Abstract
Viruses encode for structural proteins that participate in virion formation and include capsid and envelope proteins. In addition, viruses encode for an array of non-structural accessory proteins important for replication, spread, and immune evasion in the host and are often linked to virus pathogenesis. Most virus accessory proteins are non-essential for growth in cell culture because of the simplicity of the infection barriers or because they have roles only during a state of the infection that does not exist in cell cultures (i.e., tissue-specific functions), or finally because host factors in cell culture can complement their absence. For these reasons, the study of most nonessential viral factors is more complex and requires development of suitable cell culture systems and in vivo models. Approximately half of the proteins encoded by the herpes simplex virus 1 (HSV-1) genome have been classified as non-essential. These proteins have essential roles in vivo in counteracting antiviral responses, facilitating the spread of the virus from the sites of initial infection to the peripheral nervous system, where it establishes lifelong reservoirs, virus pathogenesis, and other regulatory roles during infection. Understanding the functions of the non-essential proteins of herpesviruses is important to understand mechanisms of viral pathogenesis but also to harness properties of these viruses for therapeutic purposes. Here, we have provided a comprehensive summary of the functions of HSV-1 non-essential proteins.
Keywords: HSV-1 non-essential proteins, HSV-1 egress, HSV-1 envelopment, innate immunity, HSV-1 based therapies, gene silencing
1. Introduction
The large family of DNA viruses, Herpesviridae, have co-evolved with mammals for millions of years [1,2]. The family Herpesviridae is further divided into three subfamilies, Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae. Herpes simplex virus type 1 (HSV-1), a member of Alphaherpesvirinae, is one of the most well-studied representatives of this family of viruses and will be the focus of this review.
HSV-1 is an enveloped dsDNA virus, which has a genome size of about 152 kb and virion size of about 150–300 nm in diameter [3]. The virion contains the envelope decorated with viral glycoproteins and a proteinaceous layer known as the tegument, which surrounds the capsid of the virus containing the genome. HSV-1 is an important human pathogen, with approximately 80% of the human population infected [3]. Symptoms of HSV-1 infection vary, from lesions in the oral-facial region (“cold sores”), to herpes keratitis, the leading cause of infectious blindness, to herpes encephalitis, which can be fatal. When HSV-1 encounters a host, it will first infect the mucosal epithelial cells in the oral-facial region (although HSV-1 also causes genital infections). It is in these cells that the virus undergoes lytic replication. The virus will then infect innervating sensory neurons, travel anterograde to the trigeminal ganglia (TG), and establish latency, where it will remain for the life of the infected individual. When HSV-1 undergoes latency there are very few genes expressed, including an 8.3-kb region known as the latency-associated transcript (LAT), which is a long non-coding regulatory RNA spliced to about 1.5- and 2-kb introns that have regulatory roles on viral genes expression, and a 6.3-kb exon encoding multiple microRNAs, which target many of the IE genes and other lytic genes, thus suppressing viral replication [4,5,6,7,8,9,10]. It seems that LAT may be important for reactivation of HSV-1 from latency and for blocking apoptosis [11,12,13,14,15,16,17,18]. Periodically, HSV-1 will reactivate from latency due to stress, immunosuppression, or other stimuli, and newly produced virions will travel retrograde to the initial site of infection. There is currently no cure for HSV-1 and no vaccine.
There are three classes of viral genes for HSV-1 and they are expressed in a cascade fashion [19,20]. The virus first encodes the immediate-early (IE) or alpha (α) genes (ICP0, ICP4, ICP27, ICP22, or ICP47) whose products are important for expression of the next class of viral genes, the early (E) or beta (β) genes. The early genes encode proteins largely involved in viral DNA replication and, along with the immediate-early genes, facilitate the expression of the late class of viral genes. The late (L) or gamma (γ) class of viral genes express proteins involved in virion assembly and egress. HSV-1 genes are also divided based on stretches of unique sequences in the genome. Therefore, there are a class of unique long (UL) and unique short (US) genes depending on which region of the genome the gene is expressed from. These unique regions are flanked by inverted repeats. Thus, the HSV-1 genome is structured as follows: TRL-UL-IRL-IRS-US-TRS. There are about 58 UL genes and about 13 US genes that have been characterized for functionality, though there are more viral genes that have not been well characterized or described (Dolan 1998).
Interestingly, although HSV-1 is known to encode 80 genes, it has also been found that about half of these genes are non-essential for viral replication in cell culture [21,22]. Essential genes of HSV-1 are involved in viral DNA replication, the transcription of certain viral genes, genes encoding capsid proteins, genes encoding viral DNA packaging proteins, and some envelope glycoproteins. HSV-1 genes determined to be non-essential are involved in nucleic acid metabolism, combating various host responses to infection, facilitating optimal viral replication, facilitating primary envelopment, virus pathogenesis, or other functions that are not yet characterized (Table 1). While deletion of the non-essential genes in cell culture does not inhibit viral replication, these genes are generally essential for replication in the natural human host as mutant viruses deleted of non-essential genes have rarely been isolated from a patient. One example are mutants in the viral glycoprotein gC that have been recovered from patients with recurrent herpes keratitis [23,24].
Table 1.
Non-essential genes of HSV-1, corresponding proteins, their location on the HSV-1 virion, and their function. Pink: tegument proteins, blue: accessory proteins, yellow: envelope proteins, green: capsid proteins.
| Gene | Protein | Location on Virion | Function |
|---|---|---|---|
| RL1 or γ134.5 | ICP34.5 | tegument | Prevents host translational shutoff and autophagy |
| RL2 or α0 | ICP0 | tegument | Promiscuous transactivator of genes, disrupts repressor complexes, E3 ubiquitin ligase, inhibits innate immunity, modulates endocytosis, etc. |
| UL2 | uracil-DNA glycosylase | accessory | nucleic acid metabolism |
| UL3 | accessory | ||
| UL4 | accessory | ||
| UL7 | tegument | Virion assembly and egress | |
| UL10 | gM | envelope | Host and viral protein trafficking |
| UL11 | tegument | Cytoplasmic envelopment | |
| UL12 | accessory | Nucleic acid metabolism | |
| UL12.5 | accessory | Involved in depleting mtDNA | |
| UL13 | Ser/thr protein kinase | tegument | Blocking innate immune responses, supporting viral protein synthesis |
| UL16 | tegument | Cytoplasmic envelopment | |
| UL20 | envelope | Glycoprotein trafficking | |
| UL21 | tegument | Promotes capsid egress to the cytoplasm | |
| UL23 | thymidine kinase (TK) | tegument | Broad spectrum nucleoside kinase |
| UL24 | accessory | Glycoprotein trafficking, nucleolus dispersal | |
| UL31 | accessory | Component of the nuclear egress complex (NEC), promotes primary nuclear envelopment | |
| UL34 | accessory | Component of the nuclear egress complex (NEC), promotes primary nuclear envelopment | |
| UL35 | VP26 | capsid | Affects DNA packaging, mediates capsid assembly, trafficking post viral entry |
| UL39 | RR1 (ribonucleotide reductase) | accessory | Part of the ribonucleotide reductase (RR) complex, converts ribonucleotide diphosphates to corresponding deoxyribonucleotides, allowing for virus replication particularly in non-dividing cells |
| UL40 | RR2 (ribonucleotide reductase) | accessory | Part of the ribonucleotide reductase (RR) complex, converts ribonucleotide diphosphates to corresponding deoxyribonucleotides, allowing for virus replication particularly in non-dividing cells |
| UL41 | VHS | tegument | Viral RNase, degrades host transcripts and blocks antiviral responses |
| L43 | tegument | ||
| UL44 | gC | envelope | Mediates viral binding to heparan sulfate, regulates entry by a low-pH pathway |
| UL45 | envelope | Required for syncytia formation during HSV-1 gB syn infection | |
| UL46 | VP11/12 | tegument | Regulation of transcription, activates pathways for cell survival, blocks pathways for innate immunity activation |
| UL47 | VP13/14 | tegument | Regulation of transcription, modulating post-transcriptional processing of mRNAs |
| UL49 | VP22 | tegument | Facilitates viral gene expression, protein expression, and DNA replication; inhibits inflammasome |
| UL49.5 | gN | envelope | Binding partner of gM |
| UL50 | tegument | Nucleic acid metabolism | |
| UL51 | tegument | Participates in cytoplasmic envelopment; facilitates virus spread from cell-to-cell; recruits UL7 to tegument | |
| UL53 | gK | envelope | Participates in virion egress from host cell; regulates virus entry and fusogenic activity of the virion; complexes with UL20 |
| UL55 | tegument | Participates in cytoplasmic envelopment | |
| UL56 | tegument | Participates in cytoplasmic envelopment | |
| US1 | ICP22 | accessory | Regulates viral late gene expression; facilitates formation of complexes important for protein folding; participates in primary envelopment; blocks immune responses |
| US1.5 | accessory | Participates in viral gene transcription | |
| US2 | tegument | Protein trafficking | |
| US3 | Ser/thr protein kinase | tegument | Blocks apoptosis, enhances viral gene expression, facilitates capsid nuclear egress, phosphorylates numerous substrates |
| US3.5 | Ser/thr protein kinase | tegument | Phosphorylates substrates but cannot block apoptosis and does not facilitate nuclear egress |
| US4 | gG | envelope | Regulation of chemokines |
| US5 | gJ | envelope | Inhibits apoptosis and cell stress pathways |
| US7 | gI | envelope | Enhances virus spread from cell-to-cell; facilitates anterograde transport of virions after reactivation from latency; important for neurovirulence |
| US8 | gE | envelope | Enhances virus spread from cell-to-cell; facilitates anterograde transport of virions after reactivation from latency; important for neurovirulence |
| US8.5 | accessory | Localizes in the nucleoli | |
| US9 | tegument | Enhances virus spread from cell-to-cell; facilitates anterograde transport of virions after reactivation from latency; important for neurovirulence | |
| US10 | tegument | Important for neurovirulence | |
| US11 | tegument | Block PKR activation and shutoff of host translation; block IFN induction; regulation of virus genes expression; trafficking of unenveloped capsids | |
| US12 | ICP47 | accessory | Prevents MHC I antigen presentation, supports neurovirulence |
It is of great interest to understand the roles of non-essential genes to better understand virus–host interactions. Moreover, the non-essential genes have properties that make them attractive for the development of therapeutics. There are varying degrees of deficiency of viruses mutated for non-essential genes when grown in cell culture, and for some of these genes, the defect is cell type specific [25,26]. There is still much to learn about the non-essential genes of HSV-1. Here, we present a comprehensive analysis of the current understanding of the roles of non-essential genes of HSV-1. We explore the functions ascribed to these genes and their corresponding proteins, the potential treatment and therapeutic avenues that can be explored based on the functions and characterization of select HSV-1 non-essential genes, and the complex and intricate roles of non-essential genes in HSV-1 infection.
2. Repressors of Gene Silencing, Viral Transactivators, and Host Evasion Factors
2.1. RL2 or α0 (ICP0)
The infected cell protein 0 (ICP0) of HSV-1 was first reported as a nuclear phosphoprotein with an essential role in cell cultures only at low multiplicity of infection (MOI). ICP0 was deemed to be non-essential at high multiplicities of infection in cell cultures, but viral gene expression was reduced [19,27,28,29,30,31,32,33]. In certain cell lines, particularly cancer cell lines, such as the human osteosarcoma (U2OS), an ICP0-null virus replicates as efficiently as wild-type virus, which may be due to impaired recruitment of antiviral factors to the sites of viral gene transcription and DNA replication and/or due to lack of certain restriction factors [26,28,30,32,34,35]. Genes coding for ICP0 are present in the genomes of simplex and varicelloviruses, but they are absent from the mardivirus genus. These proteins show strong sequence homology to ICP0 within the RING (Really Interesting New Gene) finger domain. Orthologs of ICP0 are also present in lymphocryptoviruses (e.g., EBV) and the cytomegalovirus (CMV) [36,37,38]. The functions of ICP0 are broad, from activation of transcription and chromatin remodeling, to evasion of antiviral responses, cell cycle effects, interfering with DNA damage responses, and endocytosis.
In early studies, ICP0 was found with ICP4 to stimulate ICP8 expression in transfection assays [39,40]. Furthermore, it was shown to function as a potent transactivator of different genes introduced into cells by transfection or infection, including the viral thymidine kinase (TK) gene and ICP6 gene, the human immunodeficiency virus (HIV) LTR, and several human papillomavirus (HPV) genes [41,42,43,44,45,46,47,48,49,50]. In fact, ICP0 was found to stimulate the expression of all three classes of HSV genes [31,51]. Therefore, ICP0 was proposed to be a promiscuous transactivator of gene expression.
ICP0 also functions as an E3 ubiquitin ligase and most substrates ubiquitinated by ICP0 appear to be targeted for degradation (Figure 1A). This activity of ICP0 was mapped to residues 116–156, where there is a Zn2+-binding RING finger domain [52,53,54,55]. To exert its E3 ubiquitin ligase function, ICP0 forms a complex with different ubiquitin conjugation enzymes, including UbcH5a and UbcH6 [52,56,57,58,59,60]. Major targets of ICP0 are components of the nuclear domain 10 (ND10) bodies. As a DNA virus, the genome of HSV-1 transcribes and replicates in the nucleus. The host attempts to block viral gene expression and replication by entrapping the viral DNA in promyelocytic leukemia (PML)-nuclear bodies (NBs) and depositing histones and other repressor complexes on it. The main protein that orchestrates the formation of ND10 bodies is the PML. Other components of ND10s include the Sp100, Daxx, Mre 11, ATM, ATRX, p53, and others. ICP0 disrupts the ND10s by causing degradation of the different isoforms of PML, Sp100, and potentially of other proteins (Figure 1A) [34,59,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] Notably, several components of the ND10 bodies are interferon inducible genes, which underscores the synergy between gene silencing mechanisms and innate immunity in suppressing HSV-1 gene expression. ICP0-null viruses or E3 ubiquitin ligase mutants have viral DNA entrapped in PML-NBs at low MOI and display reduced transactivation activity and ability to block antiviral responses [34,64,78,79,80,81,82,83]. ICP0 E3 ligase-deficient viruses are hypersensitive to interferon, replicate poorly, and fail to reactivate efficiently from neuronal latency [25,55,67,68,69,70,71]. Based, on these observations, Dr. Kalamvoki’s group recently developed a high-throughput assay to screen for ICP0-E3 ubiquitin ligases inhibitors [72]. This assay is proximity based and takes advantage of the fact that ICP0 is autoubiquitinated and degraded during infection and that this ICP0 autoubiquitination can occur in vitro using the purified protein encoded by the exon II of ICP0 (contains the RF domain), UbcH5a, and Ub [60,73,74]. Screening a small compound library, Dr. Kalamvoki’s group identified potential scaffolds that can interfere with the ICP0 E3 ubiquitin ligase activity [72].
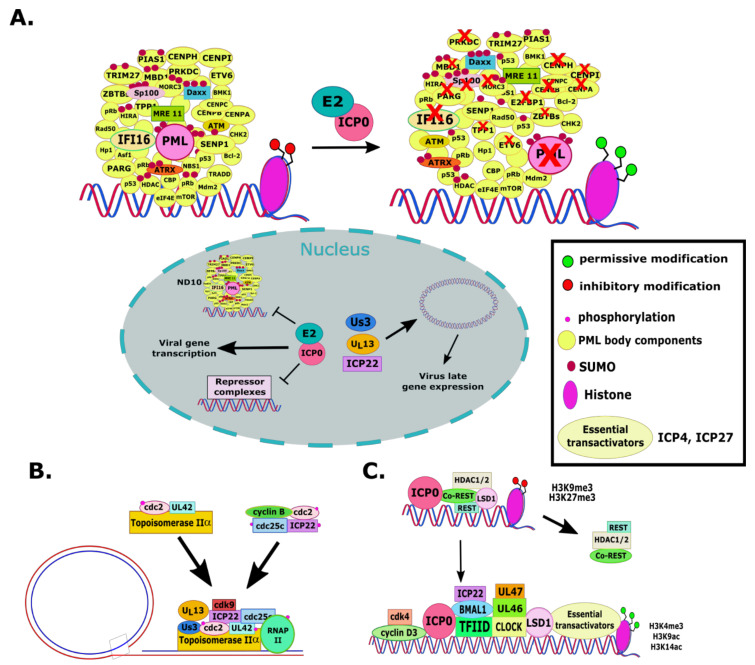
Figure 1.
Nuclear functions of non-essential HSV-1 proteins. HSV-1 encodes multiple proteins able to counteract antiviral host responses within the nucleus. (A): ICP0 functions as an E3 ligase ubiquitin ligase that degrades ND10 components that encapsulate the viral genome in the nucleus, including PML, Daxx, SP100, centromeric proteins, and others. The degradation of IFI16 involves multiple factors. These events facilitate initiation of viral gene transcription. (B): The viral protein ICP0 is also known to disrupt repressor complexes that silence the viral genome, as well as recruit factors to enable viral gene transcription. Altogether, ICP0 facilitates permissive histone modifications, while suppressing silencing modifications, to enable for viral gene expression. (C): The viral kinases US3 and UL13, with ICP22, are known to facilitate viral late gene expression, which occurs through the recruitment of host factors, such as Topoisomerase IIα and RNA polymerase II, to the sites of DNA replication in the nucleus. Together, these non-essential viral proteins are important for optimal expression of other viral genes and for viral DNA replication.
ICP0 has seven SUMO-interacting motif (SIM)-like sequences (SLSs), and multiple ND10 components, including PML and SP100, are SUMOylated; therefore, ICP0 could bind to them (Figure 1A) [34,56,75,76,77,78,79,80,81]. It has been found that inhibition of cellular ubiquitination led to an increase of SUMOylated proteins that ended up accumulating at PML-NBs [82]. ICP0 utilizes both SUMO-dependent and SUMO-independent mechanisms to degrade Sp100 and multiple PML isoforms in an effort to prevent restriction of the virus by the host [34,56,75,76,78,79,83,84]. Other proteins could also be the target of SUMO-dependent degradation by ICP0 [75,77,81]. Specifically, SUMO-dependent degradation of MORC3 by ICP0, which associates with Sp100, has been observed and this occurs in a RING-finger-dependent manner and appears to diminish the association of PML-NBs with viral DNA [85]. Additionally, there has been a function ascribed to ICP0 SUMO–SIM interactions at the ND10s to modulate the DNA damage response (DDR) during infection [86,87]. For example, the DNA repair function of the DNA-dependent protein kinase (DNA-PK) is inhibited by ICP0 through degradation of its catalytic subunit and this facilitates virus replication [88,89,90]. Additionally, ICP0 mediates the degradation of two E3 ubiquitin ligases RFN8 and RFN168 that act as mediators of the ATM pathway and trigger recruitment of downstream effectors to sites of double-strand DNA breaks [91,92,93,94,95]. More work will need to be done to characterize the degradation of SUMOylated proteins by ICP0 that are not related to the ND10s. The ability for ICP0 to interrupt SUMO interactions and to degrade SUMOylated proteins during infection is likely a strategy to modify the cellular proteome to both prevent antiviral responses and promote the infection [34,76,83].
In tandem with the dispersion of ND10 bodies, ICP0 activates the viral chromatin (Figure 1B). Immediately after its release in the nucleus, HSV-1 DNA associates with repressive histones and other repressor complexes [96,97,98]. However, markers of active gene expression label the viral chromatin during lytic infection, such as tri-methylation of histone H3 at lysine 4 (H3K4) and acetylation of H3 at lysine 9 and lysine 14 [97,98,99], while suppressive epigenetic modifications of histone H3 (H3K9me3 and H3K27me3) are removed in an ICP0-dependent manner (Figure 1B) [65,100,101]. ICP0 was also found to associate with class II HDACs in vitro and control their repressor activity [102,103]. In addition, ICP0 seems to promote histone acetylation, as demonstrated using inhibitors of histone deacetylases [103,104,105]. This is also supported by the fact that ICP0 recruits to the viral genome the histone acetyltransferase CLOCK through interaction with the circadian regulator protein BMAL1. This leads to recruitment of additional viral transactivators ICP4, ICP22, ICP27, and part of the host transcription complex TFIID [106,107,108]. Tandemly, ICP0 disrupts repressor complexes, such as the REST/CoREST/HDAC complex and LSD1 [109,110,111,112]. ICP0 disperses the REST/CoREST/HDAC1/2/LSD1 through interaction with CoREST in an effort to promote HSV-1 gene expression and DNA replication (Figure 1B) [78,112,113,114,115]. It was also found that the interferon-inducible gene 16 (IFI16), Daxx, and ATRX proteins serve to restrict the virus, likely through sensing of viral DNA and obstructing replication and causing deposition of silencing histone H3 [34,65,100,116,117,118,119,120]. ICP0 induces the degradation of ATRX and IFI16 [121,122,123,124,125]. Degradation of ATRX seems to be secondary to PML degradation, while depletion of ICP0 appears to be both ICP0 dependent and independent.
ICP0 has also been shown to harness cell cycle components to support the infection. Thus, ICP0 was found to recruit cyclin D3 and the kinase cdk4 to ND10s to enable viral gene transcription and DNA replication, which was also supported by the fact that ICP0 nuclear-to-cytoplasmic translocation was enabled by cyclin D3 (Figure 1B) [108,126,127]. ICP0 has been found to arrest cells in the G2/M phase to promote virus replication by activating the checkpoint kinase 2 (Chk2) [128]. Consistent with these roles of ICP0, it was also found to degrade the centromere proteins CENP-A, CENP-B, and CENP-C, inducing the interphase centromere damage response (iCDR) (Figure 1A) [129,130,131,132]. In addition to this disturbance to the cell cycle, it has been found that ICP0 degrades the DNA-interacting protein TPP1, leading to transcription of telomere repeat-containing RNA activation (TERRA) and increased viral replication [133].
As mentioned earlier, there are interwoven relationships between gene silencing and innate immunity and it is not coincidence the ICP0 targets them both. ICP0-null and other ICP0 mutant viruses displayed increased sensitivity to interferon both in vivo and in vitro [32,70,134,135]. As discussed above, ICP0 blocks the nuclear pattern recognition receptors (PRRs) IFI16 and DNA-PKs, which may also impact the cGAS and STING DNA sensing pathway [119,122,123,124,136,137]. Inhibition of STING-dependent immune responses involves ICP0 as ICP0-null virus growth is partially rescued in cells with impaired STING signaling [25,26]. Furthermore, ICP0 was found to reduce the levels of the Toll-like receptor 2 (TLR2) adaptors MyD88 (myeloid differentiation factor 88) and the Mal (MyD88 adaptor-like protein) TIRAP (TIR domain-containing adaptor protein), thus blocking immune responses through this pathway (Figure 2A) [138]. Overall, ICP0 has been proposed to inhibit IRF3 and IRF7-dependent immune responses to sequester these proteins away from host chromatin [139,140,141]. ICP0 was also recently found to have a role in autophagy inhibition through causing the downregulation of p62/SQSTM1 and OPTN autophagy adaptor proteins in a proteasome-dependent and RING finger-independent mechanism (Figure 2C) [142]. It was also demonstrated that the cytoplasmic ICP0 is most likely involved in this function. Another target of ICP0 is the deubiquitylating enzyme USP7 (ubiquitin-specific protease 7) or HAUSP. USP7 appears to bind and stabilize ICP0, but ICP0 degrades USP7 late during infection in a RING finger-dependent manner [58,78,79,143,144]. One reason why the virus could promote degradation of USP7 is because it has a major role in TLR- and TNFa receptor (TNFR)-induced gene expression [145].
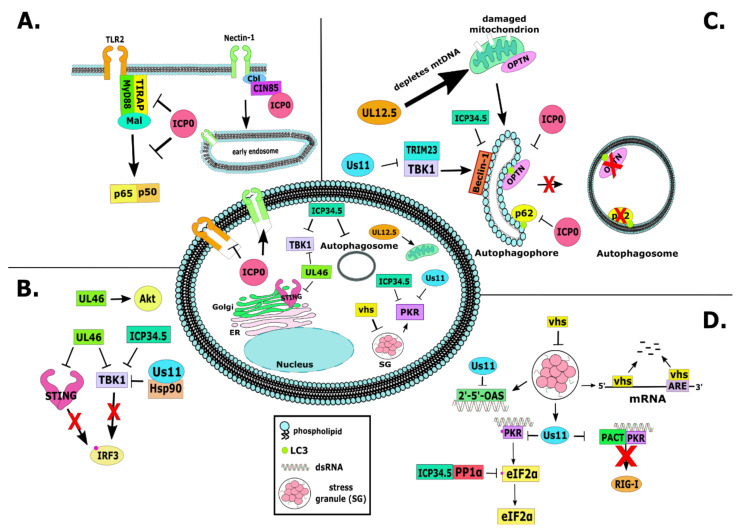
Figure 2.
Cytoplasmic functions of some non-essential HSV-1 proteins. (A): ICP0 participates in two major functions in the cytoplasm. First, ICP0 degrades the TLR2 adaptors TIRAP and Mal, thus blocking NF-κB activity. ICP0 also binds to the endocytosis adaptor CIN85 and along with Cbl promotes internalization of the viral entry receptor Nectin-1. This is a mechanism to promote progeny virus spread to uninfected cells. (B): The tegument protein UL46 blocks STING and TBK1, which prevents stimulation of interferon-regulated genes. ICP34.5 and US11 are also involved in blocking TBK1, emphasizing the importance of blocking TBK1 activity during HSV-1 infection. (C): The autophagy pathway is blocked during HSV-1 infection through binding of ICP34.5 to Beclin-1, thus preventing maturation of the autophagophore to an autophagosome. ICP0 has also been found to cause downregulation of p62 and OPTN proteins during infection, which may also serve as another mechanism of blocking selective autophagy. It has also been found that the protein encoded by UL12.5 causes depletion of mtDNA during infection, which causes damage to mitochondria. (D): HSV-1 prevents host translational shutoff from occurring during infection. One mechanism is through ICP34.5 binding to both PP1a and eIF2α, causing dephosphorylation of eIF2α and preventing shutoff of translation. HSV-1 also encodes vhs, which is a viral RNase that degrades AU-rich element (ARE) containing mRNAs. It has also been shown that vhs prevents the formation of cytoplasmic stress granules (SGs) during infection, which contain dsRNA that would otherwise cause PKR activation. HSV-1 also encodes US11, which blocks PKR, thus blocking host translational shutoff and innate immunity activation, as well as blocking PKR and PACT-induced activation of RIG-I during infection.
Most functions of ICP0 discussed above are performed while in the nucleus. However, ICP0 translocates to the cytoplasm after enabling viral gene expression, where it remains for the reminder of the infection. The cytoplasmic functions of ICP0 remain unexplored. Dr. Roizman’s group first described an interaction of ICP0 with the endocytosis adaptor CIN85 [146]. Dr. Kalamvoki’s group has built upon these findings and reported that ICP0 promotes endocytosis of the viral entry receptor Nectin-1 (Figure 2A) [147]. This is perhaps a mechanism that ensures spread of progeny viruses to uninfected cells. CIN85 forms a complex with the Cbl E3 ligase that is involved in endocytosis of multiple surface components. Thus, ICP0 through CIN85 and Cbl could modulate the surface of infected cells to suppress antiviral responses.
Finally, the role of ICP0 has also been investigated during the latent stage of the virus. ICP0 appears to be important for efficient virus reactivation from latently infected trigeminal ganglia (TG) in mouse ocular infections [67,68,71,148,149,150,151]. ICP0 is also required for VP16-dependent viral reactivation [67,152]. While ICP0 is important for balancing lytic and latent infection, it is still not fully understood what its specific role is in this process.
2.2. UL46 and UL47 (VP11/12 and VP13/14)
The HSV-1 genes UL46 and UL47 were first described to encode proteins that modulate UL48 function [153,154,155]. It was then determined that UL46 encodes the phosphoprotein, VP11/12, and UL47 encodes the phosphoprotein VP13/14, which are also glycosylated, and both are non-essential in cell culture, although they have roles in enhancing the transactivation of viral genes by VP16 [156,157,158,159,160,161]. It was also found that UL47, but not UL46, deletion mutants of HSV-1 demonstrated a significant defect in alpha-TIF (UL48)-mediated expression of the TK gene, suggesting a supportive role in transcription of viral genes [161]. Homologs of UL46 and UL47 have been identified in multiple alphaherpesviruses, including pseudorabies virus, HSV-2, Marek’s disease virus, and VZV [153,157,162,163,164]. Homologs of UL47 have been found in bovine herpesvirus and equine herpesvirus, and UL47 has been found to be abundantly expressed in the viral tegument [153,165,166]. T cell responses specific to UL46 and UL47 have both been found in patients with intraocular HSV-1 infection [167].
VP11/12 has since been found to be tyrosine phosphorylated during HSV-1 infection in multiple lymphocyte cell types but not in epithelia or fibroblasts, and this was found to at least partly be due to the activity of lymphocyte-specific Src family kinase, Lck [168]. It was then found that HSV-1 infection increases the amount of phosphorylation of Lck at Y394 in Jurkat T cells, which occurred in a UL46-dependent manner [169]. Moreover, it was found that the Akt pathway is activated through the PI3 kinase, and UL46 was found to be involved in the activation of this pathway in HSV-1-infected HEL fibroblast cells (Figure 2B) [170,171]. VP11/12 was then found to bind to the Src family kinases Grb2, p85, and Shc, thus leading to Akt activation in a T cell lymphoma cell line, and in support of this, it was found that infection with UL46-deficient viruses in HFFs led to reduced Akt activation as compared to infection with wild-type virus [163,172]. In neuronal cells, it was also found that VP11/12 was necessary but not sufficient during infection to induce the phosphorylation and activation of the small GTPase Dynamin 2 [173]. UL46 has also been found to interact with the small GTPase Rab27a during HSV-1 infection of oligodendrocytes, as well as with gH and gD, but the significance of these interactions has not yet been investigated farther than reduced viral growth and infectivity in Rab27a-depleted cells [174].
It was demonstrated that HSV-1 infection of T cells led to the phosphorylation and degradation of adaptor complex protein Dok-2, which is involved in T cell regulation [175,176]. The degradation of Dok-2 occurred in a UL46-dependent manner, but the significance of this degradation has not yet been fully elucidated beyond a potential immune evasion strategy [177]. It was also recently found that UL46 works with Us3 to activate mTORC1 in fibroblasts, which supports virus growth [178]. Further supporting a role for UL46 in both immune evasion and viral growth was the observation that UL46 interacts with the DNA sensor STING (STimulator of INterferon Genes) at its C-terminus, which prevents the induction of interferon stimulatory genes and blocks type I interferon response to infection in fibroblasts (Figure 2B) [179]. Furthermore, it was found that UL46 binds to TBK1, TANK-binding kinase 1, at its N-terminus and this was found to also play a role in blocking the interferon pathway in fibroblasts (Figure 2B) [179,180]. In support of this, the pseudorabies homolog of UL46 was found to interact with STING [181]. Together, these results show strong evidence for a role of UL46 in blocking innate immune responses to HSV-1 infection.
Less work has been done to characterize UL47 or its gene product, VP13/14. It has been found that VP13/14 is involved in nuclear egress through interactions with the viral proteins UL34, UL31, and US3 [182]. UL47 was observed in the nucleus of infected cells during early times post-infection, which is consistent with the interaction of UL47 with UL48, but was also detected in the cytoplasm at late times post-infection [183,184,185].
UL47 has also been found, with ICP27, to regulate mRNA processing and transport by redistributing polyadenylate binding protein (PABP) to the nucleus during infection of HeLa cells, further supporting a role for UL47 in modulating post-transcriptional processing of mRNAs [186]. UL47 has also been implicated in the regulation of the viral RNase vhs, which is discussed later in this review [187]. More work is needed to characterize fully the role of VP13/14 during HSV-1 infection, but so far it seems that UL47 facilitates viral infection by supporting viral gene transcription, mRNA processing, and transport.
2.3. UL49 (VP22)
VP22 is a 301-aa tegument protein (Figure 3) that is encoded by the UL49 gene [188], and has been associated with multiple functions during infection, which result from its interactions with host and cellular factors [189]. It localizes in multiple areas of the cell depending on the time point of the infection, even though its functional role is not always clear.
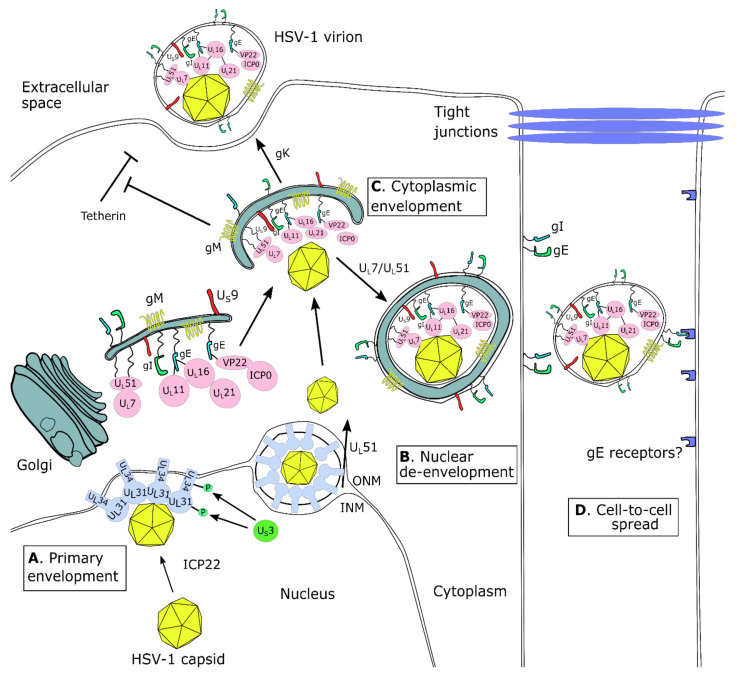
Figure 3.
HSV-1 egress and relevant non-essential proteins. Essential proteins are not depicted. (A) UL31 and UL34 form the Nuclear Egress Complex (NEC), which drives the vesiculation of the inner nuclear membrane (INM) and primary envelopment of HSV-1 capsids. (B) Nuclear de-envelopment is mediated by UL51. Tegument and envelope proteins assemble in a complex network on membranes derived from the trans-Golgi network. (C) HSV-1 capsids undergo cytoplasmic envelopment in a process regulated by multiple non-essential proteins, which involves functional redundancy. After cytoplasmic envelopment, enveloped virions are sorted to the extracellular space, which requires gK. HSV-1 release from the cell membrane can be inhibited by tetherin, which is counteracted by gM. (D) Depending on cell type (e.g., polarized epithelial cells), enveloped virions can disseminate through cell-to-cell spread, in a process that requires UL7/UL51 for sorting of virions towards parts of the membrane that contain gE/gI. Virions may spread to adjacent cells by binding to gE/gI receptors in adjacent cells. However, such receptors are unknown.
Early during infection, VP22 is mostly in the cytoplasm but eventually accumulates in the nucleus. VP22 is phosphorylated after entry into the cell, and this has been suggested to trigger its translocation into the nucleus [190,191]. Inside the nucleus, VP22 may be involved in modulation of nucleosome deposition and repression, which will affect virus life cycle progression [192]. VP22 may also have a function in the nucleolus, since it localizes there during early infection, even though it is not required for chromatin marginalization and HSV-1 replication [193].
VP22 associates with ICP0 in the nucleus, and its overexpression affects the transcription of gC and TK1 in the nucleus, suggesting that VP22 affects transcription though an interaction with ICP0 [194]. VP22 regulates the proper subcellular localization of VP16, VP26, ICP0, ICP4, ICP27, and Hsc-70 in infected cells [195]. These proteins localize to the nucleus early during infection and are then translocated to the cytoplasm later in infection. This translocation relies on specific dileucine motifs on VP22 [195].
VP22 also seems to be required for the expression of the vhs RNase, suggesting that expression of vhs in the absence of VP22 is lethal [196]. VP22-null mutants accumulate spontaneous secondary mutations in the UL41 (vhs) gene, therefore VP22 and vhs may have competitive functions [187]. There is a protein synthesis defect in the absence of VP22, which can result in a compensatory frameshift mutation in vhs. Mechanistically, VP22 and vhs interplay functionally at the level of accumulation and translation of viral mRNAs, indicated by the decrease in mRNA levels and polysome assembly when VP22 is absent. This phenotype can be rescued by the abovementioned complementary mutations in vhs [197]. VP22 is required for optimal protein synthesis at late times during infection, and the accumulation of gE, gD, and vhs mRNAs during early infection [198].
VP22 is not required for the accumulation of other tegument proteins, for virion assembly, or productive HSV-1 replication, but the size of the plaques of VP22 mutant HSV-1 strains (lacking the C-terminus) are smaller than the wild type (WT). Therefore, VP22 is probably required for efficient spread [191]. This effect may stem from the multiple interactions of VP22 as a tegument protein at sites of HSV-1 cytoplasmic envelopment, namely the TGN membranes [199]. Optimal packaging of VP22 in virions requires the amino acids 43–86, which facilitate localization of the protein to the TGN [200], but the exact order of VP22 packaging may be flexible. Overexpression of VP22 after infection with a recombinant HSV-1 that has two VP22 copies, resulted in 2–3-fold higher incorporation of VP22 into nascent virions [201]. In any case, the TGN VP22 mediates proper cytoplasmic envelopment as it interacts with the cytoplasmic tail of gD, therefore bridging the viral capsid and the envelope (Figure 3) [202]. Additionally, VP22 interacts with UL16 and deletion of UL16 results in dramatic reductions of VP22 in released virions [203].
Furthermore, it interacts with gE [204,205]. Deletion of VP22 results in reduced amounts of ICP0, gE, and gD in the extracellular infectious virions, whose number is also reduced [206]. Additionally, VP22 bridges a complex between gE/gI and gM, which is selective in its formation, since it does not include VP16, a close partner of VP22 [207]. This VP22/gE/gI/gM complex also recruits ICP0 in a VP22-dependent fashion. None of those proteins is absolutely required for the formation of a subcomplex; however, optimal complex formation results in efficient virus formation (Figure 3) [208].
One important aspect of VP22 is its involvement in cytoskeleton reorganization. VP22 requires microtubule reorganization for its translocation to the nucleus [209], and it also affects the reorganization and polymerization of the microtubules late during infection, suggesting a late trafficking role [185]. This occurs independently of virus replication or other viral factors [209,210].
VP22 localization is also affected by motor proteins, like the actin-associated motor protein non-muscle myosin IIA (NMIIA). Inhibition of the ATPase activity of NMIIA impaired the perinuclear vesicular pattern of VP22 and the release of virus into the extracellular space, but it did not affect the cell-associated virus. VP22-containing particles line up along NMIIA-containing filaments that run through protrusions, which can emanate from infected cells [211]. The interactions of VP22 with the cytoskeleton probably affect CD1d-mediated activation of natural killer T (NKT) cells. CD1d is an MHC class I-like molecule that mediates self and microbial lipid presentation to NKT cells. HSV-1 can inhibit CD1d-mediated antigen presentation to NKT cells by suppressing CD1d recycling to the cell surface. VP22 is required for this inhibition of CD1d recycling, which probably occurs because of the reorganization of the cytoskeleton that VP22 promotes, which consequently affects CD1d recycling to the plasma surface [212].
An important host factor that VP22 interacts with is the AIM2 inflammasome, promoting the evasion of AIM2-dependent inflammasome activation during infection. The AIM2 inflammasome is normally activated by DNA, which would be available during HSV-1 infection since the HSV-1 nucleocapsid has been reported to be degraded in the cytoplasm [213]. However, HSV-1 infection induces AIM2-independent inflammasome activation, which is inhibited by VP22. VP22 interacts with AIM2 and prevents its oligomerization, which is the first step in AIM2 inflammasome activation. Mice that lack AIM2 can support infection of a VP22-null HSV-1 [214]. Considering that VP22 can move between cells [215], this would be an efficient manner to block inflammatory responses in uninfected cells adjacent to the infection.
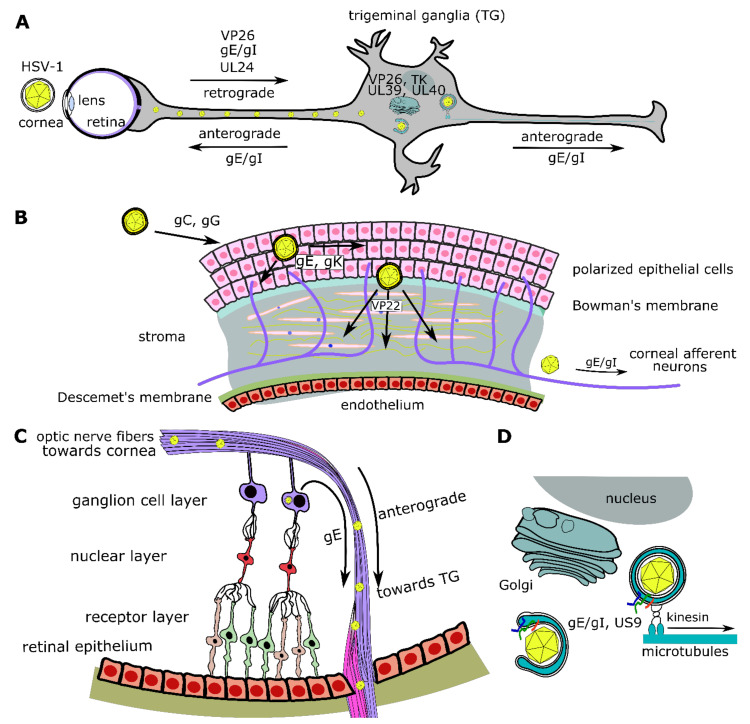
Another property of VP22 is that it can be transported intercellularly using a Golgi-independent mechanism, which involves the actin cytoskeleton since it is sensitive to cytochalasin D [215]. The importance of VP22 in spread is most evident in animal models. VP22 is required for efficient development of corneal lesions in mice following ocular inoculation (Figure 4) and it is also important for neurovirulence, through two possible mechanisms [206]. First, two dileucine motifs of VP22 (at positions 235–236 aa and 251–252 aa) are required for spread of viral antigens in the mouse brain and efficient virulence. These two motifs have been associated with proper cytoplasmic localization of other viral proteins, and VP22 may mediate neurovirulence though that function [195]. Second, a VP22 mutant HSV-1 exhibits impaired viral replication (about 1000-fold) and spread in the brains of infected mice, supporting the importance of VP22 for virus spread in neurons [195]. VP22 may exert its proviral effect in neuronal spread through blocking of AIM2-dependent inflammasome activation, as explained above. Infection of AIM2-/- mice with a VP22-null HSV-1 results in 3 logs higher viral yield than infection of AIM2+/+ mice, suggesting that VP22 promotes neuronal spread by inhibiting an AIM-2-dependent host response against HSV-1 infection [214].
Figure 4.
HSV-1 ocular infection based on data in mouse models. (A) HSV-1 can infect the eye through the cornea, where it can establish a productive infection in the corneal epithelium. The virus can then spread through the innervating sensory neurons to the trigeminal ganglia (TG), moving retrograde along axons towards the neuronal bodies. VP26 mediates migration to the TG. gE/gI and UL24 are important for trafficking of the virion along the axons towards the neuronal bodies. UL39/UL40 and TK are required for replication in cells that do not actively divide, such as neurons. gE/gI affect both retrograde and anterograde movement of the virion. (B) HSV-1 infection of the cornea: The virus can infect the upper layer of polarized epithelial cells, which requires gC and gG. Dissemination of the ocular infection requires the function of gE and gK, and further spread of the virus towards the underlying stromal layers of the cornea requires the function of VP22. HSV-1 can infect corneal afferent neurons and then spread towards the TG utilizing gE/gI. (C) HSV-1 can establish an infection in the retinal neuronal cells and can then move retrograde towards the cornea or anterograde towards the central nervous system (CNS). This migration requires gE, and presumably gI since they function in a complex. (D) Anterograde trafficking of virions inside neurons requires gE/gI and US9. Based on data in pseudorabies virus (PRV), US9 interacts with kinesins that regulate the anterograde movement of virions along microtubules towards axonal termini. It is also possible that gE/gI and US9 are required for proper cytoplasmic envelopment and sorting to axons.
The role of VP22 in spread is also used for the transport of viral RNAs during infection to adjacent non-infected cells, but this function can also be utilized for the delivery of products, such as chimeric polypeptides [216]. For example, a chimera consisting of VP22 linked to p53 can spread between cells and accumulate in recipient cell nuclei, while inducing apoptosis in p53-negative osteosarcoma cells [217]. There have been other chimeras that are described in the literature, but the potential of this strategy is not definitive. Not all types of cargo can be carried by VP22, and in vitro data may not translate well in animal models [218,219,220,221,222]. Additionally, VP22 conjugated cargo may be transported, but it might not be functional [223]. These conflicting results suggest that VP22 is not ideal for carrying all proteins, either due to inefficient transport of the chimeras or structural effects of the chimera on VP22. After mapping the functional domains of VP22, engineered versions of VP22 with higher transport efficiency could be investigated [189]. Nonetheless, VP22 chimeras are a promising tool and could be used in cancer, gene therapy, and vaccines. Notably, we have not detected VP22 in CD63+ EVs or ESCRT+ EVs derived from HSV-1 infected cells, suggesting that the intercellular transfer of VP22 does not depend on light extracellular vesicles (lighter than virions) derived from infected cells [224,225].
A potential avenue for utilizing VP22 transport in cancer therapy involves the introduction into target cells of a nontoxic drug and an enzyme that can convert to it to toxic. For example, introduction in cancer cells of the thymidine kinase (TK) gene and ganciclovir (GCV) results in phosphorylation of GCV, turning it into a nucleoside analogue that kills cells by inhibiting chain extension during deoxyribonucleic acid synthesis. Cytotoxicity is also observed in adjacent cells of a tumor. However, the levels of prodrug that need to be administered to kill adjacent cells in a solid tumor end up being toxic for the patient. These problems of efficient TK and GCV delivery can be resolved through VP22 [226]. VP22 can enhance intercellular trafficking of TK and can amplify the killing effect of the TK/GCV combination, making the fusion of TK and VP22 an attractive candidate for cancer therapy [227]. Another anticancer treatment is the use of the bacterial enzyme cytosine deaminase (CD) with the prodrug 5-fluorocytosine (5-FC), which is converted by CD to the highly toxic 5-fluorouracil. The efficacy of this combination can be enhanced through fusion of CD and VP22. The CD–VP22 fusion has a higher cytotoxicity in mouse models when compared to administration of CD alone [228,229].
VP22 could also be used in the context of gene therapy as a carrier [230]. For example, intraocular administration in mice of an adenoviral vector carrying VP22 fused to GFP showed a dramatic increase in the number of CNS neurons expressing GFP versus when administering an adenovirus with just GFP [231]. Other potential uses of VP22 to enhance adenovirus-based gene transfer have been noted in the literature [232]. VP22 can also enhance DNA vaccine protection against Pseudomonas aeruginosa in mice [233]. It is possible to fuse VP22 to other antigens of interest inside a DNA vaccine and that can enhance antigen-specific responses and antitumor effects [234].
2.4. US1 (ICP22)
The US1 immediate-early gene product, ICP22, is a 420-amino-acid protein. Mutant viruses lacking ICP22 display reduced virus yields in some cell lines, including primary human and rodent cell lines, but not in others, such as Vero (African green monkey) and HEp-2 cells (human epithelial), implying cell type-dependent effects [19,20,235,236,237,238,239,240,241,242]. Using different models of infection in mice and guinea pigs, a virus deleted of ICP22 caused reduced virulence and displayed reduced replication during an acute ocular infection and reduced neurovirulence [238,243,244,245,246]. Homologs of ICP22 are found in other herpesviruses, though the importance of ICP22 in infection seems to differ between viruses [247,248,249,250,251] ICP22 is guanylylated, adenylylated, and is phosphorylated by UL13 and US3 [252,253,254,255,256]. Phosphorylation of ICP22 at tyrosine 116 has been found to be important for ocular infection, affecting virulence, but the kinase responsible has not yet been specified [245].
ICP22 contains two nuclear import signals and has been implicated in viral gene expression [238,239,257,258]. Particularly, the carboxyl-terminal domain (CTD) of ICP22, in conjunction with the viral UL13 protein kinase, was found to enhance the synthesis of a subset of late (γ2) proteins exemplified by the products of the UL38, UL41, and US11 genes (Figure 1C). ICP22 and the UL13 protein kinase mediate the activation of cdc2 and degradation of its partners, cyclins A and B. Cdc2 and its new partner, the viral DNA polymerase accessory factor (UL42), bind topoisomerase IIα in an ICP22-dependent manner (Figure 1C) [259,260,261,262]. Although topoisomerase II is required for viral DNA synthesis, ICP22 is not, suggesting that the ICP22/topoisomerase II interplay has another role during HSV-1 infection. Indeed, topoisomerase II appears to be required for untangling concatemeric DNA progeny for optimal transcription of late genes.
Regarding the role of UL13 in the abovementioned complex, it was found that ICP22 and UL13 are involved in a common pathway that alters RNAP II phosphorylation, and in some cell lines, this change promotes viral late transcription, and also involves US1.5, a shorter gene encoded from the US1 ORF (Figure 1C) [263,264,265,266]. This ICP22/UL13-mediated phosphorylation of RNAP II resulted in an “intermediate” electrophoretic mobility between that of hyperphosphorylated (RNAP IIo) and hypophosphorylated (RNAP IIa) states [267]. Furthering this work, it was found that UL13 and the C-terminus of ICP22 are both required for RNAP II phosphorylation [267,268,269]. In cells infected with mutants from which UL13 had been deleted, ICP22 fails to aggregate in the nuclear structures containing nascent DNA, ICP4, RNA polymerase II, and other factors, implying a role of this UL13-mediated phosphorylation in viral late gene expression (Figure 1C) [270,271,272,273].
ICP22 was also found to bind the cyclin-dependent kinase 9 (cdk9) but not cdk7, and this complex in conjunction with viral protein kinases (UL13 and US3) phosphorylates the carboxyl terminus of RNAP II. The primary function of cdk9 and its partners, the cyclin T variants, is in the elongation of RNA transcripts, although functions related to the initiation and processing of transcripts have also been reported. Cdk9 was found to be important for optimization of the expression of genes regulated by ICP22. Therefore, one function of cdk9 during HSV-1 infection may be to bring ICP22 into the RNAP II transcription complex [274,275,276,277]. In support of these findings, it was reported that ICP22 binds to positive transcription elongation factor b (P-TEFb) and to RNAP II, and along with cdk9 they could suppress the expression of host genes, offering an advantage to the virus [278,279]. In fact, ICP22 represses transcription from all classes of viral genes but selectively upregulates expression of some late (γ2) genes [280,281]. Thus, ICP22 may be important to either repress or activate viral genes at different stages of the viral life cycle.
Besides its functions in viral gene transcription, a regulatory role has been proposed for ICP22 that involves the differential expression of two transcripts produced by the US3 open reading frame. The US3 gene was reported to encode two proteins. In wild-type virus-infected cells, the predominant form is the full-length Us3. However, in ICP22-null virus-infected cells, a shorter form of US3 is produced that initiates from methionine 77 and has been named US3.5 [282]. Like US3, the US3.5 mediates the phosphorylation of HDAC1, HDAC2, the protein kinase A regulatory IIα subunit (PKA RIIα), and the UL31 protein. Additionally, both kinases cofractionate with mitochondria. However, the US3.5 failed to block apoptosis (a well-established role of US3) and does not enable efficient release of virus particles from nuclei. Thus, the two proteins differ in the range of functions they exhibit [103].
Another role that has been attributed to ICP22 is involved in the host cell chaperone machinery by facilitating the formation of virus-induced chaperon-enriched (VICE) domains in the nucleus of some infected cells. Recent studies suggested that ICP22 mimics a cellular type II J protein, which is a co-chaperone in the nucleus [283,284,285]. The VICE domains are usually formed adjacent to the viral replication compartments, they contain several host chaperones (Hsp70, Hsp40, Hsp90), proteasomal components, ubiquitinated proteins, and at least one viral protein. These domains are hypothesized to play a role in protein quality control and remodeling, and during infection, they may participate in the formation of viral replication compartments and transcriptional regulation. VICE domains may also allow for correct folding of proteins participating in macromolecular assemblies. VICE domains may have a greater role in certain cell lines, where ICP22 expression is essential [273,286,287,288,289,290].
ICP22 was also found to form a complex with the HSV-1 proteins UL31, UL34, UL47, and US3 (Figure 3). These proteins, as discussed elsewhere, are important for viral egress through the nuclear membrane. ICP22 colocalizes with UL31 and UL34 at the nuclear membrane in WT virus-infected cells. In UL31-null virus-infected cells, targeting of ICP22 to the nuclear membrane is inhibited. In ICP22-null virus-infected cells, UL31 and UL34 mis-localized in the ER and the nuclear membrane, and significantly reduced the numbers of primary enveloped virions that were observed in the perinuclear space, although capsids accumulated in the nuclei. These data suggest that ICP22 plays a role in HSV-1 primary envelopment by interacting with the nuclear egress complex [291].
More recently, roles for ICP22 in combating the immune system have been proposed. The T cell co-stimulatory molecule CD80 was found to be downregulated in DCs in a manner dependent on ICP22 binding to the CD80 promoter, which seems to limit the pathogenesis of the virus as well as delaying the immune response to infection [292,293,294]. Recent studies with HSV-2 and transfection assays have found that ICP22 may be important in blocking type I IFN responses during infection [295]. Additionally, ICP22 may regulate host E3 ubiquitin ligases. Cumulatively, the findings regarding ICP22 are that it is important for the expression of the late (γ2) class of viral genes, formation of viral replication compartment and VICE domains in the nucleus, binding to host transcripts thereby altering host responses to the virus, and even by facilitating nuclear egress of viral capsids.
3. Host Evasion Factors
3.1. RL1 or γ134.5 (ICP34.5)
The HSV-1 γ134.5 gene product was first described in 1986 [296,297], and HSV-1 and HSV-2 are the only members of the alphaherpesviruses expressing ICP34.5 [298,299]. Deletion of the ICP34.5 gene abolished the capacity of the virus to spread from peripheral mucosal sites to the central nervous system (CNS) or replicate in the CNS, and diminished the capacity of the virus to replicate at mucosal sites and, subsequently, establish latency, or be able to be reactivated ex vivo [300]. In support of this, an ICP34.5-null virus displayed reduced neurovirulence following intracerebral inoculation into mice [298,299,301,302,303,304,305,306,307]. Furthermore, in an ocular model of infection, ICP34.5-null virus did not cause corneal disease [308]. In mouse embryonic dorsal root ganglia (DRG) three-dimensional cultures for HSV-1 latency, a virus with a deletion in ICP34.5 rendered the virus incapable of reactivation, even though the virus was clearly able to replicate and persist in a quiescent form in the DRG neurons [309].
The requirement of ICP34.5 for viral growth is cell type and status dependent, including an inability to replicate in non-dividing cells [303,310,311,312]. The ICP34.5 protein contains a domain homologous to GADD34/MyD116, which functions during growth arrest or DNA damage [313,314,315,316]. In cell culture, this domain of ICP34.5 was shown to be required for preventing the shutoff of host translation through the reversal of phosphorylation of eukaryotic translation initiation factor alpha (eIF2α), which occurs in a PKR-dependent fashion (Figure 2D) [317,318,319,320,321,322]. It was later found that ICP34.5 associates with the host protein phosphatase 1α via its C-terminus and redirects it to dephosphorylate eIF2α (Figure 2D) [317,318,323].
In interferon (IFN)-α/β receptor knockout mice, the ICP34.5-null virus showed a rescue to near wild-type replication levels in the trigeminal ganglia, and this sensitivity to IFN-α/β was found to occur in a manner dependent on the RNA sensor protein kinase R (PKR) [135,324,325]. It was also found that mouse embryonic fibroblasts (MEFs) infected with an ICP34.5-null virus induced higher expression of innate immunity genes and phosphorylation of the transcription factor IRF3, which was partially dependent on TANK-binding kinase 1 (TBK1) binding (Figure 2B) [326]. The binding site of TBK1 to ICP34.5 was found to be dispensable for blocking IRF3 phosphorylation, though ICP34.5 was still demonstrated to be important for this function [327].
Another major role for the ICP34.5 protein is blocking autophagy through its interaction with Beclin-1, which has been observed largely in mouse embryonic fibroblasts (MEFs) (Figure 2C) [321,328,329]. Viruses lacking the Beclin-binding domain (BBD) of ICP34.5 were attenuated [328]. Mice infected with the mutant virus missing the BBD of ICP34.5 replicated less in brain and corneal tissue, but the BBD was found to be dispensable for reactivation [330]. The control of autophagy by ICP34.5 was also implicated in preventing MHC-class I antigen presentation of gB from HSV-1-infected macrophages to CD8+ T cells through autophagy [331]. In support of this, the BBD of ICP34.5 was found to be important to antagonize autophagy and prevent MHC-class II antigen presentation in dendritic cells (DCs) [332]. Consistently, there seemed to be an increase in CD4+ T cell responses, including IFN-γ and IL-2 production in mice infected with HSV-1 lacking the ICP34.5 BBD [330]. However, in MEFs lacking the gene Atg5, which is essential for autophagy, the infection with ICP34.5 deleted for the BBD was not rescued compared to wild-type MEFs and no improvement in virus replication was noticed [333,334]. This suggests that the primary role of ICP34.5 is to counteract PKR activation rather than xenophagy. In support of this, the growth of ICP34.5-deficient virus was completely rescued in PKR-/- MEF cells. The discrepancies in the importance of the BBD and the fact that certain dendritic cell lines and neuroblastoma cells actually exhibiting higher autophagy activation when infected with an ICP34.5-null virus highlight the cell type-dependent role of ICP34.5 [321,328,333,335,336,337]. It was recently described that the expression of the ICP0 protein of HSV-1 was not sustained in the ICP34.5-deleted virus, which complicates our understanding of cellular effects by this virus [327]. More work is needed to clarify how cell type and cell status influences infection with ICP34.5-mutant viruses, and more specifically the ways in which ICP34.5 is able to modulate autophagy in different cells.
3.2. US12 (ICP47)
Infection of fibroblasts with HSV-1 renders the cells resistant to lysis by CD8+ cytotoxic T lymphocytes (CTLs), which normally recognize cell surface MHC I proteins presenting viral antigens. ICP47 can block the transport of MHC I proteins to the surface, and in this way inhibits lysis of infected cells by CTLs [338]. This explains why using ICP47 in a vaccine vector cannot confer protective immunity in vivo, since it prevents MHC I CTL induction [339]. To prevent the presentation of MHC I molecules on the cell surface, ICP47 binds to the transport-associated with antigen processing (TAP) factor. TAP mediates the transport of peptides destined for presentation by MHC I from the cytosol to the ER [340]. ICP47 binds with high affinity to the substrate-binding site of TAP, preventing the binding of other peptides [341,342], and this binding is species specific, since binding to murine TAP is much weaker [343,344]. This suggests that mice are not the optimal animal model to study the CD8+ T cell protective effect of ICP47, but pigs, dogs, or monkeys appear more suitable [345]. Alternatively, recombinant HSV-1 strains that contain murine MHC I complex-binding proteins can be used, and they effectively restrict MHC I antigen presentation in murine models. Work on such models has demonstrated that preventing MHC I antigen presentation increases neurovirulence of HSV-1 since viral entry, replication, and survival in the CNS is possible [346].
Preventing MHC I presentation can also be used to enhance the potency of oncolytic mutant strains, such as those based on ICP34.5-null mutants [347,348]. When using oncolytic viruses for cancer immunotherapy, there are issues with the immunogenicity against viral vectors that carry antigens and the memory response that arises after repeated injection of the vector during prime-boost regimens. CTL responses against the vectors prevent build-up of an immune response against the antigen of interest, which in the case of cancer immunotherapy is a peptide that is expressed in tumors. To solve this issue, a tumor peptide can be fused with part of the adenovirus 19K-derived leader sequence (MRYMILGLLALAAVCSA), which is an ER-targeting sequence, so that when expressed in APCs, the fusion product bypasses TAP and traffics to the ER. In the ER, the tumor antigen can be trimmed by aminopeptidases [349], loaded on MHC I molecules, and presented on the cell surface in a TAP-independent manner [350]. In parallel, US12 can be expressed from the viral vector and its expression restricts TAP and TAP-dependent MHC I presentation. In the end, viral vector peptides will not be presented through the TAP pathway and the immunogenicity against the vector will be restricted [351].
This protective role of ICP47 against CD8+ T cells is responsible for enhancing HSV-1 neuropathology in vivo. While an ICP47-null virus and WT replicate similarly in corneal epithelial tissues, the ICP47-null virus causes little to no neurologic disease and encephalitis [352]. Mice depleted of T cells can support WT levels of neurovirulence, but mice survival is decreased after exogenous delivery of CD8+ T cells [353], suggesting that CTLs can control HSV-1-associated disease. Presentation of HSV-1 peptides to CTLs through TAP is inhibited by ICP47, thus absence of ICP47 restricts neurovirulence whereas absence of TAP does not [354]. Additionally, TAP expression in the brain of infected mice is increased, suggesting that it has a host defense role. Infection with an ICP47-null HSV-1 virus does not trigger an increase of TAP, likely because this virus does not invade the brain [354].
4. Nucleic Acid Metabolism and Endonucleases
4.1. UL2, UL12, UL12.5, UL50
HSV-1 has been shown to encode multiple proteins for the metabolism of nucleic acids in the host cell. One such protein is encoded by UL2, and is the uracil DNA glycosylase, which is an important enzyme for removing uracil from DNA [355,356,357,358,359]. The uracil DNA glycosylase activity was described to be important in adult neurons for the replication of the viral genome [360,361]. In support of this, infection of mice with mutant viruses lacking uracil DNA glycosylase activity had significantly reduced viral load in peripheral and central nervous system tissues, as well as reduced reactivation from latency [361]. Later, UL2-encoded uracil DNA glycosylase activity was found, with cellular factors and with the viral DNA polymerase, to participate in base excision repair coupled with DNA replication, supporting a role for UL2 during genome replication [362,363,364,365]. Moreover, UL2 nuclear localization was found to be important for efficient viral replication [366]. UL2 was found to be nonessential in cell culture [367]. There is a homolog for this protein in other herpes viruses [368,369,370,371].
HSV-1 also encodes an alkaline nuclease or deoxyribonuclease, which is a phosphoprotein with endo- and exonuclease activity, which localizes to the nucleus of infected cells [372,373,374,375]. The alkaline nuclease is encoded by the UL12 gene of HSV-1 [22,374,376,377,378,379]. The UL12 gene of HSV-1 is highly related in sequence to proteins from other herpes viruses [380,381,382,383]. UL12 is not essential for viral DNA replication [384]. UL12 is important for viral capsids to egress from the nucleus [385,386]. It was found that cells infected with a mutant virus lacking UL12 released many particles with genomes incapable of undergoing new rounds of infection [387]. This increase in defective particles released in the absence of UL12 during infection was later found to specifically be due to the nuclease activity of the protein [388].
Mutant viruses lacking alkaline nuclease activity have reduced growth in cell culture, which may be due to a role for UL12 in processing viral DNA replication intermediates and packaging the DNA into the capsid [389,390,391]. More specifically, UL12 has been found with ICP8, the ssDNA binding protein of the virus, to mediate strand exchange during DNA replication through increased nuclease function by UL12 [392,393,394]. UL12 also seems to be involved in single-strand annealing during homologous DNA repair in infected cells, which also involves ICP8 as the single-strand annealing protein [395,396]. UL12 was also found to interact with components of the MRN (homologous recombination repair complex containing the proteins Mre11, Rad50, and Nbs1) in the nucleus during infection [397]. Together, these reports support a role for UL12 in the processing of viral DNA during infection.
The UL12.5 protein of HSV-1 has not been fully characterized, but its ORF is known to overlap that of UL12. UL12.5 lacks the first 126 aa of UL12, it retains the nuclease and the ICP8 binding activities of UL12, and was initially described as a capsid nuclease [398,399]. However, UL12.5 does not accumulate to high levels in the nucleus and cannot efficiently substitute for UL12 in promoting viral genome maturation [400]. Interestingly, the only known function of UL12.5 seems to be related to mitochondria stress, as it has been found that mitochondrial DNA is eliminated early during HSV-1 infection in a UL12.5-dependent manner, which also involves mitochondrial nucleases (Figure 2C) [401,402,403]. This role by UL12.5 in altering mitochondria stability during infection has not been further described, but it has been found to be dispensable for viral replication [404]. More work is needed to understand the role of UL12.5 during HSV-1 infection.
Another important protein involved in viral DNA replication is the protein encoded by UL50 of HSV-1. UL50 encodes a tegument protein, which is a deoxyuridine 5′-triphosphate nucleotidohydrolase (dUTPase), which is important for the synthesis of thymidine for DNA replication [153,405,406,407,408]. The dUTPase activity was found to be nonessential in cell culture; however, replication in the CNS of mice infected with a UL50-deleted virus was reduced, along with reduced neurovirulence and reactivation [358,409,410]. The dUTPase is phosphorylated by the viral kinase US3 (see below), which seems to regulate the activity of this protein in a cell type-dependent manner, as well as specifically affecting the neurovirulence and replication competency of HSV-1 in the central nervous system [411,412,413]. There are homologs for UL50 in other members of the Herpesviridae family [164,380,381,414,415,416,417]. Recent reports studying homologs of the HSV-1 UL50 from EBV or using transfections of multiple UL50 homologs show the ability of UL50 to affect TLR1/TLR2-mediated immune responses and upregulate NF-κB activity [418,419,420]. While it is still not known how this pro-inflammatory effect by the HSV-1 dUTPase affects viral infection or pathogenesis, particularly as it relates to infection of different cell types, it is worth further investigation to understand the immunomodulatory capacity of HSV-1 and the viral dUTPase.
4.2. UL39 and UL40 (RR1 and RR2)
The holoenzyme of the viral ribonucleotide reductase (RR) is composed of two subunits, the large subunit, also known as ICP6, that is encoded by the UL39 gene and the small subunit encoded by the UL40 gene. HSV-1 RR converts ribonucleotide diphosphates to corresponding deoxyribonucleotides, allowing for virus replication, particularly in non-dividing cells [421]. Both subunits of the RR are needed for enzyme activity [422], so a decrease in either subunit decreases RR activity. Knockdown of UL40 using siRNAs triggers a mild (50%) decrease in plaque size and numbers [423]. Chemical inhibitors of the ribonucleotidase activity can lead to poor viral replication, depending on the cell type [371,424,425]. Dividing cells in S phase contain an elevated dNTPs pool and are capable of supporting replication of ribonucleotide reductase-deficient virus [421,426]. RR is required in non-replicating cells, such as neurons that have a reduced dNTPs pool (Figure 4A). This attribute makes an RR-deficient virus an attractive option for use in oncolytic therapy that targets malignant gliomas, since it generates a non-replicating virus in such tissues, giving a good safety profile [427].
HSV-2 UL39 has been associated with antiapoptotic functions [428]. HSV-1 UL39 mutants exhibit a 50% reduction in protection from TNFα [429], which suggests that HSV-1 R1 is important for protection of HSV-infected cells from this death ligand, while the 50% efficiency suggests that other viral proteins contribute to this protection. Nonetheless, HSV-2 R1 interacts constitutively with caspase-8 and prevents its interaction with FADD, inhibiting TNFα-mediated apoptosis [430].
The large subunit of the RR has been shown to contribute to ocular virulence in mice [431], but null mutants can produce lesions in a guinea pig model [432]. This has also recently been observed with a naturally occurring viral mutant in mice that were impaired in acute replication in the eyes and the trigeminal ganglia of mice, and also defective in establishing a latent infection and reactivation [433]. Interestingly, this mutant cannot inhibit caspase 8-induced apoptosis as wild-type virus [433], further supporting the relevance of the antiapoptotic effects of the RR for pathogenesis. An RR-deficient virus that exhibits impaired acute replication in the eyes and the trigeminal ganglia of mice is an attractive option for a herpes prophylactic vaccine, since it appears to confer protection against HSV-1 challenge post-immunization with an RR-deficient mutant [434].
4.3. UL41 (vhs)
It was first observed that HSV-1 caused the shutoff of host protein synthesis as early as 1978 [435,436,437]. This effect was later ascribed to the UL41 gene product of HSV-1, the virion host shutoff (vhs) protein, which is found in the viral tegument [438,439,440,441,442]. There are homologs for vhs in other alphaherpesviruses, such as HSV-2, varicella-zoster virus, equine herpesvirus, and pseudorabies, which is indicative of a conserved benefit for the virus to express this protein [443,444,445]. Although vhs is dispensable in cell culture, its absence in vivo leads to reduced viral pathogenicity and severe attenuation [135,441,446,447,448,449,450]. Vhs was first described to be able to block the accumulation of both host transcripts and all three classes of viral mRNAs [438,439,440,441,451,452,453]. However, it was later suggested that the activity of vhs is regulated late during infection by the viral proteins UL47, VP16, and VP22 and this is how it spares beta and gamma viral transcripts, although it downmodulates alpha gene transcripts [187,454,455,456,457]. Vhs blocks the accumulation of mRNAs due to its role as a viral RNase [452,458,459,460]. More specifically, vhs has been identified as an endoribonuclease, with sequence similarities to the FEN-1 family of nucleases, which are found in eukaryotes and archaebacteria and are involved in DNA replication and repair [460,461,462]. Vhs displays substrate specificity similar to that of RNase A and it cleaves at the 3’ end of single-stranded cytidine or uridine residues [463]. A group of mRNAs targeted by vhs includes those with adenylate-uridylate (AU)-rich elements at the 3’ end. Several AU-rich mRNAs are stress response transcripts that are upregulated during HSV-1 infection, as they encode for hostile products, including type-I interferon-related products [457,464,465].
These mRNAs appear to be cleaved in ARE and deadenylated in a 3’-5’ decay process occurring in a vhs-dependent manner, whereas the truncated 5’ domains may persist, and this is a mechanism by which HSV-1 counteracts antiviral responses (Figure 2D). Stable host transcripts appear to be targeted by vhs in a different way that includes binding of vhs to the cap structure via its affinity for the translation initiation factor eIF4H that causes mRNA decapping and degradation of the uncapped mRNA 5’ to 3’ [465,466,467]. In addition to eIF4H, vhs also interacts with other subunits of the cap structure, including the eIF4AII isoform, the eIF4B, and perhaps other components of the translation apparatus [460,468,469,470,471,472]. These interactions may allow vhs to access some targeted mRNAs during translation initiation and regulate their expression. Overall, vhs displays specificity since it preferentially degrades translating mRNAs and not tRNAs or rRNAs [473].
By degrading host transcripts, particularly those induced by interferons, vhs has a central role in blocking antiviral responses. It was first described that vhs may be involved in blocking immune responses when it was observed that growth of a vhs-deficient virus was rescued in mice deleted of interferon signaling receptors [135]. It was then shown that there was increased cytokine production in mice infected with a UL41-deficient mutant as compared to wild-type virus-infected mice. Additionally, UL41 mutant viruses displayed increased sensitivity to interferon-α and -β compared to wild-type virus [474]. Vhs was then described to be important for blocking the activation of dendritic cells (DCs), which was found to occur in a toll-like receptor (TLR)-independent manner [475,476]. It was also demonstrated in mature DCs that vhs is required for HSV-1 to block phosphorylation of STAT1 and IFNγ signaling [477]. Infection of immunocompromised mice lacking the STAT1 gene with a vhs-deficient virus did not rescue the growth of this virus and also resulted in higher induction of cytokines than in wild-type virus-infected mice, indicating that vhs has a fundamental role in promoting virus replication and that STAT1 was required to mount an appropriate non-pathological inflammatory response [478].
In addition, by degrading transcripts, vhs was found to decrease the formation of cytoplasmic stress granules (SGs) in infected cells, thus preventing activation of PKR through accumulation of dsRNAs at the site of SGs, which would otherwise lead to innate immunity activation and translation shutoff due to phosphorylation of eIF2α (Figure 2D) [465,479,480,481,482,483]. Furthermore, vhs has been proposed, with ICP0, to block the DNA sensor IFN-γ-inducible gene IFI16 through its endoribonuclease activity, thus blocking the antiviral activities of IFI16 in multiple cell types [123].
Cumulatively, the expression of vhs has been found to benefit the virus in multiple ways, both in vitro and in vivo.
5. Viral Kinases
5.1. UL23 (TK)
The HSV-1 thymidine kinase (TK) is a 376-aa protein, encoded by UL23. TK is responsible for phosphorylating thymidine and deoxycytidine through an ATP-dependent mechanism, though it has been described to have broad substrate specificity [484,485,486,487,488,489]. TK is also known to phosphorylate the nucleoside analogs acyclovir (ACV) and ganciclovir (GCV), which have an inhibitory effect on the viral DNA polymerase, thus blocking viral replication [485,490,491]. TK activity is conserved across other herpesviruses [488,492,493,494,495,496,497,498].
In vitro studies have demonstrated that TK is dispensable for virus replication in sensory neurons derived from dorsal root ganglia of rat embryos [499]. However, in vivo studies found that TK is required for virus replication in trigeminal ganglia and the brain but not in peripheral tissues of adult mice (Figure 4A) [500,501,502,503]. This is most likely because adult neurons are post-mitotic and they do not express adequate levels of cellular TK to support the growth of HSV-1 TK-null virus, unlike dividing cells. In support of this, it was shown that substitution of the viral TK with the host TK gene enabled the recombinant virus to replicate in TG [504,505]. In addition, the absence of TK activity impairs HSV-1 reactivation from latency [426,506,507,508,509]. Particularly, it was reported that following corneal inoculation of mice, the HSV-1 TK-null virus was severely impaired for replication in TG [502,510]. However, LAT was expressed in these ganglia, suggesting that the TK-null virus can establish latency [511]. Notably, a TK-null mutant virus cannot reactivate even when latent viral loads were comparable to those that permit efficient reactivation of wild-type virus, indicating that latency establishment was not an issue [237,500,506,508,512,513].
Prolonged treatment with acyclovir and its analogs can lead to virus-acquired drug resistance because of mutations accumulating in the TK gene. Such mutant viruses are causing major problems in immunocompromised individuals in the clinic [514,515,516]. Considering also that the properties of HSV-1 TK have been explored in experimental therapies of intracranial tumors, it is important to clarify if HSV-1 TK-null viruses can establish lifelong infections in immunocompromised hosts. Using different HSV-1 TK mutants and different backgrounds of nude mice, it was demonstrated that all HSV-1 TK mutants can establish persistent infections in the TG and brain stem of nude mice [508]. This is consistent with the detection of ACV-resistant TK mutants in the CNS of immunocompromised patients with persistent infection [517].
While the role of TK in viral replication and latency in vivo has been the subject of a fair amount of investigation, a breadth of information has been obtained regarding the potential of TK expression for therapeutic purposes. More specifically, much work has been done on using the HSV-TK/GCV suicide gene therapy system for cancer treatment. This system works such that GCV is monophosphorylated by HSV-TK and further phosphorylated by host cell kinases. The triphosphate form of GCV is an analog of purine and it incorporates in the nascent DNA of the cancer cells, which are actively proliferating and synthesizing DNA. This causes the DNA polymerase to stall with subsequent termination of nuclear and mitochondrial DNA synthesis. As a consequence, DNA damage and cell cycle arrest is induced, leading to caspase-dependent cell death in cancer cells [518,519,520,521,522,523,524]. It was observed in breast cancer cells that the HSV-TK/GCV system induced p53-dependent DNA damage responses and cell cycle arrest, perturbing mitochondrial homeostasis through membrane potential dysfunction and release of cytochrome c into the cytoplasm in neuroblastoma cells [519,525,526]. Similar findings were reported in hepatocellular carcinoma cells using an adenovirus method of delivery for HSV-TK [527]. The HSV-TK/GCV system has also been seen to be effective in tumor models in mice [528,529,530,531]. This system is considered efficient because the effects of HSV-TK/GCV are also mediated through bystander effects on surrounding cells and tissues to those that uptake HSV-TK/GCV, which is thought to occur through the transfer of cytotoxic molecules between cells [532,533,534,535,536,537]. HSV-TK/GCV has since been used in several phase I/II clinical trials [538,539,540,541,542,543]. Other preclinical trials have also been published using HSV TK in other delivery systems, and frequently in combination with other antitumor therapy methods [544,545,546,547,548,549,550].
5.2. US3 and US3.5
The serine/threonine protein kinase US3 of HSV-1 has multiple significant functions, though it is non-essential in cell culture [21]. However, US3 has been found to be critical for infection of both peripheral sites and the central nervous system [237,551,552]. Furthermore, US3 has been implicated in the promotion of the viral infection in multiple ways, from blocking host responses to promoting viral replication and nuclear egress.
One function of US3 is to promote the egress of nucleocapsids from the nucleus to the cytoplasm through primary envelopment (Figure 3) [553,554]. This is supported by the observation that in US3-null virus-infected cells, aberrant HSV-1 capsids are trapped between the inner and the outer nuclear membrane [103]. Us3 deficiency also causes accumulation of viral proteins essential for cytoplasmic envelopment and viral infectivity, such as gK, with the capsids in the perinuclear space (PNS) [555]. To further promote nucleocapsids egress, US3 has been implicated in phosphorylation of lamin A/C that causes changes in its architecture, including changes in its localization and conformation [556,557,558,559]. US3 also assists in the egress of nucleocapsids through interactions with and phosphorylation of UL31 [560] and UL34 [556,561,562]. All three proteins are located within perinuclear virions and at the inner nuclear membrane (INM), suggesting that they could be incorporated into the virion during budding at the INM. US3 also phosphorylates UL47 at Ser-77, promoting its nuclear localization [563]. A proposed role of nuclear UL47 is to interact with the nuclear egress factors UL31, UL34, and US3 to regulate viral nuclear egress [291].
Another function of US3 is inhibition of apoptosis [564,565]. It has been demonstrated that expression of US3 protein outside of the context of the infection mediated post-translational modification of BAD, a proapoptotic protein, which is no longer proapoptotic upon post-translational modifications at Ser-112 and Ser-136 by US3 [566]. Consequently, PARP cleavage, BAD cleavage, and caspase 3 activity were blocked when proteins that induce apoptosis were expressed concordantly with US3 [470,566,567]. Us3 may block apoptosis in multiple ways since it blocks cell death induced after infection with various HSV-1 mutants, such as ΔICP4, but it can also protect against cell death induced after thermal or osmotic shock [564,568]. In support of this, the optimal consensus sequence of US3 peptide substrates was found to resemble the target sequence of the cellular cAMP-dependent protein kinase PKA [569]. PKA is a key enzyme important in the regulation of metabolism, survival, and proliferation of eukaryotic cells, and it mediates most of the biological effects of the second messenger cAMP. The pattern of proteins phosphorylated by US3 overlaps that of phosphoproteins targeted by PKA. Consistently, PKA could block apoptosis by stimuli that Us3 could block. Overall, US3 can block DNA fragmentation and cell death caused by exogenous expression of pro-apoptotic factors or a variety of other stimuli.
US3 has been implicated in promoting viral gene expression both at the level of transcription and translation and virus replication (Figure 1). US3 promotes viral gene transcription by preventing the deacetylation of histones, a function that also involves ICP0 [103,570]. The US3 kinase from VZV and PRV promotes hyperphosphorylation of HDAC2 and likely HDAC1 to reduce viral genome silencing and allow efficient viral replication [570]. US3 has also been proposed to act as the ser/thr kinase Akt, although it does not look like Akt. This Akt-like kinase function of US3 has a role in stimulating mRNA translation through activation of mTORC1 by phosphorylating tuberous sclerosis complex 2 (TSC2) on the same sites as Akt [571,572]. Activated mTORC1 negatively regulates the activity of the translation repressor 4E-BP1, enabling cap-dependent translation. Additionally, US3 phosphorylates the viral dUTPase, encoded by UL50, at Ser-187, and that causes an increase of its activity over host dUTPases, thereby promoting HSV-1 replication [411].
Finally, US3 is implicated in virus defense against the host. One example is the requirement of US3 for inactivation of CD8+ cytotoxic T lymphocytes, thus preventing cytokine production [573]. US3 also blocks TLR2 signaling early during infection by preventing TRAF6 polyubiquitination [574]. Additionally, US3 hyper-phosphorylates IRF3 at Ser175, which inhibits IFN-β production [575]. Furthermore, the Bcl-2-associated transcription factor 1 (Bclaf1) was recently shown to be degraded in a US3-dependent manner during HSV-1 infection [576]. This degradation prevented IFN-α-mediated interferon-stimulated genes expression [576]. US3 also phosphorylates both ULK1 and Beclin-1, thus blocking autophagy activation during HSV-1 infection in a manner independent of ICP34.5 [577]. In a different approach to promoting the viral infection, US3 has also been found to affect the subcellular localization of certain viral proteins by affecting their phosphorylation status. Particularly, US3 causes a decrease in the amount of gB found on the cell surface by phosphorylating its cytoplasmic tail at Thr-887 and perhaps enhancing its endocytosis [578]. This phosphorylation of gB has been implicated in virus pathogenesis since mutation of Thr-887 significantly impaired viral replication in the mouse cornea and the development of herpes stromal keratitis and periocular skin disease [578]. US3 has also been found, with gB, to be involved in downregulation of the major histocompatibility complex class I-like antigen-presenting molecule, CD1d, through prevention of recycling of CD1d to the cell surface [579].
A shorter version of Us3 named Us3.5 was discussed earlier together with ICP22.
Overall, US3 plays many important roles in the viral life cycle and in viral infectivity.
5.3. UL13
In addition to the US3 kinase, HSV-1 encodes a second serine/threonine protein kinase UL13, which functions in the cell nuclei and is present in the virion as a tegument protein associated with the capsid [580,581,582,583,584]. UL13 is conserved across members of alpha-, beta-, and gamma-herpesviruses [380,585,586,587,588,589]. UL13 is non-essential in cell culture, but it seems to have a role in counteracting antiviral responses and is important for optimal viral replication in cell culture [590,591,592]. In a mouse model, a UL13-deleted virus was sensitive to type I IFN, suggesting an important role for UL13 in blocking host responses to infection [593].
UL13 phosphorylates multiple viral proteins, including itself, the immediate-early viral protein ICP22 and US1.5, ICP0, and numerous tegument and envelope proteins (Figure 1) [190,242,253,254,592,594,595]. It seems that phosphorylation of ICP0 by UL13 is important for stabilizing ICP0 protein during infection [596]. This is supported by the fact that ICP0 is degraded both early and late in cells infected with a mutant lacking the UL13 protein kinase. Furthermore, it was found that ICP0 encoded by wild-type virus or the UL13-null mutant is stable in cells transfected with a plasmid encoding UL13 before infection [596]. Phosphorylation of UL46 by UL13 leads to Akt activation to promote cell survival [597]. UL13 also phosphorylates VP22 at casein kinase II consensus sites, but it was found that UL13 modulates cellular localization of VP22 in a phosphorylation-independent manner [590,595,598]. The significance of phosphorylation of VP22 by UL13 has not been investigated. UL13 phosphorylates the viral Fc receptor gE/gI, though it seems that a host kinase may also phosphorylate gE/gI [599]. Phosphorylation of gE by UL13 is thought to facilitate packaging of UL13 into the virion [599]. However, this phosphorylation could also impact gE trafficking or other functions. UL13 expression was found to be downregulated by the US11 RNA-binding protein. US11 was found to bind to the RNA sequence, designated as 12/14, which is present in the coterminal HSV-1 mRNAs UL12, UL13, and UL14. This binding led to reduced UL13 kinase activity due to reduced mRNA levels [600]. While the exact mechanism of downregulation of UL13 transcripts by US11 is unknown, it is thought that this occurs through nucleocytoplasmic export of the transcript [600]. The kinase activity of UL13 and US3 was found to be important for the viral glycoproteins gC and gD to be modified and expressed late during infection, as loss of both UL13 and US3 diminished virion release, showing a role for UL13 in assembly and egress of the virion [601]. Thus, UL13 modifies multiple viral proteins both to promote assembly and release of the virus, and to affect the stability and localization of viral proteins.
UL13 plays roles in modulating host responses to infection. For example, interferon stimulation and production of cytokines are modulated by UL13 during infection. UL13 has been found to be important for the induction of a set of suppression of cytokine signaling (SOCS) genes late during infection, which is important for blocking the interferon response during HSV-1 infection of cells [602,603,604,605]. Recently, UL13 was found with the other viral kinase US3 to be important for regulating phosphorylation of protein kinase R (a nucleic acid sensor) during infection [606]. UL13 was also found to hyperphosphorylate an important host factor for the elongation of peptide chains during mRNA translation, eF-1δ during HSV-1 infection, supporting viral protein synthesis, and also preventing apoptosis [607,608,609]. UL13 was found to phosphorylate the cellular casein kinase II β subunit (CKIIβ), though the significance of this phosphorylation has not been explored [610]. However, it was found that UL13 is able to phosphorylate proteins at similar residues as the cellular cdc2 cyclin kinase [610]. It was also determined that UL13, with ICP22, is responsible for activating cdc2 during infection, which is required for optimal expression of viral late genes [259,260]. UL13 was also found with ICP22 to phosphorylate the RNA polymerase II (RNAP II), supporting virus late gene expression [267]. Overall, phosphorylation of host proteins by UL13 supports viral infection by supporting virus late gene expression, blocking innate immune responses, supporting viral protein synthesis, and activating cellular proteins for the benefit of the virus.
6. Virion Morphogenesis, Egress, Cell-to-Cell Spread, and Host Evasion
6.1. UL3 and UL4
The HSV-1 UL3 and UL4 are late proteins that have only minorly been studied, though they have been determined to be nonessential in cell culture and UL4 was also found to be nonessential in mouse models of HSV-1 infection for latency, reactivation, and pathogenesis [421,611,612]. The UL3 phosphoprotein and the UL4 protein have homologs in other members of the Herpesviridae family [84,153,368,369,370,613,614,615,616,617,618,619,620]. UL3 appears to localize perinuclearly early during infection and in nuclear puncta at late times post infection [613,621,622]. UL3 and UL4 have also been found in the nuclei of infected cells and found to co-localize with ICP22 in nuclear bodies, which may involve recruitment by ICP22 [271,623,624,625].
6.2. UL7 and UL51
HSV-1 pUL7 is a 296-aa tegument protein [626] that lacks a putative transmembrane sequence or motifs that could facilitate its membrane anchor. Therefore, its association with membranes is mediated through the interaction with a membrane protein, i.e., UL51. UL7 forms a complex with UL51, and this is required for the recruitment of UL7 to cytoplasmic membranes and into the virion tegument (Figure 3C) [627]. pUL7 and pUL51 form a stable and direct protein-to-protein interaction [628], and they function as a complex in infected cells. Both are important for HSV-1 assembly and plaque formation. Their individual ablation results in similarly lower yields with a double UL7/UL51 knockdown, suggesting that UL7 and UL51 work in the same pathway [628], which is likely related to cytoplasmic envelopment of HSV-1 virions since many unenveloped capsids next to membranes can be seen in cells infected with UL7/UL51 mutants [628].
UL7 ablation can affect HSV-1 infection at earlier times, too. UL7 absence results in viruses with lower yields in vitro and lower pathogenic effects in vivo [629]. Mice infected with a UL7 mutant HSV-1 exhibit longer survival than those infected with WT HSV-1. A decrease in LAT mRNA expression was observed in the CNS and trigeminal ganglia of mice infected with a UL7 mutant HSV-1, suggesting decreased viral gene transcription in the absence of UL7. This is supported by data that show that UL7 may participate in the complex that is involved in the transcription of ICP4 [629]. It is not clear if UL7 is directly involved in the interaction between the promoter of ICP4 and the transcriptional complex or is involved perhaps in the chromatin remodeling process that enables transcription of ICP4. UL7 can be detected through cell fractionation and fluorescence microscopy in the nucleus [630], although it is primarily seen in the cytoplasm. It is not clear if its function in the nucleus is derived from UL7 delivered as part of the incoming virions [627] or from nascently expressed UL7.
UL7 and UL51 can also affect cell-to-cell spread of HSV-1. pUL51 interacts with pUL7 and gE/gI in infected cells, and deletion of part of UL51 or deletion of UL7 results in failure of gE to concentrate at junctional surfaces of Vero cells (Figure 3). This suggests a role for a UL51/UL7 complex in cell-to-cell spread of HSV-1; however, this may depend on the cell line. A pUL7/pUL51/gE/gI method for cell-to-cell spread can occur in polarized epithelial cells, such as HaCaT, but different cell-to-cell spread mechanisms may be utilized in non-polarized cells, such as Vero [631]. Additionally, the UL7/UL51 complex can affect cell-to-cell spread by localizing to focal adhesions in infected cells. Focal adhesions are contact sites between the cytoplasm and the extracellular matrix. They are dynamic and respond to extracellular stimuli and play a role in cell attachment and movement. Ablation of the UL7/UL51 complex results in destabilization of focal adhesions and diminished cell integrity [628]. UL7 and UL51 seem to mediate the stability of focal adhesions during infection, perhaps to maintain the proximity of infected and non-infected cells so that cell-to-cell spread can be promoted. This still remains to be demonstrated.
An additional function of UL7 may be exhibited on the mitochondria. UL7 has been identified as a partner of the adenine nucleotide translocase (ANT2) through a mass spectrometry approach combined with affinity purification. ANT2 localizes in the inner mitochondrial membrane and is a member of the permeability transposition pore complex. In physiological conditions, it exchanges ATP and ADP on the inner mitochondrial membrane and is essential for maintaining the cell metabolism exchange of cytosolic ADP for mitochondrial ATP. HSV-1 infection can affect mitochondrial function, and UL7 may be one of the proteins that are important for this function [632].
UL51 is a late gene that is expressed as three phosphoproteins with sizes of 27, 29, and 30 kDa. It can be detected in extracellular HSV-1 virions [633], is phosphorylated on five sites [634], and phosphorylation on the Ser-184 site has been described as important for HSV-1 replication in vitro and pathogenicity in vivo after ocular infection of mice [634]. UL51 localizes in the cytoplasm [633], mostly in the perinuclear area, but part of it also localizes to the Golgi. Golgi localization requires the N-terminus of UL51, which is palmitoylated to mediate sorting to Golgi membranes. UL51 is packaged in virions, on the inside of the viral envelope [633]. UL51 internalization into vesicles and virions may occur during cytoplasmic envelopment in infected cells [633]. Infections with a UL51-null HSV-1 yields smaller plaques and a growth of 2 logs lower than a WT HSV-1. UL51-null infections exhibit enveloped virions at the perinuclear space, as opposed to enveloped virions at membranes at the TGN during WT HSV-1 infections [635]. The membranes that encapsulate those UL51-null virions resemble nuclear membranes through electron microscopy. Such membranes are tightly wrapped around nucleocapsids and do not appear as fuzzy as the membrane of the extracellular virions. Additionally, nucleocapsids are found intranuclearly adjacent to the inner nuclear membrane (INM) with membranes with the same appearance, further supporting that these perinuclear enveloped virions are enveloped with membranes derived from the nuclear cisternae. This suggests that UL51 acts at a post-inner nuclear membrane envelopment step, possibly during the outer nuclear membrane de-envelopment process (Figure 3B).
As mentioned above, UL51 has a role in cell-to-cell spread that is dependent on cell type. UL51 colocalizes with gE in infected cells and it can be immunoprecipitated together with gE, in addition to affecting gE localization to cell junctions [636]. It is possible that UL51 functions as a trafficking mediator while is present on the cytoplasmic side of Golgi membranes.
UL51 recruits UL7 into the nascent virion tegument (Figure 3) [627]. Their colocalization is incomplete though, suggesting that they have other independent functions [627].
UL51 also interacts with UL14 in infected cells, as shown by affinity purification [637]. Three amino acids on UL51 are required for this interaction, and their mutation results in decreased viral replication and accumulation of unenveloped and partially enveloped capsids in the cytoplasm. The localization of both UL51 and UL14 depends on their reciprocal interaction. These data suggest the UL51-UL14 complex regulates cytoplasmic envelopment of HSV-1 [637].
6.3. UL10 and UL49.5 (gM and gN)
Glycoprotein M (gM) is an integral viral envelope membrane protein that spans the membrane eight times [638]. Its deletion results in only a small decrease in viral yields in cell culture [611,639], thus it is defined as non-essential. Even though a gM-null virus can establish a latent infection in mice, it is impaired for growth within the nervous system versus the wild-type virus [638].
gM localizes within the leaflets of the nuclear membrane, at the Golgi and the TGN, and the envelopes of cytoplasmic and extracellular virus particles [640]. Infection with a US3-null HSV-1 results in punctate extensions and invaginations of the nuclear membrane, on which gM localizes [640]. This suggests that gM becomes incorporated into the virion envelope upon budding through the nuclear membrane. Transfection of gM leads to its localization to the TGN and plasma membrane (PM) [641,642]; however, this pattern changes during infection. When gM is expressed, it is recruited to nuclear membranes and then to perinuclear virions once they are formed. This occurs before HSV-1 induces reorganization of the TGN and before gM localization to the TGN. Consistent with these observations, confirmed partners of gM, such as gH/gL, gN, VP22, UL31, and UL34 [643,644,645,646], do not colocalize with gM early during infection. Therefore, it has been proposed that the function of gM early during infection in the nuclear membranes is separate from its function during viral egress [641]. Characterization of gM domains showed that its trafficking to the TGN requires its transmembrane domains, while its C-terminal trafficking motifs are dispensable. The requirement of the transmembrane domains suggests that gM may associate with other transmembrane proteins for trafficking (Figure 3C) [647], but this remains to be shown.
The role of gM in the TGN is related to the trafficking of host and viral proteins during infection. For example, co-expression of gB, gD, gH, and gL can trigger fusion of cell membranes of transfected cells. Such fusion can be inhibited by the additional co-expression of gM [643]. In this context, gD and gH/gL can be seen to relocalize from the plasma membrane to the TGN, suggesting that inhibition of fusion is triggered through the removal of the fusion glycoproteins from the surface [643]. These data suggest that gM is involved in either retaining viral glycoproteins at the TGN or causing their translocalization from the plasma membrane to the TGN, supporting virion maturation at the TGN [648]. Further supporting data show that an absence of gM results in reduced gH/gL internalization from the PM of infected cells, and reduced incorporation in produced virions [649].
gM can interact with gN, resulting in altered intracellular targeting of both proteins. Co-immunoprecipitations in transfected or infected cells indicate that gM and gN form a complex [642], and gN overexpression seems to mediate the formation of syncytia in infected cells, which are inhibited normally by gM [642,643,650]. Syncytia occurs when cell membranes fuse, forming large multinucleated cells. This suggests a strict regulation of fusion that can be deregulated by altered gN expression possibly through altering the localization of gD and gH/gL from the plasma membrane to the TGN that is triggered by gM [643]. gN is an ER-resident protein that in the presence of gM is translocated to the TGN. gM and gN are covalently linked between two cysteines, and exit of gN from the ER requires the N-terminus of gM but not the C-terminus. gN is non-essential and its deletion does not seem to affect viral growth [644].
While gM can inhibit syncytium formation in transfected cells [643] and reduces the surface expression of proteins involved in fusion, only gN and UL46 have been identified as partners of HSV-1 gM. Proteomics studies with an emphasis on host proteins identified the host extended synaptotagmin 1 (E-Syt1) as a gM partner [651]. E-Syt proteins promote the close apposition of the ER and the plasma membrane (PM), and the transfer of lipids between the ER and the PM. Functions of several synaptotagmins remain to be determined, but they seem to engage and regulate SNARE proteins (the core cellular fusion machinery) and act as Ca2+ sensors.
It was found that during HSV-1 infection, knocking down E-Syt1 triggered the release of the virus into the extracellular space, at the expense of cell-associated infectious particles. Conversely, overexpressing E-Syt1 led to reduced levels of mature virions in the medium, hinting at a negative regulation. E-Syt1 did not act alone but in combination with the related E-Syt3, which exhibited a similar phenotype. Most interestingly, these E-Syt proteins impacted viral entry, as well as cell–cell fusion (syncytia) and viral plaque size (cell-to-cell spread), suggesting they acted on the viral fusion machinery [651]. One possible mechanism of action might involve deregulation of Ca2+ signaling that occurs during HSV-1 infection [652,653], and which could affect E-Syt Ca2+-dependent function [651].
A BioID proteomics approach identified multiple gM partners, with 35% of those being involved in protein transport. XPO6, an exportin, is required for gM to be released from the nucleus to the TGN [654].
Another interesting way that gM is involved in affecting host trafficking is through modulation of tetherin (Figure 3). Tetherin is an effective cellular factor against a variety of enveloped viruses. Its antiviral activity stems from its ability to form a tether between a host membrane and a budding viral envelope, inhibiting the release of budding virions [655]. Tetherin can also target HSV-1, as the overexpression of tetherin led to accumulation of HSV-1 particles to the cell surface, suggesting inhibition of HSV-1 release [656]. HSV-1 counteracts tetherin function through gM, which interacts with tetherin and removes it from the plasma membrane, thus preventing virion tethering to the plasma membrane. This antagonistic effect might be due to gM preventing tetherin reaching the cell surface or relocalizing tetherin away from the plasma membrane [656], perhaps in a similar manner with gD and gH/Gl [643].
6.4. UL11
UL11 is an early expressed 96-aa myristylated and palmitoylated tegument protein [657,658,659] that is not required for HSV-1 replication in cell culture. A UL11-null virus exhibits smaller plaques and displays about one log decrease in progeny virus production [660]. Additionally, the palmitoylation and myristylation of UL11 is not required for viral growth, since a non-myristylated UL11 mutant HSV-1 can rescue the growth of a UL11-null HSV-1 [661]. The palmitoylation of UL11 is required though for association of UL11 with the cytoplasmic faces of Golgi membranes of infected cells [658,660].
UL11 can interact with several viral proteins, including UL16, as observed through immunoprecipitation, mass spectrometry, and yeast-two-hybrid assays [155,662]. There are dileucine and acidic cluster motifs on UL11 that are required for the UL11–UL16 interaction [659], as well as the free cysteines of UL16 [663].
It is not clear what the mechanism of the packaging of UL11 in the tegument of nascent virions is [659]. Tandem affinity purification (TAP) supports that UL11 interacts specifically with the cytoplasmic domain of gD and gE [664]. In the absence of the cytoplasmic tail of gE, virion packaging of UL11 was reduced by 80% [665]. Similarly, gE packaging is reduced 85% in the absence of UL11, as gE packaging requires the UL11 acidic cluster [665]. These data highlight the importance of UL11 in recruiting glycoprotein-enriched membranes for cytoplasmic envelopment of the virus and could have implications for gE/gI-mediated cell-to-cell spread of HSV-1 (Figure 3C,D) [665]. Interestingly, deletion of the gD cytoplasmic domain still allows partial binding of UL11 to the ectodomain of gD, suggesting that UL11 can be highly adherent (“sticky”) [664]. This would support that adherent tegument proteins can support extensive protein–protein interactions, which would mediate the bridging of the viral capsid to the envelope. UL11-null virus infections result in accumulation of unenveloped capsids in the cytoplasm surrounded by electron-dense material (most likely other tegument proteins) [666].
The “stickiness” of UL11 can be explained by its description as an intrinsically disordered protein (IDP). IDPs contain amino acids and elements that cause them to exhibit hallmarks of a disordered structure, such as slower electrophoretic mobility than expected based on length, reduced size exclusion chromatography mobility due to reduced protein compaction, and low proportion of hydrophobic amino acids with a high proportion of charged and hydrophilic amino acids. The result is a protein that cannot fold spontaneously into a stable conformation and fluctuates rapidly through a range of conformations. Such proteins frequently interact and function within protein–protein interaction networks [667]. As a result, UL11 can undergo phase separation in vitro and form biomolecular membrane-less condensates. Such condensates can contain one or many different kinds of proteins. Specific conditions may mediate the formation of condensates by UL11 in cells, such as binding to gE, UL16, or clustering within lipid rafts. Other HSV-1 tegument proteins also have IDP regions, indicating that phase separation may be used for tegument packaging during HSV-1 cytoplasmic envelopment [668].
If one deletes the dileucine and acidic cluster (AC) motifs of UL11, then UL11 increases its association with detergent-resistant membranes (DRMs), which are enriched in cholesterol and sphingolipids. One possibility is that the deletion of dileucine motifs and ACs results in palmitoylation and myristylation to recruit UL11 to DRMs [669].
6.5. UL16
UL16 is an unusual gene because it is contained within the intron of the UL15 gene and is transcribed antisense to the UL15 gene [611]. UL16 has a bewildering number of interactions with gE, UL11, UL21, VP22 (Figure 3) [203], gD, and mitochondria [670]. These interactions are probably regulated temporally but also structurally by different domains of UL16.
UL16 resides on cytoplasmic capsids [671] and participates in a bridging interaction with membrane-bound UL11 [203,662]. This suggests a role for UL16 in HSV-1 cytoplasmic envelopment, which became clear in electron microscopy studies of cells infected with UL16-null HSV-1 [203]. No defects in the transport of capsids to cytoplasmic membranes were observed, but the wrapping of capsids with membranes was delayed. Moreover, clusters of cytoplasmic capsids were observed but only near membranes where they were wrapped, resulting in multiple capsids within a single envelope. Post-envelopment egress does not require UL16, and viruses released in the supernatant were not affected by UL16 ablation [203]. Additionally, less gE and less gD were packaged in UL16-null viruses, which is expected since UL16 interacts with both [672]. These data support a role for UL16 in cytoplasmic envelopment. Cell-to-cell spread is also blocked during UL16-null virus infection, which may be due to mislocalization of gE, since gE and UL16 form a complex [672].
The structural modulation of UL16 interactions became clear in the studies of the UL16/UL11/UL21/gE complex. UL16 directly interacts with UL11, which resides on the cytoplasmic side of the TGN. This interaction requires most of the UL16 sequence except the first 40 aa, and does so in a manner that requires free cysteines on UL16 [663]. Covalent modification of the UL16 free cysteines with N-ethylmaleimide blocks binding to UL11 but not to UL21 [673], suggesting a binding site on UL16 for UL11 and another for UL21 (Figure 3C).
Interestingly, UL16 is released from capsids upon binding of HSV-1 virions to cells, but it is not clear if it maintains its interaction with UL11 [674]. For UL16 to receive a signal from outside the virion, it must interface in some manner with glycoproteins on the surface of the virion. One possibility is through gE, with which UL16 interacts, as discussed above. The N-terminus of UL16 can bind gE but the full length cannot, indicating a possible regulatory effect of the UL16 C-terminus on the UL16–gE interaction [675]. This interaction may have multiple effects, including effects on cytoplasmic envelopment described above. Additionally, since gE is involved in cell-to-cell spread, it may be involved in rearrangements that occur upon binding of virions to cell entry receptors, which results in release of UL16 from the viral capsid [663].
The inconsistency between the N-terminus and the full-length binding of UL16 with gE became clearer when it was shown that UL21 binding to UL16 reveals the UL11-binding free cysteine-requiring site of UL16. Then, UL11 binds to UL16 and this event activates the UL16–gE interaction. Importantly, the function of gE is dependent on UL11, UL16, and UL21, as evidenced by infections with HSV-1 gBsyn mutants that lack UL11, UL16, or UL21. The syncytial phenotype of gBsyn HSV-1 infections requires functional gE, and syncytia cannot form in the absence of UL11, UL16, and UL21. Cell-to-cell spread involves the localization of gE to junctions at the cell surface, and in the absence of UL11, UL16, or UL21, gE cannot localize there. Collectively, these data suggest that these proteins work as a complex during HSV-1 infection [676].
One interesting aspect is the species-specific requirement for the UL16 protein when comparing HSV-1 and HSV-2 [677]. Depletion of UL16 in HSV-2 results in 50- to 100-fold lower viral yields, with defects in both nuclear egress and cytoplasmic envelopment. In contrast, depletion of UL16 in HSV-1 results in a 10-fold replication deficiency and defects in cytoplasmic envelopment of viral capsids. HSV-1 UL16 can promote the nuclear egress of HSV-2 UL16-null mutants, suggesting that HSV-2 lacks an activity that can promote nuclear egress in the absence of UL16, as opposed to HSV-1 [677].
A UL16-null virus is greatly diminished in its ability to package gD. UL16 binds directly to the cytoplasmic tail of gD. If the cytoplasmic tail of gD is removed, UL16 is still packaged into virions [678]. This non-reciprocal interaction suggests that packaging of UL16 on capsids is independent of gD, but recruitment of gD during cytoplasmic envelopment may require UL16.
6.6. UL53 (gK) and UL20
Glycoprotein K (gK) is a highly hydrophobic 338-amino-acid protein that is encoded by the UL53 gene [679]. gK is highly embedded on the membrane [680], which makes its study difficult. Its structure is composed of three or four transmembrane domains, as shown by tag insertions in multiple domains of gK [680].
Deletion of HSV-1 gK results in a small plaque phenotype, lower viral yields, and accumulation of non-enveloped virion particles in the perinuclear space [681,682]. These data suggest that gK has a role in the egress of virus from infected cells (Figure 3). Further work demonstrated that deletion of gK triggers a collapse of the Golgi to the ER, in a manner similar to brefeldin A [680]. Virion entrapment in this perinuclear space may occur due to this Golgi collapse, and it has been suggested that this collapse may be partially due to an antifusogenic role of gK during egress.
The antifusogenic role of gK has been studied extensively. Cell-to-cell transmission of HSV-1 occurs by either release of virions to the extracellular space or virus-induced cell-to-cell fusion. Certain spontaneous mutants of HSV-1 have been found to induce the formation of large multinucleated cells or syncytia. Such mutations have been identified in gK and in other viral glycoproteins, such as gB, but those in gK are more frequently observed [683]. The syncytial gK mutants of HSV-1 have been used in multiple studies to investigate the function of gK during infection.
When gK is expressed outside the context of the infection, it localizes in the ER and the perinuclear space. However, infection of syncytial gK-transfected cells with a gK-null virus triggered expression of gK on the cell surface and cell fusion [680]. Wild-type gK can also inhibit fusion that is triggered by other HSV-1 glycoproteins outside the context of the infection. Co-transfection of the four viral glycoproteins gD, gB, gH, and gL triggers cell-to-cell fusion and leads to syncytia. However, co-expression of wild-type gK with these glycoproteins reduces dramatically the formation of syncytia [684]. Therefore, it was suggested that gK is part of the mechanism through which HSV-1 regulates its own fusogenic activity. The anti-fusogenic activity of gK might prevent fusion of the viral envelope with the membrane of exocytic vesicles as the virus leaves the cell. In a similar manner, it could prevent the collapse of Golgi to the ER that was described above. BFA works by blocking vesicle and protein transport from the ER to the Golgi, and gB could affect Golgi integrity by blocking vesicles feeding into the Golgi from the ER [680].
The antifusogenic role of gK can also be seen during the absence of UL20 in UL20-null virus-infected cells. This virus produced smaller plaques, and electron microscopy of infected cells showed accumulated capsids in the cytoplasm with few enveloped virions inside cytoplasmic vesicles [685]. A gK syncytial mutant in a UL20-null genetic background did not allow cell fusion as seen previously. Additionally, multiple virion capsids within a single envelope were seen in the cytoplasm, further supporting the anti-fusogenic role of gK during viral egress and also indicating an indirect role for UL20 in membrane fusion [685].
The UL20 gene encodes a 222-amino-acid non-glycosylated transmembrane protein that is conserved in all herpesviruses. It was thought that UL20 was essential for virus replication since deleting UL20 prevented replication. However, experiments utilizing 143TK- cell lines indicated cell type-dependent replication of UL20-null HSV-1 (F). Electron microscopy images of this virus in non-permissive Vero cells revealed a profound entrapment of viral particles in the perinuclear space. UL20 is required for intracellular transport and cell surface expression of gK in transient expression experiments, indicating a role in virus-specified glycoprotein trafficking. During infection, UL20 protein is required for gK transport to the surface, which is necessary for virus-induced cell fusion that is caused by syncytial mutations in either gB or gK (i.e., mutations in the gB and gK genes that allow for formation of syncytia) [685].
Additionally, gK is a virion component that is important for virus entry into cells [686]. The role of gK during entry might stem from its interaction with the viral glycoproteins that mediate virus entry. gK forms a functional protein complex with UL20, which is required for gK and UL20-associated functions in the life cycle of HSV-1 [686,687]. Coimmunoprecipitation experiments showed that UL20 forms a complex with gB and gH in infected cells but not with gD [688]. Additionally, gK has a functional amino-terminal domain [689] that can interact with the extracellular portions of gB and gH [688]. These results suggest that the gK/UL20 complex may modulate the fusogenic properties of gB and gH via direct physical interactions. Treatment of virions with a protease that cleaves the gB-binding domain of gK results in reduced infectivity of the treated virions [690], which further supports the presence of gK on the surface of the virion and its role in mediating virus entry. Other data further support that gK is required for proper localization of gD and gH/gL on HSV-1 assembly compartments [691]. Additionally, gK is required for gB binding to Akt during entry into neuroblastoma cells, release of calcium, and fusion of the viral envelope with host membranes. In the absence of the N-terminal functional domain of gK, entry into cells occurs through endocytosis [692]. Virus entry is therefore modulated by gK at multiple levels.
An interesting sequence of clinically relevant papers show the importance of gK in ocular infection with HSV-1 (Figure 4B). While deletion of the N-terminal domain of gK does not affect growth in Vero cells, it reduced cell-to-cell spread. Ocular infection of mice with a mutant HSV-1 that lacks the N-terminus of gK produced no significant ocular disease symptoms, versus infection with a wild-type strain. Additionally, the viral genome could not be amplified from ganglionic neurons that were infected with the mutant versus the wild-type HSV-1 [693]. Therefore, the N-terminus of gK is essential for neuroinvasiveness and herpes keratitis in the mouse ocular model. Work expanding on this paper showed that a virus lacking the N-terminal domain of gK can attach to cell surfaces of Vero cells and ganglionic axons as efficiently as wild-type HSV-1; however, the mutant virus cannot enter into the cytoplasm of ganglionic neurons [692]. These data are in agreement with data showing decreased corneal scarring in ocularly infected mice with a gK mutant virus [694].
6.7. UL21
UL21 is an accessory gene that encodes a 535-aa protein of the tegument. It was reported by Baines et al. to be dispensable for viral replication in cell culture. UL21 promotes the growth of long cellular protrusions when over-expressed in non-neuronal cells and is associated with microtubules [695]. Additionally, UL21 forms a complex with UL11, UL16, UL21, and gE in transfected cells, and is necessary for the UL11–UL16 interaction [676].
UL21 is non-essential, but viral growth kinetics with a UL21-null virus showed that the overall viral yield is lower [696]. Most UL21-interacting proteins were found to be cytoskeletal proteins expressed in the central nervous system, such as the glial fibrillary acidic protein (GFAP). The distribution of GFAP is also altered in UL21-null virus-infected glial cells, when compared to WT-virus-infected cells. These results suggest that UL21 is involved in capsid transport through interacting with cytoskeletal proteins. The altered distribution of GFAP has only been reported in glial cells, so it is not clear if UL21 can affect trafficking in neurons and no follow-up studies are available [696].
Infection with a UL21-null HSV-1 resulted in a delay in the onset of immediate early gene expression. Additionally, a reduced number of capsids were found in the cytoplasm after UL21-null virus infection although DNA-containing capsids were formed in the nucleus [697]. These data suggest that UL21 has an early function that facilitates viral gene expression, as well as a late function that promotes the exit of capsids from the nucleus to the cytoplasm. The early function is supported by the crystal structure of the C-terminal domain of UL21 [698]. Based on these studies, it is shown that UL21 can bind E. coli RNA, which suggests a role for UL21 in transcription or translation, but further work is needed. Regarding the late function of UL21, multiple empty capsids have been observed in the cytoplasm of UL21-null-infected cells [699]. Therefore, it was suggested that UL21 either retains capsids in the nucleus until they receive DNA, and disruption of UL21 allows empty capsids to be transported to the cytoplasm, or that UL21 protects DNA-filled capsids and its absence results in empty capsids in the cytoplasm [699].
6.8. UL24
UL24 is a late viral gene that is expressed as a predominantly nucleus-associated 30 kDa protein [700] but localizes to the cytoplasm as well [701]. It is encoded by mRNAs with two different 5’ ends. The majority of UL24 is encoded by the mRNA that contains the first initiation codon of the ORF [702]. It is unclear why UL24 is transcribed from different sets composing six transcripts [702]. The third initiation codon in the UL24 ORF leads to the expression of a protein termed UL24.5 with a size of 18 kDa [703]. A UL24.5-null HSV-1 exhibits viral growth similar to a WT virus but does not trigger dispersal of nucleolar proteins as WT [703].
A UL24-null virus yields mildly lower titers in cells and slightly smaller plaque sizes. Corneal infection in mice with a UL24-null virus results in 1 log lower viral load versus WT, but there is a 4 logs lower viral growth in the trigeminal ganglia. These data suggest that UL24 is important for the dissemination of HSV-1 from the cornea to the trigeminal ganglia in mice (Figure 4A) [700,704]. UL24 may be important for virulence in murine and guinea pig models of intravaginal infection with HSV-2; however, the similarities between HSV-1 and HSV-2 UL24 are difficult to assess [705].
UL24 is one of four genes that when mutated can confer a syncytial (syn) phenotype [701]. It is not known how mutations in the UL24 gene confer syncytia. Mechanistically, lack of UL24 during late infection results in mislocalization of gB and gD with respect to actin [701], which are proteins involved in fusion. UL24 mutations that confer syncytia may work through the effect of UL24 on the localization of these fusogenic viral glycoproteins.
UL24 was found through bioinformatics to contain PD-(D/E)XK endonuclease signature sequences [706]. These sequences are required for the dispersal of nucleolin that occurs during infection, since their deletion or mutagenesis prevents nucleolin dispersal that occurs normally during infection. This suggests that UL24 is involved in nucleolin dispersal through its endonuclease motif [707,708]. Mutating the endonuclease motif also causes one log lower viral growth in the eye and the trigeminal ganglia of an ocular mouse model, indicating that the effect of UL24 endonuclease function is involved in dissemination of HSV-1 from the eye to the ganglia in vivo [709]. Another nucleolar component that is dispersed due to the endonuclease function of UL24 is the B23 nucleolar protein [710], which is a multifunctional protein that participates in ribosome biogenesis, mRNA processing, chromatin remodeling, and maintains genome stability [711]. UL24 also mediates nuclear egress of HSV-1 nucleocapsids and this effect most likely occurs through the abovementioned effect of UL24 on dispersion of nucleolar proteins [712].
UL24 may also play a role in immune evasion, through a function unrelated to its endonuclease motif [713]. Exogenous UL24 can bind with the p65 and p50 components of NF-κB and prevent their translocation to the nucleus. Therefore, it impairs the production of IFN-β and pro-inflammatory chemokines and may mediate immune evasion during HSV-1 infection [713].
6.9. UL31 and UL34
pUL34 is a type 2 integral membrane protein with a 247-aa nucleoplasmic domain that binds pUL31 and holds it in close approximation to the inner nuclear membrane (INM) [714,715]. UL34 is anchored to the INM by a C-terminal transmembrane helix, with several residues extending into the perinuclear space [714]. UL34 retention at the INM requires the presence of UL31 [716], and both UL34 and UL31 localization is dependent on their co-expression and interaction [562]. UL31 and UL34 form a complex, which has been termed the nuclear egress complex (NEC), and it is required for efficient exit of nascent HSV-1 capsids from the nucleus (Figure 3A).
Deletion of UL31 results in 3–4 logs lower viral yields compared to WT HSV-1, slightly decreased levels of total viral DNA, and a 3–5-fold reduction in the ratio of monomeric to concatemeric DNA, suggesting minor roles in both DNA replication and processing or packaging of viral DNA [717].
Deletion of UL34 results in 2–5 logs lower yields of HSV-1 in cell culture. While a UL34-null virus can assemble DNA-containing capsids, they accumulate in the nucleus and are unable to bud through the inner nuclear membrane [718].
The role of UL31 and UL34 initiates with the disruption of nuclear lamina during HSV-1 replication. Formation of HSV-1 replication compartments (RCs) and annexation of space in the nucleus results in cellular chromatin marginalization and compression [719]. The phase of chromatin marginalization occurs during the initial phase of RC formation, and this does not require UL31 and UL34. However, later during infection, RCs penetrate the host chromatin and the nuclear lamina, and reach a region of the nucleus close to the INM. In co-transfection experiments of UL31 and UL34, marginalization of host chromatin was not observed; however, during infection, both UL31 and UL34 are required for alteration of the distribution of lamina components [720], which suggests that other viral proteins cooperate with NEC for lamina disruption. Nonetheless, UL34 can interact directly with lamin A/C in vitro [721]. This disruption of lamina is a regulated process during infection since the viral protein US3 is involved [557,721,722]. The kinase activity of US3 was not necessary for the redistribution and disruption of lamin A/C or lamin B [559], indicating that US3 spatial interaction with NEC may be the regulatory mechanism. However, US3 phosphorylates lamin A/C during HSV-1 infection [558].
The N-terminus of pUL31 also harbors multiple phosphorylation sites of the viral US3 kinase. Preventing the phosphorylation of pUL31 mimics the growth defect of a US3-null virus, with 1-2 logs lower viral yields. The importance of the N-terminus and its phosphorylation is highlighted by the following observations: First, pUL31 that lacks the N-terminus is retained in the cytoplasm if co-expressed with UL34, suggesting that they prematurely interact before they enter the nucleus. This is probably why UL31 and UL34 utilize different transport routes to the nucleus, averting their premature interaction. Second, the phosphorylation of the N-terminus of pUL31 is necessary for the proper localization of the pUL31/pUL34 complex in the nuclear rim and the optimal egress of virions from the perinuclear space [560]. UL31 and UL34 distribution is even across the nuclear rim, but this requires US3 expression [562]. In the absence of US3, UL31 and UL34 localize in small punctate areas at the nuclear rim. This supports that UL31 and UL34 form a complex that accumulates at the nuclear membrane and plays an important role in HSV-1 nucleocapsid envelopment at the inner nuclear membrane. Absence of US3 causes accumulation of capsids in nuclear membrane invaginations, delayed onset of virus production, and reduced virus titers [556]. UL31 and UL34 associate with perinuclear virions but not with extracellular virions, supporting the de-envelopment/re-envelopment model of viral egress [556].
NEC by itself is sufficient to drive the vesiculation of the nuclear envelope in transfected cells in the absence of any other viral proteins [723,724,725]. Using purified HSV-1 NEC components and synthetic liposomes, it was shown that NEC has an intrinsic ability to vesiculate membranes in vitro [726]. NEC formed a coat-like hexagonal lattice on the inner surface of the budded vesicles, which suggested that it vesiculated membranes without the help of other proteins by creating a hexagonal scaffold inside the bud [727]. Further structural characterization showed that HSV-1 UL31 and UL34 form the NEC heterodimer through extensive interactions that involve residues distributed throughout UL31 and UL34. The heterodimers are further organized to form oligomeric structures. Mutagenesis of their oligomeric interfaces reduced NEC-mediated budding in vitro, supporting that NEC oligomerization drives capsid budding during nuclear egress of herpesviruses [726]. However, other cellular and viral factors may still participate in NEC oligomerization and nuclear budding in cells, such as US3 mentioned above.
Besides NEC oligomerization on the INM, a host factor mediating primary envelopment of HSV-1 is the endosomal sorting complex required for transport-III (ESCRT-III). ESCRT-III promotes primary envelopment by mediating scission during HSV-1 budding through the INM [728]. UL34 interacts with ALIX but not other ESCRT-III proteins, suggesting that ALIX acts as an adaptor for the recruitment of ESCRT-III proteins by UL34, resulting in scission of the budding vesicles formed by the NEC. The mechanism of fusion of the primary envelope with the outer nuclear membrane is still not clear.
UL31 also functions in another interesting manner in order to conserve viral resources. Three major types of HSV-1 capsids have been described, the empty capsids (A capsids), capsids that lack viral DNA (B capsids), and viral DNA-containing capsids (C capsids) [729]. Type C capsids are preferentially selected compared to A and B to undergo primary envelopment at the INM, and the mechanism of their selective involvement involves UL31, UL17, and UL25. UL17 and UL25 interact and form a stable complex. The different types of capsids contain different copies of this complex. C capsids contain 75 copies, while B capsids contain 25 copies [730]. Because of its enrichment in C capsids, the UL25/UL17 complex is termed C capsid-specific complex (CCSC). While it is possible that the CCSC binds more efficiently to C capsids, an interaction has been identified between UL31 and CCSC in infected cells [731]. This supports a model of egress in which the CCSC is added to capsids after DNA is inserted and engages UL31 either in the nucleus, or within the NEC at the INM. The end result is an elegant way to conserve cellular resources by selecting only capsids that have the potential to produce infectious virions for primary envelopment [731].
6.10. UL35 (VP26)
The HSV-1 UL35 gene encodes for a 12-kDa capsid protein designated VP26, which is located on the outer surface of the viral capsid, on the tips of the hexons that constitute the capsid shell [732]. The HSV-1 capsid has an icosahedral structure, and the major capsid protein is VP5. VP5 forms both the pentons and the hexons of the capsid, which are composed of five or six VP5 monomers [733]. Pentons are located at the icosahedral vertices, while hexons form the faces and the edges of the capsid structure. VP26 is attached to VP5 molecules that make up the hexons. The C-terminus of VP26 interacts with the upper domain (UD) of VP5 [734], and their interaction has been well characterized [734,735]. VP5 and VP26 interaction is required for localization of VP26 to the sites of capsid assembly in the nucleus [736]. However, in vitro reconstitution of HSV-1 capsids showed that VP26 is not required for proper capsid assembly [732,737].
These data agree with reports that show that VP26 is non-essential for viral growth in vitro [738] but influences the production of infectious virus in vivo. In a mouse ocular infection, a UL35-null virus yields 2-fold less virus in the eye but 30–100-fold less virus in the trigeminal ganglia (Figure 4A). VP26 does not seem to affect the transport of the virus from the eye to the ganglia but is important for the replication of the virus in the ganglia [738]. Similar results have shown in vitro that a UL35-null virus exhibits reduced viral yields when cultured in neuroblastoma cell lines. A potential reason is mislocalization of the main capsid protein VP5, which exhibits a punctate distribution during UL35-null virus infection as opposed to a diffuse distribution during a WT infection [739].
Another way that VP26 can affect replication is by mediating incorporation of UL25 into nucleocapsids and by extension affecting DNA packaging. This is because UL25 is part of the 3-component viral terminase complex, which transports the HSV-1 genome into the viral capsid [740]. This is supported by yeast-two-hybrid data that show interaction of VP26 and UL25 [735].
VP26 may also affect capsid delivery to the nucleus following entry of the virus into the cells by interacting with the dynein light-chain subunits DYLNT1 and DYLNT3. Cytoplasmic dynein is a molecular motor that is associated with microtubules, and each dynein complex contains two copies of either DYLNT1 or DYLNT3. VP26, DYLNT1 and DYLNT3 colocalize with microtubules, and VP26 is required for migration of HSV-1 capsids towards the nucleus [741]. These data suggest that VP26 is important for the retrograde transport of HSV-1 capsids from the plasma membrane towards the nuclear membrane after viral entry into cells. Further work described the N-terminus of VP26 as a binding region for the dynein light-chain subunits DYNLT1 and DYNLT3 [742].
6.11. UL43
UL43 has a size of about 32 kDa and based on its sequence, it may contain seven transmembrane domains composed almost entirely of alpha helixes [743]. UL43 is not present in mature extracellular virions [744]. Other functions remain unknown.
6.12. UL44 (gC)
gC is a 511-aa type I integral membrane glycoprotein that mediates HSV-1 attachment to host cell surface glycosaminoglycans. Absence of gC results in reduced binding of virus to cells, although the virus that binds can enter cells and initiate infection [745]. gC can bind to both heparin and heparan sulfate. The binding that occurs in the absence of gC is dependent on cell surface heparan sulfate [745,746]. It was shown that two areas of gC participate in heparan sulfate binding (R143, R145, R147, T150, G247). Synthetic peptides that corresponded to these two areas prevented virus binding and entry in cells, and they also agglutinated red blood cells [747]. These data suggest that these gC areas mediate the binding of virus on cell surface heparan sulfate.
Additionally, gC can regulate cell entry and infection by a low-pH pathway [748]. The presence of gC confers a higher pH threshold for acid-induced changes in gB, affecting fusion. Using a gC-null virus, it was found that there was a delay in entry relative to WT HSV-1 [745]. A research group tested infection with HSV-1 and gC-null HSV-1 on different cell lines. They observed that a gC-null virus displayed different infectivity, depending on whether these cell lines support low-pH or pH-neutral entry. Treatment with ammonium chloride did not affect gC-null HSV-1 entry into cells that support the pH-neutral pathway, suggesting that gC is dispensable for that pathway. When they assessed for infectious virus after infection in a low-pH environment, gC-null HSV-1 was lagging in intracellular transport or release from intracellular vesicles formed after endocytic entry. After treating HSV-1 ΔgC versus WT HSV-1 virions with different pH solutions, it was shown that the presence of gC increases the pH at which fusogenic conformational change of gB occurs [748].
6.13. UL45
UL45 is a late gene [749] that encodes an 18-kDa protein that is present in virions and is enriched in the envelope–tegument interface, thus associated with the viral envelope [749]. While it is non-essential for viral growth in vitro [750], it is required for efficient growth in the central nervous system (CNS) of mice when inoculating with a low viral dose [751].
UL45 is required for syncytia formation during infection with a gB mutant HSV-1 that causes syncytia (gBsyn) [752]. A UL45 C-terminus truncation prevents the formation of syncytia with gBsyn [753], suggesting that UL45 affects entry that requires gB function. However, UL45 plays a dispensable role in virus entry to cells either through pH-dependent endocytosis or pH-independent mechanisms [754].
6.14. UL55 and UL56
The UL56 gene product is a C-terminal-anchored type II membrane protein conserved among HSV-1, HSV-2, and herpes B virus. Even though UL56 is dispensable for viral growth in cultured cells, it plays an important role in the pathogenicity of HSV-1.
HSV-1 mutants lacking UL56 are substantially less pathogenic in mice but have similar growth in cell culture [755]. The role of UL56 in the severity of HSV-1 ocular disease can be seen in work based on a quantitative trait locus (QTL)-based assay, which involved infecting mice with 40 recombinant strains derived from mice infected simultaneously with two avirulent strains. Phenotypically meaningful variations could be seen in multiple genes, including UL56, whose features were associated with an increase in ocular virulence in mice [756]. However, UL55 and UL56 do not appear to have a role in the latent stage of the virus since mice that were infected with HSV-1 lacking UL55 and UL56 could still develop a latent infection [757]. Furthermore, HSV-1 mutants lacking the entire UL56 gene have been found in human samples [758] and are considered to be less pathogenic or to lack neurovirulence [755,758]. Therefore, UL56 is non-essential in cell culture, and is not implicated in latency but is important for pathogenicity in vivo.
UL56 contains a hydrophobic domain in its carboxyl-terminal tail (aa 217–234) which is embedded in the membrane, and its deletion results in reduced pathogenicity as it generates an avirulent HSV-1 strain [759]. Characterization of UL56 through immunofluorescence studies showed that UL56 localized to the Golgi and cytoplasmic vesicles in UL56-transfected or HSV-2-infected cells. The C-terminal domain is important for association with cytoplasmic membranes and the N-terminal is important for its translocation to the Golgi and the cytoplasmic vesicles. Protease digestion assays combined with fractionation through sucrose gradients suggested that UL56 is a type II membrane protein associated with lipid rafts. These data suggest that UL56 may be involved in vesicular trafficking in HSV-2-infected cells. Expanding on that work, an interaction between UL11 and UL56 was identified [760], suggesting a complex that may be involved in the cytoplasmic envelopment of HSV.
Ushijima et al. in a series of papers investigated the role of UL56 during HSV-2 infection. They first demonstrated that UL56 interacts through its PY motifs with Nedd4, an E3 ubiquitin ligase. UL56 triggered increased NEDD4 ubiquitination and its subsequent degradation during infection [761]. Additionally, they investigated potential co-localization of UL56 with Nedd4 and they found that UL56 localizes to the TGN and early endosomes but not with CD63 in late endosomes. Co-localization of UL56 with Nedd4 was observed at the TGN, but a lack of co-localization with CD63 suggested that HSV-2 is not using MVBs for cytoplasmic envelopment. Nonetheless, deletion of UL56 restricted infectious HSV-2 release. Therefore, Ushijima suggested that UL56 functions in coordination with other host or viral factors in trafficking and membrane sorting. Nedd4 may be among these factors, and this function of UL56 may be redundant with other viral proteins that have sorting functions and may also depend on the cell type [762].
Ushijima et al. in 2010 showed how UL56 interacts with Itch, which is another Nedd4-family ligase, triggering its degradation through lysosomes. Interestingly, HSV-1 does not degrade Nedd4, but it does degrade Itch [763].
6.15. US2
The US2 gene of HSV is predicted to encode a 291-aa protein of 33 kDa. It is predicted to have a hydrophobic N-terminus [764]; it is non-essential in cell culture and not involved in the pathogenesis in the CNS of mice [765]. HSV-1 Us2 is not associated with any specific phenotype [766]; however, most research so far has been done on HSV-2 Us2. Initially, US2 was observed as discrete granules late during infection within and at the periphery of the nucleus [767]. However, further analysis of US2 by immunofluorescence microscopy of infected Vero and A431 cells detected a filamentous-like cytoplasmic pattern [768]. Additional data suggested interaction of US2 with cytokeratin 18 through yeast-two-hybrid assays, and confirmed the interaction by co-immunoprecipitation, which seems to involve the N-terminus of US2 [768]. Other HSV gene products can also interact with proteins of the cytoskeleton [769,770,771]. US2 and cytokeratin 18 interaction suggests participation of US2 in trafficking during infection, but more work is needed to characterize this function.
It was later shown that HSV-2 US2 is a membrane-associated ubiquitin-interacting protein [772]. HSV-2 US2 lacks specific membrane sorting signals, and can be found at the plasma membrane, in cytoplasmic vesicles, and diffusely throughout the cytoplasm. Through a discontinuous gradient, US2 can be detected in detergent-resistant membranes, and cofractionates with caveolin-1 and ganglioside GM1. Treatment of infected cells with BFA (brefeldin A) did not affect the localization of US2, suggesting that it is not part of the ER-Golgi secretory pathway [772]. Co-localization experiments showed that US2 localizes predominantly to recycling endosomes and the plasma membrane, but the resistance to BFA treatment suggests that this localization of US2 is regulated post-translationally. Through mass spectrometry, most US2-interacting proteins were shown to be ubiquitinated. US2 could be pulled down by mono-ubiquitin conjugated agarose but not by protein G agarose, suggesting that US2 interacts specifically with ubiquitin and not with ubiquitin-conjugated proteins [772].
Lu et al. in 2017 showed that HSV-2 US2 could activate NF-κB signaling. Deficiencies in US2 decreased HSV-2 WT-mediated NF-κB activation and cytokine and chemokine production, while overexpression of US2 produced the opposite effects. Co-immunoprecipitations suggested that US2 interacts with TGF-β-activated kinase 1 (TAK1). US2 induced the phosphorylation of TAK1, resulting in the activation of TAK1-mediated downstream signaling. This role of US2 in NF-κB activation was confirmed in mice. Interestingly, HSV-1 US2 did not activate NF-κB like HSV-2 US2 [773].
6.16. US4 (gG)
Glycoprotein G (gG) is one of the least well-characterized glycoproteins of HSV-1. Infections with gG-null HSV-1 exhibit similar growth to WT virus and little attenuation in vivo [774]. When focusing on polarized epithelial cells though, gG seems to be required for infection through the apical surface. However, a gG-null virus can still infect these cells through the basal membranes and replicate normally. In vivo infection of apical surfaces of mouse corneas with a gG-null HSV-1 results in delayed scarification, but once scarification occurs, a gG-null virus has yields similar to wild-type virus [775].
An interesting observation is that inoculation of mice with a baculovirus recombinant vector carrying the gG ORF results in partial protection from lethal challenge with intraperitoneally injected HSV-1 [776,777]. This protection was not observed when using vaccinia virus as a vector [778]. This might be a result of higher expression of gG in the baculovirus system, or more intriguingly, gG in insect cells of the baculovirus system may be glycosylated in a different pattern that increases gG immunogenicity. However, this protection does not apply to corneal infection with HSV-1 after vaccination with the same gG-containing baculovirus vector [779].
The most interesting role of gG regards its interplay with chemokines. Chemokines are chemotactic cytokines that coordinate the recruitment of immune cells to infection sites, thus are important for the outcome of a viral infection [780]. Mice that are depleted of chemokine ligands or receptors are highly susceptible to genital herpes infection and neuroinvasion of the CNS due to defective leukocyte mobilization to the infected mucosa [781].
HSV-1 gG localizes on the plasma membrane [782], and it can bind chemokines with high affinity [783,784]. Binding of HSV-1 gG to chemokines while being on the plasma membrane of infected cells occurs through the glycosaminoglycan (GAG)-binding domain of the chemokine, which is required for binding to gG. Interestingly, binding of gG to chemokines does not inhibit chemokine function, rather increases it both in vitro and in vivo. Experiments show that higher migration of leukocytes occurs in the presence of gG on HSV-1-infected cells, due to increased chemokine binding to its specific receptor and downstream MAPK signaling mediated by the surface gG. It is possible that gG acts as a GAG and mediates a local increase of chemokine concentration in parts of the membrane. This will increase chemokine signaling, which will be beneficial for the virus in a number of ways [783]. First, it is possible that chemokine deregulation due to binding to gG promotes viral dissemination through MAPK signaling and NF-κB activation, which enhances viral replication [785]. An alternative hypothesis is that the increased infiltration of leukocytes to sites of infection increases the number of available cells that can then be infected by HSV-1, thus helping the virus spread in vivo [783]. The increased leukocyte migration hypothesis may still occur even through chemokines binding to HSV-1 particles, since gG is also present on the viral envelope [782,786].
6.17. US5 (gJ)
US5 is a late gene that encodes the glycoprotein J (gJ) of HSV-1. gJ localizes to multiple membrane compartments and has been little characterized [787]. gJ is another glycoprotein that mediates protection from CTL killing of infected cells. CTLs kill targets in part by inducing apoptosis either though activation of the Fas pathway and downstream activation of caspases or by releasing lytic granules that contain granzyme B, which can induce apoptosis by cleaving caspases in target cells. gJ can inhibit both pathways; however, other viral genes can compensate for deletion of gJ [788]. gJ can also bind the F0F1ATPase synthase in the mitochondrial membrane that is required for induction of ROS, which suggests that gJ may inhibit F0F1ATPase function [788].
6.18. gE/gI (US8/US7), US9
Glycoprotein E (gE) was first described as a receptor for the Fc portion of immunoglobulin G and for its role in virus spread from cell to cell [789]. Glycoprotein I (gI) is the other polypeptide of the gE/gI complex [789], and like gE, it contains a 400-aa extracellular (ET) domain and a 100-aa cytoplasmic (CT) domain. Since the majority of gE is bound to gI, these proteins are often studied in tandem.
gI can increase the affinity of gE to IgG. gE and gI mutants exhibit a small plaque phenotype in vitro compared to WT HSV-1 [774]. The number of plaques detected is not affected, suggesting that gE and gI regulate cell-to-cell spread in vitro [790]. Expression of gE/gI in human epithelial cells resulted in the localization of gE/gI at lateral surfaces of cells and colocalization with the adherens junction marker β-catenin. At subconfluent monolayers during infection, gE/gI localize at the parts of the plasma membrane that are in contact with another cell [791]. Therefore gE/gI seems to mediate cell-to-cell spread of HSV-1 across cell junctions by interacting with cell junction components (Figure 3D).
Normally, HSV-1 particles are sorted to cell junctions, whereas few virions reach the apical surfaces of polarized epithelial cells [792]. Deleting gE results in HSV-1 virions that cannot translocate to cell junctions and they leave the cell through the apical surface [792]. Work that has characterized a panel of gE mutant viruses with small insertions in the ET domain underlined the importance of this domain of gE for cell-to-cell spread. Several of these gE ET mutant proteins were able to complex with gI and be incorporated into virions, but the formed virions behaved similarly to gE-null mutants. This suggests that gE/gI promotes HSV-1 cell-to-cell spread by binding either extracellular ligands through the gE ET or components of cell junctions (Figure 3D).
Antibodies specific for HSV-1 antigens can simultaneously bind at the surface of infected cells to gE/gI via their Fc region and to a cell surface HSV-1 antigen by their antigen-binding fragments (Fabs) [793,794,795]. This process is known as antibody bipolar bridging (ABB), and may be a strategy to prevent the host from utilizing anti-HSV-1 antibody responses.
HSV-1 gE mutants show decreased neurovirulence [796], and the Fc binding portion of gE was shown to be important in vivo [797]. Introducing insertions in HSV-1 either inside or outside the Fc binding portion of gE resulted in lower yields only for the Fc domain mutant, when testing the two mutants in mice. Additionally, the Fc-binding gE mutant virus was impaired in its ability to reach the ganglia (Figure 4A) [797]. HSV-1 gE/gI-null mutants also show significantly reduced spread in the corneal epithelium of infected mice, due to the reduced ability of these mutants to undergo anterograde transport from sensory ganglia back to the cornea [798].
A major factor for the decreased neurovirulence and cornea infection of gE mutants is the less efficient anterograde transport (Figure 4A). Anterograde transport in infected neurons (e.g., after reactivation of virus from latency) involves the transport of viral particles from the neuronal-cell body along axons to axonal termini, and transfer across junctions formed between neurons and epithelial cells. gE and gI mutants displayed markedly reduced anterograde spread between neurons within the retina and from the retina to retinorecipient regions of the brain [799]. HSV-1 gE/gI and US9 possess overlapping or additive effects in anterograde axonal transport. Mutants lacking gE, gI, or US9 displayed significantly reduced transport of capsids and glycoproteins towards axonal termini, while concomitant deletion of gE and US9 produced nearly zero levels of capsids and glycoproteins even in proximal axons [800,801]. Investigation of HSV-1 infection using a mouse retina model concluded that HSV-1 US9 is required for the transport of capsids, but not viral glycoproteins, from the retina into the optic nerve (Figure 4D) [802]. US9 is a viral tegument protein [803] that has been found associated with the ER and the Golgi [802], and with unenveloped capsids. It is tail anchored, has no ectodomain, and contains a cytoplasmic domain with TGN localization signals, which are important for its function [804,805].
The fact that gE and US9 HSV-1 mutant viruses accumulate in the cytoplasm and do not enter the axons suggests either a trafficking issue or defective virion envelopment. Further characterization of the assembly of those mutants showed accumulation of unenveloped capsids in the cytoplasm of the infected cells and few enveloped virions. Both cannot enter axons in neuronal cells. Additionally, most capsids produced from gE and Us9 mutant viruses remained adhered to, or nested up against, membranes in the cytoplasm [806]. Considering that gE/gI and US9 accumulate in the TGN (a site of virus assembly), and that the gE/gI complex sorts virus particles to epithelial cell junctions [792], the loss of gE/gI and US9 might lead to misrouting of HSV-1 capsids and virions so that they do not enter axons. Alternatively, the defective cytoplasmic envelopment might also inhibit anterograde transport. These defects may depend both on gE and US9 since US9 has been shown to colocalize with capsids and not glycoproteins, whereas gE/gI colocalized with glycoproteins and not capsids [800].
It was also reported that gI can induce the formation of rod-shaped structures [807]. About 40% of gI-transfected cells expressed rod-shaped structures in the cytoplasm, besides the typical gI localization patterns (nuclear rim and cytoplasmic speckles, and junctions). The rods themselves vary in width and length. By doing immunofluorescence analysis using antibodies against different domains of gI, it was shown that the aa 110–202 of gI in the rod-shaped structures are not exposed. Since gE interacts with the aa 128–145 of gI, it will not interact with the gI of the rod-shaped structures, suggesting that gE is important for the proper function of gI. Two proline residues have been implicated in the induction of the gI rods, but they are not required for viral replication. However, these residues are important for mediating syncytia formation during infection with a UL24 mutant virus [807]. Thus, the coordination between gI and UL24 may be important for viral cell-to-cell spread and pathogenesis.
6.19. US8.5
The US8.5 gene overlaps with parts of the US8 and the US9 gene [808]. Its transcription is initiated within the coding sequence of US8 and it is transcribed earlier than US8, while the US8.5 transcript is co-terminal with the transcripts of US8 and US9 [809]. The US8.5 protein localizes in the nucleoli, but its function remains unknown.
6.20. US10
The US10 gene encodes a polypeptide of 313 aa [764] that was identified as a capsid/tegument-associated protein localizing to nuclei as foci late during infection [810]. Deletion of US9, 10, 11, or 12 has no effect on the neurovirulence and latency/reactivation potential of HSV-1, but it affects its neuroinvasiveness from peripheral sites to the CNS [810]. Fractionation studies show US10 tightly associating with the nuclear matrix. Analysis of isolated intracellular capsids showed that both phosphorylated and unphosphorylated forms of US10 were associated with the capsid/tegument. The nature of this association remains to be determined.
6.21. US11
US11 was identified as a late gene since it was shown that its expression required DNA replication [811]. Early work described the RNA-binding activity of US11 to an in vitro RNA transcript of HSV-1 [812], which was later shown to be the UL34 transcript. US11 binding to UL34 mRNA prevented its accumulation [813]. Expression of US11 in baby hamster kidney cells prevented HSV-1 infection through a gD-mediated step, but this effect was not clarified [814]. Simonin et al. in 1995 [815] showed US11 is phosphorylated independently of viral genome expression, by host kinases. The first work that connected US11 and PKR showed that US11 can bind protein kinase R in vitro and preclude the phosphorylation of eIF-2α [319]. Work by the same group showed that US11 and PKR interact in the context of viral infection and this interaction is RNA dependent (Figure 2D) [816].
Us11 is an abundant tegument protein of the virus that is released in the cells during virus entry. It can later be found in ribonucleoprotein fibrils, clusters of interchromatin granules, and in nucleoli [817]. Importantly, US11 distribution in the nucleus follows that of nucleolin. Additionally, nuclear egress of HSV-1 capsids requires nucleolin, and US11 and nucleolin associate during infection. The polyproline type II helix-containing domain of US11 is required for this interaction, and this domain is also responsible for US11 nucleolar accumulation. Nucleolin is involved in nucleocytoplasmic shuttling and US11 accumulates in the nucleolus in its absence [817]. These data suggest that nucleolin could regulate the nucleocytoplasmic shuttling of US11 during infection.
Interesting work showed that US11 protein can bind HTLV-1 and HIV-1 responsive elements and can transactivate envelope retroviral glycoprotein expression by binding to the Rex-responsive element (RexRE), which is located in the 3’ untranslated region (UTR) of the HTLV-1 env mRNA [818]. Such data raise the possibility of in vivo interactions between herpes virus and human retroviruses, possibly affecting the expression of each virus at the post-transcriptional level. Further work showed how US11 binds to HSV-1 mRNAs, such as the HSV-1 UL34 mRNA (its natural target), which results in its accumulation and might affect its trafficking. Two different US11 domains were described, a C-terminal RNA-binding domain and an N-terminal effector domain, the deletion of which created a trans-dominant negative mutant [819].
More work on the binding partners of US11 identified binding between the RNA-binding domain of US11 and a 600-bp RNA sequence that is present in the co-terminal HSV-1 mRNAs UL12, UL13, and UL14 [600]. US11 downregulates expression of the UL13 protein kinase at early times during infection [600]. UL13 is expressed with late gene kinetics but is also a component of the tegument. In the absence of UL13, there is a decrease in the accumulation of a subset of late mRNAs. US11 might downregulate UL13 during early infection to stall the accumulation of the late mRNAs. These data further support the post-transcriptional control that US11 applies to viral transcripts.
The subject of multiple studies has been the interaction of US11 with PKR. The PKR kinase is an RNA sensor that upon sensing viral RNAs, phosphorylates the alpha subunit of the translation initiation factor eukaryotic initiation factor 2 (eIF2α) and thereby inhibits protein synthesis. The viral proteins γ134.5 and US11 prevent the accumulation of phosphorylated eIF2α and consequently the translational shutoff. Particularly, the γ134.5 protein directs protein phosphatase 1α to dephosphorylate eIF2α, reversing the effects of PKR activation (Figure 3D). Us11 when expressed under an immediate early promoter can rescue the growth of a γ134.5-null virus. This requires a 68-amino-acid fragment of US11, which contains the RNA binding domain and was found to be sufficient for preventing PKR activation, thereby allowing protein synthesis and rescuing the growth of γ134.5 mutant viruses [820]. This 68-aa domain of US11 can also inhibit activation of PKR in a cell-free system, supporting the RNA binding function that has been ascribed to US11. Through its interaction with PKR, US11 can also inhibit autophagy activation by dsRNAs in a Beclin-1-independent manner [821].
The protein activator of PKR (PACT) is another restriction factor for HSV-1 as HSV-1-induced interferon production in murine cells was inhibited in the absence of PACT. Binding of PACT to PKR is a dsRNA-independent mechanism of activation of PKR. PACT-mediated PKR activation occurs only under cellular stress, such as withdrawal of growth factors or treatment with a low dose of actinomycin D [822]. US11 can prevent PACT-mediated PKR activation by binding to the dimerization domain (DD) of PKR. This allows binding of PACT to PKR but prevents the conformational change of PKR that is normally induced by PACT and activates PKR [823]. US11 was found to bind both to PKR and to PACT, but only its binding to PKR was essential for preventing PACT from activating PKR, although binding of PACT to PKR was not prevented [824]. Further work on the binding of US11 to PACT suggested that it might also prevent PACT-mediated activation of RIG-I (Figure 2D) [824].
US11 also has a proviral role through inhibiting the synthesis of 2’-5’ oligoadenylate by the oligoadenylate synthase (2’-5’-OAS) (Figure 2D). 2’-5’-OAS is another dsRNA sensor that acts to block protein synthesis and decreases RNA stability in virus-infected cells. Following RNA binding, 2’-5’-OAS can synthesize 2’-5’ oligoadenylates (OA) from ATP that activate RNase L. Subsequently, RNase L cleaves mRNAs and rRNAs, inhibiting virus infection. HSV-1 can inhibit OA synthesis in IFN-stimulated primary human cells through the action of US11. This inhibition requires the RNA-binding motif of US11, which suggests that the mechanism involves partitioning of RNAs during infection [825].
US11 can also counteract type I IFN activation by binding to Hsp90 and preventing TBK1 binding (Figure 2B). Consequently, downstream IRF3 phosphorylation and IFN induction are inhibited [826]. Further work from the same research group showed that US11 also binds to the tripartite motif protein 23 (TRIM23), which is a key regulator of autophagy-mediated antiviral defense mediated by TBK1. The formation of autophagosomes mediated by TRIM23 or TBK1 is reduced by US11 in infected cells, through the exclusion of TBK1 from the TRIM23 complex in infected cells, in mouse embryonic fibroblasts (Figure 2C) [826]. It should be noted, however, that the exclusion of TBK1 might depend on cell type, especially since TBK1 is implicated in human fibroblasts in the modulation of autophagy [827].
US11 is also involved in anterograde transport of HSV-1 in dorsal root ganglia (DRG). Kinesin is a microtubule-dependent molecular motor in cells that is used for transport of unenveloped HSV-1 nucleocapsids. US11 was identified as a kinesin-binding protein, and an interaction between US11 and the heavy chain of kinesin (uKHC) was described [828]. The 20 to 24 RXP repeats in the carboxy half of US11 that bind RNA are also where uKHC can bind. This polyproline domain may acquire a type II helix conformation with arginine residues on one side that interact with the negatively charged RNA. The other side contains hydrophobic, uncharged, or acidic chains that may provide specificity to the RNA binding and contain the uKHC binding site [829]. Another US11 binding protein is the cellular PAT1 polypeptide, which binds microtubules, is involved in the intracellular trafficking of amyloid precursor protein (APP), and contains a region homologous to kinesin light chain (KLC). The US11–PAT1 interaction also requires the carboxy-terminal RNA-binding domain of US11 [830]. This association of US11 with another molecular motor-associated protein further suggests a role for US11 in trafficking of unenveloped capsids.
The effect of US11 on neurovirulence has been further investigated in vitro and in vivo [831]. Intracranial infection of mice with a US11-null virus is pathologically like a wild-type infection. In contrast, corneal infection with a US11-null virus requires a longer time for onset of morbidity, indicating a role for US11 in neuroinvasion. Replication in trigeminal ganglia and periocular tissue was mediated by US11. However, US11 deletion does not affect latency and the frequency of reactivation from trigeminal ganglia, even though the US11-null virus reemerges with slightly slower kinetics [831].
A possible role of US11 in viral dissemination may stem from its packing in extracellular vesicles (EVs). We have detected US11 in ESCRT+ EVs that have been isolated from cells infected with HSV-1 [225]. EVs that contain US11 may reach neighboring uninfected cells and alter their status in order to regulate viral dissemination.
7. Conclusions
HSV-1 encodes a large set of genes that regulate different facets of the virus life cycle, such as virus entry, viral gene transcription and expression, DNA replication, virion formation and release, host evasion, and pathogenesis. Some of the non-essential proteins of HSV-1 have been studied more extensively than others, while the functions of some genes still remain unknown. Considering that the approximately 100 gene products encoded by the virus support both viral functions and host evasion simultaneously, it is mandatory that each gene product has multiple roles throughout the virus life cycle. This results in functional redundancy that contributes to the versatility of the virus. In this review, we summarized research that has been carried out on more than 50 non-essential proteins that function in different facets of the HSV-1 life cycle.
Non-essential genes are dispensable in vitro when ablated individually, and a lot of work to delineate their functions is based on deleting or mutating them and investigating their effects on viral processes and antiviral responses. However, many non-essential proteins work as components of functional networks in complex circuits, such as UL11/gE/gD, UL16/gE/UL11/UL21/VP22, UL31/UL34/US3, or ICP0/UL46/UL47/ICP22/US3/UL13/VP22 (described above). Therefore, it is likely that the field of HSV-1 research would benefit from investigating non-essential proteins not individually, but in tandem. An example is the work that has been done on HSV-1 mutants that trigger the formation of syncytia, in which regulatory interactions among HSV-1 proteins were described based on the effect of a non-essential protein deletion in the context of a syncytial HSV-1 mutation [672,709,832]. A potential problem that may arise by mutating non-essential genes in tandem is that the resulting virus may not be viable.
Another consideration for further research is the relevance of the model systems being used. Various non-essential proteins should be properly investigated in a relevant physiological context, depending on their function. For example, the role of non-essential proteins in cell-to-cell spread may give different insights when investigated in polarized epithelial cells, versus human fibroblasts, or the functions in trafficking will be different in neurons with long axons versus epithelial cells. Systems like organotypic brain slice cultures [833] can be used that recapitulate more closely the variable environment of a natural infection, considering that organotypic epithelial culture systems have been successfully utilized for the study of HSV-1 ribonucleotide reductase [834]. Such considerations extend to animal models as well, since the host immune regulation may be better investigated in a model other than mice. Spontaneous reactivation of latent HSV-1 occurs in humans and rabbits but not mice [835]. The human Stimulator of Interferon Genes (STING) is regulated in a different manner than the murine STING, complicating the study of non-essential protein functions on innate immunity [836,837]. It has also been described that ICP47 binding to the murine TAP is far weaker than the human one; therefore, the prevention of MHC I antigen presentation cannot be properly evaluated in mice, and this has implications for studies of neurovirulence and survival in the CNS [341,345,346]. Transgenic animal models, or alternative models, such as pigs, dogs, or monkeys [345], may have to be chosen carefully for research questions regarding the function of such non-essential proteins.
Another possibility for recapitulating a natural infection of the human CNS is the recently established brain organoids [838]. Recent advances in stem cell differentiation permit the use of human-induced pluripotent stem cells (hiPSCs) to generate three-dimensional (3D) neuron cultures, which are referred to as brain organoids. They exhibit neuronal heterogeneity and lamina-like structure [839]. Brain organoids can be infected with HSV-1 and exhibit inflammation, HSV-1 can establish hallmarks of latency in such cultures in the presence of antivirals like interferon (IFN), and HSV-1 can infect the outer laminar structure of these organoids moving further inside after infection [840,841]. All these elements make the use of brain organoids in HSV-1 research attractive. Investigation of latency can be complemented with the use of Lund human mesencephalic (LUHMES) cells [842]. These are human embryonic neuronal precursor cells that can be differentiated to postmitotic neurons and they can be used as a supplemental model to study latency and interactions of host and viral non-essential proteins. After infection of HSV-1, there is a loss of lytic gene transcription and an increase in the number of neurons that express latency-associated transcripts (LATs) [842]. The advantage of the latter system is that it does not require the presence of an antiviral factor like IFNβ to maintain latency.
The non-essential genes of HSV-1 offer unique properties that can be utilized for the development of recombinant HSV-1 viruses suitable for oncolytic therapy [843]. The first oncolytic virus to receive regulatory approval in the United States was an attenuated HSV-1 (named T-VEC) that has been engineered to lack ICP34.5, a major HSV-1 neurovirulence factor, ICP47, which normally prevents MHC I antigen presentation, and to express the human GM-CSF gene, which promotes dendritic cell accumulation at sites of inflammation and enhances APC function [844]. T-VEC was approved for the treatment of patients with local unresectable malignant melanoma. T-VEC is currently being investigated for further potential uses against Merkel cell carcinoma, and other malignancies [845,846]. The multiple roles of HSV-1 non-essential proteins in vivo make the study of manipulating their function worthwhile for therapeutic purposes [847]. For example, engineered HSV-1 lacking the UL39 gene, which is required for replication in non-dividing cells (i.e., neurons), makes it an attractive candidate for targeting gliomas [848]. For similar reasons, HSV-1 mutants lacking the function of the uracil glycosylase UL2 [849] or UL56 are less neurovirulent and good candidates for further investigation. Besides oncolytic therapy, non-essential proteins can be utilized for vaccine research. An HSV-1 strain carrying a deletion in gK cannot infect neuronal axons and establish latency. This strain has been explored as a vaccine to confer protection against lethal intravaginal HSV-1 and HSV-2 challenge in mice and rhesus macaques [850,851]. ICP0-null or vhs-null viruses combined perhaps with other mutations could be explored for vaccine strategies due to their attenuated phenotype as well.
Significant research has been carried out on the roles of non-essential proteins of HSV-1 for many decades. While many aspects of the virus have been unveiled regarding the networks of interactions and the role of individual proteins in the virus life cycle, a lot remains to be clarified regarding the role of non-essential proteins in vivo. Further investigation of the non-essential HSV-1 proteins will allow us to better understand mechanisms of HSV-1 pathogenesis and disease, and will also enable effective harnessing of HSV-1 properties for cancer, gene therapy, and vaccine strategies.
Author Contributions
Conceptualization, M.K.; Writing—Original draft preparation, C.D., H.W. and M.K.; Review and editing, M.K.; Supervision, M.K.; Funding acquisition, M.K.; C.D. and H.W. have equally contributed to the manuscript in addition to the following information. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIAID R21AI144883 and the NIGMS P20GM113117 grants, and the KUMC enhancement award Y6K00080.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGeoch D.J., Dolan A., Ralph A.C. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 2000;74:10401–10406. doi: 10.1128/JVI.74.22.10401-10406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Underdown S.J., Kumar K., Houldcroft C. Network analysis of the hominin origin of Herpes Simplex virus 2 from fossil data. Virus Evol. 2017;3 doi: 10.1093/ve/vex026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knipe D.M., Howley P. Fields Virology. 6th ed. Volume 1 Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. [Google Scholar]
- 4.Mador N., Goldenberg D., Cohen O., Panet A., Steiner I. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J. Virol. 1998;72:5067–5075. doi: 10.1128/JVI.72.6.5067-5075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umbach J.L., Kramer M.F., Jurak I., Karnowski H.W., Coen D.M., Cullen B.R. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen W., Sa e Silva M., Jaber T., Vitvitskaia O., Li S., Henderson G., Jones C. Two small RNAs encoded within the first 1.5 kilobases of the herpes simplex virus type 1 latency-associated transcript can inhibit productive infection and cooperate to inhibit apoptosis. J. Virol. 2009;83:9131–9139. doi: 10.1128/JVI.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croen K.D., Ostrove J.M., Dragovic L.J., Smialek J.E., Straus S.E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N. Engl. J. Med. 1987;317:1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 8.Mador N., Panet A., Latchman D., Steiner I. Expression and splicing of the latency-associated transcripts of herpes simplex virus type 1 in neuronal and non-neuronal cell lines. J. Biochem. 1995;117:1288–1297. doi: 10.1093/oxfordjournals.jbchem.a124857. [DOI] [PubMed] [Google Scholar]
- 9.Garber D.A., Schaffer P.A., Knipe D.M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J. Virol. 1997;71:5885–5893. doi: 10.1128/JVI.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S.H., Kramer M.F., Schaffer P.A., Coen D.M. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 1997;71:5878–5884. doi: 10.1128/JVI.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner E.K., Bloom D.C. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 1997;10:419–443. doi: 10.1128/CMR.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed M., Lock M., Miller C.G., Fraser N.W. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 2002;76:717–729. doi: 10.1128/JVI.76.2.717-729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson G., Peng W., Jin L., Perng G.-C., Nesburn A.B., Wechsler S.L., Jones C. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J. Neurovirol. 2002;8:103–111. doi: 10.1080/13550280290101085. [DOI] [PubMed] [Google Scholar]
- 14.Inman M., Perng G.-C., Henderson G., Ghiasi H., Nesburn A.B., Wechsler S.L., Jones C. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 2001;75:3636–3646. doi: 10.1128/JVI.75.8.3636-3646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L., Peng W., Perng G.-C., Brick D.J., Nesburn A.B., Jones C., Wechsler S.L. Identification of herpes simplex virus type 1 latency-associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J. Virol. 2003;77:6556–6561. doi: 10.1128/JVI.77.11.6556-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perng G.C., Ghiasi H., Slanina S.M., Nesburn A.B., Wechsler S.L. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J. Virol. 1996;70:976–984. doi: 10.1128/JVI.70.2.976-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng W., Henderson G., Perng G.-C., Nesburn A.B., Wechsler S.L., Jones C. The gene that encodes the herpes simplex virus type 1 latency-associated transcript influences the accumulation of transcripts (Bcl-xL and Bcl-xS) that encode apoptotic regulatory proteins. J. Virol. 2003;77:10714–10718. doi: 10.1128/JVI.77.19.10714-10718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perng G.-C., Jones C., Ciacci-Zanella J., Stone M., Henderson G., Yukht A., Slanina S.M., Hofman F.M., Ghiasi H., Nesburn A.B., et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 19.Honess R.W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974;14:8–19. doi: 10.1128/JVI.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honess R.W., Roizman B. Regulation of herpesvirus macromolecular synthesis: Sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987;236:573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch D.J., Dolan A., Frame M.C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986;14:3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh Y., Tanaka S., Liu Y., Hidaka Y., Mori R. Molecular characterization of naturally occuring glycoprotein C-negative herpes simplex virus type 1. Arch. Virol. 1993;129:119–130. doi: 10.1007/BF01316889. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka Y., Sakuma S., Kumano Y., Minagawa H., Mori R. Characterization of glycoprotein C-negative mutants of herpes simplex virus type 1 isolated from a patient with keratitis. Arch. Virol. 1990;113:195–207. doi: 10.1007/BF01316673. [DOI] [PubMed] [Google Scholar]
- 25.Kalamvoki M., Roizman B. HSV-1 degrades, stabilizes, requires, or is stung by STING depending on ICP0, the US3 protein kinase, and cell derivation. Proc. Natl. Acad. Sci. USA. 2014;111:E611-7. doi: 10.1073/pnas.1323414111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deschamps T., Kalamvoki M. Impaired STING pathway in human osteosarcoma U2OS cells contributes to the growth of ICP0-null mutant herpes simplex virus. J. Virol. 2017;91 doi: 10.1128/JVI.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann M., Braun D.K., Pereira L., Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 1984;52:108–118. doi: 10.1128/JVI.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stow N.D., Stow E.C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 29.Sacks W.R., Schaffer P.A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 1987;61:829–839. doi: 10.1128/JVI.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao F., Schaffer P.A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 1995;69:6249–6258. doi: 10.1128/JVI.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J. Virol. 1992;66:2916–2927. doi: 10.1128/JVI.66.5.2916-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everett R.D., Boutell C., Orr A. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 2004;78:1763–1774. doi: 10.1128/JVI.78.4.1763-1774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalamvoki M., Roizman B. Role of herpes simplex virus ICP0 in the transactivation of genes introduced by infection or transfection: A reappraisal. J. Virol. 2010;84:4222–4228. doi: 10.1128/JVI.02585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alandijany T., Roberts A.P.E., Conn K.L., Loney C., McFarlane S., Orr A., Boutell C. Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection. PLoS Pathog. 2018;14:e1006927. doi: 10.1371/journal.ppat.1006769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandri-Goldin R.M., Sekulovich R.E., Leary K. The alpha protein ICP0 does not appear to play a major role in the regulation of herpes simplex virus gene expression during infection in tissue culture. Nucleic Acids Res. 1987;15:905–919. doi: 10.1093/nar/15.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriuchi H., Moriuchi M., Straus S.E., Cohen J.I. Varicella-zoster virus (VZV) open reading frame 61 protein transactivates VZV gene promoters and enhances the infectivity of VZV DNA. J. Virol. 1993;67:4290–4295. doi: 10.1128/JVI.67.7.4290-4295.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everett R., Orr A., Elliott M. The equine herpesvirus 1 gene 63 RING finger protein partially complements Vmw110, its herpes simplex virus type 1 counterpart. J. Gen. Virol. 1995;76:2369–2374. doi: 10.1099/0022-1317-76-9-2369. [DOI] [PubMed] [Google Scholar]
- 38.Everett R.D., Boutell C., McNair C., Grant L., Orr A. Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins. J. Virol. 2010;84:3476–3487. doi: 10.1128/JVI.02544-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinlan M.P., Knipe D.M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 1985;5:957–963. doi: 10.1128/MCB.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knipe D.M., Smith J.L. A mutant herpesvirus protein leads to a block in nuclear localization of other viral proteins. Mol. Cell. Biol. 1986;6:2371–2381. doi: 10.1128/MCB.6.7.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelman I.H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA. 1985;82:5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosca J.D., Bednarik D.P., Raj N.B., Rosen C.A., Sodroski J.G., Haseltine W.A., Hayward G.S., Pitha P.M. Activation of human immunodeficiency virus by herpesvirus infection: Identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 1987;84:7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrove J.M., Leonard J., Weck K.E., Rabson A.B., Gendelman H.E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J. Virol. 1987;61:3726–3732. doi: 10.1128/JVI.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein D.J., Weller S.K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: Isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 1988;62:196–205. doi: 10.1128/JVI.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCusker C.T., Bacchetti S. The responsiveness of human papillomavirus upstream regulatory regions to herpes simplex virus immediate early proteins. Virus Res. 1988;11:199–207. doi: 10.1016/0168-1702(88)90083-4. [DOI] [PubMed] [Google Scholar]
- 46.Gius D., Laimins L.A. Activation of human papillomavirus type 18 gene expression by herpes simplex virus type 1 viral transactivators and a phorbol ester. J. Virol. 1989;63:555–563. doi: 10.1128/JVI.63.2.555-563.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolis D.M., Rabson A.B., Straus S.E., Ostrove J.M. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology. 1992;186:788–791. doi: 10.1016/0042-6822(92)90048-T. [DOI] [PubMed] [Google Scholar]
- 48.Nabel G.J., Rice S.A., Knipe D.M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988;239:1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- 49.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Hare P., Hayward G.S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J. Virol. 1985;56:723–733. doi: 10.1128/JVI.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai W., Schaffer P.A. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 1992;66:2904–2915. doi: 10.1128/JVI.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boutell C., Sadis S., Everett R.D. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Everett R.D., Barlow P., Milner A., Luisi B., Orr A., Hope G., Lyon D. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J. Mol. Biol. 1993;234:1038–1047. doi: 10.1006/jmbi.1993.1657. [DOI] [PubMed] [Google Scholar]
- 54.Grant K., Grant L., Tong L., Boutell C. Depletion of intracellular zinc inhibits the ubiquitin ligase activity of viral regulatory protein ICP0 and restricts herpes simplex virus 1 replication in cell culture. J. Virol. 2012;86:4029–4033. doi: 10.1128/JVI.06962-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lium E.K., Silverstein S. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 1997;71:8602–8614. doi: 10.1128/JVI.71.11.8602-8614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boutell C., Orr A., Everett R.D. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J. Virol. 2003;77:8686–8694. doi: 10.1128/JVI.77.16.8686-8694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boutell C., Everett R.D. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and Ubiquitinates p53. J. Biol. Chem. 2003;278:36596–36602. doi: 10.1074/jbc.M300776200. [DOI] [PubMed] [Google Scholar]
- 58.Boutell C., Canning M., Orr A., Everett R.D. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 2005;79:12342–12354. doi: 10.1128/JVI.79.19.12342-12354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu H., Roizman B. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA. 2003;100:8963–8968. doi: 10.1073/pnas.1533420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanni E., Gatherer D., Tong L., Everett R.D., Boutell C. Functional characterization of residues required for the herpes simplex virus 1 E3 ubiquitin ligase ICP0 to interact with the cellular E2 ubiquitin-conjugating enzyme UBE2D1 (UbcH5a) J. Virol. 2012;86:6323–6333. doi: 10.1128/JVI.07210-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maul G.G., Guldner H.H., Spivack J.G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0) J. Gen. Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 62.Mullen M.A., Ciufo D.M., Hayward G.S. Mapping of intracellular localization domains and evidence for colocalization interactions between the IE110 and IE175 nuclear transactivator proteins of herpes simplex virus. J. Virol. 1994;68:3250–3266. doi: 10.1128/JVI.68.5.3250-3266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Everett R.D., Maul G.G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Everett R.D., Murray J., Orr A., Preston C.M. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 2007;81:10991–11004. doi: 10.1128/JVI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen C., Corpet A., Roubille S., Maroui M.A., Poccardi N., Rousseau A., Kleijwegt C., Binda O., Texier P., Sawtell N., et al. Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 Chaperone Axis. PLoS Pathog. 2018;14:e1007313. doi: 10.1371/journal.ppat.1007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maroui M.A., Callé A., Cohen C., Streichenberger N., Texier P., Takissian J., Rousseau A., Poccardi N., Welsch J., Corpet A., et al. Latency entry of herpes simplex Virus 1 is determined by the interaction of its genome with the nuclear environment. PLoS Pathog. 2016;12:e1005834. doi: 10.1371/journal.ppat.1005834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson R.L., Sawtell N.M. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 2006;80:10919–10930. doi: 10.1128/JVI.01253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai W., Astor T.L., Liptak L.M., Cho C., Coen D.M., Schaffer P.A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 1993;67:7501–7512. doi: 10.1128/JVI.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roizman B. The checkpoints of viral gene expression in productive and latent infection: The role of the HDAC/CoREST/LSD1/REST repressor complex. J. Virol. 2011;85:7474–7482. doi: 10.1128/JVI.00180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Härle P., Sainz B.J., Carr D.J.J., Halford W.P. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology. 2002;293:295–304. doi: 10.1006/viro.2001.1280. [DOI] [PubMed] [Google Scholar]
- 71.Halford W.P., Schaffer P.A. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 2001;75:3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deschamps T., Waisner H., Dogrammatzis C., Roy A., Chacko S., Perera C., Prisinzano T.E., Kalamvoki M. Discovery of small-molecule inhibitors targeting the E3 ubiquitin ligase activity of the herpes simplex virus 1 ICP0 protein using an in vitro high-throughput screening assay. J. Virol. 2019;93 doi: 10.1128/JVI.00619-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagglund R., Roizman B. Herpes simplex virus 1 mutant in which the ICP0 HUL-1 E3 ubiquitin ligase site is disrupted stabilizes cdc34 but degrades D-type cyclins and exhibits diminished neurotoxicity. J. Virol. 2003;77:13194–13202. doi: 10.1128/JVI.77.24.13194-13202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gu H., Poon A.P., Roizman B. During its nuclear phase the multifunctional regulatory protein ICP0 undergoes proteolytic cleavage characteristic of polyproteins. Proc. Natl. Acad. Sci. USA. 2009;106:19132–19137. doi: 10.1073/pnas.0910920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boutell C., Cuchet-Lourenço D., Vanni E., Orr A., Glass M., McFarlane S., Everett R.D. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 2011;7:e1002245. doi: 10.1371/journal.ppat.1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuchet-Lourenço D., Vanni E., Glass M., Orr A., Everett R.D. Herpes simplex virus 1 ubiquitin ligase ICP0 interacts with PML isoform I and induces its SUMO-independent degradation. J. Virol. 2012;86:11209–11222. doi: 10.1128/JVI.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sloan E., Tatham M.H., Groslambert M., Glass M., Orr A., Hay R.T., Everett R.D. Analysis of the SUMO2 proteome during HSV-1 infection. PLoS Pathog. 2015;11:e1005059. doi: 10.1371/journal.ppat.1005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Everett R.D., Boutell C., Pheasant K., Cuchet-Lourenço D., Orr A. Sequences related to SUMO interaction motifs in herpes simplex virus 1 protein ICP0 act cooperatively to stimulate virus infection. J. Virol. 2014;88:2763–2774. doi: 10.1128/JVI.03417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Everett R.D., Parsy M.-L., Orr A. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 2009;83:4963–4977. doi: 10.1128/JVI.02593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Everett R.D., Freemont P., Saitoh H., Dasso M., Orr A., Kathoria M., Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 1998;72:6581–6591. doi: 10.1128/JVI.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chelbi-Alix M.K., de Thé H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18:935–941. doi: 10.1038/sj.onc.1202366. [DOI] [PubMed] [Google Scholar]
- 82.Sha Z., Blyszcz T., González-Prieto R., Vertegaal A.C.O., Goldberg A.L. Inhibiting ubiquitination causes an accumulation of SUMOylated newly synthesized nuclear proteins at PML bodies. J. Biol. Chem. 2019;294:15218–15234. doi: 10.1074/jbc.RA119.009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Everett R.D., Murray J. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 2005;79:5078–5089. doi: 10.1128/JVI.79.8.5078-5089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maroui M.A., Maarifi G., McManus F.P., Lamoliatte F., Thibault P., Chelbi-Alix M.K. Promyelocytic Leukemia Protein (PML) requirement for interferon-induced global cellular SUMOylation. Mol. Cell. Proteom. 2018;17:1196–1208. doi: 10.1074/mcp.RA117.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sloan E., Orr A., Everett R.D. MORC3, a Component of PML nuclear bodies, has a role in restricting herpes simplex virus 1 and human cytomegalovirus. J. Virol. 2016;90:8621–8633. doi: 10.1128/JVI.00621-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conn K.L., Wasson P., McFarlane S., Tong L., Brown J.R., Grant K.G., Domingues P., Boutell C. Novel role for protein inhibitor of activated STAT 4 (PIAS4) in the restriction of herpes simplex virus 1 by the cellular intrinsic antiviral immune response. J. Virol. 2016;90:4807–4826. doi: 10.1128/JVI.03055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown J.R., Conn K.L., Wasson P., Charman M., Tong L., Grant K., McFarlane S., Boutell C. SUMO ligase protein inhibitor of activated STAT1 (PIAS1) is a constituent promyelocytic leukemia nuclear body protein that contributes to the intrinsic antiviral immune response to herpes simplex virus 1. J. Virol. 2016;90:5939–5952. doi: 10.1128/JVI.00426-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lees-Miller S.P., Long M.C., Kilvert M.A., Lam V., Rice S.A., Spencer C.A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 1996;70:7471–7477. doi: 10.1128/JVI.70.11.7471-7477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parkinson J., Lees-Miller S.P., Everett R.D. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 1999;73:650–657. doi: 10.1128/JVI.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson S.A., DeLuca N.A. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Natl. Acad. Sci. USA. 2003;100:7871–7876. doi: 10.1073/pnas.1230643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lilley C.E., Chaurushiya M.S., Boutell C., Landry S., Suh J., Panier S., Everett R.D., Stewart G.S., Durocher D., Weitzman M.D. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 2010;29:943–955. doi: 10.1038/emboj.2009.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lilley C.E., Chaurushiya M.S., Boutell C., Everett R.D., Weitzman M.D. The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog. 2011;7:e1002084. doi: 10.1371/journal.ppat.1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaurushiya M.S., Lilley C.E., Aslanian A., Meisenhelder J., Scott D.C., Landry S., Ticau S., Boutell C., Yates J.R., III, Schulman B.A., et al. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: Viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol. Cell. 2012;46:79–90. doi: 10.1016/j.molcel.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagel C.-H., Albrecht N., Milovic-Holm K., Mariyanna L., Keyser B., Abel B., Weseloh B., Hofmann T.G., Eibl M.M., Hauber J. Herpes simplex virus immediate-early protein ICP0 is targeted by SIAH-1 for proteasomal degradation. J. Virol. 2011;85:7644–7657. doi: 10.1128/JVI.02207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conwell S.E., White A.E., Harper J.W., Knipe D.M. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 ICP0. J. Virol. 2015;89:220–229. doi: 10.1128/JVI.02635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deshmane S.L., Fraser N.W. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 1989;63:943–947. doi: 10.1128/JVI.63.2.943-947.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herrera F.J., Triezenberg S.J. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J. Virol. 2004;78:9689–9696. doi: 10.1128/JVI.78.18.9689-9696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kent J.R., Zeng P.-Y., Atanasiu D., Gardner J., Fraser N.W., Berger S.L. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J. Virol. 2004;78:10178–10186. doi: 10.1128/JVI.78.18.10178-10186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J., Kent J.R., Placek B., Whelan K.A., Hollow C.M., Zeng P.-Y., Fraser N.W., Berger S.L. Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1. J. Virol. 2006;80:5740–5746. doi: 10.1128/JVI.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cabral J.M., Oh H.S., Knipe D.M. ATRX promotes maintenance of herpes simplex virus heterochromatin during chromatin stress. eLife. 2018;7 doi: 10.7554/eLife.40228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J.S., Raja P., Knipe D.M. Herpesviral ICP0 protein promotes two waves of heterochromatin removal on an early viral promoter during lytic infection. mBio. 2016;7 doi: 10.1128/mBio.02007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lomonte P., Thomas J., Texier P., Caron C., Khochbin S., Epstein A.L. Functional interaction between class II histone deacetylases and ICP0 of herpes simplex virus type 1. J. Virol. 2004;78:6744–6757. doi: 10.1128/JVI.78.13.6744-6757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poon A.P.W., Gu H., Roizman B. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. USA. 2006;103:9993–9998. doi: 10.1073/pnas.0604142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hobbs W.E., II, DeLuca N.A. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 1999;73:8245–8255. doi: 10.1128/JVI.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poon A.P.W., Liang Y., Roizman B. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 2003;77:12671–12678. doi: 10.1128/JVI.77.23.12671-12678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalamvoki M., Roizman B. Circadian CLOCK histone acetyl transferase localizes at ND10 nuclear bodies and enables herpes simplex virus gene expression. Proc. Natl. Acad. Sci. USA. 2010;107:17721–17726. doi: 10.1073/pnas.1012991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalamvoki M., Roizman B. The Histone Acetyltransferase CLOCK is an essential component of the herpes simplex virus 1 transcriptome that includes TFIID, ICP4, ICP27, and ICP22. J. Virol. 2011;85:9472–9477. doi: 10.1128/JVI.00876-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalamvoki M., Roizman B. Interwoven roles of cyclin D3 and cdk4 recruited by ICP0 and ICP4 in the expression of herpes simplex virus genes. J. Virol. 2010;84:9709–9717. doi: 10.1128/JVI.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cliffe A.R., Knipe D.M. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 2008;82:12030–12038. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kutluay S.B., Triezenberg S.J. Regulation of histone deposition on the herpes simplex virus type 1 genome during lytic infection. J. Virol. 2009;83:5835–5845. doi: 10.1128/JVI.00219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Placek B.J., Huang J., Kent J.R., Dorsey J., Rice L., Fraser N.W., Berger S.L. The histone variant H3.3 regulates gene expression during lytic infection with herpes simplex virus type 1. J. Virol. 2009;83:1416–1421. doi: 10.1128/JVI.01276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gu H., Roizman B. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J. Virol. 2009;83:4376–4385. doi: 10.1128/JVI.02515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu H., Liang Y., Mandel G., Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. USA. 2005;102:7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gu H., Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. USA. 2007;104:17134–17139. doi: 10.1073/pnas.0707266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalamvoki M., Roizman B. Nuclear retention of ICP0 in cells exposed to HDAC inhibitor or transfected with DNA before infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 2008;105:20488–20493. doi: 10.1073/pnas.0810879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merkl P.E., Orzalli M.H., Knipe D.M. Mechanisms of host IFI16, PML, and Daxx protein restriction of herpes simplex virus 1 replication. J. Virol. 2018;92 doi: 10.1128/JVI.00057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lukashchuk V., Everett R.D. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 2010;84:4026–4040. doi: 10.1128/JVI.02597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Everett R.D. Dynamic response of IFI16 and promyelocytic leukemia nuclear body components to herpes simplex virus 1 infection. J. Virol. 2016;90:167–179. doi: 10.1128/JVI.02249-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diner B.A., Lum K.K., Toettcher J.E., Cristea I.M. Viral DNA sensors IFI16 and cyclic GMP-AMP synthase possess distinct functions in regulating viral gene expression, immune defenses, and apoptotic responses during herpesvirus infection. mBio. 2016;7 doi: 10.1128/mBio.01553-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McFarlane S., Orr A., Roberts A.P.E., Conn K.L., Iliev V., Loney C., Filipe A.d.S., Smollett K., Gu Q., Robertson N., et al. The histone chaperone HIRA promotes the induction of host innate immune defences in response to HSV-1 infection. PLoS Pathog. 2019;15:e1007667. doi: 10.1371/journal.ppat.1007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jurak I., Silverstein L.B., Sharma M., Coen D.M. Herpes simplex virus is equipped with RNA- and protein-based mechanisms to repress expression of ATRX, an effector of intrinsic immunity. J. Virol. 2012;86:10093–10102. doi: 10.1128/JVI.00930-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orzalli M.H., DeLuca N.A., Knipe D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. USA. 2012;109 doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orzalli M.H., Broekema N.M., Knipe D.M. Relative contributions of herpes simplex virus 1 ICP0 and vhs to loss of cellular IFI16 vary in different human cell types. J. Virol. 2016;90:8351–8359. doi: 10.1128/JVI.00939-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cuchet-Lourenço D., Anderson G., Sloan E., Orr A., Everett R.D. The viral ubiquitin ligase ICP0 is neither sufficient nor necessary for degradation of the cellular DNA sensor IFI16 during herpes simplex virus 1 infection. J. Virol. 2013;87:13422–13432. doi: 10.1128/JVI.02474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diner B.A., Lum K.K., Javitt A., Cristea I.M. Interactions of the antiviral factor interferon gamma-inducible protein 16 (IFI16) mediate immune signaling and herpes simplex virus-1 immunosuppression. Mol. Cell. Proteomics. 2015;14:2341–2356. doi: 10.1074/mcp.M114.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawaguchi Y., van Sant C., Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 1997;71:7328–7336. doi: 10.1128/JVI.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kalamvoki M., Roizman B. ICP0 enables and monitors the function of D cyclins in herpes simplex virus 1 infected cells. Proc. Natl. Acad. Sci. USA. 2009;106:14576–14580. doi: 10.1073/pnas.0906905106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li H., Baskaran R., Krisky D.M., Bein K., Grandi P., Cohen J.B., Glorioso J.C. Chk2 is required for HSV-1 ICP0-mediated G2/M arrest and enhancement of virus growth. Virology. 2008;375:13–23. doi: 10.1016/j.virol.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lomonte P., Sullivan K.F., Everett R.D. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 2001;276:5829–5835. doi: 10.1074/jbc.M008547200. [DOI] [PubMed] [Google Scholar]
- 130.Lomonte P., Morency E. Centromeric protein CENP-B proteasomal degradation induced by the viral protein ICP0. FEBS Lett. 2007;581:658–662. doi: 10.1016/j.febslet.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 131.Everett R.D., Earnshaw W.C., Findlay J., Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gross S., Catez F., Masumoto H., Lomonte P. Centromere architecture breakdown induced by the viral E3 ubiquitin ligase ICP0 protein of herpes simplex virus type 1. PLoS ONE. 2012;7:e44227. doi: 10.1371/journal.pone.0044227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng Z., Kim E.T., Vladimirova O., Dheekollu J., Wang Z., Newhart A., Liu D., Myers J.L., Hensley S.E., Moffat J., et al. HSV-1 remodels host telomeres to facilitate viral replication. Cell Rep. 2014;9:2263–2278. doi: 10.1016/j.celrep.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mossman K.L., Saffran H.A., Smiley J.R. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 2000;74:2052–2056. doi: 10.1128/JVI.74.4.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leib D.A., Harrison T.E., Laslo K.M., Machalek M.A., Moorman N.J., Virgin H.W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burleigh K., Maltbaek J.H., Cambier S., Green R., Gale M.J., James R.C., Stetson D.B. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aba4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Orzalli M.H., Broekema N.M., Diner B.A., Hancks D.C., Elde N.C., Cristea I.M., Knipe D.M. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc. Natl. Acad. Sci. USA. 2015;112:1773–1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Lint A.L., Murawski M.R., Goodbody R.E., Severa M., Fitzgerald K.A., Finberg R.W., Knipe D.M., Kurt-Jones E.A. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 2010;84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin R., Noyce R.S., Collins S.E., Everett R.D., Mossman K.L. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 2004;78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Melroe G.T., DeLuca N.A., Knipe D.M. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 2004;78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melroe G.T., Silva L., Schaffer P.A., Knipe D.M. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction. Virology. 2007;360:305–321. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Waisner H., Kalamvoki M. The ICP0 protein of herpes simplex virus 1 (HSV-1) downregulates major autophagy adaptor proteins sequestosome 1 and optineurin during the early stages of HSV-1 infection. J. Virol. 2019;93 doi: 10.1128/JVI.01258-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalamvoki M., Gu H., Roizman B. Overexpression of the ubiquitin-specific protease 7 resulting from transfection or mutations in the ICP0 binding site accelerates rather than depresses herpes simplex virus 1 gene expression. J. Virol. 2012;86:12871–12878. doi: 10.1128/JVI.01981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mostafa H.H., Thompson T.W., Davido D.J. N-terminal phosphorylation sites of herpes simplex virus 1 ICP0 differentially regulate its activities and enhance viral replication. J. Virol. 2013;87:2109–2119. doi: 10.1128/JVI.02588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Colleran A., Collins P.E., O’Carroll C., Ahmed A., Mao X., McManus B., Kiely P.A., Burstein E., Carmody R.J. Deubiquitination of NF-κB by ubiquitin-specific protease-7 promotes transcription. Proc. Natl. Acad. Sci. USA. 2013;110:618–623. doi: 10.1073/pnas.1208446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang Y., Kurakin A., Roizman B. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc. Natl. Acad. Sci. USA. 2005;102:5838–5843. doi: 10.1073/pnas.0501253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deschamps T., Dogrammatzis C., Mullick R., Kalamvoki M. Cbl E3 ligase mediates the removal of nectin-1 from the surface of herpes simplex virus 1-infected cells. J. Virol. 2017;91 doi: 10.1128/JVI.00393-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leib D.A., Coen D.M., Bogard C.L., Hicks K.A., Yager D.R., Knipe D.M., Tyler K.L., Schaffer P.A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 1989;63:759–768. doi: 10.1128/JVI.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Halford W.P., Kemp C.D., Isler J.A., Davido D.J., Schaffer P.A. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 2001;75:6143–6153. doi: 10.1128/JVI.75.13.6143-6153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harris R.A., Everett R.D., Zhu X.X., Silverstein S., Preston C.M. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 1989;63:3513–3515. doi: 10.1128/JVI.63.8.3513-3515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cliffe A.R., Arbuckle J.H., Vogel J.L., Geden M.J., Rothbart S.B., Cusack C.L., Strahl B.D., Kristie T.M., Deshmukh M. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe. 2015;18:649–658. doi: 10.1016/j.chom.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thompson R.L., Preston C.M., Sawtell N.M. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McGeoch D.J., Dalrymple M.A., Davison A.J., Dolan A., Frame M.C., McNab D., Perry L.J., Scott J.E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplx virus type 1. J. Gen. Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 154.McKnight J.L., Pellett P.E., Jenkins F.J., Roizman B. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate alpha-trans-inducing factor-dependent activation of alpha genes. J. Virol. 1987;61:992–1001. doi: 10.1128/JVI.61.4.992-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vittone V., Diefenbach E., Triffett D., Douglas M.W., Cunningham A.L., Diefenbach R.J. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 2005;79:9566–9571. doi: 10.1128/JVI.79.15.9566-9571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barker D.E., Roizman B. Identification of three genes nonessential for growth in cell culture near the right terminus of the unique sequences of long component of herpes simplex virus 1. Virology. 1990;177:684–691. doi: 10.1016/0042-6822(90)90534-X. [DOI] [PubMed] [Google Scholar]
- 157.Kato K., Daikoku T., Goshima F., Kume H., Yamaki K., Nishiyama Y. Synthesis, subcellular localization and VP16 interaction of the herpes simplex virus type 2 UL46 gene product. Arch. Virol. 2000;145:2149–2162. doi: 10.1007/s007050070045. [DOI] [PubMed] [Google Scholar]
- 158.Liu M., Tang J., Wang X., Yang T., Geller A.I. Enhanced long-term expression from helper virus-free HSV-1 vectors packaged in the presence of deletions in genes that modulate the function of VP16, U L 46 and U L 47. J. Neurosci. Methods. 2005;145:1–9. doi: 10.1016/j.jneumeth.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 159.McLean G., Rixon F., Langeland N., Haarr L., Marsden H. Identification and characterization of the virion protein products of herpes simplex virus type 1 gene UL47. J. Gen. Virol. 1990;71:2953–2960. doi: 10.1099/0022-1317-71-12-2953. [DOI] [PubMed] [Google Scholar]
- 160.Meredith D.M., Lindsay J.A., Halliburton I.W., Whittaker G.R. Post-translational modification of the tegument proteins (VP13 and VP14) of herpes simplex virus type 1 by glycosylation and phosphorylation. J. Gen. Virol. 1991;72:2771–2775. doi: 10.1099/0022-1317-72-11-2771. [DOI] [PubMed] [Google Scholar]
- 161.Zhang Y., McKnight J.L. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 1993;67:1482–1492. doi: 10.1128/JVI.67.3.1482-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kopp M., Klupp B.G., Granzow H., Fuchs W., Mettenleiter T.C. Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: Role for UL47 in virion morphogenesis in the cytoplasm. J. Virol. 2002;76:8820–8833. doi: 10.1128/JVI.76.17.8820-8833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Strunk U., Saffran H.A., Wu F.W., Smiley J.R. Role of herpes simplex virus VP11/12 tyrosine-based motifs in binding and activation of the Src family kinase Lck and recruitment of p85, Grb2, and Shc. J. Virol. 2013;87:11276–11286. doi: 10.1128/JVI.01702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yanagida N., Yoshida S., Nazerian K., Lee L.F. Nucleotide and predicted amino acid sequences of Marek’s disease virus homologues of herpes simplex virus major tegument proteins. J. Gen. Virol. 1993;74:1837–1845. doi: 10.1099/0022-1317-74-9-1837. [DOI] [PubMed] [Google Scholar]
- 165.Carpenter D.E., Misra V. The most abundant protein in bovine herpes 1 virions is a homologue of herpes simplex virus type 1 UL47. J. Gen. Virol. 1991;72:3077–3084. doi: 10.1099/0022-1317-72-12-3077. [DOI] [PubMed] [Google Scholar]
- 166.Whittaker G.R., Riggio M.P., Halliburton I.W., Killington R.A., Allen G.P., Meredith D.M. Antigenic and protein sequence homology between VP13/14, a herpes simplex virus type 1 tegument protein, and gp10, a glycoprotein of equine herpesvirus 1 and 4. J. Virol. 1991;65:2320–2326. doi: 10.1128/JVI.65.5.2320-2326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verjans G.M., Dings M.E., McLauchlan J., van der Kooi A., Hoogerhout P., Brugghe H.F., Timmermans H.A., Baarsma G.S., Osterhaus A.D. Intraocular T cells of patients with herpes simplex virus (HSV)-induced acute retinal necrosis recognize HSV tegument proteins VP11/12 and VP13/14. J. Infect. Dis. 2000;182:923–927. doi: 10.1086/315759. [DOI] [PubMed] [Google Scholar]
- 168.Zahariadis G., Wagner M.J., Doepker R.C., Maciejko J.M., Crider C.M., Jerome K.R., Smiley J.R. Cell-type-specific tyrosine phosphorylation of the herpes simplex virus tegument protein VP11/12 encoded by gene UL46. J. Virol. 2008;82:6098–6108. doi: 10.1128/JVI.02121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wagner M.J., Smiley J.R. Herpes simplex virus requires VP11/12 to induce phosphorylation of the activation loop tyrosine (Y394) of the Src family kinase Lck in T lympocytes. J. Virol. 2009;83:12452–12461. doi: 10.1128/JVI.01364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benetti L., Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wagner M.J., Smiley J.R. Herpes simplex virus requires VP11/12 to activate src family kinase-phosphoinositide 3-kinase-akt signaling. J. Virol. 2011;85:2803–2812. doi: 10.1128/JVI.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Strunk U., Ramos D.G., Saffran H.A., Smiley J.R. Role of Herpes simplex virus 1 VP11/12 tyrosine-based binding motifs for Src family kinases, p85, Grb2 and Shc in activation of the phosphoinositide 3-kinase-Akt pathway. Virology. 2016;498:31–35. doi: 10.1016/j.virol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 173.Martin C., Leyton L., Hott M., Arancibia Y., Spichiger C., McNiven M.A., Court F.A., Concha M.I., Burgos P.V., Otth C. Herpes simplex virus type 1 neuronal infection perturbs golgi apparatus integrity through activation of src tyrosine kinase and dyn-2 GTPase. Front. Cell. Infect. Microbiol. 2017;7:371. doi: 10.3389/fcimb.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bello-Morales R., Crespillo A.J., Fraile-Ramos A., Tabarés E., Alcina A., López-Guerrero J.A. Role of the small GTPase Rab27a during herpes simplex virus infection of oligodendrocytic cells. BMC Microbiol. 2012;12:265. doi: 10.1186/1471-2180-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dong S., Corre B., Foulon E., Dufour E., Veillette A., Acuto O., Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J. Exp. Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Némorin J.-G., Laporte P., Bérubé G., Duplay P. p62 dok negatively regulates CD2 signaling in jurkat cells. J. Immunol. 2001;166:4408–4415. doi: 10.4049/jimmunol.166.7.4408. [DOI] [PubMed] [Google Scholar]
- 177.Lahmidi S., Strunk U., Smiley J.R., Pearson A., Duplay P. Herpes simplex virus 1 infection of T cells causes VP11/12-dependent phosphorylation and degradation of the cellular protein Dok-2. Virology. 2017;511:66–73. doi: 10.1016/j.virol.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 178.Vink E.I., Lee S., Smiley J.R., Mohr I. Remodeling mTORC1 responsiveness to amino acids by the herpes simplex virus UL46 and Us3 gene products supports replication during nutrient insufficiency. J. Virol. 2018;92:1–10. doi: 10.1128/JVI.01377-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deschamps T., Kalamvoki M. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus 1. J. Virol. 2017;91 doi: 10.1128/JVI.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.You H., Zheng S., Huang Z., Lin Y., Shen Q., Zheng C. Herpes simplex virus 1 tegument protein ul46 inhibits tank-binding kinase 1-mediated signaling. mBio. 2019;10:1–10. doi: 10.1128/mBio.00919-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu J., Gao F., Wu J., Zheng H., Tong W., Cheng X., Liu Y., Zhu H., Fu X., Jiang Y., et al. Characterization of nucleocytoplasmic shuttling of pseudorabies virus protein UL46. Front. Vet. Sci. 2020;7:484. doi: 10.3389/fvets.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Z., Kato A., Shindo K., Noda T., Sagara H., Kawaoka Y., Arii J., Kawaguchi Y. Herpes simplex virus 1 UL47 Interacts with viral nuclear egress factors UL31, UL34, and Us3 and regulates viral nuclear egress. J. Virol. 2014;88:4657–4667. doi: 10.1128/JVI.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Donnelly M., Elliott G. Nuclear localization and shuttling of herpes simplex virus tegument protein VP13/14. J. Virol. 2001;75:2566–2574. doi: 10.1128/JVI.75.6.2566-2574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Donnelly M., Elliott G. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J. Virol. 2001;75:2575–2583. doi: 10.1128/JVI.75.6.2575-2583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yedowitz J.C., Kotsakis A., Schlegel E.F.M., Blaho J.A. Nuclear localizations of the herpes simplex virus type 1 tegument proteins VP13/14, vhs, and VP16 precede VP22-dependent microtubule reorganization and VP22 nuclear import. J. Virol. 2005;79:4730–4743. doi: 10.1128/JVI.79.8.4730-4743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dobrikova E., Shveygert M., Walters R., Gromeier M. Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its subcellular distribution. J. Virol. 2010;84:270–279. doi: 10.1128/JVI.01740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shu M., Taddeo B., Zhang W., Roizman B. Selective degradation of mRNAs by the HSV host shutoff RNase is regulated by the UL47 tegument protein. Proc. Natl. Acad. Sci. USA. 2013;110 doi: 10.1073/pnas.1305475110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Heine J.W., Honess R.W., Cassai E., Roizman B. Proteins specified by herpes simplex virus XII. The virion polypeptides of type 1 strains. J. Virol. 1974;14:640–651. doi: 10.1128/JVI.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aints A., Güven H., Gahrton G., Smith C.I.E., Dilber M.S. Mapping of herpes simplex virus-1 VP22 functional domains for inter- and subcellular protein targeting. Gene Ther. 2001;8:1051–1056. doi: 10.1038/sj.gt.3301493. [DOI] [PubMed] [Google Scholar]
- 190.Morrison E.E., Wang Y.F., Meredith D.M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 1998;72:7108–7114. doi: 10.1128/JVI.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pomeranz L.E., Blaho J.A. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length VP22. J. Virol. 2000;74:10041–10054. doi: 10.1128/JVI.74.21.10041-10054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Van Leeuwen H., Okuwaki M., Hong R., Chakravarti D., Nagata K., O’Hare P. Herpes simplex virus type 1 tegument protein VP22 interacts with TAF-I proteins and inhibits nucleosome assembly but not regulation of histone acetylation by INHAT. J. Gen. Virol. 2003;84:2501–2510. doi: 10.1099/vir.0.19326-0. [DOI] [PubMed] [Google Scholar]
- 193.López M.R., Schlegel E.F.M., Wintersteller S., Blaho J.A. The major tegument structural protein VP22 targets areas of dispersed nucleolin and marginalized chromatin during productive herpes simplex virus 1 infection. Virus Res. 2008;136:175–188. doi: 10.1016/j.virusres.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu X., Liu L., Wu L., Wang L., Dong C., Li W., Li Q. Herpes simplex virus type 1 tegument protein VP22 is capable of modulating the transcription of viral TK and gC genes via interaction with viral ICP0. Biochimie. 2010;92:1024–1030. doi: 10.1016/j.biochi.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 195.Tanaka M., Kato A., Satoh Y., Ide T., Sagou K., Kimura K., Hasegawa H., Kawaguchi Y. Herpes simplex virus 1 VP22 regulates translocation of multiple viral and cellular proteins and promotes neurovirulence. J. Virol. 2012;86:5264–5277. doi: 10.1128/JVI.06913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sciortino M.T., Taddeo B., Giuffrè-Cuculletto M., Medici M.A., Mastino A., Roizman B. Replication-competent herpes simplex virus 1 isolates selected from cells transfected with a bacterial artificial chromosome DNA lacking only the UL49 gene vary with respect to the defect in the UL41 gene encoding host shutoff RNase. J. Virol. 2007;81:10924–10932. doi: 10.1128/JVI.01239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mbong E.F., Woodley L., Dunkerley E., Schrimpf J.E., Morrison L.A., Duffy C. Deletion of the herpes simplex virus 1 UL49 gene results in mRNA and protein translation defects that are complemented by secondary mutations in UL41. J. Virol. 2012;86:12351–12361. doi: 10.1128/JVI.01975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Duffy C., Mbong E.F., Baines J.D. VP22 of herpes simplex virus 1 promotes protein synthesis at late times in infection and accumulation of a subset of viral mRNAs at early times in infection. J. Virol. 2009;83:1009–1017. doi: 10.1128/JVI.02245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brignati M.J., Loomis J.S., Wills J.W., Courtney R.J. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 2003;77:4888–4898. doi: 10.1128/JVI.77.8.4888-4898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.O’Regan K.J., Brignati M.J., Murphy M.A., Bucks M.A., Courtney R.J. Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 is facilitated by trans-Golgi network localization and is independent of interaction with glycoprotein E. Virology. 2010;405:176–192. doi: 10.1016/j.virol.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leslie J., Rixon F.J., Mclauchlan J. Overexpression of the herpes simplex virus type 1 tegument protein VP22 increases its incorporation into virus particles. Virology. 1996;68:60–68. doi: 10.1006/viro.1996.0286. [DOI] [PubMed] [Google Scholar]
- 202.Chi J.H.I., Harley C.A., Mukhopadhyay A., Wilson D.W. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 2005;86:253–261. doi: 10.1099/vir.0.80444-0. [DOI] [PubMed] [Google Scholar]
- 203.Starkey J.L., Han J., Chadha P., Marsh J.A., Wills J.W. Elucidation of the block to herpes simplex virus egress in the absence of tegument protein UL16 reveals a novel interaction with VP22. J. Virol. 2014;88:110–119. doi: 10.1128/JVI.02555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O’Regan K.J., Bucks M.A., Murphy M.A., Wills J.W., Courtney R.J. A conserved region of the herpes simplex virus type 1 tegument protein VP22 facilitates interaction with the cytoplasmic tail of glycoprotein E (gE) Virology. 2007;358:192–200. doi: 10.1016/j.virol.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 205.O’Regan K.J., Murphy M.A., Bucks M.A., Wills J.W., Courtney R.J. Incorporation of the herpes simplex virus type 1 tegument protein VP22 into the virus particle is independent of interaction with VP16. Virology. 2007;369:263–280. doi: 10.1016/j.virol.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 206.Duffy C., LaVail J.H., Tauscher A.N., Wills E.G., Blaho J.A., Baines J.D. Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea. J. Virol. 2006;80:8664–8675. doi: 10.1128/JVI.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Elliott G., Mouzakitis G., O’Hare P. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 1995;69:7932–7941. doi: 10.1128/JVI.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maringer K., Stylianou J., Elliott G. A Network of protein interactions around the herpes simplex virus tegument protein VP22. J. Virol. 2012;86:12971–12982. doi: 10.1128/JVI.01913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kotsakis A., Pomeranz L.E., Blouin A., Blaho J.A. Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of VP22, a major virion tegument protein. J. Virol. 2001;75:8697–8711. doi: 10.1128/JVI.75.18.8697-8711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Blouin A., Blaho J.A. Assessment of the subcellular localization of the herpes simplex virus structural protein VP22 in the absence of other viral gene products. Virus Res. 2001;81:57–68. doi: 10.1016/S0168-1702(01)00355-0. [DOI] [PubMed] [Google Scholar]
- 211.Van Leeuwen H., Elliott G., O’Hare P. Evidence of a role for nonmuscle myosin II in herpes simplex virus type 1 egress. J. Virol. 2002;76:3471–3481. doi: 10.1128/JVI.76.7.3471-3481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu J., Gallo R.M., Duffy C., Brutkiewicz R.R. A VP22-null HSV-1 is impaired in inhibiting CD1d-mediated antigen presentation. Viral Immunol. 2016;29:409–416. doi: 10.1089/vim.2015.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Horan K.A., Hansen K., Jakobsen M.R., Holm C.K., Søby S., Unterholzner L., Thompson M., West J.A., Iversen M.B., Rasmussen S.B., et al. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J. Immunol. 2013;190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maruzuru Y., Ichinohe T., Sato R., Miyake K., Okano T., Suzuki T., Koshiba T., Koyanagi N., Tsuda S., Watanabe M., et al. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe. 2018;23:254–265. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 215.Elliott G., O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/S0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 216.Sciortino M.T., Taddeo B., Poon A.P.W., Mastino A., Roizman B. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc. Natl. Acad. Sci. USA. 2002;99:8318–8323. doi: 10.1073/pnas.122231699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Phelan A., Elliott G., Hare P.O. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat. Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 218.Fang B., Xu B., Koch P., Roth J.A. Intercellular trafficking of VP22-GFP fusion proteins is not observed in cultured mammalian cells. Gene Ther. 1998;5:1420–1424. doi: 10.1038/sj.gt.3300741. [DOI] [PubMed] [Google Scholar]
- 219.Dilber M.S., Phelan A., Aints A., Mohamed A.J., Elliott G., Edvard Smith C.I., O’Hare P. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- 220.Sheridan P.J., Lawrie A., Crossman D.C., Holt C.M., Newman C.M. VP22-mediated intercellular transport correlates with enhanced biological activity of MybEngrailed but not (HSV-I) thymidine kinase fusion proteins in primary vascular cells following non-viral transfection. J. Gene Med. 2005;7:375–385. doi: 10.1002/jgm.679. [DOI] [PubMed] [Google Scholar]
- 221.Hakkarainen T., Wahlfors T., Meriläinen O., Loimas S., Hemminki A., Wahlfors J. VP22 does not significantly enhance enzyme prodrug cancer gene therapy as a part of a VP22-HSVTk-GFP triple fusion construct. J. Gene Med. 2005;7:898–907. doi: 10.1002/jgm.737. [DOI] [PubMed] [Google Scholar]
- 222.Beerens A.M.J., Rots M.G., de Vries E.F.J., Haisma H.J. Fusion of herpes simplex virus thymidine kinase to VP22 does not result in intercellular trafficking of the protein. Int. J. Mol. Med. 2007;19:841–849. doi: 10.3892/ijmm.19.5.841. [DOI] [PubMed] [Google Scholar]
- 223.Aints A., Dilber M.S., Smith C.I.E. Intercellular spread of GFP-VP22. J. Gene Med. 1999;1:275–279. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<275::AID-JGM44>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 224.Dogrammatzis C., Deschamps T., Kalamvoki M. Biogenesis of extracellular vesicles during herpes simplex virus 1 infection: Role of the CD63 tetraspanin. J. Virol. 2018;93 doi: 10.1128/JVI.01850-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dogrammatzis C., Saleh S., Deighan C., Kalamvoki M. Diverse populations of extracellular vesicles with opposite functions during herpes simplex virus 1 infection. J. Virol. 2020;95:e02357-20. doi: 10.1128/JVI.02357-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Greco O., Joiner M.C., Doleh A., Scott S.D. VP22-mediated intercellular transport for suicide gene therapy under oxic and hypoxic conditions. Gene Ther. 2005;12:974–979. doi: 10.1038/sj.gt.3302482. [DOI] [PubMed] [Google Scholar]
- 227.Liu C.S., Kong B.H., Xia H.H.Q., Ellem K.A.O., Wei M.Q. VP22 enhanced intercellular trafficking of HSV thymidine kinase reduced the level of ganciclovir needed to cause suicide cell death. J. Gene Med. 2001;3:145–152. doi: 10.1002/jgm.164. [DOI] [PubMed] [Google Scholar]
- 228.Wybranietz W.A., Groß C.D., Phelan A., O’Hare P., Spiegel M., Graepler F., Bitzer M., Stähler P., Gregor M., Lauer U.M. Enhanced suicide gene effect by adenoviral transduction of aVP22-cytosine deaminase (CD) fusion gene. Gene Ther. 2001;8:1654–1664. doi: 10.1038/sj.gt.3301564. [DOI] [PubMed] [Google Scholar]
- 229.Lee K.C., Hamstra D.A., Bullarayasamudram S., Bhojani M.S., Moffat B.A., Dornfeld K.J., Ross B.D., Rehemtulla A. Fusion of the HSV-1 tegument protein vp22 to cytosine deaminase confers enhanced bystander effect and increased therapeutic benefit. Gene Ther. 2006;13:127–137. doi: 10.1038/sj.gt.3302631. [DOI] [PubMed] [Google Scholar]
- 230.Cashman S.M., Sadowski S.L., Morris D.J., Frederick J., Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors—Implications for gene therapy. Mol. Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 231.Kretz A., Wybranietz W.A., Hermening S., Lauer U.M., Isenmann S. HSV-1 VP22 augments adenoviral gene transfer to CNS neurons in the retina and striatum in vivo. Mol. Ther. 2003;7:659–669. doi: 10.1016/S1525-0016(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 232.Xiong F., Xiao S., Peng F., Zheng H., Yu M., Ruan Y., Li W., Shang Y., Zhao C., Zhou W., et al. Herpes simplex virus VP22 enhances adenovirus-mediated microdystrophin gene transfer to skeletal muscles in dystrophin-deficient (mdx) mice. Hum. Gene Ther. 2007;18:490–501. doi: 10.1089/hum.2006.155. [DOI] [PubMed] [Google Scholar]
- 233.Yu X., Wang Y., Xia Y., Zhang L., Yang Q., Lei J. A DNA vaccine encoding VP22 of herpes simplex virus type I (HSV-1) and OprF confers enhanced protection from Pseudomonas aeruginosa in mice. Vaccine. 2016;34:4399–4405. doi: 10.1016/j.vaccine.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 234.Hung C.-F., Cheng W.-F., Chai C.-Y., Hsu K.-F., He L., Ling M., Wu T.-C. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J. Immunol. 2001;166:5733–5740. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 235.Honess R.W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J. Virol. 1973;12:1347–1365. doi: 10.1128/JVI.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Post L.E., Roizman B. A generalized technique for deletion of specific genes in large genomes: A gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 237.Meignier B., Longnecker R., Mavromara-Nazos P., Sears A.E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus I. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 238.Sears A.E., Halliburton I.W., Meignier B., Silver S., Roizman B. Herpes simplex virus 1 mutant deleted in the alpha 22 gene: Growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J. Virol. 1985;55:338–346. doi: 10.1128/JVI.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Poffenberger K.L., Raichlen P.E., Herman R.C. In vitro characterization of a herpes simplex virus type 1 ICP22 deletion mutant. Virus Genes. 1993;7:171–186. doi: 10.1007/BF01702397. [DOI] [PubMed] [Google Scholar]
- 240.Jacquemont B., Verrier B., Epstein A.L., Machuca I. Expression of immediate-early genes in herpes simplex virus type 1-infected XC cells: Lack of ICP22 (68K) polypeptide. J. Gen. Virol. 1984;65:1331–1340. doi: 10.1099/0022-1317-65-8-1331. [DOI] [PubMed] [Google Scholar]
- 241.Orlando J.S., Astor T.L., Rundle S.A., Schaffer P.A. The products of the herpes simplex virus type 1 immediate-early US1/US1.5 genes downregulate levels of S-phase-specific cyclins and facilitate virus replication in S-phase Vero cells. J. Virol. 2006;80:4005–4016. doi: 10.1128/JVI.80.8.4005-4016.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ogle W.O., Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J. Virol. 1999;73:4305–4315. doi: 10.1128/JVI.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Poffenberger K.L., Idowu A.D., Fraser-Smith E.B., Raichlen P.E., Herman R.C. A herpes simplex virus type 1 ICP22 deletion mutant is altered for virulence and latency in vivo. Arch. Virol. 1994;139:111–119. doi: 10.1007/BF01309458. [DOI] [PubMed] [Google Scholar]
- 244.Orlando J.S., Balliet J.W., Kushnir A.S., Astor T.L., Kosz-Vnenchak M., Rice S.A., Knipe D.M., Schaffer P.A. ICP22 is required for wild-type composition and infectivity of herpes simplex virus type 1 virions. J. Virol. 2006;80:9381–9390. doi: 10.1128/JVI.01061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brandt C.R., Kolb A.W. Tyrosine 116 of the herpes simplex virus type 1 IEalpha22 protein is an ocular virulence determinant and potential phosphorylation site. Invest. Ophthalmol. Vis. Sci. 2003;44:4601–4607. doi: 10.1167/iovs.03-0582. [DOI] [PubMed] [Google Scholar]
- 246.Bowles R.N., Blaho J.A. A truncation mutation of the neurovirulence ICP22 protein produced by a recombinant HSV-1 generated by bacterial artificial chromosome technology targets infected cell nuclei. J. Neurovirol. 2011;17:559–569. doi: 10.1007/s13365-011-0064-z. [DOI] [PubMed] [Google Scholar]
- 247.Holden V.R., Zhao Y., Thompson Y., Caughman G.B., Smith R.H., O’Callaghan D.J. Characterization of the regulatory function of the ICP22 protein of equine herpesvirus type 1. Virology. 1995;210:273–282. doi: 10.1006/viro.1995.1344. [DOI] [PubMed] [Google Scholar]
- 248.Kost R.G., Kupinsky H., Straus S.E. Varicella-zoster virus gene 63: Transcript mapping and regulatory activity. Virology. 1995;209:218–224. doi: 10.1006/viro.1995.1246. [DOI] [PubMed] [Google Scholar]
- 249.Heineman T.C., Cohen J.I. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 1995;69:7367–7370. doi: 10.1128/JVI.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gray W.L., Gusick N.J., Ek-Kommonen C., Kempson S.E., Fletcher T.M. 3rd The inverted repeat regions of the simian varicella virus and varicella-zoster virus genomes have a similar genetic organization. Virus Res. 1995;39:181–193. doi: 10.1016/0168-1702(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 251.Cai M., Jiang S., Zeng Z., Li X., Mo C., Yang Y., Chen C., Xie P., Bian Y., Wang J., et al. Probing the nuclear import signal and nuclear transport molecular determinants of PRV ICP22. Cell Biosci. 2016;6:3. doi: 10.1186/s13578-016-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Blaho J.A., Mitchell C., Roizman B. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J. Virol. 1993;67:3891–3900. doi: 10.1128/JVI.67.7.3891-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Purves F.C., Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Purves F.C., Ogle W.O., Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Blaho J.A., Zong C.S., Mortimer K.A. Tyrosine phosphorylation of the herpes simplex virus type 1 regulatory protein ICP22 and a cellular protein which shares antigenic determinants with ICP22. J. Virol. 1997;71:9828–9832. doi: 10.1128/JVI.71.12.9828-9832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.O’Toole J.M., Aubert M., Kotsakis A., Blaho J.A. Mutation of the protein tyrosine kinase consensus site in the herpes simplex virus 1 alpha22 gene alters ICP22 posttranslational modification. Virology. 2003;305:153–167. doi: 10.1006/viro.2002.1746. [DOI] [PubMed] [Google Scholar]
- 257.Stelz G., Rücker E., Rosorius O., Meyer G., Stauber R.H., Spatz M., Eibl M.M., Hauber J. Identification of two nuclear import signals in the alpha-gene product ICP22 of herpes simplex virus 1. Virology. 2002;295:360–370. doi: 10.1006/viro.2002.1384. [DOI] [PubMed] [Google Scholar]
- 258.Ng T.I., Chang Y.E., Roizman B. Infected cell protein 22 of herpes simplex virus 1 regulates the expression of virion host shutoff gene U(L)41. Virology. 1997;234:226–234. doi: 10.1006/viro.1997.8659. [DOI] [PubMed] [Google Scholar]
- 259.Advani S.J., Brandimarti R., Weichselbaum R.R., Roizman B. The disappearance of cyclins A and B and the increase in activity of the G(2)/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the alpha22/U(S)1.5 and U(L)13 viral genes. J. Virol. 2000;74:8–15. doi: 10.1128/JVI.74.1.8-15.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Advani S.J., Weichselbaum R.R., Roizman B. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA. 2000;97:10996–11001. doi: 10.1073/pnas.200375297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Advani S.J., Weichselbaum R.R., Roizman B. Herpes simplex virus 1 activates cdc2 to recruit topoisomerase II alpha for post-DNA synthesis expression of late genes. Proc. Natl. Acad. Sci. USA. 2003;100:4825–4830. doi: 10.1073/pnas.0730735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Smith-Donald B.A., Roizman B. The interaction of herpes simplex virus 1 regulatory protein ICP22 with the cdc25C phosphatase is enabled in vitro by viral protein kinases US3 and UL13. J. Virol. 2008;82:4533–4543. doi: 10.1128/JVI.02022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rice S.A., Long M.C., Lam V., Schaffer P.A., Spencer C.A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 1995;69:5550–5559. doi: 10.1128/JVI.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rice S.A., Long M.C., Lam V., Spencer C.A. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J. Virol. 1994;68:988–1001. doi: 10.1128/JVI.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Spencer C.A., Dahmus M.E., Rice S.A. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 1997;71:2031–2040. doi: 10.1128/JVI.71.3.2031-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jenkins H.L., Spencer C.A. RNA polymerase II holoenzyme modifications accompany transcription reprogramming in herpes simplex virus type 1-infected cells. J. Virol. 2001;75:9872–9884. doi: 10.1128/JVI.75.20.9872-9884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Long M.C., Leong V., Schaffer P.A., Spencer C.A., Rice S.A. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 1999;73:5593–5604. doi: 10.1128/JVI.73.7.5593-5604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bastian T.W., Rice S.A. Identification of sequences in herpes simplex virus type 1 ICP22 that influence RNA polymerase II modification and viral late gene expression. J. Virol. 2009;83:128–139. doi: 10.1128/JVI.01954-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bowman J.J., Schaffer P.A. Origin of expression of the herpes simplex virus type 1 protein U(S)1.5. J. Virol. 2009;83:9183–9194. doi: 10.1128/JVI.00984-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Leopardi R., Ward P.L., Ogle W.O., Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the U(L)13 proteinkinase. J. Virol. 1997;71:1133–1139. doi: 10.1128/JVI.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jahedi S., Markovitz N.S., Filatov F., Roizman B. Colocalization of the herpes simplex virus 1 UL4 protein with infected cell protein 22 in small, dense nuclear structures formed prior to onset of DNA synthesis. J. Virol. 1999;73:5132–5138. doi: 10.1128/JVI.73.6.5132-5136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wu N., Watkins S.C., Schaffer P.A., DeLuca N.A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J. Virol. 1996;70:6358–6369. doi: 10.1128/JVI.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cun W., Chen J., Zhang Y., Liu L., Li Q. Analysis of the cellular localization of herpes simplex virus 1 immediate-early protein ICP22. Virol. Sin. 2010;25:158–167. doi: 10.1007/s12250-010-3118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Durand L.O., Roizman B. Role of cdk9 in the optimization of expression of the genes regulated by ICP22 of herpes simplex virus 1. J. Virol. 2008;82:10591–10599. doi: 10.1128/JVI.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ou M., Sandri-Goldin R.M. Inhibition of cdk9 during herpes simplex virus 1 infection impedes viral transcription. PLoS ONE. 2013;8:e79007. doi: 10.1371/journal.pone.0079007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fraser K.A., Rice S.A. Herpes simplex virus immediate-early protein ICP22 triggers loss of serine 2-phosphorylated RNA polymerase II. J. Virol. 2007;81:5091–5101. doi: 10.1128/JVI.00184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fraser K.A., Rice S.A. Herpes simplex virus type 1 infection leads to loss of serine-2 phosphorylation on the carboxyl-terminal domain of RNA polymerase II. J. Virol. 2005;79:11323–11334. doi: 10.1128/JVI.79.17.11323-11334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Guo L., Wu W., Liu L., Wang L., Zhang Y., Wu L., Guan Y., Li Q. Herpes simplex virus 1 ICP22 inhibits the transcription of viral gene promoters by binding to and blocking the recruitment of P-TEFb. PLoS ONE. 2012;7:e45749. doi: 10.1371/journal.pone.0045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zaborowska J., Baumli S., Laitem C., O’Reilly D., Thomas P.H., O’Hare P., Murphy S. Herpes simplex virus 1 (HSV-1) ICP22 protein directly interacts with cyclin-dependent kinase (CDK)9 to inhibit RNA polymerase II transcription elongation. PLoS ONE. 2014;9:e107654. doi: 10.1371/journal.pone.0107654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Prod’hon C., Machuca I., Berthomme H., Epstein A., Jacquemont B. Characterization of regulatory functions of the HSV-1 immediate-early protein ICP22. Virology. 1996;226:393–402. doi: 10.1006/viro.1996.0667. [DOI] [PubMed] [Google Scholar]
- 281.Kwun H.J., Yim S.W., Lee D.H., Jang K.L. Activation of the thymidine kinase promoter by herpes simplex virus type 1 immediate early proteins. Mol. Cells. 1999;9:277–280. [PubMed] [Google Scholar]
- 282.Poon A.P.W., Roizman B. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 2005;79:8470–8479. doi: 10.1128/JVI.79.13.8470-8479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang Y., Yang Z., Cao Y., Zhang S., Li H., Huang Y., Ding Y.-Q., Liu X. The Hsp40 family chaperone protein DnaJB6 enhances Schlafen1 nuclear localization which is critical for promotion of cell-cycle arrest in T-cells. Biochem. J. 2008;413:239–250. doi: 10.1042/BJ20071510. [DOI] [PubMed] [Google Scholar]
- 284.Sullivan C.S., Pipas J.M. The virus-chaperone connection. Virology. 2001;287:1–8. doi: 10.1006/viro.2001.1038. [DOI] [PubMed] [Google Scholar]
- 285.Adlakha M., Livingston C.M., Bezsonova I., Weller S.K. The herpes simplex virus 1 immediate early protein ICP22 is a functional mimic of a cellular J protein. J. Virol. 2020;94 doi: 10.1128/JVI.01564-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bastian T.W., Livingston C.M., Weller S.K., Rice S.A. Herpes simplex virus type 1 immediate-early protein ICP22 is required for VICE domain formation during productive viral infection. J. Virol. 2010;84:2384–2394. doi: 10.1128/JVI.01686-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Burch A.D., Weller S.K. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J. Virol. 2005;79:10740–10749. doi: 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Burch A.D., Weller S.K. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 2004;78:7175–7185. doi: 10.1128/JVI.78.13.7175-7185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Livingston C.M., Ifrim M.F., Cowan A.E., Weller S.K. Virus-Induced Chaperone-Enriched (VICE) domains function as nuclear protein quality control centers during HSV-1 infection. PLoS Pathog. 2009;5:e1000619. doi: 10.1371/journal.ppat.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mostafa H.H., Davido D.J. Herpes simplex virus 1 ICP22 but not US 1.5 is required for efficient acute replication in mice and VICE domain formation. J. Virol. 2013;87:13510–13519. doi: 10.1128/JVI.02424-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Maruzuru Y., Shindo K., Liu Z., Oyama M., Kozuka-Hata H., Arii J., Kato A., Kawaguchi Y. Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress. J. Virol. 2014;88:7445–7454. doi: 10.1128/JVI.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Matundan H., Ghiasi H. Herpes simplex virus 1 ICP22 suppresses CD80 Expression by murine dendritic cells. J. Virol. 2019;93 doi: 10.1128/JVI.01803-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tormanen K., Wang S., Ghiasi H. CD80 plays a critical role in increased inflammatory responses in herpes simplex virus 1-infected mouse corneas. J. Virol. 2020;94 doi: 10.1128/JVI.01511-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Matundan H.H., Jaggi U., Wang S., Ghiasi H. Loss of ICP22 in HSV-1 elicits immune infiltration and maintains stromal keratitis despite reduced primary and latent virus infectivity. Invest. Ophthalmol. Vis. Sci. 2019;60:3398–3406. doi: 10.1167/iovs.19-27701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang M., Fu M., Li M., Hu H., Gong S., Hu Q. Herpes simplex virus type 2 inhibits type I IFN signaling mediated by the novel E3 ubiquitin protein ligase activity of viral protein ICP22. J. Immunol. 2020;205:1281–1292. doi: 10.4049/jimmunol.2000418. [DOI] [PubMed] [Google Scholar]
- 296.Chou J., Roizman B. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J. Virol. 1986;57:629–637. doi: 10.1128/JVI.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ackermann M., Chou J., Sarmiento M., Lerner R.A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J. Virol. 1986;58:843–850. doi: 10.1128/JVI.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Taha M., Clements G., Brown S. A variant of herpes simplex virus type 2 strain HG52 with a 1.5 kb deletion in RL between 0 to 0.02 and 0.81 to 0.83 map units is non-neurovirulent for mice. J. Gen. Virol. 1989;70:705–716. doi: 10.1099/0022-1317-70-3-705. [DOI] [PubMed] [Google Scholar]
- 299.Taha M.Y., Clements G.B., Brown S.M. The herpes simplex virus type 2 (HG52) variant JH2604 has a 1488 bp deletion which eliminates neurovirulence in mice. J. Gen. Virol. 1989;70:3073–3078. doi: 10.1099/0022-1317-70-11-3073. [DOI] [PubMed] [Google Scholar]
- 300.Whitley R.J., Kern E.R., Chatterjee S., Chou J., Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J. Clin. Investig. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.MacLean A.R., Ul-Fareed M., Robertson L., Harland J., Brown S.M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the “a” sequence. J. Gen. Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 302.Chou J., Kern E.R., Whitley R.J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 303.Robertson L.M., MacLean A.R., Brown S.M. Peripheral replication and latency reactivation kinetics of the non-neurovirulent herpes simplex virus type 1 variant 1716. J. Gen. Virol. 1992;73:967–970. doi: 10.1099/0022-1317-73-4-967. [DOI] [PubMed] [Google Scholar]
- 304.Dolan A., McKie E., MacLean A.R., McGeoch D.J. Status of the ICP34.5 gene in herpes simplex virus type 1 strain 17. J. Gen. Virol. 1992;73:971–973. doi: 10.1099/0022-1317-73-4-971. [DOI] [PubMed] [Google Scholar]
- 305.Perng G.C., Thompson R.L., Sawtell N.M., Taylor W.E., Slanina S.M., Ghiasi H., Kaiwar R., Nesburn A.B., Wechsler S.L. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J. Virol. 1995;69:3033–3041. doi: 10.1128/JVI.69.5.3033-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Thompson R.L., Stevens J.G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983;131:171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- 307.Bolovan C.A., Sawtell N.M., Thompson R.L. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 1994;68:48–55. doi: 10.1128/JVI.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Perng G.C., Ghiasi H., Slanina S.M., Nesburn A.B., Wechsler S.L. High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J. Virol. 1996;70:2883–2893. doi: 10.1128/JVI.70.5.2883-2893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mattila R.K., Harila K., Kangas S.M., Paavilainen H., Heape A.M., Mohr I.J., Hukkanen V. An investigation of herpes simplex virus type 1 latency in a novel mouse dorsal root ganglion model suggests a role for ICP34.5 in reactivation. J. Gen. Virol. 2015;96:2304–2313. doi: 10.1099/vir.0.000138. [DOI] [PubMed] [Google Scholar]
- 310.Brown S.M., Harland J., MacLean A.R., Podlech J., Clements J.B. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J. Gen. Virol. 1994;75:2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- 311.Kesari S., Lasner T.M., Balsara K.R., Randazzo B.P., Lee V.M., Trojanowski J.Q., Fraser N.W. A neuroattenuated ICP34.5-deficient herpes simplex virus type 1 replicates in ependymal cells of the murine central nervous system. J. Gen. Virol. 1998;79:525–536. doi: 10.1099/0022-1317-79-3-525. [DOI] [PubMed] [Google Scholar]
- 312.Mao H., Rosenthal K.S. Strain-dependent structural variants of herpes simplex virus type 1 ICP34.5 determine viral plaque size, efficiency of glycoprotein processing, and viral release and neuroinvasive disease potential. J. Virol. 2003;77:3409–3417. doi: 10.1128/JVI.77.6.3409-3417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Brown S.M., MacLean A.R., McKie E.A., Harland J. The herpes simplex virus virulence factor ICP34.5 and the cellular protein MyD116 complex with proliferating cell nuclear antigen through the 63-amino-acid domain conserved in ICP34.5, MyD116, and GADD34. J. Virol. 1997;71:9442–9449. doi: 10.1128/JVI.71.12.9442-9449.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Chou J., Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.McGeoch D.J., Barnett B.C. Neurovirulence factor. Nature. 1991;353:609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- 317.Chou J., Chen J.J., Gross M., Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes si. Proc. Natl. Acad. Sci. USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Cassady K.A., Gross M., Roizman B. The second-site mutation in the herpes simplex virus recombinants lacking the gamma134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2alpha. J. Virol. 1998;72:7005–7011. doi: 10.1128/JVI.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ward S.L., Scheuner D., Poppers J., Kaufman R.J., Mohr I., Leib D.A. In vivo replication of an ICP34.5 second-site suppressor mutant following corneal infection correlates with in vitro regulation of eIF2 alpha phosphorylation. J. Virol. 2003;77:4626–4634. doi: 10.1128/JVI.77.8.4626-4634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tallóczy Z., Virgin H.W., IV, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2:24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 322.Wylie K.M., Schrimpf J.E., Morrison L.A. Increased eIF2alpha phosphorylation attenuates replication of herpes simplex virus 2 vhs mutants in mouse embryonic fibroblasts and correlates with reduced accumulation of the PKR antagonist ICP34.5. J. Virol. 2009;83:9151–9162. doi: 10.1128/JVI.00886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.He B., Gross M., Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activ. Proc. Natl. Acad. Sci. USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Cheng G., Brett M.-E., He B. Val193 and Phe195 of the γ134.5 protein of herpes simplex virus 1 are required for viral resistance to interferon-α/β. Virology. 2001;290:115–120. doi: 10.1006/viro.2001.1148. [DOI] [PubMed] [Google Scholar]
- 325.Leib D.A., Machalek M.A., Williams B.R.G., Silverman R.H., Virgin H.W. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Verpooten D., Ma Y., Hou S., Yan Z., He B. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J. Biol. Chem. 2009;284:1097–1105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Manivanh R., Mehrbach J., Knipe D.M., Leib D.A. Role of herpes simplex virus 1 γ34.5 in the regulation of IRF3 signaling. J. Virol. 2017;91 doi: 10.1128/JVI.01156-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Orvedahl A., Alexander D., Tallóczy Z., Sun Q., Wei Y., Zhang W., Burns D., Leib D.A., Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 329.Tallóczy Z., Jiang W., Virgin H.W., Leib D.A., Scheuner D., Kaufman R.J., Eskelinen E.-L., Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl. Acad. Sci. USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Leib D.A., Alexander D.E., Cox D., Yin J., Ferguson T.A. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J. Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D., Alexander D., Leib D., Norbury C., Lippé R., et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Gobeil P.A.M., Leib D.A. Herpes simplex virus γ34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. mBio. 2012;3 doi: 10.1128/mBio.00267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Alexander D.E., Ward S.L., Mizushima N., Levine B., Leib D.A. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J. Virol. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Alexander D.E., Leib D.A. Xenophagy in herpes simplex virus replication and pathogenesis. Autophagy. 2008;4:101–103. doi: 10.4161/auto.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Santana S., Bullido M.J., Recuero M., Valdivieso F., Aldudo J. Herpes simplex virus type I induces an incomplete autophagic response in human neuroblastoma cells. J. Alzheimers Dis. 2012;30:815–831. doi: 10.3233/JAD-2012-112000. [DOI] [PubMed] [Google Scholar]
- 336.Rasmussen S.B., Horan K.A., Holm C.K., Stranks A.J., Mettenleiter T.C., Simon A.K., Jensen S.B., Rixon F.J., He B., Paludan S.R. Activation of autophagy by α-herpesviruses in myeloid cells is mediated by cytoplasmic viral DNA through a mechanism dependent on stimulator of IFN genes. J. Immunol. 2011;187:5268–5276. doi: 10.4049/jimmunol.1100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Budida R., Stankov M.V., Döhner K., Buch A., Panayotova-Dimitrova D., Tappe K.A., Pohlmann A., Sodeik B., Behrens G.M.N. Herpes simplex virus 1 interferes with autophagy of murine dendritic cells and impairs their ability to stimulate CD8(+) T lymphocytes. Eur. J. Immunol. 2017;47:1819–1834. doi: 10.1002/eji.201646908. [DOI] [PubMed] [Google Scholar]
- 338.York I.A., Roop C., Andrews D.W., Riddell S.R., Graham F.L., Johnson D.C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 339.Banks T.A., Nair S., Rouse B.T. Recognition by and in vitro induction of cytotoxic T lymphocytes against predicted epitopes of the immediate-early protein ICP27 of herpes simplex virus. J. Virol. 1993;67:613–616. doi: 10.1128/JVI.67.1.613-616.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Fruh K., Ahn K., Djaballah H., Sempe P., van Endert P.M., Tampé R., Peterson P.A., Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 341.Tomazin R., Hill A.B., Jugovic P., York I., van Endert P., Ploegh H.L., Andrews D.W., Johnson D.C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. doi: 10.1002/j.1460-2075.1996.tb00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Galocha B., Hill A., Barnett B.C., Dolan A., Raimondi A., Cook R.F., Brunner J., Mcgeoch D.J., Ploegh H.L. The active site of ICP47, a herpes simplex virus-encoded inhibitor of the major histocompatibility complex (MHC)-encoded peptide transported associated with antigen processing (TAP), maps to the NH2-terminal 35 residues. J. Exp. Med. 1997;185:1565–1572. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ahn K., Meyer T.H., Uebel S., Sempé P., Djaballah H., Yang Y., Peterson P.A., Früh K., Tampé R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus protein ICP47. EMBO J. 1996;15:3247–3255. doi: 10.1002/j.1460-2075.1996.tb00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tomazin R., van Schoot N.E.G., Goldsmith K., Jugovic P., Sempé P., Früh K., Johnson D.C. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J. Virol. 1998;72:2560–2563. doi: 10.1128/JVI.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jugovic P., Hill A.M., Tomazin R., Ploegh H., Johnson D.C. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J. Virol. 1998;72:5076–5084. doi: 10.1128/JVI.72.6.5076-5084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Orr M.T., Edelmann K.H., Vieira J., Corey L., Raulet D.H., Wilson C.B. Inhibition of MHC class I is a virulence factor in herpes simplex virus infection of mice. PLoS Pathog. 2005;1:e7. doi: 10.1371/journal.ppat.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Liu B.L., Robinson M., Han Z.Q., Branston R.H., English C., Reay P., McGrath Y., Thomas S.K., Thornton M., Bullock P., et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 348.Thomas S., Kuncheria L., Roulstone V., Kyula J.N., Mansfield D., Bommareddy P.K., Smith H., Kaufman H.L., Harrington K.J., Coffin R.S. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer. 2019;7:1–17. doi: 10.1186/s40425-019-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Saric T., Chang S.C., Hattori A., York I.A., Markant S., Rock K.L., Tsujimoto M., Goldberg A.L. An IFN-γ-induced aminopeptidase in the ER, ERAP I, trims precursors to MHC class I-presented peptides. Nat. Immunol. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 350.Marijt K.A., van Hall T. To TAP or not to TAP: Alternative peptides for immunotherapy of cancer. Curr. Opin. Immunol. 2020;64:15–19. doi: 10.1016/j.coi.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 351.Raafat N., Sadowski-Cron C., Mengus C., Heberer M., Spagnoli G.C., Zajac P. Preventing vaccinia virus class-I epitopes presentation by HSV-ICP47 enhances the immunogenicity of a TAP-independent cancer vaccine epitope. Int. J. Cancer. 2012;131:659–669. doi: 10.1002/ijc.27362. [DOI] [PubMed] [Google Scholar]
- 352.Nishiyama Y., Kurachi R., Daikoku T., Umene K. The US 9, 10, 11, and 12 genes of herpes simplex virus type 1 are of no importance for its neurovirulence and latency in mice. Virology. 1993;194:419–423. doi: 10.1006/viro.1993.1279. [DOI] [PubMed] [Google Scholar]
- 353.Goldsmith B.K., Chen W., Johnson D.C., Hendricks R.L. Infected Cell Protein (ICP)47 enhances herpes simpex virus neurovirulence by blocking the CD8 T cell response. J. Exp. Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Burgos J.S., Serrano-Saiz E., Sastre I., Valdivieso F. ICP47 mediates viral neuroinvasiveness by induction of TAP protein following intravenous inoculation of herpes simplex virus type 1 in mice. J. Neurovirol. 2006;12:420–427. doi: 10.1080/13550280601009546. [DOI] [PubMed] [Google Scholar]
- 355.Lindahl T. DNA repair enzymes. Annu. Rev. Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- 356.Worrad D.M., Caradonna S. Identification of the coding sequence for herpes simplex virus uracil-DNA glycosylase. J. Virol. 1988;62:4774–4777. doi: 10.1128/JVI.62.12.4774-4777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Savva R., Pearl L.H. Crystallization and preliminary X-ray analysis of the uracil-DNA glycosylase DNA repair enzyme from herpes simplex virus type 1. J. Mol. Biol. 1993;234:910–912. doi: 10.1006/jmbi.1993.1642. [DOI] [PubMed] [Google Scholar]
- 358.Caradonna S.J., Cheng Y.C. Induction of uracil-DNA glycosylase and dUTP nucleotidohydrolase activity in herpes simplex virus-infected human cells. J. Biol. Chem. 1981;256:9834–9837. [PubMed] [Google Scholar]
- 359.Caradonna S., Worrad D., Lirette R. Isolation of a herpes simplex virus cDNA encoding the DNA repair enzyme uracil-DNA glycosylase. J. Virol. 1987;61:3040–3047. doi: 10.1128/JVI.61.10.3040-3047.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Focher F., Verri A., Verzeletti S., Mazzarello P., Spadari S. Uracil in Oris of herpes simplex 1 alters its specific recognition by origin binding protein (OBP): Does virus induced uracil-DNA glycosylase play a key role in viral reactivation and replication? Chromosoma. 1992;102:67–71. doi: 10.1007/BF02451788. [DOI] [PubMed] [Google Scholar]
- 361.Pyles R.B., Thompson R.L. Evidence that the herpes simplex virus type 1 uracil DNA glycosylase is required for efficient viral replication and latency in the murine nervous system. J. Virol. 1994;68:4963–4972. doi: 10.1128/JVI.68.8.4963-4972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Bogani F., Chua C.N., Boehmer P.E. Reconstitution of uracil DNA glycosylase-initiated base excision repair in herpes simplex virus-1. J. Biol. Chem. 2009;284:16784–16790. doi: 10.1074/jbc.M109.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Bogani F., Corredeira I., Fernandez V., Sattler U., Rutvisuttinunt W., Defais M., Boehmer P.E. Association between the herpes simplex virus-1 DNA polymerase and uracil DNA glycosylase. J. Biol. Chem. 2010;285:27664–27672. doi: 10.1074/jbc.M110.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Prichard M.N., Lawlor H., Duke G.M., Mo C., Wang Z., Dixon M., Kemble G., Kern E.R. Human cytomegalovirus uracil DNA glycosylase associates with ppUL44 and accelerates the accumulation of viral DNA. Virol. J. 2005;2:55. doi: 10.1186/1743-422X-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Courcelle C.T., Courcelle J., Prichard M.N., Mocarski E.S. Requirement for uracil-DNA glycosylase during the transition to late-phase cytomegalovirus DNA replication. J. Virol. 2001;75:7592–7601. doi: 10.1128/JVI.75.16.7592-7601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Li M., Zou X., Wang Y., Xu Z., Ou X., Li Y., Liu D., Guo Y., Deng Y., Jiang S., et al. The nuclear localization signal-mediated nuclear targeting of herpes simplex virus 1 early protein UL2 is important for efficient viral production. Aging. 2020;12:2921–2938. doi: 10.18632/aging.102786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mullaney J., Moss H.W., McGeoch D.J. Gene UL2 of herpes simplex virus type 1 encodes a uracil-DNA glycosylase. J. Gen. Virol. 1989;70:449–454. doi: 10.1099/0022-1317-70-2-449. [DOI] [PubMed] [Google Scholar]
- 368.Dean H.J., Cheung A.K. A 3’ coterminal gene cluster in pseudorabies virus contains herpes simplex virus UL1, UL2, and UL3 gene homologs and a unique UL3.5 open reading frame. J. Virol. 1993;67:5955–5961. doi: 10.1128/JVI.67.10.5955-5961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Khattar S.K., van Drunen Littel-van den Hurk S., Babiuk L.A., Tikoo S.K. Identification and transcriptional analysis of a 3’-coterminal gene cluster containing UL1, UL2, UL3, and UL3.5 open reading frames of bovine herpesvirus-1. Virology. 1995;213:28–37. doi: 10.1006/viro.1995.1543. [DOI] [PubMed] [Google Scholar]
- 370.Li H., Liu S., Han Z., Shao Y., Chen S., Kong X. Comparative analysis of the genes UL1 through UL7 of the duck enteritis virus and other herpesviruses of the subfamily Alphaherpesvirinae. Genet. Mol. Biol. 2009;32:121–128. doi: 10.1590/S1415-47572009005000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Prichard M.N., Duke G.M., Mocarski E.S. Human cytomegalovirus uracil DNA glycosylase is required for the normal temporal regulation of both DNA synthesis and viral replication. J. Virol. 1996;70:3018–3025. doi: 10.1128/JVI.70.5.3018-3025.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Puvion-Dutilleul F., Pichard E. Viral alkaline nuclease in intranuclear dense bodies induced by herpes simplex infection. Biol. Cell. 1986;58:15–22. doi: 10.1111/j.1768-322X.1986.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 373.Knipe D.M. The role of viral and cellular nuclear proteins in herpes simplex virus replication. Adv. Virus Res. 1989;37:85–123. doi: 10.1016/s0065-3527(08)60833-7. [DOI] [PubMed] [Google Scholar]
- 374.Banks L.M., Halliburton I.W., Purifoy D.J., Killington R.A., Powell K.L. Studies on the herpes simplex virus alkaline nuclease: Detection of type-common and type-specific epitopes on the enzyme. J. Gen. Virol. 1985;66:1–14. doi: 10.1099/0022-1317-66-1-1. [DOI] [PubMed] [Google Scholar]
- 375.Daikoku T., Yamashita Y., Tsurumi T., Nishiyama Y. The US3 protein kinase of herpes simplex virus type 2 is associated with phosphorylation of the UL12 alkaline nuclease in vitro. Arch. Virol. 1995;140:1637–1644. doi: 10.1007/BF01322537. [DOI] [PubMed] [Google Scholar]
- 376.Costa R.H., Draper K.G., Banks L., Powell K.L., Cohen G., Eisenberg R., Wagner E.K. High-resolution characterization of herpes simplex virus type 1 transcripts encoding alkaline exonuclease and a 50,000-dalton protein tentatively identified as a capsid protein. J. Virol. 1983;48:591–603. doi: 10.1128/JVI.48.3.591-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Preston C.M., Cordingley M.G. mRNA- and DNA-directed synthesis of herpes simplex virus-coded exonuclease in Xenopus laevis oocytes. J. Virol. 1982;43:386–394. doi: 10.1128/JVI.43.2.386-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wathen M.W., Hay J. Physical mapping of the herpes simplex virus type 2 nuc- lesion affecting alkaline exonuclease activity by using herpes simplex virus type 1 deletion clones. J. Virol. 1984;51:237–241. doi: 10.1128/JVI.51.1.237-241.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Draper K.G., Devi-Rao G., Costa R.H., Blair E.D., Thompson R.L., Wagner E.K. Characterization of the genes encoding herpes simplex virus type 1 and type 2 alkaline exonucleases and overlapping proteins. J. Virol. 1986;57:1023–1036. doi: 10.1128/JVI.57.3.1023-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Baer R., Bankier A.T., Biggin M.D., Deininger P.L., Farrell P.J., Gibson T.J., Hatfull G., Hudson G.S., Satchwell S.C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 381.Grose C., Tyler S., Peters G., Hiebert J., Stephens G.M., Ruyechan W.T., Jackson W., Storlie J., Tipples G.A. Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: Evolution of genotypes with an accelerated cell spread phenotype. J. Virol. 2004;78:6799–6807. doi: 10.1128/JVI.78.13.6799-6807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.De Wind N., Peeters B.P., Zuderveld A., Gielkens A.L., Berns A.J., Kimman T.G. Mutagenesis and characterization of a 41-kilobase-pair region of the pseudorabies virus genome: Transcription map, search for virulence genes, and comparison with homologs of herpes simplex virus type 1. Virology. 1994;200:784–790. doi: 10.1006/viro.1994.1242. [DOI] [PubMed] [Google Scholar]
- 383.Gao M., Robertson B.J., McCann P.J., O’Boyle D.R., Weller S.K., Newcomb W.W., Brown J.C., Weinheimer S.P. Functional conservations of the alkaline nuclease of herpes simplex type 1 and human cytomegalovirus. Virology. 1998;249:460–470. doi: 10.1006/viro.1998.9344. [DOI] [PubMed] [Google Scholar]
- 384.Weller S.K., Seghatoleslami M.R., Shao L., Rowse D., Carmichael E.P. The herpes simplex virus type 1 alkaline nuclease is not essential for viral DNA synthesis: Isolation and characterization of a lacZ insertion mutant. J. Gen. Virol. 1990;71:2941–2952. doi: 10.1099/0022-1317-71-12-2941. [DOI] [PubMed] [Google Scholar]
- 385.Shao L., Rapp L.M., Weller S.K. Herpes simplex virus 1 alkaline nuclease is required for efficient egress of capsids from the nucleus. Virology. 1993;196:146–162. doi: 10.1006/viro.1993.1463. [DOI] [PubMed] [Google Scholar]
- 386.Sagou K., Uema M., Kawaguchi Y. Nucleolin is required for efficient nuclear egress of herpes simplex virus type 1 nucleocapsids. J. Virol. 2010;84:2110–2121. doi: 10.1128/JVI.02007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Porter I.M., Stow N.D. Virus particles produced by the herpes simplex virus type 1 alkaline nuclease null mutant ambUL12 contain abnormal genomes. J. Gen. Virol. 2004;85:583–591. doi: 10.1099/vir.0.19657-0. [DOI] [PubMed] [Google Scholar]
- 388.Grady L.M., Szczepaniak R., Murelli R.P., Masaoka T., Le Grice S.F.J., Wright D.L., Weller S.K. The exonuclease activity of herpes simplex virus 1 UL12 is required for production of viral DNA that can be packaged to produce infectious virus. J. Virol. 2017;91 doi: 10.1128/JVI.01380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Martinez R., Sarisky R.T., Weber P.C., Weller S.K. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J. Virol. 1996;70:2075–2085. doi: 10.1128/JVI.70.4.2075-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Goldstein J.N., Weller S.K. In vitro processing of herpes simplex virus type 1 DNA replication intermediates by the viral alkaline nuclease, UL12. J. Virol. 1998;72:8772–8781. doi: 10.1128/JVI.72.11.8772-8781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Henderson J.O., Ball-Goodrich L.J., Parris D.S. Structure–function analysis of the herpes simplex virus type 1 UL12 gene: Correlation of deoxyribonuclease activityin vitrowith replication function. Virology. 1998;243:247–259. doi: 10.1006/viro.1998.9054. [DOI] [PubMed] [Google Scholar]
- 392.Reuven N.B., Staire A.E., Myers R.S., Weller S.K. The herpes simplex virus type 1 alkaline nuclease and single-stranded DNA binding protein mediate strand exchange in vitro. J. Virol. 2003;77:7425–7433. doi: 10.1128/JVI.77.13.7425-7433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Reuven N.B., Willcox S., Griffith J.D., Weller S.K. Catalysis of strand exchange by the HSV-1 UL12 and ICP8 proteins: Potent ICP8 recombinase activity is revealed upon resection of dsDNA substrate by nuclease. J. Mol. Biol. 2004;342:57–71. doi: 10.1016/j.jmb.2004.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Reuven N.B., Weller S.K. Herpes simplex virus type 1 single-strand DNA binding protein ICP8 enhances the nuclease activity of the UL12 alkaline nuclease by increasing its processivity. J. Virol. 2005;79:9356–9358. doi: 10.1128/JVI.79.14.9356-9358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Schumacher A.J., Mohni K.N., Kan Y., Hendrickson E.A., Stark J.M., Weller S.K. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog. 2012;8:e1002862. doi: 10.1371/journal.ppat.1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Tolun G., Makhov A.M., Ludtke S.J., Griffith J.D. Details of ssDNA annealing revealed by an HSV-1 ICP8-ssDNA binary complex. Nucleic Acids Res. 2013;41:5927–5937. doi: 10.1093/nar/gkt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Balasubramanian N., Bai P., Buchek G., Korza G., Weller S.K. Physical interaction between the herpes simplex virus type 1 exonuclease, UL12, and the DNA double-strand break-sensing MRN complex. J. Virol. 2010;84:12504–12514. doi: 10.1128/JVI.01506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Bronstein J.C., Weller S.K., Weber P.C. The product of the UL12.5 gene of herpes simplex virus type 1 is a capsid-associated nuclease. J. Virol. 1997;71:3039–3047. doi: 10.1128/JVI.71.4.3039-3047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Reuven N.B., Antoku S., Weller S.K. The UL12.5 gene product of herpes simplex virus type 1 exhibits nuclease and strand exchange activities but does not localize to the nucleus. J. Virol. 2004;78:4599–4608. doi: 10.1128/JVI.78.9.4599-4608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Martinez R., Goldstein J.N., Weller S.K. The product of the UL12.5 gene of herpes simplex virus type 1 is not essential for lytic viral growth and is not specifically associated with capsids. Virology. 2002;298:248–257. doi: 10.1006/viro.2002.1444. [DOI] [PubMed] [Google Scholar]
- 401.Saffran H.A., Pare J.M., Corcoran J.A., Weller S.K., Smiley J.R. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep. 2007;8:188–193. doi: 10.1038/sj.embor.7400878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Duguay B.A., Smiley J.R. Mitochondrial nucleases ENDOG and EXOG participate in mitochondrial DNA depletion initiated by herpes simplex virus 1 UL12.5. J. Virol. 2013;87:11787–11797. doi: 10.1128/JVI.02306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Corcoran J.A., Saffran H.A., Duguay B.A., Smiley J.R. Herpes simplex virus UL12.5 targets mitochondria through a mitochondrial localization sequence proximal to the N terminus. J. Virol. 2009;83:2601–2610. doi: 10.1128/JVI.02087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Duguay B.A., Saffran H.A., Ponomarev A., Duley S.A., Eaton H.E., Smiley J.R. Elimination of mitochondrial DNA is not required for herpes simplex virus 1 replication. J. Virol. 2014;88:2967–2976. doi: 10.1128/JVI.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Björnberg O., Bergman A.C., Rosengren A.M., Persson R., Lehman I.R., Nyman P.O. dUTPase from herpes simplex virus type 1; purification from infected green monkey kidney (Vero) cells and from an overproducing Escherichia coli strain. Protein Expr. Purif. 1993;4:149–159. doi: 10.1006/prep.1993.1021. [DOI] [PubMed] [Google Scholar]
- 406.Preston V.G., Fisher F.B. Identification of the herpes simplex virus type 1 gene encoding the dUTPase. Virology. 1984;138:58–68. doi: 10.1016/0042-6822(84)90147-8. [DOI] [PubMed] [Google Scholar]
- 407.Caradonna S.J., Adamkiewicz D.M. Purification and properties of the deoxyuridine triphosphate nucleotidohydrolase enzyme derived from HeLa S3 cells. Comparison to a distinct dUTP nucleotidohydrolase induced in herpes simplex virus-infected HeLa S3 cells. J. Biol. Chem. 1984;259:5459–5464. [PubMed] [Google Scholar]
- 408.Williams M. V Deoxyuridine triphosphate nucleotidohydrolase induced by herpes simplex virus type 1. Purification and characterization of induced enzyme. J. Biol. Chem. 1984;259:10080–10084. [PubMed] [Google Scholar]
- 409.Pyles R.B., Sawtell N.M., Thompson R.L. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J. Virol. 1992;66:6706–6713. doi: 10.1128/JVI.66.11.6706-6713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Lirette R., Caradonna S. Inhibition of phosphorylation of cellular dUTP nucleotidohydrolase as a consequence of herpes simplex virus infection. J. Cell. Biochem. 1990;43:339–353. doi: 10.1002/jcb.240430406. [DOI] [PubMed] [Google Scholar]
- 411.Kato A., Tsuda S., Liu Z., Kozuka-Hata H., Oyama M., Kawaguchi Y. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral dUTPase and regulates its catalytic activity in infected cells. J. Virol. 2014;88:655–666. doi: 10.1128/JVI.02710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kato A., Shindo K., Maruzuru Y., Kawaguchi Y. Phosphorylation of a herpes simplex virus 1 dUTPase by a viral protein kinase, Us3, dictates viral pathogenicity in the central nervous system but not at the periphery. J. Virol. 2014;88:2775–2785. doi: 10.1128/JVI.03300-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kato A., Hirohata Y., Arii J., Kawaguchi Y. Phosphorylation of herpes simplex virus 1 dUTPase upregulated viral dUTPase activity to compensate for low cellular dUTPase activity for efficient viral replication. J. Virol. 2014;88:7776–7785. doi: 10.1128/JVI.00603-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Riggio M.P., Onions D.E. DNA sequence of a gene cluster in the equine herpesvirus-4 genome which contains a newly identified herpesvirus gene encoding a membrane protein. Arch. Virol. 1993;133:171–178. doi: 10.1007/BF01309752. [DOI] [PubMed] [Google Scholar]
- 415.Baumeister J., Klupp B.G., Mettenleiter T.C. Pseudorabies virus and equine herpesvirus 1 share a nonessential gene which is absent in other herpesviruses and located adjacent to a highly conserved gene cluster. J. Virol. 1995;69:5560–5567. doi: 10.1128/JVI.69.9.5560-5567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Williams M.V.J. Demonstration of a herpes simplex virus type 2-induced deoxyuridine triphosphate nucleotidohydrolase in infected KB cells and in biochemically transformed HeLa cells. J. Gen. Virol. 1984;65:209–213. doi: 10.1099/0022-1317-65-1-209. [DOI] [PubMed] [Google Scholar]
- 417.Liang X., Tang M., Manns B., Babiuk L.A., Zamb T.J. Identification and deletion mutagenesis of the bovine herpesvirus 1 dUTPase gene and a gene homologous to herpes simplex virus UL49.5. Virology. 1993;195:42–50. doi: 10.1006/viro.1993.1344. [DOI] [PubMed] [Google Scholar]
- 418.Ariza M.-E., Glaser R., Kaumaya P.T.P., Jones C., Williams M. V The EBV-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 2009;182:851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- 419.Glaser R., Litsky M.L., Padgett D.A., Baiocchi R.A., Yang E.V., Chen M., Yeh P.-E., Green-Church K.B., Caligiuri M.A., Williams M. V EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 420.Ariza M.E., Glaser R., Williams M. V Human herpesviruses-encoded dUTPases: A family of proteins that modulate dendritic cell function and innate immunity. Front. Microbiol. 2014;5:504. doi: 10.3389/fmicb.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Goldstein D.J., Weller S.K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: Characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 422.Bacchetti S., Evelegh M.J., Muirhead B. Identification and separation of the two subunits of the herpes simplex virus ribonucleotide reductase. J. Virol. 1986;57:1177–1181. doi: 10.1128/JVI.57.3.1177-1181.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ren Y. Ph.D. Thesis. University of Cambridge; Cambridge, UK: 2011. Glycoprotein M and ESCRT in Herpes Simplex Virus Type 1 Assembly. [Google Scholar]
- 424.Paradis H., Gaudreau P., Brazeau P., Langelier Y. Mechanism of inhibition of herpes simplex virus (HSV) ribonucleotide reductase by a nonapeptide corresponding to the carboxyl terminus of its subunit 2. Specific binding of a photoaffinity analog, [4’-azido-Phe6]HSV H2-(6-15), to subunit 1. J. Biol. Chem. 1988;263:16045–16050. [PubMed] [Google Scholar]
- 425.Spector T., Stonehuerner J.G., Biron K.K., Averett D.R. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem. Pharmacol. 1987;36:4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]
- 426.Coen D.M., Kosz-Vnenchak M., Jacobson J.G., Leib D.A., Bogard C.L., Schaffer P.A., Tyler K.L., Knipe D.M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Campadelli-Fiume G., De Giovanni C., Gatta V., Nanni P., Lollini P.L., Menotti L. Rethinking herpes simplex virus: The way to oncolytic agents. Rev. Med. Virol. 2011;21:213–226. doi: 10.1002/rmv.691. [DOI] [PubMed] [Google Scholar]
- 428.Gober M.D., Wales S.Q., Hunter J.C., Sharma B.K., Aurelian L. Stress up-regulates neuronal expression of the herpes simplex virus type 2 large subunit of ribonucleotide reductase (R1; ICP10) by activating activator protein 1. J. Neurovirol. 2005;11:329–336. doi: 10.1080/13550280591002423. [DOI] [PubMed] [Google Scholar]
- 429.Langelier Y., Bergeron S., Chabaud S., Lippens J., Guilbault C., Sasseville A.M.J., Denis S., Mosser D.D., Massie B. The R1 subunit of herpes simplex virus ribonucleotide reductase protects cells against apoptosis at, or upstream of, caspase-8 activation. J. Gen. Virol. 2002;83:2779–2789. doi: 10.1099/0022-1317-83-11-2779. [DOI] [PubMed] [Google Scholar]
- 430.Dufour F., Sasseville A.M.J., Chabaud S., Massie B., Siegel R.M., Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis. 2011;16:256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- 431.Brandt C.R., Kintner R.L., Pumfery A.M., Visalli R.J., Grau D.R. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J. Gen. Virol. 1991;72:2043–2049. doi: 10.1099/0022-1317-72-9-2043. [DOI] [PubMed] [Google Scholar]
- 432.Turk S.R., Kik N.A., Birch G.M., Chiego D.J., Jr., Shipman C., Jr. Herpes simplex virus type 1 ribonucleotide reductase null mutants induce lesions in guinea pigs. Virology. 1989;173:733–735. doi: 10.1016/0042-6822(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 433.Mostafa H.H., Thompson T.W., Konen A.J., Haenchen S.D., Hilliard J.G., Macdonald S.J., Morrison L.A., Davido D.J. Herpes simplex virus 1 mutant with point mutations in UL39 Is impaired for acute viral replication in mice, establishment of latency, and explant-induced reactivation. J. Virol. 2018;92:1–12. doi: 10.1128/JVI.01654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Davido D.J., Tu E.M., Wang H., Korom M., Gazquez Casals A., Reddy P.J., Mostafa H.H., Combs B., Haenchen S.D., Morrison L.A. Attenuated herpes simplex virus 1 (HSV-1) expressing a mutant form of ICP6 stimulates a strong immune response that protects mice against HSV-1-induced corneal disease. J. Virol. 2018;92 doi: 10.1128/JVI.01036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Fenwick M.L., Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J. Gen. Virol. 1982;61:121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- 436.Fenwick M.L., McMenamin M.M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 1984;65:1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- 437.Fenwick M.L., Walker M.J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J. Gen. Virol. 1978;41:37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- 438.Kwong A.D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Kwong A.D., Kruper J.A., Frenkel N. Herpes simplex virus virion host shutoff function. J. Virol. 1988;62:912–921. doi: 10.1128/JVI.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Oroskar A.A., Read G.S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 1989;63:1897–1906. doi: 10.1128/JVI.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Read G.S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 1983;46:498–512. doi: 10.1128/JVI.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Schek N., Bachenheimer S.L. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 1985;55:601–610. doi: 10.1128/JVI.55.3.601-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Berthomme H., Jacquemont B., Epstein A. The pseudorabies virus host-shutoff homolog gene: Nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology. 1993;193:1028–1032. doi: 10.1006/viro.1993.1221. [DOI] [PubMed] [Google Scholar]
- 444.Lu P., Jones F.E., Saffran H.A., Smiley J.R. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 2001;75:1172–1185. doi: 10.1128/JVI.75.3.1172-1185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Smith T.J., Morrison L.A., Leib D.A. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 2002;76:2054–2061. doi: 10.1128/jvi.76.5.2054-2061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Strelow L.I., Leib D.A. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 1995;69:6779–6786. doi: 10.1128/JVI.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Smibert C.A., Smiley J.R. Differential regulation of endogenous and transduced beta-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J. Virol. 1990;64:3882–3894. doi: 10.1128/JVI.64.8.3882-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Strelow L., Smith T., Leib D. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology. 1997;231:28–34. doi: 10.1006/viro.1997.8497. [DOI] [PubMed] [Google Scholar]
- 449.Strelow L.I., Leib D.A. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 1996;70:5665–5667. doi: 10.1128/JVI.70.8.5665-5667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Fenwick M.L., Everett R.D. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 1990;71:2961–2967. doi: 10.1099/0022-1317-71-12-2961. [DOI] [PubMed] [Google Scholar]
- 451.Strom T., Frenkel N. Effects of herpes simplex virus on mRNA stability. J. Virol. 1987;61:2198–2207. doi: 10.1128/JVI.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Krikorian C.R., Read G.S. In vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J. Virol. 1991;65:112–122. doi: 10.1128/JVI.65.1.112-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Oroskar A.A., Read G.S. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 1987;61:604–606. doi: 10.1128/JVI.61.2.604-606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Lam Q., Smibert C.A., Koop K.E., Lavery C., Capone J.P., Weinheimer S.P., Smiley J.R. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 1996;15:2575–2581. doi: 10.1002/j.1460-2075.1996.tb00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Taddeo B., Sciortino M.T., Zhang W., Roizman B. Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA. Proc. Natl. Acad. Sci. USA. 2007;104:12163–12168. doi: 10.1073/pnas.0705245104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Taddeo B., Zhang W., Roizman B. The herpes simplex virus host shutoff RNase degrades cellular and viral mRNAs made before infection but not viral mRNA made after infection. J. Virol. 2013;87:4516–4522. doi: 10.1128/JVI.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Esclatine A., Taddeo B., Evans L., Roizman B. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA. 2004;101:3603–3608. doi: 10.1073/pnas.0400354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sorenson C.M., Hart P.A., Ross J. Analysis of herpes simplex virus-induced mRNA destabilizing activity using an in vitro mRNA decay system. Nucleic Acids Res. 1991;19:4459–4465. doi: 10.1093/nar/19.16.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zelus B.D., Stewart R.S., Ross J. The virion host shutoff protein of herpes simplex virus type 1: Messenger ribonucleolytic activity in vitro. J. Virol. 1996;70:2411–2419. doi: 10.1128/JVI.70.4.2411-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.David N.E., Feng P., Mian I.S., Read G.S. mRNA Degradation by the virion host shutoff (vhs) protein of herpes simplex virus: Genetic and Biochemical evidence that vhs is a nuclease. J. Virol. 2002;76:8560–8571. doi: 10.1128/JVI.76.17.8560-8571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Elgadi M.M., Hayes C.E., Smiley J.R. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 1999;73:7153–7164. doi: 10.1128/JVI.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Doherty A.J., Serpell L.C., Ponting C.P. The helix-hairpin-helix DNA-binding motif: A structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Taddeo B., Roizman B. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 2006;80:9341–9345. doi: 10.1128/JVI.01008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Taddeo B., Esclatine A., Zhang W., Roizman B. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 2003;77:6178–6187. doi: 10.1128/JVI.77.11.6178-6187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Esclatine A., Taddeo B., Roizman B. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 2004;78:8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Karr B.M., Read G.S. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology. 1999;264:195–204. doi: 10.1006/viro.1999.9986. [DOI] [PubMed] [Google Scholar]
- 467.Perez-Parada J., Saffran H.A., Smiley J.R. RNA degradation induced by the herpes simplex virus vhs protein proceeds 5′ to 3′ in vitro. J. Virol. 2004;78:13391–13394. doi: 10.1128/JVI.78.23.13391-13394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Feng P., Everly D.N., Jr., Read G.S. mRNA decay during herpesvirus infections: Interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 2001;75:10272–10280. doi: 10.1128/JVI.75.21.10272-10280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Feng P., Everly D.N.J., Read G.S. mRNA decay during herpes simplex virus (HSV) infections: Protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 2005;79:9651–9664. doi: 10.1128/JVI.79.15.9651-9664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Benetti L., Munger J., Roizman B. The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J. Virol. 2003;77:6567–6573. doi: 10.1128/JVI.77.11.6567-6573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Doepker R.C., Hsu W.-L., Saffran H.A., Smiley J.R. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 2004;78:4684–4699. doi: 10.1128/JVI.78.9.4684-4699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Barzilai A., Zivony-Elbom I., Sarid R., Noah E., Frenkel N. The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery. J. Virol. 2006;80:505–513. doi: 10.1128/JVI.80.1.505-513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Shiflett L.A., Read G.S. mRNA decay during herpes simplex virus (HSV) infections: Mutations that affect translation of an mRNA influence the sites at which it is cleaved by the HSV virion host shutoff (Vhs) protein. J. Virol. 2013;87:94–109. doi: 10.1128/JVI.01557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Suzutani T., Nagamine M., Shibaki T., Ogasawara M., Yoshida I., Daikoku T., Nishiyama Y., Azuma M. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 2000;81:1763–1771. doi: 10.1099/0022-1317-81-7-1763. [DOI] [PubMed] [Google Scholar]
- 475.Samady L., Costigliola E., MacCormac L., McGrath Y., Cleverley S., Lilley C.E., Smith J., Latchman D.S., Chain B., Coffin R.S. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (hsv) relieves the viral block to dendritic cell activation: Potential of vhs- HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 2003;77:3768–3776. doi: 10.1128/JVI.77.6.3768-3776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Cotter C.R., Nguyen M.L., Yount J.S., López C.B., Blaho J.A., Moran T.M. The virion host shut-off (vhs) protein blocks a TLR-independent pathway of herpes simplex virus type 1 recognition in human and mouse dendritic cells. PLoS ONE. 2010;5:e8684. doi: 10.1371/journal.pone.0008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Eisemann J., Mühl-Zürbes P., Steinkasserer A., Kummer M. Infection of mature dendritic cells with herpes simplex virus type 1 interferes with the interferon signaling pathway. Immunobiology. 2007;212:877–886. doi: 10.1016/j.imbio.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 478.Pasieka T.J., Cilloniz C., Lu B., Teal T.H., Proll S.C., Katze M.G., Leib D.A. Host responses to wild-type and attenuated herpes simplex virus infection in the absence of Stat1. J. Virol. 2009;83:2075–2087. doi: 10.1128/JVI.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Burgess H.M., Mohr I. Defining the role of stress granules in innate immune suppression by the herpes simplex virus 1 endoribonuclease VHS. J. Virol. 2018;92 doi: 10.1128/JVI.00829-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Dauber B., Poon D., dos Santos T., Duguay B.A., Mehta N., Saffran H.A., Smiley J.R. The herpes simplex virus virion host shutoff protein enhances translation of viral true late mRNAs independently of suppressing protein kinase R and stress granule formation. J. Virol. 2016;90:6049–6057. doi: 10.1128/JVI.03180-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Sciortino M.T., Parisi T., Siracusano G., Mastino A., Taddeo B., Roizman B. The virion host shutoff RNase plays a key role in blocking the activation of protein kinase R in cells infected with herpes simplex virus 1. J. Virol. 2013;87:3271–3276. doi: 10.1128/JVI.03049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Dauber B., Saffran H.A., Smiley J.R. The herpes simplex virus 1 virion host shutoff protein enhances translation of viral late mRNAs by preventing mRNA overload. J. Virol. 2014;88:9624–9632. doi: 10.1128/JVI.01350-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Yao X.-D., Rosenthal K.L. Herpes simplex virus type 2 virion host shutoff protein suppresses innate dsRNA antiviral pathways in human vaginal epithelial cells. J. Gen. Virol. 2011;92:1981–1993. doi: 10.1099/vir.0.030296-0. [DOI] [PubMed] [Google Scholar]
- 484.Kit S., Dubbs D.R., Piekarski L.J., Hsu T.C. Deletion of thymidine kinase activity from L cells resistant to bromodeoxyuridine. Exp. Cell Res. 1963;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- 485.Deville-Bonne D., el Amri C., Meyer P., Chen Y., Agrofoglio L.A., Janin J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: Structural and catalytic properties. Antiviral Res. 2010;86:101–120. doi: 10.1016/j.antiviral.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 486.McKnight S.L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Liu Q., Summers W.C. Site-directed mutagenesis of a nucleotide-binding domain in HSV-1 thymidine kinase: Effects on catalytic activity. Virology. 1988;163:638–642. doi: 10.1016/0042-6822(88)90308-X. [DOI] [PubMed] [Google Scholar]
- 488.Gentry G.A. Viral thymidine kinases and their relatives. Pharmacol. Ther. 1992;54:319–355. doi: 10.1016/0163-7258(92)90006-L. [DOI] [PubMed] [Google Scholar]
- 489.Wild K., Bohner T., Folkers G., Schulz G.E. The structures of thymidine kinase from Herpes simplex virus type 1 in complex with substrates and a substrate analogue. Protein Sci. 1997;6:2097–2106. doi: 10.1002/pro.5560061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Elion G.B., Furman P.A., Fyfe J.A., de Miranda P., Beauchamp L., Schaeffer H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Fyfe J.A., Keller P.M., Furman P.A., Miller R.L., Elion G.B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J. Biol. Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 492.Gustafson E.A., Chillemi A.C., Sage D.R., Fingeroth J.D. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob. Agents Chemother. 1998;42:2923–2931. doi: 10.1128/AAC.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Gustafson E.A., Schinazi R.F., Fingeroth J.D. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J. Virol. 2000;74:684–692. doi: 10.1128/JVI.74.2.684-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Lock M.J., Thorley N., Teo J., Emery V.C. Azidodeoxythymidine and didehydrodeoxythymidine as inhibitors and substrates of the human herpesvirus 8 thymidine kinase. J. Antimicrob. Chemother. 2002;49:359–366. doi: 10.1093/jac/49.2.359. [DOI] [PubMed] [Google Scholar]
- 495.Solaroli N., Johansson M., Balzarini J., Karlsson A. Substrate specificity of three viral thymidine kinases (TK): Vaccinia virus TK, feline herpesvirus TK, and canine herpesvirus TK. Nucleosides Nucleotides Nucleic Acids. 2006;25:1189–1192. doi: 10.1080/15257770600894451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Ehlers B., Dural G., Marschall M., Schregel V., Goltz M., Hentschke J. Endotheliotropic elephant herpesvirus, the first betaherpesvirus with a thymidine kinase gene. J. Gen. Virol. 2006;87:2781–2789. doi: 10.1099/vir.0.81977-0. [DOI] [PubMed] [Google Scholar]
- 497.Mittal S.K., Field H.J. Analysis of the bovine herpesvirus type 1 thymidine kinase (TK) gene from wild-type virus and TK-deficient mutants. J. Gen. Virol. 1989;70:901–918. doi: 10.1099/0022-1317-70-4-901. [DOI] [PubMed] [Google Scholar]
- 498.Dong J., Bai J., Sun T., Gu Z., Wang J., Sun H., Jiang P. Comparative pathogenicity and immunogenicity of triple and double gene-deletion pseudorabies virus vaccine candidates. Res. Vet. Sci. 2017;115:17–23. doi: 10.1016/j.rvsc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 499.Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978;14:133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- 500.Field H.J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob. Agents Chemother. 1980;17:209–216. doi: 10.1128/AAC.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Field H.J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J. Hyg. 1978;81:267–277. doi: 10.1017/S0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Gordon Y.J., Gilden D.M., Becker Y. HSV-1 thymidine kinase promotes virulence and latency in the mouse. Investig. Ophthalmol. Vis. Sci. 1983;24:599–602. [PubMed] [Google Scholar]
- 503.Price R.W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect. Immun. 1981;34:571–580. doi: 10.1128/IAI.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Chen S.H., Cook W.J., Grove K.L., Coen D.M. Human thymidine kinase can functionally replace herpes simplex virus type 1 thymidine kinase for viral replication in mouse sensory ganglia and reactivation from latency upon explant. J. Virol. 1998;72:6710–6715. doi: 10.1128/JVI.72.8.6710-6715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Chen S.-H., Lin Y.-W., Griffiths A., Huang W.-Y., Chen S.-H. Competition and complementation between thymidine kinase-negative and wild-type herpes simplex virus during co-infection of mouse trigeminal ganglia. J. Gen. Virol. 2006;87:3495–3502. doi: 10.1099/vir.0.82223-0. [DOI] [PubMed] [Google Scholar]
- 506.Leist T.P., Sandri-Goldin R.M., Stevens J.G. Latent infections in spinal ganglia with thymidine kinase-deficient herpes simplex virus. J. Virol. 1989;63:4976–4978. doi: 10.1128/JVI.63.11.4976-4978.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Chen S.-H., Pearson A., Coen D.M., Chen S.-H. Failure of thymidine kinase-negative herpes simplex virus to reactivate from latency following efficient establishment. J. Virol. 2004;78:520–523. doi: 10.1128/JVI.78.1.520-523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Huang C.-Y., Yao H.-W., Wang L.-C., Shen F.-H., Hsu S.-M., Chen S.-H. Thymidine kinase-negative herpes simplex virus 1 can efficiently establish persistent infection in neural tissues of nude mice. J. Virol. 2017;91 doi: 10.1128/JVI.01979-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Stroop W.G., Banks M.C., Qavi H., Chodosh J., Brown S.M. A thymidine kinase deficient HSV-2 strain causes acute keratitis and establishes trigeminal ganglionic latency, but poorly reactivates in vivo. J. Med. Virol. 1994;43:297–309. doi: 10.1002/jmv.1890430319. [DOI] [PubMed] [Google Scholar]
- 510.Jacobson J.G., Ruffner K.L., Kosz-Vnenchak M., Hwang C.B., Wobbe K.K., Knipe D.M., Coen D.M. Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J. Virol. 1993;67:6903–6908. doi: 10.1128/JVI.67.11.6903-6908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Tenser R.B., Hay K.A., Edris W.A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J. Virol. 1989;63:2861–2865. doi: 10.1128/JVI.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Field H.J., Bell S.E., Elion G.B., Nash A.A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob. Agents Chemother. 1979;15:554–561. doi: 10.1128/AAC.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Wilcox C.L., Crnic L.S., Pizer L.I. Replication, latent infection, and reactivation in neuronal culture with a herpes simplex virus thymidine kinase-negative mutant. Virology. 1992;187:348–352. doi: 10.1016/0042-6822(92)90326-K. [DOI] [PubMed] [Google Scholar]
- 514.Morfin F., Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 2003;26:29–37. doi: 10.1016/S1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 515.Bacon T.H., Levin M.J., Leary J.J., Sarisky R.T., Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Coen D.M., Schaffer P.A. Antiherpesvirus drugs: A promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discov. 2003;2:278–288. doi: 10.1038/nrd1065. [DOI] [PubMed] [Google Scholar]
- 517.Safrin S. Treatment of acyclovir-resistant herpes simplex virus infections in patients with AIDS. J. Acquir. Immune Defic. Syndr. 1992;5:29–32. [PubMed] [Google Scholar]
- 518.Chen H., Beardsley G.P., Coen D.M. Mechanism of ganciclovir-induced chain termination revealed by resistant viral polymerase mutants with reduced exonuclease activity. Proc. Natl. Acad. Sci. USA. 2014;111:17462–17467. doi: 10.1073/pnas.1405981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Zeng Z.-J., Xiang S.-G., Xue W.-W., Li H.-D., Ma N., Ren Z.-J., Xu Z.-J., Jiao C.-H., Wang C.-Y., Hu W.-X. The cell death and DNA damages caused by the Tet-On regulating HSV-tk/GCV suicide gene system in MCF-7 cells. Biomed. Pharmacother. 2014;68:887–892. doi: 10.1016/j.biopha.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 520.Osaki T., Tanio Y., Tachibana I., Hosoe S., Kumagai T., Kawase I., Oikawa S., Kishimoto T. Gene therapy for carcinoembryonic antigen-producing human lung cancer cells by cell type-specific expression of herpes simplex virus thymidine kinase gene. Cancer Res. 1994;54:5258–5261. [PubMed] [Google Scholar]
- 521.Wei S.-J., Chao Y., Hung Y.-M., Lin W., Yang D.-M., Shih Y.-L., Ch’ang L.-Y., Whang-Peng J., Yang W.K. S- and G2-phase cell cycle arrests and apoptosis induced by ganciclovir in murine melanoma cells transduced with herpes simplex virus thymidine kinase. Exp. Cell Res. 1998;241:66–75. doi: 10.1006/excr.1998.4005. [DOI] [PubMed] [Google Scholar]
- 522.Tomicic M.T., Thust R., Kaina B. Ganciclovir-induced apoptosis in HSV-1 thymidine kinase expressing cells: Critical role of DNA breaks, Bcl-2 decline and caspase-9 activation. Oncogene. 2002;21:2141–2153. doi: 10.1038/sj.onc.1205280. [DOI] [PubMed] [Google Scholar]
- 523.Yin X., Yu B., Tang Z., He B., Ren J., Xiao X., Tang W. Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther. 2013;20:77–81. doi: 10.1038/cgt.2012.86. [DOI] [PubMed] [Google Scholar]
- 524.Qiu Y., Peng G.-L., Liu Q.-C., Li F.-L., Zou X.-S., He J.-X. Selective killing of lung cancer cells using carcinoembryonic antigen promoter and double suicide genes, thymidine kinase and cytosine deaminase (pCEA-TK/CD) Cancer Lett. 2012;316:31–38. doi: 10.1016/j.canlet.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 525.Beltinger C., Fulda S., Kammertoens T., Meyer E., Uckert W., Debatin K.-M. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc. Natl. Acad. Sci. USA. 1999;96:8699–8704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Beltinger C., Fulda S., Kammertoens T., Uckert W., Debatin K.-M. Mitochondrial amplification of death signals determines thymidine kinase/ganciclovir-triggered activation of apoptosis. Cancer Res. 2000;60:3212–3217. [PubMed] [Google Scholar]
- 527.Liu X., Wang S., Guo X., Wei F., Yin J., Zang Y., Li N., Chen D. Exogenous p53 and ASPP2 expression enhances rAdV-TK/ GCV-induced death in hepatocellular carcinoma cells lacking functional p53. Oncotarget. 2016;7:18896–18905. doi: 10.18632/oncotarget.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Moolten F.L., Wells J.M. Curability of tumors bearing herpes thymidine kinase genes transfered by retroviral vectors. J. Natl. Cancer Inst. 1990;82:297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- 529.Ram Z., Culver K.W., Walbridge S., Blaese R.M., Oldfield E.H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993;53:83–88. [PubMed] [Google Scholar]
- 530.O’Malley B.W., Chen S.-H., Schwartz M.R., Woo S.L.C. Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Res. 1995;55:1080–1085. [PubMed] [Google Scholar]
- 531.Hall S.J., Sanford M.A., Atkinson G., Chen S.-H. Induction of potent antitumor natural killer cell activity by herpes simplex virus-thymidine kinase and ganciclovir therapy in an orthotopic mouse model of prostate cancer. Cancer Res. 1998;58:3221–3225. [PubMed] [Google Scholar]
- 532.Ram Z., Walbridge S., Shawker T., Culver K.W., Blaese R.M., Oldfield E.H. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J. Neurosurg. 1994;81:256–260. doi: 10.3171/jns.1994.81.2.0256. [DOI] [PubMed] [Google Scholar]
- 533.Freeman S.M., Abboud C.N., Whartenby K.A., Packman C.H., Koeplin D.S., Moolten F.L., Abraham G.N. The “Bystander Effect”: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 534.Hamel W., Magnelli L., Chiarugi V.P., Israel M.A. Herpes simplex virus thymidine kinase/ganciclovir-mediated apoptotic death of bystander cells. Cancer Res. 1996;56:2697–2702. [PubMed] [Google Scholar]
- 535.Vile R.G., Nelson J.A., Castleden S., Chong H., Hart I.R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 536.Pastorakova A., Jakubechova J., Altanerova U., Altaner C. Suicide gene therapy mediated with exosomes produced by mesenchymal stem/stromal cells stably transduced with HSV thymidine kinase. Cancers. 2020;12:1096. doi: 10.3390/cancers12051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Tamura R., Miyoshi H., Morimoto Y., Oishi Y., Sampetrean O., Iwasawa C., Mine Y., Saya H., Yoshida K., Okano H., et al. Gene therapy using neural stem/progenitor cells derived from human induced pluripotent stem cells: Visualization of migration and bystander killing effect. Hum. Gene Ther. 2020;31:352–366. doi: 10.1089/hum.2019.326. [DOI] [PubMed] [Google Scholar]
- 538.Voges J., Reszka R., Gossmann A., Dittmar C., Richter R., Garlip G., Kracht L., Coenen H.H., Sturm V., Wienhard K., et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann. Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 539.Li N., Zhou J., Weng D., Zhang C., Li L., Wang B., Song Y., He Q., Lin D., Chen D., et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2007;13:5847–5854. doi: 10.1158/1078-0432.CCR-07-0499. [DOI] [PubMed] [Google Scholar]
- 540.Nasu Y., Saika T., Ebara S., Kusaka N., Kaku H., Abarzua F., Manabe D., Thompson T.C., Kumon H. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol. Ther. 2007;15:834–840. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- 541.Van Putten E.H.P., Dirven C.M.F., van den Bent M.J., Lamfers M.L.M. Sitimagene ceradenovec: A gene-based drug for the treatment of operable high-grade glioma. Futur. Oncol. 2010;6:1691–1710. doi: 10.2217/fon.10.134. [DOI] [PubMed] [Google Scholar]
- 542.Sangro B., Mazzolini G., Ruiz M., Ruiz J., Quiroga J., Herrero I., Qian C., Benito A., Larrache J., Olagüe C., et al. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 543.Chiocca E.A., Aguilar L.K., Bell S.D., Kaur B., Hardcastle J., Cavaliere R., McGregor J., Lo S., Ray-Chaudhuri A., Chakravarti A., et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J. Clin. Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Islam S.M.B.U., Hong Y.M., Ornella M.S.C., Ngabire D., Jang H., Cho E., Kim E.-K., Hale J.J.J., Kim C.H., Ahn S.C., et al. Engineering and preclinical evaluation of western reserve oncolytic vaccinia virus expressing A167Y mutant herpes simplex virus thymidine kinase. Biomedicines. 2020;8:426. doi: 10.3390/biomedicines8100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Hossain J.A., Ystaas L.R., Mrdalj J., Välk K., Riecken K., Fehse B., Bjerkvig R., Grønli J., Miletic H. Lentiviral HSV-Tk.007-mediated suicide gene therapy is not toxic for normal brain cells. J. Gene Med. 2016;18:234–243. doi: 10.1002/jgm.2895. [DOI] [PubMed] [Google Scholar]
- 546.Kenarkoohi A., Bamdad T., Soleimani M., Soleimanjahi H., Fallah A., Falahi S. HSV-TK Expressing mesenchymal stem cells exert inhibitory effect on cervical cancer model. Int. J. Mol. Cell. Med. 2020;9:146–154. doi: 10.22088/IJMCM.BUMS.9.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Jo E.B., Lee H., Lee K.W., Kim S.J., Hong D., Park J.B. Complete regression of metastatic de-differentiated liposarcoma with engineered mesenchymal stromal cells with dTRAIL and HSV-TK. Am. J. Transl. Res. 2020;12:3993–4000. [PMC free article] [PubMed] [Google Scholar]
- 548.Islam S.M.B.U., Lee B., Jiang F., Kim E.-K., Ahn S.C., Hwang T.-H. Engineering and characterization of oncolytic vaccinia virus expressing truncated herpes simplex virus thymidine kinase. Cancers. 2020;12:228. doi: 10.3390/cancers12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Dührsen L., Hartfuß S., Hirsch D., Geiger S., Maire C.L., Sedlacik J., Guenther C., Westphal M., Lamszus K., Hermann F.G., et al. Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget. 2019;10:6049–6061. doi: 10.18632/oncotarget.27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Li H., Du H., Zhang G., Wu Y., Qiu P., Liu J., Guo J., Liu X., Sun L., Du B., et al. Curcumin plays a synergistic role in combination with HSV-TK/GCV in inhibiting growth of murine B16 melanoma cells and melanoma xenografts. PeerJ. 2019;7:e7760. doi: 10.7717/peerj.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sagou K., Imai T., Sagara H., Uema M., Kawaguchi Y. Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by Autophosphorylation and its role in pathogenesis. J. Virol. 2009;83:5773–5783. doi: 10.1128/JVI.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Morimoto T., Arii J., Tanaka M., Sata T., Akashi H., Yamada M., Nishiyama Y., Uema M., Kawaguchi Y. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J. Virol. 2009;83:11624–11634. doi: 10.1128/JVI.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wisner T.W., Wright C.C., Kato A., Kawaguchi Y., Mou F., Baines J.D., Roller R.J., Johnson D.C. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 2009;83:3115–3126. doi: 10.1128/JVI.01462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Wild P., Leisinger S., de Oliveira A.P., Doehner J., Schraner E.M., Fraevel C., Ackermann M., Kaech A. Nuclear envelope impairment is facilitated by the herpes simplex virus 1 Us3 kinase. F1000Research. 2019;8:198. doi: 10.12688/f1000research.17802.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Tobler K., Senn C., Schraner E.M., Ackermann M., Fraefel C., Wild P. The herpes simplex virus 1 Us3 kinase is involved in assembly of membranes needed for viral envelopment and in distribution of glycoprotein K. F1000Research. 2019;8:727. doi: 10.12688/f1000research.19194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Reynolds A.E., Wills E.G., Roller R.J., Ryckman B.J., Baines J.D. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 2002;76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Ryckman B.J., Roller R.J. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 2004;78:399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Mou F., Forest T., Baines J.D. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 2007;81:6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Bjerke S.L., Roller R.J. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006;347:261–276. doi: 10.1016/j.virol.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Mou F., Wills E., Baines J.D. Phosphorylation of the U(L)31 protein of herpes simplex virus 1 by the U(S)3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J. Virol. 2009;83:5181–5191. doi: 10.1128/JVI.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Purves F.C., Spector D., Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 1991;65:5757–5764. doi: 10.1128/JVI.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Reynolds A.E., Ryckman B.J., Baines J.D., Zhou Y., Liang L., Roller R.J. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 2001;75:8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Kato A., Liu Z., Minowa A., Imai T., Tanaka M., Sugimoto K., Nishiyama Y., Arii J., Kawaguchi Y. Herpes simplex virus 1 protein kinase Us3 and major tegument protein UL47 reciprocally regulate their subcellular localization in infected cells. J. Virol. 2011;85:9599–9613. doi: 10.1128/JVI.00845-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Leopardi R., van Sant C., Roizman B. The herpes simplex virus 1 protein kinase Us3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Galvan V., Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Munger J., Roizman B. The Us3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA. 2001;98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Hagglund R., Munger J., Poon A.P.W., Roizman B. U(S)3 protein kinase of herpes simplex virus 1 blocks caspase 3 activation induced by the products of U(S)1.5 and U(L)13 genes and modulates expression of transduced U(S)1.5 open reading frame in a cell type-specific manner. J. Virol. 2002;76:743–754. doi: 10.1128/JVI.76.2.743-754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Leopardi R., Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Benetti L., Roizman B. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA. 2004;101:9411–9416. doi: 10.1073/pnas.0403160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Walters M.S., Kinchington P.R., Banfield B.W., Silverstein S. Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases. J. Virol. 2010;84:9666–9676. doi: 10.1128/JVI.00981-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Chuluunbaatar U., Roller R., Feldman M.E., Brown S., Shokat K.M., Mohr I. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Chuluunbaatar U., Mohr I. A herpesvirus kinase that masquerades as Akt: You don’t have to look like Akt, to act like it. Cell Cycle. 2011;10:2064–2068. doi: 10.4161/cc.10.13.16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Sloan D.D., Zahariadis G., Posavad C.M., Pate N.T., Kussick S.J., Jerome K.R. CTL Are inactivated by herpes simplex virus-infected cells expressing a viral protein kinase. J. Immunol. 2003;171:6733–6741. doi: 10.4049/jimmunol.171.12.6733. [DOI] [PubMed] [Google Scholar]
- 574.Sen J., Liu X., Roller R., Knipe D.M. Herpes simplex virus US3 tegument protein inhibits Toll-like receptor 2 signaling at or before TRAF6 ubiquitination. Virology. 2013;439:65–73. doi: 10.1016/j.virol.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Wang S., Wang K., Lin R., Zheng C. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J. Virol. 2013;87:12814–12827. doi: 10.1128/JVI.02355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Qin C., Zhang R., Lang Y., Shao A., Xu A., Feng W., Han J., Wang M., He W., Yu C., et al. Bclaf1 critically regulates the type I interferon response and is degraded by alphaherpesvirus US3. PLoS Pathog. 2019;15:e1007559. doi: 10.1371/journal.ppat.1007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Rubio R.M., Mohr I. Inhibition of ULK1 and Beclin1 by an α-herpesvirus Akt-like Ser/Thr kinase limits autophagy to stimulate virus replication. Proc. Natl. Acad. Sci. USA. 2019;116:26941–26950. doi: 10.1073/pnas.1915139116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Kato A., Arii J., Shiratori I., Akashi H., Arase H., Kawaguchi Y. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and Regulates its expression on the cell surface. J. Virol. 2009;83:250–261. doi: 10.1128/JVI.01451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Rao P., Pham H.T., Kulkarni A., Yang Y., Liu X., Knipe D.M., Cresswell P., Yuan W. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J. Virol. 2011;85:8093–8104. doi: 10.1128/JVI.02689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Cunningham C., Davison A.J., Dolan A., Frame M.C., McGeoch D.J., Meredith D.M., Moss H.W., Orr A.C. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 1992;73:303–311. doi: 10.1099/0022-1317-73-2-303. [DOI] [PubMed] [Google Scholar]
- 581.Smith R.F., Smith T.F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 1989;63:450–455. doi: 10.1128/JVI.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Chee M.S., Lawrence G.L., Barrell B.G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 583.Overton H.A., McMillan D.J., Klavinskis L.S., Hope L., Ritchie A.J., Wong-kai-in P. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology. 1992;190:184–192. doi: 10.1016/0042-6822(92)91204-8. [DOI] [PubMed] [Google Scholar]
- 584.Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J. Virol. 1980;35:798–811. doi: 10.1128/JVI.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Kenyon T.K., Lynch J., Hay J., Ruyechan W., Grose C. Varicella-zoster virus ORF47 protein serine kinase: Characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 2001;75:8854–8858. doi: 10.1128/JVI.75.18.8854-8858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Chee M.S., Bankier A.T., Beck S., Bohni R., Brown C.M., Cerny R., Horsnell T., Hutchison C.A., III, Kouzarides T., Martignetti J.A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 587.Lawrence G.L., Chee M., Craxton M.A., Gompels U.A., Honess R.W., Barrell B.G. Human herpesvirus 6 is closely related to human cytomegalovirus. J. Virol. 1990;64:287–299. doi: 10.1128/JVI.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Ng T.I., Talarico C., Burnette T.C., Biron K., Roizman B. Partial substitution of the functions of the herpes simplex virus 1 U(L)13 gene by the human cytomegalovirus U(L)97 gene. Virology. 1996;225:347–358. doi: 10.1006/viro.1996.0609. [DOI] [PubMed] [Google Scholar]
- 589.Daikoku T., Shibata S., Goshima F., Oshima S., Tsurumi T., Yamada H., Yamashita Y., Nishiyama Y. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology. 1997;235:82–93. doi: 10.1006/viro.1997.8653. [DOI] [PubMed] [Google Scholar]
- 590.Coulter L.J., Moss H.W., Lang J., McGeoch D.J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 1993;74:387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 591.Overton H., McMillan D., Hope L., Wong-Kai-In P. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology. 1994;202:97–106. doi: 10.1006/viro.1994.1326. [DOI] [PubMed] [Google Scholar]
- 592.Tanaka M., Nishiyama Y., Sata T., Kawaguchi Y. The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: The activity is not essential for optimal expression of UL41 and ICP0. Virology. 2005;341:301–312. doi: 10.1016/j.virol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 593.Shibaki T., Suzutani T., Yoshida I., Ogasawara M., Azuma M. Participation of type I interferon in the decreased virulence of the UL13 gene-deleted mutant of herpes simplex virus type 1. J. Interf. Cytokine Res. 2001;21:279–285. doi: 10.1089/107999001300177466. [DOI] [PubMed] [Google Scholar]
- 594.Ogle W.O., Ng T.I., Carter K.L., Roizman B. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology. 1997;235:406–413. doi: 10.1006/viro.1997.8710. [DOI] [PubMed] [Google Scholar]
- 595.Asai R., Ohno T., Kato A., Kawaguchi Y. Identification of proteins directly phosphorylated by UL13 protein kinase from herpes simplex virus 1. Microbes Infect. 2007;9:1434–1438. doi: 10.1016/j.micinf.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 596.Zhu Z., Du T., Zhou G., Roizman B. The stability of herpes simplex virus 1 ICP0 early after infection is defined by the RING finger and the UL13 protein kinase. J. Virol. 2014;88:5437–5443. doi: 10.1128/JVI.00542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Eaton H.E., Saffran H.A., Wu F.W., Quach K., Smiley J.R. Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J. Virol. 2014;88:7379–7388. doi: 10.1128/JVI.00712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Geiss B.J., Cano G.L., Tavis J.E., Morrison L.A. Herpes simplex virus 2 VP22 phosphorylation induced by cellular and viral kinases does not influence intracellular localization. Virology. 2004;330:74–81. doi: 10.1016/j.virol.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 599.Ng T.I., Ogle W.O., Roizman B. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: Glycoproteins E and I. Virology. 1998;241:37–48. doi: 10.1006/viro.1997.8963. [DOI] [PubMed] [Google Scholar]
- 600.Attrill H.L., Cumming S.A., Clements J.B., Graham S.V. The herpes simplex virus type 1 US11 protein binds the coterminal UL12, UL13, and UL14 RNAs and regulates UL13 expression in vivo. J. Virol. 2002;76:8090–8100. doi: 10.1128/JVI.76.16.8090-8100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Gershburg S., Geltz J., Peterson K.E., Halford W.P., Gershburg E. The UL13 and US3 protein kinases of herpes simplex virus 1 cooperate to promote the assembly and release of mature, infectious virions. PLoS ONE. 2015;10:e0131420. doi: 10.1371/journal.pone.0131420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Sato Y., Koshizuka T., Ishibashi K., Hashimoto K., Ishioka K., Ikuta K., Yokota S.-I., Fujii N., Suzutani T. Involvement of herpes simplex virus type 1 UL13 protein kinase in induction of SOCS genes, the negative regulators of cytokine signaling. Microbiol. Immunol. 2017;61:159–167. doi: 10.1111/1348-0421.12483. [DOI] [PubMed] [Google Scholar]
- 603.Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Frey K.G., Ahmed C.M.I., Dabelic R., Jager L.D., Noon-Song E.N., Haider S.M., Johnson H.M., Bigley N.J. HSV-1-induced SOCS-1 expression in keratinocytes: Use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J. Immunol. 2009;183:1253–1262. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Reichard A.C., Cheemarla N.R., Bigley N.J. SOCS1/3 expression levels in HSV-1-infected, cytokine-polarized and -unpolarized macrophages. J. Interf. Cytokine Res. 2015;35:32–41. doi: 10.1089/jir.2013.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Pennisi R., Musarra-Pizzo M., Lei Z., Zhou G.G., Sciortino M.T. VHS, US3 and UL13 viral tegument proteins are required for herpes simplex virus-induced modification of protein kinase R. Sci. Rep. 2020;10:5580. doi: 10.1038/s41598-020-62619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Merrick W.C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 1992;56:291–315. doi: 10.1128/MR.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Kawaguchi Y., Van Sant C., Roizman B. Eukaryotic elongation factor 1delta is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J. Virol. 1998;72:1731–1736. doi: 10.1128/JVI.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Perkins D., Pereira E.F.R., Gober M., Yarowsky P.J., Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Kawaguchi Y., Kato K., Tanaka M., Kanamori M., Nishiyama Y., Yamanashi Y. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1delta. J. Virol. 2003;77:2359–2368. doi: 10.1128/JVI.77.4.2359-2368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Baines J.D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 1991;65:938–944. doi: 10.1128/JVI.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Jun P., Strelow L., Herman R., Marsden H., Eide T., Haarr L., Leib D. The UL4 gene of herpes simplex virus type 1 is dispensable for latency, reactivation and pathogenesis in mice. J. Gen. Virol. 1998;79:1603–1611. doi: 10.1099/0022-1317-79-7-1603. [DOI] [PubMed] [Google Scholar]
- 613.Worrad D.M., Caradonna S. The herpes simplex virus type 2 UL3 open reading frame encodes a nuclear localizing phosphoprotein. Virology. 1993;195:364–376. doi: 10.1006/viro.1993.1386. [DOI] [PubMed] [Google Scholar]
- 614.Yoshida S., Lee L.F., Yanagida N., Nazerian K. Identification and characterization of a Marek’s disease virus gene homologous to glycoprotein L of herpes simplex virus. Virology. 1994;204:414–419. doi: 10.1006/viro.1994.1546. [DOI] [PubMed] [Google Scholar]
- 615.Ghiasi H., Perng G.C., Cai S., Nesburn A.B., Wechsler S.L. The UL3 open reading frame of herpes simplex virus type 1 codes for a phosphoprotein. Virus Res. 1996;44:137–142. doi: 10.1016/0168-1702(96)01330-5. [DOI] [PubMed] [Google Scholar]
- 616.McGeoch D.J., Cunningham C., McIntyre G., Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 617.Davison A.J. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J. 1983;2:2203–2209. doi: 10.1002/j.1460-2075.1983.tb01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Telford E.A.R., Watson M.S., McBride K., Davison A.J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-U. [DOI] [PubMed] [Google Scholar]
- 619.Dean H.J., Cheung A.K. Identification of the pseudorabies virus UL4 and UL5 (helicase) genes. Virology. 1994;202:962–967. doi: 10.1006/viro.1994.1419. [DOI] [PubMed] [Google Scholar]
- 620.Vlcek C., Benes V., Lu Z., Kutish G.F., Paces V., Rock D., Letchworth G.J., Schwyzer M. Nucleotide sequence analysis of a 30-kb region of the bovine herpesvirus 1 genome which exhibits a colinear gene arrangement with the UL21 to UL4 genes of herpes simplex virus. Virology. 1995;210:100–108. doi: 10.1006/viro.1995.1321. [DOI] [PubMed] [Google Scholar]
- 621.Yamada H., Jiang Y.-M., Zhu H.-Y., Inagaki-Ohara K., Nishiyama Y. Nucleolar localization of the UL3 protein of herpes simplex virus type 2. J. Gen. Virol. 1999;80:2157–2164. doi: 10.1099/0022-1317-80-8-2157. [DOI] [PubMed] [Google Scholar]
- 622.Zheng C., Lin F., Wang S., Xing J. A novel virus-encoded nucleocytoplasmic shuttling protein: The UL3 protein of herpes simplex virus type 1. J. Virol. Methods. 2011;177:206–210. doi: 10.1016/j.jviromet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 623.Eide T., Marsden H.S., Leib D.A., Cunningham C., Davison A.J., Langeland N., Haarr L. Identification of the UL4 protein of herpes simplex virus type 1. J. Gen. Virol. 1998;79:3033–3038. doi: 10.1099/0022-1317-79-12-3033. [DOI] [PubMed] [Google Scholar]
- 624.Markovitz N.S., Roizman B. Small dense nuclear bodies are the site of localization of herpes simplex virus 1 U(L)3 and U(L)4 proteins and of ICP22 only when the latter protein is present. J. Virol. 2000;74:523–528. doi: 10.1128/JVI.74.1.523-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Ward P.L., Taddeo B., Markovitz N.S., Roizman B. Identification of a novel expressed open reading frame situated between genes U(L)20 and U(L)21 of the herpes simplex virus 1 genome. Virology. 2000;266:275–285. doi: 10.1006/viro.1999.0081. [DOI] [PubMed] [Google Scholar]
- 626.Loret S., Lippé R. Biochemical analysis of infected cell polypeptide (ICP)0, ICP4, ul7 and ul23 incorporated into extracellular herpes simplex virus type 1 virions. J. Gen. Virol. 2012;93:624–634. doi: 10.1099/vir.0.039776-0. [DOI] [PubMed] [Google Scholar]
- 627.Roller R.J., Fetters R. The herpes simplex virus 1 UL51 protein interacts with the UL7 protein and plays a role in its recruitment into the virion. J. Virol. 2015;89:3112–3122. doi: 10.1128/JVI.02799-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Albecka A., Owen D.J., Ivanova L., Brun J., Liman R., Davies L., Ahmed M.F., Colaco S., Hollinshead M., Graham S.C., et al. Dual function of the pUL7-pUL51 tegument protein complex in herpes simplex virus 1 infection. J. Virol. 2017;91:1–19. doi: 10.1128/JVI.02196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Xu X., Fan S., Zhou J., Zhang Y., Che Y., Cai H., Wang L., Guo L., Liu L., Li Q. The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of α-4 gene transcription. Virol. J. 2016;13:1–12. doi: 10.1186/s12985-016-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Nozawa N., Daikoku T., Yamauchi Y., Takakuwa H., Goshima F., Yoshikawa T., Nishiyama Y. Identification and characterization of the UL7 gene product of herpes simplex virus type 2. Virus Genes. 2002;24:257–266. doi: 10.1023/A:1015332716927. [DOI] [PubMed] [Google Scholar]
- 631.Feutz E., McLeland-Wieser H., Ma J., Roller R.J. Functional interactions between herpes simplex virus pUL51, pUL7 and gE reveal cell-specific mechanisms for epithelial cell-to-cell spread. Virology. 2019;537:84–96. doi: 10.1016/j.virol.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Tanaka M., Sata T., Kawaguchi Y. The product of the Herpes simplex virus 1 UL7 gene interacts with a mitochondrial protein, adenine nucleotide translocator 2. Virol. J. 2008;5:1–13. doi: 10.1186/1743-422X-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Nozawa N., Daikoku T., Koshizuka T., Yamauchi Y., Yoshikawa T., Nishiyama Y. Subcellular Localization of herpes simplex virus type 1 UL51 protein and role of palmitoylation in golgi apparatus targeting. J. Virol. 2003;77:3204–3216. doi: 10.1128/JVI.77.5.3204-3216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Kato A., Oda S., Watanabe M., Oyama M., Kozuka-Hata H., Koyanagi N., Maruzuru Y., Arii J., Kawaguchi Y. Roles of the phosphorylation of herpes simplex virus 1 UL51 at a specific site in viral replication and pathogenicity. J. Virol. 2018;92:1–21. doi: 10.1128/JVI.01035-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Nozawa N., Kawaguchi Y., Tanaka M., Kato A., Kato A., Kimura H., Nishiyama Y. Herpes simplex virus type 1 UL51 protein is involved in maturation and egress of virus particles. J. Virol. 2005;79:6947–6956. doi: 10.1128/JVI.79.11.6947-6956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Roller R.J., Haugo A.C., Yang K., Baines J.D. The herpes simplex virus 1 UL51 gene product has cell type-specific functions in cell-to-cell spread. J. Virol. 2014;88:4058–4068. doi: 10.1128/JVI.03707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Oda S., Arii J., Koyanagi N., Kato A., Kawaguchi Y. The interaction between herpes simplex virus 1 tegument proteins UL51 and UL14 and its role in virion morphogenesis. J. Virol. 2016;90:8754–8767. doi: 10.1128/JVI.01258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Maclean C.A., Robertson L.M., Jamieson F.E. Characterization of the UL10 gene product of herpes simplex virus type and investigation of its role in vivo. J. Gen. Virol. 1993;74:975–983. doi: 10.1099/0022-1317-74-6-975. [DOI] [PubMed] [Google Scholar]
- 639.MacLean C.A., Efstathiou S., Elliott M.L., Jamieson F.E., McGeoch D.J. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J. Gen. Virol. 1991;72:897–906. doi: 10.1099/0022-1317-72-4-897. [DOI] [PubMed] [Google Scholar]
- 640.Baines J.D., Wills E., Jacob R.J., Pennington J., Roizman B. Glycoprotein M of herpes simplex virus 1 is incorporated into virions during budding at the inner nuclear membrane. J. Virol. 2007;81:800–812. doi: 10.1128/JVI.01756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Zhang J., Nagel C.-H., Sodeik B., Lippé R. Early, active, and specific localization of herpes simplex virus type 1 gM to nuclear membranes. J. Virol. 2009;83:12984–12997. doi: 10.1128/JVI.01180-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.El Kasmi I., Lippé R. Herpes simplex virus 1 gN partners with gM To modulate the viral fusion machinery. J. Virol. 2015;89:2313–2323. doi: 10.1128/JVI.03041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Crump C.M., Bruun B., Bell S., Pomeranz L.E., Minson T., Browne H.M. Alphaherpesvirus glycoprotein M causes the relocalization of plasma membrane proteins. J. Gen. Virol. 2004;85:3517–3527. doi: 10.1099/vir.0.80361-0. [DOI] [PubMed] [Google Scholar]
- 644.Striebinger H., Funk C., Raschbichler V., Bailer S.M. Subcellular trafficking and functional relationship of the HSV-1 glycoproteins N and M. Viruses. 2016;8:83. doi: 10.3390/v8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Fuchs W., Klupp B.G., Granzow H., Osterrieder N., Mettenleiter T.C. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 2002;76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Schnee M., Ruzsics Z., Bubeck A., Koszinowski U.H. Common and specific properties of Herpesvirus UL34/UL31 protein family members revealed by protein complementation assay. J. Virol. 2006;80:11658–11666. doi: 10.1128/JVI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Striebinger H., Zhang J., Ott M., Funk C., Radtke K., Duron J., Ruzsics Z., Haas J., Lippé R., Bailer S.M. Subcellular trafficking and functional importance of herpes simplex virus type 1 glycoprotein M domains. J. Gen. Virol. 2015;96:3313–3325. doi: 10.1099/jgv.0.000262. [DOI] [PubMed] [Google Scholar]
- 648.Leege T., Fuchs W., Granzow H., Kopp M., Klupp B.G., Mettenleiter T.C. Effects of simultaneous deletion of pUL11 and glycoprotein M on virion maturation of herpes simplex virus type 1. J. Virol. 2009;83:896–907. doi: 10.1128/JVI.01842-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Ren Y., Bell S., Zenner H.L., Kathy Lau S.Y., Crump C.M. Glycoprotein M is important for the efficient incorporation of glycoprotein H-L into herpes simplex virus type 1 particles. J. Gen. Virol. 2012;93:319–329. doi: 10.1099/vir.0.035444-0. [DOI] [PubMed] [Google Scholar]
- 650.Koyano S., Mar E.C., Stamey F.R., Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 2003;84:1485–1491. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- 651.El Kasmi I., Khadivjam B., Lackman M., Duron J., Bonneil E., Thibault P., Lippé R. Extended synaptotagmin 1 interacts with herpes simplex virus 1 glycoprotein M and Negatively modulates virus-induced membrane fusion. J. Virol. 2018;92 doi: 10.1128/JVI.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Cheshenko N., del Rosario B., Woda C., Marcellino D., Satlin L.M., Herold B.C. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 2003;163:283–293. doi: 10.1083/jcb.200301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Kalamvoki M., Roizman B. Bcl-2 blocks accretion or depletion of stored calcium but has no effect on the redistribution of IP3 receptor I mediated by glycoprotein E of herpes simplex virus 1. J. Virol. 2007;81:6316–6325. doi: 10.1128/JVI.00311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Boruchowicz H., Hawkins J., Cruz-Palomar K., Lippé R. The XPO6 exportin mediates herpes simplex virus 1 gM nuclear release late in infection. J. Virol. 2020;94 doi: 10.1128/JVI.00753-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Perez-Caballero D., Zang T., Ebrahimi A., McNatt M.W., Gregory D.A., Johnson M.C., Bieniasz P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Blondeau C., Pelchen-Matthews A., Mlcochova P., Marsh M., Milne R.S.B., Towers G.J. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J. Virol. 2013;87:13124–13133. doi: 10.1128/JVI.02250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.MacLean C.A., Clark B., McGeoch D.J. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 1989;70:3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- 658.Loomis J.S., Bowzard J.B., Courtney R.J., Wills J.W. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2001;75:12209–12219. doi: 10.1128/JVI.75.24.12209-12219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Loomis J.S., Courtney R.J., Wills J.W. Packaging determinants in the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2006;80:10534–10541. doi: 10.1128/JVI.01172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.MacLean C.A., Dolan A., Jamieson F.E., McGeoch D.J. The myristylated virion proteins of herpes simplex virus type 1: Investigation of their role in the virus life cycle. J. Gen. Virol. 1992;73:539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- 661.Baird N.L., Starkey J.L., Hughes D.J., Wills J.W. Myristylation and palmitylation of HSV-1 UL11 are not essential for its function. Virology. 2010;397:80–88. doi: 10.1016/j.virol.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Loomis J.S., Courtney R.J., Wills J.W. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2003;77:11417–11424. doi: 10.1128/JVI.77.21.11417-11424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Yeh P.-C., Meckes D.G., Wills J.W. Analysis of the interaction between the UL11 and UL16 tegument proteins of herpes simplex virus. J. Virol. 2008;82:10693–10700. doi: 10.1128/JVI.01230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Farnsworth A., Wisner T.W., Johnson D.C. Cytoplasmic Residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD. J. Virol. 2007;81:319–331. doi: 10.1128/JVI.01842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Han J., Chadha P., Meckes D.G., Baird N.L., Wills J.W. Interaction and Interdependent packaging of tegument protein UL11 and glycoprotein E of herpes simplex virus. J. Virol. 2011;85:9437–9446. doi: 10.1128/JVI.05207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Fulmer P.A., Melancon J.M., Baines J.D., Kousoulas K.G. UL20 protein functions precede and are required for the UL11 functions of herpes simplex virus type 1 cytoplasmic virion envelopment. J. Virol. 2007;81:3097–3108. doi: 10.1128/JVI.02201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Wright P.E., Dyson H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015;16:18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Metrick C.M., Koenigsberg A.L., Heldwein E.E. Conserved outer tegument component UL11 from herpes simplex virus 1 is an intrinsically disordered, RNA-binding protein. mBio. 2020;11:1–22. doi: 10.1128/mBio.00810-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Baird N.L., Yeh P.C., Courtney R.J., Wills J.W. Sequences in the UL11 tegument protein of herpes simplex virus that control association with detergent-resistant membranes. Virology. 2008;374:315–321. doi: 10.1016/j.virol.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Chadha P., Sarfo A., Zhang D., Abraham T., Carmichael J., Han J., Wills J.W. Domain interaction studies of herpes simplex virus 1 tegument protein UL16 reveal its interaction with mitochondria. J. Virol. 2017;91 doi: 10.1128/JVI.01995-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Nalwanga D., Rempel S., Roizman B., Baines J.D. The U(L)16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology. 1996;226:236–242. doi: 10.1006/viro.1996.0651. [DOI] [PubMed] [Google Scholar]
- 672.Carmichael J.C., Wills J.W. Differential requirements for gE, gI, and UL16 among herpes simplex virus 1 syncytial variants suggest unique modes of dysregulating the mechanism of cell-to-cell spread. J. Virol. 2019;93:1–20. doi: 10.1128/JVI.00494-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Harper A.L., Meckes D.G., Marsh J.A., Ward M.D., Yeh P.-C., Baird N.L., Wilson C.B., Semmes O.J., Wills J.W. Interaction domains of the UL16 and UL21 tegument proteins of herpes simplex virus. J. Virol. 2010;84:2963–2971. doi: 10.1128/JVI.02015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Meckes D.G., Wills J.W. Structural rearrangement within an enveloped virus upon binding to the host cell. J. Virol. 2008;82:10429–10435. doi: 10.1128/JVI.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Yeh P.-C., Han J., Chadha P., Meckes D.G., Ward M.D., Semmes O.J., Wills J.W. Direct and specific binding of the UL16 tegument protein of herpes simplex virus to the cytoplasmic tail of glycoprotein E. J. Virol. 2011;85:9425–9436. doi: 10.1128/JVI.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Han J., Chadha P., Starkey J.L., Wills J.W. Function of glycoprotein E of herpes simplex virus requires coordinated assembly of three tegument proteins on its cytoplasmic tail. Proc. Natl. Acad. Sci. USA. 2012;109:19798–19803. doi: 10.1073/pnas.1212900109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Gao J., Yan X., Banfield B.W. Comparative analysis of UL16 mutants derived from multiple strains of herpes simplex virus 2 (HSV-2) and HSV-1 reveals species-specific requirements for the UL16 protein. J. Virol. 2018;92 doi: 10.1128/JVI.00629-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Carmichael J.C., Starkey J., Zhang D., Sarfo A., Chadha P., Wills J.W., Han J. Glycoprotein D of HSV-1 is dependent on tegument protein UL16 for packaging and contains a motif that is differentially required for syncytia formation. Virology. 2019;527:64–76. doi: 10.1016/j.virol.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Hutchinson L., Goldsmith K., Snoddy D., Ghosh H., Graham F.L., Johnson D.C. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 1992;66:5603–5609. doi: 10.1128/JVI.66.9.5603-5609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Foster T.P., Alvarez X., Kousoulas K.G. Plasma membrane topology of syncytial domains of herpes simplex virus type 1 glycoprotein K (gK): The UL20 protein enables cell surface localization of gK but not gK-mediated cell-to-cell fusion. J. Virol. 2003;77:499–510. doi: 10.1128/JVI.77.1.499-510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Hutchinson L., Roop-Beauchamp C., Johnson D.C. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 1995;69:4556–4563. doi: 10.1128/JVI.69.7.4556-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Jayachandra S., Baghian A., Kousoulas K.G. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J. Virol. 1997;71:5012–5024. doi: 10.1128/JVI.71.7.5012-5024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Hutchinson L., Graham F.L., Cai W., Debroy C., Person S., Johnson D.C. Herpes simplex virus (HSV) glycoproteins B and K inhibit cell fusion induced by HSV syncytial mutants. Virology. 1993;196:514–531. doi: 10.1006/viro.1993.1507. [DOI] [PubMed] [Google Scholar]
- 684.Avitabile E., Lombardi G., Campadelli-Fiume G. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH, and gL. J. Virol. 2003;77:6836–6844. doi: 10.1128/JVI.77.12.6836-6844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Foster T.P., Melancon J.M., Baines J.D., Kousoulas K.G. The herpes simplex virus type 1 UL20 protein modulates membrane fusion events during cytoplasmic virion morphogenesis and virus-induced cell fusion. J. Virol. 2004;78:5347–5357. doi: 10.1128/JVI.78.10.5347-5357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Foster T.P., Rybachuk G.V., Kousoulas K.G. Glycoprotein K specified by herpes simplex virus type 1 is expressed on virions as a golgi complex-dependent glycosylated species and functions in virion entry. J. Virol. 2001;75:12431–12438. doi: 10.1128/JVI.75.24.12431-12438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Foster T.P., Chouljenko V.N., Kousoulas K.G. Functional and physical interactions of the herpes simplex virus type 1 UL20 membrane protein with glycoprotein K. J. Virol. 2008;82:6310–6323. doi: 10.1128/JVI.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Chouljenko V.N., Iyer A.V., Chowdhury S., Kim J., Kousoulas K.G. The herpes simplex virus type 1 UL20 protein and the amino terminus of glycoprotein K (gK) physically interact with gB. J. Virol. 2010;84:8596–8606. doi: 10.1128/JVI.00298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Chouljenko V.N., Iyer A.V., Chowdhury S., Chouljenko D.V., Kousoulas K.G. The amino terminus of herpes simplex virus type 1 glycoprotein K (gK) modulates gB-mediated virus-induced cell fusion and virion egress. J. Virol. 2009;83:12301–12313. doi: 10.1128/JVI.01329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Jambunathan N., Chowdhury S., Subramanian R., Chouljenko V.N., Walker J.D., Kousoulas K.G. Site-specific proteolytic cleavage of the amino terminus of herpes simplex virus glycoprotein K on virion particles inhibits virus entry. J. Virol. 2011;85:12910–12918. doi: 10.1128/JVI.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Lau S.Y.K., Crump C.M. HSV-1 gm and the gK/pUL20 complex are important for the localization of gD and gH/L to viral assembly sites. Viruses. 2015;7:915–938. doi: 10.3390/v7030915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Musarrat F., Jambunathan N., Rider P.J.F., Chouljenko V.N., Kousoulas K.G. The amino terminus of herpes simplex virus 1 glycoprotein K (gK) is required for gb binding to akt, release of intracellular calcium, and fusion of the viral envelope with plasma membranes. J. Virol. 2018;92 doi: 10.1128/JVI.01842-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Saied A.A., Chouljenko V.N., Subramanian R., Kousoulas K.G. A replication competent HSV-1(McKrae) with a mutation in the amino-terminus of Glycoprotein K (gK) is unable to infect mouse trigeminal ganglia after cornea infection. Curr. Eye Res. 2014;39:596–603. doi: 10.3109/02713683.2013.855238. [DOI] [PubMed] [Google Scholar]
- 694.Matundan H.H., Mott K.R., Akhtar A.A., Breunig J.J., Ghiasi H. Mutations within the pathogenic region of herpes simplex virus 1 gK signal sequences alter cell surface expression and neurovirulence. J. Virol. 2015;89:2530–2542. doi: 10.1128/JVI.03506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Takakuwa H., Goshima F., Koshizuka T., Murata T., Daikoku T., Nishiyama Y. Herpes simplex virus encodes a virion-associated protein which promotes long cellular processes in over-expressing cells. Genes Cells. 2001;6:955–966. doi: 10.1046/j.1365-2443.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 696.Muto Y., Goshima F., Ushijima Y., Kimura H., Nishiyama Y. Generation and characterization of UL21-null herpes simplex virus type 1. Front. Microbiol. 2012;3:1–6. doi: 10.3389/fmicb.2012.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Le Sage V., Jung M., Alter J.D., Wills E.G., Johnston S.M., Kawaguchi Y., Baines J.D., Banfield B.W. The herpes simplex virus 2 UL21 protein is essential for virus propagation. J. Virol. 2013;87:5904–5915. doi: 10.1128/JVI.03489-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Metrick C.M., Heldwein E.E. Novel structure and unexpected RNA-binding ability of the C-terminal domain of herpes simplex virus 1 tegument protein UL21. J. Virol. 2016;90:7007–7008. doi: 10.1128/JVI.00992-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Sarfo A., Starkey J., Mellinger E., Zhang D., Chadha P., Carmichael J., Wills J.W. The UL21 Tegument protein of herpes simplex virus 1 is differentially required for the syncytial phenotype. J. Virol. 2017;91:1–18. doi: 10.1128/JVI.01161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Jacobson J.G., Chen S.H., Cook W.J., Kramer M.F., Coen D.M. Importance of the herpes simplex virus UL24 gene for productive ganglionic infection in mice. Virology. 1998;242:161–169. doi: 10.1006/viro.1997.9012. [DOI] [PubMed] [Google Scholar]
- 701.Abdeljelil N.B., Rochette P.A., Pearson A. The UL24 protein of herpes simplex virus 1 affects the sub-cellular distribution of viral glycoproteins involved in fusion. Virology. 2013;444:263–273. doi: 10.1016/j.virol.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 702.Pearson A., Coen D.M. Identification, localization, and regulation of expression of the UL24 protein of herpes simplex virus type 1. J. Virol. 2002;76:10821–10828. doi: 10.1128/JVI.76.21.10821-10828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Dridi S., Richerioux N., Suarez C.E.G., Vanharen M., Sanabria-Solano C., Pearson A. A Mutation in the UL24 gene abolishes expression of the newly identified UL24.5 protein of herpes simplex virus 1 and leads to an increase in pathogenicity in mice. J. Virol. 2018;92 doi: 10.1128/JVI.00671-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Rochette P.A., Bourget A., Sanabria-Solano C., Lahmidi S., Lavalleé G.O., Pearson A. Mutation of UL24 impedes the dissemination of acute herpes simplex virus 1 infection from the cornea to neurons of trigeminal ganglia. J. Gen. Virol. 2015;96:2794–2805. doi: 10.1099/vir.0.000189. [DOI] [PubMed] [Google Scholar]
- 705.Blakeney S., Kowalski J., Tummolo D., DeStefano J., Cooper D., Guo M., Gangolli S., Long D., Zamb T., Natuk R.J., et al. Herpes simplex virus type 2 UL24 gene is a virulence determinant in murine and guinea pig disease models. J. Virol. 2005;79:10498–10506. doi: 10.1128/JVI.79.16.10498-10506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Kniżewski Ł., Kinch L., Grishin N.V., Rychlewski L., Ginalski K. Human herpesvirus 1 UL24 gene encodes a potential PD-(D/E)XK endonuclease. J. Virol. 2006;80:2575–2577. doi: 10.1128/JVI.80.5.2575-2577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Lymberopoulos M.H., Pearson A. Involvement of UL24 in herpes-simplex-virus-1-induced dispersal of nucleolin. Virology. 2007;363:397–409. doi: 10.1016/j.virol.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 708.Bertrand L., Leiva-Torres G.A., Hyjazie H., Pearson A. Conserved residues in the UL24 protein of herpes simplex virus 1 are important for dispersal of the nucleolar protein nucleolin. J. Virol. 2010;84:109–118. doi: 10.1128/JVI.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Leiva-Torres G.A., Rochette P.A., Pearson A. Differential importance of highly conserved residues in UL24 for herpes simplex virus 1 replication in vivo and reactivation. J. Gen. Virol. 2010;91:1109–1116. doi: 10.1099/vir.0.017921-0. [DOI] [PubMed] [Google Scholar]
- 710.Callé A., Ugrinova I., Epstein A.L., Bouvet P., Diaz J.-J., Greco A. Nucleolin is required for an efficient herpes simplex virus type 1 infection. J. Virol. 2008;82:4762–4773. doi: 10.1128/JVI.00077-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Box J.K., Paquet N., Adams M.N., Boucher D., Bolderson E., O’Byrne K.J., Richard D.J. Nucleophosmin: From structure and function to disease development. BMC Mol. Biol. 2016;17:1–12. doi: 10.1186/s12867-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Lymberopoulos M.H., Bourget A., Abdeljelil N.B., Pearson A. Involvement of the UL24 protein in herpes simplex virus 1-induced dispersal of B23 and in nuclear egress. Virology. 2011;412:341–348. doi: 10.1016/j.virol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 713.Xu H., Su C., Pearson A., Mody C.H., Zheng C. Herpes simplex virus 1 UL24 abrogates the DNA sensing signal pathway by inhibiting NF-κB activation. J. Virol. 2017;91:1–10. doi: 10.1128/JVI.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Shiba C., Daikoku T., Goshima F., Takakuwa H., Yamauchi Y., Koiwai O., Nishiyama Y. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J. Gen. Virol. 2000;81:2397–2405. doi: 10.1099/0022-1317-81-10-2397. [DOI] [PubMed] [Google Scholar]
- 715.Ye G.-J., Roizman B. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of UL34 protein. Proc. Natl. Acad. Sci. USA. 2000;97:11002–11007. doi: 10.1073/pnas.97.20.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Funk C., Ott M., Raschbichler V., Nagel C.H., Binz A., Sodeik B., Bauerfeind R., Bailer S.M. The herpes simplex virus protein pUL31 escorts nucleocapsids to sites of nuclear egress, a process coordinated by Its N-terminal domain. PLoS Pathog. 2015;11:e1004957. doi: 10.1371/journal.ppat.1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Chang Y.E., van Sant C., Krug P.W., Sears A.E., Roizman B. The null mutant of the U(L)31 gene of herpes simplex virus 1: Construction and phenotype in infected cells. J. Virol. 1997;71:8307–8315. doi: 10.1128/JVI.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Roller R.J., Zhou Y., Schnetzer R., Ferguson J., DeSalvo D. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 2000;74:117–129. doi: 10.1128/JVI.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Monier K., Armas J.C.G., Etteldorf S., Ghazal P., Sullivan K.F. Annexation of the interchromosomal space during viral infection. Nat. Cell Biol. 2000;2:661–665. doi: 10.1038/35023615. [DOI] [PubMed] [Google Scholar]
- 720.Simpson-Holley M., Baines J., Roller R., Knipe D.M. Herpes simplex virus 1 UL31 and UL34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J. Virol. 2004;78:5591–5600. doi: 10.1128/JVI.78.11.5591-5600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Reynolds A.E., Liang L., Baines J.D. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34. J. Virol. 2004;78:5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Morrison A.L., DeLassus G.S. Breach of the nuclear lamina during assembly of herpes simplex viruses. Nucleus. 2011;2:271–276. doi: 10.4161/nucl.2.4.16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Klupp B.G., Granzow H., Fuchs W., Keil G.M., Finke S., Mettenleiter T.C. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. USA. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Desai P.J., Pryce E.N., Henson B.W., Luitweiler E.M., Cothran J. Reconstitution of the Kaposi’s sarcoma-associated herpesvirus nuclear egress complex and formation of nuclear membrane vesicles by coexpression of ORF67 and ORF69 gene products. J. Virol. 2012;86:594–598. doi: 10.1128/JVI.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Luitweiler E.M., Henson B.W., Pryce E.N., Patel V., Coombs G., McCaffery J.M., Desai P.J. Interactions of the Kaposi’s sarcoma-associated herpesvirus nuclear egress complex: ORF69 is a potent factor for remodeling cellular membranes. J. Virol. 2013;87:3915–3929. doi: 10.1128/JVI.03418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Bigalke J.M., Heldwein E.E. Structural basis of membrane budding by the nuclear egress complex of herpesviruses. EMBO J. 2015;34:2921–2936. doi: 10.15252/embj.201592359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Zeev-Ben-Mordehai T., Weberruß M., Lorenz M., Cheleski J., Hellberg T., Whittle C., el Omari K., Vasishtan D., Dent K.C., Harlos K., et al. Crystal structure of the herpesvirus nuclear egress complex provides insights into inner nuclear membrane remodeling. Cell Rep. 2015;13:2645–2652. doi: 10.1016/j.celrep.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Arii J., Watanabe M., Maeda F., Tokai-Nishizumi N., Chihara T., Miura M., Maruzuru Y., Koyanagi N., Kato A., Kawaguchi Y. ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity. Nat. Commun. 2018;9:3379. doi: 10.1038/s41467-018-05889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Tandon R., Mocarski E.S., Conway J.F. The A, B, Cs of herpesvirus capsids. Viruses. 2015;7:899–914. doi: 10.3390/v7030899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Newcomb W.W., Homa F.L., Brown J.C. Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices. J. Virol. 2006;80:6286–6294. doi: 10.1128/JVI.02648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Yang K., Baines J.D. Selection of HSV capsids for envelopment involves interaction between capsid surface components pU L31, pU L17, and pU L25. Proc. Natl. Acad. Sci. USA. 2011;108:14276–14281. doi: 10.1073/pnas.1108564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Chen D.-H., Jakana J., McNab D., Mitchell J., Zhou Z.H., Dougherty M., Chiu W., Rixon F.J. The Pattern of tegument-capsid interaction in the herpes simplex virus type 1 virion is not influenced by the small hexon-associated protein VP26. J. Virol. 2001;75:11863–11867. doi: 10.1128/JVI.75.23.11863-11867.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Zhou Z.H., He J., Jakana J., Tatman J.D., Rixon F.J., Chiu W. Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids. Nature. 1995;2:1026–1030. doi: 10.1038/nsb1195-1026. [DOI] [PubMed] [Google Scholar]
- 734.Bowman B.R., Baker M.L., Rixon F.J., Chiu W., Quiocho F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003;22:757–765. doi: 10.1093/emboj/cdg086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Lee J.H., Vittone V., Diefenbach E., Cunningham A.L., Diefenbach R.J. Identification of structural protein-protein interactions of herpes simplex virus type 1. Virology. 2008;378:347–354. doi: 10.1016/j.virol.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 736.Rixon F.J., Addison C., McGregor A., Macnab S.J., Nicholson P., Preston V.G., Tatman J.D. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J. Gen. Virol. 1996;77:2251–2260. doi: 10.1099/0022-1317-77-9-2251. [DOI] [PubMed] [Google Scholar]
- 737.Thomsen D.R., Roof L.L., Homa F.L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J. Virol. 1994;68:2442–2457. doi: 10.1128/JVI.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Desai P., DeLuca N.A., Person S. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology. 1998;247:115–124. doi: 10.1006/viro.1998.9230. [DOI] [PubMed] [Google Scholar]
- 739.Kobayashi R., Kato A., Oda S., Koyanagi N., Oyama M., Kozuka-Hata H., Arii J., Kawaguchi Y. Function of the herpes simplex virus 1 small capsid protein VP26 is regulated by phosphorylation at a specific site. J. Virol. 2015;89:6141–6147. doi: 10.1128/JVI.00547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Kobayashi R., Kato A., Sagara H., Watanabe M., Maruzuru Y., Koyanagi N., Arii J., Kawaguchi Y. Herpes simplex virus 1 small capsomere-interacting protein VP26 regulates nucleocapsid maturation. J. Virol. 2017;91 doi: 10.1128/JVI.01068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Douglas M.W., Diefenbach R.J., Homa F.L., Miranda-Saksena M., Rixon F.J., Vittone V., Byth K., Cunningham A.L. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 2004;279:28522–28530. doi: 10.1074/jbc.M311671200. [DOI] [PubMed] [Google Scholar]
- 742.Apcarian A., Cunningham A.L., Diefenbach R.J. Identification of binding domains in the herpes simplex virus type 1 small capsid protein pUL35 (VP26) J. Gen. Virol. 2010;91:2659–2663. doi: 10.1099/vir.0.019984-0. [DOI] [PubMed] [Google Scholar]
- 743.Carter K.L., Ward P.L., Roizman B. Characterization of the products of the U(L)43 gene of herpes simplex virus 1: Potential implications for regulation of gene expression by antisense transcription. J. Virol. 1996;70:7663–7668. doi: 10.1128/JVI.70.11.7663-7668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Loret S., Guay G., Lippé R. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 2008;82:8605–8618. doi: 10.1128/JVI.00904-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Herold B.C., WuDunn D., Soltys N., Spear P.G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 1991;65:1090–1098. doi: 10.1128/JVI.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.WuDunn D., Spear P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989;63:52–58. doi: 10.1128/JVI.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Trybala E., Bergstrom T., Svennerholm B., Jeansson S., Glorioso J.C., Olofsson S. Localization of a functional site on herpes simplex virus type 1 glycoprotein C involved in binding to cell surface heparan sulphate. J. Gen. Virol. 1994;75:743–752. doi: 10.1099/0022-1317-75-4-743. [DOI] [PubMed] [Google Scholar]
- 748.Komala Sari T., Gianopulos K.A., Weed D.J., Schneider S.M., Pritchard S.M., Nicola A.V. Herpes simplex virus glycoprotein c regulates low-pH Entry. mSphere. 2020;5:1–16. doi: 10.1128/mSphere.00826-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Visalli R.J., Brandt C.R. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29:167–178. doi: 10.1016/0168-1702(93)90057-T. [DOI] [PubMed] [Google Scholar]
- 750.Visalli R.J., Brandt C.R. The HSV-1 UL45 gene product is not required for growth in vero cells. Virology. 1991;185:419–423. doi: 10.1016/0042-6822(91)90790-I. [DOI] [PubMed] [Google Scholar]
- 751.Visalli R.J., Brandt C.R. Mutation of the herpes simplex virus 1 KOS UL45 gene reveals dose dependent effects on central nervous system growth. Arch. Virol. 2002;147:519–532. doi: 10.1007/s007050200004. [DOI] [PubMed] [Google Scholar]
- 752.Dollery S.J., Lane K.D., Delboy M.G., Roller D.G., Nicola A.V. Role of the UL45 protein in herpes simplex virus entry via low pH-dependent endocytosis and its relationship to the conformation and function of glycoprotein B. Virus Res. 2010;149:115–118. doi: 10.1016/j.virusres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Haanes E.J., Nelson C.M., Soule C.L., Goodman J.L. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J. Virol. 1994;68:5825–5834. doi: 10.1128/JVI.68.9.5825-5834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Nicola A.V., Hou J., Major E.O., Straus S.E. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Berkowitz C., Moyal M., Rossen-Wolff A., Darai G., Becker Y. Herpes simplex virus type 1 (HSV-1) UL56 gene is involved in viral intraperitoneal pathogenicity to immunocompetent mice. Arch. Virol. 1994;134:73–83. doi: 10.1007/BF01379108. [DOI] [PubMed] [Google Scholar]
- 756.Kolb A.W., Lee K., Larsen I., Craven M., Brandt C.R. Quantitative trait locus based virulence determinant mapping of the HSV-1 genome in murine ocular infection: Genes involved in viral regulatory and innate immune networks contribute to virulence. PLoS Pathog. 2016;12:e1005499. doi: 10.1371/journal.ppat.1005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Nash T.C., Spivack J.G. The UL55 and UL56 genes of herpes simplex virus type 1 are not required for viral replication, intraperitoneal virulence, or establishment of latency in mice. Virology. 1994;204:794–798. doi: 10.1006/viro.1994.1595. [DOI] [PubMed] [Google Scholar]
- 758.Umene K., Fukumaki Y. DNA genome of spontaneously occurring deletion mutants of herpes simplex virus type 1 lacking one copy of the inverted repeat sequences of the L component. Arch. Virol. 2011;156:1305–1315. doi: 10.1007/s00705-011-0983-2. [DOI] [PubMed] [Google Scholar]
- 759.Kehm R., Gelderblom H.R., Darai G. Identification of the UL56 protein of herpes simplex virus type 1 within the virion by immune electron microscopy. Virus Genes. 1998;17:49–53. doi: 10.1023/A:1008053017716. [DOI] [PubMed] [Google Scholar]
- 760.Koshizuka T., Kawaguchi Y., Goshima F., Mori I., Nishiyama Y. Association of two membrane proteins encoded by herpes simplex virus type 2, UL11 and UL56. Virus Genes. 2006;32:153–163. doi: 10.1007/s11262-005-6871-7. [DOI] [PubMed] [Google Scholar]
- 761.Ushijima Y., Koshizuka T., Goshima F., Kimura H., Nishiyama Y. Herpes simplex virus type 2 UL56 interacts with the ubiquitin ligase Nedd4 and Increases its ubiquitination. J. Virol. 2008;82:5220–5233. doi: 10.1128/JVI.02515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Ushijima Y., Goshima F., Kimura H., Nishiyama Y. Herpes simplex virus type 2 tegument protein UL56 relocalizes ubiquitin ligase Nedd4 and has a role in transport and/or release of virions. Virol. J. 2009;6:1–13. doi: 10.1186/1743-422X-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Ushijima Y., Luo C., Kamakura M., Goshima F., Kimura H., Nishiyama Y. Herpes simplex virus UL56 interacts with and regulates the Nedd4-family ubiquitin ligase Itch. Virol. J. 2010;7:1–11. doi: 10.1186/1743-422X-7-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.McGeoch D.J. On the predictive recognition of signal peptide sequences. Virus Res. 1985;3:271–286. doi: 10.1016/0168-1702(85)90051-6. [DOI] [PubMed] [Google Scholar]
- 765.Weber P.C., Levine M., Glorioso J.C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987;236:576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- 766.Glück B., Möbius S., Pfaff F., Zell R., Sauerbrei A. Novel method for genotyping clinical herpes simplex virus type 1 isolates. Arch. Virol. 2015;160:2807–2811. doi: 10.1007/s00705-015-2568-y. [DOI] [PubMed] [Google Scholar]
- 767.Jiang Y.-M., Yamada H., Goshima F., Daikoku T., Oshima S., Wada K., Nishiyama Y. Characterization of the herpes simplex virus type 2 (HSV-2) US2 gene product and a US2-deficient HSV-2 mutant. J. Gen. Virol. 1998;79:1989–1995. doi: 10.1099/0022-1317-79-11-2777. [DOI] [PubMed] [Google Scholar]
- 768.Goshima F., Watanabe D., Suzuki H., Takakuwa H., Yamada H., Nishiyama Y. The US2 gene product of herpes simplex virus type 2 interacts with cytokeratin 18. Arch. Virol. 2001;146:2201–2209. doi: 10.1007/s007050170030. [DOI] [PubMed] [Google Scholar]
- 769.Norrild B., Lehto V.P., Virtanen I. Organization of cytoskeleton elements during herpes simplex virus type I infection of human fibroblasts: An immunofluorescence study. J. Gen. Virol. 1986;67:97–105. doi: 10.1099/0022-1317-67-1-97. [DOI] [PubMed] [Google Scholar]
- 770.Wu Y., Wei F., Tang L., Liao Q., Wang H., Shi L., Gong Z., Zhang W., Zhou M., Xiang B., et al. Herpesvirus acts with the cytoskeleton and promotes cancer progression. J. Cancer. 2019;10:2185–2193. doi: 10.7150/jca.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Mingo R.M., Han J., Newcomb W.W., Brown J.C. Replication of herpes simplex virus: Egress of progeny virus at specialized cell membrane sites. J. Virol. 2012;86:7084–7097. doi: 10.1128/JVI.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Kang M.-H., Roy B.B., Finnen R.L., le Sage V., Johnston S.M., Zhang H., Banfield B.W. The Us2 gene product of herpes simplex virus 2 is a membrane-associated ubiquitin-interacting protein. J. Virol. 2013;87:9590–9603. doi: 10.1128/JVI.00994-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Lu X., Huang C., Zhang Y., Lin Y., Wang X., Li Q., Liu S., Tang J., Zhou L. The Us2 gene product of herpes simplex virus 2 modulates NF-κB activation by targeting TAK1. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Balan P., Davis-Poynter N., Bell S., Atkinson H., Browne H., Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J. Gen. Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 775.Tran L.C., Kissner J.M., Westerman L.E., Sears A.E. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA. 2000;97:1818–1822. doi: 10.1073/pnas.020510297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Ghiasi H., Kaiwar R., Nesburn A.B., Wechsler S.L. Baculovirus-expressed glycoprotein g of herpes simplex virus type 1 partially protects vaccinated mice against lethal HSV-1 challenge. Virology. 1992;190:233–239. doi: 10.1016/0042-6822(92)91209-D. [DOI] [PubMed] [Google Scholar]
- 777.Ghiasi H., Kaiwar R., Nesburn A.B., Slanina S., Wechsler S.L. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): Comparative protection against lethal challenge in mice. J. Virol. 1994;68:2118–2126. doi: 10.1128/JVI.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Blacklaws B.A., Krishna S., Minson A.C., Nash A.A. Immunogenicity of herpes simplex virus type 1 glycoproteins expressed in vaccinia virus recombinants. Virology. 1990;177:727–736. doi: 10.1016/0042-6822(90)90539-4. [DOI] [PubMed] [Google Scholar]
- 779.Ghiasi H., Hofman F.M., Cai S., Perng G.C., Nesburn A.B., Wechsler S.L. Vaccination with different HSV-1 glycoproteins induces different patterns of ocular cytokine responses following HSV-1 challenge of vaccinated mice. Vaccine. 1999;17:2576–2582. doi: 10.1016/S0264-410X(99)00056-0. [DOI] [PubMed] [Google Scholar]
- 780.Baggiolini M. Chemokines and leukocyte traffic. Nat. Immunol. 1998;392:949–952. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 781.Thapa M., Carr D.J.J. CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: Uncoupling of CD8+ T-cell effector function but not migration. J. Virol. 2009;83:9486–9501. doi: 10.1128/JVI.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Richman D.D., Buckmaster A., Bell S., Hodgman C., Minson A.C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J. Virol. 1986;57:647–655. doi: 10.1128/JVI.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Viejo-Borbolla A., Martinez-Martín N., Nel H.J., Rueda P., Martín R., Blanco S., Arenzana-Seisdedos F., Thelen M., Fallon P.G., Alcamí A. Enhancement of chemokine function as an immunomodulatory strategy employed by human herpesviruses. PLoS Pathog. 2012;8:e1002497. doi: 10.1371/journal.ppat.1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Martinez-Martin N., Viejo-Borbolla A., Martín R., Blanco S., Benovic J.L., Thelen M., Alcamí A. Herpes simplex virus enhances chemokine function through modulation of receptor trafficking and oligomerization. Nat. Commun. 2015;6 doi: 10.1038/ncomms7163. [DOI] [PubMed] [Google Scholar]
- 785.McLean T.I., Bachenheimer S.L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 1999;73:8415–8426. doi: 10.1128/JVI.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Martínez-Martín N., Viejo-Borbolla A., Alcami A. Herpes simplex virus particles interact with chemokines and enhance cell migration. J. Gen. Virol. 2016;97:3007–3016. doi: 10.1099/jgv.0.000616. [DOI] [PubMed] [Google Scholar]
- 787.Aubert M., Chen Z., Lang R., Dang C.H., Fowler C., Sloan D.D., Jerome K.R. The antiapoptotic herpes simplex virus glycoprotein J localizes to multiple cellular organelles and induces reactive oxygen species formation. J. Virol. 2008;82:617–629. doi: 10.1128/JVI.01341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Jerome K.R., Chen Z., Lang R., Torres M.R., Hofmeister J., Smith S., Fox R., Froelich C.J., Corey L. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or fas. J. Immunol. 2001;167:3928–3935. doi: 10.4049/jimmunol.167.7.3928. [DOI] [PubMed] [Google Scholar]
- 789.Johnson D.C., Frame M.C., Ligas M.W., Cross A.M., Stow N.D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 1988;62:1347–1354. doi: 10.1128/JVI.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Dingwell K.S., Brunetti C.R., Hendricks R.L., Tang Q., Tang M., Rainbow A.J., Johnson D.C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 1994;68:834–845. doi: 10.1128/JVI.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Dingwell K.S., Johnson D.C. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 1998;72:8933–8942. doi: 10.1128/JVI.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Johnson D.C., Webb M., Wisner T.W., Brunetti C. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 2001;75:821–833. doi: 10.1128/JVI.75.2.821-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Frank I., Friedman H.M. A novel function of the herpes simplex virus type 1 Fc receptor: Participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 1989;63:4479–4488. doi: 10.1128/JVI.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Dubin G., Socolof E., Frank I., Friedman H.M. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 1991;65:7046–7050. doi: 10.1128/JVI.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Van Vliet K.E., de Graaf-Miltenburg L.A., Verhoef J., Van Strijp J.A. Direct evidence for antibody bipolar bridging on herpes simplex virus-infected cells. Immunology. 1992;77:109–115. [PMC free article] [PubMed] [Google Scholar]
- 796.Neidhardt H., Schröder C.H., Kaerner H.C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J. Virol. 1987;61:600–603. doi: 10.1128/JVI.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Saldanha C.E., Lubinski J., Martin C., Nagashunmugam T., Wang L., van der Keyl H., Tal-Singer R., Friedman H.M. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 2000;74:6712–6719. doi: 10.1128/JVI.74.15.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Polcicova K., Biswas P.S., Banerjee K., Wisner T.W., Rouse B.T., Johnson D.C. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl. Acad. Sci. USA. 2005;102:11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Wang F., Tang W., McGraw H.M., Bennett J., Enquist L.W., Friedman H.M. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J. Virol. 2005;79:13362–13372. doi: 10.1128/JVI.79.21.13362-13372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Snyder A., Polcicova K., Johnson D.C. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J. Virol. 2008;82:10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Howard P.W., Wright C.C., Howard T., Johnson D.C. Herpes simplex virus gE/gI extracellular domains promote axonal transport and spread from neurons to epithelial cells. J. Virol. 2014;88:11178–11186. doi: 10.1128/JVI.01627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.LaVail J.H., Tauscher A.N., Sucher A., Harrabi O., Brandimarti R. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146:974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Frame M.C., McGeoch D.J., Rixon F.J., Orr A.C., Marsden H.S. The 10K virion phosphoprotein encoded by gene US9 from herpes simplex virus type 1. Virology. 1986;150:321–332. doi: 10.1016/0042-6822(86)90297-7. [DOI] [PubMed] [Google Scholar]
- 804.Brideau A.D., Banfield B.W., Enquist L.W. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 1998;72:4560–4570. doi: 10.1128/JVI.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Brideau A.D., Eldridge M.G., Enquist L.W. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 2000;74:4549–4561. doi: 10.1128/JVI.74.10.4549-4561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.DuRaine G., Wisner T.W., Howard P., Williams M., Johnson D.C. Erratum for DuRaine et al., “Herpes simplex virus gE/gI and US9 promote both envelopment and sorting of virus particles in the cytoplasm of neurons, two processes that precede anterograde transport in axons”. J. Virol. 2017;91:1–20. doi: 10.1128/JVI.00933-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Zhang W., Gao P., Gui X., Zhou L., Ge X., Guo X., Wills J.W., Han J., Yang H. Induction of rod-shaped structures by herpes simplex virus glycoprotein I. J. Virol. 2020;94 doi: 10.1128/JVI.00231-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Georgopoulou U., Michaelidou A., Roizman B., Mavromara-Nazos P. Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J. Virol. 1993;67:3961–3968. doi: 10.1128/JVI.67.7.3961-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Willemse M.J., Strijdveen I.G.L., van Schooneveld S.H.B., van den Berg M.C., Sondermeijer P.J.A. Transcriptional analysis of the short segment of the feline herpesvirus type 1 genome and insertional mutagenesis of a unique reading frame. Virology. 1995;208:704–711. doi: 10.1006/viro.1995.1202. [DOI] [PubMed] [Google Scholar]
- 810.Yamada H., Daikoku T., Yamashita Y., Jiang Y.M., Tsurumi T., Nishiyama Y. The product of the US10 gene of herpes simplex virus type 1 is a capsid/tegument-associated phosphoprotein which copurifies with the nuclear matrix. J. Gen. Virol. 1997;78:2923–2931. doi: 10.1099/0022-1317-78-11-2923. [DOI] [PubMed] [Google Scholar]
- 811.Johnson P.A., MacLean C., Marsden H.S., Dalziel R.G., Everett R.D. The product of gene US11 of herpes simplex type 1 is expressed as a true late gene. J. Gen. Virol. 1986;67:871–883. doi: 10.1099/0022-1317-67-5-871. [DOI] [PubMed] [Google Scholar]
- 812.Roller R.J., Roizman B. The herpes simplex virus Us11 open reading frame encodes a sequence-specific RNA-binding protein. J. Virol. 1990;64:3463–3470. doi: 10.1128/JVI.64.7.3463-3470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Roller R.J., Roizman B. Herpes simplex virus 1 RNA-binding protein US11 negatively regulates the accumulation of a truncated viral mRNA. J. Virol. 1991;65:5873–5879. doi: 10.1128/JVI.65.11.5873-5879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Roller R.J., Roizman B. A herpes simplex virus 1 US11-expressing cell line is resistant to herpes simplex virus infection at a step in viral entry mediated by glycoprotein D. J. Virol. 1994;68:2830–2839. doi: 10.1128/JVI.68.5.2830-2839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Simonin D., Diaz J.-J., Kindbeiter K., Pernas P., Madjar J.-J. Phosphorylation of herpes simplex virus type 1 Us11 protein is independent of viral genome expression. Electrophoresis. 1995;16:1317–1322. doi: 10.1002/elps.11501601216. [DOI] [PubMed] [Google Scholar]
- 816.Cassady K.A., Gross M. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 2002;76:2029–2035. doi: 10.1128/jvi.76.5.2029-2035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Greco A., Arata L., Soler E., Gaume X., Coute Y., Hacot S., Calle A., Monier K., Epstein A.L., Sanchez J.-C., et al. Nucleolin interacts with US11 protein of herpes simplex virus 1 and is involved in its trafficking. J. Virol. 2012;86:1449–1457. doi: 10.1128/JVI.06194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Diaz J.-J., Dodon M.D., Schaerer-Uthurralt N., Simonin D., Kindbeiter K., Gazzolo L., Madjar J.-J. Post-transcriptional transctivation of human retroviral envelope glycoprotein expression by herpes simplex virus Us11 protein. Nature. 1996;379:273–277. doi: 10.1038/379273a0. [DOI] [PubMed] [Google Scholar]
- 819.Schaerer-Uthurralt N., Erard M., Kindbeiter K., Madjar J.J., Diaz J.J. Distinct domains in herpes simplex virus type 1 US11 protein mediate post-transcriptional transactivation of human T-lymphotropic virus type I envelope glycoprotein gene expression and specific binding to the Rex responsive element. J. Gen. Virol. 1998;79:1593–1602. doi: 10.1099/0022-1317-79-7-1593. [DOI] [PubMed] [Google Scholar]
- 820.Poppers J., Mulvey M., Khoo D., Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 2000;74:11215–11221. doi: 10.1128/JVI.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Lussignol M., Queval C., Bernet-Camard M.-F., Cotte-Laffitte J., Beau I., Codogno P., Esclatine A. The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J. Virol. 2013;87:859–871. doi: 10.1128/JVI.01158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Sadler A.J., Latchoumanin O., Hawkes D., Mak J., Williams B.R.G. An antiviral response directed by PKR phosphorylation of the RNA helicase A. PLoS Pathog. 2009;5:e1000311. doi: 10.1371/journal.ppat.1000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Peters G.A., Khoo D., Mohr I., Sen G.C. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 2002;76:11054–11064. doi: 10.1128/JVI.76.21.11054-11064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Kew C., Lui P.-Y., Chan C.-P., Liu X., Au S.W.N., Mohr I., Jin D.-Y., Kok K.-H. Suppression of PACT-induced type I interferon production by herpes simplex virus 1 Us11 protein. J. Virol. 2013;87:13141–13149. doi: 10.1128/JVI.02564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Sànchez R., Mohr I. Inhibition of cellular 2′-5′ oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J. Virol. 2007;81:3455–3464. doi: 10.1128/JVI.02520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Liu X., Main D., Ma Y., He B. Herpes simplex virus 1 inhibits TANK-binding kinase 1 through formation of the Us11-Hsp90 complex. J. Virol. 2018;92 doi: 10.1128/JVI.00402-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Liyana A., Vanessa S.S. The emerging role of human TBK1 in virus-induced autophagy. Autophagy. 2019;15:917–918. doi: 10.1080/15548627.2019.1580513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Diefenbach R.J., Miranda-Saksena M., Diefenbach E., Holland D.J., Boadle R.A., Armati P.J., Cunningham A.L. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 2002;76:3282–3291. doi: 10.1128/JVI.76.7.3282-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Roller R.J., Monk L.L., Stuart D., Roizman B. Structure and function in the herpes simplex virus 1 RNA-binding protein U(s)11: Mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 1996;70:2842–2851. doi: 10.1128/JVI.70.5.2842-2851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Benboudjema L., Mulvey M., Gao Y., Pimplikar S.W., Mohr I. Association of the herpes simplex virus Type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J. Virol. 2003;77:9192–9203. doi: 10.1128/JVI.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Charron A.J., Ward S.L., North B.J., Ceron S., Leib D.A. The US11 gene of herpes simplex virus 1 promotes neuroinvasion and periocular replication following corneal infection. J. Virol. 2019;93:1–19. doi: 10.1128/JVI.02246-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Melancon J.M., Luna R.E., Foster T.P., Kousoulas K.G. Herpes simplex virus type 1 gK is required for gB-mediated virus-induced cell fusion, while neither gB and gK nor gB and UL20p function redundantly in virion de-envelopment. J. Virol. 2005;79:299–313. doi: 10.1128/JVI.79.1.299-313.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Croft C.L., Noble W. Preparation of organotypic brain slice cultures for the study of Alzheimer’s disease [version 1; peer review: 3 approved] F1000Research. 2018;7 doi: 10.12688/f1000research.14500.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Visalli R.J., Courtney R.J., Meyers C. Infection and replication of herpes simplex virus type 1 in an organotypic epithelial culture system. Virology. 1997;230:236–243. doi: 10.1006/viro.1997.8484. [DOI] [PubMed] [Google Scholar]
- 835.Webre J.M., Hill J.M., Nolan N.M., Clement C., McFerrin H.E., Bhattacharjee P.S., Hsia V., Neumann D.M., Foster T.P., Lukiw W.J., et al. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Conlon J., Burdette D.L., Sharma S., Bhat N., Thompson M., Jiang Z., Rathinam V.A.K., Monks B., Jin T., Xiao T.S., et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-Dimethylxanthenone-4-Acetic acid. J. Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Cavlar T., Deimling T., Ablasser A., Hopfner K.P., Hornung V. Species-specific detection of the antiviral small-molecule compound CMA by STING. EMBO J. 2013;32:1440–1450. doi: 10.1038/emboj.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Sidhaye J., Knoblich J.A. Brain organoids: An ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Watanabe M., Buth J.E., Vishlaghi N., de la Torre-Ubieta L., Taxidis J., Khakh B.S., Coppola G., Pearson C.A., Yamauchi K., Gong D., et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat zika virus infection. Cell Rep. 2017;21:517–532. doi: 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Qiao H., Guo M., Shang J., Zhao W., Wang Z., Liu N., Li B., Zhou Y., Wu Y., Chen P. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog. 2020;16:e1008899. doi: 10.1371/journal.ppat.1008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.D’Aiuto L., Bloom D.C., Naciri J.N., Smith A., Edwards T.G., Mcclain L., Callio J.A., Jessup M., Wood J., Chowdari K., et al. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three dimensional cultures derived from induced pluripotent stem cells. J. Virol. 2019;93 doi: 10.1128/JVI.00111-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Edwards T.G., Bloom D.C. Lund human mesencephalic (LUHMES) neuronal cell line supports herpes simplex virus 1 latency in vitro. J. Virol. 2019;93:1–14. doi: 10.1128/JVI.02210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Dogrammatzis C., Waisner H., Kalamvoki M. Cloaked viruses and viral factors in cutting edge exosome-based therapies. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018;14:839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Ghonime M.G., Jackson J., Shah A., Roth J., Li M., Saunders U., Coleman J., Gillespie G.Y., Markert J.M., Cassady K.A. Chimeric HCMV/HSV-1 and Δγ134.5 oncolytic herpes simplex virus elicit immune mediated antigliomal effect and antitumor memory. Transl. Oncol. 2018;11:86–93. doi: 10.1016/j.tranon.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Chiocca E.A., Nakashima H., Kasai K., Fernandez S.A., Oglesbee M. Preclinical toxicology of rQNestin34.5v.2: An oncolytic herpes virus with transcriptional regulation of the ICP34.5 neurovirulence gene. Mol. Ther. Methods Clin. Dev. 2020;17:871–893. doi: 10.1016/j.omtm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Sokolowski N.A., Rizos H., Diefenbach R.J. Oncolytic virotherapy dovepress oncolytic virotherapy using herpes simplex virus: How far have we come? Oncolytic Virotherapy. 2015;4:207–219. doi: 10.2147/OV.S66086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Miao L., Fraefel C., Sia K.C., Newman J.P., Mohamed-Bashir S.A., Ng W.H., Lam P.Y.P. The potential application of a transcriptionally regulated oncolytic herpes simplex virus for human cancer therapy. Br. J. Cancer. 2014;110:94–106. doi: 10.1038/bjc.2013.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Pyles R.B., Warnick R.E., Chalk C.L., Szanti B.E., Parysek L.M. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum. Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 850.Stanfield B.A., Stahl J., Chouljenko V.N., Subramanian R., Charles A.S., Saied A.A., Walker J.D., Kousoulas K.G. A single intramuscular vaccination of mice with the HSV-1 VC2 virus with mutations in the glycoprotein K and the membrane protein UL20 confers full protection against lethal intravaginal challenge with virulent HSV-1 and HSV-2 strains. PLoS ONE. 2014;9:e109890. doi: 10.1371/journal.pone.0109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Stanfield B.A., Pahar B., Chouljenko V.N., Veazey R., Kousoulas K.G. Vaccination of rhesus macaques with the live-attenuated HSV-1 vaccine VC2 stimulates the proliferation of mucosal T cells and germinal center responses resulting in sustained production of highly neutralizing antibodies. Vaccine. 2017;35:536–543. doi: 10.1016/j.vaccine.2016.12.018. [DOI] [PubMed] [Google Scholar]