Abstract
The modern nitrogen cycle consists of a web of microbially mediated redox transformations. Among the most crucial reactions in this cycle is the oxidation of ammonia to nitrite, an obligately aerobic process performed by a limited number of lineages of bacteria (AOB) and archaea (AOA). As this process has an absolute requirement for O2, the timing of its evolution—especially as it relates to the Great Oxygenation Event ~ 2.3 billion years ago—remains contested and is pivotal to our understanding of nutrient cycles. To estimate the antiquity of bacterial ammonia oxidation, we performed phylogenetic and molecular clock analyses of AOB. Surprisingly, bacterial ammonia oxidation appears quite young, with crown group clades having originated during Neoproterozoic time (or later) with major radiations occurring during Paleozoic time. These results place the evolution of AOB broadly coincident with the pervasive oxygenation of the deep ocean. The late evolution AOB challenges earlier interpretations of the ancient nitrogen isotope record, predicts a more substantial role for AOA during Precambrian time, and may have implications for understanding of the size and structure of the biogeochemical nitrogen cycle through geologic time.
Subject terms: Element cycles, Environmental microbiology, Evolution
Introduction
The biogeochemical nitrogen cycle is second only to carbon in size and, arguably, importance for the biosphere (e.g.1). The nitrogen cycle supplies fixed nitrogen for biomass while also fueling diverse microbial metabolisms, with of fluxes hundreds of teramoles of nitrogen per year (e.g.2). Nitrogen primarily enters this cycle by way of reduced forms (i.e. ammonia fixed from N2 by the enzyme nitrogenase), and so biological nitrification (i.e. the oxidation of ammonia to nitrite and nitrate) is an essential step for enabling downstream processes such as anammox and denitrification3. No metabolism has yet been discovered that is capable of oxidizing ammonia in the absence of O2 or O2-derived compounds like nitrite or NO4—therefore the modern nitrogen cycle where oxidized forms are regenerated and recycled is necessarily tied to O2.
Aerobic ammonia oxidation is found in a limited, polyphyletic set of Bacteria (AOB) and Archaea (AOA). In both AOB and AOA, the first step in ammonia oxidation is performed via ammonia monooxygenase (AMO), a member of the copper membrane monooxygenase (CuMMO) family. The CuMMO family includes the related particulate methane monooxygenases (pMMO) and enzymes that oxidize other small hydrocarbons5. CuMMO enzymes have an absolute requirement for O2, leading to the hypothesis that metabolic pathways utilizing these enzymes—including ammonia oxidation—evolved after the evolution of oxygenic photosynthesis provided significant O2 to the environment. While alternative, O2-independent ammonia oxidation processes such as the coupling of ammonia oxidation to phototrophy or metal reduction have been hypothesized, no organism has ever been characterized that can perform these reactions6,7. The evolution of oxygenic photosynthesis in Cyanobacteria led to the accumulation of atmospheric O2 to biologically meaningful concentrations ~ 2.3 billion years ago (Ga) during the Great Oxygenation Event (GOE), and it has been suggested that the onset of the aerobic nitrogen cycle occurred shortly thereafter8. However, others have argued from isotopic evidence that an aerobic nitrogen cycle was in place much deeper in Earth history (e.g.9). Distinguishing between these possibilities from the rock record alone is difficult due to the poor preservation of Archean strata and the lack of a robust framework for interpreting the ancient nitrogen isotope record. Moreover, signatures in the nitrogen isotope record may reflect only the expansion to geochemical prominence or first preservation of signatures of nitrogen metabolisms, and not necessarily their initial evolutionary origin. Instead, the biological record can provide opportunities for querying the antiquity of organisms and metabolisms responsible for driving the nitrogen cycle.
Here, we estimate the antiquity of AOB via phylogenetic and molecular clock analyses. We show that ammonia oxidation in bacteria has evolved convergently at least twice and that crown group AOB clades originated < 1 Ga, with major radiations occurring within the last ~ 500 million years (Ma). This suggests that bacteria did not contribute to ammonia oxidation until late in Earth history—more than 1.5 Ga after O2 first accumulated in the atmosphere. The predicted appearance of AOB at a time when Earth surface environments underwent oxygenation to modern-like levels points to the potential role for niche expansion in fostering evolution and boosting turnover of the marine fixed nitrogen inventory. This suggests a substantial difference in scale or structure of the biogeochemical nitrogen cycle during Precambrian time, likely with a more dominant role for AOA.
Phylogenetic distribution of proteins involved in ammonia oxidation
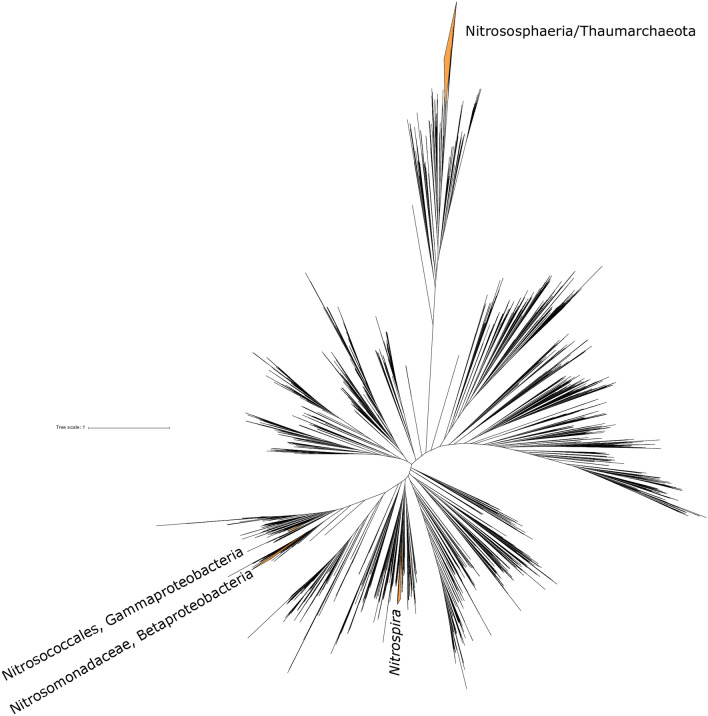
Phylogenetic analysis of the distribution of genes associated with ammonia oxidation shows that this metabolism is restricted to four clades of characterized ammonia oxidizers (Fig. 1). These include members of the Nitrosphaeria class of Crenarchaeota/Thaumarchaeota, the Nitrococcaceae family within the Gammaproteobacteria, the Nitrosomonadaceae family within the Betaproteobacteria, and some members of Nitrospira (the “comammox” bacteria, the only known organisms capable of oxidizing ammonia to nitrite and subsequently to nitrate10,11) (Fig. 2). These results are based on the presence of genes encoding ammonia monooxygenase and homologous proteins from the copper membrane monooxygenase family (Fig. 2, Supplemental Figures 1 and 2). While CuMMO sequences were recovered from diverse lineages including some that have not previously been characterized to possess the capacity for methanotrophy (e.g. members of the UBP10 and Myxococcota phyla), these proteins are most closely related to enzymes that are characterized as performing carbon oxidation (e.g. pMMO, butane monooxygenase), and no organisms outside of characterized clades of ammonia oxidizers were found to encode AMO. In all AOB lineages, hydroxylamine oxidoreductase (HAO) was found to cooccur with AMO and phylogenetic relationships among HAO proteins reflected those of AMO (Supplemental Figure 3). Downstream metabolic traits related to ammonia oxidation (such as aerobic respiration and carbon fixation) are not closely related between lineages of ammonia oxidizers; comammox Nitrospira, for instance, utilize bd O2 reductases for respiration and the rTCA pathway for carbon fixation12,13, as opposed to the heme-copper O2 reductases and Calvin cycle typical of ammonia oxidizing Proteobacteria14. This suggests that steps downstream of ammonia and hydroxylamine oxidation are not conserved among ammonia oxidizers, and instead diverse genes for these steps have been recruited to the overall ammonia oxidation pathway via vertical inheritance or horizontal gene transfer from non-ammonia oxidizers. In all cases, ammonia oxidation appears to be a derived trait, with basal members of the clades and closely related outgroups lacking the capacity for ammonia oxidation. Importantly, these clades of ammonia oxidizing microorganisms are not closely related and are phylogenetically separated by many lineages incapable of ammonia oxidation (Fig. 1).
Figure 1.
Tree of life built with concatenated ribosomal proteins following methods from15. Clades of ammonia oxidizing organisms highlighted in orange and labeled. The distribution of ammonia oxidation is polyphyletic, spread across one lineage within the Archaea (Nitrososphaeria) and three within the Bacteria (Nitrosococcales and Nitrosomonadaceae in the Proteobacteria phylum, and some members of the genus Nitrospira within the Nitrospirota phylum).
Figure 2.
Phylogeny of concatenated protein sequences of CuMMO A and B subunits (i.e. AmoA and AmoB, PmoA and PmoB), with ammonia monoxygenases highlighted in orange. Major clades are collapsed and labeled by the taxonomy of the organisms in which they are found as determined with GTDB-Tk16. The highly divergent archaeal ammonia monooxygenase is placed as an outgroup, though the placement of the root is indeterminate. Transfer Bootstrap Expectation (TBE) support values shown as calculated by BOOSTER17.
Phylogenetic analysis of proteins involved in ammonia oxidation, compared to organismal relationships among AOB, provide evidence for convergent evolution and horizontal gene transfer as major drivers for the extant diversity of organisms with the genetic capacity for ammonia oxidation. These relationships are consistent with major clades of AOB acquiring the capacity for ammonia oxidation through separate evolutionary events, followed largely by vertical inheritance within each AOB clade. These data are not consistent with a much more ancient acquisition of ammonia oxidation (e.g. in the last common ancestor of Nitrosococcales and Nitrosomonadaceae) followed by extensive loss. As a result, the age of total group Nitrosococcales, Nitrosomonadaceae, and the extant diversity of comammox Nitrospira can confidently be used to constrain the timing of acquisition of the capacity for ammonia oxidation in each lineage. Additionally, our data are consistent with hypotheses for ammonia oxidation evolving from earlier aerobic methane oxidation pathways (Supplemental Information).
Molecular clock evidence for the late evolution of ammonia oxidizing bacteria
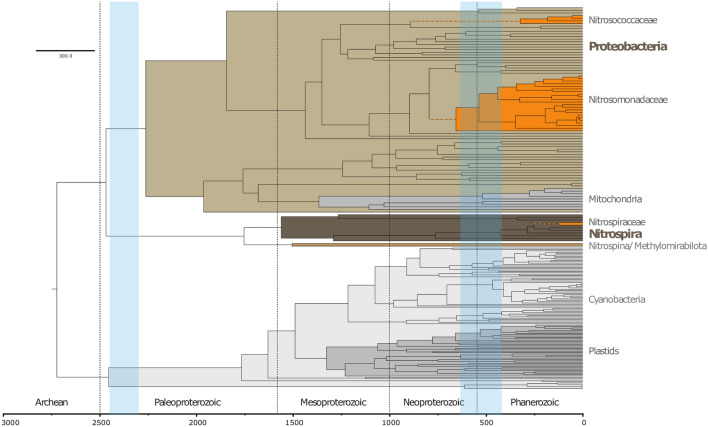
To connect the evolution history of AOB described above to events in Earth history, we performed molecular clock analyses to determine when AOB clades diverged from non-ammonia oxidizing relatives (i.e. age of total groups) and when AOB clades subsequently radiated (i.e. age of crown groups). Molecular clocks estimate the origin of each AOB clade to Neoproterozoic or Phanerozoic time, with each stem group AOB lineage emerging between 238 (comammox Nitrospira) and 894 Ma (Nitrosococcaceae) and radiation of crown groups occurring after 538 Ma (Fig. 3, Table 1). The 95% confidence intervals of divergence times introduce uncertainty of ± 150 Ma to these estimates (Table 1, Supplemental Figure 4), but in all cases firmly place the major radiation of extant AOB to Phanerozoic time even if the origin of crown groups was in late Neoproterozoic time. Between the uncertainty in the ages of total group AOB clades, and the fact that the acquisition of ammonia oxidation could in theory occur at any point along stem lineages prior to the divergence of crown groups, the range of 95% confidence intervals of ages of origin of the first AOB consistent with our data is between 1169 and 414 Ma. This age range includes scenarios involving the first evolution of ammonia oxidation in the earliest stem group Nitrosococcaceae (1169 Ma) or at the base of crown group Nitrosomonadaceae (414 Ma). This also accommodates the possibility that these groups acquired ammonia oxidation roughly simultaneously between 414 and 490 Ma. The analysis therefore does not allow for a unique determination of which proteobacterial lineage first acquired the capacity for ammonia oxidation. However, all scenarios consistent with our data involve a later acquisition of ammonia oxidation within the Nitrospira, after the radiation of ammonia oxidizing Nitrosomonadaceae and nitrite oxidizing Nitrospira.
Figure 3.
Molecular clock showing estimated age of clades of ammonia oxidizing bacteria. Phylum-level clades highlighted in gray and brown, with ammonia oxidizing clades highlighted in orange. Approximate timing of Great Oxygenation Event (~ 2.45–2.3 Ga) and Neoproterozoic/Paleozoic Oxygenation Event (~ 635–420 Ma) shown with light blue bars. While stem lineages of AOB clades may predate the NOE, the radiation of crown groups all occur broadly coincident or subsequent to the NOE, suggesting that evolutionary radiations of nitrogen-cycling organisms may have been causally linked with expansions in ocean oxygenation and/or productivity during this time period.
Table 1.
Age ranges for key divergences discussed in the text in millions of years (Ma).
| Clade | Estimated age of crown group | 95% Confidence interval | Estimated age of total group | 95% confidence interval |
|---|---|---|---|---|
| Nitrosococcaceae | 325 | 194–490 | 894 | 596–1169 |
| Nitrosomonadaceae | 538 | 414–662 | 657 | 510–821 |
| Nitrospira | 124 | 59–197 | 238 | 149–329 |
The Neoproterozoic to early Phanerozoic origin of crown group AOB suggested by our data is ~ 1.5 Ga later than previous suggestions that placed ammonium oxidation at or before the GOE ~ 2.3 Ga (e.g.8,9), suggesting that the first rock record evidence for ammonia oxidation records the activity of ammonia oxidizing archaea or other biological or abiotic processes. Our estimate for the origin of bacterial ammonia oxidation is during the time in Earth history that saw the biosphere transition from a low-productivity, exclusively microbial state characteristic of Proterozoic time18,19 to a more modern system fueled by eukaryotic algae and supporting complex multicellular organisms including animals20,21 possibly triggered by increased phosphate availability22. Net primary productivity of the biosphere is thought to have increased significantly at this time19,23, along with a rise in atmospheric oxygen concentrations to near-modern levels and the more permanent oxygenation of the deep ocean24. The timing of this final rise in atmospheric and marine O2 is not well-constrained and may have occurred as early as Ediacaran time25 or as late as ~ 420 Ma (e.g.26). The divergence of stem group proteobacterial AOB during Neoproterozoic time and the radiation of crown group proteobacterial AOB clades during Paleozoic time suggests that these evolutionary innovations may be causally linked. Increased oxygenation of the oceans would have provided additional O2 for ammonia oxidation, while higher NPP necessitates higher fluxes of fixed nitrogen through the biosphere leading to higher rates of N2 fixation to reduced forms (e.g.23). As a result, the necessary substrates for ammonia oxidation would have been more abundant after this later rise of O2 than earlier in Proterozoic time, potentially opening additional niche space that enabled the radiation of AOB. This hypothesis is further supported by the tendency of AOB to be adapted to higher ammonia (e.g.27–30) and possibly oxygen (e.g.31,32) concentrations than AOA.
The delayed evolution and radiation of AOB may also be a consequence of limited copper availability in Proterozoic oceans. Aerobic ammonia oxidation has a relatively high requirement for copper for enzyme cofactors (e.g.33), and copper availability in Proterozoic oceans may have been limited due to the insolubility of copper sulfide minerals in periodically euxinic oceans (e.g.34) and/or lower continental weathering of copper and subsequent runoff into the oceans35. The expansion of some metabolic pathways may therefore have been impeded by the availability of trace metals necessary as enzyme cofactors34. AOA have a comparable copper requirement for electron transport and nitrogen metabolic proteins as AOB (e.g.36), and so extreme copper limitation during Proterozoic time would be expected to impede AOA as well as AOB. Copper limitation may therefore have limited the expansion and potential productivity of the oxidative nitrogen cycle for much of Proterozoic time.
Implications for the Proterozoic nitrogen cycle
The late evolution of crown group AOB clades suggests that bacteria were not playing a dominant role in driving the oxidative arm of the nitrogen cycle during Proterozoic time, though it is always possible that there is a deeper history of bacterial ammonia oxidation by other lineages that remain undiscovered or that are now extinct. The nitrogen isotope record is consistent with active nitrification and denitrification through most of Proterozoic time (e.g.37–39), but does not provide direct evidence for the taxonomic affinity of organisms driving these processes. While the lack of an archaeal fossil record for calibrating molecular clocks makes estimating the antiquity of AOA challenging, recent work40 has suggested that ammonia oxidizing Thaumarchaeota originated ~ 2.3 Ga, in time to drive the aerobic nitrogen cycle shortly after the GOE (e.g.8). Consistent with this hypothesis is the lower oxygen requirements of AOA compared to proteobacterial ammonia oxidizers, leading to the continued dominance of AOA in modern oxygen minimum zones (e.g.41). This is also in keeping with AOA having evolved at a time with one to several orders of magnitude lower O2 than was present during the origin of AOB (e.g.25). The relative contribution of AOA and AOB to ammonia oxidation fluxes through time has not previously been constrained as the nitrogen isotope signatures of these groups overlap42 and the relative abundance and activity of AOA and AOB in modern environments is only determined roughly on a local scale via sequencing-based approaches that are not applicable to deep time (e.g.41). However, our results in combination with those of Ren et al., suggest that the AOA were responsible for driving all biological ammonia oxidation for most of Proterozoic time until the origin of the first AOB < 1 Ga.
It is important to note that AOA and different lineages of AOB utilize different biochemical pathways downstream of AMO, so their relative contribution to ammonia oxidation through time has significant implications for modeling of the productivity and atmospheric impact of the ancient biosphere. For example, extant AOA fix carbon using a uniquely energy-efficient O2-tolerant carbon fixation pathway (a variant of the hydroxypropionate/hydroxybutyrate pathway), while AOB typically utilize the Calvin Cycle (or, in comammox Nitrospira, the rTCA cycle)12,19,43. This allows AOA to fix 1.3 g of dry cell mass for every mole of ammonia oxidized, in contrast to only 0.8 g/mol in ammonia oxidizing Proteobacteria43. Further, nitrification currently accounts for ~ 75% of non-photosynthetic carbon fixation in aquatic environments44, so a nearly twofold difference in efficiency of carbon fixation in AOA versus AOB may lead to significant differences in predictions of net primary productivity of the biosphere through time. This is particularly important in the Proterozoic when photosynthetic carbon fixation rates are thought to have been much lower than today (e.g.19,45,46). Furthermore, the typical release of N2O by AOA is significantly lower than from AOB27, particularly under low-oxygen conditions47. As a result, an increased contribution of AOA to nitrification during Proterozoic time would likely be associated with a lower biogenic N2O flux, potentially at levels sufficiently low to prevent N2O from accumulating as an important greenhouse gas in the Proterozoic atmosphere as previously proposed (e.g.48).
Our results provide necessary constraints for establishing a timeline for the evolution of the biological nitrogen cycle. Before the origin of the first ammonia oxidizers, the nitrogen cycle would have consisted primarily of a vector toward reduced forms, with perhaps some oxidized nitrogen produced abiotically via processes like lightning (e.g.49,50). This reduced biogeochemical nitrogen cycle is thought to have persisted through Archean time (e.g.23,51) and may have continued into Proterozoic time until the evolution of the first ammonia oxidizing Archaea. Due to the stability of fixed nitrogen in the oceans as ammonia at this time, the nitrogen demands of phototrophic primary productivity would have been readily met23,51. Following the evolution of the AOA, the Proterozoic biosphere may have still been nitrogen limited, as the conversion of ammonia to nitrite/nitrate in oxygenated surface oceans would likely be followed by substantial loss of fixed nitrogen via denitrification and anammox in anoxic bottom waters (e.g.52). This extensive nitrogen loss would have maintained low concentrations of fixed nitrogen in the oceans, consistent with the relatively high substrate affinity of AOA (e.g.29) and low overall GPP predictions for that time23,45,46. The continuing dominance of AOA relative to AOB in oligotrophic environments53–55 may be a vestige of the key role of AOA in driving nitrification in nitrogen-limited Precambrian oceans.
The Earth experienced several evolutionary and environmental revolutions during Neoproterozoic and Paleozoic time including the rise of atmospheric oxygen to near-modern levels (e.g.25,26), persistent oxygenation of the deep oceans (e.g.56), the rise of eukaryotic algae and animals (e.g.20), and finally the evolution of plants and colonization of terrestrial environments (e.g.57). These events had a number of effects on weathering and geochemical cycles and may have triggered evolutionary innovations in the nitrogen cycle. For instance, higher primary productivity (e.g.19) would have increased fluxes of nitrogen through the biosphere while increased oxygenation would have increased the stability of nitrate in the oceans and subsequently allowed the accumulation of a large marine fixed nitrogen pool for the first time since the GOE (e.g.39). The colonization and expansion of terrestrial and freshwater ecosystems may have led to the development of localized nitrogen-rich copiotrophic environments like those preferred by AOB today54,58,59. These changes may have provided opportunities for the convergent evolution of multiple lineages of ammonia oxidizing bacteria, particularly Nitrosomonadaceae and Nitrosococcaceae.
Finally, it appears that comammox Nitrospira evolved last of all known lineages of ammonia oxidizers. Comammox Nitrospira appear to be derived from a larger and more ancient clade of nitrite oxidizing Nitrospirota via HGT of ammonia oxidation genes. Molecular clocks suggest that this transition occurred during Mesozoic time (Fig. 3). Comammox Nitrospira and their nitrite oxidizing relatives are adapted to low O2 concentrations (e.g.13); the apparent coincidence of the evolution of comammox Nitrospira with Mesozoic Oceanic Anoxic Events (e.g.60) may reflect the expansion of niches for ammonia oxidizers with low oxygen demands at this time.
Conclusions
The molecular clock evidence for broadly coincident radiations of multiple convergently evolved crown group AOB clades (Nitrosomonadaceae, Nitrosococcaceae, and comammox Nitrospira) shown here is largely unprecedented in molecular clock studies, which typically address the age of a single clade (e.g. acquisition of phototrophy within a bacterial phylum61) or show multiple evolutionary events scattered through time (e.g. evolution of C30 sterols in sponges and algae62). This adds strength to interpretations that the convergent evolutionary transitions to ammonia oxidation in these groups and/or their subsequent radiation may be linked to increases in ocean oxygenation at this time and highlights the interconnectedness between evolution of biogeochemically relevant microorganisms and major environmental perturbations. These interpretations are made only stronger in combination with other work indicating evolutionary and ecosystem expansion of AOA around this time40.
The necessity of reevaluating the structure and size of the Proterozoic nitrogen cycle in light of evidence for late-evolving AOB highlights a recurring problem in assessing the antiquity of microbial lineages—the rock record typically records the indirect effects of a (bio)geochemical process or the metabolism driving it, not directly the organisms that perform it. As a result, care must be taken in applying a strictly uniformitarian interpretation of the biological drivers of geochemical processes in deep time, as this overlooks evolutionary processes such as convergent evolution or horizontal gene transfer of metabolic pathways that lead to incongruent histories and potentially different combinations of traits in ancient drivers of biogeochemical cycles from the organisms responsible for these processes today.
Methods
Phylogenetic methods followed those described previously63 and summarized here. Genomes were downloaded from the NCBI Genbank and WGS databases. Completeness and contamination of metagenome-assembled genomes (MAGs) was estimated based on presence and copy number of conserved single-copy proteins by CheckM64. Protein sequences used in analyses (see below) were identified locally with the tblastn function of BLAST + 65, aligned with MUSCLE66, and manually curated in Jalview67. Positive BLAST hits were considered to be full length (e.g. > 90% the shortest reference sequence from an isolate genome) with e-values greater than 1e−20. Presence of metabolic pathways of interest in incomplete MAGs was predicted with MetaPOAP68 to check for False Positives (contamination) or False Negatives (genes present in source genome but not recovered in metagenome-assembled genomes). Phylogenetic trees were calculated using RAxML69 on the Cipres science gateway70. Transfer bootstrap support values were calculated by BOOSTER17, and trees were visualized with the Interactive Tree of Life viewer71. Taxonomic assignment was confirmed with GTDB-Tk16. Histories of vertical versus horizontal inheritance of metabolic genes was inferred by comparison of organismal and metabolic protein phylogenies72,73.
A concatenated protein alignment was generated by extracting protein sequences for marker genes from genomes of interest via the tblastn function of BLAST +65, aligning protein sequences with MUSCLE66, and then concatenating aligned sequences. Concatenated alignments were curated with Gblocks74 and manually in Jalview67. Taxa included in this alignment consist of all available AOB genomes on the NCBI GenBank and WGS databases as well as sister groups and outgroups spanning the full diversity of the Proteobacteria and Nitrospirota as well as closely related phyla (e.g. Methylomirabilota and Nitrospinota) as assessed by GTDB16 and concatenated ribosomal protein phylogenies of the tree of life (Fig. 1,15), as well as Cyanobacteria, plastids, and mitochondria. Phylogenetic markers were chosen as conserved proteins across bacteria, plastids, and mitochondria, as previously reported61,75, and consisted of AtpA, AtpB, EfTu, AtpE, AtpF, AtpH, AtpI, Rpl2, Rpl16, Rps3, and Rps12 protein sequences. Bayesian molecular clock analyses were carried out using BEAST v2.4.576 using the Cyberinfrastructure for Phylogenetic Research (CIPRES) Science Gateway v 3.3 server70. As previously reported, the CpREV model was chosen as the best-fitting amino acid substitution model for the concatenated protein dataset based on ProtTest analysis61. Cross-calibration techniques utilizing plastid and mitochondrial endosymbiosis events were used as priors, utilizing time constraints for the most recent common ancestor of Angiosperms (normal distribution with a mean of 217 Ma and sigma of 40 Ma) and of land plants (normal distribution with a mean of 477 Ma and sigma of 70 Ma) as has been previously described by Smith et al.77,78. We also constrained the most recent common ancestor of Rhodophytes with as a more recent study and precise estimate of the fossil constraint Bangiomorpha pubescens utilizing Re-Os isotopic measurements of the Bylot Supergroup of Baffin Island where the fossil was first described79. Taking into account previously reported ages of Bangiomorpha, we set this constraint as a uniform prior from 1030 to 1200 Ma, in order to account both Re-Os and Pb-Pb isotopic measurements estimating the age of Bangiomorpha80. A conservative uniform prior between 2300 and 3800 Ma was set on the divergence between Cyanobacteria and Melainabacteria, as oxygenic photosynthesis evolved prior to the Great Oxygenation Event and most likely evolved sometime after the Late Heavy Bombardment. Finally, a uniform prior for all taxa was again set conservatively between 2400 and 3800 Ma, assuming that the Last Bacterial Common Ancestor most likely evolved after the Late Heavy Bombardment. Wide uniform priors were used as a means to provide very conservative upper and lower limits. Three Markov chain Monte Carlo chains were run for 100 million generations sampling every 10,000th generation, and the first 50% of generations were discarded as burn-in. TreeAnnotator v1.7.576 was used to generate maximum clade credibility trees.
As there are no known fossils of archaea to be used as molecular clock calibrations19, calibrating archaeal molecular clocks with plant and algal fossils requires extrapolating evolutionary rates across the entire Tree of Life. Rates of molecular evolution can vary substantially across deeply diverging lineages (e.g.81) and so application of molecular clocks to inter-domain datasets in the absence of robust calibrations can introduce untenable artifacts and uncertainty (e.g.82). Recent molecular clocks spanning the full Tree of Life built with calibrations from only a single domain, for example, can produce dates for the divergence of bacteria and archaea spanning > 4 Ga between different marker sets83 and with credible intervals spanning > 1 Ga for nodes in unconstrained domains84. The methods we utilize here were therefore determined to not be viable for performing molecular clock analyses on ammonia oxidizing archaea and so these organisms were not included in our molecular clock analyses.
Supplementary Information
Acknowledgements
LMW acknowledges support from an Agouron Institute Postdoctoral Fellowship, a Simons Foundation Postdoctoral Fellowship in Marine Microbial Ecology, and an NSF XSEDE Startup Award that provided computational resources via the CIPRES Science Gateway.
Author contributions
All authors conceived the study. L.M.W. collected data and performed phylogenetic analyses. P.M.S. performed molecular clock analyses. L.M.W. and P.M.S. wrote the manuscript with contributions from D.T.J.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material availlable at 10.1038/s41598-021-81718-2.
References
- 1.Tyrrell T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999;400(6744):525. doi: 10.1038/22941. [DOI] [Google Scholar]
- 2.Canfield DE, Glazer AN, Falkowski PG. The evolution and future of Earth’s nitrogen cycle. Science. 2010;330(6001):192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 3.Zerkle AL, Mikhail S. The geobiological nitrogen cycle: from microbes to the mantle. Geobiology. 2017;15(3):343–352. doi: 10.1111/gbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Z, Wessels HJ, van Alen T, Jetten MS, Kartal B. Nitric oxide-dependent anaerobic ammonium oxidation. Nat. Commun. 2019;10:1244. doi: 10.1038/s41467-019-09268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khadka R, Clothier L, Wang L, Lim CK, Klotz MG, Dunfield PF. Evolutionary history of copper membrane monooxygenases. Front. Microbiol. 2018;9:2493. doi: 10.3389/fmicb.2018.02493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.in’t Zandt MH, de Jong AE, Slomp CP, Jetten MS. The hunt for the most-wanted chemolithoautotrophic spookmicrobes. FEMS Microbiol. Ecol. 2018;94(6):fiy064. doi: 10.1093/femsec/fiy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward, L., Shih, P.M., Hemp, J., Kakegawa, T., Fischer, W.W. and McGlynn, S.E. Phototrophic methane oxidation in a member of the chloroflexi phylum. bioRxiv, 531582 (2019).
- 8.Zerkle AL, Poulton SW, Newton RJ, Mettam C, Claire MW, Bekker A, Junium CK. Onset of the aerobic nitrogen cycle during the great oxidation event. Nature. 2017;542(7642):465. doi: 10.1038/nature20826. [DOI] [PubMed] [Google Scholar]
- 9.Garvin J, Buick R, Anbar AD, Arnold GL, Kaufman AJ. Isotopic evidence for an aerobic nitrogen cycle in the latest Archean. Science. 2009;323(5917):1045–1048. doi: 10.1126/science.1165675. [DOI] [PubMed] [Google Scholar]
- 10.Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528(7583):504. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kessel MA, Speth DR, Albertsen M, Nielsen PH, den Camp HJO, Kartal B, et al. Complete nitrification by a single microorganism. Nature. 2015;528(7583):555. doi: 10.1038/nature16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lücker S, Wagner M, Maixner F, Pelletier E, Koch H, Vacherie B, Rattei T, Damsté JSS, Spieck E, Le Paslier D, Daims H. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc. Natl. Acad. Sci. 2010;107(30):13479–13484. doi: 10.1073/pnas.1003860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomo A, Pedersen AG, Fowler SJ, Dechesne A, Sicheritz-Pontén T, Smets BF. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J. 2018;12(7):1779. doi: 10.1038/s41396-018-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein LY. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J. 2007;9(12):2993–3007. doi: 10.1038/s41396-018-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, Butterfield CN, Hernsdorf AW, Amano Y, Ise K, Suzuki Y. A new view of the tree of life. Nat. Microbiol. 2016;1(5):16048. doi: 10.1038/nmicrobiol.2016.48. [DOI] [PubMed] [Google Scholar]
- 16.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, Hugenholtz P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018;36(10):996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 17.Lemoine F, Domelevo Entfellner JB, Wilkinson E, Correia D, Davila Felipe M, De Oliveira T, Gascuel O. Renewing Felsenstein's phylogenetic bootstrap in the era of big data. Nature. 2018;556(7702):452–456. doi: 10.1038/s41586-018-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dick GJ, Grim SL, Klatt JM. Controls on O2 production in cyanobacterial mats and implications for earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 2018 doi: 10.1146/annurev-earth-082517-010035. [DOI] [Google Scholar]
- 19.Ward LM, Shih PM. The evolution and productivity of carbon fixation pathways in response to changes in oxygen concentration over geological time. Free Radic. Biol. Med. 2019;140:188–199. doi: 10.1016/j.freeradbiomed.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Brocks JJ, Jarrett AJM, Sirantoine E, Hallmann C, Hoshino Y, Liyanage T. The rise of algae in Cryogenian oceans and the emergence of animals. Nature. 2017;548(7669):578–581. doi: 10.1038/nature23457. [DOI] [PubMed] [Google Scholar]
- 21.Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 22.Laakso TA, Sperling EA, Johnston DT, Knoll AH. Ediacaran reorganization of the marine phosphorus cycle. Proc. Natl. Acad. Sci. 2020;117(22):11961–11967. doi: 10.1073/pnas.1916738117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward LM, Rasmussen B, Fischer WW. Primary productivity was limited by electron donors prior to the advent of oxygenic photosynthesis. J. Geophys. Res. Biogeosci. 2019;124(2):211–226. doi: 10.1029/2018JG004679. [DOI] [Google Scholar]
- 24.Sperling EA, Wolock CJ, Morgan AS, Gill BC, Kunzmann M, Halverson GP, Macdonald FA, Knoll AH, Johnston DT. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature. 2015;523(7561):451. doi: 10.1038/nature14589. [DOI] [PubMed] [Google Scholar]
- 25.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014 doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 26.Stolper DA, Keller CB. A record of deep-ocean dissolved O2 from the oxidation state of iron in submarine basalts. Nature. 2018;553(7688):323. doi: 10.1038/nature25009. [DOI] [PubMed] [Google Scholar]
- 27.Hink L, Gubry-Rangin C, Nicol GW, Prosser JI. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018;12(4):1084. doi: 10.1038/s41396-017-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, Daebeler A, Romano S, Albertsen M, Stein LY, Daims H. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature. 2017;549(7671):269–272. doi: 10.1038/nature23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens-Habbena W, Berube PM, Urakawa H, José R, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461(7266):976. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 30.Schleper C. Ammonia oxidation: different niches for bacteria and archaea? ISME J. 2010;4(9):1092–1094. doi: 10.1038/ismej.2010.111. [DOI] [PubMed] [Google Scholar]
- 31.Ke X, Lu W, Conrad R. High oxygen concentration increases the abundance and activity of bacterial rather than archaeal nitrifiers in rice field soil. Microb. Ecol. 2015;70(4):961–970. doi: 10.1007/s00248-015-0633-4. [DOI] [PubMed] [Google Scholar]
- 32.Qin W, Meinhardt KA, Moffett JW, Devol AH, Virginia Armbrust E, Ingalls AE, Stahl DA. Influence of oxygen availability on the activities of ammonia-oxidizing archaea. Environ. Microbiol. Rep. 2017;9(3):250–256. doi: 10.1111/1758-2229.12525. [DOI] [PubMed] [Google Scholar]
- 33.Amin SA, Moffett JW, Martens-Habbena W, Jacquot JE, Han Y, Devol A, Ingalls AE, Stahl DA, Armbrust EV. Copper requirements of the ammonia-oxidizing archaeon Nitrosopumilus maritimus SCM1 and implications for nitrification in the marine environment. Limnol. Oceanogr. 2013;58(6):2037–2045. doi: 10.4319/lo.2013.58.6.2037. [DOI] [Google Scholar]
- 34.Saito MA, Sigman DM, Morel FM. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorg. Chim. Acta. 2003;356:308–318. doi: 10.1016/S0020-1693(03)00442-0. [DOI] [Google Scholar]
- 35.Hao J, Sverjensky DA, Hazen RM. A model for late Archean chemical weathering and world average river water. Earth Planet. Sci. Lett. 2017;457:191–203. doi: 10.1016/j.epsl.2016.10.021. [DOI] [Google Scholar]
- 36.Walker CB, De La Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ, Brochier-Armanet C, Chain PSG, Chan PP, Gollabgir A, Hemp J. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kipp MA, Stüeken EE, Yun M, Bekker A, Buick R. Pervasive aerobic nitrogen cycling in the surface ocean across the Paleoproterozoic Era. Earth Planet. Sci. Lett. 2018;500:117–126. doi: 10.1016/j.epsl.2018.08.007. [DOI] [Google Scholar]
- 38.Koehler MC, Stüeken EE, Kipp MA, Buick R, Knoll AH. Spatial and temporal trends in Precambrian nitrogen cycling: a Mesoproterozoic offshore nitrate minimum. Geochim. Cosmochim. Acta. 2017;198:315–337. doi: 10.1016/j.gca.2016.10.050. [DOI] [Google Scholar]
- 39.Stüeken EE, Kipp MA, Koehler MC, Buick R. The evolution of Earth's biogeochemical nitrogen cycle. Earth Sci. Rev. 2016;160:220–239. doi: 10.1016/j.earscirev.2016.07.007. [DOI] [Google Scholar]
- 40.Ren M, Feng X, Huang Y, Wang H, Hu Z, Clingenpeel S, Swan BK, Fonseca MM, Posada D, Stepanauskas R, Hollibaugh JT. Phylogenomics suggests oxygen availability as a driving force in Thaumarchaeota evolution. ISME J. 2019;13:1. doi: 10.1038/s41396-019-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bristow LA, Dalsgaard T, Tiano L, Mills DB, Bertagnolli AD, Wright JJ, Hallam SJ, Ulloa O, Canfield DE, Revsbech NP, Thamdrup B. Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proc. Natl. Acad. Sci. 2016;113(38):10601–10606. doi: 10.1073/pnas.1600359113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santoro AE, Casciotti KL. Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation. ISME J. 2011;5(11):1796. doi: 10.1038/ismej.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Könneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T, von Borzyskowski LS, Erb TJ, Stahl DA, Berg IA. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2fixation. Proc. Natl. Acad. Sci. 2014;111(22):8239–8244. doi: 10.1073/pnas.1402028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raven JA. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 2009;56(2–3):177–192. doi: 10.3354/ame01315. [DOI] [Google Scholar]
- 45.Crockford PW, Hayles JA, Bao H, Planavsky NJ, Bekker A, Fralick PW, Halverson GP, Bui TH, Peng Y, Wing BA. Triple oxygen isotope evidence for limited mid-Proterozoic primary productivity. Nature. 2018;559(7715):613. doi: 10.1038/s41586-018-0349-y. [DOI] [PubMed] [Google Scholar]
- 46.Hodgskiss MS, Crockford PW, Peng Y, Wing BA, Horner TJ. A productivity collapse to end Earth’s Great oxidation. Proc. Natl. Acad. Sci. 2019;116(35):17207–17212. doi: 10.1073/pnas.1900325116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, Schleper C. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 2014;8(5):1135. doi: 10.1038/ismej.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buick R. Did the Proterozoic ‘Canfield Ocean’ cause a laughing gas greenhouse? Geobiology. 2007;5(2):97–100. doi: 10.1111/j.1472-4669.2007.00110.x. [DOI] [Google Scholar]
- 49.Navarro-González R, Molina MJ, Molina LT. Nitrogen fixation by volcanic lightning in the early Earth. Geophys. Res. Lett. 1998;25(16):3123–3126. doi: 10.1029/98GL02423. [DOI] [Google Scholar]
- 50.Wong ML, Charnay BD, Gao P, Yung YL, Russell MJ. Nitrogen oxides in early Earth's atmosphere as electron acceptors for life's emergence. Astrobiology. 2017;17(10):975–983. doi: 10.1089/ast.2016.1473. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Junium CK, Grassineau NV, Nisbet EG, Izon G, Mettam C, Martin A, Zerkle AL. Ammonium availability in the Late Archaean nitrogen cycle. Nat. Geosci. 2019;12(7):553–557. doi: 10.1038/s41561-019-0371-1. [DOI] [Google Scholar]
- 52.Fennel K, Follows M, Falkowski PG. The co-evolution of the nitrogen, carbon and oxygen cycles in the Proterozoic ocean. Am. J. Sci. 2005;305(6–8):526–545. doi: 10.2475/ajs.305.6-8.526. [DOI] [Google Scholar]
- 53.Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442(7104):806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 54.Straka LL, Meinhardt KA, Bollmann A, Stahl DA, Winkler MK. Affinity informs environmental cooperation between ammonia-oxidizing archaea (AOA) and anaerobic ammonia-oxidizing (Anammox) bacteria. ISME J. 2019;13(8):1997–2004. doi: 10.1038/s41396-019-0408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wuchter C, Abbas B, Coolen MJ, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. 2006;103(33):12317–12322. doi: 10.1073/pnas.0600756103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stolper DA, Bucholz CE. Neoproterozoic to early Phanerozoic rise in island arc redox state due to deep ocean oxygenation and increased marine sulfate levels. Proc. Natl. Acad. Sci. 2019;116(18):8746–8755. doi: 10.1073/pnas.1821847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibarra DE, Rugenstein JKC, Bachan A, Baresch A, Lau KV, Thomas DL, Lee JE, Boyce CK, Chamberlain CP. Modeling the consequences of land plant evolution on silicate weathering. Am. J. Sci. 2019;319(1):1–43. doi: 10.2475/01.2019.01. [DOI] [Google Scholar]
- 58.Limpiyakorn T, Fürhacker M, Haberl R, Chodanon T, Srithep P, Sonthiphand P. amoA-encoding archaea in wastewater treatment plants: a review. Appl. Microbiol. Biotechnol. 2013;97(4):1425–1439. doi: 10.1007/s00253-012-4650-7. [DOI] [PubMed] [Google Scholar]
- 59.Verhamme DT, Prosser JI, Nicol GW. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011;5(6):1067–1071. doi: 10.1038/ismej.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson SA, Heimhofer U, Hesselbo SP, Petrizzo MR. Mesozoic climates and oceans—a tribute to Hugh Jenkyns and Helmut Weissert. Sedimentology. 2017;64(1):1–15. doi: 10.1111/sed.12349. [DOI] [Google Scholar]
- 61.Shih PM, Ward LM, Fischer WW. Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proc. Natl. Acad. Sci. 2017;114(40):10749–10754. doi: 10.1073/pnas.1710798114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gold DA, Grabenstatter J, de Mendoza A, Riesgo A, Ruiz-Trillo I, Summons RE. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc. Natl. Acad. Sci. 2016;113(10):2684–2689. doi: 10.1073/pnas.1512614113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward LM, Shih PM. Granick revisited: synthesizing evolutionary and ecological evidence for the late origin of bacteriochlorophyll via ghost lineages and horizontal gene transfer. bioRxiv. 2020 doi: 10.1101/2020.09.01.277905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward LM, Shih PM, Fischer WW. MetaPOAP: presence or absence of metabolic pathways in metagenome-assembled genomes. Bioinformatics. 2018;34(24):4284–4289. doi: 10.1093/bioinformatics/bty510. [DOI] [PubMed] [Google Scholar]
- 69.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller, M. A., W. Pfeiffer and T. Schwartz (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE).
- 71.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucl. Acids Res. 2016;44(W1):W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doolittle RF. Of urfs and orfs: A Primer on How to Analyze Derived Amino Acid Sequences. Mill Valley: University Science Books; 1986. [Google Scholar]
- 73.Ward LM, Hemp J, Shih PM, McGlynn SE, Fischer WW. Evolution of phototrophy in the Chloroflexi phylum driven by horizontal gene transfer. Front. Microbiol. 2018;9:260. doi: 10.3389/fmicb.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 75.Shih PM, Hemp J, Ward LM, Matzke NJ, Fischer WW. Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology. 2017;15(1):19–29. doi: 10.1111/gbi.12200. [DOI] [PubMed] [Google Scholar]
- 76.Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15(4):e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shih PM, Matzke NJ. Primary endosymbiosis events date to the later Proterozoic with cross-calibrated phylogenetic dating of duplicated ATPase proteins. Proc. Natl. Acad. Sci. 2013;110(30):12355–12360. doi: 10.1073/pnas.1305813110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith SA, Beaulieu JM, Donoghue MJ. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl. Acad. Sci. 2010;107(13):5897–5902. doi: 10.1073/pnas.1001225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibson TM, Shih PM, Cumming VM, Fischer WW, Crockford PW, Hodgskiss MS, Wörndle S, Creaser RA, Rainbird RH, Skulski TM, Halverson GP. Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology. 2017;46(2):135–138. doi: 10.1130/G39829.1. [DOI] [Google Scholar]
- 80.Butterfield NJ. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology. 2000;26(3):386–404. doi: 10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. [DOI] [Google Scholar]
- 81.Kuo CH, Ochman H. Inferring clocks when lacking rocks: the variable rates of molecular evolution in bacteria. Biol. Direct. 2009;4(1):35. doi: 10.1186/1745-6150-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roger AJ, Hug LA. The origin and diversification of eukaryotes: problems with molecular phylogenetics and molecular clock estimation. Philos. Trans. R. Soc. B Biol. Sci. 2006;361(1470):1039–1054. doi: 10.1098/rstb.2006.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Q, Mai U, Pfeiffer W, Janssen S, Asnicar F, Sanders JG, Belda-Ferre P, Al-Ghalith GA, Kopylova E, McDonald D, Kosciolek T. Phylogenomics of 10,575 genomes reveals evolutionary proximity between domains Bacteria and Archaea. Nat. Commun. 2019;10(1):1–14. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Betts HC, Puttick MN, Clark JW, Williams TA, Donoghue PC, Pisani D. Integrated genomic and fossil evidence illuminates life’s early evolution and eukaryote origin. Nat. Ecol. Evol. 2018;2(10):1556. doi: 10.1038/s41559-018-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.