Abstract
Background: Current tobacco treatment guidelines have established the efficacy of available interventions, but they do not provide detailed guidance for common implementation questions frequently faced in the clinic. An evidence-based guideline was created that addresses several pharmacotherapy-initiation questions that routinely confront treatment teams.
Methods: Individuals with diverse expertise related to smoking cessation were empaneled to prioritize questions and outcomes important to clinicians. An evidence-synthesis team conducted systematic reviews, which informed recommendations to answer the questions. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach was used to rate the certainty in the estimated effects and the strength of recommendations.
Results: The guideline panel formulated five strong recommendations and two conditional recommendations regarding pharmacotherapy choices. Strong recommendations include using varenicline rather than a nicotine patch, using varenicline rather than bupropion, using varenicline rather than a nicotine patch in adults with a comorbid psychiatric condition, initiating varenicline in adults even if they are unready to quit, and using controller therapy for an extended treatment duration greater than 12 weeks. Conditional recommendations include combining a nicotine patch with varenicline rather than using varenicline alone and using varenicline rather than electronic cigarettes.
Conclusions: Seven recommendations are provided, which represent simple practice changes that are likely to increase the effectiveness of tobacco-dependence pharmacotherapy.
Keywords: dependence, pharmacotherapy, smoking, tobacco, treatment
Contents
Summary of Recommendations
Introduction
Guideline Scope and Target Audience
Terminology
Disclaimer
Methods
Panel Composition
Conflict-of-Interest Management
Questions and Outcomes of Interest
Literature Search
Literature Screening and Evidence Synthesis
Formulating Recommendations
Independent Review
Funding and Updating
Questions and Recommendations
Question 1: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline or a Nicotine Patch?
Question 2: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline or Bupropion?
Question 3: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline plus Nicotine-Replacement Therapy or Varenicline Alone?
Question 4: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline or an Electronic Cigarette?
Question 5: In Tobacco-Dependent Adults Who Are Not Ready to Discontinue Tobacco Use, Should Clinicians Begin Treatment with the Optimal Controller or Wait Until They Are Ready to Stop Tobacco Use?
Question 6: In Tobacco-Dependent Adults with Comorbid Psychiatric Conditions, Including Substance-Use Disorder, Depression, Anxiety, Schizophrenia, and/or Bipolar Disorder, for Whom Treatment Is Being Initiated, Should Clinicians Start with the Optimal Controller Identified for Patients without Psychiatric Conditions or Use a Nicotine Patch?
Question 7: In Tobacco-Dependent Adults for Whom Treatment Is Being Initiated with a Controller, Should They Be Treated with an Extended-Duration (>12 wk) or Standard-Duration (6–12 wk) Regimen?
Discussion
Patient Perspective
Summary of Recommendations
-
1.
For tobacco-dependent adults in whom treatment is being initiated, we recommend varenicline over a nicotine patch (strong recommendation, moderate certainty in the estimated effects). Remarks: To promote adherence to pharmacologic therapy, providers should be prepared to counsel patients about the relative safety and efficacy of varenicline treatment compared with a nicotine patch.
-
2.
For tobacco-dependent adults in whom treatment is being initiated, we recommend varenicline over bupropion (strong recommendation, moderate certainty in the estimated effects).
-
3.
For tobacco-dependent adults in whom treatment is being initiated, we suggest varenicline plus a nicotine patch over varenicline alone (conditional recommendation, low certainty in the estimated effects).
-
4.
For tobacco-dependent adults in whom treatment is being initiated, we suggest varenicline over electronic cigarettes (conditional recommendation, very low certainty in the estimated effects). Remarks: The recommendation’s strength reflects very low certainty in the effects used to derive the recommendation. After our evidence synthesis, new evidence emerged regarding serious adverse effects of electronic cigarettes. If these serious adverse effects continue to be reported, the strength of the recommendation should be reevaluated. Note that this recommendation is intended for treatment of tobacco dependence under the supervision of a clinician; it should not be extrapolated to unsupervised treatment or recreational use.
-
5.
In tobacco-dependent adults who are not ready to discontinue tobacco use, we recommend that clinicians begin treatment with varenicline rather than waiting until patients are ready to stop tobacco use (strong recommendation, moderate certainty in the estimated effects).
-
6.
For tobacco-dependent adults with comorbid psychiatric conditions, including substance-use disorder, depression, anxiety, schizophrenia, and/or bipolar disorder, for whom treatment is being initiated, we recommend varenicline over a nicotine patch (strong recommendation, moderate certainty in the estimated effects).
-
7.
For tobacco-dependent adults for whom treatment is being initiated with a controller, we recommend using extended-duration (>12 wk) over standard-duration (6–12 wk) therapy (strong recommendation, moderate certainty in the estimated effects).
Introduction
Tobacco dependence remains a pervasive clinical problem in pulmonary practice. In 1988, the U.S. Surgeon General first described tobacco use as the cardinal sign of addiction to nicotine (1). The report established that treatment of this intransigent addiction requires shifting from episodic models of care to sustained, longitudinal strategies, emphasizing long-term control over the compulsion to smoke. Eight years later, the USPHS published a comprehensive tobacco-dependence treatment guideline, establishing a new paradigm for care (2). The guideline, together with its 2008 update (3), provided the evidential basis for pharmacologic treatment and a workflow to maximize penetration of pharmacologic treatment into clinical practice. As a result, a first principle of clinical practice was established: all patients who use tobacco should receive treatment for their dependence, rather than simply being encouraged to stop.
Clinicians engage tobacco treatment infrequently, limiting the effectiveness of other pulmonary interventions (4). Several explanatory theories have been proposed, including that clinicians’ willingness to invest in the problem may be limited by their frustration with continued smoking despite respiratory symptoms (4, 5) and/or by the perceived ineffectiveness of pharmacotherapeutic interventions (4, 6). Although the USPHS guidelines have deemed interventions as efficacious, they do not provide tailored guidance for common clinical questions that impact effectiveness.
Guideline Scope and Target Audience
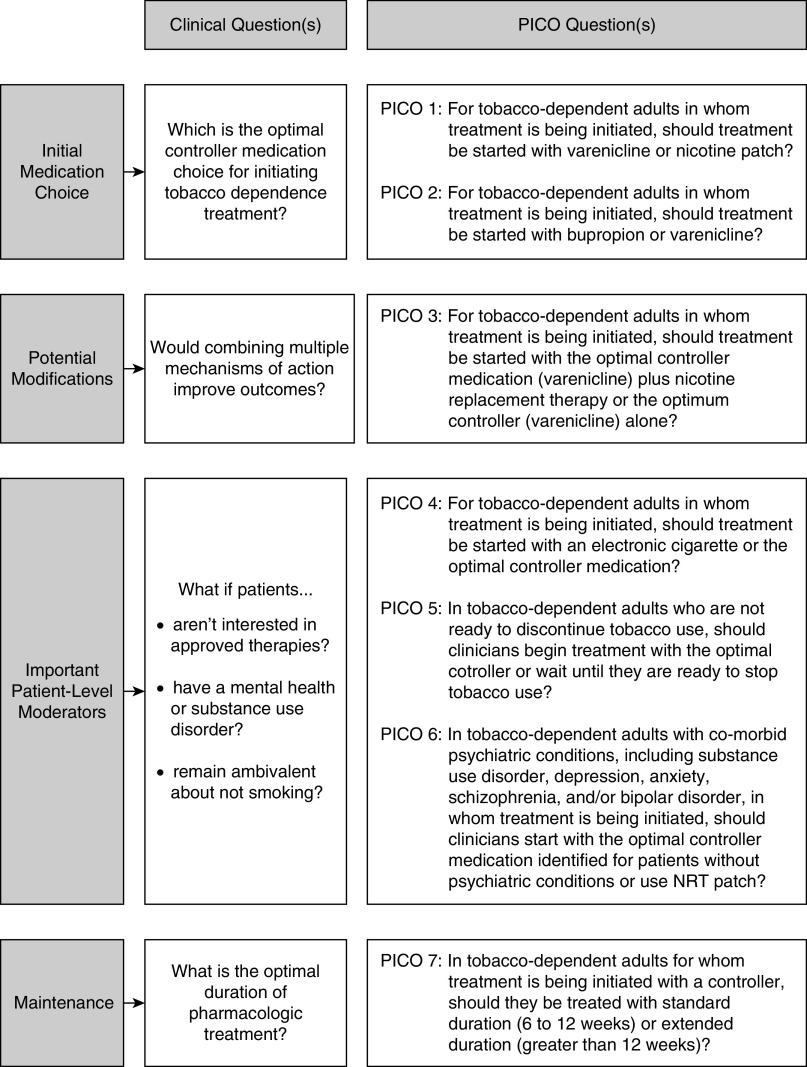
This guideline expands on the USPHS foundation. It focuses on the initial pharmacotherapy of tobacco dependence, defined by problematic patterns of tobacco use leading to clinically significant impairment or distress, in adult patients, excluding pregnant and adolescent populations. The goal is to improve patient-centered care of tobacco dependence by identifying a single evidence-based pathway that balances important outcomes, including short- and long-term tobacco abstinence and serious adverse events (SAEs), while accounting for important clinical variability (Figure 1). It was not possible to include all possible pharmacotherapy combination choices, nor was it feasible to account for all possible variations encountered in practice.
Figure 1.
Logic model for identification of important clinical questions and translation into evaluable PICO-formatted questions. NRT = nicotine-replacement therapy; PICO = Population, Intervention, Comparator, and Outcome.
The panel recognized that the epidemic of tobacco dependence involves an array of social, environmental, and behavioral determinants. However, the panel had to omit important topics, such as communication and counseling methods, healthcare system designs, epidemic-control policies, and second-line therapy, because each is sufficiently robust to warrant its own guideline. This guideline was created with the assumption that accepted foundations of tobacco-dependence treatment are already in practice (Box 1).
Box 1. Foundations of Tobacco-Dependence Treatment
-
1.
All patients should be screened for tobacco use, and the potential diagnosis of tobacco dependence should be assessed.
-
2.
The diagnosis of tobacco dependence, as well as the toxic effects of tobacco exposure, should be incorporated into the patient’s problem list.
-
3.
Simply encouraging patients to stop smoking is insufficient. All patients who use tobacco should be provided with evidence-based treatment, including pharmacotherapy, to help them stop.
-
4.
Tobacco-dependence interventions require longitudinal follow-up, akin to the longitudinal evaluation and management of other chronic illnesses.
Based on Reference 3.
The target audience for the recommendations in our guideline includes patients, physicians, other clinicians, nurses, and policy makers who inform patient decision-making, clinical practice, and health-policy decisions. The intended settings for applying all recommendations include any clinical setting where pharmacologic therapy is being initiated.
Terminology
It is well established that nicotine addiction is a compulsive disorder, characterized not by insufficient motivation to stop but also by amplified unconscious motivation to continue the maladaptive behavior (1). Dependence is a chronic relapsing and remitting disease, requiring longitudinal management. For these reasons, several deliberate language choices were used during the production of this document:
-
•
The terms treatment of tobacco dependence and treatment encompass the totality of evaluation and management services provided by clinicians, whereas the terms cessation, quit, and abstinence are limited to discussions of outcome.
-
•
Pharmacotherapy is used to describe the general class of tools used to achieve clinical objectives. The more common terms pharmacologic supports, quit-smoking medications, and cessation aids are avoided to emphasize the clinicians’ role in longitudinal management.
-
•
Although we recognize the unique role of the prescriber in pharmacotherapeutic decision-making, we also recognize the importance of associated caregivers in achieving pharmacotherapeutic goals and addressing patient concerns. The term treatment team is used to emphasize the preference for an integrated, multidisciplinary approach to treatment.
-
•
The dichotomous terms success and failure are not used, favoring instead the concept of a compulsion to smoke that exists on a therapeutic continuum from uncontrolled to controlled. Control over compulsion emphasizes the waxing and waning nature of therapeutic effects over time and highlights the need for longitudinal vigilance.
-
•
Medications have been categorized as controllers or relievers on the basis of their pharmacokinetics (7). Controller medications are expected to have a delayed onset of effect, acting to reduce the frequency and intensity of the impulse to smoke, whereas reliever medications are expected to have more acute effects, useful in relieving the impact of cue-induced cravings.
Disclaimer
It is important to realize that guidelines cannot account for all potential clinical circumstances. This guideline is not intended to supplant clinician judgment, and its recommendations should not be considered mandates. For all recommendations, we have considered the balance of desirable and undesirable effects, certainty of evidence, patients’ values and preferences, resources required, equity, acceptability, and feasibility. Clinicians are encouraged to apply the recommendations in the clinical context of each individual patient, particularly regarding the patient’s values and preferences.
Methods
Guideline recommendations were developed in accordance with principles outlined by the Institute of Medicine (now the National Academy of Medicine) (8). The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach was used to assess the certainty of the estimated effects and to rate the strength of the recommendations (9–13). Panel composition, conflict-of-interest management, external review, and organizational approval all proceeded in accordance with American Thoracic Society (ATS) policies and procedures (14).
Panel Composition
The project proposal was approved by the ATS Board of Directors. Potential panelists were identified by the co-chairs on the basis of documented expertise and training in tobacco-dependence counseling and/or treatment. The final panel included individuals with expertise in guideline methodology, behavioral health, health equity, nursing, pharmacy, and pediatrics. One member-in-training and one patient representative were included. Two committee members represented countries outside of North America. A patient representative participated in rating the importance of the outcomes and formulating the recommendations and provided a unique patient perspective on the importance of the guideline once completed.
Conflict-of-Interest Management
All potential panelists disclosed their potential conflicts of interest to the ATS. Most panelists were determined to have no substantial conflicts of interest and were approved to participate without limitation. One panelist with a relevant industry relationship participated in discussions but was recused from formulating, grading, writing, or editing recommendations.
Questions and Outcomes of Interest
Twenty-two candidate questions in the PICO (Population, Intervention, Comparator, and Outcome) format were prioritized during an in-person meeting (May 2018), with seven being chosen for inclusion in the guideline (Figure 1). One question was discarded in May of 2019 because of an absence of evidence and was replaced with an alternative question (PICO 3), leading to a recommendation based on available evidence. After comparing varenicline, nicotine patches, and bupropion in questions 1 and 2, varenicline was shown to be the best controller of the three; therefore, varenicline replaced the “optimal controller” in questions 3 through 6 when formulating recommendations.
The panel selected and defined outcomes for each question a priori and then rated the importance of each using a 9-point scale (15). The panel identified two critically important outcomes relevant to all questions: 1) abstinence, measured by biomarkers or self-report, for the 7 days before follow-up, performed at least 6 months after the target stop date, and 2) incidence of SAEs, defined by the trialists as attributable to the pharmacologic treatment. These included, but were not limited to, depression, anxiety, suicidal ideation or suicidal behavior, and neurological events such as seizures. Important outcomes also informed decision-making, including 1) abstinence during the treatment period; 2) tobacco-use relapse measured at the end of the follow-up; 3) increase or decrease in use of other substances, including alcohol, marijuana, cocaine, and opioids; 4) quality of life (QOL); 5) severity of withdrawal from the beginning of the treatment to the end of the follow-up, measured by global withdrawal scores; and 6) change in tobacco use measured by cigarettes per day.
Literature Search
A medical librarian worked with the methodologists to search for available evidence within MEDLINE, the Excerpta Medica Database (EMBASE), the Cumulative Index to Nursing and Allied Health Literature, the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews, the National Institute of Health Research Centre for Reviews and Dissemination database, the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. The initial search was not limited by publication date or language, was completed in January 2019, and was then updated through October 2019 (see online supplement). The Cochrane Scottish Intercollegiate Guidelines Network randomized-controlled-trials (RCTs) filter was used to identify RCTs, and the British Medical Journal Observational Studies filter was used to identify observational studies. Panel members reviewed identified reference lists for completeness.
Literature Screening and Evidence Synthesis
The methodology team followed the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions, using title and abstract screening, full-text screening, and data extractions performed in duplicate (16). Two independent reviewers conducted an initial screening of titles and abstracts and obtained the full texts of studies that appeared eligible according to the inclusion criteria. The screening process followed a priori inclusion criteria delineating study population, intervention, comparison, and study design. Two reviewers conducted duplicate full-text reviews and resolved any disagreement through consensus discussion.
Direct comparison meta-analyses used the Mantel-Haenszel method with random effects, with the chi-square test and I2 statistic being used to assess heterogeneity (RevMan version 5.3; The Nordic Cochrane Centre). A network meta-analysis with Bayesian statistical approaches was conducted, which included building fixed and random-effects network models by running the Markov chain Monte Carlo simulation, selecting the best-fit model on the basis of the deviance information criterion, and checking intransitivity (gemtc version 0.8-2; R Foundation for Statistical Computing) (17). Funnel-plot symmetry was used to assess publication bias (18).
Relative risks (RRs) were used to report analysis of dichotomous outcomes, mean differences (MDs) were used for continuous outcomes, and hazard ratios (HRs) were used for time-to-event outcomes; all were accompanied by the 95% confidence interval (95% CI) for the estimate. The absolute risk reduction (ARR) was estimated by multiplying the median of risks observed in control groups by the pooled risk ratio and then presenting the result in terms of the anticipated increase or decrease in patients experiencing the effect per 1,000 patients treated (19).
Within each comparison, certainty in the estimated effects for each outcome was assessed using the GRADE approach (20, 21). The lead methodologists categorized the certainty in the estimated effects into four degrees ranging from very low to high, as determined by considering the risk of bias, precision, consistency, directness, likelihood of publication bias, presence of a dose–effect relationship, and potential effect of residual and opposing confounding (11, 12, 22). The methodology team also conducted systematic reviews of patient values and preferences, as well as cost-effectiveness.
For each question, GRADE evidence profiles and the “Evidence-to-Decision” (EtD) framework were constructed to summarize the results of systematic reviews by using the GRADEpro Guideline Development Tool (www.gradepro.org) (9, 10, 13). Each EtD table included sections on effectiveness and safety, resource use, patient values and preferences, impact on health equity, acceptability, and feasibility.
The Guidelines in Intensive care, Development and Evaluation (GUIDE) Group provided methods support for this guideline.
Formulating Recommendations
Panel members reviewed the evidence profiles and EtD tables (see online supplement) at a second in-person meeting in May of 2019. Recommendations were formulated after panel members evaluated the benefits and harms, certainty in the estimated effects, assumptions about values and preferences, resource use, feasibility, acceptability, and equity impact of various courses of action. The strengths of the recommendations were rated using established GRADE criteria (23). Consensus on the direction and strength of recommendations, and on associated remarks, was achieved through discussion and iterative voting. Panelists with dissenting opinions were given the opportunity to record the rationale for their dissent.
Independent Review
The resulting final guideline was subject to review by the ATS Documents Editor, and by anonymous peer reviewers, and was approved for publication by the ATS Executive Committee, per ATS policy.
Funding and Updating
This guideline was funded by the ATS. In accordance with ATS policy, the guideline will be reevaluated by the sponsoring assembly or committee in roughly 5 years, and the need to readdress existing questions or to address new questions will be determined. The ATS did not influence the content of this guideline.
Questions and Recommendations
Question 1: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline or a Nicotine Patch?
Rationale for question
Only 3% of smokers who are untreated will achieve abstinence within a given year (24, 25); however, cessation increases significantly if pharmacotherapy is used (3). Maximizing the impact of the initial pharmacotherapy decision may promote adherence and improve outcomes (26). To identify an optimal controller medication, the panel evaluated the relative effectiveness of varenicline and a nicotine patch.
Summary of evidence
The systematic review identified 14 RCTs directly comparing varenicline with a nicotine patch. Eleven RCTs reported point-prevalence abstinence at 6 months after treatment, assessed by self-report and exhaled carbon monoxide (eCO) verification (n = 7,362) (27–38). Nine RCTs (n = 7,153) reported important outcomes, including point-prevalence abstinence during the 10- to 12-week treatment period (27–34) (Table 1).
Table 1.
Evidentiary Basis for Strong Recommendation Favoring Varenicline over Nicotine Patch, with Moderate-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline | Nicotine Patch | Relative | Absolute (per 1,000 Patients) | ||
|
Seven-day point-prevalence tobacco abstinence at 6 mo (follow-up: 6 mo; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 11 | RCT | Not serious | Not serious | Not serious | Not serious | None | 1,081/3,743 (28.9%) | 20.2% | RR, 1.20 (1.09–1.32) | 40 more (↑18–↑65) | High | Critical |
|
Point-prevalence tobacco abstinence during the treatment period (follow-up: range, 10–12 wk; assessed with self-report + exhaled carbon monoxide) | ||||||||||||
| 9 | RCT | Not serious | Not serious | Not serious | Not serious | None | 1,449/3,640 (39.8%) | 25.4% | RR, 1.40 (1.31–1.49) | 101 more (↑79–↑124) | High | Important |
|
Quality of life, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Serious adverse events (follow-up: range, 4 wk to 3 mo) | ||||||||||||
| 10 | RCT | Not serious | Not serious | Not serious | Serious | None | 61/3,799 (1.6%) | 1.1% | RR, 0.72 (0.52–1.00) | 3 fewer (↓5–↓0) | Moderate | Critical |
|
Tobacco-use relapse measured at the end of the follow-up (follow-up: range, 8 wk to 6 mo) | ||||||||||||
| 2 | RCT | Serious | Not serious | Not serious | Serious | None | —/491 | —/314 | HR, 0.93 (0.78–1.11) | N/A | Low | Important |
|
Other substance abuse, alcohol (follow-up: 6 mo; assessed with alcohol test [breath alcohol ≤ 0.02 g/dl]) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 8/49 (16.3%) | 29.0% | RR, 0.56 (0.24–1.30) | 128 fewer (↓221–↑87) | Very low | Important |
|
Other substance abuse, any drug (follow-up: 6 mo) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 18/49 (36.7%) | 25.8% | RR, 1.42 (0.71–2.87) | 108 more (↓75–↑483) | Very low | Important |
|
Severity of withdrawal, MNWS total (follow-up: 12 wk; assessed with MNWS; lower score indicates better outcome) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 14 | 14 | — | MD, 0.08 higher (↓1.98–↑2.14) | Very low | Important |
|
Severity of withdrawal, MNWS urge to smoke (follow-up: 7–12 wk; assessed with MNWS; lower score indicates better outcome) | ||||||||||||
| 2 | RCT | Serious | Serious | Not serious | Not serious | None | 381 | 380 | — | MD, 0.32 lower (↓0.33–↓0.31) | Low | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; HR = hazard ratio; MD = mean difference; MNWS = Minnesota Nicotine Withdrawal Scale; N/A = not available; RCT = randomized controlled trial; RR = relative risk.
Varenicline treatment was found to be superior to the patch in achieving continuous long-term abstinence and was associated with fewer adverse events. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
Compared with a nicotine patch, varenicline increased long-term abstinence, measured as 7-day point-prevalence abstinence at 6-month follow-up (RR, 1.20; 95% CI, 1.09 to 1.32; ARR, 40 more per 1,000 patients; 95% CI, 18 more to 65 more; high certainty in the estimated effects) and 7-day point-prevalence abstinence during the treatment period (RR, 1.40; 95% CI, 1.31 to 1.49; ARR, 101 more per 1,000 patients; 95% CI, 79 more to 124 more; high certainty in the estimated effects) (27–36). Relative impact on QOL could not be evaluated because of a paucity of data.
Harms and burdens
Varenicline likely reduced the risk of SAEs compared with a nicotine patch (RR, 0.72; 95% CI, 0.52 to 1.00; ARR, 3 fewer per 1,000 patients; 95% CI, 5 fewer to 0 fewer; moderate certainty in the estimated effects) (27, 28, 30, 31, 33–35, 37, 38). Varenicline may also reduce relapse at the end of follow-up compared with a nicotine patch (HR, 0.93; 95% CI, 0.78 to 1.11; low certainty in the estimated effects) (30, 37). Overall severity of withdrawal symptoms was assessed using Minnesota Nicotine Withdrawal Scale (MNWS) scores, with a lower score indicating a better outcome (39). It is unclear whether varenicline treatment improves an MNWS score assessed at a 12-week follow-up because of the very low certainty of the estimated effects (MD, 0.08 higher; 95% CI, 1.98 lower to 2.14 higher; very low certainty in the estimated effects) (36). Varenicline treatment might reduce the MMWS measure of the urge to smoke at follow-up Weeks 7 to 12 (MD, 0.32 lower; 95% CI, 0.33 lower to 0.31 lower; low certainty in the estimated effects) (27, 36).
Certainty in the estimated effects
Certainty in the estimated effects was consistently judged to be high for both 7-day point-prevalence abstinence at 6-month follow-up and point-prevalence abstinence during the treatment period (range, 10 to 12 wk) (27–36). Certainty in the SAE estimate during follow-up (4 wk to 3 mo) was judged as moderate because of serious imprecision (27, 28, 30, 32–35, 37, 38). Certainty in the tobacco-use relapse estimate during follow-up was judged as low on the basis of risk of bias in the open-label design of one RCT (27), with imprecision suggesting possible opposing conclusions regarding the relapse benefit of varenicline (30, 37). The overall severity of withdrawal symptoms was judged to be of very low certainty because of serious risk of bias and very serious imprecision, whereas the estimate of the urge to smoke was judged to be of low certainty because of a serious risk of bias and inconsistency.
Other considerations
Over-the-counter accessibility and lower costs were identified as important values to consider when comparing efficacy and safety (40–43). Acknowledging significant variation in varenicline cost by country and insurance coverage, the estimated direct costs for 12 weeks of medication ranged from $1,220 to $1,584 for varenicline and $170 to $240 for a nicotine patch. Cost-effectiveness analyses suggested that varenicline is cost-effective compared with a nicotine patch (30, 44–46). Uptake of varenicline was noted to be lower than that of the patch, perhaps due to underprescribing or limited availability, but was considered a feasible option (47, 48).
Panel discussion and conclusions
The panel concluded 1) that varenicline is superior in achieving continuous long-term abstinence when compared with a nicotine patch and 2) that varenicline is associated with fewer SAEs than a nicotine patch. On balance, the panel concluded that the clinical superiority of varenicline (balance of effect) outweighs its higher price and the possibly important uncertainty or variability of patients’ values and preferences. Therefore, a majority of the panel preferred a strong recommendation. Although there was unanimity about the preferred intervention, two panelists (H.J.F. and P.F.) departed from panel consensus and advocated that the recommendation be conditional rather than strong, arguing that some patients may prefer to initiate treatment with a nicotine patch because of concerns of out-of-pocket costs, over-the-counter availability, and perceptions of nonsevere adverse effects and that escalation of therapy could be considered on follow-up if a nicotine patch were not as effective as initial pharmacotherapy.
Recommendation 1
For tobacco-dependent adults in whom treatment is being initiated, we recommend varenicline over a nicotine patch (strong recommendation, moderate certainty in the estimated effects). Remarks: To promote adherence to pharmacologic therapy, providers should be prepared to counsel patients about the relative safety and efficacy of varenicline treatment compared with a nicotine patch.
What others are saying
The USPHS guidelines clearly identified an association between improved abstinence rates and health insurance benefit coverage for pharmacotherapy (3). Tobacco-dependence treatment is verifiably cost-effective when compared with treatment of other clinical disorders. To our knowledge, no clinical practice guidelines have recommended initiation with varenicline over a nicotine patch. However, the American College of Cardiology recently published a tobacco-cessation clinical pathway, recommending varenicline or combination nicotine-replacement therapies for both outpatients with stable cardiovascular disease and hospitalized patients with acute coronary syndrome upon discharge (49).
Research needs
Clinical trials to assess the long-term efficacy and relapse-prevention capabilities of both the intervention and the comparator were limited. Future research should consider measuring QOL outcomes, given the paucity of evidence on this outcome. More research is needed to evaluate effective strategies for using varenicline in relapse prevention and management. Behavioral and social-science investment in strategies for improving uptake of varenicline are warranted. An assessment of the potentially negative consequences of over-the-counter availability of pharmacotherapy would also be useful in directing future policy.
Question 2: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started With Varenicline or Bupropion?
Rationale for question
Bupropion was the first nonnicotine pharmacotherapy for the treatment of tobacco dependence and has established itself as an effective controller agent (50–52). Despite different mechanisms of action, bupropion and varenicline are sometimes considered equivalent first-line agents for tobacco-dependence treatment (53). To identify the optimal controller medication, the panel evaluated the relative effectiveness of bupropion and varenicline.
Summary of evidence
Our systematic review identified seven RCTs comparing varenicline with bupropion. Four of the trials (n = 5,626) evaluated 7-day point-prevalence abstinence rates at 6-month follow-up (28, 54–56). Five trials (n = 5,655) evaluated 7-day point-prevalence abstinence during the 8- to 12-week treatment period (28, 54–57). Two trials were identified that explored impact on QOL (54, 55, 58), and seven trials evaluated the incidence of SAEs with varenicline compared with bupropion (28, 54–57, 59, 60). Withdrawal symptoms were estimated using the MNWS and the Brief Questionnaire of Smoking Urges (QSU-brief) (54, 55, 59) (Table 2).
Table 2.
Evidentiary Basis for Strong Recommendation Favoring Varenicline over Bupropion with Moderate-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline | Bupropion | Relative | Absolute (per 1,000 Patients) | ||
|
Seven-day point-prevalence tobacco abstinence at 6 mo (follow-up: 6 mo; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 4 | RCT | Not serious | Not serious | Not serious | Not serious | None | 874/2,819 (31.0%) | 25.6% | RR, 1.30 (1.19–1.42) | 77 more (↑49–↑108) | High | Critical |
|
Seven-day point-prevalence tobacco abstinence during treatment period (follow-up: range, 8–12 wk; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 5 | RCT | Not serious | Not serious | Not serious | Not serious | None | 1,206/2,834 (42.6%) | 35.9% | RR, 1.41 (1.32–1.52) | 147 more (↑115–↑187) | High | Critical |
|
Quality of life, self-control (follow-up: 12 mo; assessed with smoking-cessation quality of life; higher score indicates better outcome) | ||||||||||||
| 2 | RCT | Serious | Not serious | Not serious | Serious | None | 448 | 398 | — | Effect size, 0.17 | Low | Critical |
|
Quality of life, health transition (follow-up: 12 mo; assessed with smoking-cessation quality of life; lower score indicates better outcome) | ||||||||||||
| 2 | RCT | Serious | Not serious | Not serious | Serious | None | 451 | 401 | — | Effect size, −0.18 | Low | Critical |
|
Serious adverse events (follow-up: range, 7 wk to 3 mo) | ||||||||||||
| 7 | RCT | Not serious | Not serious | Not serious | Serious | None | 54/2,954 (1.8%) | 1.8% | RR, 0.81 (0.57–1.16) | 3 fewer (↓8–↑3) | Moderate | Critical |
|
Tobacco-use relapse measured at the end of follow-up, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | — |
|
Other substance abuse, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | — |
|
Withdrawal symptom, urge to smoke (follow-up: 12 wk; assessed with MNWS; lower score indicates better outcome; scale of 0–4) | ||||||||||||
| 3 | RCT | Not serious | Not serious | Not serious | Serious | None | 798 | 772 | — | MD, 0.3 lower (↓0.43–↓0.17) | Moderate | Critical |
|
Withdrawal symptom, QSU-brief total craving score (follow-up: 12 wk; assessed with QSU-brief; lower score indicates better outcome) | ||||||||||||
| 3 | RCT | Not serious | Not serious | Not serious | Serious | None | 797 | 772 | — | MD, 0.23 lower (↓0.37–↓0.09) | Moderate | Critical |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; MD = mean difference; MNWS = Minnesota Nicotine Withdrawal Scale; QSU-brief = Brief Questionnaire of Smoking Urges; RCT = randomized controlled trial; RR = relative risk.
Varenicline treatment showed significant benefit compared with bupropion and showed similar risk of harm. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
Varenicline increased the 7-day point-prevalence of tobacco abstinence at 6-month follow-up compared with bupropion (RR, 1.30; 95% CI, 1.19 to 1.42; ARR, 77 more per 1,000 patients; 95% CI, 40 more to 108 more; high certainty in the estimated effects). Within the active treatment period, varenicline increased abstinence, largely measured as 7-day point-prevalence abstinence (RR, 1.41; 95% CI, 1.32 to 1.52; ARR, 147 more per 1,000 patients; 95% CI, 115 more to 187 more; high certainty in the estimated effects). Regarding QOL, treatment with varenicline may increase the self-control score compared with bupropion (effect size, 0.17, in which a higher score equals better QOL; 95% CI, not available; low certainty in the estimated effects). Varenicline may improve QOL measured as a health transition score (effect size, −0.18, in which a lower score equals better QOL; 95% CI, not available; low certainty in the estimated effects).
Harms and burdens
Seven RCTs contributed to estimates of SAEs, monitored within a range of 7 weeks to 3 months of follow-up (28, 54–57, 59, 60). Varenicline treatment probably reduced the risk of SAEs compared with bupropion (RR, 0.81; 95% CI, 0.57 to 1.16; ARR, 3 fewer per 1,000 patients; 95% CI, 8 fewer to 3 more; moderate certainty in the estimated effects). Varenicline probably reduced withdrawal symptoms as measured by both the MMWS urge to smoke (MD, 0.3 lower; 95% CI, 0.43 lower to 0.17 lower; moderate certainty in the estimated effects) and the QSU-brief total craving score (MD, 0.23 lower; 95% CI, 0.37 lower to 0.09 lower; moderate certainty in the estimated effects).
Certainty in the estimated effects
Certainty in the estimated effects was judged to be high for 7-day point-prevalence abstinence, at both the 6-month follow-up and during the active treatment periods. Tobacco abstinence was assessed by self-report and verified by eCO concentration. Certainty in the estimated effects on QOL was low because of risk of bias and imprecision. Certainty in effects regarding SAEs was moderate because of imprecision. Finally, certainty in effects on withdrawal, urge to smoke, and overall cravings was moderate because of imprecision.
Other considerations
The guideline panel considered tobacco abstinence at 6 months or later and the avoidance of SAEs to be critical outcomes. Given the availability of generic bupropion, we considered the potential cost barriers to implementation and conducted a systematic review to assess the cost-effectiveness of using varenicline compared with bupropion. We identified three cost-utility analyses, two cost-effectiveness analyses, and one cost–benefit analysis in the U.S. setting (45–47, 61–63). All economic evaluations identified suggested that varenicline is cost-effective compared with bupropion. The panel noted that, although methodologically sound, most of the U.S. economic evaluations were funded by the manufacturer of varenicline. Furthermore, the panel considered varenicline as a probably acceptable and probably feasible intervention for stakeholders, with the removal of the black-boxed warning of serious neuropsychiatric side effects after the EAGLES (Evaluating the Safety and Efficacy of Varenicline and Bupropion for Smoking Cessation in Subjects with and without a History of Psychiatric Disorders) trial (28).
Panel discussion and conclusions
The panel concluded 1) that varenicline showed a large, desirable effect in achieving abstinence compared with bupropion, with high-certainty evidence, and 2) that varenicline treatment likely results in little to no difference in SAEs compared with bupropion. As a result, the panel concluded that the clinical superiority (balance of effect) of varenicline outweighs its higher price and the possibly important uncertainty or variability of patients’ values and preferences. Therefore, all panel members preferred a strong recommendation. The panel also made important observations related to varenicline access. A qualitative interview study of Veterans Health Administration substance-abuse-program staff reported that all programs offered nicotine-replacement therapy (NRT) and some provided bupropion, but few provided varenicline (64). This pattern is unlikely to be unique to the Veterans Health Administration; payer costs appear to form barriers to availability, despite favorable cost-effectiveness (65).
Recommendation 2
For tobacco-dependent adults in whom treatment is being initiated, we recommend varenicline over bupropion (strong recommendation, moderate certainty in the estimated effects).
What others are saying
To our knowledge, no other clinical practice guideline has compared varenicline with bupropion. The American College of Chest Physicians “Tobacco Dependence Treatment Toolkit” references varenicline as the most effective monotherapy, citing the USPHS guidelines (7). However, the American College of Chest Physicians did not explicitly endorse use of varenicline over bupropion, likely because of a paucity of clinical data at the time of the Toolkit’s publication. A 2016 American College of Physicians publication discusses varenicline’s efficacy but did not directly compare bupropion with varenicline or recommend one over the other (66).
Research needs
Future trials are necessary to evaluate the relative clinical effect of varenicline and bupropion in uniquely at-risk populations, such as pregnant women, adolescents, and patients with a history of treatment unresponsiveness (57, 67, 68). The relative cost-effectiveness of these agents should be further evaluated in the contexts of both relapse prevention and retreatment after relapse (69, 70).
Question 3: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline plus Nicotine-Replacement Therapy or Varenicline Alone?
Rationale for question
Varenicline is a partial agonist of the α4β2 nicotinic acetylcholine receptor, densely expressed across the mesolimbic system and implicated in instinctive learning (71). Varenicline acts as an agonist–antagonist, with a mechanism of action believed to involve a reduction in the rewarding capacity of nicotine. A common resulting perception is that there is limited utility in combining varenicline with nicotine pharmacotherapy. However, nicotine addiction and smoking behavior are complex and likely involve multiple pathways beyond the α4β2 receptor system. After identification of the optimal controller, the panel believed it important to evaluate whether supplementing varenicline therapy with nicotine-replacement would be better than using varenicline alone.
Summary of evidence
Our review identified three treatment trials that directly compared varenicline combined with a nicotine patch with varenicline alone, two of which reported on smoking abstinence at 6 months. The combined studies enrolled 776 individuals and assessed the smoking status of individuals at baseline and at 6 months (72, 73). Three randomized trials enrolling 893 individuals compared adverse events (72–74). No studies in our review evaluated varenicline in combination with nicotine-reliever forms (i.e., gum, lozenge, inhaler, or nasal spray) (Table 3).
Table 3.
Evidentiary Basis for Conditional Recommendation Favoring Varenicline plus Patch over Patch Alone with Low-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline + Nicotine | Varenicline Alone | Relative | Absolute (per 1,000 Patients) | ||
|
Seven-day point-prevalence tobacco abstinence, 6 mo or later (follow-up: mean, 6 mo; assessed with self-report, confirmed with exhaled carbon monoxide) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 154/386 (39.9%) | 29.3% | RR, 1.36 (1.07–1.72) | 105 more (↑21–↑211) | High | Critical |
|
Seven-day point-prevalence tobacco abstinence during treatment (assessed with self-report, confirmed with exhaled carbon monoxide) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 184/386 (47.7%) | 36.2% | RR, 1.31 (1.11–1.54) | 112 more (↑40–↑196) | High | Important |
|
Quality of life, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Serious adverse events (follow-up: mean, 6 mo; as reported) | ||||||||||||
| 3 | RCT | Not serious | Not serious | Not serious | Very serious | None | 4/444 (0.9%) | 1.4% | RR, 1.06 (0.27–4.05) | 1 more (↓10–↑42) | Low | Critical |
|
Relapse, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Other substance use, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Withdrawal, composite-symptoms rating (follow-up: mean, 4 wk; assessed with Mood and Physical Symptoms Scale; lower change score indicates better outcome; scale of 2–12) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 35 | 34 | — | MD, 0.04 lower (0–0) | Very low | Important |
|
Withdrawal, craving (follow-up: mean, 4 wk; assessed with Wisconsin Withdrawal Symptom Scale; lower score indicates better outcome; scale of 0–4) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 110 | 87 | — | MD, 0.1 higher (↓0.19–↑0.39) | Low | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; MD = mean difference; RCT = randomized controlled trial; RR = relative risk.
Combination treatment using varenicline and nicotine patch showed benefit compared with varenicline and showed similar risk of severe adverse events. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
Varenicline plus a nicotine patch significantly increased abstinence compared with varenicline alone, measured as 7-day point-prevalence abstinence at 6 months or later, when assessed by self-report and confirmed by eCO (RR, 1.36; 95% CI, 1.07 to 1.72; ARR, 105 more per 1,000 patients; 95% CI, 21 more to 211 more; high certainty in the estimated effects) (72, 73). Varenicline plus a nicotine patch statistically increased abstinence, measured as 7-day point-prevalence abstinence during treatment when compared with varenicline alone (RR, 1.31; 95% CI, 1.11 to 1.54; ARR, 112 more per 1,000 patients; 95% CI, 40 more to 196 more; high certainty in the estimated effects) (72, 73).
Harms and burdens
Varenicline plus a nicotine patch might result in a trivial increase in undesirable effects. Varenicline plus a nicotine patch may increase the risk of SAEs slightly compared with varenicline alone (RR, 1.06; 95% CI, 0.27 to 4.05; ARR, 1 more per 1,000 patients; 95% CI, 10 fewer to 42 more; low certainty in the estimated effects) (72–74). Overall, the evidence was very uncertain about the effect of varenicline plus a nicotine patch on withdrawal symptoms at 4 weeks, as measured by either the Mood and Physical Symptoms Scale (MD, 0.04 lower; 95% CI, not available; very low certainty in the estimated effects) or the Wisconsin Withdrawal Symptom Scale (MD, 0.1 higher; 95% CI, 0.19 lower to 0.39 higher; low certainty in the estimated effects) when compared with varenicline alone (72, 74). In both measures, a lower score indicates a better outcome.
Certainty in estimated effects
Certainty in the effects estimated from the evidence was judged to be high for 7-day point-prevalence abstinence, both during the treatment period and at 6-month follow-up. Certainty in estimates of SAEs was judged to be low because of very serious imprecision related to a very small number of events. Certainty in estimates of withdrawal-symptom scores was judged to be low or very low because of risk of bias and imprecision in effect size (very serious imprecision for composite-score rating and serious imprecision for craving score).
Other considerations
Although combination therapy was considered feasible to implement, the panel remained concerned that prescriber and/or payer reluctance might affect feasibility. In addition, initiating two medications could complicate instructions, affect adherence, or limit patient agreement with the recommendation. Although the panel considered both interventions to be acceptable to stakeholders, combination therapy might be most acceptable if introduced sequentially, particularly if the patient had experienced monotherapy or significant withdrawal symptoms in the past. Studies have demonstrated that low-burden, clinician-directed projects aimed at improving patient awareness of the evidence can increase patient receptivity to pharmacotherapy (75). No data were identified that assessed the comparative cost-effectiveness or relapse rate of the intervention.
Panel discussion and conclusions
The panel concluded 1) that varenicline plus a nicotine patch showed a large, desirable effect compared with varenicline alone on smoking abstinence and 2) that varenicline plus a nicotine patch may increase the risk of SAEs only slightly compared with varenicline alone. As a result, the panel suggested varenicline plus a nicotine patch rather than varenicline alone for treatment of tobacco dependence. The panel chose to make a conditional recommendation because the low certainty in estimated SAEs limited confidence in the overall certainty of the evidence.
Of note, the original clinical question sought to identify the relative effect of adding a nicotine reliever to the varenicline controller to manage acute cue-induced cravings, which returned no direct evidence for analysis. The panel therefore decided to liberalize the definition of nicotine therapy to include the patch, comparing varenicline plus a nicotine patch with varenicline alone. Given the differences in pharmacokinetics between a nicotine patch and other delivery forms, the panel did not believe that a conclusion regarding the effectiveness of other forms of nicotine replacement could be made at this time.
Recommendation 3
For tobacco-dependent adults in whom treatment is being initiated, we suggest varenicline plus a nicotine patch over using varenicline alone (conditional recommendation, low certainty in the estimated effects).
What others are saying
To our knowledge, no other clinical practice guideline has compared varenicline plus a nicotine patch with varenicline alone. The tobacco-cessation clinical pathway, recently produced by the American College of Cardiology, suggests that adding nicotine to varenicline may be an option for smokers who do not succeed with NRT or varenicline alone (49).
Research needs
A lack of randomized trials made answering the more specific question regarding the efficacy of varenicline plus nicotine relievers impossible. Given the potential utility of reliever medication in managing acute, cue-induced cravings and the advantages inherent to self-regulating nicotine concentrations, such trials are necessary to identify clinically relevant differences in outcomes. In addition, data are needed on the cost-effectiveness of combination therapy and on the impact on relapse after discontinuation. Mechanistic research into how nicotine potentiates varenicline’s effect could shed light on advanced pharmacologic strategies and new therapeutic targets.
Question 4: For Tobacco-Dependent Adults in Whom Treatment Is Being Initiated, Should Treatment Be Started with Varenicline or an Electronic Cigarette?
Rationale for question
Despite the established efficacy of various pharmacologic agents for treatment of tobacco dependence, a significant number of clinicians have recommended electronic cigarettes as a means of helping their patients stop smoking (76–78). Electronic cigarettes have been used more often than pharmacologic agents by individuals in the United States trying to control smoking (79). Given varenicline’s identified optimum-controller effectiveness, the panel believed it important to evaluate whether varenicline or electronic cigarettes should be used to treat tobacco-dependent adults.
Summary of evidence
Our systematic review identified only an observational study and a conference abstract of an RCT, both comparing varenicline with electronic cigarettes. The observational study enrolled 3,093 individuals who were attempting to quit smoking, including 156 using varenicline and 200 using electronic cigarettes, and followed the individuals for a mean of 1 year (79). The RCT recruited 54 smokers with a history of acute coronary syndrome (80). Because of the paucity of direct evidence, the panel elected to consider indirect evidence. Eleven randomized trials comparing varenicline with nicotine replacement (27–36) and two randomized trials comparing electronic cigarettes with nicotine replacement (81, 82) were selected, and a network meta-analysis including 8,830 individuals was performed (Table 4).
Table 4.
Evidentiary Basis for Conditional Recommendation Favoring Varenicline over Electronic Cigarettes, with Very-Low-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline | Electronic Cigarette | Relative | Absolute (per 1,000 Patients) | ||
|
Point-prevalence tobacco abstinence, 6 mo or later (follow-up: mean, 24 wk) | ||||||||||||
| 1 | RCT | Serious | Not serious | Serious | Very serious | None | 13/27 (48.1%) | 32.5% | RR, 1.44 (0.75–2.80) | 143 more (↓81–↑585) | Very low | Critical |
|
Continuous abstinence, 6 mo or longer (follow-up: mean, 1 yr; assessed with persistent abstinence from all tobacco) | ||||||||||||
| 1 | Observational study | Serious | Not serious | Not serious | Serious | None | 156 | 200 | — | MD, 0.046 higher (↓0.018–↑0.11) | Very low | Critical |
|
Point-prevalence tobacco abstinence during treatment, not measured | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Quality of life, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Serious adverse events (follow-up: 24 wk) | ||||||||||||
| 1 | RCT | Serious | Not serious | Serious | Very serious | None | 0/27 (0.0%) | 0.0% | No estimate | — | Very low | Critical |
|
Relapse (follow-up: 1 yr) | ||||||||||||
| 1 | Observational study | Serious | Not serious | Not serious | Serious | None | 156 | 200 | — | MD, 0.065 higher (0–0) | Very low | Important |
|
Other substance use, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Withdrawal, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; MD = mean difference; RCT = randomized controlled trial; RR = relative risk.
Indirect comparisons were used to estimate the relative effect. Varenicline treatment showed an uncertain benefit compared with electronic cigarettes but had significantly fewer adverse events than electronic cigarettes. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
The observational study showed that varenicline might lead to an increase in continuous abstinence at 6 months or later when compared with electronic cigarettes, but the evidence is very uncertain (MD, +4.6%; 95% CI, −1.8% to +11%; very low certainty in the estimated effects). The RCT reported as an abstract suggested an increase in self-reported 7-day point-prevalence abstinence of 14.8% (95% CI, 3.9% to 25.8%) among individuals receiving varenicline compared with electronic cigarettes. The indirect evidence showed that varenicline might lead to a non–statistically significant decrease in abstinence at 6 months or later, but the evidence is very uncertain (RR, 0.85; 95% CI, 0.65 to 1.10; ARR, 42 fewer per 1,000 patients; 95% CI, 99 fewer to 28 more; very low certainty in the estimated effects). However, varenicline might lead to an increase in point-prevalence abstinence during treatment at 3 months when compared with electronic cigarettes, but the evidence is very uncertain (RR, 1.10; 95% CI, 0.73% to 1.60%; ARR, 22 more per 1,000 patients; 95% CI, 58 fewer to 129 more; very low certainty in the estimated effects).
Harms and burdens
The RCT reported as an abstract described no SAEs in either group, although the number enrolled was small. The indirect evidence showed that varenicline might decrease the RR of SAEs compared with electronic cigarettes, but the evidence is very uncertain (RR, 0.32; 95% CI, 0.071 to 0.82; ARR, 52 fewer per 1,000 patients; 95% CI, 72 fewer to 14 more; very low certainty in the estimated effects). Both the direct and indirect evidence showed that varenicline might be associated with a slight, but not clinically significant, increase of the rate of relapse compared with electronic cigarettes, but the evidence is very uncertain (HR, 1.1; 95% CI, 0.7 to 1.5; very low certainty in the estimated effects).
Certainty in estimated effects
For the direct evidence, certainty in the estimates from randomized evidence for abstinence at 6 months or later was judged to be very low because of a serious risk of bias, serious inconsistency, and very serious imprecision. Certainty in the effects estimated from the nonrandomized evidence was judged to be very low for both continuous abstinence at 6 months or later and relapse at 1 year because of risk of bias and imprecision. Certainty in the effects estimated from the indirect evidence was judged to be very low for abstinence at 6 months or later, adverse events, and relapse because of risk of bias, indirectness, and imprecision.
Other considerations
The panel considered both interventions to be acceptable to stakeholders, with varenicline being increasingly feasible because of the removal of the boxed warning. While acknowledging individual variability in perceived utility of persistent nicotine dependence, the panel identified significant psychosocial implications of nicotine exposure, including effects on learning, attention, mental health, and susceptibility to addiction to other drugs (83–88). This category of harms is frequently underestimated by stakeholders but may represent an additional consequence of use as the risk profile of electronic cigarettes continues to evolve (89, 90). No data assessing the cost-effectiveness of varenicline compared with electronic cigarettes were identified.
Panel discussion and conclusions
The panel concluded 1) that varenicline showed an uncertain benefit compared with electronic cigarettes in abstinence or relapse and 2) that varenicline had fewer adverse events than electronic cigarettes. As a result, the panel recommended varenicline rather than electronic cigarettes for treatment of tobacco dependence. The panel chose to make a conditional recommendation because the very low certainty in the estimated effects limited confidence in these conclusions.
The panel made three important observations related to the generalizability of the indirect comparison of varenicline with electronic cigarettes. First, significant differences in the common comparator between varenicline and electronic cigarettes likely impacted the effect estimates. Specifically, studies comparing varenicline with the nicotine patch used dummy medications and/or placebos as controls, whereas studies comparing electronic cigarettes with a nicotine patch used open-label interventions and were susceptible to performance bias. Second, the targeted outcomes were qualitatively different in the studies used for the indirect comparisons. When varenicline is compared with a nicotine patch, the outcome numerators reflect discontinuation of the smoking behavior. In contrast, when electronic cigarettes are compared with a nicotine patch, the outcome numerators reflect a substitution in the mechanism of nicotine delivery. Continuation of electronic cigarette use may indicate a continuation of the compulsive behavior, whereas cessation does not. Finally, clinical trial SAE rates are not synonymous with product safety. Electronic cigarettes appear to carry their own unique risk profile, with wide variability in effects across product categories, aerosol constituents, ages of initiation, and consumer use patterns (91). The panel was aware of large epidemiologic studies of the respiratory and cardiovascular impact of electronic cigarette use and highlighted that the overall health consequences of electronic cigarette use have become increasingly suspect (92–94); conversely, initial safety concerns over varenicline have diminished under scrutiny (95, 96). The panel emphasized that the recommendation is intended exclusively for tobacco-dependence treatment under the supervision of a trained clinician and should not be extrapolated to other contexts, such as unsupervised treatment of tobacco dependence or recreational use.
Although there was unanimity among the panel regarding the preferred intervention, four panelists (H.J.F., P.G., S. Pakhale, and M.C.P.) advocated for a strong, rather than conditional, recommendation. They were concerned about the safety and effectiveness of electronic cigarettes because of reports that were not included in the evidence synthesis (97–109). They cited reports of deaths or disability due to electronic cigarette– or vaping-associated lung injury (110–113), burns due to product explosion, acute nicotine poisoning, and seizures, as well as histopathologic injuries in laboratory studies. They noted that such concerns have prompted warnings about electronic cigarettes from numerous organizations, as described below (110, 114–117). Two nonvoting panelists later joined the dissent (P.F., T.L.), but these panelists were unavailable to participate in the panel discussions of the evidence or the formulation and grading of the recommendation.
Recommendation 4
For tobacco-dependent adults, we suggest varenicline over electronic cigarettes (conditional recommendation, very low certainty in the estimated effects). Remarks: The recommendation’s strength reflects very low certainty in the effects used to derive the recommendation. After our evidence synthesis, new evidence emerged regarding serious adverse effects of electronic cigarettes. If these serious adverse effects continue to be reported, the strength of the recommendation should be reevaluated. Note that this recommendation is intended for treatment of tobacco dependence under the supervision of a clinician; it should not be extrapolated to unsupervised treatment or recreational use.
What others are saying
To our knowledge, no other clinical practice guideline has compared varenicline with electronic cigarettes. The American College of Cardiology recently produced a tobacco-cessation clinical pathway, recommending pharmacotherapy and advocating discussion with patients who choose to use electronic cigarettes (49). Several professional societies have developed warnings or adopted policy positions regarding the electronic cigarette’s role in tobacco-dependence treatment, including the U.S. CDC (110), the European Respiratory Society (114), the Federation of International Respiratory Societies (115), the Ibero-Latino-American Respiratory Scientific Societies (116), the American Association for Cancer Research, and the American Society of Clinical Oncology (117), each recommending that clinicians rely on medications approved by the U.S. Food and Drug Administration or other regulatory agencies instead of relying on alternative modalities that lack an established evidentiary base. A 2018 National Academies of Sciences, Engineering, and Medicine evidence review of the public health impact of electronic cigarettes made specific comment on the quality of the evidence comparing electronic cigarettes with pharmacotherapy, noting insufficient evidence warranting electronic cigarettes as cessation pharmacotherapy (118). A widely cited policy statement produced by the Royal College of Physicians in 2016 did promote the widespread availability of electronic cigarettes in the United Kingdom as a public health substitute for smoking, but avoided positioning electronic cigarettes as a means of treating tobacco dependence (119).
Research needs
The primary impediment to confidently answering the question is the lack of randomized trials directly comparing varenicline with electronic cigarettes. Assuming a specific antecedent exposure causing electronic cigarette– or vaping-associated lung injury can be identified, it may become ethically feasible to conduct trials blinding participants to both the intervention and the comparator (91). In all cases of clinical trials involving electronic cigarettes, we recommend using objective measures of compensatory behaviors and long-term control over dependence, not simply counts of cigarettes consumed (120). Observational studies that account for the known variability in real-world use patterns when describing the long-term safety outcomes of electronic cigarette use are also needed (91).
Question 5: In Tobacco-Dependent Adults Who Are Not Ready to Discontinue Tobacco Use, Should Clinicians Begin Treatment with the Optimal Controller or Wait Until They Are Ready to Stop Tobacco Use?
Rationale for question
The “5A framework” of the USPHS guideline includes reminders to Ask every patient about tobacco use, Advise current smokers to stop, Assess their willingness to stop smoking, Assist with pharmacotherapy, and Arrange for follow-up care (2). The idea that “readiness to quit” should be assessed has been prominent in published treatment strategies because of near-universal initial acceptance of the transtheoretical model of behavior change (121, 122). More recently, however, the relevance of the model has come into question on the basis of observations that behavior change is dynamic (123), a significant portion of patients undergo unplanned cessation attempts with many experiencing sustained control over compulsion (124, 125), and pretreatment of tobacco users may increase the number of patients who will go on to stop smoking (126–131). Although patients may not be ready to abstain, they may be willing to try tobacco-dependence treatment (132). The panel therefore posed a question evaluating the relative effect of initiating optimal controller therapy before patients express a readiness to abstain.
Summary of evidence
Our systematic review identified four randomized, double-blind, placebo-controlled clinical trials that addressed the efficacy of initiation of tobacco-dependence treatment in smokers unready to abstain (133–136) and a fifth study that was a randomized, double-blind placebo-controlled trial evaluating the 15-day experimental effect of varenicline in non–treatment-seeking smokers (137). Three studies (n = 2,387) evaluated abstinence rates at least 6 months after treatment initiation, two of which also evaluated outcomes during the treatment period. All studies involved participants who were regular smokers but were either unable or unwilling to make a quit attempt at the time of study entry. Self-reported abstinence was biochemically confirmed with eCO (Table 5).
Table 5.
Evidentiary Basis for Strong Recommendation Favoring Pretreatment in Patients Unready to Attempt Abstinence over Waiting for Patient Readiness, with Moderate-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline Pretreatment | Wait until Patient Ready | Relative | Absolute (per 1,000 Patients) | ||
|
Point-prevalence tobacco abstinence, 6 mo or later (follow-up: range, 6 mo to 1 yr; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 3 | RCT | Not serious | Not serious | Not serious | Not serious | None | 473/1,360 (34.8%) | 17.3% | RR, 2.00 (1.70–2.35) | 173 more (↑121–↑234) | High | Critical |
|
Point-prevalence tobacco abstinence during treatment (follow-up: 24 wk; assessed with: self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 615/1,253 (49.1%) | 20.6% | RR, 2.49 (2.09–2.98) | 308 more (↑225–↑409) | High | Important |
|
Smoking reduction, week 4 (follow-up: 4 wk; assessed with reduction ≥ 50%) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Serious | Not serious | None | —/785 | 31.1% | OR, 1.95 (1.59–2.41) | 157 more (↑107–↑210) | Moderate | Important |
|
Smoking reduction (follow-up: range, 8 wk to 3 mo; assessed with reduction ≥ 50%) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Serious | Not serious | None | —/785 | 15.1% | OR, 2.03 (1.57–2.61) | 114 more (↑67–↑166) | Moderate | Important |
|
Smoking reduction in number of cigarettes/d | ||||||||||||
| 1 | RCT | Serious | Not serious | Serious | Serious | None | 77 | 76 | — | MD, 2.6 higher (0–0) | Very low | Important |
|
Motivation to quit | ||||||||||||
| 3 | RCT | Serious | Not serious | Serious | Serious | None | 456/595 (76.6%) | 169/270 (62.6%) | RR, 1.17 (0.98–1.40) | 106 more (↓13–↑250) | Very low | Important |
|
Serious adverse events | ||||||||||||
| 4 | RCT | Not serious | Not serious | Not serious | Serious | None | 34/1,369 (2.5%) | 17/1,046 (1.6%) | RR, 1.75 (0.98–3.13) | 12 more (↓0–↑35) | Moderate | Critical |
|
Withdrawal (follow-up: range, 12–15 d; assessed with Questionnaire of Smoking Urges, tonic craving; lower score indicates better outcome; scale of 1–7) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 46 | 54 | — | MD, 1.54 lower (↓2.15–↓0.93) | Low | Important |
|
Withdrawal (follow-up: range, 12–15 d; assessed with Wisconsin Smoking Withdrawal Scale, tonic craving; lower score indicates better outcome; scale of 0–8) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 46 | 54 | — | MD, 1.26 lower (↓1.34–↓1.18) | Low | Important |
|
Withdrawal (follow-up: 2 mo; assessed with MNWS, total withdrawal; lower score indicates better outcome; scale of 0–27) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 77 | 76 | — | MD, 0.1 higher (0–0) | Low | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; MD = mean difference; MNWS = Minnesota Nicotine Withdrawal Scale; OR = odds ratio; RCT = randomized controlled trial; RR = relative risk.
Initiation of varenicline treatment in smokers not ready to abstain showed a large effect on abstinence and a small increase in severe adverse events. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
More smokers were able to stop smoking when treated with varenicline, despite initial reluctance. Using 7-day point-prevalence abstinence at 6 months after treatment, varenicline increased abstinence compared with waiting for affirmation of readiness (RR, 2.00; 95% CI, 1.70 to 2.35; ARR, 173 more per 1,000 patients; 95% CI, 121 more to 234 more; high certainty in the estimated effects). Treating with varenicline also increased 7-day point-prevalence abstinence compared with waiting (RR, 2.49; 95%, CI, 2.09 to 2.98; ARR, 308 more per 1,000 patients; 95% CI, 225 more to 409 more; high certainty in the estimated effects).
Harms and burdens
The impact of treatment strategies on QOL was not reported. Varenicline likely increased SAEs associated with treatment (RR, 1.75; 95% CI, 0.98 to 3.13; ARR, 12 more per 1,000 patients; 95% CI, 0 fewer to 35 more; moderate certainty in the estimated effects). Impact on withdrawal scores was assessed using several validated metrics and suggested a small but significant reduction in symptoms among those receiving varenicline treatment. Estimates of effect on withdrawal symptoms, in which lower scores indicate better outcomes, were 1.54 points lower (95% CI, 2.15 lower to 0.93 lower; low certainty in the estimated effects) when assessed with the QSU-brief and 1.26 points lower (95% CI, 1.34 lower to 1.18 lower; low certainty in the estimated effects) when assessed with the Wisconsin Smoking Withdrawal Scale. MNWS scores remained unchanged (MD, +0.1; 95% CI, not available, low certainty in the estimated effects).
Certainty in estimated effects
Certainty in the estimated effects was judged to be high for 7-day point-prevalence abstinence both during the treatment period and at follow-up at 6 months or later. Certainty in SAE effects was judged as moderate because of imprecision. Certainty in estimates of withdrawal severity was judged to be low because of serious risk of bias and serious imprecision.
Other considerations
The cost of varenicline ranges from $2,442 to $3,096 for 24 weeks of treatment. The panel concluded that cost-effectiveness estimates favor the intervention. Parenthetically, the panel noted that the varenicline patent expires in December 2020, which could unpredictably affect cost-effectiveness. On balance, the evidence suggests that varenicline pretreatment could increase health equity, on the basis of the higher prevalence of tobacco use among people who experience poverty. In general, the evidence also suggests that varenicline used in this way would be acceptable to stakeholders (138–141). Although uptake of varenicline is low, people prefer pharmacotherapy options if they are of higher efficacy, have less-frequent side effects, and prevent weight gain (40–43, 138, 142). Cost concerns become less important in the presence of improved efficacy and safety. In addition, the panel considered starting with varenicline to be more feasible than asking patients who are not yet ready to stop smoking to quit immediately.
Panel discussion and conclusions
The panel concluded 1) that the initiation of varenicline treatment in smokers not ready to abstain showed a large effect on abstinence, with high certainty in the estimated effects, and 2) that initiation of pretreatment showed a small increase in SAEs, with moderate certainty in the estimated effects. As a result, the panel concluded that the clinical superiority (balance of effect) of varenicline in smokers not ready to abstain outweighs its higher price and the possibly important uncertainty or variability of patients’ values and preferences. Therefore, the panel preferred a strong recommendation.
The panel considered the potential threat to patient autonomy if the proactive approach is misapplied but recognized that autonomy is preserved when clinicians engage their patients in discussion, encourage pharmacotherapy with continued smoking, and respect their decision to decline treatment.
Overall, the panel judged patient values as having important variability, given that individual patients may prioritize relative efficacy, side effects, accessibility, and costs differently. Several panelists believed that the increased cost of treatment before quitting was offset by the number of smokers who would be successfully treated. The out-of-pocket costs for smokers were difficult to assess, given that prescription agents are often covered by insurance. In addition, if medication prescriptions are filled but not used, overall costs could be substantial. Administrative restrictions based on these concerns risk undermining the effectiveness of evidence-based treatment strategies.
With its capacity to be a singularly effective disease-preventing intervention, pharmacotherapy pretreatment in tobacco dependence is expected to enable 308 additional smokers per 1,000 to stop. This dramatic benefit, coupled with minor harm (28, 143), compels clinicians to consider pretreatment for every patient with tobacco dependence. The panel recognized the unconventional aspects of this recommendation and identified several workflow requirements inherent to the conclusion. First, implementing pretreatment protocols in practice will require clinicians to move their therapeutic focus away from the anticipated behavioral outcome (i.e., smoking) and focus instead on resolving the intermediate mediator of smoking (i.e., the compulsion to smoke). In this way, pharmacotherapy is no longer contingent on readiness to quit but is instead a therapeutic intervention aimed at improving readiness to stop smoking. Second, the prescriber becomes responsible for reframing the patient’s expectations and goals of therapy. No longer is the only goal cessation, but goals might also include an increasing willingness to consider an abstinence attempt. Finally, focusing pretreatment regimens on controlling the compulsion to smoke implies that variable pretreatment durations will be required before patients are able to attempt abstinence. Cessation rates steadily increase through 24 weeks of pretreatment, suggesting that attempts to set a “quit date” too early in treatment may be counterproductive (133–136).
Recommendation 5
In tobacco-dependent adults who are not ready to discontinue tobacco use, we recommend that clinicians begin treatment with varenicline rather than waiting until patients are ready to stop tobacco use (strong recommendation, moderate certainty in the estimated effects).
What others are saying
A collaborative statement on current chronic obstructive pulmonary disease (COPD) research needs, published by the ATS and the European Respiratory Society, positions pretreatment in pursuit of smoking reduction as a potential mechanism for reducing COPD respiratory symptoms but does not address the potential role of pretreatment for all patients with COPD who continue to smoke (144). The ATS research statement on smoking-cessation interventions in lung cancer screening programs identified the profound drop-off in intervention rates after assessment of quit readiness as a potential point for performance improvement but did not make specific pretreatment recommendations (145). The Canadian Cancer Society produced an educational flyer encouraging agency among smokers not ready to quit (146). It offers advice for preparing for a quit attempt but does not address the role of pharmacotherapy.
Research needs
Given the cumulative effect of varenicline on abstinence during the treatment phase, guidance regarding the optimal duration of treatment, including methods for determining when continuation is unlikely to derive further benefit, is critical to maximizing impact on outcomes. Future research needs to measure outcomes important to patients, such as QOL and other substance use, when conducting trials on patients who are unready to quit. In addition, given the identified synergistic effect of nicotine on varenicline, studies evaluating the impact of combination pharmacotherapy on treatment outcomes are warranted. Finally, research on subgroup populations with comorbidities should be considered. For example, should patients with COPD who continue to smoke receive longer-duration treatment, given their recognized dependence severity (147)? Would patients with a history of depression or alcoholism benefit from combination pretreatment with bupropion (148, 149)? Does the pharmacotherapy follow-up interval after initiation have an impact on efficacy (150, 151)?
Question 6: In Tobacco-Dependent Adults with Comorbid Psychiatric Conditions, Including Substance-Use Disorder, Depression, Anxiety, Schizophrenia, and/or Bipolar Disorder, for Whom Treatment Is Being Initiated, Should Clinicians Start with the Optimal Controller Identified for Patients without Psychiatric Conditions or Use a Nicotine Patch?
Rationale for question
Before the publication of the EAGLES clinical trial, there was a boxed warning regarding possible neuropsychiatric adverse events for both varenicline and bupropion. These concerns stemmed from case reports and postmarketing surveillance, as no RCTs found evidence for these events and early observations suggested no significant increase in neuropsychiatric adverse events with pharmacotherapy compared with placebo, even among patients with preexisting mental illness. Given the persistent stigma assigned to varenicline within the behavioral health community, the panel believed it important to evaluate the evidence guiding the clinical question of whether varenicline or nicotine should be used in adults with comorbid psychiatric conditions (152).
Summary of evidence
Our systematic review identified two randomized clinical trials (n = 2,194) that directly compared varenicline with a nicotine patch in a cohort of participants with mental illness (28, 35). Both studies assessed SAEs as well as point-prevalence abstinence at the end of the 12-week treatment period and at 6-month follow-up. Relapse of tobacco use was not reported in these two trials (Table 6).
Table 6.
Evidentiary Basis for Strong Recommendation Favoring Varenicline over Patch in Patients with Substance-Use or Psychiatric Disorders, with Moderate-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Varenicline | Nicotine Patch | Relative | Absolute (per 1,000 Patients) | ||
|
Point-prevalence tobacco abstinence at 6 mo (follow-up: 6 mo; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Not serious | None | 275/1,109 (24.8%) | 11.7% | RR, 1.31 (1.12–1.53) | 36 more (↑14–↑62) | High | Critical |
|
Point-prevalence tobacco abstinence during treatment period (follow-up: 12 wk; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Serious | None | 368/1,109 (33.2%) | 13.9% | RR, 1.78 (0.78–4.08) | 108 more (↓31–↑428) | Moderate | Important |
|
Quality of life, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Serious adverse events | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Serious | None | 23/1,103 (2.1%) | 1.2% | RR, 0.95 (0.54–1.67) | 1 fewer (↓5–↑8) | Moderate | Critical |
|
Tobacco-use relapse measured at the end of the follow-up (at 6 mo or later), not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Critical |
|
Other substance abuse, alcohol | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 8/49 (16.3%) | 29.0% | RR, 0.56 (0.24–1.30) | 128 fewer (↓221–↑87) | Very low | Important |
|
Other substance abuse, any drug | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Very serious | None | 18/49 (36.7%) | 25.8% | RR, 1.42 (0.71–2.87) | 108 more (↓75–↑483) | Very low | Important |
|
Severity of withdrawal, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; RCT = randomized controlled trial; RR = relative risk.
Varenicline treatment may result in a large benefit and may decrease serious adverse events. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
Compared with a nicotine patch, varenicline increased eCO-confirmed 7-day point-prevalence abstinence assessed at 6-month follow-up (RR, 1.31; 95% CI, 1.12 to 1.53; ARR, 36 more per 1,000 patients; 95% CI, 14 more to 62 more; high certainty in the estimated effects). Varenicline likely increased the 7-day point-prevalence abstinence at the end of the 12-week treatment period (RR, 1.78; 95% CI, 0.78 to 4.08; ARR, 108 more per 1,000 patients; 95% CI, 31 fewer to 428 more; moderate certainty in the estimated effects). Relapse rates at follow-up at 6 months or later and impacts on QOL were not reported.
Harms and burdens
Our systematic review suggested that varenicline may decreased the risk of SAEs slightly compared with a nicotine patch (RR, 0.95; 95% CI, 0.54 to 1.67; ARR, 1 fewer per 1,000 patients; 95% CI, 5 fewer to 8 more; moderate certainty in the estimated effects). The undesirable effects were judged as trivial. There was one RCT that evaluated the impact of varenicline on the use of other substances. The effect of varenicline on other substance use was unclear because of very low certainty of evidence (for alcohol use: RR, 0.56; 95% CI, 0.24 to 1.3; ARR, 128 fewer per 1,000 patients; 95% CI, 221 fewer to 87 more; very low certainty in the estimated effects; other substance use: RR, 1.42; 95% CI, 0.71 to 2.87; ARR, 108 more per 1,000 patients; 95% CI, 75 fewer to 483 more; very low certainty in the estimated effects). The effect on the severity of withdrawal was not available.
Certainty in estimated effects
Certainty in the estimated effects of varenicline was judged to be high for 7-day point-prevalence abstinence at 6-month follow-up. Certainty in the estimated effects on 7-day point-prevalence abstinence at 12-week follow-up and on SAEs were judged to be moderate because of serious imprecision, with 95% CIs that could lead to opposing conclusions. Estimates for impact of varenicline on the risks of other substance use were judged to be of very low certainty because of a serious risk of bias and very serious imprecision due to the small number of events.
Other considerations
The guideline panel considered tobacco abstinence at 6 months or later, SAE avoidance, and relapse of tobacco use to be the critical outcomes. For this question, the panel wanted to evaluate the efficacy of treatment on abstinence while being mindful of the potential increased risk of exacerbating underlying psychiatric conditions. There is variability in how individuals value the risk of addiction and the importance of being free from smoking. For patients with underlying substance-use disorders, they valued being able to continue treatment for their substance-use disorder while undergoing treatment for tobacco dependence (153). The panel considered both interventions to be acceptable to stakeholders and increasingly feasible to implement with the boxed warning removed. However, low willingness to abstain, dual dependence on other substances, lower perceived efficacy of treatments, and need for additional support may form important barriers to treatment for patients with psychiatric illness (154, 155).
Overall, patients with psychiatric illness are less likely to engage in treatment for tobacco dependence (48, 156). When psychiatric inpatient smokers attempt to quit, they are less likely to receive evidence-based treatment (157). The panel considered varenicline treatment a feasible strategy for smokers with comorbid psychiatric conditions. Targeted quality-improvement projects and provider education can improve uptake of pharmacotherapy for patients with psychiatric disorders (64, 75, 158).
Panel discussion and conclusions
When compared with nicotine patches, the panel concluded that varenicline 1) may result in a large benefit for abstinence and 2) would likely result in little to no difference in SAEs, with both results having moderate certainty in the estimated effects, in patients with substance-use or psychiatric disorders.
As a result, the panel concluded that the clinical superiority (balance of effect) of varenicline in in patients with substance-use or psychiatric disorders outweighs its higher price and the possibly important uncertainty or variability of patients’ values and preferences. Therefore, the panel preferred a strong recommendation.
As in the general population, the feasibility of this recommendation is likely to be affected by variation in access and insurance benefits. However, variation in styles of behavioral therapy and substance-use recovery, together with accompanying attitudes regarding pharmacologic support, represent additional potential barriers to implementation that are unique to this population (159–161). Persons with psychiatric illnesses may also have more severe nicotine dependence than the general population and may require more flexibility in the approach, including higher doses, longer-duration counseling, and/or more aggressive combinations of pharmacotherapy (162). In light of the tragic toll tobacco dependence currently has on this subgroup, achieving health parity will require a substantial change in the way we view tobacco interventions within this community (163).
Recommendation 6
For tobacco-dependent adults with comorbid psychiatric conditions, including substance-use disorder, depression, anxiety, schizophrenia, and/or bipolar disorder, for whom treatment is being initiated, we recommend varenicline over a nicotine patch (strong recommendation, moderate certainty in the estimated effects).
What others are saying
The Substance Abuse and Mental Health Administration has recommended treatment of tobacco dependence for individuals with psychiatric illness, advocating for the early use of pharmacotherapy, given the elevated risk of comorbid medical conditions (164). The Association for the Treatment of Tobacco Use and Dependence has also strongly recommended that providers working with individuals with psychiatric or substance-use disorders fully integrate tobacco treatment into their service programs (165).
Research needs
Patients with psychiatric and/or substance-use disorders are more likely to have tobacco dependence, with over 30% reporting current tobacco use (166). These patients account for almost 40% of cigarette consumption in the United States (167). Yet, fewer than half of mental health and substance-use treatment facilities in the United States offer evidence-based tobacco-dependence treatments (168–170). Implementation research is needed to maximize the provision of evidence-based treatments for tobacco dependence within this context.
Question 7: In Tobacco-Dependent Adults for Whom Treatment Is Being Initiated with a Controller, Should They Be Treated with an Extended-Duration (>12 wk) or Standard-Duration (6–12 wk) Regimen?
Rationale for question
Controller pharmacotherapies treat tobacco dependence effectively when taken as prescribed, but relapse after pharmacologic discontinuation is common (171). Among the various strategies aimed at preventing relapse, an extended duration of treatment has been effective at modifying sustained abstinence rates in some contexts (172). The panel found guidance on treatment duration to be of critical importance, especially the comparison between extended-duration (i.e., >12 wk) and standard therapy (8–12 wk).
Summary of evidence
Our systematic review identified 12 studies that directly compared extended (>12 wk) versus standard-duration controller therapy with varenicline, bupropion, or nicotine (173–183). Eight studies (n = 3,711) provided data for the primary analysis of 7-day point-prevalence abstinence at 12 months; five reported SAE data (n = 2,612) (178, 180, 181, 183) (Table 7).
Table 7.
Evidentiary Basis for Strong Recommendation Favoring Extended over Standard-Duration Treatment Regimens, with Moderate-Certainty Evidence
| Certainty Assessment |
Number (or Percent) |
Effect (95% CI) |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Extended Duration | Standard Duration | Relative | Absolute (per 1,000 Patients) | ||
|
Seven-day point-prevalence tobacco abstinence at 1 yr (follow-up: mean, 1 yr; assessed with self-report + exhaled carbon-monoxide concentration verification) | ||||||||||||
| 8 | RCT | Serious | Not serious | Not serious | Not serious | None | 751/1,935 (38.8%) | 24.2% | RR, 1.22 (1.07–1.39) | 53 more (↑17–↑94) | Moderate | Critical |
|
Quality of life (follow-up: 52 wk; assessed with SF-12; higher score indicates worse quality of life; scale of 12–47) | ||||||||||||
| 1 | RCT | Serious | Not serious | Serious | Serious | None | 47 | 51 | — | MD, 1.15 lower (↓3.75–↑1.45) | Very low | Important |
|
No. of cigarettes (follow-up: mean, 1 yr) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 345 | 180 | — | MD, 0.6 lower (↓1.53–↑0.33) | Low | Important |
|
Serious adverse events | ||||||||||||
| 5 | RCT | Not serious | Not serious | Not serious | Serious | None | 30/1,304 (2.3%) | 0.8% | RR, 1.37 (0.79–2.36) | 3 more (↓2–↑11) | Moderate | Critical |
|
Relapse (follow-up: range, 12–18 mo) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Serious | None | 322 | 333 | HR, 0.43 (0.29–0.64) | 0 fewer (0–0) | Moderate | Important |
|
Time to relapse (follow-up: 1 yr) | ||||||||||||
| 2 | RCT | Serious | Serious | Not serious | Not serious | None | 948 | 787 | — | MD, 22.03 d more (↑10.81–↑33.24) | Low | Important |
|
Other substance use, not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
|
Withdrawal (follow-up: range, 13–14 wk; assessed with craving and urge to smoke; low score indicates better outcome) | ||||||||||||
| 2 | RCT | Serious | Serious | Not serious | Serious | None | 601 | 585 | — | MD, 1.54 lower (↓3.94–↑0.85) | Very low | Important |
|
Withdrawal (follow-up: 25 wk; assessed with MNWS urge to smoke; lower score indicates better outcome; scale of 0–4) | ||||||||||||
| 1 | RCT | Serious | Not serious | Not serious | Serious | None | 499 | 468 | — | MD, 0.27 lower (↓0.44–↓0.1) | Low | Important |
Definition of abbreviations: ↑ = increase of; ↓ = decrease of; CI = confidence interval; HR = hazard ratio; MD = mean difference; MNWS = Minnesota Nicotine Withdrawal Scale; RCT = randomized controlled trial; RR = relative risk; SF-12 = Short-Form Health Survey.
Treatment with more than 12 weeks of pharmacotherapy provides a larger benefit than treatment courses of fewer than 12 weeks and has a similar risk of serious adverse events. For complete evidence tables, together with references, explanations of certainty assessments, and results of the Evidence-to-Decision process, see online supplement.
Benefits
Compared with standard-duration controller therapy, extended-duration therapy probably increased abstinence at 1-year follow-up, measured as 7-day point-prevalence abstinence, (RR, 1.22; 95% CI, 1.07 to 1.39; ARR, 53 more per 1,000 patients; 95% CI, 17 more to 94 more; moderate certainty in the estimated effects) (175, 178–183). Pooled estimates from two trials with 655 participants also suggested extended-duration therapy probably reduced relapse assessed at 12 to 18 months after initiation of therapy (HR, 0.43; 95% CI, 0.29 to 0.64; moderate certainty in the estimated effects) (175, 180, 181).
Harms and burdens
Compared with standard-duration controller therapy, extended-duration therapy probably increased SAEs slightly (RR, 1.37; 95% CI, 0.79 to 2.36; ARR, 3 more per 1,000 patients; 95% CI, 2 fewer to 11 more; moderate certainty in the estimated effects) (178, 180, 181, 183).
Certainty in estimated effects
Certainty in the effects estimated for 7-day point-prevalence abstinence at 12 months was judged to be moderate because of serious risk of bias. Certainty in estimated SAE effects was judged as moderate because of serious imprecision. The estimate of relapse was assessed as having moderate certainty because of serious imprecision.
Other considerations
The panel considered extended-duration therapy to be acceptable to stakeholders and feasible to implement. Cost-effectiveness has been evaluated for varenicline in two systematic reviews, and both concluded that extended treatment was cost-effective compared with standard-duration treatment (184, 185). Cost-effectiveness data are not available for bupropion and NRT. Medication costs differ significantly on the basis of drug class and availability of generic alternatives, and these differences create economic barriers to implementation for uninsured populations. For the most vulnerable populations, optimizing pharmacotherapeutic choice and duration is critical to promoting equity, as tobacco dependence is most prevalent among disadvantaged individuals. The panel considered extended therapy to be probably feasible and acceptable to both providers and patients, although there may be an additional treatment burden.
Panel discussion and conclusions
The panel concluded 1) that more than 12 weeks of pharmacotherapy provides a large benefit compared with standard treatment courses of fewer than 12 weeks, with increased abstinence and decreased relapse rates having moderate-certainty evidence, and 2) that extended-duration therapy probably does not increase or decrease SAEs compared with standard-duration therapy, with outcomes showing moderate-certainty evidence. As a result, the panel made a strong recommendation for extended-duration treatment of tobacco dependence beyond 3 months, including regimens of up to 12 months in duration, considering that the clinical superiority (balance of effect) of extended-duration treatment outweighs its higher price and the possibly important uncertainty or variability of patients’ values and preferences. Therefore, all panel members preferred a strong recommendation.
Recommendation 7
For tobacco-dependent adults for whom treatment is being initiated with a controller, we recommend using extended-duration therapy (>12 wk) over standard-duration (6–12 wk) therapy (strong recommendation, moderate certainty in the estimated effects).
What others are saying
The 2018 American College of Cardiology tobacco-cessation clinical pathway recommends at least 3 months of therapy with nicotine and 3–6 months of therapy when using varenicline or bupropion (49).
Research needs
Although extended-duration therapy for tobacco dependence is effective and safe, the optimal duration of treatment for each drug within specific populations is unknown. Benefits of pharmacotherapy are evident in studies of treatment for up to 12 months, but additional studies of long-term and maintenance therapy are needed. Studies evaluating the factors influencing long-term adherence could shed light on novel methods for improving maintenance outcomes.
Discussion
Traditionally, the clinical community has engaged tobacco use as the toxic antecedent to chronic disease, and so it follows that clinicians have focused on methods to increase the patient’s motivation to stop. Our 21st-century perspective, however, engages tobacco use as the cardinal manifestation of a disturbance in the brain’s molecular-learning mechanisms. From that perspective, the treatment team’s responsibility extends beyond facilitating quitting and includes maximizing longitudinal control over the compulsion to smoke.
The main limitation of our guideline is the limited number of recommendations included. Because our objective was to identify a functional, evidence-based pharmacotherapy pathway, we began the process by identifying an optimal controller medication on which to build additional clinical recommendations. By necessity, our guideline could not address all possible pharmacotherapy options. Future guidelines should consider optimal controller strategies for patients in whom varenicline has previously failed or who have previously refused this treatment.
The guideline addresses several limiting misconceptions, including the value of combination pharmacotherapy, the approach to patients who are reluctant to stop smoking, and the safety and efficacy of treating vulnerable behavioral health patients.
Patient Perspective
In 1967, I made a decision that I now regard as the worst I have ever made. As a bright, young college freshman, I made the decision to smoke. Admittedly, I was not thinking of the impact it would have on me during my lifetime. I can tell you from experience that the addiction to smoking is real. Every day is going to be the day to quit, but every moment of the day brings reminders that the addiction to cigarettes is stronger than the will to quit. Days, months, and years go by, and the quit date keeps getting pushed further and further into the future.
All smokers face the possibility of lung cancer, heart disease, and other debilitating illnesses. Smokers also must contend with the societal stigma that tobacco use carries. Smoking is time consuming, and it is costly. With all these negatives, it is not surprising that most smokers really do want to quit. Healthcare professionals have a prominent role to play in tobacco dependence. They have the trust of their patients, the media, and opinion leaders, and their voices are heard across a vast range of social, economic, and political arenas. Patients look to providers for rational and useful tools to assist them in their quest to become smoke free. By developing effective pathways for treating tobacco dependence, the ATS is taking important steps toward changing the impact tobacco dependence will have on future generations.
Supplementary Material
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems.
Members of the subcommittee are as follows:
Harold J. Farber, M.D., M.S.P.H.1 (Co-Chair)
Frank T. Leone, M.D., M.S.2 (Co-Chair)
Luciane Cruz-Lopes, Sc.D., M.Sc.3*
Michelle N. Eakin, Ph.D.4
A. Eden Evins, M.D., M.P.H.5
Sarah Evers-Casey, M.P.H.2
Joelle Fathi, D.N.P., M.N.6
Kathleen Fennig7‡
Patricia Folan, R.N., D.N.P.8
Izabela Fulone, Ph.D.3*
Panagis Galiatsatos, M.D., M.H.S.4
Hyma Gogineni, Pharm.D., M.Sc.9,10
Stephen Kantrow, M.D.11
Hasmeena Kathuria, M.D.12
Thomas Lamphere, B.S., R.R.T.-A.C.C.S., R.P.F.T.13
Rachael L. Murray, Ph.D.14*
Enid Neptune, M.D., Ph.D.4
Kelly K. O’Brien, M.L.I.S.15§
Manuel C. Pacheco, M.D., M.Sc.16,17,18
Smita Pakhale, M.D., M.Sc.19
Sureka Pavalagantharajah, B.H.Sc.20*
David Prezant, M.D.21
Stephanie Ross, Ph.D.22*
David P. L. Sachs, M.D.23
Benjamin Toll, Ph.D.24
Dona Upson, M.D.25
Dan Xiao, M.D., Ph.D.26
Yuan Zhang, Ph.D.22*
Yuqing Zhang, M.D., M.Sc., Ph.D.22||
Meng Zhu, Ph.D.22*
*Methodologist.
‡Patient representative.
§Medical librarian.
||Lead methodologist.
1Department of Pediatrics, Division of Pulmonary Medicine, Texas Children’s Hospital and Baylor College of Medicine, Houston, Texas; 2Comprehensive Smoking Treatment Program, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 3Pharmaceutical Science Graduate Course, University of Sorocaba, Sorocaba, São Paulo, Brazil; 4Division of Pulmonary and Critical Care Medicine, Johns Hopkins University, Baltimore, Maryland; 5Department of Psychiatry, Massachusetts General Hospital, Harvard University, Cambridge, Massachusetts; 6Department of Biobehavioral Nursing and Health Informatics, School of Nursing, University of Washington, Seattle, Washington; 7Public Advisory Roundtable, American Thoracic Society, New York, New York; 8Center for Tobacco Control, Northwell Health, Great Neck, New York; 9College of Pharmacy, Western University of Health Sciences, Pomona, California; 10Veterans Affairs Loma Linda Ambulatory Care Center, Redlands, California; 11Department of Pulmonary Medicine, School of Medicine, Louisiana State University, New Orleans, Louisiana; 12Department of Pulmonary and Critical Care Medicine, Boston University Medical Center, Boston, Massachusetts; 13Pennsylvania Society for Respiratory Care, Philadelphia, Pennsylvania; 14Division of Epidemiology and Public Health, University of Nottingham, Nottingham, United Kingdom; 15Library and Archives, American Dental Association, Chicago, Illinois; 16Department of Internal Medicine and Pulmonology, Technological University of Pereira, Pereira, Colombia; 17Colombian Association of Pulmonology and Thoracic Surgery, Bogotá, Colombia; 18Colombian Association of Internal Medicine, Bogotá, Colombia; 19Department of Pulmonology, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 20Michael G. DeGroote School of Medicine and 22Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada; 21Fire Department of New York, New York, New York; 23Palo Alto Center for Pulmonary Disease Prevention, Palo Alto, California; 24Hollings Cancer Center, Medical University of South Carolina, Charleston, South Carolina; 25Department of Pulmonary Medicine, New Mexico Veterans Affairs Health Care System, Albuquerque, New Mexico; and 26Tobacco Medicine and Tobacco Cessation Center, Center of Respiratory Medicine, China-Japan Friendship Hospital, Beijing, China
Acknowledgment
The authors thank the thousands of volunteer participants who contributed their time and effort to developing this evidence base. They thank Dr. Kevin Wilson, ATS Documents Editor, for significant methodologic contributions and guidance during the document development phase. Without his efforts, this guideline would not have been possible. They also thank Ms. Kimberly Lawrence (ATS staff) for implementation assistance and production guidance. Without her considerable abilities in managing a collaborative work environment, we could not have accomplished the goals of this project. Methodological support for this guideline was provided by the GUIDE group (https://guidecanada.org).
Footnotes
This official clinical practice guideline was approved by the American Thoracic Society May 2020
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202005-1982ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: A.E.E. received research support and served on an advisory committee for Pfizer; received support from the National Cancer Institute for a study on testing an organizational change model to address smoking in mental healthcare; received research support from the National Institute on Mental Health for a trial on integrated smoking cessation, exercise, and weight management in serious mental illness (Achieve); received research support from Patient-Centered Outcomes Research Institute for a study of facilitators and barriers to implementation of integrated smoking cessation treatment for smokers with serious mental illness; and received a Career Award from the National Institute on Drug Abuse for mentoring in addiction treatment research. H.G. received research support from Western University of Health Sciences for a study on pharmacists’ knowledge and attitudes about electronic cigarettes. S. Pakhale received research support from the Canadian Institute of Health Research for a study on nicotine reduction therapy and e-cigarettes, using the marketed e-cigarette NJOY as a study instrument. D.P. reported potential 2020 Research Support from the CDC/National Institute for Occupational Safety and Health for a study on tobacco cessation in firefighters, using varenicline as a study instrument. D.P.L.S. served on an advisory committee for Pfizer; and has noncommercialized intellectual property—U.S. patent 6,602,892—Methods for Nicotine Replacement Dosage Determination. B.T. served on an advisory committee for Pfizer; testifies on behalf of plaintiffs on litigation filed against the tobacco companies; and received research support from the National Cancer Institute. H.J.F., F.T.L., L.C.-L., M.N.E., S.E.-C., J.F., K.F., P.F., I.F., P.G., S.K., H.K., T.L., R.L.M., E.N., K.K.O’B., M.C.P., S. Pavalagantharajah, S.R., D.U., D.X., Yuan Zhang, Yuqing Zhang, and M.Z. reported no relevant commercial relationships.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Clinical Problems
References
- 1.U.S. Department of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services; Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC; 1988. The health consequences of smoking: nicotine addiction. A report of the Surgeon General. [accessed 2019 Aug 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44695/ [Google Scholar]
- 2.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Smoking Cessation Guideline PanelClinical practice guideline 18: smoking cessation.Rockville, MD: U.S. Department of Health and Human Services, USPHS, Agency for Health Care Policy and Research; 1996 [Google Scholar]
- 3.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Tobacco Use and Dependence Guideline PanelTreating tobacco use and dependence: 2008 update: clinical practice guideline.Rockville, MD: USPHS, U.S. Department of Health and Human Services; 2008 [Google Scholar]
- 4.van Eerd EAM, Bech Risør M, Spigt M, Godycki-Cwirko M, Andreeva E, Francis N, et al. Why do physicians lack engagement with smoking cessation treatment in their COPD patients? A multinational qualitative study. NPJ Prim Care Respir Med. 2017;27:41. doi: 10.1038/s41533-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers-Casey S, Schnoll R, Jenssen BP, Leone FT. Implicit attribution of culpability and impact on experience of treating tobacco dependence. Health Psychol. 2019;38:1069–1074. doi: 10.1037/hea0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone FT, Evers-Casey S, Graden S, Schnoll R. Behavioral economic insights into physician tobacco treatment decision-making. Ann Am Thorac Soc. 2015;12:364–369. doi: 10.1513/AnnalsATS.201410-467BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs D, Leone F, Farber H, Bars M, Prezant D, Schane R, et al. Tobacco-Dependence Treatment Tool Kit Committee. 3rd ed. Glenview, IL: American College of Chest Physicians; 2010. American College of Chest Physicians tobacco-dependence treatment tool Kit. [accessed 2020 Jun 20]. Available from: https://foundation.chestnet.org/wp-content/uploads/2020/04/Tobacco-Dependence-Toolkit.pdf. [Google Scholar]
- 8.Institute of Medicine. Washington, DC: National Academies Press; 2011. Clinical practice guidelines we can trust. Available from: https://www.awmf.org/fileadmin/user_upload/Leitlinien/International/IOM_CPG_lang_2011.pdf. [Google Scholar]
- 9.Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Working Group. GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Working Group. GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89–98. doi: 10.1016/j.jclinepi.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 14.ATS Documents Unit. New York, NY: American Thoracic Society; 2018. American Thoracic Society guidelines packet. [accessed 2019 Sep 30]. Available from: https://www.thoracic.org/statements/document-development/resources/gats.pdf. [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. 2nd ed. Chichester, United Kingdom: John Wiley & Sons; 2019. Cochrane handbook for systematic reviews of interventions. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 17.Vienna, Austria: R Foundation for Statistical Computing; 2019. R gemtc package. Version 0.8-2. [accessed 2019 Sep 30]. Available from: https://www.r-project.org/foundation/ [Google Scholar]
- 18.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Irwig L, Irwig J, Trevena L, Sweet M. London, United Kingdom: Hammersmith Press; 2008. Relative risk, relative and absolute risk reduction, number needed to treat and confidence intervals. In: Smart choices: making sense of health advice; pp. 213–216. [accessed 2019 Oct 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK63647/ [PubMed] [Google Scholar]
- 20.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Serv Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 25.CDC Tobacco Free. Atlanta, GA: CDC; 2019. Smoking cessation: fast facts. [accessed 2019 Oct 1]. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitting/index.htm. [Google Scholar]
- 26.Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthenelli RM, Benowitz NL, West R, St. Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 29.de Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M. Project impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. J Subst Abuse Treat. 2012;43:322–330. doi: 10.1016/j.jsat.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. 2016;315:371–379. doi: 10.1001/jama.2015.19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis. 2012;16:268–272. doi: 10.5588/ijtld.11.0183. [DOI] [PubMed] [Google Scholar]
- 32.Lerman C, Schnoll RA, Hawk LW, Jr, Cinciripini P, George TP, Wileyto EP, et al. PGRN-PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3:131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 2016;14:80. doi: 10.1186/s12916-016-0626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo-controlled, prospective study. Basic Clin Pharmacol Toxicol. 2016;119:78–84. doi: 10.1111/bcpt.12548. [DOI] [PubMed] [Google Scholar]
- 35.Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, et al. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction. 2017;112:1808–1820. doi: 10.1111/add.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study) Circ J. 2010;74:771–778. doi: 10.1253/circj.cj-09-0803. [DOI] [PubMed] [Google Scholar]
- 37.Gray KM, McClure EA, Baker NL, Hartwell KJ, Carpenter MJ, Saladin ME. An exploratory short-term double-blind randomized trial of varenicline versus nicotine patch for smoking cessation in women. Addiction. 2015;110:1027–1034. doi: 10.1111/add.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikonomidis I, Marinou M, Vlastos D, Kourea K, Andreadou I, Liarakos N, et al. Effects of varenicline and nicotine replacement therapy on arterial elasticity, endothelial glycocalyx and oxidative stress during a 3-month smoking cessation program. Atherosclerosis. 2017;262:123–130. doi: 10.1016/j.atherosclerosis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21:216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etter J-F, Schneider NG. An Internet survey of use, opinions and preferences for smoking cessation medications: nicotine, varenicline, and bupropion. Nicotine Tob Res. 2013;15:59–68. doi: 10.1093/ntr/nts084. [DOI] [PubMed] [Google Scholar]
- 41.Busch S, Falba T, Duchovny N, Jofre-Bonet M, O’Malley S, Sindelar J. Value to smokers of improved cessation products: evidence from a willingness-to-pay survey. Nicotine Tob Res. 2004;6:631–639. doi: 10.1080/14622200410001727885. [DOI] [PubMed] [Google Scholar]
- 42.Dugas EN, Wellman RJ, Kermack A, Tremblay M, O’Loughlin J. Reasons young smokers do not use NRT even when it is available free-of-charge: an exploratory study. Can J Addict. 2016;7:14–21. [Google Scholar]
- 43.Morphett K, Partridge B, Gartner C, Carter A, Hall W. Why don’t smokers want help to quit? A qualitative study of smokers’ attitudes towards assisted vs. unassisted quitting. Int J Environ Res Public Health. 2015;12:6591–6607. doi: 10.3390/ijerph120606591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monica, CA: GoodRx; 2019. Prescription prices, coupons & pharmacy information. [accessed 2019 Oct 1]. Available from: https://www.goodrx.com/ [Google Scholar]
- 45.Halpern MT, Dirani R, Schmier JK. The cost effectiveness of varenicline for smoking cessation. Manag Care Interface. 2007;20:18–25. [PubMed] [Google Scholar]
- 46.Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics. 2008;26:497–511. doi: 10.2165/00019053-200826060-00004. [DOI] [PubMed] [Google Scholar]
- 47.Knight C, Howard P, Baker CL, Marton JP. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health. 2010;13:209–214. doi: 10.1111/j.1524-4733.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 48.Rothrauff TC, Eby LT. Counselors’ knowledge of the adoption of tobacco cessation medications in substance abuse treatment programs. Am J Addict. 2011;20:56–62. doi: 10.1111/j.1521-0391.2010.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri M-A, Morris PB, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72:3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chron Obstruct Pulmon Dis. 2008;3:45–53. doi: 10.2147/copd.s1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansourati J, Borel M-L, Munier S, Guevel-Jointret A-L. Medications in smoking cessation [in French] Presse Med. 2005;34:1331–1336. doi: 10.1016/s0755-4982(05)84182-x. [DOI] [PubMed] [Google Scholar]
- 52.Ross S, Williams D. Bupropion: risks and benefits. Expert Opin Drug Saf. 2005;4:995–1003. doi: 10.1517/14740338.4.6.995. [DOI] [PubMed] [Google Scholar]
- 53.Mohanasundaram UM, Chitkara R, Krishna G. Smoking cessation therapy with varenicline. Int J Chron Obstruct Pulmon Dis. 2008;3:239–251. doi: 10.2147/copd.s1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 55.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 56.Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70:522–533. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial. Nicotine Tob Res. 2012;14:234–239. doi: 10.1093/ntr/ntr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hays JT, Croghan IT, Baker CL, Cappelleri JC, Bushmakin AG. Changes in health-related quality of life with smoking cessation treatment. Eur J Public Health. 2012;22:224–229. doi: 10.1093/eurpub/ckq137. [DOI] [PubMed] [Google Scholar]
- 59.Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 60.Bethesda, MD: ClinicalTrials.gov, National Library of Medicine, NIH; 2018. The effect of varenicline (Chantix) and bupropion (Zyban) on smoking lapse behavior. [accessed 2019 Oct 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT00580853. [Google Scholar]
- 61.Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. Clinicoecon Outcomes Res. 2018;10:67–74. doi: 10.2147/CEOR.S153897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson KC, II, Nahoopii R, Said Q, Dirani R, Brixner D. An employer-based cost-benefit analysis of a novel pharmacotherapy agent for smoking cessation. J Occup Environ Med. 2007;49:453–460. doi: 10.1097/JOM.0b013e3180459ff2. [DOI] [PubMed] [Google Scholar]
- 63.Xenakis JG, Kinter ET, Ishak KJ, Ward AJ, Marton JP, Willke RJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics. 2011;29:497–510. doi: 10.2165/11589230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Gifford E, Tavakoli S, Wisdom J, Hamlett-Berry K. Implementation of smoking cessation treatment in VHA substance use disorder residential treatment programs. Psychiatr Serv. 2015;66:295–302. doi: 10.1176/appi.ps.201400008. [DOI] [PubMed] [Google Scholar]
- 65.Koutnik-Fotopoulos E. Malvern, PA: Population Health Learning Network, HMP Global; 2017. ICER: does the industry take cost-effectiveness seriously? [accessed 2019 Nov 5]. Available from: https://www.managedhealthcareconnect.com/article/icer-does-industry-take-cost-effectiveness-seriously. [Google Scholar]
- 66.Patel MS, Steinberg MB. In the clinic: smoking cessation. Ann Intern Med. 2016;164:ITC33–ITC48. doi: 10.7326/AITC201603010. [DOI] [PubMed] [Google Scholar]
- 67.Siu AL U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services task force recommendation statement. Ann Intern Med. 2015;163:622–634. doi: 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 68.Colby SM, Gwaltney CJ. Pharmacotherapy for adolescent smoking cessation. JAMA. 2007;298:2182–2184. doi: 10.1001/jama.298.18.2182. [DOI] [PubMed] [Google Scholar]
- 69.Kautiainen K, Ekroos H, Puhakka M, Liira H, Laine J, Linden K, et al. Re-treatment with varenicline is a cost-effective aid for smoking cessation. J Med Econ. 2017;20:246–252. doi: 10.1080/13696998.2016.1249485. [DOI] [PubMed] [Google Scholar]
- 70.Taylor M, Leonardi-Bee J, Agboola S, McNeill A, Coleman T. Cost effectiveness of interventions to reduce relapse to smoking following smoking cessation. Addiction. 2011;106:1819–1826. doi: 10.1111/j.1360-0443.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 71.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 72.Koegelenberg CFN, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O’Brien JA, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312:155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- 73.Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med. 2014;12:172. doi: 10.1186/s12916-014-0172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hajek P, Smith KM, Dhanji AR, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med. 2013;11:140. doi: 10.1186/1741-7015-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen LS, Baker TB, Korpecki JM, Johnson KE, Hook JP, Brownson RC, et al. Low-burden strategies to promote smoking cessation treatment among patients with serious mental illness. Psychiatr Serv. 2018;69:849–851. doi: 10.1176/appi.ps.201700399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kandra KL, Ranney LM, Lee JGL, Goldstein AO. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PLoS One. 2014;9:e103462. doi: 10.1371/journal.pone.0103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96–98. doi: 10.1016/j.pmedr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baldassarri SR, Chupp GL, Leone FT, Warren GW, Toll BA. Practice patterns and perceptions of chest health care providers on electronic cigarette use: an in-depth discussion and report of survey results. J Smok Cessat. 2018;13:72–77. doi: 10.1017/jsc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benmarhnia T, Pierce JP, Leas E, White MM, Strong DR, Noble ML, et al. Can e-cigarettes and pharmaceutical aids increase smoking cessation and reduce cigarette consumption? Findings from a nationally representative cohort of American smokers. Am J Epidemiol. 2018;187:2397–2404. doi: 10.1093/aje/kwy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ioakeimidis N, Vlachopoulos C, Georgakopoulos C, Abdelrasoul M, Skliros N, Katsi V, et al. Smoking cessation rates with varenicline and electronic cigarettes in relapsed smokers with a history of acute coronary syndrome [abstract] Eur Heart J. 2018;39:242. [Google Scholar]
- 81.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 82.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 83.U.S. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Atlanta, GA: U.S. Department of Health and Human Services; Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC; 2014. The health consequences of smoking—50 years of progress: a report of the Surgeon General. [accessed 2014 Apr 13]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK179276/ [Google Scholar]
- 84.Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer ANM, De Vries TJ, et al. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- 85.Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- 86.Murthy VH. E-cigarette use among youth and young adults: a major public health concern. JAMA Pediatr. 2017;171:209–210. doi: 10.1001/jamapediatrics.2016.4662. [DOI] [PubMed] [Google Scholar]
- 87.Kandel ER, Kandel DB. Shattuck lecture: a molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932–943. doi: 10.1056/NEJMsa1405092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leone FT, Evers-Casey S. Developing a rational approach to tobacco use treatment in pulmonary practice: a review of the biological basis of nicotine addiction. Clin Pulm Med. 2012;19:53–61. doi: 10.1097/CPM.0b013e318247cada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. E-cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Majeed BA, Weaver SR, Gregory KR, Whitney CF, Slovic P, Pechacek TF, et al. Changing perceptions of harm of e-cigarettes among U.S. adults, 2012-2015. Am J Prev Med. 2017;52:331–338. doi: 10.1016/j.amepre.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leone FT, Carlsen K-H, Chooljian D, Crotty Alexander LE, Detterbeck FC, Eakin MN, et al. Recommendations for the appropriate structure, communication, and investigation of tobacco harm reduction claims: an official American Thoracic Society policy statement. Am J Respir Crit Care Med. 2018;198:e90–e105. doi: 10.1164/rccm.201808-1443ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li D, Sundar IK, McIntosh S, Ossip DJ, Goniewicz ML, O’Connor RJ, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2020;29:140–147. doi: 10.1136/tobaccocontrol-2018-054694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang JB, Olgin JE, Nah G, Vittinghoff E, Cataldo JK, Pletcher MJ, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13:e0198681. doi: 10.1371/journal.pone.0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedman L, Backman H, Stridsman C, Bosson JA, Lundbäck M, Lindberg A, et al. Association of electronic cigarette use with smoking habits, demographic factors, and respiratory symptoms. JAMA Netw Open. 2018;1:e180789. doi: 10.1001/jamanetworkopen.2018.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotz D, Viechtbauer W, Simpson C, van Schayck OCP, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med. 2015;3:761–768. doi: 10.1016/S2213-2600(15)00320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci. 2016;154:332–340. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71:1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 2016;94:667–679. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 100.Rossheim ME, Livingston MD, Soule EK, Zeraye HA, Thombs DL. Electronic cigarette explosion and burn injuries, US Emergency Departments 2015-2017. Tob Control. 2019;28:472–474. doi: 10.1136/tobaccocontrol-2018-054518. [DOI] [PubMed] [Google Scholar]
- 101.Harrison R, Hicklin D., Jr Electronic cigarette explosions involving the oral cavity. J Am Dent Assoc. 2016;147:891–896. doi: 10.1016/j.adaj.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 102.Khairudin MN, Mohd Zahidin AZ, Bastion M-LC. Front to back ocular injury from a vaping-related explosion. BMJ Case Rep. 2016;2016:bcr2016214964. doi: 10.1136/bcr-2016-214964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang JT, Wang B, Chang CM, Ambrose BK. National estimates of poisoning events related to liquid nicotine in young children treated in US hospital emergency departments, 2013-2017. Inj Epidemiol. 2019;6:10. doi: 10.1186/s40621-019-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morley S, Slaughter J, Smith PR. Death from ingestion of E-liquid. J Emerg Med. 2017;53:862–864. doi: 10.1016/j.jemermed.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 105.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47:15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 106.Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics. 2018;141:e20163927. doi: 10.1542/peds.2016-3927. [DOI] [PubMed] [Google Scholar]
- 107.Khan MS, Khateeb F, Akhtar J, Khan Z, Lal A, Kholodovych V, et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin Respir J. 2018;12:1295–1299. doi: 10.1111/crj.12775. [DOI] [PubMed] [Google Scholar]
- 108.Viswam D, Trotter S, Burge PS, Walters GI. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018;2018:bcr2018224350. doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bradford LE, Rebuli ME, Ring BJ, Jaspers I, Clement KC, Loughlin CE. Danger in the vapor? ECMO for adolescents with status asthmaticus after vaping. J Asthma. doi: 10.1080/02770903.2019.1643361. [online ahead of print] 27 Jul 2019. [DOI] [PubMed] [Google Scholar]
- 110.Office on Smoking and Health. Atlanta, GA: CDC; 2019. Smoking and tobacco use: electronic cigarettes. [accessed 2019 Oct 6]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. [Google Scholar]
- 111.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin: final report. N Engl J Med. 2020;382:903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 112.Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, DeSisto CL, et al. Lung Injury Response Epidemiology/Surveillance Group. Characteristics of a multistate outbreak of lung injury associated with e-cigarette use, or vaping: United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:860–864. doi: 10.15585/mmwr.mm6839e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schier JG, Meiman JG, Layden J, Mikosz CA, VanFrank B, King BA, et al. CDC 2019 Lung Injury Response Group. Severe pulmonary disease associated with electronic-cigarette-product use: interim guidance. MMWR Morb Mortal Wkly Rep. 2019;68:787–790. doi: 10.15585/mmwr.mm6836e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bals R, Boyd J, Esposito S, Foronjy R, Hiemstra PS, Jiménez-Ruiz CA, et al. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J. 2019;53:1801151. doi: 10.1183/13993003.01151-2018. [DOI] [PubMed] [Google Scholar]
- 115.Schraufnagel DE, Blasi F, Drummond MB, Lam DCL, Latif E, Rosen MJ, et al. Forum of International Respiratory Societies. Electronic cigarettes: a position statement of the forum of international respiratory societies. Am J Respir Crit Care Med. 2014;190:611–618. doi: 10.1164/rccm.201407-1198PP. [DOI] [PubMed] [Google Scholar]
- 116.Ibero-Latino-American Respiratory Scientific Societies. Montevideo, Uruguay: Latin American Thoracic Association; 2019. Declaration of the Ibero-Latino-American respiratory scientific societies on electronic nicotine delivery systems. Available from: https://alatorax.org/es/descargar/adjunto/372-ntdcsf-deln-doc-posicion-may2019-eng.pdf. [Google Scholar]
- 117.Brandon TH, Goniewicz ML, Hanna NH, Hatsukami DK, Herbst RS, Hobin JA, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin Cancer Res. 2015;21:514–525. doi: 10.1158/1078-0432.CCR-14-2544. [DOI] [PubMed] [Google Scholar]
- 118.Board on Population Health and Public Health, National Academies of Sciences, Engineering, and MedicinePublic health consequences of e-cigarettes.Washington, DC: National Academies Press; 2018775 [Google Scholar]
- 119.Royal College of Physicians. London, United Kingdom: Royal College of Physicians; 2016. Nicotine without smoke: tobacco harm reduction. [accessed 2019 Jul 12]. Available from: https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-tobacco-harm-reduction-0. [Google Scholar]
- 120.Leone FT, Baldassarri SR, Galiatsatos P, Schnoll R. Nicotine dependence: future opportunities and emerging clinical challenges. Ann Am Thorac Soc. 2018;15:1127–1130. doi: 10.1513/AnnalsATS.201802-099PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marlow SP, Stoller JK. Smoking cessation. Respir Care. 2003;48:1238–1254. [Discussion, pp. 1254–1256] [PubMed] [Google Scholar]
- 122.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 123.West R. Time for a change: putting the transtheoretical (stages of change) model to rest. Addiction. 2005;100:1036–1039. doi: 10.1111/j.1360-0443.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- 124.Medbø A, Melbye H, Rudebeck CE. “I did not intend to stop: I just could not stand cigarettes any more.” A qualitative interview study of smoking cessation among the elderly. BMC Fam Pract. 2011;12:42. doi: 10.1186/1471-2296-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.West R, Sohal T. “Catastrophic” pathways to smoking cessation: findings from national survey. BMJ. 2006;332:458–460. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bolliger CT, Zellweger J-P, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ. 2000;321:329–333. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Etter J-F, Laszlo E, Zellweger J-P, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. J Clin Psychopharmacol. 2002;22:487–495. doi: 10.1097/00004714-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 128.Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–1402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 129.Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clin Pharmacol Ther. 2005;78:689–696. doi: 10.1016/j.clpt.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 130.Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess. 2008;12:1–135. doi: 10.3310/hta12020. iii–iv, ix–xi. [DOI] [PubMed] [Google Scholar]
- 131.Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. 2009;338:b1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults: United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65:1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 133.Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687–694. doi: 10.1001/jama.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steinberg ML, Lu S-E, Williams JM. Varenicline for smoking reduction in smokers not yet ready to quit: a double-blind, proof-of-concept randomized clinical trial. Addict Behav. 2018;84:20–26. doi: 10.1016/j.addbeh.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 135.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. Flexible Quit Date Study Group. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res. 2011;13:955–964. doi: 10.1093/ntr/ntr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218:391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marti J. Assessing preferences for improved smoking cessation medications: a discrete choice experiment. Eur J Health Econ. 2012;13:533–548. doi: 10.1007/s10198-011-0333-z. [DOI] [PubMed] [Google Scholar]
- 139.Aumann I, Treskova M, Hagemann N, von der Schulenburg J-M. Analysis of driving factors of willingness to use and willingness to pay for existing pharmacological smoking cessation aids among young and middle-aged adults in Germany. Appl Health Econ Health Policy. 2016;14:441–452. doi: 10.1007/s40258-016-0239-0. [DOI] [PubMed] [Google Scholar]
- 140.Choo EK, Sullivan AF, LoVecchio F, Perret JN, Camargo CA, Jr, Boudreaux ED. Patient preferences for emergency department-initiated tobacco interventions: a multicenter cross-sectional study of current smokers. Addict Sci Clin Pract. 2012;7:4. doi: 10.1186/1940-0640-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paterson RW, Boyle KJ, Parmeter CF, Neumann JE, De Civita P. Heterogeneity in preferences for smoking cessation. Health Econ. 2008;17:1363–1377. doi: 10.1002/hec.1336. [DOI] [PubMed] [Google Scholar]
- 142.Heydari G. Is cost of medication for quit smoking important for smokers, experience of using Champix in Iranian smoking cessation program 2016. Int J Prev Med. 2017;8:63. doi: 10.4103/ijpvm.IJPVM_375_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zawertailo L. Safety of smoking cessation drugs for mentally ill patients. Lancet. 2016;387:2481–2482. doi: 10.1016/S0140-6736(16)30294-X. [DOI] [PubMed] [Google Scholar]
- 144.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, et al. ATS/ERS Task Force for COPD Research. An official American Thoracic Society/European Respiratory Society statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:e4–e27. doi: 10.1164/rccm.201501-0044ST. [DOI] [PubMed] [Google Scholar]
- 145.Kathuria H, Detterbeck FC, Fathi JT, Fennig K, Gould MK, Jolicoeur DG, et al. ATS Assembly on Thoracic Oncology. Stakeholder research priorities for smoking cessation interventions within lung cancer screening programs: an official American Thoracic Society research statement. Am J Respir Crit Care Med. 2017;196:1202–1212. doi: 10.1164/rccm.201709-1858ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canadian Cancer Society. Toronto, Canada: Canadian Cancer Society; 2013. One step at a time: for smokers who don’t want to quit. [accessed 2019 Oct 6]. Available from: https://www.smokershelpline.ca/docs/default-source/default-document-library/pdf/osaat-who_want_to_quit_en_nov2014.pdf?sfvrsn=2e986d0e_0. [Google Scholar]
- 147.Jiménez-Ruiz CA, Masa F, Miravitlles M, Gabriel R, Viejo JL, Villasante C, et al. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest. 2001;119:1365–1370. doi: 10.1378/chest.119.5.1365. [DOI] [PubMed] [Google Scholar]
- 148.Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry. 1999;174:173–178. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- 149.Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- 150.Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446–452. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 151.Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2007. p. CD001837. [DOI] [PubMed]
- 152.Rogers K.The best drug for quitting smoking can’t shake its suicide stigma VICE News 2015 Nov 24 [accessed 2019 Oct 9]. Available from: https://www.vice.com/en_us/article/vv7gym/the-best-drug-for-quitting-smoking-cant-shake-its-suicide-stigma
- 153.Richter KP, McCool RM, Okuyemi KS, Mayo MS, Ahluwalia JS. Patients’ views on smoking cessation and tobacco harm reduction during drug treatment. Nicotine Tob Res. 2002;4:S175–S182. doi: 10.1080/1462220021000032735. [DOI] [PubMed] [Google Scholar]
- 154.Fallin A, Miller A, Ashford K. Smoking among pregnant women in outpatient treatment for opioid dependence: a qualitative inquiry. Nicotine Tob Res. 2016;18:1727–1732. doi: 10.1093/ntr/ntw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wapf V, Schaub M, Klaeusler B, Boesch L, Stohler R, Eich D. The barriers to smoking cessation in Swiss methadone and buprenorphine-maintained patients. Harm Reduct J. 2008;5:10. doi: 10.1186/1477-7517-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deal H, Newcombe DAL, Walker N, Galea S. Smoking cessation in a community alcohol and drug service: acceptability and feasibility. Addict Disord Their Treat. 2014;13:199–209. [Google Scholar]
- 157.Strong DR, Uebelacker L, Fokas K, Saritelli J, Matsko S, Abrantes AM, et al. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. J Addict Med. 2014;8:77–83. doi: 10.1097/ADM.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 158.Himelhoch S, Riddle J, Goldman HH. Barriers to implementing evidence-based smoking cessation practices in nine community mental health sites. Psychiatr Serv. 2014;65:75–80. doi: 10.1176/appi.ps.201200247. [DOI] [PubMed] [Google Scholar]
- 159.Twaddle AC. Health decisions and sick role variations: an exploration. J Health Soc Behav. 1969;10:105–115. [PubMed] [Google Scholar]
- 160.Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. J Clin Psychopharmacol. 2006;26:S13–S19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- 161.White W, Kurtz E. The varieties of recovery experience: a primer for addiction treatment professionals and recovery advocates. Int J Self Help Self Care. 2005;3:21–61. [Google Scholar]
- 162.Fagerström K, Aubin H-J. Management of smoking cessation in patients with psychiatric disorders. Curr Med Res Opin. 2009;25:511–518. doi: 10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- 163.Tam J, Warner KE, Meza R. Smoking and the reduced life expectancy of individuals with serious mental illness. Am J Prev Med. 2016;51:958–966. doi: 10.1016/j.amepre.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 164.Morris C, Waxmonsky J, May M, Giese A, Martin L. Rockville, MD: U.S. Substance Abuse and Mental Health Services Administration; 2009. Mental health tobacco cessation toolkit. [accessed 2019 Oct 7]. Available from: https://www.thenationalcouncil.org/integrated-health-coe/ [Google Scholar]
- 165.Dalla Palu A, Dauer J, McDonald C, Morris C, Reyes R, Richter K, et al. Rockville, MD: U.S. Substance Abuse and Mental Health Services Administration; Integrating tobacco treatment within behavioral health. [accessed 2019 Oct 7]. Available from: https://www.integration.samhsa.gov/Integrating_Tobacco_Treatment_-_BH.pdf. [Google Scholar]
- 166.Lipari RN, Van Horn S.Smoking and mental illness among adults in the United StatesThe CBHSQ reportRockville, MD: U.S. Substance Abuse and Mental Health Services Administration; 2013 [PubMed] [Google Scholar]
- 167.U.S. Substance Abuse and Mental Health Services Administration. Rockville, MD: U.S. Substance Abuse and Mental Health Services Administration; 2013. The NSDUH report data spotlight: adults with mental illness or substance use disorder account for 40 percent of all cigarettes smoked. [accessed 2019 Oct 7]. Available from: https://msleadership.org/wp-content/uploads/docs/SAMHSA_NSDUH_March2013.pdf. [Google Scholar]
- 168.Marynak K, VanFrank B, Tetlow S, Mahoney M, Phillips E, Jamal Mbbs A, et al. Tobacco cessation interventions and smoke-free policies in mental health and substance abuse treatment facilities: United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:519–523. doi: 10.15585/mmwr.mm6718a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. J Subst Abuse Treat. 2008;34:165–172. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.U.S. Substance Abuse and Mental Health Services Administration. Rockville, MD: U.S. Substance Abuse and Mental Health Services Administration; 2013. The N-SSATS report: tobacco cessation services. [accessed 2019 Oct 7]. Available from: https://www.samhsa.gov/data/sites/default/files/N-SSATS%20Rprt%20Tobacco%20Cessation%20Services/The%20N-SSATS%20Report%20%20Tobacco%20Cessation%20Services/The%20N-SSATS%20Report%20%20Tobacco%20Cessation%20Services.htm. [Google Scholar]
- 171.Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- 172.Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao NC, et al. A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: efficacy, safety, and adherence. Psychooncology. 2019;28:561–569. doi: 10.1002/pon.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- 174.Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 175.Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 176.Pomerleau OF, Pomerleau CS, Marks JL, Snedecor SM, Mehringer AM, Namenek Brouwer RJ, et al. Prolonged nicotine patch use in quitters with past abstinence-induced depressed mood. J Subst Abuse Treat. 2003;24:13–18. doi: 10.1016/s0740-5472(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 177.Dale Horst W, Klein MW, Williams D, Werder SF. Extended use of nicotine replacement therapy to maintain smoking cessation in persons with schizophrenia. Neuropsychiatr Dis Treat. 2005;1:349–355. [PMC free article] [PubMed] [Google Scholar]
- 178.Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 179.Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82:186–195. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- 180.Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145–154. doi: 10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504–511. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. 2016;111:142–155. doi: 10.1111/add.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bolin K, Mörk A-C, Wilson K. Smoking-cessation therapy using varenicline: the cost-utility of an additional 12-week course of varenicline for the maintenance of smoking abstinence. J Eval Clin Pract. 2009;15:478–485. doi: 10.1111/j.1365-2753.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 185.Knight C, Marbaix S, Annemans L, Prignot J, Bowrin K. The cost-effectiveness of an extended course (12+12 weeks) of varenicline plus brief counselling compared with other reimbursed smoking cessation interventions in Belgium, from a public payer perspective. Acta Clin Belg. 2012;67:416–422. doi: 10.2143/ACB.67.6.2062706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.