Abstract
Background
Dupilumab blocks the shared receptor component for interleukin (IL)‐4/IL‐13, key drivers of type 2 inflammation. In phase 2b (NCT01854047) and phase 3 LIBERTY ASTHMA QUEST (NCT02414854), add‐on dupilumab 200/300 mg every 2 weeks (q2w) reduced severe exacerbations, improved prebronchodilator (pre‐BD) forced expiratory volume in 1 second (FEV1) and quality of life measures, and it was generally well tolerated in patients with uncontrolled, persistent (phase 2b), or moderate‐to‐severe (phase 3) asthma.
Methods
In patients on high‐dose inhaled corticosteroids (ICS) with type 2‐high asthma (subgroups including baseline blood eosinophils ≥150/300 cells/µL and/or fractional exhaled nitric oxide [FeNO] ≥25 ppb), annualized severe exacerbation rates over the treatment period, changes from baseline in pre‐BD FEV1 and asthma control (5‐item asthma control questionnaire [ACQ‐5]) were analyzed.
Results
In high‐dose ICS type 2‐high subgroups, dupilumab 200/300 mg q2w vs placebo in the phase 2b (24 weeks) and phase 3 (52 weeks) studies significantly reduced severe exacerbations by 55%‐69%/57%‐60% (all P<.05) and 53%‐69%/48%‐66% (all P < .001), respectively, except in patients with ≥ 300 eosinophils/µL in phase 2b study (24%/50% (P = .52/0.15). Across subgroups, pre‐BD FEV1 improved by 0.18‐0.22 L/0.19‐0.24 L (all P < .05) and 0.23‐0.36 L/0.15‐0.25 L (all P < .01) and ACQ‐5 scores were reduced by 0.46‐0.55/0.47‐0.85 (all P < .05) and 0.38‐0.50/0.24‐0.30 (all P < .05), respectively, except dupilumab 200 mg q2w in phase 2b in patients with FeNO ≥ 25 ppb (0.41; P = .09). Dupilumab was also effective in patients taking medium‐dose ICS.
Conclusion
Dupilumab significantly reduced severe exacerbations and improved lung function and asthma control in patients with type 2‐high asthma on high‐dose ICS at baseline.
Keywords: asthma control, exacerbations, inhaled corticosteroids, moderate‐to‐severe asthma, prebronchodilator FEV1
This study examines dupilumab efficacy in type 2‐high asthma patients receiving high‐dose ICS at baseline. Dupilumab reduces severe exacerbations, improves lung function and asthma control in patients on high‐dose ICS with elevated baseline blood eosinophils or FeNO. Dupilumab efficacy is rapid and sustained throughout treatment and comparable across type 2‐high asthma patients receiving high‐dose ICS at baseline. Abbreviations: ACQ‐5, 5‐item Asthma Control Questionnaire; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; ppb, parts per billion; pre‐BD, prebronchodilator.
1. INTRODUCTION
Inhaled corticosteroids (ICS) have been the mainstay treatment of persistent asthma for more than 40 years. By targeting the glucocorticoid receptor, ICS inhibit the release of cytokines and other proinflammatory mediators, decrease eosinophil and mast cell recruitment, and inhibit nuclear transcription factors, thus suppressing adhesion molecule function and inducible nitric oxide synthase. 1 , 2 , 3 , 4 However, the largest clinical benefits are seen at low‐dose ICS, with diminishing returns due to increased systemic adverse events at higher doses. 5
Uncontrolled, moderate‐to‐severe asthma accounts for approximately 20% of all asthma cases 6 , 7 and includes patients who have persistent symptoms and/or exacerbations despite the use of high‐dose ICS and controller medicines. This population is at an increased risk of exacerbations, with many patients having substantially reduced lung function and impaired quality of life, all of which culminate in considerable healthcare resource use and associated costs. 8 Type 2‐high asthma, characterized by type 2 inflammation, occurs in approximately 50% of patients with asthma. 9 It includes the allergic phenotype, characterized by increased expression of specific immunoglobulin E (IgE) to aeroallergens; eosinophilic phenotype, characterized by eosinophilia evident in the blood, airways, and/or tissue 9 , 10 , 11 ; and nonallergic, adult‐onset, intrinsic phenotype. In recent years, add‐on biologic treatments for use in patients with uncontrolled moderate‐to‐severe or persistent asthma have been developed that specifically target elements of type 2 inflammation such as interleukin (IL)‐4, IL‐5, and IL‐13, which play crucial roles in the pathogenesis of asthma. 12 , 13 One such agent, dupilumab, is a fully human VelocImmune®‐derived monoclonal antibody that blocks the shared receptor component for IL‐4 and IL‐13, key and central drivers of type 2 inflammation in multiple diseases. 14 , 15 , 16 , 17
In the phase 2 studies (NCT01312961 and NCT01854047), dupilumab‐ vs placebo‐treated patients with uncontrolled persistent asthma had a significantly reduced severe exacerbation rate and improved prebronchodilator (pre‐BD) forced expiratory volume in 1 second (FEV1). 18 , 19 Of importance, these findings were consistent irrespective of baseline blood eosinophil count, a biomarker for type 2 inflammation. 19 In the subsequent phase 3 LIBERTY ASTHMA QUEST study (NCT02414854), add‐on dupilumab significantly reduced severe asthma exacerbations and improved pre‐BD FEV1 in the overall intention‐to‐treat (ITT) population of patients with uncontrolled, moderate‐to‐severe asthma. 20 Treatment effects were of greater magnitude in subgroups of patients with elevated baseline levels of the type 2 biomarkers (blood eosinophils ≥150 cells/µL or fractional exhaled nitric oxide [FeNO] ≥25 ppb). Dupilumab was generally well tolerated by patients in each of the studies.
This analysis across the dupilumab phase 2b and phase 3 QUEST studies further assessed the effect of dupilumab on severe exacerbations, pre‐BD FEV1, and asthma control in subgroups of patients with different characteristics of type 2‐high disease who were taking high‐dose ICS at baseline. Given the evidence from previous studies and in other biologics, we expected dupilumab to also be effective in this patient population. For completeness and transparency, we also repeated the analysis in patients with elevated type 2 biomarkers taking medium‐dose ICS at baseline and have included the findings in Appendix S1.
2. METHODS
2.1. Study design and patients
The phase 2b study (NCT01854047) was a randomized, double‐blind, placebo‐controlled, parallel‐group study conducted in adults (aged ≥18 years) with an asthma diagnosis for at least 12 months based on the Global Initiative for Asthma 2009 guidelines 21 and receiving treatment with high‐ (>1000 µg/day) or medium‐ (500‐1000 µg/day) dose ICS plus long‐acting β2‐agonists. Patients were randomly assigned (1:1:1:1:1) to receive subcutaneous dupilumab 200 or 300 mg every 2 weeks (q2w) or every 4 weeks or placebo, over a 24‐week period. Full details of the study design and conduct, including inclusion and exclusion criteria, have been published previously. 19 For this post hoc analysis, only the data from patients randomized to dupilumab q2w regimens (the approved dosing regimen) and placebo were included.
LIBERTY ASTHMA QUEST was a phase 3 multinational, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study that assessed the efficacy and safety of dupilumab in patients with uncontrolled asthma treated with high‐ (>1000 µg/day) or medium‐ (500‐1000 µg/day) dose ICS (Table S1). Patients ≥12 years were randomized in a 2:2:1:1 ratio to receive add‐on subcutaneous dupilumab 200 or 300 mg q2w or volume‐matched placebo for 52 weeks. The study was open to all patients irrespective of eosinophilic status or any other biomarker requirement. Full details of study design, methodology, and eligibility criteria have been reported previously. 20 , 22
In both the phase 2b study and LIBERTY ASTHMA QUEST, atopy was self‐reported by patients; no further clinical assessments were conducted to verify.
Both studies were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation and with applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. Study conduct and documentation were monitored by local institutional review boards or ethics committees, and all the patients provided written informed consent before participating in the trial. Adolescent patients provided assent according to the ethics committee and approved standard practice for pediatric patients at each participating center.
2.2. Study endpoints
Annualized severe exacerbation rates (defined as number of severe exacerbations per patient‐year), change from baseline in pre‐BD FEV1 (L), and asthma control (using the patient‐reported 5‐item Asthma Control Questionnaire [ACQ‐5] 23 ) were assessed over the 24‐week (phase 2b study) and 52‐week (QUEST) treatment periods. A responder analysis of ACQ‐5 scores was conducted in which the proportion of patients with a response to treatment (responders) was defined as those with an improvement from baseline in ACQ‐5 score that reached or exceeded the threshold of the minimum clinically important difference (MCID) of 0.5. 24 In line with the primary analyses for these studies, patients with improvement from baseline of <0.5 or with a missing value were considered as nonresponders for that time point.
2.3. Statistical analysis
Prespecified efficacy analyses were performed on subgroups of patients in the overall ITT population categorized by ICS controller requirement at baseline (adapted from GINA 2014 25 ; Table S1), and post hoc analyses, which further stratified the groups by baseline levels of blood eosinophils or FeNO. The subgroups examined were high‐dose ICS and eosinophils ≥150 cells/µL, high‐dose ICS and eosinophils ≥300 cells/µL, high‐dose ICS and FeNO ≥25 ppb, high‐dose ICS and eosinophils ≥150 cells/µL or FeNO ≥25 ppb, and high‐dose ICS ITT subgroups. The same analyses were also performed on equivalent subgroups for completeness and transparency in patients taking medium‐dose ICS. The ITT population was defined as all patients who were randomized, and data were analyzed according to the assigned intervention and whether an intervention was received. Results are presented separately for each study.
In both studies, the annualized rate of severe asthma exacerbations was analyzed using a negative binomial regression model. 19 , 20 , 22 Change from baseline in FEV1 (L) and ACQ‐5 was analyzed using mixed‐effects models with repeated measures. Additional model details are provided in Appendix S1.
Descriptive statistics have been employed to present the data; a P value of <.05 for the comparison between each dupilumab dose and placebo (within each subgroup) was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
In the phase 2b study, 307 patients were randomized to dupilumab q2w regimens, and 158 patients were randomized to placebo (Figure S1A). Of these 465 patients, 231 (49.6%) received high‐dose ICS, and 221 (47.5%) received medium‐dose ICS at baseline. In QUEST, a total of 1902 patients were randomized (placebo: n = 638, dupilumab: n = 1264; Figure S1B). Fifteen (0.8%) patients were on low‐dose ICS at study entry (in violation of the protocol) and are not included in this analysis, resulting in an analysis population of 1887. Of these, 979 (51.9%) were on high‐dose ICS at baseline, and 908 (48.1%) patients received medium‐dose ICS (Table 1). In each study, the subgroups of patients receiving high‐dose ICS had relatively worse lung function, asthma control, and prior exacerbations, indicative of their greater disease severity and needed a higher dose of controller medication (Table 1). Furthermore, baseline demographics for each subgroup are shown in Tables S2‐5.
TABLE 1.
Baseline demographic and disease characteristics in phase 2b and phase 3 QUEST studies by baseline ICS dose
| Dupilumab phase 2b | Dupilumab phase 3 QUEST | ||||||
|---|---|---|---|---|---|---|---|
| Placebo a |
Dupilumab 200 mg q2w |
Dupilumab 300 mg q2w |
Matching placebo to dupilumab 200 mg q2w |
Dupilumab 200 mg q2w |
Matching placebo to dupilumab 300 mg q2w |
Dupilumab 300 mg q2w |
|
| High‐dose ICS at baseline, n | 77 | 75 | 79 | 172 | 317 | 167 | 323 |
| Age, mean (SD), years | 50.3 (10.9) | 54.0 (11.6) | 48.3 (12.4) | 48.9 (14.1) | 49.5 (13.8) | 49.5 (13.9) | 49.1 (14.9) |
| Age <18 y, n (%) | 0 | 0 | 0 | 6 (3.5) | 6 (1.9) | 3 (1.8) | 11 (3.4) |
| Female sex, n (%) | 54.0 (70.1) | 43 (57.3) | 52 (65.8) | 110 (64.0) | 199 (62.8) | 119 (71.3) | 203 (62.8) |
| Ongoing atopic medical condition, n (%) | 57 (75.0) | 52 (69.3) | 59 (74.7) | 147 (85.5) | 257 (81.1) | 136 (81.4) | 262 (81.1) |
| Prebronchodilator FEV1, mean (SD), L | 1.70 (0.50) | 1.73 (0.54) | 1.80 (0.53) | 1.74 (0.55) | 1.69 (0.56) | 1.65 (0.50) | 1.70 (0.60) |
| Percent predicted, mean (SD) | 58.97 (10.79) | 59.45 (11.45) | 60.14 (10.79) | 58.10 (12.77) | 56.38 (13.48) | 56.43 (13.33) | 56.37 (14.40) |
| Postbronchodilator FEV1, mean (SD), L | NA | NA | NA | 2.11 (0.62) | 2.06 (0.69) | 2.03 (0.61) | 2.11 (0.71) |
| FEV1 reversibility b , mean (SD), % | 25.79 (12.12) | 25.61 (19.02) | 27.59 (17.25) | 23.57 (15.65) | 28.23 (23.58) | 27.04 (16.43) | 28.20 (26.72) |
| Severe asthma exacerbations in past year, mean (SD), n | 2.57 (2.72) | 2.19 (1.75) | 2.77 (2.77) | 2.26 (1.77) | 2.24 (2.11) | 2.57 (2.33) | 2.22 (1.67) |
| ACQ‐5 score c , mean (SD) | 2.82 (0.82) | 2.88 (0.74) | 2.98 (0.86) | 2.82 (0.76) | 2.90 (0.87) | 2.89 (0.82) | 2.91 (0.79) |
| AQLQ(S) global score, mean (SD) | 3.84 (1.06) | 3.91 (1.16) | 3.70 (1.15) | 4.15 (1.05) | 4.18 (1.10) | 4.13 (1.04) | 4.04 (1.02) |
| Biomarker levels | |||||||
| Blood eosinophil count, median (IQR), cells/µL | 280.0 (190.0‐450.0) | 280.0 (150.0‐550.0) | 260.0 (160.0‐420.0) | 280.0 (130.0‐485.0) | 250.0 (130.0‐470.0) | 280.0 (150.0‐510.0) | 250.0 (130.0‐470.0) |
| Total IgE, median (IQR), IU/mL | 216.0 (94.0‐463.0) | 151.0 (47.0‐428.0) | 163.0 (78.0‐405.0) | 176.0 (52.0‐406.0) | 151.0 (51.0‐459.0) | 160.5 (62.0‐363.0) | 175.5 (59.5‐450.0) |
| FeNO, median (IQR), ppb | 30.0 (18.0‐41.0) | 27.0 (15.0‐41.0) | 29.0 (13.0‐62.0) | 23.0 (14.0‐38.0) | 23.0 (14.0‐39.0) | 27.0 (15.5‐43.5) | 24.0 (15.0‐42.0) |
| Medium‐dose ICS at baseline, n | 78 | 69 | 74 | 144 | 310 | 151 | 303 |
| Age, mean (SD), years | 47.4 (14.1) | 47.9 (14.6) | 46.7 (12.6) | 47.5 (17.3) | 46.2 (16.6) | 46.7 (15.5) | 46.4 (16.1) |
| Age <18 y, n (%) | 0 | 0 | 0 | 15 (10.4) | 28 (9.0) | 15 (9.9) | 23 (7.6) |
| Female sex, n (%) | 48 (61.5) | 47 (68.1) | 48 (64.9) | 87 (60.4) | 186 (60.0) | 96 (63.6) | 191 (61.6) |
| Ongoing atopic medical condition, n (%) | 55 (73.3) | 55 (80.9) | 49 (69.0) | 118 (81.9) | 249 (80.3) | 127 (84.1) | 255 (84.2) |
| Prebronchodilator FEV1, mean (SD), L | 1.95 (0.58) | 1.87 (0.52) | 1.91 (0.54) | 1.80 (0.67) | 1.88 (0.66) | 1.86 (0.62) | 1.87 (0.59) |
| Percent predicted, mean (SD) | 62.9 (10.5) | 62.9 (10.3) | 61.3 (10.2) | 58.9 (13.7) | 60.4 (13.2) | 60.4 (14.3) | 60.6 (12.2) |
| Postbronchodilator FEV1, mean (SD), L | NA | NA | NA | 2.23 (0.79) | 2.27 (0.79) | 2.25 (0.77) | 2.24 (0.73) |
| FEV1 reversibility b , mean (SD), % | 29.84 (16.08) | 27.89 (16.40) | 26.89 (15.92) | 27.17 (21.48) | 26.39 (21.95) | 25.99 (19.05) | 23.31 (20.14) |
| Severe asthma exacerbations in past year, mean (SD), n | 1.95 (1.67) | 1.45 (0.83) | 1.96 (1.59) | 1.85 (1.30) | 1.91 (3.14) | 2.03 (1.71) | 1.82 (2.03) |
| ACQ‐5 score c , mean (SD) | 2.57 (0.76) | 2.55 (0.86) | 2.64 (0.68) | 2.59 (0.68) | 2.62 (0.70) | 2.61 (0.69) | 2.62 (0.70) |
| AQLQ(S) global score d , mean (SD) | 4.35 (1.10) | 4.19 (1.15) | 4.14 (1.07) | 4.39 (0.97) | 4.43 (1.05) | 4.52 (0.98) | 4.53 (1.03) |
| Biomarker levels | |||||||
| Blood eosinophil count, median (IQR), cells/µL | 245.0 (150.0‐420.0) | 240.0 (180.0‐400.0) | 270.0 (160.0‐380.0) | 260.0 (140.0‐490.0) | 250.0 (120.0‐450.0) | 245.0 (130.0‐420.0) | 250.0 (130.0‐450.0) |
| Total IgE, median (IQR), IU/mL | 182.0 (84.0‐430.0) | 237.0 (51.0‐647.0) | 168.5 (73.0‐409.0) | 173.5 (75.0‐493.0) | 158.0 (65.0‐441.0) | 188.0 (52.5‐482.0) | 172.5 (66.0‐454.0) |
| FeNO, median (IQR), ppb | 28.0 (16.0‐53.0) | 32.0 (18.0‐52.0) | 28.0 (16.0‐48.0) | 27.0 (16.0‐51.5) | 24.0 (16.0‐47.0) | 27.0 (16.0‐51.0) | 24.0 (13.0‐43.0) |
Abbreviations: ACQ‐5, 5‐Item Asthma Control Questionnaire; AQLQ[S], Asthma Quality of Life Questionnaire [Standardized]; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; HRQoL, health‐related quality of life; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IQR, interquartile range; NA, not applicable; ppb, parts per billion; q2w, every 2 wk; SD, standard deviation.
In the phase 2b study, the same amount of placebo was given regardless of dupilumab dose (not volume‐matched).
Forced expiratory volume in 1 s (L) reversibility indicates the change in FEV1 between prebronchodilator and postbronchodilator measurements.
5‐item asthma control questionnaire is a patient‐reported measure of the adequacy of asthma control and change in asthma control that occurs either spontaneously or as a result of treatment. Scores range between 0 (totally controlled) and 6 (severely uncontrolled).
Asthma quality of life questionnaire (standardized) is a patient‐reported measure of asthma‐specific HRQoL. Higher scores indicate better HRQoL; a global score is rated on a 7‐point Likert‐like scale (7 = “not impaired at all” to 1 = “severely impaired”).
3.2. Annualized rate of severe asthma exacerbations
In the overall ITT population of the phase 2b study, dupilumab 200 and 300 mg q2w compared with placebo significantly reduced adjusted annualized severe exacerbation rates in the subgroup receiving high‐dose ICS at baseline by 56% (P = .004) and 57% (P = .002), respectively (Figure 1). Numerical reductions vs placebo were observed in the subgroup on high‐dose ICS with blood eosinophil counts ≥300 cells/µL at baseline (P = .524 and P = .149, respectively), and significant reductions vs placebo were observed in subgroups on high‐dose ICS with blood eosinophil count ≥150 cells/µL (P < .05 and P < .01), FeNO ≥25 ppb (P < .01 and P < .05), and blood eosinophil count ≥150 cells/µL or FeNO ≥25 ppb (P < .01 for both) (Figure 1). In the phase 2b study, adjusted severe exacerbation rates could not be calculated for the subgroups of patients receiving medium‐dose ICS as the number of events was deemed too small to provide valid estimates from an adjusted model; therefore, unadjusted data are presented. In the subgroup receiving medium‐dose ICS at baseline, the reductions in the unadjusted annualized severe exacerbation rate for patients receiving dupilumab 200 and 300 mg q2w vs placebo were 67.5%/62.4% vs 46.3% (Figure S2). Consistent numerical improvements vs placebo were also observed for each subgroup when further stratified by blood eosinophil and FeNO levels at baseline (Figure S2).
FIGURE 1.
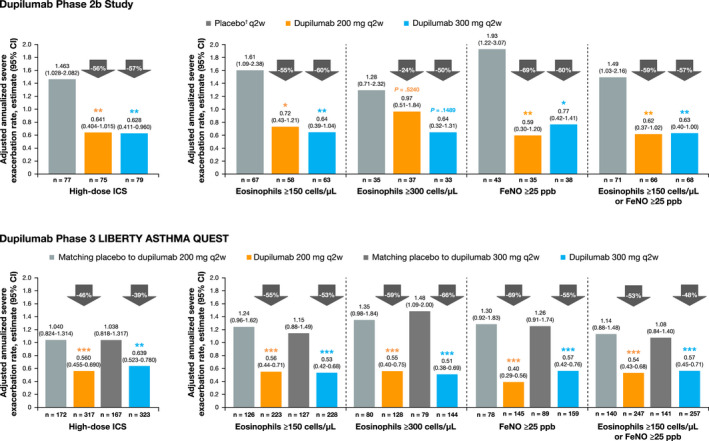
Annualized rate of severe exacerbations in dupilumab‐treated patients (q2w) vs placebo during the 24‐wk treatment period in the phase 2b study and 52‐wk treatment period in the phase 3 QUEST study on high‐dose ICS at baseline and further stratified by baseline eosinophil and FeNO levels. †In the phase 2b study, the same amount of placebo was given regardless of dupilumab dose (not volume‐matched as in the phase 3 QUEST study). ***P < .001, **P < .01, *P < .05 vs placebo. CI, confidence interval; FeNO, fractional exhaled nitic oxide; q2w, every 2 wk
In the overall ITT population of QUEST, dupilumab 200 and 300 mg q2w compared with placebo significantly reduced adjusted annualized severe exacerbation rates in patients receiving high‐dose ICS at baseline by 46% (P < .001) and 39% (P = .002), respectively (Figure 1); when further stratified by baseline type 2 biomarker status (blood eosinophil count and/or FeNO levels), a greater magnitude of treatment effect was observed in patients with type 2‐high phenotype with reductions vs placebo ranging from 48% to 69% (all P < .001) (Figure 1). In patients on medium‐dose ICS at baseline, significant reductions in adjusted annualized severe exacerbation rates by 51% (P = .0004) and 53% (P < .0001), respectively, were observed; this was also seen for each subgroup when further stratified by blood eosinophil and FeNO levels at baseline (Figure S2).
3.3. Prebronchodilator FEV1
In the overall ITT population of the phase 2b study, treatment with dupilumab 200 and 300 mg q2w vs placebo improved pre‐BD FEV1 in patients receiving high‐dose ICS at baseline by least squares (LS) mean difference (95% confidence interval [CI]) of 0.14 L (0.03‐0.24; P = .011) and 0.19 L (0.09‐0.30; P = .0002), respectively, by Week 2. This improvement was sustained up to 24 weeks (0.21 L [0.09‐0.32; P = .0005] and 0.22 L [0.11‐0.34; P = .0001]; Figure S3). When further stratified by baseline eosinophils or FeNO levels, statistically significant improvements vs placebo were consistently observed as early as Week 2 (LS mean difference range: 0.14‐0.21 L and 0.18‐0.23 L, all P < .05, respectively), with one exception (dupilumab 200 mg q2w patients with a baseline blood eosinophil count of ≥300 cells/µL [0.09 L; P = .263]), where a statistically significant improvement was reached first by Week 4. In all subgroups, these improvements were maintained throughout the 24‐week treatment duration (LS mean difference range: 0.18‐0.22 L and 0.19‐0.24 L, respectively; all P < .05) (Figure 2, Table S6). Improvements in pre‐BD FEV1 were also observed for the medium‐dose ICS group; at Week 2, the LS mean difference (95% CI) vs placebo was 0.14 L (0.03‐0.26; P = .01) and 0.11 L (−0.01‐0.22; P = .06). This numerical improvement was sustained up to 24 weeks (0.13 L [−0.01‐0.26; P = .06] and 0.09 L [−0.04‐0.22; P = .18]; Figure S3). Similar results were observed for each subgroup when further stratified by blood eosinophil and FeNO levels at baseline (Figure S4, Table S7).
FIGURE 2.
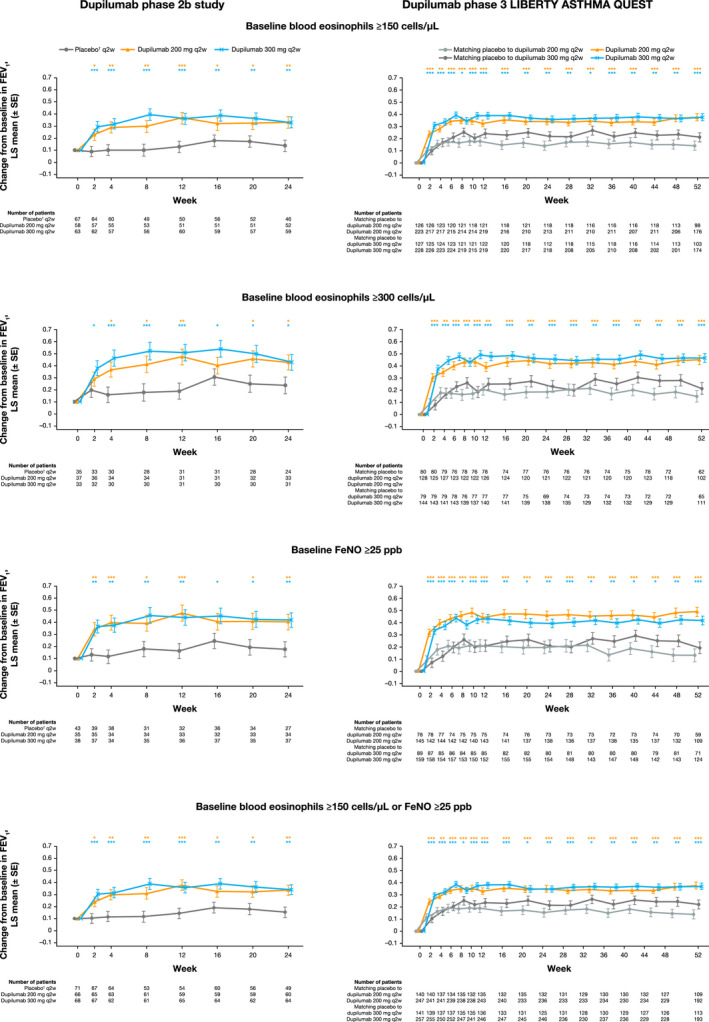
Least squares mean change from baseline in FEV1 (L) during the 24‐wk treatment period in the phase 2b study in patients with uncontrolled, persistent asthma and 52‐wk treatment period in the phase 3 QUEST study in patients with uncontrolled, moderate‐to‐severe asthma on high‐dose ICS at baseline and further stratified by baseline eosinophil and FeNO levels. †In the phase 2b study, the same amount of placebo was given regardless of dupilumab dose (not volume‐matched as in the phase 3 QUEST study). ***P < .001, **P < .01, *P < .05 vs placebo. FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LS, least squares; q2w, every 2 wk; SE, standard error
In the overall ITT population of the QUEST study, dupilumab 200 and 300 mg q2w vs placebo rapidly improved pre‐BD FEV1 in the high‐dose ICS subgroup by LS mean difference (95% CI) of 0.12 L (0.05‐0.18; P = .0004) and 0.16 L (0.09‐0.22; P < .0001), respectively, at Week 2. This improvement was sustained over the 52‐week treatment period (0.20 L [0.12‐0.28; P < .0001] and 0.13 L [0.05‐0.21; P = .002]; Figure S3). When further stratified by baseline eosinophils or FeNO levels, statistically significant improvements vs placebo were consistently observed as early as Week 2 (LS mean difference range: 0.13‐0.23 L and 0.19‐0.30 L, respectively; all P < .001) and were maintained throughout the 52‐week treatment period (LS mean difference range: 0.23‐0.36 L and 0.15‐0.25 L, respectively; all P < .05) (Figure 2, Table S6). At Week 2, treatment with dupilumab 200 and 300 mg q2w vs placebo also significantly improved pre‐BD FEV1 in the medium‐dose ICS subgroup by LS mean difference (95% CI) 0.18 L [0.11‐0.25] and 0.14 L [0.07‐0.21], respectively; both P < .0001; with sustained improvements by Week 52 of 0.20 L (0.11‐0.29; P < .0001), and 0.14 L (0.06‐0.23; P = .0009), respectively (Figure S3). Similar results were observed in subgroups further stratified by baseline eosinophils or FeNO levels (Figure S4, Table S7).
3.4. Asthma control (ACQ‐5 scores)
In the overall ITT population of the phase 2b study, treatment with dupilumab 200 and 300 mg q2w vs placebo improved asthma control in patients requiring high‐dose ICS at baseline (LS mean difference from baseline [95% CI] at Week 24: −0.49 [−0.82 to −0.16; P = .004] and −0.42 [−0.74 to −0.10; P = .01, respectively]; Figure S5). These improvements were also observed in the subgroups stratified by baseline eosinophils or FeNO (Figure 3). Improvements in ACQ‐5 scores were also seen in patients requiring medium‐dose ICS at baseline (LS mean difference from baseline [95% CI] at Week 24: −0.35 [−0.65 to −0.05; P = .02] and −0.28 [−0.57 to 0.01; P = .06], respectively; Figure S5) and in the subgroups stratified by baseline eosinophils or FeNO (Figure S6).
FIGURE 3.
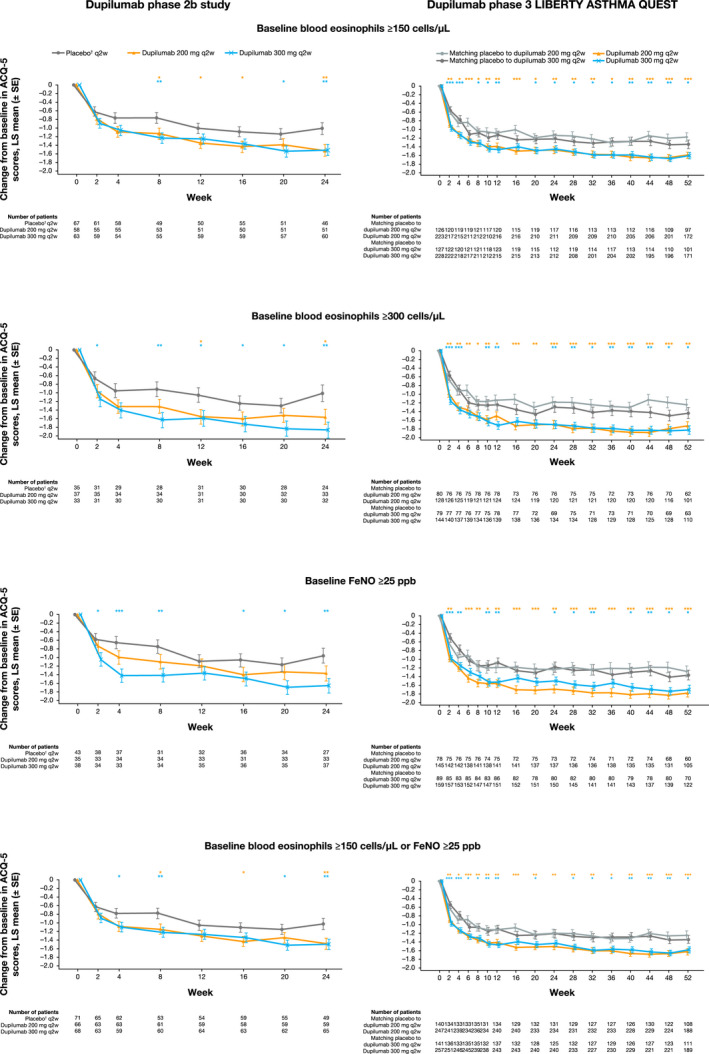
Least squares mean change from baseline in ACQ‐5 scores during the 24‐wk treatment period in the phase 2b study in patients with uncontrolled, persistent asthma and 52‐wk treatment period in the phase 3 QUEST study in patients with uncontrolled, moderate‐to‐severe asthma on high‐dose ICS at baseline and further stratified by baseline eosinophil and FeNO levels. †In the phase 2b study, the same amount of placebo was given regardless of dupilumab dose (not volume‐matched as in the phase 3 QUEST study). ***P < .001, **P < .01, *P < .05 vs placebo. ACQ‐5, 5‐item asthma control questionnaire; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LS, least squares; q2w, every 2 wk; SE, standard error
Similarly, in the overall ITT population of QUEST, consistent improvements in asthma control were observed for patients with high‐dose ICS at baseline (LS mean difference from baseline [95% CI] vs placebo at Week 24: −0.31 [−0.51 to −0.11; P = .002] and −0.13 [−0.33 to 0.07; P = .21], respectively; Week 52: −0.34 [−0.54 to −0.14; P = .0009] and −0.15 [−0.35 to 0.05; P = .14], respectively; Figure S5) and in subgroups stratified by baseline eosinophils or FeNO (Figure 3). This was also the case in patients requiring medium‐dose ICS at baseline (LS mean difference from baseline [95% CI] vs placebo at Week 24: −0.40 [−0.59 to −0.22; P = .0001] and −0.27 [−0.45 to −0.09; P = .003], respectively; Week 52: −0.44 [−0.63 to −0.24; P < .0001] and −0.29 [−0.48 to −0.10; P = .002], respectively; Figure S5) and in the subgroups stratified by baseline eosinophils or FeNO (Figure S6).
3.5. ACQ‐5 responder analysis
Many of the improvements in asthma control were clinically significant as evidenced by the differences vs placebo in ACQ‐5 scores reaching or exceeding the MCID of 0.5. 24 In the high‐dose ICS subgroup of the phase 2b study, after 24 weeks, the proportion of responders in dupilumab‐treated patients was 75% (56/75 patients; odds ratio [OR] 2.59 [1.29, 5.20]; P = .007) and 76% (60/79 patients; OR 2.85 [1.43, 5.68]; P = .003) vs 53% (41/77) of placebo‐treated patients.
In the overall ITT population of QUEST, at 24 weeks, a numerical increase in responders was observed in the high‐dose ICS at baseline subgroup treated with dupilumab 200 and 300 mg q2w vs matched placebos, respectively: 75% (238/317 patients) vs 69% (118/172 patients) (OR vs placebo [95%CI] 1.36 [0.90, 2.08]; P = .15) and 72% (231/323) vs 62% (104/167) (OR 1.51 [1.00, 2.26]; P = .05]) (Table S8). Numerical improvements in responder rates were also observed in the medium‐dose ICS subgroups for both studies (Table S9).
4. DISCUSSION
In this analysis of data from the pivotal phase 2b and phase 3 LIBERTY ASTHMA QUEST studies, dupilumab 200 and 300 mg q2w vs placebo reduced severe exacerbation rates and improved FEV1 (L), asthma control (ACQ‐5), and rate of responders (ACQ improvement of MCID ≥ 0.5) in patients with uncontrolled, type 2‐high persistent or moderate‐to‐severe asthma taking high‐dose ICS at baseline. Improvements were observed across each of the subgroups as early as the first time point assessed and were sustained until treatment end in both studies.
There are currently 5 biologic add‐on treatments for patients with uncontrolled severe asthma that target underlying type 2 inflammatory processes: dupilumab, benralizumab, reslizumab, mepolizumab, and omalizumab. As previously outlined, dupilumab blocks the shared receptor component for IL‐4/IL‐13, key and central drivers of type 2 inflammation. 14 , 15 , 16 , 17 It is approved in the EU 26 as add‐on maintenance treatment in patients aged ≥12 years with severe asthma with type 2 inflammation characterized by raised blood eosinophils and/or raised FeNO levels who are inadequately controlled with a high‐dose ICS plus another medicinal product for maintenance treatment. It is also approved in the USA 27 and other countries 28 as an add‐on maintenance treatment in patients with moderate‐to‐severe asthma aged ≥12 years with an eosinophilic phenotype or with oral corticosteroid‐dependent asthma. 19 , 20 , 29 Benralizumab, reslizumab, and mepolizumab are monoclonal antibodies targeting IL‐5, a key component in eosinophil activation, proliferation, and degranulation. Benralizumab binds the alpha chain of the IL‐5 (IL‐5α) eosinophil cell surface receptor, blocking IL‐5 binding and has been evaluated in phase 3 studies in patients ≥12 years. 30 Mepolizumab and reslizumab bind directly to IL‐5, which in turn blocks binding with IL‐5α. 31 , 32 Mepolizumab has been evaluated in patients aged ≥6 years and reslizumab in patients ≥18 years. Omalizumab, an anti‐IgE monoclonal antibody that has been evaluated in patients ≥6 years, binds IgE and inhibits basophil and mast cell release of proinflammatory mediators. 33
Consistent with findings from the parent studies and other dupilumab studies, 19 , 20 , 29 a generally greater magnitude of dupilumab efficacy was observed in patients with a type 2‐high endotype. In the QUEST study, the highest magnitude of reduction in annualized severe exacerbation rate was observed in patients with type 2‐high endotypes, with differences vs placebo of 48%‐69%, compared with 39%‐46% in all patients in the high‐dose ICS subgroup and of 58%‐74%, compared with 51%‐53% in all patients in medium‐dose ICS subgroup. Of note, in the QUEST study, in patients taking high‐dose ICS a significant reduction in the adjusted annualized severe exacerbation rate with dupilumab vs matched placebo was observed in the subgroup of patients with baseline eosinophils ≥300 µ/L; a similar trend was observed in the phase 2b study although the effect did not reach statistical significance, likely due to low patient numbers in the subgroups of the phase 2b study. These observations were also seen in patients taking medium‐dose ICS in the QUEST study; in the phase 2b study, adjusted severe exacerbation rates could not be calculated for the subgroups of patients receiving medium‐dose ICS as the number of events was deemed too small to provide valid estimates from an adjusted model, but non‐significant reductions in the unadjusted annualized severe exacerbation rate were observed with dupilumab vs matched placebo. In both the phase 2b and QUEST studies, higher magnitudes of responses were seen in change from baseline in FEV1 compared with matched placebo in patients with type 2‐high asthma taking high‐dose ICS. This result was echoed in patients taking medium‐dose ICS in the phase 3 QUEST study.
Similar findings have also been observed with the other biologic add‐on therapies in patients with type 2‐high asthma on medium‐to‐high‐dose ICS. In the benralizumab phase 3 SIROCCO and CALIMA studies, significant reductions in annual asthma exacerbation rates and improvements in FEV1 and asthma symptom scores compared with placebo were observed in patients with high‐dose ICS and baseline blood eosinophils ≥300 cells/µL. 34 , 35 The phase 3 BREATH reslizumab studies also demonstrated reductions of clinical asthma exacerbations of 50%‐59% and improvements in FEV1, asthma control, forced vital capacity, and rescue short‐acting beta agonist use compared with placebo in patients with eosinophils ≥400 cells/µL (ITT population), and with lower efficacy in patients with <400 cells/µL (ITT population). 36 , 37 Higher efficacy in reduction of severe exacerbations, and improvement of FEV1 and ACQ‐5 in patients with higher baseline blood eosinophils compared with patients with lower levels were also demonstrated in the DREAM, MENSA, and MUSCA phase 3 mepolizumab studies. 38 , 39 , 40 Finally, significantly greater reductions in asthma exacerbations were seen in patients with raised FeNO (≥19.5 ppb), eosinophils (≥260 cells/µL), and periostin (≥50 ng/mL) who were treated with omalizumab in the EXTRA phase 3 study, compared with placebo recipients. 41 Although elevated eosinophils in peripheral blood have been associated with type 2 asthma, their levels can be significantly influenced by the use of ICS. 42 Thus, peripheral blood eosinophilia as a type 2 inflammation marker should always be interpreted in view of the current dose of ICS. This could also account for the efficacy of dupilumab being demonstrated irrespective of the baseline ICS dose used by the patients.
The strength of this analysis is its randomized, double‐blind design, its large population sizes, and the inclusion of patients regardless of ICS dose at baseline. One of the limitations was that the findings are constrained by the low sample sizes in some of the subgroups, as these were not defined a priori. The studies were not powered specifically to investigate differences between patients with asthma with high/medium‐dose ICS and eosinophils ≥150 cells/µL or ≥300 cells/µL, FeNO ≥25 ppb, or eosinophils ≥150 cells/µL or FeNO ≥25 ppb. In addition, a large placebo effect on asthma control was observed in the QUEST study, possibly due to increased adherence in patients in the placebo group, which may have contributed to the differences in ACQ‐5 improvement from baseline for dupilumab vs placebo not being statistically significant. Finally, due to the timings of the trial, old versions of the GINA guidelines were used when designing the studies and this analysis.
5. CONCLUSIONS
In conclusion, the outcomes of these 2 pivotal dupilumab studies confirm that dupilumab is effective across a spectrum of patients on high‐dose ICS at baseline with raised type 2 biomarkers and 1 or 2 additional controllers. Efficacy was also demonstrated in patients with type 2‐high asthma on medium‐dose ICS at baseline with 1‐2 additional controllers. Improvements in lung function were seen within 2 weeks, sustained throughout treatment, and generally of greater magnitude in subgroups of patients with elevated baseline levels of type 2 biomarkers (blood eosinophils ≥150 cells/µL or ≥300 cells/µL or FeNO ≥25 ppb).
CONFLICT OF INTEREST
Arnaud Bourdin reports non‐financial support from GlaxoSmithKline (GSK), during the conduct of the study; personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, Chiesi, GSK, Novartis, and Sanofi‐Regeneron; other from Acceleron, Actelion, Galapagos, MSD, Nuvaira, Pulmonx, United Therapeutic, and Vertex, outside the submitted work. Alberto A. Papi reports grants, personal fees, non‐financial support and other from GlaxoSmithKline, Boehringer Ingelheim, Chiesi Farmaceutici, and TEVA; grants, personal fees and non‐financial support from AstraZeneca and Menarini; personal fees, non‐financial support and other from Mundipharma, Zambon, Novartis, and Sanofi/Regeneron; personal fees from Roche and Edmondpharma; and grants from Fondazione Maugeri and Fondazione Chiesi; outside the submitted work. Jonathan Corren reports research support from Sanofi outside the submitted work. J. Christian Virchow reports personal fees from AstraZeneca, Avontec, Bayer, Bencard, Bionorica, Boehringer Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Altana, Pfizer, Revotar, Sandoz‐Hexal, Stallergens, Teva, UCB/Schwarz‐Pharma, Zydus/Cadila, and possibly others; other for Avontec, Boehringer Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, MEDA, MSD, Mundipharma, Novartis, Regeneron, Revotar, Roche, Sanofi‐Aventis, Sandoz‐Hexal, Teva, UCB/Schwarz‐Pharma, and possibly others; and research grants from Deutsche Forschungsgesellschaft, Land Mecklenburg‐Vorpommern, GSK, and MSD. Megan S. Rice reports personal fees and other from Sanofi, outside the submitted work. Yamo Deniz reports personal fees and other from Regeneron Pharmaceuticals, Inc, outside the submitted work. Michel Djandji reports personal fees and other from Sanofi, outside the submitted work. Paul Rowe reports personal fees and other from Sanofi, outside the submitted work. Ian D. Pavord reports personal fees from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis, GlaxoSmithKline, Genentech, and Regeneron; other from Teva, Chiesi, Sanofi, Circassia, and Knopp; grants from NIHR, outside the submitted work.
AUTHOR’S CONTRIBUTIONS
MSR, YD, and MD contributed to project concept, study design, and study implementation; AB, AAP, and JC contributed to data collection; MSR contributed to data and statistical analysis; all authors contributed to data analysis and interpretation, and critical revision of the mansucript; all authors critically reviewed and approved the final version of the manuscript.
Supporting information
Fig S1A
Fig S1B
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
App S1
Bourdin A, Papi AA, Corren J, et al. Dupilumab is effective in type 2‐high asthma patients receiving high‐dose inhaled corticosteroids at baseline. Allergy.2021;76:269–280. 10.1111/all.14611
Funding information
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov numbers: NCT01854047 (phase 2b) and NCT02414854 (phase 3 LIBERTY ASTHMA QUEST). Medical writing/editorial assistance provided by Martina Fuchsberger, PhD, of Excerpta Medica, funded by Sanofi and Regeneron Pharmaceuticals, Inc
REFERENCES
- 1. Cameron SJ, Cooper EJ, Crompton GK, Hoare MV, Grant IW. Substitution of beclomethasone aerosol for oral prednisolone in the treatment of chronic asthma. Br Med J. 1973;4(5886):205‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF‐κB activity through induction of IκB synthesis. Science. 1995;270(5234):286‐290. [DOI] [PubMed] [Google Scholar]
- 3. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 Pt 2):S17‐S22. [DOI] [PubMed] [Google Scholar]
- 4. Trevor JL, Deshane JS. Refractory asthma: mechanisms, targets, and therapy. Allergy. 2014;69(7):817‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID. Inhaled corticosteroid therapy in adult asthma. Time for a new therapeutic dose terminology. Am J Respir Crit Care Med. 2019;199(12):1471‐1477. [DOI] [PubMed] [Google Scholar]
- 6. Bateman ED, Boushey HA, Bousquet J, et al. Can guideline‐defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170(8):836‐844. [DOI] [PubMed] [Google Scholar]
- 7. Hermosa JL, Sánchez CB, Rubio MC, Mínguez MM, Walther JL. Factors associated with the control of severe asthma. J Asthma. 2010;47(2):124‐130. [DOI] [PubMed] [Google Scholar]
- 8. Kerkhof M, Tran TN, Soriano JB, et al. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campo P, Rodríguez F, Sánchez‐García S, et al. Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol. 2013;23(2):76‐88. [PubMed] [Google Scholar]
- 11. Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650‐658. [DOI] [PubMed] [Google Scholar]
- 12. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 13. Voehringer D, Reese TA, Huang X, Shinkai K, Locksely RM. Type 2 immunity is controlled by IL‐4/IL‐13 expression in hematopoietic non‐eosinophilic cells of the innate immune system. J Exp Med. 2006;203(6):1435‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacDonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111(14):5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murphy AJ, MacDonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111(14):5153‐5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425‐437. [DOI] [PubMed] [Google Scholar]
- 17. Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455‐2466. [DOI] [PubMed] [Google Scholar]
- 19. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet 2016;388(10039):31‐44. [DOI] [PubMed] [Google Scholar]
- 20. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 21. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2009. http://www.ginasthma.org. Accessed April 12, 2020.
- 22. Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: Phase 3 randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate‐to‐severe asthma. Adv Ther. 2018;35(5):737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553‐558. [DOI] [PubMed] [Google Scholar]
- 25. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2014. https://ginasthma.org/wp‐content/uploads/2019/01/2014‐GINA.pdf. Accessed April 12, 2020.
- 26. EU EMA approval . New add‐on treatment for patients with severe asthma. https://www.ema.europa.eu/en/documents/press‐release/press‐release‐new‐add‐treatment‐patients‐severe‐asthma_en.pdf. Accessed April 12, 2020.
- 27. Dupixent . Prescribing information. sanofi‐aventis U.S. LLC and Regeneron Pharmaceuticals, Inc; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf. Accessed April 12, 2020.
- 28. Dupixent (dupilumab) . Japan PMDA. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/780069_4490405G1024_1_05. Accessed April 12, 2020.
- 29. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378(26):2475‐2485. [DOI] [PubMed] [Google Scholar]
- 30. Faserna . Prescribing information. AstraZeneca; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. Accessed April 12, 2020.
- 31. Nucala (mepolizumab) prescribing information . GlaxoSmithKline; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf. Accessed April 12, 2020.
- 32. Cinqair . Prescribing information. Teva Pharmaceuticals; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf. Accessed April 12, 2020.
- 33. Xolair Prescribing information . Genentech Inc; 2019. https://www.gene.com/download/pdf/xolair_prescribing.pdf. Accessed April 12, 2020.
- 34. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2115‐2127. [DOI] [PubMed] [Google Scholar]
- 35. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2128‐2141. [DOI] [PubMed] [Google Scholar]
- 36. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 37. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150(4):799‐810. [DOI] [PubMed] [Google Scholar]
- 38. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. [DOI] [PubMed] [Google Scholar]
- 39. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 40. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. [DOI] [PubMed] [Google Scholar]
- 41. Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804‐811. [DOI] [PubMed] [Google Scholar]
- 42. Lommatzsch M, Klein M, Stoll P, Virchow JC. Impact of an increase in the inhaled corticosteroid dose on blood eosinophils in asthma. Thorax. 2019;74(4):417‐418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1A
Fig S1B
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
App S1


