Abstract
Emerging adulthood (18–25 years) represents a window of opportunity to modify the trajectory of cardiometabolic disease risk into older adulthood. Not known is the extent to which rest-activity rhythms (RAR) may be related to biomarkers of cardiometabolic health in this population. In this cross-sectional, observational study, 52 healthy emerging adults wore wrist accelerometers (14 consecutive days; 24h/day) for assessment of nonparametric RAR metrics, including interdaily stability (IS; day-to-day RAR consistency), intradaily variability (IV; within-day RAR fragmentation), and relative amplitude (RA; robustness of RAR), as well as autocorrelation (correlation of rest/activity levels at 24-hour lag-times). Cardiometabolic biomarkers, including body mass index (BMI), body fat percentage, blood pressure (BP), fasting lipids, glucose, and C-reactive protein (CRP) were assessed. Additional measures including physical activity, sleep duration, and habitual caffeine and alcohol consumption were also evaluated. A series of multivariable regression models of cardiometabolic biomarkers were used to quantify associations with RAR metrics. On average, participants were 20±1 years of age (21 male, 31 female), non-obese, and non-hypertensive. All were non-smokers and free of major diseases or conditions. In separate models, which adjusted for sex, BMI, moderate-vigorous physical activity, sleep duration, caffeine, and alcohol consumption, IS was inversely associated with total cholesterol (p≤0.01) and non-HDL cholesterol (p<0.05), IV was positively associated with CRP (p<0.05), and autocorrelation was inversely associated with total cholesterol (p<0.05) and CRP (p<0.05). Conversely, associations between RA and cardiometabolic biomarkers were nonsignificant after adjustment for BMI, alcohol, and caffeine consumption. In conclusion, RAR metrics, namely a higher IS, lower IV, and higher autocorrelation, emerged as novel biomarkers associated with more favorable indices of cardiometabolic health in this sample of apparently healthy emerging adults.
Keywords: emerging adults, interdaily stability, intradaily variability, relative amplitude, cardiometabolic risk, actigraphy, body activity 24-hour rhythm
Introduction
Cardiometabolic diseases including type-2 diabetes, hypertension, and obesity represent the most prevalent and deadly set of conditions that affect one in two American adults (Benjamin et al. 2019). Currently, 12% of adults have diabetes (U.S. Department of Health and Human Services 2020), 42.4% are obese (Hales et al. 2020), and 45% are hypertensive (Ostchega et al. 2020). Poor cardiometabolic health often begins in childhood and escalates across the lifespan (Gundogan et al. 2009; Wang et al. 2018). “Emerging adulthood” is a recently defined developmental period spanning the 18–25 y of age post-high-school age-range (Arnett et al. 2006; Gilmore 2019) that may be particularly important to thwarting cardiometabolic disease initiation and progression. For example, while cardiometabolic health behaviors are poor in high-school youth (52% are physically inactive (Kapteyn et al. 2018), 32% obtain insufficient sleep (Liu et al. 2016), 91% report not eating sufficient fruits and vegetables (Lee-Kwan et al. 2017)), they decline even further in the emerging adult phase (Frech 2012). Specifically, significant declines in physical activity are reported (Li et al. 2016; Corder et al. 2019), and this age group demonstrates greater increases in overweight and obesity than in any other life stage (Ng et al. 2014). Identifying novel factors in youth, including emerging adults, that may modify their trajectory of cardiometabolic disease risk is a key research priority (NHLBI 2019).
Current recommendations for cardiometabolic health behaviors are one-dimensional in that they prioritize duration. For example, physical activity recommendations are for ≥150 min/week of moderate-intensity, ≥75 min of vigorous-intensity aerobic activity, or an equivalent combination of both (Piercy et al. 2018), while sleep duration guidelines are for ≥7h/night (Watson et al. 2015). One plausible extension of this paradigm is to also consider rhythmicity of these behaviors and their interaction with endogenous biological rhythms (Bae et al. 2019). Experimental evidence suggests that circadian misalignment, induced via rapid and extreme (e.g., 8–12h) shifts in sleep-wake cycles, may contribute to excess cardiometabolic disease risk (Scheer et al. 2009; Leproult et al. 2014; Morris et al. 2016). Subsequently, habitual irregularity in sleep timing and/or duration have emerged as potential prognostic indicators of increased cardiometabolic disease risk, even in milder contexts (e.g., 1–3h shifts), such as those experienced by community-dwelling adults in free-living settings (Huang et al. 2019; Huang et al. 2020). However, examining sleep regularity in isolation permits assumptions, rather than objective assessment, of the extent of rhythmicity in activity exhibited during wakefulness.
Instead, continuous actigraphy allows for reliable and objective estimation of diurnal patterns of 24h movement over time (i.e., “rest-activity rhythms” (Ancoli-Israel et al. 2003; Littner et al. 2003)), which may provide additional insight on the link between behavioral rhythmicity and cardiometabolic health. For example, greater interdaily stability (IS), which describes day-to-day consistency in rest-activity rhythms, has been associated with a lower odds of diabetes, hypertension, and obesity in midlife and older adults (Sohail et al. 2015; Abbott et al. 2019). Intradaily variability (IV), which depicts the frequency and extent of transitions between rest and activity within a day, has also been associated with higher odds of obesity, lower cardiorespiratory fitness, and higher metabolic risk in adolescents (Garaulet et al. 2017). Lower relative amplitude (RA; indicative of a less robust rest-activity rhythm) has been associated with a higher body mass index (BMI) in both adolescents and adults (Cespedes Feliciano et al. 2017; Quante et al. 2019). Additionally, a lower RA is associated with increased risk of cardiovascular events in older men (Paudel et al. 2011), while patients with type-2 diabetes demonstrate a significantly lower IS, higher IV, and lower RA as compared to healthy controls (Cavalcanti-Ferreira et al. 2018). While the majority of this work has been conducted in adolescence or middle-aged/older adults, it consistently culminates to suggest that low rhythmicity and irregularity in rest-activity patterns may be part of an upstream behavioral phenotype of excess risk for cardiometabolic disease.
These lines of evidence: (1) the exacting clinical and population health burden of cardiometabolic disease, (2) the potential for emerging adulthood as a window of opportunity to forestall the progression of cardiometabolic disease, (3) the need to expand paradigms of cardiometabolic health behavior prescription to be more holistic, and, (4) the potential for rest-activity rhythmicity to inform an upstream phenotype of risk for cardiometabolic disease in emerging adults, provide the conceptual premise of the current investigation. The purpose of this study was to evaluate the relation between rest-activity rhythms (derived from 14 d of actigraphy) and cardiometabolic biomarkers in a sample of generally healthy emerging adults (18–25 y of age). We expected to find that more regular and more robust rest-activity rhythms would be associated with better cardiometabolic health in this sample.
Methods
Study Participants
Participants were recruited from the University of Delaware and the surrounding Newark, DE region. Participants were healthy, full-time undergraduate college students between the ages of 18 and 25 y. Individuals were excluded from participation if they: 1) had a history of any major chronic diseases or conditions (including cardiovascular, renal, metabolic, autoimmune, or chronic respiratory diseases, cancer, or sleep disorders), 2) were currently working night-shift-work, 3) were using sleep medication, 4) were diagnosed with depression, 5) had a seated resting blood pressure (BP) >140/90 mmHg, 6) were currently pregnant, 7) or were smokers (≥1 cigarette in last month). This study was approved by the Institutional Review Board at the University of Delaware and was conducted in accordance with international ethical standards including the Declaration of Helsinki and those specific to performing biological rhythms research on humans (Portaluppi et al. 2010). All participants provided verbal and written informed consent prior to participation.
Wrist Actigraphy
Participants were provided with an accelerometer (Micro Motionlogger; Ambulatory Monitoring Inc, Ardsley, NY) to be worn on the non-dominant wrist 24h/d, except for during water-based activities (i.e., showering), for 14 consecutive days and nights. Data were collected in zero-crossing mode and were stored in 60 s epochs. To be included in final rest-activity analyses, ≥10 d of accelerometry with >1000 min of data/d were required. All actigraphy data were visually screened and analyzed by the same researcher. Since all participants were college students, monitoring procedures were performed during the school semester when all participants were enrolled in a full-time course load and excluded any periods when students were on holiday breaks or during final examinations.
Rest-Activity Rhythm Analyses
Nonparametric analyses of rest-activity data were performed, as these methods make no assumptions about the waveform (i.e., shape) of the 24h activity pattern when data are plotted as a time series (Witting et al. 1990; Van Someren et al. 1999). Three nonparametric parameters were used to estimate 24h rest-activity rhythms for each participant: 1) IS, 2) IV, and 3) RA. All computations for nonparametric analyses are based on raw-data epochs that were resampled into 1h bins prior to analysis.
IS indicates the extent to which rest-activity patterns of individual days resemble each other and may reflect better coupling of rest-activity rhythms to supposedly stable external zeitgebers (i.e., external cues for entraining circadian rhythms). Values can range from 0 to 1, with 0 indicating very unstable day-to-day rhythms and 1 indicating perfect stability. IS is calculated as the ratio between the variance of the average 24h activity pattern around the mean and the overall variance, according to the following equation:
where n is the total number of data, p is the number of data per day (i.e., 24), are the hourly means of activity, is the mean of all data, and xi represents the individual data points.
IV indicates the magnitude of the hour-to-hour (i.e., within-day) transitions between rest and activity. For example, higher IV values are likely to be observed in individuals who often nap during the daytime or who often demonstrate increased activity during the nighttime. Values can range from 0 to 2 (or greater, rarely (Van Someren et al. 1999)), where 0 represents a perfect sine wave and larger values represent more fragmentation. IV is calculated as the ratio of the mean squares of the difference between all successive hours and the mean squares around the grand mean (i.e., the overall variance), according to the following equation:
RA depicts the difference between the most active 10h period (M10; the 10h block with the largest mean activity) and the least active 5h period (L5; the 5h block with the smallest mean activity) in the average 24h pattern. Greater RA values indicate a more robust 24h rhythm, reflective of relatively higher activity during wake and/or lower activity during sleep. Additionally, RA values are normalized to the overall activity, as seen in the following equation:
In addition to nonparametric analyses, autocorrelation analysis was performed, as this method also makes no assumptions about the waveform of activity data. Autocorrelation provides a correlation coefficient which reflects the association between the level of activity at specified time-lags (in this case, 24h). For example, high autocorrelation at clock times which are 24h apart would indicate a more predictable and consistent activity rhythm. All rest-activity rhythm analyses were performed using Action4 software version 1.16 (Ambulatory Monitoring, Inc., Ardsley, NY).
Cardiometabolic Biomarkers
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2) using a calibrated scale and a wall-mounted stadiometer at the time of participant screening. Body fat percentage (%) was also determined via bioelectrical impedance analysis (Tania TBF-300A, Arlington Heights, IL).
Upon completion of the 14 d actigraphy protocol, participants returned to the lab prior to 13:00h. Participants were instructed to fast for 12h, to abstain from caffeine, alcohol, exercise for a minimum of 12h, and to withhold any anti-inflammatory medications for a minimum of 24h prior to this visit. Following at least 10 min of rest in the supine position, participants underwent three brachial BP measurements, each separated by 1 min of quiet rest, using an oscillometric cuff on the upper right arm (SphygmoCor XCEL, ATCOR Medical, Naperville, IL). Measurements were averaged to derive a final representative resting BP value. Venous blood samples were also obtained and analyzed clinically for fasting glucose, lipids (total cholesterol, high-density lipoproteins [HDL], low-density lipoproteins [LDL], non-HDL cholesterol), triglyceride concentrations via spectrophotometry (Quest Diagnostics, Inc., Philadelphia, PA). C-reactive protein (CRP) concentrations were also determined in a subset of participants. Serum samples were collected in vacuum tubes without anticoagulant and centrifuged for 15 min at 1000 x g at 4° C. Serum was aliquoted and stored at −80° C until analysis using an enzyme-linked immunosorbent assay (R&D Systems, Inc, Minneapolis, MN).
Other Measures
Wrist accelerometry data were also processed to derive estimates of mean sleep duration (h/night). Data were scored with ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) using the University of California, San Diego scoring algorithm. Use of this algorithm has been validated to produce accurate and reliable sleep estimates relative to polysomnography (Jean-Louis et al. 2001). Participants were also provided with a standardized sleep diary (Carney et al. 2012) to be used simultaneously with wrist accelerometry and were instructed to record in the diary daily. Sleep diaries were primarily used as a supplementary reference during retrospective analysis in the case of any inconsistencies with accelerometry data. Relative to recommendations which suggest >7 nights of accelerometry data be used for evaluation of sleep duration (Aili et al. 2017; Fekedulegn et al. 2020), a more conservative threshold of ≥10 nights of sleep accelerometry data was required for inclusion in final analyses.
A torso accelerometer (ActiGraph wgt3x-bt, ActiGraph L.L.C., Pensacola, FL) was provided to all participants for estimation of habitual moderate-vigorous physical activity (MVPA; mean min/d). Participants were instructed to wear the accelerometer during all waking hours for 7 consecutive days which overlapped with wrist accelerometry. ActiGraphs were analyzed using ActiLife software version 6.11.9. Wear time was validated using the Troiano algorithm (Troiano et al. 2008) and activity variables were calculated using the Freedson Combination 1998 algorithm (Freedson et al. 1998). To be included in final analyses, a minimum of 4 d with ≥10 h of wear time were required (Colley et al. 2010).
Habitual alcohol consumption was estimated for each participant via self-reported questionnaire which asked, “How many alcoholic beverages do you drink per week, on average?” with specific instructions to consider all beer, wine, and liquor beverages. Similarly, habitual caffeine consumption was estimated per self-reported responses to “How many caffeinated beverages do you drink per week, on average?” with specific instructions to consider all coffee, tea, and soft drinks.
Statistical Analyses
Subject characteristics and rest-activity rhythm parameters were summarized using means and standard deviations (SD). Four linear regression models were then used to examine associations between rest-activity rhythms and cardiometabolic biomarkers, with the first of these models (model 1) controlling only for sex.
To determine if the association between rest-activity rhythms and cardiometabolic biomarkers was independent of sleep duration and MVPA, in addition to sex, model 2 additionally adjusted for these covariates.
To determine if the association between rest-activity rhythms and cardiometabolic biomarkers was independent of habitual alcohol and caffeine consumption, in addition to sex, model 3 adjusted for these covariates.
Finally, to examine the association between rest-activity rhythms and cardiometabolic biomarkers independent of the potential influence of adiposity, model 4 adjusted for both sex and BMI. This covariate was chosen over % body fat to avoid multicollinearity (a strong point-biserial correlation was observed between sex and % body fat [rpb=−0.77, p<0.001]).
Regression results were presented using β values and 95% confidence intervals (CI) in terms of SD-units; in other words, β gives the expected change in a given outcome for a 1-SD increase in an accompanying rest-activity rhythm parameter. Significance was set at α=0.05 for all tests. All analyses were performed using the Statistical Package for the Social Sciences (SPSS version 26.0, IBM, NY).
RESULTS
Subject characteristics and rest-activity parameters
A total of 59 participants were enrolled in this study. Three participants were withdrawn prior to completion of sleep monitoring (two upon disclosure of treatment for depression, one unable to complete actigraphy monitoring), two participants provided incomplete actigraphy data (i.e., <10 d), and two participants were unable to complete the study protocol due to COVID-19-related research restrictions during their enrollment. Thus, 52 participants were included in the final analytic sample.
Participant characteristics are displayed in Table 1. Participants were 20 ± 1 y of age (21 males, 31 females; 69% white). Fasting lipids and glucose values were available for 51 participants, and CRP was available in a smaller subset (n=42). By design, participants were generally healthy, as demonstrated by mean BMI, resting BP, fasting lipids, fasting glucose, and CRP values all within normal ranges for this age group. Participants obtained an average of 7.1 ± 0.7 h/night of sleep and 67 ± 24 min/d of MVPA. Additionally, participants reported an average of 5.4 ± 5.6 alcoholic beverages and 5.1 ± 5.4 caffeinated beverages/week, respectively.
Table 1.
Descriptive Characteristics of Study Sample
| Measure | Mean ± SD (n = 52) |
|---|---|
| Subject Characteristics | |
| Age, y | 20 ± 1 |
| Sex, m/f | 21/31 |
| BMI, kg/m2 | 23.7 ± 2.4 |
| Body Fat, % | 22.8 ± 8.2 |
| Systolic BP, mmHg | 117 ± 9 |
| Diastolic BP, mmHg | 69 ± 7 |
| Blood Chemistry | |
| Total Cholesterol, mg/dL (n=51) | 158 ± 27 |
| LDL Cholesterol, mg/dL (n=51) | 85 ± 23 |
| HDL Cholesterol, mg/dL (n=51) | 58 ± 13 |
| Non-HDL Cholesterol, mg/dL (n=51) | 100 ± 24 |
| Triglycerides, mg/dL (n=51) | 72 ± 30 |
| Glucose, mg/dL (n=51) | 85 ± 7 |
| CRP, mg/L (n=42) | 0.90 ± 0.79 |
| Health Behavior Characteristics | |
| Mean Sleep Duration, h/night | 7.1 ± 0.7 |
| Mean MVPA, min/day | 67 ± 24 |
| Alcohol Consumption, drinks/week | 5.4 ± 5.6 |
| Caffeine Consumption, drinks/week | 5.1 ± 5.4 |
| Rest-Activity Rhythm Parameters (Observable Range) | |
| Interdaily Stability (0 to 1) | 0.64 ± 0.10 |
| Intradaily Variability (0 to ~2) | 0.45 ± 0.09 |
| Relative Amplitude (0 to 1) | 0.81 ± 0.06 |
| Autocorrelation at 24h (−1 to +1) | 0.44 ± 0.10 |
BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CRP, c-reactive protein; MVPA, moderate-vigorous physical activity
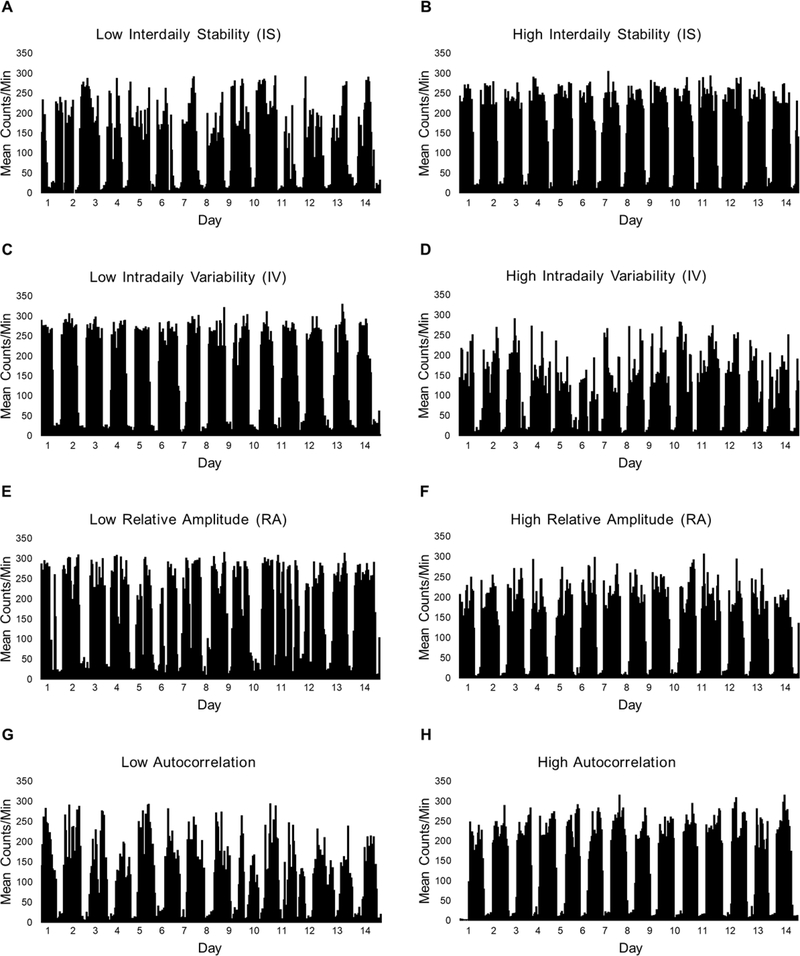
Participants in the analytic sample provided a mean of 13.5 ± 0.8 d of valid wrist actigraphy data, with a mean of 1427 ± 51 min of valid data/d. Rest-activity rhythm parameters for the sample are also displayed in Table 1. Additionally, Figure 1 illustrates eight examples of rest-activity patterns across 14 consecutive days and nights for eight emerging adults who demonstrated either low or high IS, IV, RA, or autocorrelation.
Figure 1. Activity plots illustrating rest-activity patterns in emerging adults.
Plots representing low and high interdaily stability (A, B), intradaily variability (C, D), relative amplitude (E, F), and activity 24h autocorrelation (G, H) are displayed for visualization of each rest-activity rhythm parameter. Plots depict activity levels (mean counts/min) for every hour across 14 consecutive days and nights for eight emerging adults from this sample.
Rest-activity rhythms and cardiometabolic biomarkers
Associations between rest-activity parameters and cardiometabolic biomarkers are displayed in Table 2. After adjusting for sex, every 1-SD increase in IS (i.e., increased similarity in rest-activity rhythms from one day to the next) was inversely associated with % body fat (−1.48%, 95% CI: −2.95, −0.03), total cholesterol (−10.39 mg/dL, 95% CI: −17.42, −3.37), LDL (−7.21 mg/dL, 95% CI: −13.44, −0.98), non-HDL cholesterol (−8.45 mg/dL, 95% CI: −15.05, −1.85), and triglycerides (−9.38 mg/dL, 95% CI: −17.61, −1.16). Associations were similar after adjusting for sex, MVPA, and sleep duration; however, only the associations between IS with total cholesterol and non-HDL cholesterol remained in models adjusting for sex, alcohol, and caffeine consumption (total cholesterol: −10.23 mg/dL, 95% CI: −17.71, −2.75; non-HDL cholesterol: −7.45, 95% CI: −14.44, −0.47).
Table 2.
Associations between Rest-Activity Rhythms and Cardiometabolic Biomarkers
| Rest-Activity Rhythm Parameter | Cardiometabolic Biomarker | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | ||
| Interdaily Stability | BMI, kg/m2 | −0.38 (−1.07, 0.31) | −0.16 (−0.85, 0.53) | −0.34 (−1.07, 0.40) |
| Body Fat, % | −1.48 (−2.95, −0.03)* | −1.24 (−2.75, 0.28) | −1.19 (−2.71, 0.32) | |
| Systolic BP, mmHg | −1.02 (−3.04, 1.00) | −1.10 (−3.25, 1.04) | −0.59 (−2.71, 1.52) | |
| Diastolic BP, mmHg | −0.45 (−2.36, 1.46) | −0.46 (−2.43, 1.51) | −0.04 (−2.03, 1.95) | |
| Total-C, mg/dL | −10.39 (−17.42, −3.37)† | −11.41 (−18.77, −4.06)† | −10.23 (−17.71, −2.75)† | |
| LDL-C, mg/dL | −7.21 (−13.44, −0.98)* | −7.91 (−14.53, −1.29)* | −6.43 (−13.04, 0.19) | |
| HDL-C, mg/dL | −1.94 (−5.55, 1.66) | −2.34 (−6.25, 1.58) | −2.78 (−6.50, 0.95) | |
| Non-HDL-C, mg/dL | −8.45 (−15.05, −1.85)* | −9.08 (−16.19, −1.96)* | −7.45 (−14.44, −0.47)* | |
| Triglycerides, mg/dL | −9.38 (−17.61, −1.16)* | −9.04 (−17.71, −0.37)* | −7.66 (−16.28, 0.97) | |
| Glucose, mg/dL | −0.62 (−2.49, 1.25) | −0.85 (−2.88, 1.18) | −0.49 (−2.49, 1.51) | |
| CRP, mg/L | −0.22 (−0.47, 0.02) | −0.21 (−0.47, 0.04) | −0.25 (−5.14, 0.02) | |
| Intradaily Variability | BMI, kg/m2 | 0.17 (−0.53, 0.88) | −0.17 (−0.87, 0.53) | 0.20 (−0.55, 0.96) |
| Body Fat, % | 0.41 (−1.13, 1.95) | −0.33 (−1.92, 1.26) | 0.58 (−1.00, 2.15) | |
| Systolic BP, mmHg | 0.06 (−2.02, 2.13) | 0.03 (−2.18, 2.25) | 0.07 (−2.09, 2.23) | |
| Diastolic BP, mmHg | 0.06 (−1.88, 2.00) | −0.34 (−2.36, 1.67) | 0.13 (−1.89, 2.16) | |
| Total-C, mg/dL | 7.45 (−0.06, 14.97) | 7.53 (−0.51, 13.16) | 8.57 (0.71, 16.43)* | |
| LDL-C, mg/dL | 4.36 (−2.25, 10.96) | 4.03 (−3.10, 11.17) | 4.62 (−2.30, 11.53) | |
| HDL-C, mg/dL | 1.97 (−1.72, 5.66) | 2.44 (−1.57, 6.46) | 2.87 (−0.94, 6.68) | |
| Non-HDL-C, mg/dL | 5.49 (−1.54, 12.51) | 5.09 (−2.60, 12.77) | 5.71 (−1.60, 13.01) | |
| Triglycerides, mg/dL | 7.51 (−1.09, 16.10) | 7.59 (−1.46, 16.65) | 7.76 (−1.07, 16.59) | |
| Glucose, mg/dL | 0.48 (−1.44, 2.40) | 0.45 (−1.64, 2.55) | 0.43 (−1.61, 2.48) | |
| CRP, mg/L | 0.30 (0.05, 0.56)* | 0.31 (0.04, 0.58)* | 0.38 (0.10, 0.66)† | |
| Relative Amplitude | BMI, kg/m2 | −0.69 (−1.35, −0.02)* | −0.28 (−1.03, 0.46) | −0.67 (−1.37, 0.03) |
| Body Fat, % | −1.63 (−3.07, −0.19)* | −1.49 (−3.13, 0.15) | −1.44 (−2.90, 0.02) | |
| Systolic BP, mmHg | −1.75 (−3.72, 0.22) | −2.01 (−4.29, 0.28) | −1.49 (−3.52, 0.54) | |
| Diastolic BP, mmHg | −0.69 (−2.59, 1.20) | −1.59 (−3.69, 0.51) | −0.43 (−2.37, 1.51) | |
| Total-C, mg/dL | −6.57 (−13.92, 0.77) | −8.40 (−16.89, 0.09) | −6.23 (−13.88, 1.42) | |
| LDL-C, mg/dL | −6.52 (−12.77, −0.27)* | −8.19 (−15.45, −0.94)* | −5.86 (−12.34, 0.63) | |
| HDL-C, mg/dL | 0.60 (−3.02, 4.22) | 0.63 (−3.70, 4.95) | 0.07 (−3.66, 3.79) | |
| Non-HDL-C, mg/dL | −7.17 (−13.84, −0.49)* | −9.02 (−16.87, −1.18)* | −6.29 (−13.19, 0.60) | |
| Triglycerides, mg/dL | −4.93 (−13.42, 3.55) | −6.68 (−16.38, 3.01) | −3.36 (−12.00, 0.60) | |
| Glucose, mg/dL | −0.03 (−1.90, 1.85) | 0.48 (−1.75, 2.70) | 0.11 (−1.84, 2.07) | |
| CRP, mg/L | −0.13 (−0.36, 0.10) | −0.12 (−0.40, 0.16) | −0.14 (−0.38, 0.11) | |
| Autocorrelation at 24h | BMI, kg/m2 | −0.24 (−0.93, 0.45) | 0.00 (−0.69, 0.69) | −0.21 (−0.95, 0.53) |
| Body Fat, % | −0.89 (−2.38, 0.60) | −0.48 (−2.03, 1.08) | −0.69 (−2.23, 0.85) | |
| Systolic BP, mmHg | −0.76 (−2.78, 1.26) | −0.81 (−2.97, 1.35) | −0.42 (−2.54, 1.70) | |
| Diastolic BP, mmHg | −0.84 (−2.73, 1.04) | −0.49 (−2.46, 1.48) | −0.60 (−2.58, 1.39) | |
| Total-C, mg/dL | −8.61 (−15.78, −1.63)* | −8.27 (−15.99, −0.54)* | −8.81 (−16.43, −1.18)* | |
| LDL-C, mg/dL | −5.96 (−12.26, 0.34) | −5.45 (−12.29, 1.39) | −5.34 (−12.03, 1.35) | |
| HDL-C, mg/dL | −1.60 (−5.19, 2.00) | −1.77 (−5.70, 2.17) | −2.64 (−6.37, 1.09) | |
| Non-HDL-C, mg/dL | −7.01 (−13.70, −0.32)* | −6.50 (−13.86, 0.86) | −6.16 (−13.25, 0.92) | |
| Triglycerides, mg/dL | −7.26 (−15.61, 1.09) | −7.89 (−16.65, 0.86) | −5.71 (−14.5, 3.03) | |
| Glucose, mg/dL | −0.26 (−2.72, 1.61) | −0.49 (−2.53, 1.54) | −0.09 (−2.09, 1.91) | |
| CRP, mg/L | −0.24 (−0.48, 0.00)* | −0.25 (−0.50, 0.00)* | −0.29 (−0.55, −0.02)* |
Results are presented as unstandardized β values and 95% confidence intervals (CI) for every 1-SD increase in each rest-activity rhythm parameter. Model 1 adjusts for sex; Model 2 adjusts for sex, MVPA, and sleep duration. Model 3 adjusts for sex, alcohol, and caffeine consumption.
p≤0.05
p≤0.01. BMI, body mass index; BP, blood pressure; C, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CRP, C-reactive protein
Of all cardiometabolic biomarkers tested, IV was only associated with CRP concentration, such that every 1-SD increase in IV (i.e., greater within-day fragmentation of activity rhythms) was positively associated with a 0.30 mg/L increase in CRP concentration (95% CI: 0.05, 0.56) after adjusting for sex. This association remained after including MVPA and sleep duration in the model, and again in models adjusting for sex, alcohol, and caffeine consumption.
Every 1-SD increase in RA (i.e., more robust rest-activity rhythms) was inversely associated with BMI (−0.69 kg/m2, CI: −1.35, −0.02), % body fat (−1.63%, CI: −3.07, −0.19), LDL (−6.52 mg/dL, CI: −12.77, −0.27), and non-HDL cholesterol (−7.17, CI: −13.84, −0.49). Associations between LDL and non-HDL cholesterol remained significant after adjusting for sex plus MVPA and sleep duration, while the associations between RA and adiposity measures were lost. Associations between RA and all cardiometabolic biomarkers were eventually lost in models that adjusted for sex plus alcohol and caffeine consumption.
Each 1-SD increase in the activity 24h autocorrelation lag time (i.e., correlation in activity levels at the same clock time for each day) was inversely associated with circulating lipids, namely total cholesterol (−8.61 mg/dL, CI: −15.78, −1.63) and non-HDL cholesterol (−7.01 mg/dL, CI: −13.70, −0.32), as well as lower CRP concentrations (−0.24 mg/L, CI: −0.48, 0.00). After adding MVPA and sleep duration to the model, inverse associations with total cholesterol (−8.27 mg/dL, CI: −15.99, −0.54) and CRP (−0.25 mg/dL, CI: −0.50, 0.00) remained, while the association with non-HDL cholesterol became nonsignificant (p=0.08). Results were similar in models adjusting for sex, alcohol, and caffeine consumption.
Influence of BMI
To determine if associations between rest-activity parameters and cardiometabolic biomarkers were independent of adiposity, all associations (except % body fat) were evaluated after adjustment for BMI plus sex (Table 3). IS remained significantly inversely associated with total cholesterol (−9.92 mg/dL, 95% CI: −17.05, −2.79) and non-HDL cholesterol (−7.82 mg/dL, 95% CI: −14.46, −1.17), while inverse associations with LDL (−6.51 mg/dL, 95% CI: −12.74, −0.27) and triglycerides (−10.10 mg/dL, 95% CI: −18.41, −1.79) also emerged. As seen in all prior models, IV remained positively associated with CRP concentrations (0.30 mg/L, 95% CI: 0.06, 0.55). Similarly, the activity 24h autocorrelation remained significantly inversely associated with total cholesterol (−8.24 mg/dL, 95% CI: −15.44, −1.04) and CRP concentrations (−0.24 mg/L, −0.48, −0.01). Conversely, all associations between RA and cardiometabolic biomarkers remained nonsignificant.
Table 3.
Associations between Rest-Activity Rhythms and Cardiometabolic Biomarkers after adjustment for BMI
| Rest-Activity Rhythm Parameter | Cardiometabolic Biomarker | Model 4 |
|---|---|---|
| β (95% CI) | ||
| Interdaily Stability | Systolic BP, mmHg | −0.67 (−2.63, 1.30) |
| Diastolic BP, mmHg | −0.25 (−2.17, 1.66) | |
| Total-C, mg/dL | −9.92 (−17.05, −2.79)† | |
| LDL-C, mg/dL | −6.51 (−12.74, −0.27)* | |
| HDL-C, mg/dL | −2.10 (−5.78, 1.57) | |
| Non-HDL-C, mg/dL | −7.82 (−14.46, −1.17)* | |
| Triglycerides, mg/dL | −10.10 (−18.41, −1.79)* | |
| Glucose, mg/dL | −0.51 (−2.42, 1.39) | |
| CRP, mg/L | −0.21 (−0.45, 0.04) | |
| Intradaily Variability | Systolic BP, mmHg | −0.11 (−2.10, 1.88) |
| Diastolic BP, mmHg | −0.03 (−1.96, 1.90) | |
| Total-C, mg/dL | 7.75 (−0.94, 16.39) | |
| LDL-C, mg/dL | 4.00 (−2.50, 10.50) | |
| HDL-C, mg/dL | 2.02 (−1.71, 5.75) | |
| Non-HDL-C, mg/dL | 5.15 (−1.81, 12.11) | |
| Triglycerides, mg/dL | 7.18 (−0.34, 14.69) | |
| Glucose, mg/dL | 0.43 (−1.51, 2.36) | |
| CRP, mg/L | 0.30 (0.06, 0.55)* | |
| Relative Amplitude | Systolic BP, mmHg | −1.19 (−3.17, 0.81) |
| Diastolic BP, mmHg | −0.36 (−2.33, −1.61) | |
| Total-C, mg/dL | −6.28 (−15.11, 2.55) | |
| LDL-C, mg/dL | −5.39 (−11.87, 1.09) | |
| HDL-C, mg/dL | 0.43 (−3.38, 4.24) | |
| Non-HDL-C, mg/dL | −6.17 (−13.13, 0.78) | |
| Triglycerides, mg/dL | −5.74 (−13.43, 1.95) | |
| Glucose, mg/dL | 0.20 (−1.75, 2.15) | |
| CRP, mg/L | −0.09 (−0.33, 0.14) | |
| Autocorrelation at 24h | Systolic BP, mmHg | −0.53 (−2.48, 1.41) |
| Diastolic BP, mmHg | −0.72 (−2.61, 1.16) | |
| Total-C, mg/dL | −8.24 (−15.44, −1.04)* | |
| LDL-C, mg/dL | −5.47 (−11.70, 0.76) | |
| HDL-C, mg/dL | −1.68 (−5.33, 1.96) | |
| Non-HDL-C, mg/dL | −6.56 (−13.22, 0.10) | |
| Triglycerides, mg/dL | −7.62 (−16.03, 0.78) | |
| Glucose, mg/dL | −0.19 (−2.07, 1.70) | |
| CRP, mg/L | −0.24 (−0.48, −0.01)* |
Results are presented as unstandardized β values and 95% confidence intervals (CI) for every 1-SD increase in each rest-activity rhythm parameter. Model 4 also adjusts for sex.
p≤0.05
p≤0.01. BP, blood pressure; C, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CRP, C-reactive protein
DISCUSSION
The identification of novel markers of cardiometabolic health in youth, especially those that are modifiable to intervention, represents a key research priority (NHLBI 2019). We used multivariable regression models to test associations between rest-activity rhythm parameters of IS, IV, RA, and activity 24h autocorrelation with several cardiometabolic measures of fasting cholesterol, triglycerides, and glucose, CRP, BMI, % body fat, and resting systolic and diastolic BP in a sample of emerging adults. After adjustments for several possible confounding variables, the key and novel findings were that IS was consistently inversely associated with serum lipids, namely total cholesterol and non-HDL cholesterol, IV was consistently positively associated with CRP, and activity 24h autocorrelation was consistently inversely associated with total cholesterol and CRP in this sample. These data suggest that rest-activity rhythm metrics may be novel, modifiable targets with which to attenuate the trajectory of cardiometabolic disease risk in emerging adulthood.
A central finding of this study was that increased IS and activity 24h autocorrelation were associated with a more optimal lipid profile, and these associations were independent of sex, adiposity, sleep duration, MVPA, and alcohol and caffeine consumption. Specifically, for every 1-SD increase in IS (i.e., increased similarity in rest-activity patterns from one day to the next), total cholesterol decreased by ~10 mg/dL and non-HDL cholesterol decreased by ~8 mg/dL, while every 1-SD increase in activity 24h autocorrelation (i.e., increased correlation of activity levels at 24h time lags) was associated with ~8 mg/dL lower total cholesterol levels. These changes represent a 5–8% reduction when compared to mean values. Lowering cholesterol is a high clinical priority for preventing CVD morbidity and mortality (Arnett et al. 2019), even in young adults, as higher cholesterol in young adulthood is associated with a significantly greater risk of CVD later in life (Jeong et al. 2018; Zhang et al. 2019). While a 10% reduction in serum cholesterol has been recognized for its beneficial impact on lowering CVD risk (Law et al. 1994; Gould et al. 1995), smaller changes are also relevant, as every 1% reduction in total cholesterol is estimated to yield a 2% reduction in CV events (Fager et al. 1997). Considering our data are derived from generally healthy young adults with relatively normal circulating lipid concentrations, these findings suggest that encouraging regular rest-activity rhythms may have amplified benefits for those demonstrating less optimal lipid profiles. Moreover, the full risk-reduction benefits from lowered lipid concentrations are found when maintained over longer periods (Law et al. 1994), suggesting that initiating interventions that promote rest-activity rhythmicity earlier in life (e.g., emerging adulthood) may provide more potent protection from future cardiometabolic complications than those initiated later in adulthood.
Our findings converge with previous work examining the association between IS and the activity 24h autocorrelation with cardiometabolic outcomes in several ways. For example, in a group of 83 working adults (44.3 ± 11.9 y of age) who wore a Fitbit for 21 d, greater steps-based IS was associated with a better lipid profile characterized by higher HDL and lower triglycerides (Rykov et al. 2020). In a sample of 1137 older adults, higher IS was independently associated with numerous cardiometabolic comorbidities, including a lower odds of having metabolic syndrome, diabetes, obesity, hypertension, dyslipidemia, and CVD (Sohail et al. 2015). Additionally, activity autocorrelation at the 24h lag time has previously been related to LDL-cholesterol, triglycerides, and health-related quality of life, which remained after adjustment for demographics, physical activity, and sleep disturbances in a small group of middle-aged adults (Buman et al. 2016). The current study converges with these previous studies and extends them by showing that actigraphy-derived metrics of between-day behavioral rhythmicity are sensitive biomarkers of cardiometabolic health, and particularly lipid profile, even in a sample of otherwise healthy emerging adults.
This study also showed that for every 1-SD increase in IV, CRP increased by ~0.30 mg/L, while every 1-SD increase in the activity 24h autocorrelation yielded a reduction in CRP by ~0.25 mg/L. Considering a lower autocorrelation indicates less day-to-day consistency in rest/activity levels at any given time, our autocorrelation results are comparable to the growing body of evidence that links circadian disruption with inflammation. For example, epidemiological evidence suggests that inflammatory markers may be higher in shift workers than daytime workers (Sookoian et al. 2007; Burgueno et al. 2010; Puttonen et al. 2011). Highly controlled experimental studies have also identified an independent influence of circadian misalignment on circulating inflammatory markers, including CRP, interleukin-6, TNF-α, and resistin, in both shift-working and non-shift-working adults (Leproult et al. 2014; Wright et al. 2015; Morris et al. 2016; Morris et al. 2017). The current study also compliments and extends prior sleep regularity studies suggesting that relatively mild day-to-day irregularity in behavioral rhythms may independently associate with elevated inflammatory activity (Okun et al. 2011; Park et al. 2016). To our knowledge, only one other study has specifically reported on actigraphy-derived rest-activity rhythm parameters and circulating markers of inflammation. Consistent with our findings, Buman et al. previously found autocorrelation of activity levels at 24h lag-time to be associated with circulating levels of CRP in a sample of 17 men and 3 women (49.7 ± 9.1 y of age) (Buman et al. 2016). Our finding that CRP was higher in those with higher IV is also plausible, as IV indicates the extent of fragmentation of the rest-activity rhythm and could reflect a greater occurrence of daytime naps and/or nocturnal awakenings (Cespedes Feliciano et al. 2017). Indeed, sleep disturbances (Irwin et al. 2016), poor sleep quality (Nowakowski et al. 2018; Vallat et al. 2020), and daytime napping (Leng et al. 2014; Devine et al. 2016) have previously been associated with elevated inflammatory markers in several studies, including those examining adolescents and young adults (El-Sheikh et al. 2007; Okun et al. 2009; Leng et al. 2014).
Our study conveys distinct public health relevance in light of our focus on emerging adults enrolled in higher education. Full-time college students, especially those who live on-campus, spend the majority of time in a structured school setting and are inadvertently exposed to ecological and environmental factors specific to that setting, such as artificial lighting, varying class schedules, housing conditions, public transportation, green space, food availability, access to technology, and access to recreational facilities. Consequently, school environments have a strong influence on all components of the rest-activity rhythm (i.e., sleep, wake, physical activity). Indeed, it has been reported that diurnal physical activity (Sulemana et al. 2006) and sleep-wake patterns (Onyper et al. 2012) are largely determined by school schedules. Our findings support the notion that higher education institutions have great potential to promote health behaviors (Plotnikoff et al. 2015), and suggest that integrating healthy patterns of rest and activity into the university setting could enhance efforts to improve cardiometabolic health in this life stage.
Our findings partially diverge from prior studies, as adjusted RA was not found to associate with any of the cardiometabolic metrics measured. Higher RA, which represents higher activity during wakefulness and lower activity during the night, has previously been shown to be associated with measures of adiposity in both adolescents and adults (Cespedes Feliciano et al. 2017; Quante et al. 2019). One possible reason for our null finding is a “ceiling effect.” Our young adult college student sample consisted of generally healthy non-smokers who reported a mean RA of 0.81 ± 0.06, a mean MVPA of 67 ± 24 min/d, and 7.1 ± 0.7h of sleep/night. Thus, on average, the RA was relatively high, and likely a result of examining participants who were very active and met sleep duration guidelines (Watson et al. 2015). Young adults who do not go to college are often less healthy: they are more likely to smoke, less likely to engage in regular physical activity, and less likely to eat a healthful diet (Cutler et al. 2010; Lawrence 2017). Our data underscore that to fully understand the relationship between rest-activity metrics, and particularly RA, with cardiometabolic factors in emerging adults, future studies should include those who do not go to college.
Identifying mechanisms linking rest-activity rhythms with lipid profile and inflammation is important for conceptual understanding and for developing effective interventions. One candidate is dietary intake, and in particular, meal timing. Cross-sectional observational studies have found meal irregularity to associate with higher BMI and waist circumference, low-grade inflammation, and increased risk of metabolic syndrome in adults (Sierra-Johnson et al. 2008; Pot et al. 2014; Guinter et al. 2019). Similarly, human laboratory studies have reported beneficial effects of regular mean frequency on fasting lipids and postprandial insulin profiles when compared to an irregular meal frequency (Farshchi et al. 2004; 2005). In one cohort study conducted in 1083 youth who were re-contacted across a 27 y period, irregular breakfast at age 16 y predicted metabolic syndrome at age 43 y, independent of other lifestyle and eating factors (Wennberg et al. 2016). Taken together, this literature presents the hypothesis that irregular rest-activity rhythms may be bidirectionally linked to meal irregularity (i.e., timing/frequency), which in turn could cause, or exacerbate, impairments of lipid metabolism and inflammation. Indeed, accumulating evidence suggests that meal timing may impact daily biological rhythms via an exogenous influence on behavior (Boulos et al. 1980) as well as an endogenous influence on circadian phase of peripheral tissues (Wehrens et al. 2017). Conversely, the inverse may also be true, as the timing that one sleeps (or, does not sleep) exerts a direct influence on the timing that one eats (Baron et al. 2011; Spaeth et al. 2013). The interactions between rest-activity rhythms, other behavioral rhythms (i.e., eating behavior), and cardiometabolic health represents an intriguing line of inquiry that will require further examination in future studies.
Limitations of this study include a cross-sectional design which prevents the examination of directionality between rest-activity rhythms and cardiometabolic biomarkers, and the use of a volunteer sample from a distinct Northeastern U.S. suburban university setting. Thus, our findings may not be generalizable to other geographical regions or those with differing racial demographics. Additionally, the small sample size limited our ability to include all covariates of interest into one model. However, it is important to note that associations across regression models were consistent and are in agreement with previous literature on rest-activity rhythms and cardiometabolic health in larger and more diverse samples (Sohail et al. 2015; Buman et al. 2016; Garaulet et al. 2017; Rykov et al. 2020), supporting the validity of our results. We also did not consider diet quality or eating patterns, both of which are strong determinants of cardiometabolic outcomes (Schwingshackl et al. 2015; Wennberg et al. 2016) and should be considered in future studies. Finally, nonsignificant associations between rest-activity rhythms and resting BP in this study highlight the need for future investigations to employ ambulatory BP monitoring, as nocturnal BP characteristics are strong predictors of cardiovascular disease (Hermida et al. 2018). Strengths of this study include the focus on emerging adults (18–25 y of age) who are being recognized as a previously overlooked population in the context of cardiometabolic health (Nelson et al. 2008). The use of 14 consecutive days of accelerometer data of both day and night times to provide a rich and rigorous dataset with minimal missing data that builds on previous methodologies that relied on fewer days of objective monitoring (Garaulet et al. 2017; Quante et al. 2019).
In summary, the current study provides initial evidence that metrics of rest-activity patterns in emerging adults may be a modifiable marker of future cardiometabolic disease risk. Specifically, our findings suggest that greater consistency in day-to-day patterns of rest and activity, and less fragmentation of the rest-activity rhythm, appear to independently associate with more favorable indices of cardiometabolic health in this sample of apparently healthy emerging adults. These data present several avenues for future study. For example, prospective, cohort examinations are needed to test this hypothesis in large, ecologically valid samples of emerging adults that objectively consider other determinants of cardiometabolic outcomes, such as diet and tobacco use. Such studies can also help clarify the magnitude and temporality of the relationships between rest-activity metrics with cardiometabolic outcomes. This may also provide an opportunity to examine the role of socioenvironmental factors in these relationships, as numerous socioeconomic (Armstrong et al. 2018; Patterson et al. 2018), residential (Grandner et al. 2015), and neighborhood factors (Bennett et al. 2007; Basner et al. 2018) influence sleep and physical activity duration, and may also influence their regularity. Additionally, while the main effect of rest-activity metrics on cardiometabolic measures are indicated here, the mechanisms underpinning these relationships are not clear. Elucidating the mechanisms linking rest-activity rhythms with cardiometabolic markers is necessary to inform primary interventions, and to continue advancing this line of research.
Acknowledgements
The authors would like to thank all study participants, as well as Wendy Nichols, RN, and the staff at the University of Delaware’s Nurse Managed Primary Care Center for their assistance with blood and specimen collections and with the processing of clinical labs for this project.
Sources of Funding
This study was supported, in part, by the National Institute of General Medical Sciences P20GM113125, the National Institute on Minority Health and Health Disparities R01MD012734, and the National Institute on Drug Abuse R01DA051321.
Footnotes
Disclosure Statement
The authors report no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, EKH, upon reasonable request.
References
- Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, Nyenhuis SM, Ramos AR, Shah NA, Sotres-Alvarez D, et al. 2019. Sleep timing, stability, and bp in the sueno ancillary study of the hispanic community health study/study of latinos. Chest. 155:60–68. DOI: 10.1016/j.chest.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aili K, Astrom-Paulsson S, Stoetzer U, Svartengren M, Hillert L. 2017. Reliability of actigraphy and subjective sleep measurements in adults: The design of sleep assessments. J Clin Sleep Med. 13:39–47. DOI: 10.5664/jcsm.6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. 2003. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 26:342–392. doi: 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- Armstrong S, Wong CA, Perrin E, Page S, Sibley L, Skinner A. 2018. Association of physical activity with income, race/ethnicity, and sex among adolescents and young adults in the united states: Findings from the national health and nutrition examination survey, 2007–2016. JAMA Pediatr. 172:732–740. doi: 10.1001/jamapediatrics.2018.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019. 2019 acc/aha guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 74:1376–1414. DOI: 10.1037/11381-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ, Tanner JL. 2006. Emerging adults in america: Coming of age in the 21st century. Washington, D.C.: American Psychological Association. [Google Scholar]
- Bae S-A, Fang MZ, Rustgi V, Zarbl H, Androulakis IP. 2019. At the interface of lifestyle, behavior, and circadian rhythms: Metabolic implications. Front Nutr. 6:132–132. doi: 10.3389/fnut.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron KG, Reid KJ, Kern AS, Zee PC. 2011. Role of sleep timing in caloric intake and bmi. Obesity (Silver Spring). 19:1374–1381. doi: 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- Basner M, McGuire S. 2018. Who environmental noise guidelines for the european region: A systematic review on environmental noise and effects on sleep. Int J Environ Res Public Health. 15:1–45. doi: 10.3390/ijerph15030519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. 2019. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 139:e56–e528. DOI: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bennett GG, McNeill LH, Wolin KY, Duncan DT, Puleo E, Emmons KM. 2007. Safe to walk? Neighborhood safety and physical activity among public housing residents. PLoS Med. 4:1599–1606. doi: 10.1371/journal.pmed.0040306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos Z, Terman M. 1980. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 4:119–131. doi: 10.1016/0149-7634(80)90010-X [DOI] [PubMed] [Google Scholar]
- Buman MP, Hu F, Newman E, Smeaton AF, Epstein DR. 2016. Behavioral periodicity detection from 24 h wrist accelerometry and associations with cardiometabolic risk and health-related quality of life. Biomed Res Int. 2016:1–9. doi: 10.1155/2016/4856506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgueno A, Gemma C, Gianotti TF, Sookoian S, Pirola CJ. 2010. Increased levels of resistin in rotating shift workers: A potential mediator of cardiovascular risk associated with circadian misalignment. Atherosclerosis. 210:625–629. doi: 10.1016/j.atherosclerosis.2009.12.032 [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. 2012. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 35:287–302. doi: 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti-Ferreira P, Berk L, Daher N, Campus T, Araujo J, Petrofsky J, Lohman E. 2018. A nonparametric methodological analysis of rest-activity rhythm in type 2 diabetes. Sleep Sci. 11:281–289. doi: 10.5935/1984-0063.20180044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults — United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65:137–141. DOI: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, et al. 2017. Actigraphy-derived daily rest-activity patterns and body mass index in community-dwelling adults. Sleep. 40:zsx168. doi: 10.1093/sleep/zsx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley R, Connor Gorber S, Tremblay MS. 2010. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. 21:63–69. [PubMed] [Google Scholar]
- Corder K, Winpenny E, Love R, Brown HE, White M, Sluijs EV. 2019. Change in physical activity from adolescence to early adulthood: A systematic review and meta-analysis of longitudinal cohort studies. Br J Sports Med. 53:496–503. doi: 10.1136/bjsports-2016-097330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DM, Lleras-Muney A. 2010. Understanding differences in health behaviors by education. J Health Econ. 29:1–28. doi: 10.1016/j.jhealeco.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine JK, Wolf JM. 2016. Integrating nap and night-time sleep into sleep patterns reveals differential links to health-relevant outcomes. J Sleep Res. 25:225–233. doi: 10.1111/jsr.12369 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Granger DA, Erath SA, Acebo C. 2007. The association between children’s sleep disruption and salivary interleukin-6. J Sleep Res. 16:188–197. doi: 10.1111/j.1365-2869.2007.00593.x [DOI] [PubMed] [Google Scholar]
- Fager G, Wiklund O. 1997. Cholesterol reduction and clinical benefit. Are there limits to our expectations? Arterioscler Thromb Vasc Biol. 17:3527–3533. doi: 10.1161/01.ATV.17.12.3527 [DOI] [PubMed] [Google Scholar]
- Farshchi HR, Taylor MA, Macdonald IA. 2004. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur J Clin Nutr. 58:1071–1077. doi: 10.1038/sj.ejcn.1601935 [DOI] [PubMed] [Google Scholar]
- Farshchi HR, Taylor MA, Macdonald IA. 2005. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr. 81:16–24. doi: 10.1093/ajcn/81.1.16 [DOI] [PubMed] [Google Scholar]
- Fekedulegn D, Andrew ME, Shi M, Violanti JM, Knox S, Innes KE. 2020. Actigraphy-based assessment of sleep parameters. Ann Work Expo Health. 64:350–367. doi: 10.1093/annweh/wxaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech A 2012. Healthy behavior trajectories between adolescence and young adulthood. Adv Life Course Res. 17:59–68. doi: 10.1016/j.alcr.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. 1998. Calibration of the computer science and applications, inc. Accelerometer. Med Sci Sports Exerc. 30:777–781. doi: 10.1097/00005768-199805000-0002 [DOI] [PubMed] [Google Scholar]
- Garaulet M, Martinez-Nicolas A, Ruiz JR, Konstabel K, Labayen I, Gonzalez-Gross M, Marcos A, Molnar D, Widhalm K, Casajus JA, et al. 2017. Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: The helena study. Clin Nutr. 36:1558–1566. doi: 10.1016/j.clnu.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Gilmore K 2019. Is emerging adulthood a new developmental phase? J Am Psychoanal Assoc. 67:625–653. doi: 10.1177/0003065119868680 [DOI] [PubMed] [Google Scholar]
- Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. 1995. Cholesterol reduction yields clinical benefit. A new look at old data. Circulation. 91:2274–2282. doi: 10.1161/01.CIR.91.8.2274 [DOI] [PubMed] [Google Scholar]
- Grandner MA, Jackson NJ, Izci-Balserak B, Gallagher RA, Murray-Bachmann R, Williams NJ, Patel NP, Jean-Louis G. 2015. Social and behavioral determinants of perceived insufficient sleep. Front Neurol. 6:1–14. doi: 10.3389/fneur.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinter MA, Campbell PT, Patel AV, McCullough ML. 2019. Irregularity in breakfast consumption and daily meal timing patterns in association with body weight status and inflammation. Br J Nutr. 122:1192–1200. doi: 10.1017/S0007114519002125 [DOI] [PubMed] [Google Scholar]
- Gundogan K, Bayram F, Capak M, Tanriverdi F, Karaman A, Ozturk A, Altunbas H, Gokce C, Kalkan A, Yazici C. 2009. Prevalence of metabolic syndrome in the mediterranean region of turkey: Evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metab Syndr Relat Disord. 7:427–434. doi: 10.1089/met.2008.0068 [DOI] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL. 2020. Prevalence of obesity and severe obesity among adults: United states, 2017–2018. NCHS Data Brief.1–8. [PubMed] [Google Scholar]
- Hermida RC, Crespo JJ, Otero A, Dominguez-Sardina M, Moya A, Rios MT, Castineira MC, Callejas PA, Pousa L, Sineiro E, et al. 2018. Asleep blood pressure: Significant prognostic marker of vascular risk and therapeutic target for prevention. Eur Heart J. 39:4159–4171. doi: 10.1093/eurheartj/ehy475 [DOI] [PubMed] [Google Scholar]
- Huang T, Mariani S, Redline S. 2020. Sleep irregularity and risk of cardiovascular events: The multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 75:991–999. doi: 10.1016/j.jacc.2019.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Redline S. 2019. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: The multi-ethnic study of atherosclerosis. Diabetes Care. 42:1422–1429. doi: 10.2337/dc19-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE. 2016. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 80:40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. 2001. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 105:185–191. doi: 10.1016/S0165-0270(00)00364-2 [DOI] [PubMed] [Google Scholar]
- Jeong SM, Choi S, Kim K, Kim SM, Lee G, Park SY, Kim YY, Son JS, Yun JM, Park SM. 2018. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J Am Heart Assoc. 7:e008819. doi: 10.1161/JAHA.118.008819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn A, Banks J, Hamer M, Smith JP, Steptoe A, van Soest A, Koster A, Htay Wah S. 2018. What they say and what they do: Comparing physical activity across the USA, england and the netherlands. J Epidemiol Community Health. 72:471–476. doi: 10.1136/jech-2017-209703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MR, Wald NJ, Thompson SG. 1994. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 308:367–372. doi: 10.1136/bmj.308.6925.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EM. 2017. Why do college graduates behave more healthfully than those who are less educated? J Health Soc Behav. 58:291–306. doi: 10.1177/0022146517715671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kwan SH, Moore LV, Blanck HM, Harris DM, Galuska D. 2017. Disparities in state-specific adult fruit and vegetable consumption - united states, 2015. MMWR Morb Mortal Wkly Rep. 66:1241–1247. doi: 10.15585/mmwr.mm6645a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Ahmadi-Abhari S, Wainwright NW, Cappuccio FP, Surtees PG, Luben R, Brayne C, Khaw KT. 2014. Daytime napping, sleep duration and serum c reactive protein: A population-based cohort study. BMJ Open. 4:e006071. doi: 10.1136/bmjopen-2014-006071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Holmback U, Van Cauter E. 2014. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 63:1860–1869. doi: 10.2337/db13-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Liu D, Haynie D, Gee B, Chaurasia A, Seo DC, Iannotti RJ, Simons-Morton BG. 2016. Individual, social, and environmental influences on the transitions in physical activity among emerging adults. BMC Public Health. 16:1–12. doi: 10.1186/s12889-016-3368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, et al. 2003. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep. 26(3):337–341. doi: 10.1093/sleep/26.3.337 [DOI] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K, Scheer FAJL. 2016. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 113:E1402–E1411. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. 2017. Circadian misalignment increases c-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 32:154–164. doi: 10.1177/0748730417697537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. 2008. Emerging adulthood and college-aged youth: An overlooked age for weight-related behavior change. Obesity (Silver Spring). 16:2205–2211. doi: 10.1038/oby.2008.365 [DOI] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 384:766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI. 2019. Notice of special interest (nosi): Epidemiologic studies to characterize cardiovascular health and its predictors and trajectories in diverse groups of children; not-hl-19–711. In.: National Heart, Lung, and Blood Institute. [Google Scholar]
- Nowakowski S, Matthews KA, von Kanel R, Hall MH, Thurston RC. 2018. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep. 41:zsy049. doi: 10.1093/sleep/zsy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, Hall M. 2009. Disturbed sleep is associated with increased c-reactive protein in young women. Brain Behav Immun. 23:351–354. doi: 10.1016/j.bbi.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF 3rd, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. 2011. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 73:142–150. doi: 10.1097/PSY.0b013e3182020d08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyper SV, Thacher PV, Gilbert JW, Gradess SG. 2012. Class start times, sleep, and academic performance in college: A path analysis. Chronobiol Int. 29:318–335. doi: 10.3109/07420528.2012.655868 [DOI] [PubMed] [Google Scholar]
- Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. 2020. Hypertension prevalence among adults aged 18 and over: United states, 2017–2018. NCHS Data Brief.1–8. [PubMed] [Google Scholar]
- Park H, Tsai KM, Dahl RE, Irwin MR, McCreath H, Seeman TE, Fuligni AJ. 2016. Sleep and inflammation during adolescence. Psychosom Med. 78:677–685. doi: 10.1097/PSY.0000000000000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Lozano A, Huang L, Perkett M, Beeson J, Hanlon A. 2018. Towards a demographic risk profile for sedentary behaviours in middle-aged british adults: A cross-sectional population study. BMJ Open. 8:e019639. doi: 10.1136/bmjopen-2017-019639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, Barrett-Connor E, Stefanick ML, Ensrud KE. 2011. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 28:258–266. doi: 10.3109/07420528.2011.553016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. 2018. The physical activity guidelines for americans. JAMA. 320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff RC, Costigan SA, Williams RL, Hutchesson MJ, Kennedy SG, Robards SL, Allen J, Collins CE, Callister R, Germov J. 2015. Effectiveness of interventions targeting physical activity, nutrition and healthy weight for university and college students: A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 12:45. doi: 10.1186/s12966-015-0203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–1929. doi: 10.3109/07420528.2010.516381 [DOI] [PubMed] [Google Scholar]
- Pot GK, Hardy R, Stephen AM. 2014. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a british birth cohort. Int J Obes (Lond). 38:1518–1524. doi: 10.1038/ijo.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttonen S, Viitasalo K, Harma M. 2011. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. 28:528–535. doi: 10.3109/07420528.2011.580869 [DOI] [PubMed] [Google Scholar]
- Quante M, Cespedes Feliciano EM, Rifas-Shiman SL, Mariani S, Kaplan ER, Rueschman M, Oken E, Taveras EM, Redline S. 2019. Association of daily rest-activity patterns with adiposity and cardiometabolic risk measures in teens. J Adolesc Health. 65:224–231. doi: 10.1016/j.jadohealth.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rykov Y, Thach TQ, Dunleavy G, Roberts AC, Christopoulos G, Soh CK, Car J. 2020. Activity tracker-based metrics as digital markers of cardiometabolic health in working adults: Cross-sectional study. JMIR Mhealth Uhealth. 8:e16409. doi: 10.2196/16409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 106:4453–4458. doi: 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Hoffmann G. 2015. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 115:780–800 e785. doi: 10.1016/j.jand.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M, De Faire U, Fisher RM, Hellenius ML. 2008. Eating meals irregularly: A novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring). 16:1302–1307. doi: 10.1038/oby.2008.203 [DOI] [PubMed] [Google Scholar]
- Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. 2015. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 32:802–813. doi: 10.3109/07420528.2015.1041597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD, Pirola CJ. 2007. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Dinges DF, Goel N. 2013. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 36:981–990. doi: 10.5665/sleep.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulemana H, Smolensky MH, Lai D. 2006. Relationship between physical activity and body mass index in adolescents. Med Sci Sports Exerc. 38:1182–1186. doi: 10.1249/01.mss.0000222847.35004.a5 [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. 2008. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc. 40:181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 2020. National diabetes statistics report 2020. In.: Centers for Disease Control and Prevention. [Google Scholar]
- Vallat R, Shah VD, Redline S, Attia P, Walker MP. 2020. Broken sleep predicts hardened blood vessels. PLoS Biol. 18:e3000726. doi: 10.1371/journal.pbio.3000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. 1999. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in alzheimer patients by application of nonparametric methods. Chronobiol Int. 16:505–518. doi: 10.3109/07420529908998724 [DOI] [PubMed] [Google Scholar]
- Wang LX, Filipp SL, Urbina EM, Gurka MJ, DeBoer MD. 2018. Longitudinal associations of metabolic syndrome severity between childhood and young adulthood: The bogalusa heart study. Metab Syndr Relat Disord. 16:208–214. doi: 10.1089/met.2017.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, et al. 2015. Recommended amount of sleep for a healthy adult: A joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep. 38:843–844. doi: 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. 2017. Meal timing regulates the human circadian system. Curr Biol. 27:1768–1775 e1763. doi: 10.1016/j.cub.2017.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg M, Gustafsson PE, Wennberg P, Hammarstrom A. 2016. Irregular eating of meals in adolescence and the metabolic syndrome in adulthood: Results from a 27-year prospective cohort. Public Health Nutr. 19:667–673. doi: 10.1017/S1368980015001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. 1990. Alterations in the circadian rest-activity rhythm in aging and alzheimer’s disease. Biol Psychiatry. 27:563–572. doi: 10.1016/0006-3223(90)90523-5 [DOI] [PubMed] [Google Scholar]
- Wright KP Jr., Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. 2015. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 47:24–34. doi: 10.1016/j.bbi.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, et al. 2019. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 74:330–341. doi: 10.1016/j.jacc.2019.03.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, EKH, upon reasonable request.