Abstract
Placozoa are small disc-shaped animals, representing the simplest known, possibly ancestral, organization of free-living animals. With only six morphological distinct cell types, without any recognized neurons or muscle, placozoans exhibit fast effector reactions and complex behaviors. However, little is known about electrogenic mechanisms in these animals. Here, we showed the presence of rapid action potentials in four species of placozoans (Trichoplax adhaerens [H1 haplotype], Trichoplax sp.[H2], Hoilungia hongkongensis [H13], and Hoilungia sp. [H4]). These action potentials are sodium-dependent and can be inducible. The molecular analysis suggests the presence of 5–7 different types of voltage-gated sodium channels, which showed substantial evolutionary radiation compared to many other metazoans. Such unexpected diversity of sodium channels in early-branched metazoan lineages reflect both duplication events and parallel evolution of unique behavioral integration in these nerveless animals.
Keywords: Placozoa, Trichoplax, Hoilungia, Evolution, Basal metazoa, Voltage-gated sodium channels, Calcium channels, Action potentials
1. Introduction
The origin and early evolution of electrogenic mechanisms for behavioral integration in animals are elusive [1–3]. A diverse set of voltage-gated ion channels and all-or-none action potentials have been described in ctenophores - descendants of the earliest-branching metazoans, which independently evolved neuromuscular systems [4,5]. On the other hand, Porifera might exemplify secondary simplification of features, and many sponges lost some ancestral traits such as sodium voltage-gated channels [6,7]. However, slow (likely calcium) action potentials can be recorded from glass sponges [8,9].
Thus, representatives of Placozoa (the third branch on the animal tree of life) can be critical reference models to decipher the development of integrative mechanisms in Metazoa [10–14]. Although placozoans might contain more than one hundred marine species [15], most information about the group is based on the study of Trichoplax adhaerens - the founding member of the phylum. These 0.5–2 mm disc-shaped benthic grazers are the simplest free-living animals [16], with only six morphologically recognized cell types [17].
Despite stunning morphological simplicity, placozoans have a complex behavioral repertoire [18–23], including social behaviors [24] and ultrafast contractions [25]. But the cellular bases of behavior in T. adhaerens and related species are unknown. We hypothesize that placozoans have developed all-or-none action potentials to support the rapid propagation and integration of electrical and chemical signals across cell layers. A specialized meshwork of fiber cells, located in the middle layer of cells, was considered to be an analog of the neural and muscular systems [16], but no synapses and no gap junctions have been described morphologically [16,17]. The innexins and connexins – the canonical gap junction proteins are not encoded in the sequenced genome of T.adhaerens and its kin [14,26,27]. Adherent junctions do facilitate diffusion of potential nutrients into the animals [28], but it is unknown if they participate in the propagation of any electrical signals.
Ultrasmall sizes of most of the placozoan cells (3–5 μm), their extremely fragile nature, significantly limit the application of patch-clamp protocols for direct electrophysiological studies. Furthermore, a very high level of autofluorescence also restricts the usage of voltage-sensitive dyes for these animals. Both voltage-gated T-type calcium [29] and leak sodium [30] ion channels have been recently cloned and expressed in heterologous systems. These studies provided the first insights into the placozoan electrophysiology. Also, two types of sodium voltage-gated ion channels have been indentified in Trichoplax [6,31,32], and they might belong to the Nav2-like family of the channels with possible Ca2+-selectivity [32–35]. However, no successful recordings were performed from intact animals, and it is unclear whether regenerative and/or Na+-dependent action potentials exist in placozoans.
Here, we performed electrophysiological tests on representatives of two Placozoa genera (Trichoplax and Hoilungia) and provided the evidence for the presence of rapid Na+-dependent action potentials. We also revealed a surprising diversity of sodium voltage-gated channels in all four investigated species as well as candidates for other diverse families of cationic channels. Our data suggest parallel evolution and prominent diversification of mechanisms controlling excitability in these nerveless animals.
2. Materials and methods
2.1. Animals
Three different species, Trichoplax adhaerens (haplotype H1), Trichoplax sp.(H2), and Hoilungia hongkongensis(H13) [26], were maintained in the laboratory culture [55]; animals were fed on rice grains and algae as described elsewhere [23]. Hoilungia sp. (H4) were maintained in 10–40 L marine aquaria and fed on three species of algae (Tetraselmis marina, Spirulina versicolor, Leptolyngbya ectocarpi) [36,37].
2.2. Electrophysiological experiments
In all tests, animals were placed in 30 mm Petri dishes, and experiments were conducted under differential contrast microscopy (DIC, Olympus BX51WI microscope, n = 49 specimens). Extracellular recordings were performed using glass microelectrodes. The microelectrodes were made from borosilicate glass capillary (BF150–86-10) on a Sutter p-1000 puller (Sutter,USA) and filled with artificial seawater or equimolar sodium-free solution. The extracellular signals were filtered from 300 to 10000 Hz, amplified (x50) with MultiClamp 700B amplifier (Molecular Devices, USA), digitized at 20 kHz using Digidata 1500 and recorded using PCLAMP software (Molecular Devices,USA).
To reduce movements, placozoans were immobilized in 1% agarose made either artificial seawater (ASW: NaCl-450mM, KCl-13.4mM, MgCl2-24mM, CaCl2-9.5mM, MgSO4–5mM; pH = 8.0) or sodium-free seawater, where NaCl was replaced by N-methyl-d-glucamine (NMDG, 390 mM, pH = 8.0) [38]. Initially, 1% agarose and seawater were heated to 40 °C, and then we placed a small (<0.1–0.2 mL) drop of the agarose solution in a dish at room temperature. Using a pipette with 10 mm of ASW, we transferred (<10sec) a specimen into the same dish, placed on ice, which prevented damage to the animals. Thus, we embedded a placozoan in the middle of the agar drop, and a small agar block with the animal was placed into a recording chamber filled with ASW or a given extracellular solution. The small sizes of agar block hold the animals, allowed to perform microelectrode recordings and change solutions as needed. Placozoans were monitored under DIC microscopy and were well-maintained for several hours for long-term observations and physiological tests. Statistical analyses of all electrophysiological and pharmacological tests were performed using paired Students’ t-tests.
2.3. Comparative bioinformatic analyses
We used the data from 18 genomes of basal metazoans and two choanoflagellates (Supplement 1) for the presence of sodium and calcium channels. All accession numbers, gene IDs, and sequences are listed in Supplement 2. The search for possible Nav and Cav homologs and computational annotation of predicted gene functions was performed using sequence similarity methods (BLAST) algorithm and protein domain detection(Pfam and SMART [39,40]). Human Nav and Cav protein sequences were used as queries to search target proteomes. All hits with the score at least 100 were put as queries in BLAST search against the SwissProt database to infer their family assignment and completeness.
Protein sequences were aligned in Mafft [41] with default settings. Phylogenetic trees were inferred using the Maximum Likelihood algorithm implemented in IQTREE web server [42]. Tree robustness was tested with 1000 replicates of ultrafast bootstrap.
3. Results
3.1. Induced action potentials in placozoans
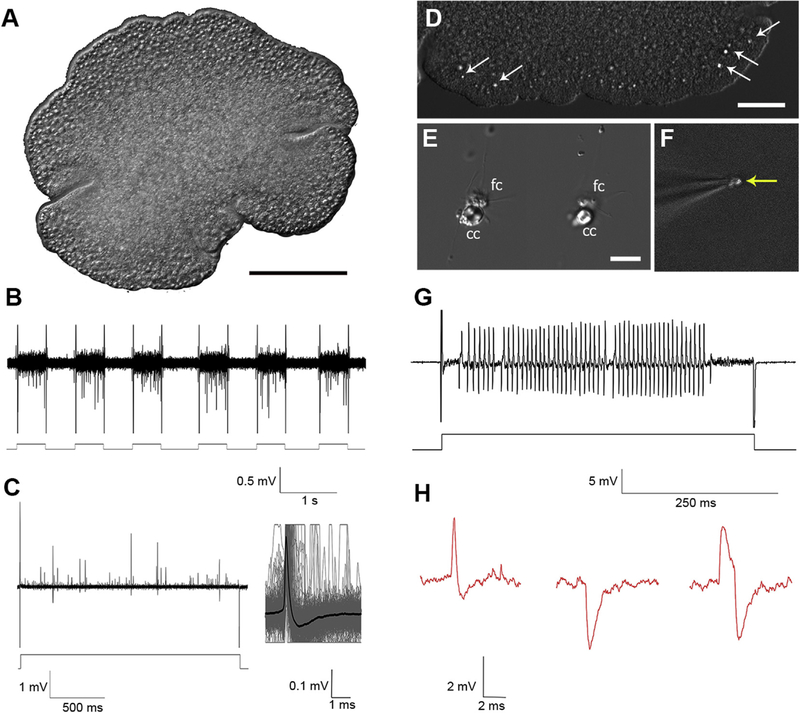
Fig. 1 shows one of the tested placozoan species (Hoilungia), which are flat animals (20 μm in‘dorso-ventral’ orientation). For electrical recordings, we used extracellular glass microelectrodes with the initial resistance 1–2MΩ. Applying negative pressure inside the pipette on contact with placozoan cells increased the resistance of microelectrodes to 6–18MΩ. In general, no spontaneous action potentials were recorded. However, electrical stimulation (10–150 nA pulses, 0.5–2sec) induced a burst of electrical signals (Fig. 1B,C,H), and these types of responses were observed in each of the four tested species of Placozoa (Fig.1S Supplement). The averaging of 50–1000 electrical responses revealed a class of regenerative electrical signals, which we recognized as action potentials. They were similar to action potentials observed in other basal metazoans and bilaterian animals (including mammals), where recording is performed either from nerves or around neuronal somata [43]. It is also possible to see different shapes of these action potentials (Fig. 1C,H,2B), which likely reflect different positions of recording electrodes. Of note, the duration of these action potentials is short (compared to the majority of invertebrates [43]), and the averaging of the signals was ~3 ms, which is comparable to some fast-spiking activity (also shorter action potentials, 1–2 ms, were recorded).
Fig. 1. Electrical activity in Placozoa.
A) A general view of Hoilungia hongkongensis (the focus is on the upper side of this disc-shaped animal). B, C) Extracellular recording from an intact animal. Depolarization by 50 nA current repetitively induced a burst of action potentials. C) Illustrative examples of different electrical responses following a single depolarization pulse; an insert shows the shape of a normalized signal, which was obtained by averaging of several dozen action potentials within a given response to a depolarization pulse (lower trace). D-G) Patch recording from a single crystal cell. D) A view of the rim area of Trichoplax sp.(H2), arrows show the position of crystal cells (gravity receptors [44]), which can easily be observed under DIC illumination. E) Isolated crystal cells (cc); these cells can be isolated together with fiber cells (fc). F) The image shows the electrode with a single crystal cell (note a visible aragonite crystal - arrow). G) Electrical recording from a single crystal cell (current pulse: 20 nA). H) Different shapes of spikes recorded from Hoilungia. Scale: A-100μm; D–50μm; E–10 μm.
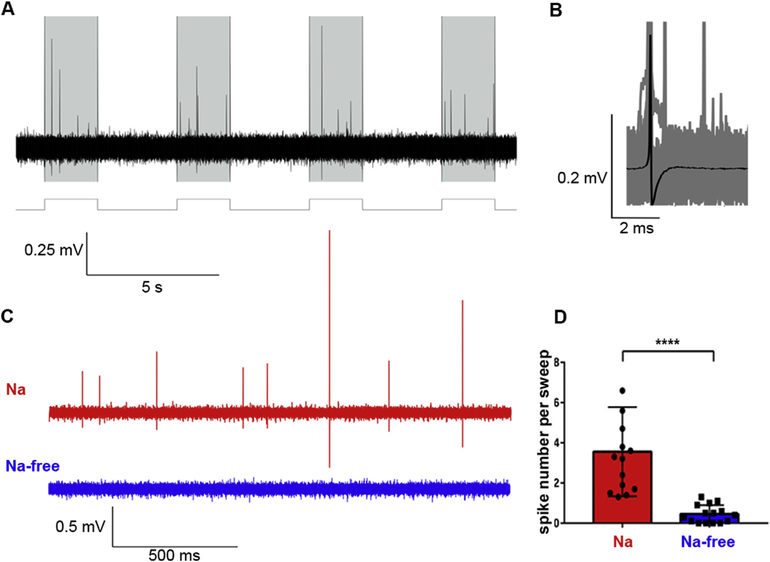
Fig. 2.
A) Action potentials in response to 150 nA current pulses (shaded area, 2s). B) The averaged action potential (AP) from all recorded action potentials (n = 911). C) An illustrated example of a typical response to a single current pulse in the artificial seawater [ASW] (with Na+, red trace) and in the Na+-free solution (NaCl was replaced by N-methyl-D-glucamine (NMDG), blue trace). D) A bar plot shows average numbers of APs to single current pulse in the presence of Na+ [red, ASW] and in the Na+-free solution (blue); Students’ paired t-test, ****p < 0.0001, n = 14. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The specialized crystal cells were recently discovered in both Trichoplax [17] and Hoilungia [26,36]; they were identified as the gravity sensors [44]. These cells are easily recognized in intact animals under differential contrast illumination due to the presence of a large aragonite crystal in each cell (Fig. 1D). These cells are also easy to isolate (Fig. 1E), and they are relatively large for single-cell recording (Fig. 1F). Fig. 1G shows a prominent burst of fast action potentials induced in the crystal cells.
3.2. Sodium-dependence of spikes in placozoans
Short durations of the recorded electrical signals in placozoans suggest that these action potentials reflect the activity of sodium-type ion channels. To test this possibility, we replaced the standard artificial seawater with sodium-free solution, were NaCl was substituted by N-methyl-d-glucamine (NMDG) to preserve the osmolarity. As in control tests – animals were immobilized in small blocks of 1% agarose, which limited movements of animals but allowed changing of solutions. The induced electrical activity was reversibly eliminated in Na-free NMDG solution, suggesting sodium-dependence of observed action potentials (Fig. 2). Of note, direct placement of free-moving placozoans in the NMDG solution induced dissociation of animals into cells within 30–60 min. Thus, as a control test, we placed animals in the 1% agar block made of Na-free solution, where the animals preserved their shape; then, we were able to use an extracellular electrode filled with different solutions to test the presence/absence of Na-dependent action potentials. Applying a small positive pressure during the recording, we carried out microapplication of the solution inside the electrode directly to the recording site. Here, we showed that when the electrode was filled with Na-free solution, no action potentials were induced by current pulses, but the spikes were restored when the electrode filled with NaCl-containing artificial seawater was used (Fig. 2C and D; n = 14).
3.3. The diversity and structure of sodium channels in placozoa
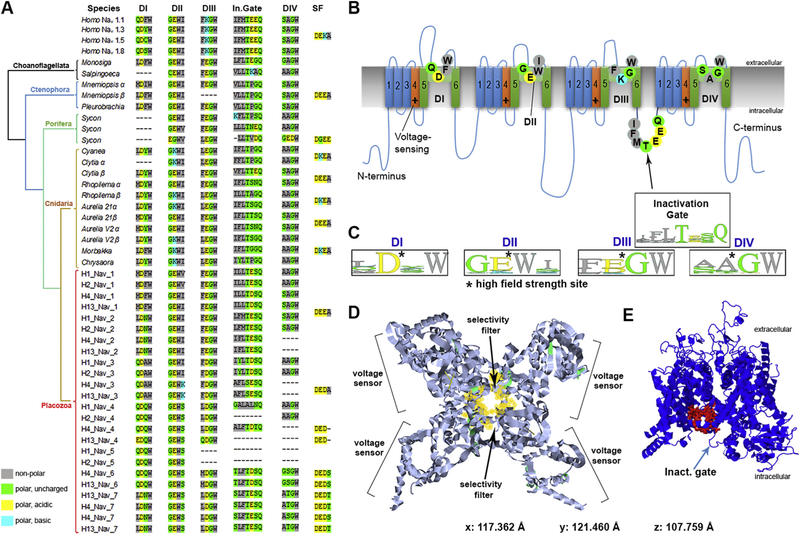
Na+-dependent action potentials in placozoans could be mediated by two classes of voltage-gated cation channels (Cav3 and Nav, Fig. 3B) with a shared topology containing 24 transmembrane (TM) helices, which are four repeats of 6TM domains [31,32,35,45,46] evolved from prokaryotic ancestors [32,47]. One sodium-conducting Cav3 and two Nav channels in Trichoplax have been reported [6,31,32,46]. Here, we identified five Nav in both Trichoplax species (H1, H2 haplotypes) and seven channels in each species of the genus Hoilungia (Fig. 3A and 4). It appears that in the lineage leading to Hoilungia, there was one additional duplication event generating novel Nav orthologs (Fig. 4). Thus, the discovered diversity of Nav-type channels in Placozoa is significantly higher than it was anticipated and observed in other basal metazoans, including ctenophores, sponges, and most of the bilaterians, except vertebrates.
Fig. 3. Voltage-gated sodium channels(Nav) in Placozoa revealed a remarkable diversity of ion selectivity pore motifs.
All metazoan’s Nav have the same 4-domain organization, acquired from ancestral eukaryotes [6,31,33,35,45,51]. Here, choanoflagellates are used as the outgroup (Fig. 4). A) The table shows critical amino acids of Nav domains (D1-DIV), which contribute to pore selectivity motifs (marked as SF–Selectivity Filter) [32]) and inactivation gates (In.Gate). Top four entries represent human Nav1; these channels contain a critical lysine (K) in the pore region, which is responsible for sodium selectivity [6,31,32]. B) Schematic organization of a generalized sodium channel. Each of the four Nav domain (I-IV) contains six transmembrane loops (1–6), the pore region with four key amino-acids (indicated by circles) responsible for ion selectivity (table A). The voltage sensor parts are indicated as +. A region responsible for inactivation is located between domains DIII and DIV. Selectivity pore motifs are from Homo Nav1 subtypes [6,31,32]. C) WebLogo representation [52] of four critical amino acids forming ion selectivity filters (SF), as calculated from table A for each of the Nav domains (DI–DIV). D-E) The reconstruction of a hypothetical 3D-structure of the Nav-1-like channel from Trichoplax adhaerens (TriadITZ_003340). D) Top Nav view is based on pdb ID: 6A90 modeling [53]). E) Side view of the same Nav-1-like Trichoplax channel generated using Phyre2 modeling [54]. The structural model is close to the human Nav1.4 type, where a ‘detection’ pocket is marked by red, and located close to the inactivation gate (arrow). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4. The diversity and evolutionary relationships of voltage-gated sodium and calcium channels in Metazoa and two species of choanoflagellates.
(Monosiga and Salpingoeca) as outgroups. 207 protein sequences were aligned with Mafft [41]. The phylogenetic tree was reconstructed using Iqtree [42] with the VT + G4 evolutionary model. Abbreviations: Nav–voltage-gated sodium channels, Cav-voltage-gated calcium channels, NALCN–sodium leak channels. Clade labeled “Putative ion channels” correspond to the group of sequences with no experimentally studied homologs, which mostly present in early-branching metazoan lineages. Placozoan species are denoted by mitochondrial haplotypes: H1–Trichoplax adhaerens, H2–Trichoplax sp., H13–Hoilungia hongkongensis, H4–Hoilungia sp. Selective groups of ctenophores, cnidaria, and sponges are collapsed here, but Fig.1S (supplement) shows the expanded version of this tree. The references for each particular species, gene and/or their sequences with relevant GeneBank accession numbers are summarized in the supplementary excel Table S1.
The pore motifs controlling calcium (EEEE/EEDD) or sodium (DEKA/DKEA) ion selectivity is highly conservative over 800 million years of evolution and can be predicted based on the presence of negatively charged amino acids such as aspartate (D) and glutamate (E) and a positively charged lysine(K) [6,31–33,48,49]. Fig. 3A illustrates a much greater diversity of the pore/ion selectivity filter motifs discovered in placozoans compared to other studied groups, including cnidarians and many bilaterians (Supplement 3–6).
Of note, some pore motifs contain polar uncharged amino acids - threonine (T) and serine (S), suggesting distinct characteristics of voltage-gated channels in Placozoa. Specifically, in both Trichoplax and Hoilungia, we identified novel combinations for selective filters responsible for Na+ pore permeability (Fig. 3A): DEDA, DEET, DEDT, DEDS, and already known from other species DEEA, characteristic for Nav2-type of the channels with less specific cation selectivity [32].
4. Discussion
The observed diversity of Nav channels and their motifs in Placozoa also implies differences in their functions, cellular and subcellular localization, and pore selectivity – all to be tested experimentally in future studies. The presence of distinct pore motifs suggests that both Na+ and Ca2+ permeability occurred in Nav-like and Cav3-like channels [29,32,46]. The involvement of Cav3-like channels in Na-dependent action potentials is highly likely, but their kinetics can be slower [29].
Some medusozoans (Cnidaria) and bilaterians (including humans) independently developed Nav1-type channels with very fast activation kinetics and high selectivity for Na+ vs. Ca2+ [6,31,32,35,45,50]. This metazoan-specific innovation is associated with the presence of a single positively-charged lysine (K) in the selective filter region, leading to DEKA and DKEA motifs for humans and jellyfishes, respectively [31,32,35,45,47]. In two species of Hoilungia (H4 and H13 haplotypes), we also identified lysine (K) but in the 4th vs. the 2nd positions (as in jellyfishes) of the second domain (DII, Fig. 3A), suggesting faster kinetics for placozoans’ Nav.
We detect genes encoding Nav-type channels in ctenophores (which is not surprising due to their predatory lifestyle and complex behaviors) and in the sessile calcareous sponge Sycon; Fig. 4 shows that additional/putative voltage-gated cationic channels can be discovered in basal metazoans (although we do not exclude the presence of possible pseudogenes). In contrast, the desmosponges likely lost voltage-gated soldium channels.
The systemic functions of ion channels in placozoans are unknown. The detection of the action potential in the crystal cells is an important indication of the role of fast signaling in the control of the geotaxis and spatial orientation. However, systematic analysis of cellular and subcellular expression of Nav as well as pharmacological and imaging studies would be essential to characterize cellular and biophysical bases of rapid (Na-dependent) responses in these animals. The diversity of predicted Nav channels might also reflect the resistance of placozoans against potential toxins.
In summary, the revealed adaptive radiation of Nav in Placozoa and the appearance of additional channel orthologs in Hoilungia (compared to Trichoplax) emphasize the importance of Placozoa for deciphering the evolution of integrative functions in animals and discoveries of novel properties of ion channels in these cryptic animals.
Supplementary Material
Acknowledgments
This work was supported, in part, by the Human Frontiers Science Program (RGP0060/2017) and National Science Foundation (1146575,1557923,1548121 and 1645219) grants to L.L.M.; and Russian Ministry of Science and High Education (2020–1902-01–312) grant to P.M.B.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2020.08.020.
References
- [1].Anderson PA, Greenberg RM, Phylogeny of ion channels: clues to structure and function, Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129 (2001) 17–28, 10.1016/s1096-4959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- [2].Cai X, Ancient origin of four-domain voltage-gated Na+ channels predates the divergence of animals and fungi, J. Membr. Biol. 245 (2012) 117–123, 10.1007/s00232-012-9415-9. [DOI] [PubMed] [Google Scholar]
- [3].Castelfranco AM, Hartline DK, Evolution of rapid nerve conduction, Brain Res. 1641 (2016) 11–33, 10.1016/j.brainres.2016.02.015. [DOI] [PubMed] [Google Scholar]
- [4].Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, Grigorenko AP, Dailey C, Berezikov E, Buckley KM, Ptitsyn A, Reshetov D, Mukherjee K, Moroz TP, Bobkova Y, Yu F, Kapitonov VV, Jurka J, Bobkov YV, Swore JJ, Girardo DO, Fodor A, Gusev F, Sanford R, Bruders R, Kittler E, Mills CE, Rast JP, Derelle R, Solovyev VV, Kondrashov FA, Swalla BJ, Sweedler JV, Rogaev EI, Halanych KM, Kohn AB, The ctenophore genome and the evolutionary origins of neural systems, Nature 510 (2014) 109–114, 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moroz LL, Kohn AB, Independent origins of neurons and synapses: insights from ctenophores, Philos. Trans. R. Soc. Lond. B Biol. Sci. 371 (2016) 20150041, 10.1098/rstb.2015.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liebeskind BJ, Hillis DM, Zakon HH, Evolution of sodium channels predates the origin of nervous systems in animals, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 9154–9159, 10.1073/pnas.1106363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS, The Amphimedon queenslandica genome and the evolution of animal complexity, Nature 466 (2010) 720–726, 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leys SP, Mackie GO, Meech RW, Impulse conduction in a sponge, J. Exp. Biol. 202 (Pt 9) (1999) 1139–1150. [DOI] [PubMed] [Google Scholar]
- [9].Leys SP, Mackie GO, Electrical recording from a glass sponge, Nature 387 (1997) 29–30, 10.1038/387029b0. [DOI] [Google Scholar]
- [10].Monk T, Paulin MG, Predation and the origin of neurones, Brain Behav. Evol. 84 (2014) 246–261, 10.1159/000368177. [DOI] [PubMed] [Google Scholar]
- [11].Telford MJ, Moroz LL, Halanych KM, Evolution: a sisterly dispute, Nature 529 (2016) 286–287, 10.1038/529286a. [DOI] [PubMed] [Google Scholar]
- [12].Moroz LL, NeuroSystematics and periodic system of neurons: model vs reference species at single-cell resolution, ACS Chem. Neurosci. 9 (2018) 1884–1903, 10.1021/acschemneuro.8b00100. [DOI] [PubMed] [Google Scholar]
- [13].Varoqueaux F, Fasshauer D, Getting nervous: an evolutionary overhaul for communication, Annu. Rev. Genet. 51 (2017) 455–476, 10.1146/annurev-genet-120116-024648. [DOI] [PubMed] [Google Scholar]
- [14].Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS, The Trichoplax genome and the nature of placozoans, Nature 454 (2008) 955–960, 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- [15].Schierwater B, DeSalle R, Placozoa, Curr Biol 28 (2018) R97–R98, 10.1016/j.cub.2017.11.042. [DOI] [PubMed] [Google Scholar]
- [16].Grell KG, Ruthmann A, Placozoa, in: Harrison FW (Ed.), Microscopic Anatomy of Invertebrates, Wiley-Liss, New York, 1991, pp. 13–27. [Google Scholar]
- [17].Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, Eitel M, Fasshauer D, Reese TS, Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens, Curr. Biol. 24 (2014) 1565–1572, 10.1016/j.cub.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Senatore A, Reese TS, Smith CL, Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses, J. Exp. Biol. 220 (2017) 3381–3390, 10.1242/jeb.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith CL, Pivovarova N, Reese TS, Coordinated feeding behavior in Trichoplax, an animal without synapses, PloS One 10 (2015), e0136098, 10.1371/journal.pone.0136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ueda T, Koya S, Maruyama YK, Dynamic patterns in the locomotion and feeding behaviors by the placozoan Trichoplax adhaerence, Biosystems 54 (1999) 65–70. [DOI] [PubMed] [Google Scholar]
- [21].Smith CL, Reese TS, Govezensky T, Barrio RA, Coherent directed movement toward food modeled in Trichoplax, a ciliated animal lacking a nervous system, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 8901–8908, 10.1073/pnas.1815655116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Romanova DY, Heyland A, Sohn D, Kohn AB, Fasshauer D, Varoqueaux F, Moroz LL, Glycine as a signaling molecule and chemoattractant in Trichoplax (Placozoa): insights into the early evolution of neurotransmitters, Neuro-report 31 (2020) 490–497, 10.1097/WNR.0000000000001436. [DOI] [PubMed] [Google Scholar]
- [23].Heyland A, Croll R, Goodall S, Kranyak J, Wyeth R, Trichoplax adhaerens, an enigmatic basal metazoan with potential, Methods Mol. Biol. 1128 (2014) 45–61, 10.1007/978-1-62703-974-1_4. [DOI] [PubMed] [Google Scholar]
- [24].Fortunato A, Aktipis A, Social feeding behavior of Trichoplax adhaerens, Front Ecol Evol 7 (2019), 10.3389/fevo.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Armon S, Bull MS, Aranda-Diaz A, Prakash M, Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) E10333–E10341, 10.1073/pnas.1802934115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eitel M, Francis WR, Varoqueaux F, Daraspe J, Osigus HJ, Krebs S, Vargas S, Blum H, Williams GA, Schierwater B, Worheide G, Comparative genomics and the nature of placozoan species, PLoS Biol. 16 (2018), e2005359, 10.1371/journal.pbio.2005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Osigus HJ, Rolfes S, Herzog R, Kamm K, Schierwater B, Polyplacotoma mediterranea is a new ramified placozoan species, Curr. Biol. 29 (2019) R148–R149, 10.1016/j.cub.2019.01.068. [DOI] [PubMed] [Google Scholar]
- [28].Smith CL, Reese TS, Adherens junctions modulate diffusion between epithelial cells in Trichoplax adhaerens, Biol. Bull. 231 (2016) 216–224, 10.1086/691069. [DOI] [PubMed] [Google Scholar]
- [29].Smith CL, Abdallah S, Wong YY, Le P, Harracksingh AN, Artinian L, Tamvacakis AN, Rehder V, Reese TS, Senatore A, Evolutionary insights into T-type Ca(2+) channel structure, function, and ion selectivity from the Trichoplax adhaerens homologue, J. Gen. Physiol. 149 (2017) 483–510, 10.1085/jgp.201611683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elkhatib W, Smith CL, Senatore A, A Na(+) leak channel cloned from Trichoplax adhaerens extends extracellular pH and Ca(2+) sensing for the DEG/ENaC family close to the base of Metazoa, J. Biol. Chem. 294 (2019) 16320–16336, 10.1074/jbc.RA119.010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zakon HH, Adaptive evolution of voltage-gated sodium channels: the first 800 million years, Proc. Natl. Acad. Sci. U. S. A. 109 (Suppl 1) (2012) 10619–10625, 10.1073/pnas.1201884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fux JE, Mehta A, Moffat J, Spafford JD, Eukaryotic voltage-gated sodium channels: on their origins, asymmetries, losses, diversification and adaptations, Front. Physiol. 9 (2018) 1406, 10.3389/fphys.2018.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moran Y, Barzilai MG, Liebeskind BJ, Zakon HH, Evolution of voltage-gated ion channels at the emergence of Metazoa, J. Exp. Biol. 218 (2015) 515–525, 10.1242/jeb.110270. [DOI] [PubMed] [Google Scholar]
- [34].Ghezzi A, Liebeskind BJ, Thompson A, Atkinson NS, Zakon HH, Ancient association between cation leak channels and Mid1 proteins is conserved in fungi and animals, Front. Mol. Neurosci. 7 (2014) 15, 10.3389/fnmol.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liebeskind BJ, Hillis DM, Zakon HH, Independent acquisition of sodium selectivity in bacterial and animal sodium channels, Curr. Biol. 23 (2013) R948–R949, 10.1016/j.cub.2013.09.025. [DOI] [PubMed] [Google Scholar]
- [36].Romanova DY, Cell types diversity of H4 haplotype Placozoa sp, Marine Biological Journal 4 (2019) 81–90, 10.21072/mbj.2019.04.1.07. [DOI] [Google Scholar]
- [37].Moroz LL, Sohn D, Romanova DY, Kohn AB, Microchemical identification of enantiomers in early-branching animals: lineage-specific diversification in the usage of D-glutamate and D-aspartate, Biochem. Biophys. Res. Commun. 527 (2020) 947–952, 10.1016/j.bbrc.2020.04.135. [DOI] [PubMed] [Google Scholar]
- [38].Thuma JB, Hooper SL, Choline and NMDG directly reduce outward currents: reduced outward current when these substances replace Na(+) is alone not evidence of Na(+)-activated K(+) currents, J. Neurophysiol. 120 (2018) 3217–3233, 10.1152/jn.00871.2017. [DOI] [PubMed] [Google Scholar]
- [39].Giles TC, Emes RD, Inferring function from homology, Methods Mol. Biol. 1526 (2017) 23–40, 10.1007/978-1-4939-6613-4_2. [DOI] [PubMed] [Google Scholar]
- [40].Letunic I, Bork P, 20 years of the SMART protein domain annotation resource, Nucleic Acids Res. 46 (2018) D493–D496, 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Katoh K, Standley DM, MAFFT multiple sequence alignment software version 7: improvements in performance and usability, Mol. Biol. Evol. 30 (2013) 772–780, 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ, W-Iq-Tree, A fast online phylogenetic tool for maximum likelihood analysis, Nucleic Acids Res. 44 (2016) W232–W235, 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bullock TH, Horridge GA, Structure and Function in the Nervous Systems of Invertebrates, Freeman, San Francisco, 1965. [Google Scholar]
- [44].Mayorova TD, Smith CL, Hammar K, Winters CA, Pivovarova NB, Aronova MA, Leapman RD, Reese TS, Cells containing aragonite crystals mediate responses to gravity in Trichoplax adhaerens (Placozoa), an animal lacking neurons and synapses, PloS One 13 (2018), e0190905, 10.1371/journal.pone.0190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liebeskind BJ, Hillis DM, Zakon HH, Convergence of ion channel genome content in early animal evolution, Proc. Natl. Acad. Sci. U. S. A. 112 (2015) E846–E851, 10.1073/pnas.1501195112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Senatore A, Raiss H, Le P, Physiology and evolution of voltage-gated calcium channels in early diverging animal phyla: Cnidaria, placozoa, Porifera and ctenophora, Front. Physiol. 7 (2016) 481, 10.3389/fphys.2016.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nishino A, Okamura Y, Evolutionary history of voltage-gated sodium channels, Handb. Exp. Pharmacol. 246 (2018) 3–32, 10.1007/164_2017_70. [DOI] [PubMed] [Google Scholar]
- [48].Zakon HH, Jost MC, Lu Y, Expansion of voltage-dependent Na+ channel gene family in early tetrapods coincided with the emergence of terrestriality and increased brain complexity, Mol. Biol. Evol. 28 (2011) 1415–1424, 10.1093/molbev/msq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pozdnyakov I, Matantseva O, Skarlato S, Diversity and evolution of four-domain voltage-gated cation channels of eukaryotes and their ancestral functional determinants, Sci. Rep. 8 (2018) 3539, 10.1038/s41598-018-21897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu FH, Catterall WA, Overview of the voltage-gated sodium channel family, Genome Biol. 4 (2003) 207, 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liebeskind BJ, Hillis DM, Zakon HH, Phylogeny unites animal sodium leak channels with fungal calcium channels in an ancient, voltage-insensitive clade, Mol. Biol. Evol. 29 (2012) 3613–3616, 10.1093/mol-bev/mss182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Crooks GE, Hon G, Chandonia JM, Brenner SE, WebLogo: a sequence logo generator, Genome Res. 14 (2004) 1188–1190, 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Berman H, Henrick K, Nakamura H, Announcing the worldwide protein data bank, Nat. Struct. Biol. 10 (2003) 980, 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- [54].Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ, The Phyre2 web portal for protein modeling, prediction and analysis, Nat. Protoc. 10 (2015) 845–858, 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moroz LL, Romanova DY, Nikitin MA, Sohn D, Kohn AB, Neveu E, Varoqueaux F, Fasshauer D, The diversification and lineage-specific expansion of nitric oxide signaling in Placozoa: insights in the evolution of gaseous transmission, Sci. Rep. 10 (1) (2020) 13020, 10.1038/s41598-020-69851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.