Abstract
Risk factors for COVID-19 patients with poorer outcomes include pre-existing conditions: obesity, type 2 diabetes mellitus, cardiovascular disease (CVD), heart failure, hypertension, low oxygen saturation capacity, cancer, elevated: ferritin, C reactive protein (CRP) and D-dimer. A common denominator, hyperinsulinaemia, provides a plausible mechanism of action, underlying CVD, hypertension and strokes, all conditions typified with thrombi. The underlying science provides a theoretical management algorithm for the frontline practitioners.
Vitamin D activation requires magnesium. Hyperinsulinaemia promotes: magnesium depletion via increased renal excretion, reduced intracellular levels, lowers vitamin D status via sequestration into adipocytes and hydroxylation activation inhibition. Hyperinsulinaemia mediates thrombi development via: fibrinolysis inhibition, anticoagulation production dysregulation, increasing reactive oxygen species, decreased antioxidant capacity via nicotinamide adenine dinucleotide depletion, haem oxidation and catabolism, producing carbon monoxide, increasing deep vein thrombosis risk and pulmonary emboli. Increased haem-synthesis demand upregulates carbon dioxide production, decreasing oxygen saturation capacity. Hyperinsulinaemia decreases cholesterol sulfurylation to cholesterol sulfate, as low vitamin D regulation due to magnesium depletion and/or vitamin D sequestration and/or diminished activation capacity decreases sulfotransferase enzyme SULT2B1b activity, consequently decreasing plasma membrane negative charge between red blood cells, platelets and endothelial cells, thus increasing agglutination and thrombosis.
Patients with COVID-19 admitted with hyperglycaemia and/or hyperinsulinaemia should be placed on a restricted refined carbohydrate diet, with limited use of intravenous dextrose solutions. Degree/level of restriction is determined by serial testing of blood glucose, insulin and ketones. Supplemental magnesium, vitamin D and zinc should be administered. By implementing refined carbohydrate restriction, three primary risk factors, hyperinsulinaemia, hyperglycaemia and hypertension, that increase inflammation, coagulation and thrombosis risk are rapidly managed.
Keywords: deep vein thrombosis, venous thromboembol, cytokines, inflammation, oxidative stress
Introduction
Individuals most at risk of poorer outcomes from SARS-CoV-2 infection causing COVID-19 include those ≥65 years of age and/or those with pre-existing conditions such as: obesity, type 2 diabetes mellitus (T2DM) or elevated D-dimer1 2 and black, Asian and minority ethnic (BAME) individuals.1 3 A common denominator of these presentations, hyperinsulinaemia, has an explanation by the underlying science that provides the theory for our proposed management algorithm for frontline practitioners.
There is a clear correlation between increasing age and increasing prevalence of metabolic disease including hypertension, stroke, T2DM, CVD and cancer occurrences. Yet this correlation is not causation. Those conditions, traditionally known as ‘diseases of ageing’, are occurring at higher rates in younger population groups.2 4 Chronic hyperinsulinaemia provides a plausible mechanism of action underlying the increase in these morbidities.5 This mechanism may also help to explain why these individuals have poorer outcomes associated with SARS-CoV-2 infections.
Hyperglycaemia increases secretion of proinflammatory cytokine interleukin 6 (IL-6) and blood coagulation via increasing hepatic clotting factors. Conversely, hyperinsulinaemia disturbs fibrinolysis by elevating plasminogen activator inhibitor type 1 (PAI-1).6 7 Increased thrombi/emboli have been detected in postmortem findings in COVID-19 cases.8–12 In fact, pulmonary thrombi may be the cause of oxygen desaturation and acute respiratory distress in many COVID-19 cases. Thus, strategies that can reduce the risk of disseminated intravascular coagulopathy may improve oxygenation and outcomes in COVID-19.13 14
Underlying science for rationale of proposed clinical management
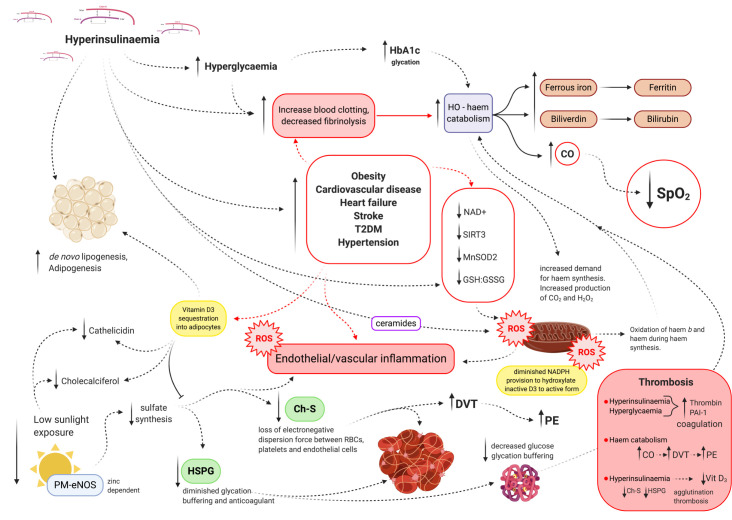
Hyperglycaemia increases haemoglobin glycation damage.15 16 A study published in Diabetes Research and Clinical Practice analysed 132 patients with COVID-19, classified into three groups based on haemoglobin A1c (HbA1c) levels; results found a significant linear negative correlation between haemoglobin oxygen saturation (SaO2)and HbA1c, p=0.01.16 Hyperinsulinaemia inhibits beta-oxidation and ketolysis, thereby ‘enforcing’ cellular ATP generation from glucose substrate.17 Glucose oxidation consumes four nicotinamide adenine dinucleotide (NAD+) to produce two acetyl moieties, whereas beta-oxidation consumes two, ketolysis one and acetoacetate none (online supplemental appendix A); consequently, glucose oxidation depletes the NAD+ intracellular pool more than the other three substrates combined, thereby decreasing NAD+ availability for mitochondrial deacetylase sirtuin 3 (SIRT3) activity.18 19 NAD+ dependent SIRT3 activates manganese superoxide dismutase 2 (MnSOD2) via acetylation of lysine residue 68 (K68) and increases the production of NADPH via isocitrate dehydrogenase (IDH2) to drive the reduction of oxidised glutathione (GSSG) to reduced glutathione (GSH). Coupled with the glucose fuelling effect on the cellular redox system, insulin increases mitochondrial production of reactive oxygen species (ROS) via generation of ceramides.20 Hyperglycaemia and hyperinsulinaemia are the twin blades of dysregulated ROS production and management (figure 1).
Figure 1.
Schematic representation of the role of hyperinsulinaemia in endothelial/vascular inflammation, red blood cell (RBC) and platelet coagulation, sequestration and/or inhibition of vitamin D activation and its downstream consequences, such as decreased cholesterol sulfate (Ch-S), heparan sulfate proteoglycans (HSPG) and cathelicidin synthesis. Carbon dioxide (CO2), carbon monoxide (CO), deep vein thrombosis (DVT), endothelial nitric oxide synthase (eNOS), reduced glutathione (GSH), oxidised glutathione (GSSG), haemoglobin A1c (HbA1c), haem-oxygenase (HO), manganese superoxide dismutase 2 (MnSOD2), nicotinamide adenine dinucleotide (NAD+), plasma membrane (PM), plasminogen activator inhibitor type 1 (PAI-1), pulmonary embolism (PE), reactive oxygen species (ROS), oxygen saturation (SpO2), sirtuin 3 (SIRT3) and type 2 diabetes mellitus (T2DM).
openhrt-2020-001356supp001.pdf (5.9MB, pdf)
The haem part of haemoglobin is synthesised in mitochondria. As a result of increased haemoglobin glycation and intracellular haem oxidative damage, an upregulated demand for synthesis of new haem to keep up the replenishment of damaged haem ensues. This may contribute to respiration independent increased carbon dioxide levels, as extramitochondrial haem synthesis step 5, produces four carbon dioxide molecules via the decarboxylation of uroporphyrinogen III to coproporphyrinogen III, further taxing the external respiration system. In addition, step 7 of haem synthesis generates the production of three hydrogen peroxide (H2O2) ROS molecules. Thus, increased haem damage that would necessitate an increased upregulation in haem synthesis may place further burden on the intracellular redox system. To compound the problem, mitochondrial electron transport chain (ETC) complexes, complex II and III require haem b (protoheme IX). Hyperglycaemia and hyperinsulinaemia increased intracellular ROS production coupled with diminished mitochondrial stress management capacity, increases mitochondrial oxidative phosphorylation (mt-OxPhos) haem oxidation, reducing mt-OxPhos capacity.21
The breakdown of damaged haem activates signals for the synthesis of new haem. Haem-oxygenase (HO) induces hepatic aminolevulinic acid synthase (ALAS1) (enzyme in the first step of haem synthesis) activity. Haem is catabolised/degraded by HO (gene Hmox1/Hmox2), producing ferrous iron, biliverdin and carbon monoxide, resulting in increased plasma ferritin and bilirubin. These markers have been shown to be significantly elevated in COVID-19 patients with poorer outcomes.22
Increased haem breakdown by HO produces endogenous carbon monoxide, which has a higher binding affinity with haemoglobin compared with oxygen. This would result in a decreased oxygen saturation capacity. Deep vein thrombosis occurrence increases significantly with elevated carbon monoxide concentrations, increasing the risk of pulmonary emboli (PE) and acute coronary syndrome.23 24 Hyperinsulinaemia drives the pathogenesis of obesity, CVD, T2DM, hypertension, increased haem oxidation, haem breakdown, endogenous carbon monoxide production and resultant increased thrombi risk. Furthermore, D-dimer found to be markedly elevated in patients with COVID-19 is a direct marker for fibrinolytic and coagulation activity.25 26 Patients with COVID-19 who have a high risk of venous thromboembolisms suffer poorer outcomes.22 Measuring carboxyhaemoglobin (COHb or HbCO) to assess carbon monoxide levels in COVID-19 positive patients is warranted and may provide further insight.
Vitamin D status has garnered great interest and debate with regards to risk/prevention of infection and prognosis in those with COVID-19.1 27 28 Evidence supports the argument that good levels of vitamin D status lowers the risk of contracting a respiratory infectious pathogen,29 possibly due to vitamin D induced increases in airway surface liquid epithelial production of antimicrobial and immunomodulatory host defence peptide, cathelicidin.30–32
In a retrospective study of 107 patients in Switzerland, results showed 25-hydroxycholecalciferol D-calcidiol (25OHD, inactive D3) levels were significantly lower in patients that had a positive PCR for SARS-CoV-2, median value 11.1 ng/mL versus COVID-19 negative patients, who had significantly higher 25OHD levels at 24.6 ng/mL, p=0.004. This study indicates vitamin D’s role in association with risk of infection, as opposed to disease severity and/or mortality, supporting an antiviral role for vitamin D3.33 Furthermore, a study published in the Irish Medical Journal found increased rates of vitamin D3 deficiency in lower latitude countries such as Spain and Italy, despite being typically sunnier. The authors attribute this to fortification and supplementation practices in their more northern European neighbours, as well as darker skin pigmentation and sun avoidance in southern warmer climate inhabitants.34 Epidemic acute respiratory infections result from a lack of sunlight exposure-generated vitamin D during winter and early spring; this most likely includes viral respiratory infection COVID-19.35 Cannell et al35 demonstrated that groups low in vitamin D levels, including: obese, elderly, hyperinsulinaemic, dark skin and those with chronic health conditions, required 5000 IU of vitamin D each day in order to obtain 125 nmol/L (50 ng/mL) plasma levels of 25OHD that appears to be protective against viral respiratory infection and sequalae. Further investigations into the relationship between vitamin D levels, age and COVID-19 outcomes would be valuable.
A 2017 meta-analysis of 25 randomised controlled trials (RCTs) published in The BMJ, studying 11 000 participants given vitamin D or placebo, concluded that vitamin D supplementation is safe and protects against acute respiratory tract infections, where the greatest benefit was seen in those most deficient and benefits were also greatest in subjects taking vitamin D daily.36 The study highlighted that only four people who are deficient in vitamin D need to be treated to prevent one case of acute infection. Additionally, critical care research has demonstrated the efficacy and importance of vitamin D contribution to survival in intensive care unit (ICU) patients with acute respiratory distress syndrome.37 Vitamin D operates by several mechanisms that are critical in immune defence, those include: maintenance of tight junctions, promotes the production of antimicrobial peptides cathelicidin and defensins in airway epithelia and macrophages31 and moderates the inflammatory response.38 The hotly debated question is: is a low vitamin D status a marker or maker of poor health?
A low vitamin D status is associated with hyperinsulinaemia’s pathologies (obesity, T2DM, CVD and metabolic cancers).39 Insulin mediates de novo lipogenesis and adipogenesis.40 Hyperinsulinaemia sequesters lipophilic vitamin D3 into adipocytes.41 Insulin stimulates bone resorption and calcium (Ca2+) and phosphate release. Elevated plasma Ca2+ and phosphate inhibits renal enzymatic 25OHD-1α-hydroxylation (CYP27B1) activation of inactive 25OHD-calcidiol to biologically active 1,25(OH)2D-calcitriol.42 CYP27B1 is a mitochondrial cytochrome P450 hydroxylase situated on the matrix side of the inner mitochondrial membrane. CYP27B1 function is dependent on OxPhos NADPH production and healthy robust mitochondrial electron transport machinery.
Hyperglycaemia and hyperinsulinaemia both increase mitochondrial ROS (mtROS) production while decreasing ROS management capacity via depletion of NAD+, resulting in diminished MnSOD2 and a decreased GSH:GSSG ratio.19 43–47 A decreased ability to hydroxylate and thus activate 25OHD-calcidiol to its active form 1,25(OH)2D-calcitriol, due to poor ETC health and diminished NADPH generation, may complicate research findings in plasma measurements that typically measure total 25OHD-calcidiol. Additionally, it does not take into account vitamin D binding protein bound 25OHD-calcidiol. Furthermore, some hydroxylation of inactive to active vitamin D occurs intracellularly within other tissues and may be inhibited by excessive mitochondrial ROS and NAD+ glucose metabolism depletion. This aspect of vitamin D metabolism may not be picked up with blood plasma measurements, where plasma total inactive 25OHD is the typical form of measure.42 Interestingly, in a year-long weight loss intervention study, 56 obese (body mass index >30 kg/m2) participants were randomised to a very low carbohydrate ketogenic diet (VLCKD) or a standard hypocaloric Mediterranean diet (SHMD). Both groups had significant increases in their serum 25OHD-calcidiol status with weight loss, as measured by chemiluminescence. However, the VLCKD group had a greater significant increase relative to the SHMD, from 18.4 to 29.3 ng/mL, p<0.0001 versus 17.5 to 21.3 ng/mL, p=0.067, as well as decreases in C reactive protein.48 These results indicate the role of dietary macronutrient distribution on insulin secretion stimulus and its consequential effect on mitochondrial vitamin D hydroxylation activity and possible inflammation mediated depletion (usage) of vitamin D.
Vitamin D may be created in the skin via exposure to ultraviolet B (UVB) radiation from sunlight and consumed in the diet. The action spectrum for vitamin D generation is UVB 280–320 nm. The best time of sun exposure for optimal vitamin D generation from sunlight, at minimal risk of cutaneous malignant melanoma, is noon.49 Other less investigated aspects of the role of vitamin D, sun exposure and blood coagulability may play a crucial role in the increased risk of poorer outcomes seen in COVID-19 high-risk individuals, whose risk factors are arguably markers of hyperinsulinaemia. Years of hyperinsulinaemia that would manifest overt pathologies such as obesity, CVD, hypertension and cancer would come with an already heavy-risk burden list, which includes: increased haemoglobin glycation damage, intracellular haem-oxidation with reduced antioxidative capacity, increased haem-oxygenase haem catabolism thus producing increased endogenous carbon monoxide production, leading to increased risk of DVT and subsequent PE and decreased mitochondrial vitamin D hydroxylase activation.
Sunlight exposure driven photo-catalyses has potential effects on other less investigated roles in human health. One is in aiding in production of cholesterol sulfate (Ch-S). The majority of Ch-S is synthesised in the epidermis and supplied to the bloodstream. Ch-S constitutes the majority of blood sterol sulfates, having an ionic negative charge that imparts its amphiphilic property, enabling water solubility and free movement through intracellular cytoplasm and extracellular plasma. Red blood cells (RBCs) and platelets produce Ch-S that aids in maintaining their extracellular side plasma membrane negative charge, thus preventing thrombi and agglutination via maintaining electrorepulsion-driven dispersion.50 Endothelial cells also synthesise Ch-S; the enzyme endothelial nitric oxide synthase (eNOS) is traditionally thought to mediate synthesis of nitric oxide (NO); however, it has been established that when eNOS is membrane bound, it is no longer able to synthesise NO.51–54 Membrane-bound eNOS lacks association with intracellular calmodulin binding; this results in a closed conformation of the heterodimer enzyme. The closed conformation of eNOS has the potential to simultaneously transfer two electrons versus the open conformation that transfers one electron. Extracellular exclusion zone water is sensitive to sunlight’s infrared 270 nm spectrum. At this frequency, sunlight exposure elevates electrons to a higher excitation level, facilitating activation of the water involved in the oxidation of thiosulfate to sulfate, the first step in producing the sulfate required for Ch-S synthesis.55 56 Impaired zinc utilisation and deficiency is associated with T2DM and CVD.57 The catalytic activity of eNOS is zinc dependent, a deficiency in zinc would result in inhibition of eNOS sulfate synthesis and inhibition of eNOS may result in increased clotting.58
Following eNOS sulfate synthesis, sulfotransferase enzyme SULT2B1b catalyses cholesterol sulfurylation, producing Ch-S. Activated vitamin 1,25(OH)2D-calcitriol induces SULT2B1b expression and activity.59 60 Thus, completing a circle, where hyperinsulinaemia decreases vitamin D bioavailability, hydroxylation activation and consequently decreasing SULT2B1b sulfurylation of sulfate to cholesterol, thereby decreasing the negative charge that aids in dispersion around RBCs, platelets and endothelial cells, thus increasing agglutination and thrombosis. Furthermore, sunlight exposure eNOS sulfate production provides for increased sulfate availability for heparan sulfate proteoglycan (HSPG) synthesis; HSPGs are robust anticoagulants and buffer glycation damage.61 Ch-S is necessary for RBCs to be able to deform in order to travel through tight vascular spaces while allowing the trafficking of cholesterol between cells to HDL-A1. In addition, sulfate provides a non-haem method of oxygen delivery to oxidative phosphorylation dependent cells. Global ‘lockdown strategies’ may have inadvertently reduced incidental sun exposure and consequently lowered vitamin D and sulfate synthesis.
Hyperinsulinaemia increases mitochondrial ROS, with consequences that are far reaching. Healthy robust mitochondria regulate intracellular magnesium (Mg2+) pools and modulate Mg2+ concentrations between the cytosol and mitochondrial compartments.62 Hyperinsulinaemia causes increased renal excretion of magnesium, and insulin resistance (IR) reduces intracellular magnesium levels.63 An Mg2+ deficiency (MgD) may exist in absence of hypomagnesaemia.64 MgD has been implicated in perturbations of pancreatic beta-cell function, hyperinsulinaemia, IR and CVD due to its diverse and essential roles in an extensive list of cellular metabolic pathways, not least counting ATP transport, DNA repair capacity and cell viability. Mg2+ is required to transport vitamin D around in the blood, and activation of vitamin D to its active form via hepatic and renal hydroxylation is Mg2+ dependent.65 Clinical manifestations of MgD include, but are not limited to, hyperinsulinaemia’s metabolic pathologies such as T2DM, osteoporosis, vitamin D metabolism disorders, CVD and hyperglycaemia.66 67 There is a strong relationship between MgD and increased oxidative stress. MgD drives decreased expression and activity of antioxidant defence enzymes glutathione peroxidase, SOD and catalase and decreased production of glutathione, further taxing mitochondrial health and subsequent increased ROS production and consequent oxidative damage to proteins, membranes and haem.68 69 MgD increases the production of cytokines: IL-1β, IL-6, tumour necrosis factor α and PAI-1.64 70 Hyperinsulinaemia increases production of PAI-1, coupled with MgD-induced increased production of PAI-1 that inhibits fibrinolysis, together they compound coagulation risk. Lower serum magnesium is associated with increased thrombotic risk and slowed fibrinolysis.71–73 Moreover, in vivo experiments have shown that magnesium has antithrombotic effects and reduces mortality in induced pulmonary thromboembolism.74
An individual whose system is in a chronic state of hyperinsulinaemia already has increased risk of thrombi/clots. CVD and strokes are typified by thrombi. Thus, the association with lower vitamin D may be both a marker and maker of poor health due to hyperinsulinaemia inducing low vitamin D status/availability, as a result of vitamin D sequestration into adipocytes and prevention of hydroxylation by mitochondrial ROS sensitive 1α−25OHD-hydroxylase. Even with supplementation or adequate sun exposure, a mismatched low vitamin K2 status needed to move Ca2+ into bones would further result in increased plasma Ca2+ inhibition of vit D activation. Hyperinsulinaemia mediates thrombi development via multiple modalities, those include but are likely not limited to: (A) inhibition of fibrinolysis, (B) increased mtROS production with decreased antioxidant capacity, leading to further oxidation of haem, (C) increasing haem breakdown product carbon monoxide that increases thrombosis and decreases oxygen saturation capacity, (D) increased demand for haem synthesis resulting in increased H2O2 and OxPhos independent CO2 production, again further burdening the external respiratory system for removal of CO2 and (E) decreased Ch-S production via sulfurylation by SULT2B1b sulfotransferase due to hyperinsulinaemia’s affect driving low activated vitamin D regulation on SULT2B1b, leading to increased RBC and platelet agglutination and thrombosis.
When systemic health is already on the edge due to hyperinsulinaemia, then additionally challenged with an extra stressor such as COVID-19, it may not be able to handle any more. Individuals already in an excess coagulable state, one more straw added in SARS-CoV-2 may be the straw that breaks the camel’s back.
Rationale for clinical management hypotheses
Given the above theories whereby hyperinsulinaemia aggravates the SARS-CoV-2 disease progress, lifestyle strategies that have been shown to be effective for managing hyperinsulinaemia and/or type 2 diabetes should also theoretically attenuate SARS-CoV-2 disease progression. Glucose restriction should be a primary focus; this leads to a decreased need/requirement for endogenous and/or exogenous insulin, thus targeting and managing two problems in one step.75 76 The importance of managing hyperglycaemia via its, largely dietary, upstream mediator, rather than management of elevated plasma glucose once it has been elevated already, cannot be overemphasised. In an RCT conducted by the ACCORD group, 10 251 patients with a mean age of 63 years and median HbA1c of 8.1% were randomised to one of two groups, one to target lowering their HbA1c to 6%, via receiving intensive therapy (insulin), and the other group to target an HbA1c of 7.0%–7.9% following standard therapy. The primary outcomes measured were non-fatal stroke, non-fatal myocardial infarction and CVD death. The intervention group receiving intensive therapy (insulin) to normalise their HbA1c had higher mortality, to the extent that after 3.5 years, this intervention was stopped. Their HbA1c was successfully lowered via the intensive therapy; however, there was no reduction in major CVD events while there was an increase in deaths.15 Implementation in management of hyperglycaemia should be targeted at what causes blood glucose to raise, its upstream effectors, the vast majority of which is from dietary sources. By restricting glucose (consideration to be given with regards to intravenous glucose), this subsequently lowers insulin secretion.75
Methods may include implementing a well-formulated ketogenic diet (WFKD), low carbohydrate healthy fat diet (LCHF), low glycaemic load (GL) diet and dietary carbohydrate restriction in as much a practicable way as possible approach. Choice in approach may vary for a myriad of reasons; while nutritional ketosis is demonstrated to be safe, it is not necessarily the primary focus in this instance. Whereas lowering hyperglycaemia and hyperinsulinaemia are at the forefront of the pathophysiological development of increasing risk factors for poorer outcomes in at risk individuals.
Implementing a method that helps get patients into a ‘fasting mimicking state’ using any of these fasting mimicking diets, which has a phenotype of healthy blood glucose (3.5–6.0 mmol/L), insulin (<200 pmol/L) and ketones (<7.0 mmol/L in healthy individuals, ketones at ≤3.0 mmol/L is a respectable concentration in metabolically unhealthy patients),77–79 where there is no drive to calorie restrict. Again bearing in mind, during acute care settings, the focus is on lowering both glucose and insulin through carbohydrate restriction, whereas the focus is not on achieving any particular ketosis goal, aside from ensuring avoidance of ketoacidosis—ketones ≥7.0 mmol/L78 and/or presence of ketones plus lowered serum bicarbonate <15–18 mmol/L, with concomitant hyperglycaemia (glucose levels>13.8 mmol/L (250 mg/dL))—the triad of diabetic ketoacidosis.80–82 Monitoring serum bicarbonate provides a sensitivity test for any metabolic acidosis with presence of ketones. Ketoacidosis does not occur unless there are presence of ketones with hyperglycaemia and bicarbonate levels start trending downwards, indicating insulin insufficiency, as seen in patients with type 1 diabetes mellitus (T1DM) who do not produce enough, if any insulin to regulate ketogenesis with hyperglycaemia.
It is important to note, in the case of T2DM, if carbohydrates are ingested, blood glucose will elevate and ketones will lower or be absent due to insulin production in T2DM hyperinsulinaemic individuals, which inhibits ketogenesis. In the case of T1DM, if carbohydrates are ingested and insulin and/or oral hypoglycaemic medications are stopped, these patients may present with both ketosis/ketoacidosis and hyperglycaemia. Although carbohydrate restriction will manage hyperglycaemia (and hyperinsulinaemia in people with T2D), it is never recommended to stop insulin in patients with T1D. However, carbohydrate restriction will likely require the reduction of basal insulin and/or the reduction/cessation of short/rapid acting insulin.
Focus in clinical management in patients with COVID-19 in hospital is to aim to keep them out of progression into the ICU. Carbohydrate restriction, with a maximum of 90 g of carbohydrate per 24 hours, LCHF and WFKD diets implemented in hospital and clinical settings have been shown to be safe and effective.79 83 In addition, parenteral and enteral glucose-restricted feeding have been shown to be safe and effective should these be required.76 84 Furthermore, coupled with remotely monitored deprescribing of medications,83 both safety and efficacy have also been demonstrated in the home setting.79 Based on the current clinical data and plausible mechanisms of action that drive an increased risk of infection, the thrombosis risk factor and possible increased thrombi ‘load’ in at risk individuals, it may be prudent for clinicians to advise stay-at-home at risk and COVID-19 positive patients to implement a carbohydrate restricted diet.
No clear reference range for insulin exists; current practice has been to use insulin concentrations relative to glucose as a proxy for determining IR, which does not account for hyperinsulinaemia. While patients with an HbA1c >40 mmol/L can be presumed to be hyperinsulinaemic by default, a healthy-appearing glucose concentration may be due to perniciously elevated insulin levels (hyperinsulinaemia) regulating blood glucose levels leading to a false negative result.85 If triglycerides are elevated (>1.7 mmol/L), or the patient has other signs of metabolic syndrome, then there should be a higher suspicion of hyperinsulinaemia. Hyperinsulinaemia precedes hyperglycaemia by up to 24 years.86 Insulin regulates lipolysis, fatty acid oxidation and consequently ketogenesis17, and while insulin-dependent T1DM requires exogenous insulin to prevent ketoacidosis where plasma ketones exceed >25.0 mmol/L concurrent with hyperglycaemia,78 a state of hyperinsulinaemia prevents ketogenesis. In healthy people, the presence of ketones below 7.0 mmol/L (absent of hyperglycaemia) have been shown to be safe and even therapeutic.19 87 However, in metabolically unstable patients, especially when ketosis may need to be induced, reducing the upper reference interval to 3.0 mmol/L may be preferable, while ensuring close monitoring of and no trending downwards of serum bicarbonate. Triaging glucose, insulin and ketone body beta-hydroxybutyrate (BHB) concentrations provides a refined ability to ascertain a patient’s physiological insulin concentration, where presence of ketones 0.5–3.0 mmol/L, fasting glucose concentration ≤5.6 mmol/L and insulin assay (if practical) results may provide the valuable information needed to determine the degree of carbohydrate restriction a patient requires. Presence of BHB even at 0.5 mmol/L with fasting glucose concentration ≤5.6 mmol/L is a proxy marker for insulin levels being inside of a range that is likely to be non-pathological for the patient77 and indicates physiological insulin homeostasis range.
Insulin has a 5–15 min pulsatile secretory pattern and an ultradian oscillatory rhythm.88 A single fasting blood sample may miss its peak; when possible, gold standard practice would be to take three samples, 5 min apart or to consider a 2-hour postprandial level, especially if fasting insulin levels are <180 pmol/L.85 In order to determine the degree of carbohydrate restriction and deprescribing of medications a patient may require, multiple blood measurements of glucose, insulin and BHB taken at regular time point intervals through the course of multiple days must be performed. This then enables the determination of an individual patient’s physiological insulin concentration while also addressing the hyperinsulinaemia and hyperglycaemia in the process. If insulin is unable to be measured, then a normal level of blood glucose (3.5–6.0 mmol/L) in combination with physiologically healthy levels of BHB (0.5–3.0 mmol/L) could be considered a proxy for good insulin control. Please note that this may not be accurate if taken within 2 hours of a meal.
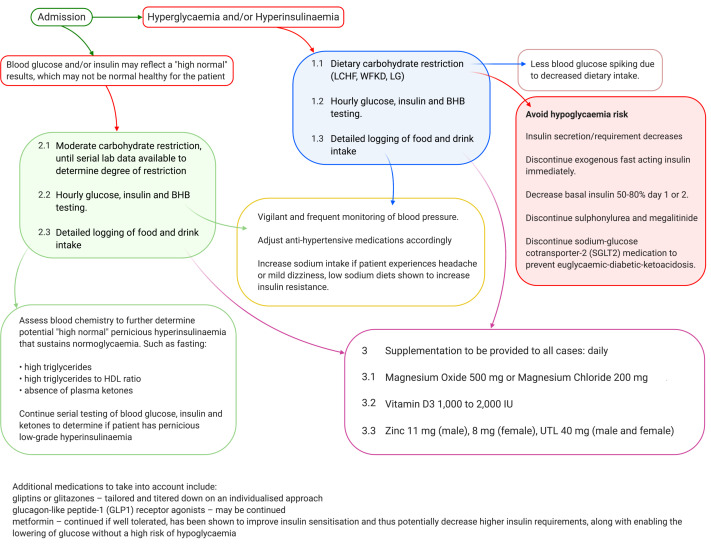
HbA1c, blood glucose, insulin, BHB and bicarbonate should be measured on entrance into hospital or ICU. Elevated HbA1c (>40 mmol/mol, 5.8%) without acidosis (lower bicarbonate <18 mmol/L)80–82 is to be treated as hyperinsulinaemia. When necessary, results are to be used to determine the degree of carbohydrate restriction required for either enteral or parenteral management. Hourly blood sampling to determine (figure 2):
Figure 2.
Clinical management schematic. Beta-hydroxybutyrate (BHB), high-density lipoprotein (HDL), low carbohydrate healthy fat diet (LCHF), low glycaemic load diet (LG), sodium–glucose cotransporter 2 (SGLT2), well-formulated ketogenic diet (WFKD) and upper tolerable limit (UTL).
Blood glucose (with detailed record of meal and drink intakes).
Ketone BHB.
Insulin if feasible (ideally obtain an average from three samples taken over 15 min).
Depending on the initial and subsequent postprandial levels, this will inform how frequently measurements should be made.
Blood glucose is expected to respond first to the carbohydrate restriction, depending on the degree of restriction, and the degree and duration of IR and hyperinsulinaemia, the insulin levels may take longer to lower.
If control appears to be underway, measurements may be tapered to every 2 hours, then 4 hours or four times per day, depending on the level of control seen.
If measured levels do not appear to be responding to the degree of carbohydrate restriction, then it may be necessary to increase the degree of restriction.
If glucose levels are going up, and there are concerns about any form of metabolic acidosis (including ketoacidosis), close serum bicarbonate monitoring is advised.
Close attention must be paid towards the need for deprescribing medications when implementing a very low carbohydrate WFKD. As determined via non-randomised interventional trials and clinical applications of very low carbohydrate diets in patients with type 2 diabetes, fast-acting insulin, sulfonylurea and megalitinide drugs are stopped immediately on induction of very low carbohydrate restriction in order to prevent sudden hypoglycaemic events. If not completely terminating prescribed basal insulin immediately, a reduction of dosage to between 50% and 80% is required.79 89 Hourly glucose readings enable close monitoring to prevent hypoglycaemia in absence of nutritional ketosis and keto-adaptation, where it has been shown considerably lower glucose values may be well tolerated in individuals who are in nutritional ketosis.90–92 Sodium–glucose cotransporter-2 (SGLT2) inhibitors medication should also be discontinued on start of carbohydrate restriction in order to prevent euglycaemic-diabetic-ketoacidosis.77
Additional medications to take into account include:
Gliptins or glitazones – tailored and titrated down on an individualised approach.
Glucagon-like peptide-1 (GLP1) receptor agonists – may be continued.
Metformin – continued if well tolerated, has been shown to improve insulin sensitisation and thus potentially decrease higher insulin requirements, along with enabling the lowering of glucose without a high risk of hypoglycaemia. If a risk of lactic acidosis presents, discontinue.
Addressing hypertension and antihypertensive medications: refined carbohydrate restriction leads to lowered endogenous and/or exogenous insulin requirements; this results in increased renal sodium secretion and diuresis resulting in hypotension. Vigilant and frequent monitoring of blood pressure to avoid hypotension is vital, with according adjustments to antihypertensive medications. Increased sodium intake and/or monitoring for hyponatraemia may likely be needed if patients experience headaches or light-headedness.79 It is also important to discontinue sodium restriction recommendations when implementing carbohydrate restriction as low sodium diets have been shown to increase MgD and IR, thus potentiating hyperglycaemia.93 94
Metformin, diuretics and proton pump inhibitors (PPI) drugs have been shown to increase incidence of hypomagnesaemia and are commonly prescribed to patients with T2DM, a condition of hyperinsulinaemia. A reduction in gastric acidity production by PPI drugs decrease Mg2+ solubility, thus decreasing absorption potential in the gastrointestinal (GI) tract; metformin causes GI Mg2+ loss, while diuretics and hyperglycaemia decrease renal tubular reabsorption of Mg2+.95–97 Patients implementing very low carbohydrate restriction, where compliance was confirmed by presence of capillary BHB, in a remote setting non-randomised intervention with online medical supervision, were prescribed daily supplementation doses for vitamin D3 and Mg2+ at: 1000–2000 IU vitamin D3, 500 mg magnesium oxide or 200 mg magnesium chloride.79
Other safe and likely beneficial therapies include zinc supplementation (11 mg for men and 8 mg for women, with an upper tolerable limit of 40 mg regardless of sex)98 and high-noon sensible sun exposure (sans sunscreen), especially given these therapies have been demonstrated to be safe. Given the rationale for the clinical management of COVID-19, it would be potentially of benefit for clinicians to advise patients and at-risk individuals who are at home to implement these practices, as prevention and earlier management will be more efficacious.76 79 83 89
Discussion
While it is not clear what concentration of insulin is ideal for an individual patient, in terms of functional concentrations that work in healthy physiological homeostasis as opposed to chronic elevated concentrations that function in pathological adaptive homeostasis, and drives inflammation and pathological cellular outcomes; healthy glucose concentrations at the cost of hyperinsulinaemia comes at a heavy price. Approaches to achieve both healthy glucose regulation and minimal insulin secretion where ‘very little does the job’ should be managed from a top-down approach, where large changes in blood glucose concentrations are primarily driven by dietary sources, resulting in the subsequent need for more insulin, consequently driving a higher baseline level of insulin. Logically restricting dietary carbohydrates results in less extreme oscillations in blood glucose levels and subsequent stimulus and need for insulin. Testing dynamic insulin responses to meals and/or controlled glucose loads need to become the normal practice, where the focus is not specifically on IR, which may be physiological glucose sparing in the case for low carbohydrate adherent individuals but to establish if hyperinsulinaemia exists and further enable fine tuning of what degree of carbohydrate restriction is ideal on an individual bases.
Hyperinsulinaemia drives mtROS production and diminishes cellular antioxidative countermeasures; the effects may be subtle yet profound. Such as decreasing NADPH production leads to a lowered ability to activate inactive 25OHD-calcidiol to its biologically active form, which subsequently affects active 1,25(OH)2D-calcitriol-dependent processes such as sulfurylation activity required to synthesise Ch-S in order to prevent agglutination and thrombosis via providing the electronegative repulsion force around RBCs, platelets and endothelial cells. Furthermore, antioxidant GSH production is dependent on NADPH. Hyperinsulinaemia affects vitamin D bioavailability as a result of sequestration into adipocytes. Vitamin D synthesis, availability and activation functions in decreasing risk of contracting respiratory infections and decreasing coagulation/thrombosis. Hyperinsulinaemia driven increased mtROS production taxes haem synthesis, resulting in less haem availability for haem dependent processes, such as maintaining a healthy ETC and for haemoglobin to carry oxygen, further driving hypoxia and decreased oxygen saturation capacity. Hyperinsulinaemia and hyperglycaemia increases oxidised haem which increases haem catabolism, resulting in chronic increased CO production. Chronic elevated CO levels in turn decrease oxygen saturation capacity and increase risk of DVT leading to increased risk of PE, while long-term lowered oxygen saturation capacity consolidates hypoxia and further mtROS.
Implementing glucose restriction aids to resolve hyperinsulinaemia and hyperglycaemia and their twin effects in poorer outcomes in patients at risk or with COVID-19. Chronic stimulus of insulin secretion results in hyperinsulinaemia and its downstream pathological sequelae. Glucose drives insulin secretion, excessive dietary high GL carbohydrates consumed frequently and chronically drives pathological development of hyperinsulinaemia. Clinical management should be focused on lowering carbohydrate consumption to manage both hyperinsulinaemia and hyperglycaemia, coupled with the lowering and describing of glucose management and antihypertensive medications, such as fast-acting and basal insulin. A decrease in dietary carbohydrate and its subsequent effects on lowering insulin secretion leads to rapid changes in blood pressure, requiring vigilant monitoring with describing of antihypertensive drugs, as carbohydrate restriction and resultant decrease in insulin facilitates return of homeostatic regulation of blood pressure. Thus, through carbohydrate restriction, three primary factors—hyperinsulinaemia, hyperglycaemia and hypertension—that increase inflammation, coagulation and thrombosis risk are rapidly managed.
Limitations
While on admission, blood glucose and/or insulin may reflect a high normal result, this may not be normal for that person. Fasting insulin does not always identify hyperinsulinaemia, a first test for glucose and insulin can appear healthy, as glucose levels sit within the healthy reference range, and insulin not above values seen in non-diabetics. Providing a hypothetical example to demonstrate the point, without any clear reference range for fasting insulin, using a hypothetical fasting insulin reference range of 21–153 pmol/L (3.5–25.5 μU/mL), where 1 μU/mL = 6 pmol/L99: a patient’s first blood test results may show, glucose 5 mmol/L with insulin 144 pmol/L (24 μU/mL), consequently this patient would not be considered hyperinsulinaemic and therefore not triaged to clinical management of carbohydrate restriction in order to decrease risk of coagulation and thrombosis complications of COVID-19. In this example, the patient may be maintaining a healthy glucose concentration via a pernicious chronically higher than ideal insulin concentration for that individual. Whereas a true non-hyperinsulinaemia individual may also have a fasting blood glucose of 5 mmol/L with insulin 24 pmol/L (4 μU/mL), where the maintenance of a healthy fasting glucose is not sustained by a higher basal insulin level but by maintaining a low carbohydrate consumption that maintains healthy glucose levels and does not frequently stimulate insulin secretion. In both instances, glucose and insulin appear healthy. The only way to know if someone is hyperinsulinaemic (excluding those who have overt hyperglycaemia and/or overtly elevated insulin levels) is by postprandial dynamic testing. In our example above, if both patients were reassessed 2 hours postprandially, the first patient’s glucose may have returned to 6.5 mmol/L, while the insulin may be elevated to >1000 pmol/L (166 μU/mL), while the second patient is more likely to have a blood glucose of 5.3 mmol/L and an insulin concentration of 144 pmol/L (24 μU/mL). The limitation of the first readings taken may lead a clinician to think a patient is neither hyperglycaemic nor hyperinsulinaemic, if they only go by the first test and do not perform multiple tests for the first days of admission. Postprandial dynamic testing is required to truly ascertain hyperinsulinaemia, therefore clinicians need to extrapolate backwards after the treatment of carbohydrate restriction to subsequently ascertain whether insulin was chronically elevated in that patient and to determine what is a healthy insulin concentration that is specific for individual patients.
It must also be emphasised that our proposed management strategy is theoretical based on biochemical principles and practical strategies from dietary control of type 2 diabetes. SARS-CoV-2 has placed unprecedented strain on many nation’s health systems but has also highlighted that metabolic health is key to good immunological health. A well-formulated dietary strategy that restricts carbohydrates should be inexpensive to implement, complementary to current treatment strategies and can be considered as part of home-based treatments. Although we may be overcautious with monitoring frequency, additional monitoring is required but may be dependent on the patient’s health status; experienced practitioners will be able to refine our recommendations.
Conclusion
Based on the underlying science, patients with COVID-19 admitted with hyperglycaemia and/or hyperinsulinaemia should be theoretically triaged to a restricted carbohydrate management algorithm either enteral or parenteral. The degree of restriction and the level to be determined by serial testing of blood glucose, insulin and ketones. Intravenous solutions containing dextrose should be limited where possible. Additionally, supplemental vitamin D, magnesium and zinc should be administered.
Acknowledgments
Many thanks to Eleonore de Bizemont for language editing and proof reading the manuscript.
Footnotes
Twitter: @I_mitochondria, @PharmacistCath, @DrAseemMalhotra, @brads.science, @Yvoni_Kyr, @kenbrookler
Contributors: IDC performed the literature search, wrote the original draft and designed the images and reviewed and edited the final manuscript. KHB contributed to writing and developing the clinical management and reviewed and edited the final manuscript. CC guided the diagnostic and drug management and reviewed and edited the final manuscript. JJD contributed to writing and editing and reviewed and edited the final manuscript. AM, BE and YK reviewed and edited the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JJD is Director of Scientific Affairs at Analyze. Invest. Develop. Partner.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: There are no data in this work.
References
- 1.Khunti K, Singh AK, Pareek M, et al. Is ethnicity linked to incidence or outcomes of Covid-19? BMJ 2020;369:m1548. 10.1136/bmj.m1548 [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–9. 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 3.Malhotra A, Kamepalli RK, Bamrah JS. Perspective: poor metabolic health is a major issue for increased COVID-19 mortality in BamE groups. The Physician 2020;6:1–4. 10.38192/1.6.2.4 [DOI] [Google Scholar]
- 4.Araújo J, Cai J, Stevens J. Prevalence of optimal metabolic health in American adults: National health and nutrition examination survey 2009-2016. Metab Syndr Relat Disord 2019;17:46–52. 10.1089/met.2018.0105 [DOI] [PubMed] [Google Scholar]
- 5.Crofts CAP. Hyperinsulinemia: a unifying theory of chronic disease? Diabesity 2015;1:34 10.15562/diabesity.2015.19 [DOI] [Google Scholar]
- 6.Stegenga ME, van der Crabben SN, Levi M, et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006;55:1807–12. 10.2337/db05-1543 [DOI] [PubMed] [Google Scholar]
- 7.Perkins JM, Joy NG, Tate DB, et al. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab 2015;309:E168–76. 10.1152/ajpendo.00064.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:NEJMoa2015432. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1-13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995–2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 2020;7:e362–3. 10.1016/S2352-3026(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, Wang X, Yang P, et al. COVID-19 complicated by acute pulmonary embolism. Radiology 2020;2:e200067 10.1148/ryct.2020200067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020;201:1299–300. 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudkerk M, Büller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for public health of the Netherlands. Radiology 2020;201629:201629. 10.1148/radiol.2020201629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstein HC, Miller ME, et al. , Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract 2020;164:108214 10.1016/j.diabres.2020.108214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 2004;70:309–19. 10.1016/j.plefa.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014;25:42–52. 10.1016/j.tem.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman JC, Verdin E. β-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr 2017;37:51–76. 10.1146/annurev-nutr-071816-064916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen ME, Tippetts TS, Anderson MC, et al. Insulin increases ceramide synthesis in skeletal muscle. J Diabetes Res 2014;2014:1–9. 10.1155/2014/765784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swenson SA, Moore CM, Marcero JR, et al. From synthesis to utilization: the Ins and outs of mitochondrial heme. Cells 2020;9:579. 10.3390/cells9030579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung W-S, Lin C-L, Kao C-H. Carbon monoxide poisoning and risk of deep vein thrombosis and pulmonary embolism: a nationwide retrospective cohort study. J Epidemiol Community Health 2015;69:557–62. 10.1136/jech-2014-205047 [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi A, Mizukami H, Sakamoto N, et al. Endogenous carbon monoxide concentration in blood elevates in acute coronary syndrome of nonsmoker population. Fukushima J Med Sci 2015;61:72–8. 10.5387/fms.2015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwose EU, Richards RS, Jelinek HF, et al. D-Dimer identifies stages in the progression of diabetes mellitus from family history of diabetes to cardiovascular complications. Pathology 2007;39:252–7. 10.1080/00313020701230658 [DOI] [PubMed] [Google Scholar]
- 26.Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol 2019;94:833–9. 10.1002/ajh.25482 [DOI] [PubMed] [Google Scholar]
- 27.Brown R, Sarkar A. Vitamin D Deficiency: A Factor in COVID-19, Progression, Severity and Mortality?-An Urgent Call for Research. MitoFit Preprint Arch 2020;1. [Google Scholar]
- 28.Brown R, Rhein H, Alipio M, et al. Rapid Response: COVID-19 ’ICU’ risk – 20-fold greater in the Vitamin D Deficient. BAME, African Americans, the Older, Institutionalised and Obese, are at greatest risk. Sun and ‘D’-supplementation – Game-changers? Research urgently required. BMJ 2020;369. [Google Scholar]
- 29.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020;12:988 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009;4:1151–65. 10.2217/fmb.09.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas Buonfiglio LG, Cano M, Pezzulo AA, et al. Effect of vitamin D3 on the antimicrobial activity of human airway surface liquid: preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Respir Res 2017;4:e000211. 10.1136/bmjresp-2017-000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Harten RM, van Woudenbergh E, van Dijk A, et al. Cathelicidins: immunomodulatory antimicrobials. Vaccines 2018;6:63. 10.3390/vaccines6030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020;12:1359. 10.3390/nu12051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laird E, Rhodes J, Kenny RA, et al. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J 2020;113:81. [PubMed] [Google Scholar]
- 35.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect 2006;134:1129–40. 10.1017/S0950268806007175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dancer RCA, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015;70:617–24. 10.1136/thoraxjnl-2014-206680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77–98. 10.1007/s00223-012-9619-0 [DOI] [PubMed] [Google Scholar]
- 39.De Pergola G, Nitti A, Bartolomeo N, et al. Possible role of hyperinsulinemia and insulin resistance in lower vitamin D levels in overweight and obese patients. Biomed Res Int 2013;2013:1–6. 10.1155/2013/921348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley RL, Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes 1999;48:272–8. 10.2337/diabetes.48.2.272 [DOI] [PubMed] [Google Scholar]
- 41.Carrelli A, Bucovsky M, Horst R, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res 2017;32:237–42. 10.1002/jbmr.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bikle D. Vitamin D: production, metabolism, and mechanisms of action. MDText.com, Inc, 2017. [Google Scholar]
- 43.Veech RL, Bradshaw PC, Clarke K, et al. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017;69:305–14. 10.1002/iub.1627 [DOI] [PubMed] [Google Scholar]
- 44.Elamin M, Ruskin DN, Masino SA, et al. Ketone-Based metabolic therapy: is increased NAD+ a primary mechanism? Front Mol Neurosci 2017;10:377 10.3389/fnmol.2017.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J, Feng Z, Wang X, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ 2018;25:229–40. 10.1038/cdd.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin L, Ipek Özlem, Beaumont M, et al. Nutritional ketosis increases NAD+/NADH ratio in healthy human brain: an in vivo study by 31P-MRS. Front Nutr 2018;5:62. 10.3389/fnut.2018.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regulation of glucose metabolism by NAD+ and ADP-ribosylation. Cells 2019;8:890 10.3390/cells8080890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perticone M, Maio R, Sciacqua A, et al. Ketogenic diet-induced weight loss is associated with an increase in vitamin D levels in obese adults. Molecules 2019;24:2499. 10.3390/molecules24132499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moan J, Dahlback A, Porojnicu AC. At what time should one go out in the sun? Adv Exp Med Biol 2008;624:86–8. 10.1007/978-0-387-77574-6_7 [DOI] [PubMed] [Google Scholar]
- 50.Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what's it all about? J Lipid Res 2003;44:1268–78. 10.1194/jlr.R300005-JLR200 [DOI] [PubMed] [Google Scholar]
- 51.Marletta MA. Another activation switch for endothelial nitric oxide synthase: why does it have to be so complicated? Trends Biochem Sci 2001;26:519–21. 10.1016/S0968-0004(01)01937-5 [DOI] [PubMed] [Google Scholar]
- 52.Dudzinski DM, Igarashi J, Greif D, et al. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 2006;46:235–76. 10.1146/annurev.pharmtox.44.101802.121844 [DOI] [PubMed] [Google Scholar]
- 53.Rafikov R, Fonseca FV, Kumar S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol 2011;210:271–84. 10.1530/JOE-11-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seneff S, Lauritzen A, Davidson R, et al. Is endothelial nitric oxide synthase a moonlighting protein whose day job is cholesterol sulfate synthesis? implications for cholesterol transport, diabetes and cardiovascular disease. Entropy 2012;14:2492–530. 10.3390/e14122492 [DOI] [Google Scholar]
- 55.Pollack GH, Figueroa X, Zhao Q. Molecules, water, and radiant energy: new clues for the origin of life. Int J Mol Sci 2009;10:1419–29. 10.3390/ijms10041419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voeikov V, Del Giudice E. Water Respiration-The basis of the living state.. Water Journal 2009;1:52–75. [Google Scholar]
- 57.Foster M, Samman S, Zinc SS. Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal 2010;13:1549–73. 10.1089/ars.2010.3111 [DOI] [PubMed] [Google Scholar]
- 58.Yanai H, Javitt NB, Higashi Y, et al. Expression of cholesterol sulfotransferase (SULT2B1b) in human platelets. Circulation 2004;109:92–6. 10.1161/01.CIR.0000108925.95658.8D [DOI] [PubMed] [Google Scholar]
- 59.Seo Y-K, Mirkheshti N, Song CS, et al. Sult2B1B sulfotransferase: induction by vitamin D receptor and reduced expression in prostate cancer. Mol Endocrinol 2013;27:925–39. 10.1210/me.2012-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elias PM, Williams ML, Choi E-H, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis. Biochim Biophys Acta 2014;1841:353–61. 10.1016/j.bbalip.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Agostini AI, Dong J-C, de Vantéry Arrighi C, et al. Human follicular fluid heparan sulfate contains abundant 3-O-sulfated chains with anticoagulant activity. J Biol Chem 2008;283:28115–24. 10.1074/jbc.M805338200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamanaka R, Tabata S, Shindo Y, et al. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci Rep 2016;6:1–12. 10.1038/srep30027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DiNicolantonio JJ, O'Keefe JH, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Heart 2018;5:e000668. 10.1136/openhrt-2017-000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci 2019;20:1351. 10.3390/ijms20061351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc 2018;118:181–9. 10.7556/jaoa.2018.037 [DOI] [PubMed] [Google Scholar]
- 66.de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev 2015;95:1–46. 10.1152/physrev.00012.2014 [DOI] [PubMed] [Google Scholar]
- 67.Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, et al. Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients 2018;10:168. 10.3390/nu10020168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbagallo M, Dominguez LJ, Tagliamonte MR, et al. Effects of glutathione on red blood cell intracellular magnesium. Hypertension 1999;34:76–82. 10.1161/01.HYP.34.1.76 [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94:329–54. 10.1152/physrev.00040.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheltova AA, Kharitonova MV, Iezhitsa IN, et al. Magnesium deficiency and oxidative stress: an update. Biomedicine 2016;6:8–14. 10.7603/s40681-016-0020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Çiçek G, Açıkgoz SK, Yayla Çağrı, et al. Magnesium as a predictor of acute stent thrombosis in patients with ST-segment elevation myocardial infarction who underwent primary angioplasty. Coron Artery Dis 2016;27:47–51. 10.1097/MCA.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 72.Gromova OA, Torshin IY, Kobalava ZD, et al. Deficit of magnesium and states of hypercoagulation: intellectual analysis of data obtained from a sample of patients aged 18-50 years from medical and preventive facilities in Russia. Kardiologiia 2018;58:22–35. 10.18087/cardio.2018.4.10106 [DOI] [PubMed] [Google Scholar]
- 73.Sobczak AIS, Phoenix FA, Pitt SJ, et al. Reduced plasma magnesium levels in type-1 diabetes associate with prothrombotic changes in fibrin clotting and fibrinolysis. Thromb Haemost 2020;120:243–52. 10.1055/s-0039-3402808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheu JR, Hsiao G, Shen MY, et al. Antithrombotic effects of magnesium sulfate in in vivo experiments. Int J Hematol 2003;77:414–9. 10.1007/BF02982655 [DOI] [PubMed] [Google Scholar]
- 75.DiNicolantonio JJ, OKeefe JH. Added sugars drive coronary heart disease via insulin resistance and hyperinsulinaemia: a new paradigm. Open Heart 2017;4:e000729. 10.1136/openhrt-2017-000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly T, Unwin D, Finucane F. Low-Carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. Int J Environ Res Public Health 2020;17:2557. 10.3390/ijerph17072557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Min SH, Oh TJ, Baek S-I, et al. Degree of ketonaemia and its association with insulin resistance after dapagliflozin treatment in type 2 diabetes. Diabetes Metab 2018;44:73–6. 10.1016/j.diabet.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 78.Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013;339:211–4. 10.1126/science.1227166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther 2018;9:583–612. 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gosmanov AR, Nematollahi LR. Diabetic Ketoacidosis - Symptoms, Diagnosis and Treatment. BMJ Best Pract 2020. [Google Scholar]
- 81.Savage MW, Sinclair-Hammersley M, Rayman G, et al. The management of diabetic ketoacidosis in adults 2010.
- 82.Seetho IW, Wilding JPH. The clinical management of diabetes mellitus. Clin Biochem Metab Clin Asp Third Ed 2014:305–32. [Google Scholar]
- 83.Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-Term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year Non-randomized clinical trial. Front Endocrinol 2019;10:348. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dressler A, Haiden N, Trimmel-Schwahofer P, et al. Ketogenic parenteral nutrition in 17 pediatric patients with epilepsy. Epilepsia Open 2018;3:30–9. 10.1002/epi4.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crofts CAP, Schofield G, Wheldon MC, et al. Determining a diagnostic algorithm for hyperinsulinaemia. Journal of Insulin Resistance 2019;4:1–7. 10.4102/jir.v4i1.49 [DOI] [Google Scholar]
- 86.Crofts C, Schofield G, Zinn C, et al. Identifying hyperinsulinaemia in the absence of impaired glucose tolerance: an examination of the Kraft database. Diabetes Res Clin Pract 2016;118:50–7. 10.1016/j.diabres.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 87.Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and Mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab 2018;2018:1–27. 10.1155/2018/5157645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Satin LS, Butler PC, Ha J, et al. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med 2015;42:61–77. 10.1016/j.mam.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murdoch C, Unwin D, Cavan D, et al. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract 2019;69:360–1. 10.3399/bjgp19X704525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cahill GF, Herrera MG, Morgan AP, et al. Hormone-fuel interrelationships during fasting. J Clin Invest 1966;45:1751–69. 10.1172/JCI105481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cahill G, Aoki T. Alternate fuel utilisation in brain. Baltimore: Williams & Wilkins, 1980. [Google Scholar]
- 92.Cahill GF, Veech RL. Ketoacids? good medicine? Trans Am Clin Climatol Assoc 2003;114:149–61. [PMC free article] [PubMed] [Google Scholar]
- 93.Garg R, Williams GH, Hurwitz S, et al. Low-Salt diet increases insulin resistance in healthy subjects. Metabolism 2011;60:965–8. 10.1016/j.metabol.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nishimuta M, Kodama N, Yoshitake Y, et al. Dietary salt (sodium chloride) requirement and adverse effects of salt restriction in humans. J Nutr Sci Vitaminol 2018;64:83–9. 10.3177/jnsv.64.83 [DOI] [PubMed] [Google Scholar]
- 95.Florentin M, Elisaf MS, Heguilen RM. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol 2012;1:151–4. 10.5527/wjn.v1.i6.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters KE, Chubb SAP, Davis WA, et al. The relationship between hypomagnesemia, metformin therapy and cardiovascular disease complicating type 2 diabetes: the Fremantle diabetes study. PLoS One 2013;8:e74355. 10.1371/journal.pone.0074355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chrysant SG. Proton pump inhibitor-induced hypomagnesemia complicated with serious cardiac arrhythmias. Expert Rev Cardiovasc Ther 2019;17:345–51. 10.1080/14779072.2019.1615446 [DOI] [PubMed] [Google Scholar]
- 98.Institute of Medicine (US) Panel on Micronutrients Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press, 2001. [PubMed] [Google Scholar]
- 99.Knopp JL, Holder-Pearson L, Chase JG. Insulin units and conversion factors: a story of truth, Boots, and faster half-truths. J Diabetes Sci Technol 2019;13:597–600. 10.1177/1932296818805074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2020-001356supp001.pdf (5.9MB, pdf)