Abstract
Purpose
Vagus nerve stimulation (VNS) is an effective adjunctive treatment for drug-resistant epilepsy (DRE) and difficult-to-treat depression (DTD). More than 125.000 patients have been implanted with VNS Therapy® System (LivaNova PLC) since initial approval. Patients with DRE often require magnetic resonance imaging (MRI) of the brain during the course of their disease. VNS Therapy System devices are labeled to allow MRI under certain conditions; however, there are no published comprehensive articles about the real-world experience using MRI in patients with implanted VNS devices.
Methods
A systematic review in accordance with PRISMA statement was performed using PubMed database. Full-length articles reporting MRI (1.5 T or 3 T scanner) of patients with implanted VNS for DRE or DTD and published since 2000 were included. The primary endpoint was a positive outcome that was defined as a technically uneventful MRI scan performed in accordance with the VNS Therapy System manufacturer guidelines and completed according to the researchers’ planned scanning protocol without harm to the patient.
Results
Twenty-six articles were eligible with 25 articles referring to the VNS Therapy System, and 216 patients were included in the analysis. No serious adverse events or serious device-related adverse events were reported. MRI scan was prematurely terminated in one patient due to a panic attack.
Conclusion
This systematic review indicates that cranial MRI of patients with an implanted VNS Therapy System can be completed satisfactorily and is tolerable and safe using 1.5 T and 3 T MRI scanners when performed in adherence to the VNS manufacturer’s guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00234-021-02705-y.
Keywords: Depression, Epilepsy, MRI, Vagus nerve stimulation, VNS, Side effects
Introduction
Epilepsy and depression are prevalent neurological and psychiatric diseases that are often associated with a pharmaco-refractory course, high long-term morbidity, and decreased quality of life [1].
In children and adults, epilepsy is associated with a drug-resistant course in more than 30% of the patients [2]. Patients with DRE [3] are at risk of physical and psychological comorbidities as well as psychosocial problems in addition to their seizure burden. Among the broad spectrum of comorbidities requiring continuous and comprehensive treatment as well as long-term interdisciplinary care, disturbances of cognition, behavior, and communication as well as psychiatric illness are common in children and adults with DRE [4–6]. A variety of brain anomalies, genetic mutations, socio-economic implications, anticonvulsive polytherapy, and inter-ictal epileptiform activity may additionally modify and aggravate the complex course of DRE of various etiologies [3, 4, 6].
In a multi-center trial of 406 patients with epilepsy and primary generalized tonic-clonic seizures and/or focal to bilateral tonic-clonic seizures, 59.6% of patients had experienced at least one seizure-related accidental injury in the last 12 months with the most common being head injuries (35.%) [7]. A quarter of these patients suffered at least one serious injury, and it has been reported that patients with epilepsy are 2.2–4.8 times more likely to die by some type of accident than the standard population [8].
Seizure-associated accidents and injuries further impair quality of life in patients with DRE and may pose an indication for magnetic resonance imaging (MRI). MRI is commonly performed for epilepsy-related injuries or status epilepticus which are major contributors to poor quality of life and mortality. Patients with DRE may also undergo repetitive MRI of the brain (cMRI) for comprehensive pre-surgical evaluation using advanced techniques of MR scanners [9]. Due to the progressive nature of the disease, changes in clinical symptoms may require performing repeated cMRI, direct intracerebral recordings (e.g., stereotactic electroencephalogram [EEG]) [10], functional MRI [11, 12], or novel minimally invasive MRI-guided laser therapies may be needed after VNS implantation [13].
Among adults with depression, more than 25% experience treatment-resistant depression (TRD) that encompasses considerable morbidity and adverse effects on quality of life. TRD comprises failure to respond to two or more antidepressants used at an appropriate dose for an adequate time frame [14]. Since 2001 in the European Union and 2005 in the USA, VNS therapy has also been approved for adjunctive treatment of patients with chronic and recurrent TRD. In addition to standard-of-care treatments with pharmacotherapy, psychotherapy, and electro-convulsive therapy, adjunctive VNS Therapy System has been shown to reduce depressive symptoms, improve quality-of-life, and prevent relapse in patients with TRD [15–17]. Major depressive disorder (MDD) is one of the most diagnosed mental disorders in most first world countries, including Europe, China, and the USA. Structural and functional brain alterations are common in patients with MDD. During the last three decades, MRI has played a critical role in deciphering the pathogenesis of this disorder [18].
Since 1994 in Europe and 1997 in the USA, VNS Therapy System has been an approved and well-accepted adjunctive treatment of patients with drug-resistant epilepsy (DRE). More than 125,000 patients have been implanted with VNS Therapy System (data on file; LivaNova PLC).
VNS Therapy Systems are labeled to allow MRI under certain conditions [19] (Supplementary material); however, no comprehensive real-world experience on the use of MRI in patients with implanted VNS systems is available in the published literature. We conducted this systematic review to analyze information on use and safety of MRI scans in patients with an implanted VNS Therapy System for DRE or TRD.
Methods
Search strategy and article selection
A literature review (systematic review) was conducted till June 2020 using the PubMed database (https://pubmed.ncbi.nlm.nih.gov/advanced/). A search syntax strategy was devised using keywords: (“MRI” OR “fMRI” OR “magnetic resonance”) AND (“VNS” OR “Vagus nerve stimulator” OR “Vagal nerve stimulator” OR “Vagus nerve stimulation”). In the query box of the PubMed Advanced Search Builder, the following search query was used: ((MRI[Title/Abstract]) OR (fMRI[Title/Abstract]) OR (magnetic resonance[Title/Abstract])) AND ((VNS[Title/Abstract]) OR (Vagus nerve stimulator [Title/Abstract]) OR (Vagal nerve stimulator [Title/Abstract]) OR (Vagus nerve stimulation [Title/Abstract])). Articles were included if they were manuscripts published in English and described MRI scan procedures on patients with DRE or TRD and implanted with a VNS device for approved indications. Review articles, nonclinical articles, and articles reporting on scans of patients with transcutaneous VNS (t-VNS) were excluded.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [20].
Data extraction
Each article was searched for clear evidence that patients underwent an MRI procedure with a 1.5 T or 3 T MRI scanner with an implanted VNS Therapy System for DRE or TRD and labeled for conditional MRI scanning. The primary endpoint was a positive outcome that was defined as a technically uneventful MRI scan performed in accordance with the VNS device manufacturer guidelines and completed according to the researchers’ planned scanning protocol without harm to the patient.
Each article was searched for any of the following scanning related adverse events:
Device-related adverse event: Any VNS Therapy System-related non-serious or serious adverse event reported to occur during MRI scanning performed in accordance with manufacturer guideline.
VNS device malfunction: Reported VNS Therapy System device malfunction during or after MRI scanning performed in accordance with manufacturer guidelines. This could include erratic stimulation or generator malfunction, destruction, or necessary re-programing as confirmed by “system diagnostics” after scanning.
Results
Included studies
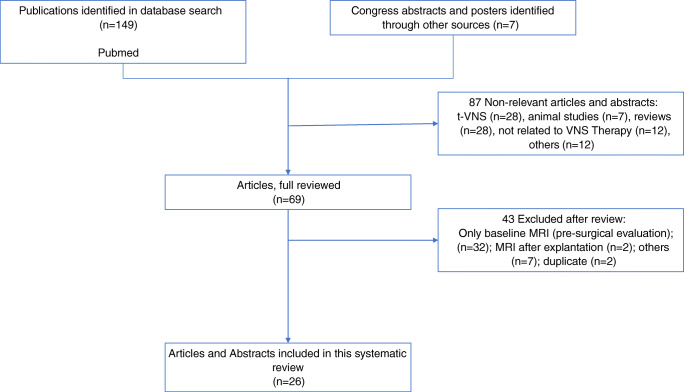
The search strategy yielded 156 publications (Fig. 1). Among these publications, 87 were excluded for not reporting on patients with VNS undergoing MRI scans and not being relevant to for the present analysis (e.g., animal studies; t-VNS; patient receives MRI before VNS implantation; not original research (review); not English; “VNS” has other meaning). A full review of the remaining 69 publications led to 43 publications being excluded for reporting only baseline MRI scans in the context of a pre-surgical evaluation, MRI scans after VNS Therapy System explanation, same patients reported in other articles (duplicates), or other reason (e.g., only positron emission tomography [PET] scans performed). Finally, 26 articles were included in this analysis and included data from 216 patients (Table 1). Not all studied specify the field strength of the MRI scanners used, nevertheless 77 patients were reported getting 1.5 T, and 58 patients received 3 T scans. Some studies described in these articles performed multiple scans on the same patient. The duration of the performed scans (exposure time) was only mentioned in a few articles for fMRI sequences and could not be meaningfully evaluated (Table 2).
Fig. 1.
Search flow leading to articles included in this review
Table 1.
Technical specifications of included MRI studies in patients with DRE and TRD receiving adjunctive VNS therapy (details about used scan sequences are listed in Table 2)
| Study | Purpose | Design | Field strength | # pts | Age range (yrs) | VNS device | VNS on/off | Coil | Scanned region | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients with drug-resistant epilepsy | ||||||||||
| Maniker A et al.; Surg Neurol. 2000 | Technical safety study | Uncontrolled observational. Prospective | 1.5 T | 4 | N/A | Livanova model 100 | On | N/A | N/A | A focus on orientation of implanted generator and “magnet mode”; no MRI-related side effects reported |
| Narayanan JT et al.; Epilepsia 2002 | Evaluation of short-term effects of VNS on brain activation and cerebral blood flow | Uncontrolled observational. Prospective | 1.5 T | 5 | 21–57 | Livanova model 102 | On | Quadrature head coil | Brain | No MRI-related side effects reported |
| Sucholeiki R et al.; Seizure 2002 | Feasibility and safety study of fMRI/BOLD signal intensity from different brain regions during VNS | Uncontrolled observational. Prospective | 1.5 T | 4 | 22–49 | Livanova model 100 | On | T/R head | Brain | No MRI-related side effects reported |
| Beitinjaneh F et al.; Epilepsia 2002 | Elective brain MRI; clinical re-evaluation right mesial temporal sclerosis | Case report | N/A | 1 | 40 | Livanova; model N/A | Off | N/A | N/A | First scan interrupted due to seizure; repeated scan completed; no MRI-related side effects reported |
| Wilfong et al.; Epilepsia 2002 | Off-label use; evaluation of gait disturbances | Case report | 1.5 T | 3 | 5–14 | Livanova model 102 | Off | Body | Spine (cervical and thoracic) | No MRI-related side effects reported |
| Liu WC et al.; J Neurol Neurosurg Psych 2003 | Evaluation of activation of brain regions in the left and right hemisphere due to VNS | Uncontrolled observational. Prospective | 1.5 T | 5 | 26–40 | Livanova model 102 | On | N/A | Brain | No MRI-related side effects reported |
| Tatum WO et al.; Epilepsy Behav. 2004 | Emergency use; electroclinical complex partial status epilepticus of right temporal lobe origin | Case report | 1.5 T | 1 | 21 | Livanova model 102 | Off | T/R head | Brain | No MRI-related side effects reported |
| Roebling R et al.; Epilepsy Res. 2009 | Off-label emergency use; rapidly progressive paraparesis | Case report | 1.5 T | 1 | 72 | Livanova model 101 | Off | Body | Spine (cervical) | No MRI-related side effects reported |
| Gorny KR et al.; J Magn Reson Imaging 2010 | Clinical safety study | Uncontrolled observational. Prospective | 3 T | 17 | N/A | Livanova models 100, 102, and 103 | Off | T/R head | Brain | No MRI-related side effects reported |
| Howell KB et al.; Epilepsia 2012 | Emergency use; acute and chronic phases of FIRES | Uncontrolled observational. Retrospective | 1.5 T or 3 T | 1 | Child | Livanova; model N/A | N/A | N/A | Brain | Patient died due to FIRES; no MRI-related side effects reported |
| Stapleton-Kotloski JR et al.; Fr Neurol. 2014 | Evaluation of localization of interictal epileptiform activity | Uncontrolled observational. Prospective | N/A | 8 | 6–63 | Livanova; model N/A | Off | N/A | Brain | No MRI-related side effects reported |
| de Jonge JC et al.; Epilepsia 2014 | Safety study of epilepsy-related etiology, pre-surgical evaluation; follow-up of tumor pathology; neuronavigation; non-epilepsy-related comorbidities; trauma | Uncontrolled observational. Prospective | 1.5 T and 3 T | 70 | 5–68 | Livanova; model N/A | Off | T/R head Body | Brain; Extremities; Orbita | 4 drop-outs due to abnormal pre-scan device diagnostic (n = 2), battery depletion (n = 1), and VNS explanation (n = 1); suspected post-scan lead break (n = 1) probably not related to MRI; body coil accidentally used (n = 1): no MRI-related side effects reported; scans with 1.5 T (n = 67); scans with 3.0 T (n = 30) |
| Rösch J et al.; Epilepsy Res. 2015 | Re-evaluation and follow-up of epileptogenic lesions in mesial temporal lobe | Uncontrolled observational. Prospective | 3 T | 15 | 26–72 | Livanova model 102 | Off | T/R head | Brain | Exclusion of additional cavernomas; Rasmussen encephalitis follow-up; no MRI-related side effects reported |
| Wang K et al.; Neuropsych Dis Treat. 2016 | Evaluation of resting-state brain network after left parietal-occipital lesion-resection surgery | Case report | 1.5 T | 1 | 17 | PINS Medical model G111 | N/A | N/A | Brain | Not a Livanova generator; no MRI-related side effects reported |
| Jiltsova E et al.; Neuromodulation 2016 | Targeting ANT | Uncontrolled observational. Prospective | 1.5 T | 3 | N/A | Livanova; model N/A | N/A | N/A | Brain | No MRI-related side effects reported |
| Cantarín-Extremera et al.; EU J Paed Neurol. 2016 | Late onset of bradycardia and drop attacks | Case report | N/A | 1 | 12 | Livanova; model N/A | N/A | N/A | N/A | No MRI-related side effects reported |
| Lehner KR et al.; J. Neurosurg. 2018 | MRI-guided LITT of epilepsy generalized or multifocal seizure onsets | Case report | 1.5 T | 1 | 29 | Livanova; model N/A | N/A | 8-Channel head | Brain | No MRI-related side effects reported |
| Casimo K et al.; J. Neurosurg Pediatr. 2018 | Evaluation of preservation of electrophysiological functional connectivity after partial corpus callosotomy | Case report | 1.5 T | 1 | 17 | Livanova; model N/A | Off | N/A | Brain | No MRI-related side effects reported |
| Huang Y et al.; J. Neurosurg Pediatr. 2019 | LITT following stereotactic laser ablation for completion corpus callosotomy | Uncontrolled observational. Retrospective | 3 T | 2 | 11–40 | Livanova; model N/A | N/A | 8-Channel head | Brain | No MRI-related side effects reported |
| Tao et al.; Epilepsia 2020 | LITT following stereotactic laser anterior corpus callosotomy (SLACC) for drop attacks in Lennox-Gastaut syndrome | Uncontrolled observational. Retrospective | 3 T | 9 | Median 33 | Livanova; model N/A | N/A | N/A | Brain | No MRI-related side effects reported |
| Zhu J. et al.; Behav Brain Res. 2020 | Evaluation of VNS effects on spontaneous brain activity in patients with DRE | Uncontrolled observational. Prospective | 3 T | 15 |
Active 19 ± 13; Control 29 ± 3 |
Livanova; model N/A | Off | T/R head | Brain | No MRI-related side effects reported |
| Patients with treatment-resistant depression | ||||||||||
| Bohning DE et al.; Invest Radiol. 2001 | Evaluation of VNS parameter induced BOLD signal changes during synchronized fMRI technique | Uncontrolled observational. Prospective | 1.5 T | 9 | 45 ± 8 | Livanova models 100 and 101 | On | T/R head | Brain | No reports of drop-outs; no MRI-related side effects reported |
| Lomarev M et al.; J. Psychiatr Res. 2002 | Evaluation of VNS parameter induced BOLD signal changes during synchronized fMRI technique | Uncontrolled observational. Prospective | 1.5 T | 9 | 50 ± 6 | Livanova models 100 and 101 | On | T/R head | Brain | Dose-dependent modulating effect of VNS on brain activity; 3 drop-outs due to technical problems: generators did not restart while in the MR scanner; no MRI-related side effects reported; four of the patients participated in a previous study (Bohning et al., 2000). |
| Mu Q et al.; Biol. Psychiatry 2004 | Evaluation of global brain activation due to different VNS parameter | Randomized-controlled. Prospective | 1.5 T | 12 | 48 ± 8 | Livanova model 102 | On | T/R head | Brain | Technical problem with 1 generator: no stimulation signal during scan; 2 patients did not tolerate the scans (not specified); 3 drop-outs |
| Critchley HD et al.; Psychosom Med. 2007 | Evaluation of VNS on emotional memory and its underlying brain activity | Case report | 1.5 T | 1 | 48 | Livanova; model N/A | On | T/R head | Brain | No MRI-related side effects reported; direct modulatory effects |
| Nahas Z et al.; Neuropsychopharm. 2007 | Evaluation of ventro-medial prefrontal cortex deactivation with greater right insula activation | Randomized-controlled. Prospective | 1.5 T | 17 | Adults | Livanova model 102 | On | T/R head | Brain | Panic attack in scanner (n = 1); technical problems: due to build-in “magnet switch” from n = 107 scans, generator not restarting within scanner (n = 11); generator not keeping pre-set duty cycle within scanner (n = 16). |
Abbreviations: FIRES, febrile infection-related epilepsy syndrome; N/A, not available
Table 2.
Addendum to Table 1: technical information about used scan sequences
| Author | Sequences |
|---|---|
|
A. Maniker et al.; Surg Neurol. 2000 [1] |
fMRI; gradient echo EPI; FOV 24 × 24 cm; TR/TE = 2000/60; 4 slices, 5 mm; matrix 64 × 64; 4 VNS cycles = 20min |
| D.E. Bohning et al.; Invest Radiol. 2001 [2] | Multi-slice single-shot gradient echo EPI-fMRI; 64 × 64 matrix; FOV = 270 mm; α = 88°; TE = 40.0 ms, slice thickness = 8.0 mm; gap = 0.0 mm; with fat saturation. 15 contiguous 8 mm thickness axial slices, parallel to AC-PC. T1-weighted structural images (TE = 20 ms, TR = 600 ms) for anatomical reference. |
| J.T. Narayanan et al.; Epilepsia 2002 [3] |
Sag T1-weighted (TR 300/TE 14/1 NEX), axial fast spin echo T2 (TR 3000/TE 91/1 NEX), axial fast FLAIR (TR 10002/TE 172/1 NEX) with inversion time (TI) of 2.2 s, axial T1 (TR 500/TE 14/1 NEX), and axial diffusion-weighted echo planar imaging (TR 6000/TE 99-100/1 NEX) with b values of 0 and 1000; 5-mm thickness; gap of 2.5 mm, a 256 × 192 matrix, the same imaging angle along the orbitomeatal line; FOV = 22 or 24 cm. DWI: 128 × 128 matrix size, 5-mm slice thickness with no gap; FOV = 22 × 22 cm; total acquisition time of 42 s. fMRI: EpiBOLD (echo planar blood oxygenation level dependent); single-shot, gradient-echo, echo planar pulse sequence (TR 3000/TE 40), flip angle of 90°, FOV 22 cm, 64 × 64 matrix (slice thickness, 5 mm with no gap). 18 contiguous slices in an axial oblique plane, parallel to the AC-PC line. After fMRI, a routine T1-weighted imaging using the same axial–oblique prescription (TR 500/TE 12/1NEX) was performed to generate corresponding anatomic images for fMRI. |
| M. Lomarev et al.; J. Psychiatr Res. 2002 [4] | Multi-slice single-shot gradient echo EPI-fMRI; 64 × 64 matrix; FOV = 270 mm; α = 88°; TE = 40.0 ms, slice thickness = 8.0 mm; gap = 0.0 mm; with fat saturation. 15 contiguous 8 mm thickness axial slices, parallel to AC-PC. Also T1-weighted structural images (TE = 20 ms, TR = 600 ms) for anatomical reference. |
| R. Sucholeiki et al.; Seizure 2002 [5] |
High resolution anatomic images, sagittal with the spoiled GRASS pulse sequence with TR = 600 ms, TE = 10 ms, FOV = 24 cm, and matrix size = 256 × 256 fMRI: 12 slices; resting acquisition a time course of images, consisting of 30 s “on” and 30 s “off” for 6 min. Gradient-recalled EPI: TE = 40 ms; FOV = 64 × 64 matrix; 8 mm slice thickness. Other imaging parameters consisted of TR = 2000 ms (flip angle [FA] = 87°); 64 × 64 matrix/8 mm cut thickness yielding voxels of 3.75 × 3.75 × 8 mm. |
| F. Beitinjaneh et al.; Epilepsia 2002 [6] | n/a |
| A.A. Wilfong et al.; Epilepsia 2002 [7] | Standard pulse sequences matrix = 256 × 192 and 2 Nex: (1) axial T1-weighted spin echo; TR = 600; TE = 9; (2) coronal T1-weighted fast spoiled GRASS (FSPGR); 60° flip angle; TR = 115, TE = 3.2; and (3) sagittal T1-weighted SE; TR = 400; TE = 8 |
| Liu WC et al.; J Neurol Neurosurg Psych 2003 [8] |
fMRI/BOLD; T1-weighted co-planar: TR/TE = 550/min; FOV = 24 cm; matrix = 256 × 256; 28 slices 5 mm slice thickness. fMRI: TR/TE = 4000/60 ms; FOV = 24 cm; matrix = 64 × 64; 28 slices 5 mm slice thickness; 3 scans á 5 min 56 s with 30 s pause between each scan. |
| Tatum WO et al.; Epilepsy Behav. 2004 [9] | fMRI; DWI; sagT2; axT2; FLAIR; corT2; TR = 10,000 ms and 8000 ms; TE = 107 ms and 83 ms; FOV 40 × 20 cm; 256 × 256 matrix |
| Mu Q et al.; Biol. Psychiatry 2004 [10] |
fMRI/BOLD T1-weighted sagittal; TR = 625 ms; TE = 20 ms; slice thickness = 5 mm; gap = 1 mm; FOV = 256 mm; # slices = 27; matrix = 256 × 256. Whole brain gradient echo planar imaging (EPI): except for a TR = 2279 ms, TE = 45 ms, 64 × 64 matrix, voxel size of 4 × 4 × 6 mm3. The fMRI session: 13 min and 40 s. |
| Critchley HD et al.; Psychosom Med. 2007 [11] | fMRI; normalized T2*-weighted echo planar; T2*-weighted EPI volumes, 2 mm slice thickness, 1 mm interslice gap, bandwidth 2298 Hz/pixel, matrix 64 × 64, FoV 192 mm, TR/TE=3960/50 ms, isotropic spatial resolution 3 mm, 90° flip angle, 30° tilt of the image slice from axial toward coronal orientation to avoid signal dropouts. |
| Nahas Z et al.; Neuropsychopharm. 2007 [12] | fMRI/BOLD; anatomical T1-weighted sagittal; TR = 625 ms, TE = 20 ms, slice thickness = 5 mm, gap = 1 mm, FOV = 256 mm, number of slices = 27, matrix = 256x256. Whole brain gradient echo planar imaging (EPI): except for a TR = 2837 ms, TE = 45 ms, 128 × 128 matrix, voxel size of 2 × 2 × 6mm3. The fMRI session: 400 images, 18 min and 54 s. |
| Roebling R et al.; Epilepsy Res. 2009 [13] | Sagittal T2-weighted |
| Gorny KR et al.; J Magn Reson Imaging 2010 [14] | Spoiled gradient echo scans with flip angles of 30° and 60° coronal plane; TR = 6000 ms, TE = 15 ms, 36 cm FOV; 256 × 256 matrix; 5-mm-thick slices. 36 sections in 26 min at each flip angle. 3-plane localizer; sagittal T1-FLAIR, coronal T1 GRE (IR FSPGR or MPRAGE); coronal T2 FLAIR, axial T2 FSE or propeller, axial T2 FSE or propeller, GRE T2* |
| Howell KB et al.; Epilepsia 2012 [15] |
Encephalitis protocol; axial and coronal T2-weighted and FLAIR sequences; T1-weighted volumetric sequence reformatted in three orthogonal planes Epilepsy protocol |
| Stapleton-Kotloski JR et al.; Fr Neurol. 2014 [16] | n/a |
| de Jonge JC et al; Epilepsia 2014 [17] | fMRI and others |
| Rösch J et al.; Epilepsy Res. 2015 [18] | T2 tse cor; T2 ax; IR; DWI ax; T2*ax; MPRAGE; T1 cor; 3D FLAIR |
| Wang K et al.; Neuropsych Dis Treat. 2016 [20] | rs-fMRI; T1-weighted 3-D magnetization-prepared rapid gradient-echo sequences and functional imaging (echo-planar imaging sequences) |
| Jiltsova E et al.; Neuromodulation 2016 [21] | MRI examinations with short tau inversion recovery (STIR); T1-weighted magnetization prepared gradient echo (MPRAGE) |
| Cantarín-Extremera et al.; EU J Paed Neurol. 2016 [22] | n/a |
| Lehner KR et al.; J. Neurosurg. 2018 [23] |
DTI: parallel imaging mode with an acceleration factor of 2. A single shot spin echo planar imaging sequence was used, with 5 images obtained without diffusion weighting and 33 isotropically distributed diffusion gradient directions. The b value in the diffusion-weighted images was 1000 s/mm2. The TE was 90.3 ms, and the TR was 14,000 ms, but may have varied up to 14,800 ms in some patients. Images were zero filled to a matrix size of 128 × 128, yielding an image resolution of 0.9 × 0.9 × 3 mm3. From the diffusion-weighted images, maps of fractional anisotropy, mean diffusivity, and V1 images (the main vector of the diffusion tensor) were calculated using FSL software. Resting functional MRI: TR 2000 ms, TE 30 ms, matrix 64 ∗ 64, field of view 240 mm, slice thickness 3 mm, and 40 continuous axial oblique slices (1 voxel = 3.75 × 3.75 × 3 mm). |
| Casimo K et al.; J. Neurosurg Pediatr. 2018 [24] | Anat. MRI and DTI: 20 directions, TR 4.33 s; TE 105 ms, flip angle 90°, slice thickness 4 mm, in-plane resolution 1.56 × 1.56 mm; sagittal T1-weighted MRI: T1-weighted MPRAGE resolution 1.3 × 1.3 mm, 1 mm slice thickness. A 1.5-T MR imager 1.3 mm, 1 mm slice thickness. |
| Huang Y et al.; J. Neurosurg Pediatr. 2019 [25] |
High resolution T1-weighted (3D FSPGR; TE = 3.72 ms; TR = 9.23–9.62 ms, depending on slice coverage; FOV = 240 × 240 mm2; acquisition matrix = 256 × 256; voxel size = 0.94 × 0.94 × 1.00 mm3; orientation = sagittal), T2-weighted (FLAIR, TE = 88.9 ms, TR = 9500 ms, FOV = 240 × 240 mm2, acquisition matrix = 320 × 256, voxel size = 0.94 × 0.94 × 1.00 mm3, orientation = axial) and diffusion-weighted images were acquired as part of the standard clinical care for surgical treatment and planning. DT-EPI sequence using ab value of 1000 s/mm2 sampling 40 isotropically distributed diffusion directions (dir) (40 dir, b = 1000, b0 = 1, TE = 80.70 ms, TR = 15,000 ms, FOV = 260 × 260 mm2, acquisition matrix = 256 × 256, voxel size = 1.02 × 1.02 × 2.50 mm3, number of excitations [NEX] = 1). For cases 4, 5, and 6, diffusion data were acquired with a DTEPI sequence (TE = 60.70 ms, TR = 8000 ms, FOV = 250 × 250 mm2, acquisition matrix = 256 × 256, voxel size = 0.98 × 0.98 × 2.00 mm3, NEX = 1) using a b value of 1000 s/mm2 sampling 30 isotropically distributed diffusion directions. For all patients, one additional volume was acquired at b = 0 at the beginning of each scan. |
| Tao et al.; Epilepsia 2020 [26] | DTI; T1-weighted contrast-enhanced; the number of gradients was 15–32 (median = 32), echo time was 82–136 (median = 96) ms, repetition time was 3.9–9.1 (median = 8.0) seconds, matrix size was 92 × 89 to 128 × 160, slice thickness was 2–5 (median = 2.5) mm, field of view was 224–260 (median = 244) mm, and voxel size was 1.8–2.5 (median = 2.0) mm3. A b-value of 1000 s/mm3 was used in all cases. |
| Zhu J. et al.; Behav Brain Res. 2020 [27] |
rs-fMRI. A: High-resolution three-dimensional turbo fast spin-echo T1WI sequence (T1W 3D-TFE) with the following parameters: repetition time =12 ms, echo time =5.9 ms, flip angle = 8°, matrix = 256 × 256, field of view = 256 × 256 mm, slice thickness = 1.6 mm, gaps = −0.8 mm, slices = 180, scanning time = 5 min 54 s. B: T2WI 3D FLAIR sequence with the following parameters: repetition time = 5000 ms; echo time = 340 ms; matrix = 252 × 290, field of view = 200 × 232 mm, slice thickness = 1.5 mm, gaps = 1.0 mm, slices = 120, scanning time = 6 min 30 s. C: Single-shot echo planar imaging for the BOLD-fMRI sequence, with the following parameters: echo time = 30 ms, repetition time = 2000 ms, flip angle = 90°, field of view = 224 × 224 mm, image matrix=64 × 64, scanning slice thickness = 3.5 mm, slice gaps = 0.5 mm, slices = 34, scanning time = 8 min. |
In 23 of the 26 articles, only cranial MRI (cMRI) was performed. Two studies report on patients receiving spinal MRI and one study includes patients receiving MRI of the extremities. Of the eligible articles, 21 studies evaluated patients with DRE and 5 studies evaluated patients with TRD. Nineteen studies addressed either a technical or clinical research question, two studies addressed MRI in the context of medical emergencies (trauma and febrile infection-related epilepsy syndrome [FIRES]), one study was conducted in the context of a diagnostic workup for bradycardia of unknown origin, and one article reviewed all patients with an implanted VNS receiving MRI at their center for any reason. The remaining 3 studies were published in the last 2 years and addressed MRI scans in the context of laser interstitial thermal therapy (LITT) for DRE.
Of the 26 eligible articles, 25 studies were in patients implanted with VNS Therapy System models from LivaNova PLC, London, UK (before Feb. 2015, Cyberonics, Inc.) and one article presented a case study of a patient implanted with a VNS system from PINS Medical (Beijing, China), which from a design point of view is comparable with the VNS Therapy System. MRI procedures included MRI-guided laser interstitial thermal therapy (LITT) in three patients (Table 1).
Adverse events
None of the included articles reported a serious adverse event or a device-related adverse event. In one patient with TRD, scanning was interrupted due to a panic attack [11], and this was described as an event of mild intensity.
The article by de Jonge et al. reported one patient, in whom high lead impedance was detected post MRI scanning [21]. However, because “system diagnostics” prior to MR scanning in this patient were not performed according to manufacturer guidelines, no temporal relationship between scanning and lead impedance could be established. In a separate article [22], two patients were reported not to tolerate MRI scanning after previous successful MRI scans; the authors did not specify further details.
Three articles reported one or more generators failing to start stimulation when in the magnetic field of the scanner when MRIs were performed contrary to instructions for use in the physician’s manual [11, 22, 23, 28]. As an example, the design of the study by Nahas and colleagues was based upon achieving uninterrupted VNS at programmed device settings during functional magnetic resonance imaging (fMRI). To achieve this, Model 101 VNS Therapy Systems were programmed to continue during MRI. Instructions for use call for programming current output to 0 mA before MRI (Physician’s Manual, 2002). During the study, all Model 101 VNS Therapy Systems performed as designed. This included resetting of stimulation parameters to factory programmed settings when exposed to magnetic and radiofrequency (RF) fields generated during MRI, or movement of the pulse generator’s reed switch when exposed to a static magnetic field to interrupt the programmed Model 101 duty cycle. While this caused inability for the investigators to maintain uninterrupted VNS during fMRI and resulted in a number of scans having to be excluded from the final analysis, no stimulation-related adverse events during fMRI and no unintentional resetting of VNS parameters to a higher output by fMRI were reported [11].
There were no reports of adverse events in children with VNS Therapy System during MRI examinations. In three case reports, the unintentional execution of a full-body MRI in patients implanted with an VNS Therapy System was described, with no reported clinical consequences for the patients [21, 24, 25].
Taken together in this analysis, one non-serious adverse event was reported among all reported patients (0.4%) and was unrelated to the VNS system implanted. No serious adverse events were reported. No VNS Therapy System malfunction was reported when MRI scanning was performed according to instructions for VNS Therapy System use in the physician’s manual.
Discussion
Clinical concerns during MRI scanning of patients with an implanted VNS Therapy Systems are typically focused based on three phenomena during MRI: heating, force, and malfunction. These could be caused by the interaction of the VNS Therapy System with the static fields, gradient fields, and the RF fields generated by the MRI scanner. The static field can exert torque and force on ferromagnetic objects, which could result in displacements; RF and gradient fields could each result in excessive heating, and all three of these fields could either separately or in combination theoretically cause generator vibration and malfunction. Clinically, these would be manifested by the presence of pain or loss of therapy. In addition to these hazards, there is also the possibility of unintended stimulation caused by either the RF or gradient fields.
Laboratory testing focusing on the functional aspects of the VNS Therapy System indicated that MRI procedures performed at 1.5 T and 3 T produced no significant alterations in the operation of the generators (Livanova, data on file). These findings coincide with results described by Shellock and coworkers for the VNS Therapy System studied under in vitro conditions in 1.5 T and 3 T scans [19]. However, Shellock and coworkers identified potentially unsafe conditions with regards to MRI-related heating, therefore MRI scans at 1.5 T and 3 T in patients implanted with VNS Therapy System should only be performed respecting the clearly defined “exclusion zones” in the manufacturers’ guidelines [19, 28].
None of the here reviewed literature on MR imaging studies in patients implanted with the VNS Therapy System did not report any symptoms or signs of pain, local discomfort, or loss of therapy. However, most are brain studies and have scanned fMRI, DTI, and 3D T1 (MP-RAGE or FSPGR), of which all are low SAR sequences. Two studies involving MRI of the spine reporting 4 patient scans in total. Especially, the work of Wilfong [24] where 3 patients underwent MR spine imaging must be assessed critically, as this is an abstract presumably concerns a conference contribution and is not widely available.
Our analysis here has demonstrated that cranial magnetic resonance imaging for soft tissue visualization can be performed safely under appropriate conditions in patients implanted with a VNS Therapy System. Such “MRI conditional” use of MRI means that a VNS Therapy System poses no known hazard in a specified magnetic resonance environment if specific conditions that are described in the physician’s manual are met (Supplemental materials).
MRI scans of the head, neck, and extremities are possible currently with 1.5 T and 3 T MRI scanners. Initial studies have demonstrated that 7 Tesla MRI may improve lesion detection in epilepsy patients [26, 27, 29, 30]. Comprehensive technical assessment will be needed in order to evaluate the safety of MRI scanning at this higher magnetic field strength in patients implanted with a VNS Therapy System.
VNS has been established as an effective, safe, and well-tolerated adjunctive therapeutic option in patients with DRE [31] and TRD [32]. This present systematic review on real-world use of MRI in patients with DRE and TRD has revealed no serious adverse events, device-related adverse events, or VNS Therapy System malfunction when MRI scanning is performed in accordance with VNS Therapy System instructions for use in the physician’s manual (LivaNova PLC, 2019; Supplemental materials).
With the first approval for VNS therapy (1994 EU and 1997 USA, Cyberonics Inc.), MRI scans were only allowed using local transmit/receive (T/R) coils. T/R coils are commonly used only for extremity imaging on modern MRI scanners. Brain MRI scan protocols nowadays often use parallel imaging and cannot be scanned unmodified using a T/R head coil. Since 2017, the MRI guidelines for VNS therapy were expanded and permit the usage of a transmit body coil, together with a receive-only local coil, respecting the International Electrotechnical Commission (IEC 60601-2-33) SAR limits (2 and 3.2 W/kg for whole body and head scans, respectively) under normal mode operation (Group A, Supplemental materials) [28]. According to these guideline extensions, 28 out of 216 patients may have been scanned with transmit body coil.
Limitations
This systematic review must be interpreted with caution as it is retrospective and evaluated a heterogenous mix of patient populations, VNS systems, and MRI techniques and methods. Most of the articles summarized experience that was based upon a small number of patients and had a length of long-term follow-up that differed across studies. These limitations notwithstanding, no safety signal emerged from this systematic literature review of real-world MRI use in patients implanted with a VNS Therapy System when MRI is performed according to instructions for MRI system use (LivaNova PLC, 2019).
Conclusion
This systematic review indicates that cranial MRI of patients with an implanted VNS Therapy System can be completed satisfactorily, is tolerable, and safe using 1.5 T and 3 T scanners, when the manufacturer guidelines are followed for MRI scanning.
Supplementary Information
(PPTX 174 kb)
(PPTX 76 kb)
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Abbreviations
- cMRI
Cranial MRI
- DRE
Drug-resistant epilepsy
- fMRI
Functional magnetic resonance imaging
- LITT
Laser interstitial thermal therapy
- MDD
Major depressive disorder
- MRI
Magnetic resonance imaging
- PRISMA guidelines
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TRD
Treatment-resistant depression
- VNS
Vagus nerve stimulation
- DTD
Difficult-to-treat depression
- RF
Radio frequency
Author’s contributions
All authors contributed to the conception and design of the review. Literature search and analysis were performed by SF, MD, and RT. SF and RT wrote the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Conflicts of interest
SF and MD are employee of LivaNova PLC Deutschland GmbH—a fully owned subsidiary of LivaNova PLC. MD holds stock options in LivaNova PLC. RT and AN have no conflicts of interest to report.
Ethical standards
This work is in compliance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The submission is a review and does not require informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 4.Laurent A, Arzimanoglou A, Panagiotakaki E, Sfaello I, Kahane P, Ryvlin P, Hirsch E, de Schonen S. Visual and auditory socio-cognitive perception in unilateral temporal lobe epilepsy in children and adolescents: a prospective controlled study. Epileptic Disord. 2014;16:456–470. doi: 10.1684/epd.2014.0716. [DOI] [PubMed] [Google Scholar]
- 5.Reilly SK, Yin J, Ayoub AE, et al. Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Sci. 2015;347:1155–1159. doi: 10.1126/science.1260943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrilla AA, Sutton BS, Leinwand BI, Parente A, Ferrari L, Wade CT. Incremental burden of mental health conditions in adult patients with focal seizures. Epilepsy Behav. 2020;112:107426. doi: 10.1016/j.yebeh.2020.107426. [DOI] [PubMed] [Google Scholar]
- 7.Salas-Puig X, Iniesta M, Abraira L, Puig J, Q.-G.s. group Accidental injuries in patients with generalized tonic-clonic seizures. A multicenter, observational, cross-sectional study (QUIN-GTC study) Epilepsy Behav. 2019;92:135–139. doi: 10.1016/j.yebeh.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Trinka E, Bauer G, Oberaigner W, Ndayisaba JP, Seppi K, Granbichler CA. Cause-specific mortality among patients with epilepsy: results from a 30-year cohort study. Epilepsia. 2013;54:495–501. doi: 10.1111/epi.12014. [DOI] [PubMed] [Google Scholar]
- 9.Berger A, Cohen N, Fahoum F, Medvedovsky M, Meller A, Ekstein D, Benifla M, Aizenstein O, Fried I, Gazit T, Strauss I (2020) Preoperative localization of seizure onset zones by magnetic source imaging, EEG-correlated functional MRI, and their combination. J Neurosurg:1–7. 10.3171/2020.3.JNS192794 [DOI] [PubMed]
- 10.Bartolomei F, Bonini F, Vidal E, Trébuchon A, Lagarde S, Lambert I, McGonigal A, Scavarda D, Carron R, Benar CG. How does vagal nerve stimulation (VNS) change EEG brain functional connectivity? Epilepsy Res. 2016;126:141–146. doi: 10.1016/j.eplepsyres.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, Walker J, Anderson B, Koola J, Kose S, Lomarev M, Bohning DE, George MS. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacol. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Xu C, Zhang X, Qiao L, Wang X, Zhang X, Yan X, Ni D, Yu T, Zhang G, Li Y. A resting-state functional MRI study on the effect of vagal nerve stimulation on spontaneous regional brain activity in drug-resistant epilepsy patients. Behav Brain Res. 2020;392:112709. doi: 10.1016/j.bbr.2020.112709. [DOI] [PubMed] [Google Scholar]
- 13.Hong J, Desai A, Thadani VM, Roberts DW. Efficacy and safety of corpus callosotomy after vagal nerve stimulation in patients with drug-resistant epilepsy. J Neurosurg. 2018;128:277–286. doi: 10.3171/2016.10.JNS161841. [DOI] [PubMed] [Google Scholar]
- 14.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacol. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, Moreno FA, Dunner DL, Lesem MD, Thompson PM, Husain M, Vine CJ, Banov MD, Bernstein LP, Lehman RB, Brannon GE, Keepers GA, O'Reardon JP, Rudolph RL, Bunker M. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6:631–640. doi: 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Bottomley JM, LeReun C, Diamantopoulos A, Mitchell S, Gaynes BN. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: a systematic review and meta-analysis. Compr Psychiatry. 2019;98:152156. doi: 10.1016/j.comppsych.2019.152156. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Bunker MT, Aaronson ST, Conway CR, Rothschild AJ, Mordenti G, Rush AJ. Durability of symptomatic responses obtained with adjunctive vagus nerve stimulation in treatment-resistant depression. Neuropsychiatr Dis Treat. 2019;15:457–468. doi: 10.2147/NDT.S196665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo C, Li G, Lin X, Jiang D, Xu Y, Tian H, Wang W, Song X. The rise and fall of MRI studies in major depressive disorder. Transl Psychiatry. 2019;9:335. doi: 10.1038/s41398-019-0680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shellock FG, Begnaud J, Inman DM. Vagus nerve stimulation therapy system: in vitro evaluation of magnetic resonance imaging-related heating and function at 1.5 and 3 Tesla. Neuromodulation. 2006;9:204–213. doi: 10.1111/j.1525-1403.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, P. Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge JC, Melis GI, Gebbink TA, de Kort GA, Leijten FS. Safety of a dedicated brain MRI protocol in patients with a vagus nerve stimulator. Epilepsia. 2014;55:e112–e115. doi: 10.1111/epi.12774. [DOI] [PubMed] [Google Scholar]
- 22.Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, Denslow S, Lomarev M, Moghadam P, Chae JH, George MS. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004;55:816–825. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Lomarev M, Denslow S, Nahas Z, Chae JH, George MS, Bohning DE. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36:219–227. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 24.Wilfong AA. Body MRI and vagus nerve stimulation. Epilepsia. 2002;43(7):347. [Google Scholar]
- 25.Roebling R, Huch K, Kassubek J, Lerche H, Weber Y. Cervical spinal MRI in a patient with a vagus nerve stimulator (VNS) Epilepsy Res. 2009;84:273–275. doi: 10.1016/j.eplepsyres.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Wang I, Oh S, Blumcke I et al (2020) Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 10.1111/epi.16682 [DOI] [PMC free article] [PubMed]
- 27.Feldman RE, Delman BN, Pawha PS, Dyvorne H, Rutland JW, Yoo J, Fields MC, Marcuse LV, Balchandani P. 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One. 2019;14:e0213642. doi: 10.1371/journal.pone.0213642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livanova, PLC, London, UK, MRI with the VNS Therapy® system, physician’s manual, October 2019; 2-26-0010-0300/2 (Worldwide). https://vnstherapy.com/safety-information. Accessed 9 Feb 2021
- 29.Veersema TJ, Ferrier CH, van Eijsden P, Gosselaar PH, Aronica E, Visser F, Zwanenburg JM, de Kort GAP, Hendrikse J, Luijten PR, Braun KPJ. Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. Epilepsia Open. 2017;2:162–171. doi: 10.1002/epi4.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. AJNR Am J Neuroradiol. 2015;36:1204–1215. doi: 10.3174/ajnr.A4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez HFJ, Yengo-Kahn A, Englot DJ. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg Clin N Am. 2019;30:219–230. doi: 10.1016/j.nec.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, Reimherr FW, Schwartz TL, Zajecka JM. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 174 kb)
(PPTX 76 kb)