Abstract
The genus Cyrtonotula Uvarov, 1939 (Blaberidae, Epilamprinae) is recorded for the first time from Hainan Island, China. Three new species, Cyrtonotula epunctata Wang & Wang, sp. nov., C. maculosa Wang & Wang, sp. nov., and C. longialata Wang & Wang, sp. nov., are described based on morphological data and a molecular analysis using Automatic Barcode Gap Discovery (ABGD). Additional barcode data of blaberid species, including these three new species, are provided to facilitate future species identification. Morphological photographs and habitat photos of these new species, as well as a key to the known species, are provided.
Keywords: ABGD, Cyrtonota , DNA barcodes, habitat, neighbor joining, species identification
Introduction
The Epilamprinae genus Cyrtonota was proposed by Hanitsch (1929), with C. lata as type species, on the basis of one single female specimen from Sumatra. It was characterized by its comparatively large pronotum, hind metatarsus length barely equal to the succeeding joints combined, and the reduced tergum with nearly truncate apex. Owing that the name is preoccupied by a genus of spider (Simon 1864), a replacement name, Cyrtonotula, was proposed by Uvarov (1939).
Since then, no more species have been reported from this genus. Roth (2003) removed it from Epilamprinae and treated it as Blaberidaeincerate sedis. Recently, Mavropulo et al. (2015) returned it back to Epilamprinae as a result of a phylogenetic analysis based on 28S and the male genital characters; this was verified by Djernæs et al. (2020) using four mitochondrial and three nuclear genes. Mavropulo et al. (2015) described two species from Indonesia and provided a description on the male genitalia of this genus for the first time. Later, a fourth species was described from the Philippines (Lucañas 2017). To date, there are four known Cyrtonotula species worldwide and none from China.
Species of Cyrtonotula are currently identified primarily on the basis of morphological characters, mainly the shortened tegmina and wings, the shape of the pronotum, and male genitalia. DNA barcoding had not been employed to explore the diversity of Cyrtonotula.
DNA barcodes (the standard COI sequence) have been proven to be a useful supplementary method in identifying cockroach species and have been effective in resolving problems, such as sexual dimorphism and the identification of nymphs (Che et al. 2017; Bai et al. 2018; Wang et al. 2018). In DNA barcoding studies of cockroaches, the Automatic Barcode Gap Discovery (ABGD) method of species delineation (Puillandre et al. 2012) is widely used and has proven effective in discerning cockroach species (Bai et al. 2018; Wang et al. 2019; Li et al. 2020). Here, Cyrtonotula is reported from China, and three new species are described, with the aid of DNA barcoding.
Material and methods
Morphological study
Type specimens are deposited in the Institute of Entomology, College of Plant Protection, Southwest University, Chongqing, China (SWU). Male genital segments were processed with 10% NaOH for maceration of the soft tissues, observed in glycerol with a Motic K400 stereomicroscope or a Leica M205A stereomicroscope, and preserved with the remainder of the specimen in ethyl alcohol. Photographs were taken with a Leica DFC digital microscope camera attached to a Leica M205A stereomicroscope. All photos and images were processed with Adobe Photoshop CS6. Species descriptions are based on the holotype male. Measurements are given according to the whole sample studied for the description. Sclerites in male genitalia are named according to Klass (1997). The terminology of venation follows Li et al. (2018). Vein abbreviations in this article are as follows:
ScP subcosta posterior;
R radius;
RA radius anterior;
RP radius posterior;
Pcu postcubitus;
M media;
CuA cubitus anterior;
CuP cubitus posterior;
V vannal.
DNA extraction, amplification, and sequencing
We used standard methods to sample cytochrome c oxidase subunit I (COI) of four species (Table 1) as follows. Total DNA was extracted using Hipure Tissue DNA Mini Kit from the hind legs of alcohol-preserved specimens according to the standard DNA barcoding methods for the cockroach. The mitochondrial COI gene was amplified by PCR using primer sets of COI-F3 (5'-CAACYAATCATAAAGANATTGGAAC-3') and COI-R3 (5'-TAAACTTCTGGRTGACCAAARAATCA-3') resulting in a fragment length of 658 bp for genetic analysis after trimming the primers from the amplified product. The amplification reaction was performed in a total volume of 25 µL, including 23 µL T3 DNA polymerase, 1 µL of each primer and 1 µL DNA template. The thermal cycling conditions were as follows: initial denaturation of 2 min at 98 °C followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 53 °C for 10 s, extension at 72 °C for 10 s, and a final extension at 72 °C for 5 min; the samples were then held at 8 °C. The amplified samples were evaluated in 1% agarose gels. Sequencing in both directions was performed by BGI Technology Solutions Co. Ltd (BGI-Tech) (Beijing, China).
Table 1.
Samples of COI genes used in this study.
| Genus | Species | Voucher number | Sequence ID | Locality (China) | Accession number |
|---|---|---|---|---|---|
| Opisthoplatia | O. orientalis | C01.1M | OpisOrie03 | Guangzhou, Guangdong | MW649981 |
| C01.3M | OpisOrie05 | Wuzhishan, Hainan | MW649982 | ||
| Cyrtonotula | C. epunctata sp. nov. | M01.1M | CyrtTest01 | Diaoluoshan, Hainan | MW649978 |
| M01.2F | CyrtTest02 | Diaoluoshan, Hainan | MW649979 | ||
| M01.3F | CyrtTest03 | Wuzhishan, Hainan | MW649980 | ||
| C. maculosa sp. nov. | K01.1M | QuadBrac01 | Yinggeling, Hainan | MW649972 | |
| K01.2M | QuadBrac02 | Yinggeling, Hainan | MW649973 | ||
| K01.3F | QuadBrac03 | Yinggeling, Hainan | MW649974 | ||
| C. longialata sp. nov. | K02.1F | QuadMacr01 | Baoting, Hainan | MW649975 | |
| K02.2M | QuadMacr02 | Dalimuling, Hainan | MW649976 | ||
| K02.3M | QuadMacr03 | Bawangling, Hainan | MW649977 | ||
| cf. Cyrtonotula sp. MNHN BL13 | KY497672 | ||||
| Pseudophoraspis | P. kabakovi | MH755938 | |||
| MH755939 | |||||
| Rhabdoblatta | R. densimaculata | MK547402 | |||
| MK547405 | |||||
| MK547406 | |||||
| R. mascifera | MK547407 | ||||
| MK547408 | |||||
| Outgroup | Mantis religiosa | KR148854 | |||
Sequence processing and phylogenetic analyses
A total of 11 mitochondrial COI sequences were obtained from four Epilamprinae species, plus one Cyrtonotula sequence, another seven Epilamprinae sequences, and one sequence representing the mantis outgroup were downloaded from NCBI for phylogenetic analyses (Table 1). Sequences were aligned in online MAFFT 7 (https://mafft.cbrc.jp/alignment/server/) using the Q-INS-i algorithm. The alignment was then manually corrected in MEGA 7 (Kumar et al. 2007). Intraspecific and interspecific genetic distances are quantified based on the Kimura 2-parameter (K2P) distance model (Kimura 1980) in MEGA 7. The neighbor joining (NJ) tree was constructed in MEGA 7 under the Kimura 2 parameter model (K2P). Statistical support was estimated with 1000 bootstrap replicates. To determine putative species in our study, we used the species delimitation approach, Automatic Barcoding Gap Discovery (ABGD), which was performed using the online webserver (http://wwwabi.snv.jussieu.fr/public/abgd/). The settings were as follows: Pmin = 0.001, Pmax = 0.1, Steps = 10, X (relative gap width) = 1.0, Nb bins = 20, and using Jukes Cantor (JC69) distance.
Results
Species delimitation based on COI and morphological data
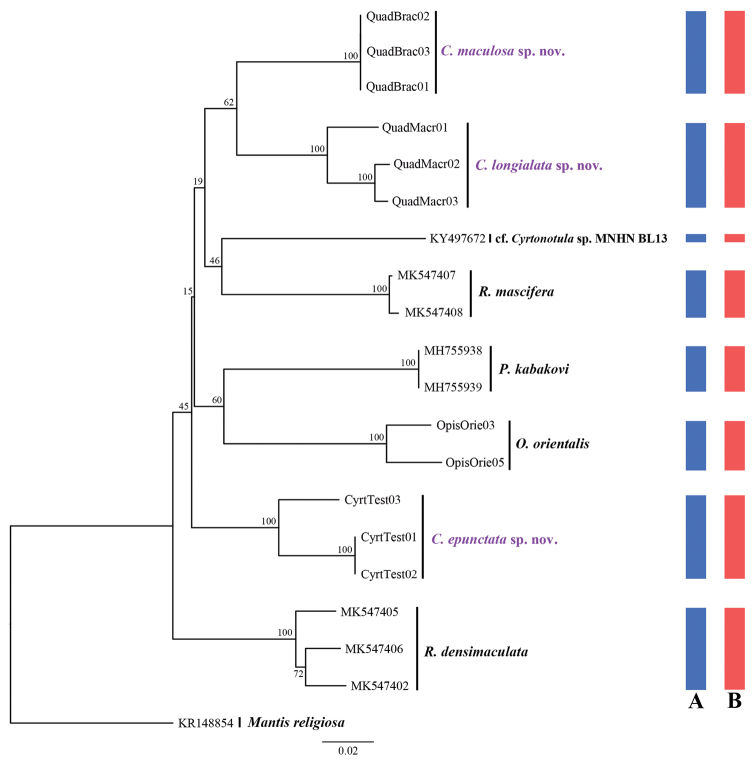
In this study, we acquired 11 COI sequences representing three Cyrtonotula and one Opisthoplatia species. All new sequences were deposited in GenBank (accession numbers MW649972 to MW649982 in Table 1). An NJ analysis revealed that clades from the same species, including male and female samples, constituted monophyletic groups with high support values (Fig. 1). We observed the lowest and highest K2P interspecies genetic distance among these species, 0.1056 for C. maculosa sp. nov. and C. longialata sp. nov., and 0.1367 for C. epunctata sp. nov. and C. maculosa sp. nov. We used the ABGD method to delimit Cyrtonotula species. Three MOTUs were detected in the ABGD analysis, which are completely consistent with the results based on morphological characters (Fig. 1) for C. epunctata sp. nov., C. maculosa sp. nov., and C. longialata sp. nov.
Figure 1.
Neighbor joining (NJ) tree derived from COI sequences based on the K2P model. Morphospecies blue; MOTUs in ABGD red.
Systematics
Cyrtonotula
Uvarov, 1939
3327F5BE-0571-5F7F-9C55-A792D4F90281
Cyrtonota Hanitsch 1929: 281. Type species: Cyrtonota lata Hanitsch, 1929.
Cyrtonotula Uravov 1939: 459, replacement name for Cyrtonota Hanitsch, 1929; Princis 1967: 662; Mavropulo et al. 2015: 18; Lucañas 2017: 132. New record from China.
Diagnosis.
Medium-sized cockroaches. Both sexes similar. Ocular distance slightly narrower than the distance between antennal sockets, greater than ocellar distance. Pronotum broad, anterior margin curved and posterior margin obtusely produced. Tegmina and wings usually brachypterous, not reaching the abdominal apex (except for macropterous C. longialata sp. nov.), their apices somewhat rounded or approximately truncated. Anteroventral margin of front femur Type B; tarsi moderately long; hind metatarsus slender, distinctly longer or nearly equal to the remaining segments combined, armed with two or less equal rows of spines and large apical pulvilli; succeeding tarsomeres armed only with spines surrounding the large pulvilli; the pretarsus with arolium, claws symmetrical and unspecialized. Supra-anal plate entire, with a median incision. Cerci elongate. Subgenital plate large, nearly symmetrical or somewhat asymmetrical. Styli cylindrical.
Male genitalia. Right phallomere Morphnini-type (Anisyutkin and Yushkova 2017): consisting of sclerites R1T, R2, R3, R4, and R5; R4 irregular plate-like, separated; R3 connected to R5. The shape of apical sclerite of L2D irregular and variable. Sclerite L3 hook apically blunt; the folded structure distinct with bristles (visible at high magnification), sclerite L4U present.
Remarks.
Based on the closely similar structure of right phallomere in the epilamprines, four genera have been recorded from China: Morphna Shelford, Pseudophoraspis Kirby, Rhabdoblatta Kirby, and Stictolampra Hanitsch (Beccaloni 2014; Anisyutkin and Yushkova 2017).
The genus Cyrtonotula differs from Rhabdoblatta, Pseudophoraspis, and Stictolampra principally by its reduction of the tegmina and wings. Additionally, C. longialata sp. nov. is morphologically somewhat similar to some Rhabdoblatta and Stictolampra species but can be distinguished by the presence of glandular specialization on the abdominal tergites, basal portion of sclerite L2D, and the non-punctate pronotum.
The genus Cyrtonotula can be distinguished from Morphna by the structure of hind tarsi: metatarsus distinctly longer or about as long as other segments combined, with relatively numerous tarsal spines (metatarsus slightly shorter or nearly equal to remaining segments combined with larger pulvilli, tarsal spines few or absent).
Key to species of Cyrtonotula worldwide
| 1 | Tegmina and wings fully developed, both extending beyond the abdominal apex | C. longialata sp. nov. |
| – | Tegmina and wings reduced, not reaching the abdominal apex | 2 |
| 2 | Pronotum testaceous without maculae | C. epunctata sp. nov. |
| – | Pronotum with scattered maculae | 3 |
| 3 | Vertex with 3 longitudinal dark lines | C. lata Hanitsch |
| – | Vertex with longitudinal lines fewer than 3 | 4 |
| 4 | Front femur Type B1 | C. maquilingensis Lucañas |
| – | Front femur Type B2 | 5 |
| 5 | First abdominal tergum specialized, sclerite L3 comparatively truncated at apex | C. maculosa sp. nov. |
| – | Abdominal tergites unspecialized, sclerite L3 with apex rounded to truncated | 6 |
| 6 | Vertex with yellowish striation, tegmina and wings strongly reduced | C. tertia Mavropulo, Anisyutkin, Zagoskin, Zagoskina, Lukyantsev & Mukha |
| – | Vertex speckled with black, tegmina and wings weakly reduced | C. secunda Mavropulo, Anisyutkin, Zagoskin, Zagoskina, Lukyantsev & Mukha |
Cyrtonotula epunctata
Wang & Wang sp. nov.
4821DB9B-9A01-5B30-8CD4-CE9023250C2A
http://zoobank.org/1B3A01E3-2D33-4263-A61F-656D79F82863
Figure 2.
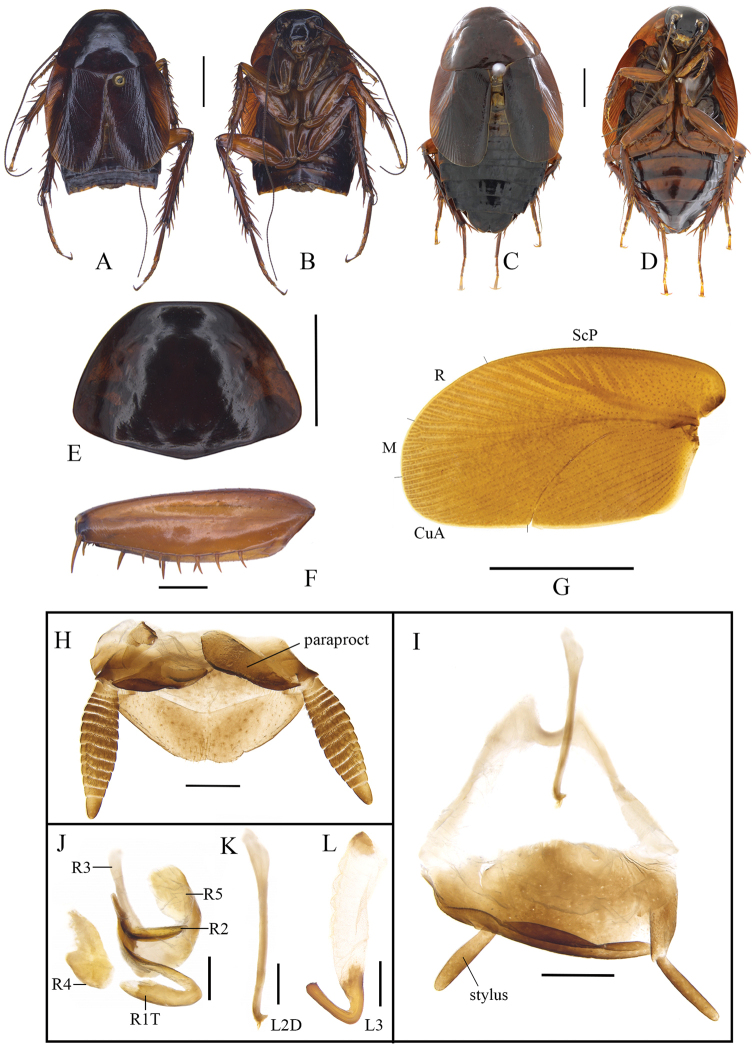
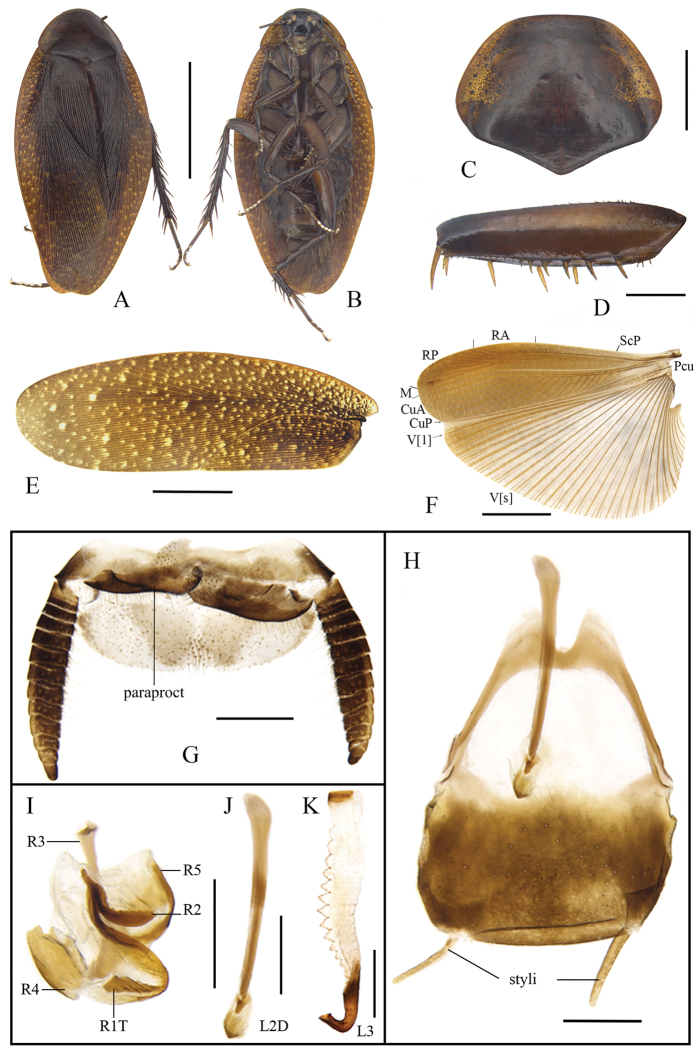
Cyrtonotula epunctata Wang & Wang, sp. nov. A, B, E–L male C, D female A paratype, dorsal view B paratype, ventral view C paratype, dorsal view D paratype, ventral view E pronotum, dorsal view F front femur, ventral view G tegmen H supra-anal plate, ventral view I subgenital plate, dorsal view J right phallomere, dorsal view K median phallomere (sclerite L2D), dorsal view L left phallomere (sclerite L3), dorsal view. Scale bars: 1.0 cm (C, D); 5.0 mm (A, B, E, G); 1.0 mm (F, H, I); 0.5 mm (J–L).
Figure 5.
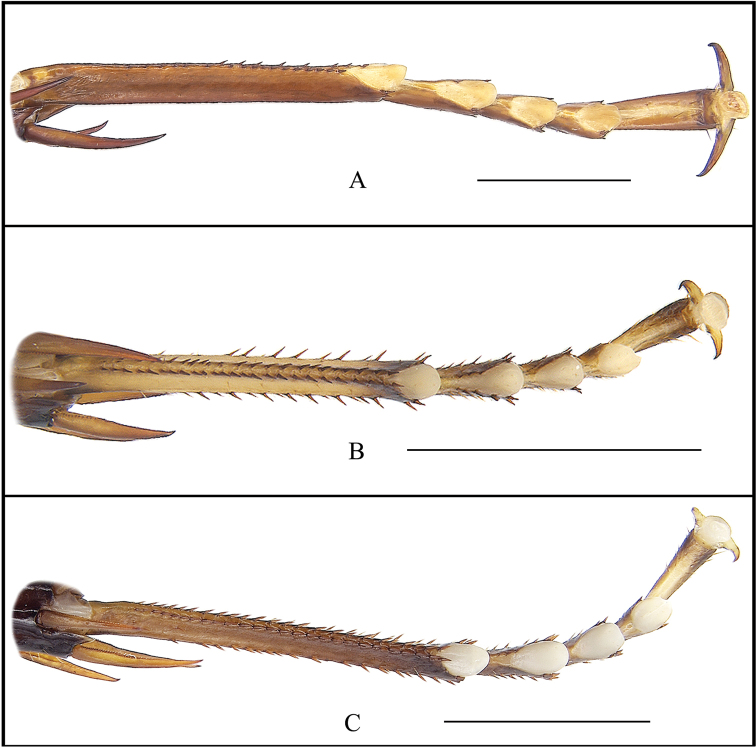
Hind tarsi ACyrtonotula epunctata Wang & Wang, sp. nov., female, paratype BCyrtonotula maculosa Wang & Wang, sp. nov., male, holotype CCyrtonotula longialata Wang & Wang, sp. nov., male, holotype. Scale bars: 2.0 mm.
Type material.
Holotype. China • male; Hainan Prov., Lingshui County, Diaoluoshan Mountain; 916 m; 16 Apr. 2015; Lu Qiu & Qi-Kun Bai leg.; SWU-B-BB100101.
Paratypes. China • 1 male & 2 females; same collection data as holotype; 18 Apr. 2015; SWU-B-BB100102 to 100104 • 1 female; Hainan Prov., Wuzhishan Nature Reserve; 795 m; 18 May 2014; Xin-Ran Li, Shun-Hua Gui & Jian-Yue Qiu leg.; SWU-B-BB100105 • 1 female; Hainan Prov., Diaoluoshan Mountain; 275 m; 25 May 2014; Xin-Ran Li, Shun-Hua Gui & Jian-Yue Qiu leg.; SWU-B-BB100106.
Differential diagnosis.
The new species readily differs from all its congeners in the spination of hind tarsi. Cyrtonotula epunctata sp. nov. resembles C. lata Hanitsch, 1929 in testaceous body color and the length of hind metatarsus, but the new species can be distinguished from C. lata by the following characters: the coloration of facial part black, with clypeo-labral area yellowish brown, and vertex without visible lines (vs deep testaceous and vertex with three longitudinal dark lines in C. lata), and tegmina only reaching to the posterior margin of the third abdominal segment (vs reaching over the sixth abdominal tergite in C. lata).
Description.
Measurements (mm). Overall length: male 20.7–21.0, female 28.9–39.5; pronotum length × width: male 6.3–6.5 × 9.3–9.5, female 8.5 × 11.3; tegmen length: male 9.3–9.6 × 5.6–5.9, female 13.0–18.6 × 8.0–11.9.
Male. Colouration testaceous. Surfaces smooth and glossy (Fig. 2A). Eyes black. Ocellar spots yellow white. Vertex, frons black; clypeus and labrum yellowish brown (Fig. 2B). Pronotum deep testaceous, without spots (Fig. 2E). Tegmina auburn, moderately punctured (Fig. 2G). Legs ferruginous. Abdomen and cerci dark brown (Fig. 2B).
Vertex concealed. Interocular space same as the width between the antennal sockets, slightly greater than ocellar distance. Pronotum nearly semicircular, anterior margin parabolic, posterior margin obtusely angled (Fig. 2E). Tegmina reduced, reaching up the 4th abdominal tergite only; apex rounded; venation distinct, all main veins (Sc, R, and CuP) present, Sc thickened (easily visible on ventral side of tegmen) (Fig. 2A, G). Wings vestigial, only reaching to the posterior margin of the 3rd abdominal segment, completely covered by tegmina. Front femur Type B2 (Fig. 2F). Hind metatarsus depressed-cylindrical, nearly equal to the succeeding segments combined, with single complete row of spines along ventral margin and several additional spines on inner side; four proximal tarsomeres with pulvilli terminal, the one on the second tarsomere occupying practically the whole length of the segment; claws symmetrical and simple; arolium present (Fig. 5A). Abdominal tergites unspecialized; knobs along the posterior margin indistinct; weak spiracle-bearing outgrowths of tergite VIII with distinct spiracle. Supra-anal plate with the posterior margin widely rounded and a weak mesal incision. Cerci distinctly segmented, densely covered with bristles. Paraprocts of blaberid type, asymmetrical (Fig. 2H). Subgenital plate rounded, slightly asymmetrical; the base of the inner plate bifurcated. Styli cylindrical, apically rounded (Fig. 2I).
Male genitalia. Right phallomere with caudal part of sclerite R1T rectangular in shape; cranial part of R1T more or less straight; R2 curved; R3 long; R4 irregular plate-like; R5 large, fused with sclerite R3 in caudal part (Fig. 2J). “chaetae-bearing membranous area” absent. Sclerite L2D not divided into basal and apical parts, slender and rod-like, with basal end tapering and a bifurcated outgrowth born near the basal end (Fig. 2K). Sclerite L3 hooked, apex slightly rounded, with a small tooth on the inner margin less distinct; folded structure present, with bristles. Sclerite L4U distinct (Fig. 2L).
Female. Similar to the male but body somewhat larger.
Etymology.
Derived from the Latin word epunctatus, referring to the lack of visible spots on the body.
Cyrtonotula maculosa
Wang & Wang sp. nov.
18349DCF-071B-5629-810D-1C91AB4A2936
http://zoobank.org/4DC34DE6-DFC6-4F5D-AE5C-B0A35CD8F97A
Figure 3.
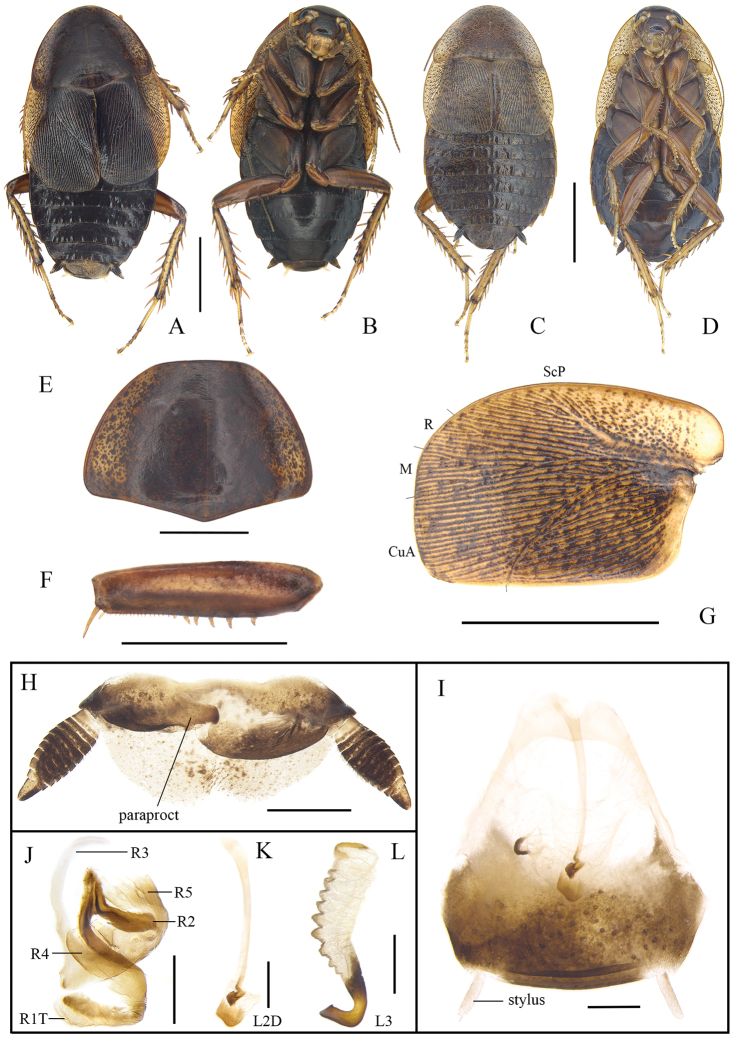
Cyrtonotula maculosa Wang & Wang, sp. nov. A, B, E–L male C, D female A holotype, dorsal view B holotype, ventral view C paratype, dorsal view D paratype, ventral view E pronotum, dorsal view F front femur, ventral view G tegmen H supra-anal plate, ventral view I subgenital plate, dorsal view J right phallomere, dorsal view K median phallomere (sclerite L2D), dorsal view L left phallomere (sclerite L3), dorsal view. Scale bars: 1.0 cm (C, D); 5.0 mm (A, B, E–G); 1.0 mm (H–L).
Figure 6.
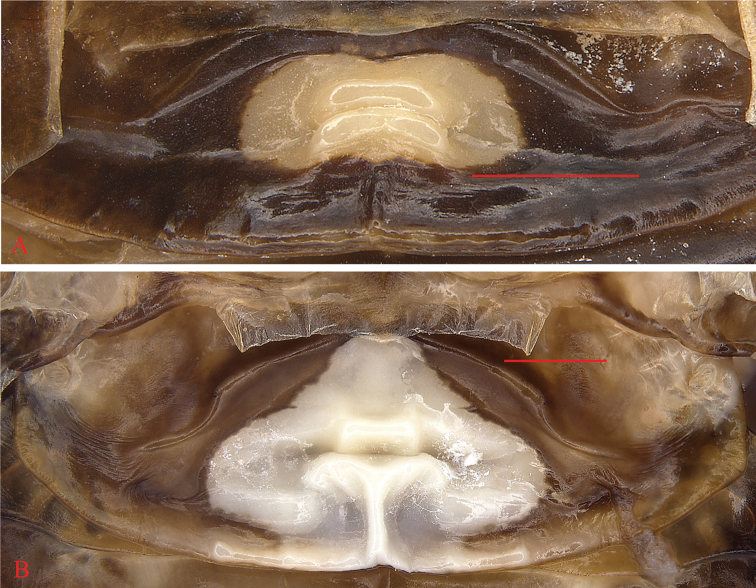
The first abdominal tergal gland ACyrtonotula maculosa Wang & Wang, sp. nov., male, paratype BCyrtonotula longialata Wang & Wang, sp. nov., male, holotype. Scale bars: 1.0 mm.
Type material.
Holotype. China • male; Hainan Prov., Yinggeling Nature Reserve, Nanfa Conservation Station; 650 m; 21 Apr. 2015; Lu Qiu & Qi-Kun Bai leg.; SWU-B-BB090101.
Paratypes. China • 11 males & 11 females; same collection data as holotype; SWU-B-BB090102 to 090123.
Differential diagnosis.
This new species is closely related to C. tertia Mavropulo, Anisyutkin, Zagoskin, Zagoskina, Lukyantsev & Mukha, 2015 in the shape of tegmina and body color, but the former can be distinguished from the latter by the specialized abdominal terga (vs unspecialized) and the shape of sclerite L3 of male genitalia, in which L3 hook is comparatively robust and posteriorly truncate distinctly (vs comparatively slender and rounded apically in C. tertia).
Description.
Measurements (mm). Overall length: male 22.5–27.0, female 31.0; pronotum length × width: male 5.5–6.4 × 7.8–8.2, female 6.3 × 9.1; tegmen length: male 20.6–21.8 × 7.9–8.3, female 25.7 × 9.3.
Male. Body yellowish brown (Fig. 3A). Eyes black. Ocellar spots light yellow. Head black except for yellowish brown clypeo-labral area, facial part of head with weak transverse wrinkles (Fig. 3B). Pronotum yellow-brown, with densely scattered irregular brown spots (Fig. 3E). Tegmina dark yellow, punctured, with brown patches spreading along the veins (Fig. 3G). Coxa, trochanter, and femur yellowish brown; tibia and tarsomere light yellow. Abdomen and cerci blackish brown (Fig. 3B).
Vertex slightly exposed, with two longitudinal yellowish-brown bands. Distance between eyes approximately equal to the width between the antennal sockets and smaller than ocellar distance (Fig. 3B). Pronotum campaniform, widely rounded along anterolateral margins, posterior margin obtusely angled (Fig. 3E). Tegmina considerably shortened, reaching the third abdominal tergite, apex subtruncate; venation distinct, all main veins (Sc, R and CuP) present, Sc thickened (easily visible on ventral side of tegmen) (Fig. 3A, G). Wings vestigial, only reaching the first abdominal tergite. Anterior margin of fore femur Type B2, with 6 or 7 spines (Fig. 3F). Hind metatarsus not quite as long as other segments combined with two rows of spines; well-developed pulvilli on all proximal tarsomeres; claws symmetrical and simple; arolium present (Fig. 5B). Abdominal tergite 1st specialized, lip-like (Fig. 6A); terga 3rd–7th with a few knobs (Fig. 3A); spiracles large, located on the posterolateral angles of tergite 8th. Supra-anal plate with the posterior margin widely rounded, a weak incision at middle. Cerci fusiform, traces of segmentation distinct. Paraprocts of blaberid type, asymmetrical (Fig. 3H). Subgenital plate entire with hind margin rounded; base of the inner plate bifurcated. Styli cylindrical (Fig. 3I).
Male genitalia. Right phallomere with caudal part of sclerite R1T nearly rectangular in shape, cranial part of R1T curved; R2 rounded; R3 elongate apically, curved inward, fused with sclerite R5; R4 plate-like, separated. Sclerite L2D divided into basal and apical parts, basal part rod-like, widened apically, with irregular apical outgrowth; apical part with fine bristles; apical membrane covered with chaetae (Fig. 3K). Sclerite L3 hooked, apically subquadrate; inner margin with a tooth-shaped convexity at apex; folded structure distinct, with bristles; Sclerite L4U present, comparatively narrow (Fig. 3L).
Female. Similar to the male. Body color lighter. Tegmina only reaching the second abdominal tergite, with apex distinctly truncated. Abdominal tergites unspecialized.
Etymology.
Derived from the Latin word maculosus, referring to the scattered with dense spots pronotum and tegmen.
Cyrtonotula longialata
Wang & Wang sp. nov.
58743CE3-1C27-59F2-BC13-3EC14C052D7D
http://zoobank.org/164B8791-1167-4341-BACC-39F40F0B1A5C
Figure 4.
A–LCyrtonotula longialata Wang & Wang, sp. nov., male A holotype, dorsal view B holotype, ventral view C pronotum, dorsal view D front femur, ventral view E tegmen F wings G supra-anal plate, ventral view H subgenital plate, dorsal view I right phallomere, dorsal view J median phallomere (sclerite L2D), dorsal view K left phallomere (sclerite L3), dorsal view. Scale bars: 1.0 cm (A, B); 5.0 mm (C, E, F); 1.0 mm (D, G–K).
Type material.
Holotype. China • male; Hainan Prov., Limuling Mountain; 18 Apr. 2015; Xin-Ran Li & Zhi-Wei Qiu leg.; SWU-B-BB090201.
Paratypes. China • 3 males; same collection data as holotype; SWU-B-BB090202 to 090204 • 6 males & 2 females; Hainan Prov., Baoting County, Maogan Township; 549–776 m; 11–12 Apr. 2015; Xin-Ran Li, Lu Qiu, Zhi-Wei Qiu & Qi-Kun Bai leg.; SWU-B-BB090205 to 090212 • 1 male; Hainan Prov., Bawangling Mountain; 600–800 m; 29 Apr. 2015; Lu Qiu & Qi-Kun Bai leg.; SWU-B-BB090213 • 2 males; Hainan Prov., Diaoluoshan Mountain; 275m; 24 May 2014; Xin-Ran Li & Shun-Hua Gui leg.; SWU-B-BB090214 and 090215.
Differential diagnosis.
The new species principally differs from all its congeners, except for C. maculosa sp. nov., in the presence of abdominal tergal glands. From C. maculosa sp. nov., C. longialata sp. nov. differs in having the completely developed tegmina and wings extending beyond the abdominal apex, the shape of tergal glands (see description below).
Description.
Measurements (mm). Overall length: male 27.0–30.0, female 31.0; pronotum length × width: male 6.2–6.4 × 7.8–8.2, female 6.3 × 9.1; tegmen length: male 23.0–25.0 × 8.6–9.2, female 25.7 × 9.3.
Male. General colour brown (Fig. 4A). Eyes black. Ocellar spots yellow-white. Head black except for brown clypeo-labral area; facial part of head with weak transverse wrinkles and paired impressions under ocelli (Fig. 4B). Pronotum russet, reddish brown at center, speckled with small, brown patches (Fig. 4C). A few yellow spots present in tegmina (Fig. 4E); wings with costal field, radial field, mediocubital field fulvous, and anal field pale brown (Fig. 4F). Legs and abdomen dark yellowish brown. Cerci dark brown (Fig. 4B).
Vertex slightly exposed. Interocular distance as wide as inter-antennal distance, slightly greater than inter-ocellar distance. Pronotum flabellate, widely rounded along anterolateral margins, posterior margin obtusely angled (Fig. 4C). Tegmina and wings completely developed, exceeding abdominal apex; tegmina with rounded apex; venation distinct, all main veins (Sc, R, and CuP) present (Fig. 4E, F). Anterior margin of fore femur B2 (Fig. 4D). Hind metatarsus distinctly longer than other segments combined, armed with two rows of spines; pulvilli large on all proximal tarsomeres; claws symmetrical and simple; arolium present (Fig. 5C). The first abdominal tergite specialized, cap-like (Fig. 6B); tergite VIII with posterolateral angles strongly expressed. Supra-anal plate with the caudal margin widely rounded and a weak median incision. Cerci robust, segmented. Paraprocts of blaberid type, asymmetrical (Fig. 4G). Subgenital plate symmetrical, rounded. Base of inner plate bifurcated. Styli long, cylindrical, apically rounded (Fig. 4H).
Male genitalia. Right phallomere with caudal part of sclerite R1T nearly rectangular, cranial part of R1T curved; R2 arched; R3 elongate and widened apically, fused with sclerite R5 in caudal part; R4 irregular plate-like, separated (Fig. 4I). Sclerite L2D divided into basal and apical parts, basal part rod-like, widened cranially; apical part trifurcate; apical membrane covered with chaetae (Fig. 4J). Sclerite L3 hooked, apically subtruncate; inner margin with apex pointed; folded structure and bristles present; sclerite L4U distinct (Fig. 4K).
Female. Similar to the male. Abdominal tergites unspecialized.
Remarks.
Currently, this is the only species of Cyrtonotula with fully developed tegmina and wings. This species is placed in Cyrtonotula because it closely resembles C. maculosa sp. nov. in having sclerite L3 hooked (apex nearly truncate and inner margin with a distinct point) and in the location of tergal gland.
Etymology.
The species epithet is derived from the Latin adjective longialatus, which refers to the well-developed wings.
Discussion
Flightless cockroaches are usually considered to persist in stable habitats, where food, shelter, and mates are easily accessible (Bell et al. 2007). All of previously known Cyrtonotula species are brachypterous, but C. longialata sp. nov. is noteworthy for being the first macropterous in this genus. Brachypterous cockroaches, including C. epunctata sp. nov. and C. maculosa sp. nov., were observed on leaf litter and scree in areas with trickling water (Fig. 7A–C), while C. longialata sp. nov., maybe a canopy species, was collected in low vegetation (Fig. 7D). We speculate that habitats of C. epunctata sp. nov. and C. maculosa sp. nov. may be different from the habitat of C. longialata sp. nov., and habitat might be one of the determining factors in variations of the wings. This study is also the first to use COI DNA barcode to evaluate diversity of Cyrtonotula species. Our results show that DNA-based species delimitation methods perform well and that individuals were correctly assigned to their corresponding species, although only 10 sequences of Cyrtonotula were included here. Therefore, taking into consideration that there are only seven species of the genus found worldwide, we expect more species of Cyrtonotula, especially macropterous ones, will be discovered and observed with further sampling, so that the knowledge of this genus Cyrtonotula could be comprehensive and explored more deeply.
Figure 7.
Living Cytonotula species from Hainan, China A female C. epunctata sp. nov. (Diaoluoshan Mountain) B male C. epunctata sp. nov. (Diaoluoshan Mountain) C female C. maculosa sp. nov. (Yinggeling Mountain) DCyrtonotula longialata sp. nov. (Baoting County). Photos: A–C by Lu Qiu D by Xin-Ran Li.
Supplementary Material
Acknowledgements
We express our sincere thanks to all the collectors of the type material. We especially thank Lu Qiu and Xin-Ran Li for providing the photos of living Cytonotula species, and we also thank John Richard Schrock for proofing the manuscript. This work was supported by the National Natural Sciences Foundation of China (no. 31872271) and the Fundamental Research Funds for the Central Universities, China (XDJK2020D040).
Citation
Wang Y-S, Chen R, Jin D-T, Che Y-L, Wang Z-Q (2021) New record of Cyrtonotula Uvarov, 1939 (Blaberidae, Epilamprinae) from China, with three new species based on morphological and COI data. ZooKeys 1021: 127–143. https://doi.org/10.3897/zookeys.1021.59526
References
- Anisyutkin LN, Yushkova OV. (2017) New data on cockroaches of the subfamily Epilamprinae (Dictyoptera: Blaberidae) from India and Sri Lanka, with descriptions of new species and the genital complex of Aptera fusca (Thunberg, 1784). Zootaxa 4236: 41–64. 10.11646/zootaxa.4236.1.2 [DOI] [PubMed] [Google Scholar]
- Bai QK, Wang LL, Wang ZQ, Lo N, Che YL. (2018) Exploring the diversity of Asian Cryptocercus (Blattodea : Cryptocercidae): species delimitation based on chromosome numbers, morphology and molecular analysis. Invertebrate Systematics 32: 69–91. 10.1071/IS17003 [DOI] [Google Scholar]
- Beccaloni GW. (2014) Cockroach Species File Online. Version 5.0/5.0. http://cockroach.speciesfile.org/ [accessed 5 December 2020]
- Bell WJ, Roth LM, Nalepa CA. (2007) Cockroaches: Ecology, Behavior, and Natural History. The Johns Hopkins University Press, Baltimore, 230 pp. [Google Scholar]
- Che YL, Gui SH, Lo N, Ritchie A, Wang ZQ. (2017) Species delimitation and phylogenetic relationships in ectobiid cockroaches (Dictyoptera, Blattodea) from China. PLoS ONE 12: e0169006. 10.1371/journal.pone.0169006 [DOI] [PMC free article] [PubMed]
- Djernæs M, Varadínová ZK, Kotyk M, Eulitz U, Klass K-D. (2020) Phylogeny and life history evolution of Blaberoidea (Blattodea). Arthropod Systematics & Phylogeny 78: 29–67. [Google Scholar]
- Hanitsch R. (1929) Fauma Sumatrensis. (Beitrag No. 63). Blattidae. Tijdschrift voor Entomologie 72: 263–302. [Google Scholar]
- Kimura M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- Klass KD. (1997) The external male genitalia and the phylogeny of Blattaria and Mantodea 42: 1–341.
- Kumar NP, Rajavel AR, Natarajan R, Jambulingam P. (2007) DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). Journal of Medical Entomology 44: 1–7. 10.1093/jmedent/41.5.01 [DOI] [PubMed] [Google Scholar]
- Li M, Zhao QY, Chen R, He JJ, Peng T, Deng WB, Che YL, Wang ZQ. (2020) Species diversity revealed in Sigmella Hebard, 1929 (Blattodea, ectobiidae) based on morphology and four molecular species delimitation methods. PLoS ONE 15: e0232821. 10.1371/journal.pone.0232821 [DOI] [PMC free article] [PubMed]
- Li XR, Zheng YH, Wang CC, Wang ZQ. (2018) Old method not old-fashioned: parallelism between wing venation and wing-pad tracheation of cockroaches and a revision of terminology. Zoomorphology 137: 519–533. 10.1007/s00435-018-0419-6 [DOI] [Google Scholar]
- Lucañas CC. (2017) Some new brachypterous cockroaches (Blattodea: Blaberidae: Epilamprinae) from the Philippines. Zootaxa 4294: 130–136. 10.11646/zootaxa.4294.1.9 [DOI] [Google Scholar]
- Mavropulo VA, Anisyutkin LN, Zagoskin MV, Zagoskina AS, Lukyantsev SV, Mukha DV. (2015) New data on the family Blaberidae (Dictyoptera) from Southeast Asia: new species, morphological diversity and phylogeny on the base of ribosomal DNA sequences. Zoosystematica Rossica 24: 14–30. 10.31610/zsr/2015.24.1.14 [DOI] [Google Scholar]
- Puillandre N, Lambert A, Brouillet S, Achaz G. (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21: 1864–1877. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- Roth LM. (2003) Systematics and phylogeny of cockroaches (Dictyoptera: Blattaria). Oriental Insects 37: 1–186. 10.1080/00305316.2003.10417344 [DOI] [Google Scholar]
- Simon EL. (1864) Histoire naturelle des araignées (aranéides). Librairie encyclopédique de Roret, Paris, 556 pp. 10.5962/bhl.title.47654 [DOI] [Google Scholar]
- Uvarov BP. (1939) Twenty-four new generic names in Orthoptera. Annals and Magazine of Natural History (Series 11) 3: 457–459. 10.1080/03745481.1939.9723626 [DOI]
- Wang LL, Liao SR, Liu ML, Deng WB, He JJ, Wang ZQ, Che YL. (2019) Chromosome number diversity in Asian Cryptocercus (Blattodea, Cryptocercidae) and implications for karyotype evolution and geographic distribution on the Western Sichuan Plateau. Systematics and Biodiversity 17: 594–608. 10.1080/14772000.2019.1659878 [DOI] [Google Scholar]
- Wang ZZ, Zhao QY, Che YL, Wang ZQ. (2018) Establishment of a new genus, Brephallus Wang et al., gen. nov. (Blattodea, Blaberidae, Epilamprinae) based on two species from Pseudophoraspis, with details of polymorphism in species of Pseudophoraspis. ZooKeys 785: 117–131. 10.3897/zookeys.785.26565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.