Abstract
Aging leads to a high burden on society, both medically and economically. Cellular senescence plays an essential role in the initiation of aging and age-related diseases. Recent studies have highlighted the therapeutic value of senescent cell deletion in natural aging and many age-related disorders. However, the therapeutic strategies for manipulating cellular senescence are still at an early stage of development. Among these strategies, therapeutic drugs that target cellular senescence are arguably the most highly anticipated. Many recent studies have demonstrated that a variety of drugs exhibit healthy aging effects. In this review, we summarize different types of drugs promoting healthy aging – such as senolytics, senescence-associated secretory phenotype (SASP) inhibitors, and nutrient signaling regulators – and provide an update on their potential therapeutic merits. Taken together, our review synthesizes recent advancements in the therapeutic potentialities of drugs promoting healthy aging with regard to their clinical implications.
Keywords: advancement, drug, healthy aging, senescence, senolytics
Introduction
The aging population is growing rapidly worldwide, leading to a great challenge for public health and societal economics.1 According to epidemiological data from the World Health Organization (WHO), elderly people (over 60 years) will account for 11–22% of the population by 2050.2 Aging is the highest risk factor for all chronic disorders, such as cardiovascular diseases, stroke, and Alzheimer’s disease, which signifies the need for developing effective healthy aging strategies.3,4 Compared with gene manipulation, therapeutic drugs targeting senescent cells have unique advantages in treatment compliance. However, currently available strategies are mostly at an early research stage. Cellular senescence plays a causative role in lifespan and in multiple diseases associated with aging.5 Cellular senescence is defined as a cell fate in which proliferating or differentiated cells undergo replication arrest and develop into a fibrotic or pro-inflammatory senescence-associated secretory phenotype (SASP).6 Cellular senescence consists of both replicative senescence and non-replicative senescence. Replicative senescence is related to the limited capacity of cellular division, relating to telomerase dysfunction. In repeated cell division, the length of telomeres may gradually shorten, which would trigger stress-induced premature senescence.7 In contrast, non-replicative senescence can be induced by a variety of factors, including DNA damage, inflammation, mitochondrial dysfunction, epigenetic disruption, and strong mitogen signaling or oncogenes. DNA damage, caused by various stress factors such as oxidative stress, ultraviolet or gamma irradiation, and chemotherapeutics, is the main cause of cellular senescence, since it may activate the p53/p21 pathways, and result in permanent cell cycle arrest.8 Unregulated inflammation also plays an important role in the pathogenesis and progression of age-related diseases.9 Besides, senescent cells could also secrete proinflammatory factors,10 which may further aggravate inflammation. Mitochondrial dysfunction may cause reactive oxygen species (ROS) accumulation and promote the formation of superoxide radicals – key players in cellular senescence and accelerated aging.11 Another factor that could induce cellular senescence is epigenetic modification, which includes DNA methylation, histone posttranslational modifications, and noncoding RNAs. Epigenetic alterations play important roles in oxidative stress, persistent inflammation, and autophagy deficiency – all important causes of cellular senescence.12 Besides, strong mitogen signaling or oncogenes can also induce the cellular senescence. The activation of oncogenes can act as a genetic stress and cause irreversible growth arrest, which is thought to be a barrier to malignant transformation because of its suppression effect on cell proliferation.13
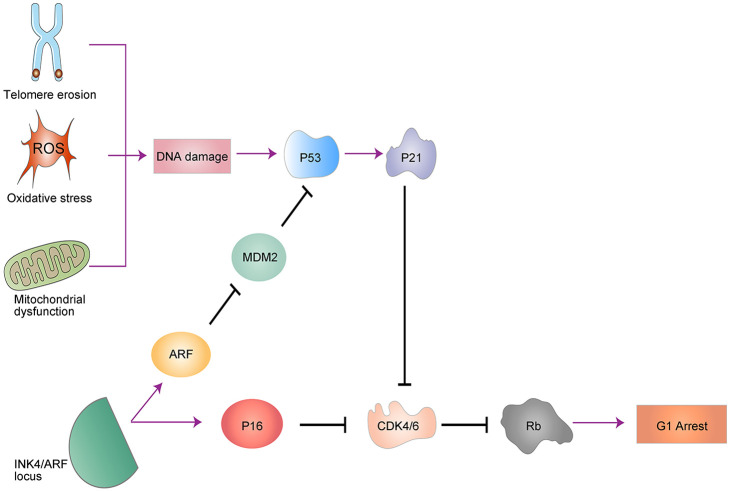
The molecular biomarkers of senescent cells include senescence-associated β-galactosidase (SA-β-gal), p16INK4a, and p53.14,15 p16INK4a/Rb and p53/p21 are the two main pathways that regulate cellular-growth arrest,16 which is the defining characteristic of senescence (Figure 1). DNA damage will increase the deposition of γH2AX and 53BP1 in chromatin, which, in turn, activates kinase cascades, which ultimately results in p53 activation. The activation of p53 induces transcription of the cyclin-dependent kinase inhibitor, p21CIP1, which blocks CDK4/6 activity and consequently causes Rb dephosphorylation and cell cycle arrest. Another related pathway is the p16INK4a/Rb pathway. p16INK4a, p15INK4b and ARF are tumor suppressors residing within the INK4/ARF locus. Furthermore, p16INK4a and p15INK4b are cyclin-dependent kinase inhibitors that inhibit CDK4/6, which also cause Rb dephosphorylation and cell cycle arrest. In addition, ARF inhibits MDM2, resulting in increased levels of p53, and thereby allowing cross talk with the p53/p21CIP1 pathways.
Figure 1.
The signaling pathways of senescence. Two main pathways, p16INK4a/Rb and p53/p21CIP1, regulate senescence-mediated growth arrest, and they both converge on repression of CDK4/6. In addition, ARF inhibits MDM2, resulting in increased levels of p53 and thereby allowing cross talk with the p53/p21CIP1 pathways.
ROS, reactive oxygen species.
Cellular senescence is an important determinant of death in the elderly,17–19 and contributes to accelerated aging. Senescent cells can develop into a SASP, arrested proliferation, and resistance to proapoptotic pathways through senescence-associated antiapoptotic pathways. The persistent presence of senescent cells results in the secretion of multiple factors including cytokines or chemokines, proteases, ROS and microRNAs, which further cause inflammation, tissue fibrosis, and stem cell dysfunction, and results in the dysfunction of multiple organs and accelerated aging.20 A study has shown that transplanting a small number of senescent cells into the knee joint region can cause osteoarthritis-like changes and impair joint function.21 In addition, transplanting senescent cells into young mice results into persistent physiological dysfunction and induces host-cell senescence, whereas transplanting fewer senescent cells into older mice reduces their survival.22 These studies show that senescent cells can induce the senescence of surrounding normal cells through a bystander effect,23,24 which will cause the continuous accumulation of senescent cells, ultimately leading to organ aging. On the contrary, clearance of p16Ink4a-positive cells delayed tumorigenesis and attenuated age-related deterioration of organ function without apparent side effects, which means that senescent cells negatively influence lifespan and promote age-dependent pathologies.25 In addition, the immune cells and immune responses undergo the phenotypic and genetic changes during aging, which is also known as immunosenescence.26,27 Immunosenescence is associated with a low-grade inflammation called inflammaging because of the impaired immune surveillance. Aging, likely via inflammaging, is associated with the emergence of many chronic diseases.28
There is growing evidence of nutrition-mediated alleviation of different aspects of cellular senescence, which may help confer a state of healthy organismal aging. Studies have shown that drugs promoting healthy aging can improve heart function and carotid vascular reactivity in aged mice,29 and provide lots of benefits in patients with idiopathic pulmonary disease and obesity-induced metabolic dysfunction.6,30 In addition, some drugs promoting healthy aging can retard the development of tumors and extend the median lifespan through elimination of senescent cells.25 The underlying mechanisms are related to many different pathways (Figures 2–4). In this review, we will discuss different kinds of drugs that target cellular senescence (Table 1), and highlight their advancement in terms of therapeutic potential. We also provide some important clues for their use in clinical applications.
Figure 2.
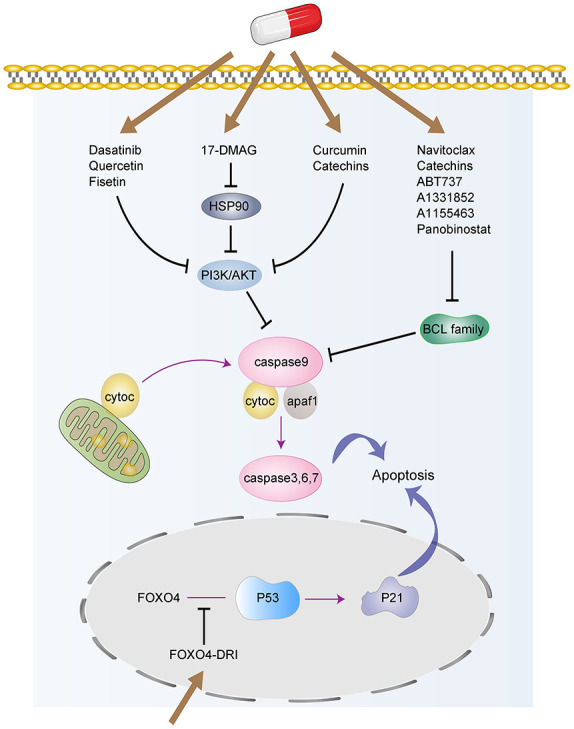
The targeted signaling pathways involved in senolytics. Senolytic drugs, such as Dasatinib, Quercetin, Fisetin, 17-DMAG, Navitoclax, Catechins, etc., induce the senescent cells apoptosis through different pathways, including the BCL-2/BCL-xL, P53, and PI3K/AKT pathways.
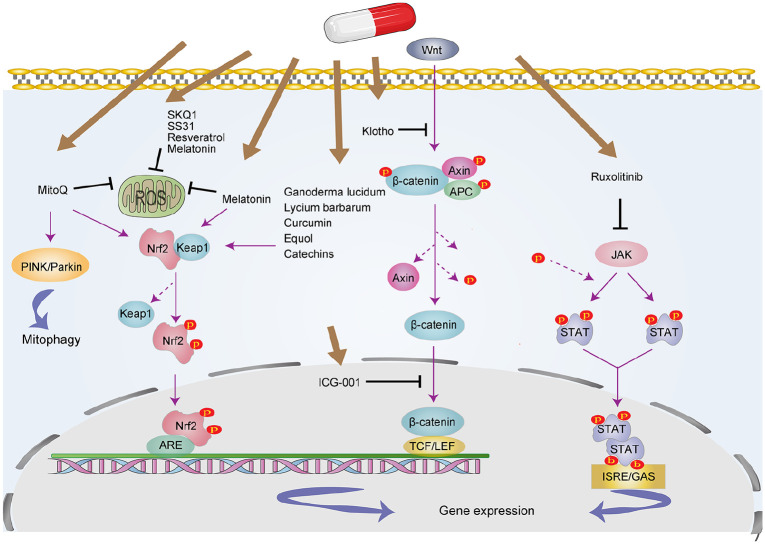
Figure 3.
Targeted signaling pathways involved in SASP inhibitor. These drugs include antioxidants, Wnts/β-catenin signaling inhibitor, JAK inhibitor, and so on. The related pathways include Nrf2, NF-κB, Wnt/β-catenin, and JAK pathways.
JAK, Janus kinase; ROS, reative oxygen species; SASP, senescence-associated secretory phenotype.
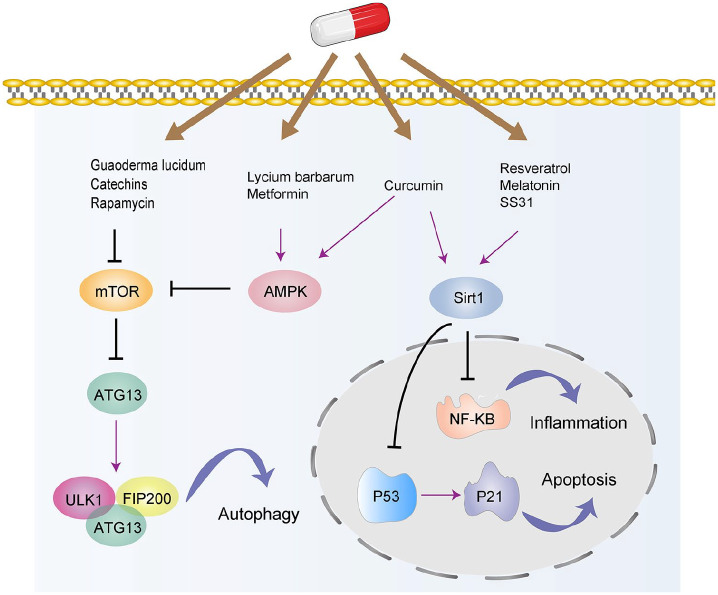
Figure 4.
The targeted signaling pathways involved in nutrient signaling regulator. These drugs include resveratrol, curcumin, metformin, rapamycin, Lycium barbarum, etc. The related signaling pathways include the Sirtuin, mTOR, and AMPK pathways.
Table 1.
Therapeutic drugs targeting of senescence.
| Compound | Model | Dose | Drug target | Comments | References | |
|---|---|---|---|---|---|---|
| Senolytics | Dasatinib and Quercetin | Aged C57BL/6 mice (24–27 months) | Treated with Dasatinib (5 mg/kg/day) and Quercetin (50 mg/kg/day) for 3 consecutive days every 2 weeks | The PI3K/AKT pathway | Eliminate senescent cells, inhibit the inflammation, alleviate physiological dysfunction, and increase survival. Increase 36% higher median post-treatment lifespan of mice. But may have side effects such as pulmonary edema | Xu et al.,22 Justice et al.,6 Hickson et al.31 |
| Fisetin | WT f1 C57BL/6:FVB mice at the age of 85 weeks | 500 mg/kg/day | The PI3K/AKT pathway | Induce apoptosis of senescent cells, reduce inflammation and oxidative stress, extend both median and maximum lifespan of mice (percentage not provided) | Zhu et al.,32 Yousefzadeh et al.33 | |
| 17-DMAG | Ercc1−/Δ mice at the age of 6 weeks | 10 mg/kg three times per week every 3 weeks | The PI3K/AKT pathway | Promote senescent cell apoptosis, reduce the incidence of age-related symptoms | Fuhrmann-Stroissnigg et al.34 | |
| Navitoclax, A1331852, A1155463, and ABT737 | Male C57BL/6J and p16-3MR transgenic mice at the age of 2–3 months | 50 mg/kg/d for 7 days per cycle for two cycles with a 2-week interval between the cycles | BCL family | Promote senescent cell apoptosis, but may have side effects such as thrombocytopenia and neutropenia | Chang et al.,35 Zhu et al.,36 Wilson et al.37 | |
| Panobinostat | A549 and FaDu cell lines | 25 nM | BCL family | As a post-chemotherapy senolytic with the potential to kill persistent senescent cells that accumulate during standard chemotherapy | Samaraweera et al.38 | |
| FOXO4-DRI | XpdTTD/TTD mice, p16::3MR mice and the f1 generation of them. At the age of 115–130 weeks | 5 mg/kg three times per day every other day for 30 days in naturally aged mice | P53-FOXO4 interaction | Restore fitness, fur density, and renal function in aging mice | Baar et al.39 | |
| Catechins | Adult bone marrow-derived human mesenchymal stem cells (hMSCs), 3T3-L1 preadipocytes | 50 or 100 µM | Bax/Bcl-2, Nrf2, and PI3K/AKT/mTOR pathways | Reduce ROS production and prevent oxidative stress-induced cellular senescence, inhibit the SASP and induce senescent cell death | Shin et al.,40 Kumar et al.41 | |
| SASP inhibitor | MitoQ | Male C57BL/6J db/db and db/m mice at the age of 12 weeks | 5 mg/kg, twice weekly for 12 weeks | Mitophagy and the Nrf2/PINK pathway | Reduce oxidative-stress damage and improve mitochondrial function | Braakhuis et al.,42 Rossman et al.,43 Xiao et al.44 |
| SS31 | Male Sprague–Dawley rats of ischemia reperfusion injury (IRI) model | 2 mg/kg administered 30 min before onset of ischemia and at the onset of reperfusion, or 2 mg at 30 min/24 h/48 h after ischemia reperfusion | SIRT1/SIRT3 and the NF-κB pathway | Reduce ROS levels, promote the recovery of ATP, alleviate mitochondrial dysfunction and prevent apoptosis | Birk et al.,45 Lee et al.,46 Cho et al.47 | |
| SKQ1 | The laboratory outbred SHR mice and three strains of inbred mice, that is, 129/sv, BALB/c, and C57Bl/6. | 5 or 50 nmol/kg/day in different groups | antioxidant | Increase the lifespan of rodents (percentage not provided) | Anisimov et al.48 | |
| Melatonin | The primary cortical neurons derived from SD rats | 0.1–1 mM | The Keap1/Nrf2/ARE pathway and SIRT1 | Reduce oxidative stress | Maity et al.,49 Chuang et al.50 | |
| Klotho | Male Spraque–Dawley rats, Tg-Kl mice and Kl+/− mice of ischemia reperfusion injury (IRI) model | 0.01 mg/kg 30 or 60 min after reperfusion; | Wnt/β-catenin pathway | Attenuate renal damage and promote recovery | Hu et al.51 | |
| ICG001 | HKC-8 cells lines or HK-2 cells lines | 5 μM | Wnt/β-catenin pathway | Attenuate renal damage and inhibit senescence | Miao et al.,52 Luo et al.53 | |
| Ruxolitinib | Zmpste24 deficient mice | Administered in subcutaneously implanted slow-release pellets | The JAK pathway | Inhibit SASP and reduce inflammation and reduce premature aging phenotypes | Xu et al.,54 Griveau et al.55 | |
| Ganoderma lucidum | Caenorhabditis elegans, male BALB/c mice at the age of 19–21 months | 100 ppm in Caenorhabditis elegans. 50 and 250 mg/kg once daily for 15 days in aged mice. | The Nrf2, mTOR, and MAPK pathways | Promote health, increase vitality, reduce ROS level, exhibit anti-inflammatory and immunomodulatory effects. Extend the lifespan of C. elegans by about 20–30% | Yun et al.,56 Sudheesh et al.,57 Bhardwaj et al.,58 Chuang et al.59 | |
| Equol | Male and OVX female SD rats | 250 ppm for 2 weeks prior to 90-min transient middle cerebral artery occlusion followed by reperfusion | The Nrf2/ARE pathway | Reduce antioxidative stress | Sekikawa et al.,60 Jing et al.,61 Ma et al.62 | |
| Nutrient signalling regulator | Rapamycin | Genetically heterogeneous mice at the age of 9 months, 3xTg-AD mice | 2.24 mg/kg/day in the study of Miller et al. At doses of 4.7, 14, or 42 ppm from age of 9 months and euthanized at 22 months of age in the study of Wilkinson et al. 2.24 mg/kg/day in 3xTg-AD mice for 10 weeks. | The mTOR signaling pathway | Delay many age-related pathological processes, improve cognitive function and retard multiple aspects of aging in mice. Extend median survival by an average of 10% in males and 18% in females in mice. But may have side effects such as immunosuppression, thrombocytopenia and so on | Wilkinson et al.,63 Caccamo et al.,64 Harrison et al.,65 Johnson et al.,66 Miller et al.67 |
| Metformin | Male C57BL/6 mice at the age of one year | 0.1% w/w in diet (~10.6 mg/kg/day) | The AMPK and NF-κB pathways | Delay the development of age-related diseases, extend 5.83% of mean lifespan of mice. | Bannister et al.,68 Martin-Montalvo et al.,69 Ng et al.70 | |
| Resveratrol | Diabetic rat model with coronary heart disease, Saccharomyces cerevisiae | 10 mg/kg/day for 8 weeks in rat model. 2–5 mM in Saccharomyces cerevisiae. | The SIRT1/NF-κB pathway | Extend the lifespans of S. cerevisiae by 70% in S. cerevisiae, prevent age-related diseases | Howitz et al.,71 Huo et al.,72 Szkudelski et al.73 | |
| Lycium barbarum | ARPE-19 cell exposed to ultraviolet B (UVB). C57BL/6J mice with a high-fat diet | Treated with L. barbarum extracts (from 0 to 200 μg/mL) for 2 h in ARPE-19 cell. 100 mg/kg LBP-supplemented diet for 24 weeks in mice. | The AMPK and Nrf2 pathways | Reduce ROS level, alleviate cellular oxidative stress, inflammation and apoptosis | Hsieh et al.,74 Xing et al.,75 Yang et al.76 | |
| Curcumin | Caenorhabditis elegans. Ten-week-old male Wistar rats with endurance training. | 20 mM in Caenorhabditis elegans. 50 or 100 mg/kg /day for 28 days in rats. | The AMPK, sirtuin, PI3K/AKT, NF-κB, and Nrf2 pathways | Reduce ROS level, have anti-apoptotic and anti-inflammatory effects. Increase in 39.3% in mean lifespan and 21.4% in maximum lifespan of C. elegans. | Liao et al.,77 Lee et al.,78 Pluta et al.,79 Ray et al.80 | |
| Spermidine | Wild-type (MAP1S+/+) and MAP1S knockout mice (MAP1S−/−). High glucose -treated HT-22 cells | 3 mM administered orally via drinking water in mice. 0.25 or 1 μM in HT-22 cells. |
The autophagy | Inhibit the oxidative stress, prevent high glucose-induced neurotoxicity and senescence, extend the lifespan. Increase 25% of the median survival time of mice. | Zhu et al.,81 Yue et al.82 |
ROS, reative oxygen species; SASP, senescence-associated secretory phenotype.
Therapeutic drugs targeting senescence
Although scientists are eager to find the best way to prevent, delay, or alleviate aging, therapeutic strategy development still has a long way to go.83,84 In this field, drug discovery is of potential interest. In this review, we will introduce current advancements in drugs promoting healthy aging. According to the mechanism and the related signaling pathways, they are classified into senolytics, SASP inhibitors, and nutrient signaling regulators.
Senolytics
Senolytics are agents that selectively induce the apoptosis of senescent cells. This type of drug can be classified into BCL family inhibitors, PI3K/AKT inhibitors, and FOXO regulators.
BCL family inhibitors
The BCL family is composed of pro-apoptotic proteins and pro-survival proteins, including BCL-2, BCL-xL, and McL-1.85,86 BCL-2/BCL-xL is one of the pro-survival pathways, meaning that targeting BCL family proteins may effectively clear senescent cells. However, BCL-xL inhibitors may have significant side effects, such as thrombocytopenia and neutropenia.35,37 At present, BCL inhibitors with healthy aging effects include mainly navitoclax (namely ABT263), A1331852, A1155463, and ABT737.87
Navitoclax
Navitoclax is a BCL-2 inhibitor that is orally bioavailable and has a high affinity for BCL-xL, BCL-2, and BCL-w.86 The healthy aging effects of navitoclax can reduce the viability of certain senescent cells, such as human umbilical vein epithelial cells, human lung fibroblasts, and mouse embryonic fibroblasts (MEFs), but not human primary preadipocytes.36 In a study by Chang et al., senescent cells in either sublethally irradiated or normally aged mice are effectively eliminated after treatment with navitoclax.35 In addition, navitoclax is also used as a therapeutic drug for the side effects of adjuvant treatment of tumors through promoting senescent-cell apoptosis generated by adjuvant therapy, thus reducing tumor recurrence and metastasis.88,89
ABT737, A1331852, and A1155463
ABT737, A1331852, and A1155463 are also BCL-2 family protein inhibitors. ABT737 – a precursor of navitoclax – is a BH3-mimetic drug that can block the interaction between anti-apoptotic family members (e.g., BCL-2, BCL-w and BCL-xL) and pro-apoptotic proteins containing the BH3 domain, and induce the apoptosis of senescent cells.90 Compared with navitoclax, ABT737 is not orally bioavailable and has a low aqueous solubility. These poor physiochemical and pharmaceutical properties greatly limit its application.86 A1331852 and A1155463 have been shown to induce senescent cell death, but have no effect on non-senescent cells.32 Compared with the less-specific BCL-2 family inhibitor navitoclax, A1331852 and A1155463 are selective BCL-xL inhibitors with lower blood toxicity,32 which makes them a better candidate for clinical application.
Panobinostat
Panobinostat is a histone deacetylase inhibitor that has anti-tumor effects.91 Panobinostat has been found to be able to kill the senescent cells that accumulate during standard chemotherapy. During tumor treatment, normal tissues will also be impaired, resulting in a senescent phenotype. Cellular senescence is one of the reasons for the survival of cancer cells after chemotherapy. A study by Samaraweera et al. discovered that panobinostat could effectively kill senescent cancer cells after chemotherapy,38 and the possible mechanism may be related to inhibition of BCL-xL. The expression of BCL-xL is increased in chemotherapy-induced senescent cells, but it is decreased after panobinostat treatment.
Catechins
Green tea has been studied extensively for its beneficial effects, and epidemiological studies have shown the association between drinking tea and beneficial effects.92 Catechins are polyphenolic compounds found in green tea, and the most abundant catechins are (–)-epigallocatechin gallate (EGCG) and (–)-epigallocatechin.93 In recent years, EGCG has attracted significant research interest due to its benefits in health effects. In a study by Kumar et al.,94 EGCG treatment could alleviate macrophage inflammation and senescence, and curb incidences of inflammatory disorders in the elderly. Besides, catechins are powerful antioxidants and radical scavengers possessing a potential role in the management of neurodegenerative diseases and cardiovascular disorders.95,96 It has also been proved that catechins have anti-tumor effects by suppressing proangiogenic factors.97 The protective effects of catechins may be related to several pathways, including Nrf2, PI3K/AKT/mTOR and Bax/Bcl-2. In the study of Shin et al.,40 EGCG pre-treatment reduces acetylated p53 and p21 protein levels in H2O2-treated hMSCs (human mesenchymal stem cells), but loses its antioxidant effect in Nrf2-knockdown hMSCs, which indicates that EGCG prevents oxidative stress-induced cellular senescence through Nrf2 activation. In another study by Kumar et al.,41 EGCG could inhibit SASP, protect against ROS production and DNA damage, and downregulate the activation of PI3K/Akt/mTOR pathway. It could also induce senescent cell death through inhibiting Bcl-2 expression. These results collectively show that EGCG could act as an mTOR inhibitor and SASP modulator as well as a potential senolytic agent, indicating its multi-faceted attributes that could be useful for developing healthy aging or age-delaying therapies.
PI3K/AKT inhibitors
The PI3K/AKT pathway is one of the pro-survival pathways in senescent cells. Studies have shown that phosphoinositide 3-kinase (PI3K) is involved in protecting cells against apoptosis,98 and one of its targets is the pleckstrin homology (PH) domain-containing serine/threonine kinase Akt, the activation of which can phosphorylate Bad, caspase-9, and FKHRL1, leading to their inactivation and cell survival.
Dasatinib and quercetin
Dasatinib (D) is a tyrosine kinase inhibitor that can affect a variety of tyrosine kinases, thus inhibit cell replication, migration, and invasion, and induce tumor cell apoptosis.99 Quercetin (Q), a rich micronutrient in daily diet, is a natural flavonol that inhibits the activity of mTOR and PI3K.100 Epidemiological studies recommend that diet plans consisting of flavonoids such as quercetin have positive health benefits, especially for the heart.101 D and Q are the first senolytic drugs to be discovered via a hypothesis-driven approach, and they have been demonstrated to relieve a variety of age-related diseases and improve survival in older mice.6 In the study of Xu et al., the combination of D and Q was proved to selectively kill senescent cells, reduce the secretion of pro-inflammatory cytokines, alleviate physiological dysfunction, and increase survival of elderly mice. The D/Q combination has been applied in clinical trials and the results show that D/Q significantly improve the physiological function in idiopathic pulmonary fibrosis (IPF) patients,6 and could effectively eliminate p16INK4a-positive cells, reduce the activity of SA-β-gal, and reduce the release of inflammatory factors in patients with diabetic nephropathy.31 Since a D/Q combination strategy inhibits tyrosine kinase and PI3K, long-term medication may affect a variety of biological pathways, which may cause serious side effects like pulmonary edema. Therefore, the current treatment via a D/Q combination involves intermittent administration, which is not only sufficient to eliminate senescent cells, but also to avoid off-target effects and reduce side effects.22
Fisetin
Fisetin is a natural flavonoid found in many fruits and vegetables, such as apples, persimmons, grapes, onions, cucumbers, and strawberries.102 Epidemiological studies have suggested that flavonoid intake has beneficial effects on vascular health, and is associated with a decreased risk of coronary heart disease and cardiovascular disease.103,104 In the nervous system, fisetin could inhibit the activity of lipoxygenase and reduce the production of pro-inflammatory eicosanoids and their by-products, and thus protect brain function in age-related neurological diseases.105 Recently, fisetin has been found to have senolytic activity. A study by Zhu et al. demonstrated that fisetin could induce apoptosis in aged human umbilical-vein endothelial cells.32 Besides, fisetin treatment could reduce the proportion of senescent cells, inflammation, and oxidative stress in premature-aging mice, while in elderly mice it could restore tissue homeostasis, reduce age-related pathological changes, and extend the median and maximum life span.33 The mechanism of fisetin in senescent-cell apoptosis may be related to its blocking on the PI3K/AKT pathway. A study has shown that fisetin could block the PI3K/AKT pathway during anti-tumor treatment,106 to lead to senescent cell death.29 Compared with the features of other senolytics, fisetin derives from natural foods, has few adverse reactions, and can act on many different types of senescent cells.33
HSP90 inhibitors
HSP90 is a highly conserved chaperone protein that plays an important role in protein stabilization and degradation. It interacts with cochaperone proteins to ensure proper folding, stabilization, and degradation of proteins involving in growth, development, and apoptosis.107,108 HSP90 affects a variety of cellular processes, improves cell survival, and promotes wound healing, and it also promotes cell survival by stabilizing AKT and/or ERK.34,108 AKT and p-AKT, as HSP90 client proteins, are key regulators of the PI3K/AKT pathway,98 and the interaction of HSP90-AKT facilitates the survival of senescent cells. 17-DMAG is an HSP90 inhibitor derived from bacteria. A previous study has shown that 17-DMAG inhibits HSP90, downregulates the PI3K/AKT pathway, reduces senescent cells, and promotes senescent cell apoptosis.34 Meanwhile, 17-DMAG treatment in Ercc1–/Δ mice can significantly reduce the incidence of age-related symptoms, including kyphosis, dystonia, tremor, loss of forelimb grip, compromised coat condition, ataxia, gait disorder, and general impairments in body condition. However, at present, only a few studies have investigated the healthy aging effects of HSP90 inhibitors.
FOXO regulators
FOXO4-DRI
FOXOs controls cell functions such as growth, survival, metabolism, and oxidative stress as transcriptional factor to regulate the expression of target genes109; FOXO4 plays an important role in FOXO function. FOXO4 can interact with p53, which is involved in the regulation of multiple target genes and controls a wide range of cellular processes, including metabolic adaptation, DNA repair, cell cycle arrest, apoptosis, and senescence.110,111 Studies have shown that FOXO4 can interact with p53, inhibit p53-mediated apoptosis, and thus maintain the vitality of senescent cells.39 In order to interfere with FOXO4-p53 interactions, a peptide named FOXO4-DRI is designed, which comprises part of the p53-interaction domain in FOXO4. Compared with the properties of FOXO4, FOXO4-DRI has a higher affinity for p53 binding, leading to the release of p53 in the nucleus to induce apoptosis. Therefore, FOXO4-DRI can effectively block p53-FOXO4 interaction, and, thus, selectively target senescent cells that depend on the p53 pathway. FOXO4-DRI has been shown to restore fitness, fur density, and renal function in both rapidly aging mice XpdTTD/TTD, and naturally aging mice.39 However, only a few relevant studies have demonstrated that FOXO4-DRI exerts a healthy aging effect.
In addition to the BCL family inhibitors, PI3K/AKT inhibitors and FOXO4-DRI, some other compounds could also eliminate senescent cells, such as UBX0101, piperlongumine, azithromycin, and roxithromycin. However, the mechanisms of these drugs inducing senescent cell apoptosis have not been fully elucidated. Jeon et al. demonstrate that intra-articular injection of UBX0101 selectively clears senescent cells that accumulate in the articular cartilage, thus reducing the development of post-traumatic osteoarthritis and promoting chondrogenesis.112 However, this study does not address the mechanism through which UBX0101 clears senescent cells, which will require further studies for its elucidation. Piperlongumine is a natural product isolated from pepper plants, and has been shown to kill senescent cells by inducing apoptosis. In addition, it has a strong synergistic effect on the senolytic activity of ABT-263, and combined use can reduce the dose of ABT-263 needed to clear senescent cells.113 Still, the mechanism by which piperlongumine induces apoptosis is not clear. Azithromycin and roxithromycin are macrolide antibiotics with anti-infection effects and were reported to have senolytic effects in a recent study.114 In this latter study, human fibroblast senescence was induced via chronic treatment with a DNA-damaging agent, and azithromycin intervention did not affect the viability in normal fibroblasts but did selectively kill senescent fibroblasts. In addition, azithromycin also protects mice from lung damage caused by radiation. The mechanism for azithromycin scavenging senescent cells has not been clarified, but may be related to drug metabolism. This study shows that azithromycin induces autophagy and glycolysis in senescent cells, and it can also affect mitochondrial activity, which may support its specific senolytic activity.
In summary, senolytics contribute to healthy aging by clearing senescent cells. Over the years, animal studies and clinical trials have shown that selectively eliminating senescent cells can reduce the burden of aging, improve symptoms of age-related diseases, and extend median lifespan. However, senolytics still have some problems that need to be addressed. Since different senolytics may target different types of senescent cells, senolytic drugs should be selected according to distinct senescent cell types. Some senolytics have significant adverse effects, so they need to be assessed to determine whether their administration is therapeutic or deleterious. Hence, more research is needed to confirm the safety and efficacy of senolytics drugs.
SASP inhibitors
Irreversible cell cycle arrest is commonly regarded as the key characteristic of senescent cells, and senolytics alleviate aging by inducing apoptosis of senescent cells. However, another major feature of senescent cells is the acquisition of SASP. Drugs that target SASP, such as antioxidants, Wnt/β-catenin inhibitors, and Janus kinase (JAK) inhibitors, also have healthy aging effects since SASP is associated with a pro-inflammatory status and a faster aging rate.
Antioxidants
MitoQ
MitoQ is an antioxidant that targets mitochondria, and has a strong effect on preventing mitochondrial oxidative damage.115,116 Studies have shown that mitoQ can reduce the production of ROS, improve mitochondrial function, and alleviate aging associated with oxidative stress.42 Heart failure can decrease mitochondrial contents in subsarcolemmal and interfibrillar area, and further reduce tissue respiration, while mitoQ can restore mitochondrial membrane potential and improve tissue respiration.117 In elderly mice, supplementation of mitoQ can improve vascular endothelial function and inhibit arterial sclerosis by reducing mitochondrial ROS.43 Moreover, a study by Xiao et al. shows that mitoQ can reduce tubular damage in diabetic nephropathy.44 All of these studies have demonstrated the protective effects of mitoQ, and the mechanism is related to mitochondrial autophagy and the Nrf2/PINK pathway. Mitochondrial autophagy helps to remove damaged mitochondria and reduce cellular senescence, while PINK1/Parkin is an important pathway for mitochondrial autophagy.118,119 Nrf2 is an antioxidant and key factor in oxidative stress and metabolism, while Keap1 is a negative regulator of Nrf2 and can be activated during oxidative stress.120 A study by Xiao et al.44 demonstrated decreased levels of LC3, PINK, Parkin, and Nrf2 in the tubular cells of db/db mice, while Keap1 was up-regulated. However, these changes were reversed after mitoQ treatment, meaning that mitoQ regulates mitochondrial autophagy through both Nrf2/Keap1 and PINK/Parkin pathways.
SS31
SS31 is a cell-permeable antioxidant peptide that targets mitochondria, which can reduce the generation of mitochondrial ROS, protect mitochondrial structure, and alleviate mitochondrial dysfunction.47,121,122 In studies of acute kidney injury (AKI) induced by ischemia-reperfusion injury, SS31 has been shown to protect cells from oxidative stress-induced mitochondrial dysfunction and apoptosis, promote the production of ATP, and improve the prognosis of the kidneys.45 The mechanism of SS31 in reducing oxidative stress and protecting mitochondria may be related to SIRT1/SIRT3, the NF-κB pathway, and CD36. A study by Lee et al. shows that SS31 upregulates the expression of SIRT1/SIRT3 and the level of ATP in H9C2 cells, as well as inhibiting oxidative stress.46 However, the protective effect of SS31 disappears when SIRT1/SIRT3 expression is inhibited via a targeted siRNA. In addition, the protective effect of SS31 may also be related to the downregulation of CD36 and NF-κB. CD36 is a glycosylated surface receptor found in the plasma membrane and mitochondria in various cells and can regulate oxidative stress and ROS production.123–126 Study has shown that SS31 can inhibit NF-κB, downregulate CD36, reduce the production of ROS, inhibit oxidative stress, improve the kidney function of db/db mice, and ameliorate high glucose-induced damage in HK-2 cells.127 In summary, the protective effect of SS31 in reducing oxidative stress and protecting mitochondria may be related to the up-regulation of SIRT1/SIRT3, inhibition of the NF-κB pathway, and downregulation of CD36.
SKQ1
SKQ1 is an antioxidant that contains plastoquinone, which targets mitochondria and has a stronger antioxidative effect than that of mitoQ.128 SKQ1 has been shown to extend the lifespan of mice,48 inhibit the development of some age-related diseases, such as cataracts and retinopathy,128 decrease arrhythmia caused by H2O2 or ischemia in isolated rat hearts,129 and reduce ROS levels and inhibit tumorigenesis in p53(–/–) mice.130 It can also inhibit oxidative stress, effectively prevent damage caused by ultraviolet light, and promote corneal wound healing after eye surgery.131 Collectively, these studies suggest that SKQ1 has the potential to become an effective antioxidant and a promising drug promoting healthy aging.
Melatonin
Melatonin is a methoxyindole whose physiological function is to convey circadian information on light and darkness.132 Epidemiological research has suggested that Melatonin has significant apoptotic, angiogenic, oncostatic, and anti-proliferative effects on various oncological cells.133 Melatonin is also found to have a higher concentration in mitochondria than in other organelles, and have the ability to scavenge oxygen radicals.134 In a study of gastric mucosa, melatonin could reduce mitochondrial oxidative stress, inhibit indomethacin-induced activation of the mitochondrial apoptotic pathway, and prevent collapse of the mitochondrial membrane potential.49 Besides, it can inhibit oxidative stress in a Parkinson’s disease model and reduce mitochondrial fragmentation and neuronal death.50 It also has a protective effect on myocardial infarction, improving mitochondrial integrity and reducing the production of ROS.135 The mechanism by which melatonin alleviates oxidative stress and protects mitochondria may be related to both the Keap1/Nrf2/ARE pathway and SIRT1 activation.136,137 Melatonin may inhibit the ubiquitination of Nrf2, thereby reduce its degradation by proteasomes. In addition, studies have shown that melatonin activates sirtuins. Since sirtuin pathways are involved in free-radical regulation, melatonin may alleviate oxidative stress by activating sirtuins.
Astaxanthin
Astaxanthin is a red pigment in the carotenoid lutein subclass, has a strong antioxidant capacity by clearing free radicals, and has great potential to fight disease.138 Studies have shown that the protective effect of astaxanthin is related to the maintenance of mitochondrial function. It can protect mitochondrial membranes and cristae from H2O2-induced structural damage, inhibit mitochondrial dysfunction caused by oxidative stress, and ultimately reduce apoptosis.139
Ganoderma lucidum
Ganoderma lucidum is a traditional Chinese medicine belonging to the white-rot fungus, and has been found to promote health, increase vitality, and prolong life.56 Epidemiological studies have demonstrated inverse correlation between mushroom intake and gastric, gastrointestinal, and breast cancer.140 In a study by Sudheesh et al.,57 the level of glutathione, glutathione peroxidase, and glutathione-s-transferase activities increased significantly after feeding aged mice with G. lucidum, while lipid peroxidation, advanced oxidation protein products (AOPP), and ROS were significantly reduced. Another study has shown that G. lucidum can enhance Krebs-cycle dehydrogenases and the activity of the mitochondrial electron transport chain complex IV in aged rats, which effectively ameliorates age-associated declines in cellular energy.141 In addition, G. lucidum exhibits anti-inflammatory and immunomodulatory effects.58 The healthy aging mechanism of G. lucidum may be related to Nrf2, mTOR and MAPK pathways. A previous study has shown that G. lucidum treatment induces the expression of Nrf2, while transfection with Nrf2 siRNA attenuates its protection effects.142 In another study, G. lucidum protects Caenorhabditis elegans from paraquat- and heavy metals-induced oxidative stress through the mTOR signaling pathway.143 In addition, Ganoderma lucidum can upregulate the Toll-interleukin 1 receptor intracellular domain and activate the MAPK pathway, which increases expression of DAF-16 – a transcription factor related to the lifespan of C. elegans, thereby extending its lifespan.59
Equol
Equol is a polyphenolic compound derived from soy isoflavones and has high antioxidant properties.60 Epidemiological evidence has shown that diets rich in phytoestrogen-containing foods could reduce the risk of a number of syndromes and chronic diseases, including cardiovascular and neurodegenerative diseases, and certain types of cancer.144 Equol may help to prevent osteoporosis in postmenopausal women,145 and is strongly associated with a lower incidence of prostate cancer in men.146 In addition, equol has been reported to have a kidney-protective effect, and reduce serum creatinine, serum phosphorus, C-reactive protein (CRP), and proteinuria levels in patients with chronic kidney diseases.61 The healthy aging mechanism of equol may be related to the reduction of oxidative stress and activation of the Nrf2/ARE pathway. In a rat model of cerebral ischemia, equol can increase the endogenous antioxidant effect, reduce the oxidative stress, and decrease the cerebral infarction area and neurological dysfunction.62 In human umbilical-vein endothelial cells, equol treatment can induce Nrf2 activation and increase gene products of heme oxygenase-1 (HO-1), improve cell survival in response to H2O2, and reduce apoptosis; however, the protective effect of equol on H2O2-induced apoptosis is reduced in cells transfected with Nrf2 siRNA.147
Wnt/β-catenin inhibitors
Wnt/β-catenin signaling is an evolutionarily conserved pathway involved in organ development and tissue repair, which is silent in normal adults but is reactivated after kidney injury in a wide range of chronic kidney disease models.53 Wnt/β-catenin signaling has been demonstrated to be related to cellular senescence, and inhibitors of the Wnt/β-catenin pathway, such as Klotho and ICG-001, have healthy aging effects.52
Klotho
Klotho is an anti-aging protein that is expressed predominantly in normal tubular cells.148 Studies have shown that a deficiency of Klotho is associated with the increased extent of vascular calcification in chronic kidney disease (CKD) patients, while supplementation of Klotho can inhibit the differentiation of vascular smooth muscle cells into osteoid or osteoblastic cells, thereby inhibiting vascular calcification.149 In a study using AKI rats, deficiency of Klotho exacerbates kidney injury, while Klotho supplementation attenuates renal damage and promotes recovery from AKI.51 The mechanism of the healthy aging effect of Klotho is related to the inhibition on Wnt/β-catenin signaling pathway. A previous study has shown that continuous Wnt exposure accelerates cellular senescence, while Klotho could bind to different types of Wnt ligands, resulting in the suppression of the downstream signaling transduction of the Wnt/β-catenin pathway.150 On the contrary, deletion of α-Klotho increases Wnt/β-catenin signaling in mice. These results suggest that Klotho may exert healthy aging effects by suppressing the Wnt signaling pathway.
ICG-001
ICG-001 is a small molecule that blocks β-catenin-mediated gene transcription in a CBP [cAMP-responsive element binding (CREB)-binding protein]-dependent manner.151 Studies have shown that ICG-001 reduces tumor growth in both in vitro and in vivo xenograft models.152 Furthermore, in a study on liver fibrogenesis, ICG-001 significantly inhibits fibrotic parameters in vitro, and significantly attenuates collagen accumulation and inhibits macrophage infiltration, intrahepatic inflammation, and angiogenesis in vivo.153 The mechanism of the healthy aging effect of ICG-001 is related to inhibition of the Wnt/β-catenin signaling pathway. By inhibiting the binding of β-catenin with the transcription coactivator CBP, ICG-001 is able to inhibit the downstream signaling transduction of the Wnt/β-catenin pathway. For example, studies have shown that ICG-001 can inhibit Wnt9a-induced p53 and p21 expression,53 and inhibition of Wnt/β-catenin by ICG-001 protects mitochondrial biogenesis and cellular proliferation, suggesting its great value in protection against age-related mitochondrial dysfunction.52
JAK inhibitors
The JAK pathway plays an important role in the regulation of cytokine production. The JAK family consists of four members, including JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), of which JAK1 and 2 are associated with inflammatory signaling.154 The JAK pathway is related to aging. Studies have shown that the JAK pathway is activated in aging adipose tissue and produces pro-inflammatory factors, while inhibition of the JAK pathway can inhibit SASP and reduce inflammation and weakness in aged mice.54 Aging is often associated with lipid metabolism disorders, and JAK inhibitors can inhibit the production of senescent cell activin A and weaken senescent-cell-mediated inhibition of adipogenesis.155
Ruxolitinib
Ruxolitinib is a JAK1/2 inhibitor, and its healthy aging effect is related to the inhibition of the JAK pathway.54,155 The healthy aging effect of ruxolitinib as a JAK inhibitor has also been demonstrated in many studies. In a study by Griveau et al.55, ruxolitinib rescues progerin-induced cell cycle arrest, cellular senescence, and disrupts nuclei in human normal fibroblasts expressing progerin. In addition, in a mouse model of progeria, ruxolitinib can reduce several premature aging phenotypes, such as bone fractures, bone mineral content, and grip strength. These results show that ruxolitinib has the potentiality to be a drug that promotes healthy aging.
In summary, SASP inhibitors contribute to healthy aging by reducing oxidative-stress damage, improving mitochondrial function, and inhibiting the bystander effect of senescent cells. We notice that some drugs may contribute to healthy aging through different mechanisms at the same time. For example, as mentioned above, catechin is a senolytic; however, it can also prevent oxidative stress-induced cellular senescence and alleviate inflammatory disorders, which shows that it can also act as an SASP inhibitor.41 Though SASP inhibitors have health benefits, careful consideration should be taken before their application. For example, proper intake of antioxidants may benefit health, while excessive intake of exogenous antioxidants may inhibit the synthesis of endogenous antioxidant enzymes and disrupt the balance between oxidative and anti-oxidative processes, which is important for maintaining homeostasis.156,157 Besides, SASP of senescent cells may also have beneficial effects in certain conditions. For example, study has shown that, in the mouse model, senescent fibroblasts and endothelial cells appear very early in response to a cutaneous wound and they accelerate wound closure by inducing myofibroblast differentiation through the secretion of platelet-derived growth factor AA, which defines a beneficial role for the SASP in tissue repair.158
Nutrient signaling regulators
Nutrients are necessary for life, as they are a crucial requirement for biological processes, and nutrient sensing signaling has been shown to regulate ageing in eukaryotic organisms from yeast to humans through dietary and pharmacological manipulation.159 Therefore, signaling systems including Sirtuin, mTOR, and AMPK, play important roles in regulating physiological decisions and the drugs that modulate these signaling pathways may help to alleviate aging and age-relate disease.
Sirtuin regulators
Resveratrol
Resveratrol is one of the polyphenols found in many red wines and has attracted considerable attention in recent years due to its healthy aging effects.160 Epidemiological studies have indicated that resveratrol plays a key role in prostate cancer prevention as dietary micronutrient.161 Resveratrol also has been reported to extend the lifespans of nematodes and yeast and to prevent age-related diseases in the elderly.71–73,162–165 Studies have shown that the antioxidant and healthy aging effects of resveratrol may be related to the Sirtuin pathway. Sirtuin 1 (SIRT1) is a NAD(+)-dependent deacetylase that targets various transcription factors, and through the deacetylation of transcription factors and histones, SIRT1 plays a variety of roles in gene silencing, anti-oxidative stress, anti-apoptosis, and inhibition of inflammation.166 SIRT1 levels and activity are reduced during chronic inflammation or aging in response to oxidative stress, while resveratrol is able to increase the levels of SIRT1.167 Another study by Liu et al. demonstrates that resveratrol significantly increases the activity of SIRT1, inhibits the NF-κB pathway, and reverses the loss of intestinal stem cells.168 However, although resveratrol has been shown to increase the lifespan of nematodes and yeast, it has not been shown to increase the lifespan of mice.67 Hence, more research is needed in the impact on longevity of resveratrol.
mTOR inhibitors
Rapamycin
Rapamycin is an inhibitor of mTOR signaling and has been shown to exert healthy aging effects by delaying many age-related pathological processes in mice.63 It can also delay pathological changes in the brain in Alzheimer’s disease, thereby improving cognitive function.64 Furthermore, feeding rapamycin to mice can also extend their lifespan, even when fed late in life.65,67 The healthy aging effect of rapamycin is related to mTOR signaling. mTOR plays an important role in the regulation of cellular growth and cancer, but increased activity of mTOR can also lead to cellular senescence.169,170 Studies have shown that rapamycin can extend the lifespan of mice by targeting mTOR.65 By inhibiting mTOR, rapamycin can induce autophagy to improve the pathologies of amyloid beta and tau, which are two primary hallmarks of Alzheimer’s disease, and ultimately improve cognitive deficits in Alzheimer’s disease.64 The inhibitory effect of rapamycin on cellular senescence may also be related to the Nrf2 pathway.171 In this study, rapamycin can reduce cytoplasmic Keap1 levels, activate the Nrf2 pathway, and reduce the expression levels of p16, p21, and pH2AX. In contrast, rapamycin has no effect on p21 or p16 levels in the Nrf2-deleted mice, while the inhibition effect of rapamycin is restored after Nrf2 transfection. Of note, rapamycin has other effects, such as immunosuppression, thrombocytopenia, delaying wound healing, altering glucose homeostasis, and increasing incidence of cataracts, which may limit its application in geriatrics.66
Spermidine
Spermidine is a polyamine synthesized by eukaryotic cells, which has anti-inflammatory properties and can maintain mitochondrial function and prevent stem cells from aging; additionally, in epidemiological studies, dietary intake of polyamines has been associated with reduced cardiovascular and cancer-related mortalities.172 Besides, recent epidemiological data also reports a positive association between nutritional spermidine uptake and human health span and lifespan.173 During the aging process, the concentration of spermidine in cells gradually decreases, and supplementation of spermidine can effectively inhibit the oxidative stress in aging mice.174 Hyperglycaemia (HG)-induced neurotoxicity leads to the pathogenesis of diabetic encephalopathy and neuronal senescence, while spermidine can prevent HG-induced neurotoxicity and senescence.81 In a kidney ischemia/reperfusion injury model, spermidine supplementation can markedly attenuate increases in plasma creatinine concentrations and tubular injury, inhibit oxidative stress, and suppress tissue necrosis.175 The healthy aging effect of spermidine may be related to the enhancement of autophagy. Studies have shown that autophagy defects in liver cells trigger oxidative stress-induced cell death, while spermidine treatment can enhance autophagy and reduce liver fibrosis and liver tumor lesions, and long-term administration of spermidine can prolong the lifespan of mice.82 In another study, oral supplementation of spermidine extends the lifespan of older mice and has a cardioprotective effect since spermidine-feeding enhances cardiac autophagy and mitochondrial autophagy; however, in mice that lack the autophagy-related protein ATG5 in cardiomyocytes, spermidine-feeding fails to provide cardioprotection.176
AMPK activators
Metformin
Metformin is a drug used for the treatment of type-2 diabetes,177 and, in recent years, it has been found to have healthy aging and life-extending effects. Epidemiological studies have documented an association between metformin and reduced cancer incidence and mortality.178 Besides, studies have shown that long-term use of metformin can reduce cognitive decline,70 reduce the oxidative damage and chronic inflammation, and prolong the health and lives.69 Patients with type-2 diabetes treated with metformin live longer than those treated with sulfonylureas.68 Moreover, metformin can also reduce vascular complications by inhibiting the damage of vascular endothelial cells caused by oxidative stress and can reduce the apoptosis of cardiomyocytes and improve the structure and function of the heart.179,180 The healthy aging mechanism of metformin may be related to the AMPK and NF-κB pathways. AMPK is a conserved cellular-energy sensor that regulates many other cellular processes, while metformin acts as an AMPK agonist.181–183 In a study by He et al.,180 the AMPK pathway is activated and hyperglycemic-induced apoptosis in H9c2 cells is reduced after intervention with metformin. Besides, Studies have shown that metformin inhibits the activation of the NF-kB pathway by preventing the translocation of NF-κB to the nucleus and inhibiting the phosphorylation of IκB and IKKα/β.184 By inhibiting the NF-κB pathway, metformin can inhibit the expression of various inflammatory cytokines during cellular senescence.
Curcumin
Curcumin is a polyphenol found in Curcuma longa, which exhibits healthy aging properties.185 It has been reported to extend the lifespan of C. elegans and Drosophila, is able to regulate the expression of genes related to aging,77,78 and has antioxidant effects.186 Epidemiologic studies also suggest a link between curcumin supplementation and cognitive benefits and highlight its neuroprotective effects.187,188 In neurodegenerative diseases, curcumin has a variety of therapeutic effects, including anti-oxidative, anti-apoptotic, anti-inflammatory, and neurogenesis-promoting properties.79 These results suggest that curcumin may be a potential preventive and therapeutic agent in neurodegenerative diseases. The healthy aging mechanism of curcumin may be related to AMPK, sirtuin, PI3K/AKT, NF-κB, and Nrf2 pathways. In mice receiving endurance training, curcumin treatment increases AMPK phosphorylation in skeletal muscle, up-regulates SIRT1 expression, and increases mitochondrial biogenesis.80 In tumor treatments, curcumin can inhibit the proliferation in a variety of tumor cells by down-regulating PI3K/AKT signaling, and can induce tumor-cell apoptosis.189 Curcumin can also activate sirtuin and inhibit the NF-κB pathway by inhibiting the acetylation of p65, thereby inhibiting the release of inflammatory cytokines. In addition, it can relieve oxidative stress and inflammation in chronic diseases through the Nrf2-keap1 pathway, inhibit the pro-inflammatory pathways related to many chronic diseases, and block the production of TNF.190,191
Lycium barbarum
Lycium barbarum is also a traditional Chinese medicine, belonging to the plant family solanaceae,192 and has been shown to exert both antioxidative and healthy aging effects. For example, a study by Hsieh et al. has shown that L. barbarum could reduce cellular oxidative stress and protect cells from apoptosis.74 In addition, it can alleviate inflammation and improve neurotransmission in neurodegenerative diseases,75 and reduce insulin resistance induced by a high-fat diet.76 The antioxidative mechanism of L. barbarum may be related to AMPK and Nrf2 pathways. Study has shown that L. barbarum could increase the activity of AMPK and reduce endoplasmic reticulum stress in db/db mice193; however, after blocking AMPK with siRNA, the protective effect of L. barbarum is eliminated. In another study, L. barbarum could induce Nrf2 nuclear translocation and increase the expression of Nrf2-dependent ARE, while the protective effect is abolished by siRNA-mediated silencing of Nrf2.194
Conclusion
In summary, we show the advancements in therapeutic drugs that target cellular senescence. Through modulating inflammation, oxidative stress, mitochondrial function, and so on, these drugs could selectively or indirectly retard the aging process. These drugs are classified into three types: senolytics, SASP inhibitors, and nutrient signaling regulators (Table 1). Besides, we also collected data on some drugs that have been applied in clinical trials (Table 2).6,31,43,195–198 In addition to promote healthy aging, these drugs could also serve as the therapeutic drugs for various age-related diseases. To improve healthy aging and longevity is a big strategy for social and economic development in the world. Living healthily with a long lifespan is the best expectation of everyone. Hence, healthy aging is increasingly recognized as a healthcare priority. Hopefully, some drugs are very promising in this regard. However, exploration of the best application of strategies in more clinical trials is also needed. Nevertheless, our review provides important clues for the use of future prospective drugs that exhibit healthy aging activities.
Table 2.
Clinical trials of drugs promoting healthy aging.
| Drug | Participants and sample size, n | Dose | Duration | Status | Main findings (completed) or research purposes (recruiting) | Reference (completed) or NCT number (recruiting) |
|---|---|---|---|---|---|---|
| Dasatinib plus Quercetin | Patients with IPF, n = 14 | Dasatinib:100 mg/day, Quercetin:1250 mg/day, three-days/week | 3 weeks | completed | Dasatinib plus Quercetin may alleviate physical dysfunction in IPF | Justice et al.,6 USA |
| Dasatinib plus Quercetin | Adults aged 50–80 years with diabetes mellitus and CKD were included, n = 9 | Dasatinib: 100 mg daily, Quercetin:1000 mg total daily (500 mg twice daily) | 3 days | completed | Dasatinib plus Quercetin treatment significantly decreased senescent cell burden in humans. | Hickson et al.,31 USA |
| Dasatinib plus Quercetin | Allogeneic HSCT patients surviving ⩾1 year post-HSCT, n = 10 | Quercetin: 1000 mg daily, Dasatinib:100 mg daily | 3 consecutive days | recruiting | Evaluate the biologic markers of premature aging and senescence in HSCT survivors and their correlation with clinical outcomes | [ClinicalTrials.gov identifier: NCT02652052] |
| Dasatinib | Patients with SSc-ILD, n = 12 | Not provided | 169 days | completed | A decrease in skin expression of SASP and other senescence-related gene sets was associated with Dasatinib treatment | Martyanov et al.,196 USA |
| curcumin | Healthy adults aged 60–85 years old, n = 60 | 80 mg per day or per time | Acute (1 and 3 h after a single dose), chronic (4 weeks) | completed | Acute: trend in improvement in sustained attention (p = 0.168) and significant improvement in working memory; chronic: significant improvement in working memory | Cox et al.,198 Australia |
| MitoQ | Healthy older adults (60–79 years) with impaired endothelial function, n = 20 | 20 mg/day | 6 weeks | completed | MitoQ and other therapeutic strategies targeting mitochondrial reactive oxygen species may hold promise for treating age-related vascular dysfunction. | Rossman et al.,43 USA |
| Fisetin | Adults aged 70 years or older, n = 40 | 20 mg/kg/day | 2 consecutive days | recruiting | Evaluate markers of frailty and markers of inflammation, insulin resistance, and bone resorption while maintaining bone formation in older adults. | [ClinicalTrials.gov identifier: NCT03675724] |
| Fisetin | Patients with osteoarthritis aged 40–80, n = 72 | 20 mg/kg per day | Orally for two consecutive days, followed by 28 days off, then 2 more consecutive days. | recruiting | To determine whether fisetin reduces senescent cells, pro-inflammatory and cartilage degenerating SASP markers, and reduces osteoarthritis-symptoms leading to improved joint health and function. | [ClinicalTrials.gov identifier: NCT04210986] |
| Metformin | Patients aged 60 years or older, with stable coronary artery disease and prediabetes disease, n = 12 | 500 mg tablet by mouth, every 6–8 h per day | 1 years | recruiting | Enhance understanding of the regenerative impact of metformin and the basis for clinical improvement in the setting of senescence. | [ClinicalTrials.gov identifier: NCT03451006] |
| Rapamycin | Participants more than 40 years of age and had no history of diabetes/hypercholesterolemia, n = 36 | Topical application of 0.5cc rapamycin cream (10 μM) to the dorsal side of each hand | 8 months | completed | Rapamycin reduced the markers of aging and clinical improvement in skin appearance was noted | Chung et al.,197 USA |
CKD, chronic kidney disease; HSCT, hematopoietic stem cell transplant; IPF, idiopathic pulmonary fibrosis; SASP, senescence-associated secretory phenotype; SSc-ILD, systemic sclerosis–associated interstitial lung disease.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China grants (82070707, 91949114, 81722011 and 81521003); National Key Research and Development Project 2019YFC2005000; the Project of Innovation Team of Chronic Kidney Disease with Integrated Traditional Chinese and Western Medicine (2019KCXTD014); Frontier Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110105004) and Outstanding Scholar Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (Grant No. 2018GZR110102004).
ORCID iD: Lili Zhou  https://orcid.org/0000-0001-5044-6965
https://orcid.org/0000-0001-5044-6965
Contributor Information
Mingsheng Zhu, State Key Laboratory of Organ Failure Research, National Clinical Research Center of Kidney Disease, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China; Department of Nephrology, The People’s Hospital of Gaozhou, Maoming, China.
Ping Meng, Department of Nephrology, Huadu District People’s Hospital, Southern Medical University, Guangzhou, China.
Xian Ling, State Key Laboratory of Organ Failure Research, National Clinical Research Center of Kidney Disease, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Lili Zhou, Division of Nephrology, Nanfang Hospital, 1838 North Guangzhou Ave, Guangzhou 510515, China; Bioland Laboratory (Guangzhou Regenerative Medicine and Health Guangdong Laboratory, Guangzhou, China.
References
- 1. Beard JR, Bloom DE. Towards a comprehensive public health response to population ageing. Lancet 2015; 385: 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nabavi SF, Braidy N, Habtemariam S, et al. Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s diseases: from chemistry to medicine. Curr Top Med Chem 2016; 16: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 3. St Sauver JL, Boyd CM, Grossardt BR, et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open 2015; 5: e6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godic A. The role of stem cells in anti-aging medicine. Clin Dermatol 2019; 37: 320–325. [DOI] [PubMed] [Google Scholar]
- 5. Khosla S, Farr JN, Tchkonia T, et al. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 2020; 16: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019; 40: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Childs BG, Baker DJ, Kirkland JL, et al. Senescence and apoptosis: dueling or complementary cell fates? Embo Rep 2014; 15: 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ou HL, Schumacher B. DNA damage responses and p53 in the aging process. Blood 2018; 131: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del PR, Ferri C. Inflammation-accelerated senescence and the cardiovascular system: mechanisms and perspectives. Int J Mol Sci 2018; 19: 3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 2015; 36: 217–228. [DOI] [PubMed] [Google Scholar]
- 11. Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal 2013; 19: 1420–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiong Y, Zhou L. The signaling of cellular senescence in diabetic nephropathy. Oxid Med Cell Longev 2019; 2019: 7495629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu XL, Ding J, Meng LH. Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin 2018; 39: 1553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 2007; 371: 21–31. [DOI] [PubMed] [Google Scholar]
- 15. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017; 16: 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 2018; 217: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine 2017; 21: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giacconi R, Malavolta M, Costarelli L, et al. Cellular senescence and inflammatory burden as determinants of mortality in elderly people until the extreme old age. EBioMedicine 2015; 2: 1316–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopes-Paciencia S, Saint-Germain E, Rowell MC, et al. The senescence-associated secretory phenotype and its regulation. Cytokine 2019; 117: 15–22. [DOI] [PubMed] [Google Scholar]
- 21. Xu M, Bradley EW, Weivoda MM, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci 2017; 72: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018; 24: 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Da SP, Ogrodnik M, Kucheryavenko O, et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 2019; 18: e12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson G, Wordsworth J, Wang C, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 2012; 11: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016; 530: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pantsulaia I, Ciszewski WM, Niewiarowska J. Senescent endothelial cells: potential modulators of immunosenescence and ageing. Ageing Res Rev 2016; 29: 13–25. [DOI] [PubMed] [Google Scholar]
- 27. Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol 2018; 105: 4–9. [DOI] [PubMed] [Google Scholar]
- 28. Fulop T, Dupuis G, Witkowski JM, et al. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin 2016; 68: 84–91. [PubMed] [Google Scholar]
- 29. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14: 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer AK, Xu M, Zhu Y, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019; 18: e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019; 47: 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017; 9: 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018; 36: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 2017; 8: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016; 22: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016; 15: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson WH, Hernandez-Ilizaliturri FJ, Dunleavy K, et al. Novel disease targets and management approaches for diffuse large B-cell lymphoma. Leuk Lymphoma 2010; 51(Suppl. 1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samaraweera L, Adomako A, Rodriguez-Gabin A, et al. A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep 2017; 7: 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baar MP, Brandt RMC, Putavet DA, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017; 169: 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shin JH, Jeon HJ, Park J, et al. Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via Nrf2. Int J Mol Med 2016; 38: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar R, Sharma A, Kumari A, et al. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019; 20: 171–189. [DOI] [PubMed] [Google Scholar]
- 42. Braakhuis AJ, Nagulan R, Somerville V. The effect of MitoQ on aging-related biomarkers: a systematic review and meta-analysis. Oxid Med Cell Longev 2018; 2018: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rossman MJ, Santos-Parker JR, Steward CAC, et al. Chronic supplementation with a Mitochondrial Antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018; 71: 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao L, Xu X, Zhang F, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 2017; 11: 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Birk AV, Liu S, Soong Y, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 2013; 24: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee F, Shao P, Wallace C, et al. Combined therapy with SS31 and mitochondria mitigates myocardial ischemia-reperfusion injury in rats. Int J Mol Sci 2018; 19: 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cho S, Szeto HH, Kim E, et al. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 2007; 282: 4634–4642. [DOI] [PubMed] [Google Scholar]
- 48. Anisimov VN, Egorov MV, Krasilshchikova MS, et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging (Albany NY) 2011; 3: 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maity P, Bindu S, Dey S, et al. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res 2009; 46: 314–323. [DOI] [PubMed] [Google Scholar]
- 50. Chuang JI, Pan IL, Hsieh CY, et al. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res 2016; 61: 230–240. [DOI] [PubMed] [Google Scholar]
- 51. Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 2010; 78: 1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miao J, Liu J, Niu J, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019; 18: e13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo C, Zhou S, Zhou Z, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J Am Soc Nephrol 2018; 29: 1238–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015; 112: E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Griveau A, Wiel C, Ziegler DV, et al. The JAK1/2 inhibitor ruxolitinib delays premature aging phenotypes. Aging Cell 2020; 19: e13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yun TK. Update from Asia. Asian studies on cancer chemoprevention. Ann N Y Acad Sci 1999; 889: 157–192. [DOI] [PubMed] [Google Scholar]
- 57. Sudheesh NP, Ajith TA, Ramnath V, et al. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin Nutr 2010; 29: 406–412. [DOI] [PubMed] [Google Scholar]
- 58. Bhardwaj N, Katyal P, Sharma AK. Suppression of inflammatory and allergic responses by pharmacologically potent fungus Ganoderma lucidum. Recent Pat Inflamm Allergy Drug Discov 2014; 8: 104–117. [DOI] [PubMed] [Google Scholar]
- 59. Chuang MH, Chiou SH, Huang CH, et al. The lifespan-promoting effect of acetic acid and Reishi polysaccharide. Bioorg Med Chem 2009; 17: 7831–7840. [DOI] [PubMed] [Google Scholar]
- 60. Sekikawa A, Ihara M, Lopez O, et al. Effect of S-equol and soy isoflavones on heart and brain. Curr Cardiol Rev 2019; 15: 114–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jing Z, Wei-Jie Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: a systematic review and meta-analysis. Clin Nutr 2016; 35: 117–124. [DOI] [PubMed] [Google Scholar]
- 62. Ma Y, Sullivan JC, Schreihofer DA. Dietary genistein and equol (4’, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am J Physiol Regul Integr Comp Physiol 2010; 299: R871–R877. [DOI] [PubMed] [Google Scholar]
- 63. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell 2012; 11: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caccamo A, Majumder S, Richardson A, et al. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 2010; 285: 13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460: 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johnson SC, Kaeberlein M. Rapamycin in aging and disease: maximizing efficacy while minimizing side effects. Oncotarget 2016; 7: 44876–44878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 2014; 16: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 69. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013; 4: 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ng TP, Feng L, Yap KB, et al. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 2014; 41: 61–68. [DOI] [PubMed] [Google Scholar]
- 71. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–196. [DOI] [PubMed] [Google Scholar]
- 72. Huo X, Zhang T, Meng Q, et al. Resveratrol effects on a diabetic rat model with coronary heart disease. Med Sci Monit 2019; 25: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta 2015; 1852: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 74. Hsieh FC, Hung CT, Cheng KC, et al. Protective effects of Lycium barbarum extracts on UVB-induced damage in human retinal pigment epithelial cells accompanied by attenuating ROS and DNA damage. Oxid Med Cell Longev 2018; 2018: 4814928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xing X, Liu F, Xiao J, et al. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med 2016; 18: 253–263. [DOI] [PubMed] [Google Scholar]
- 76. Yang Y, Li W, Li Y, et al. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid Med Cell Longev 2014; 2014: 145641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liao VH, Yu CW, Chu YJ, et al. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev 2011; 132: 480–487. [DOI] [PubMed] [Google Scholar]
- 78. Lee KS, Lee BS, Semnani S, et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res 2010; 13: 561–570. [DOI] [PubMed] [Google Scholar]
- 79. Pluta R, Ulamek-Koziol M, Czuczwar SJ. Neuroprotective and neurological/cognitive enhancement effects of curcumin after brain ischemia injury with Alzheimer’s disease phenotype. Int J Mol Sci 2018; 19: 4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ray HR, Yamada T, Ishizawa R, et al. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 2015; 64: 1334–1347. [DOI] [PubMed] [Google Scholar]
- 81. Zhu WW, Xiao F, Tang YY, et al. Spermidine prevents high glucose-induced senescence in HT-22 cells by upregulation of CB1 receptor. Clin Exp Pharmacol Physiol 2018; 45: 832–840. [DOI] [PubMed] [Google Scholar]
- 82. Yue F, Li W, Zou J, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res 2017; 77: 2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pignolo RJ, Passos JF, Khosla S, et al. Reducing senescent cell burden in aging and disease. Trends Mol Med 2020; 26: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov 2020; 19: 513–532. [DOI] [PubMed] [Google Scholar]
- 85. Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208. [DOI] [PubMed] [Google Scholar]
- 86. Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428. [DOI] [PubMed] [Google Scholar]
- 87. Kang C. Senolytics and senostatics: a two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Mol Cells 2019; 42: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Short S, Fielder E, Miwa S, et al. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 2019; 41: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Demaria M, O’Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 2017; 7: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Merino D, Khaw SL, Glaser SP, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119: 5807–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yoon S, Eom GH. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J 2016; 52: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pervin M, Unno K, Takagaki A, et al. Function of green tea catechins in the brain: epigallocatechin gallate and its metabolites. Int J Mol Sci 2019; 20: 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cai ZY, Li XM, Liang JP, et al. Bioavailability of tea catechins and its improvement. Molecules 2018; 23: 2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kumar R, Sharma A, Padwad Y, et al. Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology 2020; 21: 325–343. [DOI] [PubMed] [Google Scholar]
- 95. Farkhondeh T, Yazdi HS, Samarghandian S. The protective effects of green tea catechins in the management of neurodegenerative diseases: a review. Curr Drug Discov Technol 2019; 16: 57–65. [DOI] [PubMed] [Google Scholar]
- 96. Shaterzadeh-Yazdi H, Farkhondeh T, Samarghandian S. An overview on cardiovascular protective effects of catechins. Cardiovasc Hematol Disord Drug Targets 2017; 17: 154–160. [DOI] [PubMed] [Google Scholar]
- 97. Bernatoniene J, Kopustinskiene DM. The role of catechins in cellular responses to oxidative stress. Molecules 2018; 23: 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 2000; 97: 10832–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Montero JC, Seoane S, Ocana A, et al. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res 2011; 17: 5546–5552. [DOI] [PubMed] [Google Scholar]
- 100. Bruning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med Chem 2013; 13: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 101. Patel RV, Mistry BM, Shinde SK, et al. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem 2018; 155: 889–904. [DOI] [PubMed] [Google Scholar]
- 102. Khan N, Syed DN, Ahmad N, et al. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 2013; 19: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Terao J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem Pharmacol 2017; 139: 15–23. [DOI] [PubMed] [Google Scholar]
- 104. Kim Y, Je Y. Flavonoid intake and mortality from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. Clin Nutr ESPEN 2017; 20: 68–77. [DOI] [PubMed] [Google Scholar]
- 105. Maher P. How fisetin reduces the impact of age and disease on CNS function. Front Biosci (Schol Ed) 2015; 7: 58–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Syed DN, Adhami VM, Khan MI, et al. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem 2013; 13: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010; 11: 515–528. [DOI] [PubMed] [Google Scholar]
- 108. Trepel J, Mollapour M, Giaccone G, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010; 10: 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol 2003; 73: 689–701. [DOI] [PubMed] [Google Scholar]
- 110. Bourgeois B, Madl T. Regulation of cellular senescence via the FOXO4-p53 axis. FEBS Lett 2018; 592: 2083–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tia N, Singh AK, Pandey P, et al. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene 2018; 648: 97–105. [DOI] [PubMed] [Google Scholar]
- 112. Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017; 23: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang Y, Chang J, Liu X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016; 8: 2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ozsvari B, Nuttall JR, Sotgia F, et al. Azithromycin and roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany NY) 2018; 10: 3294–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wani WY, Gudup S, Sunkaria A, et al. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 2011; 61: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 116. Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 2010; 1201: 96–103. [DOI] [PubMed] [Google Scholar]
- 117. Ribeiro JR, Dabkowski ER, Shekar KC, et al. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 2018; 117: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Diot A, Morten K, Poulton J. Mitophagy plays a central role in mitochondrial ageing. Mamm Genome 2016; 27: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Aggarwal S, Mannam P, Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 2016; 311: L433–L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Uruno A, Furusawa Y, Yagishita Y, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 2013; 33: 2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Reddy PH, Manczak M, Kandimalla R. Mitochondria-targeted small molecule SS31: a potential candidate for the treatment of Alzheimer’s disease. Hum Mol Genet 2017; 26: 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dai DF, Chiao YA, Marcinek DJ, et al. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 2014; 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hou Y, Wu M, Wei J, et al. CD36 is involved in high glucose-induced epithelial to mesenchymal transition in renal tubular epithelial cells. Biochem Biophys Res Commun 2015; 468: 281–286. [DOI] [PubMed] [Google Scholar]
- 124. Okamura DM, Pennathur S, Pasichnyk K, et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol 2009; 20: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liani R, Halvorsen B, Sestili S, et al. Plasma levels of soluble CD36, platelet activation, inflammation, and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med 2012; 52: 1318–1324. [DOI] [PubMed] [Google Scholar]
- 126. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009; 2: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hou Y, Shi Y, Han B, et al. The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renal function in diabetic nephropathy. Nephrol Dial Transpl 2018; 33: 1908–1918. [DOI] [PubMed] [Google Scholar]
- 128. Skulachev VP, Anisimov VN, Antonenko YN, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 2009; 1787: 437–461. [DOI] [PubMed] [Google Scholar]
- 129. Bakeeva LE, Barskov IV, Egorov MV, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochemistry (Mosc) 2008; 73: 1288–1299. [DOI] [PubMed] [Google Scholar]
- 130. Agapova LS, Chernyak BV, Domnina LV, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells. Biochemistry (Mosc) 2008; 73: 1300–1316. [DOI] [PubMed] [Google Scholar]
- 131. Zernii EY, Gancharova OS, Tiulina VV, et al. Mitochondria-targeted antioxidant SKQ1 protects cornea from oxidative damage induced by ultraviolet irradiation and mechanical injury. BMC Ophthalmol 2018; 18: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie 2015; 61: 77–84. [DOI] [PubMed] [Google Scholar]
- 133. Bhattacharya S, Patel KK, Dehari D, et al. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem 2019; 462: 133–155. [DOI] [PubMed] [Google Scholar]
- 134. Reiter R, Tan D, Rosales-Corral S, et al. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018; 23: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pei HF, Hou JN, Wei FP, et al. Melatonin attenuates postmyocardial infarction injury via increasing Tom70 expression. J Pineal Res. Epub ahead of print 1 November 2016. DOI: 10.1111/jpi.12371. [DOI] [PubMed] [Google Scholar]
- 136. Manchester LC, Coto-Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 2015; 59: 403–419. [DOI] [PubMed] [Google Scholar]
- 137. Ramis MR, Esteban S, Miralles A, et al. Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev 2015; 146–148: 28–41. [DOI] [PubMed] [Google Scholar]
- 138. Yuan JP, Peng J, Yin K, et al. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 2011; 55: 150–165. [DOI] [PubMed] [Google Scholar]
- 139. Kim SH, Kim H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 2018; 10: 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sliva D, Loganathan J, Jiang J, et al. Mushroom Ganoderma lucidum prevents colitis-associated carcinogenesis in mice. PLoS One 2012; 7: e47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sudheesh NP, Ajith TA, Janardhanan KK. Ganoderma lucidum (Fr.) P. Karst enhances activities of heart mitochondrial enzymes and respiratory chain complexes in the aged rat. Biogerontology 2009; 10: 627–636. [DOI] [PubMed] [Google Scholar]
- 142. Lee YH, Kim JH, Song CH, et al. Ethanol extract of Ganoderma lucidum augments cellular anti-oxidant defense through activation of Nrf2/HO-1. J Pharmacopuncture 2016; 19: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cuong VT, Chen W, Shi J, et al. The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp Gerontol 2019; 117: 99–105. [DOI] [PubMed] [Google Scholar]
- 144. Mayo B, Vazquez L, Florez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019; 11: 2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pawlowski JW, Martin BR, McCabe GP, et al. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: a randomized crossover trial. Am J Clin Nutr 2015; 102: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Akaza H, Miyanaga N, Takashima N, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol 2004; 34: 86–89. [DOI] [PubMed] [Google Scholar]
- 147. Zhang T, Liang X, Shi L, et al. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS One 2013; 8: e79075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhou L, Li Y, Zhou D, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 2013; 24: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rattazzi M, Bertacco E, Del VA, et al. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transplant 2013; 28: 2968–2976. [DOI] [PubMed] [Google Scholar]
- 150. Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007; 317: 803–806. [DOI] [PubMed] [Google Scholar]
- 151. Hao S, He W, Li Y, et al. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 2011; 22: 1642–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci 2014; 105: 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Akcora BO, Storm G, Bansal R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 804–818. [DOI] [PubMed] [Google Scholar]
- 154. Xu M, Tchkonia T, Kirkland JL. Perspective: targeting the JAK/STAT pathway to fight age-related dysfunction. Pharmacol Res 2016; 111: 152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015; 4: e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sadowska-Bartosz I, Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed Res Int 2014; 2014: 404680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bouayed J, Bohn T. Exogenous antioxidants–double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 2010; 3: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014; 31: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Johnson SC. Nutrient sensing, signaling and ageing: the role of IGF-1 and mTOR in ageing and age-related disease. Subcell Biochem 2018; 90: 49–97. [DOI] [PubMed] [Google Scholar]
- 160. Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018; 44: 69–82. [DOI] [PubMed] [Google Scholar]
- 161. Mokbel K, Wazir U, Mokbel K. Chemoprevention of prostate cancer by natural agents: evidence from molecular and epidemiological studies. Anticancer Res 2019; 39: 5231–5259. [DOI] [PubMed] [Google Scholar]
- 162. Aghamiri S, Jafarpour A, Zandsalimi F, et al. Effect of resveratrol on the radiosensitivity of 5-FU in human breast cancer MCF-7 cells. J Cell Biochem 2019; 120: 15671–15677. [DOI] [PubMed] [Google Scholar]
- 163. Wang X, Chen L, Peng W. Protective effects of resveratrol on osteoporosis via activation of the SIRT1-NF-κB signaling pathway in rats. Exp Ther Med 2017; 14: 5032–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 2007; 26: 3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Witte AV, Kerti L, Margulies DS, et al. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci 2014; 34: 7862–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hwang JW, Yao H, Caito S, et al. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 2013; 61: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 168. Liu S, Zheng Z, Ji S, et al. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol 2018; 80: 473–479. [DOI] [PubMed] [Google Scholar]
- 169. Castilho RM, Squarize CH, Chodosh LA, et al. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 2009; 5: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lee JH, Budanov AV, Park EJ, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010; 327: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang R, Yu Z, Sunchu B, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 2017; 16: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science 2018; 359: eaan2788. [DOI] [PubMed] [Google Scholar]
- 173. Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr 2018; 108: 371–380. [DOI] [PubMed] [Google Scholar]
- 174. Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11: 1305–1314. [DOI] [PubMed] [Google Scholar]
- 175. Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res 2017; 40: 1197–1208. [DOI] [PubMed] [Google Scholar]
- 176. Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016; 22: 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dumitrescu R, Mehedintu C, Briceag I, et al. Metformin-clinical pharmacology in PCOs. J Med Life 2015; 8: 187–192. [PMC free article] [PubMed] [Google Scholar]
- 178. Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, et al. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017; 60: 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Batchuluun B, Inoguchi T, Sonoda N, et al. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 2014; 232: 156–164. [DOI] [PubMed] [Google Scholar]
- 180. He C, Zhu H, Li H, et al. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013; 62: 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179–5183. [DOI] [PubMed] [Google Scholar]
- 182. Shibata R, Ouchi N, Kihara S, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem 2004; 279: 28670–28674. [DOI] [PubMed] [Google Scholar]
- 183. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Moiseeva O, Deschenes-Simard X, St-Germain E, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF- κB activation. Aging Cell 2013; 12: 489–498. [DOI] [PubMed] [Google Scholar]
- 185. Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct 2018; 9: 705–714. [DOI] [PubMed] [Google Scholar]
- 186. Olszanecki R, Jawien J, Gajda M, et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 2005; 56: 627–635. [PubMed] [Google Scholar]
- 187. Kuszewski JC, Wong R, Howe P. Can curcumin counteract cognitive decline? Clinical trial evidence and rationale for combining omega-3 fatty acids with curcumin. Adv Nutr 2018; 9: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mazzanti G, Di Giacomo S. Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules 2016; 21: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sandur SK, Ichikawa H, Pandey MK, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 2007; 43: 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. He Y, Yue Y, Zheng X, et al. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 2015; 20: 9183–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017; 18: 447–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gao Y, Wei Y, Wang Y, et al. Lycium barbarum: a traditional Chinese herb and a promising anti-aging agent. Aging Dis 2017; 8: 778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tang L, Zhang Y, Jiang Y, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011; 236: 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li H, Li Z, Peng L, et al. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic Res 2017; 51: 200–210. [DOI] [PubMed] [Google Scholar]
- 195. Ermogenous C, Green C, Jackson T, et al. Treating age-related multimorbidity: the drug discovery challenge. Drug Discov Today 2020; 25: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 196. Martyanov V, Whitfield ML, Varga J. Senescence signature in skin biopsies from systemic sclerosis patients treated with senolytic therapy: potential predictor of clinical response? Arthritis Rheumatol 2019; 71: 1766–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chung CL, Lawrence I, Hoffman M, et al. Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience 2019; 41: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol 2015; 29: 642–651. [DOI] [PubMed] [Google Scholar]