Abstract
Rabies is transmitted to humans mainly by dogs but also by other animal species. Reliable data on the incidence of Rabies virus (RABV) in humans, dogs, and other animal species in Africa, could be essential in the implementation of a global strategic plan to eliminate the RABV by 2030 as adopted by the WHO, OIE, and FAO. We searched the Pubmed, Embase, Scopus, African Journal Online, and African Index Medicus databases for relevant studies that report data on the incidence of RABV in Africa up to February 17, 2020. Information on active and past RABV exposures in various categories of dogs, humans and other animal species were extracted. Incidence and seroprevalence estimates were pooled using a random-effect meta-analysis. We included 73 articles which provided 142 RABV incidence and seroprevalence records in 21 African countries. The estimated incidence of RABV in 222 humans, 15,600 dogs, and 12,865 other animal species was 83.4% (95% CI = 64.6–96.5), 44.1% (95% CI = 35.1–53.4), and 41.4% (95% CI = 29.6–53.8), respectively. The estimated seroprevalence of RABV in 420 humans, 3577 dogs, and 8,55 other animal species was 33.8% (95% CI = 21.9–46.8), 19.8% (95% CI = 13.3–27.3), and 3.6% (95% CI = 0.3–9.2), respectively. The incidence of RABV in general was higher in suspected rabid dogs, other animal species of the Orders Perissodactyla, Artiodactyla and Carnivora. The incidence of RABV was higher for humans in regions of West and East Africa, for dogs in urban areas and in regions of Central and South Africa, and for animals of the order Perissodactyla in urban areas. This meta-analysis demonstrated a high incidence of RABV in Africa. Itis necessary to improve surveillance system to provide reliable data on RABV in Africa, essential for the implementation of an effective control strategy.
Keywords: Rabies virus, Incidence, Africa, One health
1. Introduction
Rabies infections is associated with an estimated 59,000 human deaths every year, mostly in low-income economies of Africa and Asia [1]. Most rabies human deaths occur among children less than fifteen years old, who are less aware of rabies associated risk. Rabies represents a real public health concern and it has an impact on livestock economy.
Rabies is caused by viruses of the Lyssavirus Genus (in the Family Rhabdoviridae of the Order Mononegavirales) [2]. Rabies virus (RABV) is the most common causative agent of rabies, distributed in almost all countries of the world and can infect a wide range of mammal species. Documented host species of RABV include domestic animals, but also wild-living carnivores comprising foxes and raccoon dogs in Europe [2], foxes in the Middle East, raccoon dogs and ferret-badgers in Asia, skunks [3], foxes, coyotes and mongooses in the Americas, African civet and mongooses in Africa [4]. Owned dogs are the principal hosts involved in the transmission of RABV to human in more than 99% of cases through bites [5]. But rabies can also be transmitted by scratches, mucosal licking, and in rare cases by organ transplantation [2]. After exposure, the incubation period is very variable and can range from a few days to a year or more [5]. Rabies is almost always fatal once clinical disease develops [6]. Nevertheless, rabies is preventable through post-exposure prophylaxis (PEP), which has shown a good efficacy [7]. However, this prophylaxis costs 40 United States dollars, when the average daily allowance in Africa and Asia is 1–2 dollars per person, making it unaffordable for most population at risk. The persistence of rabies in Africa is mainly fuelled by the vicious cycle of the lack of accurate epidemiological data, the negligence of the populations and decision-makers, the lack of public information and sensitization on the risk of rabies, and the geographic and financial barriers to access rabies vaccines [8]. WHO and partners launched a global plan to eliminate dog-mediated human rabies by 2030 [9]. It has been demonstrate in developed countries that multi-annual vaccination campaigns with vaccination coverage of at least 70% of the domestic dog population should be effective in order to achieve rabies control and elimination [10]. Nevertheless, implementing a nationwide vaccination campaign is a real challenge for poor country of Africa, who generally fail to achieve the required vaccination coverage. Like suggest in a recent review, the knowledge of rabies epidemiology is crucial for implementation of more targeted and effective vaccination campaigns [11]. It would be important to know the general epidemiological data on rabies in Africa in order to be able to put in place a global plan of elimination of rabies adapted to the African context. This study aims to contribute to ongoing efforts towards elimination of dog-mediated human rabies by providing an overview of the status and occurrence of rabies in Africa through a meta-analysis of rabies incidence and seroprevalence data in various categories of human, dog, and other animal species populations.
2. Methods
2.1. Design and inclusion criteria
We used the preferred reporting items for systematic reviews and meta–analyses (PRISMA) for the present study (Supplementary Table 1) [12]. We published the study protocol in Prospero under number CRD42020169706. We defined inclusion criteria according to the Joanna Briggs Institute recommendations for systematic reviews of prevalence and incidence [13]. The population consisted of humans, dogs, and other animal species. Humans were classified as apparently healthy subjects and suspected rabies cases. Dogs were further classified into owned dogs, stray dogs, wild dogs, and unclassified dogs. The other animal species were grouped into their taxonomic Orders. The condition and the context were rabies and Africa respectively. We grouped African countries according to United Nations Statistics Division (UNSD). The types of studies were cross-sectional, community and hospital outbreaks, baseline data for cohort, and case-control. Study population were classified according to the inclusion criteria of the selected studies. We considered RABV incidence data derived from all types of samples including brain, serum, saliva, cerebrospinal fluid and any organ or tissue biopsy. We considered the RABV incidence data generated with all common diagnostic assays for antibody and antigen detection including molecular, immune-enzymatic, immunochromatographic, immunofluorescent, histopathological and culture-based assays. With respect to these assays, targets used for RABV detection were either antibodies, antigens, RNA, live virus or Negri bodies. We considered Negri bodies, live virus, RNA, and antigens as targets indicating active infection by the RABV. IgM and IgG antibodies were considered to characterize recent and past RABV infections respectively. We excluded studies carried out only on RABV laboratory confirmed cases, studies for which the abstract and/or full text were not available, case reports, reviews, comments, studies with studied population size <10 participants, studies reporting experimental RABV infections and duplicates.
2.2. Article searching strategy
The Pubmed, Embase, Scopus, African Journals Online, and African Index Medicus databases were queried for relevant studies on the subject from their inception until February 17, 2020. The languages considered were English and French. The main search strategy (Supplementary Table 2) developed for Pubmed was adapted to other databases. We also reviewed the reference lists of included studies and relevant reviews for additional inclusions. We solicited colleagues with extended access to electronic bibliographic resources to search eligible articles not accessible or unavailable in open access.
2.3. Selection and data extraction
Duplicates from different databases were removed. Two investigators (SK and JTEB) independently selected the articles retrieved from the databases. All remaining articles were distributed equally among 16 authors for full text review and data extraction for those included. Each article was reviewed by at least two study authors. Disagreements on eligibility and the data extracted were resolved by discussion between two investigators and the intervention of a third author (SK) if necessary. Data collected included: the study characteristics [study design, country, UNSD region, study period, mean or median age of participants, age range of participants, proportion of male subjects, study setting (rural versus urban and hospital versus community), hospitalisation, clinical case definition of participants], risk of bias assessment data, and key data for incidence estimation [species (human, dog or other animal species), category of humans and dogs, taxonomic Order of animals, RABV detection assays, target searched for RABV detection, type of infection (active infection, recent or past exposure), type of sample tested, number of samples tested for RABV and number of positive ones].
2.4. Study definitions
Studies performed in several hospitals or cities were defined as multicentric and those in a single hospital or city were defined as monocentric. We considered the data from each subpopulation or type of infection (active, recent or past RABV infection) of the article as unique incidence data. For studies searching for multiple infection targets representing the same type of infection (active infection, recent or past RABV exposure) in the same participant, we either combined the results of the markers into a single study of the corresponding type of infection or selected the marker with high incidence. Given the near 100% fatality of RABV, we defined the incidence in a category as the rate of RABV detection among all participants of the category in the considered study.
2.5. Evaluation of the quality of studies
The quality of the studies considered was assessed using the tool of Hoy et al. (10 questions) (Supplementary Table 3) [14]. The expected answers to the questions were “yes”, “no”, “unclear” and “not applicable” depending on the content of the articles. A score of 1 was assigned for all “yes” answers and 0 for the other ones. Articles with a total score of 0–3, 4–6, and 7–10 were considered to be respectively at high, moderate, and low risk of bias.
2.6. Data synthesis
We analysed the data as outlined in a previously published study [15]. The main outcome of the study was the combined incidence according to population categories (humans, dogs or other animal species) and the types of RABV infection (active, recent, past RABV infection). We used the metaprop command of packages (metafor and meta) in R software version 3.6.2 to estimate the combined incidences according to a random model effects [16]. The secondary outcomes of the study were to identify the socio-demographic and diagnostic factors associated with the RABV incidence with respect to the infection types (active, recent, past) and well-defined population categories (apparently healthy humans or suspected rabies case, owned, stray or wild dogs, and other animal species with known taxonomic Order). Secondary outcomes were obtained through subgroup analyses. Cochran's Q test was used to assess data heterogeneity [16]. Sources of heterogeneity were investigated by univariate and multivariate metaregression analyses. The funnel plot and Egger's test were used to assess the publication bias [17]. Egger's test p-value <0.1 were considered indicative of the presence of publication bias. A sensitivity analysis that included only cross-sectional studies was performed.
3. Results
3.1. Study selection
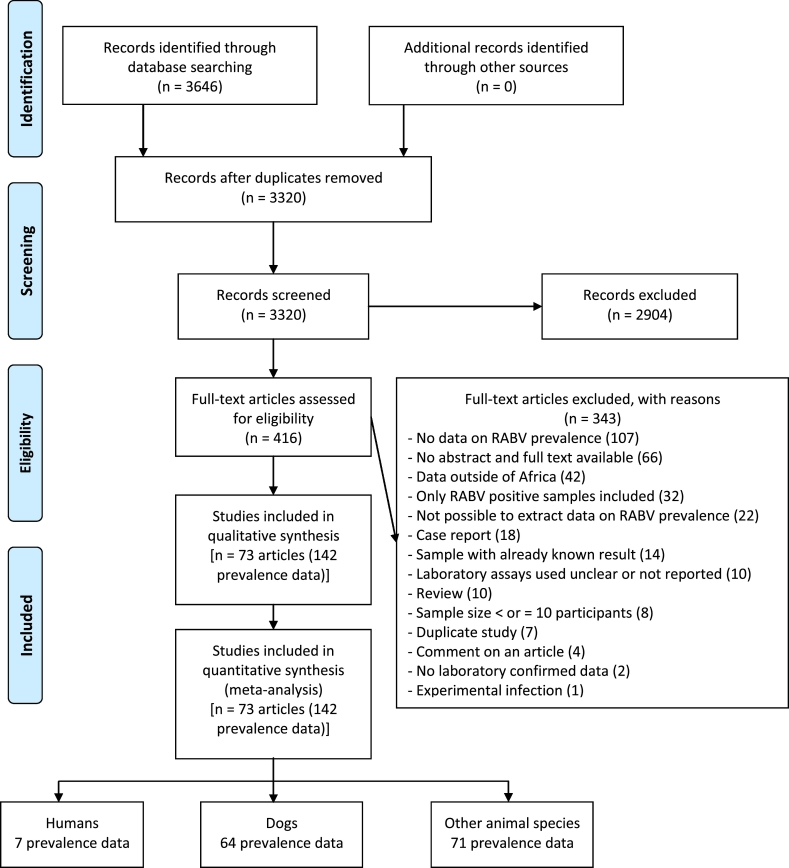
Database searches yielded a total of 3646 potentially relevant articles. A total of 326 duplicate articles and 2904 additional articles were excluded after careful review of their titles and abstracts. Examination of the full texts of the 416 remaining articles allowed to exclude 343 of them mainly due to the absence of RABV incidence data and the unavailability of their abstracts and full texts (Fig. 1, Supplementary Table 4). A group of 73 articles corresponding to 142 incidence data was finally selected for this review [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90]].
Fig. 1.
Study selection flowchart.
3.2. Quality evaluation
The quality of the studies included was assessed using the Hoy et al. tool checklist (Supplementary Table 5). The scores obtained for these studies varied from 3 to 7 with a median of 5 [4,5]. Most of the studies had a moderate risk of bias (139/142; 97.9%). Only one study had a response rate ≥ 70% and three studies were representative of the population of a country.
3.3. Baseline characteristics of included studies
The summary and individual data of the included studies are presented in Supplementary Table 6, Supplementary Table 7. The studies were published between 1966 and 2019 and the participants were recruited between 1975 and 2018. The majority of included studies were from East Africa (61/142; 43.0%) and West Africa (48/142; 33.8%). The included studies covered 21 African countries with the highest representativeness occupied by Nigeria (42/142; 29.6%) and Ethiopia (17/142; 12.0%). The direct fluorescent antibody test (82/142; 57.8%) and the brain (82/142; 57.8%) was the assay and type of sample mainly used for the detection of RABV respectively. Dogs were the most recruited animal species (64/142; 45.1%) in the included studies, but RABV were also found in humans (7/142; 4.9%) and several other animal species (71/142; 50.0%). Apart from dogs, other animal species recruited belonged to 6 Orders including Artiodactyla, Carnivora, Chiroptera, Perissodactyla, Primates, and Rodentia. Artiodactyla and Carnivora Orders were the most represented respectively; with a record of 20 (14.1%) and 19 (13.4%). Eight included studies had reported RABV pooled incidence data for several animals and did not allow their attributions to a specific taxonomic Order.
3.4. The pooled incidence of Rabies virus among humans, dogs, and other animal species in Africa
The included studies recruited 33,539 participants, including 642 humans, 19,177 dogs, and 13,720 other animal species.
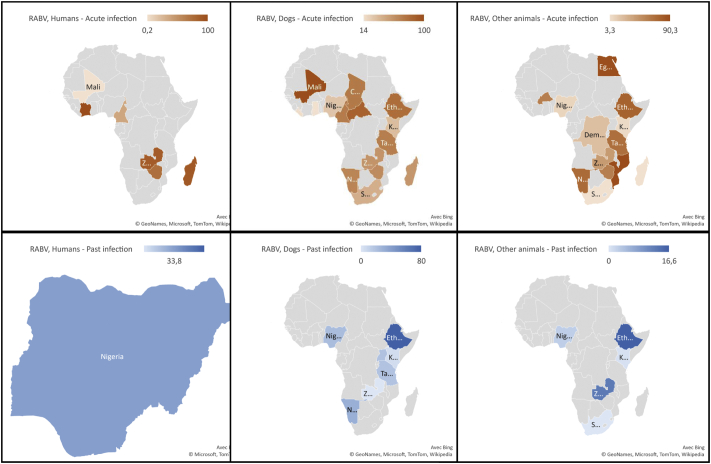
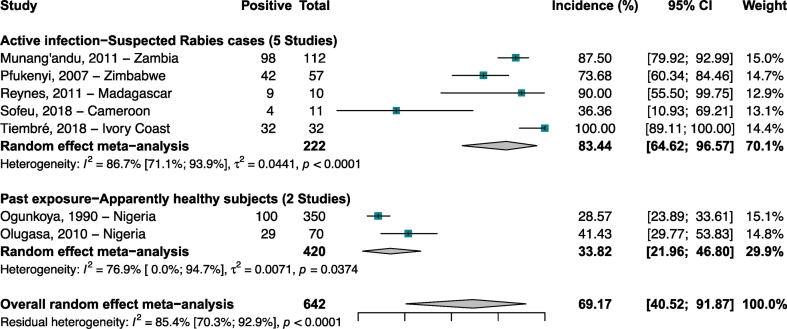
Human studies were carried out in Nigeria, Cameroon, Madagascar, Zimbabwe, Zambia, and Ivory Coast [26,29,39,52,65,67,71] (Fig. 2). The 5 included studies which enrolled humans suspected rabies cases with active infections (N = 222 participants) reported incidence ranging from 87.5 to 100% with a meta-incidence of 83.4% (CI 95%: 64.6–96.5) (Fig. 3). The seroprevalence were 33.8% (CI 95%: 21.9–46.8) in 420 apparently healthy subjects with past RABV exposure.
Fig. 2.
Rabies virus incidence in humans, dogs, and other animal species in Africa, 1966–2019.
Fig. 3.
Incidence of Rabies virus infections in humans in Africa.
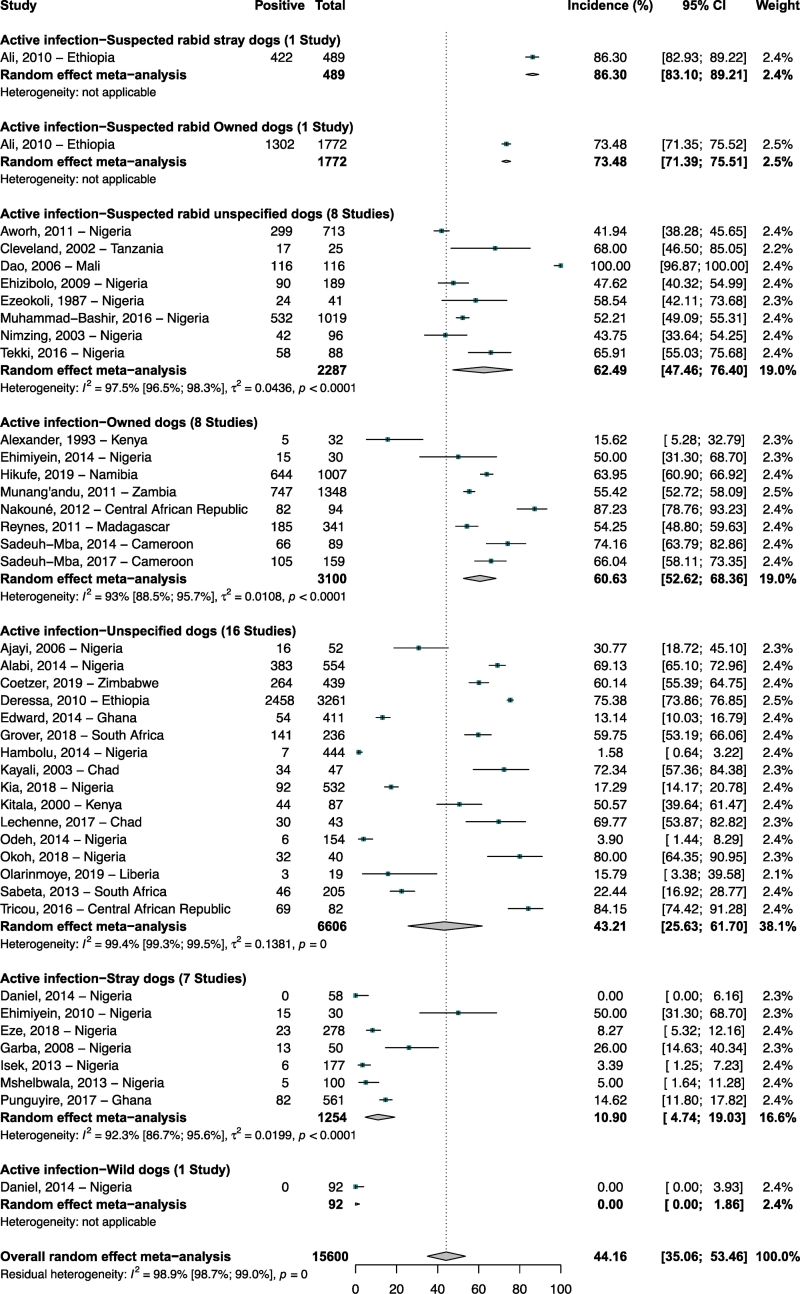
The studies in dogs were mainly carried out in Nigeria (31/64; 48.4%) (Supplementary Table 6). The 40 articles (42 incidence data) in dogs with active infection (N = 15,600 participants) reported incidence ranging from 0 to 100% with a meta-incidence of 44.1% (95% CI: 35.1–53.4) (Fig. 4) [18,[20], [21], [22],25,28,33,36,40,41,43,[45], [46], [47],[49], [50], [51], [52], [53], [54], [55],59,61,62,65,66,70,75,76,78,[80], [81], [82], [83], [84], [85], [86], [87],89,90]. RABV meta-incidence in dogs with evidence of active RABV infection ranged from 0% in wild dogs to 86.3% in suspected rabid stray dogs. The 15 articles (21 seroprevalence data) in dogs with past exposure (N = 3577 participants) reported seroprevalence ranging from 0 to 80% with a meta-seroprevalence of 19.8% (95% CI: 13.3–27.3) (Supplementary Fig. 1) [19,32,35,38,41,42,64,[68], [69], [70], [71],73,74,77,80]. RABV meta-seroprevalence in dogs with evidence of past exposure ranged from 4.9% in wild dogs to 28.4% in unspecified dogs.
Fig. 4.
Incidence of Rabies virus infections in dogs population in Africa.
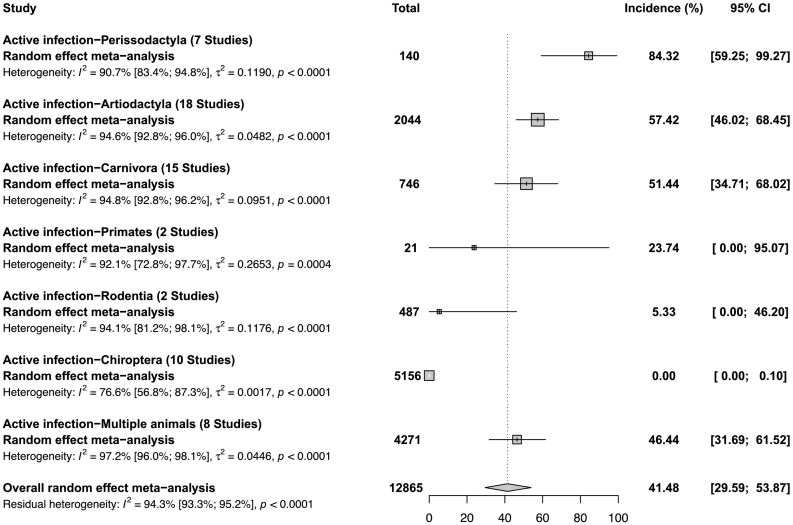
The studies in other animal species were mainly carried out in Namibia, Nigeria, and Ethiopia (Supplementary Table 6). The 24 articles (62 incidence data) in other animal species with active infection (N = 12,865 participants) reported incidence ranging from 0 to 100% with a meta-incidence of 41.4% (95% CI: 29.6–53.8) (Fig. 5, Supplementary Fig. 2) [20,21,23,24,27,30,31,34,37,40,44,46,48,52,[56], [57], [58],63,65,75,81,86,88,90]. RABV meta-incidence in other animal species with evidence of active RABV infection ranged from 0% in the Order Chiroptera to 84.3% in the Order Perissodactyla. The 9 articles (10 seroprevalence data) in other animal species with past exposure (N = 855 participants) reported incidence ranging from 0 to 40% with a meta-incidence of 3.6% (95% CI: 0.3–9.2) (Supplementary Fig. 3) [23,38,42,58,60,63,70,72,79]. RABV meta-seroprevalence in other animal species with evidence of past exposure ranged from 0.4% in the Order Chiroptera to 7.8% in the Order Carnivora.
Fig. 5.
Incidence of Rabies virus infections in other animal species in Africa.
3.5. Sensitivity, heterogeneity and publication bias analysis
All estimated incidence denoted substantial heterogeneity (H > 1, I2 > 60%, and p heterogeneity <0.05) (Table 1). The results of this sensitivity analysis showed results comparable to the overall incidence estimates. The funnel plot was asymmetrical for the estimate of the overall incidence of RABV in dogs (Supplementary Fig. 4; p Egger test <0.001), indicating the existence of publication bias. The funnel plot was symmetrical for the estimate of the overall incidence of RABV in humans (Supplementary Fig. 5; p Egger test: 0.173) and other animal species (Supplementary Fig. 6; p Egger test: 0.337), indicating the absence of publication bias.
Table 1.
Summary of meta-analysis results for incidence of Rabies virus in humans, dogs, and other animal species in Africa.
| Incidence. % (95%CI) | 95% Prediction interval | N Studies | N Participants | H (95%CI) | §I2 (95%CI) | P heterogeneity | P Egger test | |
|---|---|---|---|---|---|---|---|---|
| RABV incidence in humans | ||||||||
| Active infection | ||||||||
| Suspected Rabies cases | ||||||||
| Overall | 83.4 [64.6–96.6] | [12.4–100] | 5 | 222 | 2.7 [1.9–4] | 86.7 [71.1–93.9] | < 0.001 | 0.695 |
| Cross-sectional | 89.9 [75.4–98.9] | [14.7–100] | 4 | 211 | 2.4 [1.5–3.8] | 82.6 [55.5–93.2] | 0.001 | 0.755 |
| Past exposure | ||||||||
| Apparently healthy subjects | ||||||||
| Overall | 33.8 [22–46.8] | NA | 2 | 420 | 2.1 [1–4.4] | 76.9 [0–94.7] | 0.037 | NA |
| Cross-sectional | 33.8 [22–46.8] | NA | 2 | 420 | 2.1 [1–4.4] | 76.9 [0–94.7] | 0.037 | NA |
| RABV incidence in dogs | ||||||||
| Active infection | ||||||||
| Suspected rabid stray dogs | ||||||||
| Overall | 86.3 [83.1–89.2] | NA | 1 | 489 | NA | NA | 1 | NA |
| Cross-sectional | 86.3 [83.1–89.2] | NA | 1 | 489 | NA | NA | 1 | NA |
| Suspected rabid owned dogs | ||||||||
| Overall | 73.5 [71.4–75.5] | NA | 1 | 1772 | NA | NA | 1 | NA |
| Cross-sectional | 73.5 [71.4–75.5] | NA | 1 | 1772 | NA | NA | 1 | NA |
| Suspected rabid unspecified dogs | ||||||||
| Overall | 62.5 [47.5–76.4] | [12.3–99.2] | 8 | 2287 | 6.4 [5.3–7.6] | 97.5 [96.5–98.3] | < 0.001 | 0.299 |
| Cross-sectional | 62.5 [47.5–76.4] | [12.3–99.2] | 8 | 2287 | 6.4 [5.3–7.6] | 97.5 [96.5–98.3] | < 0.001 | 0.299 |
| Owned dogs | ||||||||
| Overall | 60.6 [52.6–68.4] | [33.3–84.8] | 8 | 3100 | 3.8 [2.9–4.8] | 93 [88.5–95.7] | < 0.001 | 0.808 |
| Cross-sectional | 57 [49.9–63.9] | [33.9–78.6] | 7 | 3006 | 3.2 [2.4–4.3] | 90.1 [82.2–94.5] | < 0.001 | 0.695 |
| Unspecified dogs | ||||||||
| Overall | 43.2 [25.6–61.7] | [0−100] | 16 | 6606 | 13.3 [12.4–14.4] | 99.4 [99.3–99.5] | < 0.001 | 0.138 |
| Cross-sectional | 43.2 [25.6–61.7] | [0–100] | 16 | 6606 | 13.3 [12.4–14.4] | 99.4 [99.3–99.5] | < 0.001 | 0.138 |
| Stray dogs | ||||||||
| Overall | 10.9 [4.7–19] | [0–45] | 7 | 1254 | 3.6 [2.7–4.7] | 92.3 [86.7–95.6] | < 0.001 | 0.98 |
| Cross-sectional | 13.7 [6.8–22.4] | [0–49.3] | 6 | 1196 | 3.5 [2.6–4.7] | 91.8 [84.9–95.5] | < 0.001 | 0.612 |
| Wild dogs | ||||||||
| Overall | 0 [0–1.9] | NA | 1 | 92 | NA | NA | 1 | NA |
| Past exposure | ||||||||
| Unspecified dogs | ||||||||
| Overall | 28.5 [17.2–41.2] | [0–87.2] | 4 | 777 | 3.8 [2.6–5.5] | 92.9 [85.2–96.6] | < 0.001 | 0.545 |
| Cross-sectional | 26.7 [11.6–45.2] | [0–100] | 3 | 499 | 4.4 [2.9–6.7] | 94.9 [88.4–97.7] | < 0.001 | 0.486 |
| Owned dogs | ||||||||
| Overall | 21.8 [11.7–33.8] | [0–70] | 10 | 2168 | 5.8 [4.9–6.8] | 97 [95.8–97.9] | < 0.001 | 0.978 |
| Cross-sectional | 22.5 [11.5–35.7] | [0–73.5] | 9 | 2088 | 6.1 [5.2–7.3] | 97.3 [96.2–98.1] | < 0.001 | 0.934 |
| Stray dogs | ||||||||
| Overall | 17.9 [2.3–42.2] | [0–100] | 4 | 535 | 5.5 [4–7.4] | 96.6 [93.9–98.2] | < 0.001 | 0.943 |
| Cross-sectional | 17.9 [2.3–42.2] | [0–100] | 4 | 535 | 5.5 [4–7.4] | 96.6 [93.9–98.2] | < 0.001 | 0.943 |
| Wild dogs | ||||||||
| Overall | 4.9 [0–27.4] | [0–100] | 3 | 97 | 2.8 [1.7–4.7] | 87.3 [64.1–95.5] | < 0.001 | 0.083 |
| Cross-sectional | 4.9 [0–27.4] | [0–100] | 3 | 97 | 2.8 [1.7–4.7] | 87.3 [64.1–95.5] | < 0.001 | 0.083 |
| RABV incidence in other animal species | ||||||||
| Active infection | ||||||||
| Perissodactyla | ||||||||
| Overall | 84.3 [59.2–99.3] | [1.6–100] | 7 | 140 | 3.3 [2.5–4.4] | 90.7 [83.4–94.8] | < 0.001 | 0.758 |
| Cross-sectional | 84.3 [59.2–99.3] | [1.6–100] | 7 | 140 | 3.3 [2.5–4.4] | 90.7 [83.4–94.8] | < 0.001 | 0.758 |
| Artiodactyla | ||||||||
| Overall | 57.4 [46–68.5] | [12.2–96.2] | 18 | 2044 | 4.3 [3.7–5] | 94.6 [92.8–96] | < 0.001 | 0.163 |
| Cross-sectional | 57.4 [46–68.5] | [12.2–96.2] | 18 | 2044 | 4.3 [3.7–5] | 94.6 [92.8–96] | < 0.001 | 0.163 |
| Carnivora | ||||||||
| Overall | 51.4 [34.7–68] | [0–100] | 15 | 746 | 4.4 [3.7–5.1] | 94.8 [92.8–96.2] | < 0.001 | 0.302 |
| Cross-sectional | 48.9 [31.8–66.2] | [0–100] | 14 | 731 | 4.5 [3.8–5.3] | 95 [93.1–96.4] | < 0.001 | 0.21 |
| Primates | ||||||||
| Overall | 23.7 [0–95.1] | NA | 2 | 21 | 3.6 [1.9–6.6] | 92.1 [72.8–97.7] | < 0.001 | NA |
| Cross-sectional | 23.7 [0–95.1] | NA | 2 | 21 | 3.6 [1.9–6.6] | 92.1 [72.8–97.7] | < 0.001 | NA |
| Rodentia | ||||||||
| Overall | 5.3 [0–46.2] | NA | 2 | 487 | 4.1 [2.3–7.3] | 94.1 [81.2–98.1] | < 0.001 | NA |
| Cross-sectional | 5.3 [0–46.2] | NA | 2 | 487 | 4.1 [2.3–7.3] | 94.1 [81.2–98.1] | < 0.001 | NA |
| Chiroptera | ||||||||
| Overall | 0 [0–0.1] | [0–1.5] | 10 | 5156 | 2.1 [1.5–2.8] | 76.6 [56.8–87.3] | < 0.001 | 0.04 |
| Cross-sectional | 0 [0–0.1] | [0–1.5] | 10 | 5156 | 2.1 [1.5–2.8] | 76.6 [56.8–87.3] | < 0.001 | 0.04 |
| Multiple animals | ||||||||
| Overall | 46.4 [31.7–61.5] | [3.5–93.3] | 8 | 4271 | 6 [5–7.2] | 97.2 [96–98.1] | < 0.001 | 0.299 |
| Cross-sectional | 46.4 [31.7–61.5] | [3.5–93.3] | 8 | 4271 | 6 [5–7.2] | 97.2 [96–98.1] | < 0.001 | 0.299 |
| Past exposure | ||||||||
| Carnivora | ||||||||
| Overall | 7.8 [0−23] | [0–72.4] | 6 | 180 | 2.6 [1.8–3.7] | 85.4 [70.1–92.8] | < 0.001 | 0.05 |
| Cross-sectional | 7.8 [0–23] | [0–72.4] | 6 | 180 | 2.6 [1.8–3.7] | 85.4 [70.1–92.8] | < 0.001 | 0.05 |
| Artiodactyla | ||||||||
| Overall | 7 [4.2–10.5] | NA | 1 | 256 | NA | NA | 1 | NA |
| Cross-sectional | 7 [4.2–10.5] | NA | 1 | 256 | NA | NA | 1 | NA |
| Chiroptera | ||||||||
| Overall | 0.4 [0–2.8] | [0–83.8] | 3 | 419 | 1.9 [1–3.5] | 72.1 [5.6–91.7] | 0.028 | 0.245 |
| Cross-sectional | 0.4 [0–2.8] | [0–83.8] | 3 | 419 | 1.9 [1–3.5] | 72.1 [5.6–91.7] | 0.028 | 0.245 |
CI: confidence interval; N: Number; 95% CI: 95% Confidence Interval; NA: not applicable. H estimates the extent of heterogeneity, a value of H = 1 indicates lack of evidence on heterogeneity of effects and a value of H > 1indicates a potential heterogeneity of effects. §: I2 describes the proportion of total variation in study estimates that is due to heterogeneity, a value >50% indicates presence of heterogeneity. All these estimates were obtained using random effect meta-analysis.
3.6. Additional subgroup analysis
In addition to the analyses by infection and population categories, we conducted an additional subgroup analysis for each category identified.
In humans, the incidence of active RABV infection in suspected rabies cases differed significantly depending on the study design (cross-sectional = 89.9%; cohort = 36.4%; p = 0.001) and the UNSD region (West Africa = 100%; East Africa = 83.5%; Central Africa = 36.4%; p < 0.001) (Supplementary Table 8).
With respect to owned dogs, the incidence of active RABV infection was significantly different depending on the time of data collection (prospective = 65.4%; retrospective = 64.6%; retroprospective = 15.6%; p < 0.001), the UNSD region (Central Africa = 76.2%; Southern Africa = 64.0%; West Africa = 50.0%; Eastern Africa = 45.9%; p = 0.002), and the recruitment setting (urban/rural = 69.5%; urban = 69.1%; rural = 15.6%; p < 0.001). In stray dogs, the incidence of active RABV infection differed significantly depending on the study design (cross-sectional = 13.7%; community outbreak = 0.0%; p < 0.001) and the time of data collection (retrospective = 14.6%; prospective = 3.9%; p < 0.001). The incidence of past RABV infections in owned dogs was significantly different by time of data collection (prospective = 23.5%; retrospective = 9.6%; p = 0.018) and country (p < 0.001). In wilds dogs, the incidence of past RABV infections was significantly different by UNSD region (West Africa = 24.1%; Eastern Africa = 0.0%; p < 0.001), country (p < 0.001), and the recruitment setting (urban/rural = 24.1%; rural = 0.0%; p = 0.003).
In other animal species of the Order of Perissodactyla, the incidence of current RABV infection was significantly different depending on the recruitment setting (urban = 71.5%; rural = 1.6%; p < 0.001) and the country. (p < 0.001). In other animal species of the Order Carnivora, the incidence of active RABV infections differed significantly depending on the study design (community outbreak = 86.7%; cross-sectional = 48.9%; p = 0.011) the time of data collection (retroprospective = 90.0%; retrospective = 53.2%; prospective = 45.1; p = 0.006) and country (p < 0.001). In other animal species of the Order Carnivora, the seroprevalence of past exposure to RABV showed a significant difference depending on the time of data collection (prospective = 14.3; retrospective = 3.6%; retroprospective = 0.0%; p = 0.039) and country (p = 0.030).
3.7. Exploration of the heterogeneity source
The sources of heterogeneity were revealed at 82.1% for the incidence of active RABV infection in suspected rabies cases, 13.4% in owned dogs, 83.5% in stray dogs, 48.4% in Artiodactyla, 63.1% in Carnivora, and 92.2% in Chiroptera (Supplementary Table 9). The sources of heterogeneity were revealed at 100% for the incidence of past RABV infection in wild dogs.
4. Discussion
This meta-analysis aimed to provide reliable combined data on the incidence of RABV in humans, dogs, and other animal species in Africa. Data from 7 articles (7 incidence records), 52 articles (63 incidence records), and 30 articles (72 incidence records) showed pooled incidences of 69.1% in humans, 35.6% in dogs, and 35.0% in other animal species respectively. The RABV incidence was substantially heterogeneous with high rates recorded in active RABV infection, suspected rabies cases, suspected rabid owned and stray dogs, Perissodactyla, Artiodactyla and Carnivora. RABV incidence in humans was also higher in West and East Africa. The highest incidence of RABV in owned dogs were in urbans setting and in Central and South Africa. The highest incidence in Perissodactyla was in urban settings.
Twenty-one of the 54 Africa countries were represented in this systematic review. However, Africa record the second high rate of human death and it estimated that rabies is present in all the Africa countries [10]. While human and animal rabies have been declared notifiable disease by the WHO and OIE, the major problem of rabies in Africa remains its negligence by the population, its non-prioritization by decision makers, and the lack of active and efficient surveillance system [91]. These situations could explain the underreporting of RABV in African countries [92]. The included studies were most perform in East and West Africa regions, which are highly endemic of RABV [1]. Nigeria and Ethiopia, belonging respectively to West and East regions of Africa, were the most represented countries in this study. Although this study provided extensive data of RABV incidence in Africa, the reported data are still largely underestimated, as it has been suggested that only 3% of human rabies cases are reported in Africa [93]. In accordance with Expert recommendations on rabies, detection of RABV in this study was mostly performed using the fluorescent antibody test using brain sample since this technique is known as the gold standard for the diagnosis of both human and animal rabies [1].
This meta-analysis confirmed dogs as the major host of RABV in many countries of Africa, and highlighted domestic dogs (owned and stray dogs) as the most concerned dog categories in the articles considered. Our findings strengthened previous analyses, and confirmed the role of dog in the persistence of the enzootic cycle of domestic rabies through dog-to-dog virus transmission [94]. The position of owned dog among the most recruited dog categories could be explained by the fact that they are more accessible for observation and sample collection compared to stray dogs which are harder to follow up. Conversely stray dogs are relatively difficult to be accessed by the quite passive surveillance and control system in most countries. Thus, stray dogs are likely less covered by rabies vaccination and this could explain what a higher rate of confirmed rabies cases was found in stray dogs compared to owned dogs (Fig. 4). This underscore the need to consider stray dogs in vaccination campaign programs rather than relying only on the awareness of owners who have to vaccinate their pets according to the regulations in force. Accordingly, a coordinated and pluriannual program of rabies control with vaccination campaigns targeting both owned and stray dogs of owned in KwaZulu-Natal resulted in outstanding success towards rabies elimination [95]. It has become evident that an efficient mass vaccination for rabies must include stray dogs in the required 70% coverage of dog population [96]. This evidence prompted Bourhy and collaborators to integrate hands on vaccination of stray dogs in all round of customized online and onsite training for rabies-control officers in endemic regions in Africa [97].
The finding of this study shows the RABV incidence in 27 other animal species, and one combined incidence among multiple animal species, thus indicating the wide range of host species of the RABV among mammals. Surprisingly order known to be the major RABV reservoirs such as Chiroptera did not show evidence of active infections while orders such as Perissodactyla and Artiodactyla which are domestic animals showed the highest incidence [98].
One major question that is still to be ruled out in rabies epidemiology remains the significance of occurrence of rabies specific antibodies in healthy, unvaccinated individuals reported in a number of studies of wildlife, domestic dogs, and humans; suggesting the potential for nonlethal rabies exposure [99]. It is tempting to explain away the presence of rabies specific antibodies in humans, dogs, and other animal species by cross-reactivity with other potentially less lethal lyssaviruses [100]. Antibody positive typically represent prior vaccination histories rather than non-productive rabies infection or recovery from rabies infection. This is particularly true for humans and domestic animals, but may be true for wildlife species as well in areas with active or experimental wildlife vaccination programs. However, it has been recently suggested that there might be individuals in which nonlethal rabies exposure occurs [99]. Improving the estimates of rabies seroprevalence in wildlife, domestic dogs and humans in rabies endemic regions could potentially provide substantial clarifications about the quite complex rabies ecology. Longitudinal studies performed in areas where there are seropositive and disease-free individuals would also be of great importance.
In contrast to WHO estimates indicating that rabies incidence would be higher in rural areas, where awareness and accessibility of the vaccine is reduced [10], The subgroup analysis revealed that RABV was more prevalent in urban than in rural areas. This observation is consistent with previous reports from Cameroon where the rate of domestic rabies was significantly high in the urban setting of the nation capital Yaounde compared to rural areas of the same region of the country [101]. But the apparent urban nature of domestic rabies suggested by this report is biased by the fact rabies cases in rural areas are underestimated as result of logistic and financial barriers to access laboratory services that are still very centralized in the major towns of African countries [93].
It is important to set up efficient active surveillance system, complemented by further extensive studies in Africa, to provide the actual burden of rabies that would inform the prioritization of rabies control and elimination in Africa. The importance of reliable and representative epidemiological data that will inform the optimal design and regular evaluations of interventions towards the global elimination of dog mediated human rabies by 2030 cannot be overemphasized. For this, the epidemiological data from laboratories should be shared between government officials, researchers and workers from different sectors at local, national, regional and global levels. To contain rabies effectively, a coordinated approach is needed between the human, animal and environmental health sectors. We should move from protecting human lives against rabies by providing access to post-exposure prophylaxis, to a paradigm shift with extensive promotion of dog vaccination which is the most cost-effective way to reduce the burden of rabies. We need to change the paradigm and strengthen community involvement and any other kind of collaboration that could benefit rabies control.
This review had several limitations. Up to 66 full texts out of 416 eligible could not be found, which could influence the incidences estimated in this meta-analysis. There is a significant residue of unexplained heterogeneity that could be for reasons such as vaccination that we did not take into account in this meta-analysis. Despite the fact that RABV has been identified as circulating in several reservoir species, there are 15 other viral species of the Lyssavirus genus that can cause rabies with clinical presentation indistinguishable from RABV-induced rabies [2]. The multiple testing methodologies used both for active infections and past exposures do not have the same sensitivities and specificities for the detection of RABV targets and are probably also sources of heterogeneity that we have not investigated. However, the evidence of RABV was performed in the majority of studies included by direct immunofluorescence, which allows the specific detection of RABV thanks to specific fluorescent antibody.
Overall, our analyses revealed a high incidence in Africa of RABV in humans, dogs and other animal species. This emphasizes the crucial need to promote responsible dog ownership and mass vaccination campaigns as key interventions to reduce the risk of domestic rabies in Africa. Outstanding examples from African countries, such as South Africa and Malawi, where vaccine coverage of dog populations at ≥70% during several years of pilot programs have shown that the global elimination of dog mediated human rabies by 2030 is an achievable goal. All the tools are available and efficient and most African countries have already developed their strategic and/or operational plans for the national elimination of dog mediated human rabies by 2030.
The following are the supplementary data related to this article.
Preferred reporting items for systematic reviews and meta-analyses checklist.
Search strategy in Medline (Pubmed).
Items for risk of bias assessment.
Main reasons of exclusion of eligible studies.
Risk of bias assessment.
Characteristics of included studies.
Individual characteristics of included studies.
Subgroup analysis of incidence of Rabies virus in humans, dogs, and other animal species in Africa.
Univariable and multivariable meta-regression analysis on the incidence of Rabies virus in humans, dogs, and other animal species in Africa.
Seroprevalence of Rabies virus infections in dogs in Africa.
Incidence of Rabies virus infections in other animal species in Africa.
Seroprevalence of Rabies virus infections in other animal species in Africa.
Funnel plot for publication for Rabies virus incidence in dogs in Africa.
Funnel plot for publication for Rabies virus incidence in humans in Africa.
Funnel plot for publication for Rabies virus incidence in other animal species in Africa.
Supplementary material
Funding
Dr. Sebastien Kenmoe is supported by AREF/EDCTP (VARIAFRICA-TMA2019PF-2705) to gain on-the job training in conducting systematic reviews and meta-analysis in the Centre for Global Health, Usher Institute, University of Edinburgh, United Kingdom and to develop an investigator group in Africa.
Acknowledgments
Acknowledgement
None.
References
- 1.WHO . World Health Organization; Geneva: 2018. WHO Expert Consultation on Rabies: Third Report. [Google Scholar]
- 2.Fooks A.R., Cliquet F., Finke S., Freuling C., Hemachudha T. Rabies. Nat. Rev. Dis. Primers. 2017;3:17091. doi: 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 3.Davis R., Nadin-Davis S.A., Moore M., Hanlon C. Genetic characterization and phylogenetic analysis of skunk-associated rabies viruses in North America with special emphasis on the central plains. Virus Res. 2013;174:27–36. doi: 10.1016/j.virusres.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Sabeta C.T., Shumba W., Mohale D.K., Miyen J.M., Wandeler A.I. Mongoose rabies and the African civet in Zimbabwe. Vet. Rec. 2008;163:580. doi: 10.1136/vr.163.19.580. [DOI] [PubMed] [Google Scholar]
- 5.Jackson A.C. Human rabies: a 2016 update. Curr. Infect. Dis. Rep. 2016;18:38. doi: 10.1007/s11908-016-0540-y. [DOI] [PubMed] [Google Scholar]
- 6.Ugolini G., Hemachudha T. Rabies: changing prophylaxis and new insights in pathophysiology. Curr. Opin. Infect. Dis. 2018;31:93–101. doi: 10.1097/QCO.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Vol. 93. 2018. Rabies vaccines: WHO position paper – April 2018 weekly epidemiological record. No 16; pp. 201–220. (18 p) [Google Scholar]
- 8.Dodet B. The fight against rabies in Africa: from recognition to action. Vaccine. 2009;27:5027–5032. doi: 10.1016/j.vaccine.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 9.World Health O, Food, Agriculture Organization of the United N, World Organisation for Animal H . World Health Organization; Geneva: 2018. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030. [Google Scholar]
- 10.WHO World Health Organization; Rabies "Epidemiology and burden of disease". 2018. https://wwwwhoint/rabies/epidemiology/en/ Available from:
- 11.Bourhy H., de Melo G.D., Tarantola A. New aspects of rabies control. Bull. Acad. Natl Med. 2020;204(2020):1000–1009. doi: 10.1016/j.banm.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.la Moher D, Tetzlaff J, Altman DG Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6. [PMC free article] [PubMed]
- 13.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Chapter 5: Systematic reviews of prevalence and incidence. In: Munn Z., editor. JBI Manual for Evidence Synthesis. 2020. [Google Scholar]
- 14.Hoy D., Brooks P., Woolf A., Blyth F., March L. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Kenmoe S., Tchatchouang S., Ebogo-Belobo J.T., Ka’e A.C., Mahamat G. Systematic review and meta-analysis of the epidemiology of Lassa virus in humans, rodents and other mammals in sub-Saharan Africa. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 17.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odeh L.E., Umoh J.U., Dzikwi A.A. Assessment of risk of possible exposure to rabies among processors and consumers of dog meat in Zaria and Kafanchan, Kaduna state, Nigeria. Global J. Health Sci. 2014;6:142–153. doi: 10.5539/gjhs.v6n1p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mtui-Malamsha N., Sallu R., Mahiti G.R., Mohamed H., Oleneselle M. Ecological and epidemiological findings associated with zoonotic rabies outbreaks and control in Moshi, Tanzania, 2017–2018. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16162816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hikufe E.H., Freuling C.M., Athingo R., Shilongo A., Ndevaetela E.E. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011-2017. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coetzer A., Gwenhure L., Makaya P., Markotter W., Nel L. Epidemiological aspects of the persistent transmission of rabies during an outbreak (2010–2017) in Harare, Zimbabwe. PLoS One. 2019;14:e0210018. doi: 10.1371/journal.pone.0210018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezeokoli C.D., Umoh J.U. Epidemiology of rabies in northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1987;81:268–272. doi: 10.1016/0035-9203(87)90237-9. [DOI] [PubMed] [Google Scholar]
- 23.Dzikwi A.A., Kuzmin I.I., Umoh J.U., Kwaga J.K.P., Ahmad A.A. Evidence of Lagos bat virus circulation among Nigerian fruit bats. J. Wildl. Dis. 2010;46:267–271. doi: 10.7589/0090-3558-46.1.267. [DOI] [PubMed] [Google Scholar]
- 24.LsN Kalemba, Niezgoda M., Gilbert A.T., Doty J.B., Wallace R.M. Exposure to Lyssaviruses in Bats of the Democratic Republic of the Congo. J. Wildl. Dis. 2017;53:408–410. doi: 10.7589/2016-06-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoh GsR, Kazeem H.M., Kia G.S.N., Ponfa Z.N. Heat induced epitope retrieval for rabies virus detection by direct fluorescent antibody test in formalin-fixed dog brain tissues. Open Vet. J. 2018;8:313–317. doi: 10.4314/ovj.v8i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiembré I., Broban A., Bénié J., Tetchi M., Druelles S. Human rabies in Côte d’Ivoire 2014-2016: results following reinforcements to rabies surveillance. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umoh J.U., Ezeokoli C.D., Okoh A.E. Immunofluorescent staining of trypsinized formalin-fixed brain smears for rabies antigen: results compared with those obtained by standard methods for 221 suspect animal cases in Nigeria. J. Hyg. 1985;94:129–134. doi: 10.1017/s0022172400061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechenne M., Mindekem R., Madjadinan S., Oussiguéré A., Moto D.D. The importance of a participatory and integrated one health approach for rabies control: the case of N’Djaména, Chad. Trop. Med. Infect. Dis. 2017;2:43. doi: 10.3390/tropicalmed2030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofeu C.L., Broban A., Njifou Njimah A., Blaise Momo J., Sadeuh-Mba S.A. Improving systematic rabies surveillance in Cameroon: a pilot initiative and results for 2014-2016. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okoh A.E. Investigation of possible rabies reservoirs in rodents in Nigeria. International journal of zoonoses. 1986;13:1–5. [PubMed] [Google Scholar]
- 31.Mebatsion T., Cox J.H., Frost J.W. Isolation and characterization of 115 street rabies virus isolates from Ethiopia by using monoclonal antibodies: Identification of 2 isolates as Mokola and Lagos bat viruses. J. Infect. Dis. 1992;166:972–977. doi: 10.1093/infdis/166.5.972. [DOI] [PubMed] [Google Scholar]
- 32.Oluwayelu D.O., Adebiyi A.I., Ohore O.G., Cadmus S.I. Lack of protection against rabies in neighbourhood dogs in some peri-urban and rural areas of Ogun and Oyo states, Nigeria. Afr. J. Med. Med. Sci. 2014;43:157–162. [PubMed] [Google Scholar]
- 33.Sadeuh-Mba S.A., Momo J.B., Besong L., Loul S., Njouom R. Molecular characterization and phylogenetic relatedness of dog-derived Rabies Viruses circulating in Cameroon between 2010 and 2016. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badiali L., Ferris D.H. A preliminary report on rabies in suspected equine encephalomyelitis cases in the United Arab Republic. Bull. World Health Organ. 1966;34:797–798. [PMC free article] [PubMed] [Google Scholar]
- 35.Olugas B.O., Aiyedun J.O., Emikpe B.O. Prevalence of antibody against rabies among confined, free-roaming and stray dogs in a transit city of Nigeria. Vet. Ital. 2011;47:453–460. [PubMed] [Google Scholar]
- 36.Alabi O., Nguku P., Chukwukere S., Gaddo A., Nsubuga P. Profile of dog bite victims in Jos plateau state, Nigeria: a review of dog bite records (2006-2008) Pan African Med. J. 2014;18:12. doi: 10.11694/pamj.supp.2014.18.1.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall D.A., Williams S.D., Kuzmin I.V., Rupprecht C.E., Tallents L.A. Rabies in endangered Ethiopian wolves. Emerg. Infect. Dis. 2004;10:2214–2217. doi: 10.3201/eid1012.040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berentsen A.R., Dunbar M.R., Becker M.S., M’Soka J., Droge E. Rabies, canine distemper, and canine parvovirus exposure in large carnivore communities from two zambian ecosystems. Vector Borne Zoonotic Dis. 2013;13:643–649. doi: 10.1089/vbz.2012.1233. [DOI] [PubMed] [Google Scholar]
- 39.Olugasa B.O., Odeniyi A.O., Adeogun A.O., Adeola O.A. Antibody levels against rabies among occupationally exposed individuals in a Nigerian University. Vet. Ital. 2010;46:21–28. [PubMed] [Google Scholar]
- 40.Kitala P.M., McDermott J.J., Kyule M.N., Gathuma J.M. Community-based active surveillance for rabies in Machakos District, Kenya. Prevent. Ve. Med. 2000;44:73–85. doi: 10.1016/s0167-5877(99)00114-2. [DOI] [PubMed] [Google Scholar]
- 41.Eze U.U., Ngoepe E.C., Anene B.M., Ezeokonkwo R.C., Nwosuh C. Detection of lyssavirus antigen and antibody levels among apparently healthy and suspected rabid dogs in South-Eastern Nigeria. BMC Res. Notes. 2018;11:920. doi: 10.1186/s13104-018-4024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mebatsion T., Sillero-Zubiri C., Gottelli D., Cox J.H. Detection of rabies antibody by ELISA and RFFIT in unvaccinated dogs and in the endangered Simien jackal (Canis simensis) of Ethiopia. Zentralbl. Veterinarmed. B. 1992;39:233–235. doi: 10.1111/j.1439-0450.1992.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 43.Mshelbwala P.P., Ogunkoya A.B., Maikai B.V. Detection of rabies antigen in the saliva and brains of apparently healthy dogs slaughtered for human consumption and its public health implications in abia state, Nigeria. ISRN Vet. Sci. 2013:468043. doi: 10.1155/2013/468043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghomo H.O., Ako-Nai A.K., Oduye O.O., Tomori O., Rupprecht C.E. Detection of rabies virus antibodies in fruit bats (Eidolon helvum) from Nigeria. J. Wildl. Dis. 1990;26:258–261. doi: 10.7589/0090-3558-26.2.258. [DOI] [PubMed] [Google Scholar]
- 45.Ehimiyein A.M., Niezgoda M., Orciari L., Osinubi M.O.V., Ehimiyein I.O. Efficacy of a direct rapid immunohistochemical test (DRIT) for rabies detection in Nigeria. Afr. J. Biomed. Res. 2014;17:101–107. [Google Scholar]
- 46.Sabeta C.T., Weyer J., Geertsma P., Mohale D., Miyen J. Emergence of rabies in the Gauteng province, South Africa: 2010-2011. J. S. Afr. Vet. Assoc. 2013;84 doi: 10.4102/jsava.v84i1.923. [DOI] [PubMed] [Google Scholar]
- 47.Dao S., Abdillahi A.M., Bougoudogo F., Toure K., Simbe C. Epidemiological aspects of human and animal rabies in the urban area of Bamako, Mali. Bull. Soc. Pathol. Exot. 2006;99:183–186. [PubMed] [Google Scholar]
- 48.Coetzer A., Coertse J., Makalo M.J., Molomo M., Markotter W. Epidemiology of rabies in Lesotho: the importance of routine surveillance and virus characterization. Trop. Med. Infect. Dis. 2017;2:30. doi: 10.3390/tropicalmed2030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleaveland S., Fèvre E.M., Kaare M., Coleman P.G. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull. World Health Organ. 2002;80:304–310. [PMC free article] [PubMed] [Google Scholar]
- 50.Kayali U., Mindekem R., Yémadji N., Oussiguéré A., Naïssengar S. Incidence of canine rabies in N’Djaména. Prevent. Vet. Med. 2003;61:227–233. doi: 10.1016/j.prevetmed.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Sadeuh-Mba S.A., Besong L., Demanou M., Loul S., Nchare A. Laboratory data of dog rabies in southern Cameroon from 2010 to 2013. BMC Res. Notes. 2014;7:905. doi: 10.1186/1756-0500-7-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynes J.-M., Andriamandimby S.F., Razafitrimo G.M., Razainirina J., Jeanmaire E.M. Laboratory surveillance of rabies in humans, domestic animals, and bats in Madagascar from 2005 to 2010. Adv. Prev. Med. 2011;2011:727821. doi: 10.4061/2011/727821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Punguyire D.T., Osei-Tutu A., Aleser E.V., Letsa T. Level and pattern of human rabies and dog bites in techiman municipality in the middle belt of Ghana: A six year retrospective records review. Pan African Med. J. 2017:28. doi: 10.11604/pamj.2017.28.281.14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olarinmoye A.O., Kamara V., Jomah N.D., Olugasa B.O., Ishola O.O. Molecular detection of rabies virus strain with n-gene that clustered with china lineage 2 co-circulating with africa lineages in monrovia, liberia: First reported case in africa. Epidemiol. Infect. 2019:147. doi: 10.1017/S0950268818003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakouné E., Digol M., Konamna X., Selekon B., Le Faou A. New introduction and spread of rabies among dog population in Bangui. Acta Trop. 2012;123:107–110. doi: 10.1016/j.actatropica.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Twabela A.T., Mweene A.S., Masumu J.M., Muma J.B., Lombe B.P. Overview of animal rabies in Kinshasa province in the democratic republic of Congo. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Benedictis P., Sow A., Fusaro A., Veggiato C., Talbi C. Phylogenetic analysis of rabies viruses from Burkina Faso, 2007. Zoonoses Public Health. 2010;57:e42–e46. doi: 10.1111/j.1863-2378.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 58.Oelofsen M.J., Smith M.S. Rabies and bats in a rabies-endemic area of southern Africa: application of two commercial test kits for antigen and antibody detection. Onderstepoort J. Vet. Res. 1993;60:257–260. [PubMed] [Google Scholar]
- 59.Hambolu S.E., Dzikwi A.A., Kwaga J.K., Kazeem H.M., Umoh J.U. Rabies and dog bites cases in Lagos state Nigeria: a prevalence and retrospective studies (2006-2011) Global J. Health Sci. 2014;6:107–114. doi: 10.5539/gjhs.v6n1p107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sillero-Zubiri C., King A.A., Macdonald D.W. Rabies and mortality in Ethiopian wolves (Canis simensis) J. Wildl. Dis. 1996;32:80–86. doi: 10.7589/0090-3558-32.1.80. [DOI] [PubMed] [Google Scholar]
- 61.Ehimiyein A., Niezgoda M., Orciari L., Kuzmin I., Osinubi M. Rabies cases in dog markets in Kaduna state, northern Nigeria. Int. J. Infect. Dis. 2010;14:e476. [Google Scholar]
- 62.Ajayi B.B., Rabo J.S., Baba S.S. Rabies in apparently healthy dogs: histological and immunohistochemical studies. Nigerian Postgraduate Med. J. 2006;13:128–134. [PubMed] [Google Scholar]
- 63.Alexander K.A., Smith J.S., Macharia M.J., King A.A. Rabies in the Masai Mara, Kenya: preliminary report. Onderstepoort J. Vet. Res. 1993;60:411–414. [PubMed] [Google Scholar]
- 64.Cleaveland S., Barrat J., Barrat M.J., Selve M., Kaare M. A rabies serosurvey of domestic dogs in rural Tanzania: results of a rapid fluorescent focus inhibition test (RFFIT) and a liquid-phase blocking ELISA used in parallel. Epidemiol. Infect. 1999;123:157–164. doi: 10.1017/s0950268899002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munang’andu H.M., Mweene A.S., Siamudaala V., Muma J.B., Matandiko W.U. Rabies status in Zambia for the period 1985–2004. Zoonoses Public Health. 2011;58:21–27. doi: 10.1111/j.1863-2378.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 66.Edward F.D., Osei-Tutu A., Gbeddy K., Quist C. A retrospective study of rabies cases at Techiman Municipal, Ghana, 2009–2012. Int. J. Infect. Dis. 2014;21:179. [Google Scholar]
- 67.Pfukenyi D.M., Pawandiwa D., Makaya P.V., Ushewokunze-Obatolu U. A retrospective study of rabies in humans in Zimbabwe, between 1992 and 2003. Acta Trop. 2007;102:190–196. doi: 10.1016/j.actatropica.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Wosu L.O., Anyanwu H.N. Seroepidemiological survey of rabies virus antibodies in non vaccinated dogs in Nsukka environs, Nigeria. Zentralbl. Veterinarmed. B. 1990;37:47–52. doi: 10.1111/j.1439-0450.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 69.Laurenson K., Van Heerden J., Stander P., Van Vuuren M.J. Seroepidemiological survey of sympatric domestic and wild dogs (Lycaon pictus) in Tsumkwe District, north-eastern Namibia. Onderstepoort J. Vet. Res. 1997;64:313–316. [PubMed] [Google Scholar]
- 70.Alexander K.A., Kat P.W., Wayne R.K., Fuller T.K. Serologic survey of selected canine pathogens among free-ranging jackals in Kenya. J. Wildl. Dis. 1994;30:486–491. doi: 10.7589/0090-3558-30.4.486. [DOI] [PubMed] [Google Scholar]
- 71.Ogunkoya A.B., Beran G.W., Umoh J.U., Gomwalk N.E., Abdulkadir IA, et al. Serological evidence of infection of dogs and man in Nigeria by lyssaviruses (family Rhabdoviridae) Trans. R. Soc. Trop. Med. Hyg. 1990;84:842–845. doi: 10.1016/0035-9203(90)90103-l. [DOI] [PubMed] [Google Scholar]
- 72.Tyem D.A., Dogonyaro B.B., Woma T.A., Ngoepe E.C. Sabeta CT (2017) Sero-Surveillance of Lyssavirus Specific Antibodies in Nigerian Fruit Bats (Eidolon helvum) Trop. Med. Infect. Dis. 2021;2:26. doi: 10.3390/tropicalmed2030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Creel S., Creel N.M., Munson L., Sanderlin D., MJU Appel. Serosurvey for selected viral diseases and demography of African wild dogs in Tanzania. J. Wildl. Dis. 1997;33:823–832. doi: 10.7589/0090-3558-33.4.823. [DOI] [PubMed] [Google Scholar]
- 74.Millán J., Chirife A.D., Kalema-Zikusoka G., Cabezón O., Muro J. Serosurvey of dogs for human, livestock, and wildlife pathogens, Uganda. Emerg. Infect. Dis. 2013;19:680–682. doi: 10.3201/eid1904.121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grover M., Bessell P.R., Conan A., Polak P., Sabeta C.T. Spatiotemporal epidemiology of rabies at an interface between domestic dogs and wildlife in South Africa. Sci. Rep. 2018;8:10864. doi: 10.1038/s41598-018-29045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tricou V., Bouscaillou J., Kamba Mebourou E., Koyanongo F.D., Nakouné E. Surveillance of canine rabies in the Central African Republic: impact on human health and molecular epidemiology. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oluwayelu D.O., Adebiyi A.I., OGU Ohore. A survey of rabies virus antibodies in confined, hunting and roaming dogs in Ogun and Oyo States, Southwestern Nigeria. Asian Pacific J. Trop. Dis. 2015;5:17–21. [Google Scholar]
- 78.Aworh M.K., Nwosuh C.I., Ajumobi O.O., Okewole P.A., Okolocha E.C. A Retrospective Study of Rabies Cases Reported at Vom Christian Hospital, Plateau State, Nigeria, 2006–2010. Niger. Vet. J. 2011;32 [Google Scholar]
- 79.Baba S.S., Bwala J.P., El-Yaguda A.D., Baba M.M. Serological evidence of rabies virus infection of slaughter camels (Camelus Dromedarus) imported to Nigeria. Trop. Vet. 2005;23:78–82. [Google Scholar]
- 80.Daniel O.O., Adebowale I.A., Obokparo G.O. Survey of rabies virus antibodies in confined, hunting and roaming dogs in Ogun and Oyo states, Nigeria. Bull. Anim. Health Prod. Africa. 2014;62:37–44. [Google Scholar]
- 81.Deressa A., Ali A., Bayene M., Selassie B.N., Yimer E. The status of rabies in Ethiopia: a retrospective record review. Ethiop. J. Health Dev. 2010;24 [Google Scholar]
- 82.Ehizibolo D.O., Nwosuh C.I., Ehizibolo E.E., Kia G.S.N. Comparison of the fluorescent antibody test and direct microscopic examination for rabies diagnosis at the National Veterinary Research Institute, Vom, Nigeria. Afr. J. Biomed. Res. 2009;12:73–76. [Google Scholar]
- 83.Garba A., Oboegbulem S.I., Elsa A.T., Junaidu A.U., Magaji A.A. A comparative rabies laboratory diagnosis: peculiar features of samples from apparently healthy dogs in Nigeria. Sokoto J. Vet. Sci. 2008;7 [Google Scholar]
- 84.Isek T.I., Umoh J.U., Dzikwi A.A. Detection of rabies antigen in the brain tissues of Apparetly healthy dogs slaughteres in Ogoja - Cross River State, Nigeria. Nigerian Vet. J. 2013;34 [Google Scholar]
- 85.Kia G.S.N., Huang Y., Zhou M., Zhou Z., Gnanadurai C.W. Molecular characterization of a rabies virus isolated from trade dogs in Plateau State, Nigeria. Sokoto J. Vet. Sci. 2018;16:54–62. [Google Scholar]
- 86.Muhammad-Bashir B., Sani A.M., Ademola O.P., Peterside K., Maryam M. Prevalence and demographic distribution of canine rabies in Plateau State, Nigeria, 2004–2009. Bull. Anim. Health Prod. Africa. 2016;64:129–138. [Google Scholar]
- 87.Nimzing L., Nanbol Z. Detection of rabies antigen in brains of suspected rabid dogs using sellers staining technique and enzyme immunoassay. Highland Med. Res. J. 2003;1:48–51. [Google Scholar]
- 88.Swai E.S., Moshy W.E., Kaaya J.E., Mtui P.F. Spatial and temporal distribution of rabies in northern Tanzania in the period of 1993-2002. Tanzania J. Health Res. 2010;12:80–85. doi: 10.4314/thrb.v12i1.56335. [DOI] [PubMed] [Google Scholar]
- 89.Tekki I.S., Ponfa Z.N., Nwosuh C.I., Kumbish P.R., Jonah C.L. Comparative assessment of seller’s staining test (SST) and direct fluorescent antibody test for rapid and accurate laboratory diagnosis of rabies. Afr. Health Sci. 2016;16:123–127. doi: 10.4314/ahs.v16i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ali A., Mengistu F., Hussen K., Getahun G., Deressa A. Overview of rabies in and around Addis Ababa, in animals examined in EHNRI zoonoses laboratory between, 2003 and 2009. Ethiopian Vet. J. 2010;14:91–101. [Google Scholar]
- 91.Mbilo C., Coetzer A., Bonfoh B., Angot A., Bebay C. Dog rabies control in West and Central Africa: a review. Acta Trop. 2020;105459 doi: 10.1016/j.actatropica.2020.105459. [DOI] [PubMed] [Google Scholar]
- 92.Dodet B., Tejiokem M.C., Aguemon A.R., Bourhy H. Human rabies deaths in Africa: breaking the cycle of indifference. Int. Health. 2015;7:4–6. doi: 10.1093/inthealth/ihu071. [DOI] [PubMed] [Google Scholar]
- 93.Knobel D.L., Cleaveland S., Coleman P.G., Fèvre E.M., Meltzer M.I. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 94.Bourhy H., Nakouné E., Hall M., Nouvellet P., Lepelletier A. Revealing the micro-scale signature of endemic zoonotic disease transmission in an African urban setting. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LeRoux K., Stewart D., Perrett K.D., Nel L.H., Kessels J.A. Rabies control in KwaZulu-Natal, South Africa. Bull. World Health Organ. 2018;96:360–365. doi: 10.2471/BLT.17.194886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sabeta C., Ngoepe E.C. Controlling dog rabies in Africa: successes, failures and prospects for the future. Rev. Sci. Tech. 2018;37:439–449. doi: 10.20506/rst.37.2.2813. [DOI] [PubMed] [Google Scholar]
- 97.Bourhy H., Troupin C., Faye O., Meslin F.X., Abela-Ridder B. Customized online and onsite training for rabies-control officers. Bull. World Health Organ. 2015;93:503–506. doi: 10.2471/BLT.14.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begeman L., GeurtsvanKessel C., Finke S., Freuling C.M., Koopmans M. Comparative pathogenesis of rabies in bats and carnivores, and implications for spillover to humans. Lancet Infect. Dis. 2018;18:e147–e159. doi: 10.1016/S1473-3099(17)30574-1. [DOI] [PubMed] [Google Scholar]
- 99.Gold S., Donnelly C.A. Vol. 14. 2020. Rabies virus-neutralising antibodies in healthy, unvaccinated individuals: what do they mean for rabies epidemiology? p. e0007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue S., Sato Y., Hasegawa H., Noguchi A., Yamada A. Cross-reactive antigenicity of nucleoproteins of lyssaviruses recognized by a monospecific antirabies virus nucleoprotein antiserum on paraffin sections of formalin-fixed tissues. Pathol. Int. 2003;53:525–533. doi: 10.1046/j.1440-1827.2003.01511.x. [DOI] [PubMed] [Google Scholar]
- 101.Sadeuh-Mba S.A., Momo J.B., Besong L., Loul S., Njouom R. Molecular characterization and phylogenetic relatedness of dog-derived Rabies Viruses circulating in Cameroon between 2010 and 2016. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred reporting items for systematic reviews and meta-analyses checklist.
Search strategy in Medline (Pubmed).
Items for risk of bias assessment.
Main reasons of exclusion of eligible studies.
Risk of bias assessment.
Characteristics of included studies.
Individual characteristics of included studies.
Subgroup analysis of incidence of Rabies virus in humans, dogs, and other animal species in Africa.
Univariable and multivariable meta-regression analysis on the incidence of Rabies virus in humans, dogs, and other animal species in Africa.
Seroprevalence of Rabies virus infections in dogs in Africa.
Incidence of Rabies virus infections in other animal species in Africa.
Seroprevalence of Rabies virus infections in other animal species in Africa.
Funnel plot for publication for Rabies virus incidence in dogs in Africa.
Funnel plot for publication for Rabies virus incidence in humans in Africa.
Funnel plot for publication for Rabies virus incidence in other animal species in Africa.
Supplementary material