Abstract
Whether vitamin D or marine omega-3 (n-3) fatty acid supplementation reduces risk of cancer or cardiovascular disease (CVD) in general populations at usual risk for these outcomes is relatively unexplored in randomized trials. The primary goal of the VITamin D and OmegA-3 TriaL (VITAL), a nationwide, randomized, placebo-controlled, 2 × 2 factorial trial of vitamin D3 (2000 IU/day) and marine n-3 fatty acids (1 g/day) in the primary prevention of cancer and CVD among 25 871 US men aged ≥50 years and women aged ≥55 years, was to fill these knowledge gaps. Studying the influence of sex and race/ethnicity on treatment-related outcomes was a prespecified goal; such analyses help ensure that important effects are not missed and contribute to the foundation for developing targeted recommendations for supplement use. To enable investigation of potential sex- and race-specific treatment effects, trial investigators enrolled an even balance of men (n = 12 786) and women (n = 13 085) and oversampled African Americans (n = 5106). Significant or suggestive variation in intervention effects according to sex, race/ethnicity, and other participant characteristics was observed for some, though not all, outcomes. Additional research is needed to determine which individuals may be most likely to derive a net benefit from vitamin D or n-3 fatty acid supplementation. (VITAL clinicaltrials.gov identifier: NCT01169259).
Keywords: cancer, cardiovascular disease, marine n-3 fatty acids, primary prevention, race and ethnicity, randomized controlled trial, sex, vitamin D
‘. . . data [on vitamin D and n-3 fatty acids] were inconsistent and insufficient to establish causality.’
Vitamin D supplementation is an established intervention for the prevention and treatment of bone disorders. 1 Marine omega-3 (n-3) fatty acid supplementation has been recommended for heart health in patients with coronary heart disease (CHD) who do not meet target intakes for fish rich in n-3 fatty acids.2,3 A decade ago, vitamin D and n-3 fatty acids were also increasingly being used for the possible prevention of cancer or a first cardiovascular event, and their US sales soared.4-6 These possible new benefits were supported by promising data from some ecologic, laboratory, and observational studies, but such data were inconsistent and insufficient to establish causality.1,7,8 With respect to vitamin D, trials that had considered cancer or cardiovascular disease (CVD) outcomes in secondary or post hoc analyses had produced generally null results but were limited by one or more of the following issues: low doses, insufficient power, short durations, and nonrigorous assessment of endpoints. 1 Because of the lack of large trials of vitamin D in doses sufficient to produce meaningful increases in 25-hydroxyvitamin D (25(OH)D) levels and designed with cancer or CVD as primary outcomes, the Institute of Medicine 1 in 2011 and the US Preventive Services Task Force 9 in 2014 concluded that the effectiveness of supplemental vitamin D for prevention of cancer, CVD, or other nonskeletal outcomes was uncertain. The Institute of Medicine called for trials of higher doses (at least twice the current recommended dietary allowance of 600-800 IU/day for bone health) to assess the benefit-risk balance in diverse populations. Supplementation may be particularly salient for Black individuals, who tend to have lower cutaneous synthesis of vitamin D in response to solar radiation than members of other racial groups. 10 With respect to n-3 fatty acids, some11-13 though not all14-16 trials in secondary prevention or high-risk settings had found CVD risk reductions, but no large trial in a general population unselected for elevated cardiovascular risk had been conducted. Moreover, previous trials had included few Black participants.
We conducted the VITamin D and OmegA-3 TriaL (VITAL) to address these knowledge gaps. VITAL is the first of only 2 large (N ≥ 10 000) trials of moderate- to high-dose vitamin D for cancer or CVD prevention; the only trial of supplemental n-3 fatty acids for primary prevention of CVD in a usual-risk population; and the only large trial of either intervention to enroll a significant number of Black participants. In this article, we summarize the rationale, design, and results of the main trial7,17-19 and available ancillary studies. Our focus is on whether intervention effects differ by sex or race/ethnicity, but we also report significant or suggestive findings from analyses of other prespecified subgroups. The results are discussed in the context of relevant research, including recent meta-analyses of randomized trial data.
VITAL Overview
Design
VITAL was a nationwide, randomized, double-blind, placebo-controlled trial of the benefits and risks of supplemental vitamin D3 (2000 IU/day) and marine n-3 fatty acids (1 g/day Omacor fish-oil capsule with 840 mg of the n-3 fatty acids eicosapentaenoic acid [EPA; 460 mg] and docosahexaenoic acid [DHA; 380 mg]) in the primary prevention of cancer and CVD among 25 871 US men and women, aged ≥50 and ≥55 years, respectively.7,17,18,20 By design, a similar number of men and women were enrolled, and African Americans were oversampled, to permit investigation of potential sex- and race-specific treatment effects. Eligible participants had no history of cancer (except non-melanoma skin cancer), myocardial infarction (MI), stroke, transient ischemic attack, or coronary revascularization. They also had to agree to limit intakes of vitamin D and calcium from outside supplemental sources, including multivitamins, to no more than 800 IU per day and 1200 mg per day, respectively (the recommended dietary allowances [RDAs] for older adults 1 ) and to refrain from taking outside fish-oil supplements. Individuals with renal failure or dialysis, severe liver disease, anticoagulant use, history of hypercalcemia or parathyroid disorders, or other conditions that could pose safety concerns were not eligible to enroll. After successfully completing a 3-month placebo run-in, participants were randomized in a 2 × 2 factorial design to vitamin D, n-3 fatty acids, both active agents, or both placebos (Figure 1). Randomization took place from November 2011 to March 2014. Randomized treatment ended as planned on December 31, 2017. The median intervention period was 5.3 years, with a range of 3.8 to 6.1 years. Postintervention follow-up is ongoing.
Figure 1.
VITAL Factorial Design.
Adapted from Bassuk et al. 20 with permission. Copyright ©2016, Elsevier.
Participants completed baseline questionnaires about clinical and lifestyle risk factors for cancer, CVD, and other conditions. They also completed annual follow-up questionnaires regarding treatment compliance and side effects, risk factor updates, and endpoint occurrence. Study physicians blinded to treatment assignment reviewed participants’ medical records to confirm or disconfirm reported endpoints using accepted criteria.21-23 Deaths were ascertained with the National Death Index-Plus and other sources. All participants were asked to provide an optional prerandomization blood sample; 16 956 participants (66% of the study population) did so, and ~6000 provided follow-up samples in years 1 to 5. Some Boston-area participants (n = 1054) visited a local Clinical and Translational Science Center clinic for detailed assessments at baseline and years 1, 2, and 4. Ancillary studies were or are being conducted to assess treatment effects on a wide range of cancer- and cardiovascular-related and other outcomes with the goal of determining the overall benefit-risk balance of supplementation. Findings regarding bone health, colorectal adenoma, heart failure, chronic knee pain, falls, depression, and diabetic kidney disease have been published and are summarized below. With the exception of clinic assessments, blood collections, and some ancillary studies, VITAL has been conducted by postal mailings and electronic communications.
Although the primary goal of VITAL was to estimate treatment effects in the overall study population, sex and race/ethnicity were prespecified as potential effect modifiers, as were other baseline characteristics, including age, traditional cardiovascular risk factors (smoking, diabetes, hypertension, high cholesterol, and parental history of premature MI), body mass index (BMI), aspirin use, statin use, serum 25(OH)D, outside use of supplemental vitamin D (in amounts allowed by the trial protocol), dietary fish intake, plasma n-3 index (EPA + DHA as a percent of total fatty acids 24 ), and concurrent randomization to the active group of the other VITAL intervention. Analyses stratified by these variables may increase the likelihood that significant effects are not overlooked and provide a basis for “precision prevention”—that is, the development of targeted guidelines for supplement use.
Findings of all analyses stratified by sex and race/ethnicity are reported below, as are significant or suggestive findings from analyses stratified by other prespecified variables.
Baseline Characteristics
Characteristics of the study population at enrollment are shown in Table 1. Of the 25 871 participants, 49% are men and 51% women. The mean age is 67.1 years. The cohort is racially diverse, with 71% self-identified non-Hispanic Whites, 20% African Americans, and the rest members of other racial/ethnic groups. As expected in this large sample, randomization balanced the characteristics between the treatment groups, rendering it unlikely that confounding factors explain the findings. 20
Table 1.
Baseline Characteristics of the 25 871 VITAL Participants, According to Randomized Treatment Assignment a .
| Baseline characteristics b | All participants | Vitamin D | n-3 fatty acids | ||
|---|---|---|---|---|---|
| Active | Placebo | Active | Placebo | ||
| Total number | 25 871 | 12 927 | 12 944 | 12 933 | 12 938 |
| Female sex, n (%) | 13 085/25 871 (50.6) | 6547 (50.6) | 6538 (50.5) | 6547 (50.6) | 6538 (50.5) |
| Age, years, mean ± SD | 67.1 ± 7.1 | 67.1 ± 7.0 | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity, n (%) c | |||||
| Non-Hispanic White | 18 046/25 304 (71.3) | 9013 (71.3) | 9033 (71.4) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106/25 304 (20.2) | 2553 (20.2) | 2553 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013/25 304 (4.0) | 516 (4.1) | 497 (3.9) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388/25 304 (1.5) | 188 (1.5) | 200 (1.6) | 200 (1.6) | 188 (1.5) |
| Native American | 228/25 304 (0.9) | 118 (0.9) | 110 (0.9) | 120 (0.9) | 108 (0.9) |
| Other or unknown | 523/25 304 (2.1) | 259 (2.0) | 264 (2.1) | 249 (2.0) | 274 (2.2) |
| Body mass index, kg/m2, mean ± SD d | 28.1 ± 5.7 | 28.1 ± 5.7 | 28.1 ± 5.7 | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking, n (%) | 1836/25 485 (7.2) | 921 (7.2) | 915 (7.2) | 920 (7.2) | 916 (7.2) |
| Hypertension treated with medication, n (%) | 12 791/25 698 (49.8) | 6352 (49.5) | 6439 (50.1) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication, current use, n (%) | 9524/25 428 (37.5) | 4822 (38.0) | 4702 (36.9) | 4788 (37.7) | 4736 (37.2) |
| Diabetes, n (%) | 3549/25 828 (13.7) | 1812 (14.0) | 1737 (13.4) | 1799 (13.9) | 1750 (13.5) |
| Current regular aspirin use, n (%) e | 11 570/25 497 (45.4) | 5756 (45.2) | 5814 (45.6) | 5771 (45.3) | 5799 (45.5) |
Abbreviation: SD, standard deviation.
There were no significant differences in the baseline characteristics between the groups.
Race and ethnic group were reported by participants.
For body mass index, data were missing for 2.4% of participants.
At least monthly.
Follow-up, Adherence, Achieved Serum 25-Hydroxyvitamin D, and Plasma n-3 Index
Yearly questionnaire response rates averaged 93%, and mortality follow-up exceeded 98% during the 5.3-year trial. 17 Adherence to randomized treatment was high.17,18 Among the ~15 500 participants with analyzable blood samples at baseline, the mean serum total 25(OH)D level was 30.8 ng/mL (standard deviation [SD], 10.0 ng/mL), with 12.7% and 32.2% having levels <20 ng/mL and 20 to <30 ng/mL, respectively, and the mean plasma n-3 index was 2.6% (SD, 0.9%). After 1 year of treatment, there was a large postrandomization difference in 25(OH)D levels between the active vitamin D and placebo groups (40% increase) and in the n-3 index between the active n-3 fatty acid and placebo groups (55% increase), which remained throughout the trial. In the subgroup with follow-up measures, the achieved serum 25(OH)D with active vitamin D exceeded 40 ng/mL and the achieved plasma n-3 index with active n-3 fatty acids was ~4.1%, without changes over time in the placebo groups.
Baseline 25(OH)D was lower in men than in women (29.7 [SE (standard error), 0.30] vs 31.4 [SE, 0.30] ng/mL; P, interaction <.0001) and in African Americans than in non-Hispanic Whites (27.9 [SE, 0.29] vs 32.5 [SE, 0.22] ng/mL; P, interaction <.0001) after adjustment for clinical factors. 25 The increases in 25(OH)D levels with 1 year of treatment were greater in women than in men (13.7 [SE 0.49] vs 11.7 [SE 0.51] ng/mL; P, interaction = .007) and in African Americans than in Whites (+15.7 [0.84] vs +12.0 [0.42] ng/mL; P, interaction <.0001). 25
The baseline plasma n-3 index was higher in African Americans than in non-Hispanic Whites (2.83% [SD 0.93] vs 2.58% [SD 0.90], P, interaction <.001). 26 Baseline fish intake was also higher in African Americans than in non-Hispanic Whites (dark-meat fish: 1.4 [SD 3.1] vs 0.9 [SD 1.1] servings per week, P, interaction <.001; white-meat fish: 1.6 [SD 4.4] vs 1.0 [SD 1.2] servings per week, P, interaction <.001). 26 Baseline n-3 index levels by sex and treatment-associated changes in fatty acid blood levels by sex and race/ethnicity have not been reported.
VITAL Results
Supplemental Vitamin D
Findings for primary, secondary, and selected other outcomes of vitamin D supplementation in the total study population are shown in Table 2. A summary of key subgroup findings is provided in Table 3.
Table 2.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) of Primary, Secondary, and Other Outcomes by Randomized Assignment to Vitamin Da,b.
| Endpoint | Vitamin D (N = 12 927) | Placebo (N = 12 944) | HR | 95% CI |
|---|---|---|---|---|
| No. of participants w/event | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventc,d | 396 | 409 | 0.97 | 0.85-1.12 |
| Expanded CVD event e | 536 | 558 | 0.96 | 0.86-1.08 |
| Total myocardial infarction (MI) | 169 | 176 | 0.96 | 0.78-1.19 |
| Total stroke | 141 | 149 | 0.95 | 0.76-1.20 |
| Cardiovascular mortality | 152 | 138 | 1.11 | 0.88-1.40 |
| Other vascular outcomes f | ||||
| Percutaneous coronary intervention (PCI) | 182 | 188 | 0.97 | 0.79-1.19 |
| Coronary artery bypass graft (CABG) | 73 | 98 | 0.75 | 0.55-1.01 |
| Fatal MI | 24 | 15 | 1.60 | 0.84-3.06 |
| Fatal stroke | 19 | 23 | 0.84 | 0.46-1.54 |
| Total invasive cancer c | 793 | 824 | 0.96 | 0.88-1.06 |
| Cancer mortality | 154 | 187 | 0.83 | 0.67-1.02 |
| All-cause mortality | 485 | 493 | 0.99 | 0.87-1.12 |
| Excluding the first 2 years of follow-up: | ||||
| Major CVD event | 274 | 296 | 0.93 | 0.79-1.09 |
| Total invasive cancer | 490 | 522 | 0.94 | 0.83-1.06 |
| Cancer mortality | 112 | 149 | 0.75 | 0.59-0.96 |
| All-cause mortality | 368 | 384 | 0.96 | 0.84-1.11 |
Adapted from Manson et al. 17 with permission. Copyright ©2019, Massachusetts Medical Society.
Analyses were from Cox regression models controlling for age, sex, and n-3 fatty acid randomization group. Analyses were not adjusted for multiple comparisons.
Primary outcome.
A composite of myocardial infarction, stroke, and cardiovascular mortality.
A composite of major cardiovascular events plus coronary revascularization (CABG + PCI).
Not prespecified as primary or secondary outcomes.
Table 3.
VITAL Vitamin D Intervention: Summary of Key Subgroup Findings.
| Total invasive cancer |
| • No significant interaction by sex |
| • No significant interaction by race/ethnicity (signal for greater benefit in African Americans) |
| • Significant interaction by body mass index (reduction in those with healthy weights but not in those with elevated body mass index) |
| Major cardiovascular disease events a |
| • No significant interaction by sex |
| • No significant interaction by race/ethnicity |
| All-cause mortality |
| • No significant interaction by sex |
| • No significant interaction by race/ethnicity |
| Other endpoints |
| • Colorectal adenoma |
| ° No significant interaction by sex or race/ethnicity |
| • Advanced colorectal adenoma |
| ° Significant interaction by baseline 25(OH)D level (greater benefit in those with levels below 30 ng/mL) |
| • Bone mineral density |
| ° No significant interaction by sex or race/ethnicity |
| ° Significant interaction by baseline free 25(OH)D level: (greater benefit in those with levels below the median) |
| • Chronic knee pain |
| ° No significant interaction by sex or race/ethnicity |
| • Falls |
| ° No significant interaction by sex or race/ethnicity |
| ° No significant interaction by body mass index (signal for greater benefit in those with healthy weights but not in those with elevated body mass index) |
| • Depression |
| ° No significant interaction by sex or race/ethnicity |
| ° No significant interaction by body mass index (signal for greater benefit in those with healthy weights but not in those with elevated body mass index) |
| • Chronic kidney disease in individuals with diabetes |
| ° No significant interaction by race/ethnicity |
A composite of myocardial infarction, stroke, and cardiovascular mortality.
Vitamin D and Cancer
Total cohort
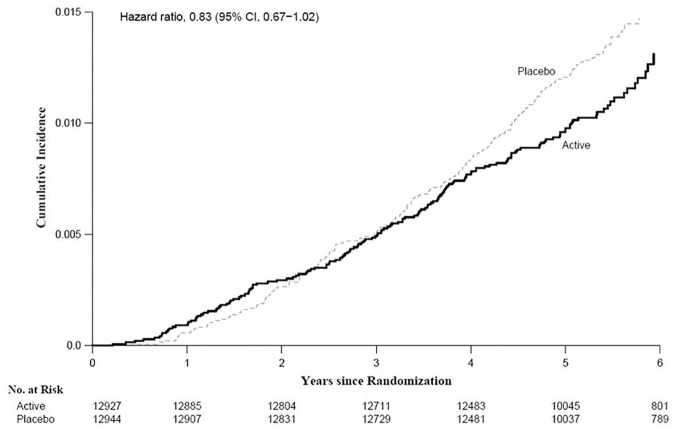
Supplemental vitamin D did not significantly reduce the primary endpoint of total invasive cancer incidence (hazard ratio [HR] = 0.96 [95% confidence interval 0.88-1.06]) but showed a promising signal for reduction in total cancer mortality (HR = 0.83 [0.67-1.02]), which was most apparent in analyses that excluded the first year (HR = 0.79 [0.63-0.99]) or first 2 years (HR = 0.75 [0.59-0.96]) of follow-up to account for latency. 17 The cumulative incidence curves for cancer mortality diverged starting at 4 years (Figure 2). The HRs for incidence of prespecified site-specific cancers were 1.02 (0.79-1.31) for breast cancer, 0.88 (0.72-1.07) for prostate cancer, and 1.09 (0.73-1.62) for colorectal cancer. In ancillary analyses, vitamin D was unrelated to incidence of colorectal adenoma or serrated colorectal polyps. 27 Preliminary analyses show that vitamin D was associated with a significant reduction in incidence of advanced-stage (metastatic or fatal) cancer (HR = 0.83 [0.69-0.99]; P = .036; unpublished data).
Figure 2.
Cumulative incidence rate of cancer mortality by year of follow-up: vitamin D versus placebo.
From Manson et al. 17 with permission. Copyright ©2019, Massachusetts Medical Society.
Sex and race/ethnicity subgroups
Vitamin D did not significantly reduce total cancer incidence in either men (HR = 0.93 [0.82-1.06]) or women (HR = 1.02 [0.87-1.18]) (P, interaction = .38). 17 For colorectal cancer incidence, there was no modification of the treatment effect by sex (unpublished data). African Americans assigned to vitamin D had a suggestive reduction in total cancer incidence (HR = 0.77 [0.59-1.01]), although the P value for interaction by race/ethnicity was not significant (non-Hispanic Whites: HR = 0.99 [0.89-1.11]; others: HR = 1.03 [0.70-1.51]; P, interaction = 0.21). 17 African Americans also had lower treatment-associated HRs for breast, prostate, and colorectal cancer than did non-Hispanic Whites, but, as for total cancer, the interactions were not significant (unpublished data). For colorectal adenoma or serrated polyps, treatment effects were not modified by sex or race. 27
Other subgroups
Individuals with normal BMI (<25 kg/m2) experienced a significant treatment-associated reduction in incidence of total cancer (HR = 0.76 [0.63-0.90]), but overweight or obese individuals did not (P, interaction = .002). 17 Compared with participants with baseline 25(OH)D serum levels of 30 ng/mL or greater, those with lower 25(OH)D levels had a greater treatment-associated reduction in conventional adenoma, particularly advanced adenoma (P, interaction of .07 and .04, respectively). 27 However, interactions by baseline 25(OH)D were not observed for total invasive cancer. 17
Vitamin D and CVD
Total cohort
Vitamin D did not reduce the co-primary endpoint of major CVD events (a composite of MI, stroke, and CVD mortality; HR = 0.97 [0.85-1.12]), nor did it reduce prespecified secondary cardiovascular endpoints, including an expanded composite of major CVD events plus coronary revascularization (HR = 0.96 [0.86-1.08]), or MI (HR = 0.96 [0.78-1.19]), stroke (HR = 0.95 [0.76-1.20]), and CVD mortality (HR = 1.11 [0.88-1.40]) considered individually. 17 Analyses of major CVD events that excluded the first year or 2 years of follow-up also yielded null findings. Vitamin D did not influence 1-year changes in lipids or inflammatory markers. 28 In ancillary analyses, there was no association between vitamin D and risk of first or recurrent hospitalization for heart failure. 29
Sex and race/ethnicity subgroups
Associations between supplemental vitamin D and risk of primary and secondary CVD endpoints did not significantly vary by sex or race/ethnicity; and vitamin D did not significantly reduce these outcomes in any sex or race/ethnicity subgroup. 17 For major CVD events, HRs in men and women were 1.01 (0.84-1.21) and 0.93 (0.76-1.14), respectively (P, interaction by sex = .57); and HRs in non-Hispanic Whites, Blacks, and members of other races/ethnicities were 0.93 (0.79-1.10), 0.91 (0.65-1.26), and 1.36 (0.80-2.31), respectively (P, interaction by race/ethnicity = .37).
Vitamin D and All-Cause Mortality
Vitamin D supplementation was unrelated to all-cause mortality (HR = 0.99 [0.87-1.12]) in analyses of the full cohort, in sex-stratified analyses, and in race/ethnicity-stratified analyses. 17 The HRs in men and women were 0.98 (0.83-1.16) and 1.00 (0.83-1.20), respectively (P, interaction by sex = .90; unpublished data); and the HRs also did not differ significantly by race/ethnicity (P, interaction = .56; unpublished data).
Vitamin D and Other Outcomes
Vitamin D supplementation did not significantly increase risk of hypercalcemia, kidney stones, or gastrointestinal symptoms. 17 In women, vitamin D was associated with a trend toward smaller decreases in bone mineral density at the spine over a 2-year interval (P = .062); a similar benefit was not seen in men (P, interaction by sex = .067). 30 Other analyses indicate that baseline levels of free 25(OH)D (but not total 25(OH)D) are useful for identifying those who derive bone mineral density benefit from supplemental vitamin D, 30 supporting the potential importance of this novel biomarker on treatment-associated outcomes. Vitamin D did not decrease risk of chronic knee pain, 31 falls, 32 or depression 33 in the total study population; neither sex nor race/ethnicity was a significant effect modifier. For the latter 2 outcomes, vitamin D tended to confer a stronger protective effect in individuals with normal BMI than in those with overweight or obesity (falls [defined as ≥2 falls]: P, interaction by BMI = .07; depression: P, interaction by BMI = .06). Among 1312 participants with type 2 diabetes, vitamin D did not prevent development or progression of chronic kidney disease (assessed by change in glomerular filtration rate estimated from serum creatinine and cystatin C from baseline to year 5), 34 with no difference in the treatment effect between non-Hispanic White and African-American participants.
Supplemental n-3 Fatty Acids
Findings for primary, secondary, and selected other outcomes of n-3 fatty acid supplementation in the total study population are shown in Table 4. A summary of key subgroup findings is provided in Table 5.
Table 4.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) of Primary, Secondary, and Other Outcomes by Randomized Assignment to n-3 Fatty Acids (n-3 FAs)a,b.
| Endpoint | n-3 FAs (N = 12 933) | Placebo (N = 12 938) | HR | 95% CI |
|---|---|---|---|---|
| No. of participants w/event | ||||
| Cardiovascular disease (CVD), primary and secondary outcomes | ||||
| Major CVD eventc,d | 386 | 419 | 0.92 | 0.80-1.06 |
| Expanded CVD event e | 527 | 567 | 0.93 | 0.82-1.04 |
| Total myocardial infarction (MI) | 145 | 200 | 0.72 | 0.59-0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83-1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76-1.21 |
| Other vascular outcomes f | ||||
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63-0.95 |
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73-1.33 |
| Fatal MI | 13 | 26 | 0.50 | 0.26-0.97 |
| Coronary heart disease (CHD) mortality | 37 | 49 | 0.76 | 0.49-1.16 |
| Total CHD g | 308 | 370 | 0.83 | 0.71-0.97 |
| Ischemic stroke | 111 | 116 | 0.96 | 0.74-1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72-2.39 |
| Fatal stroke | 22 | 20 | 1.10 | 0.60-2.01 |
| Total invasive cancer c | 820 | 797 | 1.03 | 0.93-1.13 |
| Cancer mortality | 168 | 173 | 0.97 | 0.79-1.20 |
| All-cause mortality | 493 | 485 | 1.02 | 0.90-1.15 |
| Excluding the first 2 years of follow-up: | ||||
| Cardiovascular outcomes | ||||
| Major CVD event | 269 | 301 | 0.89 | 0.76-1.05 |
| Total myocardial infarction | 94 | 131 | 0.72 | 0.55-0.93 |
| Total stroke | 103 | 112 | 0.92 | 0.70-1.20 |
| Total invasive cancer | 536 | 476 | 1.13 | 1.00-1.28 |
| Cancer mortality | 126 | 135 | 0.93 | 0.73-1.19 |
| All-cause mortality | 371 | 381 | 0.97 | 0.84-1.12 |
Adapted from Manson et al. 18 with permission. Copyright ©2019, Massachusetts Medical Society.
Analyses were from Cox regression models that were controlled for age, sex, and randomization group in the vitamin D portion of the trial. Analyses were not adjusted for multiple comparisons.
Primary outcomes.
A composite of MI, stroke, and cardiovascular mortality.
A composite of MI, stroke, cardiovascular mortality, and coronary revascularization (CABG, PCI).
Not prespecified as primary or secondary outcomes.
A composite of MI, coronary revascularization (CABG, PCI), and CHD death.
Table 5.
VITAL n-3 Fatty Acid Intervention: Summary of Key Subgroup Findings.
| Major cardiovascular disease events a |
| • No significant interaction by sex |
| • No significant interaction by race/ethnicity (signal for greater benefit in African Americans) |
| • Significant interaction by baseline dietary fish intake (reduction in those with below-median intake but not in those with above-median intake) |
| Myocardial infarction |
| • No significant interaction by sex |
| • Significant interaction by race/ethnicity (reduction in African Americans but not in members of other racial/ethnic groups) |
| • Significant interaction by baseline dietary fish intake (reduction in those with below-median intake but not in those with above-median intake) |
| • Significant interaction by traditional cardiovascular risk factors (reduction greatest in those with at least 2 risk factors) |
| Coronary revascularization |
| • Significant interaction by race/ethnicity (reduction in African Americans but not in members of other racial/ethnic groups) |
| Total coronary heart disease |
| • Significant interaction by race/ethnicity (reduction in African Americans but not in members of other racial/ethnic groups) |
| Total invasive cancer |
| • Significant interaction by sex (greater benefit in women) |
| • No significant interaction by race/ethnicity |
| • No significant interaction by baseline fish intake (signal for greater benefit in those with below-median intake) |
| All-cause mortality |
| • No significant interaction by sex |
| • No significant interaction by race/ethnicity |
| • Significant interaction by baseline dietary fish intake (trend toward reduction in those with below-median intake but not in those with above-median intake) |
| Other endpoints |
| • Colorectal adenoma |
| ° Significant interaction by race/ethnicity (reduction in African Americans but not members of other racial/ethnic groups) |
| ° Significant interaction by baseline plasma n-3 index (reduction in those with low baseline plasma n-3 index but not in those with high baseline plasma n-3 index) |
| • Chronic knee pain |
| ° No significant interaction by sex or race/ethnicity |
A composite of myocardial infarction, stroke, and cardiovascular mortality.
n-3 Fatty Acids and CVD
Total cohort
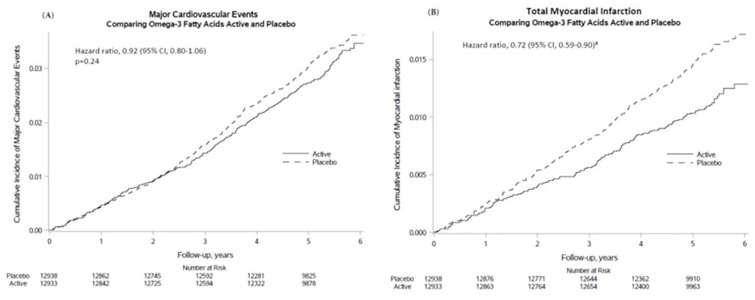
Supplemental n-3 fatty acids did not significantly reduce the primary endpoint of major CVD events (HR = 0.92 [0.80-1.06]) but did reduce total MI, a key secondary endpoint, by a significant 28% (HR = 0.72 [0.59-0.90]). 18 This benefit became apparent after the first year and lasted for the rest of the trial period (Figure 3). In addition, the intervention significantly reduced the exploratory endpoints of percutaneous coronary intervention (PCI; HR = 0.78 [0.63-0.95]), fatal MI (HR = 0.50 [0.26-0.97]), and total CHD (HR = 0.83 [0.71-0.97]). However, there were no significant reductions in risk of coronary artery bypass grafting (CABG), stroke, CVD mortality, or an expanded CVD endpoint (major CVD events plus coronary revascularization [PCI or CABG]). In analyses that excluded the first 2 years of follow-up, the HR for major CVD events strengthened slightly to 0.89 (0.76-1.05). In ancillary analyses, n-3 fatty acid supplementation was associated with a significant reduction in risk of recurrent (but not first) hospitalization for heart failure. 29 Among participants with 1-year follow-up blood samples, n-3 fatty acid supplementation was associated with a small but significant reduction in triglycerides but had no significant effect on other lipids (unpublished data) or on inflammatory markers. 28
Figure 3.
Cumulative incidence rates of major CVD events and total MI by year of follow-up, in the n-3 fatty acid group and the placebo group.
Adapted from Manson et al. 18 with permission. Copyright ©2019, Massachusetts Medical Society.
Sex and race/ethnicity subgroups
The effect of n-3 fatty acid supplementation on major CVD events and MI did not differ for men and women (major CVD events: men, HR = 0.91 [0.76-1.10]; women, HR = 0.93 [0.76-1.15]; P, interaction by sex = .88; MI: men, HR = 0.72 [0.55-0.95]; women, HR = 0.73 [0.52-1.03]; P, interaction by sex = .99). 18 Associations between n-3 fatty acids and risk of major CVD events also did not significantly vary by race/ethnicity (P, interaction = .26); and the intervention did not significantly reduce this outcome in any race/ethnicity subgroup, although there was a suggestive signal for a protective effect in African Americans (HR = 0.74 [0.53-1.03]). 18 For MI, African Americans experienced a significant 77% treatment-associated reduction (HR = 0.23 [0.11-0.47]), whereas other racial/ethnic groups had smaller reductions (non-Hispanic Whites, HR = 0.93 [0.73-1.18]; others: HR = 0.54 [0.23-1.26]; P, interaction by race/ethnicity = .001). Of note, African Americans derived this treatment benefit irrespective of their baseline dietary fish intake, whereas non-Hispanic Whites benefitted only when their fish intake was low. In African Americans, treatment-associated HRs among those with low fish intake (below the cohort median of 1½ servings/week) and those with higher fish intake were 0.23 (0.09-0.62) and 0.21 (0.06-0.74), respectively (P, interaction = .92). In non-Hispanic Whites, the corresponding HRs were 0.71 (0.51-0.98) and 1.32 (0.90-1.95) (P, interaction = .016; see also next paragraph). In addition, the treatment benefit on MI for African Americans did not diminish after adjustment for cardiovascular risk factors (HR = 0.19 [0.07-0.50]) and was more apparent than in non-Hispanic Whites across all cardiovascular risk-factor strata. African Americans also had significant treatment-associated reductions in coronary revascularization (HR = 0.51 [0.28-0.92]) and total CHD (HR = 0.61 [0.43-0.88]), whereas non-Hispanic Whites did not (HR = 1.00 [0.82-1.21], P, interaction = .001, and HR = 1.00 [0.85-1.17], P, interaction = .004, respectively).
Other subgroups
Baseline dietary fish intake significantly influenced the effect of n-3 fatty acid supplementation on major CVD events (P, interaction = .045); it also modified the intervention’s effect on MI (P = .048) and on levels of the inflammatory marker C-reactive protein (P = .06).18,28 Supplemental n-3 fatty acids led to a 19% reduction in major CVD events (HR = 0.81 [0.67-0.98]) and a 40% reduction in MI (HR = 0.60 [0.45-0.81]) among individuals with low fish intake (below the cohort median) but not among those with higher fish intake (major CVD events: HR = 1.08 [0.88-1.32]; MI: HR = 0.94 [0.67-1.31]). 18 Consistent with this finding, treatment-associated reductions in C-reactive protein were greater in those with low fish intake than in those with high fish intake. 28
Individuals with traditional cardiovascular risk factors were more likely to derive an MI benefit from n-3 fatty acids than those without risk factors. 18 Treatment-associated HRs for those with at least 2 risk factors, with 1 risk factor, and with no risk factors were 0.57 (0.41-0.81), 0.75 (0.53-1.08), and 1.01 (0.65-1.56), respectively (P, interaction = .047).
n-3 Fatty Acids and Cancer
Total cohort
Supplemental n-3 fatty acids did not significantly predict incidence of total cancer (HR = 1.03 [0.93-1.13]); breast (HR = 0.90 [0.70-1.16]), prostate (HR = 1.15 [0.94-1.39]), or colorectal cancer (HR = 1.23 [0.83-1.83]); or cancer mortality (HR = 0.97 [0.79-1.20]) in analyses of the full trial period. 18 A signal for increased cancer incidence (HR = 1.13 [1.00-1.28]), though not for cancer mortality (HR = 0.93 [0.73-1.19]), was seen in analyses that excluded the first 2 years of follow-up. In ancillary analyses, n-3 fatty acid supplementation was unrelated to incidence of colorectal adenoma or serrated colorectal polyps. 26
Sex and race/ethnicity subgroups
The effect of n-3 fatty acid supplementation on total cancer incidence was more favorable in women (HR = 0.90 [0.78-1.05]) than in men (HR = 1.13 [1.00-1.29]) (P, interaction by sex = .02), likely because of the combination of (nonsignificant) trends for risk reduction for breast cancer and risk elevation for prostate cancer. 18 The effect of n-3 fatty acid supplementation on total cancer incidence did not differ in non-Hispanic Whites (HR = 1.02 (0.91-1.13) and African Americans (HR = 1.02 [0.79-1.33]) (P, interaction = .86). 18 Ancillary analyses show significant inverse associations between n-3 fatty acid supplementation and colorectal adenoma in African Americans (OR = 0.59 [0.35-1.00]) but not in members of other racial/ethnic groups (non-Hispanic Whites: OR = 1.06 [0.89-1.28]; others: OR = 0.86 [0.48-1.52]) (P, interaction = .11). 26
Other subgroups
Baseline fish intake tended to modify the effect of supplemental n-3 fatty acids on cancer, with more favorable effects in those with low fish intake (HR = 0.96 [0.84-1.09]) than in those with high fish intake (HR = 1.13 [0.98-1.31]; P, interaction = .09). 18 In ancillary analyses, there was an inverse association between the intervention and colorectal adenoma in those with low baseline plasma n-3 index (below the cohort median of 2.5%; OR = 0.76 [0.57-1.02]) but not in those with a higher n-3 index (OR = 1.19 [0.90-1.56]; P, interaction = 0.03). 26
n-3 Fatty Acids and All-Cause Mortality
Supplemental n-3 fatty acids were not associated with all-cause mortality in analyses of the full trial period (HR = 1.02 [0.90-1.15]) or in analyses that excluded the first 2 years of follow-up (HR = 0.97 [0.84-1.12]). 18 Effects tended to be more favorable in women (HR = 0.92 [0.76-1.11]) than in men (HR = 1.11 [0.93-1.31]), and in African Americans (HR = 0.84 [0.64-1.11]) than in non-Hispanic Whites (HR = 1.09 [0.92-1.26]), although neither the sex nor the race/ethnicity interaction was significant (P, interaction by sex = .15; P, interaction by race/ethnicity = .26). 18 Baseline fish intake modified the effect of n-3 fatty acid supplementation on all-cause mortality (P, interaction = .02). 18 The intervention was associated with a trend toward a mortality reduction in those with low fish intake (HR = 0.87 [0.73-1.04]) but not in those with higher fish intake (HR = 1.19 [0.99-1.44]).
n-3 Fatty Acids and Other Outcomes
With regard to side effects, the n-3 fatty acid intervention was well tolerated, with no treatment-associated increase in bleeding or gastrointestinal symptoms. 18 The intervention did not reduce chronic knee pain in the total study population, and neither sex nor race/ethnicity was a significant effect modifier. 31 Among participants with type 2 diabetes, the intervention did not prevent development or progression of chronic kidney disease. 34
Discussion
Supplemental Vitamin D
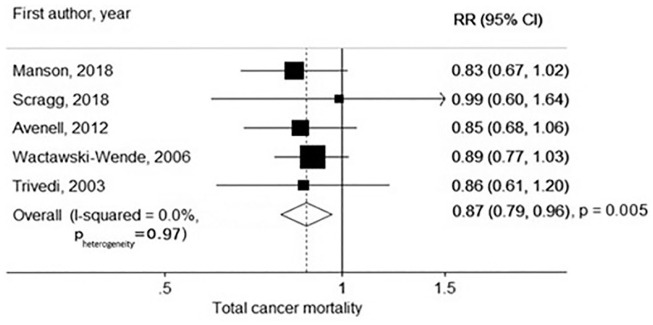
VITAL is the first large (N ≥ 10,000) trial of moderate- or high-dose vitamin D for cancer prevention. Despite methodologic limitations, several35-39 (though not all 40 ) earlier trials had also suggested stronger benefits for cancer mortality than for cancer incidence. 41 In a 2019 meta-analysis of randomized trials that included VITAL, treatment-associated relative risks for cancer mortality (5 trials, 1591 cancer deaths) and cancer incidence (10 trials, 6537 incident cancers) were 0.87 (0.79-0.96) and 0.98 (0.93-1.03), respectively (Figure 4). 42 Of note, the reduction in cancer mortality was largely attributable to interventions with daily rather than intermittent bolus dosing. The latter may lead to nonphysiological fluctuations in vitamin D blood levels. 43 Sex and race were not analyzed as modifiers of vitamin D treatment effects in this meta-analysis.
Figure 4.
Meta-analysis of randomized trials of vitamin D supplementation and cancer mortality.
Source: From Keum et al. 42 with permission. Copyright ©2019, Oxford University Press.
To understand VITAL’s promising signal of a treatment-associated cancer reduction in African Americans, elucidating the role of genetic factors may be useful. People with African ancestry differ from those with European ancestry with regard to variation in GC,44,45 a gene that encodes vitamin D binding protein, which is the major transporter of circulating vitamin D. Variation in GC; VDR, which encodes vitamin D receptor, a major determinant of the body’s responsiveness to vitamin D; and other genes involved in vitamin D synthesis, transport, and metabolism46,47 may combine with supplemental vitamin D to influence the development of cancer, CVD, and other outcomes. Although these potential interactions have rarely been studied in the setting of a randomized trial, data from the Vitamin D/Calcium Polyp Prevention Study highlight the potential utility of such investigations. 48 In that trial, 2259 participants were randomized to vitamin D, calcium, both, or placebo for prevention of recurrent colorectal adenoma. Vitamin D supplementation was unrelated to the risk of advanced colorectal adenoma in the overall study population. However, analyses stratified by VDR variation found a 64% treatment-associated reduction in risk among participants with the rs7968585 AA genotype and a 41% increase in risk among those with 1 or 2 G alleles (P, interaction <.001).
Regarding VITAL’s finding of significant modification by BMI of the treatment effect on incident cancer, there is evidence suggesting that obesity affects vitamin D bioactivity. Excess body weight correlates with lower levels of free and total 25(OH)D; 49 may disproportionately reduce the former; 50 and may influence the correlation between the 2 markers. 51 In mice, diet-induced obesity decreased free but not total 25(OH)D, and increased expression of CYP2R1, a key vitamin D–related gene. 50 Higher levels of free 25(OH)D were associated with reduced cardiovascular and all-cause mortality in CHD patients with normal weight but not in their heavier counterparts. 52 Randomized trials of vitamin D have found more favorable treatment effects on blood pressure 53 and incident diabetes 54 in individuals with normal BMI than in those who were overweight or obese. Considered together, these data lend credence to VITAL’s finding of an interaction between treatment and BMI on incident cancer.
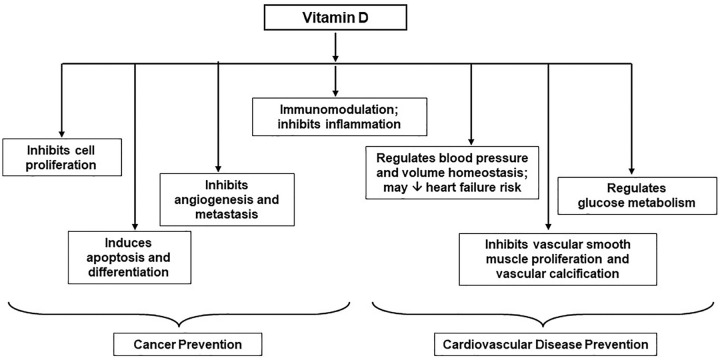
Mechanisms by which vitamin D may reduce cancer incidence and/or cancer mortality are shown in Figure 5. Laboratory data suggest that 1,25(OH)2D, the active vitamin D hormone produced from 25(OH)D, decreases tumor invasiveness, angiogenesis, and metastatic propensity.55,56 In addition, observational studies show that higher 25(OH)D levels at cancer diagnosis predict longer survival in patients.57-63 In some64-74 but not all75-79 studies, higher prediagnostic 25(OH)D or genetically determined vitamin D levels in initially cancer-free cohorts are favorably correlated with cancer mortality and/or survival following diagnosis. Two recent trials in patients with gastrointestinal cancer suggested that high-dose vitamin D may reduce disease progression and death.80,81 However, not all vitamin D trials in cancer patients have shown benefits,82,83 and supplementation may be more effective if initiated in the preclinical stage or earlier, as was done in VITAL.
Figure 5.
Mechanisms by which vitamin D may lower cancer and cardiovascular disease risk.
Adapted from Manson et al. 7 with permission. Copyright ©2012, Elsevier.
Regarding CVD, VITAL is the first large trial of moderate- or high-dose vitamin D for prevention of this outcome. Although VITAL was designed to overcome methodologic limitations of earlier trials, its null cardiovascular findings are consistent with the results of these trials,38,39,84-89 including those that tested moderate or high doses.87,89 A 2019 meta-analysis of vitamin D trials that included VITAL found that supplementation did not reduce risk of major adverse cardiovascular events (10 trials, 6243 events, 79 111 participants; relative risk [RR] = 1.00 [0.95-1.06]), MI (18 trials, 2550 events, 82 576 participants; RR = 1.00 [0.93-1.08]), stroke (15 trials, 2354 events, 82 239 participants; RR = 1.06 [0.98-1.15]), or cardiovascular mortality (10 trials, 2202 events, 76 783 participants; RR = 0.98 [0.90-1.07]). 90 There was little evidence of effect modification by sex, baseline 25(OH)D level, vitamin D dose or administration frequency, or concurrent randomization to supplemental calcium. Race was not analyzed as an effect modifier.
Mechanisms by which vitamin D may prevent CVD are shown in Figure 5. Laboratory and animal data suggest that 1,25(OH)2D inhibits vascular smooth muscle cell proliferation and vascular calcification, favorably affects volume homeostasis and blood pressure via regulation of the renin-angiotensin-aldosterone system, reduces inflammation, and improves insulin sensitivity.7,91-93 In prospective observational studies, 25(OH)D levels are inversely correlated with cardiovascular risk factors and CVD events.94-96 However, small, short-term trials have generally failed to show that vitamin D improves intermediate endpoints, including blood pressure, glucose or insulin homeostasis, inflammation, vascular function parameters, and lipids. 19 In VITAL, as noted earlier, vitamin D did not improve lipids or other CVD-related biomarkers assessed after one year.
In VITAL, the lack of cardiovascular benefit among African American and normal-weight participants contrasts with the suggestive and significant findings, respectively, for cancer reduction in these groups. The contrast may stem from the fact that vitamin D requirements for CVD and cancer prevention may be dissimilar. In observational studies, individuals with 25(OH)D levels of 20 to 25 ng/mL tend to have the lowest CVD risk, 94 whereas those with levels above 30 ng/mL tend to have the lowest risk of cancer (at least colorectal cancer). 97 Thus, many participants in VITAL (and other recent trials) may have entered the study with their vitamin D requirements for cardiovascular health already met. Although analyses restricted to participants with low baseline 25(OH)D indicated no CVD benefit in VITAL, it is possible that a trial among individuals with 25(OH)D levels well below the 20 ng/mL recommended for bone health 1 would show a greater risk reduction. However, targeting those with known vitamin D deficiency to participate in a long-term trial with a 50% chance of randomization to placebo would not be ethical.
Regarding all-cause mortality, the VITAL findings of no association between vitamin D and this outcome are in agreement with the results of previous trials, which have found no or at most modest reductions.89,98-100 In a 2019 meta-analysis of 20 vitamin D trials (6502 deaths among 83 059 participants) that included VITAL, the treatment-associated relative risk for all-cause mortality was 0.97 (0.93-1.02). 90 Longer follow-up may be necessary to detect a benefit, however.
Because VITAL tested only one vitamin D dose and administration frequency, the efficacy of other doses or dosing schedules could not be assessed. Ongoing vitamin D trials 101 may help address these questions. D-Health 102 —the only other large (N ≥ 10 000) trial of moderate- or high-dose vitamin D besides VITAL—is testing a bolus vitamin D dose of 60 000 IU/month for 5 years in 21 315 Australian men and women aged 65 to 84 years; the primary endpoints are cancer and all-cause mortality, but CVD will also be examined. Results are expected in 2021. Of note, D-Health includes few if any Black participants, precluding an examination of treatment effects in this group.
Supplemental n-3 Fatty Acids
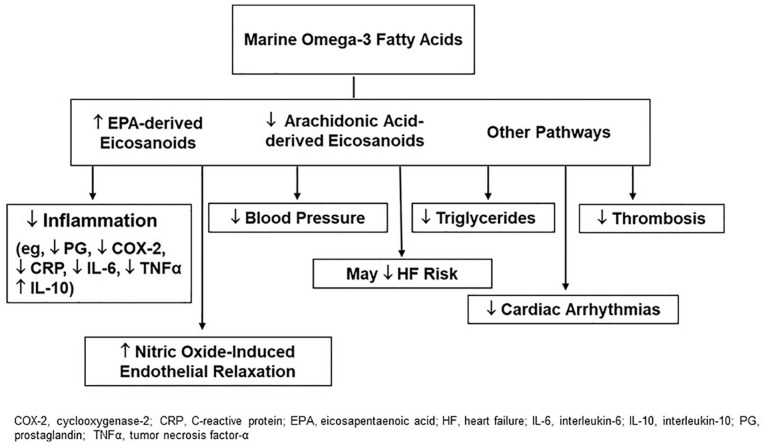
VITAL’s finding that supplemental n-3 fatty acids confer significant coronary benefits is in agreement with data from laboratory and animal studies, and small trials of intermediate cardiovascular endpoints in humans, which suggest that n-3 fatty acids reduce inflammation, thrombosis, triglycerides, blood pressure, heart rate, and atherosclerotic plaque progression; and increase nitric-oxide induced endothelial relaxation (Figure 6).7,103 Experimental studies indicate relevant molecular and gene-regulatory effects. 103 Dose-response curves for some effects plateau at n-3 fatty acid doses of 1 g/day or less. 104 In observational studies, fish intake, 105 EPA + DHA intake from food or supplements,106,107 and n-3 fatty acid biomarkers106,108 are inversely associated with coronary outcomes. However, the marked treatment benefit on MI and PCI in VITAL—the only large trial of n-3 fatty acids for primary prevention of CVD in a usual-risk population—contrasts with the findings of meta-analyses of earlier n-3 fatty acid trials in patients with or at high risk for CVD,109-111 which indicate at most a weak preventive effect on coronary death but not on other heart-related outcomes. Of note, neither VITAL nor earlier n-3 fatty acid trials found a benefit for stroke. Results from three recent trials in high-risk populations have been mixed. ASCEND, which tested the same EPA-DHA dose as in VITAL for 7.4 years in 15 480 UK adults with diabetes, reported nonsignificant results for major CVD events but a significant reduction in vascular death. 112 REDUCE-IT, which tested 4.9 years of high-dose synthetic EPA (icosapent ethyl [4 g/day]) in 8179 statin users with elevated triglycerides and at high CVD risk, found significant reductions in major CVD events (26%), and MI, stroke, and CVD death (20% to 31%). 113 Most recently, STRENGTH, which tested a high-dose intervention (n-3 carboxylic acids [4 g/day], EPA-DHA ratio of 2.75:1) in 13 086 statin users with hypertriglyceridemia, low high-density lipoprotein cholesterol, and either established atherosclerotic disease or elevated CVD risk, 114 was stopped earlier than its planned 3- to 5-year duration because preliminary trial data suggested a “low likelihood” of CVD benefit (detailed results have not yet been reported). 115 Potential reasons 18 for a greater magnitude of coronary reduction in VITAL than in the collective trials of patients with or at high risk for CVD include attenuation of incremental n-3 fatty acid benefit by CVD medications in secondary prevention settings; more advanced atherosclerotic disease in high-risk individuals, requiring more powerful interventions than n-3 fatty acids (or, as suggested by REDUCE-IT though not STRENGTH, higher n-3 fatty acid doses) to forestall clinical events; differences in dietary fish intake (few trials have examined this variable); and differences in race/ethnicity (few trials have enrolled a sizeable number of Black participants, a group with the greatest benefits in VITAL). A 2019 meta-analysis of n-3 fatty acid trials (13 trials, 127 477 participants) that included VITAL and ASCEND data reported modest but statistically significant reductions for MI, CHD death, total CHD, CVD death, and total CVD. 116 Inclusion of the REDUCE-IT findings magnified these reductions, 116 whereas inclusion of the STRENGTH findings would presumably have the opposite effect. However, results of meta-analyses that combine VITAL with trials in higher-risk cohorts may not be relevant for recommendations regarding n-3 fatty acid supplementation in usual-risk populations. Moreover, such results obscure VITAL’s promising findings in African Americans, which are of interest because of the potential for ameliorating health disparities.
Figure 6.
Mechanisms by which marine omega-3 fatty acids may lower cardiovascular disease risk.
Adapted from Manson et al. 7 with permission. Copyright ©2012, Elsevier.
That African Americans, who entered the trial with a higher plasma n-3 index and fish intake than non-Hispanic Whites, were more likely than the latter group to experience coronary benefit with n-3 fatty acid supplementation was an unexpected result that requires replication in future trials. Trials of n-3 fatty acids for CVD prevention have, with few exceptions, 12 been conducted in White populations, precluding an assessment of treatment effects by race. 110 However, REDUCE-IT, with approximately 800 non-White participants (the number of Black participants was not reported), also found a trend toward a stronger protective effect of supplementation on major CVD events in non-Whites (HR = 0.55 [0.38-0.82]) than in Whites (HR = 0.76 [0.67-0.86]); P, interaction = .13). 113 In addition, a recent pooling project of 19 observational cohorts from 16 countries reported racial differences in associations of marine- and plant-derived n-3 fatty acid biomarkers with incident coronary disease, including a significantly stronger inverse relationship between α-linolenic acid and nonfatal MI in Blacks than Whites. 108 Observational studies also suggest that genetic variation in genes related to fatty acid metabolism, including the fatty acid desaturase genes FADS1 and FADS2, the 5-lipooxygenase gene ALOX5, the 5-lipoxygenase activating protein gene ALOX5AP, and the cyclooxygenase COX-2 gene, may interact with dietary fatty acid intakes to influence risk of CHD and other health outcomes,117-119 and that people with African ancestry and those with European ancestry differ with respect to FADS variation.119-121 Elucidating the role of genetic factors may help clarify the treatment benefit for MI in African American participants. In addition, although stronger treatment benefits in African Americans than in non-Hispanic Whites persisted after accounting for traditional cardiovascular risk factors, it is possible that racial differences in other clinical, dietary, or socioenvironmental factors may help explain this finding. For example, n-3 fatty acids may ameliorate the adverse impact of air pollution, 122 an exposure that disproportionately affects African Americans 123 and raises cardiovascular risk. 124
The lack of sex differences in the effect of supplemental n-3 fatty acids on cardiovascular outcomes in VITAL is consistent with findings of meta-analyses of earlier n-3 fatty acid trials, 110 ASCEND, 112 and REDUCE-IT. 113
For cancer, the VITAL findings agree with those from n-3 fatty acid trials for secondary prevention of CVD, which indicate neutral effects or slight (but nonsignificant) elevations in cancer incidence110,112,125 and neutral effects or borderline significant reductions in cancer mortality.13,126 In contrast to the VITAL finding of greater benefit for n-3 fatty acid supplementation on cancer incidence in women than in men, a 2014 meta-analysis of 11 trials of n-3 fatty acids (1039 cancers, 39 122 participants) found no evidence of effect modification by sex. 125 For all-cause mortality, the absence of benefit in VITAL aligns with results of meta-analyses of earlier trials,109,111 ASCEND, 112 and REDUCE-IT. 113 A 2019 meta-analysis of 41 n-3 fatty acid trials (10 707 deaths, 134 034 participants) that included VITAL and ASCEND reported a RR of 0.98 (0.93-1.02) for this endpoint, 127 although extended follow-up may be required to detect a reduction. This meta-analysis did not report on sex as a potential effect modifier. However, in contrast to VITAL’s finding suggestive of a more favorable effect on all-cause mortality in women than men, a 2014 meta-analysis of 17 trials (7025 deaths, 68 705 participants) reported that n-3 fatty acid supplementation was associated with a reduction in this endpoint only in trials where the proportion of men exceeded 80%. 125 Thus, taken in aggregate, evidence for a sex difference in supplemental n-3 fatty acids on incidence of total cancer and all-cause mortality is mixed. The VITAL results of greater benefit in women than in men for these outcomes may be chance findings. Additional research is warranted.
Because only one n-3 fatty acid dose and formulation was tested in VITAL, the trial could not assess whether the effectiveness of supplementation varies according to these parameters. However, the tested dose is that recommended by the American Heart Association for cardioprotection in individuals with prior CHD2,3 and, based on fish consumption (1-2 servings/week), is at least twice the dose recommended by this organization for cardiovascular risk reduction in healthy persons.3,105 That coronary benefits of supplementation were limited to participants with low fish intake (in non-Hispanic White individuals and in the total cohort) suggests that further benefits may not accrue beyond a threshold dose. Indeed, the 2 recent trials testing a higher-dose formulation in patients with CVD or at high risk for it (REDUCE-IT and STRENGTH) found conflicting results, suggesting that the formulation of n-3 fatty acids may be more important and relevant than the dose. However, the generalizability of these findings to usual-risk populations is unknown. Additional trials of higher doses and other formulations of n-3 fatty acids in primary prevention populations are needed.
Benefit-Risk Balance, Postintervention Follow-up
In VITAL, results of ancillary investigations of mammographic density, telomere biology, diabetes, hypertension, atrial fibrillation, and cardiac structure/function (2D-echocardiograms) will soon provide a fuller picture of cancer- and cardiovascular-related effects of vitamin D and n-3 fatty acid supplementation. Additionally, forthcoming results of ancillary studies of other outcomes, including fractures, cognition, infections, pulmonary conditions, autoimmune disorders, and macular degeneration, will allow an assessment of the overall balance of benefits and risks of supplementation. Ongoing postintervention follow-up of the cohort will enable the capture of any latent and long-term treatment effects and will increase statistical power, an especially important consideration for analyses of secondary endpoints and effects within subgroups, including sex and race/ethnicity subgroups.
Overall Conclusions
In VITAL, daily high-dose vitamin D did not significantly reduce the trial’s co-primary endpoints of total invasive cancer or major CVD events but showed a promising signal for reducing cancer death. Supplemental n-3 fatty acids led to a small but statistically nonsignificant reduction in a composite endpoint of major CVD events, a significant 28% reduction in total MI, and reductions in other coronary outcomes, but no reduction in stroke or cardiovascular deaths not related to heart disease, or total invasive cancer. Race-stratified analyses revealed a promising signal for a vitamin D–associated reduction in total cancer incidence as well as a significant n-3 fatty acid–associated reduction in MI in African Americans. Sex-stratified analyses found a significantly more favorable effect of n-3 fatty acids on total cancer incidence and a trend toward a more favorable effect on all-cause mortality in women than in men. Regarding analyses stratified by other characteristics, there was variation in the effect of vitamin D on cancer, falls, and depression by BMI; the effect of n-3 fatty acids on multiple outcomes, including major CVD events, MI cancer, and all-cause mortality, by baseline dietary fish intake; and the effect of n-3 fatty acids on MI by traditional cardiovascular risk factors. These findings suggest that consideration of sex, race, and other participant characteristics is warranted in future analyses (including meta-analyses) of trials of vitamin D and n-3 fatty acids to ensure that important effects are not missed and to help provide a foundation for developing targeted recommendations for supplement use. Additional investigation is necessary to determine which individuals may be most likely to derive a net benefit from supplementation.
Supplemental Material
Supplemental material, sj-pdf-1-ajl-10.1177_1559827620972035 for The VITamin D and OmegA-3 TriaL (VITAL): Do Results Differ by Sex or Race/Ethnicity? by Shari S. Bassuk, Paulette D. Chandler, Julie E. Buring and JoAnn E. Manson in American Journal of Lifestyle Medicine
Acknowledgments
The authors thank the VITAL investigators, staff, and study participants for their dedication and commitment to the trial. A list of VITAL Research Group members is in the Supplemental Materials.
Footnotes
Authors’ Note: Data will be made available two years after publication of this manuscript. Please contact the corresponding author Dr. JoAnn Manson for details.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Shari S. Bassuk: VITAL receives grant support from the National Institutes of Health, which helps support my salary. Paulette D. Chandler: VITAL receives grant support from the National Institutes of Health, which helps support my salary. Julie E. Buring: VITAL receives grant support from the National Institutes of Health, which helps support my salary. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. My spouse serves on the Scientific Advisory Committee of Pharmavite. JoAnn E. Manson: VITAL receives grant support from the National Institutes of Health, which helps support my salary.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: VITAL is an investigator-initiated trial supported by Grants U01 CA138962 and R01 CA138962, which include support from the National Cancer Institute; National Heart; Lung and Blood Institute; Office of Dietary Supplements; National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others.
The NIH sponsors of VITAL had a role in the design and conduct of the study and interpretation of the data. Final decisions concerning the above, however, as well as data collection, management, analysis, manuscript review or approval, and decision to submit the manuscript for publication resided with VITAL investigators and the VITAL research group. The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/National Institutes of Health.
Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured the serum 25(OH)D levels and plasma n-3 index at no cost to the study.
Ethical Approval: VITAL was approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital. The study agents received Investigational New Drug Approval from the US Food and Drug Administration.
Informed Consent: All participants provided written informed consent prior to study enrollment.
Trial Registration: VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association, Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747-2757. [DOI] [PubMed] [Google Scholar]
- 3.Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135:e867-e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stipp D. Fish-oil doses can be hard to swallow. Wall Street Journal. Published January 8, 2008. Accessed October 22, 2020. https://www.wsj.com/articles/SB119975627038373627#:~:text=%22The%20kind%20of%20benefits%20seen,expert%20on%20omega%2D3's%20activity
- 5.Greider K. Has vitamin D been oversold? AARP Bulletin. Published July 5, 2012. Accessed May 22, 2020. http://www.aarp.org/health/drugs-supplements/info-07-2012/how-much-vitamin-d-is-enough.1.html
- 6.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337:1476-1478. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313:1311-1312. [DOI] [PubMed] [Google Scholar]
- 9.Moyer VA; US Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564. [DOI] [PubMed] [Google Scholar]
- 10.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126-1129. [DOI] [PubMed] [Google Scholar]
- 11.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447-455. [PubMed] [Google Scholar]
- 12.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090-1098. [DOI] [PubMed] [Google Scholar]
- 13.Tavazzi L, Maggioni AP, Marchioli R, et al. ; GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223-1230. [DOI] [PubMed] [Google Scholar]
- 14.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015-2026. [DOI] [PubMed] [Google Scholar]
- 15.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S; SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch B, Schiele R, Schneider S, et al. ; OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152-2159. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Bassuk SS, Cook NR, et al. ; VITAL Research Group. Vitamin D, marine n-3 fatty acids, and primary prevention of cardiovascular disease: current evidence. Circ Res. 2020;126:112-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD-O). 3rd ed. World Health Organization; 2000. [Google Scholar]
- 22.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41. [DOI] [PubMed] [Google Scholar]
- 24.Harris WS, Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212-220. [DOI] [PubMed] [Google Scholar]
- 25.Luttmann-Gibson H, Mora S, Camargo CA, et al. Serum 25-hydroxyvitamin D in the VITamin D and OmegA-3 TriaL (VITAL): clinical and demographic characteristics associated with baseline and change with randomized vitamin D treatment. Contemp Clin Trials. 2019;87:105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song M, Lee IM, Manson JE, et al. ; VITAL Research Group. Effect of supplementation with marine omega-3 fatty acid on risk of colorectal adenomas and serrated polyps in the US general population: a prespecified ancillary study of a randomized clinical trial. JAMA Oncol. 2019;6:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Lee IM, Manson JE, et al. No association between vitamin D supplementation and risk of colorectal adenomas or serrated polyps in a randomized trial. Clin Gastroenterol Hepatol. Published online February 13, 2020. doi: 10.1016/j.cgh.2020.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costenbader KH, MacFarlane LA, Lee IM, et al. Effects of one year of vitamin D and marine omega-3 fatty acid supplementation on biomarkers of systemic inflammation in older US adults. Clin Chem. 2019;65:1508-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djoussé L, Cook NR, Kim E, et al. ; VITAL Research Group. Supplementation with vitamin D and omega-3 fatty acids and incidence of heart failure hospitalization: VITAL-Heart Failure. Circulation. 2020;141:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeBoff MS, Chou SH, Murata EM, et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res. 2020;35:883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacFarlane LA, Cook NR, Kim E, et al. The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol. Published online June 25, 2020. doi: 10.1002/art.41416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeBoff MS, Murata EM, Cook NR, et al. VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020;105:2929-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okereke OI, Reynolds CF, 3rd, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer IH, Zelnick LR, Ruzinski J, et al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2019;322:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684-696. [DOI] [PubMed] [Google Scholar]
- 36.LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunner RL, Wactawski-Wende J, Caan BJ, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63:827-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avenell A, MacLennan GS, Jenkinson DJ, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97:614-622. [DOI] [PubMed] [Google Scholar]
- 39.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scragg R, Khaw KT, Toop L, et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the Vitamin D Assessment randomized clinical trial. JAMA Oncol. 2018;4:e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. 2019;30:733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollis BW, Wagner CL. The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30:445-456. [DOI] [PubMed] [Google Scholar]
- 45.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014;2014:981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39:28-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Kiel DP, Kraft P. The genetics of vitamin D. Bone. 2019;126:59-77. [DOI] [PubMed] [Google Scholar]
- 48.Barry EL, Peacock JL, Rees JR, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. 2017;3:628-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh JS, Evans AL, Bowles S, et al. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016;103:1465-1471. [DOI] [PubMed] [Google Scholar]
- 50.Bonnet L, Hachemi MA, Karkeni E, et al. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J Steroid Biochem Mol Biol. 2019;185:39-46. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz JB, Gallagher JC, Jorde R, et al. Determination of free 25(OH)D concentrations and their relationships to total 25(OH)D in multiple clinical populations. J Clin Endocrinol Metab. 2018;103:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu C, Xue H, Wang L, et al. Serum bioavailable and free 25-hydroxyvitamin D levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ Res. 2018;123:996-1007. [DOI] [PubMed] [Google Scholar]
- 53.Golzarand M, Shab-Bidar S, Koochakpoor G, Speakman JR, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: an updated meta-analysis. Nutr Metab Cardiovasc Dis. 2016;26:663-673. [DOI] [PubMed] [Google Scholar]
- 54.Pittas AG, Dawson-Hughes B, Sheehan P, et al. ; D2d Research Group. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700. [DOI] [PubMed] [Google Scholar]
- 56.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-357. [DOI] [PubMed] [Google Scholar]
- 57.Li M, Chen P, Li J, Chu R, Xie D, Wang H. Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:2327-2336. [DOI] [PubMed] [Google Scholar]
- 58.Vaughan-Shaw PG, O’Sullivan F, Farrington SM, et al. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116:1092-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maalmi H, Walter V, Jansen L, et al. Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients. 2018;10:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Yuan X, Tao J, Yu N, Wu R, Zhang Y. Association of circulating 25-hydroxyvitamin D levels with colorectal cancer: an updated meta-analysis. J Nutr Sci Vitaminol (Tokyo). 2018;64:432-444. [DOI] [PubMed] [Google Scholar]
- 61.Hu K, Callen DF, Li J, Zheng H. Circulating vitamin D and overall survival in breast cancer patients: a dose-response meta-analysis of cohort studies. Integr Cancer Ther. 2018;17:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song ZY, Yao Q, Zhuo Z, Ma Z, Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect. 2018;7:R294-R303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Li G, He X, et al. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35:1999-2005. [DOI] [PubMed] [Google Scholar]
- 64.Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013;57:753-764. [DOI] [PubMed] [Google Scholar]
- 65.Chowdhury R, Kunutsor S, Vitezova A, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984-2991. [DOI] [PubMed] [Google Scholar]
- 68.Fedirko V, Riboli E, Tjonneland A, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21:582-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110:2772-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shui IM, Mucci LA, Kraft P, et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104:690-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium in relation to survival following prostate cancer. Cancer Causes Control. 2016;27:669-677. [DOI] [PubMed] [Google Scholar]
- 72.Dudenkov DV, Mara KC, Petterson TM, Maxson JA, Thacher TD. Serum 25-hydroxyvitamin D values and risk of all-cause and cause-specific mortality: a population-based cohort study. Mayo Clin Proc. 2018;93:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinstein SJ, Mondul AM, Yu K, et al. Circulating 25-hydroxyvitamin D up to 3 decades prior to diagnosis in relation to overall and organ-specific cancer survival. Eur J Epidemiol. 2018;33:1087-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torfadottir JE, Aspelund T, Valdimarsdottir UA, et al. Pre-diagnostic 25-hydroxyvitamin D levels and survival in cancer patients. Cancer Causes Control. 2019;30:333-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12:e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Hilali J, de Koning EJ, van Ballegooijen AJ, et al. Vitamin D, PTH and the risk of overall and disease-specific mortality: results of the Longitudinal Aging Study Amsterdam. J Steroid Biochem Mol Biol. 2016;164:386-394. [DOI] [PubMed] [Google Scholar]
- 77.Shui IM, Mondul AM, Lindstrom S, et al. ; Breast, Prostate Cancer Cohort Consortium. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer. 2015;121:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khaw KT, Luben R, Wareham N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. 2014;100:1361-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988-2006). Cancer Res. 2010;70:8587-8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ng K, Nimeiri HS, McCleary NJ, et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019;321:1370-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urashima M, Ohdaira H, Akutsu T, et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019;321:1361-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akiba T, Morikawa T, Odaka M, et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin Cancer Res. 2018;24:4089-4097. [DOI] [PubMed] [Google Scholar]
- 83.Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin D status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846-854. [DOI] [PubMed] [Google Scholar]
- 85.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815-1822. [DOI] [PubMed] [Google Scholar]
- 86.Elamin MB, Elnour NOA, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. [DOI] [PubMed] [Google Scholar]
- 87.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. [DOI] [PubMed] [Google Scholar]
- 88.Rejnmark L, Bislev LS, Cashman KD, et al. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;12:e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol. 2017;2:608-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbarawi M, Kheiri B, Zayed Y, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4:765-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bassuk SS, Manson JE. Does vitamin D protect against cardiovascular disease? J Cardiovasc Transl Res. 2009;2:245-250. [DOI] [PubMed] [Google Scholar]
- 92.Pilz S, Tomaschitz A, Marz W, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2011;75:575-584. [DOI] [PubMed] [Google Scholar]
- 93.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10:e0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang R, Li B, Gao X, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:810-819. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grubler MR, Marz W, Pilz S, et al. Vitamin-D concentrations, cardiovascular risk and events—a review of epidemiological evidence. Rev Endocr Metab Disord. 2017;18:259-272. [DOI] [PubMed] [Google Scholar]
- 97.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2019;111:158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(10):CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307-320. [DOI] [PubMed] [Google Scholar]
- 101.Bassuk SS, Manson JE. Chapter 66: Randomized clinical trials of vitamin D, with a focus on the VITamin D and OmegA-3 TriaL (VITAL). In: Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D, Volume 2: Health, Disease, and Therapeutics. 4th ed. Academic Press; 2018:167-176. [Google Scholar]
- 102.Neale RE, Armstrong BK, Baxter C, et al. The D-Health Trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83-90. [DOI] [PubMed] [Google Scholar]
- 103.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047-2067. [DOI] [PubMed] [Google Scholar]
- 104.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885-1899. [DOI] [PubMed] [Google Scholar]
- 105.Rimm EB, Appel LJ, Chiuve SE, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138:e35-e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398-406. [DOI] [PubMed] [Google Scholar]
- 107.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15-29. [DOI] [PubMed] [Google Scholar]
- 108.Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe). Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024-1033. [DOI] [PubMed] [Google Scholar]
- 110.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;(7):CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.ASCEND Study Collaborative Group; Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540-1550. [DOI] [PubMed] [Google Scholar]
- 113.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. [DOI] [PubMed] [Google Scholar]
- 114.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.AstraZeneca. Update on Phase III STRENGTH trial for Epanova in mixed dyslipidaemia. Published January 13, 2020. Accessed October 22, 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-phase-iii-strength-trial-for-epanova-in-mixed-dyslipidaemia-13012020.html#:~:text=Following%20the%20recommendation%20from%20an,increased%20risk%20of%20cardiovascular%20(CV
- 116.Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127,477 participants. J Am Heart Assoc. 2019;8:e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med (Maywood). 2010;235:785-795. [DOI] [PubMed] [Google Scholar]
- 118.Corella D, Ordovás JM. Interactions between dietary n-3 fatty acids and genetic variants and risk of disease. Br J Nutr. 2012;107(suppl 2):S271-S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chilton FH, Dutta R, Reynolds LM, Sergeant S, Mathias RA, Seeds MC. Precision nutrition and omega-3 polyunsaturated fatty acids: a case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients. 2017;9:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mathias RA, Sergeant S, Ruczinski I, et al. The impact of FADS genetic variants on omega-6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet. 2011;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sergeant S, Hugenschmidt CE, Rudock ME, et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr. 2012;107:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 2016;1860:2891-2898. [DOI] [PubMed] [Google Scholar]
- 123.Tessum CW, Apte JS, Goodkind AL, et al. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci U S A. 2019;116:6001-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054-2070. [DOI] [PubMed] [Google Scholar]
- 125.Zhang YF, Gao HF, Hou AJ, Zhou YH. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health. 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bordeleau L, Yakubovich N, Dagenais GR, et al. ; ORIGIN Trial Investigators. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care. 2014;37:1360-1366. [DOI] [PubMed] [Google Scholar]
- 127.Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajl-10.1177_1559827620972035 for The VITamin D and OmegA-3 TriaL (VITAL): Do Results Differ by Sex or Race/Ethnicity? by Shari S. Bassuk, Paulette D. Chandler, Julie E. Buring and JoAnn E. Manson in American Journal of Lifestyle Medicine