Abstract
Development of acquired resistance to cisplatin (CDDP) is a major obstacle in the treatment of ovarian cancer patients. According to the cancer stem cell (CSC) hypothesis, the recurrence and chemoresistance are presumed to be linked to cancer stem/progenitor cells. Here, we investigated the CSC-like phenotypes and mechanism of chemoresistance in CDDP resistant ovarian cancer cells. A well-established CDDP sensitive ovarian cancer cell line A2780 and its resistant population A2780-Cp were used. We also developed a supra resistant population (SKOV3-Cp) from a naturally CDDP resistant cell line SKOV3. Both resistant/supra resistant cell lines showed significantly higher self-renewal capability than their parental counterparts. They also showed significant resistance to apoptosis and sub-G1 arrest by CDDP treatment. Stem cell marker ALDH1 positivity rates were higher both in A2780-Cp and SKOV3-Cp cell lines than in their counterparts, quantified by Aldefluor assay kit. Hoechst 33342 dye effluxing side populations were increased up to about five folds in A2780-Cp cells and two folds in SKOV3-Cp cells compared to A2780 and SKOV3 cells, respectively. Among major stemness related genes (POU5F1/OCT4, SOX2, NANOG, NES, BMI1, KLF4 and ALDH1A1), ALDH1A1 and KLF4 were significantly overexpressed in both resistant/supra resistant cells. Silencing ALDH1A1 in A2780 and A2780-Cp cells using siRNA greatly reduced the stem cell population and sensitized cells to CDDP. Moreover, silencing of ALDH1A1 reduced the transcript and protein level of its downstream target NEK-2. We also observed the downregulation of ABC transporters (ABCB1/MDR1, ABCG2 and ABCC1/MRP1) either by ALDH1A1 or NEK-2 silencing and upreguation of ABCB1/MDR1 due to the overexpression of NEK-2. Taken together, the present study suggests that stemness gene ALDH1A1 can be involved in CDDP resistance through the upregulation of NEK-2 in ovarian cancer.
Keywords: Cell biology, Cell culture, Cell death, Molecular biology, Cancer research, Cisplatin resistance, Ovarian cancer, ALDH1A1, NEK-2, ABC transporters, Stemness
Cell biology; Cell culture; Cell death; Molecular biology; Cancer research; Cisplatin resistance; Ovarian cancer; ALDH1A1; NEK-2; ABC transporters; Stemness.
1. Introduction
Ovarian cancer is considered as the most lethal gynecologic malignancy because of it's late clinical presentation and high recurrence rate (Webb and Jordan, 2017). The 5-years survival is as low as 25% among the patients with advanced disease and ovarian cancer is currently the 5th leading cause of death among women (Hu et al., 2016; Siegel et al., 2016). Platinum based regimen remain the chemotherapy backbone for the initial treatment of ovarian cancer (Wang and Wu, 2014). Unfortunately, patients frequently show drug resistance specially to cisplatin. Resistance to chemotherapy is one of the major causes of treatment failure in ovarian cancer (Agarwal and Kaye, 2003). Primary ovarian cancer, after debulking surgery and sub-sequent chemotherapy shows almost complete response in many patients with marked shrinkage in tumors. However, within few years in a higher percentage of patients relapse (Tummala and McGuire, 2005). Studies have linked such recurrence to the presence of a tiny fraction of residual tumor cells which is capable of secondary malignant growth. According to cancer stem cells (CSC) hypothesis, these resistant cells are mostly cancer stem-like cells (Lupia and Cavallaro, 2017).
A number of studies suggested an association between CSC and chemoresistant cells (Han et al., 2013; Piva et al., 2014). Piva et al., showed association of stemness related gene, SOX2 with tamoxifen resistance in breast cancer (Piva et al., 2014). Another study has found that paclitaxel resistant A2780 cells were enriched in aldehyde dehydrogenase 1 (ALDH1) (a marker for ovarian cancer stem like cells) and exhibit enhanced self rewnewal capacity (Han et al., 2013). ALDH1 family has several sub-family and members and among the members, ALDH1A1 strongly associated with drug-resistance and poor prognosis in ovarian cancer (Landen et al., 2010; Steg et al., 2012). Landen et al., assessed ALDH1A1 by the IHC analysis in 65 high-grade stage III–IV papillary serous ovarian cancer patients. They observed significantly shorter progression-free survival in patients with increasing ALDH1A1 expression. Steg et al., showed an increase of ALDH1A1 in persistent/relapsed ovarian cancer. However, there are scarcity of studies which involves ALDH1A1 in relation to the mechanism of drug-resistance in ovarian cancer. A study in multiple myeloma has shown ALDH1A1 mediated modulation of ABC transporter ABCB1/MDR1 through NEK-2 (NIMA-related expressed kinase 2) (Yang et al., 2014; Zhou et al., 2013).
NEK-2, a member of the NIMA-related family, has several putative roles in cell division, such as spindle formation and chromosome segregation (Faragher and Fry, 2003). Yang et al. showed that NEK-2 act as a downstream target of ALDH1A1 through the production of 9-cis-retinoic acid (9-CRA) in multiple myeloma. They also have showed the impact of NEK-2 on other ABC transporters such as ABCG2 and ABCC1/MRP1 (Yang et al., 2014; Zhou et al., 2013). NEK-2 also associated in drug-resistance in ovarian cancer which is evident from genomic and proteomic data (Liu et al., 2014).
In the present study, we investigated the differences of stem cell-like behavior between cisplatin-sensitive and cisplatin-resistant ovarian cancer cells and focused on the association of stemness related gene, ALDH1A1 with the resistance in relation to NEK-2 and ABC transporters.
2. Materials and methods
2.1. Reagents and antibodies
Propidium iodide (PI) and 9-CRA were purchased from Sigma Aldrich (St. Louis, MO, USA), cisplatin from Enzo life Sciences (Enzo life Sciences, NY, USA) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) from amresco (Solon, OH, USA). RPMI-1640 was obtained from Welgene (Daegu, South Korea), fetal bovine serum, trypsin–EDTA and penicillin–streptomycin were purchased from Gibco® Life Technologies (Gaithersburg, MD, USA). Fluorescein isothiocyanate (FITC)-labeled annexin V (Annexin V-FITC) kit were obtained from BD Biosciences Pharmingen (San Diego, CA, USA). Antibodies against ABCB1/MDR1 and ABCG2 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), NEK-2 from BD Transduction Laboratories™ (San Jose, CA, USA), ALDH1A1 from Abcam (Cambridge, MA, USA) and GAPDH from Millipore (Bedford, MA, USA).
2.2. Cell culture
The human ovarian cancer cell lines A2780, A2780-Cp, were kindly provided by Professor Benjamin K. Tsang at the University of Ottawa, Canada and Professor Gil Mor at the Wayne State University, USA. SKOV3 cells was purchased from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in RPMI-1640 media. All the media were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin, and maintained at 37 °C in a humidified atmosphere of 5% CO2 (Stericycle CO2 incubator, Thermo Scientific, Waltham, MA, USA).
2.3. Cytotoxicity assay
The effect of cisplatin or different siRNA on cell proliferation was determined by MTT assay as described previously (Hong et al., 2015). In brief, A2780, A2780-Cp, SKOV3 and SKOV3-Cp cells were plated onto 96-well plates at a density between 2,000 and 3,000 cells per well. The cells were cultured for 48 h in the presence of various concentrations of cisplatin (0.05 μM–40 μM) dissolved in dimethyl formamide (DMF). Cells were incubated with 50 μl MTT solution (2 mg/ml) for 3 h at 37 °C. Then MTT was removed and the cells were solubilized in 100 μl DMSO for 30 min. The optical density at 570 nm was measured using spectrophotometer (Labsystem Multiskan, Lab system, Helsinki, Finland).
2.4. 3D sphere formation assay
Sphere formation assay was performed according to previous reports (Bahmad et al., 2018) (Achilli et al., 2012) with slight modification. In brief, about 500–2,000 cells were seeded onto ultra low attachment plates (Corning, NY, USA) or poly-HEMA coated 60 mm culture dishes and cultured in serum-free Dulbecco's Modified Eagle's Medium/F12 medium containing 5 μg/ml insulin (Sigma-Aldrich, Kyunggi-Do, South Korea), 10 ng/ml basic fibroblast growth factor, B27 (0.1x) and 20 ng/ml human recombinant EGF (Invitrogen, Carlsbad, CA, USA). Cells were allowed to grow for 2 weeks to form spheres. Spheres that were more than 500 μm in diameter were counted for control and experimental groups.
2.5. Noble agar assay
Ovarian cancer cells were assayed to determine the capacity to form colonies in soft-agar as previously describe (Patel et al., 2012) with minor modification. Briefly, a total of 500–1,000 cells were suspended in DMEM containing 0.3% Noble agar (Sigma-Aldrich, Kyunggi-Do, South Korea). The bottom layer was consisted of 0.6% Noble agar in DMEM onto a 60 mm petri dish. DMEM media was added on top of agar and refreshed every 3–4 days to avoid dessication. The number of colonies per plate was counted in a phase-contrast microscope after 3 weeks of culture. Three independent experiments were carried out in triplicate.
2.6. Clonogenic assay
A total of 100–1,000 cells were seeded on the six well culture dishes at least in triplicate for 7–10 days. Cells were washed in PBS and fixed in 100% methanol. Colonies were stained with 0.5% crystal violet for 2 h and washed carefully in tap water. After air dry colonies containing more than 50 cells were counted.
2.7. Development of supra-resistant cell line
The supra-resistant SKOV-3 cells were established by exposure to six shock treatment of cisplatin with IC50 (11.5 μM) or IC90 (30 μM) doses. Cells were treated with drug for 2–4 h and allowed to grow up to 70–80% confluency between shocks. After six shocks cells were maintained in 1 μM cisplatin and expanded for experiments and cryopreservatoin.
2.8. Detection of apoptotic cells by flow cytometry
Cells were collected by trypsinization with 0.05% Trypsin-EDTA and washed twice with cold PBS. To include the floating cells, culture medium was also collected first. The cells were then stained with annexin V-FITC and PI according to the manufacturer's instructions (BD Pharmingen, CA, USA). Cells were then analyzed by flow cytometry (FACSCanto II, BD Biosciences, North Ryde, Australia) within 1 h.
2.9. Analysis of cell cycle
Cells were fixed in 70% cold ethanol (anhydrous) for overnight and stained with PI (20 μg/ml). RNAse A (40 μg/ml) treatment were performed 30 min before the addition of PI. Cells were then analyzed by flow cytometry (FACSCanto II, BD Biosciences, North Ryde, Australia) within 1 h.
2.10. siRNA mediated silencing
ALDH1A1 (siRNA-1: 5′-GGCUAAGAAGUAUAUCCUU-3′; siRNA-2: 5′-GAACAGUGUGGGUGAAUUG-3′ (Duong et al., 2012; Li et al., 2014)) and NEK-2 siRNA (siRNA-1: 5′-GGAGGGGAUCUGGCUAGUG-3′; siRNA-2: 5′-GGAAUGCCACAGACGAAGU-3′ (Kokuryo et al., 2007) and negative control siRNA were obtained from Genolution, Seoul, South Korea. Cells were seeded at 1.5× 105 cells/well in a 6-well plate in RPMI-1640 with 10% FBS without antibiotics. All siRNAs (10 nM) were transfected into cells using Lipofectamine 2000 or 3000 (Invitrogen, San Diego, CA) according to the manufacturer's protocol. After 6–8 h of siRNA transfection, media were replaced. Next day cells were replated for 2–3 days and collected for experiments.
2.11. Isolation of ALDH1+ cells
ALDH1+ cells were sorted using Aldefluor assay kit (Stemcell Technologies, Cambridge, MA, USA) according to manufacturer's instructions. An inhibitor of ALDH1, diethylamino-benzaldehyde (DEAB), was used as a negative control. A total 5 × 105 cells were stained for 30 min at 37 °C and analyzed by flow cytometry (BD FACSCanto II) within 1 h.
2.12. Isolation of side population
For side population analysis, cells were harvested and stained with 50 mg/ml Hoechst 33342 (Sigma-Aldrich, Kyunggi-Do, South Korea). The dye exclusion phenotype, via membrane efflux, was confirmed by an inhibitor, verapamil. Hoechst-stained cells were subjected to FACS using a FACSAria III (BD Biosciences, San Jose, CA, USA) for collection of dye-excluding (side population) cells. Side population (SP) and non-SP (NSP) cells were then observed for the stem cell characteristics.
2.13. Overexpression of NEK-2
NEK-2 synthetic cDNA construct was purchased from Addgene (pDONR223-NEK2; plasmid #23658). Retrovirus containing cDNA gene expression cassettes was constructed from Cosmogenetech, Seoul, Korea in to the pMXs vector. The primer sequences were NEK-2_AgeI_F: AACTGGACCGACCGGT-ATGCCTTCCCGGGCTGAG; NEK-2_SalI_R: TTATTTTATCGTCGAC-CTAAAAGATTAATGCACATAAC. Recombinant retrovirus was produced in 293T cells.
2.14. RNA extraction and cDNA preparation
Cells grown in a 60 mm and 100 mm dish were washed with PBS and collected with TRIzol® Reagent (Life Technologies, Carlsbad, CA, USA) using cell scraper. Cells were processed immediately or stored in -80 °C for extraction of RNA. Total RNA was isolated according to the manufacturer's instructions with minor modifications (Life Technologies, Carlsbad, CA, USA). The concentration of RNA was determined by the NanoDrop-2000 (Thermo Scientific, Wilmington, DE, USA). Extracted RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) to remove any genomic DNA. Reverse transcription of 1–2 μg of total RNA was done using PrimeScirpt™ reverse transcriptase cDNA synthesis kit (Takara, Tokyo, Japan). The synthesized cDNAs were kept at -20 °C until use.
2.15. Quantitative real-time PCR
The cDNAs were diluted 1:5 or 1:10 times. The cDNAs were amplified by PCR for 40 cycles (94 °C for 30 s, 55–62 °C for 30 s and 72 °C for 30 s) using the SYBR super mix (Bio-Rad, Hercules, CA, USA). After PCR amplification, melting curve analysis was performed to verify the PCR products. Gene expressions were calculated using 2−ΔΔCt method. Primers used in this study are summarized in Table 1.
Table 1.
Summary of the primer pairs for real-time RT-PCR.
| Genes | Sequence(s) Top line: forward primer 5′-3′ Bottom line: reverse primer 5′-3′ |
Gene Bank accession/reference |
|---|---|---|
| POU5F1/OCT4 | AGTGAGAGGCAACCTGGAGA ACACTCGGACCACATCCTTC |
NM_001285987.1 |
| BMI1 | AATCCCCACCTGATGTGTGT GCTGGTCTCCAGGTAACGAA |
NM_005180.8 |
| NANOG | ACCAGAACTGTGTTCTCTTCCACC CCATTGCTATTCTTCGGCCAGTTG |
AB093576.1 |
| NES | CCTGGGAAAGGGAGAGTACC TGGTCCTTCTCCACCGTATC |
NM_006617.1 |
| SOX2 | TCAGGAGTTGTCAAGGCAGAGAAG GCCGCCGCCGATGATTGTTATTAT |
NM_003106.3 |
| KLF4 | CCCAGCCAGAAAGCACTACA CAACTTCCAGTCACCCCCTT |
XM_005252305.1 |
| ALDH1A1 | TGGACCAGTGCAGCAAATCA ACGCCATAGCAATTCACCCA |
NM_000689.4 |
| NEK-2 | CATTGGCACAGGCTCCTAC GAGCCATAGTCAAGTTCTTTCCA |
NM_002497.2 (Takahashi et al., 2014) |
| ABCG2 | CAGGTCAGAGTGTGGTTTCTGTA TTGTGAGATTGACCAACAGACCAT |
NM_001257386.1 |
| ABCB1/MDR1 | CCGAACCGTTGTTTCTTTGACT ACCAAGTAGGCTCCAAACCG |
NM_000927.4 |
| ABCC1/MRP1 | TACCTCCTGTGGCTGAATCTGG CCGATTGTCTTTGCTCTTCATG |
XM_011522497.1 (Huang and Sadee, 2006) |
2.16. Western blotting
Western blotting was performed according to a previous study with a few modifications (Hong et al., 2015). In brief, after treatment, ovarian cancer cells were collected with 0.05% Trypsin-EDTA, washed with PBS and suspended in extraction buffer. The supernatant was collected following centrifugation with 13,000 rpm at 4 °C for 20 min. The protein concentration was measured using BCA protein assay kit (Pierce, Rockford, IL, USA). A total of 20 μg protein lysate was loaded and subjected to 10–12% SDS-PAGE. The proteins were transferred to nitrocellulose membrane and blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing 0.1% Tween-20. The membrane was incubated with specific primary antibodies overnight at 4 °C, and then incubated with peroxidase-conjugated secondary antibodies. Signals were visualized by fluorescence detector (Alpha Innotech, FluorChem HD2, CA, USA) using chemiluminescence detection kit (ECL™, GE Healthcare, UK). Densitometric analysis was performed using NIH ImageJ software version 1.50i (https://imagej.nih.gov/ij/docs/menus/analyze.html).
2.17. Statistical analysis
All the experiments were performed at least in triplicates and the data expressed as Mean ± SD. Student's t-test was done for statistical comparison. Microsoft excel 2010 and GraphPad Prism-5 were used for the analyses. A P value <0.05 was considered as statistically significant.
3. Results
3.1. Higher self-renewal capacity determined in cisplatin resistant or supra resistant populations of A2780 and SKOV3 ovarian cancer cells
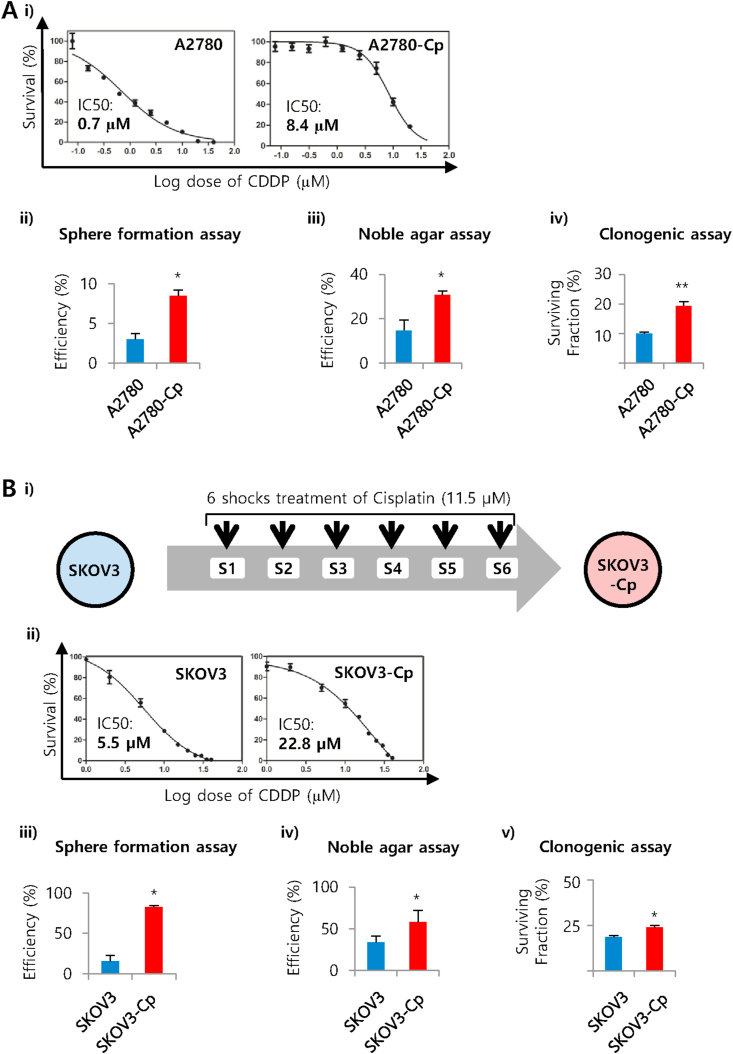
We have used well established commercially available cell line A2780, a high grade serous ovarian carcinoma cell line and developed resistant population A2780-Cp. Dose-response non-linear curve fit from MTT assay showed that A2780-Cp cells is about 12 times more resistant (IC50 value: 8.4 μM) than the original (IC50 value: 0.7 μM) (Figure 1A). Both of the cell lines were then evaluated for self-renewal capacity which is one of the most important characteristics of stem cells. We have performed three different assays for the detection of self-renewal capacity namely sphere formation assay, noble agar assay and clonogenic assay. We observed significantly higher sphere formation capacity in A2780-Cp resistant cell line compare to A2780 cells (8.5% vs 3%, 3-folds; P = 0.016). Similarly higher efficiency was observed in noble agar assay (30.7% vs 14.7%, 2-folds; P = 0.04). In clonogenic assay, we further observed the enhanced surviving fraction in the resistant cell line (19%; P = 0.0004) compared to parental cells (9.9%) (Figure 1A). Representative colonies from noble agar and clonogenic assays has shown in the supplementary information (Figure S1). Then we hypothesized that if chemoresistant is related to stemness, enhanced resistant cells will show more self renewal capacity or stem cell-like behaviors. For this purpose, we chose SKOV3 known as a chemoresistant cell line and developed a more or supra resistant population.
Figure 1.
Cisplatin resistant or supra resistant cell lines showed higher self-renewal capacity compared to parental cells. A) i) Determination of IC50 values for A2780 and A2780-Cp cells against cisplatin. Both the cell lines were treated with 0.078 μM–40 μM concentrations of cisplatin for 48 h. Dose-response non-linear curve fit was prepared from MTT assay using graphpad prism-5. ii) Spheres forming efficiency of A2780 and A2780-Cp cells. Cells (500–1,000) were allowed to grow in an ultra-low attachment, serum free culture condition in DMEM/F12 media supplemented with growth factors and developed spheres (>50 μm in diameter) counted after 2 weeks. Means of 3 experiments ±SD are shown. ∗P < 0.05 between A2780 and A2780-Cp cells. iii) Noble agar colony formation assay. Basal layers of 0.6% noble agar were prepared and the cells were seeded in 0.3% noble agar on the top of it and allowed to grow for 2 weeks. iv) Clonogenic assay for A2780 and A2780-Cp cells. Cells were seeded in 6-well plate, and allowed for 10 days to form colonies. B) Development of supra resistant SKOV3-Cp ovarian cancer cell line and its self-renewal capacity. i) Supra resistant SKOV3-Cp cell line was developed using six shocks treatment. A dose of 11.5 μM of cisplatin was given to the cells for 4 h followed by drug free culture until confluence. After confluence next shock was given and the process continued upto 6 shocks. Cells were maintained in 0.33 μM of cisplatin after 2–3 passage to maintain its resistance. A vehicle [dimethyl formamide (DMF)] treated control cells was also maintained in parallel. ii) Determination of IC50 values for SKOV3 and SKOV3-Cp cells against cisplatin as above. iii) Spheres forming efficiency of SKOV3 and SKOV3-Cp cells. iv) Noble agar colony formation assay. v) Clonogenic assay for SKOV3 and SKOV3-Cp cells.
The supra resistant population of SKOV3 was established following a pulse method using two concentrations (5 μM and 11.5 μM) of cisplatin shock treatment. A total of 6 shocks was given to mimic the six cycles of chemotherapy for the ovarian cancer patients. After 6 shocks, we found resistant population-1 (generated with 5 μM dose) is 4 times more resistant (IC50: 22.8 μM) than the parental cells (Figure 1B) and resistant population-2 (generated with 11.5 μM dose), 5 times more resistant (IC50: 27.8 μM) than the parental cells (Figure S2A). As we found similar level of resistance with lower dose shocks, we used resistant population-1 for further evaluation.
We compared the supra resistant population-1 and parental SKOV3 cells morphologically and did not find any difference. The phenotypic response of supra resistant population-1 to cisplatin was remarkable from crystal violet staining (Figures S2B-2D), where they survived in higher doses of cisplatin. Similar to A2780 resistant cell line (A2780-Cp), the supra resistant population-1 of SKOV3 (SKOV3-Cp) showed enhanced self-renewal capacity in all three self-renewal assays. We observed significantly higher level of sphere formation (15.4% and 82.4% in SKOV3 and SKOV3-Cp cells respectively; 5-folds, P = 0.005), more colonies in noble agar (34% and 58.4% in SKOV3 and SKOV3-Cp cells respectively; P = 0.049) and more surviving fraction in clonogenic assay (18% and 24% in SKOV3 and SKOV3-Cp cells respectively; P = 0.007) indicating association of chemoresistance with self renewal (Figure 1B).
3.2. Resistance to apoptotic cell death in cisplatin sensitive, resistant and supra resistant ovarian cancer cells
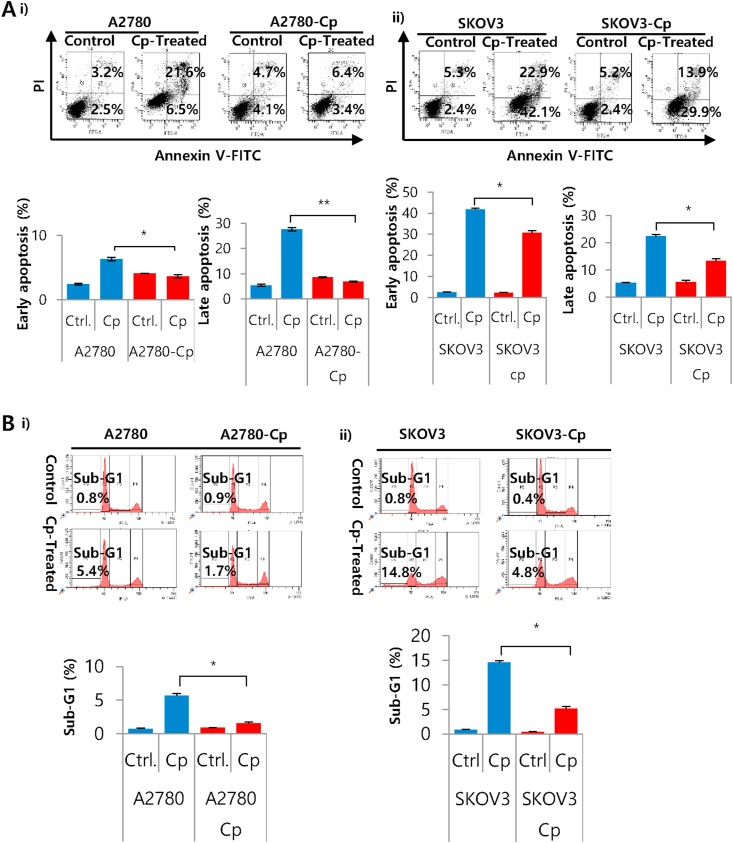
Next we evaluated the differences in apoptosis and cell cycle. We observed that cisplatin treatment causes less apoptotic cell death in resistant cell line A2780-Cp, both in early and late apoptotic phases (3.6% and 6.9% respectively) compared to parental cells (6.3% and 27.6% respectively) (Figure 2A). Similar trends were observed in supra resistant population SKOV3-Cp which showed significantly lower fraction of early (30.6%) and late apoptotic cells (13.4%) compared to parental cells (42% and 22.4% respectively) (Figure 2A). In cell cycle analysis, sub-G1 population was increased in A2780 sensitive cell line (5.6%) in response to cisplatin treatment, suggesting enhanced DNA damage. On the other hand, resistant A2780-Cp cell line showed lesser degree of DNA damage (1.5%). Similar trend also followed by SKOV3 supra resistant cells with comparatively lower number of sub-G1 cells (5.1% and 14.5% in supra resistant, SKOV3-Cp and SKOV3 cells respectively). Though earlier report showed G1 arrest with CDDP treatment (Liu et al., 2019), we did not observe G0/G1 cell cycle arrest in the cell lines we studied. Bar diagram with all cell cycle phases has shown in the supplementary information (Figure S3).
Figure 2.
Apoptotic cell death in cisplatin sensitive, resistant and supra resistant ovarian cancer cells. A) Annexin V/PI staining was performed for the detection of early and late apoptosis by flow cytometry after 24 h of cisplatin treatment. Images and graphs are the representative of three independent experiments and data were expressed as Mean ± SD. (i) A2780 and A2780-Cp cells were treated with 5 μM CDDP and (ii) SKOV3 and SKOV3-Cp cells were treated with 10 μM CDDP. ∗P < 0.05 and ∗∗P < 0.01, control versus cisplatin treatment. B) Cell cycle analysis of cisplatin sensitive, resistant and supra resistant ovarian cancer cells. PI staining was performed for the detection of sub-G1 cells by flow cytometry. (i) A2780 and A2780-Cp cells and (ii) SKOV3 and SKOV3-Cp cells. ∗P < 0.05 and ∗∗P < 0.01, control versus cisplatin treatment.
3.3. Determination of stem cell markers and gene expression in resistant and supraresistant cells compared to parental one
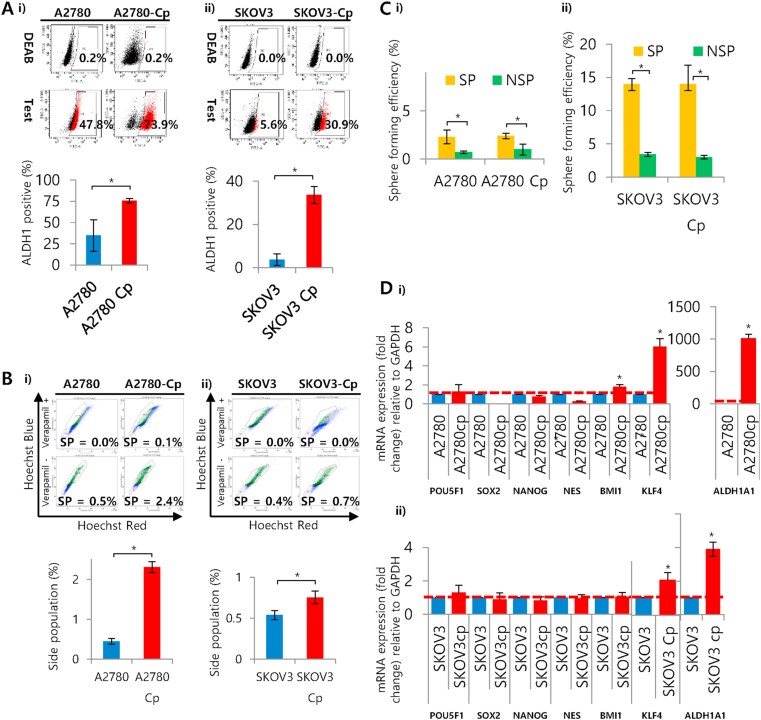
We checked stem cells marker, ALDH1 by ALDEFLUOR™ assay using flow cytometry. Both the resistant/supra resistant cells (A2780-Cp 76%, P = 0.044; SKOV3-Cp 34%, P = 0.021) showed significantly higher ALDH1 positivity rate compared to parental (A2780 35% and SKOV3 3.7%) (Figure 3A). We also isolated stem-like side population (SP) with Hoechst dye using flow cytometry equipped with UV laser. The gating strategy for the isolation of SP and NSP illustrated in Figure S4. It has been shown that SP cells isolated from ovarian cancer are tumorigenic and chemoresistant (Hu et al., 2010). Both the resistant/supra resistant cells (A2780-Cp 2.3%, P = 0.015; SKOV3-Cp 0.8%, P = 0.044) showed higher percentage of SP compared to their parental counterparts (A2780 0.45% and SKOV3 0.54%) (Figure 3B). The sphere forming efficiency of SP cells were higher in all cell lines irrespective to their resistant status (Figure 3C). A number of stemness related genes (POU5F1/OCT4, SOX2, NANOG, NES, BMI1, KLF4 (kruppel like factor 4) and ALDH1A1 (aldehyde dehydrogenase 1 subtype A1)) expression was evaluated to determine the stem-like characteristics among the resistant cells. Comparing A2780 sensitive and resistant cell lines, BMI1, KLF4 and ALDH1A1 (1.5, 6 and 1000-fold changes respectively) were signicantly overexpressed. We observed the amplification of KLF4 (2-folds) and ALDH1A1 (4-folds) in supra resistant population-1. As the expression of ALDH1A1 was much more prominent in both resistant cell lines, we focused on this gene to investigate it's involvement further with resistance. Moreover, we checked some stmeness associated genes including SOX2, NANOG, NES, ALDH1A1 in A2780 and A2780-Cp spheres and observed higher expression of NES in both (A2780, P = 0.005 and A2780-Cp, P = 0.014) compared to adherent cells (Figure S5). The ALDH1A1 expression was more than forty folds higher in A2780 spheres (P = 0.001), however, interestingly, we did not observe any change in ALDH1A1 expression in A2780-Cp spheres compared to adherent cells (Figure S5). This might be due to higher endogenous level of ALDH1A1 in A2780-Cp cells.
Figure 3.
Determination of stem cell markers and gene expression in resistant and supraresistant cells compared to parental one. A) ALDH positive cells in cisplatin sensitive, resistant and supra resistant ovarian cancer cells. Cells were stained with ALDEFLUOR™ substrate represents a positive population, which is blocked by specific inhibitor DEAB. Data were expressed as mean Mean ± SD. ∗P < 0.01 between (i) A2780 andA2780-Cp or (ii) SKOV3 and SKOV3-Cp cells. B) Determination of side populations (SP) in cisplatin sensitive, resistant and supra resistant cells by dual-filter flow cytometry. Cells were stained with 2.5 μg/ml concentration of Hoechst dye 33342. The control cells were incubated in the presence of 50 μM verapamil in addition to Hoechst dye. Verapamil-sensitive, Hoechst-dye excluding cells were considered as side population. (i) A2780 and A2780-Cp cells, and (ii) SKOV3 and SKOV3-Cp cells. C) Sphere forming efficiency of SP and Non-SP (NSP) collected from A2780 and SKOV3 cell lines. Cells were allowed to grow in ultralow attachment, serum free culture condition in DMEM/F12 media supplemented with growth factors and counted after 2 weeks. The numbers of sphere with >50 μm in diameter obtained from 500-1000 cells were considered for the determination of efficiency. Means of 3 experiments ±SD are shown. ∗P < 0.01 between SKOV3 and SKOV3-Cp cells. D) mRNA expression of stemness related genes in cisplatin sensitive, resistant and supra resistant cells. Relative expression for POU5F1/OCT4, SOX2, NANOG, NES, BMI1, KLF4 and ALDH1A1 mRNA with respect to the housekeeping gene GAPDH presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-ΔΔCt method with SYBR-green real-time PCR. (i) A2780 and A2780-Cp cells, and (ii) SKOV3 and SKOV3-Cp cells. Data were expressed as Mean ± SD. ∗P < 0.05 between A2780-A2780-Cp or SKOV3– SKOV3-Cp cells.
3.4. Silencing of ALDH1A1 and its effect on stem cell markers and cisplatin sensitivity in A2780 and A2780-Cp cell lines
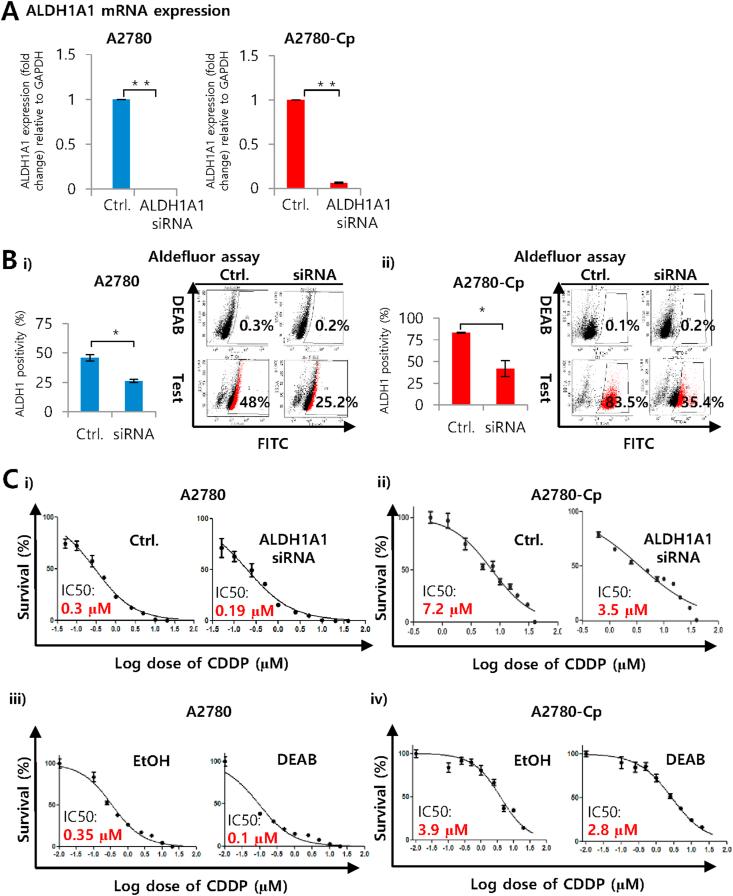
Next, we have evaluated the involvement of ALDH1A1 in the regulation of stemness. ALDH1A1 targeted siRNA efficiently silenced the gene transcriptions both A2780 sensitive (P = 0.0001) and resistant cell lines (P = 0.0001) (Figure 4A). Moreover, silencing of ALDH1A1 reduced the stem cell population detected by ALDEFLUOR™ assay suggesting involvement of ALDH1A1 in stemness regulation. In cisplatin sensitive A2780 cells, scrambled RNA (siRNA control) showed 48% of ALDH1 positivity which drops to 26% in the ALDH1A1 silenced cells (P = 0.011). In case of A2780-Cp resistant cells ALDH1 positive cells reduced from 83% to 42% in siRNA for ALDH1A1 transfected cells (P = 0.023) (Figure 4B). We also evaluated the involvement of ALDH1A1 in the regulation of cisplatin resistance. Silencing of ALDH1A1 using siRNA or inhibition of ALDH1 by DEAB (ALDH1 inhibitor) sensitized the ovarian cancer cells to cisplatin. In every treatment, the IC50 value was reduced in ALDH1A1 siRNA or DEAB group compared to siRNA control. Interestingly, in A2780 resistant cells (A2780-Cp) a more than 50% reduction in survival (IC50 7.2 μM in siRNA control to 3.5 μM in siRNA transfected group) suggests strong association of ALDH1A1 with cisplatin resistance.
Figure 4.
Silencing of ALDH1A1 and its impact on stem cell markers and cisplatin sensitivity in A2780 and A2780-Cp cell lines. A) Silencing mediated downregulation of ALDH1A1 expression in cisplatin sensitive and resistant A2780 cells. Relative expression for ALDH1A1 mRNA with respect to the gene GAPDH presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-ΔΔCt method with SYBR-green real-time PCR. Data were expressed as Mean ± SD. ∗∗P < 0.01 between control and siRNA treated cells. B) Reduction of ALDH positive population due to the silencing of ALDH1A1 gene in cisplatin resistant and supra resistant ovarian cancer cells. Cells were stained with ALDEFLUOR™ substrate represents a positive population, which is blocked by specific inhibitor DEAB. Data were expressed as Mean ± SD. ∗P < 0.05 between (i) A2780-A2780-Cp or (ii) SKOV3– SKOV3-Cp cells. C) Sensitization of ovarian cancer cells to cisplatin as a result of silencing ALDH1A1 with siRNA or inhibiting ALDH1 by DEAB. Cells were treated with 0.078 μM–40 μM concentrations of cisplatin for 48 h either treated with ALDH1A1 siRNA (i-ii) or DEAB (iii-iv). IC50 values were determined from the dose-response non-linear curve fit prepared by GraphPad prism-5 using data from MTT assay.
3.5. Silencing of ALDH1A1 and NEK-2 in A2780 cells and its effect on ABC membrane transporters
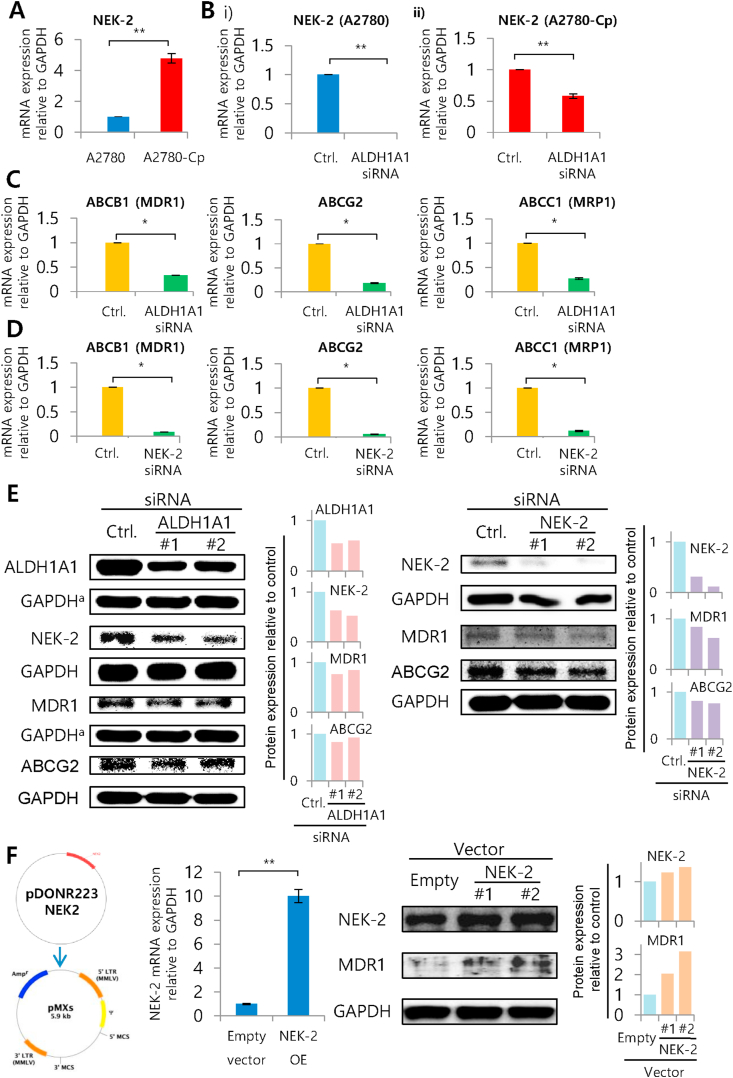
ALDH1A1 is an isotype of aldehyde dehydrogenase family out of 19 isotypes found in human. It has been shown that ALDH1A1 can cause chemoresistance through NEK-2 in Multiple myeloma. ALDH1A1 convert 9-cis-retinal to 9-CRA which then transcriptionally upregulates NEK-2 affecting ABC transporters (Yang et al., 2014). However, there is little or few evidences from solid tumor showing ALDH1A1's involvement in chemoresistance. The basal expression of NEK-2 was 5-fold higher in A2780 resistant cells compared to parental A2780 cells (P = 0.003) (Figure 5A). Treatment of A2780-Cp cells with 9-CRA for 4 h shown to induce NEK-2 as well as ABCG2 expression (Figure S6). Importantly, silencing of ALDH1A1 showed a reduction of NEK-2 in both A2780 (P = 0.0001) and A2780-Cp cells (P = 0.003), which suggests an association between ALDH1A1 and NEK-2 in ovarian cancer cells (Figure 5B). Moreover, silencing of ALDH1A1 further showed downregulation of a number of ABC transporters such as ABCB1/MDR1 (P = 0.004), ABCG2 (P = 0.002) and ABCC1/MRP1 (P = 0.003) (Figure 5C). We have also observed enhanced CDDP induced late apoptotic cell deaths after silencing ALDH1A1 (P = 0.017) or NEK-2 (P = 0.0001) in A2780 cells (Figure S7). In A2780-Cp cells, ALDH1A1 silencing failed to induce apoptosis might be due to very high endogenous level of ALDH1A1 (~1000-folds compared to parental cells), however, NEK-2 silencing showed significant increase (P = 0.021) in late apoptotic cell deaths (Figure S7).
Figure 5.
Silencing ALDH1A1 and NEK-2 in A2780 cells and its impact on ABC membrane transporters. A) NEK-2 expression in A2780 and A2780-Cp ovarian cancer cells. Relative expression for NEK-2 mRNA with respect to the gene GAPDH presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-ΔΔCt method with SYBR-green real-time PCR. Data were expressed as Mean ± SD. ∗P < 0.05 and ∗∗P < 0.01 between control and siRNA treated cells. B) Effect of ALDH1A1 silencing on NEK-2 expression in A2780 and A2780-Cp cells. C) Effect of ALDH1A1 silencing on ABC transporters expression (ABCB1, ABCG2 and ABCC1) in A2780-Cp cells. D) Effect of NEK-2 silencing on ABC transporter expression (ABCB1, ABCG2 and ABCC1) in A2780-Cp cells. E) Impact of ALDH1A1 and NEK-2 silencing on downstream molecules was assessed by western blotting. Bar diagram showing the protein expression compared to GAPDH determined by densitometric analysis of proteins bands. F) Overexpression of NEK-2 in A2780 cells, its confirmation at mRNA and protein levels and determination of ABCB1 expression. Bar diagram showing the protein expression compared to GAPDH determined by densitometric analysis of proteins bands.
To confirm whether the downregulation of ABC transporters is NEK-2 mediated or not, we performed NEK-2 silencing and checked the ABC transporter genes expression. We observed significant reduction of all selected ABC transporter gene expressions (ABCB1/MDR1, P = 0.001; ABCG2, P = 0.0005 and ABCC1/MRP1, P = 0.001) after silencing NEK-2 (Figure 5D). Silencing of ALDH1A1 showed reduction in ALDH1A1, NEK-2, ABCB1/MDR1 and ABCG2 at the protein level (Figure 5E (left panel)). Silencing of NEK-2 also showed the downregulation of NEK-2, ABCB1/MDR1 and ABCG2 at protein levels (Figure 5E (right panel)). Finally, we performed over-expression of NEK-2 in A2780 cell line using retroviral transfection. The overexpressed NEK-2 in A2780 cells was confirmed at mRNA and protein levels. NEK-2 overexpressed cells showed enhancement of ABCB1/MDR1 protein, which indicates the association of NEK-2 with ABC transporter mediated chemoresistance (Figure 5F). All original uncropped immunoblots of Figure 5E-F are presented in the supplementary figure file S8. A comprehensive resistant mechanism involving ALDH1A1, NEK-2 and ABC transporters in ovarian cancer cells is illustrated in Figure 6.
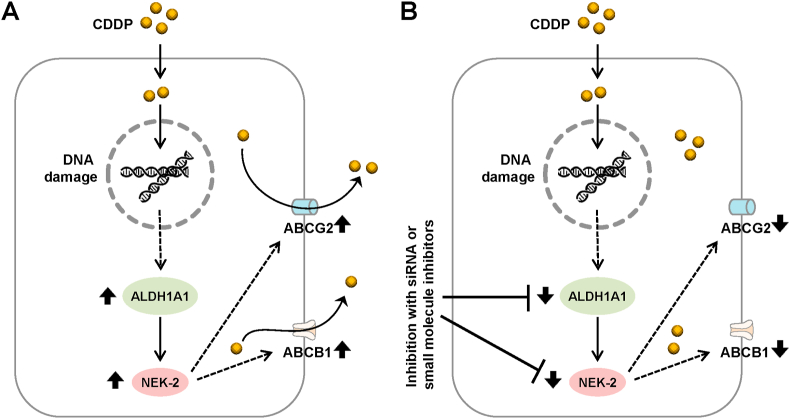
Figure 6.
Schematic diagram showing the mechanism of cisplatin (CDDP) resistance in ovarian cancer cells. A) Development of cisplatin resistance in ovarian cancer cells. Therapeutic treatment of ovarian cancer patient with cisplatin results in the development of resistant by increased expression of ALDH1A1, its downstream target NEK-2 and ABC transporters such as ABCB1/MDR1 by currently unknown mechanisms. B) Sensitization of ovarian cancer cells can achieved by targeting ALDH1A1 with siRNA or small molecule inhibitors. Inhibition of ALDH1A1 and/or NEK-2 can reduce the expression of ABC transporters which may leads to the accumulation of cisplatin inside cells resulting enhanced DNA damage and subsequent cell death.
4. Discussion
Chemoresistance in ovarian cancer remains as one of the major barriers in the clinic. Here we demonstrated that CDDP resistant ovarian cancer cells show cancer stem/progenitor cells like characteristics, overcome apoptotic cell death and modulate cancer resistance through the expression of stemness related gene, ALDH1A1. One of the potential mechanism underlying this resistance involve ABC transporters and NEK-2. Though, from a number of studies, individual association with chemoresistance shown in ALDH1A1 (Steg et al., 2012), selected ABC transporters (Fletcher et al., 2016) and NEK-2 (Yang et al., 2014), present study showed for the first time that ALDH1A1 causes ABC transporters (ABCB1, ABCG2 and ABCC1) upregulation via the modulation of NEK-2 in ovarian cancer cells.
Chemoresistance is one of the features but not the defining characteristics of CSC or progenitor cells. However, recurrence of ovarian cancer points towards a minor population of residual intrinsic cancer cells which supports the assumption that this disease is sustained or driven by CSC (Lupia and Cavallaro, 2017; Merlos-Suarez et al., 2011). One of the major characteristics of CSC is the ability to survive in anchorage independent condition (anoikis) as spheres usually in the ascites in case of advanced ovarian cancer. Thus enrichment of CSC is frequently reported in the patient ascites (Bapat et al., 2005; Di et al., 2013; Lupia and Cavallaro, 2017; Mo et al., 2015). Mimicking such situation in vitro we showed higher sphere formation capacity of resistant A2780-Cp cells as well as SKOV3-Cp developed supra resistant population under low attachment condition. This observation is consistent with earlier findings where they observed an increased sphere formation in paclitaxel and topotecan resistant W1 ovarian cancer primary cell lines (Januchowski et al., 2016). A study also found higher sphere formation in resistant cells was observed (Wang et al., 2013). In addition to sphere formation soft agar and clonogenic assay used to determine CSC-like characteristics (Choi et al., 2016; Shah et al., 2017; Xiao et al., 2017). We observed higher number of colonies in noble-agar and clonogenic assays which further confirms their potential to contain CSC. These results suggest that our resistant cells have CSC-like properties.
A number of mechanisms have been shown to cause drug resistance in CSC (Safa, 2016). Apoptosis is one of the important cancer hallmarks and CSC must avoid apoptosis to sustain a new tumor. In fact evidence suggests CSC are resistant to apoptosis (Dean, 2009) which reflected in our findings. We observed reduced rate of apoptosis in resistant and supra resistant cells. Moreover, our data showed low rate of sub-G1 population in resistant cells which suggesting the DNA protective capability. Our results are in agreement with previous report which showed no sign of apoptosis in CD133 + H460 Lung CSC after treatment with tissue factor targeting agent (Hu et al., 2017). In cell cycle analysis, studies showed enrichment of quiescent, G0/G1 or DNA synthesis, S phase population for CSC (Atashpour et al., 2015), however, we did not observe such difference in our studied resistant cells. Avoidance of both early and late apoptotic cell death and accumulation of sub-G1 population further indicates CSC phenotypes of our resistant cells.
Aldehyde dehydrogenases (ALDH1) activity and Hoechst 33342 dye effluxing side population extensively utilized for the determination of CSC (Behbod and Vivanco, 2015; Tomita et al., 2016). ALDH oxidizes aldehyde to corresponding carboxylic acids. Such detoxification processes protect CSC from reactive oxygen species (ROS) and reactive aldehyde mediated DNA damage. ALDH1 positivity considered as a CSC marker for different type of cancer (Lupia and Cavallaro, 2017). In the present study, both of our resistant and supra resistant cells, A2780-Cp and SKOV3-Cp respectively showed increased ALDH1 positivity and consistent with previous studies conducted in drug resistant patient samples or cell lines (Croker and Allan, 2012; Januchowski et al., 2016; Landen et al., 2010; Wang et al., 2013). Wang et al., 2013 and Landen et al., 2010 observed higher positivity of ALDH1 and ALDH1A1 in ovarian cancer patients. ALDH1 positivity was positively correlated lower overall survival (Wang et al., 2013) and ALDH1A1 positivity was negatively correlated with progression-free survival (Landen et al., 2010). A number of studies showed higher percentage of ALDH-positive cells in different breast and ovarian cancer cell lines resistant to CDDP, doxycycline, paclitaxel, topotecan etc. (Croker and Allan, 2012; Januchowski et al., 2016; Landen et al., 2010). These results supports the involvement of ALDH positivity and development of resistant phenotype in ovarian cancer cells.
In addition to ALDH activity, we have determined side population (SP) in our resistant and supra resistant cells. Higher percentage of SP observed in the resistant cells. Such increase in SP in drug resistant cells observed in earlier studies (Hosonuma et al., 2011; Hu et al., 2010; Richard et al., 2013; Szotek et al., 2006), which indicates the enrichment of CSC cells. The presence of CSC is frequently evaluated using the expression pattern of stemness related genes (Pozzi et al., 2015). Among major stemness related genes (POU5F1/OCT4, SOX2, NANOG, NES, BMI1, KLF4 and ALDH1A1), expression of ALDH1A1 and self-renewal capability was higher in our resistant cell lines. Januchowski et al., 2016 observed higher sphere formation capacity in ALDH1A1 overexpressing W1 ovarian primary cells resistant to paclitaxel and topotecan (Januchowski et al., 2016), which is in agreement with our findings. Our result also indicated that resistance may develops be through the expression of ALDH1A1. In fact, either siRNA mediated silencing of ALDH1A1 or inhibitor DEAB induced inhibition of ALDH1 causes downregulation of CSC marker and increases the sensitivity of cells to CDDP. Duong et al., 2012 showed sensitization of aggressive pancreatic adenocarcinoma cells MIA PaCa-2 to gemcitabine through ALDH1A1 silencing (Duong et al., 2012). Dylla et al., 2008 targeted ALDH1A1 of ESA + CD44 + colon CSC using siRNA and observed sensitivity to cyclophosphamide (Dylla et al., 2008). These and our results revealed that chemoresistance can be attributed to increased ALDH activity. Li et al., 2014 observed reduced stemness after silencing ALDH1A1 in lung adenoma stem cells. Alternatively, ALDH inhibitor DEAB mediated approach performed previously (Li et al., 2014). Januchowski et al., 2016 showed higher sensitivity to paclitaxel and topotecan in W1 primary ovarian cancer cells (Januchowski et al., 2016). Similarly Croker and Allan, 2012 observed sensitization of paclitaxel and DOX after DEAB treatment (Croker and Allan, 2012). Collectively, our findings suggests a possible association among stemness, chemoresistance and ALDH activity.
CSC overexpress ABC-family efflux multidrug transporters that flushes out different cytotoxic and targeted agents from the cells (Dean, 2009). A recent study in multiple myeloma showed association between CSC associated gene ALDH1A1 and ABC transporters through retinoic acid (RA) pathway (Yang et al., 2014). The study identified NEK-2 as a key player for such modulation. However, such evidence in solid tumor is unknown. In the present study, we have established such connection in ovarian cancer. Silencing ALDH1A1 downregulates NEK-2 both at mRNA and protein levels suggest the position of NEK-2 in the downstream of ALDH1A1 signaling. Yang et al., 2014 explains the initiation of ALDH1A1 signaling through the conversion of 9-cis-retinal to 9-CRA then through the expression of NEK-2. Similar to their finding we observed downregulation of selected ABC-transporters and apoptotic cell deaths after silencing ALDH1A1 or even NEK-2. Overexpression of NEK-2 confirms its involvement in this signaling through the upregulation of important ABC-transporter, ABCB1/MDR1. Mechanistically, cisplatin resistance largely depends on activation of drug influx system such as copper influx transporter CTR1, DNA-adduct formation etc (Holzer et al., 2006; Johnson et al., 1994). In the current study, role DNA influx pump or DNA-adduct formation has not explored and a subject of further investigation to establish the crucial role of ALDH1A1 in CDDP resistance.
Taken together, we uncovered a mechanism of CDDP resistance through up-regulation of ALDH1A1, NEK-2 and stemness in ovarian cacner cells. The findings from current study will implicate the ALDH1A1-NEK2-ABC transporters pathway in drug-resistance and may help to design specific small molecule inhibitors to overcome disease relapse in ovarian cancer.
Declarations
Author contribution statement
M. Uddin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Y. Song: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
B. Kim and U. Cho: Analyzed and interpreted the data.
A. Azmi: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Y. Song was supported by National Research Foundation of Korea (2009–0093820 and 5262–20150100) and BK21 plus program (5262–20150100). M. Uddin was supported by National Research Foundation of Korea (0431–20140010). A. Azmi was supported by National Cancer Institute (3P30CA022453).
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Professor Benjamin K. Tsang, Department of Cellular and Molecular Medicine at the University of Ottawa, Canada and Professor Gil G. Mor, Department of Obstetrics and Gynecology at the Wayne State University, USA for providing human ovarian cancer cell lines A2780 and A2780-Cp.
Contributor Information
Md. Hafiz Uddin, Email: uddinh@karmanos.org.
Yong Sang Song, Email: yssong@snu.ac.kr.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Figure S1. Colonies from noble agar and clonogenic assays. A) The phase-contrast micrograph of colonies in noble agar (original magnification X40) and B) colonies stained with crystal violet and photographed with Sony Cyber-shot DSC-W830 digital camera.
Figure S2. Development of supra resistant populations from SKOV3 cells against cisplatin using shock treatment. A) Dose-response non-linear curve fit for SKOV3 cell line after 6 shock treatment with moderate (11 µM) and high dose (30 µM) of cisplatin. Dimethylformamide used as vehicle. Resistant population-1 showed 4 times increase of IC50 value and resistant population-2 showed 5 times increase of IC50 value compared to parental SKOV3 cells. B) Morphological comparison between SKOV3 and it’s more resistant population (SKOV3-Cp). C) Preparation of cisplatin containing 1% noble-agar sphere on 100 mm culture dish. D) Phenotypic comparison between SKOV3 and resistant population, SKOV3-Cp. After 4 days of culture cells were stained with crystal violet.
Figure S3. Cell cycle phases distribution of cisplatin sensitive, resistant and supra resistant ovarian cancer cells. A) Bar diagram showing different cell cycle phases (%) of A2780 and A2780-Cp cells treated with or without 5 µM cisplatin (Cp) for 24 h. B) Bar diagram showing different cell cycle phases (%) of SKOV3 and SKOV3-Cp cells treated with or without 10 µM cisplatin (Cp) for 24 h.
Figure S4. Gating strategy for the isolation of side population (SP) and non-SP (NSP) by flow cytometry. A) Gating strategy followed sequential isolation of single cells, live cells, and Hoechst 33342 dye stained cells. B) Event statistics of different populations isolated by gating.
Figure S5. Expression of stemness associated genes SOX2, NANOG, NES, ALDH1A1 and downstream target of ALDH1A1, NEK-2 in adherent cells and in spheres of A2780 cells. Relative expressions of mRNA compared to GAPDH are presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-∆∆Ct method with SYBR-green real-time PCR. A) Expression of mRNAs in A2780 adherent cells and spheres. B) Expression of mRNAs in A2780-Cp adherent cells and spheres. Data were expressed as Mean ± SD. ∗P < 0.05 and ∗∗P < 0.01 between adherent cells and spheres.
Figure S6. Expression of NEK-2 and ABC transporters in A2780 and A2780-Cp cells induced by with 9-cis retinoic acid (9-CRA). Cells were treated with 10 µM 9-CRA for 4 h. Relative expressions of mRNA compared to GAPDH are presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-∆∆Ct method with SYBR-green real-time PCR. A) Expression of mRNAs in A2780 cells treated with or without 9-CRA. B) Expression of mRNAs in A2780-Cp cells treated with or without 9-CRA. Data were expressed as Mean ± SD. ∗P < 0.05 between control (Ctrl.) and 9-CRA.
Figure S7. Silencing of ALDH1A1 and NEK-2 and apoptotic cell death in cisplatin sensitive, resistant A2780 cells. Silencing mediated downregulation of ALDH1A1 or NEK-2 were done using RNAi technique. After 48 h of silencing, cells were treated with cisplatin (2 µM for A2780 and 10 µM for A2780-Cp cells for another 48 h. Annexin V/PI staining was performed for the detection of early and late apoptosis by flow cytometry. Images and graphs are the representative of independent experiments and data were expressed as Mean ± SD. Ai)-Aiii) representative flow cytometric images, early apoptosis and late apoptosis of A2780 cells respectively; Bi)-Biii) representative flow cytometric images, early apoptosis and late apoptosis of A2780-Cp cells respectively. ∗P < 0.05 and ∗∗P < 0.01.
Figure S8. The full original immunoblots of cropped images presented in Figure 5.
References
- Achilli T.M., Meyer J., Morgan J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expet Opin. Biol. Ther. 2012;12(10):1347–1360. doi: 10.1517/14712598.2012.707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R., Kaye S.B. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Canc. 2003;3(7):502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- Atashpour S., Fouladdel S., Movahhed T.K., Barzegar E., Ghahremani M.H., Ostad S.N., Azizi E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci. 2015;18(7):635–643. [PMC free article] [PubMed] [Google Scholar]
- Bahmad H.F., Cheaito K., Chalhoub R.M., Hadadeh O., Monzer A., Ballout F.…Abou-Kheir W. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front. Oncol. 2018;8:347. doi: 10.3389/fonc.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat S.A., Mali A.M., Koppikar C.B., Kurrey N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- Behbod F., Vivanco M.D. Side population. Methods Mol. Biol. 2015;1293:73–81. doi: 10.1007/978-1-4939-2519-3_4. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Park J.H., Han J.W., Kim E., Jae-Wook O., Lee S.Y.…Gurunathan S. Differential cytotoxic potential of silver nanoparticles in human ovarian cancer cells and ovarian cancer stem cells. Int. J. Mol. Sci. 2016;17(12) doi: 10.3390/ijms17122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker A.K., Allan A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Canc. Res. Treat. 2012;133(1):75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- Dean M. ABC transporters, drug resistance, and cancer stem cells. J. Mammary Gland Biol. Neoplasia. 2009;14(1):3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- Di J., Duiveman-de Boer T., Zusterzeel P.L., Figdor C.G., Massuger L.F., Torensma R. The stem cell markers Oct4A, Nanog and c-Myc are expressed in ascites cells and tumor tissue of ovarian cancer patients. Cell. Oncol. 2013;36(5):363–374. doi: 10.1007/s13402-013-0142-8. [DOI] [PubMed] [Google Scholar]
- Duong H.Q., Hwang J.S., Kim H.J., Kang H.J., Seong Y.S., Bae I. Aldehyde dehydrogenase 1A1 confers intrinsic and acquired resistance to gemcitabine in human pancreatic adenocarcinoma MIA PaCa-2 cells. Int. J. Oncol. 2012;41(3):855–861. doi: 10.3892/ijo.2012.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylla S.J., Beviglia L., Park I.K., Chartier C., Raval J., Ngan L.…Gurney A.L. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PloS One. 2008;3(6):e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher A.J., Fry A.M. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell. 2003;14(7):2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.I., Williams R.T., Henderson M.J., Norris M.D., Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Han X., Du F., Jiang L., Zhu Y., Chen Z., Liu Y.…Hua D. A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties. Oncol. Lett. 2013;6(5):1295–1298. doi: 10.3892/ol.2013.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer A.K., Manorek G.H., Howell S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006;70(4):1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Hong Y.H., Uddin M.H., Jo U., Kim B., Song J., Suh D.H.…Song Y.S. ROS accumulation by PEITC selectively kills ovarian cancer cells via UPR-mediated apoptosis. Front. Oncol. 2015;5:167. doi: 10.3389/fonc.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosonuma S., Kobayashi Y., Kojo S., Wada H., Seino K., Kiguchi K., Ishizuka B. Clinical significance of side population in ovarian cancer cells. Hum. Cell. 2011;24(1):9–12. doi: 10.1007/s13577-010-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., McArthur C., Jaffe R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Canc. 2010;102(8):1276–1283. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Bao J., Wang Z., Zhang Z., Gu P., Tao F.…Jiang W. The plasma lncRNA acting as fingerprint in non-small-cell lung cancer. Tumour. Biol. 2016;37(3):3497–3504. doi: 10.1007/s13277-015-4023-9. [DOI] [PubMed] [Google Scholar]
- Hu Z., Xu J., Cheng J., McMichael E., Yu L., Carson W.E., 3rd. Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget. 2017;8(1):1481–1494. doi: 10.18632/oncotarget.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Sadee W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Canc. Lett. 2006;239(2):168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Januchowski R., Wojtowicz K., Sterzyska K., Sosiska P., Andrzejewska M., Zawierucha P.…Zabel M. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int. J. Biochem. Cell Biol. 2016;78:248–259. doi: 10.1016/j.biocel.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Johnson S.W., Swiggard P.A., Handel L.M., Brennan J.M., Godwin A.K., Ozols R.F., Hamilton T.C. Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and -resistant human ovarian cancer cells. Cancer Res. 1994;54(22):5911–5916. [PubMed] [Google Scholar]
- Kokuryo T., Senga T., Yokoyama Y., Nagino M., Nimura Y., Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res. 2007;67(20):9637–9642. doi: 10.1158/0008-5472.CAN-07-1489. [DOI] [PubMed] [Google Scholar]
- Landen C.N., Jr., Goodman B., Katre A.A., Steg A.D., Nick A.M., Stone R.L.…Sood A.K. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol. Canc. Therapeut. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xiang Y., Xiang L., Xiao Y., Li F., Hao P. ALDH maintains the stemness of lung adenoma stem cells by suppressing the Notch/CDK2/CCNE pathway. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0092669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Fan J., Ai G., Liu J., Luo N., Li C., Cheng Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019;52(1):37. doi: 10.1186/s40659-019-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gao Y., Lu Y., Zhang J., Li L., Yin F. Upregulation of NEK2 is associated with drug resistance in ovarian cancer. Oncol. Rep. 2014;31(2):745–754. doi: 10.3892/or.2013.2910. [DOI] [PubMed] [Google Scholar]
- Lupia M., Cavallaro U. Ovarian cancer stem cells: still an elusive entity? Mol. Canc. 2017;16(1):64. doi: 10.1186/s12943-017-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suarez A., Barriga F.M., Jung P., Iglesias M., Cespedes M.V., Rossell D.…Batlle E. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8(5):511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Mo L., Bachelder R.E., Kennedy M., Chen P.H., Chi J.T., Berchuck A.…Pizzo S.V. Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78. Mol. Canc. Therapeut. 2015;14(3):747–756. doi: 10.1158/1535-7163.MCT-14-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.A., Ramkissoon S.H., Bryan M., Pliner L.F., Dontu G., Patel P.S.…Rameshwar P. Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Sci. Rep. 2012;2:906. doi: 10.1038/srep00906. https://www.nature.com/articles/srep00906#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva M., Domenici G., Iriondo O., Rabano M., Simoes B.M., Comaills V.…Vivanco M. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol. Med. 2014;6(1):66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi V., Sartini D., Rocchetti R., Santarelli A., Rubini C., Morganti S.…Emanuelli M. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell. Physiol. Biochem. 2015;36(2):784–798. doi: 10.1159/000430138. [DOI] [PubMed] [Google Scholar]
- Richard V., Nair M.G., Santhosh Kumar T.R., Pillai M.R. Side population cells as prototype of chemoresistant, tumor-initiating cells. BioMed Res. Int. 2013;2013:517237. doi: 10.1155/2013/517237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa A.R. Resistance to cell death and its modulation in cancer stem cells. Crit. Rev. Oncog. 2016;21(3-4):203–219. doi: 10.1615/CritRevOncog.2016016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M., Cardenas R., Wang B., Persson J., Mongan N.P., Grabowska A., Allegrucci C. HOXC8 regulates self-renewal, differentiation and transformation of breast cancer stem cells. Mol. Canc. 2017;16(1):38. doi: 10.1186/s12943-017-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. Ca - Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Steg A.D., Bevis K.S., Katre A.A., Ziebarth A., Dobbin Z.C., Alvarez R.D.…Landen C.N. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin. Canc. Res. 2012;18(3):869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szotek P.P., Pieretti-Vanmarcke R., Masiakos P.T., Dinulescu D.M., Connolly D., Foster R.…Donahoe P.K. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc. Natl. Acad. Sci. U. S. A. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Iwaya T., Sawada G., Kurashige J., Matsumura T., Uchi R.…Mimori K. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann. Surg Oncol. 2014;21(1):205–212. doi: 10.1245/s10434-013-3264-3. [DOI] [PubMed] [Google Scholar]
- Tomita H., Tanaka K., Tanaka T., Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7(10):11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala M.K., McGuire W.P. Recurrent ovarian cancer. Clin. Adv. Hematol. Oncol. 2005;3(9):723–736. [PubMed] [Google Scholar]
- Wang J., Wu G.S. Role of autophagy in cisplatin resistance in ovarian cancer cells. J. Biol. Chem. 2014;289(24):17163–17173. doi: 10.1074/jbc.M114.558288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.J., Wu S.P., Liu J.B., Shi Y.S., Huang X., Zhang Q.B., Yao K.T. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73(3):1219–1231. doi: 10.1158/0008-5472.CAN-12-1408. [DOI] [PubMed] [Google Scholar]
- Webb P.M., Jordan S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Xiao W., Gao Z., Duan Y., Yuan W., Ke Y. Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. J. Exp. Clin. Canc. Res. 2017;36(1):41. doi: 10.1186/s13046-017-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhou W., Xia J., Gu Z., Wendlandt E., Zhan X.…Zhan F. NEK2 mediates ALDH1A1-dependent drug resistance in multiple myeloma. Oncotarget. 2014;5(23):11986–11997. doi: 10.18632/oncotarget.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Yang Y., Xia J., Wang H., Salama M.E., Xiong W.…Zhan F. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Canc. Cell. 2013;23(1):48–62. doi: 10.1016/j.ccr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Colonies from noble agar and clonogenic assays. A) The phase-contrast micrograph of colonies in noble agar (original magnification X40) and B) colonies stained with crystal violet and photographed with Sony Cyber-shot DSC-W830 digital camera.
Figure S2. Development of supra resistant populations from SKOV3 cells against cisplatin using shock treatment. A) Dose-response non-linear curve fit for SKOV3 cell line after 6 shock treatment with moderate (11 µM) and high dose (30 µM) of cisplatin. Dimethylformamide used as vehicle. Resistant population-1 showed 4 times increase of IC50 value and resistant population-2 showed 5 times increase of IC50 value compared to parental SKOV3 cells. B) Morphological comparison between SKOV3 and it’s more resistant population (SKOV3-Cp). C) Preparation of cisplatin containing 1% noble-agar sphere on 100 mm culture dish. D) Phenotypic comparison between SKOV3 and resistant population, SKOV3-Cp. After 4 days of culture cells were stained with crystal violet.
Figure S3. Cell cycle phases distribution of cisplatin sensitive, resistant and supra resistant ovarian cancer cells. A) Bar diagram showing different cell cycle phases (%) of A2780 and A2780-Cp cells treated with or without 5 µM cisplatin (Cp) for 24 h. B) Bar diagram showing different cell cycle phases (%) of SKOV3 and SKOV3-Cp cells treated with or without 10 µM cisplatin (Cp) for 24 h.
Figure S4. Gating strategy for the isolation of side population (SP) and non-SP (NSP) by flow cytometry. A) Gating strategy followed sequential isolation of single cells, live cells, and Hoechst 33342 dye stained cells. B) Event statistics of different populations isolated by gating.
Figure S5. Expression of stemness associated genes SOX2, NANOG, NES, ALDH1A1 and downstream target of ALDH1A1, NEK-2 in adherent cells and in spheres of A2780 cells. Relative expressions of mRNA compared to GAPDH are presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-∆∆Ct method with SYBR-green real-time PCR. A) Expression of mRNAs in A2780 adherent cells and spheres. B) Expression of mRNAs in A2780-Cp adherent cells and spheres. Data were expressed as Mean ± SD. ∗P < 0.05 and ∗∗P < 0.01 between adherent cells and spheres.
Figure S6. Expression of NEK-2 and ABC transporters in A2780 and A2780-Cp cells induced by with 9-cis retinoic acid (9-CRA). Cells were treated with 10 µM 9-CRA for 4 h. Relative expressions of mRNA compared to GAPDH are presented as fold change. All samples were run in triplicate and relative gene expressions were determined by 2-∆∆Ct method with SYBR-green real-time PCR. A) Expression of mRNAs in A2780 cells treated with or without 9-CRA. B) Expression of mRNAs in A2780-Cp cells treated with or without 9-CRA. Data were expressed as Mean ± SD. ∗P < 0.05 between control (Ctrl.) and 9-CRA.
Figure S7. Silencing of ALDH1A1 and NEK-2 and apoptotic cell death in cisplatin sensitive, resistant A2780 cells. Silencing mediated downregulation of ALDH1A1 or NEK-2 were done using RNAi technique. After 48 h of silencing, cells were treated with cisplatin (2 µM for A2780 and 10 µM for A2780-Cp cells for another 48 h. Annexin V/PI staining was performed for the detection of early and late apoptosis by flow cytometry. Images and graphs are the representative of independent experiments and data were expressed as Mean ± SD. Ai)-Aiii) representative flow cytometric images, early apoptosis and late apoptosis of A2780 cells respectively; Bi)-Biii) representative flow cytometric images, early apoptosis and late apoptosis of A2780-Cp cells respectively. ∗P < 0.05 and ∗∗P < 0.01.
Figure S8. The full original immunoblots of cropped images presented in Figure 5.