Abstract
Background
Ketamine is a novel fast-acting antidepressant. Acute ketamine treatment can reverse microstructure deficits and normalize functional alterations in the brain, but little is known about the impacts of ketamine on brain volumes in individuals with depression.
Methods
We used 3 T magnetic resonance imaging (MRI) and tensorbased morphological methods to investigate the regional volume differences for 29 healthy control (HC) subjects and 21 subjects with major depressive disorder (MDD), including 10 subjects with comorbid post-traumatic stress disorder (PTSD). All the subjects participated in MRI scanning before and 24 h post intravenous ketamine infusion. The effects of acute ketamine administration on HC, MDD, and MDD/PTSD groups were examined separately by whole-brain voxel-wise t-tests.
Results
Our data showed smaller volume of inferior frontal gyrus (IFG, opercular part) in MDD and MDD/PTSD subjects compared to HC, and a significant correlation between opercular IFG volume and depressive severity in MDD subjects only. Ketamine administration normalized the structural alterations of opercular IFG in both MDD and MDD/PTSD groups, and significantly improved depressive and PTSD symptoms. Twenty-four hours after a single ketamine infusion, there were two clusters of voxels with volume changes in MDD subjects, including significantly increased volumes of opercular IFG. No significant structural alterations were found in the MDD/PTSD or HC groups.
Conclusion
These findings provide direct evidence that acute ketamine administration can normalize structural alterations associated with depression and highlight the importance of IFG in the guidance of future therapeutic targets.
Keywords: ketamine, depression, brain volumes, structural alterations, inferior frontal gyrus, major depressive disorder, post-traumatic stress disorder, magnetic resonance imaging
Introduction
Major depressive disorder (MDD) has become the leading cause of disability around the world.1 The proportion of the world population suffering from depression is estimated to be over 300 million people and the prevalence of depression is still increasing.2 However, first-line antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs) have limited effectiveness and slow onset of clinical response, with only one-third of depressed patients remitted after a first course of treatment.3 In contrast with traditional antidepressants, a single dose of ketamine results in a robust, sustainable antidepressant effect and can significantly reduce suicidal thoughts within 24 h.4,5 A large number of studies, mostly preclinical, report the molecular, cellular, and behavior changes associated with the effects of ketamine on the brain, significantly advancing our understanding of the biological mechanisms underlying ketamine’s therapeutic effects. However, the evaluation of acute ketamine effects on brain structure in living humans with depression is limited. Investigation of ketamine's antidepressant mechanism of action provides a promising path in the development of new therapeutic strategies for major depression.6
There are widespread microstructural abnormalities in individuals with depression,7–9 which have been related to the depression severity, development of depressive symptoms, and remission status.10–12 These microstructure alterations are reflected in the brain morphological changes in depression. For example, abnormal microstructure has been shown in the hippocampus of subjects with depression.13 Consistently, some neuroimaging studies demostrated that the hippocampus volume was abnormal in depressed patients.14,15 A meta-analysis of cortical thickness in unmedicated MDD patients has shown greater cortical thickness in some areas (posterior cingulate cortex, ventromedial prefrontal cortex, and anterior cingulate cortex) and lower cortical thickness in others (gyrus rectus, orbital segment of the superior frontal gyrus, and middle temporal gyrus) in comparison with healthy control (HC).16 Another study revealed lower grey matter volume in the right supplementary motor area, left insula, and right middle temporal gyrus of individuals with depression.17 There are other structural abnormalities in depressed subjects, including altered volumes and shapes of putamen and thalamus, and lower volumes of anterior cingulate cortex, amygdala and prefrontal cortex.18–20 Collectively, those findings suggest that depression includes structural alterations in multiple brain regions.
It has been repeatedly shown that acute ketamine administration can rapidly induce synaptic plasticity, and reverse stress-related synaptic deficits and depressive-like symptoms in animals.9,21 For example, animal studies demonstrated that ketamine administration increased dendritic arborization and soma size in certain neurons within a few hours,22 inhibited stress-induced dendritic spine loss and elicit spine formation in the brain of stressed mice.23 The synaptic alterations induced by acute ketamine treatment can be reflected by changes in microstructure and morphology in the brain. A previous neuroimaging study has shown that white matter microstructure abnormalities in depressed patients can be rapidly restored with ketamine treatment.24 Another study found that a single ketamine infusion can normalize the altered volumes of hippocampus and nucleus accumbens in depressed remitters at 24 h following treatment.25
Therefore, we hypothesized that acute ketamine administration will normalize structural alterations in multiple areas (such as frontal areas) associated with depression. To explore the effects of acute ketamine administration on brain structures, we examined the volumes of regions across the whole brain in the MDD group (including MDD/PTSD subgroup) compared to HC group and investigated whether regions with altered volumes would be normalized at 24 h following ketamine administration. Additionally, we investigated the relationship between volumes and depressive severity. To understand the structural alterations induced by ketamine administration in different subjects, we conducted a voxel-wise structural comparison between baseline and post ketamine scans for each subject, and then examined the structural alterations in groups.
Methods
Participants
Participants aged between 18 and 60 years old underwent physical and neurological examination to rule out any major medical or neurological illness. Exclusion criteria for all participants were alcohol and drug use disorder, except for nicotine dependence; positive urine toxicology or pregnancy tests before any scan; significant medical condition or magnetic resonance imaging (MRI) contraindications; psychotropic medication within the past 2 months. Additional exclusive criteria for healthy participants were current or history of any DSM-5 diagnosis except for nicotine dependence; first-degree relative with a history of psychotic, mood, or anxiety disorder; regular medication use with the past 2 months or history of psychiatric medication use. Subjects were not receiving psychotherapy or non-invasive stimulation treatment throughout the study.
The Structural Clinical Interview for DSM-IV-TR or DSM-5 was administered at screening. Mood and PTSD symptoms were additionally assessed using the Hamilton Depression Rating Scale (HAMD-24) and the PTSD Checklist (PCL-5) at baseline, 24 h after ketamine administration, and 48 h after ketamine administration. Individuals with MDD and/or PTSD had to meet current criteria for major depressive disorder in current depressive episode, and/or PTSD in order to participate in the study.
In total, 29 healthy controls (HC group) and 21 subjects with MDD (including 10 subjects comorbid with PTSD) participated in this study. All study procedures were approved by the Yale University Institutional Review Board and the Yale MRRC Protocol Review Committee. All participants provided written informed consent.
Ketamine Administration
Racemic ketamine was obtained from the Yale-New Haven Hospital Pharmacy and administered intravenously. There were 36 subjects who participated in an initial bolus of 0.23 mg/kg over 1 min followed by a constant infusion of 0.58 mg/kg over 1 hour (23 HC, 9 MDD and 4 MDD/PTSD). There were 14 subjects (6 HC, 2 MDD and 6 MDD/PTSD) who participated in an infusion of 0.5 mg/kg over 40 mins. Both ketamine doses are validated subanesthic dosing regimens frequently used in previous studies.26,27
MRI Acquisition
T1-weighted MRI scans were acquired on a 3 T Siemens Tim Trio scanner and later upgraded to a Siemens Prisma scanner (voxel size = 1 × 1 × 1 mm, FoV = 256 mm, TR = 1200 ms, TE = 2.66 ms, flip angle = 12°). All participants completed MRI scans at baseline prior to ketamine administration and 24 h following ketamine administration.
Tensor-Based Morphometry
Brain volume changes were processed via the Yale Bioimage Suite software package28 (http://www.bioimagesuite.org) running on a Linux workstation (Red Hat Inc., Raleigh, North Carolina). First, images were skull stripped and any remaining non-brain tissues was manually removed. All the images were aligned to Montreal Neurological Institute (MNI) space, using a previously validated nonrigid registration algorithm.29 This algorithm, based on a free-form deformation model and normalized mutual information, was used to compute the local volume deformations required to map the registered image to the target image. The determinant of the Jacobian of the deformation field was used to qualify local volume differences between the registered images and the MNI template. To compare the regional volume differences across whole brain between groups, the individual Jacobian maps were parcellated with automated anatomical labeling (AAL3) atlas30 and the Jacobian values were extracted across whole brain for each subject.
To examine the volume changes between baseline and 24 h post ketamine administration, this nonlinear registration algorithm was applied to compute the within-subject transformations. The degree of local volume expansion or contraction (percent change) was computed with Jacobian maps. This analysis produced Jacobian maps with a resolution of 1x1x1 mm3, where each voxel has a value representing the local volume change: 1 represents no volume change, >1 represents increased volume after ketamine administration, and <1 represents decreased volume after ketamine administration. The individual Jacobian maps were then warped to the standard Montreal Neurological Institute (MNI) space and the t-score maps of Jacobian values were extracted in MDD and MDD/PTSD groups for further analysis.
Statistical Analysis
The distribution normality of clinical variables and outcome measures was tested by Shapiro-Wilk tests. Non-parametric tests and transformations were used as necessary. SEM was provided as an estimate of variance. Significance was set at p < 0.05. Group differences between HC and MDD groups (and sub-groups) in gender, age and clinical severity were assessed using Chi-square test, and one-way ANOVA followed by Bonferroni post-hoc test. The regional volume differences between HC and MDD groups were examined with one-way ANOVA followed by Fisher’s Least Significant Difference (LSD) post-hoc test. General linear model (GLM) was used to examine the effects of diagnosis and subgroups on the brain volume, controlling for gender, age, education and smoking status. The effects of ketamine administration on the volume of interested regions and clinical severity were performed with GLM, followed by pairwise comparison between pre and post ketamine infusion. Spearman correlation analysis was conducted to examine the relationship between brain volume and clinical severity.
Within-subject Jacobian maps at each voxel throughout the brain were threshold at p < 0.001, two-tailed. To correct for multiple comparison, AFNI’s 3dClustSim program were applied to obtain a corrected significance level of p < 0.05. The MNI location of the peak maxima of significant clusters were determined and reported using xjView toolbox (http://www.alivelearn.net/xjview).
Results
There were no significant sex and age differences between MDD (12 females and 9 males, 35.80 ± 2.7 years) and HC groups (15 females and 14 males, 32.90 ± 2.3 years). Similarly, sex and age did not differ between MDD only (7 females and 4 males, 37.82 ± 4.6 years), MDD/PTSD (5 females and 5 males, 33.50 ± 2.5 years), and HC groups. All p values > 0.05 after Bonferroni correction.
Volume Differences Compared to HC
All MDD vs HC
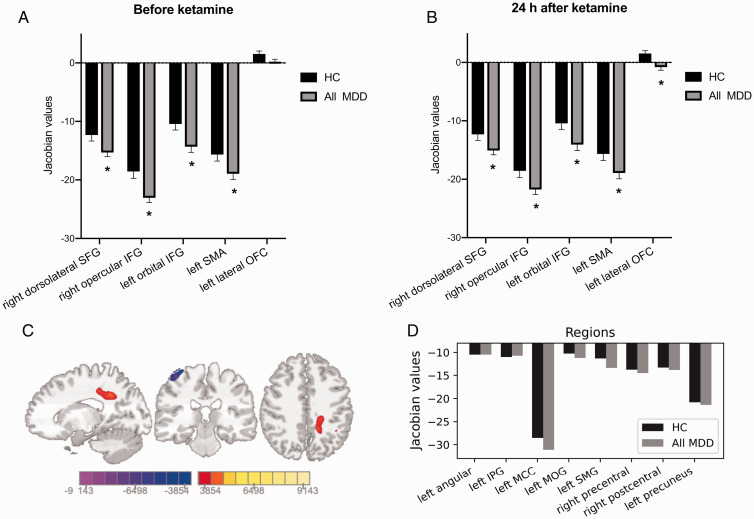
Controlling for sex, age, education, and smoking status, diagnosis (HC or All MDD) had a significant effect on the volume of right dorsolateral superior frontal gyrus (SFG; F = 3.63, p = 0.0078), right opercular inferior frontal gyrus (IFG; F = 5.29, p = 0.0007), left orbital IFG (F = 8.77, p < 0.0001), left supplementary motor area (SMA; F = 2.5, p = 0.04). All those regions in the All MDD group showed a smaller volume compared to HC before ketamine administration (all p values < 0.05, t-test, Figure 1(A)). There was no significant difference in left lateral OFC volume between the All MDD group and the HC group.
Figure 1.
Smaller volumes in the All MDD group compared to the HC group. A and B, After one single infusion of ketamine, All MDD group (including MDD and MDD/PTSD subjects) still showed smaller volume in some regions compared to HC. Additionally, left lateral OFC volume was significantly smaller in the All MDD group 24 h after ketamine administration. Bar represented mean ± SEM. Asterisks indicates significance at p < 0.05. C and D, Colored voxels showing the local volume changes in the All MDD group after ketamine administration (threshold at p < 0.001, FWE corrected). The color bar represents the t-score of Jacobian values. MNI coordinates: x= −14, y = −27, z = 41.Voxels were involved in eight regions, none of which showed significant volume difference compared to HC. IPG: inferior parietal gyrus; MCC: middle cingulate & paracingulate gyri; MOG: middle occipital gyrus; SMG: supramarginal gyrus.
After ketamine administration, right dorsolateral SFG, right opercular IFG, left orbital IFG and left SMA remained different in volumes compared to HC (all p values < 0.05, t-test, Figure 1(B)). However, left lateral OFC showed significantly decreased volume in the All MDD group after ketamine administration (p = 0.003, Figure 1(B)).
MDD, MDD/PTSD vs HC
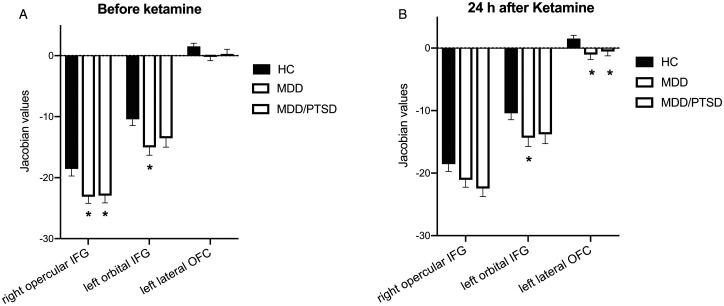
Controlling for sex, age, education, and smoke status, disease diagnosis (HC, MDD or MDD/PTSD) had a significant effect on the volume of right opercular IFG (F = 5.13, p < 0.0001), left orbital IFG (F = 8.49, p < 0.0001). Compared to HC, both MDD and MDD/PTSD groups showed a smaller volume in right opercular IFG (corrected p = 0.02 and p = 0.03, ANOVA, Figure 2(A)). The MDD group had a smaller volume in left orbital IFG compared to HC (corrected p = 0.01, ANOVA, Figure 2(A)). There was no significant difference in left lateral OFC volume between the MDD, MDD/PTSD and the HC group.
Figure 2.
Increased volume in right opercular IFG after ketamine administration. A, Before ketamine administration, both MDD and MDD/PTSD subjects showed significantly smaller volume in right opercular IFG compared to HC subjects. Besides, MDD subjects had a significantly smaller volume in left lateral OFC. B, After ketamine administration, there was no significant volume difference in right opercular IFG between HC, MDD, and MDD/PTSD groups. However, both MDD and MDD/PTSD groups showed a significantly smaller volume in left lateral OFC compared to HC. Asterisks indicates significance at p < 0.05.
After ketamine administration, there were no group differences in the volume of right opercular IFG. However, there was a trending difference in the volume of left orbital IFG between MDD and HC group (corrected p = 0.059, ANOVA). Both MDD and MDD/PTSD groups showed a lower volume in left lateral OFC (corrected p = 0.008 and p = 0.03, ANOVA, Figure 2(B)) compared to the HC group.
Ketamine Administration and Symptom Relief
Ketamine administration had a significant effect on depression severity of MDD subjects (pre-administration HAMD = 20.18 ± 2.8; 24 h post-administration HAMD = 9.27 ± 3.3, F = 6.41, p = 0.019) and MDD/PTSD subjects (pre-treatment HAMD = 25.00 ± 2.2, 24 h post-treatment HAMD = 10.3 ± 2.0, F = 23.7, p < 0.001). However, ketamine did not show significant effects on PTSD severity in MDD/PTSD subjects (pre-administration PCL = 48.25 ± 7.2, post-administration PCL = 33.25 ± 5.8, F = 3.28, p = 0.09). 24 h after ketamine infusion, 55% MDD subjects (6 out of 11) and 70% MDD/PTSD subjects (7 out of 10) met depression remission criteria. However, only 37.5% MDD/PTSD subjects met PTSD remission criteria.
All MDD Subjects
Compared to baseline, tensor-based morphometry (TBM) results indicated two clusters of voxels representing volume changes in the All MDD group 24 h after ketamine administration (Figure 1(C)), with one cluster (6347 voxels) showing significantly increased volume in six regions: left angular gyrus, left inferior parietal gyrus (IPG), left middle cingulate & paracingulate gyri (MCC), left middle occipital gyrus (MOG), left supramarginal gyrus (SMG), left precuneus and the other cluster (1762 voxels) showing with significantly decreased volume in the right precentral gyrus and right postcentral gyrus (see Supplementary Table 1). However, before ketamine administration, the volumes of those regions were not significantly different from the HC group, as shown in Figure 1(D).
MDD Subjects
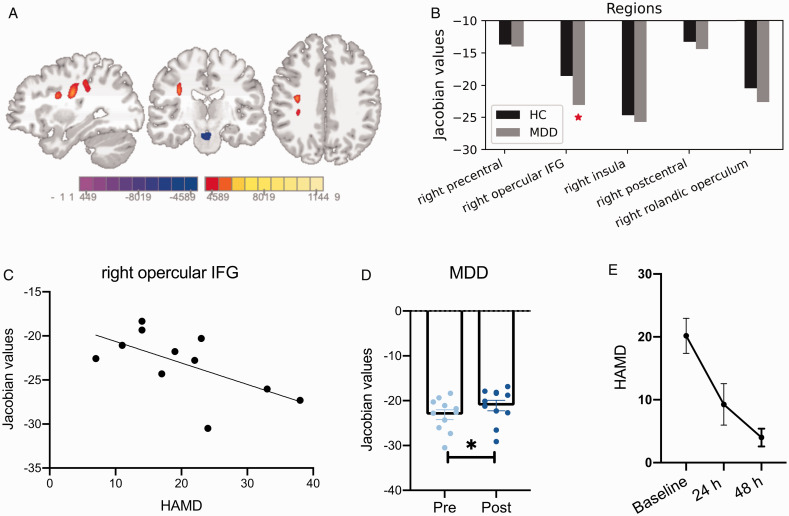
Compared to baseline, TBM results indicated two clusters of voxel changes in the MDD group 24 h after ketamine administration (Figure 3(A)), with one cluster showing increased volume in five regions: right precentral gyrus, right opercular IFG, right rolandic operculum, right insula and right postcentral gyrus, and the other cluster showing decreased volume in the areas of midbrain (see Supplementary Table 2). Before ketamine administration, the volume of those five regions were all relatively smaller in the MDD group compared to HC, especially right opercular IFG showed a significant volume decrease compared to HC (p = 0.03, Figure 3(B)).
Figure 3.
Ketamine had a significant effect on the volume of right opercular IFG and depressive symptom relief for MDD subjects. A, Colored voxels showing the local volume changes after ketamine administration (threshold at p < 0.001, FWE corrected). The color bar represents the t-score of Jacobian values. MNI coordinates: x = 32, y = −18, z = 33. B, Voxels representing increased volume located in five regions that showed a smaller volume in MDD subjects prior to ketamine administration, compared to HC. Especially, the volume of right opercular IFG was significantly smaller in comparison to HC. C and D, There was a significant correlation between the volume of right opercular IFG and HAMD scores in MDD subjects. After ketamine administration, MDD subjects had a significant volume increase in right opercular IFG. E, The depressive severity of MDD subjects were significantly improved within 24 h and 48 h after ketamine administration. Asterisks indicates significance at p < 0.05.
Before ketamine administration, the volume of right opercular IFG showed a negative correlation with depression severity in the MDD group (r = −0.62, p = 0.04, Figure 3(C)). After ketamine administration, the volume of right opercular IFG was increased (p < 0.05, paired t-test, Figure 3(D)), and depression severity of the MDD group was significantly decreased (p < 0.001, paired t-test, Figure 3(E)). There was no significant correlation between the volume of other regions indicated by VBM results and symptom severity.
MDD/PTSD Subjects
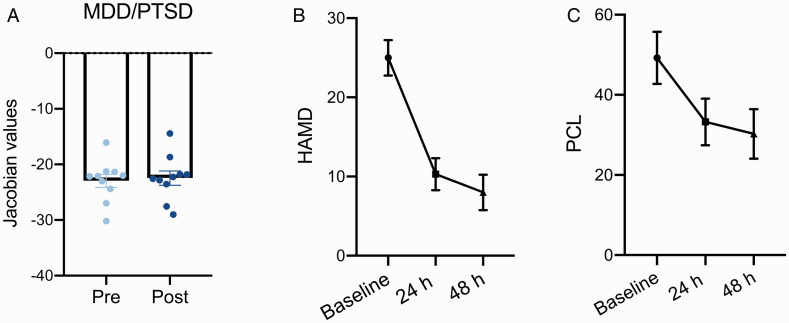
In the MDD/PTSD group, there was no significant volume change at 24 h after ketamine administration compared to baseline. The volume of right opercular IFG showed a minor increase, resulting in a non-significant volume difference compared to HC (Figures 2(B) and 4(A)). MDD/PTSD subjects demonstrated a significant reduction in depressive and PTSD symptoms 24 h after ketamine administration (HAMD p < 0.001, and PCL p = 0.01, paired t-test, Figure 4(B) and (C)). In a further exploratory analysis, we found MDD symptom relief had trend significant effects on the improvement of PTSD severity in MDD/PTSD group (F = 4.23, p = 0.058).
Figure 4.
Significant improvement of depression and PTSD symptoms in MDD/PTSD subjects. A, The volume of right opercular IFG in MDD/PTSD subjects were slightly increased after ketamine administration, which resulted in the non-significant volume differences compared to HC. B and C, The depression and PTSD severity were significantly alleviated within 24 h and 48 h after ketamine administration. The improvement of PTSD severity was significantly related to the improved HAMD severity in MDD/PTSD subjects.
HC Subjects
After a single ketamine infusion, there were no significant structural changes in any brain regions of the HC group. However, the volume of multiple brain regions had a tendency to decrease (P < 0.01, FWE corrected), which were in the cerebellum regions (see Supplementary Figure 1).
Discussion
Numerous studies have revealed brain structural abnormalities in subjects with depressive symptoms,31,32 and some of the structural alterations were improved in those who exhibited significant improvement after antidepressant treatment.25,33 Consistent with previous findings, we found smaller volumes of several regions including IFG (right opercular and left orbital parts) in MDD subjects compared to HC. After a single infusion of ketamine, MDD and MDD/PTSD subjects showed a significant improvement in clinical symptoms, and the volume of right opercular IFG was normalized. Our findings highlight that the structural abnormality of IFG might be related to the neurobiological mechanism of depressive symptoms.
The structural abnormalities of IFG in subjects with depressive symptoms have been revealed previously in many studies. For example, a meta-analysis reported grey matter volume reductions in IFG in depressed patients compared to healthy controls.34 Recent work has shown significantly lower grey matter volumes of areas located within IFG (triangular and orbital parts), and those alterations were associated with depression in MDD patients.35 Further, the structural abnormalities of IFG were also demonstrated in MDD patients with comorbidities, such as reduced IFG cortical thickness in MDD comorbid generalized anxiety disorder,36 and a smaller IFG volume in depressed patients with symptoms of anxiety.37 In line with this, our results showed that volumes of IFG (opercular and orbital parts) were lower in MDD and MDD/PTSD subjects compared to HC. Additionally, we found the volume of IFG (opercular part) in MDD subjects without PTSD comorbidity was significantly related to depressive severity. The IFG has been implicated in emotion detection, regulation, and cognitive control.38,39 A meta-analysis of shared neural phenotypes across mood and anxiety disorders indicates that abnormal activation of inferior frontal gyrus/insula is one of the common abnormalities related to social and affective processing.40 It was suggested the enhanced performance in cognitive conflict tasks was related to the enhanced activation in IFG and its communication with the dorsomedial prefrontal cortex.41 Additional evidence for the role of the IFG in cognitive and emotional processing includes the associations between increased preferential processing of negative information in depressed subjects with abnormal activation of left IFG.42 Together, these data suggested that structural alterations of IFG play an important role in the development of depression symptoms.
Ketamine is considered to exert its rapid antidepressant effects through the direct inhibition of the NMDA receptor or via its enantiomer and metabolites.43,44 In contrast with traditional antidepressant drugs, ketamine response can be elicited as early as hours, with improvement effects sustained over several days.45,46 Preclinical studies revealed cellular and behavioral changes due to acute ketamine treatment such as increased synapse number, elicited translation of brain-derived neurotrophic factor, and rapidly reversed effects of chronic stress.47,48 Ketamine-induced microstuctural changes could be reflected in alterations of brain functions and volumes, which were shown in recent studies. Some neuroimaging studies have shown ketamine-associated functional connectivity changes in depressed subjects and suggested the ketamine could normalize some of the functional abnormality.49,50 For example, a single infusion of ketamine blunted aberrant hyperreactivity of subgenual anterior cingulate cortex to positive incentives.51 In another study, the volume of left nucleus accumbens that was abnormally larger in MDD subjects compared to HC, but decreased 24 h after ketamine administration.25 Consistently, we found that ketamine exerted a significant effect on the volume of right opercular IFG that was associated with depressive severity – 24 h after ketamine administration, the volume of right opercular IFG in the MDD group was normalized, together with significantly improved depressive symptoms. The MDD/PTSD group demonstrated normalized right opercular IFG volume within 24 h after ketamine administration, with significant improvements in depressive and PTSD symptoms. Although our findings of decrease in PTSD symptoms post ketamine administration are in line with previous research,49 we show that decrease in PTSD symptoms is likely due to the decrease in depression. Further work is needed to understand ketamine effects on PTSD symptomatology.
Unexpectedly, the volume of left lateral OFC was significantly reduced in MDD and MDD/PTSD subjects after ketamine administration. Alterations in OFC resulting from antidepressants have been reported elsewhere, in which the functional connectivity of medial OFC to temporal cortex areas, the parahippocampal gyrus and fusiform gyrus showed a greater decrease in MDD patients receiving traditional antidepressants.52 The lateral OFC is implicated in reward processing53 and punishing events,54 playing a crucial role in the development of depression. It has been shown that subjects with familial risk of depression had greater activation in lateral OFC to aversive stimuli.55 In another neuroimaging study, patients receiving antidepressants showed decreased functional connectivity of lateral OFC with several regions compared to patients not receiving antidepressants, which was related to depressive symptom improvement.56 Further, it has been demonstrated that functional regulation of the lateral OFC via non-invasive brain stimulation can improve depressive symptoms.57,58 It is possible that the decreased lateral OFC volume after ketamine administration in our study might be associated with its weakened functional connectivity and thus depressive symptom relief. However, this abnormal alteration in the volume of lateral OFC could also be due to the side-effects of ketamine action, which need more attention in future clinical practice.
There are several limitations of this study. First, the sample size of MDD and MDD/PTSD groups is relatively small, which may result in under or overestimation of volume differences between HC and subgroups. However, the smaller volume of right opercular IFG was also confirmed in the comparison between HC and All MDD groups. Second, we only explored the effects of a single ketamine infusion. Although our results did not show direct ketamine effects either on brain structures or PTSD symptom improvement in MDD/PTSD subjects, repeated ketamine administration may provide with different outcomes. Third, there was a scanner upgrade through the study, which might influence the structural variables. However, each subject finished the pre- and post-ketamine infusion scan on the same scanner. The proportion of HC and all MDD subjects was not significantly different (p = 0.79, Chi-square test) before and after the upgrade. Another limitation is this study lacks a placebo control group. A similar study has shown reduced grey matter volumes of prefrontal areas in healthy and placebo subjects after a single ketamine infusion, which was considered to be possibly subject to physiological influences such as blood flow.59 Future work is still necessary to elucidate whether acute ketamine administration has placebo effects on the brain structures of MDD subjects. The structural changes after ketamine administration observed in our study should be interpreted with caution.
Conclusion
Our findings indicated lower IFG volumes (right opercular and left orbital parts) in MDD and MDD/PTSD subjects compared to HC, and that lower right opercular IFG volume was associated with more severe depressive symptoms in MDD subjects. After ketamine administration, MDD and MDD/PTSD subjects showed significant reductions in depressive and PTSD symptoms and the normalizations of right opercular IFG volumes. This is first evidence of ketamine-induced regional volume alterations being related to depressive severity, which is worthy of future attention focusing on the role of IFG in the neuropathogenesis of depression.
Supplemental Material
Supplemental material, sj-pdf-1-css-10.1177_2470547020980681 for Ketamine Normalizes the Structural Alterations of Inferior Frontal Gyrus in Depression by Dan Dai, Cheryl M. Lacadie, Sophie E. Holmes, Ryan Cool, Alan Anticevic, Chris Averill, Chadi Abdallah and Irina Esterlis in Chronic Stress
Acknowledgments
We thank the staff at the Yale Magnetic Resonance Research Center for their support with data processing and analysis. We also thank the National Center for PTSD, the Yale Center for Clinical Investigation, and the individuals who took part in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Fundings were provided by the Nancy Taylor Foundation (IE) and the Veterans Affairs National Center for PTSD (IE, CGA). CGA received support from the Beth K and Stuart Yudofsky Chair in the Neuropsychiatry of Military Post Traumatic Stress Syndrome.
ORCID iDs: Dan Dai https://orcid.org/0000-0001-8527-3232
Ryan Cool https://orcid.org/0000-0003-0655-0006
Chris Averill https://orcid.org/0000-0001-7575-6142
Chadi Abdallah https://orcid.org/0000-0001-5783-6181
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Friedrich M. Depression is the leading cause of disability around the world. JAMA. 2017; 317(15): 1517. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Depression and Other Common Mental Disorders: global Health Estimates. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 3.Kolovos S, van Tulder MW, Cuijpers P, et al. The effect of treatment as usual on major depressive disorder: a meta-analysis. J Affect Disord. 2017; 210: 72–81. [DOI] [PubMed] [Google Scholar]
- 4.Grunebaum MF, Galfalvy HC, Choo T-H, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018; 175(4): 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data Meta-analysis. Am J Psychiatry. 2018; 175(2): 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krystal JH, Abdallah CG, Sanacora G.Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019; 101(5): 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Velzen LS, Kelly S, Isaev D, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020; 25: 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise T, Radua J, Nortje G.Cleare AJ, Young AH, Arnone, D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. 2016; 79(4): 293–302. [DOI] [PubMed] [Google Scholar]
- 9.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016; 22(3): 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen X, Adams MJ, Ritakari TE.Cox SR, McIntosh AM, Whalley HC. White matter microstructure and its relation to longitudinal measures of depressive symptoms in mid-and late life. Biol Psychiatry. 2019; 86(10): 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repple J, Mauritz M, Meinert S, et al. Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder. Mol Psychiatry. 2020; 25(7): 1550–1558. [DOI] [PubMed] [Google Scholar]
- 12.Holmes SE, Scheinost D, Finnema SJ, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019; 10(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk MT, Cha J, Semanek D, et al. Altered dentate gyrus microstructure in individuals at high familial risk for depression predicts future symptoms [published online ahead of print June 21, 2020]. Biol Psychiatry: Cognit Neurosci Neuroimag. doi:10.1016/j.bpsc.2020.06.006 [DOI] [PMC free article] [PubMed]
- 14.Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol Psychiatry. 2017; 82(5): 339–350. [DOI] [PubMed] [Google Scholar]
- 15.Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2013; 18(12): 1265–1272. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Zhao Y, Chen Z, et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology. 2020; 45(4): 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neurosci Biobehav Rev. 2016; 60: 43–50. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Liang H, Han D, et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage: Clinical. 2016; 11: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bora E, Fornito A, Pantelis C.Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012; 138(1–2): 9–18. [DOI] [PubMed] [Google Scholar]
- 20.Salvadore G, Nugent AC, Lemaitre H, et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011; 54(4): 2643–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 2015; 601: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalleri L, Pich EM, Millan M, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018; 23(4): 812–823. [DOI] [PubMed] [Google Scholar]
- 23.Ng LHL, Huang Y, Han L.Chang RC-C, Chan YS, Lai CSW. Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl Psychiatry. 2018; 8(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasavada MM, Leaver AM, Espinoza RT, et al. Structural connectivity and response to ketamine therapy in major depression: a preliminary study. J Affect Disord. 2016; 190: 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdallah CG, Jackowski A, Salas R, et al. The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology. 2017; 42(8): 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdallah CG, Averill LA, Gueorguieva R, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology. 2020; 45(6): 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdallah CG, De Feyter HM, Averill LA, et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology. 2018; 43(10): 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papademetris X, Jackowski MP, Rajeevan N, et al. BioImage suite: an integrated medical image analysis suite: an update. Insight J. 2006; 2006: 209. [PMC free article] [PubMed] [Google Scholar]
- 29.Scheinost D, Holmes SE, DellaGioia N, et al. Multimodal investigation of network level effects using intrinsic functional connectivity, anatomical covariance, and structure-to-function correlations in unmedicated major depressive disorder. Neuropsychopharmacology. 2018; 43(5): 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolls ET, Huang C-C, Lin C-P.Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020; 206: 116189. [DOI] [PubMed] [Google Scholar]
- 31.Schmaal L, Hibar D, Sämann P, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry. 2017; 22(6): 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redlich R, Opel N, Bürger C, et al. The limbic system in youth depression: brain structural and functional alterations in adolescent in-patients with severe depression. Neuropsychopharmacology. 2018; 43(3): 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogovitsyn N, Muller M, Souza R, et al. Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report. Neuropsychopharmacology. 2020; 45(2): 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y-J, Du M-Y, Huang X-Q, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. 2014; 44(14): 2927–2937. [DOI] [PubMed] [Google Scholar]
- 35.Kandilarova S, Stoyanov D, Sirakov N.Maes M, Specht K. Reduced grey matter volume in frontal and temporal areas in depression: contributions from voxel-based morphometry study. Acta Neuropsychiatr. 2019; 31(5): 252–257. [DOI] [PubMed] [Google Scholar]
- 36.Canu E, Kostić M, Agosta F, et al. Brain structural abnormalities in patients with major depression with or without generalized anxiety disorder comorbidity. J Neurol. 2015; 262(5): 1255–1265. [DOI] [PubMed] [Google Scholar]
- 37.Peng W, Jia Z, Huang X, et al. Brain structural abnormalities in emotional regulation and sensory processing regions associated with anxious depression. Prog Neuropsychopharmacol Biol Psychiatry. 2019; 94: 109676. [DOI] [PubMed] [Google Scholar]
- 38.Urgesi C, Mattiassi AD, Buiatti T.Marini A. Tell it to a child! A brain stimulation study of the role of left inferior frontal gyrus in emotion regulation during storytelling. Neuroimage. 2016; 136: 26–36. [DOI] [PubMed] [Google Scholar]
- 39.Jastorff J, De Winter FL, Van den Stock J.Vandenberghe R, Giese MA, Vandenbulcke M. Functional dissociation between anterior temporal lobe and inferior frontal gyrus in the processing of dynamic body expressions: Insights from behavioral variant frontotemporal dementia. Hum Brain Mapp. 2016; 37(12): 4472–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janiri D, Moser DA, Doucet GE, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry. 2020; 77(2): 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Yang X, Zhou F, et al. Modafinil enhances cognitive, but not emotional conflict processing via enhanced inferior frontal gyrus activation and its communication with the dorsomedial prefrontal cortex. Neuropsychopharmacology. 2020; 45(6): 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gollan JK, Connolly M, Buchanan A, et al. Neural substrates of negativity bias in women with and without major depression. Biol Psychol. 2015; 109: 184–191. [DOI] [PubMed] [Google Scholar]
- 43.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016; 533(7604): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018; 23(4): 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12): 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013; 74(4): 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015; 20: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C, Yang J, Luo A.Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019; 9(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacol. 2017; 42(6): 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ionescu DF, Felicione JM, Gosai A, et al. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry. 2018; 26(6): 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris LS, Costi S, Tan A.Stern ER, Charney DS, Murrough JW. Ketamine normalizes subgenual cingulate cortex hyper-activity in depression. Neuropsychopharmacology. 2020; 45(6): 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rolls ET, Cheng W, Du J, et al. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cognit Affect Neurosci. 2020; 15(1): 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Vanni-Mercier G, Isnard J.Mauguière F, Dreher J-C. The neural dynamics of reward value and risk coding in the human orbitofrontal cortex. Brain. 2016; 139(Pt 4): 1295–1309. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Yu H, Yin Y.Zhou X. Intention modulates the effect of punishment threat in norm enforcement via the lateral orbitofrontal cortex. J Neurosci. 2016; 36(35): 9217–9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCabe C, Woffindale C, Harmer CJ.Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry. 2012; 72(7): 588–594. [DOI] [PubMed] [Google Scholar]
- 56.Cheng W, Rolls ET, Qiu J, et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016; 139(Pt 12): 3296–3309. [DOI] [PubMed] [Google Scholar]
- 57.Downar J. Orbitofrontal cortex: a ‘non-rewarding’ new treatment target in depression? Curr Biol. 2019. ; 29(2):R59–R62. [DOI] [PubMed] [Google Scholar]
- 58.Rao VR, Sellers KK, Wallace DL, et al. Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr Biol. 2018; 28(24): 3893–3902. [DOI] [PubMed] [Google Scholar]
- 59.Höflich A, Ganger S, Tik M, et al. Imaging the neuroplastic effects of ketamine with VBM and the necessity of placebo control. Neuroimage. 2017; 147: 198–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-css-10.1177_2470547020980681 for Ketamine Normalizes the Structural Alterations of Inferior Frontal Gyrus in Depression by Dan Dai, Cheryl M. Lacadie, Sophie E. Holmes, Ryan Cool, Alan Anticevic, Chris Averill, Chadi Abdallah and Irina Esterlis in Chronic Stress