Abstract
Introduction:
Plasmodium vivax causes significant public health problems in endemic regions. A vaccine to prevent disease is critical, considering the rapid spread of drug-resistant parasite strains, and the development of hypnozoites in the liver with potential for relapse. A minimally effective vaccine should prevent disease and transmission while an ideal vaccine provides sterile immunity.
Areas covered:
Despite decades of research, the complex life cycle, technical challenges and a lack of funding have hampered progress of P. vivax vaccine development. Here, we review the progress of potential P. vivax vaccine candidates from different stages of the parasite life cycle. We also highlight the challenges and important strategies for rational vaccine design. These factors can significantly increase immune effector mechanisms and improve the protective efficacy of these candidates in clinical trials to generate sustained protection over longer periods of time.
Expert opinion:
A vaccine that presents functionally-conserved epitopes from multiple antigens from various stages of the parasite life cycle is key to induce broadly neutralizing strain-transcending protective immunity to effectively disrupt parasite development and transmission.
Keywords: clinical trials, heterologous prime/boost immunizations, viral vectors, virus-like particles, vivax vaccines
1. Introduction
The development and implementation of a vaccine to eradicate smallpox triggered a renaissance in vaccine research. This resulted in one of the most economical global health interventions that led to a number of licensed vaccines in the 21st century. Despite the successes of vaccines, 20% of children especially from low- and middle-income countries do not complete the scheduled immunizations in their first year [1].
Malaria is a public health problem and a major burden to socioeconomic development in many developing countries of the world. In 2018, the WHO reported an estimated 228 million clinical cases of malaria leading to 405,000 deaths worldwide. Of these cases, 85% occur in sub-Saharan Africa and Southeast Asia. Plasmodium falciparum and Plasmodium vivax are the main causative parasites for malaria [2]. Although, P. vivax malaria is a significant public health burden in many regions, much of the associated mortality is due to P. falciparum infections in non-immune persons, especially children under five years and pregnant women of sub-Saharan Africa [2].
Plasmodium vivax has the greatest geographic distribution, accounting for 12.4% of malaria in Africa and >70% in Asia and the Americas [3–5]. P. vivax causes an estimated 14.3 million malaria episodes each year and is the leading cause of malaria in Asia and Latin America [6,7]. However, there is increasing evidence that the severity of P. vivax is underestimated as it can be non-life-threatening and self-limiting [3]. Also, endemic countries often lack broad access to affordable and accessible healthcare, intensifying the impact of this disease among poorer communities. Vivax malaria incapacitates individuals of all ages from repeated febrile episodes and severe anemia, while recurrent infections can lead to life-long impairment and increased risk for pregnant women [8]. As reports of clinical severity and lethal cases of P. vivax infections increase [9–14], together with wide-spread drug resistance [15–20] and relapse infections, the development of an effective antimalarial vaccine is considered an essential part of the overall control strategy to preventing disease [21].
A change in the disease pattern in a population often results from an epidemiological shift. Several factors that may govern this shift are age, number of immunizations, number of vaccinated individuals, different disease serotypes and immunizations for at risk individuals [22,23]. In most vivax malaria endemic areas, transmission is intermittent and acquired immunity is short-lived and biased towards being strain-specific [24,25]. Recent reports indicate that this may be further compounded by P. vivax infections and disease in Duffy-negative individuals [26–28], previously thought to be resistant. Thus, there is legitimate and increasing concern that P. vivax may adapt to or be present in populations previously considered resistant and greatly and the actual global burden is much higher than currently predicted. All these factors emphasize the need for improving public health and therapeutic strategies including the development of a vaccine against this disease.
Like vaccines against other microbial pathogens, an effective vivax vaccine should provide long-term, broadly-neutralizing, strain-transcending immunity, that eliminates clinical disease and disrupts transmission. Failure of a vaccine against an infectious disease to generate such broadly-neutralizing antibodies will result in re-infection and continued disease transmission within the affected population, as occurred in about 10% of vaccinees’ that receive the MMR vaccine [29]. Sometimes, despite the adequate levels of protective antibodies generated after vaccination, these antibodies do not persist over a long period of time resulting in disease. To circumvent this, booster doses are essential to maintain the level of protective antibodies to prevent vaccine failure.
Development of herd immunity should lead to resistance to the spread of an infectious agent amongst communities and eventually lead to disease elimination. This type of immunity can develop after infection, resulting in potentially significant morbidity and mortality, or at minimal risk in individuals who receive a vaccine such as oral polio vaccine (OPV) [30]. Scientific studies can help in the immunological and disease surveillance to generate epidemiological models for defining the threshold for herd immunity [31]. For example, cross-sectional and longitudinal studies can examine the antibodies and activated T- cells against the infectious agent. Based on the successes of vaccines that have eliminated other infectious diseases, the past decade has brought fresh impetus to the fight against malaria, driven by a growing appreciation of the humanitarian and economic magnitude of the problem and access to new funding sources.
1.1. Malaria Life Cycle
Plasmodium vivax has a complex life cycle with multiple stages of cellular differentiation and host cell types that require transmission by an anopheline mosquito. Infection begins with inoculation of sporozoites into the host’s skin by an infected mosquito during a blood meal, these then travel to the liver. In the liver, sporozoites traverse then invade and develop in hepatocytes into thousands of merozoites, which are released into the blood stream upon hepatocyte rupture. However, some parasites arrest development in hepatocytes to remain temporarily dormant (hypnozoites), enabling multiple sequential clinical relapses, termed relapse infections, and potential transmission from a single infection. Released into the blood, merozoites infect reticulocytes to initiate cyclical asexual development from rings through trophozoites to merozoites within 48 hrs. Upon maturation, the infected reticulocyte ruptures to release into the blood between 8–32 new blood-stage merozoites to re-initiate the asexual cycle [32]. Consequently, toxins released with this cycle of development lead to a tertian pattern of repeating fever, the paroxysm, characteristic of vivax malaria. Importantly, some merozoites differentiate into sexual erythrocytic stages (gametocytes), even in the first asexual generation of P. vivax coming out of the liver, leading to immediate mosquito transmission. In the mosquito, the parasite undergoes sexual reproduction to produce sporozoites, which find their way into the mosquito salivary glands where they become infective and subsequently injected into a new host during a blood meal. Given the complex nature of the parasite’s life cycle and the inadequate ability to prevent relapse infections and transmission, vaccine development should be an integral part of the overall strategy for malaria control [33–35].
1.2. Immunity to Plasmodium vivax infection
The development of a robust and persistent clinical immunity in some residents of endemic regions strongly supports the potential of an effective vaccine. Typically, acquired immunity to P. vivax blood-stage invasion ligands play a critical role in controlling blood-stage infection and disease. Studies of these ligands have revealed targets of naturally-acquired immunity (NAI) in individuals exposed to vivax infections. This immunity can trigger a robust protective immune response that inhibits sporozoite or merozoite invasion of host cells and protects against clinical disease [36]. Such targets are considered potential vaccine targets.
In endemic regions, the capacity of individuals to develop an adaptive immunity against Plasmodium infection and disease increases with age, prior exposure and transmission intensity [37]. The quality and longevity of this adaptive response is highly variable, with some individuals acquiring long-term protection following a limited number of exposures, whereas in other cases repeated exposure is needed to generate and sustain protective immunity.
In vivax malaria, the development of NAI is achieved by, exposure to both primary blood-stage and relapse infections that increases with age due to the booster effect by repeated infection [38,39]. NAI responses to P. vivax target both pre-erythrocytic and blood-stage antigens and include humoral and cellular components, although most studies have focused on very few candidates (Table 1).
Table 1:
(a) An overview of selected promising Plasmodium vivax vaccine candidates that have progressed into clinical trials with their correlates of protection and vaccine outcomes
| Candidate Vaccine/platform | Host (Clinical & preclinical studies) | Correlates of protection | Outcome | Progress |
|---|---|---|---|---|
| Blood-stage candidates | ||||
| PvDBPII | Phase 1a (UK adults) NCT01816113 | Antibodies against PvDBPII | No serious adverse events. Across all vaccinated individuals median polyclonal IgG EC50 values were comparable in PvDBPII-DARC in vitro binding. | Completed [1] |
| PvDBPII/GLA-SE | Phase 1 (34 Indian males) CTRI/2016/09/007289 |
PvDBPII-specific antibodies | No adverse events were observed. 50μg treatment, 82% mean binding-inhibitory activity was observed in PvDBPII-DARC binding assay at day 180 |
Completed by PATH/MVI [2] |
| Pre-erythrocytic candidates | ||||
| P. vivax irradiated sporozoites | Phase I/IIa (Colombia, adults) NCT01082341 |
Anti- PvCSP IgG1 levels correlates with protection | Partial protection observed in 42% of the Fy+ volunteers. | MVDDC, NHLBI/NIH [3] |
| PvCSP- derived long synthetic peptides, Allohydrogel Montanide ISA 720 51 | Phase 1b (Colombia adults) NCT01081847 | Antibody responses | Transient pain at injection site and induration occurred in the Montanide 50 μg group, PBMCs from all groups secreted IL-5 and IFNγ. | Completed by MVDDC [4] |
| VMP001-AS01B | Phase I/IIa (USA, adults) NCT01157897 |
Antibody titers | Significant delay in infection patency observed in 59% of vaccinated subjects. | Ongoing studies by WRAIR, MVI, GSK [5] |
| Sexual-stage candidates | ||||
| Pvs25- Recombinant Pvs25, Montanide ISA51 | Phase I (USA, adults) NCT00295581 |
Antibody responses correlated with transmission blocking activity | High reactogenicity observed | Completed by DIR/NIAID, JHSPH [6] |
PvCSP- Plasmodium vivax circumsporozoite protein
PvDBPII- P. vivax Duffy Binding protein region II
PvDBPII/GLA-SE- recombinant P. vivax DBP region II formulated with glucopyranosyl lipid adjuvant-stable emulsion
DARC- Duffy antigen receptor for chemokines
VMP001- Vivax malaria protein 001
PBMC- Peripheral blood mononuclear cells
cPvMSP1- Chimeric Plasmodium vivax Merozoite surface protein 1
Pvs25- Ookinete surface protein
LLPCs- Long-lived plasma cells
Generally, NAI does not lead to sterile immunity, but decreases parasite densities to reduce the frequency and severity of clinical disease. In areas of high transmission intensity, such as Papua New Guinea, NAI to P. vivax becomes prevalent early in life with suppressed parasitemia leading to only few or complete absence of clinical disease in older children and adults [40], compared to P. falciparum [41]. On the other hand, in areas of low parasite transmission that are more common for vivax malaria, adults experience disease due to lack of development of robust clinical immunity during childhood. In some low transmission regions, (Amazon Basin or South Pacific), it is common to find individuals with asymptomatic parasitemia [42,43], suggesting that NAI can also occur with relatively few infections although, some host genetic factors (e.g. Fya allele), may also play a protective role against clinical disease [44,45]. These data support the possibility of a successful P. vivax vaccine in areas of little or no parasite diversity.
1.3. Limitations to vivax vaccine development
P. vivax is classified as a neglected tropical disease, especially in terms of species-specific therapies for vaccine development and anti-relapse therapies. Compared to P. falciparum, there has been no coherent vaccine program for P. vivax. This process has been hampered by the lack of a continuous culture system for blood-stages and restricted availability of ideal animal models to study parasite biology. Secondly, the preferential infection of reticulocytes, which account for only 1–2% of total RBCs in peripheral blood, severely hinders the potential to support P. vivax ex vivo studies. Hence, many studies are limited by available access to fresh parasites from infected patients and facilities to support non-human primate (NHP) infections. Unfortunately, these NHP-adapted lines do not adequately reflect the genetic diversity of human infections [46]. This, has restricted studying the parasite’s biology and the identification of new candidates and their evaluation [47,48]. To support vivax research, most studies have relied on surrogate functional assays to study the potential invasion inhibitory effects of antibodies against P. vivax invasion ligands [41,49–53], define the structural determinants for receptor recognition [54] and the epitope targets for immune antibody neutralization [25,55].
Progress towards developing a robust long-term P. vivax culture method remains limited [47,50,56] and instead advances in in vitro culture of P. knowlesi in human RBCs, a closely related zoonotic species that naturally infects macaques, have provided an important support for more advanced laboratory studies [57] [58] opening the door to some experimental functional studies of P. vivax.
Altogether these limitations represent a major hurdle for testing vaccine efficacy and hinder the progress of P. vivax vaccine development. Controlled human malaria infections (CHMI) have been widely utilized for studying vaccine efficacy during the development of P. falciparum vaccine candidates [59–63]. However, CHMIs for P. vivax are more recent due to logistical challenges and the possibility of a relapse infection. Different groups in Australia, Colombia, and the USA have been working towards successfully developing blood-stage [64] and sporozoite (including mosquito-bite) P. vivax CHMIs [65–69]. McCarthy et al., established a parasite blood bank from an infected volunteer to overcome logistical challenges of a mosquito-bite CHMI [64]. Their team in Australia developed this infected blood-stage malaria (IBSM) inoculum as an alternative strategy to test vaccine efficacy [64,70]. Nonetheless, even as these models provide critical opportunities for testing vaccine immunogenicity and efficacy in experimentally controlled settings for early phase clinical trials prior to more costly larger-scale clinical studies, it is important to note that CHMI immunity may not fully replicate protective responses of NAI.
Genetic diversity is also an important consideration for vivax vaccine development as the genetic diversity observed in P. vivax is greater than in P. falciparum [71–73]. This may be a challenge if immunity is biased towards immunodominant variant epitopes, leading to strain-specific protective immune responses [24,55,74]. However, our studies have indicated a modified vaccine design can overcome strain immunity by focusing immune responses to conserved functional epitopes, which are otherwise less immunogenic [75]. Generally, identification of new candidates and epitopes follow P. falciparum research; however, P. vivax research comparatively has limited funds to progress.
1.4. Plasmodium vivax blood-stage vaccine candidates.
The pathology of P. vivax infections depends critically on the parasite’s ability to recognize and invade reticulocytes, a complex process dependent upon a series of highly specific, and sequential ligand-receptor interactions between merozoites and the host erythrocyte surface proteins [76–78]. Blood-stage vaccines have so far focused on inducing broadly neutralizing antibodies against parasite invasion ligands to block interactions with host cell receptors, thereby preventing invasion, growth, and clinical disease. In addition, a blood-stage vaccine has the potential to reduce gametocytemia in the host and indirectly reduce transmission. Humoral immune responses to blood-stage antigens are believed to be an important component of NAI to malaria [79,80].
Preclinical studies have characterized P. vivax merozoite antigens that might be viable potential vaccine candidates, based on their immunogenicity in animal models, recognition by NAI antibodies from vivax-exposed individuals with NAI and most importantly the ability to elicit parasite invasion-inhibitory antibodies. Leading blood-stage vaccine candidates include the Duffy-binding protein (DBP) [41,81–83], apical membrane antigen-1 (AMA1) [84], several reticulocyte binding protein (RBPs) [38,85,86], and the major merozoite surface proteins (MSPs), which include MSP1 [87–90], the MSP3 family [91–94], and MSP9 [95–97]. Of these antigens, MSP-1 and the DBP have received the most attention, but only DBP has advanced to Phase Ia clinical trials [98,99] (Table 1).
1.4.1. Plasmodium vivax Duffy binding protein
The P. vivax DBP is an apical organelle protein sequestered in the microneme and released to the merozoite surface during reticulocyte invasion. DBP belongs to the Duffy binding-like erythrocyte-binding protein (DBL-EBP) family, encoded by erythrocyte binding-like (ebl) genes [100,101], with homologs in other Plasmodium species [102–106]. Members of this family share similar molecular structures and functional characteristics [53,100–103]. In some Plasmodium spp. multiple ebl genes enable them to readily use alternative receptor pathways for invasion while P. vivax appears to be especially dependent upon on a single receptor, the Duffy antigen receptor for chemokines (DARC or Fy) [107–109].
The DBP-DARC interaction is associated with the decisive and irreversible step of junction formation between the merozoite and the host reticulocyte [51,76,78,97,108,110]; although, alternate invasion pathways now appear evident for P. vivax as discussed below [111–113]. This parasite’s strong preference for the DARC invasion pathway represents a weakness and provided early justification of DBP as a prime target for vaccine-induced immunity against asexual stages of the parasite.
The DBP receptor-binding domain termed Region II (DBPII) [100], contains the critical residues for receptor binding [24,52,114,115]. Structural studies revealed that DBPII dimerizes upon DARC engagement in a step-wise fashion to create a stable heterotetramer [114,115]. Supporting its potential as a vaccine candidate included numerous studies of individuals in endemic regions, demonstrating that naturally-acquired anti-DBPII antibodies with significant quantitative and qualitative serological responses [25,39,83,116] can block DBP-DARC interaction and inhibit invasion [25,41,50,117]. Further studies revealed that the epitope targets of natural-acquired anti-DBPII inhibitory antibodies map to the dimer interface, suggesting that interference with dimerization is a major factor underlying anti-DBP NAI [25,114]. Naturally-acquired antibodies that inhibit this interaction associate with clinical immunity [118,119].
Typically, disease-causing infections are absent in most endemic areas with a high prevalence of DARC negativity [28,120–123]. However, there are increasing reports of P. vivax infections occurring in DARC-negative individuals [26–28]. The molecular basis of these infections is yet to be resolved, although it is suggested that these infections in people carrying the null alleles may be viable due to transient expression of DARC in erythroid bone marrow precursors cells of DARC-negative individuals [124]. Alternatively, duplications of the dbp genes may allow vivax to evade host anti-DBP humoral immunity by using a secondary invasion pathway [112,125–127].
1.4.1.1. DBP-based Vaccine
DBP is so far the leading vaccine candidate for targeting the disease-causing blood stages of P. vivax malaria. The development of a DBP-based vaccine candidate based on the Sal-1 allele has progressed through pre-clinical studies [54,75,79,128–131] and two recent Phase Ia human clinical trials (Table 1) [98,99]. In preclinical studies, immunogenicity studies in laboratory animals produce anti-DBPII antibodies, which inhibit DBPII-DARC interaction. Despite being a promising vaccine candidate, the presence of immunodominant variant epitopes in DBPII misdirects immune responses that compromises vaccine efficacy in eliciting high titer neutralizing antibody to conserved strain-transcending functional epitopes [25,55,74,75].
Naturally-occurring polymorphisms in DBPII confer significant differences in sensitivity to inhibition by immune antibodies [24,55,132], with evidence of DBPII variant-specific antibody responses that correlate with homologous and not heterologous protection [133]. Even a single amino acid substitution can alter the antigenic character of a parasite antigen thus providing compelling evidence that immune selection is a driving force for allelic variation. Thus, it is critical to have a rational vaccine design and immunization strategy to focus immune responses to conserved functional epitopes that are targets of naturally occurring strain transcending anti-DBP inhibitory antibodies. Similar to approaches used for ligands of other microbial pathogens, several basic approaches have been applied to circumvent the inherent bias of eliciting a strain-specific immunity in a DBPII vaccine, including:
(i) Combination allele vaccines. The objective is to create broader specificity by directing the bulk of antibody to common epitopes within the constituent alleles that make up the vaccine. A vaccine made up of antigenically-distinct dbpII alleles elicited a higher antibody response and broader specificity to the individual antigens used in vaccine compared to the single alleles, suggesting that multiple DBPII variant alleles may be required in a vaccine for broader coverage [128]. Other studies also showed that single malaria antigens tend to induce protection against the homologous but not heterologous parasite strains [24,84,134,135], while a multiple component/allele vaccine did overcome strain-specific immunity with P. falciparum pre-clinical vaccine PfAMA1 [136–138] and the pneumococcal vaccine [139].
(ii) Immunofocusing. Epitope specificity is critical for vaccine design against malaria antigens. Dominant B-cell epitopes within DBPII are polymorphic surface-exposed motifs, [25,54], which tend to create an inherent bias towards a strain-specific immune response and limit induction of immune response towards more conserved protective epitopes [140,141]. Some elite responders in endemic regions do produce broadly inhibitory anti-DBPII antibodies [74], an indication that conserved epitope targets of strain-transcending immunity are present in DBP. An engineered DBPII vaccine, termed DEKnull-2, lacking the immunodominant variant surface epitopes, elicited broadly functional anti-DBPII antibodies to shared epitopes on multiple dbp alleles and inhibited parasite invasion of reticulocytes in vitro [75,81,129,142]. Most importantly, DEKnull-2 was recognized by naturally acquired anti-DBPII inhibitory antibodies [56], indicating that the vaccine contained conserved epitopes associated with natural protective immune response to non-dominant epitopes. This supports the strategy of targeting immune responses to conserved functional epitopes to avoid induction of strain-specific responses to dominant variant epitopes.
(iii) Sub-unit DBPII vaccine. Another practical approach to avoid strain-specific immunity is to identify minimal conserved epitopes within DBPII that elicit protective neutralizing antibodies against the intact native ligand. Stable strain-transcending immunity in elite responders in endemic regions offers the potential to identify such target epitopes to guide vaccine development [25,41,50,83]. These individuals are capable of producing high titers of invasion-inhibitory anti-DBPII antibodies [25,74,133]. By screening DBPII phage libraries or overlapping DBP peptides (mimotopes) with broadly inhibitory antibodies, subunits of the native refolded protein that are associated with protective immune response were identified [25,143]. Similarly, structure-based vaccinology is gaining ground as a new approach to identify functional epitopes for malaria vaccine development.
Structural studies revealed that DARC-binding residues are critical for DBPII-DARC dimerization upon receptor binding [114,144]. Furthermore, co-crystallization of DBP-DARC together with naturally acquired human [25,145] and vaccine-induced [146] inhibitory anti-DBP antibodies enabled identification of epitopes associated with these inhibitory antibodies. These data demonstrate that epitopes of naturally-acquired antibodies bind to the dimer interface and adjacent DARC binding groove thereby interfering with dimerization [25,114,144,145,147], while mAbs derived by vaccination with rDBPII (in mice and humans) have so far bound to epitopes within subdomain 3 (SD3) of DBPII, probably steric hindrance that indirectly hinders dimer formation [81,146].
Each strategy above has the potential to elicit antibodies that favor responses against conserved protective epitopes, with functional inhibition against broader allelic variants and diverse P. vivax strains, thus providing critical information on motifs to be included in a DBPII-based vaccine to induce broadly neutralizing and global strain-transcending protection.
(iv) Viral vectored DBPII vaccine. In a DBPII vaccine Phase Ia trial, an adenovirus serotype 36 (ChAd63) and a modified vaccinia virus ANKA (MVA) targeting DBPII-Sal1 strain were used as the delivery methods. These viral-vectored vaccines were well tolerated and demonstrated a safety profile in malaria-naïve adults, inducing DBPII specific antibodies including B cell and T cell responses [98]. Similarly, in a related Phase I randomized trial, a rDBPII vaccine formulated in GLA-SE adjuvant was safe and immunogenic in naïve adults [99]. Functional analysis demonstrated that anti-DBPII antibodies induced in both vaccine studies blocked binding of DBPII-DARC interaction in vitro. These studies further validate the vaccine potential of DBP and supports targeting parasite invasion ligands for vaccine development. However, further studies are required in P. vivax challenge models and/or ability to protect against natural infection in a Phase IIb trial in endemic regions.
1.4.2. Plasmodium vivax EBP2
A novel homolog of DBP, termed P. vivax erythrocyte binding protein 2 (PvEBP2) [71,111,148], was recently identified as a potential alternate invasion pathway ligand [100]. However, the lack of sequence similarity indicates that PvEBP2 is genetically distant from P. vivax DBP and other Plasmodium DBPs [111]. PvEBP2 is also under strong diversifying selection [149] but with lower SNPs relative to DBP [111], binds exclusively to reticulocytes, with a preference for immature (CD71high) and Duffy-positive reticulocytes with only minimal binding to DARC negative reticulocytes [112]. Despite lack of direct evidence, its conserved features and epidemiological data suggests PvEBP2 might play a role in a DARC-independent invasion of reticulocytes [112,149]. Evidence for positive diversifying selection within the PvEBP2 ligand domain similar to that on DBPII is an indication of an important biological function including reticulocyte invasion and/or a target of acquired immunity [149]. Furthermore, the role of PvEBP2 as a protein ligand is supported by recent serological analysis [150,151]. PvEBP2 is a target of NAI following natural exposure to P. vivax infection and is suggested to be a possible serological marker for detecting recent P. vivax infections [152]. In addition, anti-PvEBP2 antibody levels are shown to be positively correlated with age, cumulative exposure and are associated with protection and correlate with reduced risk of clinical disease [119,153,154]. Even though PvEBP2 has emerged as potential vaccine candidate, there is a need for more functional studies to evaluate NAI and design strategies to replicate long-term protective anti-PvEBP2 immunity.
1.4.3. Plasmodium vivax reticulocyte binding proteins
The P. vivax reticulocyte binding proteins (PvRBPs) represent additional ligand families implicated as important in the process of reticulocyte invasion. The restricted preference of P. vivax to invade reticulocytes is attributed to the RBPs [86,155,156]. It is believed that PvRBPs target reticulocytes for invasion and then trigger the release of DBP from the micronemes for the final high-affinity binding and irreversible step of junction formation just before invasion [110]. Homologs of these proteins in other Plasmodium spp. are implicated in early phase of invasion and regulate different invasion pathways [155,157–159]. In P. falciparum, these reticulocyte-binding protein homologs referred to as PfRH ligands are well characterized vaccine candidates [160]. Given their essential role in the invasion process, and the vaccine potential of its homologs in other species, RBPs are considered attractive vaccine targets against asexual blood-stage development.
There are 11 members of the rbp gene families reported in P. vivax: five full genes (rbp1a, rbp1b, rbp12a, rbp2, rbp2c), three partial genes (rbp1p1, rbp2p1, and rbp2p2), and three pseudogenes (rbp2d, rbp2e, rbp3) [111,155,161,162]. Although functional redundancy is not yet defined for the PvRBPs, similar to its homologs in P. falciparum and other species, it is suggested that the multiple PvRBPs may provide P. vivax phenotypic variation allowing the plasticity to recognize and use different receptors and pathways for invasion [155,163,164]. Likewise, it is speculated that RBPs might play a role in a DARC-independent invasion pathway for P. vivax infections in DARC negative individuals [28,165]. Thus, the generation of effective immunity to the PvRBPs may require targeting conserved functional domains of multiple members of this multi-gene family [166–169].
Despite the essential role played by the PvRBP ligands in the invasion process, only a few (RBP 1a, 2a, 2b and 2c) have received much attention [111,118,153,170–173], with the molecular function of other members currently unknown. Members of the PvRBP1 family characterized so far reveal differential binding specificities for normocytes and /or reticulocytes (reviewed in [174]). The large sizes (250–350kDa) and the limited knowledge of the receptors for members of this family have limited the progress towards vaccine development. However, PvRBP2b is implicated as the primary determinant of reticulocyte tropism of P. vivax and binds to transferrin receptor 1 (CD71), which is highly expressed on reticulocytes but not on mature erythrocytes [173].
Similar to other asexual stage vaccine candidates, the PvRBPs are genetically diverse [160,175,176], suggesting that these adhesins are under diversifying selection and hence attractive immune targets [175–177]. Similar patterns of immune selection have been observed with other microbial adhesion molecules including PfRh2 [178], DBP [24,179], PfAMA-1 [180,181], which ultimately results in antigenically-distinct variants in the population and a bias towards strain-specific immunity. This diversity is believed to provide the parasite with a host immune escape mechanism favoring its survival.
Naturally-acquired antibodies to PvRBPs are prevalent in residents of endemic regions with different transmission intensities, and similar to DBP, anti-PvRBP serological response correlates with age, cumulative parasite exposure and clinical protection [112,150,170,182–185] and may even last longer than anti-DBP antibodies in the absence of repeated exposure [175]. Similarly, vaccine induced immunity against functional regions of the PvRBPs proteins are associated with inhibition of reticulocyte binding and merozoite invasion of reticulocytes in vitro [170,172,173,186] as is the case with PfRH ligands in P. falciparum [163,187–190]. These features further strengthen PvRBPs as prime targets for blood-stage vivax malaria.
1.4.4. Plasmodium vivax Apical Membrane Antigen (PvAMA1)
AMA1 is a unique multi-stage specific vaccine target important in the host cell invasion processes of sporozoites [191–193] and merozoites [192–194]. AMA1 is a highly conserved apicomplexan ligand that is sequestered in the microneme until invasion is initiated [195] and provides a unique opportunity as a multi-stage vaccine target. It is shown to work together with proteins of the rhoptry neck protein (RON) complex. A tight junction between RON2, a rhoptry neck protein, and AMA1 is essential for invasion [196]. Similar to DBP, crystallographic studies of AMA1 have shown that AMA1 polymorphisms flanking a hydrophobic receptor-binding motif that is formed by two PAN domains help evade immune responses [197–199].
Naturally acquired antibodies against PvAMA1 block receptor binding, similar to anti-DBPII immunity. However, polymorphic residues adjacent to the receptor-binding pocket motif for the RON2 receptor are associated with strain-specific immune responses and consequently induction of strain-specific immunity may be a challenge to strain-transcending vaccine efficacy. Although analysis of neutralizing antibody responses to PvAMA1 identified the 1F9 epitope as an attractive antigenic target, it is polymorphic and may be associated with strain-limited immune protection [197].
Similar studies from rodent and non-human primate show that PvAMA1 is a target for protective immune responses [84,200]. A recombinant vaccine based on domain II of PvAMA1 in different adjuvants formulations elicited significant anti-AMA1 antibody titers in mice. Most importantly, these vaccine-induced antibodies were inhibitory against reticulocyte invasion by different Asian P. vivax isolates [192,193]. Furthermore, immune-epidemiological studies show naturally-acquired antibodies to PvAMA1 even in cases of very limited exposures. Altogether, these data indicate that PvAMA1 can be considered among the most promising blood-stage antigens to be used as a subunit malaria vaccine [201,202].
1.4.5. Plasmodium vivax Merozoite surface Protein (PvMSP1) and chimeric vaccine designs
MSP1 is a large post-translationally processed protein tethered to the merozoite surface by a C-terminal glycosylphosphatidylinositol group on its 42 kDa fragment (MSP142) [203]. During invasion, a parasite-expressed subtilisin-like protease further cleaves this MSP142 fragment resulting in MSP119 and MSP33 [204]. Some studies showed that MSP1 is highly immunogenic and naturally-acquired antibodies to the C-terminal fragment disrupted merozoite invasion [194,205,206]. Consequently, MSP1 was a component of a number of previous vaccine studies. Despite the early support as a vaccine target and data that identified PvMSP119 C-terminal fragment as a critical binding domain for erythrocytes, PvMSP1 lacks a clearly defined functional role and is now considered to be a part of the parasite’s immune evasion mechanisms.
An important contribution of these earlier vaccine studies was the use of chimeric P. berghei that expressed PvMSP19 to circumvent the lack of a challenge model which impeded preclinical testing of vivax blood-stage candidates. Mice immunized with a chimeric PvMSP119, generated a strong cytophilic antibody response along with CD4 and CD8 T cell responses against PvMSP119 making these modular chimeric T-cell epitopes as a promising strategy for inducing a protective immune response [194,207].
Other studies contributed development of a heterologous prime-boost strategy involving an adeno virus-vectored vaccine encoding two P. vivax blood-stage antigens PvAMA1 and PvMSP142 in Aotus I. lemurinus monkeys. Significant protection against blood-stage challenge in Aotus monkeys was observed, indicating the antigen delivery approach is safe and immunogenic [208]. While this regimen requires further development, the results emphasize the importance of heterologous/prime boost strategies for increasing the efficacy of blood-stage vivax vaccines.
1.5. Pre-erythrocytic vaccine
Pre-erythrocytic (PE) vaccines target the early stages from Plasmodium sporozoites infection until completion of liver stage development and breakthrough to blood-stage. This is an important bottleneck of Plasmodium life cycle and PE vaccines aim to prevent infection when the parasite burden is at its lowest. Early support for PE vaccines was based on studies with irradiated sporozoite vaccine strategies in the P. berghei rodent model that demonstrated sterile protection [209,210]. Subsequent studies in monkeys and in humans supported the potential for PE vaccine development [211,212]. However, potentially significant production, and logistical challenges have limited irradiated (whole) sporozoite vaccine approach, with much research turned to subunit vaccines. Even as PE vaccines have great potential, the requirement to induce sterile protection has long been considered a major weakness. In addition, PE vaccination against P. vivax will need to be equally effective against hypnozoite development.
1.5.1. Plasmodium vivax Circumsporozoite protein (PvCSP)
The CSP is the major surface protein of Plasmodium sporozoites and considered promising vaccine targets against PE stages of malaria parasites since they are directly exposed to host immune antibodies as sporozoites migrate to the liver during the early phase of infection (Figure 1). In P. vivax NAI and controlled human malaria infections, antibodies to PvCSP correlate with short-term protection [213]. However, unlike the rodent malaria parasites and P. falciparum, PvCSP is genetically diverse with two distinct strain types, VK210 and VK247, differing in central repeat region [214].
Despite licensure of a PfCSP vaccine, Mosquirix™ [215], only a few pre-clinical and human clinical trial studies of P. vivax PE vaccine candidates primarily evaluating CSP-based vaccines have been reported [216–222]. In addition, advancement has been hindered by the inherent complications related to the nature of the relapsing P. vivax infections. The vaccines evaluated so far include synthetic peptides, different types of recombinant proteins, and a chimeric PvCSP as summarized in Table 1 [68,223–226]. Although these P. vivax vaccines have been safe, they are poorly immunogenic and failed to elicit protection against infection by sporozoite challenge. Further optimization with different antigens and/or adjuvant formulation is required to improve its efficacy.
A chimeric PE vaccine, vivax malaria protein 1 (VMP001), incorporated the N- and C- terminal regions and truncated repeat regions from both VK210 and VK247 strains of PvCSP. VMP001 induced high titer antibodies in mice, using Montanide ISA 720 [227,228] as well as a strong cellular immune response with synthetic TLR4 (GLA-SE) [229] as an adjuvant. Similar antibody and cellular immune responses were observed in monkeys immunized with VMP001-GLA-SE [230] and VMP001 formulated in TLR9 agonist and protected against challenge infection with P. vivax sporozoites [231]. In a related study, VMP001 conjugated to a lipid enveloped polymer poly (lactide-co-glycolide) acid nanoparticles (VMP001-NP) adjuvanted in MPLA, elicited a balanced Th1/Th2 humoral response in mice with enhanced avidity and affinity toward the domains within PvCSP implicated in protection and were able to agglutinate live P. vivax sporozoites [232].
In a Phase 1/2a clinical trial, VMP001 formulated in the GSK Adjuvant System AS01B (VMP001/AS01B) was well tolerated and immunogenic, with volunteers generating robust humoral and cellular (CD4+ T cell) immune responses to the vaccine antigen (Table 1). Though the vaccine did not induce sterile protection, there was a significant delay in time to parasitemia observed in 59% of vaccinated subjects [68].
Significant progress in VLP vaccine design have led to increased PvCSP immunogenicity in recent years [220,221]. An innovative design Rv21, similar to RTS,S, improved immunogenicity of PvCSP VLP approach even at low doses (5 μg) when combined with Matrix-M adjuvant [220]. Another VLP approach using Qβ-peptides from E. coli bacteriophage showed similar outcomes by coupling fragments of PvCSP VK210 formulated in Matrix-M adjuvant [221]. Similar to earlier studies with blood-stage vaccine studies, an important advance in evaluating protective efficacy has been the introduction of transgenic P. berghei expressing PvCSP [219–221,233]. Upon challenge with these PvCSP transgenic sporozoites, improved protective efficacy was observed in these approaches [220,221].
1.5.2. Plasmodium vivax Thrombospondin-related anonymous protein (PvTRAP)
PvTRAP, also known as sporozoite surface protein-2 (SSP2), is a conserved microneme protein vaccine target involved in sporozoite motility and enables invading sporozoites to bind heparin sulfate molecules on hepatocytes [234,235]. Very few preclinical studies have evaluated the efficacy of a PvTRAP vaccine. Long synthetic peptides within the N-terminal region of the PvTRAP binding motif administered with both Freund and Montanide ISA 720 adjuvants to BALB/c mice and Aotus monkeys induced antibodies reactive to P. vivax sporozoites [236]. In Aotus monkeys, the vaccine formulated in CFA/IFA induced higher titers compared to Montanide ISA 720 and partial protection was observed upon intravenous challenge with 2×104 P. vivax sporozoites. In a related study, a heterologous prime-boost vaccine study with recombinant ChAd63 and MVA both expressing PvTRAP, respectively, induced high titer antibodies and high T cell response in immunized mice, which partially protected against challenged infection with a chimeric P. berghei expressing PvTRAP [237]. These results show PvTRAP as a potential vaccine candidate. However, further assessments in different vaccine strategies and animal models is still required.
1.5.3. Plasmodium vivax cell-traversal protein for oökinetes and sporozoites (PvCelTOS)
CelTOS is a conserved microneme protein in all Plasmodium spp. and has an essential function in sporozoites and oökinete cell traversal motility. PvCelTOS released from micronemes, targets the inner-leaflet of cell membranes allowing parasites to exit cells in mosquitoes and human hosts [238]. This function is essential for parasite motility and establishing successful infections, making it an ideal multi-stage vaccine candidate to prevent infection and transmission. In addition, PvCelTOS shows very limited genetic diversity among global clinical isolates [239].
Mice immunized with recombinant PvCelTOS induced both humoral and cell-mediated immune responses that reduced infection, while passive transfer of anti-PvCelTOS antibodies conferred protection to mice [240,241]. Efficacy with PvCelTOS vaccines with different strategies to boost immunogenicity have produced mixed results even with induction of high antibody titers [242,243]. Overcoming an important limitation of vivax malaria research efficacy in immunized mice was assessed with challenge infections of PvCelTOS-expressing transgenic P. berghei sporozoites. Promising results were provided by viral vectored PvCelTOS leading to sterile protection in 30% of ChAd63- PvCelTOS and ChAd63-VLP groups versus 10% ChAd63-MVA [244]. Further supporting a PvCelTOS vaccine, immunizations of human volunteers with irradiated sporozoites induced sterile immunity [245,246] and a strong anti-PvCelTOS response that correlated with protection [247].
1.6. Sexual-stage antigens
The current strategies to control malaria are inadequate to eradicate vivax malaria, especially relapse infections and transmission. Therefore, it is of importance to develop new tools to reduce the reproduction rate of the parasite resulting from relapse. Transmission-blocking vaccines (TBVs) are extremely important interventions to address this issue and reduce malaria transmission [248–250]. As discussed above for CelTOS, TBV antibodies against the parasite oökinete or mosquito midgut surface (oökinete receptors) antigens taken up with the bloodmeal can prevent the oökinete invasion of the midgut. Therefore, TBV antibodies can abrogate the cascade of secondary infections resulting in reduced parasitic reproducing rate and further transmission [249–251]. Below TBV candidates other than PvCelTOS are discussed.
The P. vivax oökinete surface protein (Pvs25) is a leading malaria transmission-blocking vaccine (TBV) candidate based on its high immunogenicity in animal models, transmission-blocking activity of antibodies elicited in clinical trials and high conservation among P. vivax isolates from endemic areas. In a single blinded, dose escalating, controlled Phase I study, Pvs25 expressed in S. cerevisiae and formulated in Montanide ISA 51 was terminated due to systemic reactogenicity (Table 1) [252]. However, the same antigen adjuvanted with alhydrogel, showed mild adverse reactions indicating the importance of adjuvant choice in designing clinical trials. Anti-Pvs25 vaccine-induced antibody responses were functional as they showed transmission-blocking activity in mosquito feeding assays that correlated to antibody titers [253].
In a related study in mice, Pvs25 and another TBV, Pvs28, formulated in alum induced antibodies that arrested the growth of P. vivax in mosquitoes fed with infected blood meal [254]. A field study showed that naturally acquired antibodies from P. vivax infected patients from regions with differential transmission intensities had anti-Pvs230 antibody responses [255]. Therefore, this vivax candidate, Pvs230D1-EPA, an orthologue of the P. falciparum candidate Pfs230D1- EPA has been garnering attention for clinical trials [256].
Another leading TBV candidate is the Anopheline alanyl aminopeptidase N (AnAPN1), a mosquito midgut surface protein, which mediates Plasmodium establishment in the mosquito [251,257–259]. Studies have shown that anti-AnAPN1 antibodies completely inhibit the development of naturally-circulating gametocytemic isolates of P. falciparum in Cameroon from infected volunteers in two independent transmission seasons [251]. A related study also evaluated cross-species efficacy of these antibodies against P. vivax from infected volunteers in Thailand, with only partial inhibition observed, suggesting a role of polymorphisms in immune response to AnAPN1 across different vectors [251].
The mechanism of action of TBVs in general is restricted solely to the activity of inhibitory antibodies [251,257,259]. Therefore, a successful TBV candidate should induce potent, high-titer antibodies that may be sustainable for one season of transmission.
2. Conclusions
P. vivax vaccine development has been slowed by the complex nature of the parasite’s biology, technical challenges due to the lack of in vitro culture, and limited access to experimental models to screen new vaccine candidates. Despite these challenges, vivax vaccine research has slowly progressed in recent years with development of transgenic rodent malaria models for some vaccine targets, a humanized mouse model and a new culture platform for liver stage studies, and the development of P. knowlesi lines adapted to human blood-stage culture. Recent advances in the use of these new platforms, structural vaccine design and better vaccine delivery platforms, will accelerate candidate identification and screening and enhance vaccine efficacy. With new adjuvants and delivery systems, there is a renewed interest in reestablishing the current vaccine candidates to accelerate them to clinical trials and eventual licensing. Engineered viruses tagged with recombinant proteins and VLPs could ultimately increase immunogenicity, safety and efficacy. These platforms can stimulate the antibody and cell-mediated responses and the same time serve as compatible adjuvants for human use, thus increasing the efficacy of these previously explored candidates. Also, heterologous prime-boost strategies could result in persistent immune response over longer periods of time, while multivalent-multistage vaccines will help prevent disease and transmission. Considering effective P. vivax vaccines will be essential for malaria eradication, these newer strategies will be remarkable to progress towards the clinical assessment of these vivax vaccine candidates.
3. Expert Opinion
Plasmodium invasive stages display an array of surface antigens important for initiating infection, promoting disease and enabling transmission and are, therefore, key targets for vaccine intervention. Epitope specificity is critical for vaccine efficacy. Thus, a rational vaccine design that focuses on the immune response to functionally conserved epitopes is essential to enhance induction of broadly neutralizing strain-transcending protective immunity. Such a vaccine should include functional epitopes of multiple antigens from different stages of the parasite life cycle.
Optimal immunogenicity and safety are critical outcomes of any effective vaccine. Hence, the study design, biological variables, delivery methods and route of administration are key factors to take into consideration.
Equally important are methods for novel candidate identification and validation, especially animal models for in vivo studies. Despite the availability of surrogate assays and more advanced in vitro parasite culture methods, including the use of chimeric/transgenic parasites for vivax vaccine studies, immunization challenge studies in primates remain the more direct and reliable measure of vaccine efficacy in vivo. All these factors are subject to availability of adequate funding, which has been a limiting factor for vivax vaccine studies. A full summary of challenges to P. vivax vaccine development and an action plan are summarized in Table 2.
Table 2:
Overview of P. vivax vaccine development: challenges, problems, potential actions, and 5-year plan to overcome these impediments.
| Challenges | Problem | Actions | 5-year plan |
|---|---|---|---|
| Blood-stage | |||
| Clinical infection of Duffy-negative individuals | P. vivax blood-stage merozoites use alternative ligands to invade Duffy-negative individuals | Target multiple epitopes essential for merozoite invasion | Identify novel ligands that facilitate merozoite invasion of Duffy-negative individuals |
| Low parasitemia and asymptomatic infections (lack of febrile malaria) | Parasite’s preference for Reticulocyte and host factors masks clinical infections | Better surveillance systems i.e. detection of sub-patent parasitemia | Develop tools to culture in vitro |
| Pre-erythrocytic stage | |||
| Preventing/eliminating dormant hypnozoites | P. vivax hypnozoite dormancy and reactivation | Research into hypnozonticidal strategies i.e drugs, PE vaccines | Establish in vitro liver stage assays to interrogate novel antigens/ targets such as ILSDA |
| Sexual stage | |||
| Targeting early formation of gametocytes | P. vivax sexual reproductive efficiency | Identifying blood-stage and gametocyte vaccine targets to prevent transmission | Develop in vitro tools to facilitate gametocyte cultures |
| Poor immunity conferred from natural exposure | Multiple vaccine doses within a transmission season could affect vaccine efficacy | Improving on adjuvant formulation, vaccine delivery and antigen selection to develop protective immune responses | Develop a potent single dose TBV candidate |
| Overall challenges | |||
| Lack of P. vivax sporozoites or merozoites | Parasite availability has hampered clinical trials and basic science research. | Use of in vivo models cultivate parasites and develop CHMI models (sporozoite inoculation, mosquito-bite challenges, and blood-stage inoculation). | Increase access to parasites from endemic regions. Overcome logistical challenges of mosquito-bite challenges by inoculating IBSM to test vaccine efficacy. |
| Overcoming parasite immune escape mechanisms | P. vivax genetic diversity and antigenic polymorphism | Identify conserved functional epitopes of neutralization and possibly target parasite using a multiantigen vaccine approach | Generate monoclonal antibodies (mAbs) that can neutralize multiple strain as passive immunization |
| Scarce genetic tool kit | Forward and reverse genetics tools are limited | Use transgenic or chimeric parasites to infer functions of P. vivax genes | Develop in vitro cultures, Use NHP models |
| Dearth of funding for P. vivax research | Inadequate funding for P. vivax research compared to P. falciparum | Increase funding to novel P. vivax vaccine research | |
mAbs-Monoclonal antibodies
PE-Pre-erythrocytic
ILSDA-In vitro Liver-stage Development assay
PEV- Pre-erythrocytic vaccine
TBV-Transmission blocking vaccine
CHMI-Controlled human malaria infections
IBSM- Infected blood-stage malaria
NHP-Non-human primate
Table 1:
(b) Potential Plasmodium vivax vaccine candidates targeting different stages of the life cycle.
| Different stages of life cycle | Potential P. vivax vaccine candidates | |
|---|---|---|
| Merozoite | 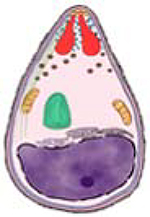 |
PvDBP [1,2,7–9] PvRBPs [10,11] PvEBP2 [12] PvMSP1[13–18] PvAMA1[14] |
| Sporozoite | 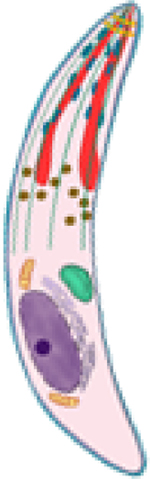 |
PvCSP [4,5,19–35] PvTRAP [22,35–37] PvCelTOS [34,35,38–40] |
| Macrogametocyte Microgametocyte |
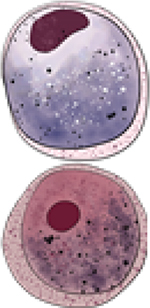 |
Pvs25 [6,41,42] Pvs28 [42] Pvs230 [43,44] |
| Oökinete |  |
|
PvDBP- P. vivax Duffy Binding protein region
PvRBPs- P. vivax Reticulocyte binding proteins
PvEBP2- P. vivax Erythrocyte binding protein2
PvMSP1- P. vivax Merozoite surface protein 1
PvAMA1- P. vivax Apical membrane antigen 1
PvCSP- P. vivax Circumsporozoite protein
PvTRAP- P. vivax Thrombospondin-related anonymous protein
PvCelTOS- P. vivax Cell-traversal protein for oökinetes and sporozoites
Pvs- P. vivax sexual stage
Article Highlights.
Plasmodium vivax malaria remains an important public health problem, yet there is no vaccine to prevent transmission and disease.
There is a need for novel candidate identification and validation.
A multivalent, multi-stage vaccine candidate elicits a stronger and robust immune response that can provide cross-strain protection.
Immune focusing and structure-based vaccine design can enhance vaccine efficacy.
Heterologous prime-boost strategy may induce a persistent, long-lasting immune response.
Different platforms such as viral-vectored subunit vaccine candidates and virus-like particles can be effective tools for vaccine delivery.
Acknowledgments
Funding
This study was supported by National Institutes of Health grant R01AI064478 (J.H.A.) and R01AI137162. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Organization WH. State of the World’s Vaccines and Immunization. World Health Organization (WHO), UNICEF, World Bank., 5–210 (2009). [Google Scholar]
- 2.Organization WH. World malaria report 2019. (2019)
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg, 77(6 Suppl), 79–87 (2007). [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra CA, Howes RE, Patil AP et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis, 4(8), e774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg, 64(1–2 Suppl), 97–106 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Ruiz W, Kroeger A. The socioeconomic impact of malaria in Colombia and Ecuador. Health Policy Plan, 9(2), 144–154 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Bonilla E, Rodriguez A. Determining malaria effects in rural Colombia. Soc Sci Med, 37(9), 1109–1114 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Smereck J Malaria in pregnancy: update on emergency management. J Emerg Med, 40(4), 393–396 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis, 11(1), 132–134 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomar AV, Vidal JE, Lomar FP, Barbas CV, de Matos GJ, Boulos M. Acute respiratory distress syndrome due to vivax malaria: case report and literature review. Braz J Infect Dis, 9(5), 425–430 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Alexandre MA, Ferreira CO, Siqueira AM et al. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis, 16(10), 1611–1614 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochar DK, Das A, Kochar SK et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg, 80(2), 194–198 (2009). [PubMed] [Google Scholar]
- 13.Sharma A, Khanduri U. How benign is benign tertian malaria? J Vector Borne Dis, 46(2), 141–144 (2009). [PubMed] [Google Scholar]
- 14.Lacerda MV, Hipolito JR, Passos LN. Chronic Plasmodium vivax infection in a patient with splenomegaly and severe thrombocytopenia. Rev Soc Bras Med Trop, 41(5), 522–523 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Rijken MJ, Boel ME, Russell B et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J, 10(1), 113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird JK, Basri H, Purnomo et al. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg, 44(5), 547–552 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Ruebush TK, Zegarra J, Cairo J et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg, 69(5), 548–552 (2003). [PubMed] [Google Scholar]
- 18.Teka H, Petros B, Yamuah L et al. Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia. Malar J, 7, 220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ketema T, Bacha K, Birhanu T, Petros B. Chloroquine-resistant Plasmodium vivax malaria in Serbo town, Jimma zone, south-west Ethiopia. Malar J, 8, 177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan K, Maithani MM. Congenital malaria due to chloroquine-resistant Plasmodium vivax: a case report. J Trop Pediatr, 56(6), 454–455 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Carlton JM, Sina BJ, Adams JH. Why is Plasmodium vivax a neglected tropical disease? PLoS Negl Trop Dis, 5(6), e1160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee A Outbreaks of rubella indicate epidemiological shift in age. Indian Pediatr, 52(2), 169 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Gioula G, Fylaktou A, Exindari M et al. Rubella immunity and vaccination coverage of the population of northern Greece in 2006. Euro Surveill, 12(11), E9–10 (2007). [DOI] [PubMed] [Google Scholar]
- 24.VanBuskirk KM, Cole-Tobian JL, Baisor M et al. Antigenic drift in the ligand domain of Plasmodium vivax duffy binding protein confers resistance to inhibitory antibodies. J Infect Dis, 190(9), 1556–1562 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Chootong P, Ntumngia FB, VanBuskirk KM et al. Mapping epitopes of the Plasmodium vivax Duffy binding protein with naturally acquired inhibitory antibodies. Infect Immun, 78(3), 1089–1095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngassa Mbenda HG, Gouado I, Das A. An additional observation of Plasmodium vivax malaria infection in Duffy-negative individuals from Cameroon. J Infect Dev Ctries, 10(6), 682–686 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Wurtz N, Mint Lekweiry K, Bogreau H et al. Vivax malaria in Mauritania includes infection of a Duffy-negative individual. Malar J, 10, 336 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menard D, Barnadas C, Bouchier C et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A, 107(13), 5967–5971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng S, Ni MY, Fang VJ et al. Characteristics of vaccine failures in a randomized placebo-controlled trial of inactivated influenza vaccine in children. Pediatr Infect Dis J, 33(2), e63–e66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med, 371(18), 1661–1663 (2014). [DOI] [PubMed] [Google Scholar]
- 31.May RM, Anderson RM. Population biology of infectious diseases: Part II. Nature, 280(5722), 455–461 (1979). [DOI] [PubMed] [Google Scholar]
- 32.Nilsson SK, Childs LM, Buckee C, Marti M. Targeting human transmission biology for malaria elimination. PLoS Pathog, 11(6), e1004871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones TR, Hoffman SL. Malaria vaccine development. Clin Microbiol Rev, 7(3), 303–310 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci, 266(3), 169–177 (1973). [DOI] [PubMed] [Google Scholar]
- 35.Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg, 68(3), 258–259 (1974). [DOI] [PubMed] [Google Scholar]
- 36.López C, Yepes-Pérez Y, Hincapié-Escobar N, Díaz-Arévalo D, Patarroyo MA. What Is known about the immune response induced by Plasmodium vivax malaria vaccine candidates? Front Immunol, 8(126) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev, 22(1), 13–36, Table of Contents (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cattani JA, Moir JS, Gibson FD et al. Small-area variations in the epidemiology of malaria in Madang Province. P N G Med J, 29(1), 11–17 (1986). [PubMed] [Google Scholar]
- 39.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol, 169(6), 3200–3207 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Lin E, Kiniboro B, Gray L et al. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLOS ONE, 5(2), e9047 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michon P, Fraser T, Adams JH. Naturally acquired and vaccine-elicited antibodies block erythrocyte cytoadherence of the Plasmodium vivax Duffy binding protein. Infect Immun, 68(6), 3164–3171 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg, 66(6), 641–648 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Harris I, Sharrock WW, Bain LM et al. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J, 9(1), 254 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King CL, Adams JH, Xianli J et al. Fy(a)/Fy(b) antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci U S A, 108(50), 20113–20118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maestre A, Muskus C, Duque V et al. Acquired antibody responses against Plasmodium vivax infection vary with host genotype for duffy antigen receptor for chemokines (DARC). PLoS One, 5(7), e11437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otto TD, Gilabert A, Crellen T et al. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat microbiol, 3(6), 687–697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J. Cultivation of Plasmodium vivax. Trends Parasitol, 24(2), 85–88 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Mueller I, Galinski MR, Baird JK et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis, 9(9), 555–566 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Tran TM, Moreno A, Yazdani SS, Chitnis CE, Barnwell JW, Galinski MR. Detection of a Plasmodium vivax erythrocyte binding protein by flow cytometry. Cytometry A, 63(1), 59–66 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Grimberg BT, Udomsangpetch R, Xainli J et al. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med, 4(12), e337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choe H, Moore MJ, Owens CM et al. Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC). Mol Microbiol, 55(5), 1413–1422 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Hans D, Pattnaik P, Bhattacharyya A et al. Mapping binding residues in the Plasmodium vivax domain that binds Duffy antigen during red cell invasion. Mol Microbiol, 55(5), 1423–1434 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med, 180(2), 497–506 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VanBuskirk KM, Sevova E, Adams JH. Conserved residues in the Plasmodium vivax Duffy-binding protein ligand domain are critical for erythrocyte receptor recognition. Proc Natl Acad Sci U S A, 101(44), 15754–15759 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ntumngia FB, Adams JH. Design and immunogenicity of a novel synthetic antigen based on the ligand domain of the Plasmodium vivax duffy binding protein. Clin Vaccine Immunol, 19(1), 30–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell B, Suwanarusk R, Borlon C et al. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood, 118(13), e74–81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moon RW, Hall J, Rangkuti F et al. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc Natl Acad Sci U S A, 110(2), 531–536 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohring F, Hart MN, Rawlinson TA et al. Rapid and iterative genome editing in the malaria parasite Plasmodium knowlesi provides new tools for P. vivax research. Elife, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol, 11(1), 57–64 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Duncan CJ, Draper SJ. Controlled human blood stage malaria infection: current status and potential applications. Am J Trop Med Hyg, 86(4), 561–565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roestenberg M, de Vlas SJ, Nieman AE, Sauerwein RW, Hermsen CC. Efficacy of preerythrocytic and blood-stage malaria vaccines can be assessed in small sporozoite challenge trials in human volunteers. J Infect Dis, 206(3), 319–323 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Herrington DA, Clyde DF, Losonsky G et al. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature, 328(6127), 257–259 (1987). [DOI] [PubMed] [Google Scholar]
- 63.Church LW, Le TP, Bryan JP et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis, 175(4), 915–920 (1997). [DOI] [PubMed] [Google Scholar]
- 64.McCarthy JS, Griffin PM, Sekuloski S et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis, 208(10), 1688–1694 (2013).**This study is established an alternative method to facilitate early testing for vaccine efficacy by inoculating infected blood-stage malaria (IBSM) to individuals.
- 65.Herrera S, Fernández O, Manzano MR et al. Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg, 81(5), 740–746 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrera S, Solarte Y, Jordán-Villegas A et al. Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg, 84(2 Suppl), 4–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arévalo-Herrera M, Forero-Peña DA, Rubiano K et al. Plasmodium vivax sporozoite challenge in malaria-naïve and semi-immune Colombian volunteers. PloS one, 9(6), e99754–e99754 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett JW, Yadava A, Tosh D et al. Phase 1/2a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLOS Negl Trop Dis, 10(2), e0004423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Payne RO, Griffin PM, McCarthy JS, Draper SJ. Plasmodium vivax controlled human malaria infection - progress and prospects. Trends Parasitol, 33(2), 141–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffin P, Pasay C, Elliott S et al. Safety and reproducibility of a clinical trial system using induced blood stage Plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLOS Negl Trop Dis, 10(12), e0005139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neafsey DE, Galinsky K, Jiang RH et al. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet, 44(9), 1046–1050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearson RD, Amato R, Auburn S et al. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet, 48(8), 959–964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hupalo DN, Luo Z, Melnikov A et al. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet, 48(8), 953–958 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King CL, Michon P, Shakri AR et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci U S A, 105(24), 8363–8368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ntumngia FB, Pires CV, Barnes SJ et al. An engineered vaccine of the Plasmodium vivax Duffy binding protein enhances induction of broadly neutralizing antibodies. Sci Rep, 7(1), 13779 (2017).***This study describes a strategy for antigen design to enhance cross-strain protection of a DBP vaccine, which can be applied other malaria vaccine candidates.
- 76.Chitnis CE. Molecular insights into receptors used by malaria parasites for erythrocyte invasion. Curr Opin Hematol, 8(2), 85–91 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell, 124(4), 755–766 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol, 34(13–14), 1413–1429 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Yazdani SS, Shakri AR, Mukherjee P, Baniwal SK, Chitnis CE. Evaluation of immune responses elicited in mice against a recombinant malaria vaccine based on Plasmodium vivax Duffy binding protein. Vaccine, 22(27–28), 3727–3737 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol, 28(1–2), 51–60 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Ntumngia FB, Schloegel J, Barnes SJ et al. Conserved and variant epitopes of Plasmodium vivax Duffy Binding Protein as targets of inhibitory monoclonal antibodies. Infect Immun, 80(3), 1203–1208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh S, Pandey K, Chattopadhayay R et al. Biochemical, biophysical, and functional characterization of bacterially expressed and refolded receptor binding domain of Plasmodium vivax duffy-binding protein. J Biol Chem, 276(20), 17111–17116 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Xainli J, Cole-Tobian JL, Baisor M et al. Epitope-specific humoral immunity to Plasmodium vivax Duffy binding protein. Infect Immun, 71(5), 2508–2515 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kocken CH, Dubbeld MA, Van Der Wel A et al. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect Immun, 67(1), 43–49 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galinski MR, Xu M, Barnwell JW. Plasmodium vivax reticulocyte binding protein-2 (PvRBP-2) shares structural features with PvRBP-1 and the Plasmodium yoelii 235 kDa rhoptry protein family. Mol Biochem Parasitol, 108(2), 257–262 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell, 69(7), 1213–1226 (1992). [DOI] [PubMed] [Google Scholar]
- 87.Dutta S, Ware LA, Barbosa A, Ockenhouse CF, Lanar DE. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect Immun, 69(9), 5464–5470 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soares IS, Levitus G, Souza JM, Del Portillo HA, Rodrigues MM. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun, 65(5), 1606–1614 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang C, Collins WE, Sullivan JS, Kaslow DC, Xiao L, Lal AA. Partial protection against Plasmodium vivax blood-stage infection in Saimiri monkeys by immunization with a recombinant C-terminal fragment of merozoite surface protein 1 in block copolymer adjuvant. Infect Immun, 67(1), 342–349 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Storti-Melo LM, Souza-Neiras WC, Cassiano GC et al. Evaluation of the naturally acquired antibody immune response to the Pv200L N-terminal fragment of Plasmodium vivax merozoite surface protein-1 in four areas of the Amazon Region of Brazil. Am J Trop Med Hyg, 84(2 Suppl), 58–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Audran R, Cachat M, Lurati F et al. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun, 73(12), 8017–8026 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sirima SB, Nébié I, Ouédraogo A et al. Safety and immunogenicity of the Plasmodium falciparum merozoite surface protein-3 long synthetic peptide (MSP3-LSP) malaria vaccine in healthy, semi-immune adult males in Burkina Faso, West Africa. Vaccine, 25(14), 2723–2732 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Galinski MR, Corredor-Medina C, Povoa M, Crosby J, Ingravallo P, Barnwell JW. Plasmodium vivax merozoite surface protein-3 contains coiled-coil motifs in an alanine-rich central domain. Mol Biochem Parasitol, 101(1–2), 131–147 (1999). [DOI] [PubMed] [Google Scholar]
- 94.Galinski MR, Ingravallo P, Corredor-Medina C, Al-Khedery B, Povoa M, Barnwell JW. Plasmodium vivax merozoite surface proteins-3beta and-3gamma share structural similarities with P. vivax merozoite surface protein-3alpha and define a new gene family. Mol Biochem Parasitol, 115(1), 41–53 (2001). [DOI] [PubMed] [Google Scholar]
- 95.Lima-Junior JC, Tran TM, Meyer EVS et al. Naturally acquired humoral and cellular immune responses to Plasmodium vivax merozoite surface protein 9 in Northwestern Amazon individuals. Vaccine, 26(51), 6645–6654 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barnwell JW, Galinski MR, DeSimone SG, Perler F, Ingravallo P. Plasmodium vivax, P. cynomolgi, and P. knowlesi: identification of homologue proteins associated with the surface of merozoites. Exp Parasitol, 91(3), 238–249 (1999). [DOI] [PubMed] [Google Scholar]
- 97.Vargas-Serrato E, Barnwell JW, Ingravallo P, Perler FB, Galinski MR. Merozoite surface protein-9 of Plasmodium vivax and related simian malaria parasites is orthologous to p101/ABRA of P. falciparum. Mol Biochem Parasitol, 120(1), 41–52 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Payne RO, Silk SE, Elias SC et al. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight, 2(12), e93683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh K, Mukherjee P, Shakri AR et al. Malaria vaccine candidate based on Duffy-binding protein elicits strain transcending functional antibodies in a Phase I trial. NPJ Vaccines, 3, 48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A, 89(15), 7085–7089 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams JH, Hudson DE, Torii M et al. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell, 63(1), 141–153 (1990). [DOI] [PubMed] [Google Scholar]
- 102.Sim BK, Orlandi PA, Haynes JD et al. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol, 111(5 Pt 1), 1877–1884 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adams JH, Blair PL, Kaneko O, Peterson DS. An expanding ebl family of Plasmodium falciparum. Trends Parasitol, 17(6), 297–299 (2001). [DOI] [PubMed] [Google Scholar]
- 104.Narum DL, Fuhrmann SR, Luu T, Sim BK. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol Biochem Parasitol, 119(2), 159–168 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Mayer DC, Kaneko O, Hudson-Taylor DE, Reid ME, Miller LH. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A, 98(9), 5222–5227 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gilberger TW, Thompson JK, Triglia T, Good RT, Duraisingh MT, Cowman AF. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem, 278(16), 14480–14486 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science, 189(4202), 561–563 (1975). [DOI] [PubMed] [Google Scholar]
- 108.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science, 187(4178), 748–750 (1975). [DOI] [PubMed] [Google Scholar]
- 109.Haynes JD, Dalton JP, Klotz FW et al. Receptor-like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med, 167(6), 1873–1881 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh AP, Ozwara H, Kocken CH, Puri SK, Thomas AW, Chitnis CE. Targeted deletion of Plasmodium knowlesi Duffy binding protein confirms its role in junction formation during invasion. Mol Microbiol, 55(6), 1925–1934 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Hester J, Chan ER, Menard D et al. De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis, 7(12), e2569 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ntumngia FB, Thomson-Luque R, Torres Lde M, Gunalan K, Carvalho LH, Adams JH. A novel erythrocyte binding protein of Plasmodium vivax suggests an alternate invasion pathway into Duffy-Positive reticulocytes. mBio, 7(4) (2016).**This study describes a new P. vivax vaccine target, with potential as an alternative ligand to DBP for reticulocyte invasion.
- 113.Gunalan K, Lo E, Hostetler JB et al. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci U S A, 113(22), 6271–6276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Batchelor JD, Zahm JA, Tolia NH. Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat Struct Mol Biol, 18(8), 908–914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adams JH, Mueller I. The biology of Plasmodium vivax. In: Malaria: Biology in the Era of Eradication. Wirth D, Alonso PL (Eds.) (Cold SPring Harbor Laboratory Press, Cold Spring Harbor, New York, 2017) 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cole-Tobian JL, Cortes A, Baisor M et al. Age-acquired immunity to a Plasmodium vivax invasion ligand, the duffy binding protein. J Infect Dis, 186(4), 531–539 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Dutta S, Daugherty JR, Ware LA, Lanar DE, Ockenhouse CF. Expression, purification and characterization of a functional region of the Plasmodium vivax Duffy binding protein. Mol Biochem Parasitol, 109(2), 179–184 (2000). [DOI] [PubMed] [Google Scholar]
- 118.Nicolete VC, Frischmann S, Barbosa S, King CL, Ferreira MU. Naturally acquired binding-inhibitory antibodies to Plasmodium vivax Duffy Binding Protein and clinical immunity to malaria in Rural Amazonians. J Infect Dis, 214(10), 1539–1546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He WQ, Shakri AR, Bhardwaj R et al. Antibody responses to Plasmodium vivax Duffy binding and Erythrocyte binding proteins predict risk of infection and are associated with protection from clinical Malaria. PLoS Negl Trop Dis, 13(2), e0006987 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Twohig KA, Pfeffer DA, Baird JK et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis, 13(1), e0007140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zimmerman PA. Plasmodium vivax Infection in Duffy-Negative People in Africa. Am J Trop Med Hyg, 97(3), 636–638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Howes RE, Reiner RC Jr., Battle KE et al. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis, 9(11), e0004222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kasehagen LJ, Mueller I, Kiniboro B et al. Reduced Plasmodium vivax erythrocyte infection in PNG Duffy-negative heterozygotes. PLoS One, 2(3), e336 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dechavanne C, Dechavanne S, Metral S et al. Duffy antigen expression in erythroid bone marrow precursor cells of genotypically Duffy negative individuals. bioRxiv, 508481 (2018). [Google Scholar]
- 125.Hostetler JB, Lo E, Kanjee U et al. Independent origin and global distribution of distinct Plasmodium vivax Duffy Binding Protein gene duplications. PLOS Negl Trop Dis, 10(10), e0005091 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Popovici J, Roesch C, Rougeron V. The enigmatic mechanisms by which Plasmodium vivax infects Duffy-negative individuals. PLOS Pathogens, 16(2), e1008258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Popovici J, Roesch C, Carias LL et al. Amplification of Duffy binding protein-encoding gene allows Plasmodium vivax to evade host anti-DBP humoral immunity. Nature Commun, 11(1), 953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ntumngia FB, Schloegel J, McHenry AM et al. Immunogenicity of single versus mixed allele vaccines of Plasmodium vivax Duffy binding protein region II. Vaccine, 31(40), 4382–4388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ntumngia FB, Barnes SJ, McHenry AM, George MT, Schloegel J, Adams JH. Immunogenicity of a synthetic vaccine based on Plasmodium vivax Duffy Binding Protein Region II. Clin Vaccine Immunol, 21(9), 1215–1223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moreno A, Caro-Aguilar I, Yazdani SS et al. Preclinical assessment of the receptor-binding domain of Plasmodium vivax Duffy-binding protein as a vaccine candidate in rhesus macaques. Vaccine, 26(34), 4338–4344 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Cassan SC, Shakri AR, Llewellyn D et al. Preclinical assessment of viral vectored and protein vaccines targeting the Duffy-Binding Protein Region II of Plasmodium Vivax. Front Immunol, 6, 348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McHenry AM, Barnes SJ, Ntumngia FB, King CL, Adams JH. Determination of the molecular basis for a limited dimorphism, N417K, in the Plasmodium vivax Duffy-binding protein. PLoS One, 6(5), e20192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cole-Tobian JL, Michon P, Biasor M et al. Strain-specific duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous plasmodium vivax strains in Papua New Guinean children. Infect Immun, 77(9), 4009–4017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renia L, Ling IT, Marussig M, Miltgen F, Holder AA, Mazier D. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect Immun, 65(11), 4419–4423 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kennedy MC, Wang J, Zhang Y et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun, 70(12), 6948–6960 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kusi KA, Faber BW, Thomas AW, Remarque EJ. Humoral immune response to mixed PfAMA1 alleles; multivalent PfAMA1 vaccines induce broad specificity. PLoS One, 4(12), e8110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kusi KA, Faber BW, Riasat V, Thomas AW, Kocken CH, Remarque EJ. Generation of humoral immune responses to multi-allele PfAMA1 vaccines; effect of adjuvant and number of component alleles on the breadth of response. PLoS One, 5(11), e15391 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duan J, Mu J, Thera MA et al. Population structure of the genes encoding the polymorphic Plasmodium falciparum apical membrane antigen 1: implications for vaccine design. Proc Natl Acad Sci U S A, 105(22), 7857–7862 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson-Adkins M, Adkins C. A new conjugate vaccine against pneumococcal disease. Nurse Pract, 26(5), 52, 55–56, 59–62 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Tobin GJ, Trujillo JD, Bushnell RV et al. Deceptive imprinting and immune refocusing in vaccine design. Vaccine, 26(49), 6189–6199 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol, 5(7), 555–563 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen E, Salinas ND, Ntumngia FB, Adams JH, Tolia NH. Structural analysis of the synthetic Duffy Binding Protein (DBP) antigen DEKnull relevant for Plasmodium vivax malaria vaccine design. PLoS Negl Trop Dis, 9(3), e0003644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.George MT, Schloegel JL, Ntumngia FB et al. Identification of an immunogenic broadly inhibitory surface epitope of the Plasmodium vivax Duffy Binding Protein ligand domain. mSphere, 4(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Batchelor JD, Malpede BM, Omattage NS, DeKoster GT, Henzler-Wildman KA, Tolia NH. Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLOS Pathog, 10(1), e1003869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Urusova D, Carias L, Huang Y et al. Structural basis for neutralization of Plasmodium vivax by naturally acquired human antibodies that target DBP. Nat Microbiol, 4(9), 1486–1496 (2019).**This paper describes the use of structural vaccinology as a tool for epitope identification for immunogen design.
- 146.Chen E, Salinas ND, Huang Y et al. Broadly neutralizing epitopes in the Plasmodium vivax vaccine candidate Duffy Binding Protein. Proc Natl Acad Sci U S A, 113(22), 6277–6282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carias LL, Dechavanne S, Nicolete VC et al. Identification and characterization of functional human monoclonal antibodies to Plasmodium vivax Duffy-Binding Protein. J Immunol, 202(9), 2648–2660 (2019).**This paper describes the use of naturally acquire immunity for developing neutralizing monoclonal antibodies with potential for use in therapeutic intervention by passive immunization.
- 148.Menard D, Chan ER, Benedet C et al. Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis, 7(11), e2489 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roesch C, Popovici J, Bin S et al. Genetic diversity in two Plasmodium vivax protein ligands for reticulocyte invasion. PLoS Negl Trop Dis, 12(10), e0006555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franca CT, He WQ, Gruszczyk J et al. Plasmodium vivax Reticulocyte Binding Proteins Are Key Targets of Naturally Acquired Immunity in Young Papua New Guinean Children. PLoS Negl Trop Dis, 10(9), e0005014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kerkhof K, Sluydts V, Willen L et al. Serological markers to measure recent changes in malaria at population level in Cambodia. Malar J, 15(1), 529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Longley RJ, White MT, Takashima E et al. Development and validation of serological markers for detecting recent Plasmodium vivax infection. Nat Med, 26(5), 741–749 (2020). [DOI] [PubMed] [Google Scholar]
- 153.Franca CT, Hostetler JB, Sharma S et al. An antibody screen of a Plasmodium vivax antigen library identifies novel merozoite proteins associated with clinical protection. PLoS Negl Trop Dis, 10(5), e0004639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Franca CT, White MT, He WQ et al. Identification of highly-protective combinations of Plasmodium vivax recombinant proteins for vaccine development. Elife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carlton JM, Adams JH, Silva JC et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature, 455(7214), 757–763 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galinski MR, Barnwell JW. Plasmodium vivax: Merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today, 12(1), 20–29 (1996). [DOI] [PubMed] [Google Scholar]
- 157.Meyer EV, Semenya AA, Okenu DM et al. The reticulocyte binding-like proteins of P. knowlesi locate to the micronemes of merozoites and define two new members of this invasion ligand family. Mol Biochem Parasitol, 165(2), 111–121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keen JK, Sinha KA, Brown KN, Holder AA. A gene coding for a high-molecular mass rhoptry protein of Plasmodium yoelii. Mol Biochem Parasitol, 65(1), 171–177 (1994). [DOI] [PubMed] [Google Scholar]
- 159.Rayner JC, Huber CS, Galinski MR, Barnwell JW. Rapid evolution of an erythrocyte invasion gene family: the Plasmodium reichenowi Reticulocyte Binding Like (RBL) genes. Mol Biochem Parasitol, 133(2), 287–296 (2004). [DOI] [PubMed] [Google Scholar]
- 160.Gruszczyk J, Lim NT, Arnott A et al. Structurally conserved erythrocyte-binding domain in Plasmodium provides a versatile scaffold for alternate receptor engagement. Proc Natl Acad Sci U S A, 113(2), E191–200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci USA, 97(17), 9648–9653 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gruner AC, Snounou G, Fuller K, Jarra W, Renia L, Preiser PR. The Py235 proteins: glimpses into the versatility of a malaria multigene family. Microbes Infect, 6(9), 864–873 (2004). [DOI] [PubMed] [Google Scholar]
- 163.Duraisingh MT, Triglia T, Ralph SA et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J, 22(5), 1047–1057 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Preiser PR, Jarra W, Capiod T, Snounou G. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature, 398(6728), 618–622 (1999). [DOI] [PubMed] [Google Scholar]
- 165.Russo G, Faggioni G, Paganotti GM et al. Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J, 16(1), 74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tomas AM, Margos G, Dimopoulos G et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J, 20(15), 3975–3983 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Persson KE, McCallum FJ, Reiling L et al. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest, 118(1), 342–351 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Omara-Opyene AL, Moura PA, Sulsona CR et al. Genetic disruption of the Plasmodium falciparum digestive vacuole plasmepsins demonstrates their functional redundancy. J Biol Chem, 279(52), 54088–54096 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Ogun SA, Tewari R, Otto TD et al. Targeted disruption of py235ebp-1: invasion of erythrocytes by Plasmodium yoelii using an alternative Py235 erythrocyte binding protein. PLoS Pathog, 7(2), e1001288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gupta ED, Anand G, Singh H et al. Naturally acquired human antibodies against reticulocyte-binding domains of Plasmodium vivax proteins, PvRBP2c and PvRBP1a, exhibit binding-inhibitory activity. J Infect Dis, 215(10), 1558–1568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Han JH, Lee SK, Wang B et al. Identification of a reticulocyte-specific binding domain of Plasmodium vivax reticulocyte-binding protein 1 that is homologous to the PfRh4 erythrocyte-binding domain. Sci Rep, 6, 26993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ntumngia FB, Thomson-Luque R, Galusic S et al. Identification and Immunological Characterization of the Ligand Domain of Plasmodium vivax Reticulocyte Binding Protein 1a. J Infect Dis, 218(7), 1110–1118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gruszczyk J, Huang RK, Chan LJ et al. Cryo-EM structure of an essential Plasmodium vivax invasion complex. Nature, 559(7712), 135–139 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Chan LJ, Dietrich MH, Nguitragool W, Tham WH. Plasmodium vivax Reticulocyte Binding Proteins for invasion into reticulocytes. Cell Microbiol, 22(1), e13110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rayner JC, Tran TM, Corredor V, Huber CS, Barnwell JW, Galinski MR. Dramatic difference in diversity between Plasmodium falciparum and Plasmodium vivax reticulocyte binding-like genes. Am J Trop Med Hyg, 72(6), 666–674 (2005). [PubMed] [Google Scholar]
- 176.Kosaisavee V, Lek-Uthai U, Suwanarusk R et al. Genetic diversity in new members of the reticulocyte binding protein family in Thai Plasmodium vivax isolates. PLoS One, 7(3), e32105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reiling L, Richards JS, Fowkes FJ et al. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol, 185(10), 6157–6167 (2010). [DOI] [PubMed] [Google Scholar]
- 178.Triglia T, Thompson J, Caruana SR, Delorenzi M, Speed T, Cowman AF. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun, 69(2), 1084–1092 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsuboi T, Kappe SH, al-Yaman F, Prickett MD, Alpers M, Adams JH. Natural variation within the principal adhesion domain of the Plasmodium vivax duffy binding protein. Infect Immun, 62(12), 5581–5586 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Polley SD, Conway DJ. Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics, 158(4), 1505–1512 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Verra F, Hughes AL. Natural selection on apical membrane antigen-1 of Plasmodium falciparum. Parassitologia, 41(1–3), 93–95 (1999). [PubMed] [Google Scholar]
- 182.Rojas Caraballo J, Delgado G, Rodriguez R, Patarroyo MA. The antigenicity of a Plasmodium vivax reticulocyte binding protein-1 (PvRBP1) recombinant fragment in humans and its immunogenicity and protection studies in Aotus monkeys. Vaccine, 25(18), 3713–3721 (2007). [DOI] [PubMed] [Google Scholar]
- 183.Tran TM, Oliveira-Ferreira J, Moreno A et al. Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg, 73(2), 244–255 (2005). [PubMed] [Google Scholar]
- 184.Hietanen J, Chim-Ong A, Chiramanewong T et al. Gene models, expression repertoire, and immune response of Plasmodium vivax reticulocyte binding proteins. Infect Immun, 84(3), 677–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Longley RJ, Franca CT, White MT et al. Asymptomatic Plasmodium vivax infections induce robust IgG responses to multiple blood-stage proteins in a low-transmission region of western Thailand. Malar J, 16(1), 178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gupta S, Singh S, Popovici J et al. Targeting a reticulocyte binding protein and duffy binding protein to inhibit reticulocyte invasion by Plasmodium vivax. Sci Rep, 8(1), 10511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rayner JC, Vargas-Serrato E, Huber CS, Galinski MR, Barnwell JW. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med, 194(11), 1571–1581 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao X, Yeo KP, Aw SS et al. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog, 4(7), e1000104 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Triglia T, Chen L, Lopaticki S et al. Plasmodium falciparum merozoite invasion is inhibited by antibodies that target the PfRh2a and b binding domains. PLoS Pathog, 7(6), e1002075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lopaticki S, Maier AG, Thompson J et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect Immun, 79(3), 1107–1117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kale S, Yadav CP, Rao PN et al. Antibody responses within two leading Plasmodium vivax vaccine candidate antigens in three geographically diverse malaria-endemic regions of India. Malar J, 18(1), 425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gentil F, Bargieri DY, Leite JA et al. A recombinant vaccine based on domain II of Plasmodium vivax Apical Membrane Antigen 1 induces high antibody titres in mice. Vaccine, 28(38), 6183–6190 (2010). [DOI] [PubMed] [Google Scholar]
- 193.Vicentin EC, Françoso KS, Rocha MV et al. Invasion-inhibitory antibodies elicited by immunization with Plasmodium vivax apical membrane antigen-1 expressed in Pichia pastoris yeast. Infect Immun, 82(3), 1296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fonseca JA, Cabrera-Mora M, Singh B et al. A chimeric protein-based malaria vaccine candidate induces robust T cell responses against Plasmodium vivax MSP119. Sci Rep, 6(1), 34527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Triglia T, Healer J, Caruana SR et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol Microbiol, 38(4), 706–718 (2000). [DOI] [PubMed] [Google Scholar]
- 196.Srinivasan P, Beatty WL, Diouf A et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13275–13280 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Coley AM, Gupta A, Murphy VJ et al. Structure of the malaria antigen AMA1 in complex with a growth-inhibitory antibody. PLoS Pathog, 3(9), 1308–1319 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bai T, Becker M, Gupta A et al. Structure of AMA1 from Plasmodium falciparum reveals a clustering of polymorphisms that surround a conserved hydrophobic pocket. Proceedings of the National Academy of Sciences of the United States of America, 102(36), 12736–12741 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pizarro JC, Normand BV-L, Chesne-Seck M-L et al. Crystal structure of the malaria vaccine candidate Apical Membrane Antigen 1. Science, 308(5720), 408–411 (2005). [DOI] [PubMed] [Google Scholar]
- 200.Narum DL, Ogun SA, Thomas AW, Holder AA. Immunization with parasite-derived apical membrane antigen 1 or passive immunization with a specific monoclonal antibody protects BALB/c mice against lethal Plasmodium yoelii yoelii YM blood-stage infection. Infect Immun, 68(5), 2899 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Seth RK, Bhat AA, Rao DN, Biswas S. Acquired immune response to defined Plasmodium vivax antigens in individuals residing in northern India. Microb Infect, 12(3), 199–206 (2010). [DOI] [PubMed] [Google Scholar]
- 202.Wickramarachchi T, Premaratne PH, Perera KLRL et al. Natural human antibody responses to Plasmodium vivax apical membrane antigen 1 under low transmission and unstable malaria conditions in Sri Lanka. Infect Immun, 74(1), 798 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gerold P, Schofield L, Blackman MJ, Holder AA, Schwarz RT. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and −2 of Plasmodium falciparum. Mol Biochem Parasitol, 75(2), 131–143 (1996). [DOI] [PubMed] [Google Scholar]
- 204.Moss DK, Remarque EJ, Faber BW et al. Plasmodium falciparum 19-kilodalton merozoite surface protein 1 (MSP1)-specific antibodies that interfere with parasite growth in vitro can inhibit MSP1 processing, merozoite invasion, and intracellular parasite development. Infect Immun, 80(3), 1280–1287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perera KL, Handunnetti SM, Holm I, Longacre S, Mendis K. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect Immun, 66(4), 1500–1506 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han H-J, Park S-G, Kim S-H et al. Epidermal growth factor-like motifs 1 and 2 of Plasmodium vivax merozoite surface protein 1 are critical domains in erythrocyte invasion. Biochem Biophys Res Commun, 320(2), 563–570 (2004). [DOI] [PubMed] [Google Scholar]
- 207.Dobrescu I, de Camargo TM, Gimenez AM et al. Protective immunity in mice immunized with P. vivax MSP119-based formulations and challenged with P. berghei expressing PvMSP119. Front Immunol, 11(28) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Obaldia N, Stockelman MG, Otero W et al. A Plasmodium vivax plasmid DNA- and adenovirus-vectored malaria vaccine encoding blood-stage antigens AMA1 and MSP142 in a prime/boost heterologous immunization regimen partially protects Aotus monkeys against blood-stage challenge. Clin Vaccine Immunol, 24(4), e00539–00516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature, 216(5111), 160–162 (1967). [DOI] [PubMed] [Google Scholar]
- 210.Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by X-irradiated Plasmodium berghei sporozoites. Nature, 222(5192), 488–489 (1969). [DOI] [PubMed] [Google Scholar]
- 211.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg, 24(3), 397–401 (1975). [DOI] [PubMed] [Google Scholar]
- 212.Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M et al. Protective efficacy of Plasmodium vivax radiation-attenuated sporozoites in Colombian volunteers: A randomized controlled trial. PLoS Negl Trop Dis, 10(10), e0005070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McCarthy VC, Clyde DF. Plasmodium vivax: correlation of circumsporozoite precipitation (CSP) reaction with sporozoite-induced protective immunity in man. Exp Parasitol, 41(1), 167–171 (1977). [DOI] [PubMed] [Google Scholar]
- 214.Lim CS, Tazi L, Ayala FJ. Plasmodium vivax: Recent world expansion and genetic identity to Plasmodium simium. Proc Natl Acad Sci U S A, 102(43), 15523 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.(CHMP). Summary of opinion: Mosquirix—Plasmodium falciparum and hepatitis B vaccine (recombinant, adjuvanted). Agency, EM; (2015) [Google Scholar]
- 216.Herrera S, De Plata C, González M et al. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing P. vivax CS epitopes in Aotus monkeys. Parasite Immunol, 19(4), 161–170 (1997). [DOI] [PubMed] [Google Scholar]
- 217.Yang C, Collins WE, Xiao L et al. Induction of protective antibodies in Saimiri monkeys by immunization with a multiple antigen construct (MAC) containing the Plasmodium vivax circumsporozoite protein repeat region and a universal T helper epitope of tetanus toxin. Vaccine, 15(4), 377–386 (1997). [DOI] [PubMed] [Google Scholar]
- 218.Collins WE, Nussenzweig RS, Ballou WR et al. Immunization of Saimiri sciureus boliviensis with recombinant vaccines based on the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg, 40(5), 455–464 (1989). [DOI] [PubMed] [Google Scholar]
- 219.de Camargo TM, de Freitas EO, Gimenez AM et al. Prime-boost vaccination with recombinant protein and adenovirus-vector expressing Plasmodium vivax circumsporozoite protein (CSP) partially protects mice against Pb/Pv sporozoite challenge. Sci Rep, 8(1), 1118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Salman AM, Montoya-Díaz E, West H et al. Rational development of a protective P. vivax vaccine evaluated with transgenic rodent parasite challenge models. Sci Rep, 7(1), 46482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Atcheson E, Reyes-Sandoval A. Protective efficacy of peptides from Plasmodium vivax circumsporozoite protein. Vaccine, 38(27), 4346–4354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Atcheson E, Bauza K, Salman AM et al. Tailoring a Plasmodium vivax vaccine to enhance efficacy through a combination of a CSP virus-like particle and TRAP viral vectors. Infect Immun, 86(9), e00114–00118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Herrington DA, Nardin EH, Losonsky G et al. Safety and immunogenicity of a recombinant sporozoite malaria vaccine against Plasmodium vivax. Am J Trop Med Hyg, 45(6), 695–701 (1991). [DOI] [PubMed] [Google Scholar]
- 224.Herrera S, Bonelo A, Liliana Perlaza B et al. Use of long synthetic peptides to study the antigenicity and immunogenicity of the Plasmodium vivax circumsporozoite protein. International journal for parasitology, 34(13), 1535–1546 (2004). [DOI] [PubMed] [Google Scholar]
- 225.Herrera S, Bonela A, Perlaza BL et al. Safety and elicitation of humoral and cellular responses in colombian malaria-naive volunteers by a Plasmodium vivax circumsporozoite protein–derived synthetic vaccine. Am J Trop Med Hyg, 73(5_suppl), 3–9 (2005). [DOI] [PubMed] [Google Scholar]
- 226.Herrera S, Fernández OL, Vera O et al. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg, 84(2 Suppl), 12–20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yadava A, Sattabongkot J, Washington MA et al. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect Immun, 75(3), 1177–1185 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bell BA, Wood JF, Bansal R et al. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine, 27(9), 1448–1453 (2009). [DOI] [PubMed] [Google Scholar]
- 229.Lumsden JM, Nurmukhambetova S, Klein JH et al. Evaluation of immune responses to a Plasmodium vivax CSP-based recombinant protein vaccine candidate in combination with second-generation adjuvants in mice. Vaccine, 30(22), 3311–3319 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Lumsden JM, Pichyangkul S, Srichairatanakul U et al. Evaluation of the safety and immunogenicity in rhesus monkeys of a recombinant malaria vaccine for Plasmodium vivax with a synthetic Toll-like receptor 4 agonist formulated in an emulsion. Infect Immun, 79(9), 3492–3500 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yadava A, Hall CE, Sullivan JS et al. Protective efficacy of a Plasmodium vivax circumsporozoite protein-based vaccine in Aotus nancymaae is associated with antibodies to the repeat region. PLoS Negl Trop Dis, 8(10), e3268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PloS one, 7(2), e31472–e31472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lima LC, Marques RF, Gimenez AM et al. A multistage formulation based on full-length CSP and AMA-1 ectodomain of Plasmodium vivax induces high antibody titers and T-cells and partially protects mice challenged with a transgenic Plasmodium berghei parasite. Microorganisms, 8(6) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rogers WO, Malik A, Mellouk S et al. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci U S A, 89(19), 9176–9180 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Templeton TJ, Kaslow DC. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol, 84(1), 13–24 (1997). [DOI] [PubMed] [Google Scholar]
- 236.Castellanos A, Arévalo-Herrera M, Restrepo N, Gulloso L, Corradin G, Herrera S. Plasmodium vivax thrombospondin related adhesion protein: immunogenicity and protective efficacy in rodents and Aotus monkeys. Mem Inst Oswaldo Cruz, 102, 411–416 (2007). [DOI] [PubMed] [Google Scholar]
- 237.Bauza K, Malinauskas T, Pfander C et al. Efficacy of a Plasmodium vivax malaria vaccine using ChAd63 and modified vaccinia Ankara expressing thrombospondin-related anonymous protein as assessed with transgenic Plasmodium berghei parasites. Infect Immun, 82(3), 1277–1286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jimah JR, Salinas ND, Sala-Rabanal M et al. Malaria parasite CelTOS targets the inner leaflet of cell membranes for pore-dependent disruption. eLife, 5, e20621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mehrizi AA, Torabi F, Zakeri S, Djadid ND. Limited genetic diversity in the global Plasmodium vivax Cell traversal protein of Ookinetes and Sporozoites (CelTOS) sequences; implications for PvCelTOS-based vaccine development. Infect Genet Evol, 53, 239–247 (2017). [DOI] [PubMed] [Google Scholar]
- 240.Bergmann-Leitner ES, Legler PM, Savranskaya T, Ockenhouse CF, Angov E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine, 29(35), 5940–5949 (2011). [DOI] [PubMed] [Google Scholar]
- 241.Bergmann-Leitner ES, Mease RM, De La Vega P et al. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLOS ONE, 5(8), e12294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bergmann-Leitner E, Hosie H, Whittington J et al. Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria. Front Immunol, 4(176) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ferraro B, Talbott KT, Balakrishnan A et al. Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infect Immun, 81(10), 3709–3720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Alves E, Salman AM, Leoratti F et al. Evaluation of Plasmodium vivax cell-traversal protein for ookinetes and sporozoites as a preerythrocytic P. vivax vaccine. Clin Vaccine Immunol, 24(4), e00501–00516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hoffman SL, Goh LM, Luke TC et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis, 185(8), 1155–1164 (2002). [DOI] [PubMed] [Google Scholar]
- 246.Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol, 11(4), 412–419 (1999). [DOI] [PubMed] [Google Scholar]
- 247.Doolan DL, Southwood S, Freilich DA et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Nat Acad Sci U S A, 100(17), 9952–9957 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carter R Transmission blocking malaria vaccines. Vaccine, 19(17–19), 2309–2314 (2001). [DOI] [PubMed] [Google Scholar]
- 249.Saul A Efficacy model for mosquito stage transmission blocking vaccines for malaria. Parasitology, 135(13), 1497–1506 (2008). [DOI] [PubMed] [Google Scholar]
- 250.Dinglasan RR, Jacobs-Lorena M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol, 24(8), 364–370 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Armistead JS, Morlais I, Mathias DK et al. Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria. Infect Immun, 82(2), 818–829 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu Y, Ellis RD, Shaffer D et al. Phase 1 Trial of Malaria Transmission Blocking Vaccine Candidates Pfs25 and Pvs25 Formulated with Montanide ISA 51. PLOS ONE, 3(7), e2636 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Malkin EM, Durbin AP, Diemert DJ et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine, 23(24), 3131–3138 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hisaeda H, Stowers AW, Tsuboi T et al. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun, 68(12), 6618 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tentokam BCN, Amaratunga C, Alani NAH et al. Naturally acquired antibody response to malaria transmission blocking vaccine candidate Pvs230 domain 1. Front Immunol, 10(2295) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. npj Vaccines, 5(1), 48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Atkinson SC, Armistead JS, Mathias DK et al. The Anopheles-midgut APN1 structure reveals a new malaria transmission-blocking vaccine epitope. Nat Struct Biol, 22(7), 532–539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dinglasan RR, Kalume DE, Kanzok SM et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci U S A, 104(33), 13461–13466 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mathias DK, Jardim JG, Parish LA et al. Differential roles of an Anopheline midgut GPI-anchored protein in mediating Plasmodium falciparum and Plasmodium vivax ookinete invasion. Infect Genet Evol, 28, 635–647 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


