Abstract
Helicobacter pylori is a common component of the human stomach microbiota, possibly dating back to the speciation of Homo sapiens. A history of pathogen evolution in allopatry has led to the development of genetically distinct H. pylori subpopulations, associated with different human populations, and more recent admixture among H. pylori subpopulations can provide information about human migrations. However, little is known about the degree to which some H. pylori genes are conserved in the face of admixture, potentially indicating host adaptation, or how virulence genes spread among different populations. We analyzed H. pylori genomes from 14 countries in the Americas, strains from the Iberian Peninsula, and public genomes from Europe, Africa, and Asia, to investigate how admixture varies across different regions and gene families. Whole-genome analyses of 723 H. pylori strains from around the world showed evidence of frequent admixture in the American strains with a complex mosaic of contributions from H. pylori populations originating in the Americas as well as other continents. Despite the complex admixture, distinctive genomic fingerprints were identified for each region, revealing novel American H. pylori subpopulations. A pan-genome Fst analysis showed that variation in virulence genes had the strongest fixation in America, compared with non-American populations, and that much of the variation constituted non-synonymous substitutions in functional domains. Network analyses suggest that these virulence genes have followed unique evolutionary paths in the American populations, spreading into different genetic backgrounds, potentially contributing to the high risk of gastric cancer in the region.
Subject terms: Population genetics, Microbial genetics
Introduction
The arrival of the conquistadors in the Americas more than 500 years ago was a major event in human evolution. Colonialism connected human European and African newcomers with Indigenous people (we capitalize Indigenous to respect the preference of some Indigenous research partners who contributed samples for this work) living in the Americas in the precolonial era [1]. So began one of the largest natural admixture events among modern human populations, that continues in the mosaic of diversity and multiculturalism observed in the Americas today [2].
Just as human populations adapt, diversify, and recombine, so too have their microbiota [3]. Nowhere is this more evident than among the stomach-dwelling obligate human pathogen Helicobacter pylori where the close relationship of host and bacterium underpins a history of co-evolution that is reflected in the ancestry of strains [4]. Unlike many gastrointestinal microorganisms, the mode of transmission of H. pylori, thought to occur principally from human-to-human through close personal contact within families and communities [5], may have led to a degree of genetic isolation and the emergence of H. pylori subpopulations that are geographically stratified in different human populations [6, 7]. This has allowed the study of recent human migrations by quantifying changes in the patterns of admixture between historically isolated H. pylori populations [8, 9].
In the Americas, rapid evolution in the 500 years since European colonization has influenced the differentiation of H. pylori subpopulations across countries [10, 11] to the extent that even individual genes can be identified as having, for example, Latin American ancestry, as observed in analyses of whole-genome ancestry [11, 12]. However, while genetic admixture among H. pylori is instructive for understanding human migrations, there is a pressing need to understand the spread of certain genes among subpopulations to better understand adaption in relation to virulence. For example, while H. pylori can colonize the stomach for decades without causing any symptoms [13], progression to serious clinical diseases, such as gastric cancer, is associated with carriage of certain genotypes of the bacteria [14, 15], in particular those that carry genes linked with virulence [16].
Here we analyze 723 H. pylori genomes, including 254 isolates we collected and sequenced as part of this and previous studies [10, 12]. Isolates were collected from 14 geographical sites across America, from Canada to Argentina, where the risk of gastric cancer is high, particularly in South and Central America. We also sequenced strains from Portugal and Spain to analyze the contribution of the most relevant European ancestral source for Latin America: the Iberian Peninsula. This allowed quantification with a previously unprecedented resolution of different ancestral sources of H. pylori in the Americas and of recent and ongoing admixture among ancestral non-American and Indigenous American subpopulations. Against this backdrop of frequent admixture and complex patterns of mosaic inheritance in H. pylori we investigated variations in admixture across the genome and the differential spread of genes through multiple ancestral backgrounds. In each regional H. pylori population, we were able to identify how important virulence genes have followed unique evolutionary paths in the Americas, potentially contributing to the high risk of gastric cancer in the region [17].
Materials and methods
Isolate sampling and genome sequencing
We sampled 149 previously undescribed H. pylori isolates from 12 countries in America, from Canada in the North to Argentina in the South and from the Iberian Peninsula (Spain and Portugal) (Supplementary Table 1). These isolates were augmented with 105 published [10, 12] genomes from samples isolated from three Latin American countries to give a total of 254 genomes. Study sites contributed samples from urban areas of mixed ancestry as well as Indigenous communities (Supplementary Table 1). Study site technicians isolated H. pylori strains from gastric biopsies of patients or research participants in a selective blood agar media under a microaerobic atmosphere and extracted DNA using the DNeasy Mini Kit (Qiagen, Hilden Germany). Purified DNA from clinical H. pylori isolates was sent from the study sites and following quality control, libraries were prepared using Illumina® TruSeq® Nano kit (San Diego, CA, USA) aiming for an insert size of 900 bp. Libraries were sequenced on the MiSeq platform using v3 chemistry, 2*300 bp paired end reads generating a coverage of on average 256-fold (22.3 min to 1063.3 max) (Supplementary Table 2).
Genome assembly, annotation, and archiving
Illumina raw reads were trimmed and filtered using TrimGalore! software v.0.3.7 (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) applying the quality cutoff Q30 and only keeping reads longer than 30 bp. Filtered reads were de novo assembled using SPAdes v.3.9.0 [18]. The resulting draft and complete genomes were annotated as described previously using the online automatic pipeline Rast annotation v.2.0 [19]. Summary statistics from the sequencing and assembly were collected using MultiQC v.1.0 [20]. All genomes with accompanying metadata were submitted to NCBI under the BioProject number PRJNA601302 [21]. The 254 genomes we sequenced were analyzed with 469 H. pylori genome sequences published by others. The final study dataset comprised 723 genome sequences that were used for subsequent analyses. Of these, 337 isolates came from populations in the Americas and 386 from populations in other continents (Supplementary Table 1). All genomic sequences were submitted to the online automatic pipeline Rast annotation.
Core and accessory genome characterization
A pan-genome list was constructed from all 723 isolates in this study by automated annotation of all genomes in the dataset using RAST [19]. Duplicate genes were removed if they exceeded a BLAST threshold of 70% nucleotide identity [22]. This reference pan-genome list comprised 2545 unique genes that appeared in at least one of our 723 genomes. Consistent with previous studies and the whole-genome MLST principle [23], the gene complement and allelic variation of each isolate were determined by comparison with the pan-genome list, with gene presence recorded as a BLAST match exceeding 70% sequence identity over 50% of sequence length. Core genes were present in >90% of the genomes and accessory genes were present in at least one isolate. Each gene was aligned individually using Mafft [24] and concatenated into a single multiFASTA alignment file for each isolate.
Analysis of genome ancestry
Genome-wide haplotype data were prepared by calling SNPs from a core-genome alignment using BEAGLE v.3.3.2 [25]. A total of 498,461 SNPs were identified in 1385 genes. The genome-wide haplotype data was used in FineSTRUCTURE v.0.02 to define isolate populations based on the similarity of the haplotype copying profiles obtained by CHROMOPAINTER v.0.02 [26]. FineSTRUCTURE was run for 100,000 iterations of both the burn-in and Markov chain Monte Carlo (MCMC) method to cluster individuals based on the co-ancestry matrix as described [27]. The results were visualized as a heat map with each cell indicating the proportion of DNA “chunks” a recipient receives from each donor. The population structure was analyzed in more detail using principal component analysis (PCA) with the co-ancestry matrix obtained previously. Multiple PCAs were calculated from 1 to 11 principal components (PCs) using R.
To identify the proportion of ancestry of H. pylori isolates from the Americas, we conducted chromosome painting using ChromoPainterV2 [26], designating both the American and non-American populations as donors (723 genomes), and American isolates as recipients (337 genomes). The algorithm identifies important haplotype information from dense data such as SNP data, and efficiently describes shared ancestry within a recombining population. Each individual is painted using all the other individuals as donors and the result is visualized in a bar plot built with R [28]. Finally, we calculated the average and standard deviation of the proportion of ancestry from each population present in each American country, which were visualized in box plots using R.
Fst and GenomegaMap analyses to identify gene variants fixed in American populations
Local adaptation in American isolates compared to non-American ancestors was evaluated using the Fst test within the R package PopGenome v.2.2.4 [29]. The Fst calculation was performed by comparing all genes of the pan-genome that were present in >50% of the isolates (1649 genes) in American versus non-American isolates. The criteria for data inclusion in the multiple alignments were as follows. First, we selected genes present in >50% of the studied strains, then the sequences that did not constitute >90% of the length of the gene were removed; finally, each position that did not constitute >50% of alignment depth was also removed. The final alignments were then use to identify polymorphisms using SNP-sites. [30]. A list was obtained by ordering the 1649 genes according to the Fst value, and the top 35 genes (Table 1) were analyzed further. The consensus nucleotide and amino acid sequence alignments of these genes from all the American and non-American isolates were calculated using the SeaView v.4 software [31] and compared against the reference strain 26695 [32] to determine synonymous or non-synonymous mutations. From the top 35 genes, we selected virulence genes with available information on the structure of the proteins. In these genes, the structure of the protein was obtained from Protein Data Bank and the Fst values of each position were visualized in the structure with gradient color, using PyMOL software (The PyMOL Molecular Graphics System, v.2.3.2 Schrödinger, LLC), highlighting positions with an Fst value above the upper 99th percentile.
Table 1.
List of the 35 genes with the highest Fst values fixed in the American populations.
| Top | Gene | No. strains with the gene | No. strains (%) | Description | Fst max value | P99 | No. positions >P99 |
|---|---|---|---|---|---|---|---|
| 1 | HP0543 | 509 | 70.40 | cag pathogenicity island protein cag22 (cagF) | 0.5360 | 0.4507 | 3 |
| 2 | HP0175 | 723 | 100.00 | Putative peptidyl-prolyl cis,trans-isomerase (PpiC) | 0.5018 | 0.2134 | 3 |
| 3 | HP0181 | 723 | 100.00 | CvpA family protein | 0.4957 | 0.4683 | 3 |
| 4 | HP0887 | 713 | 98.62 | Vacuolating cytotoxin autotransporter (vacA) | 0.4885 | 0.4226 | 13 |
| 5 | HP0537 | 511 | 70.68 | cag pathogenicity island protein cag16 (cagM) | 0.4509 | 0.3841 | 4 |
| 6 | id7111332 | 671 | 92.81 | Hypothetical protein | 0.4407 | 0.4399 | 1 |
| 7 | HP0538 | 510 | 70.54 | cag pathogenicity island protein cag17 (cagN) | 0.4394 | 0.3144 | 4 |
| 8 | id7531345 | 486 | 67.22 | Hypothetical protein | 0.4360 | 0.4304 | 1 |
| 9 | HP0524 | 515 | 71.23 | Type IV secretion system protein | 0.4311 | 0.3095 | 7 |
| 10 | HP0547 | 507 | 70.12 | cag pathogenicity island protein cag26 (cagA) | 0.4151 | 0.2604 | 9 |
| 11 | HP0539 | 510 | 70.54 | cag pathogenicity island protein cag18 (cagL) | 0.4126 | 0.2929 | 3 |
| 12 | HP0525 | 514 | 71.09 | Type IV secretion system ATPase | 0.4108 | 0.3719 | 3 |
| 13 | HP0544 | 510 | 70.54 | Type IV secretion/conjugal transfer ATPase | 0.4092 | 0.2609 | 8 |
| 14 | HP0130 | 722 | 99.86 | Hypothetical protein | 0.4039 | 0.2714 | 4 |
| 15 | HP0486 | 723 | 100.00 | Membrane protein | 0.4014 | 0.3615 | 5 |
| 16 | id10887 | 723 | 100.00 | Putative protein | 0.3974 | 0.2771 | 2 |
| 17 | HP0528 | 509 | 70.40 | cag pathogenicity island protein cag8 (cagX) | 0.3901 | 0.3389 | 4 |
| 18 | id970544 | 721 | 99.72 | Hypothetical protein | 0.3892 | 0.3860 | 1 |
| 19 | HP1524 | 723 | 100.00 | Lipoprotein | 0.3852 | 0.1972 | 2 |
| 20 | HP0686 | 709 | 98.06 | Iron(III) dicitrate transport protein (FecA) | 0.3816 | 0.2385 | 7 |
| 21 | id71034 | 624 | 86.31 | Lysine-specific permease | 0.3758 | 0.2994 | 5 |
| 22 | HP0706 | 723 | 100.00 | Membrane protein | 0.3705 | 0.3092 | 3 |
| 23 | HP0559 | 723 | 100.00 | Acyl carrier protein | 0.3693 | 0.3655 | 1 |
| 24 | HP0561 | 723 | 100.00 | 3-oxoacyl-ACP reductase | 0.3684 | 0.2786 | 3 |
| 25 | HP0523 | 517 | 71.51 | cag pathogenicity island protein cag4 (cagg) | 0.3622 | 0.2806 | 2 |
| 26 | HP0722 | 691 | 95.57 | Membrane protein | 0.3549 | 0.3023 | 5 |
| 27 | HP1012 | 722 | 99.86 | Zn-dependent protease | 0.3517 | 0.1692 | 5 |
| 28 | HP0211 | 706 | 97.65 | Beta-lactamase HcpA | 0.3512 | 0.1825 | 4 |
| 29 | HP0541 | 510 | 70.54 | cag pathogenicity island protein cag20 (cagH) | 0.3501 | 0.1942 | 4 |
| 30 | HP0554 | 723 | 100.00 | Hypothetical protein | 0.3499 | 0.2827 | 4 |
| 31 | HP0530 | 510 | 70.54 | Type IV secretion system protein (cagV) | 0.3484 | 0.3401 | 3 |
| 32 | HP0534 | 510 | 70.54 | cag pathogenicity island protein cag13 (cagS) | 0.3460 | 0.2943 | 2 |
| 33 | HP1055 | 721 | 99.72 | Membrane protein | 0.3438 | 0.2876 | 4 |
| 34 | HP1243 | 520 | 71.92 | babA | 0.3434 | 0.2758 | 4 |
| 35 | HP0522 | 520 | 71.92 | cag pathogenicity island protein cag3 (cagd) | 0.3416 | 0.2749 | 6 |
Genomegamap was used for Bayesian estimation of dN/dS ratio (also denoted KA/KS or ω) of vacA, babA, and cagA genes. MCMC was run twice for each analysis with 1000,000 iterations and a burn-in of 22,000 iterations. Both runs were compared for convergence at several parameters and merged to obtain distributions; graphs of omega data were produced using R [33]. Differences in positions under positive selection between American and non-American strains were compared using the MCMC output from the previous analyses.
Network analysis of genes with high Fst values
Genetic distance calculation
The sequence reading frame of the selected virulence genes was verified manually and aligned by reverse translation with MUSCLE, using the SeaView v.4 software. The obtained multiple alignments were used to estimate genetic distance with PAUP v.4.0a166, using maximum likelihood criteria [34].
Genetic distance normalization
Genetic distances between each strain pair were obtained from Nexus files and formatted as a triangular matrix. Each distance was normalized between 0 and 1 according to
where NWij is the normalized weights between strain i and strain j, Wij is the genetic distance between strain i and strain j, and Wij(max), Wij(min) are the maximum and minimum value, respectively, for the genetic distance in the whole network, respectively. With this normalization a value of 0 means the two strains have the highest genetic similarity of all strain pairs, and a value of 1 means the two strains are the most dissimilar.
Edge and node trimming
Since all strain pairs have a measure of genetic distance, the resulting network is fully connected and without any perceivable structure. To assess the real structure of the network, edges were removed one by one, starting from the highest values of NWij (most dissimilar) until reaching a specific number of connected components. This procedure ensures that only the most genetically similar strain pairs are conserved, while keeping the overall structure of the network based on genetic closeness. If a given edge removal breaks the network into more than one subnetwork, the number of connected components is increased by exactly the number of resulting subnetworks. This edge and node removal procedure is repeated until the number of subnetworks reaches a threshold and the separation of nodes is optimized (θ). In the trimmed networks the thresholds are core (θ = 3), cagPAI (θ = 3), cagA (θ = 8), babA (θ = 1), vacA (θ = 3), and ppiC (θ = 3). Networks were drawn using the Fruchterman Reingold [35] algorithm implemented in the network visualization program “gephi” [36].
Results
A previously undescribed Southwestern European subpopulation
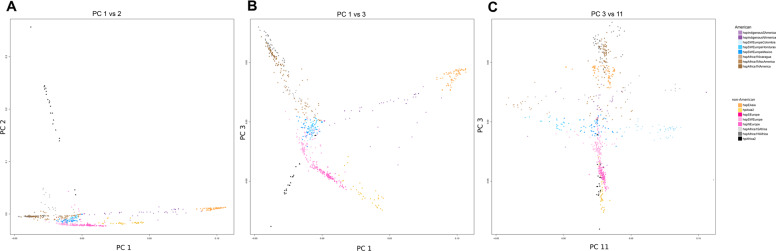
We performed co-ancestry analyses of 723 H. pylori genomes from different regions of the world to better understand the ancestry of H. pylori in Latin America with respect to ancestors from other continents. Sequenced genomes were from countries poorly represented in previous studies, including Spain and Portugal. In the dataset we also included a large number of French isolates, allowing the identification of a new European subpopulation (Fig. 1 and Supplementary Fig. 1). Isolates from the Iberian Peninsula in particular formed the previously undescribed subpopulation termed hspSWEurope, distinct from the subpopulation previously described as hspSEurope [10], which includes isolates from Belgium, Germany, Italy, France, and a few from South Asia. These were also distinct from a third European subpopulation, hspNEurope, which includes mostly isolates from Sweden, the UK, and Ireland.
Fig. 1. Co-ancestry PCA analysis of 723 H. pylori strains from different regions of the world.
a PC1 vs. PC2, showing the most distant populations hspEAsia and hspAfrica2; b PC1 vs. PC3 with the axis showing the separation of continental European and African subpopulations and the distribution of Indigenous American subpopulations; c PC3 vs. PC11 with the axis illustrating separation of European and African subpopulations in the Americas. The color code for each population is indicated to the right of the graphic.
Novel American subpopulations
The addition of 254 isolates and genomes from 14 geographical locations (eight not previously represented in whole-genome analyses) greatly improved the resolution of H. pylori populations in the Americas (Fig. 1 and Supplementary Fig. 1). This permitted the identification of novel subpopulations across the continent and an improved definition of the ancestral contribution in each regional subpopulation. Three subpopulations with European ancestry were identified, all with a hspSWEurope predominance: hspSWEuropeColombia (previously reported as hspSEuropeColombia) and two novel subpopulations, the hspSWEuropeHonduras including isolates from Honduras and neighboring countries (Nicaragua, Guatemala, and El Salvador) and hspSWEuropeMexico with isolates from Mexico, but also from North, Central, and South America. The greater depth of sampling also allowed improved resolution of the hspAfrica1NAmerica subpopulation, previously described to be present in the US and Canada. This subpopulation was found to be of predominantly West African ancestry (Supplementary Fig. 1), and to include several newly sequenced isolates from Brazil and a few from Colombia. Two other Latin American subpopulations with African, specifically hspAfrica1SAfrica, ancestry were identified: the previously described hspAfrica1Nicaragua population [10, 11] (Supplementary Fig. 1) that includes isolates predominantly from Nicaragua and neighboring Honduras; and the previously reported hspAfrica1MiscAmerica [11], comprising isolates from Mexico and Colombia (Supplementary Fig. 1). These refined descriptions of subpopulations demonstrate ongoing admixture in this region.
Population structure was further studied using PCA analysis (Fig. 1), where the first PC reflects the divergence of Asian strains with hspEAsia at one extreme and African strains at the other. The second PC, on the other hand, documents the distance of the hpAfrica2 population from all others (Fig. 1a). We also observed a component that clearly shows the separation of African and European subpopulations (Fig. 1b). Whereas hspNEurope is closer to hpAsia2, hspSWEurope is closer to European subpopulations of the Americas. The components best illustrating the relationship and distribution of American and non-American subpopulations are depicted in Fig. 1c. This shows hspSWEuropeMexico to be closer to hspSWEurope, while hspSWEuropeHonduras is closer to hspAfrica1Nicaragua, and hspSWEuropeColombia is clearly separated from the others. Separation of African subpopulations in the Americas is also clear: hspAfricaNAmerica is closer to hspAfrica1Wafrica.
The only American isolates that grouped with the Asian populations HpAsia2 and hspEAsia were Indigenous American isolates (often referred to in the literature as Amerind, a term that may have ambiguous interpretations but is generally used to refer to Indigenous Peoples of the Americas) (Fig. 1b). This supports previous suggestions that the Indigenous American H. pylori ancestors were replaced by the conquistador H. pylori European strains [37] in American populations following European colonization. Furthermore, two Indigenous subpopulations were distinguished: one including strains from Canada (hspIndigenousNAmerica) and the other with strains from South America (hspIndigenousSAmerica), formerly jointly designated hspAmerind (Supplementary Fig. 1). It has been suggested that Indigenous strains were outcompeted by European strains [38]. To address this we analyzed strains with >10% Indigenous ancestry (Supplementary Fig. 2). The figure shows strains ranging from 100% Indigenous ancestry in Canada (hspIndigenousNAmerica) and over 80% Indigenous ancestry, particularly in the hspIndigenousSAmerica subpopulation, down to around 10% Indigenous ancestry. These strains display a gradient of admixture, with chunks of European, African, and Asian ancestry consistent with in vivo competition; this pattern suggests that Indigenous strains recombined with European and African strains in mestizo populations and were gradually excluded from these populations.
Countries characterized by distinctive admixture profiles
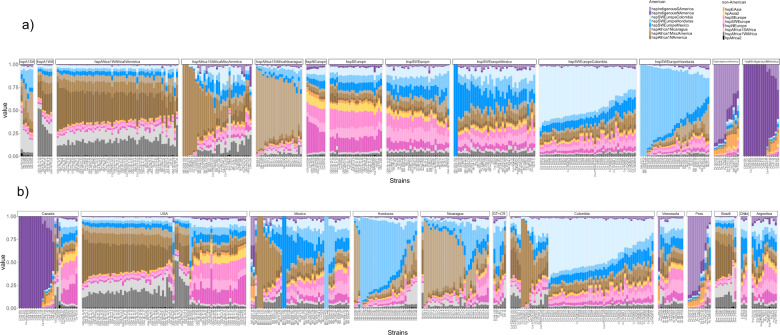
To infer recent recombination in American H. pylori subpopulations we used a reference collection of American and non-American donor genomes to paint each American recipient genome, and CHROMOPAINTER v.0.02 to infer the number of DNA chunks donated to each recipient genome from each donor source (Fig. 2a). The three American subpopulations with European ancestry and the three with African ancestry were all painted with a mixture of European and African donors from both American and non-American populations, indicative of a high level of admixture occurring in the Americas. A large proportion of these genomes were painted by donors from their own population, particularly the subpopulations hspSWEuropeColombia, hspSWEuropeHonduras, and hspAfrica1Nicaragua, where 50% or more of the chromosome was painted by their own population (Fig. 2a). This effect was most pronounced in the central parts of the continent, specifically Honduras, Colombia, and Nicaragua (Fig. 2b). The proportion of self-identity (fraction of the genome painted by strains from their own population) is variable in each subpopulation with a gradient from low to high self-identity (Fig. 2a). Admixture was lower in the two Indigenous subpopulations, particularly in the hspIndigenousNAmerica, suggesting these populations have remained genetically isolated, and both carried traces of hspEAsia (Fig. 2b).
Fig. 2. Representation of the ancestry admixture in H. pylori strains from American populations, as analyzed with chromosome painting.
Each column represents one strain and the color indicates the proportion of the corresponding ancestry in that genome. The color code for each subpopulation is shown on the right of Fig. 2. a Representation by subpopulation, from left to right, African, European, and Indigenous American subpopulations; b admixture profiles by country along the American continent, from left to right, from Canada to Argentina.
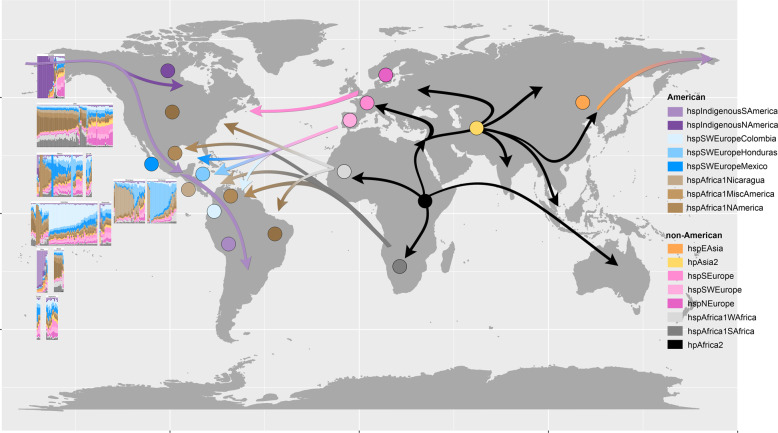
Even though some isolates in different countries showed similar ancestry, the proportion of inheritance from mixed American and non-American ancestors varied across countries (Fig. 2b and Supplementary Fig. 3), consistent with ongoing admixture between regional subpopulations. Each country can be differentiated from their European and African ancestors as well as American neighbors. Figure 3 and Supplementary Fig. 3 illustrate the composition of genomic ancestry along the continent, showing a particularly high degree of admixture in Mexico—the gateway to the Americas for the Spanish. Figure 3 also summarizes probable migrations to the Americas from other continents.
Fig. 3. World map illustrating H. pylori ancestral contributions to the Americas (colored arrows) from other continents (black arrows).
Filled circles represent the dominant H. pylori populations at the different geographical locations and the left panel shows chromosome painting results for the isolates from the respective American countries studied.
Some American isolates retain European or African ancestry
Many American isolates retained strong ancestral roots from other continents and could be classified within European or African populations (Fig. 2a). The first group included isolates from North, Central, and South America, with high levels of hspSWEurope ancestry (Fig. 2b). A second group was classified as hspSEurope, containing mostly isolates from the US, Canada, and Argentina. In addition, a small group of isolates from North America (US and Canada) but none from Latin America was classified as hspNEurope. Finally, a small number of American strains were classified within African subpopulations: some from Honduras as hspAfrica1SAfrica and seven US isolates as hspAfrica1WAfrica (Fig. 2a).
Virulence genes are fixed in American populations, driving evolution of H. pylori
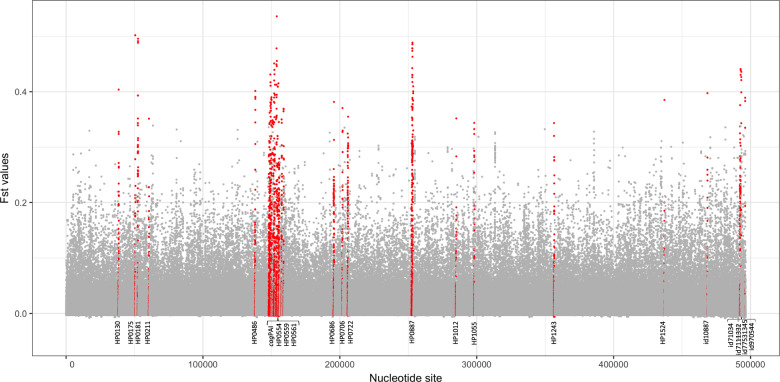
Fst analysis was used to identify genetic variants more common in the Americas than in populations in other continents. Whole-genome analysis (American vs non-American populations) was used with a relaxed threshold for gene presence to include genes present in >50% of the genomes, enabling us to include commonly known virulence genes, such as the cagPAI—which are only present in a subset of strains. The distribution of Fst values over the pan-genome (Fig. 4) was used to select 35 genes with the highest Fst values (labeled in red in Fig. 4 and Table 1). In total, there were 142 sites with significant Fst values in these 35 genes, of which 22 encode recognized virulence factors and membrane proteins, including 13 Cag pathogenicity island genes, vacA, babA, hofC, and ppiC. Furthermore, the strongest fixation index was identified in the gene encoding for CagF, a chaperone for CagA. Other genes with high numbers of significant sites were vacA (with 13 sites), cagA (with 9 sites), and the cagPAI ATPase (with 8 sites). Fifteen of the 35 genes were present in all strains studied including vacA, hofC, ppiC, three other membrane proteins, fecA and a Zn-dependent protease (Table 1). These results suggest that virulence plays a strong role in regional adaption to specific human populations.
Fig. 4. Whole-genome Fst analysis to identify genetic variants that are significantly more common in the Americas than in the rest of the world.
The X-axis indicates the nucleotide sites and the Y-axis shows the Fst value for each site. cagPAI genes include HP0522, HP0523, HP0524, HP0528, HP0525 HP0530, HP0534, HP0537, HP0538, HP0539, HP0541, HP0543, HP0544, and HP0547.
Fixed non-synonymous mutations occur in functionally important domains of virulence proteins
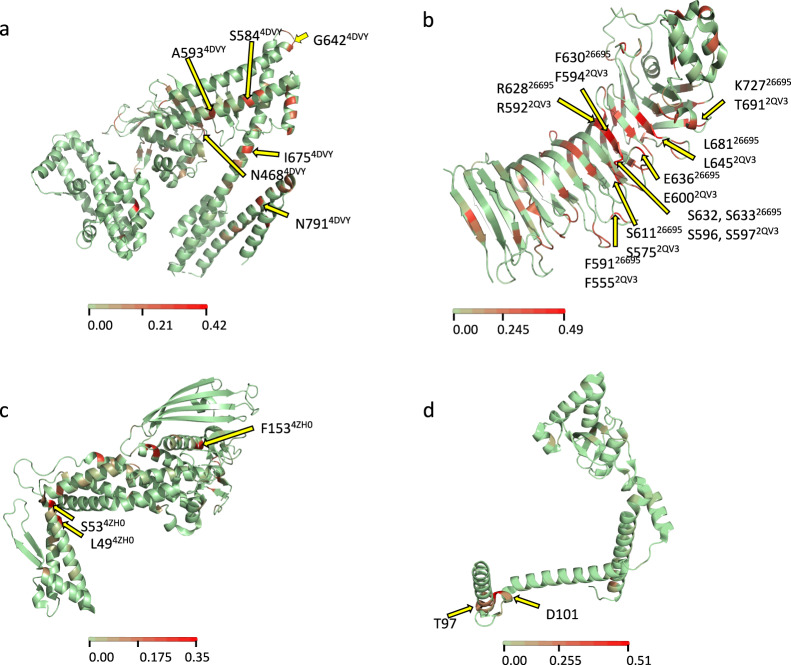
In order to relate mutations significantly fixed in American populations to specific protein domains we further characterized these mutations in virulence genes with corresponding protein structure data. In cagA six non-synonymous (NS) substitutions were identified, four in domain II and two in domain III (Fig. 5a and Supplementary Fig. 4a), both regions important for the diverse functions displayed by the protein, specifically the binding of CagA to the inner surface of the host cell and recruitment of PAR1, respectively. In vacA, nine of the significant Fst positions led to NS changes and all these amino acid changes were located in the mid region (referred to as the m region) of the protein (Fig. 5b and Supplementary Fig. 4b), the region that targets the protein to the host cell receptor. In BabA, three aa positions—aa 49, 53, and 153—showed a NS substitution (Fig. 5c and Supplementary Fig. 4c); this last residue has been implicated in sensitivity to acid. Finally, in ppiC, a peptidyl-prolyl isomerase, three NS substitutions occur in the chaperone domain (two substitutions in D101N and X97A) (Fig. 5d and Supplementary Fig. 4d). The position with the second-highest Fst value among all the genes was a synonymous substitution in aa position 100 of PpiC, also in the chaperone domain (Supplementary Fig. 4d).
Fig. 5. Localization of non-synonymous mutations fixed in the Americas in virulence proteins of H. pylori.
The available crystal structure of each protein was used to indicate the sites with amino acid changes. a CagA, b VacA, c BabA, and d PpiC. The color scale represents the Fst value for each position.
To further document specific differences in virulence genes between American and non-American strains, we studied positions under positive selection using GenomegaMap. As with Fst, results showed that major virulence genes (cagA, vacA, and babA) present several positions under strong positive selection (Supplementary Figs. 5–7). Although the regions of the genes with positions under selection were similar for American and non-American strains, the number of positions and strength of the ω values were higher for the American strains. This suggests stronger selective pressure in functionally active regions of virulence genes. In cagA most of the positions along the gene have as high as 35-fold ω values in American isolates, occurring mostly in regions representing extra EPIYA-C and CM motifs (Supplementary Fig. 5) [39]. Similar to Fst analyses, we found positions in the mid region of vacA that are under positive selection in the Americas, but not in strains from other continents (Supplementary Fig. 6). In babA, GenomegaMap showed positions in the head domain that were under positive selection (Supplementary Fig. 7), consistent with the Fst analyses.
Host-interacting genes have followed different evolutionary trajectories in H. pylori populations
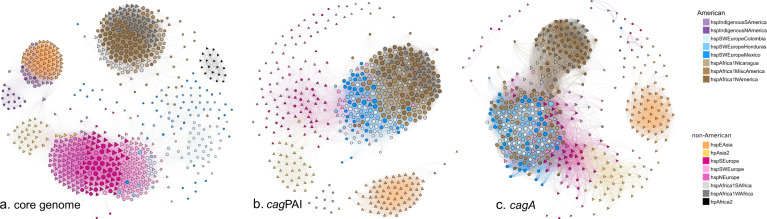
In order to analyze evolution of virulence genes in H. pylori populations, a network analysis that compared the distance in gene sequence in an all-versus-all manner to reveal nodes of relatedness was performed. For reference, the analysis was performed with the core genome, resulting in separation of the 14 subpopulations, with the non-American subpopulations grouping in tighter modules relative to the American subpopulations, consistent with a longer evolutionary history with relatively less admixture (Fig. 6a). In contrast, when analyzing cagPAI (Fig. 6b) in the Latin American subpopulations, the three European American subpopulations formed a node in close proximity to another tight node of subpopulations with African ancestry including both American and non-American strains. Whereas continental European populations presented a diffuse group close to hpAsia2 strains, the hspEAsia node was clearly apart from all other subpopulations. When analyzing the cagA gene (Fig. 6c), all Latin American subpopulations with African or European ancestry formed a large group, which also included some continental European strains. The American hspAfrica1NAmerica strains clustered with the continental African hspAfrica1WAfrica and hspAfrica1SAfrica strains, and the two Asian nodes clustered separately.
Fig. 6. Distance network analyses of the core genome and of virulence genes of the 723 H. pylori strains studied.
The networks of the core genome, the cagPAI pathogenicity island and cagA gene are presented. Circles denote American strains and triangles denote strains from other continents. The color of each strain symbol indicates the ancestry as assigned by fineSTRUCTURE and the size of the symbol is proportional to the number of connections each strain has with other strains.
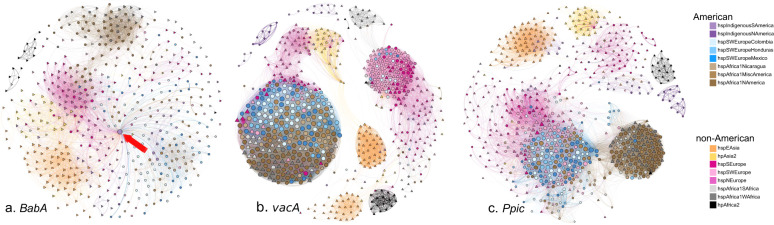
When analyzing babA, all Latin American subpopulations (with European, African, or Indigenous ancestry) formed a single node closely related to hspEAsia (Fig. 7a). In the Latin American subpopulations, one Indigenous strain showed an unusually large number of interactions with other strains (Fig. 7a, arrow). Conversely, American hspAfrica1NAmerica strains formed a separate module with continental African hspAfrica1WAfrica strains. Furthermore, the continental European strains mixed with hpAsia2 strains, whereas the continental African hpAfrica2 node was clearly distant from all other populations.
Fig. 7. Distance network analyses of virulence genes of the 723 H. pylori strains studied.
The networks of the babA, vacA, and ppiC genes are presented. Circles denote American strains and triangles denote strains from other continents. The color of each strain symbol indicates the ancestry as assigned by fineSTRUCTURE and the size of the symbol is proportional to the number of connections each strain has with other strains.
When analyzing vacA (Fig. 7b), American subpopulations with European or African ancestry clustered in a large node that also contained hspAfrica1WAfrica, hspAfrica1SAfrica, and a few hspSWEurope isolates. In contrast to the other genes, for vacA, hspEAsia split into two nodes. Although the continental European populations were dispersed, there were two defined groups, one with mostly hspNEurope strains and the other with hspSEurope, hspSWEurope, and a few Latin American strains. Within analyzing ppiC (Fig. 7c), most Latin American subpopulations were within one node that also included some hspSWEurope strains; this group was closer to continental European than to continental African subpopulations. The continental African subpopulations hspAfrica1WAfrica and hspAfrica1SAfrica grouped with the American hspAfrica1NAmerica strains.
In all genes studied, the continental African hpAfrica2 strains formed a group apart from all other populations, probably because this population has remained isolated and without exposure to other populations, thus precluding recombination [40]. Also, in all genes the American hspAfrica1NAmerica strains clustered with the continental African hspAfrica1WAfrica strains, suggesting that in these important host-interacting genes African American strains conserved their original African ancestry.
Discussion
Genomic variation among H. pylori subpopulations in the Americas reflects the ongoing adaption and admixture among strains originating in the Americas and other continents. Although high rates of admixture in human and bacterial populations would be expected to abolish local signals of population structure, distinctive patterns of inheritance and admixture were observed for each country, including small countries that share borders, like Nicaragua and Honduras in Central America. While unequal sampling in some countries may influence the number and geographical specificity of H. pylori populations, the clear regional population structure, likely influenced by environmental factors and local diets, is consistent with the history of human migration. The number of H. pylori subpopulations per country varied from four in Honduras to six in Mexico and Nicaragua, including Indigenous American (hspIndigenousSAmerica) and continental European (hspSWEurope) subpopulations. This may reflect Mexico’s history as gateway to the Americas and a cross-continental hub of human diversity [41].
The structuring of American H. pylori populations with European and African ancestry illustrates a pattern consistent with historic human migrations. For the three Latin American H. pylori subpopulations with European ancestry, the major fraction of European ancestry was hspSWEurope, consistent with historical human migrations from Spain and Portugal. For the H. pylori subpopulations with African ancestry, two had primarily hspAfrica1SAfrica ancestry while strains from Brazil and the US had primarily hspAfrica1WAfrica ancestry. This pattern is consistent with forced human migration that occurred through the slave trade from the 16th through the 19th century when millions of West Africans were brought to Brazil and the US or the colonies that became the US [42]. Similarly, recent human genomic studies in American populations show that human genomes too reflect a number of migrations, as well as admixture and adaptive processes, leading to modern human Latin American populations [43]. As with H. pylori, a Latino-specific European component was recently described in human Caribbean American populations, which significantly diverged from the ancestral Iberian source [44]; thus, the apparent rapid evolution observed in H. pylori in America seems to have occurred in humans as well.
In our analysis, some American isolates clustered within continental European and African subpopulations. This observation is consistent with more recent human migrations, such as the movement of Europeans to Canada, the US and some South American countries, including the large European immigration wave to Argentina and Chile during the 19th and 20th centuries [45]. Given the frequency of admixture observed across countries in the Americas, national boundaries cannot explain the maintenance of continental European or African subpopulations. To some degree, the maintenance of these non-American subpopulations likely resulted from reduced admixture of ancestral European or African migrants and their descendants with other American communities. However, it may also be the case that host factors influenced the success of particular H. pylori strains and the spread of genes among different subpopulations. Further sampling throughout South America will improve understanding of the importance of human demographic factors in H. pylori population structuring.
Concerning the two Indigenous American subpopulations, a broader sampling of H. pylori strains from Indigenous American and East Asian communities is needed before drawing conclusions about how they are related to American and non-American subpopulations. It should be noted, in particular, that only one Indigenous community in Canada (Aklavik, Northwest Territories) contributed samples for this work. Interestingly, Okinawan strains seem to constitute an intermediate group between hspEAsia and Indigenous American strains, which can be seen, for example, in Fig. 1a (purple circles).
Pan-genome-wide Fst analysis quantified the degree of differentiation among H. pylori genes in different subpopulations revealing variation in the patterns of gene flow (Fig. 3). Most of the genes with the highest fixation values encoded proteins that interact with the host, and in particular, known virulence factors. These included several genes of the cag pathogenicity island [46], which have high affinity interactions with proteins of the human host. Fixed nucleotide variants mainly resulted in non-synonymous mutations in functionally important domains. For example, in CagA, changes occurred in domains II and III. Domain II is known to tether CagA to the inner surface of the host cell by binding to phosphatidylserine [47] and also has a region that recognizes β1-integrin, a binding necessary to allow translocation of CagA. In domain III, one NS substitution (N791D) was in the N-terminal binding sequence (NBS). This may affect the intramolecular interaction NBS/CBS that is reported to be important in the recruitment of PAR1 [39]. PAR1 recruitment tethers two CagA proteins via the CM sequence to enhance CagA-SHP2 complex formation [48], leading to activation of the Ras-ERK MAPK pathway. This domain has also some similarity with cytoskeletal proteins that may participate in the alteration of cell–cell junctions by mimicking host proteins.
In VacA, nucleotide substitutions occurred in the m region of the protein, which recognizes the receptor on the surface of the human cell [49, 50]; changes in this domain may also change specificity to the target cell [50–52]. Interestingly, six of the NS substitutions occurred in the denominated subdomain four in p55 between β-sheets 32–35, a surface-exposed domain, which may be exposed to selection and diversification driven by the immune response [53]. In BabA, three NS mutations caused a change in aa residues 49, 53, and 153. Whereas 49 and 53 have not yet been associated with any function, it has recently been hypothesized that the change F153M causes increased acid sensitivity of Leb binding, altering the pH-responsive mechanism of adherence to the gastric mucosa [54]. Finally, in PpiC, a peptidyl-prolyl isomerase with two functionally important domains, the enzymatic activity and the chaperone function [55, 56], three NS substitutions occurred in the chaperone domain. Thus, most of the fixed base changes occurred in functionally relevant regions of virulence genes, in domains known to interact with molecules of the host. Furthermore, in the major virulence genes cagA, vacA, and babA, American strains presented higher ω values at a larger number of positions, indicating a stronger selection in the Americas, consistent with more recent host-bacteria adaption in this region. It is plausible that some of the human genes that encode proteins interacting with H. pylori proteins are under a co-evolutive process for a better co-existence of H. pylori and its human host. Along these lines, recent studies in American human populations suggest a rapid adaptive evolution in genes involved in inflammation, blood metabolites, and immune related-traits, showing that admixture is a key driving mechanism in this rapid evolution in Latin American human populations [43, 57].
Combined with an understanding of the background population structure, evidence of different evolutionary trajectories among H. pylori genes, potentially influenced by host factors, allowed investigation of the spread of virulence genes into different genetic backgrounds. Visualization of interactions between populations using gene-sequence distance network analysis revealed that among Latin American H. pylori subpopulations with European ancestry, the cagPAI is evolving to form a separate population, distinct from African and Asian populations. This contrasts with observations of the cagA gene in isolation, in which all Latin American subpopulations, regardless of African or European ancestry, grouped in a module including continental European strains. This suggests that the European ancestral variant of this gene may be preferred over the African ancestral variant in Latin American strains, allowing the former to spread more readily into multiple genetic backgrounds.
Patterns of variation at different loci provided information about the levels of conservation and diversification in multiple genetic backgrounds. For example, babA from all Latin American subpopulations comprised a single cluster, in which the Indigenous South American strains interact with both hspEAsia strains and with Latin American strains, suggesting that Indigenous South American strains conserve EAsia characteristics but also share ancestry with the contemporary Latin American subpopulations. A different structure was observed for vacA for which all American populations, except the Indigenous strains, tightly grouped with hspAfrica1WAfrica and hspAfrica1SAfrica strains, indicating that vacA retains strong African ancestry across the Americas, excluding Indigenous communites. In contrast, for ppiC there was evidence of the retention of European ancestry, given that all Latin American populations clustered with continental European strains.
Since most non-synonymous substitutions occur in regions important for interaction with the human molecular target, it is likely that selection acts upon both host and pathogen targets, as it does for some other human infections [58, 59]. Thus, since most conserved cagA mutations occur in regions interacting with β1-integrin and with PAR1, it is likely that these proteins conserve the continental European interacting motifs in Latin American mestizo populations, regardless of African or European ancestry. Considering babA, it is likely that it differentiated in Latin American mestizo populations to interact with the largely predominant type O blood group antigen [60]. In VacA, changes are in the region recognizing the cell receptor; our results suggest that in all mestizo and African American populations the receptor motif is conserved and shares homology with the West African population. Interestingly, vacA was the only gene with two clearly separated hspEAsia subpopulations, suggesting that there may be different VacA receptors or motif variants in humans with East Asian ancestry.
Extensive sampling of strains from the Iberian Peninsula and countries across the Americas has provided evidence of frequent admixture between H. pylori populations with a complex mosaic of contributions from non-American subpopulations, along with more recent ongoing contributions from American populations. Against this backdrop of population structure, which reflects the history of humans populating the Americas, there is evidence of host-pathogen co-evolution. It is known that infections represent a major selective pressure for humans and that evidence of this is often located in protein domains that interact with pathogens [59]. By identifying the different evolutionary trajectories in H. pylori pathogenicity genes and the highly specific evolutionary changes associated with different human ancestral populations, we provide a basis for considering how human migrations can lead to the emergence of novel pathogen subpopulations. Latin America is a region with one of the highest mortality rates due to gastric cancer in the world [17] and understanding the nature of the co-evolution of H. pylori virulence genes with interacting proteins in its human host will help us find ways to counteract their deleterious effects.
Supplementary information
Suppl. Fig. 1. Population structure of H. pylori strains from the Americas and other continents.
Suppl. Fig 2. Representation of the ancestry admixture in H. pylori strains from Indigenous American populations, as analysed with chromosome painting.
Suppl. Fig 3. Comparative admixture analyses of H. pylori strains in the Americas by country.
Suppl. Fig. 4. Fst analysis of individual virulence genes to identify genetic variants that are significantly more commonly in the Americas than in the rest of the world.
Suppl. Fig. 5. Analyses of positions under selective pressure in the cagA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Fig. 6. Analyses of positions under selective pressure in the vacA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Fig. 7. Analyses of positions under selective pressure in the babA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Table 1. Characteristics of the 723 worldwide H. pylori strains analyzed in this study.
Suppl. Table 2. Genome size, contigs number and coverage of the genomes from the 149 H. pylori strains sequenced in this study.
Acknowledgements
This work was supported by “Consejo Nacional de Ciencia y Tecnología” (CONACYT; no. 576951) grant to ZYM-R, and by Magnus Bergvalls Foundation and Swedish Society for Medical Research (SSMF) to KT. SKS and BP are funded by the Medical Research Council (MR/L015080/1). Supported in part by the US National Cancer Institute National Cancer Institute to DRM (P01 CA028842, R01 CA190612, K07 CA125588, P30 CA068485). We are very grateful to Daniel Falush (Unit of Statistical Genetics of Bacteria, Institut Pasteur of Shanghai) for his critical input during the analysis of the presented data. We acknowledge the Aklavik H. pylori Project Planning Committee (Northwest Territories, Canada) for providing input on our presentation of this analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kaisa Thorell, Javier Torres
Contributor Information
Kaisa Thorell, Email: kaisa.thorell@gu.se.
Javier Torres, Email: uimeip@gmail.com.
Supplementary information
The online version of this article (10.1038/s41396-020-00758-0) contains supplementary material, which is available to authorized users.
References
- 1.O’Rourke DH, Raff JA. The human genetic history of the Americas: the final frontier. Curr Biol. 2010;20:R202–7. doi: 10.1016/j.cub.2009.11.051.. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Linares A. How genes have illuminated the history of early americans and latino americans. Cold Spring Harb Perspect Biol. 2015;7:a008557. doi: 10.1101/cshperspect.a008557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biol. 2017;15:127. doi: 10.1186/s12915-017-0454-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–5. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell H, Katelaris P. Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection. Med J Aust. 2016;204:376–80. doi: 10.5694/mja16.00104.. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka Y. Helicobacter pylori typing as a tool for tracking human migration. Clin Microbiol Infect. 2009;15:829–34. doi: 10.1111/j.1469-0691.2009.02967.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth T, Wang X, Linz B, Novick RP, Lum JK, Blaser M, et al. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. PNAS. 2004;101:4746–51. doi: 10.1073/pnas.0306629101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, et al. Age of the association between Helicobacter pylori and man. Ochman H editor. PLoS Pathog. 2012;8:e1002693. doi: 10.1371/journal.ppat.1002693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–8. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz-Ramírez ZY, Mendez-Tenorio A, Kato I, Bravo MM, Rizzato C, Thorell K, et al. Whole genome sequence and phylogenetic analysis show Helicobacter pylori strains from latin america have followed a unique evolution pathway. Front Cell Infect Microbiol. 2017;7. 10.3389/fcimb.2017.00050. [DOI] [PMC free article] [PubMed]
- 11.Thorell K, Yahara K, Berthenet E, Lawson DJ, Mikhail J, Kato I, et al. Rapid evolution of distinct Helicobacter pylori subpopulations in the Americas. PLoS Genet. 2017;13:e1006730. doi: 10.1371/journal.pgen.1006730.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorell K, Hosseini S, Palacios Gonzáles RVP, Chaotham C, Graham DY, Paszat L, et al. Identification of a Latin American-specific BabA adhesin variant through whole genome sequencing of Helicobacter pylori patient isolates from Nicaragua. BMC Evol Biol. 2016;16:53. doi: 10.1186/s12862-016-0619-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson K, Letley DP, Kaneko K Molecular. Pathogenesis and signal transduction by Helicobacter pylori. Tegtmeyer N, Backert S, editors. Current topics in microbiology and immunology. vol 400. Cham: Springer International Publishing; 2017. 10.1007/978-3-319-50520-6.
- 14.Javed S, Skoog EC, Solnick JV. Impact of Helicobacter pylori virulence factors on the host immune response and gastric pathology. In: current topics in microbiology and immunology. 2019;21–52. 10.1007/978-3-030-15138-6_2. [DOI] [PubMed]
- 15.Berthenet E, Yahara K, Thorell K, Pascoe B, Meric G, Mikhail JM, et al. A GWAS on Helicobacter pylori strains points to genetic variants associated with gastric cancer risk. BMC Biol. 2018;16:84. doi: 10.1186/s12915-018-0550-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olbermann P, Josenhans C, Moodley Y, Uhr M, Stamer C, Vauterin M, et al. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010;6:e1001069. 10.1371/journal.pgen.1001069. [DOI] [PMC free article] [PubMed]
- 17.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492.. [DOI] [PubMed] [Google Scholar]
- 18.Bankevich A, Nurk S, Antipov D, Gurevich AAA, Dvorkin M, Kulikov ASS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. doi: 10.1089/cmb.2012.0021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–8. doi: 10.1093/bioinformatics/btw354.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolley KA, Maiden MCJ. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11:595. doi: 10.1186/1471-2105-11-595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Méric G, Yahara K, Mageiros L, Pascoe B, Maiden MCJ, Jolley KA, et al. A reference pan-genome approach to comparative bacterial genomics: identification of novel epidemiological markers in pathogenic campylobacter. Bereswill S, editor. PLoS One. 2014;9:e92798. doi: 10.1371/journal.pone.0092798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheppard SK, Jolley KA, Maiden MCJ. A gene-by-gene approach to bacterial population genomics: whole genome MLST of campylobacter. Genes. 2012;3:261–77. doi: 10.3390/genes3020261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh K. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66. doi: 10.1093/nar/gkf436.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84:210–23. doi: 10.1016/j.ajhg.2009.01.005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson DJ, Hellenthal G, Myers S, Falush D. Inference of population structure using dense haplotype data. Copenhaver GP, editor. PLoS Genet. 2012;8:e1002453. doi: 10.1371/journal.pgen.1002453.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahara K, Furuta Y, Oshima K, Yoshida M, Azuma T, Hattori M, et al. Chromosome painting in silico in a bacterial species reveals fine population structure. Mol Biol Evol. 2013;30:1454–64. doi: 10.1093/molbev/mst055.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org/.
- 29.Pfeifer B, Wittelsbürger U, Ramos-Onsins SE, Lercher MJ. PopGenome: an efficient swiss army knife for population genomic analyses in R. Mol Biol Evol. 2014;31:1929–36. doi: 10.1093/molbev/msu136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Micro Genom. 2016;2:1–5. doi: 10.1099/mgen.0.000056.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–4. doi: 10.1093/molbev/msp259.. [DOI] [PubMed] [Google Scholar]
- 32.Tomb J, White O, Kerlavage A, Clayton R, Sutton G, Fleischmann R, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–47. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 33.Wilson DJ. GenomegaMap: within-species genome-wide dN/dS estimation from over 10,000 genomes. Rosenberg M, editor. Mol Biol Evol. 2020; 1–23. 10.1093/molbev/msaa069. [DOI] [PMC free article] [PubMed]
- 34.Swofford, DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, Massachusetts: Sinauer Associates; 2003.
- 35.Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Softw Pr Exp. 1991;21:1129–64. doi: 10.1002/spe.4380211102.. [DOI] [Google Scholar]
- 36.Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. In: Proceedings of the International AAAI Conference on Weblogs and Social Media. Association for the Advancement of Artificial Intelligence.
- 37.Domínguez-Bello MG, Pérez ME, Bortolini MC, Salzano FM, Pericchi LR, Zambrano-Guzmán O, et al. Amerindian Helicobacter pylori strains go extinct, as european strains expand their host range. Buckling A, editor. PLoS One. 2008;3:e3307. 10.1371/journal.pone.0003307. [DOI] [PMC free article] [PubMed]
- 38.Kersulyte D, Kalia A, Gilman R, Mendez M, Herrera P, Cabrera L, et al. Helicobacter pylori from Peruvian Amerindians: traces of human migrations in strains from remote Amazon, and genome sequence of an Amerind strain. PLoS One. 2010;5:e15076. 10.1371/journal.pone.0015076. [DOI] [PMC free article] [PubMed]
- 39.Hayashi T, Senda M, Morohashi H, Higashi H, Horio M, Kashiba Y, et al. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012;12:20–33. doi: 10.1016/j.chom.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Suerbaum S, Achtman M. Evolution of Helicobacter pylori: the role of recombination. Trends Microbiol. 1999;7:182–4. doi: 10.1016/S0966-842X(99)01505-X.. [DOI] [PubMed] [Google Scholar]
- 41.Adhikari K, Chacón-Duque JC, Mendoza-Revilla J, Fuentes-Guajardo M, Ruiz-Linares A. The genetic diversity of the Americas. Annu Rev Genomics Hum Genet. 2017;18:277–96. doi: 10.1146/annurev-genom-083115-022331.. [DOI] [PubMed] [Google Scholar]
- 42.Eltis D. “A brief overview of the trans-atlantic slave trade,” voyages: the trans-Atlantic slave trade database. https://www.slavevoyages.org/voyage/about. Accessed 2 October 2019.
- 43.Norris ET, Rishishwar L, Chande AT, Conley AB, Ye K, Valderrama-Aguirre A, et al. Admixture-enabled selection for rapid adaptive evolution in the Americas. Genome Biol. 2020;21:29. doi: 10.1186/s13059-020-1946-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno-Estrada A, Gravel S, Zakharia F, McCauley JL, Byrnes JK, Gignoux CR, et al. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homburger JR, Moreno-Estrada A, Gignoux CR, Nelson D, Sanchez E, Ortiz-Tello P, et al. Genomic insights into the ancestry and demographic history of south America. Tarazona-Santos E, editor. PLOS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Backert S, Tegtmeyer N, Fischer W. Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Futur Microbiol. 2015;10:955–65. doi: 10.2217/fmb.15.32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399–411. doi: 10.1016/j.chom.2010.04.005.. [DOI] [PubMed] [Google Scholar]
- 48.Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–7. doi: 10.1016/j.mib.2007.12.003.. [DOI] [PubMed] [Google Scholar]
- 49.Garner JA, Cover TL. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–203. doi: 10.1128/IAI.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji X, Frati F, Barone S, Pagliaccia C, Burroni D, Xu G, et al. Evolution of functional polymorphism in the gene coding for the Helicobacter pylori cytotoxin. FEMS Microbiol Lett. 2002;206:253–8. doi: 10.1111/j.1574-6968.2002.tb11018.x.. [DOI] [PubMed] [Google Scholar]
- 51.Letley DP, Rhead JL, Twells RJ, Dove B, Atherton JC. Determinants of non-toxicity in the gastric pathogen Helicobacter pylori. J Biol Chem. 2003;278:26734–41. doi: 10.1074/jbc.M304071200.. [DOI] [PubMed] [Google Scholar]
- 52.Pagliaccia C, de Bernard M, Lupetti P, Ji X, Burroni D, Cover TL, et al. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc Natl Acad Sci USA. 1998;95:10212–7. doi: 10.1073/pnas.95.17.10212.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gangwer KA, Mushrush DJ, Stauff DL, Spiller B, McClain MS, Cover TL, et al. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci USA. 2007;104:16293–8. doi: 10.1073/pnas.0707447104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bugaytsova JA, Björnham O, Chernov YA, Gideonsson P, Henriksson S, Mendez M, et al. Helicobacter pylori adapts to chronic infection and gastric disease via pH-responsive BabA-mediated adherence. Cell Host Microbe. 2017;21:376–89. doi: 10.1016/j.chom.2017.02.013.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basak C, Pathak SK, Bhattacharyya A, Pathak S, Basu J, Kundu M. The secreted peptidyl prolyl cis,trans -isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J Immunol. 2005;174:5672–80. doi: 10.4049/jimmunol.174.9.5672.. [DOI] [PubMed] [Google Scholar]
- 56.Yaseen A, Audette GF. Structural flexibility in the Helicobacter pylori peptidyl-prolyl cis,trans-isomerase HP0175 is achieved through an extension of the chaperone helices. J Struct Biol. 2018;204:261–9. doi: 10.1016/j.jsb.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Norris ET, Wang L, Conley AB, Rishishwar L, Mariño-Ramírez L, Valderrama-Aguirre A, et al. Genetic ancestry, admixture and health determinants in Latin America. BMC Genom. 2018;19:861. doi: 10.1186/s12864-018-5195-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in african americans. Science. 2010;329:841–5. doi: 10.1126/science.1193032.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sironi M, Cagliani R, Forni D, Clerici M. Evolutionary insights into host-pathogen interactions from mammalian sequence data. Nat Rev Genet. 2015;16:224–36. doi: 10.1038/nrg3905.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aspholm-Hurtig M. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–22. doi: 10.1126/science.1098801.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Population structure of H. pylori strains from the Americas and other continents.
Suppl. Fig 2. Representation of the ancestry admixture in H. pylori strains from Indigenous American populations, as analysed with chromosome painting.
Suppl. Fig 3. Comparative admixture analyses of H. pylori strains in the Americas by country.
Suppl. Fig. 4. Fst analysis of individual virulence genes to identify genetic variants that are significantly more commonly in the Americas than in the rest of the world.
Suppl. Fig. 5. Analyses of positions under selective pressure in the cagA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Fig. 6. Analyses of positions under selective pressure in the vacA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Fig. 7. Analyses of positions under selective pressure in the babA gene of strains from the Americas and other continents using GenomegaMap.
Suppl. Table 1. Characteristics of the 723 worldwide H. pylori strains analyzed in this study.
Suppl. Table 2. Genome size, contigs number and coverage of the genomes from the 149 H. pylori strains sequenced in this study.