Abstract
Tumorigenicity of induced pluripotent stem cells (iPSCs) is anticipated when cells derived from iPSCs are transplanted. It has been reported that iPSCs formed a teratoma in vivo in autologous transplantation in a nonhuman primate model without immunosuppression. However, there has been no study on tumorigenicity in major histocompatibility complex (MHC)-matched allogeneic iPSC transplantation with immune-competent hosts. To examine the tumorigenicity of allogeneic iPSCs, we generated four iPSC clones carrying a homozygous haplotype of the MHC. Two clones were derived from female fibroblasts by using a retrovirus and the other two clones were derived from male peripheral blood mononuclear cells by using Sendai virus (episomal approach). The iPSC clones were transplanted into allogenic MHC-matched immune-competent cynomolgus macaques. After transplantation of the iPSCs into subcutaneous tissue of an MHC-matched female macaque and into four testes of two MHC-matched male macaques, histological analysis showed no tumor, inflammation, or regenerative change in the excised tissues 3 months after transplantation, despite the results that iPSCs formed teratomas in immune-deficient mice and in autologous transplantation as previously reported. The results in the present study suggest that there is no tumorigenicity of iPSCs in MHC-matched allogeneic transplantation in clinical application.
Keywords: iPSCs, tumorigenicity, allogenic transplantation, MHC, cynomolgus macaque
Introduction
Induced pluripotent stem cells (iPSCs) will be useful not only in regenerative medicine but also in cancer immunotherapy and drug discovery1–6. Since the generation of autologous iPSCs is a time-consuming process with a high expense, allogeneic iPSCs originating from healthy individuals carrying a homozygous human leukocyte antigen (HLA) haplotype have been developed and stored as a national initiative in Japan7,8. It has been reported that allogeneic transplantation of cells and tissues generated from HLA haplotype-homozygous iPSCs into an HLA-matched recipient minimized the allogenic immunoreaction and enabled doses of immunosuppressive agents to be reduced7–13.
Tumorigenicity of iPSCs is anticipated when cells derived from iPSCs are transplanted. The tumorigenic potential of iPSC-derived cells is due to contamination with undifferentiated cells14–17, which are intermediate products having proliferative potentials. Various strategies for removal of immature cells from transplanted cells have been proposed15,18. To examine contamination of tumorigenic or immature cells, transplantation assays have been performed using immune-deficient animal models18–21. However, these studies did not reflect the practical clinical therapeutics in regenerative medicine in which cells are transplanted into recipients with a functional immune system rejecting the transformed cells and tumor cells22,23. There has been no study to clarify the tumorigenicity in major histocompatibility complex (MHC)-matched allogeneic iPSC transplantation with immune-competent nonhuman primates, while transplantation of MHC-matched allogenic iPSCs did not result in the formation of teratomas in pigs and mice16,17, although it had been reported that undifferentiated autologous iPSCs formed teratomas in a dose-dependent manner in rhesus monkeys without immunosuppression24,25.
In the present study, to examine the tumorigenicity of iPSCs in allogeneic transplantation, firstly, we transplanted an iPSC derived from female macaque skin fibroblasts into the parental autologous cynomolgus macaque and an MHC-matched immune-competent cynomolgus macaque, which are experimental animals genetically close to humans. Histological analysis showed no tumor, inflammation, or regenerative change in the excised tissues 3 months after allogeneic transplantation, despite the fact that the iPSC formed teratomas in autologous transplantation as previously reported. We next transplanted four iPSC clones into four testicles of two allogeneic MHC-matched male macaques. The two iPSC clones were generated from female macaque skin fibroblasts and the two other iPSC clones were generated from male macaque peripheral blood mononuclear cells. These iPSCs showed no tumorigenicity with very low immunogenicity in MHC-matched macaques. Therefore, cells derived from iPSCs may not form tumors in patients even if the graft is contaminated with immature iPSCs.
Materials and Methods
iPSC Culture
Two iPSCs clones, CMF1/1 -1 and CMF1/1-2, were established from skin fibroblasts of a 3-year-old female MHC homozygous cynomolgus macaque by transducing human OCT3/4, SOX2, KLF4, and c-MYC using lentivirus vectors, as previously reported1,26. Briefly, macaque fibroblast cells expressing the mouse solute carrier family 7 (cationic amino acid transporter, y+, system), member 1 (Slc7a1) gene were transduced with human OCT3/4, SOX2, KLF4, and c-MYC by using lentivirus vectors. Other iPSC clones, CMT1/1-4 and CMT1/1-6, were established from peripheral blood T cells of a male MHC homozygous cynomolgus macaque. The T cells were activated by anti-CD3/CD28 antibody-coated beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and transduced with reprogramming factors via Sendai virus (SeV) vectors. The established iPSC clones were transfected with small interfering RNA L527 using Lipofectamine RNAi Max (Invitrogen, Waltham, MA, USA) for removal of SeV vectors from the cytoplasm (Supplemental Table 1)27. Cynomolgus monkey embryonic stem cells (ESCs) were reported previously26,28.
The iPSCs and the ESCs were cultured with mouse embryonic fibroblasts (MEFs; ReproCELL, Yokohama, Japan) as feeder cells in primate ES medium (ReproCELL) supplemented with 4 ng/ml of recombinant human basic fibroblast growth factor (WAKO, Osaka, Japan) on a 0.1% gelatin (Sigma-Aldrich, Saint Louis, MO, USA)-coated dish at 37% in a 5% CO2 atmosphere. The medium was changed every day. The macaque iPSCs were passaged every 5–7 days by enzymatic digestion in CTK [0.6 mg/ml collagenase, 0.06% trypsin, 2.5 mM CaCl2, and 25% Knockout™ Serum Replacement (KSR, Thermo Fisher Scientific, Waltham, MA, USA)] solution at 37°C for 5 min. Detached colonies containing MEFs were collected and cultured on the 0.1% gelatin-coated dish at 37°C for 15 to 30 min to remove MEFs again (attached–remove method). After removing MEFs, the iPSCs were resuspended in a fresh medium and transferred onto fresh MEFs. For flow cytometric analysis, iPSCs were cultured in a feeder-free condition using the CellartisÒ DEF-CS™ 500 Culture system (TAKARA Bio, Kusatsu, Japan) according to the manufacturer’s instructions.
Karyotyping Analysis of iPSCs
Karyotyping analyses of the four iPSC lines were performed by Chromocenter Inc., Yonago, Japan. Preparation of metaphase chromosomes of iPSCs was performed by standard protocols. The slides were stained with Hoechst 33258 for Q-banding karyotyping. The passage numbers of CMF1/1 -1, CMF1/1-2, CMT1/1-4, and CMT1/1-6 cells for karyotyping analysis were 34, 20, 24, and 20, respectively. The results of karyotyping analysis are shown in Supplemental Table 1.
Reverse Transcription Polymerase Chain Reaction of iPSCs
Total RNA was isolated using TRIZOL® Reagent (Life Technologies, Carlsbad, CA, USA). First-strand cDNA was made from total RNA by SuperScript™ IV Reverse Transcriptase (Life Technologies). Reverse transcription polymerase chain reaction (RT-PCR) was performed with KOD One® PCR Master Mix (TOYOBO Co., Ltd., Osaka, Japan) according to the manufacturer’s instructions using primer sets shown in Supplemental Table 2. The conditions of RT-PCR were as follows: prewarming at 94°C for 2 min followed by 35 cycles consisting of denaturation for 30 s at 98°C, annealing for 30 s at variable temperatures shown in Supplemental Table 2, and extension for 30 s at 68°C. A 7-min extension at 68°C was added after the final cycle.
Differentiation of Mesenchymal Stem Cell–like Cells from CMF1/1 -1 Cells
Mesenchymal stem cell-like cells named iMSCs were derived from CMF1/1 -1 iPSC. Three days after embryonal body (EB) formation under the condition of mesodermal differentiation [recombinant human bone morphogenetic protein 4 (BMP-4), 100 ng/ml; PeproTech, Rocky Hill, NJ, USA], the mesodermal EBs were cocultured for 14 days on a mouse mesenchymal stem cell line, C3 H 10T1/2, as feeder cells in the presence of vascular endothelial growth factor (50 ng/ml; PeproTech) and stem cell factor (100 ng/ml; PeproTech) to generate hematopoietic stem cells and iMSC progenitor cells27. CD45− CD34− cells were sorted by a FACS Aria instrument (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and cultured with 20% fetal bovine serum–containing Iscove’s Modified Dulbecco’s Medium (Thermo Fisher Scientific) at 37°C in a 5% CO2 atmosphere. The number of attached cells was increased 7 days later.
Animals and iPSC Transplantation
All animal experiments were approved by the Shiga University of Medical Science Animal Experiment Committee (Permit numbers: 2012-6-3 H, 2017-1-6H2, 2019-1-6, and 2019-8 -1) and were carried out in strict accordance with the Guidelines for the Husbandry and Management of Laboratory Animals of the Research Center for Animal Life Science at Shiga University of Medical Science, the guidelines of an Institutional Animal Care and Use Committee (IACUC), and Standards Relating to the Care and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. MHC homozygous and MHC heterozygous cynomolgus macaques were identified in the Filipino macaque population, which carries a particular Mafa haplotype allele called HT1 (Supplemental Table 3)29–31. Regular veterinary care and monitoring, balanced nutrition, and environmental enrichment for the animals were provided by the Research Center for Animal Life Science at Shiga University of Medical Science. The temperature and humidity in the animal rooms were maintained at 25 ± 2°C and 50 ± 5%, respectively. The cynomolgus macaques were housed individually in cages. The light cycle was 12 h of artificial light from 8:00 to 20:00. Each animal was fed 20 g/kg/day of commercial pellet macaque chow (CMK-2, CLEA Japan, Tokyo, Japan) in the morning and supplemented with 20 to 50 g of sweet potato and half of a banana in the afternoon. Water was supplied ad libitum by an automatic supplier. Identification number, age, and detailed information on transplanted iPSCs of each monkey were as follows. Monkey #733, a 15-year-old female monkey bearing the Mafa HT1 homozygous allele and the parental monkey of the iPSC line CMF1/1 -1, was used for autologous transplantation. CMF1/1 -1 cells (1 × 107 cells) at passage 24 and iMSCs (1 × 107 cells) at passage 5 derived from CMF1/1 -1 cells were embedded in 50% Matrigel (BD Bioscience, San Diego, CA, USA) and autologously injected into the subcutaneous tissue of each shoulder of monkey #733. Monkey #1497, a 12-year-old female monkey, was used for MHC-matched allogenic transplantation. CMF1/1 -1 cells (1 × 107 cells) at passage 41 and iMSCs (1 × 107 cells) at passage 4 derived from CMF1/1 -1 cells were embedded in 50% Matrigel (BD Bioscience) and subcutaneously injected into each shoulder. Monkeys #303 and #307, 14-year-old male monkeys, were used for MHC-matched allogenic transplantation into testicles without Matrigel. CMF1/1 -1 cells (1.1 × 107 cells), CMF1/1-2 cells (1.2 × 107 cells), CMT1/1-4 cells (0.9 × 107 cells), and CMT1/1-6 cells (1.5 × 107 cells) were respectively injected into each testicle under the guidance of ultrasonography. All monkeys were healthy at the time of transplantation and all transplantations were performed under ketamine/xylazine anesthesia. All injected monkeys were observed for 12 weeks. Genetic information on Mafa haplotypes of the monkeys, iPSCs, and iMSCs is shown in Supplemental Table 3.
NOD.CB17-Prkdcscid/J mice (NOD-SCID mice) and NOD/Shi-scid, IL-2Rγnull mice (NOG mice) were purchased from CLEA Japan and the Central Institute for Experimental Animals (CIEA, Kawasaki, Japan), respectively. iPSC clones were suspended in 50% Matrigel (BD Bioscience) and were injected subcutaneously into the necks of 6- to 8-week-old NOG mice and NOD-SCID mice.
Histological Analysis and Immunocytochemistry
The sections and cells were stained with hematoxylin and eosin (HE), alcian blue, alkaline phosphatase, and Oil-red O using a standard protocol. For immunohistochemistry, after incubation overnight with primary antibodies (Supplemental Table 4), a secondary antibody conjugated with horseradish peroxidase (NICHIREI Bioscience, Tokyo, Japan) and a peroxidase substrate, 3,3′-diaminobenzidine tetrahydrochloride (NICHIREI Bioscience), was used for color development for immunohistochemistry. To perform immunocytochemistry of iPSCs, the cells were fixed in 4% paraformaldehyde and were permeabilized with 0.5% saponin in phosphate buffered saline (PBS) containing 0.1% bovine serum albumin. After incubation with primary antibodies (Supplemental Table 3), cyanin3 (Cy3)-conjugated goat anti-mouse immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, PA, USA) or Cy3-conjugated goat anti-rabbit IgG (Abcam, Cambridge, UK) was used as a secondary antibody. The All-in-One Fluorescence Microscope BZ-X710 (KEYENCE, Osaka, Japan) was used for observation.
Flow Cytometry
A single cell suspension of iPSCs cultured in a feeder-free condition and iMSCs were stained with purified primary antibodies and fluorescein-conjugated primary antibodies (Supplemental Table 4). Cy3-conjugated goat anti-mouse IgG or Cy3-conjugated goat anti-rabbit IgG was used as a secondary antibody when purified antibodies were used. Dead cells were labeled with 2 μg/ml propidium iodide (Sigma-Aldrich). The cells were analyzed by a FACS Calibur instrument (Becton, Dickinson and Company).
Enzyme-linked Immunosorbent Assay
The concentrations of TGF-β1 in culture supernatants of iMSCs were measured using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Detection of IgG in Plasma of the Macaques After Transplantation
After heat inactivation, plasma collected from the iPSC-transplanted macaques was diluted 10 times in PBS before use. The diluted plasma was added to iPSCs cultured in a feeder-free condition as a primary antibody. Fluorescein isothiocyanate–labeled goat polyclonal anti-macaque IgG (Fc) (Nordic Immunological Laboratories, Susteren, The Netherlands) was used as a secondary antibody to detect the attached IgG on the surface of the cells.
ELISPOT Assay
A monkey interferon (IFN)-γ/ interleukin (IL)-2 FluoroSpot kit (MABTECH, Nacka Strand, Sweden) was used to detect cellular immune responses against the transplanted cells according to the manufacturer’s instructions. Briefly, heparinized peripheral blood was collected from monkeys #733 and #1497 before and after transplantation. Purified blood cells (5 × 105 cells) were cultured in an ELISPOT plate with each cell lysate made from 5 × 104 cells. After culture for 3 days, the number of spots positive for IFN-γ and IL-2 was counted by the ImmunoSpot analyzer (Cellular Technology Limited, Shaker Heights, OH, USA). Stimulation indices were calculated by the following formula: number of spots in culture of the blood cells plus cell lysate/number of spots in culture of the blood cells only.
Sialic Acid Staining on the Surface of iPSCs
For detection of sialic acid on the surface of iPSCs, cells were fixed with 4% paraformaldehyde. Then the cells were stained with biotinylated MAACKIA AMURENSIS LECTIN II (MALII, Vector Laboratories, Inc., Burlingame, CA, USA) and biotinylated ELDERBERRY BARK LECTIN (SNA, Vector Laboratories, Inc.), which specifically recognize α2-3 and α2-6 sialic acids, respectively32,33. To cleave sialic acids, cells were treated with sialidase in acetate buffer pH 5.5 (2.05 g sodium acetate in 500 ml distilled water) at 37°C for 20 h before lectin staining. Streptavidin conjugated with Alexa flour 488 (Invitrogen) was used to detect biotinylated lectins.
Results
iPSCs Induced by Lentiviral and Sendai Virus Vectors Form Teratomas in Immune-deficient Mice
We prepared four macaque iPSC lines carrying a homozygous MHC haplotype (Supplemental Table 3) for MHC-matched allogeneic transplantation. All of the iPSCs were positive for the immature makers TRA-1-60, SSEA4, OCT3/4, and NANOG as detected in monkey ESCs (Fig. 1A and Supplemental Fig. 1). All of the iPSCs expressed endogenous genes such as c-Myc, Klf4, Sox2, and Oct3/4 (Supplemental Fig. 2). The iPSCs that were subcutaneously transplanted into immune-deficient NOD/Shi-scid, IL-2Rγnull mice (NOG mice) showed pluripotency and tumorigenicity by formation of teratomas with components of three germ layers (AFP: endoderm, αSMA: mesoderm, and β3-tubulin: ectoderm) 10 to 20 weeks later (Fig. 1B). The karyotypes of the two lines CMF1/1 -1 and CMT1/1-4 were normal in Q-band analysis, while the other two lines CMF1/1-2 and CMT1/1-6 had abnormal karyotypes, which had additional chromosomal fragments of unknown origins in chromosome 1 and chromosome 17, respectively (Supplemental Table 1).
Figure 1.
Macaque iPSCs used in the present study show an immature phenotype with pluripotency. (A) Flow cytometric analysis of iPSCs. Lines indicate cells stained with antibodies against indicated molecules. Gray histograms indicate negative controls stained with isotype-matched control antibodies. *: Passage number. (B) HE-stained sections and immunohistochemistry of each teratoma derived from iPSCs in NOG mice after subcutaneous injection. The magnifications of the left panel and right panel of HE-stained sections are ×1 and ×40, respectively. The right panels are magnified images of the insets of the left panel. The magnifications of AFP (endoderm), αSMA (mesoderm), and β3-tubulin (ectoderm) are ×400. HE: hematoxylin and eosin; iPSC: induced pluripotent stem cell.
Teratoma Formation by iPSC in the Donor of iPSCs but not in the MHC-matched Allogenic Recipient
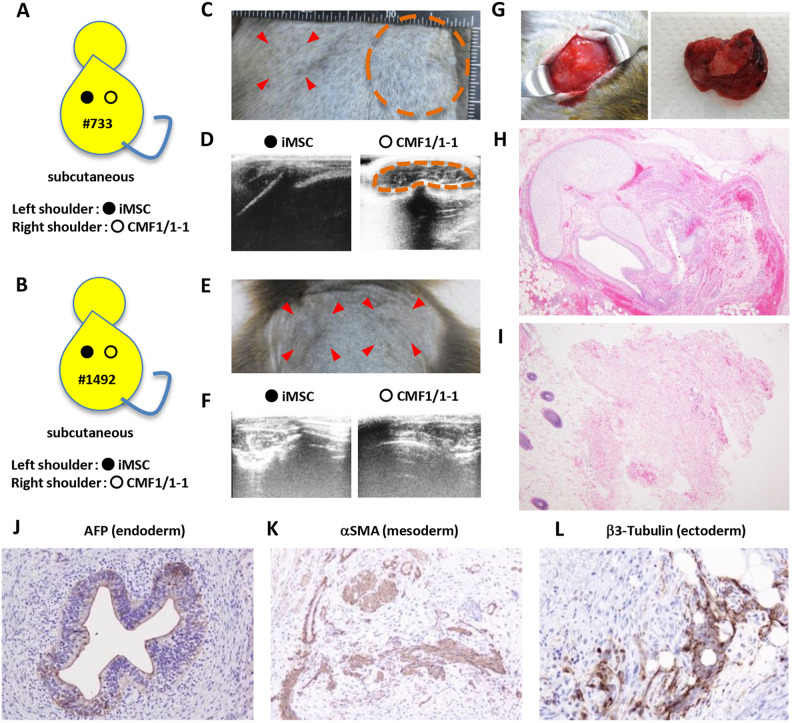
To examine the tumorigenicity of iPSCs in immune-competent cynomolgus macaques, CMF1/1 -1 cells, which were derived from fibroblasts of female monkey #733 carrying the homozygous haplotype of MHC, and mesenchymal stem cell–like cells derived from CMF1/1 -1 cells (iMSCs) were autologously transplanted into the subcutaneous tissue of monkey #733 and the MHC-matched female monkey #1497 carrying an identical MHC haplotype heterozygously as previously reported (Fig. 2A, B and Supplemental Fig. 3)25. iMSCs positive for CD105, CD73, CD44, CD90, and CD29 that were derived from iPSCs were transplanted for comparison (Supplemental Fig. 3A–C). The iMSCs differentiated into chondrocytes, osteoblasts, and adipocytes and produced TGF-β in vitro (Supplemental Fig. 3D, E), being consistent with the definition given by the Mesenchymal and Tissue Stem Cell Committee of ISCT34,35. Four weeks after transplantation, a hard mass was detected as a heteroechoic lesion by ultrasonography at the transplantation site of iPSCs in autologous monkey #733 but not at the iMSC-transplanted site or at the iPSC and iMSC-transplanted sites in allogeneic MHC-matched monkey #1497 (Fig. 2C–F). The tumor in monkey #733 was excised 6 weeks after transplantation because of its increasing size (Fig. 2G). The tumor was a teratoma with cells positive for AFP (endoderm), αSMA (mesoderm), and β3-tubulin (exoderm) (Fig. 2 H and J–L). Sparsely distributed fibrosis without any inflammatory cells was observed in an HE-stained section of excised tissue of the iMSC-transplanted site 3 months after autologous transplantation (Fig. 2I). No tumor or tissue derived from iPSCs and iMSCs was detected in histological sections of both sites in allogeneic MHC-matched monkey #1497 (data not shown).
Figure 2.
Macaque iPSCs form a teratoma in autologous transplantation but not in MHC-matched allogeneic transplantation. Monkeys #733 and #1497 were transplanted with CMF1/1 -1 cells in subcutaneous tissue of the shoulders and were observed for 12 weeks. (A) Schema of the transplantation. Autologous iPSCs, CMF1/1 -1 cells at passage 24, and iMSCs at passage 5 derived from CMF1/1 -1 cells were injected into subcutaneous tissue of the right shoulder (ˆ) and left shoulder (•) of macaque #733 with 50% Matrigel, respectively. (B) Schema of the transplantation. MHC-matched allogeneic iPSCs, CMF1/1 -1 cells at passage 41, and iMSCs at passage 4 derived from CMF1/1 -1 cells were respectively injected into subcutaneous tissue of the right shoulder (ˆ) and left shoulder (•) of macaque #1497 with 50% Matrigel. (C) The right shoulder of the iPSC transplantation site was swollen at 6 weeks after transplantation (circled), while the left shoulder of the iMSC transplantation site (arrows) was not swollen. (D) Ultrasonography revealed a heteroechoic mass (circled) in subcutaneous tissue on the scapula of the right shoulder but not that of the left shoulder 3 months after transplantation. (E) Neither shoulder of the cell transplantation site (arrows) was swollen 3 months after transplantation. (F) Ultrasonography revealed no mass in subcutaneous tissue in both shoulders 3 months after transplantation. (G) Gross appearance of the teratoma in the right shoulder of monkey #733. (H) HE-stained section of the teratoma in monkey #733. The magnification was ×40. (I) HE-stained section of biopsy specimens of the left shoulder of monkey #733 at 3 months after transplantation. The magnification was ×40. (J, K, and L) Immunohistochemistry of the teratoma. The magnifications of AFP (endoderm), αSMA (mesoderm), and β3-tubulin (ectoderm) were ×200, ×200 and ×400, respectively. HE: hematoxylin and eosin; iPSC: induced pluripotent stem cell; MHC: major histocompatibility complex.
Humoral and Cellular Immune Responses Against Transplanted Cells in the Recipient Monkeys
To detect a humoral immune response against the transplanted cells, we measured levels of antibodies against the cells in monkey plasma by using a flow cytometer (Fig. 3A, B). IgG against the autologously transplanted cells in monkey #733 was not detected, but IgG against MEFs, which were used as feeder cells in the iPSC culture and might have been transplanted with iPSCs (average ratio of MEF contamination: 3.3 ± 0.36%), was detected (Fig. 3A). Immune cells including CD4+ T cells, CD8+ T cells, and CD20+ B cells infiltrated into the teratoma in autologously transplanted monkey #733, while a focal infiltration of a small number of natural killer (NK) cells was detected in the tissue (Supplemental Fig. 4A, B and Supplemental Table 5). No inflammatory cells were found in the iMSC-transplanted site (Fig. 2I).
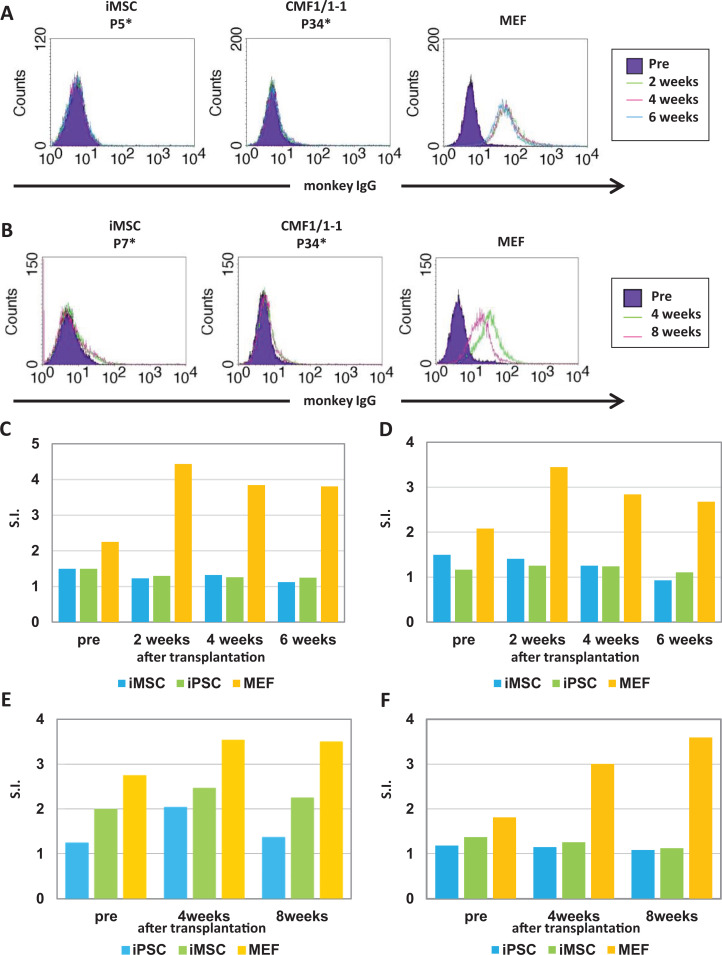
Figure 3.
Immunological reaction of the transplanted cells in monkeys #733 and #1497. (A, B) iPSCs were cultured without MEFs to avoid the absorption of MEF antigens for a flow cytometric assay. CMF1/1 -1 cells, iMSCs, and MEFs were incubated with the plasma of monkeys #733 (A) and #1497 (B) as a primary antibody. Fluorescein-conjugated anti-monkey IgG was used to detect IgG specific for the cells in plasma as a secondary antibody. Filled histograms indicate cells stained with the plasma collected before transplantation. Colored lines indicate cells stained with the plasma collected after transplantation. *: Passage number. C, D, E, and F: ELISPOT assay of IFN-γ (C, E) and IL-2 (D, F). Peripheral blood cells in autologous transplantation in monkey #733 (C, D) and in MHC-matched allogeneic transplantation in monkey #1497 (E, F) were cultured with the lysate of indicated cells. The experiments were usually performed in duplicate wells for each condition. Results are shown as S.I. HE: hematoxylin and eosin; IFN: interferon; IgG: immunoglobulin G; IL: interleukin; iPSC: induced pluripotent stem cell; MEF: mouse embryonic fibroblast; MHC: major histocompatibility complex; S.I.: stimulation indices.
We examined a cellular immune response against the transplanted cells (Fig. 3C, D). Ratios of both IFN-γ-producing and IL-2-producing cells responding only in MEFs, but not in iPSCs and iMSCs, were higher after transplantation than before transplantation (Fig. 3C, D). Neither humoral immune responses nor cellular immune responses against autologous iPSCs and iMSCs were detected in the autologous transplantation in monkey #733. These results suggest that inflammatory cells infiltrating the teratoma react with a small number of MEFs contaminated in the transplanted iPSCs. On the other hand, weak IgG and IFN-γ responses against MHC-matched allogeneic cells were detected (Fig. 3B, E, and F). Inflammatory cells did not remain 3 months after transplantation in monkey #1497 (data not shown).
No Tumor or Tissue Derived From iPSCs in MHC-matched Testes 3 Months After Transplantation in Macaques
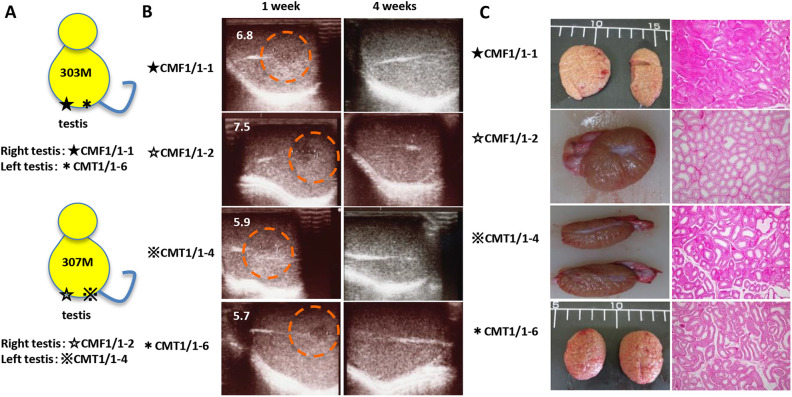
To examine the tumorigenicity of MHC-matched allogeneic iPSCs in an immune-privileged site of immunocompetent and MHC-matched hosts, four iPSC lines with the MHC homozygous allele, CMF1/1 -1, CMF1/1-2, CMT1/1-4, and CMT1/1-6, were transplanted into each testicle of MHC heterozygous macaques #303 and #307 (Fig. 4A and Supplemental Table 3). Low-echoic lesions were observed by ultrasonography 1 week after transplantation, while the lesions were diminished in size 4 weeks after transplantation as well as after the subcutaneous transplantation in monkey #1497 (Fig. 4B). Three months after transplantation, no tumor or tissue derived from the iPSCs was histologically detected in any of the testicles excised from monkeys #303 and #307, even though the testicles were immune-privileged sites (Fig. 4C).
Figure 4.
Allogeneic iPSCs form no tumors or tissues in the testicles of MHC-matched macaques. (A) Schema of the transplantation. CMF1/1 -1 and CMT1/1-6 cells were injected into the right testis (★) and left testis (⋆) of macaque #303, respectively. CMF1/1-2 and CMT1/1-4 cells were injected into the right testis (☆) and left testis (※) of macaque #307, respectively. All iPSC clones were injected without Matrigel, and the monkeys were observed for 12 weeks after transplantation. (B) Ultrasonographic images of the testicles after iPSC transplantation. Circles indicate low-echogenic areas where iPSCs were injected. The greater diameters of the low-echogenic areas (mm) are indicated in images taken 1 week after transplantation. (C) Gross appearance of the resected testicles and microscopic findings of HE-stained sections of each testicle excised 3 months after transplantation. The magnification of HE-stained sections is ×40. HE: hematoxylin and eosin; iPSC: induced pluripotent stem cell; MHC: major histocompatibility complex.
Plasma IgG of the Macaques After Transplantation Reacts with iPSCs
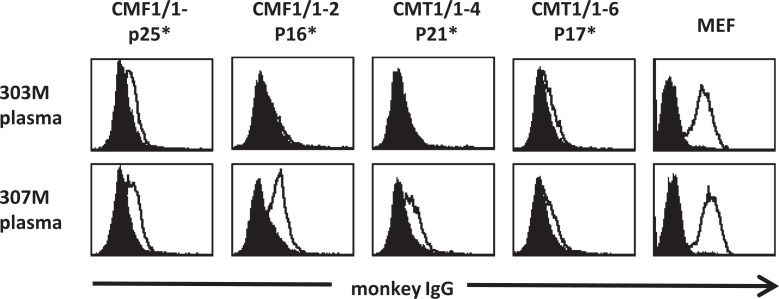
Immune responses against iPSCs in recipient macaques were examined. Low levels of IgG specific for the MHC-matched allogeneic iPSCs were detected in the plasma of iPSC-transplanted macaques #303 and #307, but the fluorescence intensity of IgG against the transplanted iPSCs was lower than that of IgG against MEFs as was also observed in monkey #1497 (Fig. 5). Minor histocompatibility antigens on the surface of iPSCs and/or embryonic antigens such as SSEA4 and OCT3/4 might be the targets of the acquired immune responses36,37. However, no binding of IgG specific against the antigens on MHC-matched #733 peripheral blood mononuclear cells and embryonic antigens expressed on the surface of MHC-matched embryonic carcinoma31 were detected in the plasma collected from macaques #303 and #307 after MHC-matched allogeneic transplantation, whereas a very weak IgG response specific for embryonic carcinoma cells was detected only in the plasma of macaques #1497 (Supplemental Fig. 5).
Figure 5.
IgG against transplanted iPSCs was detected in the plasma of iPSC-transplanted macaques. iPSCs were cultured without MEFs to avoid the absorption of MEF antigens. The iPSCs and MEFs were stained with the plasma of iPSC-transplanted macaques #303 and #307. Filled histograms indicate cells stained with the plasma collected before transplantation. Lines indicate cells stained with the plasma collected 4 weeks after transplantation. *: Passage number. IgG: immunoglobulin G; iPSC: induced pluripotent stem cell; MEF: mouse embryonic fibroblast.
Since immature cells have been shown to be depleted by innate immunity including natural killer cells38,39, macrophages13,40,41, and an alternative pathway of complements16,42, we studied the reaction of the host immune system to the transplanted iPSCs in MHC-matched macaques. The transplanted iPSCs expressed detectable levels of MHC class I molecules including HLA-G and HLA-E, which are known as ligands of suppressive receptors for NK cell activity (Supplemental Fig. 6A). This was supported by the formation of teratomas in NOD.CB17-Prkdcscid/J mice (NOD-SCID mice) carrying NK cells as well as in NOG mice lacking NK cells (Supplemental Table 6), while a small number of NK cells was found around the teratoma in NOD-SCID mice (Supplemental Fig. 4C). The transplanted iPSCs also expressed CD47, which is known as a “Don’t eat me signal” to avoid phagocytosis by macrophages (Supplemental Fig. 6B). To assess the rejection by an alternative pathway of complements, we examined the expression of sialic acids on the surface of iPSCs, which has been shown to prevent activation of complements42. Sialic acids linked to galactose with α2-3 and α2-6 linkages were expressed on the surface of iPSCs, suggesting low susceptibility to activation of an alternative pathway of complements (Supplemental Fig. 6C).
Discussion
We examined the tumorigenicity of iPSCs in immune-competent macaques with MHC-matched allogeneic transplantation. In immune-competent hosts, MHC-homozygous iPSCs formed teratomas in autologous transplantation but did not form any tumors in MHC-matched allogeneic transplantation, although the cells had an abnormal karyotype and were injected into an immune-privileged site such as the testis. MHC-matched transplantation of allogenic iPSCs induced a low level of immune responses against the transplanted cells. Our results using cynomolgus macaques are consistent with the results of previous studies showing no teratoma formation in immune-competent pigs and mice in which MHC-matched iPSCs were transplanted16,17. These results suggest that cells derived from MHC-matched allogeneic iPSCs would not form tumors in patients even if the graft is contaminated with immature iPSCs.
In a previous study, porcine iPSCs induced by Yamanaka factors using a retrovirus were transplanted into MHC-matched immune-competent pigs, and the iPSCs were rejected by innate immunity including NK cells16. On the other hand, we used four clones of iPSCs induced by introduction of Yamanaka factors using either a retrovirus or Sendai virus (episomal approach) to compare the frequencies of tumor formation when different methods for iPSC induction were used, and no tumor was observed in any of the macaques injected with the iPSCs. Thus, the methods of iPSC induction might not affect the tumorigenicity of iPSCs in MHC-matched allogenic transplantation.
One of the reasons for transplanted iPSCs not forming a teratoma in MHC-matched allogeneic transplantation was that the transplanted iPSCs were immunologically rejected depending on weak acquired immune responses against transplanted iPSCs in recipient monkeys. However, the immunogenicity of iPSCs was less potent than that of MEFs in MHC-matched allogeneic transplantations, even though the number of contaminated MEFs was smaller than that of transplanted iPSCs, as previously reported3,43,44. Although an inflammatory response in the immune-privileged testis is milder than that in other body organs, immune cells might access the testis and resident macrophages in the testis can evoke acquired immune responses. Innate immunity including NK cells, macrophages, and complements would also be involved in the rejection of immature cells as previously described16,38–42. However, the four transplanted iPSC clones expressed inhibitory molecules of NK cells, macrophages, and complements. In addition, the iPSC clones except for CMT1/1-4 would not be rejected by NK cells because the iPSCs formed teratomas in both NOG mice not possessing NK activity and NOD/SCID mice possessing NK activity. Therefore, the transplanted iPSCs in the present study seemed to have low susceptibility to host innate immune activity.
The tumor-forming ability of iPSCs is important for teratoma formation in MHC-matched allogeneic transplantation. CMT1/1-4 cells did not form a teratoma in the presence of NK cells in NOD-SCID mice, although the expression levels of inhibitory molecules of NK cell activity, HLA-G and HLA-E, on the cell surface were not reduced. At least, CMF1/1-4 cells did not form a teratoma under the condition of the presence of NK cells.
There is a possibility that the number of iPSCs was not sufficient to form a teratoma even though we transplanted 5 × 106 to 1 × 107 iPSCs dissociated by CTK solution. When dissociated cells were transplanted, a larger number of cells was necessary to form teratomas in mice. It has been reported that the incidence of teratoma formation varied substantially depending on the transplanted cells and recipients45,46. From this point of view, transplantation of differentiated cells originating from iPSCs may be safe since contamination of more than 1 × 107 immature cells is not practical in clinical transplantation.
Matrigel is always used for a tumorigenicity assay of iPSCs because Matrigel is known as a scaffold to enhance the tumorigenicity of iPSCs in NOG mice45–47. CMF1/1 -1 cells in Matrigel formed teratomas in NOG mice and autologous monkey #733, but the iPSCs did not form a teratoma in MHC-matched allogenic monkey #1497 transplanted with Matrigel or in monkey #303 transplanted without Matrigel. Thus, CMF1/1 -1 cells have tumorigenicity in vivo but failed to form teratomas in MHC-matched allogenic recipients with or without Matrigel. Since Matrigel containing xenogeneic proteins is not used for treatment in humans, the possibility of transplanted cells derived from iPSCs forming tumors in allogeneic recipients is speculated to be low.
The microenvironment at the transplantation site also affects the tumorigenicity of iPSCs18,19,46,47. Subcutaneous transplantation to examine tumorigenicity of iPSCs is considered to ensure the absence of tumorigenic cells of the final cell products18,47, while intramuscular injection resulted in the formation of a teratoma with a smaller number of pluripotent cells than that in the case of subcutaneous injection18,19,45,47. We injected iPSCs into subcutaneous tissue and testicles, where we could easily assess tumor formation; however, we cannot exclude the possibility of tumor formation in other sites such as the muscle and brain18,19,45–47.
Conclusion
We showed no tumorigenicity and minimal immunogenicity of MHC-matched allogeneic iPSC transplantation in cynomolgus macaques. Although the number of experiments was limited, the result indicated that transplantation of differentiated cells derived from iPSCs might be safer than previously anticipated in the aspect of tumorigenicity.
Supplemental Material
Supplemental Material, sj-pdf-1-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pdf-2-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-1-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-2-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-3-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-4-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-5-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-6-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Acknowledgments
We thank Ms Naoko Kitagawa for support in the culture of cells; Mr Akira Yokoe for making sections and staining; and Dr Hideaki Tsuchiya, Mr Takahiro Nakagawa, and Mr Ikuo Kawamoto for animal care.
Footnotes
Ethical Approval: This study was approved by the Shiga University of Medical Science Animal Experiment Committee (Permit numbers: 2012-6-3 H, 2017-1-6H2, 2019-1-6, and 2019-8 -1).
Statement of Human and Animal Rights: All experiments using iPSCs and animal experiments were carried out in strict accordance with the Guidelines for the Husbandry and Management of Laboratory Animals of the Research Center for Animal Life Science at Shiga University of Medical Science, the guidelines of an Institutional Animal Care and Use Committee (IACUC) and Standards Relating to the Care and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Authors’ Contribution: HI and VLP contributed equally to this work.
Data Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by grants from the Japan Agency for Medical Research and Development (AMED) under Grant Number 16bm0404007. CTN is supported by the Sato Yo International Scholarship Foundation.
ORCID iD: Hirohito Ishigaki  https://orcid.org/0000-0002-5249-8598
https://orcid.org/0000-0002-5249-8598
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 2. Sugita S, Iwasaki Y, Makabe K, Kimura T, Futagami T, Suegami S, Takahashi M. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Reports. 2016;7(4):619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 2013;1(4):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawamura T, Miyagawa S, Fukushima S, Maeda A, Kashiyama N, Kawamura A, Miki K, Okita K, Yoshida Y, Shiina T, Ogasawara K, et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Reports 2016;6(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Cell. 2013;12(1):31–36. [DOI] [PubMed] [Google Scholar]
- 6. Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, Woltjen K, Nakagawa M, Asada T, Arai T, Kawakatsu S, et al. iPSC-Based compound screening and In vitro trials identify a synergistic anti-amyloid β combination for Alzheimer’s disease. Cell Rep. 2017;21(8):2304–2312. [DOI] [PubMed] [Google Scholar]
- 7. Kaneko S, Yamanaka S. To be immunogenic, or not to be: that’s the iPSC question. Cell Stem Cell. 2013:12(4);385–386. [DOI] [PubMed] [Google Scholar]
- 8. Ichise H, Nagano S, Maeda T, Miyazaki M, Miyazaki Y, Kojima H, Yawata N, Yawata M, Tanaka H, Saji H, Masuda K, et al. NK Cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Reports. 2017;9(3):853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakatsuji N, Nakajima F, Tokunaga K. HLA-haplotype banking and iPS cells. Nat Biotechnol. 2008:26(7);739–740. [DOI] [PubMed] [Google Scholar]
- 10. Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. [DOI] [PubMed] [Google Scholar]
- 11. Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, Itoh Y, et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–391. [DOI] [PubMed] [Google Scholar]
- 13. Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA- matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11(2):147–152. [DOI] [PubMed] [Google Scholar]
- 14. Liu Z, Tang Y, Lü S, Zhou J, Du Z, Duan C, Li Z, Wang C. The tumourigenicity of iPS cells and their differentiated derivates. J Cell Mol Med. 2013;17(6):782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sougawa N, Miyagawa S, Fukushima S, Kawamura A, Yokoyama J, Ito E, Harada A, Okimoto K, Mochizuki-Oda N, Saito A, Sawa Y. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Sci Rep. 2018;8(1):3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mizukami Y, Abe T, Shibata H, Makimura Y, Fujishiro SH, Yanase K, Hishikawa S, Kobayashi E, Hanazono Y. MHC-matched induced pluripotent stem cells can attenuate cellular and humoral immune responses but are still susceptible to innate immunity in pigs. PLoS One. 2014;9(6);e98319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamura A, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ito E, Watabe T, Masuda S, Toda K, Hatazawa J, Morii E, et al. Teratocarcinomas arising from allogeneic induced pluripotent stem cell-derived cardiac tissue constructs provoked host immune rejection in mice. Sci Rep. 2016;6;19464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, Kawamata S. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014;9(1):e85336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2(3):198–210. [DOI] [PubMed] [Google Scholar]
- 20. Müller FJ, Goldmann J, Löser P, Loring JF. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6(5);412–414. [DOI] [PubMed] [Google Scholar]
- 21. Prockop DJ. Defining the probability that a cell therapy will produce a malignancy. Mol Ther. 2010;18(7):1249–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pardoll D. T cells and tumours. Nature. 2001;411(6841):1010–1012. [DOI] [PubMed] [Google Scholar]
- 24. Ebeling M, Küng E, See A, Broger C, Steiner G, Berrera M, Heckel T, Iniguez L, Albert T, Schmucki R, Biller H, et al. Genome-based analysis of the nonhuman primate Macaca fascicularis as a model for drug safety assessment. Genome Res. 2011;21(10):1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hong SG, Winkler T, Wu C, Guo V, Pittaluga S, Nicolae A, Donahue RE, Metzger ME, Price SD, Uchida N, Kuznetsov SA, et al. Path to the clinic: assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014;7(4):1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okahara-Narita J, Umeda R, Nakamura S, Mori T, Noce T, Torii R. Induction of pluripotent stem cells from fetal and adult cynomolgus macaque fibroblasts using four human transcription factors. Primates. 2012;53(2):205–213. [DOI] [PubMed] [Google Scholar]
- 27. Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, Takayama N, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(1):114–126. [DOI] [PubMed] [Google Scholar]
- 28. Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, Imahie H, Kondo Y, Iritani A, Nakatsuji N. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev Dyn. 2001;222(2):273–279. [DOI] [PubMed] [Google Scholar]
- 29. Shiina T, Yamada Y, Aarnink A, Suzuki S, Masuya A, Ito S, Ido D, Yamanaka H, Iwatani C, Tsuchiya H, Ishigaki H, et al. Discovery of novel MHC-class I alleles and haplotypes in Filipino cynomolgus macaques (Macaca fascicularis) by pyrosequencing and Sanger sequencing: Mafa-class I polymorphism. Immunogenetics. 2015;67(10):563–578. [DOI] [PubMed] [Google Scholar]
- 30. Ishigaki H, Shiina T, Ogasawara K. MHC-identical and transgenic cynomolgus macaques for preclinical studies. Inflamm Regen. 2018;38:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishigaki H, Maeda T, Inoue H, Akagi T, Sasamura T, Ishida H, Inubushi T, Okahara J, Shiina T, Nakayama M, Itoh Y, et al. Transplantation of iPS-derived tumor cells with a homozygous MHC haplotype induces GRP94 antibody production in MHC-matched macaques. Cancer Res. 2017;77(21):6001–6010. [DOI] [PubMed] [Google Scholar]
- 32. Geisler C, Jarvis DL. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 2011;21(8):988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac (alpha 2-6) Gal/GalNAc sequence. J Biol Chem. 1987;262(4):1596–1601. [PubMed] [Google Scholar]
- 34. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 35. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 36. Lee HY, Chen CY, Tsai TI, Li ST, Lin KH, Cheng YY, Ren CT, Cheng TJ, Wu CY, Wong CH. Immunogenicity study of Globo H analogues with modification at the reducing or nonreducing end of the tumor antigen. J Am Chem Soc. 2014;136(48):16844–16853. [DOI] [PubMed] [Google Scholar]
- 37. Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, Zebroski H, Dhodapkar MV. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci USA. 2010;107(19);8718–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dressel R, Nolte J, Elsner L, Novota P, Guan K, Streckfuss-Bömeke K, Hasenfuss G, Jaenisch R, Engel W. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 2010;24(7):2164–2177. [DOI] [PubMed] [Google Scholar]
- 39. Dressel R, Schindehütte J, Kuhlmann T, Elsner L, Novota P, Baier PC, Schillert A, Bickeböller H, Herrmann T, Trenkwalder C, Paulus W, et al. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients’ immune response. PLoS One. 2008;3(7):e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26(12):2538–2545. [DOI] [PubMed] [Google Scholar]
- 42. Koch CA, Jordan CE, Platt JL. Complement-dependent control of teratoma formation by embryonic stem cells. J Immunol. 2006;177(7);4803–4809. [DOI] [PubMed] [Google Scholar]
- 43. Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. [DOI] [PubMed] [Google Scholar]
- 44. Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. [DOI] [PubMed] [Google Scholar]
- 45. Nara Y, Muramatsu S, Takino N, Kodera M, Nakayama T, Inoue N, Kakiuchi T, Tukada H, Ono H, Terao K, Okuno T, et al. Brain tumor formation after allogeneic transplantation of monkey embryonic stem cells. Neuropathology. 2005;25(2):17. [Google Scholar]
- 46. Kishi Y, Tanaka Y, Shibata H, Nakamura S, Takeuchi K, Masuda S, Ikeda T, Muramatsu S, Hanazono Y. Variation in the incidence of teratomas after the transplantation of nonhuman primate ES cells into immunodeficient mice. Cell Transplant. 2008;17(9);1095–1102. [PubMed] [Google Scholar]
- 47. Kawamata S, Kanemura H, Sakai N, Takahashi M, Go MJ. Design of a tumorigenicity test for induced pluripotent stem cell (iPSC)-derived cell products. J Clin Med. 2015;4(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pdf-2-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-1-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-2-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-3-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-4-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-5-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation
Supplemental Material, sj-pptx-6-cll-10.1177_0963689721992066 for No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques by Hirohito Ishigaki, Van Loi Pham, Jun Terai, Takako Sasamura, Cong Thanh Nguyen, Hideaki Ishida, Junko Okahara, Shin Kaneko, Takashi Shiina, Misako Nakayama, Yasushi Itoh and Kazumasa Ogasawara in Cell Transplantation