Abstract
Systematic reviews apply rigorous methodologies to address a pre-specified, clearly formulated clinical research question. The conclusion that results is often cited to more robustly inform decision-making by clinicians, third-party payers and managed care organizations about the clinical question of interest. While systematic reviews provide a rigorous standard, they may be unfeasible when the task is to create general disease-focused guidelines comprised of multiple clinical practice questions versus a single major clinical practice question. Collaborating transplantation and cellular therapy societal committees also recognize that the quantity and or quality of reference sources may be insufficient for a meaningful systematic review. As the conduct of systematic reviews has evolved over time in terms of grading systems, reporting requirements and use of technology, here we provide current guidance in methodologies, resources for reviewers, and approaches to overcome challenges in conducting systematic reviews in transplantation and cellular therapy.
Keywords: Systematic reviews, hematopoietic cell transplantation, cellular therapy
Introduction
Reviews based on a systematic assessment of evidence from the literature help providers make informed clinical decisions and educate third-party payers and managed care organizations about best practices.1,2 These evidence-based, or systematic reviews, include an a priori stated research question, a well-described comprehensive literature search, a justification for the inclusion or exclusion of the available evidence based on independent reviewers, a risk of bias assessment, an appraisal of the quality of collected data, and a systematic presentation and synthesis of the findings of included studies.3–8 If the quality of evidence permits, a quantitative analysis (meta-analysis) may also be performed.9 This systematic approach minimizes individual bias and increases the reliability and quality of the conclusions drawn,8 making systematic reviews the gold standard in evidence-based medicine.10
In 1999, the American Society for Blood and Marrow Transplantation (ASBMT, now the American Society for Transplantation and Cellular Therapy [ASTCT]) launched an initiative to conduct reviews of the role of transplantation in select diseases.1 In 2000, the Evidence-Based Review Steering Committee published the methodology to be followed, with inclusion criteria to be determined by the expert panel, the quality of the body of evidence to be graded based per Shipp et al,11 and the strength of recommendations to be graded per Chalmers et al.12 The methodology was revised in 2005 to incorporate four standard inclusion/exclusion criteria. Studies had to be published in 1990 or later, have a minimum of 70% of subjects with the disease under review or results stratified by the disease, have 25 or more subjects unless exclusion of such studies would profoundly affect treatment recommendations, and meeting abstracts and data from non-peer reviewed journals were to be excluded.13 The Scottish Intercollegiate Guidelines Network (SIGN) guidelines were to be used to assess the levels of evidence and to grade the strength of treatment recommendations; methodology for conducting a meta-analysis was included.13,14 As of 2009, seven reviews had been published,15–21 at which time the Steering Committee recommended that reviews be updated at 5-year intervals.22
Many systematic reviews have been performed by ASTCT, the European Society for Blood and Marrow Transplantation (EBMT), the Center for International Blood and Marrow Transplantation Research (CIBMTR), the National Institutes of Health (NIH), other professional societies, and investigators throughout the world on transplant and cellular therapy (TCT)-related topics using various methodologies. For example, over 50 systematic reviews and meta-analyses were published from January 1, 2018 to July 31, 2020 (MeSH terms: “hematopoietic stem cell transplantation” AND “systematic review” AND “meta-analysis”; MEDLINE search and review independently conducted by AS and LJB).
Since the 2005 ASBMT statement, the conduct of systematic reviews has evolved with the use of new grading systems, adoption of uniform reporting requirements, and development of software programs that facilitate international collaborative reviews. Here we review how the current standards for conducting systematic reviews apply to the field of TCT, provide useful resources for professionals undertaking such reviews, and give guidance on how to overcome common pitfalls along with some practical considerations.
Conduct of a Systematic Review
Suggested steps for conducting the review are shown in Figure 1 with details provided in the following sections. Examples of software and websites that can assist throughout these steps are provided in Table 1.
Figure 1.
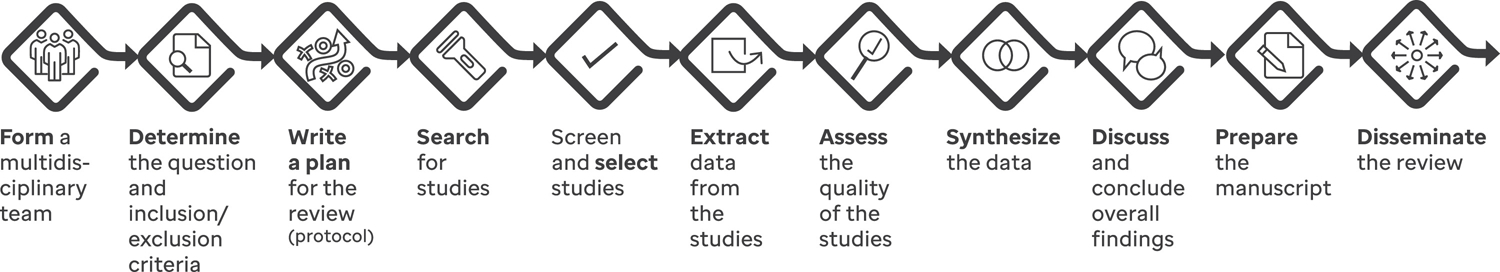
A flow diagram of the various steps involved in developing a systematic review.
Table 1.
Examples of software and websites that can assist in developing and managing a systematic review.
| Product | Cost | Functionality | |||||
|---|---|---|---|---|---|---|---|
| Citation manager | Deduplication | Screening studies | Documentation of review | Data extraction | Statistical analysis | ||
| Abstrackr http://abstrackr.cebm.brown.edu |
Free | × | |||||
| CADIMA https://www.cadima.info |
Free | × | × | × | |||
| Colandr https://www.colandrapp.com |
Free | × | × | × | |||
| Covidence https://www.covidence.org |
Free limited version. Full version requires subscription. | × | × | × | |||
| DistillerSR https://www.evidencepartners.com/products/distillersr-systematic-reviewsoftware/ |
Subscription required | × | × | × | × | ||
| EndNote https://endnote.com |
Free online version lacks some functionality. Desktop version requires subscription. | × | × | × | |||
| EPPI-Reviewer https://eppi.ioe.ac.uk/CMS/Default.aspx?alias=eppi.ioe.ac.uk/cms/er4& |
Subscription required | × | × | × | × | × | |
| Rayyan https://rayyan.qcri.org |
Free | × | × | ||||
| RevMan https://training.cochrane.org/resource/introduction-revman |
Free | × | × | × | × | ||
| Systematic Review Data Repository (SRDR) https://ser.ahrq.gov |
Free | × | × | × | |||
| System for the Unified Management, Assessment and Review of Information (SUMARI) http://www.jbisumari.og |
Subscription required | × | × | × | × | × | |
| Zotero https://www.zotero.org |
Free | × | × | × | |||
Form a multidisciplinary team
Prior to beginning the review process, a multidisciplinary study team should be assembled based on the intended purpose and target audience. Expertise in data search and information retrieval, the clinical topic, and knowledge synthesis methods should be represented in such a team. A library information sciences specialist is an asset to facilitate design of a comprehensive search strategy and enable retrieval of all relevant studies across different databases. Specific expertise in biostatistics should be included if quantitative data synthesis is planned. Ideally, stakeholders linked to the research topic (such as clinicians, patients, caregivers, donors, and policy makers), should be included to enhance usage of review findings6, 23–25 plus their inclusion may be mandated by some funding bodies.26 All team members should disclose relevant conflicts of interest and professional or intellectual biases in order to critically appraise their potential contribution to the team.
Determine the question and inclusion/exclusion criteria
A clear, concise, specific, and answerable research question is critical in developing a high-quality review. In preparation to question development, the team should first evaluate what research has been conducted previously or is ongoing, noting what findings will be relevant to the target audience.27, 28 It may be optimal to update a previous review if there have been considerable advances in the literature or methodological advances.29–34
A common acronym used to refine research questions is PICOS: P stands for population or problem of interest; I for intervention, exposure, or indicator to be evaluated; C for comparison or control group; O for the outcome measure being assessed; and S for study design.25 All five components may not be applicable but considering each will enable the team to refine the question. In TCT, one can define the study population based on standard characteristics such as: (1) demographics including participants’ age, sex, race, and ethnicity; (2) disease-related factors, such as disease status (e.g., in remission or with relapsed or refractory disease) and disease risk (e.g., early, intermediate, high, very high);35–37 and (3) transplant and cell therapy-related factors, including type (e.g., allogeneic or autologous), donor source (e.g., human leukocyte antigen [HLA]-matched related/unrelated donor or haploidentical donor), graft source (e.g., bone marrow, peripheral blood–derived stem cells, or cord blood). Similarly, comparison groups may be broadly (e.g., HLA-matched related graft recipients versus HLA-matched unrelated graft recipients) or more specifically defined (e.g., transplant recipients receiving calcineurin inhibitor–based graft-versus-host disease [GVHD] prophylaxis versus others).
Study inclusion/exclusion criteria should be defined before initiating the literature search in order to be unbiased by the literature available. Any necessary revisions based on practical aspects of the data available following the initial search should be applied consistently throughout the review process.28,38 Criteria should state years of study publication, language restrictions (if applicable) and specific items linked to the research question. Accepted study designs that may best answer the research question should also be predefined.39 Studies may be of varying designs but should be studying the same outcome. Generally, when conducting a systematic review measuring an outcome of an intervention, data from randomized controlled trials (RCT) and quasi-RCT are preferred. However, high-quality non-randomized studies and observational studies may be included as they provide evidence of “real world” data. Case series and case reports are usually excluded because they are associated with a high potential for bias27 but may be included if the research question is related to a rare event or outcome. Review articles, abstracts, meeting presentations, editorials, viewpoints and other data that have not been peer reviewed are typically excluded.
Write a plan for the review (protocol)
The plan for the review should be summarized into a protocol guided by consensus recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.40 The protocol should include relevant background, rationale for conducting the review, the research question, data collection methods (literature search plan, inclusion and exclusion criteria and data extraction methods), risk of bias assessment methods, and planned data synthesis and/or analysis methods. The protocol should be published to maintain transparency, prevent duplicative efforts by other investigators, ensure reproducibility, and reduce bias.6 This can be done through online registration in databases such as PROSPERO,41 Cochrane Library,42 F1000 Research,43 Joanna Briggs Institute,44 Open Science Framework registries,45 or Zenodo46 or published in a peer-reviewed journal. Any protocol amendments should be recorded. An example of such a protocol recently initiated as a collaborative effort between the CIBMTR and the EBMT is available in the PROSPERO database (registration number: CRD42020147640).47
Search for studies
As bibliographic databases differ with respect to their coverage of scholarly disciplines, publication types, dates of publication, journals and books indexed, geographic locations, languages, and search syntaxes, it is a best practice to use more than one database.48 In addition to the commonly used MEDLINE, other medical databases include Embase, Web of Science, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and the Cochrane Library. Most databases require subscriptions; access may be available through academic libraries.
The search strategies should be constructed in collaboration with a library information sciences specialist to ensure that they are well-constructed, thorough and reproducible.49 The team should identify keywords, name variations, synonyms, and subject headings for the key concepts of the research question. Researchers from different institutions, fields of discipline, or geographic locations may use different terminology to describe the same idea; therefore, the search strategy should be as inclusive as possible. For instance, hematopoietic stem cell transplantation may be referred to as HSCT, hematopoietic cell transplantation, bone marrow transplantation, stem cell transplantation, cord blood transplantation, or peripheral blood transplantation. Terms are joined using Boolean operators (AND, OR, NOT). An example of a search strategy in TCT is included as Supplementary Table 1. Search strategies should also be adjusted by using the advanced search features specific to the database searched and peer-reviewed to ensure quality.50 The search strategy should be pilot tested to ensure that the major articles considered relevant are being captured. If key articles are missing, the search string should be modified and retested prior to finalization.
Screen and select studies
Specialized software applications have simplified the process of screening, removing duplications, and selecting studies (Table 1). All references should undergo screening in two stages by at least two independent members of the study team.6,25 The first stage involves screening the title and abstract to exclude articles that clearly do not meet the inclusion criteria. In the second stage, the full text of all potentially eligible articles is reviewed to ensure that the studies meet all inclusion and exclusion criteria. If there are unresolved disagreements, a discussion with a third reviewer may help reach a consensus. For studies chosen for inclusion, the reference list of each article and/or any publications referring to that study can be hand-searched for further relevant studies (the ‘snowball method’). The study flow and the reasons for excluding full-text papers should be documented in an adapted PRISMA flow diagram (a hypothetical example diagram is shown in Figure 2).51, 52
Figure 2.
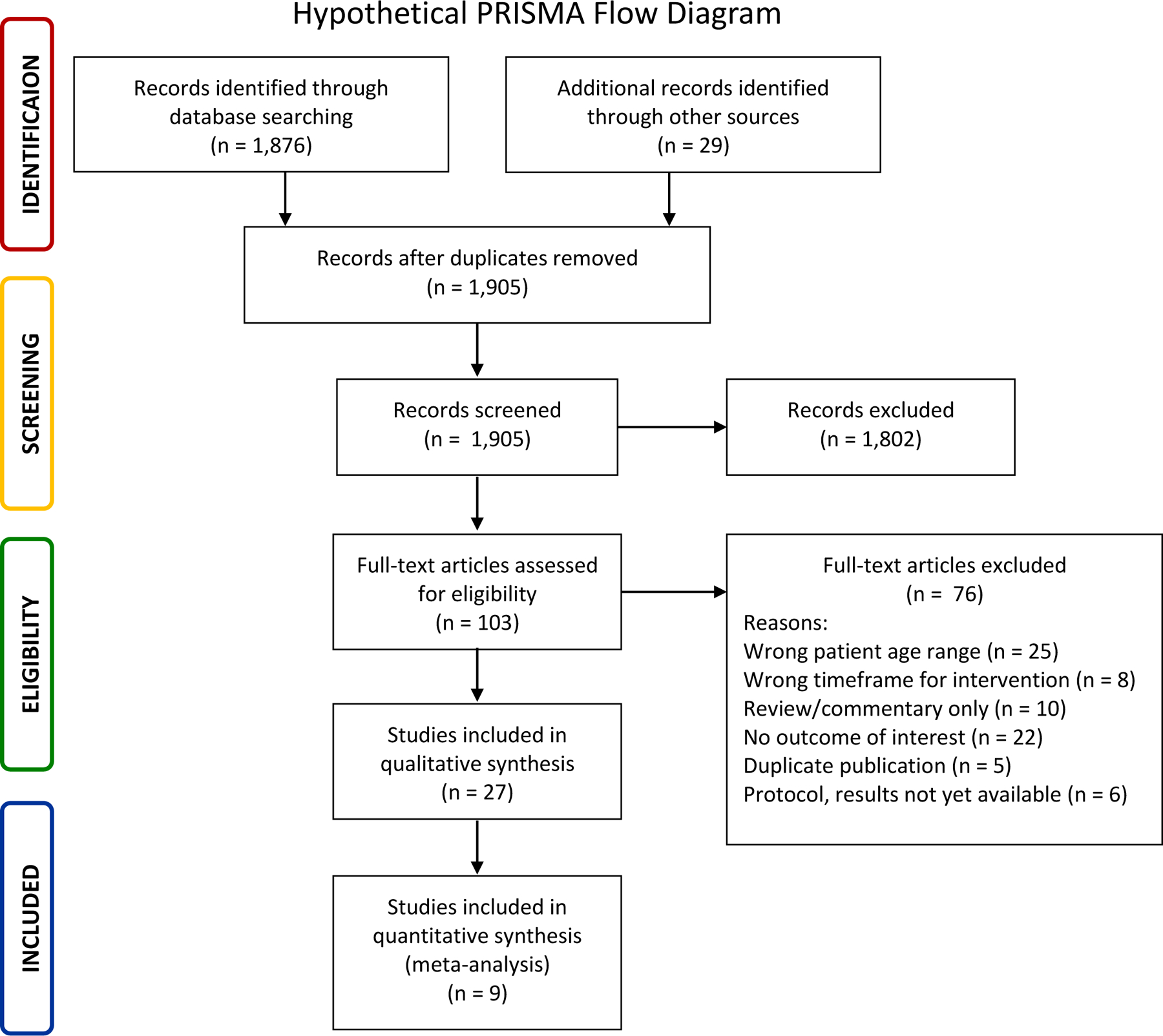
Hypothetical PRISMA flow diagram.
Source: Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.
Extract data from the studies
The use of a standardized data collection form ensures the uniformity of data acquisition. Piloting the form will ensure that all relevant data are collected, and adjustments made to avoid unnecessary details. Free-text fields should be limited, and data coded in pre-defined numerical, fixed-text (yes/no), or drop-down menus or lists to avoid discrepancies and ambiguity at the time of data synthesis. Software applications are available that support the creation of custom data-collection forms (Table 1), although word-processing or spreadsheet software programs may also be used. A minimum of two independent reviewers should collect data from all included studies. The level of inter-rater agreement may be calculated using the kappa statistic and reported.53 Any disagreements should be resolved by discussion with a third reviewer.
Assess the quality of the studies
The quality of evidence and risk of bias of the included studies should be evaluated. Table 2 provides the quality ratings and their definitions of several frameworks that have been used in TCT reviews depending on the scope and research question. Over 100 global organizations have adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE)54–56 framework for evaluating the quality of a body of evidence.57 The GRADE framework has four levels of quality; several factors may impact the quality levels.58 Factors decreasing the quality of a review include inadequate design of included studies, lack of direct evidence, unexplained heterogeneity or inconsistency of results, lack of precision of results or publication bias (i.e., when the outcome influences the decision to publish). Inclusion of several large studies or studies reporting a large magnitude of effect or dose-response gradient in a review increase quality of the evidence. The quality rating may decrease or increase by one level for each factor up to a maximum of three levels. Therefore, the GRADE framework addresses a common challenge reviewers face when assessing the quality of evidence from a wide range of studies with heterogeneity in design, populations, interventions and/or outcomes. While quality of evidence from case reports and case series is very low, they may be incorporated in decision making based on the GRADE approach when no other higher level of evidence is available.58, 59
Table 2.
Frameworks for assessing quality of evidence.
| Framework | Quality rating | Definition |
|---|---|---|
| AHRQ74 | Good | Design and conduct of study addresses risk of bias with appropriate measurement of outcomes and analytic methods |
| Fair | Do not meet criteria for good quality, no flaw likely to cause major bias, missing information often drives rating | |
| Poor | Inappropriate design, conduct, analysis, or reporting | |
| GRADE54–56 | High | Randomized trials; or double-upgraded observational studies. |
| Moderate | Downgraded randomized trials; or upgraded observational studies. | |
| Low | Double-downgraded randomized trials; or observational studies. | |
| Very low | Triple-downgraded randomized trials; or downgraded observational studies; or case series/case reports. | |
| NCCN75 | High | Based upon factors of quality (e.g., trial design and how the results/observations were derives), quantity of data (e.g., number of trials, size of trials, clinical observations only), and consistency of data (e.g., similar or conflicting results across available studies of observations), |
| Lower | ||
| Any | ||
| SIGN14 | 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of RCTs, or RCTs with a low risk of bias | |
| 2++ | High-quality systematic reviews of case-control or cohort studies; high-quality case-control or cohort studies with a very low risk of confound, bias, or chance and a high probability that the relationship is causal. | |
| 2+ | Well-conducted case-control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal. | |
| 2− | Case-control or cohort studies with a high risk of confounding, bias, or chance and a significant risk that the relationship is not causal | |
| 3 | Nonanalytic studies, e.g., case reports of case series | |
| 4 | Expert opinion | |
| USPHS/IDSA76 | I | Evidence from ≥1 properly randomized, controlled trial. |
| II | Evidence for ≥1 well-designed clinical trial without randomization, from cohort or case-controlled analytic studies (preferable from >1 center), or from multiple time series or dramatic results from uncontrolled experiments. | |
| III | Evidence from opinions of respected authorities based on clinical experience, descriptive. |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NCCN, National Comprehensive Cancer Network; RCTs, randomized controlled trials; SIGN, Scottish Intercollegiate Guidelines Network; USPHS/IDSA, United States Public Health Service and Infectious Disease Society of America
Several tools are available for evaluating the risk of bias within a study and across studies. The Cochrane risk-of-bias tool may be used for RCTs,60, 61 ROBINS-I for non-randomized studies of interventions,62 and the Newcastle-Ottawa Scale for observational studies.63–65 Case reports and small case series cannot be assessed for risk of bias.
Synthesize the data
All systematic reviews include a systematic presentation and synthesis of the findings from the studies. Software programs that facilitate the synthesis of data are included in Table 1. Developing a table with similar information for each study may identify patterns and lead to further thematic clustering of studies and tabulation of data.66 Calculating the frequency of different types of results and/or outcomes is another way to create an initial description of the pattern of the included studies, but does not take into account the sample size, the precision level, or the size of the effect.67, 68
If the quality of evidence permits, a quantitative synthesis of the data (meta-analysis) may be performed to evaluate effect-size more accurately.9 A meta-analysis combines results of separate RCTs or other high-quality studies using statistical methods.69 Weighted study effect sizes are used to give greater weight to studies with higher precision.70 Results are usually presented in the form of a forest plot (example shown in Supplementary Figure 1), which not only gives an overview of individual studies but also shows the degree to which they are statistically heterogeneous.71 If there is a statistical heterogeneity among the included studies, a subgroup analysis may be explored.72 Funnel plots are frequently used to detect potential publication bias where studies with negative results or no statistically significant findings are less likely to be published in peer reviewed journals (example shown in Supplementary Figure 2).
Including only randomized or quasi-randomized studies in a systematic review may be possible for a well-studied topic.73 However, it still may be appropriate to perform a meta-analysis with few RCT61 and/or retrospective cohort studies if the quality of the studies is high.65 However, pooling data from poor quality studies into a meta-analysis is not recommended because errors and biases from individual studies will be compounded, yielding misleading results and conclusions.
Discuss and conclude overall findings
Team member discussion is critical to reach valid conclusions and, if relevant, establish the strength of recommendation for an intervention. Multiple frameworks have been used in TCT reviews for assessing strength of a recommendation (Table 3). While GRADE is frequently used,56,57 other frameworks for assessing the strength ofrecommendations may be considered based on the focus and scope of the review.14, 74–76
Table 3.
Frameworks for assessing strength of recommendations.
| Framework | Strength | Definition |
|---|---|---|
| AHRQ74 | High | We are very confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. |
| Moderate | We are moderately confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has some deficiencies. | |
| Low | We have limited confidence that the estimate of effect lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). | |
| Insufficient | We have no evidence, we are unable to estimate an effect, or we have not confidence in the estimate of effect for this outcome. No evidence is available, or the body of evidence has unacceptable deficiencies, precluding reaching a conclusion. | |
| GRADE54–56 | Strong | When the desirable effects of an intervention clearly outweigh the undesirable effects, or clearly do not |
| Weak | When the trade-offs are less certain - either because of low quality evidence or because evidence suggests that desirable and undesirable effects are closely balanced | |
| NCCN75 | 1 | Based on high-level of evidence, there is uniform NCCN consensus that the intervention is appropriate |
| 2A | Based on lower level of evidence, there is uniform NCCN consensus that the intervention is appropriate | |
| 2B | Based on lower level of evidence, there is NCCN consensus that the intervention is appropriate | |
| C | Based on high-level of evidence, there is major NCCN disagreement that the intervention is appropriate | |
| SIGN14 | A | At least 1 meta-analysis, systematic review, or RCT rated as 1++ and directly applicable to the target population or a systematic review of RCTs or a body of evidence consisting principally of studies rated as 1+, directly applicable to the target population, and demonstrating overall consistency of results |
| B | A body of evidence including studies rates as 2++, directly applicable to the target population, and demonstrating overall consistency of results of extrapolated evidence from studies rated as 1++ or 1+ | |
| C | Body of evidence including studies rated as 2+, directly applicable to the target populations, and demonstrating overall consistence of results or extrapolated evidence from studies rated as 2++ | |
| D | Evidence level 3 or 4 or extrapolated evidence from studies rated as 2+. | |
| USPHS/IDSA76 | A | Should always be offered. |
| B | Should generally be offered. | |
| C | Evidence for efficacy is insufficient to support a recommendation for or against, or evidence for efficacy might not outweigh adverse consequences, or cost of the approach. Optional | |
| D | Moderate evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should generally not be offered. | |
| E | Good evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should never be offered. |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NCCN, National Comprehensive Cancer Network; SIGN, Scottish Intercollegiate Guidelines Network; USPHS/IDSA, United States Public Health Service and Infectious Disease Society of America
Factors that affect the strength of a recommendation in GRADE include the quality of the evidence, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patient values and preferences, and uncertainty about whether the intervention represents a wide use of resources. Strong recommendations suggest that all or almost all individuals would choose that intervention, and therefore, it may not be necessary to present several therapeutic options to patients. Weak recommendations imply that there is likely to be important variation in the decision that informed individuals are likely to make, and that engaging patients and families in a shared decision-making process is essential. Recommendations are more likely to be weak rather than strong when the quality of evidence is low, when evidence suggests that desirable and undesirable consequences are closely balanced,58 when there is substantial variation or uncertainty in patient values and preferences, and when interventions require considerable resources.
Prepare the manuscript
The PRISMA statement includes a 27-item checklist that covers the steps of a systematic review and meta-analysis that should be included with reviews submitted for peer review.77 Other specialized guidelines and checklists that may be utilized depending on the review include ENhancing Transparency in REporting the synthesis of Qualitative research (ENTREQ),78 Meta-Analysis Reporting Standards (MARS)79 and Meta-analysis Of Observational Studies in Epidemiology (MOOSE).80
Disseminate the review to enhance uptake of findings
The effective dissemination of study findings has a significant impact on how evidence-based findings are incorporated into clinical practice.81,82 The study team should develop a plan early in the process for dissemination of their findings, including but not limited to, publication in a peer-reviewed journal. Once published, additional aspects of the dissemination plan should be executed, including presentations at conferences, promotion on social media, and distributing the findings in an appropriate format to all other relevant stakeholders, such as policy makers, payers, professional organizations, patients/caregivers and advocates, and hospital administrators. Dissemination of findings can be enhanced by ensuring that multidisciplinary stakeholders are involved from the beginning.
Approaches to Systematic Reviews when there is a Lack of High-Quality Evidence
The study team may consider alternative approaches when the amount or quality of evidence is too low to form conclusions. Scoping reviews are a valid method of summarizing the literature when the literature on a particular topic is very scarce or when reviewers want to address a broad research question.83, 84 This type of review entails the same searching process as a systematic review but does not require a formal evaluation of the quality of studies and risk of bias assessment.
Clinical questions regarding recently introduced therapeutic approaches may be addressed (e.g., clinical practice recommendations for chimeric antigen receptor T cell therapy 85,86 or the literature summarized to serve as a basis for future research (e.g., conditioning intensity in obese patients).87 Another option is to avoid a full formal systematic review but use the modified Delphi method for determining the degree of consensus among experts,88 which may then be combined with a grading system for assigning a strength of the recommendation.89–94
Conclusion
Systematic reviews are critical pieces of medical literature that summarize the totality of evidence to enable sound clinical and policy decisions and identify avenues for future research when applied to a single clinical research question of interest. Transparency of methods throughout their conduct enables reproducibility and a critical assessment of the results by and for all stakeholders. The best practices in methodologic frameworks reviewed here serve as a basis for the conduct of high-quality systematic reviews and meta-analyses in TCT and should be updated as they evolve. The use of evidence-based recommendations, informed by rigorous systematic reviews, can be a powerful approach to improve patients’ health outcomes.
Supplementary Material
Acknowledgements
We thank Keith A. Laycock, PhD, ELS, for scientific editing and Matthew J. Page, PhD for critical review of the manuscript. The National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health supported this publication under Award Number K23HL150232 (S Badawy). Akshay Sharma is the recipient of an American Society of Scholar Award and a paid consultant for Spotlight Therapeutics and CRISPR Therapeutics/Vertex Pharmaceuticals and Novartis. Between 2017 and 2020, Hélène Schoemans has participated in advisory boards for Incyte (2018) and Janssen & Novartis (2020). She has received speaker’s fees from Jazz Pharmaceuticals (2017); Novartis & Incyte (2018) and Incyte, Jazz Pharmaceuticals & Takeda (2019). She has received travel grants from EBMT (2017); EBMT, Celgene & Abbvie (2018); EBMT & Incyte (2019) and EBMT & Gilead (2020). Research funding was also received from Novartis for an investigator-initiated study and consultancy (2020). None of the other authors has a financial disclosure to make. The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the NHLBI and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; HHSH250201700006C, SC1MC31881–01–00 and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014–18–1–2850, N00014–18–1–2888, and N00014–20–1–2705 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612 and BARDA. The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, HRSA or any other agency of the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evidence-based reviews and the role of blood and marrow transplantation in the treatment of selected disease: an ASBMT policy statement. American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2000;6:523. [PubMed] [Google Scholar]
- 2.Jones R, Horowitz M, Wall D, et al. ASBMT policy statement regarding the methodology of evidence-based reviews in evaluating the role of blood and marrow transplantation in the treatment of selected diseases. American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2000;6:524–5. [PubMed] [Google Scholar]
- 3.Schmidt LM, Gotzsche PC. Of mites and men: reference bias in narrative review articles: a systematic review. J Fam Pract 2005;54:334–8. [PubMed] [Google Scholar]
- 4.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 1997;126:376–80. [DOI] [PubMed] [Google Scholar]
- 5.Petticrew M Systematic reviews from astronomy to zoology: myths and misconceptions. BMJ 2001;322:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garritty C, Stevens A, Hamel C, Golfam M, Hutton B, Wolfe D. Knowledge synthesis in evidence-based medicine. Semin Nucl Med 2019;49:136–44. [DOI] [PubMed] [Google Scholar]
- 7.Sargeant JM, O’Connor AM. Introduction to systematic reviews in animal agriculture and veterinary medicine. Zoonoses Public Health 2014;61Suppl 1:3–9. [DOI] [PubMed] [Google Scholar]
- 8.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91–108. [DOI] [PubMed] [Google Scholar]
- 9.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic press; 1985. [Google Scholar]
- 10.OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine Oxford, UK; 2011. [Google Scholar]
- 11.Shipp MA, Abeloff MD, Antman KH, et al. International Consensus Conference on High-Dose Therapy with Hematopoietic Stem Cell Transplantation in Aggressive Non-Hodgkin’s Lymphomas: report of the jury. J Clin Oncol 1999;17:423–9. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers TC, Berrier J, Sacks HS, Levin H, Reitman D, Nagalingam R. Meta-analysis of clinical trials as a scientific discipline. II: Replicate variability and comparison of studies that agree and disagree. Stat Med 1987;6:733–44. [DOI] [PubMed] [Google Scholar]
- 13.Jones R, Nieto Y, Rizzo JD, et al. The evolution of the evidence-based review: evaluating the science enhances the art of medicine--statement of the Steering Committee for Evidence-Based Reviews of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2005;11:819–22. [DOI] [PubMed] [Google Scholar]
- 14.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001;323:334–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn T, Wolff SN, Czuczman M, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of diffuse large cell B-cell non-Hodgkin’s lymphoma: an evidence-based review. Biol Blood Marrow Transplant 2001;7:308–31. [DOI] [PubMed] [Google Scholar]
- 16.Hahn T, Wingard JR, Anderson KC, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: an evidence-based review. Biol Blood Marrow Transplant 2003;9:4–37. [DOI] [PubMed] [Google Scholar]
- 17.Hahn T, Wall D, Camitta B, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 2005;11:823–61. [DOI] [PubMed] [Google Scholar]
- 18.Hahn T, Wall D, Camitta B, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant 2006;12:1–30. [DOI] [PubMed] [Google Scholar]
- 19.Oliansky DM, Rizzo JD, Aplan PD, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 2007;13:1–25. [DOI] [PubMed] [Google Scholar]
- 20.Oliansky DM, Appelbaum F, Cassileth PA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant 2008;14:137–80. [DOI] [PubMed] [Google Scholar]
- 21.Oliansky DM, Antin JH, Bennett JM, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant 2009;15:137–72. [DOI] [PubMed] [Google Scholar]
- 22.Jones RB, Nieto Y, Wall D, et al. Methodology for updating published evidence-based reviews evaluating the role of blood and marrow transplantation in the treatment of selected diseases: a policy statement by the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2009;15:761–2. [DOI] [PubMed] [Google Scholar]
- 23.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:iii–iv, 1–72. [DOI] [PubMed] [Google Scholar]
- 24.Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2009:CD001055. [DOI] [PMC free article] [PubMed]
- 25.University of York NHS Centre for Reviews Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York, UK: Centre for Reviews and Dissemination, University of York; 2009. [Google Scholar]
- 26.Graham ID, Tetroe J, Gagnon M. Lost in translation: just lost or beginning to find our way? Ann Emerg Med 2009;54:313–4; discussion 4. [DOI] [PubMed] [Google Scholar]
- 27.Morton S, Berg A, Levit L, Eden J. Finding what works in health care: standards for systematic reviews: National Academies Press; 2011. [PubMed] [Google Scholar]
- 28.Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Chapter 2: Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 6 ed: Cochrane; 2019. [Google Scholar]
- 29.Oliansky DM, Larson RA, Weisdorf D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukemia: update of the 2006 evidence-based review. Biol Blood Marrow Transplant 2012;18:16–7. [DOI] [PubMed] [Google Scholar]
- 30.Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant 2012;18:505–22. [DOI] [PubMed] [Google Scholar]
- 31.Shah N, Callander N, Ganguly S, et al. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2015;21:1155–66. [DOI] [PubMed] [Google Scholar]
- 32.Oliansky DM, Czuczman M, Fisher RI, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transplant 2011;17:20–47. [DOI] [PubMed] [Google Scholar]
- 33.DeFilipp Z, Advani AS, Bachanova V, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the American Society of Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25:2113–23. [DOI] [PubMed] [Google Scholar]
- 34.Dholaria B, Savani GN, Hamilton BK, et al. Hematopoietic cell transplantation in thetreatment of newly disgnosed adult acute myeloid leukemia: an evidence-based review from the American Society of Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020. 10.1016/j.bbmt.2020.09.020 [DOI]
- 35.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 2008;112:1992–2001. [DOI] [PubMed] [Google Scholar]
- 36.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol 2007;25:4246–54. [DOI] [PubMed] [Google Scholar]
- 37.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014; 123:3665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton S, Berg A, Levit L, Eden J. Standards for Initiating a Systematic Review. Finding what works in health care: standards for systematic reviews. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 39.McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J.. Chapter 3: Defining the criteria for including studies and how that will be grouped for synthesis. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 6 ed: Cochrane; 2019. [Google Scholar]
- 40.Moher D, Shamseer, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRIMSA-P) 2015 statement. Syst Rev. 2015: 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PROSPERO: International prospective register of systematic reviews. (AccessedAug 13, 2020, at https://www.crd.york.ac.uk/prospero/) [DOI] [PMC free article] [PubMed]
- 42.Cochrane Library. (AccessedAug 13, 2020, at https://www.cochranelibrary.com/.)
- 43.F1000 Research. (AccessedAug 13, 2020, at https://f1000research.com/.)
- 44.Joanna Briggs Institute. (AccessedAug 13, 2020, at https://joannabriggs.org/.)
- 45.OSF registries. at https://osf.io/registries.)
- 46.Zenodo (Accessed Aug 13, 2020, at https://zenodo.org/.)
- 47.Male-specific late effects after hematopoietic cell transplantation. 2020. (AccessedAug 13, 2020, at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020147640.)
- 48.Wu YP, Aylward BS, Roberts MC, Evans SC. Searching the scientific literature: implications for quantitative and qualitative reviews. Clin Psychol Rev 2012;32:553–7. [DOI] [PubMed] [Google Scholar]
- 49.Morton S, Berg A, Levit L, Eden J. Standards for Finding and Assessing Individual Studies. Finding what works in health care: standards for systematic reviews. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 50.Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol 2009;62:944–52. [DOI] [PubMed] [Google Scholar]
- 51.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altman DG. Measuring agreement. In: Altman DG, ed. Practical Statistics for Medical Research. London, UK: CRC press; 1991. [Google Scholar]
- 54.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 55.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso-Coello P, Schunemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 58.Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterne JAC, Savovic J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019:366:I44898. [DOI] [PubMed] [Google Scholar]
- 61.Rashidi A, Meybodi MA, Cao W, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation in myelodysplastic syndromes: systematic review and meta-analysis. Biol Blood Marrow Transplant 2020;26:e138–e41. [DOI] [PubMed] [Google Scholar]
- 62.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute. 2014. [Google Scholar]
- 64.Aulakh S, Reljic T, Yassine F, et al. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: A systematic review and meta-analysis. Hematol Oncol Stem Cell Ther 2020. [DOI] [PMC free article] [PubMed]
- 65.Parrondo RD, Reljic T, Iqbal M, et al. Efficacy of autologous and allogeneic hematopoietic cell transplantation in Waldenstrom Macroglobulinemia: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk 2020. [DOI] [PubMed]
- 66.Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme Version 1April2006;1:b92. [Google Scholar]
- 67.Bushman BJ, Wang MC. Vote-counting procedures in meta-analysis. In: Cooper HHL, Valentine JC, eds, ed. The Handbook of Research Synthesis and Meta-Analysis, 2nd ed. New York, NY, US: Russell Sage Foundation; 2009:207–20. [Google Scholar]
- 68.Cwikel J, Behar L, Rabson-Hare J. A comparison of a vote count and a meta-analysis review of intervention research with adult cancer patients. Research on Social Work Practice 2000;10:139–58. [Google Scholar]
- 69.Lipsey MW, Wilson DB. Practical meta-analysis: SAGE publications, Inc; 2001. [Google Scholar]
- 70.Verbeek J, Ruotsalainen J, Hoving JL. Synthesizing study results in a systematic review. Scand J Work Environ Health 2012;38:282–90. [DOI] [PubMed] [Google Scholar]
- 71.Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: an overview with suggestions for practice. Res Synth Methods 2010;1:66–80. [DOI] [PubMed] [Google Scholar]
- 72.Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med 2002;21:1503–11. [DOI] [PubMed] [Google Scholar]
- 73.Lehrnbecher T, Fisher BT, Phillips B, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis 2020;71:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol 2015;68:1312–24. [DOI] [PubMed] [Google Scholar]
- 75.Development and Update of the NCCN Guidelines. (AccessedAug 13, 2020, at https://www.nccn.org/professionals/development.aspx.)
- 76.USPHS/IDSA Prevention of Opportunistic Infections Working Group. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA). MMWR Recomm Rep 1999;48:1–59, 61–6. [PubMed] [Google Scholar]
- 77.Page M, McKenzie J, Bossuty P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. (AccessedOct 18, 2020) at https://osf.io/preprints/metaarxiv/v7gm2 [DOI] [PMC free article] [PubMed]
- 78.Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol 2012;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.APA Publications Communications Board Working Group on Journal Article Reporting Standards. Reporting standards for research in psychology: why do we need them? What might they be? Am Psychol 2008;63:839–51.19086746 [Google Scholar]
- 80.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 81.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003;362:1225–30. [DOI] [PubMed] [Google Scholar]
- 82.Khera N From evidence to clinical practice in blood and marrow transplantation. Blood Rev 2015;29:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 85.Jain T, Bar M, Kansagra AJ, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 2019;25:2305–21. [DOI] [PubMed] [Google Scholar]
- 86.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of chimeric antigen receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant 2019;54:1868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bubalo J, Carpenter PA, Majhail N, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant 2014;20:600–16. [DOI] [PubMed] [Google Scholar]
- 88.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montoto S, Corradini P, Dreyling M, et al. Indications for hematopoietic stem cell transplantation in patients with follicular lymphoma: a consensus project of the EBMT-Lymphoma Working Party. Haematologica 2013;98:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kroger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia 2015;29:2126–33. [DOI] [PubMed] [Google Scholar]
- 91.Kharfan-Dabaja MA, Kumar A, Hamadani M, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2016;22:2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: aninternational collaborative effort on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2017;23:1826–38. [DOI] [PubMed] [Google Scholar]
- 93.Kanate AS, Kumar A, Dreger P, et al. Maintenance therapies for Hodgkin and non-Hodgkin lymphomas after autologous transplantation: a consensus project of ASBMT, CIBMTR, and the Lymphoma Working Party of EBMT. JAMA Oncol 2019;5:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol 2020;7:e157–e67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


