Abstract
Introduction
The potential advantages of oncoplastic breast conserving surgery (BCS) have not been validated in robust studies that constitute high levels of evidence, despite oncoplastic techniques being widely adopted around the globe. There is hence the need to define the precise role of oncoplastic BCS in the treatment of early breast cancer, with consensual recommendations for clinical practice.
Methods
A panel of world-renowned breast specialists was convened to evaluate evidence, express personal viewpoints and establish recommendations for the use of oncoplastic BCS as primary treatment of unifocal early stage breast cancers using the GRADE approach.
Results
According to the results of the systematic review of literature, the panelists were asked to comment on the recommendation for use of oncoplastic BCS for treatment of operable breast cancer that is suitable for breast conserving surgery, with the GRADE approach. Based on the voting outcome, the following recommendation emerged as a consensus statement: Oncoplastic breast conserving surgery should be recommended versus standard breast conserving surgery for the treatment of operable breast cancer in adult women who are suitable candidates for breast conserving surgery (with very low certainty of evidence).
Discussion
This review has revealed a low level of evidence for most of the important outcomes in oncoplastic surgery with lack of any randomized data and absence of standard tools for evaluation of clinical outcomes and especially patients’ values.
Despite areas of controversy, about one-third (36%) of panel members expressed a strong recommendation in support of oncoplastic BCS. Presumably, this reflects a synthesis of views on the relative complexity of these techniques, associated complications, impact on quality of life and costs.
Keywords: Breast surgery, Breast conserving surgery, Oncoplastic breast surgery, GRADE method
1. Introduction
1.1. Breast cancer as a public health problem
Breast cancer is the most frequent malignancy among women, with 2.1 million new cases diagnosed worldwide each year and the leading cause of female death from cancer [1]. Although systemic therapies have an increasingly prominent role in breast cancer management, surgery remains the cornerstone of treatment for the majority of early stage breast cancer patients. The equivalence of outcomes between mastectomy and breast conserving therapy (BCT) in terms of overall survival has been confirmed by long-term follow up of seminal BCT trials [2,3]. Patients managed with BCT have improved cosmetic outcomes and quality of life compared to non-reconstructed mastectomy patients [4,5]. Nonetheless, breast conservation surgery (BCS) does not always yield favorable results, with 25–30% of women experiencing significant deformity following surgery [6]. The percentage breast volume excision is a crucial factor determining cosmetic outcome after completion of both surgery and breast irradiation [7]. Techniques for oncoplastic breast conserving surgery were developed with the aim of improving cosmetic outcomes when compared with standard breast conserving surgery [8,9], thus potentially resulting in better quality-of-life. Moreover, oncoplastic surgery can reduce rates of positive margins and need for re-excision or mastectomy due to larger volumes of excised tissue [10]. Despite being widely adopted around the globe, the potential advantages of oncoplastic surgery have not been validated in robust studies that constitute high levels of evidence [11]. There is hence the need to define the precise role of oncoplastic BCS in the treatment of early breast cancer, with consensual and unambiguous recommendations for clinical practice.
1.2. Description of the target population
The primary target population for these guidelines are adult women with either invasive or non-invasive operable breast cancer undergoing BCS as primary treatment. These guidelines do not offer information on surgical management of locally advanced breast cancer following neoadjuvant chemotherapy and subsequent BCS, nor on use of oncoplastic techniques for excision of multiple ipsilateral cancers.
1.3. Aims and objectives of these guidelines
The purpose of these guidelines is to provide evidence-based recommendations for use of oncoplastic BCS as primary treatment of unifocal early stage breast cancers. The target audience includes patients and a broad range of healthcare professionals including: 1) breast surgical oncologists and plastic surgeons; 2) radiation and medical oncologists; 3) breast radiologists; 4) psycho-oncologists; 5) breast pathologists; 6) patients’ advocacy representatives and 7) decision makers. Policy makers and other stakeholders might express interest in these guidelines as use of oncoplastic BCS can potentially impact on healthcare costs by reducing rates of recurrence, and re-operations with enhanced quality-of-life for patients.
The GRADE approach has been used to evaluate current evidence and provide surgeons with actionable suggestions and allow them to formulate recommendations [12].
2. Methods
2.1. Panel composition and coordination
The panel was composed of twenty-one academics and clinicians from across the world with expertise in the field of breast cancer management, and included breast surgical oncologists, plastic surgeons, medical and radiation oncologists, dedicated breast radiologists, pathologists, psycho-oncologists, breast care nurses, patient advocacy representatives and patients themselves (Supplemental file 1). The panelists were invited to participate in face-to-face discussions during the Milan International Oncoplastic Breast Surgery Meeting held in December 2019 [13]. The panel was selected by a designated leading group (NR, GC, MBN, GM, RDM). The panelists were recognized experts in their fields and were chosen on the basis of semiquantitative selection criteria with representation from Europe, Middle East, North and South America: familiarity with relevant specialties involved in the multidisciplinary management of breast cancer; membership of international organizations (St Gallen International Breast Cancer Conference; San Antonio Breast Cancer Symposium; European Society of Surgical Oncology (ESSO)) [[14], [15], [16]]; authorship of peer-reviewed papers on breast cancer multidisciplinary management in high-impact factor journals; other specialists working in comprehensive breast units with regular participation in multidisciplinary team meetings. The panel chair and co-chair were both breast surgical oncologists (NR and GC respectively). The leading group comprised a panel chair and co-chair, the meeting president (MBN) and two other breast surgical oncologists (GM and RDM) with specific training in GRADE methodology.
The leading group of the consensus conference first met in May 2019 at Mario Negri Institute for Pharmacologic Research IRCCS in Milan, where a course was organized by members of the working group (IM and MC) to specifically train the leading group (NR, GC, MBN, GM and RDM) who defined the main questions in the guidelines, compiled a list of outcomes and chose panel members. All panelists were asked to declare any conflicts of interest prior to all meetings.
Using GRADEpro software [17] the proposed list of outcomes was shared amongst the panelists who provided comments and suggested appropriate changes. After integrating feedback from panelists, the outcome list was modified accordingly and outcomes then graded based on perceived importance (on a scale from 1 to 9: 1–3 low importance; 4–6 important but not critical for decision making; 7–9 critical for decision making).
The draft version of the document was sent to external reviewers prior to the finalization. The external reviewers were nominated by the Group for Reconstructive and Therapeutic Advancements (G.Re.T.A) [18] and other scientific bodies (European Society of Surgical Oncology (ESSO) [16]).
The Mario Negri Institute for Pharmacological Research IRCCS conducted the systematic review of evidence and coordinated the process of guideline development applying the GRADE methodology [12].
2.2. Guideline funding and management of conflicts of interest
Conflicts of interest for all participants were managed with advice from the Institute of Medicine [19]. No conflicts of interest were declared at each meeting by the chair, co-chair and other members of the guideline panel.
2.3. Development of clinical question
A single key clinical question was developed according to the P.I.C.O. acronym based on definition of population (P), intervention (I), comparison (C) and outcomes (O).
The guideline panel unanimously agreed to address the following clinical question:
Should oncoplastic breast-conserving surgery vs. standard breast-conserving surgery be used for the treatment of operable breast cancer in adult women who are suitable candidates for breast conserving surgery?
2.4. Identification of outcomes
The guideline panel used the GRADEpro Guideline Development Tool [17] to deliberate and then prioritize outcomes of interest.
The panel identified the following outcomes of benefit:
-
1.
Quality of life
-
2.
Patient’s satisfaction with aesthetic outcome
-
3.
Loco-regional recurrence
-
4.
Re-excision rate (defined as re-excision of positive margins)
-
5.
Conversion to mastectomy (defined as mastectomy for positive margins)
-
6.
Overall survival
-
7.
Margin positivity rate
-
8.
Disease-free survival
-
9.
Depression
-
10.
Anxiety
-
11.
Aesthetic outcome non-patient-reported
-
12.
Insomnia
-
13.
Fatigue
The following outcomes were judged as “critical” for decision-making in oncoplastic breast surgery:
-
1.
Quality of life
-
2.
Patient’s satisfaction with aesthetic outcome
-
3.
Loco-regional recurrence
-
4.
Re-excision rate (defined as re-excision of positive margins)
-
5.
Conversion to mastectomy (defined as mastectomy for positive margins)
-
6.
Overall survival
-
7.
Margin positivity rate
-
8.
Disease-free survival
-
9.
Depression
The panel identified the following adverse outcomes that potentially cause harm to patients:
-
1.
Surgical complications (defined as post-operative complication presenting within one month after the surgical procedure).
-
2.
Post-operative need for second level exams (defined as the need of second level exams in the follow-up due to imaging abnormalities deriving from the surgical procedure)
-
3.
Pain (defined as post-operative pain)
-
4.
Time to adjuvant treatment (defined as time from the surgical procedure to the start of any adjuvant therapy)
-
5.
Return to operating theatre within 7 days (defined as any re-intervention for early post-operative complication)
-
6.
Operative time (defined as the duration of the surgical procedure)
“Surgical complications” was the only outcome judged to be “critical” for decision-making, with the other outcomes being classified as “important”.
2.5. Search strategy and selection of evidence
A systematic literature review was performed by searching PubMed, Embase and Cochrane Library, Psychinfo, and Lilacs (up to July 19, 2020) without date or language restrictions. The full search strategy is available as Supplemental File 2. Main articles were cross-referenced to ensure that all relevant publications were identified. The PRISMA flow-chart is reported in Supplemental file 3. Information relating to study design, patient characteristics, treatment received, and study results were documented.
2.6. Certainty of evidence
For each selected outcome, an evaluation of the certainty of evidence was performed based on the GRADE approach. GRADE encompasses five main domains: study limitations, imprecision, indirectness, inconsistency, and publication bias. Based on the study design, the certainty level starts at a pre-specified level (high certainty for randomized controlled trials). The detection of limitations in one or more of the five domains can lead to downgrading the certainty of evidence. The final judgment can be one of the following: high, moderate, low and very low. A summary of the certainty of evidence and a quantitative synthesis of effects for each outcome are shown in an evidence profile table (Supplemental file 4).
2.7. Evidence to decision (EtD) framework
The EtD framework provides a transparent and structured approach to support the decision-making process [12]. It allows evidence to be summarized in relation to prioritization of the problem, substantiality of desirable and undesirable effects, balance of the effects, certainty of evidence, patients values and preference, use of resources, equity, acceptability and feasibility.
2.8. Benefit/harm balance and clinical recommendation
During the face-to-face meeting held in Milan (December 2019), the panelists were asked to express their opinion on each of the domains of the EtD (priority of the problem, substantiality of desirable and undesirable effects, balance of the effects, certainty of evidence, patients values and preferences, equity, acceptability and feasibility).
At this point of the decision-making process, the panel voted on the relative balance between benefits and harms for the intervention (oncoplastic BCS) and its comparator (standard BCS) with the options of 7 categories: favors the comparison, possibly favors the comparison, does not favor either the comparison or the intervention, possibly favors the intervention, favors the intervention, varies, don’t know. In addition, the strength of the recommendation was evaluated with the option of 5 judgments: strong recommendation against the intervention, conditional recommendation against the intervention, conditional recommendation for either the comparison or the intervention, conditional recommendation for the intervention, strong recommendation for the intervention.
The AGREE-reporting checklist was followed to guide the reporting of the present recommendation [20].
3. Results
3.1. Search strategy results and details of the identified relevant studies
The literature search yielded 18,699 items after elimination of duplicate records. A total of 47 full-text articles were assessed for eligibility, amongst which 37 studies met pre-defined criteria with 29 studies included in meta-analyses [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]] (Fig. 1).
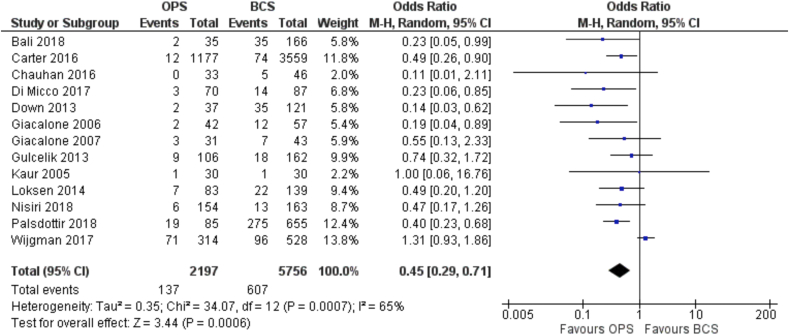
Fig. 1.
Forest Plot. Margin positivity.
A comprehensive search strategy was applied and for each of the other GRADE domains titles and abstracts were screened with the aim of identifying potentially eligible articles. Full texts for the latter were sought and results of systematic reviews in EtD are reported herein (Supplemental file 5).
3.2. Prioritization of outcomes
The panel deemed the following outcomes to be critical (mean value):
-
1.
Quality of life (8.40)
-
2.
Patient’s satisfaction with aesthetic outcome (8.14)
-
3.
Loco-regional recurrence (7.53)
-
4.
Re-excision rate (7.50)
-
5.
Conversion to mastectomy (7.50)
-
6.
Overall survival (7.07)
-
7.
Margin positivity rate (6.92)
-
8.
Disease-free survival (6.80)
-
9.
Depression (6.73)
-
10.
Surgical Complications (6.71)
3.3. Included studies
The analysis included studies conducted in the United States, the United Kingdom, Europe, South America, China, India, Canada, Israel and Iran with involvement of 193,833 patients in total and a mean of 6683 women per trial.
All studies included patients with invasive carcinoma or ductal carcinoma in situ (DCIS). These were managed with various techniques of oncoplastic breast-conserving surgery (level I (excision of less than 20% of breast volume without skin excision - parenchymal rearrangements) and level II (excision of 20–50% of breast volume - chest wall perforator flaps; therapeutic mammoplasties) [51] that were compared with standard BCS (wide local excision, lumpectomy, quadrantectomy).
Study design included prospective cohorts, case-control studies, cross-sectional studies, and database analyses.
The main features of primary studies are summarized in Supplemental file 6.
There were 7 studies [22,24,26,28,36,38,49] that included variable proportions of patients (range 2.1%–28.9%) undergoing neoadjuvant systemic treatments before any surgical intervention.
3.4. Effects of intervention
All results were presented in the Summary of Findings Table (Supplemental file 7).
3.4.1. Margin positivity
A total of 13 studies reported data on margin positivity rates [22,24,25,28,[30], [31], [32], [33],35,38,40,42,49] and these were all designed as observational studies. They included more than 2000 patients having oncoplastic BCS and almost 3 times as many (5756) patients with standard BCS. Positive margins were reported in 137/2197 (6.2%) patients in the oncoplastic BCS group and 607/5756 (10.5%) patients in the standard BCS group.
The OR for margin positivity was 0.45 (95% C.I. 0.29 to 0.71; I-squared 93%) in favor of oncoplastic surgery (Fig. 1), representing an absolute reduction of 55 fewer cases of positive margins per 1000 patients (from fewer 72 fewer to 28 fewer) (certainty of evidence = low).
3.4.2. Re-excision of positive margins
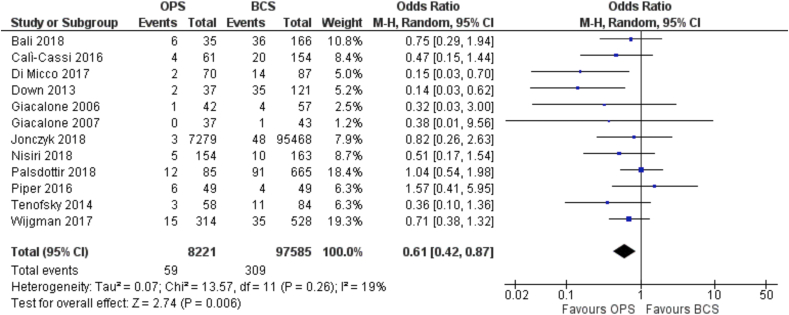
Similarly, a total of 12 studies reported data on rates of re-excision for positive margins rates [22,23,28,[30], [31], [32],34,40,42,43,48,49] and these were also observational studies. They included more than 8000 patients managed with oncoplastic BCS and almost 100,000 (97585) patients treated with standard BCS. This translates into an OR for margin re-excision of 0.61 (95% C.I. 0.42 to 0.87) in favor of the oncoplastic BCS group (Fig. 2), for which the absolute risk is 1 less case of re-excision per 1000 patients.
Fig. 2.
Forest Plot. Re-excision.
(level of certainty = very low).
3.4.3. Conversion to mastectomy
Rates of conversion to mastectomy were reported in 8 studies, all of which were observational [22,25,[29], [30], [31],38,43,49].
These included a total of 665 patients who underwent oncoplastic BCS and 1220 patients with standard BCS. Fewer than 10% of patients in both the oncoplastic (48/665; 7.2%) and standard (94/1220; 7.7%) groups required completion mastectomy for positive margins.
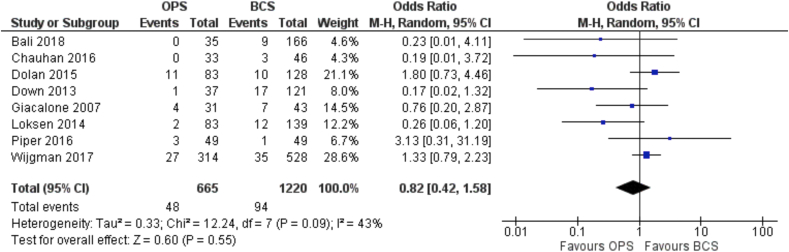
The OR for conversion to mastectomy for positive margins was 0.82 (95% C.I. 0.42 to 1.58) showing a not significant difference between the oncoplastic over standard BCS (Fig. 3). This represents an absolute reduction in conversion to mastectomy of 13 cases per 1000 patients (from 43 fewer to 39 more). (level of certainty = very low).
Fig. 3.
Forest Plot. Conversion to mastectomy.
3.4.4. Loco-regional recurrence
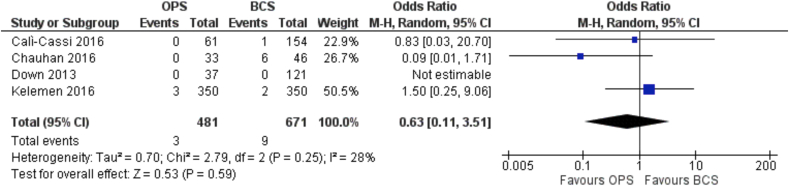
Only 4 studies included data on loco-regional recurrence rates [23,25,30,36] and once again were confined to all observational studies. Collectively, these studies involved a total of 504 patients who underwent an oncoplastic procedure and 723 patients treated with standard BCS. After a follow up period ranging from 18 to 76 months, rates of loco-regional recurrence were 1.2% for the oncoplastic BCS group (6/504) and 2.2% in the standard BCS group (16/723).
The OR for loco-regional recurrence was 0.63 (95% C.I. 0.11 to 3.51) showing a not significant difference between the two groups (Fig. 4). (level of certainty = very low).
Fig. 4.
Forest Plot. Locoregional recurrence.
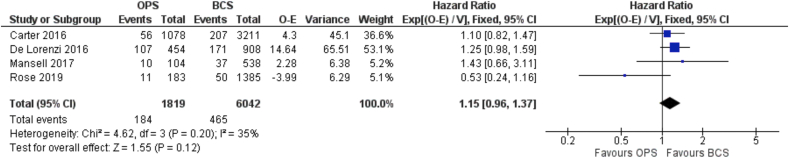
3.4.5. Disease-free survival
Disease-free survival data was available from 4 observational studies [24,27,39,44]. These included a total of 1864 patients who underwent oncoplastic BCS and 6256 patients treated with standard BCS. Any form of recurrence was documented in 10% of patients in the oncoplastic surgery group (187/1864) compared with 7.7% for standard BCS (482/6256). There was a broad range in duration of follow-up from 41 months to 86 months. The HR for recurrence was 1.15 (95% C.I. 0.96 to 1.37) (Fig. 5). This translates in the absence of significant difference between the two groups for this outcome. (level of certainty = very low).
Fig. 5.
Forest Plot. Disease-free survival.
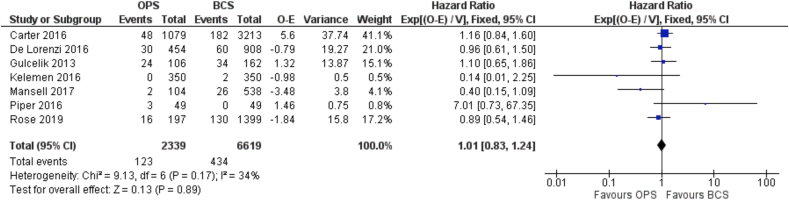
3.4.6. Overall survival
Overall survival data was available in 7 observational studies but these were observational and prone to confounding [24,27,33,36,39,43,44].
These included a total of 2384 patients who underwent an oncoplastic breast conserving surgery and 6833 patients with standard BCS. The proportion of deaths in these 2 surgical groups were similar: 5.3% (127/2384) for the oncoplastic group and 6.5% (446/6833) for the standard BCS group. Follow-up ranged from 41 months to 86 months. The HR for death was 1.01 (95% C.I. 0.83 to 1.24) (Fig. 6). This translates in the absence of significant difference between the two groups for this outcome (level of certainty = very low).
Fig. 6.
Forest Plot. Overall survival.
3.4.7. Quality of life
Information on quality of life (QoL) was available in 4 studies each of which were observational in design [28,45,47,49]. However, high levels of heterogeneity precluded any meaningful meta-analysis and therefore these were reported narratively.
Veiga and colleagues reported improvement in several QoL domains for oncoplastic BCS patients at 12 months including physical and psychological well-being, health perception, vitality, role-, emotional-, and social functioning [49]. Furthermore, these authors documented how much more positively patients viewed the future and demonstrated better mental health and self-esteem (assessed with the Short Form-36 and the Rosenberg-EPM Self-Esteem Scale) [52,53]. Nonetheless, there was omission of data on two out of the six QoL domains (body image and sexual functioning).
Di Micco and colleagues found that psychological functioning was not significantly worse in patients undergoing oncoplastic BCS [28], whilst improved psychological and sexual well-being was reported by Schechter and colleagues for oncoplastic patients [47]. Both these studies used the breast Q [54] tool and the latter authors found no significant differences for other BREAST-Q domains tested. Likewise Rose and colleagues reported significantly better psychosocial well-being for patients undergoing oncoplastic compared with standard BCS (OR 2.15; CI 1.25–3.69), with no differences for the domains of physical and sexual well-being. (level of certainty = low) [45].
3.4.8. Patient’s satisfaction with aesthetic outcome
Data was provided on patient satisfaction with aesthetic outcome in 7 studies all with an observational design [21,28,41,42,[46], [47], [48]]. As for QoL, there were high levels of heterogeneity between these studies, and meta-analyses of data was not possible on account of this. Acosta-Marin and colleagues assessed patient-reported aesthetic outcome using a scale from 1 (bad) to 5 (excellent) [21]. The average cosmetic score at 6 months for the oncoplastic BCS group was 4.4 compared with 4.2 for those having standard BCS. The final mean cosmetic scores reported by patients for these two groups at 12 months were 4.5 and 4.2 respectively.
Di Micco and colleagues evaluated patient-reported aesthetic outcome using BREAST Q and reported a mean score of 68 and 80 for oncoplastic and standard BCS groups respectively [28].
Schechter and colleagues also used BREAST-Q to assess patient reported aesthetic outcome and found mean scores of 75.18 (satisfaction with breast domain) for the oncoplastic group but a rather lower score of 39.64 for the standard BCS group [47].
Interestingly, using a scale from 1 (excellent) to 4 (unsatisfactory), Santos and colleagues reported a higher proportion of patients with excellent results in the standard group (69.2%) versus 61.4% in the oncoplastic group (total number of patients in each group 45 and 35 respectively) [46].
In contrast to the above studies, Tenofsky and colleagues acquired patient-reported aesthetic outcome data from the patient charts without use of a validated breast questionnaire and reported excellent results in 13.8% of patients having an oncoplastic procedure compared with only 7.1% for the standard BCS group [48].
Ojala and colleagues evaluated patient-reported aesthetic outcome using the Breast Cancer Treatment Outcome Scale (BCTOS) [55] and reported good outcomes in 61% of patients in the oncoplastic BCS group and 81% in the standard BCS group (p < 0.001) [41]. The mean BCTOS aesthetic score was worse after oncoplastic (1.62) than standard (1.84) BCS (p = 0.002). Moreover, oncoplastic resection appears to score worse for almost every aesthetics category using this instrument.
Plasdottir and colleagues employed a non-standardized scale to assess patient-reported aesthetic outcomes by asking patients directly whether they were happy with the appearance and shape of their breast(s) following surgery [42]. There were high levels of patient satisfaction rate with cosmetic outcomes for both surgical groups, although a slightly higher proportion of women in the oncoplastic group gave the responses “I agree very much” or “I agree for most parts” (no statistically significant difference).
(level of certainty = very low).
3.4.9. Depression
None of the studies included any data on post-operative depressive illness following either type of BCS.
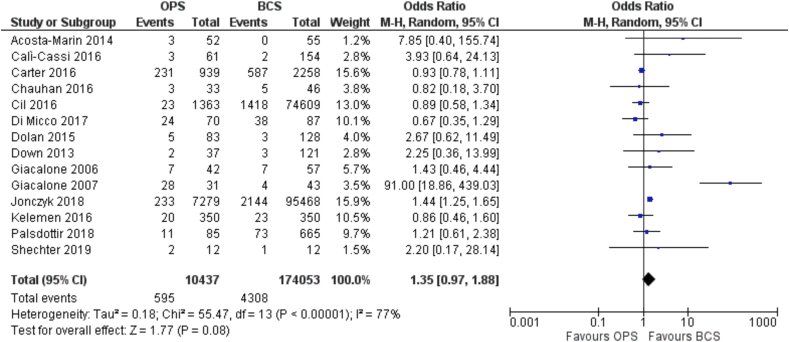
3.4.10. Surgical complications
There were 14 studies within this systematic review that reported data on surgical complications [21,[23], [24], [25], [26],[28], [29], [30], [31], [32],34,36,42,47] and these involved a total of 10437 patients treated with oncoplastic BCS and 174053 patients treated with standard BCS. The overall incidence of post-operative complications was 595/10437 (5.7%) for patients in the oncoplastic group and 4308/174053 (2.5%) for patients in the standard BCS group. These included infection, seroma, hematoma, non-healing wound, wound dehiscence, skin necrosis, pain, and abscess formation.
The OR for surgical complications was 1.35 (95% C.I. 0.97 to 1.88) (Fig. 7) representing a not significant. difference between the two groups for this outcome (level of certainty = very low).
Fig. 7.
Forest Plot. Surgical complications.
3.5. Values and preference, equity, acceptability, feasibility
A summary of evidence levels and panelist’s judgments are presented in EtD (Supplemental file 5).
3.6. Resource use and cost-effectiveness
Only one study on Complete Economic Evaluation (CEE) was identified during this systematic review and indicated an overall cost-effectiveness of large volume oncoplastic surgery when compared to standard lumpectomy [59]. The cost-effectiveness analysis is described in the attached document (Supplemental file 8).
Half the panelists (50%) considered the certainty of evidence for resources to support use of oncoplastic surgery to be very low. Although no studies compared resource requirements for the two surgical approaches in terms of direct and indirect costs, three-quarters (75%) of the panel considered these to be moderate, with oncoplastic BCS not incurring larger increments in procedural costs compared to standard BCS approaches. Nonetheless, it was recognized that some level II procedures demand longer operative times and correspondingly higher costs associated with theatre staffing and occupancy.
Just over one-third (37.5%) of the panel considered the cost-effectiveness of oncoplastic BCS probably favors whilst a quarter (25%) stated that it definitely favors the intervention.
3.7. Recommendation
In the light of summarized judgments for each domain in EtD, panelists were asked to comment on the recommendation for use of oncoplastic BCS for treatment of operable breast cancer that is suitable for breast conserving surgery.
Almost half (43%) of the panelists offered a conditional recommendation, whilst just over one-third (36%) a strong recommendation for the intervention (oncoplastic BCS). Notably one-fifth of panelists (21%) expressed a conditional recommendation for either the intervention or the comparator (standard BCS).
Based on this voting outcome, the following recommendation emerged as a consensus statement: Oncoplastic breast conserving surgery should be recommended versus standard breast conserving surgery for the treatment of operable breast cancer in adult women who are suitable candidates for breast conserving surgery (with very low certainty of evidence).
4. Discussion
The current evidence base for oncoplastic BCS derives mainly from observational studies and single-center case-series with few studies directly comparing oncoplastic to standard BCS in matched cohorts of patients. Despite low levels of evidence for most studies, there is an emergent theme that some of the more obvious and anticipated benefits of oncoplastic BCS are not supported by the evidence with this meta-analysis failing to yield odds ratios significantly in favor of oncoplastic BCS. Moreover, critical outcomes relating to QoL have not been investigated in the majority of studies and available data is often based on assessment with non-standardized scales or inappropriate use of standardized scales. More than three-quarters (79%) of panelists offered a conditional or strong recommendation in favor of adopting oncoplastic techniques versus standard BCS for surgical management of adult women with operable breast cancer who are otherwise suitable candidates for breast conserving surgery (without any clear indication for mastectomy).
According to this systematic review of the literature, oncoplastic BCS is associated with both a significant reduction in rates of positive margins (with a low certainty of evidence) and re-excision (with a very low certainty of evidence) when compared to standard forms of BCS that generally involve resection of smaller volumes of tissue.
However, other potentially desirable effects of oncoplastic BCS are not apparent from results of this meta-analysis comparing the two surgical approaches of oncoplastic with standard BCS.
This meta-analysis provides tentative support for the intuitive assumption that rates of loco-regional recurrence are lower for oncoplastic BCS that tends to be associated with wider margins of resection. However, local recurrence is determined by multiple factors including tumour size and oncoplastic procedures may be biassed towards larger tumours. This benefit for oncoplastic versus standard BCS was not significant with a very low level of certainty. Patients undergoing oncoplastic BCS had slightly worse disease-free and overall survival, although once again there was a very low level of certainty for this outcome and hazard ratios were close to unity.
This meta-analysis did not include the key parameters QoL and patient-reported aesthetic outcomes as few studies contained data on these outcomes and methods of assessment were highly heterogeneous.
Notwithstanding these shortcomings, more than half (54%) of panelists anticipated that the desirable effects would be favourably affected by oncoplastic BCS.
Surgical complications were considered to be the only undesirable effect of oncoplastic versus standard breast conserving approaches. Oncoplastic surgery was associated with a slight (non-significant) increase in rates of complications but the certainty of evidence was very low. Just over one-third of the panel expressed the opinion that any undesirable anticipated effects were small.
A possible reason why more than three-quarters of panelists offered a conditional or strong recommendation in favor of adopting oncoplastic techniques versus standard BCS for surgical management of adult women with operable breast cancer who are otherwise suitable candidates for breast conserving surgery even though no strong evidence was available in literature for most of the highly prioritized outcomes is that they strongly relied on the significant impact of oncoplastic techniques on the reduction of positive margins and excision rates.
4.1. Limitations of the study
One of the limitations of the study relates to the composition of the panel that include some surgeons who did not perform oncoplastic procedures and may therefore have been intrinsically biased against this approach.
Not all panelists engaged in the feedback process, both in terms of prioritization of outcomes phase and the pre-voting session.
Although all members of the panel considered oncoplastic BCS to be a research priority, some breast cancer specialists are skeptical as to whether this needs formal evidence-based validation; it could be viewed as simply a variant form of breast conserving surgery that has already been validated in randomized controlled trials [2,3].
Other limitations relate to a paucity of high quality publications that either fail to address key outcomes or include poorly designed studies with much heterogeneity or sub-standard methodology for assessment of outcomes.
The panel prioritized outcomes in the preliminary phase of the GRADE process and identified quality-of-life and patient-reported aesthetic outcome as the two most relevant outcomes for oncoplastic BCS, although ironically these outcomes were excluded from most studies comparing oncoplastic and standard BCS. Only three studies assessed QoL, with one of these using standard measurement tools inappropriately by not applying all the domains. Amongst those studies assessing patient-reported aesthetic outcome, only half used standardized tools with others using non-validated methods.
Complications have likewise often been assessed without use of standardized tools with much variation in definition and assessment of surgical complications across different studies. Currently available tools for assessment of complications should be refined and adapted.
Another limitation of this meta-analysis relates to recent consensus on definition of a negative margin. A minimum margin of clearance (no ink on tumor for invasive carcinoma and 2 mm for DCIS) is now acceptable following wide excision of both invasive and non-invasive disease [60,61]. Less stringent margin policies have arguably detracted from the benefit of oncoplastic surgery in terms of both rates of margin positivity and re-excision (although rates of re-excision are very low in both cohorts). The apparent adverse effect of oncoplastic surgery on disease-free and overall survival, may reflect the poor quality of published studies to-date with introduction of substantial bias and confounding variables (no information is available on biology and stage of cancers in the two cohorts of oncoplastic and standard surgery).
4.2. Subgroup considerations
Data available in the literature did not allow any subgroup analysis for level I versus level II oncoplastic procedures. Similarly, data could not be extracted on patients with locally advanced breast cancer managed with primary systemic treatments prior to either oncoplastic or standard BCS. Also it was not possible to stratify results based on age categories (<40, 41–65, >65 years of age).
4.3. Research priorities
The generation of robust evidence is challenging for surgery and limited by standardization of techniques and tailored approaches to treatment.
Paramount amongst the knowledge gaps in breast cancer research and treatment is the need to carefully evaluate the effectiveness of oncoplastic BCS which represents an escalation of surgical complexity. There are areas of controversy to be resolved, especially relating to complications, cost-effectiveness and patient reported outcomes. The UK Association of Breast Surgery have analyzed key research gaps [62], whilst a European consensus conference revealed significant variation in several aspects of oncoplastic practice [63]. The Oncoplastic Breast Consortium (OPBC) [64] performed a structured study following a Delphi process [65] with the aim of prioritizing knowledge gaps and defining optimal methodological strategies to address these.
The OPBC consensus panel recommended a prospective multi-center cohort study with propensity score matching and satisfaction with breast (assessed with BREAST-Q) as the primary endpoint to address the impact of oncoplastic surgery on QoL. Similar study design with alternative endpoints was also recommended for evaluating complications, return to the operating room and delays in commencement of adjuvant therapies.
4.4. Monitoring and evaluation
Outcomes in breast oncoplastic surgery should be evaluated using standard tools. Those currently available should be refined and adapted.
4.5. Implementation considerations
Lack of access to well-structured training in oncoplastic breast surgery was identified as a principal barrier to more widespread adoption of these techniques across the world. Dedicated training programs should be established by postgraduate medical education systems under the aegis of professional associations allied to breast cancer management.
5. Conclusions
The GRADE system was employed to analyze published data and seek support for oncoplastic BCS as the new “state of the art” surgical approach to early stage breast cancer compared to standard BCS. A panel of world-renowned breast specialists was convened to evaluate evidence, express personal viewpoints and establish recommendations.
This review has revealed a low level of evidence for most of the important outcomes in oncoplastic surgery with lack of any randomized data and absence of standard tools for evaluation of clinical outcomes and especially patients’ values.
There is substantial uncertainty in the balance between beneficial and adverse effects of oncoplastic versus standard BCS and no formal assessment of patients’ values and preferences has been performed. This may lead to surgical over-treatment in some cases when patients are less interested in potential benefits in terms of margin status and re-excision. Others may specifically wish to avoid disruption of an otherwise normal contralateral breast.
The outcome rated as most important by the panelists in the prioritization process was QoL that has been sparsely investigated to-date; data on cosmetic outcomes is heterogeneous with assessment based on either subjective or objective methodologies. There is an urgent need for appropriate and validated tools that can routinely be applied in daily practice.
Poor access to accredited training programs hampers more widespread implementation of a comprehensive oncoplastic service, although these techniques are unlikely to impact equity of health systems.
Despite areas of controversy, about one-third (36%) of panel members expressed a strong recommendation in support of oncoplastic BCS. Presumably, this reflects a synthesis of views on the relative complexity of these techniques, associated complications, impact on quality of life and costs. Patients should be informed of the following recommendation before consenting to oncoplastic procedures for breast conserving surgery:
“Oncoplastic Breast Conserving surgery should be recommended versus standard breast conserving surgery for the treatment of operable breast cancer in adult women who are suitable candidates for breast conserving surgery (with very low certainty of evidence)”.
Authors’ contribution
Our manuscript lists more than 8 authors.
All listed authors made substantial contributions to conception and design of the study and/or acquisition of data and/or analysis and interpretation of data.
All listed authors participated in drafting the article and/or revised it critically for important intellectual content.
All listed authors gave final approval of the version to be published.
Financial disclosures
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Funding source
No fundings have been received for the preparation of this manuscript.
Ethical approval
Ethical approval was not requested for this study.
Declaration of competing interest
None of the authors have conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.02.013.
Contributor Information
Nicola Rocco, Email: nicola.rocco@greta-oncoplastic.com, nicolarocco2003@gmail.com.
Nahid Nafissi, Email: nahid.nafissi@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World health organization. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ Accessed September 2020.
- 2.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Arndt V., Stegmaier C., Ziegler H., Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. J Canc Res Clin Oncol. 2008;134:1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PubMed] [Google Scholar]
- 5.Jagsi R., Li Y., Morrow M., Janz N., Alderman A., Graf J., Hamilton A., Katz S., Hawley S. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction. Ann Surg. 2015;261:1198–1206. doi: 10.1097/SLA.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clough K.B., Cuminet J., Fitoussi A., Nos C., Mosseri V. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg. 1998;41:471–481. doi: 10.1097/00000637-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane R.A., Valasiadou P., Wilson A.R.M., Al-Ghazal S.K., Macmillan R.D. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90:1505–1509. doi: 10.1002/bjs.4344. [DOI] [PubMed] [Google Scholar]
- 8.Clough K.B., Kroll S.S., Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999;104(2):409–420. doi: 10.1097/00006534-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Macmillan R.D., McCulley S.J. Oncoplastic breast surgery: what, when and for whom? Curr Breast Cancer Rep. 2016;8:112–117. doi: 10.1007/s12609-016-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audretsch W. Commentary on: the oncoplastic reduction approach to breast conservation therapy: benefits for margin control. Aesthetic Surg J. 2014;34(8) doi: 10.1177/1090820X14546892. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Huang Y., Zhang L., Yang C., Wang K. Comparison of oncoplastic breast-conserving surgery and breast-conserving surgery alone: a meta-analysis. J Breast Cancer. 2018;21(3):321–329. doi: 10.4048/jbc.2018.21.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oncoplastic breast meeting. https://oncoplasticbc.org/documents/mbn-2019-programme.pdf
- 14.St gallen international breast cancer conference. https://www.oncoconferences.ch/events/bcc-2021
- 15.San Antonio breast cancer Symposium. https://www.sabcs.org
- 16.European society of surgical Oncology. https://www.essoweb.org
- 17.GRADEpro. www.gradepro.org
- 18.Group for reconstructive and therapeutic Advancements (G.Re.T.A) https://greta.maurizionava.it
- 19.Bernard lo and marilyn J field. Institute of medicine (US) committee on conflict of interest in medical research, education, and practice. National Academies Press (US); Washington (DC): 2009. Conflict of interest in medical research, education, and practice. -13: 978-0-309-13188-9. [PubMed] [Google Scholar]
- 20.Brouwers M.C., Kerkvliet K., Spithoff K. On behalf of the AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Marin V., Acosta-Freites V., Contreras A. Oncoplastic breast surgery: initial experience at the Centro Clinico de Estereotaxia-CECLINES, Caracas, Venezuela. Ecancermedicalscience. 2014;8:470. doi: 10.3332/ecancer.2014.470. Published 2014 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bali R., Kankam H.K.N., Borkar N., Provenzano E., Agrawal A. Wide local excision versus oncoplastic breast surgery: differences in surgical outcome for an assumed margin (0, 1, or 2 mm) distance. Clin Breast Canc. 2018;18(5):e1053–e1057. doi: 10.1016/j.clbc.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Calì Cassi L., Vanni G., Petrella G. Comparative study of oncoplastic versus non-oncoplastic breast conserving surgery in a group of 211 breast cancer patients. Eur Rev Med Pharmacol Sci. 2016;20(14):2950–2954. [PubMed] [Google Scholar]
- 24.Carter S.A., Lyons G.R., Kuerer H.M. Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol. 2016;23(10):3190–3198. doi: 10.1245/s10434-016-5407-9. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan A., Sharma M.M. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Med J Armed Forces India. 2016;72(1):12–18. doi: 10.1016/j.mjafi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cil T.D., Cordeiro E. Complications of oncoplastic breast surgery involving soft tissue transfer versus breast-conserving surgery: an analysis of the NSQIP database. Ann Surg Oncol. 2016;23(10):3266–3271. doi: 10.1245/s10434-016-5477-8. [DOI] [PubMed] [Google Scholar]
- 27.De Lorenzi F., Hubner G., Rotmensz N., Bagnardi V., Loschi P., Maisonneuve P., Venturino M., Orecchia R., Galimberti V., Veronesi P. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. EJSO. 2016;42:71–77. doi: 10.1016/j.ejso.2015.08.160. [DOI] [PubMed] [Google Scholar]
- 28.Di Micco R., O’Connell R.L., Barry P.A., Roche N., MacNeill F.A., Rusby J.E. Standard wide local excision or bilateral reduction mammoplasty in large-breasted women with small tumours: surgical and patient-reported outcomes. Eur J Surg Oncol. 2017;43(4):636–641. doi: 10.1016/j.ejso.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Dolan R., Patel M., Weiler-Mithoff E. Imaging results following oncoplastic and standard breast conserving surgery. Breast Care. 2015;10(5):325–329. doi: 10.1159/000437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Down S.K., Jha P.K., Burger A., Hussien M.I. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J. 2013;19(1):56–63. doi: 10.1111/tbj.12047. [DOI] [PubMed] [Google Scholar]
- 31.Giacalone P.L., Roger P., Dubon O. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2007;14(2):605–614. doi: 10.1245/s10434-006-9098-5. [DOI] [PubMed] [Google Scholar]
- 32.Giacalone P.L., Roger P., Dubon O., El Gareh N., Daurés J.P., Laffargue F. Traitement conservateur des cancers du sein: zonectomie vs oncoplastie. Etude prospective à propos de 99 patientes [Lumpectomy vs oncoplastic surgery for breast-conserving therapy of cancer. A prospective study about 99 patients] Ann Chir. 2006;131(4):256–261. doi: 10.1016/j.anchir.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Gulcelik M.A., Dogan L., Yuksel M., Camlibel M., Ozaslan C., Reis E. Comparison of outcomes of standard and oncoplastic breast-conserving surgery. J Breast Cancer. 2013;16(2):193–197. doi: 10.4048/jbc.2013.16.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonczyk M.M., Jean J., Graham R., Chatterjee A. Trending towards safer breast cancer surgeries? Examining acute complication rates from A 13-year NSQIP analysis. Cancers. 2019;11(2):253. doi: 10.3390/cancers11020253. Published 2019 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur N., Petit J.Y., Rietjens M. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2005;12(7):539–545. doi: 10.1245/ASO.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 36.Kelemen P., Pukancsik D., Újhelyi M. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: a single-centre retrospective study. Eur J Surg Oncol. 2019;45(2):118–124. doi: 10.1016/j.ejso.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 37.De Lorenzi F., Hubner G., Rotmensz N. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77. doi: 10.1016/j.ejso.2015.08.160. [DOI] [PubMed] [Google Scholar]
- 38.Losken A., Pinell-White X., Hart A.M., Freitas A.M., Carlson G.W., Styblo T.M. The oncoplastic reduction approach to breast conservation therapy: benefits for margin control. Aesthetic Surg J. 2014;34(8):1185–1191. doi: 10.1177/1090820X14545618. [DOI] [PubMed] [Google Scholar]
- 39.Mansell J., Weiler-Mithoff E., Stallard S., Doughty J.C., Mallon E., Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast. 2017;32:179–185. doi: 10.1016/j.breast.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Nisiri A., Pour R.O., Zadeh H.M., Ramim T. Comparison of surgical margin after breast cancer surgery between oncoplastic technique and conventional breast-conserving surgery. Int J Cancer Manag. 2018;11(4) [Google Scholar]
- 41.Ojala K., Meretoja T.J., Leidenius M.H. Aesthetic and functional outcome after breast conserving surgery - comparison between conventional and oncoplastic resection. Eur J Surg Oncol. 2017;43(4):658–664. doi: 10.1016/j.ejso.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Palsdottir E.P., Lund S.H.L., Asgeirsson K.S.A. Oncoplastic breast-conserving surgery in Iceland: a population-based study. Scand J Surg. 2018;107(3):224–229. doi: 10.1177/1457496918766686. [DOI] [PubMed] [Google Scholar]
- 43.Piper M., Peled A.W., Sbitany H., Foster R.D., Esserman L.J., Price E.R. Comparison of mammographic Findings following oncoplastic mammoplasty and lumpectomy without reconstruction. Ann Surg Oncol. 2016;23(1):65–71. doi: 10.1245/s10434-015-4611-3. [DOI] [PubMed] [Google Scholar]
- 44.Rose M., Svensson H., Handler J., Hoyer U., Ringberg A., Manjer J. Oncoplastic breast surgery compared to conventional breast-conserving surgery with regard to oncologic outcome. Clin Breast Canc. 2019;19(6) doi: 10.1016/j.clbc.2019.05.016. 423-432.e5. [DOI] [PubMed] [Google Scholar]
- 45.Rose M., Svensson H., Handler J., Hoyer U., Ringberg A., Manjer J. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Canc Res Treat. 2020;180(1):247–256. doi: 10.1007/s10549-020-05544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos G., Urban C., Edelweiss M.I. Long-term comparison of aesthetical outcomes after oncoplastic surgery and lumpectomy in breast cancer patients. Ann Surg Oncol. 2015;22(8):2500–2508. doi: 10.1245/s10434-014-4301-6. [DOI] [PubMed] [Google Scholar]
- 47.Shechter S., Friedman O., Inbal A. Oncoplastic partial breast reconstruction improves patient satisfaction and aesthetic outcome for central breast tumours. ANZ J Surg. 2019;89(5):536–540. doi: 10.1111/ans.15078. [DOI] [PubMed] [Google Scholar]
- 48.Tenofsky P.L., Dowell P., Topalovski T., Helmer S.D. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg. 2014;207(3):398–402. doi: 10.1016/j.amjsurg.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Veiga D., Veiga-Filho J., Ribeiro L.M., Archangelo-Arcangelo-Junior I. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg. 2010;125(3):811–817. doi: 10.1097/PRS.0b013e3181ccdac5. [DOI] [PubMed] [Google Scholar]
- 50.Wijgman D.J., Ten Wolde B., van Groesen N.R., Keemers-Gels M.E., van den Wildenberg F.J., Strobbe L.J. Short term safety of oncoplastic breast conserving surgery for larger tumors. Eur J Surg Oncol. 2017;43(4):665–671. doi: 10.1016/j.ejso.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Clough K.B., Kaufman G.J., Nos C., Buccimazza I., Sarfati I.M. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol. 2010 May;17(5):1375–1391. doi: 10.1245/s10434-009-0792-y. [DOI] [PubMed] [Google Scholar]
- 52.RAND health care SF-36. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
- 53.Rosenberg M. Princeton University Press; Princeton, NJ: 1965. Society and the adolescent self-image. [Google Scholar]
- 54.Pusic A.L., Klassen A.F., Scott A.M., Klok J.A., Cordeiro P.G., Cano S.J. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 55.Stanton A.L., Krishnan L., Collins C.A. Form or function? Part 1. subjective cosmetic andfunctional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91:2273–2281. [PubMed] [Google Scholar]
- 59.Chatterjee A., Offodile A.C., II, Asban A., Minasian R.A., Losken A., Graham R., Chen L., Czerniecki B.J., Fisher C. A cost-utility analysis comparing oncoplastic breast surgery to standard lumpectomy in large breasted women. Adv Breast Canc Res. 2018;7(2) [Google Scholar]
- 60.Moran M.S., Schnitt S.J., Giuliano A.E. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553–564. doi: 10.1016/j.ijrobp.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrow M., Van Zee K.J., Solin L.J. Society of surgical Oncology-American society for radiation Oncology-American society of clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23(12):3801–3810. doi: 10.1245/s10434-016-5449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cutress R.I., McIntosh S., Potter S. Opportunities and priorities for breast surgical research. Lancet Oncol. 2018;19(10):e521–e533. doi: 10.1016/S1470-2045(18)30511-4. [DOI] [PubMed] [Google Scholar]
- 63.Weber W.P., Soysal S.D., El-Tamer M. First International Consensus Conference on standardization of oncoplastic breast conserving surgery. Breast Canc Res Treat. 2017;165(1):139–149. doi: 10.1007/s10549-017-4314-5. [DOI] [PubMed] [Google Scholar]
- 64.Oncoplastic breast Consortium. www.oncoplasticbc.org
- 65.Weber W.P., Morrow M., Boniface J., Pusic A., Montagna G., Kappos E.A., Ritter M., Haug M., Kurzeder C., Saccilotto R., Schulz A., Benson J., Fitzal F., Matrai Z., Shaw J., Peeters M.V., Potter S., Heil J. Oncoplastic Breast Consortium.Knowledge gaps in oncoplastic breast surgery. Lancet Oncol. 2020 Aug;21(8):e375–e385. doi: 10.1016/S1470-2045(20)30084-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.