffmann HJ. Early improvement in basophil sensitivity predicts symptom relief with grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:741–744.e5. [DOI] [PubMed] [Google Scholar]
- 972.Ozdemir SK, Guloglu D, Sin BA, Elhan AH, Ikinciogullari A, Misirligil Z. Reliability of basophil activation test using CD203c expression in diagnosis of pollen allergy. Am J Rhinol Allergy. 2011;25:e225–e231. [DOI] [PubMed] [Google Scholar]
- 973.Leśniak M, Dyga W, Porebski G, Czarnobilska E. [Basophil activation test—a practical approach to diagnosis of common respiratory allergy]. Przegl Lek. 2015;72:725–730. Polish. [PubMed] [Google Scholar]
- 974.Lesniak M, Dyga W, Rusinek B, Mazur M, Czarnobilska E. Comparison of the basophil activation test versus the nasal provocation test in establishing eligibility for specific immunotherapy. Pol Arch Med Wewn. 2016;126:521–529. [DOI] [PubMed] [Google Scholar]
- 975.Nopp A, Johansson SG, Ankerst J, et al. Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy. 2006;61:298–302. [DOI] [PubMed] [Google Scholar]
- 976.Zidarn M, Kosnik M, Silar M, Grahek A, Korosec P. Rhinitis symptoms caused by grass pollen are associated with elevated basophile allergen sensitivity and a larger grass-specific immunoglobulin E fraction. Clin Exp Allergy. 2012;42:49–57. [DOI] [PubMed] [Google Scholar]
- 977.Zidarn M, Kosnik M, Silar M, Bajrovic N, Korosec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen-specific basophil response; a real-life, nonrandomized controlled study. Allergy. 2015;70:547–555. [DOI] [PubMed] [Google Scholar]
- 978.Van Overtvelt L, Baron-Bodo V, Horiot S, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011;66:1530–1537. [DOI] [PubMed] [Google Scholar]
- 979.Ando N, Nakamura Y, Ishimaru K, et al. Allergen-specific basophil reactivity exhibits daily variations in seasonal allergic rhinitis. Allergy. 2015;70:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. [DOI] [PubMed] [Google Scholar]
- 981.Sastre J Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40:1442–1460. [DOI] [PubMed] [Google Scholar]
- 982.Sastre-Ibanez M, Sastre J. Molecular allergy diagnosis for the clinical characterization of asthma. Expert Rev Mol Diagn. 2015;15:789–799. [DOI] [PubMed] [Google Scholar]
- 983.Canonica GW, Ansotegui IJ, Pawankar R, et al. A WAO-ARIA-GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Sastre J, Sastre-Ibanez M. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2016;16:565–570. [DOI] [PubMed] [Google Scholar]
- 985.Sastre J Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol. 2013;13:646–650. [DOI] [PubMed] [Google Scholar]
- 986.Scala E, Abeni D, Pomponi D, et al. Ole e 1, Ole e 7, and Ole e 9: identifying distinct clinical subsets of olive tree-allergic patients. J Allergy Clin Immunol. 2016;137:629–631.e3. [DOI] [PubMed] [Google Scholar]
- 987.Sastre J, Rodriguez F, Campo P, Laffond E, Marin A, Alonso MD. Adverse reactions to immunotherapy are associated with different patterns of sensitization to grass allergens. Allergy. 2015;70:598–600. [DOI] [PubMed] [Google Scholar]
- 988.Bronnert M, Mancini J, Birnbaum J, et al. Component-resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. 2012;42:1406–1415. [DOI] [PubMed] [Google Scholar]
- 989.Barber D, Arias J, Boquete M, et al. Analysis of mite allergic patients in a diverse territory by improved diagnostic tools. Clin Exp Allergy. 2012;42:1129–1138. [DOI] [PubMed] [Google Scholar]
- 990.Carvalho Kdos A, de Melo-Neto OP, Magalhaes FB, et al. Blomia tropicalis Blo t 5 and Blo t 21 recombinant allergens might confer higher specificity to serodiagnostic assays than whole mite extract. BMC Immunol. 2013;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. [DOI] [PubMed] [Google Scholar]
- 992.Gamez C, Sanchez-Garcia S, Ibanez MD, et al. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy. 2011;66:1375–1383. [DOI] [PubMed] [Google Scholar]
- 993.Saarelainen S, Taivainen A, Rytkonen-Nissinen M, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004;34:1576–1582. [DOI] [PubMed] [Google Scholar]
- 994.Mattsson L, Lundgren T, Everberg H, Larsson H, Lidholm J. Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol. 2009;123:362–368. [DOI] [PubMed] [Google Scholar]
- 995.Uriarte SA, Sastre J. Clinical relevance of molecular diagnosis in pet allergy. Allergy. 2016;71:1066–1068. [DOI] [PubMed] [Google Scholar]
- 996.Eder K, Becker S, San Nicolo M, Berghaus A, Groger M. Usefulness of component resolved analysis of cat allergy in routine clinical practice. Allergy Asthma Clin Immunol. 2016;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Cabanas R, Lopez-Serrano MC, Carreira J, et al. Importance of albumin in cross-reactivity among cat, dog and horse allergens. J Investig Allergol Clin Immunol. 2000;10:71–77. [PubMed] [Google Scholar]
- 998.Smith W, Butler AJ, Hazell LA, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–1738. [DOI] [PubMed] [Google Scholar]
- 999.Saarelainen S, Rytkonen-Nissinen M, Rouvinen J, et al. Animal-derived lipocalin allergens exhibit immunoglobulin E cross-reactivity. Clin Exp Allergy. 2008;38:374–381. [DOI] [PubMed] [Google Scholar]
- 1000.Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107:419–428. [DOI] [PubMed] [Google Scholar]
- 1001.Postigo I, Gutierrez-Rodriguez A, Fernandez J, Guisantes JA, Sunen E, Martinez J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to Pleosporaceae. Clin Exp Allergy. 2011;41:443–451. [DOI] [PubMed] [Google Scholar]
- 1002.Barber D, Moreno C, Ledesma A, et al. Degree of olive pollen exposure and sensitization patterns. Clinical implications. J Investig Allergol Clin Immunol. 2007;17(Suppl 1):11–16. [PubMed] [Google Scholar]
- 1003.Letran A, Espinazo M, Moreno F. Measurement of IgE to pollen allergen components is helpful in selecting patients for immunotherapy. Ann Allergy Asthma Immunol. 2013;111:295–297. [DOI] [PubMed] [Google Scholar]
- 1004.Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Carroll WD, Lenney W, Child F, et al. Asthma severity and atopy: how clear is the relationship? Arch Dis Child. 2006;91:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Simpson BM, Custovic A, Simpson A, et al. NAC Manchester Asthma and Allergy Study (NAC-MAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy. 2001;31:391–399. [DOI] [PubMed] [Google Scholar]
- 1007.Dreborg S, Frew A. Position paper: allergen standardization and skin tests. Allergy. 1993;48:49–82. [PubMed] [Google Scholar]
- 1008.Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. [DOI] [PubMed] [Google Scholar]
- 1009.Del Giacco SR, Bakirtas A, Bel E, et al. Allergy in severe asthma. Allergy. 2017;72:207–220. [DOI] [PubMed] [Google Scholar]
- 1010.Roberts G, Ollert M, Aalberse R, et al. A new framework for the interpretation of IgE sensitization tests. Allergy. 2016;71:1540–1551. [DOI] [PubMed] [Google Scholar]
- 1011.Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–1116. [DOI] [PubMed] [Google Scholar]
- 1012.Custovic A, Johnston SL, Pavord I, et al. EAACI position statement on asthma exacerbations and severe asthma. Allergy. 2013;68:1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013;13:110–117. [DOI] [PubMed] [Google Scholar]
- 1014.Patelis A, Borres MP, Kober A, Berthold M. Multiplex component-based allergen microarray in recent clinical studies. Clin Exp Allergy. 2016;46:1022–1032. [DOI] [PubMed] [Google Scholar]
- 1015.Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Gronlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. 1999;29:896–904. [DOI] [PubMed] [Google Scholar]
- 1016.Asarnoj A, Hamsten C, Waden K, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. 2016;137:813–821.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Prosperi MC, Belgrave D, Buchan I, Simpson A, Custovic A. Challenges in interpreting allergen microarrays in relation to clinical symptoms: a machine learning approach. Pediatr Allergy Immunol. 2014;25:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Simpson A, Lazic N, Belgrave DC, et al. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol. 2015;136:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1019.Custovic A, Sonntag HJ, Buchan IE, Belgrave D, Simpson A, Prosperi MC. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136:1645–1652.e8. [DOI] [PubMed] [Google Scholar]
- 1020.Posa D, Perna S, Resch Y, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139:541–549.e8. [DOI] [PubMed] [Google Scholar]
- 1021.Custovic A, Lazic N, Simpson A. Pediatric asthma and development of atopy. Curr Opin Allergy Clin Immunol. 2013;13:173–180. [DOI] [PubMed] [Google Scholar]
- 1022.Lazic N, Roberts G, Custovic A, et al. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68:764–770. [DOI] [PubMed] [Google Scholar]
- 1023.Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. [DOI] [PubMed] [Google Scholar]
- 1024.Holt PG, Strickland D, Bosco A, et al. Distinguishing benign from pathologic TH2 immunity in atopic children. J Allergy Clin Immunol. 2016;137:379–387. [DOI] [PubMed] [Google Scholar]
- 1025.Rosner-Friese K, Kaul S, Vieths S, Pfaar O. Environmental exposure chambers in allergen immunotherapy trials: current status and clinical validation needs. J Allergy Clin Immunol. 2015;135:636–643. [DOI] [PubMed] [Google Scholar]
- 1026.Werfel T, Heratizadeh A, Niebuhr M, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136:96–103.e9. [DOI] [PubMed] [Google Scholar]
- 1027.Badorrek P, Dick M, Emmert L, et al. Pollen starch granules in bronchial inflammation. Ann Allergy Asthma Immunol. 2012;109:208–214.e6. [DOI] [PubMed] [Google Scholar]
- 1028.Ahuja SK, Manoharan MS, Harper NL, et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol. 2017;139:844–854. [DOI] [PubMed] [Google Scholar]
- 1029.Ellis AK, Steacy LM, Hobsbawn B, Conway CE, Walker TJ. Clinical validation of controlled grass pollen challenge in the Environmental Exposure Unit (EEU). Allergy Asthma Clin Immunol. 2015;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Ellis AK, Soliman M, Steacy LM, Adams DE, Hobsbawn B, Walker TJ. Clinical validation of controlled exposure to birch pollen in the Environmental Exposure Unit (EEU). Allergy Asthma Clin Immunol. 2016;12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Enomoto T, Ide T, Ogino S. Construction of an environmental exposure unit and investigation of the effects of cetirizine hydrochloride on symptoms of cedar pollinosis in Japan. J Investig Allergol Clin Immunol. 2007;17:173–181. [PubMed] [Google Scholar]
- 1032.Hashiguchi K, Tang H, Fujita T, et al. Validation study of the OHIO Chamber in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2009;149:141–149. [DOI] [PubMed] [Google Scholar]
- 1033.Jacobs RL, Ramirez DA, Andrews CP. Validation of the biogenics research chamber for Juniperus ashei (mountain cedar) pollen. Ann Allergy Asthma Immunol. 2011;107:133–138. [DOI] [PubMed] [Google Scholar]
- 1034.Krug N, Hohlfeld JM, Larbig M, et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003;33:1667–1674. [DOI] [PubMed] [Google Scholar]
- 1035.Lueer K, Biller H, Casper A, et al. Safety, efficacy and repeatability of a novel house dust mite allergen challenge technique in the Fraunhofer allergen challenge chamber. Allergy. 2016;71:1693–1700. [DOI] [PubMed] [Google Scholar]
- 1036.Ronborg SM, Mosbech H, Poulsen LK. Exposure chamber for allergen challenge. A placebo-controlled, double-blind trial in house-dust-mite asthma. Allergy. 1997;52:821–828. [DOI] [PubMed] [Google Scholar]
- 1037.Zuberbier T, Abelson MB, Akdis CA, et al. Validation of the Global Allergy and Asthma European Network (GA2LEN) chamber for trials in allergy: innovation of a mobile allergen exposure chamber. J Allergy Clin Immunol. 2017;139:1158–1166. [DOI] [PubMed] [Google Scholar]
- 1038.Hohlfeld JM, Holland-Letz T, Larbig M, et al. Diagnostic value of outcome measures following allergen exposure in an environmental challenge chamber compared with natural conditions. Clin Exp Allergy. 2010;40:998–1006. [DOI] [PubMed] [Google Scholar]
- 1039.Krug N, Gupta A, Badorrek P, et al. Efficacy of the oral chemoattractant receptor homologous molecule on TH2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133:414–419. [DOI] [PubMed] [Google Scholar]
- 1040.Horak F, Jäger S, Nirnberger G, et al. Pharmacodynamic dose finding of dimetindene in a sustained release formulation. Arzneimittelforschung. 1993;43:1193–1195. [PubMed] [Google Scholar]
- 1041.Horak FF, Jäger S, Nirnberger G, et al. Dose-related control of allergic rhinitis symptoms by a H1-receptor antagonist. Finding the proper doses [correction of dosis] of dimethindene maleate in patients with allergic rhinitis. Int Arch Allergy Immunol. 1994;103:298–302. [DOI] [PubMed] [Google Scholar]
- 1042.Day JH, Briscoe MP, Ratz JD, Ellis AK, Yao R, Danzig M. Onset of action of loratadine/montelukast in seasonal allergic rhinitis subjects exposed to ragweed pollen in the Environmental Exposure Unit. Allergy Asthma Proc. 2009;30:270–276. [DOI] [PubMed] [Google Scholar]
- 1043.Horak F, Zieglmayer P, Zieglmayer R, Lemell P. Onset of action of loratadine/montelukast in seasonal allergic rhinitis patients exposed to grass pollen. Arzneimittelforschung. 2010;60:249–255. [DOI] [PubMed] [Google Scholar]
- 1044.Berkowitz RB, Woodworth GG, Lutz C, et al. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Ann Allergy Asthma Immunol. 2002;89:38–45. [DOI] [PubMed] [Google Scholar]
- 1045.Day JH, Briscoe MP, Rafeiro E, Ratz JD. Comparative clinical efficacy, onset and duration of action of levocetirizine and desloratadine for symptoms of seasonal allergic rhinitis in subjects evaluated in the Environmental Exposure Unit (EEU). Int J Clin Pract. 2004;58:109–118. [DOI] [PubMed] [Google Scholar]
- 1046.Horak F, Zieglmayer UP, Zieglmayer R, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22:151–157. [DOI] [PubMed] [Google Scholar]
- 1047.Day JH, Briscoe MP, Rafeiro E, Hewlett D Jr, Chapman D, Kramer B. Randomized double-blind comparison of cetirizine and fexofenadine after pollen challenge in the Environmental Exposure Unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25:59–68. [PubMed] [Google Scholar]
- 1048.Murdoch RD, Bareille P, Ignar D, et al. Once-daily dosing of levocabastine has comparable efficacy to twice-daily dosing in the treatment of allergic rhinitis assessed in an allergen challenge chamber. Int J Clin Pharmacol Ther. 2015;53:811–818. [DOI] [PubMed] [Google Scholar]
- 1049.Horak F, Zieglmayer PU, Zieglmayer R, Kavina A, Lemell P. Levocetirizine has a longer duration of action on improving total nasal symptoms score than fexofenadine after single administration. Br J Clin Pharmacol. 2005;60:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Badorrek P, Dick M, Schauerte A, et al. A combination of cetirizine and pseudoephedrine has therapeutic benefits when compared to single drug treatment in allergic rhinitis. Int J Clin Pharmacol Ther. 2009;47:71–77. [DOI] [PubMed] [Google Scholar]
- 1051.Barchuk WT, Salapatek AM, Ge T, D’Angelo P, Liu X. A proof-of-concept study of the effect of a novel H3-receptor antagonist in allergen-induced nasal congestion. J Allergy Clin Immunol. 2013;132:838–846.e6. [DOI] [PubMed] [Google Scholar]
- 1052.Horak F, Toth J, Marks B, et al. Efficacy and safety relative to placebo of an oral formulation of cetirizine and sustained-release pseudoephedrine in the management of nasal congestion. Allergy. 1998;53:849–856. [DOI] [PubMed] [Google Scholar]
- 1053.Yonekura S, Okamoto Y, Yamamoto H, et al. Randomized double-blind study of prophylactic treatment with an antihistamine for seasonal allergic rhinitis. Int Arch Allergy Immunol. 2013;162:71–78. [DOI] [PubMed] [Google Scholar]
- 1054.Krug N, Hohlfeld JM, Geldmacher H, et al. Effect of loteprednol etabonate nasal spray suspension on seasonal allergic rhinitis assessed by allergen challenge in an environmental exposure unit. Allergy. 2005;60:354–359. [DOI] [PubMed] [Google Scholar]
- 1055.Salapatek AM, Patel P, Gopalan G, Varghese ST. Mometasone furoate nasal spray provides early, continuing relief of nasal congestion and improves nasal patency in allergic patients. Am J Rhinol Allergy. 2010;24:433–438. [DOI] [PubMed] [Google Scholar]
- 1056.Zieglmayer P, Zieglmayer R, Bareille P, Rousell V, Salmon E, Horak F. Fluticasone furoate versus placebo in symptoms of grass-pollen allergic rhinitis induced by exposure in the Vienna Challenge Chamber. Curr Med Res Opin. 2008;24:1833–1840. [DOI] [PubMed] [Google Scholar]
- 1057.Bareille P, Murdoch RD, Denyer J, et al. The effects of a TRPV1 antagonist, SB-705498, in the treatment of seasonal allergic rhinitis. Int J Clin Pharmacol Ther. 2013;51:576–584. [DOI] [PubMed] [Google Scholar]
- 1058.Corren J, Wood RA, Patel D, et al. Effects of omalizumab on changes in pulmonary function induced by controlled cat room challenge. J Allergy Clin Immunol. 2011;127:398–405. [DOI] [PubMed] [Google Scholar]
- 1059.Horak F VTX-1463, a novel TLR8 agonist for the treatment of allergic rhinitis. Expert Opin Investig Drugs. 2011;20:981–986. [DOI] [PubMed] [Google Scholar]
- 1060.Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–1579. [DOI] [PubMed] [Google Scholar]
- 1061.Xiao JZ, Kondo S, Yanagisawa N, et al. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int. 2007;56:67–75. [DOI] [PubMed] [Google Scholar]
- 1062.Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–477.e1. [DOI] [PubMed] [Google Scholar]
- 1063.Meyer W, Narkus A, Salapatek AM, Hafner D. Double-blind, placebo-controlled, dose-ranging study of new recombinant hypoallergenic Bet v 1 in an environmental exposure chamber. Allergy. 2013;68:724–731. [DOI] [PubMed] [Google Scholar]
- 1064.Nolte H, Maloney J, Nelson HS, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–1501.e6. [DOI] [PubMed] [Google Scholar]
- 1065.Patel D, Couroux P, Hickey P, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–109.e7. [DOI] [PubMed] [Google Scholar]
- 1066.Patel P, Holdich T, Fischer von Weikersthal-Drachenberg KJ, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133:121–129.e2. [DOI] [PubMed] [Google Scholar]
- 1067.Committee for Medicinal Products for Human Use (CHMP). Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. Pre-authorisation evaluation of medicines for human use. November 20, 2008. Doc. Ref. CHMP/EWP/18504/2006. European Medicines Agency; 2008:S1–S13. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf Accessed December 19, 2017.
- 1068.Department of Health U.S. and Human Services. U.S. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for Industry. Allergic rhinitis: clinical development programs for drug products. Draft: February 2016. Clinical/Medical Revision 1. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071293.pdf. Accessed December 19, 2017.
- 1069.Agache I, Bilo M, Braunstahl GJ, et al. In vivo diagnosis of allergic diseases—allergen provocation tests. Allergy. 2015;70:355–365. [DOI] [PubMed] [Google Scholar]
- 1070.Riechelmann H, Epple B, Gropper G. Comparison of conjunctival and nasal provocation test in allergic rhinitis to house dust mite. Int Arch Allergy Immunol. 2003;130:51–59. [DOI] [PubMed] [Google Scholar]
- 1071.Dordal MT, Lluch-Bernal M, Sánchez MC, et al. ; SEAIC Rhinoconjunctivitis Committee. Allergen-specific nasal provocation testing: review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J Investig Allergol Clin Immunol. 2011;21:1–12. [PubMed] [Google Scholar]
- 1072.Malm L, Gerth van Wijk R, Bachert C. Guidelines for nasal provocations with aspects on nasal patency, airflow, and airflow resistance. International Committee on Objective Assessment of the Nasal Airways, International Rhinologic Society. Rhinology. 2000;38:1–6. [PubMed] [Google Scholar]
- 1073.Gosepath J, Amedee RG, Mann WJ. Nasal provocation testing as an international standard for evaluation of allergic and nonallergic rhinitis. Laryngoscope. 2005;115:512–516. [DOI] [PubMed] [Google Scholar]
- 1074.Casset A, Khayath N, de Blay F. How in vitro assays contribute to allergy diagnosis. Curr Allergy Asthma Rep. 2016;16:82. [DOI] [PubMed] [Google Scholar]
- 1075.Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70:1393–1405. [DOI] [PubMed] [Google Scholar]
- 1076.Airaksinen L, Tuomi T, Vanhanen M, Voutilainen R, Toskala E. Use of nasal provocation test in the diagnostics of occupational rhinitis. Rhinology. 2007;45:40–46. [PubMed] [Google Scholar]
- 1077.Campo P, Salas M, Blanca-Lopez N, Rondon C. Local allergic rhinitis. Immunol Allergy Clin North Am. 2016;36:321–332. [DOI] [PubMed] [Google Scholar]
- 1078.Incorvaia C, Fuiano N, Canonica GW. Seeking allergy when it hides: which are the best fitting tests? World Allergy Organ J. 2013;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079.Rondon C, Campo P, Herrera R, et al. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J Allergy Clin Immunol. 2011;128:1192–1197. [DOI] [PubMed] [Google Scholar]
- 1080.Moller C, Bjorksten B, Nilsson G, Dreborg S. The precision of the conjunctival provocation test. Allergy. 1984;39:37–41. [DOI] [PubMed] [Google Scholar]
- 1081.Bertel F, Mortemousque B, Sicard H, Andre C. [Conjunctival provocation test with Dermatophagoides pteronyssinus in the diagnosis of allergic conjunctivitis from house mites]. J Fr Ophtalmol. 2001;24:581–589. French. [PubMed] [Google Scholar]
- 1082.Fauquert JL, Jedrzejczak-Czechowicz M, Rondon C, et al. Conjunctival allergen provocation test: guidelines for daily practice. Allergy. 2017;72:43–54. [DOI] [PubMed] [Google Scholar]
- 1083.Agarwal G, Hernandez D, Citardi MJ, Fakhri S, Luong A. End-organ testing for allergic rhinitis with fungi is poorly correlated with fungal sensitivity. Otolaryngol Head Neck Surg. 2013;148:391–395. [DOI] [PubMed] [Google Scholar]
- 1084.Jang TY, Kim YH. Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy. 2015;29:e100–e104. [DOI] [PubMed] [Google Scholar]
- 1085.de Blay F, Doyen V, Lutz C, et al. A new, faster, and safe nasal provocation test method for diagnosing mite allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115:385–390.e1. [DOI] [PubMed] [Google Scholar]
- 1086.Krzych-Falta E, Furmanczyk K, Samolinski B. Specificity and sensitivity assessment of selected nasal provocation testing techniques. Postepy Dermatol Alergol. 2016;33:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. NASAL cytology: practical aspects and clinical relevance. Clin Exp Allergy. 2016;46:785–792. [DOI] [PubMed] [Google Scholar]
- 1088.Waecker NJ Jr, Shope TR, Weber PA, Buck ML, Domingo RC, Hooper DG. The Rhino-Probe nasal curette for detecting respiratory syncytial virus in children. Pediatr Infect Dis J. 1993;12:326–329. [DOI] [PubMed] [Google Scholar]
- 1089.Gelardi M, Passalacqua G, Fiorella ML, Quaranta N. Assessment of biofilm by nasal cytology in different forms of rhinitis and its functional correlations. Eur Ann Allergy Clin Immunol. 2013;45:25–29. [PubMed] [Google Scholar]
- 1090.Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. [DOI] [PubMed] [Google Scholar]
- 1091.Canakcioglu S, Tahamiler R, Saritzali G, et al. Evaluation of nasal cytology in subjects with chronic rhinitis: a 7-year study. Am J Otolaryngol. 2009;30:312–317. [DOI] [PubMed] [Google Scholar]
- 1092.Di Lorenzo G, Pacor ML, Amodio E, et al. Differences and similarities between allergic and nonallergic rhinitis in a large sample of adult patients with rhinitis symptoms. Int Arch Allergy Immunol. 2011;155:263–270. [DOI] [PubMed] [Google Scholar]
- 1093.Gelardi M, Ciprandi G, Incorvaia C, et al. Allergic rhinitis phenotypes based on mono-allergy or poly-allergy. Inflamm Res. 2015;64:373–375. [DOI] [PubMed] [Google Scholar]
- 1094.Gelardi M, Incorvaia C, Passalacqua G, Quaranta N, Frati F. The classification of allergic rhinitis and its cytological correlate. Allergy. 2011;66:1624–1625. [DOI] [PubMed] [Google Scholar]
- 1095.Gelardi M, Peroni DG, Incorvaia C, et al. Seasonal changes in nasal cytology in mite-allergic patients. J Inflamm Res. 2014;7:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1096.Shah R, McGrath KG. Chapter 6: Nonallergic rhinitis. Allergy Asthma Proc. 2012;33(Suppl 1):S19–S21. [DOI] [PubMed] [Google Scholar]
- 1097.Gelardi M, Luigi Marseglia G, Licari A, et al. Nasal cytology in children: recent advances. Ital J Pediatr. 2012;38:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Comoglu S, Keles N, Deger K. Inflammatory cell patterns in the nasal mucosa of patients with idiopathic rhinitis. Am J Rhinol Allergy. 2012;26:e55–e62. [DOI] [PubMed] [Google Scholar]
- 1099.Gelardi M “Overlapped” rhinitis: a real trap for rhinoallergologists. Eur Ann Allergy Clin Immunol. 2014;46:234–236. [PubMed] [Google Scholar]
- 1100.Gelardi M, Quaranta N, Passalacqua G. When sneezing indicates the cell type. Int Forum Allergy Rhinol. 2013;3:393–398. [DOI] [PubMed] [Google Scholar]
- 1101.Spector SL, English G, Jones L. Clinical and nasal biopsy response to treatment of perennial rhinitis. J Allergy Clin Immunol. 1980;66:129–137. [DOI] [PubMed] [Google Scholar]
- 1102.Howarth PH, Persson CG, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 2005;115:S414–S441. [DOI] [PubMed] [Google Scholar]
- 1103.Sivam A, Jeswani S, Reder L, et al. Olfactory cleft inflammation is present in seasonal allergic rhinitis and is reduced with intranasal steroids. Am J Rhinol Allergy. 2010;24:286–290. [DOI] [PubMed] [Google Scholar]
- 1104.Uller L, Emanuelsson CA, Andersson M, Erjefalt JS, Greiff L, Persson CG. Early phase resolution of mucosal eosinophilic inflammation in allergic rhinitis. Respir Res. 2010;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105.Yang SH, Yu CL, Chen YL, Chiao SL, Chen ML. Traditional Chinese medicine, Xin-yi-san, reduces nasal symptoms of patients with perennial allergic rhinitis by its diverse immunomodulatory effects. Int Immunopharmacol. 2010;10:951–958. [DOI] [PubMed] [Google Scholar]
- 1106.Asai K, Foley SC, Sumi Y, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergol Int. 2008;57:377–381. [DOI] [PubMed] [Google Scholar]
- 1107.Rak S, Heinrich C, Scheynius A. Comparison of nasal immunohistology in patients with seasonal rhinoconjunctivitis treated with topical steroids or specific allergen immunotherapy. Allergy. 2005;60:643–649. [DOI] [PubMed] [Google Scholar]
- 1108.Plewako H, Arvidsson M, Petruson K, et al. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002;110:68–71. [DOI] [PubMed] [Google Scholar]
- 1109.Pullerits T, Linden A, Malmhall C, Lotvall J. Effect of seasonal allergen exposure on mucosal IL-16 and CD4+ cells in patients with allergic rhinitis. Allergy. 2001;56:871–877. [PubMed] [Google Scholar]
- 1110.Wilson DR, Nouri-Aria KT, Walker SM, et al. Grass pollen immunotherapy: symptomatic improvement correlates with reductions in eosinophils and IL-5 mRNA expression in the nasal mucosa during the pollen season. J Allergy Clin Immunol. 2001;107:971–976. [DOI] [PubMed] [Google Scholar]
- 1111.Kujundzic M, Babarovic E, Petkovic M, Pavlovic-Ruzic I, Coklo M, Zamolo G. Mometasone furoate and nasal vascularisation in allergic patients. Coll Antropol. 2013;37:127–130. [PubMed] [Google Scholar]
- 1112.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–1472.e1. [DOI] [PubMed] [Google Scholar]
- 1113.Till SJ, Jacobson MR, O’Brien F, et al. Recruitment of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic rhinitis and effects of topical corticosteroid therapy. Allergy. 2001;56:126–131. [DOI] [PubMed] [Google Scholar]
- 1114.Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–165. [DOI] [PubMed] [Google Scholar]
- 1115.Lund V, Aaronsen D, Bousquet J, Dahl R, Davies RJ, Durham S. International consensus report on the diagnosis and management of rhinitis. Allergy. 1994;49:S1–S34. [PubMed] [Google Scholar]
- 1116.Mackay IS, Durham SR. ABC of allergies. Perennial rhinitis. BMJ. 1998;316:917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1117.Woodcock A, Custovic A. ABC of allergies. Avoiding exposure to indoor allergens. BMJ. 1998;316:1075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118.Krouse HJ. Environmental controls and avoidance measures. Int Forum Allergy Rhinol. 2014;4(Suppl 2):S32–S34. [DOI] [PubMed] [Google Scholar]
- 1119.Geller-Bernstein C, Pibourdin JM, Dornelas A, Fondarai J. Efficacy of the acaricide: acardust for the prevention of asthma and rhinitis due to dust mite allergy, in children. Allerg Immunol (Paris). 1995;27:147–154. [PubMed] [Google Scholar]
- 1120.Ghazala L, Schmid F, Helbling A, Pichler WJ, Pichler CE. Efficacy of house dust mite and allergen impermeable encasings in patients with house dust mite allergy. Allergologie. 2004;27:26–34. [Google Scholar]
- 1121.Kniest FM, Wolfs BJ, Vos H, et al. Mechanisms and patient compliance of dust-mite avoidance regimens in dwellings of mite-allergic rhinitic patients. Clin Exp Allergy. 1992;22:681–689. [DOI] [PubMed] [Google Scholar]
- 1122.Moon JS, Choi SO. Environmental controls in reducing house dust mites and nasal symptoms in patients with allergic rhinitis. Yonsei Med J. 1999;40:238–243. [DOI] [PubMed] [Google Scholar]
- 1123.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85:1050–1057. [DOI] [PubMed] [Google Scholar]
- 1124.Terreehorst I, Hak E, Oosting AJ, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. 2003;349:237–246. [DOI] [PubMed] [Google Scholar]
- 1125.Antonicelli L, Bilo MB, Pucci S, Schou C, Bonifazi F. Efficacy of an air-cleaning device equipped with a high efficiency particulate air filter in house dust miterespiratoryallergy. Allergy.1991;46:594–600. [DOI] [PubMed] [Google Scholar]
- 1126.Sheikh A, Hurwitz B, Nurmatov U, van Schayck CP. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Stillerman A, Nachtsheim C, Li W, Albrecht M, Waldman J. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Ann Allergy Asthma Immunol. 2010;104:440–449. [DOI] [PubMed] [Google Scholar]
- 1128.Brehler R, Kniest FM. Encasing study in mite-allergic patients: one-year, double-blind, placebo and environmental-controlled investigation. Allergy and Clinical Immunology International - Journal of the World Allergy Organization. 2006;18:15–19. [Google Scholar]
- 1129.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. [DOI] [PubMed] [Google Scholar]
- 1130.Chew GL. Assessment of environmental cockroach allergen exposure. Curr Allergy Asthma Rep. 2012;12:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Coleman AT, Rettiganti M, Bai S, Brown RH, Perry TT. Mouse and cockroach exposure in rural Arkansas Delta region homes. Ann Allergy Asthma Immunol. 2014;112:256–260. [DOI] [PubMed] [Google Scholar]
- 1132.Le Cann P, Paulus H, Glorennec P, Le Bot B, Frain S, Gangneux JP. Home environmental interventions for the prevention or control of allergic and respiratory diseases: what really works. J Allergy Clin Immunol Pract. 2017;5:66–79. [DOI] [PubMed] [Google Scholar]
- 1133.Sever ML, Arbes SJ Jr, Gore JC, et al. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J Allergy Clin Immunol. 2007;120:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1134.McConnell R, Milam J, Richardson J, et al. Educational intervention to control cockroach allergen exposure in the homes of hispanic children in Los Angeles: results of the La Casa study. Clin Exp Allergy. 2005;35:426–433. [DOI] [PubMed] [Google Scholar]
- 1135.Arbes SJ Jr, Sever M, Mehta J, et al. Abatement of cockroach allergens (Bla g 1 and Bla g 2) in low-income, urban housing: month 12 continuation results. J Allergy Clin Immunol. 2004;113:109–114. [DOI] [PubMed] [Google Scholar]
- 1136.McConnell R, Jones C, Milam J, et al. Cockroach counts and house dust allergen concentrations after professional cockroach control and cleaning. Ann Allergy Asthma Immunol. 2003;91:546–552. [DOI] [PubMed] [Google Scholar]
- 1137.Wood RA, Eggleston PA, Rand C, Nixon WJ, Kanchanaraksa S. Cockroach allergen abatement with extermination and sodium hypochlorite cleaning in inner-city homes. Ann Allergy Asthma Immunol. 2001;87:60–64. [DOI] [PubMed] [Google Scholar]
- 1138.Gergen PJ, Mortimer KM, Eggleston PA, et al. Results of the National Cooperative Inner-City Asthma Study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner-city homes. J Allergy Clin Immunol. 1999;103:501–506. [DOI] [PubMed] [Google Scholar]
- 1139.Eggleston PA, Wood RA, Rand C, Nixon WJ, Chen PH, Lukk P. Removal of cockroach allergen from inner-city homes. J Allergy Clin Immunol. 1999;104:842–846. [DOI] [PubMed] [Google Scholar]
- 1140.Williams LW, Reinfried P, Brenner RJ. Cockroach extermination does not rapidly reduce allergen in settled dust. J Allergy Clin Immunol. 1999;104:702–703. [DOI] [PubMed] [Google Scholar]
- 1141.Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95:518–524. [DOI] [PubMed] [Google Scholar]
- 1142.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. [DOI] [PubMed] [Google Scholar]
- 1143.Sever ML, Salo PM, Haynes AK, Zeldin DC. Innercity environments and mitigation of cockroach allergen. Am J Prev Med. 2011;41:S55–S56. [DOI] [PubMed] [Google Scholar]
- 1144.Custovic A, Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update (in collaboration with GA(2)LEN). Allergy. 2005;60:1112–1115. [DOI] [PubMed] [Google Scholar]
- 1145.Portnoy J, Kennedy K, Sublett J, et al. Environmental assessment and exposure control: a practice parameter—furry animals. Ann Allergy Asthma Immunol. 2012;108:223.e1–223.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146.Sánchez J, Díez S, Cardona R. Pet avoidance in allergy cases: Is it possible to implement it? Biomedica. 2015;35:357–362. [DOI] [PubMed] [Google Scholar]
- 1147.Bjornsdottir US, Jakobinudottir S, Runarsdottir V, Juliusson S. The effect of reducing levels of cat allergen (Fel d 1) on clinical symptoms in patients with cat allergy. Ann Allergy Asthma Immunol. 2003;91:189–194. [DOI] [PubMed] [Google Scholar]
- 1148.Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158:115–120. [DOI] [PubMed] [Google Scholar]
- 1149.Avner DB, Perzanowski MS, Platts-Mills TA, Woodfolk JA. Evaluation of different techniques for washing cats: quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J Allergy Clin Immunol. 1997;100:307–312. [DOI] [PubMed] [Google Scholar]
- 1150.Hodson T, Custovic A, Simpson A, Chapman M, Woodcock A, Green R. Washing the dog reduces dog allergen levels, but the dog needs to be washed twice a week. J Allergy Clin Immunol. 1999;103:581–585. [DOI] [PubMed] [Google Scholar]
- 1151.Wood RA, Chapman MD, Adkinson NF Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83:730–734. [DOI] [PubMed] [Google Scholar]
- 1152.Vredegoor DW, Willemse T, Chapman MD, Heederik DJ, Krop EJ. Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. J Allergy Clin Immunol. 2012;130:904–909 e907. [DOI] [PubMed] [Google Scholar]
- 1153.Arshad SH. Environmental control for secondary prevention of asthma. Clin Exp Allergy. 2010;40:2–4. [DOI] [PubMed] [Google Scholar]
- 1154.National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. [DOI] [PubMed] [Google Scholar]
- 1155.Kalyoncu A A new approach to an old problem: controversial issues in seasonal rhinoconjunctivitis. J Allergy Ther. 2014;5:164–166. [Google Scholar]
- 1156.D’Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. [DOI] [PubMed] [Google Scholar]
- 1157.Bastl K, Berger M, Bergmann KC, Kmenta M, Berger U. The medical and scientific responsibility of pollen information services. Wien Klin Wochenschr. 2017;129:70–74. [DOI] [PubMed] [Google Scholar]
- 1158.Kiotseridis H, Cilio CM, Bjermer L, Tunsater A, Jacobsson H, Dahl A. Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin Transl Allergy. 2013;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Ferguson BJ. Environmental controls of allergies. Otolaryngol Clin North Am. 2008;41:411–417, viii-ix. [DOI] [PubMed] [Google Scholar]
- 1160.Reisacher WR. Allergy treatment: environmental control strategies. Otolaryngol Clin North Am. 2011;44:711–725, x. [DOI] [PubMed] [Google Scholar]
- 1161.Comert S, Karakaya G, Kalyoncu AF. Wraparound eyeglasses improve symptoms and quality of life in patients with seasonal allergic rhinoconjunctivitis. Int Forum Allergy Rhinol. 2016;6:722–730. [DOI] [PubMed] [Google Scholar]
- 1162.Kenney P, Hilberg O, Pedersen H, Nielsen OB, Sigsgaard T. Nasal filters for the treatment of allergic rhinitis: a randomized, double-blind, placebo-controlled crossover clinical trial. J Allergy Clin Immunol. 2014;133:1477–1480.e13. [DOI] [PubMed] [Google Scholar]
- 1163.Kenney P, Hilberg O, Laursen AC, Peel RG, Sigsgaard T. Preventive effect of nasal filters on allergic rhinitis: a randomized, double-blind, placebo-controlled crossover park study. J Allergy Clin Immunol. 2015;136:1566–1572.e5. [DOI] [PubMed] [Google Scholar]
- 1164.Ellenbecker MJ. Engineering controls as an intervention to reduce worker exposure. Am J Ind Med. 1996;29:303–307. [DOI] [PubMed] [Google Scholar]
- 1165.Castano R, Trudeau C, Castellanos L, Malo JL. Prospective outcome assessment of occupational rhinitis after removal from exposure. J Occup Environ Med. 2013;55:579–585. [DOI] [PubMed] [Google Scholar]
- 1166.Casale TB, Blaiss MS, Gelfand E, et al. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111:S835–S842. [DOI] [PubMed] [Google Scholar]
- 1167.Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. [DOI] [PubMed] [Google Scholar]
- 1168.Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–1536. [PubMed] [Google Scholar]
- 1169.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1170.Bousquet J, Bindslev-Jensen C, Canonica GW, et al. The ARIA/EAACI criteria for antihistamines: an assessment of the efficacy, safety and pharmacology of desloratadine. Allergy. 2004;59(Suppl 77):4–16. [DOI] [PubMed] [Google Scholar]
- 1171.Compalati E, Canonica GW. Efficacy and safety of rupatadine for allergic rhino-conjunctivitis: a systematic review of randomized, double-blind, placebo-controlled studies with meta-analysis. Curr Med Res Opin. 2013;29:1539–1551. [DOI] [PubMed] [Google Scholar]
- 1172.Blaiss MS. Cost-effectiveness of H1-antihistamines. Clin Allergy Immunol. 2002;17:319–336. [PubMed] [Google Scholar]
- 1173.Consumer Reports Best Buy Drugs&trade. Using the antihistamines to treat: allergies, hay fever, & hives. Comparing effectiveness, safety, and price. Yonkers, NY: Consumer Reports of United States, Inc.; 2013. https://www.consumerreports.org/content/dam/cro/news_articles/health/PDFs/Antihistamines_Full_Report.pdf. Accessed December 19, 2017. [Google Scholar]
- 1174.Ridolo E, Montagni M, Bonzano L, Incorvaia C, Canonica GW. Bilastine: new insight into antihistamine treatment. Clin Mol Allergy. 2015;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1175.Mullol J, Bousquet J, Bachert C, et al. Update on rupatadine in the management of allergic disorders. Allergy. 2015;70(Suppl 100):1–24. [DOI] [PubMed] [Google Scholar]
- 1176.Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.Mosges R, Konig V, Koberlein J. The effectiveness of modern antihistamines for treatment of allergic rhinitis—an IPD meta-analysis of 140,853 patients. Allergol Int. 2013;62:215–222. [DOI] [PubMed] [Google Scholar]
- 1178.Compalati E, Baena-Cagnani R, Penagos M, et al. Systematic review on the efficacy of fexofenadine in seasonal allergic rhinitis: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Arch Allergy Immunol. 2011;156:1–15. [DOI] [PubMed] [Google Scholar]
- 1179.Ferrer M Pharmacokinetic evaluation of levocetirizine. Expert Opin Drug Metab Toxicol. 2011;7:1035–1047. [DOI] [PubMed] [Google Scholar]
- 1180.Mosges R, Konig V, Koberlein J. The effectiveness of levocetirizine in comparison with loratadine in treatment of allergic rhinitis—a meta-analysis. Allergol Int. 2011;60:541–546. [DOI] [PubMed] [Google Scholar]
- 1181.Katiyar S, Prakash S. Pharmacological profile, efficacy and safety of rupatadine in allergic rhinitis. Prim Care Respir J. 2009;18:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Bachert C A review of the efficacy of desloratadine, fexofenadine, and levocetirizine in the treatment of nasal congestion in patients with allergic rhinitis. Clin Ther. 2009;31:921–944. [DOI] [PubMed] [Google Scholar]
- 1183.Bachert C, van Cauwenberge P. Desloratadine treatment for intermittent and persistent allergic rhinitis: a review. Clin Ther. 2007;29:1795–1802. [DOI] [PubMed] [Google Scholar]
- 1184.Canonica GW, Tarantini F, Compalati E, Penagos M. Efficacy of desloratadine in the treatment of allergic rhinitis: a meta-analysis of randomized, double-blind, controlled trials. Allergy. 2007;62:359–366. [DOI] [PubMed] [Google Scholar]
- 1185.Patou J, De Smedt H, van Cauwenberge P, Bachert C. Pathophysiology of nasal obstruction and meta-analysis of early and late effects of levocetirizine. Clin Exp Allergy. 2006;36:972–981. [DOI] [PubMed] [Google Scholar]
- 1186.Schenkel EJ. Effect of desloratadine on the control of morning symptoms in patients with seasonal and perennial allergic rhinitis. Allergy Asthma Proc. 2006;27:465–472. [DOI] [PubMed] [Google Scholar]
- 1187.Hore I, Georgalas C, Scadding G. Oral antihistamines for the symptom of nasal obstruction in persistent allergic rhinitis—a systematic review of randomized controlled trials. Clin Exp Allergy. 2005;35:207–212. [DOI] [PubMed] [Google Scholar]
- 1188.Passalacqua G, Canonica GW. A review of the evidence from comparative studies of levocetirizine and desloratadine for the symptoms of allergic rhinitis. Clin Ther. 2005;27:979–992. [DOI] [PubMed] [Google Scholar]
- 1189.Greisner WA 3rd. Onset of action for the relief of allergic rhinitis symptoms with second-generation antihistamines. Allergy Asthma Proc. 2004;25:81–83. [PubMed] [Google Scholar]
- 1190.Limon L, Kockler DR. Desloratadine: a nonsedating antihistamine. Ann Pharmacother. 2003;37:237–246; quiz 313-236. [DOI] [PubMed] [Google Scholar]
- 1191.Bojkowski CJ, Gibbs TG, Hellstern KH, Major EW, Mullinger B. Acrivastine in allergic rhinitis: a review of clinical experience. J Int Med Res. 1989;17(Suppl 2):54B–68B. [PubMed] [Google Scholar]
- 1192.Penston J, Wormsley KG. Adverse reactions and interactions with H2-receptor antagonists. Med Toxicol. 1986;1:192–216. [DOI] [PubMed] [Google Scholar]
- 1193.Wood-Baker R, Lau L, Howarth PH. Histamine and the nasal vasculature: the influence of H1 and H2-histamine receptor antagonism. Clin Otolaryngol Allied Sci. 1996;21:348–352. [DOI] [PubMed] [Google Scholar]
- 1194.Taylor-Clark T, Sodha R, Warner B, Foreman J. Histamine receptors that influence blockage of the normal human nasal airway. Br J Pharmacol. 2005;144:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Wang D, Clement P, Smitz J. Effect of H1 and H2 antagonists on nasal symptoms and mediator release in atopic patients after nasal allergen challenge during the pollen season. Acta Otolaryngol. 1996;116:91–96. [DOI] [PubMed] [Google Scholar]
- 1196.Juliusson S, Bende M. Effect of systemically administered H1- and H2-receptor antagonists on nasal blood flow as measured with laser Doppler flowmetry in a provoked allergic reaction. Rhinology. 1996;34:24–27. [PubMed] [Google Scholar]
- 1197.Brooks CD, Butler D, Metzler C. Effect of H2 blockade in the challenged allergic nose. J Allergy Clin Immunol. 1982;70:373–376. [DOI] [PubMed] [Google Scholar]
- 1198.Carpenter GB, Bunker-Soler AL, Nelson HS. Evaluation of combined H1- and H2-receptor blocking agents in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1983;71:412–417. [DOI] [PubMed] [Google Scholar]
- 1199.Carr WW, Ratner P, Munzel U, et al. Comparison of intranasal azelastine to intranasal fluticasone propionate for symptom control in moderate-to-severe seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33:450–458. [DOI] [PubMed] [Google Scholar]
- 1200.Han D, Chen L, Cheng L, et al. A multicenter randomized double-blind 2-week comparison study of azelastine nasal spray 0.1% versus levocabastine nasal spray 0.05% in patients with moderate-to-severe allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2011;73:260–265. [DOI] [PubMed] [Google Scholar]
- 1201.Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in patients with allergy to Texas mountain cedar pollen. Int Forum Allergy Rhinol. 2011;1:275–279. [DOI] [PubMed] [Google Scholar]
- 1202.Meltzer EO, Blaiss M, Fairchild CJ. Comprehensive report of olopatadine 0.6% nasal spray as treatment for children with seasonal allergic rhinitis. Allergy Asthma Proc. 2011;32:213–220. [DOI] [PubMed] [Google Scholar]
- 1203.Kalpaklioglu AF, Kavut AB. Comparison of azelastine versus triamcinolone nasal spray in allergic and nonallergic rhinitis. Am J Rhinol Allergy. 2010;24:29–33. [DOI] [PubMed] [Google Scholar]
- 1204.Berger WE, Ratner PH, Casale TB, Meltzer EO, Wall GM. Safety and efficacy of olopatadine hydrochloride nasal spray 0.6% in pediatric subjects with allergic rhinitis. Allergy Asthma Proc. 2009;30:612–623. [DOI] [PubMed] [Google Scholar]
- 1205.Bernstein JA, Prenner B, Ferguson BJ, Portnoy J, Wheeler WJ, Sacks HJ. Double-blind, placebo-controlled trial of reformulated azelastine nasal spray in patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2009;23:512–517. [DOI] [PubMed] [Google Scholar]
- 1206.Kaliner MA, Storms W, Tilles S, et al. Comparison of olopatadine 0.6% nasal spray versus fluticasone propionate 50 microg in the treatment of seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30:255–262. [DOI] [PubMed] [Google Scholar]
- 1207.Shah S, Berger W, Lumry W, La Force C, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray and azelastine 0.10% nasal spray in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30:628–633. [DOI] [PubMed] [Google Scholar]
- 1208.Shah SR, Nayak A, Ratner P, Roland P, Michael Wall G. Effects of olopatadine hydrochloride nasal spray 0.6% in the treatment of seasonal allergic rhinitis: a phase III, multicenter, randomized, double-blind, active- and placebo-controlled study in adolescents and adults. Clin Ther. 2009;31:99–107. [DOI] [PubMed] [Google Scholar]
- 1209.van Bavel J, Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30:512–518. [DOI] [PubMed] [Google Scholar]
- 1210.Meltzer EO, Garadi R, Laforce C, et al. Comparative study of sensory attributes of two antihistamine nasal sprays: olopatadine 0.6% and azelastine 0.1%. Allergy Asthma Proc. 2008;29:659–668. [DOI] [PubMed] [Google Scholar]
- 1211.Pipkorn P, Costantini C, Reynolds C, et al. The effects of the nasal antihistamines olopatadine and azelastine in nasal allergen provocation. Ann Allergy Asthma Immunol. 2008;101:82–89. [DOI] [PubMed] [Google Scholar]
- 1212.Lumry W, Prenner B, Corren J, Wheeler W. Efficacy and safety of azelastine nasal spray at a dose of 1 spray per nostril twice daily. Ann Allergy Asthma Immunol. 2007;99:267–272. [DOI] [PubMed] [Google Scholar]
- 1213.Patel P, D’Andrea C, Sacks HJ. Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol. 2007;21:499–503. [DOI] [PubMed] [Google Scholar]
- 1214.Patel D, Garadi R, Brubaker M, et al. Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc. 2007;28:592–599. [DOI] [PubMed] [Google Scholar]
- 1215.Berger W, Hampel F Jr, Bernstein J, Shah S, Sacks H, Meltzer EO. Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:375–381. [DOI] [PubMed] [Google Scholar]
- 1216.Hampel FC Jr, Ratner PH, Amar NJ, et al. Improved quality of life among seasonal allergic rhinitis patients treated with olopatadine HCl nasal spray 0.4% and olopatadine HCl nasal spray 0.6% compared with vehicle placebo. Allergy Asthma Proc. 2006;27:202–207. [DOI] [PubMed] [Google Scholar]
- 1217.Corren J, Storms W, Bernstein J, et al. Effectiveness of azelastine nasal spray compared with oral cetirizine in patients with seasonal allergic rhinitis. Clin Ther. 2005;27:543–553. [DOI] [PubMed] [Google Scholar]
- 1218.Meltzer EO, Hampel FC, Ratner PH, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:600–606. [DOI] [PubMed] [Google Scholar]
- 1219.Ratner PH, Hampel FC, Amar NJ, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis to mountain cedar. Ann Allergy Asthma Immunol. 2005;95:474–479. [DOI] [PubMed] [Google Scholar]
- 1220.LaForce CF, Corren J, Wheeler WJ, Berger WE, Rhinitis Study Group. Efficacy of azelastine nasal spray in seasonal allergic rhinitis patients who remain symptomatic after treatment with fexofenadine. Ann Allergy Asthma Immunol. 2004;93:154–159. [DOI] [PubMed] [Google Scholar]
- 1221.Berger WE, White MV, Rhinitis Study Group. Efficacy of azelastine nasal spray in patients with an unsatisfactory response to loratadine. Ann Allergy Asthma Immunol. 2003;91:205–211. [DOI] [PubMed] [Google Scholar]
- 1222.Saengpanich S, Assanasen P, deTineo M, Haney L, Naclerio RM, Baroody FM. Effects of intranasal azelastine on the response to nasal allergen challenge. Laryngoscope. 2002;112:47–52. [DOI] [PubMed] [Google Scholar]
- 1223.Falser N, Wober W, Rahlfs VW, Baehre M. Comparative efficacy and safety of azelastine and levocabastine nasal sprays in patients with seasonal allergic rhinitis. Arzneimittelforschung. 2001;51:387–393. [DOI] [PubMed] [Google Scholar]
- 1224.Berlin JM, Golden SJ, Teets S, Lehman EB, Lucas T, Craig TJ. Efficacy of a steroid nasal spray compared with an antihistamine nasal spray in the treatment of perennial allergic rhinitis. J Am Osteopath Assoc. 2000;100:S8–S13. [PubMed] [Google Scholar]
- 1225.Golden S, Teets SJ, Lehman EB, et al. Effect of topical nasal azelastine on the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2000;85:53–57. [DOI] [PubMed] [Google Scholar]
- 1226.Berger WE, Fineman SM, Lieberman P, Miles RM. Double-blind trials of azelastine nasal spray monotherapy versus combination therapy with loratadine tablets and beclomethasone nasal spray in patients with seasonal allergic rhinitis. Rhinitis Study Groups. Ann Allergy Asthma Immunol. 1999;82:535–541. [DOI] [PubMed] [Google Scholar]
- 1227.Stern MA, Wade AG, Ridout SM, Cambell LM. Nasal budesonide offers superior symptom relief in perennial allergic rhinitis in comparison to nasal azelastine. Ann Allergy Asthma Immunol. 1998;81:354–358. [DOI] [PubMed] [Google Scholar]
- 1228.Herman D, Garay R, Le Gal M. A randomized double-blind placebo controlled study of azelastine nasal spray in children with perennial rhinitis. Int J Pediatr Otorhinolaryngol. 1997;39:1–8. [DOI] [PubMed] [Google Scholar]
- 1229.Newson-Smith G, Powell M, Baehre M, Garnham SP, MacMahon MT. A placebo controlled study comparing the efficacy of intranasal azelastine and beclomethasone in the treatment of seasonal allergic rhinitis. Eur Arch Otorhinolaryngol. 1997;254:236–241. [DOI] [PubMed] [Google Scholar]
- 1230.Weiler JM, Meltzer EO. Azelastine nasal spray as adjunctive therapy to azelastine tablets in the management of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:327–332. [DOI] [PubMed] [Google Scholar]
- 1231.LaForce C, Dockhorn RJ, Prenner BM, et al. Safety and efficacy of azelastine nasal spray (Astelin NS) for seasonal allergic rhinitis: a 4-week comparative multicenter trial. Ann Allergy Asthma Immunol. 1996;76:181–188. [DOI] [PubMed] [Google Scholar]
- 1232.Charpin D, Godard P, Garay RP, Baehre M, Herman D, Michel FB. A multicenter clinical study of the efficacy and tolerability of azelastine nasal spray in the treatment of seasonal allergic rhinitis: a comparison with oral cetirizine. Eur Arch Otorhinolaryngol. 1995;252:455–458. [DOI] [PubMed] [Google Scholar]
- 1233.Pelucchi A, Chiapparino A, Mastropasqua B, Marazzini L, Hernandez A, Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to methacholine in allergic rhinitis in response to grass pollens. J Allergy Clin Immunol. 1995;95:515–523. [DOI] [PubMed] [Google Scholar]
- 1234.Gastpar H, Nolte D, Aurich R, et al. Comparative efficacy of azelastine nasal spray and terfenadine in seasonal and perennial rhinitis. Allergy. 1994;49:152–158. [DOI] [PubMed] [Google Scholar]
- 1235.Meltzer EO, Weiler JM, Dockhorn RJ, Widlitz MD, Freitag JJ. Azelastine nasal spray in the management of seasonal allergic rhinitis. Ann Allergy. 1994;72:354–359. [PubMed] [Google Scholar]
- 1236.Passali D, Piragine F. A comparison of azelastine nasal spray and cetirizine tablets in the treatment of allergic rhinitis. J Int Med Res. 1994;22:17–23. [DOI] [PubMed] [Google Scholar]
- 1237.Ratner PH, Findlay SR, Hampel F Jr, van Bavel J, Widlitz MD, Freitag JJ. A double-blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitis. J Allergy Clin Immunol. 1994;94:818–825. [DOI] [PubMed] [Google Scholar]
- 1238.Davies RJ, Lund VJ, Harten-Ash VJ. The effect of intranasal azelastine and beclomethasone on the symptoms and signs of nasal allergy in patients with perennial allergic rhinitis. Rhinology. 1993;31:159–164. [PubMed] [Google Scholar]
- 1239.Dorow P, Aurich R, Petzold U. Efficacy and tolerability of azelastine nasal spray in patients with allergic rhinitis compared to placebo and budesonide. Arzneimittelforschung. 1993;43:909–912. [PubMed] [Google Scholar]
- 1240.Gambardella R A comparison of the efficacy of azelastine nasal spray and loratidine tablets in the treatment of seasonal allergic rhinitis. J Int Med Res. 1993;21:268–275. [DOI] [PubMed] [Google Scholar]
- 1241.Gastpar H, Aurich R, Petzold U, et al. Intranasal treatment of perennial allergic rhinitis. Comparison of azelastine nasal spray and budesonide nasal aerosol. Arzneimittelforschung. 1993;43:475–479. [PubMed] [Google Scholar]
- 1242.Bascom R, Pipkorn U, Lichtenstein LM, Naclerio RM. The influx of inflammatory cells into nasal washings during the late response to antigen challenge. Effect of systemic steroid pretreatment. Am Rev Respir Dis. 1988;138:406–412. [DOI] [PubMed] [Google Scholar]
- 1243.Bascom R, Pipkorn U, Proud D, et al. Major basic protein and eosinophil-derived neurotoxin concentrations in nasal-lavage fluid after antigen challenge: effect of systemic corticosteroids and relationship to eosinophil influx. J Allergy Clin Immunol. 1989;84:338–346. [DOI] [PubMed] [Google Scholar]
- 1244.Schwartz E, Levin L, Leibowitz H, et al. Oral cortisone therapy in ragweed hay fever. J Allergy. 1952;23:32–38. [DOI] [PubMed] [Google Scholar]
- 1245.Schiller IW, Lowell FC. Oral cortisone in the treatment of hay fever. J Allergy. 1953;24:297–301. [DOI] [PubMed] [Google Scholar]
- 1246.Schwartz E Oral hydrocortisone therapy in bronchial asthma and bay fever. J Allergy. 1954;25:112–119. [DOI] [PubMed] [Google Scholar]
- 1247.Brooks CD, Karl KJ, Francom SF. Oral methylprednisolone acetate (Medrol Tablets) for seasonal rhinitis: examination of dose and symptom response. J Clin Pharmacol. 1993;33:816–822. [DOI] [PubMed] [Google Scholar]
- 1248.Kwaselow A, McLean J, Busse W, et al. A comparison of intranasal and oral flunisolide in the therapy of allergic rhinitis. Evidence for a topical effect. Allergy. 1985;40:363–367. [DOI] [PubMed] [Google Scholar]
- 1249.Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013;40:277–281. [DOI] [PubMed] [Google Scholar]
- 1250.Kronholm A Injectable depot corticosteroid therapy in hay fever. J Int Med Res. 1979;7:314–317. [DOI] [PubMed] [Google Scholar]
- 1251.Ohlander BO, Hansson RE, Karlsson KE. A comparison of three injectable corticosteroids for the treatment of patients with seasonal hay fever. J Int Med Res. 1980;8:63–69. [DOI] [PubMed] [Google Scholar]
- 1252.Laursen LC, Faurschou P, Pals H, Svendsen UG, Weeke B. Intramuscular betamethasone dipropionate vs. oral prednisolone in hay fever patients. Allergy. 1987;42:168–172. [DOI] [PubMed] [Google Scholar]
- 1253.Laursen LC, Faurschou P, Munch EP. Intramuscular betamethasone dipropionate vs. topical beclomethasone dipropionate and placebo in hay fever. Allergy. 1988;43:420–424. [DOI] [PubMed] [Google Scholar]
- 1254.Borum P, Gronborg H, Mygind N. Seasonal allergic rhinitis and depot injection of a corticosteroid. Evaluation of the efficacy of medication early and late in the season based on detailed symptom recording. Allergy. 1987;42:26–32. [DOI] [PubMed] [Google Scholar]
- 1255.Aasbjerg K, Torp-Pedersen C, Vaag A, Backer V. Treating allergic rhinitis with depot-steroid injections increase risk of osteoporosis and diabetes. Respir Med. 2013;107:1852–1858. [DOI] [PubMed] [Google Scholar]
- 1256.Mygind N, Laursen LC, Dahl M. Systemic corticosteroid treatment for seasonal allergic rhinitis: a common but poorly documented therapy. Allergy. 2000;55:11–15. [DOI] [PubMed] [Google Scholar]
- 1257.Wall JW, Shure N. Intranasal cortisone; preliminary study. AMA Arch Otolaryngol. 1952;56:172–176. [PubMed] [Google Scholar]
- 1258.Sidi E, Tardif R. [Treatment of allergic rhinitis accompanied by eczema with hydrocortisone acetate injected into nasal mucous membrane]. Sem Hop. 1955;31:1922–1923. French. [PubMed] [Google Scholar]
- 1259.Simmons MW. Intranasal injection of corticosteroids. Calif Med. 1960;92:155–158. [PMC free article] [PubMed] [Google Scholar]
- 1260.Baker DC Jr, Strauss RB. Intranasal injections of long acting corticosteroids. Ann Otol Rhinol Laryngol. 1962;71:525–531. [DOI] [PubMed] [Google Scholar]
- 1261.Mabry RL. Intraturbinal steroid injection: indications, results, and complications. South Med J. 1978;71:789–791, 794. [DOI] [PubMed] [Google Scholar]
- 1262.Yang TY, Jung YG, Kim YH, Jang TY. A comparison of the effects of botulinum toxin A and steroid injection on nasal allergy. Otolaryngol Head Neck Surg. 2008;139:367–371. [DOI] [PubMed] [Google Scholar]
- 1263.Mabry RL. Intranasal corticosteroid injection: indications, technique, and complications. Otolaryngol Head Neck Surg (1979). 1979;87:207–211. [DOI] [PubMed] [Google Scholar]
- 1264.Rowe RJ, Dusler TW, Kinkella AM. Visual changes and triamcinolone. JAMA. 1967;201:333. [Google Scholar]
- 1265.Byers B Blindness secondary to steroid injections into the nasal turbinates. Arch Ophthalmol. 1979;97:79–80. [DOI] [PubMed] [Google Scholar]
- 1266.Martin PA, Church CA, Petti GH Jr, Hedayi R. Visual loss after intraturbinate steroid injection. Otolaryngol Head Neck Surg. 2003;128:280–281. [DOI] [PubMed] [Google Scholar]
- 1267.Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988;81:580–589. [PubMed] [Google Scholar]
- 1268.Meltzer EO, Jalowayski AA, Orgel HA, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102:39–49. [DOI] [PubMed] [Google Scholar]
- 1269.Baroody FM, Cruz AA, Lichtenstein LM, Kagey-Sobotka A, Proud D, Naclerio RM. Intranasal beclomethasone inhibits antigen-induced nasal hyperresponsiveness to histamine. J Allergy Clin Immunol. 1992;90:373–376. [DOI] [PubMed] [Google Scholar]
- 1270.Meyer P, Andersson M, Persson CG, Greiff L. Steroid-sensitive indices of airway inflammation in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2003;14:60–65. [DOI] [PubMed] [Google Scholar]
- 1271.Penagos M, Compalati E, Tarantini F, Baena-Cagnani CE, Passalacqua G, Canonica GW. Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta-analysis of randomized, double-blind, placebo-controlled, clinical trials. Allergy. 2008;63:1280–1291. [DOI] [PubMed] [Google Scholar]
- 1272.Rodrigo GJ, Neffen H. Efficacy of fluticasone furoate nasal spray vs. placebo for the treatment of ocular and nasal symptoms of allergic rhinitis: a systematic review. Clin Exp Allergy. 2011;41:160–170. [DOI] [PubMed] [Google Scholar]
- 1273.Herman H Once-daily administration of intranasal corticosteroids for allergic rhinitis: a comparative review of efficacy, safety, patient preference, and cost. Am J Rhinol. 2007;21:70–79. [DOI] [PubMed] [Google Scholar]
- 1274.Rachelefsky G, Farrar JR. A control model to evaluate pharmacotherapy for allergic rhinitis in children. JAMA Pediatr. 2013;167:380–386. [DOI] [PubMed] [Google Scholar]
- 1275.Craig TJ, Mende C, Hughes K, Kakumanu S, Lehman EB, Chinchilli V. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc. 2003;24:53–58. [PubMed] [Google Scholar]
- 1276.Meltzer EO, Munafo DA, Chung W, Gopalan G, Varghese ST. Intranasal mometasone furoate therapy for allergic rhinitis symptoms and rhinitis-disturbed sleep. Ann Allergy Asthma Immunol. 2010;105:65–74. [DOI] [PubMed] [Google Scholar]
- 1277.Meltzer EO. Formulation considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2007;98:12–21. [DOI] [PubMed] [Google Scholar]
- 1278.van Bavel JH, Ratner PH, Amar NJ, et al. Efficacy and safety of once-daily treatment with beclomethasone dipropionate nasalaerosol in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33:386–396. [DOI] [PubMed] [Google Scholar]
- 1279.Meltzer EO, Jacobs RL, LaForce CF, Kelley CL, Dunbar SA, Tantry SK. Safety and efficacy of once-daily treatment with beclomethasone dipropionate nasal aerosol in subjects with perennial allergic rhinitis. Allergy Asthma Proc. 2012;33:249–257. [DOI] [PubMed] [Google Scholar]
- 1280.Ratner PH, Andrews C, Martin B, et al. A study of the efficacy and safety of ciclesonide hydrofluoroalkane nasal aerosol in patients with seasonal allergic rhinitis from mountain cedar pollen. Allergy Asthma Proc. 2012;33:27–35. [DOI] [PubMed] [Google Scholar]
- 1281.LaForce C, van Bavel J, Meltzer EO, Wingertzahn MA. Efficacy and safety of ciclesonide hydrofluoroalkane nasal aerosol once daily for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2009;103:166–173. [DOI] [PubMed] [Google Scholar]
- 1282.Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Akerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105:489–494. [DOI] [PubMed] [Google Scholar]
- 1283.Fokkens WJ, Cserhati E, dos Santos JM, et al. Budesonide aqueous nasal spray is an effective treatment in children with perennial allergic rhinitis, with an onset of action within 12 hours. Ann Allergy Asthma Immunol. 2002;89:279–284. [DOI] [PubMed] [Google Scholar]
- 1284.Kaiser HB, Naclerio RM, Given J, Toler TN, Ellsworth A, Philpot EE. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119:1430–1437. [DOI] [PubMed] [Google Scholar]
- 1285.Day J, Carrillo T. Comparison of the efficacy of budesonide and fluticasone propionate aqueous nasal spray for once daily treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 1998;102:902–908. [DOI] [PubMed] [Google Scholar]
- 1286.Juniper EF, Guyatt GH, O’Byrne PM, Viveiros M. Aqueous beclomethasone diproprionate nasal spray: regular versus “as required” use in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1990;86:380–386. [DOI] [PubMed] [Google Scholar]
- 1287.Juniper EF, Guyatt GH, Archer B, Ferrie PJ. Aqueous beclomethasone dipropionate in the treatment of ragweed pollen-induced rhinitis: further exploration of “as needed” use. J Allergy Clin Immunol. 1993;92:66–72. [DOI] [PubMed] [Google Scholar]
- 1288.Jen A, Baroody F, de Tineo M, Haney L, Blair C, Naclerio R. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;105:732–738. [DOI] [PubMed] [Google Scholar]
- 1289.Dykewicz MS, Kaiser HB, Nathan RA, et al. Fluticasone propionate aqueous nasal spray improves nasal symptoms of seasonal allergic rhinitis when used as needed (prn). Ann Allergy Asthma Immunol. 2003;91:44–48. [DOI] [PubMed] [Google Scholar]
- 1290.DeWester J, Philpot EE, Westlund RE, Cook CK, Rickard KA. The efficacy of intranasal fluticasone propionate in the relief of ocular symptoms associated with seasonal allergic rhinitis. Allergy Asthma Proc. 2003;24:331–337. [PubMed] [Google Scholar]
- 1291.Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta-analysis. Allergy. 2011;66:686–693. [DOI] [PubMed] [Google Scholar]
- 1292.Ratner P, Van Bavel J, Mohar D, et al. Efficacy of daily intranasal fluticasone propionate on ocular symptoms associated with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2015;114:141–147. [DOI] [PubMed] [Google Scholar]
- 1293.Baroody FM, Shenaq D, DeTineo M, Wang J, Naclerio RM. Fluticasone furoate nasal spray reduces the nasal-ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol. 2009;123:1342–1348. [DOI] [PubMed] [Google Scholar]
- 1294.Keith PK, Scadding GK. Are intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhinitis? Curr Med Res Opin. 2009;25:2021–2041. [DOI] [PubMed] [Google Scholar]
- 1295.Taramarcaz P, Gibson PG. Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis. Cochrane Database Syst Rev. 2003;(4):CD003570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1296.Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy. 2013;68:569–579. [DOI] [PubMed] [Google Scholar]
- 1297.Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1298.Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89:479–484. [DOI] [PubMed] [Google Scholar]
- 1299.Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010;104:13–29. [DOI] [PubMed] [Google Scholar]
- 1300.Wilson AM, O’Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004;116:338–344. [DOI] [PubMed] [Google Scholar]
- 1301.Maspero JF, Rosenblut A, Finn A Jr, Lim J, Wu W, Philpot E. Safety and efficacy of fluticasone furoate in pediatric patients with perennial allergic rhinitis. Otolaryngol Head Neck Surg. 2008;138:30–37. [DOI] [PubMed] [Google Scholar]
- 1302.Meltzer EO, Tripathy I, Maspero JF, Wu W, Philpot E. Safety and tolerability of fluticasone furoate nasal spray once daily in paediatric patients aged 6–11 years with allergic rhinitis: subanalysis of three randomized, double-blind, placebo-controlled, multicentre studies. Clin Drug Investig. 2009;29:79–86. [DOI] [PubMed] [Google Scholar]
- 1303.Rosenblut A, Bardin PG, Muller B, et al. Long-term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitis. Allergy. 2007;62:1071–1077. [DOI] [PubMed] [Google Scholar]
- 1304.Ratner PH, Meltzer EO, Teper A. Mometasone furoate nasal spray is safe and effective for 1-year treatment of children with perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2009;73:651–657. [DOI] [PubMed] [Google Scholar]
- 1305.Verkerk MM, Bhatia D, Rimmer J, Earls P, Sacks R, Harvey RJ. Intranasal steroids and the myth of mucosal atrophy: a systematic review of original histological assessments. Am J Rhinol Allergy. 2015;29:3–18. [DOI] [PubMed] [Google Scholar]
- 1306.van As A, Bronsky EA, Dockhorn RJ, et al. Once daily fluticasone propionate is as effective for perennial allergic rhinitis as twice daily beclomethasone diproprionate. J Allergy Clin Immunol. 1993;91:1146–1154. [DOI] [PubMed] [Google Scholar]
- 1307.Brannan MD, Herron JM, Reidenberg P, Affrime MB. Lack of hypothalamic-pituitary-adrenal axis suppression with once-daily or twice-daily beclomethasone dipropionate aqueous nasal spray administered to patients with allergic rhinitis. Clin Ther. 1995;17:637–647. [DOI] [PubMed] [Google Scholar]
- 1308.Vargas R, Dockhorn RJ, Findlay SR, Korenblat PE, Field EA, Kral KM. Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic-pituitary-adrenal axis. J Allergy Clin Immunol. 1998;102:191–197. [DOI] [PubMed] [Google Scholar]
- 1309.Howland WC 3rd, Dockhorn R, Gillman S, et al. A comparison of effects of triamcinolone acetonide aqueous nasal spray, oral prednisone, and placebo on adrenocortical function in male patients with allergic rhinitis. J Allergy Clin Immunol. 1996;98:32–38. [DOI] [PubMed] [Google Scholar]
- 1310.Nayak AS, Ellis MH, Gross GN, et al. The effects of triamcinolone acetonide aqueous nasal spray on adrenocortical function in children with allergic rhinitis. J Allergy Clin Immunol. 1998;101:157–162. [DOI] [PubMed] [Google Scholar]
- 1311.Galant SP, Melamed IR, Nayak AS, et al. Lack of effect of fluticasone propionate aqueous nasal spray on the hypothalamic-pituitary-adrenal axis in 2- and 3-year-old patients. Pediatrics. 2003;112:96–100. [DOI] [PubMed] [Google Scholar]
- 1312.Kim K, Weiswasser M, Nave R, et al. Safety of once-daily ciclesonide nasal spray in children 2 to 5 years of age with perennial allergic rhinitis. Pediatr Asthma Allergy Immunol. 2007;20:229–242. [Google Scholar]
- 1313.Chervinsky P, Kunjibettu S, Miller DL, et al. Long-term safety and efficacy of intranasal ciclesonide in adult and adolescent patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2007;99:69–76. [DOI] [PubMed] [Google Scholar]
- 1314.Patel D, Ratner P, Clements D, Wu W, Faris M, Philpot E. Lack of effect on adult and adolescent hypothalamic-pituitary-adrenal axis function with use of fluticasone furoate nasal spray. Ann Allergy Asthma Immunol. 2008;100:490–496. [DOI] [PubMed] [Google Scholar]
- 1315.Weinstein S, Qaqundah P, Georges G, Nayak A. Efficacy and safety of triamcinolone acetonide aqueous nasal spray in children aged 2 to 5 years with perennial allergic rhinitis: a randomized, double-blind, placebo-controlled study with an open-label extension. Ann Allergy Asthma Immunol. 2009;102:339–347. [DOI] [PubMed] [Google Scholar]
- 1316.Tripathy I, Levy A, Ratner P, Clements D, Wu W, Philpot E. HPA axis safety of fluticasone furoate nasal spray once daily in children with perennial allergic rhinitis. Pediatr Allergy Immunol. 2009;20:287–294. [DOI] [PubMed] [Google Scholar]
- 1317.Hampel FC Jr, Nayak NA, Segall N, Small CJ, Li J, Tantry SK. No hypothalamic-pituitary-adrenal function effect with beclomethasone dipropionate nasal aerosol, based on 24-hour serum cortisol in pediatric allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115:137–142. [DOI] [PubMed] [Google Scholar]
- 1318.Liu A, Manche EE. Bilateral posterior subcapsular cataracts associated with long-term intranasal steroid use. J Cataract Refract Surg. 2011;37:1555–1558. [DOI] [PubMed] [Google Scholar]
- 1319.Ahmadi N, Snidvongs K, Kalish L, et al. Intranasal corticosteroids do not affect intraocular pressure or lens opacity: a systematic review of controlled trials. Rhinology. 2015;53:290–302. [DOI] [PubMed] [Google Scholar]
- 1320.Mener DJ, Shargorodsky J, Varadhan R, Lin SY. Topical intranasal corticosteroids and growth velocity in children: a meta-analysis. Int Forum Allergy Rhinol. 2015;5:95–103. [DOI] [PubMed] [Google Scholar]
- 1321.Mucha SM, deTineo M, Naclerio RM, Baroody FM. Comparison of montelukast and pseudoephedrine in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2006;132:164–172. [DOI] [PubMed] [Google Scholar]
- 1322.Horak F, Zieglmayer P, Zieglmayer R, et al. A placebo-controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2009;102:116–120. [DOI] [PubMed] [Google Scholar]
- 1323.Meltzer EO, Ratner PH, McGraw T. Oral phenylephrine HCl for nasal congestion in seasonal allergic rhinitis: a randomized, open-label, placebo-controlled study. J Allergy Clin Immunol Pract. 2015;3:702–708. [DOI] [PubMed] [Google Scholar]
- 1324.Salerno SM, Jackson JL, Berbano EP. The impact of oral phenylpropanolamine on blood pressure: a meta-analysis and review of the literature. J Hum Hypertens. 2005;19:643–652. [DOI] [PubMed] [Google Scholar]
- 1325.Salerno SM, Jackson JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart rate: a meta-analysis. Arch Intern Med. 2005;165:1686–1694. [DOI] [PubMed] [Google Scholar]
- 1326.Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–1832. [DOI] [PubMed] [Google Scholar]
- 1327.Vernacchio L, Kelly JP, Kaufman DW, Mitchell AA. Pseudoephedrine use among US children, 1999–2006: results from the Slone survey. Pediatrics. 2008;122:1299–1304. [DOI] [PubMed] [Google Scholar]
- 1328.Roberge RJ, Hirani KH, Rowland PL 3rd, Berkeley R, Krenzelok EP. Dextromethorphan- and pseudoephedrine-induced agitated psychosis and ataxia: case report. J Emerg Med. 1999;17:285–288. [DOI] [PubMed] [Google Scholar]
- 1329.Sauder KL, Brady WJ Jr, Hennes H. Visual hallucinations in a toddler: accidental ingestion of a sympathomimetic over-the-counter nasal decongestant. Am J Emerg Med. 1997;15:521–526. [DOI] [PubMed] [Google Scholar]
- 1330.Barnes ML, Biallosterski BT, Gray RD, Fardon TC, Lipworth BJ. Decongestant effects of nasal xylometazoline and mometasone furoate in persistent allergic rhinitis. Rhinology. 2005;43:291–295. [PubMed] [Google Scholar]
- 1331.Watanabe H, Foo TH, Djazaeri B, Duncombe P, Mackay IS, Durham SR. Oxymetazoline nasal spray three times daily for four weeks in normal subjects is not associated with rebound congestion or tachyphylaxis. Rhinology. 2003;41:167–174. [PubMed] [Google Scholar]
- 1332.Devillier P, Dreyfus JF, Demoly P, Calderon MA. A meta-analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen-induced seasonal allergic rhinoconjunctivitis. BMC Med. 2014;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333.Grainger J, Drake-Lee A. Montelukast in allergic rhinitis: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:360–367. [DOI] [PubMed] [Google Scholar]
- 1334.Rodrigo GJ, Yanez A. The role of antileukotriene therapy in seasonal allergic rhinitis: a systematic review of randomized trials. Ann Allergy Asthma Immunol. 2006;96:779–786. [DOI] [PubMed] [Google Scholar]
- 1335.Gonyeau MJ, Partisan AM. A clinical review of montelukast in the treatment of seasonal allergic rhinitis. Formulary. 2003;38:368–378. [Google Scholar]
- 1336.Endo S, Gotoh M, Okubo K, Hashiguchi K, Suzuki H, Masuyama K. Trial of pranlukast inhibitory effect for cedar exposure using an OHIO chamber. J Drug Assess. 2012;1:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1337.Wakabayashi K, Hashiguchi K, Kanzaki S, et al. Pranlukast dry syrup inhibits symptoms of Japanese cedar pollinosis in children using OHIO Chamber. Allergy Asthma Proc. 2012;33:102–109. [DOI] [PubMed] [Google Scholar]
- 1338.Day JH, Briscoe MP, Ratz JD. Efficacy of levocetirizine compared with montelukast in subjects with ragweed-induced seasonal allergic rhinitis in the Environmental Exposure Unit. Allergy Asthma Proc. 2008;29:304–312. [DOI] [PubMed] [Google Scholar]
- 1339.Patel P, Philip G, Yang W, et al. Randomized, double-blind, placebo-controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:551–557. [DOI] [PubMed] [Google Scholar]
- 1340.Chervinsky P, Philip G, Malice MP, et al. Montelukast for treating fall allergic rhinitis: effect of pollen exposure in 3 studies. Ann Allergy Asthma Immunol. 2004;92:367–373. [DOI] [PubMed] [Google Scholar]
- 1341.Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20:1549–1558. [DOI] [PubMed] [Google Scholar]
- 1342.van Adelsberg J, Philip G, Pedinoff AJ, et al. Montelukast improves symptoms of seasonal allergic rhinitis over a 4-week treatment period. Allergy. 2003;58:1268–1276. [DOI] [PubMed] [Google Scholar]
- 1343.van Adelsberg J, Philip G, LaForce CF, et al. Randomized controlled trial evaluating the clinical benefit of montelukast for treating spring seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90:214–222. [DOI] [PubMed] [Google Scholar]
- 1344.Philip G, Malmstrom K, Hampel FC, et al. Montelukast for treating seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32:1020–1028. [DOI] [PubMed] [Google Scholar]
- 1345.Ratner PH, Howland WC 3rd, Arastu R, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nighttime nasal symptoms of seasonal allergic rhinitis compared with montelukast. Ann Allergy Asthma Immunol. 2003;90:536–542. [DOI] [PubMed] [Google Scholar]
- 1346.Pullerits T, Praks L, Skoogh BE, Ani R, Lotvall J. Randomized placebo-controlled study comparing a leukotriene receptor antagonist and a nasal glucocorticoid in seasonal allergic rhinitis. Am J Respir Crit Care Med. 1999;159:1814–1818. [DOI] [PubMed] [Google Scholar]
- 1347.Goodman MJ, Jhaveri M, Saverno K, Meyer K, Nightengale B. Cost-effectiveness of second-generation antihistamines and montelukast in relieving allergic rhinitis nasal symptoms. Am Health Drug Benefits. 2008;1:26–34. [PMC free article] [PubMed] [Google Scholar]
- 1348.Jiang RS. Efficacy of a leukotriene receptor antagonist in the treatment of perennial allergic rhinitis. J Otolaryngol. 2006;35:117–121. [DOI] [PubMed] [Google Scholar]
- 1349.Altounyan RE. Review of clinical activity and mode of action of sodium cromoglycate. Clin Allergy. 1980;10 Suppl:481–489. [DOI] [PubMed] [Google Scholar]
- 1350.Kay AB, Walsh GM, Moqbel R, et al. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol. 1987;80:1–8. [DOI] [PubMed] [Google Scholar]
- 1351.Edwards AM. Chromones. Chem Immunol Allergy. 2014;100:317–322. [DOI] [PubMed] [Google Scholar]
- 1352.Kuriyama K, Hiyama Y, Nagatahira R, Okuda T, Saito K, Ito K. An antiallergic activity of disodium cromoglycate unrelated to mast cell activation. Agents Actions. 1986;18:473–478. [DOI] [PubMed] [Google Scholar]
- 1353.Murphy S, Kelly HW. Cromolyn sodium: a review of mechanisms and clinical use in asthma. Drug Intell Clin Pharm. 1987;21:22–35. [DOI] [PubMed] [Google Scholar]
- 1354.Holgate ST. Reflections on the mechanism(s) of action of sodium cromoglycate (Intal) and the role of mast cells in asthma. Respir Med. 1989;83(Suppl A):25–31. [DOI] [PubMed] [Google Scholar]
- 1355.NasalCrom. Cromolyn nasal. Drug monograph. Dosing. Epocrates, Inc.; 2017. http://online.epocrates.com/drugs/otcs/211701/NasalCrom/Dosing. Accessed December 19, 2017. [Google Scholar]
- 1356.Lejeune M, Lefebvre PP, Delvenne P, El-Shazly AE. Nasal sodium cromoglycate (Lomusol) modulates the early phase reaction of mild to moderate persistent allergic rhinitis in patients mono-sensitized to house dust mite: a preliminary study. Int Immunopharmacol. 2015;26:272–276. [DOI] [PubMed] [Google Scholar]
- 1357.Tandon MK, Strahan EG. Double-blind crossover trial comparing beclomethasone dipropionate and sodium cromoglycate in perennial allergic rhinitis. Clin Allergy. 1980;10:459–462. [DOI] [PubMed] [Google Scholar]
- 1358.McDowell MK, Spitz E. Treatment of chronic perennial allergic rhinitis: a double-blind trial of cromolyn sodium. Ann Allergy. 1977;39:169–174. [PubMed] [Google Scholar]
- 1359.Warland A, Kapstad B. The effect of disodium cromoglycate in perennial allergic rhinitis. A controlled clinical study. Acta Allergol. 1977;32:195–199. [DOI] [PubMed] [Google Scholar]
- 1360.Cohan RH, Bloom FL, Rhoades RB, Wittig HJ, Haugh LD. Treatment of perennial allergic rhinitis with cromolyn sodium. Double-blind study on 34 adult patients. J Allergy Clin Immunol. 1976;58:121–128. [DOI] [PubMed] [Google Scholar]
- 1361.Lange B, Lukat KF, Rettig K, Holtappels G, Bachert C. Efficacy, cost-effectiveness, and tolerability of mometasone furoate, levocabastine, and disodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:272–282. [DOI] [PubMed] [Google Scholar]
- 1362.Fisher WG. Comparison of budesonide and disodium cromoglycate for the treatment of seasonal allergic rhinitis in children. Ann Allergy. 1994;73:515–520. [PubMed] [Google Scholar]
- 1363.Bousquet J, Chanal I, Alquie MC, et al. Prevention of pollen rhinitis symptoms: comparison of fluticasone propionate aqueous nasal spray and disodium cromoglycate aqueous nasal spray. A multicenter, double-blind, double-dummy, parallel-group study. Allergy. 1993;48:327–333. [DOI] [PubMed] [Google Scholar]
- 1364.Welsh PW, Stricker WE, Chu CP, et al. Efficacy of beclomethasone nasal solution, flunisolide, and cromolyn in relieving symptoms of ragweed allergy. Mayo Clin Proc. 1987;62:125–134. [DOI] [PubMed] [Google Scholar]
- 1365.Bjerrum P, Illum P. Treatment of seasonal allergic rhinitis with budesonide and disodium cromoglycate. A double-blind clinical comparison between budesonide and disodium cromoglycate. Allergy. 1985;40:65–69. [DOI] [PubMed] [Google Scholar]
- 1366.Morrow-Brown H, Jackson FA, Pover GM. A comparison of beclomethasone dipropionate aqueous nasal spray and sodium cromoglycate nasal spray in the management of seasonal allergic rhinitis. Allergol Immunopathol (Madr). 1984;12:355–361. [PubMed] [Google Scholar]
- 1367.Brown HM, Engler C, English JR. A comparative trial of flunisolide and sodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Clin Allergy. 1981;11:169–173. [DOI] [PubMed] [Google Scholar]
- 1368.Wilson JA, Walker SR. A clinical study of the prophylactic use of betamethasone valerate and sodium cromoglycate in the treatment of seasonal allergic rhinitis. J Laryngol Otol. 1976;90:201–206. [DOI] [PubMed] [Google Scholar]
- 1369.Frankland AW, Walker SR. A comparison of intranasal betamethasone valerate and sodium cromoglycate in seasonal allergic rhinitis. Clin Allergy. 1975;5:295–300. [DOI] [PubMed] [Google Scholar]
- 1370.Meltzer EO; NasalCrom Study Group. Efficacy and patient satisfaction with cromolyn sodium nasal solution in the treatment of seasonal allergic rhinitis: a placebo-controlled study. Clin Ther. 2002;24:942–952. [DOI] [PubMed] [Google Scholar]
- 1371.Schuller DE, Selcow JE, Joos TH, et al. A multicenter trial of nedocromil sodium, 1% nasal solution, compared with cromolyn sodium and placebo in ragweed seasonal allergic rhinitis. J Allergy Clin Immunol. 1990;86:554–561. [DOI] [PubMed] [Google Scholar]
- 1372.Chandra RK, Heresi G, Woodford G. Double-blind controlled crossover trial of 4% intranasal sodium cromoglycate solution in patients with seasonal allergic rhinitis. Ann Allergy. 1982;49:131–134. [PubMed] [Google Scholar]
- 1373.Craig S, Rubinstein E, Reisman RE, Arbesman CE. Treatment of ragweed hay fever with intranasally administered disodium cromoglycate. Clin Allergy. 1977;7:569–576. [DOI] [PubMed] [Google Scholar]
- 1374.Handelman NI, Friday GA, Schwartz HJ, et al. Cromolyn sodium nasal solution in the prophylactic treatment of pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 1977;59:237–242. [DOI] [PubMed] [Google Scholar]
- 1375.Nizami RM, Baboo MT. Efficacy double-blind, crossover study of sodium cromoglycate in patients with seasonal allergic rhinitis. Ann Allergy. 1977;38:42–45. [PubMed] [Google Scholar]
- 1376.Posey WC, Nelson HS. Controlled trials with four per cent cromolyn spray in seasonal allergic rhinitis. Clin Allergy. 1977;7:485–496. [DOI] [PubMed] [Google Scholar]
- 1377.Knight A, Underdown BJ, Demanuele F, Hargreave FE. Disodium cromoglycate in ragweed-allergic rhinitis. J Allergy Clin Immunol. 1976;58:278–283. [DOI] [PubMed] [Google Scholar]
- 1378.Kim KT, Kerwin E, Landwehr L, et al. Use of 0.06% ipratropium bromide nasal spray in children aged 2 to 5 years with rhinorrhea due to a common cold or allergies. Ann Allergy Asthma Immunol. 2005;94:73–79. [DOI] [PubMed] [Google Scholar]
- 1379.Kaiser HB, Findlay SR, Georgitis JW, et al. The anticholinergic agent, ipratropium bromide, is useful in the treatment of rhinorrhea associated with perennial allergic rhinitis. Allergy Asthma Proc. 1998;19:23–29. [DOI] [PubMed] [Google Scholar]
- 1380.Ensing K, de Zeeuw RA, Nossent GD, Koeter GH, Cornelissen PJ. Pharmacokinetics of ipratropium bromide after single dose inhalation and oral and intravenous administration. Eur J Clin Pharmacol. 1989;36:189–194. [DOI] [PubMed] [Google Scholar]
- 1381.Dockhorn R, Aaronson D, Bronsky E, et al. Ipratropium bromide nasal spray 0.03% and beclomethasone nasal spray alone and in combination for the treatment of rhinorrhea in perennial rhinitis. Ann Allergy Asthma Immunol. 1999;82:349–359. [DOI] [PubMed] [Google Scholar]
- 1382.Finn AF Jr, Aaronson D, Korenblat P, et al. Ipratropium bromide nasal spray 0.03% provides additional relief from rhinorrhea when combined with terfenadine in perennial rhinitis patients; a randomized, double-blind, active-controlled trial. Am J Rhinol. 1998;12:441–449. [DOI] [PubMed] [Google Scholar]
- 1383.Meltzer EO, Orgel HA, Biondi R, et al. Ipratropium nasal spray in children with perennial rhinitis. Ann Allergy Asthma Immunol. 1997;78:485–491. [DOI] [PubMed] [Google Scholar]
- 1384.Gorski P, Pazdrak K, Ruta U. Effect of ipratropium on nasal reactivity to histamine and eosinophil influx in perennial allergic rhinitis. Eur J Clin Pharmacol. 1993;44:545–547. [DOI] [PubMed] [Google Scholar]
- 1385.Meltzer EO, Orgel HA, Bronsky EA, et al. Ipratropium bromide aqueous nasal spray for patients with perennial allergic rhinitis: a study of its effect on their symptoms, quality of life, and nasal cytology. J Allergy Clin Immunol. 1992;90:242–249. [DOI] [PubMed] [Google Scholar]
- 1386.Sanwikarja S, Schmitz PI, Dieges PH. The effect of locally applied ipratropium aerosol on the nasal methacholine challenge in patients with allergic and non-allergic rhinitis. Ann Allergy. 1986;56:162–166. [PubMed] [Google Scholar]
- 1387.Schultz Larsen F, Mygind N, Larsen FS. Ipratropium treatment for rhinorrhoea in patients with perennial rhinitis. An open follow-up study of efficacy and safety. Clin Otolaryngol Allied Sci. 1983;8:267–272. [DOI] [PubMed] [Google Scholar]
- 1388.Borum P, Mygind N, Schultz Larsen F. Intranasal ipratropium: a new treatment for perennial rhinitis. Clin Otolaryngol Allied Sci. 1979;4:407–411. [DOI] [PubMed] [Google Scholar]
- 1389.Milgrom H, Biondi R, Georgitis JW, et al. Comparison of ipratropium bromide 0.03% with beclomethasone dipropionate in the treatment of perennial rhinitis in children. Ann Allergy Asthma Immunol. 1999;83:105–111. [DOI] [PubMed] [Google Scholar]
- 1390.Kaiser HB, Findlay SR, Georgitis JW, et al. Long-term treatment of perennial allergic rhinitis with ipratropium bromide nasal spray 0.06%. J Allergy Clin Immunol. 1995;95:1128–1132. [DOI] [PubMed] [Google Scholar]
- 1391.Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–340.e1. [DOI] [PubMed] [Google Scholar]
- 1392.Adelroth E, Rak S, Haahtela T, et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;106:253–259. [DOI] [PubMed] [Google Scholar]
- 1393.Casale TB, Condemi J, LaForce C, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–2967. [DOI] [PubMed] [Google Scholar]
- 1394.Chervinsky P, Casale T, Townley R, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:160–167. [DOI] [PubMed] [Google Scholar]
- 1395.Okubo K, Ogino S, Nagakura T, Ishikawa T. Omalizumab is effective and safe in the treatment of Japanese cedar pollen-induced seasonal allergic rhinitis. Allergol Int. 2006;55:379–386. [DOI] [PubMed] [Google Scholar]
- 1396.Casale TB, Bernstein IL, Busse WW, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:110–121. [DOI] [PubMed] [Google Scholar]
- 1397.Corren J, Diaz-Sanchez D, Saxon A, et al. Effects of omalizumab, a humanized monoclonal anti-IgE antibody, on nasal reactivity to allergen and local IgE synthesis. Ann Allergy Asthma Immunol. 2004;93:243–248. [DOI] [PubMed] [Google Scholar]
- 1398.Bez C, Schubert R, Kopp M, et al. Effect of anti-immunoglobulin E on nasal inflammation in patients with seasonal allergic rhinoconjunctivitis. Clin Exp Allergy. 2004;34:1079–1085. [DOI] [PubMed] [Google Scholar]
- 1399.Nagakura T, Ogino S, Okubo K, Sato N, Takahashi M, Ishikawa T. Omalizumab is more effective than suplatast tosilate in the treatment of Japanese cedar pollen-induced seasonal allergic rhinitis. Clin Exp Allergy. 2008;38:329–337. [DOI] [PubMed] [Google Scholar]
- 1400.Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:274–280. [DOI] [PubMed] [Google Scholar]
- 1401.Rolinck-Werninghaus C, Hamelmann E, Keil T, et al. The co-seasonal application of anti-IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59:973–979. [DOI] [PubMed] [Google Scholar]
- 1402.Casale TB, Busse WW, Kline JN, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117:134–140. [DOI] [PubMed] [Google Scholar]
- 1403.Kopp MV, Hamelmann E, Zielen S, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and comorbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–279. [DOI] [PubMed] [Google Scholar]
- 1404.Kopp MV, Hamelmann E, Bendiks M, et al. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol. 2013;24:427–433. [DOI] [PubMed] [Google Scholar]
- 1405.Chaker AM, Shamji MH, Dumitru FA, et al. Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: A randomized controlled trial. J Allergy Clin Immunol. 2016;137:452–461.e9. [DOI] [PubMed] [Google Scholar]
- 1406.Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J. 2005;84:426–430. [PubMed] [Google Scholar]
- 1407.Rogkakou A, Guerra L, Massacane P, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis—a randomized controlled study. Eur Ann Allergy Clin Immunol. 2005;37:353–356. [PubMed] [Google Scholar]
- 1408.Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol. 2009;123:517–521. [DOI] [PubMed] [Google Scholar]
- 1409.Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53–56. [DOI] [PubMed] [Google Scholar]
- 1410.Garavello W, Romagnoli M, Sordo L, Gaini RM, Di Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatr Allergy Immunol. 2003;14:140–143. [DOI] [PubMed] [Google Scholar]
- 1411.Garavello W, Di Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol. 2005;137:310–314. [DOI] [PubMed] [Google Scholar]
- 1412.Li H, Sha Q, Zuo K, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec. 2009;71:50–55. [DOI] [PubMed] [Google Scholar]
- 1413.Marchisio P, Varricchio A, Baggi E, et al. Hypertonic saline is more effective than normal saline in seasonal allergic rhinitis in children. Int J Immunopathol Pharmacol. 2012;25:721–730. [DOI] [PubMed] [Google Scholar]
- 1414.Satdhabudha A, Poachanukoon O. Efficacy of buffered hypertonic saline nasal irrigation in children with symptomatic allergic rhinitis: a randomized double-blind study. Int J Pediatr Otorhinolaryngol. 2012;76:583–588. [DOI] [PubMed] [Google Scholar]
- 1415.Chen JR, Jin L, Li XY. The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children. Int J Pediatr Otorhinolaryngol. 2014;78:1115–1118. [DOI] [PubMed] [Google Scholar]
- 1416.Hermelingmeier KE, Weber RK, Hellmich M, Heubach CP, Mosges R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. 2012;26:e119–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1417.Psaltis AJ, Foreman A, Wormald PJ, Schlosser RJ. Contamination of sinus irrigation devices: a review of the evidence and clinical relevance. Am J Rhinol Allergy. 2012;26:201–203. [DOI] [PubMed] [Google Scholar]
- 1418.Ozdemir O Various effects of different probiotic strains in allergic disorders: an update from laboratory and clinical data. Clin Exp Immunol. 2010;160:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1419.Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. [DOI] [PubMed] [Google Scholar]
- 1420.Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol. 2015;5:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1421.Guvenc IA, Muluk NB, Mutlu FS, et al. Do probiotics have a role in the treatment of allergic rhinitis?: A comprehensive systematic review and meta analysis. Am J Rhinol Allergy. 2016. [DOI] [PubMed] [Google Scholar]
- 1422.Lue KH, Sun HL, Lu KH, et al. A trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7–12 years. Int J Pediatr Otorhinolaryngol. 2012;76:994–1001. [DOI] [PubMed] [Google Scholar]
- 1423.Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol. 2004;15:152–158. [DOI] [PubMed] [Google Scholar]
- 1424.Peng GC, Hsu CH. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol. 2005;16:433–438. [DOI] [PubMed] [Google Scholar]
- 1425.Costa DJ, Marteau P, Amouyal M, et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study). Eur J Clin Nutr. 2014;68:602–607. [DOI] [PubMed] [Google Scholar]
- 1426.Lin TY, Chen CJ, Chen LK, Wen SH, Jan RH. Effect of probiotics on allergic rhinitis in Df, Dp or dust-sensitive children: a randomized double blind controlled trial. Indian Pediatr. 2013;50:209–213. [DOI] [PubMed] [Google Scholar]
- 1427.Kawase M, He F, Kubota A, et al. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int J Food Microbiol. 2009;128:429–434. [DOI] [PubMed] [Google Scholar]
- 1428.Giovannini M, Agostoni C, Riva E, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. 2007;62:215–220. [DOI] [PubMed] [Google Scholar]
- 1429.Tamura M, Shikina T, Morihana T, et al. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2007;143:75–82. [DOI] [PubMed] [Google Scholar]
- 1430.Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002;57:243–246. [DOI] [PubMed] [Google Scholar]
- 1431.Nagata Y, Yoshida M, Kitazawa H, Araki E, Gomyo T. Improvements in seasonal allergic disease with Lactobacillus plantarum No. 14. Biosci Biotechnol Biochem. 2010;74:1869–1877. [DOI] [PubMed] [Google Scholar]
- 1432.Chen YS, Jan RL, Lin YL, Chen HH, Wang JY. Randomized placebo-controlled trial of Lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. 2010;45:1111–1120. [DOI] [PubMed] [Google Scholar]
- 1433.Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol. 2009;15:3261–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434.Lin WY, Fu LS, Lin HK, Shen CY, Chen YJ. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6-13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. 2014;55:181–188. [DOI] [PubMed] [Google Scholar]
- 1435.Yonekura S, Okamoto Y, Okawa T, et al. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30:397–405. [DOI] [PubMed] [Google Scholar]
- 1436.Ishida Y, Nakamura F, Kanzato H, et al. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: a double-blind, placebo-controlled study. J Dairy Sci. 2005;88:527–533. [DOI] [PubMed] [Google Scholar]
- 1437.Aldinucci C, Bellussi L, Monciatti G, et al. Effects of dietary yoghurt on immunological and clinical parameters of rhinopathic patients. Eur J Clin Nutr. 2002;56:1155–1161. [DOI] [PubMed] [Google Scholar]
- 1438.Jan RH, Chen CJ, Chen LK, Wen SH, Lin TY. Is the effect of probiotics on allergic rhnitis confined to Dermatophagoides farinae, Dermatophagoides pteronyssinus, or dust-sensitive children? A randomized prospective double-blind controlled trial. Ci Ji Yi Xue Za Zhi. 2011;23:51–54. http://www.sciencedirect.com/science/article/pii/S1016319011000474. Accessed December 19, 2017. [Google Scholar]
- 1439.Gotoh M, Sashihara T, Ikegami S, et al. Efficacy of oral administration of a heat-killed Lactobacillus gasseri OLL2809 on patients of Japanese cedar pollinosis with high Japanese-cedar pollen-specific IgE. Biosci Biotechnol Biochem. 2009;73:1971–1977. [DOI] [PubMed] [Google Scholar]
- 1440.Ivory K, Chambers SJ, Pin C, Prieto E, Arques JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38:1282–1289. [DOI] [PubMed] [Google Scholar]
- 1441.Singh A, Hacini-Rachinel F, Gosoniu ML, et al. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: an exploratory, randomized, placebo-controlled clinical trial. Eur J Clin Nutr. 2013;67:161–167. [DOI] [PubMed] [Google Scholar]
- 1442.Xiao JZ, Kondo S, Yanagisawa N, et al. Effect of probiotic Bifidobacterium longum BB536 [corrected] in relieving clinical symptoms and modulating plasma cytokine levels of Japanese cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial. J Investig Allergol Clin Immunol. 2006;16:86–93. [PubMed] [Google Scholar]
- 1443.Xiao JZ, Kondo S, Yanagisawa N, et al. Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo-controlled trial. Clin Exp Allergy. 2006;36:1425–1435. [DOI] [PubMed] [Google Scholar]
- 1444.Nishimura I, Igarashi T, Enomoto T, Dake Y, Okuno Y, Obata A. Clinical efficacy of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi for perennial allergic rhinitis. Allergol Int. 2009;58:179–185. [DOI] [PubMed] [Google Scholar]
- 1445.Dolle S, Berg J, Rasche C, Worm M. Tolerability and clinical outcome of coseasonal treatment with Escherichia coli strain Nissle 1917 in grass pollen-allergic subjects. Int Arch Allergy Immunol. 2014;163:29–35. [DOI] [PubMed] [Google Scholar]
- 1446.Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii effects in children with allergic rhinitis. Allergy. 2005;60:702–703. [DOI] [PubMed] [Google Scholar]
- 1447.Johnson DA, Hricik JG. The pharmacology of alpha-adrenergic decongestants. Pharmacotherapy. 1993;13:110S–115S; discussion 143S-146S. [PubMed] [Google Scholar]
- 1448.Nielsen LP, Mygind N, Dahl R. Intranasal corticosteroids for allergic rhinitis: superior relief? Drugs. 2001;61:1563–1579. [DOI] [PubMed] [Google Scholar]
- 1449.Zieglmayer UP, Horak F, Toth J, Marks B, Berger UE, Burtin B. Efficacy and safety of an oral formulation of cetirizine and prolonged-release pseudoephedrine versus budesonide nasal spray in the management of nasal congestion in allergic rhinitis. Treat Respir Med. 2005;4:283–287. [DOI] [PubMed] [Google Scholar]
- 1450.Kaiser HB, Banov CH, Berkowitz RR, et al. Comparative efficacy and safety of once-daily versus twice-daily loratadine-pseudoephedrine combinations versus placebo in seasonal allergic rhinitis. Am J Ther. 1998;5:245–251. [DOI] [PubMed] [Google Scholar]
- 1451.Nathan RA, Finn AF Jr, LaForce C, et al. Comparison of cetirizine-pseudoephedrine and placebo in patients with seasonal allergic rhinitis and concomitant mild-to-moderate asthma: randomized, double-blind study. Ann Allergy Asthma Immunol. 2006;97:389–396. [DOI] [PubMed] [Google Scholar]
- 1452.McFadden EA, Gungor A, Ng B, Mamikoglu B, Moinuddin R, Corey J. Loratadine/pseudoephedrine for nasal symptoms in seasonal allergic rhinitis: a double-blind, placebo-controlled study. Ear Nose Throat J. 2000;79:254, 257–258, 260 passim. [PubMed] [Google Scholar]
- 1453.Serra HA, Alves O, Rizzo LF, Devoto FM, Ascierto H. Loratadine-pseudoephedrine in children with allergic rhinitis, a controlled double-blind trial. Br J Clin Pharmacol. 1998;45:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1454.Corren J, Harris AG, Aaronson D, et al. Efficacy and safety of loratadine plus pseudoephedrine in patients with seasonal allergic rhinitis and mild asthma. J Allergy Clin Immunol. 1997;100:781–788. [DOI] [PubMed] [Google Scholar]
- 1455.Bronsky E, Boggs P, Findlay S, et al. Comparative efficacy and safety of a once-daily loratadine-pseudoephedrine combination versus its components alone and placebo in the management of seasonal allergic rhinitis. J Allergy Clin Immunol. 1995;96:139–147. [DOI] [PubMed] [Google Scholar]
- 1456.Grossman J, Bronsky EA, Lanier BQ, et al. Loratadine-pseudoephedrine combination versus placebo in patients with seasonal allergic rhinitis. Ann Allergy. 1989;63:317–321. [PubMed] [Google Scholar]
- 1457.Sussman GL, Mason J, Compton D, Stewart J, Ricard N. The efficacy and safety of fexofenadine HCl and pseudoephedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol. 1999;104:100–106. [DOI] [PubMed] [Google Scholar]
- 1458.Bertrand B, Jamart J, Marchal JL, Arendt C. Cetirizine and pseudoephedrine retard alone and in combination in the treatment of perennial allergic rhinitis: a double-blind multicentre study. Rhinology. 1996;34:91–96. [PubMed] [Google Scholar]
- 1459.Grosclaude M, Mees K, Pinelli ME, Lucas M, Van de Venne H. Cetirizine and pseudoephedrine retard, given alone or in combination, in patients with seasonal allergic rhinitis. Rhinology. 1997;35:67–73. [PubMed] [Google Scholar]
- 1460.Pleskow W, Grubbe R, Weiss S, Lutsky B. Efficacy and safety of an extended-release formulation of desloratadine and pseudoephedrine vs the individual components in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;94:348–354. [DOI] [PubMed] [Google Scholar]
- 1461.Chervinsky P, Nayak A, Rooklin A, Danzig M. Efficacy and safety of desloratadine/pseudoephedrine tablet, 2.5/120 mg two times a day, versus individual components in the treatment of patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2005;26:391–396. [PubMed] [Google Scholar]
- 1462.Grubbe RE, Lumry WR, Anolik R. Efficacy and safety of desloratadine/pseudoephedrine combination vs its components in seasonal allergic rhinitis. J Investig Allergol Clin Immunol. 2009;19:117–124. [PubMed] [Google Scholar]
- 1463.Chiang YC, Shyur SD, Chen TL, et al. A randomized controlled trial of cetirizine plus pseudoephedrine versus loratadine plus pseudoephedrine for perennial allergic rhinitis. Asian Pac J Allergy Immunol. 2006;24:97–103. [PubMed] [Google Scholar]
- 1464.Chen YA, Chang KP, Lin YS, Hao SP. A randomized, double-blind, parallel-group study to compare the efficacy and safety of a once-daily loratadine-pseudoephedrine combination with that of a twice-daily loratadine-pseudoephedrine combination in the treatment of allergic rhinitis. Eur Arch Otorhinolaryngol. 2007;264:1019–1025. [DOI] [PubMed] [Google Scholar]
- 1465.Yau WP, Mitchell AA, Lin KJ, Werler MM, Hernandez-Diaz S. Use of decongestants during pregnancy and the risk of birth defects. Am J Epidemiol. 2013;178:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1466.Hampton LM, Nguyen DB, Edwards JR, Budnitz DS. Cough and cold medication adverse events after market withdrawal and labeling revision. Pediatrics. 2013;132:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1467.Moinuddin R, deTineo M, Maleckar B, Naclerio RM, Baroody FM. Comparison of the combinations of fexofenadine-pseudoephedrine and loratadine-montelukast in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:73–79. [DOI] [PubMed] [Google Scholar]
- 1468.Stubner UP, Toth J, Marks B, Berger UE, Burtin B, Horak F. Efficacy and safety of an oral formulation of cetirizine and prolonged-release pseudoephedrine versus xylometazoline nasal spray in nasal congestion. Arzneimittelforschung. 2001;51:904–910. [DOI] [PubMed] [Google Scholar]
- 1469.Wilson A, Dempsey OJ, Sims EJ, Coutie WJ, Paterson MC, Lipworth BJ. Evaluation of treatment response in patients with seasonal allergic rhinitis using domiciliary nasal peak inspiratory flow. Clin Exp Allergy. 2000;30:833–838. [DOI] [PubMed] [Google Scholar]
- 1470.Barnes ML, Ward JH, Fardon TC, Lipworth BJ. Effects of levocetirizine as add-on therapy to fluticasone in seasonal allergic rhinitis. Clin Exp Allergy. 2006;36:676–684. [DOI] [PubMed] [Google Scholar]
- 1471.Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract. 1998;47:118–125. [PubMed] [Google Scholar]
- 1472.Di Lorenzo G, Pacor ML, Pellitteri ME, et al. Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in mono-therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis. Clin Exp Allergy. 2004;34:259–267. [DOI] [PubMed] [Google Scholar]
- 1473.Pinar E, Eryigit O, Oncel S, Calli C, Yilmaz O, Yuksel H. Efficacy of nasal corticosteroids alone or combined with antihistamines or montelukast in treatment of allergic rhinitis. Auris Nasus Larynx. 2008;35:61–66. [DOI] [PubMed] [Google Scholar]
- 1474.Feng S, Fan Y, Liang Z, Ma R, Cao W. Concomitant corticosteroid nasal spray plus antihistamine (oral or local spray) for the symptomatic management of allergic rhinitis. Eur Arch Otorhinolaryngol. 2016;273:3477–3486. [DOI] [PubMed] [Google Scholar]
- 1475.Ciebiada M, Barylski M, Gorska Ciebiada M. Nasal eosinophilia and serum soluble intercellular adhesion molecule 1 in patients with allergic rhinitis treated with montelukast alone or in combination with desloratadine or levocetirizine. Am J Rhinol Allergy. 2013;27:e58–e62. [DOI] [PubMed] [Google Scholar]
- 1476.Yamamoto H, Yamada T, Sakashita M, et al. Efficacy of prophylactic treatment with montelukast and montelukast plus add-on loratadine for seasonal allergic rhinitis. Allergy Asthma Proc. 2012;33:e17–e22. [DOI] [PubMed] [Google Scholar]
- 1477.Cingi C, Gunhan K, Gage-White L, Unlu H. Efficacy of leukotriene antagonists as concomitant therapy in allergic rhinitis. Laryngoscope. 2010;120:1718–1723. [DOI] [PubMed] [Google Scholar]
- 1478.Li AM, Abdullah VJ, Tsen CS, et al. Leukotriene receptor antagonist in the treatment of childhood allergic rhinitis—a randomized placebo-controlled study. Pediatr Pulmonol. 2009;44:1085–1092. [DOI] [PubMed] [Google Scholar]
- 1479.Lu S, Malice MP, Dass SB, Reiss TF. Clinical studies of combination montelukast and loratadine in patients with seasonal allergic rhinitis. J Asthma. 2009;46:878–883. [DOI] [PubMed] [Google Scholar]
- 1480.Watanasomsiri A, Poachanukoon O, Vichyanond P. Efficacy of montelukast and loratadine as treatment for allergic rhinitis in children. Asian Pac J Allergy Immunol. 2008;26:89–95. [PubMed] [Google Scholar]
- 1481.Saengpanich S, deTineo M, Naclerio RM, Baroody FM. Fluticasone nasal spray and the combination of loratadine and montelukast in seasonal allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2003;129:557–562. [DOI] [PubMed] [Google Scholar]
- 1482.Nayak AS, Philip G, Lu S, Malice MP, Reiss TF; Montelukast Fall Rhinitis Investigator Group. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88:592–600. [DOI] [PubMed] [Google Scholar]
- 1483.Meltzer EO, Malmstrom K, Lu S, et al. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J Allergy Clin Immunol. 2000;105:917–922. [DOI] [PubMed] [Google Scholar]
- 1484.Wilson AM, Orr LC, Sims EJ, Lipworth BJ. Effects of monotherapy with intra-nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clin Exp Allergy. 2001;31:61–68. [PubMed] [Google Scholar]
- 1485.Prenner BM, Lu S, Danzig MR. Safety of fixed-dose loratadine/montelukast in subjects with allergic rhinitis. Allergy Asthma Proc. 2010;31:493–498. [DOI] [PubMed] [Google Scholar]
- 1486.Berger W, Meltzer EO, Amar N, et al. Efficacy of MP-AzeFlu in children with seasonal allergic rhinitis: Importance of paediatric symptom assessment. Pediatr Allergy Immunol. 2016;27:126–133. [DOI] [PubMed] [Google Scholar]
- 1487.Meltzer E, Ratner P, Bachert C, et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161:369–377. [DOI] [PubMed] [Google Scholar]
- 1488.Price D, Shah S, Bhatia S, et al. A new therapy (MP29-02) is effective for the long-term treatment of chronic rhinitis. J Investig Allergol Clin Immunol. 2013;23:495–503. [PubMed] [Google Scholar]
- 1489.Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129:1282–1289.e10. [DOI] [PubMed] [Google Scholar]
- 1490.Meltzer EO, LaForce C, Ratner P, Price D, Ginsberg D, Carr W. MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial of efficacy and safety. Allergy Asthma Proc. 2012;33:324–332. [DOI] [PubMed] [Google Scholar]
- 1491.Salapatek AM, Lee J, Patel D, et al. Solubilized nasal steroid (CDX-947) when combined in the same solution nasal spray with an antihistamine (CDX-313) provides improved, fast-acting symptom relief in patients with allergic rhinitis. Allergy Asthma Proc. 2011;32:221–229. [DOI] [PubMed] [Google Scholar]
- 1492.Hampel FC, Ratner PH, Van Bavel J, et al. Double-blind, placebo-controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann Allergy Asthma Immunol. 2010;105:168–173. [DOI] [PubMed] [Google Scholar]
- 1493.LaForce CF, Carr W, Tilles SA, et al. Evaluation of olopatadine hydrochloride nasal spray, 0.6%, used in combination with an intranasal corticosteroid in seasonal allergic rhinitis. Allergy Asthma Proc. 2010;31:132–140. [DOI] [PubMed] [Google Scholar]
- 1494.Ratner PH, Hampel F, Van Bavel J, et al. Combination therapy with azelastine hydrochloride nasal spray and fluticasone propionate nasal spray in the treatment of patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2008;100:74–81. [DOI] [PubMed] [Google Scholar]
- 1495.Berger W, Bousquet J, Fox AT, et al. MP-AzeFlu is more effective than fluticasone propionate for the treatment of allergic rhinitis in children. Allergy. 2016;71:1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1496.Klimek L, Bachert C, Stjarne P, et al. MP-AzeFlu provides rapid and effective allergic rhinitis control in real life: a pan-European study. Allergy Asthma Proc. 2016;37:376–386. [DOI] [PubMed] [Google Scholar]
- 1497.Klimek L, Bachert C, Mosges R, et al. Effectiveness of MP29-02 for the treatment of allergic rhinitis in real-life: results from a noninterventional study. Allergy Asthma Proc. 2015;36:40–47. [DOI] [PubMed] [Google Scholar]
- 1498.Ma KW. Acupuncture: its place in the history of Chinese medicine. Acupunct Med. 2002;18:88–99. [Google Scholar]
- 1499.Zijlstra FJ, van den Berg-de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1500.Roberts J, Huissoon A, Dretzke J, Wang D, Hyde C. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1501.Feng S, Han M, Fan Y, et al. Acupuncture for the treatment of allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. 2015;29:57–62. [DOI] [PubMed] [Google Scholar]
- 1502.Petti FB, Liguori A, Ippoliti F. Study on cytokines IL-2, IL-6, IL-10 in patients of chronic allergic rhinitis treated with acupuncture. J Tradit Chin Med. 2002;22:104–111. [PubMed] [Google Scholar]
- 1503.Duddukuri GR, Kumar PS, Kumar VB, Athota RR. Immunosuppressive effect of honey on the induction of allergen-specific humoral antibody response in mice. Int Arch Allergy Immunol. 1997;114:385–388. [DOI] [PubMed] [Google Scholar]
- 1504.Ishikawa Y, Tokura T, Nakano N, et al. Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J Med Food. 2008;11:14–20. [DOI] [PubMed] [Google Scholar]
- 1505.Ishikawa Y, Tokura T, Ushio H, et al. Lipid-soluble components of honeybee-collected pollen exert antiallergic effect by inhibiting IgE-mediated mast cell activation in vivo. Phytother Res. 2009;23:1581–1586. [DOI] [PubMed] [Google Scholar]
- 1506.Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157–161. [DOI] [PubMed] [Google Scholar]
- 1507.Al-Waili NS, Boni NS. Natural honey lowers plasma prostaglandin concentrations in normal individuals. J Med Food. 2003;6:129–133. [DOI] [PubMed] [Google Scholar]
- 1508.Asha’ari ZA, Ahmad MZ, Jihan WS, Che CM, Leman I. Ingestion of honey improves the symptoms of allergic rhinitis: evidence from a randomized placebo-controlled trial in the East coast of Peninsular Malaysia. Ann Saudi Med. 2013;33:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1509.Saarinen K, Jantunen J, Haahtela T. Birch pollen honey for birch pollen allergy—a randomized controlled pilot study. Int Arch Allergy Immunol. 2011;155:160–166. [DOI] [PubMed] [Google Scholar]
- 1510.Rajan TV, Tennen H, Lindquist RL, Cohen L, Clive J. Effect of ingestion of honey on symptoms of rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2002;88:198–203. [DOI] [PubMed] [Google Scholar]
- 1511.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27:677–689. [DOI] [PubMed] [Google Scholar]
- 1512.Matkovic Z, Zivkovic V, Korica M, Plavec D, Pecanic S, Tudoric N. Efficacy and safety of Astragalus membranaceus in the treatment of patients with seasonal allergic rhinitis. Phytother Res. 2010;24:175–181. [DOI] [PubMed] [Google Scholar]
- 1513.D’Souza P, Amit A, Saxena VS, Bagchi D, Bagchi M, Stohs SJ. Antioxidant properties of Aller-7, a novel polyherbal formulation for allergic rhinitis. Drugs Exp Clin Res. 2004;30:99–109. [PubMed] [Google Scholar]
- 1514.Pratibha N, Saxena VS, Amit A, D’Souza P, Bagchi M, Bagchi D. Anti-inflammatory activities of Aller-7, a novel polyherbal formulation for allergic rhinitis. Int J Tissue React. 2004;26:43–51. [PubMed] [Google Scholar]
- 1515.Amit A, Saxena VS, Pratibha N, et al. Mast cell stabilization, lipoxygenase inhibition, hyaluronidase inhibition, antihistaminic and antispasmodic activities of Aller-7, a novel botanical formulation for allergic rhinitis. Drugs Exp Clin Res. 2003;29:107–115. [PubMed] [Google Scholar]
- 1516.Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483–495. [DOI] [PubMed] [Google Scholar]
- 1517.Suzuki M, Yoshino K, Maeda-Yamamoto M, Miyase T, Sano M. Inhibitory effects of tea catechins and O-methylated derivatives of (−)− epigallocatechin3-O-gallate on mouse type IV allergy. J Agric Food Chem. 2000;48:5649–5653. [DOI] [PubMed] [Google Scholar]
- 1518.Maeda-Yamamoto M, Inagaki N, Kitaura J, et al. O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. 2004;172:4486–4492. [DOI] [PubMed] [Google Scholar]
- 1519.Masuda S, Maeda-Yamamoto M, Usui S, Fujisawa T. ‘Benifuuki’ green tea containing o-methylated catechin reduces symptoms of Japanese cedar pollinosis: a randomized, double-blind, placebo-controlled trial. Allergol Int. 2014;63:211–217. [DOI] [PubMed] [Google Scholar]
- 1520.Hu G, Walls RS, Bass D, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12-week clinical trial. Ann Allergy Asthma Immunol. 2002;88:478–487. [DOI] [PubMed] [Google Scholar]
- 1521.Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, Yoshikawa M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J Agric Food Chem. 2006;54:2915–2920. [DOI] [PubMed] [Google Scholar]
- 1522.Russell LC, Burchiel KJ. Neurophysiological effects of capsaicin. Brain Res. 1984;320:165–176. [DOI] [PubMed] [Google Scholar]
- 1523.Philip G, Baroody FM, Proud D, Naclerio RM, Togias AG. The human nasal response to capsaicin. J Allergy Clin Immunol. 1994;94:1035–1045. [DOI] [PubMed] [Google Scholar]
- 1524.Cheng J, Yang XN, Liu X, Zhang SP. Capsaicin for allergic rhinitis in adults. Cochrane Database Syst Rev. 2006;(2):CD004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1525.Corren J, Lemay M, Lin Y, Rozga L, Randolph RK. Clinical and biochemical effects of a combination botanical product (ClearGuard) for allergy: a pilot randomized double-blind placebo-controlled trial. Nutr J. 2008;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1526.Ohmori Y, Ito M, Kishi M, Mizutani H, Katada T, Konishi H. Antiallergic constituents from oolong tea stem. Biol Pharm Bull. 1995;18:683–686. [DOI] [PubMed] [Google Scholar]
- 1527.Bernstein DI, Bernstein CK, Deng C, et al. Evaluation of the clinical efficacy and safety of grape-seed extract in the treatment of fall seasonal allergic rhinitis: a pilot study. Ann Allergy Asthma Immunol. 2002;88:272–278. [DOI] [PubMed] [Google Scholar]
- 1528.Chakravarty N Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70:237–242. [PubMed] [Google Scholar]
- 1529.El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6:1135–1142. [DOI] [PubMed] [Google Scholar]
- 1530.Kalus U, Pruss A, Bystron J, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17:1209–1214. [DOI] [PubMed] [Google Scholar]
- 1531.Nikakhlagh S, Rahim F, Aryani FH, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol. 2011;32:402–407. [DOI] [PubMed] [Google Scholar]
- 1532.Alsamarai AM, Abdulsatar M, Ahmed Alobaidi AH. Evaluation of topical black seed oil in the treatment of allergic rhinitis. Antiinflamm Antiallergy Agents Med Chem. 2014;13:75–82. [DOI] [PubMed] [Google Scholar]
- 1533.Rotondo S, Rajtar G, Manarini S, et al. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123:1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534.Varilek GW, Yang F, Lee EY, et al. Green tea polyphenol extract attenuates inflammation in interleukin 2-deficient mice, a model of autoimmunity. J Nutr. 2001;131:2034–2039. [DOI] [PubMed] [Google Scholar]
- 1535.Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–2340. [DOI] [PubMed] [Google Scholar]
- 1536.Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–243. [DOI] [PubMed] [Google Scholar]
- 1537.Takano H, Osakabe N, Sanbongi C, et al. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood). 2004;229:247–254. [DOI] [PubMed] [Google Scholar]
- 1538.Lenon GB, Xue CC, Story DF, Thien FC, McPhee S, Li CG. Inhibition of release of inflammatory mediators in primary and cultured cells by a Chinese herbal medicine formula for allergic rhinitis. Chin Med. 2007;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1539.Lenon GB, Li CG, Xue CC, Thien FC, Story DF. Inhibition of release of vasoactive and inflammatory mediators in airway and vascular tissues and macrophages by a chinese herbal medicine formula for allergic rhinitis. Evid Based Complement Alternat Med. 2007;4:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1540.Xue CC, Thien FC, Zhang JJ, Da Costa C, Li CG. Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial. Altern Ther Health Med. 2003;9:80–87. [PubMed] [Google Scholar]
- 1541.Mao TK, Van de Water J, Gershwin ME. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food. 2005;8:27–30. [DOI] [PubMed] [Google Scholar]
- 1542.Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1543.Cingi C, Conk-Dalay M, Cakli H, Bal C. The effects of spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;265:1219–1223. [DOI] [PubMed] [Google Scholar]
- 1544.Ishikura Y, Sumwa Y, Okada T. Anti-allergic effects of Rubus suavissimus extract. Japanese J Inflamm. 1995;15:167–173. [Google Scholar]
- 1545.Yonekura S, Okamoto Y, Yamasaki K, et al. A randomized, double-blind, placebo-controlled study of ten-cha (Rubus suavissimus) on house dust mite allergic rhinitis. Auris Nasus Larynx. 2011;38:600–607. [DOI] [PubMed] [Google Scholar]
- 1546.Das AK, Mizuguchi H, Kodama M, et al. Shoseiryu-to suppresses histamine signaling at the transcriptional level in TDI-sensitized nasal allergy model rats. Allergol Int. 2009;58:81–88. [DOI] [PubMed] [Google Scholar]
- 1547.Baba S Double-blind clinical tiral of Sho-seiryuto (TJ-19) for perennial nasal allergy. Pract Otol. 1995;88:389–405. [Google Scholar]
- 1548.Badar VA, Thawani VR, Wakode PT, et al. Efficacy of Tinospora cordifolia in allergic rhinitis. J Ethnopharmacol. 2005;96:445–449. [DOI] [PubMed] [Google Scholar]
- 1549.Roscheck B, Fink RC, McMichael A. Nettle extract (Urtica dioica) affects key receptor and enzymes associated with allergic rhinitis. Phytother Res. 2009;23:920–926. [DOI] [PubMed] [Google Scholar]
- 1550.Randomized Mittman P., double-blind study of freeze-dried Urtica dioica in the treatment of allergic rhinitis. Planta Med. 1990;56:44–47. [DOI] [PubMed] [Google Scholar]
- 1551.Passali D, Lauriello M, Anselmi M, Bellussi L. Treatment of hypertrophy of the inferior turbinate: long-term results in 382 patients randomly assigned to therapy. Ann Otol Rhinol Laryngol. 1999;108:569–575. [DOI] [PubMed] [Google Scholar]
- 1552.Jose J, Coatesworth AP. Inferior turbinate surgery for nasal obstruction in allergic rhinitis after failed medical treatment. Cochrane Database Syst Rev. 2010;(12):CD005235. [DOI] [PubMed] [Google Scholar]
- 1553.Costa DJ, Sanford T, Janney C, Cooper M, Sindwani R. Radiographic and anatomic characterization of the nasal septal swell body. Arch Otolaryngol Head Neck Surg. 2010;136:1107–1110. [DOI] [PubMed] [Google Scholar]
- 1554.Karatzanis AD, Fragiadakis G, Moshandrea J, Zenk J, Iro H, Velegrakis GA. Septoplasty outcome in patients with and without allergic rhinitis. Rhinology. 2009;47:444–449. [DOI] [PubMed] [Google Scholar]
- 1555.Kim YH, Kim BJ, Bang KH, Hwang Y, Jang TY. Septoplasty improves life quality related to allergy in patients with septal deviation and allergic rhinitis. Otolaryngol Head Neck Surg. 2011;145:910–914. [DOI] [PubMed] [Google Scholar]
- 1556.Mori S, Fujieda S, Yamada T, Kimura Y, Takahashi N, Saito H. Long-term effect of submucous turbinectomy in patients with perennial allergic rhinitis. Laryngoscope. 2002;112:865–869. [DOI] [PubMed] [Google Scholar]
- 1557.Chen YL, Tan CT, Huang HM. Long-term efficacy of microdebrider-assisted inferior turbinoplasty with lateralization for hypertrophic inferior turbinates in patients with perennial allergic rhinitis. Laryngoscope. 2008;118:1270–1274. [DOI] [PubMed] [Google Scholar]
- 1558.Caffier PP, Scherer H, Neumann K, Luck S, Enzmann H, Haisch A. Diode laser treatment in therapy-resistant allergic rhinitis: impact on nasal obstruction and associated symptoms. Lasers Med Sci. 2011;26:57–67. [DOI] [PubMed] [Google Scholar]
- 1559.Chang CW, Ries WR. Surgical treatment of the inferior turbinate: new techniques. Curr Opin Otolaryngol Head Neck Surg. 2004;12:53–57. [DOI] [PubMed] [Google Scholar]
- 1560.Hytonen ML, Back LJ, Malmivaara AV, Roine RP. Radiofrequency thermal ablation for patients with nasal symptoms: a systematic review of effectiveness and complications. Eur Arch Otorhinolaryngol. 2009;266:1257–1266. [DOI] [PubMed] [Google Scholar]
- 1561.Li KK, Powell NB, Riley RW, Troell RJ, Guilleminault C. Radiofrequency volumetric tissue reduction for treatment of turbinate hypertrophy: a pilot study. Otolaryngol Head Neck Surg. 1998;119:569–573. [DOI] [PubMed] [Google Scholar]
- 1562.Lin HC, Lin PW, Friedman M, et al. Long-term results of radiofrequency turbinoplasty for allergic rhinitis refractory to medical therapy. Arch Otolaryngol Head Neck Surg. 2010;136:892–895. [DOI] [PubMed] [Google Scholar]
- 1563.Siméon R, Soufflet B, Souchal Delacour I. Coblation turbinate reduction in childhood allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127:77–82. [DOI] [PubMed] [Google Scholar]
- 1564.Aksoy F, Yildirim YS, Veyseller B, Ozturan O, Demirhan H. Midterm outcomes of outfracture of the inferior turbinate. Otolaryngol Head Neck Surg. 2010;143:579–584. [DOI] [PubMed] [Google Scholar]
- 1565.Chhabra N, Houser SM. The diagnosis and management of empty nose syndrome. Otolaryngol Clin North Am. 2009;42:311–330, ix. [DOI] [PubMed] [Google Scholar]
- 1566.Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138:492–497. [DOI] [PubMed] [Google Scholar]
- 1567.Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ. A systematic review of the evidence base for vidian neurectomy in managing rhinitis. J Laryngol Otol. 2016;130(Suppl 4):S7–S28. [DOI] [PubMed] [Google Scholar]
- 1568.Kobayashi T, Hyodo M, Nakamura K, Komobuchi H, Honda N. Resection of peripheral branches of the posterior nasal nerve compared to conventional posterior neurectomy in severe allergic rhinitis. Auris Nasus Larynx. 2012;39:593–596. [DOI] [PubMed] [Google Scholar]
- 1569.Osguthorpe JD. The evolution of understanding inhalant allergy. Otolaryngol Clin North Am. 2011;44:519–535, vii. [DOI] [PubMed] [Google Scholar]
- 1570.Noon L Prophylactic inoculation against hayfever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 1571.Mason WW, Ward WA. Standardized extracts. Otolaryngol Clin North Am. 1992;25:101–117. [PubMed] [Google Scholar]
- 1572.Zimmer J, Vieths S, Kaul S. Standardization and regulation of allergen products in the European Union. Curr Allergy Asthma Rep. 2016;16:21. [DOI] [PubMed] [Google Scholar]
- 1573.Carnes J, Iraola V, Gallego M, Leonor JR. Control process for manufacturing and standardization of allergenic molecules. Curr Allergy Asthma Rep. 2015;15:37. [DOI] [PubMed] [Google Scholar]
- 1574.Park KH, Son M, Choi SY, et al. In vitro evaluation of allergen potencies of commercial house dust mite sublingual immunotherapy reagents. Allergy Asthma Immunol Res. 2015;7:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1575.Thomsen GF, Schlunssen V, Skadhauge LR, et al. Are allergen batch differences and the use of double skin prick test important? BMC Pulm Med. 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1576.Slater JE. Standardized allergen extracts in the United States. Clin Allergy Immunol. 2004;18:421–432. [PubMed] [Google Scholar]
- 1577.Jutel M, Agache I, Bonini S, et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358–368. [DOI] [PubMed] [Google Scholar]
- 1578.Fernández-Caldas E, Zakzuk J, Lockey RF. Allergen Standardization. Posted September 2009. Milwaukee, WI: World Allergy Organization; 2009. http://www.worldallergy.org/professional/allergic_diseases_center/allergen_standardization/ Accessed December 19, 2017. [Google Scholar]
- 1579.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial Timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–1408. [DOI] [PubMed] [Google Scholar]
- 1580.Creticos PS. Allergen immunotherapy: vaccine modification. Immunol Allergy Clin North Am. 2016;36:103–124. [DOI] [PubMed] [Google Scholar]
- 1581.Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1582.Worm M, Patel D, Creticos PS. Cat peptide antigen desensitisation for treating cat allergic rhinoconjunctivitis. Expert Opin Investig Drugs. 2013;22:1347–1357. [DOI] [PubMed] [Google Scholar]
- 1583.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. [DOI] [PubMed] [Google Scholar]
- 1584.Nony E, Bouley J, Le Mignon M, et al. Development and evaluation of a sublingual tablet based on recombinant Bet v 1 in birch pollen-allergic patients. Allergy. 2015;70:795–804. [DOI] [PubMed] [Google Scholar]
- 1585.Klimek L, Schendzielorz P, Pinol R, Pfaar O. Specific subcutaneous immunotherapy with recombinant grass pollen allergens: first randomized dose-ranging safety study. Clin Exp Allergy. 2012;42:936–945. [DOI] [PubMed] [Google Scholar]
- 1586.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. [DOI] [PubMed] [Google Scholar]
- 1587.Circassia Pharmaceuticals. Circassia Announces Top-Line Results from Cat Allergy Phase III Study. Press release. Oxford, UK: Circassia Pharmaceuticals; June 20, 2016. http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study/. Accessed December 19, 2017. [Google Scholar]
- 1588.Circassia Pharmaceuticals. Circassia Announces Top-Line Results from House Dust Mite Allergy Field Study. Press release. Oxford, UK: Circassia Pharmaceuticals; April 18, 2017. http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-house-dust-mite-allergy-field-study/. Accessed December 19, 2017. [Google Scholar]
- 1589.Spertini F, DellaCorte G, Kettner A, et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase IIb study. J Allergy Clin Immunol. 2016;138:162–168. [DOI] [PubMed] [Google Scholar]
- 1590.Norman PS, Lichtenstein LM, Marsh DG. Studies on allergoids from naturally occurring allergens. IV. Efficacy and safety of long-term allergoid treatment of ragweed hay fever. J Allergy Clin Immunol. 1981;68:460–470. [DOI] [PubMed] [Google Scholar]
- 1591.Grammer LC, Zeiss CR, Suszko IM, Shaughnessy MA, Patterson R. A double-blind, placebo-controlled trial of polymerized whole ragweed for immunotherapy of ragweed allergy. J Allergy Clin Immunol. 1982;69:494–499. [DOI] [PubMed] [Google Scholar]
- 1592.Grammer LC, Shaughnessy MA, Suszko IM, Shaughnessy JJ, Patterson R. A double-blind histamine placebo-controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol. 1983;72:448–453. [DOI] [PubMed] [Google Scholar]
- 1593.Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272–279. [DOI] [PubMed] [Google Scholar]
- 1594.Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented-polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013;68:1306–1313. [DOI] [PubMed] [Google Scholar]
- 1595.Francis JN, Durham SR. Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr Opin Allergy Clin Immunol. 2004;4:543–548. [DOI] [PubMed] [Google Scholar]
- 1596.Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. [DOI] [PubMed] [Google Scholar]
- 1597.Busse W, Gross G, Korenblat P, Nayak N, Tarpay M, Levitt D. Phase 2/3 study of the novel vaccine Amb a 1 immunostimulatory oligodeoxyribonucleotide conjugate AIC in ragweed allergic adults. J Allergy Clin Immunol. 2006;117:S88–S89. [Google Scholar]
- 1598.DuBuske LM, Frew AJ, Horak F, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–247. [DOI] [PubMed] [Google Scholar]
- 1599.Couroux P, Patel D, Armstrong K, Larche M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–981. [DOI] [PubMed] [Google Scholar]
- 1600.Purohit A, Niederberger V, Kronqvist M, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–1525. [DOI] [PubMed] [Google Scholar]
- 1601.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. [DOI] [PubMed] [Google Scholar]
- 1602.Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–231. [DOI] [PubMed] [Google Scholar]
- 1603.Norman PS, Ohman JL Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–1628. [DOI] [PubMed] [Google Scholar]
- 1604.Litwin A, Pesce AJ, Fischer T, Michael M, Michael JG. Regulation of the human immune response to ragweed pollen by immunotherapy. A controlled trial comparing the effect of immunosuppressive peptic fragments of short ragweed with standard treatment. Clin Exp Allergy. 1991;21:457–465. [DOI] [PubMed] [Google Scholar]
- 1605.Klimek L, Uhlig J, Mosges R, Rettig K, Pfaar O. A high polymerized grass pollen extract is efficacious and safe in a randomized double-blind, placebo-controlled study using a novel up-dosing cluster-protocol. Allergy. 2014;69:1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1606.Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A, Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60:801–807. [DOI] [PubMed] [Google Scholar]
- 1607.Bousquet J, Hejjaoui A, Soussana M, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. IV. Comparison of the safety and efficacy of two dosages of a high-molecular-weight allergoid. J Allergy Clin Immunol. 1990;85:490–497. [DOI] [PubMed] [Google Scholar]
- 1608.Bousquet J, Maasch HJ, Hejjaoui A, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. III. Efficacy and safety of unfractionated and high-molecular-weight preparations in rhinoconjunctivitis and asthma. J Allergy Clin Immunol. 1989;84:546–556. [DOI] [PubMed] [Google Scholar]
- 1609.Pfaar O, Nell MJ, Boot JD, et al. A randomized, 5-arm dose finding study with a mite allergoid SCIT in allergic rhinoconjunctivitis patients. Allergy. 2016;71:967–976. [DOI] [PubMed] [Google Scholar]
- 1610.Tulic MK, Fiset PO, Christodoulopoulos P, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113:235–241. [DOI] [PubMed] [Google Scholar]
- 1611.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. [DOI] [PubMed] [Google Scholar]
- 1612.Senti G, Johansen P, Haug S, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39:562–570. [DOI] [PubMed] [Google Scholar]
- 1613.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. [DOI] [PubMed] [Google Scholar]
- 1614.Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1615.Passalacqua G, Canonica GW. Allergen immunotherapy: history and future developments. Immunol Allergy Clin North Am. 2016;36:1–12. [DOI] [PubMed] [Google Scholar]
- 1616.Bachert C, Larche M, Bonini S, et al. Allergen immunotherapy on the way to product-based evaluation—a WAO statement. World Allergy Organ J. 2015;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1617.Meadows A, Kaambwa B, Novielli N, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess. 2013;17:vi, xi-xiv, 1–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1618.Lin SY, Erekosima N, Suarez-Cuervo C, et al. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review. Comparative Effectiveness Reviews, No. 111. Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://www.ncbi.nlm.nih.gov/books/NBK133239/. Accessed December 19, 2017. [PubMed] [Google Scholar]
- 1619.Purkey MT, Smith TL, Ferguson BJ, et al. Subcutaneous immunotherapy for allergic rhinitis: an evidence based review of the recent literature with recommendations. Int Forum Allergy Rhinol. 2013;3:519–531. [DOI] [PubMed] [Google Scholar]
- 1620.Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. [DOI] [PubMed] [Google Scholar]
- 1621.Rajakulasingam K Early improvement of patients’ condition during allergen-specific subcutaneous immunotherapy with a high-dose hypoallergenic 6-grass pollen preparation. Eur Ann Allergy Clin Immunol. 2012;44:128–134. [PubMed] [Google Scholar]
- 1622.Bozek A, Kolodziejczyk K, Krajewska-Wojtys A, Jarzab J. Pre-seasonal, subcutaneous immunotherapy: a double-blinded, placebo-controlled study in elderly patients with an allergy to grass. Ann Allergy Asthma Immunol. 2016;116:156–161. [DOI] [PubMed] [Google Scholar]
- 1623.Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–S55. [DOI] [PubMed] [Google Scholar]
- 1624.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. [DOI] [PubMed] [Google Scholar]
- 1625.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–1397. [DOI] [PubMed] [Google Scholar]
- 1626.Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not. A retrospective study. Clin Exp Allergy. 2001;31:1295–1302. [DOI] [PubMed] [Google Scholar]
- 1627.Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909. [DOI] [PubMed] [Google Scholar]
- 1628.Cox L, Jacobsen L. Comparison of allergen immunotherapy practice patterns in the United States and Europe. Ann Allergy Asthma Immunol. 2009;103:451–459; quiz 459–461, 495. [DOI] [PubMed] [Google Scholar]
- 1629.Franklin W, Lowell FC. Comparison of two dosages of ragweed extract in the treatment of pollenosis. JAMA. 1967;201:915–917. [PubMed] [Google Scholar]
- 1630.Johnstone DE, Dutton A. The value of hyposensitization therapy for bronchial asthma in children—a 14-year study. Pediatrics. 1968;42:793–802. [PubMed] [Google Scholar]
- 1631.Frew AJ, Powell RJ, Corrigan CJ, Durham SR; UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–325. [DOI] [PubMed] [Google Scholar]
- 1632.Nelson HS. Subcutaneous injection immunotherapy for optimal effectiveness. Immunol Allergy Clin North Am. 2011;31:211–226, viii. [DOI] [PubMed] [Google Scholar]
- 1633.Nelson H, Blaiss M, Nolte H, Wurtz SO, Andersen JS, Durham SR. Efficacy and safety of the SQ-standardized grass allergy immunotherapy tablet in mono- and polysensitized subjects. Allergy. 2013;68:252–255. [DOI] [PubMed] [Google Scholar]
- 1634.Calderon MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012;129:929–934. [DOI] [PubMed] [Google Scholar]
- 1635.Blume SW, Yeomans K, Allen-Ramey F, et al. Administration and burden of subcutaneous immunotherapy for allergic rhinitis in U.S. and Canadian clinical practice. J Manag Care Spec Pharm. 2015;21:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1636.van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:116–134. [DOI] [PubMed] [Google Scholar]
- 1637.Lowell FC, Franklin W. A double-blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N Engl J Med. 1965;273:675–679. [DOI] [PubMed] [Google Scholar]
- 1638.Reid MJ, Moss RB, Hsu YP, Kwasnicki JM, Commerford TM, Nelson BL. Seasonal asthma in northern California: allergic causes and efficacy of immunotherapy. J Allergy Clin Immunol. 1986;78:590–600. [DOI] [PubMed] [Google Scholar]
- 1639.Esch RE. Allergen immunotherapy: what can and cannot be mixed? J Allergy Clin Immunol. 2008;122:659–660. [DOI] [PubMed] [Google Scholar]
- 1640.Grier TJ, LeFevre DM, Duncan EA, Esch RE, Coyne TC. Allergen stabilities and compatibilities in mixtures of high-protease fungal and insect extracts. Ann Allergy Asthma Immunol. 2012;108:439–447. [DOI] [PubMed] [Google Scholar]
- 1641.Weber RW. Patterns of pollen cross-allergenicity. J Allergy Clin Immunol. 2003;112:229–239; quiz 240. [DOI] [PubMed] [Google Scholar]
- 1642.Van Metre TE Jr, Rosenberg GL, Vaswani SK, Ziegler SR, Adkinson NF. Pain and dermal reaction caused by injected glycerin in immunotherapy solutions. J Allergy Clin Immunol. 1996;97:1033–1039. [DOI] [PubMed] [Google Scholar]
- 1643.Plunkett G Update: stability of allergen extracts to establish expiration dating. Curr Opin Otolaryngol Head Neck Surg. 2016;24:261–269. [DOI] [PubMed] [Google Scholar]
- 1644.Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. Risk factors for fatal and nonfatal reactions to subcutaneous immunotherapy: national surveillance study on allergen immunotherapy (2008-2013). Ann Allergy Asthma Immunol. 2016;116:354–359.e2. [DOI] [PubMed] [Google Scholar]
- 1645.Schaffer FM, Garner LM, Ebeling M, Adelglass JM, Hulsey TC, Naples AR. The efficacy assessment of a self-administered immunotherapy protocol. Int Forum Allergy Rhinol. 2016;6:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1646.Nanda A, O’Connor M, Anand M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339–1344. [DOI] [PubMed] [Google Scholar]
- 1647.Lent AM, Harbeck R, Strand M, et al. Immunologic response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118:1249–1256. [DOI] [PubMed] [Google Scholar]
- 1648.Feng S, Xu Y, Ma R, Sun Y, Luo X, Li H. Cluster subcutaneous allergen specific immunotherapy for the treatment of allergic rhinitis: a systematic review and meta-analysis. PLoS One. 2014;9:e86529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1649.Winslow AW, Turbyville JC, Sublett JW, Sublett JL, Pollard SJ. Comparison of systemic reactions in rush, cluster, and standard-build aeroallergen immunotherapy. Ann Allergy Asthma Immunol. 2016;117:542–545. [DOI] [PubMed] [Google Scholar]
- 1650.Tabar AI, Echechipia S, Garcia BE, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116:109–118. [DOI] [PubMed] [Google Scholar]
- 1651.Pfaar O, Klimek L, Fischer I, et al. Safety of two cluster schedules for subcutaneous immunotherapy in allergic rhinitis or asthma patients sensitized to inhalant allergens. Int Arch Allergy Immunol. 2009;150:102–108. [DOI] [PubMed] [Google Scholar]
- 1652.Calabria CW, Cox L. Accelerated immunotherapy schedules and premedication. Immunol Allergy Clin North Am. 2011;31:251–263, ix. [DOI] [PubMed] [Google Scholar]
- 1653.Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int. 2013;62:403–413. [DOI] [PubMed] [Google Scholar]
- 1654.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–1214. [DOI] [PubMed] [Google Scholar]
- 1655.Schmitt J, Schwarz K, Stadler E, Wustenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136:1511–1516. [DOI] [PubMed] [Google Scholar]
- 1656.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. [DOI] [PubMed] [Google Scholar]
- 1657.Ebner C, Kraft D, Ebner H. Booster immunotherapy (BIT). Allergy. 1994;49:38–42. [DOI] [PubMed] [Google Scholar]
- 1658.Arroabarren E, Tabar AI, Echechipia S, Cambra K, Garcia BE, Alvarez-Puebla MJ. Optimal duration of allergen immunotherapy in children with dust mite respiratory allergy. Pediatr Allergy Immunol. 2015;26:34–41. [DOI] [PubMed] [Google Scholar]
- 1659.Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. 1993;92:6–15. [DOI] [PubMed] [Google Scholar]
- 1660.Bernstein DI, Wanner M, Borish L, Liss GM, American Academy of Allergy Asthma and Immunology Immunotherapy Committee, Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004;113:1129–1136. [DOI] [PubMed] [Google Scholar]
- 1661.Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000;106:840–843. [DOI] [PubMed] [Google Scholar]
- 1662.Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004;92:225–227. [DOI] [PubMed] [Google Scholar]
- 1663.Kennedy JL, Robinson D, Christophel J, Borish L, Payne S. Decision-making analysis for allergen immunotherapy versus nasal steroids in the treatment of nasal steroid-responsive allergic rhinitis. Am J Rhinol Allergy. 2014;28:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1664.Keiding H, Jorgensen KP. A cost-effectiveness analysis of immunotherapy with SQ allergen extract for patients with seasonal allergic rhinoconjunctivitis in selected European countries. Curr Med Res Opin. 2007;23:1113–1120. [DOI] [PubMed] [Google Scholar]
- 1665.Hankin CS, Cox L, Bronstone A, Wang Z. Allergy immunotherapy: reduced health care costs in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2013;131:1084–1091. [DOI] [PubMed] [Google Scholar]
- 1666.Larenas Linnemann DE. One hundred years of immunotherapy: review of the first landmark studies. Allergy Asthma Proc. 2012;33:122–128. [DOI] [PubMed] [Google Scholar]
- 1667.Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin Allergy. 1986;16:483–491. [DOI] [PubMed] [Google Scholar]
- 1668.Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;(12):CD002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1669.de Bot CM, Moed H, Berger MY, Roder E, van Wijk RG, van der Wouden JC. Sublingual immunotherapy in children with allergic rhinitis: quality of systematic reviews. Pediatr Allergy Immunol. 2011;22:548–558. [DOI] [PubMed] [Google Scholar]
- 1670.Roder E, Berger MY, de Groot H, van Wijk RG. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol. 2008;19:197–207. [DOI] [PubMed] [Google Scholar]
- 1671.Larenas-Linnemann D, Blaiss M, Van Bever HP, Compalati E, Baena-Cagnani CE. Pediatric sublingual immunotherapy efficacy: evidence analysis, 2009–2012. Ann Allergy Asthma Immunol. 2013;110:402–415 e409. [DOI] [PubMed] [Google Scholar]
- 1672.Kim JM, Lin SY, Suarez-Cuervo C, et al. Allergen-specific immunotherapy for pediatric asthma and rhinoconjunctivitis: a systematic review. Pediatrics. 2013;131:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1673.Durham SR, Creticos PS, Nelson HS, et al. Treatment effect of sublingual immunotherapy tablets and pharmacotherapies for seasonal and perennial allergic rhinitis: pooled analyses. J Allergy Clin Immunol. 2016;138:1081–1088.e4. [DOI] [PubMed] [Google Scholar]
- 1674.Calderon MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–311. [DOI] [PubMed] [Google Scholar]
- 1675.Maloney J, Durham S, Skoner D, et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70:302–309. [DOI] [PubMed] [Google Scholar]
- 1676.Creticos PS, Bernstein DI, Casale TB, Lockey RF, Maloney J, Nolte H. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194–1204.e4. [DOI] [PubMed] [Google Scholar]
- 1677.Oykhman P, Kim HL, Ellis AK. Allergen immunotherapy in pregnancy. Allergy Asthma Clin Immunol. 2015;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1678.Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101:206–211. [DOI] [PubMed] [Google Scholar]
- 1679.Valovirta E, Berstad AK, de Blic J, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537–1546. [DOI] [PubMed] [Google Scholar]
- 1680.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–975. [DOI] [PubMed] [Google Scholar]
- 1681.Larenas-Linnemann D How does the efficacy and safety of Oralair® compare to other products on the market? Ther Clin Risk Manag. 2016;12:831–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1682.Larenas-Linnemann D Direct comparison of efficacy of sublingual immunotherapy tablets for rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2016;116:274–286. [DOI] [PubMed] [Google Scholar]
- 1683.Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy. 2006;61:1177–1183. [DOI] [PubMed] [Google Scholar]
- 1684.Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125:660–666.e4. [DOI] [PubMed] [Google Scholar]
- 1685.Creticos PS, Esch RE, Couroux P, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–758. [DOI] [PubMed] [Google Scholar]
- 1686.Creticos PS, Maloney J, Bernstein DI, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131:1342–1349.e6. [DOI] [PubMed] [Google Scholar]
- 1687.Nolte H, Amar N, Bernstein DI, et al. Safety and tolerability of a short ragweed sublingual immunotherapy tablet. Ann Allergy Asthma Immunol. 2014;113:93–100.e3. [DOI] [PubMed] [Google Scholar]
- 1688.Cortellini G, Spadolini I, Patella V, et al. Sublingual immunotherapy for Alternaria-induced allergic rhinitis: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol. 2010;105:382–386. [DOI] [PubMed] [Google Scholar]
- 1689.Bergmann KC, Demoly P, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–1614.e6. [DOI] [PubMed] [Google Scholar]
- 1690.Swamy RS, Reshamwala N, Hunter T, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130:215–224.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1691.Amar SM, Harbeck RJ, Sills M, Silveira LJ, O’Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009;124:150–156.e5. [DOI] [PubMed] [Google Scholar]
- 1692.Leatherman BD, Khalid A, Lee S, et al. Dosing of sublingual immunotherapy for allergic rhinitis: evidence-based review with recommendations. Int Forum Allergy Rhinol. 2015;5:773–783. [DOI] [PubMed] [Google Scholar]
- 1693.Makatsori M, Scadding GW, Lombardo C, et al. Dropouts in sublingual allergen immunotherapy trials—a systematic review. Allergy. 2014;69:571–580. [DOI] [PubMed] [Google Scholar]
- 1694.Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinocon junctivitis and asthma: a systematic review. JAMA. 2013;309:1278–1288. [DOI] [PubMed] [Google Scholar]
- 1695.Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740–752. [DOI] [PubMed] [Google Scholar]
- 1696.Di Bona D, Plaia A, Scafidi V, Leto-Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010;126:558–566. [DOI] [PubMed] [Google Scholar]
- 1697.Chelladurai Y, Suarez-Cuervo C, Erekosima N, et al. Effectiveness of subcutaneous versus sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. J Allergy Clin Immunol Pract. 2013;1:361–369. [DOI] [PubMed] [Google Scholar]
- 1698.Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol. 2012;130:1097–1107.e2. [DOI] [PubMed] [Google Scholar]
- 1699.Nelson H, Cartier S, Allen-Ramey F, Lawton S, Calderon MA. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256–266.e3. [DOI] [PubMed] [Google Scholar]
- 1700.Aasbjerg K, Dalhoff KP, Backer V. Adverse events during immunotherapy against grass pollen-induced allergic rhinitis—differences between subcutaneous and sublingual treatment. Basic Clin Pharmacol Toxicol. 2015;117:73–84. [DOI] [PubMed] [Google Scholar]
- 1701.Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract. 2014;20:225–238. [DOI] [PubMed] [Google Scholar]
- 1702.Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132:1322–1336. [DOI] [PubMed] [Google Scholar]
- 1703.Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–1366. [DOI] [PubMed] [Google Scholar]
- 1704.Hoeks SB, de Groot H, Hoekstra MO. [Sublingual immunotherapy in children with asthma or rhinoconjunctivitis: not enough evidence because of poor quality of the studies; a systematic review of literature]. Ned Tijdschr Geneeskd. 2008;152:261–268. Dutch. [PubMed] [Google Scholar]
- 1705.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725.e5. [DOI] [PubMed] [Google Scholar]
- 1706.Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1707.Larsson O, Hellkvist L, Peterson-Westin U, Cardell LO. Novel strategies for the treatment of grass pollen-induced allergic rhinitis. Expert Opin Biol Ther. 2016;16:1143–1150. [DOI] [PubMed] [Google Scholar]
- 1708.Senti G, Kundig TM. Novel delivery routes for allergy immunotherapy: intralymphatic, epicutaneous, and intradermal. Immunol Allergy Clin North Am. 2016;36:25–37. [DOI] [PubMed] [Google Scholar]
- 1709.Cox L, Compalati E, Kundig T, Larche M. New directions in immunotherapy. Curr Allergy Asthma Rep. 2013;13:178–195. [DOI] [PubMed] [Google Scholar]
- 1710.Garaczi E, Szabo K, Francziszti L, et al. DermAll nanomedicine for allergen-specific immunotherapy. Nanomedicine. 2013;9:1245–1254. [DOI] [PubMed] [Google Scholar]
- 1711.Phillips EW. Relief of hay-fever by intradermal injections of pollen extract. JAMA. 1926;86:182–184. 10.1001/jama.1926.02670290022008. [DOI] [Google Scholar]
- 1712.Senti G, Graf N, Haug S, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;124:997–1002. [DOI] [PubMed] [Google Scholar]
- 1713.Agostinis F, Forti S, Di Berardino F. Grass transcutaneous immunotherapy in children with seasonal rhinoconjunctivitis. Allergy. 2010;65:410–411. [DOI] [PubMed] [Google Scholar]
- 1714.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. [DOI] [PubMed] [Google Scholar]
- 1715.Senti G, von Moos S, Tay F, Graf N, Johansen P, Kundig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy. 2015;70:707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1716.Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1717.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–1296. [DOI] [PubMed] [Google Scholar]
- 1718.Hylander T, Latif L, Petersson-Westin U, Cardell LO. Intralymphatic allergen-specific immunotherapy: an effective and safe alternative treatment route for pollen-induced allergic rhinitis. J Allergy Clin Immunol. 2013;131:412–420. [DOI] [PubMed] [Google Scholar]
- 1719.Hylander T, Larsson O, Petersson-Westin U, et al. Intralymphatic immunotherapy of pollen-induced rhinoconjunctivitis: a double-blind placebo-controlled trial. Respir Res. 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1720.Patterson AM, Bonny AE, Shiels WE, Erwin EA 2nd. Three-injection intralymphatic immunotherapy in adolescents and young adults with grass pollen rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2016;116:168–170. [DOI] [PubMed] [Google Scholar]
- 1721.Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013;132:1248–1252.e5. [DOI] [PubMed] [Google Scholar]
- 1722.Schmid JM, Nezam H, Madsen HH, Schmitz A, Hoffmann HJ. Intralymphatic immunotherapy induces allergen specific plasmablasts and increases tolerance to skin prick testing in a pilot study. Clin Transl Allergy. 2016;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1723.Taudorf E, Laursen LC, Lanner A, et al. Oral immunotherapy in birch pollen hay fever. J Allergy Clin Immunol. 1987;80:153–161. [DOI] [PubMed] [Google Scholar]
- 1724.Oppenheimer J, Areson JG, Nelson HS. Safety and efficacy of oral immunotherapy with standardized cat extract. J Allergy Clin Immunol. 1994;93:61–67. [DOI] [PubMed] [Google Scholar]
- 1725.Van Deusen MA, Angelini BL, Cordoro KM, Seiler BA, Wood L, Skoner DP. Efficacy and safety of oral immunotherapy with short ragweed extract. Ann Allergy Asthma Immunol. 1997;78:573–580. [DOI] [PubMed] [Google Scholar]
- 1726.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–1269. [DOI] [PubMed] [Google Scholar]
- 1727.Allam JP, Stojanovski G, Friedrichs N, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–727. [DOI] [PubMed] [Google Scholar]
- 1728.Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1729.Reisacher WR, Suurna MV, Rochlin K, Bremberg MG, Tropper G. Oral mucosal immunotherapy for allergic rhinitis: a pilot study. Allergy Rhinol (Providence). 2016;7:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1730.Passalacqua G, Albano M, Ruffoni S, et al. Nasal immunotherapy to Parietaria: evidence of reduction of local allergic inflammation. Am J Respir Crit Care Med. 1995;152:461–466. [DOI] [PubMed] [Google Scholar]
- 1731.Pajno GB, Vita D, Caminiti L, et al. Children’s compliance with allergen immunotherapy according to administration routes. J Allergy Clin Immunol. 2005;116:1380–1381. [DOI] [PubMed] [Google Scholar]
- 1732.Tari MG, Mancino M, Monti G. Immunotherapy by inhalation of allergen in powder in house dust allergic asthma—a double-blind study. J Investig Allergol Clin Immunol. 1992;2:59–67. [PubMed] [Google Scholar]
- 1733.Hamelmann E, Rolinck-Werninghaus C, Wahn U. Is there a role for anti-IgE in combination with specific allergen immunotherapy? Curr Opin Allergy Clin Immunol. 2003;3:501–510. [DOI] [PubMed] [Google Scholar]
- 1734.Klunker S, Saggar LR, Seyfert-Margolis V, et al. Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120:688–695. [DOI] [PubMed] [Google Scholar]
- 1735.Kopp MV, Brauburger J, Riedinger F, et al. The effect of anti-IgE treatment on in vitro leukotriene release in children with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;110:728–735. [DOI] [PubMed] [Google Scholar]
- 1736.Massanari M, Nelson H, Casale T, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–389. [DOI] [PubMed] [Google Scholar]
- 1737.Portnoy J, King K, Kanarek H, Horner S. Incidence of systemic reactions during rush immunotherapy. Ann Allergy. 1992;68:493–498. [PubMed] [Google Scholar]
- 1738.Lockey RF, Nicoara-Kasti GL, Theodoropoulos DS, Bukantz SC. Systemic reactions and fatalities associated with allergen immunotherapy. Ann Allergy Asthma Immunol. 2001;87:47–55. [DOI] [PubMed] [Google Scholar]
- 1739.Begin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1740.Schulze J, Rose M, Zielen S. Beekeepers anaphylaxis: successful immunotherapy covered by omalizumab. Allergy. 2007;62:963–964. [DOI] [PubMed] [Google Scholar]
- 1741.Galera C, Soohun N, Zankar N, Caimmi S, Gallen C, Demoly P. Severe anaphylaxis to bee venom immunotherapy: efficacy of pretreatment and concurrent treatment with omalizumab. J Investig Allergol Clin Immunol. 2009;19:225–229. [PubMed] [Google Scholar]
- 1742.Cox L, Platts-Mills TA, Finegold I, et al. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007;120:1373–1377. [DOI] [PubMed] [Google Scholar]
- 1743.Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007;120:1378–1381. [DOI] [PubMed] [Google Scholar]
- 1744.Global Initiative for Asthma. Global strategy for asthma management and prevention. Global Initiative for Asthma; 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed December 19, 2017. [Google Scholar]
- 1745.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR3). Guidelines for the diagnosis and management of asthma. https://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf. Accessed December 19, 2017.
- 1746.British guideline on the management of asthma Edinburgh: UK: Scottish Intercollegiate Guidelines Network; London, UK: British Thoracic Society. 2016. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016. December 19, 2017. [Google Scholar]
- 1747.Plaza Moral V, Alonso Mostaza S, Alvarez Rodriguez C, et al. Spanish guideline on the management of asthma. J Investig Allergol Clin Immunol. 2016;26(Suppl 1):1–92. [DOI] [PubMed] [Google Scholar]
- 1748.Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26:710–715. [DOI] [PubMed] [Google Scholar]
- 1749.Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. [DOI] [PubMed] [Google Scholar]
- 1750.Krouse JH, Brown RW, Fineman SM, et al. Asthma and the unified airway. Otolaryngol Head Neck Surg. 2007;136:S75–S106. [DOI] [PubMed] [Google Scholar]
- 1751.Antonicelli L, Micucci C, Voltolini S, et al. Allergic rhinitis and asthma comorbidity: ARIA classification of rhinitis does not correlate with the prevalence of asthma. Clin Exp Allergy. 2007;37:954–960. [DOI] [PubMed] [Google Scholar]
- 1752.Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T; Copenhagen Allergy Study. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57:1048–1052. [DOI] [PubMed] [Google Scholar]
- 1753.Leynaert B, Neukirch C, Kony S, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004;113:86–93. [DOI] [PubMed] [Google Scholar]
- 1754.Ohta K, Bousquet PJ, Aizawa H, et al. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy. 2011;66:1287–1295. [DOI] [PubMed] [Google Scholar]
- 1755.Ponte EV, Franco R, Nascimento HF, et al. Lack of control of severe asthma is associated with coexistence of moderate-to-severe rhinitis. Allergy. 2008;63:564–569. [DOI] [PubMed] [Google Scholar]
- 1756.Valero A, Pereira C, Loureiro C, et al. Interrelationship between skin sensitization, rhinitis, and asthma in patients with allergic rhinitis: a study of Spain and Portugal. J Investig Allergol Clin Immunol. 2009;19:167–172. [PubMed] [Google Scholar]
- 1757.Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. [DOI] [PubMed] [Google Scholar]
- 1758.ten Brinke A, Grootendorst DC, Schmidt JT, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. [DOI] [PubMed] [Google Scholar]
- 1759.Bousquet J, Gaugris S, Kocevar VS, et al. Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: a subgroup analysis of the investigation of montelukast as a partner agent for complementary therapy [corrected]. Clin Exp Allergy. 2005;35:723–727. [DOI] [PubMed] [Google Scholar]
- 1760.Price D, Zhang Q, Kocevar VS, Yin DD, Thomas M. Effect of a concomitant diagnosis of allergic rhinitis on asthma-related health care use by adults. Clin Exp Allergy. 2005;35:282–287. [DOI] [PubMed] [Google Scholar]
- 1761.Sazonov Kocevar V, Thomas J 3rd, Jonsson L, et al. Association between allergic rhinitis and hospital resource use among asthmatic children in Norway. Allergy. 2005;60:338–342. [DOI] [PubMed] [Google Scholar]
- 1762.Thomas M, Kocevar VS, Zhang Q, Yin DD, Price D. Asthma-related health care resource use among asthmatic children with and without concomitant allergic rhinitis. Pediatrics. 2005;115:129–134. [DOI] [PubMed] [Google Scholar]
- 1763.Gaugris S, Sazonov-Kocevar V, Thomas M. Burden of concomitant allergic rhinitis in adults with asthma. J Asthma. 2006;43:1–7. [DOI] [PubMed] [Google Scholar]
- 1764.Ibanez MD, Valero AL, Montoro J, et al. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr Allergy Immunol. 2013;24:678–684. [DOI] [PubMed] [Google Scholar]
- 1765.Latvala J, von Hertzen L, Lindholm H, Haahtela T. Trends in prevalence of asthma and allergy in Finnish young men: nationwide study, 1966–2003. BMJ. 2005;330:1186–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1766.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy. 2004;34:38–43. [DOI] [PubMed] [Google Scholar]
- 1767.Lee SL, Wong W, Lau YL. Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood). Pediatr Allergy Immunol. 2004;15:72–78. [DOI] [PubMed] [Google Scholar]
- 1768.Selnes A, Nystad W, Bolle R, Lund E. Diverging prevalence trends of atopic disorders in Norwegian children. Results from three cross-sectional studies. Allergy. 2005;60:894–899. [DOI] [PubMed] [Google Scholar]
- 1769.Anderson HR, Ruggles R, Strachan DP, et al. Trends in prevalence of symptoms of asthma, hay fever, and eczema in 12–14 year olds in the British Isles, 1995–2002: questionnaire survey. BMJ. 2004;328:1052–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1770.Huurre TM, Aro HM, Jaakkola JJ. Incidence and prevalence of asthma and allergic rhinitis: a cohort study of Finnish adolescents. J Asthma. 2004;41:311–317. [DOI] [PubMed] [Google Scholar]
- 1771.Robertson CF, Roberts MF, Kappers JH. Asthma prevalence in Melbourne schoolchildren: have we reached the peak? Med J Aust. 2004;180:273–276. [DOI] [PubMed] [Google Scholar]
- 1772.Teeratakulpisarn J, Wiangnon S, Kosalaraksa P, Heng S. Surveying the prevalence of asthma, allergic rhinitis and eczema in school-children in Khon Kaen, Northeastern Thailand using the ISAAC questionnaire: phase III. Asian Pac J Allergy Immunol. 2004;22:175–181. [PubMed] [Google Scholar]
- 1773.Vellinga A, Droste JH, Vermeire PA, et al. Changes in respiratory and allergic symptoms in schoolchildren from 1996 to 2002, results from the ISAAC surveys in Antwerp (Belgium). Acta Clin Belg.2005;60:219–225. [DOI] [PubMed] [Google Scholar]
- 1774.Galassi C, De Sario M, Biggeri A, et al. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994-2002. Pediatrics. 2006;117:34–42. [DOI] [PubMed] [Google Scholar]
- 1775.Braun-Fahrlander C, Gassner M, Grize L, et al. No further increase in asthma, hay fever and atopic sensitisation in adolescents living in Switzerland. Eur Respir J. 2004;23:407–413. [DOI] [PubMed] [Google Scholar]
- 1776.Settipane RJ, Settipane GA. IgE and the allergy-asthma connection in the 23-year follow-up of Brown University students. Allergy Asthma Proc. 2000;21:221–225. [DOI] [PubMed] [Google Scholar]
- 1777.Bodtger U, Poulsen LK, Linneberg A. Rhinitis symptoms and IgE sensitization as risk factors for development of later allergic rhinitis in adults. Allergy. 2006;61:712–716. [DOI] [PubMed] [Google Scholar]
- 1778.Plaschke PP, Janson C, Norrman E, Bjornsson E, Ellbjar S, Jarvholm B. Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am J Respir Crit Care Med. 2000;162:920–924. [DOI] [PubMed] [Google Scholar]
- 1779.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–425. [DOI] [PubMed] [Google Scholar]
- 1780.Toren K, Olin AC, Hellgren J, Hermansson BA. Rhinitis increase the risk for adult-onset asthma—a Swedish population-based case-control study (MAP-study). Respir Med. 2002;96:635–641. [DOI] [PubMed] [Google Scholar]
- 1781.Porsbjerg C, von Linstow ML, Ulrik CS, Nepper-Christensen S, Backer V. Risk factors for onset of asthma: a 12-year prospective follow-up study. Chest. 2006;129:309–316. [DOI] [PubMed] [Google Scholar]
- 1782.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106:S201–S205. [DOI] [PubMed] [Google Scholar]
- 1783.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–1057. [DOI] [PubMed] [Google Scholar]
- 1784.Shaaban R, Zureik M, Soussan D, et al. Allergic rhinitis and onset of bronchial hyperresponsiveness: a population-based study. Am J Respir Crit Care Med. 2007;176:659–666. [DOI] [PubMed] [Google Scholar]
- 1785.Rochat MK, Illi S, Ege MJ, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. 2010;126:1170–1175.e2. [DOI] [PubMed] [Google Scholar]
- 1786.Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863–869. [DOI] [PubMed] [Google Scholar]
- 1787.Sibbald B, Rink E. Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax. 1991;46:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1788.Corren J, Adinoff AD, Buchmeier AD, Irvin CG. Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 1992;90:250–256. [DOI] [PubMed] [Google Scholar]
- 1789.Kersten ET, van Leeuwen JC, Brand PL, et al. Effect of an intranasal corticosteroid on exercise induced bronchoconstriction in asthmatic children. Pediatr Pulmonol. 2012;47:27–35. [DOI] [PubMed] [Google Scholar]
- 1790.Reed CE, Marcoux JP, Welsh PW. Effects of topical nasal treatment on asthma symptoms. J Allergy Clin Immunol. 1988;81:1042–1047. [DOI] [PubMed] [Google Scholar]
- 1791.Chyrek-Borowska S, Siergiejko Z, Michalska I. The effects of a new generation of H1 antihistamines (cetirizine and loratadine) on histamine release and the bronchial response to histamine in atopic patients. J Investig Allergol Clin Immunol. 1995;5:103–107. [PubMed] [Google Scholar]
- 1792.Wasserfallen JB, Leuenberger P, Pecoud A. Effect of cetirizine, a new H1 antihistamine, on the early and late allergic reactions in a bronchial provocation test with allergen. J Allergy Clin Immunol. 1993;91:1189–1197. [DOI] [PubMed] [Google Scholar]
- 1793.Nishimura M, Koga T, Kamimura T, et al. Comparison of leukotriene receptor antagonists and antihistamines as an add-on therapy in patients with asthma complicated by allergic rhinitis. Kurume Med J. 2011;58:9–14. [DOI] [PubMed] [Google Scholar]
- 1794.Suissa S, Ernst P. Bias in observational study of the effectiveness of nasal corticosteroids in asthma. J Allergy Clin Immunol. 2005;115:714–719. [DOI] [PubMed] [Google Scholar]
- 1795.Grembiale RD, Camporota L, Naty S, Tranfa CM, Djukanovic R, Marsico SA. Effects of specific immunotherapy in allergic rhinitic individuals with bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162:2048–2052. [DOI] [PubMed] [Google Scholar]
- 1796.Rak S, Lowhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol. 1988;82:470–480. [DOI] [PubMed] [Google Scholar]
- 1797.Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. 2002;109:251–256. [DOI] [PubMed] [Google Scholar]
- 1798.Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–857. [DOI] [PubMed] [Google Scholar]
- 1799.Gotzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;(2):CD001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1800.Terreehorst I, Duivenvoorden HJ, Tempels-Pavlica Z, et al. The effect of encasings on quality of life in adult house dust mite allergic patients with rhinitis, asthma and/or atopic dermatitis. Allergy. 2005;60:888–893. [DOI] [PubMed] [Google Scholar]
- 1801.National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. [DOI] [PubMed] [Google Scholar]
- 1802.Egan M, Bunyavanich S. Allergic rhinitis: the “Ghost Diagnosis” in patients with asthma. Asthma Res Pract. 2015;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1803.Simons FE. Is antihistamine (H1-receptor antagonist) therapy useful in clinical asthma? Clin Exp Allergy. 1999;29(Suppl 3):98–104. [DOI] [PubMed] [Google Scholar]
- 1804.Aubier M, Neukirch C, Peiffer C, Melac M. Effect of cetirizine on bronchial hyperresponsiveness in patients with seasonal allergic rhinitis and asthma. Allergy. 2001;56:35–42. [DOI] [PubMed] [Google Scholar]
- 1805.Bousquet J, Emonot A, Germouty J, et al. Double-blind multicenter study of cetirizine in grass-pollen-induced asthma. Ann Allergy. 1990;65:504–508. [PubMed] [Google Scholar]
- 1806.Van Ganse E, Kaufman L, Derde MP, Yernault JC, Delaunois L, Vincken W. Effects of antihistamines in adult asthma: a meta-analysis of clinical trials. Eur Respir J. 1997;10:2216–2224. [DOI] [PubMed] [Google Scholar]
- 1807.Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomised, placebo-controlled trial: first results of ETAC. Early Treatment of the Atopic Child. Pediatr Allergy Immunol. 1998;9:116–124. [PubMed] [Google Scholar]
- 1808.Anvari S, Vyhlidal CA, Dai H, Jones BL. Genetic variation along the histamine pathway in children with allergic versus nonallergic asthma. Am J Respir Cell Mol Biol. 2015;53:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1809.Bhargava S, Prakash A, Rehan HS, Gupta LK. Effect of systemic corticosteroids on serum apoptotic markers and quality of life in patients with asthma. Allergy Asthma Proc. 2015;36:275–282. [DOI] [PubMed] [Google Scholar]
- 1810.Henriksen JM, Wenzel A. Effect of an intranasally administered corticosteroid (budesonide) on nasal obstruction, mouth breathing, and asthma. Am Rev Respir Dis. 1984;130:1014–1018. [DOI] [PubMed] [Google Scholar]
- 1811.Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993;91:97–101. [DOI] [PubMed] [Google Scholar]
- 1812.Gani F, Pozzi E, Crivellaro MA, et al. The role of patient training in the management of seasonal rhinitis and asthma: clinical implications. Allergy. 2001;56:65–68. [DOI] [PubMed] [Google Scholar]
- 1813.Meltzer EO. Role for cysteinyl leukotriene receptor antagonist therapy in asthma and their potential role in allergic rhinitis based on the concept of “one linked airway disease”. Ann Allergy Asthma Immunol. 2000;84:176–185; quiz 185-177. [DOI] [PubMed] [Google Scholar]
- 1814.Bousquet J, Reid J, van Weel C, et al. Allergic rhinitis management pocket reference 2008. Allergy. 2008;63:990–996. [DOI] [PubMed] [Google Scholar]
- 1815.Nowak D Management of asthma with anti-immunoglobulin E: a review of clinical trials of omalizumab. Respir Med. 2006;100:1907–1917. [DOI] [PubMed] [Google Scholar]
- 1816.Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–308. [DOI] [PubMed] [Google Scholar]
- 1817.D’Amato G, Salzillo A, Piccolo A, D’Amato M, Liccardi G. A review of anti-IgE monoclonal antibody (omalizumab) as add on therapy for severe allergic (IgE-mediated) asthma. Ther Clin Risk Manag. 2007;3:613–619. [PMC free article] [PubMed] [Google Scholar]
- 1818.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1819.Humbert M, Boulet LP, Niven RM, Panahloo Z, Blogg M, Ayre G. Omalizumab therapy: patients who achieve greatest benefit for their asthma experience greatest benefit for rhinitis. Allergy. 2009;64:81–84. [DOI] [PubMed] [Google Scholar]
- 1820.Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59:709–717. [DOI] [PubMed] [Google Scholar]
- 1821.Lai T, Wang S, Xu Z, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1822.Erekosima N, Suarez-Cuervo C, Ramanathan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124:616–627. [DOI] [PubMed] [Google Scholar]
- 1823.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. [DOI] [PubMed] [Google Scholar]
- 1824.Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. [DOI] [PubMed] [Google Scholar]
- 1825.Inal A, Altintas DU, Yilmaz M, Karakoc GB, Kendirli SG, Sertdemir Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J Investig Allergol Clin Immunol. 2007;17:85–91. [PubMed] [Google Scholar]
- 1826.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–859. [DOI] [PubMed] [Google Scholar]
- 1827.Pasquali M, Baiardini I, Rogkakou A, et al. Levocetirizine in persistent allergic rhinitis and asthma: effects on symptoms, quality of life and inflammatory parameters. Clin Exp Allergy. 2006;36:1161–1167. [DOI] [PubMed] [Google Scholar]
- 1828.Baena-Cagnani CE, Berger WE, DuBuske LM, et al. Comparative effects of desloratadine versus montelukast on asthma symptoms and use of beta 2-agonists in patients with seasonal allergic rhinitis and asthma. Int Arch Allergy Immunol. 2003;130:307–313. [DOI] [PubMed] [Google Scholar]
- 1829.Berger WE, Schenkel EJ, Mansfield LE; Desloratadine Study Group. Safety and efficacy of desloratadine 5 mg in asthma patients with seasonal allergic rhinitis and nasal congestion. Ann Allergy Asthma Immunol. 2002;89:485–491. [DOI] [PubMed] [Google Scholar]
- 1830.Aaronson DW. Evaluation of cetirizine in patients with allergic rhinitis and perennial asthma. Ann Allergy Asthma Immunol. 1996;76:447–446. [DOI] [PubMed] [Google Scholar]
- 1831.Grant JA, Nicodemus CF, Findlay SR, et al. Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1995;95:923–932. [DOI] [PubMed] [Google Scholar]
- 1832.Jindal A, Suriyan S, Sagadevan S, et al. Comparison of oral montelukast and intranasal fluticasone in patients with asthma and allergic rhinitis. J Clin Diagn Res. 2016;10:OC06–OC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1833.Baiardini I, Villa E, Rogkakou A, et al. Effects of mometasone furoate on the quality of life: a randomized placebo-controlled trial in persistent allergic rhinitis and intermittent asthma using the Rhinasthma questionnaire. Clin Exp Allergy. 2011;41:417–423. [DOI] [PubMed] [Google Scholar]
- 1834.Nair A, Vaidyanathan S, Clearie K, Williamson P, Meldrum K, Lipworth BJ. Steroid sparing effects of intranasal corticosteroids in asthma and allergic rhinitis. Allergy. 2010;65:359–367. [DOI] [PubMed] [Google Scholar]
- 1835.Agondi RC, Machado ML, Kalil J, Giavina-Bianchi P Intranasal corticosteroid administration reduces nonspecific bronchial hyperresponsiveness and improves asthma symptoms. J Asthma. 2008;45:754–757. [DOI] [PubMed] [Google Scholar]
- 1836.Pedroletti C, Lundahl J, Alving K, Hedlin G. Effect of nasal steroid treatment on airway inflammation determined by exhaled nitric oxide in allergic schoolchildren with perennial rhinitis and asthma. Pediatr Allergy Immunol. 2008;19:219–226. [DOI] [PubMed] [Google Scholar]
- 1837.Dahl R, Nielsen LP, Kips J, et al. Intranasal and inhaled fluticasone propionate for pollen-induced rhinitis and asthma. Allergy. 2005;60:875–881. [DOI] [PubMed] [Google Scholar]
- 1838.Nathan RA, Yancey SW, Waitkus-Edwards K, et al. Fluticasone propionate nasal spray is superior to montelukast for allergic rhinitis while neither affects overall asthma control. Chest. 2005;128:1910–1920. [DOI] [PubMed] [Google Scholar]
- 1839.Stelmach R, do Patrocinio TNM, Ribeiro M, Cukier A. Effect of treating allergic rhinitis with corticosteroids in patients with mild-to-moderate persistent asthma. Chest. 2005;128:3140–3147. [DOI] [PubMed] [Google Scholar]
- 1840.Thio BJ, Slingerland GL, Fredriks AM, et al. Influence of intranasal steroids during the grass pollen season on bronchial responsiveness in children and young adults with asthma and hay fever. Thorax. 2000;55:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1841.Katial RK, Oppenheimer JJ, Ostrom NK, et al. Adding montelukast to fluticasone propionate/salmeterol for control of asthma and seasonal allergic rhinitis. Allergy Asthma Proc. 2010;31:68–75. [DOI] [PubMed] [Google Scholar]
- 1842.Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006;61:737–742. [DOI] [PubMed] [Google Scholar]
- 1843.Arbes SJ Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. [DOI] [PubMed] [Google Scholar]
- 1844.Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121:1126–1132 e1127. [DOI] [PubMed] [Google Scholar]
- 1845.Baroody FM, Mucha SM, deTineo M, Naclerio RM. Evidence of maxillary sinus inflammation in seasonal allergic rhinitis. Otolaryngol Head Neck Surg. 2012;146:880–886. [DOI] [PubMed] [Google Scholar]
- 1846.Naclerio RM, deTineo ML, Baroody FM. Ragweed allergic rhinitis and the paranasal sinuses. A computed tomographic study. Arch Otolaryngol Head Neck Surg. 1997;123:193–196. [DOI] [PubMed] [Google Scholar]
- 1847.Savolainen S Allergy in patients with acute maxillary sinusitis. Allergy. 1989;44:116–122. [DOI] [PubMed] [Google Scholar]
- 1848.Chen CF, Wu KG, Hsu MC, Tang RB. Prevalence and relationship between allergic diseases and infectious diseases. J Microbiol Immunol Infect. 2001;34:57–62. [PubMed] [Google Scholar]
- 1849.Holzmann D, Willi U, Nadal D. Allergic rhinitis as a risk factor for orbital complication of acute rhinosinusitis in children. Am J Rhinol. 2001;15:387–390. [PubMed] [Google Scholar]
- 1850.Yu X, Sperling A, Blair C, Thompson K, Naclerio R. Antigen stimulation of TH2 cells augments acute bacterial sinusitis in mice. J Allergy Clin Immunol. 2004;114:328–334. [DOI] [PubMed] [Google Scholar]
- 1851.Naclerio R, Blair C, Yu X, Won YS, Gabr U, Baroody FM. Allergic rhinitis augments the response to a bacterial sinus infection in mice: a review of an animal model. Am J Rhinol. 2006;20:524–533. [DOI] [PubMed] [Google Scholar]
- 1852.Kalfa VC, Spector SL, Ganz T, Cole AM. Lysozyme levels in the nasal secretions of patients with perennial allergic rhinitis and recurrent sinusitis. Ann Allergy Asthma Immunol. 2004;93:288–292. [DOI] [PubMed] [Google Scholar]
- 1853.Melvin TA, Lane AP, Nguyen MT, Lin SY. Allergic rhinitis patients with recurrent acute sinusitis have increased sinonasal epithelial cell TLR9 expression. Otolaryngol Head Neck Surg. 2010;142:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1854.Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2014;4:93–103. [DOI] [PubMed] [Google Scholar]
- 1855.Li QC, Cheng KJ, Wang F, Zhou SH. Role of atopy in chronic rhinosinusitis with nasal polyps: does an atopic condition affect the severity and recurrence of disease? J Laryngol Otol. 2016;130:640–644. [DOI] [PubMed] [Google Scholar]
- 1856.Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections in adults with atopic disease and IgE antibodies to common aeroallergens. PLoS One. 2013;8:e68582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1857.Frerichs KA, Nigten G, Romeijn K, Kaper NM, Grolman W, van der Heijden GJ. Inconclusive evidence for allergic rhinitis to predict a prolonged or chronic course of acute rhinosinusitis. Otolaryngol Head Neck Surg. 2014;150:22–27. [DOI] [PubMed] [Google Scholar]
- 1858.Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1859.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1860.Gelincik A, Buyukozturk S, Aslan I, et al. Allergic vs nonallergic rhinitis: which is more predisposing to chronic rhinosinusitis? Ann Allergy Asthma Immunol. 2008;101:18–22. [DOI] [PubMed] [Google Scholar]
- 1861.Kirtsreesakul V, Ruttanaphol S. The relationship between allergy and rhinosinusitis. Rhinology. 2008;46:204–208. [PubMed] [Google Scholar]
- 1862.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20:625–628. [DOI] [PubMed] [Google Scholar]
- 1863.Alho OP, Karttunen R, Karttunen TJ. Nasal mucosa in natural colds: effects of allergic rhinitis and susceptibility to recurrent sinusitis. Clin Exp Immunol. 2004;137:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1864.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–983. [DOI] [PubMed] [Google Scholar]
- 1865.Berrettini S, Carabelli A, Sellari-Franceschini S, et al. Perennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factors. Allergy. 1999;54:242–248. [DOI] [PubMed] [Google Scholar]
- 1866.Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope. 2008;118:1521–1527. [DOI] [PubMed] [Google Scholar]
- 1867.Al-Qudah M Food sensitization in medically resistant chronic rhinosinusitis with or without nasal polyposis. Int Arch Allergy Immunol. 2016;169:40–44. [DOI] [PubMed] [Google Scholar]
- 1868.Gorgulu O, Ozdemir S, Canbolat EP, Sayar C, Olgun MK, Akbas Y. Analysis of the roles of smoking and allergy in nasal polyposis. Ann Otol Rhinol Laryngol. 2012;121:615–619. [DOI] [PubMed] [Google Scholar]
- 1869.Lill C, Loader B, Seemann R, et al. Milk allergy is frequent in patients with chronic sinusitis and nasal polyposis. Am J Rhinol Allergy. 2011;25:e221–e224. [DOI] [PubMed] [Google Scholar]
- 1870.Munoz del Castillo F, Jurado-Ramos A, Fernandez-Conde BL, et al. Allergenic profile of nasal polyposis. J Investig Allergol Clin Immunol. 2009;19:110–116. [PubMed] [Google Scholar]
- 1871.Collins MM, Loughran S, Davidson P, Wilson JA. Nasal polyposis: prevalence of positive food and inhalant skin tests. Otolaryngol Head Neck Surg. 2006;135:680–683. [DOI] [PubMed] [Google Scholar]
- 1872.Kirtsreesakul V Role of allergy in the therapeutic response of nasal polyps. Asian Pac J Allergy Immunol. 2002;20:141–146. [PubMed] [Google Scholar]
- 1873.Voegels RL, Santoro P, Butugan O, Formigoni LG. Nasal polyposis and allergy: is there a correlation? Am J Rhinol. 2001;15:9–14. [DOI] [PubMed] [Google Scholar]
- 1874.Asero R, Bottazzi G. Nasal polyposis: a study of its association with airborne allergen hypersensitivity. Ann Allergy Asthma Immunol. 2001;86:283–285. [DOI] [PubMed] [Google Scholar]
- 1875.Asero R, Bottazzi G. Hypersensitivity to molds in patients with nasal polyposis: a clinical study. J Allergy Clin Immunol. 2000;105:186–188. [DOI] [PubMed] [Google Scholar]
- 1876.Pang YT, Eskici O, Wilson JA. Nasal polyposis: role of subclinical delayed food hypersensitivity. Otolaryngol Head Neck Surg. 2000;122:298–301. [DOI] [PubMed] [Google Scholar]
- 1877.Pumhirun P, Limitlaohapanth C, Wasuwat P. Role of allergy in nasal polyps of Thai patients. Asian Pac J Allergy Immunol. 1999;17:13–15. [PubMed] [Google Scholar]
- 1878.Keith PK, Conway M, Evans S, et al. Nasal polyps: effects of seasonal allergen exposure. J Allergy Clin Immunol. 1994;93:567–574. [DOI] [PubMed] [Google Scholar]
- 1879.Bonfils P, Malinvaud D. Influence of allergy in patients with nasal polyposis after endoscopic sinus surgery. Acta Otolaryngol. 2008;128:186–192. [DOI] [PubMed] [Google Scholar]
- 1880.Erbek SS, Erbek S, Topal O, Cakmak O. The role of allergy in the severity of nasal polyposis. Am J Rhinol. 2007;21:686–690. [DOI] [PubMed] [Google Scholar]
- 1881.Bonfils P, Avan P, Malinvaud D. Influence of allergy on the symptoms and treatment of nasal polyposis. Acta Otolaryngol. 2006;126:839–844. [DOI] [PubMed] [Google Scholar]
- 1882.Berger WE, Granet DB, Kabat AG. Diagnosis and management of allergic conjunctivitis in pediatric patients. Allergy Asthma Proc. 2017;38:16–27. [DOI] [PubMed] [Google Scholar]
- 1883.Strachan D, Sibbald B, Weiland S, et al. World-wide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allergy Immunol. 1997;8:161–176. [DOI] [PubMed] [Google Scholar]
- 1884.Kim DH, Park YS, Jang HJ, Kim JH, Lim DH. Prevalence and allergen of allergic rhinitis in Korean children. Am J Rhinol Allergy. 2016;30:72–78. [DOI] [PubMed] [Google Scholar]
- 1885.Alexandropoulos T, Haidich AB, Pilalas D, Dardavessis T, Daniilidis M, Arvanitidou M. Characteristics of patients with allergic rhinitis in an outpatient clinic: a retrospective study. Allergol Immunopathol(Madr). 2013;41:194–200. [DOI] [PubMed] [Google Scholar]
- 1886.Gradman J, Wolthers OD. Allergic conjunctivitis in children with asthma, rhinitis and eczema in a secondary outpatient clinic. Pediatr Allergy Immunol. 2006;17:524–526. [DOI] [PubMed] [Google Scholar]
- 1887.Kosrirukvongs P, Visitsunthorn N, Vichyanond P, Bunnag C. Allergic conjunctivitis. Asian Pac J Allergy Immunol. 2001;19:237–244. [PubMed] [Google Scholar]
- 1888.Almaliotis D, Michailopoulos P, Gioulekas D, et al. Allergic conjunctivitis and the most common allergens in Northern Greece. World Allergy Organ J. 2013;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1889.Han DH, Ahn JC, Mun SJ, Park SK, Oh SY, Rhee CS. Novel risk factors for allergic rhinitis in Korean elementary school children: ARCO-kids phase II in a community. Allergy Asthma Immunol Res. 2015;7:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1890.Navarro A, Colas C, Anton E, et al. Epidemiology of allergic rhinitis in allergy consultations in Spain: Alergologica-2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):7–13. [PubMed] [Google Scholar]
- 1891.Bielory L, Skoner DP, Blaiss MS, et al. Ocular and nasal allergy symptom burden in America: the Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Allergy Asthma Proc. 2014;35:211–218. [DOI] [PubMed] [Google Scholar]
- 1892.Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295–299 e291-e227. [DOI] [PubMed] [Google Scholar]
- 1893.Drucker AM. Atopic dermatitis: burden of illness, quality of life, and associated complications. Allergy Asthma Proc. 2017;38:3–8. [DOI] [PubMed] [Google Scholar]
- 1894.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S127. [DOI] [PubMed] [Google Scholar]
- 1895.The ISAAC story. The International Study of Allergies and Asthma in Childhood. ISAAC Steering Committee; 2017. http://isaac.auckland.ac.nz/story/index.html. Accessed December 19, 2017.
- 1896.Williams H, Robertson C, Stewart A, et al. World-wide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. [DOI] [PubMed] [Google Scholar]
- 1897.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681–687.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1898.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176–180. [DOI] [PubMed] [Google Scholar]
- 1899.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis—a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. [DOI] [PubMed] [Google Scholar]
- 1900.Schneider L, Hanifin J, Boguniewicz M, et al. Study of the atopic march: development of atopic comorbidities. Pediatr Dermatol. 2016;33:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1901.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836–845. [DOI] [PubMed] [Google Scholar]
- 1902.Sybilski AJ, Raciborski F, Lipiec A, et al. Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study. J Dermatol. 2015;42:140–147. [DOI] [PubMed] [Google Scholar]
- 1903.Bozek A, Jarzab J. Epidemiology of IgE-dependent allergic diseases in elderly patients in Poland. Am J Rhinol Allergy. 2013;27:e140–e145. [DOI] [PubMed] [Google Scholar]
- 1904.Hon KL, Wang SS, Leung TF. The atopic march: from skin to the airways. Iran J Allergy Asthma Immunol. 2012;11:73–77. [PubMed] [Google Scholar]
- 1905.Batlles Garrido J, Torres-Borrego J, Bonillo Perales A, et al. Prevalence and factors linked to atopic eczema in 10- and 11-year-old schoolchildren. Isaac 2 in Almeria, Spain. Allergol Immunopathol (Madr). 2010;38:174–180. [DOI] [PubMed] [Google Scholar]
- 1906.Peroni DG, Piacentini GL, Bodini A, Rigotti E, Pigozzi R, Boner AL. Prevalence and risk factors for atopic dermatitis in preschool children. Br J Dermatol. 2008;158:539–543. [DOI] [PubMed] [Google Scholar]
- 1907.Lowe AJ, Hosking CS, Bennett CM, et al. Skin prick test can identify eczematous infants at risk of asthma and allergic rhinitis. Clin Exp Allergy. 2007;37:1624–1631. [DOI] [PubMed] [Google Scholar]
- 1908.Karaman O, Turgut CS, Uzuner N, et al. The determination of asthma, rhinitis, eczema, and atopy prevalence in 9- to 11-year-old children in the city of Izmir. Allergy Asthma Proc. 2006;27:319–324. [DOI] [PubMed] [Google Scholar]
- 1909.Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. 2005;116:1067–1072. [DOI] [PubMed] [Google Scholar]
- 1910.Kidon MI, Chiang WC, Liew WK, et al. Sensitization to dust mites in children with allergic rhinitis in Singapore: does it matter if you scratch while you sneeze? Clin Exp Allergy. 2005;35:434–440. [DOI] [PubMed] [Google Scholar]
- 1911.Yemaneberhan H, Flohr C, Lewis SA, et al. Prevalence and associated factors of atopic dermatitis symptoms in rural and urban Ethiopia. Clin Exp Allergy. 2004;34:779–785. [DOI] [PubMed] [Google Scholar]
- 1912.Min YG, Choi BY, Kwon SK, et al. Multicenter study on the prevalence of perennial allergic rhinitis and allergy-associated disorders. J Korean Med Sci. 2001;16:697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1913.Ozdemir N, Ucgun I, Metintas S, Kolsuz M, Metintas M. The prevalence of asthma and allergy among university freshmen in Eskisehir, Turkey. Respir Med. 2000;94:536–541. [DOI] [PubMed] [Google Scholar]
- 1914.Garcia-Gonzalez JJ Vega-Chicote JM, Rico P, et al. Prevalence of atopy in students from Malaga, Spain. Ann Allergy Asthma Immunol. 1998;80:237–244. [DOI] [PubMed] [Google Scholar]
- 1915.Leung R, Ho P. Asthma, allergy, and atopy in three South-East Asian populations. Thorax. 1994;49:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1916.Inuo C, Kondo Y, Tanaka K, et al. Japanese cedar pollen-based subcutaneous immunotherapy decreases tomato fruit-specific basophil activation. Int Arch Allergy Immunol. 2015;167:137–145. [DOI] [PubMed] [Google Scholar]
- 1917.Kondo Y, Urisu A. Oral allergy syndrome. Allergol Int. 2009;58:485–491. [DOI] [PubMed] [Google Scholar]
- 1918.Ebner C, Birkner T, Valenta R, et al. Common epitopes of birch pollen and apples—studies by western and northern blot. J Allergy Clin Immunol. 1991;88:588–594. [DOI] [PubMed] [Google Scholar]
- 1919.Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy. 1993;71:470–476. [PubMed] [Google Scholar]
- 1920.Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115:341–384. [DOI] [PubMed] [Google Scholar]
- 1921.Skamstrup Hansen K, Vestergaard H, Stahl Skov P, et al. Double-blind, placebo-controlled food challenge with apple. Allergy. 2001;56:109–117. [DOI] [PubMed] [Google Scholar]
- 1922.Bohle B, Zwolfer B, Heratizadeh A, et al. Cooking birch pollen-related food: divergent consequences for IgE- and T cell-mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. 2006;118:242–249. [DOI] [PubMed] [Google Scholar]
- 1923.Bindslev-Jensen C, Vibits A, Stahl Skov P, Weeke Oral allergy syndrome: the effect of astemizole. Allergy. 1991;46:610–613. [DOI] [PubMed] [Google Scholar]
- 1924.Asero R Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28:1368–1373. [DOI] [PubMed] [Google Scholar]
- 1925.Bolhaar ST, Tiemessen MM, Zuidmeer L, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–769. [DOI] [PubMed] [Google Scholar]
- 1926.Mauro M, Russello M, Incorvaia C, et al. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156:416–422. [DOI] [PubMed] [Google Scholar]
- 1927.Asero R How long does the effect of birch pollen injection SIT on apple allergy last? Allergy. 2003;58:435–438. [DOI] [PubMed] [Google Scholar]
- 1928.Krouse JH. Allergy and Immunology: An Otolaryngic Approach. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 1929.Bircher AJ, Van Melle G, Haller E, Curty B, Frei PC. IgE to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994;24:367–374. [DOI] [PubMed] [Google Scholar]
- 1930.Marseglia GL, Poddighe D, Caimmi D, et al. Role of adenoids and adenoiditis in children with allergy and otitis media. Curr Allergy Asthma Rep. 2009;9:460–464. [DOI] [PubMed] [Google Scholar]
- 1931.Cassano P, Gelardi M, Cassano M, Fiorella ML, Fiorella R. Adenoid tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: a novel approach to therapeutic management. Int J Pediatr Otorhinolaryngol. 2003;67:1303–1309. [DOI] [PubMed] [Google Scholar]
- 1932.Zhang L, Mendoza-Sassi RA, Cesar JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008:CD006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1933.Evcimik MF, Dogru M, Cirik AA, Nepesov MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. 2015;79:694–697. [DOI] [PubMed] [Google Scholar]
- 1934.Dogru M, Evcimik MF, Calim OF. Does adenoid hypertrophy affect disease severity in children with allergic rhinitis? Eur Arch Otorhinolaryngol. 2017;274:209–213. [DOI] [PubMed] [Google Scholar]
- 1935.Modrzynski M, Zawisza E. The influence of birch pollination on the adenoid size in children with intermittent allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2007;71:1017–1023. [DOI] [PubMed] [Google Scholar]
- 1936.Atan Sahin O, Kececioglu N, Serdar M, Ozpinar A. The association of residential mold exposure and adenotonsillar hypertrophy in children living in damp environments. Int J Pediatr Otorhinolaryngol, 2016;88:233–238. [DOI] [PubMed] [Google Scholar]
- 1937.Huang SW, Giannoni C. The risk of adenoid hypertrophy in children with allergic rhinitis. Ann Allergy Asthma Immunol. 2001;87:350–355. [DOI] [PubMed] [Google Scholar]
- 1938.Karaca CT, Toros SZ, Noseri H, et al. Role of allergy in children with adenotonsillar hypertrophy. J Craniofac Surg. 2012;23:e611–e613. [DOI] [PubMed] [Google Scholar]
- 1939.Ameli F, Brocchetti F, Tosca MA, Signori A, Ciprandi G. Adenoidal hypertrophy and allergic rhinitis: is there an inverse relationship? Am J Rhinol Allergy. 2013;27:e5–e10. [DOI] [PubMed] [Google Scholar]
- 1940.Sadeghi-Shabestari M, Jabbari Moghaddam Y, Ghaharri H. Is there any correlation between allergy and adenotonsillar tissue hypertrophy? Int J Pediatr Otorhinolaryngol. 2011;75:589–591. [DOI] [PubMed] [Google Scholar]
- 1941.Eren E, Arslanoglu S, Erdem SB, et al. Chicken or the egg: the dilemma of allergic rhinitis versus adenoid hypertrophy. Rhinology. 2015;53:154–159. [DOI] [PubMed] [Google Scholar]
- 1942.Ni K, Zhao L, Wu J, Chen W, Hongya Yang, Li X. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2015;79:1448–1454. [DOI] [PubMed] [Google Scholar]
- 1943.Masieri S, Trabattoni D, Incorvaia C, et al. A role for Waldeyer’s ring in immunological response to allergens. Curr Med Res Opin. 2014;30:203–205. [DOI] [PubMed] [Google Scholar]
- 1944.Warman M, Granot E, Halperin D. Improvement in allergic and nonallergic rhinitis: a secondary benefit of adenoidectomy in children. Ear Nose Throat J. 2015;94:220, 222, 224–227. https://www.entjournal.com/article/improvement-allergic-and-nonallergic-rhinitis-secondary-benefit-adenoidectomy-children. Accessed December 19, 2017. [DOI] [PubMed] [Google Scholar]
- 1945.Scadding G Non-surgical treatment of adenoidal hypertrophy: the role of treating IgE-mediated inflammation. Pediatr Allergy Immunol. 2010;21:1095–1106. [DOI] [PubMed] [Google Scholar]
- 1946.Chohan A, Lal A, Chohan K, Chakravarti A, Gomber S. Systematic review and meta-analysis of randomized controlled trials on the role of mometasone in adenoid hypertrophy in children. Int J Pediatr Otorhinolaryngol. 2015;79:1599–1608. [DOI] [PubMed] [Google Scholar]
- 1947.Pagella F, De Amici M, Pusateri A, et al. Adenoids and clinical symptoms: Epidemiology of a cohort of 795 pediatric patients. Int J Pediatr Otorhinolaryngol. 2015;79:2137–2141. [DOI] [PubMed] [Google Scholar]
- 1948.Friedman RA, Doyle WJ, Casselbrant ML, Bluestone C, Fireman P. Immunologic-mediated eustachian tube obstruction: a double-blind crossover study. J Allergy Clin Immunol. 1983;71:442–447. [DOI] [PubMed] [Google Scholar]
- 1949.Skoner DP, Doyle WJ, Chamovitz AH, Fireman P. Eustachian tube obstruction after intranasal challenge with house dust mite. Arch Otolaryngol Head Neck Surg. 1986;112:840–842. [DOI] [PubMed] [Google Scholar]
- 1950.Skoner DP, Doyle WJ, Fireman P. Eustachian tube obstruction (ETO) after histamine nasal provocation—a double-blind dose-response study. J Allergy Clin Immunol. 1987;79:27–31. [DOI] [PubMed] [Google Scholar]
- 1951.Bluestone CD, Cantekin EI. Current clinical methods, indications and interpretation of eustachian tube function tests. Ann Otol Rhinol Laryngol. 1981;90:552–562. [DOI] [PubMed] [Google Scholar]
- 1952.O’Connor RD, Ort H, Leong AB, Cook DA, Street D, Hamburger RN. Tympanometric changes following nasal antigen challenge in children with allergic rhinitis. Ann Allergy. 1984;53:468–471. [PubMed] [Google Scholar]
- 1953.Lazo-Saenz JG, Galvan-Aguilera AA, Martinez-Ordaz VA, Velasco-Rodriguez VM, Nieves-Renteria A, Rincon-Castaneda C. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132:626–629. [DOI] [PubMed] [Google Scholar]
- 1954.Knight LC, Eccles R, Morris S. Seasonal allergic rhinitis and its effects on eustachian tube function and middle ear pressure. Clin Otolaryngol Allied Sci. 1992;17:308–312. [DOI] [PubMed] [Google Scholar]
- 1955.Osur SL, Volovitz B, Dickson S, Enck DC, Bernstein JM. Eustachian tube dysfunction in children with ragweed hayfever during natural pollen exposure. Allergy Proc. 1989;10:133–139. [DOI] [PubMed] [Google Scholar]
- 1956.Bernstein JM, Lee J, Conboy K, Ellis E, Li P. Further observations on the role of IgE-mediated hypersensitivity in recurrent otitis media with effusion. Otolaryngol Head Neck Surg. 1985;93:611–615. [DOI] [PubMed] [Google Scholar]
- 1957.Bernstein JM, Lee J, Conboy K, Ellis E, Li P. The role of IgE-mediated hypersensitivity in recurrent otitis media with effusion. Am J Otol. 1983;5:66–69. [PubMed] [Google Scholar]
- 1958.Bernstein JM, Ellis E, Li P. The role of IgE-mediated hypersensitivity in otitis media with effusion. Otolaryngol Head Neck Surg. 1981;89:874–878. [DOI] [PubMed] [Google Scholar]
- 1959.Caffarelli C, Savini E, Giordano S, Gianlupi G, Cavagni G. Atopy in children with otitis media with effusion. Clin Exp Allergy. 1998;28:591–596. [DOI] [PubMed] [Google Scholar]
- 1960.Yeo SG, Park DC, Eun YG, Cha CI. The role of allergic rhinitis in the development of otitis media with effusion: effect on eustachian tube function. Am J Otolaryngol. 2007;28:148–152. [DOI] [PubMed] [Google Scholar]
- 1961.Chantzi FM, Kafetzis DA, Bairamis T, et al. IgE sensitization, respiratory allergy symptoms, and heritability independently increase the risk of otitis media with effusion. Allergy. 2006;61:332–336. [DOI] [PubMed] [Google Scholar]
- 1962.Tomonaga K, Kurono Y, Mogi G. The role of nasal allergy in otitis media with effusiom. Acta Otolaryngol. 1998;458:s41–s47. [DOI] [PubMed] [Google Scholar]
- 1963.Atopy Borge P. and secretory otitis media. Immunological studies and responses to topical corticosteroid therapy. J Laryngol Otol. 1983;97:117–129. [DOI] [PubMed] [Google Scholar]
- 1964.Corey JP, Adham RE, Abbass AH, Seligman I. The role of IgE-mediated hypersensitivity in otitis media with effusion. Am J Otolaryngol. 1994;15:138–144. [DOI] [PubMed] [Google Scholar]
- 1965.Kreiner-Moller E, Chawes BL, Caye-Thomasen P, Bonnelykke K, Bisgaard H. Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clin Exp Allergy. 2012;42:1615–1620. [DOI] [PubMed] [Google Scholar]
- 1966.Hurst DS. Efficacy of allergy immunotherapy as a treatment for patients with chronic otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2008;72:1215–1223. [DOI] [PubMed] [Google Scholar]
- 1967.Hurst DS. Association of otitis media with effusion and allergy as demonstrated by intradermal skin testing and eosinophil cationic protein levels in both middle ear effusions and mucosal biopsies. Laryngoscope. 1996;106:1128–1137. [DOI] [PubMed] [Google Scholar]
- 1968.Hurst DS. Allergy management of refractory serous otitis media. Otolaryngol Head Neck Surg. 1990;102:664–669. [DOI] [PubMed] [Google Scholar]
- 1969.Alles R, Parikh A, Hawk L, Darby Y, Romero JN, Scadding G. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol. 2001;12:102–106. [DOI] [PubMed] [Google Scholar]
- 1970.McMahan JT, Calenoff E, Croft DJ, Barenholtz L, Weber LD. Chronic otitis media with effusion and allergy: modified RAST analysis of 119 cases. Otolaryngol Head Neck Surg. 1981;89:427–431. [DOI] [PubMed] [Google Scholar]
- 1971.Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion - a double-blind study. Int J Pediatr Otorhinolaryngol. 1982;4:133–137. [DOI] [PubMed] [Google Scholar]
- 1972.Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy. 1982;49:81–85. [PubMed] [Google Scholar]
- 1973.Williamson I, Benge S, Barton S, et al. Topical intranasal corticosteroids in 4–11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomised placebo controlled trial. BMJ. 2009;339:b4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1974.Rosenfeld RM, Shin JJ, Schwartz SR, et al. Clinical practice guideline: otitis media with effusion (update). Otolaryngol Head Neck Surg. 2016;154:S1–S41. [DOI] [PubMed] [Google Scholar]
- 1975.Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 2008;372:406–414. [DOI] [PubMed] [Google Scholar]
- 1976.Derebery MJ. Allergic and immunologic aspects of Meniere’s disease. Otolaryngol Head Neck Surg. 1996;114:360–365. [DOI] [PubMed] [Google Scholar]
- 1977.Singh S, Nagarkar AN, Bansal S, Vir D, Gupta AK. Audiological manifestations of allergic rhinitis. J Laryngol Otol. 2011;125:906–910. [DOI] [PubMed] [Google Scholar]
- 1978.Derebery MJ, Berliner KI. Prevalence of allergy in Meniere’s disease. Otolaryngol Head Neck Surg. 2000;123:69–75. [DOI] [PubMed] [Google Scholar]
- 1979.Derebery MJ. Allergic management of Meniere’s disease: an outcome study. Otolaryngol Head Neck Surg. 2000;122:174–182. [DOI] [PubMed] [Google Scholar]
- 1980.Derebery MJ, Valenzuela S. Meniere’s syndrome and allergy. Otolaryngol Clin North Am. 1992;25:213–224. [PubMed] [Google Scholar]
- 1981.Keles E, Godekmerdan A, Kalidag T, et al. Meniere’s disease and allergy: allergens and cytokines. J Laryngol Otol. 2004;118:688–693. [DOI] [PubMed] [Google Scholar]
- 1982.Hsu L, Zhu XN, Zhao YS. Immunoglobulin E and circulating immune complexes in endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1990;99:535–538. [DOI] [PubMed] [Google Scholar]
- 1983.Gibbs SR, Mabry RL, Roland PS, Shoup AG, Mabry CS. Electrocochleographic changes after intranasal allergen challenge: a possible diagnostic tool in patients with Meniere’s disease. Otolaryngol Head Neck Surg. 1999;121:283–284. [DOI] [PubMed] [Google Scholar]
- 1984.Viscomi GJ, Bojrab DI. Use of electrocochleography to monitor antigenic challenge in Meniere’s disease. Otolaryngol Head Neck Surg. 1992;107:733–737. [DOI] [PubMed] [Google Scholar]
- 1985.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1986.Passali D, Benedetto de F, Benedetto de M, et al. Rhino-Bronchial Syndrome. The SIO-AIMAR (Italian Society of Otorhinolaryngology, Head Neck Surgery-Interdisciplinary Scientific Association for the Study of the Respiratory Diseases) survey. Acta Otorhinolaryngol Ital. 2011;31:27–34. [PMC free article] [PubMed] [Google Scholar]
- 1987.Lin HC, Cho SH, Ghoshal AG, et al. Respiratory diseases and the impact of cough in Taiwan: results from the APBORD observational study. Medicine (Baltimore). 2016;95:e3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1988.Ghoshal AG, Ravindran GD, Gangwal P, et al. The burden of segregated respiratory diseases in India and the quality of care in these patients: results from the Asia-Pacific Burden of Respiratory Diseases study. Lung India. 2016;33:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1989.Krzych-Falta E, Piekarska B, Sybilski A, Wojas O, Samolinski B. The safety of nasal allergen challenge test assessed in lower airways. Iran J Allergy Asthma Immunol. 2015;14:581–588. [PubMed] [Google Scholar]
- 1990.Chakir J, Laviolette M, Turcotte H, Boutet M, Boulet LP. Cytokine expression in the lower airways of nonasthmatic subjects with allergic rhinitis: influence of natural allergen exposure. J Allergy Clin Immunol. 2000;106:904–910. [DOI] [PubMed] [Google Scholar]
- 1991.Chakir J, Laviolette M, Boutet M, Laliberte R, Dube J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996;75:735–744. [PubMed] [Google Scholar]
- 1992.Buday T, Gavliakova S, Mokry J, Medvedova I, Kavalcikova-Bogdanova N, Plevkova J. The guinea pig sensitized by house dust mite: a model of experimental cough studies. Adv Exp Med Biol. 2016;905:87–95. [DOI] [PubMed] [Google Scholar]
- 1993.Cho SH, Lin HC, Ghoshal AG, et al. Respiratory disease in the Asia-Pacific region: cough as a key symptom. Allergy Asthma Proc. 2016;37:131–140. [DOI] [PubMed] [Google Scholar]
- 1994.He S, Li YJ, Chen J. Clinical features of allergic rhinitis in children of Shanghai, China. Genet Mol Res. 2016;15. [DOI] [PubMed] [Google Scholar]
- 1995.Roth DF, Ferguson BJ. Vocal allergy: recent advances in understanding the role of allergy in dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2010;18:176–181. [DOI] [PubMed] [Google Scholar]
- 1996.Millqvist E, Bende M, Brynnel M, Johansson I, Kappel S, Ohlsson AC. Voice change in seasonal allergic rhinitis. J Voice. 2008;22:512–515. [DOI] [PubMed] [Google Scholar]
- 1997.Koc EA, Koc B, Erbek S. Comparison of acoustic and stroboscopic findings and voice handicap index between allergic rhinitis patients and controls. Balkan Med J. 2014;31:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1998.Krouse JH, Dworkin JP, Carron MA, Stachler RJ. Baseline laryngeal effects among individuals with dust mite allergy. Otolaryngol Head Neck Surg. 2008;139:149–151. [DOI] [PubMed] [Google Scholar]
- 1999.Randhawa PS, Nouraei S, Mansuri S, Rubin JS. Allergic laryngitis as a cause of dysphonia: a preliminary report. Logoped Phoniatr Vocol. 2010;35:169–174. [DOI] [PubMed] [Google Scholar]
- 2000.Hamdan AL, Sibai A, Youssef M, Deeb R, Zaitoun F. The use of a screening questionnaire to determine the incidence of allergic rhinitis in singers with dysphonia. Arch Otolaryngol Head Neck Surg. 2006;132:547–549. [DOI] [PubMed] [Google Scholar]
- 2001.Turley R, Cohen SM, Becker A, Ebert CS Jr. Role of rhinitis in laryngitis: another dimension of the unified airway. Ann Otol Rhinol Laryngol. 2011;120:505–510. [DOI] [PubMed] [Google Scholar]
- 2002.Simberg S, Sala E, Tuomainen J, Ronnemaa AM. Vocal symptoms and allergy—a pilot study. J Voice. 2009;23:136–139. [DOI] [PubMed] [Google Scholar]
- 2003.Randhawa PS, Mansuri S, Rubin JS. Is dysphonia due to allergic laryngitis being misdiagnosed as laryngopharyngeal reflux? Logoped Phoniatr Vocol. 2010;35:1–5. [DOI] [PubMed] [Google Scholar]
- 2004.Brook CD, Platt MP, Reese S, Noordzij JP. Utility of allergy testing in patients with chronic laryn-gopharyngeal symptoms: is it allergic laryngitis? Otolaryngol Head Neck Surg. 2016;154:41–45. [DOI] [PubMed] [Google Scholar]
- 2005.Eren E, Arslanoglu S, Aktas A, et al. Factors confusing the diagnosis of laryngopharyngeal reflux: the role of allergic rhinitis and inter-rater variability of laryngeal findings. Eur Arch Otorhinolaryngol. 2014;271:743–747. [DOI] [PubMed] [Google Scholar]
- 2006.Belafsky PC, Peake J, Smiley-Jewell SM, Verma SP, Dworkin-Valenti J, Pinkerton KE. Soot and house dust mite allergen cause eosinophilic laryngitis in an animal model. Laryngoscope. 2016;126:108–112. [DOI] [PubMed] [Google Scholar]
- 2007.Mouadeb DA, Belafsky PC, Birchall M, Hood C, Konia T, Pinkerton KE. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngol Head Neck Surg. 2009;140:493–497. [DOI] [PubMed] [Google Scholar]
- 2008.Roth DF, Abbott KV, Carroll TL, Ferguson BJ. Evidence for primary laryngeal inhalant allergy: a randomized, double-blinded crossover study. Int Forum Allergy Rhinol. 2013;3:10–18. [DOI] [PubMed] [Google Scholar]
- 2009.Reidy PM, Dworkin JP, Krouse JH. Laryngeal effects of antigen stimulation challenge with perennial allergen Dermatophagoides pteronyssinus. Otolaryngol Head Neck Surg. 2003;128:455–462. [DOI] [PubMed] [Google Scholar]
- 2010.Dworkin JP, Reidy PM, Stachler RJ, Krouse JH. Effects of sequential Dermatophagoides pteronyssinus antigen stimulation on anatomy and physiology of the larynx. Ear Nose Throat J. 2009;88:793–799. [PubMed] [Google Scholar]
- 2011.Brook C, Noordzij JP, Russell K, Aliphas A, Platt M. Predictive findings of allergic disease in fiberoptic nasolaryngoscopy. Laryngoscope. 2015;125:286–290. [DOI] [PubMed] [Google Scholar]
- 2012.Jackson-Menaldi CA, Dzul AI, Holland RW. Allergies and vocal fold edema: a preliminary report. J Voice. 1999;13:113–122. [DOI] [PubMed] [Google Scholar]
- 2013.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. [DOI] [PubMed] [Google Scholar]
- 2014.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–535. [DOI] [PubMed] [Google Scholar]
- 2015.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. [DOI] [PubMed] [Google Scholar]
- 2016.Assa’ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. [DOI] [PubMed] [Google Scholar]
- 2017.Plaza-Martin AM, Jimenez-Feijoo R, Andaluz C, et al. Polysensitization to aeroallergens and food in eosinophilic esophagitis in a pediatric population. Allergol Immunopathol (Madr). 2007;35:35–37. [DOI] [PubMed] [Google Scholar]
- 2018.Sugnanam KK, Collins JT, Smith PK, et al. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy. 2007;62:1257–1260. [DOI] [PubMed] [Google Scholar]
- 2019.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. [DOI] [PubMed] [Google Scholar]
- 2020.Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil-associated gastrointestinal disorders: a world-wide-web based registry. J Pediatr. 2002;141:576–581. [DOI] [PubMed] [Google Scholar]
- 2021.Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. 2010;31:509–515. [DOI] [PubMed] [Google Scholar]
- 2022.Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–833. [DOI] [PubMed] [Google Scholar]
- 2023.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41:451–453. [DOI] [PubMed] [Google Scholar]
- 2024.Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. 2003;112:796–797. [DOI] [PubMed] [Google Scholar]
- 2025.Ramirez RM, Jacobs RL. Eosinophilic esophagitis treated with immunotherapy to dust mites. J Allergy Clin Immunol. 2013;132:503–504. [DOI] [PubMed] [Google Scholar]
- 2026.Shedden A Impact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online survey. Treat Respir Med. 2005;4:439–446. [DOI] [PubMed] [Google Scholar]
- 2027.Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124:S43–S70. [DOI] [PubMed] [Google Scholar]
- 2028.Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117:158–162. [DOI] [PubMed] [Google Scholar]
- 2029.Craig TJ, Hanks CD, Fisher LH. How do topical nasal corticosteroids improve sleep and daytime somnolence in allergic rhinitis? J Allergy Clin Immunol. 2005;116:1264–1266. [DOI] [PubMed] [Google Scholar]
- 2030.Sherkat AA, Sardana N, Safaee S, Lehman EB, Craig TJ. The role of pseudoephedrine on day-time somnolence in patients suffering from perennial allergic rhinitis (PAR). Ann Allergy Asthma Im-munol. 2011;106:97–102. [DOI] [PubMed] [Google Scholar]
- 2031.Ferguson BJ. Influences of allergic rhinitis on sleep. Otolaryngol Head Neck Surg. 2004;130:617–629. [DOI] [PubMed] [Google Scholar]
- 2032.Mann RD, Pearce GL, Dunn N, Shakir S. Sedation with “non-sedating” antihistamines: four prescription-event monitoring studies in general practice. BMJ. 2000;320:1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2033.Hindmarch I, Shamsi Z. Antihistamines: models to assess sedative properties, assessment of sedation, safety and other side-effects. Clin Exp Allergy. 1999;29(Suppl 3):133–142. [DOI] [PubMed] [Google Scholar]
- 2034.Ishman SL, Smith DF, Benke JR, Nguyen MT, Lin SY. The prevalence of sleepiness and the risk of sleep-disordered breathing in children with positive allergy test. Int Forum Allergy Rhinol. 2012;2:139–143. [DOI] [PubMed] [Google Scholar]
- 2035.Yuksel H, Sogut A, Yilmaz H, Yilmaz O, Dinc G. Sleep actigraphy evidence of improved sleep after treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2009;103:290–294. [DOI] [PubMed] [Google Scholar]
- 2036.Benninger MS, Benninger RM. The impact of allergic rhinitis on sexual activity, sleep, and fatigue. Allergy Asthma Proc. 2009;30:358–365. [DOI] [PubMed] [Google Scholar]
- 2037.Meltzer EO, Nathan R, Derebery J, et al. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009;30:244–254. [DOI] [PubMed] [Google Scholar]


