Abstract
Individuals with overweight or obesity (OW/OB) are at increased risk for significant physical and psychological comorbidities. The current treatment for OW/OB is behavioral weight loss, which provides psychoeducation on nutrition and physical activity, as well as behavior therapy skills. However, behavioral weight loss is not effective for the majority of the individuals who participate. Research suggests that overeating, or eating past nutritional needs, is one of the leading causes of weight gain. Accumulating evidence suggests that appetitive traits, such as food cue responsiveness and satiety responsiveness, are associated with overeating and weight in youth and adults. The following review presents the current literature on the relationship between food cue responsiveness, satiety responsiveness, overeating, and OW/OB. Research suggests that higher food cue responsiveness and lower satiety responsiveness are associated with overeating and OW/OB cross-sectionally and longitudinally. Emerging data suggest that food cue responsiveness and satiety responsiveness may exist along the same continuum, and can be targeted to manage overeating and reduce weight. We have developed a treatment model targeting food cue responsiveness and satiety responsiveness, to reduce overeating and weight and have preliminary feasibility, acceptability, and efficacy data, with testing currently being conducting in larger trials. Through programs targeting appetitive traits we hope to develop an alternative weight loss model to assist individuals with a propensity to overeat.
Keywords: Overeating, Obesity, Food cue responsivity, Satiety responsivity, Appetite, Weight loss, Regulation of Cues
1. Introduction
Obesity is highly prevalent in the United States as approximately one third of children and two thirds of adults have overweight or obesity (OW/OB). (1, 2) OW/OB is associated with cardiovascular disease, type 2 diabetes, cancer, osteoarthritis, psychological impairment, poor quality of life and all-cause mortality. (3, 4) Medical care for individuals with OB costs nearly $150 billion across the United States, (5) and these costs are expected to rise by $48–66 billion per year by the year 2030. (6) Considering the high prevalence rates, rising medical costs, and significant comorbidities, it is essential that more potent models are developed to treat OW/OB effectively.
The current empirically supported treatment for adults with OW/OB is behavioral weight loss (BWL). (7–12) BWL includes dietary and caloric recommendations, guidelines for physical activity, and behavior therapy skills to adhere to treatment recommendations. Although BWL is effective and provides clinically significant weight loss for some adults, (10) BWL is not effective for all individuals. Up to 50% of participants in BWL treatment programs fail to achieve meaningful weight loss. (11) Moreover, BWL is even less effective in providing long-term weight-loss maintenance, as 65% of participants no longer meet the initial 7% weight-loss goal 4 years post-treatment. (12) These results could be due to heterogeneity and a lack of consideration for individual characteristics that can impact treatment response. (13, 14) Thus, it is imperative to understand the individual characteristics that predict treatment outcomes in the short and long term, and to develop novel treatments to address mechanisms associated with non-response.
Eating past nutritional needs, or overeating, is one of the most proximal drivers of OB rates (15, 16) and is considered more important than metabolic changes. (17–19) Rates of overeating are especially high in OW/OB samples, with up to 80% of adults with OW/OB endorsing overeating regularly. (20) Overeating is a complex process which is influenced by individual behavior, the environment, genes, physiology, and neural processes. However, societal advances have created an “obesogenic” environment that encourages excess energy intake and discourages energy expenditure. (21–24) Calorically dense foods are easily available, highly variable, tasty, relatively inexpensive, and portable. (25–29) Overeating in today’s environment is incredibly easy, especially for those who have a propensity to overeat.
2. Behavioral susceptibility to overeating
Interestingly, not everyone who lives in this environment has OW/OB. In fact, within the same family there can be weight discordant children. It is possible that some individuals have a behavioral susceptibility to overeat. Behavioral susceptibility was first described by Stanley Schachter who hypothesized that individuals with OW/OB, compared to individuals with a healthy weight, are more reactive to external cues to eat and less sensitive to internal satiety signals. (30, 31) Jane Wardle and colleagues extended this theory and developed the behavioral susceptibility theory (BST), (32–35) which proposes that genetically determined appetitive traits interact with the environment and lead to overeating and weight gain in individuals with these risk factors. The BST focuses on two important aspects of appetite, eating onset and eating offset. Within the context of BST, food cue responsiveness (FR) is considered the primary driver of eating onset, while satiety responsiveness (SR) is the primary driver of eating offset. FR conceptually relates to biological, cognitive and emotional responses to food cues, and includes concepts such as cravings, emotional eating, and reward-based eating. SR refers to perceiving and stopping eating based on satiety cues. The following review of the literature takes the perspective of the BST and briefly summarizes data to date on genetics, appetitive traits, interactions with the environment, overeating and body weight.
The BST states that genetic risk factors interact with appetitive traits and the environment to influence overeating. (35) Decades of research have shown that human body weight is highly heritable, with estimates ranging from 32% (36) to 90%, (37) with a median estimate of 73%. (38) The Collaborative Project of Development of Anthropometrical Measures in Twins (CODATwins), which includes over 200,000 twin pairs from 22 countries, (39) showed that the influence of genetic factors on BMI is lowest at age 4 years (40%) and highest at age 19 years (75%). (39, 40) Interestingly, the influence of the shared environment is not observed after age 15. These results suggest that over time, the genetic influence on BMI strengthens and the shared environment weakens, pointing to the importance of an individual’s interaction with the obesogenic environment. (35) Although the BST is an important theory, it does not specifically describe how these appetitive traits develop and more importantly, how the environment interacts with individual level variables. Theoretically, by decreasing FR (eating onset) and improving SR (eating offset), individuals could learn how to reduce overeating in the current obesogenic food environment.
3. Assessments of Food Cue Responsiveness (FR) and Satiety Responsiveness (SR)
A variety of measures exist that assess FR and SR. Jane Wardle and colleagues developed the Child Eating Behavior Questionnaire (CEBQ) (41) several years before the BST was formally articulated. The CEBQ was initially designed to be a parent report of children’s eating, and includes 8 subscales, FR, SR, emotional over-eating, enjoyment of food, desire to drink, slowness in eating, emotional under-eating and food fussiness. The FR subscale includes four items that assess desires to eat outside of physiological hunger and the SR subscale includes five items that evaluate a child’s sensitivity to stop eating or choosing not to eat in response to feeling full. The initial validation of the CEBQ was with 4–5 year olds in the US. (42) Since the initial publication, the CEBQ has been adapted to assess eating behaviors in babies (Baby Eating Behavior Questionnaire (43)) and adults (Adult Eating Behavior Questionnaire (44)).
FR can be assessed using neuroimaging techniques, such as MRI. Typically a food cue, either as a picture or as a taste, is presented and Blood Oxygenated Level Dependent (BOLD) response either in anticipation of the cue or delivery of the cue is assessed. Neural areas associated with the motivation to eat past nutritional requirements are the dopamine reward/motivation circuitry involving striatal limbic and cortical substrates. (45, 46) Key components of this hedonic pathway are located in the cortico-limbic areas of the brain and include the nucleus accumbens and caudate nucleus (dopaminergic reward pathways which govern anticipation and motivation); amygdala and hippocampus (learning); anterior insula (sensory processing); and orbitofrontal cortex (reward value appraisal, executive control, and decision-making). (47) Consumption of palatable food (those high in fat and sugar) releases dopamine in the ventral and dorsal striatum. The release of dopamine in the dorsal striatum is proportional to the self-reported level of pleasure gained by eating the food. (48) High fat and high sugar foods may differentially affect these brain reward regions. One study compared high sugar milkshakes and high fat milkshakes on BOLD response and showed high fat milkshakes show higher levels of BOLD responses in caudate and somatosensory regions, but no significant bilateral insular changes while high sugar milkshakes showed higher levels of BOLD responses in putamen and gustatory regions and increased bilateral insula. (49)
Data suggest that these reward circuitry dysregulations differentiated those who have OW/OB and those who have a healthy weight. (50–52) For instance, children with OW/OB compared to those with healthy weight show higher levels of BOLD responses in the bilateral insula and bilateral amygdala in response to taste of sugar compared to water. (53) Another study compared children with OW/OB to children with healthy weight and showed greater BOLD responses in right insula, operculum, bilateral precuneus, and posterior cingulate cortex following milkshake consumption. (54) Studies also demonstrate greater increase in fronto-striatal circuitry activation during anticipation of high-caloric foods as compared with those with healthy weight. (55, 56) After eating a 500 calorie meal, participants with obesity, compared to participants with healthy weight, show greater activation in the medial prefrontal cortex, superior frontal gyrus, caudate, and hippocampus. (57) In addition, over time, individuals with obesity, compared to individuals with healthy weight, demonstrated greater activation in the corticolimbic regions (lateral orbital frontal cortex, caudate, anterior cingulate), suggesting that individuals with OB have sustained responses in brain regions implicated in reward, even after eating (58). Importantly, for individuals with OW/OB, greater activation of the hedonic pathways in response to food images has been shown to predict short-term weight loss (59, 60) and was associated with successful maintenance of ≥ 13.6 kg (30 lbs.) weight loss over 3 years or more. (61) Hypothesized mechanisms of action include impulsivity or lack of self-control, which when coupled with higher levels of reward, may contribute to the drive to overeat. (62, 63)
In addition to using the CEBQ and brain imaging, concepts related to FR and SR can also be assessed with a variety of other questionnaires and tasks. The eating in the absence of hunger (EAH) paradigm (64) and the EAH questionnaire (65, 66) assess eating when exposed to food when physically satiated. The EAH behavioral paradigm has been the focus of considerable research to understand appetitive mechanisms. Children with higher FR and lower SR have higher caloric intake during this paradigm. (42, 67) Furthermore, greater caloric consumption during EAH has been linked with weight gain in children and adults. (68, 69) Attention bias, or the direction and strength of attention associated with a cue, can also be considered an assessment of FR. Attentional bias can be measured by reaction time, eye movements, or brain activity in response to salient stimuli, using event related potentials (ERPs; see (70) for review).
The remaining measures are self-report questionnaires that assess constructs closely related to FR. The Power of Food scale (PFS) (71) assesses appetite for high-palatable foods, and includes three levels of proximity, food available, food present and food tasted. The Food Craving Questionnaire (FCQ) (72, 73) assesses cravings using a multidimensional approach, with one subscale that queries about cues that may trigger eating. One study showed that individuals with higher FR, as assessed via physiological responsivity to a food cue, indicated higher subjective craving rating of those foods. (74) The Intuitive Eating Scale-2 (IES-2) assesses eating when physically hungry and stopping when full, with one subscale that focuses on reliance on hunger and satiety cues. The water load test (75, 76) evaluates how much water is consumed until perceived satiation and then again until maximum fullness. More research is needed to understand how these assessments are interrelated and what aspects of FR and SR are measured.
4. Development of Food Cue Responsiveness (FR)
Changes in FR over time is considered a product of both genetic and environmental influences. Emerging quantitative genetic modeling suggests that FR may be up to 75% heritable, (77) with the most evidence to date focusing on the “high risk” FTO alleles at rs9939609 in children. (78) Presence of this FTO minor allele at rs9939609 was associated with greater consumption during an EAH task compared with children without any risk allele in multiple samples. (79–81) As these studies were conducted in children, how the potential genetic risk for high FR changes over the life course remains to be fully elucidated.
Beyond genetic susceptibility, overeating develops through basic learning processes, including Pavlovian conditioning and operant conditioning. (82, 83) In today’s obesogenic food environment there are a plethora of opportunities to overeat through associations of cues in the environment with food, and over time, these cues can trigger responding (i.e., FR). Through Pavlovian conditioning, these food cues become directly associated with food intake and can elicit arousal, cravings, expectancies, thoughts, urges, motivation to eat. (84) There are also opportunities for operant conditioning, where the association of food seeking actions or eating are paired with the reinforcing effects of eating. (85) Importantly, these two learning processes act in concert (86) and the presentation of Pavlovian food cues can increase the strength or of operant eating or food-seeking through Pavlovian Instrumental Transfer. (87, 88)
Food cues can also acquire secondary reinforcing properties through their direct association with food. (89) Discriminative stimuli, those stimuli that are present when operant actions are reinforced, can increase operant responding by “setting the occasion” for the action–outcome relationship rather than eliciting or motivating behavior through their simple direct association with food. (90) Finally, with repetition and practice, stimuli associated with operant responding can eventually elicit the operant behavior directly. (91–93)
Increased FR may also provide opportunities for basic learning processes to take place. Ferriday and Brunstrom (94) suggest that one mechanism by which FR can increase consumption is by individuals planning to consumer larger amounts, resulting in overeating. Another potential mechanism linking FR and consumption is the association between FR and attention bias towards food cues, as there is some evidence that greater FR is associated with an increased attention bias toward foods. (95, 96) Increased attention to food cues may provide more opportunities for basic learning processes to occur, and lead to increased number of cues leading to consumption.
5. Development of Satiety Responsiveness (SR)
Conceptually, individuals will continue to eat in response to conditioned food-related cues until the intake is terminated by interoceptive satiety cues, (97) suggesting that SR is an inhibitory mechanism. SR develops early in life and is also considered an interaction of both genetic influences and factors in the environment. Several studies suggest that SR could be up to 63–72% heritable. (77, 98) Genetic mutations (such as in the leptin gene and melanocortin 4 receptor (MC4R) gene) are believed to contribute to OW/OB by disrupting satiety signaling. (99) One study evaluated the mechanisms by which a 16p11.2 deletion impacts BMI and found that altered SR preceded the development of obesity. (100) Research on FTO in children showed that the polymorphism rs9939609 is associated with reduced SR and that SR may mediate the relationship between FTO and BMI. (101) A more recent study (102) using participants from the same sample created a polygenetic risk score using 28 of 34 known obesity single-nucleotide polymorphisms (SNPs) identified in meta-analyses (103, 104) of children and adults. Results showed the polygenetic risk score to be positively associated with adiposity and negatively associated with SR, with SR mediating the association between the polygenetic risk score and adiposity. It is possible that decreased SR is a mechanism through which genetic risk accelerates weight gain and contributes to the development of OW/OB.
Some youth are born with this genetic risk factor of poorer SR. Beyond genetics, some data suggest that heightened SR during infancy may be promoted through breastfeeding. Research showed that breastfeeding during the first year of life (of at least 6 weeks in duration) is related to greater SR at 18–24 months (105) and that baby-led weaning compared to standard weaning is also related to greater SR at 18–24 months. (106) A study utilizing a sample from Amsterdam demonstrated that exclusively breastfed infants in the first four months have greater SR at age 5 than those who were introduced to solid foods during the first four months. (107) There is some conflicting research regarding breastfeeding, SR, and BMI such that one study found that breastfeeding exposure is related to higher SR at age 3–6, but not related to BMI change; (108) yet another study found breastfeeding intensity is related to obesity risk but not SR. (109)
It is also possible that factors in the environment can impact SR. Davidson and colleagues outline how interaction with the obesogenic environment can interfere with associative mechanisms that underlie the learned control of energy regulation. (110) Consistent with this analysis, overeating and the resulting excess weight gain is considered the result of physiological inhibitory signals (i.e. hormone cholecystokinin, leptin, Glucagon-like peptide-1) failing to suppress the capacity of cues in the environment (sight, smell, perceived palatability of a desirable food) from continuing to stimulate appetitive behaviors. (97) Excess energy intake and body weight gain is triggered by reducing the ability to inhibit appetitive and consummatory responses to food-related cues. (111–114) Practically, consumption of a westernized diet that is high in sugar and saturated fat may promote higher FR because a reduction in inhibitory stimulus control by satiety signals can enable food cues to be more excitatory within a Pavlovian framework. (110)
A number of factors in the environment can contribute to this mechanism, although a full review is available elsewhere. (110) Research in animals showed that consumption of non-caloric sweeteners can reduce the validity of sweet tastes as predictors of post-ingestive outcomes; (114, 115) however, data in humans tends to be more mixed. (116) Data suggest that SR depends, at least in part, on the hippocampus. (117) The hippocampus is involved in the encoding and retrieval of spatial relations among objects in the environment and the formation and recall of memories about events and facts. (118) However, more recently, the hippocampus has been associated with the utilization of hunger signals (119) and resolving “predictable ambiguities” when a single stimulus signals different outcomes dependent on other cues. (120) Data suggest that a diet that is high in sugar and saturated fat impacts the function of the blood brain barrier and the hippocampus itself. (121–123) Thus, the consumption of a western diet may promote overeating by affecting the hippocampus and other brain substrates that are involved in Pavlovian learning, ultimately reducing the ability of satiety cues to be inhibitory. (123, 124)
6. Relationship between appetitive traits, eating behavior, and body weight
In both children and adults, studies suggest that FR is associated with greater food consumption, while SR is associated with less food consumption. In children, higher FR is related to faster eating rate and increased energy intake during an EAH paradigm, while higher SR is associated with lower energy intake in the EAH paradigm and lower overall energy intake measured across 5 days. (42) The Gemini Birth Cohort collected data from over 1000 families with children 16 to 21 months old, and revealed that higher FR is associated with more frequent meals, but not larger meals. (125) Furthermore, it suggested that SR is inversely related to meal size (125) and is associated with consumption and post-meal satiety. (126) A study employing ecological momentary assessment found a significant positive relationship between food cues and snacking behavior, with FR moderating the relationship. (127)
Studies among toddlers show an inverse relationship between SR and energy intake during a lunch meal; but not during subsequent snack intake (EAH). (128) Similarly, adolescents classified as having greater SR (based on latent profile analyses) consume less food in an EAH paradigm. (129) There is also some evidence that SR, as measured by the Satiety Quotient, is related to subsequent ad libitum eating and self-reported food intake. (130, 131) These data provide a wide breadth of literature supporting the positive association between FR and eating behaviors and negative association between SR and eating behaviors across the lifespan.
FR is also consistently associated with higher body weight, while SR is inversely associated with BMI. (34, 109, 128, 132–135) Higher FR and lower SR are related to higher standardized child BMI, (136) with these associations demonstrated in multiple samples, including school aged Latino children, (137) preschool children of families of low socioeconomic status, (138) and Dutch children aged 6–7. (139) Similarly higher FR is associated with higher BMI in adults, (140) while SR in adults is inversely associated with BMI. (44, 141) Further, self-reported intuitive eating is consistently inversely associated with BMI. (142–149) Longitudinal investigations of these associations are more limited but reflect the same effect. Higher FR in children at the age of 6 is a significant predictor of a greater increase in BMI from age 6 to 8. (135) The same study showed that higher BMIz at age 4 predicted lower SR at age 6, and the increase in BMIz from age 4 to age 6 predicted decreased SR between ages 6 and 8. (135) In a sample children ages 7 to 9, one year changes in BMI were significantly predicted by scores on a relative reinforcing value of food task at baseline. (150) In infants, SR at age 3 months is inversely related to weight SD scores at age 15 months independent of weight at 3 months. (151) One study demonstrated that SR at age 2 is negatively associated with BMIz at age 4 controlling for birth weight Z score. (128) A separate study found that SR at 5–6 years is negatively associated with BMI at age 7–8 while controlling for birth weight. (152) Taken together, sufficient evidence suggests that SR is inversely related to BMI, while higher FR is associated with higher BMI. Longitudinal associations suggest the predictive power of FR and SR on BMI but additional studies are warranted.
Furthermore, data suggest that adults with OW/OB, compared to those with healthy weight, differentially respond to external food cues, with increases in both subjective ratings (increased desire to eat) and physiological responses (increased salivary response) to food cues. (153, 154) Our group demonstrated a stronger conditioned salivary response to innocuous food cues for individuals with obesity compared to those with healthy weight. (155) Several studies have shown that individuals with obesity, and those with binge eating, compared to those with healthy weight, display greater attention bias to food words and pictures. (156–164)
Emerging research also suggests that SR is related to weight loss. (165) Our group conducted a secondary data analysis among 150 children enrolled in family-based treatment for weight loss. (166) Latent class analyses revealed 3 trajectories of appetitive traits: high SR group (47.4%), high FR group (34.6%), and high emotional eating group (18.0%). (167) Interestingly, children in all three trajectories lost weight during treatment, however, only the children in the high SR group maintained their weight loss at the 12- and the 24-month follow-ups. Similarly, a study in adult men demonstrated that those with poorer SR lose less weight than those with high SR immediately following a 16-week randomized control trial of a satiating diet (higher protein; lower carbohydrate) to control diet (lower protein, higher carbohydrate) irrespective of group assignment. (165)
More recently, emerging research suggests that FR and SR may exist along the same continuum. Data in children and adults consistently show a significant negative relationship between FR and SR. (44, 168, 169) Additionally, more recent understandings suggest that the brain circuitry involved in both energy homeostasis and hedonic eating overlap and are less distinct than previously considered. (170) Thus, it is possible that these two appetitive traits may influence the impact of each other on overeating. This is similar to models in addiction research between reward (FR) and inhibition (SR). Considering this profile of appetitive traits, there may be intervention opportunities related to decreasing FR and increasing SR.
In summary, appetitive traits are associated with overeating and body weight. The BST outlined the interaction between appetitive traits, genetics and the environment. As part of this paper, we extended the BST by elucidating pathways showing how the environment interacts with individual appetitive characteristics. Figure 1 presents our extension of the original BST (solid lines) with the addition of these pathways that further explain the mechanisms of how genetics and environment interact with appetitive traits.
Figure 1:
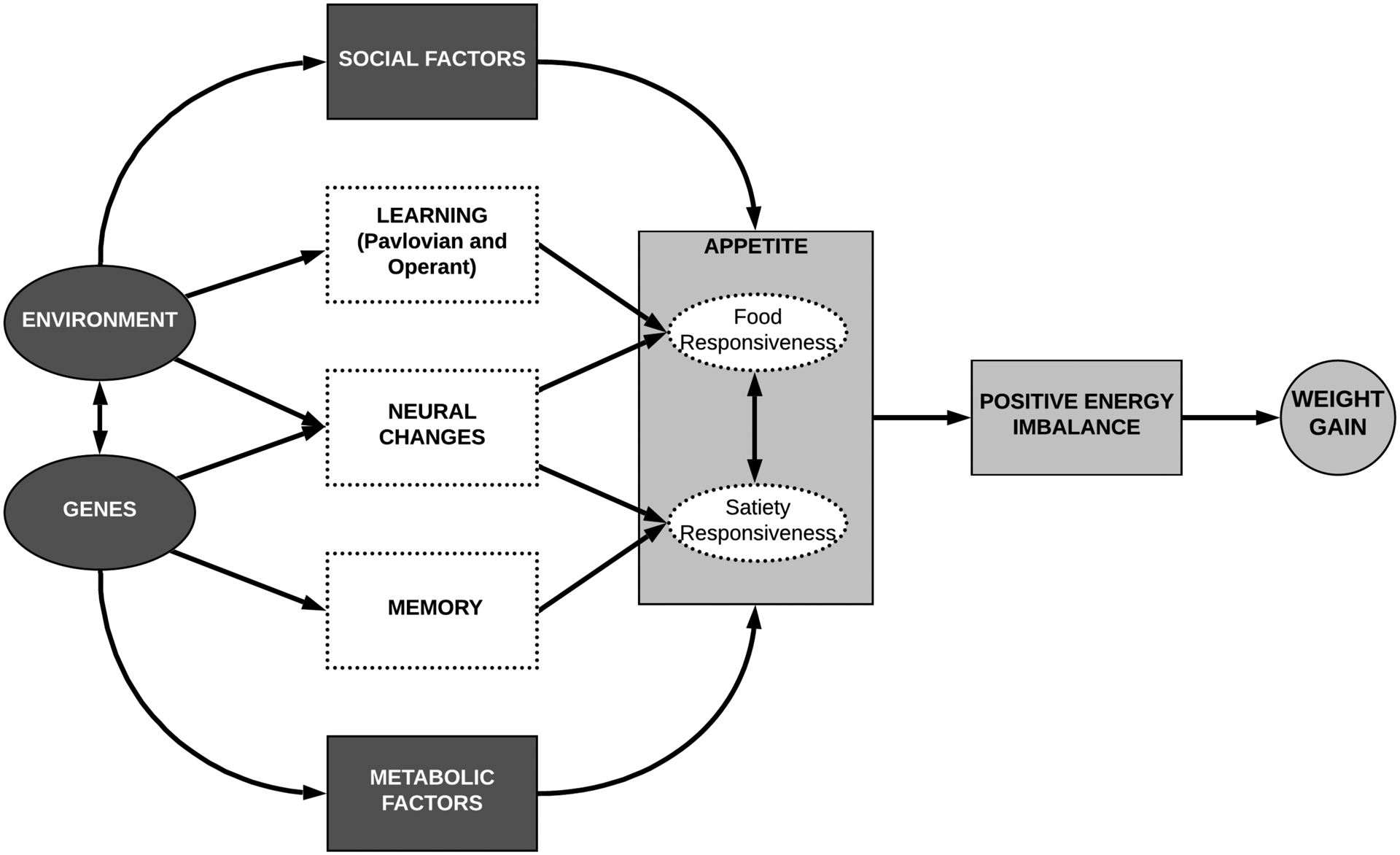
Extension of the Behavioral Susceptibility Theory which elaborates on the mechanisms of the development of higher food cue responsiveness (FR) and lower satiety responsiveness (SR) and relation to overeating and weight gain (adapted from Llewellyn & Fildes, 2017 (35))
7. Treatment development of a program designed to target FR and SR
Based on the theory and existing literature, we believe that FR and SR are emerging, important variables related to overeating and OW/OB and could be potential mechanisms for the development of a novel model for weight loss. We developed a treatment program that specifically targets both FR and SR, called Regulation of Cues (ROC). To target improvement in SR, we adapted Appetite Awareness Training (AAT). AAT focuses on rating hunger and satiety and learning to stop eating when physically full, and has been tested in children and adults. (171–173) To target decreases in FR, we developed a cue-exposure treatment for food (CET-Food) to reduce eating in response to food cues while sated. CET-Food involves exposure to food without consuming it while sated. CET-Food can teach people to resist eating when they are not physically hungry even though food is present by improving inhibitory learning. (174–177) ROC incorporates these two skill sets with psychoeducation, coping skills, experiential learning and parenting skills (when applied with children). Our pilot studies to date have shown feasibility, acceptability and initial efficacy with children (178, 179) and adults. (180) Large randomized control trials are needed to establish ROC as an evidence-based treatment for obesity, overeating, and/or binge eating. Two large studies among adults in our lab are currently underway (NCT02516839, NCT03678766 (181)) and will help elucidate whether ROC is an effective weight-loss treatment targeting the mechanisms of FR and SR. To date, it is unclear whether ROC can be a stand-alone treatment or if it can be used to improve the potency of BWL. It is also unclear how to optimize inhibitory learning with food cues. (175–177)
8. Conclusions
In summary, we believe that FR and SR are important variables related to overeating and weight gain, and it is possible that these two appetitive characteristics may exist along the same continuum. We have outlined the methods for the development of these appetitive traits and how they contribute to overeating in the current food environment. In particular, we have outlined options for intervening with these two mechanisms, by focusing on improving SR by training participants to respond to their appetite and on changing responses to FR by training inhibitory learning. We have developed the ROC program which targets these two mechanisms and are testing this program in larger trials. We believe that by targeting mechanisms of overeating, we may be able to develop more potent and durable interventions to decrease overeating and weight.
Acknowledgements
This manuscript is based on work presented during the 2019 Annual Meeting of the Society for the Study of Ingestive Behavior, July 9-13, 2019 in Utrecht, Netherlands.
Funding
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive and Kidney Diseases of the National Institutes of Health and the Department of Defense under Award Numbers, R01DK103554, R01DK094475, DOD W81XWH-18-1-0220, R01DK114794, K23DK114480. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- 1.Ogden C, Carroll M, Kit B, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner A, Ravanbakht S, Skelton J, et al. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3):e20173459. doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon J The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–8. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Flegal K, Kit B, Graubard B. Overweight, obesity, and all-cause mortality--reply. JAMA. 2013;309(16):1681–2. doi: 10.1001/jama.2013.3101. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Basu A. Estimating the medical care costs of obesity in the United States: Systematic review, meta-analysis, and empirical analysis. Value Health. 2016;19(5):602–13. doi: 10.1016/j.jval.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with Type 2 diabetes: One-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden T, Butryn M, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Wing R, Hamman R, Bray G, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadden T, West D, Neiberg R, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17(4):713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadden TA, Neiberg RH, Wing R, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19(10):1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field A, Carmargo C Jr, Ogino S. The merits of subtyping obesity: one size does not fit all. JAMA. 2013;310(20):2147–8. doi: 10.1001/jama.2013.281501. [DOI] [PubMed] [Google Scholar]
- 14.Kelly A, Marcus M, Yanovski J, et al. Working toward precision medicine approaches to treat severe obesity in adolescents: report of an NIH workshop. Int J Obes. 2018;42(11):1834–44. doi: 10.1038/s41366-018-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swinburn B, Sacks G, Lo S, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr. 2009;89(6):1723–8. doi: 10.3945/ajcn.2008.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffery R, Harnack L. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes. 2007;56(11):2673–6. doi: 10.2337/db07-1029. [DOI] [PubMed] [Google Scholar]
- 17.Small D Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes. 2009;33:Suppl 2:S44–8. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud H, Lenard N, Shin A. Food reward, hyperphagia, and obesity. AM J Physiol Regul Integr Comp Physiol. 2011;300(6):R1266–R77. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas J, Doshi S, Crosby RD, et al. Ecological momentary assessment of obesogenic eating behavior: combining person-specific and environmental predictors. Obesity. 2011;9(8):1574–9. doi: 10.1038/oby.2010.335. [DOI] [PubMed] [Google Scholar]
- 21.Brownell K Fast food and obesity in children. Pediatrics. 2004;113(1 Pt 1):132. doi: 10.1542/peds.113.1.132. [DOI] [PubMed] [Google Scholar]
- 22.Lowe M Self-regulation of energy intake in the prevention and treatment of obesity: is it feasible? Obes Res. 2003;11:Suppl 44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- 23.Kessler D The end of overeating: Taking control of the insatiable American appetite. New York, NY: Rodale. 2009 [Google Scholar]
- 24.Berthoud H Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43(3):315–7. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Drewnowski A. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med. 2004;27(Suppl 3):154–62. doi: 10.1016/j.amepre.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Drewnowski A The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181–8. doi: 10.3945/ajcn.2010.29300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffey K, Popkin B. Causes of increased energy intake among children in the U.S., 1977–2010. Am J Prev Med. 2013;44(2):e1–8. doi: 10.1016/j.amepre.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen S, Popkin B. Patterns and trends in food portion sizes, 1977–1998. JAMA. 2003;289(4):450–3. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 29.Popkin B, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol Behav. 2005;86(5):603–13. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 30.Schachter S Some extraordinary facts about obese humans and rats. Am Psychol. 1971;26(2):129–44. doi: 10.1037/h0030817. [DOI] [PubMed] [Google Scholar]
- 31.Schachter S, Rodin J. Obese humans and rats. Hillsdale NJ. Erlbaum. 1974 [Google Scholar]
- 32.Carnell S, Benson L, Pryor K, et al. Appetitive traits from infancy to adolescence: using behavioral and neural measures to investigate obesity risk. Physiol Behav. 2013;121:79–88. doi: 10.1016/j.physbeh.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llewellyn C, Wardle J. Behavioral susceptibility to obesity: Gene-environment interplay in the development of weight. Physiol Behav. 2015;152(Pt B):494–501. doi: 10.1016/j.physbeh.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Carnell S, Wardle J. Appetite and adiposity in children: Evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88(1):22–9. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 35.Llewellyn C, Fildes A. Behavioural susceptibility theory: Professor Jane Wardle and the role of appetite in genetic risk of obesity. Curr Obes Rep. 2017;6(1):38–45. doi: 10.1007/s13679-017-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silventoinen K, Bartels M, Posthuma D, et al. Genetic regulation of growth in height and weight from 3 to 12 years of age: a longitudinal study of Dutch twin children. Twin Res Hum Genet. 2007;10(2):354–63. doi: 10.1375/twin.10.2.354. [DOI] [PubMed] [Google Scholar]
- 37.Poulsen P, Vaag A, Kyvik K, et al. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44(5):537–43. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 38.Min J, Chiu D, Wang Y. Variation in the heritability of body mass index based on diverse twin studies: a systematic review. Obes Rev. 2013;134(11):871–82. doi: 10.1111/obr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silventoinen K, Jelenkovic A, Sund R, et al. The CODATwins project: The cohort description of collaborative project of development of anthropometrical measures in twins to study macro-environmental variation in genetic and environmental effects on anthropometric traits. Twins Res Hum Genet. 2015;18(4):348–60. doi: 10.1017/thg.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silventoinen K, Jelenkovic A, Sund R, et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the Collaborative project of development of anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr. 2016;104(2):371–9. doi: 10.3945/ajcn.116.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardle J, Guthrie C, Sanderson S, et al. Development of the Children’s Eating Behaviour Questionnaire. J Child Adol Psychiatry. 2001;42(7):963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 42.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: Validation of the Child Eating Behaviour Questionnaire. Appetite. 2007;48(1):104–13. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 43.Llewellyn C, van Jaarsveld C, Johnson L, et al. Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite. 2011;57(2):388–96. doi: 10.1016/j.appet.2011.05.324. [DOI] [PubMed] [Google Scholar]
- 44.Hunot C, Fildes A, Croker H, et al. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite. 2016;105:356–63. doi: 10.1016/j.appet.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkow N, Wise R, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci. 2017;18(12):741–52. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 46.Hoch T, Pischetsrieder M, Hess A. Snack food intake in ad libitum fed rats is triggered by the combination of fat and carbohydrates. Front Psychol. 2014;5:250. doi: 10.3389/fpsyg.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berthoud H. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21(6):888–96. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small D, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–15. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 49.Stice E, Burger K, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr. 2013;98(6):1377–84. doi: 10.3945/ajcn.113.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G, Volkow N, Thanos P, et al. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 51.Barry R, Byun N, Williams J, et al. Brief exposure to obesogenic diet disrupts brain dopamine networks. PLoS One. 2018;13(4):e0191299. doi: 10.1371/journal.pone.0191299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsiö J, Olsewski P, Norback A, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171(3):779–87. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Boutelle KN, Wierenga C, Bischoff-Grethe A, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes 2015;39(4):620–8. doi: 10.1038/ijo.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohon C Brain response to taste in overweight children: A pilot feasibility study. PLoS One. 2017;12(2):e0172604. doi: 10.1371/journal.pone.0172604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stice E, Spoor S, Bohon C, et al. Relation between obesity and blunted striatal response to foods is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoeckel L, Weller R, Cook E, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 57.Martin L, Holsen LM, Chambes RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18(2):254–60. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 58.Dimitropoulos A, Tkach J, Ho A, et al. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58(1):303–12. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paolini B, Laurienti P, Simpson S, et al. Global integration of the hot-state brain network of appetite predicts short term weight loss in older adult. Front Aging Neurosci. 2015;7:70. doi: 10.3389/fnagi.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murdaugh D, Cox J, Cook E 3rd, et al. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–21. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCaffery J, Haley A, Sweet L, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90(4):926–34. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence N, Hinton E, Parkinson J, et al. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63(1):415–522. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 63.Babbs R, Sun X, Felsted J, et al. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol Behav. 2013;121:103–11. doi: 10.1016/j.physbeh.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fisher J, Birch L. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold T, Johnston C, Lee C, et al. Eating in the absence of hunger in college students. Appetite. 2015;92:51–6. doi: 10.1016/j.appet.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Tanofsky-Kraff M, Ranzenhofer L, Yanovski S, et al. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite. 2008;51(1):148–55. doi: 10.1016/j.appet.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lansigan R, Edmond J, Gilbert-Diamond D. Understanding eating in the absence of hunger among young children: A systematic review of existing studies. Appetite. 2015;85:36–47. doi: 10.1016/j.appet.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asta K, Miller A, Retzloff L, et al. Eating in the absence of hunger and weight gain in low-income toddlers. Pediatrics. 2016;137(5):e20153786. doi: 10.1542/peds.2015-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feig E, Piers A, Kral T, et al. Eating in the absence of hunger is related to loss-of-control eating, hedonic hunger, and short-term weight gain in normal-weight women. Appetite. 2018;123:317–24. doi: 10.1016/j.appet.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Hagan K, Alsasmar A, Exum A, et al. A systematic review and meta-analysis of attentional bias toward food in individuals with overweight and obesity. Appetite. 2020;151:104710. doi: 10.1016/j.appet.2020.104710. [DOI] [PubMed] [Google Scholar]
- 71.Lowe M, Butryn M, Didie E, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–8. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Cepeda-Benita A, Gleaves D, Williams T, et al. The development and validation of the State and Trait food Cravings Questionnaires. Behav Therapy. 2000;31:151–73. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 73.Cepeda-Benito D, Gleaves D, Fernandez M, et al. The development and validation of Spanish versions of the State and Trait Food Cravings Questionnaires. Behav Res & Ther. 2000;38(11):1125–38. doi: 10.1016/S0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 74.Nederkoorn C, Smulders F, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35(1):45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- 75.Jones M Satiety testing: ready for the clinic? World J Gastroenterol. 2008;14(35):5371–6. doi: 10.3748/wjg.14.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Dyke Z, Vogele C, Blechert J, et al. The Water Load Test as a measure of gastric interoception: development of a two-stage protocol and application to a healthy female population. PloS One. 2016;11(9):e0163574. doi: 10.1371/journal.pone.0163574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carnell S, Haworth C, Plomin R, et al. Genetic influence on appetite in children. Int J Obes. 2008;32(10):1468–73. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 78.Velders P, De Wit J, Jansen P, et al. FTO at rs9939609, food responsiveness, emotional control and symptoms of ADHD in preschool children. PLoS One. 2012;7(11):e49131. doi: 10.1371/journal.pone.0049131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haworth C, Davis O, Plomin R. Twins Early Development Study (TEDS): a genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Res Hum Genet. 2013;16(1):117–25. doi: 10.1017/thg.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trouton A, Spinath F, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res 2002;5(5):444–8. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 81.Wardle J, Llewellyn C, Sanderson S, et al. The FTO gene and measured food intake in children. Int J Obes. 2009;33(1):42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 82.Berridge K, Ho C, Richard J, et al. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boutelle K, Bouton M. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015;93:62–74. doi: 10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watson P, Wiers R, Hommel B, et al. Working for food you don’t desire. Cues interfere with goal-directed food-seeking. Appetite. 2014;79:139–48. doi: 10.1016/j.appet.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Bouton M. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103(1):51–8. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 86.Bouton M. Learning theory. In: Sadock BJ, Sadock VA, Ruiz P, (eds) Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. New York: Lippincott Williams & Wilkins. 2009:647–58. [Google Scholar]
- 87.Holmes N, Marchand A, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34(8):1277–95. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Rescorla R, Solomon R. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74(3):151–82. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 89.Williams B Conditioned reinforcement: Experimental and theoretical issues. Behav Anal. 1994;17(2):261–85. doi: 10.1007/bf03392675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hogarth L, Retzler C, Munafo M, et al. Extinction of cue-evoked drug-seeking relies on degrading hierarchical instrumental expectancies. Behav Res & Ther. 2014;59:61–70. doi: 10.1016/j.brat.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balleine B, O’Doherty J. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharm. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thrailkill E, Bouton M. Contextual control of instrumental actions and habits. J Exp Psychol Anim Learn Cogn. 2015;41(1):69–80. doi: 10.1037/xan0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tricomi E, Balleine B, O’Doherty J. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29(11):2225–32. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferriday D, Brunstrom J. How does food-cue exposure lead to larger meal sizes? Br J Nutr. 2008;100(6):1325–32. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- 95.Hou R, Mogg K, Bradley B, et al. External eating, impulsivity and attentional bias to food cues. Appetite. 2011;56(2):424–7. doi: 10.1016/j.appet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 96.Hardman C, Rogers P, Etchells K, et al. The effects of food-related attentional bias training on appetite and food intake. Appetite. 2013;71:295–300. doi: 10.1016/j.appet.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woods S Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- 98.Llewellyn C, Cornelia H, van Jaarsveld C, et al. Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. Am J Clin Nutr. 2010;91(5):1172–9. doi: 10.3945/ajcn.2009.28868. [DOI] [PubMed] [Google Scholar]
- 99.O’Rahilly S, Farooqi I. Human obesity: A heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57(11):2905–10. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maillard A, Hippolyte L, Rodriguez-Herreros B, et al. 16p11.2 Locus modulates response to satiety before the onset of obesity. Int J Obes. 2016;40(5):870–6. doi: 10.1038/ijo.2015.247. [DOI] [PubMed] [Google Scholar]
- 101.Wardle J, Carnell S, Haworth C, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrin and Metab. 2008;93(9):3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 102.LLewellyn C, Trzaskowski M, van Jaarsveld C, et al. Satiety mechanisms in genetic risk of obesity. JAMA Pediatr. 2014;168(4):338–44. doi: 10.1001/jamapediatrics.2013.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bradfield J, Taal H, Timpson N, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2010;42(11):937–48. doi: 10.1038/ng.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Speliotes E, Willer C, Berndt S, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown A, Lee M. Breastfeeding during the first year promotes satiety responsiveness in children aged 18–24 months. Pediatr Obes. 2012;7:382–90. doi: 10.1111/j.2047-6310.2012.00071.x. [DOI] [PubMed] [Google Scholar]
- 106.Brown A, Lee M. Early influences on child satiety-responsiveness: the role of weaning style. Pediatr Obes. 2015;10(1):57–66. doi: 10.1111/j.2047-6310.2013.00207.x. [DOI] [PubMed] [Google Scholar]
- 107.Moller L, de Hoog M, van Eijsden M, et al. Infant nutrition in relation to eating behaviour and fruit and vegetable intake at age 5 years. Br J Nutr. 2013;109(3):564–71. doi: 10.1017/S0007114512001237. [DOI] [PubMed] [Google Scholar]
- 108.Disantis K, Collins B, Fisher J, et al. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? Int J Behav Nutr Phys Act. 2011;8:89. doi: 10.1186/1479-5868-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hathcock A, Krause K, Viera A, et al. Satiety responsiveness and the relationship between breastfeeding and weight status of toddlers of overweight and obese women. Matern Child Health. 2014;19:1023–30. doi: 10.1007/s10995-013-1331-9. [DOI] [PubMed] [Google Scholar]
- 110.Davidson T, Tracy A, Schier L, et al. A view of obesity as a learning and memory disorder. J Exp Psychol Anim Learn Cogn. 2014;40(3):261–79. doi: 10.1037/xan0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davidson T, Kanoski S, Walls E, et al. Memory inhibition and energy regulation. Physiol Behav. 2005;86(5):731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Davidson T, Kanoski S, Schier L, et al. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7(6):613–6. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davidson T Do impaired memory and body weight regulation originate in childhood with diet-induced hippocampal dysfunction? Am J Clin Nutr. 2014;99(5):971–2. doi: 10.3945/ajcn.114.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davidson T, Sample C, Swithers S. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem. 2014;108:172–84. doi: 10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davidson T, Martin A, Clark K, et al. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove). 2011;64(7):1430–144. doi: 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Swithers S Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013;24(9):431–41. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davidson T, Kanoski S, Chan K, et al. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124(1):97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Squire L Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 119.Davidson T, Jarrard L. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59(2):167–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 120.Andersen P, Morris R, Amaral D, et al. Theories of hippocampal function in Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. (eds.), The Hippocampus Book. Oxford University Press; 2006:581–713. [Google Scholar]
- 121.Hargrave S, Davidson T, Zheng W, et al. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav Neurosci. 2016;130(1):123–35. doi: 10.1037/bne0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Attuquayefio T, Stevenson R, Boakes R, et al. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J Exp Psychol Anim Learn Cogn. 2016;42(4):415–528. doi: 10.1037/xan0000118. [DOI] [PubMed] [Google Scholar]
- 123.Davidson T, Hargrave S, Swithers S, et al. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–22. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davidson T, Monnot A, Neal A, et al. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Syrad H, Johnson L, Wardle J, et al. Appetitive traits and food intake patterns in early life. Am J Clin Nutr. 2016;103(1):231–5. doi: 10.3945/ajcn.115.117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hinton E, Comlek SL, D, Hamilton-Shield J. Satiety responsiveness: is it a predictor of food intake in adults and can it be enhanced to control consumption? Appetite. 2018;123:460. doi: 10.1016/j.appet.2017.11.048. [DOI] [Google Scholar]
- 127.Schuz B, Schuz N, Ferguson S. It’s the power of food: individual differences in food cue responsiveness and snacking in everyday life. Int J Behav Nutr Phys Act. 2015;12:149. doi: 10.1186/s12966-015-0312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mallan K, Nambiar S, Magarey A, et al. Satiety responsiveness in toddlerhood predicts energy intake and weight status at four years of age. Appetite. 2014;74(79–85). doi: 10.1016/j.appet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Reyes M, Hoyos V, Martinez S, et al. Satiety responsiveness and eating behavior among Chilean adolescents and the role of breastfeeding. Int J Obes. 2014;38(4):552–7. doi: 10.1038/ijo.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drapeau V, King N, Hetherington M, et al. Appetite sensations and satiety quotient: Predictors of energy intake and weight loss. Appetite. 2007;48(2):159–66. doi: 10.1016/j.appet.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Dalton M, Hollingworth S, Blundell J, et al. Weak satiety responsiveness is a reliable trait associated with hedonic risk factors for overeating among women. Nutrients. 2015;7(8):7421–36. doi: 10.3390/nu7095345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Viana V, Sinde S, Saxton J. Children’s Eating Behaviour Questionnaire: associations with BMI in Portuguese children. Br J Nutr. 2008;100(2):445–50. doi: 10.1017/S0007114508894391. [DOI] [PubMed] [Google Scholar]
- 133.Webber L, Hill C, Saxton J, et al. Eating behaviour and weight in children. Int J Obes. 2009;33(1):21–8. doi: 10.1038/ijo.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sanchez U, Weisstaub G, Santos J, et al. GOCS cohort: Children’s eating behavior scores and BMI. Eur J Clin Nutr. 2016;70(8):925–8. doi: 10.1038/ejcn.2016.18. [DOI] [PubMed] [Google Scholar]
- 135.Steinbekk S, Wichstrom L. Predictors of change in BMI from the age of 4 to 8. J Pediatr Psychol. 2015;40(10):1056–64. doi: 10.1093/jpepsy/jsv052. [DOI] [PubMed] [Google Scholar]
- 136.French S, Epstein L, Jeffery R, et al. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite. 2012;59(2):541–9. doi: 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Behar A, Crespo N, Garcia M, et al. Validation of a shortened version of the Children’s Eating Behavior Questionnaire and associations with BMI in a clinical sample of Latino children. J Nutr Educ Behav. 2018;50(4):372–8. doi: 10.1016/j.jneb.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 138.Domoff S, Miller A, Kaciroti N, et al. Validation of the Children’s Eating Behaviour Questionnaire in a low-income preschool-aged sample in the United States. Appetite. 2015;95:415–20. doi: 10.1016/j.appet.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sleddens E, Kremers S, Thijs C. The children’s eating behaviour questionnaire: factorial validity and association with Body Mass Index in Dutch children aged 6–7. Int J Behav Nutr Phys Act. 2008;4:49. doi: 10.1186/1479-5868-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Price M, Higgs S, Lee M. Self-reported eating traits: Underlying components of food responsivity and dietary restriction are positively related to BMI. Appetite. 2015;95:203–10. doi: 10.1016/j.appet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 141.Mallan KM, Fildes A, de la Piedad Garcia X, et al. Appetitive traits associated with higher and lower body mass index: evaluating the validity of the adult eating behaviour questionnaire in an Australian sample. Int J Behav Nutr Phys Act. 2017;14(1):130. doi: 10.1186/s12966-017-0587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Augustus-Horvath C, Tylka T. The acceptance model of intuitive eating: a comparison of women in emerging adulthood, early adulthood, and middle adulthood. J Couns Psychol. 2011;58(1):110–25. doi: 10.1037/a0022129. [DOI] [PubMed] [Google Scholar]
- 143.Camilleri G, Mejean C, Bellisle F, et al. Intuitive eating is inversely associated with body weight status in the general population-based NutriNet-Santé study. Obesity. 2016;24:1154–61. doi: 10.1002/oby.21440. [DOI] [PubMed] [Google Scholar]
- 144.Gast J, Campbell Nielson A, Hunt A, et al. Intuitive eating: associations with physical activity motivation and BMI. Am J Health Promot. 2015;28(3):e91–e9. doi: 10.4278/ajhp.130305-QUAN-97. [DOI] [PubMed] [Google Scholar]
- 145.Gast J, Madanat H, Nielson A. Are men more intuitive when it comes to eating and physical activity? Am J Mens Health. 2012;6(2):164–71. doi: 10.1177/1557988311428090. [DOI] [PubMed] [Google Scholar]
- 146.Madden C, Leong S, Gray A, et al. Eating in response to hunger and satiety signals is related to BMI in a nationwide sample of 1601 mid-age New Zealand women. Public Health Nutr. 2012;15(12):2272–9. doi: 10.1017/S1368980012000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tylka T. Development and psychometric evaluation of a measure of intuitive eating. J Coun Psychol. 2006;53(2):226–40. doi: 10.1037/0022-0167.53.2.226. [DOI] [Google Scholar]
- 148.Tylka T, Calogero R, Dandielsdottir S. Is intuitive eating the same as flexible dietary control? Their links to each other and well-being could provide an answer. Appetite. 2015;95:166–75. doi: 10.1016/j.appet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 149.Van Dyke N, Drinkwater E. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17(8):1757–66. doi: 10.1017/S1368980013002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hill C, Saxton J, Webber L, et al. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7--10-y-old children. Am J Clin Nutr. 2009;90(2):276–91. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 151.Van Jaarsveld C, Llewellyn C, Johnson L, et al. Prospective associations between appetitive traits and weight gain in infancy. Am J Clin Nutr. 2011;94:1562–7. doi: 10.3945/ajcn.111.015818. [DOI] [PubMed] [Google Scholar]
- 152.Parkinson K, Drewett R, Le Couteur A, et al. Do maternal ratings of appetite in infants predict later Child Eating Behaviour Questionnaire scores and body mass index? Appetite. 2010;54(2):186–90. doi: 10.1016/j.appet.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 153.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes. 2011;35(1):142–9. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- 154.Tetley AC, Brunstrom JM, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52(3):614–20. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 155.Meyer M, Risbrough V, Liang J, et al. Pavlovian conditioning of hedonic food cues in overweight and lean individuals. Paper presented at the Society for the Study of Ingestive Behavior, New Orleans, LA. 2013 [Google Scholar]
- 156.Castellanos E, Charboneau E, Dietrich M, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes. 2009;33(9):1063–73. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 157.Werthmann J, Roefs A, Nederkoorn C, et al. Can(not) take my eyes off it: attention bias for food in overweight participants Health Psychology. 2011;30(5):561–9. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- 158.Nijs I, Franken I, Muris P. Food-related Stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eat Behav. 2010;11(4):258–65. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 159.Nijs I, Muris P, Euser AS, et al. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–54. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 160.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19(9):1775–83. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nummenmaa L, Hietanen J, Calvo M, et al. Food catches the eye but not for everyone: a BMI-contingent attentional bias in rapid detection of nutriments. PloS One. 2011;6(5):e19215. doi: 10.1371/journal.pone.0019215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Doolan K, Breslin G, Hanna D, et al. Attentional bias to food-related visual cues: is there a role in obesity? Proc Nutr Soc. 2015;74(1):37–45. doi: 10.1017/S002966511400144X. [DOI] [PubMed] [Google Scholar]
- 163.Hendrikse J, Cachia R, Kothe E, et al. Attentional biases for food cues in overweight and individuals with obesity: a systematic review of the literature. Obes Rev. 2015;16(5):424–32. doi: 10.1111/obr.12265. [DOI] [PubMed] [Google Scholar]
- 164.Stojek M, Shank L, Vannucci A, et al. A systematic review of attentional biases in disorders involving binge eating. Appetite. 2018;123:367–89. doi: 10.1016/j.appet.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arguin H, Tremblay A, Blundell J, et al. Impact of a non-restrictive satiating diet on anthropometrics, satiety responsiveness and eating behaviour traits in obese men displaying a high or a low satiety phenotype. Br J Nutr. 2017;118(9):750–60. doi: 10.1017/S0007114517002549. [DOI] [PubMed] [Google Scholar]
- 166.Boutelle KN, Rhee K, Liang J, et al. Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: A randomized clinical trial. JAMA Pediatr. 2017;171(7):622–8. doi: 10.1001/jamapediatrics.2017.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boutelle KN, Kang Sim D, Manzano M, et al. Role of appetitive phenotype trajectory groups on child body weight during a family-based treatment for children with overweight or obesity. Int J Obes. 2019;43(11):2302–8. doi: 10.1038/s41366-019-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hunot-Alexander C, Beeken R, Goodman W, et al. Confirmation of the factor structure and reliability of the ‘Adult Eating Behavior Questionnaire’ in an adolescent sample. Front Psychol. 2019;10:1991. doi: 10.3389/fpsyg.2019.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zickgraf H, Rigby A. The Adult Eating Behaviour Questionnaire in a bariatric surgery-seeking sample: Factor structure, convergent validity, and associations with BMI. Eur Eat Disord Rev. 2019;27(1):97–104. doi: 10.1002/erv.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Williams D Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194–9. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Craighead L, Allen H. Appetite awareness training: A cognitive behavioral intervention for binge eating. Cogn Behav Pract. 1995;2:249–70. doi: 10.1016/S1077-7229(95)80013-1. [DOI] [Google Scholar]
- 172.Bloom T, Sharpe L, Mullan B, et al. A pilot evaluation of appetite-awareness training in the treatment of childhood overweight and obesity: a preliminary investigation. Int J Eat Disord. 2013;46(1):47–51. doi: 10.1002/eat.22041. [DOI] [PubMed] [Google Scholar]
- 173.Allen H, Craighead L. Appetite monitoring in the treatment of binge eating disorder. Behav Therapy. 1999;30:253–72. doi: 10.1016/S0005-7894(99)80007-0. [DOI] [Google Scholar]
- 174.Craske MG, Kircanski K, Zelikowsky M, et al. Optimizing inhibitory learning during exposure therapy. Behav Res & Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 175.Jacoby R, Abramowitz J. Inhibitory learning approaches to exposure therapy: A critical review and translation to obsessive-compulsive disorder. Clin Psychol Rev. 2016;69:28–40. doi: 10.1016/j.cpr.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 176.Weisman JS, Rodebaugh TL. Exposure therapy augmentation: A review and extension of techniques informed by an inhibitory learning approach. Clin Psychol Rev. 2018;59:41–51. doi: 10.1016/j.cpr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 177.Craske M, Treanor M, Conway C, et al. Maximizing exposure therapy: an inhibitory learning approach. Behav Res & Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boutelle KN, Zucker N, Peterson C, et al. Two novel treatments to reduce overeating in overweight children: a randomized controlled trial. J Consult Clin Psychol. 2011;79(6):759–71. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Boutelle KN, Zucker N, Peterson C, et al. An intervention based on Schachter’s externality theory for overweight children: the regulation of cues pilot. J Pediatr Psychol. 2014;39(4):405–17. doi: 10.1093/jpepsy/jst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boutelle KN, Knatz S, Carlson J, et al. An open trial targeting food cue reactivity and satiety sensitivity in overweight and obese binge eaters. Cogn & Behav Pract. 2017;24(3):363–73. doi: 10.1016/j.cbpra.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boutelle KN, Eichen D, Peterson C, et al. Design of the PACIFIC study: A randomized controlled trial evaluating a novel treatment for adults with overweight and obesity. Contemp Clin Trials. 2019;84:105824. doi: 10.1016/j.cct.2019.105824. [DOI] [PMC free article] [PubMed] [Google Scholar]


