INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a chronic and heterogeneous neurodevelopmental disorder, characterized by hyperactivity, impulsivity and/or reduction of sustained attention (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition – DSM-5; 11th revision of the International Classification of Diseases and Related Health Problems - ICD‐11) (Posner et al., 2020; Reed et al., 2019) ADHD is also associated with memory deficits, which are related to distractibility, reduced attention, and working memory impairments. (Owens and Hoza, 2003; Posner et al., 2020; Steinau, 2013) While the precise etiology remains under study, ADHD is thought to result from the interaction of genetic and environmental factors, resulting in pervasive maturational and functional impairments of the prefrontal cortex and associated neural networks.(Arnsten and Li, 2005; Gallo and Posner, 2016; Lange et al., 2010; Posner et al., 2020)
ADHD stands out as the most prevalent neurodevelopmental disorder of childhood. (Feldman and Reiff, 2014; Owens and Hoza, 2003; Steinau, 2013) For example, Polanczyk et al. estimated an 3.4% (95% Confidence Interval [CI], 2.6–4.5%), while a recent systematic review indicated of 7.2% (95% CI 6.7–7.8), with differences likely reflecting heterogeneity in study methodology. (Polanczyk et al., 2015; Sayal et al., 2018) In adults, these numbers are more modest, with an estimated prevalence of 2.5% (95% CI 2.1–3.1), possibly due to the maturation of the cerebral cortex, more specifically of prefrontal areas, and to epigenetic mechanisms. (Polanczyk et al., 2007; Posner et al., 2020; Simon et al., 2009)
Current therapeutic approaches for ADHD are associated with significant clinical challenges. Although studies demonstrated the effectiveness of stimulant drugs (e.g., methylphenidate) to reduce ADHD symptoms, there are many aspects of concern, such as: tachyphylaxis, particularly when chronically used; (Volkow and Swanson, 2013), significant side effects; (Feldman and Reiff, 2014; Matza et al., 2005; Okie, 2006; Volkow and Swanson, 2013) the risks of abuse and addiction; (Shier et al., 2013) and unclear long-term cost-effectiveness. (Gilmore and Milne, 2001; Matza et al., 2005; Torrance, 1986)
In this context, novel therapeutic strategies are currently under investigation for ADHD. Transcranial direct current stimulation (tDCS) has emerged as a promising tool in modulating spontaneous neural network excitability. (Brunoni et al., 2012; DaSilva et al., 2011; Stagg and Nitsche, 2011) tDCS uses low-intensity electrical stimulation to modulate targeted brain regions, such that it can increase or decrease excitability of the neural tissue. (Philip et al., 2017) Recent studies have found significant improvement in attention and behavioral inhibition in children with ADHD following tDCS treatment. (Bandeira et al., 2016a; Breitling et al., 2016; Cachoeira et al., 2017; Soltaninejad et al., 2019) In other domains of development, such as memory, no robust effects have been observed. (Nejati et al., 2017; Soff et al., 2017; Sotnikova et al., 2017)
Therefore, in order to better understand the potential modulatory effects of tDCS in individuals with ADHD, and to summarize and discuss the level of evidence available to inform the design of future clinical trials, we analyzed the current state of the literature through this systematic review.
Background
Neurobiological basis of ADHD
Although pathophysiological mechanisms of ADHD remain unknown, the available literature suggests that symptoms arise from the combination of complex etiological processes described above. (Arnsten and Li, 2005; Gallo and Posner, 2016; Lange et al., 2010; Posner et al., 2020)
Neuroimaging studies have shown structural and functional brain impairments in individuals with ADHD, including cortical and subcortical volumetric abnormities. (Castellanos et al., 2001; Castellanos et al., 1996; Dickstein et al., 2006; Lukito et al., 2020; Norman et al., 2016) Deficits in cognitive processing have been associated with hypoactivation and decreased volume in the prefrontal area, caudate nucleus and cerebellum. (Antshel et al., 2011; Brown et al., 2006; Lukito et al., 2020; Puig and Gulledge, 2011; Spencer-Smith and Anderson, 2009) This is generally attributed to a deviation from typical development, resulting in a delay in structural maturation. (Posner et al., 2020; Rubia et al., 2014; Shaw et al., 2007; Spencer-Smith and Anderson, 2009) Using a longitudinal approach using cortical thickness as an index of maturation, Shaw et al. found that the median age for children with ADHD to reach 50% of cortical thickness was 10.5 years compared to 7.5 years in typically developing children; findings were more pronounced in the middle prefrontal cortex. (Shaw et al., 2007) This observation supports the higher prevalence of ADHD in childhood when compared to adults, however it does not explain the persistence of symptoms in some adults.
Brain structural, neuroimmunological and neurochemical abnormalities have also been implicated in the pathophysiological framework of ADHD. One study found ADHD symptoms were associated with a reduction in prefrontal and anterior cingulate grey matter volume, as well as reductions in the amygdala, hippocampus, striatal and temporoparietal regions. (Gallo and Posner, 2016; Hoogman et al., 2017; Hoogman et al., 2019; Hoogman et al., 2012; Norman et al., 2017)
Likewise, neuroinflammation has been investigated as a potential mechanism in ADHD. Although this area of research appears preliminary, it indicates that impaired neurotransmitter action, increased oxidative stress, abnormal neuronal development, and damage to the blood-brain barrier (with associated glial activation) are related to impaired brain development and maturation, and consequently the unfolding of neurodevelopmental disorders such as ADHD. (Dunn et al., 2019; Leffa et al., 2018b)
Deficits in the levels of monoaminergic neurotransmitters such as dopamine, norepinephrine, and serotonin in tracts associated with attention, and motivation have also been reported. (Arnsten and Li, 2005; Castellanos and Proal, 2012; Volkow and Swanson, 2013) These investigations were empirically motivated by the positive response of patients with ADHD to psychostimulants. (Arnsten and Pliszka, 2011; Prince, 2008; Sharma and Couture, 2014), and are bolstered by significant research indicating involvement of dopamine transporters. (Gallo and Posner, 2016; Sharma and Couture, 2014) Genetic studies have reinforced this hypothesis, revealing polymorphisms in genes that encode dopamine transporters in addition to genes responsible for the expression of dopamine receptors, resulting in impairment of dopaminergic circuits, which are in turn intrinsically related to noradrenergic and serotonergic signaling. (Arnsten and Pliszka, 2011; Gallo and Posner, 2016; Sharma and Couture, 2014) Furthermore, disruption of dopaminergic pathways in ADHD have also been associated with dysfunctional glutamatergic and GABAergic networks, ensuing impaired modulation of inhibitory response. (Prince, 2008)
In addition to the mesocorticolimbic pathways, involvement of broader cortical networks, such as the default mode network (DMN) and executive control network, have also been implicated in ADHD. (Castellanos and Proal, 2012; Posner et al., 2020; Volkow and Swanson, 2013) The DMN is involved in introspective processes associated with the brain’s resting-state, and includes the ventral medial prefrontal cortex, posterior cingulate cortex and inferior parietal lobe. (Buckner et al., 2008; Mak et al., 2017) In a recent study, Bozhilova et al. proposed that ADHD-related cognitive dysfunction could be explained by an overactive DMN, culminating in exaggerated spontaneous internal distractibility. (Bozhilova et al., 2018)
One unifying feature of the existing literature is the involvement of the prefrontal cortex in nearly all ADHD symptoms. The prefrontal cortex is the main brain region associated with executive functions, (Puig and Gulledge, 2011) corresponding to neurocognitive skills related to planning and performing intentional and self-organized actions. (Arnsten and Li, 2005; Spencer-Smith and Anderson, 2009) Among the executive functions, attention and inhibitory control are the most-often affected in ADHD. There is further evidence of a physiological association between the prefrontal cortex and modulation of inhibitory control and attention. (Arnsten and Li, 2005; Brown et al., 2006) The interplay between functional and anatomical abnormalities related to connectivity and the development of ADHD is also an area of intense interest.
Therapeutic approaches to modulate neuronal activity in prefrontal area as well as to reduce impulsivity, hyperactivity, inattention symptoms and associated cognitive dysfunctions remain a challenge. (Antshel et al., 2011)
Technical aspects of tDCS
Transcranial direct current stimulation is recognized as a technically simple method, involving the application of a weak galvanic current on the scalp, which moves from the anode electrode (positive pole) to the cathode (negative pole), thereby forming an electrical circuit. (Nitsche and Paulus, 2000; Stagg and Nitsche, 2011) The stimulation promotes spontaneous modulation of neuronal activity, increasing or reducing cortical excitability, by facilitating the neuronal depolarization and hyperpolarization, respectively, according to the electric current direction in relation to the axonal orientation. (Lefaucheur et al., 2017; Nitsche et al., 2008; Nitsche and Paulus, 2000; Stagg and Nitsche, 2011) Its mechanisms of action are likely based in the modulation of synaptic plasticity of glutamatergic and GABAergic pathways (Lefaucheur et al., 2017; Nitsche et al., 2004)
Specific polarity effects in tDCS have been widely investigated. (Liebetanz et al., 2002; Nitsche et al., 2008; Nitsche et al., 2003c; Nitsche and Paulus, 2000) Overall, anodal stimulation is thought to promote increased cortical excitability by neuronal depolarization, whereas cathodal stimulation is considered to induce hyperpolarization, and a consequent reduction of neuronal activity. (Liebetanz et al., 2002; Nitsche et al., 2003c; Nitsche and Paulus, 2000) However, this notion is likely inconsistent as the effect of tDCS is more complex and depends on many factors, such as location and orientation of cells and associated dendritic trees, and also the rate and nature of local neuronal activity. As an example, in a clinical trial conducted by Nitsche and Paulus in 2000, neuronal spatial positioning and current direction were each found to present polarity-dependent effects. (Nitsche and Paulus, 2000)
Transcranial direct current stimulation effects may be increased by changes in electrode size and montage. (Nitsche et al., 2008) Small electrodes may induce effects more restricted to the stimulated area, and cephalic montage (i.e. stimulatory and reference electrodes placed on the scalp) promotes a more extensive modulation. (Nitsche et al., 2008)
tDCS stands out among other brain stimulation techniques, such as transcranial magnetic stimulation and deep brain stimulation, due to its ability to modulate spontaneous neuronal activity, rather than directly inducing action potentials. (Liebetanz et al., 2002; Nitsche et al., 2008; Nitsche et al., 2003c; Nitsche and Paulus, 2000) In addition to its favorable safety profile, tDCS is low cost, and a simple technique to administer. (Brunoni et al., 2011a; Nitsche et al., 2008; Nitsche et al., 2003c; Nitsche and Paulus, 2000)
Over the last decade, there is some indication that tDCS may be efficacious to reduce symptoms for neurological and psychiatric disorders such as major depressive disorder, (Lefaucheur et al., 2017; Palm et al., 2016; Sharafi et al., 2019; Wang, 2019) obsessive-compulsive disorder (OCD), (D’Urso et al., 2016; Gowda et al., 2019) addiction, (Batista et al., 2015; Lefaucheur et al., 2017) fibromyalgia, (Khedr et al., 2017) neuropathic pain, (Ngernyam et al., 2015; Yoon et al., 2014) and ADHD as extensively discussed below.
In addition to its application in clinical studies, the efficacy of tDCS to modulate ADHD neuroimmune mechanisms in cognitive domains has been evaluated in animal models with encouraging results. (Leffa et al., 2018a; Leffa et al., 2016) Improvement in long-term memory has been observed in spontaneously hypertensive rats, an animal model of ADHD, in addition to downregulation of pro-inflammatory cytokines, increased production of reactive oxygen species and levels of glutathione (antioxidant). (Leffa et al., 2018a) As a comprehensive approach of animal studies applying tDCS for ADHD modulation is beyond the scope of this review, please see the following references for further discussion. (Leffa et al., 2018a; Leffa et al., 2016)
METHODS
Search Strategy
A systematic approach was applied to perform the current review. To achieve a comprehensive overview of the our review topic, a search on Medline/PubMed, Cochrane Library, Web of Science, ScienceDirect and Embase was conducted using a combination of the following descriptors and its contractions, including the Medical Subject Headings (MeSH) terms: (a) “attention-deficit/hyperactivity disorder” or “ADHD”; and (b) “transcranial direct current stimulation” or “tDCS”. Additionally, we searched the references of included manuscripts. The final date of all searches was November 20, 2019.
Eligibility Criteria
The inclusion criteria used to select the studies were: a) application of tDCS in individuals with ADHD; b) use of cognitive tasks, or neurophysiological assessments, to assess ADHD symptoms before and after tDCS; c) presentation of mean and standard deviation of outcomes analyzed; d) studies published in indexed periodicals; and e) publications written in English. The following exclusion criteria were applied: a) review/meta-analysis articles and/or study protocols; b) trials not investigating tDCS in ADHD population; and c) studies in animals.
Studies Selection
Two experienced researchers independently performed the search and selection of studies. A total of 374 records were identified through the database searching, and an additional nine were identified after reviewing references of included manuscripts. Following the removal of duplicates, 45 studies were assessed for eligibility. Titles and abstracts from 45 articles were revised, and twelve addressing the application and/or physiological mechanisms of tDCS in ADHD individuals were eligible. The screening resulted in the exclusion of 33 manuscripts: review/meta-analysis articles and/or study protocols (n=20); animal studies (n=2), and trials not investigating tDCS applied to ADHD population (n=11). Following review of the identified manuscripts, eleven clinical studies fulfilled the eligibility criteria and were included. Figure 1 presents a flowchart of the review search process adapted from PRISMA flow diagram. (Moher et al., 2009) Of note, we considered performing a meta-analysis, but concluded the methods and outcomes of the available papers were too varied to provide meaningful meta-analytic information.
Figure 1.
Flowchart of review search adapted from PRISMA flow diagram. (Moher et al., 2009)
Data Extraction and Synthesis
One author extracted data from the included studies for scoping review. Table 1 summarizes the characteristics and findings of the clinical studies included, all addressing the effects of tDCS in ADHD. The trials were published between 2015 and 2019 - two in 2015, (Cosmo et al., 2015a; Cosmo et al., 2015b) other two in 2016, (Bandeira et al., 2016b; Breitling et al., 2016) four in 2017, (Cachoeira et al., 2017; Nejati et al., 2017; Soff et al., 2017; Sotnikova et al., 2017) two in 2018, (Allenby et al., 2018; Jacoby and Lavidor, 2018) and one in 2019. (Soltaninejad et al., 2019); and conducted in Brazil (4 publications, two by the same research group), (Bandeira et al., 2016b; Cachoeira et al., 2017; Cosmo et al., 2015a; Cosmo et al., 2015b) Germany (3 studies, two by the same team at Philipps-University), (Breitling et al., 2016; Soff et al., 2017; Sotnikova et al., 2017) Iran (2 articles from the same group, one in collaboration with researchers from Germany and UK), (Nejati et al., 2017; Soltaninejad et al., 2019) Israel (1 trial), (Jacoby and Lavidor, 2018) and US (1 study performed by researchers from the University of Pennsylvania). (Allenby et al., 2018)
Table 1.
Characteristics and findings of clinical studies assessing the effects of tDCS in ADHD
| Stimulation Protocol | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Population | Montage* | Current intensity (density)/duration | tDCS design | Number of sessions | Results | Adverse events | Conclusions |
|
Cosmo et al. (Cosmo et al., 2015a; Cosmo et al., 2015b) |
60 adults with ADHD |
Anode: L DLPFC (F3) Cathode: R DLPFC (F4) |
1mA (0.03mA/cm2) 20 minutes |
Offline |
1 active 1 sham |
A statistically significant difference was found comparing the weighted node degree prior to and following active tDCS, in the electrodes located over left DLPFC and correlated areas No significant differences between tDCS and sham groups in the Go/No-Go tasks |
None |
Anodal tDCS increased functional brain connectivity in individuals with ADHD compared to baseline resting state No improvement in inhibitory control by tDCS application over L DLPFC |
| Breitling et al. (Breitling et al., 2016) | 21 adolescents with ADHD and 21 healthy controls |
Experiment 1:
Anode: R IFG (F8) Cathode: posterior to L mastoid Experiment 2: Anode: posterior to L mastoid Cathode: R IFG (F8) |
1mA (0.03mA/cm2) 20min |
Online | 2 active 1 sham |
Reduction in commission errors and variability in RT in the modified Eriksen Flanker tasks after anodal stimulation, when compared to sham | Skin sensations | Improved interference control following anodal tDCS over R inferior frontal gyrus |
| Bandeira et al. (Bandeira et al., 2016) | 9 children and adolescents with ADHD | Anode: L DLPFC (F3) Cathode: R supraorbital area (Fp2) |
2mA (0.06mA/cm2) 30 minutes |
Online | 5 active |
Decrease in errors by omission in TAVIS-3, as well as in uncorrected and total errors in switching task and completion time in the naming task of the NEPSY-II, when comparing post and pre intervention results. No significant differences in the Digit Span subtest of the WISC-III | Mild and moderate tingling, itching and burning sensation. Sense of shock, mild headache, neck pain, local redness and mild sleepiness | Anodal tDCS enhanced selective attention and inhibitory control after 5 sessions. |
| Nejati et al. (Nejati et al., 2017) | 25 children with ADHD Experiment 1 (N=15) Experiment 2 (N=10) |
Experiment 1: Anode: L DLPFC (F3) Cathode: R DLPFC (F4) Experiment 2: Montage 1: Anode: L DLPFC (F3) Cathode: R OFC (Fp2) Montage 2: Anode: R OFC (Fp2) Cathode: L DLPFC (F3) |
1mA (0.04mA/cm2) 15min |
Offline |
Experiment 1: 1 active 1 sham Experiment 2: 2 active 1 sham |
Improved N-back test performance in both experiments when anodal tDCS was applied over L DLPFC Experiment 1: improved interference response inhibition. No differences on Go/No-Go and WCST Experiment 2: improved performance on Go/No-Go with montage 2, and on WCST for both montages (more pronounced in the montage 1) |
Mild side effects (itching and tingling) | Anodal L DLPFC stimulation improved working memory. Anodal tDCS over L DLPFC and cathodal on R DLPFC enhanced interference response inhibition Increased inhibitory control and cognitive flexibility were seen after anodal stimulation over R OFC |
| Soff et al. (Soff et al., 2017) | 15 adolescents with ADHD | Anode: L DLPFC (F3) Cathode: vertex (Cz) |
1mA Anode (0.08mA/cm2) Cathode (0.29mA/cm2) 20min |
Online | 5 active 5 sham |
Improvement in clinical parameters of inattention, impulsivity, and Qb-test after tDCS, when compared to sham | Mild side effects (tingling and itching) | Anodal tDCS caused a significant reduction in clinical symptoms of inattention and impulsivity. It also improved hyperactivity, with a more robust reduction seen by the 7th day following the treatment |
| Sotnikova et al. (Sotnikova et al., 2017) | 16 adolescents with ADHD | Anode: L DLPFC (F3) Cathode: vertex (Cz). |
1mA Anode (0.29mA/cm2) Cathode (0.08mA/cm2) 20min |
Online | 1 session | Improvement in RT in the working memory paradigm in the active group More omission errors and less accuracy in the active group, when compared to sham Increase in connectivity and neuronal activation in the L DLPFC and surrounding regions in the active group |
Mild side effects (tingling and itching) | Anodal stimulation over L DLPFC improved motor performance, but worsened accuracy in the working memory paradigm Anodal tDCS increased neuronal activation and connectivity in the L DLPFC with propagation to L premotor cortex, L supplementary motor area and precuneus |
| Cachoeira et al. (Cachoeira et al., 2017) | 17 adults with ADHD | Anode: R DLPFC (F4) Cathode: L DLPFC (F3) |
2 mA (0.06mA/cm2) 20 minutes |
Offline | 5 active 5 sham |
Significant lower ASRS inattention and SDS scores after active tDCS, compared to sham |
Tingling, itching, burning sensation, headache, fatigue, anxiety, visual symptoms, nausea, insomnia and acute mood change | Anodal tDCS over R DLPFC significantly improved attention and functional impairment, but not hyperactivity/impulsivity |
| Jacoby and Lavidor (Jacoby and Lavidor, 2018) | 21 adults with ADHD/ 16 healthy controls | Anode: L and R DLPFC (F3 and F4) Cathode: cerebellum |
1.8mA Anode (0.2mA/cm2) Cathode (0.05mA/cm2) 20 minutes |
Offline | 1 active 1 sham |
No tDCS effects in most MOXO-CPT domains, except for hyperactivity | None | Double anodal prefrontal tDCS reduced hyperactivity, however no effects were observed in attention and impulsivity domains |
| Allenby et al. (Allenby et al., 2018) | 37 adults with ADHD | Anode: L DLPFC (F3) Cathode: R supra-orbital area (Fp2) |
2mA (0.08mA/cm2) 20 minutes |
Online | 2 periods (active vs. sham) of 3 sessions | Decrease in false positive errors on CPT before and immediately after active tDCS No significant improvement in SST performance following active tDCS |
Mild side effects (mostly itching, burning and tingling) | Repeated anodal tDCS over L DLPFC improved impulsivity, however the effects were transient (< 3 days) |
| Soltaninejad et al. (Soltaninejad et al., 2019) | 20 adolescents with ADHD |
Experiment 1: Anode: L DLPFC (F3) Cathode: R supraorbital area (Fp2) Experiment 2: Anode: R supraorbital area (Fp2) Cathode: L DLPFC (F3) |
1.5mA (0.04mA/ cm2) 15 minutes |
Online | 2 active 1 sham |
Cathodal tDCS and anodal stimulation over the L DLPFC enhanced inhibition accuracy and prepotent response inhibition in the Go/No-Go task, respectively No significant difference was seen in interference inhibition measured by the Stroop task |
None. Reported in methods, but no results available | tDCS over the left DLPFC improved inhibitory control |
Stimulated areas are described in accordance with the 10–20 International EEG system.
ADHD: attention-deficit/hyperactivity disorder; tDCS: transcranial direct current stimulation; L: left; R: right; DLPFC: dorsolateral prefrontal cortex; OFC: orbitofrontal cortex; IFG: inferior frontal gyrus; RT: reaction time; ASRS: Adult ADHD Self-Report Scale Symptom; WISC-III: Wechsler Intelligence Scale for Children; NEPSY-II: Neuropsychological Development Assessment; TAVIS-3: Visual Attention Test; WCST: Wisconsin Card Sorting Test; Qb-Test: Quantified Behavioral Test; SDS: Sheehan Disability Scale; CPT: Conners’ Continuous Performance Task; SST: Stop Signal Task; MOXO-CPT: MOXO Continuous Performance Test.
The included manuscripts addressed the application of tDCS in individuals with ADHD for the modulation of attention (n=5), (Bandeira et al., 2016b; Cachoeira et al., 2017; Jacoby and Lavidor, 2018; Nejati et al., 2017; Soff et al., 2017) working memory (n=4), (Bandeira et al., 2016b; Nejati et al., 2017; Soff et al., 2017; Sotnikova et al., 2017) inhibitory control/impulsivity (n=8), (Allenby et al., 2018; Bandeira et al., 2016b; Breitling et al., 2016; Cachoeira et al., 2017; Cosmo et al., 2015a; Nejati et al., 2017; Soff et al., 2017; Soltaninejad et al., 2019) and 2 articles assessed neurophysiological parameters; (Cosmo et al., 2015b; Sotnikova et al., 2017) Most articles assessed adults (n=5), (Allenby et al., 2018; Cachoeira et al., 2017; Cosmo et al., 2015a; Cosmo et al., 2015b; Jacoby and Lavidor, 2018) four studies included adolescents, (Breitling et al., 2016; Soff et al., 2017; Soltaninejad et al., 2019; Sotnikova et al., 2017) one evaluated children, (Nejati et al., 2017) and one study included adolescents and children. (Bandeira et al., 2016b)
Risk of bias assessment
The Cochrane Collaboration’s tool for assessing risk of bias was applied to evaluate included trials in six domains - random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). (Higgins and Thomas, 2019) The results are summarized in Figure 2.
Figure 2.
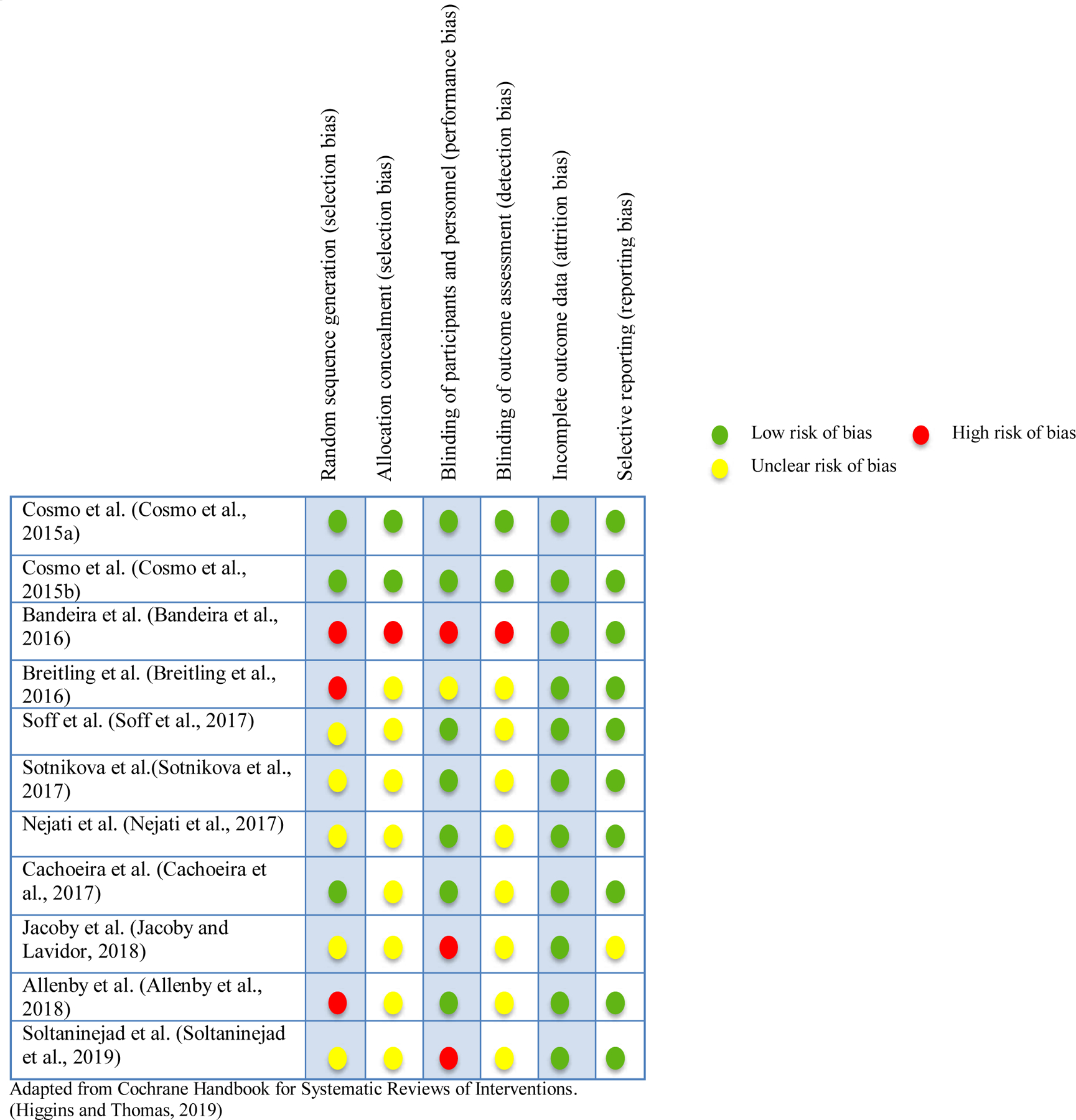
Risk of bias assessment
Of the 11 manuscripts included in this systematic review, 27.3% had a high risk of selection and performance bias, and 9.1% a high risk of detection bias. The risk of attrition bias was low in all trials. The proportion of studies with an unclear risk of bias was higher for allocation concealment (72.7%) and blinding of outcome assessment (72.7%) than in other domains.
RESULTS
To date, few studies have investigated the application of tDCS in individuals with ADHD. The trials selected for the current review address the effects of transcranial direct current stimulation in the modulation of ADHD inhibitory control symptoms, attention, working memory and neurophysiological parameters. (Table 1)
Inhibitory control, Attention, and Working Memory findings
In a recent randomized, sham-controlled trial, Cosmo et al. assessed adults with ADHD regarding the modulation of inhibitory control following anodal stimulation or sham. (Cosmo et al., 2015a) The assessment of n=60 ADHD subjects who underwent a single 20-minute 1mA offline session (anodal tDCS over the left DLPFC and cathodal at the right DLPFC) or sham with similar montage revealed no significant effects on inhibitory control in the two Go/No-Go tasks applied. (Cosmo et al., 2015a)
Soltaninejad et al. showed an improvement in inhibitory control after 15 minutes of online tDCS application at 1.5mA over left DLPFC. (Soltaninejad et al., 2019) Following a single cathodal stimulation, n=20 adolescents with ADHD presented improved performance during Go/No-Go task regarding no-go stimuli, compared to anodal and sham. However, anodal tDCS (1 session) over the same area resulted in increased correct go responses, but no significant results for no-go stimulus, compared to sham. No differences were found in accuracy and reaction time of the Stroop task. This crossover, single-blind and sham-controlled study concluded that left cathodal tDCS and left anodal stimulation over L DLPFC could enhance inhibition accuracy and prepotent response inhibition, respectively, resulting in improved inhibitory control. (Soltaninejad et al., 2019)
Breitling et al. investigated the use of tDCS in the right inferior frontal gyrus (rIFG), with reference electrode placed posterior to the left mastoid area, on interference control (a component of the inhibitory control) in ADHD adolescent patients, compared to healthy individuals. (Breitling et al., 2016) Subjects underwent three sessions of stimulation in a random order: anodal, cathodal and sham, with one-week interval between interventions. Overall, this study showed no group differences. However, after noticing a learning effect, the authors performed an exploratory analysis that evaluated only the first session of each subject; using this approach they reported a reduction in the number of commission errors and variability in reaction time in the modified Eriksen Flanker tasks among ADHD patients during anodal stimulation, compared to sham. No significant findings were observed following cathodal stimulation. (Breitling et al., 2016)
In a within-subject crossover trial, adults (n=37) with ADHD received two intervention periods with tDCS or sham stimulation, each one composed by three sessions, separated by a 2-week washout period. (Allenby et al., 2018) Active stimulation was applied online, combined with a visual working memory training task, delivering a current density of 0.08mA/cm2 for 20 minutes with anode positioned on the left DLPFC and cathode on the right supraorbital area, with the aim to improve impulsivity, estimated by the performance in the Conners’ Continuous Performance Task (CPT) scores and Stop Signal Task (SST). A decrease in false positive errors (commission errors, β= −0.36, 95% CI: −0.54 to −0.18, p<.001) was noticed on the CPT between the baseline session and end of active tDCS treatment, however the effect did not last up to the follow up visit (3 days after the last stimulation). No significant differences were seen for true positive errors (omission errors), true positive response time, or SST by stimulation condition or by session interaction. Based in these findings, they suggested that three sessions of anodal tDCS over the left DLPFC might transiently decrease impulsivity in individuals with ADHD. (Allenby et al., 2018)
In a recent crossover, double-blind, sham-controlled study, Sotnikova et al. analyzed the effect of tDCS to improve working memory in n=16 adolescents with ADHD. (Sotnikova et al., 2017) A current density of 0.029mA/cm2 was delivered for 20 minutes with the anodal electrode over left DLPFC and cathodal placed in the vertex. For the working memory assessment, they used an adapted test that combined N-back and Go/No-Go tasks features. tDCS was applied simultaneously to the cognitive task and functional magnetic resonance imaging (fMRI). Surprisingly, more omission errors and less accuracy were observed in the active group when compared to sham, despite an improvement in reaction time. The authors hypothesized that, in adolescents with ADHD, anodal stimulation may improve motor performance, but at the cost of poorer precision. (Sotnikova et al., 2017)
A study applying double anodal tDCS was performed to assess the sustained attention in adults with ADHD. In a crossover design with three sessions (baseline, active tDCS and sham), healthy individuals (n=16) and ADHD participants (n=21) were evaluated with regard to their performance in the MOXO Continuous Performance Test (MOXO-CPT), a cognitive test that assess attention, impulsivity, timing and hyperactivity. (Jacoby and Lavidor, 2018) For the active tDCS session, subjects received 1.8mA bilateral offline anodal stimulation for 20 minutes over the right (R) and left DLPFC, with cathode placed over the cerebellum (1cm below the inion); this montage was designed to obtain a spreading effect and modulate a broader cortical area. For sham, an identical montage was used, however the stimulator was turned off after 30 seconds of ramp up. However, they found no group differences or enhanced performance, and lack of effect was attributed to learning and repetition effects that potentially concealed stimulation response. (Jacoby and Lavidor, 2018)
In a pilot study, Bandeira et al. applied five daily sessions of anodal tDCS online at 2mA for five 30-minute sessions, over the left DLPFC, with return electrode on the right supraorbital area, in n=9 children and adolescents with ADHD. (Bandeira et al., 2016a) A decrease in uncorrected and total errors in switching task, and completion time in the naming task of the Neuropsychological Development Assessment (NEPSY-II), an inhibitory control subtest, were observed when comparing pre and post intervention results. In addition to enhancement in inhibitory control, a decrease in the omission errors using the Visual Attention Test (TAVIS-3) was observed when comparing post- and pre-intervention results, indicating an improvement in attention tDCS. No significant differences were found in the Digit Span subtest of the Wechsler Intelligence Scale for Children (WISC-III). Based on these findings, the authors proposed that tDCS could increase selective attention, reduce attention deficits and improve inhibitory control. (Bandeira et al., 2016a)
Nejati et al. performed a crossover, double-blinded, sham-controlled trial to investigate the effects of tDCS over the DLPFC and orbitofrontal cortex (OFC) on executive functioning in 25 children with ADHD. (Nejati et al., 2017) Subjects were divided into two experiments: 1) Included n=15 individuals that underwent anodal stimulation over L DLPFC with cathode placed on R DLPFC, then after 3 days the same montage was applied for the sham intervention; and 2) Delivered active tDCS, in n=10 children, with anode over the L DLPFC and cathode on the R OFC (montage 1), followed by 72hrs of washout. After that, anode was positioned on R OFC and cathode over the L DLPFC (montage 2), with subsequent sham stimulation after 3 days from prior session. For both experiments, active tDCS was delivered at 1mA (current density of 0.04mA/cm2) for 15 minutes, and during sham stimulation the current was ramped up for 30 seconds then turned off. With regards to working memory, they observed a significant decrease in reaction time using the N-back following anodal stimulation over the L DLPFC with cathode on the R DLPFC (experiment 1) compared to sham (F=21.01, p<.01), but no difference was observed in the number of accurate responses (F=0.21, p=.65). In experiment 2, only the montage with anode over the L DLPFC and cathode on the R OFC significantly increased accuracy and decreased RT (p<.01), compared to montage 2 and sham. In addition to examining working memory, the authors investigated the role of tDCS in inhibitory control, interference control and cognitive flexibility, using the Go/No-Go, Stroop task and the Wisconsin Card Sorting Test (WCST), respectively. (Nejati et al., 2017) For the experiment 1, no significant differences were found in the Go/No-Go task between the active and sham interventions, suggesting no enhanced inhibitory control in individuals with ADHD following anodal L DLPFC and cathodal R DLPFC stimulation. Additionally, no improvement was observed in the WCST between groups. However, a significant effect on interference response inhibition was noted in the performance of the Stroop task with improved accuracy (F=9.01, p<.01 and RT (F=7.7, p<.02). As for the experiment 2, the second montage (anode on R OFC/cathode over L DLPFC) demonstrated a robust increase of no-go accuracy compared to sham (p<.01). There was a significant reduction in perseverative errors, completed categories and total errors (p<.01) on WCST following stimulation with both montages, with more effective findings associated to the first montage, implying that increased activity of left DLPFC and down modulation of right OFC enhances cognitive flexibility and reduces impulsivity. The authors concluded that anodal L DLPFC stimulation improved working memory in children with ADHD. Furthermore, an improved interference response inhibition was also observed when applying anodal tDCS over L DLPFC with reference electrode on R DLPFC (experiment 1), however no differences were seen on Go/No-Go and WCST for this montage. As for experiment 2, an enhanced inhibitory control was noted following active stimulation over R OFC (montage 2), as well as improved cognitive flexibility when using both montages (more pronounced in the montage 1). (Nejati et al., 2017)
Soff et al. in a randomized double-blind sham-controlled crossover trial indicated improvements in cognition in adolescents with ADHD with tDCS. (Soff et al., 2017) This study assessed inattention and impulsivity by the application of Quantified Behavioral Test (Qb-Test), a neurophysiological test that aims to assess three domains - inattention, hyperactivity and impulsivity. It includes N-back working memory assessment and a measurement of patient’s activity. In this paradigm, inattention was assessed based on increased omission errors, slower reaction time and greater reaction time variability. In this trial, patients (n=15) placed in one group received 20 minutes of anodal 1mA stimulation (current density: 0.029mA/cm2) over the left (L) dorsolateral prefrontal cortex (DLPFC), with return electrode placed in the vertex, once daily for 5 days. Then, after two weeks, they underwent sham procedure with the same montage. Initially, group 2 received sham procedure, and, after two weeks, they received active treatment. With regards to cognition assessment, patients in both groups performed Qb-Test from days 2 to 5. The same cognitive tests were additionally conducted seven days after the end of application sessions. This study demonstrated active stimulation was associated with improvement in inattention and N-back performance, with significant improvement in impulsivity associated with active tDCS compared to sham based on Qb-Test performance, when compared to the sham stimulation. In addition, this trial aimed to analyze the lasting clinical effect of the active treatment, and there was an even more robust reduction in hyperactivity by the seventh day following the treatment end. (Soff et al., 2017)
Cachoeira et al. in a randomized sham-controlled trial demonstrated improvement in ADHD symptoms in n=17 adults with ADHD who received active tDCS. (Cachoeira et al., 2017) The authors utilized the Adult ADHD Self-Report Scale Symptom Checklist-1 v1 (ASRS) to evaluate inattentiveness and hyperactive/impulsive symptoms. Functional impairment in three domains (school, work and family life) was evaluated by using Sheehan Disability Scale (SDS). In this study, the anodal electrode was placed over the right DLPFC and cathode over the left DLPFC, and delivered 2 mA for 20 minutes during five consecutive days. Sham procedures were identical, except that during sham stimulation the device was turned off after one minute of active tDCS. The authors found significant lower ASRS inattention and SDS scores after active tDCS in comparison with sham, suggesting improvement in inattention and functional impairment, respectively; although effects were transient, and attenuated over the following 4 weeks (Cachoeira et al., 2017)
Neurophysiological findings
In regard to neurophysiological mechanisms, a mathematical model was applied to examine the modulation of cortical connectivity by tDCS in ADHD subjects. (Cosmo et al., 2015b) This sophisticated computational algorithm was based on electroencephalographic activity recorded before and immediately after a single active or sham 20-minute tDCS session at 1mA over the left DLPFC. The authors found significant differences when comparing the functional cortical networks (measured as weighted node degree) within the active group, what was not noted in the sham intervention. The results revealed an increase in cortical connectivity following anodal stimulation in the stimulated area and correlates, suggesting a spreading of the modulatory activity. (Cosmo et al., 2015b)
Consistent with these findings, Sotnikova et al. reported increased in fMRI metrics of connectivity and neuronal activation in the stimulated area, which extended to other regions of the brain. Subjects were submitted to 20 minutes of tDCS (active or sham) synchronized to the fMRI scanning and working memory task, described as a combination of the N-back and Go/No-Go tasks, followed by a 10 minutes resting state fMRI. In addition to the aforementioned findings related to the modulation of working memory performance, the authors observed through the fMRI results that the active anodal tDCS promoted significant hemodynamic changes in the left DLPFC (target area) with propagation of the activation of neural networks involving the left premotor cortex, left supplementary motor area and precuneus. (Sotnikova et al., 2017)
Safety aspects
tDCS has been recognized as a safe technique with transient and mostly mild adverse events. (Matsumoto and Ugawa, 2017) Multiple studies have documented brief and minor side effects such as itching, tingling, burning sensation, skin redness under electrodes, mild headache, fatigue, or insomnia. (Iyer et al., 2005; Nitsche et al., 2003a; Nitsche et al., 2003b; Poreisz et al., 2007) However, some trials reported concerning adverse events, mainly when applying tDCS in individuals with depression. Mania and hypomania have been observed following tDCS, (Arul-Anandam et al., 2010; Brunoni et al., 2011b; Galvez et al., 2011) suggesting a potential cognitive impairment or even harm associated to the technique. In accordance to this hypothesis, in the study performed by Sotnikova et al., the authors found that anodal tDCS resulted in improved motor performance in adolescents, however poor precision with more omission errors and decreased accuracy were noted when compared to sham, (Sotnikova et al., 2017) raising concerns for worsening of impulsivity.
Regarding the use of tDCS in ADHD, no major safety concerns have been identified. To date, no serious adverse events have been observed. Seven studies reported adverse events, mostly mild itching, tingling sensation, or headache. (Allenby et al., 2018; Bandeira et al., 2016a; Breitling et al., 2016; Cachoeira et al., 2017; Nejati et al., 2017; Soff et al., 2017; Sotnikova et al., 2017) One study had an uncommon side effect described as acute mood change (sadness, tension and hypobulia), lasting one day. (Cachoeira et al., 2017) This variability in the occurrence, type, duration and severity of side effects might be explained by different aspects of the stimulation protocol such as montage, current density, skin impedance, duration of stimulation, number of sessions, poor technical compliance, individual predisposition/sensitivity, (Matsumoto and Ugawa, 2017) as well as by the disorder under study and the brain structural and functional abnormalities associated to it. Therefore, while tDCS for ADHD appears safe, this is based on generally small studies, and as larger trials are conducted the field should expect some degree of significant adverse events, particularly because of the associated comorbidity between ADHD and major depressive disorder.
As aforesaid, side effects are one of the main challenges in the ADHD pharmacological treatment. Therefore, the investigation of tDCS as a potential therapeutic tool to modulate ADHD symptoms is reinforced by its overall safe profile.
DISCUSSION
To obtain accurate information about the application of tDCS in ADHD, its potential indications and limitations, it is necessary to comment on specific aspects if the protocols described above. The main differences noted across studies included variation in the current density, polarity-dependent findings, number of sessions, tDCS online or offline application, and electrodes montage/target area. Furthermore, even when using well-established parameters, differences may be attributed to variations in study designs, possibly explaining the distinct, and even controversial, findings.
Variability in the applied current density is likely a major contributor to the mixed findings reported above. As current density stands for the current intensity divided by the electrode size, a reduction in the electrode dimensions results in increased current density, and may increase focality. (Faria et al., 2011) In prior trials, brain focality has been translated into better cognitive performance. (Naka et al., 2018; Nikolin et al., 2015) However, in the setting of the complex ADHD pathophysiological mechanisms with impairment of mesocorticolimbic pathways, in addition to the involvement of broader cortical networks (Castellanos and Proal, 2012; Dickstein et al., 2006; Posner et al., 2020; Volkow and Swanson, 2013) it is unlikely that a focal stimulation approach would be sufficient. This hypothesis is supported by Jacoby and Lavidor’s trial that, among the studies included in this review, applied one of the highest current density (0.2mA/cm2) and yet showed no improvement in cognitive performance in ADHD. (Jacoby and Lavidor, 2018) In contrast, Breitling et al. delivered the lowest current density (0.03mA/cm2) and found a reduction in the number of commission errors and variability in reaction time among ADHD subjects that received anodal stimulation, (Breitling et al., 2016) implying greater cognitive effects could be associated with reduced focality.
Polarity dependent effects were also an important feature of the reviewed studies. While one trial applying anodal stimulation at 1mA over the left DLPFC did not find significantly improvement in inhibitory control, (Cosmo et al., 2015a) another study achieved this using cathodal tDCS at 1.5mA over the same area. (Soltaninejad et al., 2019) These results are consistent with the idea that the traditional mechanistic understanding (i.e., anodal tDCS is excitatory and cathodal tDCS is inhibitory) is likely incorrect. A possible explanation for these findings is supported by Batsikadze et al., which revealed that specific polarity-dependent effects were observed only when applying stimulation at 1 mA. (Batsikadze et al., 2013) Thus, at 1.5mA, cathodal stimulation might have enhanced neuronal depolarization rather than hyperpolarization, resulting in increased inhibitory control.
Other important factors include the number of sessions, and online vs. offline tDCS application. Regarding the session frequency, three main approaches were observed. Two studies included five tDCS sessions, (Bandeira et al., 2016a; Cachoeira et al., 2017) another one had three sessions, (Allenby et al., 2018) while others applied only a single treatment. (Cosmo et al., 2015a; Cosmo et al., 2015b; Jacoby and Lavidor, 2018; Nejati et al., 2017; Soltaninejad et al., 2019) Significant cognitive results were generally found in the studies utilizing multiple sessions, underscoring the importance of cumulative exposure. Nonetheless, improved inhibitory control was observed in a study with a single tDCS session, (Soltaninejad et al., 2019) although this trial utilized online tDCS, and prior studies have demonstrated greater effects following online tDCS application. (Martin et al., 2014; Stagg et al., 2013) Likely, the stimulation synchronized with the cognitive task might result in the further activation of brain networks required for response, resulting in enhanced brain excitability and improving cognitive performance. Supporting this hypothesis, in the trial performed by Cosmo et al., subjects underwent either active or sham stimulation, without simultaneous application of cognitive task, which may explain their findings. (Cosmo et al., 2015a) Similar results were observed in a trial applying 10 sessions of tDCS offline in patients with depression, were no improvement in cognitive performance was detected. (Loo et al., 2010) Together, these findings raise the possibility that the use of online tDCS might play a more robust role than the number of sessions as a clinical effectiveness modifier and predictor of response.
Relevant to the discussion of number of tDCS sessions, the study by Allenby et al., demonstrated a reduction impulsivity following three sessions of active tDCS. (Allenby et al., 2018) However, the effect was quite limited in duration (i.e., less than three days). Cachoeira et al. observed enhanced attention and functional capacity after five tDCS sessions, with reduced inattention sustained for up to two weeks; these effects are likely attributable to the greater number of sessions. (Cachoeira et al., 2017) Interestingly, Soff et al. observed a reduction in inattention and impulsivity as well as improved hyperactivity, following five consecutive anodal tDCS sessions, with a more robust hyperactivity reduction seen by the 7th day after the treatment, implying a long-lasting tDCS effect when applying repeated sessions. (Soff et al., 2017) Prior studies have shown that tDCS physiological effects might last 30–90 minutes following a single stimulation session, depending on stimulation duration and current intensity; (Cirillo et al., 2017; Nitsche and Paulus, 2000, 2001) with potential for long-lasting neuroplastic changes after multiple sessions, likely due to changes in the synaptic strength induced by long-term potentiation (LTP)-like response and metaplasticity mechanisms. (Cirillo et al., 2017; Cohen Kadosh et al., 2010) Considering the hypoactivity of prefrontal area, caudate nucleus and cerebellum shown in prior ADHD studies, (Antshel et al., 2011; Brown et al., 2006; Lukito et al., 2020; Puig and Gulledge, 2011; Spencer-Smith and Anderson, 2009) it is unlikely that a single or even a small number of sessions, would be sufficient to induce changes required to yield long-lasting cognitive improvement. Based on these observations, further studies with multiple tDCS sessions and longer follow-up periods are recommended to determine the optimal therapeutic tDCS protocol, to accomplish more consistent and sustained effects, an essential step to establish this technique as a therapeutic tool for ADHD.
Regarding the stimulation montage, electrode placement was highly variable in the reviewed trials. Some studies applied stimulation over the left dorsolateral prefrontal cortex, in others bifrontal anodal montage was used, modulating right and left DLPFC simultaneously (Jacoby and Lavidor, 2018); while two trials applied active stimulation respectively over the right inferior frontal gyrus (rIFG) and orbitofrontal cortex. Although all studies stimulated the prefrontal cortex, the position of the reference electrode was highly variable; since the reference electrode also contributes to tDCS’ neuromodulatory activity, this variability likely impacted observed results. To this point, in the study performed by Jacoby and Lavidor, bifrontal anodal montage was applied with cathode over the cerebellum, with the goal to spread the stimulation effects, and yielded null results. (Jacoby and Lavidor, 2018) In this scenario, it is possible that current spreading resulted in the dissipation of potentially therapeutic effects, by reducing the electric field strength in the target areas. (Cosmo et al., 2015b; Opitz et al., 2015) Furthermore, controversial findings were found among studies regarding target area for inhibitory control. In the study from Nejati et al., active cathodal tDCS was delivered over the left DLPFC resulting in increased inhibitory control as measured by the Go/No-Go task. (Nejati et al., 2017) The authors suggested that this outcome might be explained not by reduced cortical excitability when applying cathodal stimulation, but for supposed indirectly enhanced activity in the right DLPFC by decreased transcallosal inhibition. (Nejati et al., 2017) However, the placement of the anodal on the R OFC (Brodmann area 11- BA11) might have also contributed to enhanced depolarization in the adjacent right inferior frontal gyrus (Brodmann area 47), a known anatomical target for improving interference control (important component of inhibitory control). This hypothesis is supported by the exploratory analysis from Breitling et al. that found improved interference control in individuals with ADHD exposed to anodal tDCS over the rIFG. (Breitling et al., 2016) Enhanced inhibitory control was also observed by Soltaninejad et al. and Cachoeira et al., here both studies applied anodal stimulation on the right DLPFC. (Cachoeira et al., 2017; Soltaninejad et al., 2019) Interestingly, in the trial performed by Bandeira et al., anodal electrode was placed on the left DLPFC yielded improved inhibitory control. (Bandeira et al., 2016a) Allenby et al. and Soff et al., reported similar findings, as both detected improvement in impulsivity using anodal tDCS to the left DLPFC. (Allenby et al., 2018; Soff et al., 2017) Furthermore, a recent meta-analysis assessed the effectiveness of tDCS on inhibitory control in ADHD trials. Salehinejad et al. concluded that this neuromodulation technique improved inhibitory control when applying anodal tDCS over the dorsolateral prefrontal cortex (DLPFC), albeit with a small effect size. (Salehinejad et al., 2019)
The results from the above-mentioned trials suggest that both right and left prefrontal cortices play an important role in improving inhibitory control, consequently decreasing impulsivity in ADHD. In the light of these findings, possibly more robust enhancement of inhibitory control might be obtained from the application of double anodal tDCS, with one electrode over the left DLPFC (F3, according to the 10–20 International EEG system) and another one on the rIFG (F8), with placement of the reference electrode in a cephalic position, for example the vertex.
In addition to optimizing the stimulation protocol, another significant challenge in tDCS trials is to establish biomarkers that integrating the biophysical elements of tDCS with the neurobiological targets of interest in ADHD. To date, only two ADHD trials have investigated neurophysiological parameters as potential markers of tDCS effects. Cosmo et al. applied a mathematical model that aimed to characterize dynamic spatial parameters of cortical activation resulting from active stimulation, using EEG-based connectivity between electrode pairs over time. (Cosmo et al., 2015b) Using this model, they described network evolution associated with tDCS, described as the number of times that an electrode was connected to others over time, thus providing objective measurements that described the spatial effects of anodal stimulation. This analysis revealed that, in addition to the modulation of the target area (left DLPFC), current spreading over time was responsible for the observation of increased cortical connectivity in regions distal to the site of stimulation, including occipital, left and right temporal and centroparietal areas. (Cosmo et al., 2015b) Similar findings were documented by Sotnikova et al. (Sotnikova et al., 2017) By acquiring fMRI data, the authors found an increase in connectivity and neuronal activation in the stimulated area (left DLPFC) with extension of the modulatory effects to the left premotor area, left SMA and precuneus - neuronal network areas associated with working memory performance. The spreading effect of tDCS found in both studies might be explained by the cortical connections between the stimulated areas, to the transcallosal modulation, in addition to the type of montage applied. (Cosmo et al., 2015b) Previous studies have shown more diffuse tDCS effects when using a cephalic montage, (Nitsche et al., 2008) whereas when using an extracephalic reference, there was more focal tDCS activity under the electrode. In this context, the cephalic montage seems to be more suitable to ADHD trials given that this a chronic neurodevelopmental disorder with complex neurophysiological mechanisms that functionally compromises frontal areas and correlates, as discussed above. Therefore, increases in brain excitability beyond the region under the stimulating electrode could lead to enhanced neuronal activity in correlated cortical networks with consequent improvement of cognitive performance.
The main limitation of this systematic review was the small number of studies investigating the application of tDCS in ADHD. Additionally, as highlighted above, initially we considered performing a meta-analysis, however the methods and outcomes were too varied among the trials to provide a meaningful meta-analytic approach.
In summary, this review of tDCS in individuals with ADHD revealed the potential efficacy of this technique in modulating executive functions, particularly attention, working memory and inhibitory control - domains heterogeneously and dynamically affected in this disorder. Furthermore, an increase in connectivity and neuronal activation was observed in the stimulated areas and correlates, suggesting a spreading of the modulatory activity. Because of the significant diversity of the included trials, it is not possible to definitely propose a standardized protocol for the application of tDCS in ADHD; however based in the review of the aforementioned findings and the specialized literature, future studies could consider multiple sessions of tDCS, online application, double anodal stimulation over left DLPFC and rIFG, with extracephalic reference placement for more focal modulation. Likely, this stimulation approach would reveal more robust, long-lasting cognitive and neurophysiological effects in subjects with ADHD.
As a final comment, the assessment of tDCS as a therapeutic tool for the treatment of ADHD is recent and, as such, there is a general paucity of articles about this topic in the literature. Additional studies are needed in order to clarify several questions, including efficacy, long-lasting effects, and further optimization of stimulation parameters in this population.
FUNDING SOURCES
Camila Cosmo is supported by R25 MH101076. Effort on this paper was also supported by the VA RR&D Center for Neurorestoration and Neurotechnology (NSP). The funders had no role in the conduct of the study, manuscript preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funders.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflict of interests to report.
REFERENCES
- Allenby C, Falcone M, Bernardo L, Wileyto EP, Rostain A, Ramsay JR, Lerman C, Loughead J, 2018. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul 11, 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Hargrave TM, Simonescu M, Kaul P, Hendricks K, Faraone SV, 2011. Advances in understanding and treating ADHD. BMC Med 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Li BM, 2005. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR, 2011. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav 99, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arul-Anandam AP, Loo C, Mitchell P, 2010. Induction of hypomanic episode with transcranial direct current stimulation. J ECT 26, 68–69. [DOI] [PubMed] [Google Scholar]
- Bandeira ID, Guimaraes RS, Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, Argollo N, Lucena R, 2016a. Transcranial Direct Current Stimulation in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder (ADHD): A Pilot Study. J Child Neurol 31, 918–924. [DOI] [PubMed] [Google Scholar]
- Bandeira ID, Guimaraes RS, Jagersbacher JG, Barretto TL, de Jesus-Silva JR, Santos SN, Argollo N, Lucena R, 2016b. Transcranial Direct Current Stimulation in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder (ADHD): A Pilot Study. J Child Neurol. [DOI] [PubMed] [Google Scholar]
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2015. A Randomized Placebo-Controlled Trial of Targeted Prefrontal Cortex Modulation with Bilateral tDCS in Patients with Crack-Cocaine Dependence. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA, 2013. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591, 1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova NS, Michelini G, Kuntsi J, Asherson P, 2018. Mind wandering perspective on attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 92, 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling C, Zaehle T, Dannhauer M, Bonath B, Tegelbeckers J, Flechtner HH, Krauel K, 2016. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS). Front Cell Neurosci 10, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Buckley J, Gibbs D, Kaplan G, Lofton K, McDonagh K, Schrader A, Vaughn A, Whetsell G, Wade R, 2006. Executive dialogue series. Performance improvement in the era of quality improvement. Hosp Health Netw 80, 80–89. [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F, 2011a. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14, 1133–1145. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F, 2012. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Zanao T, de Oliveira JF, Bensenor IM, Fregni F, 2011b. Manic psychosis after sertraline and transcranial direct-current stimulation. J Neuropsychiatry Clin Neurosci 23, E4–5. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cachoeira CT, Leffa DT, Mittelstadt SD, Mendes LST, Brunoni AR, Pinto JV, Blazius V, Machado V, Bau CHD, Rohde LA, Grevet EH, Schestatsky P, 2017. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder - A pilot randomized controlled study. Psychiatry Res 247, 28–32. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal JD, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL, 2001. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 58, 289–295. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL, 1996. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 53, 607–616. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E, 2012. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 16, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V, 2017. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 10, 1–18. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V, 2010. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr Biol 20, 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmo C, Baptista AF, de Araujo AN, do Rosario RS, Miranda JG, Montoya P, de Sena EP, 2015a. A Randomized, Double-Blind, Sham-Controlled Trial of Transcranial Direct Current Stimulation in Attention-Deficit/Hyperactivity Disorder. PLoS One 10, e0135371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmo C, Ferreira C, Miranda JG, do Rosario RS, Baptista AF, Montoya P, de Sena EP, 2015b. Spreading Effect of tDCS in Individuals with Attention-Deficit/Hyperactivity Disorder as Shown by Functional Cortical Networks: A Randomized, Double-Blind, Sham-Controlled Trial. Front Psychiatry 6, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K, 2011. Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Res 193, 17–27. [DOI] [PubMed] [Google Scholar]
- D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A, 2016. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety 33, 1132–1140. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Volz MS, Bikson M, Fregni F, 2011. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP, 2006. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 47, 1051–1062. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Nigg JT, Sullivan EL, 2019. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav 182, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P, 2011. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry 69, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Faria P, Hallett M, Miranda PC, 2011. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng 8, 066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Reiff MI, 2014. Clinical practice. Attention deficit-hyperactivity disorder in children and adolescents. N Engl J Med 370, 838–846. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J, 1997. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology 48, 589–601. [DOI] [PubMed] [Google Scholar]
- Gallo EF, Posner J, 2016. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry 3, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez V, Alonzo A, Martin D, Mitchell PB, Sachdev P, Loo CK, 2011. Hypomania induction in a patient with bipolar II disorder by transcranial direct current stimulation (tDCS). J ECT 27, 256–258. [DOI] [PubMed] [Google Scholar]
- Gilmore A, Milne R, 2001. Methylphenidate in children with hyperactivity: review and cost-utility analysis. Pharmacoepidemiol Drug Saf 10, 85–94. [DOI] [PubMed] [Google Scholar]
- Gowda SM, Narayanaswamy JC, Hazari N, Bose A, Chhabra H, Balachander S, Bhaskarapillai B, Shivakumar V, Venkatasubramanian G, Reddy YCJ, 2019. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: A randomized, double blinded, sham controlled trial. Brain Stimul 12, 922–929. [DOI] [PubMed] [Google Scholar]
- Haavik J, Halmoy A, Lundervold AJ, Fasmer OB, 2010. Clinical assessment and diagnosis of adults with attention-deficit/hyperactivity disorder. Expert Rev Neurother 10, 1569–1580. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, 2019. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N, Zeeuw P, Szekely E, Sudre G, Wolfers T, Onnink AMH, Dammers JT, Mostert JC, Vives-Gilabert Y, Kohls G, Oberwelland E, Seitz J, Schulte-Ruther M, Ambrosino S, Doyle AE, Hovik MF, Dramsdahl M, Tamm L, van Erp TGM, Dale A, Schork A, Conzelmann A, Zierhut K, Baur R, McCarthy H, Yoncheva YN, Cubillo A, Chantiluke K, Mehta MA, Paloyelis Y, Hohmann S, Baumeister S, Bramati I, Mattos P, Tovar-Moll F, Douglas P, Banaschewski T, Brandeis D, Kuntsi J, Asherson P, Rubia K, Kelly C, Martino AD, Milham MP, Castellanos FX, Frodl T, Zentis M, Lesch KP, Reif A, Pauli P, Jernigan TL, Haavik J, Plessen KJ, Lundervold AJ, Hugdahl K, Seidman LJ, Biederman J, Rommelse N, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Oosterlaan J, Polier GV, Konrad K, Vilarroya O, Ramos-Quiroga JA, Soliva JC, Durston S, Buitelaar JK, Faraone SV, Shaw P, Thompson PM, Franke B, 2017. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, Jahanshad N, Sudre G, Wolfers T, Earl EA, Soliva Vila JC, Vives-Gilabert Y, Khadka S, Novotny SE, Hartman CA, Heslenfeld DJ, Schweren LJS, Ambrosino S, Oranje B, de Zeeuw P, Chaim-Avancini TM, Rosa PGP, Zanetti MV, Malpas CB, Kohls G, von Polier GG, Seitz J, Biederman J, Doyle AE, Dale AM, van Erp TGM, Epstein JN, Jernigan TL, Baur-Streubel R, Ziegler GC, Zierhut KC, Schrantee A, Hovik MF, Lundervold AJ, Kelly C, McCarthy H, Skokauskas N, O’Gorman Tuura RL, Calvo A, Lera-Miguel S, Nicolau R, Chantiluke KC, Christakou A, Vance A, Cercignani M, Gabel MC, Asherson P, Baumeister S, Brandeis D, Hohmann S, Bramati IE, Tovar-Moll F, Fallgatter AJ, Kardatzki B, Schwarz L, Anikin A, Baranov A, Gogberashvili T, Kapilushniy D, Solovieva A, El Marroun H, White T, Karkashadze G, Namazova-Baranova L, Ethofer T, Mattos P, Banaschewski T, Coghill D, Plessen KJ, Kuntsi J, Mehta MA, Paloyelis Y, Harrison NA, Bellgrove MA, Silk TJ, Cubillo AI, Rubia K, Lazaro L, Brem S, Walitza S, Frodl T, Zentis M, Castellanos FX, Yoncheva YN, Haavik J, Reneman L, Conzelmann A, Lesch KP, Pauli P, Reif A, Tamm L, Konrad K, Oberwelland Weiss E, Busatto GF, Louza MR, Durston S, Hoekstra PJ, Oosterlaan J, Stevens MC, Ramos-Quiroga JA, Vilarroya O, Fair DA, Nigg JT, Thompson PM, Buitelaar JK, Faraone SV, Shaw P, Tiemeier H, Bralten J, Franke B, 2019. Brain Imaging of the Cortex in ADHD: A Coordinated Analysis of Large-Scale Clinical and Population-Based Samples. Am J Psychiatry 176, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Rijpkema M, Janss L, Brunner H, Fernandez G, Buitelaar J, Franke B, Arias-Vasquez A, 2012. Current self-reported symptoms of attention deficit/hyperactivity disorder are associated with total brain volume in healthy adults. PLoS One 7, e31273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM, 2005. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64, 872–875. [DOI] [PubMed] [Google Scholar]
- Jacoby N, Lavidor M, 2018. Null tDCS Effects in a Sustained Attention Task: The Modulating Role of Learning. Front Psychol 9, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H, Galal H, Osman AM, Farghaly HSM, Karim AA, Ahmed GA, 2017. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul 10, 893–901. [DOI] [PubMed] [Google Scholar]
- Lange KW, Reichl S, Lange KM, Tucha L, Tucha O, 2010. The history of attention deficit hyperactivity disorder. Atten Defic Hyperact Disord 2, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W, 2017. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128, 56–92. [DOI] [PubMed] [Google Scholar]
- Leffa DT, Bellaver B, Salvi AA, de Oliveira C, Caumo W, Grevet EH, Fregni F, Quincozes-Santos A, Rohde LA, Torres ILS, 2018a. Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimul 11, 743–751. [DOI] [PubMed] [Google Scholar]
- Leffa DT, de Souza A, Scarabelot VL, Medeiros LF, de Oliveira C, Grevet EH, Caumo W, de Souza DO, Rohde LAP, Torres ILS, 2016. Transcranial direct current stimulation improves short-term memory in an animal model of attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 26, 368–377. [DOI] [PubMed] [Google Scholar]
- Leffa DT, Torres ILS, Rohde LA, 2018b. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 25, 328–333. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W, 2002. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247. [DOI] [PubMed] [Google Scholar]
- Loo CK, Sachdev P, Martin D, Pigot M, Alonzo A, Malhi GS, Lagopoulos J, Mitchell P, 2010. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 13, 61–69. [DOI] [PubMed] [Google Scholar]
- Lukito S, Norman L, Carlisi C, Radua J, Hart H, Simonoff E, Rubia K, 2020. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundervold AJ, Adolfsdottir S, Halleland H, Halmoy A, Plessen K, Haavik J, 2011. Attention Network Test in adults with ADHD--the impact of affective fluctuations. Behav Brain Funct 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R, 2017. The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Connect 7, 25–33. [DOI] [PubMed] [Google Scholar]
- Martin DM, Liu R, Alonzo A, Green M, Loo CK, 2014. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp Brain Res 232, 3345–3351. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ugawa Y, 2017. Adverse events of tDCS and tACS: A review. Clin Neurophysiol Pract 2, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matza LS, Paramore C, Prasad M, 2005. A review of the economic burden of ADHD. Cost Eff Resour Alloc 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka M, Matsuzawa D, Ishii D, Hamada H, Uchida T, Sugita K, Sutoh C, Shimizu E, 2018. Differential effects of high-definition transcranial direct current stimulation on verbal working memory performance according to sensory modality. Neurosci Lett 687, 131–136. [DOI] [PubMed] [Google Scholar]
- Nejati V, Salehinejad MA, Nitsche MA, Najian A, Javadi AH, 2017. Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. J Atten Disord, 1087054717730611. [DOI] [PubMed] [Google Scholar]
- Ngernyam N, Jensen MP, Arayawichanon P, Auvichayapat N, Tiamkao S, Janjarasjitt S, Punjaruk W, Amatachaya A, Aree-uea B, Auvichayapat P, 2015. The effects of transcranial direct current stimulation in patients with neuropathic pain from spinal cord injury. Clin Neurophysiol 126, 382–390. [DOI] [PubMed] [Google Scholar]
- Nikolin S, Loo CK, Bai S, Dokos S, Martin DM, 2015. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage 117, 11–19. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A, 2008. Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1, 206–223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W, 2003a. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol 56, 255–276. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W, 2003b. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114, 2220–2222; author reply 2222–2223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, Lang N, Henning S, Paulus W, Tergau F, 2004. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19, 2720–2726. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W, 2003c. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114, 600–604. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2001. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, Rubia K, 2016. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA Psychiatry 73, 815–825. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Carlisi CO, Christakou A, Chantiluke K, Murphy C, Simmons A, Giampietro V, Brammer M, Mataix-Cols D, Rubia K, 2017. Neural dysfunction during temporal discounting in paediatric Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. Psychiatry Res Neuroimaging 269, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S, 2006. ADHD in adults. N Engl J Med 354, 2637–2641. [DOI] [PubMed] [Google Scholar]
- Opitz A, Paulus W, Will S, Antunes A, Thielscher A, 2015. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 109, 140–150. [DOI] [PubMed] [Google Scholar]
- Owens J, Hoza B, 2003. Diagnostic utility of DSM-IV-TR symptoms in the prediction of DSM-IV-TR ADHD subtypes and ODD. J Atten Disord 7, 11–27; discussion 29. [DOI] [PubMed] [Google Scholar]
- Palm U, Hasan A, Strube W, Padberg F, 2016. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci 266, 681–694. [DOI] [PubMed] [Google Scholar]
- Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL, 2017. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am J Psychiatry 174, 628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA, 2007. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164, 942–948. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA, 2015. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry 56, 345–365. [DOI] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W, 2007. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72, 208–214. [DOI] [PubMed] [Google Scholar]
- Posner J, Polanczyk GV, Sonuga-Barke E, 2020. Attention-deficit hyperactivity disorder. Lancet 395, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J, 2008. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol 28, S39–45. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT, 2011. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 44, 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranby KW, Boynton MH, Kollins SH, McClernon FJ, Yang C, Fuemmeler BF, 2012. Understanding the phenotypic structure of adult retrospective ADHD symptoms during childhood in the United States. J Clin Child Adolesc Psychol 41, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, Maj M, Stein DJ, Maercker A, Tyrer P, Claudino A, Garralda E, Salvador-Carulla L, Ray R, Saunders JB, Dua T, Poznyak V, Medina-Mora ME, Pike KM, Ayuso-Mateos JL, Kanba S, Keeley JW, Khoury B, Krasnov VN, Kulygina M, Lovell AM, de Jesus Mari J, Maruta T, Matsumoto C, Rebello TJ, Roberts MC, Robles R, Sharan P, Zhao M, Jablensky A, Udomratn P, Rahimi-Movaghar A, Rydelius PA, Bahrer-Kohler S, Watts AD, Saxena S, 2019. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 18, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Brinson H, 2014. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol 58 Suppl 1, S3–16. [PubMed] [Google Scholar]
- Salehinejad MA, Wischnewski M, Nejati V, Vicario CM, Nitsche MA, 2019. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits. PLoS One 14, e0215095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Prasad V, Daley D, Ford T, Coghill D, 2018. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 5, 175–186. [DOI] [PubMed] [Google Scholar]
- Sharafi E, Taghva A, Arbabi M, Dadarkhah A, Ghaderi J, 2019. Transcranial Direct Current Stimulation for Treatment-Resistant Major Depression: A Double-Blind Randomized Sham-Controlled Trial. Clin EEG Neurosci 50, 375–382. [DOI] [PubMed] [Google Scholar]
- Sharma A, Couture J, 2014. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann Pharmacother 48, 209–225. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL, 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A 104, 19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier AC, Reichenbacher T, Ghuman HS, Ghuman JK, 2013. Pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: clinical strategies. J Cent Nerv Syst Dis 5, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I, 2009. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 194, 204–211. [DOI] [PubMed] [Google Scholar]
- Soff C, Sotnikova A, Christiansen H, Becker K, Siniatchkin M, 2017. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm (Vienna) 124, 133–144. [DOI] [PubMed] [Google Scholar]
- Soltaninejad Z, Nejati V, Ekhtiari H, 2019. Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. J Atten Disord 23, 325–332. [DOI] [PubMed] [Google Scholar]
- Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M, 2017. Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topogr 30, 656–672. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M, Anderson V, 2009. Healthy and abnormal development of the prefrontal cortex. Dev Neurorehabil 12, 279–297. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I, 2013. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci 33, 11425–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA, 2011. Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53. [DOI] [PubMed] [Google Scholar]
- Steinau S, 2013. Diagnostic Criteria in Attention Deficit Hyperactivity Disorder - Changes in DSM 5. Front Psychiatry 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance GW, 1986. Measurement of health state utilities for economic appraisal. J Health Econ 5, 1–30. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, 2013. Clinical practice: Adult attention deficit-hyperactivity disorder. N Engl J Med 369, 1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, 2019. Transcranial direct current stimulation for the treatment of major depressive disorder: A meta-analysis of randomized controlled trials. Psychiatry Res 276, 186–190. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI, 2014. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabil Neural Repair 28, 250–259. [DOI] [PubMed] [Google Scholar]


