Abstract
The α7-type nicotinic acetylcholine receptor is one of the most unique and interesting of all the members of the cys-loop superfamily of ligand-gated ion channels. Since it was first identified initially as a binding site for α-bungarotoxin in mammalian brain and later as a functional homomeric receptor with relatively high calcium permeability, it has been pursued as a potential therapeutic target for numerous indications, from Alzheimer disease to asthma. In this review, we discuss the history and state of the art for targeting α7 receptors, beginning with subtype-selective agonists and the basic pharmacophore for the selective activation of α7 receptors. A key feature of α7 receptors is their rapid desensitization by standard “orthosteric” agonist, and we discuss insights into the conformational landscape of α7 receptors that has been gained by the development of ligands binding to allosteric sites. Some of these sites are targeted by positive allosteric modulators that have a wide range of effects on the activation profile of the receptors. Other sites are targeted by direct allosteric agonist or antagonists. We include a perspective on the potential importance of α7 receptors for metabotropic as well as ionotropic signaling. We outline the challenges that exist for future development of drugs to target this important receptor and approaches that may be considered to address those challenges.
Significance Statement
The α7-type nicotinic acetylcholine receptor (nAChR) is acknowledged as a potentially important therapeutic target with functional properties associated with both ionotropic and metabotropic signaling. The functional properties of α7 nAChR can be regulated in diverse ways with the variety of orthosteric and allosteric ligands described in this review.
I. Introduction
“To move is all mankind can do and for such, the sole executant is muscle, whether in whispering a syllable or felling a forest.” These words by Charles Sherrington (Sherrington, 1947) draw attention to the most accessible and critically important synapses of the body: neuromuscular junctions. These synapses provide the starting point for all our studies of synaptic physiology and pharmacology. The nicotinic acetylcholine receptors (nAChRs) of the neuromuscular junction are the key mediators of this fundamental connection between the integrated output of the brain and our ability to manifest the desired output of our brain. These receptors were the first ligand-gated channels to be cloned and studied at the level of their single-channel current [reviewed in (Papke 2014)]. An appreciation that nicotine was one of the most widely used and subtle but psychologically compelling drugs to which we are exposed motivated great interest in looking for homologous receptors in the brain.
II. Diversity of Nicotinic Acetylcholine Receptor
Early studies that probed the brain with radioligands identified two distinct and largely nonoverlapping populations of candidate receptors, with one population binding nicotine and acetylcholine (ACh) with high affinity and the other binding the snake toxin α-bungarotoxin (α-BTX) (Clarke et al., 1985). The biochemical isolation of the high-affinity nicotine-binding proteins of brain (Whiting and Lindstrom, 1986) was achieved at about the same time as the subunits for these receptors were cloned and heterologously expressed in Xenopus oocytes (Boulter et al., 1987). Despite having distinct pharmacological properties from the nAChRs of the neuromuscular junction, the high-affinity nicotine receptors of the brain were in many ways similar nAChRs. Functional receptors form as complexes of five subunits, which are heteromeric, requiring at least two different types of subunits (Cooper et al., 1991). One type, designated α subunits, contains essential primary elements of the ACh binding site, whereas other subunits contain complementary elements of the binding sites, which form at subunit interfaces. Each subunit in the nAChR pentameric complex has an extracellular domain followed by three transmembrane helices, a variable hydrophilic intracellular loop (Stokes et al., 2015), and a fourth hydrophobic transmembrane span. Eight different genes (CHRNA2, CHRNA3, CHRNA4, CHRNA5, CHRNA6, CHRNB2, CHRNB3, and CHRNB4) have been cloned from mammalian brain coding for the nAChR subunit proteins of these heteromeric neuronal receptors: α2, α3, α4, α5, α6, β2, β3, and β4 (Wang et al., 1996; Forsayeth and Kobrin, 1997; Gerzanich et al., 1997). Notably, α9 and α10 subunits have also been cloned (CHRNA9, and CHRNA10), and although α9 forms functional receptors when expressed alone, together these subunits can also form heteromeric receptors in unique locations outside the brain (Elgoyhen et al., 1994, 2001). Functional heteromeric neuronal-type receptors containing α2, α3, α4, or α6 must also contain a β subunit (β2 or β4) (Wang et al., 1996; Gerzanich et al., 1997, 1998; Dowell et al., 2003).
Although a relatively minor subtype of nAChR in the brain, receptors containing α3 subunits are of primary importance in autonomic ganglia, where they mediate the synaptic transmission through the ganglia (Wang et al., 2002). In the brain though, most high-affinity heteromeric receptors contain α4 subunits usually in combination with β2. Although these high-affinity nAChRs of the brain certainly have high structural and sequence homology to the receptors of the neuromuscular junction, they are not easily amenable to study in situ (Heinemann et al., 1990) due to fact that they are primarily located at presynaptic terminals (Wonnacott, 1997; Dani, 2001). The functional analogs of nAChRs in the brain that mediate the majority of fast excitatory transmission are structurally unrelated receptors activated by glutamate (Traynelis et al., 2010).
Although we began to gain some understanding about the high-affinity receptors in the brain facilitated by the use of heterologous expression systems (Deneris et al., 1988; Wada et al., 1988; Duvoisin et al., 1989; Papke et al., 1989a,b; Luetje et al., 1990; Papke, 2014), for a number of years the nature of the α-BTX binding sites in the brain remained a mystery (Carbonetto et al., 1978; Hunt and Schmidt, 1978; Oswald and Freeman, 1981; Marks et al., 1986; Wonnacott, 1986; Schoepfer et al., 1990) until the cloning of the α7-subunit gene (CHRNA7) (Bertrand et al., 1992; Seguela et al., 1993). An additional α-BTX neuronal nAChR subunit, α8, was also discovered (Gotti et al., 1994). It is expressed in chick retina where it forms functional receptors, but there is no mammalian homolog.
One of the first unique properties noted for α7 receptors was that they formed functional receptors without the coexpression of additional complementary subunits, suggesting the potential presence of five low-affinity ACh binding sites at the α7−α7 subunit interfaces (Palma et al., 1996). It has been shown that α7 receptors have intrinsically low probability of opening in response to ACh alone because of the existence of desensitized states associated with high levels of agonist occupancy (Uteshev et al., 2002; Williams et al., 2011a,b; Williams et al., 2012; Andersen et al., 2013), as reviewed in Papke and Lindstrom (2020). When activated by ACh alone, the α7 nAChR has other unique physiologic and pharmacological properties that distinguish it, including a high permeability to calcium (ratio of calcium to sodium permeability ≈ 10), rapid and reversible desensitization, and pronounced inward rectification (Seguela et al., 1993). In contrast, the ratio of calcium to sodium permeability of the nAChR in rat ganglionic neurons (Adams and Nutter, 1992) has been shown to be only 0.65:1.
The α7 subunit is highly expressed in the hippocampus and hypothalamus (Seguela et al., 1993; Dominguez del Toro et al., 1994) and has functionally important expression in non-neuronal tissues, such as cells of the immune system (Wang et al., 2003). α7 receptors are also selectively activated by choline (Papke et al., 1996) and are therefore ideally suited to respond to manifestly different kinds of signals, including localized tissue damage and paracrine signals. Human α7 receptors expressed in Xenopus oocytes have functional properties that correspond well to those of α7 responses of cultured hippocampal neurons (Lindstrom et al., 1984; Alkondon et al., 1994; Alkondon and Albuquerque, 1995; Papke and Porter Papke, 2002) and native neuronal tissues (Uteshev et al., 2002). However, functional expression of α7 receptors in transfected cells was found to be difficult to achieve until the discovery of the molecular chaperone resistance to cholinesterase 3 (RIC-3) (Halevi et al., 2003), which allowed for functional expression in a variety of cell lines (Williams et al., 2005). Subsequently, NACHO, an alternative chaperone protein, was discovered (Gu et al., 2016), which may be at least as important as RIC-3 for nAChR function in the brain (Matta et al., 2017; Deshpande et al., 2020).
In this review, we focus primarily on pharmacological tools used to study α7 nAChRs. However, it should also be noted that transgenic animals and gene-delivery methodology provide alternative supplementary approaches for the study of α7 function in vivo. α7 knockout mice have widely been used, both for the study of α7 in the central nervous system (Stoker and Markou, 2013; Koukouli et al., 2016) and in the periphery (Alsharari et al., 2013). Additionally, conditional knockouts of α7 have been generated using the Cre-Lox approach (Hernandez et al., 2014). α7 has also been studied with animals made suitable for optogenetic stimulation of cholinergic fibers (Grybko et al., 2011) and with α7 gene delivery to increase α7 expression in specific brain regions (Ren et al., 2007). Immunohistochemistry is a common tool used to sort out the roles for specific receptor subtypes, but the use of the α7 knockout mice has revealed that α7 antibodies should be used with caution since they detect putative α7 protein signals in knockout animals (Herber et al., 2004; Garg and Loring, 2017). As α7 antibodies have questionable reliability, fluorescently tagged α7 proteins (Palma et al., 2002) have been shown to be useful tools (Lee et al., 2009; Rogers et al., 2012).
III. α7 Receptors as Therapeutic Targets
Alzheimer disease, Parkinson disease, Lewy-body dementia, and schizophrenia are all characterized by decreased expression of nAChRs in the brain (Schröder et al., 1991a,b; Lange et al., 1993; Freedman et al., 1995; James and Nordberg, 1995; Perry et al., 1995; Nordberg et al., 1997; Spurden et al., 1997; Gotti et al., 2006). Normal aging results in a loss of cholinergic function and an impairment in normal learning ability that can be temporarily modulated by nicotine or nicotinic compounds (Arendash et al., 1995; Levin and Torry, 1996; Prendergast et al., 1997). Based on these types of data, a number of attempts are ongoing to develop clinical strategies for treatment of both disease-related and senile dementia that target neuronal nAChRs (Bhat et al., 1990; Weinstock, 1995; Wilson et al., 1995; Snaedal et al., 1996; Kihara et al., 1997; Robbins et al., 1997; Woodruff-Pak and Hinchliffe, 1997; Zamani et al., 1997; Russo et al., 2012, 2014). Unfortunately, to date, no trials have been successful at bringing a drug to market. In some cases, this may have been due to lack of efficacy, and in other cases it may have been due to unforeseen adverse effects (Yang et al., 2017; Manetti et al., 2018; Terry and Callahan, 2019, 2020). It remains to be the case that new discoveries and research directions are required to provide some hope that future trial outcomes might be improved.
Drugs that appear active in preclinical models for cognitive disorders typically have significant efficacy for activation of the α7 ion channel (Briggs et al., 2009; Pieschl et al., 2017). A second major new direction for the development of α7-based therapeutics is for the treatment of inflammatory diseases and pain (Wang et al., 2003). Research in this area began with the discovery of the role of α7 nAChR in the vagal-mediated cholinergic anti-inflammatory pathways (CAPs) (Borovikova et al., 2000; van Westerloo et al., 2006; Pavlov et al., 2007; Rosas-Ballina et al., 2009; Rosas-Ballina and Tracey, 2009). Discovery of the CAPs provided impetus to discover drugs for inflammatory diseases and inflammation-related pain. This also gave compelling motivation to reconsider our view of α7 and other nAChRs strictly as mediators of transmembrane signals that rely on channel-mediated ion flux. The non-neuronal cells that mediate α7's control of inflammation have not been shown to generate α7-mediated currents. Moreover, some α7-targeting ligands that can effectively control inflammation are “silent agonists,” ligands with little or no efficacy for ion-channel activation but the ability to induce nonconduction states that may be associated with signal transduction (Thomsen and Mikkelsen, 2012a; Clark et al., 2014; Papke et al., 2015a; van Maanen et al., 2015; Quadri et al., 2018a). The role of α7 in CAP involves signaling through the Jak2/STAT3 pathway; decreasing levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 through inhibition of nuclear factor κB activation; and increasing levels of anti-inflammatory cytokines, such as IL-10 (de Jonge et al., 2005; Chatterjee et al., 2009; Marrero and Bencherif, 2009; Egea et al., 2015; Zhang et al., 2017). Evidence for the role of the Jak2/STAT3 signaling in CAP has come primarily from studies that have shown a correlation between the effects of nicotine (Li et al., 2020b) or α7-selective agonists (Krafft et al., 2017; Zhang et al., 2020b) on inflammation-associated cytokines and the relative levels of phosphorylated and nonphosphorylated Jak2 and STAT3 with Western blot analyses. These effects were shown to be sensitive to α7 antagonists (de Jonge et al., 2005; Li et al., 2020b; Zhang et al., 2020b), small interfering RNA knockdown of α7 (Fei et al., 2017), or the Jak2 antagonist AG490 (de Jonge et al., 2005; Fei et al., 2017; Krafft et al., 2017). However, α7 nAChR has a large and diverse intracellular interactome (Paulo et al., 2009), and it remains to be determined whether there is a direct interaction of the α7 nAChR protein itself with the Jak2/STAT3 pathway or whether the effects rely on other intracellular intermediates.
Even the α7 agonists that are most efficacious for producing channel activation elicit only brief and infrequent ion-channel currents and are far more effective at inducing and, in some cases, maintaining the receptors in nonconducting states, which have traditionally been dismissed as desensitized and functionally unimportant (Williams et al., 2011b). However, accumulating data suggest that the prejudice that the ligand-bound nonconducting states of nAChRs are all functionally unimportant should be discarded. Just as conformational changes promoted by ligand binding extend through the transmembrane domains, they must also extend into the intracellular domain and likely regulate signal-transduction processes in both neuronal and non-neuronal cells.
In this review, we will cover multiple pharmacological approaches to the therapeutic targeting of α7 nAChRs and how they have evolved as our perspectives have improved over the last 2 decades to include targeting the orthosteric agonist (i.e., ACh) binding site as well as more recently discovered sites for allosteric modulators (Williams et al., 2011c) and activators (Horenstein et al., 2016; Gulsevin et al., 2019; Toma et al., 2019), also considering metabotropic as well as ionotropic signaling.
IV. α7-Selective Agonists
A. Older Ligands and Structures
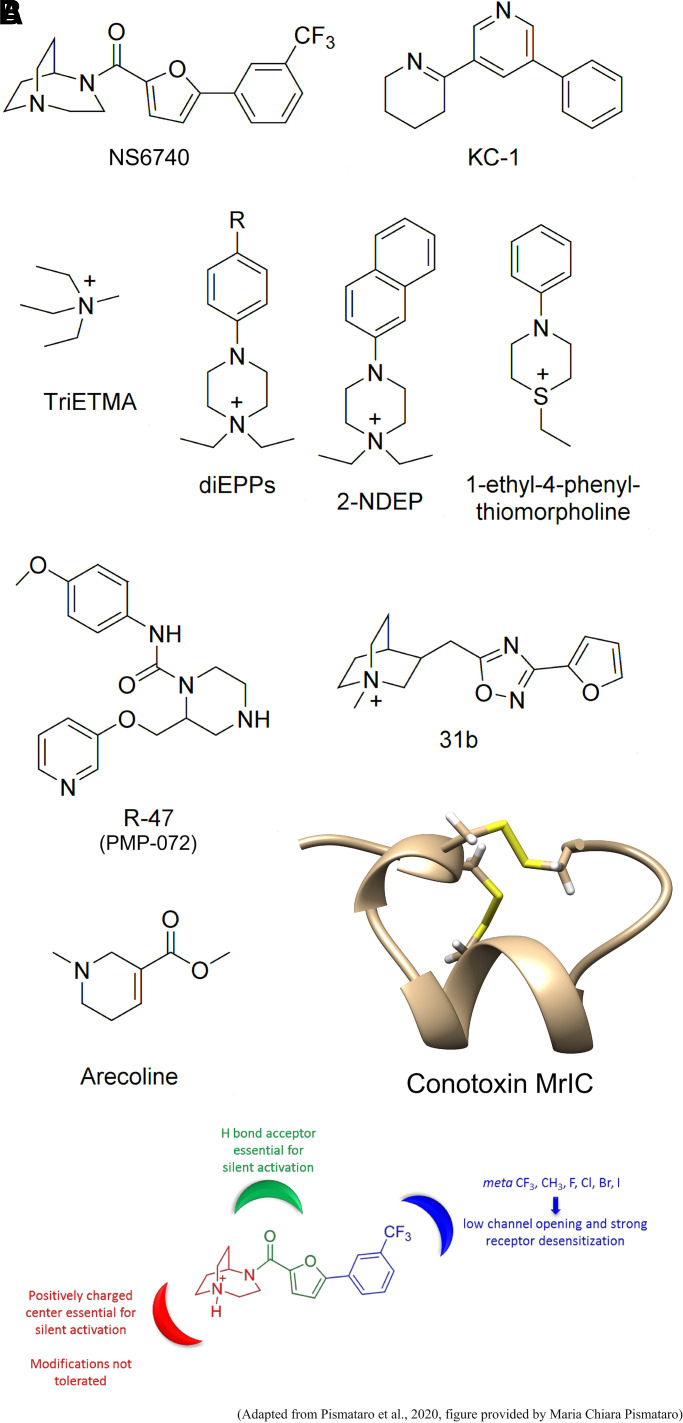
The first and arguably most direct approach for the selective targeting of α7 was with the identification of α7-selective agonists that activated α7 receptors but not other nAChR subtypes. One of the first such agents to be identified was GTS-21 (3-[(2,4-Dimethoxy)benzylidene]-anabaseine, GTS-21 is a benzylidene anabaseine, Fig. 2, top right, where R1 and R2 are OCH3 (methoxy) groups) (Meyer et al., 1997). GTS-21 is a partial agonist for α7 receptors that has remained one of the standard drugs in the field, with more than 20 PubMed citations in 2020 alone. However, it should be noted that GTS-21 is something of a complicated drug in that it inhibits 5HT3 receptors (Gurley and Lanthorn, 1998) and other nAChR subtypes (Briggs et al., 1997) and produces protracted desensitization of α7 receptors after activation (Papke et al., 2009). As we will discuss later, some of these unusual properties may very well be why the drug continues to be useful as the field is expanding the extent of potential indications.
Fig. 2.
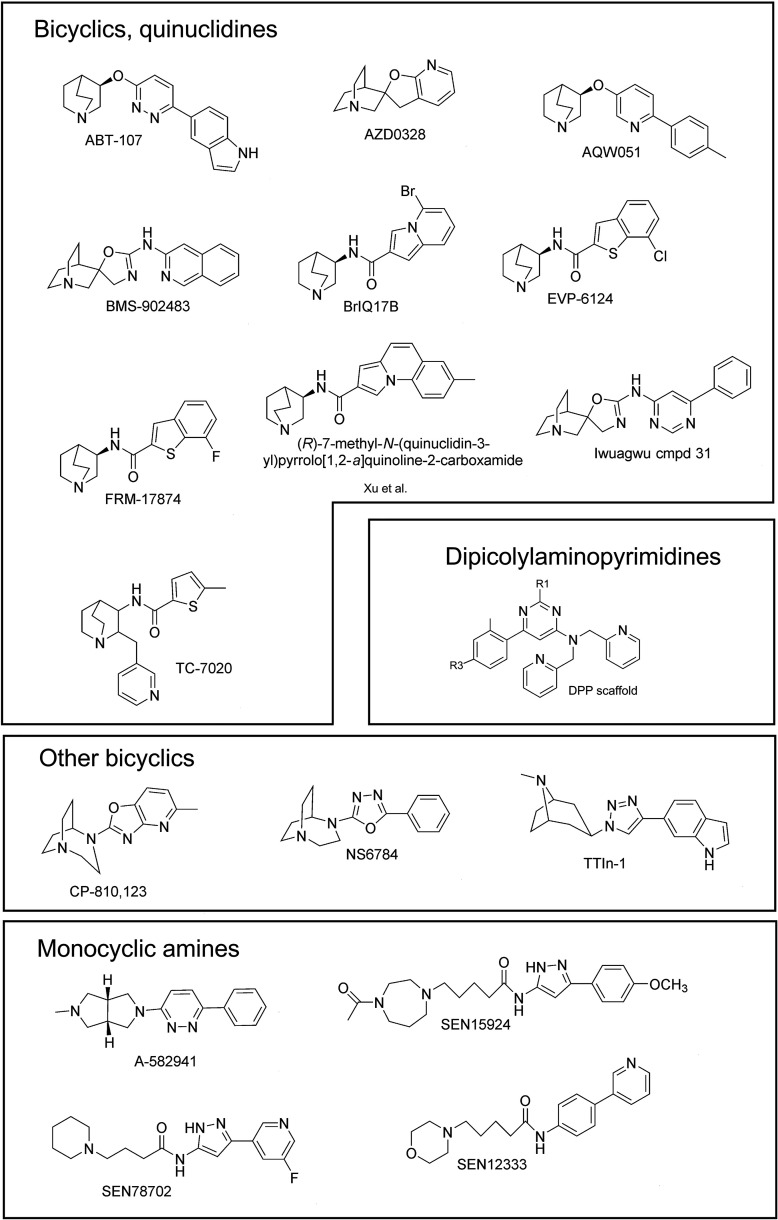
Recently identified putative α7-selective agonists (see Table 1). A-582941 (2-methyl-5-[6-phenylpyridazin-3-yl]octa- hydropyrrolo[3,4-c]pyrrole) (Tietje et al., 2008); ABT-107, 5-(6-[(3R)-1-azabicyclo[2,2,2]oct-3-yloxy] pyridazin-3-yl)-1H-indole (Malysz et al., 2010); ABT-126, (4s)-4-(5-phenyl-1, 3, 4-thiadiazol-2-yloxy)-1-azatricyclo[3.3.1.13, 7]decane (Haig et al., 2018); AQW051, (R)-3-(6-p-tolyl-pyridin-3-yloxy)-1-aza-bicyclo (2.2.2)octane (Feuerbach et al., 2015); AZD0328, (20 R)-spiro-[1-azabicyclo[2.2.2]octane-3,20 (30 H)-furo[2,3-b]pyridine] d-tartrate (Sydserff et al., 2009); BMS-933043, (2R)-N-(6-(1H-imidazol-1-yl)-4-pyrimidinyl)-4′H-spiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxazol]-2′-amine (Cook et al., 2016; Pieschl et al., 2017); BMS-910731, N-(6-methyl-1,3-benzoxazol-2-yl)-3′,5′-dihydro-4-azaspiro[bicyclo[2.2.2]octane-2,4'-imidazole]-2'-amine (Hill et al., 2017); BMS-902483, (1S,2R,4S)-N-isoquinolin-3-yl)-4′H-4-azaspiro[bicyclo[2.2.2]octane-2,5′oxazol]-2′-amine (Hill et al., 2016; 28105289) (Cook et al., 2016); Br-IQ17B, N-[(3R)-1- azabicyclo[2,2,2]oct-3-yl]-5-bromoindolizine-2-carboxamide (Tang et al., 2015); CP-810,123, 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (O'Donnell et al., 2010); EVP-6124 (Prickaerts et al., 2012); FRM-17874 (Stoiljkovic et al., 2015); NS6784, 2-(1,4-diazabicyclo[3.2.2] nonan-4-yl)-5-phenyl-1,3,4-oxadiazole (Briggs et al., 2009); SEN12333, WAY-317538 5-morpholin-4-yl-pentanoic acid (4-pyridin-3-yl-phenyl)-amide (Roncarati et al., 2009); SEN15924, WAY-361789, 5-(4-acetyl[1,4]diazepan-1-yl)pentanoic acid [5-(4-methoxyphenyl)-1H-pyrazol-3-yl] amide (Zanaletti et al., 2012b); SEN78702, WYE-308775, N‐[5-(5-fluoropyridin-3-yl)‐1H‐pyrazol-3-yl]-4-piperidin-1-ylbutyramide (Zanaletti et al., 2012a); TC-7020, [5-methyl-N-[2-(pyridin- 3-ylmethyl)-1-azabicyclo[2.2.2]oct-3-yl]thiophene-2-carbox-amide (Marrero et al., 2010); and 5-(1-((1S,3R)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)-1H-1,2,3-triazol-4-yl)-1H-indole (TTIn-1) and related compounds (Arunrungvichian et al., 2015).
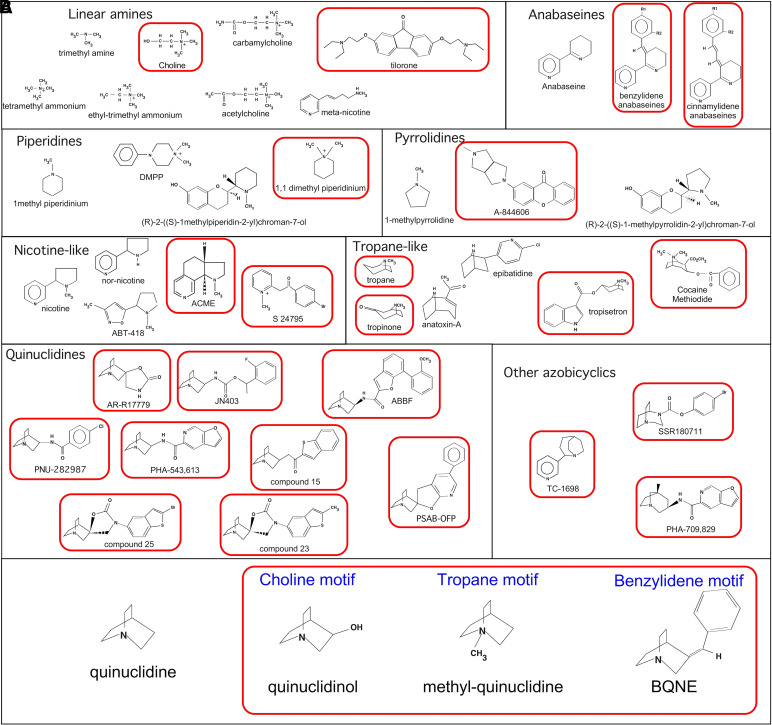
The range of α7-selective agonists widened rapidly after the identification of GTS-21, as numerous drug companies established programs in the area. Progress in the field was presented in a paper published in 2008 (Horenstein et al., 2008) that discussed numerous published structures (Fig. 1A) and, by comparing selective and nonselective drugs of multiple structural families, identified three structural motifs that could be applied to a nonselective agonist to produce an analog that was α7-selective. One motif was associated with the hydroxyl group that was present in the α7-selective agonist choline but not present in the nonselective agonist ethyl-trimethyl-ammonium (Papke et al., 1996). A second was identified as the “tropane motif” based on the structural dissection of tropisetron (Papke et al., 2005a). The third, “benzylidene motif,” was identified in distinguishing the α7-selective GTS-21 from the parent compound anabaseine, which activates multiple nAChR subtypes (Kem et al., 1997). In the 2008 study, it was shown that the nonselective agonist quinuclidine could be modified with any of the three motifs identified to generate a new α7-selective compound (Fig. 1B) (Horenstein et al., 2008).
Fig. 1.
Compounds used to determine the structural motifs of α7-selective agonists as presented in (Horenstein et al. 2008). (A) α7-Selective agonists are in red boxes compared with related compounds that are not selective for α7. The highlighted compounds are tilorone (2,7-bis[2-(diethylamino)ethoxy]fluoren-9-one dihydrochloride) (Briggs et al.,2008); A-844606 (2-(5-methyl-hexahydro-pyrrolo[3,4-c]pyrrol-2-yl)-xanthen-9-one) (Briggs et al., 2008); ACME (cis-1-methyl-2,3,3a,4,5,9b,-hexahydro-1H-pyrrolo[3,2-h]isoquinoline) (Papke et al., 2005b); S 24795 (2-[2-(4-bromophenyl)-2-oxoethyl]-1-methyl pyridinium) (Lopez-Hernandez et al., 2007); tropane ((1R,5S)-8-methyl-8-azabicyclo[3.2.1]octane) (Papke et al., 2005a); tropinone (8-methyl-8-azabicyclo[3.2.1]octan-3-one) (Papke et al., 2005a); tropisetron ([(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 1H-indole-3-carboxylate) (Macor et al., 2001; Papke et al., 2005a); cocaine methiodide, (methyl (1R,2R,3S,5S)-3-benzoyloxy-8,8-dimethyl-8-azoniabicyclo[3.2.1]octane-2-carboxylate) (Francis et al., 2001); AR-R17779 ((−)-Spiro-1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2'-one) (Mullen et al., 2000; Papke et al., 2004); JN403 ((S)-(1-azabicyclo[2.2.2]oct-3-yl)-carbamic acid (S)-1-(2-fluoro-phenyl)-ethyl ester) (Feuerbach et al., 2007); ABBF (N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide) (Boess et al., 2007); PNU-282987 (Bodnar et al., 2005); PHA-543,613 (Acker et al., 2008); compound 15b ((+)-3-[2-(benzo[b]thiophen-2-yl)-2- oxoethyl]-1-azabicyclo[2.2.2]octane) (Tatsumi et al., 2004); compound 25 ((R)-3′-(5-chlorothiophen-2-yl)spiro-1-azabicyclo[2.2.2]octane-3,5′-[1',3′]oxazolidin-2'-one) (Tatsumi et al., 2004); compound 23 ((R)-3′-(5-iodothiophen-2-yl)spiro [1-azabicyclo[2.2.2]octane-3,5′-[1',3′]oxazolidin]-2'-one) (Tatsumi et al., 2004); PSAB-OFP, ((R)-(-)-5′phenylspiro[1-azabicyclo[2.2.2] octane-3,2'-(3′H)furo[2,3-b]pyridine) (Broad et al., 2002); SSR-180711 (1,4-diazabicyclo[3.2.2]nonane-4-carboxylic acid, 4-bromophenyl ester) (Biton et al., 2007); TC-1698 (2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane) (Marrero et al., 2004); and PHA-709829 (Acker et al., 2008). (B) Modified quinuclidine α7-selective agonists: quinuclidinol (1-azabicyclo[2.2.2]octan-3-ol) (Horenstein et al., 2008); methyl-quinuclidine (1-methyl-1-azoniabicyclo[2.2.2]octane iodide) (Horenstein et al., 2008); and BQNE ((E)-3-benzylidene-1-azoniabicyclo-[2.2.2]octane chloride) (Horenstein et al., 2008).
B. Identification via Compound Screening
The process of identifying selective agonists typically involves many steps, and with large-scale programs the first step is running radioligand screens with cells or tissues expressing the target receptor and off-target receptors of interest. This first step, which identifies high-affinity ligands but does not distinguish between agonist and antagonist, must then be followed up with functional assays. Large-scale programs have generally relied on high-throughput screening with automated measurements using transfected cell lines and fluorescent indicators that typically measure changes in intracellular calcium, which is presumed to be a downstream reporter of receptor activation. In some cases, especially in smaller studies, these are followed up with patch-clamp or voltage-clamp studies. However, in most large-scale studies no actual raw data are provided, only tabulated summaries. Although these approaches are generally thought to be amenable to the study of heteromeric receptors expressed in cell lines, they are less suitable for the study of α7 receptors. Even when applied to heteromeric receptors, these approaches can lead to erroneous conclusions due to the pharmacological differences in receptors with varying subunit stoichiometry, a factor that cannot be directly controlled in transfected cells. For example, the initial characterization of Sazetidine-A (Xiao et al., 2006) claimed that it desensitized α4β2 nAChRs without activating them. However, it was later shown that this was only the case for the receptor configuration with three α subunits and two β subunits (Zwart et al., 2008). For receptors with the reverse subunit ratio, Sazetidine-A is a potent full agonist.
Because of its special properties discussed above, α7 nAChRs remain difficult to study with high-throughput cell-based assays, which has often led to compromised approaches, such as the use of nondesensitizing α7-5HT3 chimeric receptors (Craig et al., 2004; O'Donnell et al., 2010) or by amplifying responses with an allosteric modulator (Arunrungvichian et al., 2015; Kaczanowska et al., 2017). However, both of these approaches yield receptors with properties atypical of native α7 receptors activated by ACh (Dinklo et al., 2006; Gee et al., 2007; Miller et al., 2020; Papke and Lindstrom, 2020). Likewise, high-throughput Fluorescent Imaging Plate Reader (FLIPR) assays (Dunlop et al., 2007), which rely on calcium signals (Skidmore et al., 2012; Zanaletti et al., 2012b; Hill et al., 2016; Iwuagwu et al., 2017), are most likely reporting downstream signaling and not ion-channel currents (King et al., 2018; Miller et al., 2020) and may suggest a significantly higher potency than what may be obtained with traditional electrophysiological methods (Haydar et al., 2009). Because of these limitations, many of both older studies (Horenstein et al., 2008) and more recent work (Tietje et al., 2008; Malysz et al., 2010; Marrero et al., 2010; Prickaerts et al., 2012; Yamauchi et al., 2012; Zanaletti et al., 2012a; Feuerbach et al., 2015; Tang et al., 2015) identifying α7-selective agonists rely on receptors expressed in Xenopus oocytes. Although α7 receptors give large reliable responses when expressed in oocytes, there are nonetheless also special concerns that are not always well addressed in these studies. For example, most often responses are measured in terms of peak currents only, and in the case of α7 receptor responses, the amplitude of peak currents is more a function of the synchronization to receptor activation that occurs in advance of the full drug application than it is a measure of the concentration dependence of receptor activation (Papke and Thinschmidt, 1998; Papke and Porter Papke, 2002). Additionally, the reversibility of drug-induced desensitization and the cumulative effects of desensitization with repeated drug applications are concerns that are seldom well addressed or even considered [for example see (Prickaerts et al. 2012)].
The basic methods and conclusions of the studies that characterized the compounds in Fig. 1 have been previously summarized (Horenstein et al., 2008). Although some of these compounds like cis-1-methyl-2,3,3a,4,5,9b,-hexahydro-1H-pyrrolo[3,2-h]isoquinoline (Papke et al., 2005b), PHA-709829 (Acker et al., 2008), and the cinnamylidene anabaseines (de Fiebre et al., 1995; Meyer et al., 1998) have had relatively little impact on the field, others like GTS-21 and PNU-282987 (Bodnar et al., 2005) have proven to be useful experimental tools and are cited in 129 and 165 papers, respectively. Additionally, as a drug already approved for use in humans, tropisetron has been tested with humans suffering from schizophrenia for its ability to improve deficiencies in auditory gating (Koike et al., 2005; Zhang et al., 2012).
As will be discussed in detail below, two forms of α7 activity, channel-based and signal-transduction, may point separately to cognitive functions and regulation of the immune system, respectively (Briggs et al., 2009; Horenstein and Papke, 2017). One application that may fall in between is in regard to the symptomatic management of schizophrenia, in which the desensitizing partial agonist GTS-21 has received particular attention (Martin et al., 2004; Martin and Freedman, 2007; Kem et al., 2018). Although smoking is on a slow decline in the general population, the incidence of smoking remains especially high in people with schizophrenia (Mallet et al., 2017), in which it seems that smoking serves as a sort of self-mediation, providing some of the relief that might be obtained with α7-based therapies (Mackowick et al., 2012). Unfortunately, the population of schizophrenics that smoke probably have developed the same kind of dependence that normal smokers must deal with, a dependence that is normally associated with the effects of nicotine on the heteromeric receptors in the brain (Papke et al., 2020a). Therefore, the management of the smoking behavior in schizophrenics may require novel cessation therapies that address both α7 stimulation and attenuation of the dependence that is due to the heteromeric nAChRs.
C. New Compounds and Structures
Shown in Fig. 2 are α7-selective agonists that have been identified since the 2008 study. Data related to these compounds are summarized in Table 1. It should be noted that this survey omits two agents that are reputed to be α7-selective agonists and have actually been used in clinical trials, (4s)-4-(5-phenyl-1, 3, 4-thiadiazol-2-yloxy)-1-azatricyclo[3.3.1.13, 7]decane (Haig et al., 2018) and R3487/MEM3454 (Huang et al., 2014), because there are no published structures or basic research published to establish their α7 activity. It should also be noted that many of the compounds in Fig. 2 are the leads from studies of multiple compounds in the studies referenced in Table 1, as indicated. The 19 compounds shown and listed were drawn from a total of roughly 400 actually reported. A common structural feature of α7-selective agonists is the presence of a nitrogen center that is sufficiently basic to be protonated. The resulting ammonium group is what traditionally has been considered the minimal pharmacophoric element. However, a few possible exceptions to this “rule” have emerged with the DPP compounds discussed below. Some of the members of this family feature a core aminopyrimidine ring, which has been considered to have sufficiently weak basicity based on NMR titrations, that they may bind to the receptor in unprotonated form. In addition to those compounds presented in Fig. 2 and described in Table 1, there have been several other notable medicinal chemistry characterizations, including an in situ click-chemistry study using acetylcholine binding protein (AChBP) (Yamauchi et al., 2012), a family of 4-heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes] (Hill et al., 2016), a series of spirocyclic quinuclidinyl-d2-isoxazoline derivatives (Dallanoce et al., 2011), spiroguanidine-derived α7 neuronal nicotinic receptor partial agonists (Hill et al., 2017), and a series of agonists with a 1,3,4-oxadiazol-2-amine core. These studies account for an additional 124 compounds. With so many potential compounds available, an important question is whether any of them really stand out as major new discoveries.
TABLE 1.
Putative α7-selective agonists (see Figure 2 for structures)
| Compound | Summary* | Reference |
|---|---|---|
| A-582941 |
Expression system:
Xenopus oocytes and GH4C1 cells for α7. α3* and α4* receptors in HEK cells with Ca2+ FLIPR assay. Binding studies with human brain membranes. Effects on 5HT3 receptors: not studied. Summary: partial agonist of α7 with relatively little activation of other nAChR tested. Positive cognitive effects (inhibitory avoidance) in rats blocked by NS6740. {Total of 12 compounds evaluated.} |
(Tietje et al., 2008) (Briggs et al., 2009) |
| ABT-107 |
Expression system: oocyte α7 compared with α3β4 α4β2 and α4β4 in cell lines. Also tested in brain slices. Effects on 5HT3 receptors: no activity. Summary: efficacious (80%) partial agonist for human α7 (EC50 = 50–90 nM). Protected cultured cortical neurons from glutamate toxicity. Numerous follow-up studies. |
(Malysz et al., 2010) |
| AZD0328 |
Expression systems: receptor binding with transfected HEK cells compared with nicotine binding in rat brain, PC12 cells, or muscle-type BC3H1 cells. Xenopus oocytes rat and human α7, rat and human α4β2, and human α3β4. Effects on 5HT3 receptors: partial agonist (12%) EC50 = 474 ± 173 nM. Summary: efficacious (64%) partial agonist for human α7 (EC50 = 150 ± 40 nM). Low efficacy on α4β2 receptors. Positive effects in NOR. Increases activity of midbrain dopamine neurons. Some follow-up studies on memory and dopaminergic denervation. |
(Sydserff et al., 2009) |
| AQW051 |
Expression system: binding studies in SH-SY5Y cells and rat brain membranes. Xenopus oocytes voltage clamp for α7, all others FLIPR from cell lines. No actual data shown. nAChR subtypes studied: α7, α2β2, α3β2, α3β4,α2β4,α3β4, α4β4. Effects on 5HT3 receptors: nature of activity ill-defined, claimed 500-fold less potent than for α7. Summary: claimed efficacy for α7 of 73%, but no data shown, claimed EC50 ≈ 40 nM. Positive effects with NOR and water-maze performance with aged rats. Pharmacokinetics and tolerability were evaluated in three phase I placebo-controlled studies in 180 healthy subjects with relatively few adverse effects. Numerous follow-up studies. |
(Feuerbach et al., 2015) |
| BMS-933043 |
Expression system: cell line FLIPR. Methods described only in supplemental material, and actual no data shown in manuscript or supplement. Binding in HEK cell membranes. Electrophysiology with patch-clamp and dynaflow (Cellectricon) perfusion system. nAChR subtypes studied: α1β1γε, α3β4, α4β2, α7. Effects on 5HT3 receptors: putatively low potency compared with α7. Summary: impossible to evaluate the quality of the data. This is a particular concern of the α7 electrophysiology. Positive effects reported with NOR. {Total of three compounds evaluated.} |
(King et al., 2017a) |
| BMS-902483 |
Expression system: Binding with α7-transfected cells. Electrophysiology on α7 with patch-clamp and dynaflow (Cellectricon) perfusion system. Limited data shown. nAChR subtypes studied: α1β1γε, α3β4, α4β4, α7. Binding data only for non-α7. Effects on 5HT3 receptors: sntagonist IC50 = 0.51 µM. Summary: α7 partial agonist (62%) EC50 = 0.24 µM. Limited data on selectivity. Positive effects on NOR, auditory gating, and other behavioral tests. Augmented LTP. NOR effects blocked by NS6740. {Total of 58 compounds evaluated.} |
(Pieschl et al., 2017) (Cook et al., 2016) |
| BrIQ17B |
Expression system:
Xenopus oocytes for α7 (peak currents) and other subtypes. Radioisotope ligand binding, Western blots, whole-cell recordings of hippocampal culture neurons also used. nAChR subtypes studied: α3β4, α4β2, α7, and GABAA receptors. Effects on 5HT3 receptors: inhibition only at high conc. Summary: partial (64%) agonist, EC50 1.8 ± 0.2 (based on peak currents). Lower conc. inhibited ACh-evoked responses. Inconsistency in data since in one case 0.3 µM produced no apparent response when applied prior to ACh, yet in CRC study 0.3 µM produced approximately 7% maximal response. |
(Tang et al., 2015) |
| CP-810,123 |
Expression system: binding assay for rat r7 nAChRs expressed in GH4C1 cells using [125I]BTX as the radioligand. High-throughput FLIPR-based functional assay that used SH-EP1 cells expressing the α7/5-HT3 chimera. nAChR subtypes studied: only α7/5HT3 chimera studied directly α4β2, and α3β4 inferred from binding studies with rat brain or IMR32 cells, respectively. Effects on 5HT3 receptors: binding assay for human 5-HT3 receptors expressed in HEK293 cells using [3H]LY278584 as the radioligand. Summary: Large family off compounds studies with CP-810,123 identified as most promising lead. Data based on chimera reported an EC50 on this unnatural receptor of 16.4 nM with an Imax 195% that of 50 µM nicotine. No data are shown. Tested in auditory gating yielded inconclusive results. {Total of 43 compounds evaluated.} |
(O'Donnell et al., 2010) |
| DPP compounds |
Expression system: Binding with AChBPs, transfected cells, and Xenopus oocytes. nAChR subtypes studied: α7 α4β2. Effects on 5HT3 receptors: confirmed no activity with cell-based neurotransmitter fluorescent engineered reporters. Summary: Compounds have been described as “noncanonical agonists” since their structures defy normal models of the nAChR pharmacophore. They were initially identified by their binding to molluscan AChBP. Activity and selectivity confirmed with cell-based fluorescence activity with the PAM PNU-120596 used to increase α7 signals. Selective activation of α7 confirmed with TEVC in Xenopus oocytes for a subset of the compounds. Efficacy ranged from 40%–80% with submicromolar potencies with a range of desensitizing activities. {Total of 75 compounds evaluated.} |
(Kaczanowska et al., 2017; Camacho-Hernandez et al., 2019) |
| EVP-6124 (encenicline) |
Expression system: binding with rat brain membranes, TEVC in Xenopus oocytes. nAChR subtypes studied: α7, α4β2, α3β4, and muscle-type receptors. Effects on 5HT3 receptors: Binding studies showed that EVP-6124 inhibited the 5-HT3 receptor by 51% at 10 nM. Summary: a reported EC50 of 0.16 µM based on peak currents, suggesting more potent activity on 5HT receptors than α7. IMax estimation limited by protocol, which permitted cumulative desensitization. Claimed to have potentiating activity at low conc.; however, see Figure 3. Active in NOR and other cognitive tests. Numerous follow-up studies. |
(Prickaerts et al., 2012) |
| FRM-17874 |
Expression systems: binding studies and Xenopus oocyte TEVC. nAChR subtypes studied: α7 only. Effects on 5HT3 receptors: Evaluated in binding studies that showed significant inhibition. TEVC in oocytes showed an IC50 of 3.2 ± 2.4 nM. Summary: analog of EVP-6124, also reputed to have a potentiating effect at low conc. TEVC in oocytes indicated a EC50 of 0.42 ± 0.17 µM, but data were of insufficient quality to estimate an Imax. FRM-17874 improved novel object recognition in rats and enhanced memory acquisition and reversal learning in the mouse water T-maze and enhanced hippocampal LTP. |
(Stoiljkovic et al., 2015) |
| Iwuagwu et al. Compound 31 |
Expression systems: FLIPR assays of transfected cells and patch clamp for HERG and reportedly for α7, although no patch data are shown either, and no patch-clamp results reported for α7. nAChR subtypes studied: α7 only. Effects on 5HT3 receptors: IC50 for 5HT3 = 9.2 µM from FLIPR. Summary: Lead compound from a study of 4-heteroarylamino-10-azaspiro [oxazole-5,30-bicyclo[2.2.2]octanes]. EC50 for α7 of 11 nM from FLIPR. Positive effect in NOR. No apparent follow-up publications. {Total of 31 compounds evaluated.} |
(Iwuagwu et al., 2017) |
| NS6784 |
Expression system: Xenopus oocytes and GH4C1 cells for α7. α3* and α4* receptors in HEK cells with Ca2+ FLIPR assay. Binding studies with human brain membranes. nAChR subtypes studied: putative α3*, putative α4*, α7. Effects on 5HT3 receptors: not studied. Summary: Efficacious agonist of α7 with relatively little activation of other nAChR tested. {Total of three compounds evaluated.} |
(Briggs et al., 2009) |
|
SEN12333 (WAY-317538) |
Expression system: GH4C1 cell line for a7 FLIPR and patch-clamp studies. Binding studies with transfected cells. nAChR subtypes studied: putative α3*, putative α4*. Effects on 5HT3 receptors: claimed inactive, no data shown. Summary: EC50 = 687 nM in FLIPR assay and 42 µM in patch-clamp study of peak currents. Data on numerous other analogs reported. {Total of 81 compounds evaluated.} |
(Beinat et al., 2012, 2015) (Haydar et al., 2009) |
|
SEN15924 (WAY-361789) |
Expression system: FLIPR assays GH4C1 cells for α7. Transfected HEK cells for 5HT3, SH-SY5Y for putative ganglionic (α3*) receptors, and TE671 for muscle-type. nAChR subtypes studied: putative α3*, α1β1γδ, and α7. Effects on 5HT3 receptors: inhibitory activity at >30 µM. Summary: Large study of numerous analogs. Lead compound does something in FLIPR assay (no actual data shown) EC50 = 0.18 µM ± 0.01. Positive effects in NOR and auditory gating reported. {Total of 25 compounds evaluated.} |
(Zanaletti et al., 2012b) |
|
SEN78702 (WYE-308775) |
Expression system: FLIPR assays: GH4C1 cells for α7. Transfected HEK cells for 5HT3, SH-SY5Y for putative ganglionic (α3*) receptors and TE671 for muscle-type transfected CHO cells for HERG channels. nAChR subtypes studied: putative α1β1γδ, α3*4, α7. Effects on 5HT3 receptors: reportedly no agonist activity. Antagonist activity not studied. Summary: hypothetically, a full agonist in FLIPR assay, but relative to what standard is not clear EC50 = 125 ± 70 nM. Potency values from such assays are typically at least 10-fold higher than those from electrophysiology. No agonist activity detected on other subtypes. Antagonist activity not studied. Positive effects in NOR, and acoustic startle response reported. {Total of 12 compounds evaluated.} |
(Zanaletti et al., 2012b) |
| (R)-7-methyl-N-quinuclidin-3-yl)pyrrolo[1,2-a]quinoline-2-carboxamide (Compound 10a) |
Expression system:
Xenopus oocytes. nAChR subtypes studied: α3β4, α4β2, and α7. Effects on 5HT3 receptors: Very effective antagonist of 5HT3a expressed in oocytes. IC50 ≈ 800 nM, full inhibition with 10 µM. Summary: Very little data presented. EC50 for α7 of approximately 2 µM with roughly 70% efficacy (peak currents). MLA blocks, PNU-120596 potentiates, and MLA. Preapplications of low conc. inhibited ACh responses with an IC50 of 21. 2 ± 1.3 nM. No apparent follow-up publications at the time of this writing. {Total of 32 compounds evaluated.} |
(Xue et al., 2019) |
| TC-7020 |
Expression system:
Xenopus oocytes for α7. TE671 and SH-SY5Y and SH-EP1 cells for other nAChRs in FLIPR assays. Also, brain membranes for binding studies. nAChR subtypes studied: α1β1γδ (TE671 cells), putative α3 (SH-SY5Y) α4* (SH-EP1), and α7 in oocytes. Effects on 5HT3 receptors: not studied. Summary: Authors state that TC-7020 is an efficacious partial (68%) agonist for α7 net charge responses, but data are not shown, nor is an EC50 provided. Oocyte work was done in the laboratory of an author of this review (R.L.P.), and although Marrero et al. say that an EC50 was not determined, the readers of this review may be assured that it was. There were minimal responses of other subtypes in FLIPR assay compared with nicotine. It may be noted that in regard to the results with TE671 cells, muscle-type receptors are not calcium permeable, and nicotine is a weak agonist for this subtype. Effects measured on mediators of CAP, TNF-α, and JAK-2. |
(Marrero et al., 2010) |
| TTln-1 |
Expression system: fluorescence resonance energy transfer assay using cell lines expressing transfected cDNAs and a fluorescence cell reporter as well as ligand binding. nAChR subtypes studied: α4β2 and α7, also the α7/5ht chimera. Effects on 5HT3 receptors: antagonist, IC50 = 5 = 4.9 ± 2.7 µM. Summary: This is a study evaluating a family of analogs for receptor activity with a novel approach. α7 data are from either the nondesensitizing α7/5HT chimera or α7 in the presence of the PAM PNU-120596. Therefore, the estimated EC50 of 570 ± 130 nM is probably too low by at least a factor of 10, and efficacy cannot be evaluated. {Total of 24 compounds evaluated.} |
(Arunrungvichian et al., 2015) |
BTX, bungarotoxin; CRC, concentration response curve; HEK, human embryonic kidney; HERG, human Ether-à-go-go-Related Gene; LTP, long-term potentiation; TEVC, two-electrode voltage-clamp.
D. Functional Properties of α7-Selective Agonists
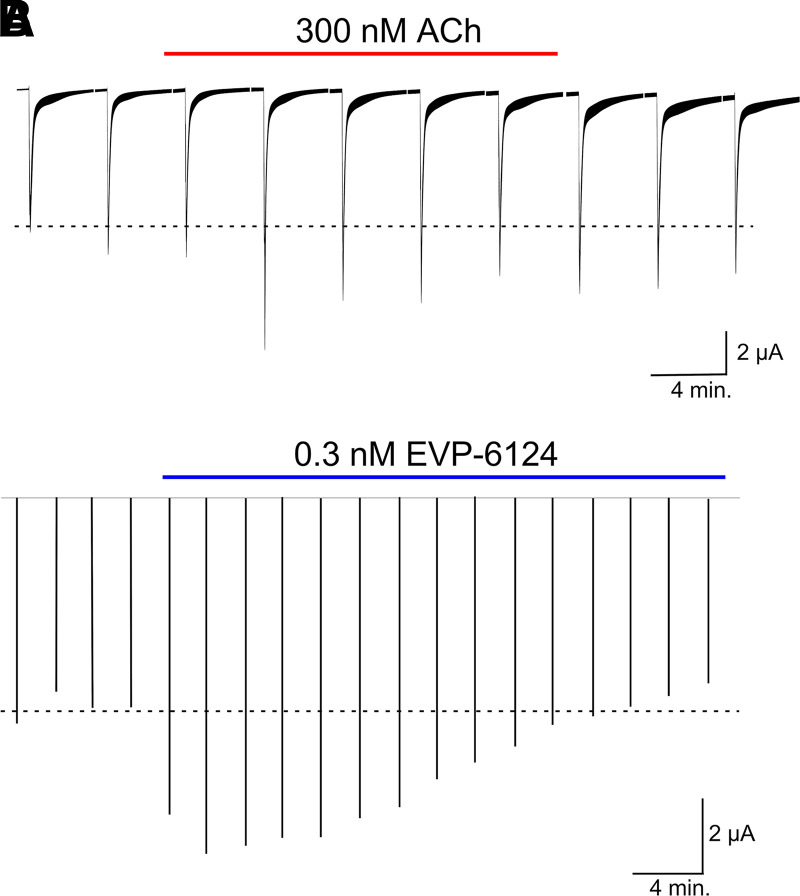
One compound that has drawn a fair amount of attention since it was first published in 2012 and actually advanced to clinical trials for Alzheimer disease (Barbier et al., 2015) and schizophrenia (Preskorn et al., 2014) is EVP-6124. One thing that made EVP-6124 stand out in its initial characterization was the claim that EVP-6124 (as well as its derivative FRM-17874), based on the study of peak currents in Xenopus oocytes in addition to its acting as an agonist of α7, could at low concentration potentiate the activity of the normal neurotransmitter ACh. As noted above, there are caveats and limitations to the analysis of α7 peak currents that are not always appreciated. In the case of the putative potentiation of ACh responses by EVP-6124, as shown in Fig. 3, this is not a special property of EVP-6124 but rather a special property of α7 receptors. Essentially the same effect can be obtained by priming the ACh responses with a low concentration of ACh to give a larger (i.e., more synchronized) peak current response.
Fig. 3.
Apparent potentiation of α7 peak current responses by tonic low concentrations of agonist. (A) Averaged normalized responses (±S.E.M.) of oocytes (n = 7) expressing human α7 to repeated applications of ACh. After the first two control applications, the bath solution was switched to Ringer's solution containing 300 nM ACh. (B) Peak current response data of “representative” (i.e., n = 1) responses of an α7-expressing oocyte extrapolated from Figure 6B of (Prickaerts et al. 2012).
For the most part, all of the recent characterizations of α7 agonists have focused solely on receptor ion-channel activation. One lesson that might be learned from GTS-21, a compound used in well over 200 studies, is that there is more to a potentially useful drug than how well it produces transient activation of the channel. Like all nAChRs, α7 receptors have multiple conformational states, including several nonconducting states that, although classified as desensitized, may be associated with the signal-transduction processes that underlie CAP, which is something that will later be discussed in greater detail under the topic of silent agonists. As noted above, in addition to activating α7 receptors, GTS-21 produces desensitization that persists for a significant period of time (Papke et al., 2009). Of all of the studies referenced in Table 1, the desensitizing properties of the agents were only considered of interest with the DPP compounds (Camacho-Hernandez et al., 2019). Note that these compounds were originally introduced as 4,6-disubstituted-2-aminopyrimidines; however, with further consideration of their structures, (P. Taylor personal communication) the nomenclature of these compounds should be based on their core structure as N,N-dipicolyl amino pyrimidines. The family can further be divided into “DPP” compounds and “2-amino-dipicolylaminopyrimidine” compounds, wherein the prefix stands for an additional amino substitution at position 2 of the pyrimidine ring.
E. Translational Development
Notwithstanding the DPP compounds, which certainly merit more detailed studies and evaluation with in vivo models, it is unclear whether the hundreds of new α7-selective agonists identified since 2008 have really advanced the field very far. None have really proven themselves in clinical trials, and as experimental tools, it remains to be seen whether any will surpass the utility of agents like PNU-282987, PHA-543,613, and GTS-21, which are already commonly used. Moreover, it is rumored that many of the programs in this area by the large pharmaceutical companies like Pfizer (Malysz et al., 2010; O'Donnell et al., 2010; Zanaletti et al., 2012b), Abbott (Tietje et al., 2008; Briggs et al., 2009), AstraZeneca (Sydserff et al., 2009), Bristol-Myers Squibb (Cook et al., 2016; Hill et al., 2016, 2017; Iwuagwu et al., 2017; Pieschl et al., 2017), Wyeth (Haydar et al., 2009), Novartis (Feuerbach et al., 2015), Bayer (in partnership with EnVivo) (Prickaerts et al., 2012), Targacept (Marrero and Bencherif, 2009), GlaxoSmithKline (Skidmore et al., 2012), and Servier (Beracochea et al., 2008) have been discontinued. Although some have left the field of nicotinic receptor research entirely, others have shifted their efforts away from targeting the orthosteric agonist binding site and toward allosteric modulators.
V. α7-Positive Allosteric Modulators
A. Functional Modulation and α7 Nicotinic Acetylcholine Receptor Structure
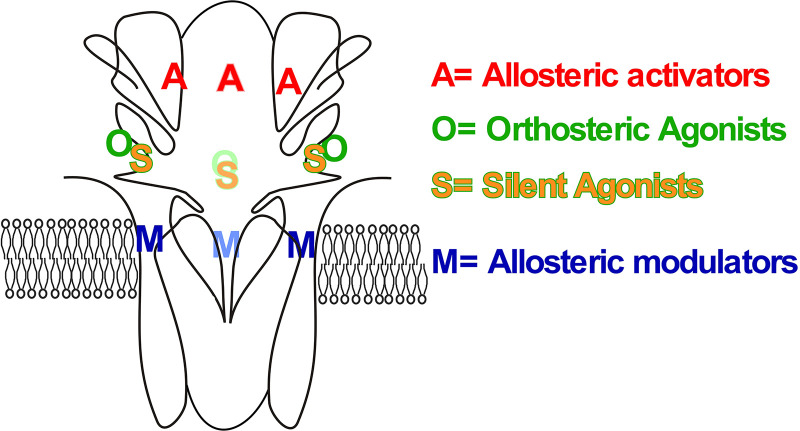
Like all nAChRs, the α7 receptor is an allosteric protein [reviewed in (Papke and Lindstrom 2020)] with multiple ligand binding sites that interact to determine the conformational and functional dynamics of the receptor. Considering first the ACh or orthosteric binding sites, as mentioned earlier, these are configured in the extracellular domain at the subunit interfaces. Early mutagenesis studies with heteromeric muscle-type nAChRs [reviewed in (Papke 2014)] inferred the existence of three critical subdomains on the primary face of the ligand binding site in the α subunit, which are referred to as the A, B, and C loops. A pair of disulfide-linked vicinal cysteines at the tip of the C-loop is a defining feature of all α subunits. In heteromeric nAChRs, subunits that lack these vicinal cysteines form the complementary face of the orthosteric binding sites. Specialized subdomains referred to as the D, E, and F loops are present in the muscle subunits that provide the complementary surface of the ACh binding sites (δ, γ, and ε, the ε subunit substituting for γ in adult muscle-type receptors). In heteromeric neuronal receptors these specialized subdomains are present in the β2 and β4 subunits (Papke and Lindstrom, 2020). Early electron micrographic studies of the nAChR of the Torpedo electric ray homologous to muscle-type receptors supported the presence of these functional subdomains (Unwin, 1993; 2005) that more recently have been definitively identified in high-resolution structures on neuronal α4β2 (Morales-Perez et al., 2016) and α3β4 (Morales-Perez et al., 2016) receptor subunit complexes. Support for the hypothesis that the α7 subunits of homomeric α7 receptors contain homologs of both the primary and complementary surfaces of the orthosteric binding sites at alternating subunit interfaces has come from mutation analyses (Papke, 2014) and the crystal structures of molluscan AChBPs (Brejc et al., 2001). AChBPs are soluble proteins secreted by the glial cells in the ganglia of various invertebrates, and they are formed as pentamers of proteins that are homologous to the extracellular domain of nAChR α subunits (Camacho-Hernandez and Taylor, 2020).
Since no crystal structures of α7 receptors are available at present, homomeric pentamers of AChBP mutants have been developed as models for α7 (Gulsevin, 2020; Gulsevin et al., 2020a,b). Even if we begin with the parsimonious and possibly naive assumption that each α7 receptor has five functionally equivalent orthosteric activation (OA) agonist binding sites (Palma et al., 1996), early studies with the AChBPs suggested that as ligands begin to bind, at least in regard to some ligands, the binding sites become nonequivalent (Hibbs et al., 2009). Crystal structures with the α7-selective partial agonist GTS-21 (see above) showed that the ligand crystallized in different orientations at some interfaces compared with others. Although those studies could not determine whether the difference between binding sites represented a starting condition or was an emergent property of the crystallization process, recent in silico studies that begin with symmetrically configured subunits suggest that when these are allowed dynamic relaxations, the subunit interfaces quickly become asymmetric (Henchman et al., 2003; Gulsevin, 2020; Gulsevin et al., 2020a,b).
Considering what we know about the dynamics of α7 activation by orthosteric agonists (Papke and Lindstrom, 2020), regardless of whether all of the five subunit interfaces start out as functionally equivalent, as long as one or more of them bind agonist, it is clear that dynamic conformational changes affect the entire receptor. The activation of the α7 ion channel by orthosteric agonist occurs at low probability and only with low levels of agonist site occupancy (Uteshev et al., 2002; Williams et al., 2011a; Williams et al., 2012). Further levels of agonist binding serve only to induce the concentration-dependent form of desensitization that is unique to α7 (Papke and Lindstrom, 2020).
B. Desensitization and Allostericism
Desensitization (Katz and Thesleff, 1957) is a feature common to all nAChR, and for heteromeric nAChR, coincident with desensitization, the orthosteric binding site adopts a conformation that binds agonists with high affinity (Papke, 2014). It was this feature that allowed the early radioligand binding studies to identify the heteromeric nAChR as high-affinity receptors for ACh and nicotine (Clarke et al., 1985). Although α7 receptors desensitize so rapidly that the currents evoked by the application of high concentrations of AChs are terminated before the drug application can even be completed (Papke and Porter Papke, 2002; Papke, 2010; Williams et al., 2012), the orthosteric binding sites do not adopt a conformation with high affinity for ACh, and, in general, α7 receptor desensitization is rapidly reversible. There are, however, exceptions to this in which a particular ligand like GTS-21 can induce relatively stable desensitization. The possible functional significance of this will be discussed further in the section on silent agonists.
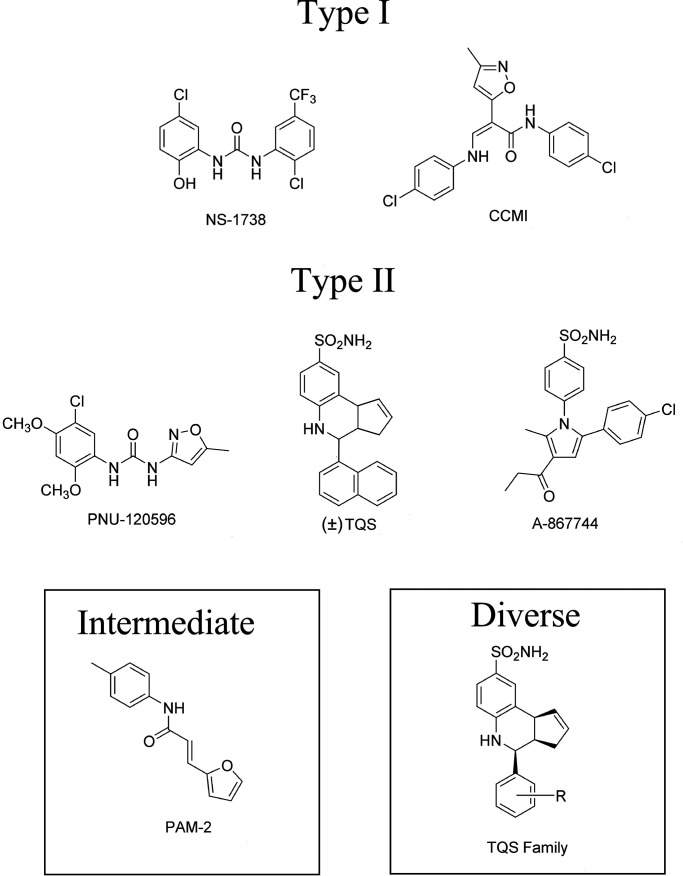
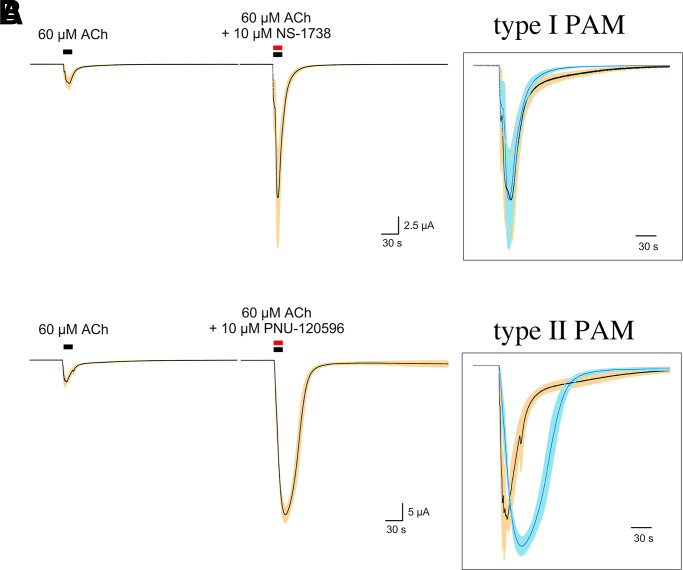
As noted above, nAChRs have a long history of being considered allosteric proteins (Changeux, 1981), and as such, their function is regulated by ligands binding to allosteric sites as well as the sites for orthosteric agonists (Changeux and Revah, 1987; Papke, 2014). In recent years, some of the most striking effects for allosteric ligands have been described for positive allosteric modulators (PAMs) of α7 receptors (Williams et al., 2011c). As noted above, in general, α7 receptors have only a low probability of ion-channel activation by ACh or other agonists working through the orthosteric binding sites. Two basic types of PAMs have been identified that differ in the degree to which they synergize with orthosteric agonists to overcome the intrinsic limitations on α7-channel activation (Fig. 4) (Gronlien et al., 2007). Type I PAMs like NS-1738 (Timmermann et al., 2007) increase channel activation during the phase that precedes the induction of more stable desensitized states, so that responses are increased in amplitude but not very much in duration (Fig. 5A). Type II PAMs like PNU-120596 (Hurst et al., 2005; Gronlien et al., 2007) (Fig. 5B) increase channel currents by additionally destabilizing conformations associated with desensitized states of the receptor (Williams et al., 2011b). PAMs of this type when coapplied with agonist will not only stimulate prolonged currents during the coapplication if receptors have been desensitized by a previous drug application, but type II PAMs applied alone will reactivate receptors (Papke et al., 2009) (see also discussion of allosteric antagonists below).
Fig. 4.
Structures of commonly used α7 PAMs. See Table 2 for chemical names and references. NS-1738 and N-(4-chlorophenyl)-α-[[(4-chloro-phenyl)amino]methylene]-3-methyl-5-isoxazoleacet-amide (CCMI) are classified as type I (see text and Figure 5), whereas PNU-120596, (±)TQS, and A-867744 are type II PAMs. PAM-2 activity is intermediate between the two types (see text). The scaffold of TQS has provided the basis for many different allosteric ligands with diverse functions (Gill et al., 2012; Gill-Thind et al., 2015), as discussed in the text.
Fig. 5.
Representative data from prototypical type I (NS-1738) and type II (PNU-120596) PAMs. (A) Averaged normalized responses (±S.E.M.) of oocytes (n = 4) expressing human α7 to 60 µM ACh or 60 µM ACh coapplied with 10 µM NS-1738. The insert shows the control and potentiated responses scaled to the same peak amplitude. (B) Averaged normalized responses (±S.E.M.) of oocytes (n = 4) expressing human α7 to 60 µM ACh or 60 µM ACh coapplied with 10 µM PNU-120596. The insert shows the control and potentiated responses scaled to the same peak amplitude.
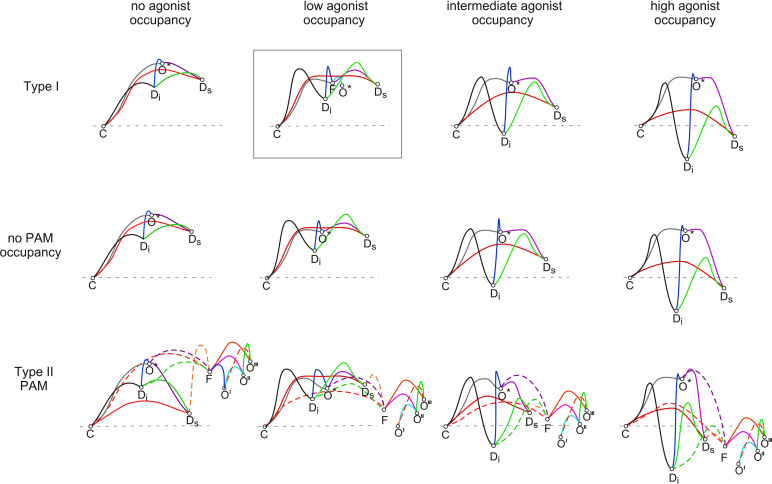
As shown in a schematic representation of the conformational dynamics of α7 activation and desensitization as regulated by agonists and PAMs [Fig. 6, adapted from (Williams et al. 2011b) and modified based on (Quadri et al. 2019)], when bound by orthosteric agonist alone, site occupancy is low, and the receptor has only a low probability of entering a relatively unstable open state for brief durations. The effects of a type I PAM would be consistent with an increase in single-channel conductance; however, single-channel studies (Andersen et al., 2016) have shown that the primary effects are to stabilize the open state and to permit reopening when the OA site occupancy is low (box in Fig. 6) without changing the transitions to the desensitized states that develop over time or with changes in OA site occupancy.
Fig. 6.
Schematic illustration of energy landscapes for the conformational states associated with α7 activation, desensitization, and modulation [adapted from (Quadri et al. 2019) and (Williams et al. 2011b)] illustrating their relative energy levels and transition rates. Under equilibrium conditions, the distributions of receptors into the resting closed, open, and desensitized states will be determined by the relative free energy of the states (represented by vertical displacements). Dynamically, the transition rates between the states will be inversely related to the log of the energy barriers between the states. In the absence of any PAM (center row), the primary effect of agonist binding is to shift the equilibrium between the conformational states from the resting closed (C) state toward the desensitized states Ds and Di with a small probability of opening only at relatively low levels of agonist occupancy. The shallow energy well assigned to the open state (O*) is consistent with the brief opening observed in single-channel recordings and the high energy barriers into the O* state consistent with the low Popen observed. With the binding of a type I PAM (upper row), the primary effect is to deepen the well for the open state and to permit repeated transitions between the threshold activation (Flip) state and the open state, consistent with observations of single-channel currents in the presence of the type I PAM (Andersen et al., 2016). Note that this effect is only seen at low levels of agonist occupancy (in box). In the presence of the type II PAM (lower row, the Ds state is connected to another Flip state that then permits many reopenings to full and subconductance open states (O', O'', and O''').
Single-channel studies of α7 receptors potentiated by type II PAMs (Williams et al., 2011b; Williams et al., 2012; Peng et al., 2013; Andersen et al., 2016; Quadri et al., 2019) have indicated that the increased channel activation is associated with transitions between desensitized states and an unstable intermediate flip state (Lape et al., 2008) that is then able to convert repeatedly between two or more novel open-channel states in bursts that can persist for many seconds. These bursts represent bouts of single-channel activation typically more than a hundred thousand times greater than the single-channel currents stimulated by ACh alone. Comparing then the PAM effects on the macroscopic (whole-cell) current, which are increased on the scale of 50–100-fold, with the single-channel effects, we see that the net effects of these PAMs is to generate very large bursts of currents from a very limited fraction of the channels at any one time. Because of this stochastic nature of the large effects on a small fraction of channels typically in a given experiment, there is a great deal of variability among the responses in a group of cells.
Studies of mutants and chimeras localized the binding sites for α7 PAMs (Bertrand et al., 2008; Young et al., 2008) to the upper portion of the second transmembrane domain, with an especially important role attributed to a methionine residue in the 15' position (Young et al., 2008). The presence of a methionine residue in this position is unique to α7 among all the nAChR subunits, and not only does mutation of this residue to leucine (the most common residue in other subunits) lead to a loss of sensitivity for α7 to PAM potentiation, but substitution of this residue into the sequence of β2 or β4 subunits generates heteromeric receptors that are sensitive to potentiation by many α7 PAMs (Stokes et al., 2019).
With a relatively large potentiating ligand bound within one or more of the transmembrane domains, it is perhaps not surprising that the ion conduction pathways that form in PAM-potentiated receptors are qualitatively different from the channels formed when the receptor is activated by ACh alone. Channels activated by ACh have relatively high calcium permeability and inward rectifying current-voltage relations, which are features that are not typical of PAM-potentiated currents (Sitzia et al., 2011; Miller et al., 2020). Specific PAMs may each generate their own unique conduction pathway (Miller et al., 2020), a differing set of full and subconductance states, and varying sensitivity to channel-blocking antagonists (Quadri et al., 2019).
C. Ligands and Structures
Compounds identified as α7 PAMs are listed in Table 2, and the structures of the most commonly used ones are shown in Fig. 4. Earlier known compounds are described in more detail in a previous review (Williams et al., 2011c). The first α7 PAM to be identified, 5-hydroxyindole (Gurley et al., 2000), is classified as type I but has not been widely used since it works with very low potency. The effects of NS1738, a more potent type I PAM, are shown in Fig. 5 compared with the effects of the widely used type II PAM, PNU-120596. The cholinesterase inhibitor, galantamine, which was approved for the treatment of Alzheimer disease, was initially claimed to be an α7 PAM (Samochocki et al., 2003); however, this claim has recently been shown to be invalid (Kowal et al., 2018).
TABLE 2.
α7 PAMs (see Figure 4 for select structures)
| PAM | Chemical Name | Type | Reference |
|---|---|---|---|
| 5-HI | 5-Hydroxyindole | I | (Zwart et al., 2002) |
| CCMI | N-(4-Chlorophenyl)-α-[[(4-chloro-phenyl)amino]methylene]-3-methyl-5-isoxazoleacet-amide | I | (Ng et al., 2007) |
| NS-1738 | 1-(5-Chloro-2-hydroxy-phenyl)-3-(2-chloro-5-trifluoromethyl-phenyl)-urea | I | (Timmermann et al., 2007) |
| PNU-120596 | 1-(5-Chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea | II | (Hurst et al., 2005) |
| TQS | 4-Naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide | II | (Gronlien et al., 2007) |
| A-867744 | 4-(5-(4-Chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide | II | (Faghih et al., 2009) |
| SB-206553 | 3,5-Dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b']di pyrrole-1(2H)-carboxamide | II | (Dunlop et al., 2009) |
| JNJ-1930942 | 2-[[4-Fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol | II | (Dinklo et al., 2011) |
| Genestein | 5,7-Dihydroxy-3-(4-hydroxyphenyl)chromen-4-one | I | (Gronlien et al., 2007) |
| Ivermectin | 22,23-Dihydroavermectin B1a + 22,23-dihydroavermectin B1b | I | (Collins and Millar, 2010) |
| LL-00066471 | 4-(5-(4-Chlorophenyl)-2-(2-cyclopropylacetyl)-l, 4-dimethyl-lh-pyrrol-3-yl)benzenesulfonamide | N.D. | (Verma et al., 2021) |
| PAM-2 | (E)-3-furan-2-yl-N-p-tolyl-acrylamide | II | (Targowska-Duda et al., 2019) |
| BNC375 | 4-((1R,3R)-3-(((5-Chloro-2-methoxyphenyl)amino)methyl)-2,2-dimethylcyclopropyl)benzenesulfonamide | I | (Harvey et al., 2019) |
| Compound 28 | 4-(5-(4-Chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide | N.D. | (Sinha et al., 2020) |
| RGM079 | 1-(2',5′-Dihydroxyphenyl)-3-(2-fluoro-4-hydroxyphenyl)-1-propanone | II | (Perez de Vega et al., 2019) |
| JWX-A0108 | 6-(2-Chloro-6-methylphenyl)-2-((3-fluoro-4-methylphenyl)amino)thiazolo[4,5-d]pyrimidin-7(6H)-one | I | (Sun et al., 2019) |
| AVL-3288 | N‐(4‐Chlorophenyl)‐α‐[[(4‐chloro‐phenyl)amino]methylene]‐3‐methyl‐5‐isoxazoleacet‐amide | I | (Thomsen and Mikkelsen, 2012b) |
| B-973 | 3-(3,4-Difluorophenyl)-N-(1-(6-(4-(pyridin-2-yl)piperazin-1-yl)pyrazin-2-yl)ethyl)propanamide | II | (Post-Munson et al., 2017) |
| Compound 111 | 2,4,2′,5′-Tetrahydroxychalcone | II | (Balsera et al., 2014) |
| RO5126946 | 5-Chloro-N-[(1S,3R)-2,2-dimethyl-3-(4-sulfamoyl-phenyl)-cyclopropyl]-2-methoxy-benzamide | I | (Sahdeo et al., 2014) |
| Lu AF58801 | (1S,2S)-2-Phenyl-cyclopropanecarboxylic acid [α(R)-(4-ethoxy-phenyl)-2-hydroxy-ethyl]-amide | I | (Eskildsen et al., 2014) |
N.D., not determined.
α7 PAMs have been shown to be active in many of the same animal models that have been used with the identification of α7-selective agonists. For example, LL-00066471 (Verma et al., 2021) and BNC375 (Wang et al., 2020b) were shown to improve performance in novel object recognition (NOR) and other cognitive tests. Likewise, RO5126946 (Sahdeo et al., 2014), NS1738 (Timmermann et al., 2007), and Lu AF58801, (1S,2S)-2-phenyl-cyclopropanecarboxylic acid [α(R)-(4-ethoxy-phenyl)-2-hydroxy-ethyl]-amide (Eskildsen et al., 2014) were also active in cognitive tests, and BNC375 enhanced long-term potentiation (Wang et al., 2020b). LL-00066471, JWX-A0108 (Sun et al., 2019), and JNJ-1930942 (Dinklo et al., 2011) improved acoustic startle reflex or genetic defects believed to be associated with hippocampal auditory gating. PAM2 (Arias et al., 2020), 1-(2',5′-dihydroxyphenyl)-3-(2-fluoro-4-hydroxyphenyl)-1-propanone (Perez de Vega et al., 2019), TQS (Abbas et al., 2017), and PNU-120596 (Bagdas et al., 2018b) were effective in models of inflammatory or neuropathic pain. Some PAMs are advancing toward clinical trials [(Gee et al. 2017), reviewed in (Yang et al. 2017)]. In 1997, AVL-3288 advanced into a phase I clinical trial for schizophrenia and schizoaffective disorder. However, more recently it has been reported that primary clinical outcomes were negative in follow-up trials (Kantrowitz et al., 2020).
Because of the large currents promoted by α7 PAMs and the reportedly high calcium permeability of α7 receptors when activated by ACh (Seguela et al., 1993), it has been a concern that the use of α7 PAMs and especially type II PAMs in vivo might lead to large potentially cytotoxic increases in intracellular calcium (Williams et al., 2012; Guerra-Alvarez et al., 2015) [see also (Uteshev 2016)]. However, other studies suggest the opposite to be true, that PAMs can be cytoprotective (2009; Kalappa et al., 2013). Also, as noted previously, PAM-potentiated currents in general lack the high calcium permeability reported for α7 receptors activated by ACh alone (Miller et al., 2020) and lose much of their channel-potentiating activity at temperatures approaching body temperature (Sitzia et al., 2011).
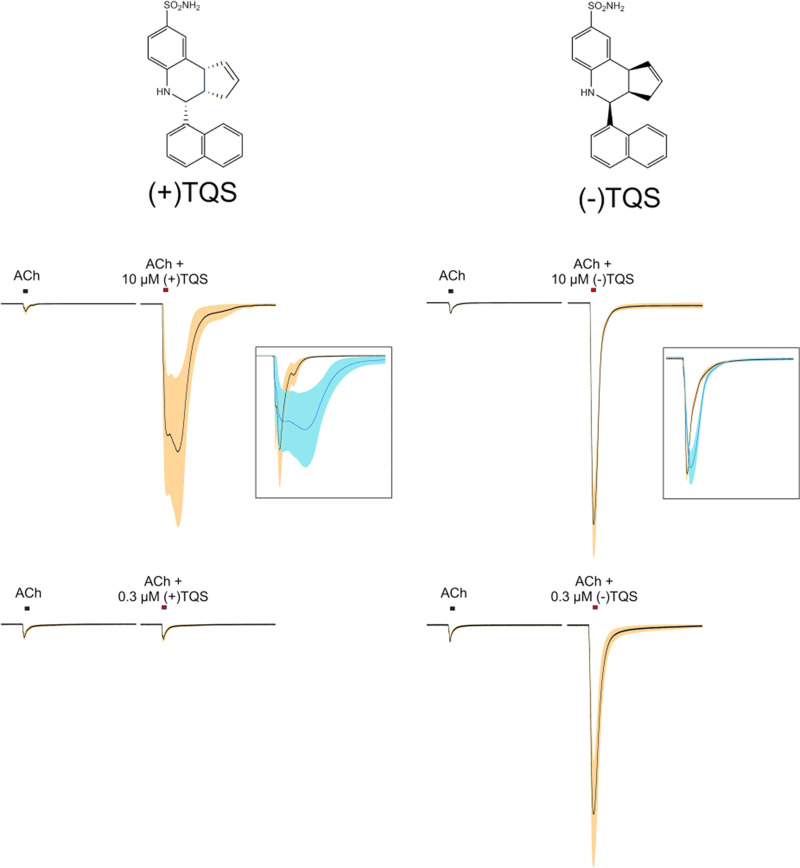
Of all the α7 PAMs identified to date, the chemical scaffold of TQS has been shown to be one of the more interesting and a potential starting point for a large number of novel compounds with very diverse properties. Work done by Neil Millar and colleagues (Gill et al., 2012; Gill-Thind et al., 2015) described multiple analogs variously as allosteric agonists (see below), type I PAMs, type II PAMs, noncompetitive antagonists, and silent allosteric modulators, the last of which we would identify as “allosteric antagonists” (Papke et al., 2020b). We have been fortunate to have Dr. Ganesh Thakur as a collaborator, and he likewise has generated a large library of mostly unpublished TQS-related compounds that we have been able to use for our studies of allosteric mechanisms (Horenstein et al., 2016). The basic syntheses for compounds in this family generates racemic mixtures of stereoisomers, and Dr. Thakur's work has brought to light the fact that the isomers of these TQS analogs can differ greatly in their biologic activity (Thakur et al., 2013; Stokes et al., 2019; Papke et al., 2020b). The separation of the TQS isomers, for example, revealed that the (+) isomer behaves like a type II PAM but only at relatively high concentrations, whereas the (−) isomer is much more potent and functions more like a type I PAM (Fig. 7). The use of racemic TQS therefore amounts to the simultaneous use of two distinctly different PAMs. To date, only two other TQS-related compounds have had their stereoisomers studied separately, and those isomers too were shown to have distinctly different activity profiles (discussed below), suggesting that there is more room for discovery in the characterization of the compounds in this structural family.
Fig. 7.
TQS isomers. Shown on top are the structures of the two isomers of TQS (Stokes et al., 2019). The upper traces are averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 60 µM ACh or 60 µM ACh coapplied with 10 µM (+)TQS or (−)TQS (n equal to 3 and 4, respectively). The lower traces are averaged normalized responses (±S.E.M.) of oocytes (n = 8) expressing human α7 to 60 µM ACh or 60 µM ACh coapplied with 0.3 µM (+)TQS or (−)TQS. The data for the 0.3 µM responses have previously been published in bar graph format (Stokes et al., 2019).
D. Allosteric Activators (Ago–Positive Allosteric Modulator)
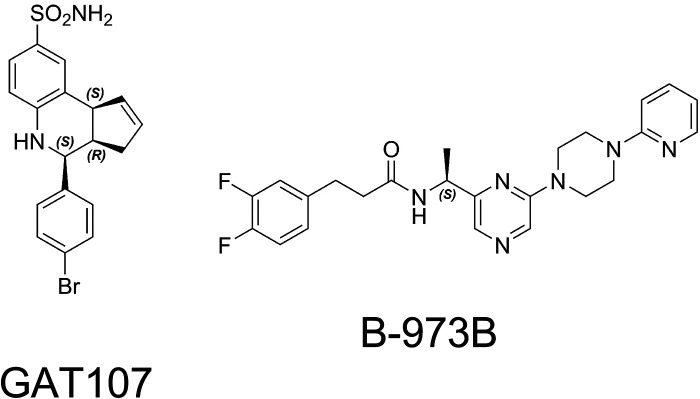
By definition, a PAM is an agent that does not activate the (wild-type) α7 receptor when applied alone but does increase the activation produced by an orthosteric agonist when the two are coapplied or otherwise work in concert (perhaps by preapplication of the PAM). Among the compounds described by the Millar group were agents that behaved as allosteric agonists—that is, they produced channel activation when applied alone without the coapplication of an orthosteric agonist (Gill et al., 2012; Pałczyńska et al., 2012). This activation appeared to rely on the same putative binding site in the second transmembrane domain required for PAM activity (Gill et al., 2011). Additionally, the agents increased activation by orthosteric agonists, supporting their classification as “ago-PAMs,” a term first applied to activators of metabotropic glutamate receptors (Noetzel et al., 2012). One of the first compounds of this type to be identified was 4BP-TQS (Gill et al., 2011). The Thakur laboratory subsequently isolated the isomers of 4BP-TQS (Thakur et al., 2013), and it was shown that all of the activity was accounted for by the (+) isomer, which has subsequently been identified in the literature as “GAT107” (Papke et al., 2014b) (Fig. 8). Additional allosteric activators utilizing the TQS scaffold were identified by the Millar laboratory (Gill-Thind et al., 2015), but these have not been studied in detail, nor have their isomers been separated. More recently, B-973 (Fig. 8) was identified as an ago-PAM (Post-Munson et al., 2017) with a structure that is not related to the TQS scaffold. The isomers of B-973 were isolated, and “B-973B” was identified as the active form (Garai et al., 2018).
Fig. 8.
Ago-PAM structures. GAT107 (Thakur et al., 2013), which is the active isomer of 4BP-TQS (Gill et al., 2011), and B-973B (Garai et al., 2018), which is the active isomer of B-973, 3-(3,4-difluorophenyl)-N-(1-(6-(4-(pyridin-2-yl)piperazin-1-yl)pyrazin-2-yl)ethyl)propanamide (Post-Munson et al., 2017).
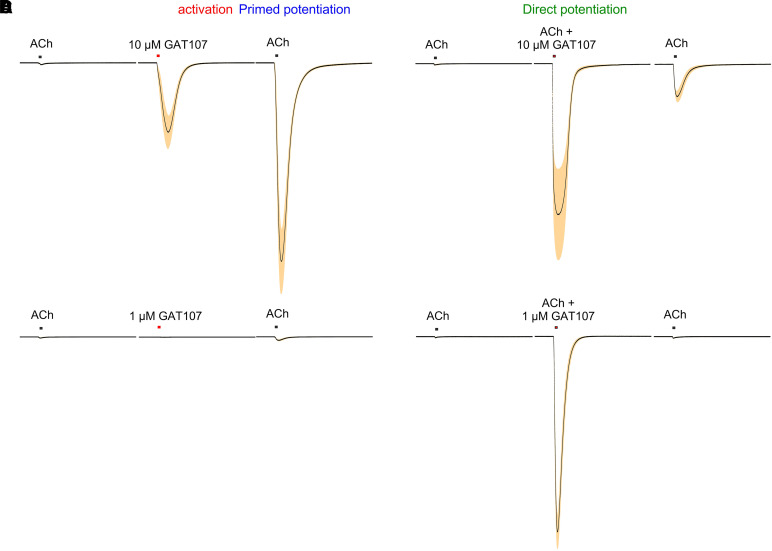
We have characterized three forms of GAT107 activity (Fig. 9). When the compound is applied alone at a sufficiently high concentration, there is “direct allosteric activation” (Fig. 9A). This activity is transient and decays with the washout of free compound from the bath. However, GAT107 appears to remain bound to the PAM binding sites in the transmembrane domains so that after an application of GAT107 alone, a subsequent application of ACh is greatly increased in amplitude, a phenomenon we refer to as “primed potentiation” (Papke et al., 2014b) (Fig. 9A, second ACh response). In oocyte experiments, GAT107-primed potentiation can persist for up to an hour. Responses are, of course, also very large when GAT107 is coapplied with agonist (Fig. 9B), in which case it directly potentiates the ACh response, acting like a typical PAM (“direct potentiation”). The potency of GAT107 as a PAM is greater than its potency as an allosteric agonist so that the application of 1 µM alone does not activate receptors and produces relatively little primed potentiation (Fig. 9C). However, when coapplied with ACh, it does very effectively activate receptors and potentiate the ACh response (Fig. 9D). It should also be noted that there is relatively little primed potentiation after an episode of direct potentiation compared with after the application of GAT107 alone. This suggests that after activation by the simultaneous application of ACh and GAT107 either the receptor adopts a PAM-insensitive state (Williams et al., 2011b) or the GAT107 is less tightly bound to the transmembrane PAM sites during this form of activation. Although differing somewhat in duration and concentration dependence, the functional properties of B-973B are basically similar to those of GAT107 on the level of macroscopic current (Quadri et al., 2019). On the microscopic level, however, although the two ago-PAMs each promote protracted bursts of single-channel opening, each have their own distinct fingerprint of full and subconductance states, and the agents differ in their sensitivity to the noncompetitive antagonist mecamylamine (Quadri et al., 2019; Miller et al., 2020).
Fig. 9.
Concentration and protocol dependence of responses to GAT107. (A) The traces are the averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 10 µM GAT107 applied alone and followed by an application of 60 µM, as compared with the initial responses to ACh alone (n = 5). (B) The traces shown are the averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 10 µM GAT107 coapplied with 60 µM ACh and followed by an application of 60 µM, as compared with the initial responses to ACh alone (n = 5). (C) The traces shown are the averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 1 µM GAT107 applied by itself and followed by an application of 60 µM, as compared with the initial responses to ACh alone (n = 7). (D) The traces shown are the averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 1 µM GAT107 coapplied with 60 µM ACh and followed by an application of 60 µM, as compared with the initial responses to ACh alone (n = 7).
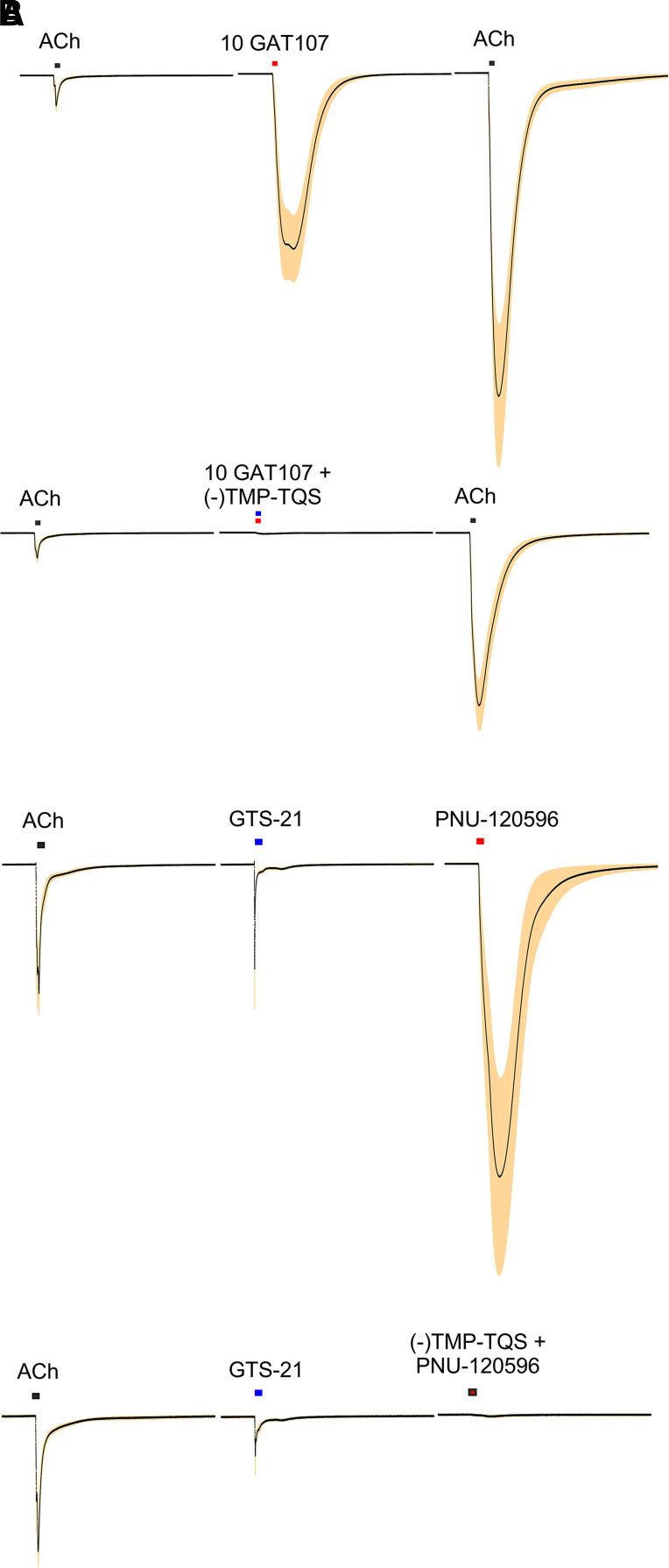
Although it was originally proposed that 4BP-TQS produced allosteric activation solely by binding to the transmembrane PAM site (Gill et al., 2011), several lines of evidence argue for GAT107 binding to additional allosteric sites in the extracellular domain as well as the transmembrane PAM site (Papke et al., 2014b; Horenstein et al., 2016). As mentioned above, there is a clear kinetic difference in transient allosteric activation by GAT107 and its prolonged effects as a PAM. There are also distinct structural epitopes in the receptor that affect allosteric activation without major effects on the PAM activity of GAT107. For example, α7D101A mutants show virtually no allosteric activation by GAT107, whereas the ACh responses are still well potentiated (Horenstein et al., 2016). The TQS analog, 2,3,5,6TMP-TQS (TMP-TQS) was first identified by the Millar laboratory as a silent allosteric modulator because it had no apparent PAM activity yet was also able to antagonize allosteric activation. Subsequent isolation of the TMP-TQS isomers showed that although the (+) isomer was a weak PAM, the (−) isomer was a potent antagonist of GAT107 allosteric activation with relatively little effect on the primed potentiation produced by a GAT107 application (Papke et al., 2020b) (Fig. 10A). This pharmacological and structural separation of the two forms of GAT107 and TMP-TQS supports the existence of specific binding sites for allosteric activation. Therefore, another way in which α7 receptors may be developed as pharmacologic targets is by identifying small ligands that would bind to these allosteric sites and couple with conventional PAMs to produce activation (Gulsevin et al., 2019) or induce other conformational changes. This concept will be discussed in more detail in the section on silent agonists below.
Fig. 10.
Allosteric antagonism by (−)TMP-TQS. (A) The upper traces show the averaged normalized responses (±S.E.M.) of oocytes expressing human α7 to 10 µM GAT107 applied alone and followed by an application of 60 µM, as compared with the initial responses to ACh alone (n = 5) using the same protocol illustrated in Figure 9. The lower traces show the averaged normalized responses (±S.E.M.) obtained with the same protocol but with the coapplication of 100 µM (−)TQS with 10 µM GAT107 (n = 7). (B) Inhibition of responses to PNU-120596 after desensitization produced by 100 µM GTS-21. The upper traces show the averaged normalized responses (±S.E.M.) of oocytes (n = 4) expressing human α7 to a control application of ACh, an application of 100 µM GTS-21 and then the application of 10 µM PNU-120596 alone. The lower traces show the averaged normalized responses (±S.E.M.) obtained with the same protocol but with the coapplication of 100 µM (−)TQS with 10 µM PNU-120596 (n = 6).
The blockade of GAT107 allosteric activation by (−)TMP-TQS (Fig. 10A), would be consistent with this analog functioning as a competitive antagonist at the allosteric activation binding sites. However, it is actually likely that it is also capable of functioning as an inverse agonist at that site. As mentioned earlier, GTS-21 is a partial agonist that produces a significant amount of residual desensitization. Applications of PNU-120596 alone after GTS-21 applications can reactivate channels and produce a current (Papke et al., 2009) (Fig. 10B). Applications of (−)TMP-TQS suppress this reactivation of desensitized channels (Fig. 10B).
VI. Silent Agonists
A. Conditional Activation of α7
In addition to the effects of agonists and allosteric ligands, the conformational states of α7 nAChR can be regulated by the binding of agents identified as “silent agonists” (Chojnacka et al., 2013; Papke et al., 2014a). Even efficacious agonists are relatively inefficient at inducing the open-channel state of α7 and are far more effective at stabilizing agonist-dependent nonconducting states, which are traditionally referred to as “desensitized” (Katz and Thesleff, 1957), a term that may be correct only when referring to ionotropic function. As evidence accumulates for α7 having metabotropic activity (Horenstein and Papke, 2017; Kabbani and Nichols, 2018), we see with ligands like NS6740 a dissociation between channel activation and metabotropic function so that receptors with “desensitized” ion channels may be metabotropically active (Thomsen and Mikkelsen, 2012a; Papke et al., 2015a).
Although there are likely to be more, we can distinguish two classes of agonist-induced nonconducting states: Ds, which can be converted to open-channel states with PAMs, and Di, which is insensitive to PAMs (Williams et al., 2011b). Extending the concept of full and partial agonists, silent agonists bind competitively with efficacious agonists, but with such low probability of inducing channel activation that they appear as antagonists unless coapplied with a PAM. Although silent agonists are relatively ineffective at activating the channel, they do induce Ds and Di unlike the classic competitive antagonist methyllycaconitine (MLA). Signal-transduction studies in non-neuronal cells (Thomsen and Mikkelsen, 2012a; Boulet et al., 2015; Papke et al., 2015a; Yue et al., 2015; Zanetti et al., 2016; King et al., 2017b; Maldifassi et al., 2018) have implicated silent agonists and the Ds and/or Di states of the receptor as likely to mediate channel-independent signaling. Developing specific therapeutics for the treatment of inflammatory diseases and pain may therefore come from defining the structural features of drugs that predict silent agonism. It is not sufficient merely to detect the induction of Ds and Di states but to appreciate the receptor’s dynamic nature and how the distribution of conformational states evolves and changes over time as agonist and/or PAM applications perturb the population of receptors (Papke et al., 2015a; Papke et al., 2018b). Although Di is ultimately favored by high concentrations of PAM and agonists (Fig. 6), Ds may be favored only intermittently, and these dynamics can be differentially regulated by specific ligands (Williams et al., 2011b).
B. Ligands and Structures
Just as there are multiple motifs that may make ligands selective activators of the α7 ion channel (Horenstein et al., 2008), we (Chojnacka et al., 2013; Papke et al., 2014a, 2015a; van Maanen et al., 2015; Quadri et al., 2016, 2017a,b, 2018b) and others (Briggs et al., 2009) identified multiple classes of structurally distinct silent agonists (Fig. 11). One of the first silent agonists to be identified, NS6740 (Briggs et al., 2009), is also arguably one of the most interesting and may point to a fundamental dichotomy in the modes of α7 receptor function for therapeutic purposes both ion channel–mediated and ion channel–independent, corresponding to cognitive function and the CAP, respectively. The efficacy of NS6740 for channel activation is no more than 20% that of ACh (Pismataro et al., 2020), but it very effectively induces nonconducting states that have been shown in oocyte studies to be stable for long periods of time after a single application of NS6740. While this desensitization persists, the receptors are unable to be activated by more efficacious agonists. Throughout this period the desensitization can be perturbed by applications of a PAM like PNU-120596, and interestingly, sequential applications of NS6740 and the long-acting ago-PAM GAT107 can generate large currents persistently for an hour (Papke et al., 2018b).
Fig. 11.
Silent agonists. (A) Structures: NS6740, 1,4-diazabicyclo[3.2.2]non-4-yl[5-[3-(trifluoromethyl)phenyl]-2-furanyl]methanone hydrochloride (Pismataro et al., 2020); KC-1 (Chojnacka et al., 2013); TriETMA, triethylmethylammonium (Papke et al., 2014a); diEPP (Papke et al., 2014a); DMPP, 1,1-dimethyl-4-phenylpiperazin-1-ium iodide (Quadri et al., 2016); 2-NDEP, 1,1-diethyl-4(naphthalene-2-yl)piperazin-1-ium (Gulsevin et al., 2019); R-47 (PMP-072), (R)-N-(4-methoxyphenyl)-2-((pyridin-3-yloxy)methyl)piperazine-1-carboxamide (Clark et al., 2014); 31b, 3-(furan-2-yl)-5-(quinuclidin-3-ylmethyl)-1,2,4-oxadiazole methiodide (Quadri et al., 2018b); and a-conotoxin MrIC (image provided by Dr. Alican Gulsevin). (B) Model for NS6740 pharmacophore adapted from (Pismataro et al., 2020) (image provided by Dr. Maria Chiara Pismataro).
A negative effect of NS6740 on the α7-mediated cognitive effects of a more efficacious α7 agonist was shown by (Briggs et al. 2009), who used NS6740 to block the effects of A-582941 in a mouse model of inhibitory avoidance. Likewise, it was later shown that NS6740 could block the effects of BMS-902483 in NOR (Pieschl et al., 2017). In rat hippocampal slices, NS6740 reduced synaptic plasticity (Papke et al., 2018a). However, in regard to CAP, NS6740 and the desensitizing partial agonist GTS-21 were both shown to effectively reduce the release of TNF-α from microglial cells exposed to the bacterial endotoxin lipopolysaccharide (Thomsen and Mikkelsen, 2012a). Numerous in vivo and in vitro studies have confirmed the activity of GTS-21 as a regulator of the CAP (Kox et al., 2011; Yue et al., 2015; Kashiwagi et al., 2017; Kong et al., 2018; Schaller et al., 2018; Wang et al., 2019; Sitapara et al., 2020; Wang et al., 2020a), and although it is less well studied, NS6740 was also shown to induce significant dose- and time-dependent antinociceptive activity in formalin- and acetic acid–induced nociceptive behaviors as well as in the chronic constrictive nerve injury model for neuropathic pain (Papke et al., 2015a).
C. Function In Vivo and In Vitro
Results like those described above have motivated other studies to identify other silent agonists for potential development as treatments for inflammatory disease and neuropathic pain (Horenstein and Papke, 2017; Bagdas et al., 2018a; Manetti et al., 2018). The compound identified as “KC-1” (5′-phenylanabaseine, 6'-phenyl-3,4,5,6-tetrahydro-2,2'-bipyridine) (Chojnacka et al., 2013) was developed in the laboratory of Nicole Horenstein using an anabaseine scaffold related to GTS-21. In a systematic analysis of linear amines, we identified triethyl methylammonium as a minimally sized silent agonist (Papke et al., 2014a) created by the addition of a methyl group the to the minimally sized α7-selective agonist ethyl dimethyl ammonium (Horenstein et al., 2008). A similar approach was used to generate additional families of silent agonists and to implicate a critical difference in the size of the cationic nitrogen group to produce a shift from active partial agonism to silent agonism (Papke et al., 2014a). One particularly interesting group was the diEPP family based on the ganglionic agonist dimethylphenylpiperazinium with the switch from methyl to larger ethyl subgroups. This family was further developed (Quadri et al., 2016) and led to the identification of two analogs that were subsequently shown to be active in vivo for reducing inflammatory pain [para trifluoromethyl N,N-diethyl-N'-phenylpiperazine (Quadri et al., 2016)] and attenuating inflammation in an animal model of multiple sclerosis [1-ethyl-4-(3-(bromo)phenyl)piperazine (Godin et al., 2020)].
Although the basic assumption based on the pharmacophore studies (Papke et al., 2014a) was that silent agonists work primarily through an extension of the site for orthosteric agonists that is more permissive of the somewhat larger ammonium group, we also investigated the hypothesis that silent agonism, as revealed by the application of PAMs, might also come from ligands that bound to the allosteric activation site implicated in our studies of GAT107. Using in silico screening of our library of diEPP compounds, we identified 1,1-diethyl-4(naphthalene-2-yl)piperazin-1-ium iodide (2-NDEP) as a candidate allosteric silent agonist and confirmed that it generated PNU-120596–dependent currents in the α7C190A mutant, which has an inactivated orthosteric agonist binding site (Gulsevin et al., 2019). Testing the hypothesis that a sulfonium could function as a surrogate for ammonium in a nicotinic agonist led to the identification of 1-ethyl-4-phenylthiomorpholin-1-ium triflate as a silent agonist (Quadri et al., 2017b).
The compound R-47 (also PMP-072) has an interesting history. It was first developed as a proprietary compound that was passed on as intellectual property through a series of now defunct or inactive companies, eventually ending up with Targacept. Once it was finally released, it was published as “R-47” by the team of chemists who originally synthesized it (Clark et al., 2014) and as “PMP-072” by groups who also collaborated with the company that first developed it (van Maanen et al., 2015). The data on PMP-072's activity in a collagen-induced model of rheumatoid arthritis were actually published as part of a Ph.D. thesis 6 years prior to the time when it was permitted to publish the structure. The paper by (Clark et al., 2014) showed that R-47 significantly inhibited the cellular infiltration in a murine model of allergic lung inflammation. More recently, R-47 has been shown to prevent and reverse paclitaxel-induced peripheral neuropathy (Toma et al., 2019).
The compound identified as “31b” is the lead compound from a study of compounds with a methyl-quinuclidine core pharmacophore (Quadri et al., 2018b). This compound can be classified as a silent agonist based on its electrophysiological properties but has not yet been tested with in vivo models. Arecoline, on the other hand, is a silent agonist (Papke et al., 2015b) that is self-administered by hundreds of millions of people on a daily basis because it is probably the most active alkaloid in the areca nut (Gupta et al., 2020), the key ingredient in betel quids (betel nut). Betel (areca) is the fourth most commonly used addictive substance in the world (World Health Organization, 2004; Papke et al., 2020c; Singh et al., 2020).
D. Other Novel Silent Agonists
The functional and structural diversity of conotoxins is enormous, and several have been identified as selective antagonists of α7 nAChRs (see below). Interestingly though, conotoxin MrIC has been implicated to be an α7 silent agonist in cell-based assays (Jin et al., 2014; Mueller et al., 2015). Although it has been reported to be an antagonist of α7 expressed in oocytes (Jin et al., 2014), using a commercially available sample of MrIC (Alomone Laboratories, Jerusalem, Israel) we saw that, although 50 µM MrIC applied alone did not activate α7 receptors, when it was coapplied with 30 µM PNU-120596, substantial currents were stimulated (Fig. 12A). Presumably, if MrIC were coapplied with a standard agonist to α7-expressing cells, it would behave as a competitive antagonist since this a basic property of silent agonists (Papke et al., 2014a). It may be the case that other conotoxins that have been classified as antagonists might have similar silent agonist properties if they were tested with PAM coapplications.
Fig. 12.
Silent agonists revealed by PNU-120596 applications. (A) In the upper traces are the averaged normalized responses (±S.E.M.) of oocytes (n = 3) expressing human α7 to a control application of 60 µM ACh followed by an application of 50 µM conotoxin MrIC (Alomone, Jerusalem Israel). In the lower traces are the averaged normalized responses (±S.E.M.) of oocytes (n = 7) expressing human α7 to a control application of 60 µM ACh followed by an application of 50 µM conotoxin MrIC (Alomone, Jerusalem, Israel) coapplied with 30 µM PNU-120596. (B) The upper traces show the averaged normalized responses (±S.E.M.) of oocytes (n = 5) expressing human α7 to a control application of 60 µM ACh followed by an application of 1 ml room-temperature coffee. Cells were then stimulated with ACh again prior to a coapplication of coffee with 10 µM PNU-120596. The lower traces show the averaged normalized responses (±S.E.M.) of oocytes (n = 7) expressing human α7 to a control application of 60 µM ACh followed by an application of 1 mM of the coffee alkaloid N-methylpyridinium (insert) coapplied with 10 µM PNU-120596.
It is interesting to consider what other foods in our diet might also have silent effects of α7 receptors. For example, we made the somewhat serendipitous observation that, although coffee had no apparent effects on α7 receptors, responses observed when coffee was coapplied to α7 receptors with PNU-120596 (Fig. 12B, upper traces) suggest that there are also previously unknown silent agonists in this widely consumed beverage. There are many biologically active molecules in coffee, and we confirmed that neither caffeine nor the alkaloid trigonelline were α7 silent agonists (not shown). However, N-methylpyridinium, another plentiful alkaloid in coffee (Burton et al., 2020) that is a urinary biomarker for coffee consumption (Lang et al., 2011), is an effective silent agonist (Fig. 12B, lower traces).
VII. α7 Antagonists
A. Snake Toxin Antagonists and Their Analogs
Prior to the cloning of the α7 gene, the associated receptors in brain were identified simply as “α-BTX binding sites” (Jumblatt et al., 1981; Schulz et al., 1991), “α-BTX receptors” (Clarke et al., 1991), or “α-BTX sensitive neuronal nAChR” (Zorumski et al., 1992; Castro and Albuquerque, 1995), and α-BTX is, of course, an excellent antagonist for α7-mediated responses (Uteshev et al., 1996; Alkondon et al., 1998; Kempsill et al., 1999; Drisdel and Green, 2000; Kaiser and Wonnacott, 2000; Xiao et al., 2009). However, α-BTX is also a potent and nearly irreversible antagonist of the nAChR at the neuromuscular junction (Sarvey et al., 1978), so although it has utility for the sorts of binding studies that were used to identify α7-selective agonists (see above) and confirm the presence of α7 receptors in cell lines (Williams et al., 2012) and tissues (Rasmussen and Perry, 2006; Xiao et al., 2009), it has no utility for in vivo studies.
A search for mammalian homologs to the snake toxin that bind to muscle-type and α7 nAChRs brought to light the existence of a large family identified as “three-finger proteins” based on structures that are homologous to important domains in the snake toxins (Nirthanan, 2020). One analog, secreted mammalian Ly-6/urokinase plasminogen activator receptor related protein-1, is secreted by epithelial cells; related proteins in the brain are membrane-tethered via glycosylphosphatidylinositol anchors. They appear to function as endogenous regulators of nAChRs, but the details remain somewhat uncertain [reviewed in Vasilyeva et al. 2017; Tsetlin et al. 2020]. Soluble synthetic proteins of the toxin-like domains of several of these proteins have been shown to be able to modulate the function of numerous proteins, including α7 receptors. Although the secreted mammalian Ly-6/urokinase plasminogen activator receptor related protein-1 protein appears to antagonize α7 function (Shulepko et al., 2020), the water-soluble synthetic variant of human Lynx1 has been reported to upregulate α7 function (Shenkarev et al., 2020).
The conotoxin α-CTx ImII has been reported to be a selective antagonist of α7 nAChR (Ellison et al., 2003). Additional α7-selective conotoxins have been developed by making mutations in the α-conotoxins PnIA (Hopping et al., 2014), ImI (Armishaw et al., 2006), or ArIB (Innocent et al., 2008). Structures of the AChBPs alone or complexed with either an agonist or the conotoxin ImI suggest that although the binding of agonists led to the closing down of the C-loop over the orthosteric ligand binding site from the more open “apo” (resting) configuration, binding of the conotoxin had the effect of pushing the C-loop further back, in the opposite direction as what occurs with the binding of agonists, in addition to blocking the binding site itself (Hansen et al., 2005).
B. α7 Channel Blockers
α7 receptors are sensitive to a variety of open-channel blockers, including the local anesthetics QX-314 and tetracaine as well as the larger slowly reversible antagonists bis-(2,2,6,6-tetramethyl-4-piperidinyl) sebacate and 2,2,6,6-tetramethylpiperidin-4-yl heptanoate. Inhibition by these agents, however, varied depending on whether the channels were activated by ACh alone or ACh in combination with the PAM PNU-120596 (Peng et al., 2013). The anticholinesterase ASS234 (Fig. 13), which was developed as a therapeutic for Alzheimer disease (Romero et al., 2020), was shown to be a noncompetitive antagonist of α7 and was used as a starting point to develop additional antagonists, with compound 38 proposed as a new lead compound (Criado et al., 2016). Compound 7i (Fig. 13) was identified as a lead compound in a study to identify α7 antagonists that might have utility as antidotes for organophosphorus nerve agent intoxication (Peng et al., 2010). However, there has not been much follow-up on either of these studies. A more recent study of piperidine-spirooxadiazole derivatives (Zhang et al., 2020a) identified compound B10 (Fig. 13) as a noncompetitive antagonist of α7 with an IC50 of 5.4 µM and reasonably good selectivity relative to α4β2 and α3β4 nAChRs.
Fig. 13.
Antagonist structures. ASS234, N-({5-[3-(1-benzyl-4-piperidinyl)propoxy]-1-methyl-1H-indol-2-yl}methyl)-N-methyl-2-propyn-1-amine (Criado et al., 2016); compound 38, 2-(1-benzylpiperidin-4-yl)ethan-1-amine (Criado et al., 2016); compound 7i, N-((1-benzylpiperidin-4-yl)methyl)-1-(2-chlorobenzyl)-N-methyl-1H-1,2,3-triazole-4-carboxamide (Peng et al., 2010); B10, 3-(4-bromophenyl)-8-methyl-1-oxa-2,4,8-triazaspiro[4.5]dec-2-ene (Zhang et al., 2020a); mecamylamine, N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine;hydrochloride; tkP3BzPB, 1,2,4,5-tetra-{5-[1-(3-benzyl)pyridinium]pent-1-yl}benzene tetrabromide; and MLA.
The widely used, relatively nonselective nAChR antagonist mecamylamine (Fig. 13) inhibits α7 currents activated by ACh alone, with an IC50 of 10 ± 1 µM and currents activated by ACh coapplied with PNU-120596 with an IC50 of 4.8 ± 0.7 µM (Peng et al., 2013), which was roughly an order of magnitude lower than its potency for inhibiting heteromeric neuronal nAChR (Papke et al., 2013). As noted earlier, α7 currents activated by the ago-PAM B-973B are largely insensitive to mecamylamine (Quadri et al., 2019). 1,2,4,5-Tetra-{5-[1-(3-benzyl)pyridinium]pent-1-yl}benzene tetrabromide (Fig. 13) is a potent α7-selective noncompetitive antagonist that produces a slowly reversible block of both open and closed channels. It has an IC50 of 1.0 ± 0.1 µM for α7 and a time constant for recovery of 26 minutes. It is 48-fold less potent for blocking α4β2 nAChRs and 10-fold less potent for α3β4 nAChRs, and the block of these heteromeric receptors is readily reversible (Lopez-Hernandez et al., 2009). It may be noted, though, that with the exception of mecamylamine, which is readily available and widely used, the other α7-selective noncompetitive antagonists are neither easily available nor commonly used.
C. Methyllycaconitine
By far the most commonly used α7 antagonist is MLA (methyllycaconitine), which was first isolated from Delphinium brownii (Aiyar et al., 1979). Although initially described as having low nanomolar potency for inhibiting the α7 responses of cultured hippocampal neurons (Alkondon et al., 1992) and of approximately 100 nM in brain slices (Frazier et al., 1998), in oocyte studies MLA has a potency of 1.2 ± 0.2 µM for the inhibition of α7 and was a 30-fold less potent antagonist of α4β2 nAChRs but only 2-fold less potent for inhibiting α3β4 nAChRs (Lopez-Hernandez et al., 2009). As well as being active in vitro (Alkondon et al., 1992; Donnelly-Roberts et al., 1996; Alkondon et al., 1998; Virginio et al., 2002; Lopez-Hernandez et al., 2009), MLA has also been used in many in vivo studies to evaluate the role of α7 receptors in various central nervous system functions (Rao et al., 1996; Felix and Levin, 1997; Damaj et al., 1999; Klink et al., 2001; Markou and Paterson, 2001; Levin et al., 2002; Andreasen et al., 2009). These differences in the apparent potency of MLA are curious but may relate to differences in the methodology used. The high potencies reported for the hippocampal culture and slice experiments ( Alkondon et al.,1992; Frazier et al., 1998; 2880) were associated with prolonged bath applications of MLA at low concentration, whereas in the oocyte studies it was acutely applied without preincubation. It may be the case that the receptors acquire high affinity for the ligand with prolonged exposure. This would be analogous to the behavior of nicotine with its “high-affinity receptors” that only bind nicotine with nanomolar affinity after the receptors equilibrate into desensitized states over time. Nicotine’s potency for transient activation of α4β2 receptors is 2.5 µM (Papke et al., 2007), which is much lower than the 4.6 nM affinity reported in binding studies of α4β2 receptors expressed in oocytes (Parker et al., 1998).
MLA is commonly used to confirm the role of α7 receptors in CAP (Tasaka et al., 2015; Bagdas et al., 2016; Donvito et al., 2017; Krafft et al., 2017; Gao et al., 2018; Papke et al., 2018b; Quadri et al., 2018a; Yin et al., 2019; Li et al., 2020a; Pinheiro et al., 2020). The dosage used in these studies has typically been around 3 mg/kg (Gao et al., 2018; Yin et al., 2019; Li et al., 2020a) but in some cases was as low as 1 mg/kg (Tasaka et al., 2015; Pinheiro et al., 2020) and, by one group, was as high as 10 mg/kg (Bagdas et al., 2016; Donvito et al., 2017; Papke et al., 2018b; Quadri et al., 2018a), wherein it likely had significant effects on the α3* receptors of autonomic ganglia as well as on the α7 receptors on cells of the immune system. An alternative approach for showing the critical role of α7 in CAP has been the use of α7-knockout mice (Bagdas et al., 2016; Li et al., 2018; Fang et al., 2019; Shao et al., 2019).
MLA is generally considered a competitive antagonist of α7 activation by orthosteric agonists, and it has been used as an alternative ligand to identify neuronal α-BTX binding sites (Yum et al., 1996) shown to bind in the same sites as α-BTX but with more rapid kinetics of association and disassociation (Davies et al., 1999). However, the binding of MLA appears to do more than simply block the access of orthosteric agonists to the binding sites, especially in regard to CAP and the allosteric activation of α7. Like NS6740 and GTS-21, MLA was also shown to decrease the microglia response to lipopolysaccharide stimulation of TNF-α (Thomsen and Mikkelsen, 2012a), which to some degree might confound its use as an antagonist in the in vivo studies of CAP.
In regard to allosterically modulated receptors, MLA appears to be more of an inverse agonist than a simple blocker of the ACh binding site. When α7 receptors in outside-out patch-clamp experiments were activated by a solution containing ACh and PNU-120596, the long bursts stimulated by the drug exposure continued on average for another 2.57 seconds after the drugs were removed, suggesting that no further binding was required to maintain the bursting behavior. However, when bursting channels were exposed to a solution containing MLA, the bursts ceased on average in 220 milliseconds, suggesting that MLA actively suppressed channel reopening (Williams et al., 2011b). Under conditions in which persistent activation of α7 receptors was achieved by application of PNU-120596 to receptors that had covalently bound (tethered) agonists, applications of MLA nonetheless produced transient, concentration-dependent decreases in current. When persistent currents were generated by sequential applications of NS6740 and GAT107, applications of MLA at high concentrations (≥100 nM) reduced current, whereas lower concentrations actually produced concentration-dependent increases in current (Papke et al., 2018b).
VIII. Discussion and Conclusions
α7 nAChRs are marvelously complex and challenging drug targets with a rich array of conformational states regulated by both orthosteric and allosteric binding sites (Fig. 14). Although ligands working at the orthosteric sites give us a mere glimpse at ion-channel activation, and silent agonists binding in the extended orthosteric site give us not even that much, both classes of ligands take the receptors into nonconducting states that may function in ways that we are only beginning to understand. The drugs binding to the allosteric modulator sites have revealed something of the complex conformational landscape associated with the nonconducting states, which themselves provide an entirely new dimension of potentially functional states. On top of this already complex matrix of interacting states, we have yet to appreciate in any real detail how this matrix expands as ligands bind at multiple sites that may initially be similar but dynamically change with increasing levels of agonist occupancy.
Fig. 14.
Binding sites for therapeutic ligands on α7 nAChRs. Cartoon of a cut-away view of an α7 receptor subunit complex (two subunits removed) and the approximate locations of the binding sites for the ligands discussed in this review. Located in the extracellular vestibule are putative binding sites (A) for allosteric ligands, such as ago-PAMs, allosteric agonists (2NDEP), and allosteric antagonists/inverse agonists, such as (−)TMP-TQS. Located at subunit interfaces on the outer surface of the extracellular domain are the binding sites (O) for ACh and other orthosteric agonists. These sites will also overlap the sites (S) that bind somewhat larger silent agonists. The binding site for PAMs and other allosteric modulators (M) is within the transmembrane domain and requires specific residues at the outer end of the second transmembrane domain. This figure is adapted from (Papke and Lindstrom 2020).
In this review, we have necessarily focused on channel activation as a reporter of α7 conformational dynamics. However, the addition of CAP to the profile of α7 therapeutics means that one of the greatest challenges in in the future will be to understand how conformational changes regulated by ligand binding to extracellular and transmembrane sites translate to intracellular systems of signal transduction that exist in α7-expressing cells of the immune system, some of which apparently do not even have the capacity for channel activation. We are only beginning to understand the mechanisms connecting ligand binding to channel activation in heteromeric receptors for which structures of extracellular and transmembrane domains are available (Morales-Perez et al., 2016; Walsh et al., 2018; Gharpure et al., 2019). However, each nAChR has a unique intracellular domain, and we have very limited understanding (Stokes et al., 2015) of their functions. The intracellular domain of α7 receptor subunits has features that have been well conserved through evolution and hold promise for bettering our understanding of the function of α7 receptors.
Although the α7-selective agonists discussed are clearly active in the various assays that were used to identify them, continued work in this area would benefit from more consideration of how they will be presented to the receptors in vivo. In most of the systems used, detection of any response at all required the rapid application of relatively high concentrations of agonist to evoke a coordinated response from a significant fraction of the receptors. This mode of delivery and synchronized activation of receptors are largely irrelevant to the therapeutic delivery of drugs, which would typically be associated with slow delivery of low concentrations of drug (Papke et al., 2011). Although an attempt was made to model this with EVP-6124 and perhaps gave misleading results (Fig. 3), it should be kept in mind for all fast-acting, nondesensitizing agonists that their activity in vivo should be characterized with more relevant protocols.
As noted above, of all the recently characterized α7 agonists, the DPP family stands out as the most novel both in structure and functional diversity. They present a challenge to our conventional models of the nAChR pharmacophore, and because of their wide range of desensitizing activities, studies of their activity in vivo may help determine the relative significance of desensitization for specific indications. Another area in which additional work would be beneficial is in regard to separation of racemic compounds into component isomers of differing activity. This will not only provide for more selective drugs with specific activities, but also, as structural models continue to be developed, knowledge of the stereochemistry of active versus inactive compounds will permit more productive structure-based design of new drugs.
Clearly the study of α7 nAChR presents unique challenges that when appreciated and met will allow for greatly improved therapeutic targeting for particular indications. We need to better understand the conformational dynamics of the receptor as regulated by ligand binding. This can be accomplished by continuing to generate new chemical tools and characterizing the time- and concentration-dependent effects of those ligands on both the conducting and nonconducting states of the receptor. Those data can then also be used to inform the experimental design in a variety of functional assays. Through understanding how specific ligands manipulate all of the conformational states of α7, we will be able to target individual elements in the intracellular cascades associated with inflammatory disease using drugs, such as silent agonists and our recently discovered class of allosteric agonists, as well as to hopefully further develop new therapeutics for cognitive disorders and dementias.
Acknowledgments
We thank Clare Stokes for retrieving data from the laboratory archives, proofreading, and other important comments on the manuscript. We also thank Drs. Alican Gulsevin and Maria Chiara Pismataro for contributing images to Figure 11.
Abbreviations
- ACh
acetylcholine
- AChBP
acetylcholine binding protein
- α-BTX
α-bungarotoxin
- CAP
cholinergic anti-inflammatory pathway
- diEPP
1,1-diethyl-4-phenylpiperazinium
- DPP
dipicolylaminopyrimdine
- EVP-6124
(R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide
- FLIPR
Fluorescent Imaging Plate Reader
- FRM-17874
(R)-7-fluoro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide
- GAT107
4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide
- IL
interleukin
- KC-1
5′-phenylanabaseine, 6'-phenyl-3,4,5,6-tetrahydro-2,2'-bipyridine
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- 2-NDEP
1,1-diethyl-4(naphthalene-2-yl)piperazin-1-ium iodide
- NOR
novel object recognition
- OA
orthosteric activation
- PAM
positive allosteric modulator
- PHA-543,613
N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide
- PHA-709829
N-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide
- PNU-282987
N-[(3R)-1-azabicyclo[2.2.2]octan-3-yl]-4-chlorobenzamide
- PNU-120596
1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea
- RIC-3
resistance to cholinesterase 3
- STAT3
signal transducer and activator of transcription 3
- TNF
tumor necrosis factor-α.
Appendix
Figure preparation: To illustrate the functional properties of α7-targeting ligands discussed in this review, we have drawn upon the large archive of data in the Papke laboratory. Except when noted, much of the data were used in the preparation of papers that are cited in the review or come from unpublished experiments with the same protocols. Therefore, these figures are to a degree adapted from those prior papers, and when appropriate, statistical analyses are provided in the original papers. However, the data for the illustrations in this review were all generated from fresh analyses of the original pClamp data files.
Expression in Xenopus Oocytes
The human α7 nAChR clone was obtained from Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA). The human resistance to cholinesterase 3 clone was obtained from Dr. M. Treinin (Hebrew University, Jerusalem, Israel) and coinjected with α7 to improve the level and speed of α7 receptor expression without affecting the pharmacological properties of the receptors (Halevi et al., 2003). Subsequent to linearization and purification of the plasmid cDNAs, complementary RNAs were prepared using the mMessage mMachine in vitro RNA transcription kit (Ambion, Austin, TX).
Oocytes were surgically removed from mature female Xenopus laevis frogs (Nasco, Ft. Atkinson, WI). Frogs were maintained in the Animal Care Service facility of the University of Florida, and all procedures were approved by the University of Florida Institutional Animal Care and Use Committee. In brief, the frog was first anesthetized for 15–20 minutes in 1.5-liter frog tank water containing 1 g of 3-aminobenzoate methanesulfonate buffered with sodium bicarbonate. The harvested oocytes were treated with 1.4 mg/ml type 1 collagenase (Worthington Biochemicals, Freehold NJ) for 2–4 hours at room temperature in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES, and 12 mg/l tetracycline, pH 7.6) to remove the ovarian tissue and the follicular layers. Stage V oocytes were subsequently isolated and injected with 4–6 ng α7 RNA and 2–3 ng RIC-3 RNA (2:1 ratio) in 50 nl water. Oocytes were maintained in Barth’s solution with calcium [additional 0.32 mM Ca(NO3)2 and 0.41 mM CaCl2], and recordings were carried out 1–14 days after injection.
Two-Electrode Voltage-Clamp Electrophysiology
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City, CA) (Papke and Stokes, 2010). Both the voltage and current electrodes were filled with 3 M KCl. Oocytes were voltage-clamped at −60 mV at room temperature (24°C). The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, and 1 μM atropine, pH 7.2) at 2 ml/min. The control ACh concentrations were 60 μM.
Solutions were applied from 96-well plates via disposable tips. Drug applications were 12 seconds in duration followed by 181-second washout periods. The responses were calculated as both peak current amplitudes and net charge, as previously described (Papke and Porter Papke, 2002). Data were collected at 50 Hz, filtered at 20 Hz, and analyzed by Clampfit 9.2 or 10.0 (Molecular Devices) and Excel (Microsoft, Redmond, WA). Data were expressed as mean ± S.E.M. from at least four oocytes for each experiment and plotted with Kaleidagraph 4.5.2 (Abelbeck Software, Reading, PA). Multicell averages were calculated for comparisons of complex responses. Averages of the normalized data were calculated for each of the 10,322 points in each of the 206.44-second traces (acquired at 50 Hz) as well as the S.E. for those averages.
Authorship Contributions
Participated in research design: Papke, Horenstein.
Conducted experiments: Papke.
Contributed new reagents or analytic tools: Horenstein.
Performed data analysis: Papke.
Wrote or contributed to the writing of the manuscript: Papke, Horenstein.
Footnotes
The authors are supported by National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM57481]. This grant was first funded in 2000 and bears the same title as this review.
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Abbas M, Alzarea S, Papke RL, Rahman S (2017) The α7 nicotinic acetylcholine receptor positive allosteric modulator attenuates lipopolysaccharide-induced activation of hippocampal IκB and CD11b gene expression in mice. Drug Discov Ther 11:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker BAJacobsen EJRogers BNWishka DGReitz SCPiotrowski DWMyers JKWolfe MLGroppi VEThornburgh BA, et al. (2008) Discovery of N-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide as an agonist of the alpha7 nicotinic acetylcholine receptor: in vitro and in vivo activity. Bioorg Med Chem Lett 18:3611–3615. [DOI] [PubMed] [Google Scholar]
- Adams DJ, Nutter TJ (1992) Calcium permeability and modulation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J Physiol Paris 86:67–76. [DOI] [PubMed] [Google Scholar]
- Aiyar VN, Benn MH, Hanna T, Jacyno J, Roth SH, Wilkens JL (1979) The principal toxin of Delphinium brownii Rydb., and its mode of action. Experientia 35:1367–1368. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX (1995) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. III. Agonist actions of the novel alkaloid epibatidine and analysis of type II current. J Pharmacol Exp Ther 274:771–782. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX (1998) alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810:257–263. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Wonnacott S, Albuquerque EX (1992) Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol 41:802–808. [PubMed] [Google Scholar]
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX (1994) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonist-elicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther 271:494–506. [PubMed] [Google Scholar]
- Alsharari SD, Freitas K, Damaj MI (2013) Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem Pharmacol 86:1201–1207. [DOI] [PubMed] [Google Scholar]
- Andersen N, Corradi J, Sine SM, Bouzat C (2013) Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc Natl Acad Sci USA 110:20819–20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR, Bouzat C (2016) Exploring the positive allosteric modulation of human α7 nicotinic receptors from a single-channel perspective. Neuropharmacology 107:189–200. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP (2009) Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 23:797–804. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR (1995) Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res 674:252–259. [DOI] [PubMed] [Google Scholar]
- Arias HRGhelardini CLucarini ETae HSYousuf AMarcovich IManetti DRomanelli MNElgoyhen ABAdams DJ, et al. (2020) (E)-3-Furan-2-yl-N-p-tolyl-acrylamide and its derivative DM489 decrease neuropathic pain in mice predominantly by α7 nicotinic acetylcholine receptor potentiation. ACS Chem Neurosci 11:3603–3614. [DOI] [PubMed] [Google Scholar]
- Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF (2006) Alpha-selenoconotoxins, a new class of potent alpha7 neuronal nicotinic receptor antagonists. J Biol Chem 281:14136–14143. [DOI] [PubMed] [Google Scholar]
- Arunrungvichian K, Fokin VV, Vajragupta O, Taylor P (2015) Selectivity optimization of substituted 1,2,3-triazoles as α7 nicotinic acetylcholine receptor agonists. ACS Chem Neurosci 6:1317–1330. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI (2018a) New insights on neuronal nicotinic acetylcholine receptors as targets for pain and inflammation: A focus on α7 nAChRs. Curr Neuropharmacol 16:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Meade JA, Alkhlaif Y, Muldoon PP, Carroll FI, Damaj MI (2018b) Effect of nicotine and alpha-7 nicotinic modulators on visceral pain-induced conditioned place aversion in mice. Eur J Pain. 22:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI (2016) The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol 173:2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera BMulet JFernández-Carvajal Ade la Torre-Martínez RFerrer-Montiel AHernández-Jiménez JGEstévez-Herrera JBorges RFreitas AELópez MG, et al. (2014) Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: a new target for a privileged structure. Eur J Med Chem 86:724–739. [DOI] [PubMed] [Google Scholar]
- Barbier AJHilhorst MVan Vliet ASnyder PPalfreyman MGGawryl MDgetluck NMassaro MTiessen RTimmerman W, et al. (2015) Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin Ther 37:311–324. [DOI] [PubMed] [Google Scholar]
- Beinat CBanister SDvan Prehn SDoddareddy MRHibbs DSako MChebib MTran TAl-Muhtasib NXiao Y, et al. (2012) Consequences of linker length alteration of the α7 nicotinic acetylcholine receptor (nAChR) agonist, SEN12333. Bioorg Med Chem Lett 22:2380–2384. [DOI] [PubMed] [Google Scholar]
- Beinat CReekie TBanister SDO’Brien-Brown JXie TOlson TTXiao YHarvey AO’Connor SColes C, et al. (2015) Structure-activity relationship studies of SEN12333 analogues: determination of the optimal requirements for binding affinities at α7 nAChRs through incorporation of known structural motifs. Eur J Med Chem 95:277–301. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Boucard A, Trocme-Thibierge C, Morain P (2008) Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology (Berl) 196:555–564. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M (1992) Pharmacological properties of the homomeric alpha 7 receptor. Neurosci Lett 146:87–90. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, Gopalakrishnan M (2008) Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2-M3 segment. Mol Pharmacol 74:1407–1416. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Turner SL, Marks MJ, Collins AC (1990) Selective changes in sensitivity to cholinergic agonists and receptor changes elicited by continuous physostigmine infusion. J Pharmacol Exp Ther 255:187–196. [PubMed] [Google Scholar]
- Biton BBergis OEGalli FNedelec ALochead AWJegham SGodet DLanneau CSantamaria RChesney F, et al. (2007) SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology 32:1–16. [DOI] [PubMed] [Google Scholar]
- Bodnar ALCortes-Burgos LACook KKDinh DMGroppi VEHajos MHigdon NRHoffmann WEHurst RSMyers JK, et al. (2005) Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem 48:905–908. [DOI] [PubMed] [Google Scholar]
- Boess FGDe Vry JErb CFlessner THendrix MLuithle JMethfessel CRiedl BSchnizler Kvan der Staay FJ, et al. (2007) The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. J Pharmacol Exp Ther 321:716–725. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. [DOI] [PubMed] [Google Scholar]
- Boulet LP, Gauvreau GM, Cockcroft DW, Davis B, Vachon L, Cormier Y, O’Byrne PM (2015) Effects of ASM-024, a modulator of acetylcholine receptor function, on airway responsiveness and allergen-induced responses in patients with mild asthma. Can Respir J 22:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J (1987) Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci USA 84:7763–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276. [DOI] [PubMed] [Google Scholar]
- Briggs CAAnderson DJBrioni JDBuccafusco JJBuckley MJCampbell JEDecker MWDonnelly-Roberts DElliot RLGopalakrishnan M, et al. (1997) Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav 57:231–241. [DOI] [PubMed] [Google Scholar]
- Briggs CAGrønlien JHCurzon PTimmermann DBWeen HThorin-Hagene KKerr PAnderson DJMalysz JDyhring T, et al. (2009) Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors. Br J Pharmacol 158:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CASchrimpf MRAnderson DJGubbins EJGrønlien JHHåkerud MWeen HThorin-Hagene KMalysz JLi J, et al. (2008) Alpha7 nicotinic acetylcholine receptor agonist properties of tilorone and related tricyclic analogues. Br J Pharmacol 153:1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad LMFelthouse CZwart RMcPhie GIPearson KHCraig PJWallace LBroadmore RJBoot JRKeenan M, et al. (2002) PSAB-OFP, a selective alpha 7 nicotinic receptor agonist, is also a potent agonist of the 5-HT3 receptor. Eur J Pharmacol 452:137–144. [DOI] [PubMed] [Google Scholar]
- Burton IW, Martinez Farina CF, Ragupathy S, Arunachalam T, Newmaster S, Berrué F (2020) Quantitative NMR methodology for the authentication of roasted coffee and prediction of blends. J Agric Food Chem 68:14643–14651. [DOI] [PubMed] [Google Scholar]
- Camacho-Hernandez GA, Stokes C, Duggan BM, Kaczanowska K, Brandao-Araiza S, Doan L, Papke RL, Taylor P (2019) Synthesis, pharmacological characterization, and structure-activity relationships of noncanonical selective agonists for α7 nAChRs. J Med Chem 62:10376–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Hernandez GA, Taylor P (2020) Lessons from nature: Structural studies and drug design driven by a homologous surrogate from invertebrates, AChBP. Neuropharmacology 179:108108. [DOI] [PubMed] [Google Scholar]
- Carbonetto ST, Fambrough DM, Muller KJ (1978) Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci USA 75:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX (1995) alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J 68:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P (1981) The acetylcholine receptor: an “allosteric” membrane protein, Academic Press Inc., New York. [PubMed] [Google Scholar]
- Changeux J-P, Revah F (1987) The acetylcholine receptor molecule: allosteric sites and the ion channel. Trends Neurosci 10:245–250. [Google Scholar]
- Chatterjee PK, Al-Abed Y, Sherry B, Metz CN (2009) Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation. Am J Physiol Cell Physiol 297:C1294–C1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K, Papke RL, Horenstein NA (2013) Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett 23:4145–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB, Lamppu D, Libertine L, McDonough A, Kumar A, LaRosa G, Rush R, Elbaum D (2014) Discovery of novel 2-((pyridin-3-yloxy)methyl)piperazines as α7 nicotinic acetylcholine receptor modulators for the treatment of inflammatory disorders. J Med Chem 57:3966–3983. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Hamill GS, Nadi NS, Jacobowitz DM, Pert A (1991) 3H-nicotine-and 125I-alpha-bungarotixin-labeled nicotinic receptors in the interpeduncular nucleus of rats. II. Effects of habenular deafferenation. J Comput Neurosci 251:407–413. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz RD, Paul SM, Pert CB, Pert A (1985) Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci 5:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Millar NS (2010) Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol Pharmacol 78:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JZusi FCMcDonald IMKing DHill MDIwuagwu CMate RAFang HZhao RWang B, et al. (2016) Design and synthesis of a new series of 4-heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes as α7 nicotinic receptor agonists. 1. Development of pharmacophore and early structure-activity relationship. J Med Chem 59:11171–11181. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M (1991) Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature 350:235–238. [DOI] [PubMed] [Google Scholar]
- Craig PJBose SZwart RBeattie REFolly EAJohnson LRBell EEvans NMBenedetti GPearson KH, et al. (2004) Stable expression and characterisation of a human alpha 7 nicotinic subunit chimera: a tool for functional high-throughput screening. Eur J Pharmacol 502:31–40. [DOI] [PubMed] [Google Scholar]
- Criado M, Mulet J, Sala F, Sala S, Colmena I, Gandía L, Bautista-Aguilera OM, Samadi A, Chioua M, Marco-Contelles J (2016) N-Benzylpiperidine derivatives as α7 nicotinic receptor antagonists. ACS Chem Neurosci 7:1157–1165. [DOI] [PubMed] [Google Scholar]
- Dallanoce CMagrone PMatera CFrigerio FGrazioso GDe Amici MFucile SPiccari VFrydenvang KPucci L, et al. (2011) Design, synthesis, and pharmacological characterization of novel spirocyclic quinuclidinyl-Δ2-isoxazoline derivatives as potent and selective agonists of α7 nicotinic acetylcholine receptors. ChemMedChem 6:889–903. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Dukat M, Martin BR (1999) Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther 291:1284–1291. [PubMed] [Google Scholar]
- Dani JA (2001) Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 49:166–174. [DOI] [PubMed] [Google Scholar]
- Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Wonnacott S (1999) Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology 38:679–690. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Zoltewicz J, Henry JC, Muraskin S, Kem WR, Papke RL (1995) Characterization of a series of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative is a selective agonist at neuronal nicotinic alpha 7/[125I]a-bungarotoxin receptor subtypes. Mol Pharm 47:164–171. [PubMed] [Google Scholar]
- de Jonge WJvan der Zanden EPThe FOBijlsma MFvan Westerloo DJBennink RJBerthoud HRUematsu SAkira Svan den Wijngaard RM, et al. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6:844–851. [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M (1994) Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol 349:325–342. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Connolly J, Boulter J, Wada E, Wada K, Swanson LW, Patrick J, Heinemann S (1988) Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron 1:45–54. [DOI] [PubMed] [Google Scholar]
- Deshpande AVinayakamoorthy RMGarg BKThummapudi JPOza GAdhikari KAgarwal ADalvi PIyer SThulasi Raman S, et al. (2020) Why does knocking out NACHO, but not RIC3, completely block expression of α7 nicotinic receptors in mouse brain? Biomolecules 10:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinklo T, Lesage AS, Grantham CG (2006) Desensitization characteristics of the human alpha7nAChR/5HT3A chimera receptor. J Mol Neurosci 30:109–110. [DOI] [PubMed] [Google Scholar]
- Dinklo TShaban HThuring JWLavreysen HStevens KEZheng LMackie CGrantham CVandenberk IMeulders G, et al. (2011) Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther 336:560–574. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Xue IC, Arneric SP, Sullivan JP (1996) In vitro neuroprotective properties of the novel cholinergic channel activator (ChCA), ABT-418. Brain Res 719:36–44. [DOI] [PubMed] [Google Scholar]
- Donvito GBagdas DToma WRahimpour EJackson AMeade JAAlSharari SKulkarni ARIvy Carroll FLichtman AH, et al. (2017) The interaction between alpha 7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α represents a new antinociceptive signaling pathway in mice. Exp Neurol 295:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM (2003) Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J Neurosci 23:8445–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Green WN (2000) Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J Neurosci 20:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop JLock TJow BSitzia FGrauer SJow FKramer ABowlby MRRandall AKowal D, et al. (2009) Old and new pharmacology: positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor by the 5-hydroxytryptamine(2B/C) receptor antagonist SB-206553 (3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b′]di pyrrole-1(2H)-carboxamide). J Pharmacol Exp Ther 328:766–776. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Roncarati R, Jow B, Bothmann H, Lock T, Kowal D, Bowlby M, Terstappen GC (2007) In vitro screening strategies for nicotinic receptor ligands. Biochem Pharmacol 74:1172–1181. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S (1989) The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron 3:487–496. [DOI] [PubMed] [Google Scholar]
- Egea J, Buendia I, Parada E, Navarro E, León R, Lopez MG (2015) Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol 97:463–472. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S (1994) Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79:705–715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J (2001) Alpha10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98:3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M, McIntosh JM, Olivera BM (2003) Alpha-conotoxins ImI and ImII. Similar alpha 7 nicotinic receptor antagonists act at different sites. J Biol Chem 278:757–764. [DOI] [PubMed] [Google Scholar]
- Eskildsen J, Redrobe JP, Sams AG, Dekermendjian K, Laursen M, Boll JB, Papke RL, Bundgaard C, Frederiksen K, Bastlund JF (2014) Discovery and optimization of Lu AF58801, a novel, selective and brain penetrant positive allosteric modulator of alpha-7 nicotinic acetylcholine receptors: attenuation of subchronic phencyclidine (PCP)-induced cognitive deficits in rats following oral administration. Bioorg Med Chem Lett 24:288–293. [DOI] [PubMed] [Google Scholar]
- Faghih RGopalakrishnan SMGronlien JHMalysz JBriggs CAWetterstrand CWeen HCurtis MPSarris KAGfesser GA, et al. (2009) Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J Med Chem 52:3377–3384. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang J, Chen F, Xu Y, Zhang H, Wang Y (2019) α7nAChR deletion aggravates myocardial infarction and enhances systemic inflammatory reaction via mTOR-signaling-related autophagy. Inflammation 42:1190–1202. [DOI] [PubMed] [Google Scholar]
- Fei R, Zhang Y, Wang S, Xiang T, Chen W (2017) α7 Nicotinic acetylcholine receptor in tumor-associated macrophages inhibits colorectal cancer metastasis through the JAK2/STAT3 signaling pathway. Oncol Rep 38:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R, Levin ED (1997) Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience 81:1009–1017. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Nozulak J, Lingenhoehl K, McAllister K, Hoyer D (2007) JN403, in vitro characterization of a novel nicotinic acetylcholine receptor alpha7 selective agonist. Neurosci Lett 416:61–65. [DOI] [PubMed] [Google Scholar]
- Feuerbach DPezous NWeiss MShakeri-Nejad KLingenhoehl KHoyer DHurth KBilbe GPryce CRMcAllister K, et al. (2015) AQW051, a novel, potent and selective α7 nicotinic ACh receptor partial agonist: pharmacological characterization and phase I evaluation. Br J Pharmacol 172:1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth JR, Kobrin E (1997) Formation of oligomers containing the beta3 and beta4 subunits of the rat nicotinic receptor. J Neurosci 17:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Cheng EY, Weiland GA, Oswald RE (2001) Specific activation of the alpha 7 nicotinic acetylcholine receptor by a quaternary analog of cocaine. Mol Pharmacol 60:71–79. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV (1998) Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci 18:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33. [DOI] [PubMed] [Google Scholar]
- Gao X, Sun Q, Zhang W, Jiang Y, Li R, Ye J (2018) Anti-inflammatory effect and mechanism of the spirocyclopiperazinium salt compound LXM-15 in rats and mice. Inflamm Res 67:363–370. [DOI] [PubMed] [Google Scholar]
- Garai S, Raja KS, Papke RL, Deschamps JR, Damaj MI, Thakur GA (2018) B-973, a novel α7 nAChR Ago-PAM: Racemic and asymmetric synthesis, electrophysiological studies, and in vivo evaluation. ACS Med Chem Lett 9:1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg BK, Loring RH (2017) Evaluating commercially available antibodies for rat α7 nicotinic acetylcholine receptors. J Histochem Cytochem 65:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KWOlincy AKanner RJohnson LHogenkamp DHarris JTran MEdmonds SASauer WYoshimura R, et al. (2017) First in human trial of a type I positive allosteric modulator of alpha7-nicotinic acetylcholine receptors: Pharmacokinetics, safety, and evidence for neurocognitive effect of AVL-3288. J Psychopharmacol 31:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee VJ, Kracun S, Cooper ST, Gibb AJ, Millar NS (2007) Identification of domains influencing assembly and ion channel properties in alpha 7 nicotinic receptor and 5-HT3 receptor subunit chimaeras. Br J Pharmacol 152:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Kuryatov A, Anand R, Lindstrom J (1997) “Orphan” alpha6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol Pharmacol 51:320–327. [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J (1998) Alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther 286:311–320. [PubMed] [Google Scholar]
- Gharpure ATeng JZhuang YNoviello CMWalsh RM Jr, Cabuco R, Howard RJ, Zaveri NT, Lindahl EHibbs RE (2019) Agonist selectivity and ion permeation in the α3β4 ganglionic nicotinic receptor. Neuron 104:501–511.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS (2012) A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol Pharmacol 81:710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS (2011) Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 108:5867–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill-Thind JK, Dhankher P, D’Oyley JM, Sheppard TD, Millar NS (2015) Structurally similar allosteric modulators of α7 nicotinic acetylcholine receptors exhibit five distinct pharmacological effects. J Biol Chem 290:3552–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin JRRoy PQuadri MBagdas DToma WNarendrula-Kotha RKishta OADamaj MIHorenstein NAPapke RL, et al. (2020) A silent agonist of α7 nicotinic acetylcholine receptors modulates inflammation ex vivo and attenuates EAE. Brain Behav Immun 87:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Hanke W, Maury K, Moretti M, Ballivet M, Clementi F, Bertrand D (1994) Pharmacology and biophysical properties of alpha 7 and alpha 7-alpha 8 alpha-bungarotoxin receptor subtypes immunopurified from the chick optic lobe. Eur J Neurosci 6:1281–1291. [DOI] [PubMed] [Google Scholar]
- Gotti CMoretti MBohr IZiabreva IVailati SLonghi RRiganti LGaimarri AMcKeith IGPerry RH, et al. (2006) Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol Dis 23:481–489. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J (2007) Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724. [DOI] [PubMed] [Google Scholar]
- Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S (2011) A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci 33:1786–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Matta JA, Lord B, Harrington AW, Sutton SW, Davini WB, Bredt DS (2016) Brain α7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron 89:948–955. [DOI] [PubMed] [Google Scholar]
- Guerra-Álvarez M, Moreno-Ortega AJ, Navarro E, Fernández-Morales JC, Egea J, López MG, Cano-Abad MF (2015) Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca(2+) release from the endoplasmic reticulum. J Neurochem 133:309–319. [DOI] [PubMed] [Google Scholar]
- Gulsevin A (2020) Nicotinic receptor pharmacology in silico: Insights and challenges. Neuropharmacology 177:108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsevin A, Meiler J, Horenstein NA (2020a) A computational analysis of the factors governing the dynamics of α7 nAChR and its homologs. Biophys J 119:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsevin A, Papke RL, Horenstein N (2020b) In silico modeling of the α7 nicotinic acetylcholine receptor: New pharmacological challenges associated with multiple modes of signaling. Mini Rev Med Chem 20:841–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsevin A, Papke RL, Stokes C, Garai S, Thakur GA, Quadri M, Horenstein NA (2019) Allosteric agonism of α7 nicotinic acetylcholine receptors: Receptor modulation outside the orthosteric site. Mol Pharmacol 95:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Tulsyan S, Thakur N, Sharma V, Sinha DN, Mehrotra R (2020) Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul Toxicol Pharmacol 110:104548. [DOI] [PubMed] [Google Scholar]
- Gurley D, Harris EW, Li C, Johnson EC, Lanthorn T (2000) 5-Hydroxyindole potentiates the nicotinic acetylcholine receptor alpha7 subtype. Soc Neurosci Abs 716:15. [Google Scholar]
- Gurley DA, Lanthorn TH (1998) Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci Lett 247:107–110. [DOI] [PubMed] [Google Scholar]
- Haig GM, Wang D, Zhao J, Othman AA, Bain EE (2018) Efficacy and safety of the α7-nicotinic acetylcholine receptor agonist ABT-126 in the treatment of cognitive impairment associated with schizophrenia: Results from a phase 2b randomized controlled study in smokers. J Clin Psychiatry 79:16m11162. [DOI] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278:34411–34417. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24:3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJAvery TDSchaeffer LJoseph CHuff BCSingh RMorice CGiethlen BGrishin AAColes CJ, et al. (2019) Discovery of BNC375, a potent, selective, and orally available type I positive allosteric modulator of α7 nAChRs. ACS Med Chem Lett 10:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar SNGhiron CBettinetti LBothmann HComery TADunlop JLa Rosa SMicco IPollastrini MQuinn J, et al. (2009) SAR and biological evaluation of SEN12333/WAY-317538: Novel alpha 7 nicotinic acetylcholine receptor agonist. Bioorg Med Chem 17:5247–5258. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, Patrick J (1990) The brain nicotinic acetylcholine receptor gene family. Prog Brain Res 86:195–203. [DOI] [PubMed] [Google Scholar]
- Henchman RH, Wang HL, Sine SM, Taylor P, McCammon JA (2003) Asymmetric structural motions of the homomeric alpha7 nicotinic receptor ligand binding domain revealed by molecular dynamics simulation. Biophys J 85:3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Severance EG, Cuevas J, Morgan D, Gordon MN (2004) Biochemical and histochemical evidence of nonspecific binding of alpha7nAChR antibodies to mouse brain tissue. J Histochem Cytochem 52:1367–1376. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Cortez I, Gu Z, Colón-Sáez JO, Lamb PW, Wakamiya M, Yakel JL, Dineley KT (2014) Research tool: Validation of floxed α7 nicotinic acetylcholine receptor conditional knockout mice using in vitro and in vivo approaches. J Physiol 592:3201–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RE, Sulzenbacher G, Shi J, Talley TT, Conrod S, Kem WR, Taylor P, Marchot P, Bourne Y (2009) Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal alpha7 nicotinic acetylcholine receptor. EMBO J 28:3040–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MDFang HDigavalli SVHealy FLGallagher LPost-Munson DChen PNatale JBenitex YMorgan D, et al. (2017) Development of spiroguanidine-derived α7 neuronal nicotinic receptor partial agonists. Bioorg Med Chem Lett 27:578–581. [DOI] [PubMed] [Google Scholar]
- Hill MDFang HKing HDIwuagwu CIMcDonald IMCook JZusi FCMate RAKnox RJPost-Munson D, et al. (2016) Development of 4-heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes] as α7 nicotinic receptor agonists. ACS Med Chem Lett 8:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopping G, Wang CI, Hogg RC, Nevin ST, Lewis RJ, Adams DJ, Alewood PF (2014) Hydrophobic residues at position 10 of α-conotoxin PnIA influence subtype selectivity between α7 and α3β2 neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 91:534–542. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL (2008) Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol 74:1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL (2017) Anti-inflammatory silent agonists. ACS Med Chem Lett 8:989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL, Kulkarni AR, Chaturbhuj GU, Stokes C, Manther K, Thakur GA (2016) Critical molecular determinants of α7 nicotinic acetylcholine receptor allosteric activation: separation of direct allosteric activation and positive allosteric modulation. J Biol Chem 291:5049–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Gopalakrishnan M, Li J (2009) Positive allosteric modulation of alpha7 neuronal nicotinic acetylcholine receptors: lack of cytotoxicity in PC12 cells and rat primary cortical neurons. Br J Pharmacol 158:1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Felix AR, Kwon S, Lowe D, Wallace T, Santarelli L, Meltzer HY (2014) The alpha-7 nicotinic receptor partial agonist/5-HT3 antagonist RG3487 enhances cortical and hippocampal dopamine and acetylcholine release. Psychopharmacology (Berl) 231:2199–2210. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Schmidt J (1978) The electron microscopic autoradiographic localization of alpha-bungarotoxin binding sites within the central nervous system of the rat. Brain Res 142:152–159. [DOI] [PubMed] [Google Scholar]
- Hurst RSHajós MRaggenbass MWall TMHigdon NRLawson JARutherford-Root KLBerkenpas MBHoffmann WEPiotrowski DW, et al. (2005) A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocent N, Livingstone PD, Hone A, Kimura A, Young T, Whiteaker P, McIntosh JM, Wonnacott S (2008) {alpha}Conotoxin ArIB[V11L,V16D] is a potent and selective antagonist at rat and human native {alpha}7 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 327:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwuagwu CKing DMcDonald IMCook JZusi FCHill MDMate RAFang HKnox RGallagher L, et al. (2017) Design and synthesis of a novel series of 4-heteroarylamino-1′-azaspiro[oxazole-5,3′-bicyclo[2.2.2]octanes as α7 nicotinic receptor agonists 2. Development of 4-heteroaryl SAR. Bioorg Med Chem Lett 27:1261–1266. [DOI] [PubMed] [Google Scholar]
- James JR, Nordberg A (1995) Genetic and environmental aspects of the role of nicotinic receptors in neurodegenerative disorders: emphasis on Alzheimer’s disease and Parkinson’s disease. Behav Genet 25:149–159. [DOI] [PubMed] [Google Scholar]
- Jin AH, Vetter I, Dutertre S, Abraham N, Emidio NB, Inserra M, Murali SS, Christie MJ, Alewood PF, Lewis RJ (2014) MrIC, a novel α-conotoxin agonist in the presence of PNU at endogenous α7 nicotinic acetylcholine receptors. Biochemistry 53:1–3. [DOI] [PubMed] [Google Scholar]
- Jumblatt JE, Marquis JK, Mautner HG (1981) On the specificity of 125-I-alpha-bungarotoxin binding to axonal membranes. J Neurochem 37:392–400. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA (2018) Beyond the channel: Metabotropic signaling by nicotinic receptors. Trends Pharmacol Sci 39:354–366. [DOI] [PubMed] [Google Scholar]
- Kaczanowska K, Camacho Hernandez GA, Bendiks L, Kohs L, Cornejo-Bravo JM, Harel M, Finn MG, Taylor P (2017) Substituted 2-aminopyrimidines selective for α7-nicotinic acetylcholine receptor activation and association with acetylcholine binding proteins. J Am Chem Soc 139:3676–3684. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S (2000) Alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol 58:312–318. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Sun F, Johnson SR, Jin K, Uteshev VV (2013) A positive allosteric modulator of α7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br J Pharmacol 169:1862–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JTJavitt DCFreedman RSehatpour PKegeles LSCarlson MSobeih TWall MMChoo THVail B, et al. (2020) Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology 45:1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S, Khan MA, Yasuhara S, Goto T, Kem WR, Tompkins RG, Kaneki M, Martyn JA (2017) Prevention of burn-induced inflammatory responses and muscle wasting by GTS-21, a specific agonist for α7 nicotinic acetylcholine receptors. Shock 47:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Thesleff S (1957) A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 138:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Papke RL, Lingle CJ (1997) Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther 283:979–992. [PubMed] [Google Scholar]
- Kem WR, Olincy A, Johnson L, Harris J, Wagner BD, Buchanan RW, Christians U, Freedman R (2018) Pharmacokinetic limitations on effects of an alpha7-nicotinic receptor agonist in schizophrenia: Randomized trial with an extended-release formulation. Neuropsychopharmacology 43:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempsill FE, Covernton PJ, Whiting PJ, Connolly JG (1999) Agonist activation and alpha-bungarotoxin inhibition of wild type and mutant alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol 383:347–359. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, Maeda T, Akaike A (1997) Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann Neurol 42:159–163. [DOI] [PubMed] [Google Scholar]
- King DIwuagwu CCook JMcDonald IMMate RZusi FCHill MDFang HZhao RWang B, et al. (2017a) BMS-933043, a selective α7 nAChR partial agonist for the treatment of cognitive deficits associated with schizophrenia. ACS Med Chem Lett 8:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Gillevet TC, Kabbani N (2017b) A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Bio 7:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Ullah A, Bak E, Jafri MS, Kabbani N (2018) Ionotropic and metabotropic mechanisms of allosteric modulation of α7 nicotinic receptor intracellular calcium. Mol Pharmacol 93:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike KHashimoto KTakai NShimizu EKomatsu NWatanabe HNakazato MOkamura NStevens KEFreedman R, et al. (2005) Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res 76:67–72. [DOI] [PubMed] [Google Scholar]
- Kong WKang KGao YLiu HMeng XCao YYang SLiu WZhang JYu K, et al. (2018) GTS-21 protected against LPS-induced sepsis myocardial injury in mice through α7nAChR. Inflammation 41:1073–1083. [DOI] [PubMed] [Google Scholar]
- Koukouli F, Rooy M, Changeux JP, Maskos U (2016) Nicotinic receptors in mouse prefrontal cortex modulate ultraslow fluctuations related to conscious processing. Proc Natl Acad Sci USA 113:14823–14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal NM, Ahring PK, Liao VWY, Indurti DC, Harvey BS, O’Connor SM, Chebib M, Olafsdottir ES, Balle T (2018) Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors. Br J Pharmacol 175:2911–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kox M, Pompe JC, Peters E, Vaneker M, van der Laak JW, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW, Pickkers P (2011) α7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-α production and lung injury. Br J Anaesth 107:559–566. [DOI] [PubMed] [Google Scholar]
- Krafft PR, McBride D, Rolland WB, Lekic T, Flores JJ, Zhang JH (2017) α7 Nicotinic acetylcholine receptor stimulation attenuates neuroinflammation through JAK2-STAT3 activation in murine models of intracerebral hemorrhage. BioMed Res Int 2017:8134653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Wahl A, Stark T, Hofmann T (2011) Urinary N-methylpyridinium and trigonelline as candidate dietary biomarkers of coffee consumption. Mol Nutr Food Res 55:1613–1623. [DOI] [PubMed] [Google Scholar]
- Lange KW, Wells FR, Jenner P, Marsden CD (1993) Altered muscarinic and nicotinic receptor densities in cortical and subcortical brain regions in Parkinson’s disease. J Neurochem 60:197–203. [DOI] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG (2008) On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Gwalani L, Mishra V, Anandjiwala P, Sala F, Sala S, Ballesta JJ, O’Malley D, Criado M, Loring RH (2009) Investigating the role of protein folding and assembly in cell-type dependent expression of alpha7 nicotinic receptors using a green fluorescent protein chimera. Brain Res 1259:7–16. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N (2002) Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience 109:757–765. [DOI] [PubMed] [Google Scholar]
- Levin ED, Torry D (1996) Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology (Berl) 123:88–97. [DOI] [PubMed] [Google Scholar]
- Li BWu JBao JHan XShen SYe XDai JWu ZNiu MHe Y, et al. (2020a) Activation of α7nACh receptor protects against acute pancreatitis through enhancing TFEB-regulated autophagy. Biochim Biophys Acta Mol Basis Dis 1866:165971. [DOI] [PubMed] [Google Scholar]
- Li DJ, Fu H, Tong J, Li YH, Qu LF, Wang P, Shen FM (2018) Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol 15:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang X, Jin T, Hao J (2020b) Nicotine promotes activation of human pancreatic stellate cells through inducing autophagy via α7nAChR-mediated JAK2/STAT3 signaling pathway. Life Sci 243:117301. [DOI] [PubMed] [Google Scholar]
- Lindstrom J, Criado M, Hochschwender S, Fox JL, Sarin V (1984) Immunochemical tests of acetylcholine receptor subunit models. Nature 311:573–575. [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez G, Placzek AN, Thinschmidt JS, Lestage P, Trocme-Thibierge C, Morain P, Papke RL (2007) Partial agonist and neuromodulatory activity of S 24795 for alpha7 nAChR responses of hippocampal interneurons. Neuropharmacology 53:134–144. [DOI] [PubMed] [Google Scholar]
- López-Hernández GY, Thinschmidt JS, Zheng G, Zhang Z, Crooks PA, Dwoskin LP, Papke RL (2009) Selective inhibition of acetylcholine-evoked responses of alpha7 neuronal nicotinic acetylcholine receptors by novel tris- and tetrakis-azaaromatic quaternary ammonium antagonists. Mol Pharmacol 76:652–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Wada K, Rogers S, Abramson SN, Heinemann S, Patrick J (1990) Neurotoxins distinguish between different neuronal nicotinic acetylcholine receptor subunit combinations. J Neurochem 55:632–640. [DOI] [PubMed] [Google Scholar]
- Mackowick KM, Lynch MJ, Weinberger AH, George TP (2012) Treatment of tobacco dependence in people with mental health and addictive disorders. Curr Psychiatry Rep 14:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC (2001) The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett 11:319–321. [DOI] [PubMed] [Google Scholar]
- Maldifassi MCMartín-Sánchez CAtienza GCedillo JLArnalich FBordas AZafra FGiménez CExtremera MRenart J, et al. (2018) Interaction of the α7-nicotinic subunit with its human-specific duplicated dupα7 isoform in mammalian cells: Relevance in human inflammatory responses. J Biol Chem 293:13874–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet JLe Strat YSchürhoff FMazer NPortalier CAndrianarisoa MAouizerate BBerna FBrunel LCapdevielle D, et al. ; FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) group (2017) Cigarette smoking and schizophrenia: a specific clinical and therapeutic profile? Results from the FACE-Schizophrenia cohort. Prog Neuropsychopharmacol Biol Psychiatry 79 (Pt B):332–339. [DOI] [PubMed] [Google Scholar]
- Malysz JAnderson DJGrønlien JHJi JBunnelle WHHåkerud MThorin-Hagene KWeen HHelfrich RHu M, et al. (2010) In vitro pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J Pharmacol Exp Ther 334:863–874. [DOI] [PubMed] [Google Scholar]
- Manetti D, Bellucci C, Chiaramonte N, Dei S, Teodori E, Romanelli MN (2018) Designing selective modulators for the nicotinic receptor subtypes: challenges and opportunities. Future Med Chem 10:433–459. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res 3:361–373. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC (1986) Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and alpha-bungarotoxin. Mol Pharmacol 30:427–436. [PubMed] [Google Scholar]
- Marrero MB, Bencherif M (2009) Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res 1256:1–7. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M (2010) An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther 332:173–180. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M (2004) The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther 309:16–27. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R (2007) Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 78:225–246. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R (2004) Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 174:54–64. [DOI] [PubMed] [Google Scholar]
- Matta JA, Gu S, Davini WB, Lord B, Siuda ER, Harrington AW, Bredt DS (2017) NACHO mediates nicotinic acetylcholine receptor function throughout the brain. Cell Rep 19:688–696. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang G, de Fiebre CM (1997) 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res 768:49–56. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Zoltewicz JA, Meyers C, King MA, Papke RL, De Fiebre CM (1998) Neuroprotective and memory-related actions of novel alpha-7 nicotinic agents with different mixed agonist/antagonist properties. J Pharmacol Exp Ther 284:1026–1032. [PubMed] [Google Scholar]
- Miller DR, Khoshbouei H, Garai S, Cantwell LN, Stokes C, Thakur G, Papke RL (2020) Allosterically potentiated α7 nicotinic acetylcholine Receptors: Reduced Calcium Permeability and Current-Independent Control of Intracellular Calcium. Mol Pharmacol 98:695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, Hibbs RE (2016) X-ray structure of the human α4β2 nicotinic receptor. Nature 538:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Starobova H, Inserra MC, Jin AH, Deuis JR, Dutertre S, Lewis RJ, Alewood PF, Daly NL, Vetter I (2015) α-Conotoxin MrIC is a biased agonist at α7 nicotinic acetylcholine receptors. Biochem Pharmacol 94:155–163. [DOI] [PubMed] [Google Scholar]
- Mullen GNapier JBalestra MDeCory THale GMacor JMack RLoch J 3rd, Wu E, Kover A, et al. (2000) (-)-Spiro[1-azabicyclo[2.2.2]octane-3,5′-oxazolidin-2′-one], a conformationally restricted analogue of acetylcholine, is a highly selective full agonist at the alpha 7 nicotinic acetylcholine receptor. J Med Chem 43:4045–4050. [DOI] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW (2007) Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci USA 104:8059–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirthanan S (2020) Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: molecules, mechanisms and medicine. Biochem Pharmacol 181:114168. [DOI] [PubMed] [Google Scholar]
- Noetzel MJRook JMVinson PNCho HPDays EZhou YRodriguez ALLavreysen HStauffer SRNiswender CM, et al. (2012) Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol 81:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A, Lundqvist H, Hartvig P, Andersson J, Johansson M, Hellstrŏm-Lindahi E, Långström B (1997) Imaging of nicotinic and muscarinic receptors in Alzheimer’s disease: effect of tacrine treatment. Dement Geriatr Cogn Disord 8:78–84. [DOI] [PubMed] [Google Scholar]
- O’Donnell CJRogers BNBronk BSBryce DKCoe JWCook KKDuplantier AJEvrard EHajós MHoffmann WE, et al. (2010) Discovery of 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (CP-810,123), a novel alpha 7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J Med Chem 53:1222–1237. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2004) Betel-Quid and Areca-Nut Chewing, in Monographs (Cancer IAfRo ed) vol 85, pp 1–240. [Google Scholar]
- Oswald RE, Freeman JA (1981) Alpha-bungarotoxin binding and central nervous system nicotinic acetylcholine receptors. Neuroscience 6:1–14. [DOI] [PubMed] [Google Scholar]
- Pałczyńska MM, Jindrichova M, Gibb AJ, Millar NS (2012) Activation of α7 nicotinic receptors by orthosteric and allosteric agonists: influence on single-channel kinetics and conductance. Mol Pharmacol 82:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D (1996) Neuronal nicotinic alpha 7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol 491:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Mileo AM, Martinez-Torres A, Eusebi F, Miledi R (2002) Some properties of human neuronal alpha 7 nicotinic acetylcholine receptors fused to the green fluorescent protein. Proc Natl Acad Sci USA 99:3950–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL (2010) Tricks of perspective: insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. J Mol Neurosci 40:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL (2014) Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol 89:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari SD, Thakur GA, Damaj MI (2015a) The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology 91:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P (1996) An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett 213:201–204. [DOI] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S (1989a) Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron 3:589–596. [DOI] [PubMed] [Google Scholar]
- Papke RL, Brunzell DH, De Biasi M (2020a) Cholinergic receptors and addiction. Curr Top Behav Neurosci 45:123–151. [DOI] [PubMed] [Google Scholar]
- Papke RL, Chojnacka K, Horenstein NA (2014a) The minimal pharmacophore for silent agonism of the α7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther 350:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Duvoisin R, Boulter J, Heinemann S (1989b) The possible importance of the neuronal nicotinic subunit b4 to the kinetic properties of the adrenal chromaffin cell acetylcholine receptor. 19th Annual Meeting of the Society for Neuroscience. 333.12. [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA (2007) The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem 101:160–167. [DOI] [PubMed] [Google Scholar]
- Papke RL, Garai S, Stokes C, Horenstein NA, Zimmerman AD, Abboud KA, Thakur GA (2020b) Differing activity profiles of the stereoisomers of 2,3,5,6TMP-TQS, a putative silent allosteric modulator of α7 nAChR. Mol Pharmacol 98:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Hatsukami DK, Herzog TA (2020c) Betel quid, health, and addiction. Subst Use Misuse 55:1528–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Kulkarni AR, Stokes C, Corrie LW, Maeng CY, Thakur GA (2014b) The activity of GAT107, an allosteric activator and positive modulator of α7 nicotinic acetylcholine receptors (nAChR), is regulated by aromatic amino acids that span the subunit interface. J Biol Chem 289:4515–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Stokes C (2015b) Nicotinic activity of arecoline, the psychoactive element of “betel nuts”, suggests a basis for habitual use and anti-inflammatory activity. PLoS One 10:e0140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA (2009) Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Lindstrom JM (2020) Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 168:108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK, Rose GM (2004) Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett 14:1849–1853. [DOI] [PubMed] [Google Scholar]
- Papke RL, Peng C, Kumar A, Stokes C (2018a) NS6740, an α7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci Lett 677:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Schiff HC, Jack BA, Horenstein NA (2005a) Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci Lett 378:140–144. [DOI] [PubMed] [Google Scholar]
- Papke RL, Stokes C (2010) Working with OpusXpress: methods for high volume oocyte experiments. Methods 51:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Damaj MI, Thakur GA, Manther K, Treinin M, Bagdas D, Kulkarni AR, Horenstein NA (2018b) Persistent activation of α7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br J Pharmacol 175:1838–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Muldoon P, Imad Damaj M (2013) Similar activity of mecamylamine stereoisomers in vitro and in vivo. Eur J Pharmacol 720:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Thinschmidt JS (1998) The correction of alpha7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci Lett 256:163–166. [DOI] [PubMed] [Google Scholar]
- Papke RL, Trocmé-Thibierge C, Guendisch D, Al Rubaiy SA, Bloom SA (2011) Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther 337:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Zheng G, Horenstein NA, Dwoskin LP, Crooks PA (2005b) The characterization of a novel rigid nicotine analog with alpha7-selective nAChR agonist activity and modulation of agonist properties by boron inclusion. Bioorg Med Chem Lett 15:3874–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MJ, Beck A, Luetje CW (1998) Neuronal nicotinic receptor beta2 and beta4 subunits confer large differences in agonist binding affinity. Mol Pharmacol 54:1132–1139. [PubMed] [Google Scholar]
- Paulo JA, Brucker WJ, Hawrot E (2009) Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J Proteome Res 8:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VAOchani MYang LHGallowitsch-Puerta MOchani KLin XLevi JParrish WRRosas-Ballina MCzura CJ, et al. (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35:1139–1144. [DOI] [PubMed] [Google Scholar]
- Peng C, Kimbrell MR, Tian C, Pack TF, Crooks PA, Fifer EK, Papke RL (2013) Multiple modes of α7 nAChR noncompetitive antagonism of control agonist-evoked and allosterically enhanced currents. Mol Pharmacol 84:459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Zhang Q, Snyder GL, Zhu H, Yao W, Tomesch J, Papke RL, O’Callaghan JP, Welsh WJ, Wennogle LP (2010) Discovery of novel alpha7 nicotinic receptor antagonists. Bioorg Med Chem Lett 20:4825–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Vega MJFernandez-Mendivil Cde la Torre Martínez RGonzález-Rodríguez SMullet JSala FSala SCriado MMoreno-Fernández SMiguel M, et al. (2019) 1-(2′,5′-Dihydroxyphenyl)-3-(2-fluoro-4-hydroxyphenyl)-1-propanone (RGM079): A positive allosteric modulator of α7 nicotinic receptors with analgesic and neuroprotective activity. ACS Chem Neurosci 10:3900–3909. [DOI] [PubMed] [Google Scholar]
- Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, Irving D, Brown A, Perry RH (1995) Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuroscience 64:385–395. [DOI] [PubMed] [Google Scholar]
- Pieschl RLMiller RJones KMPost-Munson DJChen PNewberry KBenitex YMolski TMorgan DMcDonald IM, et al. (2017) Effects of BMS-902483, an α7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur J Pharmacol 807:1–11. [DOI] [PubMed] [Google Scholar]
- Pinheiro NMMiranda CJCPSantana FRBittencourt-Mernak MArantes-Costa FMOlivo CPerini AFesta SCaperuto LCTibério IFLC, et al. (2020) Effects of VAChT reduction and α7nAChR stimulation by PNU-282987 in lung inflammation in a model of chronic allergic airway inflammation. Eur J Pharmacol 882:173239. [DOI] [PubMed] [Google Scholar]
- Pismataro MC, Horenstein NA, Stokes C, Quadri M, De Amici M, Papke RL, Dallanoce C (2020) Design, synthesis, and electrophysiological evaluation of NS6740 derivatives: Exploration of the structure-activity relationship for alpha7 nicotinic acetylcholine receptor silent activation. Eur J Med Chem 205:112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Munson DJPieschl RLMolski TFGraef JDHendricson AWKnox RJMcDonald IMOlson REMacor JEWeed MR, et al. (2017) B-973, a novel piperazine positive allosteric modulator of the α7 nicotinic acetylcholine receptor. Eur J Pharmacol 799:16–25. [DOI] [PubMed] [Google Scholar]
- Prendergast MATerry AV Jr, Jackson WJ, Marsh KC, Decker MW, Arneric SPBuccafusco JJ (1997) Improvement in accuracy of delayed recall in aged and non-aged, mature monkeys after intramuscular or transdermal administration of the CNS nicotinic receptor agonist ABT-418. Psychopharmacology (Berl) 130:276–284. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC (2014) Normalizing effects of EVP-6124, an α-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract 20:12–24. [DOI] [PubMed] [Google Scholar]
- Prickaerts Jvan Goethem NPChesworth RShapiro GBoess FGMethfessel CReneerkens OAFlood DGHilt DGawryl M, et al. (2012) EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology 62:1099–1110. [DOI] [PubMed] [Google Scholar]
- Quadri M, Bagdas D, Toma W, Stokes C, Horenstein NA, Damaj MI, Papke RL (2018a) The antinociceptive and anti-inflammatory properties of the alpha7 nAChR weak partial agonist p-CF3N,N-diethyl-N'-phenylpiperazine. J Pharmacol Exp Ther. 367:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Garai S, Thakur GA, Stokes C, Gulsevin A, Horenstein NA, Papke RL (2019) Macroscopic and microscopic activation of α7 nicotinic acetylcholine receptors by the structurally unrelated allosteric agonist-positive allosteric modulators (ago-PAMs) B-973B and GAT107. Mol Pharmacol 95:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Matera C, Silnović A, Pismataro MC, Horenstein NA, Stokes C, Papke RL, Dallanoce C (2017a) Identification of α7 nicotinic acetylcholine receptor silent agonists based on the spirocyclic quinuclidine-Δ2 -isoxazoline scaffold: Synthesis and electrophysiological evaluation. ChemMedChem 12:1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Papke RL, Horenstein NA (2016) Dissection of N,N-diethyl-N'-phenylpiperazines as α7 nicotinic receptor silent agonists. Bioorg Med Chem 24:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Silnović A, Matera C, Horenstein NA, Stokes C, De Amici M, Papke RL, Dallanoce C (2018b) Novel 5-(quinuclidin-3-ylmethyl)-1,2,4-oxadiazoles to investigate the activation of the α7 nicotinic acetylcholine receptor subtype: Synthesis and electrophysiological evaluation. Eur J Med Chem 160:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Stokes C, Gulsevin A, Felts ACJ, Abboud KA, Papke RL, Horenstein NA (2017b) Sulfonium as a surrogate for ammonium: A new α7 nicotinic acetylcholine receptor partial agonist with desensitizing activity. J Med Chem 60:7928–7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TS, Correa LD, Reid RT, Lloyd GK (1996) Evaluation of anti-nociceptive effects of neuronal nicotinic acetylcholine receptor (NAChR) ligands in the rat tail-flick assay. Neuropharmacology 35:393–405. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC (2006) An autoradiographic analysis of [125I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett 404:9–14. [DOI] [PubMed] [Google Scholar]
- Ren K, Thinschmidt J, Liu J, Ai L, Papke RL, King MA, Hughes JA, Meyer EM (2007) alpha7 Nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience 145:314–322. [DOI] [PubMed] [Google Scholar]
- Robbins TW, McAlonan G, Muir JL, Everitt BJ (1997) Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer’s disease. Behav Brain Res 83:15–23. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Myers EJ, Gahring LC (2012) The expression of nicotinic receptor alpha7 during cochlear development. Brain Behav 2:628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A, Marco-Contelles J, Ramos E (2020) Highlights of ASS234: a novel and promising therapeutic agent for Alzheimer’s disease therapy. Neural Regen Res 15:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarati RScali CComery TAGrauer SMAschmi SBothmann HJow BKowal DGianfriddo MKelley C, et al. (2009) Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther 329:459–468. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ (2009) The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ (2009) Cholinergic control of inflammation. J Intern Med 265:663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, Bufalo AD, Frustaci A, Fini M, Cesario A (2014) Beyond Acetylcholinesterase Inhibitors for Treating Alzheimer's Disease: 7-nAChR Agonists in Human Clinical Trials. Curr Pharm Des. 20:6014–6021. [DOI] [PubMed] [Google Scholar]
- Russo P, Cardinale A, Shuller H (2012) A new “era” for the α7-nAChR. Curr Drug Targets 13:721–725. [DOI] [PubMed] [Google Scholar]
- Sahdeo SWallace THirakawa RKnoflach FBertrand DMaag HMisner DTombaugh GCSantarelli LBrameld K, et al. (2014) Characterization of RO5126946, a Novel α7 nicotinic acetylcholine receptor-positive allosteric modulator. J Pharmacol Exp Ther 350:455–468. [DOI] [PubMed] [Google Scholar]
- Samochocki MHöffle AFehrenbacher AJostock RLudwig JChristner CRadina MZerlin MUllmer CPereira EF, et al. (2003) Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305:1024–1036. [DOI] [PubMed] [Google Scholar]
- Sarvey JM, Albuquerque EX, Eldefrawi AT, Eldefrawi M (1978) Effects of alpha-bungarotoxin and reversible cholinergic ligands on normal and denervated mammalian skeletal muscle. Membr Biochem 1:131–157. [DOI] [PubMed] [Google Scholar]
- Schaller SJNagashima MSchönfelder MSasakawa TSchulz FKhan MASKem WRSchneider GSchlegel JLewald H, et al. (2018) GTS-21 attenuates loss of body mass, muscle mass, and function in rats having systemic inflammation with and without disuse atrophy. Pflugers Arch 470:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J (1990) Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron 5:35–48. [DOI] [PubMed] [Google Scholar]
- Schröder H, Giacobini E, Struble RG, Zilles K, Maelicke A (1991a) Nicotinic cholinoceptive neurons of the frontal cortex are reduced in Alzheimer’s disease. Neurobiol Aging 12:259–262. [DOI] [PubMed] [Google Scholar]
- Schröder H, Giacobini E, Struble RG, Zilles K, Maelicke A, Luiten PG, Strosberg AD (1991b) Cellular distribution and expression of cortical acetylcholine receptors in aging and Alzheimer’s disease. Ann N Y Acad Sci 640:189–192. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Loring RH, Aizenman E, Zigmond RE (1991) Autoradiographic localization of putative nicotinic receptors in the rat brain using 125I-neuronal bungarotoxin. J Neurosci 11:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao BZ, Wang SL, Fang J, Li ZS, Bai Y, Wu K (2019) Alpha7 nicotinic acetylcholine receptor alleviates inflammatory bowel disease through induction of AMPK-mTOR-p70S6K-mediated autophagy. Inflammation 42:1666–1679. [DOI] [PubMed] [Google Scholar]
- Shenkarev ZOShulepko MABychkov MLKulbatskii DSShlepova OVVasilyeva NAAndreev-Andrievskiy AAPopova ASLagereva EALoktyushov EV, et al. (2020) Water-soluble variant of human Lynx1 positively modulates synaptic plasticity and ameliorates cognitive impairment associated with α7-nAChR dysfunction. J Neurochem 155:45–61. [DOI] [PubMed] [Google Scholar]
- Sherrington CS (1947) The integrative action of the nervous system, Cambridge University Press, Cambridge. [Google Scholar]
- Shulepko MA, Bychkov ML, Shlepova OV, Shenkarev ZO, Kirpichnikov MP, Lyukmanova EN (2020) Human secreted protein SLURP-1 abolishes nicotine-induced proliferation, PTEN down-regulation and α7-nAChR expression up-regulation in lung cancer cells. Int Immunopharmacol 82:106303. [DOI] [PubMed] [Google Scholar]
- Singh A, Dikshit R, Chaturvedi P (2020) Betel nut use: The South Asian story. Subst Use Misuse 55:1545–1551. [DOI] [PubMed] [Google Scholar]
- Sinha NKarche NPVerma MKWalunj SSNigade PBJana GKurhade SPHajare AKTilekar ARJadhav GR, et al. (2020) Discovery of novel, potent, brain-permeable, and orally efficacious positive allosteric modulator of α7 nicotinic acetylcholine receptor [4-(5-(4-chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide]: Structure-activity relationship and preclinical characterization. J Med Chem 63:944–960. [DOI] [PubMed] [Google Scholar]
- Sitapara RAGauthier AGValdés-Ferrer SILin MPatel VWang MMartino ATPerron JCAshby CR Jr, Tracey KJ, et al. (2020) The α7 nicotinic acetylcholine receptor agonist, GTS-21, attenuates hyperoxia-induced acute inflammatory lung injury by alleviating the accumulation of HMGB1 in the airways and the circulation. Mol Med 26:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J (2011) Voltage- and temperature-dependent allosteric modulation of α7 nicotinic receptors by PNU120596. Front Pharmacol 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore JAtcha ZBoucherat ECastelletti LChen DWCoppo FTCutler LDunsdon RMHeath BMHutchings R, et al. (2012) The discovery of 2-fluoro-N-(3-fluoro-4-(5-((4-morpholinobutyl)amino)-1,3,4-oxadiazol-2-yl)phenyl)benzamide, a full agonist of the alpha-7 nicotinic acetylcholine receptor showing efficacy in the novel object recognition model of cognition enhancement. Bioorg Med Chem Lett 22:3531–3534. [DOI] [PubMed] [Google Scholar]
- Snaedal J, Johannesson T, Jonsson JE, Gylfadottir G (1996) The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer’s disease. Dementia 7:47–52. [DOI] [PubMed] [Google Scholar]
- Spurden DP, Court JA, Lloyd S, Oakley A, Perry R, Pearson C, Pullen RG, Perry EK (1997) Nicotinic receptor distribution in the human thalamus: autoradiographical localization of [3H]nicotine and [125I] alpha-bungarotoxin binding. J Chem Neuroanat 13:105–113. [DOI] [PubMed] [Google Scholar]
- Stoiljkovic MLeventhal LChen AChen TDriscoll RFlood DHodgdon HHurst RNagy DPiser T, et al. (2015) Concentration-response relationship of the α7 nicotinic acetylcholine receptor agonist FRM-17874 across multiple in vitro and in vivo assays. Biochem Pharmacol 97:576–589. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A (2013) Unraveling the neurobiology of nicotine dependence using genetically engineered mice. Curr Opin Neurobiol 23:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Garai S, Kulkarni AR, Cantwell LN, Noviello CM, Hibbs RE, Horenstein NA, Abboud KA, Thakur GA, Papke RL (2019) Heteromeric neuronal nicotinic acetylcholine receptors with mutant β subunits acquire sensitivity to α7-selective positive allosteric modulators. J Pharmacol Exp Ther 370:252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Treinin M, Papke RL (2015) Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci 36:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LLYang TYWei NNLu WJiao WXZhou QQMiao YZGao QWang XTSun Q, et al. (2019) Pharmacological characterization of JWX-A0108 as a novel type I positive allosteric modulator of α7 nAChR that can reverse acoustic gating deficits in a mouse prepulse inhibition model. Acta Pharmacol Sin 40:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydserff SSutton EJSong DQuirk MCMaciag CLi CJonak GGurley DGordon JCChristian EP, et al. (2009) Selective alpha7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem Pharmacol 78:880–888. [DOI] [PubMed] [Google Scholar]
- Tang JS, Xie BX, Bian XL, Xue Y, Wei NN, Zhou JH, Hao YC, Li G, Zhang LR, Wang KW (2015) Identification and in vitro pharmacological characterization of a novel and selective α7 nicotinic acetylcholine receptor agonist, Br-IQ17B. Acta Pharmacol Sin 36:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targowska-Duda KM, Budzynska B, Michalak A, Jozwiak K, Biala G, Arias HR (2019) 3-Furan-2-yl-N-p-tolyl-acrylamide, a highly selective positive allosteric modulator of α7 nicotinic receptors, produces anxiolytic-like activity in mice. J Psychopharmacol 33:558–567. [DOI] [PubMed] [Google Scholar]
- Tasaka Y, Yasunaga D, Kiyoi T, Tanaka M, Tanaka A, Suemaru K, Araki H (2015) Involvement of stimulation of α7 nicotinic acetylcholine receptors in the suppressive effect of tropisetron on dextran sulfate sodium-induced colitis in mice. J Pharmacol Sci 127:275–283. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Seio K, Fujio M, Katayama J, Horikawa T, Hashimoto K, Tanaka H (2004) (+)-3-[2-(Benzo[b]thiophen-2-yl)-2-oxoethyl]-1-azabicyclo[2.2.2]octane as potent agonists for the alpha7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett 14:3781–3784. [DOI] [PubMed] [Google Scholar]
- Terry AV, Callahan PM (2019) Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob Res 21:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr, Callahan PM (2020) α7 Nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 170:108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur GA, Kulkarni AR, Deschamps JR, Papke RL (2013) Expeditious synthesis, enantiomeric resolution, and enantiomer functional characterization of (4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS): an allosteric agonist-positive allosteric modulator of α7 nicotinic acetylcholine receptors. J Med Chem 56:8943–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD (2012a) The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol 251:65–72. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD (2012b) Type I and II positive allosteric modulators differentially modulate agonist-induced up-regulation of α7 nicotinic acetylcholine receptors. J Neurochem 123:73–83. [DOI] [PubMed] [Google Scholar]
- Tietje KRAnderson DJBitner RSBlomme EABrackemeyer PJBriggs CABrowman KEBury DCurzon PDrescher KU, et al. (2008) Preclinical characterization of A-582941: A novel alpha7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neurosci Ther 14:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann DBGrønlien JHKohlhaas KLNielsen EODam EJørgensen TDAhring PKPeters DHolst DChristensen JK, et al. (2007) An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther 323:294–307. [DOI] [PubMed] [Google Scholar]
- Toma WKyte SLBagdas DJackson AMeade JARahman FChen ZJDel Fabbro ECantwell LKulkarni A, et al. (2019) The α7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal. Exp Neurol 320:113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetlin VI, Kasheverov IE, Utkin YN (2020) Three-finger proteins from snakes and humans acting on nicotinic receptors: Old and new. J Neurochem, in press. [DOI] [PubMed] [Google Scholar]
- Unwin N (1993) Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol 229:1101–1124. [DOI] [PubMed] [Google Scholar]
- Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346:967–989. [DOI] [PubMed] [Google Scholar]
- Uteshev V (2016) Are positive allosteric modulators of α7 nAChRs clinically safe? J Neurochem 136:217–219. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL (2002) Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res 948:33–46. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Stevens DR, Haas HL (1996) Alpha-bungarotoxin-sensitive nicotinic responses in rat tuberomammillary neurons. Pflugers Arch 432:607–613. [DOI] [PubMed] [Google Scholar]
- van Maanen MAPapke RLKoopman FAKoepke JBevaart LClark RLamppu DElbaum DLaRosa GJTak PP, et al. (2015) Two novel α7 nicotinic acetylcholine receptor ligands: in vitro properties and their efficacy in collagen-induced arthritis in mice. PLoS One 10:e0116227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T (2006) The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130:1822–1830. [DOI] [PubMed] [Google Scholar]
- Vasilyeva NA, Loktyushov EV, Bychkov ML, Shenkarev ZO, Lyukmanova EN (2017) Three-finger proteins from the Ly6/uPAR family: Functional diversity within one structural motif. Biochemistry (Mosc) 82:1702–1715. [DOI] [PubMed] [Google Scholar]
- Verma MKGoel RNBokare AMDandekar MPKoul SDesai STota SSingh NNigade PBPatil VB, et al. (2021) LL-00066471, a novel positive allosteric modulator of α7 nicotinic acetylcholine receptor ameliorates cognitive and sensorimotor gating deficits in animal models: Discovery and preclinical characterization. Eur J Pharmacol 891:173685. [DOI] [PubMed] [Google Scholar]
- Virginio C, Giacometti A, Aldegheri L, Rimland JM, Terstappen GC (2002) Pharmacological properties of rat alpha 7 nicotinic receptors expressed in native and recombinant cell systems. Eur J Pharmacol 445:153–161. [DOI] [PubMed] [Google Scholar]
- Wada K, Ballivet M, Boulter J, Connolly J, Wada E, Deneris ES, Swanson LW, Heinemann S, Patrick J (1988) Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science 240:330–334. [DOI] [PubMed] [Google Scholar]
- Walsh RM Jr, Roh SH, Gharpure A, Morales-Perez CL, Teng JHibbs RE (2018) Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 557:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J (1996) Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem 271:17656–17665. [DOI] [PubMed] [Google Scholar]
- Wang H, Cai D, Chen Z, Wang Y (2020a) GTS-21 promotes α7 nAChR to alleviate intestinal ischemia-reperfusion-induced apoptosis and inflammation of enterocytes. Med Sci Monit 26:e921618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HYu MOchani MAmella CATanovic MSusarla SLi JHWang HYang HUlloa L, et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388. [DOI] [PubMed] [Google Scholar]
- Wang J, Li R, Peng Z, Zhou W, Hu B, Rao X, Yang X, Li J (2019) GTS-21 reduces inflammation in acute lung injury by regulating M1 polarization and function of alveolar macrophages. Shock 51:389–400. [DOI] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Korczyn AD (2002) The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice. Prog Neurobiol 68:341–360. [DOI] [PubMed] [Google Scholar]
- Wang X, Daley C, Gakhar V, Lange H, Vardigan JD, Pearson M, Zhou X, Warren L, Miller CO, Belden M, Harvey AJ, Grishin AA, Coles CJ, O'Connor SM, Thomson F, Duffy JL, Bell IM, Uslaner JM (2020b) Pharmacological characterization of the novel and selective alpha7 nicotinic acetylcholine receptor positive allosteric modulator BNC375. J Pharmacol Exp Ther. 373:311–324 [DOI] [PubMed] [Google Scholar]
- Weinstock M (1995) The pharmacotherapy of Alzheimer’s disease based on the cholinergic hypothesis: an update. Neurodegeneration 4:349–356. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM (1986) Purification and characterization of a nicotinic acetylcholine receptor from chick brain. Biochemistry 25:2082–2093. [DOI] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol Pharmacol 82:746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL (2011a) The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137:369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL (2011b) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL (2011c) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, Cohen CJ, Aiyar J (2005) Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J Biol Chem 280:1257–1263. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, Kovera C, McCarten JR (1995) Nicotine patches in Alzheimer’s disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav 51:509–514. [DOI] [PubMed] [Google Scholar]
- Wonnacott S (1986) alpha-Bungarotoxin binds to low-affinity nicotine binding sites in rat brain. J Neurochem 47:1706–1712. [DOI] [PubMed] [Google Scholar]
- Wonnacott S (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci 20:92–98. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Hinchliffe RM (1997) Mecamylamine- or scopolamine-induced learning impairment: ameliorated by nefiracetam. Psychopharmacology (Berl) 131:130–139. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Abdrakhmanova GR, Baydyuk M, Hernandez S, Kellar KJ (2009) Rat neuronal nicotinic acetylcholine receptors containing alpha7 subunit: pharmacological properties of ligand binding and function. Acta Pharmacol Sin 30:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ (2006) Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460. [DOI] [PubMed] [Google Scholar]
- Xue Y, He X, Yang T, Wang Y, Liu Z, Zhang G, Wang Y, Wang K, Zhang L, Zhang L (2019) Discovery of fused heterocyclic carboxamide derivatives as novel α7-nAChR agonists: Synthesis, preliminary SAR and biological evaluation. Eur J Med Chem 182:111618. [DOI] [PubMed] [Google Scholar]
- Yamauchi JGGomez KGrimster NDufouil MNemecz AFotsing JRHo KYTalley TTSharpless KBFokin VV, et al. (2012) Synthesis of selective agonists for the α7 nicotinic acetylcholine receptor with in situ click-chemistry on acetylcholine-binding protein templates. Mol Pharmacol 82:687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Xiao T, Sun Q, Wang K (2017) The current agonists and positive allosteric modulators of α7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B 7:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Zhao X, Wang L, Xie X, Geng H, Zhan X, Teng J (2019) Sevoflurane-induced inflammation development: involvement of cholinergic anti-inflammatory pathway. Behav Pharmacol 30:730–737. [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu R, Cheng W, Hu Y, Li J, Pan X, Peng J, Zhang P (2015) GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-κB signaling pathway through the α7 nicotinic acetylcholine receptor. Int Immunopharmacol 29:504–512. [DOI] [PubMed] [Google Scholar]
- Yum L, Wolf KM, Chiappinelli VA (1996) Nicotinic acetylcholine receptors in separate brain regions exhibit different affinities for methyllycaconitine. Neuroscience 72:545–555. [DOI] [PubMed] [Google Scholar]
- Zamani MR, Allen YS, Owen GP, Gray JA (1997) Nicotine modulates the neurotoxic effect of beta-amyloid protein(25-35)) in hippocampal cultures. Neuroreport 8:513–517. [DOI] [PubMed] [Google Scholar]
- Zanaletti RBettinetti LCastaldo CCeccarelli ICocconcelli GComery TADunlop JGenesio EGhiron CHaydar SN, et al. (2012a) N-[5-(5-fluoropyridin-3-yl)-1H-pyrazol-3-yl]-4-piperidin-1-ylbutyramide (SEN78702, WYE-308775): a medicinal chemistry effort toward an α7 nicotinic acetylcholine receptor agonist preclinical candidate. J Med Chem 55:10277–10281. [DOI] [PubMed] [Google Scholar]
- Zanaletti RBettinetti LCastaldo CCocconcelli GComery TDunlop JGaviraghi GGhiron CHaydar SNJow F, et al. (2012b) Discovery of a novel alpha-7 nicotinic acetylcholine receptor agonist series and characterization of the potent, selective, and orally efficacious agonist 5-(4-acetyl[1,4]diazepan-1-yl)pentanoic acid [5-(4-methoxyphenyl)-1H-pyrazol-3-yl] amide (SEN15924, WAY-361789). J Med Chem 55:4806–4823. [DOI] [PubMed] [Google Scholar]
- Zanetti SR, Ziblat A, Torres NI, Zwirner NW, Bouzat C (2016) Expression and functional role of α7 nicotinic receptor in human cytokine-stimulated natural killer (NK) cells. J Biol Chem 291:16541–16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HHe XWang XYu BZhao SJiao PJin HLiu ZWang KZhang L, et al. (2020a) Design, synthesis and biological activities of piperidine-spirooxadiazole derivatives as α7 nicotinic receptor antagonists. Eur J Med Chem 207:112774. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lu Y, Bian H, Guo L, Zhu H (2017) Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res 9:971–985. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mao G, Zhang Z, Zhang Y, Guo Z, Chen J, Ding W (2020b) Activating α7nAChRs enhances endothelial progenitor cell function partially through the JAK2/STAT3 signaling pathway. Microvasc Res 129:103975. [DOI] [PubMed] [Google Scholar]
- Zhang XYLiu LLiu SHong XChen DCXiu MHYang FDZhang ZZhang XKosten TA, et al. (2012) Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry 169:974–981. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Thio LL, Isenberg KE, Clifford DB (1992) Nicotinic acetylcholine currents in cultured postnatal rat hippocampal neurons. Mol Pharmacol 41:931–936. [PubMed] [Google Scholar]
- Zwart RCarbone ALMoroni MBermudez IMogg AJFolly EABroad LMWilliams ACZhang DDing C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol 73:1838–1843. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, Sher E (2002) 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology 43:374–384. [DOI] [PubMed] [Google Scholar]