Abstract
Bidirectional relationships between inflammation and metabolic dysfunction may contribute to the pathophysiology of psychiatric illnesses like depression. Metabolic disturbances drive inflammation, which in turn exacerbate metabolic outcomes including insulin resistance. Both inflammatory (e.g. endotoxin, vaccination) and metabolic challenges (e.g. glucose ingestion) have been shown to affect activity and functional connectivity (FC) in brain regions that subserve reward and motor processing. We previously reported relationships between elevated concentrations of endogenous inflammatory markers including C-reactive protein (CRP) and low corticostriatal FC, which correlated with symptoms of anhedonia and motor slowing in major depression (MD). Herein, we examined whether similar relationships were observed between plasma markers related to glucose metabolism (non-fasting concentrations of glucose, insulin, leptin, adiponectin and resistin) in 42 medically-stable, unmedicated MD outpatients who underwent fMRI. A targeted, hypothesis-driven approach was used to assess FC between seeds in subdivisions of the ventral and dorsal striatum and a region in ventromedial prefrontal cortex (VS-vmPFC), which was previously found to correlate with both inflammation and symptoms of anhedonia and motor slowing. Associations between FC and gene expression signatures were also explored. A composite score of all 5 glucose-related markers (with increasing values reflecting higher concentrations) was negatively correlated with both ventral striatum (VS)-vmPFC (r=−0.33, p<0.05) and dorsal caudal putamen (dcP)-vmPFC (r=−0.51, p<0.01) FC, and remained significant after adjusting for covariates including body mass index (p<0.05). Moreover, an interaction between the glucose-related composite score and CRP was observed for these relationships (F[2,33]=4.3, p<0.05) whereby significant correlations between the glucose-related metabolic markers and FC was found only in patients with high plasma CRP (>3 mg/L; r=−0.61 to −0.81, p<0.05). Insulin and resistin were the individual markers most predictive of VS-vmPFC and dcP-mPFC FC, respectively, and insulin, resistin and CRP clustered together and in association with both LV-vmPFC and dcP-vmPFC in principal component analyses. Exploratory whole blood gene expression analyses also confirmed that gene probes negatively associated with FC were enriched for both inflammatory and metabolic pathways (FDR p<0.05). These results provide preliminary evidence that inflammation and metabolic dysfunction contribute jointly to deficits in reward and motor circuits in MD. Future studies using fasting samples and longitudinal and interventional approaches are required to further elucidate the respective contributions of inflammation and metabolic dysfunction to circuits and symptoms relevant to motivation and motor activity, which may have treatment implications for patients with psychiatric illnesses like depression.
Keywords: glucose metabolism, inflammation, insulin, C-reactive protein, functional connectivity, fMRI, gene expression
1. Introduction
A significant portion of patients with depression (30–50% depending on the sample) have increased inflammatory markers in both the peripheral blood and cerebrospinal fluid, including inflammatory cytokines and the acute phase reactant C-reactive protein (CRP)(Dantzer et al., 2008; Felger et al., 2018; Goldsmith et al., 2016b; Zunszain et al., 2013). Both obesity and its associated metabolic disturbances are important drivers of systemic inflammation, in part through activation of resident macrophages around expanding adipocytes (Park et al., 2005; Weisberg et al., 2003). However, increases in circulating insulin and free fatty acids also activate macrophages (Ieronymaki et al., 2019a; Ieronymaki et al., 2019b), and the associated release of inflammatory cytokines is a major contributor to insulin resistance (Hirosumi et al., 2002; Hotamisligil et al., 1994; Shi et al., 2006). Accordingly, a high percentage of patients with depression also exhibit evidence of metabolic dysfunction including insulin resistance (IR) (Hamer et al., 2019; Li et al., 2016).
Of further clinical relevance to depression is the mounting evidence that increased inflammation, obesity and metabolic disturbances have all been associated with reduced responsiveness to standard antidepressant medications (Furman et al., 2018; Haroon et al., 2018b; Jha et al., 2018; Strawbridge et al., 2015; Vogelzangs et al., 2014). With regard to novel antidepressants, work from our group for example showed that, in addition to inflammatory pathways, differential expression of genes related to glucose and lipid metabolism were predictive of the subsequent antidepressant response to the tumor necrosis factor (TNF) antagonist, infliximab (Mehta et al., 2013), which was observed in depressed patients with higher levels of plasma CRP (Raison et al., 2013). These findings were validated by measurement of circulating glucose and lipid-related biomarkers (Bekhbat et al., 2018), and both metabolism-related genes and plasma biomarkers were affected by administration of infliximab in these patients, further exemplifying bidirectional relationships between inflammation and metabolism.
This relationship between inflammation and metabolism and antidepressant response is not surprising considering that both systemic inflammation and metabolic disturbances like insulin resistance can have consequences on organs and tissues such as the brain. Indeed, numerous studies in humans and laboratory animals have demonstrated that administration of inflammatory stimuli can impact dopamine release and affect the neural activation of key basal ganglia and prefrontal regions that subserve reward processing, motivation, and motor speed (Brydon et al., 2008; Capuron et al., 2012; Eisenberger et al., 2010; Felger et al., 2015; Felger et al., 2013; Felger and Treadway, 2017; Harrison et al., 2016; Moieni et al., 2019; Yohn et al., 2016). Likewise, oral glucose challenge has been shown to alter the cerebral blood flow and functional connectivity (FC) of cortical and limbic brain regions including basal ganglia (Al-Zubaidi et al., 2018; Jastreboff et al., 2016; Page et al., 2013; van Opstal et al., 2018).
Increasing data from our group and others has demonstrated evidence that endogenous inflammation in patients with major depression (MD) is also associated with alterations in the structure and function of reward and motor-relevant cortical and subcortical structures (Felger et al., 2016b; Haroon et al., 2018a; Mehta et al., 2018a; Meier et al., 2016; Opel et al., 2019; Savitz et al., 2012; Yin et al., 2019). For example, our previous work using resting-state functional magnetic resonance imaging (rfMRI) showed that plasma concentrations of CRP (as well as inflammatory cytokines and their soluble receptors) were negatively corelated with FC in dopaminergic corticostriatal reward and motor circuits in association with anhedonia and motor slowing in whole-brain analyses (Felger et al., 2016a; Yin et al., 2019). Using a targeted, hypothesis-driven FC approach, reduced FC between the left ventral striatum (VS) and right dorsal caudal putamen (dcP) and the ventromedial prefrontal cortex (vmPFC) corelated with CRP (Felger et al., 2016b). Corticostriatal FC involving VS was in turn selectively associated with anhedonia, a core symptom of depression, and FC involving dcP predicted motor slowing, a prominent symptom in depression and other psychiatric disorders. Symptoms of anhedonia and motor slowing have been associated with alterations in reward and motor circuits involving the basal ganglia and prefrontal cortex (Drysdale et al., 2017; Dunlop and Nemeroff, 2007; Treadway and Pizzagalli, 2014) and with inflammation (Felger and Treadway, 2017; Medeiros et al., 2020). However, whether deficits in reward and motor circuits are similarly associated with highly related markers of metabolic dysfunction in MD has not been examined.
Herein, we examined relationships between non-fasting levels of glucose-related plasma metabolic markers (Bekhbat et al., 2018) and targeted FC in reward and motor circuits found to be related to anhedonia and motor slowing in our previous work (Felger et al., 2016b; Mehta et al., 2020b). The glucose-related metabolic panel from our previous work was comprised of glucose and insulin as well as the adipokines leptin, resistin, and adiponectin, which are associated with glucose homeostasis and insulin resistance as well as with depression (Bryson et al., 1999; Lehto et al., 2010; Li et al., 2016; Singh and Saxena, 2010). Multivariate linear models and principal components analyses (PCA) including CRP were used to determine whether metabolic and inflammatory markers exhibited joint or independent relationships with FC. Because gene expression has been previously associated with brain structure in MD and is predictive of antidepressant response (Cattaneo et al., 2016; Cattaneo et al., 2013; Mehta et al., 2013; Mora et al., 2018; Savitz et al., 2013), we also examined whether inflammatory and metabolic whole blood immune cell gene expression pathways were associated with FC.
2. Methods
2.1. Participants
Forty-eight participants (18–65 years) with a primary diagnosis of major depressive disorder or bipolar disorder, current episode depressed as determined by Structured Clinical Interview for Diagnostic and Statistical Manual-IV-TR (SCID-IV) were studied, as previously described (Felger et al., 2016b), all of whom had rfMRI, CRP and gene expression data. Forty-two of these 48 participants also had additional plasma samples available for analysis of metabolic markers. Briefly, subjects were free of psychotropic medications or medications known to affect the immune system, and were tested for drugs of abuse at screening and on the day of the scan. Medications for other medical conditions were allowed per patients’ treating physicians, although patients were required to be medically-stable as determined by medical history, physical exam and laboratory testing (see Supplementary Information). No patients had non-fasting glucose levels out of normal range and only one subject was taking a medication used to treat diabetes. CRP was assessed 2–4 time at screening (1–4 weeks apart) to ensure stable levels of inflammation (two values within 25%). Participants with active infections were excluded until medically-stable. Subjects were recruited from a parent study on phenotyping depressed patients with increased inflammation (ClinicalTrials.gov NCT01426997) which involved a single inpatient visit to the Emory Clinical Research Network clinic to collect MRI, biomarker, behavioral and sleep data. All procedures were approved a priori by the Institutional Review Board of Emory University. All participants provided written informed consent.
2.2. Resting-state fMRI data acquisition and preprocessing
Data was acquired on a 3T Magnetom Trio scanner (Siemens, USA) with a 20-channel head coil at the Emory Center for Systems Imaging in the afternoon (3PM±2 hours). Anatomic images (1mm3 isotropic) were obtained using a T1-weighted, magnetization prepared rapid gradient echo (MPRAGE) sequence (Mugler and Brookeman, 1990). Wakeful rfMRI images were acquired using a Z-SAGA pulse sequence for recovering susceptibility signal losses regularly seen in T2-weighted fMRI (Heberlein and Hu, 2004) (See Supplementary Information). Analysis of FC was conducted with AFNI (http://afni.nimh.nih.gov/). Pre-processing (Li et al., 2019b; Li et al., 2017; Yin et al., 2019) included voxel-wise outlier detection (~5.5× median absolute deviation), despiking, slice timing correction, volume alignment, anatomy-to-EPI co-registration (Saad et al., 2009), nuisance signal (head motion, cerebrospinal fluid, and white matter) regression, band pass filtering (0.009Hz<f<0.08Hz), and Gaussian (FWHM=5mm) spatial smoothing. Regarding head motion, no participant had movement >3.4mm/degree in translation/rotation, consistent with previous studies (Felger et al., 2016b; Oathes et al., 2015; Wacker et al., 2009). Individual’s 4D fMRI data was spatially normalized into a standard stereotaxic space, the Montreal Neurological Institute (MNI) template, with 1mm3 resolution.
2.3. Analysis of functional connectivity
Associations between circulating metabolic and gene expression markers and corticostriatal resting-state FC were examined using targeted VS and dcP to vmPFC FC relationships previously identified to be associated with both inflammation and behavior (anhedonia and motor slowing, respectively)(Felger et al., 2016b). Targeted seed-to-ROI FC was calculated as signal correlations between spherical seeds (r=3mm) centered on the left inferior VS (including nucleus accumbens; MNI coordinates: x=−14, y=8, z=−9) or the right dcP (x=28, y=1, z=3)(Capuron et al., 2012; Di Martino et al., 2008; Felger et al., 2016b) and a ROI in vmPFC (MNI coordinates: x=0, y=44, z=−8 and volume=1408mm3). This vmPFC ROI was previously reported to be associated with neural activation in response to receipt of reward versus loss in a meta-analysis of neuroimaging studies (Diekhof et al., 2012) and with decreased cortico-limbic FC in our previous work (Felger et al., 2016b; Mehta et al., 2018b; Mehta et al., 2020b). From this vmPFC ROI, subject-level FC scores (Fisher’s Z values) were extracted for correlation with metabolic markers and CRP (Felger et al., 2016b; Mehta et al., 2018b; Mehta et al., 2020b).
2.4. Blood collection
Whole blood was collected on the day after the scan through indwelling catheters after participants had at least 30 minutes of rest. For plasma isolation, blood was collected into EDTA-containing vacutainer tubes approximately three hours from a meal provided at the research clinic (3pm±1hour). Plasma was obtained by centrifugation at 1000×g for 10 minutes at 4°C, aliquoted into siliconized polypropylene tubes, and stored at −80°C until assay. Whole blood for gene expression analysis was collected in Tempus Blood RNA Tubes (Applied Biosystems, Carlsbad, CA) at 9am and stored at −80°C until RNA extraction by the Emory Cancer Genomics Core for microarray analysis.
2.6. Measurement of glucose-related metabolic markers and CRP
Plasma markers related to glucose metabolism were measured in the Biomarker Core Lab of the Foundation for Atlanta Veterans Education and Research. Plasma leptin, resistin and adiponectin were measured by ELISA (Boster Biological, Pleasanton, CA). Assay detection limits were 10 pg/ml, 3 pg/ml and 0.06 ng/ml, respectively. Plasma glucose (colorimetric), insulin (immunoturbidometric) and high sensitivity (hs)-CRP (immunoturbidimetric) were assayed using reagents from Sekisui Diagnostics (Exton, PA) implemented on the AU480 chemistry analyzer (Beckman Coulter). Mean inter- and intra-assay coefficients of variation were reliably <10% (Bekhbat et al., 2018; Felger et al., 2016b; Le et al., 2000; Mehta et al., 2013). To examine the shared contribution of all glucose and insulin-related circulating metabolic markers to the relationship with FC, a composite score was created from the sum of Z-scores of all markers, a common method for examining the contribution of multiple related metabolic (Agostinis-Sobrinho et al., 2017; Eisenmann, 2008; Erdembileg et al., 2015) or inflammatory (Erdembileg et al., 2015; Felger et al., 2018; Haroon et al., 2020; Hopkins et al., 2012; Mehta et al., 2020a) markers or risk factors, and referred to as the glucose-related composite score.
2.7. Microarray
Extraction of RNA was performed using Ambion Tempus RNA Spin kit (Thermo Fisher, Asheville, NC, USA) according to manufacturer instructions. RNA concentrations and A260/280 ratio (Tecan iControl, Life Sciences, Switzerland) and sample quality (Agilent Bioanalyzer 2100 RNA Nano assay, Agilent, Santa Clara, CA, USA) was determined. Each sample was linearly amplified by TotalPrep-96 RNA Amplification Kit for Array Analysis (Illumina, San Diego CA, USA). On the same day, all samples were hybridization to Illumina Human HT-12 Expression BeadChips using the Whole-Genome Gene Expression Direct Hybridization Assay (Illumina). BeadChips were scanned on the Illumina HiScan to determine raw probe fluorescence intensity.
2.8. Gene by functional connectivity associations and pathway analyses
Raw expression data were quantile-normalized in Illumina Genome Studio and 16,969 probes passed the filter criteria of Illumina probe detection p-value of <0.01 in at least 10% of the samples. Data were deposited in NCBI Gene Expression Omnibus as series GSE135524. The average signal intensity data were log2-transformed in R (http://www.R-project.org) (Mehta et al., 2013; Xiao et al., 2016). The association between FC Z-scores and gene expression was assessed while adjusting for age, sex, race and body mass index (BMI) using linear models in R (Barfield et al., 2012; Xiao et al., 2016). To explore relationships between FC and gene pathways, a set of probes was first identified to be associated with FC based on a threshold of |Rpartial|>0.30 (after controlling for covariates in multiple linear regression) consistent with a biologically relevant, medium effect size (Cole et al., 2003; Han et al., 2016; Mellon et al., 2016; Miller et al., 2018; Ross et al., 2019; Torres et al., 2013), combined with nominal p<0.05 (Guo et al., 2006; Han et al., 2016; Torres et al., 2013), which together has been shown to be more reliable across pathway analyses than use of FDR p-value alone (Cole et al., 2003; Guo et al., 2006; Patterson et al., 2006). Identified genes were then applied to the stringency of secondary statistical analyses (Cole et al., 2003; Guo et al., 2006; Patterson et al., 2006) at FDR<0.05 to assess their functional annotation within curated pathways and networks in GeneGo MetaCore (St. Joseph, MI, USA) (Ekins et al., 2007; Han et al., 2016; Mehta et al., 2013). Of note, the percentage of peripheral blood immune cell subsets from complete blood count (CBC) was stable across the range of FC Z-scores (STable 1).
2.9. Statistics
Clinical characteristics were summarized using mean and standard deviation (SD) for continuous variables and percent for categorical variables, which were examined with respect to both the glucose composite score and plasma CRP. Associations between the glucose composite score and FC Z-scores were assessed in linear regression models with and without clinical covariates that may modify relationships between peripheral biomarkers and brain circuits, including age, sex, race, and body mass index (BMI). To determine whether relationships between metabolic markers and FC depended on inflammation, an interaction term was created for the glucose composite score and level of plasma CRP concentration (as per American Heart Association/Center for Disease Control definitions of low, moderate and high risk for cardiovascular and metabolic disease, <1, 1–3 and >3 mg/L CRP coded as 1, 2 and 3, respectively)(Ridker, 2003), which was examined in multivariate general linear models including glucose scores and CRP with and without clinical covariates. To determine which individual metabolic markers contributed most to the relationships between glucose scores and FC, individual metabolic markers and FC Z-scores were entered into backward and forward linear regression models using the same criteria for entry and removal. Consistent with previous reports (Bekhbat et al., 2018; Felger et al., 2018; Haroon et al., 2014; Raison et al., 2009; Torres et al., 2013), markers were natural log (ln) transformed to improve normality for parametric statistical modeling. PCA was also used to determine whether individual metabolic markers and CRP clustered together and with FC Z-scores. Varimax rotation with Kaiser Normalization was used to simplify factor structure, as determined by Eigen values >1, and only individual variable contributions of >0.5 qualified for loading components (Grimsholm et al., 2005). Tests of significance were two-tailed, conducted in IBM SPSS Statistics 24.
3. Results
3.1. Sociodemographic, clinical, and biological characteristics of the sample
Characteristics of the study sample, including demographic variables as well as concentrations of CRP and metabolic markers from 48 patients (42 with metabolic markers), are summarized in Table 1. CRP was significantly correlated with the glucose composite score (r=0.374, p=0.015), as well as with a number of the individual metabolic markers, including insulin (r=0.454, p=0.003), leptin (r=0.532, p<0.001), and resistin (r=0.391, p=0.01). BMI was also significantly correlated with CRP (r=0.652, p<0.001), the glucose composite score (r=0.426, p=0.005), leptin (r=0.623, p<0.001), and insulin (r=0.369, p=0.016). As such, BMI was included in all analyses involving covariates to control for the potential confounding of additional factors related to metabolism and/or obesity that were not measured herein.
Table 1.
Demographic and clinical variables of the study sample, and their relationship to the glucose-related composite score and peripheral inflammation as measured by plasma CRP.
| Variable | Mean (s.d.) (n=48) | Correlation with Glucose Composite Score, r (p) | Correlation with CRP, r (p) |
|---|---|---|---|
| Demographic and CRP | |||
| Age (years) | 38.3 (10.9) | 0.314 (0.043) | −0.086 (0.561) |
| Sex, Male (n, %) Race |
14 (29.2) | 0.164 (0.301) | 0.129 (0.382) |
| Caucasian (n, %) | 18 (37.5) | −0.287 (0.065) | −0.071 (0.629) |
| African American (n, %) | 30 (62.5) | ||
| BMI (kg m−2) | 31.3 (7.6) | 0.426. (0.005) | 0.619 (<0.001) |
| CRP (mg/L) Metabolic marker (n=42) |
1.5 (1.6) | 0.328 (0.034) | - |
| Glucose Composite Score | 0.0 (2.43) | - | 0.328 (0.034) |
| Glucose (mg/dL) | 110.8 (19.6) | 0.330 (0.033) | −0.198 (0.208) |
| Insulin (U/mL) | 54.3 (34.4) | 0.427 (0.005) | 0.454 (0.003) |
| Leptin (pg/mL) | 13957.5 (5227.0) | 0.521 (<0.001) | 0.532 (<0.001) |
| Adiponectin (ng/mL) | 1272.7 (430.7) | 0.554 (<0.001) | −0.022 (0.891) |
| Resistin (pg/mL) | 2379.9 (2319.7) | 0.652 (<0.001) | 0.391 (0.01) |
Abbreviations: BMI - Body Mass Index; CRP - C-reactive protein
3.2. Relationships between glucose-related metabolic markers and functional connectivity in reward and motor circuits and their interaction with CRP
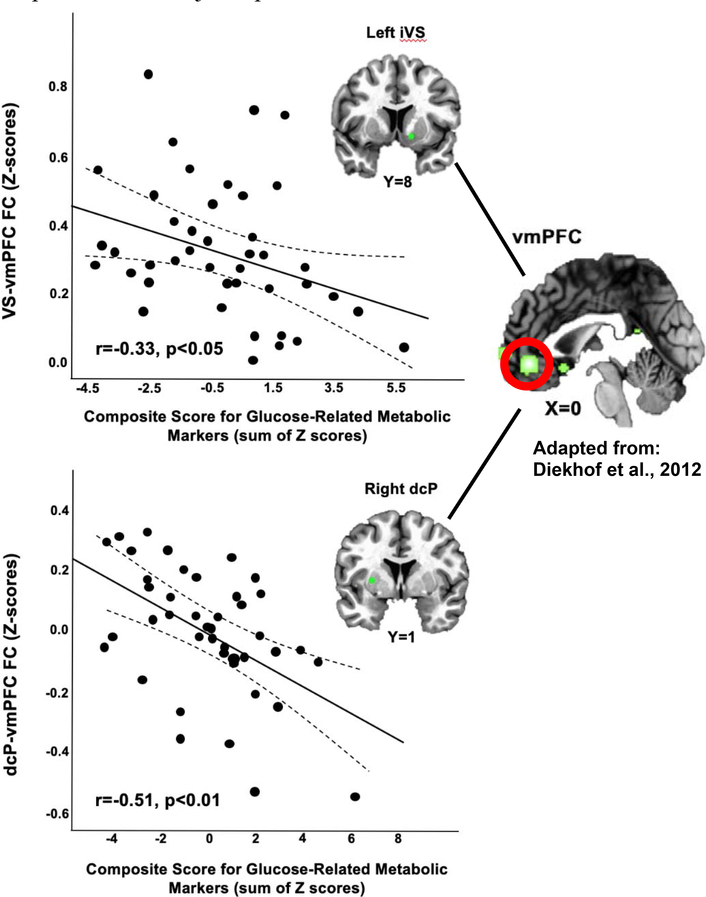
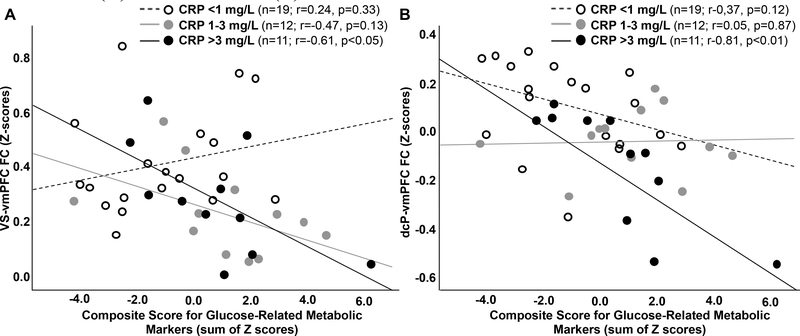
Glucose composite scores, with increasing values representing higher concentrations of each marker (Figure S1) and reflective of greater metabolic dysfunction, were negatively associated with both VS to vmPFC (VS-vmPFC; r=−0.330, p=0.033) and dcP-vmPFC (r=−0.508, p=0.001) FC (Figure 1). Both relationships remained significant after adjusting for covariates (p<0.05 for VS-vmPFC, p<0.01 for dcP-vmPFC). To determine whether associations between glucose-related metabolic markers and FC depended on inflammation, relationships between FC and an interaction term for the glucose composite score by level of plasma CRP concentration (low <1, moderate 1–3 and high >3 mg/L) was examined in multivariate general linear models including the glucose composite score and plasma CRP level. Main effects were observed for CRP level (F[4,74]=3.2, p=0.018) and the glucose score by CRP interaction term (F[2,33]=4.3, p=0.017); the interaction term (but not level of plasma CRP, p=0.122) remained significant (p<0.05) when controlling for clinical covariates, and univariate sub-analyses indicated that the interaction term was significant for VS-vmPFC FC (F[1,33]=4.7, p=0.037) with a trend for dcP-vmPFC FC (F[1,33]=3.9, p=0.058). To illustrate these results, correlations between glucose scores and FC were examined and plotted for each level of CRP, and indicated that significant relationships were observed only in patients with high CRP (>3 mg/L; n=11) for both VS-vmPFC (r=−0.613, p=0.045) and dcP-vmPFC (r=−0.813, p=0.002) FC (Figure 2).
Figure 1. Glucose composite score was negatively associated with ventral striatum (VS)-ventromedial prefrontal cortex (vmPFC) and dorsal caudal putamen (dcP)-vmPFC functional connectivity (FC).
A composite score for glucose-related markers, with increasing values reflective of greater concentrations of these markers (see Figure S1), was created as the sum of Z-scores for non-fasting plasma concentrations of glucose, insulin, leptin, adiponectin and resistin. Glucose composite score was negatively associated with functional connectivity between left VS and the vmPFC [image reprinted from Diekhof et al. with permission from Elsevier, copyright 2012] and right dcP and the vmPFC, in 42 medically-stable, medication free outpatients with major depression.
Figure 2. Relationships between the glucose composite score and ventral striatum (VS)-ventromedial prefrontal cortex (vmPFC) and dorsal caudal putamen (dcP)-vmPFC functional connectivity (FC) depended on plasma levels of C-reactive protein (CRP).
Specifically, an interaction between the glucose-related metabolic markers and level of plasma CRP concentration was observed whereby significant correlations between the glucose composite score and FC was found only in patients with high plasma CRP (>3 mg/L) for both VS-vmPFC (A) and dcP-vmPFC (B) FC.
3.3. Individual metabolic markers, insulin and resistin cluster together and with CRP in association with functional connectivity in reward and motor circuits
Although no individual metabolic markers were associated with VS-vmPFC connectivity at p<0.05 (all r=−0.002 to −0.221), insulin was found to be the strongest predictor of VS-vmPFC FC (r=−0.221, p =0.160) in backward and forward linear regression models using the same criteria for entry and removal. Resistin was the strongest predictor of dcP-vmPFC FC (r=−0.422, p = 0.005), as confirmed in backward and forward linear regression using the same criteria for entry and removal. Insulin also exhibited a non-significant trend to correlate with dcP-vmPFC FC (r=−0.272, p=0.081). To confirm and extend the above results from linear regression indicating a metabolism (glucose composite score) by inflammation (plasma CRP) interaction with respect to their relationship with FC, PCA was used to examine whether metabolic markers and CRP clustered together and in association with FC. In PCA including all 5 metabolic markers and CRP along with VS-vmPFC FC, the first principle component (eigenvalues contributing 29.68% of the overall variance) was comprised of VS-vmPFC FC, insulin, resistin, and CRP (Table 2). Using all markers with dcP-vmPFC FC, the first principle component (eigenvalues contributing 31.36% of the overall variance) similarly included dCP-vmPFC FC, insulin, resistin, and CRP (Table 2).
Table 2.
Loading factors for Principle Component Analysis of FC, CRP, and metabolic markers
| Marker | 1 | VS-vmPFC Factor 2 |
3 | 1 | dcP-vmPFC Factor 2 |
3 |
|---|---|---|---|---|---|---|
| FC | −0.535 | −0.764 | ||||
| CRP | 0.785 | 0.756 | ||||
| Leptin | −0.725 | 0.740 | ||||
| Insulin | 0.780 | 0.698 | ||||
| Glucose | 0.908 | −0.901 | ||||
| Adiponectin | 0.920 | 0.907 | ||||
| Resistin | 0.505 | 0.504 | 0.593 | 0.531 |
Data reflect factors after Varimax rotation with Kaiser Normalization and variable contributions CRP - C-reactive protein; dcP- dorsal caudal putamen; FC - functional connectivity; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum
3.4. Inflammatory and metabolic gene expression pathways are associated with functional connectivity in reward and motor circuits: Exploratory analyses
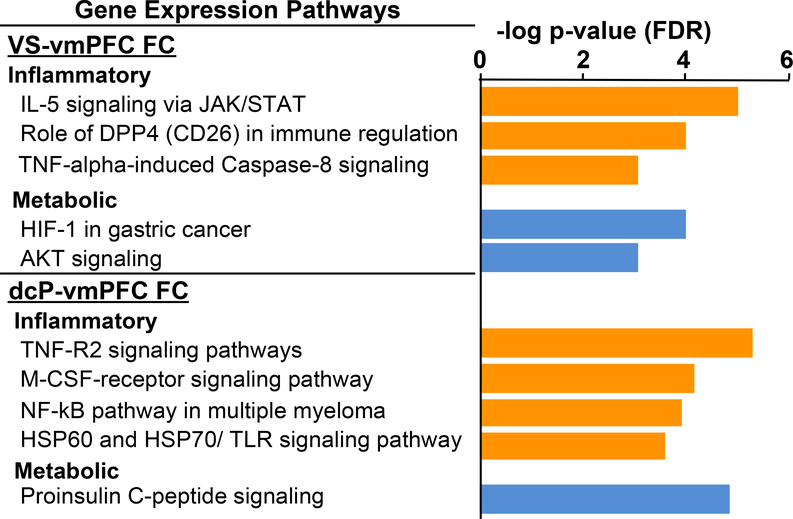
To explore whether both inflammatory and metabolic signaling pathways in immune cells were related to deficits in reward and motor-related pathways, targeted corticostrital FC described above was used to identify transcriptional pathways in peripheral blood immune cells that were negatively associated with VS and dcP to vmPFC FC in all 48 patients. In linear models controlling for clinical covariates (age, race, sex and BMI), 266 probes were identified to be negatively correlated with VS-vmPFC FC, whereas 836 were negatively associated with dcP-vmPFC FC (Rpartial>−0.3, p<0.05) (SData 1). Of the 12 significant pathways (FDR p<0.05) enriched in the 266 transcripts that negatively correlated with VS-vmPFC (SData 2), 7 were related to the immune system or inflammation (e.g. JAK/STAT, DPP4, and TNF signaling) and two were related to metabolism (HIF-1 and AKT signaling) (Figure 3). Similarly, of the top 20 of 87 pathways enriched in the 836 transcripts negatively correlated with dcP-vmPFC FC (SData 2), 11 were related to the immune system or inflammation (e.g. M-CSF, NFkB, and TLR signaling) and one to metabolism (proinsulin signaling) (Figure 3). Networks of canonical pathways built from the genes identified from the top ten pathways enriched in transcripts negatively correlated with VS-vmPFC FC and dcP-vmPFC FC (network objects; n=29 for VS and n=47 dcP) were also primarily related to inflammatory and metabolic processes (Figure S2). Of the 16 pathways enriched in the 191 genes positively associated with VS-vmPFC FC, 6 were related to immune responses (e.g. interleukin (IL) 2/3/6 and M-CSF/G-CSF signaling) but were primarily driven by a single network object, PI3K cat class IA (SData 2) representing the transcript PIK3CD from the dataset (ILMN_1766275); of the only 9 pathways significantly enriched in 992 genes positively correlated with dcP-vmPFC, the top 5 were interestingly related to interferon and IL-4 signaling (see SData 1 and 2).
Figure 3. Gene expression pathways enriched in genes negatively associated with ventral striatum (VS)-ventromedial prefrontal cortex (vmPFC) and dorsal caudal putamen (dcP)-vmPFC functional connectivity (FC).
Pathways related to both inflammation and metabolism were in the top pathways significantly enriched (FDR p<0.05) in the genes negatively correlated with VS-vmPFC and dcP-vmPFC FC in 48 medically-stable, medication free outpatients with major depression. Orange = inflammatory and blue = metabolic-related pathways.
4. Discussion
This study demonstrated that peripheral biomarkers of metabolic disturbance relevant to glucose metabolism are associated with low FC in reward and motor circuits in MD. Moreover, a significant interaction was observed between the glucose composite score and level of CRP concentration whereby significant correlations between the glucose-related metabolic markers and FC was found only in patients with high plasma CRP (>3 mg/L). Interestingly, insulin and resistin were the individual markers most predictive of VS-vmPFC and dcP-mPFC FC, respectively, and insulin, resistin and CRP clustered together and in association with both LV-vmPFC and dcP-vmPFC in PCA. Together, the interaction and PCA results provide support for the idea that plasma inflammatory and metabolic markers contributed jointly to decreased FC in corticostriatal reward and motor pathways in depression. These findings were mirrored in exploratory gene expression analyses indicating that both inflammatory and metabolic pathways in peripheral blood immune cells were negatively correlated with VS-vmPFC and dcP-vmPFC FC. While relationships between metabolism and inflammation are complex, this study provides some preliminary findings regarding their potential roles in circuit deficits that contribute to MD, which can inform future longitudinal and interventional studies.
The composite scores for the five glucose-related plasma markers were negatively correlated with VS-vmPFC and dcP-vmPFC FC Z-scores (which were associated with symptoms of anhedonia and motor slowing in our previous work), whereby MD patients with higher levels of plasma markers had lower levels of FC. An interaction was also uncovered indicating that the relationship between glucose score and FC depended on the level of plasma CRP, and patients with both high glucose scores and high CRP exhibited low levels of FC. Despite a strong relationship between the glucose score and BMI, these relationships, including a metabolism by inflammation interaction, remained significant when controlling for BMI and other covariates. This finding suggests that connectivity in reward and motor circuits may be sensitive to metabolic disturbances involving glucose metabolism that are independent of variability in BMI and adiposity. With regard to individual markers that contributed to the relationship between the glucose score and FC, insulin was the strongest metabolic marker associated with VS-vmPFC FC and resistin was the most significant metabolic predictor of dcP-vmPFC FC. Interestingly, both insulin and resistin in conjunction with CRP were clustered together with FC in PCA.
The above findings are interesting when further considering the relationships between both resistin and insulin, and resistin and inflammation. The role of resistin as a factor produced in adipose tissue that contributes to insulin resistance (Tilg and Moschen, 2006) is now thought to be true primarily in rodents (Steppan et al., 2001). While the primary action of resistin in humans is controversial, initially pointing to metabolic disturbances in obesity (Ukkola, 2002), recent evidence shows that it is produced in tissues other than adipocytes and supports a second role of resistin (as well as other adipokines) in inflammation (Kusminski et al., 2005). Indeed, resistin has been shown to induce production of inflammatory cytokines (Beier et al., 2008; Silswal et al., 2005), and may serve as a key intermediate link between disturbances in glucose metabolism/insulin resistance and inflammatory processes that jointly impact the brain and behavior in depression. Our results interpreted in this light illuminate a scenario whereby adiposity and metabolic disturbances cause rising insulin levels that increase inflammatory mediators such as adipokines and CRP, which may together drive activation of immune cells and inflammatory cytokines on the brain and behavior (Capuron et al., 2017; Luppino et al., 2010), while feeding back on adipose cells and other tissues to promote further metabolic dysfunction.
Another area relevant to the intersection of metabolism and inflammation, and which may have treatment implications for depression, is the intracellular shift in metabolism required to sustain immune cell activation, i.e. “immunometabolism” (O’Neill et al., 2016; Treadway et al., 2019). In states of chronic low-grade inflammation, such as in depression, increased energy demands and activation of inflammatory pathways may cause immune cells to shift from more energy-efficient oxidative phosphorylation to glycolysis, a less-efficient but more rapid producer of energy (Lacourt et al., 2018; O’Neill et al., 2016). This shift in metabolic pathways may be evidenced in gene expression findings from our previous studies demonstrating that glucose-related genes predict treatment response to inflximab in individuals with high inflammation (Bekhbat et al., 2018; Mehta et al., 2013), and that both high CRP and anhedonia were required to observe shifts in immunometabolic transcripts in peripheral blood immune cells in patients with depression (Bekhbat et al., 2020). These studies are complemented by the gene expression findings herein supporting associations between inflammatory and metabolic gene transcripts and low FC in reward and motor circuits.
Gene pathways related to both inflammation and metabolism were among the top of those that were enriched in gene transcripts negatively correlated with both vs-vmPFC and dcP-vmPFC FC. For example, NFkB signaling pathways have consistently been shown to be related to immune and inflammatory responses (Liu et al., 2017; Mitchell et al., 2016), though may also play a role in energy homeostasis, regulation of glycolysis, and maintenance of inflammation in metabolic disorders (Hasegawa et al., 2012; Moretti et al., 2012). We have previously shown that TNF signaling pathways are predictive of the antidepressant response to infliximab, a TNF antagonist, in addition to a number of metabolic transcripts (Mehta et al., 2013), and TNF is a primary driver of insulin resistance (Shi et al., 2006). DPP4 is widely expressed in immune cells and in the setting of obesity, may promote inflammation and insulin resistance (Ghorpade et al., 2018). DPP4 inhibitors may also be of potential benefit in metabolic disorders such as diabetes (Barnett, 2006). HIF-1 and AKT pathways may contribute to the Warburg effect whereby cells rapidly shift glucose metabolism from the slow, energy-maximizing oxidative phosphorylation to the rapid but less energy-yielding glycolysis (Courtnay et al., 2015; Nagao et al., 2019), and both have been implicated in the pathophysiology and treatment of depression (Li et al., 2019a; Matsuda et al., 2019; Shibata et al., 2013). Of note, although 6 immune pathways (interleukin (IL) 2/3/6 and M-CSF/G-CSF signaling) were enriched in the transcripts positively associated with VS-vmPFC FC, they were represented by genes that were repeated across these and the other pathways, all of which contained PIK3CD. Unlike other class IA PI3Ks, PIK3CD is expressed primarily in leukocytes and involved in regulation of acquired immune responses (Clayton et al., 2002; Rolf et al., 2010). This was consistent with significant pathways for acquired immune cytokines (IL-4, interferon-gamma) enriched in genes positively correlated with dcP-vmPFC FC.
Activated inflammatory and metabolic intracellular signaling pathways may reflect processes that sustain low-grade peripheral inflammation in patients with depression who exhibit high CRP and metabolic disturbances, thus leading to cytokine release that impacts dopamine signaling in the brain to drive symptoms relevant to motivational deficits and psychomotor slowing (Capuron et al., 2012; Felger et al., 2016b; Felger and Treadway, 2017; Goldsmith et al., 2016a). Furthermore, evidence exists that dopaminergic neurons may be sensitive to metabolic signals that may relate important information about metabolic states in the periphery to guide reward-related decision making (Treadway et al., 2019). For example, there may be bidirectional communication between peripheral insulin and striatal dopamine that guide food choices and energy utilization (Stouffer et al., 2015; Ter Horst et al., 2018), which may, in part, explain why insulin was the strongest predictor of vs-vmPFC FC herein.
One strength of this study was the use of an a priori, targeted method of examining corticostriatal FC using seeds and a ROI from the literature (which we had previously shown to be related to anhedonia and motor slowing)(Felger et al., 2016b), allowing examination of relationships with metabolic and gene markers without bias of ROIs derived by variables within the dataset. Data also support our previous findings demonstrating relationships between immune and metabolic markers/pathways with depression and antidepressant response (Bekhbat et al., 2018; Mehta et al., 2013). Primary limitations of this study were the cross-sectional nature and limited sample size, which did not permit higher level modeling such as a formal path or mediation analysis to disentangle complex causal relationships among highly related metabolic and inflammatory variables, or examination of sex differences. A healthy control group was also not studied, however patients with low glucose composite scores (and low CRP) had similar positive FC in the assessed corticostriatal circuits as frequently observed in controls groups in numerous studies (Barnes et al., 2010; Di Martino et al., 2008; Kelly et al., 2009), and glucose-related markers were consistently elevated in patients with high glucose composite scores and in association with high plasma CRP. Another limitation is that the blood samples were not collected under fasting conditions so that basal measures of insulin resistance (e.g. HOMA-IR) could not be calculated and hemoglobin A1c was not measured. However, it should be noted that conditions were standardized across participants; blood was collected ~3 ±1 hours from the last meal and at the same time as the rfMRI scan (conducted ~24 hours apart). Therefore, the study captured approximately “normal” circulating levels of insulin, leptin, adiponectin and resistin for these subjects on a given day, but may have led to glucose levels not contributing to the association with FC due to increased variability. Future work involving fasting sample collection in longitudinal and interventional studies is necessary to isolate causal relationships between inflammation, metabolism, and corticostriatal FC in depression. Strategies that employ metabolic and inflammatory challenge paradigms may help elucidate these complex relationships. Whether these relationships are present in other psychiatric populations that exhibit motivation and motor-related symptoms remains unknown. Nevertheless, findings suggest that both inflammation and metabolic disturbances represent potential novel treatment targets to improve FC in circuits that underlie motivational deficits and psychomotor slowing, providing a framework for future investigation.
Supplementary Material
Highlights.
Metabolism and inflammation may interact to contribute to depression (MD)
Inflammation has predicted low reward circuit functional connectivity (FC) in MD
Glucose-related metabolic markers also negatively correlated with FC in MD
Relationship between metabolic markers and FC depended on level of CRP concentration
Inflammatory and metabolic gene pathways were also associated with FC
Acknowledgments
Funding Sources: This work was supported by funds from the National Natural Science Foundation of China (grant number 31920103009, 31671169, 31530031 to Z.L.), the National Institute of Mental Health (grant number R01MH109637 to J.C.F., grant number K23MH114037 D.R.G, and grants R01MH112076, R01MH107033, K23MH091254 to E.H.), the Brain and Behavioral Research Foundation and Dana Foundation (grants BBRF22296, CADF49143 to J.C.F.). In addition, the study was supported in part by Public Health Service Grants (grant number UL1TR000454, UL1TR002378 and KL2TR000455) from the Clinical and Translational Science Award program, and by the National Institute of Health/National Cancer Institute under award number P30CA138292, and the Emory Integrated Genomics Core (EIGC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities.
Footnotes
Financial Disclosure: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostinis-Sobrinho CA, Moreira C, Abreu S, Lopes L, Sardinha LB, Oliveira-Santos J, Oliveira A, Mota J, Santos R, 2017. Muscular fitness and metabolic and inflammatory biomarkers in adolescents: Results from LabMed Physical Activity Study. Scand J Med Sci Sports 27, 1873–1880. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidi A, Heldmann M, Mertins A, Jauch-Chara K, Munte TF, 2018. Influences of Hunger, Satiety and Oral Glucose on Functional Brain Connectivity: A Multimethod RestingState fMRI Study. Neuroscience 382, 80–92. [DOI] [PubMed] [Google Scholar]
- Barfield RT, Kilaru V, Smith AK, Conneely KN, 2012. CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinformatics 28, 1280–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM, Petersen SE, Schlaggar BL, 2010. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Frontiers in systems neuroscience 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett A, 2006. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. International journal of clinical practice 60, 1454–1470. [DOI] [PubMed] [Google Scholar]
- Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE, 2008. New role of resistin in lipopolysaccharide-induced liver damage in mice. The Journal of pharmacology and experimental therapeutics 325, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Chu K, Le NA, Woolwine BJ, Haroon E, Miller AH, Felger JC, 2018. Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology 98, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, Felger JC, 2020. Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD, 2008. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry 63, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson JM, Phuyal JL, Swan V, Caterson ID, 1999. Leptin has acute effects on glucose and lipid metabolism in both lean and gold thioglucose-obese mice. Am J Physiol 277, E417–422. [DOI] [PubMed] [Google Scholar]
- Capuron L, Lasselin J, Castanon N, 2017. Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacology 42, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH, 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Ferrari C, Uher R, Bocchio-Chiavetto L, Riva MA, Consortium MRCI, Pariante CM, 2016. Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-beta mRNA Levels Accurately Predict Treatment Response in Depressed Patients. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM, 2013. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M, 2002. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. The Journal of experimental medicine 196, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Galic Z, Zack JA, 2003. Controlling false-negative errors in microarray differential expression analysis: a PRIM approach. Bioinformatics 19, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC, 2015. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Molecular biology reports 42, 841–851. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP, 2008. Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex 18, 2735–2747. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O, 2012. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50, 1252–1266. [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C, 2017. Erratum: Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature medicine 23, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB, 2007. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64, 327–337. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann JC, 2008. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Nikolsky Y, Bugrim A, Kirillov E, Nikolskaya T, 2007. Pathway mapping tools for analysis of high content data. Methods in molecular biology 356, 319–350. [DOI] [PubMed] [Google Scholar]
- Erdembileg A, Mirsoian A, Enkhmaa B, Zhang W, Beckett LA, Murphy WJ, Berglund LF, 2015. Attenuated age-impact on systemic inflammatory markers in the presence of a metabolic burden. PLoS One 10, e0121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le NA, Feinberg R, Tansey MG, Miller AH, 2018. What does plasma CRP tell us about peripheral and central inflammation in depression? Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Hernandez CR, Miller AH, 2015. Levodopa reverses cytokine-induced reductions in striatal dopamine release. The international journal of neuropsychopharmacology 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016a. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016b. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH, 2013. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JL, Soyombo A, Czysz AH, Jha MK, Carmody TJ, Mason BL, Scherer PE, Trivedi MH, 2018. Adiponectin Moderates Antidepressant Treatment Outcome in the Combining Medications to Enhance Depression Outcomes Randomized Clinical Trial. Pers Med Psychiatry 9–10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, Bluher M, Czech MP, Tabas I, 2018. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 555, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, Treadway MT, Felger JC, Miller AH, 2016a. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain, behavior, and immunity 56, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ, 2016b. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular psychiatry 21, 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsholm O, Rantapaa-Dahlqvist S, Forsgren S, 2005. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther 7, R416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L, 2006. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nature biotechnology 24, 1162–1169. [DOI] [PubMed] [Google Scholar]
- Hamer JA, Testani D, Mansur RB, Lee Y, Subramaniapillai M, McIntyre RS, 2019. Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Experimental neurology 315, 1–8. [DOI] [PubMed] [Google Scholar]
- Han TJ, Felger JC, Lee A, Mister D, Miller AH, Torres MA, 2016. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 25, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, Felger JC, Miller AH, 2018a. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC, Miller AH, 2018b. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, Hu XP, Miller AH, 2014. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH, 2020. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 45, 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD, 2016. A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biological psychiatry 80, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H, 2012. Blockade of the nuclear factor-kappaB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 125, 1122–1133. [DOI] [PubMed] [Google Scholar]
- Heberlein KA, Hu X, 2004. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 51, 212–216. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS, 2002. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336. [DOI] [PubMed] [Google Scholar]
- Hopkins MH, Flanders WD, Bostick RM, 2012. Associations of circulating inflammatory biomarkers with risk factors for colorectal cancer in colorectal adenoma patients. Biomark Insights 7, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM, 1994. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America 91, 4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieronymaki E, Daskalaki MG, Lyroni K, Tsatsanis C, 2019a. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front Immunol 10, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieronymaki E, Theodorakis EM, Lyroni K, Vergadi E, Lagoudaki E, Al-Qahtani A, Aznaourova M, Neofotistou-Themeli E, Eliopoulos AG, Vaporidi K, Tsatsanis C, 2019b. Insulin Resistance in Macrophages Alters Their Metabolism and Promotes an M2-Like Phenotype. Journal of immunology 202, 1786–1797. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Arora J, Giannini C, Kubat J, Malik S, Van Name MA, Santoro N, Savoye M, Duran EJ, Pierpont B, Cline G, Constable RT, Sherwin RS, Caprio S, 2016. Altered Brain Response to Drinking Glucose and Fructose in Obese Adolescents. Diabetes 65, 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Wakhlu S, Dronamraju N, Minhajuddin A, Greer TL, Trivedi MH, 2018. Validating pre-treatment body mass index as moderator of antidepressant treatment outcomes: Findings from CO-MED trial. Journal of affective disorders 234, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K, 2009. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Kumar S, 2005. Role of resistin in obesity, insulin resistance and Type II diabetes. Clinical science (London, England : 1979) 109, 243–256. [DOI] [PubMed] [Google Scholar]
- Lacourt TE, Vichaya EG, Chiu GS, Dantzer R, Heijnen CJ, 2018. The High Costs of Low-Grade Inflammation: Persistent Fatigue as a Consequence of Reduced Cellular-Energy Availability and Non-adaptive Energy Expenditure. Front Behav Neurosci 12, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NA, Innis-Whitehouse W, Li X, Bakker-Arkema R, Black D, Brown WV, 2000. Lipid and apolipoprotein levels and distribution in patients with hypertriglyceridemia: effect of triglyceride reductions with atorvastatin. Metabolism 49, 167–177. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Herzig KH, Viinamaki H, Hintikka J, 2010. Serum adiponectin and resistin levels in major depressive disorder. Acta psychiatrica Scandinavica 121, 209–215. [DOI] [PubMed] [Google Scholar]
- Li G, Zhao M, Cheng X, Zhao T, Feng Z, Zhao Y, Fan M, Zhu L, 2019a. FG-4592 Improves Depressive-Like Behaviors through HIF-1-Mediated Neurogenesis and Synapse Plasticity in Rats. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shelton RC, Chassan RA, Hammond JC, Gower BA, Garvey TW, 2016. Impact of Major Depressive Disorder on Prediabetes by Impairing Insulin Sensitivity. J Diabetes Metab 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lei K, Coles CD, Lynch ME, Hu X, 2019b. Longitudinal changes of amygdala functional connectivity in adolescents prenatally exposed to cocaine. Drug and alcohol dependence 200, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Prudente CN, Stilla R, Sathian K, Jinnah HA, Hu X, 2017. Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Human brain mapping 38, 4098–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC, 2017. NF-kappaB signaling in inflammation. Signal transduction and targeted therapy 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG, 2010. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Ikeda Y, Murakami M, Nakagawa Y, Tsuji A, Kitagishi Y, 2019. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases (Basel, Switzerland) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros GC, Rush AJ, Jha M, Carmody T, Furman JL, Czysz AH, Trombello JM, Cooper CM, Trivedi MH, 2020. Positive and negative valence systems in major depression have distinct clinical features, response to antidepressants, and relationships with immunomarkers. Depression and anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Raison CL, Woolwine BJ, Haroon E, Binder EB, Miller AH, Felger JC, 2013. Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav Immun 31, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC, 2018a. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC, 2018b. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, behavior, and immunity 73, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, Felger JC, 2020a. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, Felger JC, 2020b. Inflammation, Reward Circuitry and Symptoms of Anhedonia and PTSD in Trauma-Exposed Womenn. Social Cognitive and Affective Neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain, behavior, and immunity 53, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Wolkowitz OM, Schonemann MD, Epel ES, Rosser R, Burke HB, Mahan L, Reus VI, Stamatiou D, Liew CC, Cole SW, 2016. Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment. Transl Psychiatry 6, e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Shalowitz MU, Story RE, Leigh AKK, Ham P, Arevalo JMG, Cole SW, 2018. Divergent transcriptional profiles in pediatric asthma patients of low and high socioeconomic status. Pediatric pulmonology 53, 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Vargas J, Hoffmann A, 2016. Signaling via the NFkappaB system. Wiley interdisciplinary reviews. Systems biology and medicine 8, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Tan KM, Inagaki TK, Muscatell KA, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI, 2019. Sex Differences in the Relationship Between Inflammation and Reward Sensitivity: A Randomized Controlled Trial of Endotoxin. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C, Zonca V, Riva MA, Cattaneo A, 2018. Blood biomarkers and treatment response in major depression. Expert Rev Mol Diagn 18, 513–529. [DOI] [PubMed] [Google Scholar]
- Moretti M, Bennett J, Tornatore L, Thotakura AK, Franzoso G, 2012. Cancer: NF-kappaB regulates energy metabolism. The international journal of biochemistry & cell biology 44, 2238–2243. [DOI] [PubMed] [Google Scholar]
- Mugler JP 3rd, Brookeman JR, 1990. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 15, 152–157. [DOI] [PubMed] [Google Scholar]
- Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H, 2019. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. International journal of molecular sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Kishton RJ, Rathmell J, 2016. A guide to immunometabolism for immunologists. Nature reviews. Immunology 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes DJ, Patenaude B, Schatzberg AF, Etkin A, 2015. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry 77, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, Cearns M, Clark S, Toben C, Grotegerd D, Heindel W, Kugel H, Teuber A, Minnerup H, Berger K, Dannlowski U, Baune BT, 2019. Large-scale evidence for an association between low-grade peripheral inflammation and brain structural alterations in major depression in the BiDirect study. Journal of psychiatry & neuroscience : JPN 44, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS, 2013. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA : the journal of the American Medical Association 309, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R, 2005. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69, 29–35. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Lobenhofer EK, Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, Fang H, Kawasaki ES, Hager J, Tikhonova IR, Walker SJ, Zhang L, Hurban P, de Longueville F, Fuscoe JC, Tong W, Shi L, Wolfinger RD, 2006. Performance comparison of one-color and two-color platforms within the MicroArray Quality Control (MAQC) project. Nature biotechnology 24, 1140–1150. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH, 2009. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biological psychiatry 65, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, 2003. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107, 363–369. [DOI] [PubMed] [Google Scholar]
- Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, Santinelli S, Saunders T, Hebeis B, Killeen N, Okkenhaug K, Turner M, 2010. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. Journal of immunology 185, 4042–4052. [DOI] [PubMed] [Google Scholar]
- Ross KM, Carroll JE, Dunkel Schetter C, Hobel C, Cole SW, 2019. Pro-inflammatory immune cell gene expression during the third trimester of pregnancy is associated with shorter gestational length and lower birthweight. Am J Reprod Immunol 82, e13190. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW, 2009. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. NeuroImage 44, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC, 2012. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC, 2013. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain, behavior, and immunity 31, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS, 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation 116, 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Yamagata H, Uchida S, Otsuki K, Hobara T, Higuchi F, Abe N, Watanabe Y, 2013. The alteration of hypoxia inducible factor-1 (HIF-1) and its target genes in mood disorder patients. Progress in neuro-psychopharmacology & biological psychiatry 43, 222–229. [DOI] [PubMed] [Google Scholar]
- Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ, 2005. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochemical and biophysical research communications 334, 1092–1101. [DOI] [PubMed] [Google Scholar]
- Singh B, Saxena A, 2010. Surrogate markers of insulin resistance: A review. World J Diabetes 1, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA, 2001. The hormone resistin links obesity to diabetes. Nature 409, 307–312. [DOI] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, Carr KD, Rice ME, 2015. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nature communications 6, 8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Ter Horst KW, Lammers NM, Trinko R, Opland DM, Figee M, Ackermans MT, Booij J, van den Munckhof P, Schuurman PR, Fliers E, Denys D, DiLeone RJ, la Fleur SE, Serlie MJ, 2018. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Science translational medicine 10. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR, 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews. Immunology 6, 772–783. [DOI] [PubMed] [Google Scholar]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH, Kohn JN, Barsevick AM, Long Q, Miller AH, 2013. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer 119, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Cooper JA, Miller AH, 2019. Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends in cognitive sciences 23, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Pizzagalli DA, 2014. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biology of mood & anxiety disorders 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkola O, 2002. Resistin - a mediator of obesity-associated insulin resistance or an innocent bystander? European journal of endocrinology 147, 571–574. [DOI] [PubMed] [Google Scholar]
- van Opstal AM, Hafkemeijer A, van den Berg-Huysmans AA, Hoeksma M, Blonk C, Pijl H, Rombouts S, van der Grond J, 2018. Brain activity and connectivity changes in response to glucose ingestion. Nutritional neuroscience, 1–8. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, van Reedt Dortland AK, Schoevers RA, Giltay EJ, de Jonge P, Penninx BW, 2014. Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 39, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA, 2009. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage 46, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr., 2003. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Beitler JJ, Higgins KA, Conneely K, Dwivedi B, Felger J, Wommack EC, Shin DM, Saba NF, Ong LY, Kowalski J, Bruner DW, Miller AH, 2016. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain, behavior, and immunity 52, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Xu X, Chen G, Mehta ND, Haroon E, Miller AH, Luo Y, Li Z, Felger JC, 2019. Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain, behavior, and immunity 80, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Muller CE, Miguel NS, Correa M, Salamone JD, 2016. Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology 233, 3575–3586. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM, 2013. Inflammation and depression. Current topics in behavioral neurosciences 14, 135–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.