Abstract
Diabetic retinopathy (DR), the most common microvascular complication of diabetes and leading cause of visual impairment in adults worldwide, is suggested to be linked to abnormal lipid metabolism. The present study aims to comprehensively investigate the relationship between n-6 polyunsaturated fatty acids (PUFAs) and DR. This was a propensity score matching based case–control study, including 69 pairs of DR patients and type 2 diabetic patients without DR with mean age of 56.7 ± 9.2 years. Five n-6 PUFAs were determined by UPLC-ESI-MS/MS system. Principle component regression (PCR) and multiple conditional logistic regression models were used to investigate the association of DR risk with n-6 PUFAs depending on independent training and testing sets, respectively. According to locally weighted regression model, we observed obvious negative correlation between levels of five n-6 PUFAs (linoleic acid, γ-linolenic acid, eicosadienoic acid, dihomo-γ-linolenic acid and arachidonicacid) and DR. Based on multiple PCR model, we also observed significant negative association between the five n-6 PUFAs and DR with adjusted OR (95% CI) as 0.62 (0.43,0.87). When being evaluated depending on the testing set, the association was still existed, and PCR model had excellent classification performance, in which area under the curve (AUC) was 0.88 (95% CI: 0.78, 0.99). In addition, the model also had valid calibration with a non-significant Hosmer–Lemeshow Chi-square of 9.44 (P = 0.307) in the testing set. n-6 PUFAs were inversely associated with the presence of DR, and the principle component could be potential indicator in distinguishing DR from other T2D patients.
Keywords: diabetic retinopathy, n-6 polyunsaturated fatty acids, propensity score matching, principal component regression
Introduction
It has been reported that about 415 million people worldwide have been affected by diabetes mellitus (DM) in 2015, and the number will reach 642 million by 2040 (1). Diabetic retinopathy (DR) has emerged as the most common microvascular complication of diabetes (2) and the leading cause of blindness and visual impairment in adults worldwide (3, 4, 5). It is characterized by progressive loss of vascular cells and slow dissolution of inter-vascular junctions, which result in vascular leakage and retinal edema (6). Later stages of the disease are identified with inflammatory cell infiltration, tissue destruction and neovascularization (7, 8). With the rising prevalence of diabetes and increasing numbers of people with diabetes living longer, the number of people with DR and visual impairment due to this disease is rising rapidly worldwide (4). From 1980 to 2018, the annual incidence of DR ranged from 2.2 to 12.7% and progression from 3.4 to 12.3% (9). The public health burden due to DR would thus increase correspondingly.
Indeed, current dietary guideline suggests people to intake more polyunsaturated fatty acids (PUFAs) for health, and PUFAs have been demonstrated to be associated with the pathogenesis of proliferative and degenerative retinal diseases because of their effects of anti-angiogenic, anti-vasoproliferative, and neuroprotective (10). It is reported that PUFAs include n-3, n-6 and others. Similar to n-3 fats, n-6 PUFAs are also one of the most important classes of PUFAs, and rich in many plant oils, particularly linoleic acid (LA) (11). Both clinical and experimental studies have shown that increased dietary intake of n-3 PUFAs reduces pathological retinal angiogenesis (12, 13) by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function (14, 15). However, the epidemiological data of n-6 PUFAs on diabetic retinopathy risk remain inconclusive (11) and disputed (16). Some researchers suggest that n-6 PUFAs might be harmful because their metabolites promote inflammation and angiogenesis, which are strongly associated with the pathogenesis of clinical DR (17, 18). While an in vitro experiment reveals that linoleic acid, the most abundant form of n-6 PUFAs, can inhibit hyperglycemia-induced excess proliferation of retinal vascular endothelial cells (15). And the products of n-6 PUFAs such as lipoxins, resolvins and protectins may be beneficial on the development of diabetes since their anti-inflammatory, anti-VEGF (vascular endothelial growth factor), suppression of tumor necrosis factor-α (TNF-α) production and inhibiting endothelial cell proliferation in pathological retinal angiogenesis (19).
So far, the research on the effects of n-6 PUFAs on human diabetic retinopathy is very rare, and their relationship is not yet clear. To address these critical knowledge gaps, we aimed to comprehensively investigate the association between n-6 PUFAs levels and DR based on a widelytargeted metabolomics approach, which had been popularly accepted as an useful way to identify a specific disease early and better understand the related pathogenesis depending on the unbiased monitoring of changes in endogenous metabolism-related physiological processes (20).
Design and methods
Study population
The participants were enrolled from two clinical centers, the Second Affiliated Hospital of Wenzhou Medical University and the First Affiliated Hospital of Anhui Medical University. In brief, 955 volunteers (755 health controls, 112 DM patients without DR and 83 DR cases) aged over 35 years with free of preexisting diseases (i.e. type 1 diabetes, cardiovascular disease, cerebrovascular disease, hyperkalemia, cancer, infectious disease or other chronic systemic diseases) were recruited during August 2017 to June 2018. The procedures of the present study strictly followed the tenets of the Declaration of Helsinki. The protocol had also been carefully reviewed and approved by Ethics Committee of the Eye hospital of WMU before the study (Number: KYK (2017) 46) and confirmed by the two associated hospitals. All participants in the current study were voluntary and provided written informed consent.
In the multicenter based case-control study, we matched 69 pairs of cases (DR group) and controls (DM group) for the main study at a 1:1 ratio based on age, sex, BMI and glycosylated hemoglobin using the propensity score matching (PSM) approach to adjust for potential confounding effects on the results. The DR group included 60 patients with non-proliferative diabetic retinopathy (NPDR: 9 mild, 31 moderate and 20 severe) and nine patients with proliferative diabetic retinopathy (PDR). Each case or control received careful ophthalmic examination as well as retinal photographs by two independent trained ophthalmologists. In particular, for further fully examine the effects of n-6 PUFAs on the T2D and DR, healthy volunteers were also matched with T2D patients using PSM at a 1:1 ratio as the blank control based on T2D controls in accordance with age, gender and BMI. Then they were randomly splitted into a training set and other independent testing set at a 7:3 ratio based on the pairs. The training set was used to investigate the relationship between n-6 PUFAs intensity and the risk to develop DR. To measure the robustness of the relationship, it was additionally assessed based on the testing set.
Baseline clinical examinations and biochemical analysis
All participants received anthropometric and blood pressure measurements. The BMI was calculated as: BMI = weight (kg)/height (m)2. After at least 8 (8–10) h fasting, a total of 6 mL venous blood sample was collected under complete aseptic precautions from each study participant with tubes containing ethylene-diamine tetra-acetic acid (for plasma) or none (for serum) at 08:00 to 10:00 h in the morning of the enrollment. Then the fasting blood samples were separated within 15 min of collection, analyzed within 30 min or stored at −86°C in a freezer for further measurement in the central laboratory by a well-trained professional technician. Fasting blood glucose (FPG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and other routine biochemical assessments were detected by the automatic biochemical analyzer (Roche, Cobas c311). Information on age, sex, course of the disease, medical history, smoking, drinking, occupation, education as well as life habits and life styles were collected by a well-trained investigator in each hospital with the face-to-face interview based on the specifically designed standardized structure-questionnaires. The collection of other data containing clinical manifestation and biochemical assessments was also performed by two systematically trained investigators strictly following the specific standardized operation procedures (SOP) of the current study.
Metabolic profiling and quality control
The serum samples were thawed on ice, one volume of ice-cold methanol was added thereto and spun for 3 min, and then centrifuged at 12,000 r/min for 10 min at 4°C. The supernatant was then collected and centrifuged at 12,000 r/min for 5 min at 4°C. Finally, supernatant samples were collected again and subjected to qualitative and relative quantitative assessments of widely targeted metabolites using an ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS) system by the central laboratory of Metware, Inc, a professional and experienced metabolomics institution in Wuhan city, China.
Serum PUFAs were measured using a UPLC-ESI-MS/MS system as described previously. Five n-6 PUFAs (linoleic acid (LA), gamma-linolenic acid (GLA), eicosadienoic acid (EDA), dihomo-gamma-linolenic acid (DGLA), arachidonic acid (AA)) and five n-3 PUFAs (alpha-linolenic acid (ALA), stearidonic acid(S), eicosatrienoic acid (ETA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)) were identified.
Quality control (QC) samples were prepared from the mixture of sample extracts and used to monitor the reproducibility of analyzed samples under the same processing methods. During sample testing, a QC sample was inserted into every 20 test samples.
Data processing
Software 1.6.3 (AB Sciex), were preprocessed (conversion, peak detection, retention time correction, and peak alignment) by MultiQuant™ Software (AB Sciex), and processed using MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/) and RStudio Version 1.2.5033 (Copyright 2009-2019 Rstudio, Inc.).
Peaks with coefficient of variation (CV) greater than 30% in the QC samples were not considered for further analysis (21). Meanwhile, peaks with missing values in more than 20% of the samples were also discarded (22). While, for those peaks with missing values in less than 20% of the samples, half of the lowest detected peak areas were used to fill the missed data. Finally, Z-score normalization was used for the raw peak area for further data analysis.
Statistical analysis
All data management, analyses and visualization were performed using STATA MP for windows version 15.1 (Copyright 1985-2017 StataCorp LLC, Texas, USA) and RStudio Version 1.2.5033 (Copyright 2009-2019 Rstudio, Inc.).
The normality of continuous data was assessed using both QQ-plots and Shapiro–Wilk test in the demographic and clinical data. Paired t-tests were applied to assess the differences between these PSM matched cases and controls when they were normal or similar normal distributed. Otherwise, the Wilcoxon signed-rank tests would be used. Meanwhile, differences in the proportion of categorical variables between the two groups were evaluated using the McNemar–Bowker test, which was one of the commonly used statistical approaches for pair-designed study. Similarly, paired t-test and Wilcoxon signed-rank test were conducted to compare n-6 PUFAs in the DR cases with T2D controls. In addition, we also examined the relationships of n-6 PUFAs and progressive grade of DR using a commonly used nonparametric regression approach named locally weighted regression (LOESS) model. Considering that basically n-3 and n-6 PUFAs are competing each other and are working together as well (11). The content of n-6 PUFAs were expressed as ratios with n-3 PUFAs to correct the influence of n-3 PUFAs. In addition, the activities of desaturase and elongase are closely linked to the metabolism of n-6 PUFAs, so we have also explored the activities of desaturase and elongase, in which these activities are estimated from the product-to-precursor ratios of some individual fatty acids in serum (23). The 18:3 n-6/18:2 n-6 (GLA/LA), 20:4 n-6/20:3 n-6 (AA/DGLA) and 20:3 n-6/18:3 n-6 (DGLA/GLA) are commonly suggested to represent the activities of D6D, delta 5-desaturase (D5D) and elongase, respectively (24).
As increased systolic blood pressure (SBP) and diabetes duration were known risk factors of DR (4, 25), we further investigated the relationship between DR and these five detected n-6 PUFAs repeatedly based on multivariable conditional logistic regression models in which SBP and diabetes duration were adjusted. Considering the potential collinearity of predictors included in the above-mentioned multiple models, principal component regression was also applied for further examination of the association between risk of DR and n-6 PUFAs. To avoid potential overfitting, the number of components included in the models would be determined by their cumulative variation ratio, which should be generally more than 80%. To comprehensively evaluate the discriminating power of models established based on the principal component regression models, the area under the curve (AUC) in the receiver operating characteristic (ROC) analysis was assessed in both the train and testing sets, respectively. Furthermore, the performance of the predictive model was also assessed by the net reclassification improvement (NRI) and integrated discrimination improvement (IDI), respectively. In addition, the model’s good calibration (predictive accuracy) was determined by a non-significant Hosmer–Lemeshow goodness of fit test.
Results
Data quality
The stability of UPLC-ESI-MS/MS system and reliability of the testing results were carefully assessed depending on the QC sample. The CV values of the LA, GLA, EDA, DGLA, AA, ALA, SA, ETA, EPA and DHA peaks are 17.8, 13.3, 18.4, 15.7, 17.3, 11.0, 24.7, 20.3, 15.6 and 16.3% (Supplementary Table 1, see section on supplementary materials given at the end of this article), respectively. In metabolomics studies, if the CV value of QC samples is less than 30%, the data quality is generally considered reliable (21). So, the data quality of the present study is acceptable and will induce credible findings.
Study participants and baseline characteristics
A total of 69 pairs of participants (69 cases vs 69 controls) were included in this study and randomly splitted into independent training set (49 pairs) and testing set (20 pairs) at a ratio of 7:3. The proportions of women in the train and testing sets were 43/98 and 21/40, respectively. The mean ± standard deviation (s.d.) of age in the cases and controls were 58.0 ± 10.2 and 53.4 ± 9.4 years in the training set, and 57.0 ± 10.0 and 56.0 ± 12.0 years in the testing set. The detailed baseline characteristics of participants were summarized in Table 1. Except for age, systolic blood pressure (SBP) and duration of disease, no significant differences (P > 0.05) between the cases and controls were observed in the demographic indicators (history, education, occupation, etc.) and biochemical examination indicators (blood glucose, blood lipids, etc.).
Table 1.
Clinical and demographic characteristics of the study population.
| Variables | Training set | Testing set | ||||
|---|---|---|---|---|---|---|
| DM/DR | DM | DR | DM/DR | DM | DR | |
| Age, years | 49/49 | 53.4 ± 9.4 | 58.0 ± 10.2 | 20/20 | 56.0 ± 12.0 | 57.0 ± 10.0 |
| Gender, n/N | ||||||
| Male | 29/49 | 26/49 | 9/20 | 10/20 | ||
| Female | 20/49 | 23/49 | 11/20 | 10/20 | ||
| BMI, kg/m2 | 49/49 | 23.9 (22.1, 27.4) | 24.1 (22.4, 27.0) | 20/20 | 24.8 (23.1, 26.1) | 25.2 (22.4, 26.2) |
| FPG, mmol/L | 49/49 | 8.3 (6.9, 12.0) | 8.9 (6.7, 10.9) | 20/20 | 9.2 ± 2.7 | 7.8 ± 2.6 |
| HbA1c, % | 49/49 | 10.2 ± 2.4 | 9.7 ± 1.9 | 20/20 | 9.9 ± 2.0 | 10.3 ± 1.9 |
| LDL, mmol/L | 49/49 | 2.7 ± 0.9 | 2.6 ± 1.1 | 19/20 | 2.4 ± 1.2 | 2.5 ± 1.0 |
| HDL, mmol/L | 49/47 | 1.1 (0.8, 1.4) | 1.1 (0.9, 1.4) | 19/19 | 1.0 ± 0.3 | 1.0 ± 0.3 |
| TG, mmol/L | 49/49 | 1.6 (1.0, 2.1) | 1.4 (1.0, 1.7) | 19/20 | 1.8 (1.5, 2.4) | 1.5 (1.1, 2.3) |
| TC, mmol/L | 49/49 | 4.8 ± 1.1 | 4.6 ± 1.5 | 19/20 | 4.5 ± 1.3 | 4.3 ± 1.3 |
| SBP, mmHg | 49/49 | 125 ± 14 | 137 ± 20 | 20/20 | 135 ± 18 | 140 ± 25 |
| DBP, mmHg | 49/49 | 78 (73, 86) | 76 (70, 80) | 20/20 | 83 ± 10 | 81 ± 12 |
| Duration of diabetes, years | 41/44 | 8.1 ± 6.2 | 12.2 ± 6.1 | 14/19 | 9.9 ± 6.6 | 12.1 ± 7.8 |
| Education, n/N | ||||||
| Junior high school or below | 27/49 | 27/49 | 11/20 | 13/20 | ||
| High school or above | 22/49 | 22/49 | 9/20 | 7/20 | ||
| Occupation, n/N | ||||||
| Manual workers | 23/47 | 22/44 | 8/18 | 12/20 | ||
| Mental worker | 10/47 | 8/44 | 5/18 | 3/20 | ||
| Both | 14/47 | 14/44 | 5/18 | 5/20 | ||
| History of diabetes, n/N | ||||||
| Yes | 17/49 | 25/49 | 10/20 | 8/20 | ||
| No | 32/49 | 24/49 | 10/20 | 12/20 | ||
| Smoking habits, n/N | ||||||
| Non-smokers | 29/47 | 23/45 | 12/19 | 13/20 | ||
| Current smokers | 13/47 | 16/45 | 6/19 | 5/20 | ||
| Ex-smokers | 5/47 | 6/45 | 1/19 | 2/20 | ||
| Alcohol consumption, n/N | ||||||
| Non-drinkers | 22/47 | 20/45 | 11/19 | 9/20 | ||
| Current drinkers | 23/47 | 19/45 | 7/19 | 8/20 | ||
| Ex-drinkers | 2/47 | 6/45 | 1/19 | 3/20 | ||
| Center, n/N | ||||||
| Wenzhou | 24/49 | 33/49 | 12/20 | 15/20 | ||
| Hefei | 25/49 | 16/49 | 8/20 | 5/20 | ||
Continuous data obeying normal or similar normal distribution were described as mean ± standard deviation (s.d.) and the paired t-test was applied to compare the differences between the two groups. Otherwise, median (1st quartile, 3rd quartile) and Wilcoxon signed-rank tests were used. Categorical data were presented as n/N and McNemar–Bowker test was utilized to compare the differences of the cases and controls.
n, the number of exposed persons in each group; N, group size.
DBP, diastolic blood pressure; DM, type 2 diabetes (T2D) without diabetic retinopathy; DR, T2D with diabetic retinopathy; DM/DR= Number of DM and DR groups; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglyceride; TC, total cholesterol; SBP, systolic blood pressure.
n-6 PUFAs and DR
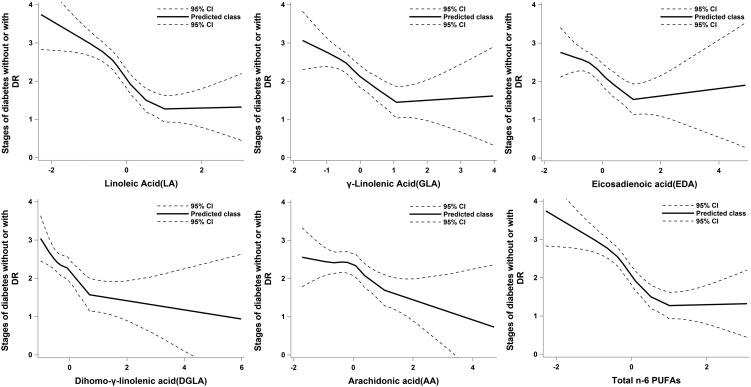
As can be seen clearly in Fig. 1, obvious negative correlation relationships existed between the progressive grade of DR and n-6 PUFAs level based on the LOESS models. The relationship between the progressive grade of DR and total n-6 PUFAs (sum of LA, GLA, EDA, DGLA and AA) also revealed a similar trend. Compared to DM controls, patients with DR had significantly lower levels of LA, GLA, EDA, DGLA, AA and total n-6 PUFAs in both training set and testing set (Table 2). All of these results indicated that elevated intensities of n-6 PUFAs were significantly related to decreased risk to develop DR in T2D patients. In addition, the desaturase and elongase related to n-6 PUFAs metabolism did not differ significantly (P > 0.05) between the DM and DR groups.
Figure 1.
Relationship between standard transformed serum n-6 PUFAs and the progressive stages of diabetic retinopathy (DR). Stages: 0, diabetic patients without DR; 1, mild non-proliferative DR patients; 2, moderate non-proliferative DR patients; 3, severe non-proliferative DR patients; 4, proliferative DR patients.
Table 2.
Standard transformed serum n-6 PUFAs, desaturase and elongase activity between DR and DM.
| Training set | Testing set | |||||
|---|---|---|---|---|---|---|
| DM (n = 49) | DR (n = 49) | P-value | DM (n = 20) | DR (n = 20) | P-value | |
| n-6 PUFAs | ||||||
| 18:2 n-6 (LA) | 0.4 (−0.2, 0.9) | −0.6 (−1.0, −0.3) | <0.001 | 0.5 (−0.2, 1.6) | −0.4 (−0.7, −0.2) | <0.001 |
| 18:3 n-6 (GLA) | 0.1 (−0.5, 0.8) | −0.5 (−0.9, −0.2) | <0.001 | 0.5 ± 0.9 | −0.2 ± 0.6 | 0.005 |
| 20:2 n-6 (EDA) | 0.1 (−0.6, 0.7) | −0.5 (−0.8, −0.1) | 0.002 | 0.5 ± 0.9 | −0.3 ± 0.7 | 0.007 |
| 20:3 n-6 (DGLA) | −0.2 (−0.5, 0.4) | −0.4 (−0.7, −0.2) | 0.008 | 0.5 ± 0.8 | −0.3 ± 0.5 | 0.001 |
| 20:4 n-6 (AA) | −0.1 (−0.6, 0.8) | −0.3 (−0.9, −0.0) | 0.028 | 0.5 ± 0.9 | −0.2 ± 0.8 | 0.016 |
| Total n-6 PUFAs | 0.4 (−0.2, 0.9) | −0.6 (−1.0, −0.3) | <0.001 | 0.5 (−0.2, 1.6) | −0.4 (−0.7, −0.2) | <0.001 |
| Desaturase and elongase activity | ||||||
| ∆6-desaturase activity | −0.3 (−0.7, 0.5) | −0.3 (−0.9, 0.3) | 0.738 | 0.1 ± 0.8 | 0.2 ± 0.9 | 0.832 |
| Elongase activity | −0.3 (−0.7, 0.1) | −0.1 (−0.6, 0.3) | 0.156 | −0.2 (−0.4, 0.3) | −0.1 (−0.6, 0.1) | 0.433 |
| ∆5-desaturase activity | −0.0 ± 1.1 | 0.2 ± 0.8 | 0.344 | −0.4 (−0.9, −0.1) | −0.4 (−0.6, 0.6) | 0.204 |
Data obeying normal or similar normal distribution were described as mean ± standard deviation (s.d.) and the paired t-test was applied to compare the differences between the two groups. Otherwise, median (1st quartile, 3rd quartile) and Wilcoxon signed-rank tests were used. Total n-6 PUFA is the sum of LA, GLA, EDA, DGLA and AA. ∆6-desaturase activity = GLA/LA; elongase activity = DGLA/GLA; ∆5-desaturase activity = AA/DGLA.
AA, arachidonic acid;DGLA, dihomo-gamma-linolenic acid;DM, type 2 diabetes (T2D) without diabetic retinopathy; DR, T2D with diabetic retinopathy;EDA, eicosadienoic acid;GLA, gamma-linolenic acid; LA, linoleic acid; PUFA, polyunsaturated fatty acid.
Associations between the odds of DR and n-6 PUFAs intensities based on unadjusted and adjusted conditional logistic regression models were presented in Table 3. After adjusting for major risk factors for DR, the individual LA, GLA, DGLA and total n-6 PUFAs in the training set were associated with a 71% (adjusted odds ratio (aOR) 0.29; 95% CI 0.13–0.63), 43% (aOR 0.57; 95% CI 0.34–0.96), 63% (aOR 0.37; 95% CI 0.15–0.87) and 71% (aOR 0.29; 95% CI 0.13–0.64) lower risk of DR, respectively; however ,the significance did not persist in the testing set. Among fatty acids ratios (n-6 PUFAs/ total n-3 PUFAs) (26), the ratio of AA was not significantly associated, whereas other n-6 PUFAs ratios were differentially associated with DR risk. Specifically, the LA (/total n-3 PUFAs) and total n-6 PUFAs (/total n-3 PUFAs) in testing set were associated with a 80% (aOR 0.20; 95% CI 0.04–0.96), 80% (aOR 0.20; 95% CI 0.04–0.96) lower risk of DR, respectively.
Table 3.
Association of serum n-6 PUFAs, PUFA ratios, desaturase and elongase activity with DR.
| Training set | Testing set | |||
|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| n-6 PUFAs | ||||
| 18:2 n-6 (LA) | 0.25 (0.12, 0.53) | 0.29 (0.13, 0.63) | 0.20 (0.04, 0.90) | 0.20 (0.04, 1.01) |
| 18:3 n-6 (GLA) | 0.50 (0.30, 0.82) | 0.57 (0.34, 0.96) | 0.31 (0.10, 0.96) | 0.34 (0.09, 1.34) |
| 20:2 n-6 (EDA) | 0.58 (0.36, 0.95) | 0.59 (0.34, 1.02) | 0.28 (0.08, 1.00) | 0.26 (0.06, 1.14) |
| 20:3 n-6 (DGLA) | 0.41 (0.19, 0.88) | 0.37 (0.15, 0.87) | 0.16 (0.03, 0.85) | 0.16 (0.02, 1.04) |
| 20:4 n-6 (AA) | 0.51 (0.29, 0.90) | 0.54 (0.27, 1.07) | 0.53 (0.26, 1.06) | 0.58 (0.27, 1.24) |
| Total n-6 PUFAs | 0.25 (0.12, 0.53) | 0.29 (0.13, 0.64) | 0.20 (0.04, 0.91) | 0.20 (0.04, 1.01) |
| Fatty acids ratios | ||||
| LA/Total n-3 PUFAs | 0.25 (0.12, 0.53) | 0.28 (0.12, 0.65) | 0.28 (0.10, 0.78) | 0.20 (0.04, 0.96) |
| GLA/Total n-3 PUFAs | 0.47 (0.28, 0.78) | 0.60 (0.36, 0.98) | 0.31 (0.11, 0.86) | 0.30 (0.08, 1.07) |
| EDA/Total n-3 PUFAs | 0.54 (0.34, 0.86) | 0.56 (0.32, 0.98) | 0.21 (0.05, 0.84) | 0.13 (0.02, 1.08) |
| DGLA/Total n-3 PUFAs | 0.44 (0.24, 0.81) | 0.46 (0.23, 0.93) | 0.17 (0.04, 0.82) | 0.12 (0.01, 1.04) |
| AA/Total n-3 PUFAs | 0.55 (0.34, 0.90) | 0.71 (0.41, 1.25) | 0.39 (0.16, 0.92) | 0.41 (0.16, 1.01) |
| Total n-6/n-3 PUFAs | 0.25 (0.12, 0.53) | 0.28 (0.12, 0.65) | 0.28 (0.10, 0.78) | 0.20 (0.04, 0.96) |
| Desaturase and elongase activity | ||||
| ∆6-desaturase activity | 0.88 (0.61, 1.26) | 0.93 (0.60, 1.44) | 1.09 (0.51, 2.35) | 2.10 (0.63, 6.97) |
| Elongase activity | 1.02 (0.73, 1.42) | 0.94 (0.63, 1.39) | 0.64 (0.26, 1.57) | 0.34 (0.11, 1.07) |
| ∆5-desaturase activity | 1.33 (0.81, 2.17) | 1.89 (0.94, 3.80) | 1.72 (0.83, 3.55) | 1.59 (0.69, 3.69) |
Data were presented with odds ratio (OR) and 95% CI .
*Adjusted forsystolic blood pressure (SBP) and duration of diabetes. ∆6-desaturase activity = GLA/LA; elongase activity = DGLA/GLA; ∆5-desaturase activity = AA/DGLA.
AA, arachidonic acid;DGLA, dihomo-gamma-linolenic acid; EDA, eicosadienoic acid; GLA, gamma-linolenic acid; LA, linoleic acid; PUFA, polyunsaturated fatty acid. Total n-6 PUFA is the sum of LA, GLA, EDA, DGLA and AA. Total n-3 PUFA is the sum of alpha-linolenic acid (ALA), docosahexaenoic acid (DHA); eicosapentaenoic acid (EPA), eicosatrienoic acid (ETA), stearidonic acid (SA).
To avoid impacts due to the collinearity of independent variables and to weaken influence of n-3 PUFAs on the associations, we additionally performed a multiple principal component analysis (PCA) on n-6 PUFAs to total n-3 PUFAs. As we could see in Fig. 2, the top two principle components (PC) could explain 80.8% (62.0 and 18.8% for the 1st and 2nd PC, respectively) of the contributions of the five n-6 PUFAs. To decrease the potential over-fitting, we further investigated the relationship between the likelihood of DR in T2D and n-6 PUFAs using a multivariable generalized linear regression model including the top two principle components and the above-mentioned two major risk factors. The adjusted OR (95% CI) for the first principle component were 0.62 (0.43, 0.87) in the training set and 0.23 (0.04, 1.31) in the testing set, respectively. These results suggested again that elevated n-6 PUFAs levels were significantly associated with decreased risk of DR in T2D.
Figure 2.
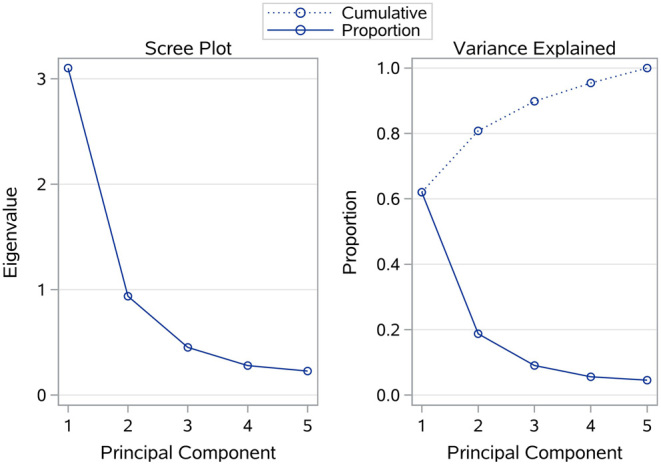
Screen plot and variance explained of principal component analysis.
In addition, the number of participants with prescriptions for each class of diabetes medication was presented in Supplementary Table 2. From the results of Supplementary Table 3 training set, insulin, metformin, gliclazide, acarbose, pioglitazone and other are not significant covariates for the first principal component.
Optimal model selection
To screen an optimal model included these detected n-6 PUFAs in indicating the risk to get DR in T2D, we constructed a total of seven forecasting models and comprehensively evaluated their performance. Total n-6/n-3 PUFAs, commonly used indicator in the research of polyunsaturated fatty acids (26), and SBP, diabetes duration constituted Model 7 (reference model). As can be seen from Fig. 3, the reference model could show excellent recognition ability in both the training set and testing set with AUC (95% CI) of 0.84 (0.76, 0.92) and 0.85 (0.73, 0.98), respectively.
Figure 3.
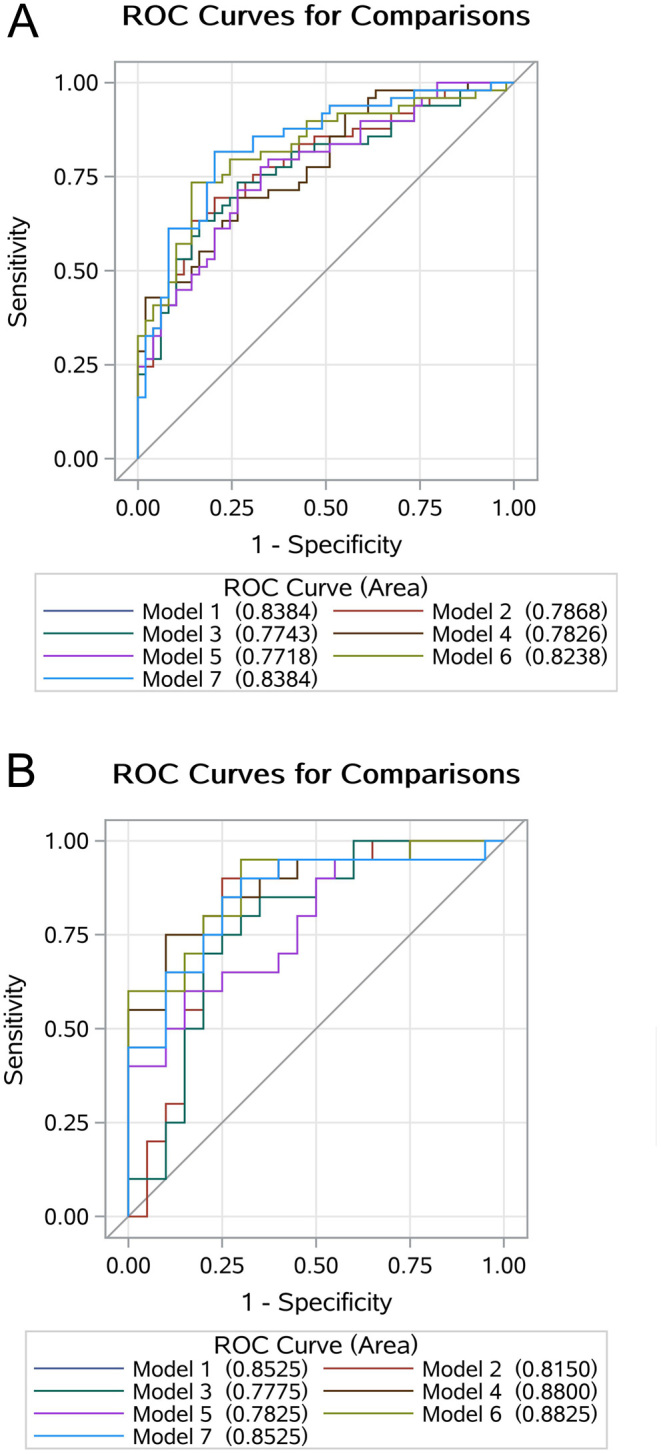
Comparisons of the performance of each model using receiver operating characteristic (ROC) analysis in the training set (A) and testing set (B). LA, linoleic acid; GLA, gamma-linolenic acid; EDA, eicosadienoic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic acid; PUFA, polyunsaturated fatty acid; PC, principal component. Model 1: LA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 2: GLA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 3: EDA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 4: DGLA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 5: AA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 6: the 1st PC + the 2nd PC + systolic BP + duration of diabetes; Model 7: Total n-6/n-3 PUFAs + systolic BP + duration of diabetes.
According to the testing set, the dataset to evaluate the generalization ability of the model, the AUC of Model 4 (0.88, 95%CI (0.77, 0.99)) and Model 6 (0.88, 95%CI (0.78, 0.99)) were greater than that of Model 7, but the difference was not significant (Table 4). In particular, model 6 had better performance than that of model 7 in both NRI (NRI = 1.00, 95% CI: 0.48–1.52, P = 0.002) and IDI (IDI = 0.04, 95% CI: 0.01–0.16, P = 0.033). In addition, the model 6 also had good calibration in both the train and testing sets with a non-significant Hosmer–Lemeshow Chi-square of 7.57 (P = 0.477) and 9.44 (P = 0.307), respectively.
Table 4.
Selection of the optimal model for identifying diabetic retinopathy (DR).
| Contrast | Difference in AUC (95% CI) | P-value | IDI (95% CI) | P-value | NRI (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Training set | ||||||
| Model1–Model7 | 0 | – | <−0.01 (<−0.01, <0.01) | 0.170 | −0.04 (−0.44, 0.35) | 0.840 |
| Model2–Model7 | −0.05 (−0.10, <0.01) | 0.053 | −0.10 (−0.15, −0.04) | <0.001 | −0.69 (−1.06, −0.32) | <0.001 |
| Model3–Model7 | −0.06 (−0.12, −0.01) | 0.021 | −0.11 (−0.16, −0.05) | <0.001 | −0.61 (−0.99, −0.24) | 0.002 |
| Model4–Model7 | −0.06 (−0.12, 0.01) | 0.096 | −0.09 (−0.16, −0.02) | 0.011 | −0.41 (−0.80, −0.02) | 0.043 |
| Model5–Model7 | −0.07 (−0.13, −0.01) | 0.027 | −0.12 (−0.18, −0.05) | <0.001 | −0.57 (−0.95, −0.19) | 0.005 |
| Model6–Model7 | −0.01 (−0.05, 0.02) | 0.447 | −0.03 (−0.07, 0.02) | 0.228 | −0.29 (−0.68, 0.11) | 0.157 |
| Testing set | ||||||
| Model1–Model6 | 0 | – | <−0.01 (<−0.01, <−0.01) | <0.001 | −1.10 (−1.61, 0.59) | <0.001 |
| Model2–Model6 | −0.04 (−0.16, 0.09) | 0.560 | −0.09 (−0.21, 0.03) | 0.134 | −0.50 (−1.09, 0.09) | 0.114 |
| Model3–Model6 | −0.08 (−0.18, 0.03) | 0.161 | −0.11 (−0.22, <0.01) | 0.057 | −0.80 (−1.36, −0.24) | 0.011 |
| Model4–Model6 | 0.03 (−0.08, 0.13) | 0.606 | 0.06 (−0.08, 0.20) | 0.394 | 0.60 (<0.01, 1.19) | 0.058 |
| Model5–Model6 | −0.07 (−0.22, 0.08) | 0.367 | −0.14 (−0.29, 0.01) | 0.074 | −0.40 (−1.00, 0.20) | 0.206 |
| Model6–Model7 | 0.03 (−0.03, 0.09) | 0.304 | 0.04 (<0.01, 0.16) | 0.033 | 1.00 (0.48, 1.52) | 0.002 |
Model 1: LA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 2: GLA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 3: EDA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 4: DGLA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 5: AA/total n-3 PUFAs + systolic BP + duration of diabetes; Model 6: the 1st PC + the 2nd PC + systolic BP + duration of diabetes; Model 7: Total n-6/n-3 PUFAs + systolic BP + duration of diabetes.
AUC, the area under the receiver-operating-characteristic (ROC) curve; IDI, the integrated discrimination improvement; NRI, the net reclassification improvement.
Discussion
In this propensity score matching (PSM) based case–control study, we provided, to the best of our knowledge, relatively extensive evaluation to five individual serum n-6 PUFAs with risk of DR, and used total five n-3 PUFAs to calculate the ratios of n-6 PUFAs and n-3 PUFAs. Overall, the LA, which is primarily diet-derived PUFA, was significantly associated with DR risk. Among primarily endogenous PUFAs, GLA, EDA, DGLA and total n-6 PUFAs were negatively associated with DR risk, whereas the negative correlation between AA and DR risk was not significant. In this study, considering the impact of early DR patients’ misclassification as DM without recognition, not only 69 diabetic participants without DR were set up as the control, but also 69 participants without diabetes were also selected as another healthy control. As can be seen from Supplementary Fig. 1, these five detected n-6 PUFAs showed an apparent downward trend from the healthy control, T2D control to DR group in both the training and testing set.
Comparison with other studies on DR or DM
Previous data on serum n-6 PUFAs in relation to risk of DR were scarce, especially epidemiological data. Most research is based on animal experiments and in vitro cell experiments. We compared our study with an lipidomics research by Philippe Koehrer and colleagues with a small sample size (18 control subjects, 14 diabetic patients without DR, 12 mild non-proliferative DR patients, 12 moderate non-proliferative DR patients, 22 severe non-proliferative DR patients, and 24 proliferative DR patients) (27). Consistent with our findings, Philippe Koehrer and colleagues reported a significant decrease in levels of AA in DR patients was observed. As we performed propensity score matching (PSM), the power of the test was improved, so the levels of LA, GLA, EDA, DGLA and total n-6 PUFAs were significantly reduced in serum of diabetic patients with or without retinopathy. In addition, we comprehensively considered the relationship between n-6 PUFAs and DR risk, using the total n-6/n-3 ratio for consideration. On the other hand, linoleic acid, the most abundant form of n-6 PUFAs, accounted for 98.9% of the total PUFA in our research. In order to avoid weakening the impact of other fatty acids due to differences in magnitude, we considered using the principal component of n-6 PUFAs as a new comprehensive evaluation index. In a food frequency questionnaire-based epidemiological study, Mariko Sasaki and colleagues reported that although it was not significantly associated with DR risk in the pooled analysis, PUFAs were detected to be negatively correlated to the likelihood of DR in those whose HbA1c under 7% (28). Their findings indicate that the relationship between PUFAs and the risk of DR would be obviously modified by HbA1c. However, although 128 of 138 participants in the present study have HbA1c greater than 7.0%, higher level of n-6 PUFAs was observed to be significantly associated with lower likelihood of DR, which is inconsistent of Sasaki’s finding. We speculated that this inconsistency may be mainly due to much higher accuracy of our PUFAs data, which were carefully assessed using UPLC-ESI-MS/MS system. In statistics, under the same circumstances, more accurate data will achieve higher power when comparing with inaccurate data. In this condition, the probability of detecting the association will be obviously improved.
It is reported that D6D, elongase and D5D play a key role in the metabolic pathway of LA (29). Abundant evidences containing animal experiment (30), cross-sectional research (23) and cohort study (24) reveal that abnormal PUFAs metabolism in diabetic patients is closely related to the activities of desaturation and elongase depending on the comparison between diabetic patients and their counterparts. In the current study, the association between the levels of PUFAs and the presence of DR do not achieve significant level. The possible reason may be due to the participants in both DM and DR groups having very similar HbA1c, which was matched using propensity score matching approach. However, when comparing with healthy controls, all diabetic patients including DM and DR all have obvious lower levels of D6D, elongase and D5D, which is consistent with above -mentioned studies.
Biological plausibility and implications
DR is due to neovascularization as a result of increased production of growth hormone, insulin-like growth factor (IGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β), hepatocyte growth factor, placental growth factor, tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) in response to hypoxia and ischemia of the inner retinal layers (19). Current evidence reveals that n-6 PUFAs and their products lipoxins, resolvins and protectins have anti-inflammatory effects, can effectively inhibit the production of VEGF and TNF-α, and restrict endothelial cell proliferation that may account for their beneficial effects in pathological retinal angiogenesis (19).
Although previous studies have suggested that LA, GLA and AA were involved in the development of DR, no evidence on the linkage between DR risk and EDA as well as DGLA have been detected. Linoleic acid (LA) is known as the major dietary n-6 PUFA and an essential fatty acid. A long-term prospective study which was conducted 40 years ago revealed that LA had a strong inhibitory effect on the development of retinopathy in diabetic patients (31). Jason H Y Wu's study (32), which consists of 20 prospective cohort studies, showed that the risk of the incident T2D was inversely associated with levels of LA because of efficient improving blood sugar, insulin resistance, insulin secretion capacity (33) and reduce inflammation (34). At the same time, high blood sugar and inflammation levels are detected to be independent risk factors of diabetic microangiopathy (15). Meanwhile, early studies have shown that supplements of γ-linolenic acid (GLA) and other substances can effectively improve hyperglycemia induced disturbance of vascular endothelial function, which reflects the disturbance of microcirculation perfusion and is of great importance for the pathogenesis of DR (35). Another study also reports that arachidonic acid (AA) metabolites are involved in regulating angiogenesis (36), and the lipoxygenase pathway of arachidonic acid metabolism is reported to be involved in mediating retinal NV via the VEGF/PEDF balance disruption (37).
Strengths and limitations
Our study has some notable strengths. The used of propensity score matching (PSM) can not only effectively balance the confounding bias in non-randomized controlled studies, make the research results close to the effect of randomized controlled studies, but also improve the test efficacy of the study. The two-stage research idea was adopted to effectively improve the credibility of the conclusion, in which the association was detected based on a training set and evaluated in another independent testing set, respectively. In addition, principal component regression was used to avoid the collinearity among independent variables and potential over-fitting, and the integrated principal component index may become a new indicator. Similar to other researches, the present study also had limitations. First, the sample size was not too large which would like to affect the reliability of the results, to some extent, since it will lead to insufficient power to reject the null hypothesis. However, it does not mean that the current sample size is insufficient since it has been carefully estimated during the study design period. Moreover, suppose the sample size is really inadequate, the association between the likelihood of DR with n-6 PUFAs we detected in the present study will be more robust when we have enough participants. This study is a cross-sectional study, and the conclusion needs to be verified by a large cohort study.
Conclusions
In summary, our results clearly revealed that alterations in five n-6 PUFAs (LA, GLA, EDA, DGLA and AA) occurred from DM to DR. Elevated n-6 PUFAs were independent protective factors of DR and their combination may be optimal multiple network biomarkers to predict the risk to get DR in T2D. Our findings will be beneficial on the administration of T2D in practice.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was funded by Zhejiang Basic Public Welfare Research Project (LGF19H260011), Zhejiang University Student Science and Technology Innovation Activity Plan (2019R413073), Wenzhou Basic Public Welfare Research Project (Y20180201), the Initial Scientific Research Fund (KYQD170301), the Major Project of the Eye Hospital of Wenzhou Medical University (YNZD201602) and the academician’s science and technology innovation program in Zhejiang province (2018R413182). Part of this work was also funded by the National Nature Science Foundation of China (81670777) and Natural Science Foundation of Zhejiang Province (LZ19H020001). The sponsor or funding organization had no role in the design or conduct of this research.
STROBE Statement
The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Acknowledgements
Jushuang Li and Tao Wang conducted the research, analyzed the data and wrote the manuscript. Jingjing Zuo and Chengnan Guo analyzed the data. Fang Peng, Shuzheng Zhao, Huihui Li, Xiangqing Hou and Yuan Lan contributed to the epidemiological investigation, sample handling, data management, and analysis. Yaping Wei repeated the data analysis independently. Guangyun Mao, Chao Zheng, and Honglin Hu designed the study, thoroughly reviewed and edited the manuscript.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE.Idf diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice 2017. 128 40–50. ( 10.1016/j.diabres.2017.03.024) [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Cheung CM, Larsen M, Sharma S, Simó R.Diabetic retinopathy. Nature Reviews: Disease Primers 2016. 2 16012. ( 10.1038/nrdp.2016.12) [DOI] [PubMed] [Google Scholar]
- 3.Tan GS, Cheung N, Simó R, Cheung GC, Wong TY.Diabetic macular oedema. Lancet: Diabetes and Endocrinology 2017. 5 143–155. ( 10.1016/S2213-8587(1630052-3) [DOI] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J.et al Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012. 35 556–564. ( 10.2337/dc11-1909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K.et al Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet: Global Health 2013. 1 e339–e349. ( 10.1016/S2214-109X(1370113-X) [DOI] [PubMed] [Google Scholar]
- 6.Antonetti DA, Klein R, Gardner TW.Diabetic retinopathy. New England Journal of Medicine 2012. 366 1227–1239. ( 10.1056/NEJMra1005073) [DOI] [PubMed] [Google Scholar]
- 7.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS.Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Disease Models and Mechanisms 2012. 5 444–456. ( 10.1242/dmm.009597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaassen I, Van Noorden CJ, Schlingemann RO.Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress in Retinal and Eye Research 2013. 34 19–48. ( 10.1016/j.preteyeres.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 9.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK.et al Incidence and progression of diabetic retinopathy: a systematic review. Lancet: Diabetes and Endocrinology 2019. 7 140–149. ( 10.1016/S2213-8587(1830128-1) [DOI] [PubMed] [Google Scholar]
- 10.SanGiovanni JP, Chew EY.The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Progress in Retinal and Eye Research 2005. 24 87–138. ( 10.1016/j.preteyeres.2004.06.002) [DOI] [PubMed] [Google Scholar]
- 11.Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L.Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ 2019. 366 l4697. ( 10.1136/bmj.l4697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D.et al Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature Medicine 2007. 13 868–873. ( 10.1038/nm1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorusupudi A, Chang FY, Nelson K, Hageman GS, Bernstein PS.n-3 PUFA supplementation alters retinal very-long-chain-PUFA levels and ratios in diabetic animal models. Molecular Nutrition and Food Research 2019. 63 e1801058. ( 10.1002/mnfr.201801058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM, Sochacki AL, Faber MS, Hazra S, Duclos S.et al N-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS ONE 2013. 8 e55177. ( 10.1371/journal.pone.0055177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Shen S, Das UN, Xu G.Effect of essential fatty acids on glucose-induced cytotoxicity to retinal vascular endothelial cells. Lipids in Health and Disease 2012. 11 90. ( 10.1186/1476-511X-11-90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR.Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota coronary experiment (1968–73). BMJ 2016. 353 i1246. ( 10.1136/bmj.i1246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong Y, Fu Z, Liegl R, Chen J, Hellström A, Smith LE.Ω-3 and ω-6 long-chain pufas and their enzymatic metabolites in neovascular eye diseases. American Journal of Clinical Nutrition 2017. 106 16–26. ( 10.3945/ajcn.117.153825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Jump DB, Grant MB, Esselman WJ, Busik JV.Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Investigative Ophthalmology and Visual Science 2003. 44 5016–5022. ( 10.1167/iovs.03-0418) [DOI] [PubMed] [Google Scholar]
- 19.Das U.Polyunsaturated fatty acids in pathological retinal angiogenesis. Current Nutrition and Food Science 2009. 5 94–111. ( 10.2174/157340109788185562) [DOI] [Google Scholar]
- 20.Wei Y, Jia C, Lan Y, Hou X, Zuo J, Li J, Wang T, Mao G.The association of tryptophan and phenylalanine are associated with arsenic-induced skin lesions in a Chinese population chronically exposed to arsenic via drinking water: a case-control study. BMJ Open 2019. 9 e025336. ( 10.1136/bmjopen-2018-025336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Cheng CY, Choi H, Ikram MK, Sabanayagam C, Tan GS, Tian D, Zhang L, Venkatesan G, Tai ES.et al Plasma metabonomic profiling of diabetic retinopathy. Diabetes 2016. 65 1099–1108. ( 10.2337/db15-0661) [DOI] [PubMed] [Google Scholar]
- 22.Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, Smilde AK.Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Analytical Chemistry 2006. 78 567–574. ( 10.1021/ac051495j) [DOI] [PubMed] [Google Scholar]
- 23.Shetty SS, Kumari N S, Shetty PK.Ω-6/ω-3 fatty acid ratio as an essential predictive biomarker in the management of type 2 diabetes mellitus. Nutrition 2020. 79–80 110968 ( 10.1016/j.nut.2020.110968) [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Li M, Rahman ML, Hinkle SN, Wu J, Weir NL, Lin Y, Yang H, Tsai MY, Ferrara A.et al Plasma phospholipid n-3 and n-6 polyunsaturated fatty acids in relation to cardiometabolic markers and gestational diabetes: a longitudinal study within the prospective nichd fetal growth studies. PLoS Medicine 2019. 16 e1002910. ( 10.1371/journal.pmed.1002910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan GS, Ikram MK, Wong TY.Traditional and novel risk factors of diabetic retinopathy and research challenges. Current Medicinal Chemistry 2013. 20 3189–3199. ( 10.2174/09298673113209990023) [DOI] [PubMed] [Google Scholar]
- 26.Strandvik B.The omega-6/omega-3 ratio is of importance! Prostaglandins, Leukotrienes, and Essential Fatty Acids 2011. 85 405–406. ( 10.1016/j.plefa.2011.09.001) [DOI] [PubMed] [Google Scholar]
- 27.Koehrer P, Saab S, Berdeaux O, Isaïco R, Grégoire S, Cabaret S, Bron AM, Creuzot-Garcher CP, Bretillon L, Acar N.Erythrocyte phospholipid and polyunsaturated fatty acid composition in diabetic retinopathy. PLoS ONE 2014. 9 e106912. ( 10.1371/journal.pone.0106912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki M, Kawasaki R, Rogers S, Man RE, Itakura K, Xie J, Flood V, Tsubota K, Lamoureux E, Wang JJ.The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetes. Investigative Ophthalmology and Visual Science 2015. 56 7473–7479. ( 10.1167/iovs.15-17485) [DOI] [PubMed] [Google Scholar]
- 29.Ross A, Caballero B, Cousins R, Tucker K, Ziegler T. Modern Nutrition in Health and Disease. Philadelphia, PA, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 30.Ramsammy LS, Haynes B, Josepovitz C, Kaloyanides GJ.Mechanism of decreased arachidonic acid in the renal cortex of rats with diabetes mellitus. Lipids 1993. 28 433–439. ( 10.1007/BF02535942) [DOI] [PubMed] [Google Scholar]
- 31.Houtsmuller AJ, van Hal-Ferwerda J, Zahn KJ, Henkes HE.Favorable influences of linoleic acid on the progression of diabetic micro- and macroangiopathy in adult onset diabetes mellitus. Progress in Lipid Research 1981. 20 377–386. ( 10.1016/0163-7827(8190070-9) [DOI] [PubMed] [Google Scholar]
- 32.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kröger J.et al Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet: Diabetes and Endocrinology 2017. 5 965–974. ( 10.1016/S2213-8587(1730307-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fumiaki I, Renata M, Wu Jason HY, de Oliveira Otto Marcia Otite Fadar CO, Abioye Ajibola I, Mozaffarian Dariush, Ma Ronald CW.Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Medicine 13 e1002087 ( 10.1371/journal.pmed.1002087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M.et al Effects of n-6 pufas compared with sfas on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. American Journal of Clinical Nutrition 2012. 95 1003–1012. ( 10.3945/ajcn.111.030114) [DOI] [PubMed] [Google Scholar]
- 35.McCarty MF.Nitric oxide deficiency, leukocyte activation, and resultant ischemia are crucial to the pathogenesis of diabetic retinopathy/neuropathy – preventive potential of antioxidants, essential fatty acids, chromium, ginkgolides, and pentoxifylline. Medical Hypotheses 1998. 50 435–449. ( 10.1016/s0306-9877(9890217-1) [DOI] [PubMed] [Google Scholar]
- 36.Nie D, Honn KV.Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cellular and Molecular Life Sciences 2002. 59 799–807. ( 10.1007/s00018-002-8468-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Shabrawey M, Mussell R, Kahook K, Tawfik A, Eladl M, Sarthy V, Nussbaum J, El-Marakby A, Park SY, Gurel Z.et al Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes 2011. 60 614–624. ( 10.2337/db10-0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a