Abstract
Objectives
We investigated the effects of an enhanced partner notification (PN) counselling intervention with the offer of provider-assisted referral among people diagnosed with STI in a Cape Town public clinic.
Methods
Participants were adults diagnosed with STI at a community clinic. After the standard STI consultation, participants were randomly allocated in a 1:1:1 ratio to (1) ‘HE’: 20 min health education; (2) ‘RR’: 45 min risk reduction skills counselling; or (3) ‘ePN’: 45 min enhanced partner notification communication skills counselling and the offer of provider-assisted referral. The primary outcome was the incidence of repeat STI diagnoses during the 12 months after recruitment, and the secondary outcome was participants’ reports 2 weeks after diagnosis of notifying recent partners. Incidence rate ratios (IRRs) were used to compare the incidence rates between arms using a Poisson regression model.
Results
The sample included 1050 participants, 350 per group, diagnosed with STI between June 2014 and August 2017. We reviewed 1048 (99%) participant records, and identified 136 repeat STI diagnoses in the ePN arm, 138 in the RR arm and 141 in the HE arm. There was no difference in the annual incidence of STI diagnosis between the ePN and HE arms (IRR: 1.0; 95% CI 0.7 to 1.3), or between the RR and HE arms (IRR: 0.9; 95% CI 0.7 to 1.2). There was a greater chance of a partner being notified in the ePN condition compared with the HE condition, 64.3% compared with 53.8%, but no difference between the RR and HE arms.
Conclusions
PN counselling and education with provider-assisted services has the potential to change the behaviour of people diagnosed with STIs, increasing the number of partners they notify by more than 10%. However, these changes in behaviour did not lead to a reduction of repeat STI diagnoses.
Trial registration number
PACTR201606001682364.
Keywords: sexual behaviour, contact tracing, partner notification, sexual networks
Background
Partner notification (PN) is a process by which a person with STI informs sexual partners of their possible exposure and the need to be tested or to obtain treatment. STI PN and treatment can interrupt STI transmission and prevent reinfection of index patients, thus decreasing the pool of infectious people. STI PN and treatment are important in the context of the HIV epidemic because people with non-HIV STIs are more susceptible to HIV acquisition or transmission,1 2 especially in the context of low coverage of pre-exposure prophylaxis (PrEP) or antiretroviral therapy (ART). Current methods of PN only reach a small proportion of partners.3 4
Public sector clinics in South Africa offer STI partner services based on patient referral, where the clinician encourages the patient to refer partners for treatment without provider assistance. When index patients bear the sole responsibility for PN, the potential barriers include inadequate knowledge about or motivation for PN, stigma, and fear of blame, abandonment or violence from partners.5–7 There is evidence people are less likely to notify casual partners (compared with main partners).8
We investigated the effects of enhancing patient referral with counselling, education and the offer of provider-assisted referral among people diagnosed with STI in a Cape Town public clinic. The outcomes included the annual incidence of STI diagnoses in index patients (primary) and index patients’ reports of PN (secondary). We also examined the impact of the intervention on adverse partner responses to PN (abandonment and intimate partner violence (IPV)).
Methods
Based in a public clinic in a poor Cape Town community, we conducted a three-armed randomised controlled trial (RCT). Eligible participants were 18 years of age and older diagnosed with an STI using a syndromic approach (the standard in South Africa). They were recruited on the day of their diagnosis and participated that day, or the next, except for 45 participants who were enrolled in the study more than 1 day later (up to 3 days).
After the standard clinic STI consultation, the clinician referred potentially eligible patients to our study. Those who were eligible and consented completed a baseline questionnaire, after which we randomly allocated them in a 1:1:1 ratio to one of three interventions using a block randomisation schedule generated by computer and sequentially numbered, opaque envelopes to conceal allocation. We also invited participants to complete 2-week and 3-month, 6-month and 9-month follow-up surveys, but we only report on the 2-week survey. Participants were reimbursed approximately US$10 for each assessment.
Conditions
One of two female lay counsellors employed by the research team delivered the interventions, guided by manuals and flip charts (online supplementary appendices 1–6). Counsellors delivered all three conditions, crossing conditions, allowing for tests for balance. All participants were offered condoms.
sextrans-2020-054499supp001.pdf (12.9MB, pdf)
sextrans-2020-054499supp002.pdf (227.9KB, pdf)
sextrans-2020-054499supp003.pdf (25.1MB, pdf)
sextrans-2020-054499supp004.pdf (417.4KB, pdf)
sextrans-2020-054499supp005.pdf (13.1MB, pdf)
sextrans-2020-054499supp006.pdf (200.5KB, pdf)
Enhanced partner notification (45 min)
Grounded in the information-motivation-behavioural skills (IMB) model,9 in enhanced partner notification (ePN) condition the counsellors provided information about STIs including the importance of condom use in prevention; demonstrated condom use and for participants who were unsure how to use a condom; used interactive, motivation-enhancing and skills-building exercises (eg, a board game to demonstrate STI spread in a network); offered a menu of PN options including patient referral (face-to-face communication, phone call, text message, email, a referral card, invite partner to attend the clinic together with index patient) and provider-assisted options undertaken by the counsellor, maintaining participant anonymity (phone call, email, text message, mailing referral card); assisted the participant in developing a PN plan for each partner, considering the risk of adverse reactions; invited the participant to role-play the communication with partner, providing feedback; and gave the participant a brochure with their plan (online supplementary appendix 7).
sextrans-2020-054499supp007.pdf (83.2KB, pdf)
Risk reduction counselling (45 min)
Informed by the IMB model,9 the risk reduction (RR) condition, previously shown to be an efficacious STI prevention intervention,10 focused on safe sex and condom use. The counsellor provided information about STIs including the importance of condom use in prevention; engaged participants in a risk continuum activity to increase motivation to prevent STIs; facilitated participants to identify triggers for risky sex and a personalised risk reduction plan; invited participants to role-play negotiating condom use, providing feedback; guided them to practise condom use with a penis model; and gave a brochure to participants with their plan (online supplementary appendix 8).
sextrans-2020-054499supp008.pdf (76.3KB, pdf)
Health education (20 min)
The health education (HE) condition, designed to provide a standardised version of counsellor activities in the standard of care, comprised health education during which the counsellor provided information about STIs; answered participants’ questions; corrected misconceptions; and guided participants to practise condom use with a penis model if they were unsure.
Measures
At baseline, we asked participants their age, sex, education, STI symptoms, HIV status, and alcohol and drug use. Participants were classified as potentially hazardous alcohol users if, for men, they had an Alcohol Use Disorders Identification Test (AUDIT)-C score of greater than 3, and for women they had an AUDIT-C score of greater than 2.11 If they used any of the following drugs in the 3 prior months, they were classified as drug users: marijuana, Mandrax, cocaine, methamphetamine or any drug injected with a needle.
At baseline participants reported up to five sexual partners in the past 3 months, and classified each as ‘main’, ‘casual’ (‘someone you have sex with on a regular basis who is not a main partner’) or ‘one-night stand’ (‘someone who you may have only had sex with once or twice, not someone you have sex with on a regular basis’). Partners’ first or nick names were entered into the questionnaire system, and questions about sex partners included the partner name. Participants self-completed the baseline in English or isiXhosa as an audio-assisted survey (first 330 participants) or an interviewer-administered, paper survey (remaining participants). An armed robbery necessitated the change in the assessment method.
The primary outcome was the 12-month incidence of STI diagnosis in participants, measured using City of Cape Town electronic records. The secondary outcome was participants’ reports at the 2-week survey of whether each partner was notified by any method (in person, by phone or text message, or with a third party’s help) or whether the participant had referred the partner for STI testing or treatment. In the 2-week assessment, we also measured the partner’s reaction to the notification (appreciative, angry, violent, abandoned the index patient or no reaction) and whether the participant had had condomless sex with the partner during the 2 weeks after diagnosis. We asked four items to measure knowledge of STIs (that they can be asymptomatic, that transmission can occur when they are asymptomatic, that treatment should not be stopped when symptoms disappear and that PN can interrupt the spread of STIs), and we composed a knowledge score with a point for each correct answer (score range 0–4). We asked six questions to measure PN self-efficacy (eg, how confident are you that you can tell your main partner that you have an STI; how confident are you that you can ask your casual partner to go to the clinic to get tested for an STI) and two questions to measure condom use self-efficacy (eg, how confident are you that you can talk to your main partner about the need to use condoms). For the self-efficacy items, participants used a scale from 0 to 10, where 0 reflected no confidence at all and 10 reflected the highest level of confidence. The 2-week assessments were administered by an assessor blinded to trial condition.
Randomisation and blinding
Study assessors enrolled participants and assigned them to a time slot available for a baseline assessment. Participants who presented for baseline assessment were randomly allocated to condition using an assignment scheme pregenerated by the investigators. Randomisation was not breached throughout the trial. Recruitment, screening and assessment staff remained blinded to condition throughout the study, and the counsellors never conducted assessments with participants they had counselled.
Adverse events
There was one adverse event: a male participant reported he had had sex with a 13-year-old girl, which is defined as statutory rape in the South African law.
Analysis
The trial was designed with 90% power to detect a 20% reduction in incidence of STI diagnosis assuming an underlying annual incidence of 15%. We planned a sample size of 1050 people, 350 in each arm. In all analyses, we compared the ePN arm with the HE arm, and the RR arm with the HE arm. Statistical significance was defined as p<0.05. In analyses at the index patient level, we used an intention-to-treat approach. In all analyses at the named partner level, we used a modified intention-to-treat (mITT) approach excluding from the analysis 24 participants who, at baseline, did not report any sex partners in the 3 months prior to diagnosis.
Primary outcome
For the primary outcome, the 12-month incidence of repeat STI diagnosis in index patients was calculated and incidence rate ratios (IRRs) were used to compare the incidence rates between arms using a Poisson regression model. A subgroup analysis was performed for gender (this was preplanned and stated in the protocol), and the significance of the interaction between condition and gender was tested.
Secondary outcome
For the secondary outcome, index patients’ reports of PN, first we performed an analysis at the index patient level in which we compared the mean number of partners referred by condition, adjusting for clustering of partners by index patient, and weighting by the number of partners reported at baseline, which could range between 1 and 5. Second, we performed an analysis at the named partner level using a binomial regression model to model the probability of a partner being notified. We estimated risk differences between arms adjusting for the clustering of partners within each index patient. Subgroup analyses were performed for gender (prespecified in the protocol) and for partner type (post-hoc analysis).
Other outcomes
At the level of the index patient, we compared the following intermediate outcomes across arms, by gender: STI knowledge using ordinal logistic regression reporting OR with 95% CI; condom use and PN self-efficacy using a quantile regression model; and use of a third party (nurse, counsellor or other party) to assist with PN for at least one partner using logistic regression. At the partner level, to measure STI transmission or acquisition risk, we compared the probability of condomless sex by partner during the 2 weeks after diagnosis (vs no sex with partner or sex with a condom every time), using a binomial regression model estimating risk differences. We compared the risk of a partner perpetrating IPV or abandoning the index patient, using binomial regression models.
Results
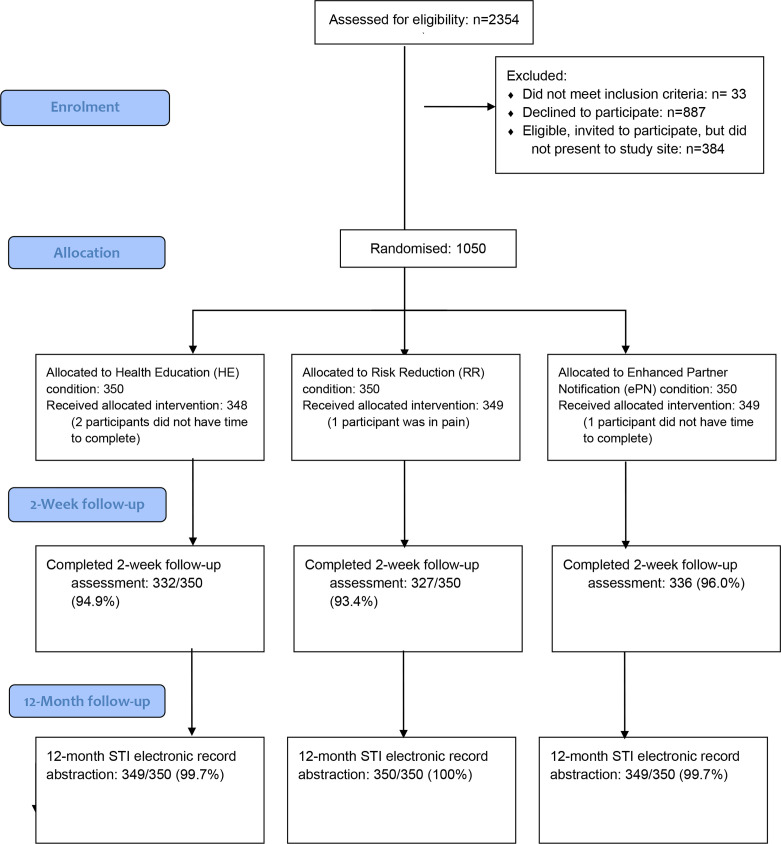
We assessed for eligibility 2354 people diagnosed with an STI between June 2014 and August 2017, of which 1050 were enrolled (figure 1). Declinations were mostly due to time constraints.
Figure 1.
STI partner notification trial flow diagram
At baseline, the arms were well balanced (table 1).
Table 1.
Participant characteristics at baseline by trial condition
| HE | RR | ePN | |
| Frequency (%) | Frequency (%) | Frequency (%) | |
| Women | 173 (49.9) | 174 (49.4) | 175 (50.0) |
| Married | 32 (9.1) | 24 (6.9) | 23 (6.6) |
| High school completion | 150 (42.9) | 172 (49.1) | 151 (43.1) |
| STI symptoms* | |||
| Genital sore | 49 (14.0) | 48 (13.7) | 57 (16.3) |
| Genital discharge | 181 (51.7) | 174 (49.7) | 181 (51.7) |
| Pain on urination | 173 (49.4) | 176 (50.3) | 184 (52.6) |
| HIV-positive (self-report) | 73 (20.9) | 64 (18.3) | 65 (18.6) |
| Hazardous alcohol use† | 213 (60.9) | 240 (68.6) | 232 (66.3) |
| Drug use (any) | 77 (22.0) | 82 (23.4) | 79 (22.6) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 30.0 | 29.2 | 29.9 |
| Total‡; mean§ (SD) | Total‡; mean§ (SD) | Total‡; mean§ (SD) | |
| Number of partners: all participants | 745; 2.1 (1.6) | 801; 2.3 (1.9) | 818; 2.3 (3.1) |
| Number of partners: male participants¶ | 481; 2.8 (1.9) | 505; 2.9 (2.0) | 561; 3.2 (4.2) |
| Number of partners: female participants¶ | 261; 1.5 (1.0) | 296; 1.7 (1.6) | 257; 1.5 (0.9) |
| All participants: number of main partners | 325; 0.9 (0.4) | 336; 1.0 (0.4) | 348; 1.0 (0.5) |
| All participants: number of casual partners | 272; 0.8 (1.2) | 285; 0.8 (1.2) | 279; 0.8 (1.4) |
| All participants: number of once-off partners | 151; 0.4 (1.0) | 187; 0.5 (1.4) | 194; 0.6 (1.9) |
*Participants could report more than one symptom.
†Hazardous alcohol use for men was an AUDIT-C score greater than 3, and for women an AUDIT-C score greater than 2.
‡Total partners during 3 months prior to STI diagnosis restricted to naming of five partners.
§Mean number of partners per index patient.
¶One person in the health education arm had gender missing.
ePN, enhanced partner notification; HE, health education; RR, risk reduction.
Primary outcome
We reviewed 1048 (99%) participant electronic medical records (figure 1). During 12 months after diagnosis, there were 136 repeat STI diagnoses in the ePN arm, 138 in the RR arm and 141 in the HE arm (table 2). No subsequent STI diagnoses were recorded for 71% of participants, 21% had one diagnosis and 7.8% had two to four diagnoses. There was no difference in the incidence of STI diagnosis between the ePN and HE arms (IRR: 0.97; 95% CI 0.74 to 1.26), or between the RR and HE arms (IRR: 0.91; 95% CI 0.70 to 1.19) (table 2). There were no differences in the incidence between arms for men and for women (table 2) and no significant interaction between gender and study arm in the annual incidence of STI diagnosis (p=0.17).
Table 2.
Annual incidence of STI diagnosis by trial condition for all participants and by gender
| HE | RR | ePN | Effect size: ePN vs HE | Effect size: RR vs HE | |
| Number of incident STI diagnoses; incidence per 100 person-years (py) | IRR (95% CI) | IRR (95% CI) | |||
| All participants | |||||
| 141/350; 40.3 per 100 py | 128/350; 36.6 per 100 py | 136/350; 38.9 per 100 py | 1.0 (0.7 to 1.3), p=0.8 | 0.9 (0.7 to 1.2), p=0.5 | |
| Gender* | |||||
| Male | 55/175; 44.0 per 100 py | 61/177; 44.1 per 100 py | 45/175; 36.5 per 100 py | 0.8 (0.6 to 1.2), p=0.4 | 1.0 (0.7 to 1.4), p=0.9 |
| Female | 51/173; 36.2 per 100 py | 35/174; 28.9 per 100 py | 53/175; 41.1 per 100 py | 1.1 (0.8 to 1.6), p=0.5 | 0.8 (0.5 to 1.2), p=0.3 |
*One participant in the HE arm had missing gender.
ePN, enhanced partner notification; HE, health education; IRR, incidence rate ratio; RR, risk reduction.
Other outcomes
By 2 weeks, we had retained over 90% of participants (figure 1). The mITT population of 1026 index patients named 2178 partners within the study restriction of 5 partners.
Secondary outcome
By 2 weeks, the mean number of partners referred per index patient was 1.68 in the ePN condition, 1.29 in the RR condition and 1.24 in the HE condition (table 3).
Table 3.
Partner notification and condom use self-efficacy, STI knowledge, partner notification, condom use, third-party assistance with PN, and harmful partner reactions, 2 weeks after STI diagnosis
| HE | RR | ePN | Effect size: ePN vs HE | Effect size: RR vs HE | |
| Partner notification self-efficacy: index patient-level analysis; median (IQR) | Median difference (95% CI), p value | ||||
| All participants | 8.2 (6.7–9.8) | 8.5 (6.7–9.7) | 8.5 (7.3–9.7) | 0.3 (−0.1 to 0.7), p=0.09 | 0.3 (−0.1 to 0.7), p=0.09 |
| Male participants | 8.2 (6.7–9.8) | 8.5 (7.0–9.7) | 8.5 (7.2–9.7) | 0.3 (−0.3 to 1.0), p=0.3 | 0.3 (−0.3 to 1.0), p=0.3 |
| Female participants | 8.3 (6.7–9.8) | 8.3 (6,7–9.7) | 8.7 (7.3–9.7) | 0.3 (−0.2 to 0.9), p=0.2 | 0.0 (−0.6 to 0.6), p=1.0 |
| Condom use self-efficacy: index patient-level analysis; median (IQR) | |||||
| All participants | 10.0 (8.0–10.0) | 10.0 (8.5–10.0) | 10.0 (9.0–10.0) | 0.0 (−0.2 to 0.2), p=1.0 | 0.0 (−0.2 to 0.2), p=1.0 |
| Male participants | 10.0 (8.5–10.0) | 10.0 (8.5–10.0) | 10.0 (9.0–10.0) | 0.0 (−0.3 to 0.3), p=1.0 | 0.0 (−0.3 to 0.3), p=1.0 |
| Female participants | 10.0 (8.0–10.0) | 10.0 (9.0–10.0) | 10.0 (9.0–10.0) | 0.0 (−0.3 to 0.3), p=1.0 | 0.0 (−0.3 to 0.3), p=1.0 |
| STI knowledge: index patient-level analysis; median (IQR) | OR (95% CI), p value | ||||
| All participants | 3 (2–4) | 3 (2–4) | 3 (2–4) | 1.0 (0.8 to 1.3), p=0.8 | 1.1 (0.8 to 1.4), p=0.5 |
| Male participants | 3 (2–4) | 3 (2–4) | 3 (2–4) | 1.1 (0.7 to 1.8), p=0.8 | 1.1 (0.8 to 1.6), p=0.6 |
| Female participants | 3 (2–4) | 3 (2–4) | 3 (2–4) | 1.1 (0.7 to 1.5), p=0.8 | 1.1 (0.8 to 1.6), p=0.6 |
| Participant reports of assistance from a third party in notifying partner (from nurse, counsellor or other persons): index patient-level analysis; frequency (%) | OR (95% CI), p value | ||||
| All participants | 13 (3.7) | 16 (4.6) | 16 (4.6) | 1.2 (0.6 to 2.6), p=0.6 | 1.2 (0.6 to 2.6), p=0.6 |
| Male participants | 8 (4.6) | 11 (6.2) | 10 (5.7) | * | * |
| Female participants | 5 (2.9) | 5 (2.9) | 6 (3.4) | * | * |
| Partners notified per index patient at 2 weeks: index patient-level analysis; mean (95% CI) | |||||
| All participants | 1.24 (1.10 to 1.37) | 1.29 (1.15 to 1.42) | 1.68 (1.49 to 1.87) | † | † |
| Male participants | 1.38 (1.18 to 1.58) | 1.32 (1.32 to 1.52) | 1.96 (1.68 to 2.24) | † | † |
| Female participants | 1.01 (0.89 to 1.14) | 1.22 (1.07 to 1.38) | 1.15 (1.03 to 1.28) | † | † |
| Risk of partner being notified: partner-level analysis; notified/all partners (%) | Risk difference‡ (95% CI), p value | ||||
| All participants | 384/714 (53.8) | 378/743 (50.9) | 464/721 (64.3) | 10.6% (4.0% to 16.1%), p=0.001 | −2.9% (−9.1% to 3.3%), p=0.36 |
| Male participants | 205/453 (45.3) | 213/461 (46.2) | 286/468 (61.1) | 15.9% (−24.3% to −7.4%), p<0.001 | −1.0% (−8.8% to 6.9%), p=0.8 |
| Female participants | 178/258 (69.0) | 165/282 (58.5) | 178/253 (70.4) | +1.4% (−10.6% to 7.8%), p=0.7 | −10.5% (−1.1% to −19.9%), p=0.03 |
| Risk of partner being notified stratified by partner type: partner-level analysis; notified/all partners of specified type (%) | Risk difference‡ (95% CI), p value | ||||
| Main partners | 262/328 (79.9) | 254/336 (75.6) | 288/350 (82.3) | 2.4% (−3.7% to 8.5%), p=0.4 | −4.2% (−11.0% to 2.4%), p=0.2 |
| Casual partners | 94/265 (35.5) | 96/270 (35.6) | 137/242 (56.6) | 21.1% (11.0% to 31.3%), p=0.00 | 0.0% (−9.0% to 9.2%), p=0.9 |
| Once-off partners | 28/121 (23.1) | 28/137 (20.4) | 39/129 (30.2) | 7.1% (−5.3% to 19.5%), p=0.3 | −3.5% (−27.2% to 20.2%), p=0.8 |
| Risk of condomless sex with partner (vs sex with condom or no sex): partner-level analysis; frequency (%) | Risk difference‡ (95% CI), p value | ||||
| All participants | 283/714 (39.6) | 338/743 (45.5) | 275/721 (38.1) | −1.5% (−8.6% to 5.6%), p=0.68 | 5.9% (−1.4% to 13.1%), p=0.12 |
| Male participants§ | 175/453 (38.6) | 210/461 (45.6) | 166/468 (35.5) | −3.2% (−12.6% to 6.3%), p=0.51 | 6.9% (−2.8% to 16.7%), p=0.165 |
| Female participants§ | 105/258 (40.7) | 128/282 (45.4) | 109/253 (43.1) | 2.4% (−7.9% to 12.7%), p=0.65 | 4.7% (−5.8% to 15.2%), p=0.38 |
| Risk of partner perpetrated IPV: partner-level analysis; frequency (%) | Risk difference‡ (95% CI), p value | ||||
| All participants | 4/714 (0.6) | 9/743 (1.2) | 8/721 (1.1) | 0.5% (−0.5% to 1.5%), p=0.28 | 0.7% (−0.3% to 1.6%), p=0.17 |
| Male participants | 3/453 (<1) | 8/461 (1.7) | 6/468 (1.3) | * | * |
| Female participants | 1/258 (<1) | 1/282 (<1) | 2/253 (<1) | * | * |
| Risk of abandonment by partner: partner-level analysis; frequency (%) | Risk difference‡ (95% CI), p value | ||||
| All participants | 7/714 (1.0) | 9/743 (1.2) | 20/721 (2.8) | 1.7% (0.2% to 3.3%), p=0.02 | 0.2% (−0.9% to 1.3%), p=0.41 |
| Male participants | 4/453 | 9/461 | 16/468 | * | * |
| Female participants | 3/258 | 0/282 | 4/253 | * | * |
*Numbers too small to perform gender-stratified model.
†Inference performed at the partner level only.
‡Based on binomial regression model to model the probability of a partner being notified, or the risk of condomless sex with a partner, or the risk of a harmful partner reaction, adjusting for the clustering of partners within each index patient.
§One case who had three partners had missing gender.
ePN, enhanced partner notification; HE, health education; IPV, intimate partner violence; PN, partner notification; RR, risk reduction.
In analyses at the partner level, there was a greater chance of a partner being notified in the ePN arm compared with the HE arm, but no significant difference between the RR and HE arms. Among men, there was a greater chance of a partner being notified in the ePN compared with the HE arm, but no difference among women. Female participants in the RR arm had a lower chance of a partner being notified compared with the HE arm (table 3). The interaction between gender and study arm in the risk of notifying a partner was not significant (p=0.054).
Other outcomes
There were no differences between conditions in STI knowledge, PN self-efficacy, condom use self-efficacy reports of PN assistance from a third party, condomless sex or partner perpetration of IPV (table 3). The intervention counsellors reported that only one participant in the ePN arm requested provider assistance for PN (requesting a text message be sent to the partner). In the ePN arm, there was a greater risk of a partner abandoning the participant compared with the HE arm.
Interaction between intervention effect and partner type (post-hoc analyses)
There was an interaction between condition and partner type on PN (p=0.016). There was no intervention effect on the notification of main partners (p=0.13) or once-off partners (p=0.78), but we found a significant intervention effect in the casual partner subgroup (table 3).
Discussion
To our knowledge, our study is the only RCT to measure the effects of enhancing patient referral with counselling and education combined with the offer of provider-assisted referral. Our premise was that merging PN interventions for combined delivery is likely to maximise impact. Recent advances in electronic communication technologies have enabled innovations in provider-assisted referral, such as those offered in the ePN arm of our trial,12 13 rendering them more feasible in settings such as Cape Town. In the ePN arm of our study, the offer of provider assistance was almost never taken up, indicating that there is not widespread acceptability of such services. This is possibly because of the fear that such intervention will disrupt valued relationships, as observed elsewhere.14 Participants in all three arms reported third-party assistance, suggesting people with STIs are able to obtain help with PN in the absence of provider-assisted referral. There are other PN interventions that could be considered for testing in a combination intervention, such as recall cues, shown in an RCT to increase the elicitation of partners and improve sexual network ascertainment.15
We found no difference between arms in the annual incidence of STI diagnosis. Despite the existence of many RCTs measuring the effects of various PN strategies on index patient reinfection including several assessing patient-delivered partner therapy,3 16 only one other RCT compared the effects of counselling and education strategies with standard of care on STI reinfection rates, showing these to be effective among people with Chlamydia trachomatis and Neisseria gonorrhoeae in the USA.17 Given the suboptimal validity of syndromic STI diagnoses,18 our measure of STI reinfection would have been less sensitive and specific than testing urine specimens as in the US trial. This might explain the different findings. Provider-assisted partner services have been shown to have a small beneficial effect on PN or partner treatment.3 19
Participants who received ePN referred more (>10%) partners for treatment compared with those receiving RR and HE. Among the few other trials that have investigated the effects of PN counselling and education, one Zimbabwean study has shown that it led to a small increase in the rate of partners notified,3 20 while another South African study showed that it led to a small increase in the number of partners treated.3 Our study contributes to the small body of evidence that PN counselling and education can change the behaviour of people diagnosed with STIs.
The participants in our trial showed accurate knowledge about STIs and high levels of PN self-efficacy, and there was no intervention effect. It is possible that our measures were not sensitive enough to capture changes in critical aspects of knowledge and self-efficacy that lead to PN behaviour changes.
Th ePN arm showed benefit in the notification of ‘casual’ partners. Many studies report that people diagnosed with STIs are more willing to notify ‘main’ partners, compared with non-main partners.8 This reluctance or difficulty in notifying non-main partners is reflected in our findings, with the proportion of main partners notified ranging between 75% and 82%, and non-main partners between 20% and 57%. On average, successful PN needs to be achieved with more than one partner per index case to prevent onward transmission,4 and it has been proposed that PN success with casual or ex-regular partners is more efficient at preventing onward transmission relative to success with regular partners.4 This suggests that if the reports of participants in our trial were valid, and if the partners they notified were infected with an STI, and if notified partners sought and received treatment, the ePN intervention would decrease the pool of infectious partners. However, the differential benefits of PN with different partner types will depend on a range of factors, such as frequency of sexual contacts, partner concurrency and sexual network structure, which we have not measured.
Between 1.0% and 2.8% of participants reported being abandoned by their partner, and participants in the ePN intervention were at greater risk. PN counselling interventions need to carefully assess risks of adverse outcomes and assist people at risk to find alternative ways to prevent reinfection. This was included in the ePN counselling. It is possible that some participants in our trial did not accurately assess the risk of abandonment, or that they chose this risk over potential reinfection.
Rates of condomless sex with partners during 2 weeks after STI diagnosis ranged from 38% to 46%, indicating risk of STI transmission or reinfection. All three conditions focused on increasing condom use, but the RR condition was modelled on an intervention shown to be effective in South Africa at reducing incident STIs and increasing condom use.10 It is unclear why we were not able to reproduce the findings of the previous trial, and further research needs to identify effective ways to reduce condomless sex among people diagnosed with STIs.
Limitations
Syndromic management has suboptimal validity for diagnosing STIs,18 and it would have been preferable to include a biomedical outcome of recurrent STI. The secondary outcome, PN, was based on participant self-report, and was not confirmed by interviewing partner(s). We only included electronic health records from the City of Cape Town Health Department and not those of the Provincial Health Department. We cannot distinguish in the records whether a participant was an ‘index case’ or referred by a partner. Most participants (78.8%) were enrolled on the day of their STI diagnosis (81.1%, 76.6% and 78.6% in the ePN, RR and HE arms, respectively) or 1 day later (16.9%).
Conclusions
This study contributes to the small body of evidence that, in settings such as Cape Town, PN counselling and education can change the behaviour of people diagnosed with STIs. Changes in behaviour did not lead to a reduction of STI diagnoses and research is needed to identify effective ways of reducing the incidence of STIs.
Key messages.
People with STIs who received partner notification counselling and offers of provider-assisted partner services notified more partners than those receiving STI prevention counselling or education.
STI partner notification counselling with offers of provider-assisted partner services did not decrease the annual incidence of STI diagnosis among people with STIs.
People with STIs receiving partner notification counselling and provider-assisted partner services reported a slightly greater risk of abandonment by their partner after notifying the partner.
People with STIs receiving the offer of provider-assisted partner services reported a very low uptake of such services, indicating these services were not widely valued.
Acknowledgments
We thank the men and women who participated in this study. We also thank Yolisa Mtshizana, Brenda Skonje and Neziswa Titi for assisting with collecting the data, Tamar Grebler for programming the electronic questionnaire, Ria Laubscher for assisting with analyses, Koena Nkoko, Ruberto Isaaks, Sr Matebeni and Sr Mavume for supporting research in the public health services they manage, and the clinicians who referred their patients to our study. We thank the two anonymous reviewers for helpful reviews.
Footnotes
Handling editor: Joseph D Tucker
Contributors: SCK and CM conceptualised the study. MK, EB, JD, MB, SaD, SeD, AM and TM contributed to the acquisition of data for the study. CL and SCK performed the statistical analyses. CM drafted the manuscript. All authors contributed to revising it and approved the final version.
Funding: This research was supported by a grant from the National Institutes of Health under award number R01HD074560.
Disclaimer: This funding source had no role in the design of this study, its execution, in the analyses and interpretation of data, or in the decision to submit the results.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Ethics Committee of the South African Medical Research Council (EC018-10/2013) and the University of Connecticut Institutional Review Board (H12-340).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available for reuse upon reasonable request. Deidentified participant data are available from CM (South African Medical Research Council, catherine.mathews@mrc.ac.za).
References
- 1. Boily M-C, Baggaley RF, Wang L, et al. . Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis 2009;9:118–29. 10.1016/S1473-3099(09)70021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Royce RA, Seña A, Cates W, et al. . Sexual transmission of HIV. N Engl J Med 1997;336:1072–8. 10.1056/NEJM199704103361507 [DOI] [PubMed] [Google Scholar]
- 3. Ferreira A, Young T, Mathews C, et al. . Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013:CD002843. 10.1002/14651858.CD002843.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Althaus CL, Turner KME, Mercer CH, et al. . Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess 2014;18:1–100, vii–viii. 10.3310/hta18020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wood JM, Harries J, Kalichman M, et al. . Exploring motivation to notify and barriers to partner notification of sexually transmitted infections in South Africa: a qualitative study. BMC Public Health 2018;18:980. 10.1186/s12889-018-5909-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medley A, Garcia-Moreno C, McGill S, et al. . Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 7. Rujumba J, Neema S, Byamugisha R, et al. . "Telling my husband I have HIV is too heavy to come out of my mouth": pregnant women's disclosure experiences and support needs following antenatal HIV testing in eastern Uganda. J Int AIDS Soc 2012;15:17429. 10.7448/IAS.15.2.17429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alam N, Chamot E, Vermund SH, et al. . Partner notification for sexually transmitted infections in developing countries: a systematic review. BMC Public Health 2010;10:19. 10.1186/1471-2458-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull 1992;111:455–74. 10.1037/0033-2909.111.3.455 [DOI] [PubMed] [Google Scholar]
- 10. Kalichman SC, Cain D, Eaton L, et al. . Randomized clinical trial of brief risk reduction counseling for sexually transmitted infection clinic patients in Cape Town, South Africa. Am J Public Health 2011;101:e9–17. 10.2105/AJPH.2011.300236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morojele NK, Nkosi S, Kekwaletswe CT, et al. . Utility of brief versions of the alcohol use disorders identification test (AUDIT) to identify excessive drinking among patients in HIV care in South Africa. J Stud Alcohol Drugs 2017;78:88–96. 10.15288/jsad.2017.78.88 [DOI] [PubMed] [Google Scholar]
- 12. Pellowski J, Mathews C, Kalichman MO, et al. . Advancing partner notification through electronic communication technology: a review of acceptability and utilization research. J Health Commun 2016;21:629–37. 10.1080/10810730.2015.1128020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bilardi JE, Fairley CK, Hopkins CA, et al. . Let them know: evaluation of an online partner notification service for Chlamydia that offers e-mail and SMS messaging. Sex Transm Dis 2010;37:563–5. 10.1097/OLQ.0b013e3181d707f1 [DOI] [PubMed] [Google Scholar]
- 14. Lichtenstein B, Schwebke JR. Partner notification methods for African American men being treated for trichomoniasis: a consideration of main men, second hitters, and third players. Med Anthropol Q 2005;19:383–401. 10.1525/maq.2005.19.4.383 [DOI] [PubMed] [Google Scholar]
- 15. Brewer DD, Potterat JJ, Muth SQ, et al. . Randomized trial of supplementary interviewing techniques to enhance recall of sexual partners in contact interviews. Sex Transm Dis 2005;32:189–93. 10.1097/01.olq.0000154492.98350.90 [DOI] [PubMed] [Google Scholar]
- 16. Trelle S, Shang A, Nartey L, et al. . Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ 2007;334:354. 10.1136/bmj.39079.460741.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson TE, Hogben M, Malka ES, et al. . A randomized controlled trial for reducing risks for sexually transmitted infections through enhanced patient-based partner notification. Am J Public Health 2009;99 (Suppl 1):S104–10. 10.2105/AJPH.2007.112128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnabas SL, Dabee S, Passmore J-AS, et al. . Converging epidemics of sexually transmitted infections and bacterial vaginosis in southern African female adolescents at risk of HIV. Int J STD AIDS 2018;29:531–9. 10.1177/0956462417740487 [DOI] [PubMed] [Google Scholar]
- 19. Faxelid E, Tembo G, Ndulo J, et al. . Individual counseling of patients with sexually transmitted diseases. A way to improve partner notification in a Zambian setting? Sex Transm Dis 1996;23:289–92. [PubMed] [Google Scholar]
- 20. Moyo W, Chirenje ZM, Mandel J, et al. . Impact of a single session of counseling on partner referral for sexually transmitted disease treatment, Harare, Zimbabwe. AIDS Behav 2002;6:237–43. 10.1023/A:1019891808383 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2020-054499supp001.pdf (12.9MB, pdf)
sextrans-2020-054499supp002.pdf (227.9KB, pdf)
sextrans-2020-054499supp003.pdf (25.1MB, pdf)
sextrans-2020-054499supp004.pdf (417.4KB, pdf)
sextrans-2020-054499supp005.pdf (13.1MB, pdf)
sextrans-2020-054499supp006.pdf (200.5KB, pdf)
sextrans-2020-054499supp007.pdf (83.2KB, pdf)
sextrans-2020-054499supp008.pdf (76.3KB, pdf)